User login
MDedge conference coverage features onsite reporting of the latest study results and expert perspectives from leading researchers.
Food insecurity drives poor glycemic control
People with diabetes who had a poor-quality diet and food insecurity were significantly more likely to have poor glycemic and cholesterol control than were those with a healthier diet and food security, based on data from a national study of more than 2,000 individuals.
The American Diabetes Association recommends a high-quality diet for people with diabetes (PWD) to achieve treatment goals; however, roughly 18% of PWD in the United States are food insecure and/or have a poor-quality diet, Sarah S. Casagrande, PhD, of DLH Corporation, Silver Spring, Md., and colleagues wrote in a poster presented at the annual scientific sessions of the ADA in New Orleans.
To examine the impact of food insecurity and diet quality on diabetes and lipid management, the researchers reviewed data from 2,075 adults with self-reported diabetes who completed the National Health and Nutrition Examination Surveys between 2013 and 2018.
Diet quality was divided into quartiles based on the 2015 Healthy Eating Index. Food insecurity was assessed using a standard 10-item questionnaire including questions about running out of food and not being able to afford more, reducing meal sizes, eating less or not at all, and going hungry because of lack of money for food.
The logistic regression analysis controlled for factors including sociodemographics, health care use, smoking, diabetes medications, blood pressure medication use, cholesterol medication use, and body mass index.
Overall, 17.6% of the participants were food insecure and had a low-quality diet, 14.2% were food insecure with a high-quality diet, 33.1% were food secure with a low-quality diet, and 35.2% were food secure with a high-quality diet.
PWD in the food insecure/low-quality diet group were significantly more likely to be younger, non-Hispanic black or Hispanic, and uninsured compared to those in the food secure/high-quality diet group (P < .001 for all).
When the researchers examined glycemic control, they found that PWD in the food insecurity/low-quality diet groups were significantly more likely than were those with food security/high-quality diets to have hemoglobin A1c of at least 7.0% (adjusted odds ratio, 1.85), A1c of at least 8.0% (aOR, 1.79), low HDL cholesterol (aOR, 1.69), and high triglycerides (aOR, 3.26).
PWD with food insecurity but a high-quality diet also were significantly more likely than were those with food security and a high quality diet to have A1c of at least 7.0% (aOR, 1.69), A1c of at least 8.0% (aOR, 1.83), and high triglycerides (aOR, 2.44). PWD with food security but a low-quality diet were significantly more likely than was the food security/high-quality diet group to have A1c of at least 7% (aOR, 1.55).
The study findings were limited by several factors including the cross-sectional design, reliance on self-reports, and inability to distinguish between type 1 and type 2 diabetes, the researchers wrote.
However, the results were strengthened by the large, nationally representative sample and the inclusion of multiple clinical outcomes in the patient assessment, they said.
The results suggest that food insecurity had a significant impact on both glycemic control and cholesterol management independent of diet quality, the researchers noted. Based on these findings, health care providers treating PWD may wish to assess their patients’ food security status, and “interventions could address disparities in food security,” they concluded.
Food insecurity a growing problem
“With more communities being pushed into state of war, drought, and famine globally, it is important to track impact of food insecurity and low quality food on common medical conditions like diabetes in our vulnerable communities,” Romesh K. Khardori, MD, professor of medicine: endocrinology, and metabolism at Eastern Virginia Medical School, Norfolk, said in an interview.
Dr. Khardori, who was not involved in the study, said he was not surprised by the current study findings.
“Type of food, amount of food, and quality of food have been stressed in diabetes management for more than 100 years,” he said. “Organizations charged with recommendations, such as the ADA and American Dietetic Association, have regularly updated their recommendations,” he noted. “It was not surprising, therefore, to find food insecurity and low quality tied to poor glycemic control.”
The take-home message for clinicians is to consider the availability and quality of food that their patients are exposed to when evaluating barriers to proper glycemic control, Dr. Khardori emphasized.
However, additional research is needed to explore whether the prescription of a sufficient amount of good quality food would alleviate the adverse impact seen in the current study, he said.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers and Dr. Khardori had no financial conflicts to disclose.
People with diabetes who had a poor-quality diet and food insecurity were significantly more likely to have poor glycemic and cholesterol control than were those with a healthier diet and food security, based on data from a national study of more than 2,000 individuals.
The American Diabetes Association recommends a high-quality diet for people with diabetes (PWD) to achieve treatment goals; however, roughly 18% of PWD in the United States are food insecure and/or have a poor-quality diet, Sarah S. Casagrande, PhD, of DLH Corporation, Silver Spring, Md., and colleagues wrote in a poster presented at the annual scientific sessions of the ADA in New Orleans.
To examine the impact of food insecurity and diet quality on diabetes and lipid management, the researchers reviewed data from 2,075 adults with self-reported diabetes who completed the National Health and Nutrition Examination Surveys between 2013 and 2018.
Diet quality was divided into quartiles based on the 2015 Healthy Eating Index. Food insecurity was assessed using a standard 10-item questionnaire including questions about running out of food and not being able to afford more, reducing meal sizes, eating less or not at all, and going hungry because of lack of money for food.
The logistic regression analysis controlled for factors including sociodemographics, health care use, smoking, diabetes medications, blood pressure medication use, cholesterol medication use, and body mass index.
Overall, 17.6% of the participants were food insecure and had a low-quality diet, 14.2% were food insecure with a high-quality diet, 33.1% were food secure with a low-quality diet, and 35.2% were food secure with a high-quality diet.
PWD in the food insecure/low-quality diet group were significantly more likely to be younger, non-Hispanic black or Hispanic, and uninsured compared to those in the food secure/high-quality diet group (P < .001 for all).
When the researchers examined glycemic control, they found that PWD in the food insecurity/low-quality diet groups were significantly more likely than were those with food security/high-quality diets to have hemoglobin A1c of at least 7.0% (adjusted odds ratio, 1.85), A1c of at least 8.0% (aOR, 1.79), low HDL cholesterol (aOR, 1.69), and high triglycerides (aOR, 3.26).
PWD with food insecurity but a high-quality diet also were significantly more likely than were those with food security and a high quality diet to have A1c of at least 7.0% (aOR, 1.69), A1c of at least 8.0% (aOR, 1.83), and high triglycerides (aOR, 2.44). PWD with food security but a low-quality diet were significantly more likely than was the food security/high-quality diet group to have A1c of at least 7% (aOR, 1.55).
The study findings were limited by several factors including the cross-sectional design, reliance on self-reports, and inability to distinguish between type 1 and type 2 diabetes, the researchers wrote.
However, the results were strengthened by the large, nationally representative sample and the inclusion of multiple clinical outcomes in the patient assessment, they said.
The results suggest that food insecurity had a significant impact on both glycemic control and cholesterol management independent of diet quality, the researchers noted. Based on these findings, health care providers treating PWD may wish to assess their patients’ food security status, and “interventions could address disparities in food security,” they concluded.
Food insecurity a growing problem
“With more communities being pushed into state of war, drought, and famine globally, it is important to track impact of food insecurity and low quality food on common medical conditions like diabetes in our vulnerable communities,” Romesh K. Khardori, MD, professor of medicine: endocrinology, and metabolism at Eastern Virginia Medical School, Norfolk, said in an interview.
Dr. Khardori, who was not involved in the study, said he was not surprised by the current study findings.
“Type of food, amount of food, and quality of food have been stressed in diabetes management for more than 100 years,” he said. “Organizations charged with recommendations, such as the ADA and American Dietetic Association, have regularly updated their recommendations,” he noted. “It was not surprising, therefore, to find food insecurity and low quality tied to poor glycemic control.”
The take-home message for clinicians is to consider the availability and quality of food that their patients are exposed to when evaluating barriers to proper glycemic control, Dr. Khardori emphasized.
However, additional research is needed to explore whether the prescription of a sufficient amount of good quality food would alleviate the adverse impact seen in the current study, he said.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers and Dr. Khardori had no financial conflicts to disclose.
People with diabetes who had a poor-quality diet and food insecurity were significantly more likely to have poor glycemic and cholesterol control than were those with a healthier diet and food security, based on data from a national study of more than 2,000 individuals.
The American Diabetes Association recommends a high-quality diet for people with diabetes (PWD) to achieve treatment goals; however, roughly 18% of PWD in the United States are food insecure and/or have a poor-quality diet, Sarah S. Casagrande, PhD, of DLH Corporation, Silver Spring, Md., and colleagues wrote in a poster presented at the annual scientific sessions of the ADA in New Orleans.
To examine the impact of food insecurity and diet quality on diabetes and lipid management, the researchers reviewed data from 2,075 adults with self-reported diabetes who completed the National Health and Nutrition Examination Surveys between 2013 and 2018.
Diet quality was divided into quartiles based on the 2015 Healthy Eating Index. Food insecurity was assessed using a standard 10-item questionnaire including questions about running out of food and not being able to afford more, reducing meal sizes, eating less or not at all, and going hungry because of lack of money for food.
The logistic regression analysis controlled for factors including sociodemographics, health care use, smoking, diabetes medications, blood pressure medication use, cholesterol medication use, and body mass index.
Overall, 17.6% of the participants were food insecure and had a low-quality diet, 14.2% were food insecure with a high-quality diet, 33.1% were food secure with a low-quality diet, and 35.2% were food secure with a high-quality diet.
PWD in the food insecure/low-quality diet group were significantly more likely to be younger, non-Hispanic black or Hispanic, and uninsured compared to those in the food secure/high-quality diet group (P < .001 for all).
When the researchers examined glycemic control, they found that PWD in the food insecurity/low-quality diet groups were significantly more likely than were those with food security/high-quality diets to have hemoglobin A1c of at least 7.0% (adjusted odds ratio, 1.85), A1c of at least 8.0% (aOR, 1.79), low HDL cholesterol (aOR, 1.69), and high triglycerides (aOR, 3.26).
PWD with food insecurity but a high-quality diet also were significantly more likely than were those with food security and a high quality diet to have A1c of at least 7.0% (aOR, 1.69), A1c of at least 8.0% (aOR, 1.83), and high triglycerides (aOR, 2.44). PWD with food security but a low-quality diet were significantly more likely than was the food security/high-quality diet group to have A1c of at least 7% (aOR, 1.55).
The study findings were limited by several factors including the cross-sectional design, reliance on self-reports, and inability to distinguish between type 1 and type 2 diabetes, the researchers wrote.
However, the results were strengthened by the large, nationally representative sample and the inclusion of multiple clinical outcomes in the patient assessment, they said.
The results suggest that food insecurity had a significant impact on both glycemic control and cholesterol management independent of diet quality, the researchers noted. Based on these findings, health care providers treating PWD may wish to assess their patients’ food security status, and “interventions could address disparities in food security,” they concluded.
Food insecurity a growing problem
“With more communities being pushed into state of war, drought, and famine globally, it is important to track impact of food insecurity and low quality food on common medical conditions like diabetes in our vulnerable communities,” Romesh K. Khardori, MD, professor of medicine: endocrinology, and metabolism at Eastern Virginia Medical School, Norfolk, said in an interview.
Dr. Khardori, who was not involved in the study, said he was not surprised by the current study findings.
“Type of food, amount of food, and quality of food have been stressed in diabetes management for more than 100 years,” he said. “Organizations charged with recommendations, such as the ADA and American Dietetic Association, have regularly updated their recommendations,” he noted. “It was not surprising, therefore, to find food insecurity and low quality tied to poor glycemic control.”
The take-home message for clinicians is to consider the availability and quality of food that their patients are exposed to when evaluating barriers to proper glycemic control, Dr. Khardori emphasized.
However, additional research is needed to explore whether the prescription of a sufficient amount of good quality food would alleviate the adverse impact seen in the current study, he said.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers and Dr. Khardori had no financial conflicts to disclose.
FROM ADA 2022
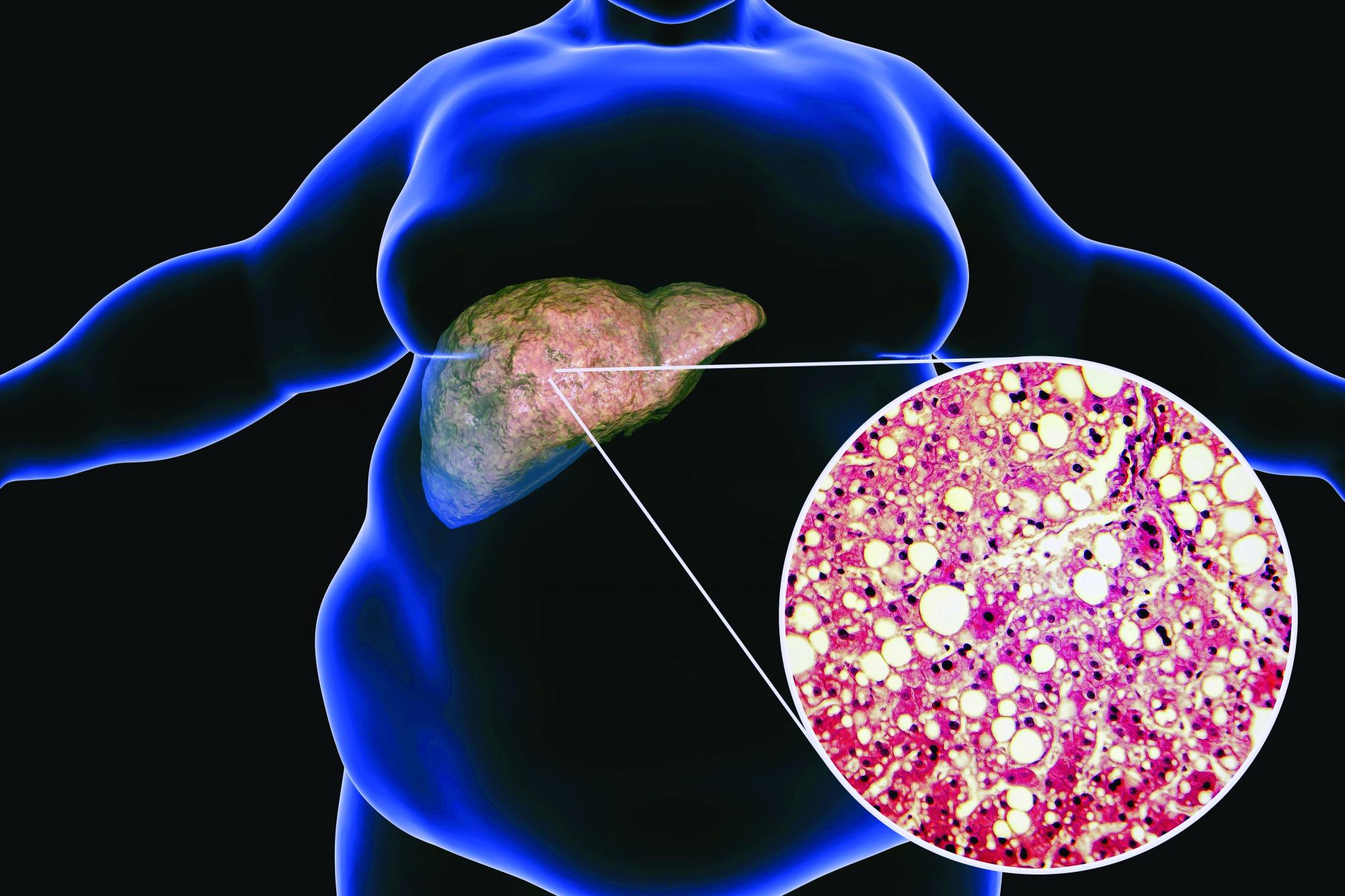
Low-carb, high-fat diet improves A1c, reduces liver fat
LONDON – A low-carbohydrate, high-fat (LCHF) diet reduced the progression of nonalcoholic fatty liver disease (NAFLD), and despite no calorie restriction, participants with both NAFLD and type 2 diabetes lost 5.8% of their body weight, according to a randomized controlled study.
“Based on these results, the LCHF diet may be recommended to people with NAFLD and type 2 diabetes,” said Camilla Dalby Hansen, MD, department of gastroenterology and hepatology, Odense University Hospital, Denmark, who presented the data at the International Liver Congress (ILC) 2022.
“Basically, if you have fat in your liver, you will benefit from eating fat,” she said.
The LCHF diet was compared with a low-fat, high-carbohydrate diet more typically followed for these conditions. The low-fat diet was also found to reduce the progression of NAFLD, but to a lesser extent than the LCHF diet.
Dr. Dalby Hansen called their study one of the most extensive investigations of the LCHF diet in patients with type 2 diabetes and fatty liver disease.
“Combining this [reduction in NAFLD score] with the huge weight loss, the lower HbA1c [blood sugar], the lowering of blood pressure in women, the rise in HDL levels, and reduction in triglycerides – all in all, this diet is very promising,” she said.
Stephen Harrison, MD, visiting professor, University of Oxford, United Kingdom, medical director of Pinnacle Clinical Research and president of Summit Clinical Research, San Antonio, commended Dr. Dalby Hansen on her methodology, which included before-and-after liver biopsies. “It’s a heinous effort to do paired liver biopsies in a lifestyle modification trial. That’s huge.”
“This study tells me that the way we manage patients doesn’t change – it is still lifestyle modification,” said Dr. Harrison, who was not involved with the study. “It’s eat less [rather] than more. It’s exercise and try to lose weight. In the long term, we give patients benefit, and we show that the disease has improved, and we offer something that means they can maintain a healthy life.”
He added that the relatively small and short trial was informative.
“They improved the NAFLD activity score [NAS],” he said. “I don’t know by how much. There was no change in fibrosis, but we wouldn’t expect this at 6 months.”
“It’s provocative work, and it gives us healthy information about how we can help manage our patients from a lifestyle perspective,” he concluded.
‘Do not lose weight. Eat until you are full’
In the study, 110 participants with type 2 diabetes and NAFLD, aged 18-78 years, were allocated to the LCHF diet, and 55 were allocated to the low-fat diet for 6 months.
The researchers performed liver biopsies at baseline and 6 months, which were blinded for scoring.
Participants had ongoing dietitian consultations, with follow-up visits at 3 and 6 months. Compliance was reported continuously through an online food diary platform.
The primary endpoint was change in glycemic control as measured by A1c level over 6 months. The secondary endpoints comprised the proportion of participants with changes in the NAS of at least 2 points over 6 months. Both these measures were compared between the two dietary groups.
The two groups were matched at baseline, with a mean age of 55-57 years, 58% were women, 89% with metabolic syndrome, and a mean BMI 34 kg/m2.
In baseline liver disease, F1 level fibrosis was the most common (58%), followed by hepatic steatosis (S1, 47%; S2, 32%), with a median NAS of 3, and 19% had nonalcoholic steatohepatitis.
The special thing about these diets was that participants were told to “not lose weight, but eat until you are full,” remarked Dr. Dalby Hansen.
Those on the LCHF diet consumed an average of 61% energy from fat, 13% from carbohydrates, and 23% from protein, compared with the low-fat diet, which comprised an average of 29% energy from fat, 46% from carbohydrates, and 21% from protein.
“It’s a lot of fat and corresponds to a quarter of a liter of olive oil per day,” said Dr. Dalby Hansen. “They really had to change their mindset a lot, because it was difficult for them to start eating all these fats, especially since we’ve all been told for decades that it isn’t good. But we supported them, and they got into it.”
The LCHF diet was primarily comprised of unsaturated fats – for example, avocado, oil, nuts, and seeds – but also included saturated fats, such as cheese, cream, and high-fat dairy products. Participants were free to eat unsaturated and saturated fats, but Dr. Dalby Hansen and her team advised participants that “good” unsaturated fats were preferable.
“Also, this diet contained vegetables but no bread, no potatoes, no rice, and no pasta. It was low in carbohydrates, below 20%,” she added.
Improved glycemic control, reduced liver fat
“We found that the LCHF diet improved diabetes control, it reduced the fat in the liver, and, even though they’re eating as many calories as they were used to until they were full, they lost 5.8% of body weight,” said Dr. Dalby Hansen in reporting the results. Participants in the low-fat group lost only 1.8% of body weight.
However, mean calorie intake dropped in both groups, by –2.2% in the LCHF group and –8.7% in the low-fat group.
“The LCHF diet improved the primary outcome of A1c by 9.5 mmol/mol, which is similar to some anti-diabetic medications, such as DPP-4 inhibitors and SGLT2 inhibitors,” she said.
The low-fat group reduced A1c by 3.4 mmol/mol, resulting in a between-group difference of 6.1 mmol/mol.
“Upon follow-up of 3 months, after stopping the diets, on average the participants in both groups returned their HbA1c levels to nearly baseline values,” she said. Results were adjusted for weight loss and baseline values.
Both diets also improved the NAS. The proportion of participants who improved their NAS score by 2 or more points was 22% in the LCHF group versus 17% in the low-fat group (P = 0.58). Additionally, in the LCHF group, 70% of participants improved their score by 1 or more points, compared with 49% in the low-fat group and fewer in the LCHF group experienced a worsening of their score (1% vs. 23%, respectively).
One participant on LCHF had high triglycerides of 12 mmol/L after 3 months. Overall, the low-density lipoprotein increased marginally by 0.2 mmol per liter in the high-fat group, said Dr. Dalby Hansen.
Dr. Dalby Hansen noted some limitations. The findings might not be applicable in more severe NAFLD, dietary assessment relied on self-reporting, no food was provided, and participants had to cook themselves. It was also an open-label study because of the nature of the intervention.
Some hope for more sustainable dieting
Many diets are difficult to adhere to, remarked Dr. Dalby Hansen. “We thought this [diet] might be easier to comply with in the longer term, and we hope that these results might provide patients with more options.”
She added that most people who started the diet adapted and complied with it. “However, it might not be for everyone, but I think we can say that if people try, and it fits into their lives, then they go for it.”
However, “it is not about going out and eating whatever fat and how much of it you want. It’s important that you cut the carbohydrates too,” she said. “With this approach, we really saw amazing results.”
Dr. Dalby Hansen added that having various diets available, including the LCHF one, meant that as clinicians they could empower patients to take control of their metabolic health.
“We can ask them directly, ‘What would fit into their life?’” she said. “We know that one size does not fit at all, and I believe that if we could engage patients more, then they can take control of their own situation.”
Asked whether these findings were enough to change guidelines, Zobair Younossi, MD, professor and chairman, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., remarked that it was the sugar at work here.
“Dietary fat – it’s not the same as fat in the liver, and this diet has more to do with the sugar levels,” he said.
“I’m always reluctant to take results from a short-term study without long-term follow-up,” Dr. Younossi said. “I want to know will patients live longer, and long-term data are needed for this. Until I have that strong evidence that outcomes are going to change, or at least some sign that the outcome is going to change, it is too early to change any guidelines.”
Dr. Dalby Hansen reports no relevant financial relationships. Dr. Harrison reported financial relationships with numerous pharmaceutical companies. Dr. Younossi reports the following financial relationships: research funds and/or consultant to Abbott, Allergan, Bristol Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
LONDON – A low-carbohydrate, high-fat (LCHF) diet reduced the progression of nonalcoholic fatty liver disease (NAFLD), and despite no calorie restriction, participants with both NAFLD and type 2 diabetes lost 5.8% of their body weight, according to a randomized controlled study.
“Based on these results, the LCHF diet may be recommended to people with NAFLD and type 2 diabetes,” said Camilla Dalby Hansen, MD, department of gastroenterology and hepatology, Odense University Hospital, Denmark, who presented the data at the International Liver Congress (ILC) 2022.
“Basically, if you have fat in your liver, you will benefit from eating fat,” she said.
The LCHF diet was compared with a low-fat, high-carbohydrate diet more typically followed for these conditions. The low-fat diet was also found to reduce the progression of NAFLD, but to a lesser extent than the LCHF diet.
Dr. Dalby Hansen called their study one of the most extensive investigations of the LCHF diet in patients with type 2 diabetes and fatty liver disease.
“Combining this [reduction in NAFLD score] with the huge weight loss, the lower HbA1c [blood sugar], the lowering of blood pressure in women, the rise in HDL levels, and reduction in triglycerides – all in all, this diet is very promising,” she said.
Stephen Harrison, MD, visiting professor, University of Oxford, United Kingdom, medical director of Pinnacle Clinical Research and president of Summit Clinical Research, San Antonio, commended Dr. Dalby Hansen on her methodology, which included before-and-after liver biopsies. “It’s a heinous effort to do paired liver biopsies in a lifestyle modification trial. That’s huge.”
“This study tells me that the way we manage patients doesn’t change – it is still lifestyle modification,” said Dr. Harrison, who was not involved with the study. “It’s eat less [rather] than more. It’s exercise and try to lose weight. In the long term, we give patients benefit, and we show that the disease has improved, and we offer something that means they can maintain a healthy life.”
He added that the relatively small and short trial was informative.
“They improved the NAFLD activity score [NAS],” he said. “I don’t know by how much. There was no change in fibrosis, but we wouldn’t expect this at 6 months.”
“It’s provocative work, and it gives us healthy information about how we can help manage our patients from a lifestyle perspective,” he concluded.
‘Do not lose weight. Eat until you are full’
In the study, 110 participants with type 2 diabetes and NAFLD, aged 18-78 years, were allocated to the LCHF diet, and 55 were allocated to the low-fat diet for 6 months.
The researchers performed liver biopsies at baseline and 6 months, which were blinded for scoring.
Participants had ongoing dietitian consultations, with follow-up visits at 3 and 6 months. Compliance was reported continuously through an online food diary platform.
The primary endpoint was change in glycemic control as measured by A1c level over 6 months. The secondary endpoints comprised the proportion of participants with changes in the NAS of at least 2 points over 6 months. Both these measures were compared between the two dietary groups.
The two groups were matched at baseline, with a mean age of 55-57 years, 58% were women, 89% with metabolic syndrome, and a mean BMI 34 kg/m2.
In baseline liver disease, F1 level fibrosis was the most common (58%), followed by hepatic steatosis (S1, 47%; S2, 32%), with a median NAS of 3, and 19% had nonalcoholic steatohepatitis.
The special thing about these diets was that participants were told to “not lose weight, but eat until you are full,” remarked Dr. Dalby Hansen.
Those on the LCHF diet consumed an average of 61% energy from fat, 13% from carbohydrates, and 23% from protein, compared with the low-fat diet, which comprised an average of 29% energy from fat, 46% from carbohydrates, and 21% from protein.
“It’s a lot of fat and corresponds to a quarter of a liter of olive oil per day,” said Dr. Dalby Hansen. “They really had to change their mindset a lot, because it was difficult for them to start eating all these fats, especially since we’ve all been told for decades that it isn’t good. But we supported them, and they got into it.”
The LCHF diet was primarily comprised of unsaturated fats – for example, avocado, oil, nuts, and seeds – but also included saturated fats, such as cheese, cream, and high-fat dairy products. Participants were free to eat unsaturated and saturated fats, but Dr. Dalby Hansen and her team advised participants that “good” unsaturated fats were preferable.
“Also, this diet contained vegetables but no bread, no potatoes, no rice, and no pasta. It was low in carbohydrates, below 20%,” she added.
Improved glycemic control, reduced liver fat
“We found that the LCHF diet improved diabetes control, it reduced the fat in the liver, and, even though they’re eating as many calories as they were used to until they were full, they lost 5.8% of body weight,” said Dr. Dalby Hansen in reporting the results. Participants in the low-fat group lost only 1.8% of body weight.
However, mean calorie intake dropped in both groups, by –2.2% in the LCHF group and –8.7% in the low-fat group.
“The LCHF diet improved the primary outcome of A1c by 9.5 mmol/mol, which is similar to some anti-diabetic medications, such as DPP-4 inhibitors and SGLT2 inhibitors,” she said.
The low-fat group reduced A1c by 3.4 mmol/mol, resulting in a between-group difference of 6.1 mmol/mol.
“Upon follow-up of 3 months, after stopping the diets, on average the participants in both groups returned their HbA1c levels to nearly baseline values,” she said. Results were adjusted for weight loss and baseline values.
Both diets also improved the NAS. The proportion of participants who improved their NAS score by 2 or more points was 22% in the LCHF group versus 17% in the low-fat group (P = 0.58). Additionally, in the LCHF group, 70% of participants improved their score by 1 or more points, compared with 49% in the low-fat group and fewer in the LCHF group experienced a worsening of their score (1% vs. 23%, respectively).
One participant on LCHF had high triglycerides of 12 mmol/L after 3 months. Overall, the low-density lipoprotein increased marginally by 0.2 mmol per liter in the high-fat group, said Dr. Dalby Hansen.
Dr. Dalby Hansen noted some limitations. The findings might not be applicable in more severe NAFLD, dietary assessment relied on self-reporting, no food was provided, and participants had to cook themselves. It was also an open-label study because of the nature of the intervention.
Some hope for more sustainable dieting
Many diets are difficult to adhere to, remarked Dr. Dalby Hansen. “We thought this [diet] might be easier to comply with in the longer term, and we hope that these results might provide patients with more options.”
She added that most people who started the diet adapted and complied with it. “However, it might not be for everyone, but I think we can say that if people try, and it fits into their lives, then they go for it.”
However, “it is not about going out and eating whatever fat and how much of it you want. It’s important that you cut the carbohydrates too,” she said. “With this approach, we really saw amazing results.”
Dr. Dalby Hansen added that having various diets available, including the LCHF one, meant that as clinicians they could empower patients to take control of their metabolic health.
“We can ask them directly, ‘What would fit into their life?’” she said. “We know that one size does not fit at all, and I believe that if we could engage patients more, then they can take control of their own situation.”
Asked whether these findings were enough to change guidelines, Zobair Younossi, MD, professor and chairman, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., remarked that it was the sugar at work here.
“Dietary fat – it’s not the same as fat in the liver, and this diet has more to do with the sugar levels,” he said.
“I’m always reluctant to take results from a short-term study without long-term follow-up,” Dr. Younossi said. “I want to know will patients live longer, and long-term data are needed for this. Until I have that strong evidence that outcomes are going to change, or at least some sign that the outcome is going to change, it is too early to change any guidelines.”
Dr. Dalby Hansen reports no relevant financial relationships. Dr. Harrison reported financial relationships with numerous pharmaceutical companies. Dr. Younossi reports the following financial relationships: research funds and/or consultant to Abbott, Allergan, Bristol Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
LONDON – A low-carbohydrate, high-fat (LCHF) diet reduced the progression of nonalcoholic fatty liver disease (NAFLD), and despite no calorie restriction, participants with both NAFLD and type 2 diabetes lost 5.8% of their body weight, according to a randomized controlled study.
“Based on these results, the LCHF diet may be recommended to people with NAFLD and type 2 diabetes,” said Camilla Dalby Hansen, MD, department of gastroenterology and hepatology, Odense University Hospital, Denmark, who presented the data at the International Liver Congress (ILC) 2022.
“Basically, if you have fat in your liver, you will benefit from eating fat,” she said.
The LCHF diet was compared with a low-fat, high-carbohydrate diet more typically followed for these conditions. The low-fat diet was also found to reduce the progression of NAFLD, but to a lesser extent than the LCHF diet.
Dr. Dalby Hansen called their study one of the most extensive investigations of the LCHF diet in patients with type 2 diabetes and fatty liver disease.
“Combining this [reduction in NAFLD score] with the huge weight loss, the lower HbA1c [blood sugar], the lowering of blood pressure in women, the rise in HDL levels, and reduction in triglycerides – all in all, this diet is very promising,” she said.
Stephen Harrison, MD, visiting professor, University of Oxford, United Kingdom, medical director of Pinnacle Clinical Research and president of Summit Clinical Research, San Antonio, commended Dr. Dalby Hansen on her methodology, which included before-and-after liver biopsies. “It’s a heinous effort to do paired liver biopsies in a lifestyle modification trial. That’s huge.”
“This study tells me that the way we manage patients doesn’t change – it is still lifestyle modification,” said Dr. Harrison, who was not involved with the study. “It’s eat less [rather] than more. It’s exercise and try to lose weight. In the long term, we give patients benefit, and we show that the disease has improved, and we offer something that means they can maintain a healthy life.”
He added that the relatively small and short trial was informative.
“They improved the NAFLD activity score [NAS],” he said. “I don’t know by how much. There was no change in fibrosis, but we wouldn’t expect this at 6 months.”
“It’s provocative work, and it gives us healthy information about how we can help manage our patients from a lifestyle perspective,” he concluded.
‘Do not lose weight. Eat until you are full’
In the study, 110 participants with type 2 diabetes and NAFLD, aged 18-78 years, were allocated to the LCHF diet, and 55 were allocated to the low-fat diet for 6 months.
The researchers performed liver biopsies at baseline and 6 months, which were blinded for scoring.
Participants had ongoing dietitian consultations, with follow-up visits at 3 and 6 months. Compliance was reported continuously through an online food diary platform.
The primary endpoint was change in glycemic control as measured by A1c level over 6 months. The secondary endpoints comprised the proportion of participants with changes in the NAS of at least 2 points over 6 months. Both these measures were compared between the two dietary groups.
The two groups were matched at baseline, with a mean age of 55-57 years, 58% were women, 89% with metabolic syndrome, and a mean BMI 34 kg/m2.
In baseline liver disease, F1 level fibrosis was the most common (58%), followed by hepatic steatosis (S1, 47%; S2, 32%), with a median NAS of 3, and 19% had nonalcoholic steatohepatitis.
The special thing about these diets was that participants were told to “not lose weight, but eat until you are full,” remarked Dr. Dalby Hansen.
Those on the LCHF diet consumed an average of 61% energy from fat, 13% from carbohydrates, and 23% from protein, compared with the low-fat diet, which comprised an average of 29% energy from fat, 46% from carbohydrates, and 21% from protein.
“It’s a lot of fat and corresponds to a quarter of a liter of olive oil per day,” said Dr. Dalby Hansen. “They really had to change their mindset a lot, because it was difficult for them to start eating all these fats, especially since we’ve all been told for decades that it isn’t good. But we supported them, and they got into it.”
The LCHF diet was primarily comprised of unsaturated fats – for example, avocado, oil, nuts, and seeds – but also included saturated fats, such as cheese, cream, and high-fat dairy products. Participants were free to eat unsaturated and saturated fats, but Dr. Dalby Hansen and her team advised participants that “good” unsaturated fats were preferable.
“Also, this diet contained vegetables but no bread, no potatoes, no rice, and no pasta. It was low in carbohydrates, below 20%,” she added.
Improved glycemic control, reduced liver fat
“We found that the LCHF diet improved diabetes control, it reduced the fat in the liver, and, even though they’re eating as many calories as they were used to until they were full, they lost 5.8% of body weight,” said Dr. Dalby Hansen in reporting the results. Participants in the low-fat group lost only 1.8% of body weight.
However, mean calorie intake dropped in both groups, by –2.2% in the LCHF group and –8.7% in the low-fat group.
“The LCHF diet improved the primary outcome of A1c by 9.5 mmol/mol, which is similar to some anti-diabetic medications, such as DPP-4 inhibitors and SGLT2 inhibitors,” she said.
The low-fat group reduced A1c by 3.4 mmol/mol, resulting in a between-group difference of 6.1 mmol/mol.
“Upon follow-up of 3 months, after stopping the diets, on average the participants in both groups returned their HbA1c levels to nearly baseline values,” she said. Results were adjusted for weight loss and baseline values.
Both diets also improved the NAS. The proportion of participants who improved their NAS score by 2 or more points was 22% in the LCHF group versus 17% in the low-fat group (P = 0.58). Additionally, in the LCHF group, 70% of participants improved their score by 1 or more points, compared with 49% in the low-fat group and fewer in the LCHF group experienced a worsening of their score (1% vs. 23%, respectively).
One participant on LCHF had high triglycerides of 12 mmol/L after 3 months. Overall, the low-density lipoprotein increased marginally by 0.2 mmol per liter in the high-fat group, said Dr. Dalby Hansen.
Dr. Dalby Hansen noted some limitations. The findings might not be applicable in more severe NAFLD, dietary assessment relied on self-reporting, no food was provided, and participants had to cook themselves. It was also an open-label study because of the nature of the intervention.
Some hope for more sustainable dieting
Many diets are difficult to adhere to, remarked Dr. Dalby Hansen. “We thought this [diet] might be easier to comply with in the longer term, and we hope that these results might provide patients with more options.”
She added that most people who started the diet adapted and complied with it. “However, it might not be for everyone, but I think we can say that if people try, and it fits into their lives, then they go for it.”
However, “it is not about going out and eating whatever fat and how much of it you want. It’s important that you cut the carbohydrates too,” she said. “With this approach, we really saw amazing results.”
Dr. Dalby Hansen added that having various diets available, including the LCHF one, meant that as clinicians they could empower patients to take control of their metabolic health.
“We can ask them directly, ‘What would fit into their life?’” she said. “We know that one size does not fit at all, and I believe that if we could engage patients more, then they can take control of their own situation.”
Asked whether these findings were enough to change guidelines, Zobair Younossi, MD, professor and chairman, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., remarked that it was the sugar at work here.
“Dietary fat – it’s not the same as fat in the liver, and this diet has more to do with the sugar levels,” he said.
“I’m always reluctant to take results from a short-term study without long-term follow-up,” Dr. Younossi said. “I want to know will patients live longer, and long-term data are needed for this. Until I have that strong evidence that outcomes are going to change, or at least some sign that the outcome is going to change, it is too early to change any guidelines.”
Dr. Dalby Hansen reports no relevant financial relationships. Dr. Harrison reported financial relationships with numerous pharmaceutical companies. Dr. Younossi reports the following financial relationships: research funds and/or consultant to Abbott, Allergan, Bristol Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
AT ILC 2022
At-home colorectal cancer testing and follow-up vary by ethnicity
Doctors were significantly less likely to order colorectal cancer screening with the at-home test Cologuard (Exact Sciences) for Black patients and were more likely to order the test for Asian patients, new evidence reveals.
Investigators retrospectively studied 557,156 patients in the Mayo Clinic health system from 2012 to 2022. They found that Cologuard was ordered for 8.7% of Black patients, compared to 11.9% of White patients and 13.1% of Asian patients.
Both minority groups were less likely than White patients to undergo a follow-up colonoscopy within 1 year of Cologuard testing. Cologuard tests the stool for blood and DNA markers associated with colorectal cancer.
Although the researchers did not examine the reasons driving the disparities, lead investigator Ahmed Ouni, MD, told this news organization that “it could be patient preferences ... or there could be some bias as providers ourselves in how we present the data to patients.”
Dr. Ouni presented the findings on May 22 at the annual Digestive Disease Week® (DDW), held in person in San Diego and virtually.
Breakdown by physician specialty
“We looked at the specialty of physicians ordering these because we wanted to see where the disparity was coming from, if there was a disparity,” said Dr. Ouni, a gastroenterologist at Mayo Clinic, Jacksonville, Florida.
Just over half (51%) of the patients received care from family medicine physicians, 27% received care from internists, and 22% were seen by gastroenterologists.
Family physicians ordered Cologuard testing for 8.7% of Black patients, compared with 16.1% of White patients, a significant difference (P < .001). Internists ordered the test for 10.5% of Black patients and 11.1% of White patients (P < .001). Gastroenterologists ordered Cologuard screening for 2.4% of Black patients and 3.2% of White patients (P = .009).
Gastroenterologists were 47% more likely to order Cologuard for Asian patients, and internists were 16% more likely to order it for this population than for White patients. However, the findings were not statistically significant for the overall cohort of Asian patients when the researchers adjusted for age and sex (P = 0.52).
Black patients were 25% less likely to have a follow-up colonoscopy within 1 year of undergoing a Cologuard test (odds ratio, 0.75; 95% confidence interval, 0.60-0.94), and Asian patients were 35% less likely (OR, 0.65; 95% CI, 0.52-0.82).
Ongoing and future research
Of the total study population, only 2.9% self-identified as Black; according to the 2020 U.S. Census, 12.4% of the population of the United States are Black persons.
When asked about the relatively low proportion of Black persons in the study, Dr. Ouni replied that the investigators are partnering with a Black physician group in the Jacksonville, Fla., area to expand the study to a more diverse population.
Additional plans include assessing how many positive Cologuard test results led to follow-up colonoscopies.
The investigators are also working with family physicians at the Mayo Clinic to examine how physicians explain colorectal cancer screening options to patients and are studying patient preferences regarding screening options, which include Cologuard, fecal immunochemical test (FIT)/fecal occult blood testing, CT colonography, and colonoscopy.
“We’re analyzing the data by ZIP code to see if this could be related to finances,” Dr. Ouni added. “So, if you’re Black or White and more financially impoverished, how does that affect how you view Cologuard and colorectal cancer screening?”
Some unanswered questions
“Overall this study supports other studies of a disparity in colorectal cancer screening for African Americans,” John M. Carethers, MD, told this news organization when asked to comment. “This is known for FIT and colonoscopy, and Cologuard, which is a genetic test in addition to FIT, appears to be in that same realm.”
“Noninvasive tests will have a role to reach populations who may not readily have access to colonoscopy,” said Dr. Carethers, John G. Searle Professor and chair of the department of internal medicine and professor of human genetics at the University of Michigan, Ann Arbor, and president of the American Gastroenterological Association. “The key here is if the test is positive, it needs to be followed up with a colonoscopy.”
Dr. Carethers added that the study raises some unanswered questions; for example, does the cost difference between testing options make a difference?
“FIT is under $20, but Cologuard is generally $300 or more,” he said. What percentage of the study population were offered other options, such as FIT? How does insurance status affect screening in different populations?”
“The findings should be taken in context of what other screening options were offered to or elected by patients,” agreed Gregory S. Cooper, MD, professor of medicine and population and quantitative health sciences at Case Western Reserve University and a gastroenterologist at University Hospitals Cleveland Medical Center.
According to guidelines, patients can be offered a menu of options, including FIT, colonoscopy, and Cologuard, Dr. Cooper said in an interview.
“If more African Americans elected colonoscopy, for example, the findings may balance out,” said Dr. Cooper, who was not affiliated with the study. “It would also be of interest to know if the racial differences changed over time. With the pandemic, the use of noninvasive options, such as Cologuard, have increased.”
“I will note that specifically for colonoscopy in the United States, the disparity gap had been closing from about 15% to 18% 20 years ago to about 3% in 2020 pre-COVID,” Dr. Carethers added. “I am fearful that COVID may have led to a widening of that gap again as we get more data.”
“It is important that noninvasive tests for screening be a part of the portfolio of offerings to patients, as about 35% of eligible at-risk persons who need to be screened are not screened in the United States,” Dr. Carethers said.
The study was not industry sponsored. Dr. Ouni and Dr. Carethers report no relevant financial relationships. Dr. Cooper has received consulting fees from Exact Sciences.
To help your patients understand their colorectal cancer screening options, send them to the AGA GI Patient Center.
A version of this article first appeared on Medscape.com.
Doctors were significantly less likely to order colorectal cancer screening with the at-home test Cologuard (Exact Sciences) for Black patients and were more likely to order the test for Asian patients, new evidence reveals.
Investigators retrospectively studied 557,156 patients in the Mayo Clinic health system from 2012 to 2022. They found that Cologuard was ordered for 8.7% of Black patients, compared to 11.9% of White patients and 13.1% of Asian patients.
Both minority groups were less likely than White patients to undergo a follow-up colonoscopy within 1 year of Cologuard testing. Cologuard tests the stool for blood and DNA markers associated with colorectal cancer.
Although the researchers did not examine the reasons driving the disparities, lead investigator Ahmed Ouni, MD, told this news organization that “it could be patient preferences ... or there could be some bias as providers ourselves in how we present the data to patients.”
Dr. Ouni presented the findings on May 22 at the annual Digestive Disease Week® (DDW), held in person in San Diego and virtually.
Breakdown by physician specialty
“We looked at the specialty of physicians ordering these because we wanted to see where the disparity was coming from, if there was a disparity,” said Dr. Ouni, a gastroenterologist at Mayo Clinic, Jacksonville, Florida.
Just over half (51%) of the patients received care from family medicine physicians, 27% received care from internists, and 22% were seen by gastroenterologists.
Family physicians ordered Cologuard testing for 8.7% of Black patients, compared with 16.1% of White patients, a significant difference (P < .001). Internists ordered the test for 10.5% of Black patients and 11.1% of White patients (P < .001). Gastroenterologists ordered Cologuard screening for 2.4% of Black patients and 3.2% of White patients (P = .009).
Gastroenterologists were 47% more likely to order Cologuard for Asian patients, and internists were 16% more likely to order it for this population than for White patients. However, the findings were not statistically significant for the overall cohort of Asian patients when the researchers adjusted for age and sex (P = 0.52).
Black patients were 25% less likely to have a follow-up colonoscopy within 1 year of undergoing a Cologuard test (odds ratio, 0.75; 95% confidence interval, 0.60-0.94), and Asian patients were 35% less likely (OR, 0.65; 95% CI, 0.52-0.82).
Ongoing and future research
Of the total study population, only 2.9% self-identified as Black; according to the 2020 U.S. Census, 12.4% of the population of the United States are Black persons.
When asked about the relatively low proportion of Black persons in the study, Dr. Ouni replied that the investigators are partnering with a Black physician group in the Jacksonville, Fla., area to expand the study to a more diverse population.
Additional plans include assessing how many positive Cologuard test results led to follow-up colonoscopies.
The investigators are also working with family physicians at the Mayo Clinic to examine how physicians explain colorectal cancer screening options to patients and are studying patient preferences regarding screening options, which include Cologuard, fecal immunochemical test (FIT)/fecal occult blood testing, CT colonography, and colonoscopy.
“We’re analyzing the data by ZIP code to see if this could be related to finances,” Dr. Ouni added. “So, if you’re Black or White and more financially impoverished, how does that affect how you view Cologuard and colorectal cancer screening?”
Some unanswered questions
“Overall this study supports other studies of a disparity in colorectal cancer screening for African Americans,” John M. Carethers, MD, told this news organization when asked to comment. “This is known for FIT and colonoscopy, and Cologuard, which is a genetic test in addition to FIT, appears to be in that same realm.”
“Noninvasive tests will have a role to reach populations who may not readily have access to colonoscopy,” said Dr. Carethers, John G. Searle Professor and chair of the department of internal medicine and professor of human genetics at the University of Michigan, Ann Arbor, and president of the American Gastroenterological Association. “The key here is if the test is positive, it needs to be followed up with a colonoscopy.”
Dr. Carethers added that the study raises some unanswered questions; for example, does the cost difference between testing options make a difference?
“FIT is under $20, but Cologuard is generally $300 or more,” he said. What percentage of the study population were offered other options, such as FIT? How does insurance status affect screening in different populations?”
“The findings should be taken in context of what other screening options were offered to or elected by patients,” agreed Gregory S. Cooper, MD, professor of medicine and population and quantitative health sciences at Case Western Reserve University and a gastroenterologist at University Hospitals Cleveland Medical Center.
According to guidelines, patients can be offered a menu of options, including FIT, colonoscopy, and Cologuard, Dr. Cooper said in an interview.
“If more African Americans elected colonoscopy, for example, the findings may balance out,” said Dr. Cooper, who was not affiliated with the study. “It would also be of interest to know if the racial differences changed over time. With the pandemic, the use of noninvasive options, such as Cologuard, have increased.”
“I will note that specifically for colonoscopy in the United States, the disparity gap had been closing from about 15% to 18% 20 years ago to about 3% in 2020 pre-COVID,” Dr. Carethers added. “I am fearful that COVID may have led to a widening of that gap again as we get more data.”
“It is important that noninvasive tests for screening be a part of the portfolio of offerings to patients, as about 35% of eligible at-risk persons who need to be screened are not screened in the United States,” Dr. Carethers said.
The study was not industry sponsored. Dr. Ouni and Dr. Carethers report no relevant financial relationships. Dr. Cooper has received consulting fees from Exact Sciences.
To help your patients understand their colorectal cancer screening options, send them to the AGA GI Patient Center.
A version of this article first appeared on Medscape.com.
Doctors were significantly less likely to order colorectal cancer screening with the at-home test Cologuard (Exact Sciences) for Black patients and were more likely to order the test for Asian patients, new evidence reveals.
Investigators retrospectively studied 557,156 patients in the Mayo Clinic health system from 2012 to 2022. They found that Cologuard was ordered for 8.7% of Black patients, compared to 11.9% of White patients and 13.1% of Asian patients.
Both minority groups were less likely than White patients to undergo a follow-up colonoscopy within 1 year of Cologuard testing. Cologuard tests the stool for blood and DNA markers associated with colorectal cancer.
Although the researchers did not examine the reasons driving the disparities, lead investigator Ahmed Ouni, MD, told this news organization that “it could be patient preferences ... or there could be some bias as providers ourselves in how we present the data to patients.”
Dr. Ouni presented the findings on May 22 at the annual Digestive Disease Week® (DDW), held in person in San Diego and virtually.
Breakdown by physician specialty
“We looked at the specialty of physicians ordering these because we wanted to see where the disparity was coming from, if there was a disparity,” said Dr. Ouni, a gastroenterologist at Mayo Clinic, Jacksonville, Florida.
Just over half (51%) of the patients received care from family medicine physicians, 27% received care from internists, and 22% were seen by gastroenterologists.
Family physicians ordered Cologuard testing for 8.7% of Black patients, compared with 16.1% of White patients, a significant difference (P < .001). Internists ordered the test for 10.5% of Black patients and 11.1% of White patients (P < .001). Gastroenterologists ordered Cologuard screening for 2.4% of Black patients and 3.2% of White patients (P = .009).
Gastroenterologists were 47% more likely to order Cologuard for Asian patients, and internists were 16% more likely to order it for this population than for White patients. However, the findings were not statistically significant for the overall cohort of Asian patients when the researchers adjusted for age and sex (P = 0.52).
Black patients were 25% less likely to have a follow-up colonoscopy within 1 year of undergoing a Cologuard test (odds ratio, 0.75; 95% confidence interval, 0.60-0.94), and Asian patients were 35% less likely (OR, 0.65; 95% CI, 0.52-0.82).
Ongoing and future research
Of the total study population, only 2.9% self-identified as Black; according to the 2020 U.S. Census, 12.4% of the population of the United States are Black persons.
When asked about the relatively low proportion of Black persons in the study, Dr. Ouni replied that the investigators are partnering with a Black physician group in the Jacksonville, Fla., area to expand the study to a more diverse population.
Additional plans include assessing how many positive Cologuard test results led to follow-up colonoscopies.
The investigators are also working with family physicians at the Mayo Clinic to examine how physicians explain colorectal cancer screening options to patients and are studying patient preferences regarding screening options, which include Cologuard, fecal immunochemical test (FIT)/fecal occult blood testing, CT colonography, and colonoscopy.
“We’re analyzing the data by ZIP code to see if this could be related to finances,” Dr. Ouni added. “So, if you’re Black or White and more financially impoverished, how does that affect how you view Cologuard and colorectal cancer screening?”
Some unanswered questions
“Overall this study supports other studies of a disparity in colorectal cancer screening for African Americans,” John M. Carethers, MD, told this news organization when asked to comment. “This is known for FIT and colonoscopy, and Cologuard, which is a genetic test in addition to FIT, appears to be in that same realm.”
“Noninvasive tests will have a role to reach populations who may not readily have access to colonoscopy,” said Dr. Carethers, John G. Searle Professor and chair of the department of internal medicine and professor of human genetics at the University of Michigan, Ann Arbor, and president of the American Gastroenterological Association. “The key here is if the test is positive, it needs to be followed up with a colonoscopy.”
Dr. Carethers added that the study raises some unanswered questions; for example, does the cost difference between testing options make a difference?
“FIT is under $20, but Cologuard is generally $300 or more,” he said. What percentage of the study population were offered other options, such as FIT? How does insurance status affect screening in different populations?”
“The findings should be taken in context of what other screening options were offered to or elected by patients,” agreed Gregory S. Cooper, MD, professor of medicine and population and quantitative health sciences at Case Western Reserve University and a gastroenterologist at University Hospitals Cleveland Medical Center.
According to guidelines, patients can be offered a menu of options, including FIT, colonoscopy, and Cologuard, Dr. Cooper said in an interview.
“If more African Americans elected colonoscopy, for example, the findings may balance out,” said Dr. Cooper, who was not affiliated with the study. “It would also be of interest to know if the racial differences changed over time. With the pandemic, the use of noninvasive options, such as Cologuard, have increased.”
“I will note that specifically for colonoscopy in the United States, the disparity gap had been closing from about 15% to 18% 20 years ago to about 3% in 2020 pre-COVID,” Dr. Carethers added. “I am fearful that COVID may have led to a widening of that gap again as we get more data.”
“It is important that noninvasive tests for screening be a part of the portfolio of offerings to patients, as about 35% of eligible at-risk persons who need to be screened are not screened in the United States,” Dr. Carethers said.
The study was not industry sponsored. Dr. Ouni and Dr. Carethers report no relevant financial relationships. Dr. Cooper has received consulting fees from Exact Sciences.
To help your patients understand their colorectal cancer screening options, send them to the AGA GI Patient Center.
A version of this article first appeared on Medscape.com.
Acute hepatitis cases in children show declining trend; adenovirus, COVID-19 remain key leads
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2022
Pemvidutide promising for fatty liver disease
LONDON – Weight loss, lipid reductions, and “robust improvements” in lipid species associated with nonalcoholic fatty liver disease were achieved in patients who were treated with pemvidutide in a first-in-human, phase 1 clinical trial reported at the annual International Liver Congress, sponsored by the European Association for the Study of the Liver.
The presenting study investigator, Stephen A. Harrison, MD, said that pemvidutide, which is also being developed for the treatment of obesity, appeared to be well tolerated. There were no serious or severe adverse events, and no patient had to discontinue treatment because of side effects.
Overall, “pemvidutide represents a promising new agent,” said Dr. Harrison, medical director of Pinnacle Research in San Antonio, Texas.
Dual incretin effect
Pemvidutide is a “balanced” dual agonist of glucagon-like peptide 1 (GLP-1) and glucagon, Dr. Harrison explained in his oral abstract.
“With glucagon, we are working to drive energy expenditure up, and with GLP-1, we’re decreasing food intake,” Dr. Harrison said.
What might set pemvidutide apart from other incretins lies within its structure, Dr. Harrison suggested. The structure has two main regions – one with greater GLP-1 specificity and the other with greater glucagon specificity, and these two areas are linked by a propriety technology called a EuPort™ domain. This is an area which allows the drug to bind to albumin, which increases its serum half-life and enables weekly dosing while slowing its entry into the bloodstream.
“Ultimately, we think that this has impacts, hypothetically, on tolerability and potentially mitigating the need for dose escalation,” said Dr. Harrison.
Weight loss results
The phase 1 study Dr. Harrison presented had a randomized, double-blind, placebo-controlled design with single and multiple ascending doses (SAD/MAD) of pemvidutide being tested. He presented data on the MAD phase only, noting that the SAD phase had been used to determine what doses to use in the latter.
Seventy individuals with a body mass index of between 25 and 40 kg/m2 were recruited and 34 of these were enrolled in the MAD phase of the study. Three doses of pemvidutide were used, given subcutaneously once a week for 12 weeks: Seven participants received 1.2 mg, 9 were given 1.8 mg, 11 had 2.4 mg, and 7 subjects were treated with placebo. Dr. Harrison noted that there were no caloric restrictions in the trial and no lifestyle modifications or interventions.
The average age of study participants ranged from 27 to 35 years and the mean BMI was 30-31 kg/m2 across each group, with their lipid parameters in the upper range of normal.
Clear weight loss reductions were seen across all the pemvidutide groups versus placebo, with the greatest percentage changes in weight loss seen with the two higher doses used. At week 12, there was a 4.9%, 10.3% and 9.0% weight loss in the 1.2-mg, 1.8-mg and 2.4-mg pemvidutide groups compared to 1.6% in placebo-treated individuals.
All patients in the 1.8-mg group achieved a 5% or greater weight loss, Dr. Harrison observed, but there “was a plateauing” effect with the 2.4-mg dose with 89% of patients achieving this target. In comparison, a third of patients on the lowest dose and 20% of those on placebo achieved this target.
The trajectory of weight loss seen in the trial suggests that “the rate of weight loss would continue beyond 12 weeks if we were to continue the therapy” Dr. Harrison said.
Lipid changes and liver fat reductions
Levels of serum lipids from baseline to week 12 fell to a greater extent with pemvidutide treatment than with placebo, in the range of –27% for total cholesterol in the two highest dose groups, –25% for LDL-cholesterol for those groups, –37% for triglycerides for the 1.2- and 1.8-mg groups, and reductions in apolipoprotein B were seen.
“We saw an initial decline in HDL [high-density lipoprotein],” Dr. Harrison said, noting that “this is consistent with prior studies looking at rapid weight loss, and over time, this mitigates as you continue to treat at least based on other mechanisms of action or other drugs with similar mechanisms.”
Pemvidutide treatment was also associated with increased lipid oxidation and decreased lipid synthesis, and “there was a robust decrease in lipids implicated in NASH inflammation,” Dr. Harrison pointed out.
Importantly, in five of eight participants who had high levels of liver fat at baseline – defined as a 5% or greater magnetic resonance imaging–derived proton-density-fat-fraction (MRI-PDFF) – showed a decrease to undetectable limits (1.5% or less). This was a greater than 90% reduction in liver fat, Dr. Harrison said. All five patients were in the 1.8-mg and 2.4-mg groups.
As for side effects, these were “predominantly upper GI, with nausea and vomiting.” These were mild in most cases, but he pointed out that five patients treated with the 1.8-mg dose experienced moderate nausea and three experienced moderate vomiting. Mild diarrhea and constipation were also seen in two of patients given this dose but was not reported in any of the other groups.
During the discussion following the presentation, it was pointed out that there was no clear dose-dependent effect considering the 1.8-mg dose seemed to have a stronger effect in some areas than the 2.4-mg dose. That’s a fair point, Dr. Harrison responded, reiterating it was a small study with a short treatment duration, but that there did look like a plateauing effect, “at least in patients with a mean BMI of between 30 and 31.”
Dr. Harrison was asked about potential effects on insulin levels and if that was a worry because, if glucagon is stimulated, it could increase insulin. That in turn might encourage insulin resistance and promote worse outcomes.
“If you look outside of just this program, glucagon agonism has been dosed in a lot of patients over time, and we haven’t seen that,” Dr. Harrison replied. Pemvidutide is an agonist rather than antagonist, so perhaps the [nonalcoholic steatohepatitis]–inducing effects seen before with glucagon antagonism won’t occur, he suggested.
Dr. Harrison disclosed ties to Altimmune (the study sponsor), Akero, Axcella, Bristol Myers Squibb, Cirius, CiVi Biopharma, Conatus, Corcept, CymaBay, Enyo, Galectin, Genentech, Genfit, Gilead, Hepion, Hightide, HistoIndex, Intercept, Madrigal, Metacrine, NGM Bio, Novartis, Novo Nordisk, NorthSea, Pfizer, Sagimet, Viking, and 89Bio.
LONDON – Weight loss, lipid reductions, and “robust improvements” in lipid species associated with nonalcoholic fatty liver disease were achieved in patients who were treated with pemvidutide in a first-in-human, phase 1 clinical trial reported at the annual International Liver Congress, sponsored by the European Association for the Study of the Liver.
The presenting study investigator, Stephen A. Harrison, MD, said that pemvidutide, which is also being developed for the treatment of obesity, appeared to be well tolerated. There were no serious or severe adverse events, and no patient had to discontinue treatment because of side effects.
Overall, “pemvidutide represents a promising new agent,” said Dr. Harrison, medical director of Pinnacle Research in San Antonio, Texas.
Dual incretin effect
Pemvidutide is a “balanced” dual agonist of glucagon-like peptide 1 (GLP-1) and glucagon, Dr. Harrison explained in his oral abstract.
“With glucagon, we are working to drive energy expenditure up, and with GLP-1, we’re decreasing food intake,” Dr. Harrison said.
What might set pemvidutide apart from other incretins lies within its structure, Dr. Harrison suggested. The structure has two main regions – one with greater GLP-1 specificity and the other with greater glucagon specificity, and these two areas are linked by a propriety technology called a EuPort™ domain. This is an area which allows the drug to bind to albumin, which increases its serum half-life and enables weekly dosing while slowing its entry into the bloodstream.
“Ultimately, we think that this has impacts, hypothetically, on tolerability and potentially mitigating the need for dose escalation,” said Dr. Harrison.
Weight loss results
The phase 1 study Dr. Harrison presented had a randomized, double-blind, placebo-controlled design with single and multiple ascending doses (SAD/MAD) of pemvidutide being tested. He presented data on the MAD phase only, noting that the SAD phase had been used to determine what doses to use in the latter.
Seventy individuals with a body mass index of between 25 and 40 kg/m2 were recruited and 34 of these were enrolled in the MAD phase of the study. Three doses of pemvidutide were used, given subcutaneously once a week for 12 weeks: Seven participants received 1.2 mg, 9 were given 1.8 mg, 11 had 2.4 mg, and 7 subjects were treated with placebo. Dr. Harrison noted that there were no caloric restrictions in the trial and no lifestyle modifications or interventions.
The average age of study participants ranged from 27 to 35 years and the mean BMI was 30-31 kg/m2 across each group, with their lipid parameters in the upper range of normal.
Clear weight loss reductions were seen across all the pemvidutide groups versus placebo, with the greatest percentage changes in weight loss seen with the two higher doses used. At week 12, there was a 4.9%, 10.3% and 9.0% weight loss in the 1.2-mg, 1.8-mg and 2.4-mg pemvidutide groups compared to 1.6% in placebo-treated individuals.
All patients in the 1.8-mg group achieved a 5% or greater weight loss, Dr. Harrison observed, but there “was a plateauing” effect with the 2.4-mg dose with 89% of patients achieving this target. In comparison, a third of patients on the lowest dose and 20% of those on placebo achieved this target.
The trajectory of weight loss seen in the trial suggests that “the rate of weight loss would continue beyond 12 weeks if we were to continue the therapy” Dr. Harrison said.
Lipid changes and liver fat reductions
Levels of serum lipids from baseline to week 12 fell to a greater extent with pemvidutide treatment than with placebo, in the range of –27% for total cholesterol in the two highest dose groups, –25% for LDL-cholesterol for those groups, –37% for triglycerides for the 1.2- and 1.8-mg groups, and reductions in apolipoprotein B were seen.
“We saw an initial decline in HDL [high-density lipoprotein],” Dr. Harrison said, noting that “this is consistent with prior studies looking at rapid weight loss, and over time, this mitigates as you continue to treat at least based on other mechanisms of action or other drugs with similar mechanisms.”
Pemvidutide treatment was also associated with increased lipid oxidation and decreased lipid synthesis, and “there was a robust decrease in lipids implicated in NASH inflammation,” Dr. Harrison pointed out.
Importantly, in five of eight participants who had high levels of liver fat at baseline – defined as a 5% or greater magnetic resonance imaging–derived proton-density-fat-fraction (MRI-PDFF) – showed a decrease to undetectable limits (1.5% or less). This was a greater than 90% reduction in liver fat, Dr. Harrison said. All five patients were in the 1.8-mg and 2.4-mg groups.
As for side effects, these were “predominantly upper GI, with nausea and vomiting.” These were mild in most cases, but he pointed out that five patients treated with the 1.8-mg dose experienced moderate nausea and three experienced moderate vomiting. Mild diarrhea and constipation were also seen in two of patients given this dose but was not reported in any of the other groups.
During the discussion following the presentation, it was pointed out that there was no clear dose-dependent effect considering the 1.8-mg dose seemed to have a stronger effect in some areas than the 2.4-mg dose. That’s a fair point, Dr. Harrison responded, reiterating it was a small study with a short treatment duration, but that there did look like a plateauing effect, “at least in patients with a mean BMI of between 30 and 31.”
Dr. Harrison was asked about potential effects on insulin levels and if that was a worry because, if glucagon is stimulated, it could increase insulin. That in turn might encourage insulin resistance and promote worse outcomes.
“If you look outside of just this program, glucagon agonism has been dosed in a lot of patients over time, and we haven’t seen that,” Dr. Harrison replied. Pemvidutide is an agonist rather than antagonist, so perhaps the [nonalcoholic steatohepatitis]–inducing effects seen before with glucagon antagonism won’t occur, he suggested.
Dr. Harrison disclosed ties to Altimmune (the study sponsor), Akero, Axcella, Bristol Myers Squibb, Cirius, CiVi Biopharma, Conatus, Corcept, CymaBay, Enyo, Galectin, Genentech, Genfit, Gilead, Hepion, Hightide, HistoIndex, Intercept, Madrigal, Metacrine, NGM Bio, Novartis, Novo Nordisk, NorthSea, Pfizer, Sagimet, Viking, and 89Bio.
LONDON – Weight loss, lipid reductions, and “robust improvements” in lipid species associated with nonalcoholic fatty liver disease were achieved in patients who were treated with pemvidutide in a first-in-human, phase 1 clinical trial reported at the annual International Liver Congress, sponsored by the European Association for the Study of the Liver.
The presenting study investigator, Stephen A. Harrison, MD, said that pemvidutide, which is also being developed for the treatment of obesity, appeared to be well tolerated. There were no serious or severe adverse events, and no patient had to discontinue treatment because of side effects.
Overall, “pemvidutide represents a promising new agent,” said Dr. Harrison, medical director of Pinnacle Research in San Antonio, Texas.
Dual incretin effect
Pemvidutide is a “balanced” dual agonist of glucagon-like peptide 1 (GLP-1) and glucagon, Dr. Harrison explained in his oral abstract.
“With glucagon, we are working to drive energy expenditure up, and with GLP-1, we’re decreasing food intake,” Dr. Harrison said.
What might set pemvidutide apart from other incretins lies within its structure, Dr. Harrison suggested. The structure has two main regions – one with greater GLP-1 specificity and the other with greater glucagon specificity, and these two areas are linked by a propriety technology called a EuPort™ domain. This is an area which allows the drug to bind to albumin, which increases its serum half-life and enables weekly dosing while slowing its entry into the bloodstream.
“Ultimately, we think that this has impacts, hypothetically, on tolerability and potentially mitigating the need for dose escalation,” said Dr. Harrison.
Weight loss results
The phase 1 study Dr. Harrison presented had a randomized, double-blind, placebo-controlled design with single and multiple ascending doses (SAD/MAD) of pemvidutide being tested. He presented data on the MAD phase only, noting that the SAD phase had been used to determine what doses to use in the latter.
Seventy individuals with a body mass index of between 25 and 40 kg/m2 were recruited and 34 of these were enrolled in the MAD phase of the study. Three doses of pemvidutide were used, given subcutaneously once a week for 12 weeks: Seven participants received 1.2 mg, 9 were given 1.8 mg, 11 had 2.4 mg, and 7 subjects were treated with placebo. Dr. Harrison noted that there were no caloric restrictions in the trial and no lifestyle modifications or interventions.
The average age of study participants ranged from 27 to 35 years and the mean BMI was 30-31 kg/m2 across each group, with their lipid parameters in the upper range of normal.
Clear weight loss reductions were seen across all the pemvidutide groups versus placebo, with the greatest percentage changes in weight loss seen with the two higher doses used. At week 12, there was a 4.9%, 10.3% and 9.0% weight loss in the 1.2-mg, 1.8-mg and 2.4-mg pemvidutide groups compared to 1.6% in placebo-treated individuals.
All patients in the 1.8-mg group achieved a 5% or greater weight loss, Dr. Harrison observed, but there “was a plateauing” effect with the 2.4-mg dose with 89% of patients achieving this target. In comparison, a third of patients on the lowest dose and 20% of those on placebo achieved this target.
The trajectory of weight loss seen in the trial suggests that “the rate of weight loss would continue beyond 12 weeks if we were to continue the therapy” Dr. Harrison said.
Lipid changes and liver fat reductions
Levels of serum lipids from baseline to week 12 fell to a greater extent with pemvidutide treatment than with placebo, in the range of –27% for total cholesterol in the two highest dose groups, –25% for LDL-cholesterol for those groups, –37% for triglycerides for the 1.2- and 1.8-mg groups, and reductions in apolipoprotein B were seen.
“We saw an initial decline in HDL [high-density lipoprotein],” Dr. Harrison said, noting that “this is consistent with prior studies looking at rapid weight loss, and over time, this mitigates as you continue to treat at least based on other mechanisms of action or other drugs with similar mechanisms.”
Pemvidutide treatment was also associated with increased lipid oxidation and decreased lipid synthesis, and “there was a robust decrease in lipids implicated in NASH inflammation,” Dr. Harrison pointed out.
Importantly, in five of eight participants who had high levels of liver fat at baseline – defined as a 5% or greater magnetic resonance imaging–derived proton-density-fat-fraction (MRI-PDFF) – showed a decrease to undetectable limits (1.5% or less). This was a greater than 90% reduction in liver fat, Dr. Harrison said. All five patients were in the 1.8-mg and 2.4-mg groups.
As for side effects, these were “predominantly upper GI, with nausea and vomiting.” These were mild in most cases, but he pointed out that five patients treated with the 1.8-mg dose experienced moderate nausea and three experienced moderate vomiting. Mild diarrhea and constipation were also seen in two of patients given this dose but was not reported in any of the other groups.
During the discussion following the presentation, it was pointed out that there was no clear dose-dependent effect considering the 1.8-mg dose seemed to have a stronger effect in some areas than the 2.4-mg dose. That’s a fair point, Dr. Harrison responded, reiterating it was a small study with a short treatment duration, but that there did look like a plateauing effect, “at least in patients with a mean BMI of between 30 and 31.”
Dr. Harrison was asked about potential effects on insulin levels and if that was a worry because, if glucagon is stimulated, it could increase insulin. That in turn might encourage insulin resistance and promote worse outcomes.
“If you look outside of just this program, glucagon agonism has been dosed in a lot of patients over time, and we haven’t seen that,” Dr. Harrison replied. Pemvidutide is an agonist rather than antagonist, so perhaps the [nonalcoholic steatohepatitis]–inducing effects seen before with glucagon antagonism won’t occur, he suggested.
Dr. Harrison disclosed ties to Altimmune (the study sponsor), Akero, Axcella, Bristol Myers Squibb, Cirius, CiVi Biopharma, Conatus, Corcept, CymaBay, Enyo, Galectin, Genentech, Genfit, Gilead, Hepion, Hightide, HistoIndex, Intercept, Madrigal, Metacrine, NGM Bio, Novartis, Novo Nordisk, NorthSea, Pfizer, Sagimet, Viking, and 89Bio.
AT ILC 2022
Fatty liver disease drives rise in liver cancer deaths
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the International Liver Congress (ILC) 2022.
“NAFLD and NASH [nonalcoholic steatohepatitis] are rapidly becoming the main causes of cirrhosis and liver cancer in the world,” Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009-2019, the annual percent change (APC) of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense Liver Research Centre at SDU and Odense University Hospital, Denmark, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around two billion cases of chronic liver disease globally, he said. He noted that over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that globally, the overall prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policymakers is very low. “From my perspective, raising awareness of NAFLD is the number one priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The WHO [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Krag has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the International Liver Congress (ILC) 2022.
“NAFLD and NASH [nonalcoholic steatohepatitis] are rapidly becoming the main causes of cirrhosis and liver cancer in the world,” Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009-2019, the annual percent change (APC) of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense Liver Research Centre at SDU and Odense University Hospital, Denmark, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around two billion cases of chronic liver disease globally, he said. He noted that over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that globally, the overall prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policymakers is very low. “From my perspective, raising awareness of NAFLD is the number one priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The WHO [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Krag has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the International Liver Congress (ILC) 2022.
“NAFLD and NASH [nonalcoholic steatohepatitis] are rapidly becoming the main causes of cirrhosis and liver cancer in the world,” Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009-2019, the annual percent change (APC) of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense Liver Research Centre at SDU and Odense University Hospital, Denmark, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around two billion cases of chronic liver disease globally, he said. He noted that over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that globally, the overall prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policymakers is very low. “From my perspective, raising awareness of NAFLD is the number one priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The WHO [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Krag has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2022
COVID-19 tied to increased risk for Alzheimer’s disease and Parkinson’s disease
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN NEUROLOGY
Pandemic stress tied to increased headache burden in teens
Contrary to previous research findings, the stress of the COVID-19 pandemic has been linked to an increased headache burden in teens.
Investigators found factors contributing to headache for preteens and teens during the pandemic included increased screen time for online learning, depression, anxiety, female sex, and weight gain.
“The stressors and pressures of the pandemic may have eventually taken their toll,” lead author Ayşe Nur Özdağ Acarli, MD, Ermenek State Hospital, department of neurology, Karaman, Turkey, told this news organization.
“Limiting screen time and providing more psychosocial supports would help lessen the burden of the COVID-19 pandemic on adolescents with headache.”
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2022.
Most common neurological problem in kids
Headache is the most common neurological problem in children and adolescents. Potential factors contributing to headache in this population include lack of sleep and physical activity, mental health problems, and socioeconomic conditions.
The COVID-19 pandemic has had a “striking” impact on every aspect of life for young people, said Dr. Acarli.
Some studies reported an improvement in headache prevalence among adolescents during COVID-19, which was attributed to less school-related stress. However, said Dr. Acarli in her personal clinical experience, young patients suffered more frequent and severe headaches during the pandemic.
She noted previous research examining the impact of the pandemic on headache in youth was conducted only in the early days of the pandemic and examined shorter-term effects. Research examining the long-term effects of the pandemic on headache in this patient population has been “lacking,” she said.
The study included 851 participants aged 10-18 years (mean age 14.9 years and 62% female) who were seen at a neurology or pediatric outpatient clinic from August-December 2021. The study excluded subjects with neurological problems, intellectual deficits, autism spectrum disorder, and epilepsy.
Participants completed detailed questionnaires providing data on demographics, exposure to COVID-19, and electronics, as well as information on depressive symptoms as assessed by the Patient Health Questionnaire-9 and anxiety symptoms using the Generalized Anxiety Disorder-7 and COVID-related anxiety.
“We used two distinct scales for anxiety: one for generalized anxiety and the other for COVID-related anxiety,” said Dr. Acarli.
Of the total study population, 756 (89%) reported headaches. This headache prevalence in children and adolescents is like that found in other studies.
Dr. Acarli noted several differences in the headache group versus the non-headache group. The female/male ratio was 2:1 versus 1:1, the mean age was 15.0 versus 14.4, and depression and generalized anxiety scores were significantly higher. There was no significant difference in COVID-19 history in those with and without headache.
Researchers categorized those with headache into four groups: worsening headaches (27%), improved headaches (3%), new onset headaches (10%), and stable headaches (61%).
Compared with the other groups, the worsened headache group included significantly more females and older individuals with more severe and frequent headaches. This group also had more participants reporting at least 15 headache attacks a month and using painkillers at least once a month.
The study showed headache severity was significantly increased with age, headache duration, depression, generalized anxiety (all P < .001), and COVID-19 anxiety (P < .01). Headache frequency, measured as attacks per month, was significantly increased with age, depression, and generalized anxiety (all P < .001).
Worsening headache outcomes during the pandemic were associated with longer exposure to computer screens (odds ratio, 1.7; 95% confidence interval, 1.2-2.3; P < .01), lack of suitable conditions for online learning (OR, 2.6; 95% CI, 1.8-3.8; P < .001), depression (OR, 2.0; 95% CI, 1.4-2.8; P < .001); and COVID-19 anxiety (OR, 3.2; 95% CI, 1.3-8.0; P < .01). Other contributing factors included school exams, living in a city, female sex, and weight gain.
There may be a link between COVID-related headaches and anxiety or depression, but it’s unclear what’s causing what. “We don’t know which is the chicken and which is the egg,” said Dr. Acarli.
Headache triggers
Commenting for this news organization, Raquel Gil-Gouveia, MD, PhD, head of the neurology department, Hospital da Luz, Lisbon, Portugal, who co-chaired the session where the research was presented, said the information collected for the study was “extensive.”
Some results were expected, including the fact that patients with headaches were more anxious and depressed, said Dr. Gil-Gouveia.
“Anxiety and depression are frequent comorbidities of headache and can act as a triggering factor for headache attacks but can also be a consequence of intense or chronic pain,” she said.
She agreed the new results differ from those of studies carried out during the first pandemic lockdown, which showed an improvement in headache, but noted online learning was not fully implemented at that time, “so it was much like being on vacation.”
In addition to isolation, anxiety, and prolonged screen exposure, the lack of peer contact and fewer sports and leisure activities may also have contributed to worsening headaches during the COVID lockdown, but these were not explored in this study, said Dr. Gil-Gouveia.
The study was supported by the Global Migraine and Pain Society. The investigators and Dr. Gil-Gouveia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to previous research findings, the stress of the COVID-19 pandemic has been linked to an increased headache burden in teens.
Investigators found factors contributing to headache for preteens and teens during the pandemic included increased screen time for online learning, depression, anxiety, female sex, and weight gain.
“The stressors and pressures of the pandemic may have eventually taken their toll,” lead author Ayşe Nur Özdağ Acarli, MD, Ermenek State Hospital, department of neurology, Karaman, Turkey, told this news organization.
“Limiting screen time and providing more psychosocial supports would help lessen the burden of the COVID-19 pandemic on adolescents with headache.”
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2022.
Most common neurological problem in kids
Headache is the most common neurological problem in children and adolescents. Potential factors contributing to headache in this population include lack of sleep and physical activity, mental health problems, and socioeconomic conditions.
The COVID-19 pandemic has had a “striking” impact on every aspect of life for young people, said Dr. Acarli.
Some studies reported an improvement in headache prevalence among adolescents during COVID-19, which was attributed to less school-related stress. However, said Dr. Acarli in her personal clinical experience, young patients suffered more frequent and severe headaches during the pandemic.
She noted previous research examining the impact of the pandemic on headache in youth was conducted only in the early days of the pandemic and examined shorter-term effects. Research examining the long-term effects of the pandemic on headache in this patient population has been “lacking,” she said.
The study included 851 participants aged 10-18 years (mean age 14.9 years and 62% female) who were seen at a neurology or pediatric outpatient clinic from August-December 2021. The study excluded subjects with neurological problems, intellectual deficits, autism spectrum disorder, and epilepsy.
Participants completed detailed questionnaires providing data on demographics, exposure to COVID-19, and electronics, as well as information on depressive symptoms as assessed by the Patient Health Questionnaire-9 and anxiety symptoms using the Generalized Anxiety Disorder-7 and COVID-related anxiety.
“We used two distinct scales for anxiety: one for generalized anxiety and the other for COVID-related anxiety,” said Dr. Acarli.
Of the total study population, 756 (89%) reported headaches. This headache prevalence in children and adolescents is like that found in other studies.
Dr. Acarli noted several differences in the headache group versus the non-headache group. The female/male ratio was 2:1 versus 1:1, the mean age was 15.0 versus 14.4, and depression and generalized anxiety scores were significantly higher. There was no significant difference in COVID-19 history in those with and without headache.
Researchers categorized those with headache into four groups: worsening headaches (27%), improved headaches (3%), new onset headaches (10%), and stable headaches (61%).
Compared with the other groups, the worsened headache group included significantly more females and older individuals with more severe and frequent headaches. This group also had more participants reporting at least 15 headache attacks a month and using painkillers at least once a month.
The study showed headache severity was significantly increased with age, headache duration, depression, generalized anxiety (all P < .001), and COVID-19 anxiety (P < .01). Headache frequency, measured as attacks per month, was significantly increased with age, depression, and generalized anxiety (all P < .001).
Worsening headache outcomes during the pandemic were associated with longer exposure to computer screens (odds ratio, 1.7; 95% confidence interval, 1.2-2.3; P < .01), lack of suitable conditions for online learning (OR, 2.6; 95% CI, 1.8-3.8; P < .001), depression (OR, 2.0; 95% CI, 1.4-2.8; P < .001); and COVID-19 anxiety (OR, 3.2; 95% CI, 1.3-8.0; P < .01). Other contributing factors included school exams, living in a city, female sex, and weight gain.
There may be a link between COVID-related headaches and anxiety or depression, but it’s unclear what’s causing what. “We don’t know which is the chicken and which is the egg,” said Dr. Acarli.
Headache triggers
Commenting for this news organization, Raquel Gil-Gouveia, MD, PhD, head of the neurology department, Hospital da Luz, Lisbon, Portugal, who co-chaired the session where the research was presented, said the information collected for the study was “extensive.”
Some results were expected, including the fact that patients with headaches were more anxious and depressed, said Dr. Gil-Gouveia.
“Anxiety and depression are frequent comorbidities of headache and can act as a triggering factor for headache attacks but can also be a consequence of intense or chronic pain,” she said.
She agreed the new results differ from those of studies carried out during the first pandemic lockdown, which showed an improvement in headache, but noted online learning was not fully implemented at that time, “so it was much like being on vacation.”
In addition to isolation, anxiety, and prolonged screen exposure, the lack of peer contact and fewer sports and leisure activities may also have contributed to worsening headaches during the COVID lockdown, but these were not explored in this study, said Dr. Gil-Gouveia.
The study was supported by the Global Migraine and Pain Society. The investigators and Dr. Gil-Gouveia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to previous research findings, the stress of the COVID-19 pandemic has been linked to an increased headache burden in teens.
Investigators found factors contributing to headache for preteens and teens during the pandemic included increased screen time for online learning, depression, anxiety, female sex, and weight gain.
“The stressors and pressures of the pandemic may have eventually taken their toll,” lead author Ayşe Nur Özdağ Acarli, MD, Ermenek State Hospital, department of neurology, Karaman, Turkey, told this news organization.
“Limiting screen time and providing more psychosocial supports would help lessen the burden of the COVID-19 pandemic on adolescents with headache.”
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2022.
Most common neurological problem in kids
Headache is the most common neurological problem in children and adolescents. Potential factors contributing to headache in this population include lack of sleep and physical activity, mental health problems, and socioeconomic conditions.
The COVID-19 pandemic has had a “striking” impact on every aspect of life for young people, said Dr. Acarli.
Some studies reported an improvement in headache prevalence among adolescents during COVID-19, which was attributed to less school-related stress. However, said Dr. Acarli in her personal clinical experience, young patients suffered more frequent and severe headaches during the pandemic.
She noted previous research examining the impact of the pandemic on headache in youth was conducted only in the early days of the pandemic and examined shorter-term effects. Research examining the long-term effects of the pandemic on headache in this patient population has been “lacking,” she said.
The study included 851 participants aged 10-18 years (mean age 14.9 years and 62% female) who were seen at a neurology or pediatric outpatient clinic from August-December 2021. The study excluded subjects with neurological problems, intellectual deficits, autism spectrum disorder, and epilepsy.
Participants completed detailed questionnaires providing data on demographics, exposure to COVID-19, and electronics, as well as information on depressive symptoms as assessed by the Patient Health Questionnaire-9 and anxiety symptoms using the Generalized Anxiety Disorder-7 and COVID-related anxiety.
“We used two distinct scales for anxiety: one for generalized anxiety and the other for COVID-related anxiety,” said Dr. Acarli.
Of the total study population, 756 (89%) reported headaches. This headache prevalence in children and adolescents is like that found in other studies.
Dr. Acarli noted several differences in the headache group versus the non-headache group. The female/male ratio was 2:1 versus 1:1, the mean age was 15.0 versus 14.4, and depression and generalized anxiety scores were significantly higher. There was no significant difference in COVID-19 history in those with and without headache.
Researchers categorized those with headache into four groups: worsening headaches (27%), improved headaches (3%), new onset headaches (10%), and stable headaches (61%).
Compared with the other groups, the worsened headache group included significantly more females and older individuals with more severe and frequent headaches. This group also had more participants reporting at least 15 headache attacks a month and using painkillers at least once a month.
The study showed headache severity was significantly increased with age, headache duration, depression, generalized anxiety (all P < .001), and COVID-19 anxiety (P < .01). Headache frequency, measured as attacks per month, was significantly increased with age, depression, and generalized anxiety (all P < .001).
Worsening headache outcomes during the pandemic were associated with longer exposure to computer screens (odds ratio, 1.7; 95% confidence interval, 1.2-2.3; P < .01), lack of suitable conditions for online learning (OR, 2.6; 95% CI, 1.8-3.8; P < .001), depression (OR, 2.0; 95% CI, 1.4-2.8; P < .001); and COVID-19 anxiety (OR, 3.2; 95% CI, 1.3-8.0; P < .01). Other contributing factors included school exams, living in a city, female sex, and weight gain.
There may be a link between COVID-related headaches and anxiety or depression, but it’s unclear what’s causing what. “We don’t know which is the chicken and which is the egg,” said Dr. Acarli.
Headache triggers
Commenting for this news organization, Raquel Gil-Gouveia, MD, PhD, head of the neurology department, Hospital da Luz, Lisbon, Portugal, who co-chaired the session where the research was presented, said the information collected for the study was “extensive.”
Some results were expected, including the fact that patients with headaches were more anxious and depressed, said Dr. Gil-Gouveia.
“Anxiety and depression are frequent comorbidities of headache and can act as a triggering factor for headache attacks but can also be a consequence of intense or chronic pain,” she said.
She agreed the new results differ from those of studies carried out during the first pandemic lockdown, which showed an improvement in headache, but noted online learning was not fully implemented at that time, “so it was much like being on vacation.”
In addition to isolation, anxiety, and prolonged screen exposure, the lack of peer contact and fewer sports and leisure activities may also have contributed to worsening headaches during the COVID lockdown, but these were not explored in this study, said Dr. Gil-Gouveia.
The study was supported by the Global Migraine and Pain Society. The investigators and Dr. Gil-Gouveia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EAN 2022
‘Superior’ CLL regimen cuts chemo in half
“Overall, our data suggests that [the chemoimmunotherapy] regimen is very effective and appears superior to published six cycles of chemotherapy regimen for the same favorable risk features,” first author Dr. Nitin Jain, an associate professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston, told MDedge.
Chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab (FCR) has been a standard frontline treatment for young, fit patients with CLL, resulting in 10-year PFS rates above 55% in patients with mutated IGHV status, said coauthor Dr. Alessandra Ferrajoli, also of the MD Anderson Cancer Center, in presenting the findings at the European Hematology Association annual congress.
The authors sought to investigate the efficacy of a targeted therapy combination of ibrutinib and obinutuzumab with fludarabine and cyclophosphamide (iFCG). They also sought to determine whether a three-cycle regimen of the chemotherapy, as compared to six cycles, could reduce the risk of myelodysplastic syndrome (MDS), which increases with chemotherapy in CLL patients who have mutated IGHV status.
For the phase 2 study, 45 previously untreated patients with CLL, who had mutated IGHV and an absence of del(17p)/TP53 mutation (both of which are associated with more favorable outcomes in CLL) were enrolled between March 2016 and August 2018. The patients were deemed fit for chemotherapy and had a median age of 60.
All patients were initially treated with three cycles of the iFCG regimen, and among them, 39 (87%) achieved undetectable measurable residual disease (MRD) in their bone marrow.
After the three cycles, an MRD-driven strategy was then used to determine subsequent treatment: All patients received nine courses of ibrutinib, and for those achieving complete remission (CR) or CR with incomplete count recovery (CRi) and undetectable MRD, three cycles of obinutuzumab were administered, while all others received nine additional cycles of obinutuzumab.
At completion of the 12 courses, those who still had MRD positivity continued on ibrutinib, while those with undetectable MRD discontinued ibrutinib.
By cycle six of iFCG, 40 (89%) of the patients achieved undetectable MRD. Overall, 44 of the 45 patients (98%) achieved undetectable MRD as their best response at any time during the study, with 69% of patients achieving CR/CRi. Four patients came off the study prior to cycle 12, including one death, one infection, and one patient who opted to pursue treatment locally. With a median follow-up of 59.6 months, there were no cases of CLL progression or Richter transformation and the lone death was from heart failure.
One patient developed treatment-related myelodysplastic syndrome (MDS), and that patient has maintained normal blood counts over 38 months of monitoring and has not required MDS therapy, Dr. Ferrajoli reported.
Over the follow-up, the six patients who were MRD positive after the completion of three cycles experienced a recurrence of MRD, defined as two consecutive values of 0.01% or higher in peripheral blood by flow cytometry, at a median of 27.2 months after stopping all therapy.
“Not unexpectedly, MRD recurrence during follow-up correlated with MRD positivity during therapy,” Dr. Ferrajoli said.
She noted that all six of the patients were being monitored, with no clinical progression or active therapy. However, with a median follow-up of 5 years, the progression-free survival (PFS) rate among the 45 patients was 97.7%, and the overall survival (OS) rate was 97.8%. Dr. Ferrajoli noted that, while the study population was clearly different, the results compare favorably with CLL clinical trial results that have previously shown a 5-year PFS of approximately 65% with FCR alone; approximately 70% with ibrutinib; and 81% with ibrutinib among patients with mutated IGHV status.
Furthermore, the rate of undetectable MRD status in mutated IGHV patients being 95% in evaluable patients in the current study is notably higher than rates of 51% through 67% reported in five other trials of CLL treatment with six cycles of FCR and with a rate of 79% in the DFCI trial of six-cycle chemotherapy plus ibrutinib.
And the current study’s undetectable MRD rate of 89% in the intention-to-treat population compares with just 13% though 40% in the five other chemotherapy trials and 79% in the DFCI trial, the authors note.
The current trial was the only one of any of their comparisons to utilize the three-cycle regimen.
Asked at the meeting about concerns of toxicities reported with obinutuzumab and chemotherapy, Dr. Ferrajoli said “the treatment was very well tolerated.”
“Myelosuppression is a concern with this combination, but we did make the use of prophylactic growth-factor mandatory in the study, so we were able to control that,” she said.
Dr. Jain noted that, while treatment trends have moved largely to chemo-free regimens, particularly in the United States because of concerns about the MDS, the current study’s results importantly shed light on a potentially beneficial approach of just three cycles of chemotherapy.
“In Europe and the rest of the world where chemo use is still common, this regimen could be considered,” he told MDedge. “The findings show that if you still use chemo in your practice, this regimen uses 50% less chemotherapy, yet seems to give higher response rates.”
“While MDS and acute myeloid leukemia (AML) remain a concern with any chemotherapy regimen, it is possible that 50% less chemo will lead to less risk of MDS AML, but longer-term follow-up [is needed],” he said.
Dr. Ferrajoli reported that she has received research support from Astra-Zeneca and Beigene. Dr. Jain has received research funding and honoraria from Genentech and Pharmacyclics.
“Overall, our data suggests that [the chemoimmunotherapy] regimen is very effective and appears superior to published six cycles of chemotherapy regimen for the same favorable risk features,” first author Dr. Nitin Jain, an associate professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston, told MDedge.
Chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab (FCR) has been a standard frontline treatment for young, fit patients with CLL, resulting in 10-year PFS rates above 55% in patients with mutated IGHV status, said coauthor Dr. Alessandra Ferrajoli, also of the MD Anderson Cancer Center, in presenting the findings at the European Hematology Association annual congress.
The authors sought to investigate the efficacy of a targeted therapy combination of ibrutinib and obinutuzumab with fludarabine and cyclophosphamide (iFCG). They also sought to determine whether a three-cycle regimen of the chemotherapy, as compared to six cycles, could reduce the risk of myelodysplastic syndrome (MDS), which increases with chemotherapy in CLL patients who have mutated IGHV status.
For the phase 2 study, 45 previously untreated patients with CLL, who had mutated IGHV and an absence of del(17p)/TP53 mutation (both of which are associated with more favorable outcomes in CLL) were enrolled between March 2016 and August 2018. The patients were deemed fit for chemotherapy and had a median age of 60.
All patients were initially treated with three cycles of the iFCG regimen, and among them, 39 (87%) achieved undetectable measurable residual disease (MRD) in their bone marrow.
After the three cycles, an MRD-driven strategy was then used to determine subsequent treatment: All patients received nine courses of ibrutinib, and for those achieving complete remission (CR) or CR with incomplete count recovery (CRi) and undetectable MRD, three cycles of obinutuzumab were administered, while all others received nine additional cycles of obinutuzumab.
At completion of the 12 courses, those who still had MRD positivity continued on ibrutinib, while those with undetectable MRD discontinued ibrutinib.
By cycle six of iFCG, 40 (89%) of the patients achieved undetectable MRD. Overall, 44 of the 45 patients (98%) achieved undetectable MRD as their best response at any time during the study, with 69% of patients achieving CR/CRi. Four patients came off the study prior to cycle 12, including one death, one infection, and one patient who opted to pursue treatment locally. With a median follow-up of 59.6 months, there were no cases of CLL progression or Richter transformation and the lone death was from heart failure.
One patient developed treatment-related myelodysplastic syndrome (MDS), and that patient has maintained normal blood counts over 38 months of monitoring and has not required MDS therapy, Dr. Ferrajoli reported.
Over the follow-up, the six patients who were MRD positive after the completion of three cycles experienced a recurrence of MRD, defined as two consecutive values of 0.01% or higher in peripheral blood by flow cytometry, at a median of 27.2 months after stopping all therapy.
“Not unexpectedly, MRD recurrence during follow-up correlated with MRD positivity during therapy,” Dr. Ferrajoli said.
She noted that all six of the patients were being monitored, with no clinical progression or active therapy. However, with a median follow-up of 5 years, the progression-free survival (PFS) rate among the 45 patients was 97.7%, and the overall survival (OS) rate was 97.8%. Dr. Ferrajoli noted that, while the study population was clearly different, the results compare favorably with CLL clinical trial results that have previously shown a 5-year PFS of approximately 65% with FCR alone; approximately 70% with ibrutinib; and 81% with ibrutinib among patients with mutated IGHV status.
Furthermore, the rate of undetectable MRD status in mutated IGHV patients being 95% in evaluable patients in the current study is notably higher than rates of 51% through 67% reported in five other trials of CLL treatment with six cycles of FCR and with a rate of 79% in the DFCI trial of six-cycle chemotherapy plus ibrutinib.
And the current study’s undetectable MRD rate of 89% in the intention-to-treat population compares with just 13% though 40% in the five other chemotherapy trials and 79% in the DFCI trial, the authors note.
The current trial was the only one of any of their comparisons to utilize the three-cycle regimen.
Asked at the meeting about concerns of toxicities reported with obinutuzumab and chemotherapy, Dr. Ferrajoli said “the treatment was very well tolerated.”
“Myelosuppression is a concern with this combination, but we did make the use of prophylactic growth-factor mandatory in the study, so we were able to control that,” she said.
Dr. Jain noted that, while treatment trends have moved largely to chemo-free regimens, particularly in the United States because of concerns about the MDS, the current study’s results importantly shed light on a potentially beneficial approach of just three cycles of chemotherapy.
“In Europe and the rest of the world where chemo use is still common, this regimen could be considered,” he told MDedge. “The findings show that if you still use chemo in your practice, this regimen uses 50% less chemotherapy, yet seems to give higher response rates.”
“While MDS and acute myeloid leukemia (AML) remain a concern with any chemotherapy regimen, it is possible that 50% less chemo will lead to less risk of MDS AML, but longer-term follow-up [is needed],” he said.
Dr. Ferrajoli reported that she has received research support from Astra-Zeneca and Beigene. Dr. Jain has received research funding and honoraria from Genentech and Pharmacyclics.
“Overall, our data suggests that [the chemoimmunotherapy] regimen is very effective and appears superior to published six cycles of chemotherapy regimen for the same favorable risk features,” first author Dr. Nitin Jain, an associate professor in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston, told MDedge.
Chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab (FCR) has been a standard frontline treatment for young, fit patients with CLL, resulting in 10-year PFS rates above 55% in patients with mutated IGHV status, said coauthor Dr. Alessandra Ferrajoli, also of the MD Anderson Cancer Center, in presenting the findings at the European Hematology Association annual congress.
The authors sought to investigate the efficacy of a targeted therapy combination of ibrutinib and obinutuzumab with fludarabine and cyclophosphamide (iFCG). They also sought to determine whether a three-cycle regimen of the chemotherapy, as compared to six cycles, could reduce the risk of myelodysplastic syndrome (MDS), which increases with chemotherapy in CLL patients who have mutated IGHV status.
For the phase 2 study, 45 previously untreated patients with CLL, who had mutated IGHV and an absence of del(17p)/TP53 mutation (both of which are associated with more favorable outcomes in CLL) were enrolled between March 2016 and August 2018. The patients were deemed fit for chemotherapy and had a median age of 60.
All patients were initially treated with three cycles of the iFCG regimen, and among them, 39 (87%) achieved undetectable measurable residual disease (MRD) in their bone marrow.
After the three cycles, an MRD-driven strategy was then used to determine subsequent treatment: All patients received nine courses of ibrutinib, and for those achieving complete remission (CR) or CR with incomplete count recovery (CRi) and undetectable MRD, three cycles of obinutuzumab were administered, while all others received nine additional cycles of obinutuzumab.
At completion of the 12 courses, those who still had MRD positivity continued on ibrutinib, while those with undetectable MRD discontinued ibrutinib.
By cycle six of iFCG, 40 (89%) of the patients achieved undetectable MRD. Overall, 44 of the 45 patients (98%) achieved undetectable MRD as their best response at any time during the study, with 69% of patients achieving CR/CRi. Four patients came off the study prior to cycle 12, including one death, one infection, and one patient who opted to pursue treatment locally. With a median follow-up of 59.6 months, there were no cases of CLL progression or Richter transformation and the lone death was from heart failure.
One patient developed treatment-related myelodysplastic syndrome (MDS), and that patient has maintained normal blood counts over 38 months of monitoring and has not required MDS therapy, Dr. Ferrajoli reported.
Over the follow-up, the six patients who were MRD positive after the completion of three cycles experienced a recurrence of MRD, defined as two consecutive values of 0.01% or higher in peripheral blood by flow cytometry, at a median of 27.2 months after stopping all therapy.
“Not unexpectedly, MRD recurrence during follow-up correlated with MRD positivity during therapy,” Dr. Ferrajoli said.
She noted that all six of the patients were being monitored, with no clinical progression or active therapy. However, with a median follow-up of 5 years, the progression-free survival (PFS) rate among the 45 patients was 97.7%, and the overall survival (OS) rate was 97.8%. Dr. Ferrajoli noted that, while the study population was clearly different, the results compare favorably with CLL clinical trial results that have previously shown a 5-year PFS of approximately 65% with FCR alone; approximately 70% with ibrutinib; and 81% with ibrutinib among patients with mutated IGHV status.
Furthermore, the rate of undetectable MRD status in mutated IGHV patients being 95% in evaluable patients in the current study is notably higher than rates of 51% through 67% reported in five other trials of CLL treatment with six cycles of FCR and with a rate of 79% in the DFCI trial of six-cycle chemotherapy plus ibrutinib.
And the current study’s undetectable MRD rate of 89% in the intention-to-treat population compares with just 13% though 40% in the five other chemotherapy trials and 79% in the DFCI trial, the authors note.
The current trial was the only one of any of their comparisons to utilize the three-cycle regimen.
Asked at the meeting about concerns of toxicities reported with obinutuzumab and chemotherapy, Dr. Ferrajoli said “the treatment was very well tolerated.”
“Myelosuppression is a concern with this combination, but we did make the use of prophylactic growth-factor mandatory in the study, so we were able to control that,” she said.
Dr. Jain noted that, while treatment trends have moved largely to chemo-free regimens, particularly in the United States because of concerns about the MDS, the current study’s results importantly shed light on a potentially beneficial approach of just three cycles of chemotherapy.
“In Europe and the rest of the world where chemo use is still common, this regimen could be considered,” he told MDedge. “The findings show that if you still use chemo in your practice, this regimen uses 50% less chemotherapy, yet seems to give higher response rates.”
“While MDS and acute myeloid leukemia (AML) remain a concern with any chemotherapy regimen, it is possible that 50% less chemo will lead to less risk of MDS AML, but longer-term follow-up [is needed],” he said.
Dr. Ferrajoli reported that she has received research support from Astra-Zeneca and Beigene. Dr. Jain has received research funding and honoraria from Genentech and Pharmacyclics.
FROM EHA 2022
Obesity linked to smaller testes and possible infertility
new data suggest.
Testicular volume is a fertility marker directly related to sperm count that has halved in the past 40 years worldwide for unknown reasons. At the same time, childhood obesity has risen dramatically and infertility appears to have risen as well, Rossella Cannarella, MD, of the department of endocrinology and andrology, University of Catania (Italy), said at the annual meeting of the Endocrine Society.
According to recent Italian studies, between 14% and 23% of young men aged 18-19 had testicular hypotrophy. “Worryingly, we don’t know the reason for this hypotrophy. And therefore, they are at risk for future infertility,” Dr. Cannarella said during a press briefing.
Her study, which included a total of 264 male children and adolescents, also linked lower testicular volume to hyperinsulinemia and insulin resistance. “The testis is not quiescent in childhood and is sensitive to the hormone insulin. Obesity and metabolic impairment actually can have an effect and negative impact on Sertoli cell proliferation,” Dr. Cannarella said.
Screen testicular volume at all visits
If other studies confirm these results, she said that pediatricians should begin routinely assessing testicular volume at all visits as is now done with height and weight to identify early deflection of the testicular growth curve.
In addition, “include male infertility as a possible consequence of obesity in counseling of male obese children,” she advised.
Asked to comment, Amin Sedaghat Herati, MD, director of male infertility and men’s health at Johns Hopkins Hospital, and assistant professor of urology at Johns Hopkins Medicine, both in Baltimore, said in an interview: “I think what’s really interesting about this study is the association that they’ve made between testicular volume and obesity.”
But, he noted, “it does not implicate necessarily the development of infertility. It’s an extrapolation. So it’s a step towards the link between obesity and infertility, and it’s an important study to establish the association, but changes in testicular volume and even changes in semen panel don’t necessarily indicate fertility or infertility.”
The findings are “consistent with what we know as far as what obesity can potentially do to the activity of the cells in the testes. The authors are postulating that it’s more the support cells, called Sertoli cells, but I would say it’s probably all of the cells that are being affected by obesity and specifically elevated leptin levels,” Dr. Herati said.
He agrees with the recommendation that pediatricians screen all boys for testicular volume. “I agree it’s a good idea so they don’t miss any cases in which the testes don’t develop the way they should or any other conditions,” Dr. Herati said. “I think in general it’s a good practice, especially in the peripubertal stage, to make sure that kids are on the same growth curve and that they’re meeting their Tanner staging. [Pediatricians] should be looking at the size of the testes and tracking, maybe not at every visit, but at least on an annual basis.”
And, he noted, “I think any study that establishes a link that we can point to when we’re educating patients and parents is important.”
Links found between overweight/obesity, testicular hypotrophy
The study population included 61 male children and adolescents with normal weight, 53 with overweight, and 150 with obesity. Insulin resistance (Homeostatic Model Assessment for Insulin Resistance index ≥ 2.5) was present in 97 participants, 22 had prediabetes, and 3 had type 2 diabetes. Clinical data were collected retrospectively.
Among the boys aged 9-14 years, those with overweight and obesity had significantly lower testicular volume, compared with those of normal weight.
Those who were in Tanner Stage 1 were more likely to have overweight and obesity than those with normal weight, suggesting that “overweight and obese adolescents start puberty later than those of normal weight,” Dr. Cannarella said.
In the 14- to 16-year-old age group, those with insulin resistance had lower testicular volume, compared with those without insulin resistance (HOMA index < 2.5). The number of insulin-resistant adolescents was greater than that of controls in the Tanner stage 2 group.
In both the prepubertal (< 9 years) and pubertal (14-16 years) groups, hyperinsulinemia was associated with lower levels of testicular volume.
Hyperinsulinemia did not influence the timing of puberty onset.
No way to quantify the effect of obesity on fertility just yet
During a press briefing, Dr. Cannarella commented that obesity is likely just one of several factors influencing what appears to be an increase in male infertility over time. “It isn’t of course the only reason, but many factors in our environment have drastically changed, compared to 40 years ago, including the prevalence of heavy metals and endocrine disruptors, and of course, the change in habits and higher prevalence of metabolic disease. All of this has an impact on the proliferation of Sertoli cells in childhood and this may explain the trend toward the decline of sperm concentration and count.”
Longitudinal data are needed to establish cause and effect, she noted. “We need longitudinal studies that link the degrees of testicular volume with the degree of the sperm concentration and count starting from childhood and ending with the adult age. This is the missing link so far.”
Dr. Cannarella has reported no relevant financial relationships. Dr. Herati has reported being an advisor for Dadi, LiNA Medical, and Teleflex.
A version of this article first appeared on Medscape.com.
new data suggest.
Testicular volume is a fertility marker directly related to sperm count that has halved in the past 40 years worldwide for unknown reasons. At the same time, childhood obesity has risen dramatically and infertility appears to have risen as well, Rossella Cannarella, MD, of the department of endocrinology and andrology, University of Catania (Italy), said at the annual meeting of the Endocrine Society.
According to recent Italian studies, between 14% and 23% of young men aged 18-19 had testicular hypotrophy. “Worryingly, we don’t know the reason for this hypotrophy. And therefore, they are at risk for future infertility,” Dr. Cannarella said during a press briefing.
Her study, which included a total of 264 male children and adolescents, also linked lower testicular volume to hyperinsulinemia and insulin resistance. “The testis is not quiescent in childhood and is sensitive to the hormone insulin. Obesity and metabolic impairment actually can have an effect and negative impact on Sertoli cell proliferation,” Dr. Cannarella said.
Screen testicular volume at all visits
If other studies confirm these results, she said that pediatricians should begin routinely assessing testicular volume at all visits as is now done with height and weight to identify early deflection of the testicular growth curve.
In addition, “include male infertility as a possible consequence of obesity in counseling of male obese children,” she advised.
Asked to comment, Amin Sedaghat Herati, MD, director of male infertility and men’s health at Johns Hopkins Hospital, and assistant professor of urology at Johns Hopkins Medicine, both in Baltimore, said in an interview: “I think what’s really interesting about this study is the association that they’ve made between testicular volume and obesity.”
But, he noted, “it does not implicate necessarily the development of infertility. It’s an extrapolation. So it’s a step towards the link between obesity and infertility, and it’s an important study to establish the association, but changes in testicular volume and even changes in semen panel don’t necessarily indicate fertility or infertility.”
The findings are “consistent with what we know as far as what obesity can potentially do to the activity of the cells in the testes. The authors are postulating that it’s more the support cells, called Sertoli cells, but I would say it’s probably all of the cells that are being affected by obesity and specifically elevated leptin levels,” Dr. Herati said.
He agrees with the recommendation that pediatricians screen all boys for testicular volume. “I agree it’s a good idea so they don’t miss any cases in which the testes don’t develop the way they should or any other conditions,” Dr. Herati said. “I think in general it’s a good practice, especially in the peripubertal stage, to make sure that kids are on the same growth curve and that they’re meeting their Tanner staging. [Pediatricians] should be looking at the size of the testes and tracking, maybe not at every visit, but at least on an annual basis.”
And, he noted, “I think any study that establishes a link that we can point to when we’re educating patients and parents is important.”
Links found between overweight/obesity, testicular hypotrophy
The study population included 61 male children and adolescents with normal weight, 53 with overweight, and 150 with obesity. Insulin resistance (Homeostatic Model Assessment for Insulin Resistance index ≥ 2.5) was present in 97 participants, 22 had prediabetes, and 3 had type 2 diabetes. Clinical data were collected retrospectively.
Among the boys aged 9-14 years, those with overweight and obesity had significantly lower testicular volume, compared with those of normal weight.
Those who were in Tanner Stage 1 were more likely to have overweight and obesity than those with normal weight, suggesting that “overweight and obese adolescents start puberty later than those of normal weight,” Dr. Cannarella said.
In the 14- to 16-year-old age group, those with insulin resistance had lower testicular volume, compared with those without insulin resistance (HOMA index < 2.5). The number of insulin-resistant adolescents was greater than that of controls in the Tanner stage 2 group.
In both the prepubertal (< 9 years) and pubertal (14-16 years) groups, hyperinsulinemia was associated with lower levels of testicular volume.
Hyperinsulinemia did not influence the timing of puberty onset.
No way to quantify the effect of obesity on fertility just yet
During a press briefing, Dr. Cannarella commented that obesity is likely just one of several factors influencing what appears to be an increase in male infertility over time. “It isn’t of course the only reason, but many factors in our environment have drastically changed, compared to 40 years ago, including the prevalence of heavy metals and endocrine disruptors, and of course, the change in habits and higher prevalence of metabolic disease. All of this has an impact on the proliferation of Sertoli cells in childhood and this may explain the trend toward the decline of sperm concentration and count.”
Longitudinal data are needed to establish cause and effect, she noted. “We need longitudinal studies that link the degrees of testicular volume with the degree of the sperm concentration and count starting from childhood and ending with the adult age. This is the missing link so far.”
Dr. Cannarella has reported no relevant financial relationships. Dr. Herati has reported being an advisor for Dadi, LiNA Medical, and Teleflex.
A version of this article first appeared on Medscape.com.
new data suggest.
Testicular volume is a fertility marker directly related to sperm count that has halved in the past 40 years worldwide for unknown reasons. At the same time, childhood obesity has risen dramatically and infertility appears to have risen as well, Rossella Cannarella, MD, of the department of endocrinology and andrology, University of Catania (Italy), said at the annual meeting of the Endocrine Society.
According to recent Italian studies, between 14% and 23% of young men aged 18-19 had testicular hypotrophy. “Worryingly, we don’t know the reason for this hypotrophy. And therefore, they are at risk for future infertility,” Dr. Cannarella said during a press briefing.
Her study, which included a total of 264 male children and adolescents, also linked lower testicular volume to hyperinsulinemia and insulin resistance. “The testis is not quiescent in childhood and is sensitive to the hormone insulin. Obesity and metabolic impairment actually can have an effect and negative impact on Sertoli cell proliferation,” Dr. Cannarella said.
Screen testicular volume at all visits
If other studies confirm these results, she said that pediatricians should begin routinely assessing testicular volume at all visits as is now done with height and weight to identify early deflection of the testicular growth curve.
In addition, “include male infertility as a possible consequence of obesity in counseling of male obese children,” she advised.
Asked to comment, Amin Sedaghat Herati, MD, director of male infertility and men’s health at Johns Hopkins Hospital, and assistant professor of urology at Johns Hopkins Medicine, both in Baltimore, said in an interview: “I think what’s really interesting about this study is the association that they’ve made between testicular volume and obesity.”
But, he noted, “it does not implicate necessarily the development of infertility. It’s an extrapolation. So it’s a step towards the link between obesity and infertility, and it’s an important study to establish the association, but changes in testicular volume and even changes in semen panel don’t necessarily indicate fertility or infertility.”
The findings are “consistent with what we know as far as what obesity can potentially do to the activity of the cells in the testes. The authors are postulating that it’s more the support cells, called Sertoli cells, but I would say it’s probably all of the cells that are being affected by obesity and specifically elevated leptin levels,” Dr. Herati said.
He agrees with the recommendation that pediatricians screen all boys for testicular volume. “I agree it’s a good idea so they don’t miss any cases in which the testes don’t develop the way they should or any other conditions,” Dr. Herati said. “I think in general it’s a good practice, especially in the peripubertal stage, to make sure that kids are on the same growth curve and that they’re meeting their Tanner staging. [Pediatricians] should be looking at the size of the testes and tracking, maybe not at every visit, but at least on an annual basis.”
And, he noted, “I think any study that establishes a link that we can point to when we’re educating patients and parents is important.”
Links found between overweight/obesity, testicular hypotrophy
The study population included 61 male children and adolescents with normal weight, 53 with overweight, and 150 with obesity. Insulin resistance (Homeostatic Model Assessment for Insulin Resistance index ≥ 2.5) was present in 97 participants, 22 had prediabetes, and 3 had type 2 diabetes. Clinical data were collected retrospectively.
Among the boys aged 9-14 years, those with overweight and obesity had significantly lower testicular volume, compared with those of normal weight.
Those who were in Tanner Stage 1 were more likely to have overweight and obesity than those with normal weight, suggesting that “overweight and obese adolescents start puberty later than those of normal weight,” Dr. Cannarella said.
In the 14- to 16-year-old age group, those with insulin resistance had lower testicular volume, compared with those without insulin resistance (HOMA index < 2.5). The number of insulin-resistant adolescents was greater than that of controls in the Tanner stage 2 group.
In both the prepubertal (< 9 years) and pubertal (14-16 years) groups, hyperinsulinemia was associated with lower levels of testicular volume.
Hyperinsulinemia did not influence the timing of puberty onset.
No way to quantify the effect of obesity on fertility just yet
During a press briefing, Dr. Cannarella commented that obesity is likely just one of several factors influencing what appears to be an increase in male infertility over time. “It isn’t of course the only reason, but many factors in our environment have drastically changed, compared to 40 years ago, including the prevalence of heavy metals and endocrine disruptors, and of course, the change in habits and higher prevalence of metabolic disease. All of this has an impact on the proliferation of Sertoli cells in childhood and this may explain the trend toward the decline of sperm concentration and count.”
Longitudinal data are needed to establish cause and effect, she noted. “We need longitudinal studies that link the degrees of testicular volume with the degree of the sperm concentration and count starting from childhood and ending with the adult age. This is the missing link so far.”
Dr. Cannarella has reported no relevant financial relationships. Dr. Herati has reported being an advisor for Dadi, LiNA Medical, and Teleflex.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022