User login
MDedge conference coverage features onsite reporting of the latest study results and expert perspectives from leading researchers.
Artificial intelligence presents opportunities, challenges in neurologic practice
PHOENIX – and it presents opportunities for increased production and automation of some tasks. However, it is prone to error and ‘hallucinations’ despite an authoritative tone, so its conclusions must be verified.
Those were some of the messages from a talk by John Morren, MD, an associate professor of neurology at Case Western Reserve University, Cleveland, who spoke about AI at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
He encouraged attendees to get involved in the conversation of AI, because it is here to stay and will have a big impact on health care. “If we’re not around the table making decisions, decisions will be made for us in our absence and won’t be in our favor,” said Dr. Morren.
He started out his talk by asking if anyone in the room had used AI. After about half raised their hands, he countered that nearly everyone likely had. Voice assistants like SIRI and Alexa, social media with curated feeds, online shopping tools that provide product suggestions, and content recommendations from streaming services like Netflix all rely on AI technology.
Within medicine, AI is already playing a role in various fields, including medical imaging, disease diagnosis, drug discovery and development, predictive analytics, personalized medicine, telemedicine, and health care management.
It also has potential to be used on the job. For example, ChatGPT can generate and refine conversations towards a specific length, format, style, and level of detail. Alternatives include Bing AI from Microsoft, Bard AI from Google, Writesonic, Copy.ai, SpinBot, HIX.AI, and Chatsonic.
Specific to medicine, Consensus is a search engine that uses AI to search for, summarize, and synthesize studies from peer-reviewed literature.
Trust, but verify
Dr. Morren presented some specific use cases, including patient education and responses to patient inquiries, as well as generating letters to insurance companies appealing denial of coverage claims. He also showed an example where he asked Bing AI to explain to a patient, at a sixth- to seventh-grade reading level, the red-flag symptoms of myasthenic crisis.
AI can generate summaries of clinical evidence of previous studies. Asked by this reporter how to trust the accuracies of the summaries if the user hasn’t thoroughly read the papers, he acknowledged the imperfection of AI. “I would say that if you’re going to make a decision that you would not have made normally based on the summary that it’s giving, if you can find the fact that you’re anchoring the decision on, go into the article yourself and make sure that it’s well vetted. The AI is just good to tap you on your shoulder and say, ‘hey, just consider this.’ That’s all it is. You should always trust, but verify. If the AI is forcing you to say something new that you would not say, maybe don’t do it – or at least research it to know that it’s the truth and then you elevate yourself and get yourself to the next level.”
Limitations
The need to verify can create its own burden, according to one attendee. “I often find I end up spending more time verifying [what ChatGPT has provided]. This seems to take more time than a traditional way of going to PubMed or UpToDate or any of the other human generated consensus way,” he said.
Dr. Morren replied that he wouldn’t recommend using ChatGPT to query medical literature. Instead he recommended Consensus, which only searches the peer-reviewed medical literature.
Another key limitation is that most AI programs are date limited: For example, ChatGPT doesn’t include information after September 2021, though this may change with paid subscriptions. He also starkly warned the audience to never enter sensitive information, including patient identifiers.
There are legal and ethical considerations to AI. Dr. Morren warned against overreliance on AI, as this could undermine compassion and lead to erosion of trust, which makes it important to disclose any use of AI-generated content.
Another attendee raised concerns that AI may be generating research content, including slides for presentations, abstracts, titles, or article text. Dr. Morren said that some organizations, such as the International Committee of Medical Journal Editors, have incorporated AI in their recommendations, stating that authors should disclose any contributions of AI to their publications. However, there is little that can be done to identify AI-generated content, leaving it up to the honor code.
Asked to make predictions about how AI will evolve in the clinic over the next 2-3 years, Dr. Morren suggested that it will likely be embedded in electronic medical records. He anticipated that it will save physicians time so that they can spend more time interacting directly with patients. He quoted Eric Topol, MD, professor of medicine at Scripps Research Translational Institute, La Jolla, Calif., as saying that AI could save 20% of a physician’s time, which could be spent with patients. Dr. Morren saw it differently. “I know where that 20% of time liberated is going to go. I’m going to see 20% more patients. I’m a realist,” he said, to audience laughter.
He also predicted that AI will be found in wearables and devices, allowing health care to expand into the patient’s home in real time. “A lot of what we’re wearing is going to be an extension of the doctor’s office,” he said.
For those hoping for more guidance, Dr. Morren noted that he is the chairman of the professional practice committee of AANEM, and the group will be putting out a position statement within the next couple of months. “It will be a little bit of a blueprint for the path going forward. There are specific things that need to be done. In research, for example, you have to ensure that datasets are diverse enough. To do that we need to have inter-institutional collaboration. We have to ensure patient privacy. Consent for this needs to be a little more explicit because this is a novel area. Those are things that need to be stipulated and ratified through a task force.”
Dr. Morren has no relevant financial disclosures.
PHOENIX – and it presents opportunities for increased production and automation of some tasks. However, it is prone to error and ‘hallucinations’ despite an authoritative tone, so its conclusions must be verified.
Those were some of the messages from a talk by John Morren, MD, an associate professor of neurology at Case Western Reserve University, Cleveland, who spoke about AI at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
He encouraged attendees to get involved in the conversation of AI, because it is here to stay and will have a big impact on health care. “If we’re not around the table making decisions, decisions will be made for us in our absence and won’t be in our favor,” said Dr. Morren.
He started out his talk by asking if anyone in the room had used AI. After about half raised their hands, he countered that nearly everyone likely had. Voice assistants like SIRI and Alexa, social media with curated feeds, online shopping tools that provide product suggestions, and content recommendations from streaming services like Netflix all rely on AI technology.
Within medicine, AI is already playing a role in various fields, including medical imaging, disease diagnosis, drug discovery and development, predictive analytics, personalized medicine, telemedicine, and health care management.
It also has potential to be used on the job. For example, ChatGPT can generate and refine conversations towards a specific length, format, style, and level of detail. Alternatives include Bing AI from Microsoft, Bard AI from Google, Writesonic, Copy.ai, SpinBot, HIX.AI, and Chatsonic.
Specific to medicine, Consensus is a search engine that uses AI to search for, summarize, and synthesize studies from peer-reviewed literature.
Trust, but verify
Dr. Morren presented some specific use cases, including patient education and responses to patient inquiries, as well as generating letters to insurance companies appealing denial of coverage claims. He also showed an example where he asked Bing AI to explain to a patient, at a sixth- to seventh-grade reading level, the red-flag symptoms of myasthenic crisis.
AI can generate summaries of clinical evidence of previous studies. Asked by this reporter how to trust the accuracies of the summaries if the user hasn’t thoroughly read the papers, he acknowledged the imperfection of AI. “I would say that if you’re going to make a decision that you would not have made normally based on the summary that it’s giving, if you can find the fact that you’re anchoring the decision on, go into the article yourself and make sure that it’s well vetted. The AI is just good to tap you on your shoulder and say, ‘hey, just consider this.’ That’s all it is. You should always trust, but verify. If the AI is forcing you to say something new that you would not say, maybe don’t do it – or at least research it to know that it’s the truth and then you elevate yourself and get yourself to the next level.”
Limitations
The need to verify can create its own burden, according to one attendee. “I often find I end up spending more time verifying [what ChatGPT has provided]. This seems to take more time than a traditional way of going to PubMed or UpToDate or any of the other human generated consensus way,” he said.
Dr. Morren replied that he wouldn’t recommend using ChatGPT to query medical literature. Instead he recommended Consensus, which only searches the peer-reviewed medical literature.
Another key limitation is that most AI programs are date limited: For example, ChatGPT doesn’t include information after September 2021, though this may change with paid subscriptions. He also starkly warned the audience to never enter sensitive information, including patient identifiers.
There are legal and ethical considerations to AI. Dr. Morren warned against overreliance on AI, as this could undermine compassion and lead to erosion of trust, which makes it important to disclose any use of AI-generated content.
Another attendee raised concerns that AI may be generating research content, including slides for presentations, abstracts, titles, or article text. Dr. Morren said that some organizations, such as the International Committee of Medical Journal Editors, have incorporated AI in their recommendations, stating that authors should disclose any contributions of AI to their publications. However, there is little that can be done to identify AI-generated content, leaving it up to the honor code.
Asked to make predictions about how AI will evolve in the clinic over the next 2-3 years, Dr. Morren suggested that it will likely be embedded in electronic medical records. He anticipated that it will save physicians time so that they can spend more time interacting directly with patients. He quoted Eric Topol, MD, professor of medicine at Scripps Research Translational Institute, La Jolla, Calif., as saying that AI could save 20% of a physician’s time, which could be spent with patients. Dr. Morren saw it differently. “I know where that 20% of time liberated is going to go. I’m going to see 20% more patients. I’m a realist,” he said, to audience laughter.
He also predicted that AI will be found in wearables and devices, allowing health care to expand into the patient’s home in real time. “A lot of what we’re wearing is going to be an extension of the doctor’s office,” he said.
For those hoping for more guidance, Dr. Morren noted that he is the chairman of the professional practice committee of AANEM, and the group will be putting out a position statement within the next couple of months. “It will be a little bit of a blueprint for the path going forward. There are specific things that need to be done. In research, for example, you have to ensure that datasets are diverse enough. To do that we need to have inter-institutional collaboration. We have to ensure patient privacy. Consent for this needs to be a little more explicit because this is a novel area. Those are things that need to be stipulated and ratified through a task force.”
Dr. Morren has no relevant financial disclosures.
PHOENIX – and it presents opportunities for increased production and automation of some tasks. However, it is prone to error and ‘hallucinations’ despite an authoritative tone, so its conclusions must be verified.
Those were some of the messages from a talk by John Morren, MD, an associate professor of neurology at Case Western Reserve University, Cleveland, who spoke about AI at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
He encouraged attendees to get involved in the conversation of AI, because it is here to stay and will have a big impact on health care. “If we’re not around the table making decisions, decisions will be made for us in our absence and won’t be in our favor,” said Dr. Morren.
He started out his talk by asking if anyone in the room had used AI. After about half raised their hands, he countered that nearly everyone likely had. Voice assistants like SIRI and Alexa, social media with curated feeds, online shopping tools that provide product suggestions, and content recommendations from streaming services like Netflix all rely on AI technology.
Within medicine, AI is already playing a role in various fields, including medical imaging, disease diagnosis, drug discovery and development, predictive analytics, personalized medicine, telemedicine, and health care management.
It also has potential to be used on the job. For example, ChatGPT can generate and refine conversations towards a specific length, format, style, and level of detail. Alternatives include Bing AI from Microsoft, Bard AI from Google, Writesonic, Copy.ai, SpinBot, HIX.AI, and Chatsonic.
Specific to medicine, Consensus is a search engine that uses AI to search for, summarize, and synthesize studies from peer-reviewed literature.
Trust, but verify
Dr. Morren presented some specific use cases, including patient education and responses to patient inquiries, as well as generating letters to insurance companies appealing denial of coverage claims. He also showed an example where he asked Bing AI to explain to a patient, at a sixth- to seventh-grade reading level, the red-flag symptoms of myasthenic crisis.
AI can generate summaries of clinical evidence of previous studies. Asked by this reporter how to trust the accuracies of the summaries if the user hasn’t thoroughly read the papers, he acknowledged the imperfection of AI. “I would say that if you’re going to make a decision that you would not have made normally based on the summary that it’s giving, if you can find the fact that you’re anchoring the decision on, go into the article yourself and make sure that it’s well vetted. The AI is just good to tap you on your shoulder and say, ‘hey, just consider this.’ That’s all it is. You should always trust, but verify. If the AI is forcing you to say something new that you would not say, maybe don’t do it – or at least research it to know that it’s the truth and then you elevate yourself and get yourself to the next level.”
Limitations
The need to verify can create its own burden, according to one attendee. “I often find I end up spending more time verifying [what ChatGPT has provided]. This seems to take more time than a traditional way of going to PubMed or UpToDate or any of the other human generated consensus way,” he said.
Dr. Morren replied that he wouldn’t recommend using ChatGPT to query medical literature. Instead he recommended Consensus, which only searches the peer-reviewed medical literature.
Another key limitation is that most AI programs are date limited: For example, ChatGPT doesn’t include information after September 2021, though this may change with paid subscriptions. He also starkly warned the audience to never enter sensitive information, including patient identifiers.
There are legal and ethical considerations to AI. Dr. Morren warned against overreliance on AI, as this could undermine compassion and lead to erosion of trust, which makes it important to disclose any use of AI-generated content.
Another attendee raised concerns that AI may be generating research content, including slides for presentations, abstracts, titles, or article text. Dr. Morren said that some organizations, such as the International Committee of Medical Journal Editors, have incorporated AI in their recommendations, stating that authors should disclose any contributions of AI to their publications. However, there is little that can be done to identify AI-generated content, leaving it up to the honor code.
Asked to make predictions about how AI will evolve in the clinic over the next 2-3 years, Dr. Morren suggested that it will likely be embedded in electronic medical records. He anticipated that it will save physicians time so that they can spend more time interacting directly with patients. He quoted Eric Topol, MD, professor of medicine at Scripps Research Translational Institute, La Jolla, Calif., as saying that AI could save 20% of a physician’s time, which could be spent with patients. Dr. Morren saw it differently. “I know where that 20% of time liberated is going to go. I’m going to see 20% more patients. I’m a realist,” he said, to audience laughter.
He also predicted that AI will be found in wearables and devices, allowing health care to expand into the patient’s home in real time. “A lot of what we’re wearing is going to be an extension of the doctor’s office,” he said.
For those hoping for more guidance, Dr. Morren noted that he is the chairman of the professional practice committee of AANEM, and the group will be putting out a position statement within the next couple of months. “It will be a little bit of a blueprint for the path going forward. There are specific things that need to be done. In research, for example, you have to ensure that datasets are diverse enough. To do that we need to have inter-institutional collaboration. We have to ensure patient privacy. Consent for this needs to be a little more explicit because this is a novel area. Those are things that need to be stipulated and ratified through a task force.”
Dr. Morren has no relevant financial disclosures.
AT AANEM 2023
Survey: 42% of PCPs not familiar with biologics for asthma
ANAHEIM, CALIF. – Patients with uncontrolled asthma are seen more often by primary care providers (PCPs) than by allergists, but a survey has found that
Bijalben Patel, MD, with the department of internal medicine, University of South Florida, Tampa, said in an interview that in addition to the considerable lack of knowledge of biologics in primary care, she was surprised that 77% of survey participants stated they only referred patients to specialists after two or more exacerbations.
“This is important because these patients are considered to have exacerbation-prone asthma, which should be managed by specialists,” she said.
She said that being “unfamiliar” with biologics meant that the healthcare provider may have heard of biologics but did not know the various types, initiation criteria, or side effects.
The researchers administered a REDCap (Research Electronic Data Capture) survey by email to primary care attending and resident physicians in the departments of internal medicine, family medicine, and pediatrics, and 85 responded. Responses were compared using Chi-square tests.
Patel presented the results of the survey at the annual meeting of the American College of Allergy, Asthma & Immunology.
82% do not order labs
Familiarity did not vary in primary care with number of patients with asthma seen per month, the researchers noted.
“Also, the frequency of PCP referrals to a specialist did not change familiarity with biologics (P = .260) or eligibility criteria (P = .393),” the researchers said.
In addition, they found that 82% of those surveyed do not order labs, and 90% do not use absolute eosinophil count to guide care.
Dr. Patel explained that lab work such as obtaining IgE levels and a complete blood count with a differential and examining the absolute eosinophil count help identify patients who are at high risk for future exacerbation and also treatable phenotypic traits, which can be targeted with biologic therapy.
Angela Duff Hogan, MD, vice chair of the ACAAI Asthma Committee and professor of pediatrics at Eastern Virginia Medical School, Norfolk, said in an interview that she finds the delay on referrals the most concerning finding in the survey results.
“I’m not as concerned they are not obtaining labs,” said Dr. Hogan, who was not part of the study. “The specialist can do that. It’s more concerning they wait so long to refer a patient with poorly controlled asthma. We know that asthma patients treated by an allergist have better asthma control, better quality of life, and reduced health care costs.”
Asthma specialists ‘need better marketing’
Dr. Hogan said that the results show the need for more studies to demonstrate that asthma specialists can improve outcomes and reduce healthcare costs.
“Objective data is more convincing than subjective data,” she noted. “As a specialty, we need to disseminate more information about asthma management, the “new” asthma guidelines, SMART/MART therapy, and the importance of biologicals in asthma. We need better marketing as a specialty in asthma care.”
Dr. Patel said that their goal with the study is to raise awareness about the available asthma biologic therapies, which have been improving care for 2 decades.
“The results of the survey point to the need to improve the communication between primary care physicians and asthma care specialists, including regarding use of biologics,” senior author Juan Carlos Cardet, MD, MPH, also an allergy specialist at USF, added in a press release. “Biologics have become an important tool in the treatment of asthma and other allergic diseases such as atopic dermatitis (eczema), chronic rhinosinusitis with nasal polyps and eosinophilic esophagitis, and can prevent substantial ill results from occurring in patients who are eligible for them.”
The study authors and Dr. Hogan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. – Patients with uncontrolled asthma are seen more often by primary care providers (PCPs) than by allergists, but a survey has found that
Bijalben Patel, MD, with the department of internal medicine, University of South Florida, Tampa, said in an interview that in addition to the considerable lack of knowledge of biologics in primary care, she was surprised that 77% of survey participants stated they only referred patients to specialists after two or more exacerbations.
“This is important because these patients are considered to have exacerbation-prone asthma, which should be managed by specialists,” she said.
She said that being “unfamiliar” with biologics meant that the healthcare provider may have heard of biologics but did not know the various types, initiation criteria, or side effects.
The researchers administered a REDCap (Research Electronic Data Capture) survey by email to primary care attending and resident physicians in the departments of internal medicine, family medicine, and pediatrics, and 85 responded. Responses were compared using Chi-square tests.
Patel presented the results of the survey at the annual meeting of the American College of Allergy, Asthma & Immunology.
82% do not order labs
Familiarity did not vary in primary care with number of patients with asthma seen per month, the researchers noted.
“Also, the frequency of PCP referrals to a specialist did not change familiarity with biologics (P = .260) or eligibility criteria (P = .393),” the researchers said.
In addition, they found that 82% of those surveyed do not order labs, and 90% do not use absolute eosinophil count to guide care.
Dr. Patel explained that lab work such as obtaining IgE levels and a complete blood count with a differential and examining the absolute eosinophil count help identify patients who are at high risk for future exacerbation and also treatable phenotypic traits, which can be targeted with biologic therapy.
Angela Duff Hogan, MD, vice chair of the ACAAI Asthma Committee and professor of pediatrics at Eastern Virginia Medical School, Norfolk, said in an interview that she finds the delay on referrals the most concerning finding in the survey results.
“I’m not as concerned they are not obtaining labs,” said Dr. Hogan, who was not part of the study. “The specialist can do that. It’s more concerning they wait so long to refer a patient with poorly controlled asthma. We know that asthma patients treated by an allergist have better asthma control, better quality of life, and reduced health care costs.”
Asthma specialists ‘need better marketing’
Dr. Hogan said that the results show the need for more studies to demonstrate that asthma specialists can improve outcomes and reduce healthcare costs.
“Objective data is more convincing than subjective data,” she noted. “As a specialty, we need to disseminate more information about asthma management, the “new” asthma guidelines, SMART/MART therapy, and the importance of biologicals in asthma. We need better marketing as a specialty in asthma care.”
Dr. Patel said that their goal with the study is to raise awareness about the available asthma biologic therapies, which have been improving care for 2 decades.
“The results of the survey point to the need to improve the communication between primary care physicians and asthma care specialists, including regarding use of biologics,” senior author Juan Carlos Cardet, MD, MPH, also an allergy specialist at USF, added in a press release. “Biologics have become an important tool in the treatment of asthma and other allergic diseases such as atopic dermatitis (eczema), chronic rhinosinusitis with nasal polyps and eosinophilic esophagitis, and can prevent substantial ill results from occurring in patients who are eligible for them.”
The study authors and Dr. Hogan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. – Patients with uncontrolled asthma are seen more often by primary care providers (PCPs) than by allergists, but a survey has found that
Bijalben Patel, MD, with the department of internal medicine, University of South Florida, Tampa, said in an interview that in addition to the considerable lack of knowledge of biologics in primary care, she was surprised that 77% of survey participants stated they only referred patients to specialists after two or more exacerbations.
“This is important because these patients are considered to have exacerbation-prone asthma, which should be managed by specialists,” she said.
She said that being “unfamiliar” with biologics meant that the healthcare provider may have heard of biologics but did not know the various types, initiation criteria, or side effects.
The researchers administered a REDCap (Research Electronic Data Capture) survey by email to primary care attending and resident physicians in the departments of internal medicine, family medicine, and pediatrics, and 85 responded. Responses were compared using Chi-square tests.
Patel presented the results of the survey at the annual meeting of the American College of Allergy, Asthma & Immunology.
82% do not order labs
Familiarity did not vary in primary care with number of patients with asthma seen per month, the researchers noted.
“Also, the frequency of PCP referrals to a specialist did not change familiarity with biologics (P = .260) or eligibility criteria (P = .393),” the researchers said.
In addition, they found that 82% of those surveyed do not order labs, and 90% do not use absolute eosinophil count to guide care.
Dr. Patel explained that lab work such as obtaining IgE levels and a complete blood count with a differential and examining the absolute eosinophil count help identify patients who are at high risk for future exacerbation and also treatable phenotypic traits, which can be targeted with biologic therapy.
Angela Duff Hogan, MD, vice chair of the ACAAI Asthma Committee and professor of pediatrics at Eastern Virginia Medical School, Norfolk, said in an interview that she finds the delay on referrals the most concerning finding in the survey results.
“I’m not as concerned they are not obtaining labs,” said Dr. Hogan, who was not part of the study. “The specialist can do that. It’s more concerning they wait so long to refer a patient with poorly controlled asthma. We know that asthma patients treated by an allergist have better asthma control, better quality of life, and reduced health care costs.”
Asthma specialists ‘need better marketing’
Dr. Hogan said that the results show the need for more studies to demonstrate that asthma specialists can improve outcomes and reduce healthcare costs.
“Objective data is more convincing than subjective data,” she noted. “As a specialty, we need to disseminate more information about asthma management, the “new” asthma guidelines, SMART/MART therapy, and the importance of biologicals in asthma. We need better marketing as a specialty in asthma care.”
Dr. Patel said that their goal with the study is to raise awareness about the available asthma biologic therapies, which have been improving care for 2 decades.
“The results of the survey point to the need to improve the communication between primary care physicians and asthma care specialists, including regarding use of biologics,” senior author Juan Carlos Cardet, MD, MPH, also an allergy specialist at USF, added in a press release. “Biologics have become an important tool in the treatment of asthma and other allergic diseases such as atopic dermatitis (eczema), chronic rhinosinusitis with nasal polyps and eosinophilic esophagitis, and can prevent substantial ill results from occurring in patients who are eligible for them.”
The study authors and Dr. Hogan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ACAAI 2023
For AFib cardioversion in obesity, dual energy might be the answer
PHILADELPHIA – , a multicenter randomized trial shows.
When treated with dual direct current cardioversion (DCCV), only 2% of patients with obesity failed to cardiovert on the first shock versus 14% (P = .002) of those treated with a conventional single DCCV, reported Joshua D. Aymond, MD, a fellow in electrophysiology at Ochsner Health, New Orleans.
Of the 14 patients in the single DCCV arm who did not convert on the first shock, 12 cardioverted when switched to dual energy. The remaining two cardioverted on the second dual shock.
In the dual DCCV group, of the two patients who did not cardiovert on the first dual shock, one did on the second. The other also cardioverted on a second shock, but this second shock was not delivered for 2 weeks, during which time the patient received a course of amiodarone-based anti-arrhythmic therapy.
No disadvantages seen with dual energy
The greater efficacy of a first shock with dual DCCV was achieved with no apparent disadvantages. There were no differences in post-procedure chest discomfort and no procedure-related adverse events in either arm, Dr. Aymond said.
The rising prevalence of obesity in the United States has created the need for a more effective first-line strategy for AF, noted Dr. Aymond, who presented the results of this study at the annual scientific sessions of the American Heart Association.
Cardioversion, which he characterized as the treatment of choice for AF, “fails to restore sinus rhythm in 20% to 35% of obese patients versus less than 10% of non-obese patients,” he said. The higher failure rate in patients with obesity is becoming a more common clinical issue not only due to the rising rates of obesity but a corresponding rise in AF, which is a related phenomenon.
“The risk of atrial fibrillation is increased by 50% relative to those who are not obese,” Dr. Aymond explained.
In this study, 200 patients at three participating centers were randomized to single DCCV or double DCCV after exclusions that included ventricular tachycardia and respiratory instability. The baseline characteristics were comparable. All 101 patients in the single DCCV group and 99 patients in the dual DCCV group were available for the intention-to-treat analysis.
200 vs. 400 joules delivered across the heart
In the study protocol, patients were fitted with four chest pads, two located adjacent but above the heart and two adjacent but below the heart. For single DCCV, 200 joules of energy were delivered from the upper right pad to the lower left pad across the heart. For dual DCCV, another 200 joules were delivered simultaneously from the upper left to the lower right across the heart. The total dose in the dual DCCV group was 400 joules.
The primary outcome was restoration of sinus rhythm of any duration immediately after DCCV. Safety, including clinical events, was a secondary outcome. Only the patients were blinded to the energy they received.
On univariate analysis, the odds ratio for successful cardioversion with dual DCCV was nearly eightfold higher (OR 7.8; P = .008) than single DCCV. On a simple multivariable analysis, when the researchers controlled for just age, sex, and body mass index, the odds ratio rose (OR 8.5; P = .007).
On a comprehensive multivariable analysis adding control for such characteristics as left ventricular ejection fraction (LVEF), obstructive sleep apnea, and antiarrhythmic drugs, the advantage of dual DCCV climbed above 12-fold (OR 12.6; P = .03).
The study is addressing a relevant and persistent question, said the AHA-invited discussant Jose A. Joglar, MD, program director, Clinical Cardiac Electrophysiology Fellowship, University of Texas Southwestern Medical Center, Dallas.
Dr. Joglar pointed out that alternatives to single DCCV for patients more difficult to cardiovert have been “sought for decades.” He noted that a variety of techniques, including dual DCCV, have been evaluated in small studies and case reports.
Alternatives for obese outlined
Several have shown promise, Dr. Joglar said. As one of several examples, he cited a 20-patient study that randomized patients to adhesive patches, like those employed in the Aymond trial, or handheld paddles. Both patches and paddles were applied with manual pressure while a 200-joule shock was delivered. The proportion of patients who cardioverted on the first shock was almost two times higher in the group after the first shock with the paddles (50% vs. 27%; P = .01). Dr. Joglar said the study supports the principle that 200 joules delivered by adhesive patches is inadequate for treatment of AF in many patients with obesity.
Dr. Joglar also cited studies suggesting that single DCCV delivered with higher energy than 200 joules appears to improve cardioversion success rates, but he indicated that this study with dual DCCV in the front-line setting provides evidence for another alternative.
“This is the first such trial with dual defibrillators as an initial strategy,” he said, calling the groups well matched and the superiority of dual DCCV “impressive.” He cautioned that the study size was well powered for the endpoint but perhaps small for evaluating relative safety.
Yet, “the study adds credibility and confidence for the use of dual DCCV, especially in difficult or refractory patients,” he said. He is less certain that it establishes dual DCCV as a standard first-line therapy in all patients with obesity. This would require additional studies to compare it to other types of strategies such as those he mentioned.
As an option for improving cardioversion in first-line treatment, dual DCCV “can be added to a list of other techniques, such as manual pressure or a higher initial dose with single DCCV,” he said.
Dr. Aymond and Dr. Joglar report no potential conflicts of interest.
PHILADELPHIA – , a multicenter randomized trial shows.
When treated with dual direct current cardioversion (DCCV), only 2% of patients with obesity failed to cardiovert on the first shock versus 14% (P = .002) of those treated with a conventional single DCCV, reported Joshua D. Aymond, MD, a fellow in electrophysiology at Ochsner Health, New Orleans.
Of the 14 patients in the single DCCV arm who did not convert on the first shock, 12 cardioverted when switched to dual energy. The remaining two cardioverted on the second dual shock.
In the dual DCCV group, of the two patients who did not cardiovert on the first dual shock, one did on the second. The other also cardioverted on a second shock, but this second shock was not delivered for 2 weeks, during which time the patient received a course of amiodarone-based anti-arrhythmic therapy.
No disadvantages seen with dual energy
The greater efficacy of a first shock with dual DCCV was achieved with no apparent disadvantages. There were no differences in post-procedure chest discomfort and no procedure-related adverse events in either arm, Dr. Aymond said.
The rising prevalence of obesity in the United States has created the need for a more effective first-line strategy for AF, noted Dr. Aymond, who presented the results of this study at the annual scientific sessions of the American Heart Association.
Cardioversion, which he characterized as the treatment of choice for AF, “fails to restore sinus rhythm in 20% to 35% of obese patients versus less than 10% of non-obese patients,” he said. The higher failure rate in patients with obesity is becoming a more common clinical issue not only due to the rising rates of obesity but a corresponding rise in AF, which is a related phenomenon.
“The risk of atrial fibrillation is increased by 50% relative to those who are not obese,” Dr. Aymond explained.
In this study, 200 patients at three participating centers were randomized to single DCCV or double DCCV after exclusions that included ventricular tachycardia and respiratory instability. The baseline characteristics were comparable. All 101 patients in the single DCCV group and 99 patients in the dual DCCV group were available for the intention-to-treat analysis.
200 vs. 400 joules delivered across the heart
In the study protocol, patients were fitted with four chest pads, two located adjacent but above the heart and two adjacent but below the heart. For single DCCV, 200 joules of energy were delivered from the upper right pad to the lower left pad across the heart. For dual DCCV, another 200 joules were delivered simultaneously from the upper left to the lower right across the heart. The total dose in the dual DCCV group was 400 joules.
The primary outcome was restoration of sinus rhythm of any duration immediately after DCCV. Safety, including clinical events, was a secondary outcome. Only the patients were blinded to the energy they received.
On univariate analysis, the odds ratio for successful cardioversion with dual DCCV was nearly eightfold higher (OR 7.8; P = .008) than single DCCV. On a simple multivariable analysis, when the researchers controlled for just age, sex, and body mass index, the odds ratio rose (OR 8.5; P = .007).
On a comprehensive multivariable analysis adding control for such characteristics as left ventricular ejection fraction (LVEF), obstructive sleep apnea, and antiarrhythmic drugs, the advantage of dual DCCV climbed above 12-fold (OR 12.6; P = .03).
The study is addressing a relevant and persistent question, said the AHA-invited discussant Jose A. Joglar, MD, program director, Clinical Cardiac Electrophysiology Fellowship, University of Texas Southwestern Medical Center, Dallas.
Dr. Joglar pointed out that alternatives to single DCCV for patients more difficult to cardiovert have been “sought for decades.” He noted that a variety of techniques, including dual DCCV, have been evaluated in small studies and case reports.
Alternatives for obese outlined
Several have shown promise, Dr. Joglar said. As one of several examples, he cited a 20-patient study that randomized patients to adhesive patches, like those employed in the Aymond trial, or handheld paddles. Both patches and paddles were applied with manual pressure while a 200-joule shock was delivered. The proportion of patients who cardioverted on the first shock was almost two times higher in the group after the first shock with the paddles (50% vs. 27%; P = .01). Dr. Joglar said the study supports the principle that 200 joules delivered by adhesive patches is inadequate for treatment of AF in many patients with obesity.
Dr. Joglar also cited studies suggesting that single DCCV delivered with higher energy than 200 joules appears to improve cardioversion success rates, but he indicated that this study with dual DCCV in the front-line setting provides evidence for another alternative.
“This is the first such trial with dual defibrillators as an initial strategy,” he said, calling the groups well matched and the superiority of dual DCCV “impressive.” He cautioned that the study size was well powered for the endpoint but perhaps small for evaluating relative safety.
Yet, “the study adds credibility and confidence for the use of dual DCCV, especially in difficult or refractory patients,” he said. He is less certain that it establishes dual DCCV as a standard first-line therapy in all patients with obesity. This would require additional studies to compare it to other types of strategies such as those he mentioned.
As an option for improving cardioversion in first-line treatment, dual DCCV “can be added to a list of other techniques, such as manual pressure or a higher initial dose with single DCCV,” he said.
Dr. Aymond and Dr. Joglar report no potential conflicts of interest.
PHILADELPHIA – , a multicenter randomized trial shows.
When treated with dual direct current cardioversion (DCCV), only 2% of patients with obesity failed to cardiovert on the first shock versus 14% (P = .002) of those treated with a conventional single DCCV, reported Joshua D. Aymond, MD, a fellow in electrophysiology at Ochsner Health, New Orleans.
Of the 14 patients in the single DCCV arm who did not convert on the first shock, 12 cardioverted when switched to dual energy. The remaining two cardioverted on the second dual shock.
In the dual DCCV group, of the two patients who did not cardiovert on the first dual shock, one did on the second. The other also cardioverted on a second shock, but this second shock was not delivered for 2 weeks, during which time the patient received a course of amiodarone-based anti-arrhythmic therapy.
No disadvantages seen with dual energy
The greater efficacy of a first shock with dual DCCV was achieved with no apparent disadvantages. There were no differences in post-procedure chest discomfort and no procedure-related adverse events in either arm, Dr. Aymond said.
The rising prevalence of obesity in the United States has created the need for a more effective first-line strategy for AF, noted Dr. Aymond, who presented the results of this study at the annual scientific sessions of the American Heart Association.
Cardioversion, which he characterized as the treatment of choice for AF, “fails to restore sinus rhythm in 20% to 35% of obese patients versus less than 10% of non-obese patients,” he said. The higher failure rate in patients with obesity is becoming a more common clinical issue not only due to the rising rates of obesity but a corresponding rise in AF, which is a related phenomenon.
“The risk of atrial fibrillation is increased by 50% relative to those who are not obese,” Dr. Aymond explained.
In this study, 200 patients at three participating centers were randomized to single DCCV or double DCCV after exclusions that included ventricular tachycardia and respiratory instability. The baseline characteristics were comparable. All 101 patients in the single DCCV group and 99 patients in the dual DCCV group were available for the intention-to-treat analysis.
200 vs. 400 joules delivered across the heart
In the study protocol, patients were fitted with four chest pads, two located adjacent but above the heart and two adjacent but below the heart. For single DCCV, 200 joules of energy were delivered from the upper right pad to the lower left pad across the heart. For dual DCCV, another 200 joules were delivered simultaneously from the upper left to the lower right across the heart. The total dose in the dual DCCV group was 400 joules.
The primary outcome was restoration of sinus rhythm of any duration immediately after DCCV. Safety, including clinical events, was a secondary outcome. Only the patients were blinded to the energy they received.
On univariate analysis, the odds ratio for successful cardioversion with dual DCCV was nearly eightfold higher (OR 7.8; P = .008) than single DCCV. On a simple multivariable analysis, when the researchers controlled for just age, sex, and body mass index, the odds ratio rose (OR 8.5; P = .007).
On a comprehensive multivariable analysis adding control for such characteristics as left ventricular ejection fraction (LVEF), obstructive sleep apnea, and antiarrhythmic drugs, the advantage of dual DCCV climbed above 12-fold (OR 12.6; P = .03).
The study is addressing a relevant and persistent question, said the AHA-invited discussant Jose A. Joglar, MD, program director, Clinical Cardiac Electrophysiology Fellowship, University of Texas Southwestern Medical Center, Dallas.
Dr. Joglar pointed out that alternatives to single DCCV for patients more difficult to cardiovert have been “sought for decades.” He noted that a variety of techniques, including dual DCCV, have been evaluated in small studies and case reports.
Alternatives for obese outlined
Several have shown promise, Dr. Joglar said. As one of several examples, he cited a 20-patient study that randomized patients to adhesive patches, like those employed in the Aymond trial, or handheld paddles. Both patches and paddles were applied with manual pressure while a 200-joule shock was delivered. The proportion of patients who cardioverted on the first shock was almost two times higher in the group after the first shock with the paddles (50% vs. 27%; P = .01). Dr. Joglar said the study supports the principle that 200 joules delivered by adhesive patches is inadequate for treatment of AF in many patients with obesity.
Dr. Joglar also cited studies suggesting that single DCCV delivered with higher energy than 200 joules appears to improve cardioversion success rates, but he indicated that this study with dual DCCV in the front-line setting provides evidence for another alternative.
“This is the first such trial with dual defibrillators as an initial strategy,” he said, calling the groups well matched and the superiority of dual DCCV “impressive.” He cautioned that the study size was well powered for the endpoint but perhaps small for evaluating relative safety.
Yet, “the study adds credibility and confidence for the use of dual DCCV, especially in difficult or refractory patients,” he said. He is less certain that it establishes dual DCCV as a standard first-line therapy in all patients with obesity. This would require additional studies to compare it to other types of strategies such as those he mentioned.
As an option for improving cardioversion in first-line treatment, dual DCCV “can be added to a list of other techniques, such as manual pressure or a higher initial dose with single DCCV,” he said.
Dr. Aymond and Dr. Joglar report no potential conflicts of interest.
AT AHA 2023
Blood pressure lowering reduces dementia risk
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM AHA 2023
Pregnancy in rheumatic disease quadruples risk of cardiovascular events
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
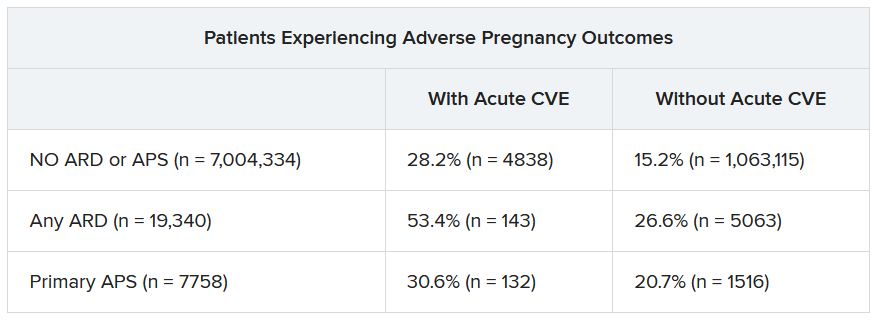
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Pregnancies with low anti-SSA/Ro autoantibody levels: Forgo fetal heart rhythm monitoring?
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Alpha-gal syndrome: Red meat is ‘just the beginning,’ expert says
ANAHEIM, CALIF. – allergist and immunologist Scott P. Commins, MD, PhD, told attendees at the annual meeting of the American College of Allergy, Asthma, and Immunology (ACAAI) annual meeting.
Dr. Commins, associate chief for allergy and immunology at the University of North Carolina at Chapel Hill, has made alpha-gal, a potentially fatal allergy, which, in the United States is tied to the bite of the Lone Star tick, his primary research focus.
Beyond red meat, “there are some people who are allergic to all things mammal,” he explained. Dairy products from mammals, medical devices made from mammalian products, vaccines and medicines that contain gelatin, and even commercial products such as perfumes and cosmetics may be behind an AGS reaction.
“The derived products from pigs and cows really find their way into a lot of our day-to-day products,” he said. “I try to keep an open mind about these exposures.”
Physicians should also be aware that “this can happen to kids,” said Dr. Commins. “It looks very similar to adults’ [AGS]. They can end up in the emergency department.”
He also had clinical advice about food challenges for AGS. He explained that there’s more alpha-gal in beef than in other red meats (including pork, venison, and lamb) with the exception of pork kidney. Pork kidney, he said, “has the most alpha-gal that we can find in the lab.”
Dr. Commins said he has stopped using beef for AGS food challenges and has switched to pork sausage patties with a high fat content microwaved in the clinic because they have less alpha-gal in general and he views them as safer.
Long delay in symptom onset
AGS symptoms typically take 2-6 hours to appear after eating red meat or being exposed to mammalian products, but Dr. Commins related a story about a patient he sent home who had very mild symptoms (some lower back itching) after he had spent the day at the clinic after a pork sausage food challenge for AGS.
The patient had returned home. Eight hours after the food challenge, his wife sent Dr. Commins a picture of her husband’s back, which was riddled with welts and was itching badly.
“I learned that if you’re going to do these food challenges, if there is a hint of symptoms at the clinic at 6 hours, keep them in the clinic, because it may really take that long to evolve,” Dr. Commins said.
One of the early signs he’s discovered is palmar erythema (redness and swelling of the hands).
Research has shown that AGS has been heavily concentrated in the Southeast, where Lone Star tick populations are clustered, but research has shown that from 2017 to 2022, it moved up the East Coast to the central United States and Upper Midwest.
“We are seeing increasing diagnoses of AGS in places that are not, perhaps, where we first thought this allergy existed,” said Dr. Commins. “Stay aware,” he cautioned.
The allergy is not exclusive to the United States, he noted. In Europe and Australia, for example, AGS is not thought to be tied to the Lone Star tick, which doesn’t inhabit those regions.
“It is a global phenomenon,” Dr. Commins said.
In August, the CDC alerted physicians to emerging cases of alpha-gal allergy after an article in Morbidity and Mortality Weekly Report indicated that health care providers have little knowledge about the allergy. Of the 1,500 health care providers surveyed, 42% had never heard of the syndrome, and another 35% were not confident in diagnosing or managing affected patients.
Matthew Lau, MD, an allergist with Kaiser Permanente in Honolulu who listened to Dr. Commins’ talk, told this news organization, “It’s important to raise awareness in primary care particularly, he said, as “allergists see only a fraction of the [AGS] patients.”
Allergists can help raise awareness
“Allergists have a role to alert the general community” and to drive more referrals, he said. That includes emergency departments, where physicians commonly see anaphylaxis.
Dr. Lau said he expects the incidence of AGS to increase, because global warming will likely lengthen warmer seasons and cause the geographic distribution to change.
Jay Lieberman, MD, a pediatric allergist at Le Bonheur Children’s Hospital in Memphis, Tenn., told this news organization, “There’s still a lot of confusion, and hearing from an expert like Dr. Commins helps tease out the not-obvious things about patients who are having more mild symptoms,” such as from allergy to dairy or medicines or vaccines that contain gelatin.
As a pediatric allergist, Dr. Lieberman said he sees less alpha-gal than his colleagues, but, he said, “On the adult side in Tennessee, it’s rampant.”
Dr. Commins, Dr. Lieberman, and Dr. Lau report no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. – allergist and immunologist Scott P. Commins, MD, PhD, told attendees at the annual meeting of the American College of Allergy, Asthma, and Immunology (ACAAI) annual meeting.
Dr. Commins, associate chief for allergy and immunology at the University of North Carolina at Chapel Hill, has made alpha-gal, a potentially fatal allergy, which, in the United States is tied to the bite of the Lone Star tick, his primary research focus.
Beyond red meat, “there are some people who are allergic to all things mammal,” he explained. Dairy products from mammals, medical devices made from mammalian products, vaccines and medicines that contain gelatin, and even commercial products such as perfumes and cosmetics may be behind an AGS reaction.
“The derived products from pigs and cows really find their way into a lot of our day-to-day products,” he said. “I try to keep an open mind about these exposures.”
Physicians should also be aware that “this can happen to kids,” said Dr. Commins. “It looks very similar to adults’ [AGS]. They can end up in the emergency department.”
He also had clinical advice about food challenges for AGS. He explained that there’s more alpha-gal in beef than in other red meats (including pork, venison, and lamb) with the exception of pork kidney. Pork kidney, he said, “has the most alpha-gal that we can find in the lab.”
Dr. Commins said he has stopped using beef for AGS food challenges and has switched to pork sausage patties with a high fat content microwaved in the clinic because they have less alpha-gal in general and he views them as safer.
Long delay in symptom onset
AGS symptoms typically take 2-6 hours to appear after eating red meat or being exposed to mammalian products, but Dr. Commins related a story about a patient he sent home who had very mild symptoms (some lower back itching) after he had spent the day at the clinic after a pork sausage food challenge for AGS.
The patient had returned home. Eight hours after the food challenge, his wife sent Dr. Commins a picture of her husband’s back, which was riddled with welts and was itching badly.
“I learned that if you’re going to do these food challenges, if there is a hint of symptoms at the clinic at 6 hours, keep them in the clinic, because it may really take that long to evolve,” Dr. Commins said.
One of the early signs he’s discovered is palmar erythema (redness and swelling of the hands).
Research has shown that AGS has been heavily concentrated in the Southeast, where Lone Star tick populations are clustered, but research has shown that from 2017 to 2022, it moved up the East Coast to the central United States and Upper Midwest.
“We are seeing increasing diagnoses of AGS in places that are not, perhaps, where we first thought this allergy existed,” said Dr. Commins. “Stay aware,” he cautioned.
The allergy is not exclusive to the United States, he noted. In Europe and Australia, for example, AGS is not thought to be tied to the Lone Star tick, which doesn’t inhabit those regions.
“It is a global phenomenon,” Dr. Commins said.
In August, the CDC alerted physicians to emerging cases of alpha-gal allergy after an article in Morbidity and Mortality Weekly Report indicated that health care providers have little knowledge about the allergy. Of the 1,500 health care providers surveyed, 42% had never heard of the syndrome, and another 35% were not confident in diagnosing or managing affected patients.
Matthew Lau, MD, an allergist with Kaiser Permanente in Honolulu who listened to Dr. Commins’ talk, told this news organization, “It’s important to raise awareness in primary care particularly, he said, as “allergists see only a fraction of the [AGS] patients.”
Allergists can help raise awareness
“Allergists have a role to alert the general community” and to drive more referrals, he said. That includes emergency departments, where physicians commonly see anaphylaxis.
Dr. Lau said he expects the incidence of AGS to increase, because global warming will likely lengthen warmer seasons and cause the geographic distribution to change.
Jay Lieberman, MD, a pediatric allergist at Le Bonheur Children’s Hospital in Memphis, Tenn., told this news organization, “There’s still a lot of confusion, and hearing from an expert like Dr. Commins helps tease out the not-obvious things about patients who are having more mild symptoms,” such as from allergy to dairy or medicines or vaccines that contain gelatin.
As a pediatric allergist, Dr. Lieberman said he sees less alpha-gal than his colleagues, but, he said, “On the adult side in Tennessee, it’s rampant.”
Dr. Commins, Dr. Lieberman, and Dr. Lau report no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. – allergist and immunologist Scott P. Commins, MD, PhD, told attendees at the annual meeting of the American College of Allergy, Asthma, and Immunology (ACAAI) annual meeting.
Dr. Commins, associate chief for allergy and immunology at the University of North Carolina at Chapel Hill, has made alpha-gal, a potentially fatal allergy, which, in the United States is tied to the bite of the Lone Star tick, his primary research focus.
Beyond red meat, “there are some people who are allergic to all things mammal,” he explained. Dairy products from mammals, medical devices made from mammalian products, vaccines and medicines that contain gelatin, and even commercial products such as perfumes and cosmetics may be behind an AGS reaction.
“The derived products from pigs and cows really find their way into a lot of our day-to-day products,” he said. “I try to keep an open mind about these exposures.”
Physicians should also be aware that “this can happen to kids,” said Dr. Commins. “It looks very similar to adults’ [AGS]. They can end up in the emergency department.”
He also had clinical advice about food challenges for AGS. He explained that there’s more alpha-gal in beef than in other red meats (including pork, venison, and lamb) with the exception of pork kidney. Pork kidney, he said, “has the most alpha-gal that we can find in the lab.”
Dr. Commins said he has stopped using beef for AGS food challenges and has switched to pork sausage patties with a high fat content microwaved in the clinic because they have less alpha-gal in general and he views them as safer.
Long delay in symptom onset
AGS symptoms typically take 2-6 hours to appear after eating red meat or being exposed to mammalian products, but Dr. Commins related a story about a patient he sent home who had very mild symptoms (some lower back itching) after he had spent the day at the clinic after a pork sausage food challenge for AGS.
The patient had returned home. Eight hours after the food challenge, his wife sent Dr. Commins a picture of her husband’s back, which was riddled with welts and was itching badly.
“I learned that if you’re going to do these food challenges, if there is a hint of symptoms at the clinic at 6 hours, keep them in the clinic, because it may really take that long to evolve,” Dr. Commins said.
One of the early signs he’s discovered is palmar erythema (redness and swelling of the hands).
Research has shown that AGS has been heavily concentrated in the Southeast, where Lone Star tick populations are clustered, but research has shown that from 2017 to 2022, it moved up the East Coast to the central United States and Upper Midwest.
“We are seeing increasing diagnoses of AGS in places that are not, perhaps, where we first thought this allergy existed,” said Dr. Commins. “Stay aware,” he cautioned.
The allergy is not exclusive to the United States, he noted. In Europe and Australia, for example, AGS is not thought to be tied to the Lone Star tick, which doesn’t inhabit those regions.
“It is a global phenomenon,” Dr. Commins said.
In August, the CDC alerted physicians to emerging cases of alpha-gal allergy after an article in Morbidity and Mortality Weekly Report indicated that health care providers have little knowledge about the allergy. Of the 1,500 health care providers surveyed, 42% had never heard of the syndrome, and another 35% were not confident in diagnosing or managing affected patients.
Matthew Lau, MD, an allergist with Kaiser Permanente in Honolulu who listened to Dr. Commins’ talk, told this news organization, “It’s important to raise awareness in primary care particularly, he said, as “allergists see only a fraction of the [AGS] patients.”
Allergists can help raise awareness
“Allergists have a role to alert the general community” and to drive more referrals, he said. That includes emergency departments, where physicians commonly see anaphylaxis.
Dr. Lau said he expects the incidence of AGS to increase, because global warming will likely lengthen warmer seasons and cause the geographic distribution to change.
Jay Lieberman, MD, a pediatric allergist at Le Bonheur Children’s Hospital in Memphis, Tenn., told this news organization, “There’s still a lot of confusion, and hearing from an expert like Dr. Commins helps tease out the not-obvious things about patients who are having more mild symptoms,” such as from allergy to dairy or medicines or vaccines that contain gelatin.
As a pediatric allergist, Dr. Lieberman said he sees less alpha-gal than his colleagues, but, he said, “On the adult side in Tennessee, it’s rampant.”
Dr. Commins, Dr. Lieberman, and Dr. Lau report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Obinutuzumab promotes renal preservation in lupus nephritis
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.
Sustained reductions in Lp(a) achieved with novel siRNA drug
In an early phase multicenter clinical study,
The reductions in serum Lp(a) in patients receiving lepodisiran were dose dependent but adverse events were not, said Steven E. Nissen, MD, professor of medicine at the Cleveland Clinic Lerner College of Medicine.
Rather, drug-related adverse events “were uncommon and generally similar across all lepodisiran doses and the placebo group,” reported Dr. Nissen, who pointed out that safety and tolerability were the primary endpoints and purpose of this phase 1 study.
Lp(a) strongly associated with CV risk
Similar to LDL cholesterol (LDL-C), elevated levels of serum Lp(a) have been associated with major adverse cardiac events (MACE). In a 2022 review article that summarized pathophysiological, observational, and genetic studies, Lp(a) was found to be implicated in vascular inflammation, atherogenesis, calcification, and thrombosis.
Furthermore, Lp(a) has been associated with residual risk of cardiovascular (CV) events even after tight control of other risk factors, including elevated LDL-C, Dr. Nissen said.
So far, no well-tolerated therapy has been found to be effective for reducing Lp(a), but siRNA is a novel and attractive approach, according to Dr. Nissen, who presented these results at the annual scientific sessions of the American Heart Association. They were also published online in JAMA.
By silencing target genes, siRNA therapies can inhibit a basic step in a given pathological process. In this case, lepodisiran silences the LPA gene to halt encoding of apolipoprotein(a), which plays a key role in Lp(a) production.
Lepodisiran is not the only treatment in development for Lp(a), noted the AHA-invited discussant Michelle L. O’Donoghue, MD, chair in cardiology, Brigham and Women’s Hospital, Boston. She mentioned several other siRNA therapies, including olpasiran that was effective in a phase 2 trial she led and published in the New England Journal of Medicine.
Drugs with different mechanisms, such as the antisense oligonucleotide pelacarsen, showed activity when tested earlier this year in a phase 1 study. No study has yet been conducted to link reductions in Lp(a) with CV event risk reduction.
The current study with lepodisiran was conducted with the participation of five clinical research sites in the United States and Singapore. Participants between the ages of 18 and 65 years were enrolled if they had a serum Lp(a) of at least 75 nmol/L (30 mg/dL), which is considered moderately elevated.
They were excluded if they had CV disease or significant risk factors, including a blood pressure greater than 160/40 mm Hg, impaired renal function (eGFR < 60 mL/min per 1.73 m2), or tobacco use (> 10 cigarettes/day).
Of 340 candidates screened, 48 were randomly assigned to one placebo or six lepodisiran groups. There were 12 participants in the placebo group and 6 in each of the lepodisiran dosing groups (4 mg, 12 mg, 32 mg, 96 mg, 304 mg, and 608 mg). All doses and placebo were administered subcutaneously one time with a planned follow-up of up to 48 weeks.
Safety profile is placebo-like
The single most common adverse event, shared by those randomly assigned to placebo, was injection-site reaction. There were no adverse events, including laboratory abnormalities, that were persistent and clearly different for those assigned to any dose of lepodisiran relative to placebo.
The maximum median percentage change in serum Lp(a) out to day 337 of follow-up was 5% reduction in the placebo group. In the active treatment groups, the reductions were 41% on 4 mg, 59% on 12 mg, 76% on 32 mg, 96% on 304 mg, and 97% on 608 mg.
These reductions were generally sustained for as long as therapy was maintained. Maximal reductions were reached at day 85 in the 4-mg group but were achieved by day 29 in the 605-mg group, Dr. Nissen reported. In fact, serum Lp(a) was undetectable in the 605-mg group at day 29 and remained so until day 281.
Currently, there is no practical treatment for Lp(a). The only potential exception, apheresis, is “cumbersome” to perform and must be repeated for sustained reductions. Niacin and PCSK9 inhibitors are known to provide modest reductions in Lp(a), but Dr. Nissen said they are too modest to expect a meaningful clinical benefit.
Lp(a) not responsive to lifestyle changes
Statins as well as all lifestyle modifications, including diet, have been shown to have “little or no effect,” Dr. Nissen said.
The safety and the evidence so far of sustained Lp(a) lowering has already led to a phase 2 trial, according to Dr. Nissen, but the more important test for the future of lepodisiran will be studies powered to confirm reductions in MACE. Lepodisiran may finally allow that hypothesis to be tested.
“I think a lot of us have been waiting a long time for evidence that we can reliably reduce Lp(a),” said Karol Watson, MD, PhD, who has a research interest in lipids and is a professor of medicine at the University of California, Los Angeles.
Although she conceded that the overwhelming evidence that Lp(a) is a risk factor does not ensure that any specific Lp(a)-lowering therapy will be clinically viable, she suggested this drug is a promising candidate to move this field forward.
“At the highest doses, lepodisiran is not just lowering Lp(a), it appears to be getting rid of it,” she said.
Dr. O’Donoghue said that the phase 1 results suggest lepodisiran might have a somewhat longer duration of action than other siRNA therapies studied for Lp(a) so far, but said larger trials are needed to determine whether the growing number of drugs in this class differ in ways that are clinically meaningful.
Overall, the excitement in this field is probably mostly driven by the fact that there are so many promising therapies for Lp(a) that address the target in so many unique ways. Dr. O’Donoghue cited, as an example, a gene-editing therapy called CTX320 that showed impressive effects in an animal study presented at the AHA meeting as a poster. She called the pipeline for treating Lp(a) “rich.”
Elevated Lp(a) is genetically determined, so levels do not generally change over time, said Donald Lloyd-Jones, MD, chair of the department of preventive medicine, Northwestern Medicine, Chicago.
“It is not affected by your diet. It is not affected by your exercise. What your level is will be the level you will have for the rest of your life,” he said. Generally, it is recommended to have Lp(a) measured just once to more accurately calculate cardiovascular risk, but Dr. Lloyd-Jones predicted that this lipid subfraction might be measured more frequently to verify control if a therapeutic becomes available.
Dr. Nissen agreed. Estimating that 64 million people in the United States have significantly elevated Lp(a), he expects this risk to be addressed as a specific and independent target in CV risk management when and if it becomes treatable.
Dr. Nissen reported financial relationships with Novartis, Silence Therapeutics, and Eli Lilly, which provided funding for this trial. Dr. Watson reported financial relationships with Amgen, Boehringer Ingelheim, Lilly, and Novartis. Dr. Lloyd-Jones disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an early phase multicenter clinical study,
The reductions in serum Lp(a) in patients receiving lepodisiran were dose dependent but adverse events were not, said Steven E. Nissen, MD, professor of medicine at the Cleveland Clinic Lerner College of Medicine.
Rather, drug-related adverse events “were uncommon and generally similar across all lepodisiran doses and the placebo group,” reported Dr. Nissen, who pointed out that safety and tolerability were the primary endpoints and purpose of this phase 1 study.
Lp(a) strongly associated with CV risk
Similar to LDL cholesterol (LDL-C), elevated levels of serum Lp(a) have been associated with major adverse cardiac events (MACE). In a 2022 review article that summarized pathophysiological, observational, and genetic studies, Lp(a) was found to be implicated in vascular inflammation, atherogenesis, calcification, and thrombosis.
Furthermore, Lp(a) has been associated with residual risk of cardiovascular (CV) events even after tight control of other risk factors, including elevated LDL-C, Dr. Nissen said.
So far, no well-tolerated therapy has been found to be effective for reducing Lp(a), but siRNA is a novel and attractive approach, according to Dr. Nissen, who presented these results at the annual scientific sessions of the American Heart Association. They were also published online in JAMA.
By silencing target genes, siRNA therapies can inhibit a basic step in a given pathological process. In this case, lepodisiran silences the LPA gene to halt encoding of apolipoprotein(a), which plays a key role in Lp(a) production.
Lepodisiran is not the only treatment in development for Lp(a), noted the AHA-invited discussant Michelle L. O’Donoghue, MD, chair in cardiology, Brigham and Women’s Hospital, Boston. She mentioned several other siRNA therapies, including olpasiran that was effective in a phase 2 trial she led and published in the New England Journal of Medicine.
Drugs with different mechanisms, such as the antisense oligonucleotide pelacarsen, showed activity when tested earlier this year in a phase 1 study. No study has yet been conducted to link reductions in Lp(a) with CV event risk reduction.
The current study with lepodisiran was conducted with the participation of five clinical research sites in the United States and Singapore. Participants between the ages of 18 and 65 years were enrolled if they had a serum Lp(a) of at least 75 nmol/L (30 mg/dL), which is considered moderately elevated.
They were excluded if they had CV disease or significant risk factors, including a blood pressure greater than 160/40 mm Hg, impaired renal function (eGFR < 60 mL/min per 1.73 m2), or tobacco use (> 10 cigarettes/day).
Of 340 candidates screened, 48 were randomly assigned to one placebo or six lepodisiran groups. There were 12 participants in the placebo group and 6 in each of the lepodisiran dosing groups (4 mg, 12 mg, 32 mg, 96 mg, 304 mg, and 608 mg). All doses and placebo were administered subcutaneously one time with a planned follow-up of up to 48 weeks.
Safety profile is placebo-like
The single most common adverse event, shared by those randomly assigned to placebo, was injection-site reaction. There were no adverse events, including laboratory abnormalities, that were persistent and clearly different for those assigned to any dose of lepodisiran relative to placebo.
The maximum median percentage change in serum Lp(a) out to day 337 of follow-up was 5% reduction in the placebo group. In the active treatment groups, the reductions were 41% on 4 mg, 59% on 12 mg, 76% on 32 mg, 96% on 304 mg, and 97% on 608 mg.
These reductions were generally sustained for as long as therapy was maintained. Maximal reductions were reached at day 85 in the 4-mg group but were achieved by day 29 in the 605-mg group, Dr. Nissen reported. In fact, serum Lp(a) was undetectable in the 605-mg group at day 29 and remained so until day 281.
Currently, there is no practical treatment for Lp(a). The only potential exception, apheresis, is “cumbersome” to perform and must be repeated for sustained reductions. Niacin and PCSK9 inhibitors are known to provide modest reductions in Lp(a), but Dr. Nissen said they are too modest to expect a meaningful clinical benefit.
Lp(a) not responsive to lifestyle changes
Statins as well as all lifestyle modifications, including diet, have been shown to have “little or no effect,” Dr. Nissen said.
The safety and the evidence so far of sustained Lp(a) lowering has already led to a phase 2 trial, according to Dr. Nissen, but the more important test for the future of lepodisiran will be studies powered to confirm reductions in MACE. Lepodisiran may finally allow that hypothesis to be tested.
“I think a lot of us have been waiting a long time for evidence that we can reliably reduce Lp(a),” said Karol Watson, MD, PhD, who has a research interest in lipids and is a professor of medicine at the University of California, Los Angeles.
Although she conceded that the overwhelming evidence that Lp(a) is a risk factor does not ensure that any specific Lp(a)-lowering therapy will be clinically viable, she suggested this drug is a promising candidate to move this field forward.
“At the highest doses, lepodisiran is not just lowering Lp(a), it appears to be getting rid of it,” she said.
Dr. O’Donoghue said that the phase 1 results suggest lepodisiran might have a somewhat longer duration of action than other siRNA therapies studied for Lp(a) so far, but said larger trials are needed to determine whether the growing number of drugs in this class differ in ways that are clinically meaningful.
Overall, the excitement in this field is probably mostly driven by the fact that there are so many promising therapies for Lp(a) that address the target in so many unique ways. Dr. O’Donoghue cited, as an example, a gene-editing therapy called CTX320 that showed impressive effects in an animal study presented at the AHA meeting as a poster. She called the pipeline for treating Lp(a) “rich.”
Elevated Lp(a) is genetically determined, so levels do not generally change over time, said Donald Lloyd-Jones, MD, chair of the department of preventive medicine, Northwestern Medicine, Chicago.
“It is not affected by your diet. It is not affected by your exercise. What your level is will be the level you will have for the rest of your life,” he said. Generally, it is recommended to have Lp(a) measured just once to more accurately calculate cardiovascular risk, but Dr. Lloyd-Jones predicted that this lipid subfraction might be measured more frequently to verify control if a therapeutic becomes available.
Dr. Nissen agreed. Estimating that 64 million people in the United States have significantly elevated Lp(a), he expects this risk to be addressed as a specific and independent target in CV risk management when and if it becomes treatable.
Dr. Nissen reported financial relationships with Novartis, Silence Therapeutics, and Eli Lilly, which provided funding for this trial. Dr. Watson reported financial relationships with Amgen, Boehringer Ingelheim, Lilly, and Novartis. Dr. Lloyd-Jones disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an early phase multicenter clinical study,
The reductions in serum Lp(a) in patients receiving lepodisiran were dose dependent but adverse events were not, said Steven E. Nissen, MD, professor of medicine at the Cleveland Clinic Lerner College of Medicine.
Rather, drug-related adverse events “were uncommon and generally similar across all lepodisiran doses and the placebo group,” reported Dr. Nissen, who pointed out that safety and tolerability were the primary endpoints and purpose of this phase 1 study.
Lp(a) strongly associated with CV risk
Similar to LDL cholesterol (LDL-C), elevated levels of serum Lp(a) have been associated with major adverse cardiac events (MACE). In a 2022 review article that summarized pathophysiological, observational, and genetic studies, Lp(a) was found to be implicated in vascular inflammation, atherogenesis, calcification, and thrombosis.
Furthermore, Lp(a) has been associated with residual risk of cardiovascular (CV) events even after tight control of other risk factors, including elevated LDL-C, Dr. Nissen said.
So far, no well-tolerated therapy has been found to be effective for reducing Lp(a), but siRNA is a novel and attractive approach, according to Dr. Nissen, who presented these results at the annual scientific sessions of the American Heart Association. They were also published online in JAMA.
By silencing target genes, siRNA therapies can inhibit a basic step in a given pathological process. In this case, lepodisiran silences the LPA gene to halt encoding of apolipoprotein(a), which plays a key role in Lp(a) production.
Lepodisiran is not the only treatment in development for Lp(a), noted the AHA-invited discussant Michelle L. O’Donoghue, MD, chair in cardiology, Brigham and Women’s Hospital, Boston. She mentioned several other siRNA therapies, including olpasiran that was effective in a phase 2 trial she led and published in the New England Journal of Medicine.
Drugs with different mechanisms, such as the antisense oligonucleotide pelacarsen, showed activity when tested earlier this year in a phase 1 study. No study has yet been conducted to link reductions in Lp(a) with CV event risk reduction.
The current study with lepodisiran was conducted with the participation of five clinical research sites in the United States and Singapore. Participants between the ages of 18 and 65 years were enrolled if they had a serum Lp(a) of at least 75 nmol/L (30 mg/dL), which is considered moderately elevated.
They were excluded if they had CV disease or significant risk factors, including a blood pressure greater than 160/40 mm Hg, impaired renal function (eGFR < 60 mL/min per 1.73 m2), or tobacco use (> 10 cigarettes/day).
Of 340 candidates screened, 48 were randomly assigned to one placebo or six lepodisiran groups. There were 12 participants in the placebo group and 6 in each of the lepodisiran dosing groups (4 mg, 12 mg, 32 mg, 96 mg, 304 mg, and 608 mg). All doses and placebo were administered subcutaneously one time with a planned follow-up of up to 48 weeks.
Safety profile is placebo-like
The single most common adverse event, shared by those randomly assigned to placebo, was injection-site reaction. There were no adverse events, including laboratory abnormalities, that were persistent and clearly different for those assigned to any dose of lepodisiran relative to placebo.
The maximum median percentage change in serum Lp(a) out to day 337 of follow-up was 5% reduction in the placebo group. In the active treatment groups, the reductions were 41% on 4 mg, 59% on 12 mg, 76% on 32 mg, 96% on 304 mg, and 97% on 608 mg.
These reductions were generally sustained for as long as therapy was maintained. Maximal reductions were reached at day 85 in the 4-mg group but were achieved by day 29 in the 605-mg group, Dr. Nissen reported. In fact, serum Lp(a) was undetectable in the 605-mg group at day 29 and remained so until day 281.
Currently, there is no practical treatment for Lp(a). The only potential exception, apheresis, is “cumbersome” to perform and must be repeated for sustained reductions. Niacin and PCSK9 inhibitors are known to provide modest reductions in Lp(a), but Dr. Nissen said they are too modest to expect a meaningful clinical benefit.
Lp(a) not responsive to lifestyle changes
Statins as well as all lifestyle modifications, including diet, have been shown to have “little or no effect,” Dr. Nissen said.
The safety and the evidence so far of sustained Lp(a) lowering has already led to a phase 2 trial, according to Dr. Nissen, but the more important test for the future of lepodisiran will be studies powered to confirm reductions in MACE. Lepodisiran may finally allow that hypothesis to be tested.
“I think a lot of us have been waiting a long time for evidence that we can reliably reduce Lp(a),” said Karol Watson, MD, PhD, who has a research interest in lipids and is a professor of medicine at the University of California, Los Angeles.
Although she conceded that the overwhelming evidence that Lp(a) is a risk factor does not ensure that any specific Lp(a)-lowering therapy will be clinically viable, she suggested this drug is a promising candidate to move this field forward.
“At the highest doses, lepodisiran is not just lowering Lp(a), it appears to be getting rid of it,” she said.
Dr. O’Donoghue said that the phase 1 results suggest lepodisiran might have a somewhat longer duration of action than other siRNA therapies studied for Lp(a) so far, but said larger trials are needed to determine whether the growing number of drugs in this class differ in ways that are clinically meaningful.
Overall, the excitement in this field is probably mostly driven by the fact that there are so many promising therapies for Lp(a) that address the target in so many unique ways. Dr. O’Donoghue cited, as an example, a gene-editing therapy called CTX320 that showed impressive effects in an animal study presented at the AHA meeting as a poster. She called the pipeline for treating Lp(a) “rich.”
Elevated Lp(a) is genetically determined, so levels do not generally change over time, said Donald Lloyd-Jones, MD, chair of the department of preventive medicine, Northwestern Medicine, Chicago.
“It is not affected by your diet. It is not affected by your exercise. What your level is will be the level you will have for the rest of your life,” he said. Generally, it is recommended to have Lp(a) measured just once to more accurately calculate cardiovascular risk, but Dr. Lloyd-Jones predicted that this lipid subfraction might be measured more frequently to verify control if a therapeutic becomes available.
Dr. Nissen agreed. Estimating that 64 million people in the United States have significantly elevated Lp(a), he expects this risk to be addressed as a specific and independent target in CV risk management when and if it becomes treatable.
Dr. Nissen reported financial relationships with Novartis, Silence Therapeutics, and Eli Lilly, which provided funding for this trial. Dr. Watson reported financial relationships with Amgen, Boehringer Ingelheim, Lilly, and Novartis. Dr. Lloyd-Jones disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
Dropping aspirin cuts bleeding in LVAD patients: ARIES-HM3
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
AT AHA 2023