User login
MDedge latest news is breaking news from medical conferences, journals, guidelines, the FDA and CDC.
On a Quest To Reduce Stigmas about Anal Cancer
“I think gastroenterologists are uniquely positioned to help with diagnosing anal diseases, in particular anal cancer,” she said. “It is part of the digestive tract, and my mission is to help gastroenterologists remember that.”
Dr. Korman is a gastroenterologist with Capital Digestive Care in Washington D.C., where she serves as chair of its Women’s Committee and as a member of the board of managers. She’s also the medical director of the Endoscopy Center of Washington D.C.
A recipient of the 2025 AGA Distinguished Clinician Award in Private Practice, Dr. Korman has dedicated her career to educating clinicians on anal cancer screening and anal human papillomavirus. On the research front, she participated as an investigator in the ANAL Cancer-HSIL Outcomes Research (ANCHOR) trial, which led to international anal cancer screening guidelines.
She also co-directs the International Anal Neoplasia Society (IANS) Standard High Resolution Anoscopy course.
When she’s not serving her patients, Dr. Korman speaks in the community about anal cancer awareness and screening. In the last few years, Dr. Korman has presented grand rounds at various institutions and speaks at major medical conferences. “I just try to advocate and help gastroenterologists understand who is at risk, how to look for anal cancer, how to screen, and who to refer. If anyone invites me to speak, I generally will do it,” said Dr. Korman.
In an interview, she talked about the outcomes of the ANCHOR trial and how it may inform future research, and her work to reduce bias and stigma for LGBTQ+ patients.
You decided to become a physician after studying in Egypt and Israel and volunteering with Physicians for Human Rights. Can you talk about that journey?
Dr. Korman: I majored in Religion and Middle East studies, and I minored in Arabic. I thought I was going to become a professor of religious studies. But during my time studying abroad and volunteering for Physicians for Human Rights, I was deeply moved by how physicians connect with the core of our shared humanity. Becoming a physician allows one to meet the most fundamental of human needs—caring for another’s health—in a direct and meaningful way.
My father is a physician, a gastroenterologist, but I never considered it as a career option growing up. The year after I graduated college, I accompanied my parents to my father’s medical school reunion and I thought, ‘Why did I never think about this?’ I decided to go back to school to take the pre-med requirements. Gastroenterology seemed to combine the ability to work with my hands, do procedures, have long-term relationships with patients, and think about complex problems.
GI medicine often involves detective work. What is the most challenging case you’ve encountered?
Dr. Korman: Sometimes the patients who have very severe disorders of gut-brain interaction can be the most challenging because finding treatments for them or getting them to a place where they accept certain types of treatment can be really difficult. And of course, you have to put your detective hat on and make sure you have ruled out all the “zebras.” It can take years to build the level of trust where patients are willing to accept the diagnosis and then pursue appropriate treatment.
I always try my best, but I don’t like to give up. I will refer a patient to a colleague if they have a problem and I can’t figure out what the diagnosis is or find a treatment that works. I believe in second and third opinions. I recognize that there’s a limit to what my brain can do and that we all have blind spots. Maybe someone will look at the case with fresh eyes and think of something else.
What was the most impactful outcomes of the ANAL Cancer-HSIL Outcomes Research (ANCHOR) trial?
Dr. Korman: This was a National Institutes of Health (NIH)-sponsored, randomized controlled trial with 26 clinical sites. We studied people living with human immunodeficiency virus (HIV), as they are the most at-risk group for anal cancer.
We were looking to prove that treating high grade squamous intraepithelial lesions (HSIL) of the anal canal would lead to a significant reduction in the rates of anal cancer. No one in the medical community would accept guidelines or recommendations about what to do with anal pre-cancers until we proved that treatment worked.
We published the findings in 2022. The study concluded when we met our endpoint earlier than expected. We were able to prove that treating high grade anal dysplasia does indeed lead to a very significant reduction in progression to anal cancer. That ultimately led to guidelines. The International Anal Neoplasia Society came out with consensus guidelines on screening for anal cancer in January 2024. In August 2024, NIH, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America came out with screening guidelines for people living with HIV.
Were there any other outcomes from this research?
Dr. Korman: One of the great things about the study is that we accumulated a bank of tissue and biologic specimens. There were about 4,500 patients randomized into the trial, but about 10,000 patients screened. So, we have a massive collection of biospecimens that we can use to ask questions about the progression of HSIL to anal cancer. We would like to understand more about viral and host molecular mechanisms and hopefully find biomarkers that will identify individuals at particularly high risk of progression. It’s a more precision medicine type of approach.
Education has been a cornerstone of your career. What’s the most rewarding part of teaching the IANS standard high resolution endoscopy course?
Dr. Korman: I first took the course in 2010, and that’s when I started my journey of learning how to perform high resolution endoscopy. Last year I was asked to help co-direct the course. It is now virtual and asynchronous where everything is recorded. But it was exciting to help reorganize the course, update the lectures, and make sure that everything is current. We get to answer questions from participants from all over the world. I think there are participants from 23 countries who have taken the course, which is amazing.
Could you share your work with the LGBTQIA+ population? What specific needs/challenges does this population have with GI care?
Dr. Korman: Many people in the sexual and gender minority community have experienced discrimination in health care settings or know of someone who has. For these reasons, LGBTQIA+ people may approach health care with the expectation of a negative encounter, or they may avoid accessing care altogether. Because anal cancer disproportionately affects sexual and gender minority communities, creating a warm, inclusive environment is key to identifying who is at risk, building trust, and ensuring patients receive the care they need. When you’re talking about anal cancer, there’s a lot of stigma and shame. I think people are afraid to seek care.
Gastroenterology has traditionally been an “old boys club” but that is changing. We’re trying to work on educating people on how to recognize their own biases and move beyond them to provide care that’s affirming and where people feel that they have a safe space to talk about their concerns. Men who have sex with men, in particular living with HIV, are at the highest risk of developing anal cancer. If you don’t know that your patient is a man who has sex with men, or they don’t want to disclose that they’re living with HIV, you don’t know to screen them, and then you’re missing an opportunity to potentially prevent a cancer.
What advice would you give to aspiring medical students interested in GI?
Dr. Korman: GI is the most exciting and interesting field. We take care of so many different organs, and we’re never bored. If medical students want to get into GI, I recommend that they try to be in an office or an endoscopy center and see if it’s really for them and get some hands-on experience if possible. To be truly great at this profession, you really must see it as a calling – jump in with your whole heart and not see it as just a job. If you can do that, you’ll succeed.
How do you handle stress and maintain work-life balance?
Dr. Korman: Exercise. I try to work out at least five days a week. I can’t live without it. That keeps me going. What do I do for fun? I spend time with my family and my friends. I enjoy going to new restaurants and being outdoors, especially near a body of water. I travel, and I love watching movies. I am also guilty of binge-watching TV on a regular basis as well.
Lightning Round
Coffee or tea?
Coffee, 100%
What’s your favorite book?
I can’t say I have just one, but I recently read Tomorrow and Tomorrow and Tomorrow and loved it
Beach vacation or mountain retreat?
Beach
Early bird or night owl?
Early bird
What’s your go-to comfort food?
Anything with bananas
If you could travel anywhere, where would you go?
Vietnam or African safari
What’s your favorite childhood memory?
Swim team when I was a kid
If you could instantly learn any skill, what would it be?
Playing the drums
Are you a planner or more spontaneous?
Planner, although it’s not my strong suit, if I’m being honest.
“I think gastroenterologists are uniquely positioned to help with diagnosing anal diseases, in particular anal cancer,” she said. “It is part of the digestive tract, and my mission is to help gastroenterologists remember that.”
Dr. Korman is a gastroenterologist with Capital Digestive Care in Washington D.C., where she serves as chair of its Women’s Committee and as a member of the board of managers. She’s also the medical director of the Endoscopy Center of Washington D.C.
A recipient of the 2025 AGA Distinguished Clinician Award in Private Practice, Dr. Korman has dedicated her career to educating clinicians on anal cancer screening and anal human papillomavirus. On the research front, she participated as an investigator in the ANAL Cancer-HSIL Outcomes Research (ANCHOR) trial, which led to international anal cancer screening guidelines.
She also co-directs the International Anal Neoplasia Society (IANS) Standard High Resolution Anoscopy course.
When she’s not serving her patients, Dr. Korman speaks in the community about anal cancer awareness and screening. In the last few years, Dr. Korman has presented grand rounds at various institutions and speaks at major medical conferences. “I just try to advocate and help gastroenterologists understand who is at risk, how to look for anal cancer, how to screen, and who to refer. If anyone invites me to speak, I generally will do it,” said Dr. Korman.
In an interview, she talked about the outcomes of the ANCHOR trial and how it may inform future research, and her work to reduce bias and stigma for LGBTQ+ patients.
You decided to become a physician after studying in Egypt and Israel and volunteering with Physicians for Human Rights. Can you talk about that journey?
Dr. Korman: I majored in Religion and Middle East studies, and I minored in Arabic. I thought I was going to become a professor of religious studies. But during my time studying abroad and volunteering for Physicians for Human Rights, I was deeply moved by how physicians connect with the core of our shared humanity. Becoming a physician allows one to meet the most fundamental of human needs—caring for another’s health—in a direct and meaningful way.
My father is a physician, a gastroenterologist, but I never considered it as a career option growing up. The year after I graduated college, I accompanied my parents to my father’s medical school reunion and I thought, ‘Why did I never think about this?’ I decided to go back to school to take the pre-med requirements. Gastroenterology seemed to combine the ability to work with my hands, do procedures, have long-term relationships with patients, and think about complex problems.
GI medicine often involves detective work. What is the most challenging case you’ve encountered?
Dr. Korman: Sometimes the patients who have very severe disorders of gut-brain interaction can be the most challenging because finding treatments for them or getting them to a place where they accept certain types of treatment can be really difficult. And of course, you have to put your detective hat on and make sure you have ruled out all the “zebras.” It can take years to build the level of trust where patients are willing to accept the diagnosis and then pursue appropriate treatment.
I always try my best, but I don’t like to give up. I will refer a patient to a colleague if they have a problem and I can’t figure out what the diagnosis is or find a treatment that works. I believe in second and third opinions. I recognize that there’s a limit to what my brain can do and that we all have blind spots. Maybe someone will look at the case with fresh eyes and think of something else.
What was the most impactful outcomes of the ANAL Cancer-HSIL Outcomes Research (ANCHOR) trial?
Dr. Korman: This was a National Institutes of Health (NIH)-sponsored, randomized controlled trial with 26 clinical sites. We studied people living with human immunodeficiency virus (HIV), as they are the most at-risk group for anal cancer.
We were looking to prove that treating high grade squamous intraepithelial lesions (HSIL) of the anal canal would lead to a significant reduction in the rates of anal cancer. No one in the medical community would accept guidelines or recommendations about what to do with anal pre-cancers until we proved that treatment worked.
We published the findings in 2022. The study concluded when we met our endpoint earlier than expected. We were able to prove that treating high grade anal dysplasia does indeed lead to a very significant reduction in progression to anal cancer. That ultimately led to guidelines. The International Anal Neoplasia Society came out with consensus guidelines on screening for anal cancer in January 2024. In August 2024, NIH, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America came out with screening guidelines for people living with HIV.
Were there any other outcomes from this research?
Dr. Korman: One of the great things about the study is that we accumulated a bank of tissue and biologic specimens. There were about 4,500 patients randomized into the trial, but about 10,000 patients screened. So, we have a massive collection of biospecimens that we can use to ask questions about the progression of HSIL to anal cancer. We would like to understand more about viral and host molecular mechanisms and hopefully find biomarkers that will identify individuals at particularly high risk of progression. It’s a more precision medicine type of approach.
Education has been a cornerstone of your career. What’s the most rewarding part of teaching the IANS standard high resolution endoscopy course?
Dr. Korman: I first took the course in 2010, and that’s when I started my journey of learning how to perform high resolution endoscopy. Last year I was asked to help co-direct the course. It is now virtual and asynchronous where everything is recorded. But it was exciting to help reorganize the course, update the lectures, and make sure that everything is current. We get to answer questions from participants from all over the world. I think there are participants from 23 countries who have taken the course, which is amazing.
Could you share your work with the LGBTQIA+ population? What specific needs/challenges does this population have with GI care?
Dr. Korman: Many people in the sexual and gender minority community have experienced discrimination in health care settings or know of someone who has. For these reasons, LGBTQIA+ people may approach health care with the expectation of a negative encounter, or they may avoid accessing care altogether. Because anal cancer disproportionately affects sexual and gender minority communities, creating a warm, inclusive environment is key to identifying who is at risk, building trust, and ensuring patients receive the care they need. When you’re talking about anal cancer, there’s a lot of stigma and shame. I think people are afraid to seek care.
Gastroenterology has traditionally been an “old boys club” but that is changing. We’re trying to work on educating people on how to recognize their own biases and move beyond them to provide care that’s affirming and where people feel that they have a safe space to talk about their concerns. Men who have sex with men, in particular living with HIV, are at the highest risk of developing anal cancer. If you don’t know that your patient is a man who has sex with men, or they don’t want to disclose that they’re living with HIV, you don’t know to screen them, and then you’re missing an opportunity to potentially prevent a cancer.
What advice would you give to aspiring medical students interested in GI?
Dr. Korman: GI is the most exciting and interesting field. We take care of so many different organs, and we’re never bored. If medical students want to get into GI, I recommend that they try to be in an office or an endoscopy center and see if it’s really for them and get some hands-on experience if possible. To be truly great at this profession, you really must see it as a calling – jump in with your whole heart and not see it as just a job. If you can do that, you’ll succeed.
How do you handle stress and maintain work-life balance?
Dr. Korman: Exercise. I try to work out at least five days a week. I can’t live without it. That keeps me going. What do I do for fun? I spend time with my family and my friends. I enjoy going to new restaurants and being outdoors, especially near a body of water. I travel, and I love watching movies. I am also guilty of binge-watching TV on a regular basis as well.
Lightning Round
Coffee or tea?
Coffee, 100%
What’s your favorite book?
I can’t say I have just one, but I recently read Tomorrow and Tomorrow and Tomorrow and loved it
Beach vacation or mountain retreat?
Beach
Early bird or night owl?
Early bird
What’s your go-to comfort food?
Anything with bananas
If you could travel anywhere, where would you go?
Vietnam or African safari
What’s your favorite childhood memory?
Swim team when I was a kid
If you could instantly learn any skill, what would it be?
Playing the drums
Are you a planner or more spontaneous?
Planner, although it’s not my strong suit, if I’m being honest.
“I think gastroenterologists are uniquely positioned to help with diagnosing anal diseases, in particular anal cancer,” she said. “It is part of the digestive tract, and my mission is to help gastroenterologists remember that.”
Dr. Korman is a gastroenterologist with Capital Digestive Care in Washington D.C., where she serves as chair of its Women’s Committee and as a member of the board of managers. She’s also the medical director of the Endoscopy Center of Washington D.C.
A recipient of the 2025 AGA Distinguished Clinician Award in Private Practice, Dr. Korman has dedicated her career to educating clinicians on anal cancer screening and anal human papillomavirus. On the research front, she participated as an investigator in the ANAL Cancer-HSIL Outcomes Research (ANCHOR) trial, which led to international anal cancer screening guidelines.
She also co-directs the International Anal Neoplasia Society (IANS) Standard High Resolution Anoscopy course.
When she’s not serving her patients, Dr. Korman speaks in the community about anal cancer awareness and screening. In the last few years, Dr. Korman has presented grand rounds at various institutions and speaks at major medical conferences. “I just try to advocate and help gastroenterologists understand who is at risk, how to look for anal cancer, how to screen, and who to refer. If anyone invites me to speak, I generally will do it,” said Dr. Korman.
In an interview, she talked about the outcomes of the ANCHOR trial and how it may inform future research, and her work to reduce bias and stigma for LGBTQ+ patients.
You decided to become a physician after studying in Egypt and Israel and volunteering with Physicians for Human Rights. Can you talk about that journey?
Dr. Korman: I majored in Religion and Middle East studies, and I minored in Arabic. I thought I was going to become a professor of religious studies. But during my time studying abroad and volunteering for Physicians for Human Rights, I was deeply moved by how physicians connect with the core of our shared humanity. Becoming a physician allows one to meet the most fundamental of human needs—caring for another’s health—in a direct and meaningful way.
My father is a physician, a gastroenterologist, but I never considered it as a career option growing up. The year after I graduated college, I accompanied my parents to my father’s medical school reunion and I thought, ‘Why did I never think about this?’ I decided to go back to school to take the pre-med requirements. Gastroenterology seemed to combine the ability to work with my hands, do procedures, have long-term relationships with patients, and think about complex problems.
GI medicine often involves detective work. What is the most challenging case you’ve encountered?
Dr. Korman: Sometimes the patients who have very severe disorders of gut-brain interaction can be the most challenging because finding treatments for them or getting them to a place where they accept certain types of treatment can be really difficult. And of course, you have to put your detective hat on and make sure you have ruled out all the “zebras.” It can take years to build the level of trust where patients are willing to accept the diagnosis and then pursue appropriate treatment.
I always try my best, but I don’t like to give up. I will refer a patient to a colleague if they have a problem and I can’t figure out what the diagnosis is or find a treatment that works. I believe in second and third opinions. I recognize that there’s a limit to what my brain can do and that we all have blind spots. Maybe someone will look at the case with fresh eyes and think of something else.
What was the most impactful outcomes of the ANAL Cancer-HSIL Outcomes Research (ANCHOR) trial?
Dr. Korman: This was a National Institutes of Health (NIH)-sponsored, randomized controlled trial with 26 clinical sites. We studied people living with human immunodeficiency virus (HIV), as they are the most at-risk group for anal cancer.
We were looking to prove that treating high grade squamous intraepithelial lesions (HSIL) of the anal canal would lead to a significant reduction in the rates of anal cancer. No one in the medical community would accept guidelines or recommendations about what to do with anal pre-cancers until we proved that treatment worked.
We published the findings in 2022. The study concluded when we met our endpoint earlier than expected. We were able to prove that treating high grade anal dysplasia does indeed lead to a very significant reduction in progression to anal cancer. That ultimately led to guidelines. The International Anal Neoplasia Society came out with consensus guidelines on screening for anal cancer in January 2024. In August 2024, NIH, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America came out with screening guidelines for people living with HIV.
Were there any other outcomes from this research?
Dr. Korman: One of the great things about the study is that we accumulated a bank of tissue and biologic specimens. There were about 4,500 patients randomized into the trial, but about 10,000 patients screened. So, we have a massive collection of biospecimens that we can use to ask questions about the progression of HSIL to anal cancer. We would like to understand more about viral and host molecular mechanisms and hopefully find biomarkers that will identify individuals at particularly high risk of progression. It’s a more precision medicine type of approach.
Education has been a cornerstone of your career. What’s the most rewarding part of teaching the IANS standard high resolution endoscopy course?
Dr. Korman: I first took the course in 2010, and that’s when I started my journey of learning how to perform high resolution endoscopy. Last year I was asked to help co-direct the course. It is now virtual and asynchronous where everything is recorded. But it was exciting to help reorganize the course, update the lectures, and make sure that everything is current. We get to answer questions from participants from all over the world. I think there are participants from 23 countries who have taken the course, which is amazing.
Could you share your work with the LGBTQIA+ population? What specific needs/challenges does this population have with GI care?
Dr. Korman: Many people in the sexual and gender minority community have experienced discrimination in health care settings or know of someone who has. For these reasons, LGBTQIA+ people may approach health care with the expectation of a negative encounter, or they may avoid accessing care altogether. Because anal cancer disproportionately affects sexual and gender minority communities, creating a warm, inclusive environment is key to identifying who is at risk, building trust, and ensuring patients receive the care they need. When you’re talking about anal cancer, there’s a lot of stigma and shame. I think people are afraid to seek care.
Gastroenterology has traditionally been an “old boys club” but that is changing. We’re trying to work on educating people on how to recognize their own biases and move beyond them to provide care that’s affirming and where people feel that they have a safe space to talk about their concerns. Men who have sex with men, in particular living with HIV, are at the highest risk of developing anal cancer. If you don’t know that your patient is a man who has sex with men, or they don’t want to disclose that they’re living with HIV, you don’t know to screen them, and then you’re missing an opportunity to potentially prevent a cancer.
What advice would you give to aspiring medical students interested in GI?
Dr. Korman: GI is the most exciting and interesting field. We take care of so many different organs, and we’re never bored. If medical students want to get into GI, I recommend that they try to be in an office or an endoscopy center and see if it’s really for them and get some hands-on experience if possible. To be truly great at this profession, you really must see it as a calling – jump in with your whole heart and not see it as just a job. If you can do that, you’ll succeed.
How do you handle stress and maintain work-life balance?
Dr. Korman: Exercise. I try to work out at least five days a week. I can’t live without it. That keeps me going. What do I do for fun? I spend time with my family and my friends. I enjoy going to new restaurants and being outdoors, especially near a body of water. I travel, and I love watching movies. I am also guilty of binge-watching TV on a regular basis as well.
Lightning Round
Coffee or tea?
Coffee, 100%
What’s your favorite book?
I can’t say I have just one, but I recently read Tomorrow and Tomorrow and Tomorrow and loved it
Beach vacation or mountain retreat?
Beach
Early bird or night owl?
Early bird
What’s your go-to comfort food?
Anything with bananas
If you could travel anywhere, where would you go?
Vietnam or African safari
What’s your favorite childhood memory?
Swim team when I was a kid
If you could instantly learn any skill, what would it be?
Playing the drums
Are you a planner or more spontaneous?
Planner, although it’s not my strong suit, if I’m being honest.

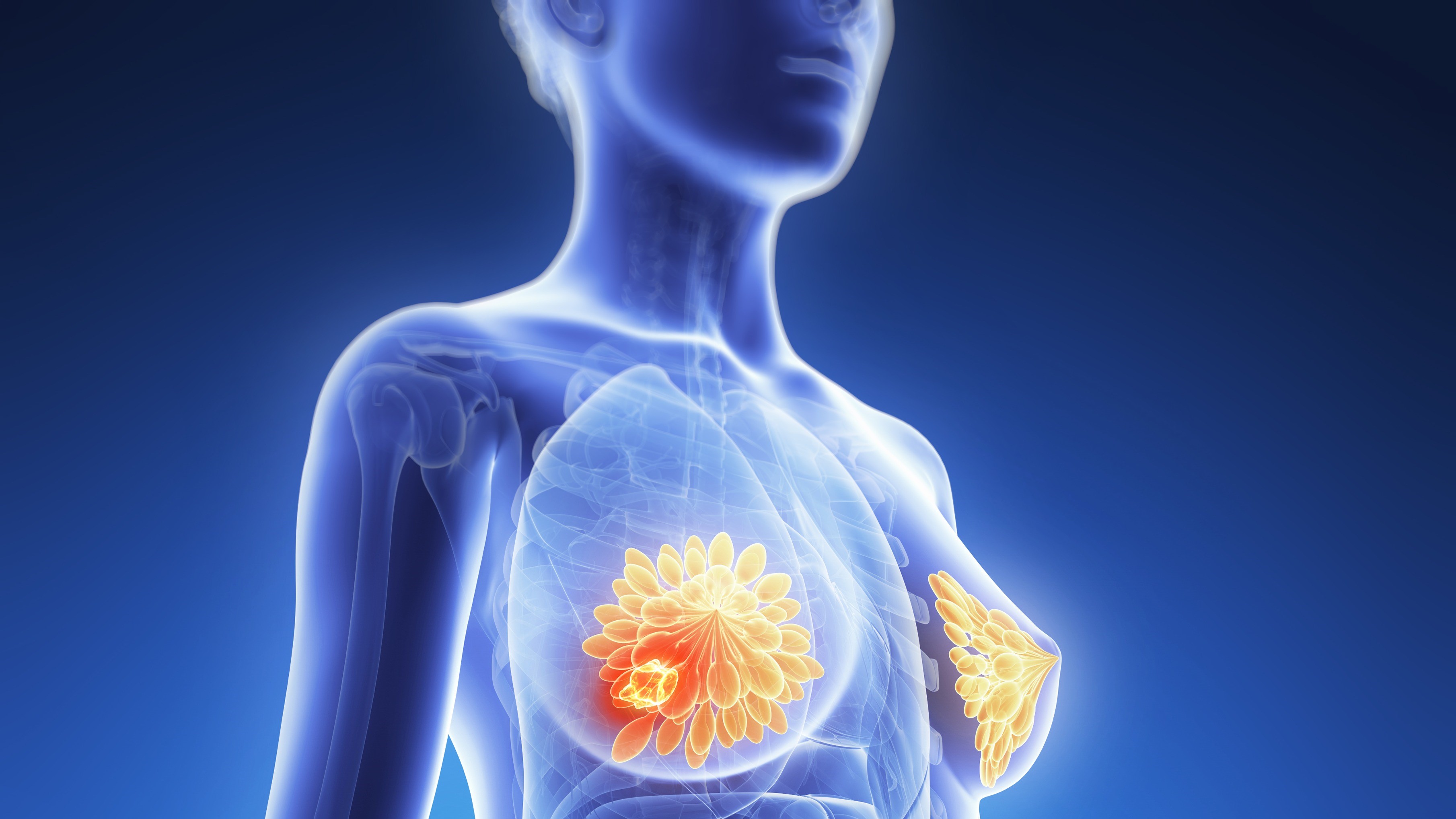
Artificial Intelligence Shows Promise in Detecting Missed Interval Breast Cancer on Screening Mammograms
TOPLINE:
An artificial intelligence (AI) system flagged high-risk areas on mammograms for potentially missed interval breast cancers (IBCs), which radiologists had also retrospectively identified as abnormal. Moreover, the AI detected a substantial number of IBCs that manual review had overlooked.
METHODOLOGY:
- Researchers conducted a retrospective analysis of 119 IBC screening mammograms of women (mean age, 57.3 years) with a high breast density (Breast Imaging Reporting and Data System [BI-RADS] c/d, 63.0%) using data retrieved from Cancer Registries of Eastern Switzerland and Grisons-Glarus databases.
- A recorded tumour was classified as IBC when an invasive or in situ BC was diagnosed within 24 months after a normal screening mammogram.
- Three radiologists retrospectively assessed the mammograms for visible signs of BC, which were then classified as either potentially missed IBCs or IBCs without retrospective abnormalities on the basis of consensus conference recommendations of radiologists.
- An AI system generated two scores (a scale of 0 to 100): a case score reflecting the likelihood that the mammogram currently harbours cancer and a risk score estimating the probability of a BC diagnosis within 2 years.
TAKEAWAY:
- Radiologists classified 68.9% of IBCs as those having no retrospective abnormalities and assigned significantly higher BI-RADS scores to the remaining 31.1% of potentially missed IBCs (P < .05).
- Potentially missed IBCs received significantly higher AI case scores (mean, 54.1 vs 23.1; P < .05) and were assigned to a higher risk category (48.7% vs 14.6%; P < .05) than IBCs without retrospective abnormalities.
- Of all IBC cases, 46.2% received an AI case score > 25, 25.2% scored > 50, and 13.4% scored > 75.
- Potentially missed IBCs scored widely between low and high risk and case scores, whereas IBCs without retrospective abnormalities scored low case and risk scores. Specifically, 73.0% of potentially missed IBCs vs 34.1% of IBCs without retrospective abnormalities had case scores > 25, 51.4% vs 13.4% had case scores > 50, and 29.7% vs 6.1% had case scores > 75.
IN PRACTICE:
“Our research highlights that an AI system can identify BC signs in relevant portions of IBC screening mammograms and thus potentially reduce the number of IBCs in an MSP [mammography screening program] that currently does not utilize an AI system,” the authors of the study concluded, adding that “it can identify some IBCs that are not visible to humans (IBCs without retrospective abnormalities).”
SOURCE:
This study was led by Jonas Subelack, Chair of Health Economics, Policy and Management, School of Medicine, University of St. Gallen, St. Gallen, Switzerland. It was published online in European Radiology.
LIMITATIONS:
The retrospective study design inherently limited causal conclusions. Without access to diagnostic mammograms or the detailed position of BC, researchers could not evaluate whether AI-marked lesions corresponded to later detected BCs.
DISCLOSURES:
This research was funded by the Cancer League of Eastern Switzerland. One author reported receiving consulting and speaker fees from iCAD.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI) system flagged high-risk areas on mammograms for potentially missed interval breast cancers (IBCs), which radiologists had also retrospectively identified as abnormal. Moreover, the AI detected a substantial number of IBCs that manual review had overlooked.
METHODOLOGY:
- Researchers conducted a retrospective analysis of 119 IBC screening mammograms of women (mean age, 57.3 years) with a high breast density (Breast Imaging Reporting and Data System [BI-RADS] c/d, 63.0%) using data retrieved from Cancer Registries of Eastern Switzerland and Grisons-Glarus databases.
- A recorded tumour was classified as IBC when an invasive or in situ BC was diagnosed within 24 months after a normal screening mammogram.
- Three radiologists retrospectively assessed the mammograms for visible signs of BC, which were then classified as either potentially missed IBCs or IBCs without retrospective abnormalities on the basis of consensus conference recommendations of radiologists.
- An AI system generated two scores (a scale of 0 to 100): a case score reflecting the likelihood that the mammogram currently harbours cancer and a risk score estimating the probability of a BC diagnosis within 2 years.
TAKEAWAY:
- Radiologists classified 68.9% of IBCs as those having no retrospective abnormalities and assigned significantly higher BI-RADS scores to the remaining 31.1% of potentially missed IBCs (P < .05).
- Potentially missed IBCs received significantly higher AI case scores (mean, 54.1 vs 23.1; P < .05) and were assigned to a higher risk category (48.7% vs 14.6%; P < .05) than IBCs without retrospective abnormalities.
- Of all IBC cases, 46.2% received an AI case score > 25, 25.2% scored > 50, and 13.4% scored > 75.
- Potentially missed IBCs scored widely between low and high risk and case scores, whereas IBCs without retrospective abnormalities scored low case and risk scores. Specifically, 73.0% of potentially missed IBCs vs 34.1% of IBCs without retrospective abnormalities had case scores > 25, 51.4% vs 13.4% had case scores > 50, and 29.7% vs 6.1% had case scores > 75.
IN PRACTICE:
“Our research highlights that an AI system can identify BC signs in relevant portions of IBC screening mammograms and thus potentially reduce the number of IBCs in an MSP [mammography screening program] that currently does not utilize an AI system,” the authors of the study concluded, adding that “it can identify some IBCs that are not visible to humans (IBCs without retrospective abnormalities).”
SOURCE:
This study was led by Jonas Subelack, Chair of Health Economics, Policy and Management, School of Medicine, University of St. Gallen, St. Gallen, Switzerland. It was published online in European Radiology.
LIMITATIONS:
The retrospective study design inherently limited causal conclusions. Without access to diagnostic mammograms or the detailed position of BC, researchers could not evaluate whether AI-marked lesions corresponded to later detected BCs.
DISCLOSURES:
This research was funded by the Cancer League of Eastern Switzerland. One author reported receiving consulting and speaker fees from iCAD.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI) system flagged high-risk areas on mammograms for potentially missed interval breast cancers (IBCs), which radiologists had also retrospectively identified as abnormal. Moreover, the AI detected a substantial number of IBCs that manual review had overlooked.
METHODOLOGY:
- Researchers conducted a retrospective analysis of 119 IBC screening mammograms of women (mean age, 57.3 years) with a high breast density (Breast Imaging Reporting and Data System [BI-RADS] c/d, 63.0%) using data retrieved from Cancer Registries of Eastern Switzerland and Grisons-Glarus databases.
- A recorded tumour was classified as IBC when an invasive or in situ BC was diagnosed within 24 months after a normal screening mammogram.
- Three radiologists retrospectively assessed the mammograms for visible signs of BC, which were then classified as either potentially missed IBCs or IBCs without retrospective abnormalities on the basis of consensus conference recommendations of radiologists.
- An AI system generated two scores (a scale of 0 to 100): a case score reflecting the likelihood that the mammogram currently harbours cancer and a risk score estimating the probability of a BC diagnosis within 2 years.
TAKEAWAY:
- Radiologists classified 68.9% of IBCs as those having no retrospective abnormalities and assigned significantly higher BI-RADS scores to the remaining 31.1% of potentially missed IBCs (P < .05).
- Potentially missed IBCs received significantly higher AI case scores (mean, 54.1 vs 23.1; P < .05) and were assigned to a higher risk category (48.7% vs 14.6%; P < .05) than IBCs without retrospective abnormalities.
- Of all IBC cases, 46.2% received an AI case score > 25, 25.2% scored > 50, and 13.4% scored > 75.
- Potentially missed IBCs scored widely between low and high risk and case scores, whereas IBCs without retrospective abnormalities scored low case and risk scores. Specifically, 73.0% of potentially missed IBCs vs 34.1% of IBCs without retrospective abnormalities had case scores > 25, 51.4% vs 13.4% had case scores > 50, and 29.7% vs 6.1% had case scores > 75.
IN PRACTICE:
“Our research highlights that an AI system can identify BC signs in relevant portions of IBC screening mammograms and thus potentially reduce the number of IBCs in an MSP [mammography screening program] that currently does not utilize an AI system,” the authors of the study concluded, adding that “it can identify some IBCs that are not visible to humans (IBCs without retrospective abnormalities).”
SOURCE:
This study was led by Jonas Subelack, Chair of Health Economics, Policy and Management, School of Medicine, University of St. Gallen, St. Gallen, Switzerland. It was published online in European Radiology.
LIMITATIONS:
The retrospective study design inherently limited causal conclusions. Without access to diagnostic mammograms or the detailed position of BC, researchers could not evaluate whether AI-marked lesions corresponded to later detected BCs.
DISCLOSURES:
This research was funded by the Cancer League of Eastern Switzerland. One author reported receiving consulting and speaker fees from iCAD.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
VHA Facilities Report Severe Staffing Shortages
VHA Facilities Report Severe Staffing Shortages
For > 10 years, the US Department of Veterans Affairs (VA) Office of Inspector General (OIG) has annually surveyed Veterans Health Administration (VHA) facilities about staffing. Its recently released report is the 8th to find severe shortages—in this case, across the board. There were 4434 severe staffing shortages reported across all 139 VHA facilities in fiscal year (FY) 2025, a 50% increase from FY 2024.
In the OIG report lexicon, a severe shortage refers to "particular occupations that are difficult to fill," and is not necessarily an indication of vacancies. Vacancy refers to a "specific unoccupied position and is distinct from the designation of a severe shortage." For example, a facility could identify an occupation as a severe occupational shortage, which could have no vacant positions or 100 vacant positions.
Nearly all facilities (94%) had severe shortages for medical officers, and 79% had severe shortages for nurses even with VHA's ability to make noncompetitive appointments for those occupations. Psychology was the most frequently reported severe clinical occupational staffing shortage, reported by 79 facilities (57%), down slightly from FY 2024 (61%). One facility reported 116 clinical occupational shortages.
The report notes that the OIG does not verify or otherwise confirm the questionnaire responses, but it appears to support other data. In the first 9 months of FY 2024, the VA added 223 physicians and 3196 nurses compared with a deficit of 781 physicians and 2129 nurses over the same period in FY 2025.
VHA facilities are finding it hard to reverse the trend. According to internal documents examined by ProPublica, nearly 4 in 10 of the roughly 2000 doctors offered jobs from January through March 2025 turned them down, 4 times the rate in the same time period in 2024. VHA also lost twice as many nurses as it hired between January and June. Many potential candidates reportedly were worried about the stability of VA employment.
VA spokesperson Peter Kasperowicz did not dispute the ProPublica findings but accused the news outlet of bias and "cherry-picking issues that are mostly routine." A nationwide shortage of health care workers has made hiring and retention difficult, he said.
Kasperowicz said the VA is "working to address" the number of doctors declining job offers by speeding up the hiring process and that the agency "has several strategies to navigate shortages." Those include referring veterans to telehealth and private clinicians.
In a statement released Aug. 12, Sen Richard Blumenthal (D-CT), ranking member of the Senate Committee on Veterans' Affairs, said, "This report confirms what we've warned for months—this Administration is driving dedicated VA employees to the private sector at untenable rates."
The OIG survey did not ask about facilities' rationales for identifying shortages. Moreover, the OIG says the responses don't reflect the possible impacts of "workforce reshaping efforts," such as the Deferred Resignation Program announced on January 28, 2025.
In response to the OIG report, Kasperowicz said it is "not based on actual VA health care facility vacancies and therefore is not a reliable indicator of staffing shortages." In a statement to CBS News, he added, "The report simply lists occupations facilities feel are difficult for which to recruit and retain, so the results are completely subjective, not standardized, and unreliable." According to Kasperowicz, the system-wide vacancy rates for doctors and nurses are 14% and 10%, respectively, which are in line with historical averages.
The OIG made no recommendations but "encourages VA leaders to use these review results to inform staffing initiatives and organizational change."
For > 10 years, the US Department of Veterans Affairs (VA) Office of Inspector General (OIG) has annually surveyed Veterans Health Administration (VHA) facilities about staffing. Its recently released report is the 8th to find severe shortages—in this case, across the board. There were 4434 severe staffing shortages reported across all 139 VHA facilities in fiscal year (FY) 2025, a 50% increase from FY 2024.
In the OIG report lexicon, a severe shortage refers to "particular occupations that are difficult to fill," and is not necessarily an indication of vacancies. Vacancy refers to a "specific unoccupied position and is distinct from the designation of a severe shortage." For example, a facility could identify an occupation as a severe occupational shortage, which could have no vacant positions or 100 vacant positions.
Nearly all facilities (94%) had severe shortages for medical officers, and 79% had severe shortages for nurses even with VHA's ability to make noncompetitive appointments for those occupations. Psychology was the most frequently reported severe clinical occupational staffing shortage, reported by 79 facilities (57%), down slightly from FY 2024 (61%). One facility reported 116 clinical occupational shortages.
The report notes that the OIG does not verify or otherwise confirm the questionnaire responses, but it appears to support other data. In the first 9 months of FY 2024, the VA added 223 physicians and 3196 nurses compared with a deficit of 781 physicians and 2129 nurses over the same period in FY 2025.
VHA facilities are finding it hard to reverse the trend. According to internal documents examined by ProPublica, nearly 4 in 10 of the roughly 2000 doctors offered jobs from January through March 2025 turned them down, 4 times the rate in the same time period in 2024. VHA also lost twice as many nurses as it hired between January and June. Many potential candidates reportedly were worried about the stability of VA employment.
VA spokesperson Peter Kasperowicz did not dispute the ProPublica findings but accused the news outlet of bias and "cherry-picking issues that are mostly routine." A nationwide shortage of health care workers has made hiring and retention difficult, he said.
Kasperowicz said the VA is "working to address" the number of doctors declining job offers by speeding up the hiring process and that the agency "has several strategies to navigate shortages." Those include referring veterans to telehealth and private clinicians.
In a statement released Aug. 12, Sen Richard Blumenthal (D-CT), ranking member of the Senate Committee on Veterans' Affairs, said, "This report confirms what we've warned for months—this Administration is driving dedicated VA employees to the private sector at untenable rates."
The OIG survey did not ask about facilities' rationales for identifying shortages. Moreover, the OIG says the responses don't reflect the possible impacts of "workforce reshaping efforts," such as the Deferred Resignation Program announced on January 28, 2025.
In response to the OIG report, Kasperowicz said it is "not based on actual VA health care facility vacancies and therefore is not a reliable indicator of staffing shortages." In a statement to CBS News, he added, "The report simply lists occupations facilities feel are difficult for which to recruit and retain, so the results are completely subjective, not standardized, and unreliable." According to Kasperowicz, the system-wide vacancy rates for doctors and nurses are 14% and 10%, respectively, which are in line with historical averages.
The OIG made no recommendations but "encourages VA leaders to use these review results to inform staffing initiatives and organizational change."
For > 10 years, the US Department of Veterans Affairs (VA) Office of Inspector General (OIG) has annually surveyed Veterans Health Administration (VHA) facilities about staffing. Its recently released report is the 8th to find severe shortages—in this case, across the board. There were 4434 severe staffing shortages reported across all 139 VHA facilities in fiscal year (FY) 2025, a 50% increase from FY 2024.
In the OIG report lexicon, a severe shortage refers to "particular occupations that are difficult to fill," and is not necessarily an indication of vacancies. Vacancy refers to a "specific unoccupied position and is distinct from the designation of a severe shortage." For example, a facility could identify an occupation as a severe occupational shortage, which could have no vacant positions or 100 vacant positions.
Nearly all facilities (94%) had severe shortages for medical officers, and 79% had severe shortages for nurses even with VHA's ability to make noncompetitive appointments for those occupations. Psychology was the most frequently reported severe clinical occupational staffing shortage, reported by 79 facilities (57%), down slightly from FY 2024 (61%). One facility reported 116 clinical occupational shortages.
The report notes that the OIG does not verify or otherwise confirm the questionnaire responses, but it appears to support other data. In the first 9 months of FY 2024, the VA added 223 physicians and 3196 nurses compared with a deficit of 781 physicians and 2129 nurses over the same period in FY 2025.
VHA facilities are finding it hard to reverse the trend. According to internal documents examined by ProPublica, nearly 4 in 10 of the roughly 2000 doctors offered jobs from January through March 2025 turned them down, 4 times the rate in the same time period in 2024. VHA also lost twice as many nurses as it hired between January and June. Many potential candidates reportedly were worried about the stability of VA employment.
VA spokesperson Peter Kasperowicz did not dispute the ProPublica findings but accused the news outlet of bias and "cherry-picking issues that are mostly routine." A nationwide shortage of health care workers has made hiring and retention difficult, he said.
Kasperowicz said the VA is "working to address" the number of doctors declining job offers by speeding up the hiring process and that the agency "has several strategies to navigate shortages." Those include referring veterans to telehealth and private clinicians.
In a statement released Aug. 12, Sen Richard Blumenthal (D-CT), ranking member of the Senate Committee on Veterans' Affairs, said, "This report confirms what we've warned for months—this Administration is driving dedicated VA employees to the private sector at untenable rates."
The OIG survey did not ask about facilities' rationales for identifying shortages. Moreover, the OIG says the responses don't reflect the possible impacts of "workforce reshaping efforts," such as the Deferred Resignation Program announced on January 28, 2025.
In response to the OIG report, Kasperowicz said it is "not based on actual VA health care facility vacancies and therefore is not a reliable indicator of staffing shortages." In a statement to CBS News, he added, "The report simply lists occupations facilities feel are difficult for which to recruit and retain, so the results are completely subjective, not standardized, and unreliable." According to Kasperowicz, the system-wide vacancy rates for doctors and nurses are 14% and 10%, respectively, which are in line with historical averages.
The OIG made no recommendations but "encourages VA leaders to use these review results to inform staffing initiatives and organizational change."
VHA Facilities Report Severe Staffing Shortages
VHA Facilities Report Severe Staffing Shortages
VA Workforce Shrinking as it Loses Collective Bargaining Rights
VA Workforce Shrinking as it Loses Collective Bargaining Rights
The US Department of Veterans Affairs (VA) is on pace to cut nearly 30,000 positions by the end of fiscal year 2025, an initiative driven by a federal hiring freeze, deferred resignations, retirements, and normal attrition. According to the VA Workforce Dashboard, health care experienced the most significant net change through the first 9 months of fiscal year 2025. That included 2129 fewer registered nurses, 751 fewer physicians, and drops of 565 licensed practical nurses, 564 nurse assistants, and 1294 medical support assistants. In total, nearly 17,000 VA employees have left their jobs and 12,000 more are expected to leave by the end of September 2025.
According to VA Secretary Doug Collins, the departures have eliminated the need for the "large-scale" reduction-in-force that he proposed earlier in 2025.
The VA also announced that in accordance with an Executive Order issued by President Donald Trump, it is terminating collective bargaining rights for most of its employees, including most clinical staff not in leadership positions. The order includes the National Nurses Organizing Committee/National Nurses United, which represents 16,000 VA nurses, and the American Federation of Government Employees, which represents 320,000 VA employees. The order exempted police officers, firefighters, and security guards. The Unions have indicated they will continue to fight the changes.
VA staffing has undergone significant reversals over the past year. The VA added 223 physicians and 3196 nurses in the first 9 months of fiscal year 2024 before reversing course this year. According to the Workforce Dashboard, the VA and Veterans Health Administration combined to hire 26,984 employees in fiscal year 2025. Cumulative losses, however, totaled 54,308.
During exit interviews, VA employees noted a variety of reasons for their departure. "Personal/family matters" and "geographic relocation" were cited by many job categories. In addition, medical and dental workers also noted "poor working relationship with supervisor or coworker(s)," "desired work schedule not offered," and "job stress/pressure" among the causes. The VA has lost 148 psychologists in fiscal year 2025 who cited "lack of trust/confidence in senior leaders," as well as "policy or technology barriers to getting the work done," and "job stress/pressure" among their reasons for departure.
The US Department of Veterans Affairs (VA) is on pace to cut nearly 30,000 positions by the end of fiscal year 2025, an initiative driven by a federal hiring freeze, deferred resignations, retirements, and normal attrition. According to the VA Workforce Dashboard, health care experienced the most significant net change through the first 9 months of fiscal year 2025. That included 2129 fewer registered nurses, 751 fewer physicians, and drops of 565 licensed practical nurses, 564 nurse assistants, and 1294 medical support assistants. In total, nearly 17,000 VA employees have left their jobs and 12,000 more are expected to leave by the end of September 2025.
According to VA Secretary Doug Collins, the departures have eliminated the need for the "large-scale" reduction-in-force that he proposed earlier in 2025.
The VA also announced that in accordance with an Executive Order issued by President Donald Trump, it is terminating collective bargaining rights for most of its employees, including most clinical staff not in leadership positions. The order includes the National Nurses Organizing Committee/National Nurses United, which represents 16,000 VA nurses, and the American Federation of Government Employees, which represents 320,000 VA employees. The order exempted police officers, firefighters, and security guards. The Unions have indicated they will continue to fight the changes.
VA staffing has undergone significant reversals over the past year. The VA added 223 physicians and 3196 nurses in the first 9 months of fiscal year 2024 before reversing course this year. According to the Workforce Dashboard, the VA and Veterans Health Administration combined to hire 26,984 employees in fiscal year 2025. Cumulative losses, however, totaled 54,308.
During exit interviews, VA employees noted a variety of reasons for their departure. "Personal/family matters" and "geographic relocation" were cited by many job categories. In addition, medical and dental workers also noted "poor working relationship with supervisor or coworker(s)," "desired work schedule not offered," and "job stress/pressure" among the causes. The VA has lost 148 psychologists in fiscal year 2025 who cited "lack of trust/confidence in senior leaders," as well as "policy or technology barriers to getting the work done," and "job stress/pressure" among their reasons for departure.
The US Department of Veterans Affairs (VA) is on pace to cut nearly 30,000 positions by the end of fiscal year 2025, an initiative driven by a federal hiring freeze, deferred resignations, retirements, and normal attrition. According to the VA Workforce Dashboard, health care experienced the most significant net change through the first 9 months of fiscal year 2025. That included 2129 fewer registered nurses, 751 fewer physicians, and drops of 565 licensed practical nurses, 564 nurse assistants, and 1294 medical support assistants. In total, nearly 17,000 VA employees have left their jobs and 12,000 more are expected to leave by the end of September 2025.
According to VA Secretary Doug Collins, the departures have eliminated the need for the "large-scale" reduction-in-force that he proposed earlier in 2025.
The VA also announced that in accordance with an Executive Order issued by President Donald Trump, it is terminating collective bargaining rights for most of its employees, including most clinical staff not in leadership positions. The order includes the National Nurses Organizing Committee/National Nurses United, which represents 16,000 VA nurses, and the American Federation of Government Employees, which represents 320,000 VA employees. The order exempted police officers, firefighters, and security guards. The Unions have indicated they will continue to fight the changes.
VA staffing has undergone significant reversals over the past year. The VA added 223 physicians and 3196 nurses in the first 9 months of fiscal year 2024 before reversing course this year. According to the Workforce Dashboard, the VA and Veterans Health Administration combined to hire 26,984 employees in fiscal year 2025. Cumulative losses, however, totaled 54,308.
During exit interviews, VA employees noted a variety of reasons for their departure. "Personal/family matters" and "geographic relocation" were cited by many job categories. In addition, medical and dental workers also noted "poor working relationship with supervisor or coworker(s)," "desired work schedule not offered," and "job stress/pressure" among the causes. The VA has lost 148 psychologists in fiscal year 2025 who cited "lack of trust/confidence in senior leaders," as well as "policy or technology barriers to getting the work done," and "job stress/pressure" among their reasons for departure.
VA Workforce Shrinking as it Loses Collective Bargaining Rights
VA Workforce Shrinking as it Loses Collective Bargaining Rights
U.S. Health Chief Kennedy Targets Vaccine Injury Compensation Program
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
AGA President Brings Forth “Message Of Inclusivity”
“I was always interested in medicine. From a relatively early age I thought that’s what I would be doing,” said Dr. Kim. When his father became disillusioned with his own career as a pathologist, he encouraged his son to look in other directions.
“In college I had the opportunity to study and learn broadly and I became interested in public policy and eventually majored in that discipline,” he said.
The mentorship of the late Uwe Reinhardt, a well-respected health economist at Princeton University, had a major impact on Dr. Kim during his senior year of college. Reinhardt told him that physicians are afforded a special position in society. “They have a moral responsibility to take the lead in terms of guiding and shaping healthcare. His message made a big impression upon me,” said Dr. Kim.
Ultimately, he decided to go into clinical medicine, but maintained his interest in healthcare policy. Experiences outside of the standard approach to medicine “helped me stay in the big picture of healthcare, to make a difference beyond just my individual patients. And that’s played a big part in keeping me involved in organized medicine,” said Dr. Kim, who began his term as AGA president in May 2025.
Dr. Kim is also a partner at South Denver Gastroenterology, a 33-provider, independent gastroenterology practice in Colorado. As the first physician in Colorado with fellowship training in endoscopic ultrasound, he introduced this service line into South Denver’s advanced endoscopy practice.
Dr. Kim has served in numerous roles with AGA, among them the co-director of the AGA Clinical Congress, the Partners in Quality program, and the Nurse Practitioner and Physician Assistant Course. He is a Digestive Disease Week® abstract reviewer, has served as AGA representative to the Accreditation Association for Ambulatory Health Care and to the Alliance of Specialty Medicine. He has also served on the AGA Governing Board as clinical private practice councilor and secretary treasurer.
He discussed the high points of his career in an interview, revealing his plans as AGA president for unifying the sectors of GI medicine and fostering GI innovation and technology.
As the new AGA president, what are your goals for the society?
Dr. Kim: I want to put out a message of inclusivity. I think what’s special about AGA is that we’re the society for all gastroenterologists. Among all the other GI organizations, I think we really have the biggest tent and we work to unite clinicians, educators, and researchers – all gastroenterologists, regardless of their individual practice situation. These days, there is a tendency toward tribalism. People are starting to gravitate toward limiting their interactions to others that are from the same backgrounds. But as gastroenterologists we have more that unites us than divides us. It’s only by working together that we can make things better for everyone.
I think the second point is that we’re on the cusp of some important transformations in gastroenterology. The screening colonoscopy model that has sustained our specialty for decades is rapidly evolving. In addition, there is an increasing ability for patients as consumers to direct their own care through advances in technology, such as virtual health platforms. We’re seeing this as patients increasingly adopt things like complementary and alternative medicine outside of the standard model of physician-directed healthcare. These are two important trends that gastroenterologists need to be aware of and learn how to manage and to adapt to. I think AGA’s role is to help guide that evolution and to give physicians the tools to be able to respond.
We want to focus on innovation and we want to focus on practical solutions.
In terms of fostering innovation in gastroenterology, we’re the first medical professional society to create an incubator for new technologies. Not only do we provide that resource to our members, but we’re also putting our money where our mouth is. Through venture capital initiatives such as our GI Opportunity Fund, we directly invest in companies that we’re helping to develop.
On the practice side, we have been engaging directly with payers to foster improved communication and address pain points on both sides. I think we’re the only medical society that’s taking this type of approach and moving away from the traditional adversarial approach to dealing with payers. Recently, we had a very productive discussion with UnitedHealthcare around some of their upcoming formulary changes for inflammatory bowel disease. We used that opportunity to highlight how nonmedical switching between existing therapies can adversely impact patients, as well as increasing burden of red tape for practices.
Your practice was one of the original groups that formed the Digestive Health Physicians Association (DPHA). What accomplishments of the association are you most proud of?
Dr. Kim: DHPA formed about 10 years ago as an advocacy organization to combat a specific perceived threat, which was the in-office ancillary exception. This is the legislative pathway that allows gastroenterologists to provide ancillary services within their practice. An example of this is pathology for endoscopic procedures, which is an incredible value to patients and improves quality of care. This was under a significant legislative threat at that time. As independent physicians, DHPA took the lead in advocating against eliminating that exception.
I think the larger accomplishment was it demonstrated that gastroenterologists, specifically independent community practice gastroenterologists, could come together successfully and advocate for issues that were of importance to our specialty. AGA and DHPA have worked very well together, collaborating on shared policy interests and have worked closely on both legislative as well as regulatory issues. We’ve sponsored joint meetings that we’ve programmed together and we’re looking forward to continuing a robust partnership.
You have introduced several new clinical practice and practice management models. Can you discuss the part-time partnership model and what it has achieved?
Dr. Kim: Like many practices, South Denver Gastroenterology historically required physician partners to work full time. This conflicted with our desire and our need to attract more women gastroenterologists into our practice. The process involved careful analysis of our direct and indirect expenses, but more importantly it required a negotiation and a meeting of the minds among our partners. A lot of this ultimately came down to trust. It helped a great deal that our practice has always had strong cohesiveness. That helped us to build that trust that partners would stay engaged in the practice even if they worked part time.
Our practice has also always prioritized work-life balance. We were able to come up with a formula that allows partners to work three days per week, retaining their partnership interest and their participation in practice decisions. They stay involved but are also financially sustainable for the practice. It’s been very successful. It’s been a big draw, not just for women, but it has allowed us to create a situation where women are fully one third of our partnership. It’s something we’re all extremely proud of.
How did you get involved in AGA?
Dr. Kim: One of the first projects I participated in was the Roadmap to the Future of GI Practice. This was an initiative to help prepare GI practices for value-based care. We did things like develop quality measure sets for GI conditions such as inflammatory bowel disease and hepatitis C. We published a bundled payment model for screening colonoscopies. We also created a model for obesity management by gastroenterologists. This was 15 years ago, and I think it was about 15 years ahead of its time! It’s interesting to see how many of these changes in GI practice that we envisioned are slowly coming to pass.
I saw that AGA was interested in me as a community-based clinician. They focused on trying to develop those practical tools to help me succeed. It’s one of the reasons I’ve stayed engaged.
What is your approach to patient communication and education?
Dr. Kim: There are two things that I always tell both my staff as well as young people who come to me asking for advice. I think the first and most important is that you should always strive to treat your patients the way that you would want your family treated. Of course, we’re not perfect, but when that doesn’t happen, look at your behavior, the way that you’re interacting, but also the way the system is treating your patients and try to improve things within your own practice. And then the other thing that I tell folks is try to spend more time listening to your patients than talking or speaking at them.
What do you think is the biggest misconception about GI?
Dr. Kim: We’re not just about colonoscopies! I went into GI not just because I enjoy performing procedures, but because our specialty covers such a broad spectrum of physiology and diseases. We also have the ability as gastroenterologists to develop long-term relationships with our patients. I’ve been in practice now more than 25 years, and the greatest satisfaction in my career doesn’t come from the endoscopy center, although I still enjoy performing procedures. It comes from the clinic; it comes from the patients whom I’ve known for decades, and the interaction and conversations that I can have with them, the ability to see their families, their parents, and now in some cases their kids or even their grandkids. It’s incredibly satisfying. It makes my job fun.
What advice would you give to aspiring medical students?
Dr. Kim: One of the things I would say is stay involved in organized medicine. As physicians, we are endowed with great trust. We also have a great responsibility to help shape our healthcare care system. If we work together, we really can make a difference, not just for our profession, but also for society at large and for the patients whom we serve.
I really hope that young people don’t lose their optimism. We hear a lot these days about how much negativity and pessimism there is about the future, especially among young people in our society. But I think it’s a great time to be in medicine. Advances in medical science have made huge strides in our ability to make real differences for our patients. And the pace of technology progress is only going to continue to accelerate. Sure, there are lots of shortcomings in the practice of medicine, but honestly, that’s always been the case. I have faith that as a profession, we are smart people, we’re committed people, and we will be successful in overcoming those challenges. That’s the message that I have for young folks.
Lightning Round
Coffee or tea?
Coffee, black
What’s one hobby you’d like to pick up?
Anything except pickleball
What’s your favorite season of the year?
Winter, I’m a skier
What’s your favorite way to spend a weekend?
Doing anything outside
If you could have dinner with any historical figure, who would it be?
Ben Franklin
What’s your go-to karaoke song?
You don’t want to hear me sing
What’s one thing on your bucket list?
Skiing in South America
What’s the best piece of advice you’ve ever received?
Follow your heart
“I was always interested in medicine. From a relatively early age I thought that’s what I would be doing,” said Dr. Kim. When his father became disillusioned with his own career as a pathologist, he encouraged his son to look in other directions.
“In college I had the opportunity to study and learn broadly and I became interested in public policy and eventually majored in that discipline,” he said.
The mentorship of the late Uwe Reinhardt, a well-respected health economist at Princeton University, had a major impact on Dr. Kim during his senior year of college. Reinhardt told him that physicians are afforded a special position in society. “They have a moral responsibility to take the lead in terms of guiding and shaping healthcare. His message made a big impression upon me,” said Dr. Kim.
Ultimately, he decided to go into clinical medicine, but maintained his interest in healthcare policy. Experiences outside of the standard approach to medicine “helped me stay in the big picture of healthcare, to make a difference beyond just my individual patients. And that’s played a big part in keeping me involved in organized medicine,” said Dr. Kim, who began his term as AGA president in May 2025.
Dr. Kim is also a partner at South Denver Gastroenterology, a 33-provider, independent gastroenterology practice in Colorado. As the first physician in Colorado with fellowship training in endoscopic ultrasound, he introduced this service line into South Denver’s advanced endoscopy practice.
Dr. Kim has served in numerous roles with AGA, among them the co-director of the AGA Clinical Congress, the Partners in Quality program, and the Nurse Practitioner and Physician Assistant Course. He is a Digestive Disease Week® abstract reviewer, has served as AGA representative to the Accreditation Association for Ambulatory Health Care and to the Alliance of Specialty Medicine. He has also served on the AGA Governing Board as clinical private practice councilor and secretary treasurer.
He discussed the high points of his career in an interview, revealing his plans as AGA president for unifying the sectors of GI medicine and fostering GI innovation and technology.
As the new AGA president, what are your goals for the society?
Dr. Kim: I want to put out a message of inclusivity. I think what’s special about AGA is that we’re the society for all gastroenterologists. Among all the other GI organizations, I think we really have the biggest tent and we work to unite clinicians, educators, and researchers – all gastroenterologists, regardless of their individual practice situation. These days, there is a tendency toward tribalism. People are starting to gravitate toward limiting their interactions to others that are from the same backgrounds. But as gastroenterologists we have more that unites us than divides us. It’s only by working together that we can make things better for everyone.
I think the second point is that we’re on the cusp of some important transformations in gastroenterology. The screening colonoscopy model that has sustained our specialty for decades is rapidly evolving. In addition, there is an increasing ability for patients as consumers to direct their own care through advances in technology, such as virtual health platforms. We’re seeing this as patients increasingly adopt things like complementary and alternative medicine outside of the standard model of physician-directed healthcare. These are two important trends that gastroenterologists need to be aware of and learn how to manage and to adapt to. I think AGA’s role is to help guide that evolution and to give physicians the tools to be able to respond.
We want to focus on innovation and we want to focus on practical solutions.
In terms of fostering innovation in gastroenterology, we’re the first medical professional society to create an incubator for new technologies. Not only do we provide that resource to our members, but we’re also putting our money where our mouth is. Through venture capital initiatives such as our GI Opportunity Fund, we directly invest in companies that we’re helping to develop.
On the practice side, we have been engaging directly with payers to foster improved communication and address pain points on both sides. I think we’re the only medical society that’s taking this type of approach and moving away from the traditional adversarial approach to dealing with payers. Recently, we had a very productive discussion with UnitedHealthcare around some of their upcoming formulary changes for inflammatory bowel disease. We used that opportunity to highlight how nonmedical switching between existing therapies can adversely impact patients, as well as increasing burden of red tape for practices.
Your practice was one of the original groups that formed the Digestive Health Physicians Association (DPHA). What accomplishments of the association are you most proud of?
Dr. Kim: DHPA formed about 10 years ago as an advocacy organization to combat a specific perceived threat, which was the in-office ancillary exception. This is the legislative pathway that allows gastroenterologists to provide ancillary services within their practice. An example of this is pathology for endoscopic procedures, which is an incredible value to patients and improves quality of care. This was under a significant legislative threat at that time. As independent physicians, DHPA took the lead in advocating against eliminating that exception.
I think the larger accomplishment was it demonstrated that gastroenterologists, specifically independent community practice gastroenterologists, could come together successfully and advocate for issues that were of importance to our specialty. AGA and DHPA have worked very well together, collaborating on shared policy interests and have worked closely on both legislative as well as regulatory issues. We’ve sponsored joint meetings that we’ve programmed together and we’re looking forward to continuing a robust partnership.
You have introduced several new clinical practice and practice management models. Can you discuss the part-time partnership model and what it has achieved?
Dr. Kim: Like many practices, South Denver Gastroenterology historically required physician partners to work full time. This conflicted with our desire and our need to attract more women gastroenterologists into our practice. The process involved careful analysis of our direct and indirect expenses, but more importantly it required a negotiation and a meeting of the minds among our partners. A lot of this ultimately came down to trust. It helped a great deal that our practice has always had strong cohesiveness. That helped us to build that trust that partners would stay engaged in the practice even if they worked part time.
Our practice has also always prioritized work-life balance. We were able to come up with a formula that allows partners to work three days per week, retaining their partnership interest and their participation in practice decisions. They stay involved but are also financially sustainable for the practice. It’s been very successful. It’s been a big draw, not just for women, but it has allowed us to create a situation where women are fully one third of our partnership. It’s something we’re all extremely proud of.
How did you get involved in AGA?
Dr. Kim: One of the first projects I participated in was the Roadmap to the Future of GI Practice. This was an initiative to help prepare GI practices for value-based care. We did things like develop quality measure sets for GI conditions such as inflammatory bowel disease and hepatitis C. We published a bundled payment model for screening colonoscopies. We also created a model for obesity management by gastroenterologists. This was 15 years ago, and I think it was about 15 years ahead of its time! It’s interesting to see how many of these changes in GI practice that we envisioned are slowly coming to pass.
I saw that AGA was interested in me as a community-based clinician. They focused on trying to develop those practical tools to help me succeed. It’s one of the reasons I’ve stayed engaged.
What is your approach to patient communication and education?
Dr. Kim: There are two things that I always tell both my staff as well as young people who come to me asking for advice. I think the first and most important is that you should always strive to treat your patients the way that you would want your family treated. Of course, we’re not perfect, but when that doesn’t happen, look at your behavior, the way that you’re interacting, but also the way the system is treating your patients and try to improve things within your own practice. And then the other thing that I tell folks is try to spend more time listening to your patients than talking or speaking at them.
What do you think is the biggest misconception about GI?
Dr. Kim: We’re not just about colonoscopies! I went into GI not just because I enjoy performing procedures, but because our specialty covers such a broad spectrum of physiology and diseases. We also have the ability as gastroenterologists to develop long-term relationships with our patients. I’ve been in practice now more than 25 years, and the greatest satisfaction in my career doesn’t come from the endoscopy center, although I still enjoy performing procedures. It comes from the clinic; it comes from the patients whom I’ve known for decades, and the interaction and conversations that I can have with them, the ability to see their families, their parents, and now in some cases their kids or even their grandkids. It’s incredibly satisfying. It makes my job fun.
What advice would you give to aspiring medical students?
Dr. Kim: One of the things I would say is stay involved in organized medicine. As physicians, we are endowed with great trust. We also have a great responsibility to help shape our healthcare care system. If we work together, we really can make a difference, not just for our profession, but also for society at large and for the patients whom we serve.
I really hope that young people don’t lose their optimism. We hear a lot these days about how much negativity and pessimism there is about the future, especially among young people in our society. But I think it’s a great time to be in medicine. Advances in medical science have made huge strides in our ability to make real differences for our patients. And the pace of technology progress is only going to continue to accelerate. Sure, there are lots of shortcomings in the practice of medicine, but honestly, that’s always been the case. I have faith that as a profession, we are smart people, we’re committed people, and we will be successful in overcoming those challenges. That’s the message that I have for young folks.
Lightning Round
Coffee or tea?
Coffee, black
What’s one hobby you’d like to pick up?
Anything except pickleball
What’s your favorite season of the year?
Winter, I’m a skier
What’s your favorite way to spend a weekend?
Doing anything outside
If you could have dinner with any historical figure, who would it be?
Ben Franklin
What’s your go-to karaoke song?
You don’t want to hear me sing
What’s one thing on your bucket list?
Skiing in South America
What’s the best piece of advice you’ve ever received?
Follow your heart
“I was always interested in medicine. From a relatively early age I thought that’s what I would be doing,” said Dr. Kim. When his father became disillusioned with his own career as a pathologist, he encouraged his son to look in other directions.
“In college I had the opportunity to study and learn broadly and I became interested in public policy and eventually majored in that discipline,” he said.
The mentorship of the late Uwe Reinhardt, a well-respected health economist at Princeton University, had a major impact on Dr. Kim during his senior year of college. Reinhardt told him that physicians are afforded a special position in society. “They have a moral responsibility to take the lead in terms of guiding and shaping healthcare. His message made a big impression upon me,” said Dr. Kim.
Ultimately, he decided to go into clinical medicine, but maintained his interest in healthcare policy. Experiences outside of the standard approach to medicine “helped me stay in the big picture of healthcare, to make a difference beyond just my individual patients. And that’s played a big part in keeping me involved in organized medicine,” said Dr. Kim, who began his term as AGA president in May 2025.
Dr. Kim is also a partner at South Denver Gastroenterology, a 33-provider, independent gastroenterology practice in Colorado. As the first physician in Colorado with fellowship training in endoscopic ultrasound, he introduced this service line into South Denver’s advanced endoscopy practice.
Dr. Kim has served in numerous roles with AGA, among them the co-director of the AGA Clinical Congress, the Partners in Quality program, and the Nurse Practitioner and Physician Assistant Course. He is a Digestive Disease Week® abstract reviewer, has served as AGA representative to the Accreditation Association for Ambulatory Health Care and to the Alliance of Specialty Medicine. He has also served on the AGA Governing Board as clinical private practice councilor and secretary treasurer.
He discussed the high points of his career in an interview, revealing his plans as AGA president for unifying the sectors of GI medicine and fostering GI innovation and technology.
As the new AGA president, what are your goals for the society?
Dr. Kim: I want to put out a message of inclusivity. I think what’s special about AGA is that we’re the society for all gastroenterologists. Among all the other GI organizations, I think we really have the biggest tent and we work to unite clinicians, educators, and researchers – all gastroenterologists, regardless of their individual practice situation. These days, there is a tendency toward tribalism. People are starting to gravitate toward limiting their interactions to others that are from the same backgrounds. But as gastroenterologists we have more that unites us than divides us. It’s only by working together that we can make things better for everyone.
I think the second point is that we’re on the cusp of some important transformations in gastroenterology. The screening colonoscopy model that has sustained our specialty for decades is rapidly evolving. In addition, there is an increasing ability for patients as consumers to direct their own care through advances in technology, such as virtual health platforms. We’re seeing this as patients increasingly adopt things like complementary and alternative medicine outside of the standard model of physician-directed healthcare. These are two important trends that gastroenterologists need to be aware of and learn how to manage and to adapt to. I think AGA’s role is to help guide that evolution and to give physicians the tools to be able to respond.
We want to focus on innovation and we want to focus on practical solutions.
In terms of fostering innovation in gastroenterology, we’re the first medical professional society to create an incubator for new technologies. Not only do we provide that resource to our members, but we’re also putting our money where our mouth is. Through venture capital initiatives such as our GI Opportunity Fund, we directly invest in companies that we’re helping to develop.
On the practice side, we have been engaging directly with payers to foster improved communication and address pain points on both sides. I think we’re the only medical society that’s taking this type of approach and moving away from the traditional adversarial approach to dealing with payers. Recently, we had a very productive discussion with UnitedHealthcare around some of their upcoming formulary changes for inflammatory bowel disease. We used that opportunity to highlight how nonmedical switching between existing therapies can adversely impact patients, as well as increasing burden of red tape for practices.
Your practice was one of the original groups that formed the Digestive Health Physicians Association (DPHA). What accomplishments of the association are you most proud of?
Dr. Kim: DHPA formed about 10 years ago as an advocacy organization to combat a specific perceived threat, which was the in-office ancillary exception. This is the legislative pathway that allows gastroenterologists to provide ancillary services within their practice. An example of this is pathology for endoscopic procedures, which is an incredible value to patients and improves quality of care. This was under a significant legislative threat at that time. As independent physicians, DHPA took the lead in advocating against eliminating that exception.
I think the larger accomplishment was it demonstrated that gastroenterologists, specifically independent community practice gastroenterologists, could come together successfully and advocate for issues that were of importance to our specialty. AGA and DHPA have worked very well together, collaborating on shared policy interests and have worked closely on both legislative as well as regulatory issues. We’ve sponsored joint meetings that we’ve programmed together and we’re looking forward to continuing a robust partnership.
You have introduced several new clinical practice and practice management models. Can you discuss the part-time partnership model and what it has achieved?
Dr. Kim: Like many practices, South Denver Gastroenterology historically required physician partners to work full time. This conflicted with our desire and our need to attract more women gastroenterologists into our practice. The process involved careful analysis of our direct and indirect expenses, but more importantly it required a negotiation and a meeting of the minds among our partners. A lot of this ultimately came down to trust. It helped a great deal that our practice has always had strong cohesiveness. That helped us to build that trust that partners would stay engaged in the practice even if they worked part time.
Our practice has also always prioritized work-life balance. We were able to come up with a formula that allows partners to work three days per week, retaining their partnership interest and their participation in practice decisions. They stay involved but are also financially sustainable for the practice. It’s been very successful. It’s been a big draw, not just for women, but it has allowed us to create a situation where women are fully one third of our partnership. It’s something we’re all extremely proud of.
How did you get involved in AGA?
Dr. Kim: One of the first projects I participated in was the Roadmap to the Future of GI Practice. This was an initiative to help prepare GI practices for value-based care. We did things like develop quality measure sets for GI conditions such as inflammatory bowel disease and hepatitis C. We published a bundled payment model for screening colonoscopies. We also created a model for obesity management by gastroenterologists. This was 15 years ago, and I think it was about 15 years ahead of its time! It’s interesting to see how many of these changes in GI practice that we envisioned are slowly coming to pass.
I saw that AGA was interested in me as a community-based clinician. They focused on trying to develop those practical tools to help me succeed. It’s one of the reasons I’ve stayed engaged.
What is your approach to patient communication and education?
Dr. Kim: There are two things that I always tell both my staff as well as young people who come to me asking for advice. I think the first and most important is that you should always strive to treat your patients the way that you would want your family treated. Of course, we’re not perfect, but when that doesn’t happen, look at your behavior, the way that you’re interacting, but also the way the system is treating your patients and try to improve things within your own practice. And then the other thing that I tell folks is try to spend more time listening to your patients than talking or speaking at them.
What do you think is the biggest misconception about GI?
Dr. Kim: We’re not just about colonoscopies! I went into GI not just because I enjoy performing procedures, but because our specialty covers such a broad spectrum of physiology and diseases. We also have the ability as gastroenterologists to develop long-term relationships with our patients. I’ve been in practice now more than 25 years, and the greatest satisfaction in my career doesn’t come from the endoscopy center, although I still enjoy performing procedures. It comes from the clinic; it comes from the patients whom I’ve known for decades, and the interaction and conversations that I can have with them, the ability to see their families, their parents, and now in some cases their kids or even their grandkids. It’s incredibly satisfying. It makes my job fun.
What advice would you give to aspiring medical students?
Dr. Kim: One of the things I would say is stay involved in organized medicine. As physicians, we are endowed with great trust. We also have a great responsibility to help shape our healthcare care system. If we work together, we really can make a difference, not just for our profession, but also for society at large and for the patients whom we serve.
I really hope that young people don’t lose their optimism. We hear a lot these days about how much negativity and pessimism there is about the future, especially among young people in our society. But I think it’s a great time to be in medicine. Advances in medical science have made huge strides in our ability to make real differences for our patients. And the pace of technology progress is only going to continue to accelerate. Sure, there are lots of shortcomings in the practice of medicine, but honestly, that’s always been the case. I have faith that as a profession, we are smart people, we’re committed people, and we will be successful in overcoming those challenges. That’s the message that I have for young folks.
Lightning Round
Coffee or tea?
Coffee, black
What’s one hobby you’d like to pick up?
Anything except pickleball
What’s your favorite season of the year?
Winter, I’m a skier
What’s your favorite way to spend a weekend?
Doing anything outside
If you could have dinner with any historical figure, who would it be?
Ben Franklin
What’s your go-to karaoke song?
You don’t want to hear me sing
What’s one thing on your bucket list?
Skiing in South America
What’s the best piece of advice you’ve ever received?
Follow your heart

'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
For patients with cancer, the determining factor in whether they pursue mental health services is often whether their oncologist explicitly says it is a good idea, a psychologist said during the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, on treating veterans with renal cell carcinoma (RCC).
Kysa Christie, PhD, of the West Los Angeles Veterans Affairs Medical Center, presented findings from a 2018 study in which researchers asked Swiss patients with cancer whether their oncologist discussed their emotional health with them.
In terms of boosting intake, it did not matter if oncologists acknowledged distress or pointed out that psychosocial services existed. Instead, a direct recommendation made a difference, increasing the likelihood of using the services over a 4-month period after initial assessment (odds ratio, 6.27).
“What it took was, ‘I really recommend this. This is something that I would want you to try,’” Christie said.
Oncologists are crucial links between patients and mental health services, Christie said: “If people don’t ask about [distress], you’re not going to see it, but it’s there. Distress is the norm, right? It is not a weakness. It is something that we expect to see.”
Christie noted that an estimated 20% of cancer patients have major depressive disorder, and 35% to 40% have a diagnosable psychiatric condition. RCC shows disproportionately high rates of mental strain. According to Christie, research suggests that about three-fourths of the population report elevated levels of distress as evidenced by patients who scored ≥ 5 on the NCCN Distress Thermometer. Patients with cancer have an estimated 20% higher risk of suicide, especially during the first 12 months after diagnosis and at end of life, she added.
“Early during a diagnosis phase, where you’re having a lot of tests being done, you know something is happening. But you don’t know what,” Christie said. “It could be very serious. That’s just a lot of stress to hold and not know how to plan for.”
After diagnosis, routine could set in and lower distress, she said. Then terminal illness may spike it back up again. Does mental health treatment work in patients with cancer?
“There’s a really strong body of evidence-based treatments for depression, anxiety, adjustment disorders, and coping with different cancers,” Christie said. But it is a step too far to expect patients to ask for help while they are juggling appointments, tests, infusions, and more. “It’s a big ask, right? It’s setting people up for failure.”
To help, Christie said she is embedded with a medical oncology team and routinely talks with the staff about which patients may need help. “One thing I like to do is try to have brief visits with veterans and introduce myself when they come to clinic. I treat it like an opt-out rather than an opt-in program: I’ll just pop into the exam room. They don’t have to ask to see me.”
Christie focuses on open-ended questions and talks about resources ranging from support groups and brief appointments to extensive individual therapy.
Another approach is a strategy known as the “warm handoff,” when an oncologist directly introduces a patient to a mental health professional. “It’s a transfer of care in front of the veteran: It’s much more time-efficient than putting in a referral.”
Christie explained how this can work. A clinician will ask her to meet with a patient during an appointment, perhaps in a couple minutes.
“Then I pop into the room, and the oncologist says, ‘Thanks for joining us. This is Mr. Jones. He has been experiencing feelings of anxiety and sadness, and we’d appreciate your help in exploring some options that might help.’ I turn to the patient and ask, ‘What more would you add?’ Then I either take Mr. Jones back to my office or stay in clinic, and we’re off to the races.”
Christie reported no disclosures.
For patients with cancer, the determining factor in whether they pursue mental health services is often whether their oncologist explicitly says it is a good idea, a psychologist said during the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, on treating veterans with renal cell carcinoma (RCC).
Kysa Christie, PhD, of the West Los Angeles Veterans Affairs Medical Center, presented findings from a 2018 study in which researchers asked Swiss patients with cancer whether their oncologist discussed their emotional health with them.
In terms of boosting intake, it did not matter if oncologists acknowledged distress or pointed out that psychosocial services existed. Instead, a direct recommendation made a difference, increasing the likelihood of using the services over a 4-month period after initial assessment (odds ratio, 6.27).
“What it took was, ‘I really recommend this. This is something that I would want you to try,’” Christie said.
Oncologists are crucial links between patients and mental health services, Christie said: “If people don’t ask about [distress], you’re not going to see it, but it’s there. Distress is the norm, right? It is not a weakness. It is something that we expect to see.”
Christie noted that an estimated 20% of cancer patients have major depressive disorder, and 35% to 40% have a diagnosable psychiatric condition. RCC shows disproportionately high rates of mental strain. According to Christie, research suggests that about three-fourths of the population report elevated levels of distress as evidenced by patients who scored ≥ 5 on the NCCN Distress Thermometer. Patients with cancer have an estimated 20% higher risk of suicide, especially during the first 12 months after diagnosis and at end of life, she added.
“Early during a diagnosis phase, where you’re having a lot of tests being done, you know something is happening. But you don’t know what,” Christie said. “It could be very serious. That’s just a lot of stress to hold and not know how to plan for.”
After diagnosis, routine could set in and lower distress, she said. Then terminal illness may spike it back up again. Does mental health treatment work in patients with cancer?
“There’s a really strong body of evidence-based treatments for depression, anxiety, adjustment disorders, and coping with different cancers,” Christie said. But it is a step too far to expect patients to ask for help while they are juggling appointments, tests, infusions, and more. “It’s a big ask, right? It’s setting people up for failure.”
To help, Christie said she is embedded with a medical oncology team and routinely talks with the staff about which patients may need help. “One thing I like to do is try to have brief visits with veterans and introduce myself when they come to clinic. I treat it like an opt-out rather than an opt-in program: I’ll just pop into the exam room. They don’t have to ask to see me.”
Christie focuses on open-ended questions and talks about resources ranging from support groups and brief appointments to extensive individual therapy.
Another approach is a strategy known as the “warm handoff,” when an oncologist directly introduces a patient to a mental health professional. “It’s a transfer of care in front of the veteran: It’s much more time-efficient than putting in a referral.”
Christie explained how this can work. A clinician will ask her to meet with a patient during an appointment, perhaps in a couple minutes.
“Then I pop into the room, and the oncologist says, ‘Thanks for joining us. This is Mr. Jones. He has been experiencing feelings of anxiety and sadness, and we’d appreciate your help in exploring some options that might help.’ I turn to the patient and ask, ‘What more would you add?’ Then I either take Mr. Jones back to my office or stay in clinic, and we’re off to the races.”
Christie reported no disclosures.
For patients with cancer, the determining factor in whether they pursue mental health services is often whether their oncologist explicitly says it is a good idea, a psychologist said during the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, on treating veterans with renal cell carcinoma (RCC).
Kysa Christie, PhD, of the West Los Angeles Veterans Affairs Medical Center, presented findings from a 2018 study in which researchers asked Swiss patients with cancer whether their oncologist discussed their emotional health with them.
In terms of boosting intake, it did not matter if oncologists acknowledged distress or pointed out that psychosocial services existed. Instead, a direct recommendation made a difference, increasing the likelihood of using the services over a 4-month period after initial assessment (odds ratio, 6.27).
“What it took was, ‘I really recommend this. This is something that I would want you to try,’” Christie said.
Oncologists are crucial links between patients and mental health services, Christie said: “If people don’t ask about [distress], you’re not going to see it, but it’s there. Distress is the norm, right? It is not a weakness. It is something that we expect to see.”
Christie noted that an estimated 20% of cancer patients have major depressive disorder, and 35% to 40% have a diagnosable psychiatric condition. RCC shows disproportionately high rates of mental strain. According to Christie, research suggests that about three-fourths of the population report elevated levels of distress as evidenced by patients who scored ≥ 5 on the NCCN Distress Thermometer. Patients with cancer have an estimated 20% higher risk of suicide, especially during the first 12 months after diagnosis and at end of life, she added.
“Early during a diagnosis phase, where you’re having a lot of tests being done, you know something is happening. But you don’t know what,” Christie said. “It could be very serious. That’s just a lot of stress to hold and not know how to plan for.”
After diagnosis, routine could set in and lower distress, she said. Then terminal illness may spike it back up again. Does mental health treatment work in patients with cancer?
“There’s a really strong body of evidence-based treatments for depression, anxiety, adjustment disorders, and coping with different cancers,” Christie said. But it is a step too far to expect patients to ask for help while they are juggling appointments, tests, infusions, and more. “It’s a big ask, right? It’s setting people up for failure.”
To help, Christie said she is embedded with a medical oncology team and routinely talks with the staff about which patients may need help. “One thing I like to do is try to have brief visits with veterans and introduce myself when they come to clinic. I treat it like an opt-out rather than an opt-in program: I’ll just pop into the exam room. They don’t have to ask to see me.”
Christie focuses on open-ended questions and talks about resources ranging from support groups and brief appointments to extensive individual therapy.
Another approach is a strategy known as the “warm handoff,” when an oncologist directly introduces a patient to a mental health professional. “It’s a transfer of care in front of the veteran: It’s much more time-efficient than putting in a referral.”
Christie explained how this can work. A clinician will ask her to meet with a patient during an appointment, perhaps in a couple minutes.
“Then I pop into the room, and the oncologist says, ‘Thanks for joining us. This is Mr. Jones. He has been experiencing feelings of anxiety and sadness, and we’d appreciate your help in exploring some options that might help.’ I turn to the patient and ask, ‘What more would you add?’ Then I either take Mr. Jones back to my office or stay in clinic, and we’re off to the races.”
Christie reported no disclosures.
'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
Michigan GI Designs a Simple Tool For a Common Problem
Patients sometimes drive hundreds of miles to see their GI physicians for problems that never seem to resolve. Constipation is one of those ailments that can affect quality of life.
The advice is, “Try this diet or laxative. Get a colonoscopy. Often, that’s not getting at the root problem,” said Eric Dinesh Shah, MD, MBA, a gastroenterologist at the University of Michigan, Ann Arbor.
Such methods aren’t equipped to test the pelvic floor, said Dr. Shah, who worked with clinical experts to develop a simple point-of-care device called RED (rectal expulsion device) that makes it easier to diagnose and predict treatment options for constipation.
The device uses a foam-filled balloon to evaluate pelvic floor problems related to constipation, after a digital rectal exam during an office visit. Because the procedure can be performed during a patient’s initial office visit, it can eliminate the need for referrals to far-away specialists for many patients.
In 2019, Dr. Shah received the AGA-Shire Research Scholar Award in Functional GI and Motility Disorders from the AGA Research Foundation for developing RED, and the device was recently cleared by the Food and Drug Administration.
GI doctors don’t always have the answers, he acknowledged in an interview, but this creates the opportunity for new advancements such as RED. utilizing local and regional workshops as well as national conferences to meet like-minded people at similar career stages, and to look for funding opportunities to explore those ideas.
What is the most challenging case you’ve encountered?
Dr. Shah: The most challenging cases to me have been the ones where I wish we could have helped people years ago. It’s not that anyone did anything wrong or was poorly intentioned. It’s quite the opposite: There sometimes is no real avenue to offer testing locally with current technology, even though the local clinical teams completely understand what should be done in a perfect world. That creates challenges where patients go hours out of their way to see specialists, just to find an answer that might have been 1 mile down the road all along.
What has been your solution to help these patients?
Dr. Shah: My work has been about helping patients who drive a hundred miles or routinely go hours out of their way for their care. Usually that’s a sign that things just aren’t working locally. Patients have lost trust in their ability to get care with the teams they have. Or the teams themselves just need help. I think a major part of the job is to reinforce the bond between the patient and their local team by giving them the tools and expertise so that the patients can get that care locally.
There’s been this trend toward this ‘hub and spoke’ model in care where all the patients are filtering into these large hospital-owned mega practices. I wonder about the sustainability of that model because it takes away the ability of patients to see doctors who are invested in their local community. What we need to be doing is trying to flip that.
I’d love to discuss the RED device and how was this device conceived?
Dr. Shah: I partnered with experts, including William Chey, MD, AGAF, at the University of Michigan, who dedicate their entire careers toward creating robust science in large academic medical centers. In understanding the best ways to care for patients today, I could focus my own career on how to translate that level of care for the patients of tomorrow. I would encourage GI trainees to find senior and peer mentors who share perspective on this approach as an anchor to shared success.
For the RED device, the problem in constipation is that patients see their gastroenterologist over and over and over. It’s ‘try this diet, try this laxative, try this drug, try this other treatment,’ and we’re not getting at the root problem. Patients might go through a series of colonoscopies to reassure them but also to reassure their doctor that they’re not missing something. What we haven’t had is a way to test and evaluate the pelvic floor locally because those technologies are high tech and live in these big academic medical centers.
What are plans for its distribution and use in the consumer space?
Dr. Shah: The device is now available in the United States (https://www.red4constipation.com).
As an AGA Research Scholar Award winner, how might AGA play a role in supporting GI doctors?
Dr. Shah: The AGA Research Scholar Award enabled me to learn how RED predicted outcomes for patients seeing general gastroenterologists who then see pelvic floor physical therapy in the community to treat constipation. The availability of pelvic floor physical therapy and the field at large, has exploded in recent years across the country (https://www.pelvicrehab.com), making it easier for patients to get the local care they need.
In looking at what this award did for my own career and those of others in my cohort, I think the AGA Research Scholar Award mechanism serves as an example of what other GI trainees can do across the many areas of GI that are ripe for transformation.
What other AGA workshops are useful to GI doctors?
Dr. Shah: The AGA Tech Summit and Innovation Fellows programs give access to a positive learning environment to network with people across career stages who are seeking to advance the field in this way. These programs are particularly successful because they focus on helping GI trainees find peer success and professional satisfaction in the shared journey, rather than focusing on the accolades. I would strongly encourage GI trainees who have an interest but don’t know where to start to apply for these programs.
What do you think is the biggest misconception about your specialty?
Dr. Shah: That gastroenterologists have all the answers with current technology. There’s a lot we still don’t know. What gives me reassurance is the momentum around new ways of thinking that GI trainees and early-stage gastroenterologists continually bring forward to improve how we care for patients.
Lightning Round
Do you prefer coffee or tea?
Coffee
Are you an early bird or night owl?
Early bird
What’s your go-to comfort food?
Tex Mex
If you could travel anywhere, where would you go?
Antarctica
What’s your favorite TV show?
Below Deck
What’s one hobby you’d like to pick up?
Painting
What’s your favorite way to spend a weekend?
A lazy weekend
If you could have dinner with any historical figure, who would it be?
Winston Churchill
What’s your go-to karaoke song?
Our endoscopy nurses give no choice other than Taylor Swift, Green Day, and the Backstreet Boys
Patients sometimes drive hundreds of miles to see their GI physicians for problems that never seem to resolve. Constipation is one of those ailments that can affect quality of life.
The advice is, “Try this diet or laxative. Get a colonoscopy. Often, that’s not getting at the root problem,” said Eric Dinesh Shah, MD, MBA, a gastroenterologist at the University of Michigan, Ann Arbor.
Such methods aren’t equipped to test the pelvic floor, said Dr. Shah, who worked with clinical experts to develop a simple point-of-care device called RED (rectal expulsion device) that makes it easier to diagnose and predict treatment options for constipation.
The device uses a foam-filled balloon to evaluate pelvic floor problems related to constipation, after a digital rectal exam during an office visit. Because the procedure can be performed during a patient’s initial office visit, it can eliminate the need for referrals to far-away specialists for many patients.
In 2019, Dr. Shah received the AGA-Shire Research Scholar Award in Functional GI and Motility Disorders from the AGA Research Foundation for developing RED, and the device was recently cleared by the Food and Drug Administration.
GI doctors don’t always have the answers, he acknowledged in an interview, but this creates the opportunity for new advancements such as RED. utilizing local and regional workshops as well as national conferences to meet like-minded people at similar career stages, and to look for funding opportunities to explore those ideas.
What is the most challenging case you’ve encountered?
Dr. Shah: The most challenging cases to me have been the ones where I wish we could have helped people years ago. It’s not that anyone did anything wrong or was poorly intentioned. It’s quite the opposite: There sometimes is no real avenue to offer testing locally with current technology, even though the local clinical teams completely understand what should be done in a perfect world. That creates challenges where patients go hours out of their way to see specialists, just to find an answer that might have been 1 mile down the road all along.
What has been your solution to help these patients?
Dr. Shah: My work has been about helping patients who drive a hundred miles or routinely go hours out of their way for their care. Usually that’s a sign that things just aren’t working locally. Patients have lost trust in their ability to get care with the teams they have. Or the teams themselves just need help. I think a major part of the job is to reinforce the bond between the patient and their local team by giving them the tools and expertise so that the patients can get that care locally.
There’s been this trend toward this ‘hub and spoke’ model in care where all the patients are filtering into these large hospital-owned mega practices. I wonder about the sustainability of that model because it takes away the ability of patients to see doctors who are invested in their local community. What we need to be doing is trying to flip that.
I’d love to discuss the RED device and how was this device conceived?
Dr. Shah: I partnered with experts, including William Chey, MD, AGAF, at the University of Michigan, who dedicate their entire careers toward creating robust science in large academic medical centers. In understanding the best ways to care for patients today, I could focus my own career on how to translate that level of care for the patients of tomorrow. I would encourage GI trainees to find senior and peer mentors who share perspective on this approach as an anchor to shared success.
For the RED device, the problem in constipation is that patients see their gastroenterologist over and over and over. It’s ‘try this diet, try this laxative, try this drug, try this other treatment,’ and we’re not getting at the root problem. Patients might go through a series of colonoscopies to reassure them but also to reassure their doctor that they’re not missing something. What we haven’t had is a way to test and evaluate the pelvic floor locally because those technologies are high tech and live in these big academic medical centers.
What are plans for its distribution and use in the consumer space?
Dr. Shah: The device is now available in the United States (https://www.red4constipation.com).
As an AGA Research Scholar Award winner, how might AGA play a role in supporting GI doctors?
Dr. Shah: The AGA Research Scholar Award enabled me to learn how RED predicted outcomes for patients seeing general gastroenterologists who then see pelvic floor physical therapy in the community to treat constipation. The availability of pelvic floor physical therapy and the field at large, has exploded in recent years across the country (https://www.pelvicrehab.com), making it easier for patients to get the local care they need.
In looking at what this award did for my own career and those of others in my cohort, I think the AGA Research Scholar Award mechanism serves as an example of what other GI trainees can do across the many areas of GI that are ripe for transformation.
What other AGA workshops are useful to GI doctors?
Dr. Shah: The AGA Tech Summit and Innovation Fellows programs give access to a positive learning environment to network with people across career stages who are seeking to advance the field in this way. These programs are particularly successful because they focus on helping GI trainees find peer success and professional satisfaction in the shared journey, rather than focusing on the accolades. I would strongly encourage GI trainees who have an interest but don’t know where to start to apply for these programs.
What do you think is the biggest misconception about your specialty?
Dr. Shah: That gastroenterologists have all the answers with current technology. There’s a lot we still don’t know. What gives me reassurance is the momentum around new ways of thinking that GI trainees and early-stage gastroenterologists continually bring forward to improve how we care for patients.
Lightning Round
Do you prefer coffee or tea?
Coffee
Are you an early bird or night owl?
Early bird
What’s your go-to comfort food?
Tex Mex
If you could travel anywhere, where would you go?
Antarctica
What’s your favorite TV show?
Below Deck
What’s one hobby you’d like to pick up?
Painting
What’s your favorite way to spend a weekend?
A lazy weekend
If you could have dinner with any historical figure, who would it be?
Winston Churchill
What’s your go-to karaoke song?
Our endoscopy nurses give no choice other than Taylor Swift, Green Day, and the Backstreet Boys
Patients sometimes drive hundreds of miles to see their GI physicians for problems that never seem to resolve. Constipation is one of those ailments that can affect quality of life.
The advice is, “Try this diet or laxative. Get a colonoscopy. Often, that’s not getting at the root problem,” said Eric Dinesh Shah, MD, MBA, a gastroenterologist at the University of Michigan, Ann Arbor.
Such methods aren’t equipped to test the pelvic floor, said Dr. Shah, who worked with clinical experts to develop a simple point-of-care device called RED (rectal expulsion device) that makes it easier to diagnose and predict treatment options for constipation.
The device uses a foam-filled balloon to evaluate pelvic floor problems related to constipation, after a digital rectal exam during an office visit. Because the procedure can be performed during a patient’s initial office visit, it can eliminate the need for referrals to far-away specialists for many patients.
In 2019, Dr. Shah received the AGA-Shire Research Scholar Award in Functional GI and Motility Disorders from the AGA Research Foundation for developing RED, and the device was recently cleared by the Food and Drug Administration.
GI doctors don’t always have the answers, he acknowledged in an interview, but this creates the opportunity for new advancements such as RED. utilizing local and regional workshops as well as national conferences to meet like-minded people at similar career stages, and to look for funding opportunities to explore those ideas.
What is the most challenging case you’ve encountered?
Dr. Shah: The most challenging cases to me have been the ones where I wish we could have helped people years ago. It’s not that anyone did anything wrong or was poorly intentioned. It’s quite the opposite: There sometimes is no real avenue to offer testing locally with current technology, even though the local clinical teams completely understand what should be done in a perfect world. That creates challenges where patients go hours out of their way to see specialists, just to find an answer that might have been 1 mile down the road all along.
What has been your solution to help these patients?
Dr. Shah: My work has been about helping patients who drive a hundred miles or routinely go hours out of their way for their care. Usually that’s a sign that things just aren’t working locally. Patients have lost trust in their ability to get care with the teams they have. Or the teams themselves just need help. I think a major part of the job is to reinforce the bond between the patient and their local team by giving them the tools and expertise so that the patients can get that care locally.
There’s been this trend toward this ‘hub and spoke’ model in care where all the patients are filtering into these large hospital-owned mega practices. I wonder about the sustainability of that model because it takes away the ability of patients to see doctors who are invested in their local community. What we need to be doing is trying to flip that.
I’d love to discuss the RED device and how was this device conceived?
Dr. Shah: I partnered with experts, including William Chey, MD, AGAF, at the University of Michigan, who dedicate their entire careers toward creating robust science in large academic medical centers. In understanding the best ways to care for patients today, I could focus my own career on how to translate that level of care for the patients of tomorrow. I would encourage GI trainees to find senior and peer mentors who share perspective on this approach as an anchor to shared success.
For the RED device, the problem in constipation is that patients see their gastroenterologist over and over and over. It’s ‘try this diet, try this laxative, try this drug, try this other treatment,’ and we’re not getting at the root problem. Patients might go through a series of colonoscopies to reassure them but also to reassure their doctor that they’re not missing something. What we haven’t had is a way to test and evaluate the pelvic floor locally because those technologies are high tech and live in these big academic medical centers.
What are plans for its distribution and use in the consumer space?
Dr. Shah: The device is now available in the United States (https://www.red4constipation.com).
As an AGA Research Scholar Award winner, how might AGA play a role in supporting GI doctors?
Dr. Shah: The AGA Research Scholar Award enabled me to learn how RED predicted outcomes for patients seeing general gastroenterologists who then see pelvic floor physical therapy in the community to treat constipation. The availability of pelvic floor physical therapy and the field at large, has exploded in recent years across the country (https://www.pelvicrehab.com), making it easier for patients to get the local care they need.
In looking at what this award did for my own career and those of others in my cohort, I think the AGA Research Scholar Award mechanism serves as an example of what other GI trainees can do across the many areas of GI that are ripe for transformation.
What other AGA workshops are useful to GI doctors?
Dr. Shah: The AGA Tech Summit and Innovation Fellows programs give access to a positive learning environment to network with people across career stages who are seeking to advance the field in this way. These programs are particularly successful because they focus on helping GI trainees find peer success and professional satisfaction in the shared journey, rather than focusing on the accolades. I would strongly encourage GI trainees who have an interest but don’t know where to start to apply for these programs.
What do you think is the biggest misconception about your specialty?
Dr. Shah: That gastroenterologists have all the answers with current technology. There’s a lot we still don’t know. What gives me reassurance is the momentum around new ways of thinking that GI trainees and early-stage gastroenterologists continually bring forward to improve how we care for patients.
Lightning Round
Do you prefer coffee or tea?
Coffee
Are you an early bird or night owl?
Early bird
What’s your go-to comfort food?
Tex Mex
If you could travel anywhere, where would you go?
Antarctica
What’s your favorite TV show?
Below Deck
What’s one hobby you’d like to pick up?
Painting
What’s your favorite way to spend a weekend?
A lazy weekend
If you could have dinner with any historical figure, who would it be?
Winston Churchill
What’s your go-to karaoke song?
Our endoscopy nurses give no choice other than Taylor Swift, Green Day, and the Backstreet Boys

Rurality and Age May Shape Phone-Only Mental Health Care Access Among Veterans
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Ergonomic ‘Timeouts’ Make Endoscopy Easier For GIs
Amandeep Shergill, MD, MS, AGAF, always thought she had good hand-eye coordination until she entered her gastroenterology fellowship.
“You’re learning how to scope and the endoscope just feels so awkward in the hands. It can be such a difficult instrument to both learn and to use,” said Dr. Shergill, professor of clinical medicine at University of California, San Francisco.
Her attendings and mentors couldn’t give her the feedback she needed.
“I was told that I wasn’t holding it right. But every time I tried to do something that someone was trying to tell me, it seemed like my hands were too small. I couldn’t hold it the way that they were teaching me to hold it.” She began to wonder: Was this about her or the tool itself?
A deep dive into hand tool interactions and medical device designs led her to human factors and ergonomics. Her fellowship mentor, Ken McQuaid, MD, AGAF, had gone to medical school with David Rempel, MD, MPH who was one of the top-funded ergonomists in the country. “He emailed David and wrote: I have a fellow who’s interested in learning more about ergonomics and applying it to endoscopy,” said Dr. Shergill.
Through her work with Dr. Rempel, she was able to uncover the mechanisms that lead to musculoskeletal disorders in endoscopists.
Over time, she has become a trailblazer in this field, helming the UC Berkeley Center for Ergonomic Endoscopy with Carisa Harris-Adamson PhD, CPE, her ergonomics collaborator. In an interview, she described the unique “timeout” algorithm she created to ease the process of endoscopy for GI physicians.
What is your favorite aspect of being a GI physician?
I really love the diversity of patients and cases. You’re always learning something new. It’s an internal medicine subspecialty and a cognitive field, so we must think about differential diagnoses, risks and benefits of procedures for patients. But as a procedural field, we get to diagnose and immediately treat certain disorders. What’s exciting about GI right now is there’s still so much to learn. I think that we’re still discovering more about how the brain-gut interaction works every day. There’s been additional research about the microbiome and the immense influence it has on both health and disease. The field is continuing to evolve rapidly. There’s always something new to learn, and I think it keeps us fresh.
Tell me about your work in ergonomics and endoscopy.
Ken McQuaid connected me with David Rempel. I worked with David to approach this problem of endoscopy ergonomics from a very rigorous ergonomics perspective. Early in my fellowship, endoscopy ergonomics wasn’t well known. There were few survey-based studies, including one from the American Society for Gastrointestinal Endoscopy (ASGE) that documented a high prevalence of endoscopist injury. But not a lot was known about what was causing injury in endoscopists.
What were the risk factors for endoscopist injury? Instead of just doing another survey, I wanted to show that there was this potential for causation given the design of the endoscopes. I worked with David to do a pilot study where we collected some pinch forces and forearm muscle loads. I was able to collect some pilot data that I used to apply for the ASGE Endoscopic Research Award. And luckily, ASGE supported that work.
Another award I received, the ASGE Career Development Award, was instrumental in allowing me to become more proficient in the science of ergonomics. I was able to leverage that career development award to go back to school. I went to UC Berkeley and got a master’s in environmental health sciences with a focus on ergonomics. It really helped me to lay the foundation and understanding for ergonomics and then apply that to endoscopy to generate a more rigorous scientific background for endoscopy ergonomics and start that conversation within the field of GI.
What leads to musculoskeletal disorders in endoscopists and how can it be prevented?
Musculoskeletal disorders are associated with the repetitive procedures that we’re performing, often utilizing high forces and in non-neutral postures. This is because of how we’re interacting with our tools and how we’re interacting with our environments. The studies I have done with Carisa Harris-Adamson have been able to demonstrate and document the high forces that are required to interact with the endoscope. To turn the control section dials and to torque and manipulate the insertion tube, there are really high distal upper extremity muscle loads that are being applied.
We were able to compare the loads and the forces we were seeing to established risk thresholds from the ergonomics literature and demonstrate that performing endoscopy was associated with moderate to high risk of development of distal upper extremity disorders.
What research are you doing now?
We’re trying to focus more on interventions. We’ve done some studies on engineering controls we can utilize to decrease the loads of holding the scope. First, it was an anti-gravity support arm. More recently we’re hoping to publish data on whether a scope stand can alleviate some of those left distal upper extremity loads because the stand is holding the scope instead of the hand holding the scope. Can we decrease injury risk by decreasing static loading?
Neck and back injuries, which have a high prevalence in endoscopists, are usually associated with how the room is set up. One of the things that I’ve tried to help promote is a pre-procedure ergonomic “timeout.” Before an endoscopist does a procedure, we’re supposed to perform a timeout focused on the patient’s safety. We should also try to advocate for physician safety and an ergonomic timeout. I developed a mnemonic device utilizing the word “MYSELF” to help endoscopists remember the ergonomic timeout checklist: M = monitor, Y = upside-down Y stance, S = scope, E = elbow/ bed position, L = lower extremities, F = free movement of endoscope/ processor placement.
First, thinking about the monitor, “M”, and fixing the monitor height so that the neck is in neutral position. Then, thinking of an upside down “Y” standing straight with the feet either hip width or shoulder width apart, so that the physician has a stable, neutral standing posture. Then “S” is for checking the scope to ensure you have a scope with optimal angulation that’s working properly.
“E” is for elbows — adjusting the bed to an optimal position so that elbows and shoulders are in neutral position. “L” is for lower extremities — are the foot pedals within an easy reach? Do you have comfortable shoes on, an anti-fatigue floor mat if you need it? And then the “F” in “MYSELF” is for the processor placement, to ensure “free movement” of the scope. By placing the processor directly behind you and lining up the processor with the orifice to be scoped, you can ensure free movement of the scope so that you can leverage large movements of the control section to result in tip deflection.
We studied the MYSELF mnemonic device for a pre-procedure ergonomic timeout in a simulated setting and presented our results at Digestive Disease Week (DDW) 2024, where we showed a reduction in ergonomic risk scores based on the Rapid Entire Body Assessment tool.
We presented the results of the scope stand study at DDW 2025 in San Diego this May.
What has been the feedback from physicians who use these supportive tools?
While physicians are very grateful for bringing attention to this issue, and many have found utility in some of the tools that I proposed, I think we still have so much work to do. We’re just all hoping to continue to move this field forward for better tools that are designed more with the breadth of endoscopists in mind.
How do you handle stress and maintain work-life balance?
A few years ago, during DDW I gave a talk entitled “Achieving Work-Life Harmony.” I disclosed at the beginning of the talk that I had not achieved work-life harmony. It’s definitely a difficult thing to do, especially in our field as GI proceduralists, where we’re frequently on call and there are potentially on-call emergencies.
One of the key things that I’ve tried to do is create boundaries to prioritize both things in my personal life and my professional life and really try to stay true to the things that are important to me. For instance, things like family time and mealtimes, I think that’s so critical. Trying to be home on evenings for dinnertime is so important.
One of my GI colleagues, Raj Keswani, MD, MS gave a talk about burnout and described imagining life as juggling balls; trying to figure out which balls are glass balls and need to be handled with care, and which balls are rubber balls.
More often, work is the rubber ball. If you drop it, it’ll bounce back and the work that you have will still be there the next day. Family, friends, our health, those are the glass balls that if they fall, they can get scuffed or shatter sometimes. That image helps me think in the moment. If I need to decide between two competing priorities, which one will still be here tomorrow? Which is the one that’s going to be more resilient, and which is the one that I need to focus on? That’s been a helpful image for me.
I also want to give a shout out to my amazing colleagues. We all pitch in with the ‘juggling’ and help to keep everyone’s ‘balls’ in the air, and cover for each other. Whether it’s a sick patient or whatever’s going on in our personal lives, we always take care of each other.
What advice would you give to aspiring GI fellows or graduating fellows?
GI is such an amazing field and many people end up focusing on the procedural aspect of it. What I think defines an exceptional gastroenterologist and physician in general is adopting both a “growth mindset” and a “mastery mindset.” And really, it starts out with when you’re exploring an area of focus, listening to what consistently draws your attention, what you’re excited about learning more about.
Finding mentors, getting involved in projects, doing deep learning, and really trying to develop an expertise in that area through additional training, coursework, and education. I think that idea of a mastery mindset will really help set you up for becoming deeply knowledgeable about a field.
Lightning Round
Coffee or tea?
Coffee
What’s your favorite book?
Project Hail Mary (audiobook)
Beach vacation or mountain retreat?
Mountain retreat
Early bird or night owl?
Night owl
What’s your go-to comfort food?
Chaat (Indian street food)
Do you prefer dogs or cats?
Dogs
What’s one hobby you’d like to pick up?
Sewing
If you could have dinner with any historical figure, who would it be?
Ruth Bader Ginsburg
What’s your go-to karaoke song?
I Wanna Dance with Somebody
What’s one thing on your bucket list?
To see the Northern Lights
Amandeep Shergill, MD, MS, AGAF, always thought she had good hand-eye coordination until she entered her gastroenterology fellowship.
“You’re learning how to scope and the endoscope just feels so awkward in the hands. It can be such a difficult instrument to both learn and to use,” said Dr. Shergill, professor of clinical medicine at University of California, San Francisco.
Her attendings and mentors couldn’t give her the feedback she needed.
“I was told that I wasn’t holding it right. But every time I tried to do something that someone was trying to tell me, it seemed like my hands were too small. I couldn’t hold it the way that they were teaching me to hold it.” She began to wonder: Was this about her or the tool itself?
A deep dive into hand tool interactions and medical device designs led her to human factors and ergonomics. Her fellowship mentor, Ken McQuaid, MD, AGAF, had gone to medical school with David Rempel, MD, MPH who was one of the top-funded ergonomists in the country. “He emailed David and wrote: I have a fellow who’s interested in learning more about ergonomics and applying it to endoscopy,” said Dr. Shergill.
Through her work with Dr. Rempel, she was able to uncover the mechanisms that lead to musculoskeletal disorders in endoscopists.
Over time, she has become a trailblazer in this field, helming the UC Berkeley Center for Ergonomic Endoscopy with Carisa Harris-Adamson PhD, CPE, her ergonomics collaborator. In an interview, she described the unique “timeout” algorithm she created to ease the process of endoscopy for GI physicians.
What is your favorite aspect of being a GI physician?
I really love the diversity of patients and cases. You’re always learning something new. It’s an internal medicine subspecialty and a cognitive field, so we must think about differential diagnoses, risks and benefits of procedures for patients. But as a procedural field, we get to diagnose and immediately treat certain disorders. What’s exciting about GI right now is there’s still so much to learn. I think that we’re still discovering more about how the brain-gut interaction works every day. There’s been additional research about the microbiome and the immense influence it has on both health and disease. The field is continuing to evolve rapidly. There’s always something new to learn, and I think it keeps us fresh.
Tell me about your work in ergonomics and endoscopy.
Ken McQuaid connected me with David Rempel. I worked with David to approach this problem of endoscopy ergonomics from a very rigorous ergonomics perspective. Early in my fellowship, endoscopy ergonomics wasn’t well known. There were few survey-based studies, including one from the American Society for Gastrointestinal Endoscopy (ASGE) that documented a high prevalence of endoscopist injury. But not a lot was known about what was causing injury in endoscopists.
What were the risk factors for endoscopist injury? Instead of just doing another survey, I wanted to show that there was this potential for causation given the design of the endoscopes. I worked with David to do a pilot study where we collected some pinch forces and forearm muscle loads. I was able to collect some pilot data that I used to apply for the ASGE Endoscopic Research Award. And luckily, ASGE supported that work.
Another award I received, the ASGE Career Development Award, was instrumental in allowing me to become more proficient in the science of ergonomics. I was able to leverage that career development award to go back to school. I went to UC Berkeley and got a master’s in environmental health sciences with a focus on ergonomics. It really helped me to lay the foundation and understanding for ergonomics and then apply that to endoscopy to generate a more rigorous scientific background for endoscopy ergonomics and start that conversation within the field of GI.
What leads to musculoskeletal disorders in endoscopists and how can it be prevented?
Musculoskeletal disorders are associated with the repetitive procedures that we’re performing, often utilizing high forces and in non-neutral postures. This is because of how we’re interacting with our tools and how we’re interacting with our environments. The studies I have done with Carisa Harris-Adamson have been able to demonstrate and document the high forces that are required to interact with the endoscope. To turn the control section dials and to torque and manipulate the insertion tube, there are really high distal upper extremity muscle loads that are being applied.
We were able to compare the loads and the forces we were seeing to established risk thresholds from the ergonomics literature and demonstrate that performing endoscopy was associated with moderate to high risk of development of distal upper extremity disorders.
What research are you doing now?
We’re trying to focus more on interventions. We’ve done some studies on engineering controls we can utilize to decrease the loads of holding the scope. First, it was an anti-gravity support arm. More recently we’re hoping to publish data on whether a scope stand can alleviate some of those left distal upper extremity loads because the stand is holding the scope instead of the hand holding the scope. Can we decrease injury risk by decreasing static loading?
Neck and back injuries, which have a high prevalence in endoscopists, are usually associated with how the room is set up. One of the things that I’ve tried to help promote is a pre-procedure ergonomic “timeout.” Before an endoscopist does a procedure, we’re supposed to perform a timeout focused on the patient’s safety. We should also try to advocate for physician safety and an ergonomic timeout. I developed a mnemonic device utilizing the word “MYSELF” to help endoscopists remember the ergonomic timeout checklist: M = monitor, Y = upside-down Y stance, S = scope, E = elbow/ bed position, L = lower extremities, F = free movement of endoscope/ processor placement.
First, thinking about the monitor, “M”, and fixing the monitor height so that the neck is in neutral position. Then, thinking of an upside down “Y” standing straight with the feet either hip width or shoulder width apart, so that the physician has a stable, neutral standing posture. Then “S” is for checking the scope to ensure you have a scope with optimal angulation that’s working properly.
“E” is for elbows — adjusting the bed to an optimal position so that elbows and shoulders are in neutral position. “L” is for lower extremities — are the foot pedals within an easy reach? Do you have comfortable shoes on, an anti-fatigue floor mat if you need it? And then the “F” in “MYSELF” is for the processor placement, to ensure “free movement” of the scope. By placing the processor directly behind you and lining up the processor with the orifice to be scoped, you can ensure free movement of the scope so that you can leverage large movements of the control section to result in tip deflection.
We studied the MYSELF mnemonic device for a pre-procedure ergonomic timeout in a simulated setting and presented our results at Digestive Disease Week (DDW) 2024, where we showed a reduction in ergonomic risk scores based on the Rapid Entire Body Assessment tool.
We presented the results of the scope stand study at DDW 2025 in San Diego this May.
What has been the feedback from physicians who use these supportive tools?
While physicians are very grateful for bringing attention to this issue, and many have found utility in some of the tools that I proposed, I think we still have so much work to do. We’re just all hoping to continue to move this field forward for better tools that are designed more with the breadth of endoscopists in mind.
How do you handle stress and maintain work-life balance?
A few years ago, during DDW I gave a talk entitled “Achieving Work-Life Harmony.” I disclosed at the beginning of the talk that I had not achieved work-life harmony. It’s definitely a difficult thing to do, especially in our field as GI proceduralists, where we’re frequently on call and there are potentially on-call emergencies.
One of the key things that I’ve tried to do is create boundaries to prioritize both things in my personal life and my professional life and really try to stay true to the things that are important to me. For instance, things like family time and mealtimes, I think that’s so critical. Trying to be home on evenings for dinnertime is so important.
One of my GI colleagues, Raj Keswani, MD, MS gave a talk about burnout and described imagining life as juggling balls; trying to figure out which balls are glass balls and need to be handled with care, and which balls are rubber balls.
More often, work is the rubber ball. If you drop it, it’ll bounce back and the work that you have will still be there the next day. Family, friends, our health, those are the glass balls that if they fall, they can get scuffed or shatter sometimes. That image helps me think in the moment. If I need to decide between two competing priorities, which one will still be here tomorrow? Which is the one that’s going to be more resilient, and which is the one that I need to focus on? That’s been a helpful image for me.
I also want to give a shout out to my amazing colleagues. We all pitch in with the ‘juggling’ and help to keep everyone’s ‘balls’ in the air, and cover for each other. Whether it’s a sick patient or whatever’s going on in our personal lives, we always take care of each other.
What advice would you give to aspiring GI fellows or graduating fellows?
GI is such an amazing field and many people end up focusing on the procedural aspect of it. What I think defines an exceptional gastroenterologist and physician in general is adopting both a “growth mindset” and a “mastery mindset.” And really, it starts out with when you’re exploring an area of focus, listening to what consistently draws your attention, what you’re excited about learning more about.
Finding mentors, getting involved in projects, doing deep learning, and really trying to develop an expertise in that area through additional training, coursework, and education. I think that idea of a mastery mindset will really help set you up for becoming deeply knowledgeable about a field.
Lightning Round
Coffee or tea?
Coffee
What’s your favorite book?
Project Hail Mary (audiobook)
Beach vacation or mountain retreat?
Mountain retreat
Early bird or night owl?
Night owl
What’s your go-to comfort food?
Chaat (Indian street food)
Do you prefer dogs or cats?
Dogs
What’s one hobby you’d like to pick up?
Sewing
If you could have dinner with any historical figure, who would it be?
Ruth Bader Ginsburg
What’s your go-to karaoke song?
I Wanna Dance with Somebody
What’s one thing on your bucket list?
To see the Northern Lights
Amandeep Shergill, MD, MS, AGAF, always thought she had good hand-eye coordination until she entered her gastroenterology fellowship.
“You’re learning how to scope and the endoscope just feels so awkward in the hands. It can be such a difficult instrument to both learn and to use,” said Dr. Shergill, professor of clinical medicine at University of California, San Francisco.
Her attendings and mentors couldn’t give her the feedback she needed.
“I was told that I wasn’t holding it right. But every time I tried to do something that someone was trying to tell me, it seemed like my hands were too small. I couldn’t hold it the way that they were teaching me to hold it.” She began to wonder: Was this about her or the tool itself?
A deep dive into hand tool interactions and medical device designs led her to human factors and ergonomics. Her fellowship mentor, Ken McQuaid, MD, AGAF, had gone to medical school with David Rempel, MD, MPH who was one of the top-funded ergonomists in the country. “He emailed David and wrote: I have a fellow who’s interested in learning more about ergonomics and applying it to endoscopy,” said Dr. Shergill.
Through her work with Dr. Rempel, she was able to uncover the mechanisms that lead to musculoskeletal disorders in endoscopists.
Over time, she has become a trailblazer in this field, helming the UC Berkeley Center for Ergonomic Endoscopy with Carisa Harris-Adamson PhD, CPE, her ergonomics collaborator. In an interview, she described the unique “timeout” algorithm she created to ease the process of endoscopy for GI physicians.
What is your favorite aspect of being a GI physician?
I really love the diversity of patients and cases. You’re always learning something new. It’s an internal medicine subspecialty and a cognitive field, so we must think about differential diagnoses, risks and benefits of procedures for patients. But as a procedural field, we get to diagnose and immediately treat certain disorders. What’s exciting about GI right now is there’s still so much to learn. I think that we’re still discovering more about how the brain-gut interaction works every day. There’s been additional research about the microbiome and the immense influence it has on both health and disease. The field is continuing to evolve rapidly. There’s always something new to learn, and I think it keeps us fresh.
Tell me about your work in ergonomics and endoscopy.
Ken McQuaid connected me with David Rempel. I worked with David to approach this problem of endoscopy ergonomics from a very rigorous ergonomics perspective. Early in my fellowship, endoscopy ergonomics wasn’t well known. There were few survey-based studies, including one from the American Society for Gastrointestinal Endoscopy (ASGE) that documented a high prevalence of endoscopist injury. But not a lot was known about what was causing injury in endoscopists.
What were the risk factors for endoscopist injury? Instead of just doing another survey, I wanted to show that there was this potential for causation given the design of the endoscopes. I worked with David to do a pilot study where we collected some pinch forces and forearm muscle loads. I was able to collect some pilot data that I used to apply for the ASGE Endoscopic Research Award. And luckily, ASGE supported that work.
Another award I received, the ASGE Career Development Award, was instrumental in allowing me to become more proficient in the science of ergonomics. I was able to leverage that career development award to go back to school. I went to UC Berkeley and got a master’s in environmental health sciences with a focus on ergonomics. It really helped me to lay the foundation and understanding for ergonomics and then apply that to endoscopy to generate a more rigorous scientific background for endoscopy ergonomics and start that conversation within the field of GI.
What leads to musculoskeletal disorders in endoscopists and how can it be prevented?
Musculoskeletal disorders are associated with the repetitive procedures that we’re performing, often utilizing high forces and in non-neutral postures. This is because of how we’re interacting with our tools and how we’re interacting with our environments. The studies I have done with Carisa Harris-Adamson have been able to demonstrate and document the high forces that are required to interact with the endoscope. To turn the control section dials and to torque and manipulate the insertion tube, there are really high distal upper extremity muscle loads that are being applied.
We were able to compare the loads and the forces we were seeing to established risk thresholds from the ergonomics literature and demonstrate that performing endoscopy was associated with moderate to high risk of development of distal upper extremity disorders.
What research are you doing now?
We’re trying to focus more on interventions. We’ve done some studies on engineering controls we can utilize to decrease the loads of holding the scope. First, it was an anti-gravity support arm. More recently we’re hoping to publish data on whether a scope stand can alleviate some of those left distal upper extremity loads because the stand is holding the scope instead of the hand holding the scope. Can we decrease injury risk by decreasing static loading?
Neck and back injuries, which have a high prevalence in endoscopists, are usually associated with how the room is set up. One of the things that I’ve tried to help promote is a pre-procedure ergonomic “timeout.” Before an endoscopist does a procedure, we’re supposed to perform a timeout focused on the patient’s safety. We should also try to advocate for physician safety and an ergonomic timeout. I developed a mnemonic device utilizing the word “MYSELF” to help endoscopists remember the ergonomic timeout checklist: M = monitor, Y = upside-down Y stance, S = scope, E = elbow/ bed position, L = lower extremities, F = free movement of endoscope/ processor placement.
First, thinking about the monitor, “M”, and fixing the monitor height so that the neck is in neutral position. Then, thinking of an upside down “Y” standing straight with the feet either hip width or shoulder width apart, so that the physician has a stable, neutral standing posture. Then “S” is for checking the scope to ensure you have a scope with optimal angulation that’s working properly.
“E” is for elbows — adjusting the bed to an optimal position so that elbows and shoulders are in neutral position. “L” is for lower extremities — are the foot pedals within an easy reach? Do you have comfortable shoes on, an anti-fatigue floor mat if you need it? And then the “F” in “MYSELF” is for the processor placement, to ensure “free movement” of the scope. By placing the processor directly behind you and lining up the processor with the orifice to be scoped, you can ensure free movement of the scope so that you can leverage large movements of the control section to result in tip deflection.
We studied the MYSELF mnemonic device for a pre-procedure ergonomic timeout in a simulated setting and presented our results at Digestive Disease Week (DDW) 2024, where we showed a reduction in ergonomic risk scores based on the Rapid Entire Body Assessment tool.
We presented the results of the scope stand study at DDW 2025 in San Diego this May.
What has been the feedback from physicians who use these supportive tools?
While physicians are very grateful for bringing attention to this issue, and many have found utility in some of the tools that I proposed, I think we still have so much work to do. We’re just all hoping to continue to move this field forward for better tools that are designed more with the breadth of endoscopists in mind.
How do you handle stress and maintain work-life balance?
A few years ago, during DDW I gave a talk entitled “Achieving Work-Life Harmony.” I disclosed at the beginning of the talk that I had not achieved work-life harmony. It’s definitely a difficult thing to do, especially in our field as GI proceduralists, where we’re frequently on call and there are potentially on-call emergencies.
One of the key things that I’ve tried to do is create boundaries to prioritize both things in my personal life and my professional life and really try to stay true to the things that are important to me. For instance, things like family time and mealtimes, I think that’s so critical. Trying to be home on evenings for dinnertime is so important.
One of my GI colleagues, Raj Keswani, MD, MS gave a talk about burnout and described imagining life as juggling balls; trying to figure out which balls are glass balls and need to be handled with care, and which balls are rubber balls.
More often, work is the rubber ball. If you drop it, it’ll bounce back and the work that you have will still be there the next day. Family, friends, our health, those are the glass balls that if they fall, they can get scuffed or shatter sometimes. That image helps me think in the moment. If I need to decide between two competing priorities, which one will still be here tomorrow? Which is the one that’s going to be more resilient, and which is the one that I need to focus on? That’s been a helpful image for me.
I also want to give a shout out to my amazing colleagues. We all pitch in with the ‘juggling’ and help to keep everyone’s ‘balls’ in the air, and cover for each other. Whether it’s a sick patient or whatever’s going on in our personal lives, we always take care of each other.
What advice would you give to aspiring GI fellows or graduating fellows?
GI is such an amazing field and many people end up focusing on the procedural aspect of it. What I think defines an exceptional gastroenterologist and physician in general is adopting both a “growth mindset” and a “mastery mindset.” And really, it starts out with when you’re exploring an area of focus, listening to what consistently draws your attention, what you’re excited about learning more about.
Finding mentors, getting involved in projects, doing deep learning, and really trying to develop an expertise in that area through additional training, coursework, and education. I think that idea of a mastery mindset will really help set you up for becoming deeply knowledgeable about a field.
Lightning Round
Coffee or tea?
Coffee
What’s your favorite book?
Project Hail Mary (audiobook)
Beach vacation or mountain retreat?
Mountain retreat
Early bird or night owl?
Night owl
What’s your go-to comfort food?
Chaat (Indian street food)
Do you prefer dogs or cats?
Dogs
What’s one hobby you’d like to pick up?
Sewing
If you could have dinner with any historical figure, who would it be?
Ruth Bader Ginsburg
What’s your go-to karaoke song?
I Wanna Dance with Somebody
What’s one thing on your bucket list?
To see the Northern Lights