User login
MDedge latest news is breaking news from medical conferences, journals, guidelines, the FDA and CDC.
New Weight Loss Drugs May Fight Obesity-Related Cancer, Too
The latest glucagon-like peptide 1 (GLP-1) receptor agonists have been heralded for their potential to not only boost weight loss and glucose control but also improve cardiovascular, gastric, hepatic, and renal values.
Throughout 2024, research has also indicated GLP-1 drugs may reduce risks for obesity-related cancer.
In a US study of more than 1.6 million patients with type 2 diabetes, cancer researchers found that patients who took a GLP-1 drug had significant risk reductions for 10 of 13 obesity-associated cancers, as compared with patients who only took insulin.
They also saw a declining risk for stomach cancer, though it wasn’t considered statistically significant, but not a reduced risk for postmenopausal breast cancer or thyroid cancer.
The associations make sense, particularly because GLP-1 drugs have unexpected effects on modulating immune functions linked to obesity-associated cancers.
“The protective effects of GLP-1s against obesity-associated cancers likely stem from multiple mechanisms,” said lead author Lindsey Wang, a medical student and research scholar at Case Western Reserve University in Cleveland.
“These drugs promote substantial weight loss, reducing obesity-related cancer risks,” she said. “They also enhance insulin sensitivity and lower insulin levels, decreasing cancer cell growth signals.”
Additional GLP-1 Studies
The Case Western team also published a study in December 2023 that found people with type 2 diabetes who took GLP-1s had a 44% lower risk for colorectal cancer than those who took insulin and a 25% lower risk than those who took metformin. The research suggested even greater risk reductions among those with overweight or obesity, with GLP-1 users having a 50% lower risk than those who took insulin and a 42% lower risk than those who took metformin.
In another recent Case Western study, both bariatric surgery and GLP-1 drugs reduced the risk for obesity-related cancers. While those who had bariatric surgery had a 22% risk reduction over 10 years, as compared with those who received no treatment, those taking GLP-1 had a 39% risk reduction.
Other studies worldwide have looked at GLP-1 drugs and tumor effects among various cancer cell lines. In a study using pancreatic cancer cell lines, GLP-1 liraglutide suppressed cancer cell growth and led to cell death. Similarly, a study using breast cancer cells found liraglutide reduced cancer cell viability and the ability for cells to migrate.
As researchers identify additional links between GLP-1s and improvements across organ systems, the knock-on effects could lead to lower cancer risks as well. For example, studies presented at The Liver Meeting in San Diego in November pointed to GLP-1s reducing fatty liver disease, which can slow the progression to liver cancer.
“Separate from obesity, having higher levels of body fat is associated with an increased risk of several forms of cancer,” said Neil Iyengar, MD, an oncologist at Memorial Sloan Kettering Cancer Center in New York City. Iyengar researches the relationship between obesity and cancer.
“I foresee that this class of drugs will revolutionize obesity and the cancer burden that comes with it, if people can get access,” he said. “This really is an exciting development.”
Ongoing GLP-1 Research
On the other hand, cancer researchers have also expressed concerns about potential associations between GLP-1s and increased cancer risks. In the obesity-associated cancer study by Case Western researchers, patients with type 2 diabetes taking a GLP-1 drug appeared to have a slightly higher risk for kidney cancer than those taking metformin.
In addition, GLP-1 studies in animals have indicated that the drugs may increase the risks for medullary thyroid cancer and pancreatic cancer. However, the data on increased risks in humans remain inconclusive, and more recent studies refute these findings.
For instance, cancer researchers in India conducted a systematic review and meta-analysis of semaglutide and cancer risks, finding that 37 randomized controlled trials and 19 real-world studies didn’t find increased risks for any cancer, including pancreatic and thyroid cancers.
In another systematic review by Brazilian researchers, 50 trials found GLP-1s didn’t increase the risk for breast cancer or benign breast neoplasms.
In 2025, new retrospective studies will show more nuanced data, especially as more patients — both with and without type 2 diabetes — take semaglutide, tirzepatide, and new GLP-1 drugs in the research pipeline.
“The holy grail has always been getting a medication to treat obesity,” said Anne McTiernan, MD, PhD, an epidemiologist and obesity researcher at the Fred Hutchinson Cancer Center in Seattle.
“There have been trials focused on these medications’ effects on diabetes and cardiovascular disease treatment, but no trials have tested their effects on cancer risk,” she said. “Usually, many years of follow-up of large numbers of patients are needed to see cancer effects of a carcinogen or cancer-preventing intervention.”
Those clinical trials are likely coming soon, she said. Researchers will need to conduct prospective clinical trials to examine the direct relationship between GLP-1 drugs and cancer risks, as well as the underlying mechanisms linked to cancer cell growth, activation of immune cells, and anti-inflammatory properties.
Because GLP-1 medications aren’t intended to be taken forever, researchers will also need to consider the associations with long-term cancer risks. Even so, weight loss and other obesity-related improvements could contribute to overall lower cancer risks in the end.
“If taking these drugs for a limited amount of time can help people lose weight and get on an exercise plan, then that’s helping lower cancer risk long-term,” said Sonali Thosani, MD, associate professor of endocrine neoplasia and hormonal disorders at the University of Texas MD Anderson Cancer Center in Houston.
“But it all comes back to someone making lifestyle changes and sticking to them, even after they stop taking the drugs,” she said. “If they can do that, then you’ll probably see a net positive for long-term cancer risks and other long-term health risks.”
A version of this article appeared on Medscape.com.
The latest glucagon-like peptide 1 (GLP-1) receptor agonists have been heralded for their potential to not only boost weight loss and glucose control but also improve cardiovascular, gastric, hepatic, and renal values.
Throughout 2024, research has also indicated GLP-1 drugs may reduce risks for obesity-related cancer.
In a US study of more than 1.6 million patients with type 2 diabetes, cancer researchers found that patients who took a GLP-1 drug had significant risk reductions for 10 of 13 obesity-associated cancers, as compared with patients who only took insulin.
They also saw a declining risk for stomach cancer, though it wasn’t considered statistically significant, but not a reduced risk for postmenopausal breast cancer or thyroid cancer.
The associations make sense, particularly because GLP-1 drugs have unexpected effects on modulating immune functions linked to obesity-associated cancers.
“The protective effects of GLP-1s against obesity-associated cancers likely stem from multiple mechanisms,” said lead author Lindsey Wang, a medical student and research scholar at Case Western Reserve University in Cleveland.
“These drugs promote substantial weight loss, reducing obesity-related cancer risks,” she said. “They also enhance insulin sensitivity and lower insulin levels, decreasing cancer cell growth signals.”
Additional GLP-1 Studies
The Case Western team also published a study in December 2023 that found people with type 2 diabetes who took GLP-1s had a 44% lower risk for colorectal cancer than those who took insulin and a 25% lower risk than those who took metformin. The research suggested even greater risk reductions among those with overweight or obesity, with GLP-1 users having a 50% lower risk than those who took insulin and a 42% lower risk than those who took metformin.
In another recent Case Western study, both bariatric surgery and GLP-1 drugs reduced the risk for obesity-related cancers. While those who had bariatric surgery had a 22% risk reduction over 10 years, as compared with those who received no treatment, those taking GLP-1 had a 39% risk reduction.
Other studies worldwide have looked at GLP-1 drugs and tumor effects among various cancer cell lines. In a study using pancreatic cancer cell lines, GLP-1 liraglutide suppressed cancer cell growth and led to cell death. Similarly, a study using breast cancer cells found liraglutide reduced cancer cell viability and the ability for cells to migrate.
As researchers identify additional links between GLP-1s and improvements across organ systems, the knock-on effects could lead to lower cancer risks as well. For example, studies presented at The Liver Meeting in San Diego in November pointed to GLP-1s reducing fatty liver disease, which can slow the progression to liver cancer.
“Separate from obesity, having higher levels of body fat is associated with an increased risk of several forms of cancer,” said Neil Iyengar, MD, an oncologist at Memorial Sloan Kettering Cancer Center in New York City. Iyengar researches the relationship between obesity and cancer.
“I foresee that this class of drugs will revolutionize obesity and the cancer burden that comes with it, if people can get access,” he said. “This really is an exciting development.”
Ongoing GLP-1 Research
On the other hand, cancer researchers have also expressed concerns about potential associations between GLP-1s and increased cancer risks. In the obesity-associated cancer study by Case Western researchers, patients with type 2 diabetes taking a GLP-1 drug appeared to have a slightly higher risk for kidney cancer than those taking metformin.
In addition, GLP-1 studies in animals have indicated that the drugs may increase the risks for medullary thyroid cancer and pancreatic cancer. However, the data on increased risks in humans remain inconclusive, and more recent studies refute these findings.
For instance, cancer researchers in India conducted a systematic review and meta-analysis of semaglutide and cancer risks, finding that 37 randomized controlled trials and 19 real-world studies didn’t find increased risks for any cancer, including pancreatic and thyroid cancers.
In another systematic review by Brazilian researchers, 50 trials found GLP-1s didn’t increase the risk for breast cancer or benign breast neoplasms.
In 2025, new retrospective studies will show more nuanced data, especially as more patients — both with and without type 2 diabetes — take semaglutide, tirzepatide, and new GLP-1 drugs in the research pipeline.
“The holy grail has always been getting a medication to treat obesity,” said Anne McTiernan, MD, PhD, an epidemiologist and obesity researcher at the Fred Hutchinson Cancer Center in Seattle.
“There have been trials focused on these medications’ effects on diabetes and cardiovascular disease treatment, but no trials have tested their effects on cancer risk,” she said. “Usually, many years of follow-up of large numbers of patients are needed to see cancer effects of a carcinogen or cancer-preventing intervention.”
Those clinical trials are likely coming soon, she said. Researchers will need to conduct prospective clinical trials to examine the direct relationship between GLP-1 drugs and cancer risks, as well as the underlying mechanisms linked to cancer cell growth, activation of immune cells, and anti-inflammatory properties.
Because GLP-1 medications aren’t intended to be taken forever, researchers will also need to consider the associations with long-term cancer risks. Even so, weight loss and other obesity-related improvements could contribute to overall lower cancer risks in the end.
“If taking these drugs for a limited amount of time can help people lose weight and get on an exercise plan, then that’s helping lower cancer risk long-term,” said Sonali Thosani, MD, associate professor of endocrine neoplasia and hormonal disorders at the University of Texas MD Anderson Cancer Center in Houston.
“But it all comes back to someone making lifestyle changes and sticking to them, even after they stop taking the drugs,” she said. “If they can do that, then you’ll probably see a net positive for long-term cancer risks and other long-term health risks.”
A version of this article appeared on Medscape.com.
The latest glucagon-like peptide 1 (GLP-1) receptor agonists have been heralded for their potential to not only boost weight loss and glucose control but also improve cardiovascular, gastric, hepatic, and renal values.
Throughout 2024, research has also indicated GLP-1 drugs may reduce risks for obesity-related cancer.
In a US study of more than 1.6 million patients with type 2 diabetes, cancer researchers found that patients who took a GLP-1 drug had significant risk reductions for 10 of 13 obesity-associated cancers, as compared with patients who only took insulin.
They also saw a declining risk for stomach cancer, though it wasn’t considered statistically significant, but not a reduced risk for postmenopausal breast cancer or thyroid cancer.
The associations make sense, particularly because GLP-1 drugs have unexpected effects on modulating immune functions linked to obesity-associated cancers.
“The protective effects of GLP-1s against obesity-associated cancers likely stem from multiple mechanisms,” said lead author Lindsey Wang, a medical student and research scholar at Case Western Reserve University in Cleveland.
“These drugs promote substantial weight loss, reducing obesity-related cancer risks,” she said. “They also enhance insulin sensitivity and lower insulin levels, decreasing cancer cell growth signals.”
Additional GLP-1 Studies
The Case Western team also published a study in December 2023 that found people with type 2 diabetes who took GLP-1s had a 44% lower risk for colorectal cancer than those who took insulin and a 25% lower risk than those who took metformin. The research suggested even greater risk reductions among those with overweight or obesity, with GLP-1 users having a 50% lower risk than those who took insulin and a 42% lower risk than those who took metformin.
In another recent Case Western study, both bariatric surgery and GLP-1 drugs reduced the risk for obesity-related cancers. While those who had bariatric surgery had a 22% risk reduction over 10 years, as compared with those who received no treatment, those taking GLP-1 had a 39% risk reduction.
Other studies worldwide have looked at GLP-1 drugs and tumor effects among various cancer cell lines. In a study using pancreatic cancer cell lines, GLP-1 liraglutide suppressed cancer cell growth and led to cell death. Similarly, a study using breast cancer cells found liraglutide reduced cancer cell viability and the ability for cells to migrate.
As researchers identify additional links between GLP-1s and improvements across organ systems, the knock-on effects could lead to lower cancer risks as well. For example, studies presented at The Liver Meeting in San Diego in November pointed to GLP-1s reducing fatty liver disease, which can slow the progression to liver cancer.
“Separate from obesity, having higher levels of body fat is associated with an increased risk of several forms of cancer,” said Neil Iyengar, MD, an oncologist at Memorial Sloan Kettering Cancer Center in New York City. Iyengar researches the relationship between obesity and cancer.
“I foresee that this class of drugs will revolutionize obesity and the cancer burden that comes with it, if people can get access,” he said. “This really is an exciting development.”
Ongoing GLP-1 Research
On the other hand, cancer researchers have also expressed concerns about potential associations between GLP-1s and increased cancer risks. In the obesity-associated cancer study by Case Western researchers, patients with type 2 diabetes taking a GLP-1 drug appeared to have a slightly higher risk for kidney cancer than those taking metformin.
In addition, GLP-1 studies in animals have indicated that the drugs may increase the risks for medullary thyroid cancer and pancreatic cancer. However, the data on increased risks in humans remain inconclusive, and more recent studies refute these findings.
For instance, cancer researchers in India conducted a systematic review and meta-analysis of semaglutide and cancer risks, finding that 37 randomized controlled trials and 19 real-world studies didn’t find increased risks for any cancer, including pancreatic and thyroid cancers.
In another systematic review by Brazilian researchers, 50 trials found GLP-1s didn’t increase the risk for breast cancer or benign breast neoplasms.
In 2025, new retrospective studies will show more nuanced data, especially as more patients — both with and without type 2 diabetes — take semaglutide, tirzepatide, and new GLP-1 drugs in the research pipeline.
“The holy grail has always been getting a medication to treat obesity,” said Anne McTiernan, MD, PhD, an epidemiologist and obesity researcher at the Fred Hutchinson Cancer Center in Seattle.
“There have been trials focused on these medications’ effects on diabetes and cardiovascular disease treatment, but no trials have tested their effects on cancer risk,” she said. “Usually, many years of follow-up of large numbers of patients are needed to see cancer effects of a carcinogen or cancer-preventing intervention.”
Those clinical trials are likely coming soon, she said. Researchers will need to conduct prospective clinical trials to examine the direct relationship between GLP-1 drugs and cancer risks, as well as the underlying mechanisms linked to cancer cell growth, activation of immune cells, and anti-inflammatory properties.
Because GLP-1 medications aren’t intended to be taken forever, researchers will also need to consider the associations with long-term cancer risks. Even so, weight loss and other obesity-related improvements could contribute to overall lower cancer risks in the end.
“If taking these drugs for a limited amount of time can help people lose weight and get on an exercise plan, then that’s helping lower cancer risk long-term,” said Sonali Thosani, MD, associate professor of endocrine neoplasia and hormonal disorders at the University of Texas MD Anderson Cancer Center in Houston.
“But it all comes back to someone making lifestyle changes and sticking to them, even after they stop taking the drugs,” she said. “If they can do that, then you’ll probably see a net positive for long-term cancer risks and other long-term health risks.”
A version of this article appeared on Medscape.com.
Both High and Low HDL Levels Linked to Increased Risk for Age-Related Macular Degeneration
TOPLINE:
This study also identified a potential novel single-nucleotide polymorphism linked to an elevated risk for the retina condition.
METHODOLOGY:
- Researchers conducted a cross-sectional retrospective analysis using data from the All of Us research program to assess the association between lipoprotein and the risk for AMD.
- They analyzed data from 2328 patients with AMD (mean age, 75.5 years; 46.7% women; 84.2% White individuals) and 5028 matched controls (mean age, 75.6 years; 52.5% women; 82.9% White individuals).
- Data were extracted for smoking status, history of hyperlipidemia, use of statins (categorized as hepatically and non-hepatically metabolized), and laboratory values for total triglyceride, low-density lipoprotein (LDL), and HDL levels.
- Data for single-nucleotide polymorphisms associated with the dysregulation of LDL and HDL metabolism were extracted using the PLINK toolkit.
TAKEAWAY:
- Both high and low HDL levels were associated with an increased risk for AMD (adjusted odds ratio [aOR], 1.28 for both; both P < .001), whereas low and high levels of triglyceride and LDL did not demonstrate a statistically significant association with the risk for AMD.
- A history of smoking and statin use showed significant associations with an increased risk for AMD (aOR, 1.30 and 1.36, respectively; both P < .001).
- Single-nucleotide polymorphisms in the genes associated with HDL metabolism, ABCA1 and LIPC, were negatively associated with the risk for AMD (aOR, 0.88; P = .04 and aOR, 0.86; P = .001, respectively).
- Lipoprotein(a) or Lp(a) was identified as a novel single nucleotide polymorphism linked to an increased risk for AMD (aOR, 1.37; P = .007).
IN PRACTICE:
“Despite conflicting evidence regarding the relationship with elevated HDL and AMD risk, this is to our knowledge the first time a U-shaped relationship with low and high HDL and AMD has been described. In fact, the presence of a U-shaped relationship may explain inconsistency in prior analyses comparing mean HDL levels in AMD and control populations,” the study authors wrote.
SOURCE:
The study was led by Jimmy S. Chen, MD, of the Viterbi Family Department of Ophthalmology and Shiley Eye Institute at the University of California, San Diego. It was published online on January 3, 2025, in Ophthalmology.
LIMITATIONS:
The study was limited by the retrospective collection and analysis of data. The use of billing codes for diagnosis extraction may have introduced documentation inaccuracies. The subgroup analysis by severity of AMD was not performed.
DISCLOSURES:
One of the authors was funded by grants from the National Eye Institute (NEI), Research to Prevent Blindness Career Development Award, Robert Machemer MD and International Retinal Research Foundation, and the UC San Diego Academic Senate. Another author reported receiving a grant from the National Heart, Lung, and Blood Institute, while a third author received funding from the National Institutes of Health (NIH), NEI, and Research to Prevent Blindness. The All of Us Research Program was supported by grants from the NIH and other sources. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
This study also identified a potential novel single-nucleotide polymorphism linked to an elevated risk for the retina condition.
METHODOLOGY:
- Researchers conducted a cross-sectional retrospective analysis using data from the All of Us research program to assess the association between lipoprotein and the risk for AMD.
- They analyzed data from 2328 patients with AMD (mean age, 75.5 years; 46.7% women; 84.2% White individuals) and 5028 matched controls (mean age, 75.6 years; 52.5% women; 82.9% White individuals).
- Data were extracted for smoking status, history of hyperlipidemia, use of statins (categorized as hepatically and non-hepatically metabolized), and laboratory values for total triglyceride, low-density lipoprotein (LDL), and HDL levels.
- Data for single-nucleotide polymorphisms associated with the dysregulation of LDL and HDL metabolism were extracted using the PLINK toolkit.
TAKEAWAY:
- Both high and low HDL levels were associated with an increased risk for AMD (adjusted odds ratio [aOR], 1.28 for both; both P < .001), whereas low and high levels of triglyceride and LDL did not demonstrate a statistically significant association with the risk for AMD.
- A history of smoking and statin use showed significant associations with an increased risk for AMD (aOR, 1.30 and 1.36, respectively; both P < .001).
- Single-nucleotide polymorphisms in the genes associated with HDL metabolism, ABCA1 and LIPC, were negatively associated with the risk for AMD (aOR, 0.88; P = .04 and aOR, 0.86; P = .001, respectively).
- Lipoprotein(a) or Lp(a) was identified as a novel single nucleotide polymorphism linked to an increased risk for AMD (aOR, 1.37; P = .007).
IN PRACTICE:
“Despite conflicting evidence regarding the relationship with elevated HDL and AMD risk, this is to our knowledge the first time a U-shaped relationship with low and high HDL and AMD has been described. In fact, the presence of a U-shaped relationship may explain inconsistency in prior analyses comparing mean HDL levels in AMD and control populations,” the study authors wrote.
SOURCE:
The study was led by Jimmy S. Chen, MD, of the Viterbi Family Department of Ophthalmology and Shiley Eye Institute at the University of California, San Diego. It was published online on January 3, 2025, in Ophthalmology.
LIMITATIONS:
The study was limited by the retrospective collection and analysis of data. The use of billing codes for diagnosis extraction may have introduced documentation inaccuracies. The subgroup analysis by severity of AMD was not performed.
DISCLOSURES:
One of the authors was funded by grants from the National Eye Institute (NEI), Research to Prevent Blindness Career Development Award, Robert Machemer MD and International Retinal Research Foundation, and the UC San Diego Academic Senate. Another author reported receiving a grant from the National Heart, Lung, and Blood Institute, while a third author received funding from the National Institutes of Health (NIH), NEI, and Research to Prevent Blindness. The All of Us Research Program was supported by grants from the NIH and other sources. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
This study also identified a potential novel single-nucleotide polymorphism linked to an elevated risk for the retina condition.
METHODOLOGY:
- Researchers conducted a cross-sectional retrospective analysis using data from the All of Us research program to assess the association between lipoprotein and the risk for AMD.
- They analyzed data from 2328 patients with AMD (mean age, 75.5 years; 46.7% women; 84.2% White individuals) and 5028 matched controls (mean age, 75.6 years; 52.5% women; 82.9% White individuals).
- Data were extracted for smoking status, history of hyperlipidemia, use of statins (categorized as hepatically and non-hepatically metabolized), and laboratory values for total triglyceride, low-density lipoprotein (LDL), and HDL levels.
- Data for single-nucleotide polymorphisms associated with the dysregulation of LDL and HDL metabolism were extracted using the PLINK toolkit.
TAKEAWAY:
- Both high and low HDL levels were associated with an increased risk for AMD (adjusted odds ratio [aOR], 1.28 for both; both P < .001), whereas low and high levels of triglyceride and LDL did not demonstrate a statistically significant association with the risk for AMD.
- A history of smoking and statin use showed significant associations with an increased risk for AMD (aOR, 1.30 and 1.36, respectively; both P < .001).
- Single-nucleotide polymorphisms in the genes associated with HDL metabolism, ABCA1 and LIPC, were negatively associated with the risk for AMD (aOR, 0.88; P = .04 and aOR, 0.86; P = .001, respectively).
- Lipoprotein(a) or Lp(a) was identified as a novel single nucleotide polymorphism linked to an increased risk for AMD (aOR, 1.37; P = .007).
IN PRACTICE:
“Despite conflicting evidence regarding the relationship with elevated HDL and AMD risk, this is to our knowledge the first time a U-shaped relationship with low and high HDL and AMD has been described. In fact, the presence of a U-shaped relationship may explain inconsistency in prior analyses comparing mean HDL levels in AMD and control populations,” the study authors wrote.
SOURCE:
The study was led by Jimmy S. Chen, MD, of the Viterbi Family Department of Ophthalmology and Shiley Eye Institute at the University of California, San Diego. It was published online on January 3, 2025, in Ophthalmology.
LIMITATIONS:
The study was limited by the retrospective collection and analysis of data. The use of billing codes for diagnosis extraction may have introduced documentation inaccuracies. The subgroup analysis by severity of AMD was not performed.
DISCLOSURES:
One of the authors was funded by grants from the National Eye Institute (NEI), Research to Prevent Blindness Career Development Award, Robert Machemer MD and International Retinal Research Foundation, and the UC San Diego Academic Senate. Another author reported receiving a grant from the National Heart, Lung, and Blood Institute, while a third author received funding from the National Institutes of Health (NIH), NEI, and Research to Prevent Blindness. The All of Us Research Program was supported by grants from the NIH and other sources. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Highly Contagious Norovirus Cases Spike This Season
Current data from the CDC’s NoroSTAT monitoring system show 495 reported outbreaks during the period from August 1, 2024, to December 11, 2024, compared with 363 outbreaks during the same period last year. In addition, the total number of norovirus outbreaks in the current season are higher than those reported in the seasonal years: 2012-2020 and 2021-2024.
Circulating strains of norovirus change over time, which can affect disease burden and potential disease severity, Sara Mirza, MD, an epidemiologist in the CDC’s Division of Viral Diseases, said in an interview.
The numbers for the 2024 norovirus season (considered approximately November to April) have reached or exceeded the case numbers seen before the COVID-19 pandemic, Mirza said.
The increase in cases may be caused in part by a new predominant strain of norovirus. “For the fall/winter of 2024-2025 season, genogroup 2, genotype 17, known as GII.17, has become the most detected genotype (strain) in the US among laboratory confirmed outbreaks reported to CDC,” said Mirza. “At this time, there is no indication that GII.17 causes more severe illness or affects one population more than another, but we are continuing to conduct surveillance to assess,” she added.
Clinical Takeaways
“Norovirus affects all ages, but young children and older adults are most at risk from more severe outcomes,” said Mirza.
“Clinicians treating older patients for acute gastroenteritis should be aware of these elevated risks and be sure to include norovirus as a potentially serious diagnosis, particularly in vulnerable patients with other diseases and those living in congregate settings, such as nursing homes,” she said.
When treating a patient with norovirus during an outbreak, use soap and water for hand hygiene after caring for patients with suspected or confirmed norovirus gastroenteritis, said Mirza. If norovirus infection is suspected, PPE use is recommended for individuals in the patient care area, she added.
Although state, local, and territorial health departments are not required to report individual cases of norovirus to the CDC, healthcare providers are encouraged to report all outbreaks of acute gastroenteritis, including suspected outbreaks of norovirus, to the appropriate state, local, or territorial health department, said Mirza. “Health departments are encouraged to report all suspected and confirmed norovirus outbreaks through the National Outbreak Reporting System and CaliciNet,” she added.
“Infection control measures, such as thorough hand washing, cleaning and disinfecting surfaces with bleach, and patient isolation and contact precautions in congregate or healthcare settings are the best ways to prevent norovirus and keep it from spreading to others,” Mirza said.
Remind patients that alcohol-based hand sanitizer is ineffective against norovirus, because the virus’s protective protein shell prevents the alcohol from penetrating and inactivating the virus, Mirza emphasized. “Soap and water work to remove germs from hands,” she said.
Cruise Ship Considerations
Cruise ships continue to be sources of increased risk for norovirus, according the CDC. The CDC’s Vessel Sanitation Program (VSP) was created to help the cruise industry prevent public health issues such as norovirus outbreaks, and to provide guidance for actions to take in the event of outbreaks.
For example, the most recently reported outbreak of norovirus on a cruise ship reported to the VSP was January 4, 2025, and occurred on a Holland America cruise from December 30, 2024, through January 8, 2025. Overall, 4.0% of passengers and 1.0% of crew members reported illness. Following VSP guidance, the ship reported increased cleaning and disinfection procedures and the collection of stool specimens for testing, and isolation of ill passengers and crew.
Clinical Perspective
In clinical practice, the number of norovirus cases is significantly exceeding previous years, and the trend seems to be consistent nationwide, David J. Cennimo, MD, associate professor of medicine and pediatrics at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“Norovirus is incredibly contagious and spreads very quickly, which is how you get entire cruise ships infected at once,” he said. Norovirus is notoriously difficult to disinfect or kill, he added.
One possible contributor to the surge in cases is increased travel, especially during the holiday season, when people are coming together and sharing food, Cennimo noted. “We have seen many infections such as pneumonia return to levels approaching the period before the COVID-19 pandemic,” he said.
For norovirus prevention, strict attention to sanitation and handwashing is a must at home or when traveling, said Cennimo. For clinicians, it is important to report outbreaks of GI illness so appropriate control measures can be taken, he said.
Visit the CDC’s website on norovirus prevention for more information.
Mirza and Cennimo had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Current data from the CDC’s NoroSTAT monitoring system show 495 reported outbreaks during the period from August 1, 2024, to December 11, 2024, compared with 363 outbreaks during the same period last year. In addition, the total number of norovirus outbreaks in the current season are higher than those reported in the seasonal years: 2012-2020 and 2021-2024.
Circulating strains of norovirus change over time, which can affect disease burden and potential disease severity, Sara Mirza, MD, an epidemiologist in the CDC’s Division of Viral Diseases, said in an interview.
The numbers for the 2024 norovirus season (considered approximately November to April) have reached or exceeded the case numbers seen before the COVID-19 pandemic, Mirza said.
The increase in cases may be caused in part by a new predominant strain of norovirus. “For the fall/winter of 2024-2025 season, genogroup 2, genotype 17, known as GII.17, has become the most detected genotype (strain) in the US among laboratory confirmed outbreaks reported to CDC,” said Mirza. “At this time, there is no indication that GII.17 causes more severe illness or affects one population more than another, but we are continuing to conduct surveillance to assess,” she added.
Clinical Takeaways
“Norovirus affects all ages, but young children and older adults are most at risk from more severe outcomes,” said Mirza.
“Clinicians treating older patients for acute gastroenteritis should be aware of these elevated risks and be sure to include norovirus as a potentially serious diagnosis, particularly in vulnerable patients with other diseases and those living in congregate settings, such as nursing homes,” she said.
When treating a patient with norovirus during an outbreak, use soap and water for hand hygiene after caring for patients with suspected or confirmed norovirus gastroenteritis, said Mirza. If norovirus infection is suspected, PPE use is recommended for individuals in the patient care area, she added.
Although state, local, and territorial health departments are not required to report individual cases of norovirus to the CDC, healthcare providers are encouraged to report all outbreaks of acute gastroenteritis, including suspected outbreaks of norovirus, to the appropriate state, local, or territorial health department, said Mirza. “Health departments are encouraged to report all suspected and confirmed norovirus outbreaks through the National Outbreak Reporting System and CaliciNet,” she added.
“Infection control measures, such as thorough hand washing, cleaning and disinfecting surfaces with bleach, and patient isolation and contact precautions in congregate or healthcare settings are the best ways to prevent norovirus and keep it from spreading to others,” Mirza said.
Remind patients that alcohol-based hand sanitizer is ineffective against norovirus, because the virus’s protective protein shell prevents the alcohol from penetrating and inactivating the virus, Mirza emphasized. “Soap and water work to remove germs from hands,” she said.
Cruise Ship Considerations
Cruise ships continue to be sources of increased risk for norovirus, according the CDC. The CDC’s Vessel Sanitation Program (VSP) was created to help the cruise industry prevent public health issues such as norovirus outbreaks, and to provide guidance for actions to take in the event of outbreaks.
For example, the most recently reported outbreak of norovirus on a cruise ship reported to the VSP was January 4, 2025, and occurred on a Holland America cruise from December 30, 2024, through January 8, 2025. Overall, 4.0% of passengers and 1.0% of crew members reported illness. Following VSP guidance, the ship reported increased cleaning and disinfection procedures and the collection of stool specimens for testing, and isolation of ill passengers and crew.
Clinical Perspective
In clinical practice, the number of norovirus cases is significantly exceeding previous years, and the trend seems to be consistent nationwide, David J. Cennimo, MD, associate professor of medicine and pediatrics at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“Norovirus is incredibly contagious and spreads very quickly, which is how you get entire cruise ships infected at once,” he said. Norovirus is notoriously difficult to disinfect or kill, he added.
One possible contributor to the surge in cases is increased travel, especially during the holiday season, when people are coming together and sharing food, Cennimo noted. “We have seen many infections such as pneumonia return to levels approaching the period before the COVID-19 pandemic,” he said.
For norovirus prevention, strict attention to sanitation and handwashing is a must at home or when traveling, said Cennimo. For clinicians, it is important to report outbreaks of GI illness so appropriate control measures can be taken, he said.
Visit the CDC’s website on norovirus prevention for more information.
Mirza and Cennimo had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Current data from the CDC’s NoroSTAT monitoring system show 495 reported outbreaks during the period from August 1, 2024, to December 11, 2024, compared with 363 outbreaks during the same period last year. In addition, the total number of norovirus outbreaks in the current season are higher than those reported in the seasonal years: 2012-2020 and 2021-2024.
Circulating strains of norovirus change over time, which can affect disease burden and potential disease severity, Sara Mirza, MD, an epidemiologist in the CDC’s Division of Viral Diseases, said in an interview.
The numbers for the 2024 norovirus season (considered approximately November to April) have reached or exceeded the case numbers seen before the COVID-19 pandemic, Mirza said.
The increase in cases may be caused in part by a new predominant strain of norovirus. “For the fall/winter of 2024-2025 season, genogroup 2, genotype 17, known as GII.17, has become the most detected genotype (strain) in the US among laboratory confirmed outbreaks reported to CDC,” said Mirza. “At this time, there is no indication that GII.17 causes more severe illness or affects one population more than another, but we are continuing to conduct surveillance to assess,” she added.
Clinical Takeaways
“Norovirus affects all ages, but young children and older adults are most at risk from more severe outcomes,” said Mirza.
“Clinicians treating older patients for acute gastroenteritis should be aware of these elevated risks and be sure to include norovirus as a potentially serious diagnosis, particularly in vulnerable patients with other diseases and those living in congregate settings, such as nursing homes,” she said.
When treating a patient with norovirus during an outbreak, use soap and water for hand hygiene after caring for patients with suspected or confirmed norovirus gastroenteritis, said Mirza. If norovirus infection is suspected, PPE use is recommended for individuals in the patient care area, she added.
Although state, local, and territorial health departments are not required to report individual cases of norovirus to the CDC, healthcare providers are encouraged to report all outbreaks of acute gastroenteritis, including suspected outbreaks of norovirus, to the appropriate state, local, or territorial health department, said Mirza. “Health departments are encouraged to report all suspected and confirmed norovirus outbreaks through the National Outbreak Reporting System and CaliciNet,” she added.
“Infection control measures, such as thorough hand washing, cleaning and disinfecting surfaces with bleach, and patient isolation and contact precautions in congregate or healthcare settings are the best ways to prevent norovirus and keep it from spreading to others,” Mirza said.
Remind patients that alcohol-based hand sanitizer is ineffective against norovirus, because the virus’s protective protein shell prevents the alcohol from penetrating and inactivating the virus, Mirza emphasized. “Soap and water work to remove germs from hands,” she said.
Cruise Ship Considerations
Cruise ships continue to be sources of increased risk for norovirus, according the CDC. The CDC’s Vessel Sanitation Program (VSP) was created to help the cruise industry prevent public health issues such as norovirus outbreaks, and to provide guidance for actions to take in the event of outbreaks.
For example, the most recently reported outbreak of norovirus on a cruise ship reported to the VSP was January 4, 2025, and occurred on a Holland America cruise from December 30, 2024, through January 8, 2025. Overall, 4.0% of passengers and 1.0% of crew members reported illness. Following VSP guidance, the ship reported increased cleaning and disinfection procedures and the collection of stool specimens for testing, and isolation of ill passengers and crew.
Clinical Perspective
In clinical practice, the number of norovirus cases is significantly exceeding previous years, and the trend seems to be consistent nationwide, David J. Cennimo, MD, associate professor of medicine and pediatrics at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“Norovirus is incredibly contagious and spreads very quickly, which is how you get entire cruise ships infected at once,” he said. Norovirus is notoriously difficult to disinfect or kill, he added.
One possible contributor to the surge in cases is increased travel, especially during the holiday season, when people are coming together and sharing food, Cennimo noted. “We have seen many infections such as pneumonia return to levels approaching the period before the COVID-19 pandemic,” he said.
For norovirus prevention, strict attention to sanitation and handwashing is a must at home or when traveling, said Cennimo. For clinicians, it is important to report outbreaks of GI illness so appropriate control measures can be taken, he said.
Visit the CDC’s website on norovirus prevention for more information.
Mirza and Cennimo had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Daycare Providers’ Little Helper
It is no secret that we have a daycare problem in this country. Twenty percent of families spend more than $36,000 for child care annually. Three quarters of a single parent’s income is spent on infant care. The result is that more than $122 billion is syphoned out of our economy in lost productivity and income.
How we got into this situation is less clear. Women who once were stay-at-home moms have moved into the workplace. Families are more mobile and grandparents who had been a source of childcare may live hours away. And, when they are nearby grandparents may themselves been forced to remain employed for economic reasons.
Despite the increase demand the market has failed to respond with more daycare providers because with a median hourly wage of less than $15.00 it is difficult to attract applicants from a pool of potential employees that is already in great demand.
And, let’s be honest, long hours cooped up inside with infants and toddlers isn’t the right job for everyone. For the most successful, although maybe not financially, providing daycare is truly a labor of love. There are high school and community college courses taught on child development and day care management. Experienced providers can be a source of tips-of-the trade to those just starting out. But, when there are three infants crying, two diapers to be changed, and a toddler heading toward a tantrum, two experienced providers may not be enough to calm the turbulent waters.
A recent article in my local newspaper provided stark evidence of how serious our daycare situation has become. Although the daycare owner denies the allegation, the Department of Health and Human Service told the parents that the investigation currently supports their complaints that the children had been given melatonin gummies without their permission. Final action is pending but it is likely the daycare will lose its license. Not surprisingly the parents have already removed their children.
Curious about whether this situation was an isolated event, it didn’t take Google too long to find evidence of other daycares in which children had been given sleep-related medications without their parents’ permission. In May 2024 a daycare provider and three of her employees in Manchester, New Hampshire, were arrested and charged with endangering the welfare of a child after allegedly spiking their charges food with melatonin. Lest you think drugging infants in daycare is just a New England thing, my research found a news story dating back to 2003 that reported on several cases in which daycare providers had been administering diphenhydramine without parents permission. In one instance there was a fatal outcome. While melatonin does not pose a health risk on a par with diphenhydramine, the issue is the fact that the parents were not consulted.
I suspect that these two incidents in Maine and New Hampshire are not isolated events and melatonin has replaced diphenhydramine as the daycare provider’s “little helper” nationwide. It’s not clear how we as pediatricians can help police this practice, other than suggesting to parents that they initiate dialogues about napping strategies with their daycare providers. Not with an accusatory tone but more of a sharing about what tricks each party uses to make napping happen. It may be that the daycare provider has some valuable and sound advice that the parents can adapt to their home situation. However, if the daycare provider’s explanation for why the child naps well doesn’t sound right or the child is unusually drowsy after daycare visits they should share their concerns with us a pediatric health care advisors.
Dr Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
It is no secret that we have a daycare problem in this country. Twenty percent of families spend more than $36,000 for child care annually. Three quarters of a single parent’s income is spent on infant care. The result is that more than $122 billion is syphoned out of our economy in lost productivity and income.
How we got into this situation is less clear. Women who once were stay-at-home moms have moved into the workplace. Families are more mobile and grandparents who had been a source of childcare may live hours away. And, when they are nearby grandparents may themselves been forced to remain employed for economic reasons.
Despite the increase demand the market has failed to respond with more daycare providers because with a median hourly wage of less than $15.00 it is difficult to attract applicants from a pool of potential employees that is already in great demand.
And, let’s be honest, long hours cooped up inside with infants and toddlers isn’t the right job for everyone. For the most successful, although maybe not financially, providing daycare is truly a labor of love. There are high school and community college courses taught on child development and day care management. Experienced providers can be a source of tips-of-the trade to those just starting out. But, when there are three infants crying, two diapers to be changed, and a toddler heading toward a tantrum, two experienced providers may not be enough to calm the turbulent waters.
A recent article in my local newspaper provided stark evidence of how serious our daycare situation has become. Although the daycare owner denies the allegation, the Department of Health and Human Service told the parents that the investigation currently supports their complaints that the children had been given melatonin gummies without their permission. Final action is pending but it is likely the daycare will lose its license. Not surprisingly the parents have already removed their children.
Curious about whether this situation was an isolated event, it didn’t take Google too long to find evidence of other daycares in which children had been given sleep-related medications without their parents’ permission. In May 2024 a daycare provider and three of her employees in Manchester, New Hampshire, were arrested and charged with endangering the welfare of a child after allegedly spiking their charges food with melatonin. Lest you think drugging infants in daycare is just a New England thing, my research found a news story dating back to 2003 that reported on several cases in which daycare providers had been administering diphenhydramine without parents permission. In one instance there was a fatal outcome. While melatonin does not pose a health risk on a par with diphenhydramine, the issue is the fact that the parents were not consulted.
I suspect that these two incidents in Maine and New Hampshire are not isolated events and melatonin has replaced diphenhydramine as the daycare provider’s “little helper” nationwide. It’s not clear how we as pediatricians can help police this practice, other than suggesting to parents that they initiate dialogues about napping strategies with their daycare providers. Not with an accusatory tone but more of a sharing about what tricks each party uses to make napping happen. It may be that the daycare provider has some valuable and sound advice that the parents can adapt to their home situation. However, if the daycare provider’s explanation for why the child naps well doesn’t sound right or the child is unusually drowsy after daycare visits they should share their concerns with us a pediatric health care advisors.
Dr Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
It is no secret that we have a daycare problem in this country. Twenty percent of families spend more than $36,000 for child care annually. Three quarters of a single parent’s income is spent on infant care. The result is that more than $122 billion is syphoned out of our economy in lost productivity and income.
How we got into this situation is less clear. Women who once were stay-at-home moms have moved into the workplace. Families are more mobile and grandparents who had been a source of childcare may live hours away. And, when they are nearby grandparents may themselves been forced to remain employed for economic reasons.
Despite the increase demand the market has failed to respond with more daycare providers because with a median hourly wage of less than $15.00 it is difficult to attract applicants from a pool of potential employees that is already in great demand.
And, let’s be honest, long hours cooped up inside with infants and toddlers isn’t the right job for everyone. For the most successful, although maybe not financially, providing daycare is truly a labor of love. There are high school and community college courses taught on child development and day care management. Experienced providers can be a source of tips-of-the trade to those just starting out. But, when there are three infants crying, two diapers to be changed, and a toddler heading toward a tantrum, two experienced providers may not be enough to calm the turbulent waters.
A recent article in my local newspaper provided stark evidence of how serious our daycare situation has become. Although the daycare owner denies the allegation, the Department of Health and Human Service told the parents that the investigation currently supports their complaints that the children had been given melatonin gummies without their permission. Final action is pending but it is likely the daycare will lose its license. Not surprisingly the parents have already removed their children.
Curious about whether this situation was an isolated event, it didn’t take Google too long to find evidence of other daycares in which children had been given sleep-related medications without their parents’ permission. In May 2024 a daycare provider and three of her employees in Manchester, New Hampshire, were arrested and charged with endangering the welfare of a child after allegedly spiking their charges food with melatonin. Lest you think drugging infants in daycare is just a New England thing, my research found a news story dating back to 2003 that reported on several cases in which daycare providers had been administering diphenhydramine without parents permission. In one instance there was a fatal outcome. While melatonin does not pose a health risk on a par with diphenhydramine, the issue is the fact that the parents were not consulted.
I suspect that these two incidents in Maine and New Hampshire are not isolated events and melatonin has replaced diphenhydramine as the daycare provider’s “little helper” nationwide. It’s not clear how we as pediatricians can help police this practice, other than suggesting to parents that they initiate dialogues about napping strategies with their daycare providers. Not with an accusatory tone but more of a sharing about what tricks each party uses to make napping happen. It may be that the daycare provider has some valuable and sound advice that the parents can adapt to their home situation. However, if the daycare provider’s explanation for why the child naps well doesn’t sound right or the child is unusually drowsy after daycare visits they should share their concerns with us a pediatric health care advisors.
Dr Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Red Wine May Not Be a Health Tonic, But Is It a Cancer Risk?
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.
Earlier this month, US surgeon general Vivek Murthy, MD, issued an advisory, calling for alcoholic beverages to carry a warning label about cancer risk. The advisory flagged alcohol as the third leading preventable cause of cancer in the United States, after tobacco and obesity, and highlighted people’s limited awareness about the relationship between alcohol and cancer risk.
But, when it comes to cancer risk, are all types of alcohol created equal?
For many years, red wine seemed to be an outlier, with studies indicating that, in moderation, it might even be good for you. Red wine has anti-inflammatory and antioxidant properties — most notably, it contains the antioxidant resveratrol. Starting in the 1990s, research began to hint that the compound might protect against heart disease, aging, and cancer, though much of this work was done in animals or test tubes.
The idea that red wine carries health benefits, however, has been called into question more recently. A recent meta-analysis, for instance, suggests that many previous studies touting the health benefits of more moderate drinking were likely biased, potentially leading to “misleading positive health associations.” And one recent study found that alcohol consumption, largely red wine and beer, at all levels was linked to an increased risk for cardiovascular disease.
Although wine’s health halo is dwindling, there might be an exception: Cancer risk.
Overall, research shows that even light to moderate drinking increases the risk for at least seven types of cancer, but when focusing on red wine, in particular, that risk calculus can look different.
“It’s very complicated and nuanced,” said Timothy Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, Boston. “And ‘complicated and nuanced’ doesn’t work very well in public health messages.”
The Knowns About Alcohol and Cancer Risk
Some things about the relationship between alcohol and cancer risk are crystal clear. “There’s no question that alcohol is a group 1 carcinogen,” Rebbeck said. “Alcohol can cause cancer.”
Groups including the International Agency for Research on Cancer (IARC) and American Cancer Society agree that alcohol use is an established cause of seven types of cancer: Those of the oral cavity, larynx, pharynx, esophagus (squamous cell carcinoma), liver (hepatocellular carcinoma), breast, and colon/rectum. Heavy drinking — at least 8 standard drinks a week for women and 15 for men — and binge drinking — 4 or more drinks in 2 hours for women and 5 or more for men — only amplify that risk. (A “standard” drink has 14 g of alcohol, which translates to a 5-oz glass of wine.)
“We’re most concerned about high-risk drinking — more than 2 drinks a day — and/or binge drinking,” said Noelle LoConte, MD, of the Division of Hematology, Medical Oncology and Palliative Care, University of Wisconsin School of Medicine and Public Health, Madison, who authored a 2018 statement on alcohol and cancer risk from the American Society of Clinical Oncology (ASCO).
Compared with not drinking, heavy drinking is linked with a roughly fivefold increase in the risk for oral cavity, pharyngeal, and esophageal cancers, and a 61% increase in the risk for breast cancer, according to LoConte and colleagues.
Things get murkier when it comes to moderate drinking — defined as up to 1 standard drink per day for women and 2 per day for men. There is evidence, LoConte said, that moderate drinking is associated with increased cancer risks, though the magnitude is generally much less than heavier drinking.
Cancer type also matters. One analysis found that the risk for breast cancer increased with even light to moderate alcohol consumption. Compared with no drinking, light to moderate drinking has also been linked to increased risks for oral cavity, pharynx, larynx, and esophageal cancers.
As for whether the type of alcoholic beverage matters, LoConte said, there’s no clear physiological reason that wine would be less risky than beer or liquor. Research indicates that ethanol is the problematic ingredient: Once ingested, it’s metabolized into acetaldehyde, a DNA-damaging substance that’s considered a probable human carcinogen. Ethanol can also alter circulating levels of estrogens and androgens, LoConte said, which is thought to drive its association with breast cancer risk.
“It likely doesn’t matter how you choose to get your ethanol,” she said. “It’s a question of volume.”
Hints That Wine Is an Outlier
Still, some studies suggest that how people ingest ethanol could make a difference.
A study published in August in JAMA Network Open is a case in point. The study found that, among older adults, light to heavy drinkers had an increased risk of dying from cancer, compared with occasional drinkers (though the increased risk among light to moderate drinkers occurred only among people who also had chronic health conditions, such as diabetes or high blood pressure, or were of lower socioeconomic status).
Wine drinkers fared differently. Most notably, drinkers who “preferred” wine — consuming over 80% of total ethanol from wine — or those who drank only with meals showed a small reduction in their risk for cancer mortality and all-cause mortality (hazard ratio [HR], 0.94 for both). The small protective association was somewhat stronger among people who reported both patterns (HR, 0.88), especially if they were of lower socioeconomic status (HR, 0.79).
The findings are in line with other research suggesting that wine drinkers may be outliers when it comes to cancer risk. A 2023 meta-analysis of 26 observational studies, for instance, found no association between wine consumption and any cancer type, with the caveat that there was «substantial» heterogeneity among the studies.
This heterogeneity caveat speaks to the inherent limitations of observational research, said Tim Stockwell, PhD, of the Canadian Institute for Substance Use Research, University of Victoria in British Columbia, Canada.
“Individual studies of alcohol and cancer risk do find differences by type of drink, or patterns of drinking,” Stockwell said. “But it’s so hard to unpack the confounding that goes along with the type of person who’s a wine drinker or a beer drinker or a spirit drinker. The beverage of choice seems to come with a lot of baggage.”
Compared with people who favor beer or liquor, he noted, wine aficionados are typically higher-income, exercise more often, smoke less, and have different diets, for example. The “best” studies, Rebbeck said, try to adjust for those differences, but it’s challenging.
The authors of the 2023 meta-analysis noted that “many components in wine could have anticarcinogenic effects” that theoretically could counter the ill effects of ethanol. Besides resveratrol, which is mainly found in red wine, the list includes anthocyanins, quercetin, and tannins. However, the authors also acknowledged that they couldn’t account for whether other lifestyle habits might explain why wine drinkers, overall, showed no increased cancer risks and sometimes lower risks.
Still, groups such as the IARC and ASCO hold that there is no known “safe” level, or type, of alcohol when it comes to cancer.
In the latest Canadian guidelines on alcohol use, the scientific panel calculated that people who have 6 drinks a week throughout adulthood (whatever the source of the alcohol) could shave 11 weeks from their life expectancy, on average, said Stockwell, who was on the guideline panel. Compare that with heavy drinking, where 4 drinks a day could rob the average person of 2 or 3 years. “If you’re drinking a lot, you could get huge benefits from cutting down,” Stockwell explained. “If you’re a moderate drinker, the benefits would obviously be less.”
Stockwell said that choices around drinking and breast cancer risk, specifically, can be “tough.” Unlike many of the other alcohol-associated cancers, he noted, breast cancer is common — so even small relative risk increases may be concerning. Based on a 2020 meta-analysis of 22 cohort studies, the risk for breast cancer rises by about 10%, on average, for every 10 g of alcohol a woman drinks per day. This study also found no evidence that wine is any different from other types of alcohol.
In real life, the calculus around wine consumption and cancer risk will probably vary widely from person to person, Rebbeck said. One woman with a family history of breast cancer might decide that having wine with dinner isn’t worth it. Another with the same family history might see that glass of wine as a stress reliever and opt to focus on other ways to reduce her breast cancer risk — by exercising and maintaining a healthy weight, for example.
“The bottom line is, in human studies, the data on light to moderate drinking and cancer are limited and messy, and you can’t draw firm conclusions from them,” Rebbeck said. “It probably raises risk in some people, but we don’t know who those people are. And the risk increases are relatively small.”
A Conversation Few Are Having
Even with many studies highlighting the connection between alcohol consumption and cancer risk, most people remain unaware about this risk.
A 2023 study by the National Cancer Institute found that only a minority of US adults knew that drinking alcohol is linked to increased cancer risk, and they were much less likely to say that was true of wine: Only 20% did, vs 31% who said that liquor can boost cancer risk. Meanwhile, 10% believed that wine helps prevent cancer. Other studies show that even among cancer survivors and patients undergoing active cancer treatment, many drink — often heavily.
“What we know right now is, physicians almost never talk about this,” LoConte said.
That could be due to time constraints, according to Rebbeck, or clinicians’ perceptions that the subject is too complicated and/or their own confusion about the data. There could also be some “cognitive dissonance” at play, LoConte noted, because many doctors drink alcohol.
It’s critical, she said, that conversations about drinking habits become “normalized,” and that should include informing patients that alcohol use is associated with certain cancers. Again, LoConte said, it’s high-risk drinking that’s most concerning and where reducing intake could have the biggest impact on cancer risk and other health outcomes.
“From a cancer prevention standpoint, it’s probably best not to drink,” she said. “But people don’t make choices based solely on cancer risk. We don’t want to come out with recommendations saying no one should drink. I don’t think the data support that, and people would buck against that advice.”
Rebbeck made a similar point. Even if there’s uncertainty about the risks for a daily glass of wine, he said, people can use that information to make decisions. “Everybody’s preferences and choices are going to be different,” Rebbeck said. “And that’s all we can really do.”
A version of this article appeared on Medscape.com.
Dementia Risk Higher for Stroke Survivors
TOPLINE:
Risk for dementia is nearly 80% higher in stroke survivors than in those without stroke, a new study reveals. The data suggest risk declines within 1 year after stroke but remains elevated for up to 20 years.
METHODOLOGY:
- Researchers conducted a population-wide analysis of over 15 million people in Canada between 2002 and 2022. The study focused on adults hospitalized for ischemic stroke, intracerebral hemorrhage, or acute myocardial infarction (AMI).
- Of 175,980 stroke survivors, 99% were matched 1:1 to residents without stroke on the basis of age, sex, rural residence, neighborhood deprivation, and vascular comorbidities. In addition, 90% of patients were matched to those with AMI.
- Incident dementia diagnoses were tracked starting 90 days after stroke until death, emigration, or the end of the study, using a validated algorithm based on hospitalization for dementia, prescriptions for cholinesterase inhibitors, or physician claims within 2 years.
- The mean follow-up duration was 5.6 years.
TAKEAWAY:
- Among stroke survivors, 19% were diagnosed with dementia vs 12.5% in the reference population. The dementia rate per 100 person-years was higher among stroke survivors than in the reference population over the entire follow-up period (3.34 vs 1.89).
- Over the entire study period, dementia was 76% more likely among stroke patients (hazard ratio [HR], 1.76; 95% CI, 1.73-1.79) and 82% more likely in the AMI cohort (HR, 1.82; 95% CI, 1.79-1.85) than in the reference population.
- Time-varying analysis revealed that dementia risk was highest within the first year after stroke, with a > 2.5-fold increase at 6 months (HR, 2.51; 95% CI, 2.42-2.59), which decreased to a 1.5-fold increase at 5 years (HR, 1.51; 95% CI, 1.48-1.56) but remained elevated compared with the reference population even 20 years after the index stroke.
- Recurrent stroke was associated with an approximately threefold increased risk for dementia (single recurrent stroke adjusted HR, 2.64; 95% CI, 2.54-2.74; multiple recurrent strokes adjusted HR, 3.05; 95% CI, 2.81-3.33).
IN PRACTICE:
“While much research has been focused on reducing the risk of a second stroke, our findings make it clear that more research also is needed on developing interventions to help prevent dementia after stroke,” lead author Raed A. Joundi, MD, DPhil, McMaster University, Hamilton, Ontario, Canada, said in a press release.
“There is a need to accelerate the implementation of promising interventions or multipronged approaches into large randomized controlled trials to lower the risk of dementia,” the investigators wrote.
SOURCE:
The study was published online on December 4 in Neurology.
LIMITATIONS:
The study’s limitations included reliance on administrative coding without imaging data, potential underestimation of mild dementia, and lack of granular information on stroke severity, disability, and prestroke cognitive decline. While adjustments were made for healthcare contact and secondary prevention medications, residual biases may have persisted.
DISCLOSURES:
This study received funding from the Canada Brain Research Fund, Heart & Stroke Foundation of Canada, and Canadian Stroke Consortium. Two authors hold awards and positions from national organizations and academic institutions in Canada. Additional details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Risk for dementia is nearly 80% higher in stroke survivors than in those without stroke, a new study reveals. The data suggest risk declines within 1 year after stroke but remains elevated for up to 20 years.
METHODOLOGY:
- Researchers conducted a population-wide analysis of over 15 million people in Canada between 2002 and 2022. The study focused on adults hospitalized for ischemic stroke, intracerebral hemorrhage, or acute myocardial infarction (AMI).
- Of 175,980 stroke survivors, 99% were matched 1:1 to residents without stroke on the basis of age, sex, rural residence, neighborhood deprivation, and vascular comorbidities. In addition, 90% of patients were matched to those with AMI.
- Incident dementia diagnoses were tracked starting 90 days after stroke until death, emigration, or the end of the study, using a validated algorithm based on hospitalization for dementia, prescriptions for cholinesterase inhibitors, or physician claims within 2 years.
- The mean follow-up duration was 5.6 years.
TAKEAWAY:
- Among stroke survivors, 19% were diagnosed with dementia vs 12.5% in the reference population. The dementia rate per 100 person-years was higher among stroke survivors than in the reference population over the entire follow-up period (3.34 vs 1.89).
- Over the entire study period, dementia was 76% more likely among stroke patients (hazard ratio [HR], 1.76; 95% CI, 1.73-1.79) and 82% more likely in the AMI cohort (HR, 1.82; 95% CI, 1.79-1.85) than in the reference population.
- Time-varying analysis revealed that dementia risk was highest within the first year after stroke, with a > 2.5-fold increase at 6 months (HR, 2.51; 95% CI, 2.42-2.59), which decreased to a 1.5-fold increase at 5 years (HR, 1.51; 95% CI, 1.48-1.56) but remained elevated compared with the reference population even 20 years after the index stroke.
- Recurrent stroke was associated with an approximately threefold increased risk for dementia (single recurrent stroke adjusted HR, 2.64; 95% CI, 2.54-2.74; multiple recurrent strokes adjusted HR, 3.05; 95% CI, 2.81-3.33).
IN PRACTICE:
“While much research has been focused on reducing the risk of a second stroke, our findings make it clear that more research also is needed on developing interventions to help prevent dementia after stroke,” lead author Raed A. Joundi, MD, DPhil, McMaster University, Hamilton, Ontario, Canada, said in a press release.
“There is a need to accelerate the implementation of promising interventions or multipronged approaches into large randomized controlled trials to lower the risk of dementia,” the investigators wrote.
SOURCE:
The study was published online on December 4 in Neurology.
LIMITATIONS:
The study’s limitations included reliance on administrative coding without imaging data, potential underestimation of mild dementia, and lack of granular information on stroke severity, disability, and prestroke cognitive decline. While adjustments were made for healthcare contact and secondary prevention medications, residual biases may have persisted.
DISCLOSURES:
This study received funding from the Canada Brain Research Fund, Heart & Stroke Foundation of Canada, and Canadian Stroke Consortium. Two authors hold awards and positions from national organizations and academic institutions in Canada. Additional details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Risk for dementia is nearly 80% higher in stroke survivors than in those without stroke, a new study reveals. The data suggest risk declines within 1 year after stroke but remains elevated for up to 20 years.
METHODOLOGY:
- Researchers conducted a population-wide analysis of over 15 million people in Canada between 2002 and 2022. The study focused on adults hospitalized for ischemic stroke, intracerebral hemorrhage, or acute myocardial infarction (AMI).
- Of 175,980 stroke survivors, 99% were matched 1:1 to residents without stroke on the basis of age, sex, rural residence, neighborhood deprivation, and vascular comorbidities. In addition, 90% of patients were matched to those with AMI.
- Incident dementia diagnoses were tracked starting 90 days after stroke until death, emigration, or the end of the study, using a validated algorithm based on hospitalization for dementia, prescriptions for cholinesterase inhibitors, or physician claims within 2 years.
- The mean follow-up duration was 5.6 years.
TAKEAWAY:
- Among stroke survivors, 19% were diagnosed with dementia vs 12.5% in the reference population. The dementia rate per 100 person-years was higher among stroke survivors than in the reference population over the entire follow-up period (3.34 vs 1.89).
- Over the entire study period, dementia was 76% more likely among stroke patients (hazard ratio [HR], 1.76; 95% CI, 1.73-1.79) and 82% more likely in the AMI cohort (HR, 1.82; 95% CI, 1.79-1.85) than in the reference population.
- Time-varying analysis revealed that dementia risk was highest within the first year after stroke, with a > 2.5-fold increase at 6 months (HR, 2.51; 95% CI, 2.42-2.59), which decreased to a 1.5-fold increase at 5 years (HR, 1.51; 95% CI, 1.48-1.56) but remained elevated compared with the reference population even 20 years after the index stroke.
- Recurrent stroke was associated with an approximately threefold increased risk for dementia (single recurrent stroke adjusted HR, 2.64; 95% CI, 2.54-2.74; multiple recurrent strokes adjusted HR, 3.05; 95% CI, 2.81-3.33).
IN PRACTICE:
“While much research has been focused on reducing the risk of a second stroke, our findings make it clear that more research also is needed on developing interventions to help prevent dementia after stroke,” lead author Raed A. Joundi, MD, DPhil, McMaster University, Hamilton, Ontario, Canada, said in a press release.
“There is a need to accelerate the implementation of promising interventions or multipronged approaches into large randomized controlled trials to lower the risk of dementia,” the investigators wrote.
SOURCE:
The study was published online on December 4 in Neurology.
LIMITATIONS:
The study’s limitations included reliance on administrative coding without imaging data, potential underestimation of mild dementia, and lack of granular information on stroke severity, disability, and prestroke cognitive decline. While adjustments were made for healthcare contact and secondary prevention medications, residual biases may have persisted.
DISCLOSURES:
This study received funding from the Canada Brain Research Fund, Heart & Stroke Foundation of Canada, and Canadian Stroke Consortium. Two authors hold awards and positions from national organizations and academic institutions in Canada. Additional details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
High Radon Levels Linked to Gestational Diabetes
New data link higher county-level radon exposure to gestational diabetes (GD) in women who haven’t previously given birth, emphasizing the need to consider environmental risks in maternal and fetal healthcare.
Yijia Zhang, PhD, with the Department of Obstetrics and Gynecology, Vagelos College of Physicians and Surgeons at Columbia University Irving Medical Center in New York, and colleagues found in a study of 9107 nulliparous pregnant women that those living in US counties with higher radon levels (2 picocuries [pCi]/L) had higher odds of developing GD than those in counties with lower (< 1 pCi/L) radon levels (odds ratio [OR], 1.37; 95% CI, 1.02-1.84.) The researchers used three radon categories, and the middle level was 1 to < 2 pCi/L.
Findings were published online on January 10 in JAMA Network Open. The researchers used data from The Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a multicenter, prospective cohort study that examines factors associated with pregnancy-related outcomes.
“To our knowledge, this is the first study to examine the association between radon exposure and the risk of GD,” the authors wrote.
GD Affects 10% of Pregnancies
GD affects about 10% of pregnancies every year in the United States, according to the Centers for Disease Control and Prevention, and can affect women and offspring long term as it raises mothers’ risk of type 2 diabetes and cardiovascular disease and raises the risk for childhood obesity. Radon exposure’s link with lung cancer risk has been well established, but its link to other health risks is uncertain, the authors note.
The authors said their findings are hypothesis-generating and said, “It is vital to conduct studies that incorporate individual-level indoor radon exposure data,” to get closer to understanding the underlying mechanisms.
Individual-Level Exposure Measures Needed
They note that the average radon level in a county might not reflect an individual’s exposure and individual-level residential factors involved with radon exposure, such as household mitigation, and whether a dwelling has a basement, for instance, “are crucial for enhancing the precision of exposure assessment.”
In an invited commentary, Alberto Ruano-Ravina, PhD, and Lucía Martín-Gisbert, MSc, both with the Department of Preventive Medicine and Public Health at the University of Santiago de Compostela in Galicia, Spain, also urged that individual-level studies be conducted to further investigate radon’s link to health risks, noting that “[r]adon is possibly the most prevalent indoor carcinogen to which human beings are exposed.”
“There is no reason for not having these studies once we have some evidence of an association from ecological studies,” they wrote. They point out that reliable radon assessments are easy and inexpensive.
“The potential association of radon exposure with gestational diabetes or any other disease should be better analyzed using exclusively radon-prone areas. An observance of a dose-response effect may be indicative of a causal relationship, and it could be easily evidenced in radon-prone areas should such a relationship exist,” the commenters wrote.
Such areas have low, medium, high, and extremely high concentration levels, the commenters wrote. Zhang’s team, they point out, had to use only three exposure levels because the number of residents in high-exposure areas (exceeding 3 pCi/L) was too small.
“It is time now to move forward and really understand the full implications of radon exposure for health,” they concluded.
One coauthor reported serving on the board of directors for Merck for Mothers and as a board member for March for Moms outside the submitted work. One coauthor reported grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health (NIH) during the conduct of the study. Four coauthors reported grants from the NIH during the conduct of the study. One coauthor reported grants from the NIH during the conduct of the study and being a cofounder of Naima Health and receiving personal fees from Organon outside the submitted work. Both commenters reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
New data link higher county-level radon exposure to gestational diabetes (GD) in women who haven’t previously given birth, emphasizing the need to consider environmental risks in maternal and fetal healthcare.
Yijia Zhang, PhD, with the Department of Obstetrics and Gynecology, Vagelos College of Physicians and Surgeons at Columbia University Irving Medical Center in New York, and colleagues found in a study of 9107 nulliparous pregnant women that those living in US counties with higher radon levels (2 picocuries [pCi]/L) had higher odds of developing GD than those in counties with lower (< 1 pCi/L) radon levels (odds ratio [OR], 1.37; 95% CI, 1.02-1.84.) The researchers used three radon categories, and the middle level was 1 to < 2 pCi/L.
Findings were published online on January 10 in JAMA Network Open. The researchers used data from The Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a multicenter, prospective cohort study that examines factors associated with pregnancy-related outcomes.
“To our knowledge, this is the first study to examine the association between radon exposure and the risk of GD,” the authors wrote.
GD Affects 10% of Pregnancies
GD affects about 10% of pregnancies every year in the United States, according to the Centers for Disease Control and Prevention, and can affect women and offspring long term as it raises mothers’ risk of type 2 diabetes and cardiovascular disease and raises the risk for childhood obesity. Radon exposure’s link with lung cancer risk has been well established, but its link to other health risks is uncertain, the authors note.
The authors said their findings are hypothesis-generating and said, “It is vital to conduct studies that incorporate individual-level indoor radon exposure data,” to get closer to understanding the underlying mechanisms.
Individual-Level Exposure Measures Needed
They note that the average radon level in a county might not reflect an individual’s exposure and individual-level residential factors involved with radon exposure, such as household mitigation, and whether a dwelling has a basement, for instance, “are crucial for enhancing the precision of exposure assessment.”
In an invited commentary, Alberto Ruano-Ravina, PhD, and Lucía Martín-Gisbert, MSc, both with the Department of Preventive Medicine and Public Health at the University of Santiago de Compostela in Galicia, Spain, also urged that individual-level studies be conducted to further investigate radon’s link to health risks, noting that “[r]adon is possibly the most prevalent indoor carcinogen to which human beings are exposed.”
“There is no reason for not having these studies once we have some evidence of an association from ecological studies,” they wrote. They point out that reliable radon assessments are easy and inexpensive.
“The potential association of radon exposure with gestational diabetes or any other disease should be better analyzed using exclusively radon-prone areas. An observance of a dose-response effect may be indicative of a causal relationship, and it could be easily evidenced in radon-prone areas should such a relationship exist,” the commenters wrote.
Such areas have low, medium, high, and extremely high concentration levels, the commenters wrote. Zhang’s team, they point out, had to use only three exposure levels because the number of residents in high-exposure areas (exceeding 3 pCi/L) was too small.
“It is time now to move forward and really understand the full implications of radon exposure for health,” they concluded.
One coauthor reported serving on the board of directors for Merck for Mothers and as a board member for March for Moms outside the submitted work. One coauthor reported grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health (NIH) during the conduct of the study. Four coauthors reported grants from the NIH during the conduct of the study. One coauthor reported grants from the NIH during the conduct of the study and being a cofounder of Naima Health and receiving personal fees from Organon outside the submitted work. Both commenters reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
New data link higher county-level radon exposure to gestational diabetes (GD) in women who haven’t previously given birth, emphasizing the need to consider environmental risks in maternal and fetal healthcare.
Yijia Zhang, PhD, with the Department of Obstetrics and Gynecology, Vagelos College of Physicians and Surgeons at Columbia University Irving Medical Center in New York, and colleagues found in a study of 9107 nulliparous pregnant women that those living in US counties with higher radon levels (2 picocuries [pCi]/L) had higher odds of developing GD than those in counties with lower (< 1 pCi/L) radon levels (odds ratio [OR], 1.37; 95% CI, 1.02-1.84.) The researchers used three radon categories, and the middle level was 1 to < 2 pCi/L.
Findings were published online on January 10 in JAMA Network Open. The researchers used data from The Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a multicenter, prospective cohort study that examines factors associated with pregnancy-related outcomes.
“To our knowledge, this is the first study to examine the association between radon exposure and the risk of GD,” the authors wrote.
GD Affects 10% of Pregnancies
GD affects about 10% of pregnancies every year in the United States, according to the Centers for Disease Control and Prevention, and can affect women and offspring long term as it raises mothers’ risk of type 2 diabetes and cardiovascular disease and raises the risk for childhood obesity. Radon exposure’s link with lung cancer risk has been well established, but its link to other health risks is uncertain, the authors note.
The authors said their findings are hypothesis-generating and said, “It is vital to conduct studies that incorporate individual-level indoor radon exposure data,” to get closer to understanding the underlying mechanisms.
Individual-Level Exposure Measures Needed
They note that the average radon level in a county might not reflect an individual’s exposure and individual-level residential factors involved with radon exposure, such as household mitigation, and whether a dwelling has a basement, for instance, “are crucial for enhancing the precision of exposure assessment.”
In an invited commentary, Alberto Ruano-Ravina, PhD, and Lucía Martín-Gisbert, MSc, both with the Department of Preventive Medicine and Public Health at the University of Santiago de Compostela in Galicia, Spain, also urged that individual-level studies be conducted to further investigate radon’s link to health risks, noting that “[r]adon is possibly the most prevalent indoor carcinogen to which human beings are exposed.”
“There is no reason for not having these studies once we have some evidence of an association from ecological studies,” they wrote. They point out that reliable radon assessments are easy and inexpensive.
“The potential association of radon exposure with gestational diabetes or any other disease should be better analyzed using exclusively radon-prone areas. An observance of a dose-response effect may be indicative of a causal relationship, and it could be easily evidenced in radon-prone areas should such a relationship exist,” the commenters wrote.
Such areas have low, medium, high, and extremely high concentration levels, the commenters wrote. Zhang’s team, they point out, had to use only three exposure levels because the number of residents in high-exposure areas (exceeding 3 pCi/L) was too small.
“It is time now to move forward and really understand the full implications of radon exposure for health,” they concluded.
One coauthor reported serving on the board of directors for Merck for Mothers and as a board member for March for Moms outside the submitted work. One coauthor reported grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health (NIH) during the conduct of the study. Four coauthors reported grants from the NIH during the conduct of the study. One coauthor reported grants from the NIH during the conduct of the study and being a cofounder of Naima Health and receiving personal fees from Organon outside the submitted work. Both commenters reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Nutrition, Drugs, or Bariatric Surgery: What’s the Best Approach for Sustained Weight Loss?
Given that more than 100 million US adults have obesity, including 22 million with severe obesity, physicians regularly see patients with the condition in their practices.
Fortunately, doctors have more tools than ever to help their patients. But the question remains: Which method is the safest and most effective? Is it diet and lifestyle changes, one of the recently approved anti-obesity medications (AOMs), bariatric surgery, or a combination approach?
There are no head-to-head trials comparing these three approaches, said Vanita Rahman, MD, clinic director of the Barnard Medical Center, Washington, DC, at the International Conference on Nutrition in Medicine, sponsored by the Physicians Committee for Responsible Medicine.
Instead, doctors must evaluate the merits and drawbacks of each intervention and decide with their patients which treatment is best for them, she told Medscape Medical News. When she sees patients, Rahman shares the pertinent research with them, so they are able to make an informed choice.
Looking at the Options
In her presentation at the conference, Rahman summarized the guidelines issued by the American Heart Association/American College of Cardiology/The Obesity Society for Management of Overweight and Obesity in Adults and the American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity, including lifestyle changes, AOMs, and bariatric surgery (Table 1).
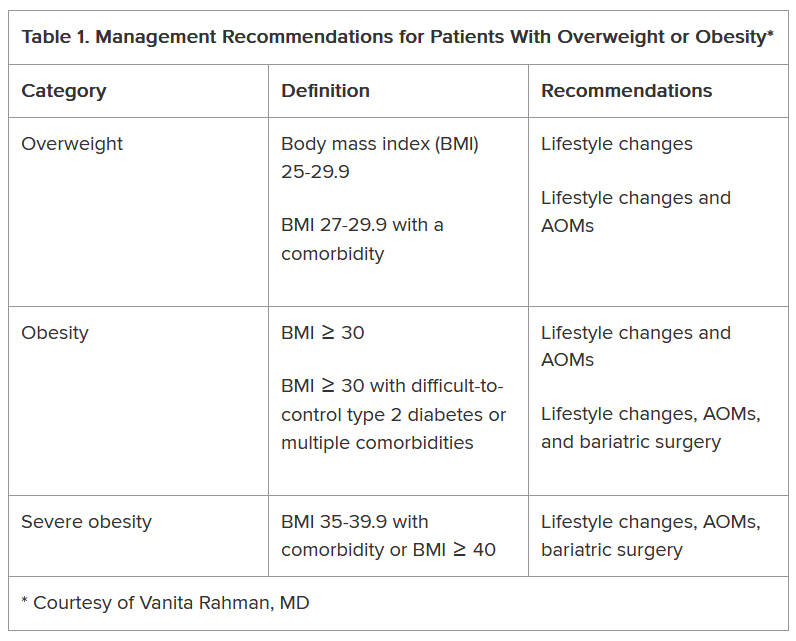
As shown, the current clinical guidelines offer recommendations that consider such factors as the patient’s BMI and presence of one or more comorbidities. Generally, they begin with lifestyle changes for people with overweight, the possibility of an AOM for those with obesity, and bariatric surgery as an option for those with severe obesity-related complications.
“In obesity, we traditionally thought the process was ‘either-or’ — either lifestyle or surgery or medication — and somehow lifestyle is better,” Sheethal Reddy, PhD, a psychologist at the Bariatric Center at Emory University Hospital Midtown, Atlanta, told Medscape Medical News.
Now physicians often use a combination of methods, but lifestyle is foundational to all of them, she said.
“If you don’t make lifestyle changes, none of the approaches will ultimately be effective,” said Reddy, who also is an assistant professor in the Division of General and GI Surgery at Emory School of Medicine, Atlanta.
Lifestyle changes don’t just involve diet and nutrition but include physical exercise.
“Being sedentary affects everything — sleep quality, appetite regulation, and metabolism. Without sufficient exercise, the body isn’t functioning well enough to have a healthy metabolism,” Reddy said.
How Durable Are the Interventions?
Although bariatric surgery has demonstrated effectiveness in helping patients lose weight, many of them regain some or most of it, Rahman said.
A systematic review and meta-analysis found weight regain in 49% of patients who underwent bariatric surgery patients, with the highest prevalence after Roux-en-Y gastric bypass.
Another study of approximately 45,000 patients who underwent bariatric surgery found differences not only in the percentage of total weight loss among Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric band procedures but also in how much of that weight stayed off between 1 and 5 years following the procedure (Table 2).
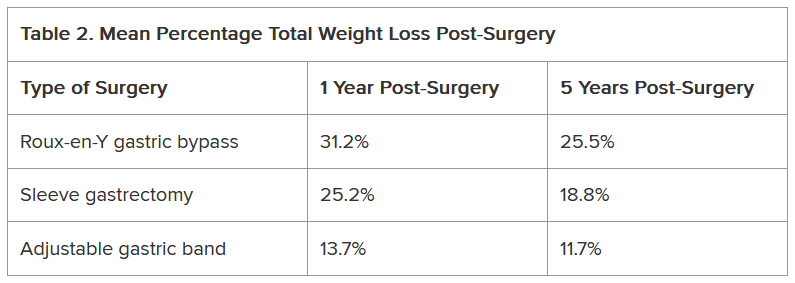
Weight regain also is a risk with AOMs, if they’re discontinued.
The STEP 1 trial tested the effectiveness of semaglutide — a glucagon-like peptide 1 (GLP-1) receptor agonist — as an adjunct to lifestyle intervention for weight loss in patients with obesity or with overweight and at least one comorbidity but not diabetes. Mean weight loss with semaglutide was 17.3% but that figure dropped 11.6 percentage points after treatment was discontinued.
Other studies also have found that patients regain weight after GLP-1 discontinuation.
Tirzepatide, a GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) combination, has shown efficacy with weight reduction, but patients experienced some weight regain upon discontinuation. In one study, patients experienced a mean weight loss of 20.9% after 36 weeks of tirzepatide. In the study’s subsequent 52-week double-blind, placebo-controlled period, patients who stopped taking the medication experienced a weight regain of 14%, whereas those who remained on the medication lost an additional 5.5% of weight.
GLP-1 and GLP-1/GIP medications do not address the factors that contribute to overweight and obesity, Rahman said. “They simply suppress the appetite; therefore, weight gain occurs after stopping them.”
Patients may stop taking anti-obesity drugs for a variety of reasons, including side effects. Rahman noted that the common side effects include nausea, vomiting, and constipation, whereas rare side effects include gastroparesis, gallbladder and biliary disease, thyroid cancer, and suicidal thoughts. GLP-1 and GLP-1/GIP medications also carry a risk for non-arteritic anterior ischemic optic neuropathy, she said.
Moreover, health insurance does not always cover these medications, which likely affects patient access to the drugs and compliance rates.
“Given the side effects and frequent lack of insurance coverage, significant questions remain about long-term safety and feasibility of these agents,” Rahman said.
What About Nutritional Approaches?
The lifestyle interventions in the semaglutide and tirzepatide studies included 500 kcal/d deficit diets, which is difficult for people to maintain, noted Rahman, who is the author of the book Simply Plant Based: Fabulous Food for a Healthy Life.
Additionally, bariatric surgery has been associated with long-term micronutrient deficiencies, including deficiencies in vitamins A, D, E, K, B1, and B12, as well as folate, iron, zinc, copper, selenium, and calcium, she said.
The best approach to food from a patient compliance standpoint and to avoid nutrient deficiencies is a whole-food, plant-based diet, Rahman said. She advocates this nutritional approach, along with physical activity, for patients regardless of whether they’ve selected lifestyle intervention alone or combined with an AOM or bariatric surgery to address obesity.
Rahman cited a 5-year heart disease study comparing an intensive lifestyle program involving a vegetarian diet, aerobic exercise, stress management training, smoking cessation, and group psychosocial support to treatment as usual. Patients in the lifestyle group lost 10.9 kg at 1 year and sustained weight loss of 5.8 kg at 5 years, whereas weight in the control group remained relatively unchanged from baseline.
She also pointed to the findings of a study of patients with obesity or with overweight and at least one comorbidity that compared standard care with a low-fat, whole-food, plant-based diet with vitamin B12 supplementation. At 6 months, mean BMI reduction was greater in the intervention group than the standard care group (−4.4 vs −0.4).
In her practice, Rahman has seen the benefits of a whole-food, plant-based diet for patients with obesity.
If people are committed to this type of dietary approach and are given the tools and resources to do it effectively, “their thinking changes, their taste buds change, and they grow to enjoy this new way of eating,” she said. “They see results, and it’s a lifestyle that can be sustained long-term.”
Addressing Drivers of Weight Gain
Patients also need help addressing the various factors that may contribute to overweight and obesity, including overconsumption of ultra-processed foods, substandard nutritional quality of restaurant foods, increasing portion sizes, distraction during eating, emotional eating, late-night eating, and cultural/traditional values surrounding food, Rahman noted.
Supatra Tovar, PsyD, RD, a clinical psychologist with a practice in Pasadena, California, agreed that identifying the reasons for weight gain is critical for treatment.
“If you’re not addressing underlying issues, such as a person’s relationship with food, behaviors around food, the tendency to mindlessly eat or emotionally eat or eat to seek comfort, the person’s weight problems won’t ultimately be fully solved by any of the three approaches — dieting, medications, or bariatric surgery,” she said.
Some of her patients “engage in extreme dieting and deprivation, and many who use medications or have had bariatric surgery hardly eat and often develop nutritional deficiencies,” said Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life.
The key to healthy and sustained weight loss is to “become attuned to the body’s signals, learn how to honor hunger, stop eating when satisfied, and eat more healthful foods, such as fruits and vegetables, whole grains, lean proteins — especially plant-based proteins — and the body gives signals that this is what it wants,” she said.
Tovar doesn’t give her clients a specific diet or set of portions.
“I teach them to listen to their bodies,” she said. “They’ve lost significant amounts of weight and continued to keep it off because they’ve done this kind of work.”
When Lifestyle Changes Aren’t Enough
For many patients, lifestyle interventions are insufficient to address the degree of overweight and obesity and common comorbidities, said W. Timothy Garvey, MD, associate director and professor, Department of Nutrition Sciences, School of Health Professions, University of Alabama at Birmingham.
“Of course, nutritional approaches are very important, not only for weight but also for general health-related reasons,” said Garvey, lead author of the 2016 American Association of Clinical Endocrinologists obesity guidelines. “We’ve seen that the Mediterranean and some plant-based diets can prevent progression from prediabetes to diabetes and improve other parameters that reflect metabolic health.”
However, it’s “not common that patients can follow these diets, lose weight, and keep it off,” Garvey cautioned. Up to 50% of weight that’s lost through lifestyle changes is typically regained by 1-year follow-up, with almost all remaining lost weight subsequently regained in the majority of individuals because the person “has to fight against pathophysiological process that drive weight regain,” he noted.
Weight-loss medications can address these pathophysiologic processes by “addressing interactions of satiety hormones with feeding centers in the brain, suppressing the appetite, and making it easier for patients to adhere to a reduced-calorie diet.”
Garvey views the weight-loss medications in the same light as drugs for diabetes and hypertension, in that people need to keep taking them to sustain the benefit.
There’s still a role for bariatric surgery because not everyone can tolerate the AOMs or achieve sufficient weight loss.
“Patients with very high BMI who have trouble ambulating might benefit from a combination of bariatric surgery and medication,” Garvey said.
While some side effects are associated with AOMs, being an “alarmist” about them can be detrimental to patients, he warned.
Rahman and Tovar are authors of books about weight loss. Reddy reported no relevant financial relationships. Garvey is a consultant on advisory boards for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, Fractyl Health, Alnylam Pharmaceuticals, Inogen, Zealand, Allurion, Carmot/Roche, Terns Pharmaceuticals, Neurocrine, Keros Therapeutics, and Regeneron. He is the site principal investigator for multi-centered clinical trials sponsored by his university and funded by Novo Nordisk, Eli Lilly, Epitomee, Neurovalens, and Pfizer. He serves as a consultant on the advisory board for the nonprofit Milken Foundation and is a member of the Data Monitoring Committee for phase 3 clinical trials conducted by Boehringer-Ingelheim and Eli Lilly.
A version of this article first appeared on Medscape.com.
Given that more than 100 million US adults have obesity, including 22 million with severe obesity, physicians regularly see patients with the condition in their practices.
Fortunately, doctors have more tools than ever to help their patients. But the question remains: Which method is the safest and most effective? Is it diet and lifestyle changes, one of the recently approved anti-obesity medications (AOMs), bariatric surgery, or a combination approach?
There are no head-to-head trials comparing these three approaches, said Vanita Rahman, MD, clinic director of the Barnard Medical Center, Washington, DC, at the International Conference on Nutrition in Medicine, sponsored by the Physicians Committee for Responsible Medicine.
Instead, doctors must evaluate the merits and drawbacks of each intervention and decide with their patients which treatment is best for them, she told Medscape Medical News. When she sees patients, Rahman shares the pertinent research with them, so they are able to make an informed choice.
Looking at the Options
In her presentation at the conference, Rahman summarized the guidelines issued by the American Heart Association/American College of Cardiology/The Obesity Society for Management of Overweight and Obesity in Adults and the American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity, including lifestyle changes, AOMs, and bariatric surgery (Table 1).

As shown, the current clinical guidelines offer recommendations that consider such factors as the patient’s BMI and presence of one or more comorbidities. Generally, they begin with lifestyle changes for people with overweight, the possibility of an AOM for those with obesity, and bariatric surgery as an option for those with severe obesity-related complications.
“In obesity, we traditionally thought the process was ‘either-or’ — either lifestyle or surgery or medication — and somehow lifestyle is better,” Sheethal Reddy, PhD, a psychologist at the Bariatric Center at Emory University Hospital Midtown, Atlanta, told Medscape Medical News.
Now physicians often use a combination of methods, but lifestyle is foundational to all of them, she said.
“If you don’t make lifestyle changes, none of the approaches will ultimately be effective,” said Reddy, who also is an assistant professor in the Division of General and GI Surgery at Emory School of Medicine, Atlanta.
Lifestyle changes don’t just involve diet and nutrition but include physical exercise.
“Being sedentary affects everything — sleep quality, appetite regulation, and metabolism. Without sufficient exercise, the body isn’t functioning well enough to have a healthy metabolism,” Reddy said.
How Durable Are the Interventions?
Although bariatric surgery has demonstrated effectiveness in helping patients lose weight, many of them regain some or most of it, Rahman said.
A systematic review and meta-analysis found weight regain in 49% of patients who underwent bariatric surgery patients, with the highest prevalence after Roux-en-Y gastric bypass.
Another study of approximately 45,000 patients who underwent bariatric surgery found differences not only in the percentage of total weight loss among Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric band procedures but also in how much of that weight stayed off between 1 and 5 years following the procedure (Table 2).

Weight regain also is a risk with AOMs, if they’re discontinued.
The STEP 1 trial tested the effectiveness of semaglutide — a glucagon-like peptide 1 (GLP-1) receptor agonist — as an adjunct to lifestyle intervention for weight loss in patients with obesity or with overweight and at least one comorbidity but not diabetes. Mean weight loss with semaglutide was 17.3% but that figure dropped 11.6 percentage points after treatment was discontinued.
Other studies also have found that patients regain weight after GLP-1 discontinuation.
Tirzepatide, a GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) combination, has shown efficacy with weight reduction, but patients experienced some weight regain upon discontinuation. In one study, patients experienced a mean weight loss of 20.9% after 36 weeks of tirzepatide. In the study’s subsequent 52-week double-blind, placebo-controlled period, patients who stopped taking the medication experienced a weight regain of 14%, whereas those who remained on the medication lost an additional 5.5% of weight.
GLP-1 and GLP-1/GIP medications do not address the factors that contribute to overweight and obesity, Rahman said. “They simply suppress the appetite; therefore, weight gain occurs after stopping them.”
Patients may stop taking anti-obesity drugs for a variety of reasons, including side effects. Rahman noted that the common side effects include nausea, vomiting, and constipation, whereas rare side effects include gastroparesis, gallbladder and biliary disease, thyroid cancer, and suicidal thoughts. GLP-1 and GLP-1/GIP medications also carry a risk for non-arteritic anterior ischemic optic neuropathy, she said.
Moreover, health insurance does not always cover these medications, which likely affects patient access to the drugs and compliance rates.
“Given the side effects and frequent lack of insurance coverage, significant questions remain about long-term safety and feasibility of these agents,” Rahman said.
What About Nutritional Approaches?
The lifestyle interventions in the semaglutide and tirzepatide studies included 500 kcal/d deficit diets, which is difficult for people to maintain, noted Rahman, who is the author of the book Simply Plant Based: Fabulous Food for a Healthy Life.
Additionally, bariatric surgery has been associated with long-term micronutrient deficiencies, including deficiencies in vitamins A, D, E, K, B1, and B12, as well as folate, iron, zinc, copper, selenium, and calcium, she said.
The best approach to food from a patient compliance standpoint and to avoid nutrient deficiencies is a whole-food, plant-based diet, Rahman said. She advocates this nutritional approach, along with physical activity, for patients regardless of whether they’ve selected lifestyle intervention alone or combined with an AOM or bariatric surgery to address obesity.
Rahman cited a 5-year heart disease study comparing an intensive lifestyle program involving a vegetarian diet, aerobic exercise, stress management training, smoking cessation, and group psychosocial support to treatment as usual. Patients in the lifestyle group lost 10.9 kg at 1 year and sustained weight loss of 5.8 kg at 5 years, whereas weight in the control group remained relatively unchanged from baseline.
She also pointed to the findings of a study of patients with obesity or with overweight and at least one comorbidity that compared standard care with a low-fat, whole-food, plant-based diet with vitamin B12 supplementation. At 6 months, mean BMI reduction was greater in the intervention group than the standard care group (−4.4 vs −0.4).
In her practice, Rahman has seen the benefits of a whole-food, plant-based diet for patients with obesity.
If people are committed to this type of dietary approach and are given the tools and resources to do it effectively, “their thinking changes, their taste buds change, and they grow to enjoy this new way of eating,” she said. “They see results, and it’s a lifestyle that can be sustained long-term.”
Addressing Drivers of Weight Gain
Patients also need help addressing the various factors that may contribute to overweight and obesity, including overconsumption of ultra-processed foods, substandard nutritional quality of restaurant foods, increasing portion sizes, distraction during eating, emotional eating, late-night eating, and cultural/traditional values surrounding food, Rahman noted.
Supatra Tovar, PsyD, RD, a clinical psychologist with a practice in Pasadena, California, agreed that identifying the reasons for weight gain is critical for treatment.
“If you’re not addressing underlying issues, such as a person’s relationship with food, behaviors around food, the tendency to mindlessly eat or emotionally eat or eat to seek comfort, the person’s weight problems won’t ultimately be fully solved by any of the three approaches — dieting, medications, or bariatric surgery,” she said.
Some of her patients “engage in extreme dieting and deprivation, and many who use medications or have had bariatric surgery hardly eat and often develop nutritional deficiencies,” said Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life.
The key to healthy and sustained weight loss is to “become attuned to the body’s signals, learn how to honor hunger, stop eating when satisfied, and eat more healthful foods, such as fruits and vegetables, whole grains, lean proteins — especially plant-based proteins — and the body gives signals that this is what it wants,” she said.
Tovar doesn’t give her clients a specific diet or set of portions.
“I teach them to listen to their bodies,” she said. “They’ve lost significant amounts of weight and continued to keep it off because they’ve done this kind of work.”
When Lifestyle Changes Aren’t Enough
For many patients, lifestyle interventions are insufficient to address the degree of overweight and obesity and common comorbidities, said W. Timothy Garvey, MD, associate director and professor, Department of Nutrition Sciences, School of Health Professions, University of Alabama at Birmingham.
“Of course, nutritional approaches are very important, not only for weight but also for general health-related reasons,” said Garvey, lead author of the 2016 American Association of Clinical Endocrinologists obesity guidelines. “We’ve seen that the Mediterranean and some plant-based diets can prevent progression from prediabetes to diabetes and improve other parameters that reflect metabolic health.”
However, it’s “not common that patients can follow these diets, lose weight, and keep it off,” Garvey cautioned. Up to 50% of weight that’s lost through lifestyle changes is typically regained by 1-year follow-up, with almost all remaining lost weight subsequently regained in the majority of individuals because the person “has to fight against pathophysiological process that drive weight regain,” he noted.
Weight-loss medications can address these pathophysiologic processes by “addressing interactions of satiety hormones with feeding centers in the brain, suppressing the appetite, and making it easier for patients to adhere to a reduced-calorie diet.”
Garvey views the weight-loss medications in the same light as drugs for diabetes and hypertension, in that people need to keep taking them to sustain the benefit.
There’s still a role for bariatric surgery because not everyone can tolerate the AOMs or achieve sufficient weight loss.
“Patients with very high BMI who have trouble ambulating might benefit from a combination of bariatric surgery and medication,” Garvey said.
While some side effects are associated with AOMs, being an “alarmist” about them can be detrimental to patients, he warned.
Rahman and Tovar are authors of books about weight loss. Reddy reported no relevant financial relationships. Garvey is a consultant on advisory boards for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, Fractyl Health, Alnylam Pharmaceuticals, Inogen, Zealand, Allurion, Carmot/Roche, Terns Pharmaceuticals, Neurocrine, Keros Therapeutics, and Regeneron. He is the site principal investigator for multi-centered clinical trials sponsored by his university and funded by Novo Nordisk, Eli Lilly, Epitomee, Neurovalens, and Pfizer. He serves as a consultant on the advisory board for the nonprofit Milken Foundation and is a member of the Data Monitoring Committee for phase 3 clinical trials conducted by Boehringer-Ingelheim and Eli Lilly.
A version of this article first appeared on Medscape.com.
Given that more than 100 million US adults have obesity, including 22 million with severe obesity, physicians regularly see patients with the condition in their practices.
Fortunately, doctors have more tools than ever to help their patients. But the question remains: Which method is the safest and most effective? Is it diet and lifestyle changes, one of the recently approved anti-obesity medications (AOMs), bariatric surgery, or a combination approach?
There are no head-to-head trials comparing these three approaches, said Vanita Rahman, MD, clinic director of the Barnard Medical Center, Washington, DC, at the International Conference on Nutrition in Medicine, sponsored by the Physicians Committee for Responsible Medicine.
Instead, doctors must evaluate the merits and drawbacks of each intervention and decide with their patients which treatment is best for them, she told Medscape Medical News. When she sees patients, Rahman shares the pertinent research with them, so they are able to make an informed choice.
Looking at the Options
In her presentation at the conference, Rahman summarized the guidelines issued by the American Heart Association/American College of Cardiology/The Obesity Society for Management of Overweight and Obesity in Adults and the American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity, including lifestyle changes, AOMs, and bariatric surgery (Table 1).

As shown, the current clinical guidelines offer recommendations that consider such factors as the patient’s BMI and presence of one or more comorbidities. Generally, they begin with lifestyle changes for people with overweight, the possibility of an AOM for those with obesity, and bariatric surgery as an option for those with severe obesity-related complications.
“In obesity, we traditionally thought the process was ‘either-or’ — either lifestyle or surgery or medication — and somehow lifestyle is better,” Sheethal Reddy, PhD, a psychologist at the Bariatric Center at Emory University Hospital Midtown, Atlanta, told Medscape Medical News.
Now physicians often use a combination of methods, but lifestyle is foundational to all of them, she said.
“If you don’t make lifestyle changes, none of the approaches will ultimately be effective,” said Reddy, who also is an assistant professor in the Division of General and GI Surgery at Emory School of Medicine, Atlanta.
Lifestyle changes don’t just involve diet and nutrition but include physical exercise.
“Being sedentary affects everything — sleep quality, appetite regulation, and metabolism. Without sufficient exercise, the body isn’t functioning well enough to have a healthy metabolism,” Reddy said.
How Durable Are the Interventions?
Although bariatric surgery has demonstrated effectiveness in helping patients lose weight, many of them regain some or most of it, Rahman said.
A systematic review and meta-analysis found weight regain in 49% of patients who underwent bariatric surgery patients, with the highest prevalence after Roux-en-Y gastric bypass.
Another study of approximately 45,000 patients who underwent bariatric surgery found differences not only in the percentage of total weight loss among Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric band procedures but also in how much of that weight stayed off between 1 and 5 years following the procedure (Table 2).

Weight regain also is a risk with AOMs, if they’re discontinued.
The STEP 1 trial tested the effectiveness of semaglutide — a glucagon-like peptide 1 (GLP-1) receptor agonist — as an adjunct to lifestyle intervention for weight loss in patients with obesity or with overweight and at least one comorbidity but not diabetes. Mean weight loss with semaglutide was 17.3% but that figure dropped 11.6 percentage points after treatment was discontinued.
Other studies also have found that patients regain weight after GLP-1 discontinuation.
Tirzepatide, a GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) combination, has shown efficacy with weight reduction, but patients experienced some weight regain upon discontinuation. In one study, patients experienced a mean weight loss of 20.9% after 36 weeks of tirzepatide. In the study’s subsequent 52-week double-blind, placebo-controlled period, patients who stopped taking the medication experienced a weight regain of 14%, whereas those who remained on the medication lost an additional 5.5% of weight.
GLP-1 and GLP-1/GIP medications do not address the factors that contribute to overweight and obesity, Rahman said. “They simply suppress the appetite; therefore, weight gain occurs after stopping them.”
Patients may stop taking anti-obesity drugs for a variety of reasons, including side effects. Rahman noted that the common side effects include nausea, vomiting, and constipation, whereas rare side effects include gastroparesis, gallbladder and biliary disease, thyroid cancer, and suicidal thoughts. GLP-1 and GLP-1/GIP medications also carry a risk for non-arteritic anterior ischemic optic neuropathy, she said.
Moreover, health insurance does not always cover these medications, which likely affects patient access to the drugs and compliance rates.
“Given the side effects and frequent lack of insurance coverage, significant questions remain about long-term safety and feasibility of these agents,” Rahman said.
What About Nutritional Approaches?
The lifestyle interventions in the semaglutide and tirzepatide studies included 500 kcal/d deficit diets, which is difficult for people to maintain, noted Rahman, who is the author of the book Simply Plant Based: Fabulous Food for a Healthy Life.
Additionally, bariatric surgery has been associated with long-term micronutrient deficiencies, including deficiencies in vitamins A, D, E, K, B1, and B12, as well as folate, iron, zinc, copper, selenium, and calcium, she said.
The best approach to food from a patient compliance standpoint and to avoid nutrient deficiencies is a whole-food, plant-based diet, Rahman said. She advocates this nutritional approach, along with physical activity, for patients regardless of whether they’ve selected lifestyle intervention alone or combined with an AOM or bariatric surgery to address obesity.
Rahman cited a 5-year heart disease study comparing an intensive lifestyle program involving a vegetarian diet, aerobic exercise, stress management training, smoking cessation, and group psychosocial support to treatment as usual. Patients in the lifestyle group lost 10.9 kg at 1 year and sustained weight loss of 5.8 kg at 5 years, whereas weight in the control group remained relatively unchanged from baseline.
She also pointed to the findings of a study of patients with obesity or with overweight and at least one comorbidity that compared standard care with a low-fat, whole-food, plant-based diet with vitamin B12 supplementation. At 6 months, mean BMI reduction was greater in the intervention group than the standard care group (−4.4 vs −0.4).
In her practice, Rahman has seen the benefits of a whole-food, plant-based diet for patients with obesity.
If people are committed to this type of dietary approach and are given the tools and resources to do it effectively, “their thinking changes, their taste buds change, and they grow to enjoy this new way of eating,” she said. “They see results, and it’s a lifestyle that can be sustained long-term.”
Addressing Drivers of Weight Gain
Patients also need help addressing the various factors that may contribute to overweight and obesity, including overconsumption of ultra-processed foods, substandard nutritional quality of restaurant foods, increasing portion sizes, distraction during eating, emotional eating, late-night eating, and cultural/traditional values surrounding food, Rahman noted.
Supatra Tovar, PsyD, RD, a clinical psychologist with a practice in Pasadena, California, agreed that identifying the reasons for weight gain is critical for treatment.
“If you’re not addressing underlying issues, such as a person’s relationship with food, behaviors around food, the tendency to mindlessly eat or emotionally eat or eat to seek comfort, the person’s weight problems won’t ultimately be fully solved by any of the three approaches — dieting, medications, or bariatric surgery,” she said.
Some of her patients “engage in extreme dieting and deprivation, and many who use medications or have had bariatric surgery hardly eat and often develop nutritional deficiencies,” said Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life.
The key to healthy and sustained weight loss is to “become attuned to the body’s signals, learn how to honor hunger, stop eating when satisfied, and eat more healthful foods, such as fruits and vegetables, whole grains, lean proteins — especially plant-based proteins — and the body gives signals that this is what it wants,” she said.
Tovar doesn’t give her clients a specific diet or set of portions.
“I teach them to listen to their bodies,” she said. “They’ve lost significant amounts of weight and continued to keep it off because they’ve done this kind of work.”
When Lifestyle Changes Aren’t Enough
For many patients, lifestyle interventions are insufficient to address the degree of overweight and obesity and common comorbidities, said W. Timothy Garvey, MD, associate director and professor, Department of Nutrition Sciences, School of Health Professions, University of Alabama at Birmingham.
“Of course, nutritional approaches are very important, not only for weight but also for general health-related reasons,” said Garvey, lead author of the 2016 American Association of Clinical Endocrinologists obesity guidelines. “We’ve seen that the Mediterranean and some plant-based diets can prevent progression from prediabetes to diabetes and improve other parameters that reflect metabolic health.”
However, it’s “not common that patients can follow these diets, lose weight, and keep it off,” Garvey cautioned. Up to 50% of weight that’s lost through lifestyle changes is typically regained by 1-year follow-up, with almost all remaining lost weight subsequently regained in the majority of individuals because the person “has to fight against pathophysiological process that drive weight regain,” he noted.
Weight-loss medications can address these pathophysiologic processes by “addressing interactions of satiety hormones with feeding centers in the brain, suppressing the appetite, and making it easier for patients to adhere to a reduced-calorie diet.”
Garvey views the weight-loss medications in the same light as drugs for diabetes and hypertension, in that people need to keep taking them to sustain the benefit.
There’s still a role for bariatric surgery because not everyone can tolerate the AOMs or achieve sufficient weight loss.
“Patients with very high BMI who have trouble ambulating might benefit from a combination of bariatric surgery and medication,” Garvey said.
While some side effects are associated with AOMs, being an “alarmist” about them can be detrimental to patients, he warned.
Rahman and Tovar are authors of books about weight loss. Reddy reported no relevant financial relationships. Garvey is a consultant on advisory boards for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, Fractyl Health, Alnylam Pharmaceuticals, Inogen, Zealand, Allurion, Carmot/Roche, Terns Pharmaceuticals, Neurocrine, Keros Therapeutics, and Regeneron. He is the site principal investigator for multi-centered clinical trials sponsored by his university and funded by Novo Nordisk, Eli Lilly, Epitomee, Neurovalens, and Pfizer. He serves as a consultant on the advisory board for the nonprofit Milken Foundation and is a member of the Data Monitoring Committee for phase 3 clinical trials conducted by Boehringer-Ingelheim and Eli Lilly.
A version of this article first appeared on Medscape.com.
Are Patients On GLP-1s Getting the Right Nutrients?
As the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) continues to exponentially expand obesity treatment, concerns have arisen regarding their impact on nutrition in people who take them.
While the medications’ dampening effects on appetite result in an average weight reduction ≥ 15%, they also pose a risk for malnutrition.
“It’s important to eat a balanced diet when taking these medications,” Deena Adimoolam, MD, an endocrinologist based in New York City and a member of the national advisory committees for the Endocrine Society and the American Diabetes Association, said in an interview.
The decreased caloric intake resulting from the use of GLP-1 RAs makes it essential for patients to consume nutrient-dense foods. Clinicians can help patients achieve a healthy diet by anticipating nutrition problems, advising them on recommended target ranges of nutrient intake, and referring them for appropriate counseling.
Where to Begin
The task begins with “setting the right expectations before the patient starts treatment,” said Scott Isaacs, MD, president-elect of the American Association of Clinical Endocrinology.
To that end, it’s important to explain to patients how the medications affect appetite and how to adapt. GLP-1 RAs don’t completely turn off the appetite, and the effect at the beginning will likely be very mild, Isaacs said in an interview.
Some patients don’t notice a change for 2-3 months, although others see an effect sooner.
“Typically, people will notice that the main impact is on satiation, meaning they’ll fill up more quickly,” said Isaacs, who is an adjunct associate professor at Emory University School of Medicine, Atlanta, Georgia. “It’s important to tell them to stop eating when they feel full because eating when full can increase the side effects, such as nausea, vomiting, diarrhea, and constipation.”
A review article, written by lead author Jaime Almandoz, MD, University of Texas Southwestern Medical Center, Dallas, in Obesity offers a “5 A’s model” as a guide on how to begin discussing overweight or obesity with patients. This involves asking for permission to discuss weight and asking about food and vitamin/supplement intake; assessing the patient’s medical history and root causes of obesity, and conducting a physical examination; advising the patient regarding treatment options and reasonable expectations; agreeing on treatment and lifestyle goals; and assisting the patient to address challenges, referring them as needed to for additional support (eg, a dietitian), as well as arranging for follow-up.
Impact of GLP-1 RAs on Food Preferences
Besides reducing hunger and increasing satiety, GLP-1 RAs may affect food preferences, according to a research review published in The International Journal of Obesity. It cites a 2014 study that found that people taking GLP-1 RAs displayed decreased neuronal responses to images of food measured by functional magnetic resonance imaging in the areas of brain associated with appetite and reward. This might affect taste preferences and food intake.
Additionally, a 2023 study suggested that during the weight-loss phase of treatment (as opposed to the maintenance phase), patients may experience reduced cravings for dairy and starchy food, less desire to eat salty or spicy foods, and less difficulty controlling eating and resisting cravings.
“Altered food preferences, decreased food cravings, and reduced food intake may contribute to long-term weight loss,” according to the research review. Tailored treatments focusing on the weight maintenance phase are needed, the authors wrote.
Are Patients Vulnerable to Malnutrition?
A recent review found that total caloric intake was reduced by 16%-39% in patients taking a GLP-1 RA or dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA, but few studies evaluated the composition of these patients’ diets. Research that examines the qualitative changes in macronutrient and micronutrient intake of patients on these medications is needed, the authors wrote.
They outlined several nutritional concerns, including whether GLP-1 RA or GIP/ GLP-1 RA use could result in protein intake insufficient for maintaining muscle strength, mass, and function or in inadequate dietary quality (ie, poor intake of micronutrients, fiber, and fluid).
“Although we don’t necessarily see ‘malnutrition’ in our practice, we do see patients who lose too much weight after months and months of treatment, patients who aren’t hungry and don’t eat all day and have one big meal at the end of the day because they don’t feel like eating, and people who continue to eat unhealthy foods,” Isaacs said.
Some patients, however, have medical histories placing them at a greater risk for malnutrition. “Identification of these individuals may help prevent more serious nutritional and medical complications that might occur with decreased food intake associated with AOMs [anti-obesity medications],” Almandoz and colleagues noted in their review.
What Should Patients Eat?
Nutritional needs vary based on the patient’s age, sex, body weight, physical activity, and other factors, Almandoz and colleagues wrote. For this reason, energy intake during weight loss should be “personalized.”
The authors also recommended specific sources of the various dietary components and noted red flags signaling potential deficiencies
Nutritional needs vary based on the degree of appetite suppression in the patient, Adimoolam said. “I recommend at least two servings of fruits and vegetables daily, and drinking plenty of water throughout the day,” she added.
Protein in particular is a “key macronutrient,” and insufficient intake can lead to a variety of adverse effects, including sarcopenia — which is already a concern in individuals being treated with GLP-1 RAs. Meal replacement products (eg, shakes or bars) can supplement diets to help meet protein needs, especially if appetite is significantly reduced.
“There are definitely concerns for sarcopenia, so we have our patients taking these drugs try to eat healthy lean proteins – 100 g/d — and exercise,” Isaacs said. Exercise, including resistance training, not only improves muscle mass but also potentiates the effects of the GLP-1 RAs in patients with obesity and type 2 diabetes.
Adequate hydration is essential for patients taking GLP-1 RAs. “One of the commonly described side effects is fatigue, but there’s no biological reason why these medications should cause fatigue. My opinion is that these patients are dehydrated, and that may be causing the fatigue,” Isaacs said.
Some patients taking GLP-1 RAs lose interest in food. Isaacs regarded this as an “adverse reaction to the medication, which necessitates either stopping it altogether, changing the dose, or adjusting the diet.” There are “many different solutions, and one size doesn’t fit all,” he said.
Dietary and Behavioral Counseling
The drugs don’t necessarily motivate a person to eat healthier food, only to eat less food, Isaacs noted.
“The person might be eating low-volume but high-calorie food, such as bag of chips or a cookie instead of an apple,” Isaacs said. Patients who are losing weight “may not realize that weight loss isn’t the only important outcome. Because they’re losing weight, they think it’s okay to eat junk food.”
Patients need education and guidance about how to eat while on these medications. Most patients find counseling about meal planning helpful, he said.
Isaacs gives nutritional guidance to his patients when he prescribes a weight loss medication. “But most physicians don’t have time to offer that type of specific counseling on an ongoing basis,” he said. Isaacs refers patients requiring more detailed and long-term guidance to a dietitian.
Patients with monotonous diets of poor quality are at increased risk for nutrition deficiencies, and counseling by a registered dietitian could help improve their dietary quality.
Registered dietitians can develop a multifaceted approach not only focusing on medication management but also on customizing the patient’s diet, assisting with lifestyle adjustments, and addressing the mental health issues surrounding obesity and its management.
People seeking obesity treatment often have psychiatric conditions, psychological distress, or disordered eating patterns, and questions and concerns have emerged about how GLP-1 RA use might affect existing mental health problems. For example, if the medication suppresses the feeling of gratification a person once got from eating high-energy dense foods, that individual may “seek rewards or pleasure elsewhere, and possibly from unhealthy sources.”
Psychological issues also may emerge as a result of weight loss, so it’s helpful to take a multidisciplinary approach that includes mental health practitioners to support patients who are being treated with GLP-1 RAs. Patients taking these agents should be monitored for the emergence or worsening of psychiatric conditions, such as depression and suicidal ideation.
Achieving significant weight loss may lead to “unexpected changes” in the dynamics of patients’ relationship with others, “which can be distressing.” Clinicians should be “sensitive to patients’ social and emotional needs” and provide support or refer patients for help with coping strategies.
GLP-1 RAs have enormous potential to improve health outcomes in patients with obesity. Careful patient selection, close monitoring, and support for patients with nutrition and other lifestyle issues can increase the chances that these agents will fulfill their potential.
Isaacs declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
As the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) continues to exponentially expand obesity treatment, concerns have arisen regarding their impact on nutrition in people who take them.
While the medications’ dampening effects on appetite result in an average weight reduction ≥ 15%, they also pose a risk for malnutrition.
“It’s important to eat a balanced diet when taking these medications,” Deena Adimoolam, MD, an endocrinologist based in New York City and a member of the national advisory committees for the Endocrine Society and the American Diabetes Association, said in an interview.
The decreased caloric intake resulting from the use of GLP-1 RAs makes it essential for patients to consume nutrient-dense foods. Clinicians can help patients achieve a healthy diet by anticipating nutrition problems, advising them on recommended target ranges of nutrient intake, and referring them for appropriate counseling.
Where to Begin
The task begins with “setting the right expectations before the patient starts treatment,” said Scott Isaacs, MD, president-elect of the American Association of Clinical Endocrinology.
To that end, it’s important to explain to patients how the medications affect appetite and how to adapt. GLP-1 RAs don’t completely turn off the appetite, and the effect at the beginning will likely be very mild, Isaacs said in an interview.
Some patients don’t notice a change for 2-3 months, although others see an effect sooner.
“Typically, people will notice that the main impact is on satiation, meaning they’ll fill up more quickly,” said Isaacs, who is an adjunct associate professor at Emory University School of Medicine, Atlanta, Georgia. “It’s important to tell them to stop eating when they feel full because eating when full can increase the side effects, such as nausea, vomiting, diarrhea, and constipation.”
A review article, written by lead author Jaime Almandoz, MD, University of Texas Southwestern Medical Center, Dallas, in Obesity offers a “5 A’s model” as a guide on how to begin discussing overweight or obesity with patients. This involves asking for permission to discuss weight and asking about food and vitamin/supplement intake; assessing the patient’s medical history and root causes of obesity, and conducting a physical examination; advising the patient regarding treatment options and reasonable expectations; agreeing on treatment and lifestyle goals; and assisting the patient to address challenges, referring them as needed to for additional support (eg, a dietitian), as well as arranging for follow-up.
Impact of GLP-1 RAs on Food Preferences
Besides reducing hunger and increasing satiety, GLP-1 RAs may affect food preferences, according to a research review published in The International Journal of Obesity. It cites a 2014 study that found that people taking GLP-1 RAs displayed decreased neuronal responses to images of food measured by functional magnetic resonance imaging in the areas of brain associated with appetite and reward. This might affect taste preferences and food intake.
Additionally, a 2023 study suggested that during the weight-loss phase of treatment (as opposed to the maintenance phase), patients may experience reduced cravings for dairy and starchy food, less desire to eat salty or spicy foods, and less difficulty controlling eating and resisting cravings.
“Altered food preferences, decreased food cravings, and reduced food intake may contribute to long-term weight loss,” according to the research review. Tailored treatments focusing on the weight maintenance phase are needed, the authors wrote.
Are Patients Vulnerable to Malnutrition?
A recent review found that total caloric intake was reduced by 16%-39% in patients taking a GLP-1 RA or dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA, but few studies evaluated the composition of these patients’ diets. Research that examines the qualitative changes in macronutrient and micronutrient intake of patients on these medications is needed, the authors wrote.
They outlined several nutritional concerns, including whether GLP-1 RA or GIP/ GLP-1 RA use could result in protein intake insufficient for maintaining muscle strength, mass, and function or in inadequate dietary quality (ie, poor intake of micronutrients, fiber, and fluid).
“Although we don’t necessarily see ‘malnutrition’ in our practice, we do see patients who lose too much weight after months and months of treatment, patients who aren’t hungry and don’t eat all day and have one big meal at the end of the day because they don’t feel like eating, and people who continue to eat unhealthy foods,” Isaacs said.
Some patients, however, have medical histories placing them at a greater risk for malnutrition. “Identification of these individuals may help prevent more serious nutritional and medical complications that might occur with decreased food intake associated with AOMs [anti-obesity medications],” Almandoz and colleagues noted in their review.
What Should Patients Eat?
Nutritional needs vary based on the patient’s age, sex, body weight, physical activity, and other factors, Almandoz and colleagues wrote. For this reason, energy intake during weight loss should be “personalized.”
The authors also recommended specific sources of the various dietary components and noted red flags signaling potential deficiencies
Nutritional needs vary based on the degree of appetite suppression in the patient, Adimoolam said. “I recommend at least two servings of fruits and vegetables daily, and drinking plenty of water throughout the day,” she added.
Protein in particular is a “key macronutrient,” and insufficient intake can lead to a variety of adverse effects, including sarcopenia — which is already a concern in individuals being treated with GLP-1 RAs. Meal replacement products (eg, shakes or bars) can supplement diets to help meet protein needs, especially if appetite is significantly reduced.
“There are definitely concerns for sarcopenia, so we have our patients taking these drugs try to eat healthy lean proteins – 100 g/d — and exercise,” Isaacs said. Exercise, including resistance training, not only improves muscle mass but also potentiates the effects of the GLP-1 RAs in patients with obesity and type 2 diabetes.
Adequate hydration is essential for patients taking GLP-1 RAs. “One of the commonly described side effects is fatigue, but there’s no biological reason why these medications should cause fatigue. My opinion is that these patients are dehydrated, and that may be causing the fatigue,” Isaacs said.
Some patients taking GLP-1 RAs lose interest in food. Isaacs regarded this as an “adverse reaction to the medication, which necessitates either stopping it altogether, changing the dose, or adjusting the diet.” There are “many different solutions, and one size doesn’t fit all,” he said.
Dietary and Behavioral Counseling
The drugs don’t necessarily motivate a person to eat healthier food, only to eat less food, Isaacs noted.
“The person might be eating low-volume but high-calorie food, such as bag of chips or a cookie instead of an apple,” Isaacs said. Patients who are losing weight “may not realize that weight loss isn’t the only important outcome. Because they’re losing weight, they think it’s okay to eat junk food.”
Patients need education and guidance about how to eat while on these medications. Most patients find counseling about meal planning helpful, he said.
Isaacs gives nutritional guidance to his patients when he prescribes a weight loss medication. “But most physicians don’t have time to offer that type of specific counseling on an ongoing basis,” he said. Isaacs refers patients requiring more detailed and long-term guidance to a dietitian.
Patients with monotonous diets of poor quality are at increased risk for nutrition deficiencies, and counseling by a registered dietitian could help improve their dietary quality.
Registered dietitians can develop a multifaceted approach not only focusing on medication management but also on customizing the patient’s diet, assisting with lifestyle adjustments, and addressing the mental health issues surrounding obesity and its management.
People seeking obesity treatment often have psychiatric conditions, psychological distress, or disordered eating patterns, and questions and concerns have emerged about how GLP-1 RA use might affect existing mental health problems. For example, if the medication suppresses the feeling of gratification a person once got from eating high-energy dense foods, that individual may “seek rewards or pleasure elsewhere, and possibly from unhealthy sources.”
Psychological issues also may emerge as a result of weight loss, so it’s helpful to take a multidisciplinary approach that includes mental health practitioners to support patients who are being treated with GLP-1 RAs. Patients taking these agents should be monitored for the emergence or worsening of psychiatric conditions, such as depression and suicidal ideation.
Achieving significant weight loss may lead to “unexpected changes” in the dynamics of patients’ relationship with others, “which can be distressing.” Clinicians should be “sensitive to patients’ social and emotional needs” and provide support or refer patients for help with coping strategies.
GLP-1 RAs have enormous potential to improve health outcomes in patients with obesity. Careful patient selection, close monitoring, and support for patients with nutrition and other lifestyle issues can increase the chances that these agents will fulfill their potential.
Isaacs declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
As the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) continues to exponentially expand obesity treatment, concerns have arisen regarding their impact on nutrition in people who take them.
While the medications’ dampening effects on appetite result in an average weight reduction ≥ 15%, they also pose a risk for malnutrition.
“It’s important to eat a balanced diet when taking these medications,” Deena Adimoolam, MD, an endocrinologist based in New York City and a member of the national advisory committees for the Endocrine Society and the American Diabetes Association, said in an interview.
The decreased caloric intake resulting from the use of GLP-1 RAs makes it essential for patients to consume nutrient-dense foods. Clinicians can help patients achieve a healthy diet by anticipating nutrition problems, advising them on recommended target ranges of nutrient intake, and referring them for appropriate counseling.
Where to Begin
The task begins with “setting the right expectations before the patient starts treatment,” said Scott Isaacs, MD, president-elect of the American Association of Clinical Endocrinology.
To that end, it’s important to explain to patients how the medications affect appetite and how to adapt. GLP-1 RAs don’t completely turn off the appetite, and the effect at the beginning will likely be very mild, Isaacs said in an interview.
Some patients don’t notice a change for 2-3 months, although others see an effect sooner.
“Typically, people will notice that the main impact is on satiation, meaning they’ll fill up more quickly,” said Isaacs, who is an adjunct associate professor at Emory University School of Medicine, Atlanta, Georgia. “It’s important to tell them to stop eating when they feel full because eating when full can increase the side effects, such as nausea, vomiting, diarrhea, and constipation.”
A review article, written by lead author Jaime Almandoz, MD, University of Texas Southwestern Medical Center, Dallas, in Obesity offers a “5 A’s model” as a guide on how to begin discussing overweight or obesity with patients. This involves asking for permission to discuss weight and asking about food and vitamin/supplement intake; assessing the patient’s medical history and root causes of obesity, and conducting a physical examination; advising the patient regarding treatment options and reasonable expectations; agreeing on treatment and lifestyle goals; and assisting the patient to address challenges, referring them as needed to for additional support (eg, a dietitian), as well as arranging for follow-up.
Impact of GLP-1 RAs on Food Preferences
Besides reducing hunger and increasing satiety, GLP-1 RAs may affect food preferences, according to a research review published in The International Journal of Obesity. It cites a 2014 study that found that people taking GLP-1 RAs displayed decreased neuronal responses to images of food measured by functional magnetic resonance imaging in the areas of brain associated with appetite and reward. This might affect taste preferences and food intake.
Additionally, a 2023 study suggested that during the weight-loss phase of treatment (as opposed to the maintenance phase), patients may experience reduced cravings for dairy and starchy food, less desire to eat salty or spicy foods, and less difficulty controlling eating and resisting cravings.
“Altered food preferences, decreased food cravings, and reduced food intake may contribute to long-term weight loss,” according to the research review. Tailored treatments focusing on the weight maintenance phase are needed, the authors wrote.
Are Patients Vulnerable to Malnutrition?
A recent review found that total caloric intake was reduced by 16%-39% in patients taking a GLP-1 RA or dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA, but few studies evaluated the composition of these patients’ diets. Research that examines the qualitative changes in macronutrient and micronutrient intake of patients on these medications is needed, the authors wrote.
They outlined several nutritional concerns, including whether GLP-1 RA or GIP/ GLP-1 RA use could result in protein intake insufficient for maintaining muscle strength, mass, and function or in inadequate dietary quality (ie, poor intake of micronutrients, fiber, and fluid).
“Although we don’t necessarily see ‘malnutrition’ in our practice, we do see patients who lose too much weight after months and months of treatment, patients who aren’t hungry and don’t eat all day and have one big meal at the end of the day because they don’t feel like eating, and people who continue to eat unhealthy foods,” Isaacs said.
Some patients, however, have medical histories placing them at a greater risk for malnutrition. “Identification of these individuals may help prevent more serious nutritional and medical complications that might occur with decreased food intake associated with AOMs [anti-obesity medications],” Almandoz and colleagues noted in their review.
What Should Patients Eat?
Nutritional needs vary based on the patient’s age, sex, body weight, physical activity, and other factors, Almandoz and colleagues wrote. For this reason, energy intake during weight loss should be “personalized.”
The authors also recommended specific sources of the various dietary components and noted red flags signaling potential deficiencies
Nutritional needs vary based on the degree of appetite suppression in the patient, Adimoolam said. “I recommend at least two servings of fruits and vegetables daily, and drinking plenty of water throughout the day,” she added.
Protein in particular is a “key macronutrient,” and insufficient intake can lead to a variety of adverse effects, including sarcopenia — which is already a concern in individuals being treated with GLP-1 RAs. Meal replacement products (eg, shakes or bars) can supplement diets to help meet protein needs, especially if appetite is significantly reduced.
“There are definitely concerns for sarcopenia, so we have our patients taking these drugs try to eat healthy lean proteins – 100 g/d — and exercise,” Isaacs said. Exercise, including resistance training, not only improves muscle mass but also potentiates the effects of the GLP-1 RAs in patients with obesity and type 2 diabetes.
Adequate hydration is essential for patients taking GLP-1 RAs. “One of the commonly described side effects is fatigue, but there’s no biological reason why these medications should cause fatigue. My opinion is that these patients are dehydrated, and that may be causing the fatigue,” Isaacs said.
Some patients taking GLP-1 RAs lose interest in food. Isaacs regarded this as an “adverse reaction to the medication, which necessitates either stopping it altogether, changing the dose, or adjusting the diet.” There are “many different solutions, and one size doesn’t fit all,” he said.
Dietary and Behavioral Counseling
The drugs don’t necessarily motivate a person to eat healthier food, only to eat less food, Isaacs noted.
“The person might be eating low-volume but high-calorie food, such as bag of chips or a cookie instead of an apple,” Isaacs said. Patients who are losing weight “may not realize that weight loss isn’t the only important outcome. Because they’re losing weight, they think it’s okay to eat junk food.”
Patients need education and guidance about how to eat while on these medications. Most patients find counseling about meal planning helpful, he said.
Isaacs gives nutritional guidance to his patients when he prescribes a weight loss medication. “But most physicians don’t have time to offer that type of specific counseling on an ongoing basis,” he said. Isaacs refers patients requiring more detailed and long-term guidance to a dietitian.
Patients with monotonous diets of poor quality are at increased risk for nutrition deficiencies, and counseling by a registered dietitian could help improve their dietary quality.
Registered dietitians can develop a multifaceted approach not only focusing on medication management but also on customizing the patient’s diet, assisting with lifestyle adjustments, and addressing the mental health issues surrounding obesity and its management.
People seeking obesity treatment often have psychiatric conditions, psychological distress, or disordered eating patterns, and questions and concerns have emerged about how GLP-1 RA use might affect existing mental health problems. For example, if the medication suppresses the feeling of gratification a person once got from eating high-energy dense foods, that individual may “seek rewards or pleasure elsewhere, and possibly from unhealthy sources.”
Psychological issues also may emerge as a result of weight loss, so it’s helpful to take a multidisciplinary approach that includes mental health practitioners to support patients who are being treated with GLP-1 RAs. Patients taking these agents should be monitored for the emergence or worsening of psychiatric conditions, such as depression and suicidal ideation.
Achieving significant weight loss may lead to “unexpected changes” in the dynamics of patients’ relationship with others, “which can be distressing.” Clinicians should be “sensitive to patients’ social and emotional needs” and provide support or refer patients for help with coping strategies.
GLP-1 RAs have enormous potential to improve health outcomes in patients with obesity. Careful patient selection, close monitoring, and support for patients with nutrition and other lifestyle issues can increase the chances that these agents will fulfill their potential.
Isaacs declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
High-Dose Atropine Curbs Myopia in Kids Despite Side Effects
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of the 3-year results of the MOSAIC trial to investigate the efficacy and safety of different atropine regimens in treatment-naive children aged 6-16 years with a spherical equivalent ≤ −0.50 diopters (D).
- They analyzed data of 199 children in Europe with myopia (mean age, 13.9 years; 60.8% girls) who were randomly assigned to either group 1 (nightly placebo for 2 years followed by 0.05% atropine eye drops for 1 year; n = 66) or group 2 (nightly 0.01% atropine eye drops for 2 years followed by another random assignment to nightly placebo, tapering placebo, or tapering of 0.01% atropine eye drops for 1 year; n = 133).
- The nightly and tapered placebo groups were combined as a single treatment group for the sake of analysis.
- The primary outcome measures included observed changes in the progression of myopia, assessed using cycloplegic spherical equivalent refraction and axial length from month 24 to month 36.
TAKEAWAY:
- Children in the 0.01% atropine then placebo groups showed greater spherical equivalent progression (adjusted difference, –0.13 D; P = .01) and axial elongation (adjusted difference, 0.06 mm; P = .008) than those in the placebo then 0.05% atropine group.
- Children in the placebo then 0.05% atropine group also experienced less axial elongation (P = .04) than those in the 0.01% atropine then tapering 0.01% atropine group.
- Among participants using 0.05% atropine, 15% reported blurred near vision and 8% reported photophobia, whereas 3% reported blurred near vision and 0% reported photophobia in the 0.01% atropine then tapering 0.01% atropine group.
- Despite experiencing adverse events, no participants in the placebo then 0.05% atropine group discontinued treatment, with 92% completing the 36-month visit and 81% adhering to the treatment regimen.
IN PRACTICE:
“Recognizing a 2-year delay in treatment initiation in the group of children originally assigned to placebo, 0.05% atropine eyedrops slowed both myopia progression and axial eye growth over the course of a 1-year period,” the authors of the study wrote.
SOURCE:
This study was led by James Loughman, PhD, of the Centre for Eye Research Ireland, Dublin. It was published online in JAMA Ophthalmology.
LIMITATIONS:
Limitations included smaller sample sizes across treatment groups in year 3 and potential carry-over effects for participants transitioning from 0.01% atropine to placebo or tapered dosing. Because the study lacked an untreated control group, rebound myopia progression could be measured based only on the expected third-year results from the 0.01% atropine then placebo groups. The age of participants during the third year may have affected the ability to detect rebound progression.
DISCLOSURES:
This study was supported partly by a grant from the Health Research Board; Fighting Blindness, Ireland; and Vyluma. Some authors reported receiving grants, nonfinancial support, or consultant fees or having several other ties with Vyluma and other sources.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of the 3-year results of the MOSAIC trial to investigate the efficacy and safety of different atropine regimens in treatment-naive children aged 6-16 years with a spherical equivalent ≤ −0.50 diopters (D).
- They analyzed data of 199 children in Europe with myopia (mean age, 13.9 years; 60.8% girls) who were randomly assigned to either group 1 (nightly placebo for 2 years followed by 0.05% atropine eye drops for 1 year; n = 66) or group 2 (nightly 0.01% atropine eye drops for 2 years followed by another random assignment to nightly placebo, tapering placebo, or tapering of 0.01% atropine eye drops for 1 year; n = 133).
- The nightly and tapered placebo groups were combined as a single treatment group for the sake of analysis.
- The primary outcome measures included observed changes in the progression of myopia, assessed using cycloplegic spherical equivalent refraction and axial length from month 24 to month 36.
TAKEAWAY:
- Children in the 0.01% atropine then placebo groups showed greater spherical equivalent progression (adjusted difference, –0.13 D; P = .01) and axial elongation (adjusted difference, 0.06 mm; P = .008) than those in the placebo then 0.05% atropine group.
- Children in the placebo then 0.05% atropine group also experienced less axial elongation (P = .04) than those in the 0.01% atropine then tapering 0.01% atropine group.
- Among participants using 0.05% atropine, 15% reported blurred near vision and 8% reported photophobia, whereas 3% reported blurred near vision and 0% reported photophobia in the 0.01% atropine then tapering 0.01% atropine group.
- Despite experiencing adverse events, no participants in the placebo then 0.05% atropine group discontinued treatment, with 92% completing the 36-month visit and 81% adhering to the treatment regimen.
IN PRACTICE:
“Recognizing a 2-year delay in treatment initiation in the group of children originally assigned to placebo, 0.05% atropine eyedrops slowed both myopia progression and axial eye growth over the course of a 1-year period,” the authors of the study wrote.
SOURCE:
This study was led by James Loughman, PhD, of the Centre for Eye Research Ireland, Dublin. It was published online in JAMA Ophthalmology.
LIMITATIONS:
Limitations included smaller sample sizes across treatment groups in year 3 and potential carry-over effects for participants transitioning from 0.01% atropine to placebo or tapered dosing. Because the study lacked an untreated control group, rebound myopia progression could be measured based only on the expected third-year results from the 0.01% atropine then placebo groups. The age of participants during the third year may have affected the ability to detect rebound progression.
DISCLOSURES:
This study was supported partly by a grant from the Health Research Board; Fighting Blindness, Ireland; and Vyluma. Some authors reported receiving grants, nonfinancial support, or consultant fees or having several other ties with Vyluma and other sources.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of the 3-year results of the MOSAIC trial to investigate the efficacy and safety of different atropine regimens in treatment-naive children aged 6-16 years with a spherical equivalent ≤ −0.50 diopters (D).
- They analyzed data of 199 children in Europe with myopia (mean age, 13.9 years; 60.8% girls) who were randomly assigned to either group 1 (nightly placebo for 2 years followed by 0.05% atropine eye drops for 1 year; n = 66) or group 2 (nightly 0.01% atropine eye drops for 2 years followed by another random assignment to nightly placebo, tapering placebo, or tapering of 0.01% atropine eye drops for 1 year; n = 133).
- The nightly and tapered placebo groups were combined as a single treatment group for the sake of analysis.
- The primary outcome measures included observed changes in the progression of myopia, assessed using cycloplegic spherical equivalent refraction and axial length from month 24 to month 36.
TAKEAWAY:
- Children in the 0.01% atropine then placebo groups showed greater spherical equivalent progression (adjusted difference, –0.13 D; P = .01) and axial elongation (adjusted difference, 0.06 mm; P = .008) than those in the placebo then 0.05% atropine group.
- Children in the placebo then 0.05% atropine group also experienced less axial elongation (P = .04) than those in the 0.01% atropine then tapering 0.01% atropine group.
- Among participants using 0.05% atropine, 15% reported blurred near vision and 8% reported photophobia, whereas 3% reported blurred near vision and 0% reported photophobia in the 0.01% atropine then tapering 0.01% atropine group.
- Despite experiencing adverse events, no participants in the placebo then 0.05% atropine group discontinued treatment, with 92% completing the 36-month visit and 81% adhering to the treatment regimen.
IN PRACTICE:
“Recognizing a 2-year delay in treatment initiation in the group of children originally assigned to placebo, 0.05% atropine eyedrops slowed both myopia progression and axial eye growth over the course of a 1-year period,” the authors of the study wrote.
SOURCE:
This study was led by James Loughman, PhD, of the Centre for Eye Research Ireland, Dublin. It was published online in JAMA Ophthalmology.
LIMITATIONS:
Limitations included smaller sample sizes across treatment groups in year 3 and potential carry-over effects for participants transitioning from 0.01% atropine to placebo or tapered dosing. Because the study lacked an untreated control group, rebound myopia progression could be measured based only on the expected third-year results from the 0.01% atropine then placebo groups. The age of participants during the third year may have affected the ability to detect rebound progression.
DISCLOSURES:
This study was supported partly by a grant from the Health Research Board; Fighting Blindness, Ireland; and Vyluma. Some authors reported receiving grants, nonfinancial support, or consultant fees or having several other ties with Vyluma and other sources.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
