User login
Victorious endurance: To pass the breaking point and not break
I’ve been thinking a lot about endurance recently.
COVID-19 is surging in the United States. Health care workers exhausted from the first and second waves are quickly reaching the verge of collapse. I’m seeing more and more heartbreaking articles about the bone-deep fatigue, fear, and frustration health care workers are facing, and I weep. As horrible as it is to be fighting this terrifying, little-understood, invisible virus, health care workers are also fighting an equally distressing war against misinformation, recklessness, apathy, and outright denial.
As if that wasn’t enough, we are also dealing with racial and social unrest not seen in decades. The most significant cultural divisions and political animosity perhaps since the Civil War. A contested election. The fraying of our democratic institutions and our standing in the global community. The weakest economy since the Great Depression. Record unemployment. Many individuals and families facing or already experiencing eviction and food insecurity. Record-setting fires, hurricanes, and other natural disasters that are only projected to intensify due to climate change.
That’s a lot to endure. And we don’t have much choice other than to live through it. Some of us will break under the strain; others will disengage by giving up clinical work or even leaving health care altogether. Some of us will pack it in and retire, walk away from relationships with family members or longtime friends, or even emigrate to another country (New Zealand, anyone?). Some of us will passively hunker down, letting the challenges of this time overwhelm us and just hoping we can hang on long enough to emerge, albeit beaten and scarred, on the other side.
But some of us will experience victorious endurance – the kind that doesn’t just accept suffering but finds a way to triumph over it. I came across the concept of victorious endurance in the Bible, but its origin is earlier, from classical Greece. It comes from the ancient Greek word hupomone, which literally means “abiding under” – as in disciplining oneself to bear up under a trial when one would more naturally rebel, or just give up. The ancient Greeks were big on virtues like self-control, long-suffering, and perseverance in the face of seemingly insurmountable difficulties; Odysseus was a poster child for hupomone. I believe the concept of victorious endurance can be applicable for people across many belief systems, philosophies, and ways of life.
The late William Barclay, former professor of divinity and biblical criticism at the University of Glasgow, Scotland, said of hupomone:
It is untranslatable. It does not describe the frame of mind which can sit down with folded hands and bowed head and let a torrent of troubles sweep over it in passive resignation. It describes the ability to bear things in such a triumphant way that it transfigures them. Chrysostom has a great panegyric on this hupomone. He calls it “the root of all goods, the mother of piety, the fruit that never withers, a fortress that is never taken, a harbour that knows no storms” and “the queen of virtues, the foundation of right actions, peace in war, calm in tempest, security in plots.” It is the courageous and triumphant ability to pass the breaking-point and not to break and always to greet the unseen with a cheer. It is the alchemy which transmutes tribulation into strength and glory.
Barclay further noted that “Cicero defines patientia, its Latin equivalent, as: ‘The voluntary and daily suffering of hard and difficult things, for the sake of honour and usefulness.”
In the midst of the most challenging public health emergency of our lifetimes, I am seeing hospitalists – and nurses, respiratory therapists, and countless other health care workers – doing exactly this, every day. I’m so incredibly proud of you all, and thankful beyond words.
I doubt that victorious endurance comes naturally to any of us; it’s something we work at, pursue and nurture. What’s the secret to cultivating victorious endurance in the midst of unimaginable stress? I’m pretty sure there’s no specific formula. I don’t mean to sound like a Pollyanna or to make light of the tumult and turmoil of these times, but here are a few things that, based on my own experiences, may help cultivate this valuable virtue.
Be part of a support network. In the midst of great stress, and especially during this time of social distancing, it’s especially tempting to just hunker down, close in on ourselves, and shut others out – sometimes even our closest friends and loved ones. Maintaining relationships is just too exhausting. But you need people who can come alongside you and offer words of encouragement when you are at your lowest. And there’s nothing that will bring out the best in you like being there to encourage and support someone else. We all need to both receive and to give emotional support at a time like this.
Take the long view. When we’re in the middle of a serious crisis, it seems like the problems we’re facing will last forever. There’s no light at the end of the tunnel, no port in the storm. But even this pandemic won’t last forever. If we can keep in mind the fact that things will eventually get better and that the current situation isn’t permanent, it can help us maintain our perspective and have more patience with the current dysfunction.
Focus on who you want to be in this moment. This is the hardest time most of us have ever lived through, both professionally and personally. But let me throw you a challenge. When you look back on this time from the perspective of five years from now, or maybe ten, how will you want to remember yourself? Who will you want to have been during this time? Looking back, what will make you proud of how you handled this challenge? Be that person.
Look for things to be thankful for. In the midst of the chaos that is our lives and our work right now, I believe we can still occasionally see moments of grace if we keep our eyes open for them. If we aren’t looking for them, we may miss them entirely. And those small moments of love, touches of compassion, displays of selflessness, and even flashes of victorious endurance in yourself or others are gifts to be treasured and held on to – to give thanks for.
Embrace a cause greater than yourself. May I suggest that one thing that might help our efforts to cultivate the virtue of victorious endurance during difficult times might be to embrace a cause that is bigger than yourself; that is, one that lures you to focus beyond your immediate circumstances? What are you passionate about, outside of your life’s normal routine?
If you don’t have a passion, consider what you might become passionate about, with a little effort. For some of us, like me, this will be our faith in God. For others it may be advocating for an end to racism or for broader social justice issues. Maybe it’s working to overcome our cultural and political divisions or to strengthen the institutions of our democracy. Perhaps it’s getting involved with efforts to mitigate climate change. Maybe it’s reaching out to the homeless or hungry in your own community or mentoring a child who is being left behind by the demands of remote learning.
Or perhaps what you embrace is even closer to home: maybe it’s working to eliminate health disparities in your institution or health system, or figuring out how to use technology and resources differently to improve how care is being delivered during or after this pandemic. Maybe it’s as simple as re-committing yourself to personally care for every patient you see today with the very best you have to offer, and with patience, compassion, and grace.
Find something that sets your heart on fire. Something that makes you want to take this difficult time and “transmute tribulation into strength and glory.” Something that, when you look back on these days, will make you thankful that you didn’t just hunker down and subsist through them. Instead, you accomplished great things; you learned; you contributed; and you grew stronger and better.
That’s victorious endurance.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey. This essay was published initially on The Hospital Leader, the official blog of SHM.
I’ve been thinking a lot about endurance recently.
COVID-19 is surging in the United States. Health care workers exhausted from the first and second waves are quickly reaching the verge of collapse. I’m seeing more and more heartbreaking articles about the bone-deep fatigue, fear, and frustration health care workers are facing, and I weep. As horrible as it is to be fighting this terrifying, little-understood, invisible virus, health care workers are also fighting an equally distressing war against misinformation, recklessness, apathy, and outright denial.
As if that wasn’t enough, we are also dealing with racial and social unrest not seen in decades. The most significant cultural divisions and political animosity perhaps since the Civil War. A contested election. The fraying of our democratic institutions and our standing in the global community. The weakest economy since the Great Depression. Record unemployment. Many individuals and families facing or already experiencing eviction and food insecurity. Record-setting fires, hurricanes, and other natural disasters that are only projected to intensify due to climate change.
That’s a lot to endure. And we don’t have much choice other than to live through it. Some of us will break under the strain; others will disengage by giving up clinical work or even leaving health care altogether. Some of us will pack it in and retire, walk away from relationships with family members or longtime friends, or even emigrate to another country (New Zealand, anyone?). Some of us will passively hunker down, letting the challenges of this time overwhelm us and just hoping we can hang on long enough to emerge, albeit beaten and scarred, on the other side.
But some of us will experience victorious endurance – the kind that doesn’t just accept suffering but finds a way to triumph over it. I came across the concept of victorious endurance in the Bible, but its origin is earlier, from classical Greece. It comes from the ancient Greek word hupomone, which literally means “abiding under” – as in disciplining oneself to bear up under a trial when one would more naturally rebel, or just give up. The ancient Greeks were big on virtues like self-control, long-suffering, and perseverance in the face of seemingly insurmountable difficulties; Odysseus was a poster child for hupomone. I believe the concept of victorious endurance can be applicable for people across many belief systems, philosophies, and ways of life.
The late William Barclay, former professor of divinity and biblical criticism at the University of Glasgow, Scotland, said of hupomone:
It is untranslatable. It does not describe the frame of mind which can sit down with folded hands and bowed head and let a torrent of troubles sweep over it in passive resignation. It describes the ability to bear things in such a triumphant way that it transfigures them. Chrysostom has a great panegyric on this hupomone. He calls it “the root of all goods, the mother of piety, the fruit that never withers, a fortress that is never taken, a harbour that knows no storms” and “the queen of virtues, the foundation of right actions, peace in war, calm in tempest, security in plots.” It is the courageous and triumphant ability to pass the breaking-point and not to break and always to greet the unseen with a cheer. It is the alchemy which transmutes tribulation into strength and glory.
Barclay further noted that “Cicero defines patientia, its Latin equivalent, as: ‘The voluntary and daily suffering of hard and difficult things, for the sake of honour and usefulness.”
In the midst of the most challenging public health emergency of our lifetimes, I am seeing hospitalists – and nurses, respiratory therapists, and countless other health care workers – doing exactly this, every day. I’m so incredibly proud of you all, and thankful beyond words.
I doubt that victorious endurance comes naturally to any of us; it’s something we work at, pursue and nurture. What’s the secret to cultivating victorious endurance in the midst of unimaginable stress? I’m pretty sure there’s no specific formula. I don’t mean to sound like a Pollyanna or to make light of the tumult and turmoil of these times, but here are a few things that, based on my own experiences, may help cultivate this valuable virtue.
Be part of a support network. In the midst of great stress, and especially during this time of social distancing, it’s especially tempting to just hunker down, close in on ourselves, and shut others out – sometimes even our closest friends and loved ones. Maintaining relationships is just too exhausting. But you need people who can come alongside you and offer words of encouragement when you are at your lowest. And there’s nothing that will bring out the best in you like being there to encourage and support someone else. We all need to both receive and to give emotional support at a time like this.
Take the long view. When we’re in the middle of a serious crisis, it seems like the problems we’re facing will last forever. There’s no light at the end of the tunnel, no port in the storm. But even this pandemic won’t last forever. If we can keep in mind the fact that things will eventually get better and that the current situation isn’t permanent, it can help us maintain our perspective and have more patience with the current dysfunction.
Focus on who you want to be in this moment. This is the hardest time most of us have ever lived through, both professionally and personally. But let me throw you a challenge. When you look back on this time from the perspective of five years from now, or maybe ten, how will you want to remember yourself? Who will you want to have been during this time? Looking back, what will make you proud of how you handled this challenge? Be that person.
Look for things to be thankful for. In the midst of the chaos that is our lives and our work right now, I believe we can still occasionally see moments of grace if we keep our eyes open for them. If we aren’t looking for them, we may miss them entirely. And those small moments of love, touches of compassion, displays of selflessness, and even flashes of victorious endurance in yourself or others are gifts to be treasured and held on to – to give thanks for.
Embrace a cause greater than yourself. May I suggest that one thing that might help our efforts to cultivate the virtue of victorious endurance during difficult times might be to embrace a cause that is bigger than yourself; that is, one that lures you to focus beyond your immediate circumstances? What are you passionate about, outside of your life’s normal routine?
If you don’t have a passion, consider what you might become passionate about, with a little effort. For some of us, like me, this will be our faith in God. For others it may be advocating for an end to racism or for broader social justice issues. Maybe it’s working to overcome our cultural and political divisions or to strengthen the institutions of our democracy. Perhaps it’s getting involved with efforts to mitigate climate change. Maybe it’s reaching out to the homeless or hungry in your own community or mentoring a child who is being left behind by the demands of remote learning.
Or perhaps what you embrace is even closer to home: maybe it’s working to eliminate health disparities in your institution or health system, or figuring out how to use technology and resources differently to improve how care is being delivered during or after this pandemic. Maybe it’s as simple as re-committing yourself to personally care for every patient you see today with the very best you have to offer, and with patience, compassion, and grace.
Find something that sets your heart on fire. Something that makes you want to take this difficult time and “transmute tribulation into strength and glory.” Something that, when you look back on these days, will make you thankful that you didn’t just hunker down and subsist through them. Instead, you accomplished great things; you learned; you contributed; and you grew stronger and better.
That’s victorious endurance.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey. This essay was published initially on The Hospital Leader, the official blog of SHM.
I’ve been thinking a lot about endurance recently.
COVID-19 is surging in the United States. Health care workers exhausted from the first and second waves are quickly reaching the verge of collapse. I’m seeing more and more heartbreaking articles about the bone-deep fatigue, fear, and frustration health care workers are facing, and I weep. As horrible as it is to be fighting this terrifying, little-understood, invisible virus, health care workers are also fighting an equally distressing war against misinformation, recklessness, apathy, and outright denial.
As if that wasn’t enough, we are also dealing with racial and social unrest not seen in decades. The most significant cultural divisions and political animosity perhaps since the Civil War. A contested election. The fraying of our democratic institutions and our standing in the global community. The weakest economy since the Great Depression. Record unemployment. Many individuals and families facing or already experiencing eviction and food insecurity. Record-setting fires, hurricanes, and other natural disasters that are only projected to intensify due to climate change.
That’s a lot to endure. And we don’t have much choice other than to live through it. Some of us will break under the strain; others will disengage by giving up clinical work or even leaving health care altogether. Some of us will pack it in and retire, walk away from relationships with family members or longtime friends, or even emigrate to another country (New Zealand, anyone?). Some of us will passively hunker down, letting the challenges of this time overwhelm us and just hoping we can hang on long enough to emerge, albeit beaten and scarred, on the other side.
But some of us will experience victorious endurance – the kind that doesn’t just accept suffering but finds a way to triumph over it. I came across the concept of victorious endurance in the Bible, but its origin is earlier, from classical Greece. It comes from the ancient Greek word hupomone, which literally means “abiding under” – as in disciplining oneself to bear up under a trial when one would more naturally rebel, or just give up. The ancient Greeks were big on virtues like self-control, long-suffering, and perseverance in the face of seemingly insurmountable difficulties; Odysseus was a poster child for hupomone. I believe the concept of victorious endurance can be applicable for people across many belief systems, philosophies, and ways of life.
The late William Barclay, former professor of divinity and biblical criticism at the University of Glasgow, Scotland, said of hupomone:
It is untranslatable. It does not describe the frame of mind which can sit down with folded hands and bowed head and let a torrent of troubles sweep over it in passive resignation. It describes the ability to bear things in such a triumphant way that it transfigures them. Chrysostom has a great panegyric on this hupomone. He calls it “the root of all goods, the mother of piety, the fruit that never withers, a fortress that is never taken, a harbour that knows no storms” and “the queen of virtues, the foundation of right actions, peace in war, calm in tempest, security in plots.” It is the courageous and triumphant ability to pass the breaking-point and not to break and always to greet the unseen with a cheer. It is the alchemy which transmutes tribulation into strength and glory.
Barclay further noted that “Cicero defines patientia, its Latin equivalent, as: ‘The voluntary and daily suffering of hard and difficult things, for the sake of honour and usefulness.”
In the midst of the most challenging public health emergency of our lifetimes, I am seeing hospitalists – and nurses, respiratory therapists, and countless other health care workers – doing exactly this, every day. I’m so incredibly proud of you all, and thankful beyond words.
I doubt that victorious endurance comes naturally to any of us; it’s something we work at, pursue and nurture. What’s the secret to cultivating victorious endurance in the midst of unimaginable stress? I’m pretty sure there’s no specific formula. I don’t mean to sound like a Pollyanna or to make light of the tumult and turmoil of these times, but here are a few things that, based on my own experiences, may help cultivate this valuable virtue.
Be part of a support network. In the midst of great stress, and especially during this time of social distancing, it’s especially tempting to just hunker down, close in on ourselves, and shut others out – sometimes even our closest friends and loved ones. Maintaining relationships is just too exhausting. But you need people who can come alongside you and offer words of encouragement when you are at your lowest. And there’s nothing that will bring out the best in you like being there to encourage and support someone else. We all need to both receive and to give emotional support at a time like this.
Take the long view. When we’re in the middle of a serious crisis, it seems like the problems we’re facing will last forever. There’s no light at the end of the tunnel, no port in the storm. But even this pandemic won’t last forever. If we can keep in mind the fact that things will eventually get better and that the current situation isn’t permanent, it can help us maintain our perspective and have more patience with the current dysfunction.
Focus on who you want to be in this moment. This is the hardest time most of us have ever lived through, both professionally and personally. But let me throw you a challenge. When you look back on this time from the perspective of five years from now, or maybe ten, how will you want to remember yourself? Who will you want to have been during this time? Looking back, what will make you proud of how you handled this challenge? Be that person.
Look for things to be thankful for. In the midst of the chaos that is our lives and our work right now, I believe we can still occasionally see moments of grace if we keep our eyes open for them. If we aren’t looking for them, we may miss them entirely. And those small moments of love, touches of compassion, displays of selflessness, and even flashes of victorious endurance in yourself or others are gifts to be treasured and held on to – to give thanks for.
Embrace a cause greater than yourself. May I suggest that one thing that might help our efforts to cultivate the virtue of victorious endurance during difficult times might be to embrace a cause that is bigger than yourself; that is, one that lures you to focus beyond your immediate circumstances? What are you passionate about, outside of your life’s normal routine?
If you don’t have a passion, consider what you might become passionate about, with a little effort. For some of us, like me, this will be our faith in God. For others it may be advocating for an end to racism or for broader social justice issues. Maybe it’s working to overcome our cultural and political divisions or to strengthen the institutions of our democracy. Perhaps it’s getting involved with efforts to mitigate climate change. Maybe it’s reaching out to the homeless or hungry in your own community or mentoring a child who is being left behind by the demands of remote learning.
Or perhaps what you embrace is even closer to home: maybe it’s working to eliminate health disparities in your institution or health system, or figuring out how to use technology and resources differently to improve how care is being delivered during or after this pandemic. Maybe it’s as simple as re-committing yourself to personally care for every patient you see today with the very best you have to offer, and with patience, compassion, and grace.
Find something that sets your heart on fire. Something that makes you want to take this difficult time and “transmute tribulation into strength and glory.” Something that, when you look back on these days, will make you thankful that you didn’t just hunker down and subsist through them. Instead, you accomplished great things; you learned; you contributed; and you grew stronger and better.
That’s victorious endurance.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey. This essay was published initially on The Hospital Leader, the official blog of SHM.
How has the pandemic affected rural and urban cancer patients?
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Research has shown that, compared with their urban counterparts, rural cancer patients have higher cancer-related mortality and other negative treatment outcomes.
Among other explanations, the disparity has been attributed to lower education and income levels, medical and behavioral risk factors, differences in health literacy, and lower confidence in the medical system among rural residents (JCO Oncol Pract. 2020 Jul;16(7):422-30).
A new survey has provided some insight into how the COVID-19 pandemic has impacted rural and urban cancer patients differently.
The survey showed that urban patients were more likely to report changes to their daily lives, thought themselves more likely to become infected with SARS-CoV-2, and were more likely to take measures to mitigate the risk of infection. However, there were no major differences between urban and rural patients with regard to changes in social interaction.
Bailee Daniels of the University of Utah in Salt Lake City, presented these results at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S04-03).
The COVID-19 and Oncology Patient Experience Consortium
Ms. Daniels explained that the COVID-19 and Oncology Patient Experience (COPES) Consortium was created to investigate various aspects of the patient experience during the pandemic. Three cancer centers – Moffitt Cancer Center, Huntsman Cancer Institute, and the Sylvester Comprehensive Cancer Center – participate in COPES.
At Huntsman, investigators studied social and health behaviors of cancer patients to assess whether there was a difference between those from rural and urban areas. The researchers looked at the impact of the pandemic on psychosocial outcomes, preventive measures patients implemented, and their perceptions of the risk of SARS-CoV-2 infection.
The team’s hypothesis was that rural patients might be more vulnerable than urban patients to the effects of social isolation, emotional distress, and health-adverse behaviors, but the investigators noted that there has been no prior research on the topic.
Assessing behaviors, attitudes, and outcomes
Between August and September 2020, the researchers surveyed 1,328 adult cancer patients who had visited Huntsman in the previous 4 years and who were enrolled in Huntsman’s Total Cancer Care or Precision Exercise Prescription studies.
Patients completed questionnaires that encompassed demographic and clinical factors, employment status, health behaviors, and infection preventive measures. Questionnaires were provided in electronic, paper, or phone-based formats. Information regarding age, race, ethnicity, and tumor stage was abstracted from Huntsman’s electronic health record.
Modifications in daily life and social interaction were assessed on a 5-point scale. Changes in exercise habits and alcohol consumption were assessed on a 3-point scale. Infection mitigation measures (the use of face masks and hand sanitizer) and perceptions about the likelihood of SARS-CoV-2 infection were measured.
The rural-urban community area codes system, which classifies U.S. census tracts by measures of population density, urbanization, and daily commuting, was utilized to categorize patients into rural and urban residences.
Characteristics of urban and rural cancer patients
There were 997 urban and 331 rural participants. The mean age was 60.1 years in the urban population and 62.6 years in the rural population (P = .01). There were no urban-rural differences in sex, ethnicity, cancer stage, or body mass index.
More urban than rural participants were employed full- or part-time (45% vs. 37%; P = .045). The rural counties had more patients who were not currently employed, primarily due to retirement (77% vs. 69% urban; P < .001).
“No health insurance coverage” was reported by 2% of urban and 4% of rural participants (P = .009), and 85% of all patients reported “good” to “excellent” overall health. Cancer patients in rural counties were significantly more likely to have ever smoked (37% vs. 25% urban; P = .001). In addition, alcohol consumption in the previous year was higher in rural patients. “Every day to less than once monthly” alcohol usage was reported by 44% of urban and 60% of rural patients (P < .001).
Changes in daily life and health-related behavior during the pandemic
Urban patients were more likely to report changes in their daily lives due to the pandemic. Specifically, 35% of urban patients and 26% of rural patients said the pandemic had changed their daily life “a lot” (P = .001).
However, there were no major differences between urban and rural patients when it came to changes in social interaction in the past month or feeling lonely in the past month (P = .45 and P = .88, respectively). Similarly, there were no significant differences for changes in alcohol consumption between the groups (P = .90).
Changes in exercise habits due to the pandemic were more common among patients in urban counties (51% vs. 39% rural; P < .001), though similar percentages of patients reported exercising less (44% urban vs. 45% rural) or more frequently (24% urban vs. 20% rural).
In terms of infection mitigation measures, urban patients were more likely to use face masks “very often” (83% vs. 66% rural; P < .001), while hand sanitizer was used “very often” among 66% of urban and 57% of rural participants (P = .05).
Urban participants were more likely than were their rural counterparts to think themselves “somewhat” or “very” likely to develop COVID-19 (22% vs. 14%; P = .04).
It might be short-sighted for oncology and public health specialists to be dismissive of differences in infection mitigation behaviors and perceptions of vulnerability to SARS-CoV-2 infection. Those behaviors and perceptions of risk could lead to lower vaccination rates in rural areas. If that occurs, there would be major negative consequences for the long-term health of rural communities and their medically vulnerable residents.
Future directions
Although the first 6 months of the COVID-19 pandemic had disparate effects on cancer patients living in rural and urban counties, the reasons for the disparities are complex and not easily explained by this study.
It is possible that sequential administration of the survey during the pandemic would have uncovered greater variances in attitude and health-related behaviors.
As Ms. Daniels noted, when the survey was performed, Utah had not experienced a high frequency of COVID-19 cases. Furthermore, different levels of restrictions were implemented on a county-by-county basis, potentially influencing patients’ behaviors, psychosocial adjustment, and perceptions of risk.
In addition, there may have been differences in unmeasured endpoints (infection rates, medical care utilization via telemedicine, hospitalization rates, late effects, and mortality) between the urban and rural populations.
As the investigators concluded, further research is needed to better characterize the pandemic’s short- and long-term effects on cancer patients in rural and urban settings and appropriate interventions. Such studies may yield insights into the various facets of the well-documented “rural health gap” in cancer outcomes and interventions that could narrow the gap in spheres beyond the COVID-19 pandemic.
Ms. Daniels reported having no relevant disclosures.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR: COVID-19 AND CANCER 2021
What Tom Brady and Patrick Mahomes can teach us about physicians
Warning: This article will be about Tom Brady. If you love Tom Brady, hate Tom Brady, previously loved and now hate Tom Brady, I’m just warning you so you’ll be in the right frame of mind to continue. (If you don’t know who Tom Brady is, he’s Gisele’s husband).
Brady, who plays for the Tampa Bay Buccaneers, has played in the NFL for 21 seasons, an unbelievable number given the average career for a quarterback is 3 years. He’s 43 years old and was the oldest player in a Super Bowl, ever. He faced Patrick Mahomes, the quarterback for the opposing Kansas City Chiefs. Mahomes is one of the most athletic and talented quarterbacks of all time, and Mahomes is nearly 20 years younger than Brady. Yet, in a shot heard around the NFL world, Brady won.
But, was a Brady victory so shocking? Hot-shot residents may have a lot of moxie and talent, but experienced doctors often prevail by simply making sound decisions and avoiding mistakes. In our department, we’ve been discussing this lately: We’re hiring two dermatologists and we’re fortunate to have some amazing candidates apply. Some, like Mahomes, are young all-stars with outstanding ability and potential, right out of residency. Others, Brady-like, have been in practice for years and are ready to move to a new franchise.
Our medical group’s experiences are probably similar to many practices: New physicians out of residency often bring energy, inspiration, and ease with the latest therapies, devices, and surgical techniques. Yet, they sometimes struggle with efficiency and unforced errors. Experienced physicians might not know what’s hot, but they can often see where the best course of action lies, understanding not only the physiology but also the patient in ways that only experience can teach you. Fortunately, for those like me who’ve crossed midlife, there doesn’t seem to be an upper limit to experience – it is possible to keep getting better. Yes, I’m just like Tom Brady. (I wrote this article just to print that line.)
Some of the best doctors I’ve ever seen in action were emeritus physicians. In medical school at Wake Forest University, one of my professors was Dr. Eben Alexander. A retired neurosurgeon, he taught a case-based critical thinking skills class. I recall his brilliant insight and coaching, working through cases that had nothing to do with the brain or with surgery. He used his vast experience and wisdom to teach us how to practice medicine. He was, at that time, nearly 90 years old. Despite having been retired for decades, he was still writing articles and editing journals. He was inspiring. For a minute, he had me thinking I’d like to be a neurosurgeon, so I could be just like Eben Alexander. I did not, but I learned things from him that still impact my practice as a dermatologist today.
I’m sure you’ve had similar experiences of older colleagues or mentors who were the best doctor in the clinic or the O.R. They are the Dr. Anthony Faucis, not just practicing, but leading while in their 8th or 9th decade. We are all so fortunate that they keep playing.
We’ve not made our final choices on whom to hire, but with two positions, I expect we’ll choose both a young doctor and an experienced one to add to our team. It will be fun to watch and learn from them. Just like it will be fun to watch Tom Brady in the Super Bowl again next year.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Warning: This article will be about Tom Brady. If you love Tom Brady, hate Tom Brady, previously loved and now hate Tom Brady, I’m just warning you so you’ll be in the right frame of mind to continue. (If you don’t know who Tom Brady is, he’s Gisele’s husband).
Brady, who plays for the Tampa Bay Buccaneers, has played in the NFL for 21 seasons, an unbelievable number given the average career for a quarterback is 3 years. He’s 43 years old and was the oldest player in a Super Bowl, ever. He faced Patrick Mahomes, the quarterback for the opposing Kansas City Chiefs. Mahomes is one of the most athletic and talented quarterbacks of all time, and Mahomes is nearly 20 years younger than Brady. Yet, in a shot heard around the NFL world, Brady won.
But, was a Brady victory so shocking? Hot-shot residents may have a lot of moxie and talent, but experienced doctors often prevail by simply making sound decisions and avoiding mistakes. In our department, we’ve been discussing this lately: We’re hiring two dermatologists and we’re fortunate to have some amazing candidates apply. Some, like Mahomes, are young all-stars with outstanding ability and potential, right out of residency. Others, Brady-like, have been in practice for years and are ready to move to a new franchise.
Our medical group’s experiences are probably similar to many practices: New physicians out of residency often bring energy, inspiration, and ease with the latest therapies, devices, and surgical techniques. Yet, they sometimes struggle with efficiency and unforced errors. Experienced physicians might not know what’s hot, but they can often see where the best course of action lies, understanding not only the physiology but also the patient in ways that only experience can teach you. Fortunately, for those like me who’ve crossed midlife, there doesn’t seem to be an upper limit to experience – it is possible to keep getting better. Yes, I’m just like Tom Brady. (I wrote this article just to print that line.)
Some of the best doctors I’ve ever seen in action were emeritus physicians. In medical school at Wake Forest University, one of my professors was Dr. Eben Alexander. A retired neurosurgeon, he taught a case-based critical thinking skills class. I recall his brilliant insight and coaching, working through cases that had nothing to do with the brain or with surgery. He used his vast experience and wisdom to teach us how to practice medicine. He was, at that time, nearly 90 years old. Despite having been retired for decades, he was still writing articles and editing journals. He was inspiring. For a minute, he had me thinking I’d like to be a neurosurgeon, so I could be just like Eben Alexander. I did not, but I learned things from him that still impact my practice as a dermatologist today.
I’m sure you’ve had similar experiences of older colleagues or mentors who were the best doctor in the clinic or the O.R. They are the Dr. Anthony Faucis, not just practicing, but leading while in their 8th or 9th decade. We are all so fortunate that they keep playing.
We’ve not made our final choices on whom to hire, but with two positions, I expect we’ll choose both a young doctor and an experienced one to add to our team. It will be fun to watch and learn from them. Just like it will be fun to watch Tom Brady in the Super Bowl again next year.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Warning: This article will be about Tom Brady. If you love Tom Brady, hate Tom Brady, previously loved and now hate Tom Brady, I’m just warning you so you’ll be in the right frame of mind to continue. (If you don’t know who Tom Brady is, he’s Gisele’s husband).
Brady, who plays for the Tampa Bay Buccaneers, has played in the NFL for 21 seasons, an unbelievable number given the average career for a quarterback is 3 years. He’s 43 years old and was the oldest player in a Super Bowl, ever. He faced Patrick Mahomes, the quarterback for the opposing Kansas City Chiefs. Mahomes is one of the most athletic and talented quarterbacks of all time, and Mahomes is nearly 20 years younger than Brady. Yet, in a shot heard around the NFL world, Brady won.
But, was a Brady victory so shocking? Hot-shot residents may have a lot of moxie and talent, but experienced doctors often prevail by simply making sound decisions and avoiding mistakes. In our department, we’ve been discussing this lately: We’re hiring two dermatologists and we’re fortunate to have some amazing candidates apply. Some, like Mahomes, are young all-stars with outstanding ability and potential, right out of residency. Others, Brady-like, have been in practice for years and are ready to move to a new franchise.
Our medical group’s experiences are probably similar to many practices: New physicians out of residency often bring energy, inspiration, and ease with the latest therapies, devices, and surgical techniques. Yet, they sometimes struggle with efficiency and unforced errors. Experienced physicians might not know what’s hot, but they can often see where the best course of action lies, understanding not only the physiology but also the patient in ways that only experience can teach you. Fortunately, for those like me who’ve crossed midlife, there doesn’t seem to be an upper limit to experience – it is possible to keep getting better. Yes, I’m just like Tom Brady. (I wrote this article just to print that line.)
Some of the best doctors I’ve ever seen in action were emeritus physicians. In medical school at Wake Forest University, one of my professors was Dr. Eben Alexander. A retired neurosurgeon, he taught a case-based critical thinking skills class. I recall his brilliant insight and coaching, working through cases that had nothing to do with the brain or with surgery. He used his vast experience and wisdom to teach us how to practice medicine. He was, at that time, nearly 90 years old. Despite having been retired for decades, he was still writing articles and editing journals. He was inspiring. For a minute, he had me thinking I’d like to be a neurosurgeon, so I could be just like Eben Alexander. I did not, but I learned things from him that still impact my practice as a dermatologist today.
I’m sure you’ve had similar experiences of older colleagues or mentors who were the best doctor in the clinic or the O.R. They are the Dr. Anthony Faucis, not just practicing, but leading while in their 8th or 9th decade. We are all so fortunate that they keep playing.
We’ve not made our final choices on whom to hire, but with two positions, I expect we’ll choose both a young doctor and an experienced one to add to our team. It will be fun to watch and learn from them. Just like it will be fun to watch Tom Brady in the Super Bowl again next year.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
When the X-Waiver gets X’ed: Implications for hospitalists
There are two pandemics permeating the United States: COVID-19 and addiction. To date, more than 468,000 people have died from COVID-19 in the U.S. In the 12-month period ending in May 2020, over 80,000 died from a drug related cause – the highest number ever recorded in a year. Many of these deaths involved opioids.
COVID-19 has worsened outcomes for people with addiction. There is less access to treatment, increased isolation, and worsening psychosocial and economic stressors. These factors may drive new, increased, or more risky substance use and return to use for people in recovery. As hospitalists, we have been responders in both COVID-19 and our country’s worsening overdose and addiction crisis.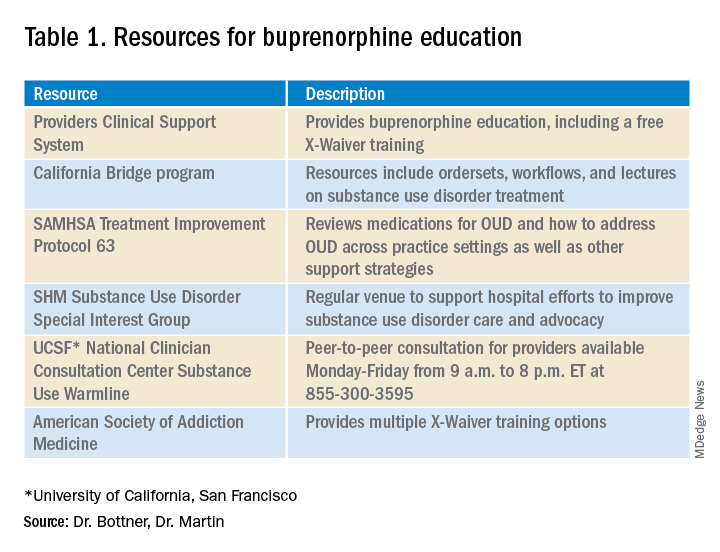
In December 2020’s Journal of Hospital Medicine article “Converging Crises: Caring for hospitalized adults with substance use disorder in the time of COVID-19”, Dr. Honora Englander and her coauthors called on hospitalists to actively engage patients with substance use disorders during hospitalization. The article highlights the colliding crises of addiction and COVID-19 and provides eight practical approaches for hospitalists to address substance use disorders during the pandemic, including initiating buprenorphine for opioid withdrawal and prescribing it for opioid use disorder (OUD) treatment.
Buprenorphine effectively treats opioid withdrawal, reduces OUD-related mortality, and decreases hospital readmissions related to OUD. To prescribe buprenorphine for OUD in the outpatient setting or on hospital discharge, providers need an X-Waiver. The X-Waiver is a result of the Drug Addiction Treatment Act 2000 (DATA 2000), which was enacted in 2000. It permits physicians to prescribe buprenorphine for OUD treatment after an 8-hour training. In 2016, the Comprehensive Addiction and Recovery Act extended buprenorphine prescribing to physician assistants (PAs) and advanced-practice nurses (APNs). However, PAs and APNs are required to complete a 24-hour training to receive the waiver.
On Jan. 14, 2021, the U.S. Department of Health and Human Services under the Trump administration announced it was removing the X-Waiver training previously required for physicians to prescribe this life-saving medication. However, on Jan. 20, 2021, the Biden administration froze the training requirement removal pending a 60-day review. The excitement about the waiver’s eradication further dampened on Jan. 25, when the plan was halted due to procedural factors coupled with the concern that HHS may not have the authority to void requirements mandated by Congress.
Many of us continue to be hopeful that the X-Waiver will soon be gone. The Substance Abuse and Mental Health Services Administration has committed to working with federal agencies to increase access to buprenorphine. The Biden administration also committed to addressing our country’s addiction crisis, including a plan to “make effective prevention, treatment, and recovery services available to all, including through a $125 billion federal investment.”
Despite the pause on HHS’s recent attempt to “X the X-Waiver,” we now have renewed attention and interest in this critical issue and an opportunity for greater and longer-lasting legislative impact. SHM supports that Congress repeal the legislative requirement for buprenorphine training dictated by DATA 2000 so that it cannot be rolled back by future administrations. To further increase access to buprenorphine treatment, the training requirement should be removed for all providers who care for individuals with OUD.
The X-Waiver has been a barrier to hospitalist adoption of this critical, life-saving medication. HHS’s stance to nix the waiver, though fleeting, should be interpreted as an urgent call to the medical community, including us as hospitalists, to learn about buprenorphine with the many resources available (see table 1). As hospital medicine providers, we can order buprenorphine for patients with OUD during hospitalization. It is discharge prescriptions that have been limited to providers with an X-Waiver.
What can we do now to prepare for the eventual X-Waiver training removal? We can start by educating ourselves with the resources listed in table 1. Those of us who are already buprenorphine champions could lead trainings in our home institutions. In a future without the waiver there will be more flexibility to develop hospitalist-focused buprenorphine trainings, as the previous ones were geared for outpatient providers. Hospitalist organizations could support hospitalist-specific buprenorphine trainings and extend the models to include additional medications for addiction.
There is a large body of evidence regarding buprenorphine’s safety and efficacy in OUD treatment. With a worsening overdose crisis, there have been increasing opioid-related hospitalizations. When new medications for diabetes, hypertension, or DVT treatment become available, as hospitalists we incorporate them into our toolbox. As buprenorphine becomes more accessible, we can be leaders in further adopting it (and other substance use disorder medications while we are at it) as our standard of care for people with OUD.
Dr. Bottner is a physician assistant in the Division of Hospital Medicine at Dell Medical School at The University of Texas at Austin and director of the hospital’s Buprenorphine Team. Dr. Martin is a board-certified addiction medicine physician and hospitalist at University of California, San Francisco, and director of the Addiction Care Team at San Francisco General Hospital. Dr. Bottner and Dr. Martin colead the SHM Substance Use Disorder Special Interest Group.
There are two pandemics permeating the United States: COVID-19 and addiction. To date, more than 468,000 people have died from COVID-19 in the U.S. In the 12-month period ending in May 2020, over 80,000 died from a drug related cause – the highest number ever recorded in a year. Many of these deaths involved opioids.
COVID-19 has worsened outcomes for people with addiction. There is less access to treatment, increased isolation, and worsening psychosocial and economic stressors. These factors may drive new, increased, or more risky substance use and return to use for people in recovery. As hospitalists, we have been responders in both COVID-19 and our country’s worsening overdose and addiction crisis.
In December 2020’s Journal of Hospital Medicine article “Converging Crises: Caring for hospitalized adults with substance use disorder in the time of COVID-19”, Dr. Honora Englander and her coauthors called on hospitalists to actively engage patients with substance use disorders during hospitalization. The article highlights the colliding crises of addiction and COVID-19 and provides eight practical approaches for hospitalists to address substance use disorders during the pandemic, including initiating buprenorphine for opioid withdrawal and prescribing it for opioid use disorder (OUD) treatment.
Buprenorphine effectively treats opioid withdrawal, reduces OUD-related mortality, and decreases hospital readmissions related to OUD. To prescribe buprenorphine for OUD in the outpatient setting or on hospital discharge, providers need an X-Waiver. The X-Waiver is a result of the Drug Addiction Treatment Act 2000 (DATA 2000), which was enacted in 2000. It permits physicians to prescribe buprenorphine for OUD treatment after an 8-hour training. In 2016, the Comprehensive Addiction and Recovery Act extended buprenorphine prescribing to physician assistants (PAs) and advanced-practice nurses (APNs). However, PAs and APNs are required to complete a 24-hour training to receive the waiver.
On Jan. 14, 2021, the U.S. Department of Health and Human Services under the Trump administration announced it was removing the X-Waiver training previously required for physicians to prescribe this life-saving medication. However, on Jan. 20, 2021, the Biden administration froze the training requirement removal pending a 60-day review. The excitement about the waiver’s eradication further dampened on Jan. 25, when the plan was halted due to procedural factors coupled with the concern that HHS may not have the authority to void requirements mandated by Congress.
Many of us continue to be hopeful that the X-Waiver will soon be gone. The Substance Abuse and Mental Health Services Administration has committed to working with federal agencies to increase access to buprenorphine. The Biden administration also committed to addressing our country’s addiction crisis, including a plan to “make effective prevention, treatment, and recovery services available to all, including through a $125 billion federal investment.”
Despite the pause on HHS’s recent attempt to “X the X-Waiver,” we now have renewed attention and interest in this critical issue and an opportunity for greater and longer-lasting legislative impact. SHM supports that Congress repeal the legislative requirement for buprenorphine training dictated by DATA 2000 so that it cannot be rolled back by future administrations. To further increase access to buprenorphine treatment, the training requirement should be removed for all providers who care for individuals with OUD.
The X-Waiver has been a barrier to hospitalist adoption of this critical, life-saving medication. HHS’s stance to nix the waiver, though fleeting, should be interpreted as an urgent call to the medical community, including us as hospitalists, to learn about buprenorphine with the many resources available (see table 1). As hospital medicine providers, we can order buprenorphine for patients with OUD during hospitalization. It is discharge prescriptions that have been limited to providers with an X-Waiver.
What can we do now to prepare for the eventual X-Waiver training removal? We can start by educating ourselves with the resources listed in table 1. Those of us who are already buprenorphine champions could lead trainings in our home institutions. In a future without the waiver there will be more flexibility to develop hospitalist-focused buprenorphine trainings, as the previous ones were geared for outpatient providers. Hospitalist organizations could support hospitalist-specific buprenorphine trainings and extend the models to include additional medications for addiction.
There is a large body of evidence regarding buprenorphine’s safety and efficacy in OUD treatment. With a worsening overdose crisis, there have been increasing opioid-related hospitalizations. When new medications for diabetes, hypertension, or DVT treatment become available, as hospitalists we incorporate them into our toolbox. As buprenorphine becomes more accessible, we can be leaders in further adopting it (and other substance use disorder medications while we are at it) as our standard of care for people with OUD.
Dr. Bottner is a physician assistant in the Division of Hospital Medicine at Dell Medical School at The University of Texas at Austin and director of the hospital’s Buprenorphine Team. Dr. Martin is a board-certified addiction medicine physician and hospitalist at University of California, San Francisco, and director of the Addiction Care Team at San Francisco General Hospital. Dr. Bottner and Dr. Martin colead the SHM Substance Use Disorder Special Interest Group.
There are two pandemics permeating the United States: COVID-19 and addiction. To date, more than 468,000 people have died from COVID-19 in the U.S. In the 12-month period ending in May 2020, over 80,000 died from a drug related cause – the highest number ever recorded in a year. Many of these deaths involved opioids.
COVID-19 has worsened outcomes for people with addiction. There is less access to treatment, increased isolation, and worsening psychosocial and economic stressors. These factors may drive new, increased, or more risky substance use and return to use for people in recovery. As hospitalists, we have been responders in both COVID-19 and our country’s worsening overdose and addiction crisis.
In December 2020’s Journal of Hospital Medicine article “Converging Crises: Caring for hospitalized adults with substance use disorder in the time of COVID-19”, Dr. Honora Englander and her coauthors called on hospitalists to actively engage patients with substance use disorders during hospitalization. The article highlights the colliding crises of addiction and COVID-19 and provides eight practical approaches for hospitalists to address substance use disorders during the pandemic, including initiating buprenorphine for opioid withdrawal and prescribing it for opioid use disorder (OUD) treatment.
Buprenorphine effectively treats opioid withdrawal, reduces OUD-related mortality, and decreases hospital readmissions related to OUD. To prescribe buprenorphine for OUD in the outpatient setting or on hospital discharge, providers need an X-Waiver. The X-Waiver is a result of the Drug Addiction Treatment Act 2000 (DATA 2000), which was enacted in 2000. It permits physicians to prescribe buprenorphine for OUD treatment after an 8-hour training. In 2016, the Comprehensive Addiction and Recovery Act extended buprenorphine prescribing to physician assistants (PAs) and advanced-practice nurses (APNs). However, PAs and APNs are required to complete a 24-hour training to receive the waiver.
On Jan. 14, 2021, the U.S. Department of Health and Human Services under the Trump administration announced it was removing the X-Waiver training previously required for physicians to prescribe this life-saving medication. However, on Jan. 20, 2021, the Biden administration froze the training requirement removal pending a 60-day review. The excitement about the waiver’s eradication further dampened on Jan. 25, when the plan was halted due to procedural factors coupled with the concern that HHS may not have the authority to void requirements mandated by Congress.
Many of us continue to be hopeful that the X-Waiver will soon be gone. The Substance Abuse and Mental Health Services Administration has committed to working with federal agencies to increase access to buprenorphine. The Biden administration also committed to addressing our country’s addiction crisis, including a plan to “make effective prevention, treatment, and recovery services available to all, including through a $125 billion federal investment.”
Despite the pause on HHS’s recent attempt to “X the X-Waiver,” we now have renewed attention and interest in this critical issue and an opportunity for greater and longer-lasting legislative impact. SHM supports that Congress repeal the legislative requirement for buprenorphine training dictated by DATA 2000 so that it cannot be rolled back by future administrations. To further increase access to buprenorphine treatment, the training requirement should be removed for all providers who care for individuals with OUD.
The X-Waiver has been a barrier to hospitalist adoption of this critical, life-saving medication. HHS’s stance to nix the waiver, though fleeting, should be interpreted as an urgent call to the medical community, including us as hospitalists, to learn about buprenorphine with the many resources available (see table 1). As hospital medicine providers, we can order buprenorphine for patients with OUD during hospitalization. It is discharge prescriptions that have been limited to providers with an X-Waiver.
What can we do now to prepare for the eventual X-Waiver training removal? We can start by educating ourselves with the resources listed in table 1. Those of us who are already buprenorphine champions could lead trainings in our home institutions. In a future without the waiver there will be more flexibility to develop hospitalist-focused buprenorphine trainings, as the previous ones were geared for outpatient providers. Hospitalist organizations could support hospitalist-specific buprenorphine trainings and extend the models to include additional medications for addiction.
There is a large body of evidence regarding buprenorphine’s safety and efficacy in OUD treatment. With a worsening overdose crisis, there have been increasing opioid-related hospitalizations. When new medications for diabetes, hypertension, or DVT treatment become available, as hospitalists we incorporate them into our toolbox. As buprenorphine becomes more accessible, we can be leaders in further adopting it (and other substance use disorder medications while we are at it) as our standard of care for people with OUD.
Dr. Bottner is a physician assistant in the Division of Hospital Medicine at Dell Medical School at The University of Texas at Austin and director of the hospital’s Buprenorphine Team. Dr. Martin is a board-certified addiction medicine physician and hospitalist at University of California, San Francisco, and director of the Addiction Care Team at San Francisco General Hospital. Dr. Bottner and Dr. Martin colead the SHM Substance Use Disorder Special Interest Group.
What to do if an employee tests positive for COVID-19
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
X-ray vision: Using AI to maximize the value of radiographic images
Artificial intelligence (AI) is expected to one day affect the entire continuum of cancer care – from screening and risk prediction to diagnosis, risk stratification, treatment selection, and follow-up, according to an expert in the field.
Hugo J.W.L. Aerts, PhD, director of the AI in Medicine Program at Brigham and Women’s Hospital in Boston, described studies using AI for some of these purposes during a presentation at the AACR Virtual Special Conference: Artificial Intelligence, Diagnosis, and Imaging (Abstract IA-06).
In one study, Dr. Aerts and colleagues set out to determine whether a convolutional neural network (CNN) could extract prognostic information from chest radiographs. The researchers tested this theory using patients from two trials – the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial and the National Lung Screening Trial (NLST).
The team developed a CNN, called CXR-risk, and tested whether it could predict the longevity and prognosis of patients in the PLCO (n = 52,320) and NLST (n = 5,493) trials over a 12-year time period, based only on chest radiographs. No clinical information, demographics, radiographic interpretations, duration of follow-up, or censoring were provided to the deep-learning system.
CXR-risk output was stratified into five categories of radiographic risk scores for probability of death, from 0 (very low likelihood of mortality) to 1 (very high likelihood of mortality).
The investigators found a graded association between radiographic risk score and mortality. The very-high-risk group had mortality rates of 53.0% (PLCO) and 33.9% (NLST). In both trials, this was significantly higher than for the very-low-risk group. The unadjusted hazard ratio was 18.3 in the PCLO data set and 15.2 in the NLST data set (P < .001 for both).
This association was maintained after adjustment for radiologists’ findings (e.g., a lung nodule) and risk factors such as age, gender, and comorbid illnesses like diabetes. The adjusted HR was 4.8 in the PCLO data set and 7.0 in the NLST data set (P < .001 for both).
In both data sets, individuals in the very-high-risk group were significantly more likely to die of lung cancer. The aHR was 11.1 in the PCLO data set and 8.4 in the NSLT data set (P < .001 for both).
This might be expected for people who were interested in being screened for lung cancer. However, patients in the very-high-risk group were also more likely to die of cardiovascular illness (aHR, 3.6 for PLCO and 47.8 for NSLT; P < .001 for both) and respiratory illness (aHR, 27.5 for PLCO and 31.9 for NLST; P ≤ .001 for both).
With this information, a clinician could initiate additional testing and/or utilize more aggressive surveillance measures. If an oncologist considered therapy for a patient with newly diagnosed cancer, treatment choices and stratification for adverse events would be more intelligently planned.
Using AI to predict the risk of lung cancer
In another study, Dr. Aerts and colleagues developed and validated a CNN called CXR-LC, which was based on CXR-risk. The goal of this study was to see if CXR-LC could predict long-term incident lung cancer using data available in the EHR, including chest radiographs, age, sex, and smoking status.
The CXR-LC model was developed using data from the PLCO trial (n = 41,856) and was validated in smokers from the PLCO trial (n = 5,615; 12-year follow-up) as well as heavy smokers from the NLST trial (n = 5,493; 6-year follow-up).
Results showed that CXR-LC was able to predict which patients were at highest risk for developing lung cancer.
CXR-LC had better discrimination for incident lung cancer than did Medicare eligibility in the PLCO data set (area under the curve, 0.755 vs. 0.634; P < .001). And the performance of CXR-LC was similar to that of the PLCOM2012 risk score in both the PLCO data set (AUC, 0.755 vs. 0.751) and the NLST data set (AUC, 0.659 vs. 0.650).
When they were compared in screening populations of equal size, CXR-LC was more sensitive than Medicare eligibility criteria in the PLCO data set (74.9% vs. 63.8%; P = .012) and missed 30.7% fewer incident lung cancer diagnoses.
AI as a substitute for specialized testing and consultation
In a third study, Dr. Aerts and colleagues used a CNN to predict cardiovascular risk by assessing coronary artery calcium (CAC) from clinically obtained, readily available CT scans.
Ordinarily, identifying CAC – an accurate predictor of cardiovascular events – requires specialized expertise (manual measurement and cardiologist interpretation), time (estimated at 20 minutes/scan), and equipment (ECG-gated cardiac CT scan and special software).
In this study, the researchers used a fully end-to-end automated system with analytic time measured in less than 2 seconds.
The team trained and tuned their CNN using the Framingham Heart Study Offspring and Third Generation cohorts (n = 1,636), which included asymptomatic patients with high-quality, cardiac-gated CT scans for CAC quantification.
The researchers then tested the CNN on two asymptomatic and two symptomatic cohorts:
- Asymptomatic Framingham Heart Study participants (n = 663) in whom the outcome measures were cardiovascular disease and death.
- Asymptomatic NLST participants (n = 14,959) in whom the outcome measure was atherosclerotic cardiovascular death.
- Symptomatic PROMISE study participants with stable chest pain (n = 4,021) in whom the outcome measures were all-cause mortality, MI, and hospitalization for unstable angina.
- Symptomatic ROMICAT-II study patients with acute chest pain (n = 441) in whom the outcome measure was acute coronary syndrome at 28 days.
Among 5,521 subjects across all testing cohorts with cardiac-gated and nongated chest CT scans, the CNN and expert reader interpretations agreed on the CAC risk scores with a high level of concordance (kappa, 0.71; concordance rate, 0.79).
There was a very high Spearman’s correlation of 0.92 (P < .0001) and substantial agreement between automatically and manually calculated CAC risk groups, substantiating robust risk prediction for cardiovascular disease across multiple clinical scenarios.
Dr. Aerts commented that, among the NLST participants who had the highest risk of developing lung cancer, the risk of cardiovascular death was as high as the risk of death from lung cancer.
Using AI to assess patient outcomes
In an unpublished study, Dr. Aerts and colleagues used AI in an attempt to determine whether changes in measurements of subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), and skeletal muscle mass would provide clues about treatment outcomes in lung cancer patients.
The researchers developed a deep learning model using data from 1,129 patients at Massachusetts General and Brigham and Women’s Hospitals, measuring SAT, VAT, and muscle mass. The team applied the measurement system to a population of 12,128 outpatients and calculated z scores for SAT, VAT, and muscle mass to determine “normal” values.
When they applied the norms to surgical lung cancer data sets from the Boston Lung Cancer Study (n = 437) and TRACERx study (n = 394), the researchers found that smokers had lower adiposity and lower muscle mass than never-smokers.
More importantly, over time, among lung cancer patients who lost greater than 5% of VAT, SAT, and muscle mass, those patients with the greatest SAT loss (P < .0001) or VAT loss (P = .0015) had the lowest lung cancer–specific survival in the TRACERx study. There was no significant impairment of lung cancer-specific survival for patients who experienced skeletal muscle loss (P = .23).
The same observation was made for overall survival among patients enrolled in the Boston Lung Cancer Study, using the 5% threshold. Overall survival was significantly worse with increasing VAT loss (P = .0023) and SAT loss (P = .0082) but not with increasing skeletal muscle loss (P = .3).
The investigators speculated about whether the correlation between body composition and clinical outcome could yield clues about tumor biology. To test this, the researchers used the RNA sequencing–based ORACLE risk score in lung cancer patients from TRACERx. There was a high correlation between higher ORACLE risk scores and lower VAT and SAT, suggesting that measures of adiposity on CT were reflected in tumor biology patterns on an RNA level in lung cancer patients. There was no such correlation between ORACLE risk scores and skeletal muscle mass.
Wonderment ... tempered by concern and challenges
AI has awe-inspiring potential to yield actionable and prognostically important information from data mining the EHR and extracting the vast quantities of information from images. In some cases (like CAC), it is information that is “hiding in plain sight.” However, Dr. Aerts expressed several cautions, some of which have already plagued AI.
He referenced the Gartner Hype Cycle, which provides a graphic representation of five phases in the life cycle of emerging technologies. The “innovation trigger” is followed by a “peak of inflated expectations,” a “trough of disillusionment,” a “slope of enlightenment,” and a “plateau of productivity.”
Dr. Aerts noted that, in recent years, AI has seemed to fall into the trough of disillusionment, but it may be entering the slope of enlightenment on the way to the plateau of productivity.
His research highlighted several examples of productivity in radiomics in cancer patients and those who are at high risk of developing cancer.
In Dr. Aerts’s opinion, a second concern is replication of AI research results. He noted that, among 400 published studies, only 6% of authors shared the codes that would enable their findings to be corroborated. About 30% shared test data, and 54% shared “pseudocodes,” but transparency and reproducibility are problems for the acceptance and broad implementation of AI.
Dr. Aerts endorsed the Modelhub initiative (www.modelhub.ai), a multi-institutional initiative to advance reproducibility in the AI field and advance its full potential.
However, there are additional concerns about the implementation of radiomics and, more generally, data mining from clinicians’ EHRs to personalize care.
Firstly, it may be laborious and difficult to explain complex, computer-based risk stratification models to patients. Hereditary cancer testing is an example of a risk assessment test that requires complicated explanations that many clinicians relegate to genetics counselors – when patients elect to see them. When a model is not explainable, it undermines the confidence of patients and their care providers, according to an editorial related to the CXR-LC study.
Another issue is that uptake of lung cancer screening, in practice, has been underutilized by individuals who meet current, relatively straightforward Medicare criteria. Despite the apparently better accuracy of the CXR-LC deep-learning model, its complexity and limited access could constitute an additional barrier for the at-risk individuals who should avail themselves of screening.
Furthermore, although age and gender are accurate in most circumstances, there is legitimate concern about the accuracy of, for example, smoking history data and comorbid conditions in current EHRs. Who performs the laborious curation of the input in an AI model to assure its accuracy for individual patients?
Finally, it is unclear how scalable and applicable AI will be to medically underserved populations (e.g., smaller, community-based, free-standing, socioeconomically disadvantaged or rural health care institutions). There are substantial initial and maintenance costs that may limit AI’s availability to some academic institutions and large health maintenance organizations.
As the concerns and challenges are addressed, it will be interesting to see where and when the plateau of productivity for AI in cancer care occurs. When it does, many cancer patients will benefit from enhanced care along the continuum of the complex disease they and their caregivers seek to master.
Dr. Aerts disclosed relationships with Onc.AI outside the presented work.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Artificial intelligence (AI) is expected to one day affect the entire continuum of cancer care – from screening and risk prediction to diagnosis, risk stratification, treatment selection, and follow-up, according to an expert in the field.
Hugo J.W.L. Aerts, PhD, director of the AI in Medicine Program at Brigham and Women’s Hospital in Boston, described studies using AI for some of these purposes during a presentation at the AACR Virtual Special Conference: Artificial Intelligence, Diagnosis, and Imaging (Abstract IA-06).
In one study, Dr. Aerts and colleagues set out to determine whether a convolutional neural network (CNN) could extract prognostic information from chest radiographs. The researchers tested this theory using patients from two trials – the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial and the National Lung Screening Trial (NLST).
The team developed a CNN, called CXR-risk, and tested whether it could predict the longevity and prognosis of patients in the PLCO (n = 52,320) and NLST (n = 5,493) trials over a 12-year time period, based only on chest radiographs. No clinical information, demographics, radiographic interpretations, duration of follow-up, or censoring were provided to the deep-learning system.
CXR-risk output was stratified into five categories of radiographic risk scores for probability of death, from 0 (very low likelihood of mortality) to 1 (very high likelihood of mortality).
The investigators found a graded association between radiographic risk score and mortality. The very-high-risk group had mortality rates of 53.0% (PLCO) and 33.9% (NLST). In both trials, this was significantly higher than for the very-low-risk group. The unadjusted hazard ratio was 18.3 in the PCLO data set and 15.2 in the NLST data set (P < .001 for both).
This association was maintained after adjustment for radiologists’ findings (e.g., a lung nodule) and risk factors such as age, gender, and comorbid illnesses like diabetes. The adjusted HR was 4.8 in the PCLO data set and 7.0 in the NLST data set (P < .001 for both).
In both data sets, individuals in the very-high-risk group were significantly more likely to die of lung cancer. The aHR was 11.1 in the PCLO data set and 8.4 in the NSLT data set (P < .001 for both).
This might be expected for people who were interested in being screened for lung cancer. However, patients in the very-high-risk group were also more likely to die of cardiovascular illness (aHR, 3.6 for PLCO and 47.8 for NSLT; P < .001 for both) and respiratory illness (aHR, 27.5 for PLCO and 31.9 for NLST; P ≤ .001 for both).
With this information, a clinician could initiate additional testing and/or utilize more aggressive surveillance measures. If an oncologist considered therapy for a patient with newly diagnosed cancer, treatment choices and stratification for adverse events would be more intelligently planned.
Using AI to predict the risk of lung cancer
In another study, Dr. Aerts and colleagues developed and validated a CNN called CXR-LC, which was based on CXR-risk. The goal of this study was to see if CXR-LC could predict long-term incident lung cancer using data available in the EHR, including chest radiographs, age, sex, and smoking status.
The CXR-LC model was developed using data from the PLCO trial (n = 41,856) and was validated in smokers from the PLCO trial (n = 5,615; 12-year follow-up) as well as heavy smokers from the NLST trial (n = 5,493; 6-year follow-up).
Results showed that CXR-LC was able to predict which patients were at highest risk for developing lung cancer.
CXR-LC had better discrimination for incident lung cancer than did Medicare eligibility in the PLCO data set (area under the curve, 0.755 vs. 0.634; P < .001). And the performance of CXR-LC was similar to that of the PLCOM2012 risk score in both the PLCO data set (AUC, 0.755 vs. 0.751) and the NLST data set (AUC, 0.659 vs. 0.650).
When they were compared in screening populations of equal size, CXR-LC was more sensitive than Medicare eligibility criteria in the PLCO data set (74.9% vs. 63.8%; P = .012) and missed 30.7% fewer incident lung cancer diagnoses.
AI as a substitute for specialized testing and consultation
In a third study, Dr. Aerts and colleagues used a CNN to predict cardiovascular risk by assessing coronary artery calcium (CAC) from clinically obtained, readily available CT scans.
Ordinarily, identifying CAC – an accurate predictor of cardiovascular events – requires specialized expertise (manual measurement and cardiologist interpretation), time (estimated at 20 minutes/scan), and equipment (ECG-gated cardiac CT scan and special software).
In this study, the researchers used a fully end-to-end automated system with analytic time measured in less than 2 seconds.
The team trained and tuned their CNN using the Framingham Heart Study Offspring and Third Generation cohorts (n = 1,636), which included asymptomatic patients with high-quality, cardiac-gated CT scans for CAC quantification.
The researchers then tested the CNN on two asymptomatic and two symptomatic cohorts:
- Asymptomatic Framingham Heart Study participants (n = 663) in whom the outcome measures were cardiovascular disease and death.
- Asymptomatic NLST participants (n = 14,959) in whom the outcome measure was atherosclerotic cardiovascular death.
- Symptomatic PROMISE study participants with stable chest pain (n = 4,021) in whom the outcome measures were all-cause mortality, MI, and hospitalization for unstable angina.
- Symptomatic ROMICAT-II study patients with acute chest pain (n = 441) in whom the outcome measure was acute coronary syndrome at 28 days.
Among 5,521 subjects across all testing cohorts with cardiac-gated and nongated chest CT scans, the CNN and expert reader interpretations agreed on the CAC risk scores with a high level of concordance (kappa, 0.71; concordance rate, 0.79).
There was a very high Spearman’s correlation of 0.92 (P < .0001) and substantial agreement between automatically and manually calculated CAC risk groups, substantiating robust risk prediction for cardiovascular disease across multiple clinical scenarios.
Dr. Aerts commented that, among the NLST participants who had the highest risk of developing lung cancer, the risk of cardiovascular death was as high as the risk of death from lung cancer.
Using AI to assess patient outcomes
In an unpublished study, Dr. Aerts and colleagues used AI in an attempt to determine whether changes in measurements of subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), and skeletal muscle mass would provide clues about treatment outcomes in lung cancer patients.
The researchers developed a deep learning model using data from 1,129 patients at Massachusetts General and Brigham and Women’s Hospitals, measuring SAT, VAT, and muscle mass. The team applied the measurement system to a population of 12,128 outpatients and calculated z scores for SAT, VAT, and muscle mass to determine “normal” values.
When they applied the norms to surgical lung cancer data sets from the Boston Lung Cancer Study (n = 437) and TRACERx study (n = 394), the researchers found that smokers had lower adiposity and lower muscle mass than never-smokers.
More importantly, over time, among lung cancer patients who lost greater than 5% of VAT, SAT, and muscle mass, those patients with the greatest SAT loss (P < .0001) or VAT loss (P = .0015) had the lowest lung cancer–specific survival in the TRACERx study. There was no significant impairment of lung cancer-specific survival for patients who experienced skeletal muscle loss (P = .23).
The same observation was made for overall survival among patients enrolled in the Boston Lung Cancer Study, using the 5% threshold. Overall survival was significantly worse with increasing VAT loss (P = .0023) and SAT loss (P = .0082) but not with increasing skeletal muscle loss (P = .3).
The investigators speculated about whether the correlation between body composition and clinical outcome could yield clues about tumor biology. To test this, the researchers used the RNA sequencing–based ORACLE risk score in lung cancer patients from TRACERx. There was a high correlation between higher ORACLE risk scores and lower VAT and SAT, suggesting that measures of adiposity on CT were reflected in tumor biology patterns on an RNA level in lung cancer patients. There was no such correlation between ORACLE risk scores and skeletal muscle mass.
Wonderment ... tempered by concern and challenges
AI has awe-inspiring potential to yield actionable and prognostically important information from data mining the EHR and extracting the vast quantities of information from images. In some cases (like CAC), it is information that is “hiding in plain sight.” However, Dr. Aerts expressed several cautions, some of which have already plagued AI.
He referenced the Gartner Hype Cycle, which provides a graphic representation of five phases in the life cycle of emerging technologies. The “innovation trigger” is followed by a “peak of inflated expectations,” a “trough of disillusionment,” a “slope of enlightenment,” and a “plateau of productivity.”
Dr. Aerts noted that, in recent years, AI has seemed to fall into the trough of disillusionment, but it may be entering the slope of enlightenment on the way to the plateau of productivity.
His research highlighted several examples of productivity in radiomics in cancer patients and those who are at high risk of developing cancer.
In Dr. Aerts’s opinion, a second concern is replication of AI research results. He noted that, among 400 published studies, only 6% of authors shared the codes that would enable their findings to be corroborated. About 30% shared test data, and 54% shared “pseudocodes,” but transparency and reproducibility are problems for the acceptance and broad implementation of AI.
Dr. Aerts endorsed the Modelhub initiative (www.modelhub.ai), a multi-institutional initiative to advance reproducibility in the AI field and advance its full potential.
However, there are additional concerns about the implementation of radiomics and, more generally, data mining from clinicians’ EHRs to personalize care.
Firstly, it may be laborious and difficult to explain complex, computer-based risk stratification models to patients. Hereditary cancer testing is an example of a risk assessment test that requires complicated explanations that many clinicians relegate to genetics counselors – when patients elect to see them. When a model is not explainable, it undermines the confidence of patients and their care providers, according to an editorial related to the CXR-LC study.
Another issue is that uptake of lung cancer screening, in practice, has been underutilized by individuals who meet current, relatively straightforward Medicare criteria. Despite the apparently better accuracy of the CXR-LC deep-learning model, its complexity and limited access could constitute an additional barrier for the at-risk individuals who should avail themselves of screening.
Furthermore, although age and gender are accurate in most circumstances, there is legitimate concern about the accuracy of, for example, smoking history data and comorbid conditions in current EHRs. Who performs the laborious curation of the input in an AI model to assure its accuracy for individual patients?
Finally, it is unclear how scalable and applicable AI will be to medically underserved populations (e.g., smaller, community-based, free-standing, socioeconomically disadvantaged or rural health care institutions). There are substantial initial and maintenance costs that may limit AI’s availability to some academic institutions and large health maintenance organizations.
As the concerns and challenges are addressed, it will be interesting to see where and when the plateau of productivity for AI in cancer care occurs. When it does, many cancer patients will benefit from enhanced care along the continuum of the complex disease they and their caregivers seek to master.
Dr. Aerts disclosed relationships with Onc.AI outside the presented work.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Artificial intelligence (AI) is expected to one day affect the entire continuum of cancer care – from screening and risk prediction to diagnosis, risk stratification, treatment selection, and follow-up, according to an expert in the field.
Hugo J.W.L. Aerts, PhD, director of the AI in Medicine Program at Brigham and Women’s Hospital in Boston, described studies using AI for some of these purposes during a presentation at the AACR Virtual Special Conference: Artificial Intelligence, Diagnosis, and Imaging (Abstract IA-06).
In one study, Dr. Aerts and colleagues set out to determine whether a convolutional neural network (CNN) could extract prognostic information from chest radiographs. The researchers tested this theory using patients from two trials – the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial and the National Lung Screening Trial (NLST).
The team developed a CNN, called CXR-risk, and tested whether it could predict the longevity and prognosis of patients in the PLCO (n = 52,320) and NLST (n = 5,493) trials over a 12-year time period, based only on chest radiographs. No clinical information, demographics, radiographic interpretations, duration of follow-up, or censoring were provided to the deep-learning system.
CXR-risk output was stratified into five categories of radiographic risk scores for probability of death, from 0 (very low likelihood of mortality) to 1 (very high likelihood of mortality).
The investigators found a graded association between radiographic risk score and mortality. The very-high-risk group had mortality rates of 53.0% (PLCO) and 33.9% (NLST). In both trials, this was significantly higher than for the very-low-risk group. The unadjusted hazard ratio was 18.3 in the PCLO data set and 15.2 in the NLST data set (P < .001 for both).
This association was maintained after adjustment for radiologists’ findings (e.g., a lung nodule) and risk factors such as age, gender, and comorbid illnesses like diabetes. The adjusted HR was 4.8 in the PCLO data set and 7.0 in the NLST data set (P < .001 for both).
In both data sets, individuals in the very-high-risk group were significantly more likely to die of lung cancer. The aHR was 11.1 in the PCLO data set and 8.4 in the NSLT data set (P < .001 for both).
This might be expected for people who were interested in being screened for lung cancer. However, patients in the very-high-risk group were also more likely to die of cardiovascular illness (aHR, 3.6 for PLCO and 47.8 for NSLT; P < .001 for both) and respiratory illness (aHR, 27.5 for PLCO and 31.9 for NLST; P ≤ .001 for both).
With this information, a clinician could initiate additional testing and/or utilize more aggressive surveillance measures. If an oncologist considered therapy for a patient with newly diagnosed cancer, treatment choices and stratification for adverse events would be more intelligently planned.
Using AI to predict the risk of lung cancer
In another study, Dr. Aerts and colleagues developed and validated a CNN called CXR-LC, which was based on CXR-risk. The goal of this study was to see if CXR-LC could predict long-term incident lung cancer using data available in the EHR, including chest radiographs, age, sex, and smoking status.
The CXR-LC model was developed using data from the PLCO trial (n = 41,856) and was validated in smokers from the PLCO trial (n = 5,615; 12-year follow-up) as well as heavy smokers from the NLST trial (n = 5,493; 6-year follow-up).
Results showed that CXR-LC was able to predict which patients were at highest risk for developing lung cancer.
CXR-LC had better discrimination for incident lung cancer than did Medicare eligibility in the PLCO data set (area under the curve, 0.755 vs. 0.634; P < .001). And the performance of CXR-LC was similar to that of the PLCOM2012 risk score in both the PLCO data set (AUC, 0.755 vs. 0.751) and the NLST data set (AUC, 0.659 vs. 0.650).
When they were compared in screening populations of equal size, CXR-LC was more sensitive than Medicare eligibility criteria in the PLCO data set (74.9% vs. 63.8%; P = .012) and missed 30.7% fewer incident lung cancer diagnoses.
AI as a substitute for specialized testing and consultation
In a third study, Dr. Aerts and colleagues used a CNN to predict cardiovascular risk by assessing coronary artery calcium (CAC) from clinically obtained, readily available CT scans.
Ordinarily, identifying CAC – an accurate predictor of cardiovascular events – requires specialized expertise (manual measurement and cardiologist interpretation), time (estimated at 20 minutes/scan), and equipment (ECG-gated cardiac CT scan and special software).
In this study, the researchers used a fully end-to-end automated system with analytic time measured in less than 2 seconds.
The team trained and tuned their CNN using the Framingham Heart Study Offspring and Third Generation cohorts (n = 1,636), which included asymptomatic patients with high-quality, cardiac-gated CT scans for CAC quantification.
The researchers then tested the CNN on two asymptomatic and two symptomatic cohorts:
- Asymptomatic Framingham Heart Study participants (n = 663) in whom the outcome measures were cardiovascular disease and death.
- Asymptomatic NLST participants (n = 14,959) in whom the outcome measure was atherosclerotic cardiovascular death.
- Symptomatic PROMISE study participants with stable chest pain (n = 4,021) in whom the outcome measures were all-cause mortality, MI, and hospitalization for unstable angina.
- Symptomatic ROMICAT-II study patients with acute chest pain (n = 441) in whom the outcome measure was acute coronary syndrome at 28 days.
Among 5,521 subjects across all testing cohorts with cardiac-gated and nongated chest CT scans, the CNN and expert reader interpretations agreed on the CAC risk scores with a high level of concordance (kappa, 0.71; concordance rate, 0.79).
There was a very high Spearman’s correlation of 0.92 (P < .0001) and substantial agreement between automatically and manually calculated CAC risk groups, substantiating robust risk prediction for cardiovascular disease across multiple clinical scenarios.
Dr. Aerts commented that, among the NLST participants who had the highest risk of developing lung cancer, the risk of cardiovascular death was as high as the risk of death from lung cancer.
Using AI to assess patient outcomes
In an unpublished study, Dr. Aerts and colleagues used AI in an attempt to determine whether changes in measurements of subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), and skeletal muscle mass would provide clues about treatment outcomes in lung cancer patients.
The researchers developed a deep learning model using data from 1,129 patients at Massachusetts General and Brigham and Women’s Hospitals, measuring SAT, VAT, and muscle mass. The team applied the measurement system to a population of 12,128 outpatients and calculated z scores for SAT, VAT, and muscle mass to determine “normal” values.
When they applied the norms to surgical lung cancer data sets from the Boston Lung Cancer Study (n = 437) and TRACERx study (n = 394), the researchers found that smokers had lower adiposity and lower muscle mass than never-smokers.
More importantly, over time, among lung cancer patients who lost greater than 5% of VAT, SAT, and muscle mass, those patients with the greatest SAT loss (P < .0001) or VAT loss (P = .0015) had the lowest lung cancer–specific survival in the TRACERx study. There was no significant impairment of lung cancer-specific survival for patients who experienced skeletal muscle loss (P = .23).
The same observation was made for overall survival among patients enrolled in the Boston Lung Cancer Study, using the 5% threshold. Overall survival was significantly worse with increasing VAT loss (P = .0023) and SAT loss (P = .0082) but not with increasing skeletal muscle loss (P = .3).
The investigators speculated about whether the correlation between body composition and clinical outcome could yield clues about tumor biology. To test this, the researchers used the RNA sequencing–based ORACLE risk score in lung cancer patients from TRACERx. There was a high correlation between higher ORACLE risk scores and lower VAT and SAT, suggesting that measures of adiposity on CT were reflected in tumor biology patterns on an RNA level in lung cancer patients. There was no such correlation between ORACLE risk scores and skeletal muscle mass.
Wonderment ... tempered by concern and challenges
AI has awe-inspiring potential to yield actionable and prognostically important information from data mining the EHR and extracting the vast quantities of information from images. In some cases (like CAC), it is information that is “hiding in plain sight.” However, Dr. Aerts expressed several cautions, some of which have already plagued AI.
He referenced the Gartner Hype Cycle, which provides a graphic representation of five phases in the life cycle of emerging technologies. The “innovation trigger” is followed by a “peak of inflated expectations,” a “trough of disillusionment,” a “slope of enlightenment,” and a “plateau of productivity.”
Dr. Aerts noted that, in recent years, AI has seemed to fall into the trough of disillusionment, but it may be entering the slope of enlightenment on the way to the plateau of productivity.
His research highlighted several examples of productivity in radiomics in cancer patients and those who are at high risk of developing cancer.
In Dr. Aerts’s opinion, a second concern is replication of AI research results. He noted that, among 400 published studies, only 6% of authors shared the codes that would enable their findings to be corroborated. About 30% shared test data, and 54% shared “pseudocodes,” but transparency and reproducibility are problems for the acceptance and broad implementation of AI.
Dr. Aerts endorsed the Modelhub initiative (www.modelhub.ai), a multi-institutional initiative to advance reproducibility in the AI field and advance its full potential.
However, there are additional concerns about the implementation of radiomics and, more generally, data mining from clinicians’ EHRs to personalize care.
Firstly, it may be laborious and difficult to explain complex, computer-based risk stratification models to patients. Hereditary cancer testing is an example of a risk assessment test that requires complicated explanations that many clinicians relegate to genetics counselors – when patients elect to see them. When a model is not explainable, it undermines the confidence of patients and their care providers, according to an editorial related to the CXR-LC study.
Another issue is that uptake of lung cancer screening, in practice, has been underutilized by individuals who meet current, relatively straightforward Medicare criteria. Despite the apparently better accuracy of the CXR-LC deep-learning model, its complexity and limited access could constitute an additional barrier for the at-risk individuals who should avail themselves of screening.
Furthermore, although age and gender are accurate in most circumstances, there is legitimate concern about the accuracy of, for example, smoking history data and comorbid conditions in current EHRs. Who performs the laborious curation of the input in an AI model to assure its accuracy for individual patients?
Finally, it is unclear how scalable and applicable AI will be to medically underserved populations (e.g., smaller, community-based, free-standing, socioeconomically disadvantaged or rural health care institutions). There are substantial initial and maintenance costs that may limit AI’s availability to some academic institutions and large health maintenance organizations.
As the concerns and challenges are addressed, it will be interesting to see where and when the plateau of productivity for AI in cancer care occurs. When it does, many cancer patients will benefit from enhanced care along the continuum of the complex disease they and their caregivers seek to master.
Dr. Aerts disclosed relationships with Onc.AI outside the presented work.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR: AI, DIAGNOSIS, AND IMAGING 2021
The importance of family acceptance for LGBTQ youth
It is well established that LGBTQ individuals experience more health disparities compared with their cisgender, heterosexual counterparts. In general, LGBTQ adolescents and young adults have higher levels of depression, suicide attempts, and substance use than those of their heterosexual peers. However, a key protective factor is family acceptance and support. By encouraging families to modify and change behaviors that are experienced by their LGBTQ children as rejecting and to engage in supportive and affirming behaviors, providers can help families to decrease risk and promote healthy outcomes for LGBTQ youth and young adults. 
We all know that a supportive family can make a difference for any child, but this is especially true for LGBTQ youth and is critical during a pandemic when young people are confined with families and separated from peers and supportive adults outside the home. Several research studies show that family support can improve outcomes related to suicide, depression, homelessness, drug use, and HIV in LGBTQ young people. Family acceptance improves health outcomes, while rejection undermines family relationships and worsens both health and other serious outcomes such as homelessness and placement in custodial care. Pediatricians can help their patients by educating parents and caregivers with LGBTQ children about the critical role of family support – both those who see themselves as accepting and those who believe that being gay or transgender is wrong and are struggling with parenting a child who identifies as LGBTQ or who is gender diverse.
The Family Acceptance Project (FAP) at San Francisco State University conducted the first research on LGBTQ youth and families, developed the first evidence-informed family support model, and has published a range of studies and evidence-based resources that demonstrate the harm caused by family rejection, validate the importance of family acceptance, and provide guidance to increase family support. FAP’s research found that parents and caregivers that engage in rejecting behaviors are typically motivated by care and concern and by trying to protect their children from harm. They believe such behaviors will help their LGBTQ children fit in, have a good life, meet cultural and religious expectations, and be respected by others.1 FAP’s research identified and measured more than 50 rejecting behaviors that parents and caregivers use to respond to their LGBTQ children. Some of these commonly expressed rejecting behaviors include ridiculing and making disparaging comments about their child and other LGBTQ people; excluding them from family activities; blaming their child when others mistreat them because they are LGBTQ; blocking access to LGBTQ resources including friends, support groups, and activities; and trying to change their child’s sexual orientation and gender identity.2 LGBTQ youth experience these and other such behaviors as hurtful, harmful, and traumatic and may feel that they need to hide or repress their identity which can affect their self-esteem, increase isolation, depression, and risky behaviors.3 Providers working with families of LGBTQ youth should focus on shared goals, such as reducing risk and having a happy, healthy child. Most parents love their children and fear for their well-being. However, many are uninformed about their child’s gender identity and sexual orientation and don’t know how to nurture and support them.
In FAP’s initial study, LGB young people who reported higher levels of family rejection had substantially higher rates of attempted suicide, depression, illegal drug use, and unprotected sex.4 These rates were even more significant among Latino gay and bisexual men.4 Those who are rejected by family are less likely to want to have a family or to be parents themselves5 and have lower educational and income levels.6
To reduce risk, pediatricians should ask LGBTQ patients about family rejecting behaviors and help parents and caregivers to identify and understand the effect of such behaviors to reduce health risks and conflict that can lead to running away, expulsion, and removal from the home. Even decreasing rejecting behaviors to moderate levels can significantly improve negative outcomes.5
Caitlin Ryan, PhD, and her team also identified and measured more than 50 family accepting behaviors that help protect against risk and promote well-being. They found that young adults who experience high levels of family acceptance during adolescence report significantly higher levels of self-esteem, social support, and general health with much lower levels of depression, suicidality, and substance abuse.7 Family accepting and supportive behaviors include talking with the child about their LGBTQ identity; advocating for their LGBTQ child when others mistreat them; requiring other family members to treat their LGBTQ child with respect; and supporting their child’s gender identity.5 FAP has developed an evidence-informed family support model and multilingual educational resources for families, providers, youth and religious leaders to decrease rejection and increase family support. These are available in print copies and for download at familyproject.sfsu.edu.
In addition, Dr. Ryan and colleagues1,4,8 recommend the following guidance for providers:
- Ask LGBTQ adolescents about family reactions to their sexual orientation, gender identity, and expression, and refer to LGBTQ community support programs and for supportive counseling, as needed.
- Identify LGBTQ community support programs and online resources to educate parents about how to help their children. Parents need culturally relevant peer support to help decrease rejection and increase family support.
- Advise parents that negative reactions to their adolescent’s LGBTQ identity may negatively impact their child’s health and mental health while supportive and affirming reactions promote well-being.
- Advise parents and caregivers to modify and change family rejecting behaviors that increase their child’s risk for suicide, depression, substance abuse ,and risky sexual behaviors.
- Expand anticipatory guidance to include information on the need for support and the link between family rejection and negative health problems.
- Provide guidance on sexual orientation and gender identity as part of normative child development during well-baby and early childhood care.
- Use FAP’s multilingual family education booklets and Healthy Futures poster series in family and patient education and provide these materials in clinical and community settings. FAP’s Healthy Futures posters include a poster guidance, a version on family acceptance, a version on family rejection and a family acceptance version for conservative families and settings. They are available in camera-ready art in four sizes in English and Spanish and are forthcoming in five Asian languages: familyproject.sfsu.edu/poster.
Dr. Lawlis is assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures.
Resources
• Family Acceptance Project – consultation and training; evidence-based educational materials for families, providers, religious leaders and youth.
• PFLAG – peer support for parents and friends with LGBTQ children in all states and several other countries.
References
1. Ryan C. Generating a revolution in prevention, wellness & care for LGBT children & youth. Temple Political & Civil Rights Law Review. 2014;23(2):331-44.
2. Ryan C. Healthy Futures Poster Series – Family Accepting & Rejecting Behaviors That Impact LGBTQ Children’s Health & Well-Being. In: Family Acceptance Project Marian Wright Edelman Institute SFSU, ed. San Francisco, CA2019.
3. Ryan C. Family Acceptance Project: Culturally grounded framework for supporting LGBTQ children and youth. J Am Acad Child Adolesc Psychiatr. 2019;58(10):S58-9.
4. Ryan C et al. Family rejection as a predictor of negative health outcomes in White and Latino lesbian, gay, and bisexual young adults. Pediatrics. 2009;123(1):346-52.
5. Ryan C. Supportive families, healthy children: Helping families with lesbian, gay, bisexual & transgender children. In: Family Acceptance Project Marian Wright Edelman Institute SFSU, ed. San Francisco, CA2009.
6. Ryan C et al. Parent-initiated sexual orientation change efforts with LGBT adolescents: Implications for young adult mental health and adjustment. J Homosexuality. 2020;67(2):159-73.
7. Ryan C et al. Family acceptance in adolescence and the health of LGBT young adults. J Child Adolesc Psychiatr Nursing. 2010;23(4):205-13. 8. Substance Abuse and Mental Health Services Administration. A Practitioner’s Guide: Helping Families to Support Their LGBT Children. In: Administration SAaMhS, ed. Vol PEP14-LGBTKIDS. Rockville, MD: HHS Publication; 2014.
It is well established that LGBTQ individuals experience more health disparities compared with their cisgender, heterosexual counterparts. In general, LGBTQ adolescents and young adults have higher levels of depression, suicide attempts, and substance use than those of their heterosexual peers. However, a key protective factor is family acceptance and support. By encouraging families to modify and change behaviors that are experienced by their LGBTQ children as rejecting and to engage in supportive and affirming behaviors, providers can help families to decrease risk and promote healthy outcomes for LGBTQ youth and young adults. 
We all know that a supportive family can make a difference for any child, but this is especially true for LGBTQ youth and is critical during a pandemic when young people are confined with families and separated from peers and supportive adults outside the home. Several research studies show that family support can improve outcomes related to suicide, depression, homelessness, drug use, and HIV in LGBTQ young people. Family acceptance improves health outcomes, while rejection undermines family relationships and worsens both health and other serious outcomes such as homelessness and placement in custodial care. Pediatricians can help their patients by educating parents and caregivers with LGBTQ children about the critical role of family support – both those who see themselves as accepting and those who believe that being gay or transgender is wrong and are struggling with parenting a child who identifies as LGBTQ or who is gender diverse.
The Family Acceptance Project (FAP) at San Francisco State University conducted the first research on LGBTQ youth and families, developed the first evidence-informed family support model, and has published a range of studies and evidence-based resources that demonstrate the harm caused by family rejection, validate the importance of family acceptance, and provide guidance to increase family support. FAP’s research found that parents and caregivers that engage in rejecting behaviors are typically motivated by care and concern and by trying to protect their children from harm. They believe such behaviors will help their LGBTQ children fit in, have a good life, meet cultural and religious expectations, and be respected by others.1 FAP’s research identified and measured more than 50 rejecting behaviors that parents and caregivers use to respond to their LGBTQ children. Some of these commonly expressed rejecting behaviors include ridiculing and making disparaging comments about their child and other LGBTQ people; excluding them from family activities; blaming their child when others mistreat them because they are LGBTQ; blocking access to LGBTQ resources including friends, support groups, and activities; and trying to change their child’s sexual orientation and gender identity.2 LGBTQ youth experience these and other such behaviors as hurtful, harmful, and traumatic and may feel that they need to hide or repress their identity which can affect their self-esteem, increase isolation, depression, and risky behaviors.3 Providers working with families of LGBTQ youth should focus on shared goals, such as reducing risk and having a happy, healthy child. Most parents love their children and fear for their well-being. However, many are uninformed about their child’s gender identity and sexual orientation and don’t know how to nurture and support them.
In FAP’s initial study, LGB young people who reported higher levels of family rejection had substantially higher rates of attempted suicide, depression, illegal drug use, and unprotected sex.4 These rates were even more significant among Latino gay and bisexual men.4 Those who are rejected by family are less likely to want to have a family or to be parents themselves5 and have lower educational and income levels.6
To reduce risk, pediatricians should ask LGBTQ patients about family rejecting behaviors and help parents and caregivers to identify and understand the effect of such behaviors to reduce health risks and conflict that can lead to running away, expulsion, and removal from the home. Even decreasing rejecting behaviors to moderate levels can significantly improve negative outcomes.5
Caitlin Ryan, PhD, and her team also identified and measured more than 50 family accepting behaviors that help protect against risk and promote well-being. They found that young adults who experience high levels of family acceptance during adolescence report significantly higher levels of self-esteem, social support, and general health with much lower levels of depression, suicidality, and substance abuse.7 Family accepting and supportive behaviors include talking with the child about their LGBTQ identity; advocating for their LGBTQ child when others mistreat them; requiring other family members to treat their LGBTQ child with respect; and supporting their child’s gender identity.5 FAP has developed an evidence-informed family support model and multilingual educational resources for families, providers, youth and religious leaders to decrease rejection and increase family support. These are available in print copies and for download at familyproject.sfsu.edu.
In addition, Dr. Ryan and colleagues1,4,8 recommend the following guidance for providers:
- Ask LGBTQ adolescents about family reactions to their sexual orientation, gender identity, and expression, and refer to LGBTQ community support programs and for supportive counseling, as needed.
- Identify LGBTQ community support programs and online resources to educate parents about how to help their children. Parents need culturally relevant peer support to help decrease rejection and increase family support.
- Advise parents that negative reactions to their adolescent’s LGBTQ identity may negatively impact their child’s health and mental health while supportive and affirming reactions promote well-being.
- Advise parents and caregivers to modify and change family rejecting behaviors that increase their child’s risk for suicide, depression, substance abuse ,and risky sexual behaviors.
- Expand anticipatory guidance to include information on the need for support and the link between family rejection and negative health problems.
- Provide guidance on sexual orientation and gender identity as part of normative child development during well-baby and early childhood care.
- Use FAP’s multilingual family education booklets and Healthy Futures poster series in family and patient education and provide these materials in clinical and community settings. FAP’s Healthy Futures posters include a poster guidance, a version on family acceptance, a version on family rejection and a family acceptance version for conservative families and settings. They are available in camera-ready art in four sizes in English and Spanish and are forthcoming in five Asian languages: familyproject.sfsu.edu/poster.
Dr. Lawlis is assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures.
Resources
• Family Acceptance Project – consultation and training; evidence-based educational materials for families, providers, religious leaders and youth.
• PFLAG – peer support for parents and friends with LGBTQ children in all states and several other countries.
References
1. Ryan C. Generating a revolution in prevention, wellness & care for LGBT children & youth. Temple Political & Civil Rights Law Review. 2014;23(2):331-44.
2. Ryan C. Healthy Futures Poster Series – Family Accepting & Rejecting Behaviors That Impact LGBTQ Children’s Health & Well-Being. In: Family Acceptance Project Marian Wright Edelman Institute SFSU, ed. San Francisco, CA2019.
3. Ryan C. Family Acceptance Project: Culturally grounded framework for supporting LGBTQ children and youth. J Am Acad Child Adolesc Psychiatr. 2019;58(10):S58-9.
4. Ryan C et al. Family rejection as a predictor of negative health outcomes in White and Latino lesbian, gay, and bisexual young adults. Pediatrics. 2009;123(1):346-52.
5. Ryan C. Supportive families, healthy children: Helping families with lesbian, gay, bisexual & transgender children. In: Family Acceptance Project Marian Wright Edelman Institute SFSU, ed. San Francisco, CA2009.
6. Ryan C et al. Parent-initiated sexual orientation change efforts with LGBT adolescents: Implications for young adult mental health and adjustment. J Homosexuality. 2020;67(2):159-73.
7. Ryan C et al. Family acceptance in adolescence and the health of LGBT young adults. J Child Adolesc Psychiatr Nursing. 2010;23(4):205-13. 8. Substance Abuse and Mental Health Services Administration. A Practitioner’s Guide: Helping Families to Support Their LGBT Children. In: Administration SAaMhS, ed. Vol PEP14-LGBTKIDS. Rockville, MD: HHS Publication; 2014.
It is well established that LGBTQ individuals experience more health disparities compared with their cisgender, heterosexual counterparts. In general, LGBTQ adolescents and young adults have higher levels of depression, suicide attempts, and substance use than those of their heterosexual peers. However, a key protective factor is family acceptance and support. By encouraging families to modify and change behaviors that are experienced by their LGBTQ children as rejecting and to engage in supportive and affirming behaviors, providers can help families to decrease risk and promote healthy outcomes for LGBTQ youth and young adults. 
We all know that a supportive family can make a difference for any child, but this is especially true for LGBTQ youth and is critical during a pandemic when young people are confined with families and separated from peers and supportive adults outside the home. Several research studies show that family support can improve outcomes related to suicide, depression, homelessness, drug use, and HIV in LGBTQ young people. Family acceptance improves health outcomes, while rejection undermines family relationships and worsens both health and other serious outcomes such as homelessness and placement in custodial care. Pediatricians can help their patients by educating parents and caregivers with LGBTQ children about the critical role of family support – both those who see themselves as accepting and those who believe that being gay or transgender is wrong and are struggling with parenting a child who identifies as LGBTQ or who is gender diverse.
The Family Acceptance Project (FAP) at San Francisco State University conducted the first research on LGBTQ youth and families, developed the first evidence-informed family support model, and has published a range of studies and evidence-based resources that demonstrate the harm caused by family rejection, validate the importance of family acceptance, and provide guidance to increase family support. FAP’s research found that parents and caregivers that engage in rejecting behaviors are typically motivated by care and concern and by trying to protect their children from harm. They believe such behaviors will help their LGBTQ children fit in, have a good life, meet cultural and religious expectations, and be respected by others.1 FAP’s research identified and measured more than 50 rejecting behaviors that parents and caregivers use to respond to their LGBTQ children. Some of these commonly expressed rejecting behaviors include ridiculing and making disparaging comments about their child and other LGBTQ people; excluding them from family activities; blaming their child when others mistreat them because they are LGBTQ; blocking access to LGBTQ resources including friends, support groups, and activities; and trying to change their child’s sexual orientation and gender identity.2 LGBTQ youth experience these and other such behaviors as hurtful, harmful, and traumatic and may feel that they need to hide or repress their identity which can affect their self-esteem, increase isolation, depression, and risky behaviors.3 Providers working with families of LGBTQ youth should focus on shared goals, such as reducing risk and having a happy, healthy child. Most parents love their children and fear for their well-being. However, many are uninformed about their child’s gender identity and sexual orientation and don’t know how to nurture and support them.
In FAP’s initial study, LGB young people who reported higher levels of family rejection had substantially higher rates of attempted suicide, depression, illegal drug use, and unprotected sex.4 These rates were even more significant among Latino gay and bisexual men.4 Those who are rejected by family are less likely to want to have a family or to be parents themselves5 and have lower educational and income levels.6
To reduce risk, pediatricians should ask LGBTQ patients about family rejecting behaviors and help parents and caregivers to identify and understand the effect of such behaviors to reduce health risks and conflict that can lead to running away, expulsion, and removal from the home. Even decreasing rejecting behaviors to moderate levels can significantly improve negative outcomes.5
Caitlin Ryan, PhD, and her team also identified and measured more than 50 family accepting behaviors that help protect against risk and promote well-being. They found that young adults who experience high levels of family acceptance during adolescence report significantly higher levels of self-esteem, social support, and general health with much lower levels of depression, suicidality, and substance abuse.7 Family accepting and supportive behaviors include talking with the child about their LGBTQ identity; advocating for their LGBTQ child when others mistreat them; requiring other family members to treat their LGBTQ child with respect; and supporting their child’s gender identity.5 FAP has developed an evidence-informed family support model and multilingual educational resources for families, providers, youth and religious leaders to decrease rejection and increase family support. These are available in print copies and for download at familyproject.sfsu.edu.
In addition, Dr. Ryan and colleagues1,4,8 recommend the following guidance for providers:
- Ask LGBTQ adolescents about family reactions to their sexual orientation, gender identity, and expression, and refer to LGBTQ community support programs and for supportive counseling, as needed.
- Identify LGBTQ community support programs and online resources to educate parents about how to help their children. Parents need culturally relevant peer support to help decrease rejection and increase family support.
- Advise parents that negative reactions to their adolescent’s LGBTQ identity may negatively impact their child’s health and mental health while supportive and affirming reactions promote well-being.
- Advise parents and caregivers to modify and change family rejecting behaviors that increase their child’s risk for suicide, depression, substance abuse ,and risky sexual behaviors.
- Expand anticipatory guidance to include information on the need for support and the link between family rejection and negative health problems.
- Provide guidance on sexual orientation and gender identity as part of normative child development during well-baby and early childhood care.
- Use FAP’s multilingual family education booklets and Healthy Futures poster series in family and patient education and provide these materials in clinical and community settings. FAP’s Healthy Futures posters include a poster guidance, a version on family acceptance, a version on family rejection and a family acceptance version for conservative families and settings. They are available in camera-ready art in four sizes in English and Spanish and are forthcoming in five Asian languages: familyproject.sfsu.edu/poster.
Dr. Lawlis is assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures.
Resources
• Family Acceptance Project – consultation and training; evidence-based educational materials for families, providers, religious leaders and youth.
• PFLAG – peer support for parents and friends with LGBTQ children in all states and several other countries.
References
1. Ryan C. Generating a revolution in prevention, wellness & care for LGBT children & youth. Temple Political & Civil Rights Law Review. 2014;23(2):331-44.
2. Ryan C. Healthy Futures Poster Series – Family Accepting & Rejecting Behaviors That Impact LGBTQ Children’s Health & Well-Being. In: Family Acceptance Project Marian Wright Edelman Institute SFSU, ed. San Francisco, CA2019.
3. Ryan C. Family Acceptance Project: Culturally grounded framework for supporting LGBTQ children and youth. J Am Acad Child Adolesc Psychiatr. 2019;58(10):S58-9.
4. Ryan C et al. Family rejection as a predictor of negative health outcomes in White and Latino lesbian, gay, and bisexual young adults. Pediatrics. 2009;123(1):346-52.
5. Ryan C. Supportive families, healthy children: Helping families with lesbian, gay, bisexual & transgender children. In: Family Acceptance Project Marian Wright Edelman Institute SFSU, ed. San Francisco, CA2009.
6. Ryan C et al. Parent-initiated sexual orientation change efforts with LGBT adolescents: Implications for young adult mental health and adjustment. J Homosexuality. 2020;67(2):159-73.
7. Ryan C et al. Family acceptance in adolescence and the health of LGBT young adults. J Child Adolesc Psychiatr Nursing. 2010;23(4):205-13. 8. Substance Abuse and Mental Health Services Administration. A Practitioner’s Guide: Helping Families to Support Their LGBT Children. In: Administration SAaMhS, ed. Vol PEP14-LGBTKIDS. Rockville, MD: HHS Publication; 2014.
Child ‘Mis’behavior – What’s ‘mis’ing?
“What kind of parent are you? Why don’t you straighten him out!” rants the woman being jostled in the grocery store by your patient. “Easy for you to say,” thinks your patient’s frazzled and now insulted parent.
Blaming the parent for an out-of-control child has historically been a common refrain of neighbors, relatives, and even strangers. But considering child behavior as resulting from both parent and child factors is central to the current transactional model of child development. In this model, mismatch of the parent’s and child’s response patterns is seen as setting them up for chronically rough interactions around parent requests/demands. A parent escalating quickly from a briefly stated request to a tirade may create more tension paired with an anxious child who takes time to act, for example. Once a parent (and ultimately the child) recognize patterns in what leads to conflict, they can become more proactive in predicting and negotiating these situations. Ross Greene, PhD, explains this in his book “The Explosive Child,” calling the method Collaborative Problem Solving (now Collaborative & Proactive Solutions or CPS).
While there are general principles parents can use to modify what they consider “mis”behaviors, these methods often do not account for the “missing” skills of the individual child (and parent) predisposing to those “mis”takes. Thinking of misbehaviors as being because of a kind of “learning disability” in the child rather than willful defiance can help cool off interactions by instead focusing on solving the underlying problem.
What kinds of “gaps in skills” set a child up for defiant or explosive reactions? If you think about what features of children, and parent-child relationships are associated with harmonious interactions this becomes evident. Children over 3 who are patient, easygoing, flexible or adaptable, and good at transitions and problem-solving can delay gratification and tolerate frustration, regulate their emotions, explain their desires, and multitask. They are better at reading the parent’s needs and intent and tend to interpret requests as positive or at least neutral and are more likely to comply with parent requests without a fuss.
What? No kid you know is great at all of these? These skills, at best variable, develop with maturation. Some are part of temperament, considered normal variation in personality. For example, so-called difficult temperament includes low adaptability, high-intensity reactions, low regularity, tendency to withdraw, and negative mood. But in the extreme, weaknesses in these skills are core to or comorbid with diagnosable mental health disorders. Defiance and irritable responses are criteria for oppositional defiant disorder (ODD), and less severe categories called aggressive/oppositional problem or variation. ODD is often found in children diagnosed with ADHD (65%), Tourette’s (15%-65%), depression (70% if severe), bipolar disorder (85%), OCD, anxiety (45%), autism, and language-processing disorders (55%), or trauma. These conditions variably include lower emotion regulation, poorer executive functioning including poor task shifting and impulsivity, obsessiveness, lower expressive and receptive communication skills, and less social awareness that facilitates harmonious problem solving.
The basic components of the CPS approach to addressing parent-child conflict sound intuitive but defining them clearly is important when families are stuck. There are three levels of plans. If the problem is an emergency or nonnegotiable, e.g., child hurting the cat, it may call for Plan A – parent-imposed solutions, sometimes with consequences or rewards. As children mature, Plan A should be used less frequently. If solving the problem is not a top life priority, Plan C – postponing action, may be appropriate. Plan C highlights that behavior change is a long-term project and “picking your fights” is important.
The biggest value of CPS for resolving behavior problems comes from intermediate Plan B. In Plan B the first step of problem solving for parents facing child defiance or upset is to empathically and nonjudgmentally figure out the child’s concern. Questions such as “I’ve noticed that when I remind you that it is trash night you start shouting. What’s up with that?” then patiently asking about the who, what, where, and when of their concern and checking to ensure understanding. Specificity is important as well as noting times when the reaction occurs or not.
Once the child’s concern is clear, e.g., feeling that the demand to take out the trash now interrupts his games during the only time his friends are online, the parents should echo the child’s concern then express their own concern about how the behavior is affecting them and others, potentially including the child; e.g., mother is so upset by the shouting that she can’t sleep, and worry that the child is not learning responsibility, and then checking for child understanding.
Finally, the parent invites brainstorming for a solution that addresses both of their concerns, first asking the child for suggestions, aiming for a strategy that is realistic and specific. Children reluctant to make suggestions may need more time and the parent may be wondering “if there is a way for both of our concerns to be addressed.” Solutions chosen are then tried for several weeks, success tracked, and needed changes negotiated.
For parents, using a collaborative approach to dealing with their child’s behavior takes skills they may not have at the moment, or ever. Especially under the stresses of COVID-19 lockdown, taking a step back from an encounter to consider lack of a skill to turn off the video game promptly when a Zoom meeting starts is challenging. Parents may also genetically share the child’s predisposing ADHD, anxiety, depression, OCD, or weakness in communication or social sensitivity.
Sometimes part of the solution for a conflict is for the parent to reduce expectations. This requires understanding and accepting the child’s cognitive or emotional limitations. Reducing expectations is ideally done before a request rather than by giving in after it, which reinforces protests. For authoritarian adults rigid in their belief that parents are boss, changing expectations can be tough and can feel like losing control or failing as a leader. One benefit of working with a CPS coach (see livesinthebalance.org or ThinkKids.org) is to help parents identify their own limitations.
Predicting the types of demands that tend to create conflict, such as to act immediately or be flexible about options, allows parents to prioritize those requests for calmer moments or when there is more time for discussion. Reviewing a checklist of common gaps in skills and creating a list of expectations and triggers that are difficult for the child helps the family be more proactive in developing solutions. Authors of CPS have validated a checklist of skill deficits, “Thinking Skills Inventory,” to facilitate detection of gaps that is educational plus useful for planning specific solutions.
CPS has been shown in randomized trials with both parent groups and in home counseling to be as effective as Parent Training in reducing oppositional behavior and reducing maternal stress, with effects lasting even longer.
CPS Plan B notably has no reward or punishment components as it assumes the child wants to behave acceptably but can’t; has the “will but not the skill.” When skill deficits are worked around the child is satisfied with complying and pleasing the parents. The idea of a “function” of the misbehavior for the child of gaining attention or reward or avoiding consequences is reinterpreted as serving to communicate the problem the child is having trouble in meeting the parent’s demand. When the parent understands and helps the child solve the problem his/her misbehavior is no longer needed. A benefit of the communication and mutual problem solving used in CPS is on not only improving behavior but empowering parents and children, building parental empathy, and improving child skills.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at pdnews@mdedge.com.
Reference
Greene RW et al. A transactional model of oppositional behavior: Underpinnings of the Collaborative Problem Solving approach. J Psychosom Res. 2003;55(1):67-75.
“What kind of parent are you? Why don’t you straighten him out!” rants the woman being jostled in the grocery store by your patient. “Easy for you to say,” thinks your patient’s frazzled and now insulted parent.
Blaming the parent for an out-of-control child has historically been a common refrain of neighbors, relatives, and even strangers. But considering child behavior as resulting from both parent and child factors is central to the current transactional model of child development. In this model, mismatch of the parent’s and child’s response patterns is seen as setting them up for chronically rough interactions around parent requests/demands. A parent escalating quickly from a briefly stated request to a tirade may create more tension paired with an anxious child who takes time to act, for example. Once a parent (and ultimately the child) recognize patterns in what leads to conflict, they can become more proactive in predicting and negotiating these situations. Ross Greene, PhD, explains this in his book “The Explosive Child,” calling the method Collaborative Problem Solving (now Collaborative & Proactive Solutions or CPS).
While there are general principles parents can use to modify what they consider “mis”behaviors, these methods often do not account for the “missing” skills of the individual child (and parent) predisposing to those “mis”takes. Thinking of misbehaviors as being because of a kind of “learning disability” in the child rather than willful defiance can help cool off interactions by instead focusing on solving the underlying problem.
What kinds of “gaps in skills” set a child up for defiant or explosive reactions? If you think about what features of children, and parent-child relationships are associated with harmonious interactions this becomes evident. Children over 3 who are patient, easygoing, flexible or adaptable, and good at transitions and problem-solving can delay gratification and tolerate frustration, regulate their emotions, explain their desires, and multitask. They are better at reading the parent’s needs and intent and tend to interpret requests as positive or at least neutral and are more likely to comply with parent requests without a fuss.
What? No kid you know is great at all of these? These skills, at best variable, develop with maturation. Some are part of temperament, considered normal variation in personality. For example, so-called difficult temperament includes low adaptability, high-intensity reactions, low regularity, tendency to withdraw, and negative mood. But in the extreme, weaknesses in these skills are core to or comorbid with diagnosable mental health disorders. Defiance and irritable responses are criteria for oppositional defiant disorder (ODD), and less severe categories called aggressive/oppositional problem or variation. ODD is often found in children diagnosed with ADHD (65%), Tourette’s (15%-65%), depression (70% if severe), bipolar disorder (85%), OCD, anxiety (45%), autism, and language-processing disorders (55%), or trauma. These conditions variably include lower emotion regulation, poorer executive functioning including poor task shifting and impulsivity, obsessiveness, lower expressive and receptive communication skills, and less social awareness that facilitates harmonious problem solving.
The basic components of the CPS approach to addressing parent-child conflict sound intuitive but defining them clearly is important when families are stuck. There are three levels of plans. If the problem is an emergency or nonnegotiable, e.g., child hurting the cat, it may call for Plan A – parent-imposed solutions, sometimes with consequences or rewards. As children mature, Plan A should be used less frequently. If solving the problem is not a top life priority, Plan C – postponing action, may be appropriate. Plan C highlights that behavior change is a long-term project and “picking your fights” is important.
The biggest value of CPS for resolving behavior problems comes from intermediate Plan B. In Plan B the first step of problem solving for parents facing child defiance or upset is to empathically and nonjudgmentally figure out the child’s concern. Questions such as “I’ve noticed that when I remind you that it is trash night you start shouting. What’s up with that?” then patiently asking about the who, what, where, and when of their concern and checking to ensure understanding. Specificity is important as well as noting times when the reaction occurs or not.
Once the child’s concern is clear, e.g., feeling that the demand to take out the trash now interrupts his games during the only time his friends are online, the parents should echo the child’s concern then express their own concern about how the behavior is affecting them and others, potentially including the child; e.g., mother is so upset by the shouting that she can’t sleep, and worry that the child is not learning responsibility, and then checking for child understanding.
Finally, the parent invites brainstorming for a solution that addresses both of their concerns, first asking the child for suggestions, aiming for a strategy that is realistic and specific. Children reluctant to make suggestions may need more time and the parent may be wondering “if there is a way for both of our concerns to be addressed.” Solutions chosen are then tried for several weeks, success tracked, and needed changes negotiated.
For parents, using a collaborative approach to dealing with their child’s behavior takes skills they may not have at the moment, or ever. Especially under the stresses of COVID-19 lockdown, taking a step back from an encounter to consider lack of a skill to turn off the video game promptly when a Zoom meeting starts is challenging. Parents may also genetically share the child’s predisposing ADHD, anxiety, depression, OCD, or weakness in communication or social sensitivity.
Sometimes part of the solution for a conflict is for the parent to reduce expectations. This requires understanding and accepting the child’s cognitive or emotional limitations. Reducing expectations is ideally done before a request rather than by giving in after it, which reinforces protests. For authoritarian adults rigid in their belief that parents are boss, changing expectations can be tough and can feel like losing control or failing as a leader. One benefit of working with a CPS coach (see livesinthebalance.org or ThinkKids.org) is to help parents identify their own limitations.
Predicting the types of demands that tend to create conflict, such as to act immediately or be flexible about options, allows parents to prioritize those requests for calmer moments or when there is more time for discussion. Reviewing a checklist of common gaps in skills and creating a list of expectations and triggers that are difficult for the child helps the family be more proactive in developing solutions. Authors of CPS have validated a checklist of skill deficits, “Thinking Skills Inventory,” to facilitate detection of gaps that is educational plus useful for planning specific solutions.
CPS has been shown in randomized trials with both parent groups and in home counseling to be as effective as Parent Training in reducing oppositional behavior and reducing maternal stress, with effects lasting even longer.
CPS Plan B notably has no reward or punishment components as it assumes the child wants to behave acceptably but can’t; has the “will but not the skill.” When skill deficits are worked around the child is satisfied with complying and pleasing the parents. The idea of a “function” of the misbehavior for the child of gaining attention or reward or avoiding consequences is reinterpreted as serving to communicate the problem the child is having trouble in meeting the parent’s demand. When the parent understands and helps the child solve the problem his/her misbehavior is no longer needed. A benefit of the communication and mutual problem solving used in CPS is on not only improving behavior but empowering parents and children, building parental empathy, and improving child skills.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at pdnews@mdedge.com.
Reference
Greene RW et al. A transactional model of oppositional behavior: Underpinnings of the Collaborative Problem Solving approach. J Psychosom Res. 2003;55(1):67-75.
“What kind of parent are you? Why don’t you straighten him out!” rants the woman being jostled in the grocery store by your patient. “Easy for you to say,” thinks your patient’s frazzled and now insulted parent.
Blaming the parent for an out-of-control child has historically been a common refrain of neighbors, relatives, and even strangers. But considering child behavior as resulting from both parent and child factors is central to the current transactional model of child development. In this model, mismatch of the parent’s and child’s response patterns is seen as setting them up for chronically rough interactions around parent requests/demands. A parent escalating quickly from a briefly stated request to a tirade may create more tension paired with an anxious child who takes time to act, for example. Once a parent (and ultimately the child) recognize patterns in what leads to conflict, they can become more proactive in predicting and negotiating these situations. Ross Greene, PhD, explains this in his book “The Explosive Child,” calling the method Collaborative Problem Solving (now Collaborative & Proactive Solutions or CPS).
While there are general principles parents can use to modify what they consider “mis”behaviors, these methods often do not account for the “missing” skills of the individual child (and parent) predisposing to those “mis”takes. Thinking of misbehaviors as being because of a kind of “learning disability” in the child rather than willful defiance can help cool off interactions by instead focusing on solving the underlying problem.
What kinds of “gaps in skills” set a child up for defiant or explosive reactions? If you think about what features of children, and parent-child relationships are associated with harmonious interactions this becomes evident. Children over 3 who are patient, easygoing, flexible or adaptable, and good at transitions and problem-solving can delay gratification and tolerate frustration, regulate their emotions, explain their desires, and multitask. They are better at reading the parent’s needs and intent and tend to interpret requests as positive or at least neutral and are more likely to comply with parent requests without a fuss.
What? No kid you know is great at all of these? These skills, at best variable, develop with maturation. Some are part of temperament, considered normal variation in personality. For example, so-called difficult temperament includes low adaptability, high-intensity reactions, low regularity, tendency to withdraw, and negative mood. But in the extreme, weaknesses in these skills are core to or comorbid with diagnosable mental health disorders. Defiance and irritable responses are criteria for oppositional defiant disorder (ODD), and less severe categories called aggressive/oppositional problem or variation. ODD is often found in children diagnosed with ADHD (65%), Tourette’s (15%-65%), depression (70% if severe), bipolar disorder (85%), OCD, anxiety (45%), autism, and language-processing disorders (55%), or trauma. These conditions variably include lower emotion regulation, poorer executive functioning including poor task shifting and impulsivity, obsessiveness, lower expressive and receptive communication skills, and less social awareness that facilitates harmonious problem solving.
The basic components of the CPS approach to addressing parent-child conflict sound intuitive but defining them clearly is important when families are stuck. There are three levels of plans. If the problem is an emergency or nonnegotiable, e.g., child hurting the cat, it may call for Plan A – parent-imposed solutions, sometimes with consequences or rewards. As children mature, Plan A should be used less frequently. If solving the problem is not a top life priority, Plan C – postponing action, may be appropriate. Plan C highlights that behavior change is a long-term project and “picking your fights” is important.
The biggest value of CPS for resolving behavior problems comes from intermediate Plan B. In Plan B the first step of problem solving for parents facing child defiance or upset is to empathically and nonjudgmentally figure out the child’s concern. Questions such as “I’ve noticed that when I remind you that it is trash night you start shouting. What’s up with that?” then patiently asking about the who, what, where, and when of their concern and checking to ensure understanding. Specificity is important as well as noting times when the reaction occurs or not.
Once the child’s concern is clear, e.g., feeling that the demand to take out the trash now interrupts his games during the only time his friends are online, the parents should echo the child’s concern then express their own concern about how the behavior is affecting them and others, potentially including the child; e.g., mother is so upset by the shouting that she can’t sleep, and worry that the child is not learning responsibility, and then checking for child understanding.
Finally, the parent invites brainstorming for a solution that addresses both of their concerns, first asking the child for suggestions, aiming for a strategy that is realistic and specific. Children reluctant to make suggestions may need more time and the parent may be wondering “if there is a way for both of our concerns to be addressed.” Solutions chosen are then tried for several weeks, success tracked, and needed changes negotiated.
For parents, using a collaborative approach to dealing with their child’s behavior takes skills they may not have at the moment, or ever. Especially under the stresses of COVID-19 lockdown, taking a step back from an encounter to consider lack of a skill to turn off the video game promptly when a Zoom meeting starts is challenging. Parents may also genetically share the child’s predisposing ADHD, anxiety, depression, OCD, or weakness in communication or social sensitivity.
Sometimes part of the solution for a conflict is for the parent to reduce expectations. This requires understanding and accepting the child’s cognitive or emotional limitations. Reducing expectations is ideally done before a request rather than by giving in after it, which reinforces protests. For authoritarian adults rigid in their belief that parents are boss, changing expectations can be tough and can feel like losing control or failing as a leader. One benefit of working with a CPS coach (see livesinthebalance.org or ThinkKids.org) is to help parents identify their own limitations.
Predicting the types of demands that tend to create conflict, such as to act immediately or be flexible about options, allows parents to prioritize those requests for calmer moments or when there is more time for discussion. Reviewing a checklist of common gaps in skills and creating a list of expectations and triggers that are difficult for the child helps the family be more proactive in developing solutions. Authors of CPS have validated a checklist of skill deficits, “Thinking Skills Inventory,” to facilitate detection of gaps that is educational plus useful for planning specific solutions.
CPS has been shown in randomized trials with both parent groups and in home counseling to be as effective as Parent Training in reducing oppositional behavior and reducing maternal stress, with effects lasting even longer.
CPS Plan B notably has no reward or punishment components as it assumes the child wants to behave acceptably but can’t; has the “will but not the skill.” When skill deficits are worked around the child is satisfied with complying and pleasing the parents. The idea of a “function” of the misbehavior for the child of gaining attention or reward or avoiding consequences is reinterpreted as serving to communicate the problem the child is having trouble in meeting the parent’s demand. When the parent understands and helps the child solve the problem his/her misbehavior is no longer needed. A benefit of the communication and mutual problem solving used in CPS is on not only improving behavior but empowering parents and children, building parental empathy, and improving child skills.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at pdnews@mdedge.com.
Reference
Greene RW et al. A transactional model of oppositional behavior: Underpinnings of the Collaborative Problem Solving approach. J Psychosom Res. 2003;55(1):67-75.
The lost year – even for common respiratory viruses
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.
The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
Puppy love: Is losing a pet too hard for children?
The big news in the Wilkoff household is that Marilyn and I will be celebrating the arrival of a granddog into our nuclear family. Our younger daughter and her husband will be welcoming into their home a golden retriever puppy the first week in March. This may not seem like big news to some families and is certainly a step down on the priority list to the arrival of the four grandchildren that we already claim on our resume. But, you must understand that no one in our family has ever owned a dog.
Although my wife’s family had a dog, she apparently never really bonded with the canine. My pleas and occasional whining from our three children to get a dog were always met with my wife’s concerns about cleanliness and hygiene. We did have an antisocial cat who lived under a bed in the guest room or in the basement. His passing after 16 years when the kids were in college was not an event marked with any emotion beyond relief.
I think I harbored an unspoken concern about how I and our children might respond emotionally and psychologically to the inevitable death of what would likely have become our family’s best friend. Dispatching a belly-up goldfish after a month or two is small potatoes compared to putting down a tail-wagging, frisbee-catching, four-footed member of the family.
It turns out that my concerns about the mental health of our children may not have been unfounded. A recently published study from the Harvard Medical School and Massachusetts General Hospital found that children who had experienced the death of a loved pet were more likely to exhibit symptoms of psychopathology than were those who had loved a pet who was still alive (Crawford et al. Eur Child Adolesc Psychiatry. 2020 Sep 10. doi: 10.1007/s00787-020-01594-5). The observed effect of the loss was more pronounced in boys. There was also no statistical difference between the psychopathology symptoms of those children who had loved and lost and those children who had never loved a pet.
By the time I left for college I had grown up with five different dogs. I had endured the loss of sweet Mary, the boxer, when we moved to a small apartment and had to send her to a “farm.” I had watched 2-year-old Blackie experience a seizure that heralded his fatal bout with distemper. I shared the struggle with my parents as we made the decision to send my much loved inveterate car chasing “Butch” back to the pound.
However, I survived these losses and wonder whether they in some way prepared me for some of the emotional challenges that would come later in life. This study from Harvard sampled only children from birth to age 8 years. For those of us in primary care a more interesting study might be one that looked for any long-term associations between pet loss as a young child with adolescent and adult mental health. With the surge in pet ownership that has surfaced during the pandemic, there should be an abundance of clinical material to mine. The Harvard researchers’ findings should make us aware of the potential for psychopathology in a child who has suffered the loss of a pet. Each family must decide whether the plusses of pet ownership are worth the risk. However, I side with Tennyson who said it is better to have loved and lost than never to have loved at all.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
The big news in the Wilkoff household is that Marilyn and I will be celebrating the arrival of a granddog into our nuclear family. Our younger daughter and her husband will be welcoming into their home a golden retriever puppy the first week in March. This may not seem like big news to some families and is certainly a step down on the priority list to the arrival of the four grandchildren that we already claim on our resume. But, you must understand that no one in our family has ever owned a dog.
Although my wife’s family had a dog, she apparently never really bonded with the canine. My pleas and occasional whining from our three children to get a dog were always met with my wife’s concerns about cleanliness and hygiene. We did have an antisocial cat who lived under a bed in the guest room or in the basement. His passing after 16 years when the kids were in college was not an event marked with any emotion beyond relief.
I think I harbored an unspoken concern about how I and our children might respond emotionally and psychologically to the inevitable death of what would likely have become our family’s best friend. Dispatching a belly-up goldfish after a month or two is small potatoes compared to putting down a tail-wagging, frisbee-catching, four-footed member of the family.
It turns out that my concerns about the mental health of our children may not have been unfounded. A recently published study from the Harvard Medical School and Massachusetts General Hospital found that children who had experienced the death of a loved pet were more likely to exhibit symptoms of psychopathology than were those who had loved a pet who was still alive (Crawford et al. Eur Child Adolesc Psychiatry. 2020 Sep 10. doi: 10.1007/s00787-020-01594-5). The observed effect of the loss was more pronounced in boys. There was also no statistical difference between the psychopathology symptoms of those children who had loved and lost and those children who had never loved a pet.
By the time I left for college I had grown up with five different dogs. I had endured the loss of sweet Mary, the boxer, when we moved to a small apartment and had to send her to a “farm.” I had watched 2-year-old Blackie experience a seizure that heralded his fatal bout with distemper. I shared the struggle with my parents as we made the decision to send my much loved inveterate car chasing “Butch” back to the pound.
However, I survived these losses and wonder whether they in some way prepared me for some of the emotional challenges that would come later in life. This study from Harvard sampled only children from birth to age 8 years. For those of us in primary care a more interesting study might be one that looked for any long-term associations between pet loss as a young child with adolescent and adult mental health. With the surge in pet ownership that has surfaced during the pandemic, there should be an abundance of clinical material to mine. The Harvard researchers’ findings should make us aware of the potential for psychopathology in a child who has suffered the loss of a pet. Each family must decide whether the plusses of pet ownership are worth the risk. However, I side with Tennyson who said it is better to have loved and lost than never to have loved at all.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
The big news in the Wilkoff household is that Marilyn and I will be celebrating the arrival of a granddog into our nuclear family. Our younger daughter and her husband will be welcoming into their home a golden retriever puppy the first week in March. This may not seem like big news to some families and is certainly a step down on the priority list to the arrival of the four grandchildren that we already claim on our resume. But, you must understand that no one in our family has ever owned a dog.
Although my wife’s family had a dog, she apparently never really bonded with the canine. My pleas and occasional whining from our three children to get a dog were always met with my wife’s concerns about cleanliness and hygiene. We did have an antisocial cat who lived under a bed in the guest room or in the basement. His passing after 16 years when the kids were in college was not an event marked with any emotion beyond relief.
I think I harbored an unspoken concern about how I and our children might respond emotionally and psychologically to the inevitable death of what would likely have become our family’s best friend. Dispatching a belly-up goldfish after a month or two is small potatoes compared to putting down a tail-wagging, frisbee-catching, four-footed member of the family.
It turns out that my concerns about the mental health of our children may not have been unfounded. A recently published study from the Harvard Medical School and Massachusetts General Hospital found that children who had experienced the death of a loved pet were more likely to exhibit symptoms of psychopathology than were those who had loved a pet who was still alive (Crawford et al. Eur Child Adolesc Psychiatry. 2020 Sep 10. doi: 10.1007/s00787-020-01594-5). The observed effect of the loss was more pronounced in boys. There was also no statistical difference between the psychopathology symptoms of those children who had loved and lost and those children who had never loved a pet.
By the time I left for college I had grown up with five different dogs. I had endured the loss of sweet Mary, the boxer, when we moved to a small apartment and had to send her to a “farm.” I had watched 2-year-old Blackie experience a seizure that heralded his fatal bout with distemper. I shared the struggle with my parents as we made the decision to send my much loved inveterate car chasing “Butch” back to the pound.
However, I survived these losses and wonder whether they in some way prepared me for some of the emotional challenges that would come later in life. This study from Harvard sampled only children from birth to age 8 years. For those of us in primary care a more interesting study might be one that looked for any long-term associations between pet loss as a young child with adolescent and adult mental health. With the surge in pet ownership that has surfaced during the pandemic, there should be an abundance of clinical material to mine. The Harvard researchers’ findings should make us aware of the potential for psychopathology in a child who has suffered the loss of a pet. Each family must decide whether the plusses of pet ownership are worth the risk. However, I side with Tennyson who said it is better to have loved and lost than never to have loved at all.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.















