User login
Changing terminology in LGBTQ+ spaces: How to keep up with the lingo
For those of us who see adolescent patients on a regular basis, it seems that they use new vocabulary almost every day. While you may not need to know what “lit” means, you probably do need to understand terms used to describe your patients’ identities. At times it feels like we, as providers, have to be on TikTok to keep up with our patients, and while this may be an amusing way to educate ourselves, a judicious Google search can be much more helpful. The interesting part about LGBTQ+ terminology is that it stems from the community and thus is frequently updated to reflect our evolving understanding of gender, sexuality, and identity. That being said, it can make it difficult for those who are not plugged in to the community to keep up to date. While we have learned in medicine to use accurate terminology and appropriate three-letter acronyms (or “TLAs” as one of my residents referenced them when I was a medical student) to describe medical conditions, the LGBTQ+ community has its own set of terms and acronyms. These new words may seem daunting, but they are often based in Latin roots or prefixes such as a-, demi-, poly-, and pan-, which may be familiar to those of us who use plenty of other Latin-based terms in medicine and our everyday lives. By paying attention to how people define and use terminology, we can better recognize their true identities and become better providers.
The first, and perhaps most important, piece of advice is to maintain cultural humility. Know when to admit you don’t recognize a term and politely ask the definition. For example, the first time I heard the term “demiboy” I said “I’m not familiar with that word. Can you explain what it means to you?” Phrasing the question as such is also helpful in that it gives the individuals a chance to really define their identity. In addition, some words may be used differently by various individuals and by asking what the word means to them, you can have a better understanding of how they are using the terminology. In this particular instance, the patient felt more masculine, but not 100%, partway between agender (meaning having no gender identity) and being “all male.” By embracing cultural humility, we place the patients in the role of expert on their own identity and orientation. According to Maria Ruud, DNP, of the University of Minnesota, Minneapolis, cultural humility is the “ongoing self-reflection and education …[seeking] to gain an awareness of their own assumptions and biases that may contribute to health disparities.”1
Another reason it is important to keep up on the language is that some adolescents, particularly younger adolescents, may not be using the terminology correctly. It can be very helpful to know the difference between polyamorous and pansexual when a 12-year-old describes themselves as polyamorous (having consenting, nonmonogamous relationships) but provides the definition for pansexual (being attracted to all gender identities). Yes, this has happened to me, and yes, my resident was appropriately confused. Correcting someone else’s vocabulary can be tricky and even inappropriate or condescending; therefore, tread cautiously. It may be appropriate, however, to correct colleagues’ or even patients’ family members’ language if they are using terms that may be hurtful to your patients. I do not allow slurs in my clinic, and when parents are using incorrect pronouns on purpose, I will often let them know that it is my job to respect their child’s identity where it is in the moment and that they have asked me to use specific pronouns, so I will continue to refer to their child with those pronouns. Reflecting the language of the patient can be a powerful statement providing them with the autonomy that they deserve as burgeoning adults navigating the complicated journey of identity.
As providers who often have to defend ourselves against “Dr. Google,” we may be leery of just searching randomly for the definition of a new word and hoping a site is credible. One site that I have used repeatedly is www.itspronouncedmetrosexual.com by Sam Killermann,2 a gender and sexuality educator.
Mr. Killermann has also produced an E-book that is regularly updated to reflect changing terminology, which can be obtained for a small donation. As Mr. Killermann explains, “New language can be intimidating, and the language of gender and sexuality is often that.”3 In reality, the definitions aren’t scary and often the words can describe something you already know exists but didn’t recognize had a specific term. Not everyone can know every term and its definition; in fact, many members of the LGBTQ+ community don’t know or even understand every term. Below is a shortened list with some of the more common terms you may encounter; however, individuals may use them differently so it is never out of place to clarify your understanding of the term’s definition.
With these resources, along with cultural humility and reflection of others’ language, we can all start to have more meaningful conversations with our patients around their identity and relationships with others.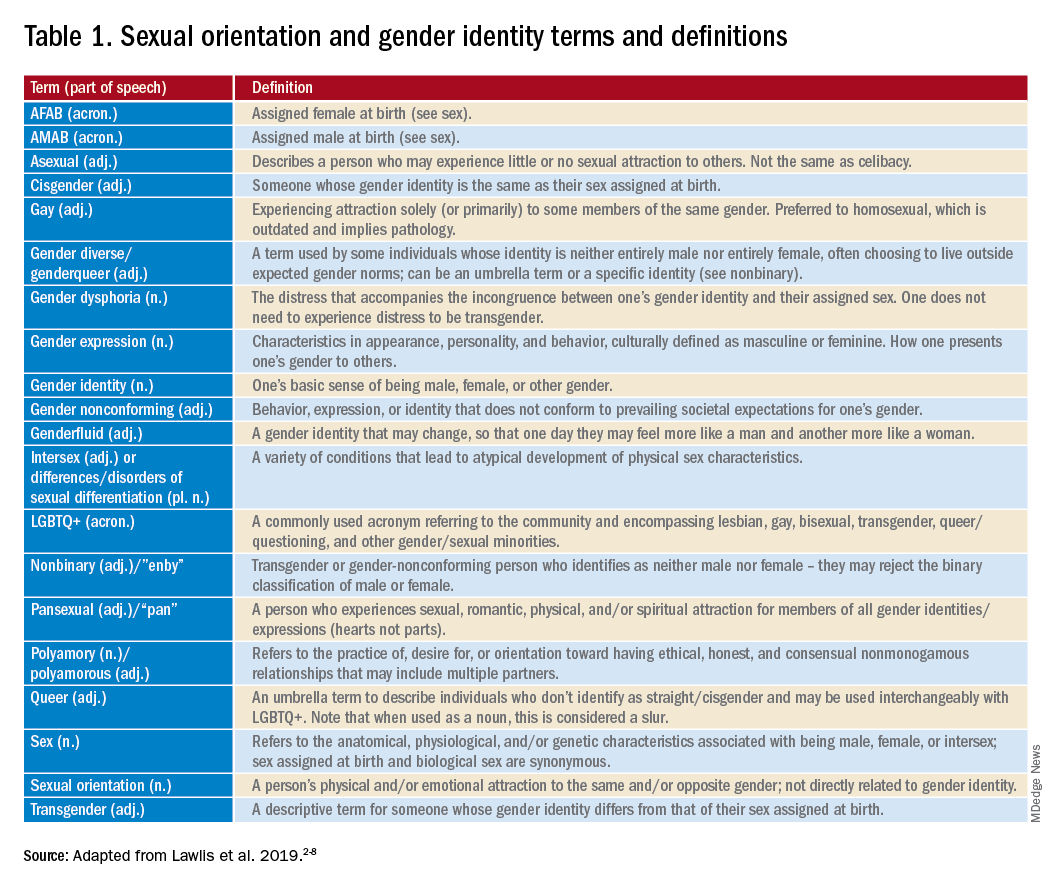
Dr. Lawlis is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Ruud M. Nursing for women’s health. 2018;22(3):255-63.
2. Killermann S. It’s Pronounced Metrosexual. 2020.
3. Killermann S. Defining LGBTQ+: A guide to gender and sexuality terminology. 2019, Feb 25.
4. The Joint Commission. Advancing effective communication, cultural competence, and patient- and family-centered care for the lesbian, gay, bisexual, and transgender (LGBT) community: A field guide. Oak Brook, Ill. 2011.
5. LGBT health disparities. American Psychiatric Association Public Interest Government Relations Office. 2013 May.
6. Lawlis S et al. Health services for LGBTQ+ patients. Psychiatr Ann. 2019;49(10):426-35.
7. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
8. Center of Excellence for Transgender Health, department of family and community medicine, UCSF. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. 2016 Jun 17.
For those of us who see adolescent patients on a regular basis, it seems that they use new vocabulary almost every day. While you may not need to know what “lit” means, you probably do need to understand terms used to describe your patients’ identities. At times it feels like we, as providers, have to be on TikTok to keep up with our patients, and while this may be an amusing way to educate ourselves, a judicious Google search can be much more helpful. The interesting part about LGBTQ+ terminology is that it stems from the community and thus is frequently updated to reflect our evolving understanding of gender, sexuality, and identity. That being said, it can make it difficult for those who are not plugged in to the community to keep up to date. While we have learned in medicine to use accurate terminology and appropriate three-letter acronyms (or “TLAs” as one of my residents referenced them when I was a medical student) to describe medical conditions, the LGBTQ+ community has its own set of terms and acronyms. These new words may seem daunting, but they are often based in Latin roots or prefixes such as a-, demi-, poly-, and pan-, which may be familiar to those of us who use plenty of other Latin-based terms in medicine and our everyday lives. By paying attention to how people define and use terminology, we can better recognize their true identities and become better providers.
The first, and perhaps most important, piece of advice is to maintain cultural humility. Know when to admit you don’t recognize a term and politely ask the definition. For example, the first time I heard the term “demiboy” I said “I’m not familiar with that word. Can you explain what it means to you?” Phrasing the question as such is also helpful in that it gives the individuals a chance to really define their identity. In addition, some words may be used differently by various individuals and by asking what the word means to them, you can have a better understanding of how they are using the terminology. In this particular instance, the patient felt more masculine, but not 100%, partway between agender (meaning having no gender identity) and being “all male.” By embracing cultural humility, we place the patients in the role of expert on their own identity and orientation. According to Maria Ruud, DNP, of the University of Minnesota, Minneapolis, cultural humility is the “ongoing self-reflection and education …[seeking] to gain an awareness of their own assumptions and biases that may contribute to health disparities.”1
Another reason it is important to keep up on the language is that some adolescents, particularly younger adolescents, may not be using the terminology correctly. It can be very helpful to know the difference between polyamorous and pansexual when a 12-year-old describes themselves as polyamorous (having consenting, nonmonogamous relationships) but provides the definition for pansexual (being attracted to all gender identities). Yes, this has happened to me, and yes, my resident was appropriately confused. Correcting someone else’s vocabulary can be tricky and even inappropriate or condescending; therefore, tread cautiously. It may be appropriate, however, to correct colleagues’ or even patients’ family members’ language if they are using terms that may be hurtful to your patients. I do not allow slurs in my clinic, and when parents are using incorrect pronouns on purpose, I will often let them know that it is my job to respect their child’s identity where it is in the moment and that they have asked me to use specific pronouns, so I will continue to refer to their child with those pronouns. Reflecting the language of the patient can be a powerful statement providing them with the autonomy that they deserve as burgeoning adults navigating the complicated journey of identity.
As providers who often have to defend ourselves against “Dr. Google,” we may be leery of just searching randomly for the definition of a new word and hoping a site is credible. One site that I have used repeatedly is www.itspronouncedmetrosexual.com by Sam Killermann,2 a gender and sexuality educator.
Mr. Killermann has also produced an E-book that is regularly updated to reflect changing terminology, which can be obtained for a small donation. As Mr. Killermann explains, “New language can be intimidating, and the language of gender and sexuality is often that.”3 In reality, the definitions aren’t scary and often the words can describe something you already know exists but didn’t recognize had a specific term. Not everyone can know every term and its definition; in fact, many members of the LGBTQ+ community don’t know or even understand every term. Below is a shortened list with some of the more common terms you may encounter; however, individuals may use them differently so it is never out of place to clarify your understanding of the term’s definition.
With these resources, along with cultural humility and reflection of others’ language, we can all start to have more meaningful conversations with our patients around their identity and relationships with others.
Dr. Lawlis is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Ruud M. Nursing for women’s health. 2018;22(3):255-63.
2. Killermann S. It’s Pronounced Metrosexual. 2020.
3. Killermann S. Defining LGBTQ+: A guide to gender and sexuality terminology. 2019, Feb 25.
4. The Joint Commission. Advancing effective communication, cultural competence, and patient- and family-centered care for the lesbian, gay, bisexual, and transgender (LGBT) community: A field guide. Oak Brook, Ill. 2011.
5. LGBT health disparities. American Psychiatric Association Public Interest Government Relations Office. 2013 May.
6. Lawlis S et al. Health services for LGBTQ+ patients. Psychiatr Ann. 2019;49(10):426-35.
7. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
8. Center of Excellence for Transgender Health, department of family and community medicine, UCSF. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. 2016 Jun 17.
For those of us who see adolescent patients on a regular basis, it seems that they use new vocabulary almost every day. While you may not need to know what “lit” means, you probably do need to understand terms used to describe your patients’ identities. At times it feels like we, as providers, have to be on TikTok to keep up with our patients, and while this may be an amusing way to educate ourselves, a judicious Google search can be much more helpful. The interesting part about LGBTQ+ terminology is that it stems from the community and thus is frequently updated to reflect our evolving understanding of gender, sexuality, and identity. That being said, it can make it difficult for those who are not plugged in to the community to keep up to date. While we have learned in medicine to use accurate terminology and appropriate three-letter acronyms (or “TLAs” as one of my residents referenced them when I was a medical student) to describe medical conditions, the LGBTQ+ community has its own set of terms and acronyms. These new words may seem daunting, but they are often based in Latin roots or prefixes such as a-, demi-, poly-, and pan-, which may be familiar to those of us who use plenty of other Latin-based terms in medicine and our everyday lives. By paying attention to how people define and use terminology, we can better recognize their true identities and become better providers.
The first, and perhaps most important, piece of advice is to maintain cultural humility. Know when to admit you don’t recognize a term and politely ask the definition. For example, the first time I heard the term “demiboy” I said “I’m not familiar with that word. Can you explain what it means to you?” Phrasing the question as such is also helpful in that it gives the individuals a chance to really define their identity. In addition, some words may be used differently by various individuals and by asking what the word means to them, you can have a better understanding of how they are using the terminology. In this particular instance, the patient felt more masculine, but not 100%, partway between agender (meaning having no gender identity) and being “all male.” By embracing cultural humility, we place the patients in the role of expert on their own identity and orientation. According to Maria Ruud, DNP, of the University of Minnesota, Minneapolis, cultural humility is the “ongoing self-reflection and education …[seeking] to gain an awareness of their own assumptions and biases that may contribute to health disparities.”1
Another reason it is important to keep up on the language is that some adolescents, particularly younger adolescents, may not be using the terminology correctly. It can be very helpful to know the difference between polyamorous and pansexual when a 12-year-old describes themselves as polyamorous (having consenting, nonmonogamous relationships) but provides the definition for pansexual (being attracted to all gender identities). Yes, this has happened to me, and yes, my resident was appropriately confused. Correcting someone else’s vocabulary can be tricky and even inappropriate or condescending; therefore, tread cautiously. It may be appropriate, however, to correct colleagues’ or even patients’ family members’ language if they are using terms that may be hurtful to your patients. I do not allow slurs in my clinic, and when parents are using incorrect pronouns on purpose, I will often let them know that it is my job to respect their child’s identity where it is in the moment and that they have asked me to use specific pronouns, so I will continue to refer to their child with those pronouns. Reflecting the language of the patient can be a powerful statement providing them with the autonomy that they deserve as burgeoning adults navigating the complicated journey of identity.
As providers who often have to defend ourselves against “Dr. Google,” we may be leery of just searching randomly for the definition of a new word and hoping a site is credible. One site that I have used repeatedly is www.itspronouncedmetrosexual.com by Sam Killermann,2 a gender and sexuality educator.
Mr. Killermann has also produced an E-book that is regularly updated to reflect changing terminology, which can be obtained for a small donation. As Mr. Killermann explains, “New language can be intimidating, and the language of gender and sexuality is often that.”3 In reality, the definitions aren’t scary and often the words can describe something you already know exists but didn’t recognize had a specific term. Not everyone can know every term and its definition; in fact, many members of the LGBTQ+ community don’t know or even understand every term. Below is a shortened list with some of the more common terms you may encounter; however, individuals may use them differently so it is never out of place to clarify your understanding of the term’s definition.
With these resources, along with cultural humility and reflection of others’ language, we can all start to have more meaningful conversations with our patients around their identity and relationships with others.
Dr. Lawlis is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Ruud M. Nursing for women’s health. 2018;22(3):255-63.
2. Killermann S. It’s Pronounced Metrosexual. 2020.
3. Killermann S. Defining LGBTQ+: A guide to gender and sexuality terminology. 2019, Feb 25.
4. The Joint Commission. Advancing effective communication, cultural competence, and patient- and family-centered care for the lesbian, gay, bisexual, and transgender (LGBT) community: A field guide. Oak Brook, Ill. 2011.
5. LGBT health disparities. American Psychiatric Association Public Interest Government Relations Office. 2013 May.
6. Lawlis S et al. Health services for LGBTQ+ patients. Psychiatr Ann. 2019;49(10):426-35.
7. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
8. Center of Excellence for Transgender Health, department of family and community medicine, UCSF. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. 2016 Jun 17.
The child with hypertension: Diagnosis and management
This transcript has been edited for clarity. The transcript and an accompanying video first appeared on Medscape.com.
Justin L. Berk, MD, MPH, MBA: Welcome back to The Cribsiders, our video recap of our pediatric medicine podcast. We interview leading experts in the field to bring clinical pearls and practice-changing knowledge, and answer lingering questions about core topics in pediatric medicine. Chris, what is our topic today?
Christopher J. Chiu, MD: I was really happy to be able to talk about our recent episode with Dr. Carissa Baker-Smith, a pediatric cardiologist and director of the Nemours preventive cardiology program. She helped us review the pediatric screening guidelines for blood pressure, including initial workup and treatment.
Dr. Berk: This was a really great episode that a lot of people found really helpful. What were some of the key takeaway pearls that you think listeners would be interested in?
Dr. Chiu: We talked about when and how we should be checking blood pressures in children. Blood pressure should be checked at every well-child visit starting at age 3. But if they have other risk factors like kidney disease or a condition such as coarctation of the aorta, then blood pressure should be checked at every visit.
Dr. Berk: One thing she spoke about was how blood pressures should be measured. How should we be checking blood pressures in the clinic?
Dr. Chiu: Clinic blood pressures are usually checked with oscillometric devices. They can differ by manufacturer, but basically they find a mean arterial pressure and then each device has a method of calculating systolic and diastolic pressures. Now after that, if the child’s blood pressure is maybe abnormal, you want to double-check a manual blood pressure using Korotkoff sounds to confirm the blood pressure.
She reminded us that blood pressure should be measured with the child sitting with their back supported, feet flat on the floor, and arm at the level of the heart. Make sure you use the right size cuff. The bladder of the cuff should be 40% of the width of the arm, and about 80%-100% of the arm circumference. She recommends sizing up if you have to.
Dr. Berk: Accuracy of blood pressure management was a really important point, especially for diagnosis at this stage. Can you walk us through what we learned about diagnosis of hypertension?
Dr. Chiu: The definitions of hypertension come from the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Up until the age of 13, they define prehypertension as systolic and diastolic blood pressures between the 90th and 95th percentile, or if the blood pressure exceeds 120/80 mm Hg. Hypertension is defined when blood pressure reaches the 95th percentile. Now age 13 is when it gets a little hazy. Many changes in the guidelines happen at age 13, when hypertension starts being defined by adult guidelines. The 2017 adult hypertension guidelines define stage 1 hypertension as 130/89 to 139/89, and stage 2 hypertension as greater than 140/90.
Dr. Berk: How about workup of hypertension? The work of pediatric hypertension is always a little bit complex. What are some of the pearls you took away?
Dr. Chui: She talked about tailoring the workup to the child. So when we’re doing our workup, obviously physical exam should be the first thing we do. You have to assess and compare pulses, which is one of the most important parts of the initial evaluation. Obviously, looking at coarctation of the aorta, but also looking for things like a cushingoid appearance. If the child is less than 6 years of age, she recommends a referral to nephrology for more comprehensive renovascular workup, which probably will include renal ultrasound, urinalysis, metabolic panel, and thyroid studies.
We have to be cognizant of secondary causes of hypertension, such as endocrine tumors, hyperthyroidism, aortic disease, or even medication-induced hypertension. She told us that in the majority of these cases, especially with our obese older children, primary hypertension or essential hypertension is the most likely cause.
Dr. Berk: That was my big takeaway. If they’re really young, they need a big workup, but otherwise it is likely primary hypertension. What did we learn about treatment?
Dr. Chui: Just as we tailor our assessment to the child, we also have to tailor treatment. We know that lifestyle modification is usually the first line of treatment, especially for primary hypertension, and Dr. Baker-Smith tells us that we really need to perform counseling that meets the patient where they are. So if they like dancing to the newest TikTok trends or music videos, maybe we can encourage them to move more that way. Using our motivational interviewing skills is really key here.
If you want to start medication, Dr. Baker-Smith uses things like low-dose ACE inhibitors or calcium channel blockers, but obviously it’ll be tailored to the patient and any underlying conditions.
Dr. Berk: That’s great – a lot of wonderful pearls on the diagnosis and management of pediatric hypertension. Thank you for joining us for another video recap of The Cribsiders pediatric podcast. You can download the full podcast, Off the Cuff: Managing Pediatric Hypertension in Your Primary Care Clinic, on any podcast player, or check out our website at www.theCribsiders.com.
Christopher J. Chiu, MD, is assistant professor, department of internal medicine, division of general internal medicine, Ohio State University, Columbus; lead physician, general internal medicine, OSU Outpatient Care East; department of internal medicine, division of general internal medicine, Ohio State University Wexner Medical Center. Dr. Chiu has disclosed no relevant financial relationships. Justin L. Berk, MD, MPH, MBA, is assistant professor, department of medicine; assistant professor, department of pediatrics, Brown University, Providence, R.I.
This transcript has been edited for clarity. The transcript and an accompanying video first appeared on Medscape.com.
Justin L. Berk, MD, MPH, MBA: Welcome back to The Cribsiders, our video recap of our pediatric medicine podcast. We interview leading experts in the field to bring clinical pearls and practice-changing knowledge, and answer lingering questions about core topics in pediatric medicine. Chris, what is our topic today?
Christopher J. Chiu, MD: I was really happy to be able to talk about our recent episode with Dr. Carissa Baker-Smith, a pediatric cardiologist and director of the Nemours preventive cardiology program. She helped us review the pediatric screening guidelines for blood pressure, including initial workup and treatment.
Dr. Berk: This was a really great episode that a lot of people found really helpful. What were some of the key takeaway pearls that you think listeners would be interested in?
Dr. Chiu: We talked about when and how we should be checking blood pressures in children. Blood pressure should be checked at every well-child visit starting at age 3. But if they have other risk factors like kidney disease or a condition such as coarctation of the aorta, then blood pressure should be checked at every visit.
Dr. Berk: One thing she spoke about was how blood pressures should be measured. How should we be checking blood pressures in the clinic?
Dr. Chiu: Clinic blood pressures are usually checked with oscillometric devices. They can differ by manufacturer, but basically they find a mean arterial pressure and then each device has a method of calculating systolic and diastolic pressures. Now after that, if the child’s blood pressure is maybe abnormal, you want to double-check a manual blood pressure using Korotkoff sounds to confirm the blood pressure.
She reminded us that blood pressure should be measured with the child sitting with their back supported, feet flat on the floor, and arm at the level of the heart. Make sure you use the right size cuff. The bladder of the cuff should be 40% of the width of the arm, and about 80%-100% of the arm circumference. She recommends sizing up if you have to.
Dr. Berk: Accuracy of blood pressure management was a really important point, especially for diagnosis at this stage. Can you walk us through what we learned about diagnosis of hypertension?
Dr. Chiu: The definitions of hypertension come from the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Up until the age of 13, they define prehypertension as systolic and diastolic blood pressures between the 90th and 95th percentile, or if the blood pressure exceeds 120/80 mm Hg. Hypertension is defined when blood pressure reaches the 95th percentile. Now age 13 is when it gets a little hazy. Many changes in the guidelines happen at age 13, when hypertension starts being defined by adult guidelines. The 2017 adult hypertension guidelines define stage 1 hypertension as 130/89 to 139/89, and stage 2 hypertension as greater than 140/90.
Dr. Berk: How about workup of hypertension? The work of pediatric hypertension is always a little bit complex. What are some of the pearls you took away?
Dr. Chui: She talked about tailoring the workup to the child. So when we’re doing our workup, obviously physical exam should be the first thing we do. You have to assess and compare pulses, which is one of the most important parts of the initial evaluation. Obviously, looking at coarctation of the aorta, but also looking for things like a cushingoid appearance. If the child is less than 6 years of age, she recommends a referral to nephrology for more comprehensive renovascular workup, which probably will include renal ultrasound, urinalysis, metabolic panel, and thyroid studies.
We have to be cognizant of secondary causes of hypertension, such as endocrine tumors, hyperthyroidism, aortic disease, or even medication-induced hypertension. She told us that in the majority of these cases, especially with our obese older children, primary hypertension or essential hypertension is the most likely cause.
Dr. Berk: That was my big takeaway. If they’re really young, they need a big workup, but otherwise it is likely primary hypertension. What did we learn about treatment?
Dr. Chui: Just as we tailor our assessment to the child, we also have to tailor treatment. We know that lifestyle modification is usually the first line of treatment, especially for primary hypertension, and Dr. Baker-Smith tells us that we really need to perform counseling that meets the patient where they are. So if they like dancing to the newest TikTok trends or music videos, maybe we can encourage them to move more that way. Using our motivational interviewing skills is really key here.
If you want to start medication, Dr. Baker-Smith uses things like low-dose ACE inhibitors or calcium channel blockers, but obviously it’ll be tailored to the patient and any underlying conditions.
Dr. Berk: That’s great – a lot of wonderful pearls on the diagnosis and management of pediatric hypertension. Thank you for joining us for another video recap of The Cribsiders pediatric podcast. You can download the full podcast, Off the Cuff: Managing Pediatric Hypertension in Your Primary Care Clinic, on any podcast player, or check out our website at www.theCribsiders.com.
Christopher J. Chiu, MD, is assistant professor, department of internal medicine, division of general internal medicine, Ohio State University, Columbus; lead physician, general internal medicine, OSU Outpatient Care East; department of internal medicine, division of general internal medicine, Ohio State University Wexner Medical Center. Dr. Chiu has disclosed no relevant financial relationships. Justin L. Berk, MD, MPH, MBA, is assistant professor, department of medicine; assistant professor, department of pediatrics, Brown University, Providence, R.I.
This transcript has been edited for clarity. The transcript and an accompanying video first appeared on Medscape.com.
Justin L. Berk, MD, MPH, MBA: Welcome back to The Cribsiders, our video recap of our pediatric medicine podcast. We interview leading experts in the field to bring clinical pearls and practice-changing knowledge, and answer lingering questions about core topics in pediatric medicine. Chris, what is our topic today?
Christopher J. Chiu, MD: I was really happy to be able to talk about our recent episode with Dr. Carissa Baker-Smith, a pediatric cardiologist and director of the Nemours preventive cardiology program. She helped us review the pediatric screening guidelines for blood pressure, including initial workup and treatment.
Dr. Berk: This was a really great episode that a lot of people found really helpful. What were some of the key takeaway pearls that you think listeners would be interested in?
Dr. Chiu: We talked about when and how we should be checking blood pressures in children. Blood pressure should be checked at every well-child visit starting at age 3. But if they have other risk factors like kidney disease or a condition such as coarctation of the aorta, then blood pressure should be checked at every visit.
Dr. Berk: One thing she spoke about was how blood pressures should be measured. How should we be checking blood pressures in the clinic?
Dr. Chiu: Clinic blood pressures are usually checked with oscillometric devices. They can differ by manufacturer, but basically they find a mean arterial pressure and then each device has a method of calculating systolic and diastolic pressures. Now after that, if the child’s blood pressure is maybe abnormal, you want to double-check a manual blood pressure using Korotkoff sounds to confirm the blood pressure.
She reminded us that blood pressure should be measured with the child sitting with their back supported, feet flat on the floor, and arm at the level of the heart. Make sure you use the right size cuff. The bladder of the cuff should be 40% of the width of the arm, and about 80%-100% of the arm circumference. She recommends sizing up if you have to.
Dr. Berk: Accuracy of blood pressure management was a really important point, especially for diagnosis at this stage. Can you walk us through what we learned about diagnosis of hypertension?
Dr. Chiu: The definitions of hypertension come from the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Up until the age of 13, they define prehypertension as systolic and diastolic blood pressures between the 90th and 95th percentile, or if the blood pressure exceeds 120/80 mm Hg. Hypertension is defined when blood pressure reaches the 95th percentile. Now age 13 is when it gets a little hazy. Many changes in the guidelines happen at age 13, when hypertension starts being defined by adult guidelines. The 2017 adult hypertension guidelines define stage 1 hypertension as 130/89 to 139/89, and stage 2 hypertension as greater than 140/90.
Dr. Berk: How about workup of hypertension? The work of pediatric hypertension is always a little bit complex. What are some of the pearls you took away?
Dr. Chui: She talked about tailoring the workup to the child. So when we’re doing our workup, obviously physical exam should be the first thing we do. You have to assess and compare pulses, which is one of the most important parts of the initial evaluation. Obviously, looking at coarctation of the aorta, but also looking for things like a cushingoid appearance. If the child is less than 6 years of age, she recommends a referral to nephrology for more comprehensive renovascular workup, which probably will include renal ultrasound, urinalysis, metabolic panel, and thyroid studies.
We have to be cognizant of secondary causes of hypertension, such as endocrine tumors, hyperthyroidism, aortic disease, or even medication-induced hypertension. She told us that in the majority of these cases, especially with our obese older children, primary hypertension or essential hypertension is the most likely cause.
Dr. Berk: That was my big takeaway. If they’re really young, they need a big workup, but otherwise it is likely primary hypertension. What did we learn about treatment?
Dr. Chui: Just as we tailor our assessment to the child, we also have to tailor treatment. We know that lifestyle modification is usually the first line of treatment, especially for primary hypertension, and Dr. Baker-Smith tells us that we really need to perform counseling that meets the patient where they are. So if they like dancing to the newest TikTok trends or music videos, maybe we can encourage them to move more that way. Using our motivational interviewing skills is really key here.
If you want to start medication, Dr. Baker-Smith uses things like low-dose ACE inhibitors or calcium channel blockers, but obviously it’ll be tailored to the patient and any underlying conditions.
Dr. Berk: That’s great – a lot of wonderful pearls on the diagnosis and management of pediatric hypertension. Thank you for joining us for another video recap of The Cribsiders pediatric podcast. You can download the full podcast, Off the Cuff: Managing Pediatric Hypertension in Your Primary Care Clinic, on any podcast player, or check out our website at www.theCribsiders.com.
Christopher J. Chiu, MD, is assistant professor, department of internal medicine, division of general internal medicine, Ohio State University, Columbus; lead physician, general internal medicine, OSU Outpatient Care East; department of internal medicine, division of general internal medicine, Ohio State University Wexner Medical Center. Dr. Chiu has disclosed no relevant financial relationships. Justin L. Berk, MD, MPH, MBA, is assistant professor, department of medicine; assistant professor, department of pediatrics, Brown University, Providence, R.I.
My favorite physical exam pearls
I would like to start the new year off by returning to the past – when the physical exam was emphasized and utilized in decision making. I think a big reason that its use has diminished in recent years is due to the physical exam not having been emphasized in training.
For those seeking to increase their comfort with conducting the physical exam, below are several methods I have found helpful to use in practice.
Examining the pharynx
We were usually taught to ask the patient to say ahhh, with or without a nasty tongue depressor.
When I was on my pediatrics rotation, I was taught to ask the patients to roar like a lion, which always gave a nice look at their posterior pharynx. The kids also really liked doing this, but it might seem a little strange to ask adults to do this.
A technique I have found that works well with adults is to ask them to yawn. I have found that this get me a great look at the pharynx for about half of my patients.
Auscultatory percussion for pleural effusions
Guarino and colleagues described a technique that is easily mastered and very effective for determining the presence of pleural effusions.1 It involves placing the stethoscope 3 cm below the last rib in the mid clavicular line and tapping from the apex down to the last rib.
For patients without effusion, a sharp change to a loud percussion note will occur at the last rib.
If the patient has an effusion, the loud percussion note will start at the top of the effusion.
This method was remarkably successful at finding pleural effusions. In the study, Dr. Guarino found a sensitivity of 96% and a specificity of 100%.
Physical exam for anemia
Look at the nails and see if they look pale. How can we do this?
The first step is to know what your own hematocrit is. You can then compare the color of your nail to that of the patient.
If you have a normal hematocrit and the patient’s nail bed color is lighter than yours, the patient likely has anemia. If you do this frequently, you will get good at estimating hematocrit. This is especially important if you do not have labs readily available.
Another way to assess for anemia is to look at the color tint of the lower conjunctiva. The best way to look for this is to look at whether there is a generous amount of visible capillaries in the lower conjunctiva. Patients without anemia have a darker red color because of these vessels, whereas patients with anemia are a lighter pink.
Strobach and colleagues2 looked at both nail bed rubor and color tint of the lower conjunctiva and found that both reliably predicted presence and degree of anemia.
Determining if clubbing is present
Most physicians are aware of Shamroth sign, and use it to evaluate for clubbing. Shamroth sign is the loss of the diamond that is created by placing the back surfaces of opposite terminal phalanges together.
I have found that it’s easier to diagnose mild clubbing by looking at the finger in profile. If the ratio of the distal phalangeal depth compared to the depth across the distal interphalangeal joint is greater than 1:1, then clubbing is present.3
Pearls
1. Have the patient try yawning to better see the pharynx without using a tongue blade.
2. Try the technique of auscultatory percussion to be more accurate at picking up pleural effusions.
3. Know your hematocrit, so you can better use color shade to assess for anemia.
4. Try looking at fingers in profile to pick up clubbing.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Guarino JR and Guarino JC. Auscultatory percussion: A simple method to detect pleural effusion. J Gen Intern Med. 1994 Feb;9(2):71-4.
2. Strobach RS et al. The value of the physical examination in the diagnosis of anemia. Correlation of the physical findings and the hemoglobin concentration. Arch Intern Med. 1988 Apr;148(4):831-2.
3. Spicknall KE et al. Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance. J Am Acad Dermatol. 2005 Jun;52(6):1020-8.
I would like to start the new year off by returning to the past – when the physical exam was emphasized and utilized in decision making. I think a big reason that its use has diminished in recent years is due to the physical exam not having been emphasized in training.
For those seeking to increase their comfort with conducting the physical exam, below are several methods I have found helpful to use in practice.
Examining the pharynx
We were usually taught to ask the patient to say ahhh, with or without a nasty tongue depressor.
When I was on my pediatrics rotation, I was taught to ask the patients to roar like a lion, which always gave a nice look at their posterior pharynx. The kids also really liked doing this, but it might seem a little strange to ask adults to do this.
A technique I have found that works well with adults is to ask them to yawn. I have found that this get me a great look at the pharynx for about half of my patients.
Auscultatory percussion for pleural effusions
Guarino and colleagues described a technique that is easily mastered and very effective for determining the presence of pleural effusions.1 It involves placing the stethoscope 3 cm below the last rib in the mid clavicular line and tapping from the apex down to the last rib.
For patients without effusion, a sharp change to a loud percussion note will occur at the last rib.
If the patient has an effusion, the loud percussion note will start at the top of the effusion.
This method was remarkably successful at finding pleural effusions. In the study, Dr. Guarino found a sensitivity of 96% and a specificity of 100%.
Physical exam for anemia
Look at the nails and see if they look pale. How can we do this?
The first step is to know what your own hematocrit is. You can then compare the color of your nail to that of the patient.
If you have a normal hematocrit and the patient’s nail bed color is lighter than yours, the patient likely has anemia. If you do this frequently, you will get good at estimating hematocrit. This is especially important if you do not have labs readily available.
Another way to assess for anemia is to look at the color tint of the lower conjunctiva. The best way to look for this is to look at whether there is a generous amount of visible capillaries in the lower conjunctiva. Patients without anemia have a darker red color because of these vessels, whereas patients with anemia are a lighter pink.
Strobach and colleagues2 looked at both nail bed rubor and color tint of the lower conjunctiva and found that both reliably predicted presence and degree of anemia.
Determining if clubbing is present
Most physicians are aware of Shamroth sign, and use it to evaluate for clubbing. Shamroth sign is the loss of the diamond that is created by placing the back surfaces of opposite terminal phalanges together.
I have found that it’s easier to diagnose mild clubbing by looking at the finger in profile. If the ratio of the distal phalangeal depth compared to the depth across the distal interphalangeal joint is greater than 1:1, then clubbing is present.3
Pearls
1. Have the patient try yawning to better see the pharynx without using a tongue blade.
2. Try the technique of auscultatory percussion to be more accurate at picking up pleural effusions.
3. Know your hematocrit, so you can better use color shade to assess for anemia.
4. Try looking at fingers in profile to pick up clubbing.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Guarino JR and Guarino JC. Auscultatory percussion: A simple method to detect pleural effusion. J Gen Intern Med. 1994 Feb;9(2):71-4.
2. Strobach RS et al. The value of the physical examination in the diagnosis of anemia. Correlation of the physical findings and the hemoglobin concentration. Arch Intern Med. 1988 Apr;148(4):831-2.
3. Spicknall KE et al. Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance. J Am Acad Dermatol. 2005 Jun;52(6):1020-8.
I would like to start the new year off by returning to the past – when the physical exam was emphasized and utilized in decision making. I think a big reason that its use has diminished in recent years is due to the physical exam not having been emphasized in training.
For those seeking to increase their comfort with conducting the physical exam, below are several methods I have found helpful to use in practice.
Examining the pharynx
We were usually taught to ask the patient to say ahhh, with or without a nasty tongue depressor.
When I was on my pediatrics rotation, I was taught to ask the patients to roar like a lion, which always gave a nice look at their posterior pharynx. The kids also really liked doing this, but it might seem a little strange to ask adults to do this.
A technique I have found that works well with adults is to ask them to yawn. I have found that this get me a great look at the pharynx for about half of my patients.
Auscultatory percussion for pleural effusions
Guarino and colleagues described a technique that is easily mastered and very effective for determining the presence of pleural effusions.1 It involves placing the stethoscope 3 cm below the last rib in the mid clavicular line and tapping from the apex down to the last rib.
For patients without effusion, a sharp change to a loud percussion note will occur at the last rib.
If the patient has an effusion, the loud percussion note will start at the top of the effusion.
This method was remarkably successful at finding pleural effusions. In the study, Dr. Guarino found a sensitivity of 96% and a specificity of 100%.
Physical exam for anemia
Look at the nails and see if they look pale. How can we do this?
The first step is to know what your own hematocrit is. You can then compare the color of your nail to that of the patient.
If you have a normal hematocrit and the patient’s nail bed color is lighter than yours, the patient likely has anemia. If you do this frequently, you will get good at estimating hematocrit. This is especially important if you do not have labs readily available.
Another way to assess for anemia is to look at the color tint of the lower conjunctiva. The best way to look for this is to look at whether there is a generous amount of visible capillaries in the lower conjunctiva. Patients without anemia have a darker red color because of these vessels, whereas patients with anemia are a lighter pink.
Strobach and colleagues2 looked at both nail bed rubor and color tint of the lower conjunctiva and found that both reliably predicted presence and degree of anemia.
Determining if clubbing is present
Most physicians are aware of Shamroth sign, and use it to evaluate for clubbing. Shamroth sign is the loss of the diamond that is created by placing the back surfaces of opposite terminal phalanges together.
I have found that it’s easier to diagnose mild clubbing by looking at the finger in profile. If the ratio of the distal phalangeal depth compared to the depth across the distal interphalangeal joint is greater than 1:1, then clubbing is present.3
Pearls
1. Have the patient try yawning to better see the pharynx without using a tongue blade.
2. Try the technique of auscultatory percussion to be more accurate at picking up pleural effusions.
3. Know your hematocrit, so you can better use color shade to assess for anemia.
4. Try looking at fingers in profile to pick up clubbing.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Guarino JR and Guarino JC. Auscultatory percussion: A simple method to detect pleural effusion. J Gen Intern Med. 1994 Feb;9(2):71-4.
2. Strobach RS et al. The value of the physical examination in the diagnosis of anemia. Correlation of the physical findings and the hemoglobin concentration. Arch Intern Med. 1988 Apr;148(4):831-2.
3. Spicknall KE et al. Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance. J Am Acad Dermatol. 2005 Jun;52(6):1020-8.
Surgeon General releases child mental health call to action
The nation’s Surgeon General, Vice Admiral Vivek H. Murthy, MD, MBA, recently released an advisory report on the current state of youth mental health and recommendations to improve well-being. This action follows a number of emergency declarations that have been made by professional organizations such as the American Academy of Child and Adolescent Psychiatry (AACAP), the American Academy of Pediatrics (AAP), and other health care groups to raise awareness about the alarming increase of depression, suicide, anxiety, and other mental health problems in youth.
These reports can be helpful in focusing attention and resources for important public health problems. Many still reference the 1999 report from former Surgeon General David Satcher, MD, PhD, which offered a number of eye-opening statistics regarding the prevalence of mental health conditions and the amount of disability associated with them.
Sadly, the present report indicates that many of these indices have grown worse in the past 20 years. For example, the advisory notes that, even before COVID-19, fully half of female high school students reported persistent feelings of sadness or hopelessness (up 40% from 2009). The report then goes on to cite a number of studies documenting even further rises in youth mental health problems associated with the pandemic.
Most of the advisory, however, is devoted to actions that can be taken by different groups, including young people themselves, parents, educators, the government, and even social media and video game companies, to support mental health and well-being. Multiple online resources are provided at the end of each of these sections.
One of the segments is aimed at health care organizations and professionals. While first making a fairly sweeping statement that “our health care system today is not set up optimally to support the mental health and well-being of children and youth,” this part then outlines five broad recommendations that might help improve the fit. These include the following.
- Increase prevention efforts, such as coordination to enrichment programs and referrals for economic and legal supports for families in need.
- Screen routinely for mental health conditions and link those who screen in with appropriate care.
- Identify mental health needs in parents and caregivers such as depression and substance use that can have negative effects on children.
- Increase partnerships between health care groups and community organizations.
- Build multidisciplinary teams that are culturally appropriate and maximally engage children and caretakers in the decision-making process.
The current report is downloadable for free (see reference below) and it is certainly worthwhile for pediatricians to take a look. Dr. Murthy writes, regarding the current state of mental health, that “it would be a tragedy if we beat back one public health crisis only to allow another to grow in its place.”
The report also outlines specific areas where additional research is needed, such as data on racial and sexual minorities and research on innovative and scalable therapies. In addition to the online resources that are provided, the report is backed by over 250 references.
Since its release, the report has generally been well received, and, indeed, there is much to support. The well-known Child Mind Institute in New York tweeted that “this document is a wake-up call for the country and a long-overdue statement of leadership from the federal government.”
Many of the recommendations are admittedly somewhat commons sense, but there are some that are much less so. For example, one recommendation to youth themselves is to serve others – something that may first come across as counterintuitive but can indeed help children and adolescents develop a sense of purpose and self-worth. The call for pediatric health care professionals to screen parents in addition to the patients themselves will likely result in some debate as well. The recommendation to reduce access to lethal means, including the specific naming of firearms, is also a welcome addition. This report also rightly puts a spotlight on the role of societal factors such as racism and poverty in the development of mental health problems and in getting access to quality treatment.
Also worth noting is how much of the advisory examined the role of media in both the problem and the solution. While recognizing that technology, smartphones, and social media are here to stay, a number of suggestions were given to parents, media organizations, journalists, and entertainment companies to reduce the negative impacts these mediums can have. Explicitly recognized in the report is that “there can be tension between what’s best for the technology company and what’s best for the individual user or society.” Also acknowledged was that the link between media of various types and mental health is complex and inconsistent with there being a strong need for additional work in this area when it comes to academic research as well as product development within these companies themselves.
Yet while there is much to like about the advisory, there remain some areas that seem lacking. For example, the text about what causes mental health conditions gets a little dualistic in mentioning biological and environmental factors without much appreciation that these are hardly independent domains. Perhaps more substantially, there was surprisingly little airtime devoted to an enormous issue that underlies so many other challenges related to mental health care – namely an inadequate workforce that gets smaller by the minute. The topic was treated much too superficially with lots of vague calls to “expand” the workforce that lacked substance or detail.
Overall, however, the new Surgeon General’s Advisory is a welcome document that offers updated knowledge of our current challenges and provides practical responses that truly could make a difference. Now all we have to do is put these recommendations into action.
Dr. Rettew is a child and adolescent psychiatrist and medical director of Lane County Behavioral Health in Eugene, Ore. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.” You can follow him on Twitter and Facebook @PediPsych.
Reference
“Protecting Youth Mental Health – The U.S. Surgeon General’s Advisory,” U.S. Department of Health & Human Services (2021).
The nation’s Surgeon General, Vice Admiral Vivek H. Murthy, MD, MBA, recently released an advisory report on the current state of youth mental health and recommendations to improve well-being. This action follows a number of emergency declarations that have been made by professional organizations such as the American Academy of Child and Adolescent Psychiatry (AACAP), the American Academy of Pediatrics (AAP), and other health care groups to raise awareness about the alarming increase of depression, suicide, anxiety, and other mental health problems in youth.
These reports can be helpful in focusing attention and resources for important public health problems. Many still reference the 1999 report from former Surgeon General David Satcher, MD, PhD, which offered a number of eye-opening statistics regarding the prevalence of mental health conditions and the amount of disability associated with them.
Sadly, the present report indicates that many of these indices have grown worse in the past 20 years. For example, the advisory notes that, even before COVID-19, fully half of female high school students reported persistent feelings of sadness or hopelessness (up 40% from 2009). The report then goes on to cite a number of studies documenting even further rises in youth mental health problems associated with the pandemic.
Most of the advisory, however, is devoted to actions that can be taken by different groups, including young people themselves, parents, educators, the government, and even social media and video game companies, to support mental health and well-being. Multiple online resources are provided at the end of each of these sections.
One of the segments is aimed at health care organizations and professionals. While first making a fairly sweeping statement that “our health care system today is not set up optimally to support the mental health and well-being of children and youth,” this part then outlines five broad recommendations that might help improve the fit. These include the following.
- Increase prevention efforts, such as coordination to enrichment programs and referrals for economic and legal supports for families in need.
- Screen routinely for mental health conditions and link those who screen in with appropriate care.
- Identify mental health needs in parents and caregivers such as depression and substance use that can have negative effects on children.
- Increase partnerships between health care groups and community organizations.
- Build multidisciplinary teams that are culturally appropriate and maximally engage children and caretakers in the decision-making process.
The current report is downloadable for free (see reference below) and it is certainly worthwhile for pediatricians to take a look. Dr. Murthy writes, regarding the current state of mental health, that “it would be a tragedy if we beat back one public health crisis only to allow another to grow in its place.”
The report also outlines specific areas where additional research is needed, such as data on racial and sexual minorities and research on innovative and scalable therapies. In addition to the online resources that are provided, the report is backed by over 250 references.
Since its release, the report has generally been well received, and, indeed, there is much to support. The well-known Child Mind Institute in New York tweeted that “this document is a wake-up call for the country and a long-overdue statement of leadership from the federal government.”
Many of the recommendations are admittedly somewhat commons sense, but there are some that are much less so. For example, one recommendation to youth themselves is to serve others – something that may first come across as counterintuitive but can indeed help children and adolescents develop a sense of purpose and self-worth. The call for pediatric health care professionals to screen parents in addition to the patients themselves will likely result in some debate as well. The recommendation to reduce access to lethal means, including the specific naming of firearms, is also a welcome addition. This report also rightly puts a spotlight on the role of societal factors such as racism and poverty in the development of mental health problems and in getting access to quality treatment.
Also worth noting is how much of the advisory examined the role of media in both the problem and the solution. While recognizing that technology, smartphones, and social media are here to stay, a number of suggestions were given to parents, media organizations, journalists, and entertainment companies to reduce the negative impacts these mediums can have. Explicitly recognized in the report is that “there can be tension between what’s best for the technology company and what’s best for the individual user or society.” Also acknowledged was that the link between media of various types and mental health is complex and inconsistent with there being a strong need for additional work in this area when it comes to academic research as well as product development within these companies themselves.
Yet while there is much to like about the advisory, there remain some areas that seem lacking. For example, the text about what causes mental health conditions gets a little dualistic in mentioning biological and environmental factors without much appreciation that these are hardly independent domains. Perhaps more substantially, there was surprisingly little airtime devoted to an enormous issue that underlies so many other challenges related to mental health care – namely an inadequate workforce that gets smaller by the minute. The topic was treated much too superficially with lots of vague calls to “expand” the workforce that lacked substance or detail.
Overall, however, the new Surgeon General’s Advisory is a welcome document that offers updated knowledge of our current challenges and provides practical responses that truly could make a difference. Now all we have to do is put these recommendations into action.
Dr. Rettew is a child and adolescent psychiatrist and medical director of Lane County Behavioral Health in Eugene, Ore. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.” You can follow him on Twitter and Facebook @PediPsych.
Reference
“Protecting Youth Mental Health – The U.S. Surgeon General’s Advisory,” U.S. Department of Health & Human Services (2021).
The nation’s Surgeon General, Vice Admiral Vivek H. Murthy, MD, MBA, recently released an advisory report on the current state of youth mental health and recommendations to improve well-being. This action follows a number of emergency declarations that have been made by professional organizations such as the American Academy of Child and Adolescent Psychiatry (AACAP), the American Academy of Pediatrics (AAP), and other health care groups to raise awareness about the alarming increase of depression, suicide, anxiety, and other mental health problems in youth.
These reports can be helpful in focusing attention and resources for important public health problems. Many still reference the 1999 report from former Surgeon General David Satcher, MD, PhD, which offered a number of eye-opening statistics regarding the prevalence of mental health conditions and the amount of disability associated with them.
Sadly, the present report indicates that many of these indices have grown worse in the past 20 years. For example, the advisory notes that, even before COVID-19, fully half of female high school students reported persistent feelings of sadness or hopelessness (up 40% from 2009). The report then goes on to cite a number of studies documenting even further rises in youth mental health problems associated with the pandemic.
Most of the advisory, however, is devoted to actions that can be taken by different groups, including young people themselves, parents, educators, the government, and even social media and video game companies, to support mental health and well-being. Multiple online resources are provided at the end of each of these sections.
One of the segments is aimed at health care organizations and professionals. While first making a fairly sweeping statement that “our health care system today is not set up optimally to support the mental health and well-being of children and youth,” this part then outlines five broad recommendations that might help improve the fit. These include the following.
- Increase prevention efforts, such as coordination to enrichment programs and referrals for economic and legal supports for families in need.
- Screen routinely for mental health conditions and link those who screen in with appropriate care.
- Identify mental health needs in parents and caregivers such as depression and substance use that can have negative effects on children.
- Increase partnerships between health care groups and community organizations.
- Build multidisciplinary teams that are culturally appropriate and maximally engage children and caretakers in the decision-making process.
The current report is downloadable for free (see reference below) and it is certainly worthwhile for pediatricians to take a look. Dr. Murthy writes, regarding the current state of mental health, that “it would be a tragedy if we beat back one public health crisis only to allow another to grow in its place.”
The report also outlines specific areas where additional research is needed, such as data on racial and sexual minorities and research on innovative and scalable therapies. In addition to the online resources that are provided, the report is backed by over 250 references.
Since its release, the report has generally been well received, and, indeed, there is much to support. The well-known Child Mind Institute in New York tweeted that “this document is a wake-up call for the country and a long-overdue statement of leadership from the federal government.”
Many of the recommendations are admittedly somewhat commons sense, but there are some that are much less so. For example, one recommendation to youth themselves is to serve others – something that may first come across as counterintuitive but can indeed help children and adolescents develop a sense of purpose and self-worth. The call for pediatric health care professionals to screen parents in addition to the patients themselves will likely result in some debate as well. The recommendation to reduce access to lethal means, including the specific naming of firearms, is also a welcome addition. This report also rightly puts a spotlight on the role of societal factors such as racism and poverty in the development of mental health problems and in getting access to quality treatment.
Also worth noting is how much of the advisory examined the role of media in both the problem and the solution. While recognizing that technology, smartphones, and social media are here to stay, a number of suggestions were given to parents, media organizations, journalists, and entertainment companies to reduce the negative impacts these mediums can have. Explicitly recognized in the report is that “there can be tension between what’s best for the technology company and what’s best for the individual user or society.” Also acknowledged was that the link between media of various types and mental health is complex and inconsistent with there being a strong need for additional work in this area when it comes to academic research as well as product development within these companies themselves.
Yet while there is much to like about the advisory, there remain some areas that seem lacking. For example, the text about what causes mental health conditions gets a little dualistic in mentioning biological and environmental factors without much appreciation that these are hardly independent domains. Perhaps more substantially, there was surprisingly little airtime devoted to an enormous issue that underlies so many other challenges related to mental health care – namely an inadequate workforce that gets smaller by the minute. The topic was treated much too superficially with lots of vague calls to “expand” the workforce that lacked substance or detail.
Overall, however, the new Surgeon General’s Advisory is a welcome document that offers updated knowledge of our current challenges and provides practical responses that truly could make a difference. Now all we have to do is put these recommendations into action.
Dr. Rettew is a child and adolescent psychiatrist and medical director of Lane County Behavioral Health in Eugene, Ore. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.” You can follow him on Twitter and Facebook @PediPsych.
Reference
“Protecting Youth Mental Health – The U.S. Surgeon General’s Advisory,” U.S. Department of Health & Human Services (2021).
Are GI hospitalists the future of inpatient care?
Dear colleagues and friends,
After an excellent debate on the future of telemedicine in GI in our most recent Perspectives column, we continue to explore changes in the way we traditionally provide care. In this issue, we discuss the GI hospitalist service, a relatively new but growing model of providing inpatient care. Is this the new ideal, allowing for more efficient care? Or are traditional or alternative models more appropriate? As with most things, the answer often lies somewhere in the middle, driven by local needs and infrastructure. Dr. Tau and Dr. Mehendiratta explore the pros and cons of these different approaches to providing inpatient GI care. I look forward to hearing your thoughts and experiences on the AGA Community forum and by email (ginews@gastro.org).
Gyanprakash A. Ketwaroo, MD, MSc, is an assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
The dedicated GI hospitalist: Taking ownership not ‘call’
By J. Andy Tau, MD
In my experience, a GI hospitalist provides mutual benefit to patients, employers, and consulting physicians. The patient benefits from more expedient consultations and expert endoscopic therapy, which translates to shorter hospitalizations and improved outcomes. The employer enjoys financial benefits as busy outpatient providers can stay busy without interruption. Consulting physicians enjoy having to only call a single phone number for trusted help from a familiar physician who does not rotate off service. Personally, the position provides the volume to develop valuable therapeutic endoscopy skills and techniques. With one stable physician at the helm, a sense of ownership can develop, rather than a sense of survival until “call” is over.
As a full-time GI hospitalist for a large single-specialty group, I provide inpatient GI and hepatology consultation from 7 a.m. to 5 p.m., Monday-Friday. I do not rotate off service. I cover three hospitals with a total of 1,000 beds with two advanced practice providers and one part-time physician. Except for endoscopic ultrasound, I perform all other endoscopic procedures. The census is usually 25-35 with an average of 10-15 new consults per day.
The most important benefit of a dedicated GI hospitalist is providing expedited consultation and expert endoscopy for patients. I can offer emergent (<6 hour) endoscopy for any patient. An esophageal food impaction is usually resolved within an hour of arrival to the ED during the day. I can help a surgeon intraoperatively on very short notice. As for acute GI bleeding cases, I oversee resuscitative efforts, while the endoscopy team prepares my preferred endoscopic equipment, eliminating surprises and delays before endoscopy. I have developed an expertise in hemostasis and managing esophageal perforations, along with a risk tolerance that cannot be matured in any setting other than daily emergency.
I have enacted evidence-based protocols for GI bleeding, iron-deficiency anemia, colonic pseudo-obstruction, pancreatitis, and liver decompensation, which internists have adopted over time, reducing phone calls and delays in prep or resuscitation.
While the day is unstructured and filled with interruptions, it is also very flexible. As opposed to the set time intervals of an outpatient clinic visit, I can spend an hour in a palliative care meeting or revisit high-risk patients multiple times a day to detect pending deterioration. Combined endoscopic and surgical cases are logistically easy to schedule given my flexibility. For example, patients with choledocholithiasis often can have a combined cholecystectomy and supine endoscopic retrograde cholangiopancreatography (ERCP) in the OR, shaving a day off admission.
My employer benefits financially as the outpatient doctors can stay busy without interruption from the hospital. With secure group messaging, we are able to make joint decisions and arrange close follow up. The relative value units earned from the hospital are high. Combined with proceeds from the professional service agreement with the hospital, they are more than enough to cover my compensation.
Any physician in need of a GI consult needs only to call one number for help. I make it as easy as possible to obtain a consult and never push back, as banal as any consult may seem. I stake my reputation on providing a service that is able, affable, and available. By teaching a consistent message to consulting physicians, I have now effected best evidence-based practices for GI conditions even without engaging me. The most notable examples include antibiotics for variceal bleeding, fluid resuscitation and early feeding for acute pancreatitis, risk stratification for choledocholithiasis, and last but not least, abandoning the inpatient fecal occult blood test.
I am on a first-name basis with every nurse in the hospital now. In exchange for my availability and cell phone number, they place orders for me and protect me from avoidable nuisances.
Many physician groups cover the inpatient service by rotating a week at a time. There can be at times a reluctance to take ownership over a difficult patient and instead a sense of “survival of the call”. However, in my job, “the buck stops with me” even if it is in the form of readmission. For example, I have to take some ownership of indigent patients who cannot easily follow up. Who will remove the stent I placed? How will they pay for Helicobacter pylori eradication or biologic therapy? Another example is diverticular bleeding. While 80% stop on their own, I take extraordinary efforts to endoscopically find and halt the bleeding in order to reduce the recurrence rate. I must find durable solutions because these high-risk patients are my responsibility again when they bounce back to me via the ED.
By way of volume alone, this position has allowed me to develop many therapeutic skills outside of a standard 3-year GI fellowship. While I did only 200 ERCPs in fellowship, I have become proficient in ERCP with around 400 cases per year (mostly native papilla) and have grown comfortable with the needle knife. I have learned endoscopic suturing, luminal stenting, and endoscopic vacuum-assisted therapy for perforation closure independently. Out of necessity, I developed a novel technique in optimizing the use of hemostatic powder by using a bone-wax plug. As endoscopy chief, I can purchase a wide variety of endoscopy equipment, compare brands, and understand the nuances of each.
In conclusion, the dedicated GI hospitalist indirectly improves the efficiency of an outpatient practice, while directly improving inpatient outcomes, collegiality, and even one’s own skills as an endoscopist. While it can be challenging and hectic, with the right mentality towards ownership of the service, it is also an incredibly rewarding position.
Dr. Tau practices with Austin Gastroenterology in Austin, Tex. He disclosed relationships with Cook Medical and Conmed.
Inpatient-only GI hospitalist: Not so fast
By Vaibhav Mehendiratta, MD
Over the past 2 decades, the medical hospitalist system has assumed care of hospitalized patients with the promise of reduced length of stay (LOS) and improved outcomes. Although data on LOS is promising, there have been conflicting results in terms of total medical costs and resource utilization. Inpatient care for patients with complex medical histories often requires regular communication with other subspecialties and outpatient providers to achieve better patient-centered outcomes.
Providing inpatient gastrointestinal care is complicated. Traditional models rely on physicians trying to balance outpatient obligations with inpatient rounding and procedures, which can result in delayed endoscopy and an inability to participate fully in multidisciplinary rounds and family meetings. The complexity of hospitalized patients often requires a multidisciplinary approach with coordination of care that is hard to accomplish in between seeing outpatients. GI groups, both private practice and academics, need to adopt a strategy for inpatient care that is tailored to the hospital system in which they operate.
As one of the largest private practice groups in New England, our experience can provide a framework for others to follow. We provide inpatient GI care at eight hospitals across northern Connecticut. Our inpatient service at the largest tertiary care hospital is composed of one general gastroenterologist, one advanced endoscopist, one transplant hepatologist, two advanced practitioners, and two fellows in training. Each practitioner provides coverage on a rotating basis, typically 1 week at a time every 4-8 weeks. This model also offers flexibility, such that we can typically accommodate urgent outpatient endoscopy for patients who may otherwise require inpatient care. Coverage at the other seven hospitals is tailored to local needs and ranges from half-day to whole-day coverage by general gastroenterologists and advanced practitioners. We believe that our model is financially viable and, based on our experience, inpatient relative value units generated are quite similar to a typical day in outpatient GI practice.
Inpatient GI care accounts for a substantial portion of overall inpatient care in the United States. Endoscopy delays have been the focus of many research articles looking at inpatient GI care. The delays are caused by many factors, including endoscopy unit/staff availability, anesthesia availability, and patient factors. While having a dedicated inpatient GI Hospitalist offers the potential to streamline access for hospital consultations and endoscopy, an exclusive inpatient GI hospitalist may be less familiar with a patient’s chronic GI illness and have different (and perhaps, conflicting) priorities regarding a patient’s care. Having incomplete access to outpatient records or less familiarity with the intricacies of outpatient care could also lead to duplication of work and increase the number of inpatient procedures that may have otherwise been deferred to the outpatient setting.
Additionally, with physician burnout on the rise and particularly in the inpatient setting, one must question the sustainability of an exclusively inpatient GI practice. That is, the hours and demands of inpatient care typically do not allow the quality of life that outpatient care provides. Our model provides time for dedicated inpatient care, while allowing each practitioner ample opportunity to build a robust outpatient practice.
Some health care organizations are adopting an extensivist model to provide comprehensive care to patients with multiple medical problems. Extensivists are outpatient primary care providers who take the time to coordinate with inpatient hospitalists to provide comprehensive care to their patients. Constant contact with outpatient providers during admission is expected to improve patient satisfaction, reduce hospital readmissions, and decrease inpatient resource utilization.
In conclusion, our experience highlights sustained benefits, and distinct advantages, of providing inpatient GI care without a GI hospitalist model. The pendulum in inpatient care keeps swinging and with progress arise new challenges and questions. Close collaboration between gastroenterologists and health systems to develop a program that fits local needs and allows optimal resource allocation will ensure delivery of high-quality inpatient GI care.
Dr. Mehendiratta is a gastroenterologist with Connecticut GI PC, Hartford, and assistant clinical professor in the department of medicine at the University of Connecticut, Farmington. He has no relevant conflicts of interest to disclose.
Dear colleagues and friends,
After an excellent debate on the future of telemedicine in GI in our most recent Perspectives column, we continue to explore changes in the way we traditionally provide care. In this issue, we discuss the GI hospitalist service, a relatively new but growing model of providing inpatient care. Is this the new ideal, allowing for more efficient care? Or are traditional or alternative models more appropriate? As with most things, the answer often lies somewhere in the middle, driven by local needs and infrastructure. Dr. Tau and Dr. Mehendiratta explore the pros and cons of these different approaches to providing inpatient GI care. I look forward to hearing your thoughts and experiences on the AGA Community forum and by email (ginews@gastro.org).
Gyanprakash A. Ketwaroo, MD, MSc, is an assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
The dedicated GI hospitalist: Taking ownership not ‘call’
By J. Andy Tau, MD
In my experience, a GI hospitalist provides mutual benefit to patients, employers, and consulting physicians. The patient benefits from more expedient consultations and expert endoscopic therapy, which translates to shorter hospitalizations and improved outcomes. The employer enjoys financial benefits as busy outpatient providers can stay busy without interruption. Consulting physicians enjoy having to only call a single phone number for trusted help from a familiar physician who does not rotate off service. Personally, the position provides the volume to develop valuable therapeutic endoscopy skills and techniques. With one stable physician at the helm, a sense of ownership can develop, rather than a sense of survival until “call” is over.
As a full-time GI hospitalist for a large single-specialty group, I provide inpatient GI and hepatology consultation from 7 a.m. to 5 p.m., Monday-Friday. I do not rotate off service. I cover three hospitals with a total of 1,000 beds with two advanced practice providers and one part-time physician. Except for endoscopic ultrasound, I perform all other endoscopic procedures. The census is usually 25-35 with an average of 10-15 new consults per day.
The most important benefit of a dedicated GI hospitalist is providing expedited consultation and expert endoscopy for patients. I can offer emergent (<6 hour) endoscopy for any patient. An esophageal food impaction is usually resolved within an hour of arrival to the ED during the day. I can help a surgeon intraoperatively on very short notice. As for acute GI bleeding cases, I oversee resuscitative efforts, while the endoscopy team prepares my preferred endoscopic equipment, eliminating surprises and delays before endoscopy. I have developed an expertise in hemostasis and managing esophageal perforations, along with a risk tolerance that cannot be matured in any setting other than daily emergency.
I have enacted evidence-based protocols for GI bleeding, iron-deficiency anemia, colonic pseudo-obstruction, pancreatitis, and liver decompensation, which internists have adopted over time, reducing phone calls and delays in prep or resuscitation.
While the day is unstructured and filled with interruptions, it is also very flexible. As opposed to the set time intervals of an outpatient clinic visit, I can spend an hour in a palliative care meeting or revisit high-risk patients multiple times a day to detect pending deterioration. Combined endoscopic and surgical cases are logistically easy to schedule given my flexibility. For example, patients with choledocholithiasis often can have a combined cholecystectomy and supine endoscopic retrograde cholangiopancreatography (ERCP) in the OR, shaving a day off admission.
My employer benefits financially as the outpatient doctors can stay busy without interruption from the hospital. With secure group messaging, we are able to make joint decisions and arrange close follow up. The relative value units earned from the hospital are high. Combined with proceeds from the professional service agreement with the hospital, they are more than enough to cover my compensation.
Any physician in need of a GI consult needs only to call one number for help. I make it as easy as possible to obtain a consult and never push back, as banal as any consult may seem. I stake my reputation on providing a service that is able, affable, and available. By teaching a consistent message to consulting physicians, I have now effected best evidence-based practices for GI conditions even without engaging me. The most notable examples include antibiotics for variceal bleeding, fluid resuscitation and early feeding for acute pancreatitis, risk stratification for choledocholithiasis, and last but not least, abandoning the inpatient fecal occult blood test.
I am on a first-name basis with every nurse in the hospital now. In exchange for my availability and cell phone number, they place orders for me and protect me from avoidable nuisances.
Many physician groups cover the inpatient service by rotating a week at a time. There can be at times a reluctance to take ownership over a difficult patient and instead a sense of “survival of the call”. However, in my job, “the buck stops with me” even if it is in the form of readmission. For example, I have to take some ownership of indigent patients who cannot easily follow up. Who will remove the stent I placed? How will they pay for Helicobacter pylori eradication or biologic therapy? Another example is diverticular bleeding. While 80% stop on their own, I take extraordinary efforts to endoscopically find and halt the bleeding in order to reduce the recurrence rate. I must find durable solutions because these high-risk patients are my responsibility again when they bounce back to me via the ED.
By way of volume alone, this position has allowed me to develop many therapeutic skills outside of a standard 3-year GI fellowship. While I did only 200 ERCPs in fellowship, I have become proficient in ERCP with around 400 cases per year (mostly native papilla) and have grown comfortable with the needle knife. I have learned endoscopic suturing, luminal stenting, and endoscopic vacuum-assisted therapy for perforation closure independently. Out of necessity, I developed a novel technique in optimizing the use of hemostatic powder by using a bone-wax plug. As endoscopy chief, I can purchase a wide variety of endoscopy equipment, compare brands, and understand the nuances of each.
In conclusion, the dedicated GI hospitalist indirectly improves the efficiency of an outpatient practice, while directly improving inpatient outcomes, collegiality, and even one’s own skills as an endoscopist. While it can be challenging and hectic, with the right mentality towards ownership of the service, it is also an incredibly rewarding position.
Dr. Tau practices with Austin Gastroenterology in Austin, Tex. He disclosed relationships with Cook Medical and Conmed.
Inpatient-only GI hospitalist: Not so fast
By Vaibhav Mehendiratta, MD
Over the past 2 decades, the medical hospitalist system has assumed care of hospitalized patients with the promise of reduced length of stay (LOS) and improved outcomes. Although data on LOS is promising, there have been conflicting results in terms of total medical costs and resource utilization. Inpatient care for patients with complex medical histories often requires regular communication with other subspecialties and outpatient providers to achieve better patient-centered outcomes.
Providing inpatient gastrointestinal care is complicated. Traditional models rely on physicians trying to balance outpatient obligations with inpatient rounding and procedures, which can result in delayed endoscopy and an inability to participate fully in multidisciplinary rounds and family meetings. The complexity of hospitalized patients often requires a multidisciplinary approach with coordination of care that is hard to accomplish in between seeing outpatients. GI groups, both private practice and academics, need to adopt a strategy for inpatient care that is tailored to the hospital system in which they operate.
As one of the largest private practice groups in New England, our experience can provide a framework for others to follow. We provide inpatient GI care at eight hospitals across northern Connecticut. Our inpatient service at the largest tertiary care hospital is composed of one general gastroenterologist, one advanced endoscopist, one transplant hepatologist, two advanced practitioners, and two fellows in training. Each practitioner provides coverage on a rotating basis, typically 1 week at a time every 4-8 weeks. This model also offers flexibility, such that we can typically accommodate urgent outpatient endoscopy for patients who may otherwise require inpatient care. Coverage at the other seven hospitals is tailored to local needs and ranges from half-day to whole-day coverage by general gastroenterologists and advanced practitioners. We believe that our model is financially viable and, based on our experience, inpatient relative value units generated are quite similar to a typical day in outpatient GI practice.
Inpatient GI care accounts for a substantial portion of overall inpatient care in the United States. Endoscopy delays have been the focus of many research articles looking at inpatient GI care. The delays are caused by many factors, including endoscopy unit/staff availability, anesthesia availability, and patient factors. While having a dedicated inpatient GI Hospitalist offers the potential to streamline access for hospital consultations and endoscopy, an exclusive inpatient GI hospitalist may be less familiar with a patient’s chronic GI illness and have different (and perhaps, conflicting) priorities regarding a patient’s care. Having incomplete access to outpatient records or less familiarity with the intricacies of outpatient care could also lead to duplication of work and increase the number of inpatient procedures that may have otherwise been deferred to the outpatient setting.
Additionally, with physician burnout on the rise and particularly in the inpatient setting, one must question the sustainability of an exclusively inpatient GI practice. That is, the hours and demands of inpatient care typically do not allow the quality of life that outpatient care provides. Our model provides time for dedicated inpatient care, while allowing each practitioner ample opportunity to build a robust outpatient practice.
Some health care organizations are adopting an extensivist model to provide comprehensive care to patients with multiple medical problems. Extensivists are outpatient primary care providers who take the time to coordinate with inpatient hospitalists to provide comprehensive care to their patients. Constant contact with outpatient providers during admission is expected to improve patient satisfaction, reduce hospital readmissions, and decrease inpatient resource utilization.
In conclusion, our experience highlights sustained benefits, and distinct advantages, of providing inpatient GI care without a GI hospitalist model. The pendulum in inpatient care keeps swinging and with progress arise new challenges and questions. Close collaboration between gastroenterologists and health systems to develop a program that fits local needs and allows optimal resource allocation will ensure delivery of high-quality inpatient GI care.
Dr. Mehendiratta is a gastroenterologist with Connecticut GI PC, Hartford, and assistant clinical professor in the department of medicine at the University of Connecticut, Farmington. He has no relevant conflicts of interest to disclose.
Dear colleagues and friends,
After an excellent debate on the future of telemedicine in GI in our most recent Perspectives column, we continue to explore changes in the way we traditionally provide care. In this issue, we discuss the GI hospitalist service, a relatively new but growing model of providing inpatient care. Is this the new ideal, allowing for more efficient care? Or are traditional or alternative models more appropriate? As with most things, the answer often lies somewhere in the middle, driven by local needs and infrastructure. Dr. Tau and Dr. Mehendiratta explore the pros and cons of these different approaches to providing inpatient GI care. I look forward to hearing your thoughts and experiences on the AGA Community forum and by email (ginews@gastro.org).
Gyanprakash A. Ketwaroo, MD, MSc, is an assistant professor of medicine at Baylor College of Medicine, Houston. He is an associate editor for GI & Hepatology News.
The dedicated GI hospitalist: Taking ownership not ‘call’
By J. Andy Tau, MD
In my experience, a GI hospitalist provides mutual benefit to patients, employers, and consulting physicians. The patient benefits from more expedient consultations and expert endoscopic therapy, which translates to shorter hospitalizations and improved outcomes. The employer enjoys financial benefits as busy outpatient providers can stay busy without interruption. Consulting physicians enjoy having to only call a single phone number for trusted help from a familiar physician who does not rotate off service. Personally, the position provides the volume to develop valuable therapeutic endoscopy skills and techniques. With one stable physician at the helm, a sense of ownership can develop, rather than a sense of survival until “call” is over.
As a full-time GI hospitalist for a large single-specialty group, I provide inpatient GI and hepatology consultation from 7 a.m. to 5 p.m., Monday-Friday. I do not rotate off service. I cover three hospitals with a total of 1,000 beds with two advanced practice providers and one part-time physician. Except for endoscopic ultrasound, I perform all other endoscopic procedures. The census is usually 25-35 with an average of 10-15 new consults per day.
The most important benefit of a dedicated GI hospitalist is providing expedited consultation and expert endoscopy for patients. I can offer emergent (<6 hour) endoscopy for any patient. An esophageal food impaction is usually resolved within an hour of arrival to the ED during the day. I can help a surgeon intraoperatively on very short notice. As for acute GI bleeding cases, I oversee resuscitative efforts, while the endoscopy team prepares my preferred endoscopic equipment, eliminating surprises and delays before endoscopy. I have developed an expertise in hemostasis and managing esophageal perforations, along with a risk tolerance that cannot be matured in any setting other than daily emergency.
I have enacted evidence-based protocols for GI bleeding, iron-deficiency anemia, colonic pseudo-obstruction, pancreatitis, and liver decompensation, which internists have adopted over time, reducing phone calls and delays in prep or resuscitation.
While the day is unstructured and filled with interruptions, it is also very flexible. As opposed to the set time intervals of an outpatient clinic visit, I can spend an hour in a palliative care meeting or revisit high-risk patients multiple times a day to detect pending deterioration. Combined endoscopic and surgical cases are logistically easy to schedule given my flexibility. For example, patients with choledocholithiasis often can have a combined cholecystectomy and supine endoscopic retrograde cholangiopancreatography (ERCP) in the OR, shaving a day off admission.
My employer benefits financially as the outpatient doctors can stay busy without interruption from the hospital. With secure group messaging, we are able to make joint decisions and arrange close follow up. The relative value units earned from the hospital are high. Combined with proceeds from the professional service agreement with the hospital, they are more than enough to cover my compensation.
Any physician in need of a GI consult needs only to call one number for help. I make it as easy as possible to obtain a consult and never push back, as banal as any consult may seem. I stake my reputation on providing a service that is able, affable, and available. By teaching a consistent message to consulting physicians, I have now effected best evidence-based practices for GI conditions even without engaging me. The most notable examples include antibiotics for variceal bleeding, fluid resuscitation and early feeding for acute pancreatitis, risk stratification for choledocholithiasis, and last but not least, abandoning the inpatient fecal occult blood test.
I am on a first-name basis with every nurse in the hospital now. In exchange for my availability and cell phone number, they place orders for me and protect me from avoidable nuisances.
Many physician groups cover the inpatient service by rotating a week at a time. There can be at times a reluctance to take ownership over a difficult patient and instead a sense of “survival of the call”. However, in my job, “the buck stops with me” even if it is in the form of readmission. For example, I have to take some ownership of indigent patients who cannot easily follow up. Who will remove the stent I placed? How will they pay for Helicobacter pylori eradication or biologic therapy? Another example is diverticular bleeding. While 80% stop on their own, I take extraordinary efforts to endoscopically find and halt the bleeding in order to reduce the recurrence rate. I must find durable solutions because these high-risk patients are my responsibility again when they bounce back to me via the ED.
By way of volume alone, this position has allowed me to develop many therapeutic skills outside of a standard 3-year GI fellowship. While I did only 200 ERCPs in fellowship, I have become proficient in ERCP with around 400 cases per year (mostly native papilla) and have grown comfortable with the needle knife. I have learned endoscopic suturing, luminal stenting, and endoscopic vacuum-assisted therapy for perforation closure independently. Out of necessity, I developed a novel technique in optimizing the use of hemostatic powder by using a bone-wax plug. As endoscopy chief, I can purchase a wide variety of endoscopy equipment, compare brands, and understand the nuances of each.
In conclusion, the dedicated GI hospitalist indirectly improves the efficiency of an outpatient practice, while directly improving inpatient outcomes, collegiality, and even one’s own skills as an endoscopist. While it can be challenging and hectic, with the right mentality towards ownership of the service, it is also an incredibly rewarding position.
Dr. Tau practices with Austin Gastroenterology in Austin, Tex. He disclosed relationships with Cook Medical and Conmed.
Inpatient-only GI hospitalist: Not so fast
By Vaibhav Mehendiratta, MD
Over the past 2 decades, the medical hospitalist system has assumed care of hospitalized patients with the promise of reduced length of stay (LOS) and improved outcomes. Although data on LOS is promising, there have been conflicting results in terms of total medical costs and resource utilization. Inpatient care for patients with complex medical histories often requires regular communication with other subspecialties and outpatient providers to achieve better patient-centered outcomes.
Providing inpatient gastrointestinal care is complicated. Traditional models rely on physicians trying to balance outpatient obligations with inpatient rounding and procedures, which can result in delayed endoscopy and an inability to participate fully in multidisciplinary rounds and family meetings. The complexity of hospitalized patients often requires a multidisciplinary approach with coordination of care that is hard to accomplish in between seeing outpatients. GI groups, both private practice and academics, need to adopt a strategy for inpatient care that is tailored to the hospital system in which they operate.
As one of the largest private practice groups in New England, our experience can provide a framework for others to follow. We provide inpatient GI care at eight hospitals across northern Connecticut. Our inpatient service at the largest tertiary care hospital is composed of one general gastroenterologist, one advanced endoscopist, one transplant hepatologist, two advanced practitioners, and two fellows in training. Each practitioner provides coverage on a rotating basis, typically 1 week at a time every 4-8 weeks. This model also offers flexibility, such that we can typically accommodate urgent outpatient endoscopy for patients who may otherwise require inpatient care. Coverage at the other seven hospitals is tailored to local needs and ranges from half-day to whole-day coverage by general gastroenterologists and advanced practitioners. We believe that our model is financially viable and, based on our experience, inpatient relative value units generated are quite similar to a typical day in outpatient GI practice.
Inpatient GI care accounts for a substantial portion of overall inpatient care in the United States. Endoscopy delays have been the focus of many research articles looking at inpatient GI care. The delays are caused by many factors, including endoscopy unit/staff availability, anesthesia availability, and patient factors. While having a dedicated inpatient GI Hospitalist offers the potential to streamline access for hospital consultations and endoscopy, an exclusive inpatient GI hospitalist may be less familiar with a patient’s chronic GI illness and have different (and perhaps, conflicting) priorities regarding a patient’s care. Having incomplete access to outpatient records or less familiarity with the intricacies of outpatient care could also lead to duplication of work and increase the number of inpatient procedures that may have otherwise been deferred to the outpatient setting.
Additionally, with physician burnout on the rise and particularly in the inpatient setting, one must question the sustainability of an exclusively inpatient GI practice. That is, the hours and demands of inpatient care typically do not allow the quality of life that outpatient care provides. Our model provides time for dedicated inpatient care, while allowing each practitioner ample opportunity to build a robust outpatient practice.
Some health care organizations are adopting an extensivist model to provide comprehensive care to patients with multiple medical problems. Extensivists are outpatient primary care providers who take the time to coordinate with inpatient hospitalists to provide comprehensive care to their patients. Constant contact with outpatient providers during admission is expected to improve patient satisfaction, reduce hospital readmissions, and decrease inpatient resource utilization.
In conclusion, our experience highlights sustained benefits, and distinct advantages, of providing inpatient GI care without a GI hospitalist model. The pendulum in inpatient care keeps swinging and with progress arise new challenges and questions. Close collaboration between gastroenterologists and health systems to develop a program that fits local needs and allows optimal resource allocation will ensure delivery of high-quality inpatient GI care.
Dr. Mehendiratta is a gastroenterologist with Connecticut GI PC, Hartford, and assistant clinical professor in the department of medicine at the University of Connecticut, Farmington. He has no relevant conflicts of interest to disclose.
Are we failing to diagnose and treat the many faces of catatonia?
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
Is anosognosia a delusion, a negative symptom, or a cognitive deficit?
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.
Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
Tap of the brakes on gender-affirming care
In the November 2021 issue of Pediatric News are two stories that on the surface present viewpoints that couldn’t be more divergent. On page 1 under the headline “Gender dysphoria” you will read about a position statement by the Royal Australian and New Zealand College of Psychiatrists (RANZCP) in which they strongly recommend a mental health evaluation for any child or adolescent with gender dysphoria “before any firm decisions are made on whether to prescribe hormonal treatments to transition, or perform surgeries.”
On page 6 is another story titled “Gender-affirming care ‘can save lives’ new research shows” that reports on a research study in which transgender and binary young people who received a year of gender-affirming care experienced less depression and fewer suicidal thoughts. Dr. David J. Inwards-Breland, chief of adolescent and young adult medicine at Rady Children’s Hospital in San Diego and one of the authors of the study is quoted as saying “The younger we can provide gender-affirming care, the less likely [our patients are] to have depression and then negative outcomes.” One can’t avoid the impression that he is in favor of moving ahead without delay in prescribing gender-affirming care.
Where does the new recommendation by the RANZCP fit in with this sense of urgency? Does requiring a mental health evaluation constitute a delay in the institution of gender-affirming care that could increase the risk of negative mental health outcomes for gender dysphoric patients?
In one of the final paragraphs in the Pediatric News article one learns that Dr. Inwards-Breland would agree with the folks of RANZCP. He acknowledges that his study relied on screening and not diagnostic testing and says that “future studies should look at a mental health evaluation and diagnosis by a mental health provider.”
When we drill into the details there are two issues that demand clarification. First, what kind of time course are we talking about for a mental health evaluation? Are we talking weeks, or months, hopefully not years? This of course depends on the availability of mental health services for the specific patient and the depth of the evaluation required. How long a delay is acceptable?
Second, will the evaluation be performed by a provider free of bias? Can it be performed without creating the impression that the patient needs to see a mental health provider because there is something wrong with being trans and we can fix it? One would hope these evaluations would be performed in the spirit of wanting to learn more about the patient with the goal of making the process go more smoothly.
Listening to neighborhood discussions around the fire pit I find that the RANZCP plea for a broader and deeper look at each gender-dysphoric child strikes a chord with the general population. More and more people are realizing that gender-dysphoria happens and that for too long it was closeted with unfortunate consequences. However, there is a feeling, in fact one in which I share, that the rapid rise in its prevalence contains an element of social contagion. And, some irreversible decisions are being made without sufficient consideration. This may or not be a valid concern but it seems to me a thorough and sensitively done mental health evaluation might minimize the collateral damage from some gender-affirming care and at least help those patients for whom it is prescribed transition more smoothly.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
In the November 2021 issue of Pediatric News are two stories that on the surface present viewpoints that couldn’t be more divergent. On page 1 under the headline “Gender dysphoria” you will read about a position statement by the Royal Australian and New Zealand College of Psychiatrists (RANZCP) in which they strongly recommend a mental health evaluation for any child or adolescent with gender dysphoria “before any firm decisions are made on whether to prescribe hormonal treatments to transition, or perform surgeries.”
On page 6 is another story titled “Gender-affirming care ‘can save lives’ new research shows” that reports on a research study in which transgender and binary young people who received a year of gender-affirming care experienced less depression and fewer suicidal thoughts. Dr. David J. Inwards-Breland, chief of adolescent and young adult medicine at Rady Children’s Hospital in San Diego and one of the authors of the study is quoted as saying “The younger we can provide gender-affirming care, the less likely [our patients are] to have depression and then negative outcomes.” One can’t avoid the impression that he is in favor of moving ahead without delay in prescribing gender-affirming care.
Where does the new recommendation by the RANZCP fit in with this sense of urgency? Does requiring a mental health evaluation constitute a delay in the institution of gender-affirming care that could increase the risk of negative mental health outcomes for gender dysphoric patients?
In one of the final paragraphs in the Pediatric News article one learns that Dr. Inwards-Breland would agree with the folks of RANZCP. He acknowledges that his study relied on screening and not diagnostic testing and says that “future studies should look at a mental health evaluation and diagnosis by a mental health provider.”
When we drill into the details there are two issues that demand clarification. First, what kind of time course are we talking about for a mental health evaluation? Are we talking weeks, or months, hopefully not years? This of course depends on the availability of mental health services for the specific patient and the depth of the evaluation required. How long a delay is acceptable?
Second, will the evaluation be performed by a provider free of bias? Can it be performed without creating the impression that the patient needs to see a mental health provider because there is something wrong with being trans and we can fix it? One would hope these evaluations would be performed in the spirit of wanting to learn more about the patient with the goal of making the process go more smoothly.
Listening to neighborhood discussions around the fire pit I find that the RANZCP plea for a broader and deeper look at each gender-dysphoric child strikes a chord with the general population. More and more people are realizing that gender-dysphoria happens and that for too long it was closeted with unfortunate consequences. However, there is a feeling, in fact one in which I share, that the rapid rise in its prevalence contains an element of social contagion. And, some irreversible decisions are being made without sufficient consideration. This may or not be a valid concern but it seems to me a thorough and sensitively done mental health evaluation might minimize the collateral damage from some gender-affirming care and at least help those patients for whom it is prescribed transition more smoothly.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
In the November 2021 issue of Pediatric News are two stories that on the surface present viewpoints that couldn’t be more divergent. On page 1 under the headline “Gender dysphoria” you will read about a position statement by the Royal Australian and New Zealand College of Psychiatrists (RANZCP) in which they strongly recommend a mental health evaluation for any child or adolescent with gender dysphoria “before any firm decisions are made on whether to prescribe hormonal treatments to transition, or perform surgeries.”
On page 6 is another story titled “Gender-affirming care ‘can save lives’ new research shows” that reports on a research study in which transgender and binary young people who received a year of gender-affirming care experienced less depression and fewer suicidal thoughts. Dr. David J. Inwards-Breland, chief of adolescent and young adult medicine at Rady Children’s Hospital in San Diego and one of the authors of the study is quoted as saying “The younger we can provide gender-affirming care, the less likely [our patients are] to have depression and then negative outcomes.” One can’t avoid the impression that he is in favor of moving ahead without delay in prescribing gender-affirming care.
Where does the new recommendation by the RANZCP fit in with this sense of urgency? Does requiring a mental health evaluation constitute a delay in the institution of gender-affirming care that could increase the risk of negative mental health outcomes for gender dysphoric patients?
In one of the final paragraphs in the Pediatric News article one learns that Dr. Inwards-Breland would agree with the folks of RANZCP. He acknowledges that his study relied on screening and not diagnostic testing and says that “future studies should look at a mental health evaluation and diagnosis by a mental health provider.”
When we drill into the details there are two issues that demand clarification. First, what kind of time course are we talking about for a mental health evaluation? Are we talking weeks, or months, hopefully not years? This of course depends on the availability of mental health services for the specific patient and the depth of the evaluation required. How long a delay is acceptable?
Second, will the evaluation be performed by a provider free of bias? Can it be performed without creating the impression that the patient needs to see a mental health provider because there is something wrong with being trans and we can fix it? One would hope these evaluations would be performed in the spirit of wanting to learn more about the patient with the goal of making the process go more smoothly.
Listening to neighborhood discussions around the fire pit I find that the RANZCP plea for a broader and deeper look at each gender-dysphoric child strikes a chord with the general population. More and more people are realizing that gender-dysphoria happens and that for too long it was closeted with unfortunate consequences. However, there is a feeling, in fact one in which I share, that the rapid rise in its prevalence contains an element of social contagion. And, some irreversible decisions are being made without sufficient consideration. This may or not be a valid concern but it seems to me a thorough and sensitively done mental health evaluation might minimize the collateral damage from some gender-affirming care and at least help those patients for whom it is prescribed transition more smoothly.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Benefits of low-dose CT scanning for lung cancer screening explained
According to the Centers for Disease Control and Prevention, lung cancer is the third-most common cancer in the United States and the leading cause of cancer deaths in both men and women. Approximately, 150,000 Americans die every year from this disease.
In fact, it has been shown that low-dose CT scan screening can reduce lung cancer deaths by 20%-30% in high-risk populations.
In the United States, low-dose CT scan screening for lung cancer has largely become the norm. In July 2021, CHEST released new clinical guidelines. These guidelines cover 18 evidence-based recommendations as well as inclusion of further evidence regarding the benefits, risks, and use of CT screening.
In doing the risk assessment of low-dose CT scan as a method of lung cancer screening, meta-analyses were performed on evidence obtained through a literature search using PubMed, Embase, and the Cochrane Library. It was concluded that the benefits outweigh the risks as a method of lung cancer screening and can be utilized in reducing lung cancer deaths.
Low-dose CT scan screening was recommended for the following patients:
- Asymptomatic individuals aged 55-77 years with a history of smoking 30 or more pack-years. (This includes those who continue to smoke or who have quit in the previous 15 years. Annual screening is advised.)
- Asymptomatic individuals aged 55-80 years with a history of smoking 20-30 pack-years who either continue to smoke or have quit in the previous 15 years.
- For asymptomatic individuals who do not meet the above criteria but are predicted to benefit based on life-year gained calculations.
Don’t screen these patients
CT scan screening should not be performed on any person who does not meet any of the above three criteria.
Additionally, if a person has significant comorbidities that would limit their life expectancy, it is recommended not to do CT scan screening. Symptomatic patients should have appropriate diagnostic testing rather than screening.
Additional recommendations from the updated guidelines include developing appropriate counseling strategies as well as deciding what constitutes a positive test.
A positive test should be anything that warrants further evaluation rather than a return to annual screening. It was also advised that overtreatment strategies should be implemented. Additionally, smoking cessation treatment should be provided.
CHEST suggested undertaking a comprehensive approach involving multiple specialists including pulmonologists, radiologists, oncologists, etc. Strategies to ensure compliance with annual screening should also be devised, the guidelines say.
USPSTF’s updated guidelines
It should be noted that the U.S. Preventative Task Force released their own set of updated guidelines in March 2021. In these guidelines, the age at which lung cancer screening should be started was lowered from 55 years to 50 years.
Also, the USPSTF lowered the minimum required smoking history in order to be screened from 30 to 20 pack-years. Their purpose for doing this was to include more high-risk women as well as minorities.
With the changes, 14.5 million individuals living in the United States would be eligible for lung cancer screening by low-dose CT scan, an increase of 6.5 million people, compared with the previous guidelines.
While only small differences exist between the set of guidelines issued by CHEST and the ones issues by the USPSTF, lung cancer screening is still largely underutilized.
One of the barriers to screening may be patients’ lacking insurance coverage for it. As physicians, we need to advocate for these screening tools to be covered.
Other barriers include lack of patient knowledge regarding low-dose CT scans as a screening tool, patient time, and patient visits with their doctors being too short.
Key message
Part of the duties of physicians is to give our patients the best information. We can reduce lung cancer mortality in high risk patients by performing annual low-dose CT scans.
Whichever set of guidelines we chose to follow, we fail our patients if we don’t follow either set of them. The evidence is clear that a low-dose CT scan is a valuable screening tool to add to our practice of medicine.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at fpnews@mdedge.com.
According to the Centers for Disease Control and Prevention, lung cancer is the third-most common cancer in the United States and the leading cause of cancer deaths in both men and women. Approximately, 150,000 Americans die every year from this disease.
In fact, it has been shown that low-dose CT scan screening can reduce lung cancer deaths by 20%-30% in high-risk populations.
In the United States, low-dose CT scan screening for lung cancer has largely become the norm. In July 2021, CHEST released new clinical guidelines. These guidelines cover 18 evidence-based recommendations as well as inclusion of further evidence regarding the benefits, risks, and use of CT screening.
In doing the risk assessment of low-dose CT scan as a method of lung cancer screening, meta-analyses were performed on evidence obtained through a literature search using PubMed, Embase, and the Cochrane Library. It was concluded that the benefits outweigh the risks as a method of lung cancer screening and can be utilized in reducing lung cancer deaths.
Low-dose CT scan screening was recommended for the following patients:
- Asymptomatic individuals aged 55-77 years with a history of smoking 30 or more pack-years. (This includes those who continue to smoke or who have quit in the previous 15 years. Annual screening is advised.)
- Asymptomatic individuals aged 55-80 years with a history of smoking 20-30 pack-years who either continue to smoke or have quit in the previous 15 years.
- For asymptomatic individuals who do not meet the above criteria but are predicted to benefit based on life-year gained calculations.
Don’t screen these patients
CT scan screening should not be performed on any person who does not meet any of the above three criteria.
Additionally, if a person has significant comorbidities that would limit their life expectancy, it is recommended not to do CT scan screening. Symptomatic patients should have appropriate diagnostic testing rather than screening.
Additional recommendations from the updated guidelines include developing appropriate counseling strategies as well as deciding what constitutes a positive test.
A positive test should be anything that warrants further evaluation rather than a return to annual screening. It was also advised that overtreatment strategies should be implemented. Additionally, smoking cessation treatment should be provided.
CHEST suggested undertaking a comprehensive approach involving multiple specialists including pulmonologists, radiologists, oncologists, etc. Strategies to ensure compliance with annual screening should also be devised, the guidelines say.
USPSTF’s updated guidelines
It should be noted that the U.S. Preventative Task Force released their own set of updated guidelines in March 2021. In these guidelines, the age at which lung cancer screening should be started was lowered from 55 years to 50 years.
Also, the USPSTF lowered the minimum required smoking history in order to be screened from 30 to 20 pack-years. Their purpose for doing this was to include more high-risk women as well as minorities.
With the changes, 14.5 million individuals living in the United States would be eligible for lung cancer screening by low-dose CT scan, an increase of 6.5 million people, compared with the previous guidelines.
While only small differences exist between the set of guidelines issued by CHEST and the ones issues by the USPSTF, lung cancer screening is still largely underutilized.
One of the barriers to screening may be patients’ lacking insurance coverage for it. As physicians, we need to advocate for these screening tools to be covered.
Other barriers include lack of patient knowledge regarding low-dose CT scans as a screening tool, patient time, and patient visits with their doctors being too short.
Key message
Part of the duties of physicians is to give our patients the best information. We can reduce lung cancer mortality in high risk patients by performing annual low-dose CT scans.
Whichever set of guidelines we chose to follow, we fail our patients if we don’t follow either set of them. The evidence is clear that a low-dose CT scan is a valuable screening tool to add to our practice of medicine.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at fpnews@mdedge.com.
According to the Centers for Disease Control and Prevention, lung cancer is the third-most common cancer in the United States and the leading cause of cancer deaths in both men and women. Approximately, 150,000 Americans die every year from this disease.
In fact, it has been shown that low-dose CT scan screening can reduce lung cancer deaths by 20%-30% in high-risk populations.
In the United States, low-dose CT scan screening for lung cancer has largely become the norm. In July 2021, CHEST released new clinical guidelines. These guidelines cover 18 evidence-based recommendations as well as inclusion of further evidence regarding the benefits, risks, and use of CT screening.
In doing the risk assessment of low-dose CT scan as a method of lung cancer screening, meta-analyses were performed on evidence obtained through a literature search using PubMed, Embase, and the Cochrane Library. It was concluded that the benefits outweigh the risks as a method of lung cancer screening and can be utilized in reducing lung cancer deaths.
Low-dose CT scan screening was recommended for the following patients:
- Asymptomatic individuals aged 55-77 years with a history of smoking 30 or more pack-years. (This includes those who continue to smoke or who have quit in the previous 15 years. Annual screening is advised.)
- Asymptomatic individuals aged 55-80 years with a history of smoking 20-30 pack-years who either continue to smoke or have quit in the previous 15 years.
- For asymptomatic individuals who do not meet the above criteria but are predicted to benefit based on life-year gained calculations.
Don’t screen these patients
CT scan screening should not be performed on any person who does not meet any of the above three criteria.
Additionally, if a person has significant comorbidities that would limit their life expectancy, it is recommended not to do CT scan screening. Symptomatic patients should have appropriate diagnostic testing rather than screening.
Additional recommendations from the updated guidelines include developing appropriate counseling strategies as well as deciding what constitutes a positive test.
A positive test should be anything that warrants further evaluation rather than a return to annual screening. It was also advised that overtreatment strategies should be implemented. Additionally, smoking cessation treatment should be provided.
CHEST suggested undertaking a comprehensive approach involving multiple specialists including pulmonologists, radiologists, oncologists, etc. Strategies to ensure compliance with annual screening should also be devised, the guidelines say.
USPSTF’s updated guidelines
It should be noted that the U.S. Preventative Task Force released their own set of updated guidelines in March 2021. In these guidelines, the age at which lung cancer screening should be started was lowered from 55 years to 50 years.
Also, the USPSTF lowered the minimum required smoking history in order to be screened from 30 to 20 pack-years. Their purpose for doing this was to include more high-risk women as well as minorities.
With the changes, 14.5 million individuals living in the United States would be eligible for lung cancer screening by low-dose CT scan, an increase of 6.5 million people, compared with the previous guidelines.
While only small differences exist between the set of guidelines issued by CHEST and the ones issues by the USPSTF, lung cancer screening is still largely underutilized.
One of the barriers to screening may be patients’ lacking insurance coverage for it. As physicians, we need to advocate for these screening tools to be covered.
Other barriers include lack of patient knowledge regarding low-dose CT scans as a screening tool, patient time, and patient visits with their doctors being too short.
Key message
Part of the duties of physicians is to give our patients the best information. We can reduce lung cancer mortality in high risk patients by performing annual low-dose CT scans.
Whichever set of guidelines we chose to follow, we fail our patients if we don’t follow either set of them. The evidence is clear that a low-dose CT scan is a valuable screening tool to add to our practice of medicine.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at fpnews@mdedge.com.
Physician gender pay gap isn’t news; health inequity is rampant
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.














