User login
Pan-Pseudothrombocytopenia in COVID-19: A Harbinger for Lethal Arterial Thrombosis?
In late 2019 a new pandemic started in Wuhan, China, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to its similarities with the virus responsible for the SARS outbreak of 2003. The disease manifestations are named coronavirus disease 2019 (COVID-19).1
Pseudothrombocytopenia, or platelet clumping, visualized on the peripheral blood smear, is a common cause for artificial thrombocytopenia laboratory reporting and is frequently attributed to laboratory artifact. In this case presentation, a critically ill patient with COVID-19 developed pan-pseudothrombocytopenia (ethylenediaminetetraacetic acid [EDTA], sodium citrate, and heparin tubes) just prior to his death from a ST-segment elevation myocardial infarction (STEMI) in the setting of therapeutic anticoagulation during a prolonged hospitalization. This case raises the possibility that pseudothrombocytopenia in the setting of COVID-19 critical illness may represent an ominous feature of COVID-19-associated coagulopathy (CAC). Furthermore, it prompts the question whether pseudothrombocytopenia in this setting is representative of increased platelet aggregation activity in vivo.
Case Presentation
A 50-year-old African American man who was diagnosed with COVID-19 3 days prior to admission presented to the emergency department of the W.G. (Bill) Hefner VA Medical Center in Salisbury, North Carolina, with worsening dyspnea and fever. His primary chronic medical problems included obesity (body mass index, 33), type 2 diabetes mellitus (hemoglobin A1c 2 months prior of 6.6%), migraine headaches, and obstructive sleep apnea. Shortly after presentation, his respiratory status declined, requiring intubation. He was admitted to the medical intensive care unit for further management.
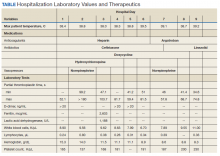
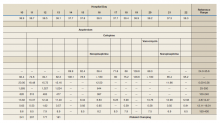
Notable findings at admission included > 20 mcg/mL FEU D-dimer (normal range, 0-0.56 mcg/mL FEU), 20.4 mg/dL C-reactive protein (normal range, < 1 mg/dL), 30 mm/h erythrocyte sedimentation rate (normal range, 0-25 mm/h), and 3.56 ng/mL procalcitonin (normal range, 0.05-1.99 ng/mL). Patient’s hemoglobin and platelet counts were normal. Empiric antimicrobial therapy was initiated with ceftriaxone (2 g IV daily) and doxycycline (100 mg IV twice daily) due to concern of superimposed infection in the setting of an elevated procalcitonin.
A heparin infusion was initiated (5,000 U IV bolus followed by continuous infusion with goal partial thromboplastin time [PTT] of 1.5x the upper limit of normal) on admission to treat CAC. Renal function worsened requiring intermittent renal replacement therapy on day 3. His lactate dehydrogenase was elevated to 1,188 U/L (normal range: 100-240 U/L) and ferritin was elevated to 2,603 ng/mL (normal range: 25-350 ng/mL) (Table). Initial neuromuscular blockade and prone positioning maneuvers were instituted to optimize oxygenation based on the latest literature for respiratory distress in the COVID-19 management.2
Intermittent norepinephrine infusion (5 mcg/min with a 2 mcg/min titration every 5 minutes as needed to maintain mean arterial pressure of > 65 mm Hg) was required for hemodynamic support throughout the patient’s course. Several therapies for COVID-19 were considered and were a reflection of the rapidly evolving literature during the care of patients with this disease. The patient originally received hydroxychloroquine (200 mg by mouth twice daily) in accordance with the US Department of Veterans Affairs (VA) institutional protocol between day 2 and day 4; however, hydroxychloroquine was stopped due to concerns of QTc prolongation. The patient also received 1 unit of convalescent plasma on day 6 after being enrolled in the expanded access program.3 The patient was not a candidate for remdesivir due to his unstable renal function and need for vasopressors. Finally, interleukin-6 inhibitors also were considered; however, the risk of superimposed infection precluded its use.
On day 7 antimicrobial therapy was transitioned to linezolid (600 mg IV twice daily) due to the persistence of fever and a portable chest radiograph revealing diffuse infiltrates throughout the bilateral lungs, worse compared with prior radiograph on day 5, suggesting a worsening of pneumonia. On day 12, the patient was transitioned to cefepime (1 gram IV daily) to broaden antimicrobial coverage and was continued thereafter. Blood cultures were negative throughout his hospitalization.
Given his worsening clinical scenario there was a question about whether or not the patient was still shedding virus for prognostic and therapeutic implications. Therefore, his SARS-CoV-2 test by polymerase chain reaction nasopharyngeal was positive again on day 18. On day 20, the patient developed leukocytosis, his fever persisted, and a portable chest radiograph revealed extensive bilateral pulmonary opacities with focal worsening in left lower base. Due to this constellation of findings, a vancomycin IV (1,500 mg once) was started for empirical treatment of hospital-acquired pneumonia. Sputum samples obtained on day 20 revealed Staphylococcus aureus on subsequent days.
From a hematologic perspective, on day 9 due to challenges to maintain a therapeutic level of anticoagulation with heparin infusion thought to be related to antithrombin deficiency, anticoagulation was changed to argatroban infusion (0.5 mcg/kg/min targeting a PTT of 70-105 seconds) for ongoing management of CAC. Although D-dimer was > 20 mcg/mL FEU on admission and on days 4 and 5, D-dimer trended down to 12.5 mcg/mL FEU on day 16.
Throughout the patient’s hospital stay, no significant bleeding was seen. Hemoglobin was 15.2 g/dL on admission, but anemia developed with a nadir of 6.5 g/dL, warranting transfusion of red blood cells on day 22. Platelet count was 165,000 per microliter on admission and remained within normal limits until platelet clumping was noted on day 15 laboratory collection.
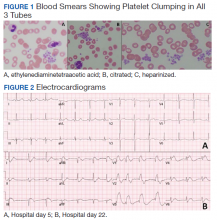
Hematology was consulted on day 20 to obtain an accurate platelet count. A peripheral blood smear from a sodium citrate containing tube was remarkable for prominent platelet clumping, particularly at the periphery of the slide (Figure 1). Platelet clumping was reproduced in samples containing EDTA and heparin. Other features of the peripheral blood smear included the presence of echinocytes with rare schistocytes. To investigate for presence of disseminated intravascular coagulation on day 22, fibrinogen was found to be mildly elevated at 538 mg/dL (normal range: 243-517 mg/dL) and a D-dimer value of 11.96 mcg/mL FEU.
On day 22, the patient’s ventilator requirements escalated to requiring 100% FiO2 and 10 cm H20 of positive end-expiratory pressure with mean arterial pressures in the 50 to 60 mm Hg range. Within 30 minutes an electrocardiogram (EKG) obtained revealed a STEMI (Figure 2). Troponin was measured at 0.65 ng/mL (normal range: 0.02-0.06 ng/mL). Just after an EKG was performed, the patient developed a ventricular fibrillation arrest and was unable to obtain return of spontaneous circulation. The patient was pronounced dead. The family declined an autopsy.
Discussion
Pseudothrombocytopenia, or platelet clumping (agglutination), is estimated to be present in up to 2% of hospitalized patients.4 Pseudothrombocytopenia was found to be the root cause of thrombocytopenia hematology consultations in up to 4% of hospitalized patients.5 The etiology is commonly ascribed to EDTA inducing a conformational change in the GpIIb-IIIa platelet complex, rendering it susceptible to binding of autoantibodies, which cause subsequent platelet agglutination.6 In most cases (83%), the use of a non-EDTA anticoagulant, such as sodium citrate, resolves the platelet agglutination and allows for accurate platelet count reporting.4 Pseudothrombocytopenia in most cases is considered an in vitro finding without clinical relevance.7 However, in this patient’s case, his pan-pseudothrombocytopenia was temporally associated with an arterial occlusive event (STEMI) leading to his demise despite therapeutic anticoagulation in the setting of CAC. This temporal association raises the possibility that pseudothrombocytopenia seen on the peripheral blood smear is an accurate representation of in vivo activity.
Pseudothrombocytopenia has been associated with sepsis from bacterial and viral causes as well as autoimmune and medication effect.4,8-10 Li and colleagues reported transient EDTA-dependent pseudothrombocytopenia in a patient with COVID-19 infection; however, platelet clumping resolved with use of a citrate tube, and the EDTA-dependent pseudothrombocytopenia phenomenon resolved with patient recovery.11 The frequency of COVID-19-related pseudothrombocytopenia is currently unknown.
Although the understanding of COVID-19-associated CAC continues to evolve, it seems that initial reports support the idea that hemostatic dysfunction tends to more thrombosis than to bleeding.12 Rather than overt disseminated intravascular coagulation with reduced fibrinogen and bleeding, CAC is more closely associated with blood clotting, as demonstrated by autopsy studies revealing microvascular thrombosis in the lungs.13 The D-dimer test has been identified as the most useful biomarker by the International Society of Thrombosis and Hemostasis to screen for CAC and stratify patients who warrant admission or closer monitoring.12 Other identified features of CAC include prolonged prothrombin time and thrombocytopenia.12
There have been varying clinical approaches to CAC management. A retrospective review found that prophylactic heparin doses were associated with improved mortality in those with elevated D-dimer > 3.0 mg/L.14 There continues to be a diversity of varying clinical approaches with many medical centers advocating for an intensified prophylactic twice daily low molecular-weight heparin compared with others advocating for full therapeutic dose anticoagulation for patients with elevated D-dimer.15 This patient was treated aggressively with full-dose anticoagulation, and despite his having a down-trend in D-dimer, he suffered a lethal arterial thrombosis in the form of a STEMI.
Varatharajah and Rajah
Conclusions
This patient’s case highlights the presence of pan-pseudothrombocytopenia despite the use of a sodium citrate and heparin containing tube in a COVID-19 infection with multiorgan dysfunction. This developed 1 week prior to the patient suffering a STEMI despite therapeutic anticoagulation. Although the exact nature of CAC remains to be worked out, it is possible that platelet agglutination/clumping seen on the peripheral blood smear is representative of in vivo activity and serves as a harbinger for worsening thrombosis. The frequency of such phenomenon and efficacy of further interventions has yet to be explored.
1. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it. Accessed July 15, 2020.
2. Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8(1):e48. Published 2020 April 11.
3. National Library of Medicine, Clinicaltrials.gov. Expanded access to convalescent plasma for the treatment of patients with COVID-19. NCT04338360. https://clinicaltrials.gov/ct2/show/nct04338360. Update April 20, 2020. Accessed July 15, 2020.
4. Tan GC, Stalling M, Dennis G, Nunez M, Kahwash SB. Pseudothrombocytopenia due to platelet clumping: a case report and brief review of the literature. Case Rep Hematol. 2016;2016:3036476. doi:10.1155/2016/3036476
5. Boxer M, Biuso TJ. Etiologies of thrombocytopenia in the community hospital: the experience of 1 hematologist. Am J Med. 2020;133(5):e183-e186. doi:10.1016/j.amjmed.2019.10.027
6. Fiorin F, Steffan A, Pradella P, Bizzaro N, Potenza R, De Angelis V. IgG platelet antibodies in EDTA-dependent pseudothrombocytopenia bind to platelet membrane glycoprotein IIb. Am J Clin Pathol. 1998;110(2):178-183. doi:10.1093/ajcp/110.2.178
7. Nagler M, Keller P, Siegrist S, Alberio L. A case of EDTA-Dependent pseudothrombocytopenia: simple recognition of an underdiagnosed and misleading phenomenon. BMC Clin Pathol. 2014;14:19. doi:10.1186/1472-6890-14-19
8. Mori M, Kudo H, Yoshitake S, Ito K, Shinguu C, Noguchi T. Transient EDTA-dependent pseudothrombocytopenia in a patient with sepsis. Intensive Care Med. 2000;26(2):218-220. doi:10.1007/s001340050050.
9. Choe W-H, Cho Y-U, Chae J-D, Kim S-H. 2013. Pseudothrombocytopenia or platelet clumping as a possible cause of low platelet count in patients with viral infection: a case series from single institution focusing on hepatitis A virus infection. Int J Lab Hematol. 2013;35(1):70-76. doi:10.1111/j.1751-553x.2012.01466.
10. Hsieh AT, Chao TY, Chen YC. Pseudothrombocytopenia associated with infectious mononucleosis. Arch Pathol Lab Med. 2003;127(1):e17-e18. doi:10.1043/0003-9985(2003)1272.0.CO;2
11. Li H, Wang B, Ning L, Luo Y, Xiang S. Transient appearance of EDTA dependent pseudothrombocytopenia in a patient with 2019 novel coronavirus pneumonia [published online ahead of print, 2020 May 5]. Platelets. 2020;1-2. doi:10.1080/09537104.2020.1760231
12. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
13. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. doi:10.1111/jth.14817
15. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;125(23):2033-2040. doi.org/10.1182/blood.2020006000.
16. Varatharajah N, Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge von Willebrand factor (eULVWF) Decorated-Platelet Strings. Fed Pract. 2020;37(6):258-259. doi:10.12788/fp.0001
17. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562-570. doi:10.1111/j.1538-7836.2005.01122.x
In late 2019 a new pandemic started in Wuhan, China, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to its similarities with the virus responsible for the SARS outbreak of 2003. The disease manifestations are named coronavirus disease 2019 (COVID-19).1
Pseudothrombocytopenia, or platelet clumping, visualized on the peripheral blood smear, is a common cause for artificial thrombocytopenia laboratory reporting and is frequently attributed to laboratory artifact. In this case presentation, a critically ill patient with COVID-19 developed pan-pseudothrombocytopenia (ethylenediaminetetraacetic acid [EDTA], sodium citrate, and heparin tubes) just prior to his death from a ST-segment elevation myocardial infarction (STEMI) in the setting of therapeutic anticoagulation during a prolonged hospitalization. This case raises the possibility that pseudothrombocytopenia in the setting of COVID-19 critical illness may represent an ominous feature of COVID-19-associated coagulopathy (CAC). Furthermore, it prompts the question whether pseudothrombocytopenia in this setting is representative of increased platelet aggregation activity in vivo.
Case Presentation
A 50-year-old African American man who was diagnosed with COVID-19 3 days prior to admission presented to the emergency department of the W.G. (Bill) Hefner VA Medical Center in Salisbury, North Carolina, with worsening dyspnea and fever. His primary chronic medical problems included obesity (body mass index, 33), type 2 diabetes mellitus (hemoglobin A1c 2 months prior of 6.6%), migraine headaches, and obstructive sleep apnea. Shortly after presentation, his respiratory status declined, requiring intubation. He was admitted to the medical intensive care unit for further management.
Notable findings at admission included > 20 mcg/mL FEU D-dimer (normal range, 0-0.56 mcg/mL FEU), 20.4 mg/dL C-reactive protein (normal range, < 1 mg/dL), 30 mm/h erythrocyte sedimentation rate (normal range, 0-25 mm/h), and 3.56 ng/mL procalcitonin (normal range, 0.05-1.99 ng/mL). Patient’s hemoglobin and platelet counts were normal. Empiric antimicrobial therapy was initiated with ceftriaxone (2 g IV daily) and doxycycline (100 mg IV twice daily) due to concern of superimposed infection in the setting of an elevated procalcitonin.
A heparin infusion was initiated (5,000 U IV bolus followed by continuous infusion with goal partial thromboplastin time [PTT] of 1.5x the upper limit of normal) on admission to treat CAC. Renal function worsened requiring intermittent renal replacement therapy on day 3. His lactate dehydrogenase was elevated to 1,188 U/L (normal range: 100-240 U/L) and ferritin was elevated to 2,603 ng/mL (normal range: 25-350 ng/mL) (Table). Initial neuromuscular blockade and prone positioning maneuvers were instituted to optimize oxygenation based on the latest literature for respiratory distress in the COVID-19 management.2
Intermittent norepinephrine infusion (5 mcg/min with a 2 mcg/min titration every 5 minutes as needed to maintain mean arterial pressure of > 65 mm Hg) was required for hemodynamic support throughout the patient’s course. Several therapies for COVID-19 were considered and were a reflection of the rapidly evolving literature during the care of patients with this disease. The patient originally received hydroxychloroquine (200 mg by mouth twice daily) in accordance with the US Department of Veterans Affairs (VA) institutional protocol between day 2 and day 4; however, hydroxychloroquine was stopped due to concerns of QTc prolongation. The patient also received 1 unit of convalescent plasma on day 6 after being enrolled in the expanded access program.3 The patient was not a candidate for remdesivir due to his unstable renal function and need for vasopressors. Finally, interleukin-6 inhibitors also were considered; however, the risk of superimposed infection precluded its use.
On day 7 antimicrobial therapy was transitioned to linezolid (600 mg IV twice daily) due to the persistence of fever and a portable chest radiograph revealing diffuse infiltrates throughout the bilateral lungs, worse compared with prior radiograph on day 5, suggesting a worsening of pneumonia. On day 12, the patient was transitioned to cefepime (1 gram IV daily) to broaden antimicrobial coverage and was continued thereafter. Blood cultures were negative throughout his hospitalization.
Given his worsening clinical scenario there was a question about whether or not the patient was still shedding virus for prognostic and therapeutic implications. Therefore, his SARS-CoV-2 test by polymerase chain reaction nasopharyngeal was positive again on day 18. On day 20, the patient developed leukocytosis, his fever persisted, and a portable chest radiograph revealed extensive bilateral pulmonary opacities with focal worsening in left lower base. Due to this constellation of findings, a vancomycin IV (1,500 mg once) was started for empirical treatment of hospital-acquired pneumonia. Sputum samples obtained on day 20 revealed Staphylococcus aureus on subsequent days.
From a hematologic perspective, on day 9 due to challenges to maintain a therapeutic level of anticoagulation with heparin infusion thought to be related to antithrombin deficiency, anticoagulation was changed to argatroban infusion (0.5 mcg/kg/min targeting a PTT of 70-105 seconds) for ongoing management of CAC. Although D-dimer was > 20 mcg/mL FEU on admission and on days 4 and 5, D-dimer trended down to 12.5 mcg/mL FEU on day 16.
Throughout the patient’s hospital stay, no significant bleeding was seen. Hemoglobin was 15.2 g/dL on admission, but anemia developed with a nadir of 6.5 g/dL, warranting transfusion of red blood cells on day 22. Platelet count was 165,000 per microliter on admission and remained within normal limits until platelet clumping was noted on day 15 laboratory collection.
Hematology was consulted on day 20 to obtain an accurate platelet count. A peripheral blood smear from a sodium citrate containing tube was remarkable for prominent platelet clumping, particularly at the periphery of the slide (Figure 1). Platelet clumping was reproduced in samples containing EDTA and heparin. Other features of the peripheral blood smear included the presence of echinocytes with rare schistocytes. To investigate for presence of disseminated intravascular coagulation on day 22, fibrinogen was found to be mildly elevated at 538 mg/dL (normal range: 243-517 mg/dL) and a D-dimer value of 11.96 mcg/mL FEU.
On day 22, the patient’s ventilator requirements escalated to requiring 100% FiO2 and 10 cm H20 of positive end-expiratory pressure with mean arterial pressures in the 50 to 60 mm Hg range. Within 30 minutes an electrocardiogram (EKG) obtained revealed a STEMI (Figure 2). Troponin was measured at 0.65 ng/mL (normal range: 0.02-0.06 ng/mL). Just after an EKG was performed, the patient developed a ventricular fibrillation arrest and was unable to obtain return of spontaneous circulation. The patient was pronounced dead. The family declined an autopsy.
Discussion
Pseudothrombocytopenia, or platelet clumping (agglutination), is estimated to be present in up to 2% of hospitalized patients.4 Pseudothrombocytopenia was found to be the root cause of thrombocytopenia hematology consultations in up to 4% of hospitalized patients.5 The etiology is commonly ascribed to EDTA inducing a conformational change in the GpIIb-IIIa platelet complex, rendering it susceptible to binding of autoantibodies, which cause subsequent platelet agglutination.6 In most cases (83%), the use of a non-EDTA anticoagulant, such as sodium citrate, resolves the platelet agglutination and allows for accurate platelet count reporting.4 Pseudothrombocytopenia in most cases is considered an in vitro finding without clinical relevance.7 However, in this patient’s case, his pan-pseudothrombocytopenia was temporally associated with an arterial occlusive event (STEMI) leading to his demise despite therapeutic anticoagulation in the setting of CAC. This temporal association raises the possibility that pseudothrombocytopenia seen on the peripheral blood smear is an accurate representation of in vivo activity.
Pseudothrombocytopenia has been associated with sepsis from bacterial and viral causes as well as autoimmune and medication effect.4,8-10 Li and colleagues reported transient EDTA-dependent pseudothrombocytopenia in a patient with COVID-19 infection; however, platelet clumping resolved with use of a citrate tube, and the EDTA-dependent pseudothrombocytopenia phenomenon resolved with patient recovery.11 The frequency of COVID-19-related pseudothrombocytopenia is currently unknown.
Although the understanding of COVID-19-associated CAC continues to evolve, it seems that initial reports support the idea that hemostatic dysfunction tends to more thrombosis than to bleeding.12 Rather than overt disseminated intravascular coagulation with reduced fibrinogen and bleeding, CAC is more closely associated with blood clotting, as demonstrated by autopsy studies revealing microvascular thrombosis in the lungs.13 The D-dimer test has been identified as the most useful biomarker by the International Society of Thrombosis and Hemostasis to screen for CAC and stratify patients who warrant admission or closer monitoring.12 Other identified features of CAC include prolonged prothrombin time and thrombocytopenia.12
There have been varying clinical approaches to CAC management. A retrospective review found that prophylactic heparin doses were associated with improved mortality in those with elevated D-dimer > 3.0 mg/L.14 There continues to be a diversity of varying clinical approaches with many medical centers advocating for an intensified prophylactic twice daily low molecular-weight heparin compared with others advocating for full therapeutic dose anticoagulation for patients with elevated D-dimer.15 This patient was treated aggressively with full-dose anticoagulation, and despite his having a down-trend in D-dimer, he suffered a lethal arterial thrombosis in the form of a STEMI.
Varatharajah and Rajah
Conclusions
This patient’s case highlights the presence of pan-pseudothrombocytopenia despite the use of a sodium citrate and heparin containing tube in a COVID-19 infection with multiorgan dysfunction. This developed 1 week prior to the patient suffering a STEMI despite therapeutic anticoagulation. Although the exact nature of CAC remains to be worked out, it is possible that platelet agglutination/clumping seen on the peripheral blood smear is representative of in vivo activity and serves as a harbinger for worsening thrombosis. The frequency of such phenomenon and efficacy of further interventions has yet to be explored.
In late 2019 a new pandemic started in Wuhan, China, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to its similarities with the virus responsible for the SARS outbreak of 2003. The disease manifestations are named coronavirus disease 2019 (COVID-19).1
Pseudothrombocytopenia, or platelet clumping, visualized on the peripheral blood smear, is a common cause for artificial thrombocytopenia laboratory reporting and is frequently attributed to laboratory artifact. In this case presentation, a critically ill patient with COVID-19 developed pan-pseudothrombocytopenia (ethylenediaminetetraacetic acid [EDTA], sodium citrate, and heparin tubes) just prior to his death from a ST-segment elevation myocardial infarction (STEMI) in the setting of therapeutic anticoagulation during a prolonged hospitalization. This case raises the possibility that pseudothrombocytopenia in the setting of COVID-19 critical illness may represent an ominous feature of COVID-19-associated coagulopathy (CAC). Furthermore, it prompts the question whether pseudothrombocytopenia in this setting is representative of increased platelet aggregation activity in vivo.
Case Presentation
A 50-year-old African American man who was diagnosed with COVID-19 3 days prior to admission presented to the emergency department of the W.G. (Bill) Hefner VA Medical Center in Salisbury, North Carolina, with worsening dyspnea and fever. His primary chronic medical problems included obesity (body mass index, 33), type 2 diabetes mellitus (hemoglobin A1c 2 months prior of 6.6%), migraine headaches, and obstructive sleep apnea. Shortly after presentation, his respiratory status declined, requiring intubation. He was admitted to the medical intensive care unit for further management.
Notable findings at admission included > 20 mcg/mL FEU D-dimer (normal range, 0-0.56 mcg/mL FEU), 20.4 mg/dL C-reactive protein (normal range, < 1 mg/dL), 30 mm/h erythrocyte sedimentation rate (normal range, 0-25 mm/h), and 3.56 ng/mL procalcitonin (normal range, 0.05-1.99 ng/mL). Patient’s hemoglobin and platelet counts were normal. Empiric antimicrobial therapy was initiated with ceftriaxone (2 g IV daily) and doxycycline (100 mg IV twice daily) due to concern of superimposed infection in the setting of an elevated procalcitonin.
A heparin infusion was initiated (5,000 U IV bolus followed by continuous infusion with goal partial thromboplastin time [PTT] of 1.5x the upper limit of normal) on admission to treat CAC. Renal function worsened requiring intermittent renal replacement therapy on day 3. His lactate dehydrogenase was elevated to 1,188 U/L (normal range: 100-240 U/L) and ferritin was elevated to 2,603 ng/mL (normal range: 25-350 ng/mL) (Table). Initial neuromuscular blockade and prone positioning maneuvers were instituted to optimize oxygenation based on the latest literature for respiratory distress in the COVID-19 management.2
Intermittent norepinephrine infusion (5 mcg/min with a 2 mcg/min titration every 5 minutes as needed to maintain mean arterial pressure of > 65 mm Hg) was required for hemodynamic support throughout the patient’s course. Several therapies for COVID-19 were considered and were a reflection of the rapidly evolving literature during the care of patients with this disease. The patient originally received hydroxychloroquine (200 mg by mouth twice daily) in accordance with the US Department of Veterans Affairs (VA) institutional protocol between day 2 and day 4; however, hydroxychloroquine was stopped due to concerns of QTc prolongation. The patient also received 1 unit of convalescent plasma on day 6 after being enrolled in the expanded access program.3 The patient was not a candidate for remdesivir due to his unstable renal function and need for vasopressors. Finally, interleukin-6 inhibitors also were considered; however, the risk of superimposed infection precluded its use.
On day 7 antimicrobial therapy was transitioned to linezolid (600 mg IV twice daily) due to the persistence of fever and a portable chest radiograph revealing diffuse infiltrates throughout the bilateral lungs, worse compared with prior radiograph on day 5, suggesting a worsening of pneumonia. On day 12, the patient was transitioned to cefepime (1 gram IV daily) to broaden antimicrobial coverage and was continued thereafter. Blood cultures were negative throughout his hospitalization.
Given his worsening clinical scenario there was a question about whether or not the patient was still shedding virus for prognostic and therapeutic implications. Therefore, his SARS-CoV-2 test by polymerase chain reaction nasopharyngeal was positive again on day 18. On day 20, the patient developed leukocytosis, his fever persisted, and a portable chest radiograph revealed extensive bilateral pulmonary opacities with focal worsening in left lower base. Due to this constellation of findings, a vancomycin IV (1,500 mg once) was started for empirical treatment of hospital-acquired pneumonia. Sputum samples obtained on day 20 revealed Staphylococcus aureus on subsequent days.
From a hematologic perspective, on day 9 due to challenges to maintain a therapeutic level of anticoagulation with heparin infusion thought to be related to antithrombin deficiency, anticoagulation was changed to argatroban infusion (0.5 mcg/kg/min targeting a PTT of 70-105 seconds) for ongoing management of CAC. Although D-dimer was > 20 mcg/mL FEU on admission and on days 4 and 5, D-dimer trended down to 12.5 mcg/mL FEU on day 16.
Throughout the patient’s hospital stay, no significant bleeding was seen. Hemoglobin was 15.2 g/dL on admission, but anemia developed with a nadir of 6.5 g/dL, warranting transfusion of red blood cells on day 22. Platelet count was 165,000 per microliter on admission and remained within normal limits until platelet clumping was noted on day 15 laboratory collection.
Hematology was consulted on day 20 to obtain an accurate platelet count. A peripheral blood smear from a sodium citrate containing tube was remarkable for prominent platelet clumping, particularly at the periphery of the slide (Figure 1). Platelet clumping was reproduced in samples containing EDTA and heparin. Other features of the peripheral blood smear included the presence of echinocytes with rare schistocytes. To investigate for presence of disseminated intravascular coagulation on day 22, fibrinogen was found to be mildly elevated at 538 mg/dL (normal range: 243-517 mg/dL) and a D-dimer value of 11.96 mcg/mL FEU.
On day 22, the patient’s ventilator requirements escalated to requiring 100% FiO2 and 10 cm H20 of positive end-expiratory pressure with mean arterial pressures in the 50 to 60 mm Hg range. Within 30 minutes an electrocardiogram (EKG) obtained revealed a STEMI (Figure 2). Troponin was measured at 0.65 ng/mL (normal range: 0.02-0.06 ng/mL). Just after an EKG was performed, the patient developed a ventricular fibrillation arrest and was unable to obtain return of spontaneous circulation. The patient was pronounced dead. The family declined an autopsy.
Discussion
Pseudothrombocytopenia, or platelet clumping (agglutination), is estimated to be present in up to 2% of hospitalized patients.4 Pseudothrombocytopenia was found to be the root cause of thrombocytopenia hematology consultations in up to 4% of hospitalized patients.5 The etiology is commonly ascribed to EDTA inducing a conformational change in the GpIIb-IIIa platelet complex, rendering it susceptible to binding of autoantibodies, which cause subsequent platelet agglutination.6 In most cases (83%), the use of a non-EDTA anticoagulant, such as sodium citrate, resolves the platelet agglutination and allows for accurate platelet count reporting.4 Pseudothrombocytopenia in most cases is considered an in vitro finding without clinical relevance.7 However, in this patient’s case, his pan-pseudothrombocytopenia was temporally associated with an arterial occlusive event (STEMI) leading to his demise despite therapeutic anticoagulation in the setting of CAC. This temporal association raises the possibility that pseudothrombocytopenia seen on the peripheral blood smear is an accurate representation of in vivo activity.
Pseudothrombocytopenia has been associated with sepsis from bacterial and viral causes as well as autoimmune and medication effect.4,8-10 Li and colleagues reported transient EDTA-dependent pseudothrombocytopenia in a patient with COVID-19 infection; however, platelet clumping resolved with use of a citrate tube, and the EDTA-dependent pseudothrombocytopenia phenomenon resolved with patient recovery.11 The frequency of COVID-19-related pseudothrombocytopenia is currently unknown.
Although the understanding of COVID-19-associated CAC continues to evolve, it seems that initial reports support the idea that hemostatic dysfunction tends to more thrombosis than to bleeding.12 Rather than overt disseminated intravascular coagulation with reduced fibrinogen and bleeding, CAC is more closely associated with blood clotting, as demonstrated by autopsy studies revealing microvascular thrombosis in the lungs.13 The D-dimer test has been identified as the most useful biomarker by the International Society of Thrombosis and Hemostasis to screen for CAC and stratify patients who warrant admission or closer monitoring.12 Other identified features of CAC include prolonged prothrombin time and thrombocytopenia.12
There have been varying clinical approaches to CAC management. A retrospective review found that prophylactic heparin doses were associated with improved mortality in those with elevated D-dimer > 3.0 mg/L.14 There continues to be a diversity of varying clinical approaches with many medical centers advocating for an intensified prophylactic twice daily low molecular-weight heparin compared with others advocating for full therapeutic dose anticoagulation for patients with elevated D-dimer.15 This patient was treated aggressively with full-dose anticoagulation, and despite his having a down-trend in D-dimer, he suffered a lethal arterial thrombosis in the form of a STEMI.
Varatharajah and Rajah
Conclusions
This patient’s case highlights the presence of pan-pseudothrombocytopenia despite the use of a sodium citrate and heparin containing tube in a COVID-19 infection with multiorgan dysfunction. This developed 1 week prior to the patient suffering a STEMI despite therapeutic anticoagulation. Although the exact nature of CAC remains to be worked out, it is possible that platelet agglutination/clumping seen on the peripheral blood smear is representative of in vivo activity and serves as a harbinger for worsening thrombosis. The frequency of such phenomenon and efficacy of further interventions has yet to be explored.
1. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it. Accessed July 15, 2020.
2. Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8(1):e48. Published 2020 April 11.
3. National Library of Medicine, Clinicaltrials.gov. Expanded access to convalescent plasma for the treatment of patients with COVID-19. NCT04338360. https://clinicaltrials.gov/ct2/show/nct04338360. Update April 20, 2020. Accessed July 15, 2020.
4. Tan GC, Stalling M, Dennis G, Nunez M, Kahwash SB. Pseudothrombocytopenia due to platelet clumping: a case report and brief review of the literature. Case Rep Hematol. 2016;2016:3036476. doi:10.1155/2016/3036476
5. Boxer M, Biuso TJ. Etiologies of thrombocytopenia in the community hospital: the experience of 1 hematologist. Am J Med. 2020;133(5):e183-e186. doi:10.1016/j.amjmed.2019.10.027
6. Fiorin F, Steffan A, Pradella P, Bizzaro N, Potenza R, De Angelis V. IgG platelet antibodies in EDTA-dependent pseudothrombocytopenia bind to platelet membrane glycoprotein IIb. Am J Clin Pathol. 1998;110(2):178-183. doi:10.1093/ajcp/110.2.178
7. Nagler M, Keller P, Siegrist S, Alberio L. A case of EDTA-Dependent pseudothrombocytopenia: simple recognition of an underdiagnosed and misleading phenomenon. BMC Clin Pathol. 2014;14:19. doi:10.1186/1472-6890-14-19
8. Mori M, Kudo H, Yoshitake S, Ito K, Shinguu C, Noguchi T. Transient EDTA-dependent pseudothrombocytopenia in a patient with sepsis. Intensive Care Med. 2000;26(2):218-220. doi:10.1007/s001340050050.
9. Choe W-H, Cho Y-U, Chae J-D, Kim S-H. 2013. Pseudothrombocytopenia or platelet clumping as a possible cause of low platelet count in patients with viral infection: a case series from single institution focusing on hepatitis A virus infection. Int J Lab Hematol. 2013;35(1):70-76. doi:10.1111/j.1751-553x.2012.01466.
10. Hsieh AT, Chao TY, Chen YC. Pseudothrombocytopenia associated with infectious mononucleosis. Arch Pathol Lab Med. 2003;127(1):e17-e18. doi:10.1043/0003-9985(2003)1272.0.CO;2
11. Li H, Wang B, Ning L, Luo Y, Xiang S. Transient appearance of EDTA dependent pseudothrombocytopenia in a patient with 2019 novel coronavirus pneumonia [published online ahead of print, 2020 May 5]. Platelets. 2020;1-2. doi:10.1080/09537104.2020.1760231
12. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
13. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. doi:10.1111/jth.14817
15. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;125(23):2033-2040. doi.org/10.1182/blood.2020006000.
16. Varatharajah N, Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge von Willebrand factor (eULVWF) Decorated-Platelet Strings. Fed Pract. 2020;37(6):258-259. doi:10.12788/fp.0001
17. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562-570. doi:10.1111/j.1538-7836.2005.01122.x
1. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it. Accessed July 15, 2020.
2. Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8(1):e48. Published 2020 April 11.
3. National Library of Medicine, Clinicaltrials.gov. Expanded access to convalescent plasma for the treatment of patients with COVID-19. NCT04338360. https://clinicaltrials.gov/ct2/show/nct04338360. Update April 20, 2020. Accessed July 15, 2020.
4. Tan GC, Stalling M, Dennis G, Nunez M, Kahwash SB. Pseudothrombocytopenia due to platelet clumping: a case report and brief review of the literature. Case Rep Hematol. 2016;2016:3036476. doi:10.1155/2016/3036476
5. Boxer M, Biuso TJ. Etiologies of thrombocytopenia in the community hospital: the experience of 1 hematologist. Am J Med. 2020;133(5):e183-e186. doi:10.1016/j.amjmed.2019.10.027
6. Fiorin F, Steffan A, Pradella P, Bizzaro N, Potenza R, De Angelis V. IgG platelet antibodies in EDTA-dependent pseudothrombocytopenia bind to platelet membrane glycoprotein IIb. Am J Clin Pathol. 1998;110(2):178-183. doi:10.1093/ajcp/110.2.178
7. Nagler M, Keller P, Siegrist S, Alberio L. A case of EDTA-Dependent pseudothrombocytopenia: simple recognition of an underdiagnosed and misleading phenomenon. BMC Clin Pathol. 2014;14:19. doi:10.1186/1472-6890-14-19
8. Mori M, Kudo H, Yoshitake S, Ito K, Shinguu C, Noguchi T. Transient EDTA-dependent pseudothrombocytopenia in a patient with sepsis. Intensive Care Med. 2000;26(2):218-220. doi:10.1007/s001340050050.
9. Choe W-H, Cho Y-U, Chae J-D, Kim S-H. 2013. Pseudothrombocytopenia or platelet clumping as a possible cause of low platelet count in patients with viral infection: a case series from single institution focusing on hepatitis A virus infection. Int J Lab Hematol. 2013;35(1):70-76. doi:10.1111/j.1751-553x.2012.01466.
10. Hsieh AT, Chao TY, Chen YC. Pseudothrombocytopenia associated with infectious mononucleosis. Arch Pathol Lab Med. 2003;127(1):e17-e18. doi:10.1043/0003-9985(2003)1272.0.CO;2
11. Li H, Wang B, Ning L, Luo Y, Xiang S. Transient appearance of EDTA dependent pseudothrombocytopenia in a patient with 2019 novel coronavirus pneumonia [published online ahead of print, 2020 May 5]. Platelets. 2020;1-2. doi:10.1080/09537104.2020.1760231
12. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
13. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. doi:10.1111/jth.14817
15. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;125(23):2033-2040. doi.org/10.1182/blood.2020006000.
16. Varatharajah N, Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge von Willebrand factor (eULVWF) Decorated-Platelet Strings. Fed Pract. 2020;37(6):258-259. doi:10.12788/fp.0001
17. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562-570. doi:10.1111/j.1538-7836.2005.01122.x
Antibiotic resistance: Personal responsibility in somewhat short supply
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.

Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.

Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.

Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
FROM OPEN FORUM INFECTIOUS DISEASES
Guidance covers glycemia in dexamethasone-treated COVID-19 patients
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.
Study: Immune checkpoint inhibitors don’t increase risk of death in cancer patients with COVID-19
The study included 113 cancer patients who had laboratory-confirmed COVID-19 within 12 months of receiving immune checkpoint inhibitor therapy. The patients did not receive chemotherapy within 3 months of testing positive for COVID-19.
In all, 33 patients were admitted to the hospital, including 6 who were admitted to the ICU, and 9 patients died.
“Nine out of 113 patients is a mortality rate of 8%, which is in the middle of the earlier reported rates for cancer patients in general [7.6%-12%],” said Aljosja Rogiers, MD, PhD, of the Melanoma Institute Australia in Sydney.
COVID-19 was the primary cause of death in seven of the patients, including three of those who were admitted to the ICU, Dr. Rogiers noted.
He reported these results during the AACR virtual meeting: COVID-19 and Cancer.
Study details
Patients in this study were treated at 19 hospitals in North America, Europe, and Australia, and the data cutoff was May 15, 2020. Most patients (64%) were treated in Europe, which was the epicenter for the COVID-19 pandemic at the time of data collection, Dr. Rogiers noted. A third of patients were in North America, and 3% were in Australia.
The patients’ median age was 63 years (range, 27-86 years). Most patients were men (65%), and most had Eastern Cooperative Oncology Group performance scores of 0-1 (90%).
The most common malignancies were melanoma (57%), non–small cell lung cancer (17%), and renal cell carcinoma (9%). Treatment was for early cancer in 26% of patients and for advanced cancer in 74%. Comorbidities included cardiovascular disease in 27% of patients, diabetes in 15%, pulmonary disease in 12%, and renal disease in 5%.
Immunosuppressive therapy equivalent to a prednisone dose of 10 mg or greater daily was given in 13% of patients, and other immunosuppressive therapies, such as infliximab, were given in 3%.
Among the 60% of patients with COVID-19 symptoms, 68% had fever, 59% had cough, 34% had dyspnea, and 15% had myalgia. Most of the 40% of asymptomatic patients were tested because they had COVID-19–positive contact, Dr. Rogiers noted.
Immune checkpoint inhibitor treatment included monotherapy with a programmed death–1/PD–ligand 1 inhibitor in 82% of patients, combination anti-PD-1 and anti-CTLA4 therapy in 13%, and other therapy – usually a checkpoint inhibitor combined with a different type of targeted agent – in 5%.
At the time of COVID-19 diagnosis, 30% of patients had achieved a partial response, complete response, or had no evidence of disease, 18% had stable disease, and 15% had progression. Response data were not available in 37% of cases, usually because treatment was only recently started prior to COVID-19 diagnosis, Dr. Rogiers said.
Treatments administered for COVID-19 included antibiotic therapy in 25% of patients, oxygen therapy in 20%, glucocorticoids in 10%, antiviral drugs in 6%, and intravenous immunoglobulin or anti–interleukin-6 in 2% each.
Among patients admitted to the ICU, 3% required mechanical ventilation, 2% had vasopressin, and 1% received renal replacement therapy.
At the data cutoff, 20 of 33 hospitalized patients (61%) had been discharged, and 4 (12%) were still in the hospital.
Mortality results
Nine patients died. The rate of death was 8% overall and 27% among hospitalized patients.
“The mortality rate of COVID-19 in the general population without comorbidities is about 1.4%,” Dr. Rogiers said. “For cancer patients, this is reported to be in the range of 7.6%-12%. To what extent patients on immune checkpoint inhibition are at a higher risk of mortality is currently unknown.”
Theoretically, immune checkpoint inhibition could either mitigate or exacerbate COVID-19 infection. It has been hypothesized that immune checkpoint inhibitors could increase the risk of severe acute lung injury or other complications of COVID-19, Dr. Rogiers said, explaining the rationale for the study.
The study shows that the patients who died had a median age of 72 years (range, 49-81 years), which is slightly higher than the median overall age of 63 years. Six patients were from North America, and three were from Italy.
“Two melanoma patients and two non–small cell lung cancer patients died,” Dr. Rogiers said. He noted that two other deaths were in patients with renal cell carcinoma, and three deaths were in other cancer types. All patients had advanced or metastatic disease.
Given that 57% of patients in the study had melanoma and 17% had NSCLC, this finding may indicate that COVID-19 has a slightly higher mortality rate in NSCLC patients than in melanoma patients, but the numbers are small, Dr. Rogiers said.
Notably, six of the patients who died were not admitted to the ICU. In four cases, this was because of underlying malignancy; in the other two cases, it was because of a constrained health care system, Dr. Rogiers said.
Overall, the findings show that the mortality rate of patients with COVID-19 and cancer treated with immune checkpoint inhibitors is similar to the mortality rate reported in the general cancer population, Dr. Rogiers said.
“Treatment with immune checkpoint inhibition does not seem to pose an additional mortality risk for cancer patients with COVID-19,” he concluded.
Dr. Rogiers reported having no conflicts of interest. There was no funding disclosed for the study.
SOURCE: Rogiers A et al. AACR: COVID-19 and Cancer, Abstract S02-01.
The study included 113 cancer patients who had laboratory-confirmed COVID-19 within 12 months of receiving immune checkpoint inhibitor therapy. The patients did not receive chemotherapy within 3 months of testing positive for COVID-19.
In all, 33 patients were admitted to the hospital, including 6 who were admitted to the ICU, and 9 patients died.
“Nine out of 113 patients is a mortality rate of 8%, which is in the middle of the earlier reported rates for cancer patients in general [7.6%-12%],” said Aljosja Rogiers, MD, PhD, of the Melanoma Institute Australia in Sydney.
COVID-19 was the primary cause of death in seven of the patients, including three of those who were admitted to the ICU, Dr. Rogiers noted.
He reported these results during the AACR virtual meeting: COVID-19 and Cancer.
Study details
Patients in this study were treated at 19 hospitals in North America, Europe, and Australia, and the data cutoff was May 15, 2020. Most patients (64%) were treated in Europe, which was the epicenter for the COVID-19 pandemic at the time of data collection, Dr. Rogiers noted. A third of patients were in North America, and 3% were in Australia.
The patients’ median age was 63 years (range, 27-86 years). Most patients were men (65%), and most had Eastern Cooperative Oncology Group performance scores of 0-1 (90%).
The most common malignancies were melanoma (57%), non–small cell lung cancer (17%), and renal cell carcinoma (9%). Treatment was for early cancer in 26% of patients and for advanced cancer in 74%. Comorbidities included cardiovascular disease in 27% of patients, diabetes in 15%, pulmonary disease in 12%, and renal disease in 5%.
Immunosuppressive therapy equivalent to a prednisone dose of 10 mg or greater daily was given in 13% of patients, and other immunosuppressive therapies, such as infliximab, were given in 3%.
Among the 60% of patients with COVID-19 symptoms, 68% had fever, 59% had cough, 34% had dyspnea, and 15% had myalgia. Most of the 40% of asymptomatic patients were tested because they had COVID-19–positive contact, Dr. Rogiers noted.
Immune checkpoint inhibitor treatment included monotherapy with a programmed death–1/PD–ligand 1 inhibitor in 82% of patients, combination anti-PD-1 and anti-CTLA4 therapy in 13%, and other therapy – usually a checkpoint inhibitor combined with a different type of targeted agent – in 5%.
At the time of COVID-19 diagnosis, 30% of patients had achieved a partial response, complete response, or had no evidence of disease, 18% had stable disease, and 15% had progression. Response data were not available in 37% of cases, usually because treatment was only recently started prior to COVID-19 diagnosis, Dr. Rogiers said.
Treatments administered for COVID-19 included antibiotic therapy in 25% of patients, oxygen therapy in 20%, glucocorticoids in 10%, antiviral drugs in 6%, and intravenous immunoglobulin or anti–interleukin-6 in 2% each.
Among patients admitted to the ICU, 3% required mechanical ventilation, 2% had vasopressin, and 1% received renal replacement therapy.
At the data cutoff, 20 of 33 hospitalized patients (61%) had been discharged, and 4 (12%) were still in the hospital.
Mortality results
Nine patients died. The rate of death was 8% overall and 27% among hospitalized patients.
“The mortality rate of COVID-19 in the general population without comorbidities is about 1.4%,” Dr. Rogiers said. “For cancer patients, this is reported to be in the range of 7.6%-12%. To what extent patients on immune checkpoint inhibition are at a higher risk of mortality is currently unknown.”
Theoretically, immune checkpoint inhibition could either mitigate or exacerbate COVID-19 infection. It has been hypothesized that immune checkpoint inhibitors could increase the risk of severe acute lung injury or other complications of COVID-19, Dr. Rogiers said, explaining the rationale for the study.
The study shows that the patients who died had a median age of 72 years (range, 49-81 years), which is slightly higher than the median overall age of 63 years. Six patients were from North America, and three were from Italy.
“Two melanoma patients and two non–small cell lung cancer patients died,” Dr. Rogiers said. He noted that two other deaths were in patients with renal cell carcinoma, and three deaths were in other cancer types. All patients had advanced or metastatic disease.
Given that 57% of patients in the study had melanoma and 17% had NSCLC, this finding may indicate that COVID-19 has a slightly higher mortality rate in NSCLC patients than in melanoma patients, but the numbers are small, Dr. Rogiers said.
Notably, six of the patients who died were not admitted to the ICU. In four cases, this was because of underlying malignancy; in the other two cases, it was because of a constrained health care system, Dr. Rogiers said.
Overall, the findings show that the mortality rate of patients with COVID-19 and cancer treated with immune checkpoint inhibitors is similar to the mortality rate reported in the general cancer population, Dr. Rogiers said.
“Treatment with immune checkpoint inhibition does not seem to pose an additional mortality risk for cancer patients with COVID-19,” he concluded.
Dr. Rogiers reported having no conflicts of interest. There was no funding disclosed for the study.
SOURCE: Rogiers A et al. AACR: COVID-19 and Cancer, Abstract S02-01.
The study included 113 cancer patients who had laboratory-confirmed COVID-19 within 12 months of receiving immune checkpoint inhibitor therapy. The patients did not receive chemotherapy within 3 months of testing positive for COVID-19.
In all, 33 patients were admitted to the hospital, including 6 who were admitted to the ICU, and 9 patients died.
“Nine out of 113 patients is a mortality rate of 8%, which is in the middle of the earlier reported rates for cancer patients in general [7.6%-12%],” said Aljosja Rogiers, MD, PhD, of the Melanoma Institute Australia in Sydney.
COVID-19 was the primary cause of death in seven of the patients, including three of those who were admitted to the ICU, Dr. Rogiers noted.
He reported these results during the AACR virtual meeting: COVID-19 and Cancer.
Study details
Patients in this study were treated at 19 hospitals in North America, Europe, and Australia, and the data cutoff was May 15, 2020. Most patients (64%) were treated in Europe, which was the epicenter for the COVID-19 pandemic at the time of data collection, Dr. Rogiers noted. A third of patients were in North America, and 3% were in Australia.
The patients’ median age was 63 years (range, 27-86 years). Most patients were men (65%), and most had Eastern Cooperative Oncology Group performance scores of 0-1 (90%).
The most common malignancies were melanoma (57%), non–small cell lung cancer (17%), and renal cell carcinoma (9%). Treatment was for early cancer in 26% of patients and for advanced cancer in 74%. Comorbidities included cardiovascular disease in 27% of patients, diabetes in 15%, pulmonary disease in 12%, and renal disease in 5%.
Immunosuppressive therapy equivalent to a prednisone dose of 10 mg or greater daily was given in 13% of patients, and other immunosuppressive therapies, such as infliximab, were given in 3%.
Among the 60% of patients with COVID-19 symptoms, 68% had fever, 59% had cough, 34% had dyspnea, and 15% had myalgia. Most of the 40% of asymptomatic patients were tested because they had COVID-19–positive contact, Dr. Rogiers noted.
Immune checkpoint inhibitor treatment included monotherapy with a programmed death–1/PD–ligand 1 inhibitor in 82% of patients, combination anti-PD-1 and anti-CTLA4 therapy in 13%, and other therapy – usually a checkpoint inhibitor combined with a different type of targeted agent – in 5%.
At the time of COVID-19 diagnosis, 30% of patients had achieved a partial response, complete response, or had no evidence of disease, 18% had stable disease, and 15% had progression. Response data were not available in 37% of cases, usually because treatment was only recently started prior to COVID-19 diagnosis, Dr. Rogiers said.
Treatments administered for COVID-19 included antibiotic therapy in 25% of patients, oxygen therapy in 20%, glucocorticoids in 10%, antiviral drugs in 6%, and intravenous immunoglobulin or anti–interleukin-6 in 2% each.
Among patients admitted to the ICU, 3% required mechanical ventilation, 2% had vasopressin, and 1% received renal replacement therapy.
At the data cutoff, 20 of 33 hospitalized patients (61%) had been discharged, and 4 (12%) were still in the hospital.
Mortality results
Nine patients died. The rate of death was 8% overall and 27% among hospitalized patients.
“The mortality rate of COVID-19 in the general population without comorbidities is about 1.4%,” Dr. Rogiers said. “For cancer patients, this is reported to be in the range of 7.6%-12%. To what extent patients on immune checkpoint inhibition are at a higher risk of mortality is currently unknown.”
Theoretically, immune checkpoint inhibition could either mitigate or exacerbate COVID-19 infection. It has been hypothesized that immune checkpoint inhibitors could increase the risk of severe acute lung injury or other complications of COVID-19, Dr. Rogiers said, explaining the rationale for the study.
The study shows that the patients who died had a median age of 72 years (range, 49-81 years), which is slightly higher than the median overall age of 63 years. Six patients were from North America, and three were from Italy.
“Two melanoma patients and two non–small cell lung cancer patients died,” Dr. Rogiers said. He noted that two other deaths were in patients with renal cell carcinoma, and three deaths were in other cancer types. All patients had advanced or metastatic disease.
Given that 57% of patients in the study had melanoma and 17% had NSCLC, this finding may indicate that COVID-19 has a slightly higher mortality rate in NSCLC patients than in melanoma patients, but the numbers are small, Dr. Rogiers said.
Notably, six of the patients who died were not admitted to the ICU. In four cases, this was because of underlying malignancy; in the other two cases, it was because of a constrained health care system, Dr. Rogiers said.
Overall, the findings show that the mortality rate of patients with COVID-19 and cancer treated with immune checkpoint inhibitors is similar to the mortality rate reported in the general cancer population, Dr. Rogiers said.
“Treatment with immune checkpoint inhibition does not seem to pose an additional mortality risk for cancer patients with COVID-19,” he concluded.
Dr. Rogiers reported having no conflicts of interest. There was no funding disclosed for the study.
SOURCE: Rogiers A et al. AACR: COVID-19 and Cancer, Abstract S02-01.
FROM AACR: COVID-19 AND CANCER
Novel probiotic shows promise in treating type 2 diabetes
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
All NSAIDs raise post-MI risk but some are safer than others: Next chapter
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New psoriasis guidelines focus on topical and alternative treatments, and severity measures
and the National Psoriasis Foundation.
The guidelines, published in the Journal of the American Academy of Dermatology, focus on treatment for adults, and follow the release of other AAD-NPF guidelines on biologics for psoriasis, psoriasis-related comorbidities, pediatric psoriasis, and phototherapy in 2019, and earlier this year, guidelines for systemic nonbiologic treatments. The latest guidelines’ section on topical treatment outlines evidence for the efficacy, effectiveness, and adverse events related to topical steroids, topical tacrolimus and pimecrolimus, vitamin D analogues, tazarotene, moisturizers, salicylic acid, anthralin, coal tar, combinations with biologic agents, and combinations with nonbiologic treatments (methotrexate, cyclosporine, acitretin, and apremilast).
The guidelines noted the “key role” of topical corticosteroids in treating psoriasis “especially for localized disease,” and include a review of the data on low-, moderate-, high-, and ultrahigh-potency topical steroids for psoriasis.
In general, all topical steroids can be used in combination with biologics, according to the guidelines, but the strongest recommendations based on the latest evidence include the addition of an ultra-high potency topical corticosteroid to standard dose etanercept for 12 weeks. Currently, 11 biologics are approved by the Food and Drug Administration for the treatment of psoriasis.
In addition, “while not FDA approved for psoriasis, the topical calcineurin inhibitors tacrolimus and pimecrolimus are often employed in the treatment of psoriasis,” can be helpful for “thinner skin such as facial and intertriginous areas,” and can be steroid sparing when used for more than 4 weeks, according to the guidelines.
Don’t discount the role of patient preferences when choosing topical treatments, the authors noted. “The optimal vehicle choice is the one the patient is mostly likely to use.”
The guidelines also address the evidence for effectiveness, and adverse events in the use of several alternative medicines for psoriasis including traditional Chinese medicine, and the herbal therapies aloe vera and St. John’s wort, as well as the potential role of dietary supplements including fish oil, vitamin D, turmeric, and zinc in managing psoriasis, and the potential role of a gluten-free diet.
In general, research on the efficacy, effectiveness, and potential adverse effects of these strategies are limited, according to the guidelines, although many patients express interest in supplements and herbal products. For example, “Many patients ask about the overall role of vitamin D in skin health. Rather than adding oral vitamin D supplementation, topical therapy with vitamin D agents is effective for the treatment of psoriasis,” the authors noted.
In addition, they noted that mind/body strategies, namely hypnosis and stress reduction or meditation techniques, have been shown to improve symptoms and can be helpful for some patients, but clinical evidence is limited.
The guidelines also addressed methods for assessing disease severity in psoriasis. They recommended using body surface area (BSA) to assess psoriasis severity and patient response to treatment in the clinical setting. However, BSA is a provider assessment tool that “does not take into account location on the body, clinical characteristics of the plaques, symptoms, or quality of life issues,” the authors noted. The Psoriasis Area and Severity Index (PASI) measures erythema, induration, and scaling and is more suited to assessing psoriasis severity and response to treatment in clinical trials rather than in practice, they said.
Prior AAD guidelines on psoriasis were published more than 10 years ago, and major developments including the availability of new biologic drugs and new data on comorbidities have been recognized in the past decade, working group cochair and author of the guidelines Alan Menter, MD, said in an interview.
The key game-changers from previous guidelines include the full section published on comorbidities plus the development of two new important cytokine classes: three IL-17 drugs and three new IL-23 drugs now available for moderate to severe psoriasis, said Dr. Menter, chairman of the division of dermatology at Baylor University Medical Center, Dallas.
Barriers to implementing the guidelines in practice may occur when “third party payers make the decision on which of the 11 biologic drugs now approved for moderate to severe psoriasis should be used,” he noted.
As for next steps in psoriasis studies, “new biomarker research is currently underway,” Dr. Menter said. With 11 biologic agents new formally approved by the FDA for moderate to severe psoriasis, the next steps are to determine which drug is likely to be the most appropriate for each individual patient.
Dr. Menter disclosed relationships with multiple companies that develop and manufacture psoriasis therapies, including Abbott Labs, AbbVie, Amgen, Eli Lilly and Company, Galderma USA, Janssen Pharmaceuticals, LEO Pharma US, Menlo Therapeutics, and Novartis. The updated guidelines were designed by a multidisciplinary work group of psoriasis experts including dermatologists, a rheumatologist, a cardiologist, and representatives from a patient advocacy organization.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2020 Jul 29. doi: 10.1016/j.jaad.2020.07.087.
and the National Psoriasis Foundation.
The guidelines, published in the Journal of the American Academy of Dermatology, focus on treatment for adults, and follow the release of other AAD-NPF guidelines on biologics for psoriasis, psoriasis-related comorbidities, pediatric psoriasis, and phototherapy in 2019, and earlier this year, guidelines for systemic nonbiologic treatments. The latest guidelines’ section on topical treatment outlines evidence for the efficacy, effectiveness, and adverse events related to topical steroids, topical tacrolimus and pimecrolimus, vitamin D analogues, tazarotene, moisturizers, salicylic acid, anthralin, coal tar, combinations with biologic agents, and combinations with nonbiologic treatments (methotrexate, cyclosporine, acitretin, and apremilast).
The guidelines noted the “key role” of topical corticosteroids in treating psoriasis “especially for localized disease,” and include a review of the data on low-, moderate-, high-, and ultrahigh-potency topical steroids for psoriasis.
In general, all topical steroids can be used in combination with biologics, according to the guidelines, but the strongest recommendations based on the latest evidence include the addition of an ultra-high potency topical corticosteroid to standard dose etanercept for 12 weeks. Currently, 11 biologics are approved by the Food and Drug Administration for the treatment of psoriasis.
In addition, “while not FDA approved for psoriasis, the topical calcineurin inhibitors tacrolimus and pimecrolimus are often employed in the treatment of psoriasis,” can be helpful for “thinner skin such as facial and intertriginous areas,” and can be steroid sparing when used for more than 4 weeks, according to the guidelines.
Don’t discount the role of patient preferences when choosing topical treatments, the authors noted. “The optimal vehicle choice is the one the patient is mostly likely to use.”
The guidelines also address the evidence for effectiveness, and adverse events in the use of several alternative medicines for psoriasis including traditional Chinese medicine, and the herbal therapies aloe vera and St. John’s wort, as well as the potential role of dietary supplements including fish oil, vitamin D, turmeric, and zinc in managing psoriasis, and the potential role of a gluten-free diet.
In general, research on the efficacy, effectiveness, and potential adverse effects of these strategies are limited, according to the guidelines, although many patients express interest in supplements and herbal products. For example, “Many patients ask about the overall role of vitamin D in skin health. Rather than adding oral vitamin D supplementation, topical therapy with vitamin D agents is effective for the treatment of psoriasis,” the authors noted.
In addition, they noted that mind/body strategies, namely hypnosis and stress reduction or meditation techniques, have been shown to improve symptoms and can be helpful for some patients, but clinical evidence is limited.
The guidelines also addressed methods for assessing disease severity in psoriasis. They recommended using body surface area (BSA) to assess psoriasis severity and patient response to treatment in the clinical setting. However, BSA is a provider assessment tool that “does not take into account location on the body, clinical characteristics of the plaques, symptoms, or quality of life issues,” the authors noted. The Psoriasis Area and Severity Index (PASI) measures erythema, induration, and scaling and is more suited to assessing psoriasis severity and response to treatment in clinical trials rather than in practice, they said.
Prior AAD guidelines on psoriasis were published more than 10 years ago, and major developments including the availability of new biologic drugs and new data on comorbidities have been recognized in the past decade, working group cochair and author of the guidelines Alan Menter, MD, said in an interview.
The key game-changers from previous guidelines include the full section published on comorbidities plus the development of two new important cytokine classes: three IL-17 drugs and three new IL-23 drugs now available for moderate to severe psoriasis, said Dr. Menter, chairman of the division of dermatology at Baylor University Medical Center, Dallas.
Barriers to implementing the guidelines in practice may occur when “third party payers make the decision on which of the 11 biologic drugs now approved for moderate to severe psoriasis should be used,” he noted.
As for next steps in psoriasis studies, “new biomarker research is currently underway,” Dr. Menter said. With 11 biologic agents new formally approved by the FDA for moderate to severe psoriasis, the next steps are to determine which drug is likely to be the most appropriate for each individual patient.
Dr. Menter disclosed relationships with multiple companies that develop and manufacture psoriasis therapies, including Abbott Labs, AbbVie, Amgen, Eli Lilly and Company, Galderma USA, Janssen Pharmaceuticals, LEO Pharma US, Menlo Therapeutics, and Novartis. The updated guidelines were designed by a multidisciplinary work group of psoriasis experts including dermatologists, a rheumatologist, a cardiologist, and representatives from a patient advocacy organization.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2020 Jul 29. doi: 10.1016/j.jaad.2020.07.087.
and the National Psoriasis Foundation.
The guidelines, published in the Journal of the American Academy of Dermatology, focus on treatment for adults, and follow the release of other AAD-NPF guidelines on biologics for psoriasis, psoriasis-related comorbidities, pediatric psoriasis, and phototherapy in 2019, and earlier this year, guidelines for systemic nonbiologic treatments. The latest guidelines’ section on topical treatment outlines evidence for the efficacy, effectiveness, and adverse events related to topical steroids, topical tacrolimus and pimecrolimus, vitamin D analogues, tazarotene, moisturizers, salicylic acid, anthralin, coal tar, combinations with biologic agents, and combinations with nonbiologic treatments (methotrexate, cyclosporine, acitretin, and apremilast).
The guidelines noted the “key role” of topical corticosteroids in treating psoriasis “especially for localized disease,” and include a review of the data on low-, moderate-, high-, and ultrahigh-potency topical steroids for psoriasis.
In general, all topical steroids can be used in combination with biologics, according to the guidelines, but the strongest recommendations based on the latest evidence include the addition of an ultra-high potency topical corticosteroid to standard dose etanercept for 12 weeks. Currently, 11 biologics are approved by the Food and Drug Administration for the treatment of psoriasis.
In addition, “while not FDA approved for psoriasis, the topical calcineurin inhibitors tacrolimus and pimecrolimus are often employed in the treatment of psoriasis,” can be helpful for “thinner skin such as facial and intertriginous areas,” and can be steroid sparing when used for more than 4 weeks, according to the guidelines.
Don’t discount the role of patient preferences when choosing topical treatments, the authors noted. “The optimal vehicle choice is the one the patient is mostly likely to use.”
The guidelines also address the evidence for effectiveness, and adverse events in the use of several alternative medicines for psoriasis including traditional Chinese medicine, and the herbal therapies aloe vera and St. John’s wort, as well as the potential role of dietary supplements including fish oil, vitamin D, turmeric, and zinc in managing psoriasis, and the potential role of a gluten-free diet.
In general, research on the efficacy, effectiveness, and potential adverse effects of these strategies are limited, according to the guidelines, although many patients express interest in supplements and herbal products. For example, “Many patients ask about the overall role of vitamin D in skin health. Rather than adding oral vitamin D supplementation, topical therapy with vitamin D agents is effective for the treatment of psoriasis,” the authors noted.
In addition, they noted that mind/body strategies, namely hypnosis and stress reduction or meditation techniques, have been shown to improve symptoms and can be helpful for some patients, but clinical evidence is limited.
The guidelines also addressed methods for assessing disease severity in psoriasis. They recommended using body surface area (BSA) to assess psoriasis severity and patient response to treatment in the clinical setting. However, BSA is a provider assessment tool that “does not take into account location on the body, clinical characteristics of the plaques, symptoms, or quality of life issues,” the authors noted. The Psoriasis Area and Severity Index (PASI) measures erythema, induration, and scaling and is more suited to assessing psoriasis severity and response to treatment in clinical trials rather than in practice, they said.
Prior AAD guidelines on psoriasis were published more than 10 years ago, and major developments including the availability of new biologic drugs and new data on comorbidities have been recognized in the past decade, working group cochair and author of the guidelines Alan Menter, MD, said in an interview.
The key game-changers from previous guidelines include the full section published on comorbidities plus the development of two new important cytokine classes: three IL-17 drugs and three new IL-23 drugs now available for moderate to severe psoriasis, said Dr. Menter, chairman of the division of dermatology at Baylor University Medical Center, Dallas.
Barriers to implementing the guidelines in practice may occur when “third party payers make the decision on which of the 11 biologic drugs now approved for moderate to severe psoriasis should be used,” he noted.
As for next steps in psoriasis studies, “new biomarker research is currently underway,” Dr. Menter said. With 11 biologic agents new formally approved by the FDA for moderate to severe psoriasis, the next steps are to determine which drug is likely to be the most appropriate for each individual patient.
Dr. Menter disclosed relationships with multiple companies that develop and manufacture psoriasis therapies, including Abbott Labs, AbbVie, Amgen, Eli Lilly and Company, Galderma USA, Janssen Pharmaceuticals, LEO Pharma US, Menlo Therapeutics, and Novartis. The updated guidelines were designed by a multidisciplinary work group of psoriasis experts including dermatologists, a rheumatologist, a cardiologist, and representatives from a patient advocacy organization.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2020 Jul 29. doi: 10.1016/j.jaad.2020.07.087.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Most younger MI patients wouldn’t get statins under guidelines
Clinical guidelines for cholesterol management may have two blind spots when it comes to heart attack prevention: Most younger adults with premature coronary artery disease who’ve had a myocardial infarction don’t meet guideline criteria for preventative statin therapy, and survivors under age 55 don’t meet the criteria for continuing nonstatin lipid-lowering treatments, a large single-center retrospective study has shown.
“The classic approach we’ve taken to identifying young adults for prevention is inadequate in younger adults,” corresponding author Ann Marie Navar, MD, PhD, of Duke University, Durham, N.C., said in an interview. “While awaiting more definitive research we should at minimum be using all the tools at our disposal, including broader use of coronary artery calcium [CAC] scoring, to identify young people who may benefit from statin therapy.”
The retrospective observational study analyzed records of 6,639 adults who had cardiac catheterization at Duke University Medical Center from 1995 to 2012 for a first myocardial infarction with obstructive coronary artery disease. The study considered those under age 55 years as “younger” patients, comprising 41% of the study group (2,733); 35% were “middle-aged” at 55-65 years (2,324) and 24% were “older,” at 66-75 years (1,582).
The report, published online Aug. 3 in the Journal of the American College of Cardiology, noted that most of the adults with premature CAD did not meet criteria for preventative statin therapy before their first MI based on ACC/American Heart Association clinical guidelines from 2013 and 2018. It also noted that younger MI survivors are also less frequently eligible for secondary prevention with intensive nonstatin lipid-lowering therapies than are older adults despite a much longer potential life span – and opportunity for another MI – for the former.
The researchers sought to evaluate the real-world implications of changes made in the 2018 guideline for adults who develop premature ischemic heart disease, and found that fewer younger patients qualify for preventative statin therapy under the 2018 guidelines.
“Younger individuals with very high-risk criteria are at higher risk of major adverse cardiovascular events, a finding supporting the appropriate implementation of intensive lipid-lowering therapies in these patients,” wrote lead author Michel Zeitouni, MD, MSc, and colleagues.
Key findings
The investigators reported that younger adults were significantly less likely to meet a class I recommendation for statins under the 2013 guideline (42.9%), compared with their middle-aged (70%) and older (82.5%) counterparts; and under the 2018 guideline, at 39.4%, 59.5%, and 77.4%, respectively (both P < .001).
Similarly, when both class I and class IIa recommendations were accounted for, younger patients were significantly less likely than were middle-aged and older patients to be eligible for statins before their index MI under both the 2013 (56.7%, 79.5%, and 85.2%, respectively and 2018 guidelines (46.4%, 73.5%, and 88.2%, respectively (both P < .01).
After their first MI, one in four younger patients (28.3%) met the very high-risk criteria compared with 40% of middle-aged and 81.4% of older patients (P trend < .001). In 8 years of follow-up, patients with very high-risk criteria based on the 2018 guideline had twice the rate of death, nonfatal MI, or stroke (hazard ratio [HR]: 2.15; 95% confidence interval, 1.98-2.33; P < .001).
The researchers acknowledged that the 2018 guideline took the important step of implementing risk enhancers – patient characteristics such as obesity and metabolic syndrome – along with the 10-year atherosclerotic cardiovascular disease (ASCVD) risk score to better identify high-risk young individuals who need statins. However, they also noted that the ability of the guidelines to identify young adults before their first MI “remains suboptimal.”
How to protect younger patients
“The 2018 guidelines will be most effective if we as providers do our best to identify risk enhancers and if we can use CAC scoring more broadly,” Dr. Navar said, noting that although CAC scoring has been shown to improve risk prediction, insurance coverage can be problematic.
“We also need to be careful to screen for the presence of the risk enhancers, such as inflammatory disease, family history, and women-specific risk factors, to make sure we aren’t missing an important high-risk group,” she added.
Other solutions to better identify at-risk younger adults include considering upgrades to the guidelines’ class IIb recommendation to class IIa to emphasize the importance of recognizing lower-risk younger adults, and recommending statins for patients at higher lifetime risk than age- and sex-matched peers, the researchers noted. “In our cohort, young individuals admitted for a first MI had a higher lifetime ASCVD risk score than did patients in the older age categories,” Dr. Zeitouni and colleagues wrote.
Dr. Navar said that these findings are a reminder that guidelines aren’t mandates. “Guidelines are meant to be a starting point for patients and physicians,” she said. “The absence of a recommendation doesn’t mean something isn’t recommended, but that there is not enough data to say one way or another.”
The study “provides important evidence” that the 2018 guidelines exempted about half of the younger adults who had a first MI from preventative statin therapy, Ron Blankstein, MD, and Avinainder Singh, MD, MMSc, noted in an editorial (J Am Coll Cardiol. 2020;76:665-8).
“Data from both the Duke and Young-MI registries should force us to reexamine how we allocate statin use among young individuals,” they noted. Dr. Blankstein is with Brigham and Women’s Hospital, Harvard Medical School, Boston. Dr. Singh is with Yale University, New Haven, Conn.
Dr. Zeitouni reported receiving lecture fees from Bristol-Myers Squibb/Pfizer. Dr. Navar reported financial relationships with Amarin, Janssen, Amgen, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Esperion, Novo Nordisk, Novartis, The Medicine Company, New Amsterdam, Cerner and Pfizer. Dr. Blankstein reported receiving research support from Amgen. Dr. Singh has no relevant financial relationships to report.
SOURCE: M. Zeitouni et al. J Am Coll Cardiol 2020 Aug 3;76:653-64.
Clinical guidelines for cholesterol management may have two blind spots when it comes to heart attack prevention: Most younger adults with premature coronary artery disease who’ve had a myocardial infarction don’t meet guideline criteria for preventative statin therapy, and survivors under age 55 don’t meet the criteria for continuing nonstatin lipid-lowering treatments, a large single-center retrospective study has shown.
“The classic approach we’ve taken to identifying young adults for prevention is inadequate in younger adults,” corresponding author Ann Marie Navar, MD, PhD, of Duke University, Durham, N.C., said in an interview. “While awaiting more definitive research we should at minimum be using all the tools at our disposal, including broader use of coronary artery calcium [CAC] scoring, to identify young people who may benefit from statin therapy.”
The retrospective observational study analyzed records of 6,639 adults who had cardiac catheterization at Duke University Medical Center from 1995 to 2012 for a first myocardial infarction with obstructive coronary artery disease. The study considered those under age 55 years as “younger” patients, comprising 41% of the study group (2,733); 35% were “middle-aged” at 55-65 years (2,324) and 24% were “older,” at 66-75 years (1,582).
The report, published online Aug. 3 in the Journal of the American College of Cardiology, noted that most of the adults with premature CAD did not meet criteria for preventative statin therapy before their first MI based on ACC/American Heart Association clinical guidelines from 2013 and 2018. It also noted that younger MI survivors are also less frequently eligible for secondary prevention with intensive nonstatin lipid-lowering therapies than are older adults despite a much longer potential life span – and opportunity for another MI – for the former.
The researchers sought to evaluate the real-world implications of changes made in the 2018 guideline for adults who develop premature ischemic heart disease, and found that fewer younger patients qualify for preventative statin therapy under the 2018 guidelines.
“Younger individuals with very high-risk criteria are at higher risk of major adverse cardiovascular events, a finding supporting the appropriate implementation of intensive lipid-lowering therapies in these patients,” wrote lead author Michel Zeitouni, MD, MSc, and colleagues.
Key findings
The investigators reported that younger adults were significantly less likely to meet a class I recommendation for statins under the 2013 guideline (42.9%), compared with their middle-aged (70%) and older (82.5%) counterparts; and under the 2018 guideline, at 39.4%, 59.5%, and 77.4%, respectively (both P < .001).
Similarly, when both class I and class IIa recommendations were accounted for, younger patients were significantly less likely than were middle-aged and older patients to be eligible for statins before their index MI under both the 2013 (56.7%, 79.5%, and 85.2%, respectively and 2018 guidelines (46.4%, 73.5%, and 88.2%, respectively (both P < .01).
After their first MI, one in four younger patients (28.3%) met the very high-risk criteria compared with 40% of middle-aged and 81.4% of older patients (P trend < .001). In 8 years of follow-up, patients with very high-risk criteria based on the 2018 guideline had twice the rate of death, nonfatal MI, or stroke (hazard ratio [HR]: 2.15; 95% confidence interval, 1.98-2.33; P < .001).
The researchers acknowledged that the 2018 guideline took the important step of implementing risk enhancers – patient characteristics such as obesity and metabolic syndrome – along with the 10-year atherosclerotic cardiovascular disease (ASCVD) risk score to better identify high-risk young individuals who need statins. However, they also noted that the ability of the guidelines to identify young adults before their first MI “remains suboptimal.”
How to protect younger patients
“The 2018 guidelines will be most effective if we as providers do our best to identify risk enhancers and if we can use CAC scoring more broadly,” Dr. Navar said, noting that although CAC scoring has been shown to improve risk prediction, insurance coverage can be problematic.
“We also need to be careful to screen for the presence of the risk enhancers, such as inflammatory disease, family history, and women-specific risk factors, to make sure we aren’t missing an important high-risk group,” she added.
Other solutions to better identify at-risk younger adults include considering upgrades to the guidelines’ class IIb recommendation to class IIa to emphasize the importance of recognizing lower-risk younger adults, and recommending statins for patients at higher lifetime risk than age- and sex-matched peers, the researchers noted. “In our cohort, young individuals admitted for a first MI had a higher lifetime ASCVD risk score than did patients in the older age categories,” Dr. Zeitouni and colleagues wrote.
Dr. Navar said that these findings are a reminder that guidelines aren’t mandates. “Guidelines are meant to be a starting point for patients and physicians,” she said. “The absence of a recommendation doesn’t mean something isn’t recommended, but that there is not enough data to say one way or another.”
The study “provides important evidence” that the 2018 guidelines exempted about half of the younger adults who had a first MI from preventative statin therapy, Ron Blankstein, MD, and Avinainder Singh, MD, MMSc, noted in an editorial (J Am Coll Cardiol. 2020;76:665-8).
“Data from both the Duke and Young-MI registries should force us to reexamine how we allocate statin use among young individuals,” they noted. Dr. Blankstein is with Brigham and Women’s Hospital, Harvard Medical School, Boston. Dr. Singh is with Yale University, New Haven, Conn.
Dr. Zeitouni reported receiving lecture fees from Bristol-Myers Squibb/Pfizer. Dr. Navar reported financial relationships with Amarin, Janssen, Amgen, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Esperion, Novo Nordisk, Novartis, The Medicine Company, New Amsterdam, Cerner and Pfizer. Dr. Blankstein reported receiving research support from Amgen. Dr. Singh has no relevant financial relationships to report.
SOURCE: M. Zeitouni et al. J Am Coll Cardiol 2020 Aug 3;76:653-64.
Clinical guidelines for cholesterol management may have two blind spots when it comes to heart attack prevention: Most younger adults with premature coronary artery disease who’ve had a myocardial infarction don’t meet guideline criteria for preventative statin therapy, and survivors under age 55 don’t meet the criteria for continuing nonstatin lipid-lowering treatments, a large single-center retrospective study has shown.
“The classic approach we’ve taken to identifying young adults for prevention is inadequate in younger adults,” corresponding author Ann Marie Navar, MD, PhD, of Duke University, Durham, N.C., said in an interview. “While awaiting more definitive research we should at minimum be using all the tools at our disposal, including broader use of coronary artery calcium [CAC] scoring, to identify young people who may benefit from statin therapy.”
The retrospective observational study analyzed records of 6,639 adults who had cardiac catheterization at Duke University Medical Center from 1995 to 2012 for a first myocardial infarction with obstructive coronary artery disease. The study considered those under age 55 years as “younger” patients, comprising 41% of the study group (2,733); 35% were “middle-aged” at 55-65 years (2,324) and 24% were “older,” at 66-75 years (1,582).
The report, published online Aug. 3 in the Journal of the American College of Cardiology, noted that most of the adults with premature CAD did not meet criteria for preventative statin therapy before their first MI based on ACC/American Heart Association clinical guidelines from 2013 and 2018. It also noted that younger MI survivors are also less frequently eligible for secondary prevention with intensive nonstatin lipid-lowering therapies than are older adults despite a much longer potential life span – and opportunity for another MI – for the former.
The researchers sought to evaluate the real-world implications of changes made in the 2018 guideline for adults who develop premature ischemic heart disease, and found that fewer younger patients qualify for preventative statin therapy under the 2018 guidelines.
“Younger individuals with very high-risk criteria are at higher risk of major adverse cardiovascular events, a finding supporting the appropriate implementation of intensive lipid-lowering therapies in these patients,” wrote lead author Michel Zeitouni, MD, MSc, and colleagues.
Key findings
The investigators reported that younger adults were significantly less likely to meet a class I recommendation for statins under the 2013 guideline (42.9%), compared with their middle-aged (70%) and older (82.5%) counterparts; and under the 2018 guideline, at 39.4%, 59.5%, and 77.4%, respectively (both P < .001).
Similarly, when both class I and class IIa recommendations were accounted for, younger patients were significantly less likely than were middle-aged and older patients to be eligible for statins before their index MI under both the 2013 (56.7%, 79.5%, and 85.2%, respectively and 2018 guidelines (46.4%, 73.5%, and 88.2%, respectively (both P < .01).
After their first MI, one in four younger patients (28.3%) met the very high-risk criteria compared with 40% of middle-aged and 81.4% of older patients (P trend < .001). In 8 years of follow-up, patients with very high-risk criteria based on the 2018 guideline had twice the rate of death, nonfatal MI, or stroke (hazard ratio [HR]: 2.15; 95% confidence interval, 1.98-2.33; P < .001).
The researchers acknowledged that the 2018 guideline took the important step of implementing risk enhancers – patient characteristics such as obesity and metabolic syndrome – along with the 10-year atherosclerotic cardiovascular disease (ASCVD) risk score to better identify high-risk young individuals who need statins. However, they also noted that the ability of the guidelines to identify young adults before their first MI “remains suboptimal.”
How to protect younger patients
“The 2018 guidelines will be most effective if we as providers do our best to identify risk enhancers and if we can use CAC scoring more broadly,” Dr. Navar said, noting that although CAC scoring has been shown to improve risk prediction, insurance coverage can be problematic.
“We also need to be careful to screen for the presence of the risk enhancers, such as inflammatory disease, family history, and women-specific risk factors, to make sure we aren’t missing an important high-risk group,” she added.
Other solutions to better identify at-risk younger adults include considering upgrades to the guidelines’ class IIb recommendation to class IIa to emphasize the importance of recognizing lower-risk younger adults, and recommending statins for patients at higher lifetime risk than age- and sex-matched peers, the researchers noted. “In our cohort, young individuals admitted for a first MI had a higher lifetime ASCVD risk score than did patients in the older age categories,” Dr. Zeitouni and colleagues wrote.
Dr. Navar said that these findings are a reminder that guidelines aren’t mandates. “Guidelines are meant to be a starting point for patients and physicians,” she said. “The absence of a recommendation doesn’t mean something isn’t recommended, but that there is not enough data to say one way or another.”
The study “provides important evidence” that the 2018 guidelines exempted about half of the younger adults who had a first MI from preventative statin therapy, Ron Blankstein, MD, and Avinainder Singh, MD, MMSc, noted in an editorial (J Am Coll Cardiol. 2020;76:665-8).
“Data from both the Duke and Young-MI registries should force us to reexamine how we allocate statin use among young individuals,” they noted. Dr. Blankstein is with Brigham and Women’s Hospital, Harvard Medical School, Boston. Dr. Singh is with Yale University, New Haven, Conn.
Dr. Zeitouni reported receiving lecture fees from Bristol-Myers Squibb/Pfizer. Dr. Navar reported financial relationships with Amarin, Janssen, Amgen, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Esperion, Novo Nordisk, Novartis, The Medicine Company, New Amsterdam, Cerner and Pfizer. Dr. Blankstein reported receiving research support from Amgen. Dr. Singh has no relevant financial relationships to report.
SOURCE: M. Zeitouni et al. J Am Coll Cardiol 2020 Aug 3;76:653-64.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
FDA okays new indication for esketamine nasal spray
The Food and Drug Administration has approved the supplemental new drug application for esketamine nasal spray (Spravato, Janssen Pharmaceuticals) to treat depressive symptoms in adults with major depressive disorder (MDD) and acute suicidal ideation or behavior.
The FDA approved esketamine nasal spray for treatment-resistant depression in March 2019, as reported by Medscape Medical News.
– which evaluated the efficacy and safety of the nasal spray in addition to a comprehensive standard of care in adults with MDD who had active suicidal ideation with intent.
The standard of care included initial hospitalization, a newly initiated or optimized oral antidepressant, and twice-weekly treatment visits for 4 weeks. During that time, patients received esketamine nasal spray 84 mg or placebo nasal spray.
Results from the trials showed that the active treatment significantly reduced depressive symptoms within 24 hours, with some patients starting to respond as early as 4 hours after the first dose.
“Traditional oral antidepressants need weeks or more to take effect, so the availability of a medicine that can begin providing relief within a day is potentially life changing,” Theresa Nguyen, chief program officer at Mental Health America, said in a company news release.
“The clinical trials supporting this new indication provide compelling evidence that esketamine may offer clinicians a new way to provide support to patients quickly in the midst of an urgent depressive episode and help set them on the path to remission,” Gerard Sanacora, MD, PhD, director of the Yale Depression Research Program, New Haven, Conn., and esketamine clinical trial investigator, said in the same release.
A full course of treatment for MDD with acute suicidal ideation or behavior is twice weekly for 4 weeks, “after which evidence of therapeutic benefit should be evaluated to determine need for continued treatment,” the company said.
Because of the risk for serious adverse events, including sedation and dissociation, and the potential for abuse or misuse, esketamine nasal spray is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS).
The patient self-administers esketamine nasal spray only in REMS-certified health care settings. Patients are not permitted to take the drug home.
Full prescribing information is available online.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved the supplemental new drug application for esketamine nasal spray (Spravato, Janssen Pharmaceuticals) to treat depressive symptoms in adults with major depressive disorder (MDD) and acute suicidal ideation or behavior.
The FDA approved esketamine nasal spray for treatment-resistant depression in March 2019, as reported by Medscape Medical News.
– which evaluated the efficacy and safety of the nasal spray in addition to a comprehensive standard of care in adults with MDD who had active suicidal ideation with intent.
The standard of care included initial hospitalization, a newly initiated or optimized oral antidepressant, and twice-weekly treatment visits for 4 weeks. During that time, patients received esketamine nasal spray 84 mg or placebo nasal spray.
Results from the trials showed that the active treatment significantly reduced depressive symptoms within 24 hours, with some patients starting to respond as early as 4 hours after the first dose.
“Traditional oral antidepressants need weeks or more to take effect, so the availability of a medicine that can begin providing relief within a day is potentially life changing,” Theresa Nguyen, chief program officer at Mental Health America, said in a company news release.
“The clinical trials supporting this new indication provide compelling evidence that esketamine may offer clinicians a new way to provide support to patients quickly in the midst of an urgent depressive episode and help set them on the path to remission,” Gerard Sanacora, MD, PhD, director of the Yale Depression Research Program, New Haven, Conn., and esketamine clinical trial investigator, said in the same release.
A full course of treatment for MDD with acute suicidal ideation or behavior is twice weekly for 4 weeks, “after which evidence of therapeutic benefit should be evaluated to determine need for continued treatment,” the company said.
Because of the risk for serious adverse events, including sedation and dissociation, and the potential for abuse or misuse, esketamine nasal spray is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS).
The patient self-administers esketamine nasal spray only in REMS-certified health care settings. Patients are not permitted to take the drug home.
Full prescribing information is available online.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved the supplemental new drug application for esketamine nasal spray (Spravato, Janssen Pharmaceuticals) to treat depressive symptoms in adults with major depressive disorder (MDD) and acute suicidal ideation or behavior.
The FDA approved esketamine nasal spray for treatment-resistant depression in March 2019, as reported by Medscape Medical News.
– which evaluated the efficacy and safety of the nasal spray in addition to a comprehensive standard of care in adults with MDD who had active suicidal ideation with intent.
The standard of care included initial hospitalization, a newly initiated or optimized oral antidepressant, and twice-weekly treatment visits for 4 weeks. During that time, patients received esketamine nasal spray 84 mg or placebo nasal spray.
Results from the trials showed that the active treatment significantly reduced depressive symptoms within 24 hours, with some patients starting to respond as early as 4 hours after the first dose.
“Traditional oral antidepressants need weeks or more to take effect, so the availability of a medicine that can begin providing relief within a day is potentially life changing,” Theresa Nguyen, chief program officer at Mental Health America, said in a company news release.
“The clinical trials supporting this new indication provide compelling evidence that esketamine may offer clinicians a new way to provide support to patients quickly in the midst of an urgent depressive episode and help set them on the path to remission,” Gerard Sanacora, MD, PhD, director of the Yale Depression Research Program, New Haven, Conn., and esketamine clinical trial investigator, said in the same release.
A full course of treatment for MDD with acute suicidal ideation or behavior is twice weekly for 4 weeks, “after which evidence of therapeutic benefit should be evaluated to determine need for continued treatment,” the company said.
Because of the risk for serious adverse events, including sedation and dissociation, and the potential for abuse or misuse, esketamine nasal spray is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS).
The patient self-administers esketamine nasal spray only in REMS-certified health care settings. Patients are not permitted to take the drug home.
Full prescribing information is available online.
A version of this article originally appeared on Medscape.com.
Real-world data show SGLT2 inhibitors for diabetes triple DKA risk
according to a new large database analysis.
The findings, which include data on the use of three different SGLT2 inhibitors in Canada and the United Kingdom and suggest a class effect, were published online July 27 in Annals of Internal Medicine by Antonios Douros, MD, PhD, of McGill University and the Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, and colleagues.
“Our results provide robust evidence that SGLT2 inhibitors are associated with an increased risk for DKA. Of note, increased risks were observed in all molecule-specific analyses, with canagliflozin [Invokana, Janssen] showing the highest effect estimate,” they noted.
And because the beneficial effects of SGLT2 inhibitors in the prevention of cardiovascular and renal disease will probably increase their uptake in the coming years, “Physicians should be aware of DKA as a potential adverse effect,” Dr. Douros and colleagues wrote.
Analysis “generally confirms what has already been published”
Asked for comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, said that the study “generally confirms what has already been published” on the topic. He noted that overall “the risk of SGLT2 inhibitor–induced ketoacidosis is quite low in type 2 diabetes, perhaps on the order of 1 episode per 1000 patient-years.”
However, Dr. Taylor cautioned: “Published evidence suggests that the risk of DKA is increased if patients are unable to eat,” such as when hospitalized patients are not permitted to eat.
“In that setting, it is probably prudent to discontinue an SGLT2 inhibitor. Also, it may be prudent not to prescribe SGLT2 inhibitors to patients with a history of DKA,” he added.
Dr. Taylor also advised: “Although not necessarily supported by this publication, I think that caution should be exercised in prescribing SGLT2 inhibitors to insulin-dependent type 2 diabetes patients. ... Some late-stage type 2 diabetes patients may have severe insulin deficiency, and their physiology may resemble that of a type 1 diabetes patient.”
Dr. Taylor has previously advised against using SGLT2 inhibitors altogether in patients with type 1 diabetes.
Increased DKA risk seen across all SGLT2 inhibitors
The study involved electronic health care databases from seven Canadian provinces and the United Kingdom, from which 208,757 new users of SGLT2 inhibitors were propensity-matched 1:1 to new dipeptidyl peptidase-4 (DPP-4) inhibitor users.
Of those taking an SGLT2 inhibitor, 42.3% took canagliflozin, 30.7% dapagliflozin (Farxiga/Forxiga, AstraZeneca), and 27.0% empagliflozin (Jardiance, Boehringer Ingelheim).
Over a mean 0.9-year follow-up, 521 patients were hospitalized with DKA, for an overall incidence rate of 1.41 per 1,000 person-years.
The rate with SGLT2 inhibitors, 2.03 per 1,000 person-years, was nearly three times that seen with DPP-4 inhibitors, at 0.75 per 1,000 person-years, a significant difference (hazard ratio, 2.85).
By individual SGLT2 inhibitor, the hazard ratios compared with DPP-4 inhibitors were 1.86 for dapagliflozin, 2.52 for empagliflozin, and 3.58 for canagliflozin, all statistically significant. Stratification by age, sex, and incident versus prevalent user did not change the association between SGLT2 inhibitors and DKA.
Asked about the higher rate for canagliflozin, Dr. Taylor commented: “It is hard to know whether there are real and reproducible differences in the risks of DKA among the various SGLT2 inhibitors. The differences are not huge and the populations are not well matched.”
But, he noted, “If canagliflozin triggers more glucosuria, it is not surprising that it would also induce more ketosis and possibly ketoacidosis.”
He also noted that the threefold relative increase in DKA with canagliflozin versus comparators is consistent with Janssen’s data, published in 2015.
“It is, of course, reassuring that both [randomized clinical trials] and epidemiology produce similar estimates of the risk of drug-induced adverse events. Interestingly, the incidence of DKA is approximately threefold higher in the Canadian [data] as compared to Janssen’s clinical trials.”
Dr. Taylor also pointed out that, in the Janssen studies, the risk of canagliflozin-induced DKA appeared to be higher among patients with anti-islet antibodies, which suggests that some may have actually had autoimmune (type 1) diabetes. “So the overall risk of SGLT2 inhibitor-induced DKA may depend at least in part on the mix of patients.”
In the current study, individuals who never used insulin had a greater relative increase in risk of DKA with SGLT2 inhibitors, compared with DPP-4 inhibitors, than did those who did use insulin (hazard ratios, 3.96 vs. 2.24, both compared with DPP-4 inhibitors). However, just among those taking SGLT2 inhibitors, the absolute risk for DKA was higher for those with prior insulin use (3.52 vs. 1.43 per 1,000 person-years).
The results of sensitivity analyses were consistent with those of the primary analysis.
The study was funded by the Canadian Institutes of Health Research and supported by ICES. Dr. Douros has reported receiving a salary support award from Fonds de recherche du Quebec – sante. Dr. Taylor was previously employed at Bristol-Myers Squibb. He is currently a consultant for Ionis Pharmaceuticals and has reported receiving research support provided to the University of Maryland School of Medicine by Regeneron.
A version of this article originally appeared on Medscape.com.
according to a new large database analysis.
The findings, which include data on the use of three different SGLT2 inhibitors in Canada and the United Kingdom and suggest a class effect, were published online July 27 in Annals of Internal Medicine by Antonios Douros, MD, PhD, of McGill University and the Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, and colleagues.
“Our results provide robust evidence that SGLT2 inhibitors are associated with an increased risk for DKA. Of note, increased risks were observed in all molecule-specific analyses, with canagliflozin [Invokana, Janssen] showing the highest effect estimate,” they noted.
And because the beneficial effects of SGLT2 inhibitors in the prevention of cardiovascular and renal disease will probably increase their uptake in the coming years, “Physicians should be aware of DKA as a potential adverse effect,” Dr. Douros and colleagues wrote.
Analysis “generally confirms what has already been published”
Asked for comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, said that the study “generally confirms what has already been published” on the topic. He noted that overall “the risk of SGLT2 inhibitor–induced ketoacidosis is quite low in type 2 diabetes, perhaps on the order of 1 episode per 1000 patient-years.”
However, Dr. Taylor cautioned: “Published evidence suggests that the risk of DKA is increased if patients are unable to eat,” such as when hospitalized patients are not permitted to eat.
“In that setting, it is probably prudent to discontinue an SGLT2 inhibitor. Also, it may be prudent not to prescribe SGLT2 inhibitors to patients with a history of DKA,” he added.
Dr. Taylor also advised: “Although not necessarily supported by this publication, I think that caution should be exercised in prescribing SGLT2 inhibitors to insulin-dependent type 2 diabetes patients. ... Some late-stage type 2 diabetes patients may have severe insulin deficiency, and their physiology may resemble that of a type 1 diabetes patient.”
Dr. Taylor has previously advised against using SGLT2 inhibitors altogether in patients with type 1 diabetes.
Increased DKA risk seen across all SGLT2 inhibitors
The study involved electronic health care databases from seven Canadian provinces and the United Kingdom, from which 208,757 new users of SGLT2 inhibitors were propensity-matched 1:1 to new dipeptidyl peptidase-4 (DPP-4) inhibitor users.
Of those taking an SGLT2 inhibitor, 42.3% took canagliflozin, 30.7% dapagliflozin (Farxiga/Forxiga, AstraZeneca), and 27.0% empagliflozin (Jardiance, Boehringer Ingelheim).
Over a mean 0.9-year follow-up, 521 patients were hospitalized with DKA, for an overall incidence rate of 1.41 per 1,000 person-years.
The rate with SGLT2 inhibitors, 2.03 per 1,000 person-years, was nearly three times that seen with DPP-4 inhibitors, at 0.75 per 1,000 person-years, a significant difference (hazard ratio, 2.85).
By individual SGLT2 inhibitor, the hazard ratios compared with DPP-4 inhibitors were 1.86 for dapagliflozin, 2.52 for empagliflozin, and 3.58 for canagliflozin, all statistically significant. Stratification by age, sex, and incident versus prevalent user did not change the association between SGLT2 inhibitors and DKA.
Asked about the higher rate for canagliflozin, Dr. Taylor commented: “It is hard to know whether there are real and reproducible differences in the risks of DKA among the various SGLT2 inhibitors. The differences are not huge and the populations are not well matched.”
But, he noted, “If canagliflozin triggers more glucosuria, it is not surprising that it would also induce more ketosis and possibly ketoacidosis.”
He also noted that the threefold relative increase in DKA with canagliflozin versus comparators is consistent with Janssen’s data, published in 2015.
“It is, of course, reassuring that both [randomized clinical trials] and epidemiology produce similar estimates of the risk of drug-induced adverse events. Interestingly, the incidence of DKA is approximately threefold higher in the Canadian [data] as compared to Janssen’s clinical trials.”
Dr. Taylor also pointed out that, in the Janssen studies, the risk of canagliflozin-induced DKA appeared to be higher among patients with anti-islet antibodies, which suggests that some may have actually had autoimmune (type 1) diabetes. “So the overall risk of SGLT2 inhibitor-induced DKA may depend at least in part on the mix of patients.”
In the current study, individuals who never used insulin had a greater relative increase in risk of DKA with SGLT2 inhibitors, compared with DPP-4 inhibitors, than did those who did use insulin (hazard ratios, 3.96 vs. 2.24, both compared with DPP-4 inhibitors). However, just among those taking SGLT2 inhibitors, the absolute risk for DKA was higher for those with prior insulin use (3.52 vs. 1.43 per 1,000 person-years).
The results of sensitivity analyses were consistent with those of the primary analysis.
The study was funded by the Canadian Institutes of Health Research and supported by ICES. Dr. Douros has reported receiving a salary support award from Fonds de recherche du Quebec – sante. Dr. Taylor was previously employed at Bristol-Myers Squibb. He is currently a consultant for Ionis Pharmaceuticals and has reported receiving research support provided to the University of Maryland School of Medicine by Regeneron.
A version of this article originally appeared on Medscape.com.
according to a new large database analysis.
The findings, which include data on the use of three different SGLT2 inhibitors in Canada and the United Kingdom and suggest a class effect, were published online July 27 in Annals of Internal Medicine by Antonios Douros, MD, PhD, of McGill University and the Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, and colleagues.
“Our results provide robust evidence that SGLT2 inhibitors are associated with an increased risk for DKA. Of note, increased risks were observed in all molecule-specific analyses, with canagliflozin [Invokana, Janssen] showing the highest effect estimate,” they noted.
And because the beneficial effects of SGLT2 inhibitors in the prevention of cardiovascular and renal disease will probably increase their uptake in the coming years, “Physicians should be aware of DKA as a potential adverse effect,” Dr. Douros and colleagues wrote.
Analysis “generally confirms what has already been published”
Asked for comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, said that the study “generally confirms what has already been published” on the topic. He noted that overall “the risk of SGLT2 inhibitor–induced ketoacidosis is quite low in type 2 diabetes, perhaps on the order of 1 episode per 1000 patient-years.”
However, Dr. Taylor cautioned: “Published evidence suggests that the risk of DKA is increased if patients are unable to eat,” such as when hospitalized patients are not permitted to eat.
“In that setting, it is probably prudent to discontinue an SGLT2 inhibitor. Also, it may be prudent not to prescribe SGLT2 inhibitors to patients with a history of DKA,” he added.
Dr. Taylor also advised: “Although not necessarily supported by this publication, I think that caution should be exercised in prescribing SGLT2 inhibitors to insulin-dependent type 2 diabetes patients. ... Some late-stage type 2 diabetes patients may have severe insulin deficiency, and their physiology may resemble that of a type 1 diabetes patient.”
Dr. Taylor has previously advised against using SGLT2 inhibitors altogether in patients with type 1 diabetes.
Increased DKA risk seen across all SGLT2 inhibitors
The study involved electronic health care databases from seven Canadian provinces and the United Kingdom, from which 208,757 new users of SGLT2 inhibitors were propensity-matched 1:1 to new dipeptidyl peptidase-4 (DPP-4) inhibitor users.
Of those taking an SGLT2 inhibitor, 42.3% took canagliflozin, 30.7% dapagliflozin (Farxiga/Forxiga, AstraZeneca), and 27.0% empagliflozin (Jardiance, Boehringer Ingelheim).
Over a mean 0.9-year follow-up, 521 patients were hospitalized with DKA, for an overall incidence rate of 1.41 per 1,000 person-years.
The rate with SGLT2 inhibitors, 2.03 per 1,000 person-years, was nearly three times that seen with DPP-4 inhibitors, at 0.75 per 1,000 person-years, a significant difference (hazard ratio, 2.85).
By individual SGLT2 inhibitor, the hazard ratios compared with DPP-4 inhibitors were 1.86 for dapagliflozin, 2.52 for empagliflozin, and 3.58 for canagliflozin, all statistically significant. Stratification by age, sex, and incident versus prevalent user did not change the association between SGLT2 inhibitors and DKA.
Asked about the higher rate for canagliflozin, Dr. Taylor commented: “It is hard to know whether there are real and reproducible differences in the risks of DKA among the various SGLT2 inhibitors. The differences are not huge and the populations are not well matched.”
But, he noted, “If canagliflozin triggers more glucosuria, it is not surprising that it would also induce more ketosis and possibly ketoacidosis.”
He also noted that the threefold relative increase in DKA with canagliflozin versus comparators is consistent with Janssen’s data, published in 2015.
“It is, of course, reassuring that both [randomized clinical trials] and epidemiology produce similar estimates of the risk of drug-induced adverse events. Interestingly, the incidence of DKA is approximately threefold higher in the Canadian [data] as compared to Janssen’s clinical trials.”
Dr. Taylor also pointed out that, in the Janssen studies, the risk of canagliflozin-induced DKA appeared to be higher among patients with anti-islet antibodies, which suggests that some may have actually had autoimmune (type 1) diabetes. “So the overall risk of SGLT2 inhibitor-induced DKA may depend at least in part on the mix of patients.”
In the current study, individuals who never used insulin had a greater relative increase in risk of DKA with SGLT2 inhibitors, compared with DPP-4 inhibitors, than did those who did use insulin (hazard ratios, 3.96 vs. 2.24, both compared with DPP-4 inhibitors). However, just among those taking SGLT2 inhibitors, the absolute risk for DKA was higher for those with prior insulin use (3.52 vs. 1.43 per 1,000 person-years).
The results of sensitivity analyses were consistent with those of the primary analysis.
The study was funded by the Canadian Institutes of Health Research and supported by ICES. Dr. Douros has reported receiving a salary support award from Fonds de recherche du Quebec – sante. Dr. Taylor was previously employed at Bristol-Myers Squibb. He is currently a consultant for Ionis Pharmaceuticals and has reported receiving research support provided to the University of Maryland School of Medicine by Regeneron.
A version of this article originally appeared on Medscape.com.