User login
Aspirin linked to reduced bladder, breast cancer mortality
However, the treatment – particularly with frequency of at least three times a week – is associated with reductions in mortality in bladder cancer and breast cancer, new observational research shows.
“The results presented here add to the accumulating evidence that aspirin may improve survival for some cancers,” the authors write in their cohort study that uses data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. The new research was published online Jan. 15 in JAMA Network Open.
“Although prior research has been most heavily concentrated in gastrointestinal cancers, our analysis extends the advantages associated with aspirin use to other cancers, such as bladder and breast cancers,” they explained.
In commenting on the study, John J. McNeil, MBBS, PhD, head of the department of epidemiology and preventive medicine at Monash University in Melbourne, said the findings, though intriguing, are not necessarily conclusive.
“The data was derived from a very large and well-conducted study,” Dr. McNeil, who has led other research on aspirin use and the elderly, said in an interview.
“But these conclusions were drawn from the observational component of the study and therefore potentially confounded by other characteristics that differentiate aspirin users from nonusers.”
Aspirin/cancer research in older people lacking
With well-known reports of decreased risks of heart disease, stroke, cancer – particularly gastrointestinal cancers – and all-cause mortality, as many as 25%-50% of adults in the U.S. report taking aspirin daily or every other day.
However, evidence of the benefits relating to cancer, specifically in older people, has been inconsistent, with one recent notable study, the randomized, double-blind ASPREE trial, showing no effect of aspirin on cancer incidence, but a higher mortality rate in elderly patients randomly assigned to aspirin for primary prevention.
To further investigate the effects in older patients, first author Holli A. Loomans-Kropp, PhD, and colleagues with the National Cancer Institute evaluated data on patients who were either 65 years or older at baseline or who had reached aged 65 during follow-up in the PLCO Cancer Screening Trial, which had enrollment from 1993 to 2001.
The authors identified 139,896 individuals with a mean age at baseline of 66.4 years; about half were women and 88.5% were non-Hispanic White.
Follow-up took place until the time of death, December 2014 for those who consented to follow-up, or December 2009 for those who refused consent to follow-up. The authors reported that there were 32,580 incident cancers, including 5.4% bladder, 14% breast, 1% esophageal, 1.2% gastric, 2.7% pancreatic, and 2.2% uterine cancers.
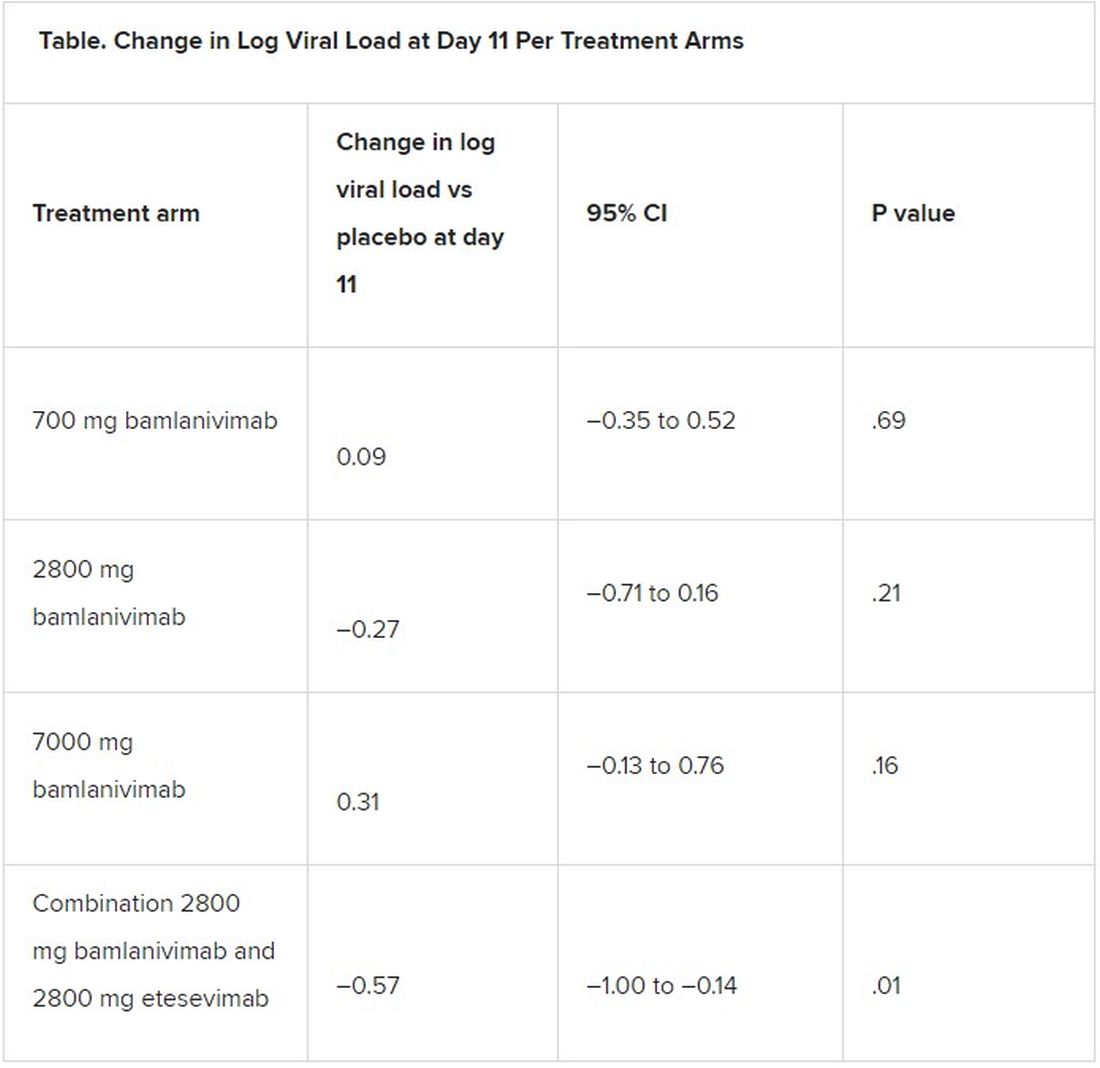
The study showed no association between aspirin use and the incidence of any of the cancer types included in the study among those over age 65.
However, further multivariate analysis of survival showed that, with follow-up adjusted to until the time of death, Dec. 31, 2015, or earlier refusal to consent, the use of aspirin at least three times per week was associated with reduced mortality in those with bladder (hazard ratio, 0.67) and breast (HR, 0.75) cancers, whereas no significant associations were observed with esophageal, gastric, pancreatic, or uterine cancer.
A similar association of any aspirin use (less than three times per week) with bladder (HR, 0.75) and breast (HR, 0.79) cancer survival was observed, the authors noted.
“These results may indicate that, for some cancer types, any aspirin use may be advantageous; however, greater benefit may be observed with increased frequency of use,” the authors wrote.
Mechanism speculation focuses on COX-2 pathway
Theories of the mechanisms behind a potential benefit of aspirin for those with bladder cancer include that urothelial cancer has increased RNA and protein expression of cyclooxygenase-2 (COX-2) and urinary prostaglandin E2, “suggesting up-regulation of the COX-2 pathway during cancer progression,” the authors wrote.
In breast cancer, a similar elevated expression of COX-2 has been shown to predict disease outcomes, including progression and decreased survival.
“This may be partly due to the mechanistic interplay between angiogenesis, cell proliferation, apoptosis, and inflammatory processes,” the authors noted.
The study isn’t the first to show a benefit specifically with bladder cancer; other studies include recent research (J Urol. 2018 Nov;200[5]:1014-21) showing that daily aspirin use among patients with bladder cancer was associated with increased 5-year survival following radical cystectomy, the authors noted.
Dr. McNeil noted that the new findings from the U.S. researchers, particularly regarding bladder cancer, are of interest. “The reduction in mortality from breast cancer is modest, but the reduction in mortality from bladder cancer was more impressive,” he said.
“However, given the fact that this finding is observational data and was a sole finding among multiple comparisons, it must be seen as suggestive rather than proven.”
Regarding possible mechanisms, Dr. McNeil added that, like the bulk of the prior research, many questions remain.
“There have been many suggestions about ways that aspirin might work at a molecular and cellular level, but no firm consensus has been reached.”
The study authors and Dr. McNeil disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, the treatment – particularly with frequency of at least three times a week – is associated with reductions in mortality in bladder cancer and breast cancer, new observational research shows.
“The results presented here add to the accumulating evidence that aspirin may improve survival for some cancers,” the authors write in their cohort study that uses data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. The new research was published online Jan. 15 in JAMA Network Open.
“Although prior research has been most heavily concentrated in gastrointestinal cancers, our analysis extends the advantages associated with aspirin use to other cancers, such as bladder and breast cancers,” they explained.
In commenting on the study, John J. McNeil, MBBS, PhD, head of the department of epidemiology and preventive medicine at Monash University in Melbourne, said the findings, though intriguing, are not necessarily conclusive.
“The data was derived from a very large and well-conducted study,” Dr. McNeil, who has led other research on aspirin use and the elderly, said in an interview.
“But these conclusions were drawn from the observational component of the study and therefore potentially confounded by other characteristics that differentiate aspirin users from nonusers.”
Aspirin/cancer research in older people lacking
With well-known reports of decreased risks of heart disease, stroke, cancer – particularly gastrointestinal cancers – and all-cause mortality, as many as 25%-50% of adults in the U.S. report taking aspirin daily or every other day.
However, evidence of the benefits relating to cancer, specifically in older people, has been inconsistent, with one recent notable study, the randomized, double-blind ASPREE trial, showing no effect of aspirin on cancer incidence, but a higher mortality rate in elderly patients randomly assigned to aspirin for primary prevention.
To further investigate the effects in older patients, first author Holli A. Loomans-Kropp, PhD, and colleagues with the National Cancer Institute evaluated data on patients who were either 65 years or older at baseline or who had reached aged 65 during follow-up in the PLCO Cancer Screening Trial, which had enrollment from 1993 to 2001.
The authors identified 139,896 individuals with a mean age at baseline of 66.4 years; about half were women and 88.5% were non-Hispanic White.
Follow-up took place until the time of death, December 2014 for those who consented to follow-up, or December 2009 for those who refused consent to follow-up. The authors reported that there were 32,580 incident cancers, including 5.4% bladder, 14% breast, 1% esophageal, 1.2% gastric, 2.7% pancreatic, and 2.2% uterine cancers.
The study showed no association between aspirin use and the incidence of any of the cancer types included in the study among those over age 65.
However, further multivariate analysis of survival showed that, with follow-up adjusted to until the time of death, Dec. 31, 2015, or earlier refusal to consent, the use of aspirin at least three times per week was associated with reduced mortality in those with bladder (hazard ratio, 0.67) and breast (HR, 0.75) cancers, whereas no significant associations were observed with esophageal, gastric, pancreatic, or uterine cancer.
A similar association of any aspirin use (less than three times per week) with bladder (HR, 0.75) and breast (HR, 0.79) cancer survival was observed, the authors noted.
“These results may indicate that, for some cancer types, any aspirin use may be advantageous; however, greater benefit may be observed with increased frequency of use,” the authors wrote.
Mechanism speculation focuses on COX-2 pathway
Theories of the mechanisms behind a potential benefit of aspirin for those with bladder cancer include that urothelial cancer has increased RNA and protein expression of cyclooxygenase-2 (COX-2) and urinary prostaglandin E2, “suggesting up-regulation of the COX-2 pathway during cancer progression,” the authors wrote.
In breast cancer, a similar elevated expression of COX-2 has been shown to predict disease outcomes, including progression and decreased survival.
“This may be partly due to the mechanistic interplay between angiogenesis, cell proliferation, apoptosis, and inflammatory processes,” the authors noted.
The study isn’t the first to show a benefit specifically with bladder cancer; other studies include recent research (J Urol. 2018 Nov;200[5]:1014-21) showing that daily aspirin use among patients with bladder cancer was associated with increased 5-year survival following radical cystectomy, the authors noted.
Dr. McNeil noted that the new findings from the U.S. researchers, particularly regarding bladder cancer, are of interest. “The reduction in mortality from breast cancer is modest, but the reduction in mortality from bladder cancer was more impressive,” he said.
“However, given the fact that this finding is observational data and was a sole finding among multiple comparisons, it must be seen as suggestive rather than proven.”
Regarding possible mechanisms, Dr. McNeil added that, like the bulk of the prior research, many questions remain.
“There have been many suggestions about ways that aspirin might work at a molecular and cellular level, but no firm consensus has been reached.”
The study authors and Dr. McNeil disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, the treatment – particularly with frequency of at least three times a week – is associated with reductions in mortality in bladder cancer and breast cancer, new observational research shows.
“The results presented here add to the accumulating evidence that aspirin may improve survival for some cancers,” the authors write in their cohort study that uses data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. The new research was published online Jan. 15 in JAMA Network Open.
“Although prior research has been most heavily concentrated in gastrointestinal cancers, our analysis extends the advantages associated with aspirin use to other cancers, such as bladder and breast cancers,” they explained.
In commenting on the study, John J. McNeil, MBBS, PhD, head of the department of epidemiology and preventive medicine at Monash University in Melbourne, said the findings, though intriguing, are not necessarily conclusive.
“The data was derived from a very large and well-conducted study,” Dr. McNeil, who has led other research on aspirin use and the elderly, said in an interview.
“But these conclusions were drawn from the observational component of the study and therefore potentially confounded by other characteristics that differentiate aspirin users from nonusers.”
Aspirin/cancer research in older people lacking
With well-known reports of decreased risks of heart disease, stroke, cancer – particularly gastrointestinal cancers – and all-cause mortality, as many as 25%-50% of adults in the U.S. report taking aspirin daily or every other day.
However, evidence of the benefits relating to cancer, specifically in older people, has been inconsistent, with one recent notable study, the randomized, double-blind ASPREE trial, showing no effect of aspirin on cancer incidence, but a higher mortality rate in elderly patients randomly assigned to aspirin for primary prevention.
To further investigate the effects in older patients, first author Holli A. Loomans-Kropp, PhD, and colleagues with the National Cancer Institute evaluated data on patients who were either 65 years or older at baseline or who had reached aged 65 during follow-up in the PLCO Cancer Screening Trial, which had enrollment from 1993 to 2001.
The authors identified 139,896 individuals with a mean age at baseline of 66.4 years; about half were women and 88.5% were non-Hispanic White.
Follow-up took place until the time of death, December 2014 for those who consented to follow-up, or December 2009 for those who refused consent to follow-up. The authors reported that there were 32,580 incident cancers, including 5.4% bladder, 14% breast, 1% esophageal, 1.2% gastric, 2.7% pancreatic, and 2.2% uterine cancers.
The study showed no association between aspirin use and the incidence of any of the cancer types included in the study among those over age 65.
However, further multivariate analysis of survival showed that, with follow-up adjusted to until the time of death, Dec. 31, 2015, or earlier refusal to consent, the use of aspirin at least three times per week was associated with reduced mortality in those with bladder (hazard ratio, 0.67) and breast (HR, 0.75) cancers, whereas no significant associations were observed with esophageal, gastric, pancreatic, or uterine cancer.
A similar association of any aspirin use (less than three times per week) with bladder (HR, 0.75) and breast (HR, 0.79) cancer survival was observed, the authors noted.
“These results may indicate that, for some cancer types, any aspirin use may be advantageous; however, greater benefit may be observed with increased frequency of use,” the authors wrote.
Mechanism speculation focuses on COX-2 pathway
Theories of the mechanisms behind a potential benefit of aspirin for those with bladder cancer include that urothelial cancer has increased RNA and protein expression of cyclooxygenase-2 (COX-2) and urinary prostaglandin E2, “suggesting up-regulation of the COX-2 pathway during cancer progression,” the authors wrote.
In breast cancer, a similar elevated expression of COX-2 has been shown to predict disease outcomes, including progression and decreased survival.
“This may be partly due to the mechanistic interplay between angiogenesis, cell proliferation, apoptosis, and inflammatory processes,” the authors noted.
The study isn’t the first to show a benefit specifically with bladder cancer; other studies include recent research (J Urol. 2018 Nov;200[5]:1014-21) showing that daily aspirin use among patients with bladder cancer was associated with increased 5-year survival following radical cystectomy, the authors noted.
Dr. McNeil noted that the new findings from the U.S. researchers, particularly regarding bladder cancer, are of interest. “The reduction in mortality from breast cancer is modest, but the reduction in mortality from bladder cancer was more impressive,” he said.
“However, given the fact that this finding is observational data and was a sole finding among multiple comparisons, it must be seen as suggestive rather than proven.”
Regarding possible mechanisms, Dr. McNeil added that, like the bulk of the prior research, many questions remain.
“There have been many suggestions about ways that aspirin might work at a molecular and cellular level, but no firm consensus has been reached.”
The study authors and Dr. McNeil disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The jury’s still out on trifluridine/tipiracil plus bevacizumab in mCRC
The median progression-free survival (PFS) in the phase 2 trial showed a difference of 1.41 months favoring TT-B over C-B, but this difference was not statistically significant.
The median overall survival was 4.64 months longer with TT-B than with C-B. However, the final analysis of TASCO1 was not designed to be comparative for overall survival, “so no formal statistical analysis is presented, and survival is a secondary endpoint,” noted investigator Eric Van Cutsem, MD, PhD, of University Hospital Gasthuisberg in Leuven, Belgium.
Dr. Van Cutsem presented the final results of TASCO1 at the 2021 Gastrointestinal Cancers Symposium (abstract 14).
Prior results from the trial were reported last year (Ann Oncol. 2020 Sep;31[9]:1160-68).
About trifluridine/tipiracil
Trifluridine/tipiracil is an oral drug combining the thymidine analogue trifluridine with tipiracil, an inhibitor of trifluridine degradation. The drug was approved by the Food and Drug Administration in 2015 under the trade name Lonsurf for the treatment of refractory metastatic colorectal cancer, and in 2019 for patients with metastatic gastric cancer or gastroesophageal junction cancer that had been treated with at least two lines of chemotherapy.
Trifluridine/tipiracil was associated with a brief but statistically significant survival benefit when compared with placebo in patients with heavily pretreated metastatic gastric cancer in the TAS-102 Gastric Study (Lancet Oncol. 2018 Nov;19[11]:1437-48).
In a separate analysis of the study, trifluridine/tipiracil was associated with significantly better overall survival and PFS than placebo in patients who had undergone gastrectomy (JAMA Oncol. 2019 Oct 10;6[1]:e193531).
TASCO1 details
In TASCO1, investigators enrolled patients with colorectal cancer who had never received systemic therapy for unresectable metastatic disease, and who were judged to be ineligible for intensive therapy due to advanced age, low tumor burden, poor performance status, comorbidities, or other clinical reasons.
After stratification by RAS status, performance status, and region, patients were randomly assigned to receive TT-B (n = 77) or C-B (n = 76).
TT-B consisted of oral trifluridine/tipiracil at 35 mg/m2 twice daily on days 1-5 and 8-12 every 4 weeks plus bevacizumab at 5 mg/kg intravenously on days 1 and 15 every 4 weeks.
C-B consisted of oral capecitabine at 1,250 or 1,000 mg/m2 twice a day on days 1-14 every 3 weeks plus bevacizumab at 7.5 mg/kg IV on day 1 every 3 weeks.
Final results
The median PFS, the primary endpoint, was 9.23 months with TT-B and 7.82 months with C-B. The difference was not statistically significant, with the upper limit of the 95% confidence interval crossing 1.
The median overall survival was 22.31 months with TT-B and 17.67 months with C-B (hazard ratio, 0.78; 95% CI, 0.55-1.10).
Dr. Van Cutsem pointed out that more patients in the TT-B arm had subsequent therapies compared with patients in the C-B arm – 59.7% vs. 40.8%.
He also noted that the safety profile of TT-B “remains unchanged from the initial analysis.”
Grade 3 or greater neutropenia, decreased neutrophil count, anemia, and decreased white blood cell count were all higher among patients on TT-B, but grade 3 or greater febrile neutropenia was similar between the groups.
Patients in the TT-B arm had more frequent grade 3 or greater nausea, vomiting, and hypertension. Grade 3 or higher hand-foot syndrome and diarrhea were both more common with C-B.
At the study cutoff date in September 2020, 66 patients in each arm had died.
Dr. Van Cutsem said more data on the efficacy of TT-B vs. C-B will come from the ongoing phase 3 SOLSTICE trial. Results from this trial are expected in late 2022.
‘The jury is still out’
The final results from TASCO1 suggest there may be some benefit from TT-B in patients with treatment-naive metastatic colorectal cancer, “but we don’t use it in the first line,” said Jeffery Clark, MD, an oncologist who was not involved in the study.
The trial supports the benefit of combining trifluridine/tipiracil with bevacizumab, and the results were “somewhat better” than he had expected, said Dr. Clark, director of clinical trials support at Mass General Cancer Center in Boston.
“Even though the results are encouraging, there were a couple of things about the trial that one has to at least think about,” Dr. Clark said in an interview.
He noted, for example, that a higher proportion of patients assigned to TT-B had prior adjuvant therapy (27.3% vs. 19.7%), and patients in the TT-B arm were also more likely to have second lines of systemic therapy, which could have skewed the results in favor of the experimental arm.
“I think, basically, the jury is still out until we see the results of the SOLSTICE trial,” he said.
The TASCO1 study was funded by Servier and Taiho. Dr. Van Cutsem has received research funding and served on an advisory board for Servier and other companies. Dr. Clark reported no relevant disclosures.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The median progression-free survival (PFS) in the phase 2 trial showed a difference of 1.41 months favoring TT-B over C-B, but this difference was not statistically significant.
The median overall survival was 4.64 months longer with TT-B than with C-B. However, the final analysis of TASCO1 was not designed to be comparative for overall survival, “so no formal statistical analysis is presented, and survival is a secondary endpoint,” noted investigator Eric Van Cutsem, MD, PhD, of University Hospital Gasthuisberg in Leuven, Belgium.
Dr. Van Cutsem presented the final results of TASCO1 at the 2021 Gastrointestinal Cancers Symposium (abstract 14).
Prior results from the trial were reported last year (Ann Oncol. 2020 Sep;31[9]:1160-68).
About trifluridine/tipiracil
Trifluridine/tipiracil is an oral drug combining the thymidine analogue trifluridine with tipiracil, an inhibitor of trifluridine degradation. The drug was approved by the Food and Drug Administration in 2015 under the trade name Lonsurf for the treatment of refractory metastatic colorectal cancer, and in 2019 for patients with metastatic gastric cancer or gastroesophageal junction cancer that had been treated with at least two lines of chemotherapy.
Trifluridine/tipiracil was associated with a brief but statistically significant survival benefit when compared with placebo in patients with heavily pretreated metastatic gastric cancer in the TAS-102 Gastric Study (Lancet Oncol. 2018 Nov;19[11]:1437-48).
In a separate analysis of the study, trifluridine/tipiracil was associated with significantly better overall survival and PFS than placebo in patients who had undergone gastrectomy (JAMA Oncol. 2019 Oct 10;6[1]:e193531).
TASCO1 details
In TASCO1, investigators enrolled patients with colorectal cancer who had never received systemic therapy for unresectable metastatic disease, and who were judged to be ineligible for intensive therapy due to advanced age, low tumor burden, poor performance status, comorbidities, or other clinical reasons.
After stratification by RAS status, performance status, and region, patients were randomly assigned to receive TT-B (n = 77) or C-B (n = 76).
TT-B consisted of oral trifluridine/tipiracil at 35 mg/m2 twice daily on days 1-5 and 8-12 every 4 weeks plus bevacizumab at 5 mg/kg intravenously on days 1 and 15 every 4 weeks.
C-B consisted of oral capecitabine at 1,250 or 1,000 mg/m2 twice a day on days 1-14 every 3 weeks plus bevacizumab at 7.5 mg/kg IV on day 1 every 3 weeks.
Final results
The median PFS, the primary endpoint, was 9.23 months with TT-B and 7.82 months with C-B. The difference was not statistically significant, with the upper limit of the 95% confidence interval crossing 1.
The median overall survival was 22.31 months with TT-B and 17.67 months with C-B (hazard ratio, 0.78; 95% CI, 0.55-1.10).
Dr. Van Cutsem pointed out that more patients in the TT-B arm had subsequent therapies compared with patients in the C-B arm – 59.7% vs. 40.8%.
He also noted that the safety profile of TT-B “remains unchanged from the initial analysis.”
Grade 3 or greater neutropenia, decreased neutrophil count, anemia, and decreased white blood cell count were all higher among patients on TT-B, but grade 3 or greater febrile neutropenia was similar between the groups.
Patients in the TT-B arm had more frequent grade 3 or greater nausea, vomiting, and hypertension. Grade 3 or higher hand-foot syndrome and diarrhea were both more common with C-B.
At the study cutoff date in September 2020, 66 patients in each arm had died.
Dr. Van Cutsem said more data on the efficacy of TT-B vs. C-B will come from the ongoing phase 3 SOLSTICE trial. Results from this trial are expected in late 2022.
‘The jury is still out’
The final results from TASCO1 suggest there may be some benefit from TT-B in patients with treatment-naive metastatic colorectal cancer, “but we don’t use it in the first line,” said Jeffery Clark, MD, an oncologist who was not involved in the study.
The trial supports the benefit of combining trifluridine/tipiracil with bevacizumab, and the results were “somewhat better” than he had expected, said Dr. Clark, director of clinical trials support at Mass General Cancer Center in Boston.
“Even though the results are encouraging, there were a couple of things about the trial that one has to at least think about,” Dr. Clark said in an interview.
He noted, for example, that a higher proportion of patients assigned to TT-B had prior adjuvant therapy (27.3% vs. 19.7%), and patients in the TT-B arm were also more likely to have second lines of systemic therapy, which could have skewed the results in favor of the experimental arm.
“I think, basically, the jury is still out until we see the results of the SOLSTICE trial,” he said.
The TASCO1 study was funded by Servier and Taiho. Dr. Van Cutsem has received research funding and served on an advisory board for Servier and other companies. Dr. Clark reported no relevant disclosures.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The median progression-free survival (PFS) in the phase 2 trial showed a difference of 1.41 months favoring TT-B over C-B, but this difference was not statistically significant.
The median overall survival was 4.64 months longer with TT-B than with C-B. However, the final analysis of TASCO1 was not designed to be comparative for overall survival, “so no formal statistical analysis is presented, and survival is a secondary endpoint,” noted investigator Eric Van Cutsem, MD, PhD, of University Hospital Gasthuisberg in Leuven, Belgium.
Dr. Van Cutsem presented the final results of TASCO1 at the 2021 Gastrointestinal Cancers Symposium (abstract 14).
Prior results from the trial were reported last year (Ann Oncol. 2020 Sep;31[9]:1160-68).
About trifluridine/tipiracil
Trifluridine/tipiracil is an oral drug combining the thymidine analogue trifluridine with tipiracil, an inhibitor of trifluridine degradation. The drug was approved by the Food and Drug Administration in 2015 under the trade name Lonsurf for the treatment of refractory metastatic colorectal cancer, and in 2019 for patients with metastatic gastric cancer or gastroesophageal junction cancer that had been treated with at least two lines of chemotherapy.
Trifluridine/tipiracil was associated with a brief but statistically significant survival benefit when compared with placebo in patients with heavily pretreated metastatic gastric cancer in the TAS-102 Gastric Study (Lancet Oncol. 2018 Nov;19[11]:1437-48).
In a separate analysis of the study, trifluridine/tipiracil was associated with significantly better overall survival and PFS than placebo in patients who had undergone gastrectomy (JAMA Oncol. 2019 Oct 10;6[1]:e193531).
TASCO1 details
In TASCO1, investigators enrolled patients with colorectal cancer who had never received systemic therapy for unresectable metastatic disease, and who were judged to be ineligible for intensive therapy due to advanced age, low tumor burden, poor performance status, comorbidities, or other clinical reasons.
After stratification by RAS status, performance status, and region, patients were randomly assigned to receive TT-B (n = 77) or C-B (n = 76).
TT-B consisted of oral trifluridine/tipiracil at 35 mg/m2 twice daily on days 1-5 and 8-12 every 4 weeks plus bevacizumab at 5 mg/kg intravenously on days 1 and 15 every 4 weeks.
C-B consisted of oral capecitabine at 1,250 or 1,000 mg/m2 twice a day on days 1-14 every 3 weeks plus bevacizumab at 7.5 mg/kg IV on day 1 every 3 weeks.
Final results
The median PFS, the primary endpoint, was 9.23 months with TT-B and 7.82 months with C-B. The difference was not statistically significant, with the upper limit of the 95% confidence interval crossing 1.
The median overall survival was 22.31 months with TT-B and 17.67 months with C-B (hazard ratio, 0.78; 95% CI, 0.55-1.10).
Dr. Van Cutsem pointed out that more patients in the TT-B arm had subsequent therapies compared with patients in the C-B arm – 59.7% vs. 40.8%.
He also noted that the safety profile of TT-B “remains unchanged from the initial analysis.”
Grade 3 or greater neutropenia, decreased neutrophil count, anemia, and decreased white blood cell count were all higher among patients on TT-B, but grade 3 or greater febrile neutropenia was similar between the groups.
Patients in the TT-B arm had more frequent grade 3 or greater nausea, vomiting, and hypertension. Grade 3 or higher hand-foot syndrome and diarrhea were both more common with C-B.
At the study cutoff date in September 2020, 66 patients in each arm had died.
Dr. Van Cutsem said more data on the efficacy of TT-B vs. C-B will come from the ongoing phase 3 SOLSTICE trial. Results from this trial are expected in late 2022.
‘The jury is still out’
The final results from TASCO1 suggest there may be some benefit from TT-B in patients with treatment-naive metastatic colorectal cancer, “but we don’t use it in the first line,” said Jeffery Clark, MD, an oncologist who was not involved in the study.
The trial supports the benefit of combining trifluridine/tipiracil with bevacizumab, and the results were “somewhat better” than he had expected, said Dr. Clark, director of clinical trials support at Mass General Cancer Center in Boston.
“Even though the results are encouraging, there were a couple of things about the trial that one has to at least think about,” Dr. Clark said in an interview.
He noted, for example, that a higher proportion of patients assigned to TT-B had prior adjuvant therapy (27.3% vs. 19.7%), and patients in the TT-B arm were also more likely to have second lines of systemic therapy, which could have skewed the results in favor of the experimental arm.
“I think, basically, the jury is still out until we see the results of the SOLSTICE trial,” he said.
The TASCO1 study was funded by Servier and Taiho. Dr. Van Cutsem has received research funding and served on an advisory board for Servier and other companies. Dr. Clark reported no relevant disclosures.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
FROM GI CANCERS SYMPOSIUM 2021
Naltrexone cuts hospitalization, deaths in alcohol use disorder
Naltrexone reduces the risk for hospitalization for alcohol use disorder (AUD), regardless of whether it is used alone or in conjunction with disulfiram or acamprosate, research suggests.
Investigators analyzed 10-year data on more than 125,000 Swedish residents with AUD and found that naltrexone, used as monotherapy or combined with acamprosate or disulfiram, was associated with significantly lower risk for AUD hospitalization or all-cause hospitalization in comparison with patients who did not use AUD medication. The patients ranged in age from 16 to 64 years.
By contrast, benzodiazepines and acamprosate monotherapy were associated with increased risk for AUD hospitalization.
“The take-home message for practicing clinicians would be that especially naltrexone use is associated with favorable treatment outcomes and should be utilized as part of the treatment protocol for AUD,” study investigator Milja Heikkinen, MD, specialist in forensic psychiatry and addiction medicine, University of Eastern Finland, Kuopio, told this news organization.
On the other hand, “benzodiazepines should be avoided and should not be administered other than for alcohol withdrawal symptoms,” she said.
The study was published online Jan. 4 in Addiction.
Real-world data
Previous research has shown that disulfiram, acamprosate, naltrexone, and nalmefene are efficacious in treating AUD, but most studies have been randomized controlled trials or meta-analyses, the authors write.
“Very little is known about overall health outcomes (such as risks of hospitalization and mortality) associated with specific treatments in real-world circumstances,” they write.
“The study was motivated by the fact that, although AUD is a significant public health concern, very little is known, especially about the comparative effectiveness of medications indicated in AUD,” said Dr. Heikkinen.
who had been diagnosed with AUD (62.5% men; mean [standard deviation] age, 38.1 [15.9] years). They followed the cohort over a median of 4.6 years (interquartile range, 2.1-.2 years).
During the follow-up period, roughly one-fourth of patients (25.6) underwent treatment with one or more drugs.

The main outcome measure was AUD-related hospitalization. Secondary outcomes were hospitalization for any cause and for alcohol-related somatic causes; all-cause mortality; and work disability.
Two types of analyses were conducted. The within-individual analyses, designed to eliminate selection bias, compared the use of a medication to periods during which the same individual was not using the medication.
Between-individual analyses (adjusted for sex, age, educational level, number of previous AUD-related hospitalizations, time since first AUD diagnosis, comorbidities, and use of other medications) utilized a “traditional” multivariate-adjusted Cox hazards regression model.
AUD pharmacotherapy ‘underutilized’
Close to one-fourth of patients (23.9%) experienced the main outcome event (AUD-related hospitalization) during the follow-up period.
The within-individual analysis showed that naltrexone – whether used as monotherapy or adjunctively with disulfiram or acamprosate – “was associated with a significantly lower risk of AUD-related hospitalization, compared to those time periods in which the same individual did not use any AUD medication,” the authors report.
By contrast, they state, acamprosate monotherapy and benzodiazepines were associated with a significantly higher risk for AUD-related hospitalization.
Similar results were obtained in the between-individual analysis. Longer duration of naltrexone use was associated with lower risk for AUD-related hospitalization.
The pattern was also found when the outcome was hospitalization for any cause. However, unlike the findings of the within-individual model, the second model found that acamprosate monotherapy was not associated with a higher risk for any-cause hospitalization.
Polytherapy, including combinations of the four AUD medications, as well as disulfiram monotherapy were similarly associated with lower risk for hospitalization for alcohol-related somatic causes.
Of the overall cohort, 6.2% died during the follow-up period. No association was found between disulfiram, acamprosate, nalmefene, and naltrexone use and all-cause mortality. By contrast, benzodiazepine use was associated with a significantly higher mortality rate (hazard ratio, 1.11; 95% confidence interval, 1.04-1.19).
“AUD drugs are underutilized, despite AUD being a significant public health concern,” Dr. Heikkinen noted. On the other hand, benzodiazepine use is “very common.”
‘Ravages’ of benzodiazepines
Commenting on the study in an interview, John Krystal, MD, professor and chair of psychiatry and director of the Center for the Translational Neuroscience of Alcoholism, Yale University, New Haven, Conn., said, “The main message from the study for practicing clinicians is that treatment works.”
Dr. Krystal, who was not involved with the study, noted that “many practicing clinicians are discouraged by the course of their patients with AUD, and this study highlights that naltrexone, perhaps in combination with other medications, may be effective in preventing hospitalization and, presumably, other hospitalization-related complications of AUD.”
Also commenting on the study, Raymond Anton, MD, professor, department of psychiatry and behavioral sciences, Medical University of South Carolina, Charleston, suggested that the “clinical knowledge of the harm of benzodiazepines in those with AUD is reinforced by these findings.”
In fact, the harm of benzodiazepines might be the study’s “most important message ... [a message that was] recently highlighted by the Netflix series “The Queen’s Gambit”, which shows the ravages of using both together, or how one leads to potential addiction with the other,” said Dr. Anton, who was not involved with the study.
The other “big take-home message is that naltrexone should be used more frequently,” said Dr. Anton, distinguished professor of psychiatry at the university and scientific director of the Charleston Alcohol Research Center. He noted that there are “recent data suggesting some clinical and genetic indicators that predict responsiveness to these medications, improving efficacy.”
The study was funded by the Finnish Ministry of Social Affairs and Health. Dr. Heikkinen reports no relevant financial relationships. The other authors’ disclosures are listed on the original article. Dr. Krystal consults for companies currently developing other treatments for AUDs and receives medications to test from AstraZeneca and Novartis for NIAAA-funded research programs. Dr. Anton has consulted for Alkermes, Lipha, and Lundbeck in the past. He is also chair of the Alcohol Clinical Trials Initiative, which is a public-private partnership partially sponsored by several companies and has received grant funding from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism to study pharmacotherapies, including naltrexone, nalmefene, and acamprosate.
A version of this article first appeared on Medscape.com.
Naltrexone reduces the risk for hospitalization for alcohol use disorder (AUD), regardless of whether it is used alone or in conjunction with disulfiram or acamprosate, research suggests.
Investigators analyzed 10-year data on more than 125,000 Swedish residents with AUD and found that naltrexone, used as monotherapy or combined with acamprosate or disulfiram, was associated with significantly lower risk for AUD hospitalization or all-cause hospitalization in comparison with patients who did not use AUD medication. The patients ranged in age from 16 to 64 years.
By contrast, benzodiazepines and acamprosate monotherapy were associated with increased risk for AUD hospitalization.
“The take-home message for practicing clinicians would be that especially naltrexone use is associated with favorable treatment outcomes and should be utilized as part of the treatment protocol for AUD,” study investigator Milja Heikkinen, MD, specialist in forensic psychiatry and addiction medicine, University of Eastern Finland, Kuopio, told this news organization.
On the other hand, “benzodiazepines should be avoided and should not be administered other than for alcohol withdrawal symptoms,” she said.
The study was published online Jan. 4 in Addiction.
Real-world data
Previous research has shown that disulfiram, acamprosate, naltrexone, and nalmefene are efficacious in treating AUD, but most studies have been randomized controlled trials or meta-analyses, the authors write.
“Very little is known about overall health outcomes (such as risks of hospitalization and mortality) associated with specific treatments in real-world circumstances,” they write.
“The study was motivated by the fact that, although AUD is a significant public health concern, very little is known, especially about the comparative effectiveness of medications indicated in AUD,” said Dr. Heikkinen.
who had been diagnosed with AUD (62.5% men; mean [standard deviation] age, 38.1 [15.9] years). They followed the cohort over a median of 4.6 years (interquartile range, 2.1-.2 years).
During the follow-up period, roughly one-fourth of patients (25.6) underwent treatment with one or more drugs.

The main outcome measure was AUD-related hospitalization. Secondary outcomes were hospitalization for any cause and for alcohol-related somatic causes; all-cause mortality; and work disability.
Two types of analyses were conducted. The within-individual analyses, designed to eliminate selection bias, compared the use of a medication to periods during which the same individual was not using the medication.
Between-individual analyses (adjusted for sex, age, educational level, number of previous AUD-related hospitalizations, time since first AUD diagnosis, comorbidities, and use of other medications) utilized a “traditional” multivariate-adjusted Cox hazards regression model.
AUD pharmacotherapy ‘underutilized’
Close to one-fourth of patients (23.9%) experienced the main outcome event (AUD-related hospitalization) during the follow-up period.
The within-individual analysis showed that naltrexone – whether used as monotherapy or adjunctively with disulfiram or acamprosate – “was associated with a significantly lower risk of AUD-related hospitalization, compared to those time periods in which the same individual did not use any AUD medication,” the authors report.
By contrast, they state, acamprosate monotherapy and benzodiazepines were associated with a significantly higher risk for AUD-related hospitalization.
Similar results were obtained in the between-individual analysis. Longer duration of naltrexone use was associated with lower risk for AUD-related hospitalization.
The pattern was also found when the outcome was hospitalization for any cause. However, unlike the findings of the within-individual model, the second model found that acamprosate monotherapy was not associated with a higher risk for any-cause hospitalization.
Polytherapy, including combinations of the four AUD medications, as well as disulfiram monotherapy were similarly associated with lower risk for hospitalization for alcohol-related somatic causes.
Of the overall cohort, 6.2% died during the follow-up period. No association was found between disulfiram, acamprosate, nalmefene, and naltrexone use and all-cause mortality. By contrast, benzodiazepine use was associated with a significantly higher mortality rate (hazard ratio, 1.11; 95% confidence interval, 1.04-1.19).
“AUD drugs are underutilized, despite AUD being a significant public health concern,” Dr. Heikkinen noted. On the other hand, benzodiazepine use is “very common.”
‘Ravages’ of benzodiazepines
Commenting on the study in an interview, John Krystal, MD, professor and chair of psychiatry and director of the Center for the Translational Neuroscience of Alcoholism, Yale University, New Haven, Conn., said, “The main message from the study for practicing clinicians is that treatment works.”
Dr. Krystal, who was not involved with the study, noted that “many practicing clinicians are discouraged by the course of their patients with AUD, and this study highlights that naltrexone, perhaps in combination with other medications, may be effective in preventing hospitalization and, presumably, other hospitalization-related complications of AUD.”
Also commenting on the study, Raymond Anton, MD, professor, department of psychiatry and behavioral sciences, Medical University of South Carolina, Charleston, suggested that the “clinical knowledge of the harm of benzodiazepines in those with AUD is reinforced by these findings.”
In fact, the harm of benzodiazepines might be the study’s “most important message ... [a message that was] recently highlighted by the Netflix series “The Queen’s Gambit”, which shows the ravages of using both together, or how one leads to potential addiction with the other,” said Dr. Anton, who was not involved with the study.
The other “big take-home message is that naltrexone should be used more frequently,” said Dr. Anton, distinguished professor of psychiatry at the university and scientific director of the Charleston Alcohol Research Center. He noted that there are “recent data suggesting some clinical and genetic indicators that predict responsiveness to these medications, improving efficacy.”
The study was funded by the Finnish Ministry of Social Affairs and Health. Dr. Heikkinen reports no relevant financial relationships. The other authors’ disclosures are listed on the original article. Dr. Krystal consults for companies currently developing other treatments for AUDs and receives medications to test from AstraZeneca and Novartis for NIAAA-funded research programs. Dr. Anton has consulted for Alkermes, Lipha, and Lundbeck in the past. He is also chair of the Alcohol Clinical Trials Initiative, which is a public-private partnership partially sponsored by several companies and has received grant funding from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism to study pharmacotherapies, including naltrexone, nalmefene, and acamprosate.
A version of this article first appeared on Medscape.com.
Naltrexone reduces the risk for hospitalization for alcohol use disorder (AUD), regardless of whether it is used alone or in conjunction with disulfiram or acamprosate, research suggests.
Investigators analyzed 10-year data on more than 125,000 Swedish residents with AUD and found that naltrexone, used as monotherapy or combined with acamprosate or disulfiram, was associated with significantly lower risk for AUD hospitalization or all-cause hospitalization in comparison with patients who did not use AUD medication. The patients ranged in age from 16 to 64 years.
By contrast, benzodiazepines and acamprosate monotherapy were associated with increased risk for AUD hospitalization.
“The take-home message for practicing clinicians would be that especially naltrexone use is associated with favorable treatment outcomes and should be utilized as part of the treatment protocol for AUD,” study investigator Milja Heikkinen, MD, specialist in forensic psychiatry and addiction medicine, University of Eastern Finland, Kuopio, told this news organization.
On the other hand, “benzodiazepines should be avoided and should not be administered other than for alcohol withdrawal symptoms,” she said.
The study was published online Jan. 4 in Addiction.
Real-world data
Previous research has shown that disulfiram, acamprosate, naltrexone, and nalmefene are efficacious in treating AUD, but most studies have been randomized controlled trials or meta-analyses, the authors write.
“Very little is known about overall health outcomes (such as risks of hospitalization and mortality) associated with specific treatments in real-world circumstances,” they write.
“The study was motivated by the fact that, although AUD is a significant public health concern, very little is known, especially about the comparative effectiveness of medications indicated in AUD,” said Dr. Heikkinen.
who had been diagnosed with AUD (62.5% men; mean [standard deviation] age, 38.1 [15.9] years). They followed the cohort over a median of 4.6 years (interquartile range, 2.1-.2 years).
During the follow-up period, roughly one-fourth of patients (25.6) underwent treatment with one or more drugs.

The main outcome measure was AUD-related hospitalization. Secondary outcomes were hospitalization for any cause and for alcohol-related somatic causes; all-cause mortality; and work disability.
Two types of analyses were conducted. The within-individual analyses, designed to eliminate selection bias, compared the use of a medication to periods during which the same individual was not using the medication.
Between-individual analyses (adjusted for sex, age, educational level, number of previous AUD-related hospitalizations, time since first AUD diagnosis, comorbidities, and use of other medications) utilized a “traditional” multivariate-adjusted Cox hazards regression model.
AUD pharmacotherapy ‘underutilized’
Close to one-fourth of patients (23.9%) experienced the main outcome event (AUD-related hospitalization) during the follow-up period.
The within-individual analysis showed that naltrexone – whether used as monotherapy or adjunctively with disulfiram or acamprosate – “was associated with a significantly lower risk of AUD-related hospitalization, compared to those time periods in which the same individual did not use any AUD medication,” the authors report.
By contrast, they state, acamprosate monotherapy and benzodiazepines were associated with a significantly higher risk for AUD-related hospitalization.
Similar results were obtained in the between-individual analysis. Longer duration of naltrexone use was associated with lower risk for AUD-related hospitalization.
The pattern was also found when the outcome was hospitalization for any cause. However, unlike the findings of the within-individual model, the second model found that acamprosate monotherapy was not associated with a higher risk for any-cause hospitalization.
Polytherapy, including combinations of the four AUD medications, as well as disulfiram monotherapy were similarly associated with lower risk for hospitalization for alcohol-related somatic causes.
Of the overall cohort, 6.2% died during the follow-up period. No association was found between disulfiram, acamprosate, nalmefene, and naltrexone use and all-cause mortality. By contrast, benzodiazepine use was associated with a significantly higher mortality rate (hazard ratio, 1.11; 95% confidence interval, 1.04-1.19).
“AUD drugs are underutilized, despite AUD being a significant public health concern,” Dr. Heikkinen noted. On the other hand, benzodiazepine use is “very common.”
‘Ravages’ of benzodiazepines
Commenting on the study in an interview, John Krystal, MD, professor and chair of psychiatry and director of the Center for the Translational Neuroscience of Alcoholism, Yale University, New Haven, Conn., said, “The main message from the study for practicing clinicians is that treatment works.”
Dr. Krystal, who was not involved with the study, noted that “many practicing clinicians are discouraged by the course of their patients with AUD, and this study highlights that naltrexone, perhaps in combination with other medications, may be effective in preventing hospitalization and, presumably, other hospitalization-related complications of AUD.”
Also commenting on the study, Raymond Anton, MD, professor, department of psychiatry and behavioral sciences, Medical University of South Carolina, Charleston, suggested that the “clinical knowledge of the harm of benzodiazepines in those with AUD is reinforced by these findings.”
In fact, the harm of benzodiazepines might be the study’s “most important message ... [a message that was] recently highlighted by the Netflix series “The Queen’s Gambit”, which shows the ravages of using both together, or how one leads to potential addiction with the other,” said Dr. Anton, who was not involved with the study.
The other “big take-home message is that naltrexone should be used more frequently,” said Dr. Anton, distinguished professor of psychiatry at the university and scientific director of the Charleston Alcohol Research Center. He noted that there are “recent data suggesting some clinical and genetic indicators that predict responsiveness to these medications, improving efficacy.”
The study was funded by the Finnish Ministry of Social Affairs and Health. Dr. Heikkinen reports no relevant financial relationships. The other authors’ disclosures are listed on the original article. Dr. Krystal consults for companies currently developing other treatments for AUDs and receives medications to test from AstraZeneca and Novartis for NIAAA-funded research programs. Dr. Anton has consulted for Alkermes, Lipha, and Lundbeck in the past. He is also chair of the Alcohol Clinical Trials Initiative, which is a public-private partnership partially sponsored by several companies and has received grant funding from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism to study pharmacotherapies, including naltrexone, nalmefene, and acamprosate.
A version of this article first appeared on Medscape.com.
Monoclonal antibody drops fat, ups muscle in obesity, diabetes
In a phase 2 randomized clinical trial of adults with type 2 diabetes and obesity, investigational drug bimagrumab (BYM338, Novartis) – a monoclonal antibody that blocks activin type II receptors and stimulates skeletal muscle growth – led to big reductions in total body fat mass and A1c and significant increases in lean mass compared with placebo.
The efficacy and safety findings “suggest that blockade of the activin receptor with bimagrumab could provide a novel pharmacologic approach for managing patients with type 2 diabetes with excess adiposity,” Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and colleagues reported in their study, published online Jan. 13 in JAMA Network Open.
Preliminary findings from the study of 75 patients treated for 48 weeks – in which neither group ate less despite intensive nutrition advice – were presented at Obesity Week in 2019.
As reported then, Lee M. Kaplan, MD, PhD, noted that the 6.5% weight loss in the bimagrumab group was similar to that seen with antiobesity medications that suppress appetite.
“What it suggests,” he said in an interview, “is that there may be a completely new mechanism at play here,” because patients receiving bimagrumab weren’t eating less but were losing the same amount of weight as reported for weight-loss drugs that work by decreasing appetite.
“Is this going to be the kind of complementary drug with a different mechanism that’s going to augment the effects of other drugs?” wondered Dr. Kaplan, director of the Obesity, Metabolism & Nutrition Institute at Massachusetts General Hospital, Boston, who has previously served as a scientific consultant to Novartis.
Asked about future plans for bimagrumab, a Novartis spokesperson said in an interview, “We are currently reviewing the program strategy and considering next steps.”
Four FDA-approved weight-loss drugs now approved
The Food and Drug Administration approval for lorcaserin (Belviq, Belviq XR, Eisai) for weight loss was rescinded on Feb. 13, 2020, when a postmarketing trial revealed an increased occurrence of cancer, leaving four drugs approved for weight loss in the United States, plus several drugs in development, Dr. Heymsfield and colleagues wrote.
The current phase 2 trial was designed to determine the safety and efficacy of bimagrumab – which had originally been studied to see if it would increase lean muscle mass in people with sarcopenia – on total body fat mass and glycemic control in patients with type 2 diabetes and overweight or obesity.
Researchers enrolled 75 adults at eight sites in the United States and one in Wales, United Kingdom, from 2017 to 2019.
On average, patients were 60 years old with an A1c of 7.8% and a body mass index of 32.9 kg/m2; they weighed 93.6 kg and had a fat mass of 35 kg.
Patients received an intravenous infusion of bimagrumab (10 mg/kg up to 1,200 mg in 5% dextrose solution) or placebo (5% dextrose solution) every 4 weeks for 48 weeks. They met with a registered dietitian at each monthly study visit and had a virtual check-in between visits.
Participants were advised to follow a diet that would cut 500 calories a day and encouraged to follow the American Diabetes Association walking program.
Body fat mass was measured by dual-energy x-ray absorptiometry (DEXA).
There were more women in the bimagrumab group than in the placebo group (62% vs. 32%), but baseline BMI, total body fat mass, and A1c were similar in both groups.
Same caloric intake, less fat tissue, more muscle, smaller waist
At 48 weeks in the bimagrumab vs. placebo group, there was on average (all P < .001):
- A loss of 20.5% vs. 0.5% (−7.5 vs. −0.2 kg) of total body fat mass.
- A loss of 6.5% vs. 0.8% (−5.9 vs. −0.8 kg) of body weight.
- A gain of 3.6% vs. a loss of 0.8% (1.7 vs. −0.4 kg) of lean mass.
Similarly, the relatively large between-group differences in total body fat mass and body weight at 48 weeks with bimagrumab were accompanied by favorable differences in BMI (−2.19 vs. −0.28 kg/m2; P < .001) and waist circumference (−9.0 vs. 0.5 cm; P < .001), the investigators pointed out.
Moreover, the reduction of abdominal visceral adipose tissue and waist circumference with bimagrumab “was nearly twice that observed in a recently published study of patients with type 2 diabetes treated with an intensive lifestyle program and the glucagon-like peptide 1 (GLP-1) agonist liraglutide,” they noted.
This highlights “the importance of moving away from body weight as a primary efficacy marker of drugs to more metabolically relevant endpoints.”
Also, A1c decreased by 0.76% in the bimagrumab group and increased by 0.04% in the placebo group (P = .005).
Serious adverse events occurred in three patients (8%) in the bimagrumab group (elevated lipase, epigastric pain, pancreatitis, pneumonia) and three patients (8%) in the placebo group (cellulitis, acute coronary syndrome, acute myocardial infarction, worsening gastroparesis, thermal burn).
Adverse events were reported by 31 of 37 patients in the bimagrumab group, most often mild diarrhea (41%) and muscle spasms (41%), and 31 of 38 patients in the placebo group, most often headache (13%) and upper respiratory tract infection (13%).
The study was funded by Novartis. Dr. Heymsfield has reported receiving personal fees from Tanita and Medifast outside the submitted work. Disclosures for the other authors are listed in the article. Dr. Kaplan has reported previously serving as a scientific consultant to Novartis.
A version of this article first appeared on Medscape.com.
In a phase 2 randomized clinical trial of adults with type 2 diabetes and obesity, investigational drug bimagrumab (BYM338, Novartis) – a monoclonal antibody that blocks activin type II receptors and stimulates skeletal muscle growth – led to big reductions in total body fat mass and A1c and significant increases in lean mass compared with placebo.
The efficacy and safety findings “suggest that blockade of the activin receptor with bimagrumab could provide a novel pharmacologic approach for managing patients with type 2 diabetes with excess adiposity,” Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and colleagues reported in their study, published online Jan. 13 in JAMA Network Open.
Preliminary findings from the study of 75 patients treated for 48 weeks – in which neither group ate less despite intensive nutrition advice – were presented at Obesity Week in 2019.
As reported then, Lee M. Kaplan, MD, PhD, noted that the 6.5% weight loss in the bimagrumab group was similar to that seen with antiobesity medications that suppress appetite.
“What it suggests,” he said in an interview, “is that there may be a completely new mechanism at play here,” because patients receiving bimagrumab weren’t eating less but were losing the same amount of weight as reported for weight-loss drugs that work by decreasing appetite.
“Is this going to be the kind of complementary drug with a different mechanism that’s going to augment the effects of other drugs?” wondered Dr. Kaplan, director of the Obesity, Metabolism & Nutrition Institute at Massachusetts General Hospital, Boston, who has previously served as a scientific consultant to Novartis.
Asked about future plans for bimagrumab, a Novartis spokesperson said in an interview, “We are currently reviewing the program strategy and considering next steps.”
Four FDA-approved weight-loss drugs now approved
The Food and Drug Administration approval for lorcaserin (Belviq, Belviq XR, Eisai) for weight loss was rescinded on Feb. 13, 2020, when a postmarketing trial revealed an increased occurrence of cancer, leaving four drugs approved for weight loss in the United States, plus several drugs in development, Dr. Heymsfield and colleagues wrote.
The current phase 2 trial was designed to determine the safety and efficacy of bimagrumab – which had originally been studied to see if it would increase lean muscle mass in people with sarcopenia – on total body fat mass and glycemic control in patients with type 2 diabetes and overweight or obesity.
Researchers enrolled 75 adults at eight sites in the United States and one in Wales, United Kingdom, from 2017 to 2019.
On average, patients were 60 years old with an A1c of 7.8% and a body mass index of 32.9 kg/m2; they weighed 93.6 kg and had a fat mass of 35 kg.
Patients received an intravenous infusion of bimagrumab (10 mg/kg up to 1,200 mg in 5% dextrose solution) or placebo (5% dextrose solution) every 4 weeks for 48 weeks. They met with a registered dietitian at each monthly study visit and had a virtual check-in between visits.
Participants were advised to follow a diet that would cut 500 calories a day and encouraged to follow the American Diabetes Association walking program.
Body fat mass was measured by dual-energy x-ray absorptiometry (DEXA).
There were more women in the bimagrumab group than in the placebo group (62% vs. 32%), but baseline BMI, total body fat mass, and A1c were similar in both groups.
Same caloric intake, less fat tissue, more muscle, smaller waist
At 48 weeks in the bimagrumab vs. placebo group, there was on average (all P < .001):
- A loss of 20.5% vs. 0.5% (−7.5 vs. −0.2 kg) of total body fat mass.
- A loss of 6.5% vs. 0.8% (−5.9 vs. −0.8 kg) of body weight.
- A gain of 3.6% vs. a loss of 0.8% (1.7 vs. −0.4 kg) of lean mass.
Similarly, the relatively large between-group differences in total body fat mass and body weight at 48 weeks with bimagrumab were accompanied by favorable differences in BMI (−2.19 vs. −0.28 kg/m2; P < .001) and waist circumference (−9.0 vs. 0.5 cm; P < .001), the investigators pointed out.
Moreover, the reduction of abdominal visceral adipose tissue and waist circumference with bimagrumab “was nearly twice that observed in a recently published study of patients with type 2 diabetes treated with an intensive lifestyle program and the glucagon-like peptide 1 (GLP-1) agonist liraglutide,” they noted.
This highlights “the importance of moving away from body weight as a primary efficacy marker of drugs to more metabolically relevant endpoints.”
Also, A1c decreased by 0.76% in the bimagrumab group and increased by 0.04% in the placebo group (P = .005).
Serious adverse events occurred in three patients (8%) in the bimagrumab group (elevated lipase, epigastric pain, pancreatitis, pneumonia) and three patients (8%) in the placebo group (cellulitis, acute coronary syndrome, acute myocardial infarction, worsening gastroparesis, thermal burn).
Adverse events were reported by 31 of 37 patients in the bimagrumab group, most often mild diarrhea (41%) and muscle spasms (41%), and 31 of 38 patients in the placebo group, most often headache (13%) and upper respiratory tract infection (13%).
The study was funded by Novartis. Dr. Heymsfield has reported receiving personal fees from Tanita and Medifast outside the submitted work. Disclosures for the other authors are listed in the article. Dr. Kaplan has reported previously serving as a scientific consultant to Novartis.
A version of this article first appeared on Medscape.com.
In a phase 2 randomized clinical trial of adults with type 2 diabetes and obesity, investigational drug bimagrumab (BYM338, Novartis) – a monoclonal antibody that blocks activin type II receptors and stimulates skeletal muscle growth – led to big reductions in total body fat mass and A1c and significant increases in lean mass compared with placebo.
The efficacy and safety findings “suggest that blockade of the activin receptor with bimagrumab could provide a novel pharmacologic approach for managing patients with type 2 diabetes with excess adiposity,” Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and colleagues reported in their study, published online Jan. 13 in JAMA Network Open.
Preliminary findings from the study of 75 patients treated for 48 weeks – in which neither group ate less despite intensive nutrition advice – were presented at Obesity Week in 2019.
As reported then, Lee M. Kaplan, MD, PhD, noted that the 6.5% weight loss in the bimagrumab group was similar to that seen with antiobesity medications that suppress appetite.
“What it suggests,” he said in an interview, “is that there may be a completely new mechanism at play here,” because patients receiving bimagrumab weren’t eating less but were losing the same amount of weight as reported for weight-loss drugs that work by decreasing appetite.
“Is this going to be the kind of complementary drug with a different mechanism that’s going to augment the effects of other drugs?” wondered Dr. Kaplan, director of the Obesity, Metabolism & Nutrition Institute at Massachusetts General Hospital, Boston, who has previously served as a scientific consultant to Novartis.
Asked about future plans for bimagrumab, a Novartis spokesperson said in an interview, “We are currently reviewing the program strategy and considering next steps.”
Four FDA-approved weight-loss drugs now approved
The Food and Drug Administration approval for lorcaserin (Belviq, Belviq XR, Eisai) for weight loss was rescinded on Feb. 13, 2020, when a postmarketing trial revealed an increased occurrence of cancer, leaving four drugs approved for weight loss in the United States, plus several drugs in development, Dr. Heymsfield and colleagues wrote.
The current phase 2 trial was designed to determine the safety and efficacy of bimagrumab – which had originally been studied to see if it would increase lean muscle mass in people with sarcopenia – on total body fat mass and glycemic control in patients with type 2 diabetes and overweight or obesity.
Researchers enrolled 75 adults at eight sites in the United States and one in Wales, United Kingdom, from 2017 to 2019.
On average, patients were 60 years old with an A1c of 7.8% and a body mass index of 32.9 kg/m2; they weighed 93.6 kg and had a fat mass of 35 kg.
Patients received an intravenous infusion of bimagrumab (10 mg/kg up to 1,200 mg in 5% dextrose solution) or placebo (5% dextrose solution) every 4 weeks for 48 weeks. They met with a registered dietitian at each monthly study visit and had a virtual check-in between visits.
Participants were advised to follow a diet that would cut 500 calories a day and encouraged to follow the American Diabetes Association walking program.
Body fat mass was measured by dual-energy x-ray absorptiometry (DEXA).
There were more women in the bimagrumab group than in the placebo group (62% vs. 32%), but baseline BMI, total body fat mass, and A1c were similar in both groups.
Same caloric intake, less fat tissue, more muscle, smaller waist
At 48 weeks in the bimagrumab vs. placebo group, there was on average (all P < .001):
- A loss of 20.5% vs. 0.5% (−7.5 vs. −0.2 kg) of total body fat mass.
- A loss of 6.5% vs. 0.8% (−5.9 vs. −0.8 kg) of body weight.
- A gain of 3.6% vs. a loss of 0.8% (1.7 vs. −0.4 kg) of lean mass.
Similarly, the relatively large between-group differences in total body fat mass and body weight at 48 weeks with bimagrumab were accompanied by favorable differences in BMI (−2.19 vs. −0.28 kg/m2; P < .001) and waist circumference (−9.0 vs. 0.5 cm; P < .001), the investigators pointed out.
Moreover, the reduction of abdominal visceral adipose tissue and waist circumference with bimagrumab “was nearly twice that observed in a recently published study of patients with type 2 diabetes treated with an intensive lifestyle program and the glucagon-like peptide 1 (GLP-1) agonist liraglutide,” they noted.
This highlights “the importance of moving away from body weight as a primary efficacy marker of drugs to more metabolically relevant endpoints.”
Also, A1c decreased by 0.76% in the bimagrumab group and increased by 0.04% in the placebo group (P = .005).
Serious adverse events occurred in three patients (8%) in the bimagrumab group (elevated lipase, epigastric pain, pancreatitis, pneumonia) and three patients (8%) in the placebo group (cellulitis, acute coronary syndrome, acute myocardial infarction, worsening gastroparesis, thermal burn).
Adverse events were reported by 31 of 37 patients in the bimagrumab group, most often mild diarrhea (41%) and muscle spasms (41%), and 31 of 38 patients in the placebo group, most often headache (13%) and upper respiratory tract infection (13%).
The study was funded by Novartis. Dr. Heymsfield has reported receiving personal fees from Tanita and Medifast outside the submitted work. Disclosures for the other authors are listed in the article. Dr. Kaplan has reported previously serving as a scientific consultant to Novartis.
A version of this article first appeared on Medscape.com.
COVID-19 risks linked to medications in IBD
Multicenter and population cohort studies suggest that patients with inflammatory bowel disease (IBD) are not at unique risk of contracting COVID-19 or experiencing worse outcomes, with the exception of a few risk factors such as corticosteroid use and combination therapy that appear tied to greater risk of hospitalization and mortality. The findings line up well with previous experience with infectious disease and are reassuring, but they also underscore the need to taper steroids and de-escalate from combination therapy, when possible.
“There is not a clear increased risk of getting COVID-19 among IBD patients compared to the general population, and that seems to hold even if you look at certain medication types, [even] if patients are on immunosuppressives like thiopurines or anti-TNF [anti–tumor necrosis factor] drugs,” Ryan C. Ungaro, MD, said in an interview. Dr. Ungaro, who is with the Icahn School of Medicine at Mount Sinai, New York, discussed IBD and COVID-19 risks at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
A systematic review showed that 0.3% of IBD patients contracted COVID-19 during study periods, compared with 0.2%-4.0% of the general population, and a matched-cohort analysis of a national Veterans Affairs database showed an infection prevalence of 0.23% among patients with IBD versus 0.20% among those without (P = .29). The analysis also showed use of anti-TNF therapies or thiopurines was not associated with an increased risk.
Studies show that patients with IBD in general do not appear to be at greater risk of severe disease outcomes such as hospitalization or 30-day mortality. For example, a U.S. national database study of more than 40 million patients compared 232 patients with IBD who were diagnosed with COVID-19 with 19,776 non-IBD patients and found that, after propensity matching, there were no significant association between IBD and worse outcomes (risk ratio, 0.93; 95% confidence interval, 0.68-1.27; P = .86) or hospitalizations (RR, 1.10; 95% CI, 0.74-1.40; P = .91)).
However, some risk factors could be red flags. Data from the international SECURE-IBD registry showed an association between combined endpoint of ICU, requiring a ventilator, or death and advanced age (adjusted odds ratio, 1.04; 95% CI, 1.01-1.06; P < .01) and two or more comorbidities (aOR, 2.87; 95% CI, 1.05-7.85; P < .04). More specifically to IBD, severe COVID-19 was associated with use of corticosteroids (aOR, 6.87; 95% CI, 2.30-20.51; P < .001). In terms of other therapies, another study found a similar effect with thiopurines (compared with TNF monotherapy; aOR, 4.08; 95% CI, 1.65-9.78; Bonferroni adjusted P = .008), and combined use of anti-TNF drugs and a thiopurine (compared with TNF monotherapy; aOR, 4.01; 95% CI, 1.73-9.61; Bonferroni adjusted P = .013), but anti-TNF therapies alone trended toward a protective effect (compared with no anti-TNF therapy; aOR, 0.69; Bonferroni adjusted P = .52). That study found no significant association between severe outcomes and anti-IL 12/23 (compared with anti-TNF monotherapy; aOR, 0.98; 95% CI, 0.12-8.06; P = .98) or anti-integrin biologics (compared with anti-TNF monotherapy; aOR, 2.42; 95% CI, 0.59-9.96; P = .22).
Overall, the data are “generally consistent with prior data on infections and IBD: That steroids and combination therapy increase the risk of infection and bad outcomes and that interestingly biologic monotherapy may actually confer a little bit of protection against emergent outcomes and at a minimum appears to be neutral,” said Dr. Ungaro.
He noted that the recommendations from the IOIBD COVID-19 Task Force were based on expert opinion, but the new data have largely supported them overall. He did suggest some potential modifications, including reducing thiopurine use among patients on combination therapy. According to Dr. Ungaro, the recommendations do call for withholding all IBD therapy for 10 days after positive SARS-CoV-2 tests, whether the patient is symptomatic or not. “I think the recent data is reassuring that potentially in asymptomatic and maybe even mild cases, the monotherapy biologics – we can consider not delaying administering those. I think we need more data about that, but it’s reassuring that patients on those had no worse outcomes and [in fact did] slightly better,” Dr. Ungaro said during the presentation.
The data reinforced the need to consider tapering patients off corticosteroids or combination therapies, if possible. “It’s something we were doing in regular IBD care beforehand, but the COVID-19 pandemic offers another reason to limit the use of steroids and evaluate if patients are able to de-escalate from combination therapies,” said Dr. Ungaro.
On the other hand, there was concern among some patients early in the pandemic that their immunotherapy drugs may put them at risk of contracting COVID-19, which led some to discontinue medications. Ongoing studies are illustrating the problem with this, according to David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the congress’s organizing committee. “The data do not in general suggest you should do that to protect yourself. In fact, being on the therapies may have a better outcome. Patients always want to come off their therapies, [but] during the pandemic that is a risk not worth taking. Getting sick from your Crohn’s disease or colitis, when there are limited health care resources and, in some places, limited hospital beds and where the rescue therapy might include steroids, is a risky proposition. It’s not the time to do this,” said Dr. Rubin.
With respect to vaccines, it appears so far that there is no increased risk of adverse events associated with IBD. Patients who are on immunosuppressive drugs may experience a lower response to immunization, which has been seen with other vaccines. “The benefits likely outweigh the risks based on our prior experience with other vaccinations. It’s an area of ongoing study, but I do think we should recommend that our IBD patients get the COVID-19 vaccine, especially if they have risk factors for severe disease,” said Dr. Ungaro.
Dr. Ungaro is on the advisory board for Bristol-Myers Squibb, Janssen, Pfizer, and Takeda. He has received funding from AbbVie, Boehringer Ingelheim, Eli Lilly, and Pfizer. He has been a speaker or received consulting fees from AbbVie and Eli Lilly. Dr. Rubin is a consultant for Janssen, Pfizer, Takeda, and AbbVie.
This article was updated Jan. 27, 2021.
Multicenter and population cohort studies suggest that patients with inflammatory bowel disease (IBD) are not at unique risk of contracting COVID-19 or experiencing worse outcomes, with the exception of a few risk factors such as corticosteroid use and combination therapy that appear tied to greater risk of hospitalization and mortality. The findings line up well with previous experience with infectious disease and are reassuring, but they also underscore the need to taper steroids and de-escalate from combination therapy, when possible.
“There is not a clear increased risk of getting COVID-19 among IBD patients compared to the general population, and that seems to hold even if you look at certain medication types, [even] if patients are on immunosuppressives like thiopurines or anti-TNF [anti–tumor necrosis factor] drugs,” Ryan C. Ungaro, MD, said in an interview. Dr. Ungaro, who is with the Icahn School of Medicine at Mount Sinai, New York, discussed IBD and COVID-19 risks at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
A systematic review showed that 0.3% of IBD patients contracted COVID-19 during study periods, compared with 0.2%-4.0% of the general population, and a matched-cohort analysis of a national Veterans Affairs database showed an infection prevalence of 0.23% among patients with IBD versus 0.20% among those without (P = .29). The analysis also showed use of anti-TNF therapies or thiopurines was not associated with an increased risk.
Studies show that patients with IBD in general do not appear to be at greater risk of severe disease outcomes such as hospitalization or 30-day mortality. For example, a U.S. national database study of more than 40 million patients compared 232 patients with IBD who were diagnosed with COVID-19 with 19,776 non-IBD patients and found that, after propensity matching, there were no significant association between IBD and worse outcomes (risk ratio, 0.93; 95% confidence interval, 0.68-1.27; P = .86) or hospitalizations (RR, 1.10; 95% CI, 0.74-1.40; P = .91)).
However, some risk factors could be red flags. Data from the international SECURE-IBD registry showed an association between combined endpoint of ICU, requiring a ventilator, or death and advanced age (adjusted odds ratio, 1.04; 95% CI, 1.01-1.06; P < .01) and two or more comorbidities (aOR, 2.87; 95% CI, 1.05-7.85; P < .04). More specifically to IBD, severe COVID-19 was associated with use of corticosteroids (aOR, 6.87; 95% CI, 2.30-20.51; P < .001). In terms of other therapies, another study found a similar effect with thiopurines (compared with TNF monotherapy; aOR, 4.08; 95% CI, 1.65-9.78; Bonferroni adjusted P = .008), and combined use of anti-TNF drugs and a thiopurine (compared with TNF monotherapy; aOR, 4.01; 95% CI, 1.73-9.61; Bonferroni adjusted P = .013), but anti-TNF therapies alone trended toward a protective effect (compared with no anti-TNF therapy; aOR, 0.69; Bonferroni adjusted P = .52). That study found no significant association between severe outcomes and anti-IL 12/23 (compared with anti-TNF monotherapy; aOR, 0.98; 95% CI, 0.12-8.06; P = .98) or anti-integrin biologics (compared with anti-TNF monotherapy; aOR, 2.42; 95% CI, 0.59-9.96; P = .22).
Overall, the data are “generally consistent with prior data on infections and IBD: That steroids and combination therapy increase the risk of infection and bad outcomes and that interestingly biologic monotherapy may actually confer a little bit of protection against emergent outcomes and at a minimum appears to be neutral,” said Dr. Ungaro.
He noted that the recommendations from the IOIBD COVID-19 Task Force were based on expert opinion, but the new data have largely supported them overall. He did suggest some potential modifications, including reducing thiopurine use among patients on combination therapy. According to Dr. Ungaro, the recommendations do call for withholding all IBD therapy for 10 days after positive SARS-CoV-2 tests, whether the patient is symptomatic or not. “I think the recent data is reassuring that potentially in asymptomatic and maybe even mild cases, the monotherapy biologics – we can consider not delaying administering those. I think we need more data about that, but it’s reassuring that patients on those had no worse outcomes and [in fact did] slightly better,” Dr. Ungaro said during the presentation.
The data reinforced the need to consider tapering patients off corticosteroids or combination therapies, if possible. “It’s something we were doing in regular IBD care beforehand, but the COVID-19 pandemic offers another reason to limit the use of steroids and evaluate if patients are able to de-escalate from combination therapies,” said Dr. Ungaro.
On the other hand, there was concern among some patients early in the pandemic that their immunotherapy drugs may put them at risk of contracting COVID-19, which led some to discontinue medications. Ongoing studies are illustrating the problem with this, according to David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the congress’s organizing committee. “The data do not in general suggest you should do that to protect yourself. In fact, being on the therapies may have a better outcome. Patients always want to come off their therapies, [but] during the pandemic that is a risk not worth taking. Getting sick from your Crohn’s disease or colitis, when there are limited health care resources and, in some places, limited hospital beds and where the rescue therapy might include steroids, is a risky proposition. It’s not the time to do this,” said Dr. Rubin.
With respect to vaccines, it appears so far that there is no increased risk of adverse events associated with IBD. Patients who are on immunosuppressive drugs may experience a lower response to immunization, which has been seen with other vaccines. “The benefits likely outweigh the risks based on our prior experience with other vaccinations. It’s an area of ongoing study, but I do think we should recommend that our IBD patients get the COVID-19 vaccine, especially if they have risk factors for severe disease,” said Dr. Ungaro.
Dr. Ungaro is on the advisory board for Bristol-Myers Squibb, Janssen, Pfizer, and Takeda. He has received funding from AbbVie, Boehringer Ingelheim, Eli Lilly, and Pfizer. He has been a speaker or received consulting fees from AbbVie and Eli Lilly. Dr. Rubin is a consultant for Janssen, Pfizer, Takeda, and AbbVie.
This article was updated Jan. 27, 2021.
Multicenter and population cohort studies suggest that patients with inflammatory bowel disease (IBD) are not at unique risk of contracting COVID-19 or experiencing worse outcomes, with the exception of a few risk factors such as corticosteroid use and combination therapy that appear tied to greater risk of hospitalization and mortality. The findings line up well with previous experience with infectious disease and are reassuring, but they also underscore the need to taper steroids and de-escalate from combination therapy, when possible.
“There is not a clear increased risk of getting COVID-19 among IBD patients compared to the general population, and that seems to hold even if you look at certain medication types, [even] if patients are on immunosuppressives like thiopurines or anti-TNF [anti–tumor necrosis factor] drugs,” Ryan C. Ungaro, MD, said in an interview. Dr. Ungaro, who is with the Icahn School of Medicine at Mount Sinai, New York, discussed IBD and COVID-19 risks at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
A systematic review showed that 0.3% of IBD patients contracted COVID-19 during study periods, compared with 0.2%-4.0% of the general population, and a matched-cohort analysis of a national Veterans Affairs database showed an infection prevalence of 0.23% among patients with IBD versus 0.20% among those without (P = .29). The analysis also showed use of anti-TNF therapies or thiopurines was not associated with an increased risk.
Studies show that patients with IBD in general do not appear to be at greater risk of severe disease outcomes such as hospitalization or 30-day mortality. For example, a U.S. national database study of more than 40 million patients compared 232 patients with IBD who were diagnosed with COVID-19 with 19,776 non-IBD patients and found that, after propensity matching, there were no significant association between IBD and worse outcomes (risk ratio, 0.93; 95% confidence interval, 0.68-1.27; P = .86) or hospitalizations (RR, 1.10; 95% CI, 0.74-1.40; P = .91)).
However, some risk factors could be red flags. Data from the international SECURE-IBD registry showed an association between combined endpoint of ICU, requiring a ventilator, or death and advanced age (adjusted odds ratio, 1.04; 95% CI, 1.01-1.06; P < .01) and two or more comorbidities (aOR, 2.87; 95% CI, 1.05-7.85; P < .04). More specifically to IBD, severe COVID-19 was associated with use of corticosteroids (aOR, 6.87; 95% CI, 2.30-20.51; P < .001). In terms of other therapies, another study found a similar effect with thiopurines (compared with TNF monotherapy; aOR, 4.08; 95% CI, 1.65-9.78; Bonferroni adjusted P = .008), and combined use of anti-TNF drugs and a thiopurine (compared with TNF monotherapy; aOR, 4.01; 95% CI, 1.73-9.61; Bonferroni adjusted P = .013), but anti-TNF therapies alone trended toward a protective effect (compared with no anti-TNF therapy; aOR, 0.69; Bonferroni adjusted P = .52). That study found no significant association between severe outcomes and anti-IL 12/23 (compared with anti-TNF monotherapy; aOR, 0.98; 95% CI, 0.12-8.06; P = .98) or anti-integrin biologics (compared with anti-TNF monotherapy; aOR, 2.42; 95% CI, 0.59-9.96; P = .22).
Overall, the data are “generally consistent with prior data on infections and IBD: That steroids and combination therapy increase the risk of infection and bad outcomes and that interestingly biologic monotherapy may actually confer a little bit of protection against emergent outcomes and at a minimum appears to be neutral,” said Dr. Ungaro.
He noted that the recommendations from the IOIBD COVID-19 Task Force were based on expert opinion, but the new data have largely supported them overall. He did suggest some potential modifications, including reducing thiopurine use among patients on combination therapy. According to Dr. Ungaro, the recommendations do call for withholding all IBD therapy for 10 days after positive SARS-CoV-2 tests, whether the patient is symptomatic or not. “I think the recent data is reassuring that potentially in asymptomatic and maybe even mild cases, the monotherapy biologics – we can consider not delaying administering those. I think we need more data about that, but it’s reassuring that patients on those had no worse outcomes and [in fact did] slightly better,” Dr. Ungaro said during the presentation.
The data reinforced the need to consider tapering patients off corticosteroids or combination therapies, if possible. “It’s something we were doing in regular IBD care beforehand, but the COVID-19 pandemic offers another reason to limit the use of steroids and evaluate if patients are able to de-escalate from combination therapies,” said Dr. Ungaro.
On the other hand, there was concern among some patients early in the pandemic that their immunotherapy drugs may put them at risk of contracting COVID-19, which led some to discontinue medications. Ongoing studies are illustrating the problem with this, according to David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the congress’s organizing committee. “The data do not in general suggest you should do that to protect yourself. In fact, being on the therapies may have a better outcome. Patients always want to come off their therapies, [but] during the pandemic that is a risk not worth taking. Getting sick from your Crohn’s disease or colitis, when there are limited health care resources and, in some places, limited hospital beds and where the rescue therapy might include steroids, is a risky proposition. It’s not the time to do this,” said Dr. Rubin.
With respect to vaccines, it appears so far that there is no increased risk of adverse events associated with IBD. Patients who are on immunosuppressive drugs may experience a lower response to immunization, which has been seen with other vaccines. “The benefits likely outweigh the risks based on our prior experience with other vaccinations. It’s an area of ongoing study, but I do think we should recommend that our IBD patients get the COVID-19 vaccine, especially if they have risk factors for severe disease,” said Dr. Ungaro.
Dr. Ungaro is on the advisory board for Bristol-Myers Squibb, Janssen, Pfizer, and Takeda. He has received funding from AbbVie, Boehringer Ingelheim, Eli Lilly, and Pfizer. He has been a speaker or received consulting fees from AbbVie and Eli Lilly. Dr. Rubin is a consultant for Janssen, Pfizer, Takeda, and AbbVie.
This article was updated Jan. 27, 2021.
FROM THE CROHN’S & COLITIS CONGRESS
First monthly injectable HIV treatment approved by FDA
Cabenuva (cabotegravir and rilpivirine, a once-per-month injectable formulation) was approved by the Food and Drug Administration as a complete regimen for treatment of HIV-1 infection in adults. It is intended to replace current antiretroviral regimens in those patients who are virologically suppressed with no history of treatment failure and with no known or suspected resistance to either of the two component drugs.
Cabenuva is the first FDA-approved monthly injectable, complete regimen for HIV-infected adults, according to the agency’s announcement.
In addition, the FDA-approved Vocabria (cabotegravir, tablet formulation), a preparatory treatment intended to be taken in combination with oral rilpivirine (Edurant) for 1 month prior to starting treatment with Cabenuva to ensure the medications are well tolerated before switching to the extended-release injectable formulation. The FDA granted the approval of Cabenuva and Vocabria to ViiV Healthcare.
Cabotegravir is as an integrase strand transfer inhibitor that blocks HIV integrase by attaching to the active integrase site and inhibiting retroviral DNA integration, which is necessary in order for HIV to replicate. In contrast, rilpivirine acts as a diarylpyrimidine nonnucleoside reverse transcriptase inhibitor of HIV-1.
Approval of Cabenuva was based upon two randomized, open-label, controlled clinical trials in 1,182 HIV-infected adults who were virologically suppressed (HIV-1 RNA less than 50 copies/mL) before initiation of treatment with Cabenuva. The two pivotal phase three clinical studies were: Antiretroviral Therapy as Long-Acting Suppression (ATLAS; NCT02951052) and First Long-Acting Injectable Regimen (FLAIR; NCT02938520). Patients in both trials continued to show virologic suppression at the conclusion of each study, and no clinically relevant change from baseline in CD4+ cell counts was observed, according to the FDA announcement.
Adverse reactions with Cabenuva included injection-site reactions, fever, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. The FDA warned that Cabenuva should not be used if there is a known previous hypersensitivity reaction to cabotegravir or rilpivirine, or in patients who are not virally suppressed (HIV-1 RNA greater than 50 copies/mL).
Cabenuva and Vocabria were granted Fast Track and Priority Review designation by the FDA. Prescribing information for Cabenuva is available on the ViiV Healthcare website.
Cabenuva (cabotegravir and rilpivirine, a once-per-month injectable formulation) was approved by the Food and Drug Administration as a complete regimen for treatment of HIV-1 infection in adults. It is intended to replace current antiretroviral regimens in those patients who are virologically suppressed with no history of treatment failure and with no known or suspected resistance to either of the two component drugs.
Cabenuva is the first FDA-approved monthly injectable, complete regimen for HIV-infected adults, according to the agency’s announcement.
In addition, the FDA-approved Vocabria (cabotegravir, tablet formulation), a preparatory treatment intended to be taken in combination with oral rilpivirine (Edurant) for 1 month prior to starting treatment with Cabenuva to ensure the medications are well tolerated before switching to the extended-release injectable formulation. The FDA granted the approval of Cabenuva and Vocabria to ViiV Healthcare.
Cabotegravir is as an integrase strand transfer inhibitor that blocks HIV integrase by attaching to the active integrase site and inhibiting retroviral DNA integration, which is necessary in order for HIV to replicate. In contrast, rilpivirine acts as a diarylpyrimidine nonnucleoside reverse transcriptase inhibitor of HIV-1.
Approval of Cabenuva was based upon two randomized, open-label, controlled clinical trials in 1,182 HIV-infected adults who were virologically suppressed (HIV-1 RNA less than 50 copies/mL) before initiation of treatment with Cabenuva. The two pivotal phase three clinical studies were: Antiretroviral Therapy as Long-Acting Suppression (ATLAS; NCT02951052) and First Long-Acting Injectable Regimen (FLAIR; NCT02938520). Patients in both trials continued to show virologic suppression at the conclusion of each study, and no clinically relevant change from baseline in CD4+ cell counts was observed, according to the FDA announcement.
Adverse reactions with Cabenuva included injection-site reactions, fever, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. The FDA warned that Cabenuva should not be used if there is a known previous hypersensitivity reaction to cabotegravir or rilpivirine, or in patients who are not virally suppressed (HIV-1 RNA greater than 50 copies/mL).
Cabenuva and Vocabria were granted Fast Track and Priority Review designation by the FDA. Prescribing information for Cabenuva is available on the ViiV Healthcare website.
Cabenuva (cabotegravir and rilpivirine, a once-per-month injectable formulation) was approved by the Food and Drug Administration as a complete regimen for treatment of HIV-1 infection in adults. It is intended to replace current antiretroviral regimens in those patients who are virologically suppressed with no history of treatment failure and with no known or suspected resistance to either of the two component drugs.
Cabenuva is the first FDA-approved monthly injectable, complete regimen for HIV-infected adults, according to the agency’s announcement.
In addition, the FDA-approved Vocabria (cabotegravir, tablet formulation), a preparatory treatment intended to be taken in combination with oral rilpivirine (Edurant) for 1 month prior to starting treatment with Cabenuva to ensure the medications are well tolerated before switching to the extended-release injectable formulation. The FDA granted the approval of Cabenuva and Vocabria to ViiV Healthcare.
Cabotegravir is as an integrase strand transfer inhibitor that blocks HIV integrase by attaching to the active integrase site and inhibiting retroviral DNA integration, which is necessary in order for HIV to replicate. In contrast, rilpivirine acts as a diarylpyrimidine nonnucleoside reverse transcriptase inhibitor of HIV-1.
Approval of Cabenuva was based upon two randomized, open-label, controlled clinical trials in 1,182 HIV-infected adults who were virologically suppressed (HIV-1 RNA less than 50 copies/mL) before initiation of treatment with Cabenuva. The two pivotal phase three clinical studies were: Antiretroviral Therapy as Long-Acting Suppression (ATLAS; NCT02951052) and First Long-Acting Injectable Regimen (FLAIR; NCT02938520). Patients in both trials continued to show virologic suppression at the conclusion of each study, and no clinically relevant change from baseline in CD4+ cell counts was observed, according to the FDA announcement.
Adverse reactions with Cabenuva included injection-site reactions, fever, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. The FDA warned that Cabenuva should not be used if there is a known previous hypersensitivity reaction to cabotegravir or rilpivirine, or in patients who are not virally suppressed (HIV-1 RNA greater than 50 copies/mL).
Cabenuva and Vocabria were granted Fast Track and Priority Review designation by the FDA. Prescribing information for Cabenuva is available on the ViiV Healthcare website.
NEWS FROM THE FDA
ColCORONA: Colchicine reduces complications in outpatient COVID-19
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.
Full-dose anticoagulation reduces need for life support in COVID-19
Full-dose anticoagulation was superior to low, prophylactic doses in reducing the need for vital organ support such as ventilation in moderately ill patients hospitalized for COVID-19, according to a report released Jan. 22 by the National Institutes of Health (NIH).
“This is a major advance for patients hospitalized with COVID. Full dose of anticoagulation in these non-ICU patients improved outcomes and there’s a trend toward a reduction in mortality,” Judith Hochman, MD, director of the Cardiovascular Clinical Research Center at NYU Langone Medical Center, New York, said in an interview.
“We have treatments that are improving outcomes but not as many that reduce mortality, so we’re hopeful when the full dataset comes in that will be confirmed,” she said.
The observation of increased rates of blood clots and inflammation among COVID-19 patients, which can lead to complications such as lung failure, heart attack, and stroke, has given rise to various anticoagulant treatment protocols and a need for randomized data on routinely administering increased doses of anticoagulation to hospitalized patients.
Today’s top-line findings come from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – examining the safety and efficacy of full-dose anticoagulation to treat moderately ill or critically ill adults hospitalized with COVID-19 compared with a lower dose typically used to prevent blood clots in hospitalized patients.
In December 2020, all three trials paused enrollment of the critically ill subgroup after results showed that full-dose anticoagulation started in the intensive care unit (ICU) was not beneficial and may have been harmful in some patients.
Moderately ill patients with COVID-19, defined as those who did not require ICU care or organ support, made up 80% of participants at enrollment in the three trials, Dr. Hochman said.
Among more than 1,000 moderately ill patients reviewed as of the data cut with the data safety monitoring board, full doses of low molecular weight or unfractionated heparin were superior to low prophylactic doses for the primary endpoint of need for ventilation or other organ supportive interventions at 21 days after randomization.
This met the predefined threshold for 99% probability of superiority and recruitment was stopped, Dr. Hochman reported. “Obviously safety figured into this decision. The risk/benefit ratio was very clear.”
The results do not pertain to patients with a previous indication for anticoagulation, who were excluded from the trials.
Data from an additional 1,000 patients will be reviewed and the data published sometime in the next 2-3 months, she said.
With large numbers of COVID-19 patients requiring hospitalization, the outcomes could help reduce the overload on intensive care units around the world, the NIH noted.
The results also highlight the critical role of timing in the course of COVID-19.
“We believe that full anticoagulation is effective early in the disease course,” Dr. Hochman said. “Based on the results so far from these three platform trials, those that were very, very sick at the time of enrollment really didn’t benefit and we needed to have caught them at an earlier stage.
“It’s possible that the people in the ICU are just different and the minute they get sick they need the ICU; so we haven’t clearly demonstrated this time course and when to intervene, but that’s the implication of the findings.”
The question of even earlier treatment is being examined in the partner ACTIV-4B trial, which is enrolling patients with COVID-19 illness not requiring hospitalization and randomizing them to the direct oral anticoagulant apixaban or aspirin or placebo.
“It’s a very important trial and we really want to get the message out that patients should volunteer for it,” said Dr. Hochman, principal investigator of the ACTIV-4 trial.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The REMAP-CAP, ACTIV-4, and ATTACC study platforms span five continents in more than 300 hospitals and are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (United Kingdom), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this article first appeared on Medscape.com.
Full-dose anticoagulation was superior to low, prophylactic doses in reducing the need for vital organ support such as ventilation in moderately ill patients hospitalized for COVID-19, according to a report released Jan. 22 by the National Institutes of Health (NIH).
“This is a major advance for patients hospitalized with COVID. Full dose of anticoagulation in these non-ICU patients improved outcomes and there’s a trend toward a reduction in mortality,” Judith Hochman, MD, director of the Cardiovascular Clinical Research Center at NYU Langone Medical Center, New York, said in an interview.
“We have treatments that are improving outcomes but not as many that reduce mortality, so we’re hopeful when the full dataset comes in that will be confirmed,” she said.
The observation of increased rates of blood clots and inflammation among COVID-19 patients, which can lead to complications such as lung failure, heart attack, and stroke, has given rise to various anticoagulant treatment protocols and a need for randomized data on routinely administering increased doses of anticoagulation to hospitalized patients.
Today’s top-line findings come from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – examining the safety and efficacy of full-dose anticoagulation to treat moderately ill or critically ill adults hospitalized with COVID-19 compared with a lower dose typically used to prevent blood clots in hospitalized patients.
In December 2020, all three trials paused enrollment of the critically ill subgroup after results showed that full-dose anticoagulation started in the intensive care unit (ICU) was not beneficial and may have been harmful in some patients.
Moderately ill patients with COVID-19, defined as those who did not require ICU care or organ support, made up 80% of participants at enrollment in the three trials, Dr. Hochman said.
Among more than 1,000 moderately ill patients reviewed as of the data cut with the data safety monitoring board, full doses of low molecular weight or unfractionated heparin were superior to low prophylactic doses for the primary endpoint of need for ventilation or other organ supportive interventions at 21 days after randomization.
This met the predefined threshold for 99% probability of superiority and recruitment was stopped, Dr. Hochman reported. “Obviously safety figured into this decision. The risk/benefit ratio was very clear.”
The results do not pertain to patients with a previous indication for anticoagulation, who were excluded from the trials.
Data from an additional 1,000 patients will be reviewed and the data published sometime in the next 2-3 months, she said.
With large numbers of COVID-19 patients requiring hospitalization, the outcomes could help reduce the overload on intensive care units around the world, the NIH noted.
The results also highlight the critical role of timing in the course of COVID-19.
“We believe that full anticoagulation is effective early in the disease course,” Dr. Hochman said. “Based on the results so far from these three platform trials, those that were very, very sick at the time of enrollment really didn’t benefit and we needed to have caught them at an earlier stage.
“It’s possible that the people in the ICU are just different and the minute they get sick they need the ICU; so we haven’t clearly demonstrated this time course and when to intervene, but that’s the implication of the findings.”
The question of even earlier treatment is being examined in the partner ACTIV-4B trial, which is enrolling patients with COVID-19 illness not requiring hospitalization and randomizing them to the direct oral anticoagulant apixaban or aspirin or placebo.
“It’s a very important trial and we really want to get the message out that patients should volunteer for it,” said Dr. Hochman, principal investigator of the ACTIV-4 trial.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The REMAP-CAP, ACTIV-4, and ATTACC study platforms span five continents in more than 300 hospitals and are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (United Kingdom), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this article first appeared on Medscape.com.
Full-dose anticoagulation was superior to low, prophylactic doses in reducing the need for vital organ support such as ventilation in moderately ill patients hospitalized for COVID-19, according to a report released Jan. 22 by the National Institutes of Health (NIH).
“This is a major advance for patients hospitalized with COVID. Full dose of anticoagulation in these non-ICU patients improved outcomes and there’s a trend toward a reduction in mortality,” Judith Hochman, MD, director of the Cardiovascular Clinical Research Center at NYU Langone Medical Center, New York, said in an interview.
“We have treatments that are improving outcomes but not as many that reduce mortality, so we’re hopeful when the full dataset comes in that will be confirmed,” she said.
The observation of increased rates of blood clots and inflammation among COVID-19 patients, which can lead to complications such as lung failure, heart attack, and stroke, has given rise to various anticoagulant treatment protocols and a need for randomized data on routinely administering increased doses of anticoagulation to hospitalized patients.
Today’s top-line findings come from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – examining the safety and efficacy of full-dose anticoagulation to treat moderately ill or critically ill adults hospitalized with COVID-19 compared with a lower dose typically used to prevent blood clots in hospitalized patients.
In December 2020, all three trials paused enrollment of the critically ill subgroup after results showed that full-dose anticoagulation started in the intensive care unit (ICU) was not beneficial and may have been harmful in some patients.
Moderately ill patients with COVID-19, defined as those who did not require ICU care or organ support, made up 80% of participants at enrollment in the three trials, Dr. Hochman said.
Among more than 1,000 moderately ill patients reviewed as of the data cut with the data safety monitoring board, full doses of low molecular weight or unfractionated heparin were superior to low prophylactic doses for the primary endpoint of need for ventilation or other organ supportive interventions at 21 days after randomization.
This met the predefined threshold for 99% probability of superiority and recruitment was stopped, Dr. Hochman reported. “Obviously safety figured into this decision. The risk/benefit ratio was very clear.”
The results do not pertain to patients with a previous indication for anticoagulation, who were excluded from the trials.
Data from an additional 1,000 patients will be reviewed and the data published sometime in the next 2-3 months, she said.
With large numbers of COVID-19 patients requiring hospitalization, the outcomes could help reduce the overload on intensive care units around the world, the NIH noted.
The results also highlight the critical role of timing in the course of COVID-19.
“We believe that full anticoagulation is effective early in the disease course,” Dr. Hochman said. “Based on the results so far from these three platform trials, those that were very, very sick at the time of enrollment really didn’t benefit and we needed to have caught them at an earlier stage.
“It’s possible that the people in the ICU are just different and the minute they get sick they need the ICU; so we haven’t clearly demonstrated this time course and when to intervene, but that’s the implication of the findings.”
The question of even earlier treatment is being examined in the partner ACTIV-4B trial, which is enrolling patients with COVID-19 illness not requiring hospitalization and randomizing them to the direct oral anticoagulant apixaban or aspirin or placebo.
“It’s a very important trial and we really want to get the message out that patients should volunteer for it,” said Dr. Hochman, principal investigator of the ACTIV-4 trial.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The REMAP-CAP, ACTIV-4, and ATTACC study platforms span five continents in more than 300 hospitals and are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (United Kingdom), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this article first appeared on Medscape.com.
Controversy flares over ivermectin for COVID-19
The National Institutes of Health has dropped its recommendation against the inexpensive antiparasitic drug ivermectin for treatment of COVID-19, and the agency now advises it can’t recommend for or against its use, leaving the decision to physicians and their patients.
“Results from adequately powered, well-designed, and well-conducted clinical trials are needed to provide more specific, evidence-based guidance on the role of ivermectin for the treatment of COVID-19,” according to new NIH guidance released last week.
Passionate arguments have been waged for and against the drug’s use.
The NIH update disappointed members of the Front Line COVID-19 Critical Care Alliance (FLCCC), which outlined its case for endorsing ivermectin in a public statement Jan. 18. Point by point, the group of 10 physicians argued against each limitation that drove the NIH’s ruling.
The group’s members said that, although grateful the recommendation against the drug was dropped, a neutral approach is not acceptable as total U.S. deaths surpassed 400,000 since last spring – and currently approach 4,000 a day. Results from research are enough to support its use, and the drug will immediately save lives, they say.
“Patients do not have time to wait,” they write, “and we as health care providers in society do not have that time either.”
NIH, which in August had recommended against ivermectin’s use, invited the group to present evidence to its treatment guidance panel on Jan. 6 to detail the emerging science surrounding ivermectin. The group cited rapidly growing evidence of the drug’s effectiveness.
Pierre Kory, MD, president/cofounder of FLCCC and a pulmonary and critical care specialist at Aurora St. Luke’s Medical Center in Milwaukee, also spoke before a Senate panel on Dec. 8 in a widely shared impassioned video, touting ivermectin as a COVID-19 “miracle” drug, a term he said he doesn’t use lightly.
Dr. Kory pleaded with the NIH to consider the emerging data. “Please, I’m just asking that they review our manuscript,” he told the senators.
“We have immense amounts of data to show that ivermectin must be implemented and implemented now,” he said.
Some draw parallels to hydroxychloroquine
Critics have said there’s not enough data to institute a protocol, and some draw parallels to another repurposed drug – hydroxychloroquine (HCQ) – which was once considered a promising treatment for COVID-19, based on flawed and incomplete evidence, and now is not recommended.
Paul Sax, MD, a professor of medicine at Harvard and clinical director of the HIV program and division of infectious diseases at Brigham and Women’s Hospital in Boston, wrote in a blog post earlier this month in the New England Journal of Medicine Journal Watch that ivermectin has more robust evidence for it than HCQ ever did.
“But we’re not quite yet at the ‘practice changing’ level,” he writes. “Results from at least five randomized clinical trials are expected soon that might further inform the decision.”
He said the best argument for the drug is seen in this explanation of a meta-analysis of studies of between 100 and 500 patients by Andrew Hill, MD, with the department of pharmacology, University of Liverpool (England).
Dr. Sax advises against two biases in considering ivermectin. One is assuming that because HCQ failed, other antiparasitic drugs will too.
The second bias to avoid, he says, is discounting studies done in low- and middle-income countries because “they weren’t done in the right places.”
“That’s not just bias,” he says. “It’s also snobbery.”
Ivermectin has been approved by the U.S. Food and Drug Administration for treatment of onchocerciasis (river blindness) and strongyloidiasis, but is not FDA-approved for the treatment of any viral infection. It also is sometimes used to treat animals.
In dropping the recommendation against ivermectin, the NIH gave it the same neutral declaration as monoclonal antibodies and convalescent plasma.
Some physicians say they won’t prescribe it
Some physicians say they won’t be recommending it to their COVID-19 patients.
Amesh Adalja, MD, an infectious disease expert and senior scholar at the Johns Hopkins University Center for Health Security in Baltimore,said in an interview that the NIH update hasn’t changed his mind and he isn’t prescribing it for his patients.
He said although “there’s enough of a signal” that he would like to see more data, “we haven’t seen anything in terms of a really robust study.”
He noted that the Infectious Diseases Society of America has 15 recommendations for COVID-19 treatment “and not one of them has to do with ivermectin.”
He added, “It’s not enough to see if it works, but we need to see who it works in and when it works in them.”
He also acknowledged that “some prominent physicians” are recommending it.
Among them is Paul Marik, MD, endowed professor of medicine and chief of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk. A cofounder of FLCCC, Dr. Marik has championed ivermectin and developed a protocol for its use to prevent and treat COVID-19.
The data surrounding ivermectin have met with hope, criticism, and warnings.
Australian researchers published a study ahead of print in Antiviral Research that found ivermectin inhibited the replication of SARS-CoV-2 in a laboratory setting.
The study concluded that the drug resulted post infection in a 5,000-fold reduction in viral RNA at 48 hours. After that study, however, the FDA in April warned consumers not to self-medicate with ivermectin products intended for animals.
The NIH acknowledged that several randomized trials and retrospective studies of ivermectin use in patients with COVID-19 have now been published in peer-reviewed journals or on preprint servers.
“Some clinical studies showed no benefits or worsening of disease after ivermectin use, whereas others reported shorter time to resolution of disease manifestations attributed to COVID-19, greater reduction in inflammatory markers, shorter time to viral clearance, or lower mortality rates in patients who received ivermectin than in patients who received comparator drugs or placebo,” the NIH guidance reads.
The NIH acknowledges limitations: the studies have been small; doses of ivermectin have varied; some patients were taking other medications at the same time (including doxycycline, hydroxychloroquine, azithromycin, zinc, and corticosteroids, which may be potential confounders); and patients’ severity of COVID was not always clearly described in the studies.
Nasia Safdar, MD, medical director of infection prevention at the University of Wisconsin Hospital in Madison, told this news organization she agrees more research is needed before ivermectin is recommended by regulatory bodies for COVID-19.
That said, Dr. Safdar added, “in individual circumstances if a physician is confronted with a patient in dire straits and you’re not sure what to do, might you consider it? I think after a discussion with the patient, perhaps, but the level of evidence certainly doesn’t rise to the level of a policy.”
A downside of recommending a treatment without conclusive data, even if harm isn’t the primary concern, she said, is that supplies could dwindle for its intended use in other diseases. Also, premature approval can limit the robust research needed to see not only whether it works better for prevention or treatment, but also if it’s effective depending on patient populations and the severity of COVID-19.
Dr. Adalja and Dr. Safdar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The National Institutes of Health has dropped its recommendation against the inexpensive antiparasitic drug ivermectin for treatment of COVID-19, and the agency now advises it can’t recommend for or against its use, leaving the decision to physicians and their patients.
“Results from adequately powered, well-designed, and well-conducted clinical trials are needed to provide more specific, evidence-based guidance on the role of ivermectin for the treatment of COVID-19,” according to new NIH guidance released last week.
Passionate arguments have been waged for and against the drug’s use.
The NIH update disappointed members of the Front Line COVID-19 Critical Care Alliance (FLCCC), which outlined its case for endorsing ivermectin in a public statement Jan. 18. Point by point, the group of 10 physicians argued against each limitation that drove the NIH’s ruling.
The group’s members said that, although grateful the recommendation against the drug was dropped, a neutral approach is not acceptable as total U.S. deaths surpassed 400,000 since last spring – and currently approach 4,000 a day. Results from research are enough to support its use, and the drug will immediately save lives, they say.
“Patients do not have time to wait,” they write, “and we as health care providers in society do not have that time either.”
NIH, which in August had recommended against ivermectin’s use, invited the group to present evidence to its treatment guidance panel on Jan. 6 to detail the emerging science surrounding ivermectin. The group cited rapidly growing evidence of the drug’s effectiveness.
Pierre Kory, MD, president/cofounder of FLCCC and a pulmonary and critical care specialist at Aurora St. Luke’s Medical Center in Milwaukee, also spoke before a Senate panel on Dec. 8 in a widely shared impassioned video, touting ivermectin as a COVID-19 “miracle” drug, a term he said he doesn’t use lightly.
Dr. Kory pleaded with the NIH to consider the emerging data. “Please, I’m just asking that they review our manuscript,” he told the senators.
“We have immense amounts of data to show that ivermectin must be implemented and implemented now,” he said.
Some draw parallels to hydroxychloroquine
Critics have said there’s not enough data to institute a protocol, and some draw parallels to another repurposed drug – hydroxychloroquine (HCQ) – which was once considered a promising treatment for COVID-19, based on flawed and incomplete evidence, and now is not recommended.
Paul Sax, MD, a professor of medicine at Harvard and clinical director of the HIV program and division of infectious diseases at Brigham and Women’s Hospital in Boston, wrote in a blog post earlier this month in the New England Journal of Medicine Journal Watch that ivermectin has more robust evidence for it than HCQ ever did.
“But we’re not quite yet at the ‘practice changing’ level,” he writes. “Results from at least five randomized clinical trials are expected soon that might further inform the decision.”
He said the best argument for the drug is seen in this explanation of a meta-analysis of studies of between 100 and 500 patients by Andrew Hill, MD, with the department of pharmacology, University of Liverpool (England).
Dr. Sax advises against two biases in considering ivermectin. One is assuming that because HCQ failed, other antiparasitic drugs will too.
The second bias to avoid, he says, is discounting studies done in low- and middle-income countries because “they weren’t done in the right places.”
“That’s not just bias,” he says. “It’s also snobbery.”
Ivermectin has been approved by the U.S. Food and Drug Administration for treatment of onchocerciasis (river blindness) and strongyloidiasis, but is not FDA-approved for the treatment of any viral infection. It also is sometimes used to treat animals.
In dropping the recommendation against ivermectin, the NIH gave it the same neutral declaration as monoclonal antibodies and convalescent plasma.
Some physicians say they won’t prescribe it
Some physicians say they won’t be recommending it to their COVID-19 patients.
Amesh Adalja, MD, an infectious disease expert and senior scholar at the Johns Hopkins University Center for Health Security in Baltimore,said in an interview that the NIH update hasn’t changed his mind and he isn’t prescribing it for his patients.
He said although “there’s enough of a signal” that he would like to see more data, “we haven’t seen anything in terms of a really robust study.”
He noted that the Infectious Diseases Society of America has 15 recommendations for COVID-19 treatment “and not one of them has to do with ivermectin.”
He added, “It’s not enough to see if it works, but we need to see who it works in and when it works in them.”
He also acknowledged that “some prominent physicians” are recommending it.
Among them is Paul Marik, MD, endowed professor of medicine and chief of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk. A cofounder of FLCCC, Dr. Marik has championed ivermectin and developed a protocol for its use to prevent and treat COVID-19.
The data surrounding ivermectin have met with hope, criticism, and warnings.
Australian researchers published a study ahead of print in Antiviral Research that found ivermectin inhibited the replication of SARS-CoV-2 in a laboratory setting.
The study concluded that the drug resulted post infection in a 5,000-fold reduction in viral RNA at 48 hours. After that study, however, the FDA in April warned consumers not to self-medicate with ivermectin products intended for animals.
The NIH acknowledged that several randomized trials and retrospective studies of ivermectin use in patients with COVID-19 have now been published in peer-reviewed journals or on preprint servers.
“Some clinical studies showed no benefits or worsening of disease after ivermectin use, whereas others reported shorter time to resolution of disease manifestations attributed to COVID-19, greater reduction in inflammatory markers, shorter time to viral clearance, or lower mortality rates in patients who received ivermectin than in patients who received comparator drugs or placebo,” the NIH guidance reads.
The NIH acknowledges limitations: the studies have been small; doses of ivermectin have varied; some patients were taking other medications at the same time (including doxycycline, hydroxychloroquine, azithromycin, zinc, and corticosteroids, which may be potential confounders); and patients’ severity of COVID was not always clearly described in the studies.
Nasia Safdar, MD, medical director of infection prevention at the University of Wisconsin Hospital in Madison, told this news organization she agrees more research is needed before ivermectin is recommended by regulatory bodies for COVID-19.
That said, Dr. Safdar added, “in individual circumstances if a physician is confronted with a patient in dire straits and you’re not sure what to do, might you consider it? I think after a discussion with the patient, perhaps, but the level of evidence certainly doesn’t rise to the level of a policy.”
A downside of recommending a treatment without conclusive data, even if harm isn’t the primary concern, she said, is that supplies could dwindle for its intended use in other diseases. Also, premature approval can limit the robust research needed to see not only whether it works better for prevention or treatment, but also if it’s effective depending on patient populations and the severity of COVID-19.
Dr. Adalja and Dr. Safdar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The National Institutes of Health has dropped its recommendation against the inexpensive antiparasitic drug ivermectin for treatment of COVID-19, and the agency now advises it can’t recommend for or against its use, leaving the decision to physicians and their patients.
“Results from adequately powered, well-designed, and well-conducted clinical trials are needed to provide more specific, evidence-based guidance on the role of ivermectin for the treatment of COVID-19,” according to new NIH guidance released last week.
Passionate arguments have been waged for and against the drug’s use.
The NIH update disappointed members of the Front Line COVID-19 Critical Care Alliance (FLCCC), which outlined its case for endorsing ivermectin in a public statement Jan. 18. Point by point, the group of 10 physicians argued against each limitation that drove the NIH’s ruling.
The group’s members said that, although grateful the recommendation against the drug was dropped, a neutral approach is not acceptable as total U.S. deaths surpassed 400,000 since last spring – and currently approach 4,000 a day. Results from research are enough to support its use, and the drug will immediately save lives, they say.
“Patients do not have time to wait,” they write, “and we as health care providers in society do not have that time either.”
NIH, which in August had recommended against ivermectin’s use, invited the group to present evidence to its treatment guidance panel on Jan. 6 to detail the emerging science surrounding ivermectin. The group cited rapidly growing evidence of the drug’s effectiveness.
Pierre Kory, MD, president/cofounder of FLCCC and a pulmonary and critical care specialist at Aurora St. Luke’s Medical Center in Milwaukee, also spoke before a Senate panel on Dec. 8 in a widely shared impassioned video, touting ivermectin as a COVID-19 “miracle” drug, a term he said he doesn’t use lightly.
Dr. Kory pleaded with the NIH to consider the emerging data. “Please, I’m just asking that they review our manuscript,” he told the senators.
“We have immense amounts of data to show that ivermectin must be implemented and implemented now,” he said.
Some draw parallels to hydroxychloroquine
Critics have said there’s not enough data to institute a protocol, and some draw parallels to another repurposed drug – hydroxychloroquine (HCQ) – which was once considered a promising treatment for COVID-19, based on flawed and incomplete evidence, and now is not recommended.
Paul Sax, MD, a professor of medicine at Harvard and clinical director of the HIV program and division of infectious diseases at Brigham and Women’s Hospital in Boston, wrote in a blog post earlier this month in the New England Journal of Medicine Journal Watch that ivermectin has more robust evidence for it than HCQ ever did.
“But we’re not quite yet at the ‘practice changing’ level,” he writes. “Results from at least five randomized clinical trials are expected soon that might further inform the decision.”
He said the best argument for the drug is seen in this explanation of a meta-analysis of studies of between 100 and 500 patients by Andrew Hill, MD, with the department of pharmacology, University of Liverpool (England).
Dr. Sax advises against two biases in considering ivermectin. One is assuming that because HCQ failed, other antiparasitic drugs will too.
The second bias to avoid, he says, is discounting studies done in low- and middle-income countries because “they weren’t done in the right places.”
“That’s not just bias,” he says. “It’s also snobbery.”
Ivermectin has been approved by the U.S. Food and Drug Administration for treatment of onchocerciasis (river blindness) and strongyloidiasis, but is not FDA-approved for the treatment of any viral infection. It also is sometimes used to treat animals.
In dropping the recommendation against ivermectin, the NIH gave it the same neutral declaration as monoclonal antibodies and convalescent plasma.
Some physicians say they won’t prescribe it
Some physicians say they won’t be recommending it to their COVID-19 patients.
Amesh Adalja, MD, an infectious disease expert and senior scholar at the Johns Hopkins University Center for Health Security in Baltimore,said in an interview that the NIH update hasn’t changed his mind and he isn’t prescribing it for his patients.
He said although “there’s enough of a signal” that he would like to see more data, “we haven’t seen anything in terms of a really robust study.”
He noted that the Infectious Diseases Society of America has 15 recommendations for COVID-19 treatment “and not one of them has to do with ivermectin.”
He added, “It’s not enough to see if it works, but we need to see who it works in and when it works in them.”
He also acknowledged that “some prominent physicians” are recommending it.
Among them is Paul Marik, MD, endowed professor of medicine and chief of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk. A cofounder of FLCCC, Dr. Marik has championed ivermectin and developed a protocol for its use to prevent and treat COVID-19.
The data surrounding ivermectin have met with hope, criticism, and warnings.
Australian researchers published a study ahead of print in Antiviral Research that found ivermectin inhibited the replication of SARS-CoV-2 in a laboratory setting.
The study concluded that the drug resulted post infection in a 5,000-fold reduction in viral RNA at 48 hours. After that study, however, the FDA in April warned consumers not to self-medicate with ivermectin products intended for animals.
The NIH acknowledged that several randomized trials and retrospective studies of ivermectin use in patients with COVID-19 have now been published in peer-reviewed journals or on preprint servers.
“Some clinical studies showed no benefits or worsening of disease after ivermectin use, whereas others reported shorter time to resolution of disease manifestations attributed to COVID-19, greater reduction in inflammatory markers, shorter time to viral clearance, or lower mortality rates in patients who received ivermectin than in patients who received comparator drugs or placebo,” the NIH guidance reads.
The NIH acknowledges limitations: the studies have been small; doses of ivermectin have varied; some patients were taking other medications at the same time (including doxycycline, hydroxychloroquine, azithromycin, zinc, and corticosteroids, which may be potential confounders); and patients’ severity of COVID was not always clearly described in the studies.
Nasia Safdar, MD, medical director of infection prevention at the University of Wisconsin Hospital in Madison, told this news organization she agrees more research is needed before ivermectin is recommended by regulatory bodies for COVID-19.
That said, Dr. Safdar added, “in individual circumstances if a physician is confronted with a patient in dire straits and you’re not sure what to do, might you consider it? I think after a discussion with the patient, perhaps, but the level of evidence certainly doesn’t rise to the level of a policy.”
A downside of recommending a treatment without conclusive data, even if harm isn’t the primary concern, she said, is that supplies could dwindle for its intended use in other diseases. Also, premature approval can limit the robust research needed to see not only whether it works better for prevention or treatment, but also if it’s effective depending on patient populations and the severity of COVID-19.
Dr. Adalja and Dr. Safdar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monoclonal antibody combo treatment reduces viral load in mild to moderate COVID-19
A combination treatment of neutralizing monoclonal antibodies bamlanivimab and etesevimab was associated with a statistically significant reduction in SARS-CoV-2 at day 11 compared with placebo among nonhospitalized patients who had mild to moderate COVID-19, new data indicate.
However, bamlanivimab alone in three different single-infusion doses showed no significant reduction in viral load, compared with placebo, according to the phase 2/3 study by Robert L. Gottlieb, MD, PhD, of the Baylor University Medical Center and the Baylor Scott & White Research Institute, both in Dallas, and colleagues.
Findings from the Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) study were published online Jan. 21 in JAMA. The results represent findings through Oct. 6, 2020.
BLAZE-1 was funded by Eli Lilly, which makes both of the antispike neutralizing antibodies. The trial was conducted at 49 U.S. centers and included 613 outpatients who tested positive for SARS-CoV-2 and had one or more mild to moderate symptoms.
Patients were randomized to one of five groups (four treatment groups and a placebo control), and researchers analyzed between-group differences.
All four treatment arms suggest a trend toward reduction in viral load, which was the primary endpoint of the trial, but only the combination showed a statistically significant reduction.
The average age of patients was 44.7 years, 54.6% were female, 42.5% were Hispanic, and 67.1% had at least one risk factor for severe COVID-19 (aged ≥55 years, body mass index of at least 30, or relevant comorbidity such as hypertension).
Among secondary outcomes, there were no consistent differences between the monotherapy groups or the combination group versus placebo for the other measures of viral load or clinical symptom scores.
The proportion of patients who had COVID-19–related hospitalizations or ED visits was 5.8% (nine events) for placebo; 1.0% (one event) for the 700-mg group; 1.9% (two events) for 2,800 mg; 2.0% (two events) for 7,000 mg; and 0.9% (one event) for combination treatment.
“Combining these two neutralizing monoclonal antibodies in clinical use may enhance viral load reduction and decrease treatment-emergent resistant variants,” the authors concluded.
Safety profile comparison
As for adverse events, immediate hypersensitivity reactions were reported in nine patients (six bamlanivimab, two combination treatment, and one placebo). No deaths occurred during the study.
Serious adverse events unrelated to SARS-CoV-2 infection or considered related to the study drug occurred in 0% (0/309) of patients in the bamlanivimab monotherapy groups; in 0.9% (1/112) of patients in the combination group; and in 0.6% (1/156) of patients in the placebo group.
The serious adverse event in the combination group was a urinary tract infection deemed unrelated to the study drug, the authors wrote.
The two most frequently reported side effects were nausea (3.0% for the 700-mg group; 3.7% for the 2,800-mg group; 5.0% for the 7,000-mg group; 3.6% for the combination group; and 3.8% for the placebo group) and diarrhea (1.0%, 1.9%, 5.9%, 0.9%, and 4.5%, respectively).
The authors included in the study’s limitations that the primary endpoint at day 11 may have been too late to best detect treatment effects.
“All patients, including those who received placebo, demonstrated substantial viral reduction by day 11,” they noted. “An earlier time point like day 3 or day 7 could possibly have been more appropriate to measure viral load.”
Currently, only remdesivir has been approved by the Food and Drug Administration for treating COVID-19, but convalescent plasma and neutralizing monoclonal antibodies have been granted emergency-use authorization.
In an accompanying editor’s note, Preeti N. Malani, MD, with the division of infectious diseases at the University of Michigan, Ann Arbor, and associate editor of JAMA, and Robert M. Golub, MD, deputy editor of JAMA, pointed out that these results differ from an earlier interim analysis of BLAZE-1 data.
A previous publication by Peter Chen, MD, with the department of medicine at Cedars Sinai Medical Center, Los Angeles, compared the three monotherapy groups (no combination group) with placebo, and in that study the 2,800-mg dose of bamlanivimab versus placebo achieved statistical significance for reduction in viral load from baseline at day 11, whereas the other two doses did not.
The editors explain that, in the study by Dr. Chen, “Follow-up for the placebo group was incomplete at the time of the database lock on Sept. 5, 2020. In the final analysis reported in the current article, the database was locked on Oct. 6, 2020, and the longer follow-up for the placebo group, which is now complete, resulted in changes in the primary outcome among that group.”
They concluded: “The comparison of the monotherapy groups against the final results for the placebo group led to changes in the effect sizes,” and the statistical significance of the 2,800-mg group was erased.
The editors pointed out that monoclonal antibodies are likely to benefit certain patients but definitive answers regarding which patients will benefit and under what circumstances will likely take more time than clinicians have to make decisions on treatment.
Meanwhile, as this news organization reported, the United States has spent $375 million on bamlanivimab and $450 million on Regeneron’s monoclonal antibody cocktail of casirivimab plus imdevimab, with the promise to spend billions more.
However, 80% of the 660,000 doses delivered by the two companies are still sitting on shelves, federal officials said in a press briefing last week, because of doubts about efficacy, lack of resources for infusion centers, and questions on reimbursement.
“While the world waits for widespread administration of effective vaccines and additional data on treatments, local efforts should work to improve testing access and turnaround time and reduce logistical barriers to ensure that monoclonal therapies can be provided to patients who are most likely to benefit,” Dr. Malani and Dr. Golub wrote.
This trial was sponsored and funded by Eli Lilly. Dr. Gottlieb disclosed personal fees and nonfinancial support (medication for another trial) from Gilead Sciences and serving on an advisory board for Sentinel. Several coauthors have financial ties to Eli Lilly. Dr. Malani reported serving on the National Institute of Allergy and Infectious Diseases COVID-19 Preventive Monoclonal Antibody data and safety monitoring board but was not compensated. Dr. Golub disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A combination treatment of neutralizing monoclonal antibodies bamlanivimab and etesevimab was associated with a statistically significant reduction in SARS-CoV-2 at day 11 compared with placebo among nonhospitalized patients who had mild to moderate COVID-19, new data indicate.
However, bamlanivimab alone in three different single-infusion doses showed no significant reduction in viral load, compared with placebo, according to the phase 2/3 study by Robert L. Gottlieb, MD, PhD, of the Baylor University Medical Center and the Baylor Scott & White Research Institute, both in Dallas, and colleagues.
Findings from the Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) study were published online Jan. 21 in JAMA. The results represent findings through Oct. 6, 2020.
BLAZE-1 was funded by Eli Lilly, which makes both of the antispike neutralizing antibodies. The trial was conducted at 49 U.S. centers and included 613 outpatients who tested positive for SARS-CoV-2 and had one or more mild to moderate symptoms.
Patients were randomized to one of five groups (four treatment groups and a placebo control), and researchers analyzed between-group differences.
All four treatment arms suggest a trend toward reduction in viral load, which was the primary endpoint of the trial, but only the combination showed a statistically significant reduction.
The average age of patients was 44.7 years, 54.6% were female, 42.5% were Hispanic, and 67.1% had at least one risk factor for severe COVID-19 (aged ≥55 years, body mass index of at least 30, or relevant comorbidity such as hypertension).
Among secondary outcomes, there were no consistent differences between the monotherapy groups or the combination group versus placebo for the other measures of viral load or clinical symptom scores.
The proportion of patients who had COVID-19–related hospitalizations or ED visits was 5.8% (nine events) for placebo; 1.0% (one event) for the 700-mg group; 1.9% (two events) for 2,800 mg; 2.0% (two events) for 7,000 mg; and 0.9% (one event) for combination treatment.
“Combining these two neutralizing monoclonal antibodies in clinical use may enhance viral load reduction and decrease treatment-emergent resistant variants,” the authors concluded.
Safety profile comparison
As for adverse events, immediate hypersensitivity reactions were reported in nine patients (six bamlanivimab, two combination treatment, and one placebo). No deaths occurred during the study.
Serious adverse events unrelated to SARS-CoV-2 infection or considered related to the study drug occurred in 0% (0/309) of patients in the bamlanivimab monotherapy groups; in 0.9% (1/112) of patients in the combination group; and in 0.6% (1/156) of patients in the placebo group.
The serious adverse event in the combination group was a urinary tract infection deemed unrelated to the study drug, the authors wrote.
The two most frequently reported side effects were nausea (3.0% for the 700-mg group; 3.7% for the 2,800-mg group; 5.0% for the 7,000-mg group; 3.6% for the combination group; and 3.8% for the placebo group) and diarrhea (1.0%, 1.9%, 5.9%, 0.9%, and 4.5%, respectively).
The authors included in the study’s limitations that the primary endpoint at day 11 may have been too late to best detect treatment effects.
“All patients, including those who received placebo, demonstrated substantial viral reduction by day 11,” they noted. “An earlier time point like day 3 or day 7 could possibly have been more appropriate to measure viral load.”
Currently, only remdesivir has been approved by the Food and Drug Administration for treating COVID-19, but convalescent plasma and neutralizing monoclonal antibodies have been granted emergency-use authorization.
In an accompanying editor’s note, Preeti N. Malani, MD, with the division of infectious diseases at the University of Michigan, Ann Arbor, and associate editor of JAMA, and Robert M. Golub, MD, deputy editor of JAMA, pointed out that these results differ from an earlier interim analysis of BLAZE-1 data.
A previous publication by Peter Chen, MD, with the department of medicine at Cedars Sinai Medical Center, Los Angeles, compared the three monotherapy groups (no combination group) with placebo, and in that study the 2,800-mg dose of bamlanivimab versus placebo achieved statistical significance for reduction in viral load from baseline at day 11, whereas the other two doses did not.
The editors explain that, in the study by Dr. Chen, “Follow-up for the placebo group was incomplete at the time of the database lock on Sept. 5, 2020. In the final analysis reported in the current article, the database was locked on Oct. 6, 2020, and the longer follow-up for the placebo group, which is now complete, resulted in changes in the primary outcome among that group.”
They concluded: “The comparison of the monotherapy groups against the final results for the placebo group led to changes in the effect sizes,” and the statistical significance of the 2,800-mg group was erased.
The editors pointed out that monoclonal antibodies are likely to benefit certain patients but definitive answers regarding which patients will benefit and under what circumstances will likely take more time than clinicians have to make decisions on treatment.
Meanwhile, as this news organization reported, the United States has spent $375 million on bamlanivimab and $450 million on Regeneron’s monoclonal antibody cocktail of casirivimab plus imdevimab, with the promise to spend billions more.
However, 80% of the 660,000 doses delivered by the two companies are still sitting on shelves, federal officials said in a press briefing last week, because of doubts about efficacy, lack of resources for infusion centers, and questions on reimbursement.
“While the world waits for widespread administration of effective vaccines and additional data on treatments, local efforts should work to improve testing access and turnaround time and reduce logistical barriers to ensure that monoclonal therapies can be provided to patients who are most likely to benefit,” Dr. Malani and Dr. Golub wrote.
This trial was sponsored and funded by Eli Lilly. Dr. Gottlieb disclosed personal fees and nonfinancial support (medication for another trial) from Gilead Sciences and serving on an advisory board for Sentinel. Several coauthors have financial ties to Eli Lilly. Dr. Malani reported serving on the National Institute of Allergy and Infectious Diseases COVID-19 Preventive Monoclonal Antibody data and safety monitoring board but was not compensated. Dr. Golub disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A combination treatment of neutralizing monoclonal antibodies bamlanivimab and etesevimab was associated with a statistically significant reduction in SARS-CoV-2 at day 11 compared with placebo among nonhospitalized patients who had mild to moderate COVID-19, new data indicate.
However, bamlanivimab alone in three different single-infusion doses showed no significant reduction in viral load, compared with placebo, according to the phase 2/3 study by Robert L. Gottlieb, MD, PhD, of the Baylor University Medical Center and the Baylor Scott & White Research Institute, both in Dallas, and colleagues.
Findings from the Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) study were published online Jan. 21 in JAMA. The results represent findings through Oct. 6, 2020.
BLAZE-1 was funded by Eli Lilly, which makes both of the antispike neutralizing antibodies. The trial was conducted at 49 U.S. centers and included 613 outpatients who tested positive for SARS-CoV-2 and had one or more mild to moderate symptoms.
Patients were randomized to one of five groups (four treatment groups and a placebo control), and researchers analyzed between-group differences.
All four treatment arms suggest a trend toward reduction in viral load, which was the primary endpoint of the trial, but only the combination showed a statistically significant reduction.
The average age of patients was 44.7 years, 54.6% were female, 42.5% were Hispanic, and 67.1% had at least one risk factor for severe COVID-19 (aged ≥55 years, body mass index of at least 30, or relevant comorbidity such as hypertension).
Among secondary outcomes, there were no consistent differences between the monotherapy groups or the combination group versus placebo for the other measures of viral load or clinical symptom scores.
The proportion of patients who had COVID-19–related hospitalizations or ED visits was 5.8% (nine events) for placebo; 1.0% (one event) for the 700-mg group; 1.9% (two events) for 2,800 mg; 2.0% (two events) for 7,000 mg; and 0.9% (one event) for combination treatment.
“Combining these two neutralizing monoclonal antibodies in clinical use may enhance viral load reduction and decrease treatment-emergent resistant variants,” the authors concluded.
Safety profile comparison
As for adverse events, immediate hypersensitivity reactions were reported in nine patients (six bamlanivimab, two combination treatment, and one placebo). No deaths occurred during the study.
Serious adverse events unrelated to SARS-CoV-2 infection or considered related to the study drug occurred in 0% (0/309) of patients in the bamlanivimab monotherapy groups; in 0.9% (1/112) of patients in the combination group; and in 0.6% (1/156) of patients in the placebo group.
The serious adverse event in the combination group was a urinary tract infection deemed unrelated to the study drug, the authors wrote.
The two most frequently reported side effects were nausea (3.0% for the 700-mg group; 3.7% for the 2,800-mg group; 5.0% for the 7,000-mg group; 3.6% for the combination group; and 3.8% for the placebo group) and diarrhea (1.0%, 1.9%, 5.9%, 0.9%, and 4.5%, respectively).
The authors included in the study’s limitations that the primary endpoint at day 11 may have been too late to best detect treatment effects.
“All patients, including those who received placebo, demonstrated substantial viral reduction by day 11,” they noted. “An earlier time point like day 3 or day 7 could possibly have been more appropriate to measure viral load.”
Currently, only remdesivir has been approved by the Food and Drug Administration for treating COVID-19, but convalescent plasma and neutralizing monoclonal antibodies have been granted emergency-use authorization.
In an accompanying editor’s note, Preeti N. Malani, MD, with the division of infectious diseases at the University of Michigan, Ann Arbor, and associate editor of JAMA, and Robert M. Golub, MD, deputy editor of JAMA, pointed out that these results differ from an earlier interim analysis of BLAZE-1 data.
A previous publication by Peter Chen, MD, with the department of medicine at Cedars Sinai Medical Center, Los Angeles, compared the three monotherapy groups (no combination group) with placebo, and in that study the 2,800-mg dose of bamlanivimab versus placebo achieved statistical significance for reduction in viral load from baseline at day 11, whereas the other two doses did not.
The editors explain that, in the study by Dr. Chen, “Follow-up for the placebo group was incomplete at the time of the database lock on Sept. 5, 2020. In the final analysis reported in the current article, the database was locked on Oct. 6, 2020, and the longer follow-up for the placebo group, which is now complete, resulted in changes in the primary outcome among that group.”
They concluded: “The comparison of the monotherapy groups against the final results for the placebo group led to changes in the effect sizes,” and the statistical significance of the 2,800-mg group was erased.
The editors pointed out that monoclonal antibodies are likely to benefit certain patients but definitive answers regarding which patients will benefit and under what circumstances will likely take more time than clinicians have to make decisions on treatment.
Meanwhile, as this news organization reported, the United States has spent $375 million on bamlanivimab and $450 million on Regeneron’s monoclonal antibody cocktail of casirivimab plus imdevimab, with the promise to spend billions more.
However, 80% of the 660,000 doses delivered by the two companies are still sitting on shelves, federal officials said in a press briefing last week, because of doubts about efficacy, lack of resources for infusion centers, and questions on reimbursement.
“While the world waits for widespread administration of effective vaccines and additional data on treatments, local efforts should work to improve testing access and turnaround time and reduce logistical barriers to ensure that monoclonal therapies can be provided to patients who are most likely to benefit,” Dr. Malani and Dr. Golub wrote.
This trial was sponsored and funded by Eli Lilly. Dr. Gottlieb disclosed personal fees and nonfinancial support (medication for another trial) from Gilead Sciences and serving on an advisory board for Sentinel. Several coauthors have financial ties to Eli Lilly. Dr. Malani reported serving on the National Institute of Allergy and Infectious Diseases COVID-19 Preventive Monoclonal Antibody data and safety monitoring board but was not compensated. Dr. Golub disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.