User login
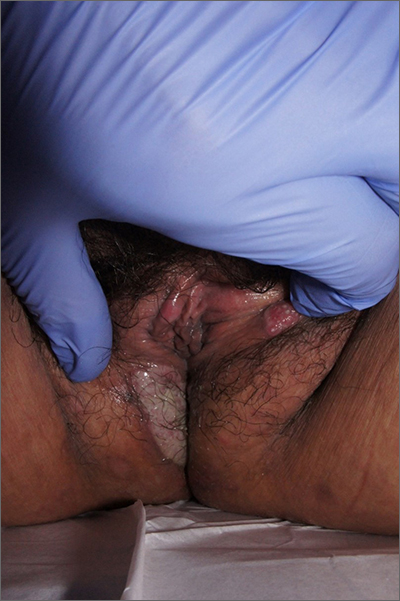
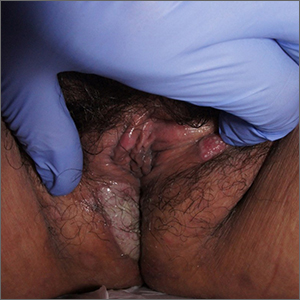
Labial growth
White-to-pink friable plaques occurring acutely in the vulva is concerning for one form of secondary syphilis that affects mucous membranes: condyloma lata.
Known as the great imitator for its variety of clinical presentations, syphilis is a sexually transmitted infection (STI) caused by the spirochete Treponema pallidum. Three to 10 days following contact with the spirochete, a painless ulcer or chancre forms and subsequently resolves—sometimes without notice.
Secondary syphilis develops from hematogenous spread of bacteria taking many forms—most commonly a widespread rash over the whole body of many (although sometimes faint) macules or papules up to about 1 cm in size and haphazardly spread out about every 1 cm. Palms and soles may be affected, even if faintly. Another, less common form of secondary syphilis includes the friable plaques (often in the anogenital area, as pictured) that are highly concentrated with bacteria. These occur 3 to 12 weeks after the appearance of a primary chancre and are variably symptomatic.
The differential diagnosis includes genital warts, vulvar carcinoma, and pemphigus vegetans. The relatively rapid, multifocal presentation helps to separate this disorder from vulvar carcinoma. A biopsy can distinguish the 2. However, diagnosis is better made with serology using nontreponemal tests, such as the rapid plasma reagin (RPR) test. Treponemal tests (assaying immunoglobulin [Ig]M and IgG to Treponema pallidum) are also an option and are very specific. Following this, an RPR titer can help guide treatment. Darkfield microscopy, which can reveal spirochetes directly, isn’t readily available but could be used to diagnose condyloma lata.
Patients who have been given a diagnosis of syphilis should be offered screening for other STIs, including HIV. Anyone who has had sexual contact with the patient within the previous 90 days should be notified, tested, and treated. Patients with primary or secondary syphilis should be treated with 2.4 million units of intramuscular (IM) benzathine penicillin G in a single dose—regardless of whether they test positive for HIV. To exclude tertiary syphilis, a careful neurologic exam should take place at the time of diagnosis and again 6 and 12 months after treatment (sooner if follow-up may be uncertain). Consider treatment failure if RPR titers haven’t fallen fourfold in 12 months. In 2022, the Centers for Disease Control and Prevention released a notice that COVID-19-vaccinated patients may have false-positive RPR titers performed from Bio-Rad Laboratories (BioPlex 2200 Syphilis Total & RPR kit).1
In this case, the patient tested positive for treponemal antibodies and had an RPR titer of 1:128. She was treated with IM benzathine penicillin with lasting clearance.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Centers for Disease Control and Prevention. Sexually Transmitted Infection Treatment Guidelines, 2021. Reviewed December 22, 2021. Accessed February 25, 2022. www.cdc.gov/std/treatment-guidelines/syphilis.htm
White-to-pink friable plaques occurring acutely in the vulva is concerning for one form of secondary syphilis that affects mucous membranes: condyloma lata.
Known as the great imitator for its variety of clinical presentations, syphilis is a sexually transmitted infection (STI) caused by the spirochete Treponema pallidum. Three to 10 days following contact with the spirochete, a painless ulcer or chancre forms and subsequently resolves—sometimes without notice.
Secondary syphilis develops from hematogenous spread of bacteria taking many forms—most commonly a widespread rash over the whole body of many (although sometimes faint) macules or papules up to about 1 cm in size and haphazardly spread out about every 1 cm. Palms and soles may be affected, even if faintly. Another, less common form of secondary syphilis includes the friable plaques (often in the anogenital area, as pictured) that are highly concentrated with bacteria. These occur 3 to 12 weeks after the appearance of a primary chancre and are variably symptomatic.
The differential diagnosis includes genital warts, vulvar carcinoma, and pemphigus vegetans. The relatively rapid, multifocal presentation helps to separate this disorder from vulvar carcinoma. A biopsy can distinguish the 2. However, diagnosis is better made with serology using nontreponemal tests, such as the rapid plasma reagin (RPR) test. Treponemal tests (assaying immunoglobulin [Ig]M and IgG to Treponema pallidum) are also an option and are very specific. Following this, an RPR titer can help guide treatment. Darkfield microscopy, which can reveal spirochetes directly, isn’t readily available but could be used to diagnose condyloma lata.
Patients who have been given a diagnosis of syphilis should be offered screening for other STIs, including HIV. Anyone who has had sexual contact with the patient within the previous 90 days should be notified, tested, and treated. Patients with primary or secondary syphilis should be treated with 2.4 million units of intramuscular (IM) benzathine penicillin G in a single dose—regardless of whether they test positive for HIV. To exclude tertiary syphilis, a careful neurologic exam should take place at the time of diagnosis and again 6 and 12 months after treatment (sooner if follow-up may be uncertain). Consider treatment failure if RPR titers haven’t fallen fourfold in 12 months. In 2022, the Centers for Disease Control and Prevention released a notice that COVID-19-vaccinated patients may have false-positive RPR titers performed from Bio-Rad Laboratories (BioPlex 2200 Syphilis Total & RPR kit).1
In this case, the patient tested positive for treponemal antibodies and had an RPR titer of 1:128. She was treated with IM benzathine penicillin with lasting clearance.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
White-to-pink friable plaques occurring acutely in the vulva is concerning for one form of secondary syphilis that affects mucous membranes: condyloma lata.
Known as the great imitator for its variety of clinical presentations, syphilis is a sexually transmitted infection (STI) caused by the spirochete Treponema pallidum. Three to 10 days following contact with the spirochete, a painless ulcer or chancre forms and subsequently resolves—sometimes without notice.
Secondary syphilis develops from hematogenous spread of bacteria taking many forms—most commonly a widespread rash over the whole body of many (although sometimes faint) macules or papules up to about 1 cm in size and haphazardly spread out about every 1 cm. Palms and soles may be affected, even if faintly. Another, less common form of secondary syphilis includes the friable plaques (often in the anogenital area, as pictured) that are highly concentrated with bacteria. These occur 3 to 12 weeks after the appearance of a primary chancre and are variably symptomatic.
The differential diagnosis includes genital warts, vulvar carcinoma, and pemphigus vegetans. The relatively rapid, multifocal presentation helps to separate this disorder from vulvar carcinoma. A biopsy can distinguish the 2. However, diagnosis is better made with serology using nontreponemal tests, such as the rapid plasma reagin (RPR) test. Treponemal tests (assaying immunoglobulin [Ig]M and IgG to Treponema pallidum) are also an option and are very specific. Following this, an RPR titer can help guide treatment. Darkfield microscopy, which can reveal spirochetes directly, isn’t readily available but could be used to diagnose condyloma lata.
Patients who have been given a diagnosis of syphilis should be offered screening for other STIs, including HIV. Anyone who has had sexual contact with the patient within the previous 90 days should be notified, tested, and treated. Patients with primary or secondary syphilis should be treated with 2.4 million units of intramuscular (IM) benzathine penicillin G in a single dose—regardless of whether they test positive for HIV. To exclude tertiary syphilis, a careful neurologic exam should take place at the time of diagnosis and again 6 and 12 months after treatment (sooner if follow-up may be uncertain). Consider treatment failure if RPR titers haven’t fallen fourfold in 12 months. In 2022, the Centers for Disease Control and Prevention released a notice that COVID-19-vaccinated patients may have false-positive RPR titers performed from Bio-Rad Laboratories (BioPlex 2200 Syphilis Total & RPR kit).1
In this case, the patient tested positive for treponemal antibodies and had an RPR titer of 1:128. She was treated with IM benzathine penicillin with lasting clearance.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Centers for Disease Control and Prevention. Sexually Transmitted Infection Treatment Guidelines, 2021. Reviewed December 22, 2021. Accessed February 25, 2022. www.cdc.gov/std/treatment-guidelines/syphilis.htm
1. Centers for Disease Control and Prevention. Sexually Transmitted Infection Treatment Guidelines, 2021. Reviewed December 22, 2021. Accessed February 25, 2022. www.cdc.gov/std/treatment-guidelines/syphilis.htm
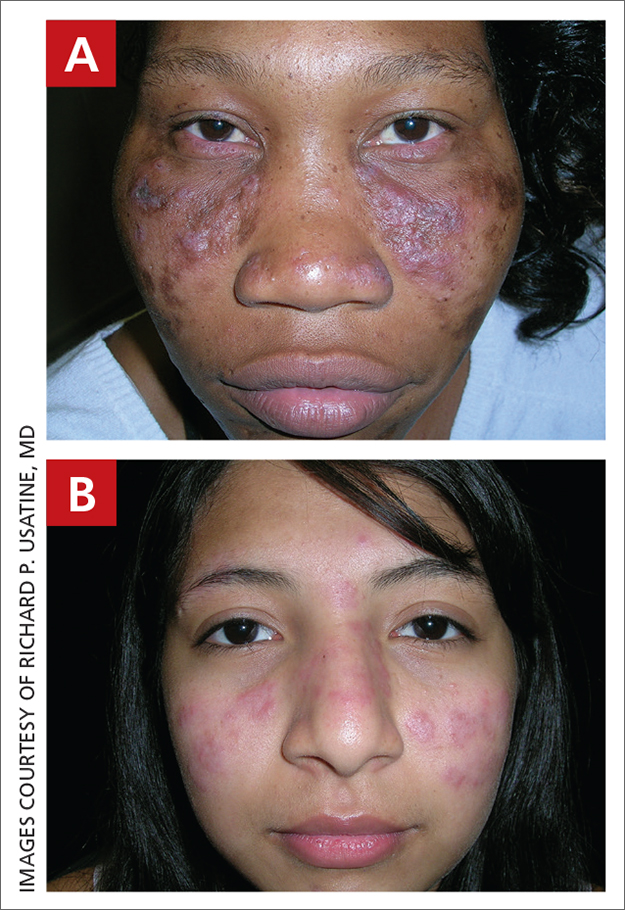
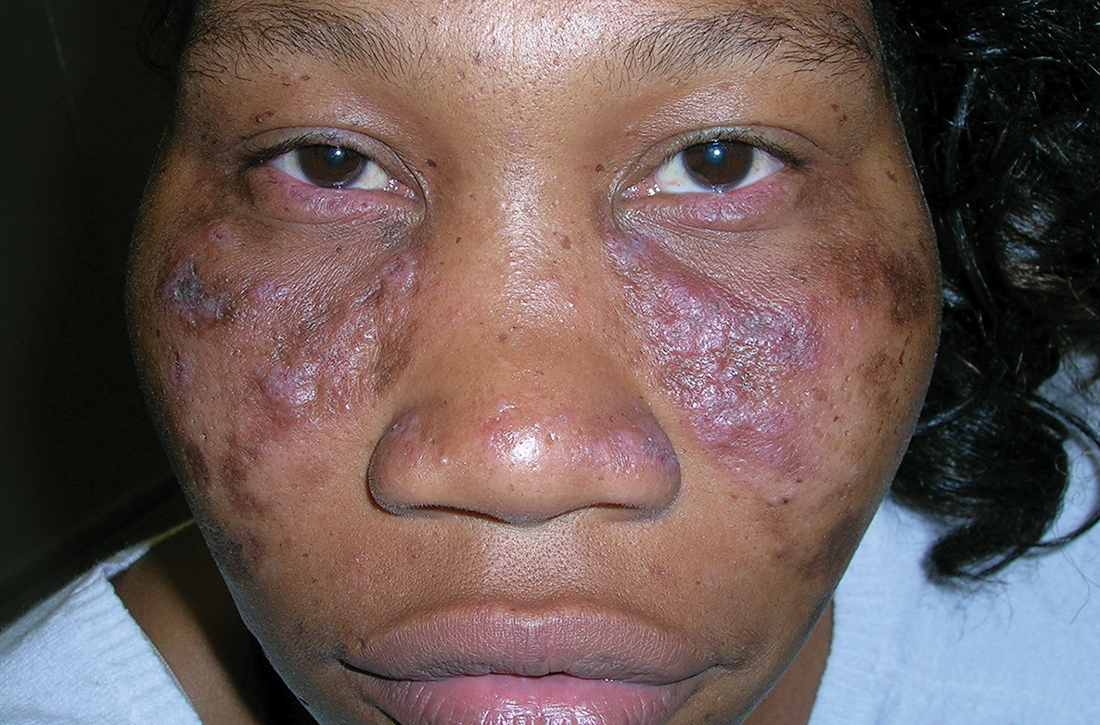
Discoid lupus
THE COMPARISON
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1
Epidemiology
DLE is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
DLE lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. DLE lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8
1. Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
2. Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
3. Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
4. McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
5. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
6. Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
7. Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
8. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
THE COMPARISON
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1
Epidemiology
DLE is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
DLE lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. DLE lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8
THE COMPARISON
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1
Epidemiology
DLE is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
DLE lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. DLE lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8
1. Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
2. Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
3. Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
4. McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
5. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
6. Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
7. Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
8. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
1. Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
2. Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
3. Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
4. McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
5. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
6. Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
7. Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
8. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
Complex link between gut microbiome and immunotherapy response in advanced melanoma
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large-scale than previously thought.
Overall, researchers identified a panel of species, including Roseburia spp. and Akkermansia muciniphila, associated with responses to ICI therapy. However, no single species was a “fully consistent biomarker” across the studies, the authors explain.
This “machine learning analysis confirmed the link between the microbiome and overall response rates (ORRs) and progression-free survival (PFS) with ICIs but also revealed limited reproducibility of microbiome-based signatures across cohorts,” Karla A. Lee, PhD, a clinical research fellow at King’s College London, and colleagues report. The results suggest that “the microbiome is predictive of response in some, but not all, cohorts.”
The findings were published online Feb. 28 in Nature Medicine.
Despite recent advances in targeted therapies for melanoma, less than half of the those who receive a single-agent ICI respond, and those who receive combination ICI therapy often suffer from severe drug toxicity problems. That is why finding patients more likely to respond to a single-agent ICI has become a priority.
Previous studies have identified the gut microbiome as “a potential biomarker of response, as well as a therapeutic target” in melanoma and other malignancies, but “little consensus exists on which microbiome characteristics are associated with treatment responses in the human setting,” the authors explain.
To further clarify the microbiome–immunotherapy relationship, the researchers performed metagenomic sequencing of stool samples collected from 165 ICI-naive patients with unresectable stage III or IV cutaneous melanoma from 5 observational cohorts in the Netherlands, United Kingdom, and Spain. These data were integrated with 147 samples from publicly available datasets.
First, the authors highlighted the variability in findings across these observational studies. For instance, they analyzed stool samples from one UK-based observational study of patients with melanoma (PRIMM-UK) and found a small but statistically significant difference in the microbiome composition of immunotherapy responders versus nonresponders (P = .05) but did not find such an association in a parallel study in the Netherlands (PRIMM-NL, P = .61).
The investigators also explored biomarkers of response across different cohorts and found several standouts. In trials using ORR as an endpoint, two uncultivated Roseburia species (CAG:182 and CAG:471) were associated with responses to ICIs. For patients with available PFS data, Phascolarctobacterium succinatutens and Lactobacillus vaginalis were “enriched in responders” across 7 datasets and significant in 3 of the 8 meta-analysis approaches. A muciniphila and Dorea formicigenerans were also associated with ORR and PFS at 12 months in several meta-analyses.
However, “no single bacterium was a fully consistent biomarker of response across all datasets,” the authors wrote.
Still, the findings could have important implications for the more than 50% of patients with advanced melanoma who don’t respond to single-agent ICI therapy.
“Our study shows that studying the microbiome is important to improve and personalize immunotherapy treatments for melanoma,” study coauthor Nicola Segata, PhD, principal investigator in the Laboratory of Computational Metagenomics, University of Trento, Italy, said in a press release. “However, it also suggests that because of the person-to-person variability of the gut microbiome, even larger studies must be carried out to understand the specific gut microbial features that are more likely to lead to a positive response to immunotherapy.”
Coauthor Tim Spector, PhD, head of the Department of Twin Research & Genetic Epidemiology at King’s College London, added that “the ultimate goal is to identify which specific features of the microbiome are directly influencing the clinical benefits of immunotherapy to exploit these features in new personalized approaches to support cancer immunotherapy.”
In the meantime, he said, “this study highlights the potential impact of good diet and gut health on chances of survival in patients undergoing immunotherapy.”
This study was coordinated by King’s College London, CIBIO Department of the University of Trento and European Institute of Oncology in Italy, and the University of Groningen in the Netherlands, and was funded by the Seerave Foundation. Dr. Lee, Dr. Segata, and Dr. Spector have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Methotrexate plus leflunomide proves effective for PsA
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
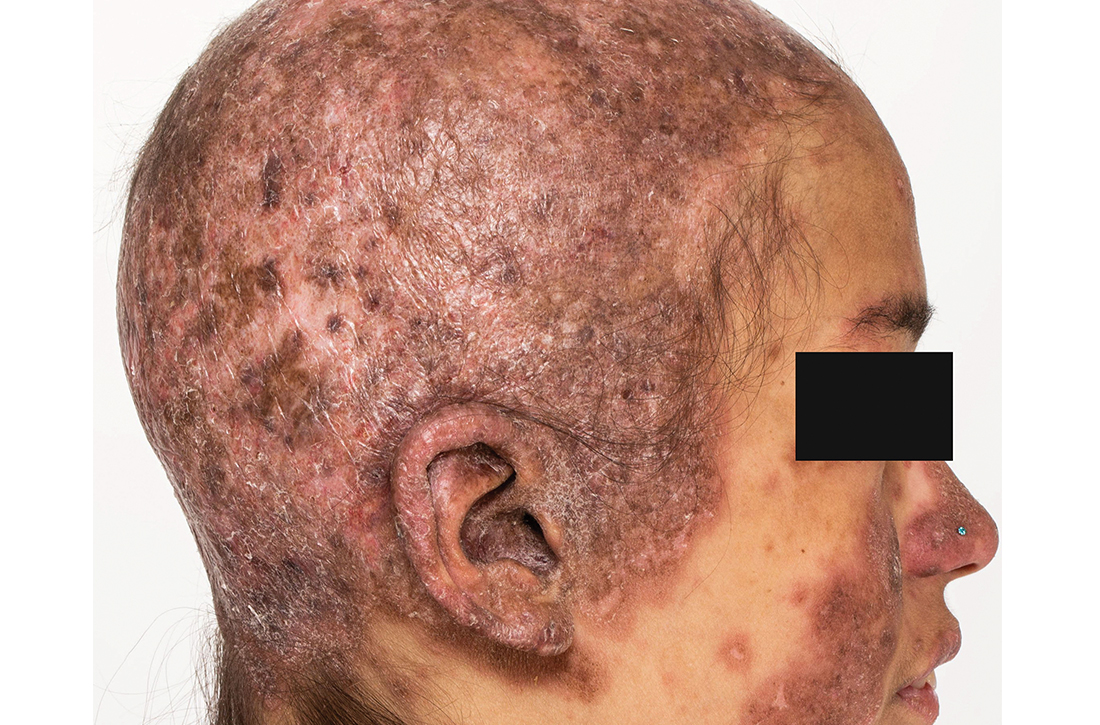
Extensive scarring alopecia and widespread rash
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
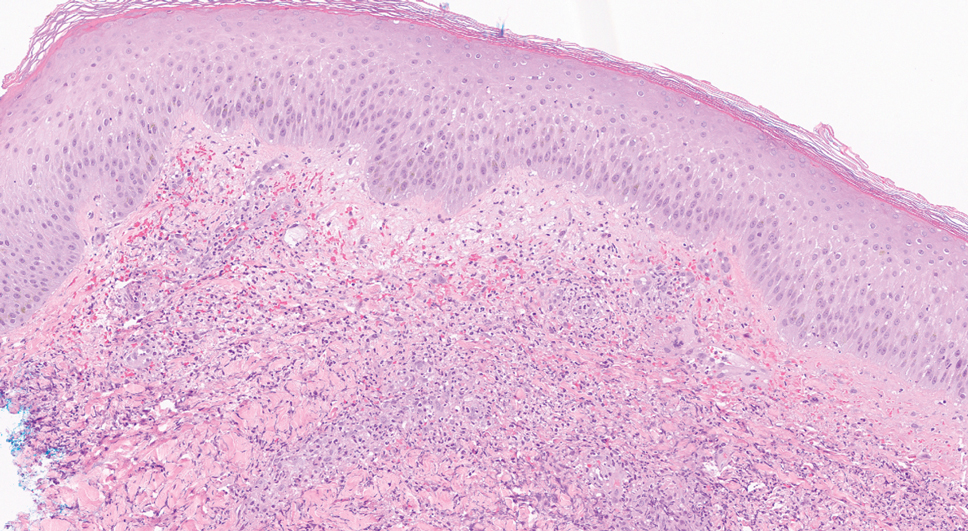
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226
Managing overuse of food IgE panels: Multiple approaches needed
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
Rapidly Enlarging Bullous Plaque
The Diagnosis: Bullous Pyoderma Gangrenosum
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.
First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
The Diagnosis: Bullous Pyoderma Gangrenosum
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.
First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
The Diagnosis: Bullous Pyoderma Gangrenosum
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.
First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
A 26-year-old previously healthy man presented to the emergency department with a new asymptomatic enlarging lesion on the lower leg that had appeared 4 days prior as a self-described “pimple” and rapidly evolved. The patient also reported chills, fatigue, and decreased appetite during that time. Physical examination revealed a red to violaceous, well-demarcated, bullous plaque involving much of the left lower leg. Laboratory studies demonstrated a hemoglobin level of 8.1 g/dL (reference range, 14.0–17.5 g/dL), hematocrit level of 23.7% (reference range, 41%–50%), platelet count of 26×103 /μL (reference range, 150–350×103 /μL), and a population of circulating blast cells and metamyelocytes.
One-third of psoriatic arthritis patients could have metabolic syndrome, data analysis finds
of 724 individuals, as did approximately 23%-63% of patients across multiple studies, investigators from Spain report.
Previous studies of people with PsA in particular suggest they are at an increased risk of cardiovascular disease and have a higher prevalence of metabolic syndrome, prompting recommendations on cardiovascular risk management for patients with PsA, wrote the authors, Ana Urruticoechea-Arana, MD, of the department of rheumatology, Hospital Can Misses, Ibiza, Spain, and colleagues.
However, assessing the prevalence of metabolic syndrome remains a challenge because the definition varies across studies, they noted.
For a more thorough assessment of the prevalence of metabolic syndrome in this population, the researchers conducted a study using two sources: a systematic literature review of 18 studies published up to March 2019, and data on patients with PsA enrolled in the CARMA (Spanish Cardiovascular in Rheumatology) project, a longitudinal cohort observational study of adults with inflammatory diseases in Spain. The findings were published March 1 in the Journal of Clinical Rheumatology.
The literature review included a total of a total of 2,452 patients with PsA, with a mean age between 42 and 59 years, and a mean disease duration ranging from 3 to 14 years.
The definitions of metabolic syndrome varied; the most common was the definition from the National Cholesterol Education Program (NECP ATP III). Other definitions used in the studies included those issued by the International Diabetes Federation, the World Health Organization, and the American Heart Association.
Across these studies, the rate of metabolic syndrome ranged from 23.5% to 62.9%. Prevalence was similar between men and women. One study that included patients with a PsA disease duration of only 3 years showed a prevalence of 38%, similar to the average prevalence overall. Another study showed a significantly higher prevalence of metabolic syndrome in patients with PsA and cutaneous psoriasis, compared with those without psoriasis (40.8% vs. 13.16%; P = .006).
The CARMA study included 724 patients with PsA; 45.4% were women and 21.8% were smokers. The mean age of the population in this study was 51 years, and the mean disease duration was 9 years. Overall, 222 patients (30.7%) met at least three criteria for metabolic syndrome, based on the NCEP ATP III definition. The most common abnormal findings for traditional cardiovascular risk factors in the CARMA cohort were high blood pressure (66.8%), hyperglycemia (42.6%), and hypertriglyceridemia (30.6%).
Despite the variation in prevalence of metabolic syndrome, depending on the definition used, the authors wrote, “It can be stated that the rate of [metabolic syndrome] in patients with PsA is in general very high, especially if we take into account the mean age of patients included in the studies.”
“These findings support the hypotheses that this increase in the inflammatory pathway in PsA may contribute a higher risk of cardiovascular events and [metabolic syndrome] in patients with PsA than patients with psoriasis alone, the risk being even higher in severe PsA,” and that insulin resistance, metabolic syndrome, and atherosclerotic events “may have a common inflammatory basis,” the researchers wrote in their discussion of the results.
The study findings were limited by several factors, most importantly the variation in definitions of metabolic syndrome in the literature review, which limits the generalizability of the results, the researchers said. Limitations of the CARMA study include the focus only on patients who were being cared for in hospitals, which might yield an overestimation of metabolic syndrome, they added.
However, the results support findings from previous studies and highlight the need for proper assessment of body weight and cardiovascular risk factors in patients with PsA at the onset of disease, they said.
“Furthermore, it is necessary to conduct more research to standardize (and modify as appropriate) the definition of [metabolic syndrome] and establish the best strategy for managing it in these patients,” they concluded.
The study was funded by an independent grant from UCB Pharma. One author disclosed receiving grants from Pfizer, Abbvie, Novartis, Roche, UCB, Sanofi, BMS, Lilly, MSD, and Janssen. Lead author Dr. Urruticoechea-Arana and the other authors had no disclosures.
of 724 individuals, as did approximately 23%-63% of patients across multiple studies, investigators from Spain report.
Previous studies of people with PsA in particular suggest they are at an increased risk of cardiovascular disease and have a higher prevalence of metabolic syndrome, prompting recommendations on cardiovascular risk management for patients with PsA, wrote the authors, Ana Urruticoechea-Arana, MD, of the department of rheumatology, Hospital Can Misses, Ibiza, Spain, and colleagues.
However, assessing the prevalence of metabolic syndrome remains a challenge because the definition varies across studies, they noted.
For a more thorough assessment of the prevalence of metabolic syndrome in this population, the researchers conducted a study using two sources: a systematic literature review of 18 studies published up to March 2019, and data on patients with PsA enrolled in the CARMA (Spanish Cardiovascular in Rheumatology) project, a longitudinal cohort observational study of adults with inflammatory diseases in Spain. The findings were published March 1 in the Journal of Clinical Rheumatology.
The literature review included a total of a total of 2,452 patients with PsA, with a mean age between 42 and 59 years, and a mean disease duration ranging from 3 to 14 years.
The definitions of metabolic syndrome varied; the most common was the definition from the National Cholesterol Education Program (NECP ATP III). Other definitions used in the studies included those issued by the International Diabetes Federation, the World Health Organization, and the American Heart Association.
Across these studies, the rate of metabolic syndrome ranged from 23.5% to 62.9%. Prevalence was similar between men and women. One study that included patients with a PsA disease duration of only 3 years showed a prevalence of 38%, similar to the average prevalence overall. Another study showed a significantly higher prevalence of metabolic syndrome in patients with PsA and cutaneous psoriasis, compared with those without psoriasis (40.8% vs. 13.16%; P = .006).
The CARMA study included 724 patients with PsA; 45.4% were women and 21.8% were smokers. The mean age of the population in this study was 51 years, and the mean disease duration was 9 years. Overall, 222 patients (30.7%) met at least three criteria for metabolic syndrome, based on the NCEP ATP III definition. The most common abnormal findings for traditional cardiovascular risk factors in the CARMA cohort were high blood pressure (66.8%), hyperglycemia (42.6%), and hypertriglyceridemia (30.6%).
Despite the variation in prevalence of metabolic syndrome, depending on the definition used, the authors wrote, “It can be stated that the rate of [metabolic syndrome] in patients with PsA is in general very high, especially if we take into account the mean age of patients included in the studies.”
“These findings support the hypotheses that this increase in the inflammatory pathway in PsA may contribute a higher risk of cardiovascular events and [metabolic syndrome] in patients with PsA than patients with psoriasis alone, the risk being even higher in severe PsA,” and that insulin resistance, metabolic syndrome, and atherosclerotic events “may have a common inflammatory basis,” the researchers wrote in their discussion of the results.
The study findings were limited by several factors, most importantly the variation in definitions of metabolic syndrome in the literature review, which limits the generalizability of the results, the researchers said. Limitations of the CARMA study include the focus only on patients who were being cared for in hospitals, which might yield an overestimation of metabolic syndrome, they added.
However, the results support findings from previous studies and highlight the need for proper assessment of body weight and cardiovascular risk factors in patients with PsA at the onset of disease, they said.
“Furthermore, it is necessary to conduct more research to standardize (and modify as appropriate) the definition of [metabolic syndrome] and establish the best strategy for managing it in these patients,” they concluded.
The study was funded by an independent grant from UCB Pharma. One author disclosed receiving grants from Pfizer, Abbvie, Novartis, Roche, UCB, Sanofi, BMS, Lilly, MSD, and Janssen. Lead author Dr. Urruticoechea-Arana and the other authors had no disclosures.
of 724 individuals, as did approximately 23%-63% of patients across multiple studies, investigators from Spain report.
Previous studies of people with PsA in particular suggest they are at an increased risk of cardiovascular disease and have a higher prevalence of metabolic syndrome, prompting recommendations on cardiovascular risk management for patients with PsA, wrote the authors, Ana Urruticoechea-Arana, MD, of the department of rheumatology, Hospital Can Misses, Ibiza, Spain, and colleagues.
However, assessing the prevalence of metabolic syndrome remains a challenge because the definition varies across studies, they noted.
For a more thorough assessment of the prevalence of metabolic syndrome in this population, the researchers conducted a study using two sources: a systematic literature review of 18 studies published up to March 2019, and data on patients with PsA enrolled in the CARMA (Spanish Cardiovascular in Rheumatology) project, a longitudinal cohort observational study of adults with inflammatory diseases in Spain. The findings were published March 1 in the Journal of Clinical Rheumatology.
The literature review included a total of a total of 2,452 patients with PsA, with a mean age between 42 and 59 years, and a mean disease duration ranging from 3 to 14 years.
The definitions of metabolic syndrome varied; the most common was the definition from the National Cholesterol Education Program (NECP ATP III). Other definitions used in the studies included those issued by the International Diabetes Federation, the World Health Organization, and the American Heart Association.
Across these studies, the rate of metabolic syndrome ranged from 23.5% to 62.9%. Prevalence was similar between men and women. One study that included patients with a PsA disease duration of only 3 years showed a prevalence of 38%, similar to the average prevalence overall. Another study showed a significantly higher prevalence of metabolic syndrome in patients with PsA and cutaneous psoriasis, compared with those without psoriasis (40.8% vs. 13.16%; P = .006).
The CARMA study included 724 patients with PsA; 45.4% were women and 21.8% were smokers. The mean age of the population in this study was 51 years, and the mean disease duration was 9 years. Overall, 222 patients (30.7%) met at least three criteria for metabolic syndrome, based on the NCEP ATP III definition. The most common abnormal findings for traditional cardiovascular risk factors in the CARMA cohort were high blood pressure (66.8%), hyperglycemia (42.6%), and hypertriglyceridemia (30.6%).
Despite the variation in prevalence of metabolic syndrome, depending on the definition used, the authors wrote, “It can be stated that the rate of [metabolic syndrome] in patients with PsA is in general very high, especially if we take into account the mean age of patients included in the studies.”
“These findings support the hypotheses that this increase in the inflammatory pathway in PsA may contribute a higher risk of cardiovascular events and [metabolic syndrome] in patients with PsA than patients with psoriasis alone, the risk being even higher in severe PsA,” and that insulin resistance, metabolic syndrome, and atherosclerotic events “may have a common inflammatory basis,” the researchers wrote in their discussion of the results.
The study findings were limited by several factors, most importantly the variation in definitions of metabolic syndrome in the literature review, which limits the generalizability of the results, the researchers said. Limitations of the CARMA study include the focus only on patients who were being cared for in hospitals, which might yield an overestimation of metabolic syndrome, they added.
However, the results support findings from previous studies and highlight the need for proper assessment of body weight and cardiovascular risk factors in patients with PsA at the onset of disease, they said.
“Furthermore, it is necessary to conduct more research to standardize (and modify as appropriate) the definition of [metabolic syndrome] and establish the best strategy for managing it in these patients,” they concluded.
The study was funded by an independent grant from UCB Pharma. One author disclosed receiving grants from Pfizer, Abbvie, Novartis, Roche, UCB, Sanofi, BMS, Lilly, MSD, and Janssen. Lead author Dr. Urruticoechea-Arana and the other authors had no disclosures.
FROM JOURNAL OF CLINICAL RHEUMATOLOGY
Beware of the latest TikTok trend: Nasal spray tans
Although nasal spray tanning is being described as a new “viral” trend, it seems to have gotten its start as early as the spring of 2021. The tanning method appears to be especially popular in the United Kingdom, where self-tanning product brands have TikTok videos promoting nasal sprays.
The rising concerns of this and other viral TikTok trends has now prompted a bipartisan group of seven state attorneys general to launch an investigation.
“As children and teens already grapple with issues of anxiety, social pressure, and depression, we cannot allow social media to further harm their physical health and mental wellbeing,” Massachusetts Attorney General Maura Healey, a Democrat, said in a statement. “State attorneys general have an imperative to protect young people and seek more information about how companies like TikTok are influencing their daily lives.”
Ms. Healey, along with colleagues from California, Florida, Kentucky, Nebraska, New Jersey, Tennessee, and Vermont, will whether Chinese-based TikTok violates state consumer protection laws.
The trend of people shooting spray tan up their nose is just the latest in a long line of so-called TikTok challenges that have caused controversy, and often, injury.
In a February TikTok, put out by the British company So Tanned (@_sotanned), a young woman appears with text stating that she uses nasal spray “morning and night” and then adds self-tanning oral drops a half hour before getting into a tanning bed.
But, dermatologist Lily Talakoub, MD, of McLean, Va., posted a TikTok with the bold warning “DO NOT USE NASAL TANNING SPRAY!” In the video, the white coat–clad Dr. Talakoub is in the foreground of the TikTok made by @Sashawoodx.
“Don’t try this at home,” said Dr. Talakoub.
“Don’t try this even if you think it can make you tanner. It can cause nausea, vomiting, very bad side effects,” she said, adding “this can be very dangerous to your health.”
It’s also worth mentioning that self-tanning products are not approved by the Food and Drug Administration for inhalation.
Still, another U.K. company, 2btanned, posted a TikTok showing a user spraying the product up his nose and, in the comments, @2btanned suggested that the spray should be used at least a week or two before sun exposure “in order to get full effects.”
@Sashawoodx tells her viewers: “Don’t walk ... RUN for these products,” as she shows herself in several different outfits, squirting 2btanned spray up her nose. As of March 2, the TikTok video had been viewed over 212,000 times.
TikTokker @giannaarose, who has 125,000 followers, said in a video that she uses two to three sprays up the nose before stepping into the tanning bed. A commenter said, “this is scary but where do I buy it”.
The main ingredient in self-tanning products is dihydroxyacetone, or DHA. DHA, which is FDA-approved for use on skin, causes a chemical reaction when heat is applied, and a pigment is deposited on the skin.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said, given that self-tanning products were never meant to be inhaled and that nasal sprays of any kind must be approved by the FDA, a company promoting the products is engaged in a dangerous game.
“People could go to jail over this,” said Dr. Friedman. What’s more, the products are unlikely to produce a tan.
“Because of the way self-tanners work, it would make no sense,” said Dr. Friedman.
“It’s purely a camouflage,” he said, adding that it does not produce melanin. Self-tanners were never intended to be inhaled, “so who knows what those ingredients would do to a different anatomical site like the inner passages of the nose.”
At a minimum, spraying into the nose could at cause irritation. But it could also potentially lead to acute or long-term damage, he said.
Some other spray ingredients, such as tyrosine and tyrosinase, are involved in producing melanin, but they only act within skin cells. If sprayed into the nose, the ingredients might produce melanin inside the nose, but not on the skin.
“This is not going to work,” said Dr. Friedman. “If anything, it could be dangerous.”
He added that there’s no such thing as a safe tan, and that self-tanning products offer no protection from dangerous ultraviolet rays. The nasal sprays are “quick fixes” that are not going to work.
“At the end of the day, just don’t inhale,” Dr. Friedman said.
A version of this article first appeared on WebMD.com.
Although nasal spray tanning is being described as a new “viral” trend, it seems to have gotten its start as early as the spring of 2021. The tanning method appears to be especially popular in the United Kingdom, where self-tanning product brands have TikTok videos promoting nasal sprays.
The rising concerns of this and other viral TikTok trends has now prompted a bipartisan group of seven state attorneys general to launch an investigation.
“As children and teens already grapple with issues of anxiety, social pressure, and depression, we cannot allow social media to further harm their physical health and mental wellbeing,” Massachusetts Attorney General Maura Healey, a Democrat, said in a statement. “State attorneys general have an imperative to protect young people and seek more information about how companies like TikTok are influencing their daily lives.”
Ms. Healey, along with colleagues from California, Florida, Kentucky, Nebraska, New Jersey, Tennessee, and Vermont, will whether Chinese-based TikTok violates state consumer protection laws.
The trend of people shooting spray tan up their nose is just the latest in a long line of so-called TikTok challenges that have caused controversy, and often, injury.
In a February TikTok, put out by the British company So Tanned (@_sotanned), a young woman appears with text stating that she uses nasal spray “morning and night” and then adds self-tanning oral drops a half hour before getting into a tanning bed.
But, dermatologist Lily Talakoub, MD, of McLean, Va., posted a TikTok with the bold warning “DO NOT USE NASAL TANNING SPRAY!” In the video, the white coat–clad Dr. Talakoub is in the foreground of the TikTok made by @Sashawoodx.
“Don’t try this at home,” said Dr. Talakoub.
“Don’t try this even if you think it can make you tanner. It can cause nausea, vomiting, very bad side effects,” she said, adding “this can be very dangerous to your health.”
It’s also worth mentioning that self-tanning products are not approved by the Food and Drug Administration for inhalation.
Still, another U.K. company, 2btanned, posted a TikTok showing a user spraying the product up his nose and, in the comments, @2btanned suggested that the spray should be used at least a week or two before sun exposure “in order to get full effects.”
@Sashawoodx tells her viewers: “Don’t walk ... RUN for these products,” as she shows herself in several different outfits, squirting 2btanned spray up her nose. As of March 2, the TikTok video had been viewed over 212,000 times.
TikTokker @giannaarose, who has 125,000 followers, said in a video that she uses two to three sprays up the nose before stepping into the tanning bed. A commenter said, “this is scary but where do I buy it”.
The main ingredient in self-tanning products is dihydroxyacetone, or DHA. DHA, which is FDA-approved for use on skin, causes a chemical reaction when heat is applied, and a pigment is deposited on the skin.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said, given that self-tanning products were never meant to be inhaled and that nasal sprays of any kind must be approved by the FDA, a company promoting the products is engaged in a dangerous game.
“People could go to jail over this,” said Dr. Friedman. What’s more, the products are unlikely to produce a tan.
“Because of the way self-tanners work, it would make no sense,” said Dr. Friedman.
“It’s purely a camouflage,” he said, adding that it does not produce melanin. Self-tanners were never intended to be inhaled, “so who knows what those ingredients would do to a different anatomical site like the inner passages of the nose.”
At a minimum, spraying into the nose could at cause irritation. But it could also potentially lead to acute or long-term damage, he said.
Some other spray ingredients, such as tyrosine and tyrosinase, are involved in producing melanin, but they only act within skin cells. If sprayed into the nose, the ingredients might produce melanin inside the nose, but not on the skin.
“This is not going to work,” said Dr. Friedman. “If anything, it could be dangerous.”
He added that there’s no such thing as a safe tan, and that self-tanning products offer no protection from dangerous ultraviolet rays. The nasal sprays are “quick fixes” that are not going to work.
“At the end of the day, just don’t inhale,” Dr. Friedman said.
A version of this article first appeared on WebMD.com.
Although nasal spray tanning is being described as a new “viral” trend, it seems to have gotten its start as early as the spring of 2021. The tanning method appears to be especially popular in the United Kingdom, where self-tanning product brands have TikTok videos promoting nasal sprays.
The rising concerns of this and other viral TikTok trends has now prompted a bipartisan group of seven state attorneys general to launch an investigation.
“As children and teens already grapple with issues of anxiety, social pressure, and depression, we cannot allow social media to further harm their physical health and mental wellbeing,” Massachusetts Attorney General Maura Healey, a Democrat, said in a statement. “State attorneys general have an imperative to protect young people and seek more information about how companies like TikTok are influencing their daily lives.”
Ms. Healey, along with colleagues from California, Florida, Kentucky, Nebraska, New Jersey, Tennessee, and Vermont, will whether Chinese-based TikTok violates state consumer protection laws.
The trend of people shooting spray tan up their nose is just the latest in a long line of so-called TikTok challenges that have caused controversy, and often, injury.
In a February TikTok, put out by the British company So Tanned (@_sotanned), a young woman appears with text stating that she uses nasal spray “morning and night” and then adds self-tanning oral drops a half hour before getting into a tanning bed.
But, dermatologist Lily Talakoub, MD, of McLean, Va., posted a TikTok with the bold warning “DO NOT USE NASAL TANNING SPRAY!” In the video, the white coat–clad Dr. Talakoub is in the foreground of the TikTok made by @Sashawoodx.
“Don’t try this at home,” said Dr. Talakoub.
“Don’t try this even if you think it can make you tanner. It can cause nausea, vomiting, very bad side effects,” she said, adding “this can be very dangerous to your health.”
It’s also worth mentioning that self-tanning products are not approved by the Food and Drug Administration for inhalation.
Still, another U.K. company, 2btanned, posted a TikTok showing a user spraying the product up his nose and, in the comments, @2btanned suggested that the spray should be used at least a week or two before sun exposure “in order to get full effects.”
@Sashawoodx tells her viewers: “Don’t walk ... RUN for these products,” as she shows herself in several different outfits, squirting 2btanned spray up her nose. As of March 2, the TikTok video had been viewed over 212,000 times.
TikTokker @giannaarose, who has 125,000 followers, said in a video that she uses two to three sprays up the nose before stepping into the tanning bed. A commenter said, “this is scary but where do I buy it”.
The main ingredient in self-tanning products is dihydroxyacetone, or DHA. DHA, which is FDA-approved for use on skin, causes a chemical reaction when heat is applied, and a pigment is deposited on the skin.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said, given that self-tanning products were never meant to be inhaled and that nasal sprays of any kind must be approved by the FDA, a company promoting the products is engaged in a dangerous game.
“People could go to jail over this,” said Dr. Friedman. What’s more, the products are unlikely to produce a tan.
“Because of the way self-tanners work, it would make no sense,” said Dr. Friedman.
“It’s purely a camouflage,” he said, adding that it does not produce melanin. Self-tanners were never intended to be inhaled, “so who knows what those ingredients would do to a different anatomical site like the inner passages of the nose.”
At a minimum, spraying into the nose could at cause irritation. But it could also potentially lead to acute or long-term damage, he said.
Some other spray ingredients, such as tyrosine and tyrosinase, are involved in producing melanin, but they only act within skin cells. If sprayed into the nose, the ingredients might produce melanin inside the nose, but not on the skin.
“This is not going to work,” said Dr. Friedman. “If anything, it could be dangerous.”
He added that there’s no such thing as a safe tan, and that self-tanning products offer no protection from dangerous ultraviolet rays. The nasal sprays are “quick fixes” that are not going to work.
“At the end of the day, just don’t inhale,” Dr. Friedman said.
A version of this article first appeared on WebMD.com.
High early recurrence rates with Merkel cell carcinoma
, and more than half of all patients with stage IV disease will have a recurrence within 1 year of definitive therapy, results of a new study show.
A study of 618 patients with MCC who were enrolled in a Seattle-based data repository shows that among all patients the 5-year recurrence rate was 40%. The risk of recurrence within the first year was 11% for patients with pathologic stage I disease, 33% for those with stage IIA/IIB disease, 45% for those with stage IIIB disease, and 58% for patients with pathologic stage IV MCC.
Approximately 95% of all recurrences happened within 3 years of the initial diagnosis, report Aubriana McEvoy, MD, from the University of Washington in Seattle, and colleagues.
“This cohort study indicates that the highest yield (and likely most cost-effective) time period for detecting MCC recurrence is 1-3 years after diagnosis,” they write in a study published online in JAMA Dermatology.
The estimated annual incidence of MCC in the United States in 2018 was 2,000 according to the American Cancer Society. The annual incidence rate is rising rapidly, however, and is estimated to reach 3,284 by 2025, McEvoy and colleagues write.
Although MCC is known to have high recurrence rates and is associated with a higher mortality rate than malignant melanoma, recurrence rate data are not captured by either the Surveillance, Epidemiology, and End Results (SEER) database or by the National Cancer Database. As a result, estimates of recurrence rates with MCC have been all over the map, ranging from 27% to 77%, depending on the population studied.
But as senior author Paul Nghiem, MD, PhD, professor and chair of dermatology at the University of Washington, Seattle, told this news organization, recurrence rates over time in their study were remarkably consistent.
“The biggest surprise to me was that, when we broke our nearly 20-year cohort into three 5- or 6-year chunks, every one of the groups had a 40% recurrence rate, within 1%. So we feel really confident that’s the right number,” he said.
Dr. Nghiem and colleagues report that, in contrast to patients with MCC, approximately 19% of patients with melanoma will have a recurrence, as will an estimated 5%-9% of patients with squamous cell carcinoma and 1%-10% of patients with basal cell carcinoma.
The fact that recurrence rates of MCC have remained stable over time despite presumed improvements in definitive therapy is disappointing, Dr. Nghiem acknowledged. He noted that it’s still unclear whether immunotherapy will have the same dramatic effect on survival rates for patients with MCC as it has for patients with malignant melanoma.
The high recurrence rates following definitive therapy for patients with early-stage disease was a novel finding, commented Shawn Demehri, MD, PhD, director of the high-risk skin cancer clinic at Massachusetts General Hospital in Boston.
“When you’re looking at patients with stage I or stage II, and they have definitive surgery but still have recurrences at a higher rate than melanoma, it brings home the point that these are among the most aggressive tumors of the skin,” he said in an interview.
The high recurrence rates seen with MCC are attributable to a variety of factors.
“This is a rare cancer of mostly older individuals with a lot of comorbidities, and also a cancer that, even though it is a primary cancer, might be detected a little later than even a melanoma primary tumor, just because of the nature of the neuroendocrine tumor cells,” he said.
Dr. Demehri was not involved in the study.
Prospective cohort
The study cohort consisted of 618 patients with MCC. The median age of the patients was 69, and 227 (37%) were women. The patients were enrolled within 6 months of their diagnosis in the prospective data repository from 2003 through 2019. Of this group, 223 had a recurrence of MCC.
As noted, there was a high risk of recurrence within 1 year, ranging from 11% for patients with pathologic stage I tumors to 58% for those with stage IV disease, and 95% of all recurrences occurred within 3 years of definitive therapy.
To get a better picture of the natural history of MCC recurrence, the investigators studied a cohort of patients with pathologically confirmed MCC who were prospectively enrolled from January 2003 through April 2019 in a data repository maintained at the University of Washington.
In addition to disease stage, factors associated with increased recurrence risk in univariable analyses include immunosuppression (hazard ratio, 2.4; P < .001), male sex (HR, 1.9; P < .001), known primary lesion among patients with clinically detectable nodal disease (HR, 2.3; P = .001), and older age (HR, 1.1, P = .06 for each 10-year increase).
Of the 187 patients in the cohort who died during the study, 121 died from MCC. At 4 years after diagnosis, MCC-specific survival rates were 95% for patients with pathologic stage I, 84% with stage IIA/IIB, 80% with stage IIIA, 58% with stage IIIB, and 41% with stage IV.
Evidence supports close monitoring within the first 3 years for patients with stage I-II MCC. Local recurrence within or adjacent to the primary tumor scar was associated with a 5-year MCC-specific survival rate of 85%, compared with 88% of patients with stage I or II disease who did not have recurrences.
“Because more than 90% of MCC recurrences arise within 3 years, it is appropriate to adjust surveillance intensity accordingly. Stage- and time-specific recurrence data can assist in appropriately focusing surveillance resources on patients and time intervals in which recurrence risk is highest,” the authors wrote.
“If you’re a patient who has not had your cancer come back for 3, 4, or 5 years, you can really cut down on the intensity of your follow-up and scans,” Dr. Nghiem said.
“We do now have two excellent blood tests that are working very well, and we have really good ways to detect the cancer coming back early, and that’s important, because we have potentially curative therapies that tend to work better if you catch the cancer early,” he said.
The study was supported by the National Institutes of Health. Dr. Nghiem reported personal fees and institutional support outside the study from several companies and patents for Merkel cell therapies with the University of Washington and University of Denmark. Dr. Demehri has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and more than half of all patients with stage IV disease will have a recurrence within 1 year of definitive therapy, results of a new study show.
A study of 618 patients with MCC who were enrolled in a Seattle-based data repository shows that among all patients the 5-year recurrence rate was 40%. The risk of recurrence within the first year was 11% for patients with pathologic stage I disease, 33% for those with stage IIA/IIB disease, 45% for those with stage IIIB disease, and 58% for patients with pathologic stage IV MCC.
Approximately 95% of all recurrences happened within 3 years of the initial diagnosis, report Aubriana McEvoy, MD, from the University of Washington in Seattle, and colleagues.
“This cohort study indicates that the highest yield (and likely most cost-effective) time period for detecting MCC recurrence is 1-3 years after diagnosis,” they write in a study published online in JAMA Dermatology.
The estimated annual incidence of MCC in the United States in 2018 was 2,000 according to the American Cancer Society. The annual incidence rate is rising rapidly, however, and is estimated to reach 3,284 by 2025, McEvoy and colleagues write.
Although MCC is known to have high recurrence rates and is associated with a higher mortality rate than malignant melanoma, recurrence rate data are not captured by either the Surveillance, Epidemiology, and End Results (SEER) database or by the National Cancer Database. As a result, estimates of recurrence rates with MCC have been all over the map, ranging from 27% to 77%, depending on the population studied.
But as senior author Paul Nghiem, MD, PhD, professor and chair of dermatology at the University of Washington, Seattle, told this news organization, recurrence rates over time in their study were remarkably consistent.
“The biggest surprise to me was that, when we broke our nearly 20-year cohort into three 5- or 6-year chunks, every one of the groups had a 40% recurrence rate, within 1%. So we feel really confident that’s the right number,” he said.
Dr. Nghiem and colleagues report that, in contrast to patients with MCC, approximately 19% of patients with melanoma will have a recurrence, as will an estimated 5%-9% of patients with squamous cell carcinoma and 1%-10% of patients with basal cell carcinoma.
The fact that recurrence rates of MCC have remained stable over time despite presumed improvements in definitive therapy is disappointing, Dr. Nghiem acknowledged. He noted that it’s still unclear whether immunotherapy will have the same dramatic effect on survival rates for patients with MCC as it has for patients with malignant melanoma.
The high recurrence rates following definitive therapy for patients with early-stage disease was a novel finding, commented Shawn Demehri, MD, PhD, director of the high-risk skin cancer clinic at Massachusetts General Hospital in Boston.
“When you’re looking at patients with stage I or stage II, and they have definitive surgery but still have recurrences at a higher rate than melanoma, it brings home the point that these are among the most aggressive tumors of the skin,” he said in an interview.
The high recurrence rates seen with MCC are attributable to a variety of factors.
“This is a rare cancer of mostly older individuals with a lot of comorbidities, and also a cancer that, even though it is a primary cancer, might be detected a little later than even a melanoma primary tumor, just because of the nature of the neuroendocrine tumor cells,” he said.
Dr. Demehri was not involved in the study.
Prospective cohort
The study cohort consisted of 618 patients with MCC. The median age of the patients was 69, and 227 (37%) were women. The patients were enrolled within 6 months of their diagnosis in the prospective data repository from 2003 through 2019. Of this group, 223 had a recurrence of MCC.
As noted, there was a high risk of recurrence within 1 year, ranging from 11% for patients with pathologic stage I tumors to 58% for those with stage IV disease, and 95% of all recurrences occurred within 3 years of definitive therapy.
To get a better picture of the natural history of MCC recurrence, the investigators studied a cohort of patients with pathologically confirmed MCC who were prospectively enrolled from January 2003 through April 2019 in a data repository maintained at the University of Washington.
In addition to disease stage, factors associated with increased recurrence risk in univariable analyses include immunosuppression (hazard ratio, 2.4; P < .001), male sex (HR, 1.9; P < .001), known primary lesion among patients with clinically detectable nodal disease (HR, 2.3; P = .001), and older age (HR, 1.1, P = .06 for each 10-year increase).
Of the 187 patients in the cohort who died during the study, 121 died from MCC. At 4 years after diagnosis, MCC-specific survival rates were 95% for patients with pathologic stage I, 84% with stage IIA/IIB, 80% with stage IIIA, 58% with stage IIIB, and 41% with stage IV.
Evidence supports close monitoring within the first 3 years for patients with stage I-II MCC. Local recurrence within or adjacent to the primary tumor scar was associated with a 5-year MCC-specific survival rate of 85%, compared with 88% of patients with stage I or II disease who did not have recurrences.
“Because more than 90% of MCC recurrences arise within 3 years, it is appropriate to adjust surveillance intensity accordingly. Stage- and time-specific recurrence data can assist in appropriately focusing surveillance resources on patients and time intervals in which recurrence risk is highest,” the authors wrote.
“If you’re a patient who has not had your cancer come back for 3, 4, or 5 years, you can really cut down on the intensity of your follow-up and scans,” Dr. Nghiem said.
“We do now have two excellent blood tests that are working very well, and we have really good ways to detect the cancer coming back early, and that’s important, because we have potentially curative therapies that tend to work better if you catch the cancer early,” he said.
The study was supported by the National Institutes of Health. Dr. Nghiem reported personal fees and institutional support outside the study from several companies and patents for Merkel cell therapies with the University of Washington and University of Denmark. Dr. Demehri has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and more than half of all patients with stage IV disease will have a recurrence within 1 year of definitive therapy, results of a new study show.
A study of 618 patients with MCC who were enrolled in a Seattle-based data repository shows that among all patients the 5-year recurrence rate was 40%. The risk of recurrence within the first year was 11% for patients with pathologic stage I disease, 33% for those with stage IIA/IIB disease, 45% for those with stage IIIB disease, and 58% for patients with pathologic stage IV MCC.
Approximately 95% of all recurrences happened within 3 years of the initial diagnosis, report Aubriana McEvoy, MD, from the University of Washington in Seattle, and colleagues.
“This cohort study indicates that the highest yield (and likely most cost-effective) time period for detecting MCC recurrence is 1-3 years after diagnosis,” they write in a study published online in JAMA Dermatology.
The estimated annual incidence of MCC in the United States in 2018 was 2,000 according to the American Cancer Society. The annual incidence rate is rising rapidly, however, and is estimated to reach 3,284 by 2025, McEvoy and colleagues write.
Although MCC is known to have high recurrence rates and is associated with a higher mortality rate than malignant melanoma, recurrence rate data are not captured by either the Surveillance, Epidemiology, and End Results (SEER) database or by the National Cancer Database. As a result, estimates of recurrence rates with MCC have been all over the map, ranging from 27% to 77%, depending on the population studied.
But as senior author Paul Nghiem, MD, PhD, professor and chair of dermatology at the University of Washington, Seattle, told this news organization, recurrence rates over time in their study were remarkably consistent.
“The biggest surprise to me was that, when we broke our nearly 20-year cohort into three 5- or 6-year chunks, every one of the groups had a 40% recurrence rate, within 1%. So we feel really confident that’s the right number,” he said.
Dr. Nghiem and colleagues report that, in contrast to patients with MCC, approximately 19% of patients with melanoma will have a recurrence, as will an estimated 5%-9% of patients with squamous cell carcinoma and 1%-10% of patients with basal cell carcinoma.
The fact that recurrence rates of MCC have remained stable over time despite presumed improvements in definitive therapy is disappointing, Dr. Nghiem acknowledged. He noted that it’s still unclear whether immunotherapy will have the same dramatic effect on survival rates for patients with MCC as it has for patients with malignant melanoma.
The high recurrence rates following definitive therapy for patients with early-stage disease was a novel finding, commented Shawn Demehri, MD, PhD, director of the high-risk skin cancer clinic at Massachusetts General Hospital in Boston.
“When you’re looking at patients with stage I or stage II, and they have definitive surgery but still have recurrences at a higher rate than melanoma, it brings home the point that these are among the most aggressive tumors of the skin,” he said in an interview.
The high recurrence rates seen with MCC are attributable to a variety of factors.
“This is a rare cancer of mostly older individuals with a lot of comorbidities, and also a cancer that, even though it is a primary cancer, might be detected a little later than even a melanoma primary tumor, just because of the nature of the neuroendocrine tumor cells,” he said.
Dr. Demehri was not involved in the study.
Prospective cohort
The study cohort consisted of 618 patients with MCC. The median age of the patients was 69, and 227 (37%) were women. The patients were enrolled within 6 months of their diagnosis in the prospective data repository from 2003 through 2019. Of this group, 223 had a recurrence of MCC.
As noted, there was a high risk of recurrence within 1 year, ranging from 11% for patients with pathologic stage I tumors to 58% for those with stage IV disease, and 95% of all recurrences occurred within 3 years of definitive therapy.
To get a better picture of the natural history of MCC recurrence, the investigators studied a cohort of patients with pathologically confirmed MCC who were prospectively enrolled from January 2003 through April 2019 in a data repository maintained at the University of Washington.
In addition to disease stage, factors associated with increased recurrence risk in univariable analyses include immunosuppression (hazard ratio, 2.4; P < .001), male sex (HR, 1.9; P < .001), known primary lesion among patients with clinically detectable nodal disease (HR, 2.3; P = .001), and older age (HR, 1.1, P = .06 for each 10-year increase).
Of the 187 patients in the cohort who died during the study, 121 died from MCC. At 4 years after diagnosis, MCC-specific survival rates were 95% for patients with pathologic stage I, 84% with stage IIA/IIB, 80% with stage IIIA, 58% with stage IIIB, and 41% with stage IV.
Evidence supports close monitoring within the first 3 years for patients with stage I-II MCC. Local recurrence within or adjacent to the primary tumor scar was associated with a 5-year MCC-specific survival rate of 85%, compared with 88% of patients with stage I or II disease who did not have recurrences.
“Because more than 90% of MCC recurrences arise within 3 years, it is appropriate to adjust surveillance intensity accordingly. Stage- and time-specific recurrence data can assist in appropriately focusing surveillance resources on patients and time intervals in which recurrence risk is highest,” the authors wrote.
“If you’re a patient who has not had your cancer come back for 3, 4, or 5 years, you can really cut down on the intensity of your follow-up and scans,” Dr. Nghiem said.
“We do now have two excellent blood tests that are working very well, and we have really good ways to detect the cancer coming back early, and that’s important, because we have potentially curative therapies that tend to work better if you catch the cancer early,” he said.
The study was supported by the National Institutes of Health. Dr. Nghiem reported personal fees and institutional support outside the study from several companies and patents for Merkel cell therapies with the University of Washington and University of Denmark. Dr. Demehri has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY