User login
Patient survey results highlight disease burden in atopic dermatitis
More than half of the patients with moderate to severe atopic dermatitis (AD) had inadequately controlled disease, which was associated with a higher patient-reported disease burden compared with those who had adequately controlled disease, in a cross-sectional study of adults with AD.
Disease control aside, patient-reported burden was generally higher in those with moderate to severe AD versus patients with mild AD, according to Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, and his coauthors.
“These results highlight beyond using measures of disease activity,” the researchers wrote. The study, published in JAMA Dermatology, was conducted before the introduction of dupilumab (Dupixent), the first biologic approved by the Food and Drug Administration for treatment of moderate to severe AD, the authors noted. (The study was supported by the manufacturers of dupilumab.)
The patients were in the Adults With Atopic Dermatitis Reporting on Their Experience (AD-AWARE) study, a cross-sectional analysis of burden of illness in adults with AD in clinical practices at six U.S. academic medical centers. The 1,519 patients completed a self-administered, Internet-based questionnaire during 2013-2014. Among these patients, 830 (54.6%) had moderate to severe AD.
A total of 185 patients with moderate to severe disease received systemic immunomodulators or phototherapy, and of those, more than half (103, or 55.7%) reported inadequate disease control, according to the survey results.
Regardless of disease control, the patients with moderate to severe AD had a greater burden of disease compared with patients with mild AD, according to the investigators. Those burdens included more severe pain and itching, sleep effects, anxiety and depression, and impairment of health-related quality of life, they reported.
Those with moderate to severe disease had a mean of 5.7 days per week with itchy skin, and 22.8% reported itch lasting for more than half a day, compared with a mean of 2.7 days and 2.9%, respectively, for those with mild disease, all significant differences.
Those with moderate to severe disease also reported more trouble sleeping, along with more frequent sleep disturbances, longer time transitioning into sleep, and more use of nonprescription sleep medications than those with mild disease.
Among those with moderate to severe disease, those who were inadequately controlled had a higher level of itch intensity and more frequent itching (a mean of 6.3 days per week), compared with those who were controlled (a mean of 5.7 days per week).
In a previous study looking at patient burden in a phase 2b clinical trial of dupilumab, Dr. Simpson and his coinvestigators found that adults with moderate to severe AD reported a “multidimensional burden” of disease that included disease activity, patient-reported symptoms, quality-of-life impact, and comorbidities (J Am Acad Dermatol. 2016 Mar;74[3]:491-8).
The current analysis based on the AD-AWARE study was supported by dupilumab manufacturers Regeneron Pharmaceuticals and Sanofi. Dr. Simpson reported disclosures related to Amgen, Anacor, Asubio, Celgene, Chugai, Galderma, Genentech, Medicis, Merck, and Regeneron; five of the 15 authors were employees of Sanofi or Regeneron. Other authors reported disclosures related to these and other companies.
SOURCE: Simpson EL et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2018.1572.
More than half of the patients with moderate to severe atopic dermatitis (AD) had inadequately controlled disease, which was associated with a higher patient-reported disease burden compared with those who had adequately controlled disease, in a cross-sectional study of adults with AD.
Disease control aside, patient-reported burden was generally higher in those with moderate to severe AD versus patients with mild AD, according to Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, and his coauthors.
“These results highlight beyond using measures of disease activity,” the researchers wrote. The study, published in JAMA Dermatology, was conducted before the introduction of dupilumab (Dupixent), the first biologic approved by the Food and Drug Administration for treatment of moderate to severe AD, the authors noted. (The study was supported by the manufacturers of dupilumab.)
The patients were in the Adults With Atopic Dermatitis Reporting on Their Experience (AD-AWARE) study, a cross-sectional analysis of burden of illness in adults with AD in clinical practices at six U.S. academic medical centers. The 1,519 patients completed a self-administered, Internet-based questionnaire during 2013-2014. Among these patients, 830 (54.6%) had moderate to severe AD.
A total of 185 patients with moderate to severe disease received systemic immunomodulators or phototherapy, and of those, more than half (103, or 55.7%) reported inadequate disease control, according to the survey results.
Regardless of disease control, the patients with moderate to severe AD had a greater burden of disease compared with patients with mild AD, according to the investigators. Those burdens included more severe pain and itching, sleep effects, anxiety and depression, and impairment of health-related quality of life, they reported.
Those with moderate to severe disease had a mean of 5.7 days per week with itchy skin, and 22.8% reported itch lasting for more than half a day, compared with a mean of 2.7 days and 2.9%, respectively, for those with mild disease, all significant differences.
Those with moderate to severe disease also reported more trouble sleeping, along with more frequent sleep disturbances, longer time transitioning into sleep, and more use of nonprescription sleep medications than those with mild disease.
Among those with moderate to severe disease, those who were inadequately controlled had a higher level of itch intensity and more frequent itching (a mean of 6.3 days per week), compared with those who were controlled (a mean of 5.7 days per week).
In a previous study looking at patient burden in a phase 2b clinical trial of dupilumab, Dr. Simpson and his coinvestigators found that adults with moderate to severe AD reported a “multidimensional burden” of disease that included disease activity, patient-reported symptoms, quality-of-life impact, and comorbidities (J Am Acad Dermatol. 2016 Mar;74[3]:491-8).
The current analysis based on the AD-AWARE study was supported by dupilumab manufacturers Regeneron Pharmaceuticals and Sanofi. Dr. Simpson reported disclosures related to Amgen, Anacor, Asubio, Celgene, Chugai, Galderma, Genentech, Medicis, Merck, and Regeneron; five of the 15 authors were employees of Sanofi or Regeneron. Other authors reported disclosures related to these and other companies.
SOURCE: Simpson EL et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2018.1572.
More than half of the patients with moderate to severe atopic dermatitis (AD) had inadequately controlled disease, which was associated with a higher patient-reported disease burden compared with those who had adequately controlled disease, in a cross-sectional study of adults with AD.
Disease control aside, patient-reported burden was generally higher in those with moderate to severe AD versus patients with mild AD, according to Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, and his coauthors.
“These results highlight beyond using measures of disease activity,” the researchers wrote. The study, published in JAMA Dermatology, was conducted before the introduction of dupilumab (Dupixent), the first biologic approved by the Food and Drug Administration for treatment of moderate to severe AD, the authors noted. (The study was supported by the manufacturers of dupilumab.)
The patients were in the Adults With Atopic Dermatitis Reporting on Their Experience (AD-AWARE) study, a cross-sectional analysis of burden of illness in adults with AD in clinical practices at six U.S. academic medical centers. The 1,519 patients completed a self-administered, Internet-based questionnaire during 2013-2014. Among these patients, 830 (54.6%) had moderate to severe AD.
A total of 185 patients with moderate to severe disease received systemic immunomodulators or phototherapy, and of those, more than half (103, or 55.7%) reported inadequate disease control, according to the survey results.
Regardless of disease control, the patients with moderate to severe AD had a greater burden of disease compared with patients with mild AD, according to the investigators. Those burdens included more severe pain and itching, sleep effects, anxiety and depression, and impairment of health-related quality of life, they reported.
Those with moderate to severe disease had a mean of 5.7 days per week with itchy skin, and 22.8% reported itch lasting for more than half a day, compared with a mean of 2.7 days and 2.9%, respectively, for those with mild disease, all significant differences.
Those with moderate to severe disease also reported more trouble sleeping, along with more frequent sleep disturbances, longer time transitioning into sleep, and more use of nonprescription sleep medications than those with mild disease.
Among those with moderate to severe disease, those who were inadequately controlled had a higher level of itch intensity and more frequent itching (a mean of 6.3 days per week), compared with those who were controlled (a mean of 5.7 days per week).
In a previous study looking at patient burden in a phase 2b clinical trial of dupilumab, Dr. Simpson and his coinvestigators found that adults with moderate to severe AD reported a “multidimensional burden” of disease that included disease activity, patient-reported symptoms, quality-of-life impact, and comorbidities (J Am Acad Dermatol. 2016 Mar;74[3]:491-8).
The current analysis based on the AD-AWARE study was supported by dupilumab manufacturers Regeneron Pharmaceuticals and Sanofi. Dr. Simpson reported disclosures related to Amgen, Anacor, Asubio, Celgene, Chugai, Galderma, Genentech, Medicis, Merck, and Regeneron; five of the 15 authors were employees of Sanofi or Regeneron. Other authors reported disclosures related to these and other companies.
SOURCE: Simpson EL et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2018.1572.
FROM JAMA DERMATOLOGY
Key clinical point: Consider the burden of disease in patients, in addition to severity, when evaluating patients with atopic dermatitis.
Major finding: Patients with moderate to severe AD experienced itchy skin a mean of 5.7 days per week, with 22.8% reporting itch lasting for more than half a day, vs. a mean of 2.7 days and 2.9%, respectively, among those with mild disease (P less than .001 for both measures).
Study details: A cross-sectional study of 1,519 adult patients with AD, who answered a questionnaire related to disease burden.
Disclosures: Dr. Simpson reported disclosures related to Amgen, Anacor, Asubio, Celgene, Chugai, Galderma, Genentech, Medicis, Merck, and Regeneron. Five of the 15 authors were employees of Sanofi or Regeneron. Other authors reported disclosures related to these and/or other companies.
Source: Simpson EL et al. JAMA Dermatol. 2018 Jul 3. doi: 10.1001/jamadermatol.2018.1572.
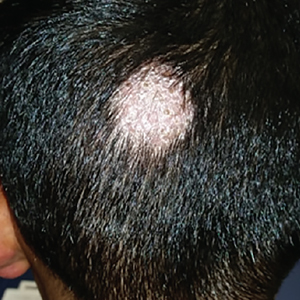
Body-wide, pruritic, papular rash • scalp lesion • excoriation • Dx?
THE CASE
A 7-year-old boy presented with a one-week history of a pruritic rash, which first appeared on his back and continued to spread across his entire body. The patient’s medical history was significant for a scalp lesion (FIGURE 1) that was being treated with oral griseofulvin (started 3 days earlier). He had no history of seasonal allergies, asthma, recent illness, or recent immunizations.
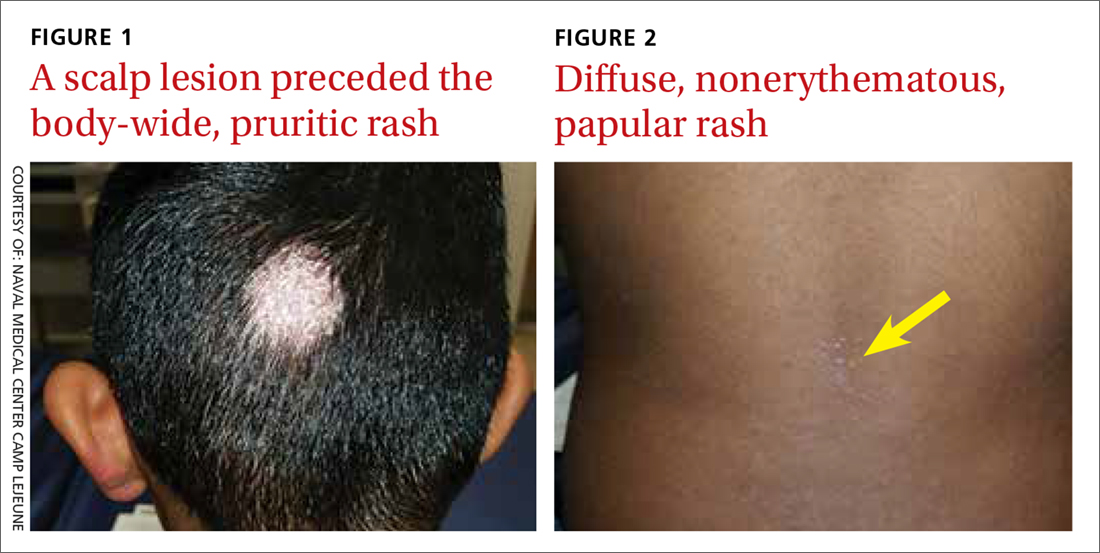
The physical exam was significant for a body-wide, nonerythematous, papular rash (FIGURE 2). There was evidence of excoriation due to itching. No mucosal involvement was appreciated. The remainder of the examination was unremarkable.
QUESTION
Based on the patient’s history and physical exam, which of the following is the most likely diagnosis?
A. Gianotti-Crosti syndrome
B. Atopic dermatitis
C. Dermatophytid reaction
D. Morbilliform drug eruption.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
The answer is C, dermatophytid reaction.
DISCUSSION
A dermatophytid reaction is a type of id reaction, or autoeczematization. An id reaction is when a localized dermatitis becomes a generalized pruritic eruption.1 In this case, the patient’s dermatitis was the result of a dermatophyte infection (tinea capitis), but an id reaction can also occur in response to noninfectious dermatitides and may be of an atopic, contact, or seborrheic nature.1
Dermatophytid reactions occur in up to 5% of all dermatophyte infections (most commonly tinea pedis) and are proposed to be type IV hypersensitivity reactions to the release of fungal antigens.1 These reactions can occur either before or after the initiation of antifungal treatment. They manifest as symmetric, pruritic, papulovesicular eruptions with fine scaling and commonly affect the face, trunk, extremities, palms, and interdigital spaces.1
What about other possible diagnoses?
Gianotti-Crosti syndrome is an asymptomatic, symmetric, papulovesicular dermatosis that involves the face, limbs, and buttocks of children 2 to 6 years of age.2 The lesions develop in response to a respiratory or gastrointestinal illness.2 They are typically associated with Epstein-Barr virus, hepatitis B, cytomegalovirus, respiratory syncytial virus, and coxsackievirus, but can occur with bacterial infections or following administration of routine immunizations.2
The lesions are self-limited and resolve within 2 months.2 Symptomatic lesions may be treated with oral antihistamines or steroids (topical or systemic).2
Continue to: Atopic dermatitis
Atopic dermatitis is characterized by symmetric involvement of the flexural surfaces of the body with a pruritic, erythematous rash that may have a fine scale.3 It usually manifests prior to 2 years of age, is recurrent, and is commonly associated with allergic rhinitis and asthma.3 Treatment involves trigger avoidance, topical emollients, topical corticosteroids, dilute bleach baths, and topical calcineurin inhibitors.3,4 For patients with significant nocturnal symptoms and sleep loss, oral antihistamines may be helpful.4
Morbilliform drug eruptions are the most common type of dermatologic drug reaction.5 These rashes occur approximately one to 2 weeks after exposure to a causative drug; they consist of pruritic, erythematous papules or macules that start centrally and may spread to the proximal extremities.5 Treatment involves discontinuation of the offending agent. Symptomatic relief may be achieved with oral antihistamines or topical or systemic corticosteroids.5
Treatment of dermatophytid reactions
While the initial impulse in the treatment of a dermatophytid reaction may be to discontinue oral antifungals, these treatments actually help resolve the underlying dermatophyte infection and should be continued. For children with tinea capitis, at least 6 weeks of treatment with an oral antifungal agent is warranted. Medications approved by the US Food and Drug Administration include terbinafine (for patients >4 years of age) and griseofulvin (for patients >2 years of age). Dosages are weight-based. (Fluconazole and itraconazole are not approved for this indication.) Lubricants, topical corticosteroids, and oral antihistamines can be used for acute management of pruritus.1
Our patient was treated successfully with griseofulvin and an oral antihistamine. However, he experienced headaches attributed to griseofulvin and was switched to terbinafine 5 mg/kg/d for 4 weeks. His tinea capitis was resolved at 8 weeks.
CORRESPONDENCE
Richard Temple, MD, CAPT, MC, USN. Department of Family Medicine, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547; Richard.w.temple2.mil@mail.mil.
1. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
2. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
3. Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86:35-42.
4. Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015;136:554-565.
5. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
THE CASE
A 7-year-old boy presented with a one-week history of a pruritic rash, which first appeared on his back and continued to spread across his entire body. The patient’s medical history was significant for a scalp lesion (FIGURE 1) that was being treated with oral griseofulvin (started 3 days earlier). He had no history of seasonal allergies, asthma, recent illness, or recent immunizations.

The physical exam was significant for a body-wide, nonerythematous, papular rash (FIGURE 2). There was evidence of excoriation due to itching. No mucosal involvement was appreciated. The remainder of the examination was unremarkable.
QUESTION
Based on the patient’s history and physical exam, which of the following is the most likely diagnosis?
A. Gianotti-Crosti syndrome
B. Atopic dermatitis
C. Dermatophytid reaction
D. Morbilliform drug eruption.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
The answer is C, dermatophytid reaction.
DISCUSSION
A dermatophytid reaction is a type of id reaction, or autoeczematization. An id reaction is when a localized dermatitis becomes a generalized pruritic eruption.1 In this case, the patient’s dermatitis was the result of a dermatophyte infection (tinea capitis), but an id reaction can also occur in response to noninfectious dermatitides and may be of an atopic, contact, or seborrheic nature.1
Dermatophytid reactions occur in up to 5% of all dermatophyte infections (most commonly tinea pedis) and are proposed to be type IV hypersensitivity reactions to the release of fungal antigens.1 These reactions can occur either before or after the initiation of antifungal treatment. They manifest as symmetric, pruritic, papulovesicular eruptions with fine scaling and commonly affect the face, trunk, extremities, palms, and interdigital spaces.1
What about other possible diagnoses?
Gianotti-Crosti syndrome is an asymptomatic, symmetric, papulovesicular dermatosis that involves the face, limbs, and buttocks of children 2 to 6 years of age.2 The lesions develop in response to a respiratory or gastrointestinal illness.2 They are typically associated with Epstein-Barr virus, hepatitis B, cytomegalovirus, respiratory syncytial virus, and coxsackievirus, but can occur with bacterial infections or following administration of routine immunizations.2
The lesions are self-limited and resolve within 2 months.2 Symptomatic lesions may be treated with oral antihistamines or steroids (topical or systemic).2
Continue to: Atopic dermatitis
Atopic dermatitis is characterized by symmetric involvement of the flexural surfaces of the body with a pruritic, erythematous rash that may have a fine scale.3 It usually manifests prior to 2 years of age, is recurrent, and is commonly associated with allergic rhinitis and asthma.3 Treatment involves trigger avoidance, topical emollients, topical corticosteroids, dilute bleach baths, and topical calcineurin inhibitors.3,4 For patients with significant nocturnal symptoms and sleep loss, oral antihistamines may be helpful.4
Morbilliform drug eruptions are the most common type of dermatologic drug reaction.5 These rashes occur approximately one to 2 weeks after exposure to a causative drug; they consist of pruritic, erythematous papules or macules that start centrally and may spread to the proximal extremities.5 Treatment involves discontinuation of the offending agent. Symptomatic relief may be achieved with oral antihistamines or topical or systemic corticosteroids.5
Treatment of dermatophytid reactions
While the initial impulse in the treatment of a dermatophytid reaction may be to discontinue oral antifungals, these treatments actually help resolve the underlying dermatophyte infection and should be continued. For children with tinea capitis, at least 6 weeks of treatment with an oral antifungal agent is warranted. Medications approved by the US Food and Drug Administration include terbinafine (for patients >4 years of age) and griseofulvin (for patients >2 years of age). Dosages are weight-based. (Fluconazole and itraconazole are not approved for this indication.) Lubricants, topical corticosteroids, and oral antihistamines can be used for acute management of pruritus.1
Our patient was treated successfully with griseofulvin and an oral antihistamine. However, he experienced headaches attributed to griseofulvin and was switched to terbinafine 5 mg/kg/d for 4 weeks. His tinea capitis was resolved at 8 weeks.
CORRESPONDENCE
Richard Temple, MD, CAPT, MC, USN. Department of Family Medicine, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547; Richard.w.temple2.mil@mail.mil.
THE CASE
A 7-year-old boy presented with a one-week history of a pruritic rash, which first appeared on his back and continued to spread across his entire body. The patient’s medical history was significant for a scalp lesion (FIGURE 1) that was being treated with oral griseofulvin (started 3 days earlier). He had no history of seasonal allergies, asthma, recent illness, or recent immunizations.

The physical exam was significant for a body-wide, nonerythematous, papular rash (FIGURE 2). There was evidence of excoriation due to itching. No mucosal involvement was appreciated. The remainder of the examination was unremarkable.
QUESTION
Based on the patient’s history and physical exam, which of the following is the most likely diagnosis?
A. Gianotti-Crosti syndrome
B. Atopic dermatitis
C. Dermatophytid reaction
D. Morbilliform drug eruption.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
The answer is C, dermatophytid reaction.
DISCUSSION
A dermatophytid reaction is a type of id reaction, or autoeczematization. An id reaction is when a localized dermatitis becomes a generalized pruritic eruption.1 In this case, the patient’s dermatitis was the result of a dermatophyte infection (tinea capitis), but an id reaction can also occur in response to noninfectious dermatitides and may be of an atopic, contact, or seborrheic nature.1
Dermatophytid reactions occur in up to 5% of all dermatophyte infections (most commonly tinea pedis) and are proposed to be type IV hypersensitivity reactions to the release of fungal antigens.1 These reactions can occur either before or after the initiation of antifungal treatment. They manifest as symmetric, pruritic, papulovesicular eruptions with fine scaling and commonly affect the face, trunk, extremities, palms, and interdigital spaces.1
What about other possible diagnoses?
Gianotti-Crosti syndrome is an asymptomatic, symmetric, papulovesicular dermatosis that involves the face, limbs, and buttocks of children 2 to 6 years of age.2 The lesions develop in response to a respiratory or gastrointestinal illness.2 They are typically associated with Epstein-Barr virus, hepatitis B, cytomegalovirus, respiratory syncytial virus, and coxsackievirus, but can occur with bacterial infections or following administration of routine immunizations.2
The lesions are self-limited and resolve within 2 months.2 Symptomatic lesions may be treated with oral antihistamines or steroids (topical or systemic).2
Continue to: Atopic dermatitis
Atopic dermatitis is characterized by symmetric involvement of the flexural surfaces of the body with a pruritic, erythematous rash that may have a fine scale.3 It usually manifests prior to 2 years of age, is recurrent, and is commonly associated with allergic rhinitis and asthma.3 Treatment involves trigger avoidance, topical emollients, topical corticosteroids, dilute bleach baths, and topical calcineurin inhibitors.3,4 For patients with significant nocturnal symptoms and sleep loss, oral antihistamines may be helpful.4
Morbilliform drug eruptions are the most common type of dermatologic drug reaction.5 These rashes occur approximately one to 2 weeks after exposure to a causative drug; they consist of pruritic, erythematous papules or macules that start centrally and may spread to the proximal extremities.5 Treatment involves discontinuation of the offending agent. Symptomatic relief may be achieved with oral antihistamines or topical or systemic corticosteroids.5
Treatment of dermatophytid reactions
While the initial impulse in the treatment of a dermatophytid reaction may be to discontinue oral antifungals, these treatments actually help resolve the underlying dermatophyte infection and should be continued. For children with tinea capitis, at least 6 weeks of treatment with an oral antifungal agent is warranted. Medications approved by the US Food and Drug Administration include terbinafine (for patients >4 years of age) and griseofulvin (for patients >2 years of age). Dosages are weight-based. (Fluconazole and itraconazole are not approved for this indication.) Lubricants, topical corticosteroids, and oral antihistamines can be used for acute management of pruritus.1
Our patient was treated successfully with griseofulvin and an oral antihistamine. However, he experienced headaches attributed to griseofulvin and was switched to terbinafine 5 mg/kg/d for 4 weeks. His tinea capitis was resolved at 8 weeks.
CORRESPONDENCE
Richard Temple, MD, CAPT, MC, USN. Department of Family Medicine, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547; Richard.w.temple2.mil@mail.mil.
1. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
2. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
3. Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86:35-42.
4. Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015;136:554-565.
5. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
1. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
2. Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54:136-145.
3. Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86:35-42.
4. Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015;136:554-565.
5. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
FDA approves topical anticholinergic for primary axillary hyperhidrosis
The in adults and children aged 9 years and older.
Glycopyrronium, which is formulated in a cloth wipe, will be marketed as Qbrexza and is expected to be available in October, according to the approval announcement June 29, which was made by Dermira, the manufacturer. The instructions for use section in the prescribing information states that patients are advised to use one cloth to apply the medication to both axillae, wiping the cloth across each underarm. Each cloth, intended for single use, is pre-moistened with 2.4% glycopyrronium solution.
Glycopyrronium blocks sweat production “by inhibiting the interaction between acetylcholine and the cholinergic receptors responsible for sweat gland activation,” the company said in a February press release.
The approval was based on the results of two phase 3 clinical studies, ATMOS-1 and ATMOS-2, which were multicenter, randomized, double-blind, vehicle-controlled, 4-week studies in patients 9 years of age or older with primary axillary hyperhidrosis for 6 months or longer. Study subjects had production of at least 50 mg of underarm sweat over 5 minutes, and scores of four or higher on the 11-point Axillary Sweating Daily Diary (ASDD) or the Children’s ASDD (ASDD-C) – an instrument developed by the company in consultation with the FDA – and scores of three or four on the four-grade Hyperhidrosis Disease Severity Scale (HDSS). In total, 463 patients were randomized to receive glycopyrronium and 234 to vehicle. Forty four of these patients were aged 9-16 years, with 25 receiving glycopyrronium and 19 receiving vehicle.
According to the February release, the ASDD/ASDD-C severity scale responder rates were about 60% in the pediatric and adult groups treated with glycopyrronium, compared with 13.0% and 28.8% for children and adults, respectively, in the vehicle group. At week 4, the median absolute change in sweat production was a reduction of 64.2 mg in children and a reduction of 80.6 mg among adults treated with glycopyrronium, compared with reductions of 53.7 mg and 62 mg among those in the vehicle group, respectively.
In the glycopyrronium-treated group, almost 80% of the pediatric patients and 74.3% of the adults experienced at least a 50% reduction in sweat production at week 4, compared with almost 55% and 53%, respectively, in the vehicle group.
Among pediatric patients, the mean decrease from baseline in the Children’s Dermatology Quality of Life Index was 8.1 in glycopyrronium-treated patients, compared with 1.9 in the vehicle group. In adults, scores on the Dermatology Life Quality Index measure were reduced by 8.4 and 4.7 in glycopyrronium- and vehicle-treated adult patients, respectively.
Nearly 57% of adults and 44% of pediatric patients treated with glycopyrronium experienced treatment-emergent adverse events, compared with 34.3% of adults and 10.5% of the pediatric patients in the vehicle group. The majority were related to anticholinergic activity and were mild, and rarely led to drug discontinuation, according to the company.
The press release announcing the approval stated that the most common adverse effects observed after application of glycopyrronium were dry mouth, mydriasis, sore throat, headache, urinary hesitation, blurred vision, dry nose, dry throat, dry eye, dry skin and constipation. Erythema, burning/stinging, and pruritus were the most common skin reactions.
The product is contraindicated in patients with glaucoma, paralytic ileus, and other medical conditions that can be exacerbated by its anticholinergic effects, according to the prescribing information. Patients should be advised that they should wash their hands thoroughly after application, and that it can cause temporary dilation of the pupils and blurred vision if glycopyrronium comes into contact with their eyes.
The in adults and children aged 9 years and older.
Glycopyrronium, which is formulated in a cloth wipe, will be marketed as Qbrexza and is expected to be available in October, according to the approval announcement June 29, which was made by Dermira, the manufacturer. The instructions for use section in the prescribing information states that patients are advised to use one cloth to apply the medication to both axillae, wiping the cloth across each underarm. Each cloth, intended for single use, is pre-moistened with 2.4% glycopyrronium solution.
Glycopyrronium blocks sweat production “by inhibiting the interaction between acetylcholine and the cholinergic receptors responsible for sweat gland activation,” the company said in a February press release.
The approval was based on the results of two phase 3 clinical studies, ATMOS-1 and ATMOS-2, which were multicenter, randomized, double-blind, vehicle-controlled, 4-week studies in patients 9 years of age or older with primary axillary hyperhidrosis for 6 months or longer. Study subjects had production of at least 50 mg of underarm sweat over 5 minutes, and scores of four or higher on the 11-point Axillary Sweating Daily Diary (ASDD) or the Children’s ASDD (ASDD-C) – an instrument developed by the company in consultation with the FDA – and scores of three or four on the four-grade Hyperhidrosis Disease Severity Scale (HDSS). In total, 463 patients were randomized to receive glycopyrronium and 234 to vehicle. Forty four of these patients were aged 9-16 years, with 25 receiving glycopyrronium and 19 receiving vehicle.
According to the February release, the ASDD/ASDD-C severity scale responder rates were about 60% in the pediatric and adult groups treated with glycopyrronium, compared with 13.0% and 28.8% for children and adults, respectively, in the vehicle group. At week 4, the median absolute change in sweat production was a reduction of 64.2 mg in children and a reduction of 80.6 mg among adults treated with glycopyrronium, compared with reductions of 53.7 mg and 62 mg among those in the vehicle group, respectively.
In the glycopyrronium-treated group, almost 80% of the pediatric patients and 74.3% of the adults experienced at least a 50% reduction in sweat production at week 4, compared with almost 55% and 53%, respectively, in the vehicle group.
Among pediatric patients, the mean decrease from baseline in the Children’s Dermatology Quality of Life Index was 8.1 in glycopyrronium-treated patients, compared with 1.9 in the vehicle group. In adults, scores on the Dermatology Life Quality Index measure were reduced by 8.4 and 4.7 in glycopyrronium- and vehicle-treated adult patients, respectively.
Nearly 57% of adults and 44% of pediatric patients treated with glycopyrronium experienced treatment-emergent adverse events, compared with 34.3% of adults and 10.5% of the pediatric patients in the vehicle group. The majority were related to anticholinergic activity and were mild, and rarely led to drug discontinuation, according to the company.
The press release announcing the approval stated that the most common adverse effects observed after application of glycopyrronium were dry mouth, mydriasis, sore throat, headache, urinary hesitation, blurred vision, dry nose, dry throat, dry eye, dry skin and constipation. Erythema, burning/stinging, and pruritus were the most common skin reactions.
The product is contraindicated in patients with glaucoma, paralytic ileus, and other medical conditions that can be exacerbated by its anticholinergic effects, according to the prescribing information. Patients should be advised that they should wash their hands thoroughly after application, and that it can cause temporary dilation of the pupils and blurred vision if glycopyrronium comes into contact with their eyes.
The in adults and children aged 9 years and older.
Glycopyrronium, which is formulated in a cloth wipe, will be marketed as Qbrexza and is expected to be available in October, according to the approval announcement June 29, which was made by Dermira, the manufacturer. The instructions for use section in the prescribing information states that patients are advised to use one cloth to apply the medication to both axillae, wiping the cloth across each underarm. Each cloth, intended for single use, is pre-moistened with 2.4% glycopyrronium solution.
Glycopyrronium blocks sweat production “by inhibiting the interaction between acetylcholine and the cholinergic receptors responsible for sweat gland activation,” the company said in a February press release.
The approval was based on the results of two phase 3 clinical studies, ATMOS-1 and ATMOS-2, which were multicenter, randomized, double-blind, vehicle-controlled, 4-week studies in patients 9 years of age or older with primary axillary hyperhidrosis for 6 months or longer. Study subjects had production of at least 50 mg of underarm sweat over 5 minutes, and scores of four or higher on the 11-point Axillary Sweating Daily Diary (ASDD) or the Children’s ASDD (ASDD-C) – an instrument developed by the company in consultation with the FDA – and scores of three or four on the four-grade Hyperhidrosis Disease Severity Scale (HDSS). In total, 463 patients were randomized to receive glycopyrronium and 234 to vehicle. Forty four of these patients were aged 9-16 years, with 25 receiving glycopyrronium and 19 receiving vehicle.
According to the February release, the ASDD/ASDD-C severity scale responder rates were about 60% in the pediatric and adult groups treated with glycopyrronium, compared with 13.0% and 28.8% for children and adults, respectively, in the vehicle group. At week 4, the median absolute change in sweat production was a reduction of 64.2 mg in children and a reduction of 80.6 mg among adults treated with glycopyrronium, compared with reductions of 53.7 mg and 62 mg among those in the vehicle group, respectively.
In the glycopyrronium-treated group, almost 80% of the pediatric patients and 74.3% of the adults experienced at least a 50% reduction in sweat production at week 4, compared with almost 55% and 53%, respectively, in the vehicle group.
Among pediatric patients, the mean decrease from baseline in the Children’s Dermatology Quality of Life Index was 8.1 in glycopyrronium-treated patients, compared with 1.9 in the vehicle group. In adults, scores on the Dermatology Life Quality Index measure were reduced by 8.4 and 4.7 in glycopyrronium- and vehicle-treated adult patients, respectively.
Nearly 57% of adults and 44% of pediatric patients treated with glycopyrronium experienced treatment-emergent adverse events, compared with 34.3% of adults and 10.5% of the pediatric patients in the vehicle group. The majority were related to anticholinergic activity and were mild, and rarely led to drug discontinuation, according to the company.
The press release announcing the approval stated that the most common adverse effects observed after application of glycopyrronium were dry mouth, mydriasis, sore throat, headache, urinary hesitation, blurred vision, dry nose, dry throat, dry eye, dry skin and constipation. Erythema, burning/stinging, and pruritus were the most common skin reactions.
The product is contraindicated in patients with glaucoma, paralytic ileus, and other medical conditions that can be exacerbated by its anticholinergic effects, according to the prescribing information. Patients should be advised that they should wash their hands thoroughly after application, and that it can cause temporary dilation of the pupils and blurred vision if glycopyrronium comes into contact with their eyes.
Elderly patients with psoriasis can benefit from biologics with low rates of adverse events
according to a new retrospective study.
Among 266 older patients, 65% achieved a 75% improvement in Psoriasis Area Severity Index score (PASI 75) after 1 year of therapy; 50% reached a PASI 90, and 40% a PASI 100, Francesca Prignano MD, PhD, and her colleagues reported in the Journal of the European Academy of Dermatology and Venereology. The rate of serious adverse events was less than 10%.
Elderly patients – those aged 65 years and older – are commonly excluded from studies on biologic treatments because they have more medical comorbidities and are thought to be more at risk for serious adverse events, like infections and malignancy, wrote Dr. Prignano of the dermatology unit, University of Florence, Italy, and her colleagues.
As a result, they noted, there is a “lack of information concerning safety and effectiveness of available treatments for psoriasis in the elderly, particularly about new biologic agents. Disease remission should be an objective for both younger patients and older patients, and biologic therapy should be considered a treatment option for all patients.”
To examine both the benefit and risk of biologics in this population, the team reviewed the records of 266 elderly psoriasis patients; everyone had been on a biologic treatment for at least 1 year.
The primary outcome was PASI score at weeks 8, 16, 28, and 52. The secondary outcomes were the rate and types of biologic-associated adverse events.
The study comprised 266 patients (mean age 72 years). Their mean psoriasis duration was 25.7 years. Comorbidities included psoriatic arthritis; hypertension and dyslipidemia; diabetes mellitus; cardiovascular, gastrointestinal and respiratory diseases; osteoporosis; thyroid dysfunction; depression; and cancer.
Adalimumab was the most commonly prescribed biologic (31%), followed by ustekinumab (28.9%), etanercept (20%), and secukinumab (15%). A smaller proportion of patients were taking infliximab, golimumab, or certolizumab pegol.
The mean baseline PASI was 16.5, although the range was wide (4-54). At the time of review, the average biologic treatment duration was 44 months. Almost half of the cohort (128) were on their second biologic, and 20 more had been on three biologics. A few patients were taking concomitant medications, including steroids, cyclosporine, and acitretin.
The mean PASI scores decreased to 3.7 at week 16, 1.6 at week 28, and 1.2 at week 52. The group exhibited a rapid response to biologic treatment. By 16 weeks, about 55% had achieved a PASI 75, about 28% a PASI 90, and about 20% a PASI 100. By 28 weeks, these numbers were about 64%, 45%, and 35%, respectively. At 1 year, they were about 65%, 50%, and 40%, respectively.
The rate of adverse events was 9.4%. There were 25 events in the cohort, the majority of which (48%) were infections; these included four respiratory infections, three urinary tract infections, two cases of mucocutaneous candidiasis, two cases of herpes zoster infection, and one case of erysipelas.
There were four malignancies: three nonmelanoma skin cancers and one vocal cord cancer.
Noting that, to date, their study represented “the broadest experience on the use of biological drugs” for elderly patients with psoriasis, they wrote that while “comorbidities should be taken into consideration when a long-term treatment is proposed, for the higher risk of side effects and drug interactions,” they wrote, noting that none of the 266 patients had a serious infection and the malignancy rate was low (1.5%).
None of the authors had financial disclosures, and the study had no funding source.
SOURCE: Ricceri F et al. J Eur Acad Dermatol Venereol. 2018 Jun 15. doi: 10.1111/jdv.15139.
according to a new retrospective study.
Among 266 older patients, 65% achieved a 75% improvement in Psoriasis Area Severity Index score (PASI 75) after 1 year of therapy; 50% reached a PASI 90, and 40% a PASI 100, Francesca Prignano MD, PhD, and her colleagues reported in the Journal of the European Academy of Dermatology and Venereology. The rate of serious adverse events was less than 10%.
Elderly patients – those aged 65 years and older – are commonly excluded from studies on biologic treatments because they have more medical comorbidities and are thought to be more at risk for serious adverse events, like infections and malignancy, wrote Dr. Prignano of the dermatology unit, University of Florence, Italy, and her colleagues.
As a result, they noted, there is a “lack of information concerning safety and effectiveness of available treatments for psoriasis in the elderly, particularly about new biologic agents. Disease remission should be an objective for both younger patients and older patients, and biologic therapy should be considered a treatment option for all patients.”
To examine both the benefit and risk of biologics in this population, the team reviewed the records of 266 elderly psoriasis patients; everyone had been on a biologic treatment for at least 1 year.
The primary outcome was PASI score at weeks 8, 16, 28, and 52. The secondary outcomes were the rate and types of biologic-associated adverse events.
The study comprised 266 patients (mean age 72 years). Their mean psoriasis duration was 25.7 years. Comorbidities included psoriatic arthritis; hypertension and dyslipidemia; diabetes mellitus; cardiovascular, gastrointestinal and respiratory diseases; osteoporosis; thyroid dysfunction; depression; and cancer.
Adalimumab was the most commonly prescribed biologic (31%), followed by ustekinumab (28.9%), etanercept (20%), and secukinumab (15%). A smaller proportion of patients were taking infliximab, golimumab, or certolizumab pegol.
The mean baseline PASI was 16.5, although the range was wide (4-54). At the time of review, the average biologic treatment duration was 44 months. Almost half of the cohort (128) were on their second biologic, and 20 more had been on three biologics. A few patients were taking concomitant medications, including steroids, cyclosporine, and acitretin.
The mean PASI scores decreased to 3.7 at week 16, 1.6 at week 28, and 1.2 at week 52. The group exhibited a rapid response to biologic treatment. By 16 weeks, about 55% had achieved a PASI 75, about 28% a PASI 90, and about 20% a PASI 100. By 28 weeks, these numbers were about 64%, 45%, and 35%, respectively. At 1 year, they were about 65%, 50%, and 40%, respectively.
The rate of adverse events was 9.4%. There were 25 events in the cohort, the majority of which (48%) were infections; these included four respiratory infections, three urinary tract infections, two cases of mucocutaneous candidiasis, two cases of herpes zoster infection, and one case of erysipelas.
There were four malignancies: three nonmelanoma skin cancers and one vocal cord cancer.
Noting that, to date, their study represented “the broadest experience on the use of biological drugs” for elderly patients with psoriasis, they wrote that while “comorbidities should be taken into consideration when a long-term treatment is proposed, for the higher risk of side effects and drug interactions,” they wrote, noting that none of the 266 patients had a serious infection and the malignancy rate was low (1.5%).
None of the authors had financial disclosures, and the study had no funding source.
SOURCE: Ricceri F et al. J Eur Acad Dermatol Venereol. 2018 Jun 15. doi: 10.1111/jdv.15139.
according to a new retrospective study.
Among 266 older patients, 65% achieved a 75% improvement in Psoriasis Area Severity Index score (PASI 75) after 1 year of therapy; 50% reached a PASI 90, and 40% a PASI 100, Francesca Prignano MD, PhD, and her colleagues reported in the Journal of the European Academy of Dermatology and Venereology. The rate of serious adverse events was less than 10%.
Elderly patients – those aged 65 years and older – are commonly excluded from studies on biologic treatments because they have more medical comorbidities and are thought to be more at risk for serious adverse events, like infections and malignancy, wrote Dr. Prignano of the dermatology unit, University of Florence, Italy, and her colleagues.
As a result, they noted, there is a “lack of information concerning safety and effectiveness of available treatments for psoriasis in the elderly, particularly about new biologic agents. Disease remission should be an objective for both younger patients and older patients, and biologic therapy should be considered a treatment option for all patients.”
To examine both the benefit and risk of biologics in this population, the team reviewed the records of 266 elderly psoriasis patients; everyone had been on a biologic treatment for at least 1 year.
The primary outcome was PASI score at weeks 8, 16, 28, and 52. The secondary outcomes were the rate and types of biologic-associated adverse events.
The study comprised 266 patients (mean age 72 years). Their mean psoriasis duration was 25.7 years. Comorbidities included psoriatic arthritis; hypertension and dyslipidemia; diabetes mellitus; cardiovascular, gastrointestinal and respiratory diseases; osteoporosis; thyroid dysfunction; depression; and cancer.
Adalimumab was the most commonly prescribed biologic (31%), followed by ustekinumab (28.9%), etanercept (20%), and secukinumab (15%). A smaller proportion of patients were taking infliximab, golimumab, or certolizumab pegol.
The mean baseline PASI was 16.5, although the range was wide (4-54). At the time of review, the average biologic treatment duration was 44 months. Almost half of the cohort (128) were on their second biologic, and 20 more had been on three biologics. A few patients were taking concomitant medications, including steroids, cyclosporine, and acitretin.
The mean PASI scores decreased to 3.7 at week 16, 1.6 at week 28, and 1.2 at week 52. The group exhibited a rapid response to biologic treatment. By 16 weeks, about 55% had achieved a PASI 75, about 28% a PASI 90, and about 20% a PASI 100. By 28 weeks, these numbers were about 64%, 45%, and 35%, respectively. At 1 year, they were about 65%, 50%, and 40%, respectively.
The rate of adverse events was 9.4%. There were 25 events in the cohort, the majority of which (48%) were infections; these included four respiratory infections, three urinary tract infections, two cases of mucocutaneous candidiasis, two cases of herpes zoster infection, and one case of erysipelas.
There were four malignancies: three nonmelanoma skin cancers and one vocal cord cancer.
Noting that, to date, their study represented “the broadest experience on the use of biological drugs” for elderly patients with psoriasis, they wrote that while “comorbidities should be taken into consideration when a long-term treatment is proposed, for the higher risk of side effects and drug interactions,” they wrote, noting that none of the 266 patients had a serious infection and the malignancy rate was low (1.5%).
None of the authors had financial disclosures, and the study had no funding source.
SOURCE: Ricceri F et al. J Eur Acad Dermatol Venereol. 2018 Jun 15. doi: 10.1111/jdv.15139.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: Patients aged 65 years and older responded well to biologics and had a low rate of serious adverse events.
Major finding: At 1 year, 65% achieved a PASI 75, 50% achieved a reached PASI 90, and 40% achieved a PASI 100, with a 9.4% rate of serious adverse events.
Study details: The retrospective study comprised 266 patients aged 65 years and older treated with biologics for psoriasis.
Disclosures: None of the authors had financial disclosures, and the study had no funding source.
Source: Ricceri F et al. J Eur Acad Dermatol Venereol. 2018. doi: 10.1111/jdv.15139.
Changing growth on scalp
The FP thought the flat lesion (arrow) might be a nevus sebaceous (NS) and that the new area could be a malignant transformation.
The FP explained that a biopsy would be needed to learn more about the lesion. He explained that he would remove the area that was friable and bleeding along with part of the original flat lesion. After injecting the area with 1% lidocaine and epinephrine, a shave biopsy was performed using a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The bleeding was stopped using aluminum chloride in water and some electrosurgery. The pathology results revealed syringocystadenoma papilliferum growing within an NS. This benign tumor is rare, but may develop within an NS.
The FP reassured the family that there was no skin cancer. The FP also referred the patient for full removal of the NS and any remnant of the syringocystadenoma papilliferum to avoid future growth and prevent additional bleeding.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP thought the flat lesion (arrow) might be a nevus sebaceous (NS) and that the new area could be a malignant transformation.
The FP explained that a biopsy would be needed to learn more about the lesion. He explained that he would remove the area that was friable and bleeding along with part of the original flat lesion. After injecting the area with 1% lidocaine and epinephrine, a shave biopsy was performed using a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The bleeding was stopped using aluminum chloride in water and some electrosurgery. The pathology results revealed syringocystadenoma papilliferum growing within an NS. This benign tumor is rare, but may develop within an NS.
The FP reassured the family that there was no skin cancer. The FP also referred the patient for full removal of the NS and any remnant of the syringocystadenoma papilliferum to avoid future growth and prevent additional bleeding.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP thought the flat lesion (arrow) might be a nevus sebaceous (NS) and that the new area could be a malignant transformation.
The FP explained that a biopsy would be needed to learn more about the lesion. He explained that he would remove the area that was friable and bleeding along with part of the original flat lesion. After injecting the area with 1% lidocaine and epinephrine, a shave biopsy was performed using a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The bleeding was stopped using aluminum chloride in water and some electrosurgery. The pathology results revealed syringocystadenoma papilliferum growing within an NS. This benign tumor is rare, but may develop within an NS.
The FP reassured the family that there was no skin cancer. The FP also referred the patient for full removal of the NS and any remnant of the syringocystadenoma papilliferum to avoid future growth and prevent additional bleeding.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Epidermal nevus and nevus sebaceous. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine, 2nd ed. New York, NY: McGraw-Hill; 2013:958-962.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Bullae associated with pediatric human parvovirus B19 infection
reported Shoko Yoshii, MD, and his associates at the National Center for Child Health and Development in Tokyo.
In a case study, a 2-year-old boy was admitted to an ED with swelling of both lower limbs. In the 2 weeks previous, he had had a fever that lasted 3 days followed by erythema on the cheeks and limbs. A physical examination reveled edematous erythema on his lower limbs with a predominance of them on the left leg. Doctors analyzed his laboratory results and found that the boy’s white blood cell count was in the normal range, with C-reactive protein level of 3.2 mg/L. The boy was treated with cefazolin for suspected bacterial cellulitis, but this did little; erythema and edema progressed on the left leg and multiple bullae developed 2 days after admission. Within a week, the bullae spontaneously ruptured and resolved.
Parvovirus B19 infection was suspected, and parvovirus B19 IgM was positive on the first day of admission. The boy ultimately recovered and has had no further episodes within 1 year of follow-up.Bullae or vesicles are considered rare manifestations of parvovirus B19 infection, which more typically presents with a “slapped-cheek” appearance and lacy exanthema, sometimes called erythema infectiosum. In adults with parvovirus infection, bullae or vesicles develop at the same time as papular purpuric gloves-and-socks syndrome.
This case did not follow this pattern, with lesions appearing on the lower legs with no involvement of the hands or feet. The few cases of parvovirus infection that have been reported with bulbous skin lesions in children generally were not associated with papular purpuric gloves-and-socks syndrome, which is widely considered a textbook manifestation of parvovirus infection, the authors wrote.
This case study was supported by a grant from National Center for Child Health and Development. No disclosures were reported.
SOURCE: Yoshii S et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.05.038.
reported Shoko Yoshii, MD, and his associates at the National Center for Child Health and Development in Tokyo.
In a case study, a 2-year-old boy was admitted to an ED with swelling of both lower limbs. In the 2 weeks previous, he had had a fever that lasted 3 days followed by erythema on the cheeks and limbs. A physical examination reveled edematous erythema on his lower limbs with a predominance of them on the left leg. Doctors analyzed his laboratory results and found that the boy’s white blood cell count was in the normal range, with C-reactive protein level of 3.2 mg/L. The boy was treated with cefazolin for suspected bacterial cellulitis, but this did little; erythema and edema progressed on the left leg and multiple bullae developed 2 days after admission. Within a week, the bullae spontaneously ruptured and resolved.
Parvovirus B19 infection was suspected, and parvovirus B19 IgM was positive on the first day of admission. The boy ultimately recovered and has had no further episodes within 1 year of follow-up.Bullae or vesicles are considered rare manifestations of parvovirus B19 infection, which more typically presents with a “slapped-cheek” appearance and lacy exanthema, sometimes called erythema infectiosum. In adults with parvovirus infection, bullae or vesicles develop at the same time as papular purpuric gloves-and-socks syndrome.
This case did not follow this pattern, with lesions appearing on the lower legs with no involvement of the hands or feet. The few cases of parvovirus infection that have been reported with bulbous skin lesions in children generally were not associated with papular purpuric gloves-and-socks syndrome, which is widely considered a textbook manifestation of parvovirus infection, the authors wrote.
This case study was supported by a grant from National Center for Child Health and Development. No disclosures were reported.
SOURCE: Yoshii S et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.05.038.
reported Shoko Yoshii, MD, and his associates at the National Center for Child Health and Development in Tokyo.
In a case study, a 2-year-old boy was admitted to an ED with swelling of both lower limbs. In the 2 weeks previous, he had had a fever that lasted 3 days followed by erythema on the cheeks and limbs. A physical examination reveled edematous erythema on his lower limbs with a predominance of them on the left leg. Doctors analyzed his laboratory results and found that the boy’s white blood cell count was in the normal range, with C-reactive protein level of 3.2 mg/L. The boy was treated with cefazolin for suspected bacterial cellulitis, but this did little; erythema and edema progressed on the left leg and multiple bullae developed 2 days after admission. Within a week, the bullae spontaneously ruptured and resolved.
Parvovirus B19 infection was suspected, and parvovirus B19 IgM was positive on the first day of admission. The boy ultimately recovered and has had no further episodes within 1 year of follow-up.Bullae or vesicles are considered rare manifestations of parvovirus B19 infection, which more typically presents with a “slapped-cheek” appearance and lacy exanthema, sometimes called erythema infectiosum. In adults with parvovirus infection, bullae or vesicles develop at the same time as papular purpuric gloves-and-socks syndrome.
This case did not follow this pattern, with lesions appearing on the lower legs with no involvement of the hands or feet. The few cases of parvovirus infection that have been reported with bulbous skin lesions in children generally were not associated with papular purpuric gloves-and-socks syndrome, which is widely considered a textbook manifestation of parvovirus infection, the authors wrote.
This case study was supported by a grant from National Center for Child Health and Development. No disclosures were reported.
SOURCE: Yoshii S et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.05.038.
FROM THE JOURNAL OF PEDIATRICS
Adalimumab strikes out for aortic inflammation in psoriasis
ORLANDO – Adalimumab (Humira) did not reduce aortic inflammation in a year-long, randomized trial that pitted the tumor necrosis factor (TNF) blocker against phototherapy and placebo in 97 psoriasis patients.
a risk factor for cardiovascular events, said senior investigator Joel M. Gelfand, MD, a dermatology professor at the University of Pennsylvania, Philadelphia.
The other trial, from Canada, also found no effect in the ascending aorta after 52 weeks of treatment, but it did find a modest increase in carotid inflammation (J Invest Dermatol. 2017 Aug;137[8]:1638-45).
Both studies used positron emission tomography/computed tomography to assess vascular inflammation.
“We know patients with psoriasis are at increased risk for cardiovascular disease. We think the same kind of inflammation that occurs in atherosclerosis occurs in psoriasis, but we are still teasing out the impact of therapy and which one is most likely to lower the risk of heart attacks, strokes, and things of that nature,” Dr. Gelfand said at the International Investigative Dermatology meeting. The study was published when he gave his presentation (Circ Cardiovasc Imaging. 2018 Jun. doi: 10.1161/CIRCIMAGING.117.007394).
Although it didn’t reduce aortic inflammation, adalimumab had a positive effect on glycoprotein acetylation, a marker of inflammation and subclinical cardiovascular disease in psoriasis. Observational studies have also reported a drop in cardiovascular events with adalimumab. Taken together, the mixed findings “give us pause for thought,” Dr. Gelfand said.
In a previous trial, he and his colleagues had found that the interleukin blocker ustekinumab (Stelara) reduced aortic inflammation in psoriasis by about 19%, but Dr. Gelfand said at the meeting that it’s too early to opt for ustekinumab over adalimumab for cardiovascular protection: “I don’t think we are quite there yet; we are still not certain.”
Following washout of psoriasis treatments, the 97 subjects were randomized to either adalimumab for 12 months or ultraviolet B phototherapy or placebo for 12 weeks, followed by adalimumab for 12 months.
Aortic inflammation was used as a proxy for cardiovascular events because trials to assess actual event rates would require thousands of patients treated for several years. “They’re not likely to be done in psoriasis any time soon,” Dr. Gelfand said.
At both 12 and 52 weeks, adalimumab patients had no change in aortic inflammation, compared with placebo and baseline. Phototherapy patients had a 4% drop from baseline at 12 weeks, but it was not statistically significant when compared with placebo.
Both adalimumab and phototherapy decreased systemic inflammation as gauged by serum C-reactive protein and interleukin-6 levels, but only adalimumab reduced TNF levels and glycoprotein acetylation at 12 and 52 weeks. Neither treatment affected insulin, adiponectin, or leptin levels. Adalimumab dropped HDL cholesterol a bit, a known side effect, while phototherapy increased it.
About half of the patients in both treatment arms had a 75% reduction in the Psoriasis Area and Severity Index at 12 weeks, compared with 7% of those on placebo. Subjects were aged 43 years, on average, and more than two-thirds were men. They had a mean psoriasis duration of 17 years and a baseline PASI score of 19.
The work was funded by the National Institutes of Health and AbbVie, adalimumab’s maker. Among many industry ties, Dr. Gelfand is a consultant for Janssen, maker of ustekinumab, and receives research grants from Janssen and AbbVie.
SOURCE: Gelfand JM et al. IID 2018, Abstract 393.
ORLANDO – Adalimumab (Humira) did not reduce aortic inflammation in a year-long, randomized trial that pitted the tumor necrosis factor (TNF) blocker against phototherapy and placebo in 97 psoriasis patients.
a risk factor for cardiovascular events, said senior investigator Joel M. Gelfand, MD, a dermatology professor at the University of Pennsylvania, Philadelphia.
The other trial, from Canada, also found no effect in the ascending aorta after 52 weeks of treatment, but it did find a modest increase in carotid inflammation (J Invest Dermatol. 2017 Aug;137[8]:1638-45).
Both studies used positron emission tomography/computed tomography to assess vascular inflammation.
“We know patients with psoriasis are at increased risk for cardiovascular disease. We think the same kind of inflammation that occurs in atherosclerosis occurs in psoriasis, but we are still teasing out the impact of therapy and which one is most likely to lower the risk of heart attacks, strokes, and things of that nature,” Dr. Gelfand said at the International Investigative Dermatology meeting. The study was published when he gave his presentation (Circ Cardiovasc Imaging. 2018 Jun. doi: 10.1161/CIRCIMAGING.117.007394).
Although it didn’t reduce aortic inflammation, adalimumab had a positive effect on glycoprotein acetylation, a marker of inflammation and subclinical cardiovascular disease in psoriasis. Observational studies have also reported a drop in cardiovascular events with adalimumab. Taken together, the mixed findings “give us pause for thought,” Dr. Gelfand said.
In a previous trial, he and his colleagues had found that the interleukin blocker ustekinumab (Stelara) reduced aortic inflammation in psoriasis by about 19%, but Dr. Gelfand said at the meeting that it’s too early to opt for ustekinumab over adalimumab for cardiovascular protection: “I don’t think we are quite there yet; we are still not certain.”
Following washout of psoriasis treatments, the 97 subjects were randomized to either adalimumab for 12 months or ultraviolet B phototherapy or placebo for 12 weeks, followed by adalimumab for 12 months.
Aortic inflammation was used as a proxy for cardiovascular events because trials to assess actual event rates would require thousands of patients treated for several years. “They’re not likely to be done in psoriasis any time soon,” Dr. Gelfand said.
At both 12 and 52 weeks, adalimumab patients had no change in aortic inflammation, compared with placebo and baseline. Phototherapy patients had a 4% drop from baseline at 12 weeks, but it was not statistically significant when compared with placebo.
Both adalimumab and phototherapy decreased systemic inflammation as gauged by serum C-reactive protein and interleukin-6 levels, but only adalimumab reduced TNF levels and glycoprotein acetylation at 12 and 52 weeks. Neither treatment affected insulin, adiponectin, or leptin levels. Adalimumab dropped HDL cholesterol a bit, a known side effect, while phototherapy increased it.
About half of the patients in both treatment arms had a 75% reduction in the Psoriasis Area and Severity Index at 12 weeks, compared with 7% of those on placebo. Subjects were aged 43 years, on average, and more than two-thirds were men. They had a mean psoriasis duration of 17 years and a baseline PASI score of 19.
The work was funded by the National Institutes of Health and AbbVie, adalimumab’s maker. Among many industry ties, Dr. Gelfand is a consultant for Janssen, maker of ustekinumab, and receives research grants from Janssen and AbbVie.
SOURCE: Gelfand JM et al. IID 2018, Abstract 393.
ORLANDO – Adalimumab (Humira) did not reduce aortic inflammation in a year-long, randomized trial that pitted the tumor necrosis factor (TNF) blocker against phototherapy and placebo in 97 psoriasis patients.
a risk factor for cardiovascular events, said senior investigator Joel M. Gelfand, MD, a dermatology professor at the University of Pennsylvania, Philadelphia.
The other trial, from Canada, also found no effect in the ascending aorta after 52 weeks of treatment, but it did find a modest increase in carotid inflammation (J Invest Dermatol. 2017 Aug;137[8]:1638-45).
Both studies used positron emission tomography/computed tomography to assess vascular inflammation.
“We know patients with psoriasis are at increased risk for cardiovascular disease. We think the same kind of inflammation that occurs in atherosclerosis occurs in psoriasis, but we are still teasing out the impact of therapy and which one is most likely to lower the risk of heart attacks, strokes, and things of that nature,” Dr. Gelfand said at the International Investigative Dermatology meeting. The study was published when he gave his presentation (Circ Cardiovasc Imaging. 2018 Jun. doi: 10.1161/CIRCIMAGING.117.007394).
Although it didn’t reduce aortic inflammation, adalimumab had a positive effect on glycoprotein acetylation, a marker of inflammation and subclinical cardiovascular disease in psoriasis. Observational studies have also reported a drop in cardiovascular events with adalimumab. Taken together, the mixed findings “give us pause for thought,” Dr. Gelfand said.
In a previous trial, he and his colleagues had found that the interleukin blocker ustekinumab (Stelara) reduced aortic inflammation in psoriasis by about 19%, but Dr. Gelfand said at the meeting that it’s too early to opt for ustekinumab over adalimumab for cardiovascular protection: “I don’t think we are quite there yet; we are still not certain.”
Following washout of psoriasis treatments, the 97 subjects were randomized to either adalimumab for 12 months or ultraviolet B phototherapy or placebo for 12 weeks, followed by adalimumab for 12 months.
Aortic inflammation was used as a proxy for cardiovascular events because trials to assess actual event rates would require thousands of patients treated for several years. “They’re not likely to be done in psoriasis any time soon,” Dr. Gelfand said.
At both 12 and 52 weeks, adalimumab patients had no change in aortic inflammation, compared with placebo and baseline. Phototherapy patients had a 4% drop from baseline at 12 weeks, but it was not statistically significant when compared with placebo.
Both adalimumab and phototherapy decreased systemic inflammation as gauged by serum C-reactive protein and interleukin-6 levels, but only adalimumab reduced TNF levels and glycoprotein acetylation at 12 and 52 weeks. Neither treatment affected insulin, adiponectin, or leptin levels. Adalimumab dropped HDL cholesterol a bit, a known side effect, while phototherapy increased it.
About half of the patients in both treatment arms had a 75% reduction in the Psoriasis Area and Severity Index at 12 weeks, compared with 7% of those on placebo. Subjects were aged 43 years, on average, and more than two-thirds were men. They had a mean psoriasis duration of 17 years and a baseline PASI score of 19.
The work was funded by the National Institutes of Health and AbbVie, adalimumab’s maker. Among many industry ties, Dr. Gelfand is a consultant for Janssen, maker of ustekinumab, and receives research grants from Janssen and AbbVie.
SOURCE: Gelfand JM et al. IID 2018, Abstract 393.
REPORTING FROM IID 2018
Key clinical point: Adalimumab does not appear to reduce aortic inflammation in psoriasis, a risk factor for cardiovascular events.
Major finding: After a year of treatment, patients had no change in aortic inflammation, compared with placebo and baseline.
Study details: Randomized, controlled trial of 97 patients
Disclosures: The National Institutes of Health and AbbVie, adalimumab’s maker, funded the work. Among many industry ties, Dr. Gelfand is a consultant for Janssen, maker of ustekinumab, and receives research grants from Janssen and AbbVie.
Source: Gelfand JM et al. IID 2018, abstract 393
Consider potty seats when you see contact dermatitis on toddler bottoms
In such cases, be on the alert for contact dermatitis, reported Claire O. Dorfman, DO, of Lehigh Valley Health Network, Allentown, Pa., and her associates at Hershey (Pa.) Medical Center.
A 3-year-old white boy with a 6-month history of a pruritic rash on his buttocks and bilateral posterior thighs was treated without improvement at the pediatric dermatology clinic with low-potency topical corticosteroids, as well as topical antibiotic and antifungal agents.
Only mild improvement was seen once disposable paper toilet seat covers were added to treatment regimen. Following the purchase of a new potty seat through an online retailer, the child’s mother discovered a number of consumer product reviews also detailing similar complaints about the manufacturer, Prince Lionheart WeePOD Basix, by more than 30 other consumers. Photos highlighting identical rash presentation in other toddlers confirmed that the toilet seat was responsible for the allergic reaction. A warning had been posted by the manufacturer but this warning was not provided by the online retailer.
Use of the seat was immediately discontinued, and complete resolution of lesions was achieved within 1 month; subsequently, a report to the Consumer Product Safety Commission was made.
Allergic contact dermatitis to toilet seats is becoming increasingly common, the authors noted. Although the source of allergies is varied, wood historically has been identified as the most common material associated with the condition. Polypropylene and polyurethane foam also have been found to cause irritation. However, in the case reported by Dr. Dorfman and her associates, the precise irritant could not be identified because of the atypical pattern of the lesions and their irregular presentation on the buttocks and thighs. They speculated that this irregularity could be attributed to “the small, round shape of the seat and the squirmy behavior of a toddler,” because the typical arciform distribution was not present. Relief was not achieved with the paper liners because they did not completely cover the seat.
Because the rash resolved when the seat was replaced, parents declined patch testing. As a result, it was not possible to identify the specific allergenic component of the polyurethane. The polyurethanes used to make the seats are synthetic polymers that contain isocyanates, and frequently diaminodiphenylmethane, a curing agent. Possible allergy to the dyes used during manufacture also was considered but the presenting rash was reported in all four of the available colors made.
Although it was speculated that exposure to cleansers could be to blame for possible irritant dermatitis given reports of cracking of the potty seat, the mother and several online reviews indicated only soap and water were used, not harsh cleaning agents.
The clinicians had no relevant financial disclosures.
SOURCE: Dorfman CO et al. Pediatr Dermatol. 2018 May 29. doi: 10.1111/pde.13534.
In such cases, be on the alert for contact dermatitis, reported Claire O. Dorfman, DO, of Lehigh Valley Health Network, Allentown, Pa., and her associates at Hershey (Pa.) Medical Center.
A 3-year-old white boy with a 6-month history of a pruritic rash on his buttocks and bilateral posterior thighs was treated without improvement at the pediatric dermatology clinic with low-potency topical corticosteroids, as well as topical antibiotic and antifungal agents.
Only mild improvement was seen once disposable paper toilet seat covers were added to treatment regimen. Following the purchase of a new potty seat through an online retailer, the child’s mother discovered a number of consumer product reviews also detailing similar complaints about the manufacturer, Prince Lionheart WeePOD Basix, by more than 30 other consumers. Photos highlighting identical rash presentation in other toddlers confirmed that the toilet seat was responsible for the allergic reaction. A warning had been posted by the manufacturer but this warning was not provided by the online retailer.
Use of the seat was immediately discontinued, and complete resolution of lesions was achieved within 1 month; subsequently, a report to the Consumer Product Safety Commission was made.
Allergic contact dermatitis to toilet seats is becoming increasingly common, the authors noted. Although the source of allergies is varied, wood historically has been identified as the most common material associated with the condition. Polypropylene and polyurethane foam also have been found to cause irritation. However, in the case reported by Dr. Dorfman and her associates, the precise irritant could not be identified because of the atypical pattern of the lesions and their irregular presentation on the buttocks and thighs. They speculated that this irregularity could be attributed to “the small, round shape of the seat and the squirmy behavior of a toddler,” because the typical arciform distribution was not present. Relief was not achieved with the paper liners because they did not completely cover the seat.
Because the rash resolved when the seat was replaced, parents declined patch testing. As a result, it was not possible to identify the specific allergenic component of the polyurethane. The polyurethanes used to make the seats are synthetic polymers that contain isocyanates, and frequently diaminodiphenylmethane, a curing agent. Possible allergy to the dyes used during manufacture also was considered but the presenting rash was reported in all four of the available colors made.
Although it was speculated that exposure to cleansers could be to blame for possible irritant dermatitis given reports of cracking of the potty seat, the mother and several online reviews indicated only soap and water were used, not harsh cleaning agents.
The clinicians had no relevant financial disclosures.
SOURCE: Dorfman CO et al. Pediatr Dermatol. 2018 May 29. doi: 10.1111/pde.13534.
In such cases, be on the alert for contact dermatitis, reported Claire O. Dorfman, DO, of Lehigh Valley Health Network, Allentown, Pa., and her associates at Hershey (Pa.) Medical Center.
A 3-year-old white boy with a 6-month history of a pruritic rash on his buttocks and bilateral posterior thighs was treated without improvement at the pediatric dermatology clinic with low-potency topical corticosteroids, as well as topical antibiotic and antifungal agents.
Only mild improvement was seen once disposable paper toilet seat covers were added to treatment regimen. Following the purchase of a new potty seat through an online retailer, the child’s mother discovered a number of consumer product reviews also detailing similar complaints about the manufacturer, Prince Lionheart WeePOD Basix, by more than 30 other consumers. Photos highlighting identical rash presentation in other toddlers confirmed that the toilet seat was responsible for the allergic reaction. A warning had been posted by the manufacturer but this warning was not provided by the online retailer.
Use of the seat was immediately discontinued, and complete resolution of lesions was achieved within 1 month; subsequently, a report to the Consumer Product Safety Commission was made.
Allergic contact dermatitis to toilet seats is becoming increasingly common, the authors noted. Although the source of allergies is varied, wood historically has been identified as the most common material associated with the condition. Polypropylene and polyurethane foam also have been found to cause irritation. However, in the case reported by Dr. Dorfman and her associates, the precise irritant could not be identified because of the atypical pattern of the lesions and their irregular presentation on the buttocks and thighs. They speculated that this irregularity could be attributed to “the small, round shape of the seat and the squirmy behavior of a toddler,” because the typical arciform distribution was not present. Relief was not achieved with the paper liners because they did not completely cover the seat.
Because the rash resolved when the seat was replaced, parents declined patch testing. As a result, it was not possible to identify the specific allergenic component of the polyurethane. The polyurethanes used to make the seats are synthetic polymers that contain isocyanates, and frequently diaminodiphenylmethane, a curing agent. Possible allergy to the dyes used during manufacture also was considered but the presenting rash was reported in all four of the available colors made.
Although it was speculated that exposure to cleansers could be to blame for possible irritant dermatitis given reports of cracking of the potty seat, the mother and several online reviews indicated only soap and water were used, not harsh cleaning agents.
The clinicians had no relevant financial disclosures.
SOURCE: Dorfman CO et al. Pediatr Dermatol. 2018 May 29. doi: 10.1111/pde.13534.
FROM THE JOURNAL OF PEDIATRIC DERMATOLOGY
Key clinical point: Suspect contact dermatitis in cases of unexplained pruritic rash.
Major finding: Allergic contact dermatitis to toilet seats is becoming increasingly common.
Study details: A case study.
Disclosures: The authors had no relevant financial disclosures.
Source: Dorfman CO et al. Pediatr Dermatol. 2018 May 29. doi: 10.1111/pde.13534.
Ozenoxacin is a novel treatment option for impetigo
Ozenoxacin (Xepi cream), a potent novel topical antibacterial agent, is effective and well tolerated for treatment of impetigo in patients aged 2 months and older, according to results of a phase 3 randomized, double-blind, vehicle-controlled clinical trial.
A 1% cream formulation of ozenoxacin had a clinical and microbiologic response that was rapid and superior to placebo, investigators reported in this second phase 3 pivotal study, which demonstrated the efficacy of ozenoxacin cream in impetigo patients.
“With concerns over widespread antibiotic resistance, ozenoxacin is an important potential treatment option with an expanded spectrum against bacterial pathogens, including those resistant to mupirocin, ciprofloxacin, and methicillin, including MRSA [methicillin-resistant Staphylococcus aureus],” Dr. Rosen and his colleagues wrote in a report on their study in JAMA Dermatology.
The clinical trial included 412 patients aged 2 months or older with impetigo. Clinical success, defined as complete absence of the treated lesions, was seen after 5 days of treatment in 112 of the 206 (54.4%) patients randomized to ozenoxacin versus 78 of 206 patients (37.9%) who received placebo (P = .001), which confirmed the superiority of ozenoxacin, Dr. Rosen and his coauthors wrote.
In addition, microbiologic response was seen in 87.2% of patients in the ozenoxacin group during therapy (day 3) versus 63.9% in the placebo group (P = .002); by the end of therapy (day 6), response rates were 92.0% and 73.1%, respectively (P = .005).
All patients with drug-resistant infections had clinical cure or improvement at the end of treatment, including 10 patients with mupirocin-resistant S. aureus and 8 patients with MRSA, investigators wrote.
Adverse event rates were low for both ozenoxacin and placebo groups, at 3.9% and 3.4%, respectively. One patient in the ozenoxacin group experienced a serious adverse event “at least potentially related” to treatment (rosacea and seborrheic dermatitis).
“The lack of systemic adverse effects is consistent with previous studies that demonstrated that topically applied ozenoxacin has negligible systemic absorption,” Dr. Rosen and his coauthors wrote.
The study was partly supported by Ferrer Internacional; two coauthors of the study reported employment with that company. Dr. Rosen reported consulting work with Medimetriks Pharmaceuticals, which developed the agent.
SOURCE: Rosen T et al. JAMA Dermatol. 2018 Jun 13. doi: 10.1001/jamadermatol.2018.1103.
Ozenoxacin (Xepi cream), a potent novel topical antibacterial agent, is effective and well tolerated for treatment of impetigo in patients aged 2 months and older, according to results of a phase 3 randomized, double-blind, vehicle-controlled clinical trial.
A 1% cream formulation of ozenoxacin had a clinical and microbiologic response that was rapid and superior to placebo, investigators reported in this second phase 3 pivotal study, which demonstrated the efficacy of ozenoxacin cream in impetigo patients.
“With concerns over widespread antibiotic resistance, ozenoxacin is an important potential treatment option with an expanded spectrum against bacterial pathogens, including those resistant to mupirocin, ciprofloxacin, and methicillin, including MRSA [methicillin-resistant Staphylococcus aureus],” Dr. Rosen and his colleagues wrote in a report on their study in JAMA Dermatology.
The clinical trial included 412 patients aged 2 months or older with impetigo. Clinical success, defined as complete absence of the treated lesions, was seen after 5 days of treatment in 112 of the 206 (54.4%) patients randomized to ozenoxacin versus 78 of 206 patients (37.9%) who received placebo (P = .001), which confirmed the superiority of ozenoxacin, Dr. Rosen and his coauthors wrote.
In addition, microbiologic response was seen in 87.2% of patients in the ozenoxacin group during therapy (day 3) versus 63.9% in the placebo group (P = .002); by the end of therapy (day 6), response rates were 92.0% and 73.1%, respectively (P = .005).
All patients with drug-resistant infections had clinical cure or improvement at the end of treatment, including 10 patients with mupirocin-resistant S. aureus and 8 patients with MRSA, investigators wrote.
Adverse event rates were low for both ozenoxacin and placebo groups, at 3.9% and 3.4%, respectively. One patient in the ozenoxacin group experienced a serious adverse event “at least potentially related” to treatment (rosacea and seborrheic dermatitis).
“The lack of systemic adverse effects is consistent with previous studies that demonstrated that topically applied ozenoxacin has negligible systemic absorption,” Dr. Rosen and his coauthors wrote.
The study was partly supported by Ferrer Internacional; two coauthors of the study reported employment with that company. Dr. Rosen reported consulting work with Medimetriks Pharmaceuticals, which developed the agent.
SOURCE: Rosen T et al. JAMA Dermatol. 2018 Jun 13. doi: 10.1001/jamadermatol.2018.1103.
Ozenoxacin (Xepi cream), a potent novel topical antibacterial agent, is effective and well tolerated for treatment of impetigo in patients aged 2 months and older, according to results of a phase 3 randomized, double-blind, vehicle-controlled clinical trial.
A 1% cream formulation of ozenoxacin had a clinical and microbiologic response that was rapid and superior to placebo, investigators reported in this second phase 3 pivotal study, which demonstrated the efficacy of ozenoxacin cream in impetigo patients.
“With concerns over widespread antibiotic resistance, ozenoxacin is an important potential treatment option with an expanded spectrum against bacterial pathogens, including those resistant to mupirocin, ciprofloxacin, and methicillin, including MRSA [methicillin-resistant Staphylococcus aureus],” Dr. Rosen and his colleagues wrote in a report on their study in JAMA Dermatology.
The clinical trial included 412 patients aged 2 months or older with impetigo. Clinical success, defined as complete absence of the treated lesions, was seen after 5 days of treatment in 112 of the 206 (54.4%) patients randomized to ozenoxacin versus 78 of 206 patients (37.9%) who received placebo (P = .001), which confirmed the superiority of ozenoxacin, Dr. Rosen and his coauthors wrote.
In addition, microbiologic response was seen in 87.2% of patients in the ozenoxacin group during therapy (day 3) versus 63.9% in the placebo group (P = .002); by the end of therapy (day 6), response rates were 92.0% and 73.1%, respectively (P = .005).
All patients with drug-resistant infections had clinical cure or improvement at the end of treatment, including 10 patients with mupirocin-resistant S. aureus and 8 patients with MRSA, investigators wrote.
Adverse event rates were low for both ozenoxacin and placebo groups, at 3.9% and 3.4%, respectively. One patient in the ozenoxacin group experienced a serious adverse event “at least potentially related” to treatment (rosacea and seborrheic dermatitis).
“The lack of systemic adverse effects is consistent with previous studies that demonstrated that topically applied ozenoxacin has negligible systemic absorption,” Dr. Rosen and his coauthors wrote.
The study was partly supported by Ferrer Internacional; two coauthors of the study reported employment with that company. Dr. Rosen reported consulting work with Medimetriks Pharmaceuticals, which developed the agent.
SOURCE: Rosen T et al. JAMA Dermatol. 2018 Jun 13. doi: 10.1001/jamadermatol.2018.1103.
FROM JAMA DERMATOLOGY
Key clinical point: Ozenoxacin, a novel topical antibacterial agent, is effective and well tolerated for treatment of impetigo
Major finding: Clinical response was seen in 54.4% of ozenoxacin-treated patients and 37.9% of placebo-treated patients (P = .001).
Study details: A phase 3 randomized, double-blind, vehicle-controlled clinical trial that included 411 patients aged 2 months or older with impetigo.
Disclosures: Partial support came from Ferrer Internacional; two coauthors worked for that company. The study’s first author reported consulting work with Medimetriks, the developer of the agent.
Source: Rosen T et al. JAMA Dermatol. 2018 Jun 13. doi: 10.1001/jamadermatol.2018.1103.
JAK inhibitor therapy promising for refractory dermatomyositis
ORLANDO – The according to Ruth Ann Vleugels, MD, director of the autoimmune skin diseases program at Brigham and Women’s Hospital, Boston.
“We will have patients who essentially fail all of our typical therapies and are still coming to us for help. This is a huge challenge,” said Dr. Vleugels, who, several years ago, started to use tofacitinib to treat these patients. “Similar to my colleagues who use tofacitinib to treat alopecia areata, we often have to push” beyond the dose used to treat rheumatoid arthritis, to 10 mg twice a day, she said at the International Conference on Cutaneous Lupus Erythematosus. Tofacitinib helps counter the overexpression of interferon in DM.
Getting insurance coverage for this off-label indication can be tough, however, but Dr. Vleugels said she’s had success when she tells insurers that tofacitinib will likely reduce the need for IVIg.
It’s safe to keep patients on methotrexate if there are concerns about muscle involvement while their skin is brought under control with tofacitinib. In terms of side effects, “we see increased shingles,” so recommending the shingles vaccine for these patients is a good idea, she added.
It’s also important to counsel DM patients that they are at particular risk for skin reactions with antimalarials, which can be serious, so that, “if there is a drug reaction that develops, it’s noticed right away” and the drug can be stopped, she said. “If you have a patient who has very severe disease, I might skip over an antimalarial altogether,” she commented.
Methotrexate is the next option, especially if there are work ups for cancer or the patients have cancer, but Dr. Femia, director of inpatient dermatology at NYU, said she leans towards mycophenolate if there’s concern about lung involvement.
The next step, if necessary, is IVIg, which she said is “particularly helpful” for recalcitrant skin disease and can help some patients discontinue other immunosuppressives. To counter headache, a common side effect, she will space dosing out over 3 days, instead of the usual 2, and have a bag of saline administered before and after the infusion to keep patients hydrated; this counters the headache-inducing viscosity of IVIg.
Patients often see a result after the first infusion, but if there’s no benefit by the third cycle, “it’s probably time to move on,” she said. “If you have a refractory muscle disease patient and skin isn’t the main issue, rituximab is reasonable to try,” she added, noting that the benefit of tumor necrosis factor blockers, “at best, is very mixed in the DM population. They are very low down in the treatment algorithm.”
Dr. Vleugels and Dr. Femia are both Pfizer investigators.
ORLANDO – The according to Ruth Ann Vleugels, MD, director of the autoimmune skin diseases program at Brigham and Women’s Hospital, Boston.
“We will have patients who essentially fail all of our typical therapies and are still coming to us for help. This is a huge challenge,” said Dr. Vleugels, who, several years ago, started to use tofacitinib to treat these patients. “Similar to my colleagues who use tofacitinib to treat alopecia areata, we often have to push” beyond the dose used to treat rheumatoid arthritis, to 10 mg twice a day, she said at the International Conference on Cutaneous Lupus Erythematosus. Tofacitinib helps counter the overexpression of interferon in DM.
Getting insurance coverage for this off-label indication can be tough, however, but Dr. Vleugels said she’s had success when she tells insurers that tofacitinib will likely reduce the need for IVIg.
It’s safe to keep patients on methotrexate if there are concerns about muscle involvement while their skin is brought under control with tofacitinib. In terms of side effects, “we see increased shingles,” so recommending the shingles vaccine for these patients is a good idea, she added.
It’s also important to counsel DM patients that they are at particular risk for skin reactions with antimalarials, which can be serious, so that, “if there is a drug reaction that develops, it’s noticed right away” and the drug can be stopped, she said. “If you have a patient who has very severe disease, I might skip over an antimalarial altogether,” she commented.
Methotrexate is the next option, especially if there are work ups for cancer or the patients have cancer, but Dr. Femia, director of inpatient dermatology at NYU, said she leans towards mycophenolate if there’s concern about lung involvement.
The next step, if necessary, is IVIg, which she said is “particularly helpful” for recalcitrant skin disease and can help some patients discontinue other immunosuppressives. To counter headache, a common side effect, she will space dosing out over 3 days, instead of the usual 2, and have a bag of saline administered before and after the infusion to keep patients hydrated; this counters the headache-inducing viscosity of IVIg.
Patients often see a result after the first infusion, but if there’s no benefit by the third cycle, “it’s probably time to move on,” she said. “If you have a refractory muscle disease patient and skin isn’t the main issue, rituximab is reasonable to try,” she added, noting that the benefit of tumor necrosis factor blockers, “at best, is very mixed in the DM population. They are very low down in the treatment algorithm.”
Dr. Vleugels and Dr. Femia are both Pfizer investigators.
ORLANDO – The according to Ruth Ann Vleugels, MD, director of the autoimmune skin diseases program at Brigham and Women’s Hospital, Boston.
“We will have patients who essentially fail all of our typical therapies and are still coming to us for help. This is a huge challenge,” said Dr. Vleugels, who, several years ago, started to use tofacitinib to treat these patients. “Similar to my colleagues who use tofacitinib to treat alopecia areata, we often have to push” beyond the dose used to treat rheumatoid arthritis, to 10 mg twice a day, she said at the International Conference on Cutaneous Lupus Erythematosus. Tofacitinib helps counter the overexpression of interferon in DM.
Getting insurance coverage for this off-label indication can be tough, however, but Dr. Vleugels said she’s had success when she tells insurers that tofacitinib will likely reduce the need for IVIg.
It’s safe to keep patients on methotrexate if there are concerns about muscle involvement while their skin is brought under control with tofacitinib. In terms of side effects, “we see increased shingles,” so recommending the shingles vaccine for these patients is a good idea, she added.
It’s also important to counsel DM patients that they are at particular risk for skin reactions with antimalarials, which can be serious, so that, “if there is a drug reaction that develops, it’s noticed right away” and the drug can be stopped, she said. “If you have a patient who has very severe disease, I might skip over an antimalarial altogether,” she commented.
Methotrexate is the next option, especially if there are work ups for cancer or the patients have cancer, but Dr. Femia, director of inpatient dermatology at NYU, said she leans towards mycophenolate if there’s concern about lung involvement.
The next step, if necessary, is IVIg, which she said is “particularly helpful” for recalcitrant skin disease and can help some patients discontinue other immunosuppressives. To counter headache, a common side effect, she will space dosing out over 3 days, instead of the usual 2, and have a bag of saline administered before and after the infusion to keep patients hydrated; this counters the headache-inducing viscosity of IVIg.
Patients often see a result after the first infusion, but if there’s no benefit by the third cycle, “it’s probably time to move on,” she said. “If you have a refractory muscle disease patient and skin isn’t the main issue, rituximab is reasonable to try,” she added, noting that the benefit of tumor necrosis factor blockers, “at best, is very mixed in the DM population. They are very low down in the treatment algorithm.”
Dr. Vleugels and Dr. Femia are both Pfizer investigators.
EXPERT ANALYSIS FROM ICCLE 2018