User login
Hidradenitis suppurativa
THE COMPARISON
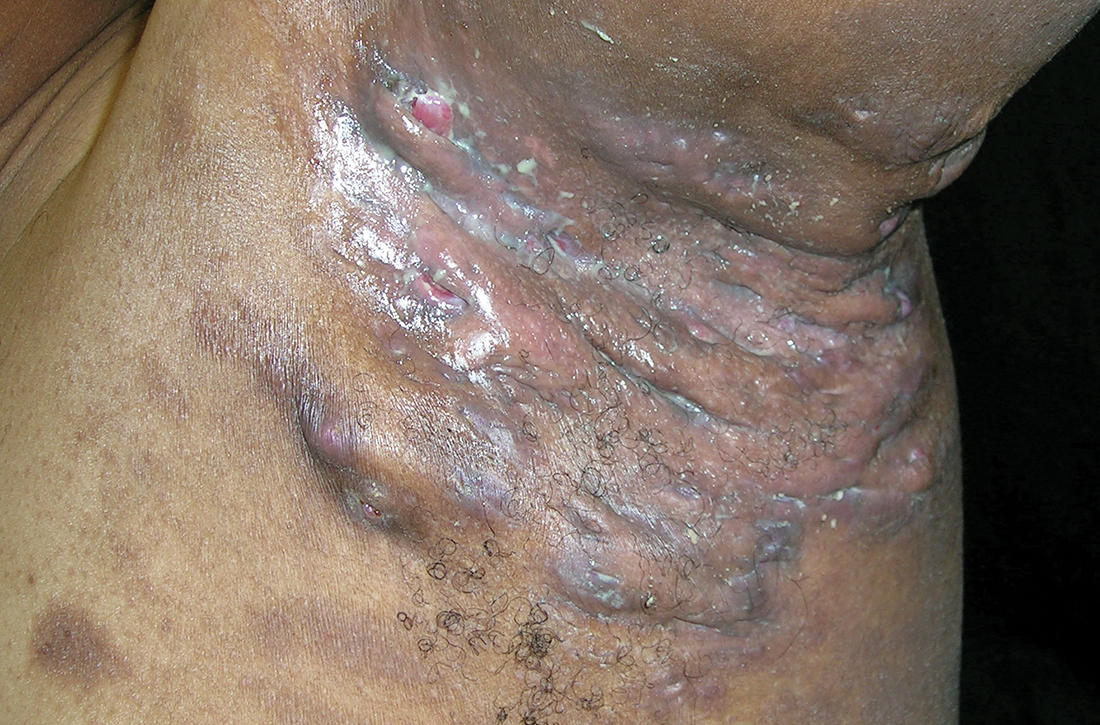
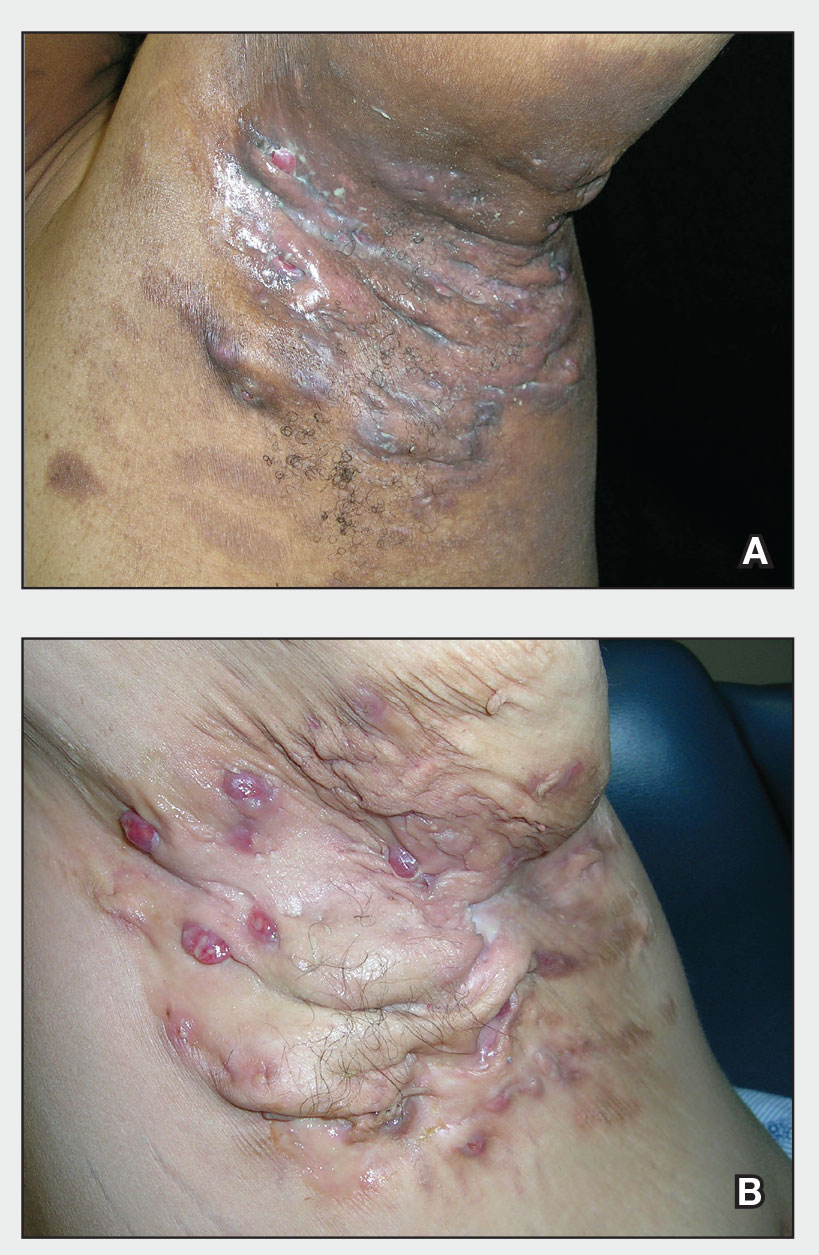
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
THE COMPARISON
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
THE COMPARISON
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
People of color bearing brunt of long COVID, doctors say
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
Hidradenitis Suppurativa
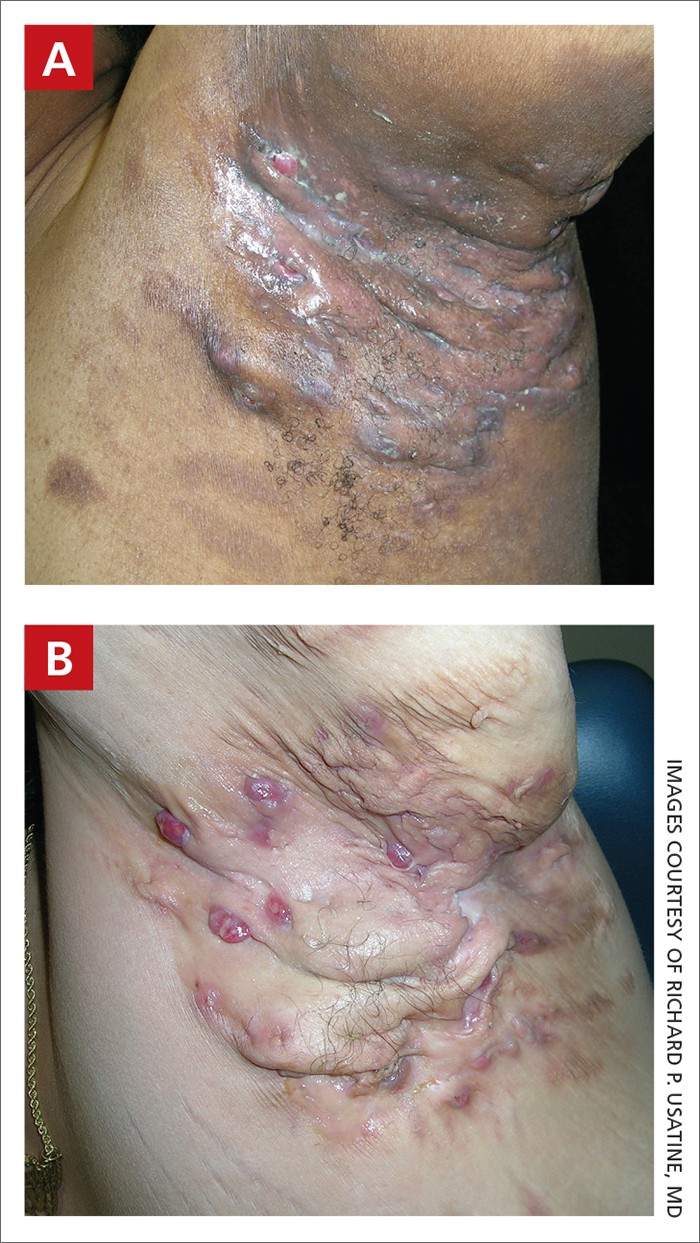
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
Health Literacy in Dermatology Patients: How to Level the Playing Field
Health literacy is a multifaceted construct that encompasses the knowledge of health and health systems, utilization of information related to health, and ability to maintain health.1 Low health literacy impairs health outcomes, disproportionately affecting socioeconomically disadvantaged populations, including racial minorities and the older population. Consistently, it is associated with fewer vaccinations and screenings, higher health care utilization, and poorer ability to take medications or interpret health information.2
With growing utilization of the Internet for health information,3 much patient education now occurs outside the clinic. Differential utilization of the Internet can exacerbate disparities in health outcomes: people with a lower family income more frequently engage in health information and dialogue online.3 Despite opportunities to improve literacy and narrow gaps in care, a lack of awareness, advocacy, and funding limit patient- and community-based initiatives. Herein, we discuss health literacy challenges in dermatology, offer potential solutions, and propose ways that stakeholders can prioritize health literacy advocacy to improve outcomes.
The Importance of Health Literacy in Dermatology
Dermatology patients often face challenges that demand greater health literacy. Active participation in health promotion, protection, and maintenance can remarkably improve outcomes. When patients understand disease pathogenesis and the rationale behind treatment choices, adherence to a treatment regimen might improve.
However, understanding dermatologic diseases and disorders can be challenging. First, many are chronic inflammatory conditions that require intricate treatment regimens. Second, the complexity of those diseases and disorders continues to grow in the era of new research and unprecedented expansion of treatment options.
For chronic conditions that require ongoing complex management, researchers have developed advanced patient tools. For instance, the eczema action plan helps atopic dermatitis patients manage conditions from home.4 However, patients with greater literacy and the ability to participate will better utilize such tools and have fewer uncontrolled flares. Patient tools meant to improve outcomes might, instead, widen gaps in care. Even with nonchronic conditions, such as nonmelanoma skin cancer, continued awareness and the need for preventive care, timely diagnosis, and appropriate intervention remain critical.
Limited Accessibility of Patient Education Materials
Patient education in dermatology occurs through several formats. Because online health resources are more readily available to those with less access to health care, the potential for such resources to narrow health disparities is immense. However, online resources have not adequately taken advantage of the opportunity to make health information openly accessible to its users. The readability of online patient education materials on a large expanse of dermatologic conditions is far too advanced.5 The readability level of some resources is as high as 17th grade (graduate school), which is much higher than the American Medical Association recommendation6 that patient education materials be presented at a 6th-grade level or less. Furthermore, the quality and comprehensiveness of content is highly variable. Rather than serving as an equalizer, the Internet may widen the gap as low health literacy continues to impair the accessibility of health information.
Solutions to Level the Playing Field
What can be done to increase the readability of patient education materials? Leveling the playing field begins with creating materials at an appropriate readability level, including online content, printed handouts, and after-visit summaries in the clinic. Writers of patient education materials should be cognizant of their choice of language and routinely use a free readability checker (https://readabilityformulas.com). Patient education materials should reflect the American Medical Association’s recommended 6th-grade level. Creators should maintain a high standard of quality and comprehensiveness; prior studies note no inverse correlation between readability and quality.5 In the age of multimedia presentation, non–print-based materials can be explored, such as audio or video for online content, podcasts, and webinars. Providers also should take the opportunity to be mindful of health literacy in clinic. Beyond assessing the readability of written resources for a patient, assessing that patient’s health literacy and tailoring one’s language will maximize engagement.
Systemic Change Is Needed
Ultimately, systemic change is needed to address the root causes of health literacy disparity, requiring advocacy for social welfare, public health, and public policy initiatives. In recognizing existing efforts, such as community outreach teams and hospital committees to evaluate health literacy materials, numerous barriers remain. Despite the notable impact of health literacy on health outcomes, there is a lack of advocacy and funds to conduct health literacy–related work.7 Because dermatologists provide holistic care and remain mindful of patients’ health literacy in the clinic, they should continue to advocate for increased awareness, improved funding, and support for local and federal initiatives.
Final Thoughts
With more opportunities to narrow gaps in care, it is more pertinent than ever to acknowledge the impact of health literacy on dermatology outcomes. Leveling the playing field begins with (1) an awareness of health literacy and (2) creating readable and comprehensible patient education content. Greater advocacy from community and professional organizations; increased funding from nonprofit organizations, industry, and federal institutions; and increased involvement by dermatologists in bringing greater attention to health literacy will improve outcomes in dermatology.
- Liu C, Wang D, Liu C, et al. What is the meaning of health literacy? a systematic review and qualitative synthesis. Fam Med Community Health. 2020;8:e000351. doi:10.1136/fmch-2020-000351
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107. doi:10.7326/0003-4819-155-2-201107190-00005
- Rice RE. Influences, usage, and outcomes of Internet health information searching: multivariate results from the Pew surveys. Int J Med Inform. 2006;75:8-28. doi:10.1016/j.ijmedinf.2005.07.032
- Brown J, Weitz NW, Liang A, et al. Does an eczema action plan improve atopic dermatitis? a single-site randomized controlled trial. Clin Pediatr (Phila). 2018;57:1624-1629. doi:10.1177/0009922818795906
- De DR, Shih T, Katta R, et al. Readability, quality, and timeliness of patient online health resources for contact dermatitis and patch testing. Dermatitis. 2022;33:155-160. doi:10.1097/DER.0000000000000789
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association, American Medical Foundation; 2003.
- Nutbeam D, McGill B, Premkumar P. Improving health literacy in community populations: a review of progress. Health Promot Int. 2018;33:901-911. doi:10.1093/heapro/dax015
Health literacy is a multifaceted construct that encompasses the knowledge of health and health systems, utilization of information related to health, and ability to maintain health.1 Low health literacy impairs health outcomes, disproportionately affecting socioeconomically disadvantaged populations, including racial minorities and the older population. Consistently, it is associated with fewer vaccinations and screenings, higher health care utilization, and poorer ability to take medications or interpret health information.2
With growing utilization of the Internet for health information,3 much patient education now occurs outside the clinic. Differential utilization of the Internet can exacerbate disparities in health outcomes: people with a lower family income more frequently engage in health information and dialogue online.3 Despite opportunities to improve literacy and narrow gaps in care, a lack of awareness, advocacy, and funding limit patient- and community-based initiatives. Herein, we discuss health literacy challenges in dermatology, offer potential solutions, and propose ways that stakeholders can prioritize health literacy advocacy to improve outcomes.
The Importance of Health Literacy in Dermatology
Dermatology patients often face challenges that demand greater health literacy. Active participation in health promotion, protection, and maintenance can remarkably improve outcomes. When patients understand disease pathogenesis and the rationale behind treatment choices, adherence to a treatment regimen might improve.
However, understanding dermatologic diseases and disorders can be challenging. First, many are chronic inflammatory conditions that require intricate treatment regimens. Second, the complexity of those diseases and disorders continues to grow in the era of new research and unprecedented expansion of treatment options.
For chronic conditions that require ongoing complex management, researchers have developed advanced patient tools. For instance, the eczema action plan helps atopic dermatitis patients manage conditions from home.4 However, patients with greater literacy and the ability to participate will better utilize such tools and have fewer uncontrolled flares. Patient tools meant to improve outcomes might, instead, widen gaps in care. Even with nonchronic conditions, such as nonmelanoma skin cancer, continued awareness and the need for preventive care, timely diagnosis, and appropriate intervention remain critical.
Limited Accessibility of Patient Education Materials
Patient education in dermatology occurs through several formats. Because online health resources are more readily available to those with less access to health care, the potential for such resources to narrow health disparities is immense. However, online resources have not adequately taken advantage of the opportunity to make health information openly accessible to its users. The readability of online patient education materials on a large expanse of dermatologic conditions is far too advanced.5 The readability level of some resources is as high as 17th grade (graduate school), which is much higher than the American Medical Association recommendation6 that patient education materials be presented at a 6th-grade level or less. Furthermore, the quality and comprehensiveness of content is highly variable. Rather than serving as an equalizer, the Internet may widen the gap as low health literacy continues to impair the accessibility of health information.
Solutions to Level the Playing Field
What can be done to increase the readability of patient education materials? Leveling the playing field begins with creating materials at an appropriate readability level, including online content, printed handouts, and after-visit summaries in the clinic. Writers of patient education materials should be cognizant of their choice of language and routinely use a free readability checker (https://readabilityformulas.com). Patient education materials should reflect the American Medical Association’s recommended 6th-grade level. Creators should maintain a high standard of quality and comprehensiveness; prior studies note no inverse correlation between readability and quality.5 In the age of multimedia presentation, non–print-based materials can be explored, such as audio or video for online content, podcasts, and webinars. Providers also should take the opportunity to be mindful of health literacy in clinic. Beyond assessing the readability of written resources for a patient, assessing that patient’s health literacy and tailoring one’s language will maximize engagement.
Systemic Change Is Needed
Ultimately, systemic change is needed to address the root causes of health literacy disparity, requiring advocacy for social welfare, public health, and public policy initiatives. In recognizing existing efforts, such as community outreach teams and hospital committees to evaluate health literacy materials, numerous barriers remain. Despite the notable impact of health literacy on health outcomes, there is a lack of advocacy and funds to conduct health literacy–related work.7 Because dermatologists provide holistic care and remain mindful of patients’ health literacy in the clinic, they should continue to advocate for increased awareness, improved funding, and support for local and federal initiatives.
Final Thoughts
With more opportunities to narrow gaps in care, it is more pertinent than ever to acknowledge the impact of health literacy on dermatology outcomes. Leveling the playing field begins with (1) an awareness of health literacy and (2) creating readable and comprehensible patient education content. Greater advocacy from community and professional organizations; increased funding from nonprofit organizations, industry, and federal institutions; and increased involvement by dermatologists in bringing greater attention to health literacy will improve outcomes in dermatology.
Health literacy is a multifaceted construct that encompasses the knowledge of health and health systems, utilization of information related to health, and ability to maintain health.1 Low health literacy impairs health outcomes, disproportionately affecting socioeconomically disadvantaged populations, including racial minorities and the older population. Consistently, it is associated with fewer vaccinations and screenings, higher health care utilization, and poorer ability to take medications or interpret health information.2
With growing utilization of the Internet for health information,3 much patient education now occurs outside the clinic. Differential utilization of the Internet can exacerbate disparities in health outcomes: people with a lower family income more frequently engage in health information and dialogue online.3 Despite opportunities to improve literacy and narrow gaps in care, a lack of awareness, advocacy, and funding limit patient- and community-based initiatives. Herein, we discuss health literacy challenges in dermatology, offer potential solutions, and propose ways that stakeholders can prioritize health literacy advocacy to improve outcomes.
The Importance of Health Literacy in Dermatology
Dermatology patients often face challenges that demand greater health literacy. Active participation in health promotion, protection, and maintenance can remarkably improve outcomes. When patients understand disease pathogenesis and the rationale behind treatment choices, adherence to a treatment regimen might improve.
However, understanding dermatologic diseases and disorders can be challenging. First, many are chronic inflammatory conditions that require intricate treatment regimens. Second, the complexity of those diseases and disorders continues to grow in the era of new research and unprecedented expansion of treatment options.
For chronic conditions that require ongoing complex management, researchers have developed advanced patient tools. For instance, the eczema action plan helps atopic dermatitis patients manage conditions from home.4 However, patients with greater literacy and the ability to participate will better utilize such tools and have fewer uncontrolled flares. Patient tools meant to improve outcomes might, instead, widen gaps in care. Even with nonchronic conditions, such as nonmelanoma skin cancer, continued awareness and the need for preventive care, timely diagnosis, and appropriate intervention remain critical.
Limited Accessibility of Patient Education Materials
Patient education in dermatology occurs through several formats. Because online health resources are more readily available to those with less access to health care, the potential for such resources to narrow health disparities is immense. However, online resources have not adequately taken advantage of the opportunity to make health information openly accessible to its users. The readability of online patient education materials on a large expanse of dermatologic conditions is far too advanced.5 The readability level of some resources is as high as 17th grade (graduate school), which is much higher than the American Medical Association recommendation6 that patient education materials be presented at a 6th-grade level or less. Furthermore, the quality and comprehensiveness of content is highly variable. Rather than serving as an equalizer, the Internet may widen the gap as low health literacy continues to impair the accessibility of health information.
Solutions to Level the Playing Field
What can be done to increase the readability of patient education materials? Leveling the playing field begins with creating materials at an appropriate readability level, including online content, printed handouts, and after-visit summaries in the clinic. Writers of patient education materials should be cognizant of their choice of language and routinely use a free readability checker (https://readabilityformulas.com). Patient education materials should reflect the American Medical Association’s recommended 6th-grade level. Creators should maintain a high standard of quality and comprehensiveness; prior studies note no inverse correlation between readability and quality.5 In the age of multimedia presentation, non–print-based materials can be explored, such as audio or video for online content, podcasts, and webinars. Providers also should take the opportunity to be mindful of health literacy in clinic. Beyond assessing the readability of written resources for a patient, assessing that patient’s health literacy and tailoring one’s language will maximize engagement.
Systemic Change Is Needed
Ultimately, systemic change is needed to address the root causes of health literacy disparity, requiring advocacy for social welfare, public health, and public policy initiatives. In recognizing existing efforts, such as community outreach teams and hospital committees to evaluate health literacy materials, numerous barriers remain. Despite the notable impact of health literacy on health outcomes, there is a lack of advocacy and funds to conduct health literacy–related work.7 Because dermatologists provide holistic care and remain mindful of patients’ health literacy in the clinic, they should continue to advocate for increased awareness, improved funding, and support for local and federal initiatives.
Final Thoughts
With more opportunities to narrow gaps in care, it is more pertinent than ever to acknowledge the impact of health literacy on dermatology outcomes. Leveling the playing field begins with (1) an awareness of health literacy and (2) creating readable and comprehensible patient education content. Greater advocacy from community and professional organizations; increased funding from nonprofit organizations, industry, and federal institutions; and increased involvement by dermatologists in bringing greater attention to health literacy will improve outcomes in dermatology.
- Liu C, Wang D, Liu C, et al. What is the meaning of health literacy? a systematic review and qualitative synthesis. Fam Med Community Health. 2020;8:e000351. doi:10.1136/fmch-2020-000351
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107. doi:10.7326/0003-4819-155-2-201107190-00005
- Rice RE. Influences, usage, and outcomes of Internet health information searching: multivariate results from the Pew surveys. Int J Med Inform. 2006;75:8-28. doi:10.1016/j.ijmedinf.2005.07.032
- Brown J, Weitz NW, Liang A, et al. Does an eczema action plan improve atopic dermatitis? a single-site randomized controlled trial. Clin Pediatr (Phila). 2018;57:1624-1629. doi:10.1177/0009922818795906
- De DR, Shih T, Katta R, et al. Readability, quality, and timeliness of patient online health resources for contact dermatitis and patch testing. Dermatitis. 2022;33:155-160. doi:10.1097/DER.0000000000000789
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association, American Medical Foundation; 2003.
- Nutbeam D, McGill B, Premkumar P. Improving health literacy in community populations: a review of progress. Health Promot Int. 2018;33:901-911. doi:10.1093/heapro/dax015
- Liu C, Wang D, Liu C, et al. What is the meaning of health literacy? a systematic review and qualitative synthesis. Fam Med Community Health. 2020;8:e000351. doi:10.1136/fmch-2020-000351
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107. doi:10.7326/0003-4819-155-2-201107190-00005
- Rice RE. Influences, usage, and outcomes of Internet health information searching: multivariate results from the Pew surveys. Int J Med Inform. 2006;75:8-28. doi:10.1016/j.ijmedinf.2005.07.032
- Brown J, Weitz NW, Liang A, et al. Does an eczema action plan improve atopic dermatitis? a single-site randomized controlled trial. Clin Pediatr (Phila). 2018;57:1624-1629. doi:10.1177/0009922818795906
- De DR, Shih T, Katta R, et al. Readability, quality, and timeliness of patient online health resources for contact dermatitis and patch testing. Dermatitis. 2022;33:155-160. doi:10.1097/DER.0000000000000789
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association, American Medical Foundation; 2003.
- Nutbeam D, McGill B, Premkumar P. Improving health literacy in community populations: a review of progress. Health Promot Int. 2018;33:901-911. doi:10.1093/heapro/dax015
Pivotal trials in blood cancers don’t mirror patient populations
, a new study concludes.
“Our analysis shows that, over the past 10 years, participation in pivotal clinical trials investigating therapies for leukemias and MM is unrepresentative of the U.S. population,” say the authors, led by Jorge E. Cortes, MD, of the Georgia Cancer Center at Augusta University, Ga. “Trials should represent the population with the disease,” they comment.
The study was published in the Journal of Clinical Oncology.
“This study confirms that the U.S. cancer population for select hematologic malignancies was inadequately racially and ethnically represented in studies leading to drug approval,” comment the authors of an accompanying editorial.
“The results from this study should lead to questions about the generalizability of drug safety and efficacy in populations we serve as medical hematologists and oncologists,” say Mikkael A. Sekeres, MD, along with Namrata S. Chandhok, MD, both of the division of hematology, Sylvester Comprehensive Cancer Center, University of Miami.
They pose the question, for instance, as physicians practicing in South Florida, where most of their patients are Hispanic, “can we apply the results of these pivotal studies – and drug labels – to them, without any sense of whether they metabolize the drug the same way as those included in the study or have the same biologic targets?”
Analysis of pivotal trials
For their study, Dr. Cortes and colleagues analyzed 61 pivotal trials for leukemia and MM leading to approval of the drugs from the U.S. Food and Drug Administration between 2011 and 2021.
They found that only two-thirds (67.2%) of these trials reported data pertaining to race, while about half (48.8%) reported on ethnicity.
The trials that did report data on race involved a total of 13,731 patients. The vast majority (81.6%) were White, and Black patients represented only 3.8%. Asian/Pacific Islanders made up 9.1%, and American Indians or Alaskan Natives made up just 0.12% of participants, with 1.5% categorized as other.
Among the trials reporting on ethnicity, 4.7% of patients were Hispanic, with 11.5% being Hispanic in acute lymphoblastic leukemia (ALL) trials and 7.6% Hispanic in chronic myeloid leukemia (CML) trials.
Slightly more than half (54.8%) of all trial participants were male, and patients’ average ages ranged from 41.7 to 67.3 years across all malignancies.
Of the minority groups, Asian/Pacific Islanders and Black people had the highest representation in trials involving CML, at 12.7% and 5.3%, respectively.
Their lowest representation was in chronic lymphocytic leukemia (CLL), at 3% and 1.1%, respectively.
Among the trials reporting ethnicity, Hispanic people were the highest representation, with percentages ranging from 3.8% of MM trials to 11.5% in ALL trials.
Inconsistent with patient populations
Next, the researchers compared the proportions of race/ethnic groups that were found among the participants of these pivotal trials with the proportions that would be expected in patient populations for each of these blood cancers (according to the U.S. Surveillance, Epidemiology, and End Results [SEER] database).
For example, White people made up 80.3% of participants in clinical trials of MM, whereas they represent 68.7% of patients with MM, a difference that was statistically significant (P < .0001).
The finding was similar for CML, with White people accounting for 90.5% of participants in clinical trials versus 82.5% of the patient population (P < .0001).
For AML, the difference was smaller, with respective percentages of 79.6 versus 77.3% (P = .0389).
For Black people, Asian/Pacific Islanders and Hispanic people, across all five cancer types that were analyzed, the proportion of participants in clinical trials was significantly lower than the proportion in the patient population.
The analysis also showed that females were overrepresented in clinical trials for two blood cancers. For MM, trial participation was 44.7%, while disease incidence was 41.7% (P < .0001), and for CML the proportions were 44.7% versus 39.5% (P = .0009). However, females were underrepresented in a third blood cancer: in AML, the proportions were 44.7% versus 60.5% (P < .0001).
Geographic location of trials often inaccessible
The study also highlighted an obstacle to minorities participating in clinical trials: geography.
For this analysis, the researchers looked at mortality rates for the various blood cancers.
For AML, they found mortality rates were high across the whole of the United States, but centers conducting AML clinical trials were primarily in the Northeast, with no centers in the Midwest.
Key regions with high rates of AML mortality, low access to trials, and high minority representation were notably clustered in areas including east of the Carolinas, South Georgia, Alabama, and Mississippi, the authors noted.
“In many instances, trials were absent in areas with high mortality,” they report. “This makes access to clinical trials difficult, if not impossible, to patients who do not have the financial means for travel.”
Further action needed
Racial and ethnic disparities in clinical trials have been widely reported in numerous previous studies, the authors note.
Various initiatives have been launched in recent years to tackle the problem, including the National Institutes of Health Revitalization Act, FDA race and ethnicity guidance, and the International Conference for Harmonization guidance.
For oncology, the American Society of Clinical Oncology has also taken steps with the release of the new Equity, Diversity, and Inclusion Action Plan in 2021 to improve representation of minorities in research.
Dr. Cortes and colleagues suggest another step that is needed is standardized reporting of demographics of clinical trial participants.
“More importantly, efforts to increase representation of minorities and disadvantaged populations in clinical trials should be prioritized,” they say.
Dr. Cortes reports a consulting role and receiving research funding from many pharmaceutical companies. No other coauthors have financial disclosures. Dr. Chandhok reports honoraria from Healio, Clinical Care Options, and a consulting role with Servier. Dr. Sekeres reports a consulting role with Celgene, Millennium, Pfizer, Novartis, Syros Pharmaceuticals, Kurome Therapeutics, and institutional research funding from Takeda, Pfizer, Bristol Myers Squibb, Actuate Therapeutics, Sellas Life Sciences, and Bio-Path Holdings.
A version of this article first appeared on Medscape.com.
, a new study concludes.
“Our analysis shows that, over the past 10 years, participation in pivotal clinical trials investigating therapies for leukemias and MM is unrepresentative of the U.S. population,” say the authors, led by Jorge E. Cortes, MD, of the Georgia Cancer Center at Augusta University, Ga. “Trials should represent the population with the disease,” they comment.
The study was published in the Journal of Clinical Oncology.
“This study confirms that the U.S. cancer population for select hematologic malignancies was inadequately racially and ethnically represented in studies leading to drug approval,” comment the authors of an accompanying editorial.
“The results from this study should lead to questions about the generalizability of drug safety and efficacy in populations we serve as medical hematologists and oncologists,” say Mikkael A. Sekeres, MD, along with Namrata S. Chandhok, MD, both of the division of hematology, Sylvester Comprehensive Cancer Center, University of Miami.
They pose the question, for instance, as physicians practicing in South Florida, where most of their patients are Hispanic, “can we apply the results of these pivotal studies – and drug labels – to them, without any sense of whether they metabolize the drug the same way as those included in the study or have the same biologic targets?”
Analysis of pivotal trials
For their study, Dr. Cortes and colleagues analyzed 61 pivotal trials for leukemia and MM leading to approval of the drugs from the U.S. Food and Drug Administration between 2011 and 2021.
They found that only two-thirds (67.2%) of these trials reported data pertaining to race, while about half (48.8%) reported on ethnicity.
The trials that did report data on race involved a total of 13,731 patients. The vast majority (81.6%) were White, and Black patients represented only 3.8%. Asian/Pacific Islanders made up 9.1%, and American Indians or Alaskan Natives made up just 0.12% of participants, with 1.5% categorized as other.
Among the trials reporting on ethnicity, 4.7% of patients were Hispanic, with 11.5% being Hispanic in acute lymphoblastic leukemia (ALL) trials and 7.6% Hispanic in chronic myeloid leukemia (CML) trials.
Slightly more than half (54.8%) of all trial participants were male, and patients’ average ages ranged from 41.7 to 67.3 years across all malignancies.
Of the minority groups, Asian/Pacific Islanders and Black people had the highest representation in trials involving CML, at 12.7% and 5.3%, respectively.
Their lowest representation was in chronic lymphocytic leukemia (CLL), at 3% and 1.1%, respectively.
Among the trials reporting ethnicity, Hispanic people were the highest representation, with percentages ranging from 3.8% of MM trials to 11.5% in ALL trials.
Inconsistent with patient populations
Next, the researchers compared the proportions of race/ethnic groups that were found among the participants of these pivotal trials with the proportions that would be expected in patient populations for each of these blood cancers (according to the U.S. Surveillance, Epidemiology, and End Results [SEER] database).
For example, White people made up 80.3% of participants in clinical trials of MM, whereas they represent 68.7% of patients with MM, a difference that was statistically significant (P < .0001).
The finding was similar for CML, with White people accounting for 90.5% of participants in clinical trials versus 82.5% of the patient population (P < .0001).
For AML, the difference was smaller, with respective percentages of 79.6 versus 77.3% (P = .0389).
For Black people, Asian/Pacific Islanders and Hispanic people, across all five cancer types that were analyzed, the proportion of participants in clinical trials was significantly lower than the proportion in the patient population.
The analysis also showed that females were overrepresented in clinical trials for two blood cancers. For MM, trial participation was 44.7%, while disease incidence was 41.7% (P < .0001), and for CML the proportions were 44.7% versus 39.5% (P = .0009). However, females were underrepresented in a third blood cancer: in AML, the proportions were 44.7% versus 60.5% (P < .0001).
Geographic location of trials often inaccessible
The study also highlighted an obstacle to minorities participating in clinical trials: geography.
For this analysis, the researchers looked at mortality rates for the various blood cancers.
For AML, they found mortality rates were high across the whole of the United States, but centers conducting AML clinical trials were primarily in the Northeast, with no centers in the Midwest.
Key regions with high rates of AML mortality, low access to trials, and high minority representation were notably clustered in areas including east of the Carolinas, South Georgia, Alabama, and Mississippi, the authors noted.
“In many instances, trials were absent in areas with high mortality,” they report. “This makes access to clinical trials difficult, if not impossible, to patients who do not have the financial means for travel.”
Further action needed
Racial and ethnic disparities in clinical trials have been widely reported in numerous previous studies, the authors note.
Various initiatives have been launched in recent years to tackle the problem, including the National Institutes of Health Revitalization Act, FDA race and ethnicity guidance, and the International Conference for Harmonization guidance.
For oncology, the American Society of Clinical Oncology has also taken steps with the release of the new Equity, Diversity, and Inclusion Action Plan in 2021 to improve representation of minorities in research.
Dr. Cortes and colleagues suggest another step that is needed is standardized reporting of demographics of clinical trial participants.
“More importantly, efforts to increase representation of minorities and disadvantaged populations in clinical trials should be prioritized,” they say.
Dr. Cortes reports a consulting role and receiving research funding from many pharmaceutical companies. No other coauthors have financial disclosures. Dr. Chandhok reports honoraria from Healio, Clinical Care Options, and a consulting role with Servier. Dr. Sekeres reports a consulting role with Celgene, Millennium, Pfizer, Novartis, Syros Pharmaceuticals, Kurome Therapeutics, and institutional research funding from Takeda, Pfizer, Bristol Myers Squibb, Actuate Therapeutics, Sellas Life Sciences, and Bio-Path Holdings.
A version of this article first appeared on Medscape.com.
, a new study concludes.
“Our analysis shows that, over the past 10 years, participation in pivotal clinical trials investigating therapies for leukemias and MM is unrepresentative of the U.S. population,” say the authors, led by Jorge E. Cortes, MD, of the Georgia Cancer Center at Augusta University, Ga. “Trials should represent the population with the disease,” they comment.
The study was published in the Journal of Clinical Oncology.
“This study confirms that the U.S. cancer population for select hematologic malignancies was inadequately racially and ethnically represented in studies leading to drug approval,” comment the authors of an accompanying editorial.
“The results from this study should lead to questions about the generalizability of drug safety and efficacy in populations we serve as medical hematologists and oncologists,” say Mikkael A. Sekeres, MD, along with Namrata S. Chandhok, MD, both of the division of hematology, Sylvester Comprehensive Cancer Center, University of Miami.
They pose the question, for instance, as physicians practicing in South Florida, where most of their patients are Hispanic, “can we apply the results of these pivotal studies – and drug labels – to them, without any sense of whether they metabolize the drug the same way as those included in the study or have the same biologic targets?”
Analysis of pivotal trials
For their study, Dr. Cortes and colleagues analyzed 61 pivotal trials for leukemia and MM leading to approval of the drugs from the U.S. Food and Drug Administration between 2011 and 2021.
They found that only two-thirds (67.2%) of these trials reported data pertaining to race, while about half (48.8%) reported on ethnicity.
The trials that did report data on race involved a total of 13,731 patients. The vast majority (81.6%) were White, and Black patients represented only 3.8%. Asian/Pacific Islanders made up 9.1%, and American Indians or Alaskan Natives made up just 0.12% of participants, with 1.5% categorized as other.
Among the trials reporting on ethnicity, 4.7% of patients were Hispanic, with 11.5% being Hispanic in acute lymphoblastic leukemia (ALL) trials and 7.6% Hispanic in chronic myeloid leukemia (CML) trials.
Slightly more than half (54.8%) of all trial participants were male, and patients’ average ages ranged from 41.7 to 67.3 years across all malignancies.
Of the minority groups, Asian/Pacific Islanders and Black people had the highest representation in trials involving CML, at 12.7% and 5.3%, respectively.
Their lowest representation was in chronic lymphocytic leukemia (CLL), at 3% and 1.1%, respectively.
Among the trials reporting ethnicity, Hispanic people were the highest representation, with percentages ranging from 3.8% of MM trials to 11.5% in ALL trials.
Inconsistent with patient populations
Next, the researchers compared the proportions of race/ethnic groups that were found among the participants of these pivotal trials with the proportions that would be expected in patient populations for each of these blood cancers (according to the U.S. Surveillance, Epidemiology, and End Results [SEER] database).
For example, White people made up 80.3% of participants in clinical trials of MM, whereas they represent 68.7% of patients with MM, a difference that was statistically significant (P < .0001).
The finding was similar for CML, with White people accounting for 90.5% of participants in clinical trials versus 82.5% of the patient population (P < .0001).
For AML, the difference was smaller, with respective percentages of 79.6 versus 77.3% (P = .0389).
For Black people, Asian/Pacific Islanders and Hispanic people, across all five cancer types that were analyzed, the proportion of participants in clinical trials was significantly lower than the proportion in the patient population.
The analysis also showed that females were overrepresented in clinical trials for two blood cancers. For MM, trial participation was 44.7%, while disease incidence was 41.7% (P < .0001), and for CML the proportions were 44.7% versus 39.5% (P = .0009). However, females were underrepresented in a third blood cancer: in AML, the proportions were 44.7% versus 60.5% (P < .0001).
Geographic location of trials often inaccessible
The study also highlighted an obstacle to minorities participating in clinical trials: geography.
For this analysis, the researchers looked at mortality rates for the various blood cancers.
For AML, they found mortality rates were high across the whole of the United States, but centers conducting AML clinical trials were primarily in the Northeast, with no centers in the Midwest.
Key regions with high rates of AML mortality, low access to trials, and high minority representation were notably clustered in areas including east of the Carolinas, South Georgia, Alabama, and Mississippi, the authors noted.
“In many instances, trials were absent in areas with high mortality,” they report. “This makes access to clinical trials difficult, if not impossible, to patients who do not have the financial means for travel.”
Further action needed
Racial and ethnic disparities in clinical trials have been widely reported in numerous previous studies, the authors note.
Various initiatives have been launched in recent years to tackle the problem, including the National Institutes of Health Revitalization Act, FDA race and ethnicity guidance, and the International Conference for Harmonization guidance.
For oncology, the American Society of Clinical Oncology has also taken steps with the release of the new Equity, Diversity, and Inclusion Action Plan in 2021 to improve representation of minorities in research.
Dr. Cortes and colleagues suggest another step that is needed is standardized reporting of demographics of clinical trial participants.
“More importantly, efforts to increase representation of minorities and disadvantaged populations in clinical trials should be prioritized,” they say.
Dr. Cortes reports a consulting role and receiving research funding from many pharmaceutical companies. No other coauthors have financial disclosures. Dr. Chandhok reports honoraria from Healio, Clinical Care Options, and a consulting role with Servier. Dr. Sekeres reports a consulting role with Celgene, Millennium, Pfizer, Novartis, Syros Pharmaceuticals, Kurome Therapeutics, and institutional research funding from Takeda, Pfizer, Bristol Myers Squibb, Actuate Therapeutics, Sellas Life Sciences, and Bio-Path Holdings.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Expert shares tips on hair disorders and photoprotection for patients of color
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
AT PDA 2022
Hydroquinone, found in skin-lightening agents worldwide, linked with increased skin cancer risk
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Secondary CV prevention benefit from polypill promises global health benefit
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
FROM ESC CONGRESS 2022
Monkeypox in children and women remains rare, CDC data show
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Well-child visits rise, but disparities remain
Adherence to well-child visits in the United States increased overall over a 10-year period, but a gap of up to 20% persisted between the highest and lowest adherence groups, reflecting disparities by race and ethnicity, poverty level, geography, and insurance status.
Well-child visits are recommended to provide children with preventive health and development services, ensure immunizations, and allow parents to discuss health concerns, wrote Salam Abdus, PhD, and Thomas M. Selden, PhD, of the Agency for Healthcare Research and Quality, Rockville, Md.
“We know from prior studies that as of 2008, well-child visits were trending upward, but often fell short of recommendations among key socioeconomic groups,” they wrote.
To examine recent trends in well-child visits, the researchers conducted a cross-sectional study of data from the Medical Expenditure Panel Survey (MEPS) on children aged 0 to 18 years. The findings were published in JAMA Pediatrics.
The study population included 19,018 children in 2006 and 2007 and 17,533 children in 2016 and 2017.
Adherence was defined as the ratio of reported well-child visits divided by the recommended number of visits in a calendar year.
Overall, the mean adherence increased from 47.9% in 2006-2007 to 62.3% in 2016-2017.
However, significant gaps persisted across race and ethnicity. Notably, adherence in the Hispanic population increased by nearly 22% between the study dates, compared to a 15.3% increase among White non-Hispanic children. However, Hispanic children still trailed White children overall in 2016-2017 (58% vs. 67.8%).
The smallest increase in adherence occurred among Black non-Hispanic children (5.6%) which further widened the gap between Black and White non-Hispanic children in 2016-2017 (52.5% vs. 67.8%).
Adherence rates increased similarly for children with public and private insurance (15.5% and 13.9%, respectively), but the adherence rates for uninsured children remained stable. Adherence in 2016-2017 for children with private, public, and no insurance were 66.3%, 58.7%, and 31.1%.
Also, despite overall increases in adherence across regions, a gap of more than 20% separated the region with the highest adherence (Northeast) from the lowest (West) in both the 2006-2007 and 2016-2017 periods (69.3% vs. 38.4%, and 79.3% vs. 55.2%, respectively).
The findings show an increase in well-child visits that spanned a time period of increased recommendations, economic changes, and the impact of the Affordable Care Act, but unaddressed disparities remain, the researchers noted.
Reducing disparities and improving adherence, “will require the combined efforts of researchers, policymakers, and clinicians to improve our understanding of adherence, to implement policies improving access to care, and to increase health care professional engagement with disadvantaged communities,” they concluded.
Overall increases are encouraging, but barriers need attention
“Demographic data are critical to determine which groups of children need the most support for recommended well child care,” Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. In the current study, “it was encouraging to see how either public or private insurance significantly increased the percentage of children receiving well child care,” she said.
The level of increased adherence to AAP-recommended guidelines for well-child visits was surprising, said Dr. Boulter. The overall increase is likely attributable in part to the increased coverage for well-child visits in the wake of the Affordable Care Act, as the study authors mention, she said.
“The gains experienced by Hispanic families were especially encouraging,” she added.
However, ongoing barriers to well-child care include “lack of adequate provider numbers and mix, transportation difficulties for patients, and lack of child care and time away from work for parents so they can complete the recommended well child visit schedule,” Dr. Boulter noted. “Provider schedules and locations of care should be improved so families would have easier access. Also, social media should have more positive well-child messages to counteract the negative messaging.”
More research is needed to examine the impact of COVID-19 on well-child visits, Dr. Boulter emphasized. “Most likely, the percentages in all groups will have changed since COVID-19 has impacted office practices,” she said. “Anxiety about COVID-19 transmissibility in the pediatric office decreased routine office visits, and skepticism about vaccines, including vaccine refusal, has significantly changed the percentage of children who have received the AAP recommended vaccines,” she explained. Ideally, the study authors will review the MEPS data again to examine changes since the COVID-19 pandemic began, she told this news organization.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Adherence to well-child visits in the United States increased overall over a 10-year period, but a gap of up to 20% persisted between the highest and lowest adherence groups, reflecting disparities by race and ethnicity, poverty level, geography, and insurance status.
Well-child visits are recommended to provide children with preventive health and development services, ensure immunizations, and allow parents to discuss health concerns, wrote Salam Abdus, PhD, and Thomas M. Selden, PhD, of the Agency for Healthcare Research and Quality, Rockville, Md.
“We know from prior studies that as of 2008, well-child visits were trending upward, but often fell short of recommendations among key socioeconomic groups,” they wrote.
To examine recent trends in well-child visits, the researchers conducted a cross-sectional study of data from the Medical Expenditure Panel Survey (MEPS) on children aged 0 to 18 years. The findings were published in JAMA Pediatrics.
The study population included 19,018 children in 2006 and 2007 and 17,533 children in 2016 and 2017.
Adherence was defined as the ratio of reported well-child visits divided by the recommended number of visits in a calendar year.
Overall, the mean adherence increased from 47.9% in 2006-2007 to 62.3% in 2016-2017.
However, significant gaps persisted across race and ethnicity. Notably, adherence in the Hispanic population increased by nearly 22% between the study dates, compared to a 15.3% increase among White non-Hispanic children. However, Hispanic children still trailed White children overall in 2016-2017 (58% vs. 67.8%).
The smallest increase in adherence occurred among Black non-Hispanic children (5.6%) which further widened the gap between Black and White non-Hispanic children in 2016-2017 (52.5% vs. 67.8%).
Adherence rates increased similarly for children with public and private insurance (15.5% and 13.9%, respectively), but the adherence rates for uninsured children remained stable. Adherence in 2016-2017 for children with private, public, and no insurance were 66.3%, 58.7%, and 31.1%.
Also, despite overall increases in adherence across regions, a gap of more than 20% separated the region with the highest adherence (Northeast) from the lowest (West) in both the 2006-2007 and 2016-2017 periods (69.3% vs. 38.4%, and 79.3% vs. 55.2%, respectively).
The findings show an increase in well-child visits that spanned a time period of increased recommendations, economic changes, and the impact of the Affordable Care Act, but unaddressed disparities remain, the researchers noted.
Reducing disparities and improving adherence, “will require the combined efforts of researchers, policymakers, and clinicians to improve our understanding of adherence, to implement policies improving access to care, and to increase health care professional engagement with disadvantaged communities,” they concluded.
Overall increases are encouraging, but barriers need attention
“Demographic data are critical to determine which groups of children need the most support for recommended well child care,” Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. In the current study, “it was encouraging to see how either public or private insurance significantly increased the percentage of children receiving well child care,” she said.
The level of increased adherence to AAP-recommended guidelines for well-child visits was surprising, said Dr. Boulter. The overall increase is likely attributable in part to the increased coverage for well-child visits in the wake of the Affordable Care Act, as the study authors mention, she said.
“The gains experienced by Hispanic families were especially encouraging,” she added.
However, ongoing barriers to well-child care include “lack of adequate provider numbers and mix, transportation difficulties for patients, and lack of child care and time away from work for parents so they can complete the recommended well child visit schedule,” Dr. Boulter noted. “Provider schedules and locations of care should be improved so families would have easier access. Also, social media should have more positive well-child messages to counteract the negative messaging.”
More research is needed to examine the impact of COVID-19 on well-child visits, Dr. Boulter emphasized. “Most likely, the percentages in all groups will have changed since COVID-19 has impacted office practices,” she said. “Anxiety about COVID-19 transmissibility in the pediatric office decreased routine office visits, and skepticism about vaccines, including vaccine refusal, has significantly changed the percentage of children who have received the AAP recommended vaccines,” she explained. Ideally, the study authors will review the MEPS data again to examine changes since the COVID-19 pandemic began, she told this news organization.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Adherence to well-child visits in the United States increased overall over a 10-year period, but a gap of up to 20% persisted between the highest and lowest adherence groups, reflecting disparities by race and ethnicity, poverty level, geography, and insurance status.
Well-child visits are recommended to provide children with preventive health and development services, ensure immunizations, and allow parents to discuss health concerns, wrote Salam Abdus, PhD, and Thomas M. Selden, PhD, of the Agency for Healthcare Research and Quality, Rockville, Md.
“We know from prior studies that as of 2008, well-child visits were trending upward, but often fell short of recommendations among key socioeconomic groups,” they wrote.
To examine recent trends in well-child visits, the researchers conducted a cross-sectional study of data from the Medical Expenditure Panel Survey (MEPS) on children aged 0 to 18 years. The findings were published in JAMA Pediatrics.
The study population included 19,018 children in 2006 and 2007 and 17,533 children in 2016 and 2017.
Adherence was defined as the ratio of reported well-child visits divided by the recommended number of visits in a calendar year.
Overall, the mean adherence increased from 47.9% in 2006-2007 to 62.3% in 2016-2017.
However, significant gaps persisted across race and ethnicity. Notably, adherence in the Hispanic population increased by nearly 22% between the study dates, compared to a 15.3% increase among White non-Hispanic children. However, Hispanic children still trailed White children overall in 2016-2017 (58% vs. 67.8%).
The smallest increase in adherence occurred among Black non-Hispanic children (5.6%) which further widened the gap between Black and White non-Hispanic children in 2016-2017 (52.5% vs. 67.8%).
Adherence rates increased similarly for children with public and private insurance (15.5% and 13.9%, respectively), but the adherence rates for uninsured children remained stable. Adherence in 2016-2017 for children with private, public, and no insurance were 66.3%, 58.7%, and 31.1%.
Also, despite overall increases in adherence across regions, a gap of more than 20% separated the region with the highest adherence (Northeast) from the lowest (West) in both the 2006-2007 and 2016-2017 periods (69.3% vs. 38.4%, and 79.3% vs. 55.2%, respectively).
The findings show an increase in well-child visits that spanned a time period of increased recommendations, economic changes, and the impact of the Affordable Care Act, but unaddressed disparities remain, the researchers noted.
Reducing disparities and improving adherence, “will require the combined efforts of researchers, policymakers, and clinicians to improve our understanding of adherence, to implement policies improving access to care, and to increase health care professional engagement with disadvantaged communities,” they concluded.
Overall increases are encouraging, but barriers need attention
“Demographic data are critical to determine which groups of children need the most support for recommended well child care,” Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. In the current study, “it was encouraging to see how either public or private insurance significantly increased the percentage of children receiving well child care,” she said.
The level of increased adherence to AAP-recommended guidelines for well-child visits was surprising, said Dr. Boulter. The overall increase is likely attributable in part to the increased coverage for well-child visits in the wake of the Affordable Care Act, as the study authors mention, she said.
“The gains experienced by Hispanic families were especially encouraging,” she added.
However, ongoing barriers to well-child care include “lack of adequate provider numbers and mix, transportation difficulties for patients, and lack of child care and time away from work for parents so they can complete the recommended well child visit schedule,” Dr. Boulter noted. “Provider schedules and locations of care should be improved so families would have easier access. Also, social media should have more positive well-child messages to counteract the negative messaging.”
More research is needed to examine the impact of COVID-19 on well-child visits, Dr. Boulter emphasized. “Most likely, the percentages in all groups will have changed since COVID-19 has impacted office practices,” she said. “Anxiety about COVID-19 transmissibility in the pediatric office decreased routine office visits, and skepticism about vaccines, including vaccine refusal, has significantly changed the percentage of children who have received the AAP recommended vaccines,” she explained. Ideally, the study authors will review the MEPS data again to examine changes since the COVID-19 pandemic began, she told this news organization.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS