User login
Prompt palliative care cut hospital costs in pooled study
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Average cost savings per admission were $3,237 overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P-values less than .001).
Study details: Systematic review and meta-analysis of six cohort studies of 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease.
Disclosures: Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
Source: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
Understanding palliative care: An important part of practicing hospital medicine
according to Brett Hendel-Paterson, MD, FHM, a hospitalist at Region’s Hospital in St. Paul, Minn. and a presenter for this session.
Dr. Hendel-Paterson, Jeffrey L. Greenwald, MD, SFHM, of Massachusetts General Hospital, Boston, and Jeffrey Frank, MD, MBA, of Vituity, will each present on the topic of administering palliative care as a hospitalist and why it is important for hospitalists to better understand this area of medicine.
A common misunderstanding about palliative care is that it is end-of-life care only, a misconception within both the medical and patient community. Most people believe that palliative care is associated with the “angel of death,” as Dr. Greenwald stated. Palliative care does encompass end-of-life care but is also associated with life-limiting illness. Both areas of palliative can be improved with better patient communication and symptom management.
As frontline providers at times of critical illness, and throughout illness, hospitalists are ideally positioned to provide palliative care services, Dr. Greenwald stated during an interview.
With hospitalists in such a prominent role in providing palliative care, Dr. Hendel-Paterson offered a detailed explanation about why the information from this session is important for hospitalists.
“The majority of Americans who die in this country die in hospitals. We see and we know that patients sometimes get more aggressive care leading to greater suffering in their final days,” he said. “As hospitalists, we are expected to be the primary physicians in the hospital caring for patients with a variety of health conditions. We are expected to have a basic expertise and be able to independently manage health conditions. For example, we are expected to be able to diagnose and treat pneumonia without consulting infectious disease or pulmonology specialists for basic care. In the same way, we must be able to communicate well with our patients and families and help lead them through discussions of prognosis and advance care planning. Primary palliative care refers to the skill set that includes communications about serious illness and basic symptom management.”
Dr. Greenwald expanded on Dr. Hendel-Paterson’s point concerning the growing need for hospitalists who are competent in palliative care.
“As the population ages, this issue is going to become more and more important for our field, because there isn’t a sufficient pipeline, current state – or predicted future state – of palliative care providers in hospitals to meet the need. So there’s a gap in the need, and that need is increasing.”
According to Dr. Hendel-Paterson, he and his copresenters “hope that, after this session, participants will better understand primary palliative care, take ownership of end-of-life care of their patients, and will be motivated to increase skills in areas where they are lacking.”
Building on this idea of increasing one’s skills as a hospitalist, he emphasized the importance of understanding palliative care.
“The ability to practice high-quality primary palliative care is essential to being a competent hospitalist.”
Primary Palliative Care – What Every Hospitalist Should Know
Wednesday, 10:00-10:40 a.m.
Crystal Ballroom J1
according to Brett Hendel-Paterson, MD, FHM, a hospitalist at Region’s Hospital in St. Paul, Minn. and a presenter for this session.
Dr. Hendel-Paterson, Jeffrey L. Greenwald, MD, SFHM, of Massachusetts General Hospital, Boston, and Jeffrey Frank, MD, MBA, of Vituity, will each present on the topic of administering palliative care as a hospitalist and why it is important for hospitalists to better understand this area of medicine.
A common misunderstanding about palliative care is that it is end-of-life care only, a misconception within both the medical and patient community. Most people believe that palliative care is associated with the “angel of death,” as Dr. Greenwald stated. Palliative care does encompass end-of-life care but is also associated with life-limiting illness. Both areas of palliative can be improved with better patient communication and symptom management.
As frontline providers at times of critical illness, and throughout illness, hospitalists are ideally positioned to provide palliative care services, Dr. Greenwald stated during an interview.
With hospitalists in such a prominent role in providing palliative care, Dr. Hendel-Paterson offered a detailed explanation about why the information from this session is important for hospitalists.
“The majority of Americans who die in this country die in hospitals. We see and we know that patients sometimes get more aggressive care leading to greater suffering in their final days,” he said. “As hospitalists, we are expected to be the primary physicians in the hospital caring for patients with a variety of health conditions. We are expected to have a basic expertise and be able to independently manage health conditions. For example, we are expected to be able to diagnose and treat pneumonia without consulting infectious disease or pulmonology specialists for basic care. In the same way, we must be able to communicate well with our patients and families and help lead them through discussions of prognosis and advance care planning. Primary palliative care refers to the skill set that includes communications about serious illness and basic symptom management.”
Dr. Greenwald expanded on Dr. Hendel-Paterson’s point concerning the growing need for hospitalists who are competent in palliative care.
“As the population ages, this issue is going to become more and more important for our field, because there isn’t a sufficient pipeline, current state – or predicted future state – of palliative care providers in hospitals to meet the need. So there’s a gap in the need, and that need is increasing.”
According to Dr. Hendel-Paterson, he and his copresenters “hope that, after this session, participants will better understand primary palliative care, take ownership of end-of-life care of their patients, and will be motivated to increase skills in areas where they are lacking.”
Building on this idea of increasing one’s skills as a hospitalist, he emphasized the importance of understanding palliative care.
“The ability to practice high-quality primary palliative care is essential to being a competent hospitalist.”
Primary Palliative Care – What Every Hospitalist Should Know
Wednesday, 10:00-10:40 a.m.
Crystal Ballroom J1
according to Brett Hendel-Paterson, MD, FHM, a hospitalist at Region’s Hospital in St. Paul, Minn. and a presenter for this session.
Dr. Hendel-Paterson, Jeffrey L. Greenwald, MD, SFHM, of Massachusetts General Hospital, Boston, and Jeffrey Frank, MD, MBA, of Vituity, will each present on the topic of administering palliative care as a hospitalist and why it is important for hospitalists to better understand this area of medicine.
A common misunderstanding about palliative care is that it is end-of-life care only, a misconception within both the medical and patient community. Most people believe that palliative care is associated with the “angel of death,” as Dr. Greenwald stated. Palliative care does encompass end-of-life care but is also associated with life-limiting illness. Both areas of palliative can be improved with better patient communication and symptom management.
As frontline providers at times of critical illness, and throughout illness, hospitalists are ideally positioned to provide palliative care services, Dr. Greenwald stated during an interview.
With hospitalists in such a prominent role in providing palliative care, Dr. Hendel-Paterson offered a detailed explanation about why the information from this session is important for hospitalists.
“The majority of Americans who die in this country die in hospitals. We see and we know that patients sometimes get more aggressive care leading to greater suffering in their final days,” he said. “As hospitalists, we are expected to be the primary physicians in the hospital caring for patients with a variety of health conditions. We are expected to have a basic expertise and be able to independently manage health conditions. For example, we are expected to be able to diagnose and treat pneumonia without consulting infectious disease or pulmonology specialists for basic care. In the same way, we must be able to communicate well with our patients and families and help lead them through discussions of prognosis and advance care planning. Primary palliative care refers to the skill set that includes communications about serious illness and basic symptom management.”
Dr. Greenwald expanded on Dr. Hendel-Paterson’s point concerning the growing need for hospitalists who are competent in palliative care.
“As the population ages, this issue is going to become more and more important for our field, because there isn’t a sufficient pipeline, current state – or predicted future state – of palliative care providers in hospitals to meet the need. So there’s a gap in the need, and that need is increasing.”
According to Dr. Hendel-Paterson, he and his copresenters “hope that, after this session, participants will better understand primary palliative care, take ownership of end-of-life care of their patients, and will be motivated to increase skills in areas where they are lacking.”
Building on this idea of increasing one’s skills as a hospitalist, he emphasized the importance of understanding palliative care.
“The ability to practice high-quality primary palliative care is essential to being a competent hospitalist.”
Primary Palliative Care – What Every Hospitalist Should Know
Wednesday, 10:00-10:40 a.m.
Crystal Ballroom J1
How well do POLST forms assure that patients get the end-of-life care they requested?
EVIDENCE SUMMARY
The POLST form offers choices within 4 treatment areas: “attempt CPR” or “allow natural death” if the patient is in cardiopulmonary arrest; “comfort,” “limited,” or “full” medical interventions if pulse or breathing is present; choices of additional orders, including intravenous fluids, feeding tubes, and antibiotics; and additional written orders. Most POLST studies used cross-sectional and retrospective cohort designs and assessed whether CPR was attempted. Fewer studies also evaluated adherence to orders in the other treatment areas.
Community settings: Patients with POLST more likely to die out of hospital
The largest study of POLST use in community settings evaluated deaths in Oregon over one year.1 It found that patients who indicated “do not attempt CPR” on a POLST form were 6 times more likely to die a natural, out-of-hospital death than those who had no POLST form (TABLE1-10).
A West Virginia study found that patients with POLST forms had 30% higher out-of-hospital death rates than those with traditional advanced directives and no POLST.2 In a Wisconsin study, no decedents who indicated DNR on their POLST forms received CPR.3
One study that evaluated the consistency of actual medical interventions with POLST orders in all 4 treatment areas found it to be good in most areas (“feeding tubes,” “attempting CPR.” “antibiotics,” and “IV fluids”) except “additional written orders.4
Skilled nursing facilities: Generally high adherence to POLST orders
The largest study to evaluate the consistency of treatments with POLST orders among nursing home residents found high adherence overall (94%).5 Caregivers performed CPR on none of 299 residents who selected “DNR.” However, they did not administer CPR to 6 of 7 who chose “attempt CPR” and administered antibiotics to 32% of patients who specified “no antibiotics” on their POLST forms.5
A second study of nursing home residents who selected “comfort measures only” also found high consistency for attempting CPR, intensive care admission, and ventilator support, although physicians hospitalized 2% of patients to extend life.6 Similarly, treatments matched POLST orders well overall in a Washington state study, although one patient got a feeding tube against orders.7
POLST adherence is good, but can EMS workers find the form?
A study comparing emergency medical services (EMS) management with POLST orders in an Oregon registry found good consistency.8 EMS providers didn’t attempt or halted CPR in most patients with DNR orders who were found in cardiac arrest and initiated CPR in most patients who chose “attempt CPR.” EMS providers initiated CPR in the field on 11 patients (22%) with a DNR order but discontinued resuscitation en route to the hospital.
In a smaller study, EMS providers never located paper POLST forms at the scene in most cases.9
Hospice: POLST orders prevent unwanted Tx, except maybe antibiotics
A study evaluating management in hospice programs in 3 states found that care providers followed POLST orders for limited treatment in 98% of cases.10 No patients received unwanted CPR, intubation, or feeding tubes. POLST orders didn’t predict whether patients were treated with antibiotics, however.
1. Fromme EK, Zive D, Schmidt TA, et al. Association between physician orders for life-sustaining treatment for scope of treatment and in-hospital death in Oregon. J Am Geriatr Soc. 2014;62:1246-1251.
2. Pedraza SL, Culp S, Falkenstine EC, et al. POST forms more than advance directives associated with out-of-hospital death: insights from a state registry. J Pain Symptom Manage. 2016; 51:240-246.
3. Hammes B, Rooney BL, Gundrum JD, et al. The POLST program: a retrospective review of the demographics of use and outcomes in one community where advance directives are prevalent. J Palliative Med. 2012;15:77-85.
4. Lee MA, Brummel-Smith K, Meyer J, et al. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. J Am Geriatr Soc. 2000;48:1219-1225.
5. Hickman SE, Nelson CA, Moss AH, et al. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc. 2011;59:2091-2099.
6. Tolle SW, Tilden VP, Nelson CA, et al. A prospective study of the efficacy of the physician order form for life sustaining treatment. J Am Ger Soc.1998;46:1097-1102.
7. Meyers J, Moore C, McGrory A, et al. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Geron Nursing. 2004;30:37-46.
8. Richardson DK, Fromme E, Zive D, et al. Concordance of out-of-hospital and emergency department cardiac arrest resuscitation with documented end-of-life choices in Oregon. Ann Emerg Med. 2014;63:375-383.
9. Schmidt T, Olszewski EA, Zive D, et al. The Oregon physician orders for life-sustaining treatment registry: a preliminary study of emergency medical services utilization. J Emerg Med. 2013;44:796-805.
10. Hickman SE, Nelson CA, Moss AH, et al. Use of the physician orders for life-sustaining treatment (POLST) paradigm program in the hospice setting. J Palliat Med. 2009;12:133-141.
EVIDENCE SUMMARY
The POLST form offers choices within 4 treatment areas: “attempt CPR” or “allow natural death” if the patient is in cardiopulmonary arrest; “comfort,” “limited,” or “full” medical interventions if pulse or breathing is present; choices of additional orders, including intravenous fluids, feeding tubes, and antibiotics; and additional written orders. Most POLST studies used cross-sectional and retrospective cohort designs and assessed whether CPR was attempted. Fewer studies also evaluated adherence to orders in the other treatment areas.
Community settings: Patients with POLST more likely to die out of hospital
The largest study of POLST use in community settings evaluated deaths in Oregon over one year.1 It found that patients who indicated “do not attempt CPR” on a POLST form were 6 times more likely to die a natural, out-of-hospital death than those who had no POLST form (TABLE1-10).
A West Virginia study found that patients with POLST forms had 30% higher out-of-hospital death rates than those with traditional advanced directives and no POLST.2 In a Wisconsin study, no decedents who indicated DNR on their POLST forms received CPR.3
One study that evaluated the consistency of actual medical interventions with POLST orders in all 4 treatment areas found it to be good in most areas (“feeding tubes,” “attempting CPR.” “antibiotics,” and “IV fluids”) except “additional written orders.4
Skilled nursing facilities: Generally high adherence to POLST orders
The largest study to evaluate the consistency of treatments with POLST orders among nursing home residents found high adherence overall (94%).5 Caregivers performed CPR on none of 299 residents who selected “DNR.” However, they did not administer CPR to 6 of 7 who chose “attempt CPR” and administered antibiotics to 32% of patients who specified “no antibiotics” on their POLST forms.5
A second study of nursing home residents who selected “comfort measures only” also found high consistency for attempting CPR, intensive care admission, and ventilator support, although physicians hospitalized 2% of patients to extend life.6 Similarly, treatments matched POLST orders well overall in a Washington state study, although one patient got a feeding tube against orders.7
POLST adherence is good, but can EMS workers find the form?
A study comparing emergency medical services (EMS) management with POLST orders in an Oregon registry found good consistency.8 EMS providers didn’t attempt or halted CPR in most patients with DNR orders who were found in cardiac arrest and initiated CPR in most patients who chose “attempt CPR.” EMS providers initiated CPR in the field on 11 patients (22%) with a DNR order but discontinued resuscitation en route to the hospital.
In a smaller study, EMS providers never located paper POLST forms at the scene in most cases.9
Hospice: POLST orders prevent unwanted Tx, except maybe antibiotics
A study evaluating management in hospice programs in 3 states found that care providers followed POLST orders for limited treatment in 98% of cases.10 No patients received unwanted CPR, intubation, or feeding tubes. POLST orders didn’t predict whether patients were treated with antibiotics, however.
EVIDENCE SUMMARY
The POLST form offers choices within 4 treatment areas: “attempt CPR” or “allow natural death” if the patient is in cardiopulmonary arrest; “comfort,” “limited,” or “full” medical interventions if pulse or breathing is present; choices of additional orders, including intravenous fluids, feeding tubes, and antibiotics; and additional written orders. Most POLST studies used cross-sectional and retrospective cohort designs and assessed whether CPR was attempted. Fewer studies also evaluated adherence to orders in the other treatment areas.
Community settings: Patients with POLST more likely to die out of hospital
The largest study of POLST use in community settings evaluated deaths in Oregon over one year.1 It found that patients who indicated “do not attempt CPR” on a POLST form were 6 times more likely to die a natural, out-of-hospital death than those who had no POLST form (TABLE1-10).
A West Virginia study found that patients with POLST forms had 30% higher out-of-hospital death rates than those with traditional advanced directives and no POLST.2 In a Wisconsin study, no decedents who indicated DNR on their POLST forms received CPR.3
One study that evaluated the consistency of actual medical interventions with POLST orders in all 4 treatment areas found it to be good in most areas (“feeding tubes,” “attempting CPR.” “antibiotics,” and “IV fluids”) except “additional written orders.4
Skilled nursing facilities: Generally high adherence to POLST orders
The largest study to evaluate the consistency of treatments with POLST orders among nursing home residents found high adherence overall (94%).5 Caregivers performed CPR on none of 299 residents who selected “DNR.” However, they did not administer CPR to 6 of 7 who chose “attempt CPR” and administered antibiotics to 32% of patients who specified “no antibiotics” on their POLST forms.5
A second study of nursing home residents who selected “comfort measures only” also found high consistency for attempting CPR, intensive care admission, and ventilator support, although physicians hospitalized 2% of patients to extend life.6 Similarly, treatments matched POLST orders well overall in a Washington state study, although one patient got a feeding tube against orders.7
POLST adherence is good, but can EMS workers find the form?
A study comparing emergency medical services (EMS) management with POLST orders in an Oregon registry found good consistency.8 EMS providers didn’t attempt or halted CPR in most patients with DNR orders who were found in cardiac arrest and initiated CPR in most patients who chose “attempt CPR.” EMS providers initiated CPR in the field on 11 patients (22%) with a DNR order but discontinued resuscitation en route to the hospital.
In a smaller study, EMS providers never located paper POLST forms at the scene in most cases.9
Hospice: POLST orders prevent unwanted Tx, except maybe antibiotics
A study evaluating management in hospice programs in 3 states found that care providers followed POLST orders for limited treatment in 98% of cases.10 No patients received unwanted CPR, intubation, or feeding tubes. POLST orders didn’t predict whether patients were treated with antibiotics, however.
1. Fromme EK, Zive D, Schmidt TA, et al. Association between physician orders for life-sustaining treatment for scope of treatment and in-hospital death in Oregon. J Am Geriatr Soc. 2014;62:1246-1251.
2. Pedraza SL, Culp S, Falkenstine EC, et al. POST forms more than advance directives associated with out-of-hospital death: insights from a state registry. J Pain Symptom Manage. 2016; 51:240-246.
3. Hammes B, Rooney BL, Gundrum JD, et al. The POLST program: a retrospective review of the demographics of use and outcomes in one community where advance directives are prevalent. J Palliative Med. 2012;15:77-85.
4. Lee MA, Brummel-Smith K, Meyer J, et al. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. J Am Geriatr Soc. 2000;48:1219-1225.
5. Hickman SE, Nelson CA, Moss AH, et al. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc. 2011;59:2091-2099.
6. Tolle SW, Tilden VP, Nelson CA, et al. A prospective study of the efficacy of the physician order form for life sustaining treatment. J Am Ger Soc.1998;46:1097-1102.
7. Meyers J, Moore C, McGrory A, et al. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Geron Nursing. 2004;30:37-46.
8. Richardson DK, Fromme E, Zive D, et al. Concordance of out-of-hospital and emergency department cardiac arrest resuscitation with documented end-of-life choices in Oregon. Ann Emerg Med. 2014;63:375-383.
9. Schmidt T, Olszewski EA, Zive D, et al. The Oregon physician orders for life-sustaining treatment registry: a preliminary study of emergency medical services utilization. J Emerg Med. 2013;44:796-805.
10. Hickman SE, Nelson CA, Moss AH, et al. Use of the physician orders for life-sustaining treatment (POLST) paradigm program in the hospice setting. J Palliat Med. 2009;12:133-141.
1. Fromme EK, Zive D, Schmidt TA, et al. Association between physician orders for life-sustaining treatment for scope of treatment and in-hospital death in Oregon. J Am Geriatr Soc. 2014;62:1246-1251.
2. Pedraza SL, Culp S, Falkenstine EC, et al. POST forms more than advance directives associated with out-of-hospital death: insights from a state registry. J Pain Symptom Manage. 2016; 51:240-246.
3. Hammes B, Rooney BL, Gundrum JD, et al. The POLST program: a retrospective review of the demographics of use and outcomes in one community where advance directives are prevalent. J Palliative Med. 2012;15:77-85.
4. Lee MA, Brummel-Smith K, Meyer J, et al. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. J Am Geriatr Soc. 2000;48:1219-1225.
5. Hickman SE, Nelson CA, Moss AH, et al. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc. 2011;59:2091-2099.
6. Tolle SW, Tilden VP, Nelson CA, et al. A prospective study of the efficacy of the physician order form for life sustaining treatment. J Am Ger Soc.1998;46:1097-1102.
7. Meyers J, Moore C, McGrory A, et al. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Geron Nursing. 2004;30:37-46.
8. Richardson DK, Fromme E, Zive D, et al. Concordance of out-of-hospital and emergency department cardiac arrest resuscitation with documented end-of-life choices in Oregon. Ann Emerg Med. 2014;63:375-383.
9. Schmidt T, Olszewski EA, Zive D, et al. The Oregon physician orders for life-sustaining treatment registry: a preliminary study of emergency medical services utilization. J Emerg Med. 2013;44:796-805.
10. Hickman SE, Nelson CA, Moss AH, et al. Use of the physician orders for life-sustaining treatment (POLST) paradigm program in the hospice setting. J Palliat Med. 2009;12:133-141.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Quite well, for cardiopulmonary resuscitation (CPR). Most patients (91%-100%) who select “do not resuscitate” (DNR) on their physician’s orders for life-sustaining treatment (POLST) forms are allowed a natural death without attempted CPR across a variety of settings (community, skilled nursing facilities, emergency medical services, and hospice). Few patients (6%) who select “comfort measures only” die in the hospital, whereas more (22%) who choose “limited interventions,” and still more (34%) without a POLST form, die in the hospital (strength of recommendation [SOR]: B, large, consistent cross-sectional and cohort studies).
Most patients (84%) who select “attempt resuscitation” receive resuscitation for out-of-hospital cardiac arrest in emergency services settings (SOR: B, small retrospective cohort study).
POLST orders declining other services (intravenous fluids, intensive care, intubation, feeding tubes) are carried out in most (84%-100%) cases. POLST orders regarding antibiotic treatments are less effectively implemented (SOR: B, moderate-sized retrospective chart review).
Distress Screening and Management in an Outpatient VA Cancer Clinic: A Pilot Project Involving Ambulatory Patients Across the Disease Trajectory (FULL)
A diagnosis of cancer, its treatment, and surveillance are fraught with distress. Distress is defined by the National Comprehensive Cancer Network® (NCCN®) as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”1 Distress is known to occur at any point along the cancer-disease trajectory: during diagnosis, during treatment, at the end of treatment, at pivotal treatment decision points, from survivorship through to end of life.2 The severity of the distress can range from “common normal feelings of vulnerability, sadness, and fears to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crisis.”1 Most important, the impact of distress has been associated with reduced quality of life (QOL) and potentially reduced survival.3,4
About 33% of all persons with cancer experience severe distress.5,6 As a result of the prevalence and severity of distress, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management recommend that all patients with cancer should be screened for distress, using a standardized tool, at their initial visit, at appropriate intervals, and as clinically indicated.1 The time line for longitudinal screening of “appropriate intervals” has not been firmly established.2 However, it is well recognized that appropriate intervals include times of vulnerability such as remission, recurrence, termination of treatment, and progression.1,7 Despite efforts to improve distress screening and intervention, many institutions struggle to adhere to the NCCN Guidelines®.8,9
In 2012, the American College of Surgeons Commission on Cancer (ACoS CoC) identified distress screening as an essential accreditation standard by 2015.10 The standard mandates that patients be screened a minimum of 1 time at a “pivotal” medical visit (such as time of diagnosis, transitions in cancer treatment, recurrence, completion of cancer treatment, and progression of disease). In practice, most institutions typically screen at diagnosis.2 According to the ACoS CoC, 41 VAMCs are accredited sites that will be impacted by the implementation of this standard.10
Distress Screening Tools
A major challenge and barrier to integrating distress screening in cancer clinics is the lack of consensus on the best measurement tool in a busy ambulatory clinic. Although a number of screening tools are available for measuring cancer-related distress, they vary in efficacy and feasibility. According to Zabora and Macmurray, the perfect screening instrument for distress in persons with cancer does not exist.6 Brief screening tools demonstrate high sensitivity in identifying very distressed patients but lack specificity, resulting in false positives.8,11 More extensive screening instruments, such as the Hospital Anxiety and Depression Scale (HADS), the Brief Symptom Inventory (BSI)-18, and the Psycho-Oncology Screening Tool (POST), have lower rates of false positives but may be more burdensome for providers, especially when considering copyright and cost.6
Ambulatory cancer care requires a rapid screening method with high sensitivity and minimal burden.12 The NCCN Distress Thermometer (DT) has face validity and allows for rapid screening; however, its psychometric properties are not as robust as other instru ments, such as the Center for Epidemiological Studies Depression Scale, the Hospital Anxiety and Depression Scale, Psychological Distress Inventory, or Brief Symptom Inventory.13 Although the DT has been shown to identify clinically significant anxiety, it is not as sensitive in identifying depression.4
The NCCN DT has 2 parts to the screening: (1) an overall distressintensity score within the past week, including the current day; and (2) an accompanying problem list, grouped into 5 categories, addressing QOL domains.14 The quantitative score ranges from 0 (no distress) to 10 (extreme distress). The problem list complements the quantitative score by providing information about the source of distress and can help to tailor the intervention (Figure 1). Access to the NCCN Guideline and DT is free for clinical and personal use.
According to the NCCN Guideline, scores of ≥ 4 require distress-management intervention.1 Mild distress (score < 4) usually can be managed by the primary oncology team.15 However, if the patient’s score is moderate (4-7) or severe (8-10), urgent intervention is necessary. Depending on the source of the distress, the patient should be seen by the appropriate discipline. For patients with practical problems, such as transportation, finances, and housing issues, a referral to social work is needed. For those with distress related to mental health issues, psychology, psychiatry, or social work may be appropriate.
Patients with distressing physical symptoms should be seen by the physician or advanced practice registered nurse (APRN) from the oncology or palliative care team. With limited psychosocial resources available at many cancer clinics, identification and triage for those with the highest levels of distress are critical.5 Triage must incorporate both the total distress score and the components of the distress so that the appropriate disciplines are accessed for the plan of care. More than one discipline may be needed to address multifactorial distress.
Despite strong recommendations from NCCN, ACoS, and many other professional and accrediting agencies, numerous cancer programs face challenges implementing routine screening. This article reports on a large, inner city ambulatory clinic’s pilot project to distress screen all patients at every appointment in the Cancer Center of Excellence (CoE) at Louis Stokes Cleveland VAMC (LSCVAMC) between May 2012 and May 2014 and to provide immediate intervention from the appropriate discipline for patients scoring ≥ 4 on a 0 to 10 DT scale. Results of the screenings, feasibility of screening in an ambulatory VA cancer clinic, and impact on psychosocial resources are presented.
Center of Excellence Project
The LSCVAMC CoE Cancer Care Clinic began as a 3-year grant-funded project from the VA Offices of Specialty Care and Academic Affiliations with 2 major objectives: (1) to deliver quality patient-centered cancer care as measured by implementation of a process for distress screening and management, and development and implementation of a survivorship care plan for patients who have completed cancer treatment; and (2) to provide interprofessional education for the interdisciplinary health care professionals who participate in the clinic as part of their training experience.
Patients in this unique CoE cancer clinic have sameday access to all members of the interdisciplinary and interprofessional team. The ambulatory cancer care CoE team was originally composed of a surgical oncologist, a medical oncologist, a clinical nurse specialist (CNS) patient navigator, a nurse practitioner (NP) in survivorship care, a registered nurse (RN), a psychologist, and an oncology social worker. The project’s patient population included patients with a cancer concern (positive family history and suspicious scans) or a diagnosis of breast cancer, melanoma, sarcoma, or hematologic malignancies. The patient population for the project was based on the CoE team expertise and feasibility of implementation, with plans to roll out the model of care for all patients with any cancer diagnosis across the VAMC at the completion of the project.
The CoE made distress screening and management the leading priority for quality patient-centered care at the start of the project. The purpose of this emphasis on distress screening was to develop a process at LSCVAMC that would meet the 2015 CoC standards and to teach health care professional trainees (NP students, residents, social work students, and fellows in psychology and medical oncology) about distress screening and intervention.
A plan-do-act model of quality improvement (QI) was used to support the development and implementation of the distress-screening process. At the beginning of the project, the institutional review board (IRB) reviewed the protocol and determined that informed consent was not necessary because a QI project for a new standard of care did not require IRB approval. The CoE team met for about 4 months to develop a policy and procedure for the process, based on evidence from national guidelines, a review of the literature, and a discussion of the benefits and burdens of implementation within the current practice.
Limiting initial implementation to a single clinic day made the process more manageable. Descriptive methods analyzed the incidence and percentage of overall distress in this veteran population and quantified the incidences and percentages of each DT component. Feedback from patients and staff offered information on the feasibility of and satisfaction with the process.
From May 2012 to May 2014, all patients who attended the Monday outpatient CoE clinic with a diagnosis of cancer or a cancer concern were given the NCCN, 2.2013 DT instrument by the registration desk clerk at the time they registered for their clinic appointment. 16 Veterans who had difficulty filling out the DT or who had diminished capacity were assisted in completing the instrument by a designated family member and/or the clinic RN.
The completed instrument was evaluated by the CNS patient navigator, and any patient with a score ≥ 4 received an automatic referral to the behavioral health psychologist, social worker, NP, or all team members and their trainees, depending on the areas of distress (eg, practical, family, and emotional problems, spiritual/religious concerns, and/or physical problems) endorsed by the patient.
A psychiatrist was not embedded into the team but worked closely with the team’s oncology psychologist. The psychologist communicated directly with the psychiatrist, and the plan was shared with the team through the electronic medical record (EMR). The appropriate team member(s) and trainee(s) saw the patient at the visit to address needs in real time. Access to palliative care support and spiritual care was readily available if needed.
Distress screenings were recorded in a templated note in the patient’s EMR, which allowed the team to follow the distress scores on an individual basis across the cancer disease trajectory and to assess response to interventions. Multiple screenings of individuals resulted from the fact that many of the patients were seen monthly or every 3/6/9 months, depending on their disease and treatment status. Because levels of distress can fluctuate, distress was assessed at every visit to determine whether an intervention was needed at that visit. Once distress screenings were recorded in the patient’s EMR, the DT instrument was de-identified and given to the CoE research consultant to enter into a database file for analysis.
Trainees were educated about the use of the DT at time of diagnosis and across the disease trajectory. The 4-week CoE curriculum included 2 weeks of conference time to teach about the roles of psychologist, oncology social worker, and survivorship NP in assessing and initiating interventions to address the multidimensional components of the DT. Trainees working with a veteran who was distressed participated in the assessment(s) and intervention(s) for all components of distress that were endorsed.
Results
A total of 866 distress screenings were performed during the first 2 years of the project. Since all patients were screened at all visits, the 866 distress screenings reflect multiple screenings for 445 unique patients. Of the 866 screenings, 290 (33%) had distress scores of ≥ 4, meeting the criteria for intervention. Screenings reflected patient visits at any point in the disease trajectory. Because this was a new standard of care QI project rather than a research project, additional data, such as diagnosis or staging, were not collected, and IRB approval was not needed.
Because the NCCN Guideline recommendation for intervention is a score of ≥ 4, the descriptive statistics focused on those with moderate-to-severe distress. However, there were numerous occasions when the veteran would report a score of 0 to 3 and still endorse a number of the problems on the DT. The CNS and RN on the team discussed these findings with the appropriate discipline. For example, if the veteran reported a score of 1 but endorsed all 6 components on the emotional problem list, the nurses discussed the patient with the social worker or psychologist to determine whether behavioral health intervention was needed.
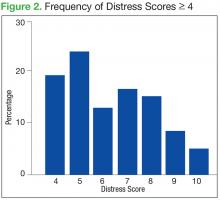
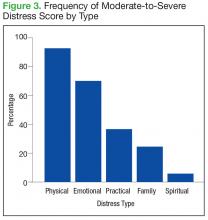
The mean distress score for the 290 screenings ≥ 4 was 6.3 on a 0 to 10 scale; median was 6.0 and mode 5.0. Two hundred ten of these screenings (72%) were categorized as moderate distress (4-7), and 80 patients (28%) reported severe distress (Figure 2). If the veteran left a box empty on the problem list, itwas recorded as missing. The frequency that patients reported each type of distress are reported in Figure 3.
The incidence of each component of distress, from those screenings with a score of ≥ 4 is described below, along with case study examples for each component. Team members involved in patient interventions provided these case studies to demonstrate clinical examples of the veteran’s distress from the problem list on the DT.
Practical/Family Distress
Practical issues were reported in 38% of the screenings (109/290). Intervention for moderate-to-severe distress associated with practical problems and family issues was provided by the team social worker. The social worker frequently addressed transportationrelated distress. Providing transportation was essential for adherence to clinic appointments, follow-up testing, treatments, and ultimately, disease management. Housing was also a problem for many veterans. It is critical that patients have access to electricity, heating, food, and water to be able to safely undergo adjuvant therapy. Thus, treatments decisions could be impacted by the veterans’ housing and transportation issues; immediate access to social work support is essential for quality cancer care.
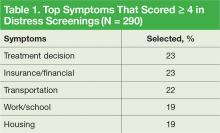
Twenty-six percent of patients that were screened expressed concerns with practical and/or family problems (75/289). Issues of domestic violence, difficulties dealing with a significant other, and concerns about children were referred to social work (Table 1).
Case Study
Ms. S. is a veteran aged 71 years with recently diagnosed breast cancer. She is being seen in the clinic for a postoperative visit following partial mastectomy and is anticipating beginning radiation therapy within the next 3 weeks. She reports a distress score of 7 and identifies concerns about work and transportation to the clinic as the sources of distress. The social worker meets with the patient and learns that she fears losing her job because of the daily travel time to and from radiation and that she cannot afford to travel 65 miles daily to LSCVAMC for radiation. The social worker listens to her concerns and assists her with a plan for short-term disability and VA housing during her radiation therapy treatments. Ms. S. was able to complete radiation at LSCVAMC with temporary housing and to return to work after therapy.
Emotional Distress
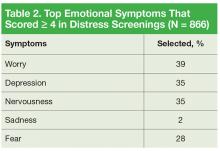
Patients who identified that their moderate-to-severe distress was related to emotional problems received same-day intervention from a psychologist skilled in providing emotional support, cognitive behavioral strategies, and assessing the need for referral to either a psychiatrist or oncology social worker. Seventy-one percent of patients reported emotional problems, such as worry, depression, and nervousness (Table 2).
Case Study
Mr. K. is a veteran aged 71 years with a new diagnosis of breast cancer. He lives on his own but has family and a few friends nearby. He reports that he doesn’t like to share his problems with others and has not told anyone of his new diagnosis. Mr. K. rates his distress a 7 and endorses worry, fear, and depression. At a treatment-planning visit, he agrees to see the psychologist for help in dealing with his distress. Treatment involves a mastectomy followed by hormonal therapy.
Mr. K. was scared about having cancer; some of his veteran colleagues have developed cancer recently, and 2 have died. He told the psychologist that he feels worthless and that this disease just makes him more of a burden on society. He has had thoughts of taking his life so that he doesn’t have to deal with cancer, but he does not have a plan. The team formulated a plan to address his anxiety and depression. Mr. K. started a serotonin reuptake inhibitor, and he met with the VA psychiatrist weekly to help develop coping strategies. The team’s psychologist worked closely with Mr. K.’s psychiatrist, and he successfully completed surgery and chemotherapy. He is now being seen in survivorship clinic, continuing care with the team and his psychologist.
Spiritual Distress
Although spiritual/religious concerns are part of DT screening, it is only a single item on the DT. Just 8% of patients (21/276) reported moderate-to-severe spiritual distress. However, there was access to a chaplain at LSCVAMC.
Case Study
Mr. H., a 63-year-old veteran with stage IV melanoma, was seen in the clinic for severe pain in his left hip and ribs (8 on a 10-point scale); he was unresponsive to escalating doses of oxycodone. During the visit, he reported that his distress level is a 10, and in addition to identifying pain as a source of distress, he indicated that he has spiritual distress. When questioned further about spiritual distress, Mr. H. reported that he deserves this pain since he caused so many others pain during his time in Vietnam. The chaplain was contacted, and the patient was seen in clinic at this visit. The chaplain gave him the opportunity to share his feelings of guilt. The importance of spiritual care when the patient is experiencing “total pain” is essential to pain management. Within 3 days, his pain score decreased to an acceptable level of 3 with no additional pharmacologic intervention.
Physical Distress
Physical problems associated with the distress scores were addressed by the surgical and medical oncologists and the APRNs (CNS patient navigator and survivorship NP). When the clinic opened, the team used the Memorial Symptom Assessment Scale to assess physical and psychological symptoms.16 However, patients reported experiencing distress at having to complete 2 tools that had a great deal of overlap. The team determined that the DT could be used as the sole screening tool for all QOL domains.
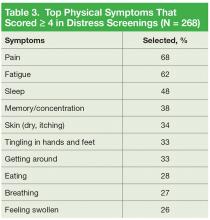
It is important to note that 92% of patients with moderate-to-severe distress reported physical symptoms as a source of distress (Table 3).
Case Study
Ms. L. is a Vietnam War veteran aged 64 years who was seen in the survivorship clinic. She was diagnosed with estrogen-receptor (ER) and progesterone-receptor (PR) breast cancer 1 year previously, had a lumpectomy followed by radiation therapy, and was on hormonal therapy. She recorded her distress score as a 6 and indicated that multiple physical symptoms were her major concern. She had difficulty with insomnia, fatigue, and hot flashes. The survivorship NP talked with Ms. L. about her symptoms and made nonpharmacologic recommendations for improving sleep, provided an exercise plan for fatigue, and initiated venlafaxine to manage the hot flashes. Ms. L. continued to be seen by the team in survivorship clinic, and during her 3-month follow-up visit, she reported improvement in sleep as the hot flashes diminished.
Multifactoral Distress
Many patients endorsed ≥ 1 component of distress. This required a team approach to intervene for the multifactorial nature of the distress.
Case Study
Mr. K. is a veteran aged 82 years who had been a farmer most of his life. He was cared for at the VA for an advancedstage squamous cell skin cancer of his scalp, which he had allowed to go untreated. The cancer has completely eroded beneath his scalp, and he wore a hat to cover the foul-smelling wound. He lived in rural Ohio with his wife of 55 years; 3 adult daughters lived in the Cleveland area. His daughters served as primary caregivers when Mr. K. came to Cleveland for daily radiation and weekly chemotherapy treatments. He had not been away from his wife since the war and misses her terribly, returning home only on weekends during the 6-week course of radiation.
His primary goal was to return home in time to harvest his farm’s produce 2 months later. He was aware that he has < 6 months to live but wanted chemotherapy and radiation to control the growth of the cancer. During this visit to the ambulatory clinic, he reported a distress score of 5 andidentified family concerns (eg, living away from his wife most of every week) and endorsed emotional concerns of fear, worry, and sadness, and reported pain, fatigue, insomnia, and constipation as physical concerns.
Mr. K. received support from the social worker, the psychologist, and the APRN for symptom management during this visit. The social worker was able to advocate for limited palliative radiation therapy treatments rather than a 6-week course; the psychologist spent 45 minutes talking with him about his fears of a painful death, worries about his wife, and sadness at not being alive for another planting season. The APRN recommended both pharmacologic and nonpharmacologic interventions for his fatigue and insomnia and initiated a pain and bowel pharmacologic regimen. The team respected Mr. K.’s wish to reconsider hospice care at the following visit after he had talked with his wife. Mr. K. died peacefully in his home with his wife and family just before the start of planting season.
Clinical Implications
Distress screening and intervention is essential for quality cancer care. While a great deal of controversy exists about the best time to screen for distress, the LSCVAMC CoE has taken on the challenge of screening and intervening in real time at every patient visit across the disease trajectory. The model of distress screening all veterans at CoE clinic visits has been rolled out to other cancer clinics at LSCVAMC.
Distress screening at each visit is not time intensive. Patients are willing to fill out the instrument while waiting for their clinic visit, and most patients find that it takes less than 5 minutes to complete. The major challenge for institutions considering screening with each visit is not the screening but access to appropriate providers able to provide timely intervention. The success of this model results, in part, because the clinic RN assesses the responses to the DT and refers to the appropriate discipline, utilizing precious resources of social work and psychology appropriately. The VA system is already committed to improving the psychosocial well-being of veterans and has established social work and psychology resources specifically for the cancer clinics.
Many patients reported to the authors that they might not have been able or willing to return to LSCVAMC to see the behavioral health specialists on another day. In addition, scheduling behavioral health appointments at another time would not allow for attending to the distress in real time. Also, from a systems standpoint, it would have been an added cost to the VA and/or the veteran for transportation for additional appointments on different days.
Finally, although the impact of the CoE project on health professional trainees has been reported elsewhere, the distress screening and intervention process were valued as being very positive for all trainees who participated in CoE clinic.17 The trainees were able to stay with the patient for the entire clinic visit, including the visits made by disciplines other than their own. For example, the family medicine residents stayed with the patient they examined to observe the distress assessments and interventions offered by the social worker and/or psychologist for the patients who scored ≥ 4 on the DT.
At the end of their rotations in the CoE, trainees reported an increased awareness of the importance of distress screening in a cancer clinic. Many were not aware of the NCCN guidelines and the ACoS CoC mandate for distress screening as a standard of cancer care. Interdisciplinary trainees rated the CoE curriculum and the conference teaching/learning sessions on distress management highly. However, observing the role of the social worker and psychologist were the most valuable to trainees, regardless of the area of practice they enter.
Conclusion
Addressing practical, psychosocial, physical, and spiritual needs will help decrease distress, support patients’ ability to tolerate treatment, and improve veterans’ QOL across the cancer-disease trajectory. Screening all patients at an outpatient cancer clinic at LSCVAMC is feasible and does not seem to be a burden for patients or providers. This pilot project has become standard of care across the LSCVAMC cancer clinics, demonstrating its sustainability.
Screening with the DT provides information about the intensity of the distress and the components contributing to the distress. The most important aspect of the screening is assessing the components of the distress and providing real-time intervention from the appropriate discipline. It is critical that the oncology team refer to the appropriate discipline based on the source of the distress rather than on only the intensity. Findings from this project indicate that physical symptoms are frequently the source of distress and may not require behavioral health intervention. However, for patients with psychosocial needs, rapid access to behavioral health care services is critical for quality veteran-centered cancer care.
Since 2015, all VA cancer centers are required to have implemented distress screening. According to the CoC, at least 1 screening must be done on every patient.10 Many institutions have begun to screen at diagnosis, but it is well known that there are many points along the cancer trajectory when patients may experience an increase in distress. Simple screening with the DT at every cancer clinic visit helps identify the veterans’ needs at any point along the disease spectrum.
At LSCVAMC, the CoE was designed as an interdisciplinary cancer clinic. With the conclusion of funding in FY 2015, the clinic has continued to function. The rollout into other clinics has continued with movement toward use of formal consult requests and continual, real-time evaluation of the process. Work on accurate, timely identification of new cancer patients and identifying pivotal cancer visits is underway. The LSCVAMC is committed to improving care and access to its veterans with cancer to ensure appropriate and adequate services across the cancer trajectory.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Holand JC, Jacobsen PB, Anderson A, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Distress Management 2.2016. © 2014 National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. Updated July 25, 2016. Accessed January 13, 2017.
2. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
3. Hamer M, Chida Y, Molloy G. Psychological distress and cancer mortality. J Psychosom Res. 2009;66(3):255-258.
4. Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2013;8(4):487-494.
5. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
6. Zabora JR, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2012;30(6):625-635.
7. Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(91):2946-2954.
8. Parry C, Padgett LS, Zebrack B. Now what? Toward an integrated research and practice agenda in distress screening. J Psychosoc Oncol. 2012;30(6):715-727.
9. Wagner LI, Spiegel D, Pearman T. Using the science of psychosocial care to implement the new American College of Surgeons Commission on Cancer distress screening standard. J Natl Compr Canc Netw. 2013;11(2):214-221.
10. American College of Surgeons Commision on Cancer. https://www.facs.org/quality-programs/cancer/coc Published 1996. Updated January 19, 2017. Accessed April 16, 2016.
11. Rohan EA. Removing the stress from selecting instruments: arming social workers to take leadership in routine distress screening implementation. J Psychosoc Oncol. 2012;30(6):667-678.
12. Merport A, Bober SL, Grose A, Recklitis CJ. Can the distress thermometer (DT) identify significant psychological distress in long-term cancer survivors? A comparison with the Brief Symptom Inventory-18 (BSI-18). Support Care Cancer. 2012;20(1):195-198.
13. Carlson LE, Bultz BD. Cancer distress screening: Needs, models, and methods. J Psychosom Res. 2003;55(5):403-409.
14. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010:8(1):4-12.
15. Jacobsen P, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
16. Portenoy R, Thaler HT, Korblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326-1336.
17. Arfons L, Mazanec P, Smith J, et al. Training health care professionals in interprofessional collaborative cancer care. Health Interprof Pract. 2015;2(3):eP1073.
A diagnosis of cancer, its treatment, and surveillance are fraught with distress. Distress is defined by the National Comprehensive Cancer Network® (NCCN®) as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”1 Distress is known to occur at any point along the cancer-disease trajectory: during diagnosis, during treatment, at the end of treatment, at pivotal treatment decision points, from survivorship through to end of life.2 The severity of the distress can range from “common normal feelings of vulnerability, sadness, and fears to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crisis.”1 Most important, the impact of distress has been associated with reduced quality of life (QOL) and potentially reduced survival.3,4
About 33% of all persons with cancer experience severe distress.5,6 As a result of the prevalence and severity of distress, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management recommend that all patients with cancer should be screened for distress, using a standardized tool, at their initial visit, at appropriate intervals, and as clinically indicated.1 The time line for longitudinal screening of “appropriate intervals” has not been firmly established.2 However, it is well recognized that appropriate intervals include times of vulnerability such as remission, recurrence, termination of treatment, and progression.1,7 Despite efforts to improve distress screening and intervention, many institutions struggle to adhere to the NCCN Guidelines®.8,9
In 2012, the American College of Surgeons Commission on Cancer (ACoS CoC) identified distress screening as an essential accreditation standard by 2015.10 The standard mandates that patients be screened a minimum of 1 time at a “pivotal” medical visit (such as time of diagnosis, transitions in cancer treatment, recurrence, completion of cancer treatment, and progression of disease). In practice, most institutions typically screen at diagnosis.2 According to the ACoS CoC, 41 VAMCs are accredited sites that will be impacted by the implementation of this standard.10
Distress Screening Tools
A major challenge and barrier to integrating distress screening in cancer clinics is the lack of consensus on the best measurement tool in a busy ambulatory clinic. Although a number of screening tools are available for measuring cancer-related distress, they vary in efficacy and feasibility. According to Zabora and Macmurray, the perfect screening instrument for distress in persons with cancer does not exist.6 Brief screening tools demonstrate high sensitivity in identifying very distressed patients but lack specificity, resulting in false positives.8,11 More extensive screening instruments, such as the Hospital Anxiety and Depression Scale (HADS), the Brief Symptom Inventory (BSI)-18, and the Psycho-Oncology Screening Tool (POST), have lower rates of false positives but may be more burdensome for providers, especially when considering copyright and cost.6
Ambulatory cancer care requires a rapid screening method with high sensitivity and minimal burden.12 The NCCN Distress Thermometer (DT) has face validity and allows for rapid screening; however, its psychometric properties are not as robust as other instru ments, such as the Center for Epidemiological Studies Depression Scale, the Hospital Anxiety and Depression Scale, Psychological Distress Inventory, or Brief Symptom Inventory.13 Although the DT has been shown to identify clinically significant anxiety, it is not as sensitive in identifying depression.4
The NCCN DT has 2 parts to the screening: (1) an overall distressintensity score within the past week, including the current day; and (2) an accompanying problem list, grouped into 5 categories, addressing QOL domains.14 The quantitative score ranges from 0 (no distress) to 10 (extreme distress). The problem list complements the quantitative score by providing information about the source of distress and can help to tailor the intervention (Figure 1). Access to the NCCN Guideline and DT is free for clinical and personal use.
According to the NCCN Guideline, scores of ≥ 4 require distress-management intervention.1 Mild distress (score < 4) usually can be managed by the primary oncology team.15 However, if the patient’s score is moderate (4-7) or severe (8-10), urgent intervention is necessary. Depending on the source of the distress, the patient should be seen by the appropriate discipline. For patients with practical problems, such as transportation, finances, and housing issues, a referral to social work is needed. For those with distress related to mental health issues, psychology, psychiatry, or social work may be appropriate.
Patients with distressing physical symptoms should be seen by the physician or advanced practice registered nurse (APRN) from the oncology or palliative care team. With limited psychosocial resources available at many cancer clinics, identification and triage for those with the highest levels of distress are critical.5 Triage must incorporate both the total distress score and the components of the distress so that the appropriate disciplines are accessed for the plan of care. More than one discipline may be needed to address multifactorial distress.
Despite strong recommendations from NCCN, ACoS, and many other professional and accrediting agencies, numerous cancer programs face challenges implementing routine screening. This article reports on a large, inner city ambulatory clinic’s pilot project to distress screen all patients at every appointment in the Cancer Center of Excellence (CoE) at Louis Stokes Cleveland VAMC (LSCVAMC) between May 2012 and May 2014 and to provide immediate intervention from the appropriate discipline for patients scoring ≥ 4 on a 0 to 10 DT scale. Results of the screenings, feasibility of screening in an ambulatory VA cancer clinic, and impact on psychosocial resources are presented.
Center of Excellence Project
The LSCVAMC CoE Cancer Care Clinic began as a 3-year grant-funded project from the VA Offices of Specialty Care and Academic Affiliations with 2 major objectives: (1) to deliver quality patient-centered cancer care as measured by implementation of a process for distress screening and management, and development and implementation of a survivorship care plan for patients who have completed cancer treatment; and (2) to provide interprofessional education for the interdisciplinary health care professionals who participate in the clinic as part of their training experience.
Patients in this unique CoE cancer clinic have sameday access to all members of the interdisciplinary and interprofessional team. The ambulatory cancer care CoE team was originally composed of a surgical oncologist, a medical oncologist, a clinical nurse specialist (CNS) patient navigator, a nurse practitioner (NP) in survivorship care, a registered nurse (RN), a psychologist, and an oncology social worker. The project’s patient population included patients with a cancer concern (positive family history and suspicious scans) or a diagnosis of breast cancer, melanoma, sarcoma, or hematologic malignancies. The patient population for the project was based on the CoE team expertise and feasibility of implementation, with plans to roll out the model of care for all patients with any cancer diagnosis across the VAMC at the completion of the project.
The CoE made distress screening and management the leading priority for quality patient-centered care at the start of the project. The purpose of this emphasis on distress screening was to develop a process at LSCVAMC that would meet the 2015 CoC standards and to teach health care professional trainees (NP students, residents, social work students, and fellows in psychology and medical oncology) about distress screening and intervention.
A plan-do-act model of quality improvement (QI) was used to support the development and implementation of the distress-screening process. At the beginning of the project, the institutional review board (IRB) reviewed the protocol and determined that informed consent was not necessary because a QI project for a new standard of care did not require IRB approval. The CoE team met for about 4 months to develop a policy and procedure for the process, based on evidence from national guidelines, a review of the literature, and a discussion of the benefits and burdens of implementation within the current practice.
Limiting initial implementation to a single clinic day made the process more manageable. Descriptive methods analyzed the incidence and percentage of overall distress in this veteran population and quantified the incidences and percentages of each DT component. Feedback from patients and staff offered information on the feasibility of and satisfaction with the process.
From May 2012 to May 2014, all patients who attended the Monday outpatient CoE clinic with a diagnosis of cancer or a cancer concern were given the NCCN, 2.2013 DT instrument by the registration desk clerk at the time they registered for their clinic appointment. 16 Veterans who had difficulty filling out the DT or who had diminished capacity were assisted in completing the instrument by a designated family member and/or the clinic RN.
The completed instrument was evaluated by the CNS patient navigator, and any patient with a score ≥ 4 received an automatic referral to the behavioral health psychologist, social worker, NP, or all team members and their trainees, depending on the areas of distress (eg, practical, family, and emotional problems, spiritual/religious concerns, and/or physical problems) endorsed by the patient.
A psychiatrist was not embedded into the team but worked closely with the team’s oncology psychologist. The psychologist communicated directly with the psychiatrist, and the plan was shared with the team through the electronic medical record (EMR). The appropriate team member(s) and trainee(s) saw the patient at the visit to address needs in real time. Access to palliative care support and spiritual care was readily available if needed.
Distress screenings were recorded in a templated note in the patient’s EMR, which allowed the team to follow the distress scores on an individual basis across the cancer disease trajectory and to assess response to interventions. Multiple screenings of individuals resulted from the fact that many of the patients were seen monthly or every 3/6/9 months, depending on their disease and treatment status. Because levels of distress can fluctuate, distress was assessed at every visit to determine whether an intervention was needed at that visit. Once distress screenings were recorded in the patient’s EMR, the DT instrument was de-identified and given to the CoE research consultant to enter into a database file for analysis.
Trainees were educated about the use of the DT at time of diagnosis and across the disease trajectory. The 4-week CoE curriculum included 2 weeks of conference time to teach about the roles of psychologist, oncology social worker, and survivorship NP in assessing and initiating interventions to address the multidimensional components of the DT. Trainees working with a veteran who was distressed participated in the assessment(s) and intervention(s) for all components of distress that were endorsed.
Results
A total of 866 distress screenings were performed during the first 2 years of the project. Since all patients were screened at all visits, the 866 distress screenings reflect multiple screenings for 445 unique patients. Of the 866 screenings, 290 (33%) had distress scores of ≥ 4, meeting the criteria for intervention. Screenings reflected patient visits at any point in the disease trajectory. Because this was a new standard of care QI project rather than a research project, additional data, such as diagnosis or staging, were not collected, and IRB approval was not needed.
Because the NCCN Guideline recommendation for intervention is a score of ≥ 4, the descriptive statistics focused on those with moderate-to-severe distress. However, there were numerous occasions when the veteran would report a score of 0 to 3 and still endorse a number of the problems on the DT. The CNS and RN on the team discussed these findings with the appropriate discipline. For example, if the veteran reported a score of 1 but endorsed all 6 components on the emotional problem list, the nurses discussed the patient with the social worker or psychologist to determine whether behavioral health intervention was needed.
The mean distress score for the 290 screenings ≥ 4 was 6.3 on a 0 to 10 scale; median was 6.0 and mode 5.0. Two hundred ten of these screenings (72%) were categorized as moderate distress (4-7), and 80 patients (28%) reported severe distress (Figure 2). If the veteran left a box empty on the problem list, itwas recorded as missing. The frequency that patients reported each type of distress are reported in Figure 3.
The incidence of each component of distress, from those screenings with a score of ≥ 4 is described below, along with case study examples for each component. Team members involved in patient interventions provided these case studies to demonstrate clinical examples of the veteran’s distress from the problem list on the DT.
Practical/Family Distress
Practical issues were reported in 38% of the screenings (109/290). Intervention for moderate-to-severe distress associated with practical problems and family issues was provided by the team social worker. The social worker frequently addressed transportationrelated distress. Providing transportation was essential for adherence to clinic appointments, follow-up testing, treatments, and ultimately, disease management. Housing was also a problem for many veterans. It is critical that patients have access to electricity, heating, food, and water to be able to safely undergo adjuvant therapy. Thus, treatments decisions could be impacted by the veterans’ housing and transportation issues; immediate access to social work support is essential for quality cancer care.
Twenty-six percent of patients that were screened expressed concerns with practical and/or family problems (75/289). Issues of domestic violence, difficulties dealing with a significant other, and concerns about children were referred to social work (Table 1).
Case Study
Ms. S. is a veteran aged 71 years with recently diagnosed breast cancer. She is being seen in the clinic for a postoperative visit following partial mastectomy and is anticipating beginning radiation therapy within the next 3 weeks. She reports a distress score of 7 and identifies concerns about work and transportation to the clinic as the sources of distress. The social worker meets with the patient and learns that she fears losing her job because of the daily travel time to and from radiation and that she cannot afford to travel 65 miles daily to LSCVAMC for radiation. The social worker listens to her concerns and assists her with a plan for short-term disability and VA housing during her radiation therapy treatments. Ms. S. was able to complete radiation at LSCVAMC with temporary housing and to return to work after therapy.
Emotional Distress
Patients who identified that their moderate-to-severe distress was related to emotional problems received same-day intervention from a psychologist skilled in providing emotional support, cognitive behavioral strategies, and assessing the need for referral to either a psychiatrist or oncology social worker. Seventy-one percent of patients reported emotional problems, such as worry, depression, and nervousness (Table 2).
Case Study
Mr. K. is a veteran aged 71 years with a new diagnosis of breast cancer. He lives on his own but has family and a few friends nearby. He reports that he doesn’t like to share his problems with others and has not told anyone of his new diagnosis. Mr. K. rates his distress a 7 and endorses worry, fear, and depression. At a treatment-planning visit, he agrees to see the psychologist for help in dealing with his distress. Treatment involves a mastectomy followed by hormonal therapy.
Mr. K. was scared about having cancer; some of his veteran colleagues have developed cancer recently, and 2 have died. He told the psychologist that he feels worthless and that this disease just makes him more of a burden on society. He has had thoughts of taking his life so that he doesn’t have to deal with cancer, but he does not have a plan. The team formulated a plan to address his anxiety and depression. Mr. K. started a serotonin reuptake inhibitor, and he met with the VA psychiatrist weekly to help develop coping strategies. The team’s psychologist worked closely with Mr. K.’s psychiatrist, and he successfully completed surgery and chemotherapy. He is now being seen in survivorship clinic, continuing care with the team and his psychologist.
Spiritual Distress
Although spiritual/religious concerns are part of DT screening, it is only a single item on the DT. Just 8% of patients (21/276) reported moderate-to-severe spiritual distress. However, there was access to a chaplain at LSCVAMC.
Case Study
Mr. H., a 63-year-old veteran with stage IV melanoma, was seen in the clinic for severe pain in his left hip and ribs (8 on a 10-point scale); he was unresponsive to escalating doses of oxycodone. During the visit, he reported that his distress level is a 10, and in addition to identifying pain as a source of distress, he indicated that he has spiritual distress. When questioned further about spiritual distress, Mr. H. reported that he deserves this pain since he caused so many others pain during his time in Vietnam. The chaplain was contacted, and the patient was seen in clinic at this visit. The chaplain gave him the opportunity to share his feelings of guilt. The importance of spiritual care when the patient is experiencing “total pain” is essential to pain management. Within 3 days, his pain score decreased to an acceptable level of 3 with no additional pharmacologic intervention.
Physical Distress
Physical problems associated with the distress scores were addressed by the surgical and medical oncologists and the APRNs (CNS patient navigator and survivorship NP). When the clinic opened, the team used the Memorial Symptom Assessment Scale to assess physical and psychological symptoms.16 However, patients reported experiencing distress at having to complete 2 tools that had a great deal of overlap. The team determined that the DT could be used as the sole screening tool for all QOL domains.
It is important to note that 92% of patients with moderate-to-severe distress reported physical symptoms as a source of distress (Table 3).
Case Study
Ms. L. is a Vietnam War veteran aged 64 years who was seen in the survivorship clinic. She was diagnosed with estrogen-receptor (ER) and progesterone-receptor (PR) breast cancer 1 year previously, had a lumpectomy followed by radiation therapy, and was on hormonal therapy. She recorded her distress score as a 6 and indicated that multiple physical symptoms were her major concern. She had difficulty with insomnia, fatigue, and hot flashes. The survivorship NP talked with Ms. L. about her symptoms and made nonpharmacologic recommendations for improving sleep, provided an exercise plan for fatigue, and initiated venlafaxine to manage the hot flashes. Ms. L. continued to be seen by the team in survivorship clinic, and during her 3-month follow-up visit, she reported improvement in sleep as the hot flashes diminished.
Multifactoral Distress
Many patients endorsed ≥ 1 component of distress. This required a team approach to intervene for the multifactorial nature of the distress.
Case Study
Mr. K. is a veteran aged 82 years who had been a farmer most of his life. He was cared for at the VA for an advancedstage squamous cell skin cancer of his scalp, which he had allowed to go untreated. The cancer has completely eroded beneath his scalp, and he wore a hat to cover the foul-smelling wound. He lived in rural Ohio with his wife of 55 years; 3 adult daughters lived in the Cleveland area. His daughters served as primary caregivers when Mr. K. came to Cleveland for daily radiation and weekly chemotherapy treatments. He had not been away from his wife since the war and misses her terribly, returning home only on weekends during the 6-week course of radiation.
His primary goal was to return home in time to harvest his farm’s produce 2 months later. He was aware that he has < 6 months to live but wanted chemotherapy and radiation to control the growth of the cancer. During this visit to the ambulatory clinic, he reported a distress score of 5 andidentified family concerns (eg, living away from his wife most of every week) and endorsed emotional concerns of fear, worry, and sadness, and reported pain, fatigue, insomnia, and constipation as physical concerns.
Mr. K. received support from the social worker, the psychologist, and the APRN for symptom management during this visit. The social worker was able to advocate for limited palliative radiation therapy treatments rather than a 6-week course; the psychologist spent 45 minutes talking with him about his fears of a painful death, worries about his wife, and sadness at not being alive for another planting season. The APRN recommended both pharmacologic and nonpharmacologic interventions for his fatigue and insomnia and initiated a pain and bowel pharmacologic regimen. The team respected Mr. K.’s wish to reconsider hospice care at the following visit after he had talked with his wife. Mr. K. died peacefully in his home with his wife and family just before the start of planting season.
Clinical Implications
Distress screening and intervention is essential for quality cancer care. While a great deal of controversy exists about the best time to screen for distress, the LSCVAMC CoE has taken on the challenge of screening and intervening in real time at every patient visit across the disease trajectory. The model of distress screening all veterans at CoE clinic visits has been rolled out to other cancer clinics at LSCVAMC.
Distress screening at each visit is not time intensive. Patients are willing to fill out the instrument while waiting for their clinic visit, and most patients find that it takes less than 5 minutes to complete. The major challenge for institutions considering screening with each visit is not the screening but access to appropriate providers able to provide timely intervention. The success of this model results, in part, because the clinic RN assesses the responses to the DT and refers to the appropriate discipline, utilizing precious resources of social work and psychology appropriately. The VA system is already committed to improving the psychosocial well-being of veterans and has established social work and psychology resources specifically for the cancer clinics.
Many patients reported to the authors that they might not have been able or willing to return to LSCVAMC to see the behavioral health specialists on another day. In addition, scheduling behavioral health appointments at another time would not allow for attending to the distress in real time. Also, from a systems standpoint, it would have been an added cost to the VA and/or the veteran for transportation for additional appointments on different days.
Finally, although the impact of the CoE project on health professional trainees has been reported elsewhere, the distress screening and intervention process were valued as being very positive for all trainees who participated in CoE clinic.17 The trainees were able to stay with the patient for the entire clinic visit, including the visits made by disciplines other than their own. For example, the family medicine residents stayed with the patient they examined to observe the distress assessments and interventions offered by the social worker and/or psychologist for the patients who scored ≥ 4 on the DT.
At the end of their rotations in the CoE, trainees reported an increased awareness of the importance of distress screening in a cancer clinic. Many were not aware of the NCCN guidelines and the ACoS CoC mandate for distress screening as a standard of cancer care. Interdisciplinary trainees rated the CoE curriculum and the conference teaching/learning sessions on distress management highly. However, observing the role of the social worker and psychologist were the most valuable to trainees, regardless of the area of practice they enter.
Conclusion
Addressing practical, psychosocial, physical, and spiritual needs will help decrease distress, support patients’ ability to tolerate treatment, and improve veterans’ QOL across the cancer-disease trajectory. Screening all patients at an outpatient cancer clinic at LSCVAMC is feasible and does not seem to be a burden for patients or providers. This pilot project has become standard of care across the LSCVAMC cancer clinics, demonstrating its sustainability.
Screening with the DT provides information about the intensity of the distress and the components contributing to the distress. The most important aspect of the screening is assessing the components of the distress and providing real-time intervention from the appropriate discipline. It is critical that the oncology team refer to the appropriate discipline based on the source of the distress rather than on only the intensity. Findings from this project indicate that physical symptoms are frequently the source of distress and may not require behavioral health intervention. However, for patients with psychosocial needs, rapid access to behavioral health care services is critical for quality veteran-centered cancer care.
Since 2015, all VA cancer centers are required to have implemented distress screening. According to the CoC, at least 1 screening must be done on every patient.10 Many institutions have begun to screen at diagnosis, but it is well known that there are many points along the cancer trajectory when patients may experience an increase in distress. Simple screening with the DT at every cancer clinic visit helps identify the veterans’ needs at any point along the disease spectrum.
At LSCVAMC, the CoE was designed as an interdisciplinary cancer clinic. With the conclusion of funding in FY 2015, the clinic has continued to function. The rollout into other clinics has continued with movement toward use of formal consult requests and continual, real-time evaluation of the process. Work on accurate, timely identification of new cancer patients and identifying pivotal cancer visits is underway. The LSCVAMC is committed to improving care and access to its veterans with cancer to ensure appropriate and adequate services across the cancer trajectory.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
A diagnosis of cancer, its treatment, and surveillance are fraught with distress. Distress is defined by the National Comprehensive Cancer Network® (NCCN®) as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”1 Distress is known to occur at any point along the cancer-disease trajectory: during diagnosis, during treatment, at the end of treatment, at pivotal treatment decision points, from survivorship through to end of life.2 The severity of the distress can range from “common normal feelings of vulnerability, sadness, and fears to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crisis.”1 Most important, the impact of distress has been associated with reduced quality of life (QOL) and potentially reduced survival.3,4
About 33% of all persons with cancer experience severe distress.5,6 As a result of the prevalence and severity of distress, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management recommend that all patients with cancer should be screened for distress, using a standardized tool, at their initial visit, at appropriate intervals, and as clinically indicated.1 The time line for longitudinal screening of “appropriate intervals” has not been firmly established.2 However, it is well recognized that appropriate intervals include times of vulnerability such as remission, recurrence, termination of treatment, and progression.1,7 Despite efforts to improve distress screening and intervention, many institutions struggle to adhere to the NCCN Guidelines®.8,9
In 2012, the American College of Surgeons Commission on Cancer (ACoS CoC) identified distress screening as an essential accreditation standard by 2015.10 The standard mandates that patients be screened a minimum of 1 time at a “pivotal” medical visit (such as time of diagnosis, transitions in cancer treatment, recurrence, completion of cancer treatment, and progression of disease). In practice, most institutions typically screen at diagnosis.2 According to the ACoS CoC, 41 VAMCs are accredited sites that will be impacted by the implementation of this standard.10
Distress Screening Tools
A major challenge and barrier to integrating distress screening in cancer clinics is the lack of consensus on the best measurement tool in a busy ambulatory clinic. Although a number of screening tools are available for measuring cancer-related distress, they vary in efficacy and feasibility. According to Zabora and Macmurray, the perfect screening instrument for distress in persons with cancer does not exist.6 Brief screening tools demonstrate high sensitivity in identifying very distressed patients but lack specificity, resulting in false positives.8,11 More extensive screening instruments, such as the Hospital Anxiety and Depression Scale (HADS), the Brief Symptom Inventory (BSI)-18, and the Psycho-Oncology Screening Tool (POST), have lower rates of false positives but may be more burdensome for providers, especially when considering copyright and cost.6
Ambulatory cancer care requires a rapid screening method with high sensitivity and minimal burden.12 The NCCN Distress Thermometer (DT) has face validity and allows for rapid screening; however, its psychometric properties are not as robust as other instru ments, such as the Center for Epidemiological Studies Depression Scale, the Hospital Anxiety and Depression Scale, Psychological Distress Inventory, or Brief Symptom Inventory.13 Although the DT has been shown to identify clinically significant anxiety, it is not as sensitive in identifying depression.4
The NCCN DT has 2 parts to the screening: (1) an overall distressintensity score within the past week, including the current day; and (2) an accompanying problem list, grouped into 5 categories, addressing QOL domains.14 The quantitative score ranges from 0 (no distress) to 10 (extreme distress). The problem list complements the quantitative score by providing information about the source of distress and can help to tailor the intervention (Figure 1). Access to the NCCN Guideline and DT is free for clinical and personal use.
According to the NCCN Guideline, scores of ≥ 4 require distress-management intervention.1 Mild distress (score < 4) usually can be managed by the primary oncology team.15 However, if the patient’s score is moderate (4-7) or severe (8-10), urgent intervention is necessary. Depending on the source of the distress, the patient should be seen by the appropriate discipline. For patients with practical problems, such as transportation, finances, and housing issues, a referral to social work is needed. For those with distress related to mental health issues, psychology, psychiatry, or social work may be appropriate.
Patients with distressing physical symptoms should be seen by the physician or advanced practice registered nurse (APRN) from the oncology or palliative care team. With limited psychosocial resources available at many cancer clinics, identification and triage for those with the highest levels of distress are critical.5 Triage must incorporate both the total distress score and the components of the distress so that the appropriate disciplines are accessed for the plan of care. More than one discipline may be needed to address multifactorial distress.
Despite strong recommendations from NCCN, ACoS, and many other professional and accrediting agencies, numerous cancer programs face challenges implementing routine screening. This article reports on a large, inner city ambulatory clinic’s pilot project to distress screen all patients at every appointment in the Cancer Center of Excellence (CoE) at Louis Stokes Cleveland VAMC (LSCVAMC) between May 2012 and May 2014 and to provide immediate intervention from the appropriate discipline for patients scoring ≥ 4 on a 0 to 10 DT scale. Results of the screenings, feasibility of screening in an ambulatory VA cancer clinic, and impact on psychosocial resources are presented.
Center of Excellence Project
The LSCVAMC CoE Cancer Care Clinic began as a 3-year grant-funded project from the VA Offices of Specialty Care and Academic Affiliations with 2 major objectives: (1) to deliver quality patient-centered cancer care as measured by implementation of a process for distress screening and management, and development and implementation of a survivorship care plan for patients who have completed cancer treatment; and (2) to provide interprofessional education for the interdisciplinary health care professionals who participate in the clinic as part of their training experience.
Patients in this unique CoE cancer clinic have sameday access to all members of the interdisciplinary and interprofessional team. The ambulatory cancer care CoE team was originally composed of a surgical oncologist, a medical oncologist, a clinical nurse specialist (CNS) patient navigator, a nurse practitioner (NP) in survivorship care, a registered nurse (RN), a psychologist, and an oncology social worker. The project’s patient population included patients with a cancer concern (positive family history and suspicious scans) or a diagnosis of breast cancer, melanoma, sarcoma, or hematologic malignancies. The patient population for the project was based on the CoE team expertise and feasibility of implementation, with plans to roll out the model of care for all patients with any cancer diagnosis across the VAMC at the completion of the project.
The CoE made distress screening and management the leading priority for quality patient-centered care at the start of the project. The purpose of this emphasis on distress screening was to develop a process at LSCVAMC that would meet the 2015 CoC standards and to teach health care professional trainees (NP students, residents, social work students, and fellows in psychology and medical oncology) about distress screening and intervention.
A plan-do-act model of quality improvement (QI) was used to support the development and implementation of the distress-screening process. At the beginning of the project, the institutional review board (IRB) reviewed the protocol and determined that informed consent was not necessary because a QI project for a new standard of care did not require IRB approval. The CoE team met for about 4 months to develop a policy and procedure for the process, based on evidence from national guidelines, a review of the literature, and a discussion of the benefits and burdens of implementation within the current practice.
Limiting initial implementation to a single clinic day made the process more manageable. Descriptive methods analyzed the incidence and percentage of overall distress in this veteran population and quantified the incidences and percentages of each DT component. Feedback from patients and staff offered information on the feasibility of and satisfaction with the process.
From May 2012 to May 2014, all patients who attended the Monday outpatient CoE clinic with a diagnosis of cancer or a cancer concern were given the NCCN, 2.2013 DT instrument by the registration desk clerk at the time they registered for their clinic appointment. 16 Veterans who had difficulty filling out the DT or who had diminished capacity were assisted in completing the instrument by a designated family member and/or the clinic RN.
The completed instrument was evaluated by the CNS patient navigator, and any patient with a score ≥ 4 received an automatic referral to the behavioral health psychologist, social worker, NP, or all team members and their trainees, depending on the areas of distress (eg, practical, family, and emotional problems, spiritual/religious concerns, and/or physical problems) endorsed by the patient.
A psychiatrist was not embedded into the team but worked closely with the team’s oncology psychologist. The psychologist communicated directly with the psychiatrist, and the plan was shared with the team through the electronic medical record (EMR). The appropriate team member(s) and trainee(s) saw the patient at the visit to address needs in real time. Access to palliative care support and spiritual care was readily available if needed.
Distress screenings were recorded in a templated note in the patient’s EMR, which allowed the team to follow the distress scores on an individual basis across the cancer disease trajectory and to assess response to interventions. Multiple screenings of individuals resulted from the fact that many of the patients were seen monthly or every 3/6/9 months, depending on their disease and treatment status. Because levels of distress can fluctuate, distress was assessed at every visit to determine whether an intervention was needed at that visit. Once distress screenings were recorded in the patient’s EMR, the DT instrument was de-identified and given to the CoE research consultant to enter into a database file for analysis.
Trainees were educated about the use of the DT at time of diagnosis and across the disease trajectory. The 4-week CoE curriculum included 2 weeks of conference time to teach about the roles of psychologist, oncology social worker, and survivorship NP in assessing and initiating interventions to address the multidimensional components of the DT. Trainees working with a veteran who was distressed participated in the assessment(s) and intervention(s) for all components of distress that were endorsed.
Results
A total of 866 distress screenings were performed during the first 2 years of the project. Since all patients were screened at all visits, the 866 distress screenings reflect multiple screenings for 445 unique patients. Of the 866 screenings, 290 (33%) had distress scores of ≥ 4, meeting the criteria for intervention. Screenings reflected patient visits at any point in the disease trajectory. Because this was a new standard of care QI project rather than a research project, additional data, such as diagnosis or staging, were not collected, and IRB approval was not needed.
Because the NCCN Guideline recommendation for intervention is a score of ≥ 4, the descriptive statistics focused on those with moderate-to-severe distress. However, there were numerous occasions when the veteran would report a score of 0 to 3 and still endorse a number of the problems on the DT. The CNS and RN on the team discussed these findings with the appropriate discipline. For example, if the veteran reported a score of 1 but endorsed all 6 components on the emotional problem list, the nurses discussed the patient with the social worker or psychologist to determine whether behavioral health intervention was needed.
The mean distress score for the 290 screenings ≥ 4 was 6.3 on a 0 to 10 scale; median was 6.0 and mode 5.0. Two hundred ten of these screenings (72%) were categorized as moderate distress (4-7), and 80 patients (28%) reported severe distress (Figure 2). If the veteran left a box empty on the problem list, itwas recorded as missing. The frequency that patients reported each type of distress are reported in Figure 3.
The incidence of each component of distress, from those screenings with a score of ≥ 4 is described below, along with case study examples for each component. Team members involved in patient interventions provided these case studies to demonstrate clinical examples of the veteran’s distress from the problem list on the DT.
Practical/Family Distress
Practical issues were reported in 38% of the screenings (109/290). Intervention for moderate-to-severe distress associated with practical problems and family issues was provided by the team social worker. The social worker frequently addressed transportationrelated distress. Providing transportation was essential for adherence to clinic appointments, follow-up testing, treatments, and ultimately, disease management. Housing was also a problem for many veterans. It is critical that patients have access to electricity, heating, food, and water to be able to safely undergo adjuvant therapy. Thus, treatments decisions could be impacted by the veterans’ housing and transportation issues; immediate access to social work support is essential for quality cancer care.
Twenty-six percent of patients that were screened expressed concerns with practical and/or family problems (75/289). Issues of domestic violence, difficulties dealing with a significant other, and concerns about children were referred to social work (Table 1).
Case Study
Ms. S. is a veteran aged 71 years with recently diagnosed breast cancer. She is being seen in the clinic for a postoperative visit following partial mastectomy and is anticipating beginning radiation therapy within the next 3 weeks. She reports a distress score of 7 and identifies concerns about work and transportation to the clinic as the sources of distress. The social worker meets with the patient and learns that she fears losing her job because of the daily travel time to and from radiation and that she cannot afford to travel 65 miles daily to LSCVAMC for radiation. The social worker listens to her concerns and assists her with a plan for short-term disability and VA housing during her radiation therapy treatments. Ms. S. was able to complete radiation at LSCVAMC with temporary housing and to return to work after therapy.
Emotional Distress
Patients who identified that their moderate-to-severe distress was related to emotional problems received same-day intervention from a psychologist skilled in providing emotional support, cognitive behavioral strategies, and assessing the need for referral to either a psychiatrist or oncology social worker. Seventy-one percent of patients reported emotional problems, such as worry, depression, and nervousness (Table 2).
Case Study
Mr. K. is a veteran aged 71 years with a new diagnosis of breast cancer. He lives on his own but has family and a few friends nearby. He reports that he doesn’t like to share his problems with others and has not told anyone of his new diagnosis. Mr. K. rates his distress a 7 and endorses worry, fear, and depression. At a treatment-planning visit, he agrees to see the psychologist for help in dealing with his distress. Treatment involves a mastectomy followed by hormonal therapy.
Mr. K. was scared about having cancer; some of his veteran colleagues have developed cancer recently, and 2 have died. He told the psychologist that he feels worthless and that this disease just makes him more of a burden on society. He has had thoughts of taking his life so that he doesn’t have to deal with cancer, but he does not have a plan. The team formulated a plan to address his anxiety and depression. Mr. K. started a serotonin reuptake inhibitor, and he met with the VA psychiatrist weekly to help develop coping strategies. The team’s psychologist worked closely with Mr. K.’s psychiatrist, and he successfully completed surgery and chemotherapy. He is now being seen in survivorship clinic, continuing care with the team and his psychologist.
Spiritual Distress
Although spiritual/religious concerns are part of DT screening, it is only a single item on the DT. Just 8% of patients (21/276) reported moderate-to-severe spiritual distress. However, there was access to a chaplain at LSCVAMC.
Case Study
Mr. H., a 63-year-old veteran with stage IV melanoma, was seen in the clinic for severe pain in his left hip and ribs (8 on a 10-point scale); he was unresponsive to escalating doses of oxycodone. During the visit, he reported that his distress level is a 10, and in addition to identifying pain as a source of distress, he indicated that he has spiritual distress. When questioned further about spiritual distress, Mr. H. reported that he deserves this pain since he caused so many others pain during his time in Vietnam. The chaplain was contacted, and the patient was seen in clinic at this visit. The chaplain gave him the opportunity to share his feelings of guilt. The importance of spiritual care when the patient is experiencing “total pain” is essential to pain management. Within 3 days, his pain score decreased to an acceptable level of 3 with no additional pharmacologic intervention.
Physical Distress
Physical problems associated with the distress scores were addressed by the surgical and medical oncologists and the APRNs (CNS patient navigator and survivorship NP). When the clinic opened, the team used the Memorial Symptom Assessment Scale to assess physical and psychological symptoms.16 However, patients reported experiencing distress at having to complete 2 tools that had a great deal of overlap. The team determined that the DT could be used as the sole screening tool for all QOL domains.
It is important to note that 92% of patients with moderate-to-severe distress reported physical symptoms as a source of distress (Table 3).
Case Study
Ms. L. is a Vietnam War veteran aged 64 years who was seen in the survivorship clinic. She was diagnosed with estrogen-receptor (ER) and progesterone-receptor (PR) breast cancer 1 year previously, had a lumpectomy followed by radiation therapy, and was on hormonal therapy. She recorded her distress score as a 6 and indicated that multiple physical symptoms were her major concern. She had difficulty with insomnia, fatigue, and hot flashes. The survivorship NP talked with Ms. L. about her symptoms and made nonpharmacologic recommendations for improving sleep, provided an exercise plan for fatigue, and initiated venlafaxine to manage the hot flashes. Ms. L. continued to be seen by the team in survivorship clinic, and during her 3-month follow-up visit, she reported improvement in sleep as the hot flashes diminished.
Multifactoral Distress
Many patients endorsed ≥ 1 component of distress. This required a team approach to intervene for the multifactorial nature of the distress.
Case Study
Mr. K. is a veteran aged 82 years who had been a farmer most of his life. He was cared for at the VA for an advancedstage squamous cell skin cancer of his scalp, which he had allowed to go untreated. The cancer has completely eroded beneath his scalp, and he wore a hat to cover the foul-smelling wound. He lived in rural Ohio with his wife of 55 years; 3 adult daughters lived in the Cleveland area. His daughters served as primary caregivers when Mr. K. came to Cleveland for daily radiation and weekly chemotherapy treatments. He had not been away from his wife since the war and misses her terribly, returning home only on weekends during the 6-week course of radiation.
His primary goal was to return home in time to harvest his farm’s produce 2 months later. He was aware that he has < 6 months to live but wanted chemotherapy and radiation to control the growth of the cancer. During this visit to the ambulatory clinic, he reported a distress score of 5 andidentified family concerns (eg, living away from his wife most of every week) and endorsed emotional concerns of fear, worry, and sadness, and reported pain, fatigue, insomnia, and constipation as physical concerns.
Mr. K. received support from the social worker, the psychologist, and the APRN for symptom management during this visit. The social worker was able to advocate for limited palliative radiation therapy treatments rather than a 6-week course; the psychologist spent 45 minutes talking with him about his fears of a painful death, worries about his wife, and sadness at not being alive for another planting season. The APRN recommended both pharmacologic and nonpharmacologic interventions for his fatigue and insomnia and initiated a pain and bowel pharmacologic regimen. The team respected Mr. K.’s wish to reconsider hospice care at the following visit after he had talked with his wife. Mr. K. died peacefully in his home with his wife and family just before the start of planting season.
Clinical Implications
Distress screening and intervention is essential for quality cancer care. While a great deal of controversy exists about the best time to screen for distress, the LSCVAMC CoE has taken on the challenge of screening and intervening in real time at every patient visit across the disease trajectory. The model of distress screening all veterans at CoE clinic visits has been rolled out to other cancer clinics at LSCVAMC.
Distress screening at each visit is not time intensive. Patients are willing to fill out the instrument while waiting for their clinic visit, and most patients find that it takes less than 5 minutes to complete. The major challenge for institutions considering screening with each visit is not the screening but access to appropriate providers able to provide timely intervention. The success of this model results, in part, because the clinic RN assesses the responses to the DT and refers to the appropriate discipline, utilizing precious resources of social work and psychology appropriately. The VA system is already committed to improving the psychosocial well-being of veterans and has established social work and psychology resources specifically for the cancer clinics.
Many patients reported to the authors that they might not have been able or willing to return to LSCVAMC to see the behavioral health specialists on another day. In addition, scheduling behavioral health appointments at another time would not allow for attending to the distress in real time. Also, from a systems standpoint, it would have been an added cost to the VA and/or the veteran for transportation for additional appointments on different days.
Finally, although the impact of the CoE project on health professional trainees has been reported elsewhere, the distress screening and intervention process were valued as being very positive for all trainees who participated in CoE clinic.17 The trainees were able to stay with the patient for the entire clinic visit, including the visits made by disciplines other than their own. For example, the family medicine residents stayed with the patient they examined to observe the distress assessments and interventions offered by the social worker and/or psychologist for the patients who scored ≥ 4 on the DT.
At the end of their rotations in the CoE, trainees reported an increased awareness of the importance of distress screening in a cancer clinic. Many were not aware of the NCCN guidelines and the ACoS CoC mandate for distress screening as a standard of cancer care. Interdisciplinary trainees rated the CoE curriculum and the conference teaching/learning sessions on distress management highly. However, observing the role of the social worker and psychologist were the most valuable to trainees, regardless of the area of practice they enter.
Conclusion
Addressing practical, psychosocial, physical, and spiritual needs will help decrease distress, support patients’ ability to tolerate treatment, and improve veterans’ QOL across the cancer-disease trajectory. Screening all patients at an outpatient cancer clinic at LSCVAMC is feasible and does not seem to be a burden for patients or providers. This pilot project has become standard of care across the LSCVAMC cancer clinics, demonstrating its sustainability.
Screening with the DT provides information about the intensity of the distress and the components contributing to the distress. The most important aspect of the screening is assessing the components of the distress and providing real-time intervention from the appropriate discipline. It is critical that the oncology team refer to the appropriate discipline based on the source of the distress rather than on only the intensity. Findings from this project indicate that physical symptoms are frequently the source of distress and may not require behavioral health intervention. However, for patients with psychosocial needs, rapid access to behavioral health care services is critical for quality veteran-centered cancer care.
Since 2015, all VA cancer centers are required to have implemented distress screening. According to the CoC, at least 1 screening must be done on every patient.10 Many institutions have begun to screen at diagnosis, but it is well known that there are many points along the cancer trajectory when patients may experience an increase in distress. Simple screening with the DT at every cancer clinic visit helps identify the veterans’ needs at any point along the disease spectrum.
At LSCVAMC, the CoE was designed as an interdisciplinary cancer clinic. With the conclusion of funding in FY 2015, the clinic has continued to function. The rollout into other clinics has continued with movement toward use of formal consult requests and continual, real-time evaluation of the process. Work on accurate, timely identification of new cancer patients and identifying pivotal cancer visits is underway. The LSCVAMC is committed to improving care and access to its veterans with cancer to ensure appropriate and adequate services across the cancer trajectory.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Holand JC, Jacobsen PB, Anderson A, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Distress Management 2.2016. © 2014 National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. Updated July 25, 2016. Accessed January 13, 2017.
2. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
3. Hamer M, Chida Y, Molloy G. Psychological distress and cancer mortality. J Psychosom Res. 2009;66(3):255-258.
4. Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2013;8(4):487-494.
5. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
6. Zabora JR, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2012;30(6):625-635.
7. Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(91):2946-2954.
8. Parry C, Padgett LS, Zebrack B. Now what? Toward an integrated research and practice agenda in distress screening. J Psychosoc Oncol. 2012;30(6):715-727.
9. Wagner LI, Spiegel D, Pearman T. Using the science of psychosocial care to implement the new American College of Surgeons Commission on Cancer distress screening standard. J Natl Compr Canc Netw. 2013;11(2):214-221.
10. American College of Surgeons Commision on Cancer. https://www.facs.org/quality-programs/cancer/coc Published 1996. Updated January 19, 2017. Accessed April 16, 2016.
11. Rohan EA. Removing the stress from selecting instruments: arming social workers to take leadership in routine distress screening implementation. J Psychosoc Oncol. 2012;30(6):667-678.
12. Merport A, Bober SL, Grose A, Recklitis CJ. Can the distress thermometer (DT) identify significant psychological distress in long-term cancer survivors? A comparison with the Brief Symptom Inventory-18 (BSI-18). Support Care Cancer. 2012;20(1):195-198.
13. Carlson LE, Bultz BD. Cancer distress screening: Needs, models, and methods. J Psychosom Res. 2003;55(5):403-409.
14. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010:8(1):4-12.
15. Jacobsen P, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
16. Portenoy R, Thaler HT, Korblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326-1336.
17. Arfons L, Mazanec P, Smith J, et al. Training health care professionals in interprofessional collaborative cancer care. Health Interprof Pract. 2015;2(3):eP1073.
1. Holand JC, Jacobsen PB, Anderson A, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Distress Management 2.2016. © 2014 National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. Updated July 25, 2016. Accessed January 13, 2017.
2. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
3. Hamer M, Chida Y, Molloy G. Psychological distress and cancer mortality. J Psychosom Res. 2009;66(3):255-258.
4. Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2013;8(4):487-494.
5. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
6. Zabora JR, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2012;30(6):625-635.
7. Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(91):2946-2954.
8. Parry C, Padgett LS, Zebrack B. Now what? Toward an integrated research and practice agenda in distress screening. J Psychosoc Oncol. 2012;30(6):715-727.
9. Wagner LI, Spiegel D, Pearman T. Using the science of psychosocial care to implement the new American College of Surgeons Commission on Cancer distress screening standard. J Natl Compr Canc Netw. 2013;11(2):214-221.
10. American College of Surgeons Commision on Cancer. https://www.facs.org/quality-programs/cancer/coc Published 1996. Updated January 19, 2017. Accessed April 16, 2016.
11. Rohan EA. Removing the stress from selecting instruments: arming social workers to take leadership in routine distress screening implementation. J Psychosoc Oncol. 2012;30(6):667-678.
12. Merport A, Bober SL, Grose A, Recklitis CJ. Can the distress thermometer (DT) identify significant psychological distress in long-term cancer survivors? A comparison with the Brief Symptom Inventory-18 (BSI-18). Support Care Cancer. 2012;20(1):195-198.
13. Carlson LE, Bultz BD. Cancer distress screening: Needs, models, and methods. J Psychosom Res. 2003;55(5):403-409.
14. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010:8(1):4-12.
15. Jacobsen P, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
16. Portenoy R, Thaler HT, Korblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326-1336.
17. Arfons L, Mazanec P, Smith J, et al. Training health care professionals in interprofessional collaborative cancer care. Health Interprof Pract. 2015;2(3):eP1073.
Ketogenic Diets and Cancer: Emerging Evidence
As early as 500 bc , fasting was used as an effective treatment for many medical ailments. Fasting continued into modern times, and in 1910, Guelpa and Marie proposed fasting as an antiepilepsy treatment. In 1921, Woodyatt noted that starvation or use of high-fat, low-carbohydrate diets in individuals with no significant medical comorbidities yielded acetone and β-hydroxybutyrate, 2 energy sources produced by the liver in the absence of glucose. A low-carbohydrate, high-fat diet was thought to be an alternative to fasting or starvation, having many of the same desired effects while continuing to nourish healthy cells. The term ketogenic diet (KD) was later coined by Wilder and Peterman, who formulated the fat-to-carbohydrate ratio that is still used today: 1 g protein per kg of body weight in children and 10 to 15 g carbohydrates daily, and fat for the remainder of calories. Both investigators reported that this diet improved their patients’ mentation and cognition.1
Use of the KD as an adjuvant to cancer therapy also began to emerge. In 1922, Braunstein noted that glucose disappeared from the urine of patients with diabetes after they were diagnosed with cancer, suggesting that glucose is recruited to cancerous areas where it is consumed at higher than normal rates. During that same time, Nobel laureate Otto Warburg found that cancer cells thrive on glycolysis, producing high lactate levels, even in the presence of abundant oxygen. Warburg conducted many in vitro and animal experiments demonstrating this outcome, known as the Warburg effect.
By the mid-20th century, KD use in epilepsy treatment and cancer research had waned. However, in the mid-to-late 1990s, with the establishment of the Charlie Foundation, the diet slowly started regaining recognition.1 Results of many in vitro and animal studies were reported, and human data also began to accumulate.
Mechanism of Action
Glucose normally stimulates pancreatic β cells to release insulin, which allows glucose to enter cells and provide energy. With high carbohydrate and glucose intake, the pancreas increasingly secretes more insulin, which promotes the interaction of growth hormone receptors and growth hormones to produce insulin-like growth factor 1 (IGF-1) in the liver—promoting cell growth and proliferation, which can be detrimental to patients with cancer. Overexpression of glucose transporters 1 and 3 (Glut-1, Glut-3) also occurs in many cancers and corresponds to the degree of glucose uptake in aggressive tumors, as seen on positron emission tomography (PET).2 Overexpression of hexokinase, the rate-limiting enzyme of glycolysis, further drives production of pyruvate and lactate, which cause reactive oxygen species damage. Translocation of the hexokinase rate-limiting enzyme from the cytosol into the outer mitochondrial membrane, where it interacts with voltage-dependent anion channels, can disrupt caspasedependent cytochrome release, which suppresses the apoptotic pathways of cancer cells and makes cancer more resistant to chemotherapy.3
When glucose is scarce, the body senses the need to make an alternative form of energy for cells. The liver then produces ketones and fatty acids, which provide for normal cells but do not benefit cancer cells. Cancer cells have dysfunctional mitochondria and possibly electron transport chain defects, which disrupt normal adenosine triphosphate (ATP) production from the mitochondria. The result is that the cancer cells become heavily dependent on ATP coming from the less efficient process of glycolysis (Figure 1).
Ketogenic diets mimic the fasting state, wherein the body responds to the lack of glucose by producing ketones for energy. Excess lactate production, which is part of the Warburg effect, compensates for ATP production defects caused by dysfunctional mitochondrial oxidative phosphorylation.2,4 The resulting tumor dependence on glucose can be exploited with KD use. Ketogenic diets selectively starve tumors by providing the fat and protein that otherwise could not be used by glucose-dependent tumor cells.
In KDs, the 4:1 ratio of high fat to low carbohydrates mimics the metabolic effects of starvation (Figure 2). These diets slow cancer by inhibiting insulin/IGF and downstream intracellular signaling pathways, such as
Ketogenic Diet Benefits
There are concerns about providing protein to patients who are at risk for renal problems. However, mouse models of diabetic nephropathy showed improved renal function with KD use. The hypothesis was that KD use, which produces prolonged elevated 3-β-hydroxybutyric acid levels, also reduces molecular responses to glucose and consequently reduces renal damage.6 Use of the diet also reduced pain and inflammation in both juvenile and adult rats. Mechanisms of action were thought to be reduced reactive oxygen species and increased central adenosine levels.7,8
Adverse Effects
Dieting is of concern to cancer patients worried about additional weight loss. The standard diet is made up predominantly of carbohydrates and is high in caloric value (Figure 3). Beck and Tisdale investigated the effect of KD use on delaying cachexia in mouse models of colon carcinoma. They found that dieting was more effective than insulin in reversing weight loss and had the added effect of reducing tumor size.7 Moreover, Tisdale and colleagues found that KD use in cachectic cancer patients could promote weight gain.8
A possible explanation is that healthy tissue nutrition selectively delays tumor growth, while cancer cells are deprived of nutrition (carbohydrates). A therapeutic weight plateau should follow initial weight loss with KD, in contrast to pathologic rapid weight loss in non-KD patients.9 Kidney stones, gout, and symptomatic hypoglycemia were also potential expected adverse effects (AEs).
Case Reports
In 1962, the New York Department of Mental Hygiene published an article about 2 women whose metastatic cancers disappeared after a series of daily hypoglycemiainduced insulin comas (brief and reversible). These patients could not undergo conventional electric shock therapy, hence, the medically induced psychotherapy. Not only did their psychotic and depressive symptoms resolve, but their cancers (grossly visible cervical cancer and metastatic melanoma) became undetectable as early as 2 months into treatment.10 Zuccoli and colleagues reported on a glioblastoma that was effectively treated with temozolomide oral chemotherapy after the patient was weaned off steroids. The patient had a radiographic response and good tumor control for about a year, before discontinuing the diet. She transitioned to chemotherapy, which included bevacizumab (antivascular endothelial growth factor), but the disease progressed and she died.11 In addition, during 8 weeks of ketogenic dieting, 2 pediatric female astrocytoma patients experienced improved mood and showed decreased glucose uptake on PET-computed tomography (PET-CT) of their tumor sites. One of these patients continued the diet and remained disease-free another 12 months.12 These early case reports provide compelling evidence for further research into the role of glucose metabolism in cancer
treatment.
Current Research
Compared with normal mice, tumor-bearing mice placed on a low-carbohydrate diet had lower glucose, insulin, and lactic acid levels.4 An in vivo microdialysis study of patients with head and neck cancers found decreased lactic acid levels in the tumor tissues after a 4-day KD.13 Most early studies and case reports involving KD in cancer focused on brain tumors.11,14-16 Human clinical studies on this diet in cancer care were limited to small, nonrandomized, brief trials (4-12 weeks) or single case studies (Table).11,17-20
Fine and colleagues conducted a 4-week feasibility study of the low-carbohydrate modified Atkins KD (≤ 20 g of carbohydrates daily) in PET-positive advanced cancer patients with solid tumors. There was a correlation between insulin levels and ketosis but not IGF. Stable disease or partial remission (measured standardized uptake value) on PET-CT correlated with 3-fold higher ketosis (but not weight loss or reduced caloric intake) relative to patients with progressive disease.17 In the ERGO trial, Rieger and colleagues studied 20 relapsed glioblastoma patients who were on KD supplemented with plant oils.18 Calories were unlimited. The primary endpoint was percentage of patients who discontinued the diet. Mean weight loss was significant, but quality of life (QOL) was maintained. The investigators also studied the effects of KD alone or combined with bevacizumab in a mouse glioblastoma model. In this mouse study, KD alone had no effect; however, it increased median survival from 52 to 58 days (P < .05) with bevacizumab.
In a pilot study, Schmidt and colleagues provided KD plus oil-protein shakes as snacks to 7 of 16 patients with a variety of advanced metastatic cancers. Mean weight loss was statistically significant, blood lipid or cholesterol levels remained stable, some QOL measures improved, and there were no severe AEs.19 Schwartz and colleagues reviewed the cases of 32 glioma patients treated with energy restricted KDs —5 from case reports, 19 from a clinical trial by Rieger and colleagues, and 8 from Champ.18,20,21 They also reported on 2 of their own glioma cases treated with the energy-restricted KD and studied tissues for expression of key ketolytic enzymes. Prolonged remission was noted from 4 months to more than 5 years in these case reports.20
The VA Pittsburgh Healthcare System safety that trial enrolled 17 patients, 11 of whom were evaluated. Mean weight loss was significant, and weight loss of ≥ 10% was noted in responders (stable or improved disease) compared with nonresponders. Three patients dieted longer than 16 weeks (survival, 80-116 weeks). One of these patients was alive at 121 weeks.22
The safety and feasibility data suggest that cancer patients can tolerate KD use. Investigators should consider combining the KD approach with standard treatment modalities, including chemotherapy and radiation.
Already investigators have conducted in vitro studies of the effect of gene expression of ketolytic and glycolytic enzymes within tumor tissue on KD response. Tumors expressing mutations in key mitochondrial oxidative phosphorylation enzymes were more likely to respond to KD use than were tumors without mutations.16,23
Ongoing Clinical Trials
Duke University has initiated a randomized study (NCT00932672) of the Atkins diet and androgen deprivation therapy for patients with prostate cancer. Tel Aviv Sourasky Medical Center in Israel is recruiting previously treated chemoradiation patients with high-grade glial tumors for an open-label study (NCT01092247) of the efficacy of KD in preventing tumor growth and recurrence. St. Joseph’s Hospital and Medical Center (Phoenix, AZ) is recruiting newly diagnosed glioblastoma patients for a phase 1/2 prospective trial (NCT02046187) involving upfront resection followed by KD with radiotherapy and concurrent temozolomide, followed by adjuvant temozolomide chemotherapy. The primary endpoint is number of patients with AEs and the secondary endpoints are overall survival, time to progression, and QOL. The University of Iowa is recruiting patients with prostate cancer and non-small cell lung cancer for 2 phase 1 studies (NCT01419483 and NCT01419587, respectively) involving KD using Nutritia KetoCal 4:1 (Gaithersburg, MD).
Conclusion
Data from case reports and trials suggest KD use is safe and tolerable for patients with cancer. Although it would be ideal to conduct a larger trial using a randomized therapeutic approach, the current emphasis on drug-based trials is a formidable obstacle. Other major obstacles are patient initiative and adherence. For now, investigators must work with anecdotal data. Examination of gene expression patterns in mitochondria and mutations in ketolytic and glycolytic enzymes may prove useful in selecting potentially responsive patients. Combining this dietary approach with standard chemotherapeutic and radiotherapeutic options may help improve tumor response, and further research is desperately needed.
Click to read the digital edition.
1. Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49(suppl 8):3-5.
2. Tian M, Zhang H, Nakasone Y, Mogi K, Endo K. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging. 2004;31(1):5-12.
3. Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40(3):171-182.
4. Ho VW, Leung K, Hsu A, et al. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71(13):4484-4493.
5. Poff AM, Ari C, Arnold P, Seyfried TN, D’Agostino DP. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int J Cancer. 2014;135(7):1711-1720.
6. Poplawski MM, Mastaitis JW, Isoda F, Grosjean F, Zheng F, Mobbs CV. Reversal of diabetic nephropathy by a ketogenic diet. PLoS One. 2011;6(4):e18604.
7. Beck SA, Tisdale MJ. Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer. 1989;59(5):677-681.
8. Tisdale MJ, Brennan RA, Fearon KC. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer. 1987;56(1):39-43.
9. Tan-Shalaby J, Seyfried T. Ketogenic diet in advanced cancer: a pilot feasibility and safety trial in the Veterans Affairs cancer patient population. J Clin Trials. 2013;3(4):149.
10. Koroljow S. Two cases of malignant tumors with metastases apparently treated successfully with hypoglycemic coma. Psychiatr Q. 1962;36:261-270.
11. Zuccoli G, Marcello N, Pisanello A, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab (Lond). 2010;7:33.
12. Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14(2):202-208.
13. Masino SA, Ruskin DN. Ketogenic diets and pain. J Child Neurol. 2013;28(8):993-1001.
14. Maroon J, Bost J, Amos A, Zuccoli G. Restricted calorie ketogenic diet for the treatment of glioblastoma multiforme. J Child Neurol. 2013;28(8):1002-1008.
15. Schroeder U, Himpe B, Pries R, Vonthein R, Nitsch S, Wollenberg B. Decline of lactate in tumor tissue after ketogenic diet: in vivo microdialysis study in patients with head and neck cancer. Nutr Cancer. 2013;65(6):843-849.
16. Chang HT, Olson LK, Schwartz KA. Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond). 2013;10(1):47.
17. Fine EJ, Segal-Isaacson CJ, Feinman RD, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28(10):1028-1035.
18. Rieger J, Bähr O, Maurer GD, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843-1852. [published correction appears in Int J Oncol. 2014;45(6):2605.
19. Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond). 2011;8(1):54.
20. Schwartz K, Chang HT, Nikolai M, et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3:3.
21. Champ CE, Palmer JD, Volek JS, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117(1):125-131.
22. Tan-Shalaby J, Carrick J, Edinger K, et al. Modified ketogenic diet in advanced malignancies—final results of a safety and feasibility trial within the Veterans Affairs Healthcare System. J Clin Oncol. 2016;34(suppl): Abstract e23173.
23. Langbein S, Zerilli M, Zur Hausen A, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94(4):578-585.
As early as 500 bc , fasting was used as an effective treatment for many medical ailments. Fasting continued into modern times, and in 1910, Guelpa and Marie proposed fasting as an antiepilepsy treatment. In 1921, Woodyatt noted that starvation or use of high-fat, low-carbohydrate diets in individuals with no significant medical comorbidities yielded acetone and β-hydroxybutyrate, 2 energy sources produced by the liver in the absence of glucose. A low-carbohydrate, high-fat diet was thought to be an alternative to fasting or starvation, having many of the same desired effects while continuing to nourish healthy cells. The term ketogenic diet (KD) was later coined by Wilder and Peterman, who formulated the fat-to-carbohydrate ratio that is still used today: 1 g protein per kg of body weight in children and 10 to 15 g carbohydrates daily, and fat for the remainder of calories. Both investigators reported that this diet improved their patients’ mentation and cognition.1
Use of the KD as an adjuvant to cancer therapy also began to emerge. In 1922, Braunstein noted that glucose disappeared from the urine of patients with diabetes after they were diagnosed with cancer, suggesting that glucose is recruited to cancerous areas where it is consumed at higher than normal rates. During that same time, Nobel laureate Otto Warburg found that cancer cells thrive on glycolysis, producing high lactate levels, even in the presence of abundant oxygen. Warburg conducted many in vitro and animal experiments demonstrating this outcome, known as the Warburg effect.
By the mid-20th century, KD use in epilepsy treatment and cancer research had waned. However, in the mid-to-late 1990s, with the establishment of the Charlie Foundation, the diet slowly started regaining recognition.1 Results of many in vitro and animal studies were reported, and human data also began to accumulate.
Mechanism of Action
Glucose normally stimulates pancreatic β cells to release insulin, which allows glucose to enter cells and provide energy. With high carbohydrate and glucose intake, the pancreas increasingly secretes more insulin, which promotes the interaction of growth hormone receptors and growth hormones to produce insulin-like growth factor 1 (IGF-1) in the liver—promoting cell growth and proliferation, which can be detrimental to patients with cancer. Overexpression of glucose transporters 1 and 3 (Glut-1, Glut-3) also occurs in many cancers and corresponds to the degree of glucose uptake in aggressive tumors, as seen on positron emission tomography (PET).2 Overexpression of hexokinase, the rate-limiting enzyme of glycolysis, further drives production of pyruvate and lactate, which cause reactive oxygen species damage. Translocation of the hexokinase rate-limiting enzyme from the cytosol into the outer mitochondrial membrane, where it interacts with voltage-dependent anion channels, can disrupt caspasedependent cytochrome release, which suppresses the apoptotic pathways of cancer cells and makes cancer more resistant to chemotherapy.3
When glucose is scarce, the body senses the need to make an alternative form of energy for cells. The liver then produces ketones and fatty acids, which provide for normal cells but do not benefit cancer cells. Cancer cells have dysfunctional mitochondria and possibly electron transport chain defects, which disrupt normal adenosine triphosphate (ATP) production from the mitochondria. The result is that the cancer cells become heavily dependent on ATP coming from the less efficient process of glycolysis (Figure 1).
Ketogenic diets mimic the fasting state, wherein the body responds to the lack of glucose by producing ketones for energy. Excess lactate production, which is part of the Warburg effect, compensates for ATP production defects caused by dysfunctional mitochondrial oxidative phosphorylation.2,4 The resulting tumor dependence on glucose can be exploited with KD use. Ketogenic diets selectively starve tumors by providing the fat and protein that otherwise could not be used by glucose-dependent tumor cells.
In KDs, the 4:1 ratio of high fat to low carbohydrates mimics the metabolic effects of starvation (Figure 2). These diets slow cancer by inhibiting insulin/IGF and downstream intracellular signaling pathways, such as
Ketogenic Diet Benefits
There are concerns about providing protein to patients who are at risk for renal problems. However, mouse models of diabetic nephropathy showed improved renal function with KD use. The hypothesis was that KD use, which produces prolonged elevated 3-β-hydroxybutyric acid levels, also reduces molecular responses to glucose and consequently reduces renal damage.6 Use of the diet also reduced pain and inflammation in both juvenile and adult rats. Mechanisms of action were thought to be reduced reactive oxygen species and increased central adenosine levels.7,8
Adverse Effects
Dieting is of concern to cancer patients worried about additional weight loss. The standard diet is made up predominantly of carbohydrates and is high in caloric value (Figure 3). Beck and Tisdale investigated the effect of KD use on delaying cachexia in mouse models of colon carcinoma. They found that dieting was more effective than insulin in reversing weight loss and had the added effect of reducing tumor size.7 Moreover, Tisdale and colleagues found that KD use in cachectic cancer patients could promote weight gain.8
A possible explanation is that healthy tissue nutrition selectively delays tumor growth, while cancer cells are deprived of nutrition (carbohydrates). A therapeutic weight plateau should follow initial weight loss with KD, in contrast to pathologic rapid weight loss in non-KD patients.9 Kidney stones, gout, and symptomatic hypoglycemia were also potential expected adverse effects (AEs).
Case Reports
In 1962, the New York Department of Mental Hygiene published an article about 2 women whose metastatic cancers disappeared after a series of daily hypoglycemiainduced insulin comas (brief and reversible). These patients could not undergo conventional electric shock therapy, hence, the medically induced psychotherapy. Not only did their psychotic and depressive symptoms resolve, but their cancers (grossly visible cervical cancer and metastatic melanoma) became undetectable as early as 2 months into treatment.10 Zuccoli and colleagues reported on a glioblastoma that was effectively treated with temozolomide oral chemotherapy after the patient was weaned off steroids. The patient had a radiographic response and good tumor control for about a year, before discontinuing the diet. She transitioned to chemotherapy, which included bevacizumab (antivascular endothelial growth factor), but the disease progressed and she died.11 In addition, during 8 weeks of ketogenic dieting, 2 pediatric female astrocytoma patients experienced improved mood and showed decreased glucose uptake on PET-computed tomography (PET-CT) of their tumor sites. One of these patients continued the diet and remained disease-free another 12 months.12 These early case reports provide compelling evidence for further research into the role of glucose metabolism in cancer
treatment.
Current Research
Compared with normal mice, tumor-bearing mice placed on a low-carbohydrate diet had lower glucose, insulin, and lactic acid levels.4 An in vivo microdialysis study of patients with head and neck cancers found decreased lactic acid levels in the tumor tissues after a 4-day KD.13 Most early studies and case reports involving KD in cancer focused on brain tumors.11,14-16 Human clinical studies on this diet in cancer care were limited to small, nonrandomized, brief trials (4-12 weeks) or single case studies (Table).11,17-20
Fine and colleagues conducted a 4-week feasibility study of the low-carbohydrate modified Atkins KD (≤ 20 g of carbohydrates daily) in PET-positive advanced cancer patients with solid tumors. There was a correlation between insulin levels and ketosis but not IGF. Stable disease or partial remission (measured standardized uptake value) on PET-CT correlated with 3-fold higher ketosis (but not weight loss or reduced caloric intake) relative to patients with progressive disease.17 In the ERGO trial, Rieger and colleagues studied 20 relapsed glioblastoma patients who were on KD supplemented with plant oils.18 Calories were unlimited. The primary endpoint was percentage of patients who discontinued the diet. Mean weight loss was significant, but quality of life (QOL) was maintained. The investigators also studied the effects of KD alone or combined with bevacizumab in a mouse glioblastoma model. In this mouse study, KD alone had no effect; however, it increased median survival from 52 to 58 days (P < .05) with bevacizumab.
In a pilot study, Schmidt and colleagues provided KD plus oil-protein shakes as snacks to 7 of 16 patients with a variety of advanced metastatic cancers. Mean weight loss was statistically significant, blood lipid or cholesterol levels remained stable, some QOL measures improved, and there were no severe AEs.19 Schwartz and colleagues reviewed the cases of 32 glioma patients treated with energy restricted KDs —5 from case reports, 19 from a clinical trial by Rieger and colleagues, and 8 from Champ.18,20,21 They also reported on 2 of their own glioma cases treated with the energy-restricted KD and studied tissues for expression of key ketolytic enzymes. Prolonged remission was noted from 4 months to more than 5 years in these case reports.20
The VA Pittsburgh Healthcare System safety that trial enrolled 17 patients, 11 of whom were evaluated. Mean weight loss was significant, and weight loss of ≥ 10% was noted in responders (stable or improved disease) compared with nonresponders. Three patients dieted longer than 16 weeks (survival, 80-116 weeks). One of these patients was alive at 121 weeks.22
The safety and feasibility data suggest that cancer patients can tolerate KD use. Investigators should consider combining the KD approach with standard treatment modalities, including chemotherapy and radiation.
Already investigators have conducted in vitro studies of the effect of gene expression of ketolytic and glycolytic enzymes within tumor tissue on KD response. Tumors expressing mutations in key mitochondrial oxidative phosphorylation enzymes were more likely to respond to KD use than were tumors without mutations.16,23
Ongoing Clinical Trials
Duke University has initiated a randomized study (NCT00932672) of the Atkins diet and androgen deprivation therapy for patients with prostate cancer. Tel Aviv Sourasky Medical Center in Israel is recruiting previously treated chemoradiation patients with high-grade glial tumors for an open-label study (NCT01092247) of the efficacy of KD in preventing tumor growth and recurrence. St. Joseph’s Hospital and Medical Center (Phoenix, AZ) is recruiting newly diagnosed glioblastoma patients for a phase 1/2 prospective trial (NCT02046187) involving upfront resection followed by KD with radiotherapy and concurrent temozolomide, followed by adjuvant temozolomide chemotherapy. The primary endpoint is number of patients with AEs and the secondary endpoints are overall survival, time to progression, and QOL. The University of Iowa is recruiting patients with prostate cancer and non-small cell lung cancer for 2 phase 1 studies (NCT01419483 and NCT01419587, respectively) involving KD using Nutritia KetoCal 4:1 (Gaithersburg, MD).
Conclusion
Data from case reports and trials suggest KD use is safe and tolerable for patients with cancer. Although it would be ideal to conduct a larger trial using a randomized therapeutic approach, the current emphasis on drug-based trials is a formidable obstacle. Other major obstacles are patient initiative and adherence. For now, investigators must work with anecdotal data. Examination of gene expression patterns in mitochondria and mutations in ketolytic and glycolytic enzymes may prove useful in selecting potentially responsive patients. Combining this dietary approach with standard chemotherapeutic and radiotherapeutic options may help improve tumor response, and further research is desperately needed.
Click to read the digital edition.
As early as 500 bc , fasting was used as an effective treatment for many medical ailments. Fasting continued into modern times, and in 1910, Guelpa and Marie proposed fasting as an antiepilepsy treatment. In 1921, Woodyatt noted that starvation or use of high-fat, low-carbohydrate diets in individuals with no significant medical comorbidities yielded acetone and β-hydroxybutyrate, 2 energy sources produced by the liver in the absence of glucose. A low-carbohydrate, high-fat diet was thought to be an alternative to fasting or starvation, having many of the same desired effects while continuing to nourish healthy cells. The term ketogenic diet (KD) was later coined by Wilder and Peterman, who formulated the fat-to-carbohydrate ratio that is still used today: 1 g protein per kg of body weight in children and 10 to 15 g carbohydrates daily, and fat for the remainder of calories. Both investigators reported that this diet improved their patients’ mentation and cognition.1
Use of the KD as an adjuvant to cancer therapy also began to emerge. In 1922, Braunstein noted that glucose disappeared from the urine of patients with diabetes after they were diagnosed with cancer, suggesting that glucose is recruited to cancerous areas where it is consumed at higher than normal rates. During that same time, Nobel laureate Otto Warburg found that cancer cells thrive on glycolysis, producing high lactate levels, even in the presence of abundant oxygen. Warburg conducted many in vitro and animal experiments demonstrating this outcome, known as the Warburg effect.
By the mid-20th century, KD use in epilepsy treatment and cancer research had waned. However, in the mid-to-late 1990s, with the establishment of the Charlie Foundation, the diet slowly started regaining recognition.1 Results of many in vitro and animal studies were reported, and human data also began to accumulate.
Mechanism of Action
Glucose normally stimulates pancreatic β cells to release insulin, which allows glucose to enter cells and provide energy. With high carbohydrate and glucose intake, the pancreas increasingly secretes more insulin, which promotes the interaction of growth hormone receptors and growth hormones to produce insulin-like growth factor 1 (IGF-1) in the liver—promoting cell growth and proliferation, which can be detrimental to patients with cancer. Overexpression of glucose transporters 1 and 3 (Glut-1, Glut-3) also occurs in many cancers and corresponds to the degree of glucose uptake in aggressive tumors, as seen on positron emission tomography (PET).2 Overexpression of hexokinase, the rate-limiting enzyme of glycolysis, further drives production of pyruvate and lactate, which cause reactive oxygen species damage. Translocation of the hexokinase rate-limiting enzyme from the cytosol into the outer mitochondrial membrane, where it interacts with voltage-dependent anion channels, can disrupt caspasedependent cytochrome release, which suppresses the apoptotic pathways of cancer cells and makes cancer more resistant to chemotherapy.3
When glucose is scarce, the body senses the need to make an alternative form of energy for cells. The liver then produces ketones and fatty acids, which provide for normal cells but do not benefit cancer cells. Cancer cells have dysfunctional mitochondria and possibly electron transport chain defects, which disrupt normal adenosine triphosphate (ATP) production from the mitochondria. The result is that the cancer cells become heavily dependent on ATP coming from the less efficient process of glycolysis (Figure 1).
Ketogenic diets mimic the fasting state, wherein the body responds to the lack of glucose by producing ketones for energy. Excess lactate production, which is part of the Warburg effect, compensates for ATP production defects caused by dysfunctional mitochondrial oxidative phosphorylation.2,4 The resulting tumor dependence on glucose can be exploited with KD use. Ketogenic diets selectively starve tumors by providing the fat and protein that otherwise could not be used by glucose-dependent tumor cells.
In KDs, the 4:1 ratio of high fat to low carbohydrates mimics the metabolic effects of starvation (Figure 2). These diets slow cancer by inhibiting insulin/IGF and downstream intracellular signaling pathways, such as
Ketogenic Diet Benefits
There are concerns about providing protein to patients who are at risk for renal problems. However, mouse models of diabetic nephropathy showed improved renal function with KD use. The hypothesis was that KD use, which produces prolonged elevated 3-β-hydroxybutyric acid levels, also reduces molecular responses to glucose and consequently reduces renal damage.6 Use of the diet also reduced pain and inflammation in both juvenile and adult rats. Mechanisms of action were thought to be reduced reactive oxygen species and increased central adenosine levels.7,8
Adverse Effects
Dieting is of concern to cancer patients worried about additional weight loss. The standard diet is made up predominantly of carbohydrates and is high in caloric value (Figure 3). Beck and Tisdale investigated the effect of KD use on delaying cachexia in mouse models of colon carcinoma. They found that dieting was more effective than insulin in reversing weight loss and had the added effect of reducing tumor size.7 Moreover, Tisdale and colleagues found that KD use in cachectic cancer patients could promote weight gain.8
A possible explanation is that healthy tissue nutrition selectively delays tumor growth, while cancer cells are deprived of nutrition (carbohydrates). A therapeutic weight plateau should follow initial weight loss with KD, in contrast to pathologic rapid weight loss in non-KD patients.9 Kidney stones, gout, and symptomatic hypoglycemia were also potential expected adverse effects (AEs).
Case Reports
In 1962, the New York Department of Mental Hygiene published an article about 2 women whose metastatic cancers disappeared after a series of daily hypoglycemiainduced insulin comas (brief and reversible). These patients could not undergo conventional electric shock therapy, hence, the medically induced psychotherapy. Not only did their psychotic and depressive symptoms resolve, but their cancers (grossly visible cervical cancer and metastatic melanoma) became undetectable as early as 2 months into treatment.10 Zuccoli and colleagues reported on a glioblastoma that was effectively treated with temozolomide oral chemotherapy after the patient was weaned off steroids. The patient had a radiographic response and good tumor control for about a year, before discontinuing the diet. She transitioned to chemotherapy, which included bevacizumab (antivascular endothelial growth factor), but the disease progressed and she died.11 In addition, during 8 weeks of ketogenic dieting, 2 pediatric female astrocytoma patients experienced improved mood and showed decreased glucose uptake on PET-computed tomography (PET-CT) of their tumor sites. One of these patients continued the diet and remained disease-free another 12 months.12 These early case reports provide compelling evidence for further research into the role of glucose metabolism in cancer
treatment.
Current Research
Compared with normal mice, tumor-bearing mice placed on a low-carbohydrate diet had lower glucose, insulin, and lactic acid levels.4 An in vivo microdialysis study of patients with head and neck cancers found decreased lactic acid levels in the tumor tissues after a 4-day KD.13 Most early studies and case reports involving KD in cancer focused on brain tumors.11,14-16 Human clinical studies on this diet in cancer care were limited to small, nonrandomized, brief trials (4-12 weeks) or single case studies (Table).11,17-20
Fine and colleagues conducted a 4-week feasibility study of the low-carbohydrate modified Atkins KD (≤ 20 g of carbohydrates daily) in PET-positive advanced cancer patients with solid tumors. There was a correlation between insulin levels and ketosis but not IGF. Stable disease or partial remission (measured standardized uptake value) on PET-CT correlated with 3-fold higher ketosis (but not weight loss or reduced caloric intake) relative to patients with progressive disease.17 In the ERGO trial, Rieger and colleagues studied 20 relapsed glioblastoma patients who were on KD supplemented with plant oils.18 Calories were unlimited. The primary endpoint was percentage of patients who discontinued the diet. Mean weight loss was significant, but quality of life (QOL) was maintained. The investigators also studied the effects of KD alone or combined with bevacizumab in a mouse glioblastoma model. In this mouse study, KD alone had no effect; however, it increased median survival from 52 to 58 days (P < .05) with bevacizumab.
In a pilot study, Schmidt and colleagues provided KD plus oil-protein shakes as snacks to 7 of 16 patients with a variety of advanced metastatic cancers. Mean weight loss was statistically significant, blood lipid or cholesterol levels remained stable, some QOL measures improved, and there were no severe AEs.19 Schwartz and colleagues reviewed the cases of 32 glioma patients treated with energy restricted KDs —5 from case reports, 19 from a clinical trial by Rieger and colleagues, and 8 from Champ.18,20,21 They also reported on 2 of their own glioma cases treated with the energy-restricted KD and studied tissues for expression of key ketolytic enzymes. Prolonged remission was noted from 4 months to more than 5 years in these case reports.20
The VA Pittsburgh Healthcare System safety that trial enrolled 17 patients, 11 of whom were evaluated. Mean weight loss was significant, and weight loss of ≥ 10% was noted in responders (stable or improved disease) compared with nonresponders. Three patients dieted longer than 16 weeks (survival, 80-116 weeks). One of these patients was alive at 121 weeks.22
The safety and feasibility data suggest that cancer patients can tolerate KD use. Investigators should consider combining the KD approach with standard treatment modalities, including chemotherapy and radiation.
Already investigators have conducted in vitro studies of the effect of gene expression of ketolytic and glycolytic enzymes within tumor tissue on KD response. Tumors expressing mutations in key mitochondrial oxidative phosphorylation enzymes were more likely to respond to KD use than were tumors without mutations.16,23
Ongoing Clinical Trials
Duke University has initiated a randomized study (NCT00932672) of the Atkins diet and androgen deprivation therapy for patients with prostate cancer. Tel Aviv Sourasky Medical Center in Israel is recruiting previously treated chemoradiation patients with high-grade glial tumors for an open-label study (NCT01092247) of the efficacy of KD in preventing tumor growth and recurrence. St. Joseph’s Hospital and Medical Center (Phoenix, AZ) is recruiting newly diagnosed glioblastoma patients for a phase 1/2 prospective trial (NCT02046187) involving upfront resection followed by KD with radiotherapy and concurrent temozolomide, followed by adjuvant temozolomide chemotherapy. The primary endpoint is number of patients with AEs and the secondary endpoints are overall survival, time to progression, and QOL. The University of Iowa is recruiting patients with prostate cancer and non-small cell lung cancer for 2 phase 1 studies (NCT01419483 and NCT01419587, respectively) involving KD using Nutritia KetoCal 4:1 (Gaithersburg, MD).
Conclusion
Data from case reports and trials suggest KD use is safe and tolerable for patients with cancer. Although it would be ideal to conduct a larger trial using a randomized therapeutic approach, the current emphasis on drug-based trials is a formidable obstacle. Other major obstacles are patient initiative and adherence. For now, investigators must work with anecdotal data. Examination of gene expression patterns in mitochondria and mutations in ketolytic and glycolytic enzymes may prove useful in selecting potentially responsive patients. Combining this dietary approach with standard chemotherapeutic and radiotherapeutic options may help improve tumor response, and further research is desperately needed.
Click to read the digital edition.
1. Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49(suppl 8):3-5.
2. Tian M, Zhang H, Nakasone Y, Mogi K, Endo K. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging. 2004;31(1):5-12.
3. Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40(3):171-182.
4. Ho VW, Leung K, Hsu A, et al. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71(13):4484-4493.
5. Poff AM, Ari C, Arnold P, Seyfried TN, D’Agostino DP. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int J Cancer. 2014;135(7):1711-1720.
6. Poplawski MM, Mastaitis JW, Isoda F, Grosjean F, Zheng F, Mobbs CV. Reversal of diabetic nephropathy by a ketogenic diet. PLoS One. 2011;6(4):e18604.
7. Beck SA, Tisdale MJ. Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer. 1989;59(5):677-681.
8. Tisdale MJ, Brennan RA, Fearon KC. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer. 1987;56(1):39-43.
9. Tan-Shalaby J, Seyfried T. Ketogenic diet in advanced cancer: a pilot feasibility and safety trial in the Veterans Affairs cancer patient population. J Clin Trials. 2013;3(4):149.
10. Koroljow S. Two cases of malignant tumors with metastases apparently treated successfully with hypoglycemic coma. Psychiatr Q. 1962;36:261-270.
11. Zuccoli G, Marcello N, Pisanello A, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab (Lond). 2010;7:33.
12. Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14(2):202-208.
13. Masino SA, Ruskin DN. Ketogenic diets and pain. J Child Neurol. 2013;28(8):993-1001.
14. Maroon J, Bost J, Amos A, Zuccoli G. Restricted calorie ketogenic diet for the treatment of glioblastoma multiforme. J Child Neurol. 2013;28(8):1002-1008.
15. Schroeder U, Himpe B, Pries R, Vonthein R, Nitsch S, Wollenberg B. Decline of lactate in tumor tissue after ketogenic diet: in vivo microdialysis study in patients with head and neck cancer. Nutr Cancer. 2013;65(6):843-849.
16. Chang HT, Olson LK, Schwartz KA. Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond). 2013;10(1):47.
17. Fine EJ, Segal-Isaacson CJ, Feinman RD, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28(10):1028-1035.
18. Rieger J, Bähr O, Maurer GD, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843-1852. [published correction appears in Int J Oncol. 2014;45(6):2605.
19. Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond). 2011;8(1):54.
20. Schwartz K, Chang HT, Nikolai M, et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3:3.
21. Champ CE, Palmer JD, Volek JS, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117(1):125-131.
22. Tan-Shalaby J, Carrick J, Edinger K, et al. Modified ketogenic diet in advanced malignancies—final results of a safety and feasibility trial within the Veterans Affairs Healthcare System. J Clin Oncol. 2016;34(suppl): Abstract e23173.
23. Langbein S, Zerilli M, Zur Hausen A, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94(4):578-585.
1. Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49(suppl 8):3-5.
2. Tian M, Zhang H, Nakasone Y, Mogi K, Endo K. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging. 2004;31(1):5-12.
3. Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40(3):171-182.
4. Ho VW, Leung K, Hsu A, et al. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71(13):4484-4493.
5. Poff AM, Ari C, Arnold P, Seyfried TN, D’Agostino DP. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int J Cancer. 2014;135(7):1711-1720.
6. Poplawski MM, Mastaitis JW, Isoda F, Grosjean F, Zheng F, Mobbs CV. Reversal of diabetic nephropathy by a ketogenic diet. PLoS One. 2011;6(4):e18604.
7. Beck SA, Tisdale MJ. Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer. 1989;59(5):677-681.
8. Tisdale MJ, Brennan RA, Fearon KC. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer. 1987;56(1):39-43.
9. Tan-Shalaby J, Seyfried T. Ketogenic diet in advanced cancer: a pilot feasibility and safety trial in the Veterans Affairs cancer patient population. J Clin Trials. 2013;3(4):149.
10. Koroljow S. Two cases of malignant tumors with metastases apparently treated successfully with hypoglycemic coma. Psychiatr Q. 1962;36:261-270.
11. Zuccoli G, Marcello N, Pisanello A, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab (Lond). 2010;7:33.
12. Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14(2):202-208.
13. Masino SA, Ruskin DN. Ketogenic diets and pain. J Child Neurol. 2013;28(8):993-1001.
14. Maroon J, Bost J, Amos A, Zuccoli G. Restricted calorie ketogenic diet for the treatment of glioblastoma multiforme. J Child Neurol. 2013;28(8):1002-1008.
15. Schroeder U, Himpe B, Pries R, Vonthein R, Nitsch S, Wollenberg B. Decline of lactate in tumor tissue after ketogenic diet: in vivo microdialysis study in patients with head and neck cancer. Nutr Cancer. 2013;65(6):843-849.
16. Chang HT, Olson LK, Schwartz KA. Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond). 2013;10(1):47.
17. Fine EJ, Segal-Isaacson CJ, Feinman RD, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28(10):1028-1035.
18. Rieger J, Bähr O, Maurer GD, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843-1852. [published correction appears in Int J Oncol. 2014;45(6):2605.
19. Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond). 2011;8(1):54.
20. Schwartz K, Chang HT, Nikolai M, et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3:3.
21. Champ CE, Palmer JD, Volek JS, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117(1):125-131.
22. Tan-Shalaby J, Carrick J, Edinger K, et al. Modified ketogenic diet in advanced malignancies—final results of a safety and feasibility trial within the Veterans Affairs Healthcare System. J Clin Oncol. 2016;34(suppl): Abstract e23173.
23. Langbein S, Zerilli M, Zur Hausen A, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94(4):578-585.
Consent and DNR orders
Question: Paramedics brought an unconscious 70-year-old man to a Florida hospital emergency department. The patient had the words “Do Not Resuscitate” tattooed onto his chest. No one accompanied him, and he had no identifications on his person. His blood alcohol level was elevated, and a few hours after his arrival, he lapsed into severe metabolic acidosis and hypotensive shock. The treating team decided to enter a DNR order, and the patient died shortly thereafter without benefit of cardiopulmonary resuscitation.
Which of the following is best?
A. An ethics consult may suggest honoring the patient’s DNR wishes, as it is reasonable to infer that the tattoo expressed an authentic preference.
B. It has been said, but remains debatable, that tattoos might represent “permanent reminders of regretted decisions made while the person was intoxicated.”
C. An earlier case report in the literature cautioned that the tattooed expression of a DNR request did not reflect that particular patient’s current wishes.
D. If this patient’s Florida Department of Health out-of-hospital DNR order confirms his DNR preference, then it is appropriate to withhold resuscitation.
E. All are correct.
ANSWER: E. The above hypothetical situation is modified from a recent case report in the correspondence section of the New England Journal of Medicine.1 It can be read as offering a sharp and dramatic focus on the issue of consent surrounding decisions to withhold CPR.
In 1983, the President’s Commission for the Study of Ethical Problems in Medicine supported DNR protocols (“no code”) based on three value considerations: self-determination, well-being, and equity.2
The physician is obligated to discuss with the patient or surrogate the procedure, risks, and benefits of CPR so that an informed choice can be made. DNR means that, in the event of a cardiac or respiratory arrest, no CPR efforts would be undertaken. DNR orders are not exclusive to the in-hospital setting, as some states, for example, Florida and Texas, have also enacted statutes that allow such orders to be valid outside the hospital.
Critics lament that problems – many surrounding the consent issue – continue to plague DNR orders.3 Discussions are often vague, and they may not meet the threshold of informed consent requirements, because they frequently omit risks and complications. A resident, rather than the attending physician, typically performs this important task. This is compounded by ill-timed discussions and wrong assumptions about patients’ preferences, which may in fact be ignored.4
Physicians sometimes extrapolate DNR orders to limit other treatments. Or, they perform CPR in contraindicated situations such as terminal illnesses, where death is expected, which amounts to “a positive violation of an individual’s right to die with dignity.” In some situations, physicians are known to override a patient’s DNR request.
Take the operating-room conundrum. There, the immediate availability of drugs, heightened skills, and in-place procedures significantly improve survival following a cardiopulmonary arrest. Studies show a 50% survival rate, versus 8%-14% elsewhere in the hospital. A Swedish study showed that 65% of the patients who had a cardiac arrest perioperatively were successfully resuscitated. When anesthesia caused the arrest, for example, esophageal intubation, disconnection from mechanical ventilation, or prolonged exposure to high concentrations of anesthetics, the recovery rate jumped to 92%.
Terminally ill patients typically disavow CPR when choosing a palliative course of action. However, surgery can be a part of palliation. In 1991, approximately 15% of patients with DNR orders had a surgical procedure, with most interventions targeting comfort and/or nursing care. When a terminally ill patient with a DNR order undergoes surgery, how should physicians deal with the patient’s no-code status, especially if an iatrogenic cardiac arrest should occur?
Because overriding a patient’s DNR wish violates the right of self-determination, a reasonable rule is to require the surgeon and/or anesthesiologist to discuss preoperatively the increased risk of a cardiac arrest during surgery, as well as the markedly improved chance of a successful resuscitation. The patient will then decide whether to retain his/her original DNR intent, or to suspend its execution in the perioperative period.5
What about iatrogenesis?
In 1999, David Casarett, MD, and Lainie F. Ross, MD, PhD, assessed whether physicians were more likely to override a DNR order if a hypothetical cardiac arrest was caused iatrogenically.6 Their survey revealed that 69% of physicians were very likely to do so. The authors suggested three explanations: 1) concern for malpractice litigation, 2) feelings of guilt or responsibility, and 3) the belief that patients do not consider the possibility of an iatrogenic cardiac arrest when they consent to a DNR order. Physicians may also believe a “properly negotiated DNR order does not apply to all foreseeable circumstances.”
However, some ethicists believe that an iatrogenic mishap does not make it permissible to override a patient’s prior refusal of treatment, because errors should not alter ethical obligations to respect a patient’s wishes to forgo treatment, including CPR.
Can a DNR order exist if it is against a patient’s wishes?7 In Gilgunn v. Massachusetts General Hospital, a 71-year-old diabetic woman with heart disease, breast cancer, and a hip fracture suffered two grand mal seizures and lapsed into a coma.8 Her daughter was the surrogate decision maker, and she made it clear that her mother always said she wanted everything done. After several weeks, the physicians decided that further treatment would be futile.
The chair of the ethics committee felt that the daughter’s opinion was not relevant because CPR was not a genuine therapeutic option and would be “medically contraindicated, inhumane, and unethical.” Accordingly, the attending physician entered a DNR order despite strong protest from the daughter. The patient died shortly thereafter without receiving CPR, and the daughter filed a negligence lawsuit against the hospital.
Still, there are state and federal statutes touching on DNR orders that warrant careful attention. For example, New York Public Health Law Section 2962, paragraph 1, states: “Every person admitted to a hospital shall be presumed to consent to the administration of cardiopulmonary resuscitation in the event of cardiac or respiratory arrest, unless there is consent to the issuance of an order not to resuscitate ...” This raises the question as to whether it is ever legally permissible in New York to enter a unilateral DNR order against the wishes of the patient.
And the federal “anti-dumping” law governing emergency treatment, widely known as EMTALA (Emergency Medical Treatment and Labor Act), requires all emergency departments to provide treatment necessary to prevent the material deterioration of the individual’s condition. This would always include the use of CPR unless specifically rejected by the patient or surrogate, as the law does not contain a “standard of care” or “futility” exception.9
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. N Engl J Med. 2017 Nov 30;377(22):2192-3.
2. President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Deciding to Forego Life-Sustaining Treatment. Washington, DC: Government Printing Office, 1983.
3. J Gen Intern Med. 2011 Jul;26(7):791-7.
4. JAMA. 1995 Nov 22-29;274(20):1591-8.
5. Hawaii Med J. 2001 Mar;60(3):64-7.
6. N Engl J Med. 1997 Jun 26;336(26):1908-10.
7. Tan SY. Futility and DNR Orders. Internal Medicine News, March 21, 2014.
8. Gilgunn v. Mass. General Hosp. No. 92-4820 (Mass. Super Ct. Apr. 21, 1995).
9. In re Baby K, 16 F.3d 590 (4th Cir. 1994).
Question: Paramedics brought an unconscious 70-year-old man to a Florida hospital emergency department. The patient had the words “Do Not Resuscitate” tattooed onto his chest. No one accompanied him, and he had no identifications on his person. His blood alcohol level was elevated, and a few hours after his arrival, he lapsed into severe metabolic acidosis and hypotensive shock. The treating team decided to enter a DNR order, and the patient died shortly thereafter without benefit of cardiopulmonary resuscitation.
Which of the following is best?
A. An ethics consult may suggest honoring the patient’s DNR wishes, as it is reasonable to infer that the tattoo expressed an authentic preference.
B. It has been said, but remains debatable, that tattoos might represent “permanent reminders of regretted decisions made while the person was intoxicated.”
C. An earlier case report in the literature cautioned that the tattooed expression of a DNR request did not reflect that particular patient’s current wishes.
D. If this patient’s Florida Department of Health out-of-hospital DNR order confirms his DNR preference, then it is appropriate to withhold resuscitation.
E. All are correct.
ANSWER: E. The above hypothetical situation is modified from a recent case report in the correspondence section of the New England Journal of Medicine.1 It can be read as offering a sharp and dramatic focus on the issue of consent surrounding decisions to withhold CPR.
In 1983, the President’s Commission for the Study of Ethical Problems in Medicine supported DNR protocols (“no code”) based on three value considerations: self-determination, well-being, and equity.2
The physician is obligated to discuss with the patient or surrogate the procedure, risks, and benefits of CPR so that an informed choice can be made. DNR means that, in the event of a cardiac or respiratory arrest, no CPR efforts would be undertaken. DNR orders are not exclusive to the in-hospital setting, as some states, for example, Florida and Texas, have also enacted statutes that allow such orders to be valid outside the hospital.
Critics lament that problems – many surrounding the consent issue – continue to plague DNR orders.3 Discussions are often vague, and they may not meet the threshold of informed consent requirements, because they frequently omit risks and complications. A resident, rather than the attending physician, typically performs this important task. This is compounded by ill-timed discussions and wrong assumptions about patients’ preferences, which may in fact be ignored.4
Physicians sometimes extrapolate DNR orders to limit other treatments. Or, they perform CPR in contraindicated situations such as terminal illnesses, where death is expected, which amounts to “a positive violation of an individual’s right to die with dignity.” In some situations, physicians are known to override a patient’s DNR request.
Take the operating-room conundrum. There, the immediate availability of drugs, heightened skills, and in-place procedures significantly improve survival following a cardiopulmonary arrest. Studies show a 50% survival rate, versus 8%-14% elsewhere in the hospital. A Swedish study showed that 65% of the patients who had a cardiac arrest perioperatively were successfully resuscitated. When anesthesia caused the arrest, for example, esophageal intubation, disconnection from mechanical ventilation, or prolonged exposure to high concentrations of anesthetics, the recovery rate jumped to 92%.
Terminally ill patients typically disavow CPR when choosing a palliative course of action. However, surgery can be a part of palliation. In 1991, approximately 15% of patients with DNR orders had a surgical procedure, with most interventions targeting comfort and/or nursing care. When a terminally ill patient with a DNR order undergoes surgery, how should physicians deal with the patient’s no-code status, especially if an iatrogenic cardiac arrest should occur?
Because overriding a patient’s DNR wish violates the right of self-determination, a reasonable rule is to require the surgeon and/or anesthesiologist to discuss preoperatively the increased risk of a cardiac arrest during surgery, as well as the markedly improved chance of a successful resuscitation. The patient will then decide whether to retain his/her original DNR intent, or to suspend its execution in the perioperative period.5
What about iatrogenesis?
In 1999, David Casarett, MD, and Lainie F. Ross, MD, PhD, assessed whether physicians were more likely to override a DNR order if a hypothetical cardiac arrest was caused iatrogenically.6 Their survey revealed that 69% of physicians were very likely to do so. The authors suggested three explanations: 1) concern for malpractice litigation, 2) feelings of guilt or responsibility, and 3) the belief that patients do not consider the possibility of an iatrogenic cardiac arrest when they consent to a DNR order. Physicians may also believe a “properly negotiated DNR order does not apply to all foreseeable circumstances.”
However, some ethicists believe that an iatrogenic mishap does not make it permissible to override a patient’s prior refusal of treatment, because errors should not alter ethical obligations to respect a patient’s wishes to forgo treatment, including CPR.
Can a DNR order exist if it is against a patient’s wishes?7 In Gilgunn v. Massachusetts General Hospital, a 71-year-old diabetic woman with heart disease, breast cancer, and a hip fracture suffered two grand mal seizures and lapsed into a coma.8 Her daughter was the surrogate decision maker, and she made it clear that her mother always said she wanted everything done. After several weeks, the physicians decided that further treatment would be futile.
The chair of the ethics committee felt that the daughter’s opinion was not relevant because CPR was not a genuine therapeutic option and would be “medically contraindicated, inhumane, and unethical.” Accordingly, the attending physician entered a DNR order despite strong protest from the daughter. The patient died shortly thereafter without receiving CPR, and the daughter filed a negligence lawsuit against the hospital.
Still, there are state and federal statutes touching on DNR orders that warrant careful attention. For example, New York Public Health Law Section 2962, paragraph 1, states: “Every person admitted to a hospital shall be presumed to consent to the administration of cardiopulmonary resuscitation in the event of cardiac or respiratory arrest, unless there is consent to the issuance of an order not to resuscitate ...” This raises the question as to whether it is ever legally permissible in New York to enter a unilateral DNR order against the wishes of the patient.
And the federal “anti-dumping” law governing emergency treatment, widely known as EMTALA (Emergency Medical Treatment and Labor Act), requires all emergency departments to provide treatment necessary to prevent the material deterioration of the individual’s condition. This would always include the use of CPR unless specifically rejected by the patient or surrogate, as the law does not contain a “standard of care” or “futility” exception.9
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. N Engl J Med. 2017 Nov 30;377(22):2192-3.
2. President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Deciding to Forego Life-Sustaining Treatment. Washington, DC: Government Printing Office, 1983.
3. J Gen Intern Med. 2011 Jul;26(7):791-7.
4. JAMA. 1995 Nov 22-29;274(20):1591-8.
5. Hawaii Med J. 2001 Mar;60(3):64-7.
6. N Engl J Med. 1997 Jun 26;336(26):1908-10.
7. Tan SY. Futility and DNR Orders. Internal Medicine News, March 21, 2014.
8. Gilgunn v. Mass. General Hosp. No. 92-4820 (Mass. Super Ct. Apr. 21, 1995).
9. In re Baby K, 16 F.3d 590 (4th Cir. 1994).
Question: Paramedics brought an unconscious 70-year-old man to a Florida hospital emergency department. The patient had the words “Do Not Resuscitate” tattooed onto his chest. No one accompanied him, and he had no identifications on his person. His blood alcohol level was elevated, and a few hours after his arrival, he lapsed into severe metabolic acidosis and hypotensive shock. The treating team decided to enter a DNR order, and the patient died shortly thereafter without benefit of cardiopulmonary resuscitation.
Which of the following is best?
A. An ethics consult may suggest honoring the patient’s DNR wishes, as it is reasonable to infer that the tattoo expressed an authentic preference.
B. It has been said, but remains debatable, that tattoos might represent “permanent reminders of regretted decisions made while the person was intoxicated.”
C. An earlier case report in the literature cautioned that the tattooed expression of a DNR request did not reflect that particular patient’s current wishes.
D. If this patient’s Florida Department of Health out-of-hospital DNR order confirms his DNR preference, then it is appropriate to withhold resuscitation.
E. All are correct.
ANSWER: E. The above hypothetical situation is modified from a recent case report in the correspondence section of the New England Journal of Medicine.1 It can be read as offering a sharp and dramatic focus on the issue of consent surrounding decisions to withhold CPR.
In 1983, the President’s Commission for the Study of Ethical Problems in Medicine supported DNR protocols (“no code”) based on three value considerations: self-determination, well-being, and equity.2
The physician is obligated to discuss with the patient or surrogate the procedure, risks, and benefits of CPR so that an informed choice can be made. DNR means that, in the event of a cardiac or respiratory arrest, no CPR efforts would be undertaken. DNR orders are not exclusive to the in-hospital setting, as some states, for example, Florida and Texas, have also enacted statutes that allow such orders to be valid outside the hospital.
Critics lament that problems – many surrounding the consent issue – continue to plague DNR orders.3 Discussions are often vague, and they may not meet the threshold of informed consent requirements, because they frequently omit risks and complications. A resident, rather than the attending physician, typically performs this important task. This is compounded by ill-timed discussions and wrong assumptions about patients’ preferences, which may in fact be ignored.4
Physicians sometimes extrapolate DNR orders to limit other treatments. Or, they perform CPR in contraindicated situations such as terminal illnesses, where death is expected, which amounts to “a positive violation of an individual’s right to die with dignity.” In some situations, physicians are known to override a patient’s DNR request.
Take the operating-room conundrum. There, the immediate availability of drugs, heightened skills, and in-place procedures significantly improve survival following a cardiopulmonary arrest. Studies show a 50% survival rate, versus 8%-14% elsewhere in the hospital. A Swedish study showed that 65% of the patients who had a cardiac arrest perioperatively were successfully resuscitated. When anesthesia caused the arrest, for example, esophageal intubation, disconnection from mechanical ventilation, or prolonged exposure to high concentrations of anesthetics, the recovery rate jumped to 92%.
Terminally ill patients typically disavow CPR when choosing a palliative course of action. However, surgery can be a part of palliation. In 1991, approximately 15% of patients with DNR orders had a surgical procedure, with most interventions targeting comfort and/or nursing care. When a terminally ill patient with a DNR order undergoes surgery, how should physicians deal with the patient’s no-code status, especially if an iatrogenic cardiac arrest should occur?
Because overriding a patient’s DNR wish violates the right of self-determination, a reasonable rule is to require the surgeon and/or anesthesiologist to discuss preoperatively the increased risk of a cardiac arrest during surgery, as well as the markedly improved chance of a successful resuscitation. The patient will then decide whether to retain his/her original DNR intent, or to suspend its execution in the perioperative period.5
What about iatrogenesis?
In 1999, David Casarett, MD, and Lainie F. Ross, MD, PhD, assessed whether physicians were more likely to override a DNR order if a hypothetical cardiac arrest was caused iatrogenically.6 Their survey revealed that 69% of physicians were very likely to do so. The authors suggested three explanations: 1) concern for malpractice litigation, 2) feelings of guilt or responsibility, and 3) the belief that patients do not consider the possibility of an iatrogenic cardiac arrest when they consent to a DNR order. Physicians may also believe a “properly negotiated DNR order does not apply to all foreseeable circumstances.”
However, some ethicists believe that an iatrogenic mishap does not make it permissible to override a patient’s prior refusal of treatment, because errors should not alter ethical obligations to respect a patient’s wishes to forgo treatment, including CPR.
Can a DNR order exist if it is against a patient’s wishes?7 In Gilgunn v. Massachusetts General Hospital, a 71-year-old diabetic woman with heart disease, breast cancer, and a hip fracture suffered two grand mal seizures and lapsed into a coma.8 Her daughter was the surrogate decision maker, and she made it clear that her mother always said she wanted everything done. After several weeks, the physicians decided that further treatment would be futile.
The chair of the ethics committee felt that the daughter’s opinion was not relevant because CPR was not a genuine therapeutic option and would be “medically contraindicated, inhumane, and unethical.” Accordingly, the attending physician entered a DNR order despite strong protest from the daughter. The patient died shortly thereafter without receiving CPR, and the daughter filed a negligence lawsuit against the hospital.
Still, there are state and federal statutes touching on DNR orders that warrant careful attention. For example, New York Public Health Law Section 2962, paragraph 1, states: “Every person admitted to a hospital shall be presumed to consent to the administration of cardiopulmonary resuscitation in the event of cardiac or respiratory arrest, unless there is consent to the issuance of an order not to resuscitate ...” This raises the question as to whether it is ever legally permissible in New York to enter a unilateral DNR order against the wishes of the patient.
And the federal “anti-dumping” law governing emergency treatment, widely known as EMTALA (Emergency Medical Treatment and Labor Act), requires all emergency departments to provide treatment necessary to prevent the material deterioration of the individual’s condition. This would always include the use of CPR unless specifically rejected by the patient or surrogate, as the law does not contain a “standard of care” or “futility” exception.9
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. N Engl J Med. 2017 Nov 30;377(22):2192-3.
2. President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Deciding to Forego Life-Sustaining Treatment. Washington, DC: Government Printing Office, 1983.
3. J Gen Intern Med. 2011 Jul;26(7):791-7.
4. JAMA. 1995 Nov 22-29;274(20):1591-8.
5. Hawaii Med J. 2001 Mar;60(3):64-7.
6. N Engl J Med. 1997 Jun 26;336(26):1908-10.
7. Tan SY. Futility and DNR Orders. Internal Medicine News, March 21, 2014.
8. Gilgunn v. Mass. General Hosp. No. 92-4820 (Mass. Super Ct. Apr. 21, 1995).
9. In re Baby K, 16 F.3d 590 (4th Cir. 1994).
A Road Map for Creating a CPRS Template for a Cancer Survivorship Treatment Summary and Care Plan (FULL)
In 2012, staff at the Comprehensive Cancer Center of VA Connecticut Healthcare System in West Haven (VACHS) decided to create a template for a Cancer Survivorship Treatment Summary and Care Plan (Survivorship Care Plan [SCP] and treatment summary are used interchangeably in this article and refer to the same document) in the VACHS Computerized Patient Record System (CPRS) to be used as one component of a Multidisciplinary Cancer Survivorship Clinic. The clinic’s providers would be advanced practice registered nurses (APRNs), based in the Comprehensive Cancer Center of VACHS. This quality improvement project was created in response to the American College of Surgeons (ACoS) Commission on Cancer (CoC) Standard 3.3, effective January 1, 2012, which mandated that the cancer committee “develops and implements a process to disseminate a comprehensive care summary and follow-up plan to patients with cancer who are completing cancer treatment.”1 According to ACoS CoC the process should be monitored, evaluated, presented, and documented at least annually to the cancer committee.
Creating the CPRS template took 9 months before the first SCPs were provided to patients in July 2013. Since that time, 210 SCPs have been provided to VACHS patients. Patient response was positive. Since implementation, patients have told their provider that they found the SCP’s list of signs and symptoms of cancer recurrence a helpful and reassuring resource.
Objective
This project is designed to be road map for other VA providers to follow by offering a review of the processes and resources that VACHS used and to share lessons learned.
The SCP is an important component of the survivorship standard of care. The CoC Standard 3.3 (version 2016) mandated that SCPs must be provided during an in-person meeting to an annually increasing percentage of patients initially diagnosed and treated for stage I, II, or III cancer in a given year—10% for those diagnosed and treated in 2015 and 25% for those diagnosed and treated in 2016 with increases in the required percentage each year thereafter. The mandated increase from 10% in 2015 to 25% in 2016 is significant and requires substantial resources to meet. Cancer centers seeking to achieve or maintain ACoS accreditation must fulfill this standard.2
It is important to establish a robust SCP process proactively. The percentage of SCPs provided that is mandated by the CoC continues to rise annually and the rate of survivorship also is expected to rise. The January 2016 CoC update clarified the phase-in of this standard over 4 years: (1) 2015: Implement a process to provide treatment summaries to at least 10% of patients treated for stage I-III cancer; (2) By end of 2016: Provide treatment summaries to at least 25% of eligible patients; (3) By end of 2017: Provide treatment summaries to at least 50% of eligible patients; and (4) By end of 2018: Provide treatment summaries to at least 75% of eligible patients.2
Background
In the fall of 2012, the project began with listening to survivors. The VA Survivorship Special Interest Group (SSIG) already had done significant work throughout the national VA system.3 The VACHS staff participated in monthly SSIG conference calls and reviewed the extensive resources created by its members, which is available through an internal VA website (Figure).
The VACHS staff reviewed the experiences of 2 VA sites using a draft CPRS survivorship care plan template. They also spent a day observing an established survivorship clinic at the VACHS academic affiliate Yale-New Haven Hospital (YNHH) in November of 2012. At that time, the YNHH clinic format was a 2-visit model for patients who had completed treatment. During their first visit to the YNHH clinic, patients meet with 4 members of an interdisciplinary team: a medical provider, a dietician, a physical therapist, and a social worker. At the end of the visit, the patient receives a SCP, a comprehensive document based on a template from the Livestrong organization.4 The second visit is scheduled 3 months later to follow up with patients and address any ongoing concerns. Patients then would be discharged from the survivorship clinic.
Given the complicated needs of the VA patient population, VACHS staff wanted to create a survivorship clinic that would provide regular, close followup by a multidisciplinary team within the existing hematology/oncology outpatient clinic. This design was believed to better serve veteran cancer survivors than a stand-alone clinic.
Clinic Creation
The VACHS chief of oncology, the cancer registrar, and the cancer care coordinator met in October 2012 to review Standard 3.3 and determine the best approach for VACHS patients. A plan to phase survivorship care into the existing hematology/oncology clinic was established. The group identified appropriate cancer survivor patients who would be followed by an APRN and a medical doctor. After reviewing the most common cancers treated at VACHS, it was decided to start the survivorship clinic with patients who had been treated for stage I lung cancer, stage I or II colorectal cancer, and/or stage I melanoma. These patients are not usually treated with chemotherapy, are less likely to relapse given early stage, and generally would be expected to be less complicated medically than would patients with more advanced disease. Patients previously treated for more advanced cancers would continue to be followed by medical doctors unless determined to be appropriate for migration to this new clinic.
The VACHS staff chose to embed this new clinic within existing APRN hematology/oncology clinics. Survivorship clinic visits were not restricted to a particular date or time in order to maximize efficiency as the workload associated with this clinic was not initially known. To track patient volume in the clinic, VACHS staff created the following note titles for patients being followed in the survivorship clinic: (1) Hem/Onc APRN Survivorship Clinic Initial Consult; (2) Hem/Onc APRN Cancer Survivorship Note; and (3) Cancer Survivor Treatment Summary. Unique note titles can be searched in VistA to create real-time reporting, thereby enabling staff to monitor the size, demographics, and workload associated with this clinic.
Vision for Care
The goals of survivorship care at VACHS were (1) to prevent, detect early, and treat complications from cancer treatment through regular clinic visits and ongoing education and support; (2) to provide holistic, individualized medical care and psychosocial support for veterans who are cancer survivors; (3) to maximize health, quality of life, and longevity; and (4) to facilitate appropriate referrals.
The VACHS clinic model incorporated: (1) regular clinic visits and follow-up with laboratories and imaging for 5 years, based on National Comprehensive Cancer Network (NCCN) guidelines; (2) monitoring for psychosocial distress at each visit, using a modified version of the NCCN Distress Thermometer, and a registered nurse or health tech documenting scores in CPRS with a templated Distress Screening note; (3) referrals to nutrition, health psychology, social work, physical therapy, smoking cessation, and the palliative care team as appropriate; (4) education about diagnosis, risk factors, and healthy living; and (5) reviewing the SCP to each patient.
The VACHS approach was designed to be patientcentered by incorporating individualized surveillance and screening guidelines, wellness education tailored to cancer type and treatment history, psychosocial support for survivors and their families through individual therapy and support groups for patients and families, and individual exercise and fitness recommendations through physical therapy and pulmonary rehabilitation referrals.
Needs Assessment
The VACHS cancer care coordinator worked with the tumor registrar to generate an initial referral patient list. Patients were identified in the tumor registry who met these criteria: diagnosed with stage I lung cancer or stage I or II colorectal cancer and treated at VACHS between 2008 and 2012. Initial research showed that there were 117 patients followed in the VACHS hematology/oncology clinic in the fall of 2012 who met the criteria. At that time, 80% of the patients identified were being followed by an attending oncology physician and 20% by an oncology APRN (Table).
Based on this initial analysis, the projected patient load for this clinic was anticipated to be 100 patients annually with between 2 and 4 annual visits each. Of note, in 2013 VACHS started a low-dose chest computed tomography screening program for patients who met high-risk criteria. The number of patients diagnosed and treated for stage I non-small cell lung cancer at VACHS during 2014 and 2015 was more than double the number treated in 2012.
Partnering With IT
The VACHS cancer care coordinator contacted the VISN 1 clinical application coordinator (CAC) in October 2012 who then reached out to her counterpart in Boston, where the sample CPRS template from the survivorship toolkit (toolkit template) was being tested. In November 2012, the VACHS CAC loaded the toolkit template into the test folder of the template drawer of VACHS CPRS. Changes were made over time to shorten the toolkit template to include the relevant information using the shortest number of words.
The template was modified by deleting 3 sections. The appointment list and the medication list were eliminated from the template as it was felt that these change with each visit. The psychosocial distress assessment also was eliminated as this score was known to change from visit to visit.
A separate initiative was undertaken simultaneously to address the need to monitor oncology patients for psychosocial distress regularly. Health psychology providers at VACHS used the NCCN Distress Thermometer to monitor distress in oncology patients. The team elected to take the distress assessment language out of the SCP and create a separate note template to record the distress scores for VACHS patients. The group chose the note titles Cancer Survivor Treatment Summary and Patient Distress Screening to clarify the purpose of the notes and to make searching CPRS or VistA for the note titles easier for providers or researchers.
CPRS Template Format
The VACHS team decided to create a short, clear, document that included diagnosis (date, location, pathology, staging), treatment (types, dates, locations, complications), disease-specific plan for surveillance, healthy living guidelines, and contact information for the survivorship provider.
The template was designed to be relatively simple for a provider to create, using check boxes that would populate the template with disease-specific care plans based on NCCN guidelines.
Review Process
The VACHS team’s original goal was to provide the first treatment summary to a patient on January 1, 2013, 10 weeks after the initial meeting with the CAC. This turned out to be overly optimistic. The VACHS Forms Committee meets once a month at VACHS. There is a formal review process for CPRS templates and all the decision makers must be present (Review Process Time Line).
The changes requested by the VACHS Forms Committee included taking out medications/appointments sections; spelling out all abbreviations; asking the educational coordinator to review educational portion of the template to make sure it complies with guidelines and reading level; and adding fields next to date of diagnosis field and, in every instance of treatment, dates for author to indicate where the diagnosis was made and where the treatment occurred.
Implementation
Starting with the list originally generated by the tumor registrar, the VACHS team set a goal of about 100 patients to be followed in an APRN survivorship clinic to focus on stage I lung cancer, stage I and II colorectal cancer, and stage I melanoma patients. As appropriate, patients were transitioned from being followed by a fellow or attending MD at the VACHS hematology/oncology clinic to the APRN survivorship clinic. As patients were seen in clinic for scheduled surveillance visits, the APRN survivorship clinic provider reviewed the process of creating a treatment summary with each patient and family as appropriate and reviewed their history with them in person to make sure that any complications related to treatment were identified. During each survivorship clinic visit, the provider verbally reviewed the plan for surveillance and signs and symptoms of recurrence to report to their clinician before providing the SCP to the patient.
Over the past 3 years, the VACHS Cancer Center has incorporated a hematology/oncology dietician, a health psychologist, a social worker, and a physical therapist into the outpatient clinic; all are usually available for sameday referrals. During regular survivorship visits, the survivorship APRN reviews any needs the veteran has and makes appropriate referrals. Palliative care personnel also are available in the cancer center during outpatient clinics for same-day consults. Survivorship patients are not automatically scheduled to see members of the team; rather, appropriate referrals are made via consults in CPRS after meeting with patients and assessing their needs.
In the past 3 years, 2 support groups were created, one for VACHS cancer center patients and one for caregivers. These groups are well attended by oncology and survivorship patients.
As part of the patient’s initial visit, the survivorship APRN reviews the patient’s information in CPRS and systematically reviews the original pathology, surgery, and tumor board notes as well as any notes related to treatments both within the VA system and in the community and creates the treatment summary CPRS note. In cases in which the patient had treatment at an outside facility, the patient signs a release of information form and original documentation of that treatment is requested. The completion of the SCP depends on the timing of when all appropriate information is available to be reviewed. As with all templates, minor editing is done to create the final note.
Once the survivorship APRN completes and signs the SCP, the patient’s primary care provider is added as a cosigner to the note. The patient receives the signed SCP at the visit. The January 2016 CoC Standard 3.3 update specifies that the SCP must be provided to the patient at an in-person visit and not mailed. As the SCP is a signed note in CPRS, it is easy to keep track of the date on which the information was reviewed and documented. If there are changes, such as a new cancer diagnoses or subsequent treatments, it is clear when the original information was documented. After providing the SCP to the patient and reviewing the document at an in-person meeting with the patient, the survivorship APRN documents the date that the SCP was provided to the patient in the progress note in CPRS. Once signed, the SCP is available to all providers within VACHS and able to be printed.
To date, 210 treatment summaries have been created for and provided to patients. Only 1 provider, the cancer care coordinator, is currently using the template, but use is not restricted. Patient feedback has been favorable: Patients state that the list of symptoms included in the treatment summary is useful. Patients report sharing the document with outside providers. The treatment summary also provides patients and families with a predictable plan for surveillance and regular in-person follow-up.
Patient Satsfaction Survey
In March 2015, VACHS conducted a patient satisfaction survey of 98 patients who had been provided with treatment summaries to better understand the impact on patients. This survey assessed quality measures, including patient’s confidence in their understanding of their cancer diagnosis, stage, treatment history, and plan for surveillance. Patient satisfaction with the resources available to them for healthy living also was measured, as was patient satisfaction with their survivorship and oncology providers and awareness that they had received a care plan.
Surveys were mailed to all VACHS survivorship patients for whom the treatment summaries were created who were still living and had not experienced a recurrence. The list of survey recipients was generated by searching VistA for the unique note title: Cancer Survivor Treatment Summary.
Sixty-six patients responded, a 67% response rate. The primary cancer diagnoses of the 66 study participants were lung (62.5%), colorectal (21.9%), melanoma (7.8%), head and neck (3.1%), and more than 1 malignancy (15.6%). Of the 66 respondents, 36.5% acknowledged receiving a treatment summary (23 patients).
Of those who acknowledge receiving a treatment summary, two-thirds stated that they have referred to the treatment summary for details about their diagnosis, treatment, plan for surveillance, and symptoms to report to practitioner. Between 73% and 76% were highly confident and between 22% and 25% were somewhat confident in their knowledge of their type of cancer, stage, treatment history and surveillance plan (> 90% positive response). The majority (66%) of patients were highly confident, and 32% were somewhat confident that there are resources available at VA to support their healthy lifestyle (98% positive response).
The survey also noted that 86% report being highly satisfied with care, and 92.4% are highly confident that their caregiver will provide compassionate care. Participants state that they have used the nutrition consults (38.5%), physical therapy (23%), health psychology (15.5%), smoking cessation (15.3%), and social work (10%). Of note, almost all those patients who reported using these services responded that they would recommend them.
Challenges
Despite the progress made at VACHS, there are significant challenges to meeting the CoC revised standard 3.3, which requires that 25% of patients treated with a stage I, II, or III cancer receive a Cancer Survivor Treatment Summary at an in-person visit in 2016. These relate primarily to multiple competing demands on provider time. In addition, 63.5% of patients who had been provided with a SCP at an in-person visit and responded to a satisfaction survey said they had not received the SCP. More research is needed to inform practice changes to optimize ongoing education and post-treatment care for veterans who are cancer survivors.
Conclusion
VA cancer centers seeking to ACoS CoC accreditation are required to provide a written summary of cancer treatment and plan for survivorship care to patients
diagnosed with at stage I, II, or III malignancy and treated at their facility. This requirement necessitates a significant ongoing investment in clinician and administrative workload to comply with the standard. The Comprehensive Cancer Center at VACHS, building on work by the SSIG, developed a concise note template in CPRS that enables oncology clinicians to create a treatment summary for each patient who meets criteria. During this process, VACHS has developed resources that may be useful to other VA cancer centers who are working to create this process. Clinicians interested in trialing the VACHS Cancer Survivor Treatment Summary template are encouraged to contact the author for additional information.
Visit www.fedprac.com/avahoupdates for an exclusive video interview with the author.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Acknowledgments
This project was a team effort. The author thanks the following VACHS colleagues for their input and support for this project: Michal Rose, MD, cancer center director; Donna Connery, tumor registrar; Renee Midgett, former clinical applications coordinator; Robert Troy Nall, health systems specialist; Forms Committee; Clarice Grens, APRN; and Jessica Barber, PhD, clinical psychologist as well as Members of VA Survivorship special interest group.
Click here to read the digital edition.
1. American College of Surgeons Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed January 18, 2017.
2. American College of Surgeons Commission on Cancer. Cancer program standards: ensuring patient-centered care 2016 Edition. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/2016%20coc%20standards%20manual_interactive%20pdf.ashx. Accessed January 18, 2017.
3. Smith J, Arfons L, Cmolik B, Moye J, Ballard E, Haggstrom D. Development and implementation of a veterans’ cancer survivorship program. Fed Pract. 2015;32(suppl 1):42S-48S..
4. National Comprehensive Cancer Network. NCCN distress thermometer and problem list for patients. http://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distress_thermometer.pdf. Updated May 6, 2016. Accessed January 18, 2016.
In 2012, staff at the Comprehensive Cancer Center of VA Connecticut Healthcare System in West Haven (VACHS) decided to create a template for a Cancer Survivorship Treatment Summary and Care Plan (Survivorship Care Plan [SCP] and treatment summary are used interchangeably in this article and refer to the same document) in the VACHS Computerized Patient Record System (CPRS) to be used as one component of a Multidisciplinary Cancer Survivorship Clinic. The clinic’s providers would be advanced practice registered nurses (APRNs), based in the Comprehensive Cancer Center of VACHS. This quality improvement project was created in response to the American College of Surgeons (ACoS) Commission on Cancer (CoC) Standard 3.3, effective January 1, 2012, which mandated that the cancer committee “develops and implements a process to disseminate a comprehensive care summary and follow-up plan to patients with cancer who are completing cancer treatment.”1 According to ACoS CoC the process should be monitored, evaluated, presented, and documented at least annually to the cancer committee.
Creating the CPRS template took 9 months before the first SCPs were provided to patients in July 2013. Since that time, 210 SCPs have been provided to VACHS patients. Patient response was positive. Since implementation, patients have told their provider that they found the SCP’s list of signs and symptoms of cancer recurrence a helpful and reassuring resource.
Objective
This project is designed to be road map for other VA providers to follow by offering a review of the processes and resources that VACHS used and to share lessons learned.
The SCP is an important component of the survivorship standard of care. The CoC Standard 3.3 (version 2016) mandated that SCPs must be provided during an in-person meeting to an annually increasing percentage of patients initially diagnosed and treated for stage I, II, or III cancer in a given year—10% for those diagnosed and treated in 2015 and 25% for those diagnosed and treated in 2016 with increases in the required percentage each year thereafter. The mandated increase from 10% in 2015 to 25% in 2016 is significant and requires substantial resources to meet. Cancer centers seeking to achieve or maintain ACoS accreditation must fulfill this standard.2
It is important to establish a robust SCP process proactively. The percentage of SCPs provided that is mandated by the CoC continues to rise annually and the rate of survivorship also is expected to rise. The January 2016 CoC update clarified the phase-in of this standard over 4 years: (1) 2015: Implement a process to provide treatment summaries to at least 10% of patients treated for stage I-III cancer; (2) By end of 2016: Provide treatment summaries to at least 25% of eligible patients; (3) By end of 2017: Provide treatment summaries to at least 50% of eligible patients; and (4) By end of 2018: Provide treatment summaries to at least 75% of eligible patients.2
Background
In the fall of 2012, the project began with listening to survivors. The VA Survivorship Special Interest Group (SSIG) already had done significant work throughout the national VA system.3 The VACHS staff participated in monthly SSIG conference calls and reviewed the extensive resources created by its members, which is available through an internal VA website (Figure).
The VACHS staff reviewed the experiences of 2 VA sites using a draft CPRS survivorship care plan template. They also spent a day observing an established survivorship clinic at the VACHS academic affiliate Yale-New Haven Hospital (YNHH) in November of 2012. At that time, the YNHH clinic format was a 2-visit model for patients who had completed treatment. During their first visit to the YNHH clinic, patients meet with 4 members of an interdisciplinary team: a medical provider, a dietician, a physical therapist, and a social worker. At the end of the visit, the patient receives a SCP, a comprehensive document based on a template from the Livestrong organization.4 The second visit is scheduled 3 months later to follow up with patients and address any ongoing concerns. Patients then would be discharged from the survivorship clinic.
Given the complicated needs of the VA patient population, VACHS staff wanted to create a survivorship clinic that would provide regular, close followup by a multidisciplinary team within the existing hematology/oncology outpatient clinic. This design was believed to better serve veteran cancer survivors than a stand-alone clinic.
Clinic Creation
The VACHS chief of oncology, the cancer registrar, and the cancer care coordinator met in October 2012 to review Standard 3.3 and determine the best approach for VACHS patients. A plan to phase survivorship care into the existing hematology/oncology clinic was established. The group identified appropriate cancer survivor patients who would be followed by an APRN and a medical doctor. After reviewing the most common cancers treated at VACHS, it was decided to start the survivorship clinic with patients who had been treated for stage I lung cancer, stage I or II colorectal cancer, and/or stage I melanoma. These patients are not usually treated with chemotherapy, are less likely to relapse given early stage, and generally would be expected to be less complicated medically than would patients with more advanced disease. Patients previously treated for more advanced cancers would continue to be followed by medical doctors unless determined to be appropriate for migration to this new clinic.
The VACHS staff chose to embed this new clinic within existing APRN hematology/oncology clinics. Survivorship clinic visits were not restricted to a particular date or time in order to maximize efficiency as the workload associated with this clinic was not initially known. To track patient volume in the clinic, VACHS staff created the following note titles for patients being followed in the survivorship clinic: (1) Hem/Onc APRN Survivorship Clinic Initial Consult; (2) Hem/Onc APRN Cancer Survivorship Note; and (3) Cancer Survivor Treatment Summary. Unique note titles can be searched in VistA to create real-time reporting, thereby enabling staff to monitor the size, demographics, and workload associated with this clinic.
Vision for Care
The goals of survivorship care at VACHS were (1) to prevent, detect early, and treat complications from cancer treatment through regular clinic visits and ongoing education and support; (2) to provide holistic, individualized medical care and psychosocial support for veterans who are cancer survivors; (3) to maximize health, quality of life, and longevity; and (4) to facilitate appropriate referrals.
The VACHS clinic model incorporated: (1) regular clinic visits and follow-up with laboratories and imaging for 5 years, based on National Comprehensive Cancer Network (NCCN) guidelines; (2) monitoring for psychosocial distress at each visit, using a modified version of the NCCN Distress Thermometer, and a registered nurse or health tech documenting scores in CPRS with a templated Distress Screening note; (3) referrals to nutrition, health psychology, social work, physical therapy, smoking cessation, and the palliative care team as appropriate; (4) education about diagnosis, risk factors, and healthy living; and (5) reviewing the SCP to each patient.
The VACHS approach was designed to be patientcentered by incorporating individualized surveillance and screening guidelines, wellness education tailored to cancer type and treatment history, psychosocial support for survivors and their families through individual therapy and support groups for patients and families, and individual exercise and fitness recommendations through physical therapy and pulmonary rehabilitation referrals.
Needs Assessment
The VACHS cancer care coordinator worked with the tumor registrar to generate an initial referral patient list. Patients were identified in the tumor registry who met these criteria: diagnosed with stage I lung cancer or stage I or II colorectal cancer and treated at VACHS between 2008 and 2012. Initial research showed that there were 117 patients followed in the VACHS hematology/oncology clinic in the fall of 2012 who met the criteria. At that time, 80% of the patients identified were being followed by an attending oncology physician and 20% by an oncology APRN (Table).
Based on this initial analysis, the projected patient load for this clinic was anticipated to be 100 patients annually with between 2 and 4 annual visits each. Of note, in 2013 VACHS started a low-dose chest computed tomography screening program for patients who met high-risk criteria. The number of patients diagnosed and treated for stage I non-small cell lung cancer at VACHS during 2014 and 2015 was more than double the number treated in 2012.
Partnering With IT
The VACHS cancer care coordinator contacted the VISN 1 clinical application coordinator (CAC) in October 2012 who then reached out to her counterpart in Boston, where the sample CPRS template from the survivorship toolkit (toolkit template) was being tested. In November 2012, the VACHS CAC loaded the toolkit template into the test folder of the template drawer of VACHS CPRS. Changes were made over time to shorten the toolkit template to include the relevant information using the shortest number of words.
The template was modified by deleting 3 sections. The appointment list and the medication list were eliminated from the template as it was felt that these change with each visit. The psychosocial distress assessment also was eliminated as this score was known to change from visit to visit.
A separate initiative was undertaken simultaneously to address the need to monitor oncology patients for psychosocial distress regularly. Health psychology providers at VACHS used the NCCN Distress Thermometer to monitor distress in oncology patients. The team elected to take the distress assessment language out of the SCP and create a separate note template to record the distress scores for VACHS patients. The group chose the note titles Cancer Survivor Treatment Summary and Patient Distress Screening to clarify the purpose of the notes and to make searching CPRS or VistA for the note titles easier for providers or researchers.
CPRS Template Format
The VACHS team decided to create a short, clear, document that included diagnosis (date, location, pathology, staging), treatment (types, dates, locations, complications), disease-specific plan for surveillance, healthy living guidelines, and contact information for the survivorship provider.
The template was designed to be relatively simple for a provider to create, using check boxes that would populate the template with disease-specific care plans based on NCCN guidelines.
Review Process
The VACHS team’s original goal was to provide the first treatment summary to a patient on January 1, 2013, 10 weeks after the initial meeting with the CAC. This turned out to be overly optimistic. The VACHS Forms Committee meets once a month at VACHS. There is a formal review process for CPRS templates and all the decision makers must be present (Review Process Time Line).
The changes requested by the VACHS Forms Committee included taking out medications/appointments sections; spelling out all abbreviations; asking the educational coordinator to review educational portion of the template to make sure it complies with guidelines and reading level; and adding fields next to date of diagnosis field and, in every instance of treatment, dates for author to indicate where the diagnosis was made and where the treatment occurred.
Implementation
Starting with the list originally generated by the tumor registrar, the VACHS team set a goal of about 100 patients to be followed in an APRN survivorship clinic to focus on stage I lung cancer, stage I and II colorectal cancer, and stage I melanoma patients. As appropriate, patients were transitioned from being followed by a fellow or attending MD at the VACHS hematology/oncology clinic to the APRN survivorship clinic. As patients were seen in clinic for scheduled surveillance visits, the APRN survivorship clinic provider reviewed the process of creating a treatment summary with each patient and family as appropriate and reviewed their history with them in person to make sure that any complications related to treatment were identified. During each survivorship clinic visit, the provider verbally reviewed the plan for surveillance and signs and symptoms of recurrence to report to their clinician before providing the SCP to the patient.
Over the past 3 years, the VACHS Cancer Center has incorporated a hematology/oncology dietician, a health psychologist, a social worker, and a physical therapist into the outpatient clinic; all are usually available for sameday referrals. During regular survivorship visits, the survivorship APRN reviews any needs the veteran has and makes appropriate referrals. Palliative care personnel also are available in the cancer center during outpatient clinics for same-day consults. Survivorship patients are not automatically scheduled to see members of the team; rather, appropriate referrals are made via consults in CPRS after meeting with patients and assessing their needs.
In the past 3 years, 2 support groups were created, one for VACHS cancer center patients and one for caregivers. These groups are well attended by oncology and survivorship patients.
As part of the patient’s initial visit, the survivorship APRN reviews the patient’s information in CPRS and systematically reviews the original pathology, surgery, and tumor board notes as well as any notes related to treatments both within the VA system and in the community and creates the treatment summary CPRS note. In cases in which the patient had treatment at an outside facility, the patient signs a release of information form and original documentation of that treatment is requested. The completion of the SCP depends on the timing of when all appropriate information is available to be reviewed. As with all templates, minor editing is done to create the final note.
Once the survivorship APRN completes and signs the SCP, the patient’s primary care provider is added as a cosigner to the note. The patient receives the signed SCP at the visit. The January 2016 CoC Standard 3.3 update specifies that the SCP must be provided to the patient at an in-person visit and not mailed. As the SCP is a signed note in CPRS, it is easy to keep track of the date on which the information was reviewed and documented. If there are changes, such as a new cancer diagnoses or subsequent treatments, it is clear when the original information was documented. After providing the SCP to the patient and reviewing the document at an in-person meeting with the patient, the survivorship APRN documents the date that the SCP was provided to the patient in the progress note in CPRS. Once signed, the SCP is available to all providers within VACHS and able to be printed.
To date, 210 treatment summaries have been created for and provided to patients. Only 1 provider, the cancer care coordinator, is currently using the template, but use is not restricted. Patient feedback has been favorable: Patients state that the list of symptoms included in the treatment summary is useful. Patients report sharing the document with outside providers. The treatment summary also provides patients and families with a predictable plan for surveillance and regular in-person follow-up.
Patient Satsfaction Survey
In March 2015, VACHS conducted a patient satisfaction survey of 98 patients who had been provided with treatment summaries to better understand the impact on patients. This survey assessed quality measures, including patient’s confidence in their understanding of their cancer diagnosis, stage, treatment history, and plan for surveillance. Patient satisfaction with the resources available to them for healthy living also was measured, as was patient satisfaction with their survivorship and oncology providers and awareness that they had received a care plan.
Surveys were mailed to all VACHS survivorship patients for whom the treatment summaries were created who were still living and had not experienced a recurrence. The list of survey recipients was generated by searching VistA for the unique note title: Cancer Survivor Treatment Summary.
Sixty-six patients responded, a 67% response rate. The primary cancer diagnoses of the 66 study participants were lung (62.5%), colorectal (21.9%), melanoma (7.8%), head and neck (3.1%), and more than 1 malignancy (15.6%). Of the 66 respondents, 36.5% acknowledged receiving a treatment summary (23 patients).
Of those who acknowledge receiving a treatment summary, two-thirds stated that they have referred to the treatment summary for details about their diagnosis, treatment, plan for surveillance, and symptoms to report to practitioner. Between 73% and 76% were highly confident and between 22% and 25% were somewhat confident in their knowledge of their type of cancer, stage, treatment history and surveillance plan (> 90% positive response). The majority (66%) of patients were highly confident, and 32% were somewhat confident that there are resources available at VA to support their healthy lifestyle (98% positive response).
The survey also noted that 86% report being highly satisfied with care, and 92.4% are highly confident that their caregiver will provide compassionate care. Participants state that they have used the nutrition consults (38.5%), physical therapy (23%), health psychology (15.5%), smoking cessation (15.3%), and social work (10%). Of note, almost all those patients who reported using these services responded that they would recommend them.
Challenges
Despite the progress made at VACHS, there are significant challenges to meeting the CoC revised standard 3.3, which requires that 25% of patients treated with a stage I, II, or III cancer receive a Cancer Survivor Treatment Summary at an in-person visit in 2016. These relate primarily to multiple competing demands on provider time. In addition, 63.5% of patients who had been provided with a SCP at an in-person visit and responded to a satisfaction survey said they had not received the SCP. More research is needed to inform practice changes to optimize ongoing education and post-treatment care for veterans who are cancer survivors.
Conclusion
VA cancer centers seeking to ACoS CoC accreditation are required to provide a written summary of cancer treatment and plan for survivorship care to patients
diagnosed with at stage I, II, or III malignancy and treated at their facility. This requirement necessitates a significant ongoing investment in clinician and administrative workload to comply with the standard. The Comprehensive Cancer Center at VACHS, building on work by the SSIG, developed a concise note template in CPRS that enables oncology clinicians to create a treatment summary for each patient who meets criteria. During this process, VACHS has developed resources that may be useful to other VA cancer centers who are working to create this process. Clinicians interested in trialing the VACHS Cancer Survivor Treatment Summary template are encouraged to contact the author for additional information.
Visit www.fedprac.com/avahoupdates for an exclusive video interview with the author.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Acknowledgments
This project was a team effort. The author thanks the following VACHS colleagues for their input and support for this project: Michal Rose, MD, cancer center director; Donna Connery, tumor registrar; Renee Midgett, former clinical applications coordinator; Robert Troy Nall, health systems specialist; Forms Committee; Clarice Grens, APRN; and Jessica Barber, PhD, clinical psychologist as well as Members of VA Survivorship special interest group.
Click here to read the digital edition.
In 2012, staff at the Comprehensive Cancer Center of VA Connecticut Healthcare System in West Haven (VACHS) decided to create a template for a Cancer Survivorship Treatment Summary and Care Plan (Survivorship Care Plan [SCP] and treatment summary are used interchangeably in this article and refer to the same document) in the VACHS Computerized Patient Record System (CPRS) to be used as one component of a Multidisciplinary Cancer Survivorship Clinic. The clinic’s providers would be advanced practice registered nurses (APRNs), based in the Comprehensive Cancer Center of VACHS. This quality improvement project was created in response to the American College of Surgeons (ACoS) Commission on Cancer (CoC) Standard 3.3, effective January 1, 2012, which mandated that the cancer committee “develops and implements a process to disseminate a comprehensive care summary and follow-up plan to patients with cancer who are completing cancer treatment.”1 According to ACoS CoC the process should be monitored, evaluated, presented, and documented at least annually to the cancer committee.
Creating the CPRS template took 9 months before the first SCPs were provided to patients in July 2013. Since that time, 210 SCPs have been provided to VACHS patients. Patient response was positive. Since implementation, patients have told their provider that they found the SCP’s list of signs and symptoms of cancer recurrence a helpful and reassuring resource.
Objective
This project is designed to be road map for other VA providers to follow by offering a review of the processes and resources that VACHS used and to share lessons learned.
The SCP is an important component of the survivorship standard of care. The CoC Standard 3.3 (version 2016) mandated that SCPs must be provided during an in-person meeting to an annually increasing percentage of patients initially diagnosed and treated for stage I, II, or III cancer in a given year—10% for those diagnosed and treated in 2015 and 25% for those diagnosed and treated in 2016 with increases in the required percentage each year thereafter. The mandated increase from 10% in 2015 to 25% in 2016 is significant and requires substantial resources to meet. Cancer centers seeking to achieve or maintain ACoS accreditation must fulfill this standard.2
It is important to establish a robust SCP process proactively. The percentage of SCPs provided that is mandated by the CoC continues to rise annually and the rate of survivorship also is expected to rise. The January 2016 CoC update clarified the phase-in of this standard over 4 years: (1) 2015: Implement a process to provide treatment summaries to at least 10% of patients treated for stage I-III cancer; (2) By end of 2016: Provide treatment summaries to at least 25% of eligible patients; (3) By end of 2017: Provide treatment summaries to at least 50% of eligible patients; and (4) By end of 2018: Provide treatment summaries to at least 75% of eligible patients.2
Background
In the fall of 2012, the project began with listening to survivors. The VA Survivorship Special Interest Group (SSIG) already had done significant work throughout the national VA system.3 The VACHS staff participated in monthly SSIG conference calls and reviewed the extensive resources created by its members, which is available through an internal VA website (Figure).
The VACHS staff reviewed the experiences of 2 VA sites using a draft CPRS survivorship care plan template. They also spent a day observing an established survivorship clinic at the VACHS academic affiliate Yale-New Haven Hospital (YNHH) in November of 2012. At that time, the YNHH clinic format was a 2-visit model for patients who had completed treatment. During their first visit to the YNHH clinic, patients meet with 4 members of an interdisciplinary team: a medical provider, a dietician, a physical therapist, and a social worker. At the end of the visit, the patient receives a SCP, a comprehensive document based on a template from the Livestrong organization.4 The second visit is scheduled 3 months later to follow up with patients and address any ongoing concerns. Patients then would be discharged from the survivorship clinic.
Given the complicated needs of the VA patient population, VACHS staff wanted to create a survivorship clinic that would provide regular, close followup by a multidisciplinary team within the existing hematology/oncology outpatient clinic. This design was believed to better serve veteran cancer survivors than a stand-alone clinic.
Clinic Creation
The VACHS chief of oncology, the cancer registrar, and the cancer care coordinator met in October 2012 to review Standard 3.3 and determine the best approach for VACHS patients. A plan to phase survivorship care into the existing hematology/oncology clinic was established. The group identified appropriate cancer survivor patients who would be followed by an APRN and a medical doctor. After reviewing the most common cancers treated at VACHS, it was decided to start the survivorship clinic with patients who had been treated for stage I lung cancer, stage I or II colorectal cancer, and/or stage I melanoma. These patients are not usually treated with chemotherapy, are less likely to relapse given early stage, and generally would be expected to be less complicated medically than would patients with more advanced disease. Patients previously treated for more advanced cancers would continue to be followed by medical doctors unless determined to be appropriate for migration to this new clinic.
The VACHS staff chose to embed this new clinic within existing APRN hematology/oncology clinics. Survivorship clinic visits were not restricted to a particular date or time in order to maximize efficiency as the workload associated with this clinic was not initially known. To track patient volume in the clinic, VACHS staff created the following note titles for patients being followed in the survivorship clinic: (1) Hem/Onc APRN Survivorship Clinic Initial Consult; (2) Hem/Onc APRN Cancer Survivorship Note; and (3) Cancer Survivor Treatment Summary. Unique note titles can be searched in VistA to create real-time reporting, thereby enabling staff to monitor the size, demographics, and workload associated with this clinic.
Vision for Care
The goals of survivorship care at VACHS were (1) to prevent, detect early, and treat complications from cancer treatment through regular clinic visits and ongoing education and support; (2) to provide holistic, individualized medical care and psychosocial support for veterans who are cancer survivors; (3) to maximize health, quality of life, and longevity; and (4) to facilitate appropriate referrals.
The VACHS clinic model incorporated: (1) regular clinic visits and follow-up with laboratories and imaging for 5 years, based on National Comprehensive Cancer Network (NCCN) guidelines; (2) monitoring for psychosocial distress at each visit, using a modified version of the NCCN Distress Thermometer, and a registered nurse or health tech documenting scores in CPRS with a templated Distress Screening note; (3) referrals to nutrition, health psychology, social work, physical therapy, smoking cessation, and the palliative care team as appropriate; (4) education about diagnosis, risk factors, and healthy living; and (5) reviewing the SCP to each patient.
The VACHS approach was designed to be patientcentered by incorporating individualized surveillance and screening guidelines, wellness education tailored to cancer type and treatment history, psychosocial support for survivors and their families through individual therapy and support groups for patients and families, and individual exercise and fitness recommendations through physical therapy and pulmonary rehabilitation referrals.
Needs Assessment
The VACHS cancer care coordinator worked with the tumor registrar to generate an initial referral patient list. Patients were identified in the tumor registry who met these criteria: diagnosed with stage I lung cancer or stage I or II colorectal cancer and treated at VACHS between 2008 and 2012. Initial research showed that there were 117 patients followed in the VACHS hematology/oncology clinic in the fall of 2012 who met the criteria. At that time, 80% of the patients identified were being followed by an attending oncology physician and 20% by an oncology APRN (Table).
Based on this initial analysis, the projected patient load for this clinic was anticipated to be 100 patients annually with between 2 and 4 annual visits each. Of note, in 2013 VACHS started a low-dose chest computed tomography screening program for patients who met high-risk criteria. The number of patients diagnosed and treated for stage I non-small cell lung cancer at VACHS during 2014 and 2015 was more than double the number treated in 2012.
Partnering With IT
The VACHS cancer care coordinator contacted the VISN 1 clinical application coordinator (CAC) in October 2012 who then reached out to her counterpart in Boston, where the sample CPRS template from the survivorship toolkit (toolkit template) was being tested. In November 2012, the VACHS CAC loaded the toolkit template into the test folder of the template drawer of VACHS CPRS. Changes were made over time to shorten the toolkit template to include the relevant information using the shortest number of words.
The template was modified by deleting 3 sections. The appointment list and the medication list were eliminated from the template as it was felt that these change with each visit. The psychosocial distress assessment also was eliminated as this score was known to change from visit to visit.
A separate initiative was undertaken simultaneously to address the need to monitor oncology patients for psychosocial distress regularly. Health psychology providers at VACHS used the NCCN Distress Thermometer to monitor distress in oncology patients. The team elected to take the distress assessment language out of the SCP and create a separate note template to record the distress scores for VACHS patients. The group chose the note titles Cancer Survivor Treatment Summary and Patient Distress Screening to clarify the purpose of the notes and to make searching CPRS or VistA for the note titles easier for providers or researchers.
CPRS Template Format
The VACHS team decided to create a short, clear, document that included diagnosis (date, location, pathology, staging), treatment (types, dates, locations, complications), disease-specific plan for surveillance, healthy living guidelines, and contact information for the survivorship provider.
The template was designed to be relatively simple for a provider to create, using check boxes that would populate the template with disease-specific care plans based on NCCN guidelines.
Review Process
The VACHS team’s original goal was to provide the first treatment summary to a patient on January 1, 2013, 10 weeks after the initial meeting with the CAC. This turned out to be overly optimistic. The VACHS Forms Committee meets once a month at VACHS. There is a formal review process for CPRS templates and all the decision makers must be present (Review Process Time Line).
The changes requested by the VACHS Forms Committee included taking out medications/appointments sections; spelling out all abbreviations; asking the educational coordinator to review educational portion of the template to make sure it complies with guidelines and reading level; and adding fields next to date of diagnosis field and, in every instance of treatment, dates for author to indicate where the diagnosis was made and where the treatment occurred.
Implementation
Starting with the list originally generated by the tumor registrar, the VACHS team set a goal of about 100 patients to be followed in an APRN survivorship clinic to focus on stage I lung cancer, stage I and II colorectal cancer, and stage I melanoma patients. As appropriate, patients were transitioned from being followed by a fellow or attending MD at the VACHS hematology/oncology clinic to the APRN survivorship clinic. As patients were seen in clinic for scheduled surveillance visits, the APRN survivorship clinic provider reviewed the process of creating a treatment summary with each patient and family as appropriate and reviewed their history with them in person to make sure that any complications related to treatment were identified. During each survivorship clinic visit, the provider verbally reviewed the plan for surveillance and signs and symptoms of recurrence to report to their clinician before providing the SCP to the patient.
Over the past 3 years, the VACHS Cancer Center has incorporated a hematology/oncology dietician, a health psychologist, a social worker, and a physical therapist into the outpatient clinic; all are usually available for sameday referrals. During regular survivorship visits, the survivorship APRN reviews any needs the veteran has and makes appropriate referrals. Palliative care personnel also are available in the cancer center during outpatient clinics for same-day consults. Survivorship patients are not automatically scheduled to see members of the team; rather, appropriate referrals are made via consults in CPRS after meeting with patients and assessing their needs.
In the past 3 years, 2 support groups were created, one for VACHS cancer center patients and one for caregivers. These groups are well attended by oncology and survivorship patients.
As part of the patient’s initial visit, the survivorship APRN reviews the patient’s information in CPRS and systematically reviews the original pathology, surgery, and tumor board notes as well as any notes related to treatments both within the VA system and in the community and creates the treatment summary CPRS note. In cases in which the patient had treatment at an outside facility, the patient signs a release of information form and original documentation of that treatment is requested. The completion of the SCP depends on the timing of when all appropriate information is available to be reviewed. As with all templates, minor editing is done to create the final note.
Once the survivorship APRN completes and signs the SCP, the patient’s primary care provider is added as a cosigner to the note. The patient receives the signed SCP at the visit. The January 2016 CoC Standard 3.3 update specifies that the SCP must be provided to the patient at an in-person visit and not mailed. As the SCP is a signed note in CPRS, it is easy to keep track of the date on which the information was reviewed and documented. If there are changes, such as a new cancer diagnoses or subsequent treatments, it is clear when the original information was documented. After providing the SCP to the patient and reviewing the document at an in-person meeting with the patient, the survivorship APRN documents the date that the SCP was provided to the patient in the progress note in CPRS. Once signed, the SCP is available to all providers within VACHS and able to be printed.
To date, 210 treatment summaries have been created for and provided to patients. Only 1 provider, the cancer care coordinator, is currently using the template, but use is not restricted. Patient feedback has been favorable: Patients state that the list of symptoms included in the treatment summary is useful. Patients report sharing the document with outside providers. The treatment summary also provides patients and families with a predictable plan for surveillance and regular in-person follow-up.
Patient Satsfaction Survey
In March 2015, VACHS conducted a patient satisfaction survey of 98 patients who had been provided with treatment summaries to better understand the impact on patients. This survey assessed quality measures, including patient’s confidence in their understanding of their cancer diagnosis, stage, treatment history, and plan for surveillance. Patient satisfaction with the resources available to them for healthy living also was measured, as was patient satisfaction with their survivorship and oncology providers and awareness that they had received a care plan.
Surveys were mailed to all VACHS survivorship patients for whom the treatment summaries were created who were still living and had not experienced a recurrence. The list of survey recipients was generated by searching VistA for the unique note title: Cancer Survivor Treatment Summary.
Sixty-six patients responded, a 67% response rate. The primary cancer diagnoses of the 66 study participants were lung (62.5%), colorectal (21.9%), melanoma (7.8%), head and neck (3.1%), and more than 1 malignancy (15.6%). Of the 66 respondents, 36.5% acknowledged receiving a treatment summary (23 patients).
Of those who acknowledge receiving a treatment summary, two-thirds stated that they have referred to the treatment summary for details about their diagnosis, treatment, plan for surveillance, and symptoms to report to practitioner. Between 73% and 76% were highly confident and between 22% and 25% were somewhat confident in their knowledge of their type of cancer, stage, treatment history and surveillance plan (> 90% positive response). The majority (66%) of patients were highly confident, and 32% were somewhat confident that there are resources available at VA to support their healthy lifestyle (98% positive response).
The survey also noted that 86% report being highly satisfied with care, and 92.4% are highly confident that their caregiver will provide compassionate care. Participants state that they have used the nutrition consults (38.5%), physical therapy (23%), health psychology (15.5%), smoking cessation (15.3%), and social work (10%). Of note, almost all those patients who reported using these services responded that they would recommend them.
Challenges
Despite the progress made at VACHS, there are significant challenges to meeting the CoC revised standard 3.3, which requires that 25% of patients treated with a stage I, II, or III cancer receive a Cancer Survivor Treatment Summary at an in-person visit in 2016. These relate primarily to multiple competing demands on provider time. In addition, 63.5% of patients who had been provided with a SCP at an in-person visit and responded to a satisfaction survey said they had not received the SCP. More research is needed to inform practice changes to optimize ongoing education and post-treatment care for veterans who are cancer survivors.
Conclusion
VA cancer centers seeking to ACoS CoC accreditation are required to provide a written summary of cancer treatment and plan for survivorship care to patients
diagnosed with at stage I, II, or III malignancy and treated at their facility. This requirement necessitates a significant ongoing investment in clinician and administrative workload to comply with the standard. The Comprehensive Cancer Center at VACHS, building on work by the SSIG, developed a concise note template in CPRS that enables oncology clinicians to create a treatment summary for each patient who meets criteria. During this process, VACHS has developed resources that may be useful to other VA cancer centers who are working to create this process. Clinicians interested in trialing the VACHS Cancer Survivor Treatment Summary template are encouraged to contact the author for additional information.
Visit www.fedprac.com/avahoupdates for an exclusive video interview with the author.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Acknowledgments
This project was a team effort. The author thanks the following VACHS colleagues for their input and support for this project: Michal Rose, MD, cancer center director; Donna Connery, tumor registrar; Renee Midgett, former clinical applications coordinator; Robert Troy Nall, health systems specialist; Forms Committee; Clarice Grens, APRN; and Jessica Barber, PhD, clinical psychologist as well as Members of VA Survivorship special interest group.
Click here to read the digital edition.
1. American College of Surgeons Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed January 18, 2017.
2. American College of Surgeons Commission on Cancer. Cancer program standards: ensuring patient-centered care 2016 Edition. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/2016%20coc%20standards%20manual_interactive%20pdf.ashx. Accessed January 18, 2017.
3. Smith J, Arfons L, Cmolik B, Moye J, Ballard E, Haggstrom D. Development and implementation of a veterans’ cancer survivorship program. Fed Pract. 2015;32(suppl 1):42S-48S..
4. National Comprehensive Cancer Network. NCCN distress thermometer and problem list for patients. http://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distress_thermometer.pdf. Updated May 6, 2016. Accessed January 18, 2016.
1. American College of Surgeons Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed January 18, 2017.
2. American College of Surgeons Commission on Cancer. Cancer program standards: ensuring patient-centered care 2016 Edition. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/2016%20coc%20standards%20manual_interactive%20pdf.ashx. Accessed January 18, 2017.
3. Smith J, Arfons L, Cmolik B, Moye J, Ballard E, Haggstrom D. Development and implementation of a veterans’ cancer survivorship program. Fed Pract. 2015;32(suppl 1):42S-48S..
4. National Comprehensive Cancer Network. NCCN distress thermometer and problem list for patients. http://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distress_thermometer.pdf. Updated May 6, 2016. Accessed January 18, 2016.
Palliative care underutilized in dementia patients with acute abdomen
Despite high rates of in-hospital mortality and nonroutine discharge, palliative care is underutilized in patients with dementia and acute abdominal emergency, according to findings published in Surgery.
“Currently little is known about palliative care utilization among patients with dementia in possible need of surgical intervention. This raises the question of whether the acute surgical emergency represents an appropriate episode during which to introduce palliative care for patients with dementia,” wrote Ana Berlin, MD, FACS, of the department of surgery at New Jersey Medical School, Newark, N.J., and her coauthors (Surgery. 2017 Dec 4. doi: 10.1016/j.surg.2017.09.048).
Of 15,209 patients aged 50 years and older with dementia and acute abdomen, 7.5% received palliative care. Patients discharged nonroutinely and patients treated operatively were less likely to receive palliative care, the researchers reported.
Dr. Berlin and her colleagues used the National Inpatient Sample database to identify patients with dementia and acute abdomen who were admitted nonelectively between 2009 and 2013. They used ICD-9 primary and secondary codes to limit surgical diagnoses to gastrointestinal obstruction, ischemia, and perforation.
Overall, 50.9% of patients were admitted for gastrointestinal obstruction, 39.8% for perforation, 5.1% for bowel ischemia, and 4.3% for mixed pathology; 17.8% of patients were managed operatively.
Patients with intestinal ischemia had the highest rate of both operation and mortality, at 22.8% and 27.6%, respectively. These patients also had the lowest rate of routine discharge, at 15.9%, the authors said. In comparison, patients with obstruction had surgical intervention and in-hospital mortality rates of 17% and 10.1%, respectively, and a routine discharge rate of 20.9%. Patients with perforation had an operation rate of 13.8%, in-hospital mortality rate of 6.8%, and routine discharge rate of 26.9%.
The palliative care utilization rate overall was 7.5%. Patients with mixed pathology who did not have surgery were most likely to receive palliative care, at 21.1%, noted Dr. Berlin and her coauthors.
Patients who died postoperatively were less likely than were those who died without surgical intervention to have received palliative care (20.9% vs. 31.4%; odds ratio = 0.63, 95% confidence interval, 0.46-0.86; P = .0039), and those who were discharged nonroutinely after an operation were less likely than were patients who were discharged nonroutinely without an operation to receive palliative care (3.7% vs. 7.0%; OR = 0.44, 95% CI, 0.34-0.57; P less than .0001).
Lastly, patients who received palliative care had shorter median hospital stays than did those who did not receive palliative care (5 days vs. 6 days; P less than .0001). These patients also had lower median hospital charges ($29,500 vs. $31,600; P = .0403).
The results identify two subsets of patients with unmet palliative care needs: patients requiring operative intervention, and patients with a diagnosis of intestinal ischemia, the authors said. In addition, the findings suggest that “surgeons should consider initiating palliative care … early in the hospital course for patients with dementia presenting with acute surgical abdomen,” they wrote.
“Both hip fracture and intensive care unit admission in patients with dementia have been described as appropriate triggers for palliative care assessment. ... Acute abdominal emergency [may also represent] an appropriate episode during which to introduce palliative care for patients with dementia,” they concluded.
The study was funded by the Rutgers New Jersey Medical School department of surgery and the New Jersey Medical School Hispanic Center of Excellence, Health Resources, and Services Administration. No other disclosures were reported.
SOURCE: Surgery. 2017 Dec 4. doi: 10.1016/j.surg.2017.09.048.
Few surgeons would argue that a patient with dementia and an acute abdomen would be less appropriate for a palliative care consultation than demented patients with a hip fracture or ICU admission who have been shown to benefit from triggered palliative care consults. However, the dynamic interaction between patient, family, and surgeon, described by Thomas Miner as the “palliative triangle,” will likely prevent universal acceptance of triggered consults (Ann Surg Oncol. 2002;9[7]:696-709). Despite the current trend of increasingly algorithmic care, there is still a need for surgeon autonomy for treatment decisions even when the recommended triggered intervention is highly appropriate. A mentor of mine put it, “I never do things routinely, but there are some things I do all the time.” As the benefits of palliative care become more evident, the referrals for palliative care for this latest indication will become “some things I do all the time” just as pharmacokinetics referrals have become for antibiotics, anticoagulation, and total parenteral nutrition.
Few surgeons would argue that a patient with dementia and an acute abdomen would be less appropriate for a palliative care consultation than demented patients with a hip fracture or ICU admission who have been shown to benefit from triggered palliative care consults. However, the dynamic interaction between patient, family, and surgeon, described by Thomas Miner as the “palliative triangle,” will likely prevent universal acceptance of triggered consults (Ann Surg Oncol. 2002;9[7]:696-709). Despite the current trend of increasingly algorithmic care, there is still a need for surgeon autonomy for treatment decisions even when the recommended triggered intervention is highly appropriate. A mentor of mine put it, “I never do things routinely, but there are some things I do all the time.” As the benefits of palliative care become more evident, the referrals for palliative care for this latest indication will become “some things I do all the time” just as pharmacokinetics referrals have become for antibiotics, anticoagulation, and total parenteral nutrition.
Few surgeons would argue that a patient with dementia and an acute abdomen would be less appropriate for a palliative care consultation than demented patients with a hip fracture or ICU admission who have been shown to benefit from triggered palliative care consults. However, the dynamic interaction between patient, family, and surgeon, described by Thomas Miner as the “palliative triangle,” will likely prevent universal acceptance of triggered consults (Ann Surg Oncol. 2002;9[7]:696-709). Despite the current trend of increasingly algorithmic care, there is still a need for surgeon autonomy for treatment decisions even when the recommended triggered intervention is highly appropriate. A mentor of mine put it, “I never do things routinely, but there are some things I do all the time.” As the benefits of palliative care become more evident, the referrals for palliative care for this latest indication will become “some things I do all the time” just as pharmacokinetics referrals have become for antibiotics, anticoagulation, and total parenteral nutrition.
Despite high rates of in-hospital mortality and nonroutine discharge, palliative care is underutilized in patients with dementia and acute abdominal emergency, according to findings published in Surgery.
“Currently little is known about palliative care utilization among patients with dementia in possible need of surgical intervention. This raises the question of whether the acute surgical emergency represents an appropriate episode during which to introduce palliative care for patients with dementia,” wrote Ana Berlin, MD, FACS, of the department of surgery at New Jersey Medical School, Newark, N.J., and her coauthors (Surgery. 2017 Dec 4. doi: 10.1016/j.surg.2017.09.048).
Of 15,209 patients aged 50 years and older with dementia and acute abdomen, 7.5% received palliative care. Patients discharged nonroutinely and patients treated operatively were less likely to receive palliative care, the researchers reported.
Dr. Berlin and her colleagues used the National Inpatient Sample database to identify patients with dementia and acute abdomen who were admitted nonelectively between 2009 and 2013. They used ICD-9 primary and secondary codes to limit surgical diagnoses to gastrointestinal obstruction, ischemia, and perforation.
Overall, 50.9% of patients were admitted for gastrointestinal obstruction, 39.8% for perforation, 5.1% for bowel ischemia, and 4.3% for mixed pathology; 17.8% of patients were managed operatively.
Patients with intestinal ischemia had the highest rate of both operation and mortality, at 22.8% and 27.6%, respectively. These patients also had the lowest rate of routine discharge, at 15.9%, the authors said. In comparison, patients with obstruction had surgical intervention and in-hospital mortality rates of 17% and 10.1%, respectively, and a routine discharge rate of 20.9%. Patients with perforation had an operation rate of 13.8%, in-hospital mortality rate of 6.8%, and routine discharge rate of 26.9%.
The palliative care utilization rate overall was 7.5%. Patients with mixed pathology who did not have surgery were most likely to receive palliative care, at 21.1%, noted Dr. Berlin and her coauthors.
Patients who died postoperatively were less likely than were those who died without surgical intervention to have received palliative care (20.9% vs. 31.4%; odds ratio = 0.63, 95% confidence interval, 0.46-0.86; P = .0039), and those who were discharged nonroutinely after an operation were less likely than were patients who were discharged nonroutinely without an operation to receive palliative care (3.7% vs. 7.0%; OR = 0.44, 95% CI, 0.34-0.57; P less than .0001).
Lastly, patients who received palliative care had shorter median hospital stays than did those who did not receive palliative care (5 days vs. 6 days; P less than .0001). These patients also had lower median hospital charges ($29,500 vs. $31,600; P = .0403).
The results identify two subsets of patients with unmet palliative care needs: patients requiring operative intervention, and patients with a diagnosis of intestinal ischemia, the authors said. In addition, the findings suggest that “surgeons should consider initiating palliative care … early in the hospital course for patients with dementia presenting with acute surgical abdomen,” they wrote.
“Both hip fracture and intensive care unit admission in patients with dementia have been described as appropriate triggers for palliative care assessment. ... Acute abdominal emergency [may also represent] an appropriate episode during which to introduce palliative care for patients with dementia,” they concluded.
The study was funded by the Rutgers New Jersey Medical School department of surgery and the New Jersey Medical School Hispanic Center of Excellence, Health Resources, and Services Administration. No other disclosures were reported.
SOURCE: Surgery. 2017 Dec 4. doi: 10.1016/j.surg.2017.09.048.
Despite high rates of in-hospital mortality and nonroutine discharge, palliative care is underutilized in patients with dementia and acute abdominal emergency, according to findings published in Surgery.
“Currently little is known about palliative care utilization among patients with dementia in possible need of surgical intervention. This raises the question of whether the acute surgical emergency represents an appropriate episode during which to introduce palliative care for patients with dementia,” wrote Ana Berlin, MD, FACS, of the department of surgery at New Jersey Medical School, Newark, N.J., and her coauthors (Surgery. 2017 Dec 4. doi: 10.1016/j.surg.2017.09.048).
Of 15,209 patients aged 50 years and older with dementia and acute abdomen, 7.5% received palliative care. Patients discharged nonroutinely and patients treated operatively were less likely to receive palliative care, the researchers reported.
Dr. Berlin and her colleagues used the National Inpatient Sample database to identify patients with dementia and acute abdomen who were admitted nonelectively between 2009 and 2013. They used ICD-9 primary and secondary codes to limit surgical diagnoses to gastrointestinal obstruction, ischemia, and perforation.
Overall, 50.9% of patients were admitted for gastrointestinal obstruction, 39.8% for perforation, 5.1% for bowel ischemia, and 4.3% for mixed pathology; 17.8% of patients were managed operatively.
Patients with intestinal ischemia had the highest rate of both operation and mortality, at 22.8% and 27.6%, respectively. These patients also had the lowest rate of routine discharge, at 15.9%, the authors said. In comparison, patients with obstruction had surgical intervention and in-hospital mortality rates of 17% and 10.1%, respectively, and a routine discharge rate of 20.9%. Patients with perforation had an operation rate of 13.8%, in-hospital mortality rate of 6.8%, and routine discharge rate of 26.9%.
The palliative care utilization rate overall was 7.5%. Patients with mixed pathology who did not have surgery were most likely to receive palliative care, at 21.1%, noted Dr. Berlin and her coauthors.
Patients who died postoperatively were less likely than were those who died without surgical intervention to have received palliative care (20.9% vs. 31.4%; odds ratio = 0.63, 95% confidence interval, 0.46-0.86; P = .0039), and those who were discharged nonroutinely after an operation were less likely than were patients who were discharged nonroutinely without an operation to receive palliative care (3.7% vs. 7.0%; OR = 0.44, 95% CI, 0.34-0.57; P less than .0001).
Lastly, patients who received palliative care had shorter median hospital stays than did those who did not receive palliative care (5 days vs. 6 days; P less than .0001). These patients also had lower median hospital charges ($29,500 vs. $31,600; P = .0403).
The results identify two subsets of patients with unmet palliative care needs: patients requiring operative intervention, and patients with a diagnosis of intestinal ischemia, the authors said. In addition, the findings suggest that “surgeons should consider initiating palliative care … early in the hospital course for patients with dementia presenting with acute surgical abdomen,” they wrote.
“Both hip fracture and intensive care unit admission in patients with dementia have been described as appropriate triggers for palliative care assessment. ... Acute abdominal emergency [may also represent] an appropriate episode during which to introduce palliative care for patients with dementia,” they concluded.
The study was funded by the Rutgers New Jersey Medical School department of surgery and the New Jersey Medical School Hispanic Center of Excellence, Health Resources, and Services Administration. No other disclosures were reported.
SOURCE: Surgery. 2017 Dec 4. doi: 10.1016/j.surg.2017.09.048.
FROM SURGERY
Key clinical point: Despite high mortality and frequent nonroutine discharges, palliative care is underutilized in dementia patients with acute abdominal emergency.
Major finding: Among dementia patients with acute abdominal emergency, 7.5% received palliative care.
Data source: A retrospective analysis of 15,209 patients aged 50 years and older from the National Inpatient Sample, for the period of 2009-2013.
Disclosures: The study was funded by the Rutgers New Jersey Medical School Department of Surgery and the New Jersey Medical School Hispanic Center of Excellence, Health Resources, and Services Administration.
SOURCE: Surgery. 2017 Dec 4. doi: http://dx.doi.org/10.1016/j.surg.2017.09.048.
The opioid epidemic, surgeons, and palliative care
Recent public and professional attention to what is now called the opioid epidemic has obvious implications for surgery and palliative care. Because of the status of “epidemic,” there is a sense of urgency within the surgical and palliative care community to reevaluate the assessment and treatment of patients for whom opioid therapy is being considered.
Although the liberal use of opioids is a common stereotype of palliative care, the use of opioids in the palliative care setting is part of a complex assessment and treatment process. Opioid use in this setting is analogous to palliative surgery in the surgical palliative care setting: It is one tool, and it is most effective and safe when based on an assessment of the more general picture. A fundamental concept of palliative care, “total pain,” provides a basis for improved pain management that goes far beyond the use and dependency on opioid therapy. Dame Cicely Saunders, who was mentored by a surgeon and later became a Fellow of the Royal College of Surgeons, defined the concept of total pain as the suffering that encompasses all of a person’s physical, psychological, social, spiritual, and practical struggles (BMJ. 2005 Jul 23;331[7510]:238). Blake Cady, a preeminent surgeon and surgical educator, once wrote that the day-to-day decisions in surgery are best made in the context of a surgical philosophy of care (J Am Coll Surg. 2005 Feb;200[2]:285-90). This applies to all interventions. Total-pain assessment provides us the opportunity to identify nonphysical factors associated with pain that might not indicate opioid use or even contraindicate their use. Existential distress or spiritual pain in a delirious or underassessed patient can be indistinguishable from physical distress. Socioeconomic factors, such as an inability to pay for medical care, can present as pain.
Surgeons are uniquely positioned as “listening posts” in the overall campaign to curb opioid misuse. They can identify patients at risk for or diagnosed with substance use disorder so they can be managed or referred for specialist treatment appropriately.
Awareness of other dimensions of pain will enhance their efficacy in this role.
Opioid sparing is a key tactic in the strategy for controlling opioid use and minimizing opioid-induced side effects. Occasionally surgical or interventional radiologic procedures are useful for this purpose.
There are immediate, specific actions surgeons can take in order to constructively participate in opioid use reform:
- Expand your patient’s pain history to include nonphysical dimensions of pain and refer appropriately.
- Know your opioids; carry an opioid conversion table. Errors in opioid conversion can result in significant undertreatment of pain but can result in overdosage just as easily.
- Know your pharmacist. Pharmacists are valuable allies in safe opioid prescribing and monitoring practices.
- Be wary of “standardized” order sets that include opioids. There is no standard dose or standard patient as we are rapidly learning from genomics.
- Utilize your state’s patient drug-monitoring program – a new pain for clinicians, but some headaches are worth it. It clearly has already put the brakes on opioid prescribing.
Given the recent public and professional attention to the problems of opioid misuse, there is a long-overdue opportunity to reassess not only the indications and management of opioid therapy but also our more general approach to the management of pain. There is now an opportunity for surgeons to play a major role in improving opioid-prescribing practice. One potentially successful approach could be better assessment and management of pain through an awareness and application of palliative care principles. Like all encounters with uncertainty, the best way out of the current opioid dilemma is the way through: Surgeons should not abandon opioids but – in conjunction with nurses, palliative care practitioners, pharmacists, and pain and anesthesia specialists – reinvent their role in the war on suffering.
Dr. Dunn is the medical director of the palliative care consultation service at the University of Pittsburgh Medical Center Hamot in Erie, Pa., and vice chair of the ACS Committee on Surgical Palliative Care.
Recent public and professional attention to what is now called the opioid epidemic has obvious implications for surgery and palliative care. Because of the status of “epidemic,” there is a sense of urgency within the surgical and palliative care community to reevaluate the assessment and treatment of patients for whom opioid therapy is being considered.
Although the liberal use of opioids is a common stereotype of palliative care, the use of opioids in the palliative care setting is part of a complex assessment and treatment process. Opioid use in this setting is analogous to palliative surgery in the surgical palliative care setting: It is one tool, and it is most effective and safe when based on an assessment of the more general picture. A fundamental concept of palliative care, “total pain,” provides a basis for improved pain management that goes far beyond the use and dependency on opioid therapy. Dame Cicely Saunders, who was mentored by a surgeon and later became a Fellow of the Royal College of Surgeons, defined the concept of total pain as the suffering that encompasses all of a person’s physical, psychological, social, spiritual, and practical struggles (BMJ. 2005 Jul 23;331[7510]:238). Blake Cady, a preeminent surgeon and surgical educator, once wrote that the day-to-day decisions in surgery are best made in the context of a surgical philosophy of care (J Am Coll Surg. 2005 Feb;200[2]:285-90). This applies to all interventions. Total-pain assessment provides us the opportunity to identify nonphysical factors associated with pain that might not indicate opioid use or even contraindicate their use. Existential distress or spiritual pain in a delirious or underassessed patient can be indistinguishable from physical distress. Socioeconomic factors, such as an inability to pay for medical care, can present as pain.
Surgeons are uniquely positioned as “listening posts” in the overall campaign to curb opioid misuse. They can identify patients at risk for or diagnosed with substance use disorder so they can be managed or referred for specialist treatment appropriately.
Awareness of other dimensions of pain will enhance their efficacy in this role.
Opioid sparing is a key tactic in the strategy for controlling opioid use and minimizing opioid-induced side effects. Occasionally surgical or interventional radiologic procedures are useful for this purpose.
There are immediate, specific actions surgeons can take in order to constructively participate in opioid use reform:
- Expand your patient’s pain history to include nonphysical dimensions of pain and refer appropriately.
- Know your opioids; carry an opioid conversion table. Errors in opioid conversion can result in significant undertreatment of pain but can result in overdosage just as easily.
- Know your pharmacist. Pharmacists are valuable allies in safe opioid prescribing and monitoring practices.
- Be wary of “standardized” order sets that include opioids. There is no standard dose or standard patient as we are rapidly learning from genomics.
- Utilize your state’s patient drug-monitoring program – a new pain for clinicians, but some headaches are worth it. It clearly has already put the brakes on opioid prescribing.
Given the recent public and professional attention to the problems of opioid misuse, there is a long-overdue opportunity to reassess not only the indications and management of opioid therapy but also our more general approach to the management of pain. There is now an opportunity for surgeons to play a major role in improving opioid-prescribing practice. One potentially successful approach could be better assessment and management of pain through an awareness and application of palliative care principles. Like all encounters with uncertainty, the best way out of the current opioid dilemma is the way through: Surgeons should not abandon opioids but – in conjunction with nurses, palliative care practitioners, pharmacists, and pain and anesthesia specialists – reinvent their role in the war on suffering.
Dr. Dunn is the medical director of the palliative care consultation service at the University of Pittsburgh Medical Center Hamot in Erie, Pa., and vice chair of the ACS Committee on Surgical Palliative Care.
Recent public and professional attention to what is now called the opioid epidemic has obvious implications for surgery and palliative care. Because of the status of “epidemic,” there is a sense of urgency within the surgical and palliative care community to reevaluate the assessment and treatment of patients for whom opioid therapy is being considered.
Although the liberal use of opioids is a common stereotype of palliative care, the use of opioids in the palliative care setting is part of a complex assessment and treatment process. Opioid use in this setting is analogous to palliative surgery in the surgical palliative care setting: It is one tool, and it is most effective and safe when based on an assessment of the more general picture. A fundamental concept of palliative care, “total pain,” provides a basis for improved pain management that goes far beyond the use and dependency on opioid therapy. Dame Cicely Saunders, who was mentored by a surgeon and later became a Fellow of the Royal College of Surgeons, defined the concept of total pain as the suffering that encompasses all of a person’s physical, psychological, social, spiritual, and practical struggles (BMJ. 2005 Jul 23;331[7510]:238). Blake Cady, a preeminent surgeon and surgical educator, once wrote that the day-to-day decisions in surgery are best made in the context of a surgical philosophy of care (J Am Coll Surg. 2005 Feb;200[2]:285-90). This applies to all interventions. Total-pain assessment provides us the opportunity to identify nonphysical factors associated with pain that might not indicate opioid use or even contraindicate their use. Existential distress or spiritual pain in a delirious or underassessed patient can be indistinguishable from physical distress. Socioeconomic factors, such as an inability to pay for medical care, can present as pain.
Surgeons are uniquely positioned as “listening posts” in the overall campaign to curb opioid misuse. They can identify patients at risk for or diagnosed with substance use disorder so they can be managed or referred for specialist treatment appropriately.
Awareness of other dimensions of pain will enhance their efficacy in this role.
Opioid sparing is a key tactic in the strategy for controlling opioid use and minimizing opioid-induced side effects. Occasionally surgical or interventional radiologic procedures are useful for this purpose.
There are immediate, specific actions surgeons can take in order to constructively participate in opioid use reform:
- Expand your patient’s pain history to include nonphysical dimensions of pain and refer appropriately.
- Know your opioids; carry an opioid conversion table. Errors in opioid conversion can result in significant undertreatment of pain but can result in overdosage just as easily.
- Know your pharmacist. Pharmacists are valuable allies in safe opioid prescribing and monitoring practices.
- Be wary of “standardized” order sets that include opioids. There is no standard dose or standard patient as we are rapidly learning from genomics.
- Utilize your state’s patient drug-monitoring program – a new pain for clinicians, but some headaches are worth it. It clearly has already put the brakes on opioid prescribing.
Given the recent public and professional attention to the problems of opioid misuse, there is a long-overdue opportunity to reassess not only the indications and management of opioid therapy but also our more general approach to the management of pain. There is now an opportunity for surgeons to play a major role in improving opioid-prescribing practice. One potentially successful approach could be better assessment and management of pain through an awareness and application of palliative care principles. Like all encounters with uncertainty, the best way out of the current opioid dilemma is the way through: Surgeons should not abandon opioids but – in conjunction with nurses, palliative care practitioners, pharmacists, and pain and anesthesia specialists – reinvent their role in the war on suffering.
Dr. Dunn is the medical director of the palliative care consultation service at the University of Pittsburgh Medical Center Hamot in Erie, Pa., and vice chair of the ACS Committee on Surgical Palliative Care.
Delivering Palliative Care in a Community Hospital: Experiences and Lessons Learned from the Front Lines
From the Division of Palliative Care, Butler Health System, Butler, PA (Drs. Stein, Reefer, Selvaggi, Ms. Doverspike); the University of Pittsburgh Medical Center, Pittsburgh, PA (Dr. Rajagopal); and the Duke Cancer Institute and Duke Fuqua School of Business, Durham, NC (Dr. Kamal).
Abstract
- Objective: To describe an approach to develop a community-centric palliative care program in a rural community health system and to review data collected over the program’s first year.
- Methods: We describe the underlying foundations of our program development including the health system’s prioritization of a palliative care program, funding opportunities, collaboration with community supports, and the importance of building a team and program that reflects a community’s needs. Data were collected through a program-maintained spreadsheet and a data monitoring system available through the Global Palliative Care Quality Alliance.
- Results: 516 new inpatient consultations were seen during the first year, for a penetration of 3.7%. The demographics of the patients who received consultation reflect that of the surrounding community. Over 50% of patients seen within the first year died, and hospice utilization at home and within facilities and inpatient hospice units increased. In addition, 79% of the patients seen by the palliative care team had a confirmed code status of do not resuscitate and do not intubate.
- Conclusions: Butler Health System’s approach to development of a palliative care program has resulted in increasing utilization of palliative care services in the hospital. Having hospital administration support, community support, and understanding the individualized needs of a community has been essential for the program’s expansion.
Key words: palliative care; program development; community hospital; rural.
Since its inception, palliative care has been committed to providing specialty-level consultation services to individuals with serious illness and their loved ones. The field has focused heavily on growth and acceptance, consistently moving upstream with regards to illness trajectory, across diseases, and across demographic variables such as age (eg, pediatric quality of life programs) and race (eg, community outreach programs addressing racial disparities in hospice use). An important frontier that remains challenging for much of the field is expansion into the community setting, where resources, implicit acceptance, and patient populations may vary.
As health system leaders appreciate the positive impacts palliative medicine on patient care and care quality, barriers to implementing palliative care programs in community hospitals must be addressed in ways tailored to the unique needs of smaller organizations and their communities. The goal of this paper is to outline the approach taken to develop Butler Health System’s community-centric palliative care program, describe our program’s underlying foundation rooted in community supports, and recount steps we have taken thus far to impact patient care in our hospital, health system, and community through the program’s first year.
Community Hospital Palliative Care—The Necessity and the Challenges
Palliative care has made strides in its growth and acceptance in the last decade; yet, the distribution of that growth has been skewed. Although 67% of hospitals now report access to specialist palliative care programs, most of the 148% growth over the last decade has been actualized in larger hospitals. Ninety percent of hospitals with greater than 300 beds report palliative care service availability whereas only 56% of small hospitals were identified to have this specialty care [1].
The inequity of access is also seen in other countries. A recent Canadian study retrospectively examined access to care of 23,860 deceased patients in Nova Scotia. Although they found 40.9% of study subjects were enrolled in a palliative care program at urban, academic centers, patients in a rural setting were only a third as likely to be enrolled in a palliative care program [2]. This access gap has important effects on patient-level outcomes, as evidence has consistently demonstrated that patients in rural settings who receive palliative care have decreased unnecessary hospitalizations and less in-hospital deaths [3].
While evidence of improved outcomes is strong, important barriers stand in the way. In a 2013 study, 374 health care providers at 236 rural hospitals in 7 states were interviewed to determine barriers to providing palliative care in rural settings. Barriers identified include a lack of administrative support, access to basic palliative care training for primary care physicians, and limited relationships to hospices [4]. Additional challenges include lack of access to tertiary-level specialty clinicians, access to and misconceptions about prescription medications, transportation for patients and providers, and incorporating a patient’s community supports [5–7].
Proposed Solutions
Techniques to improve palliative care access for rural and community centers that have been previously reviewed in the literature include videoconferencing with tertiary care experts in palliative care and education through small community-level lectures [8–10]. Goals of rural and suburban palliative care programs are broadly similar to programs at academic medical centers; however, few studies have identified impact of palliative medicine on patient care in community settings. In one suburban practice, a study found that patients were more likely to die at home if they had multiple caregivers, increased length of time under palliative care, and older age upon referral [11].
The United States has few large-scale pilot programs attempting to address the palliative needs of a more suburban or rural population. Of these, the Minnesota Rural Palliative Care Initiative developed by Stratis Health is perhaps the best publicized. Stratis Health developed and led an 18-month learning collaborative from October 2008 to April 2010 through which community teams developed or improved palliative care services. Through this initiative, a community-based health care practice model was developed that took advantage of the strong interrelationships within rural communities. After 18 months, 6 out of 10 rural Minnesota communities had formal palliative care programs, and 8 to 9 out of 10 had capabilities to at least address advance directives as well as provider and community education [12]. In another initiative, the NIH established a new suburban clinic with tertiary providers specifically for resource intensive, underserved patients [13]. The clinic was established by partnering with a service that was already in place in the community. Twenty-seven patients were seen within 7 months. The most common consults were patients with numerous comorbidities and chronic pain rather than terminal diagnoses. Given the intensive need of these patients, the authors felt that a consultation service and an interdisciplinary team that included psychosocial/spiritual/social work providers offered the most efficient method of delivering advanced palliative care needs.
The research regarding both solutions to challenges and novel methods of addressing the care gap remains sparse as evidenced by the conclusions of multiple systematic reviews and meta-analyses and the inability of the Cochrane review to find papers meeting inclusion criteria regarding techniques of community support in palliative care [14,15]. There remains a need to identify practical techniques of implementing palliative care in rural and suburban settings.
The Butler Health System Experience
In August 2015, we set out to start the first hospital-based palliative care consultation service in the Butler Health System. The health system is a nonprofit, single-hospital system anchored by Butler Memorial Hospital, a 294-bed community hospital located within a rural Pennsylvania county of 186,000 residents, 35 miles north of Pittsburgh. Butler County consists of a predominantly white, non-Hispanic population with over 15% of the residents being older than 65 years of age. The median household income is $61,000 earned primarily through blue collar occupations [16]. Driven by 53 employed primary care physicians, the health system provides services for 75,000 patients at sites covering an area of 4000 square miles. The hospital provides general medical, critical, surgical and subspecialty care and behavior health services as a regional referral center for 4 surrounding rural counties, accepting 12,500 inpatient admissions annually. A hospitalist service admits the majority of oncology patients, and the intensive care unit (ICU) is an open unit, where patients are admitted to the hospitalist, primary care, or surgical service.
While no formal needs assessment was performed prior to program development, perceptions of inadequate pain control, overuse of naloxone, underutilization of hospice services, and lack of consistent quality in end-of-life care were identified. These concerns were voiced at the levels of direct patient care on the floors, and by nursing and physician hospital leadership. Prior to our program, the chief medical officer attended the national Center to Advance Palliative Care conference to better understand the field of palliative care and its impact on improving quality of care. Concurrently, our health system was expanding its inpatient capabilities (eg, advanced neurologic and cardiac services), resulting in admissions with increased disease severity and illness complexity. With the vision of improved patient care, prioritizing quality end-of-life care and symptom management, the hospital board and administration overwhelmingly supported the development of the palliative care program, philosophically and financially.
Laying a Foundation—Funding, Collaboration, and Team Building
Funding and staffing are 2 important factors when building any program. Sources of funding for palliative care programs may include hospital support, billing revenue, grants, and philanthropy. Program development was a priority for the hospital and community. To help offset costs, efforts to raise financial support focused on utilizing the health system’s annual fundraising events. Through the generosity of individuals in the community, the hospital’s annual gala event, and donations from the hospital’s auxiliary, a total of $230,000 was raised prior to program initiation. Funds budgeted through direct hospital support and fundraising were allocated towards hiring palliative care team members and community marketing projects.
The hospital’s surrounding community is fortunate to have 2 local inpatient hospice facilities, and these relationships were imperative to providing quality end-of-life care preceding our palliative care program. A formal partnership was previously established with one while the other remains an important referral facility due to its proximity to the hospital. These hospice services are encouraged to participate in our weekly palliative care interdisciplinary team meetings. Their incorporation has improved coordination, continuity, and translation of care upon patient discharge from the acute hospital setting. Additionally, the relationships have been beneficial in tracking patients’ outcomes and data collection.
The standard structure of a palliative care team described by the Joint Commission and National Consensus Panel for Palliative Care consists of a physician, registered nurse or advanced practice provider, chaplaincy, and social work. Despite this recommendation, less than 40% of surveyed hospitals met the criteria, and less than 25% have dedicated funding to cover these positions [17]. Upon inception of our palliative care program, 2.6 funded full-time equivalents (FTEs) were allocated. These positions included a physician (1.0 FTE), a physician assistant (1.0 FTE), and a part time palliative care social worker (0.6 FTE). The 2015 National Palliative Care Registry found that 3.2 funded FTEs per 10,000 admissions is the average for hospitals with 150 to 299 beds [17]. The uncertainty of the utilization and consult volume, and the limited amount of qualified palliative care trained practitioners, resulted in the palliative program starting below this mean at 2.1 funded FTEs per 10,000 admissions. All the funded positions were located on site at the hospital. The pre-existing volunteer hospital chaplain service was identified as the pastoral care component for the program.
Increased FTEs have been associated with increased palliative care service penetration and ultimately in decreased time to consult [18]. In response to increasing consult volumes, concerns for delays in time to consult, and in preparation for expansion to an outpatient service, the palliative care department acquired an additional funded physician FTE (1.0). Ultimately the service reached a total of 3.6 FTE for inpatient services during its first 12 months; proportionately this resulted in an increase to 2.9 FTE per 10,000 admissions based on the yearly admission rate of 12,500 patients.
Educational Outreach
The success of a palliative care program depends on other clinicians’ acceptance and referral to the clinical program. We took a 2-pronged approach, focusing on both hospital-based and community-based education. The hospital-based nursing education included 30-minute presentations on general overviews of palliative care, differences between palliative care and hospice, and acute symptom management at the end of life. The palliative care team presented to all medical, surgical, and intensive care units and encompassed all shifts of nursing staff. These lectures included pre- and post-tests to assess for impact and feedback. Similar educational presentations, as well as an hour-long presentation on opioids and palliative care, were available for physicians for CME opportunities. We also distributed concise palliative care referral packets to outpatient primary care offices through the health system’s marketing team. The referral packets included examples of diagnoses, clinical scenarios, and symptoms to assist in the physicians’ understanding of palliative care services. The palliative care team also met with clinic office managers to discuss the program and answer questions.
There were also educational opportunities for patients and families in our community. Taking advantage of previously developed partnerships between the hospital system and local media outlets, the palliative care team performed local radio spots to educate the community on topics including an overview of palliative care, how to request palliative care, and the difference between palliative care and hospice care. We partnered with a local hospice agency and developed a well-received bereavement seminar for patients, family members, and employees and included the topic of advanced care planning.
Data Collection
We collect data using 2 different tools: a self-maintained spreadsheet shared between our palliative care clinicians, and a collective data tool (QDACT) included in our membership with and maintained by the Global Palliative Care Quality Alliance. Data collected and tracked in our spreadsheet includes date of consult, patient age, primary and secondary diagnoses, disposition, goals of care discussions, date of death, and 30-day readmissions. Through the QDACT data monitoring program, we are tracking and analyzing quality measures including symptom assessment and management and code status conversion. The QDACT database also provides financial data specific to our institution such as cost savings based on our billing, readmission rates, and length of stay.
Results
Projections, Volumes, and Penetration
Prior to the start of our program, our chief medical office used Center to Advance Palliative Care tools to project inpatient consultation volumes at our institution. Variables that are recommended by this center to guide projections include number of hospital admissions per year, hospital occupancy, disposition to hospice, as well as generalized estimations of inpatient mortality rates. Based on our data, it was expected that our program would receive 204 new inpatient consults in our first year, and 774 follow-up visits. Our actual new inpatient consults totaled 516, with 919 follow-up visits. Palliative care penetration (percentage of annual hospital admissions seen by the palliative care team) our first year was 3.7% (Table 1).
Consultation Demographics
The demographics of the patients seen by the palliative care team reflect that of Butler County’s Medicare fee-for-service (FFS) population (Table 2); however, differences were seen at the state and national level with regard to ethnicity (Table 2).
Almost half of consultations (49%) were placed by the hospitalist service. Since the ICU is an open unit, critical care consults are not adequately reflected by analysis of the ordering physician alone. Analysis of consultation location revealed that 27% of inpatient consults were located within the ICU.
Patient Outcomes and Disposition
Outcomes and discharge data from the first year were collected and reviewed. Ten percent of the patients seen by palliative care died in the hospital, and 51% of patients that were seen by palliative care died within the program’s first year. Thirty-seven percent of patients discharged from the hospital utilized hospice services at home, in residential nursing facility, or at an inpatient hospice unit. The remaining 53% were discharged without hospice services to home or facility (Figure).
Hospice utilization by the health system increased during our first year. Compared to the 2014 calendar year, there were a total of 263 referrals for hospice services. During the first year of the palliative care program, which started August 2015, there were a total of 293 referrals. Of the 293 total hospice referrals, 190 (64.8%) of these referrals were for patients seen by the palliative care team.
Change of Code Status
Code status and changes in codes status data were collected. Of 462 individual patients prior to or at the time of palliative care consults, 43% were full code, 4% limited code, 8% unknown status, and 45% Do Not Resuscitate. After palliative care consult, 61% of the patients who were previously full/limited/unknown converted to do not resuscitate and do not intubate status. In total, 79% of patients seen by palliative care had a confirmed code status of Do Not Resuscitate and Do Not Intubate status after consult.
Discussion
In our first year, our palliative care program exceeded the expected number of inpatient consults, corresponding with a penetration of 3.7%. With the increase of funded FTEs, preliminary data shows that the department’s penetration continues to rise remaining consistent with the data and expectations [18]. During the second year, it is anticipated that over 600 inpatient palliative care consultations will be performed with an estimated penetration of 4%. This increasing penetration reflects the rising utilization of palliative care within our hospital. Since inception of the program, the service has expanded into an outpatient clinic 2 days per week. The palliative care clinic is staffed by a registered nurse (funded 0.6 FTE) and covered by the same physicians and physician assistant providing the inpatient services. The department acquired an unfunded but designated chaplaincy volunteer to assist with patients’ spiritual needs. We believe that the success of our program during the first year was related to multiple factors: a focus of integration and education by the palliative care department, health system administration buy-in, and identification of surrounding community needs.
In addition to patient care, our palliative care department also prioritizes “tangible” impacts to better establish our contributions to the health system. We have done this through participation on hospital committees, hospital policy revision teams, and by developing innovative solutions such as a terminal extubation protocol and order set for our ICU. The health system and its administration have recognized the importance of educating nursing and physician staff on palliative care services, and have supported these continued efforts alongside our clinical obligations.
Concurrent with administration buy-in, financial supports for our palliative care services were initially supplemented by the health system. Our department understands the importance of recognizing limitations of resources in communities and their hospitals. In efforts to minimize the department’s impact on our own health system’s financial resources, we have strived to offset our costs. We helped the hospital system meet pay-for-performance palliative care metrics set by the large local insurers resulting in financial hospital reimbursement valued at $600,000 in 2016.
The question of how the program may translate into other communities raises a major limitation: the homogeneity our population. The community surrounding the hospital is primarily Caucasian, with minimal representation of minority populations. While the patient population seen by our palliative care team is reflective of our surrounding county, it does not represent Medicare FFS beneficiaries on a national level or many other types of community hospitals across the country. Variations of ethnicity, age, diagnoses, and faith are fundamental, which highlights the importance of understanding the community in which a program is developed.
The rising trajectory of our palliative care service utilization has prompted a discussion of future endeavors for our program. Expectations for a continued shortage of hospice and palliative care physicians [19] and concerns for practitioner burnout [20] underlie our thoughtful approach to expansion of inpatient and outpatient services. At this time, potential projects include a consultation trigger system and incorporation of palliative care providers in ICU rounding, as well as possible expansion of outpatient services through implantation of an advanced practitioner into surrounding nursing homes and primary care offices.
We have found a growing utilization of our program at Butler Health System. Our first year experience has highlighted the importance of identifying community and hospital administrative champions as a foundation. Additionally, understanding the specific characteristics of one’s surrounding community may allow for improved integration and acceptance of palliative care in a community setting. Our program continues to work with the health system, community, and philanthropic organizations to expand the ever-growing need for palliative care services.
1. Dumanovsky T, Augustin R, Rogers M, et al. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med 2016;19:8–15.
2. Lavergne MR, Lethbridge L, Johnston G, et al. Examining palliative care program use and place of death in rural and urban contexts: a Canadian population-based study using linked data. Rural Remote Health 2015;15:3134.
3. Seow H, Brazil K, Sussman J, et al. Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: a pooled analysis. BMJ 2014;348:g3496.
4. Fink RM, Oman KS, Youngwerth J, et al. A palliative care needs assessment of rural hospitals. J Palliat Med 2013;16:638–44.
5. Dumont S, Jacobs P, Turcotte V, et al. Palliative care costs in Canada: A descriptive comparison of studies of urban and rural patients near end of life. J Palliat Med 2015;29:908–17.
6. Kaasalainen S, Brazil K, Williams A, et al. Nurses' experiences providing palliative care to individuals living in rural communities: aspects of the physical residential setting. Rural Remote Health 2014;14:2728.
7. Ahmed N, Bestall JC, Ahmedzai SH, et al. Systematic review of the problems and issues of accessing specialist palliative care by patients, carers and health and social care professionals. J Palliat Med 2004;18:525–42.
8. Ray RA, Fried O, Lindsay D. Palliative care professional education via video conference builds confidence to deliver palliative care in rural and remote locations. BMC Health Serv Res 2014;14:272.
9. Bakitas MA, Elk R, Astin M, et al. Systematic review of palliative care in the rural setting. Cancer Control 2015;22:450–64.
10. Akiyama M, Hirai K, Takebayashi T, et al. The effects of community-wide dissemination of information on perceptions of palliative care, knowledge about opioids, and sense of security among cancer patients, their families, and the general public. Support Care Cancer 2016;24: 347–56.
11. Maida V. Factors that promote success in home palliative care: a study of a large suburban palliative care practice. J Palliat Care 2002;18:282–6.
12. Ceronsky L, Shearer J, Weng K, et al. Minnesota Rural Palliative Care Initiative: building palliative care capacity in rural Minnesota. J Palliat Med 2013;16:310–3.
13. Aggarwal SK, Ghosh A, Cheng MJ, et al. Initiating pain and palliative care outpatient services for the suburban underserved in Montgomery County, Maryland: Lessons learned at the NIH Clinical Center and MobileMed. Palliat Support Care 2015;16:1–6.
14. Rainsford S, MacLeod RD, Glasgow NJ. Place of death in rural palliative care: A systematic review. J Palliat Med 2016;30:745–63.
15. Horey D, Street AF, O'Connor M, et al. Training and supportive programs for palliative care volunteers in community settings. Cochrane Database Syst Rev 2015 Jul 20;(7):CD009500.
16. The United States Census Bureau: QuickFacts: Butler County, Pennsylvania. Accessed 10 Mar 2017 at www.census.gov/quickfacts/table/PST045216/42019,00.
17. Spetz J, Dudley N, Trupin L, et al. Few hospital palliative care programs meet national staffing recommendations. Health Aff 2016;35:1690–7.
18. Dumanovsky T, Rogers M, Spragens LH, et al. Impact of Staffing on Access to Palliative Care in U.S. Hospitals. J Palliat Med. 2015 Dec; 18(12). Pages 998-999.
19. Lupu D, American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage 2010;40:899–911.
20. Kamal AH, Bull JK, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manage 2016;51:690–6.
From the Division of Palliative Care, Butler Health System, Butler, PA (Drs. Stein, Reefer, Selvaggi, Ms. Doverspike); the University of Pittsburgh Medical Center, Pittsburgh, PA (Dr. Rajagopal); and the Duke Cancer Institute and Duke Fuqua School of Business, Durham, NC (Dr. Kamal).
Abstract
- Objective: To describe an approach to develop a community-centric palliative care program in a rural community health system and to review data collected over the program’s first year.
- Methods: We describe the underlying foundations of our program development including the health system’s prioritization of a palliative care program, funding opportunities, collaboration with community supports, and the importance of building a team and program that reflects a community’s needs. Data were collected through a program-maintained spreadsheet and a data monitoring system available through the Global Palliative Care Quality Alliance.
- Results: 516 new inpatient consultations were seen during the first year, for a penetration of 3.7%. The demographics of the patients who received consultation reflect that of the surrounding community. Over 50% of patients seen within the first year died, and hospice utilization at home and within facilities and inpatient hospice units increased. In addition, 79% of the patients seen by the palliative care team had a confirmed code status of do not resuscitate and do not intubate.
- Conclusions: Butler Health System’s approach to development of a palliative care program has resulted in increasing utilization of palliative care services in the hospital. Having hospital administration support, community support, and understanding the individualized needs of a community has been essential for the program’s expansion.
Key words: palliative care; program development; community hospital; rural.
Since its inception, palliative care has been committed to providing specialty-level consultation services to individuals with serious illness and their loved ones. The field has focused heavily on growth and acceptance, consistently moving upstream with regards to illness trajectory, across diseases, and across demographic variables such as age (eg, pediatric quality of life programs) and race (eg, community outreach programs addressing racial disparities in hospice use). An important frontier that remains challenging for much of the field is expansion into the community setting, where resources, implicit acceptance, and patient populations may vary.
As health system leaders appreciate the positive impacts palliative medicine on patient care and care quality, barriers to implementing palliative care programs in community hospitals must be addressed in ways tailored to the unique needs of smaller organizations and their communities. The goal of this paper is to outline the approach taken to develop Butler Health System’s community-centric palliative care program, describe our program’s underlying foundation rooted in community supports, and recount steps we have taken thus far to impact patient care in our hospital, health system, and community through the program’s first year.
Community Hospital Palliative Care—The Necessity and the Challenges
Palliative care has made strides in its growth and acceptance in the last decade; yet, the distribution of that growth has been skewed. Although 67% of hospitals now report access to specialist palliative care programs, most of the 148% growth over the last decade has been actualized in larger hospitals. Ninety percent of hospitals with greater than 300 beds report palliative care service availability whereas only 56% of small hospitals were identified to have this specialty care [1].
The inequity of access is also seen in other countries. A recent Canadian study retrospectively examined access to care of 23,860 deceased patients in Nova Scotia. Although they found 40.9% of study subjects were enrolled in a palliative care program at urban, academic centers, patients in a rural setting were only a third as likely to be enrolled in a palliative care program [2]. This access gap has important effects on patient-level outcomes, as evidence has consistently demonstrated that patients in rural settings who receive palliative care have decreased unnecessary hospitalizations and less in-hospital deaths [3].
While evidence of improved outcomes is strong, important barriers stand in the way. In a 2013 study, 374 health care providers at 236 rural hospitals in 7 states were interviewed to determine barriers to providing palliative care in rural settings. Barriers identified include a lack of administrative support, access to basic palliative care training for primary care physicians, and limited relationships to hospices [4]. Additional challenges include lack of access to tertiary-level specialty clinicians, access to and misconceptions about prescription medications, transportation for patients and providers, and incorporating a patient’s community supports [5–7].
Proposed Solutions
Techniques to improve palliative care access for rural and community centers that have been previously reviewed in the literature include videoconferencing with tertiary care experts in palliative care and education through small community-level lectures [8–10]. Goals of rural and suburban palliative care programs are broadly similar to programs at academic medical centers; however, few studies have identified impact of palliative medicine on patient care in community settings. In one suburban practice, a study found that patients were more likely to die at home if they had multiple caregivers, increased length of time under palliative care, and older age upon referral [11].
The United States has few large-scale pilot programs attempting to address the palliative needs of a more suburban or rural population. Of these, the Minnesota Rural Palliative Care Initiative developed by Stratis Health is perhaps the best publicized. Stratis Health developed and led an 18-month learning collaborative from October 2008 to April 2010 through which community teams developed or improved palliative care services. Through this initiative, a community-based health care practice model was developed that took advantage of the strong interrelationships within rural communities. After 18 months, 6 out of 10 rural Minnesota communities had formal palliative care programs, and 8 to 9 out of 10 had capabilities to at least address advance directives as well as provider and community education [12]. In another initiative, the NIH established a new suburban clinic with tertiary providers specifically for resource intensive, underserved patients [13]. The clinic was established by partnering with a service that was already in place in the community. Twenty-seven patients were seen within 7 months. The most common consults were patients with numerous comorbidities and chronic pain rather than terminal diagnoses. Given the intensive need of these patients, the authors felt that a consultation service and an interdisciplinary team that included psychosocial/spiritual/social work providers offered the most efficient method of delivering advanced palliative care needs.
The research regarding both solutions to challenges and novel methods of addressing the care gap remains sparse as evidenced by the conclusions of multiple systematic reviews and meta-analyses and the inability of the Cochrane review to find papers meeting inclusion criteria regarding techniques of community support in palliative care [14,15]. There remains a need to identify practical techniques of implementing palliative care in rural and suburban settings.
The Butler Health System Experience
In August 2015, we set out to start the first hospital-based palliative care consultation service in the Butler Health System. The health system is a nonprofit, single-hospital system anchored by Butler Memorial Hospital, a 294-bed community hospital located within a rural Pennsylvania county of 186,000 residents, 35 miles north of Pittsburgh. Butler County consists of a predominantly white, non-Hispanic population with over 15% of the residents being older than 65 years of age. The median household income is $61,000 earned primarily through blue collar occupations [16]. Driven by 53 employed primary care physicians, the health system provides services for 75,000 patients at sites covering an area of 4000 square miles. The hospital provides general medical, critical, surgical and subspecialty care and behavior health services as a regional referral center for 4 surrounding rural counties, accepting 12,500 inpatient admissions annually. A hospitalist service admits the majority of oncology patients, and the intensive care unit (ICU) is an open unit, where patients are admitted to the hospitalist, primary care, or surgical service.
While no formal needs assessment was performed prior to program development, perceptions of inadequate pain control, overuse of naloxone, underutilization of hospice services, and lack of consistent quality in end-of-life care were identified. These concerns were voiced at the levels of direct patient care on the floors, and by nursing and physician hospital leadership. Prior to our program, the chief medical officer attended the national Center to Advance Palliative Care conference to better understand the field of palliative care and its impact on improving quality of care. Concurrently, our health system was expanding its inpatient capabilities (eg, advanced neurologic and cardiac services), resulting in admissions with increased disease severity and illness complexity. With the vision of improved patient care, prioritizing quality end-of-life care and symptom management, the hospital board and administration overwhelmingly supported the development of the palliative care program, philosophically and financially.
Laying a Foundation—Funding, Collaboration, and Team Building
Funding and staffing are 2 important factors when building any program. Sources of funding for palliative care programs may include hospital support, billing revenue, grants, and philanthropy. Program development was a priority for the hospital and community. To help offset costs, efforts to raise financial support focused on utilizing the health system’s annual fundraising events. Through the generosity of individuals in the community, the hospital’s annual gala event, and donations from the hospital’s auxiliary, a total of $230,000 was raised prior to program initiation. Funds budgeted through direct hospital support and fundraising were allocated towards hiring palliative care team members and community marketing projects.
The hospital’s surrounding community is fortunate to have 2 local inpatient hospice facilities, and these relationships were imperative to providing quality end-of-life care preceding our palliative care program. A formal partnership was previously established with one while the other remains an important referral facility due to its proximity to the hospital. These hospice services are encouraged to participate in our weekly palliative care interdisciplinary team meetings. Their incorporation has improved coordination, continuity, and translation of care upon patient discharge from the acute hospital setting. Additionally, the relationships have been beneficial in tracking patients’ outcomes and data collection.
The standard structure of a palliative care team described by the Joint Commission and National Consensus Panel for Palliative Care consists of a physician, registered nurse or advanced practice provider, chaplaincy, and social work. Despite this recommendation, less than 40% of surveyed hospitals met the criteria, and less than 25% have dedicated funding to cover these positions [17]. Upon inception of our palliative care program, 2.6 funded full-time equivalents (FTEs) were allocated. These positions included a physician (1.0 FTE), a physician assistant (1.0 FTE), and a part time palliative care social worker (0.6 FTE). The 2015 National Palliative Care Registry found that 3.2 funded FTEs per 10,000 admissions is the average for hospitals with 150 to 299 beds [17]. The uncertainty of the utilization and consult volume, and the limited amount of qualified palliative care trained practitioners, resulted in the palliative program starting below this mean at 2.1 funded FTEs per 10,000 admissions. All the funded positions were located on site at the hospital. The pre-existing volunteer hospital chaplain service was identified as the pastoral care component for the program.
Increased FTEs have been associated with increased palliative care service penetration and ultimately in decreased time to consult [18]. In response to increasing consult volumes, concerns for delays in time to consult, and in preparation for expansion to an outpatient service, the palliative care department acquired an additional funded physician FTE (1.0). Ultimately the service reached a total of 3.6 FTE for inpatient services during its first 12 months; proportionately this resulted in an increase to 2.9 FTE per 10,000 admissions based on the yearly admission rate of 12,500 patients.
Educational Outreach
The success of a palliative care program depends on other clinicians’ acceptance and referral to the clinical program. We took a 2-pronged approach, focusing on both hospital-based and community-based education. The hospital-based nursing education included 30-minute presentations on general overviews of palliative care, differences between palliative care and hospice, and acute symptom management at the end of life. The palliative care team presented to all medical, surgical, and intensive care units and encompassed all shifts of nursing staff. These lectures included pre- and post-tests to assess for impact and feedback. Similar educational presentations, as well as an hour-long presentation on opioids and palliative care, were available for physicians for CME opportunities. We also distributed concise palliative care referral packets to outpatient primary care offices through the health system’s marketing team. The referral packets included examples of diagnoses, clinical scenarios, and symptoms to assist in the physicians’ understanding of palliative care services. The palliative care team also met with clinic office managers to discuss the program and answer questions.
There were also educational opportunities for patients and families in our community. Taking advantage of previously developed partnerships between the hospital system and local media outlets, the palliative care team performed local radio spots to educate the community on topics including an overview of palliative care, how to request palliative care, and the difference between palliative care and hospice care. We partnered with a local hospice agency and developed a well-received bereavement seminar for patients, family members, and employees and included the topic of advanced care planning.
Data Collection
We collect data using 2 different tools: a self-maintained spreadsheet shared between our palliative care clinicians, and a collective data tool (QDACT) included in our membership with and maintained by the Global Palliative Care Quality Alliance. Data collected and tracked in our spreadsheet includes date of consult, patient age, primary and secondary diagnoses, disposition, goals of care discussions, date of death, and 30-day readmissions. Through the QDACT data monitoring program, we are tracking and analyzing quality measures including symptom assessment and management and code status conversion. The QDACT database also provides financial data specific to our institution such as cost savings based on our billing, readmission rates, and length of stay.
Results
Projections, Volumes, and Penetration
Prior to the start of our program, our chief medical office used Center to Advance Palliative Care tools to project inpatient consultation volumes at our institution. Variables that are recommended by this center to guide projections include number of hospital admissions per year, hospital occupancy, disposition to hospice, as well as generalized estimations of inpatient mortality rates. Based on our data, it was expected that our program would receive 204 new inpatient consults in our first year, and 774 follow-up visits. Our actual new inpatient consults totaled 516, with 919 follow-up visits. Palliative care penetration (percentage of annual hospital admissions seen by the palliative care team) our first year was 3.7% (Table 1).
Consultation Demographics
The demographics of the patients seen by the palliative care team reflect that of Butler County’s Medicare fee-for-service (FFS) population (Table 2); however, differences were seen at the state and national level with regard to ethnicity (Table 2).
Almost half of consultations (49%) were placed by the hospitalist service. Since the ICU is an open unit, critical care consults are not adequately reflected by analysis of the ordering physician alone. Analysis of consultation location revealed that 27% of inpatient consults were located within the ICU.
Patient Outcomes and Disposition
Outcomes and discharge data from the first year were collected and reviewed. Ten percent of the patients seen by palliative care died in the hospital, and 51% of patients that were seen by palliative care died within the program’s first year. Thirty-seven percent of patients discharged from the hospital utilized hospice services at home, in residential nursing facility, or at an inpatient hospice unit. The remaining 53% were discharged without hospice services to home or facility (Figure).
Hospice utilization by the health system increased during our first year. Compared to the 2014 calendar year, there were a total of 263 referrals for hospice services. During the first year of the palliative care program, which started August 2015, there were a total of 293 referrals. Of the 293 total hospice referrals, 190 (64.8%) of these referrals were for patients seen by the palliative care team.
Change of Code Status
Code status and changes in codes status data were collected. Of 462 individual patients prior to or at the time of palliative care consults, 43% were full code, 4% limited code, 8% unknown status, and 45% Do Not Resuscitate. After palliative care consult, 61% of the patients who were previously full/limited/unknown converted to do not resuscitate and do not intubate status. In total, 79% of patients seen by palliative care had a confirmed code status of Do Not Resuscitate and Do Not Intubate status after consult.
Discussion
In our first year, our palliative care program exceeded the expected number of inpatient consults, corresponding with a penetration of 3.7%. With the increase of funded FTEs, preliminary data shows that the department’s penetration continues to rise remaining consistent with the data and expectations [18]. During the second year, it is anticipated that over 600 inpatient palliative care consultations will be performed with an estimated penetration of 4%. This increasing penetration reflects the rising utilization of palliative care within our hospital. Since inception of the program, the service has expanded into an outpatient clinic 2 days per week. The palliative care clinic is staffed by a registered nurse (funded 0.6 FTE) and covered by the same physicians and physician assistant providing the inpatient services. The department acquired an unfunded but designated chaplaincy volunteer to assist with patients’ spiritual needs. We believe that the success of our program during the first year was related to multiple factors: a focus of integration and education by the palliative care department, health system administration buy-in, and identification of surrounding community needs.
In addition to patient care, our palliative care department also prioritizes “tangible” impacts to better establish our contributions to the health system. We have done this through participation on hospital committees, hospital policy revision teams, and by developing innovative solutions such as a terminal extubation protocol and order set for our ICU. The health system and its administration have recognized the importance of educating nursing and physician staff on palliative care services, and have supported these continued efforts alongside our clinical obligations.
Concurrent with administration buy-in, financial supports for our palliative care services were initially supplemented by the health system. Our department understands the importance of recognizing limitations of resources in communities and their hospitals. In efforts to minimize the department’s impact on our own health system’s financial resources, we have strived to offset our costs. We helped the hospital system meet pay-for-performance palliative care metrics set by the large local insurers resulting in financial hospital reimbursement valued at $600,000 in 2016.
The question of how the program may translate into other communities raises a major limitation: the homogeneity our population. The community surrounding the hospital is primarily Caucasian, with minimal representation of minority populations. While the patient population seen by our palliative care team is reflective of our surrounding county, it does not represent Medicare FFS beneficiaries on a national level or many other types of community hospitals across the country. Variations of ethnicity, age, diagnoses, and faith are fundamental, which highlights the importance of understanding the community in which a program is developed.
The rising trajectory of our palliative care service utilization has prompted a discussion of future endeavors for our program. Expectations for a continued shortage of hospice and palliative care physicians [19] and concerns for practitioner burnout [20] underlie our thoughtful approach to expansion of inpatient and outpatient services. At this time, potential projects include a consultation trigger system and incorporation of palliative care providers in ICU rounding, as well as possible expansion of outpatient services through implantation of an advanced practitioner into surrounding nursing homes and primary care offices.
We have found a growing utilization of our program at Butler Health System. Our first year experience has highlighted the importance of identifying community and hospital administrative champions as a foundation. Additionally, understanding the specific characteristics of one’s surrounding community may allow for improved integration and acceptance of palliative care in a community setting. Our program continues to work with the health system, community, and philanthropic organizations to expand the ever-growing need for palliative care services.
From the Division of Palliative Care, Butler Health System, Butler, PA (Drs. Stein, Reefer, Selvaggi, Ms. Doverspike); the University of Pittsburgh Medical Center, Pittsburgh, PA (Dr. Rajagopal); and the Duke Cancer Institute and Duke Fuqua School of Business, Durham, NC (Dr. Kamal).
Abstract
- Objective: To describe an approach to develop a community-centric palliative care program in a rural community health system and to review data collected over the program’s first year.
- Methods: We describe the underlying foundations of our program development including the health system’s prioritization of a palliative care program, funding opportunities, collaboration with community supports, and the importance of building a team and program that reflects a community’s needs. Data were collected through a program-maintained spreadsheet and a data monitoring system available through the Global Palliative Care Quality Alliance.
- Results: 516 new inpatient consultations were seen during the first year, for a penetration of 3.7%. The demographics of the patients who received consultation reflect that of the surrounding community. Over 50% of patients seen within the first year died, and hospice utilization at home and within facilities and inpatient hospice units increased. In addition, 79% of the patients seen by the palliative care team had a confirmed code status of do not resuscitate and do not intubate.
- Conclusions: Butler Health System’s approach to development of a palliative care program has resulted in increasing utilization of palliative care services in the hospital. Having hospital administration support, community support, and understanding the individualized needs of a community has been essential for the program’s expansion.
Key words: palliative care; program development; community hospital; rural.
Since its inception, palliative care has been committed to providing specialty-level consultation services to individuals with serious illness and their loved ones. The field has focused heavily on growth and acceptance, consistently moving upstream with regards to illness trajectory, across diseases, and across demographic variables such as age (eg, pediatric quality of life programs) and race (eg, community outreach programs addressing racial disparities in hospice use). An important frontier that remains challenging for much of the field is expansion into the community setting, where resources, implicit acceptance, and patient populations may vary.
As health system leaders appreciate the positive impacts palliative medicine on patient care and care quality, barriers to implementing palliative care programs in community hospitals must be addressed in ways tailored to the unique needs of smaller organizations and their communities. The goal of this paper is to outline the approach taken to develop Butler Health System’s community-centric palliative care program, describe our program’s underlying foundation rooted in community supports, and recount steps we have taken thus far to impact patient care in our hospital, health system, and community through the program’s first year.
Community Hospital Palliative Care—The Necessity and the Challenges
Palliative care has made strides in its growth and acceptance in the last decade; yet, the distribution of that growth has been skewed. Although 67% of hospitals now report access to specialist palliative care programs, most of the 148% growth over the last decade has been actualized in larger hospitals. Ninety percent of hospitals with greater than 300 beds report palliative care service availability whereas only 56% of small hospitals were identified to have this specialty care [1].
The inequity of access is also seen in other countries. A recent Canadian study retrospectively examined access to care of 23,860 deceased patients in Nova Scotia. Although they found 40.9% of study subjects were enrolled in a palliative care program at urban, academic centers, patients in a rural setting were only a third as likely to be enrolled in a palliative care program [2]. This access gap has important effects on patient-level outcomes, as evidence has consistently demonstrated that patients in rural settings who receive palliative care have decreased unnecessary hospitalizations and less in-hospital deaths [3].
While evidence of improved outcomes is strong, important barriers stand in the way. In a 2013 study, 374 health care providers at 236 rural hospitals in 7 states were interviewed to determine barriers to providing palliative care in rural settings. Barriers identified include a lack of administrative support, access to basic palliative care training for primary care physicians, and limited relationships to hospices [4]. Additional challenges include lack of access to tertiary-level specialty clinicians, access to and misconceptions about prescription medications, transportation for patients and providers, and incorporating a patient’s community supports [5–7].
Proposed Solutions
Techniques to improve palliative care access for rural and community centers that have been previously reviewed in the literature include videoconferencing with tertiary care experts in palliative care and education through small community-level lectures [8–10]. Goals of rural and suburban palliative care programs are broadly similar to programs at academic medical centers; however, few studies have identified impact of palliative medicine on patient care in community settings. In one suburban practice, a study found that patients were more likely to die at home if they had multiple caregivers, increased length of time under palliative care, and older age upon referral [11].
The United States has few large-scale pilot programs attempting to address the palliative needs of a more suburban or rural population. Of these, the Minnesota Rural Palliative Care Initiative developed by Stratis Health is perhaps the best publicized. Stratis Health developed and led an 18-month learning collaborative from October 2008 to April 2010 through which community teams developed or improved palliative care services. Through this initiative, a community-based health care practice model was developed that took advantage of the strong interrelationships within rural communities. After 18 months, 6 out of 10 rural Minnesota communities had formal palliative care programs, and 8 to 9 out of 10 had capabilities to at least address advance directives as well as provider and community education [12]. In another initiative, the NIH established a new suburban clinic with tertiary providers specifically for resource intensive, underserved patients [13]. The clinic was established by partnering with a service that was already in place in the community. Twenty-seven patients were seen within 7 months. The most common consults were patients with numerous comorbidities and chronic pain rather than terminal diagnoses. Given the intensive need of these patients, the authors felt that a consultation service and an interdisciplinary team that included psychosocial/spiritual/social work providers offered the most efficient method of delivering advanced palliative care needs.
The research regarding both solutions to challenges and novel methods of addressing the care gap remains sparse as evidenced by the conclusions of multiple systematic reviews and meta-analyses and the inability of the Cochrane review to find papers meeting inclusion criteria regarding techniques of community support in palliative care [14,15]. There remains a need to identify practical techniques of implementing palliative care in rural and suburban settings.
The Butler Health System Experience
In August 2015, we set out to start the first hospital-based palliative care consultation service in the Butler Health System. The health system is a nonprofit, single-hospital system anchored by Butler Memorial Hospital, a 294-bed community hospital located within a rural Pennsylvania county of 186,000 residents, 35 miles north of Pittsburgh. Butler County consists of a predominantly white, non-Hispanic population with over 15% of the residents being older than 65 years of age. The median household income is $61,000 earned primarily through blue collar occupations [16]. Driven by 53 employed primary care physicians, the health system provides services for 75,000 patients at sites covering an area of 4000 square miles. The hospital provides general medical, critical, surgical and subspecialty care and behavior health services as a regional referral center for 4 surrounding rural counties, accepting 12,500 inpatient admissions annually. A hospitalist service admits the majority of oncology patients, and the intensive care unit (ICU) is an open unit, where patients are admitted to the hospitalist, primary care, or surgical service.
While no formal needs assessment was performed prior to program development, perceptions of inadequate pain control, overuse of naloxone, underutilization of hospice services, and lack of consistent quality in end-of-life care were identified. These concerns were voiced at the levels of direct patient care on the floors, and by nursing and physician hospital leadership. Prior to our program, the chief medical officer attended the national Center to Advance Palliative Care conference to better understand the field of palliative care and its impact on improving quality of care. Concurrently, our health system was expanding its inpatient capabilities (eg, advanced neurologic and cardiac services), resulting in admissions with increased disease severity and illness complexity. With the vision of improved patient care, prioritizing quality end-of-life care and symptom management, the hospital board and administration overwhelmingly supported the development of the palliative care program, philosophically and financially.
Laying a Foundation—Funding, Collaboration, and Team Building
Funding and staffing are 2 important factors when building any program. Sources of funding for palliative care programs may include hospital support, billing revenue, grants, and philanthropy. Program development was a priority for the hospital and community. To help offset costs, efforts to raise financial support focused on utilizing the health system’s annual fundraising events. Through the generosity of individuals in the community, the hospital’s annual gala event, and donations from the hospital’s auxiliary, a total of $230,000 was raised prior to program initiation. Funds budgeted through direct hospital support and fundraising were allocated towards hiring palliative care team members and community marketing projects.
The hospital’s surrounding community is fortunate to have 2 local inpatient hospice facilities, and these relationships were imperative to providing quality end-of-life care preceding our palliative care program. A formal partnership was previously established with one while the other remains an important referral facility due to its proximity to the hospital. These hospice services are encouraged to participate in our weekly palliative care interdisciplinary team meetings. Their incorporation has improved coordination, continuity, and translation of care upon patient discharge from the acute hospital setting. Additionally, the relationships have been beneficial in tracking patients’ outcomes and data collection.
The standard structure of a palliative care team described by the Joint Commission and National Consensus Panel for Palliative Care consists of a physician, registered nurse or advanced practice provider, chaplaincy, and social work. Despite this recommendation, less than 40% of surveyed hospitals met the criteria, and less than 25% have dedicated funding to cover these positions [17]. Upon inception of our palliative care program, 2.6 funded full-time equivalents (FTEs) were allocated. These positions included a physician (1.0 FTE), a physician assistant (1.0 FTE), and a part time palliative care social worker (0.6 FTE). The 2015 National Palliative Care Registry found that 3.2 funded FTEs per 10,000 admissions is the average for hospitals with 150 to 299 beds [17]. The uncertainty of the utilization and consult volume, and the limited amount of qualified palliative care trained practitioners, resulted in the palliative program starting below this mean at 2.1 funded FTEs per 10,000 admissions. All the funded positions were located on site at the hospital. The pre-existing volunteer hospital chaplain service was identified as the pastoral care component for the program.
Increased FTEs have been associated with increased palliative care service penetration and ultimately in decreased time to consult [18]. In response to increasing consult volumes, concerns for delays in time to consult, and in preparation for expansion to an outpatient service, the palliative care department acquired an additional funded physician FTE (1.0). Ultimately the service reached a total of 3.6 FTE for inpatient services during its first 12 months; proportionately this resulted in an increase to 2.9 FTE per 10,000 admissions based on the yearly admission rate of 12,500 patients.
Educational Outreach
The success of a palliative care program depends on other clinicians’ acceptance and referral to the clinical program. We took a 2-pronged approach, focusing on both hospital-based and community-based education. The hospital-based nursing education included 30-minute presentations on general overviews of palliative care, differences between palliative care and hospice, and acute symptom management at the end of life. The palliative care team presented to all medical, surgical, and intensive care units and encompassed all shifts of nursing staff. These lectures included pre- and post-tests to assess for impact and feedback. Similar educational presentations, as well as an hour-long presentation on opioids and palliative care, were available for physicians for CME opportunities. We also distributed concise palliative care referral packets to outpatient primary care offices through the health system’s marketing team. The referral packets included examples of diagnoses, clinical scenarios, and symptoms to assist in the physicians’ understanding of palliative care services. The palliative care team also met with clinic office managers to discuss the program and answer questions.
There were also educational opportunities for patients and families in our community. Taking advantage of previously developed partnerships between the hospital system and local media outlets, the palliative care team performed local radio spots to educate the community on topics including an overview of palliative care, how to request palliative care, and the difference between palliative care and hospice care. We partnered with a local hospice agency and developed a well-received bereavement seminar for patients, family members, and employees and included the topic of advanced care planning.
Data Collection
We collect data using 2 different tools: a self-maintained spreadsheet shared between our palliative care clinicians, and a collective data tool (QDACT) included in our membership with and maintained by the Global Palliative Care Quality Alliance. Data collected and tracked in our spreadsheet includes date of consult, patient age, primary and secondary diagnoses, disposition, goals of care discussions, date of death, and 30-day readmissions. Through the QDACT data monitoring program, we are tracking and analyzing quality measures including symptom assessment and management and code status conversion. The QDACT database also provides financial data specific to our institution such as cost savings based on our billing, readmission rates, and length of stay.
Results
Projections, Volumes, and Penetration
Prior to the start of our program, our chief medical office used Center to Advance Palliative Care tools to project inpatient consultation volumes at our institution. Variables that are recommended by this center to guide projections include number of hospital admissions per year, hospital occupancy, disposition to hospice, as well as generalized estimations of inpatient mortality rates. Based on our data, it was expected that our program would receive 204 new inpatient consults in our first year, and 774 follow-up visits. Our actual new inpatient consults totaled 516, with 919 follow-up visits. Palliative care penetration (percentage of annual hospital admissions seen by the palliative care team) our first year was 3.7% (Table 1).
Consultation Demographics
The demographics of the patients seen by the palliative care team reflect that of Butler County’s Medicare fee-for-service (FFS) population (Table 2); however, differences were seen at the state and national level with regard to ethnicity (Table 2).
Almost half of consultations (49%) were placed by the hospitalist service. Since the ICU is an open unit, critical care consults are not adequately reflected by analysis of the ordering physician alone. Analysis of consultation location revealed that 27% of inpatient consults were located within the ICU.
Patient Outcomes and Disposition
Outcomes and discharge data from the first year were collected and reviewed. Ten percent of the patients seen by palliative care died in the hospital, and 51% of patients that were seen by palliative care died within the program’s first year. Thirty-seven percent of patients discharged from the hospital utilized hospice services at home, in residential nursing facility, or at an inpatient hospice unit. The remaining 53% were discharged without hospice services to home or facility (Figure).
Hospice utilization by the health system increased during our first year. Compared to the 2014 calendar year, there were a total of 263 referrals for hospice services. During the first year of the palliative care program, which started August 2015, there were a total of 293 referrals. Of the 293 total hospice referrals, 190 (64.8%) of these referrals were for patients seen by the palliative care team.
Change of Code Status
Code status and changes in codes status data were collected. Of 462 individual patients prior to or at the time of palliative care consults, 43% were full code, 4% limited code, 8% unknown status, and 45% Do Not Resuscitate. After palliative care consult, 61% of the patients who were previously full/limited/unknown converted to do not resuscitate and do not intubate status. In total, 79% of patients seen by palliative care had a confirmed code status of Do Not Resuscitate and Do Not Intubate status after consult.
Discussion
In our first year, our palliative care program exceeded the expected number of inpatient consults, corresponding with a penetration of 3.7%. With the increase of funded FTEs, preliminary data shows that the department’s penetration continues to rise remaining consistent with the data and expectations [18]. During the second year, it is anticipated that over 600 inpatient palliative care consultations will be performed with an estimated penetration of 4%. This increasing penetration reflects the rising utilization of palliative care within our hospital. Since inception of the program, the service has expanded into an outpatient clinic 2 days per week. The palliative care clinic is staffed by a registered nurse (funded 0.6 FTE) and covered by the same physicians and physician assistant providing the inpatient services. The department acquired an unfunded but designated chaplaincy volunteer to assist with patients’ spiritual needs. We believe that the success of our program during the first year was related to multiple factors: a focus of integration and education by the palliative care department, health system administration buy-in, and identification of surrounding community needs.
In addition to patient care, our palliative care department also prioritizes “tangible” impacts to better establish our contributions to the health system. We have done this through participation on hospital committees, hospital policy revision teams, and by developing innovative solutions such as a terminal extubation protocol and order set for our ICU. The health system and its administration have recognized the importance of educating nursing and physician staff on palliative care services, and have supported these continued efforts alongside our clinical obligations.
Concurrent with administration buy-in, financial supports for our palliative care services were initially supplemented by the health system. Our department understands the importance of recognizing limitations of resources in communities and their hospitals. In efforts to minimize the department’s impact on our own health system’s financial resources, we have strived to offset our costs. We helped the hospital system meet pay-for-performance palliative care metrics set by the large local insurers resulting in financial hospital reimbursement valued at $600,000 in 2016.
The question of how the program may translate into other communities raises a major limitation: the homogeneity our population. The community surrounding the hospital is primarily Caucasian, with minimal representation of minority populations. While the patient population seen by our palliative care team is reflective of our surrounding county, it does not represent Medicare FFS beneficiaries on a national level or many other types of community hospitals across the country. Variations of ethnicity, age, diagnoses, and faith are fundamental, which highlights the importance of understanding the community in which a program is developed.
The rising trajectory of our palliative care service utilization has prompted a discussion of future endeavors for our program. Expectations for a continued shortage of hospice and palliative care physicians [19] and concerns for practitioner burnout [20] underlie our thoughtful approach to expansion of inpatient and outpatient services. At this time, potential projects include a consultation trigger system and incorporation of palliative care providers in ICU rounding, as well as possible expansion of outpatient services through implantation of an advanced practitioner into surrounding nursing homes and primary care offices.
We have found a growing utilization of our program at Butler Health System. Our first year experience has highlighted the importance of identifying community and hospital administrative champions as a foundation. Additionally, understanding the specific characteristics of one’s surrounding community may allow for improved integration and acceptance of palliative care in a community setting. Our program continues to work with the health system, community, and philanthropic organizations to expand the ever-growing need for palliative care services.
1. Dumanovsky T, Augustin R, Rogers M, et al. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med 2016;19:8–15.
2. Lavergne MR, Lethbridge L, Johnston G, et al. Examining palliative care program use and place of death in rural and urban contexts: a Canadian population-based study using linked data. Rural Remote Health 2015;15:3134.
3. Seow H, Brazil K, Sussman J, et al. Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: a pooled analysis. BMJ 2014;348:g3496.
4. Fink RM, Oman KS, Youngwerth J, et al. A palliative care needs assessment of rural hospitals. J Palliat Med 2013;16:638–44.
5. Dumont S, Jacobs P, Turcotte V, et al. Palliative care costs in Canada: A descriptive comparison of studies of urban and rural patients near end of life. J Palliat Med 2015;29:908–17.
6. Kaasalainen S, Brazil K, Williams A, et al. Nurses' experiences providing palliative care to individuals living in rural communities: aspects of the physical residential setting. Rural Remote Health 2014;14:2728.
7. Ahmed N, Bestall JC, Ahmedzai SH, et al. Systematic review of the problems and issues of accessing specialist palliative care by patients, carers and health and social care professionals. J Palliat Med 2004;18:525–42.
8. Ray RA, Fried O, Lindsay D. Palliative care professional education via video conference builds confidence to deliver palliative care in rural and remote locations. BMC Health Serv Res 2014;14:272.
9. Bakitas MA, Elk R, Astin M, et al. Systematic review of palliative care in the rural setting. Cancer Control 2015;22:450–64.
10. Akiyama M, Hirai K, Takebayashi T, et al. The effects of community-wide dissemination of information on perceptions of palliative care, knowledge about opioids, and sense of security among cancer patients, their families, and the general public. Support Care Cancer 2016;24: 347–56.
11. Maida V. Factors that promote success in home palliative care: a study of a large suburban palliative care practice. J Palliat Care 2002;18:282–6.
12. Ceronsky L, Shearer J, Weng K, et al. Minnesota Rural Palliative Care Initiative: building palliative care capacity in rural Minnesota. J Palliat Med 2013;16:310–3.
13. Aggarwal SK, Ghosh A, Cheng MJ, et al. Initiating pain and palliative care outpatient services for the suburban underserved in Montgomery County, Maryland: Lessons learned at the NIH Clinical Center and MobileMed. Palliat Support Care 2015;16:1–6.
14. Rainsford S, MacLeod RD, Glasgow NJ. Place of death in rural palliative care: A systematic review. J Palliat Med 2016;30:745–63.
15. Horey D, Street AF, O'Connor M, et al. Training and supportive programs for palliative care volunteers in community settings. Cochrane Database Syst Rev 2015 Jul 20;(7):CD009500.
16. The United States Census Bureau: QuickFacts: Butler County, Pennsylvania. Accessed 10 Mar 2017 at www.census.gov/quickfacts/table/PST045216/42019,00.
17. Spetz J, Dudley N, Trupin L, et al. Few hospital palliative care programs meet national staffing recommendations. Health Aff 2016;35:1690–7.
18. Dumanovsky T, Rogers M, Spragens LH, et al. Impact of Staffing on Access to Palliative Care in U.S. Hospitals. J Palliat Med. 2015 Dec; 18(12). Pages 998-999.
19. Lupu D, American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage 2010;40:899–911.
20. Kamal AH, Bull JK, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manage 2016;51:690–6.
1. Dumanovsky T, Augustin R, Rogers M, et al. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med 2016;19:8–15.
2. Lavergne MR, Lethbridge L, Johnston G, et al. Examining palliative care program use and place of death in rural and urban contexts: a Canadian population-based study using linked data. Rural Remote Health 2015;15:3134.
3. Seow H, Brazil K, Sussman J, et al. Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: a pooled analysis. BMJ 2014;348:g3496.
4. Fink RM, Oman KS, Youngwerth J, et al. A palliative care needs assessment of rural hospitals. J Palliat Med 2013;16:638–44.
5. Dumont S, Jacobs P, Turcotte V, et al. Palliative care costs in Canada: A descriptive comparison of studies of urban and rural patients near end of life. J Palliat Med 2015;29:908–17.
6. Kaasalainen S, Brazil K, Williams A, et al. Nurses' experiences providing palliative care to individuals living in rural communities: aspects of the physical residential setting. Rural Remote Health 2014;14:2728.
7. Ahmed N, Bestall JC, Ahmedzai SH, et al. Systematic review of the problems and issues of accessing specialist palliative care by patients, carers and health and social care professionals. J Palliat Med 2004;18:525–42.
8. Ray RA, Fried O, Lindsay D. Palliative care professional education via video conference builds confidence to deliver palliative care in rural and remote locations. BMC Health Serv Res 2014;14:272.
9. Bakitas MA, Elk R, Astin M, et al. Systematic review of palliative care in the rural setting. Cancer Control 2015;22:450–64.
10. Akiyama M, Hirai K, Takebayashi T, et al. The effects of community-wide dissemination of information on perceptions of palliative care, knowledge about opioids, and sense of security among cancer patients, their families, and the general public. Support Care Cancer 2016;24: 347–56.
11. Maida V. Factors that promote success in home palliative care: a study of a large suburban palliative care practice. J Palliat Care 2002;18:282–6.
12. Ceronsky L, Shearer J, Weng K, et al. Minnesota Rural Palliative Care Initiative: building palliative care capacity in rural Minnesota. J Palliat Med 2013;16:310–3.
13. Aggarwal SK, Ghosh A, Cheng MJ, et al. Initiating pain and palliative care outpatient services for the suburban underserved in Montgomery County, Maryland: Lessons learned at the NIH Clinical Center and MobileMed. Palliat Support Care 2015;16:1–6.
14. Rainsford S, MacLeod RD, Glasgow NJ. Place of death in rural palliative care: A systematic review. J Palliat Med 2016;30:745–63.
15. Horey D, Street AF, O'Connor M, et al. Training and supportive programs for palliative care volunteers in community settings. Cochrane Database Syst Rev 2015 Jul 20;(7):CD009500.
16. The United States Census Bureau: QuickFacts: Butler County, Pennsylvania. Accessed 10 Mar 2017 at www.census.gov/quickfacts/table/PST045216/42019,00.
17. Spetz J, Dudley N, Trupin L, et al. Few hospital palliative care programs meet national staffing recommendations. Health Aff 2016;35:1690–7.
18. Dumanovsky T, Rogers M, Spragens LH, et al. Impact of Staffing on Access to Palliative Care in U.S. Hospitals. J Palliat Med. 2015 Dec; 18(12). Pages 998-999.
19. Lupu D, American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage 2010;40:899–911.
20. Kamal AH, Bull JK, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manage 2016;51:690–6.