User login
Flu Shot Reminders Improve Use in Heart Attack Survivors
, showed the NUDGE FLU series of clinical trials.
Influenza has the potential to be a dangerous infection on its own, but it increases the risk for cardiovascular events among people with a history of heart attack, said the study’s lead author, Ankeet Bhatt, MD, a cardiologist at Kaiser Permanente San Francisco Medical Center, San Francisco.
“Yearly influenza vaccines help prevent influenza infection and, in patients with a heart attack, are potentially cardioprotective,” he said during his presentation at the American Heart Association (AHA) Scientific Sessions 2024 in Chicago. The NUDGE FLU results were simultaneously published online in JAMA Cardiology.
In Denmark, where the trials were conducted, about 80% of older adults get flu shots, but only about 40% of younger adults with chronic diseases do, Bhatt reported. In the United States, about 45% of adults and 55% of children received at least one dose of the flu vaccine in the 2023/24 flu season, according to the US Centers for Disease Control and Prevention (CDC).
The NUDGE FLU Trials
Bhatt and his colleagues conducted three related clinical trials during the 2022/23 and 2023/24 flu seasons: NUDGE-FLU and NUDGE-FLU-2 targeted older adults, whereas NUDGE-FLU-CHRONIC targeted younger adults with chronic diseases. Nearly 2 million people were involved in the three trials.
Participants were randomized to receive one of a series of different behavioral-science-informed letters, delivered through a government-run electronic communication system, or no reminder.
People who received any of the nudges had higher rates of vaccination; among heart attack survivors, there was a 1.8% improvement and among adults without a history of heart attack, there was a 1.3% improvement. But a nudge that explained the potential cardiovascular benefits of flu shots was even more effective, leading to a 3.9% increase among people with a history of heart attack and a 2% increase among those with no heart attack history.
“A simple sentence resulted in a durable improvement in the vaccination rate,” said Bhatt.
The effect was even greater among those who had not been vaccinated in the previous flu season. Among heart attack survivors, nearly 14% more people got the vaccine compared with just 1.5% more survivors who were previously vaccinated. And it was most effective among younger adults who had experienced a recent heart attack, resulting in a 26% increase.
“The impact was larger in patients with a history of acute myocardial infarction, in those who were vaccine-hesitant, and in younger people” — all groups with the most to gain from vaccination in terms of cardiovascular protection — Bhatt reported.
About 25% of people in the United States are unsure about whether to get a flu shot, said Orly Vardeny, PharmD, professor of medicine at the University of Minnesota Medical School in Minneapolis, who was not involved in the study. The fact that previously unvaccinated people were convinced by the nudges is reassuring. “That’s the group where this intervention is most likely to move the needle,” she said.
Around half of all people hospitalized for flu in the United States have cardiovascular disease, CDC data showed, so “even a small increase in the number of patients who get vaccinated has substantial public health benefits,” Vardeny said.
The NUDGE FLU series showed that nudges like this should be employed as a simple tool to improve vaccination rates, but the system would be much more difficult to implement in the United States, Bhatt said.
Denmark has a national health service and a preexisting government electronic communication system, whereas the US system is privately run and more fractured. It would be possible to make it work, he pointed out, but would take some effort.
A version of this article first appeared on Medscape.com.
, showed the NUDGE FLU series of clinical trials.
Influenza has the potential to be a dangerous infection on its own, but it increases the risk for cardiovascular events among people with a history of heart attack, said the study’s lead author, Ankeet Bhatt, MD, a cardiologist at Kaiser Permanente San Francisco Medical Center, San Francisco.
“Yearly influenza vaccines help prevent influenza infection and, in patients with a heart attack, are potentially cardioprotective,” he said during his presentation at the American Heart Association (AHA) Scientific Sessions 2024 in Chicago. The NUDGE FLU results were simultaneously published online in JAMA Cardiology.
In Denmark, where the trials were conducted, about 80% of older adults get flu shots, but only about 40% of younger adults with chronic diseases do, Bhatt reported. In the United States, about 45% of adults and 55% of children received at least one dose of the flu vaccine in the 2023/24 flu season, according to the US Centers for Disease Control and Prevention (CDC).
The NUDGE FLU Trials
Bhatt and his colleagues conducted three related clinical trials during the 2022/23 and 2023/24 flu seasons: NUDGE-FLU and NUDGE-FLU-2 targeted older adults, whereas NUDGE-FLU-CHRONIC targeted younger adults with chronic diseases. Nearly 2 million people were involved in the three trials.
Participants were randomized to receive one of a series of different behavioral-science-informed letters, delivered through a government-run electronic communication system, or no reminder.
People who received any of the nudges had higher rates of vaccination; among heart attack survivors, there was a 1.8% improvement and among adults without a history of heart attack, there was a 1.3% improvement. But a nudge that explained the potential cardiovascular benefits of flu shots was even more effective, leading to a 3.9% increase among people with a history of heart attack and a 2% increase among those with no heart attack history.
“A simple sentence resulted in a durable improvement in the vaccination rate,” said Bhatt.
The effect was even greater among those who had not been vaccinated in the previous flu season. Among heart attack survivors, nearly 14% more people got the vaccine compared with just 1.5% more survivors who were previously vaccinated. And it was most effective among younger adults who had experienced a recent heart attack, resulting in a 26% increase.
“The impact was larger in patients with a history of acute myocardial infarction, in those who were vaccine-hesitant, and in younger people” — all groups with the most to gain from vaccination in terms of cardiovascular protection — Bhatt reported.
About 25% of people in the United States are unsure about whether to get a flu shot, said Orly Vardeny, PharmD, professor of medicine at the University of Minnesota Medical School in Minneapolis, who was not involved in the study. The fact that previously unvaccinated people were convinced by the nudges is reassuring. “That’s the group where this intervention is most likely to move the needle,” she said.
Around half of all people hospitalized for flu in the United States have cardiovascular disease, CDC data showed, so “even a small increase in the number of patients who get vaccinated has substantial public health benefits,” Vardeny said.
The NUDGE FLU series showed that nudges like this should be employed as a simple tool to improve vaccination rates, but the system would be much more difficult to implement in the United States, Bhatt said.
Denmark has a national health service and a preexisting government electronic communication system, whereas the US system is privately run and more fractured. It would be possible to make it work, he pointed out, but would take some effort.
A version of this article first appeared on Medscape.com.
, showed the NUDGE FLU series of clinical trials.
Influenza has the potential to be a dangerous infection on its own, but it increases the risk for cardiovascular events among people with a history of heart attack, said the study’s lead author, Ankeet Bhatt, MD, a cardiologist at Kaiser Permanente San Francisco Medical Center, San Francisco.
“Yearly influenza vaccines help prevent influenza infection and, in patients with a heart attack, are potentially cardioprotective,” he said during his presentation at the American Heart Association (AHA) Scientific Sessions 2024 in Chicago. The NUDGE FLU results were simultaneously published online in JAMA Cardiology.
In Denmark, where the trials were conducted, about 80% of older adults get flu shots, but only about 40% of younger adults with chronic diseases do, Bhatt reported. In the United States, about 45% of adults and 55% of children received at least one dose of the flu vaccine in the 2023/24 flu season, according to the US Centers for Disease Control and Prevention (CDC).
The NUDGE FLU Trials
Bhatt and his colleagues conducted three related clinical trials during the 2022/23 and 2023/24 flu seasons: NUDGE-FLU and NUDGE-FLU-2 targeted older adults, whereas NUDGE-FLU-CHRONIC targeted younger adults with chronic diseases. Nearly 2 million people were involved in the three trials.
Participants were randomized to receive one of a series of different behavioral-science-informed letters, delivered through a government-run electronic communication system, or no reminder.
People who received any of the nudges had higher rates of vaccination; among heart attack survivors, there was a 1.8% improvement and among adults without a history of heart attack, there was a 1.3% improvement. But a nudge that explained the potential cardiovascular benefits of flu shots was even more effective, leading to a 3.9% increase among people with a history of heart attack and a 2% increase among those with no heart attack history.
“A simple sentence resulted in a durable improvement in the vaccination rate,” said Bhatt.
The effect was even greater among those who had not been vaccinated in the previous flu season. Among heart attack survivors, nearly 14% more people got the vaccine compared with just 1.5% more survivors who were previously vaccinated. And it was most effective among younger adults who had experienced a recent heart attack, resulting in a 26% increase.
“The impact was larger in patients with a history of acute myocardial infarction, in those who were vaccine-hesitant, and in younger people” — all groups with the most to gain from vaccination in terms of cardiovascular protection — Bhatt reported.
About 25% of people in the United States are unsure about whether to get a flu shot, said Orly Vardeny, PharmD, professor of medicine at the University of Minnesota Medical School in Minneapolis, who was not involved in the study. The fact that previously unvaccinated people were convinced by the nudges is reassuring. “That’s the group where this intervention is most likely to move the needle,” she said.
Around half of all people hospitalized for flu in the United States have cardiovascular disease, CDC data showed, so “even a small increase in the number of patients who get vaccinated has substantial public health benefits,” Vardeny said.
The NUDGE FLU series showed that nudges like this should be employed as a simple tool to improve vaccination rates, but the system would be much more difficult to implement in the United States, Bhatt said.
Denmark has a national health service and a preexisting government electronic communication system, whereas the US system is privately run and more fractured. It would be possible to make it work, he pointed out, but would take some effort.
A version of this article first appeared on Medscape.com.
FROM AHA 2024
Communicating the Benefits of Prenatal Vaccination to Patients
Vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) offer important protection against severe illness for pregnant people and their babies.1 However, vaccination coverage estimates among pregnant people remain suboptimal.2-5 Additionally, some measures indicate that vaccine hesitancy among pregnant people is increasing; for example, 17.5% of surveyed pregnant women reported being very hesitant about influenza vaccination during pregnancy in 2019-2020, compared with 24.7% in 2022-2023.6 Explore updated provider toolkits and prenatal vaccination patient education resources, including fact sheets, social media assets, posters, and short videos on respiratory syncytial virus (RSV), Tdap, COVID-19, influenza, and hepatitis B.
In an interview, CDC’s Haben Debessai, MD, an adjunct instructor in obstetrics and gynecology at Emory School of Medicine, Atlanta, Georgia, contextualizes the data to help healthcare professionals communicate effectively with their pregnant patients.
What can practitioners communicate to patients about why it is important to get vaccinated during their pregnancy?
When communicating with their patients, practitioners can consider opportunities to discuss how vaccines work during pregnancy, emphasizing that prenatal vaccinations are beneficial for both the pregnant person and the fetus. It can be helpful to educate patients on how a pregnant person’s immune system can develop antibodies that will then pass to the fetus during the pregnancy and confer protection during the infant’s early months of life — when they are highly susceptible to illnesses that can be severe, such as RSV-associated lower respiratory tract infections. It can also be useful to discuss pregnancy’s impact on the immune system, which contributes to pregnant people being at higher risk for severe illness from infections like COVID-19 and flu, if contracted. The outcomes of severe illness can be dire for both the pregnant person and their pregnancy, which is why vaccination is the best mitigation option. It can also be beneficial to share with patients that some vaccines, like RSV and Tdap, are specifically for neonatal benefit, which could help patients understand why some vaccines are recommended at a specific gestational age and in each pregnancy or subsequent pregnancies.
What is known about pregnant populations that experience disparities in vaccination coverage?
While vaccination coverage among pregnant people is suboptimal, coverage estimates are often lowest among Black pregnant people, some of whom report experiencing mistreatment and discrimination during pregnancy and delivery.7 It is important to recognize that there are many intersecting factors that may impact vaccination coverage. Systemic and structural factors may prohibit some patient populations from accessing vaccinations (eg, transportation barriers, difficulty accessing adequate healthcare for those on government assistance, language barriers). To be responsive to the intersectional lived realities of each of these communities, the medical and public health community continually strives to increase trustworthiness, which can lead to increased uptake of vaccinations in these populations.
What vaccines are available and recommended for pregnant people?
Four vaccines are routinely recommended during pregnancy: Tdap, COVID-19, influenza (seasonal), and RSV (seasonal). CDC recommends getting a Tdap vaccine between the 27th and 36th week of each pregnancy, preferably during the earlier part of this time period. CDC recommends that everyone 6 months or older in the United States, including pregnant people, stay up to date on COVID-19 vaccines. A COVID-19 vaccine can be given during any trimester of pregnancy. CDC recommends an annual flu vaccine during each flu season (fall/winter) for everyone 6 months or older in the United States, including pregnant people. A flu vaccine can be given during any trimester of pregnancy. For individuals who will be between 32 and 36 weeks pregnant during September through January, CDC recommends getting an RSV vaccine. RSV season and timing of vaccination may vary depending on geography. If a pregnant patient does not get the RSV vaccine during their pregnancy, CDC recommends that their baby receive an RSV monoclonal antibody (nirsevimab) to provide additional protection during the infant’s first RSV season, if they are younger than 8 months. At this time, pregnant people who received an RSV vaccine during a previous pregnancy (last year) are not recommended to receive another RSV vaccine during pregnancy. The current recommendation is for babies born during subsequent pregnancies to receive nirsevimab. Some pregnant people may also need other vaccines, such as hepatitis B.
How can practitioners approach conversations about vaccination during pregnancy amid increasing vaccine hesitancy?
Many pregnant people who do get vaccinated describe their provider’s recommendation as an important motivator toward vaccination.8-11 Communications research suggests that practitioners can further increase trustworthiness by openly discussing potential side effects of prenatal vaccinations and providing patients with a rationale for why each vaccine is recommended. Practitioners can also utilize opportunities to communicate that the risk for severe illness from whooping cough, COVID-19, flu, and RSV in pregnancy and among neonates in the first few months of life is often higher than the risk for an adverse reaction from receiving ACIP-recommended vaccines. Finally, practitioners can consider sharing tested and refined patient education resources at least one appointment prior to the recommended administration of each vaccine, providing individuals with time to process the information they need to facilitate their vaccine decision-making process.
Some patients may be more comfortable with older, well-known prenatal vaccinations but have skepticism about newer vaccines like COVID-19 and RSV. How can practitioners respond to these concerns?
As pregnant people navigate the challenges of making health decisions that could impact their developing baby, practitioners can build trust through empathetically responding to safety concerns and questions, particularly with respect to newly authorized vaccines. Vaccine confidence may be strengthened by communicating to patients that all recommended vaccinations, including those that have been newly authorized, have been rigorously tested prior to being recommended for pregnant people. Additionally, in my clinical practice, I see that patients are often more comfortable accepting vaccines when the benefit for the baby is clearly communicated. I have been pleasantly surprised that most patients I have counseled on the new maternal RSV vaccine have been receptive, making statements like, “If this will help protect my baby from getting sick, then yes, I will get it.”
As you and your staff care for pregnant patients during fall and winter virus season, remember that a provider recommendation remains one of the strongest known predictors of vaccination uptake.12 As a trusted source of information about prenatal vaccination, consider further incorporating patient education resources to help communicate how prenatal vaccination helps pregnant people share important protection against severe illnesses with their babies.
Haben Debessai, MD, is a Gilstrap Fellow at the CDC Foundation. Debessai also serves as an Emory Obstetrics/Gynecology Adjunct Instructor at Grady Health System in Atlanta, Georgia. She disclosed no relevant conflicts of interest.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131:e214-e217. doi:10.1097/AOG.0000000000002662
2. Centers for Disease Control and Prevention. Flu, Tdap, and COVID-19 vaccination coverage among pregnant women – United States, April 2024. 2024 Sep 23. 3. Centers for Disease Control and Prevention. Respiratory syncytial virus (rsv) vaccination coverage, pregnant persons. 2024 Nov 19. 4. Centers for Disease Control and Prevention. COVID-19 vaccination coverage, pregnant persons. 2024 Nov 19. 5. Centers for Disease Control and Prevention. Influenza vaccination coverage, pregnant persons. 2024 Nov 19.6. Razzaghi H et al. IMMWR Morb Mortal Wkly Rep. 2023;72:1065-1071. Published 2023 Sep 29. doi: 10.15585/mmwr.mm7239a4
7. Mohamoud YA et al. MMWR Morb Mortal Wkly Rep 2023;72:961-967. doi: https://dx.doi.org/10.15585/mmwr.mm7235e1.
8. Kiefer MK et al. Am J Obstet Gynecol MFM. 2022;4:100603. doi: 10.1016/j.ajogmf.2022.100603
9. Spires B et al. Obstet Gynecol Clin North Am. 2023;50:401-419. doi: 10.1016/j.ogc.2023.02.013
10. Wales DP et al. Public Health. 2020;179:38-44. doi: 10.1016/j.puhe.2019.10.001
11. Zimmerman M et al. J Natl Med Assoc. 2023;115:362-376. doi:10.1016/j.jnma.2023.04.003
12. Castillo E et al. Best Pract Res Clin Obstet Gynaecol. 2021;76:83-95. doi:10.1016/j.bpobgyn.2021.03.008
Vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) offer important protection against severe illness for pregnant people and their babies.1 However, vaccination coverage estimates among pregnant people remain suboptimal.2-5 Additionally, some measures indicate that vaccine hesitancy among pregnant people is increasing; for example, 17.5% of surveyed pregnant women reported being very hesitant about influenza vaccination during pregnancy in 2019-2020, compared with 24.7% in 2022-2023.6 Explore updated provider toolkits and prenatal vaccination patient education resources, including fact sheets, social media assets, posters, and short videos on respiratory syncytial virus (RSV), Tdap, COVID-19, influenza, and hepatitis B.
In an interview, CDC’s Haben Debessai, MD, an adjunct instructor in obstetrics and gynecology at Emory School of Medicine, Atlanta, Georgia, contextualizes the data to help healthcare professionals communicate effectively with their pregnant patients.
What can practitioners communicate to patients about why it is important to get vaccinated during their pregnancy?
When communicating with their patients, practitioners can consider opportunities to discuss how vaccines work during pregnancy, emphasizing that prenatal vaccinations are beneficial for both the pregnant person and the fetus. It can be helpful to educate patients on how a pregnant person’s immune system can develop antibodies that will then pass to the fetus during the pregnancy and confer protection during the infant’s early months of life — when they are highly susceptible to illnesses that can be severe, such as RSV-associated lower respiratory tract infections. It can also be useful to discuss pregnancy’s impact on the immune system, which contributes to pregnant people being at higher risk for severe illness from infections like COVID-19 and flu, if contracted. The outcomes of severe illness can be dire for both the pregnant person and their pregnancy, which is why vaccination is the best mitigation option. It can also be beneficial to share with patients that some vaccines, like RSV and Tdap, are specifically for neonatal benefit, which could help patients understand why some vaccines are recommended at a specific gestational age and in each pregnancy or subsequent pregnancies.
What is known about pregnant populations that experience disparities in vaccination coverage?
While vaccination coverage among pregnant people is suboptimal, coverage estimates are often lowest among Black pregnant people, some of whom report experiencing mistreatment and discrimination during pregnancy and delivery.7 It is important to recognize that there are many intersecting factors that may impact vaccination coverage. Systemic and structural factors may prohibit some patient populations from accessing vaccinations (eg, transportation barriers, difficulty accessing adequate healthcare for those on government assistance, language barriers). To be responsive to the intersectional lived realities of each of these communities, the medical and public health community continually strives to increase trustworthiness, which can lead to increased uptake of vaccinations in these populations.
What vaccines are available and recommended for pregnant people?
Four vaccines are routinely recommended during pregnancy: Tdap, COVID-19, influenza (seasonal), and RSV (seasonal). CDC recommends getting a Tdap vaccine between the 27th and 36th week of each pregnancy, preferably during the earlier part of this time period. CDC recommends that everyone 6 months or older in the United States, including pregnant people, stay up to date on COVID-19 vaccines. A COVID-19 vaccine can be given during any trimester of pregnancy. CDC recommends an annual flu vaccine during each flu season (fall/winter) for everyone 6 months or older in the United States, including pregnant people. A flu vaccine can be given during any trimester of pregnancy. For individuals who will be between 32 and 36 weeks pregnant during September through January, CDC recommends getting an RSV vaccine. RSV season and timing of vaccination may vary depending on geography. If a pregnant patient does not get the RSV vaccine during their pregnancy, CDC recommends that their baby receive an RSV monoclonal antibody (nirsevimab) to provide additional protection during the infant’s first RSV season, if they are younger than 8 months. At this time, pregnant people who received an RSV vaccine during a previous pregnancy (last year) are not recommended to receive another RSV vaccine during pregnancy. The current recommendation is for babies born during subsequent pregnancies to receive nirsevimab. Some pregnant people may also need other vaccines, such as hepatitis B.
How can practitioners approach conversations about vaccination during pregnancy amid increasing vaccine hesitancy?
Many pregnant people who do get vaccinated describe their provider’s recommendation as an important motivator toward vaccination.8-11 Communications research suggests that practitioners can further increase trustworthiness by openly discussing potential side effects of prenatal vaccinations and providing patients with a rationale for why each vaccine is recommended. Practitioners can also utilize opportunities to communicate that the risk for severe illness from whooping cough, COVID-19, flu, and RSV in pregnancy and among neonates in the first few months of life is often higher than the risk for an adverse reaction from receiving ACIP-recommended vaccines. Finally, practitioners can consider sharing tested and refined patient education resources at least one appointment prior to the recommended administration of each vaccine, providing individuals with time to process the information they need to facilitate their vaccine decision-making process.
Some patients may be more comfortable with older, well-known prenatal vaccinations but have skepticism about newer vaccines like COVID-19 and RSV. How can practitioners respond to these concerns?
As pregnant people navigate the challenges of making health decisions that could impact their developing baby, practitioners can build trust through empathetically responding to safety concerns and questions, particularly with respect to newly authorized vaccines. Vaccine confidence may be strengthened by communicating to patients that all recommended vaccinations, including those that have been newly authorized, have been rigorously tested prior to being recommended for pregnant people. Additionally, in my clinical practice, I see that patients are often more comfortable accepting vaccines when the benefit for the baby is clearly communicated. I have been pleasantly surprised that most patients I have counseled on the new maternal RSV vaccine have been receptive, making statements like, “If this will help protect my baby from getting sick, then yes, I will get it.”
As you and your staff care for pregnant patients during fall and winter virus season, remember that a provider recommendation remains one of the strongest known predictors of vaccination uptake.12 As a trusted source of information about prenatal vaccination, consider further incorporating patient education resources to help communicate how prenatal vaccination helps pregnant people share important protection against severe illnesses with their babies.
Haben Debessai, MD, is a Gilstrap Fellow at the CDC Foundation. Debessai also serves as an Emory Obstetrics/Gynecology Adjunct Instructor at Grady Health System in Atlanta, Georgia. She disclosed no relevant conflicts of interest.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131:e214-e217. doi:10.1097/AOG.0000000000002662
2. Centers for Disease Control and Prevention. Flu, Tdap, and COVID-19 vaccination coverage among pregnant women – United States, April 2024. 2024 Sep 23. 3. Centers for Disease Control and Prevention. Respiratory syncytial virus (rsv) vaccination coverage, pregnant persons. 2024 Nov 19. 4. Centers for Disease Control and Prevention. COVID-19 vaccination coverage, pregnant persons. 2024 Nov 19. 5. Centers for Disease Control and Prevention. Influenza vaccination coverage, pregnant persons. 2024 Nov 19.6. Razzaghi H et al. IMMWR Morb Mortal Wkly Rep. 2023;72:1065-1071. Published 2023 Sep 29. doi: 10.15585/mmwr.mm7239a4
7. Mohamoud YA et al. MMWR Morb Mortal Wkly Rep 2023;72:961-967. doi: https://dx.doi.org/10.15585/mmwr.mm7235e1.
8. Kiefer MK et al. Am J Obstet Gynecol MFM. 2022;4:100603. doi: 10.1016/j.ajogmf.2022.100603
9. Spires B et al. Obstet Gynecol Clin North Am. 2023;50:401-419. doi: 10.1016/j.ogc.2023.02.013
10. Wales DP et al. Public Health. 2020;179:38-44. doi: 10.1016/j.puhe.2019.10.001
11. Zimmerman M et al. J Natl Med Assoc. 2023;115:362-376. doi:10.1016/j.jnma.2023.04.003
12. Castillo E et al. Best Pract Res Clin Obstet Gynaecol. 2021;76:83-95. doi:10.1016/j.bpobgyn.2021.03.008
Vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) offer important protection against severe illness for pregnant people and their babies.1 However, vaccination coverage estimates among pregnant people remain suboptimal.2-5 Additionally, some measures indicate that vaccine hesitancy among pregnant people is increasing; for example, 17.5% of surveyed pregnant women reported being very hesitant about influenza vaccination during pregnancy in 2019-2020, compared with 24.7% in 2022-2023.6 Explore updated provider toolkits and prenatal vaccination patient education resources, including fact sheets, social media assets, posters, and short videos on respiratory syncytial virus (RSV), Tdap, COVID-19, influenza, and hepatitis B.
In an interview, CDC’s Haben Debessai, MD, an adjunct instructor in obstetrics and gynecology at Emory School of Medicine, Atlanta, Georgia, contextualizes the data to help healthcare professionals communicate effectively with their pregnant patients.
What can practitioners communicate to patients about why it is important to get vaccinated during their pregnancy?
When communicating with their patients, practitioners can consider opportunities to discuss how vaccines work during pregnancy, emphasizing that prenatal vaccinations are beneficial for both the pregnant person and the fetus. It can be helpful to educate patients on how a pregnant person’s immune system can develop antibodies that will then pass to the fetus during the pregnancy and confer protection during the infant’s early months of life — when they are highly susceptible to illnesses that can be severe, such as RSV-associated lower respiratory tract infections. It can also be useful to discuss pregnancy’s impact on the immune system, which contributes to pregnant people being at higher risk for severe illness from infections like COVID-19 and flu, if contracted. The outcomes of severe illness can be dire for both the pregnant person and their pregnancy, which is why vaccination is the best mitigation option. It can also be beneficial to share with patients that some vaccines, like RSV and Tdap, are specifically for neonatal benefit, which could help patients understand why some vaccines are recommended at a specific gestational age and in each pregnancy or subsequent pregnancies.
What is known about pregnant populations that experience disparities in vaccination coverage?
While vaccination coverage among pregnant people is suboptimal, coverage estimates are often lowest among Black pregnant people, some of whom report experiencing mistreatment and discrimination during pregnancy and delivery.7 It is important to recognize that there are many intersecting factors that may impact vaccination coverage. Systemic and structural factors may prohibit some patient populations from accessing vaccinations (eg, transportation barriers, difficulty accessing adequate healthcare for those on government assistance, language barriers). To be responsive to the intersectional lived realities of each of these communities, the medical and public health community continually strives to increase trustworthiness, which can lead to increased uptake of vaccinations in these populations.
What vaccines are available and recommended for pregnant people?
Four vaccines are routinely recommended during pregnancy: Tdap, COVID-19, influenza (seasonal), and RSV (seasonal). CDC recommends getting a Tdap vaccine between the 27th and 36th week of each pregnancy, preferably during the earlier part of this time period. CDC recommends that everyone 6 months or older in the United States, including pregnant people, stay up to date on COVID-19 vaccines. A COVID-19 vaccine can be given during any trimester of pregnancy. CDC recommends an annual flu vaccine during each flu season (fall/winter) for everyone 6 months or older in the United States, including pregnant people. A flu vaccine can be given during any trimester of pregnancy. For individuals who will be between 32 and 36 weeks pregnant during September through January, CDC recommends getting an RSV vaccine. RSV season and timing of vaccination may vary depending on geography. If a pregnant patient does not get the RSV vaccine during their pregnancy, CDC recommends that their baby receive an RSV monoclonal antibody (nirsevimab) to provide additional protection during the infant’s first RSV season, if they are younger than 8 months. At this time, pregnant people who received an RSV vaccine during a previous pregnancy (last year) are not recommended to receive another RSV vaccine during pregnancy. The current recommendation is for babies born during subsequent pregnancies to receive nirsevimab. Some pregnant people may also need other vaccines, such as hepatitis B.
How can practitioners approach conversations about vaccination during pregnancy amid increasing vaccine hesitancy?
Many pregnant people who do get vaccinated describe their provider’s recommendation as an important motivator toward vaccination.8-11 Communications research suggests that practitioners can further increase trustworthiness by openly discussing potential side effects of prenatal vaccinations and providing patients with a rationale for why each vaccine is recommended. Practitioners can also utilize opportunities to communicate that the risk for severe illness from whooping cough, COVID-19, flu, and RSV in pregnancy and among neonates in the first few months of life is often higher than the risk for an adverse reaction from receiving ACIP-recommended vaccines. Finally, practitioners can consider sharing tested and refined patient education resources at least one appointment prior to the recommended administration of each vaccine, providing individuals with time to process the information they need to facilitate their vaccine decision-making process.
Some patients may be more comfortable with older, well-known prenatal vaccinations but have skepticism about newer vaccines like COVID-19 and RSV. How can practitioners respond to these concerns?
As pregnant people navigate the challenges of making health decisions that could impact their developing baby, practitioners can build trust through empathetically responding to safety concerns and questions, particularly with respect to newly authorized vaccines. Vaccine confidence may be strengthened by communicating to patients that all recommended vaccinations, including those that have been newly authorized, have been rigorously tested prior to being recommended for pregnant people. Additionally, in my clinical practice, I see that patients are often more comfortable accepting vaccines when the benefit for the baby is clearly communicated. I have been pleasantly surprised that most patients I have counseled on the new maternal RSV vaccine have been receptive, making statements like, “If this will help protect my baby from getting sick, then yes, I will get it.”
As you and your staff care for pregnant patients during fall and winter virus season, remember that a provider recommendation remains one of the strongest known predictors of vaccination uptake.12 As a trusted source of information about prenatal vaccination, consider further incorporating patient education resources to help communicate how prenatal vaccination helps pregnant people share important protection against severe illnesses with their babies.
Haben Debessai, MD, is a Gilstrap Fellow at the CDC Foundation. Debessai also serves as an Emory Obstetrics/Gynecology Adjunct Instructor at Grady Health System in Atlanta, Georgia. She disclosed no relevant conflicts of interest.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131:e214-e217. doi:10.1097/AOG.0000000000002662
2. Centers for Disease Control and Prevention. Flu, Tdap, and COVID-19 vaccination coverage among pregnant women – United States, April 2024. 2024 Sep 23. 3. Centers for Disease Control and Prevention. Respiratory syncytial virus (rsv) vaccination coverage, pregnant persons. 2024 Nov 19. 4. Centers for Disease Control and Prevention. COVID-19 vaccination coverage, pregnant persons. 2024 Nov 19. 5. Centers for Disease Control and Prevention. Influenza vaccination coverage, pregnant persons. 2024 Nov 19.6. Razzaghi H et al. IMMWR Morb Mortal Wkly Rep. 2023;72:1065-1071. Published 2023 Sep 29. doi: 10.15585/mmwr.mm7239a4
7. Mohamoud YA et al. MMWR Morb Mortal Wkly Rep 2023;72:961-967. doi: https://dx.doi.org/10.15585/mmwr.mm7235e1.
8. Kiefer MK et al. Am J Obstet Gynecol MFM. 2022;4:100603. doi: 10.1016/j.ajogmf.2022.100603
9. Spires B et al. Obstet Gynecol Clin North Am. 2023;50:401-419. doi: 10.1016/j.ogc.2023.02.013
10. Wales DP et al. Public Health. 2020;179:38-44. doi: 10.1016/j.puhe.2019.10.001
11. Zimmerman M et al. J Natl Med Assoc. 2023;115:362-376. doi:10.1016/j.jnma.2023.04.003
12. Castillo E et al. Best Pract Res Clin Obstet Gynaecol. 2021;76:83-95. doi:10.1016/j.bpobgyn.2021.03.008
What to Know About Sexually Transmitted Ringworm
Ringworm (also known as tinea, jock itch, or athlete’s foot) is a common infection caused by dermatophyte fungi, known to affect skin, hair, or nails. It causes skin infections that are typically mild and are often treated with topical antifungals.
However, in recent years, newly emerging dermatophyte strains have been causing more severe and harder-to-treat ringworm. Notably, one emerging strain, Trichophyton mentagrophytes genotype VII(TMVII), is associated with sexual contact. In recent years, TMVII infections linked to sexual contact have been reported among men who have sex with men in Europe and in travelers returning from Southeast Asia. The first US case of TMVII was reported in June 2024, after which public health authorities were alerted to additional cases; all were associated with recent sexual contact. Other dermatophyte species have also been reported to cause ringworm transmitted through sexual contact.
Here are some key points to know about sexually transmitted ringworm.
Tell me more about sexually transmitted ringworm: What is causing it?
Skin-to-skin contact is a common mode of ringworm transmission. Infections with sexually transmitted TMVII commonly cause lesions on anatomical sites that may be exposed during intimate or sexual contact, such as the face, genitals, and perianal region. Sexual transmission of TMVII has been reported in Europe, predominantly among men who have sex with men, for several years. Other dermatophyte strains have been reported in association with sexual contact, including the emerging strain Trichophyton indotineae. However, sexual transmission is not the main mode of transmission for T indotineae and other dermatophyte strains.
When should clinicians suspect a potential case of sexually transmitted ringworm?
Providers should consider sexually transmitted ringworm when seeing ringworm in locations associated with intimate contact (for example, a rash on or around the genitals, perianal area, or mouth).
The typical appearance of ringworm is a raised, ring-like, erythematous rash with a scaly border that grows over time. The rash may appear pink, brown, or gray on different types of skin. Patients may note itching and flaking of the rash. In areas with hair such as the beard area, ringworm can present as pustules and be associated with hair loss.
Emerging ringworm infections can present in atypical or more severe ways, including a highly inflammatory (painful, scarring, or otherwise severe) rash, a rash affecting a large area or multiple sites, nodules, and pustules.
Sexually transmitted ringworm may be considered based on sexual history and recent sexual contact with someone with known TMVII. Recent history of travel to a region with reported sexually transmitted ringworm may increase suspicion of TMVII. In patients with a travel history to South Asia, T indotineae should be considered, especially if the rash does not improve with oral terbinafine.
How can testing help guide the diagnosis of sexually transmitted ringworm infection?
When evaluating a rash that may represent ringworm, providers should use a confirmatory test such as potassium hydroxide (KOH) preparation when possible. KOH prep can confirm the presence of a fungus that causes ringworm, but it does not identify the species or type of ringworm. Testing such as fungal culture and molecular testing can help identify specific types of ringworm, but these tests are not often performed and may take a long time to yield results.
Routine fungal cultures cannot identify TMVII and T indotineae; these tests may identify the genus Trichophyton, but only advanced molecular testing, which is available at selected US laboratories, can identify TMVII and T indotineae.
We recommend confirmatory testing because ringworm can easily be misdiagnosed as skin conditions such as psoriasis or eczema. The use of topical steroids can worsen a ringworm infection, so clinicians should be cautious about treating a rash with topical steroids if the etiology is unclear. Treatment should not be delayed if testing is not available.
Clinicians who suspect a case of TMVII infection or infection with another emerging type of severe or antifungal-resistant ringworm can contact the Centers for Disease Control and Prevention (CDC) at fungaloutbreaks@cdc.gov. More details on how clinicians can pursue testing to identify emerging strains of ringworm can be found on the American Academy of Dermatology (AAD) emerging diseases task force website.
How should clinicians treat and manage sexually transmitted ringworm?
If TMVII infection is suspected, providers can consider starting empirical treatment with oral terbinafine. Although data are limited, experience from case series suggests that TMVII may require oral antifungal treatment because it can cause severe skin infections and often does not improve with topical antifungals. Clinicians should advise patients that they may need prolonged treatment courses until the rash resolves, with possible need for treatment courses of 6-8 weeks or longer.
Any diagnosis of a sexually transmitted infection is an opportunity to engage patients in comprehensive sexual health services. Patients with suspected sexually transmitted ringworm should be evaluated for HIV and other sexually transmitted infections, including syphilis, chlamydia, and gonorrhea; clinicians should discuss and facilitate access to other preventive services, such as HIV pre-exposure prophylaxis if the patient is HIV negative and at risk for HIV. Patients should also notify their partner(s) about the diagnosis.
Is sexually transmitted ringworm a public health concern?
It is important to know that very few cases of TMVII have been reported in the United States thus far. CDC continues to monitor emerging dermatophyte strains because these types of ringworm can cause more severe or difficult-to-treat infections. Clinicians should be aware of the potential severity of sexually transmitted ringworm infections and of how diagnosis and treatment of these infections may differ from typical management of ringworm.
So far, TMVII, the dermatophyte strain most associated with spread through sexual contact, has not been documented to have antifungal resistance. More rarely, sexually transmitted ringworm may be caused by other emerging dermatophyte strains that are antifungal resistant, such as T indotineae. Itraconazole is the recommended first-line treatment for T indotineae infections.
How can clinicians counsel patients with sexually transmitted ringworm?
Ringworm can spread with skin-to-skin contact, so patients should avoid such contact with others while they have a rash. They should also avoid sharing personal items (such as razors or towels) and clothing, and launder their clothing, towels, and bedding in a high heat cycle.
People can reduce their risk of getting all types of ringworm infection by keeping their skin clean and dry, changing their socks and underwear daily, and wearing sandals in public locker rooms and other public spaces. People should avoid skin-to-skin contact with anyone with ringworm or an unexplained rash. Before having sex, people can check in with their partners and be aware of unexplained rashes on their partners’ bodies.
Where can clinicians go to learn more about sexually transmitted and other emerging types of ringworm?
CDC has partnered with the AAD to create set of online resources for clinicians for diagnosing and managing emerging dermatophyte infections. Clinicians who suspect or confirm antimicrobial resistant ringworm infection are also encouraged to submit cases to the AAD’s Emerging Diseases Registry. Clinicians wanting further guidance on how to manage suspected or confirmed ringworm infection with an emerging dermatophyte strain can also contact the CDC at fungaloutbreaks@cdc.gov. Useful information on emerging dermatophyte infections for providers and patients is also available on CDC’s website.
Relevant Reading
Zucker J et al. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.Spivack S et al. Emerg Infect Dis. 2024;30:807-809.Jabet A et al. Emerg Infect Dis. 2023;29:1411-1414.
A version of this article appeared on Medscape.com.
Dr Anand is Epidemic Intelligence Service Officer, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia. Dr Gold is Medical Officer, Mycotic Diseases Branch, Centers for Disease Control and Prevention. Dr Quilter is Medical Officer, Division of STD Prevention, Centers for Disease Control and Prevention. None reported any relevant conflicts of interest.
Ringworm (also known as tinea, jock itch, or athlete’s foot) is a common infection caused by dermatophyte fungi, known to affect skin, hair, or nails. It causes skin infections that are typically mild and are often treated with topical antifungals.
However, in recent years, newly emerging dermatophyte strains have been causing more severe and harder-to-treat ringworm. Notably, one emerging strain, Trichophyton mentagrophytes genotype VII(TMVII), is associated with sexual contact. In recent years, TMVII infections linked to sexual contact have been reported among men who have sex with men in Europe and in travelers returning from Southeast Asia. The first US case of TMVII was reported in June 2024, after which public health authorities were alerted to additional cases; all were associated with recent sexual contact. Other dermatophyte species have also been reported to cause ringworm transmitted through sexual contact.
Here are some key points to know about sexually transmitted ringworm.
Tell me more about sexually transmitted ringworm: What is causing it?
Skin-to-skin contact is a common mode of ringworm transmission. Infections with sexually transmitted TMVII commonly cause lesions on anatomical sites that may be exposed during intimate or sexual contact, such as the face, genitals, and perianal region. Sexual transmission of TMVII has been reported in Europe, predominantly among men who have sex with men, for several years. Other dermatophyte strains have been reported in association with sexual contact, including the emerging strain Trichophyton indotineae. However, sexual transmission is not the main mode of transmission for T indotineae and other dermatophyte strains.
When should clinicians suspect a potential case of sexually transmitted ringworm?
Providers should consider sexually transmitted ringworm when seeing ringworm in locations associated with intimate contact (for example, a rash on or around the genitals, perianal area, or mouth).
The typical appearance of ringworm is a raised, ring-like, erythematous rash with a scaly border that grows over time. The rash may appear pink, brown, or gray on different types of skin. Patients may note itching and flaking of the rash. In areas with hair such as the beard area, ringworm can present as pustules and be associated with hair loss.
Emerging ringworm infections can present in atypical or more severe ways, including a highly inflammatory (painful, scarring, or otherwise severe) rash, a rash affecting a large area or multiple sites, nodules, and pustules.
Sexually transmitted ringworm may be considered based on sexual history and recent sexual contact with someone with known TMVII. Recent history of travel to a region with reported sexually transmitted ringworm may increase suspicion of TMVII. In patients with a travel history to South Asia, T indotineae should be considered, especially if the rash does not improve with oral terbinafine.
How can testing help guide the diagnosis of sexually transmitted ringworm infection?
When evaluating a rash that may represent ringworm, providers should use a confirmatory test such as potassium hydroxide (KOH) preparation when possible. KOH prep can confirm the presence of a fungus that causes ringworm, but it does not identify the species or type of ringworm. Testing such as fungal culture and molecular testing can help identify specific types of ringworm, but these tests are not often performed and may take a long time to yield results.
Routine fungal cultures cannot identify TMVII and T indotineae; these tests may identify the genus Trichophyton, but only advanced molecular testing, which is available at selected US laboratories, can identify TMVII and T indotineae.
We recommend confirmatory testing because ringworm can easily be misdiagnosed as skin conditions such as psoriasis or eczema. The use of topical steroids can worsen a ringworm infection, so clinicians should be cautious about treating a rash with topical steroids if the etiology is unclear. Treatment should not be delayed if testing is not available.
Clinicians who suspect a case of TMVII infection or infection with another emerging type of severe or antifungal-resistant ringworm can contact the Centers for Disease Control and Prevention (CDC) at fungaloutbreaks@cdc.gov. More details on how clinicians can pursue testing to identify emerging strains of ringworm can be found on the American Academy of Dermatology (AAD) emerging diseases task force website.
How should clinicians treat and manage sexually transmitted ringworm?
If TMVII infection is suspected, providers can consider starting empirical treatment with oral terbinafine. Although data are limited, experience from case series suggests that TMVII may require oral antifungal treatment because it can cause severe skin infections and often does not improve with topical antifungals. Clinicians should advise patients that they may need prolonged treatment courses until the rash resolves, with possible need for treatment courses of 6-8 weeks or longer.
Any diagnosis of a sexually transmitted infection is an opportunity to engage patients in comprehensive sexual health services. Patients with suspected sexually transmitted ringworm should be evaluated for HIV and other sexually transmitted infections, including syphilis, chlamydia, and gonorrhea; clinicians should discuss and facilitate access to other preventive services, such as HIV pre-exposure prophylaxis if the patient is HIV negative and at risk for HIV. Patients should also notify their partner(s) about the diagnosis.
Is sexually transmitted ringworm a public health concern?
It is important to know that very few cases of TMVII have been reported in the United States thus far. CDC continues to monitor emerging dermatophyte strains because these types of ringworm can cause more severe or difficult-to-treat infections. Clinicians should be aware of the potential severity of sexually transmitted ringworm infections and of how diagnosis and treatment of these infections may differ from typical management of ringworm.
So far, TMVII, the dermatophyte strain most associated with spread through sexual contact, has not been documented to have antifungal resistance. More rarely, sexually transmitted ringworm may be caused by other emerging dermatophyte strains that are antifungal resistant, such as T indotineae. Itraconazole is the recommended first-line treatment for T indotineae infections.
How can clinicians counsel patients with sexually transmitted ringworm?
Ringworm can spread with skin-to-skin contact, so patients should avoid such contact with others while they have a rash. They should also avoid sharing personal items (such as razors or towels) and clothing, and launder their clothing, towels, and bedding in a high heat cycle.
People can reduce their risk of getting all types of ringworm infection by keeping their skin clean and dry, changing their socks and underwear daily, and wearing sandals in public locker rooms and other public spaces. People should avoid skin-to-skin contact with anyone with ringworm or an unexplained rash. Before having sex, people can check in with their partners and be aware of unexplained rashes on their partners’ bodies.
Where can clinicians go to learn more about sexually transmitted and other emerging types of ringworm?
CDC has partnered with the AAD to create set of online resources for clinicians for diagnosing and managing emerging dermatophyte infections. Clinicians who suspect or confirm antimicrobial resistant ringworm infection are also encouraged to submit cases to the AAD’s Emerging Diseases Registry. Clinicians wanting further guidance on how to manage suspected or confirmed ringworm infection with an emerging dermatophyte strain can also contact the CDC at fungaloutbreaks@cdc.gov. Useful information on emerging dermatophyte infections for providers and patients is also available on CDC’s website.
Relevant Reading
Zucker J et al. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.Spivack S et al. Emerg Infect Dis. 2024;30:807-809.Jabet A et al. Emerg Infect Dis. 2023;29:1411-1414.
A version of this article appeared on Medscape.com.
Dr Anand is Epidemic Intelligence Service Officer, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia. Dr Gold is Medical Officer, Mycotic Diseases Branch, Centers for Disease Control and Prevention. Dr Quilter is Medical Officer, Division of STD Prevention, Centers for Disease Control and Prevention. None reported any relevant conflicts of interest.
Ringworm (also known as tinea, jock itch, or athlete’s foot) is a common infection caused by dermatophyte fungi, known to affect skin, hair, or nails. It causes skin infections that are typically mild and are often treated with topical antifungals.
However, in recent years, newly emerging dermatophyte strains have been causing more severe and harder-to-treat ringworm. Notably, one emerging strain, Trichophyton mentagrophytes genotype VII(TMVII), is associated with sexual contact. In recent years, TMVII infections linked to sexual contact have been reported among men who have sex with men in Europe and in travelers returning from Southeast Asia. The first US case of TMVII was reported in June 2024, after which public health authorities were alerted to additional cases; all were associated with recent sexual contact. Other dermatophyte species have also been reported to cause ringworm transmitted through sexual contact.
Here are some key points to know about sexually transmitted ringworm.
Tell me more about sexually transmitted ringworm: What is causing it?
Skin-to-skin contact is a common mode of ringworm transmission. Infections with sexually transmitted TMVII commonly cause lesions on anatomical sites that may be exposed during intimate or sexual contact, such as the face, genitals, and perianal region. Sexual transmission of TMVII has been reported in Europe, predominantly among men who have sex with men, for several years. Other dermatophyte strains have been reported in association with sexual contact, including the emerging strain Trichophyton indotineae. However, sexual transmission is not the main mode of transmission for T indotineae and other dermatophyte strains.
When should clinicians suspect a potential case of sexually transmitted ringworm?
Providers should consider sexually transmitted ringworm when seeing ringworm in locations associated with intimate contact (for example, a rash on or around the genitals, perianal area, or mouth).
The typical appearance of ringworm is a raised, ring-like, erythematous rash with a scaly border that grows over time. The rash may appear pink, brown, or gray on different types of skin. Patients may note itching and flaking of the rash. In areas with hair such as the beard area, ringworm can present as pustules and be associated with hair loss.
Emerging ringworm infections can present in atypical or more severe ways, including a highly inflammatory (painful, scarring, or otherwise severe) rash, a rash affecting a large area or multiple sites, nodules, and pustules.
Sexually transmitted ringworm may be considered based on sexual history and recent sexual contact with someone with known TMVII. Recent history of travel to a region with reported sexually transmitted ringworm may increase suspicion of TMVII. In patients with a travel history to South Asia, T indotineae should be considered, especially if the rash does not improve with oral terbinafine.
How can testing help guide the diagnosis of sexually transmitted ringworm infection?
When evaluating a rash that may represent ringworm, providers should use a confirmatory test such as potassium hydroxide (KOH) preparation when possible. KOH prep can confirm the presence of a fungus that causes ringworm, but it does not identify the species or type of ringworm. Testing such as fungal culture and molecular testing can help identify specific types of ringworm, but these tests are not often performed and may take a long time to yield results.
Routine fungal cultures cannot identify TMVII and T indotineae; these tests may identify the genus Trichophyton, but only advanced molecular testing, which is available at selected US laboratories, can identify TMVII and T indotineae.
We recommend confirmatory testing because ringworm can easily be misdiagnosed as skin conditions such as psoriasis or eczema. The use of topical steroids can worsen a ringworm infection, so clinicians should be cautious about treating a rash with topical steroids if the etiology is unclear. Treatment should not be delayed if testing is not available.
Clinicians who suspect a case of TMVII infection or infection with another emerging type of severe or antifungal-resistant ringworm can contact the Centers for Disease Control and Prevention (CDC) at fungaloutbreaks@cdc.gov. More details on how clinicians can pursue testing to identify emerging strains of ringworm can be found on the American Academy of Dermatology (AAD) emerging diseases task force website.
How should clinicians treat and manage sexually transmitted ringworm?
If TMVII infection is suspected, providers can consider starting empirical treatment with oral terbinafine. Although data are limited, experience from case series suggests that TMVII may require oral antifungal treatment because it can cause severe skin infections and often does not improve with topical antifungals. Clinicians should advise patients that they may need prolonged treatment courses until the rash resolves, with possible need for treatment courses of 6-8 weeks or longer.
Any diagnosis of a sexually transmitted infection is an opportunity to engage patients in comprehensive sexual health services. Patients with suspected sexually transmitted ringworm should be evaluated for HIV and other sexually transmitted infections, including syphilis, chlamydia, and gonorrhea; clinicians should discuss and facilitate access to other preventive services, such as HIV pre-exposure prophylaxis if the patient is HIV negative and at risk for HIV. Patients should also notify their partner(s) about the diagnosis.
Is sexually transmitted ringworm a public health concern?
It is important to know that very few cases of TMVII have been reported in the United States thus far. CDC continues to monitor emerging dermatophyte strains because these types of ringworm can cause more severe or difficult-to-treat infections. Clinicians should be aware of the potential severity of sexually transmitted ringworm infections and of how diagnosis and treatment of these infections may differ from typical management of ringworm.
So far, TMVII, the dermatophyte strain most associated with spread through sexual contact, has not been documented to have antifungal resistance. More rarely, sexually transmitted ringworm may be caused by other emerging dermatophyte strains that are antifungal resistant, such as T indotineae. Itraconazole is the recommended first-line treatment for T indotineae infections.
How can clinicians counsel patients with sexually transmitted ringworm?
Ringworm can spread with skin-to-skin contact, so patients should avoid such contact with others while they have a rash. They should also avoid sharing personal items (such as razors or towels) and clothing, and launder their clothing, towels, and bedding in a high heat cycle.
People can reduce their risk of getting all types of ringworm infection by keeping their skin clean and dry, changing their socks and underwear daily, and wearing sandals in public locker rooms and other public spaces. People should avoid skin-to-skin contact with anyone with ringworm or an unexplained rash. Before having sex, people can check in with their partners and be aware of unexplained rashes on their partners’ bodies.
Where can clinicians go to learn more about sexually transmitted and other emerging types of ringworm?
CDC has partnered with the AAD to create set of online resources for clinicians for diagnosing and managing emerging dermatophyte infections. Clinicians who suspect or confirm antimicrobial resistant ringworm infection are also encouraged to submit cases to the AAD’s Emerging Diseases Registry. Clinicians wanting further guidance on how to manage suspected or confirmed ringworm infection with an emerging dermatophyte strain can also contact the CDC at fungaloutbreaks@cdc.gov. Useful information on emerging dermatophyte infections for providers and patients is also available on CDC’s website.
Relevant Reading
Zucker J et al. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.Spivack S et al. Emerg Infect Dis. 2024;30:807-809.Jabet A et al. Emerg Infect Dis. 2023;29:1411-1414.
A version of this article appeared on Medscape.com.
Dr Anand is Epidemic Intelligence Service Officer, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia. Dr Gold is Medical Officer, Mycotic Diseases Branch, Centers for Disease Control and Prevention. Dr Quilter is Medical Officer, Division of STD Prevention, Centers for Disease Control and Prevention. None reported any relevant conflicts of interest.
New Hope for Antimicrobial Peptides?
The story of antimicrobial peptides (AMPs), particularly in tackling antibiotic resistance, has been one of false dawns and unfulfilled promises. But perhaps a new generation of “smarter” compounds could see them find a wider role in clinical practice, said experts.
AMPs may be small molecules, consisting of short chains of amino acids, but these naturally occurring compounds have an important function: They are the “frontline defense” against invasive bacteria, said Henrik Franzyk, MSc Engineering, associate professor in the Department of Drug Design and Pharmacology at the University of Copenhagen in Denmark.
Multifunction Line of Defense
AMPs are cationic, meaning they are positively charged. “The reason why nature has maintained these molecules is that all the microbes out there have a negative surface charge,” explained Hans-Georg Sahl, PhD, emeritus professor of pharmaceutical microbiology at the University of Bonn in Germany.
“Thus, the content of a cell gets released, and it destroys the pathogen,” explained Paulina Szymczak, a PhD candidate in the Institute of AI for Health at Helmholtz Munich, Neuherberg, Germany.
“There are variations of that theme,” said Eefjan Breukink, PhD, professor of microbial membranes and antibiotics at Utrecht University in the Netherlands. “And then it depends on the sequence of the particular peptide,” as some can cross the cell membrane and damage the bacterium internally.
Szymczak explained that AMPs can, in this way, target the cell DNA, as both the membrane and the DNA are negatively charged. “That’s also what makes them so powerful because they don’t have just one mechanism of action, as opposed to conventional antibiotics.”
Indiscriminate Killers
But they also have another crucial function. They activate the innate immune system via so-called resident immune cells that are “sitting in the tissues and waiting for bacteria to turn up,” explained Franzyk.
“The problem with antibodies is that they typically need to replicate,” he continued, which takes between 4 and 7 days — a timeline that is much better suited to tackling a viral infection. Bacteria, on the other hand, have a replication cycle of just 30 minutes.
Another big problem is that AMPs kill cells indiscriminately, including our own.
“But the human body is clever in that it only produces these antimicrobial peptides where the bacteria are, so they are not circulating in the blood,” said Franzyk. If a small part of tissue becomes infected, the innate immune cells start producing AMPs, which may kill the bacteria, or call on other immune cells to help.
As part of this process, “they will also kill part of our own tissue, but that’s the price we have to pay,” he said.
Local Applications
It is this aspect that has, so far, limited the use of AMPs in clinical practice, certainly as a replacement for conventional antibiotics limited by bacterial resistance. The trials conducted so far have been, by and large, negative, which has dampened enthusiasm and led to the perception that the risk they pose is too great for large-scale investment.
AMPs “are not made for what we need from antibiotics in the first place,” explained Sahl. “That is, a nice, easy distribution in the body, going into abscesses” and throughout the tissues.
He continued that AMPs are “more about controlling the flora in our bodies,” and they are “really not made for being used systemically.”
Szymczak and colleagues are now working on designing active peptides with a strong antibacterial profile but limited toxicity for systematic use.
However, the “downside with these peptides is that they are not orally available, so you can’t take a pill,” Breukink said, but instead they need to be administered intravenously.
There are, nevertheless, some antibiotics in clinical use that have the same molecular features as AMPs. These include colistin, a last-resort treatment for multidrug-resistant gram-negative bacteria, and daptomycin, which is used in the treatment of systemic infections caused by gram-positive species.
Szymczak added that there have been successes in using AMPs in a more targeted way, such as using a topical cream. Another potentially promising avenue is lung infections, which are being studied in mouse models.
Less Prone to Resistance
Crucially, AMPs are markedly less prone to bacterial resistance than conventional antibiotics, partly because of their typical target: the cell membrane.
“Biologically and evolutionarily, it is a very costly operation to rebuild the membrane and change its charge,” Szymczak explained. “It’s quite hard for bacteria to learn this because it’s not a single protein that you have to mutate but the whole membrane.”
This is seen in the laboratory, where it takes around five generations, or passages, for bacteria to develop resistance when grown in the presence of antibiotics, but up to 40 passages when cultured with an AMP.
The limits of the ability of AMPs to withstand the development of bacterial resistance have been tested in the real world.
Colistin has been used widely in Asia as a growth promoter, especially in pig farming. Franzyk explained that farmers have used enormous quantities of this AMP-based antibiotic, which has indeed led to the development of resistance, including contamination of meat for human consumption, leading to resistance spreading to other parts of the world.
“The bad thing about this is it’s not something each individual bacteria needs to acquire,” he said. Because resistance is stored on small, cyclic DNA called plasmids, it “can be transferred from one bacterial species to another.”
Novel Avenues
Franzyk suggested that AMPs could nevertheless be used in combination with, or to modify, existing antibiotics to revitalize those for which there is already bacterial resistance, or to allow antibiotics that ordinarily target only gram-positive bacteria to also treat gram-negative infections, for example.
Szymczak and her colleagues are using artificial intelligence to design novel AMP candidates. Instead of manually going through compounds and checking their activity profiles in the lab, those steps are carried out computationally “so that, in the end, you synthesize as few candidates as possible” and can proceed to a mouse model “as fast as possible.”
She personally is looking at the issue of strain-specific activity to design a compound that would target, for example, only multidrug-resistant strains. “What we can do now is something that will target everything, so a kind of last resort peptide. But we are trying to make them smarter in their targets.”
Szymczak also pointed out that cancer cells are “negatively charged, similarly to bacterial cells, as opposed to mammalian cells, which are neutral.”
“So in theory, maybe we could design something that will target cancer cells but not our host cells, and that would be extremely exciting.” However, she underlined that, first, they are trying to tackle antimicrobial resistance before looking at other spaces.
Finally, Breukink is screening for small antibacterial compounds in fungi that are around half the size of a normal peptide and more hydrophobic, meaning there is a much greater chance of them being orally available.
But “you first have to test, of course,” he said, as “if you don’t have specific targets, then you will get problems with toxicity, or other issues that you do not foresee.”
No funding was declared. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
The story of antimicrobial peptides (AMPs), particularly in tackling antibiotic resistance, has been one of false dawns and unfulfilled promises. But perhaps a new generation of “smarter” compounds could see them find a wider role in clinical practice, said experts.
AMPs may be small molecules, consisting of short chains of amino acids, but these naturally occurring compounds have an important function: They are the “frontline defense” against invasive bacteria, said Henrik Franzyk, MSc Engineering, associate professor in the Department of Drug Design and Pharmacology at the University of Copenhagen in Denmark.
Multifunction Line of Defense
AMPs are cationic, meaning they are positively charged. “The reason why nature has maintained these molecules is that all the microbes out there have a negative surface charge,” explained Hans-Georg Sahl, PhD, emeritus professor of pharmaceutical microbiology at the University of Bonn in Germany.
“Thus, the content of a cell gets released, and it destroys the pathogen,” explained Paulina Szymczak, a PhD candidate in the Institute of AI for Health at Helmholtz Munich, Neuherberg, Germany.
“There are variations of that theme,” said Eefjan Breukink, PhD, professor of microbial membranes and antibiotics at Utrecht University in the Netherlands. “And then it depends on the sequence of the particular peptide,” as some can cross the cell membrane and damage the bacterium internally.
Szymczak explained that AMPs can, in this way, target the cell DNA, as both the membrane and the DNA are negatively charged. “That’s also what makes them so powerful because they don’t have just one mechanism of action, as opposed to conventional antibiotics.”
Indiscriminate Killers
But they also have another crucial function. They activate the innate immune system via so-called resident immune cells that are “sitting in the tissues and waiting for bacteria to turn up,” explained Franzyk.
“The problem with antibodies is that they typically need to replicate,” he continued, which takes between 4 and 7 days — a timeline that is much better suited to tackling a viral infection. Bacteria, on the other hand, have a replication cycle of just 30 minutes.
Another big problem is that AMPs kill cells indiscriminately, including our own.
“But the human body is clever in that it only produces these antimicrobial peptides where the bacteria are, so they are not circulating in the blood,” said Franzyk. If a small part of tissue becomes infected, the innate immune cells start producing AMPs, which may kill the bacteria, or call on other immune cells to help.
As part of this process, “they will also kill part of our own tissue, but that’s the price we have to pay,” he said.
Local Applications
It is this aspect that has, so far, limited the use of AMPs in clinical practice, certainly as a replacement for conventional antibiotics limited by bacterial resistance. The trials conducted so far have been, by and large, negative, which has dampened enthusiasm and led to the perception that the risk they pose is too great for large-scale investment.
AMPs “are not made for what we need from antibiotics in the first place,” explained Sahl. “That is, a nice, easy distribution in the body, going into abscesses” and throughout the tissues.
He continued that AMPs are “more about controlling the flora in our bodies,” and they are “really not made for being used systemically.”
Szymczak and colleagues are now working on designing active peptides with a strong antibacterial profile but limited toxicity for systematic use.
However, the “downside with these peptides is that they are not orally available, so you can’t take a pill,” Breukink said, but instead they need to be administered intravenously.
There are, nevertheless, some antibiotics in clinical use that have the same molecular features as AMPs. These include colistin, a last-resort treatment for multidrug-resistant gram-negative bacteria, and daptomycin, which is used in the treatment of systemic infections caused by gram-positive species.
Szymczak added that there have been successes in using AMPs in a more targeted way, such as using a topical cream. Another potentially promising avenue is lung infections, which are being studied in mouse models.
Less Prone to Resistance
Crucially, AMPs are markedly less prone to bacterial resistance than conventional antibiotics, partly because of their typical target: the cell membrane.
“Biologically and evolutionarily, it is a very costly operation to rebuild the membrane and change its charge,” Szymczak explained. “It’s quite hard for bacteria to learn this because it’s not a single protein that you have to mutate but the whole membrane.”
This is seen in the laboratory, where it takes around five generations, or passages, for bacteria to develop resistance when grown in the presence of antibiotics, but up to 40 passages when cultured with an AMP.
The limits of the ability of AMPs to withstand the development of bacterial resistance have been tested in the real world.
Colistin has been used widely in Asia as a growth promoter, especially in pig farming. Franzyk explained that farmers have used enormous quantities of this AMP-based antibiotic, which has indeed led to the development of resistance, including contamination of meat for human consumption, leading to resistance spreading to other parts of the world.
“The bad thing about this is it’s not something each individual bacteria needs to acquire,” he said. Because resistance is stored on small, cyclic DNA called plasmids, it “can be transferred from one bacterial species to another.”
Novel Avenues
Franzyk suggested that AMPs could nevertheless be used in combination with, or to modify, existing antibiotics to revitalize those for which there is already bacterial resistance, or to allow antibiotics that ordinarily target only gram-positive bacteria to also treat gram-negative infections, for example.
Szymczak and her colleagues are using artificial intelligence to design novel AMP candidates. Instead of manually going through compounds and checking their activity profiles in the lab, those steps are carried out computationally “so that, in the end, you synthesize as few candidates as possible” and can proceed to a mouse model “as fast as possible.”
She personally is looking at the issue of strain-specific activity to design a compound that would target, for example, only multidrug-resistant strains. “What we can do now is something that will target everything, so a kind of last resort peptide. But we are trying to make them smarter in their targets.”
Szymczak also pointed out that cancer cells are “negatively charged, similarly to bacterial cells, as opposed to mammalian cells, which are neutral.”
“So in theory, maybe we could design something that will target cancer cells but not our host cells, and that would be extremely exciting.” However, she underlined that, first, they are trying to tackle antimicrobial resistance before looking at other spaces.
Finally, Breukink is screening for small antibacterial compounds in fungi that are around half the size of a normal peptide and more hydrophobic, meaning there is a much greater chance of them being orally available.
But “you first have to test, of course,” he said, as “if you don’t have specific targets, then you will get problems with toxicity, or other issues that you do not foresee.”
No funding was declared. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
The story of antimicrobial peptides (AMPs), particularly in tackling antibiotic resistance, has been one of false dawns and unfulfilled promises. But perhaps a new generation of “smarter” compounds could see them find a wider role in clinical practice, said experts.
AMPs may be small molecules, consisting of short chains of amino acids, but these naturally occurring compounds have an important function: They are the “frontline defense” against invasive bacteria, said Henrik Franzyk, MSc Engineering, associate professor in the Department of Drug Design and Pharmacology at the University of Copenhagen in Denmark.
Multifunction Line of Defense
AMPs are cationic, meaning they are positively charged. “The reason why nature has maintained these molecules is that all the microbes out there have a negative surface charge,” explained Hans-Georg Sahl, PhD, emeritus professor of pharmaceutical microbiology at the University of Bonn in Germany.
“Thus, the content of a cell gets released, and it destroys the pathogen,” explained Paulina Szymczak, a PhD candidate in the Institute of AI for Health at Helmholtz Munich, Neuherberg, Germany.
“There are variations of that theme,” said Eefjan Breukink, PhD, professor of microbial membranes and antibiotics at Utrecht University in the Netherlands. “And then it depends on the sequence of the particular peptide,” as some can cross the cell membrane and damage the bacterium internally.
Szymczak explained that AMPs can, in this way, target the cell DNA, as both the membrane and the DNA are negatively charged. “That’s also what makes them so powerful because they don’t have just one mechanism of action, as opposed to conventional antibiotics.”
Indiscriminate Killers
But they also have another crucial function. They activate the innate immune system via so-called resident immune cells that are “sitting in the tissues and waiting for bacteria to turn up,” explained Franzyk.
“The problem with antibodies is that they typically need to replicate,” he continued, which takes between 4 and 7 days — a timeline that is much better suited to tackling a viral infection. Bacteria, on the other hand, have a replication cycle of just 30 minutes.
Another big problem is that AMPs kill cells indiscriminately, including our own.
“But the human body is clever in that it only produces these antimicrobial peptides where the bacteria are, so they are not circulating in the blood,” said Franzyk. If a small part of tissue becomes infected, the innate immune cells start producing AMPs, which may kill the bacteria, or call on other immune cells to help.
As part of this process, “they will also kill part of our own tissue, but that’s the price we have to pay,” he said.
Local Applications
It is this aspect that has, so far, limited the use of AMPs in clinical practice, certainly as a replacement for conventional antibiotics limited by bacterial resistance. The trials conducted so far have been, by and large, negative, which has dampened enthusiasm and led to the perception that the risk they pose is too great for large-scale investment.
AMPs “are not made for what we need from antibiotics in the first place,” explained Sahl. “That is, a nice, easy distribution in the body, going into abscesses” and throughout the tissues.
He continued that AMPs are “more about controlling the flora in our bodies,” and they are “really not made for being used systemically.”
Szymczak and colleagues are now working on designing active peptides with a strong antibacterial profile but limited toxicity for systematic use.
However, the “downside with these peptides is that they are not orally available, so you can’t take a pill,” Breukink said, but instead they need to be administered intravenously.
There are, nevertheless, some antibiotics in clinical use that have the same molecular features as AMPs. These include colistin, a last-resort treatment for multidrug-resistant gram-negative bacteria, and daptomycin, which is used in the treatment of systemic infections caused by gram-positive species.
Szymczak added that there have been successes in using AMPs in a more targeted way, such as using a topical cream. Another potentially promising avenue is lung infections, which are being studied in mouse models.
Less Prone to Resistance
Crucially, AMPs are markedly less prone to bacterial resistance than conventional antibiotics, partly because of their typical target: the cell membrane.
“Biologically and evolutionarily, it is a very costly operation to rebuild the membrane and change its charge,” Szymczak explained. “It’s quite hard for bacteria to learn this because it’s not a single protein that you have to mutate but the whole membrane.”
This is seen in the laboratory, where it takes around five generations, or passages, for bacteria to develop resistance when grown in the presence of antibiotics, but up to 40 passages when cultured with an AMP.
The limits of the ability of AMPs to withstand the development of bacterial resistance have been tested in the real world.
Colistin has been used widely in Asia as a growth promoter, especially in pig farming. Franzyk explained that farmers have used enormous quantities of this AMP-based antibiotic, which has indeed led to the development of resistance, including contamination of meat for human consumption, leading to resistance spreading to other parts of the world.
“The bad thing about this is it’s not something each individual bacteria needs to acquire,” he said. Because resistance is stored on small, cyclic DNA called plasmids, it “can be transferred from one bacterial species to another.”
Novel Avenues
Franzyk suggested that AMPs could nevertheless be used in combination with, or to modify, existing antibiotics to revitalize those for which there is already bacterial resistance, or to allow antibiotics that ordinarily target only gram-positive bacteria to also treat gram-negative infections, for example.
Szymczak and her colleagues are using artificial intelligence to design novel AMP candidates. Instead of manually going through compounds and checking their activity profiles in the lab, those steps are carried out computationally “so that, in the end, you synthesize as few candidates as possible” and can proceed to a mouse model “as fast as possible.”
She personally is looking at the issue of strain-specific activity to design a compound that would target, for example, only multidrug-resistant strains. “What we can do now is something that will target everything, so a kind of last resort peptide. But we are trying to make them smarter in their targets.”
Szymczak also pointed out that cancer cells are “negatively charged, similarly to bacterial cells, as opposed to mammalian cells, which are neutral.”
“So in theory, maybe we could design something that will target cancer cells but not our host cells, and that would be extremely exciting.” However, she underlined that, first, they are trying to tackle antimicrobial resistance before looking at other spaces.
Finally, Breukink is screening for small antibacterial compounds in fungi that are around half the size of a normal peptide and more hydrophobic, meaning there is a much greater chance of them being orally available.
But “you first have to test, of course,” he said, as “if you don’t have specific targets, then you will get problems with toxicity, or other issues that you do not foresee.”
No funding was declared. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
Early Oseltamivir Benefits Hospitalized Influenza Patients
TOPLINE:
Early treatment with oseltamivir on the same day as hospital admission was associated with fewer severe clinical outcomes, such as worsening pulmonary disease, need for invasive ventilation, organ failure, and in-hospital death in adults hospitalized with influenza.
METHODOLOGY:
- The 2018 guidelines from the Infectious Disease Society of America recommend prompt administration of oseltamivir to hospitalized patients with suspected or confirmed influenza, regardless of the time of symptom onset; however, variations in treatment practices and circulating virus strains may affect the effectiveness of this practice guideline.
- Researchers conducted a multicenter observational study across 24 hospitals in the United States during the 2022-2023 flu season to assess the benefits of initiating oseltamivir treatment on the same day as hospital admission for adults with acute influenza, compared with late or no treatment.
- They included 840 adults (age, ≥ 18 years) with laboratory-confirmed influenza, of which 415 patients initiated oseltamivir on the same day as hospital admission (early treatment).
- Among the 425 patients in the late/no treatment group, most (78%) received oseltamivir 1 day after admission, while 124 did not receive oseltamivir at all.
- The primary outcome was the peak pulmonary disease severity level that patients experienced during hospitalization, and secondary outcomes included hospital length of stay, ICU admission, initiation of extrapulmonary organ support using vasopressors or kidney replacement therapy, and in-hospital death.
TAKEAWAY:
- Patients in the early treatment group were less likely to experience progression and severe progression of pulmonary disease after the day of hospital admission, compared with those in the late or no treatment group (P < .001 and P = .027, respectively).
- Patients who received early oseltamivir treatment had 40% lower peak pulmonary disease severity than those who received late or no treatment (proportional adjusted odds ratio [paOR], 0.60; 95% CI, 0.49-0.72).
- They also showed lower odds of ICU admission (aOR, 0.25; 95% CI, 0.13-0.49) and use of acute kidney replacement therapy or vasopressors (aOR, 0.40; 95% CI, 0.22-0.67).
- Those in the early treatment group also had a shorter hospital length of stay (median, 4 days vs 4 days) and faced a 64% lower risk for in-hospital mortality (aOR, 0.36; 95% CI, 0.19-0.69) compared with those in the late or no treatment group.
IN PRACTICE:
“These findings support current recommendations, such as the IDSA [Infectious Disease Society of America] Influenza Clinical Practice Guidelines and CDC [Centers for Disease Control and Prevention] guidance, to initiate oseltamivir treatment as soon as possible for adult patients hospitalized with influenza,” the authors wrote.
SOURCE:
The study was led by Nathaniel M. Lewis, PhD, Influenza Division, CDC, Atlanta, Georgia, and was published online in Clinical Infectious Diseases.
LIMITATIONS:
This study may not be generalizable to seasons when influenza A(H1N1)pdm09 or B viruses are predominant as it was conducted during an influenza A(H3N2) virus–predominant season. The study lacked sufficient power to examine various oseltamivir treatment initiation timepoints or identify a potential maximum time-to-treatment threshold for effectiveness. Moreover, variables such as outpatient antiviral treatment before hospital admission and other treatments using macrolides, statins, corticosteroids, or immunomodulators before or during hospitalization were not collected, which may have influenced the study findings.
DISCLOSURES:
The study received funding from the CDC and the National Center for Immunization and Respiratory Diseases. Some authors reported receiving research support, consulting fees, funding, grants, or fees for participation in an advisory board and having other ties with certain institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Early treatment with oseltamivir on the same day as hospital admission was associated with fewer severe clinical outcomes, such as worsening pulmonary disease, need for invasive ventilation, organ failure, and in-hospital death in adults hospitalized with influenza.
METHODOLOGY:
- The 2018 guidelines from the Infectious Disease Society of America recommend prompt administration of oseltamivir to hospitalized patients with suspected or confirmed influenza, regardless of the time of symptom onset; however, variations in treatment practices and circulating virus strains may affect the effectiveness of this practice guideline.
- Researchers conducted a multicenter observational study across 24 hospitals in the United States during the 2022-2023 flu season to assess the benefits of initiating oseltamivir treatment on the same day as hospital admission for adults with acute influenza, compared with late or no treatment.
- They included 840 adults (age, ≥ 18 years) with laboratory-confirmed influenza, of which 415 patients initiated oseltamivir on the same day as hospital admission (early treatment).
- Among the 425 patients in the late/no treatment group, most (78%) received oseltamivir 1 day after admission, while 124 did not receive oseltamivir at all.
- The primary outcome was the peak pulmonary disease severity level that patients experienced during hospitalization, and secondary outcomes included hospital length of stay, ICU admission, initiation of extrapulmonary organ support using vasopressors or kidney replacement therapy, and in-hospital death.
TAKEAWAY:
- Patients in the early treatment group were less likely to experience progression and severe progression of pulmonary disease after the day of hospital admission, compared with those in the late or no treatment group (P < .001 and P = .027, respectively).
- Patients who received early oseltamivir treatment had 40% lower peak pulmonary disease severity than those who received late or no treatment (proportional adjusted odds ratio [paOR], 0.60; 95% CI, 0.49-0.72).
- They also showed lower odds of ICU admission (aOR, 0.25; 95% CI, 0.13-0.49) and use of acute kidney replacement therapy or vasopressors (aOR, 0.40; 95% CI, 0.22-0.67).
- Those in the early treatment group also had a shorter hospital length of stay (median, 4 days vs 4 days) and faced a 64% lower risk for in-hospital mortality (aOR, 0.36; 95% CI, 0.19-0.69) compared with those in the late or no treatment group.
IN PRACTICE:
“These findings support current recommendations, such as the IDSA [Infectious Disease Society of America] Influenza Clinical Practice Guidelines and CDC [Centers for Disease Control and Prevention] guidance, to initiate oseltamivir treatment as soon as possible for adult patients hospitalized with influenza,” the authors wrote.
SOURCE:
The study was led by Nathaniel M. Lewis, PhD, Influenza Division, CDC, Atlanta, Georgia, and was published online in Clinical Infectious Diseases.
LIMITATIONS:
This study may not be generalizable to seasons when influenza A(H1N1)pdm09 or B viruses are predominant as it was conducted during an influenza A(H3N2) virus–predominant season. The study lacked sufficient power to examine various oseltamivir treatment initiation timepoints or identify a potential maximum time-to-treatment threshold for effectiveness. Moreover, variables such as outpatient antiviral treatment before hospital admission and other treatments using macrolides, statins, corticosteroids, or immunomodulators before or during hospitalization were not collected, which may have influenced the study findings.
DISCLOSURES:
The study received funding from the CDC and the National Center for Immunization and Respiratory Diseases. Some authors reported receiving research support, consulting fees, funding, grants, or fees for participation in an advisory board and having other ties with certain institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Early treatment with oseltamivir on the same day as hospital admission was associated with fewer severe clinical outcomes, such as worsening pulmonary disease, need for invasive ventilation, organ failure, and in-hospital death in adults hospitalized with influenza.
METHODOLOGY:
- The 2018 guidelines from the Infectious Disease Society of America recommend prompt administration of oseltamivir to hospitalized patients with suspected or confirmed influenza, regardless of the time of symptom onset; however, variations in treatment practices and circulating virus strains may affect the effectiveness of this practice guideline.
- Researchers conducted a multicenter observational study across 24 hospitals in the United States during the 2022-2023 flu season to assess the benefits of initiating oseltamivir treatment on the same day as hospital admission for adults with acute influenza, compared with late or no treatment.
- They included 840 adults (age, ≥ 18 years) with laboratory-confirmed influenza, of which 415 patients initiated oseltamivir on the same day as hospital admission (early treatment).
- Among the 425 patients in the late/no treatment group, most (78%) received oseltamivir 1 day after admission, while 124 did not receive oseltamivir at all.
- The primary outcome was the peak pulmonary disease severity level that patients experienced during hospitalization, and secondary outcomes included hospital length of stay, ICU admission, initiation of extrapulmonary organ support using vasopressors or kidney replacement therapy, and in-hospital death.
TAKEAWAY:
- Patients in the early treatment group were less likely to experience progression and severe progression of pulmonary disease after the day of hospital admission, compared with those in the late or no treatment group (P < .001 and P = .027, respectively).
- Patients who received early oseltamivir treatment had 40% lower peak pulmonary disease severity than those who received late or no treatment (proportional adjusted odds ratio [paOR], 0.60; 95% CI, 0.49-0.72).
- They also showed lower odds of ICU admission (aOR, 0.25; 95% CI, 0.13-0.49) and use of acute kidney replacement therapy or vasopressors (aOR, 0.40; 95% CI, 0.22-0.67).
- Those in the early treatment group also had a shorter hospital length of stay (median, 4 days vs 4 days) and faced a 64% lower risk for in-hospital mortality (aOR, 0.36; 95% CI, 0.19-0.69) compared with those in the late or no treatment group.
IN PRACTICE:
“These findings support current recommendations, such as the IDSA [Infectious Disease Society of America] Influenza Clinical Practice Guidelines and CDC [Centers for Disease Control and Prevention] guidance, to initiate oseltamivir treatment as soon as possible for adult patients hospitalized with influenza,” the authors wrote.
SOURCE:
The study was led by Nathaniel M. Lewis, PhD, Influenza Division, CDC, Atlanta, Georgia, and was published online in Clinical Infectious Diseases.
LIMITATIONS:
This study may not be generalizable to seasons when influenza A(H1N1)pdm09 or B viruses are predominant as it was conducted during an influenza A(H3N2) virus–predominant season. The study lacked sufficient power to examine various oseltamivir treatment initiation timepoints or identify a potential maximum time-to-treatment threshold for effectiveness. Moreover, variables such as outpatient antiviral treatment before hospital admission and other treatments using macrolides, statins, corticosteroids, or immunomodulators before or during hospitalization were not collected, which may have influenced the study findings.
DISCLOSURES:
The study received funding from the CDC and the National Center for Immunization and Respiratory Diseases. Some authors reported receiving research support, consulting fees, funding, grants, or fees for participation in an advisory board and having other ties with certain institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Your Guide to COVID Vaccines for 2024-2025
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.
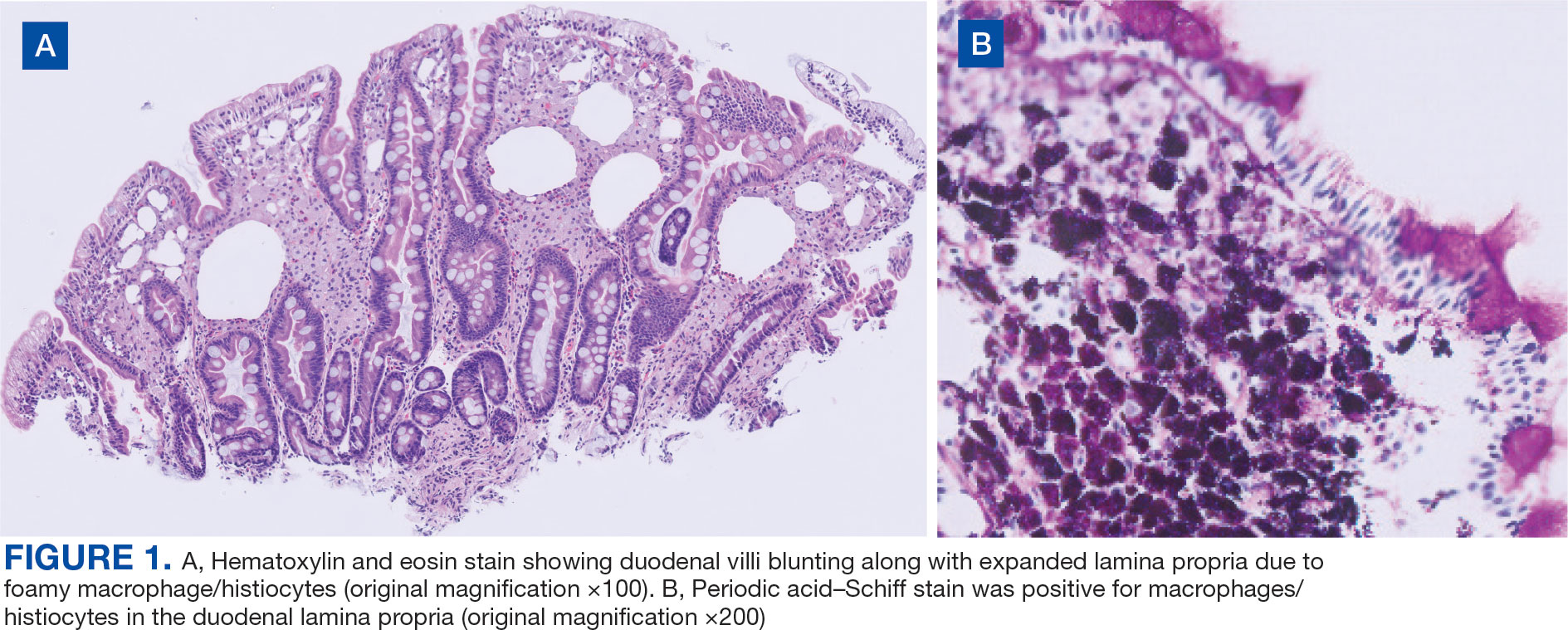
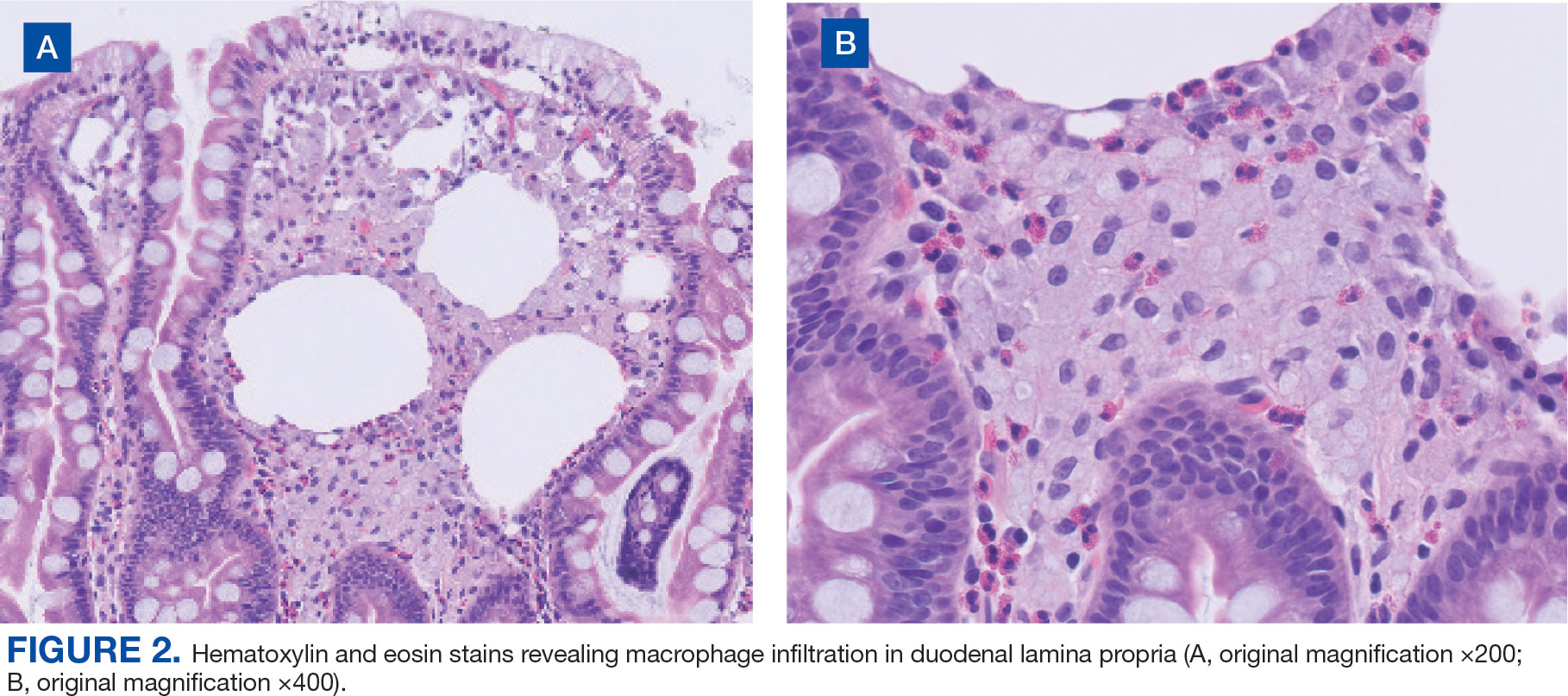
We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.
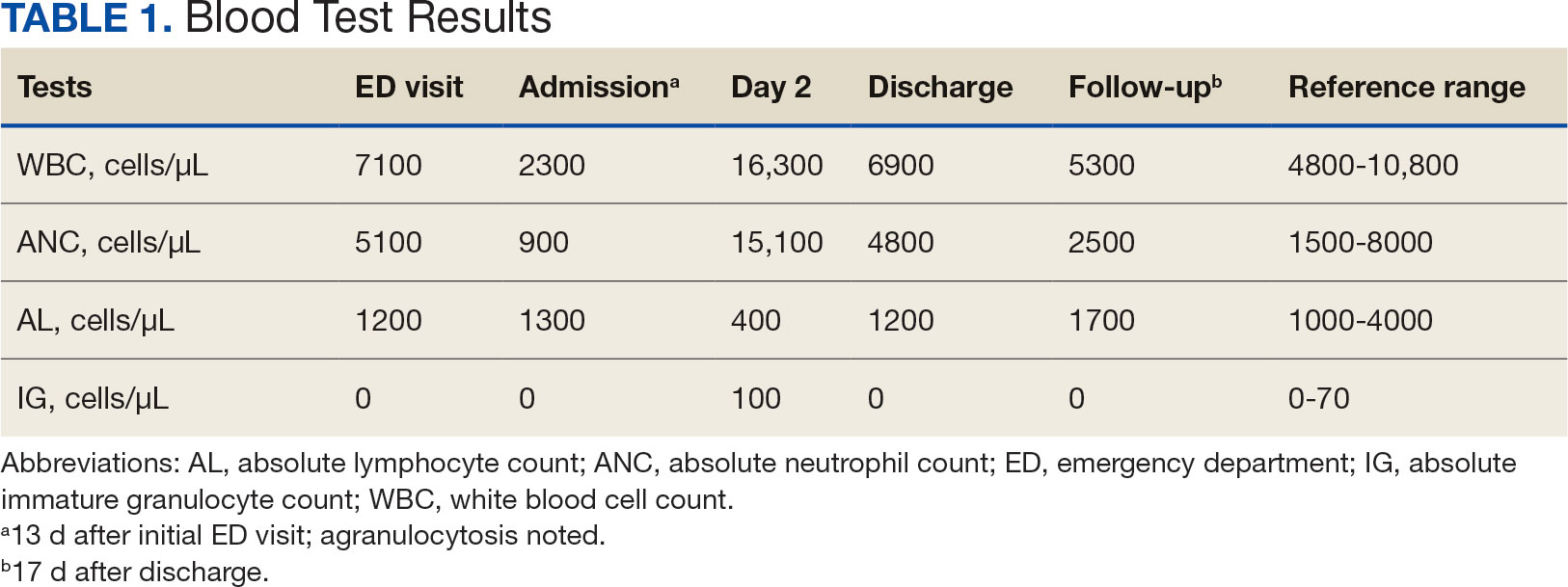
Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.

Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.

Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
The Multipronged Problem of Candida auris
The Multipronged Problem of Candida auris
Candida auris, a yeast-like fungus, is spreading globally, increasing the urgency for enhanced surveillance, new therapies, and more antimicrobial stewardship to combat its multidrug-resistant strains.
Since its discovery in 2009, C auris has been found in more than 50 countries across six continents, including Asia, Africa, and the Americas, according to the World Health Organization. In 2022, CDC reported 2377 clinical cases and 5754 screening cases of C auris in the United States.
In September, The Lancet Microbe reported on three C auris isolates from a Singapore hospital belonging to a new clade (clade six), “which is phenotypically and genotypically distinct” from the first five clades, the authors wrote. In June, Microbiology Spectrum published a study about two unusual C auris isolates from a Bangladesh NICU in 2021. They were also assigned to clade six “with potential for international transmission,” the study authors noted.
C auris has all the hallmarks of “critical pathogen,” as defined by the World Health Organization in 2022. It increases morbidity and mortality for affected patients, is difficult to eradicate in hospitals, and can be treatment resistant.
As a result, infectious disease specialists are raising more awareness and advocating for greater surveillance of C auris colonization and disease in the hospital setting for high-risk patients.
Arturo Casadevall, MD, PhD, MS, is one of them. “C auris could be a problem in your hospital as fungal diseases are getting worse every year,” said Casadevall, chair of Molecular Microbiology and Immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore. The increasing number of cases “is incremental, but when [we] look at the data over years, it is a growing problem. We may see more of these cases in the coming years.”
Expediting Diagnoses
Symptoms of C auris disease vary and can cause invasive infections, such as bloodstream or intra-abdominal infections. This is why Casadevall encourages infectious disease specialists to “always consider fungal disease when you are approaching an individual. The diagnosis is sometimes delayed because you don’t look for it,” he said.
C auris can also be misidentified in the lab “when using traditional biochemical methods for yeast identification. Accurate identification of C auris requires use of sequencing or mass spectrometry,” according to CDC.
C auris is typically found on the skin of colonized patients and can enter the body through invasive devices, incisions, wounds, and during surgery. Mostly, immunosuppressed patients are at risk for serious fungal disease, Casadevall said.
Invasive fungal disease can be life-threatening for hospitalized patients. In one review of 37 studies from 2011 to 2021, researchers found that overall mortality rates for C auris infections ranged from 29% to 62%, with 30-day mortality rates between 23% and 67%, Medical Mycology reported. Patients typically had a median hospital stay of 46-68 days, sometimes extending up to 140 days. Late-onset complications included metastatic septic issues, according to the study.
Overcoming Treatment-Resistant Strains
A resilient yeast, C auris shows higher resistance to antifungal treatments compared to other Candida species, JAMA reported. Echinocandins are the first-line treatment for adults and children over 2 months old “and some of those therapies are already resistant,” said George Thompson, MD, professor of clinical medicine at the University of California Davis School of Medicine, Davis, California. The second line is liposomal amphotericin B (5 mg/kg daily), but it has toxicity problems, Thompson said.
New therapies sans toxicity are needed to treat C auris disease. Thompson, eg, served as the principal investigator in the ReSTORE trial to study a new therapy (rezafungin for injection). In March 2023, the US Food and Drug Administration approved the treatment for candidemia and invasive candidiasis in adults with limited or no alternative treatment options.
Thompson has observed that patients with C auris disease can present with “an infection in the urinary system with burning, pain, and bladder spasms. In the majority of cases of candida sepsis, the patients will have it in their blood stream with fever, chills, and sweats,” he said. The new treatment may clear the infection quickly, said Thompson, who noted results published in The Lancet.
Infection Prevention and Antimicrobial Stewardship
Institutions like University of Michigan Health (U-M Health) in Ann Arbor, Michigan, have increased measures to tackle the issue from different angles.
To address the broader issue of treatment-resistant fungal disease, U-M Health “has a robust antimicrobial stewardship program in place,” said Laraine Lynn Washer, MD, infectious disease physician.
The program includes oversight and restriction of various antifungals to avoid potential for overuse that could lead to increased risk for antifungal resistance. Use of echinocandins, for example, “requires prior approval by our antimicrobial stewardship team members,” said Washer, who is also Clinical Professor of Infectious Diseases and the Medical Director of Infection Prevention of Epidemiology at U-M Health.
Infection prevention measures entail screening hospitalized adult patients for risk factors for C auris, such as:
- Overnight international hospitalization
- Recent stay in a long-term acute care facility
- Recent stay in a ventilator skilled nursing facility.
“If a patient has these risk factors, we perform testing to assess for colonization (presence of C auris without infection) by obtaining skin swabs from the axilla and the groin and asking our lab to perform PCR to identify genetic elements of C auris,” Washer said. “Patients who are transferred directly from another hospital ICU to our ICU also undergo testing for colonization.”
If a patient is identified with C auris, hospitals ought to perform screening tests using cultures or PCR “on other patients who may have overlapped in time and space with the patient such as hospital roommates,” Washer explained.
Once in a hospital environment, the pathogen is hard to eradicate. C auris has a unique ability to be transmitted in the healthcare environment, is relatively heat tolerant, and is resistant to some common disinfectants, Washer added. The yeast can survive for over 2 weeks on plastic and months on skin, JAMA reported.
“Hospitals should partner with local and state level public health authorities in reporting cases of Candida auris and assist in any contact investigations as requested by public health authorities,” Washer advised.
Casadevall and Washer reported no conflicts of interest. Thompson has consulted and received research funding from Astellas, Basilea, Cidara, F2G, GSK, Melinta, Mundipharma, Pfizer, and Scynexis.
A version of this article appeared on Medscape.com.
Candida auris, a yeast-like fungus, is spreading globally, increasing the urgency for enhanced surveillance, new therapies, and more antimicrobial stewardship to combat its multidrug-resistant strains.
Since its discovery in 2009, C auris has been found in more than 50 countries across six continents, including Asia, Africa, and the Americas, according to the World Health Organization. In 2022, CDC reported 2377 clinical cases and 5754 screening cases of C auris in the United States.
In September, The Lancet Microbe reported on three C auris isolates from a Singapore hospital belonging to a new clade (clade six), “which is phenotypically and genotypically distinct” from the first five clades, the authors wrote. In June, Microbiology Spectrum published a study about two unusual C auris isolates from a Bangladesh NICU in 2021. They were also assigned to clade six “with potential for international transmission,” the study authors noted.
C auris has all the hallmarks of “critical pathogen,” as defined by the World Health Organization in 2022. It increases morbidity and mortality for affected patients, is difficult to eradicate in hospitals, and can be treatment resistant.
As a result, infectious disease specialists are raising more awareness and advocating for greater surveillance of C auris colonization and disease in the hospital setting for high-risk patients.
Arturo Casadevall, MD, PhD, MS, is one of them. “C auris could be a problem in your hospital as fungal diseases are getting worse every year,” said Casadevall, chair of Molecular Microbiology and Immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore. The increasing number of cases “is incremental, but when [we] look at the data over years, it is a growing problem. We may see more of these cases in the coming years.”
Expediting Diagnoses
Symptoms of C auris disease vary and can cause invasive infections, such as bloodstream or intra-abdominal infections. This is why Casadevall encourages infectious disease specialists to “always consider fungal disease when you are approaching an individual. The diagnosis is sometimes delayed because you don’t look for it,” he said.
C auris can also be misidentified in the lab “when using traditional biochemical methods for yeast identification. Accurate identification of C auris requires use of sequencing or mass spectrometry,” according to CDC.
C auris is typically found on the skin of colonized patients and can enter the body through invasive devices, incisions, wounds, and during surgery. Mostly, immunosuppressed patients are at risk for serious fungal disease, Casadevall said.
Invasive fungal disease can be life-threatening for hospitalized patients. In one review of 37 studies from 2011 to 2021, researchers found that overall mortality rates for C auris infections ranged from 29% to 62%, with 30-day mortality rates between 23% and 67%, Medical Mycology reported. Patients typically had a median hospital stay of 46-68 days, sometimes extending up to 140 days. Late-onset complications included metastatic septic issues, according to the study.
Overcoming Treatment-Resistant Strains
A resilient yeast, C auris shows higher resistance to antifungal treatments compared to other Candida species, JAMA reported. Echinocandins are the first-line treatment for adults and children over 2 months old “and some of those therapies are already resistant,” said George Thompson, MD, professor of clinical medicine at the University of California Davis School of Medicine, Davis, California. The second line is liposomal amphotericin B (5 mg/kg daily), but it has toxicity problems, Thompson said.
New therapies sans toxicity are needed to treat C auris disease. Thompson, eg, served as the principal investigator in the ReSTORE trial to study a new therapy (rezafungin for injection). In March 2023, the US Food and Drug Administration approved the treatment for candidemia and invasive candidiasis in adults with limited or no alternative treatment options.
Thompson has observed that patients with C auris disease can present with “an infection in the urinary system with burning, pain, and bladder spasms. In the majority of cases of candida sepsis, the patients will have it in their blood stream with fever, chills, and sweats,” he said. The new treatment may clear the infection quickly, said Thompson, who noted results published in The Lancet.
Infection Prevention and Antimicrobial Stewardship
Institutions like University of Michigan Health (U-M Health) in Ann Arbor, Michigan, have increased measures to tackle the issue from different angles.
To address the broader issue of treatment-resistant fungal disease, U-M Health “has a robust antimicrobial stewardship program in place,” said Laraine Lynn Washer, MD, infectious disease physician.
The program includes oversight and restriction of various antifungals to avoid potential for overuse that could lead to increased risk for antifungal resistance. Use of echinocandins, for example, “requires prior approval by our antimicrobial stewardship team members,” said Washer, who is also Clinical Professor of Infectious Diseases and the Medical Director of Infection Prevention of Epidemiology at U-M Health.
Infection prevention measures entail screening hospitalized adult patients for risk factors for C auris, such as:
- Overnight international hospitalization
- Recent stay in a long-term acute care facility
- Recent stay in a ventilator skilled nursing facility.
“If a patient has these risk factors, we perform testing to assess for colonization (presence of C auris without infection) by obtaining skin swabs from the axilla and the groin and asking our lab to perform PCR to identify genetic elements of C auris,” Washer said. “Patients who are transferred directly from another hospital ICU to our ICU also undergo testing for colonization.”
If a patient is identified with C auris, hospitals ought to perform screening tests using cultures or PCR “on other patients who may have overlapped in time and space with the patient such as hospital roommates,” Washer explained.
Once in a hospital environment, the pathogen is hard to eradicate. C auris has a unique ability to be transmitted in the healthcare environment, is relatively heat tolerant, and is resistant to some common disinfectants, Washer added. The yeast can survive for over 2 weeks on plastic and months on skin, JAMA reported.
“Hospitals should partner with local and state level public health authorities in reporting cases of Candida auris and assist in any contact investigations as requested by public health authorities,” Washer advised.
Casadevall and Washer reported no conflicts of interest. Thompson has consulted and received research funding from Astellas, Basilea, Cidara, F2G, GSK, Melinta, Mundipharma, Pfizer, and Scynexis.
A version of this article appeared on Medscape.com.
Candida auris, a yeast-like fungus, is spreading globally, increasing the urgency for enhanced surveillance, new therapies, and more antimicrobial stewardship to combat its multidrug-resistant strains.
Since its discovery in 2009, C auris has been found in more than 50 countries across six continents, including Asia, Africa, and the Americas, according to the World Health Organization. In 2022, CDC reported 2377 clinical cases and 5754 screening cases of C auris in the United States.
In September, The Lancet Microbe reported on three C auris isolates from a Singapore hospital belonging to a new clade (clade six), “which is phenotypically and genotypically distinct” from the first five clades, the authors wrote. In June, Microbiology Spectrum published a study about two unusual C auris isolates from a Bangladesh NICU in 2021. They were also assigned to clade six “with potential for international transmission,” the study authors noted.
C auris has all the hallmarks of “critical pathogen,” as defined by the World Health Organization in 2022. It increases morbidity and mortality for affected patients, is difficult to eradicate in hospitals, and can be treatment resistant.
As a result, infectious disease specialists are raising more awareness and advocating for greater surveillance of C auris colonization and disease in the hospital setting for high-risk patients.
Arturo Casadevall, MD, PhD, MS, is one of them. “C auris could be a problem in your hospital as fungal diseases are getting worse every year,” said Casadevall, chair of Molecular Microbiology and Immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore. The increasing number of cases “is incremental, but when [we] look at the data over years, it is a growing problem. We may see more of these cases in the coming years.”
Expediting Diagnoses
Symptoms of C auris disease vary and can cause invasive infections, such as bloodstream or intra-abdominal infections. This is why Casadevall encourages infectious disease specialists to “always consider fungal disease when you are approaching an individual. The diagnosis is sometimes delayed because you don’t look for it,” he said.
C auris can also be misidentified in the lab “when using traditional biochemical methods for yeast identification. Accurate identification of C auris requires use of sequencing or mass spectrometry,” according to CDC.
C auris is typically found on the skin of colonized patients and can enter the body through invasive devices, incisions, wounds, and during surgery. Mostly, immunosuppressed patients are at risk for serious fungal disease, Casadevall said.
Invasive fungal disease can be life-threatening for hospitalized patients. In one review of 37 studies from 2011 to 2021, researchers found that overall mortality rates for C auris infections ranged from 29% to 62%, with 30-day mortality rates between 23% and 67%, Medical Mycology reported. Patients typically had a median hospital stay of 46-68 days, sometimes extending up to 140 days. Late-onset complications included metastatic septic issues, according to the study.
Overcoming Treatment-Resistant Strains
A resilient yeast, C auris shows higher resistance to antifungal treatments compared to other Candida species, JAMA reported. Echinocandins are the first-line treatment for adults and children over 2 months old “and some of those therapies are already resistant,” said George Thompson, MD, professor of clinical medicine at the University of California Davis School of Medicine, Davis, California. The second line is liposomal amphotericin B (5 mg/kg daily), but it has toxicity problems, Thompson said.
New therapies sans toxicity are needed to treat C auris disease. Thompson, eg, served as the principal investigator in the ReSTORE trial to study a new therapy (rezafungin for injection). In March 2023, the US Food and Drug Administration approved the treatment for candidemia and invasive candidiasis in adults with limited or no alternative treatment options.
Thompson has observed that patients with C auris disease can present with “an infection in the urinary system with burning, pain, and bladder spasms. In the majority of cases of candida sepsis, the patients will have it in their blood stream with fever, chills, and sweats,” he said. The new treatment may clear the infection quickly, said Thompson, who noted results published in The Lancet.
Infection Prevention and Antimicrobial Stewardship
Institutions like University of Michigan Health (U-M Health) in Ann Arbor, Michigan, have increased measures to tackle the issue from different angles.
To address the broader issue of treatment-resistant fungal disease, U-M Health “has a robust antimicrobial stewardship program in place,” said Laraine Lynn Washer, MD, infectious disease physician.
The program includes oversight and restriction of various antifungals to avoid potential for overuse that could lead to increased risk for antifungal resistance. Use of echinocandins, for example, “requires prior approval by our antimicrobial stewardship team members,” said Washer, who is also Clinical Professor of Infectious Diseases and the Medical Director of Infection Prevention of Epidemiology at U-M Health.
Infection prevention measures entail screening hospitalized adult patients for risk factors for C auris, such as:
- Overnight international hospitalization
- Recent stay in a long-term acute care facility
- Recent stay in a ventilator skilled nursing facility.
“If a patient has these risk factors, we perform testing to assess for colonization (presence of C auris without infection) by obtaining skin swabs from the axilla and the groin and asking our lab to perform PCR to identify genetic elements of C auris,” Washer said. “Patients who are transferred directly from another hospital ICU to our ICU also undergo testing for colonization.”
If a patient is identified with C auris, hospitals ought to perform screening tests using cultures or PCR “on other patients who may have overlapped in time and space with the patient such as hospital roommates,” Washer explained.
Once in a hospital environment, the pathogen is hard to eradicate. C auris has a unique ability to be transmitted in the healthcare environment, is relatively heat tolerant, and is resistant to some common disinfectants, Washer added. The yeast can survive for over 2 weeks on plastic and months on skin, JAMA reported.
“Hospitals should partner with local and state level public health authorities in reporting cases of Candida auris and assist in any contact investigations as requested by public health authorities,” Washer advised.
Casadevall and Washer reported no conflicts of interest. Thompson has consulted and received research funding from Astellas, Basilea, Cidara, F2G, GSK, Melinta, Mundipharma, Pfizer, and Scynexis.
A version of this article appeared on Medscape.com.
The Multipronged Problem of Candida auris
The Multipronged Problem of Candida auris
H5N1 Avian Influenza Spreads Across North America
It’s been a while since I’ve discussed the H5N1 avian influenza clade 2.3.4.4b and its rapid spread in North America. I hope the facts prove me wrong, but many experts have been warning for some time that ideal conditions are forming for this virus, which for now only causes zoonoses, to pose a pandemic threat.
either because they work in other medical fields or think that the experience of the COVID-19 pandemic was a worst-case scenario that is unlikely to be repeated in the short term.
The Virus Has Flown to Hawaii
According to data from the Centers for Disease Control and Prevention in Atlanta, Georgia, the infection has now affected more than 500 cattle herds in 15 states. There are about 30 outbreaks reported in poultry, equally distributed between backyard and farm-raised birds, primarily located in California. Here alone, over 3 million birds have been affected.
Wild birds are believed to have transported the highly pathogenic virus via migration routes across the Pacific, introducing it to Hawaii for the first time. Just days after wastewater analysis detected the presence of H5N1 on the island of Oahu, home to the capital Honolulu, the first outbreak was promptly reported, killing at least a dozen ducks and geese in a backyard coop. Some of these birds had been taken in early November to the Mililani Pet Fair, a sort of domestic animal festival. Local authorities recommended that anyone who attended the fair, touched a duck or goose at the event, and developed symptoms including fever, cough, sore throat, and conjunctivitis, should isolate and seek medical advice.
Meanwhile, more than 50 farmers, animal handlers, or workers involved in the slaughter of cattle or poultry across seven states have been confirmed infected, presumably contracted at their workplace. The latest case, diagnosed recently in Oregon, presented with severe conjunctivitis and mild respiratory symptoms. More than half of these patients have been identified in recent weeks in California, where active surveillance measures have been implemented. However, there is strong suspicion that the actual number of people infected with mild symptoms in the rest of the country is much, much higher.
The Red Alert Lights Up in Canada
The level of concern was raised further with news of the first severe — indeed very severe — case of H5N1 avian influenza originating from the western edge of Canada. A teenager (gender not disclosed), previously healthy and without risk factors, was hospitalized with severe respiratory failure in the intensive care unit at British Columbia Children’s Hospital in Vancouver. The source of the infection is unknown, similar to only one other case in Missouri involving an adult already hospitalized for other reasons, which was identified by chance through influenza surveillance programs. We also know that the Canadian adolescent does not live on a farm and had no known contact with potentially infected animals. The only suspicions focus on the family dog, euthanized owing to unspecified health problems in the early days of the epidemiologic investigation. Although the dog tested negative for avian influenza, a necropsy will be conducted to rule out its involvement in the transmission chain.
An initial characterization of the virus has linked it to genotype D1.1, which is circulating among wild birds and poultry farms in Canada’s westernmost province, rather than the strain typical of dairy cows in the United States. The publication of the complete viral sequence over the past weekend has, for the first time, highlighted mutations that could enhance the virus’s ability to infect human cells.
How do we know this? From the highly contested “gain-of-function” studies, which artificially modify viruses to understand which genomic points require the most surveillance — those mutations that can make the infectious agent more virulent or more transmissible between people.
Under Special Surveillance for 20 Years
The influenza A (H5N1) avian virus is not new or previously unknown, like SARS-CoV-2, and this could (in theory) give us a slight advantage. We have known about it for decades, and it began infecting humans about 20 years ago, causing pneumonia with respiratory failure. It proved lethal in about half of the cases, but only in people who had close contact with infected poultry, primarily in Southeast Asia.
Hundreds of other human cases occurred worldwide, but always in low-income countries with poor hygiene conditions and where families lived in close contact with animals. This contributed to a false sense of security in Europe and North America, where the threat has been consistently underestimated. Despite an estimated fatality rate of around 50%, the media often labeled scientists’ warnings and health authorities’ efforts to remain prepared as false alarms, tainted by suspicions of catering to the interests of pharmaceutical companies.
Some people may recall the scandal involving Tamiflu, the Roche antiviral oseltamivir, that governments stockpiled when there were fears that the avian virus might acquire the ability to spread among humans. It was dubbed “a false antidote for a false pandemic,” referring to the potential avian pandemic and the 2009 H1N1 influenza pandemic, improperly called “swine flu,” and which turned out to be less severe than expected. There was talk of €2.64 billion being “wasted” to “please” the manufacturer. Although the Cochrane Collaboration made legitimate demands for rigor and transparency in conducting and publishing clinical trials, much of the public, and the journalists who wrote the stories, cared little about these technical aspects. The prevailing message was that stockpiling drugs (or vaccines) for a disease we don’t even know will occur is a waste of taxpayers’ money rather than a prudent preventive measure.
More Vulnerable Than Ever
If we were to ascribe strategic thinking to the virus, which it is not capable of, we might argue that it chose the ideal moment to conquer the world. It began circulating in the new clade in 2020, when experts and authorities were focused on the coronavirus. It spread from birds to marine mammals and finally to cattle, exploiting the public’s post-pandemic fatigue, as people no longer wanted to hear about infectious diseases and containment measures. It ultimately rode the wave of political polarization that irrationally equates prevention with supposed cowardice on the left, and recklessness with courageous freedom on the right.
The coincidence between the future appointments announced by the incoming Trump administration and the virus’s accelerated spread deserves attention from decision-makers and health professionals worldwide. The COVID-19 pandemic experience should have taught us that ignoring a threat doesn’t make it go away, if not in our health, then at least in our wallet. The economic repercussions of a virus circulating among animals crucial to our food chain and national economies should concern everyone, well before the threat crosses the ocean, because only then can we defend ourselves.
The proposed Secretary of Health and Human Services, Robert F. Kennedy, is a proponent of the supposed benefits of raw milk, which could serve as a potent vector for the virus. He is ideologically opposed to vaccinations. It’s hard to imagine he would utilize the H5N1 vaccine stockpiles held by the US government for a campaign starting at least with farmers, as was done prophylactically in Finland with products jointly procured by 15 European countries — a group the Italian government decided not to join.
If Kennedy indeed becomes responsible for US public health, it’s reasonable to fear that, in the name of freedom, he will try to delay as much as possible — even if necessary — the obligation to undergo testing and wear masks, not to mention more restrictive infection containment measures. It’s also unlikely he would support and promote the development of new mRNA products already under study, which would become indispensable if the disease begins to spread more easily among people, as well as animals. In such a case, traditional influenza vaccine cultivation methods using chicken eggs would prove too slow and quantitatively insufficient, especially if the virus continues to circulate among poultry.
In short, let’s keep our fingers crossed, but recognize that crossing our fingers might not be enough.
This story was translated from Univadis Italy using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
It’s been a while since I’ve discussed the H5N1 avian influenza clade 2.3.4.4b and its rapid spread in North America. I hope the facts prove me wrong, but many experts have been warning for some time that ideal conditions are forming for this virus, which for now only causes zoonoses, to pose a pandemic threat.
either because they work in other medical fields or think that the experience of the COVID-19 pandemic was a worst-case scenario that is unlikely to be repeated in the short term.
The Virus Has Flown to Hawaii
According to data from the Centers for Disease Control and Prevention in Atlanta, Georgia, the infection has now affected more than 500 cattle herds in 15 states. There are about 30 outbreaks reported in poultry, equally distributed between backyard and farm-raised birds, primarily located in California. Here alone, over 3 million birds have been affected.
Wild birds are believed to have transported the highly pathogenic virus via migration routes across the Pacific, introducing it to Hawaii for the first time. Just days after wastewater analysis detected the presence of H5N1 on the island of Oahu, home to the capital Honolulu, the first outbreak was promptly reported, killing at least a dozen ducks and geese in a backyard coop. Some of these birds had been taken in early November to the Mililani Pet Fair, a sort of domestic animal festival. Local authorities recommended that anyone who attended the fair, touched a duck or goose at the event, and developed symptoms including fever, cough, sore throat, and conjunctivitis, should isolate and seek medical advice.
Meanwhile, more than 50 farmers, animal handlers, or workers involved in the slaughter of cattle or poultry across seven states have been confirmed infected, presumably contracted at their workplace. The latest case, diagnosed recently in Oregon, presented with severe conjunctivitis and mild respiratory symptoms. More than half of these patients have been identified in recent weeks in California, where active surveillance measures have been implemented. However, there is strong suspicion that the actual number of people infected with mild symptoms in the rest of the country is much, much higher.
The Red Alert Lights Up in Canada
The level of concern was raised further with news of the first severe — indeed very severe — case of H5N1 avian influenza originating from the western edge of Canada. A teenager (gender not disclosed), previously healthy and without risk factors, was hospitalized with severe respiratory failure in the intensive care unit at British Columbia Children’s Hospital in Vancouver. The source of the infection is unknown, similar to only one other case in Missouri involving an adult already hospitalized for other reasons, which was identified by chance through influenza surveillance programs. We also know that the Canadian adolescent does not live on a farm and had no known contact with potentially infected animals. The only suspicions focus on the family dog, euthanized owing to unspecified health problems in the early days of the epidemiologic investigation. Although the dog tested negative for avian influenza, a necropsy will be conducted to rule out its involvement in the transmission chain.
An initial characterization of the virus has linked it to genotype D1.1, which is circulating among wild birds and poultry farms in Canada’s westernmost province, rather than the strain typical of dairy cows in the United States. The publication of the complete viral sequence over the past weekend has, for the first time, highlighted mutations that could enhance the virus’s ability to infect human cells.
How do we know this? From the highly contested “gain-of-function” studies, which artificially modify viruses to understand which genomic points require the most surveillance — those mutations that can make the infectious agent more virulent or more transmissible between people.
Under Special Surveillance for 20 Years
The influenza A (H5N1) avian virus is not new or previously unknown, like SARS-CoV-2, and this could (in theory) give us a slight advantage. We have known about it for decades, and it began infecting humans about 20 years ago, causing pneumonia with respiratory failure. It proved lethal in about half of the cases, but only in people who had close contact with infected poultry, primarily in Southeast Asia.
Hundreds of other human cases occurred worldwide, but always in low-income countries with poor hygiene conditions and where families lived in close contact with animals. This contributed to a false sense of security in Europe and North America, where the threat has been consistently underestimated. Despite an estimated fatality rate of around 50%, the media often labeled scientists’ warnings and health authorities’ efforts to remain prepared as false alarms, tainted by suspicions of catering to the interests of pharmaceutical companies.
Some people may recall the scandal involving Tamiflu, the Roche antiviral oseltamivir, that governments stockpiled when there were fears that the avian virus might acquire the ability to spread among humans. It was dubbed “a false antidote for a false pandemic,” referring to the potential avian pandemic and the 2009 H1N1 influenza pandemic, improperly called “swine flu,” and which turned out to be less severe than expected. There was talk of €2.64 billion being “wasted” to “please” the manufacturer. Although the Cochrane Collaboration made legitimate demands for rigor and transparency in conducting and publishing clinical trials, much of the public, and the journalists who wrote the stories, cared little about these technical aspects. The prevailing message was that stockpiling drugs (or vaccines) for a disease we don’t even know will occur is a waste of taxpayers’ money rather than a prudent preventive measure.
More Vulnerable Than Ever
If we were to ascribe strategic thinking to the virus, which it is not capable of, we might argue that it chose the ideal moment to conquer the world. It began circulating in the new clade in 2020, when experts and authorities were focused on the coronavirus. It spread from birds to marine mammals and finally to cattle, exploiting the public’s post-pandemic fatigue, as people no longer wanted to hear about infectious diseases and containment measures. It ultimately rode the wave of political polarization that irrationally equates prevention with supposed cowardice on the left, and recklessness with courageous freedom on the right.
The coincidence between the future appointments announced by the incoming Trump administration and the virus’s accelerated spread deserves attention from decision-makers and health professionals worldwide. The COVID-19 pandemic experience should have taught us that ignoring a threat doesn’t make it go away, if not in our health, then at least in our wallet. The economic repercussions of a virus circulating among animals crucial to our food chain and national economies should concern everyone, well before the threat crosses the ocean, because only then can we defend ourselves.
The proposed Secretary of Health and Human Services, Robert F. Kennedy, is a proponent of the supposed benefits of raw milk, which could serve as a potent vector for the virus. He is ideologically opposed to vaccinations. It’s hard to imagine he would utilize the H5N1 vaccine stockpiles held by the US government for a campaign starting at least with farmers, as was done prophylactically in Finland with products jointly procured by 15 European countries — a group the Italian government decided not to join.
If Kennedy indeed becomes responsible for US public health, it’s reasonable to fear that, in the name of freedom, he will try to delay as much as possible — even if necessary — the obligation to undergo testing and wear masks, not to mention more restrictive infection containment measures. It’s also unlikely he would support and promote the development of new mRNA products already under study, which would become indispensable if the disease begins to spread more easily among people, as well as animals. In such a case, traditional influenza vaccine cultivation methods using chicken eggs would prove too slow and quantitatively insufficient, especially if the virus continues to circulate among poultry.
In short, let’s keep our fingers crossed, but recognize that crossing our fingers might not be enough.
This story was translated from Univadis Italy using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
It’s been a while since I’ve discussed the H5N1 avian influenza clade 2.3.4.4b and its rapid spread in North America. I hope the facts prove me wrong, but many experts have been warning for some time that ideal conditions are forming for this virus, which for now only causes zoonoses, to pose a pandemic threat.
either because they work in other medical fields or think that the experience of the COVID-19 pandemic was a worst-case scenario that is unlikely to be repeated in the short term.
The Virus Has Flown to Hawaii
According to data from the Centers for Disease Control and Prevention in Atlanta, Georgia, the infection has now affected more than 500 cattle herds in 15 states. There are about 30 outbreaks reported in poultry, equally distributed between backyard and farm-raised birds, primarily located in California. Here alone, over 3 million birds have been affected.
Wild birds are believed to have transported the highly pathogenic virus via migration routes across the Pacific, introducing it to Hawaii for the first time. Just days after wastewater analysis detected the presence of H5N1 on the island of Oahu, home to the capital Honolulu, the first outbreak was promptly reported, killing at least a dozen ducks and geese in a backyard coop. Some of these birds had been taken in early November to the Mililani Pet Fair, a sort of domestic animal festival. Local authorities recommended that anyone who attended the fair, touched a duck or goose at the event, and developed symptoms including fever, cough, sore throat, and conjunctivitis, should isolate and seek medical advice.
Meanwhile, more than 50 farmers, animal handlers, or workers involved in the slaughter of cattle or poultry across seven states have been confirmed infected, presumably contracted at their workplace. The latest case, diagnosed recently in Oregon, presented with severe conjunctivitis and mild respiratory symptoms. More than half of these patients have been identified in recent weeks in California, where active surveillance measures have been implemented. However, there is strong suspicion that the actual number of people infected with mild symptoms in the rest of the country is much, much higher.
The Red Alert Lights Up in Canada
The level of concern was raised further with news of the first severe — indeed very severe — case of H5N1 avian influenza originating from the western edge of Canada. A teenager (gender not disclosed), previously healthy and without risk factors, was hospitalized with severe respiratory failure in the intensive care unit at British Columbia Children’s Hospital in Vancouver. The source of the infection is unknown, similar to only one other case in Missouri involving an adult already hospitalized for other reasons, which was identified by chance through influenza surveillance programs. We also know that the Canadian adolescent does not live on a farm and had no known contact with potentially infected animals. The only suspicions focus on the family dog, euthanized owing to unspecified health problems in the early days of the epidemiologic investigation. Although the dog tested negative for avian influenza, a necropsy will be conducted to rule out its involvement in the transmission chain.
An initial characterization of the virus has linked it to genotype D1.1, which is circulating among wild birds and poultry farms in Canada’s westernmost province, rather than the strain typical of dairy cows in the United States. The publication of the complete viral sequence over the past weekend has, for the first time, highlighted mutations that could enhance the virus’s ability to infect human cells.
How do we know this? From the highly contested “gain-of-function” studies, which artificially modify viruses to understand which genomic points require the most surveillance — those mutations that can make the infectious agent more virulent or more transmissible between people.
Under Special Surveillance for 20 Years
The influenza A (H5N1) avian virus is not new or previously unknown, like SARS-CoV-2, and this could (in theory) give us a slight advantage. We have known about it for decades, and it began infecting humans about 20 years ago, causing pneumonia with respiratory failure. It proved lethal in about half of the cases, but only in people who had close contact with infected poultry, primarily in Southeast Asia.
Hundreds of other human cases occurred worldwide, but always in low-income countries with poor hygiene conditions and where families lived in close contact with animals. This contributed to a false sense of security in Europe and North America, where the threat has been consistently underestimated. Despite an estimated fatality rate of around 50%, the media often labeled scientists’ warnings and health authorities’ efforts to remain prepared as false alarms, tainted by suspicions of catering to the interests of pharmaceutical companies.
Some people may recall the scandal involving Tamiflu, the Roche antiviral oseltamivir, that governments stockpiled when there were fears that the avian virus might acquire the ability to spread among humans. It was dubbed “a false antidote for a false pandemic,” referring to the potential avian pandemic and the 2009 H1N1 influenza pandemic, improperly called “swine flu,” and which turned out to be less severe than expected. There was talk of €2.64 billion being “wasted” to “please” the manufacturer. Although the Cochrane Collaboration made legitimate demands for rigor and transparency in conducting and publishing clinical trials, much of the public, and the journalists who wrote the stories, cared little about these technical aspects. The prevailing message was that stockpiling drugs (or vaccines) for a disease we don’t even know will occur is a waste of taxpayers’ money rather than a prudent preventive measure.
More Vulnerable Than Ever
If we were to ascribe strategic thinking to the virus, which it is not capable of, we might argue that it chose the ideal moment to conquer the world. It began circulating in the new clade in 2020, when experts and authorities were focused on the coronavirus. It spread from birds to marine mammals and finally to cattle, exploiting the public’s post-pandemic fatigue, as people no longer wanted to hear about infectious diseases and containment measures. It ultimately rode the wave of political polarization that irrationally equates prevention with supposed cowardice on the left, and recklessness with courageous freedom on the right.
The coincidence between the future appointments announced by the incoming Trump administration and the virus’s accelerated spread deserves attention from decision-makers and health professionals worldwide. The COVID-19 pandemic experience should have taught us that ignoring a threat doesn’t make it go away, if not in our health, then at least in our wallet. The economic repercussions of a virus circulating among animals crucial to our food chain and national economies should concern everyone, well before the threat crosses the ocean, because only then can we defend ourselves.
The proposed Secretary of Health and Human Services, Robert F. Kennedy, is a proponent of the supposed benefits of raw milk, which could serve as a potent vector for the virus. He is ideologically opposed to vaccinations. It’s hard to imagine he would utilize the H5N1 vaccine stockpiles held by the US government for a campaign starting at least with farmers, as was done prophylactically in Finland with products jointly procured by 15 European countries — a group the Italian government decided not to join.
If Kennedy indeed becomes responsible for US public health, it’s reasonable to fear that, in the name of freedom, he will try to delay as much as possible — even if necessary — the obligation to undergo testing and wear masks, not to mention more restrictive infection containment measures. It’s also unlikely he would support and promote the development of new mRNA products already under study, which would become indispensable if the disease begins to spread more easily among people, as well as animals. In such a case, traditional influenza vaccine cultivation methods using chicken eggs would prove too slow and quantitatively insufficient, especially if the virus continues to circulate among poultry.
In short, let’s keep our fingers crossed, but recognize that crossing our fingers might not be enough.
This story was translated from Univadis Italy using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.