User login
For many, long COVID’s impacts go on and on, major study says
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
FROM NATURE COMMUNICATIONS
COVID-19 vaccine insights: The news beyond the headlines
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
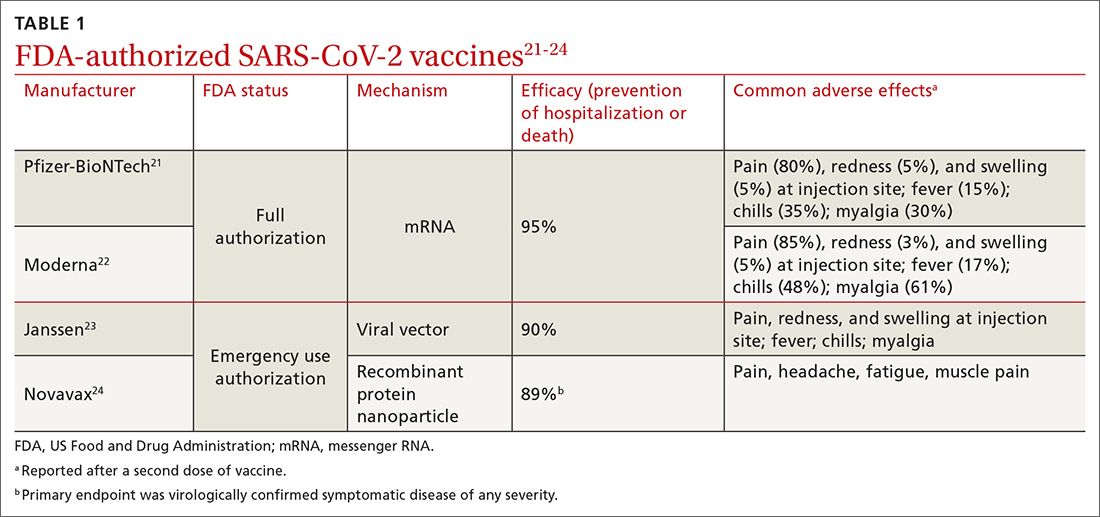
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24
Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
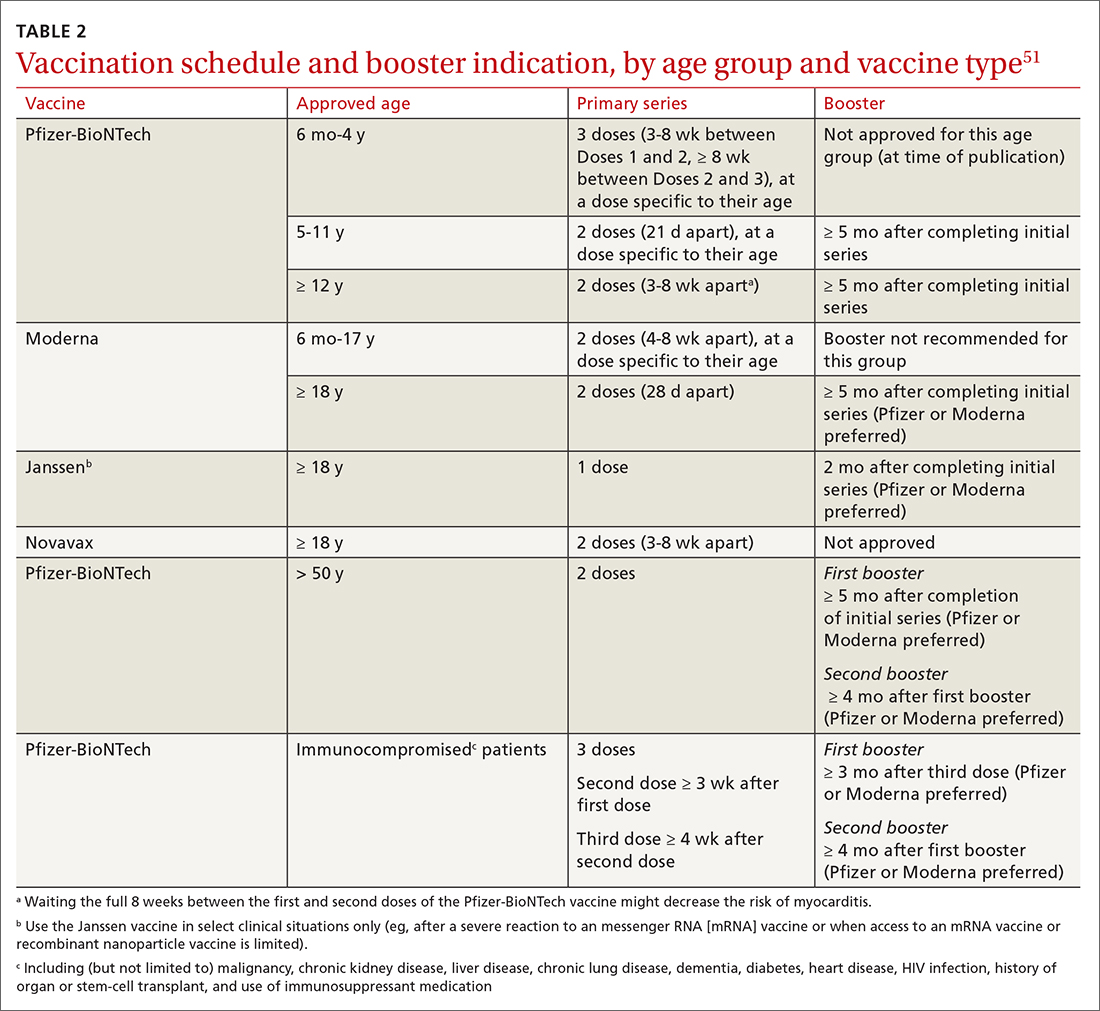
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).
Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
awww.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; john.l.kiley.mil@health.mil
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24

Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).

Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
awww.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; john.l.kiley.mil@health.mil
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24

Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).

Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
awww.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; john.l.kiley.mil@health.mil
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
PRACTICE RECOMMENDATIONS
› Vaccinate all adults (≥ 18 years) against COVID-19, based on recommendations for the initial series and boosters. A
› Vaccinate patients against COVID-19 with evidence-based assurance that doing so reduces disease-related risk of hospitalization, myocardial infarction, stroke, need for mechanical ventilation, and death. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Congenital syphilis: It’s still a significant public health problem
You’re rounding in the nursery and informed of the following about one of your new patients: He’s a 38-week-old infant delivered to a mother diagnosed with syphilis at 12 weeks’ gestation at her initial prenatal visit. Her rapid plasma reagin (RPR) was 1:64 and the fluorescent treponemal antibody–absorption (FTA-ABS) test was positive. By report she was appropriately treated. Maternal RPRs obtained at 18 and 28 weeks’ gestation were 1:16 and 1:4, respectively. Maternal RPR at delivery and the infant’s RPR obtained shortly after birth were both 1:4. The mother wants to know if her baby is infected.
One result of syphilis during pregnancy is intrauterine infection and resultant congenital disease in the infant. Before you answer this mother, let’s discuss syphilis.
Congenital syphilis is a significant public health problem. In 2021, there were a total of 2,677 cases reported for a rate of 74.1 per 100,000 live births. Between 2020 and 2021, the number of cases of congenital syphilis increased 24.1% (2,158-2,677 cases), concurrent with a 45.8% increase (10.7-15.6 per 100,000) in the rate of primary and secondary syphilis in women aged 15-44 years. Between 2012 and 2021, the number of cases of congenital syphilis increased 701.5% (334-2,677 cases) and the increase in rates of primary and secondary syphilis in women aged 15-44 was 642.9% over the same period.
Why are the rates of congenital syphilis increasing? Most cases result from a lack of prenatal care and thus no testing for syphilis. The next most common cause is inadequate maternal treatment.
Congenital syphilis usually is acquired through transplacental transmission of spirochetes in the maternal bloodstream. Occasionally, it occurs at delivery via direct contact with maternal lesions. It is not transmitted in breast milk. Transmission of syphilis:
- Can occur any time during pregnancy.
- Is more likely to occur in women with untreated primary or secondary disease (60%-100%).
- Is approximately 40% in those with early latent syphilis and less than 8% in mothers with late latent syphilis.
- Is higher in women coinfected with HIV since they more frequently receive no prenatal care and their disease is inadequately treated.
Coinfection with syphilis may also increase the rate of mother-to-child transmission of HIV.
Untreated early syphilis during pregnancy results in spontaneous abortion, stillbirth, or perinatal death in up to 40% of cases. Infected newborns with early congenital syphilis can be asymptomatic or have evidence of hepatosplenomegaly, generalized lymphadenopathy, nasal discharge that is occasionally bloody, rash, and skeletal abnormalities (osteochondritis and periostitis). Other manifestations include edema, hemolytic anemia, jaundice, pneumonia, pseudoparalysis, and thrombocytopenia. Asymptomatic infants may have abnormal cerebrospinal fluid findings including elevated CSF white cell count, elevated protein, and a reactive venereal disease research laboratory test.
Late congenital syphilis, defined as the onset of symptoms after 2 years of age is secondary to scarring or persistent inflammation and gumma formation in a variety of tissues. It occurs in up to 40% of cases of untreated maternal disease. Most cases can be prevented by maternal treatment and treatment of the infant within the first 3 months of life. Common clinical manifestations include interstitial keratitis, sensorineural hearing loss, frontal bossing, saddle nose, Hutchinson teeth, mulberry molars, perforation of the hard palate, anterior bowing of the tibia (saber shins), and other skeletal abnormalities.
Diagnostic tests. Maternal diagnosis is dependent upon knowing the results of both a nontreponemal (RPR, VDRL) and a confirmatory treponemal test (TP-PA, TP-EIA, TP-CIA, FTA-ABS,) before or at delivery. TP-PA is the preferred test. When maternal disease is confirmed, the newborn should have the same quantitative nontreponemal test as the mother. A confirmatory treponemal test is not required
Evaluation and treatment. It’s imperative that children born to mothers with a reactive test, regardless of their treatment status, have a thorough exam performed before hospital discharge. The provider must determine what additional interventions should be performed.
The American Academy of Pediatrics and the Centers for Disease Control and Prevention (www.cdc.gov/std/treatment-guidelines/congenital-syphilis.htm) have developed standard algorithms for the diagnostic approach and treatment of infants born to mothers with reactive serologic tests for syphilis. It is available in the Red Book for AAP members (https://publications.aap.org/redbook). Recommendations based on various scenarios for neonates up to 1 month of age include proven or highly probable congenital syphilis, possible congenital syphilis, congenital syphilis less likely, and congenital syphilis unlikely. It is beyond the scope of this article to list the criteria and evaluation for each scenario. The reader is referred to the algorithm.
If syphilis is suspected in infants or children older than 1 month, the challenge is to determine if it is untreated congenital syphilis or acquired syphilis. Maternal syphilis status should be determined. Evaluation for congenital syphilis in this age group includes CSF analysis for VDRL, cell count and protein, CBC with differential and platelets, hepatic panel, abdominal ultrasound, long-bone radiographs, chest radiograph, neuroimaging, auditory brain stem response, and HIV testing.
Let’s go back to your patient. The mother was diagnosed with syphilis during pregnancy. You confirm that she was treated with benzathine penicillin G, and the course was completed at least 4 weeks before delivery. Treatment with any other drug during pregnancy is not appropriate. The RPR has declined, and the infant’s titer is equal to or less than four times the maternal titer. The exam is significant for generalized adenopathy and slightly bloody nasal discharge. This infant has two findings consistent with congenital syphilis regardless of RPR titer or treatment status. This places him in the proven or highly probable congenital syphilis group. Management includes CSF analysis (VDRL, cell count, and protein), CBC with differential and platelet count, and treatment with penicillin G for 10 days. Additional tests as clinically indicated include: long-bone radiograph, chest radiography, aspartate aminotranferase and alanine aminotransferase levels, neuroimaging, ophthalmologic exam, and auditory brain stem response. Despite maternal treatment, this newborn has congenital syphilis. The same nontreponemal test should be obtained every 2-3 months until it is nonreactive. It should be nonreactive by 6 months. If the infection persists to 6-12 months post treatment, reevaluation including CSF analysis and retreatment may be indicated.
Congenital syphilis can be prevented by maternal screening, diagnosis, and treatment. When that fails it is up to us to diagnosis and adequately treat our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
You’re rounding in the nursery and informed of the following about one of your new patients: He’s a 38-week-old infant delivered to a mother diagnosed with syphilis at 12 weeks’ gestation at her initial prenatal visit. Her rapid plasma reagin (RPR) was 1:64 and the fluorescent treponemal antibody–absorption (FTA-ABS) test was positive. By report she was appropriately treated. Maternal RPRs obtained at 18 and 28 weeks’ gestation were 1:16 and 1:4, respectively. Maternal RPR at delivery and the infant’s RPR obtained shortly after birth were both 1:4. The mother wants to know if her baby is infected.
One result of syphilis during pregnancy is intrauterine infection and resultant congenital disease in the infant. Before you answer this mother, let’s discuss syphilis.
Congenital syphilis is a significant public health problem. In 2021, there were a total of 2,677 cases reported for a rate of 74.1 per 100,000 live births. Between 2020 and 2021, the number of cases of congenital syphilis increased 24.1% (2,158-2,677 cases), concurrent with a 45.8% increase (10.7-15.6 per 100,000) in the rate of primary and secondary syphilis in women aged 15-44 years. Between 2012 and 2021, the number of cases of congenital syphilis increased 701.5% (334-2,677 cases) and the increase in rates of primary and secondary syphilis in women aged 15-44 was 642.9% over the same period.
Why are the rates of congenital syphilis increasing? Most cases result from a lack of prenatal care and thus no testing for syphilis. The next most common cause is inadequate maternal treatment.
Congenital syphilis usually is acquired through transplacental transmission of spirochetes in the maternal bloodstream. Occasionally, it occurs at delivery via direct contact with maternal lesions. It is not transmitted in breast milk. Transmission of syphilis:
- Can occur any time during pregnancy.
- Is more likely to occur in women with untreated primary or secondary disease (60%-100%).
- Is approximately 40% in those with early latent syphilis and less than 8% in mothers with late latent syphilis.
- Is higher in women coinfected with HIV since they more frequently receive no prenatal care and their disease is inadequately treated.
Coinfection with syphilis may also increase the rate of mother-to-child transmission of HIV.
Untreated early syphilis during pregnancy results in spontaneous abortion, stillbirth, or perinatal death in up to 40% of cases. Infected newborns with early congenital syphilis can be asymptomatic or have evidence of hepatosplenomegaly, generalized lymphadenopathy, nasal discharge that is occasionally bloody, rash, and skeletal abnormalities (osteochondritis and periostitis). Other manifestations include edema, hemolytic anemia, jaundice, pneumonia, pseudoparalysis, and thrombocytopenia. Asymptomatic infants may have abnormal cerebrospinal fluid findings including elevated CSF white cell count, elevated protein, and a reactive venereal disease research laboratory test.
Late congenital syphilis, defined as the onset of symptoms after 2 years of age is secondary to scarring or persistent inflammation and gumma formation in a variety of tissues. It occurs in up to 40% of cases of untreated maternal disease. Most cases can be prevented by maternal treatment and treatment of the infant within the first 3 months of life. Common clinical manifestations include interstitial keratitis, sensorineural hearing loss, frontal bossing, saddle nose, Hutchinson teeth, mulberry molars, perforation of the hard palate, anterior bowing of the tibia (saber shins), and other skeletal abnormalities.
Diagnostic tests. Maternal diagnosis is dependent upon knowing the results of both a nontreponemal (RPR, VDRL) and a confirmatory treponemal test (TP-PA, TP-EIA, TP-CIA, FTA-ABS,) before or at delivery. TP-PA is the preferred test. When maternal disease is confirmed, the newborn should have the same quantitative nontreponemal test as the mother. A confirmatory treponemal test is not required
Evaluation and treatment. It’s imperative that children born to mothers with a reactive test, regardless of their treatment status, have a thorough exam performed before hospital discharge. The provider must determine what additional interventions should be performed.
The American Academy of Pediatrics and the Centers for Disease Control and Prevention (www.cdc.gov/std/treatment-guidelines/congenital-syphilis.htm) have developed standard algorithms for the diagnostic approach and treatment of infants born to mothers with reactive serologic tests for syphilis. It is available in the Red Book for AAP members (https://publications.aap.org/redbook). Recommendations based on various scenarios for neonates up to 1 month of age include proven or highly probable congenital syphilis, possible congenital syphilis, congenital syphilis less likely, and congenital syphilis unlikely. It is beyond the scope of this article to list the criteria and evaluation for each scenario. The reader is referred to the algorithm.
If syphilis is suspected in infants or children older than 1 month, the challenge is to determine if it is untreated congenital syphilis or acquired syphilis. Maternal syphilis status should be determined. Evaluation for congenital syphilis in this age group includes CSF analysis for VDRL, cell count and protein, CBC with differential and platelets, hepatic panel, abdominal ultrasound, long-bone radiographs, chest radiograph, neuroimaging, auditory brain stem response, and HIV testing.
Let’s go back to your patient. The mother was diagnosed with syphilis during pregnancy. You confirm that she was treated with benzathine penicillin G, and the course was completed at least 4 weeks before delivery. Treatment with any other drug during pregnancy is not appropriate. The RPR has declined, and the infant’s titer is equal to or less than four times the maternal titer. The exam is significant for generalized adenopathy and slightly bloody nasal discharge. This infant has two findings consistent with congenital syphilis regardless of RPR titer or treatment status. This places him in the proven or highly probable congenital syphilis group. Management includes CSF analysis (VDRL, cell count, and protein), CBC with differential and platelet count, and treatment with penicillin G for 10 days. Additional tests as clinically indicated include: long-bone radiograph, chest radiography, aspartate aminotranferase and alanine aminotransferase levels, neuroimaging, ophthalmologic exam, and auditory brain stem response. Despite maternal treatment, this newborn has congenital syphilis. The same nontreponemal test should be obtained every 2-3 months until it is nonreactive. It should be nonreactive by 6 months. If the infection persists to 6-12 months post treatment, reevaluation including CSF analysis and retreatment may be indicated.
Congenital syphilis can be prevented by maternal screening, diagnosis, and treatment. When that fails it is up to us to diagnosis and adequately treat our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
You’re rounding in the nursery and informed of the following about one of your new patients: He’s a 38-week-old infant delivered to a mother diagnosed with syphilis at 12 weeks’ gestation at her initial prenatal visit. Her rapid plasma reagin (RPR) was 1:64 and the fluorescent treponemal antibody–absorption (FTA-ABS) test was positive. By report she was appropriately treated. Maternal RPRs obtained at 18 and 28 weeks’ gestation were 1:16 and 1:4, respectively. Maternal RPR at delivery and the infant’s RPR obtained shortly after birth were both 1:4. The mother wants to know if her baby is infected.
One result of syphilis during pregnancy is intrauterine infection and resultant congenital disease in the infant. Before you answer this mother, let’s discuss syphilis.
Congenital syphilis is a significant public health problem. In 2021, there were a total of 2,677 cases reported for a rate of 74.1 per 100,000 live births. Between 2020 and 2021, the number of cases of congenital syphilis increased 24.1% (2,158-2,677 cases), concurrent with a 45.8% increase (10.7-15.6 per 100,000) in the rate of primary and secondary syphilis in women aged 15-44 years. Between 2012 and 2021, the number of cases of congenital syphilis increased 701.5% (334-2,677 cases) and the increase in rates of primary and secondary syphilis in women aged 15-44 was 642.9% over the same period.
Why are the rates of congenital syphilis increasing? Most cases result from a lack of prenatal care and thus no testing for syphilis. The next most common cause is inadequate maternal treatment.
Congenital syphilis usually is acquired through transplacental transmission of spirochetes in the maternal bloodstream. Occasionally, it occurs at delivery via direct contact with maternal lesions. It is not transmitted in breast milk. Transmission of syphilis:
- Can occur any time during pregnancy.
- Is more likely to occur in women with untreated primary or secondary disease (60%-100%).
- Is approximately 40% in those with early latent syphilis and less than 8% in mothers with late latent syphilis.
- Is higher in women coinfected with HIV since they more frequently receive no prenatal care and their disease is inadequately treated.
Coinfection with syphilis may also increase the rate of mother-to-child transmission of HIV.
Untreated early syphilis during pregnancy results in spontaneous abortion, stillbirth, or perinatal death in up to 40% of cases. Infected newborns with early congenital syphilis can be asymptomatic or have evidence of hepatosplenomegaly, generalized lymphadenopathy, nasal discharge that is occasionally bloody, rash, and skeletal abnormalities (osteochondritis and periostitis). Other manifestations include edema, hemolytic anemia, jaundice, pneumonia, pseudoparalysis, and thrombocytopenia. Asymptomatic infants may have abnormal cerebrospinal fluid findings including elevated CSF white cell count, elevated protein, and a reactive venereal disease research laboratory test.
Late congenital syphilis, defined as the onset of symptoms after 2 years of age is secondary to scarring or persistent inflammation and gumma formation in a variety of tissues. It occurs in up to 40% of cases of untreated maternal disease. Most cases can be prevented by maternal treatment and treatment of the infant within the first 3 months of life. Common clinical manifestations include interstitial keratitis, sensorineural hearing loss, frontal bossing, saddle nose, Hutchinson teeth, mulberry molars, perforation of the hard palate, anterior bowing of the tibia (saber shins), and other skeletal abnormalities.
Diagnostic tests. Maternal diagnosis is dependent upon knowing the results of both a nontreponemal (RPR, VDRL) and a confirmatory treponemal test (TP-PA, TP-EIA, TP-CIA, FTA-ABS,) before or at delivery. TP-PA is the preferred test. When maternal disease is confirmed, the newborn should have the same quantitative nontreponemal test as the mother. A confirmatory treponemal test is not required
Evaluation and treatment. It’s imperative that children born to mothers with a reactive test, regardless of their treatment status, have a thorough exam performed before hospital discharge. The provider must determine what additional interventions should be performed.
The American Academy of Pediatrics and the Centers for Disease Control and Prevention (www.cdc.gov/std/treatment-guidelines/congenital-syphilis.htm) have developed standard algorithms for the diagnostic approach and treatment of infants born to mothers with reactive serologic tests for syphilis. It is available in the Red Book for AAP members (https://publications.aap.org/redbook). Recommendations based on various scenarios for neonates up to 1 month of age include proven or highly probable congenital syphilis, possible congenital syphilis, congenital syphilis less likely, and congenital syphilis unlikely. It is beyond the scope of this article to list the criteria and evaluation for each scenario. The reader is referred to the algorithm.
If syphilis is suspected in infants or children older than 1 month, the challenge is to determine if it is untreated congenital syphilis or acquired syphilis. Maternal syphilis status should be determined. Evaluation for congenital syphilis in this age group includes CSF analysis for VDRL, cell count and protein, CBC with differential and platelets, hepatic panel, abdominal ultrasound, long-bone radiographs, chest radiograph, neuroimaging, auditory brain stem response, and HIV testing.
Let’s go back to your patient. The mother was diagnosed with syphilis during pregnancy. You confirm that she was treated with benzathine penicillin G, and the course was completed at least 4 weeks before delivery. Treatment with any other drug during pregnancy is not appropriate. The RPR has declined, and the infant’s titer is equal to or less than four times the maternal titer. The exam is significant for generalized adenopathy and slightly bloody nasal discharge. This infant has two findings consistent with congenital syphilis regardless of RPR titer or treatment status. This places him in the proven or highly probable congenital syphilis group. Management includes CSF analysis (VDRL, cell count, and protein), CBC with differential and platelet count, and treatment with penicillin G for 10 days. Additional tests as clinically indicated include: long-bone radiograph, chest radiography, aspartate aminotranferase and alanine aminotransferase levels, neuroimaging, ophthalmologic exam, and auditory brain stem response. Despite maternal treatment, this newborn has congenital syphilis. The same nontreponemal test should be obtained every 2-3 months until it is nonreactive. It should be nonreactive by 6 months. If the infection persists to 6-12 months post treatment, reevaluation including CSF analysis and retreatment may be indicated.
Congenital syphilis can be prevented by maternal screening, diagnosis, and treatment. When that fails it is up to us to diagnosis and adequately treat our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Children and COVID: Downward trend reverses with small increase in new cases
A small increase in new cases brought COVID-19’s latest losing streak to an end at 4 weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
The 40,656 new cases reported bring the U.S. cumulative count of child COVID-19 cases to over 14.8 million since the pandemic began, which represents 18.4% of all cases, the AAP and CHA said in their weekly report based on state-level data.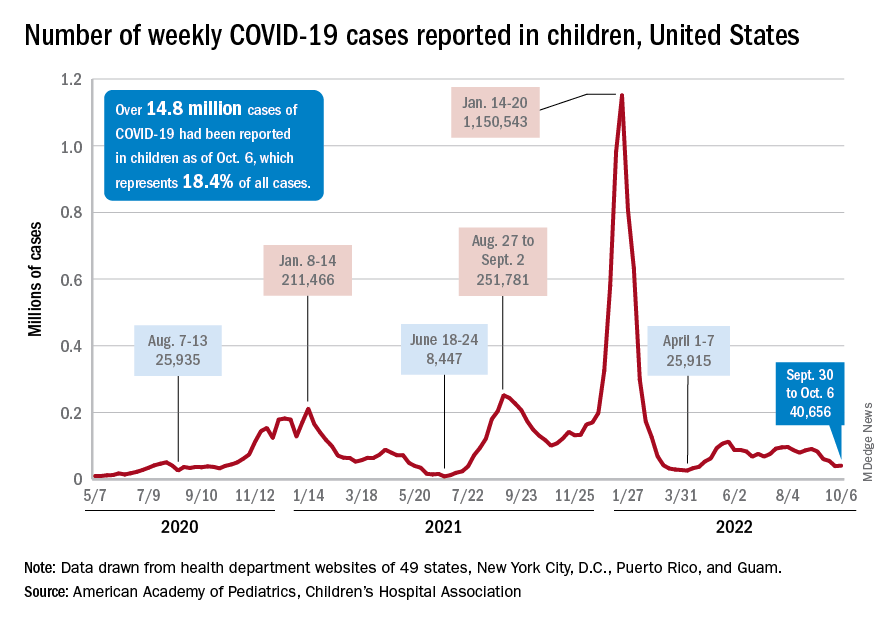
The increase in new cases was not reflected in emergency department visits or hospital admissions, which both continued sustained declines that started in August. In the week from Sept. 27 to Oct. 4, the 7-day averages for ED visits with diagnosed COVID were down by 21.5% (age 0-11), 27.3% (12-15), and 18.2% (16-17), the Centers for Disease Control and Prevention said, while the most recent 7-day average for new admissions – 127 per day for Oct. 2-8 – among children aged 0-17 years with confirmed COVID was down from 161 per day the previous week, a drop of over 21%.
The state-level data that are currently available (several states are no longer reporting) show Alaska (25.5%) and Vermont (25.4%) have the highest proportions of cumulative cases in children, and Florida (12.3%) and Utah (13.5%) have the lowest. Rhode Island has the highest rate of COVID-19 per 100,000 children at 40,427, while Missouri has the lowest at 14,252. The national average is 19,687 per 100,000, the AAP and CHA reported.
Taking a look at vaccination
Vaccinations were up slightly in children aged 12-17 years, as 20,000 initial doses were given during the week of Sept. 29 to Oct. 5, compared with 17,000 and 18,000 the previous 2 weeks. Initial vaccinations in younger children, however, continued declines dating back to August, the AAP said in its weekly vaccination trends report.
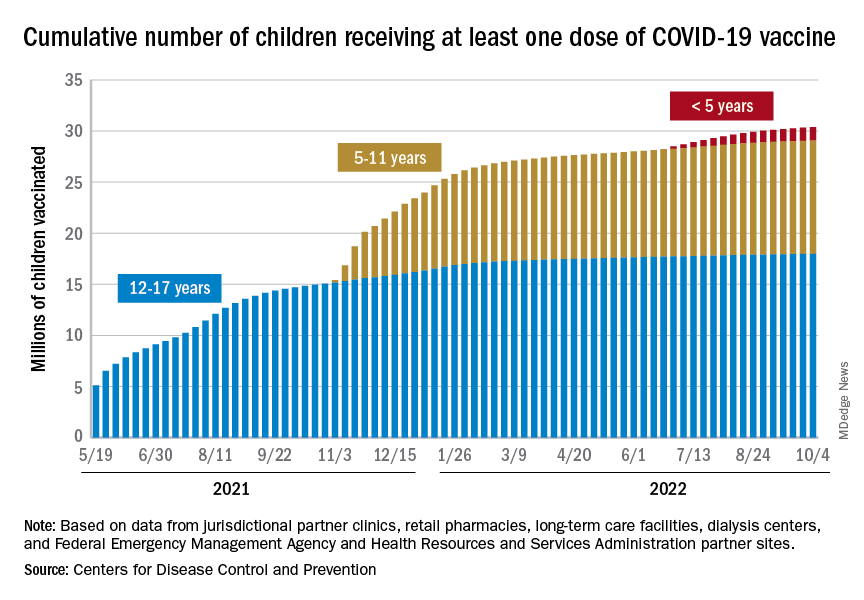
The District of Columbia and Massachusetts have the most highly vaccinated groups of 12- to 17-year-olds, as 100% and 95%, respectively, have received initial doses, while Wyoming (39%) and Idaho (42%) have the lowest. D.C. (73%) and Vermont (68%) have the highest proportions of vaccinated 5- to 11-year-olds, and Alabama (17%) and Mississippi (18%) have the lowest. For children under age 5 years, those in D.C. (33%) and Vermont (26%) are the most likely to have received an initial COVID vaccination, while Alabama, Louisiana, and Mississippi share national-low rates of 2%, the AAP said its report, which is based on CDC data.
When all states and territories are combined, 71% of children aged 12-17 have received at least one dose of vaccine, as have 38.6% of all children 5-11 years old and 6.7% of those under age 5. Almost 61% of the nation’s 16- to 17-year-olds have been fully vaccinated, along with 31.5% of those aged 5-11 and 2.4% of children younger than 5 years, the CDC said on its COVID Data Tracker.
About 42 million children – 58% of the population under the age of 18 years – have not received any vaccine yet, the AAP noted. Meanwhile, CDC data indicate that 36 children died of COVID in the last week, with pediatric deaths now totaling 1,781 over the course of the pandemic.
A small increase in new cases brought COVID-19’s latest losing streak to an end at 4 weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
The 40,656 new cases reported bring the U.S. cumulative count of child COVID-19 cases to over 14.8 million since the pandemic began, which represents 18.4% of all cases, the AAP and CHA said in their weekly report based on state-level data.
The increase in new cases was not reflected in emergency department visits or hospital admissions, which both continued sustained declines that started in August. In the week from Sept. 27 to Oct. 4, the 7-day averages for ED visits with diagnosed COVID were down by 21.5% (age 0-11), 27.3% (12-15), and 18.2% (16-17), the Centers for Disease Control and Prevention said, while the most recent 7-day average for new admissions – 127 per day for Oct. 2-8 – among children aged 0-17 years with confirmed COVID was down from 161 per day the previous week, a drop of over 21%.
The state-level data that are currently available (several states are no longer reporting) show Alaska (25.5%) and Vermont (25.4%) have the highest proportions of cumulative cases in children, and Florida (12.3%) and Utah (13.5%) have the lowest. Rhode Island has the highest rate of COVID-19 per 100,000 children at 40,427, while Missouri has the lowest at 14,252. The national average is 19,687 per 100,000, the AAP and CHA reported.
Taking a look at vaccination
Vaccinations were up slightly in children aged 12-17 years, as 20,000 initial doses were given during the week of Sept. 29 to Oct. 5, compared with 17,000 and 18,000 the previous 2 weeks. Initial vaccinations in younger children, however, continued declines dating back to August, the AAP said in its weekly vaccination trends report.

The District of Columbia and Massachusetts have the most highly vaccinated groups of 12- to 17-year-olds, as 100% and 95%, respectively, have received initial doses, while Wyoming (39%) and Idaho (42%) have the lowest. D.C. (73%) and Vermont (68%) have the highest proportions of vaccinated 5- to 11-year-olds, and Alabama (17%) and Mississippi (18%) have the lowest. For children under age 5 years, those in D.C. (33%) and Vermont (26%) are the most likely to have received an initial COVID vaccination, while Alabama, Louisiana, and Mississippi share national-low rates of 2%, the AAP said its report, which is based on CDC data.
When all states and territories are combined, 71% of children aged 12-17 have received at least one dose of vaccine, as have 38.6% of all children 5-11 years old and 6.7% of those under age 5. Almost 61% of the nation’s 16- to 17-year-olds have been fully vaccinated, along with 31.5% of those aged 5-11 and 2.4% of children younger than 5 years, the CDC said on its COVID Data Tracker.
About 42 million children – 58% of the population under the age of 18 years – have not received any vaccine yet, the AAP noted. Meanwhile, CDC data indicate that 36 children died of COVID in the last week, with pediatric deaths now totaling 1,781 over the course of the pandemic.
A small increase in new cases brought COVID-19’s latest losing streak to an end at 4 weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
The 40,656 new cases reported bring the U.S. cumulative count of child COVID-19 cases to over 14.8 million since the pandemic began, which represents 18.4% of all cases, the AAP and CHA said in their weekly report based on state-level data.
The increase in new cases was not reflected in emergency department visits or hospital admissions, which both continued sustained declines that started in August. In the week from Sept. 27 to Oct. 4, the 7-day averages for ED visits with diagnosed COVID were down by 21.5% (age 0-11), 27.3% (12-15), and 18.2% (16-17), the Centers for Disease Control and Prevention said, while the most recent 7-day average for new admissions – 127 per day for Oct. 2-8 – among children aged 0-17 years with confirmed COVID was down from 161 per day the previous week, a drop of over 21%.
The state-level data that are currently available (several states are no longer reporting) show Alaska (25.5%) and Vermont (25.4%) have the highest proportions of cumulative cases in children, and Florida (12.3%) and Utah (13.5%) have the lowest. Rhode Island has the highest rate of COVID-19 per 100,000 children at 40,427, while Missouri has the lowest at 14,252. The national average is 19,687 per 100,000, the AAP and CHA reported.
Taking a look at vaccination
Vaccinations were up slightly in children aged 12-17 years, as 20,000 initial doses were given during the week of Sept. 29 to Oct. 5, compared with 17,000 and 18,000 the previous 2 weeks. Initial vaccinations in younger children, however, continued declines dating back to August, the AAP said in its weekly vaccination trends report.

The District of Columbia and Massachusetts have the most highly vaccinated groups of 12- to 17-year-olds, as 100% and 95%, respectively, have received initial doses, while Wyoming (39%) and Idaho (42%) have the lowest. D.C. (73%) and Vermont (68%) have the highest proportions of vaccinated 5- to 11-year-olds, and Alabama (17%) and Mississippi (18%) have the lowest. For children under age 5 years, those in D.C. (33%) and Vermont (26%) are the most likely to have received an initial COVID vaccination, while Alabama, Louisiana, and Mississippi share national-low rates of 2%, the AAP said its report, which is based on CDC data.
When all states and territories are combined, 71% of children aged 12-17 have received at least one dose of vaccine, as have 38.6% of all children 5-11 years old and 6.7% of those under age 5. Almost 61% of the nation’s 16- to 17-year-olds have been fully vaccinated, along with 31.5% of those aged 5-11 and 2.4% of children younger than 5 years, the CDC said on its COVID Data Tracker.
About 42 million children – 58% of the population under the age of 18 years – have not received any vaccine yet, the AAP noted. Meanwhile, CDC data indicate that 36 children died of COVID in the last week, with pediatric deaths now totaling 1,781 over the course of the pandemic.
Vaccine update for the 2022-23 influenza season
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.
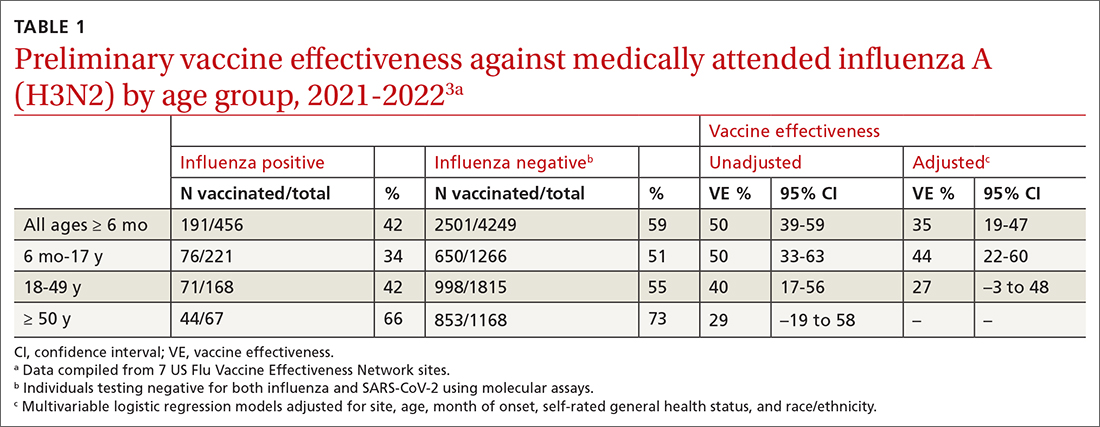
The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).
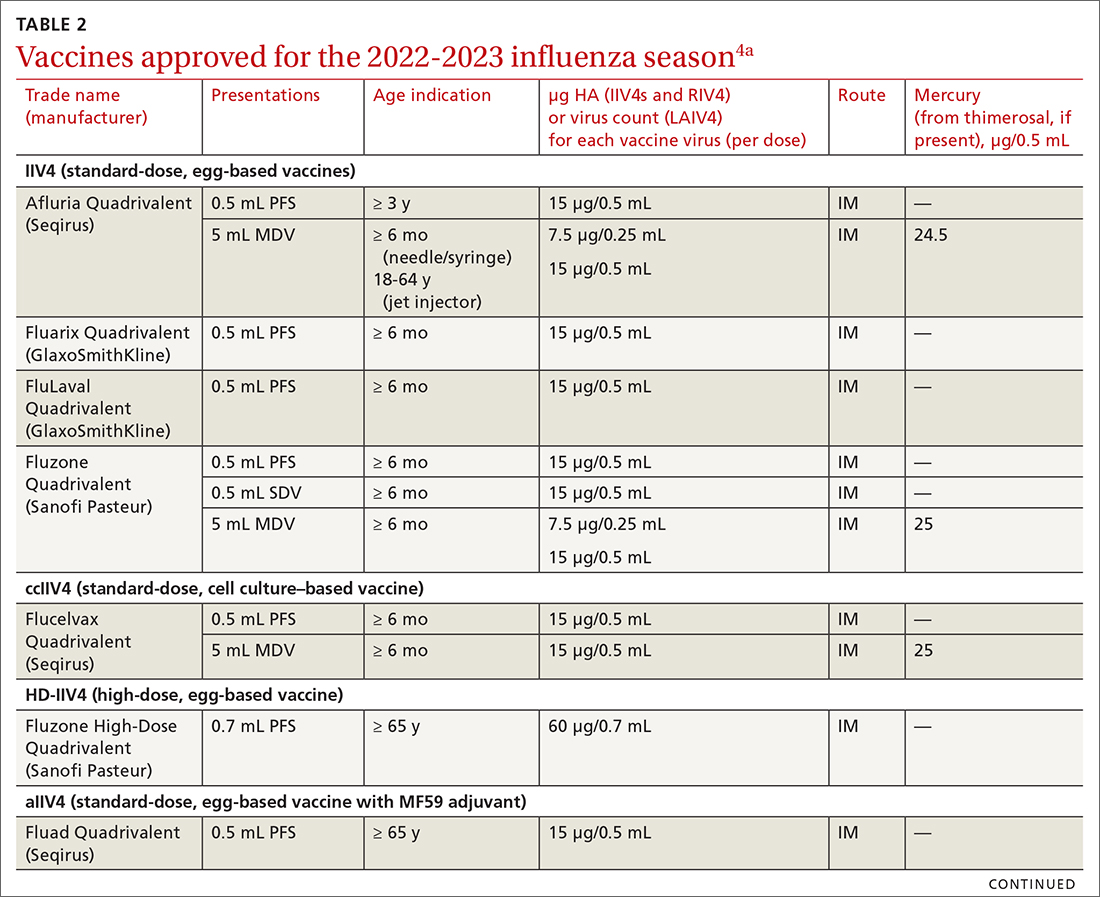
Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).
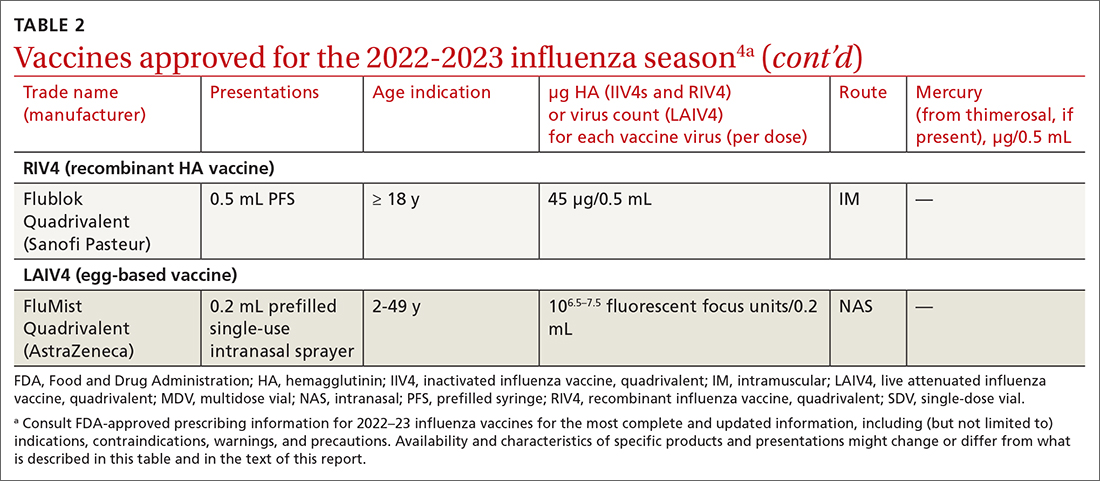
Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.
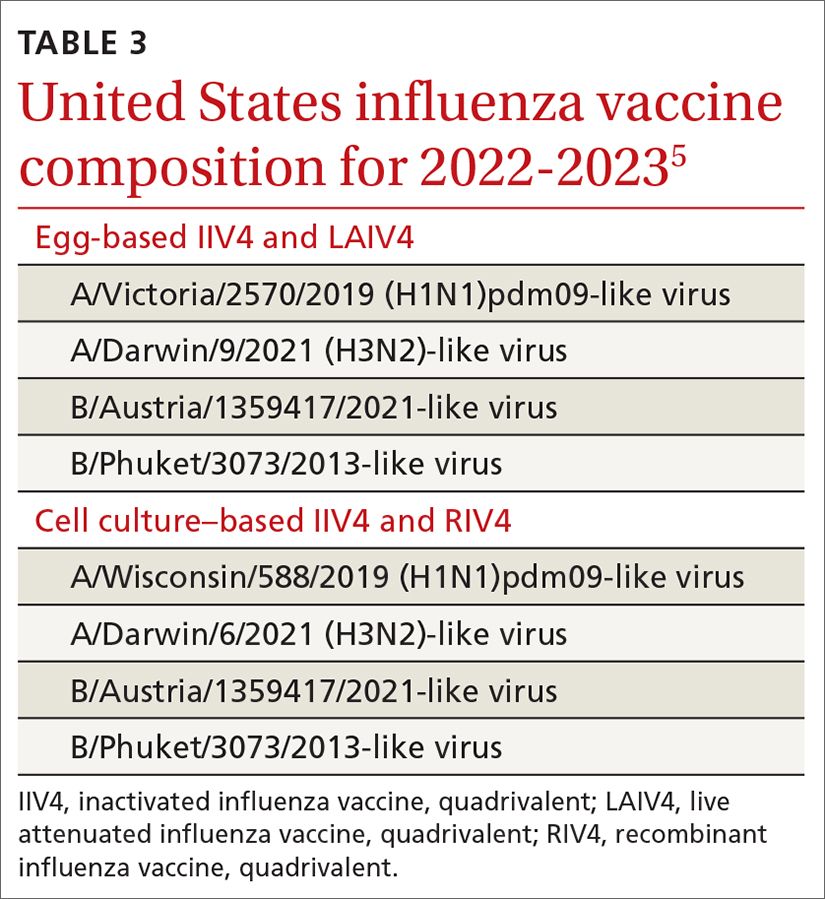
All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.
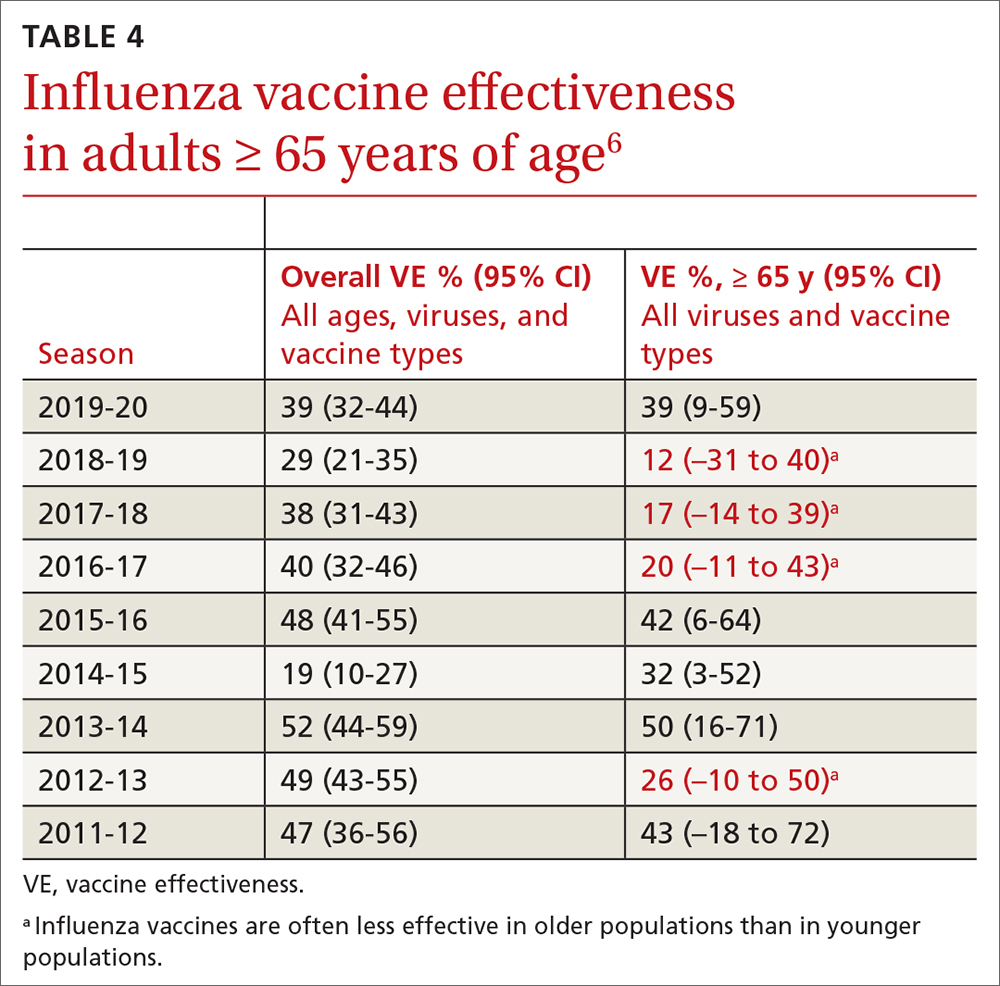
One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.

The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).

Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).

Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.

All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.

One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.

The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).

Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).

Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.

All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.

One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
Antioxidant-rich diet may reduce Helicobacter pylori risk
People who eat a balanced diet with sufficient antioxidants from fruits and vegetables may face reduced risks for Heliobacter pylori infections, according to a new report.
In particular, patients with an H. pylori infection were more likely to score lower on the Dietary Antioxidant Index (DAI), which was created to consider a diet’s entire antioxidant profile.
“Available evidence indicates that diet has an important role in developing H. pylori infection. Therefore, protective dietary factors are important from a public health point of view,” Farzad Shidfar, a professor of nutrition at the Iran University of Medical Sciences, Tehran, and member of the university’s colorectal research center, and colleagues write.
“While some nutritional research has widely focused on single nutrients or foods in diet-disease relations, the overall diet could be more informative because humans typically consume a combination of nutrients and foods,” they write. “Dietary indices such as DAI are one of the approaches for this purpose.”
The study was published online in BMC Gastroenterology.
Measuring antioxidant intake
Previous research has indicated an inverse association between the DAI and inflammatory diseases, the study authors write, including gastric cancer, colorectal cancer, nonalcoholic fatty liver disease, and obesity. Studies have also indicated that H. pylori infection is related to deficiencies in vitamins A, C, and E, which have antioxidant properties.
In a case-control study, the research team compared the dietary intake of 148 patients with H. pylori to 302 healthy controls without infection. The patients in the H. pylori–positive group were recruited between June 2021 and November 2021 from the gastroenterology clinic at Rasoul-e-Akram Hospital in Tehran, where they were newly diagnosed with active infection and not yet under treatment.
The researchers calculated the DAI based on dietary intake information from a validated, 168-item food frequency questionnaire used in Iran. The participants were asked about their dietary intake based on the average day, week, month, and year. They also discussed serving sizes of food items, and to increase the accuracy of estimates, interviewers showed household measurements or serving sizes to confirm the measurements with participants.
The average age of the study participants was 39 years, and about 60% were women. Compared with the healthy controls, those with H. pylori were significantly older, had higher body mass index, and smoked more.
Overall, patients with H. pylori had a significantly lower intake of vitamin A, vitamin E, manganese, and selenium. Other differences in dietary intake – for vitamin C and zinc – were not significant.
The average total DAI was significantly higher in the healthy controls, at 7.67, as compared with 3.57 in the patients with H. pylori. The risk for infection decreased as continuous DAI increased.
After adjusting for several variables, the researchers found that participants with less than the median DAI values had an increased risk of developing an H. pylori infection.
“A balanced diet, especially high consumption of fruits and vegetables, might protect people against the consequences of H. pylori infection,” the study authors write. “On the contrary, a diet full of carbohydrates and sweets is related to a higher H. pylori infection prevalence.”
Why a good diet may help combat infection
The findings are consistent with other studies that have noted a higher intake of fruits and vegetables among healthy people compared with those who have H. pylori infections, the study authors write. Animal studies have also indicated that taking vitamins A, C, and E and selenium can lead to a reduction in H. pylori growth.
“Several biologically plausible reasons may explain why dietary antioxidants might be, either directly or indirectly, a protective factor against H. pylori infection,” the researchers write. “It is well-known that antioxidants, with their free radical scavenging activities, can inhibit the growth of H. pylori.”
H. pylori is urease-positive and can synthesize a large amount of urease for ammonia production to neutralize gastric acid, which allows it to colonize in the stomach epithelium, the study authors write. Vitamin C inhibits urease activity and improves the stimulation of granulocytes, macrophages, lymphocytes, and immunoglobulin production. Other nutrients, such as zinc, may inhibit the urease enzyme and prevent H. pylori adhesion to gastric tissues, they write.
“Dietary elements have previously been shown to dramatically alter pathogenic responses to H. pylori infections,” Richard Peek Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
Dr. Peek, who wasn’t involved with this study, and colleagues found that iron deficiency is linked with altered bile metabolism, which can promote H. pylori–induced gastric carcinogenesis.
“The current study is important, as it suggests that shifting to a diet rich in antioxidants may be beneficial in terms of H. pylori infection,” he said.
At the same time, Dr. Peek expressed caution about generalizing the results across populations.
“Most of the persons enrolled in this study were likely infected with H. pylori as children,” he noted. “Therefore, the inverse role of antioxidant-rich diets and H. pylori infection must be interpreted with caution.”
Future studies should confirm the findings in other groups and determine whether antioxidant-rich diets limit the diseases caused by H. pylori infection, Dr. Peek added.
The study was not funded by any research center, and the authors declared no conflicts of interest. Dr. Peek reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
People who eat a balanced diet with sufficient antioxidants from fruits and vegetables may face reduced risks for Heliobacter pylori infections, according to a new report.
In particular, patients with an H. pylori infection were more likely to score lower on the Dietary Antioxidant Index (DAI), which was created to consider a diet’s entire antioxidant profile.
“Available evidence indicates that diet has an important role in developing H. pylori infection. Therefore, protective dietary factors are important from a public health point of view,” Farzad Shidfar, a professor of nutrition at the Iran University of Medical Sciences, Tehran, and member of the university’s colorectal research center, and colleagues write.
“While some nutritional research has widely focused on single nutrients or foods in diet-disease relations, the overall diet could be more informative because humans typically consume a combination of nutrients and foods,” they write. “Dietary indices such as DAI are one of the approaches for this purpose.”
The study was published online in BMC Gastroenterology.
Measuring antioxidant intake
Previous research has indicated an inverse association between the DAI and inflammatory diseases, the study authors write, including gastric cancer, colorectal cancer, nonalcoholic fatty liver disease, and obesity. Studies have also indicated that H. pylori infection is related to deficiencies in vitamins A, C, and E, which have antioxidant properties.
In a case-control study, the research team compared the dietary intake of 148 patients with H. pylori to 302 healthy controls without infection. The patients in the H. pylori–positive group were recruited between June 2021 and November 2021 from the gastroenterology clinic at Rasoul-e-Akram Hospital in Tehran, where they were newly diagnosed with active infection and not yet under treatment.
The researchers calculated the DAI based on dietary intake information from a validated, 168-item food frequency questionnaire used in Iran. The participants were asked about their dietary intake based on the average day, week, month, and year. They also discussed serving sizes of food items, and to increase the accuracy of estimates, interviewers showed household measurements or serving sizes to confirm the measurements with participants.
The average age of the study participants was 39 years, and about 60% were women. Compared with the healthy controls, those with H. pylori were significantly older, had higher body mass index, and smoked more.
Overall, patients with H. pylori had a significantly lower intake of vitamin A, vitamin E, manganese, and selenium. Other differences in dietary intake – for vitamin C and zinc – were not significant.
The average total DAI was significantly higher in the healthy controls, at 7.67, as compared with 3.57 in the patients with H. pylori. The risk for infection decreased as continuous DAI increased.
After adjusting for several variables, the researchers found that participants with less than the median DAI values had an increased risk of developing an H. pylori infection.
“A balanced diet, especially high consumption of fruits and vegetables, might protect people against the consequences of H. pylori infection,” the study authors write. “On the contrary, a diet full of carbohydrates and sweets is related to a higher H. pylori infection prevalence.”
Why a good diet may help combat infection
The findings are consistent with other studies that have noted a higher intake of fruits and vegetables among healthy people compared with those who have H. pylori infections, the study authors write. Animal studies have also indicated that taking vitamins A, C, and E and selenium can lead to a reduction in H. pylori growth.
“Several biologically plausible reasons may explain why dietary antioxidants might be, either directly or indirectly, a protective factor against H. pylori infection,” the researchers write. “It is well-known that antioxidants, with their free radical scavenging activities, can inhibit the growth of H. pylori.”
H. pylori is urease-positive and can synthesize a large amount of urease for ammonia production to neutralize gastric acid, which allows it to colonize in the stomach epithelium, the study authors write. Vitamin C inhibits urease activity and improves the stimulation of granulocytes, macrophages, lymphocytes, and immunoglobulin production. Other nutrients, such as zinc, may inhibit the urease enzyme and prevent H. pylori adhesion to gastric tissues, they write.
“Dietary elements have previously been shown to dramatically alter pathogenic responses to H. pylori infections,” Richard Peek Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
Dr. Peek, who wasn’t involved with this study, and colleagues found that iron deficiency is linked with altered bile metabolism, which can promote H. pylori–induced gastric carcinogenesis.
“The current study is important, as it suggests that shifting to a diet rich in antioxidants may be beneficial in terms of H. pylori infection,” he said.
At the same time, Dr. Peek expressed caution about generalizing the results across populations.
“Most of the persons enrolled in this study were likely infected with H. pylori as children,” he noted. “Therefore, the inverse role of antioxidant-rich diets and H. pylori infection must be interpreted with caution.”
Future studies should confirm the findings in other groups and determine whether antioxidant-rich diets limit the diseases caused by H. pylori infection, Dr. Peek added.
The study was not funded by any research center, and the authors declared no conflicts of interest. Dr. Peek reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
People who eat a balanced diet with sufficient antioxidants from fruits and vegetables may face reduced risks for Heliobacter pylori infections, according to a new report.
In particular, patients with an H. pylori infection were more likely to score lower on the Dietary Antioxidant Index (DAI), which was created to consider a diet’s entire antioxidant profile.
“Available evidence indicates that diet has an important role in developing H. pylori infection. Therefore, protective dietary factors are important from a public health point of view,” Farzad Shidfar, a professor of nutrition at the Iran University of Medical Sciences, Tehran, and member of the university’s colorectal research center, and colleagues write.
“While some nutritional research has widely focused on single nutrients or foods in diet-disease relations, the overall diet could be more informative because humans typically consume a combination of nutrients and foods,” they write. “Dietary indices such as DAI are one of the approaches for this purpose.”
The study was published online in BMC Gastroenterology.
Measuring antioxidant intake
Previous research has indicated an inverse association between the DAI and inflammatory diseases, the study authors write, including gastric cancer, colorectal cancer, nonalcoholic fatty liver disease, and obesity. Studies have also indicated that H. pylori infection is related to deficiencies in vitamins A, C, and E, which have antioxidant properties.
In a case-control study, the research team compared the dietary intake of 148 patients with H. pylori to 302 healthy controls without infection. The patients in the H. pylori–positive group were recruited between June 2021 and November 2021 from the gastroenterology clinic at Rasoul-e-Akram Hospital in Tehran, where they were newly diagnosed with active infection and not yet under treatment.
The researchers calculated the DAI based on dietary intake information from a validated, 168-item food frequency questionnaire used in Iran. The participants were asked about their dietary intake based on the average day, week, month, and year. They also discussed serving sizes of food items, and to increase the accuracy of estimates, interviewers showed household measurements or serving sizes to confirm the measurements with participants.
The average age of the study participants was 39 years, and about 60% were women. Compared with the healthy controls, those with H. pylori were significantly older, had higher body mass index, and smoked more.
Overall, patients with H. pylori had a significantly lower intake of vitamin A, vitamin E, manganese, and selenium. Other differences in dietary intake – for vitamin C and zinc – were not significant.
The average total DAI was significantly higher in the healthy controls, at 7.67, as compared with 3.57 in the patients with H. pylori. The risk for infection decreased as continuous DAI increased.
After adjusting for several variables, the researchers found that participants with less than the median DAI values had an increased risk of developing an H. pylori infection.
“A balanced diet, especially high consumption of fruits and vegetables, might protect people against the consequences of H. pylori infection,” the study authors write. “On the contrary, a diet full of carbohydrates and sweets is related to a higher H. pylori infection prevalence.”
Why a good diet may help combat infection
The findings are consistent with other studies that have noted a higher intake of fruits and vegetables among healthy people compared with those who have H. pylori infections, the study authors write. Animal studies have also indicated that taking vitamins A, C, and E and selenium can lead to a reduction in H. pylori growth.
“Several biologically plausible reasons may explain why dietary antioxidants might be, either directly or indirectly, a protective factor against H. pylori infection,” the researchers write. “It is well-known that antioxidants, with their free radical scavenging activities, can inhibit the growth of H. pylori.”
H. pylori is urease-positive and can synthesize a large amount of urease for ammonia production to neutralize gastric acid, which allows it to colonize in the stomach epithelium, the study authors write. Vitamin C inhibits urease activity and improves the stimulation of granulocytes, macrophages, lymphocytes, and immunoglobulin production. Other nutrients, such as zinc, may inhibit the urease enzyme and prevent H. pylori adhesion to gastric tissues, they write.
“Dietary elements have previously been shown to dramatically alter pathogenic responses to H. pylori infections,” Richard Peek Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
Dr. Peek, who wasn’t involved with this study, and colleagues found that iron deficiency is linked with altered bile metabolism, which can promote H. pylori–induced gastric carcinogenesis.
“The current study is important, as it suggests that shifting to a diet rich in antioxidants may be beneficial in terms of H. pylori infection,” he said.
At the same time, Dr. Peek expressed caution about generalizing the results across populations.
“Most of the persons enrolled in this study were likely infected with H. pylori as children,” he noted. “Therefore, the inverse role of antioxidant-rich diets and H. pylori infection must be interpreted with caution.”
Future studies should confirm the findings in other groups and determine whether antioxidant-rich diets limit the diseases caused by H. pylori infection, Dr. Peek added.
The study was not funded by any research center, and the authors declared no conflicts of interest. Dr. Peek reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM BMC GASTROENTEROLOGY
Emerging invasive fungal infections call for multidisciplinary cooperation
BUENOS AIRES – Emerging invasive fungal infections represent a new diagnostic and therapeutic challenge. To address their growing clinical impact on immunocompromised patients requires better local epidemiologic records, said a specialist at the XXII Congress of the Argentine Society of Infectology.
“To know that these fungal infections exist, I believe that in this respect we are falling short,” said Javier Afeltra, PhD, a mycologist at the Ramos Mejía Hospital in Buenos Aires, professor of microbiology at the School of Medicine of the University of Buenos Aires, and coordinator of the commission of immunocompromised patients of the Argentine Society of Infectious Diseases.
“There is some change in mentality that encourages professionals to report the cases they detect – for example, in scientific meetings,” Dr. Afeltra told this news orgnization. “But the problem is that there is no unified registry.
“That’s what we lack: a place to record all those isolated cases. Records where clinical and microbiological data are together within a click. Perhaps the microbiologists report their findings to the Malbrán Institute, an Argentine reference center for infectious disease research, but we do not know what the patients had. And we doctors may get together to make records of what happens clinically with the patient, but the germ data are elsewhere. We need a common registry,” he stressed.
“The main importance of a registry of this type is that it would allow a diagnostic and therapeutic decision to be made that is appropriate to the epidemiological profile of the country and the region, not looking at what they do in the North. Most likely, the best antifungal treatment for our country differs from what is indicated in the guidelines written elsewhere,” said Dr. Afeltra.
Dr. Afeltra pointed out that in the United States, when an oncohematology patient does not respond to antimicrobial treatment, the first thing that doctors think is that the patient has aspergillosis or mucormycosis, in which the fungal infection is caused by filamentous fungi.
But an analysis of data from the REMINI registry – the only prospective, observational, multicenter surveillance registry for invasive mycoses in immunocompromised patients (excluding HIV infection) in Argentina, which has been in existence since 2010 – tells a different story. The most prevalent fungal infections turned out to be those caused by Aspergillus species, followed by Fusarium species. Together, they account for more than half of cases. Mucoral infections (mucormycosis) account for less than 6%. And the initial treatments for these diseases could be different.
Changes in the local epidemiology can occur because the behavior of phytopathogenic fungi found in the environment can be modified. For example, cases of chronic mucormycosis can be detected in China but are virtually nonexistent on this side of the Greenwich meridian, Dr. Afeltra said.
“Nature is not the same in geographical areas, and the fungi … we breathe are completely different, so patients have different infections and require different diagnostic and treatment approaches,” he stressed.
Dr. Afeltra mentioned different fungi that are emerging locally and globally, including yeasts, septate, dimorphic, and pigmented hyaline fungi, that have a variable response to antifungal drugs and are associated with high mortality, “which has a lot to do with a later diagnosis,” he said, noting that reports have increased worldwide. A barrier to sharing this information more widely with the professional community, in addition to the lack of records, is the difficulty in publishing cases or series of cases in indexed journals.
Another challenge in characterizing the phenomenon is in regard to taxonomic reclassifications of fungi. Such reclassifications can mean that “perhaps we are speaking of the same pathogen in similar situations, believing that we are referring to different pathogens,” said Dr. Afeltra.
Clinical pearls related to emerging fungal pathogens
Candida auris. This organism has emerged simultaneously on several continents. It has pathogenicity factors typical of the genus, such as biofilm formation and production of phospholipases and proteinases, although it has greater thermal tolerance. In hospitals, it colonizes for weeks and months. In Argentina, it is resistant to multiple antifungal agents. Sensitivity is variable in different geographical regions. Most strains are resistant to fluconazole, and there is variable resistance to the other triazoles [which are not normally used to treat candidemia]. In the United States, in vitro resistance to amphotericin B is up to 30%, and resistance to echinocandins is up to 5%. New drugs such as rezafungin and ibrexafungerp are being studied. Infection control is similar to that used to control Clostridium difficile.
Fusarium. This genus affects immunocompromised patients, including transplant recipients of solid organs and hematopoietic progenitor cells and patients with neutropenia. The genus has various species, included within complexes, such as F. solani SC, F. oxysporum SC, and F. fujikuroi SC, with clinical manifestations similar to those of aspergillosis. In addition to the pulmonary and disseminated forms, there may be skin involvement attributable to dissemination from a respiratory focus or by contiguity from a focus of onychomycosis. In general, mortality is high, and responses to antifungal agents are variable. Some species are more sensitive to voriconazole or posaconazole, and others less so. All show in vitro resistance to itraconazole. In Argentina, voriconazole is usually used as initial treatment, and in special cases, liposomal amphotericin B or combinations. Fosmanogepix is being evaluated for the future.
Azole-resistant aspergillosis. This infection has shown resistance to itraconazole and third-generation azole drugs. In immunocompromised patients, mortlaity is high. Early detection is key. It is sensitive to amphotericin B and echinocandins. It is generally treated with liposomal amphotericin B. Olorofim and fosmanogepix are being studied.
Pulmonary aspergillosis associated with COVID-19. This infection is associated with high mortality among intubated patients. Signs and symptoms include fever, pleural effusion, hemoptysis, and chest pain, with infiltrates or cavitations on imaging. Determining the diagnosis is difficult. “We couldn’t perform lung biopsies, and it was difficult for us to get patients out of intensive care units for CT scans. We treated the proven cases. We treated the probable cases, and those that had a very low certainty of disease were also treated. We came across this emergency and tried to do the best we could,” said Dr. Afeltra. A digital readout lateral flow trial (Sona Aspergillus Galactomannan LFA) for the quantification of galactomannan, a cell wall component of the Aspergillus genus, proved to be a useful tool for screening and diagnosing patients with probable pulmonary aspergillosis associated with COVID-19. The incidence of invasive mycosis was around 10% among 185 seriously ill COVID-19 patients, according to an Argentine multicenter prospective study in which Dr. Afeltra participated.
Scedosporium and Lomentospora. These genera are rarer septate hyaline fungi. Scedosporium is a complex of species. One species, S. apiospermum, can colonize pediatric patients with cystic fibrosis. Lomentospora prolificans is a multiresistant fungus. It produces pulmonary compromise or disseminated infection. The response to antifungal agents is variable, with a high minimum inhibitory concentration for amphotericin B and isavuconazole. Patients are usually treated with voriconazole alone or in combination with terbinafine or micafungin. Olorofim is emerging as a promising treatment.
Dr. Afeltra has received fees from Biotoscana, Gador, Pfizer, Merck, and Sandoz.
This article was translated from the Medscape Spanish edition, a version appeared on Medscape.com.
BUENOS AIRES – Emerging invasive fungal infections represent a new diagnostic and therapeutic challenge. To address their growing clinical impact on immunocompromised patients requires better local epidemiologic records, said a specialist at the XXII Congress of the Argentine Society of Infectology.
“To know that these fungal infections exist, I believe that in this respect we are falling short,” said Javier Afeltra, PhD, a mycologist at the Ramos Mejía Hospital in Buenos Aires, professor of microbiology at the School of Medicine of the University of Buenos Aires, and coordinator of the commission of immunocompromised patients of the Argentine Society of Infectious Diseases.
“There is some change in mentality that encourages professionals to report the cases they detect – for example, in scientific meetings,” Dr. Afeltra told this news orgnization. “But the problem is that there is no unified registry.
“That’s what we lack: a place to record all those isolated cases. Records where clinical and microbiological data are together within a click. Perhaps the microbiologists report their findings to the Malbrán Institute, an Argentine reference center for infectious disease research, but we do not know what the patients had. And we doctors may get together to make records of what happens clinically with the patient, but the germ data are elsewhere. We need a common registry,” he stressed.
“The main importance of a registry of this type is that it would allow a diagnostic and therapeutic decision to be made that is appropriate to the epidemiological profile of the country and the region, not looking at what they do in the North. Most likely, the best antifungal treatment for our country differs from what is indicated in the guidelines written elsewhere,” said Dr. Afeltra.
Dr. Afeltra pointed out that in the United States, when an oncohematology patient does not respond to antimicrobial treatment, the first thing that doctors think is that the patient has aspergillosis or mucormycosis, in which the fungal infection is caused by filamentous fungi.
But an analysis of data from the REMINI registry – the only prospective, observational, multicenter surveillance registry for invasive mycoses in immunocompromised patients (excluding HIV infection) in Argentina, which has been in existence since 2010 – tells a different story. The most prevalent fungal infections turned out to be those caused by Aspergillus species, followed by Fusarium species. Together, they account for more than half of cases. Mucoral infections (mucormycosis) account for less than 6%. And the initial treatments for these diseases could be different.
Changes in the local epidemiology can occur because the behavior of phytopathogenic fungi found in the environment can be modified. For example, cases of chronic mucormycosis can be detected in China but are virtually nonexistent on this side of the Greenwich meridian, Dr. Afeltra said.
“Nature is not the same in geographical areas, and the fungi … we breathe are completely different, so patients have different infections and require different diagnostic and treatment approaches,” he stressed.
Dr. Afeltra mentioned different fungi that are emerging locally and globally, including yeasts, septate, dimorphic, and pigmented hyaline fungi, that have a variable response to antifungal drugs and are associated with high mortality, “which has a lot to do with a later diagnosis,” he said, noting that reports have increased worldwide. A barrier to sharing this information more widely with the professional community, in addition to the lack of records, is the difficulty in publishing cases or series of cases in indexed journals.
Another challenge in characterizing the phenomenon is in regard to taxonomic reclassifications of fungi. Such reclassifications can mean that “perhaps we are speaking of the same pathogen in similar situations, believing that we are referring to different pathogens,” said Dr. Afeltra.
Clinical pearls related to emerging fungal pathogens
Candida auris. This organism has emerged simultaneously on several continents. It has pathogenicity factors typical of the genus, such as biofilm formation and production of phospholipases and proteinases, although it has greater thermal tolerance. In hospitals, it colonizes for weeks and months. In Argentina, it is resistant to multiple antifungal agents. Sensitivity is variable in different geographical regions. Most strains are resistant to fluconazole, and there is variable resistance to the other triazoles [which are not normally used to treat candidemia]. In the United States, in vitro resistance to amphotericin B is up to 30%, and resistance to echinocandins is up to 5%. New drugs such as rezafungin and ibrexafungerp are being studied. Infection control is similar to that used to control Clostridium difficile.
Fusarium. This genus affects immunocompromised patients, including transplant recipients of solid organs and hematopoietic progenitor cells and patients with neutropenia. The genus has various species, included within complexes, such as F. solani SC, F. oxysporum SC, and F. fujikuroi SC, with clinical manifestations similar to those of aspergillosis. In addition to the pulmonary and disseminated forms, there may be skin involvement attributable to dissemination from a respiratory focus or by contiguity from a focus of onychomycosis. In general, mortality is high, and responses to antifungal agents are variable. Some species are more sensitive to voriconazole or posaconazole, and others less so. All show in vitro resistance to itraconazole. In Argentina, voriconazole is usually used as initial treatment, and in special cases, liposomal amphotericin B or combinations. Fosmanogepix is being evaluated for the future.
Azole-resistant aspergillosis. This infection has shown resistance to itraconazole and third-generation azole drugs. In immunocompromised patients, mortlaity is high. Early detection is key. It is sensitive to amphotericin B and echinocandins. It is generally treated with liposomal amphotericin B. Olorofim and fosmanogepix are being studied.
Pulmonary aspergillosis associated with COVID-19. This infection is associated with high mortality among intubated patients. Signs and symptoms include fever, pleural effusion, hemoptysis, and chest pain, with infiltrates or cavitations on imaging. Determining the diagnosis is difficult. “We couldn’t perform lung biopsies, and it was difficult for us to get patients out of intensive care units for CT scans. We treated the proven cases. We treated the probable cases, and those that had a very low certainty of disease were also treated. We came across this emergency and tried to do the best we could,” said Dr. Afeltra. A digital readout lateral flow trial (Sona Aspergillus Galactomannan LFA) for the quantification of galactomannan, a cell wall component of the Aspergillus genus, proved to be a useful tool for screening and diagnosing patients with probable pulmonary aspergillosis associated with COVID-19. The incidence of invasive mycosis was around 10% among 185 seriously ill COVID-19 patients, according to an Argentine multicenter prospective study in which Dr. Afeltra participated.
Scedosporium and Lomentospora. These genera are rarer septate hyaline fungi. Scedosporium is a complex of species. One species, S. apiospermum, can colonize pediatric patients with cystic fibrosis. Lomentospora prolificans is a multiresistant fungus. It produces pulmonary compromise or disseminated infection. The response to antifungal agents is variable, with a high minimum inhibitory concentration for amphotericin B and isavuconazole. Patients are usually treated with voriconazole alone or in combination with terbinafine or micafungin. Olorofim is emerging as a promising treatment.
Dr. Afeltra has received fees from Biotoscana, Gador, Pfizer, Merck, and Sandoz.
This article was translated from the Medscape Spanish edition, a version appeared on Medscape.com.
BUENOS AIRES – Emerging invasive fungal infections represent a new diagnostic and therapeutic challenge. To address their growing clinical impact on immunocompromised patients requires better local epidemiologic records, said a specialist at the XXII Congress of the Argentine Society of Infectology.
“To know that these fungal infections exist, I believe that in this respect we are falling short,” said Javier Afeltra, PhD, a mycologist at the Ramos Mejía Hospital in Buenos Aires, professor of microbiology at the School of Medicine of the University of Buenos Aires, and coordinator of the commission of immunocompromised patients of the Argentine Society of Infectious Diseases.
“There is some change in mentality that encourages professionals to report the cases they detect – for example, in scientific meetings,” Dr. Afeltra told this news orgnization. “But the problem is that there is no unified registry.
“That’s what we lack: a place to record all those isolated cases. Records where clinical and microbiological data are together within a click. Perhaps the microbiologists report their findings to the Malbrán Institute, an Argentine reference center for infectious disease research, but we do not know what the patients had. And we doctors may get together to make records of what happens clinically with the patient, but the germ data are elsewhere. We need a common registry,” he stressed.
“The main importance of a registry of this type is that it would allow a diagnostic and therapeutic decision to be made that is appropriate to the epidemiological profile of the country and the region, not looking at what they do in the North. Most likely, the best antifungal treatment for our country differs from what is indicated in the guidelines written elsewhere,” said Dr. Afeltra.
Dr. Afeltra pointed out that in the United States, when an oncohematology patient does not respond to antimicrobial treatment, the first thing that doctors think is that the patient has aspergillosis or mucormycosis, in which the fungal infection is caused by filamentous fungi.
But an analysis of data from the REMINI registry – the only prospective, observational, multicenter surveillance registry for invasive mycoses in immunocompromised patients (excluding HIV infection) in Argentina, which has been in existence since 2010 – tells a different story. The most prevalent fungal infections turned out to be those caused by Aspergillus species, followed by Fusarium species. Together, they account for more than half of cases. Mucoral infections (mucormycosis) account for less than 6%. And the initial treatments for these diseases could be different.
Changes in the local epidemiology can occur because the behavior of phytopathogenic fungi found in the environment can be modified. For example, cases of chronic mucormycosis can be detected in China but are virtually nonexistent on this side of the Greenwich meridian, Dr. Afeltra said.
“Nature is not the same in geographical areas, and the fungi … we breathe are completely different, so patients have different infections and require different diagnostic and treatment approaches,” he stressed.
Dr. Afeltra mentioned different fungi that are emerging locally and globally, including yeasts, septate, dimorphic, and pigmented hyaline fungi, that have a variable response to antifungal drugs and are associated with high mortality, “which has a lot to do with a later diagnosis,” he said, noting that reports have increased worldwide. A barrier to sharing this information more widely with the professional community, in addition to the lack of records, is the difficulty in publishing cases or series of cases in indexed journals.
Another challenge in characterizing the phenomenon is in regard to taxonomic reclassifications of fungi. Such reclassifications can mean that “perhaps we are speaking of the same pathogen in similar situations, believing that we are referring to different pathogens,” said Dr. Afeltra.
Clinical pearls related to emerging fungal pathogens
Candida auris. This organism has emerged simultaneously on several continents. It has pathogenicity factors typical of the genus, such as biofilm formation and production of phospholipases and proteinases, although it has greater thermal tolerance. In hospitals, it colonizes for weeks and months. In Argentina, it is resistant to multiple antifungal agents. Sensitivity is variable in different geographical regions. Most strains are resistant to fluconazole, and there is variable resistance to the other triazoles [which are not normally used to treat candidemia]. In the United States, in vitro resistance to amphotericin B is up to 30%, and resistance to echinocandins is up to 5%. New drugs such as rezafungin and ibrexafungerp are being studied. Infection control is similar to that used to control Clostridium difficile.
Fusarium. This genus affects immunocompromised patients, including transplant recipients of solid organs and hematopoietic progenitor cells and patients with neutropenia. The genus has various species, included within complexes, such as F. solani SC, F. oxysporum SC, and F. fujikuroi SC, with clinical manifestations similar to those of aspergillosis. In addition to the pulmonary and disseminated forms, there may be skin involvement attributable to dissemination from a respiratory focus or by contiguity from a focus of onychomycosis. In general, mortality is high, and responses to antifungal agents are variable. Some species are more sensitive to voriconazole or posaconazole, and others less so. All show in vitro resistance to itraconazole. In Argentina, voriconazole is usually used as initial treatment, and in special cases, liposomal amphotericin B or combinations. Fosmanogepix is being evaluated for the future.
Azole-resistant aspergillosis. This infection has shown resistance to itraconazole and third-generation azole drugs. In immunocompromised patients, mortlaity is high. Early detection is key. It is sensitive to amphotericin B and echinocandins. It is generally treated with liposomal amphotericin B. Olorofim and fosmanogepix are being studied.
Pulmonary aspergillosis associated with COVID-19. This infection is associated with high mortality among intubated patients. Signs and symptoms include fever, pleural effusion, hemoptysis, and chest pain, with infiltrates or cavitations on imaging. Determining the diagnosis is difficult. “We couldn’t perform lung biopsies, and it was difficult for us to get patients out of intensive care units for CT scans. We treated the proven cases. We treated the probable cases, and those that had a very low certainty of disease were also treated. We came across this emergency and tried to do the best we could,” said Dr. Afeltra. A digital readout lateral flow trial (Sona Aspergillus Galactomannan LFA) for the quantification of galactomannan, a cell wall component of the Aspergillus genus, proved to be a useful tool for screening and diagnosing patients with probable pulmonary aspergillosis associated with COVID-19. The incidence of invasive mycosis was around 10% among 185 seriously ill COVID-19 patients, according to an Argentine multicenter prospective study in which Dr. Afeltra participated.
Scedosporium and Lomentospora. These genera are rarer septate hyaline fungi. Scedosporium is a complex of species. One species, S. apiospermum, can colonize pediatric patients with cystic fibrosis. Lomentospora prolificans is a multiresistant fungus. It produces pulmonary compromise or disseminated infection. The response to antifungal agents is variable, with a high minimum inhibitory concentration for amphotericin B and isavuconazole. Patients are usually treated with voriconazole alone or in combination with terbinafine or micafungin. Olorofim is emerging as a promising treatment.
Dr. Afeltra has received fees from Biotoscana, Gador, Pfizer, Merck, and Sandoz.
This article was translated from the Medscape Spanish edition, a version appeared on Medscape.com.
AT SADI 2022
New technology a sepsis breakthrough?
Sepsis is among the most feared conditions for health care providers. These blood infections strike with such rapid intensity that treating them demands a mix of both clinical skill and luck – recognizing symptoms early enough while choosing the right drug to tame the bacterial culprit before the germs have overwhelmed the body’s immune system.
All too often, sepsis wins the race. According to the U.S. Centers for Disease Control and Prevention, at least 1.7 million people in this country develop sepsis annually. About 350,000 die during hospitalization or are discharged to hospice.
But new research, published in Proceedings of the National Academy of Sciences, offers hope that clinicians may one day be able to detect and treat sepsis more quickly.
The researchers broke down whole blood and dried it by heating, resulting in a solid porous structure with the bacterial DNA trapped inside. They then used chemicals – primers and enzymes – to reach inside the porous structure and amplify the target DNA.
The team was able to detect four causes of bloodstream infections – the bacteria methicillin-resistant Staphylococcus aureus (MRSA), methicillin-susceptible Staphylococcus aureus (MSSA), gram-negative Escherichia coli, and the fungal species Candida albicans. They validated their method against clinical laboratory results that used blood cultures and DNA analyses to detect sepsis.
The technique took just 2.5 hours and required roughly 1 mL of blood, according to the researchers.
“This technique can have broad applications in detection of bacterial infection and presence of bacteria in large values of blood,” Rashid Bashir, PhD, dean of the University of Illinois at Urbana-Champaign’s Grainger College of Engineering, and a co-author of the study, told this news organization.
While infection control experts and sepsis prevention advocates said the new study offers no clues about how to treat sepsis once detected, they hope the innovation eventually could save lives.
A rapid killer
Sepsis occurs when the body overreacts to an infection. The severe response can lead to tissue damage, organ failure, and death.
Thomas Heymann, MBA, president and CEO of Sepsis Alliance, an advocacy group, said mortality can rise 8% for each hour treatment is delayed.
Infants born prematurely are particularly vulnerable. Dr. Bashir and his colleagues noted that 25% of all infants admitted to the neonatal intensive care unit are diagnosed with sepsis. Of those, as many as 35% may die from infection. Sepsis is the most expensive condition treated in U.S. hospitals, accounting for $23.7 billion in costs annually, they added.
Despite high mortality rates and hospital costs, according to a Sepsis Alliance survey, only 66% of Americans are aware of the term sepsis. Only 19% can name the four primary signs of the condition: Altered body Temperature, an Infection, Mental decline, and feeling Extremely ill, or “TIME.”
Getting the appropriate antibiotics to sepsis patients quickly can greatly improve chances of survival, but Dr. Bashir said the current method of confirming the diagnosis is too slow.
Blood cultures too slow
Traditional blood cultures are among the most common methods of determining if a patient has a bloodstream infection. But the process takes about 24 hours for a culture to detect the category of bacteria and an additional day to determine exactly which bacteria is present, according to Cindy Hou, DO, infection control officer and medical director of research at Jefferson Health, Voorhees Township, New Jersey. At 72 hours, Dr. Hou said, a blood culture will finally be able to produce a “sensitivity” result, which tells doctors which antibiotics will be most effective against the pathogen.
By then, patients often are already past the point of saving. The bottom line, according to Dr. Bashir and his colleagues: Blood cultures are “too slow and cumbersome to allow for initial management of patients and thus contribute to high mortality.”
Dr. Hou called the ability to identify the type of infection in just 2.5 hours an “amazing” feat.
,” she said. “These researchers are pushing the bar for what rapid means.”
The new detection method is not yet available commercially. Dr. Bashir said he and his colleagues plan to scale their study and hope to find a way to bypass the long culture steps to identify target pathogens directly from a large volume of blood.
Dr. Hou said she believes a blood culture would still be necessary since clinicians would need sensitivity results to guide targeted treatment of infections.
“There is a lot more we need, but this paper is a call to arms for the field of rapid diagnostics to make rapid as fast as it really needs to be, but we still need to find solutions which are affordable,” Dr. Hou said.
Even without a blood culture, Dr. Bashir’s technology could improve care. Mr. Heymann said the technology could help convince clinicians worried about antibiotic resistance to prescribe treatment faster.
“We know we’re overusing antibiotics, and that’s creating a new big problem” when it comes to sepsis treatment, he said. “Getting a diagnostic read earlier is a game changer.”
Combined with a blood culture that can later confirm or help adjust the course of treatment, Dr. Hou said this new method of sepsis detection could improve care, especially in places where rapid diagnostics are not available and particularly if combined with physician education so they understand what treatment is best for various types of infection.
Mr. Heymann agreed. Sepsis Alliance also operates the Sepsis Innovation Collaborative, a group that supports public-private innovation on sepsis care.
“We’re losing someone every 90 seconds in the United States to sepsis,” Mr. Heymann said. “There is a huge opportunity to do better, and it’s this kind of innovation that is really inspiring.”
Dr. Hou is chief medical officer for Sepsis Alliance, a medical advisor for the Sepsis Innovation Collaborative, an advisor for Janssen, and a key opinion leader for T2 Biosystems. Dr. Bashir and Mr. Heymann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sepsis is among the most feared conditions for health care providers. These blood infections strike with such rapid intensity that treating them demands a mix of both clinical skill and luck – recognizing symptoms early enough while choosing the right drug to tame the bacterial culprit before the germs have overwhelmed the body’s immune system.
All too often, sepsis wins the race. According to the U.S. Centers for Disease Control and Prevention, at least 1.7 million people in this country develop sepsis annually. About 350,000 die during hospitalization or are discharged to hospice.
But new research, published in Proceedings of the National Academy of Sciences, offers hope that clinicians may one day be able to detect and treat sepsis more quickly.
The researchers broke down whole blood and dried it by heating, resulting in a solid porous structure with the bacterial DNA trapped inside. They then used chemicals – primers and enzymes – to reach inside the porous structure and amplify the target DNA.
The team was able to detect four causes of bloodstream infections – the bacteria methicillin-resistant Staphylococcus aureus (MRSA), methicillin-susceptible Staphylococcus aureus (MSSA), gram-negative Escherichia coli, and the fungal species Candida albicans. They validated their method against clinical laboratory results that used blood cultures and DNA analyses to detect sepsis.
The technique took just 2.5 hours and required roughly 1 mL of blood, according to the researchers.
“This technique can have broad applications in detection of bacterial infection and presence of bacteria in large values of blood,” Rashid Bashir, PhD, dean of the University of Illinois at Urbana-Champaign’s Grainger College of Engineering, and a co-author of the study, told this news organization.
While infection control experts and sepsis prevention advocates said the new study offers no clues about how to treat sepsis once detected, they hope the innovation eventually could save lives.
A rapid killer
Sepsis occurs when the body overreacts to an infection. The severe response can lead to tissue damage, organ failure, and death.
Thomas Heymann, MBA, president and CEO of Sepsis Alliance, an advocacy group, said mortality can rise 8% for each hour treatment is delayed.
Infants born prematurely are particularly vulnerable. Dr. Bashir and his colleagues noted that 25% of all infants admitted to the neonatal intensive care unit are diagnosed with sepsis. Of those, as many as 35% may die from infection. Sepsis is the most expensive condition treated in U.S. hospitals, accounting for $23.7 billion in costs annually, they added.
Despite high mortality rates and hospital costs, according to a Sepsis Alliance survey, only 66% of Americans are aware of the term sepsis. Only 19% can name the four primary signs of the condition: Altered body Temperature, an Infection, Mental decline, and feeling Extremely ill, or “TIME.”
Getting the appropriate antibiotics to sepsis patients quickly can greatly improve chances of survival, but Dr. Bashir said the current method of confirming the diagnosis is too slow.
Blood cultures too slow
Traditional blood cultures are among the most common methods of determining if a patient has a bloodstream infection. But the process takes about 24 hours for a culture to detect the category of bacteria and an additional day to determine exactly which bacteria is present, according to Cindy Hou, DO, infection control officer and medical director of research at Jefferson Health, Voorhees Township, New Jersey. At 72 hours, Dr. Hou said, a blood culture will finally be able to produce a “sensitivity” result, which tells doctors which antibiotics will be most effective against the pathogen.
By then, patients often are already past the point of saving. The bottom line, according to Dr. Bashir and his colleagues: Blood cultures are “too slow and cumbersome to allow for initial management of patients and thus contribute to high mortality.”
Dr. Hou called the ability to identify the type of infection in just 2.5 hours an “amazing” feat.
,” she said. “These researchers are pushing the bar for what rapid means.”
The new detection method is not yet available commercially. Dr. Bashir said he and his colleagues plan to scale their study and hope to find a way to bypass the long culture steps to identify target pathogens directly from a large volume of blood.
Dr. Hou said she believes a blood culture would still be necessary since clinicians would need sensitivity results to guide targeted treatment of infections.
“There is a lot more we need, but this paper is a call to arms for the field of rapid diagnostics to make rapid as fast as it really needs to be, but we still need to find solutions which are affordable,” Dr. Hou said.
Even without a blood culture, Dr. Bashir’s technology could improve care. Mr. Heymann said the technology could help convince clinicians worried about antibiotic resistance to prescribe treatment faster.
“We know we’re overusing antibiotics, and that’s creating a new big problem” when it comes to sepsis treatment, he said. “Getting a diagnostic read earlier is a game changer.”
Combined with a blood culture that can later confirm or help adjust the course of treatment, Dr. Hou said this new method of sepsis detection could improve care, especially in places where rapid diagnostics are not available and particularly if combined with physician education so they understand what treatment is best for various types of infection.
Mr. Heymann agreed. Sepsis Alliance also operates the Sepsis Innovation Collaborative, a group that supports public-private innovation on sepsis care.
“We’re losing someone every 90 seconds in the United States to sepsis,” Mr. Heymann said. “There is a huge opportunity to do better, and it’s this kind of innovation that is really inspiring.”
Dr. Hou is chief medical officer for Sepsis Alliance, a medical advisor for the Sepsis Innovation Collaborative, an advisor for Janssen, and a key opinion leader for T2 Biosystems. Dr. Bashir and Mr. Heymann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sepsis is among the most feared conditions for health care providers. These blood infections strike with such rapid intensity that treating them demands a mix of both clinical skill and luck – recognizing symptoms early enough while choosing the right drug to tame the bacterial culprit before the germs have overwhelmed the body’s immune system.
All too often, sepsis wins the race. According to the U.S. Centers for Disease Control and Prevention, at least 1.7 million people in this country develop sepsis annually. About 350,000 die during hospitalization or are discharged to hospice.
But new research, published in Proceedings of the National Academy of Sciences, offers hope that clinicians may one day be able to detect and treat sepsis more quickly.
The researchers broke down whole blood and dried it by heating, resulting in a solid porous structure with the bacterial DNA trapped inside. They then used chemicals – primers and enzymes – to reach inside the porous structure and amplify the target DNA.
The team was able to detect four causes of bloodstream infections – the bacteria methicillin-resistant Staphylococcus aureus (MRSA), methicillin-susceptible Staphylococcus aureus (MSSA), gram-negative Escherichia coli, and the fungal species Candida albicans. They validated their method against clinical laboratory results that used blood cultures and DNA analyses to detect sepsis.
The technique took just 2.5 hours and required roughly 1 mL of blood, according to the researchers.
“This technique can have broad applications in detection of bacterial infection and presence of bacteria in large values of blood,” Rashid Bashir, PhD, dean of the University of Illinois at Urbana-Champaign’s Grainger College of Engineering, and a co-author of the study, told this news organization.
While infection control experts and sepsis prevention advocates said the new study offers no clues about how to treat sepsis once detected, they hope the innovation eventually could save lives.
A rapid killer
Sepsis occurs when the body overreacts to an infection. The severe response can lead to tissue damage, organ failure, and death.
Thomas Heymann, MBA, president and CEO of Sepsis Alliance, an advocacy group, said mortality can rise 8% for each hour treatment is delayed.
Infants born prematurely are particularly vulnerable. Dr. Bashir and his colleagues noted that 25% of all infants admitted to the neonatal intensive care unit are diagnosed with sepsis. Of those, as many as 35% may die from infection. Sepsis is the most expensive condition treated in U.S. hospitals, accounting for $23.7 billion in costs annually, they added.
Despite high mortality rates and hospital costs, according to a Sepsis Alliance survey, only 66% of Americans are aware of the term sepsis. Only 19% can name the four primary signs of the condition: Altered body Temperature, an Infection, Mental decline, and feeling Extremely ill, or “TIME.”
Getting the appropriate antibiotics to sepsis patients quickly can greatly improve chances of survival, but Dr. Bashir said the current method of confirming the diagnosis is too slow.
Blood cultures too slow
Traditional blood cultures are among the most common methods of determining if a patient has a bloodstream infection. But the process takes about 24 hours for a culture to detect the category of bacteria and an additional day to determine exactly which bacteria is present, according to Cindy Hou, DO, infection control officer and medical director of research at Jefferson Health, Voorhees Township, New Jersey. At 72 hours, Dr. Hou said, a blood culture will finally be able to produce a “sensitivity” result, which tells doctors which antibiotics will be most effective against the pathogen.
By then, patients often are already past the point of saving. The bottom line, according to Dr. Bashir and his colleagues: Blood cultures are “too slow and cumbersome to allow for initial management of patients and thus contribute to high mortality.”
Dr. Hou called the ability to identify the type of infection in just 2.5 hours an “amazing” feat.
,” she said. “These researchers are pushing the bar for what rapid means.”
The new detection method is not yet available commercially. Dr. Bashir said he and his colleagues plan to scale their study and hope to find a way to bypass the long culture steps to identify target pathogens directly from a large volume of blood.
Dr. Hou said she believes a blood culture would still be necessary since clinicians would need sensitivity results to guide targeted treatment of infections.
“There is a lot more we need, but this paper is a call to arms for the field of rapid diagnostics to make rapid as fast as it really needs to be, but we still need to find solutions which are affordable,” Dr. Hou said.
Even without a blood culture, Dr. Bashir’s technology could improve care. Mr. Heymann said the technology could help convince clinicians worried about antibiotic resistance to prescribe treatment faster.
“We know we’re overusing antibiotics, and that’s creating a new big problem” when it comes to sepsis treatment, he said. “Getting a diagnostic read earlier is a game changer.”
Combined with a blood culture that can later confirm or help adjust the course of treatment, Dr. Hou said this new method of sepsis detection could improve care, especially in places where rapid diagnostics are not available and particularly if combined with physician education so they understand what treatment is best for various types of infection.
Mr. Heymann agreed. Sepsis Alliance also operates the Sepsis Innovation Collaborative, a group that supports public-private innovation on sepsis care.
“We’re losing someone every 90 seconds in the United States to sepsis,” Mr. Heymann said. “There is a huge opportunity to do better, and it’s this kind of innovation that is really inspiring.”
Dr. Hou is chief medical officer for Sepsis Alliance, a medical advisor for the Sepsis Innovation Collaborative, an advisor for Janssen, and a key opinion leader for T2 Biosystems. Dr. Bashir and Mr. Heymann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Evusheld PrEP may protect immunocompromised patients from severe COVID-19
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
FROM RMD OPEN
Increased HIV infection linked to pandemic-related access to PrEP
Changes to HIV pre-exposure prophylaxis (PrEP) access during the COVID-19 pandemic were linked to higher rates of HIV infection among young sexual minority men and gender-diverse individuals who identified as Black and/or Hispanic/Latino, according to a national survey.
“The public health crisis surrounding COVID-19 had clear impact on PrEP access and risk of HIV acquisition overall,” said lead investigator Ethan Morgan, PhD, College of Nursing and the Infectious Disease Institute at Ohio State University, Columbus.
he said in an interview.
The online survey was administered in four waves during the first year and a half of the pandemic, starting in March 2020. Participants were recruited through mailing lists, national networks, community partners, and social media.
Among 796 baseline respondents, 300 agreed to three follow-up surveys administered between February and March 2021, between July and August 2021, and between October and November 2021.
Inclusion required participants to identify as Black and/or Hispanic/Latino, be between ages 18-29 years, be assigned male at birth, reside in the United States, and have reported anal intercourse with a man in the past 12 months. The researchers noted that given the limited uptake of and adherence to PrEP in the targeted population, they prioritized baseline respondents who reported either current PrEP use or use at least once in their lifetime.
The researchers used separate multivariable logistic regression models to assess the association between odds of testing positive for HIV and other STIs across the four online study visits and pandemic-related changes to PrEP access, and pandemic-related changes to sexual activity.
Changes in PrEP access were reported by a total of 109 (13.8%) of baseline respondents, and HIV seroconversion was reported in 25 of 292 respondents (8.6%) who reported their HIV and other STI status at follow-up. STI positivity was reported 25.6% of the baseline cohort (n = 204).
Compared with respondents who reported no changes to PrEP access, those who did report change to access were significantly more likely to report HIV seroconversion (adjusted odds ratio, 2.80; 95% confidence interval, 1.02-7.68). However, Dr. Morgan emphasized that the study question did not ask how PrEP had changed, only if it had.
“While we presume this survey question corresponds to a diminished access to PrEP medication during the COVID-19 pandemic, the question was: ‘Has your access to PrEP been impacted by the COVID-19 pandemic?’ So, it is unfortunately unclear whether access was diminished or improved,” he explained. STI positivity was not associated with PrEP access.
The survey also asked respondents how much the pandemic had impacted their sexual activity (measured on a Likert scale of not at all, a little, moderately, quite a bit, and extremely). Respondents reporting greater impact on their sexual activity were more likely to report an STI (aOR, 1.24; 95% CI, 1.10-1.40) during the study period.
In addition, though participants reported a mean of 2.8 sexual partners in the past 3 months, those reporting a greater number were more likely to report an STI (aOR, 1.29; 95% CI, 1.21-1.38).
The researchers suggested that expansion of telehealth and mail-order prescriptions as well as structural-level interventions addressing pandemic-related unemployment and loss of health insurance could have helped preserve access to PrEP.
Commenting on the study, Monica Gandhi, MD, MPH, who was not involved in the research, noted that self-reported data can be subject to bias. “However, reduction in services for other medical care has been reported frequently throughout COVID and so this finding of reduced PrEP access, and subsequent HIV infection, is completely in line with the other studies,” she said in an interview.
Dr. Gandhi, who is director of the University of California, San Francisco Center for AIDS Research and medical director of the HIV/AIDS Clinic (“Ward 86”) at San Francisco General Hospital, added: “We knew early on in the COVID-19 pandemic that access to and uptake of PrEP was decreased based on data from Boston’s Fenway Institute.”
The Boston data, reported July 2020 at the virtual International AIDS Conference, prompted “a real attempt” by clinicians to increase PrEP access and uptake – raising community awareness, dispensing PrEP through mobile units, and changing prescribing patterns, Dr. Gandhi said. “We usually see patients every 3 months for PrEP but with HIV self-testing, we can extend that interval to every 6 months, and we did so in many centers during COVID.”
The study was funded by National Institute on Drug Abuse, part of the National Institutes of Health.
Dr. Morgan and Dr. Gandhi reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Changes to HIV pre-exposure prophylaxis (PrEP) access during the COVID-19 pandemic were linked to higher rates of HIV infection among young sexual minority men and gender-diverse individuals who identified as Black and/or Hispanic/Latino, according to a national survey.
“The public health crisis surrounding COVID-19 had clear impact on PrEP access and risk of HIV acquisition overall,” said lead investigator Ethan Morgan, PhD, College of Nursing and the Infectious Disease Institute at Ohio State University, Columbus.
he said in an interview.
The online survey was administered in four waves during the first year and a half of the pandemic, starting in March 2020. Participants were recruited through mailing lists, national networks, community partners, and social media.
Among 796 baseline respondents, 300 agreed to three follow-up surveys administered between February and March 2021, between July and August 2021, and between October and November 2021.
Inclusion required participants to identify as Black and/or Hispanic/Latino, be between ages 18-29 years, be assigned male at birth, reside in the United States, and have reported anal intercourse with a man in the past 12 months. The researchers noted that given the limited uptake of and adherence to PrEP in the targeted population, they prioritized baseline respondents who reported either current PrEP use or use at least once in their lifetime.
The researchers used separate multivariable logistic regression models to assess the association between odds of testing positive for HIV and other STIs across the four online study visits and pandemic-related changes to PrEP access, and pandemic-related changes to sexual activity.
Changes in PrEP access were reported by a total of 109 (13.8%) of baseline respondents, and HIV seroconversion was reported in 25 of 292 respondents (8.6%) who reported their HIV and other STI status at follow-up. STI positivity was reported 25.6% of the baseline cohort (n = 204).
Compared with respondents who reported no changes to PrEP access, those who did report change to access were significantly more likely to report HIV seroconversion (adjusted odds ratio, 2.80; 95% confidence interval, 1.02-7.68). However, Dr. Morgan emphasized that the study question did not ask how PrEP had changed, only if it had.
“While we presume this survey question corresponds to a diminished access to PrEP medication during the COVID-19 pandemic, the question was: ‘Has your access to PrEP been impacted by the COVID-19 pandemic?’ So, it is unfortunately unclear whether access was diminished or improved,” he explained. STI positivity was not associated with PrEP access.
The survey also asked respondents how much the pandemic had impacted their sexual activity (measured on a Likert scale of not at all, a little, moderately, quite a bit, and extremely). Respondents reporting greater impact on their sexual activity were more likely to report an STI (aOR, 1.24; 95% CI, 1.10-1.40) during the study period.
In addition, though participants reported a mean of 2.8 sexual partners in the past 3 months, those reporting a greater number were more likely to report an STI (aOR, 1.29; 95% CI, 1.21-1.38).
The researchers suggested that expansion of telehealth and mail-order prescriptions as well as structural-level interventions addressing pandemic-related unemployment and loss of health insurance could have helped preserve access to PrEP.
Commenting on the study, Monica Gandhi, MD, MPH, who was not involved in the research, noted that self-reported data can be subject to bias. “However, reduction in services for other medical care has been reported frequently throughout COVID and so this finding of reduced PrEP access, and subsequent HIV infection, is completely in line with the other studies,” she said in an interview.
Dr. Gandhi, who is director of the University of California, San Francisco Center for AIDS Research and medical director of the HIV/AIDS Clinic (“Ward 86”) at San Francisco General Hospital, added: “We knew early on in the COVID-19 pandemic that access to and uptake of PrEP was decreased based on data from Boston’s Fenway Institute.”
The Boston data, reported July 2020 at the virtual International AIDS Conference, prompted “a real attempt” by clinicians to increase PrEP access and uptake – raising community awareness, dispensing PrEP through mobile units, and changing prescribing patterns, Dr. Gandhi said. “We usually see patients every 3 months for PrEP but with HIV self-testing, we can extend that interval to every 6 months, and we did so in many centers during COVID.”
The study was funded by National Institute on Drug Abuse, part of the National Institutes of Health.
Dr. Morgan and Dr. Gandhi reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Changes to HIV pre-exposure prophylaxis (PrEP) access during the COVID-19 pandemic were linked to higher rates of HIV infection among young sexual minority men and gender-diverse individuals who identified as Black and/or Hispanic/Latino, according to a national survey.
“The public health crisis surrounding COVID-19 had clear impact on PrEP access and risk of HIV acquisition overall,” said lead investigator Ethan Morgan, PhD, College of Nursing and the Infectious Disease Institute at Ohio State University, Columbus.
he said in an interview.
The online survey was administered in four waves during the first year and a half of the pandemic, starting in March 2020. Participants were recruited through mailing lists, national networks, community partners, and social media.
Among 796 baseline respondents, 300 agreed to three follow-up surveys administered between February and March 2021, between July and August 2021, and between October and November 2021.
Inclusion required participants to identify as Black and/or Hispanic/Latino, be between ages 18-29 years, be assigned male at birth, reside in the United States, and have reported anal intercourse with a man in the past 12 months. The researchers noted that given the limited uptake of and adherence to PrEP in the targeted population, they prioritized baseline respondents who reported either current PrEP use or use at least once in their lifetime.
The researchers used separate multivariable logistic regression models to assess the association between odds of testing positive for HIV and other STIs across the four online study visits and pandemic-related changes to PrEP access, and pandemic-related changes to sexual activity.
Changes in PrEP access were reported by a total of 109 (13.8%) of baseline respondents, and HIV seroconversion was reported in 25 of 292 respondents (8.6%) who reported their HIV and other STI status at follow-up. STI positivity was reported 25.6% of the baseline cohort (n = 204).
Compared with respondents who reported no changes to PrEP access, those who did report change to access were significantly more likely to report HIV seroconversion (adjusted odds ratio, 2.80; 95% confidence interval, 1.02-7.68). However, Dr. Morgan emphasized that the study question did not ask how PrEP had changed, only if it had.
“While we presume this survey question corresponds to a diminished access to PrEP medication during the COVID-19 pandemic, the question was: ‘Has your access to PrEP been impacted by the COVID-19 pandemic?’ So, it is unfortunately unclear whether access was diminished or improved,” he explained. STI positivity was not associated with PrEP access.
The survey also asked respondents how much the pandemic had impacted their sexual activity (measured on a Likert scale of not at all, a little, moderately, quite a bit, and extremely). Respondents reporting greater impact on their sexual activity were more likely to report an STI (aOR, 1.24; 95% CI, 1.10-1.40) during the study period.
In addition, though participants reported a mean of 2.8 sexual partners in the past 3 months, those reporting a greater number were more likely to report an STI (aOR, 1.29; 95% CI, 1.21-1.38).
The researchers suggested that expansion of telehealth and mail-order prescriptions as well as structural-level interventions addressing pandemic-related unemployment and loss of health insurance could have helped preserve access to PrEP.
Commenting on the study, Monica Gandhi, MD, MPH, who was not involved in the research, noted that self-reported data can be subject to bias. “However, reduction in services for other medical care has been reported frequently throughout COVID and so this finding of reduced PrEP access, and subsequent HIV infection, is completely in line with the other studies,” she said in an interview.
Dr. Gandhi, who is director of the University of California, San Francisco Center for AIDS Research and medical director of the HIV/AIDS Clinic (“Ward 86”) at San Francisco General Hospital, added: “We knew early on in the COVID-19 pandemic that access to and uptake of PrEP was decreased based on data from Boston’s Fenway Institute.”
The Boston data, reported July 2020 at the virtual International AIDS Conference, prompted “a real attempt” by clinicians to increase PrEP access and uptake – raising community awareness, dispensing PrEP through mobile units, and changing prescribing patterns, Dr. Gandhi said. “We usually see patients every 3 months for PrEP but with HIV self-testing, we can extend that interval to every 6 months, and we did so in many centers during COVID.”
The study was funded by National Institute on Drug Abuse, part of the National Institutes of Health.
Dr. Morgan and Dr. Gandhi reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ACQUIRED IMMUNE DEFICIENCY SYNDROME