User login
For MD-IQ use only
Regular vitamin D supplements may lower melanoma risk
. They also found a trend for benefit with occasional use.
The study, published in Melanoma Research, involved almost 500 individuals attending a dermatology clinic who reported on their use of vitamin D supplements.
Regular users had a significant 55% reduction in the odds of having a past or present melanoma diagnosis, while occasional use was associated with a nonsignificant 46% reduction. The reduction was similar for all skin cancer types.
However, senior author Ilkka T. Harvima, MD, PhD, department of dermatology, University of Eastern Finland and Kuopio (Finland) University Hospital, warned there are limitations to the study.
Despite adjustment for several possible confounding factors, “it is still possible that some other, yet unidentified or untested, factors can still confound the present result,” he said.
Consequently, “the causal link between vitamin D and melanoma cannot be confirmed by the present results,” Dr. Harvima said in a statement.
Even if the link were to be proven, “the question about the optimal dose of oral vitamin D in order to for it to have beneficial effects remains to be answered,” he said.
“Until we know more, national intake recommendations should be followed.”
The incidence of cutaneous malignant melanoma and other skin cancers has been increasing steadily in Western populations, particularly in immunosuppressed individuals, the authors pointed out, and they attributed the rise to an increased exposure to ultraviolet radiation.
While ultraviolet radiation exposure is a well-known risk factor, “the other side of the coin is that public sun protection campaigns have led to alerts that insufficient sun exposure is a significant public health problem, resulting in insufficient vitamin D status.”
For their study, the team reviewed the records of 498 patients aged 21-79 years at a dermatology outpatient clinic who were deemed by an experienced dermatologist to be at risk of any type of skin cancer.
Among these patients, 295 individuals had a history of past or present cutaneous malignancy, with 100 diagnosed with melanoma, 213 with basal cell carcinoma, and 41 with squamous cell carcinoma. A further 70 subjects had cancer elsewhere, including breast, prostate, kidney, bladder, intestine, and blood cancers.
A subgroup of 96 patients were immunocompromised and were considered separately.
The 402 remaining patients were categorized, based on their self-reported use of oral vitamin D preparations, as nonusers (n = 99), occasional users (n = 126), and regular users (n = 177).
Regular use of vitamin D was associated with being more educated (P = .032), less frequent outdoor working (P = .003), lower tobacco pack years (P = .001), and more frequent solarium exposure (P = .002).
There was no significant association between vitamin D use and photoaging, actinic keratoses, nevi, basal or squamous cell carcinoma, body mass index, or self-estimated lifetime exposure to sunlight or sunburns.
However, there were significant associations between regular use of vitamin D and a lower incidence of melanoma and other cancer types.
There were significantly fewer individuals in the regular vitamin D use group with a past or present history of melanoma when compared with the nonuse group, at 18.1% vs. 32.3% (P = .021), or any type of skin cancer, at 62.1% vs. 74.7% (P = .027).
Multivariate logistic regression analysis revealed that regular vitamin D use was significantly associated with a reduced melanoma risk, at an odds ratio vs. nonuse of 0.447 (P = .016).
Occasional use was associated with a reduced, albeit nonsignificant, risk, with an odds ratio versus nonuse of 0.540 (P = .08).
For any type of skin cancers, regular vitamin D use was associated with an odds ratio vs. nonuse of 0.478 (P = .032), while that for occasional vitamin D use was 0.543 (P = .061).
“Somewhat similar” results were obtained when the investigators looked at the subgroup of immunocompromised individuals, although they note that “the number of subjects was low.”
The study was supported by the Cancer Center of Eastern Finland of the University of Eastern Finland, the Finnish Cancer Research Foundation, and the VTR-funding of Kuopio University Hospital. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
. They also found a trend for benefit with occasional use.
The study, published in Melanoma Research, involved almost 500 individuals attending a dermatology clinic who reported on their use of vitamin D supplements.
Regular users had a significant 55% reduction in the odds of having a past or present melanoma diagnosis, while occasional use was associated with a nonsignificant 46% reduction. The reduction was similar for all skin cancer types.
However, senior author Ilkka T. Harvima, MD, PhD, department of dermatology, University of Eastern Finland and Kuopio (Finland) University Hospital, warned there are limitations to the study.
Despite adjustment for several possible confounding factors, “it is still possible that some other, yet unidentified or untested, factors can still confound the present result,” he said.
Consequently, “the causal link between vitamin D and melanoma cannot be confirmed by the present results,” Dr. Harvima said in a statement.
Even if the link were to be proven, “the question about the optimal dose of oral vitamin D in order to for it to have beneficial effects remains to be answered,” he said.
“Until we know more, national intake recommendations should be followed.”
The incidence of cutaneous malignant melanoma and other skin cancers has been increasing steadily in Western populations, particularly in immunosuppressed individuals, the authors pointed out, and they attributed the rise to an increased exposure to ultraviolet radiation.
While ultraviolet radiation exposure is a well-known risk factor, “the other side of the coin is that public sun protection campaigns have led to alerts that insufficient sun exposure is a significant public health problem, resulting in insufficient vitamin D status.”
For their study, the team reviewed the records of 498 patients aged 21-79 years at a dermatology outpatient clinic who were deemed by an experienced dermatologist to be at risk of any type of skin cancer.
Among these patients, 295 individuals had a history of past or present cutaneous malignancy, with 100 diagnosed with melanoma, 213 with basal cell carcinoma, and 41 with squamous cell carcinoma. A further 70 subjects had cancer elsewhere, including breast, prostate, kidney, bladder, intestine, and blood cancers.
A subgroup of 96 patients were immunocompromised and were considered separately.
The 402 remaining patients were categorized, based on their self-reported use of oral vitamin D preparations, as nonusers (n = 99), occasional users (n = 126), and regular users (n = 177).
Regular use of vitamin D was associated with being more educated (P = .032), less frequent outdoor working (P = .003), lower tobacco pack years (P = .001), and more frequent solarium exposure (P = .002).
There was no significant association between vitamin D use and photoaging, actinic keratoses, nevi, basal or squamous cell carcinoma, body mass index, or self-estimated lifetime exposure to sunlight or sunburns.
However, there were significant associations between regular use of vitamin D and a lower incidence of melanoma and other cancer types.
There were significantly fewer individuals in the regular vitamin D use group with a past or present history of melanoma when compared with the nonuse group, at 18.1% vs. 32.3% (P = .021), or any type of skin cancer, at 62.1% vs. 74.7% (P = .027).
Multivariate logistic regression analysis revealed that regular vitamin D use was significantly associated with a reduced melanoma risk, at an odds ratio vs. nonuse of 0.447 (P = .016).
Occasional use was associated with a reduced, albeit nonsignificant, risk, with an odds ratio versus nonuse of 0.540 (P = .08).
For any type of skin cancers, regular vitamin D use was associated with an odds ratio vs. nonuse of 0.478 (P = .032), while that for occasional vitamin D use was 0.543 (P = .061).
“Somewhat similar” results were obtained when the investigators looked at the subgroup of immunocompromised individuals, although they note that “the number of subjects was low.”
The study was supported by the Cancer Center of Eastern Finland of the University of Eastern Finland, the Finnish Cancer Research Foundation, and the VTR-funding of Kuopio University Hospital. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
. They also found a trend for benefit with occasional use.
The study, published in Melanoma Research, involved almost 500 individuals attending a dermatology clinic who reported on their use of vitamin D supplements.
Regular users had a significant 55% reduction in the odds of having a past or present melanoma diagnosis, while occasional use was associated with a nonsignificant 46% reduction. The reduction was similar for all skin cancer types.
However, senior author Ilkka T. Harvima, MD, PhD, department of dermatology, University of Eastern Finland and Kuopio (Finland) University Hospital, warned there are limitations to the study.
Despite adjustment for several possible confounding factors, “it is still possible that some other, yet unidentified or untested, factors can still confound the present result,” he said.
Consequently, “the causal link between vitamin D and melanoma cannot be confirmed by the present results,” Dr. Harvima said in a statement.
Even if the link were to be proven, “the question about the optimal dose of oral vitamin D in order to for it to have beneficial effects remains to be answered,” he said.
“Until we know more, national intake recommendations should be followed.”
The incidence of cutaneous malignant melanoma and other skin cancers has been increasing steadily in Western populations, particularly in immunosuppressed individuals, the authors pointed out, and they attributed the rise to an increased exposure to ultraviolet radiation.
While ultraviolet radiation exposure is a well-known risk factor, “the other side of the coin is that public sun protection campaigns have led to alerts that insufficient sun exposure is a significant public health problem, resulting in insufficient vitamin D status.”
For their study, the team reviewed the records of 498 patients aged 21-79 years at a dermatology outpatient clinic who were deemed by an experienced dermatologist to be at risk of any type of skin cancer.
Among these patients, 295 individuals had a history of past or present cutaneous malignancy, with 100 diagnosed with melanoma, 213 with basal cell carcinoma, and 41 with squamous cell carcinoma. A further 70 subjects had cancer elsewhere, including breast, prostate, kidney, bladder, intestine, and blood cancers.
A subgroup of 96 patients were immunocompromised and were considered separately.
The 402 remaining patients were categorized, based on their self-reported use of oral vitamin D preparations, as nonusers (n = 99), occasional users (n = 126), and regular users (n = 177).
Regular use of vitamin D was associated with being more educated (P = .032), less frequent outdoor working (P = .003), lower tobacco pack years (P = .001), and more frequent solarium exposure (P = .002).
There was no significant association between vitamin D use and photoaging, actinic keratoses, nevi, basal or squamous cell carcinoma, body mass index, or self-estimated lifetime exposure to sunlight or sunburns.
However, there were significant associations between regular use of vitamin D and a lower incidence of melanoma and other cancer types.
There were significantly fewer individuals in the regular vitamin D use group with a past or present history of melanoma when compared with the nonuse group, at 18.1% vs. 32.3% (P = .021), or any type of skin cancer, at 62.1% vs. 74.7% (P = .027).
Multivariate logistic regression analysis revealed that regular vitamin D use was significantly associated with a reduced melanoma risk, at an odds ratio vs. nonuse of 0.447 (P = .016).
Occasional use was associated with a reduced, albeit nonsignificant, risk, with an odds ratio versus nonuse of 0.540 (P = .08).
For any type of skin cancers, regular vitamin D use was associated with an odds ratio vs. nonuse of 0.478 (P = .032), while that for occasional vitamin D use was 0.543 (P = .061).
“Somewhat similar” results were obtained when the investigators looked at the subgroup of immunocompromised individuals, although they note that “the number of subjects was low.”
The study was supported by the Cancer Center of Eastern Finland of the University of Eastern Finland, the Finnish Cancer Research Foundation, and the VTR-funding of Kuopio University Hospital. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MELANOMA RESEARCH
Early retirement and the terrible, horrible, no good, very bad cognitive decline
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
When Patients Make Unexpected Medical Choices
Due to advances in medicine, people are living longer with the aid of increased options for life-prolonging treatments. These treatment options may improve the quantity but not necessarily the quality of life.1
Kidney failure can be treated with renal replacement therapy (dialysis or renal transplantation) or supportive care.2 In 2017, the global prevalence of kidney failure was about 5.3 to 9.7 million.3 In the United States, about 500,000 patients are receiving maintenance dialysis for end-stage renal disease (ESRD), and about 1 in 4 will stop dialysis before death, coupled with hospice enrollment.4 ESRD is 2 times more prevalent among veterans than in nonveterans, which can be due in part to high rates of comorbid predisposing conditions, such as diabetes mellitus, hypertension, and advanced age, among others.5 The decision to discontinue dialysis and receive hospice care tends to be more difficult than choosing to withhold or forego dialysis.6
A study conducted among patients who were taken off hemodialysis before death reported that the 2 most common reasons for the withdrawal were acute medical complications and frailty.7 A retrospective study among patients with ESRD receiving hemodialysis highlighted the underutilization of hospice care in this patient population.8 The study also found that those patients who were aged > 75 years, had poor functional status, and had dialysis-related complications, such as sepsis and anemia, were more likely to elect withdrawal of hemodialysis. There was no difference in overall survival or quality of life among patients who were aged ≥ 75 years with multiple comorbidities and functional impairment who elected conservative management vs those who started dialysis.8 Long-term continuous dialysis has been associated with a lower quality of life, increased dependence on others, and a variety of symptoms, such as pain, nausea, insomnia, anxiety, or depression.9
Conservative Care vs Medical Paternalism
In the United States, it is unusual for patients with ESRD to choose conservative care, and supportive services are less available for those who do compared with patients with ESRD in Europe, Asia, Australia, and Canada.10 A study looking at a small number of US nephrologists has shown they may have limited experience in caring for patients who forego dialysis and they are not comfortable offering conservative management over dialysis.10 Another small study from Sweden also showed that many nephrologists do not feel prepared for end-of-life care and conversations.11
Patients often rely on knowledgeable recommendations from medical experts. However, medical paternalism occurs when a physician makes decisions deemed to be in the patient’s best interest but are against the patient’s wishes or when the patient is unable to give their consent.12 Hard paternalism occurs when the patient is competent to make their own medical decisions, while soft paternalism occurs when a patient is not competent to make their own medical decisions.13
Patient autonomy is widely recognized as an ethical principle in medicine. It recognizes patients as well-informed decision makers who may act without excessive influence to make intentional determinations on their own behalf.14 Autonomy can be exercised at any point during the health care process.12 Although ethical and legal guidelines encourage physicians to recommend appropriate treatment, medical opinion cannot overrule the wishes of a competent patient who refuses treatment.12
Case Presentation
Mr. S presented to the emergency department at a US Department of Veterans Affairs (VA) medical center with abdominal pain from recurrent pancreatitis. The patient aged > 65 years had a history of depression, ESRD, and was receiving hemodialysis. A computed tomography scan revealed a new pancreatic mass, and he was referred to the palliative care (PC) department nurse practitioner (NP) for a goals-of-care discussion. PC was informed to assist with hospice care initiation: The patient elected a do-not-resuscitate (DNR) code status and hospice care.
At the consultation, Mr. S stated that he had decided to forego life-prolonging treatments, including hemodialysis, and declined further evaluation for his pancreatic mass. He shared a good understanding of concerns for malignancy with his mass but did not wish to pursue further diagnostics as he knew his life expectancy was very limited without dialysis. He had been dependent on hemodialysis for the past 10 years. He had briefly received hospice care 5 years before but changed his mind and decided to pursue standard care, including life-prolonging dialysis treatments. He reported no depression, suicidal ideation, or intentions of hastening his death. He stated that he was just physically tired from his ongoing dialysis, recurrent hospitalizations, and being repeatedly subjected to diagnostic tests. Mr. S added that he had discussed his plan with his family, including his son and sister-in-law who is married to his brother. Mr. S previously identified his brother as his surrogate decision maker.
Mr. S shared that his brother had sustained a traumatic brain injury and was now unable to engage in a meaningful conversation. He shared that his family supported his decision. He also recognized that with his debility, he would need inpatient hospice care. On finding out that Mr. S’s brother was no longer able to act as the surrogate decision maker, the PC NP asked whether he wanted her to contact his son to share the outcome of their visit. The patient declined, adding that he had discussed his care plans with his family and did not feel that his health care team needed to have additional discussions with them.
Mr. S also reported chronic, recurrent right upper quadrant pain. He was prescribed oxycodone 10 mg every 4 hours as needed; however, it did little to control his pain. He also reported generalized pruritus, a complication of his renal failure.
After 1 week, Mr. S was transferred to the inpatient hospice unit. At that time, he allowed the hospice team to contact his son for medical updates and identified him as the primary point of contact for the hospice team if the need arose to reach his family. Due to the restrictions imposed by the pandemic, Mr. S had virtual video visits with his family. Mr. S developed intermittent confusion and worsening fatigue over time. His son was informed of his deteriorating condition and visited his father. Mr. S died peacefully 2 days later with his family present.
Multidisciplinary Inputs on the Case
Medicine. In discussing the case with medicine, the PC NP was informed that the goals the patient had for his care, which included stopping dialysis, having a DNR code status and pursuing hospice care, along with the patient’s pain symptoms prompted the PC consultation. The resident also shared concerns about the patient’s refusal to have his surrogate decision maker and family contacted regarding his decisions for his care.
Palliative care. After meeting with the patient and assisting in identifying goals for care, the PC NP recommended initiation of hospice care in the hospital while the patient awaited transfer to the inpatient hospice unit. The PC NP also recommended a psychiatric evaluation to rule out untreated depression that might influence the patient’s decision making. A follow-up visit with nephrology was also recommended. Optimal management of his distressing physical symptoms was recommended, including prescribing hydromorphone instead of oxycodone for his pain and starting a topical emollient for pruritus.
Nephrology. The patient’s electronic health records (EHR) showed that he informed nephrology of his desire to pursue hospice care and that he decided against further dialysis, including as-needed dialysis for comfort. The records also indicated that the patient understood the consequences of discontinuing dialysis.
Psychiatry. The patient’s EHR also showed that during his psychiatric visits, Mr. S reported he had no thoughts of suicide, and it was against his spiritual beliefs. He said he made his own medical decisions and expressed that his health care team should not attempt to change his mind. He also said he understood that stopping dialysis could lead to early death. He stated he had a close relationship with his family and discussed his medical decisions with them. He was tearful at times when he talked about his family. Mr. S shared his frustration about repeatedly being asked the same questions on succeeding visits.
After evaluation, psychiatry diagnosed Mr. S with mood disorder with depressive features and he was prescribed methylphenidate 5 mg daily and sertraline 25 mg daily. They also recommended continuing to offer dialysis in a supportive manner since the patient had changed his mind about hospice in the past. However, psychiatry followed the patient daily for 5 days and concluded that his medical decisions were not clouded by mood symptoms.
Discussion
Patients who are aged > 65 years and on dialysis are more likely to experience higher rates of hospitalization, intensive care unit admission, procedures, and death in the hospital compared with patients who have cancer or heart failure. They also use hospice services less.15 Often this is not consistent with a patient’s wishes but may occur due to limited discussion of goals, values, and preferences between physician and patient.15 Many nephrologists do not engage in these conversations for fear of upsetting patients, their perceived lack of skill in prognostication and discussing the topic, or the lack of time to have the conversation.15 It is important to have an honest and open communication with patients that allows them to be fully informed as they make their medical decisions and exercise their autonomy.
Medicare hospice guidelines also are used to help determine hospice appropriateness among veterans in the VA. Medicare requires enrollees to discontinue disease-modifying treatment for the medical condition leading to their hospice diagnosis, which can result in late hospice referrals and shorter hospice stays.16 Even though hospice referrals for patients with ESRD have increased over time, they are still happening close to the time of death, and patients’ health care utilization near the end of life remains unchanged.16 According to Medicare, patients qualify for hospice care if they are terminally ill (defined as having a life expectancy of ≤ 6 months), choose comfort care over curative care for their terminal illness, and sign a statement electing hospice care over treatments for their terminal illness.17 A DNR order is not a condition for hospice admission.18
The VA defines hospice care as comfort care provided to patients with a terminal condition, a life expectancy of ≤ 6 months, and who are no longer seeking treatment other than those that are palliative.19 Based on his ESRD, Mr. S was qualified for hospice care, and his goals for care were consistent with the hospice philosophy. Most families of patients who elected to withdraw dialysis reported a good death, using the criteria of the duration of dying, discomfort, and psychosocial circumstances.20
Role of HCPs
Health care practitioners (HCPs) are expected to help patients understand the risks and benefits of their choices and its alternative, align patients’ goals with those risks and benefits, and assist patients in making choices that promote their goals and autonomy.21 Family members are often not involved in medical decision making when patients have the capacity to make their own decisions.22 Patients will also have to give permission for protected health information to be shared with their family members.22 On the other hand, families have been shown to provide valuable emotional support to patients and are considered second patients themselves in the sense that they can be impacted by patients’ clinical situation.22 Families may also need care, time, and attention from HCPs.22
Mr. S was found capable of making his own decisions, and part of that decision was that his family not to be present for the goals-of-care discussion. He added that he would discuss the care decisions with his family. At the time of registering for VA health care services, Mr. S had provided his health care team with his brother and sister-in-law’s emergency contact information as well as named his brother surrogate decision maker. As Mr. S’s condition was expected to rapidly decline wthout dialysis, the HCPs would be able to notify family members once his condition changed, including death.
Neuroplasticity changes can contribute to chronic pain that may also lead to depression.23 Chronic pain and depression may involve the same brain structures, neurotransmitters, and signaling pathway.23 Factors leading to chronic pain and depression include decreased availability of monoamine neurotransmitters, such as serotonin, dopamine, and norepinephrine in the central nervous system, decreased brain-derived neurotrophic factor, inflammatory response, and increased glutamate activity.23 Depression and hopelessness have been associated with the desire to hasten death among patients with a terminal illness.24 Worse mental health has been associated with the desire to hasten death among patients who are older and functionally impaired.25 It was important to optimize Mr. S’s treatment for pain and depression to ensure that these factors were not influencing his medical decisions.
With increasing recognition of the need to improve quality of life, health care utilization, and provide care consistent with patients’ goals in nephrology, the concept of renal PC is emerging but remains limited.26 The need to improve supportive care or PC for patients starting on dialysis for ESRD is high as these patients tend to be older (aged > 75 years), have high rates of cardiovascular comorbidities, can have coexisting cognitive impairment and functional debility, and have an adjusted mortality rate of up to 32.5% within 1 year of starting dialysis.26 Some ways to enhance renal PC programs include incorporating PC skill development and training within nephrology fellowships, educating patients with chronic and ESRD about PC and options for medical management without dialysis, and increasing the collaboration between nephrology and PC.26
Outcomes and Implications
Respect for the ethical principle of autonomy is paramount in health care. Patients should be able to give informed consent for treatment decisions without undue influence from their HCPs and should be able to withdraw that consent at any point during treatment. Factors that may influence patients’ ability to make medical decisions should be considered, including untreated or poorly treated symptoms. The involvement of PC helps optimize symptom management, provide support, and assist in goals-of-care discussions. Advanced practice PC nurses can offer other members of the health care team additional information and support in end-of-life care. Family involvement should be encouraged even for patients who can make their own medical decisions for emotional support and to assist families in what could be a traumatic event, such as the loss of a loved one.
The desire to pursue a comfort-focused approach to terminal illness and stop disease-modifying treatments are criteria for hospice care. An interdisciplinary approach to end-of-life care is beneficial, and every specialty should be equipped to engage in honest communication and skillful prognostication. These conversations should start early in the course of a terminal illness. Multiple factors contribute to poor clinical outcomes among patients with ESRD even with renal replacement therapy, such as dialysis. There is a need to improve PC training in the field of nephrology.
Conclusions
Mr. S was able to choose to withdraw potentially life-prolonging treatments with the support of his family and HCPs. He was able to continue receiving high-quality care and treatment in accordance with his wishes and goals for his care. The provision of interdisciplinary care that focused on supporting him allowed for his peaceful and comfortable death.
1. Carr D, Luth EA. Well-being at the end of life. Annu Rev Sociol. 2019;45:515-534. doi:10.1146/annurev-soc-073018-022524
2. Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264-271. doi:10.1001/jama.2018.8981
3. Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573-585. doi:10.1038/s41581-020-0315-4
4. Richards CA, Hebert PL, Liu CF, et al. Association of family ratings of quality of end-of-life care with stopping dialysis treatment and receipt of hospice services. JAMA Netw Open. 2019;2(10):e1913115. doi:10.1001/jamanetworkopen.2019.13115
5. Fischer MJ, Kourany WM, Sovern K, Forrester K, Griffin C, Lightner N, Loftus S, Murphy K, Roth G, Palevsky PM, Crowley ST. Development, implementation and user experience of the Veterans Health Administration (VHA) dialysis dashboard. BMC Nephrol. 2020 Apr 16;21(1):136. doi:10.1186/s12882-020-01798-6
6. Schwarze ML, Schueller K, Jhagroo RA. Hospice use and end-of-life care for patients with end-stage renal disease: too little, too late. JAMA Intern Med. 2018;178(6):799-801.doi:10.1001/jamainternmed.2018.1078
7. Chen JC, Thorsteinsdottir B, Vaughan LE, et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2018;13(8):1172-1179. doi:10.2215/CJN.00590118
8. Chen HC, Wu CY, Hsieh HY, He JS, Hwang SJ, Hsieh HM. Predictors and assessment of hospice use for end-stage renal disease patients in Taiwan. Int J Environ Res Public Health. 2021;19(1):85. doi:10.3390/ijerph19010085
9. Rak A, Raina R, Suh TT, et al. Palliative care for patients with end-stage renal disease: approach to treatment that aims to improve quality of life and relieve suffering for patients (and families) with chronic illnesses. Clin Kidney J. 2017;10(1):68-73. doi.10.1093/ckj/sfw10510. Wong SPY, Boyapati S, Engelberg RA, Thorsteinsdottir B, Taylor JS, O’Hare AM. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis. 2020;75(2):167-176. doi:10.1053/j.ajkd.2019.07.006
11. Axelsson L, Benzein E, Lindberg J, Persson C. End-of-life and palliative care of patients on maintenance hemodialysis treatment: a focus group study. BMC Palliat Care. 2019;18(1):89. doi:10.1186/s12904-019-0481-y
12. Tweeddale MG. Grasping the nettle—what to do when patients withdraw their consent for treatment: (a clinical perspective on the case of Ms B). J Med Ethics. 2002;28(4):236-237. doi:10.1136/jme.28.4.236
13. Lynøe N, Engström I, Juth N. How to reveal disguised paternalism: version 2.0. BMC Med Ethics. 2021;22(1):170. doi:10.1186/s12910-021-00739-8
14. Murgic L, Hébert PC, Sovic S, Pavlekovic G. Paternalism and autonomy: views of patients and providers in a transitional (post-communist) country. BMC Med Ethics. 2015;16(1):65. doi:10.1186/s12910-015-0059-z
15. Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854-863. doi:10.2215/CJN.05760516
16. Wachterman MW, Hailpern SM, Keating NL, Kurella Tamura M, O’Hare AM. Association between hospice length of stay, health care utilization, and Medicare costs at the end of life among patients who received maintenance hemodialysis. JAMA Int Med. 2018;178(6):792-799. doi:10.1001/jamainternmed.2018.0256
17. Centers for Medicare and Medicaid Services. Hospice care. Accessed April 2, 2022. https://www.medicare.gov/coverage/hospice-care
18. National Hospice and Palliative Care Organization. Ethical behavior and consumer rights. Standards of Practice for Hospice Programs Professional Development and Resource Series. Accessed December 6, 2022. https://www.nhpco.org/wp-content/uploads/2019/04/Standards_Hospice_2018.pdf
19. US Department of Veterans Affairs. Geriatrics and extended care. Updated October 5, 2022. Accessed August 29, 2022. https://www.va.gov/geriatrics/pages/Hospice_Care.asp
20. Cohen LM, McCue JD, Germain M, Kjellstrand CM. Dialysis discontinuation. A ‘good’ death? Arch Intern Med. 1995;155(1):42-47.
21. Ubel PA, Scherr KA, Fagerlin A. Autonomy: What’s shared decision making have to do with it? Am J Bioeth. 2018;18(2):W11-W12.doi:10.1080/15265161.2017.1409844
22. Laryionava, K, Pfeil TA, Dietrich M. et al. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
23. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi:10.1155/2017/9724371
24. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911. doi:10.1001/jama.284.22.2907
25. Sullivan M, Ormel J, Kempen GIJM, Tymstra T. Beliefs concerning death, dying, and hastening death among older, functionally impaired Dutch adults: a one-year longitudinal study. J Am Gec Soc. doi:10.1111/j.1532-5415.1998.tb04541.x26. Gelfand SL, Schell J, Eneanya ND. Palliative care in nephrology: the work and the workforce. Adv Chronic Kidney Dis. 2020;27(4):350-355.e1. doi:10.1053/j.ackd.2020.02.007
Due to advances in medicine, people are living longer with the aid of increased options for life-prolonging treatments. These treatment options may improve the quantity but not necessarily the quality of life.1
Kidney failure can be treated with renal replacement therapy (dialysis or renal transplantation) or supportive care.2 In 2017, the global prevalence of kidney failure was about 5.3 to 9.7 million.3 In the United States, about 500,000 patients are receiving maintenance dialysis for end-stage renal disease (ESRD), and about 1 in 4 will stop dialysis before death, coupled with hospice enrollment.4 ESRD is 2 times more prevalent among veterans than in nonveterans, which can be due in part to high rates of comorbid predisposing conditions, such as diabetes mellitus, hypertension, and advanced age, among others.5 The decision to discontinue dialysis and receive hospice care tends to be more difficult than choosing to withhold or forego dialysis.6
A study conducted among patients who were taken off hemodialysis before death reported that the 2 most common reasons for the withdrawal were acute medical complications and frailty.7 A retrospective study among patients with ESRD receiving hemodialysis highlighted the underutilization of hospice care in this patient population.8 The study also found that those patients who were aged > 75 years, had poor functional status, and had dialysis-related complications, such as sepsis and anemia, were more likely to elect withdrawal of hemodialysis. There was no difference in overall survival or quality of life among patients who were aged ≥ 75 years with multiple comorbidities and functional impairment who elected conservative management vs those who started dialysis.8 Long-term continuous dialysis has been associated with a lower quality of life, increased dependence on others, and a variety of symptoms, such as pain, nausea, insomnia, anxiety, or depression.9
Conservative Care vs Medical Paternalism
In the United States, it is unusual for patients with ESRD to choose conservative care, and supportive services are less available for those who do compared with patients with ESRD in Europe, Asia, Australia, and Canada.10 A study looking at a small number of US nephrologists has shown they may have limited experience in caring for patients who forego dialysis and they are not comfortable offering conservative management over dialysis.10 Another small study from Sweden also showed that many nephrologists do not feel prepared for end-of-life care and conversations.11
Patients often rely on knowledgeable recommendations from medical experts. However, medical paternalism occurs when a physician makes decisions deemed to be in the patient’s best interest but are against the patient’s wishes or when the patient is unable to give their consent.12 Hard paternalism occurs when the patient is competent to make their own medical decisions, while soft paternalism occurs when a patient is not competent to make their own medical decisions.13
Patient autonomy is widely recognized as an ethical principle in medicine. It recognizes patients as well-informed decision makers who may act without excessive influence to make intentional determinations on their own behalf.14 Autonomy can be exercised at any point during the health care process.12 Although ethical and legal guidelines encourage physicians to recommend appropriate treatment, medical opinion cannot overrule the wishes of a competent patient who refuses treatment.12
Case Presentation
Mr. S presented to the emergency department at a US Department of Veterans Affairs (VA) medical center with abdominal pain from recurrent pancreatitis. The patient aged > 65 years had a history of depression, ESRD, and was receiving hemodialysis. A computed tomography scan revealed a new pancreatic mass, and he was referred to the palliative care (PC) department nurse practitioner (NP) for a goals-of-care discussion. PC was informed to assist with hospice care initiation: The patient elected a do-not-resuscitate (DNR) code status and hospice care.
At the consultation, Mr. S stated that he had decided to forego life-prolonging treatments, including hemodialysis, and declined further evaluation for his pancreatic mass. He shared a good understanding of concerns for malignancy with his mass but did not wish to pursue further diagnostics as he knew his life expectancy was very limited without dialysis. He had been dependent on hemodialysis for the past 10 years. He had briefly received hospice care 5 years before but changed his mind and decided to pursue standard care, including life-prolonging dialysis treatments. He reported no depression, suicidal ideation, or intentions of hastening his death. He stated that he was just physically tired from his ongoing dialysis, recurrent hospitalizations, and being repeatedly subjected to diagnostic tests. Mr. S added that he had discussed his plan with his family, including his son and sister-in-law who is married to his brother. Mr. S previously identified his brother as his surrogate decision maker.
Mr. S shared that his brother had sustained a traumatic brain injury and was now unable to engage in a meaningful conversation. He shared that his family supported his decision. He also recognized that with his debility, he would need inpatient hospice care. On finding out that Mr. S’s brother was no longer able to act as the surrogate decision maker, the PC NP asked whether he wanted her to contact his son to share the outcome of their visit. The patient declined, adding that he had discussed his care plans with his family and did not feel that his health care team needed to have additional discussions with them.
Mr. S also reported chronic, recurrent right upper quadrant pain. He was prescribed oxycodone 10 mg every 4 hours as needed; however, it did little to control his pain. He also reported generalized pruritus, a complication of his renal failure.
After 1 week, Mr. S was transferred to the inpatient hospice unit. At that time, he allowed the hospice team to contact his son for medical updates and identified him as the primary point of contact for the hospice team if the need arose to reach his family. Due to the restrictions imposed by the pandemic, Mr. S had virtual video visits with his family. Mr. S developed intermittent confusion and worsening fatigue over time. His son was informed of his deteriorating condition and visited his father. Mr. S died peacefully 2 days later with his family present.
Multidisciplinary Inputs on the Case
Medicine. In discussing the case with medicine, the PC NP was informed that the goals the patient had for his care, which included stopping dialysis, having a DNR code status and pursuing hospice care, along with the patient’s pain symptoms prompted the PC consultation. The resident also shared concerns about the patient’s refusal to have his surrogate decision maker and family contacted regarding his decisions for his care.
Palliative care. After meeting with the patient and assisting in identifying goals for care, the PC NP recommended initiation of hospice care in the hospital while the patient awaited transfer to the inpatient hospice unit. The PC NP also recommended a psychiatric evaluation to rule out untreated depression that might influence the patient’s decision making. A follow-up visit with nephrology was also recommended. Optimal management of his distressing physical symptoms was recommended, including prescribing hydromorphone instead of oxycodone for his pain and starting a topical emollient for pruritus.
Nephrology. The patient’s electronic health records (EHR) showed that he informed nephrology of his desire to pursue hospice care and that he decided against further dialysis, including as-needed dialysis for comfort. The records also indicated that the patient understood the consequences of discontinuing dialysis.
Psychiatry. The patient’s EHR also showed that during his psychiatric visits, Mr. S reported he had no thoughts of suicide, and it was against his spiritual beliefs. He said he made his own medical decisions and expressed that his health care team should not attempt to change his mind. He also said he understood that stopping dialysis could lead to early death. He stated he had a close relationship with his family and discussed his medical decisions with them. He was tearful at times when he talked about his family. Mr. S shared his frustration about repeatedly being asked the same questions on succeeding visits.
After evaluation, psychiatry diagnosed Mr. S with mood disorder with depressive features and he was prescribed methylphenidate 5 mg daily and sertraline 25 mg daily. They also recommended continuing to offer dialysis in a supportive manner since the patient had changed his mind about hospice in the past. However, psychiatry followed the patient daily for 5 days and concluded that his medical decisions were not clouded by mood symptoms.
Discussion
Patients who are aged > 65 years and on dialysis are more likely to experience higher rates of hospitalization, intensive care unit admission, procedures, and death in the hospital compared with patients who have cancer or heart failure. They also use hospice services less.15 Often this is not consistent with a patient’s wishes but may occur due to limited discussion of goals, values, and preferences between physician and patient.15 Many nephrologists do not engage in these conversations for fear of upsetting patients, their perceived lack of skill in prognostication and discussing the topic, or the lack of time to have the conversation.15 It is important to have an honest and open communication with patients that allows them to be fully informed as they make their medical decisions and exercise their autonomy.
Medicare hospice guidelines also are used to help determine hospice appropriateness among veterans in the VA. Medicare requires enrollees to discontinue disease-modifying treatment for the medical condition leading to their hospice diagnosis, which can result in late hospice referrals and shorter hospice stays.16 Even though hospice referrals for patients with ESRD have increased over time, they are still happening close to the time of death, and patients’ health care utilization near the end of life remains unchanged.16 According to Medicare, patients qualify for hospice care if they are terminally ill (defined as having a life expectancy of ≤ 6 months), choose comfort care over curative care for their terminal illness, and sign a statement electing hospice care over treatments for their terminal illness.17 A DNR order is not a condition for hospice admission.18
The VA defines hospice care as comfort care provided to patients with a terminal condition, a life expectancy of ≤ 6 months, and who are no longer seeking treatment other than those that are palliative.19 Based on his ESRD, Mr. S was qualified for hospice care, and his goals for care were consistent with the hospice philosophy. Most families of patients who elected to withdraw dialysis reported a good death, using the criteria of the duration of dying, discomfort, and psychosocial circumstances.20
Role of HCPs
Health care practitioners (HCPs) are expected to help patients understand the risks and benefits of their choices and its alternative, align patients’ goals with those risks and benefits, and assist patients in making choices that promote their goals and autonomy.21 Family members are often not involved in medical decision making when patients have the capacity to make their own decisions.22 Patients will also have to give permission for protected health information to be shared with their family members.22 On the other hand, families have been shown to provide valuable emotional support to patients and are considered second patients themselves in the sense that they can be impacted by patients’ clinical situation.22 Families may also need care, time, and attention from HCPs.22
Mr. S was found capable of making his own decisions, and part of that decision was that his family not to be present for the goals-of-care discussion. He added that he would discuss the care decisions with his family. At the time of registering for VA health care services, Mr. S had provided his health care team with his brother and sister-in-law’s emergency contact information as well as named his brother surrogate decision maker. As Mr. S’s condition was expected to rapidly decline wthout dialysis, the HCPs would be able to notify family members once his condition changed, including death.
Neuroplasticity changes can contribute to chronic pain that may also lead to depression.23 Chronic pain and depression may involve the same brain structures, neurotransmitters, and signaling pathway.23 Factors leading to chronic pain and depression include decreased availability of monoamine neurotransmitters, such as serotonin, dopamine, and norepinephrine in the central nervous system, decreased brain-derived neurotrophic factor, inflammatory response, and increased glutamate activity.23 Depression and hopelessness have been associated with the desire to hasten death among patients with a terminal illness.24 Worse mental health has been associated with the desire to hasten death among patients who are older and functionally impaired.25 It was important to optimize Mr. S’s treatment for pain and depression to ensure that these factors were not influencing his medical decisions.
With increasing recognition of the need to improve quality of life, health care utilization, and provide care consistent with patients’ goals in nephrology, the concept of renal PC is emerging but remains limited.26 The need to improve supportive care or PC for patients starting on dialysis for ESRD is high as these patients tend to be older (aged > 75 years), have high rates of cardiovascular comorbidities, can have coexisting cognitive impairment and functional debility, and have an adjusted mortality rate of up to 32.5% within 1 year of starting dialysis.26 Some ways to enhance renal PC programs include incorporating PC skill development and training within nephrology fellowships, educating patients with chronic and ESRD about PC and options for medical management without dialysis, and increasing the collaboration between nephrology and PC.26
Outcomes and Implications
Respect for the ethical principle of autonomy is paramount in health care. Patients should be able to give informed consent for treatment decisions without undue influence from their HCPs and should be able to withdraw that consent at any point during treatment. Factors that may influence patients’ ability to make medical decisions should be considered, including untreated or poorly treated symptoms. The involvement of PC helps optimize symptom management, provide support, and assist in goals-of-care discussions. Advanced practice PC nurses can offer other members of the health care team additional information and support in end-of-life care. Family involvement should be encouraged even for patients who can make their own medical decisions for emotional support and to assist families in what could be a traumatic event, such as the loss of a loved one.
The desire to pursue a comfort-focused approach to terminal illness and stop disease-modifying treatments are criteria for hospice care. An interdisciplinary approach to end-of-life care is beneficial, and every specialty should be equipped to engage in honest communication and skillful prognostication. These conversations should start early in the course of a terminal illness. Multiple factors contribute to poor clinical outcomes among patients with ESRD even with renal replacement therapy, such as dialysis. There is a need to improve PC training in the field of nephrology.
Conclusions
Mr. S was able to choose to withdraw potentially life-prolonging treatments with the support of his family and HCPs. He was able to continue receiving high-quality care and treatment in accordance with his wishes and goals for his care. The provision of interdisciplinary care that focused on supporting him allowed for his peaceful and comfortable death.
Due to advances in medicine, people are living longer with the aid of increased options for life-prolonging treatments. These treatment options may improve the quantity but not necessarily the quality of life.1
Kidney failure can be treated with renal replacement therapy (dialysis or renal transplantation) or supportive care.2 In 2017, the global prevalence of kidney failure was about 5.3 to 9.7 million.3 In the United States, about 500,000 patients are receiving maintenance dialysis for end-stage renal disease (ESRD), and about 1 in 4 will stop dialysis before death, coupled with hospice enrollment.4 ESRD is 2 times more prevalent among veterans than in nonveterans, which can be due in part to high rates of comorbid predisposing conditions, such as diabetes mellitus, hypertension, and advanced age, among others.5 The decision to discontinue dialysis and receive hospice care tends to be more difficult than choosing to withhold or forego dialysis.6
A study conducted among patients who were taken off hemodialysis before death reported that the 2 most common reasons for the withdrawal were acute medical complications and frailty.7 A retrospective study among patients with ESRD receiving hemodialysis highlighted the underutilization of hospice care in this patient population.8 The study also found that those patients who were aged > 75 years, had poor functional status, and had dialysis-related complications, such as sepsis and anemia, were more likely to elect withdrawal of hemodialysis. There was no difference in overall survival or quality of life among patients who were aged ≥ 75 years with multiple comorbidities and functional impairment who elected conservative management vs those who started dialysis.8 Long-term continuous dialysis has been associated with a lower quality of life, increased dependence on others, and a variety of symptoms, such as pain, nausea, insomnia, anxiety, or depression.9
Conservative Care vs Medical Paternalism
In the United States, it is unusual for patients with ESRD to choose conservative care, and supportive services are less available for those who do compared with patients with ESRD in Europe, Asia, Australia, and Canada.10 A study looking at a small number of US nephrologists has shown they may have limited experience in caring for patients who forego dialysis and they are not comfortable offering conservative management over dialysis.10 Another small study from Sweden also showed that many nephrologists do not feel prepared for end-of-life care and conversations.11
Patients often rely on knowledgeable recommendations from medical experts. However, medical paternalism occurs when a physician makes decisions deemed to be in the patient’s best interest but are against the patient’s wishes or when the patient is unable to give their consent.12 Hard paternalism occurs when the patient is competent to make their own medical decisions, while soft paternalism occurs when a patient is not competent to make their own medical decisions.13
Patient autonomy is widely recognized as an ethical principle in medicine. It recognizes patients as well-informed decision makers who may act without excessive influence to make intentional determinations on their own behalf.14 Autonomy can be exercised at any point during the health care process.12 Although ethical and legal guidelines encourage physicians to recommend appropriate treatment, medical opinion cannot overrule the wishes of a competent patient who refuses treatment.12
Case Presentation
Mr. S presented to the emergency department at a US Department of Veterans Affairs (VA) medical center with abdominal pain from recurrent pancreatitis. The patient aged > 65 years had a history of depression, ESRD, and was receiving hemodialysis. A computed tomography scan revealed a new pancreatic mass, and he was referred to the palliative care (PC) department nurse practitioner (NP) for a goals-of-care discussion. PC was informed to assist with hospice care initiation: The patient elected a do-not-resuscitate (DNR) code status and hospice care.
At the consultation, Mr. S stated that he had decided to forego life-prolonging treatments, including hemodialysis, and declined further evaluation for his pancreatic mass. He shared a good understanding of concerns for malignancy with his mass but did not wish to pursue further diagnostics as he knew his life expectancy was very limited without dialysis. He had been dependent on hemodialysis for the past 10 years. He had briefly received hospice care 5 years before but changed his mind and decided to pursue standard care, including life-prolonging dialysis treatments. He reported no depression, suicidal ideation, or intentions of hastening his death. He stated that he was just physically tired from his ongoing dialysis, recurrent hospitalizations, and being repeatedly subjected to diagnostic tests. Mr. S added that he had discussed his plan with his family, including his son and sister-in-law who is married to his brother. Mr. S previously identified his brother as his surrogate decision maker.
Mr. S shared that his brother had sustained a traumatic brain injury and was now unable to engage in a meaningful conversation. He shared that his family supported his decision. He also recognized that with his debility, he would need inpatient hospice care. On finding out that Mr. S’s brother was no longer able to act as the surrogate decision maker, the PC NP asked whether he wanted her to contact his son to share the outcome of their visit. The patient declined, adding that he had discussed his care plans with his family and did not feel that his health care team needed to have additional discussions with them.
Mr. S also reported chronic, recurrent right upper quadrant pain. He was prescribed oxycodone 10 mg every 4 hours as needed; however, it did little to control his pain. He also reported generalized pruritus, a complication of his renal failure.
After 1 week, Mr. S was transferred to the inpatient hospice unit. At that time, he allowed the hospice team to contact his son for medical updates and identified him as the primary point of contact for the hospice team if the need arose to reach his family. Due to the restrictions imposed by the pandemic, Mr. S had virtual video visits with his family. Mr. S developed intermittent confusion and worsening fatigue over time. His son was informed of his deteriorating condition and visited his father. Mr. S died peacefully 2 days later with his family present.
Multidisciplinary Inputs on the Case
Medicine. In discussing the case with medicine, the PC NP was informed that the goals the patient had for his care, which included stopping dialysis, having a DNR code status and pursuing hospice care, along with the patient’s pain symptoms prompted the PC consultation. The resident also shared concerns about the patient’s refusal to have his surrogate decision maker and family contacted regarding his decisions for his care.
Palliative care. After meeting with the patient and assisting in identifying goals for care, the PC NP recommended initiation of hospice care in the hospital while the patient awaited transfer to the inpatient hospice unit. The PC NP also recommended a psychiatric evaluation to rule out untreated depression that might influence the patient’s decision making. A follow-up visit with nephrology was also recommended. Optimal management of his distressing physical symptoms was recommended, including prescribing hydromorphone instead of oxycodone for his pain and starting a topical emollient for pruritus.
Nephrology. The patient’s electronic health records (EHR) showed that he informed nephrology of his desire to pursue hospice care and that he decided against further dialysis, including as-needed dialysis for comfort. The records also indicated that the patient understood the consequences of discontinuing dialysis.
Psychiatry. The patient’s EHR also showed that during his psychiatric visits, Mr. S reported he had no thoughts of suicide, and it was against his spiritual beliefs. He said he made his own medical decisions and expressed that his health care team should not attempt to change his mind. He also said he understood that stopping dialysis could lead to early death. He stated he had a close relationship with his family and discussed his medical decisions with them. He was tearful at times when he talked about his family. Mr. S shared his frustration about repeatedly being asked the same questions on succeeding visits.
After evaluation, psychiatry diagnosed Mr. S with mood disorder with depressive features and he was prescribed methylphenidate 5 mg daily and sertraline 25 mg daily. They also recommended continuing to offer dialysis in a supportive manner since the patient had changed his mind about hospice in the past. However, psychiatry followed the patient daily for 5 days and concluded that his medical decisions were not clouded by mood symptoms.
Discussion
Patients who are aged > 65 years and on dialysis are more likely to experience higher rates of hospitalization, intensive care unit admission, procedures, and death in the hospital compared with patients who have cancer or heart failure. They also use hospice services less.15 Often this is not consistent with a patient’s wishes but may occur due to limited discussion of goals, values, and preferences between physician and patient.15 Many nephrologists do not engage in these conversations for fear of upsetting patients, their perceived lack of skill in prognostication and discussing the topic, or the lack of time to have the conversation.15 It is important to have an honest and open communication with patients that allows them to be fully informed as they make their medical decisions and exercise their autonomy.
Medicare hospice guidelines also are used to help determine hospice appropriateness among veterans in the VA. Medicare requires enrollees to discontinue disease-modifying treatment for the medical condition leading to their hospice diagnosis, which can result in late hospice referrals and shorter hospice stays.16 Even though hospice referrals for patients with ESRD have increased over time, they are still happening close to the time of death, and patients’ health care utilization near the end of life remains unchanged.16 According to Medicare, patients qualify for hospice care if they are terminally ill (defined as having a life expectancy of ≤ 6 months), choose comfort care over curative care for their terminal illness, and sign a statement electing hospice care over treatments for their terminal illness.17 A DNR order is not a condition for hospice admission.18
The VA defines hospice care as comfort care provided to patients with a terminal condition, a life expectancy of ≤ 6 months, and who are no longer seeking treatment other than those that are palliative.19 Based on his ESRD, Mr. S was qualified for hospice care, and his goals for care were consistent with the hospice philosophy. Most families of patients who elected to withdraw dialysis reported a good death, using the criteria of the duration of dying, discomfort, and psychosocial circumstances.20
Role of HCPs
Health care practitioners (HCPs) are expected to help patients understand the risks and benefits of their choices and its alternative, align patients’ goals with those risks and benefits, and assist patients in making choices that promote their goals and autonomy.21 Family members are often not involved in medical decision making when patients have the capacity to make their own decisions.22 Patients will also have to give permission for protected health information to be shared with their family members.22 On the other hand, families have been shown to provide valuable emotional support to patients and are considered second patients themselves in the sense that they can be impacted by patients’ clinical situation.22 Families may also need care, time, and attention from HCPs.22
Mr. S was found capable of making his own decisions, and part of that decision was that his family not to be present for the goals-of-care discussion. He added that he would discuss the care decisions with his family. At the time of registering for VA health care services, Mr. S had provided his health care team with his brother and sister-in-law’s emergency contact information as well as named his brother surrogate decision maker. As Mr. S’s condition was expected to rapidly decline wthout dialysis, the HCPs would be able to notify family members once his condition changed, including death.
Neuroplasticity changes can contribute to chronic pain that may also lead to depression.23 Chronic pain and depression may involve the same brain structures, neurotransmitters, and signaling pathway.23 Factors leading to chronic pain and depression include decreased availability of monoamine neurotransmitters, such as serotonin, dopamine, and norepinephrine in the central nervous system, decreased brain-derived neurotrophic factor, inflammatory response, and increased glutamate activity.23 Depression and hopelessness have been associated with the desire to hasten death among patients with a terminal illness.24 Worse mental health has been associated with the desire to hasten death among patients who are older and functionally impaired.25 It was important to optimize Mr. S’s treatment for pain and depression to ensure that these factors were not influencing his medical decisions.
With increasing recognition of the need to improve quality of life, health care utilization, and provide care consistent with patients’ goals in nephrology, the concept of renal PC is emerging but remains limited.26 The need to improve supportive care or PC for patients starting on dialysis for ESRD is high as these patients tend to be older (aged > 75 years), have high rates of cardiovascular comorbidities, can have coexisting cognitive impairment and functional debility, and have an adjusted mortality rate of up to 32.5% within 1 year of starting dialysis.26 Some ways to enhance renal PC programs include incorporating PC skill development and training within nephrology fellowships, educating patients with chronic and ESRD about PC and options for medical management without dialysis, and increasing the collaboration between nephrology and PC.26
Outcomes and Implications
Respect for the ethical principle of autonomy is paramount in health care. Patients should be able to give informed consent for treatment decisions without undue influence from their HCPs and should be able to withdraw that consent at any point during treatment. Factors that may influence patients’ ability to make medical decisions should be considered, including untreated or poorly treated symptoms. The involvement of PC helps optimize symptom management, provide support, and assist in goals-of-care discussions. Advanced practice PC nurses can offer other members of the health care team additional information and support in end-of-life care. Family involvement should be encouraged even for patients who can make their own medical decisions for emotional support and to assist families in what could be a traumatic event, such as the loss of a loved one.
The desire to pursue a comfort-focused approach to terminal illness and stop disease-modifying treatments are criteria for hospice care. An interdisciplinary approach to end-of-life care is beneficial, and every specialty should be equipped to engage in honest communication and skillful prognostication. These conversations should start early in the course of a terminal illness. Multiple factors contribute to poor clinical outcomes among patients with ESRD even with renal replacement therapy, such as dialysis. There is a need to improve PC training in the field of nephrology.
Conclusions
Mr. S was able to choose to withdraw potentially life-prolonging treatments with the support of his family and HCPs. He was able to continue receiving high-quality care and treatment in accordance with his wishes and goals for his care. The provision of interdisciplinary care that focused on supporting him allowed for his peaceful and comfortable death.
1. Carr D, Luth EA. Well-being at the end of life. Annu Rev Sociol. 2019;45:515-534. doi:10.1146/annurev-soc-073018-022524
2. Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264-271. doi:10.1001/jama.2018.8981
3. Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573-585. doi:10.1038/s41581-020-0315-4
4. Richards CA, Hebert PL, Liu CF, et al. Association of family ratings of quality of end-of-life care with stopping dialysis treatment and receipt of hospice services. JAMA Netw Open. 2019;2(10):e1913115. doi:10.1001/jamanetworkopen.2019.13115
5. Fischer MJ, Kourany WM, Sovern K, Forrester K, Griffin C, Lightner N, Loftus S, Murphy K, Roth G, Palevsky PM, Crowley ST. Development, implementation and user experience of the Veterans Health Administration (VHA) dialysis dashboard. BMC Nephrol. 2020 Apr 16;21(1):136. doi:10.1186/s12882-020-01798-6
6. Schwarze ML, Schueller K, Jhagroo RA. Hospice use and end-of-life care for patients with end-stage renal disease: too little, too late. JAMA Intern Med. 2018;178(6):799-801.doi:10.1001/jamainternmed.2018.1078
7. Chen JC, Thorsteinsdottir B, Vaughan LE, et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2018;13(8):1172-1179. doi:10.2215/CJN.00590118
8. Chen HC, Wu CY, Hsieh HY, He JS, Hwang SJ, Hsieh HM. Predictors and assessment of hospice use for end-stage renal disease patients in Taiwan. Int J Environ Res Public Health. 2021;19(1):85. doi:10.3390/ijerph19010085
9. Rak A, Raina R, Suh TT, et al. Palliative care for patients with end-stage renal disease: approach to treatment that aims to improve quality of life and relieve suffering for patients (and families) with chronic illnesses. Clin Kidney J. 2017;10(1):68-73. doi.10.1093/ckj/sfw10510. Wong SPY, Boyapati S, Engelberg RA, Thorsteinsdottir B, Taylor JS, O’Hare AM. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis. 2020;75(2):167-176. doi:10.1053/j.ajkd.2019.07.006
11. Axelsson L, Benzein E, Lindberg J, Persson C. End-of-life and palliative care of patients on maintenance hemodialysis treatment: a focus group study. BMC Palliat Care. 2019;18(1):89. doi:10.1186/s12904-019-0481-y
12. Tweeddale MG. Grasping the nettle—what to do when patients withdraw their consent for treatment: (a clinical perspective on the case of Ms B). J Med Ethics. 2002;28(4):236-237. doi:10.1136/jme.28.4.236
13. Lynøe N, Engström I, Juth N. How to reveal disguised paternalism: version 2.0. BMC Med Ethics. 2021;22(1):170. doi:10.1186/s12910-021-00739-8
14. Murgic L, Hébert PC, Sovic S, Pavlekovic G. Paternalism and autonomy: views of patients and providers in a transitional (post-communist) country. BMC Med Ethics. 2015;16(1):65. doi:10.1186/s12910-015-0059-z
15. Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854-863. doi:10.2215/CJN.05760516
16. Wachterman MW, Hailpern SM, Keating NL, Kurella Tamura M, O’Hare AM. Association between hospice length of stay, health care utilization, and Medicare costs at the end of life among patients who received maintenance hemodialysis. JAMA Int Med. 2018;178(6):792-799. doi:10.1001/jamainternmed.2018.0256
17. Centers for Medicare and Medicaid Services. Hospice care. Accessed April 2, 2022. https://www.medicare.gov/coverage/hospice-care
18. National Hospice and Palliative Care Organization. Ethical behavior and consumer rights. Standards of Practice for Hospice Programs Professional Development and Resource Series. Accessed December 6, 2022. https://www.nhpco.org/wp-content/uploads/2019/04/Standards_Hospice_2018.pdf
19. US Department of Veterans Affairs. Geriatrics and extended care. Updated October 5, 2022. Accessed August 29, 2022. https://www.va.gov/geriatrics/pages/Hospice_Care.asp
20. Cohen LM, McCue JD, Germain M, Kjellstrand CM. Dialysis discontinuation. A ‘good’ death? Arch Intern Med. 1995;155(1):42-47.
21. Ubel PA, Scherr KA, Fagerlin A. Autonomy: What’s shared decision making have to do with it? Am J Bioeth. 2018;18(2):W11-W12.doi:10.1080/15265161.2017.1409844
22. Laryionava, K, Pfeil TA, Dietrich M. et al. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
23. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi:10.1155/2017/9724371
24. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911. doi:10.1001/jama.284.22.2907
25. Sullivan M, Ormel J, Kempen GIJM, Tymstra T. Beliefs concerning death, dying, and hastening death among older, functionally impaired Dutch adults: a one-year longitudinal study. J Am Gec Soc. doi:10.1111/j.1532-5415.1998.tb04541.x26. Gelfand SL, Schell J, Eneanya ND. Palliative care in nephrology: the work and the workforce. Adv Chronic Kidney Dis. 2020;27(4):350-355.e1. doi:10.1053/j.ackd.2020.02.007
1. Carr D, Luth EA. Well-being at the end of life. Annu Rev Sociol. 2019;45:515-534. doi:10.1146/annurev-soc-073018-022524
2. Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264-271. doi:10.1001/jama.2018.8981
3. Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573-585. doi:10.1038/s41581-020-0315-4
4. Richards CA, Hebert PL, Liu CF, et al. Association of family ratings of quality of end-of-life care with stopping dialysis treatment and receipt of hospice services. JAMA Netw Open. 2019;2(10):e1913115. doi:10.1001/jamanetworkopen.2019.13115
5. Fischer MJ, Kourany WM, Sovern K, Forrester K, Griffin C, Lightner N, Loftus S, Murphy K, Roth G, Palevsky PM, Crowley ST. Development, implementation and user experience of the Veterans Health Administration (VHA) dialysis dashboard. BMC Nephrol. 2020 Apr 16;21(1):136. doi:10.1186/s12882-020-01798-6
6. Schwarze ML, Schueller K, Jhagroo RA. Hospice use and end-of-life care for patients with end-stage renal disease: too little, too late. JAMA Intern Med. 2018;178(6):799-801.doi:10.1001/jamainternmed.2018.1078
7. Chen JC, Thorsteinsdottir B, Vaughan LE, et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2018;13(8):1172-1179. doi:10.2215/CJN.00590118
8. Chen HC, Wu CY, Hsieh HY, He JS, Hwang SJ, Hsieh HM. Predictors and assessment of hospice use for end-stage renal disease patients in Taiwan. Int J Environ Res Public Health. 2021;19(1):85. doi:10.3390/ijerph19010085
9. Rak A, Raina R, Suh TT, et al. Palliative care for patients with end-stage renal disease: approach to treatment that aims to improve quality of life and relieve suffering for patients (and families) with chronic illnesses. Clin Kidney J. 2017;10(1):68-73. doi.10.1093/ckj/sfw10510. Wong SPY, Boyapati S, Engelberg RA, Thorsteinsdottir B, Taylor JS, O’Hare AM. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis. 2020;75(2):167-176. doi:10.1053/j.ajkd.2019.07.006
11. Axelsson L, Benzein E, Lindberg J, Persson C. End-of-life and palliative care of patients on maintenance hemodialysis treatment: a focus group study. BMC Palliat Care. 2019;18(1):89. doi:10.1186/s12904-019-0481-y
12. Tweeddale MG. Grasping the nettle—what to do when patients withdraw their consent for treatment: (a clinical perspective on the case of Ms B). J Med Ethics. 2002;28(4):236-237. doi:10.1136/jme.28.4.236
13. Lynøe N, Engström I, Juth N. How to reveal disguised paternalism: version 2.0. BMC Med Ethics. 2021;22(1):170. doi:10.1186/s12910-021-00739-8
14. Murgic L, Hébert PC, Sovic S, Pavlekovic G. Paternalism and autonomy: views of patients and providers in a transitional (post-communist) country. BMC Med Ethics. 2015;16(1):65. doi:10.1186/s12910-015-0059-z
15. Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854-863. doi:10.2215/CJN.05760516
16. Wachterman MW, Hailpern SM, Keating NL, Kurella Tamura M, O’Hare AM. Association between hospice length of stay, health care utilization, and Medicare costs at the end of life among patients who received maintenance hemodialysis. JAMA Int Med. 2018;178(6):792-799. doi:10.1001/jamainternmed.2018.0256
17. Centers for Medicare and Medicaid Services. Hospice care. Accessed April 2, 2022. https://www.medicare.gov/coverage/hospice-care
18. National Hospice and Palliative Care Organization. Ethical behavior and consumer rights. Standards of Practice for Hospice Programs Professional Development and Resource Series. Accessed December 6, 2022. https://www.nhpco.org/wp-content/uploads/2019/04/Standards_Hospice_2018.pdf
19. US Department of Veterans Affairs. Geriatrics and extended care. Updated October 5, 2022. Accessed August 29, 2022. https://www.va.gov/geriatrics/pages/Hospice_Care.asp
20. Cohen LM, McCue JD, Germain M, Kjellstrand CM. Dialysis discontinuation. A ‘good’ death? Arch Intern Med. 1995;155(1):42-47.
21. Ubel PA, Scherr KA, Fagerlin A. Autonomy: What’s shared decision making have to do with it? Am J Bioeth. 2018;18(2):W11-W12.doi:10.1080/15265161.2017.1409844
22. Laryionava, K, Pfeil TA, Dietrich M. et al. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
23. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi:10.1155/2017/9724371
24. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911. doi:10.1001/jama.284.22.2907
25. Sullivan M, Ormel J, Kempen GIJM, Tymstra T. Beliefs concerning death, dying, and hastening death among older, functionally impaired Dutch adults: a one-year longitudinal study. J Am Gec Soc. doi:10.1111/j.1532-5415.1998.tb04541.x26. Gelfand SL, Schell J, Eneanya ND. Palliative care in nephrology: the work and the workforce. Adv Chronic Kidney Dis. 2020;27(4):350-355.e1. doi:10.1053/j.ackd.2020.02.007
Telehealth parent-child interaction therapy improved behavior in children with developmental delay
The children received the therapy with their parents or caregivers, who were more likely to demonstrate positive parenting behaviors than parents in the control group, authors of the new research published in JAMA Pediatrics found.
Approximately 13% of children have some form of developmental delay (DD) and more than half of these children also have at least one mental health disorder, which makes behavior problems a common and ongoing challenge, Daniel M. Bagner, PhD, a psychologist at Florida International University, Miami, and colleagues wrote.
Clinic-based interventions such as parent-child interaction therapy (PCIT) have been effective for improving behavior in children with DD, the researchers said. PCIT involves in-session caregiver coaching using a 1-way mirror and a wireless earpiece worn by the caregiver.
Barriers to the use of PCIT, especially in marginalized and low-income communities, include transportation, clinician shortages, and stigma-related concerns about a clinic visit, the researchers wrote. Technology now allows for Internet-delivered PCIT to reach more children and families, but its effectiveness for children with DD has not been well studied.
In the new study, the researchers randomized 150 children with DD and externalizing behavior problems to up to 20 weeks of Internet-delivered parent-child interaction therapy (iPCIT) or to referral as usual (RAU, the control group). The children were randomized after completion of early intervention services within 3 months of their third birthday, and participated in the sessions with a parent or caregiver. Most of the participants were from economically disadvantaged households and underrepresented ethnic backgrounds.
The iPCIT intervention was conducted weekly with a remote therapist and lasted for 1-1.5 hours; approximately half of the families received the intervention in Spanish.
The primary outcome was rating on the Child Behavior Checklist (CBCL) and assessment of children and caregivers using the Dyadic Parent-Child Interaction Coding System, fourth edition (DPICS). Assessments occurred at baseline and at week 20 (post treatment), with follow ups at 6 and 12 months.
Scores on the CBCL in the iPCIT group decreased from a mean of 61.18 at baseline to 53.83 post intervention. Scores for the control group started at 64.05 and decreased to 59.49 post intervention. At 6-12 months, the scores for both groups remained stable.
Children who received iPCIT with their parent or caregiver also showed significantly lower levels of externalizing behavior problems, compared with the RAU controls post treatment, and at 6-month and 12-month follow-ups based on the Cohen d measure of standardized effect size for differences between groups.
Significantly more children in the iPCIT group showed clinically significant improvements in externalizing problems at post treatment, compared with the RAU group (74% vs. 42%; P < .001) and at 6 months’ follow-up (73% vs. 45%; P = .002). However, the differences from baseline were not significantly different between the two groups after 12 months, which suggests that the effects may wane over time, the researchers noted.
In addition, the rate of child compliance with parent commands, as measured by a cleanup task, approximately doubled by the 12-month follow-up among children in the iPCIT group versus an increase of approximately one-third in the RAU group.
For secondary outcome measures related to caregiver behaviors, the proportion of observed positive parenting behaviors increased in the iPCIT group during the course of the intervention (postintervention odds ratio, 1.10), and the proportion of controlling and critical behaviors decreased (postintervention OR, 1.40). Harsh and inconsistent discipline decreased in both groups based on self-reports, but the decrease was steeper in iPCIT families.
iPCIT did not have a greater impact than RAU in reducing caregiver stress. The researchers wrote that they were not surprised by the lack of stress reduction “given mixed findings on the impact of parenting interventions on stress in caregivers of children with DD.”
Data support iPCIT potential
Overall, the results support findings from previous studies of clinic-based PCIT for children with DD and previous studies of telehealth interventions for typically developing children, the researchers said.
“Moreover, iPCIT-treated children not only showed reductions in behavior problems, such as aggression, but demonstrated higher rates of following directions, which is especially important for children entering kindergarten,” they wrote.
The findings were limited by several factors including the narrow focus on the primary and secondary outcomes, the use of data from a single site in a single metropolitan area – which may limit generalizability – and the lack of comparison between iPCIT and a clinic-based PCIT control group, the researchers noted. The equipment in the current study was provided to families; therefore, differences in treatment response could not be attributed to differences in technology.
The study represents the first known randomized controlled trial to evaluate a telehealth parenting intervention for children with, according to the researchers. The results suggest that technology can be leveraged to help these patients, including those from ethnic minority families who may be underserved by clinic-based care in overcoming barriers to treatment such as transportation and availability of clinicians. Use of iPCIT could be a critical resource as young children with DD complete Part C services and enter the school system.
Practical pediatric takeaways
“This was a great study, well-designed and very important and helpful for pediatric providers,” Cathy Haut, DNP, CPNP-AC, CPNP-PC, a pediatric nurse practitioner in Rehoboth Beach, Del., said in an interview.
“Young children with developmental delay and/or mental and behavioral health disorders require early identification and intervention,” said Dr. Haut. However, obstacles to intervention include stigma or parental denial of the disorder, as well as more practical challenges related to transportation, time to access a clinic or office, potential long length of treatment, and cost.
“Despite availability of state programs for young children, follow up and continued services can be challenging to complete. Once the child outgrows the state program finding alternative therapy can be difficult with the current shortage of pediatric mental health providers,” Dr. Haut noted.
“I was surprised to see that this study treatment phase was completed prior to the COVID-19 pandemic, when telehealth was not as popular a mode for health care and was not utilized to the extent that it is now, especially for pediatric care,” said Dr. Haut. “I was not surprised at the results, as the traditional mode of PCIT includes therapy and training in a space that may not be as familiar to the child as their home environment, and would include live presence of the therapist/s, which may add to anxiety for both the parent and child.”
That almost half of the parents participating in the study had graduated from college and/or completed graduate degrees “may have contributed to some of the success of this study,” Dr. Haut noted.
Benefits and barriers
“The COVID-19 pandemic brought significant change to the frequency of use and overall success of telehealth services,” Dr. Haut said. “Additional provider education in aspects such as provider technique and the use of medical devices with improved specific health care technology assisted in advancing the experience and opportunity for successful telehealth visits. Telehealth therapy offers a cost-effective option for any pediatric patients and for providers, as the time and space commitment for the patient visit can be considerably less than live office visits.
“Unfortunately, there are still overall barriers that I have personally experienced with telehealth, including interruptions in connectivity, background noise, and lack of an available computer or tablet; and with the use of cell phones not always allowing full inclusion of the caregiver and child,” said Dr. Haut. Children with DD, behavioral problems, or other mental health disorders may pose challenges for parents to manage at home while simultaneously trying to fully focus on the therapy in an online setting.
Although the current study is encouraging, “larger studies focused on specific or individual pediatric mental health and/or behavioral disorders may offer more information for providers, and better document the success of telehealth delivery of services,” Dr. Haut said.
The study was supported by the National Institute of Child Health and Human Development. Dr. Bagner disclosed funding from the National Institutes of Health. He also disclosed personal fees from PCIT International to train clinicians in PCIT supported by a grant from the Florida Department of Children and Families outside the current study. Dr. Haut had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
The children received the therapy with their parents or caregivers, who were more likely to demonstrate positive parenting behaviors than parents in the control group, authors of the new research published in JAMA Pediatrics found.
Approximately 13% of children have some form of developmental delay (DD) and more than half of these children also have at least one mental health disorder, which makes behavior problems a common and ongoing challenge, Daniel M. Bagner, PhD, a psychologist at Florida International University, Miami, and colleagues wrote.
Clinic-based interventions such as parent-child interaction therapy (PCIT) have been effective for improving behavior in children with DD, the researchers said. PCIT involves in-session caregiver coaching using a 1-way mirror and a wireless earpiece worn by the caregiver.
Barriers to the use of PCIT, especially in marginalized and low-income communities, include transportation, clinician shortages, and stigma-related concerns about a clinic visit, the researchers wrote. Technology now allows for Internet-delivered PCIT to reach more children and families, but its effectiveness for children with DD has not been well studied.
In the new study, the researchers randomized 150 children with DD and externalizing behavior problems to up to 20 weeks of Internet-delivered parent-child interaction therapy (iPCIT) or to referral as usual (RAU, the control group). The children were randomized after completion of early intervention services within 3 months of their third birthday, and participated in the sessions with a parent or caregiver. Most of the participants were from economically disadvantaged households and underrepresented ethnic backgrounds.
The iPCIT intervention was conducted weekly with a remote therapist and lasted for 1-1.5 hours; approximately half of the families received the intervention in Spanish.
The primary outcome was rating on the Child Behavior Checklist (CBCL) and assessment of children and caregivers using the Dyadic Parent-Child Interaction Coding System, fourth edition (DPICS). Assessments occurred at baseline and at week 20 (post treatment), with follow ups at 6 and 12 months.
Scores on the CBCL in the iPCIT group decreased from a mean of 61.18 at baseline to 53.83 post intervention. Scores for the control group started at 64.05 and decreased to 59.49 post intervention. At 6-12 months, the scores for both groups remained stable.
Children who received iPCIT with their parent or caregiver also showed significantly lower levels of externalizing behavior problems, compared with the RAU controls post treatment, and at 6-month and 12-month follow-ups based on the Cohen d measure of standardized effect size for differences between groups.
Significantly more children in the iPCIT group showed clinically significant improvements in externalizing problems at post treatment, compared with the RAU group (74% vs. 42%; P < .001) and at 6 months’ follow-up (73% vs. 45%; P = .002). However, the differences from baseline were not significantly different between the two groups after 12 months, which suggests that the effects may wane over time, the researchers noted.
In addition, the rate of child compliance with parent commands, as measured by a cleanup task, approximately doubled by the 12-month follow-up among children in the iPCIT group versus an increase of approximately one-third in the RAU group.
For secondary outcome measures related to caregiver behaviors, the proportion of observed positive parenting behaviors increased in the iPCIT group during the course of the intervention (postintervention odds ratio, 1.10), and the proportion of controlling and critical behaviors decreased (postintervention OR, 1.40). Harsh and inconsistent discipline decreased in both groups based on self-reports, but the decrease was steeper in iPCIT families.
iPCIT did not have a greater impact than RAU in reducing caregiver stress. The researchers wrote that they were not surprised by the lack of stress reduction “given mixed findings on the impact of parenting interventions on stress in caregivers of children with DD.”
Data support iPCIT potential
Overall, the results support findings from previous studies of clinic-based PCIT for children with DD and previous studies of telehealth interventions for typically developing children, the researchers said.
“Moreover, iPCIT-treated children not only showed reductions in behavior problems, such as aggression, but demonstrated higher rates of following directions, which is especially important for children entering kindergarten,” they wrote.
The findings were limited by several factors including the narrow focus on the primary and secondary outcomes, the use of data from a single site in a single metropolitan area – which may limit generalizability – and the lack of comparison between iPCIT and a clinic-based PCIT control group, the researchers noted. The equipment in the current study was provided to families; therefore, differences in treatment response could not be attributed to differences in technology.
The study represents the first known randomized controlled trial to evaluate a telehealth parenting intervention for children with, according to the researchers. The results suggest that technology can be leveraged to help these patients, including those from ethnic minority families who may be underserved by clinic-based care in overcoming barriers to treatment such as transportation and availability of clinicians. Use of iPCIT could be a critical resource as young children with DD complete Part C services and enter the school system.
Practical pediatric takeaways
“This was a great study, well-designed and very important and helpful for pediatric providers,” Cathy Haut, DNP, CPNP-AC, CPNP-PC, a pediatric nurse practitioner in Rehoboth Beach, Del., said in an interview.
“Young children with developmental delay and/or mental and behavioral health disorders require early identification and intervention,” said Dr. Haut. However, obstacles to intervention include stigma or parental denial of the disorder, as well as more practical challenges related to transportation, time to access a clinic or office, potential long length of treatment, and cost.
“Despite availability of state programs for young children, follow up and continued services can be challenging to complete. Once the child outgrows the state program finding alternative therapy can be difficult with the current shortage of pediatric mental health providers,” Dr. Haut noted.
“I was surprised to see that this study treatment phase was completed prior to the COVID-19 pandemic, when telehealth was not as popular a mode for health care and was not utilized to the extent that it is now, especially for pediatric care,” said Dr. Haut. “I was not surprised at the results, as the traditional mode of PCIT includes therapy and training in a space that may not be as familiar to the child as their home environment, and would include live presence of the therapist/s, which may add to anxiety for both the parent and child.”
That almost half of the parents participating in the study had graduated from college and/or completed graduate degrees “may have contributed to some of the success of this study,” Dr. Haut noted.
Benefits and barriers
“The COVID-19 pandemic brought significant change to the frequency of use and overall success of telehealth services,” Dr. Haut said. “Additional provider education in aspects such as provider technique and the use of medical devices with improved specific health care technology assisted in advancing the experience and opportunity for successful telehealth visits. Telehealth therapy offers a cost-effective option for any pediatric patients and for providers, as the time and space commitment for the patient visit can be considerably less than live office visits.
“Unfortunately, there are still overall barriers that I have personally experienced with telehealth, including interruptions in connectivity, background noise, and lack of an available computer or tablet; and with the use of cell phones not always allowing full inclusion of the caregiver and child,” said Dr. Haut. Children with DD, behavioral problems, or other mental health disorders may pose challenges for parents to manage at home while simultaneously trying to fully focus on the therapy in an online setting.
Although the current study is encouraging, “larger studies focused on specific or individual pediatric mental health and/or behavioral disorders may offer more information for providers, and better document the success of telehealth delivery of services,” Dr. Haut said.
The study was supported by the National Institute of Child Health and Human Development. Dr. Bagner disclosed funding from the National Institutes of Health. He also disclosed personal fees from PCIT International to train clinicians in PCIT supported by a grant from the Florida Department of Children and Families outside the current study. Dr. Haut had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
The children received the therapy with their parents or caregivers, who were more likely to demonstrate positive parenting behaviors than parents in the control group, authors of the new research published in JAMA Pediatrics found.
Approximately 13% of children have some form of developmental delay (DD) and more than half of these children also have at least one mental health disorder, which makes behavior problems a common and ongoing challenge, Daniel M. Bagner, PhD, a psychologist at Florida International University, Miami, and colleagues wrote.
Clinic-based interventions such as parent-child interaction therapy (PCIT) have been effective for improving behavior in children with DD, the researchers said. PCIT involves in-session caregiver coaching using a 1-way mirror and a wireless earpiece worn by the caregiver.
Barriers to the use of PCIT, especially in marginalized and low-income communities, include transportation, clinician shortages, and stigma-related concerns about a clinic visit, the researchers wrote. Technology now allows for Internet-delivered PCIT to reach more children and families, but its effectiveness for children with DD has not been well studied.
In the new study, the researchers randomized 150 children with DD and externalizing behavior problems to up to 20 weeks of Internet-delivered parent-child interaction therapy (iPCIT) or to referral as usual (RAU, the control group). The children were randomized after completion of early intervention services within 3 months of their third birthday, and participated in the sessions with a parent or caregiver. Most of the participants were from economically disadvantaged households and underrepresented ethnic backgrounds.
The iPCIT intervention was conducted weekly with a remote therapist and lasted for 1-1.5 hours; approximately half of the families received the intervention in Spanish.
The primary outcome was rating on the Child Behavior Checklist (CBCL) and assessment of children and caregivers using the Dyadic Parent-Child Interaction Coding System, fourth edition (DPICS). Assessments occurred at baseline and at week 20 (post treatment), with follow ups at 6 and 12 months.
Scores on the CBCL in the iPCIT group decreased from a mean of 61.18 at baseline to 53.83 post intervention. Scores for the control group started at 64.05 and decreased to 59.49 post intervention. At 6-12 months, the scores for both groups remained stable.
Children who received iPCIT with their parent or caregiver also showed significantly lower levels of externalizing behavior problems, compared with the RAU controls post treatment, and at 6-month and 12-month follow-ups based on the Cohen d measure of standardized effect size for differences between groups.
Significantly more children in the iPCIT group showed clinically significant improvements in externalizing problems at post treatment, compared with the RAU group (74% vs. 42%; P < .001) and at 6 months’ follow-up (73% vs. 45%; P = .002). However, the differences from baseline were not significantly different between the two groups after 12 months, which suggests that the effects may wane over time, the researchers noted.
In addition, the rate of child compliance with parent commands, as measured by a cleanup task, approximately doubled by the 12-month follow-up among children in the iPCIT group versus an increase of approximately one-third in the RAU group.
For secondary outcome measures related to caregiver behaviors, the proportion of observed positive parenting behaviors increased in the iPCIT group during the course of the intervention (postintervention odds ratio, 1.10), and the proportion of controlling and critical behaviors decreased (postintervention OR, 1.40). Harsh and inconsistent discipline decreased in both groups based on self-reports, but the decrease was steeper in iPCIT families.
iPCIT did not have a greater impact than RAU in reducing caregiver stress. The researchers wrote that they were not surprised by the lack of stress reduction “given mixed findings on the impact of parenting interventions on stress in caregivers of children with DD.”
Data support iPCIT potential
Overall, the results support findings from previous studies of clinic-based PCIT for children with DD and previous studies of telehealth interventions for typically developing children, the researchers said.
“Moreover, iPCIT-treated children not only showed reductions in behavior problems, such as aggression, but demonstrated higher rates of following directions, which is especially important for children entering kindergarten,” they wrote.
The findings were limited by several factors including the narrow focus on the primary and secondary outcomes, the use of data from a single site in a single metropolitan area – which may limit generalizability – and the lack of comparison between iPCIT and a clinic-based PCIT control group, the researchers noted. The equipment in the current study was provided to families; therefore, differences in treatment response could not be attributed to differences in technology.
The study represents the first known randomized controlled trial to evaluate a telehealth parenting intervention for children with, according to the researchers. The results suggest that technology can be leveraged to help these patients, including those from ethnic minority families who may be underserved by clinic-based care in overcoming barriers to treatment such as transportation and availability of clinicians. Use of iPCIT could be a critical resource as young children with DD complete Part C services and enter the school system.
Practical pediatric takeaways
“This was a great study, well-designed and very important and helpful for pediatric providers,” Cathy Haut, DNP, CPNP-AC, CPNP-PC, a pediatric nurse practitioner in Rehoboth Beach, Del., said in an interview.
“Young children with developmental delay and/or mental and behavioral health disorders require early identification and intervention,” said Dr. Haut. However, obstacles to intervention include stigma or parental denial of the disorder, as well as more practical challenges related to transportation, time to access a clinic or office, potential long length of treatment, and cost.
“Despite availability of state programs for young children, follow up and continued services can be challenging to complete. Once the child outgrows the state program finding alternative therapy can be difficult with the current shortage of pediatric mental health providers,” Dr. Haut noted.
“I was surprised to see that this study treatment phase was completed prior to the COVID-19 pandemic, when telehealth was not as popular a mode for health care and was not utilized to the extent that it is now, especially for pediatric care,” said Dr. Haut. “I was not surprised at the results, as the traditional mode of PCIT includes therapy and training in a space that may not be as familiar to the child as their home environment, and would include live presence of the therapist/s, which may add to anxiety for both the parent and child.”
That almost half of the parents participating in the study had graduated from college and/or completed graduate degrees “may have contributed to some of the success of this study,” Dr. Haut noted.
Benefits and barriers
“The COVID-19 pandemic brought significant change to the frequency of use and overall success of telehealth services,” Dr. Haut said. “Additional provider education in aspects such as provider technique and the use of medical devices with improved specific health care technology assisted in advancing the experience and opportunity for successful telehealth visits. Telehealth therapy offers a cost-effective option for any pediatric patients and for providers, as the time and space commitment for the patient visit can be considerably less than live office visits.
“Unfortunately, there are still overall barriers that I have personally experienced with telehealth, including interruptions in connectivity, background noise, and lack of an available computer or tablet; and with the use of cell phones not always allowing full inclusion of the caregiver and child,” said Dr. Haut. Children with DD, behavioral problems, or other mental health disorders may pose challenges for parents to manage at home while simultaneously trying to fully focus on the therapy in an online setting.
Although the current study is encouraging, “larger studies focused on specific or individual pediatric mental health and/or behavioral disorders may offer more information for providers, and better document the success of telehealth delivery of services,” Dr. Haut said.
The study was supported by the National Institute of Child Health and Human Development. Dr. Bagner disclosed funding from the National Institutes of Health. He also disclosed personal fees from PCIT International to train clinicians in PCIT supported by a grant from the Florida Department of Children and Families outside the current study. Dr. Haut had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS
Chronic pain patients swapping opioids for medical cannabis
new research shows.
“That patients report substituting cannabis for pain medicines so much really underscores the need for research on the benefits and risks of using cannabis for chronic pain,” lead author Mark C. Bicket, MD, PhD, assistant professor, department of anesthesiology, and director, Opioid Prescribing Engagement Network, University of Michigan, Ann Arbor, said in an interview.
However, he added, the question is whether they’re turning to cannabis and away from other pain treatments. “What’s not clear and one of the gaps that we wanted to address in the study was if medical cannabis use is changing the use of other treatments for chronic pain,” said Dr. Bicket.
The study was published online in JAMA Network Open.
Decreased opioid use
The survey included a representative sample of 1724 American adults aged 18 years or older with chronic noncancer pain living in areas with a medical cannabis program.
Respondents were asked about their use of three categories of pain treatments. This included medical cannabis; pharmacologic treatments including prescription opioids, nonopioid analgesics, and over-the-counter analgesics; and common nonpharmacologic treatments such as physical therapy, meditation, and cognitive-behavioral therapy (CBT).
Just over 96% of respondents completed the full survey. About 57% of the sample was female and the mean age of the study sample was 52.3 years.
Among study participants, 31% (95% CI, 28.2% - 34.1%) reported having ever used cannabis to manage pain; 25.9% (95% confidence interval, 23.2%-28.8%) reported use in the past 12 months, and 23.2% (95% CI, 20.6%-26%) reported use in the past 30 days.
“This translates into a large number of individuals who are using cannabis in an intended medical way” to treat chronic condition such as low back pain, migraine, and fibromyalgia, said Dr. Bicket.
More than half of survey respondents reported their medical cannabis use led to a decrease in prescription opioid use, prescription nonopioid use and use of over-the-counter medications.
Dr. Bicket noted “almost no one” said medical cannabis use led to higher use of these drugs.
As for nonpharmacologic treatments, 38.7% reported their use of cannabis led to decreased use of physical therapy, 19.1% to lower use of meditation, and 26% to less CBT. At the same time, 5.9%, 23.7% and 17.1%, respectively, reported it led to increased use of physical therapy, meditation, and CBT.
Medical cannabis is regulated at a state level. On a federal level, it’s considered a Schedule I substance, which means it’s deemed not to have a therapeutic use, although some groups are trying to change that categorization, said Dr. Bicket.
As a result, cannabis products “are quite variable” in terms of how they’re used (smoked, eaten etc.) and in their composition, including percentage of cannabidiol and tetrahydrocannabinol.
“We really don’t have a good sense of the relative risks and benefits that could come from cannabis as a treatment for chronic pain,” said Dr. Bicket. “As a physician, it’s difficult to have discussions with patients because I’m not able to understand the products they’re using based on this regulatory environment we have.”
He added clinicians “are operating in an area of uncertainty right now.”
What’s needed is research to determine how safe and effective medical cannabis is for chronic pain, he said.
Pain a leading indication
Commenting on the findings, Jason W. Busse, PhD, professor, department of anesthesia, and associate director, Centre for Medicinal Cannabis Research, McMaster University, Hamilton, Ont., said the study reinforces results of some prior research.
“It gives us current information certainly highlighting the high rate of use of medical cannabis among individuals with chronic pain once it becomes legally available.”
In addition, this high rate of use “means we desperately need information about the benefits and harms” of medical marijuana, he said.
Dr. Busse noted the survey didn’t provide information on the types of cannabis being used or the mode of administration. Oil drops and sprays cause less pulmonary harm than smoked versions, he said. It’s also not clear from the survey if participants are taking formulations with high levels of tetrahydrocannabinol that are associated with greater risk of harm.
He noted cannabis may interact with prescription drugs to make them less effective or, in some cases, to augment their adverse effects.
Dr. Busse pointed out some patients could be using fewer opioids because providers are under “enormous pressure” to reduce prescriptions of these drugs in the wake of spikes in opioid overdoses and deaths.
Chronic pain is “absolutely the leading indication” for medical marijuana, said Dr. Busse. U.S. reimbursement data suggest up to 65% of individuals get cannabis to treat a listed indication for chronic pain.
He said he hopes this new study will increase interest in funding new trials “so we can have better evidence to guide practice to help patients make decisions.”
The study received support from the National Institute on Drug Abuse. Dr. Bicket reported receiving personal fees from Axial Healthcare as well as grants from the National Institutes of Health, the Centers for Disease Control and Prevention, Michigan Department of Health and Human Services, Arnold Foundation, and the Patient-Centered Outcomes Research Institute outside the submitted work. Dr. Busse reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
“That patients report substituting cannabis for pain medicines so much really underscores the need for research on the benefits and risks of using cannabis for chronic pain,” lead author Mark C. Bicket, MD, PhD, assistant professor, department of anesthesiology, and director, Opioid Prescribing Engagement Network, University of Michigan, Ann Arbor, said in an interview.
However, he added, the question is whether they’re turning to cannabis and away from other pain treatments. “What’s not clear and one of the gaps that we wanted to address in the study was if medical cannabis use is changing the use of other treatments for chronic pain,” said Dr. Bicket.
The study was published online in JAMA Network Open.
Decreased opioid use
The survey included a representative sample of 1724 American adults aged 18 years or older with chronic noncancer pain living in areas with a medical cannabis program.
Respondents were asked about their use of three categories of pain treatments. This included medical cannabis; pharmacologic treatments including prescription opioids, nonopioid analgesics, and over-the-counter analgesics; and common nonpharmacologic treatments such as physical therapy, meditation, and cognitive-behavioral therapy (CBT).
Just over 96% of respondents completed the full survey. About 57% of the sample was female and the mean age of the study sample was 52.3 years.
Among study participants, 31% (95% CI, 28.2% - 34.1%) reported having ever used cannabis to manage pain; 25.9% (95% confidence interval, 23.2%-28.8%) reported use in the past 12 months, and 23.2% (95% CI, 20.6%-26%) reported use in the past 30 days.
“This translates into a large number of individuals who are using cannabis in an intended medical way” to treat chronic condition such as low back pain, migraine, and fibromyalgia, said Dr. Bicket.
More than half of survey respondents reported their medical cannabis use led to a decrease in prescription opioid use, prescription nonopioid use and use of over-the-counter medications.
Dr. Bicket noted “almost no one” said medical cannabis use led to higher use of these drugs.
As for nonpharmacologic treatments, 38.7% reported their use of cannabis led to decreased use of physical therapy, 19.1% to lower use of meditation, and 26% to less CBT. At the same time, 5.9%, 23.7% and 17.1%, respectively, reported it led to increased use of physical therapy, meditation, and CBT.
Medical cannabis is regulated at a state level. On a federal level, it’s considered a Schedule I substance, which means it’s deemed not to have a therapeutic use, although some groups are trying to change that categorization, said Dr. Bicket.
As a result, cannabis products “are quite variable” in terms of how they’re used (smoked, eaten etc.) and in their composition, including percentage of cannabidiol and tetrahydrocannabinol.
“We really don’t have a good sense of the relative risks and benefits that could come from cannabis as a treatment for chronic pain,” said Dr. Bicket. “As a physician, it’s difficult to have discussions with patients because I’m not able to understand the products they’re using based on this regulatory environment we have.”
He added clinicians “are operating in an area of uncertainty right now.”
What’s needed is research to determine how safe and effective medical cannabis is for chronic pain, he said.
Pain a leading indication
Commenting on the findings, Jason W. Busse, PhD, professor, department of anesthesia, and associate director, Centre for Medicinal Cannabis Research, McMaster University, Hamilton, Ont., said the study reinforces results of some prior research.
“It gives us current information certainly highlighting the high rate of use of medical cannabis among individuals with chronic pain once it becomes legally available.”
In addition, this high rate of use “means we desperately need information about the benefits and harms” of medical marijuana, he said.
Dr. Busse noted the survey didn’t provide information on the types of cannabis being used or the mode of administration. Oil drops and sprays cause less pulmonary harm than smoked versions, he said. It’s also not clear from the survey if participants are taking formulations with high levels of tetrahydrocannabinol that are associated with greater risk of harm.
He noted cannabis may interact with prescription drugs to make them less effective or, in some cases, to augment their adverse effects.
Dr. Busse pointed out some patients could be using fewer opioids because providers are under “enormous pressure” to reduce prescriptions of these drugs in the wake of spikes in opioid overdoses and deaths.
Chronic pain is “absolutely the leading indication” for medical marijuana, said Dr. Busse. U.S. reimbursement data suggest up to 65% of individuals get cannabis to treat a listed indication for chronic pain.
He said he hopes this new study will increase interest in funding new trials “so we can have better evidence to guide practice to help patients make decisions.”
The study received support from the National Institute on Drug Abuse. Dr. Bicket reported receiving personal fees from Axial Healthcare as well as grants from the National Institutes of Health, the Centers for Disease Control and Prevention, Michigan Department of Health and Human Services, Arnold Foundation, and the Patient-Centered Outcomes Research Institute outside the submitted work. Dr. Busse reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
“That patients report substituting cannabis for pain medicines so much really underscores the need for research on the benefits and risks of using cannabis for chronic pain,” lead author Mark C. Bicket, MD, PhD, assistant professor, department of anesthesiology, and director, Opioid Prescribing Engagement Network, University of Michigan, Ann Arbor, said in an interview.
However, he added, the question is whether they’re turning to cannabis and away from other pain treatments. “What’s not clear and one of the gaps that we wanted to address in the study was if medical cannabis use is changing the use of other treatments for chronic pain,” said Dr. Bicket.
The study was published online in JAMA Network Open.
Decreased opioid use
The survey included a representative sample of 1724 American adults aged 18 years or older with chronic noncancer pain living in areas with a medical cannabis program.
Respondents were asked about their use of three categories of pain treatments. This included medical cannabis; pharmacologic treatments including prescription opioids, nonopioid analgesics, and over-the-counter analgesics; and common nonpharmacologic treatments such as physical therapy, meditation, and cognitive-behavioral therapy (CBT).
Just over 96% of respondents completed the full survey. About 57% of the sample was female and the mean age of the study sample was 52.3 years.
Among study participants, 31% (95% CI, 28.2% - 34.1%) reported having ever used cannabis to manage pain; 25.9% (95% confidence interval, 23.2%-28.8%) reported use in the past 12 months, and 23.2% (95% CI, 20.6%-26%) reported use in the past 30 days.
“This translates into a large number of individuals who are using cannabis in an intended medical way” to treat chronic condition such as low back pain, migraine, and fibromyalgia, said Dr. Bicket.
More than half of survey respondents reported their medical cannabis use led to a decrease in prescription opioid use, prescription nonopioid use and use of over-the-counter medications.
Dr. Bicket noted “almost no one” said medical cannabis use led to higher use of these drugs.
As for nonpharmacologic treatments, 38.7% reported their use of cannabis led to decreased use of physical therapy, 19.1% to lower use of meditation, and 26% to less CBT. At the same time, 5.9%, 23.7% and 17.1%, respectively, reported it led to increased use of physical therapy, meditation, and CBT.
Medical cannabis is regulated at a state level. On a federal level, it’s considered a Schedule I substance, which means it’s deemed not to have a therapeutic use, although some groups are trying to change that categorization, said Dr. Bicket.
As a result, cannabis products “are quite variable” in terms of how they’re used (smoked, eaten etc.) and in their composition, including percentage of cannabidiol and tetrahydrocannabinol.
“We really don’t have a good sense of the relative risks and benefits that could come from cannabis as a treatment for chronic pain,” said Dr. Bicket. “As a physician, it’s difficult to have discussions with patients because I’m not able to understand the products they’re using based on this regulatory environment we have.”
He added clinicians “are operating in an area of uncertainty right now.”
What’s needed is research to determine how safe and effective medical cannabis is for chronic pain, he said.
Pain a leading indication
Commenting on the findings, Jason W. Busse, PhD, professor, department of anesthesia, and associate director, Centre for Medicinal Cannabis Research, McMaster University, Hamilton, Ont., said the study reinforces results of some prior research.
“It gives us current information certainly highlighting the high rate of use of medical cannabis among individuals with chronic pain once it becomes legally available.”
In addition, this high rate of use “means we desperately need information about the benefits and harms” of medical marijuana, he said.
Dr. Busse noted the survey didn’t provide information on the types of cannabis being used or the mode of administration. Oil drops and sprays cause less pulmonary harm than smoked versions, he said. It’s also not clear from the survey if participants are taking formulations with high levels of tetrahydrocannabinol that are associated with greater risk of harm.
He noted cannabis may interact with prescription drugs to make them less effective or, in some cases, to augment their adverse effects.
Dr. Busse pointed out some patients could be using fewer opioids because providers are under “enormous pressure” to reduce prescriptions of these drugs in the wake of spikes in opioid overdoses and deaths.
Chronic pain is “absolutely the leading indication” for medical marijuana, said Dr. Busse. U.S. reimbursement data suggest up to 65% of individuals get cannabis to treat a listed indication for chronic pain.
He said he hopes this new study will increase interest in funding new trials “so we can have better evidence to guide practice to help patients make decisions.”
The study received support from the National Institute on Drug Abuse. Dr. Bicket reported receiving personal fees from Axial Healthcare as well as grants from the National Institutes of Health, the Centers for Disease Control and Prevention, Michigan Department of Health and Human Services, Arnold Foundation, and the Patient-Centered Outcomes Research Institute outside the submitted work. Dr. Busse reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Insights From the 2020-2021 Dermatology Residency Match
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.
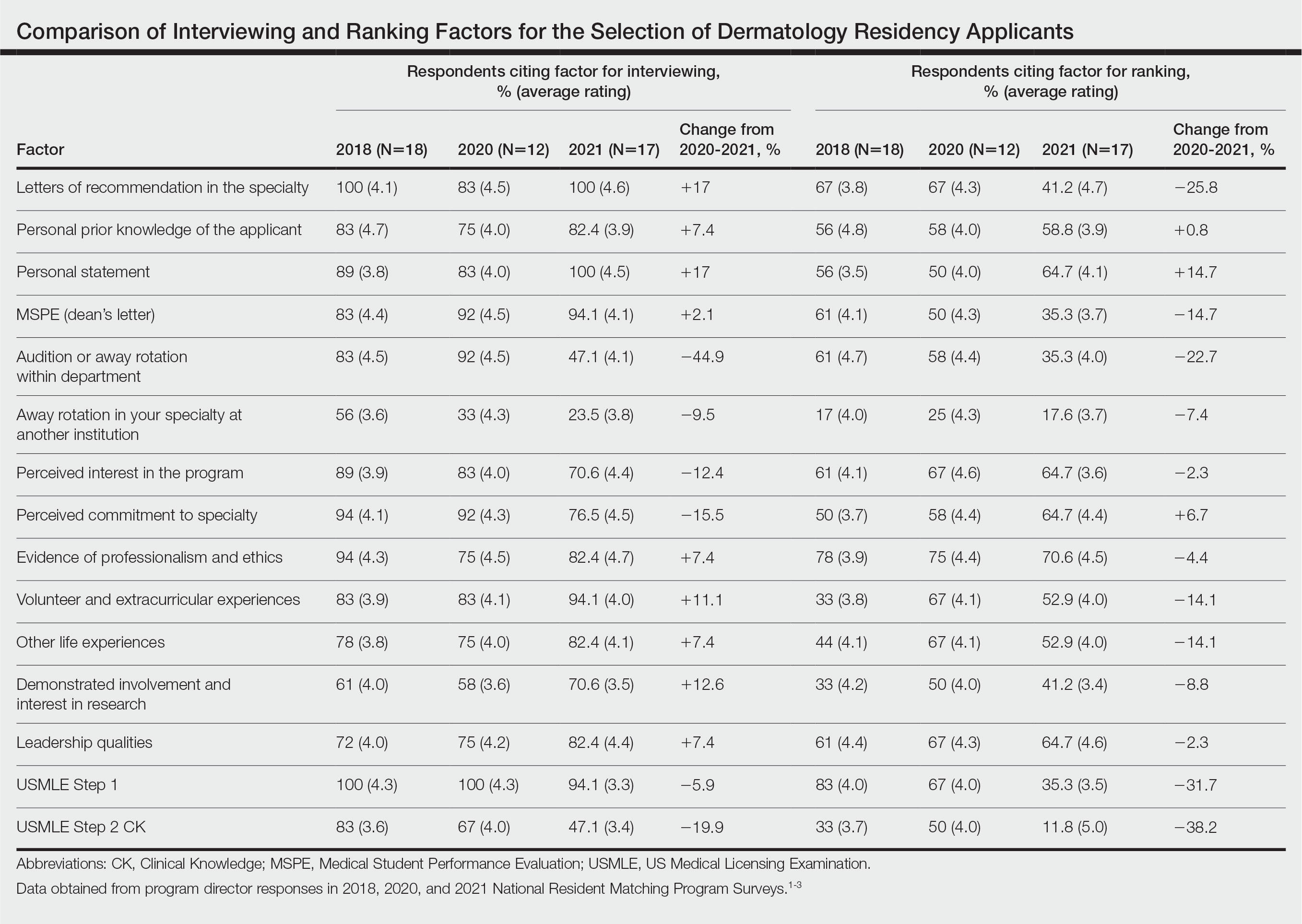
We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
PRACTICE POINTS
- Although there have been numerous changes to the dermatology interview process due to the COVID-19 pandemic, the overall fill rate for postgraduate year 2 positions remained unchanged from 2018 (prepandemic) to 2021 (postpandemic).
- Strategies to accommodate new safety recommendations for interviews may reduce the financial burden (approximately $10,000 for each senior applicant) and time constraints on applicants. These strategies should be considered for implementation in future cycles.
Immune checkpoint inhibitor–related gastrointestinal adverse events
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10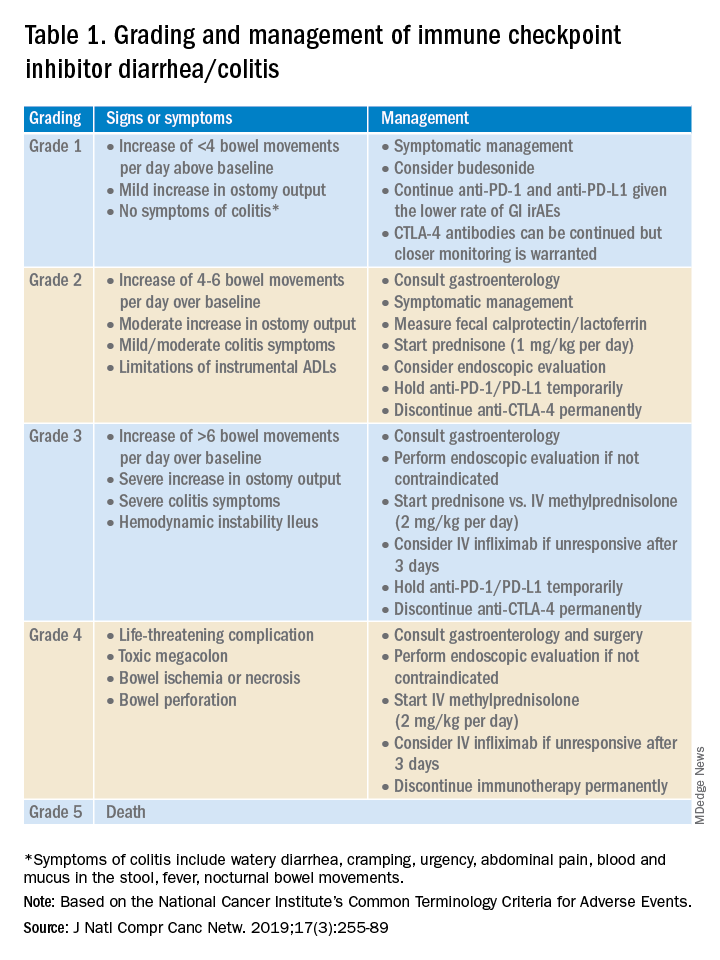
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.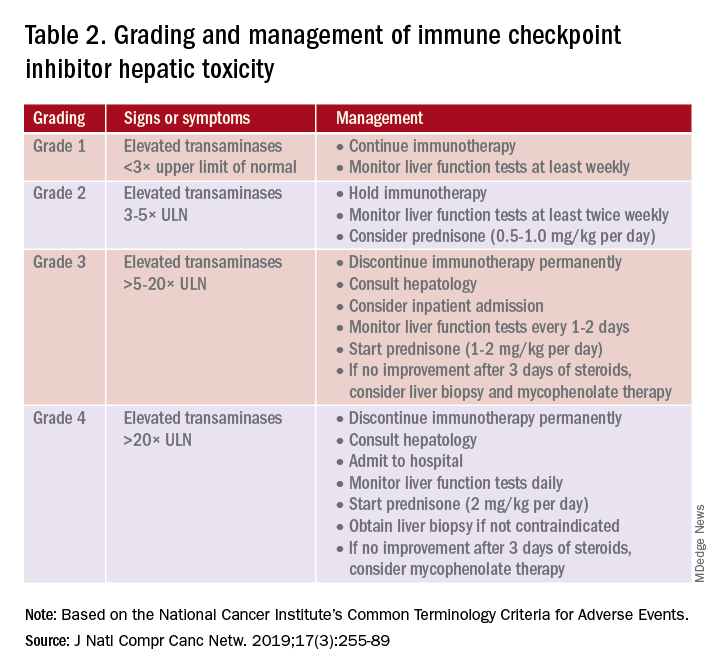
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14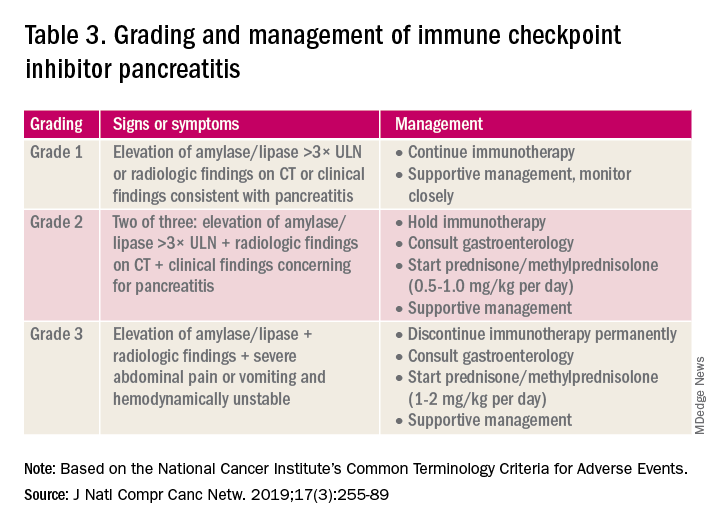
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.
New guidelines on peds obesity call for aggressive treatment
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
Screen all patients for cannabis use before surgery: Guideline
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
FROM REGIONAL ANETHESIA AND MEDICINE
Cancer clinics begin to accommodate patients demanding new cancer detection tests
Doug Flora, MD, knows the value of early cancer detection because it helped him survive kidney cancer 5 years ago. But as a medical oncologist and hematologist, and the executive medical director of oncology services at St. Elizabeth Healthcare in Edgewood, Ky., he also knows that a new era of early cancer detection testing poses big challenges for his network of six hospitals and 169 specialty and primary care offices throughout Kentucky, Ohio, and Indiana.
Multicancer early detection (MCED) tests are finally a reality and could be a potential game changer because they can screen for the possibility of up to 50 different cancers in asymptomatic individuals with one blood draw. They represent one of the fastest growing segments in medical diagnostics with a projected value of $2.77 billion by 2030, according to the market research firm Grand View Research.
These tests are different from traditional liquid biopsies, which are designed to identify actionable gene mutations to help inform treatment decisions of patients already diagnosed with cancer. Instead, MCED tests work to detect fragments of circulating free DNA that have been shed by tumors and released into the bloodstream. Detecting these cancer signals could indicate that an individual has cancer well before they ever develop symptoms.
For some cancer types, particularly those commonly diagnosed at advanced stages or those without general population screening tests, MCED testing could have a significant impact.
In its new report, Grand View Research highlights nine “prominent players” active in the MCED market; of these, two have been granted breakthrough device designation by the Food and Drug Administration: OverC MCDBT by Burning Rock on Jan. 3, 2023, and Galleri by Grail in 2019. Galleri was launched in June 2021 and can be obtained with a prescription at a cost of $949.
Yet, while patients are asking for these tests and primary care physicians are prescribing them, oncologists are grappling with how to manage the first patients whose tests tell them they may have cancer.
Ordering the tests may seem straightforward, but in reality, it is not. In fact, they are so new that most health systems have no internal guidelines for physicians. Guidelines would address when the tests should be prescribed, and whether a patient should undergo more testing or be referred to an oncologist.
Clinical trials underway
There are currently at least 17 clinical trials underway to investigate the performance and clinical utility of MCED tests. Six of these involve Grail, including NHS-Galleri, the largest study to date of 140,000 participants in the United Kingdom where participants will be followed for 3 years with annual visits at 12 and 24 months. And, the National Cancer Institute is spearheading a clinical trial of its own, according to a search of ClinicalTrials.gov.
In September 2022, Grail presented findings from its pivotal PATHFINDER study at the annual meeting of the European Society of Medical Oncology. Researchers reported that cancer signals were detected in 1.4% (92) of 6,621 participants enrolled in the study. Of the 92, 35 people were diagnosed with 36 cancers: 19 were solid tumors (2 oropharyngeal, 5 breast, l liver, 1 intrahepatic bile duct, 2 colon/rectum, 2 prostate, 1 lung, 1 pancreas, 1 small intestine, 1 uterus, 1 ovary and 1 bone) and 17 hematologic cancers (1 plasma cell myeloma/disorders, 2 lymphoid leukemia, 2 Waldenström’s macroglobulinemia, and 12 lymphoma).
Almost half of newly diagnosed cases were cancers in stage 1 or 2. Of stage 1 cancers, three were solid tumors and four were hematologic cancers. Of stage 2 cancers, three were solid tumors and four were hematologic cancers. All other cancers were in stage 3 and 4 or were listed as recurrent or no stage. Deb Schrag, MD, MPH, chair of the department of medicine at Memorial Sloan Kettering Cancer Center in New York, who presented the results from PATHFINDER at ESMO, reported that, of all diagnosed cancers, only breast, colon/rectum, prostate, and lung have established screening protocols.
The findings were so striking that the meeting scientific co-chair, Fabrice André, MD, PhD, told ESMO the oncology field must prepare for an onslaught of new patients.
“Within the next 5 years, we will need more doctors, surgeons and nurses with more diagnostic and treatment infrastructures to care for the rising number of people who will be identified by multicancer early detection tests,” said Dr. André, who is director of research at Gustave Roussy Cancer Center, Villejuif, France, and future president of ESMO (2025-2026). “We need to involve all stakeholders in deciding new pathways of care. We need to agree who will be tested and when and where tests will be carried out, and to anticipate the changes that will happen as a result of these tests.”
But first, he urged, the need for comparative trials “across all types of cancer to find out if having an early detection test affects morbidity and mortality. We also need to know how the tests benefit patients, and how to discuss the results with them,” Dr. André said.
Demand may burden health systems
Dr. Flora suggested that companies like Grail are rushing their product to market without conducting long-term sizable clinical trials.
“These diagnostic companies are a billion dollar publicly traded or venture capital-funded companies that are losing millions of dollars a quarter as they’re scaling up these tests. So, there is some pressure on the sales forces ... to start moving product long before the science has met our lowest areas for entry,” Dr. Flora said. “They are aggressively marketing to a primary care audience that knows nothing about MCEDs. It’s a sales-driven development solving a problem we all believe is real, but we don’t know if it actually solves the problem.”
There are many unanswered questions, he said. Among these include whether the tests do indeed extend survival. “What they’re suggesting – that is if the blood test detects it – that we’re going to save your life. That’s not yet been proven. This is where the providers are pushing back against these industry types to say: ‘This is the wild west right now.’ It’s very irresponsible to go out there and try to sell hundreds of millions of dollars of product to doctors who have never studied genetics,” Dr. Flora said.
Grail’s chief medical officer Jeff Venstrom, MD, however, said physicians don’t need a background in genetic testing to order or interpret Galleri because it’s not a genetic test. Genetic tests look for genetic variants associated with cancer risk, which Galleri does not. MCED tests rely on genomic profiling to identify alterations in tumors.
“Maybe there’s still confusion in the market, which is common for new technologies when they’re initially launched. This is not a 23andMe test. We do not report germline mutations that have implications for cancer risk. We’re using this blood sample to test for the presence or absence of a cancer signal. The test result is very clear and simple: One area of the report says ‘yes’ or ‘no.’ It is a binary result that says if a signal is detected or not. The second provides additional information around where that signal could be coming from,” he said.
Galleri could fill a huge unmet need in cancer prevention, Dr. Venstrom said. Not only could it detect cancer at an earlier stage, but it could serve as a screening tool for cancers like pancreatic cancer in which screening is not available.
The test is not intended to replace standard of care screening, he said. The ordering provider should have a conversation with the patient about overall cancer risk. “Are you smoking? What’s your risk of obesity-associated cancers? Do you have a family history of cancer? I think this should all be in the context of a good conversation around preventative care,” he said.
Planning and prep in Boston
In Boston, Aparna Parikh, MD, an oncologist who specializes in gastrointestinal cancers, agreed that MCED testing has forced her team at the Mass General Cancer Center global cancer care program to think outside of the box.
“We’re a major academic center and it’s not easy [because] this is all uncharted territory,” she said. “We all recognize there are more tests coming, and they are here to stay. As a health system, we have to be ready to manage not only the tests, but patient anxieties, and all the complexities that come with it. We just don’t know yet how to best navigate.”
Although Dr. Parikh’s center has set up a working group tasked with organizing an outpatient clinic for patients with positive MCED tests, the current system is haphazard.
“Right now, it gets bounced around between people,” she explained. “Sometimes, patients are getting referred to the oncology team rather than the primary care team to try to sort out where the cancer signal is coming from, that is, if it’s not immediately obvious. No one really knows who should be the right person to own it,” Dr. Parikh said. While the test is supposed to give tissue-specific results, “it’s not perfect” and sometimes imaging and other work-ups are needed to locate the source of the signa.
“A group of four or five oncologists get looped in and then we’re trying to sort it out on a case-by-case basis, but understanding that with more and more tests coming, that kind of ad hoc approach isn’t going to be sufficient. We need a happy medium between the primary care and the disease specific oncologist, someone who can kind of help think through the diagnostic workup until they have a cancer diagnosis to get them to the right place,” Dr. Parikh said.
Dr. Venstrom said Grail is committed to providing support to clinicians in these situations. “We’re doing everything we can with our medical education forums. We have this pretty intense and extensive postpositive suite of resources,” he explained. “Some of our doctors on staff call the ordering provider within 24 hours just to clarify if there are any questions or confusion from the report. For example, if it suggests the signal is coming from the lung, we provide additional support around additional workups.”
Out-of-pocket test may widen disparities in care
With the exception of a few health insurance companies that have committed to covering some of the cost for the test, Galleri is an out-of-pocket expense.
Dr. Venstrom acknowledged that broad insurance coverage for the Galleri test remains a hurdle, although “we’ve secured coverage for a handful of companies of self-insured employers and forward-thinking insurers.” This includes partnerships with Point32Health, and Alignment Health, among others, he said.
There is also growing support among more than 400 cancer organizations for the Multi-Cancer Early Detection Screening Coverage Act to accelerate coverage for Medicare beneficiaries. “We are constantly trying to understand the evidence that’s needed for payors to make sure that we get the broadest access possible for this test,” he said.
The first positive test result
Back at St. Elizabeth Healthcare where they’ve only seen one positive MCED test result thus far, Dr. Flora is more concerned about patients giving informed consent before they even get the test. “When the reps started hammering our primary care doctors, we sent communiques throughout the system saying that we would very much like to regulate this to make sure that before our patients receive accidental harm, that they at least have a conversation with somebody who understands the test,” he explained.
All 15 patients who requested the test at the hospital were first required to discuss the implications with a genetic counselor who is part of the system. “We are really pro–cancer screening,” he said, but added his hospital is “not pumped” about the Galleri test. “We’re being very cautious about overstatements made by sales guys to our primary care doctors, so we’re letting our own precision medicine people handle it.”
There’s a similar system in place at Community Health Network, a nonprofit health system with nine hospitals and 1,300 employee providers throughout Central Indiana. Patrick McGill, MD, a primary care physician and chief analytics officer for the network says they have streamlined patients with positive tests through their high-risk oncology clinic. “They don’t go straight to a medical oncologist which I know some systems are struggling with,” he said. “They get additional testing, whether it’s imaging they might need or other lab testing. We’ve had a few lung positives, and a few leukemia positives which might go straight to medical oncology. I think we had one breast that was positive so she got additional breast imaging.”
Through its foundation, CHN will offer 2,000 tests free of charge. “We decided to take cost off the table with this funding,” Dr. McGill said. “A lot of health systems I talk to are always concerned that insurance doesn’t cover it and it’s cost prohibitive. Is it creating additional disparities because only people who can afford it can get the test?”
Dr. Schrag serves as an uncompensated advisor for Grail. Previously, while with the Dana-Farber Cancer Institute, she received research funding from Grail.
Doug Flora, MD, knows the value of early cancer detection because it helped him survive kidney cancer 5 years ago. But as a medical oncologist and hematologist, and the executive medical director of oncology services at St. Elizabeth Healthcare in Edgewood, Ky., he also knows that a new era of early cancer detection testing poses big challenges for his network of six hospitals and 169 specialty and primary care offices throughout Kentucky, Ohio, and Indiana.
Multicancer early detection (MCED) tests are finally a reality and could be a potential game changer because they can screen for the possibility of up to 50 different cancers in asymptomatic individuals with one blood draw. They represent one of the fastest growing segments in medical diagnostics with a projected value of $2.77 billion by 2030, according to the market research firm Grand View Research.
These tests are different from traditional liquid biopsies, which are designed to identify actionable gene mutations to help inform treatment decisions of patients already diagnosed with cancer. Instead, MCED tests work to detect fragments of circulating free DNA that have been shed by tumors and released into the bloodstream. Detecting these cancer signals could indicate that an individual has cancer well before they ever develop symptoms.
For some cancer types, particularly those commonly diagnosed at advanced stages or those without general population screening tests, MCED testing could have a significant impact.
In its new report, Grand View Research highlights nine “prominent players” active in the MCED market; of these, two have been granted breakthrough device designation by the Food and Drug Administration: OverC MCDBT by Burning Rock on Jan. 3, 2023, and Galleri by Grail in 2019. Galleri was launched in June 2021 and can be obtained with a prescription at a cost of $949.
Yet, while patients are asking for these tests and primary care physicians are prescribing them, oncologists are grappling with how to manage the first patients whose tests tell them they may have cancer.
Ordering the tests may seem straightforward, but in reality, it is not. In fact, they are so new that most health systems have no internal guidelines for physicians. Guidelines would address when the tests should be prescribed, and whether a patient should undergo more testing or be referred to an oncologist.
Clinical trials underway
There are currently at least 17 clinical trials underway to investigate the performance and clinical utility of MCED tests. Six of these involve Grail, including NHS-Galleri, the largest study to date of 140,000 participants in the United Kingdom where participants will be followed for 3 years with annual visits at 12 and 24 months. And, the National Cancer Institute is spearheading a clinical trial of its own, according to a search of ClinicalTrials.gov.
In September 2022, Grail presented findings from its pivotal PATHFINDER study at the annual meeting of the European Society of Medical Oncology. Researchers reported that cancer signals were detected in 1.4% (92) of 6,621 participants enrolled in the study. Of the 92, 35 people were diagnosed with 36 cancers: 19 were solid tumors (2 oropharyngeal, 5 breast, l liver, 1 intrahepatic bile duct, 2 colon/rectum, 2 prostate, 1 lung, 1 pancreas, 1 small intestine, 1 uterus, 1 ovary and 1 bone) and 17 hematologic cancers (1 plasma cell myeloma/disorders, 2 lymphoid leukemia, 2 Waldenström’s macroglobulinemia, and 12 lymphoma).
Almost half of newly diagnosed cases were cancers in stage 1 or 2. Of stage 1 cancers, three were solid tumors and four were hematologic cancers. Of stage 2 cancers, three were solid tumors and four were hematologic cancers. All other cancers were in stage 3 and 4 or were listed as recurrent or no stage. Deb Schrag, MD, MPH, chair of the department of medicine at Memorial Sloan Kettering Cancer Center in New York, who presented the results from PATHFINDER at ESMO, reported that, of all diagnosed cancers, only breast, colon/rectum, prostate, and lung have established screening protocols.
The findings were so striking that the meeting scientific co-chair, Fabrice André, MD, PhD, told ESMO the oncology field must prepare for an onslaught of new patients.
“Within the next 5 years, we will need more doctors, surgeons and nurses with more diagnostic and treatment infrastructures to care for the rising number of people who will be identified by multicancer early detection tests,” said Dr. André, who is director of research at Gustave Roussy Cancer Center, Villejuif, France, and future president of ESMO (2025-2026). “We need to involve all stakeholders in deciding new pathways of care. We need to agree who will be tested and when and where tests will be carried out, and to anticipate the changes that will happen as a result of these tests.”
But first, he urged, the need for comparative trials “across all types of cancer to find out if having an early detection test affects morbidity and mortality. We also need to know how the tests benefit patients, and how to discuss the results with them,” Dr. André said.
Demand may burden health systems
Dr. Flora suggested that companies like Grail are rushing their product to market without conducting long-term sizable clinical trials.
“These diagnostic companies are a billion dollar publicly traded or venture capital-funded companies that are losing millions of dollars a quarter as they’re scaling up these tests. So, there is some pressure on the sales forces ... to start moving product long before the science has met our lowest areas for entry,” Dr. Flora said. “They are aggressively marketing to a primary care audience that knows nothing about MCEDs. It’s a sales-driven development solving a problem we all believe is real, but we don’t know if it actually solves the problem.”
There are many unanswered questions, he said. Among these include whether the tests do indeed extend survival. “What they’re suggesting – that is if the blood test detects it – that we’re going to save your life. That’s not yet been proven. This is where the providers are pushing back against these industry types to say: ‘This is the wild west right now.’ It’s very irresponsible to go out there and try to sell hundreds of millions of dollars of product to doctors who have never studied genetics,” Dr. Flora said.
Grail’s chief medical officer Jeff Venstrom, MD, however, said physicians don’t need a background in genetic testing to order or interpret Galleri because it’s not a genetic test. Genetic tests look for genetic variants associated with cancer risk, which Galleri does not. MCED tests rely on genomic profiling to identify alterations in tumors.
“Maybe there’s still confusion in the market, which is common for new technologies when they’re initially launched. This is not a 23andMe test. We do not report germline mutations that have implications for cancer risk. We’re using this blood sample to test for the presence or absence of a cancer signal. The test result is very clear and simple: One area of the report says ‘yes’ or ‘no.’ It is a binary result that says if a signal is detected or not. The second provides additional information around where that signal could be coming from,” he said.
Galleri could fill a huge unmet need in cancer prevention, Dr. Venstrom said. Not only could it detect cancer at an earlier stage, but it could serve as a screening tool for cancers like pancreatic cancer in which screening is not available.
The test is not intended to replace standard of care screening, he said. The ordering provider should have a conversation with the patient about overall cancer risk. “Are you smoking? What’s your risk of obesity-associated cancers? Do you have a family history of cancer? I think this should all be in the context of a good conversation around preventative care,” he said.
Planning and prep in Boston
In Boston, Aparna Parikh, MD, an oncologist who specializes in gastrointestinal cancers, agreed that MCED testing has forced her team at the Mass General Cancer Center global cancer care program to think outside of the box.
“We’re a major academic center and it’s not easy [because] this is all uncharted territory,” she said. “We all recognize there are more tests coming, and they are here to stay. As a health system, we have to be ready to manage not only the tests, but patient anxieties, and all the complexities that come with it. We just don’t know yet how to best navigate.”
Although Dr. Parikh’s center has set up a working group tasked with organizing an outpatient clinic for patients with positive MCED tests, the current system is haphazard.
“Right now, it gets bounced around between people,” she explained. “Sometimes, patients are getting referred to the oncology team rather than the primary care team to try to sort out where the cancer signal is coming from, that is, if it’s not immediately obvious. No one really knows who should be the right person to own it,” Dr. Parikh said. While the test is supposed to give tissue-specific results, “it’s not perfect” and sometimes imaging and other work-ups are needed to locate the source of the signa.
“A group of four or five oncologists get looped in and then we’re trying to sort it out on a case-by-case basis, but understanding that with more and more tests coming, that kind of ad hoc approach isn’t going to be sufficient. We need a happy medium between the primary care and the disease specific oncologist, someone who can kind of help think through the diagnostic workup until they have a cancer diagnosis to get them to the right place,” Dr. Parikh said.
Dr. Venstrom said Grail is committed to providing support to clinicians in these situations. “We’re doing everything we can with our medical education forums. We have this pretty intense and extensive postpositive suite of resources,” he explained. “Some of our doctors on staff call the ordering provider within 24 hours just to clarify if there are any questions or confusion from the report. For example, if it suggests the signal is coming from the lung, we provide additional support around additional workups.”
Out-of-pocket test may widen disparities in care
With the exception of a few health insurance companies that have committed to covering some of the cost for the test, Galleri is an out-of-pocket expense.
Dr. Venstrom acknowledged that broad insurance coverage for the Galleri test remains a hurdle, although “we’ve secured coverage for a handful of companies of self-insured employers and forward-thinking insurers.” This includes partnerships with Point32Health, and Alignment Health, among others, he said.
There is also growing support among more than 400 cancer organizations for the Multi-Cancer Early Detection Screening Coverage Act to accelerate coverage for Medicare beneficiaries. “We are constantly trying to understand the evidence that’s needed for payors to make sure that we get the broadest access possible for this test,” he said.
The first positive test result
Back at St. Elizabeth Healthcare where they’ve only seen one positive MCED test result thus far, Dr. Flora is more concerned about patients giving informed consent before they even get the test. “When the reps started hammering our primary care doctors, we sent communiques throughout the system saying that we would very much like to regulate this to make sure that before our patients receive accidental harm, that they at least have a conversation with somebody who understands the test,” he explained.
All 15 patients who requested the test at the hospital were first required to discuss the implications with a genetic counselor who is part of the system. “We are really pro–cancer screening,” he said, but added his hospital is “not pumped” about the Galleri test. “We’re being very cautious about overstatements made by sales guys to our primary care doctors, so we’re letting our own precision medicine people handle it.”
There’s a similar system in place at Community Health Network, a nonprofit health system with nine hospitals and 1,300 employee providers throughout Central Indiana. Patrick McGill, MD, a primary care physician and chief analytics officer for the network says they have streamlined patients with positive tests through their high-risk oncology clinic. “They don’t go straight to a medical oncologist which I know some systems are struggling with,” he said. “They get additional testing, whether it’s imaging they might need or other lab testing. We’ve had a few lung positives, and a few leukemia positives which might go straight to medical oncology. I think we had one breast that was positive so she got additional breast imaging.”
Through its foundation, CHN will offer 2,000 tests free of charge. “We decided to take cost off the table with this funding,” Dr. McGill said. “A lot of health systems I talk to are always concerned that insurance doesn’t cover it and it’s cost prohibitive. Is it creating additional disparities because only people who can afford it can get the test?”
Dr. Schrag serves as an uncompensated advisor for Grail. Previously, while with the Dana-Farber Cancer Institute, she received research funding from Grail.
Doug Flora, MD, knows the value of early cancer detection because it helped him survive kidney cancer 5 years ago. But as a medical oncologist and hematologist, and the executive medical director of oncology services at St. Elizabeth Healthcare in Edgewood, Ky., he also knows that a new era of early cancer detection testing poses big challenges for his network of six hospitals and 169 specialty and primary care offices throughout Kentucky, Ohio, and Indiana.
Multicancer early detection (MCED) tests are finally a reality and could be a potential game changer because they can screen for the possibility of up to 50 different cancers in asymptomatic individuals with one blood draw. They represent one of the fastest growing segments in medical diagnostics with a projected value of $2.77 billion by 2030, according to the market research firm Grand View Research.
These tests are different from traditional liquid biopsies, which are designed to identify actionable gene mutations to help inform treatment decisions of patients already diagnosed with cancer. Instead, MCED tests work to detect fragments of circulating free DNA that have been shed by tumors and released into the bloodstream. Detecting these cancer signals could indicate that an individual has cancer well before they ever develop symptoms.
For some cancer types, particularly those commonly diagnosed at advanced stages or those without general population screening tests, MCED testing could have a significant impact.
In its new report, Grand View Research highlights nine “prominent players” active in the MCED market; of these, two have been granted breakthrough device designation by the Food and Drug Administration: OverC MCDBT by Burning Rock on Jan. 3, 2023, and Galleri by Grail in 2019. Galleri was launched in June 2021 and can be obtained with a prescription at a cost of $949.
Yet, while patients are asking for these tests and primary care physicians are prescribing them, oncologists are grappling with how to manage the first patients whose tests tell them they may have cancer.
Ordering the tests may seem straightforward, but in reality, it is not. In fact, they are so new that most health systems have no internal guidelines for physicians. Guidelines would address when the tests should be prescribed, and whether a patient should undergo more testing or be referred to an oncologist.
Clinical trials underway
There are currently at least 17 clinical trials underway to investigate the performance and clinical utility of MCED tests. Six of these involve Grail, including NHS-Galleri, the largest study to date of 140,000 participants in the United Kingdom where participants will be followed for 3 years with annual visits at 12 and 24 months. And, the National Cancer Institute is spearheading a clinical trial of its own, according to a search of ClinicalTrials.gov.
In September 2022, Grail presented findings from its pivotal PATHFINDER study at the annual meeting of the European Society of Medical Oncology. Researchers reported that cancer signals were detected in 1.4% (92) of 6,621 participants enrolled in the study. Of the 92, 35 people were diagnosed with 36 cancers: 19 were solid tumors (2 oropharyngeal, 5 breast, l liver, 1 intrahepatic bile duct, 2 colon/rectum, 2 prostate, 1 lung, 1 pancreas, 1 small intestine, 1 uterus, 1 ovary and 1 bone) and 17 hematologic cancers (1 plasma cell myeloma/disorders, 2 lymphoid leukemia, 2 Waldenström’s macroglobulinemia, and 12 lymphoma).
Almost half of newly diagnosed cases were cancers in stage 1 or 2. Of stage 1 cancers, three were solid tumors and four were hematologic cancers. Of stage 2 cancers, three were solid tumors and four were hematologic cancers. All other cancers were in stage 3 and 4 or were listed as recurrent or no stage. Deb Schrag, MD, MPH, chair of the department of medicine at Memorial Sloan Kettering Cancer Center in New York, who presented the results from PATHFINDER at ESMO, reported that, of all diagnosed cancers, only breast, colon/rectum, prostate, and lung have established screening protocols.
The findings were so striking that the meeting scientific co-chair, Fabrice André, MD, PhD, told ESMO the oncology field must prepare for an onslaught of new patients.
“Within the next 5 years, we will need more doctors, surgeons and nurses with more diagnostic and treatment infrastructures to care for the rising number of people who will be identified by multicancer early detection tests,” said Dr. André, who is director of research at Gustave Roussy Cancer Center, Villejuif, France, and future president of ESMO (2025-2026). “We need to involve all stakeholders in deciding new pathways of care. We need to agree who will be tested and when and where tests will be carried out, and to anticipate the changes that will happen as a result of these tests.”
But first, he urged, the need for comparative trials “across all types of cancer to find out if having an early detection test affects morbidity and mortality. We also need to know how the tests benefit patients, and how to discuss the results with them,” Dr. André said.
Demand may burden health systems
Dr. Flora suggested that companies like Grail are rushing their product to market without conducting long-term sizable clinical trials.
“These diagnostic companies are a billion dollar publicly traded or venture capital-funded companies that are losing millions of dollars a quarter as they’re scaling up these tests. So, there is some pressure on the sales forces ... to start moving product long before the science has met our lowest areas for entry,” Dr. Flora said. “They are aggressively marketing to a primary care audience that knows nothing about MCEDs. It’s a sales-driven development solving a problem we all believe is real, but we don’t know if it actually solves the problem.”
There are many unanswered questions, he said. Among these include whether the tests do indeed extend survival. “What they’re suggesting – that is if the blood test detects it – that we’re going to save your life. That’s not yet been proven. This is where the providers are pushing back against these industry types to say: ‘This is the wild west right now.’ It’s very irresponsible to go out there and try to sell hundreds of millions of dollars of product to doctors who have never studied genetics,” Dr. Flora said.
Grail’s chief medical officer Jeff Venstrom, MD, however, said physicians don’t need a background in genetic testing to order or interpret Galleri because it’s not a genetic test. Genetic tests look for genetic variants associated with cancer risk, which Galleri does not. MCED tests rely on genomic profiling to identify alterations in tumors.
“Maybe there’s still confusion in the market, which is common for new technologies when they’re initially launched. This is not a 23andMe test. We do not report germline mutations that have implications for cancer risk. We’re using this blood sample to test for the presence or absence of a cancer signal. The test result is very clear and simple: One area of the report says ‘yes’ or ‘no.’ It is a binary result that says if a signal is detected or not. The second provides additional information around where that signal could be coming from,” he said.
Galleri could fill a huge unmet need in cancer prevention, Dr. Venstrom said. Not only could it detect cancer at an earlier stage, but it could serve as a screening tool for cancers like pancreatic cancer in which screening is not available.
The test is not intended to replace standard of care screening, he said. The ordering provider should have a conversation with the patient about overall cancer risk. “Are you smoking? What’s your risk of obesity-associated cancers? Do you have a family history of cancer? I think this should all be in the context of a good conversation around preventative care,” he said.
Planning and prep in Boston
In Boston, Aparna Parikh, MD, an oncologist who specializes in gastrointestinal cancers, agreed that MCED testing has forced her team at the Mass General Cancer Center global cancer care program to think outside of the box.
“We’re a major academic center and it’s not easy [because] this is all uncharted territory,” she said. “We all recognize there are more tests coming, and they are here to stay. As a health system, we have to be ready to manage not only the tests, but patient anxieties, and all the complexities that come with it. We just don’t know yet how to best navigate.”
Although Dr. Parikh’s center has set up a working group tasked with organizing an outpatient clinic for patients with positive MCED tests, the current system is haphazard.
“Right now, it gets bounced around between people,” she explained. “Sometimes, patients are getting referred to the oncology team rather than the primary care team to try to sort out where the cancer signal is coming from, that is, if it’s not immediately obvious. No one really knows who should be the right person to own it,” Dr. Parikh said. While the test is supposed to give tissue-specific results, “it’s not perfect” and sometimes imaging and other work-ups are needed to locate the source of the signa.
“A group of four or five oncologists get looped in and then we’re trying to sort it out on a case-by-case basis, but understanding that with more and more tests coming, that kind of ad hoc approach isn’t going to be sufficient. We need a happy medium between the primary care and the disease specific oncologist, someone who can kind of help think through the diagnostic workup until they have a cancer diagnosis to get them to the right place,” Dr. Parikh said.
Dr. Venstrom said Grail is committed to providing support to clinicians in these situations. “We’re doing everything we can with our medical education forums. We have this pretty intense and extensive postpositive suite of resources,” he explained. “Some of our doctors on staff call the ordering provider within 24 hours just to clarify if there are any questions or confusion from the report. For example, if it suggests the signal is coming from the lung, we provide additional support around additional workups.”
Out-of-pocket test may widen disparities in care
With the exception of a few health insurance companies that have committed to covering some of the cost for the test, Galleri is an out-of-pocket expense.
Dr. Venstrom acknowledged that broad insurance coverage for the Galleri test remains a hurdle, although “we’ve secured coverage for a handful of companies of self-insured employers and forward-thinking insurers.” This includes partnerships with Point32Health, and Alignment Health, among others, he said.
There is also growing support among more than 400 cancer organizations for the Multi-Cancer Early Detection Screening Coverage Act to accelerate coverage for Medicare beneficiaries. “We are constantly trying to understand the evidence that’s needed for payors to make sure that we get the broadest access possible for this test,” he said.
The first positive test result
Back at St. Elizabeth Healthcare where they’ve only seen one positive MCED test result thus far, Dr. Flora is more concerned about patients giving informed consent before they even get the test. “When the reps started hammering our primary care doctors, we sent communiques throughout the system saying that we would very much like to regulate this to make sure that before our patients receive accidental harm, that they at least have a conversation with somebody who understands the test,” he explained.
All 15 patients who requested the test at the hospital were first required to discuss the implications with a genetic counselor who is part of the system. “We are really pro–cancer screening,” he said, but added his hospital is “not pumped” about the Galleri test. “We’re being very cautious about overstatements made by sales guys to our primary care doctors, so we’re letting our own precision medicine people handle it.”
There’s a similar system in place at Community Health Network, a nonprofit health system with nine hospitals and 1,300 employee providers throughout Central Indiana. Patrick McGill, MD, a primary care physician and chief analytics officer for the network says they have streamlined patients with positive tests through their high-risk oncology clinic. “They don’t go straight to a medical oncologist which I know some systems are struggling with,” he said. “They get additional testing, whether it’s imaging they might need or other lab testing. We’ve had a few lung positives, and a few leukemia positives which might go straight to medical oncology. I think we had one breast that was positive so she got additional breast imaging.”
Through its foundation, CHN will offer 2,000 tests free of charge. “We decided to take cost off the table with this funding,” Dr. McGill said. “A lot of health systems I talk to are always concerned that insurance doesn’t cover it and it’s cost prohibitive. Is it creating additional disparities because only people who can afford it can get the test?”
Dr. Schrag serves as an uncompensated advisor for Grail. Previously, while with the Dana-Farber Cancer Institute, she received research funding from Grail.