User login
For MD-IQ use only
VA to Provide Services to Veterans With ‘Don’t Ask, Don’t Tell’ Discharges
The US Department of Veterans Affairs (VA) has issued a new policy statement to help ensure that active-duty service members who were discharged for their sexual orientation under the Don’t Ask, Don’t Tell policy will be able to receive full VA benefits.
“[A] great injustice was remedied and a tremendous weight was finally lifted off the shoulders of tens of thousands of dedicated American service members,” President Biden said on September 20 as the country commemorated the 10th anniversary of the repeal of “Don’t Ask, Don’t Tell,” the policy that barred lesbian, gay, bisexual, transgender, and queer (LGBTQ+) service members from serving openly.
Prior to DADT, if active-duty service members spoke out about their sexual orientation, they ran the risk of being hounded, shunned, in some cases assaulted, and discharged. If they kept it a secret, they felt they were living a lie, unable to be their whole selves. More than 100,000 were discharged because of their sexual orientation or gender identity.
Although Don’t Ask, Don’t Tell was a “compromise” law that purposed to protect them, LGBTQ+ service members were still at risk for harassment and abuse. Nor did the law protect them from discharge. Some 14,000 LGBTQ+ service members were discharged while DADT was in effect. And those who received “other than honorable” (OTH) discharges could be excluded from receiving services and benefits.
The 2011 repeal followed a “hard-fought battle,” said a release from the Human Rights Campaign, which led a coalition of members, supporters, elected officials, 70+ organizations, and 20,000 veterans to get the law overturned. HRC staff coordinated grassroots efforts and sent 19 million e-mails to members and supporters, in turn generating an “unprecedented” 625,000 e-mails and 50,000 handwritten letters to members of Congress.
After the repeal, the VA began the long process of inclusion for LGBTQ+ veterans. “At VA, we continuously work not only to meet the needs of LGBTQ+ veterans, but also to address ongoing issues that LGBTQ+ veterans face as a result of the military’s decades-long official policy of homophobia and transphobia,” Kayla Williams, assistant secretary for public affairs in VA’s Office of Public and Intergovernmental Affairs wrote in the Secretary’s blog.
The VA “recognizes that the trauma caused by the military’s decades-long policy of discrimination against LGBTQ+ people cannot be undone in a few short months,” she continued, but the Biden administration and Secretary McDonough are “taking the steps necessary to begin addressing the pain that such policies have created.”
President Biden, in his remarks, noted that as a US Senator, he had supported allowing service members to serve openly and, as Vice President, championed the repeal. He said, “I am honored to be Commander-in-Chief of the strongest and most inclusive military in our nation’s history. Today, our military doesn’t just welcome LGBTQ+ service members—it is led at the highest levels by brave LGBTQ+ veterans, including Under Secretary of the Air Force Gina Ortiz Jones and Assistant Secretary of Defense for Readiness Shawn Skelly, who served under Don’t Ask, Don’t Tell. I was gratified to appoint the first openly gay Senate-confirmed Cabinet member, Secretary Pete Buttigieg, a lieutenant in the U.S. Navy Reserve and Afghanistan veteran who joined the military under the Don’t Ask, Don’t Tell policy. And during my first week in office, I proudly delivered on my pledge to repeal the discriminatory ban on open service by patriotic transgender service members.”
In early September, Rep. Chris Pappas (D-NH), a member of the House Veterans Affairs Committee and Co-Chair of the Equality Caucus, reintroduced the SERVE (Securing the Rights our Veterans Earned) Act. The act, which is co-sponsored by Reps. Mike Levin (D-CA), Kathleen Rice (D-NY), Anthony Brown (D-MD), and Jackie Speier (D-CA), would ensure LGBTQ+ veterans who received an OTH or Entry-Level Separation discharge solely due to sexual orientation or gender identity are afforded the VA benefits they rightfully earned, including access to VA healthcare, and education, burial and memorial services, and home loans. The act includes veterans who were issued “blue discharges” during World War II and veterans discharged under former President Trump’s ban on transgender service members. The legislation has been endorsed by the Congressional LGBTQ+ Equality Caucus and is supported by more than 45 representatives.
There are processes for those veterans through which they can have their discharge papers and separation statuses reviewed and modified, Pappas said in a release, but “it can take months for the changes to take effect, and many of these veterans do not even realize they are eligible.”
Earlier this year, Veterans Affairs Secretary Denis McDonough made it a priority to ensure that LGBTQ+ veterans have the same level of access to VA care and services that all other veterans have. Among other things, he established a task force to examine how VA policies hinder or prohibit access to care and services and remove barriers that transgender veterans face in accessing gender-affirming care.
The VA is also taking steps, Williams noted, to clarify VA policy for veterans who were given OTH discharges based on homosexual conduct, gender identity or HIV status. Under this newly issued guidance, VA adjudicators shall find that all discharged service members whose separation was due to sexual orientation, gender identity or HIV status are considered “Veterans” who may be eligible for VA benefits, like VR&E, home loan guaranty, compensation and pension, health care, homeless program and/or burial benefits, so long as the record does not implicate a statutory or regulatory bar to benefits.
The policy statement does not represent a change in law, as veterans who were discharged under DADT alone have been generally eligible for benefits under current statute and regulation. However, it reiterates what constitutes eligibility for benefits under law. In addition, every Character of Discharge case that is initially considered for denial will also get a second look before that action is taken.
“Given that large numbers of LGBTQ+ veterans who were affected by previous homophobic and transphobic policies have not applied for a discharge upgrade due to the perception that the process could be onerous,” Williams wrote, “we are hopeful that this policy statement encourages more of them to contact VA to determine their eligibility for care and services.”
Today, according to the Human Right Campaign, nearly 6.1% of US military personnel self-identify as LGBTQ. Williams, herself a bisexual veteran, said she chose to present as straight during the push to repeal DADT. “I could talk credibly about how the lack of sufficient Arabic linguists harmed our effectiveness downrange, and my own identity seemed irrelevant. It took many years for me to shed the toxic legacy of having served under DADT and come back out of the closet; I’m proud to recognize this anniversary as my authentic self.”
The US Department of Veterans Affairs (VA) has issued a new policy statement to help ensure that active-duty service members who were discharged for their sexual orientation under the Don’t Ask, Don’t Tell policy will be able to receive full VA benefits.
“[A] great injustice was remedied and a tremendous weight was finally lifted off the shoulders of tens of thousands of dedicated American service members,” President Biden said on September 20 as the country commemorated the 10th anniversary of the repeal of “Don’t Ask, Don’t Tell,” the policy that barred lesbian, gay, bisexual, transgender, and queer (LGBTQ+) service members from serving openly.
Prior to DADT, if active-duty service members spoke out about their sexual orientation, they ran the risk of being hounded, shunned, in some cases assaulted, and discharged. If they kept it a secret, they felt they were living a lie, unable to be their whole selves. More than 100,000 were discharged because of their sexual orientation or gender identity.
Although Don’t Ask, Don’t Tell was a “compromise” law that purposed to protect them, LGBTQ+ service members were still at risk for harassment and abuse. Nor did the law protect them from discharge. Some 14,000 LGBTQ+ service members were discharged while DADT was in effect. And those who received “other than honorable” (OTH) discharges could be excluded from receiving services and benefits.
The 2011 repeal followed a “hard-fought battle,” said a release from the Human Rights Campaign, which led a coalition of members, supporters, elected officials, 70+ organizations, and 20,000 veterans to get the law overturned. HRC staff coordinated grassroots efforts and sent 19 million e-mails to members and supporters, in turn generating an “unprecedented” 625,000 e-mails and 50,000 handwritten letters to members of Congress.
After the repeal, the VA began the long process of inclusion for LGBTQ+ veterans. “At VA, we continuously work not only to meet the needs of LGBTQ+ veterans, but also to address ongoing issues that LGBTQ+ veterans face as a result of the military’s decades-long official policy of homophobia and transphobia,” Kayla Williams, assistant secretary for public affairs in VA’s Office of Public and Intergovernmental Affairs wrote in the Secretary’s blog.
The VA “recognizes that the trauma caused by the military’s decades-long policy of discrimination against LGBTQ+ people cannot be undone in a few short months,” she continued, but the Biden administration and Secretary McDonough are “taking the steps necessary to begin addressing the pain that such policies have created.”
President Biden, in his remarks, noted that as a US Senator, he had supported allowing service members to serve openly and, as Vice President, championed the repeal. He said, “I am honored to be Commander-in-Chief of the strongest and most inclusive military in our nation’s history. Today, our military doesn’t just welcome LGBTQ+ service members—it is led at the highest levels by brave LGBTQ+ veterans, including Under Secretary of the Air Force Gina Ortiz Jones and Assistant Secretary of Defense for Readiness Shawn Skelly, who served under Don’t Ask, Don’t Tell. I was gratified to appoint the first openly gay Senate-confirmed Cabinet member, Secretary Pete Buttigieg, a lieutenant in the U.S. Navy Reserve and Afghanistan veteran who joined the military under the Don’t Ask, Don’t Tell policy. And during my first week in office, I proudly delivered on my pledge to repeal the discriminatory ban on open service by patriotic transgender service members.”
In early September, Rep. Chris Pappas (D-NH), a member of the House Veterans Affairs Committee and Co-Chair of the Equality Caucus, reintroduced the SERVE (Securing the Rights our Veterans Earned) Act. The act, which is co-sponsored by Reps. Mike Levin (D-CA), Kathleen Rice (D-NY), Anthony Brown (D-MD), and Jackie Speier (D-CA), would ensure LGBTQ+ veterans who received an OTH or Entry-Level Separation discharge solely due to sexual orientation or gender identity are afforded the VA benefits they rightfully earned, including access to VA healthcare, and education, burial and memorial services, and home loans. The act includes veterans who were issued “blue discharges” during World War II and veterans discharged under former President Trump’s ban on transgender service members. The legislation has been endorsed by the Congressional LGBTQ+ Equality Caucus and is supported by more than 45 representatives.
There are processes for those veterans through which they can have their discharge papers and separation statuses reviewed and modified, Pappas said in a release, but “it can take months for the changes to take effect, and many of these veterans do not even realize they are eligible.”
Earlier this year, Veterans Affairs Secretary Denis McDonough made it a priority to ensure that LGBTQ+ veterans have the same level of access to VA care and services that all other veterans have. Among other things, he established a task force to examine how VA policies hinder or prohibit access to care and services and remove barriers that transgender veterans face in accessing gender-affirming care.
The VA is also taking steps, Williams noted, to clarify VA policy for veterans who were given OTH discharges based on homosexual conduct, gender identity or HIV status. Under this newly issued guidance, VA adjudicators shall find that all discharged service members whose separation was due to sexual orientation, gender identity or HIV status are considered “Veterans” who may be eligible for VA benefits, like VR&E, home loan guaranty, compensation and pension, health care, homeless program and/or burial benefits, so long as the record does not implicate a statutory or regulatory bar to benefits.
The policy statement does not represent a change in law, as veterans who were discharged under DADT alone have been generally eligible for benefits under current statute and regulation. However, it reiterates what constitutes eligibility for benefits under law. In addition, every Character of Discharge case that is initially considered for denial will also get a second look before that action is taken.
“Given that large numbers of LGBTQ+ veterans who were affected by previous homophobic and transphobic policies have not applied for a discharge upgrade due to the perception that the process could be onerous,” Williams wrote, “we are hopeful that this policy statement encourages more of them to contact VA to determine their eligibility for care and services.”
Today, according to the Human Right Campaign, nearly 6.1% of US military personnel self-identify as LGBTQ. Williams, herself a bisexual veteran, said she chose to present as straight during the push to repeal DADT. “I could talk credibly about how the lack of sufficient Arabic linguists harmed our effectiveness downrange, and my own identity seemed irrelevant. It took many years for me to shed the toxic legacy of having served under DADT and come back out of the closet; I’m proud to recognize this anniversary as my authentic self.”
The US Department of Veterans Affairs (VA) has issued a new policy statement to help ensure that active-duty service members who were discharged for their sexual orientation under the Don’t Ask, Don’t Tell policy will be able to receive full VA benefits.
“[A] great injustice was remedied and a tremendous weight was finally lifted off the shoulders of tens of thousands of dedicated American service members,” President Biden said on September 20 as the country commemorated the 10th anniversary of the repeal of “Don’t Ask, Don’t Tell,” the policy that barred lesbian, gay, bisexual, transgender, and queer (LGBTQ+) service members from serving openly.
Prior to DADT, if active-duty service members spoke out about their sexual orientation, they ran the risk of being hounded, shunned, in some cases assaulted, and discharged. If they kept it a secret, they felt they were living a lie, unable to be their whole selves. More than 100,000 were discharged because of their sexual orientation or gender identity.
Although Don’t Ask, Don’t Tell was a “compromise” law that purposed to protect them, LGBTQ+ service members were still at risk for harassment and abuse. Nor did the law protect them from discharge. Some 14,000 LGBTQ+ service members were discharged while DADT was in effect. And those who received “other than honorable” (OTH) discharges could be excluded from receiving services and benefits.
The 2011 repeal followed a “hard-fought battle,” said a release from the Human Rights Campaign, which led a coalition of members, supporters, elected officials, 70+ organizations, and 20,000 veterans to get the law overturned. HRC staff coordinated grassroots efforts and sent 19 million e-mails to members and supporters, in turn generating an “unprecedented” 625,000 e-mails and 50,000 handwritten letters to members of Congress.
After the repeal, the VA began the long process of inclusion for LGBTQ+ veterans. “At VA, we continuously work not only to meet the needs of LGBTQ+ veterans, but also to address ongoing issues that LGBTQ+ veterans face as a result of the military’s decades-long official policy of homophobia and transphobia,” Kayla Williams, assistant secretary for public affairs in VA’s Office of Public and Intergovernmental Affairs wrote in the Secretary’s blog.
The VA “recognizes that the trauma caused by the military’s decades-long policy of discrimination against LGBTQ+ people cannot be undone in a few short months,” she continued, but the Biden administration and Secretary McDonough are “taking the steps necessary to begin addressing the pain that such policies have created.”
President Biden, in his remarks, noted that as a US Senator, he had supported allowing service members to serve openly and, as Vice President, championed the repeal. He said, “I am honored to be Commander-in-Chief of the strongest and most inclusive military in our nation’s history. Today, our military doesn’t just welcome LGBTQ+ service members—it is led at the highest levels by brave LGBTQ+ veterans, including Under Secretary of the Air Force Gina Ortiz Jones and Assistant Secretary of Defense for Readiness Shawn Skelly, who served under Don’t Ask, Don’t Tell. I was gratified to appoint the first openly gay Senate-confirmed Cabinet member, Secretary Pete Buttigieg, a lieutenant in the U.S. Navy Reserve and Afghanistan veteran who joined the military under the Don’t Ask, Don’t Tell policy. And during my first week in office, I proudly delivered on my pledge to repeal the discriminatory ban on open service by patriotic transgender service members.”
In early September, Rep. Chris Pappas (D-NH), a member of the House Veterans Affairs Committee and Co-Chair of the Equality Caucus, reintroduced the SERVE (Securing the Rights our Veterans Earned) Act. The act, which is co-sponsored by Reps. Mike Levin (D-CA), Kathleen Rice (D-NY), Anthony Brown (D-MD), and Jackie Speier (D-CA), would ensure LGBTQ+ veterans who received an OTH or Entry-Level Separation discharge solely due to sexual orientation or gender identity are afforded the VA benefits they rightfully earned, including access to VA healthcare, and education, burial and memorial services, and home loans. The act includes veterans who were issued “blue discharges” during World War II and veterans discharged under former President Trump’s ban on transgender service members. The legislation has been endorsed by the Congressional LGBTQ+ Equality Caucus and is supported by more than 45 representatives.
There are processes for those veterans through which they can have their discharge papers and separation statuses reviewed and modified, Pappas said in a release, but “it can take months for the changes to take effect, and many of these veterans do not even realize they are eligible.”
Earlier this year, Veterans Affairs Secretary Denis McDonough made it a priority to ensure that LGBTQ+ veterans have the same level of access to VA care and services that all other veterans have. Among other things, he established a task force to examine how VA policies hinder or prohibit access to care and services and remove barriers that transgender veterans face in accessing gender-affirming care.
The VA is also taking steps, Williams noted, to clarify VA policy for veterans who were given OTH discharges based on homosexual conduct, gender identity or HIV status. Under this newly issued guidance, VA adjudicators shall find that all discharged service members whose separation was due to sexual orientation, gender identity or HIV status are considered “Veterans” who may be eligible for VA benefits, like VR&E, home loan guaranty, compensation and pension, health care, homeless program and/or burial benefits, so long as the record does not implicate a statutory or regulatory bar to benefits.
The policy statement does not represent a change in law, as veterans who were discharged under DADT alone have been generally eligible for benefits under current statute and regulation. However, it reiterates what constitutes eligibility for benefits under law. In addition, every Character of Discharge case that is initially considered for denial will also get a second look before that action is taken.
“Given that large numbers of LGBTQ+ veterans who were affected by previous homophobic and transphobic policies have not applied for a discharge upgrade due to the perception that the process could be onerous,” Williams wrote, “we are hopeful that this policy statement encourages more of them to contact VA to determine their eligibility for care and services.”
Today, according to the Human Right Campaign, nearly 6.1% of US military personnel self-identify as LGBTQ. Williams, herself a bisexual veteran, said she chose to present as straight during the push to repeal DADT. “I could talk credibly about how the lack of sufficient Arabic linguists harmed our effectiveness downrange, and my own identity seemed irrelevant. It took many years for me to shed the toxic legacy of having served under DADT and come back out of the closet; I’m proud to recognize this anniversary as my authentic self.”
Gastroenterology Data Trends 2021
Contents Include:
- Digital health in managing GI diseases
- Emergence of live biotherapeutic products for C. difficile and beyond
- AI and machine learning in GI practice
- Eosinophilic esophagitis: Addressing the rise in incidence and treatment options
- Racial and social diversity in GI practice
- The gut-brain connection in IBS
- Managing IBD in the backdrop of COVID-19
- Noncardia gastric cancer risk: Racial/ethnic disparity, gastric precancerous changes, and refractory H. pylori
- Rethinking management of alcohol-associated liver disease: The other fatty liver epidemic
- Endoscopic bariatric and metabolic therapies for weight loss
- The weight loss journey at University of Michigan
Contents Include:
- Digital health in managing GI diseases
- Emergence of live biotherapeutic products for C. difficile and beyond
- AI and machine learning in GI practice
- Eosinophilic esophagitis: Addressing the rise in incidence and treatment options
- Racial and social diversity in GI practice
- The gut-brain connection in IBS
- Managing IBD in the backdrop of COVID-19
- Noncardia gastric cancer risk: Racial/ethnic disparity, gastric precancerous changes, and refractory H. pylori
- Rethinking management of alcohol-associated liver disease: The other fatty liver epidemic
- Endoscopic bariatric and metabolic therapies for weight loss
- The weight loss journey at University of Michigan
Contents Include:
- Digital health in managing GI diseases
- Emergence of live biotherapeutic products for C. difficile and beyond
- AI and machine learning in GI practice
- Eosinophilic esophagitis: Addressing the rise in incidence and treatment options
- Racial and social diversity in GI practice
- The gut-brain connection in IBS
- Managing IBD in the backdrop of COVID-19
- Noncardia gastric cancer risk: Racial/ethnic disparity, gastric precancerous changes, and refractory H. pylori
- Rethinking management of alcohol-associated liver disease: The other fatty liver epidemic
- Endoscopic bariatric and metabolic therapies for weight loss
- The weight loss journey at University of Michigan
Outreach Finds Veterans Unaware of Service Connection
The US Department of Veterans Affairs (VA) Northeast Ohio Healthcare System has been eaching out directly by postal mail to hundreds of veterans with cancer who may have been exposed to Agent Orange or contaminated water at Camp Lejeune in North Carolina. Advocates say they’ve connected dozens to “service-connected” benefits that pay for 100% of the veterans’ care and can potentially provide support to their spouses after they pass away.
The details and outcomes of the outreach project were presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and in person in Denver, Colorado, from September 24 to September 26, 2021.
“Once you get a devastating diagnosis like cancer, you’ve got enough going on in your head. You shouldn’t have to worry about what the next step is in the benefit process,” said VA Northeast Ohio Healthcare System outreach coordinator Willie J. Berry in an interview. “We want you to focus on your care and not have to worry about anything else.”
Agent Orange, made up of 2,3,7,8-tetrachlorodibenzo-p-dioxin, was used to defoliate forests and kill crops during the Vietnam War. Through “100% service connection” the VA fully covers benefits for certain cancers and other diseases for veterans who are considered to have been exposed to Agent Orange in Vietnam and elsewhere.
Veterans do not need to pay copays in these cases, Berry said, and care outside the VA may be fully funded once arrangements are made.
The VA also fully covers benefits for a similar list of diseases, also including some types of cancer, for veterans who are considered to have been exposed to a contaminated water supply at Camp Lejeune in the early 1980s.
Vietnam War veterans may not be aware of the Agent Orange benefits due to a negative perception of the VA, Berry said. “They were treated poorly [by the VA] and didn’t want to have anything to do with it.”
In the first phase of the project, the VA Northeast Ohio Healthcare System tried to reach potentially eligible veterans with both cancer and possible Agent Orange exposure via phone. Seventy veterans were referred to outreach coordinators, and 16 received 100% service connection after 6 months. The latter number later grew to 34.
“The most inefficient thing were doing was calling veterans one by one,” Berry said. “We felt a mailer would be more efficient in order to reach more people.”
For the second phase, in 2021, coordinators sent informational “Dear veteran” mailers to 427 veterans with cancer who may be eligible for special Agent Orange/Camp Lejeune benefits based on their service history.
The Agent Orange letters began this way: “Through a recent medical diagnosis, VA has identified you as possibly being impacted by a change in Agent Orange Exposure legislation.” The letters then list the eligible conditions, which as of 2021 now include bladder cancer, hyperthyroidism and parkinsonism.
The letters also note that “claims often enhance a veteran’s VA compensation and reduce their cost of care. Additionally, if a veteran were to succumb to a diagnosis that they were service connected for, their spouse might be able to receive both VA health care (until the age of Medicare eligibility) as well as financial benefits for the rest of their life.”
If veterans were terminally ill, the application process for the special benefits could be expedited, Berry said. The number of veterans who received 100% service connection in the second phase of the project was not provided.
No study funding is reported. Berry has no disclosures.
The US Department of Veterans Affairs (VA) Northeast Ohio Healthcare System has been eaching out directly by postal mail to hundreds of veterans with cancer who may have been exposed to Agent Orange or contaminated water at Camp Lejeune in North Carolina. Advocates say they’ve connected dozens to “service-connected” benefits that pay for 100% of the veterans’ care and can potentially provide support to their spouses after they pass away.
The details and outcomes of the outreach project were presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and in person in Denver, Colorado, from September 24 to September 26, 2021.
“Once you get a devastating diagnosis like cancer, you’ve got enough going on in your head. You shouldn’t have to worry about what the next step is in the benefit process,” said VA Northeast Ohio Healthcare System outreach coordinator Willie J. Berry in an interview. “We want you to focus on your care and not have to worry about anything else.”
Agent Orange, made up of 2,3,7,8-tetrachlorodibenzo-p-dioxin, was used to defoliate forests and kill crops during the Vietnam War. Through “100% service connection” the VA fully covers benefits for certain cancers and other diseases for veterans who are considered to have been exposed to Agent Orange in Vietnam and elsewhere.
Veterans do not need to pay copays in these cases, Berry said, and care outside the VA may be fully funded once arrangements are made.
The VA also fully covers benefits for a similar list of diseases, also including some types of cancer, for veterans who are considered to have been exposed to a contaminated water supply at Camp Lejeune in the early 1980s.
Vietnam War veterans may not be aware of the Agent Orange benefits due to a negative perception of the VA, Berry said. “They were treated poorly [by the VA] and didn’t want to have anything to do with it.”
In the first phase of the project, the VA Northeast Ohio Healthcare System tried to reach potentially eligible veterans with both cancer and possible Agent Orange exposure via phone. Seventy veterans were referred to outreach coordinators, and 16 received 100% service connection after 6 months. The latter number later grew to 34.
“The most inefficient thing were doing was calling veterans one by one,” Berry said. “We felt a mailer would be more efficient in order to reach more people.”
For the second phase, in 2021, coordinators sent informational “Dear veteran” mailers to 427 veterans with cancer who may be eligible for special Agent Orange/Camp Lejeune benefits based on their service history.
The Agent Orange letters began this way: “Through a recent medical diagnosis, VA has identified you as possibly being impacted by a change in Agent Orange Exposure legislation.” The letters then list the eligible conditions, which as of 2021 now include bladder cancer, hyperthyroidism and parkinsonism.
The letters also note that “claims often enhance a veteran’s VA compensation and reduce their cost of care. Additionally, if a veteran were to succumb to a diagnosis that they were service connected for, their spouse might be able to receive both VA health care (until the age of Medicare eligibility) as well as financial benefits for the rest of their life.”
If veterans were terminally ill, the application process for the special benefits could be expedited, Berry said. The number of veterans who received 100% service connection in the second phase of the project was not provided.
No study funding is reported. Berry has no disclosures.
The US Department of Veterans Affairs (VA) Northeast Ohio Healthcare System has been eaching out directly by postal mail to hundreds of veterans with cancer who may have been exposed to Agent Orange or contaminated water at Camp Lejeune in North Carolina. Advocates say they’ve connected dozens to “service-connected” benefits that pay for 100% of the veterans’ care and can potentially provide support to their spouses after they pass away.
The details and outcomes of the outreach project were presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and in person in Denver, Colorado, from September 24 to September 26, 2021.
“Once you get a devastating diagnosis like cancer, you’ve got enough going on in your head. You shouldn’t have to worry about what the next step is in the benefit process,” said VA Northeast Ohio Healthcare System outreach coordinator Willie J. Berry in an interview. “We want you to focus on your care and not have to worry about anything else.”
Agent Orange, made up of 2,3,7,8-tetrachlorodibenzo-p-dioxin, was used to defoliate forests and kill crops during the Vietnam War. Through “100% service connection” the VA fully covers benefits for certain cancers and other diseases for veterans who are considered to have been exposed to Agent Orange in Vietnam and elsewhere.
Veterans do not need to pay copays in these cases, Berry said, and care outside the VA may be fully funded once arrangements are made.
The VA also fully covers benefits for a similar list of diseases, also including some types of cancer, for veterans who are considered to have been exposed to a contaminated water supply at Camp Lejeune in the early 1980s.
Vietnam War veterans may not be aware of the Agent Orange benefits due to a negative perception of the VA, Berry said. “They were treated poorly [by the VA] and didn’t want to have anything to do with it.”
In the first phase of the project, the VA Northeast Ohio Healthcare System tried to reach potentially eligible veterans with both cancer and possible Agent Orange exposure via phone. Seventy veterans were referred to outreach coordinators, and 16 received 100% service connection after 6 months. The latter number later grew to 34.
“The most inefficient thing were doing was calling veterans one by one,” Berry said. “We felt a mailer would be more efficient in order to reach more people.”
For the second phase, in 2021, coordinators sent informational “Dear veteran” mailers to 427 veterans with cancer who may be eligible for special Agent Orange/Camp Lejeune benefits based on their service history.
The Agent Orange letters began this way: “Through a recent medical diagnosis, VA has identified you as possibly being impacted by a change in Agent Orange Exposure legislation.” The letters then list the eligible conditions, which as of 2021 now include bladder cancer, hyperthyroidism and parkinsonism.
The letters also note that “claims often enhance a veteran’s VA compensation and reduce their cost of care. Additionally, if a veteran were to succumb to a diagnosis that they were service connected for, their spouse might be able to receive both VA health care (until the age of Medicare eligibility) as well as financial benefits for the rest of their life.”
If veterans were terminally ill, the application process for the special benefits could be expedited, Berry said. The number of veterans who received 100% service connection in the second phase of the project was not provided.
No study funding is reported. Berry has no disclosures.
‘Locker room talk’ about death: Time for oncologists to stop
In a recent inpatient service block, I was seeing patients alongside a resident I had gotten to know well. We were consulted on a patient with metastatic head and neck cancer who had not sought care for over a year.
When the patient presented, his voice was raspy and he could not swallow. He had lost 40 pounds. In addition to his locally advanced disease, his lungs were riddled with metastatic lesions.
When we left the room, the resident and I went to speak to the patient’s primary team, and he began to relay our recommendations.
The first words out of his mouth were, “Well, it’s pretty clear he’s going to die.”
The statement took me aback. I wasn’t alarmed by the accuracy of what he had said. The patient was obviously not doing well, and he ended up dying soon after this visit.
It was more the abrupt manner in which the resident had spoken about death. The brusque phrasing felt atypical coming from the otherwise gentle-hearted trainee. He wasn’t referring to a faceless person. We had just seen the man a few minutes ago and heard his personal struggles. I tried to see if anyone else on the team was caught off guard, but everyone was taking notes or continuing to listen, seemingly undeterred.
Oncologists’ ‘locker room talk’
And now, with the COVID pandemic forcing most of our tumor boards to go virtual, I find this locker room talk comes even more readily; phrases like “this patient is going to die” are often passed around flippantly, as if saying so will help ease the tension. During these interactions, my colleagues and I rarely acknowledge the seriousness of what a patient death will do to their family and loved ones – or what losing a patient whom we’ve known for years may do to our own psyche.
This language can even creep into how we speak with patients. We are often taught to offer prognoses coldly, ensuring that patients have a clear sense of how long they have left and to help inform their treatment choices. And yet, this training does not necessarily align with what patients want and need. For instance, in a recent survey of patients with chronic obstructive pulmonary disease, patients consistently rated physicians poorly at discussing prognosis, what dying might be like, as well as spirituality and religion.
But at the same time, these matter-of-fact statements about death probably help protect us. Death is a routine, inevitable part of an oncologist’s life, and over time, oncology training and practice hardens us to it. During medical school, I remember that a patient dying would trigger immediate reflection, sadness, and conversation with our peers. Now, unless I know a patient well, I find myself rarely reflecting on the patient behind the facts. This evolution is natural for an oncologist: If you don’t develop a tough skin about death, you may become overwhelmed with the frequency of it.
The COVID pandemic has amped our hardness toward death into overdrive. Whether we are in the intensive care unit or simply viewing death rates during the most recent COVID Delta wave, many of us cope by disassociating a face from a name.
Making time for reflection
But taking time to reflect can be therapeutic.
I recently referred a patient with metastatic prostate cancer for a phase 1 trial at an outside institution. He was one of the first patients in my genitourinary malignancies clinic when I started as an attending. The patient had progressed through several lines of therapy and was being referred for an investigational phase 1 therapy. We had discussed hospice referral, and the patient was ready for it if this therapy didn’t work out.
I did not see or hear from the man while he was on the trial. A few months later, however, the principal investigator of the trial called me to let me know the patient had progressed through the agent, suffering from significant urinary obstruction, and he was on hospice. “Unfortunately,” the investigator told me, “he’s not going to live much longer.”
When I checked in with the hospice, the patient had died.
I was surprised again at how matter-of-fact the discussion of death had been. But I was even more surprised by my own reaction. Despite the relationship I had formed with the patient, I did not feel much when I heard he had died. I didn’t have time to process the news in the moment. It was time to move on to the next patient.
It was only later, when I called the patient’s family, that I allowed my emotions to flood in. I told his family how grateful I was to know him, how strong he’d been. The patient’s family and I talked about the human, not his passing. It felt good.
Abandoning locker room talk
So how do we change how we talk about death? I don’t think the answer is massive educational programs or passing responsibility for advance care planning onto palliative care specialists. The change needs to be driven by individual oncologists. We can call out discussions of death that make us uncomfortable, gently reminding each other that we’re talking about a human life.
We can learn from our palliative care colleagues; their conversations about death routinely include a patient’s support system and personal stories. Palliative care doctors always refer to the patient by name, which helps humanize the person behind the chart.
We can emphasize a feeling of hope, a sentiment that may also be therapeutic to our patients. Even when a patient is dying, there is always something to be done. We can comfort their family, explaining what brought us to this point and how sorry we are that this is happening. We can provide options for symptom control and help patients manage those symptoms.
And we can allow ourselves to talk about how much a death affects us. We can acknowledge how much it sucks that a patient is going to die, how challenging that will be to his/her family, and how we wish it could have ended differently.
Subtle changes like these will improve our own ability to process and discuss death and will ultimately lead to better relationships with our patients. But it starts with eliminating the “locker room talk” of how we discuss death.
Ravi B. Parikh, MD, MPP, is a medical oncologist and faculty member at the University of Pennsylvania and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care (C-TAC). He has served as a director, officer, partner, employee, adviser, consultant, or trustee for GNS Healthcare, Nanology, and Cancer Study Group, and he has received research grant from Embedded Healthcare, Veterans Administration, PCF, National Palliative Care Research Center, and MUSC. A version of this article first appeared on Medscape.com.
In a recent inpatient service block, I was seeing patients alongside a resident I had gotten to know well. We were consulted on a patient with metastatic head and neck cancer who had not sought care for over a year.
When the patient presented, his voice was raspy and he could not swallow. He had lost 40 pounds. In addition to his locally advanced disease, his lungs were riddled with metastatic lesions.
When we left the room, the resident and I went to speak to the patient’s primary team, and he began to relay our recommendations.
The first words out of his mouth were, “Well, it’s pretty clear he’s going to die.”
The statement took me aback. I wasn’t alarmed by the accuracy of what he had said. The patient was obviously not doing well, and he ended up dying soon after this visit.
It was more the abrupt manner in which the resident had spoken about death. The brusque phrasing felt atypical coming from the otherwise gentle-hearted trainee. He wasn’t referring to a faceless person. We had just seen the man a few minutes ago and heard his personal struggles. I tried to see if anyone else on the team was caught off guard, but everyone was taking notes or continuing to listen, seemingly undeterred.
Oncologists’ ‘locker room talk’
And now, with the COVID pandemic forcing most of our tumor boards to go virtual, I find this locker room talk comes even more readily; phrases like “this patient is going to die” are often passed around flippantly, as if saying so will help ease the tension. During these interactions, my colleagues and I rarely acknowledge the seriousness of what a patient death will do to their family and loved ones – or what losing a patient whom we’ve known for years may do to our own psyche.
This language can even creep into how we speak with patients. We are often taught to offer prognoses coldly, ensuring that patients have a clear sense of how long they have left and to help inform their treatment choices. And yet, this training does not necessarily align with what patients want and need. For instance, in a recent survey of patients with chronic obstructive pulmonary disease, patients consistently rated physicians poorly at discussing prognosis, what dying might be like, as well as spirituality and religion.
But at the same time, these matter-of-fact statements about death probably help protect us. Death is a routine, inevitable part of an oncologist’s life, and over time, oncology training and practice hardens us to it. During medical school, I remember that a patient dying would trigger immediate reflection, sadness, and conversation with our peers. Now, unless I know a patient well, I find myself rarely reflecting on the patient behind the facts. This evolution is natural for an oncologist: If you don’t develop a tough skin about death, you may become overwhelmed with the frequency of it.
The COVID pandemic has amped our hardness toward death into overdrive. Whether we are in the intensive care unit or simply viewing death rates during the most recent COVID Delta wave, many of us cope by disassociating a face from a name.
Making time for reflection
But taking time to reflect can be therapeutic.
I recently referred a patient with metastatic prostate cancer for a phase 1 trial at an outside institution. He was one of the first patients in my genitourinary malignancies clinic when I started as an attending. The patient had progressed through several lines of therapy and was being referred for an investigational phase 1 therapy. We had discussed hospice referral, and the patient was ready for it if this therapy didn’t work out.
I did not see or hear from the man while he was on the trial. A few months later, however, the principal investigator of the trial called me to let me know the patient had progressed through the agent, suffering from significant urinary obstruction, and he was on hospice. “Unfortunately,” the investigator told me, “he’s not going to live much longer.”
When I checked in with the hospice, the patient had died.
I was surprised again at how matter-of-fact the discussion of death had been. But I was even more surprised by my own reaction. Despite the relationship I had formed with the patient, I did not feel much when I heard he had died. I didn’t have time to process the news in the moment. It was time to move on to the next patient.
It was only later, when I called the patient’s family, that I allowed my emotions to flood in. I told his family how grateful I was to know him, how strong he’d been. The patient’s family and I talked about the human, not his passing. It felt good.
Abandoning locker room talk
So how do we change how we talk about death? I don’t think the answer is massive educational programs or passing responsibility for advance care planning onto palliative care specialists. The change needs to be driven by individual oncologists. We can call out discussions of death that make us uncomfortable, gently reminding each other that we’re talking about a human life.
We can learn from our palliative care colleagues; their conversations about death routinely include a patient’s support system and personal stories. Palliative care doctors always refer to the patient by name, which helps humanize the person behind the chart.
We can emphasize a feeling of hope, a sentiment that may also be therapeutic to our patients. Even when a patient is dying, there is always something to be done. We can comfort their family, explaining what brought us to this point and how sorry we are that this is happening. We can provide options for symptom control and help patients manage those symptoms.
And we can allow ourselves to talk about how much a death affects us. We can acknowledge how much it sucks that a patient is going to die, how challenging that will be to his/her family, and how we wish it could have ended differently.
Subtle changes like these will improve our own ability to process and discuss death and will ultimately lead to better relationships with our patients. But it starts with eliminating the “locker room talk” of how we discuss death.
Ravi B. Parikh, MD, MPP, is a medical oncologist and faculty member at the University of Pennsylvania and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care (C-TAC). He has served as a director, officer, partner, employee, adviser, consultant, or trustee for GNS Healthcare, Nanology, and Cancer Study Group, and he has received research grant from Embedded Healthcare, Veterans Administration, PCF, National Palliative Care Research Center, and MUSC. A version of this article first appeared on Medscape.com.
In a recent inpatient service block, I was seeing patients alongside a resident I had gotten to know well. We were consulted on a patient with metastatic head and neck cancer who had not sought care for over a year.
When the patient presented, his voice was raspy and he could not swallow. He had lost 40 pounds. In addition to his locally advanced disease, his lungs were riddled with metastatic lesions.
When we left the room, the resident and I went to speak to the patient’s primary team, and he began to relay our recommendations.
The first words out of his mouth were, “Well, it’s pretty clear he’s going to die.”
The statement took me aback. I wasn’t alarmed by the accuracy of what he had said. The patient was obviously not doing well, and he ended up dying soon after this visit.
It was more the abrupt manner in which the resident had spoken about death. The brusque phrasing felt atypical coming from the otherwise gentle-hearted trainee. He wasn’t referring to a faceless person. We had just seen the man a few minutes ago and heard his personal struggles. I tried to see if anyone else on the team was caught off guard, but everyone was taking notes or continuing to listen, seemingly undeterred.
Oncologists’ ‘locker room talk’
And now, with the COVID pandemic forcing most of our tumor boards to go virtual, I find this locker room talk comes even more readily; phrases like “this patient is going to die” are often passed around flippantly, as if saying so will help ease the tension. During these interactions, my colleagues and I rarely acknowledge the seriousness of what a patient death will do to their family and loved ones – or what losing a patient whom we’ve known for years may do to our own psyche.
This language can even creep into how we speak with patients. We are often taught to offer prognoses coldly, ensuring that patients have a clear sense of how long they have left and to help inform their treatment choices. And yet, this training does not necessarily align with what patients want and need. For instance, in a recent survey of patients with chronic obstructive pulmonary disease, patients consistently rated physicians poorly at discussing prognosis, what dying might be like, as well as spirituality and religion.
But at the same time, these matter-of-fact statements about death probably help protect us. Death is a routine, inevitable part of an oncologist’s life, and over time, oncology training and practice hardens us to it. During medical school, I remember that a patient dying would trigger immediate reflection, sadness, and conversation with our peers. Now, unless I know a patient well, I find myself rarely reflecting on the patient behind the facts. This evolution is natural for an oncologist: If you don’t develop a tough skin about death, you may become overwhelmed with the frequency of it.
The COVID pandemic has amped our hardness toward death into overdrive. Whether we are in the intensive care unit or simply viewing death rates during the most recent COVID Delta wave, many of us cope by disassociating a face from a name.
Making time for reflection
But taking time to reflect can be therapeutic.
I recently referred a patient with metastatic prostate cancer for a phase 1 trial at an outside institution. He was one of the first patients in my genitourinary malignancies clinic when I started as an attending. The patient had progressed through several lines of therapy and was being referred for an investigational phase 1 therapy. We had discussed hospice referral, and the patient was ready for it if this therapy didn’t work out.
I did not see or hear from the man while he was on the trial. A few months later, however, the principal investigator of the trial called me to let me know the patient had progressed through the agent, suffering from significant urinary obstruction, and he was on hospice. “Unfortunately,” the investigator told me, “he’s not going to live much longer.”
When I checked in with the hospice, the patient had died.
I was surprised again at how matter-of-fact the discussion of death had been. But I was even more surprised by my own reaction. Despite the relationship I had formed with the patient, I did not feel much when I heard he had died. I didn’t have time to process the news in the moment. It was time to move on to the next patient.
It was only later, when I called the patient’s family, that I allowed my emotions to flood in. I told his family how grateful I was to know him, how strong he’d been. The patient’s family and I talked about the human, not his passing. It felt good.
Abandoning locker room talk
So how do we change how we talk about death? I don’t think the answer is massive educational programs or passing responsibility for advance care planning onto palliative care specialists. The change needs to be driven by individual oncologists. We can call out discussions of death that make us uncomfortable, gently reminding each other that we’re talking about a human life.
We can learn from our palliative care colleagues; their conversations about death routinely include a patient’s support system and personal stories. Palliative care doctors always refer to the patient by name, which helps humanize the person behind the chart.
We can emphasize a feeling of hope, a sentiment that may also be therapeutic to our patients. Even when a patient is dying, there is always something to be done. We can comfort their family, explaining what brought us to this point and how sorry we are that this is happening. We can provide options for symptom control and help patients manage those symptoms.
And we can allow ourselves to talk about how much a death affects us. We can acknowledge how much it sucks that a patient is going to die, how challenging that will be to his/her family, and how we wish it could have ended differently.
Subtle changes like these will improve our own ability to process and discuss death and will ultimately lead to better relationships with our patients. But it starts with eliminating the “locker room talk” of how we discuss death.
Ravi B. Parikh, MD, MPP, is a medical oncologist and faculty member at the University of Pennsylvania and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care (C-TAC). He has served as a director, officer, partner, employee, adviser, consultant, or trustee for GNS Healthcare, Nanology, and Cancer Study Group, and he has received research grant from Embedded Healthcare, Veterans Administration, PCF, National Palliative Care Research Center, and MUSC. A version of this article first appeared on Medscape.com.
Standing up to ‘injustice in health’: The Association of Black Gastroenterologists and Hepatologists
“Of all the forms of inequality, injustice in health is the most shocking and inhuman.” – Martin Luther King Jr., March 25, 1966. 1
This single disparity – health care injustice – too often results in needless mental anguish, physical suffering, or death. In the spring of 2020, at the peak of the COVID-19 pandemic, the convergence of injustices in health care and policing led to the disproportionate preventable physical deaths of Black men and women. This became the watershed moment for 11 gastroenterologists and hepatologists who collectively grieved but heeded the call of social responsibility to form the Association of Black Gastroenterologists and Hepatologists.
The mission of ABGH is laser focused. It is to promote health equity in Black communities, advance science, and develop the careers of Black gastroenterologists, hepatologists, and scientists. The vision is to improve gastrointestinal health outcomes in Black communities; to develop the pipeline of Black gastroenterologists and hepatologists given that currently only 4% in the United States identify as Black; to foster networking, mentoring, and sponsorship among Black students, clinical trainees, gastroenterologists, and hepatologists; and to promote the scholarship of Black gastroenterologists and hepatologists.
Through community engagement, ABGH stands to empower the Black community with knowledge and choices, which inherently strengthens the physician-patient relationship. ABGH also exists to implement positive change in long term outcome statistics in Black communities. Black Americans are 20% more likely to be diagnosed with colorectal cancer and 40% more likely to die from the disease. In addition to colorectal cancer, rates of esophageal squamous cancer, as well as cancer of the small bowel and pancreas, are highest in Black people.2 Through scientific research and clinical care, we aim to eradicate digestive health disparities.
Yet in this space, we know first-hand that, in the United States, the wellness of a community is not measured by the medical fitness of its members alone but also by the availability of equitable opportunities for fulfillment of nonmedical but health-impacting social needs. These needs, also known as social determinants of health, are made inaccessible to vulnerable populations because of systemic racism. Importantly, we recognize that dismantling racist systems is not a singular effort, nor are we pioneers in this work, but we look forward to executing health equity goals collaboratively with our fellow gastrointestinal national societies and other leading community and grassroots organizations.
The founders of ABGH are a distinctive group of practicing gastroenterologists and hepatologists from across the United States with a strong track record in DEI work through their community, clinical, and research activities. The board of directors reflects only the depth of talent shared throughout the ABGH membership. The strength of the organization lies in its diverse and energetic constituents who all exemplify outstanding training and the readiness to redefine the standard of health care delivery to the Black community. From medical students to senior level gastroenterologists, we collectively embody a considerable momentum for formation of this organization at this point in our history.
ABGH fulfills a professional career development need for budding gastroenterologists not so readily available from other organizations. The compelling impact of representing the embodiment of what many of us were told we could not become is limitless. The personal and professional growth enabled by our networking and learning from each other is both motivating and empowering since, even after overcoming the obstacles needed to become a medical provider, Black professionals are often not afforded the bandwidth, range of emotion, and protection to reveal their specific needs. For this author personally, the ABGH provides a psychological safety that allows authentic self-identity without code-switching.3 Through this authenticity has arisen formidable strength, creativity, and productivity. The leadership and innovation cultivated in ABGH stands to benefit many generations to come, both within and outside the organization.
Reflections from a junior member of ABGH: Dr. Kafayat Busari
My desire to pursue gastroenterology was bolstered by determination, curiosity, and passion, yet ironically was often met with skepticism by many in position to help advance this goal. Although projections of incertitude on members of a community that are often made to feel inadequate can diminish even the brightest of lights, conversely it can fuel the creation of an organization emboldened to specifically address GI-related health disparities. When I was a second-year internal medicine resident, I encountered a GI physician who told me GI “wasn’t something I wanted to do”—despite me expressing my interest.
Confused by the statement, I reached out to Renee L. Williams, MD, of NYU Langone Health, who I had met during my medical school training. She suggested I join a conference call later that week. On that call and the many that took place thereafter, I was introduced to Black gastroenterologists who are luminal disease experts, chair members, journal editors, transplant hepatologists, interventional endoscopists, researchers, and professors (in other words, GI professional leaders). My time on the initial call lasted perhaps less than 20 minutes, but the impact has been immeasurable.
I was provided the emotional reassurance that GI was indeed for me and told “there’s always a seat, and if it feels like there’s not, we just need to get more chairs.” Little did I know, but those metaphorical chairs were being gathered so that I and other aspiring gastroenterologists will be able to sit comfortably at these tables one day. I was witnessing these GI professional leaders set in motion the beginning of what will undoubtedly be a pivotal component in the way I approach my career as a gastroenterologist. The experience reignited my mental determination to one day attain the level of success represented by the ABGH board members and to persevere in my quest to help redefine how Black medical students and residents serve their communities as physicians.
The creation of the ABGH could not have come at a better time in my training. In the wake of recent public protests for equity of African Americans within every institution (academia, housing, banks, policing, health care, and beyond), which were fundamentally built on racism, being a junior member of ABGH has not only given me a platform to speak my truth but has also provided me with tools to help others do so as well. As someone very passionate about research, primarily in colorectal cancer, I have been given an opportunity to connect with a dream team of mentors who have taken research ideas to new levels and have challenged me to dig deeper and expand my curiosity to investigate what still needs to be uncovered. It has created opportunity after opportunity for actively building relationships, leading to meaningful collaborations and the sharing of innovative ideas and discoveries.
It is important to emphasize that ABGH is not an organization wanting to exclude themselves on the basis of ethnicity. ABGH is an example of how shared health goals within a medical discipline can be achieved when inclusion and equity is at the helm. ABGH led and represented events that raise awareness of diseases affecting all patients and aim to make the GI community more culturally competent. ABGH is future-oriented and embraces all members who align with the mission regardless of ethnicity, gender, orientation, or disability. The institution that is and will be the ABGH impresses upon me a feeling of excitement, gratitude, and humility. I look forward to continuing the mission created by the founding members and being to others what ABGH is to me.
For more information on this organization, please visit blackingastro.org.
Dr. Busari is a resident physician at Florida State University-SMH and a junior member of ABGH. Dr. Guillaume director of the Gastrointestinal Motility Center at Stony Brook (New York) University Hospital and an assistant professor of medicine at the Renaissance School of Medicine at Stony Brook University. They have no disclosures.
References
1. Galarneau C. J Health Care Poor Underserved. 2018;29(1):5-8.
2. Ashktorab H et al. Gastroenterology. 2017 Oct;153(4):910-923.
3. Blanchard AK. N Engl J Med. 2021 Jun 10;384(23):e87.
“Of all the forms of inequality, injustice in health is the most shocking and inhuman.” – Martin Luther King Jr., March 25, 1966. 1
This single disparity – health care injustice – too often results in needless mental anguish, physical suffering, or death. In the spring of 2020, at the peak of the COVID-19 pandemic, the convergence of injustices in health care and policing led to the disproportionate preventable physical deaths of Black men and women. This became the watershed moment for 11 gastroenterologists and hepatologists who collectively grieved but heeded the call of social responsibility to form the Association of Black Gastroenterologists and Hepatologists.
The mission of ABGH is laser focused. It is to promote health equity in Black communities, advance science, and develop the careers of Black gastroenterologists, hepatologists, and scientists. The vision is to improve gastrointestinal health outcomes in Black communities; to develop the pipeline of Black gastroenterologists and hepatologists given that currently only 4% in the United States identify as Black; to foster networking, mentoring, and sponsorship among Black students, clinical trainees, gastroenterologists, and hepatologists; and to promote the scholarship of Black gastroenterologists and hepatologists.
Through community engagement, ABGH stands to empower the Black community with knowledge and choices, which inherently strengthens the physician-patient relationship. ABGH also exists to implement positive change in long term outcome statistics in Black communities. Black Americans are 20% more likely to be diagnosed with colorectal cancer and 40% more likely to die from the disease. In addition to colorectal cancer, rates of esophageal squamous cancer, as well as cancer of the small bowel and pancreas, are highest in Black people.2 Through scientific research and clinical care, we aim to eradicate digestive health disparities.
Yet in this space, we know first-hand that, in the United States, the wellness of a community is not measured by the medical fitness of its members alone but also by the availability of equitable opportunities for fulfillment of nonmedical but health-impacting social needs. These needs, also known as social determinants of health, are made inaccessible to vulnerable populations because of systemic racism. Importantly, we recognize that dismantling racist systems is not a singular effort, nor are we pioneers in this work, but we look forward to executing health equity goals collaboratively with our fellow gastrointestinal national societies and other leading community and grassroots organizations.
The founders of ABGH are a distinctive group of practicing gastroenterologists and hepatologists from across the United States with a strong track record in DEI work through their community, clinical, and research activities. The board of directors reflects only the depth of talent shared throughout the ABGH membership. The strength of the organization lies in its diverse and energetic constituents who all exemplify outstanding training and the readiness to redefine the standard of health care delivery to the Black community. From medical students to senior level gastroenterologists, we collectively embody a considerable momentum for formation of this organization at this point in our history.
ABGH fulfills a professional career development need for budding gastroenterologists not so readily available from other organizations. The compelling impact of representing the embodiment of what many of us were told we could not become is limitless. The personal and professional growth enabled by our networking and learning from each other is both motivating and empowering since, even after overcoming the obstacles needed to become a medical provider, Black professionals are often not afforded the bandwidth, range of emotion, and protection to reveal their specific needs. For this author personally, the ABGH provides a psychological safety that allows authentic self-identity without code-switching.3 Through this authenticity has arisen formidable strength, creativity, and productivity. The leadership and innovation cultivated in ABGH stands to benefit many generations to come, both within and outside the organization.
Reflections from a junior member of ABGH: Dr. Kafayat Busari
My desire to pursue gastroenterology was bolstered by determination, curiosity, and passion, yet ironically was often met with skepticism by many in position to help advance this goal. Although projections of incertitude on members of a community that are often made to feel inadequate can diminish even the brightest of lights, conversely it can fuel the creation of an organization emboldened to specifically address GI-related health disparities. When I was a second-year internal medicine resident, I encountered a GI physician who told me GI “wasn’t something I wanted to do”—despite me expressing my interest.
Confused by the statement, I reached out to Renee L. Williams, MD, of NYU Langone Health, who I had met during my medical school training. She suggested I join a conference call later that week. On that call and the many that took place thereafter, I was introduced to Black gastroenterologists who are luminal disease experts, chair members, journal editors, transplant hepatologists, interventional endoscopists, researchers, and professors (in other words, GI professional leaders). My time on the initial call lasted perhaps less than 20 minutes, but the impact has been immeasurable.
I was provided the emotional reassurance that GI was indeed for me and told “there’s always a seat, and if it feels like there’s not, we just need to get more chairs.” Little did I know, but those metaphorical chairs were being gathered so that I and other aspiring gastroenterologists will be able to sit comfortably at these tables one day. I was witnessing these GI professional leaders set in motion the beginning of what will undoubtedly be a pivotal component in the way I approach my career as a gastroenterologist. The experience reignited my mental determination to one day attain the level of success represented by the ABGH board members and to persevere in my quest to help redefine how Black medical students and residents serve their communities as physicians.
The creation of the ABGH could not have come at a better time in my training. In the wake of recent public protests for equity of African Americans within every institution (academia, housing, banks, policing, health care, and beyond), which were fundamentally built on racism, being a junior member of ABGH has not only given me a platform to speak my truth but has also provided me with tools to help others do so as well. As someone very passionate about research, primarily in colorectal cancer, I have been given an opportunity to connect with a dream team of mentors who have taken research ideas to new levels and have challenged me to dig deeper and expand my curiosity to investigate what still needs to be uncovered. It has created opportunity after opportunity for actively building relationships, leading to meaningful collaborations and the sharing of innovative ideas and discoveries.
It is important to emphasize that ABGH is not an organization wanting to exclude themselves on the basis of ethnicity. ABGH is an example of how shared health goals within a medical discipline can be achieved when inclusion and equity is at the helm. ABGH led and represented events that raise awareness of diseases affecting all patients and aim to make the GI community more culturally competent. ABGH is future-oriented and embraces all members who align with the mission regardless of ethnicity, gender, orientation, or disability. The institution that is and will be the ABGH impresses upon me a feeling of excitement, gratitude, and humility. I look forward to continuing the mission created by the founding members and being to others what ABGH is to me.
For more information on this organization, please visit blackingastro.org.
Dr. Busari is a resident physician at Florida State University-SMH and a junior member of ABGH. Dr. Guillaume director of the Gastrointestinal Motility Center at Stony Brook (New York) University Hospital and an assistant professor of medicine at the Renaissance School of Medicine at Stony Brook University. They have no disclosures.
References
1. Galarneau C. J Health Care Poor Underserved. 2018;29(1):5-8.
2. Ashktorab H et al. Gastroenterology. 2017 Oct;153(4):910-923.
3. Blanchard AK. N Engl J Med. 2021 Jun 10;384(23):e87.
“Of all the forms of inequality, injustice in health is the most shocking and inhuman.” – Martin Luther King Jr., March 25, 1966. 1
This single disparity – health care injustice – too often results in needless mental anguish, physical suffering, or death. In the spring of 2020, at the peak of the COVID-19 pandemic, the convergence of injustices in health care and policing led to the disproportionate preventable physical deaths of Black men and women. This became the watershed moment for 11 gastroenterologists and hepatologists who collectively grieved but heeded the call of social responsibility to form the Association of Black Gastroenterologists and Hepatologists.
The mission of ABGH is laser focused. It is to promote health equity in Black communities, advance science, and develop the careers of Black gastroenterologists, hepatologists, and scientists. The vision is to improve gastrointestinal health outcomes in Black communities; to develop the pipeline of Black gastroenterologists and hepatologists given that currently only 4% in the United States identify as Black; to foster networking, mentoring, and sponsorship among Black students, clinical trainees, gastroenterologists, and hepatologists; and to promote the scholarship of Black gastroenterologists and hepatologists.
Through community engagement, ABGH stands to empower the Black community with knowledge and choices, which inherently strengthens the physician-patient relationship. ABGH also exists to implement positive change in long term outcome statistics in Black communities. Black Americans are 20% more likely to be diagnosed with colorectal cancer and 40% more likely to die from the disease. In addition to colorectal cancer, rates of esophageal squamous cancer, as well as cancer of the small bowel and pancreas, are highest in Black people.2 Through scientific research and clinical care, we aim to eradicate digestive health disparities.
Yet in this space, we know first-hand that, in the United States, the wellness of a community is not measured by the medical fitness of its members alone but also by the availability of equitable opportunities for fulfillment of nonmedical but health-impacting social needs. These needs, also known as social determinants of health, are made inaccessible to vulnerable populations because of systemic racism. Importantly, we recognize that dismantling racist systems is not a singular effort, nor are we pioneers in this work, but we look forward to executing health equity goals collaboratively with our fellow gastrointestinal national societies and other leading community and grassroots organizations.
The founders of ABGH are a distinctive group of practicing gastroenterologists and hepatologists from across the United States with a strong track record in DEI work through their community, clinical, and research activities. The board of directors reflects only the depth of talent shared throughout the ABGH membership. The strength of the organization lies in its diverse and energetic constituents who all exemplify outstanding training and the readiness to redefine the standard of health care delivery to the Black community. From medical students to senior level gastroenterologists, we collectively embody a considerable momentum for formation of this organization at this point in our history.
ABGH fulfills a professional career development need for budding gastroenterologists not so readily available from other organizations. The compelling impact of representing the embodiment of what many of us were told we could not become is limitless. The personal and professional growth enabled by our networking and learning from each other is both motivating and empowering since, even after overcoming the obstacles needed to become a medical provider, Black professionals are often not afforded the bandwidth, range of emotion, and protection to reveal their specific needs. For this author personally, the ABGH provides a psychological safety that allows authentic self-identity without code-switching.3 Through this authenticity has arisen formidable strength, creativity, and productivity. The leadership and innovation cultivated in ABGH stands to benefit many generations to come, both within and outside the organization.
Reflections from a junior member of ABGH: Dr. Kafayat Busari
My desire to pursue gastroenterology was bolstered by determination, curiosity, and passion, yet ironically was often met with skepticism by many in position to help advance this goal. Although projections of incertitude on members of a community that are often made to feel inadequate can diminish even the brightest of lights, conversely it can fuel the creation of an organization emboldened to specifically address GI-related health disparities. When I was a second-year internal medicine resident, I encountered a GI physician who told me GI “wasn’t something I wanted to do”—despite me expressing my interest.
Confused by the statement, I reached out to Renee L. Williams, MD, of NYU Langone Health, who I had met during my medical school training. She suggested I join a conference call later that week. On that call and the many that took place thereafter, I was introduced to Black gastroenterologists who are luminal disease experts, chair members, journal editors, transplant hepatologists, interventional endoscopists, researchers, and professors (in other words, GI professional leaders). My time on the initial call lasted perhaps less than 20 minutes, but the impact has been immeasurable.
I was provided the emotional reassurance that GI was indeed for me and told “there’s always a seat, and if it feels like there’s not, we just need to get more chairs.” Little did I know, but those metaphorical chairs were being gathered so that I and other aspiring gastroenterologists will be able to sit comfortably at these tables one day. I was witnessing these GI professional leaders set in motion the beginning of what will undoubtedly be a pivotal component in the way I approach my career as a gastroenterologist. The experience reignited my mental determination to one day attain the level of success represented by the ABGH board members and to persevere in my quest to help redefine how Black medical students and residents serve their communities as physicians.
The creation of the ABGH could not have come at a better time in my training. In the wake of recent public protests for equity of African Americans within every institution (academia, housing, banks, policing, health care, and beyond), which were fundamentally built on racism, being a junior member of ABGH has not only given me a platform to speak my truth but has also provided me with tools to help others do so as well. As someone very passionate about research, primarily in colorectal cancer, I have been given an opportunity to connect with a dream team of mentors who have taken research ideas to new levels and have challenged me to dig deeper and expand my curiosity to investigate what still needs to be uncovered. It has created opportunity after opportunity for actively building relationships, leading to meaningful collaborations and the sharing of innovative ideas and discoveries.
It is important to emphasize that ABGH is not an organization wanting to exclude themselves on the basis of ethnicity. ABGH is an example of how shared health goals within a medical discipline can be achieved when inclusion and equity is at the helm. ABGH led and represented events that raise awareness of diseases affecting all patients and aim to make the GI community more culturally competent. ABGH is future-oriented and embraces all members who align with the mission regardless of ethnicity, gender, orientation, or disability. The institution that is and will be the ABGH impresses upon me a feeling of excitement, gratitude, and humility. I look forward to continuing the mission created by the founding members and being to others what ABGH is to me.
For more information on this organization, please visit blackingastro.org.
Dr. Busari is a resident physician at Florida State University-SMH and a junior member of ABGH. Dr. Guillaume director of the Gastrointestinal Motility Center at Stony Brook (New York) University Hospital and an assistant professor of medicine at the Renaissance School of Medicine at Stony Brook University. They have no disclosures.
References
1. Galarneau C. J Health Care Poor Underserved. 2018;29(1):5-8.
2. Ashktorab H et al. Gastroenterology. 2017 Oct;153(4):910-923.
3. Blanchard AK. N Engl J Med. 2021 Jun 10;384(23):e87.
Rare hematologic malignancy may first present to a dermatologist
in about 80% of cases.
“You won’t see blastic plasmacytoid dendritic cell neoplasm listed on our primary cutaneous lymphoma classifications because it’s not technically a primary cutaneous disease,” Brittney K. DeClerck, MD, said during the annual meeting of the Pacific Dermatologic Association. “It’s a systemic disease that has secondary cutaneous manifestations. That’s a very important distinction to make, in terms of not missing the underlying disease associated with what might be commonly first seen on the skin.”
BPDCN is a malignancy of plasmacytoid dendritic cells, which capture, process, and present antigen, and allow the remainder of the immune system to be activated. “They are mainly derived from the myeloid cell lineage, and possibly from the lymphoid line in a subset of cases,” said Dr. DeClerck, associate professor of clinical pathology and dermatology at the University of Southern California, Los Angeles. “They secrete high levels of type I interferons, which is important for antiviral immunity, but they can also be implicated in severe systemic inflammatory diseases, such as systemic lupus erythematosus and systemic sclerosis.”
BPDCN involves the skin in about 80% of cases, she added, “but invariably at some point it involves the bone marrow and has an acute leukemic presentation, whether or not it happens concurrently with what we see on the skin as dermatologists. We also see variable involvement of the peripheral blood, lymph nodes, and the central nervous system.”
The classification of BPDCN has changed over time based on evolving immunohistochemical markers and technologies. For example, in 1995 it was called agranular CD4+ NK cell leukemia, in 2001 it was called blastic NK-cell lymphoma, in 2005 it was called CD4+/CD56+ hematodermic neoplasm, and in 2008 it was called BPDCN (AML subset). In 2016 it became classified as its own entity: BPDCN.
Because of changing nomenclature, the true incidence of the disease is unknown, but according to the best available literature, 75% of cases occur in men and the median age is between 60 and 70 years, “but all ages can be affected,” Dr. DeClerck said. “Cases seem to come in clusters. Our most recent cluster has been in our pediatric population. At Children’s Hospital Los Angeles, we’ve had three cases in the last couple of years. To me, that was a bit unusual.”
She added that 10%-20% of patients will have either a history of, or will develop another, hematologic malignancy, such as myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), or acute myelogenous leukemia (AML).
The general prognosis of BPDCN is poor, and the mean time from onset of lesions to an actual diagnosis is about 6.2 months, which underscores the importance of early diagnosis, Dr. DeClerck said. “There can be some nondescript solitary lesions that patients can present with, so don’t hesitate to biopsy.” The median overall survival is less than 20 months, but patients under 60 years of age have a slightly better prognosis.
Clinical presentation
Clinically, the malignancy presents with variable involvement of the skin, bone marrow, lymph nodes, peripheral blood, and central nervous system. “Patients may have one or all of these,” she said. Because 80% of patients have skin lesions, “dermatologists should be aware of this entity in order to communicate with our pathologists to understand that maybe one biopsy isn’t enough. Several biopsies may be required.”
The most common dermatologic presentation of BPDCN is erythematous to deeply violaceous nodules. Other patients may present with infiltrated ecchymotic plaques or petechial to hyperpigmented macules, patches, and plaques. Biopsy reveals a diffusely infiltrated dermis of markedly atypical large cells, but occasionally can be more subtle. “Early lesions may only be perivascular in nature, so going on high power on anything that looks atypical on low power is important in these cases,” Dr. DeClerck said.
The recommended histochemical stains for suspected BPDCN include CD123, CD4, and CD56. “We need to have other stains to rule out other things, such as negative stains that are going to exclude other T cell and B cell processes, and Merkel cell carcinoma, which can express CD56. We also want to have another confirmatory stain because other things can express CD123, CD4, and CD56. Commonly we use TCL1 or TCF4.”
The differential diagnosis of cutaneous findings includes leukemia cutis, mycosis fungoides, NK/T-cell lymphoma, and cutaneous gamma-delta T-cell lymphoma, while the differential diagnosis of biopsy findings includes AML, acute lymphoblastic leukemia, and NK/T-cell lymphoma.
Treatment of BPDCN
Historically, BPDCN was treated with multiagent high-dose chemotherapy. “Patients would frequently respond early but would relapse quickly, progress, and have a poor outcome,” Dr. DeClerck said. Now, first-line therapy is tagraxofusp-erzs (Elzonris) or multiagent chemotherapy based on where the patient is in the course of disease. Tagraxofusp-erzs is an IL-3 conjugated diphtheria toxic fusion protein which binds to CD123, which was approved by the Food and Drug Administration in 2018 for treating BPDCN. After that initial therapy, it is determined whether the patient has a complete response or failed response, she said. “If they have a complete response, they frequently go on to bone marrow transplantation, which is the only curative therapy at this point for these patients.”
According to Dr. DeClerck, an anti-BCL-2 therapy, venetoclax, can be used for patients with BPDCN as well. National Comprehensive Cancer Network (NCCN) guidelines for the treatment of BPDCN can be found on the NCCN website.
Dr. DeClerck emphasized the importance of reviewing biopsy results with a hematopathologist, “because there are complex leukemias that are beyond what dermatopathologists have been trained in.” Once a patient is diagnosed with BPDCN, she recommends rapid referral to a large center for treatment and possible bone marrow transplantation.
Dr. DeClerck disclosed that she is an adviser for tagraxofusp-erzs manufacturer Stemline Therapeutics.
in about 80% of cases.
“You won’t see blastic plasmacytoid dendritic cell neoplasm listed on our primary cutaneous lymphoma classifications because it’s not technically a primary cutaneous disease,” Brittney K. DeClerck, MD, said during the annual meeting of the Pacific Dermatologic Association. “It’s a systemic disease that has secondary cutaneous manifestations. That’s a very important distinction to make, in terms of not missing the underlying disease associated with what might be commonly first seen on the skin.”
BPDCN is a malignancy of plasmacytoid dendritic cells, which capture, process, and present antigen, and allow the remainder of the immune system to be activated. “They are mainly derived from the myeloid cell lineage, and possibly from the lymphoid line in a subset of cases,” said Dr. DeClerck, associate professor of clinical pathology and dermatology at the University of Southern California, Los Angeles. “They secrete high levels of type I interferons, which is important for antiviral immunity, but they can also be implicated in severe systemic inflammatory diseases, such as systemic lupus erythematosus and systemic sclerosis.”
BPDCN involves the skin in about 80% of cases, she added, “but invariably at some point it involves the bone marrow and has an acute leukemic presentation, whether or not it happens concurrently with what we see on the skin as dermatologists. We also see variable involvement of the peripheral blood, lymph nodes, and the central nervous system.”
The classification of BPDCN has changed over time based on evolving immunohistochemical markers and technologies. For example, in 1995 it was called agranular CD4+ NK cell leukemia, in 2001 it was called blastic NK-cell lymphoma, in 2005 it was called CD4+/CD56+ hematodermic neoplasm, and in 2008 it was called BPDCN (AML subset). In 2016 it became classified as its own entity: BPDCN.
Because of changing nomenclature, the true incidence of the disease is unknown, but according to the best available literature, 75% of cases occur in men and the median age is between 60 and 70 years, “but all ages can be affected,” Dr. DeClerck said. “Cases seem to come in clusters. Our most recent cluster has been in our pediatric population. At Children’s Hospital Los Angeles, we’ve had three cases in the last couple of years. To me, that was a bit unusual.”
She added that 10%-20% of patients will have either a history of, or will develop another, hematologic malignancy, such as myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), or acute myelogenous leukemia (AML).
The general prognosis of BPDCN is poor, and the mean time from onset of lesions to an actual diagnosis is about 6.2 months, which underscores the importance of early diagnosis, Dr. DeClerck said. “There can be some nondescript solitary lesions that patients can present with, so don’t hesitate to biopsy.” The median overall survival is less than 20 months, but patients under 60 years of age have a slightly better prognosis.
Clinical presentation
Clinically, the malignancy presents with variable involvement of the skin, bone marrow, lymph nodes, peripheral blood, and central nervous system. “Patients may have one or all of these,” she said. Because 80% of patients have skin lesions, “dermatologists should be aware of this entity in order to communicate with our pathologists to understand that maybe one biopsy isn’t enough. Several biopsies may be required.”
The most common dermatologic presentation of BPDCN is erythematous to deeply violaceous nodules. Other patients may present with infiltrated ecchymotic plaques or petechial to hyperpigmented macules, patches, and plaques. Biopsy reveals a diffusely infiltrated dermis of markedly atypical large cells, but occasionally can be more subtle. “Early lesions may only be perivascular in nature, so going on high power on anything that looks atypical on low power is important in these cases,” Dr. DeClerck said.
The recommended histochemical stains for suspected BPDCN include CD123, CD4, and CD56. “We need to have other stains to rule out other things, such as negative stains that are going to exclude other T cell and B cell processes, and Merkel cell carcinoma, which can express CD56. We also want to have another confirmatory stain because other things can express CD123, CD4, and CD56. Commonly we use TCL1 or TCF4.”
The differential diagnosis of cutaneous findings includes leukemia cutis, mycosis fungoides, NK/T-cell lymphoma, and cutaneous gamma-delta T-cell lymphoma, while the differential diagnosis of biopsy findings includes AML, acute lymphoblastic leukemia, and NK/T-cell lymphoma.
Treatment of BPDCN
Historically, BPDCN was treated with multiagent high-dose chemotherapy. “Patients would frequently respond early but would relapse quickly, progress, and have a poor outcome,” Dr. DeClerck said. Now, first-line therapy is tagraxofusp-erzs (Elzonris) or multiagent chemotherapy based on where the patient is in the course of disease. Tagraxofusp-erzs is an IL-3 conjugated diphtheria toxic fusion protein which binds to CD123, which was approved by the Food and Drug Administration in 2018 for treating BPDCN. After that initial therapy, it is determined whether the patient has a complete response or failed response, she said. “If they have a complete response, they frequently go on to bone marrow transplantation, which is the only curative therapy at this point for these patients.”
According to Dr. DeClerck, an anti-BCL-2 therapy, venetoclax, can be used for patients with BPDCN as well. National Comprehensive Cancer Network (NCCN) guidelines for the treatment of BPDCN can be found on the NCCN website.
Dr. DeClerck emphasized the importance of reviewing biopsy results with a hematopathologist, “because there are complex leukemias that are beyond what dermatopathologists have been trained in.” Once a patient is diagnosed with BPDCN, she recommends rapid referral to a large center for treatment and possible bone marrow transplantation.
Dr. DeClerck disclosed that she is an adviser for tagraxofusp-erzs manufacturer Stemline Therapeutics.
in about 80% of cases.
“You won’t see blastic plasmacytoid dendritic cell neoplasm listed on our primary cutaneous lymphoma classifications because it’s not technically a primary cutaneous disease,” Brittney K. DeClerck, MD, said during the annual meeting of the Pacific Dermatologic Association. “It’s a systemic disease that has secondary cutaneous manifestations. That’s a very important distinction to make, in terms of not missing the underlying disease associated with what might be commonly first seen on the skin.”
BPDCN is a malignancy of plasmacytoid dendritic cells, which capture, process, and present antigen, and allow the remainder of the immune system to be activated. “They are mainly derived from the myeloid cell lineage, and possibly from the lymphoid line in a subset of cases,” said Dr. DeClerck, associate professor of clinical pathology and dermatology at the University of Southern California, Los Angeles. “They secrete high levels of type I interferons, which is important for antiviral immunity, but they can also be implicated in severe systemic inflammatory diseases, such as systemic lupus erythematosus and systemic sclerosis.”
BPDCN involves the skin in about 80% of cases, she added, “but invariably at some point it involves the bone marrow and has an acute leukemic presentation, whether or not it happens concurrently with what we see on the skin as dermatologists. We also see variable involvement of the peripheral blood, lymph nodes, and the central nervous system.”
The classification of BPDCN has changed over time based on evolving immunohistochemical markers and technologies. For example, in 1995 it was called agranular CD4+ NK cell leukemia, in 2001 it was called blastic NK-cell lymphoma, in 2005 it was called CD4+/CD56+ hematodermic neoplasm, and in 2008 it was called BPDCN (AML subset). In 2016 it became classified as its own entity: BPDCN.
Because of changing nomenclature, the true incidence of the disease is unknown, but according to the best available literature, 75% of cases occur in men and the median age is between 60 and 70 years, “but all ages can be affected,” Dr. DeClerck said. “Cases seem to come in clusters. Our most recent cluster has been in our pediatric population. At Children’s Hospital Los Angeles, we’ve had three cases in the last couple of years. To me, that was a bit unusual.”
She added that 10%-20% of patients will have either a history of, or will develop another, hematologic malignancy, such as myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), or acute myelogenous leukemia (AML).
The general prognosis of BPDCN is poor, and the mean time from onset of lesions to an actual diagnosis is about 6.2 months, which underscores the importance of early diagnosis, Dr. DeClerck said. “There can be some nondescript solitary lesions that patients can present with, so don’t hesitate to biopsy.” The median overall survival is less than 20 months, but patients under 60 years of age have a slightly better prognosis.
Clinical presentation
Clinically, the malignancy presents with variable involvement of the skin, bone marrow, lymph nodes, peripheral blood, and central nervous system. “Patients may have one or all of these,” she said. Because 80% of patients have skin lesions, “dermatologists should be aware of this entity in order to communicate with our pathologists to understand that maybe one biopsy isn’t enough. Several biopsies may be required.”
The most common dermatologic presentation of BPDCN is erythematous to deeply violaceous nodules. Other patients may present with infiltrated ecchymotic plaques or petechial to hyperpigmented macules, patches, and plaques. Biopsy reveals a diffusely infiltrated dermis of markedly atypical large cells, but occasionally can be more subtle. “Early lesions may only be perivascular in nature, so going on high power on anything that looks atypical on low power is important in these cases,” Dr. DeClerck said.
The recommended histochemical stains for suspected BPDCN include CD123, CD4, and CD56. “We need to have other stains to rule out other things, such as negative stains that are going to exclude other T cell and B cell processes, and Merkel cell carcinoma, which can express CD56. We also want to have another confirmatory stain because other things can express CD123, CD4, and CD56. Commonly we use TCL1 or TCF4.”
The differential diagnosis of cutaneous findings includes leukemia cutis, mycosis fungoides, NK/T-cell lymphoma, and cutaneous gamma-delta T-cell lymphoma, while the differential diagnosis of biopsy findings includes AML, acute lymphoblastic leukemia, and NK/T-cell lymphoma.
Treatment of BPDCN
Historically, BPDCN was treated with multiagent high-dose chemotherapy. “Patients would frequently respond early but would relapse quickly, progress, and have a poor outcome,” Dr. DeClerck said. Now, first-line therapy is tagraxofusp-erzs (Elzonris) or multiagent chemotherapy based on where the patient is in the course of disease. Tagraxofusp-erzs is an IL-3 conjugated diphtheria toxic fusion protein which binds to CD123, which was approved by the Food and Drug Administration in 2018 for treating BPDCN. After that initial therapy, it is determined whether the patient has a complete response or failed response, she said. “If they have a complete response, they frequently go on to bone marrow transplantation, which is the only curative therapy at this point for these patients.”
According to Dr. DeClerck, an anti-BCL-2 therapy, venetoclax, can be used for patients with BPDCN as well. National Comprehensive Cancer Network (NCCN) guidelines for the treatment of BPDCN can be found on the NCCN website.
Dr. DeClerck emphasized the importance of reviewing biopsy results with a hematopathologist, “because there are complex leukemias that are beyond what dermatopathologists have been trained in.” Once a patient is diagnosed with BPDCN, she recommends rapid referral to a large center for treatment and possible bone marrow transplantation.
Dr. DeClerck disclosed that she is an adviser for tagraxofusp-erzs manufacturer Stemline Therapeutics.
FROM PDA 2021
Mean leadership
The differences between the mean and median of leadership data
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.
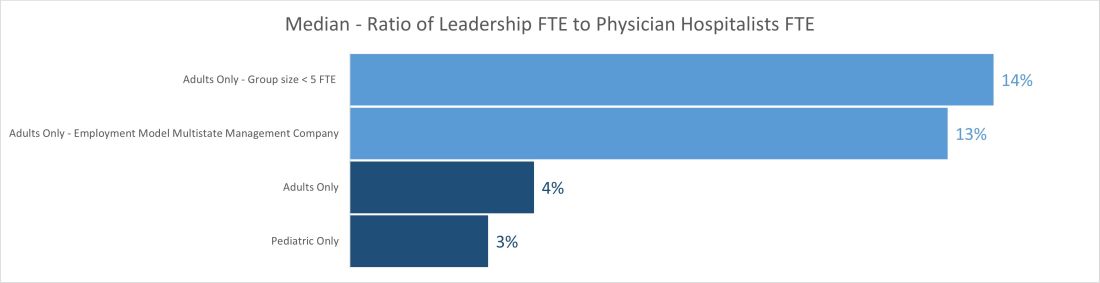
The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.
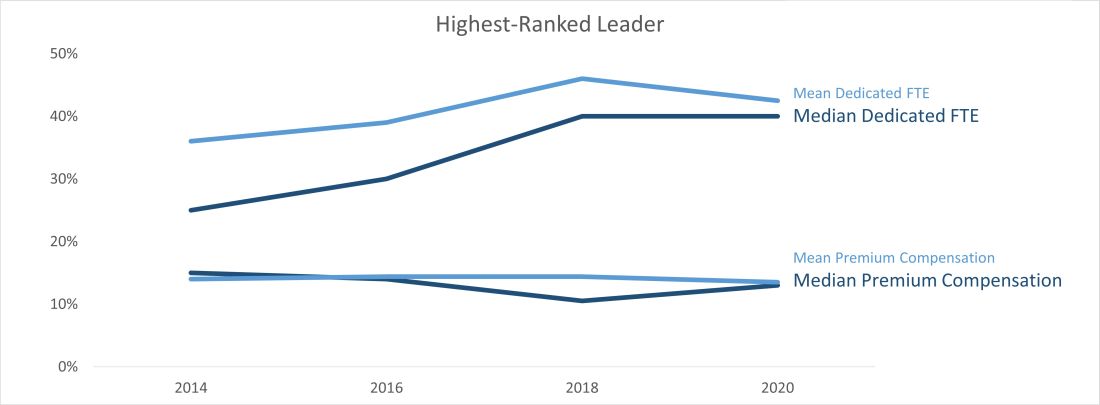
At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
The differences between the mean and median of leadership data
The differences between the mean and median of leadership data
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.

The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.

At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.

The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.

At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
Cardiogenic shock teams again tied to lower mortality
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
Botulinum Toxin for the Treatment of Intractable Raynaud Phenomenon
To the Editor:
Raynaud phenomenon (RP) is an episodic vasospasm of the digits that can lead to ulceration, gangrene, and autoamputation with prolonged ischemia. OnabotulinumtoxinA has been implemented as a treatment of intractable RP by paralyzing the muscles of the digital arteries. We report a case of a woman with severe RP secondary to systemic lupus erythematosus (SLE) who was treated with onabotulinumtoxinA injections after multiple treatment modalities failed to improve her condition. We describe the dosage and injection technique used to produce clinical improvement in our patient and compare it to prior reports in the literature.
A 33-year-old woman presented to the emergency department for worsening foot pain of 5 days' duration with dusky purple color changes concerning for impending Raynaud crisis related to RP. The patient had a history of antiphospholipid antibody syndrome (APS) and SLE with overlapping symptoms of polymyositis and scleroderma. She had been hospitalized for RP multiple times prior to the current admission. She was medically managed with nifedipine, sildenafil, losartan potassium, aspirin, alprostadil, and prostaglandin infusions, and was surgically managed with a right-hand sympathectomy and right ulnar artery bypass graft that had subsequently thrombosed. At the current presentation, she had painful dusky toes on both feet though more pronounced on the left foot. She endorsed foot pain while walking and tenderness to palpation of the fingers, which were minimally improved with intravenous prostaglandins.
Physical examination revealed blanching of the digits in both hands with pits in the right fourth and left first digits. Dusky patches overlaid all the toes as well as the superior plantar aspects of the feet (Figure 1). Given the history of APS, a punch biopsy was performed on the left medial plantar foot and results showed no histologic evidence of vasculitis or vasculopathy. Necrotic foci were present on the left and right second metatarsal bones, which were not reperfusable (Figure 2). The clinical findings and punch biopsy results favored RP as opposed to vasculopathy from APS.
Several interventions were attempted, and after 4 days with no response, the patient agreed to receive treatment with onabotulinumtoxinA. OnabotulinumtoxinA (5 U) was injected into the subcutaneous tissue of the medial and lateral aspects of each of the first and second toes near the proximal phalanges (40 U total). However, treatment could not be completed due to severe pain caused by the injections despite preprocedure regional nerve blocks to both lower extremities, preinjection icing, and lorazepam. Two days later, the patient tolerated onabotulinumtoxinA injections of all remaining digits of both feet (60 U total). She noted slight clinical improvement soon thereafter. One week after treatment of all 10 toes, she reported decreased pain and reduced duskiness of both feet (Figure 3).
One month later, the patient endorsed recurring pain in the hands and feet. Physical examination revealed reticular cyanosis and increased violaceous patches of the hands; the feet were overall unchanged from the prior hospitalization. At 4-month follow-up, there was gangrene on the left second, third, and fifth toe in addition to areas of induration noted on the fingers. She was repeatedly hospitalized over the next 6 months for pain management and gangrene of the toes, and finally underwent an amputation of the left and right second toe at the proximal and middle phalanx, respectively. She currently is continuing extensive medical management for pain and gangrene of the digits; she has not received additional onabotulinumtoxinA injections.
Raynaud phenomenon is a vascular disorder characterized by intermittent arteriolar vasospasm of the digits, often due to cold temperature or stress. Approximately 90% of RP cases are primarily idiopathic, with the remaining cases secondary to other diseases, typically systemic sclerosis, SLE, or mixed connective tissue disease.1 Symptoms present with characteristic changing of hands from white (ischemia) to blue (hypoxia) to red (reperfusion). Episodic attacks of vasospasm and ischemia can be painful and lead to digital ulcerations and necrosis of the digits or hands. Other complications including digital tuft pits, pterygium inversum unguis, or torturous nail fold capillaries with capillary dropout also may be seen.2
Although the etiology is multifactorial, the pathophysiology primarily is due to an imbalance of vasodilation and vasoconstriction. Perturbed levels of vasodilatory mediators include nitric oxide, prostacyclin, and calcitonin gene-related peptide.3 Meanwhile, abnormal neural sympathetic control of α-adrenergic receptors located on smooth muscle vasculature and subsequent endothelial hyperproliferation may contribute to inappropriate vasoconstriction.4
The first-line therapy for mild to moderate disease refractory to conservative management includes monotherapy with dihydropyridine calcium channel blockers. For severe disease, combination therapy involves addition of other classes of medications including phosphodiesterase 5 inhibitors, topical nitrates, angiotensin receptor blockers, or selective serotonin reuptake inhibitors. Intravenous prostacyclin, endothelin receptor blockers, and onabotulinumtoxinA injections may be added as third-line therapy. Finally, surgical management including sympathectomy with continued pharmacologic therapy may be needed for disease recalcitrant to the aforementioned options.2
OnabotulinumtoxinA is a neurotoxin produced by the bacterium Clostridium botulinum. The toxin’s mechanism of action involves inhibition of the release of presynaptic acetylcholine-containing vesicles at the neuromuscular junction through cleavage of sensory nerve action potential receptor proteins. In addition, it inhibits smooth muscle vasoconstriction and pain by blocking α2-adrenergic receptors on blood vessels and chronic pain-transmitting C fibers in nerves, respectively.3,5
Only recently has onabotulinumtoxinA been used for treatment of RP. Botulinum toxin is approved for the treatment of spastic and dystonic diseases such as blepharospasm, headaches in patients with chronic migraines, upper limb spasticity, cervical dystonia, torticollis, ocular strabismus, and hyperhidrosis.3 However, the versatility of its therapeutic effects is evident in its broad off-label clinical applications, including achalasia; carpal tunnel syndrome; and spasticity relating to stroke, paraplegia, and cerebral palsy, among many others.5
Few studies have analyzed the use of onabotulinumtoxinA for the treatment of RP.3,6 There is no consensus yet regarding dose, dilution, or injection sites. One vial of onabotulinumtoxinA contains 100 U and is reconstituted in 20 mL of normal saline to produce 5 U/mL. The simplest technique involves the injection of 5 U into the medial and lateral aspects of each finger at its base, at the level of or just proximal to the A1 pulley, for a total of 50 U per hand.7 In the foot, injection can be made at the base of each toe near the proximal phalanges. A regimen of 50 to 100 U per hand was used by Neumeister et al5 on 19 patients, who subsequently standardized it to 10 U on each neurovascular bundle in a follow-up study,7 giving a total volume of 2 mL per injection. Associated pain or a burning sensation initially may be experienced, which may be mitigated by a lidocaine hydrochloride wrist block prior to injection.7 This technique produced immediate and lasting pain relief, increased tissue perfusion, and resolved digital ulcers in 28 of 33 patients. Most patients reported immediate relief, and a few noted gradual reduction in pain and resolution of chronic ulcers within 2 months. Of the 33 patients, 7 (21.2%) required repeat injections for recurrent pain, but the majority were pain free up to 6 years later with a single injection schedule.7
Injection into the palmar region, wrists, and/or fingers also may be performed. Effects of using different injection sites (eg, neurovascular bundle, distal palm, proximal hand) have been explored and were not notably different between these locations.8 Lastly, the frequency of injections may be attenuated according to the spectrum and severity of the patient’s symptoms. In a report of 11 patients who received a total of 100 U of onabotulinumtoxinA per hand, 5 required repeat injections within 3 to 8 months.9
Studies have reported onabotulinumtoxinA to be a promising option for the treatment of intractable symptoms. Likewise, our patient had a notable reduction in pain with signs of clinical improvement within 24 to 48 hours after injection. The need for amputation 6 months later likely was because the patient’s toes were already necrosing prior to treatment with onabotulinumtoxinA. Thus, the timing of intervention may play a critical role in response to onabotulinumtoxinA injections, particularly because the severity of our patient’s presentation was comparable to other cases reported in the literature. Even in reports using a smaller dose—2 U injected into each toe as opposed to 10 U per toe, as in our case—follow-up showed favorable results.10 In other reports, response can be perceived within days to a week, with remarkable improvement of numbness, pain, digit color, and wound resolution, in addition to decreased frequency and severity of attacks. Moreover, greater vasodilation and subsequent tissue perfusion have been evidenced by objective measures including digital transcutaneous oxygen saturation and Doppler sonography.7,8 Side effects, which are minimal and temporary, include local pain triggering a vasospastic attack and intrinsic muscle weakness; more rarely, dysesthesia and thenar eminence atrophy have been reported.11
Available studies have shown onabotulinumtoxinA to produce favorable results in the treatment of vasospastic disease. We suspect that an earlier intervention for our patient—before necrosis of the toes developed—would have led to a more positive outcome, consistent with other reports. Treatment with onabotulinumtoxinA is an approach to consider when the standard-of-care treatments for RP have been exhausted, as timely intervention may prevent the need for surgery. The indications and appropriate dosing protocol remain to be defined, in addition to more thorough evaluation of its efficacy relative to other medical and surgical options.
- Neumeister MW. The role of botulinum toxin in vasospastic disorders of the hand. Hand Clin. 2015;31:23-37. doi:10.1016/j.hcl.2014.09.003
- Bakst R, Merola JF, Franks AG, et al. Raynaud’s phenomenon: pathogenesis and management. J Am Acad Dermatol. 2008;59:633-653. doi:10.1016/j.jaad.2008.06.004
- Iorio ML, Masden DL, Higgins JP. Botulinum toxin a treatment of Raynaud’s phenomenon: a review. Semin Arthritis Rheum. 2012;41:599-603. doi:10.1016/j.semarthrit.2011.07.006
- Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375:556-565. doi:10.1056/NEJMra1507638
- Neumeister MW, Chambers CB, Herron MS, et al. Botox therapy for ischemic digits. Plast Reconstr Surg. 2009;124:191-200. doi:10.1097/PRS.0b013e3181a80576
- Sycha T, Graninger M, Auff E, et al. Botulinum toxin in the treatment of Raynaud’s phenomenon: a pilot study. Eur J Clin Invest. 2004;34:312-313. doi:10.1016/j.jaad.2013.06.029
- Neumeister MW. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085-2092. doi:10.1016/j.jhsa.2010.09.019
- Fregene A, Ditmars D, Siddiqui A. Botulinum toxin type A: a treatment option for digital ischemia in patients with Raynaud’s phenomenon. J Hand Surg Am. 2009;34:446-452. doi:10.1016/j.jhsa.2008.11.026
- Van Beek AL, Lim PK, Gear AJL, et al. Management of vasospastic disorders with botulinum toxin A. Plast Reconstr Surg. 2007;119:217-226. doi:10.1097/01.prs.0000244860.00674.57
- Dhaliwal K, Griffin M, Denton CP, et al. The novel use of botulinum toxin A for the treatment of Raynaud’s phenomenon in the toes. BMJ Case Rep. 2018;2018:2017-2019. doi:10.1136/bcr-2017-219348
- Eickhoff JC, Smith JK, Landau ME, et al. Iatrogenic thenar eminence atrophy after Botox A injection for secondary Raynaud phenomenon. J Clin Rheumatol. 2016;22:395-396. doi:10.1097/RHU.0000000000000450
To the Editor:
Raynaud phenomenon (RP) is an episodic vasospasm of the digits that can lead to ulceration, gangrene, and autoamputation with prolonged ischemia. OnabotulinumtoxinA has been implemented as a treatment of intractable RP by paralyzing the muscles of the digital arteries. We report a case of a woman with severe RP secondary to systemic lupus erythematosus (SLE) who was treated with onabotulinumtoxinA injections after multiple treatment modalities failed to improve her condition. We describe the dosage and injection technique used to produce clinical improvement in our patient and compare it to prior reports in the literature.
A 33-year-old woman presented to the emergency department for worsening foot pain of 5 days' duration with dusky purple color changes concerning for impending Raynaud crisis related to RP. The patient had a history of antiphospholipid antibody syndrome (APS) and SLE with overlapping symptoms of polymyositis and scleroderma. She had been hospitalized for RP multiple times prior to the current admission. She was medically managed with nifedipine, sildenafil, losartan potassium, aspirin, alprostadil, and prostaglandin infusions, and was surgically managed with a right-hand sympathectomy and right ulnar artery bypass graft that had subsequently thrombosed. At the current presentation, she had painful dusky toes on both feet though more pronounced on the left foot. She endorsed foot pain while walking and tenderness to palpation of the fingers, which were minimally improved with intravenous prostaglandins.
Physical examination revealed blanching of the digits in both hands with pits in the right fourth and left first digits. Dusky patches overlaid all the toes as well as the superior plantar aspects of the feet (Figure 1). Given the history of APS, a punch biopsy was performed on the left medial plantar foot and results showed no histologic evidence of vasculitis or vasculopathy. Necrotic foci were present on the left and right second metatarsal bones, which were not reperfusable (Figure 2). The clinical findings and punch biopsy results favored RP as opposed to vasculopathy from APS.
Several interventions were attempted, and after 4 days with no response, the patient agreed to receive treatment with onabotulinumtoxinA. OnabotulinumtoxinA (5 U) was injected into the subcutaneous tissue of the medial and lateral aspects of each of the first and second toes near the proximal phalanges (40 U total). However, treatment could not be completed due to severe pain caused by the injections despite preprocedure regional nerve blocks to both lower extremities, preinjection icing, and lorazepam. Two days later, the patient tolerated onabotulinumtoxinA injections of all remaining digits of both feet (60 U total). She noted slight clinical improvement soon thereafter. One week after treatment of all 10 toes, she reported decreased pain and reduced duskiness of both feet (Figure 3).
One month later, the patient endorsed recurring pain in the hands and feet. Physical examination revealed reticular cyanosis and increased violaceous patches of the hands; the feet were overall unchanged from the prior hospitalization. At 4-month follow-up, there was gangrene on the left second, third, and fifth toe in addition to areas of induration noted on the fingers. She was repeatedly hospitalized over the next 6 months for pain management and gangrene of the toes, and finally underwent an amputation of the left and right second toe at the proximal and middle phalanx, respectively. She currently is continuing extensive medical management for pain and gangrene of the digits; she has not received additional onabotulinumtoxinA injections.
Raynaud phenomenon is a vascular disorder characterized by intermittent arteriolar vasospasm of the digits, often due to cold temperature or stress. Approximately 90% of RP cases are primarily idiopathic, with the remaining cases secondary to other diseases, typically systemic sclerosis, SLE, or mixed connective tissue disease.1 Symptoms present with characteristic changing of hands from white (ischemia) to blue (hypoxia) to red (reperfusion). Episodic attacks of vasospasm and ischemia can be painful and lead to digital ulcerations and necrosis of the digits or hands. Other complications including digital tuft pits, pterygium inversum unguis, or torturous nail fold capillaries with capillary dropout also may be seen.2
Although the etiology is multifactorial, the pathophysiology primarily is due to an imbalance of vasodilation and vasoconstriction. Perturbed levels of vasodilatory mediators include nitric oxide, prostacyclin, and calcitonin gene-related peptide.3 Meanwhile, abnormal neural sympathetic control of α-adrenergic receptors located on smooth muscle vasculature and subsequent endothelial hyperproliferation may contribute to inappropriate vasoconstriction.4
The first-line therapy for mild to moderate disease refractory to conservative management includes monotherapy with dihydropyridine calcium channel blockers. For severe disease, combination therapy involves addition of other classes of medications including phosphodiesterase 5 inhibitors, topical nitrates, angiotensin receptor blockers, or selective serotonin reuptake inhibitors. Intravenous prostacyclin, endothelin receptor blockers, and onabotulinumtoxinA injections may be added as third-line therapy. Finally, surgical management including sympathectomy with continued pharmacologic therapy may be needed for disease recalcitrant to the aforementioned options.2
OnabotulinumtoxinA is a neurotoxin produced by the bacterium Clostridium botulinum. The toxin’s mechanism of action involves inhibition of the release of presynaptic acetylcholine-containing vesicles at the neuromuscular junction through cleavage of sensory nerve action potential receptor proteins. In addition, it inhibits smooth muscle vasoconstriction and pain by blocking α2-adrenergic receptors on blood vessels and chronic pain-transmitting C fibers in nerves, respectively.3,5
Only recently has onabotulinumtoxinA been used for treatment of RP. Botulinum toxin is approved for the treatment of spastic and dystonic diseases such as blepharospasm, headaches in patients with chronic migraines, upper limb spasticity, cervical dystonia, torticollis, ocular strabismus, and hyperhidrosis.3 However, the versatility of its therapeutic effects is evident in its broad off-label clinical applications, including achalasia; carpal tunnel syndrome; and spasticity relating to stroke, paraplegia, and cerebral palsy, among many others.5
Few studies have analyzed the use of onabotulinumtoxinA for the treatment of RP.3,6 There is no consensus yet regarding dose, dilution, or injection sites. One vial of onabotulinumtoxinA contains 100 U and is reconstituted in 20 mL of normal saline to produce 5 U/mL. The simplest technique involves the injection of 5 U into the medial and lateral aspects of each finger at its base, at the level of or just proximal to the A1 pulley, for a total of 50 U per hand.7 In the foot, injection can be made at the base of each toe near the proximal phalanges. A regimen of 50 to 100 U per hand was used by Neumeister et al5 on 19 patients, who subsequently standardized it to 10 U on each neurovascular bundle in a follow-up study,7 giving a total volume of 2 mL per injection. Associated pain or a burning sensation initially may be experienced, which may be mitigated by a lidocaine hydrochloride wrist block prior to injection.7 This technique produced immediate and lasting pain relief, increased tissue perfusion, and resolved digital ulcers in 28 of 33 patients. Most patients reported immediate relief, and a few noted gradual reduction in pain and resolution of chronic ulcers within 2 months. Of the 33 patients, 7 (21.2%) required repeat injections for recurrent pain, but the majority were pain free up to 6 years later with a single injection schedule.7
Injection into the palmar region, wrists, and/or fingers also may be performed. Effects of using different injection sites (eg, neurovascular bundle, distal palm, proximal hand) have been explored and were not notably different between these locations.8 Lastly, the frequency of injections may be attenuated according to the spectrum and severity of the patient’s symptoms. In a report of 11 patients who received a total of 100 U of onabotulinumtoxinA per hand, 5 required repeat injections within 3 to 8 months.9
Studies have reported onabotulinumtoxinA to be a promising option for the treatment of intractable symptoms. Likewise, our patient had a notable reduction in pain with signs of clinical improvement within 24 to 48 hours after injection. The need for amputation 6 months later likely was because the patient’s toes were already necrosing prior to treatment with onabotulinumtoxinA. Thus, the timing of intervention may play a critical role in response to onabotulinumtoxinA injections, particularly because the severity of our patient’s presentation was comparable to other cases reported in the literature. Even in reports using a smaller dose—2 U injected into each toe as opposed to 10 U per toe, as in our case—follow-up showed favorable results.10 In other reports, response can be perceived within days to a week, with remarkable improvement of numbness, pain, digit color, and wound resolution, in addition to decreased frequency and severity of attacks. Moreover, greater vasodilation and subsequent tissue perfusion have been evidenced by objective measures including digital transcutaneous oxygen saturation and Doppler sonography.7,8 Side effects, which are minimal and temporary, include local pain triggering a vasospastic attack and intrinsic muscle weakness; more rarely, dysesthesia and thenar eminence atrophy have been reported.11
Available studies have shown onabotulinumtoxinA to produce favorable results in the treatment of vasospastic disease. We suspect that an earlier intervention for our patient—before necrosis of the toes developed—would have led to a more positive outcome, consistent with other reports. Treatment with onabotulinumtoxinA is an approach to consider when the standard-of-care treatments for RP have been exhausted, as timely intervention may prevent the need for surgery. The indications and appropriate dosing protocol remain to be defined, in addition to more thorough evaluation of its efficacy relative to other medical and surgical options.
To the Editor:
Raynaud phenomenon (RP) is an episodic vasospasm of the digits that can lead to ulceration, gangrene, and autoamputation with prolonged ischemia. OnabotulinumtoxinA has been implemented as a treatment of intractable RP by paralyzing the muscles of the digital arteries. We report a case of a woman with severe RP secondary to systemic lupus erythematosus (SLE) who was treated with onabotulinumtoxinA injections after multiple treatment modalities failed to improve her condition. We describe the dosage and injection technique used to produce clinical improvement in our patient and compare it to prior reports in the literature.
A 33-year-old woman presented to the emergency department for worsening foot pain of 5 days' duration with dusky purple color changes concerning for impending Raynaud crisis related to RP. The patient had a history of antiphospholipid antibody syndrome (APS) and SLE with overlapping symptoms of polymyositis and scleroderma. She had been hospitalized for RP multiple times prior to the current admission. She was medically managed with nifedipine, sildenafil, losartan potassium, aspirin, alprostadil, and prostaglandin infusions, and was surgically managed with a right-hand sympathectomy and right ulnar artery bypass graft that had subsequently thrombosed. At the current presentation, she had painful dusky toes on both feet though more pronounced on the left foot. She endorsed foot pain while walking and tenderness to palpation of the fingers, which were minimally improved with intravenous prostaglandins.
Physical examination revealed blanching of the digits in both hands with pits in the right fourth and left first digits. Dusky patches overlaid all the toes as well as the superior plantar aspects of the feet (Figure 1). Given the history of APS, a punch biopsy was performed on the left medial plantar foot and results showed no histologic evidence of vasculitis or vasculopathy. Necrotic foci were present on the left and right second metatarsal bones, which were not reperfusable (Figure 2). The clinical findings and punch biopsy results favored RP as opposed to vasculopathy from APS.
Several interventions were attempted, and after 4 days with no response, the patient agreed to receive treatment with onabotulinumtoxinA. OnabotulinumtoxinA (5 U) was injected into the subcutaneous tissue of the medial and lateral aspects of each of the first and second toes near the proximal phalanges (40 U total). However, treatment could not be completed due to severe pain caused by the injections despite preprocedure regional nerve blocks to both lower extremities, preinjection icing, and lorazepam. Two days later, the patient tolerated onabotulinumtoxinA injections of all remaining digits of both feet (60 U total). She noted slight clinical improvement soon thereafter. One week after treatment of all 10 toes, she reported decreased pain and reduced duskiness of both feet (Figure 3).
One month later, the patient endorsed recurring pain in the hands and feet. Physical examination revealed reticular cyanosis and increased violaceous patches of the hands; the feet were overall unchanged from the prior hospitalization. At 4-month follow-up, there was gangrene on the left second, third, and fifth toe in addition to areas of induration noted on the fingers. She was repeatedly hospitalized over the next 6 months for pain management and gangrene of the toes, and finally underwent an amputation of the left and right second toe at the proximal and middle phalanx, respectively. She currently is continuing extensive medical management for pain and gangrene of the digits; she has not received additional onabotulinumtoxinA injections.
Raynaud phenomenon is a vascular disorder characterized by intermittent arteriolar vasospasm of the digits, often due to cold temperature or stress. Approximately 90% of RP cases are primarily idiopathic, with the remaining cases secondary to other diseases, typically systemic sclerosis, SLE, or mixed connective tissue disease.1 Symptoms present with characteristic changing of hands from white (ischemia) to blue (hypoxia) to red (reperfusion). Episodic attacks of vasospasm and ischemia can be painful and lead to digital ulcerations and necrosis of the digits or hands. Other complications including digital tuft pits, pterygium inversum unguis, or torturous nail fold capillaries with capillary dropout also may be seen.2
Although the etiology is multifactorial, the pathophysiology primarily is due to an imbalance of vasodilation and vasoconstriction. Perturbed levels of vasodilatory mediators include nitric oxide, prostacyclin, and calcitonin gene-related peptide.3 Meanwhile, abnormal neural sympathetic control of α-adrenergic receptors located on smooth muscle vasculature and subsequent endothelial hyperproliferation may contribute to inappropriate vasoconstriction.4
The first-line therapy for mild to moderate disease refractory to conservative management includes monotherapy with dihydropyridine calcium channel blockers. For severe disease, combination therapy involves addition of other classes of medications including phosphodiesterase 5 inhibitors, topical nitrates, angiotensin receptor blockers, or selective serotonin reuptake inhibitors. Intravenous prostacyclin, endothelin receptor blockers, and onabotulinumtoxinA injections may be added as third-line therapy. Finally, surgical management including sympathectomy with continued pharmacologic therapy may be needed for disease recalcitrant to the aforementioned options.2
OnabotulinumtoxinA is a neurotoxin produced by the bacterium Clostridium botulinum. The toxin’s mechanism of action involves inhibition of the release of presynaptic acetylcholine-containing vesicles at the neuromuscular junction through cleavage of sensory nerve action potential receptor proteins. In addition, it inhibits smooth muscle vasoconstriction and pain by blocking α2-adrenergic receptors on blood vessels and chronic pain-transmitting C fibers in nerves, respectively.3,5
Only recently has onabotulinumtoxinA been used for treatment of RP. Botulinum toxin is approved for the treatment of spastic and dystonic diseases such as blepharospasm, headaches in patients with chronic migraines, upper limb spasticity, cervical dystonia, torticollis, ocular strabismus, and hyperhidrosis.3 However, the versatility of its therapeutic effects is evident in its broad off-label clinical applications, including achalasia; carpal tunnel syndrome; and spasticity relating to stroke, paraplegia, and cerebral palsy, among many others.5
Few studies have analyzed the use of onabotulinumtoxinA for the treatment of RP.3,6 There is no consensus yet regarding dose, dilution, or injection sites. One vial of onabotulinumtoxinA contains 100 U and is reconstituted in 20 mL of normal saline to produce 5 U/mL. The simplest technique involves the injection of 5 U into the medial and lateral aspects of each finger at its base, at the level of or just proximal to the A1 pulley, for a total of 50 U per hand.7 In the foot, injection can be made at the base of each toe near the proximal phalanges. A regimen of 50 to 100 U per hand was used by Neumeister et al5 on 19 patients, who subsequently standardized it to 10 U on each neurovascular bundle in a follow-up study,7 giving a total volume of 2 mL per injection. Associated pain or a burning sensation initially may be experienced, which may be mitigated by a lidocaine hydrochloride wrist block prior to injection.7 This technique produced immediate and lasting pain relief, increased tissue perfusion, and resolved digital ulcers in 28 of 33 patients. Most patients reported immediate relief, and a few noted gradual reduction in pain and resolution of chronic ulcers within 2 months. Of the 33 patients, 7 (21.2%) required repeat injections for recurrent pain, but the majority were pain free up to 6 years later with a single injection schedule.7
Injection into the palmar region, wrists, and/or fingers also may be performed. Effects of using different injection sites (eg, neurovascular bundle, distal palm, proximal hand) have been explored and were not notably different between these locations.8 Lastly, the frequency of injections may be attenuated according to the spectrum and severity of the patient’s symptoms. In a report of 11 patients who received a total of 100 U of onabotulinumtoxinA per hand, 5 required repeat injections within 3 to 8 months.9
Studies have reported onabotulinumtoxinA to be a promising option for the treatment of intractable symptoms. Likewise, our patient had a notable reduction in pain with signs of clinical improvement within 24 to 48 hours after injection. The need for amputation 6 months later likely was because the patient’s toes were already necrosing prior to treatment with onabotulinumtoxinA. Thus, the timing of intervention may play a critical role in response to onabotulinumtoxinA injections, particularly because the severity of our patient’s presentation was comparable to other cases reported in the literature. Even in reports using a smaller dose—2 U injected into each toe as opposed to 10 U per toe, as in our case—follow-up showed favorable results.10 In other reports, response can be perceived within days to a week, with remarkable improvement of numbness, pain, digit color, and wound resolution, in addition to decreased frequency and severity of attacks. Moreover, greater vasodilation and subsequent tissue perfusion have been evidenced by objective measures including digital transcutaneous oxygen saturation and Doppler sonography.7,8 Side effects, which are minimal and temporary, include local pain triggering a vasospastic attack and intrinsic muscle weakness; more rarely, dysesthesia and thenar eminence atrophy have been reported.11
Available studies have shown onabotulinumtoxinA to produce favorable results in the treatment of vasospastic disease. We suspect that an earlier intervention for our patient—before necrosis of the toes developed—would have led to a more positive outcome, consistent with other reports. Treatment with onabotulinumtoxinA is an approach to consider when the standard-of-care treatments for RP have been exhausted, as timely intervention may prevent the need for surgery. The indications and appropriate dosing protocol remain to be defined, in addition to more thorough evaluation of its efficacy relative to other medical and surgical options.
- Neumeister MW. The role of botulinum toxin in vasospastic disorders of the hand. Hand Clin. 2015;31:23-37. doi:10.1016/j.hcl.2014.09.003
- Bakst R, Merola JF, Franks AG, et al. Raynaud’s phenomenon: pathogenesis and management. J Am Acad Dermatol. 2008;59:633-653. doi:10.1016/j.jaad.2008.06.004
- Iorio ML, Masden DL, Higgins JP. Botulinum toxin a treatment of Raynaud’s phenomenon: a review. Semin Arthritis Rheum. 2012;41:599-603. doi:10.1016/j.semarthrit.2011.07.006
- Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375:556-565. doi:10.1056/NEJMra1507638
- Neumeister MW, Chambers CB, Herron MS, et al. Botox therapy for ischemic digits. Plast Reconstr Surg. 2009;124:191-200. doi:10.1097/PRS.0b013e3181a80576
- Sycha T, Graninger M, Auff E, et al. Botulinum toxin in the treatment of Raynaud’s phenomenon: a pilot study. Eur J Clin Invest. 2004;34:312-313. doi:10.1016/j.jaad.2013.06.029
- Neumeister MW. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085-2092. doi:10.1016/j.jhsa.2010.09.019
- Fregene A, Ditmars D, Siddiqui A. Botulinum toxin type A: a treatment option for digital ischemia in patients with Raynaud’s phenomenon. J Hand Surg Am. 2009;34:446-452. doi:10.1016/j.jhsa.2008.11.026
- Van Beek AL, Lim PK, Gear AJL, et al. Management of vasospastic disorders with botulinum toxin A. Plast Reconstr Surg. 2007;119:217-226. doi:10.1097/01.prs.0000244860.00674.57
- Dhaliwal K, Griffin M, Denton CP, et al. The novel use of botulinum toxin A for the treatment of Raynaud’s phenomenon in the toes. BMJ Case Rep. 2018;2018:2017-2019. doi:10.1136/bcr-2017-219348
- Eickhoff JC, Smith JK, Landau ME, et al. Iatrogenic thenar eminence atrophy after Botox A injection for secondary Raynaud phenomenon. J Clin Rheumatol. 2016;22:395-396. doi:10.1097/RHU.0000000000000450
- Neumeister MW. The role of botulinum toxin in vasospastic disorders of the hand. Hand Clin. 2015;31:23-37. doi:10.1016/j.hcl.2014.09.003
- Bakst R, Merola JF, Franks AG, et al. Raynaud’s phenomenon: pathogenesis and management. J Am Acad Dermatol. 2008;59:633-653. doi:10.1016/j.jaad.2008.06.004
- Iorio ML, Masden DL, Higgins JP. Botulinum toxin a treatment of Raynaud’s phenomenon: a review. Semin Arthritis Rheum. 2012;41:599-603. doi:10.1016/j.semarthrit.2011.07.006
- Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375:556-565. doi:10.1056/NEJMra1507638
- Neumeister MW, Chambers CB, Herron MS, et al. Botox therapy for ischemic digits. Plast Reconstr Surg. 2009;124:191-200. doi:10.1097/PRS.0b013e3181a80576
- Sycha T, Graninger M, Auff E, et al. Botulinum toxin in the treatment of Raynaud’s phenomenon: a pilot study. Eur J Clin Invest. 2004;34:312-313. doi:10.1016/j.jaad.2013.06.029
- Neumeister MW. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085-2092. doi:10.1016/j.jhsa.2010.09.019
- Fregene A, Ditmars D, Siddiqui A. Botulinum toxin type A: a treatment option for digital ischemia in patients with Raynaud’s phenomenon. J Hand Surg Am. 2009;34:446-452. doi:10.1016/j.jhsa.2008.11.026
- Van Beek AL, Lim PK, Gear AJL, et al. Management of vasospastic disorders with botulinum toxin A. Plast Reconstr Surg. 2007;119:217-226. doi:10.1097/01.prs.0000244860.00674.57
- Dhaliwal K, Griffin M, Denton CP, et al. The novel use of botulinum toxin A for the treatment of Raynaud’s phenomenon in the toes. BMJ Case Rep. 2018;2018:2017-2019. doi:10.1136/bcr-2017-219348
- Eickhoff JC, Smith JK, Landau ME, et al. Iatrogenic thenar eminence atrophy after Botox A injection for secondary Raynaud phenomenon. J Clin Rheumatol. 2016;22:395-396. doi:10.1097/RHU.0000000000000450
Practice Points
- Raynaud phenomenon (RP) is a vascular disorder characterized by episodic vasospasms of the digits often due to cold temperature or stress.
- OnabotulinumtoxinA has been implemented as a treatment of intractable RP after failure with traditional treatments, such as calcium channel blockers, angiotensin receptor blockers, prostaglandins, endothelin receptor blockers, and phosphodiesterase 5 inhibitors.
- A standard technique of delivery of onabotulinumtoxinA involves injection of 5 U/mL into the medial and lateral aspects of each finger at its base (near the metacarpal head) for a total of 50 U per hand or foot.
New finasteride lawsuit brings renewed attention to psychiatric, ED adverse event reports
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.
A new merit a closer look and, potentially, better counseling and monitoring from clinicians.
The nonprofit advocacy group Public Citizen filed the suit on behalf of the Post-Finasteride Syndrome Foundation (PFSF) in the U.S. District Court for the District of Columbia. The PFSF had filed a citizen’s petition in 2017 that requested that the FDA either take the 1-mg formulation off the market, or add warnings about the potential for erectile dysfunction, depression, and suicidal ideation, among other adverse reactions.
The PFSF has alleged that long-term use of Propecia (and its generic equivalents) can lead to postfinasteride syndrome (PFS), characterized by sexual dysfunction and psycho-neurocognitive symptoms. The symptoms may continue long after men stop taking the drug, according to PFSF.
Public Citizen said the FDA needs to take action in part because U.S. prescriptions of the hair loss formulation “more than doubled from 2015 to 2020,” and online and telemedicine companies such as Hims, Roman, and Keeps “aggressively market and sell generic finasteride for hair loss.” According to GoodRx, a 1-month supply of generic 1-mg tablets costs as little as $8-$10.
Both Canadian and British regulatory authorities have added warnings about depression and suicide to the Propecia label but the FDA has not changed its labeling. An agency spokesperson told this news organization that the “FDA does not comment on the status of pending citizen petitions or on pending litigation.”
Propecia’s developer, Merck, has not responded to several requests for comment from this news organization.
Why some patients develop PFS and others do not is still not understood, but some clinicians said they counsel all patients on the risks of severe and persistent side effects that have been associated with Propecia.
Robert M. Bernstein, MD, of the department of dermatology at Columbia University, New York, and a fellow of the International Society of Hair Restoration Surgery, said that 2%-4% of his patients have some side effects, similar to the original reported incidence, with sexual dysfunction being the most common.
If a man experiences an adverse effect, the drug should be stopped, Dr. Bernstein said in an interview. He noted that “there seems to be a significant increased risk of persistent side effects in people with certain psychiatric conditions, and those people should be counseled carefully before considering the medication.”
“Everybody should be warned that the risk of persistent side effects is real but in the average person it is quite uncommon,” added Dr. Bernstein, founder of Bernstein Medical, a division of Schweiger Dermatology Group focusing on the diagnosis and treatment of hair loss. “I don’t think it should be withdrawn from the market,” he said.
Alan Jacobs, MD, a Manhattan-based neuroendocrinologist and behavioral neurologist in private practice who said he has treated hundreds of men for PFS, and who is an expert witness for the plaintiff in a suit alleging that finasteride led to a man’s suicide, said that taking the drug off the market would be unfortunate because it helps so many men. “I don’t think you need to get rid of the drug per se,” he said in an interview. “But very rapidly, people need to do clinical research to find out how to predict who’s more at risk,” he added.
Michael S. Irwig, MD, associate professor of medicine at Harvard Medical School, Boston, who has studied the persistent sexual and nonsexual side effects of finasteride, said he believes there should be a boxed warning on the finasteride label to let the men who take it “know that they can have permanent persistent sexual dysfunction, and/or depression and suicide have been noted with this medicine.
“Those who prescribe it should be having a conversation with patients about the potential risks and benefits so that everybody knows about the potential before they get on the medicine,” said Dr. Irwig, who also is an endocrinologist at Beth Israel Deaconess Medical Center in Boston.
Other countries warn of psychiatric effects
The FDA approved the 1-mg form of finasteride for male pattern hair loss in 1997.
In 2012, the label and the patient insert were updated to state that side effects included less desire for sex, erectile dysfunction, and a decrease in the amount of semen produced, but that those adverse events occurred in less than 2% of men and generally went away in most men who stopped taking the drug.
That label change unleashed a flood of more than 1,000 lawsuits against Merck. The company reportedly settled at least half of them for $4.3 million in 2018. The Superior Court of New Jersey closed out the consolidated class action against Merck in May 2021, noting that all of the cases had been settled or dismissed.
The suits generally accused Merck of not giving adequate warning about sexual side effects, according to an investigation by Reuters. That 2019 special report found that Merck had understated the number of men who experienced sexual side effects and the duration of those symptoms. The news organization also reported that from 2009 to 2018, the FDA received 5,000 reports of sexual or mental health side effects – and sometimes both – in men who took finasteride. Some 350 of the men reported suicidal thoughts, and there were 50 reports of suicide.
Public Citizen’s lawsuit alleges that VigiBase, which is managed by the World Health Organization Collaborating Centre for International Drug Monitoring, lists 378 cases of suicidal ideation, 39 cases of suicide attempt, and 88 cases of completed suicide associated with finasteride use. VigiBase collects data from 153 countries on adverse reactions to medications.
In February 2021, more documents from the class action lawsuits were unsealed in response to a Reuters request. According to the news organization, the documents showed that Merck knew of reports of depression, including suicidal thoughts, as early as 2009.
However, according to Reuters, the FDA in 2011 granted Merck’s request to only note depression as a potential side effect, without including the risk of suicidal ideation.
The current FDA label notes a small incidence of sexual dysfunction, including decreased libido (1.8% in trials) and erectile dysfunction (1.3%) and mentions depression as a side effect observed during the postmarketing period.
The Canadian label has the same statistics on sexual side effects but is much stronger on mental adverse effects: “Psychiatric disorders: mood alterations and depression, decreased libido that continued after discontinuation of treatment. Mood alterations including depressed mood and, less frequently, suicidal ideation have been reported in patients treated with finasteride 1 mg. Patients should be monitored for psychiatric symptoms, and if these occur, the patient should be advised to seek medical advice.”
In the United Kingdom, patients prescribed the drug are given a leaflet, which notes that “Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Propecia,” and advises patients to stop taking the drug if they experience any of those symptoms and to discuss it with their physician.
Public Citizen noted in its lawsuit that French and German drug regulators have sent letters to clinicians advising them to inform patients of the risk of suicidal thoughts and anxiety.
Is there biological plausibility?
To bolster its argument that finasteride has dangerous psychiatric side effects, the advocacy organization cited a study first published in JAMA Dermatology in late 2020 that investigated suicidality and psychological adverse events in patients taking finasteride.
David-Dan Nguyen, MPH, and his colleagues at Brigham and Women’s Hospital in Boston, McGill University, Montreal, and the University of Montreal, examined the VigiBase database and found 356 cases of suicidality and 2,926 psychological adverse events; cases were highest from 2015 to 2019.
They documented what they called a “significant disproportionality signal for suicidality (reporting odds ratio, 1.63; 95% confidence interval, 2.90-4.15) and psychological adverse events (ROR, 4.33; 95% CI, 4.17-4.49) with finasteride, especially in younger men and those with alopecia, but not in older men or those with benign prostatic hyperplasia.
The study authors noted that some studies have suggested that men with depression have low levels of the neurosteroid allopregnanolone, which is produced by the 5-alpha reductase enzyme. Finasteride is a 5-alpha reductase inhibitor.
According to Public Citizen’s lawsuit, “The product labeling does not disclose important information about finasteride’s mechanism of action,” and “the drug inhibits multiple steroid hormone pathways that are responsible for the formation of brain neurosteroids that regulate many critical functions in the central nervous system, like sexual function, mood, sleep, cognitive function, the stress response, and motivation.”
Dr. Jacobs said that “there’s a lot of good solid high-quality research, mostly in animals, but also some on humans, showing a plausible link between blocking 5-alpha reductase in the brain, deficiency of neuroactive steroids, and depression.”
The author of an accompanying editorial, Roger S. Ho, MD, MPH, an associate professor in the department of dermatology, New York University, was skeptical. “Without a plausible biological hypothesis pharmacodynamically linking the drug and the reported adverse event, this kind of analysis may lead to false findings,” Dr. Ho said in the editorial about the Nguyen study.
Dr. Ho also wrote that he believed that the lack of a suicidality signal for dutasteride, a drug with a similar mechanism of action, but without as much media attention, “hints at a potential reporting bias unique to finasteride.”
He recommended that clinicians “conduct a full evaluation and a detailed, personalized risk-benefit assessment for patients before each prescription of finasteride.”
Important medicine, important caveats
Dr. Jacobs said that many of the men who come to him with side effects after taking finasteride have “been blown off by most of the doctors they go to see.”
Urologists dismiss them because their sexual dysfunction is not a gonad issue. They are told that it’s in their head, said Dr. Jacobs, adding that, “it is in their head, but it’s biological.”
The drug’s label advises that sexual side effects disappear when the drug is stopped. “That’s only true most of the time, not all of the time,” said Dr. Jacobs, adding that the persistence of any side effects impacts what he calls a “small subset” of men who take the drug.
“We have treated tens of thousands of patients who have benefited from the medicine and had no side effects,” said Dr. Bernstein. “But there is a lot that’s still not known about it.”
Even so, “baldness in young people is not a benign condition,” he said, adding that it can be socially debilitating. “An 18-year-old with a full head of thick hair who’s totally bald in 3 or 4 years – that can totally change his psyche,” Dr. Bernstein said. Finasteride may be the best option for those young men, and it is an important medication, he said. Does it need to be used more carefully? “Certainly you can’t argue with that,” he commented.
Dr. Bernstein and Dr. Irwig reported no conflicts. Dr. Jacobs disclosed that he is an expert witness for the plaintiffs in a suit against Propecia maker Merck.