User login
For MD-IQ use only
Teaching Tips for Dermatology Residents
Dermatology residents interact with trainees of various levels throughout the workday—from undergraduate or even high school students to postgraduate fellows. Depending on the institution’s training program, residents may have responsibilities to teach through lecture series such as Grand Rounds and didactics. Therefore, it is an integral part of resident training to become educators in addition to being learners; however, formal pedagogy education is rare in dermatology programs. 1,2 Herein, I discuss several techniques that residents can apply to their practice to cultivate ideal learning environments and outcomes for trainees.
Creating Effective Teaching and Learning Experiences
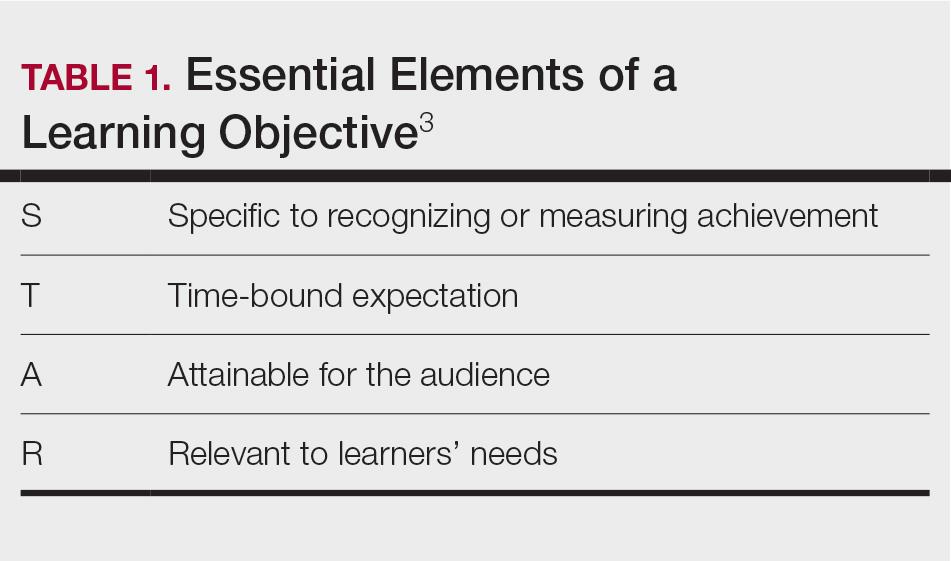
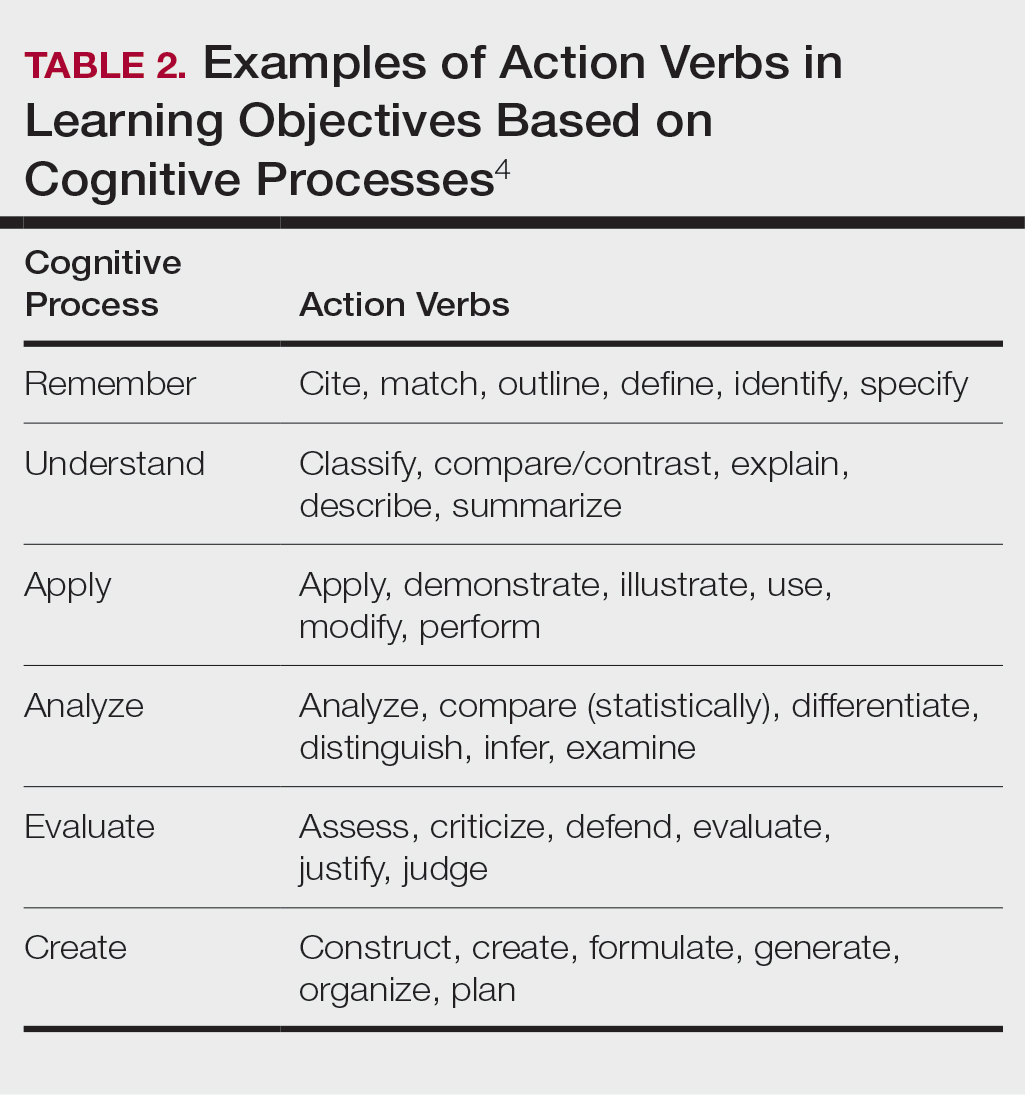
Planning to teach can be as important as teaching itself. Developing learning objectives can help to create effective teaching and learning experiences. Learning objectives should be specific, time bound, attainable, and learner centered (Table 1). It is recommended that residents aim for no more than 4 objectives per hour of learning.3 By creating clear learning objectives, residents can make connections between the content and any assessments. Bloom’s taxonomy of cognitive learning objectives gives guidance on action verbs to use in writing learning objectives depending on the cognitive process being tested (Table 2).4
Creating a Safe Educational Environment
Psychological safety is the belief that a learning environment is a safe place in which to take risks.5 A clinical learning environment that is psychologically safe can support trainee well-being and learning. Cultivating a safe educational environment may include addressing microaggressions and bias in the clinical workplace. Table 3 provides examples of statements using the 6 Ds, which can be used to mitigate these issues.6 The first 4—direct, distract, delegate, and defer—represent ways to respond to racism, microaggressions, and bias, and the last 2—display discomfort and debrief—are responses that may be utilized in any problematic incident. Residents can play an important supportive role in scenarios where learners are faced with an incident that may not be regarded as psychologically safe. This is especially true if the learner is at a lower training level than the dermatology resident. We all play a role in creating a safe workplace for our teams.

Teaching in the Clinic and Hospital
There are multiple challenges to teaching in both inpatient and outpatient environments, including limited space and time; thus, more informal teaching methods are common. For example, in an outpatient dermatology clinic, the patient schedule can become a “table of contents” of potential teaching and learning opportunities. This technique is called the focused half day.3,7 By reviewing the clinic schedule, students can focus on a specific area of interest or theme throughout the course of the day.3
Priming and framing are other focused techniques that work well in both outpatient and inpatient settings.3,8,9 Priming means alerting the trainee to upcoming learning objective(s) and focusing their attention on what to observe or do during a shared visit with a patient. Framing—instructing learners to collect information that is relevant to the diagnosis and treatment—allows trainees to help move patient care forward while the resident attends to other patients.3
Modeling involves describing a thought process out loud for a learner3,10; for example, prior to starting a patient encounter, a dermatology resident may clearly state the goal of a patient conversation to the learner, describe their thought process about the topic, summarize the important points, and ask the learner if they have any questions about what was just said. Using this technique, learners may have a better understanding of why and how to go about conducting a patient encounter after the resident models one for them.
Effectively Integrating Visual Media and Presentations
Research supported by the cognitive load theory and cognitive theory of multimedia learning has led to the assertion-evidence approach for creating presentation slides that are built around messages, not topics, and messages are supported with visuals, not bullets.3,11,12 For example, slides should be constructed with 1- to 2-line assertion statements as titles and relevant illustrations or figures as supporting evidence to enhance visual memory.3
Written text on presentation slides often is redundant with spoken narration and also decreases learning because of cognitive load. Busy background colors and/or designs consume working memory and also can be detrimental to learning. Limiting these common distractors in a presentation makes for more effective delivery and retention of knowledge.3
Final Thoughts
There are multiple avenues for teaching as a resident and not all techniques may be applicable depending on the clinical or academic scenario. This column provides a starting point for residents to augment their pedagogical skills, particularly because formal teaching on pedagogy is lacking in medical education.
- Burgin S, Zhong CS, Rana J. A resident-as-teacher program increases dermatology residents’ knowledge and confidence in teaching techniques: a pilot study. J Am Acad Dermatol. 2020;83:651-653. doi:10.1016/j.jaad.2019.12.008
- Burgin S, Homayounfar G, Newman LR, et al. Instruction in teaching and teaching opportunities for residents in US dermatology programs: results of a national survey. J Am Acad Dermatol. 2017;76:703-706. doi:10.1016/j.jaad.2016.08.043
- UNM School of Medicine Continuous Professional Learning. Residents as Educators. UNM School of Medicine; 2023.
- Bloom BS. Taxonomy of Educational Objectives. Book 1, Cognitive Domain. Longman; 1979.
- McClintock AH, Fainstad T, Blau K, et al. Psychological safety in medical education: a scoping review and synthesis of the literature. Med Teach. 2023;45:1290-1299. doi:10.1080/0142159X.2023.2216863
- Ackerman-Barger K, Jacobs NN, Orozco R, et al. Addressing microaggressions in academic health: a workshop for inclusiveexcellence. MedEdPORTAL. 2021;17:11103. doi:10.15766/mep_2374-8265.11103
- Taylor C, Lipsky MS, Bauer L. Focused teaching: facilitating early clinical experience in an office setting. Fam Med. 1998;30:547-548.
- Pan Z, Kosicki G. Framing analysis: an approach to news discourse. Polit Commun. 1993;10:55-75. doi:10.1080/10584609.1993.9962963
- Price V, Tewksbury D, Powers E. Switching trains of thought: the impact of news frames on readers’ cognitive responses. Commun Res. 1997;24:481-506. doi:10.1177/009365097024005002
- Haston W. Teacher modeling as an effective teaching strategy. Music Educators J. 2007;93:26. doi:10.2307/4127130
- Alley M. Build your scientific talk on messages, not topics. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385725653
- Alley M. Support your presentation messages with visual evidence, not bullet lists. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385729603
Dermatology residents interact with trainees of various levels throughout the workday—from undergraduate or even high school students to postgraduate fellows. Depending on the institution’s training program, residents may have responsibilities to teach through lecture series such as Grand Rounds and didactics. Therefore, it is an integral part of resident training to become educators in addition to being learners; however, formal pedagogy education is rare in dermatology programs. 1,2 Herein, I discuss several techniques that residents can apply to their practice to cultivate ideal learning environments and outcomes for trainees.
Creating Effective Teaching and Learning Experiences
Planning to teach can be as important as teaching itself. Developing learning objectives can help to create effective teaching and learning experiences. Learning objectives should be specific, time bound, attainable, and learner centered (Table 1). It is recommended that residents aim for no more than 4 objectives per hour of learning.3 By creating clear learning objectives, residents can make connections between the content and any assessments. Bloom’s taxonomy of cognitive learning objectives gives guidance on action verbs to use in writing learning objectives depending on the cognitive process being tested (Table 2).4
Creating a Safe Educational Environment
Psychological safety is the belief that a learning environment is a safe place in which to take risks.5 A clinical learning environment that is psychologically safe can support trainee well-being and learning. Cultivating a safe educational environment may include addressing microaggressions and bias in the clinical workplace. Table 3 provides examples of statements using the 6 Ds, which can be used to mitigate these issues.6 The first 4—direct, distract, delegate, and defer—represent ways to respond to racism, microaggressions, and bias, and the last 2—display discomfort and debrief—are responses that may be utilized in any problematic incident. Residents can play an important supportive role in scenarios where learners are faced with an incident that may not be regarded as psychologically safe. This is especially true if the learner is at a lower training level than the dermatology resident. We all play a role in creating a safe workplace for our teams.



Teaching in the Clinic and Hospital
There are multiple challenges to teaching in both inpatient and outpatient environments, including limited space and time; thus, more informal teaching methods are common. For example, in an outpatient dermatology clinic, the patient schedule can become a “table of contents” of potential teaching and learning opportunities. This technique is called the focused half day.3,7 By reviewing the clinic schedule, students can focus on a specific area of interest or theme throughout the course of the day.3
Priming and framing are other focused techniques that work well in both outpatient and inpatient settings.3,8,9 Priming means alerting the trainee to upcoming learning objective(s) and focusing their attention on what to observe or do during a shared visit with a patient. Framing—instructing learners to collect information that is relevant to the diagnosis and treatment—allows trainees to help move patient care forward while the resident attends to other patients.3
Modeling involves describing a thought process out loud for a learner3,10; for example, prior to starting a patient encounter, a dermatology resident may clearly state the goal of a patient conversation to the learner, describe their thought process about the topic, summarize the important points, and ask the learner if they have any questions about what was just said. Using this technique, learners may have a better understanding of why and how to go about conducting a patient encounter after the resident models one for them.
Effectively Integrating Visual Media and Presentations
Research supported by the cognitive load theory and cognitive theory of multimedia learning has led to the assertion-evidence approach for creating presentation slides that are built around messages, not topics, and messages are supported with visuals, not bullets.3,11,12 For example, slides should be constructed with 1- to 2-line assertion statements as titles and relevant illustrations or figures as supporting evidence to enhance visual memory.3
Written text on presentation slides often is redundant with spoken narration and also decreases learning because of cognitive load. Busy background colors and/or designs consume working memory and also can be detrimental to learning. Limiting these common distractors in a presentation makes for more effective delivery and retention of knowledge.3
Final Thoughts
There are multiple avenues for teaching as a resident and not all techniques may be applicable depending on the clinical or academic scenario. This column provides a starting point for residents to augment their pedagogical skills, particularly because formal teaching on pedagogy is lacking in medical education.
Dermatology residents interact with trainees of various levels throughout the workday—from undergraduate or even high school students to postgraduate fellows. Depending on the institution’s training program, residents may have responsibilities to teach through lecture series such as Grand Rounds and didactics. Therefore, it is an integral part of resident training to become educators in addition to being learners; however, formal pedagogy education is rare in dermatology programs. 1,2 Herein, I discuss several techniques that residents can apply to their practice to cultivate ideal learning environments and outcomes for trainees.
Creating Effective Teaching and Learning Experiences
Planning to teach can be as important as teaching itself. Developing learning objectives can help to create effective teaching and learning experiences. Learning objectives should be specific, time bound, attainable, and learner centered (Table 1). It is recommended that residents aim for no more than 4 objectives per hour of learning.3 By creating clear learning objectives, residents can make connections between the content and any assessments. Bloom’s taxonomy of cognitive learning objectives gives guidance on action verbs to use in writing learning objectives depending on the cognitive process being tested (Table 2).4
Creating a Safe Educational Environment
Psychological safety is the belief that a learning environment is a safe place in which to take risks.5 A clinical learning environment that is psychologically safe can support trainee well-being and learning. Cultivating a safe educational environment may include addressing microaggressions and bias in the clinical workplace. Table 3 provides examples of statements using the 6 Ds, which can be used to mitigate these issues.6 The first 4—direct, distract, delegate, and defer—represent ways to respond to racism, microaggressions, and bias, and the last 2—display discomfort and debrief—are responses that may be utilized in any problematic incident. Residents can play an important supportive role in scenarios where learners are faced with an incident that may not be regarded as psychologically safe. This is especially true if the learner is at a lower training level than the dermatology resident. We all play a role in creating a safe workplace for our teams.



Teaching in the Clinic and Hospital
There are multiple challenges to teaching in both inpatient and outpatient environments, including limited space and time; thus, more informal teaching methods are common. For example, in an outpatient dermatology clinic, the patient schedule can become a “table of contents” of potential teaching and learning opportunities. This technique is called the focused half day.3,7 By reviewing the clinic schedule, students can focus on a specific area of interest or theme throughout the course of the day.3
Priming and framing are other focused techniques that work well in both outpatient and inpatient settings.3,8,9 Priming means alerting the trainee to upcoming learning objective(s) and focusing their attention on what to observe or do during a shared visit with a patient. Framing—instructing learners to collect information that is relevant to the diagnosis and treatment—allows trainees to help move patient care forward while the resident attends to other patients.3
Modeling involves describing a thought process out loud for a learner3,10; for example, prior to starting a patient encounter, a dermatology resident may clearly state the goal of a patient conversation to the learner, describe their thought process about the topic, summarize the important points, and ask the learner if they have any questions about what was just said. Using this technique, learners may have a better understanding of why and how to go about conducting a patient encounter after the resident models one for them.
Effectively Integrating Visual Media and Presentations
Research supported by the cognitive load theory and cognitive theory of multimedia learning has led to the assertion-evidence approach for creating presentation slides that are built around messages, not topics, and messages are supported with visuals, not bullets.3,11,12 For example, slides should be constructed with 1- to 2-line assertion statements as titles and relevant illustrations or figures as supporting evidence to enhance visual memory.3
Written text on presentation slides often is redundant with spoken narration and also decreases learning because of cognitive load. Busy background colors and/or designs consume working memory and also can be detrimental to learning. Limiting these common distractors in a presentation makes for more effective delivery and retention of knowledge.3
Final Thoughts
There are multiple avenues for teaching as a resident and not all techniques may be applicable depending on the clinical or academic scenario. This column provides a starting point for residents to augment their pedagogical skills, particularly because formal teaching on pedagogy is lacking in medical education.
- Burgin S, Zhong CS, Rana J. A resident-as-teacher program increases dermatology residents’ knowledge and confidence in teaching techniques: a pilot study. J Am Acad Dermatol. 2020;83:651-653. doi:10.1016/j.jaad.2019.12.008
- Burgin S, Homayounfar G, Newman LR, et al. Instruction in teaching and teaching opportunities for residents in US dermatology programs: results of a national survey. J Am Acad Dermatol. 2017;76:703-706. doi:10.1016/j.jaad.2016.08.043
- UNM School of Medicine Continuous Professional Learning. Residents as Educators. UNM School of Medicine; 2023.
- Bloom BS. Taxonomy of Educational Objectives. Book 1, Cognitive Domain. Longman; 1979.
- McClintock AH, Fainstad T, Blau K, et al. Psychological safety in medical education: a scoping review and synthesis of the literature. Med Teach. 2023;45:1290-1299. doi:10.1080/0142159X.2023.2216863
- Ackerman-Barger K, Jacobs NN, Orozco R, et al. Addressing microaggressions in academic health: a workshop for inclusiveexcellence. MedEdPORTAL. 2021;17:11103. doi:10.15766/mep_2374-8265.11103
- Taylor C, Lipsky MS, Bauer L. Focused teaching: facilitating early clinical experience in an office setting. Fam Med. 1998;30:547-548.
- Pan Z, Kosicki G. Framing analysis: an approach to news discourse. Polit Commun. 1993;10:55-75. doi:10.1080/10584609.1993.9962963
- Price V, Tewksbury D, Powers E. Switching trains of thought: the impact of news frames on readers’ cognitive responses. Commun Res. 1997;24:481-506. doi:10.1177/009365097024005002
- Haston W. Teacher modeling as an effective teaching strategy. Music Educators J. 2007;93:26. doi:10.2307/4127130
- Alley M. Build your scientific talk on messages, not topics. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385725653
- Alley M. Support your presentation messages with visual evidence, not bullet lists. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385729603
- Burgin S, Zhong CS, Rana J. A resident-as-teacher program increases dermatology residents’ knowledge and confidence in teaching techniques: a pilot study. J Am Acad Dermatol. 2020;83:651-653. doi:10.1016/j.jaad.2019.12.008
- Burgin S, Homayounfar G, Newman LR, et al. Instruction in teaching and teaching opportunities for residents in US dermatology programs: results of a national survey. J Am Acad Dermatol. 2017;76:703-706. doi:10.1016/j.jaad.2016.08.043
- UNM School of Medicine Continuous Professional Learning. Residents as Educators. UNM School of Medicine; 2023.
- Bloom BS. Taxonomy of Educational Objectives. Book 1, Cognitive Domain. Longman; 1979.
- McClintock AH, Fainstad T, Blau K, et al. Psychological safety in medical education: a scoping review and synthesis of the literature. Med Teach. 2023;45:1290-1299. doi:10.1080/0142159X.2023.2216863
- Ackerman-Barger K, Jacobs NN, Orozco R, et al. Addressing microaggressions in academic health: a workshop for inclusiveexcellence. MedEdPORTAL. 2021;17:11103. doi:10.15766/mep_2374-8265.11103
- Taylor C, Lipsky MS, Bauer L. Focused teaching: facilitating early clinical experience in an office setting. Fam Med. 1998;30:547-548.
- Pan Z, Kosicki G. Framing analysis: an approach to news discourse. Polit Commun. 1993;10:55-75. doi:10.1080/10584609.1993.9962963
- Price V, Tewksbury D, Powers E. Switching trains of thought: the impact of news frames on readers’ cognitive responses. Commun Res. 1997;24:481-506. doi:10.1177/009365097024005002
- Haston W. Teacher modeling as an effective teaching strategy. Music Educators J. 2007;93:26. doi:10.2307/4127130
- Alley M. Build your scientific talk on messages, not topics. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385725653
- Alley M. Support your presentation messages with visual evidence, not bullet lists. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385729603
Resident Pearls
- Emphasizing specific learning objectives, prioritizing safety in the learning environment, utilizing clinical teaching techniques, and using multimedia to present messages all contribute to effective dermatology teaching by residents.
Oncology Mergers Are on the Rise. How Can Independent Practices Survive?
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
FDA Approves Topical Anticholinergic for Axillary Hyperhidrosis
The .
According to a press release from Botanix Pharmaceuticals, which developed the product and will market it under the brand name Sofdra, approval was based on results from two phase 3 studies that enrolled 710 patients with primary axillary hyperhidrosis. In the trials, patients treated with sofpironium topical gel, 12.45%, experienced “clinically and statistically meaningful changes” from baseline in the Gravimetric Sweat Production and the Hyperhidrosis Disease Severity Measure–Axillary seven-item score, according to the company.
Botanix plans to enable qualified patients to gain early access to the product in the third quarter of 2024, with commercial sales expected in the fourth quarter of 2024.
A version of this article first appeared on Medscape.com.
The .
According to a press release from Botanix Pharmaceuticals, which developed the product and will market it under the brand name Sofdra, approval was based on results from two phase 3 studies that enrolled 710 patients with primary axillary hyperhidrosis. In the trials, patients treated with sofpironium topical gel, 12.45%, experienced “clinically and statistically meaningful changes” from baseline in the Gravimetric Sweat Production and the Hyperhidrosis Disease Severity Measure–Axillary seven-item score, according to the company.
Botanix plans to enable qualified patients to gain early access to the product in the third quarter of 2024, with commercial sales expected in the fourth quarter of 2024.
A version of this article first appeared on Medscape.com.
The .
According to a press release from Botanix Pharmaceuticals, which developed the product and will market it under the brand name Sofdra, approval was based on results from two phase 3 studies that enrolled 710 patients with primary axillary hyperhidrosis. In the trials, patients treated with sofpironium topical gel, 12.45%, experienced “clinically and statistically meaningful changes” from baseline in the Gravimetric Sweat Production and the Hyperhidrosis Disease Severity Measure–Axillary seven-item score, according to the company.
Botanix plans to enable qualified patients to gain early access to the product in the third quarter of 2024, with commercial sales expected in the fourth quarter of 2024.
A version of this article first appeared on Medscape.com.
Ixekizumab Met Phase 3 Trial Endpoint in Juvenile PsA, Enthesitis-Related Arthritis
VIENNA — Ixekizumab (Taltz), an interleukin-17A inhibitor that’s already approved for the treatment of psoriatic arthritis and axial spondyloarthritis in adults appears likely to be granted the same corresponding indications for children, based on initial results from an open-label, phase 3 trial that employed adalimumab as a reference.
With a safety profile comparable with that seen in adult patients, ixekizumab “met the prespecified criterion for success at 16 weeks,” reported Athimalaipet V. Ramanan, MD, PhD, of Bristol Royal Hospital for Children and Translational Health Sciences, Bristol, England.
In this multicenter, randomized, open-label trial called COSPIRIT-JIA, which is still ongoing, investigators enrolled 101 children with active juvenile PsA (JPsA) or enthesitis-related arthritis (ERA), which is akin to spondyloarthritis in adults.
The efficacy and safety data at 16 weeks were presented as a late-breaking abstract at the annual European Congress of Rheumatology. Dr. Ramanan said that the open-label extension to 104 weeks is underway and further follow-up out to 264 weeks is planned.
Nearly 90% Achieve ACR30
The trial had an adaptive design in which the first 40 patients without biologics experience were randomized to ixekizumab or adalimumab, stratified by JPsA or ERA diagnosis, and the following 61 patients with either no biologic experience or an inadequate response or intolerance to biologics all received ixekizumab. The drugs were dosed according to weight. Dr. Ramanan explained that a placebo-controlled trial was considered unethical because of the strong evidence of benefit from biologics for JPsA and ERA.
The trial easily met its predefined threshold for success, which required ≥ 80% probability, based on Bayesian analysis, that ≥ 50% of patients would have 30% improvement in American College of Rheumatology response criteria (ACR30) at week 16. ACR30 was achieved in 88.9% of those treated with ixekizumab overall vs 95.0% of those treated with adalimumab, but the trial was not designed as a head-to-head comparison. Rather, adalimumab served as a reference.
When compared for the distinct diseases, the ACR30 rates were also numerically lower for ixekizumab relative to adalimumab for both ERA (88.9% vs 93.8%) and JPsA (88.9% vs 100%), but all of the adalimumab patients were naive to biologics. In comparison, about 75% of patients receiving ixekizumab were biologic-therapy naive.
Response rates to ixekizumab overall were numerically higher for patients without previous biologic experience than for those with experience (90.0% vs 85.7%), and this was also the case for patients with ERA (92.5% vs 78.6%). However, in the JPsA group, biologic-experienced patients had higher numerical response rates to ixekizumab (100% vs 85.0%).
An ACR30 is not a clinical goal that satisfies most patients and clinicians, Dr. Ramanan conceded, but he noted that ACR50 was reached with ixekizumab by 81.5% with ERA and 74.1% with JPsA, and ACR70 was reached by 68.5% and 55.6%, respectively. The highest responses of ACR90 (27.8% and 33.3%) and ACR100 (14.8% and 25.9%) were lower but still substantial in the ERA and JPsA groups, respectively.
Through week 16, 58.0% of those treated with ixekizumab had an adverse event considered treatment-related. Nearly half were of mild severity, and the remainder were moderate. Only 3.7% were considered serious. No patient discontinued study treatment because of an adverse event.
In this study, the presence of at least three active peripheral joints was an inclusion criterion. The median age was about 13 years in the biologic-naive adalimumab and ixekizumab groups and 14 years in the ixekizumab biologic-experienced group. The youngest patient in the study was aged 5 years, and the oldest was aged 18 years. Although about 40% of patients were women in the two biologic-naive subgroups, it was 60% in the biologic-experienced group.
On average, patients in the biologic-naive group were entered about 1 year after diagnosis. In the experienced patients, the average duration of disease at entry was nearly 4 years. About 45% of patients remained on conventional synthetic disease-modifying antirheumatic drugs while receiving ixekizumab. The proportion was 35% in the adalimumab reference arm.
Ixekizumab Might Fulfill Need for More Options
There are several biologics that have received regulatory approval or are already widely used for the treatment of JPsA or ERA, but more options are needed, according to Dr. Ramanan and the chair of the abstract session in which these data were reported. According to Caroline Ospelt, MD, PhD, a researcher at the Center for Experimental Rheumatology, University Hospital Zurich, Switzerland, regulatory approval of ixekizumab will depend on sustained efficacy and safety in longer follow-up from the COSPIRIT-JIA trial, but this trial supports continued development.
Despite a novel mechanism of action, “the data so far suggest a level of efficacy similar to that of anti-TNF [anti-tumor necrosis factor] biologics,” said Dr. Ospelt, who, in addition to moderating the late-breaking session, served as Scientific Program Chair of EULAR 2024.
While Dr. Ospelt emphasized that she is a researcher involved in translational rheumatology studies and not a clinician, she said there was consensus within the program committee to select this abstract from other high-quality latebreaker submissions on the basis of its potential clinical significance.
Dr. Ramanan reported financial relationships with AbbVie, AstraZeneca, Novartis, Pfizer, Roche, SOBI, UCB, and Eli Lilly, which provided funding for this study. Dr. Ospelt reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
VIENNA — Ixekizumab (Taltz), an interleukin-17A inhibitor that’s already approved for the treatment of psoriatic arthritis and axial spondyloarthritis in adults appears likely to be granted the same corresponding indications for children, based on initial results from an open-label, phase 3 trial that employed adalimumab as a reference.
With a safety profile comparable with that seen in adult patients, ixekizumab “met the prespecified criterion for success at 16 weeks,” reported Athimalaipet V. Ramanan, MD, PhD, of Bristol Royal Hospital for Children and Translational Health Sciences, Bristol, England.
In this multicenter, randomized, open-label trial called COSPIRIT-JIA, which is still ongoing, investigators enrolled 101 children with active juvenile PsA (JPsA) or enthesitis-related arthritis (ERA), which is akin to spondyloarthritis in adults.
The efficacy and safety data at 16 weeks were presented as a late-breaking abstract at the annual European Congress of Rheumatology. Dr. Ramanan said that the open-label extension to 104 weeks is underway and further follow-up out to 264 weeks is planned.
Nearly 90% Achieve ACR30
The trial had an adaptive design in which the first 40 patients without biologics experience were randomized to ixekizumab or adalimumab, stratified by JPsA or ERA diagnosis, and the following 61 patients with either no biologic experience or an inadequate response or intolerance to biologics all received ixekizumab. The drugs were dosed according to weight. Dr. Ramanan explained that a placebo-controlled trial was considered unethical because of the strong evidence of benefit from biologics for JPsA and ERA.
The trial easily met its predefined threshold for success, which required ≥ 80% probability, based on Bayesian analysis, that ≥ 50% of patients would have 30% improvement in American College of Rheumatology response criteria (ACR30) at week 16. ACR30 was achieved in 88.9% of those treated with ixekizumab overall vs 95.0% of those treated with adalimumab, but the trial was not designed as a head-to-head comparison. Rather, adalimumab served as a reference.
When compared for the distinct diseases, the ACR30 rates were also numerically lower for ixekizumab relative to adalimumab for both ERA (88.9% vs 93.8%) and JPsA (88.9% vs 100%), but all of the adalimumab patients were naive to biologics. In comparison, about 75% of patients receiving ixekizumab were biologic-therapy naive.
Response rates to ixekizumab overall were numerically higher for patients without previous biologic experience than for those with experience (90.0% vs 85.7%), and this was also the case for patients with ERA (92.5% vs 78.6%). However, in the JPsA group, biologic-experienced patients had higher numerical response rates to ixekizumab (100% vs 85.0%).
An ACR30 is not a clinical goal that satisfies most patients and clinicians, Dr. Ramanan conceded, but he noted that ACR50 was reached with ixekizumab by 81.5% with ERA and 74.1% with JPsA, and ACR70 was reached by 68.5% and 55.6%, respectively. The highest responses of ACR90 (27.8% and 33.3%) and ACR100 (14.8% and 25.9%) were lower but still substantial in the ERA and JPsA groups, respectively.
Through week 16, 58.0% of those treated with ixekizumab had an adverse event considered treatment-related. Nearly half were of mild severity, and the remainder were moderate. Only 3.7% were considered serious. No patient discontinued study treatment because of an adverse event.
In this study, the presence of at least three active peripheral joints was an inclusion criterion. The median age was about 13 years in the biologic-naive adalimumab and ixekizumab groups and 14 years in the ixekizumab biologic-experienced group. The youngest patient in the study was aged 5 years, and the oldest was aged 18 years. Although about 40% of patients were women in the two biologic-naive subgroups, it was 60% in the biologic-experienced group.
On average, patients in the biologic-naive group were entered about 1 year after diagnosis. In the experienced patients, the average duration of disease at entry was nearly 4 years. About 45% of patients remained on conventional synthetic disease-modifying antirheumatic drugs while receiving ixekizumab. The proportion was 35% in the adalimumab reference arm.
Ixekizumab Might Fulfill Need for More Options
There are several biologics that have received regulatory approval or are already widely used for the treatment of JPsA or ERA, but more options are needed, according to Dr. Ramanan and the chair of the abstract session in which these data were reported. According to Caroline Ospelt, MD, PhD, a researcher at the Center for Experimental Rheumatology, University Hospital Zurich, Switzerland, regulatory approval of ixekizumab will depend on sustained efficacy and safety in longer follow-up from the COSPIRIT-JIA trial, but this trial supports continued development.
Despite a novel mechanism of action, “the data so far suggest a level of efficacy similar to that of anti-TNF [anti-tumor necrosis factor] biologics,” said Dr. Ospelt, who, in addition to moderating the late-breaking session, served as Scientific Program Chair of EULAR 2024.
While Dr. Ospelt emphasized that she is a researcher involved in translational rheumatology studies and not a clinician, she said there was consensus within the program committee to select this abstract from other high-quality latebreaker submissions on the basis of its potential clinical significance.
Dr. Ramanan reported financial relationships with AbbVie, AstraZeneca, Novartis, Pfizer, Roche, SOBI, UCB, and Eli Lilly, which provided funding for this study. Dr. Ospelt reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
VIENNA — Ixekizumab (Taltz), an interleukin-17A inhibitor that’s already approved for the treatment of psoriatic arthritis and axial spondyloarthritis in adults appears likely to be granted the same corresponding indications for children, based on initial results from an open-label, phase 3 trial that employed adalimumab as a reference.
With a safety profile comparable with that seen in adult patients, ixekizumab “met the prespecified criterion for success at 16 weeks,” reported Athimalaipet V. Ramanan, MD, PhD, of Bristol Royal Hospital for Children and Translational Health Sciences, Bristol, England.
In this multicenter, randomized, open-label trial called COSPIRIT-JIA, which is still ongoing, investigators enrolled 101 children with active juvenile PsA (JPsA) or enthesitis-related arthritis (ERA), which is akin to spondyloarthritis in adults.
The efficacy and safety data at 16 weeks were presented as a late-breaking abstract at the annual European Congress of Rheumatology. Dr. Ramanan said that the open-label extension to 104 weeks is underway and further follow-up out to 264 weeks is planned.
Nearly 90% Achieve ACR30
The trial had an adaptive design in which the first 40 patients without biologics experience were randomized to ixekizumab or adalimumab, stratified by JPsA or ERA diagnosis, and the following 61 patients with either no biologic experience or an inadequate response or intolerance to biologics all received ixekizumab. The drugs were dosed according to weight. Dr. Ramanan explained that a placebo-controlled trial was considered unethical because of the strong evidence of benefit from biologics for JPsA and ERA.
The trial easily met its predefined threshold for success, which required ≥ 80% probability, based on Bayesian analysis, that ≥ 50% of patients would have 30% improvement in American College of Rheumatology response criteria (ACR30) at week 16. ACR30 was achieved in 88.9% of those treated with ixekizumab overall vs 95.0% of those treated with adalimumab, but the trial was not designed as a head-to-head comparison. Rather, adalimumab served as a reference.
When compared for the distinct diseases, the ACR30 rates were also numerically lower for ixekizumab relative to adalimumab for both ERA (88.9% vs 93.8%) and JPsA (88.9% vs 100%), but all of the adalimumab patients were naive to biologics. In comparison, about 75% of patients receiving ixekizumab were biologic-therapy naive.
Response rates to ixekizumab overall were numerically higher for patients without previous biologic experience than for those with experience (90.0% vs 85.7%), and this was also the case for patients with ERA (92.5% vs 78.6%). However, in the JPsA group, biologic-experienced patients had higher numerical response rates to ixekizumab (100% vs 85.0%).
An ACR30 is not a clinical goal that satisfies most patients and clinicians, Dr. Ramanan conceded, but he noted that ACR50 was reached with ixekizumab by 81.5% with ERA and 74.1% with JPsA, and ACR70 was reached by 68.5% and 55.6%, respectively. The highest responses of ACR90 (27.8% and 33.3%) and ACR100 (14.8% and 25.9%) were lower but still substantial in the ERA and JPsA groups, respectively.
Through week 16, 58.0% of those treated with ixekizumab had an adverse event considered treatment-related. Nearly half were of mild severity, and the remainder were moderate. Only 3.7% were considered serious. No patient discontinued study treatment because of an adverse event.
In this study, the presence of at least three active peripheral joints was an inclusion criterion. The median age was about 13 years in the biologic-naive adalimumab and ixekizumab groups and 14 years in the ixekizumab biologic-experienced group. The youngest patient in the study was aged 5 years, and the oldest was aged 18 years. Although about 40% of patients were women in the two biologic-naive subgroups, it was 60% in the biologic-experienced group.
On average, patients in the biologic-naive group were entered about 1 year after diagnosis. In the experienced patients, the average duration of disease at entry was nearly 4 years. About 45% of patients remained on conventional synthetic disease-modifying antirheumatic drugs while receiving ixekizumab. The proportion was 35% in the adalimumab reference arm.
Ixekizumab Might Fulfill Need for More Options
There are several biologics that have received regulatory approval or are already widely used for the treatment of JPsA or ERA, but more options are needed, according to Dr. Ramanan and the chair of the abstract session in which these data were reported. According to Caroline Ospelt, MD, PhD, a researcher at the Center for Experimental Rheumatology, University Hospital Zurich, Switzerland, regulatory approval of ixekizumab will depend on sustained efficacy and safety in longer follow-up from the COSPIRIT-JIA trial, but this trial supports continued development.
Despite a novel mechanism of action, “the data so far suggest a level of efficacy similar to that of anti-TNF [anti-tumor necrosis factor] biologics,” said Dr. Ospelt, who, in addition to moderating the late-breaking session, served as Scientific Program Chair of EULAR 2024.
While Dr. Ospelt emphasized that she is a researcher involved in translational rheumatology studies and not a clinician, she said there was consensus within the program committee to select this abstract from other high-quality latebreaker submissions on the basis of its potential clinical significance.
Dr. Ramanan reported financial relationships with AbbVie, AstraZeneca, Novartis, Pfizer, Roche, SOBI, UCB, and Eli Lilly, which provided funding for this study. Dr. Ospelt reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM EULAR 2024
Atopic Dermatitis: Study Compares Prevalence by Gender, Age, and Ethnic Background
than adults from other ethnic backgrounds.
Those are among the key findings from an analysis of nationally representative cross-sectional data that were presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis conference in Chicago.
“In the past few years, there has been a much-needed focus on better understanding disparities in atopic dermatitis,” one of the study authors, Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University, North Chicago, told this news organization after the conference.
“Epidemiology is one of the key ways in which we can query differences in AD at a population level.”
Drawing from the 2021 National Health Interview Survey, the researchers identified 3103 respondents who reported being diagnosed with AD or eczema. They estimated the prevalence rates of AD for the overall population and each subgroup by dividing US frequency estimates by their corresponding US population totals and used multivariable logistic regression to assess the odds of having AD.
More than half of the respondents (1643) were aged between 18 and 64 years, 522 were aged 65 years and older, and 922 were children younger than 18 years. Overall, the prevalence of AD was 7.6% in adults aged 18-64 years and 6.1% in adults aged 65 years and older, for a weighted US estimate of 15.3 and 3.2 million, respectively. The prevalence of AD varied by race/ethnicity and was highest for those from “other single and multiple races” group (12.4%), followed by Black/African American (8.5%), White (7.7%), Asian (6.5%), American Indian/Alaskan Native (4.9%), and Hispanic (4.8%) populations.
In children, race/ethnicity prevalence were highest for those from other single and multiple races (15.2.%), followed by Black/African American (14.2%), American Indian/Alaskan Native (12%), White (10.2%), Hispanic (9.5%), and Asian (9%) populations.
When the researchers combined all age groups, they observed higher prevalence rates of AD among females than among males. However, in an analysis limited to children, the prevalence rates were similar between girls and boys (10.8% vs 10.7%, respectively), for a weighted US estimate of 7.8 million children with AD.
On multiple regression, the odds of having AD were greater among women than among men (odds ratio [OR], 1.4), among adults aged 18-64 years than among those aged 65 years and older (OR, 1.4), among those younger than 18 years than among those aged 65 years and older (OR, 2.0), and among Black/African American individuals than among White individuals (OR, 1.2). Hispanic adults had a lower risk for AD than non-Hispanic White adults (OR, 0.69) as did Asian adults than White adults (OR, 0.82).
“We found AD prevalence rates were higher in children and adult females, Hispanic adults had a lower prevalence of AD than all other adult groups, and there were numerical differences in AD prevalence across racial groups,” Dr. Chovatiya said in the interview. “While there are of course limitations to the use of any nationally representative cross-sectional dataset that requires weighting to project results from a smaller sample to reflect a larger more heterogeneous group, these results are important for us to consider targeted strategies to address AD burden.”
Jonathan I. Silverberg, MD, PhD, professor of dermatology at The George Washington University, Washington, who was asked to comment on the study, said that while the prevalence of AD in children has been well documented in prior research, “this study fills an important gap by showing us that the prevalence does remain high in adults.”
In addition, “it has not shown any evidence of AD decreasing over time; if anything, it might be slightly increasing,” he said. “We’re also seeing differences [in AD] by race and ethnicity. We have seen that demonstrated in children but [has been] less clearly demonstrated in adults.”
Eli Lilly and Company funded the analysis. Dr. Chovatiya and Dr. Silverberg disclosed ties to several pharmaceutical companies, including Eli Lilly.
A version of this article appeared on Medscape.com .
than adults from other ethnic backgrounds.
Those are among the key findings from an analysis of nationally representative cross-sectional data that were presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis conference in Chicago.
“In the past few years, there has been a much-needed focus on better understanding disparities in atopic dermatitis,” one of the study authors, Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University, North Chicago, told this news organization after the conference.
“Epidemiology is one of the key ways in which we can query differences in AD at a population level.”
Drawing from the 2021 National Health Interview Survey, the researchers identified 3103 respondents who reported being diagnosed with AD or eczema. They estimated the prevalence rates of AD for the overall population and each subgroup by dividing US frequency estimates by their corresponding US population totals and used multivariable logistic regression to assess the odds of having AD.
More than half of the respondents (1643) were aged between 18 and 64 years, 522 were aged 65 years and older, and 922 were children younger than 18 years. Overall, the prevalence of AD was 7.6% in adults aged 18-64 years and 6.1% in adults aged 65 years and older, for a weighted US estimate of 15.3 and 3.2 million, respectively. The prevalence of AD varied by race/ethnicity and was highest for those from “other single and multiple races” group (12.4%), followed by Black/African American (8.5%), White (7.7%), Asian (6.5%), American Indian/Alaskan Native (4.9%), and Hispanic (4.8%) populations.
In children, race/ethnicity prevalence were highest for those from other single and multiple races (15.2.%), followed by Black/African American (14.2%), American Indian/Alaskan Native (12%), White (10.2%), Hispanic (9.5%), and Asian (9%) populations.
When the researchers combined all age groups, they observed higher prevalence rates of AD among females than among males. However, in an analysis limited to children, the prevalence rates were similar between girls and boys (10.8% vs 10.7%, respectively), for a weighted US estimate of 7.8 million children with AD.
On multiple regression, the odds of having AD were greater among women than among men (odds ratio [OR], 1.4), among adults aged 18-64 years than among those aged 65 years and older (OR, 1.4), among those younger than 18 years than among those aged 65 years and older (OR, 2.0), and among Black/African American individuals than among White individuals (OR, 1.2). Hispanic adults had a lower risk for AD than non-Hispanic White adults (OR, 0.69) as did Asian adults than White adults (OR, 0.82).
“We found AD prevalence rates were higher in children and adult females, Hispanic adults had a lower prevalence of AD than all other adult groups, and there were numerical differences in AD prevalence across racial groups,” Dr. Chovatiya said in the interview. “While there are of course limitations to the use of any nationally representative cross-sectional dataset that requires weighting to project results from a smaller sample to reflect a larger more heterogeneous group, these results are important for us to consider targeted strategies to address AD burden.”
Jonathan I. Silverberg, MD, PhD, professor of dermatology at The George Washington University, Washington, who was asked to comment on the study, said that while the prevalence of AD in children has been well documented in prior research, “this study fills an important gap by showing us that the prevalence does remain high in adults.”
In addition, “it has not shown any evidence of AD decreasing over time; if anything, it might be slightly increasing,” he said. “We’re also seeing differences [in AD] by race and ethnicity. We have seen that demonstrated in children but [has been] less clearly demonstrated in adults.”
Eli Lilly and Company funded the analysis. Dr. Chovatiya and Dr. Silverberg disclosed ties to several pharmaceutical companies, including Eli Lilly.
A version of this article appeared on Medscape.com .
than adults from other ethnic backgrounds.
Those are among the key findings from an analysis of nationally representative cross-sectional data that were presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis conference in Chicago.
“In the past few years, there has been a much-needed focus on better understanding disparities in atopic dermatitis,” one of the study authors, Raj Chovatiya, MD, PhD, clinical associate professor at Chicago Medical School, Rosalind Franklin University, North Chicago, told this news organization after the conference.
“Epidemiology is one of the key ways in which we can query differences in AD at a population level.”
Drawing from the 2021 National Health Interview Survey, the researchers identified 3103 respondents who reported being diagnosed with AD or eczema. They estimated the prevalence rates of AD for the overall population and each subgroup by dividing US frequency estimates by their corresponding US population totals and used multivariable logistic regression to assess the odds of having AD.
More than half of the respondents (1643) were aged between 18 and 64 years, 522 were aged 65 years and older, and 922 were children younger than 18 years. Overall, the prevalence of AD was 7.6% in adults aged 18-64 years and 6.1% in adults aged 65 years and older, for a weighted US estimate of 15.3 and 3.2 million, respectively. The prevalence of AD varied by race/ethnicity and was highest for those from “other single and multiple races” group (12.4%), followed by Black/African American (8.5%), White (7.7%), Asian (6.5%), American Indian/Alaskan Native (4.9%), and Hispanic (4.8%) populations.
In children, race/ethnicity prevalence were highest for those from other single and multiple races (15.2.%), followed by Black/African American (14.2%), American Indian/Alaskan Native (12%), White (10.2%), Hispanic (9.5%), and Asian (9%) populations.
When the researchers combined all age groups, they observed higher prevalence rates of AD among females than among males. However, in an analysis limited to children, the prevalence rates were similar between girls and boys (10.8% vs 10.7%, respectively), for a weighted US estimate of 7.8 million children with AD.
On multiple regression, the odds of having AD were greater among women than among men (odds ratio [OR], 1.4), among adults aged 18-64 years than among those aged 65 years and older (OR, 1.4), among those younger than 18 years than among those aged 65 years and older (OR, 2.0), and among Black/African American individuals than among White individuals (OR, 1.2). Hispanic adults had a lower risk for AD than non-Hispanic White adults (OR, 0.69) as did Asian adults than White adults (OR, 0.82).
“We found AD prevalence rates were higher in children and adult females, Hispanic adults had a lower prevalence of AD than all other adult groups, and there were numerical differences in AD prevalence across racial groups,” Dr. Chovatiya said in the interview. “While there are of course limitations to the use of any nationally representative cross-sectional dataset that requires weighting to project results from a smaller sample to reflect a larger more heterogeneous group, these results are important for us to consider targeted strategies to address AD burden.”
Jonathan I. Silverberg, MD, PhD, professor of dermatology at The George Washington University, Washington, who was asked to comment on the study, said that while the prevalence of AD in children has been well documented in prior research, “this study fills an important gap by showing us that the prevalence does remain high in adults.”
In addition, “it has not shown any evidence of AD decreasing over time; if anything, it might be slightly increasing,” he said. “We’re also seeing differences [in AD] by race and ethnicity. We have seen that demonstrated in children but [has been] less clearly demonstrated in adults.”
Eli Lilly and Company funded the analysis. Dr. Chovatiya and Dr. Silverberg disclosed ties to several pharmaceutical companies, including Eli Lilly.
A version of this article appeared on Medscape.com .
Meta-Analysis Finds Combination Cream Plus Tranexamic Acid Effective for Melasma
TOPLINE:
A meta-analysis showed that .
METHODOLOGY:
- Current treatments for melasma focus on inducing remission and preventing relapse. Tranexamic acid, an antifibrinolytic drug, has shown promise in recent studies, but its optimal use, either alone or as an adjunct to TCC, remains unclear.
- Researchers conducted a meta-analysis of four randomized controlled trials patients that compared oral tranexamic acid plus TCC (hydroquinone, retinoic acid, and hydrocortisone) and TCC alone in 480 patients with melasma, divided almost evenly into the two treatment groups.
- The main outcome was the change in the Melasma Severity Area Index (MASI) score and recurrence rate from baseline.
TAKEAWAY:
- Patients treated with oral tranexamic acid plus TCC showed a greater reduction in MASI scores compared with those who received TCC alone (mean difference, −3.10; P = .03).
- The recurrence rate of melasma was significantly lower in the tranexamic acid plus TCC group (risk ratio [RR], 0.28; P < .001).
- There was no significant difference in the incidences of erythema (RR, 0.63; P = .147) and burning (RR, 0.59; P = .131).
IN PRACTICE:
“Evidence indicates that oral tranexamic acid confers clinical benefits, contributing to the enhancement of treatment outcomes in melasma when used in conjunction with TCC therapy,” and results are promising with regards to minimizing recurrence, the authors concluded.
SOURCE:
The study was led by Ocílio Ribeiro Gonçalves, MS, of the Federal University of Piauí, Teresina, Brazil, and was published online on June 8, 2024, in Clinical and Experimental Dermatology.
LIMITATIONS:
There was heterogeneity across studies, including different methods of administration, treatment protocols (including dosage), and timing of treatment.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A meta-analysis showed that .
METHODOLOGY:
- Current treatments for melasma focus on inducing remission and preventing relapse. Tranexamic acid, an antifibrinolytic drug, has shown promise in recent studies, but its optimal use, either alone or as an adjunct to TCC, remains unclear.
- Researchers conducted a meta-analysis of four randomized controlled trials patients that compared oral tranexamic acid plus TCC (hydroquinone, retinoic acid, and hydrocortisone) and TCC alone in 480 patients with melasma, divided almost evenly into the two treatment groups.
- The main outcome was the change in the Melasma Severity Area Index (MASI) score and recurrence rate from baseline.
TAKEAWAY:
- Patients treated with oral tranexamic acid plus TCC showed a greater reduction in MASI scores compared with those who received TCC alone (mean difference, −3.10; P = .03).
- The recurrence rate of melasma was significantly lower in the tranexamic acid plus TCC group (risk ratio [RR], 0.28; P < .001).
- There was no significant difference in the incidences of erythema (RR, 0.63; P = .147) and burning (RR, 0.59; P = .131).
IN PRACTICE:
“Evidence indicates that oral tranexamic acid confers clinical benefits, contributing to the enhancement of treatment outcomes in melasma when used in conjunction with TCC therapy,” and results are promising with regards to minimizing recurrence, the authors concluded.
SOURCE:
The study was led by Ocílio Ribeiro Gonçalves, MS, of the Federal University of Piauí, Teresina, Brazil, and was published online on June 8, 2024, in Clinical and Experimental Dermatology.
LIMITATIONS:
There was heterogeneity across studies, including different methods of administration, treatment protocols (including dosage), and timing of treatment.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A meta-analysis showed that .
METHODOLOGY:
- Current treatments for melasma focus on inducing remission and preventing relapse. Tranexamic acid, an antifibrinolytic drug, has shown promise in recent studies, but its optimal use, either alone or as an adjunct to TCC, remains unclear.
- Researchers conducted a meta-analysis of four randomized controlled trials patients that compared oral tranexamic acid plus TCC (hydroquinone, retinoic acid, and hydrocortisone) and TCC alone in 480 patients with melasma, divided almost evenly into the two treatment groups.
- The main outcome was the change in the Melasma Severity Area Index (MASI) score and recurrence rate from baseline.
TAKEAWAY:
- Patients treated with oral tranexamic acid plus TCC showed a greater reduction in MASI scores compared with those who received TCC alone (mean difference, −3.10; P = .03).
- The recurrence rate of melasma was significantly lower in the tranexamic acid plus TCC group (risk ratio [RR], 0.28; P < .001).
- There was no significant difference in the incidences of erythema (RR, 0.63; P = .147) and burning (RR, 0.59; P = .131).
IN PRACTICE:
“Evidence indicates that oral tranexamic acid confers clinical benefits, contributing to the enhancement of treatment outcomes in melasma when used in conjunction with TCC therapy,” and results are promising with regards to minimizing recurrence, the authors concluded.
SOURCE:
The study was led by Ocílio Ribeiro Gonçalves, MS, of the Federal University of Piauí, Teresina, Brazil, and was published online on June 8, 2024, in Clinical and Experimental Dermatology.
LIMITATIONS:
There was heterogeneity across studies, including different methods of administration, treatment protocols (including dosage), and timing of treatment.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
VEXAS Syndrome: Study Highlights Cutaneous Symptoms
Additionally, the most common histologic findings include leukocytoclastic vasculitis, neutrophilic dermatosis, and perivascular dermatitis; different variants in the UBA1 gene are associated with specific skin manifestations.
Those are key findings from a cohort study of 112 patients with VEXAS published online in JAMA Dermatology. The study, conducted by researchers at the National Institutes of Health (NIH) and several other institutions, aimed to define the spectrum of cutaneous manifestations in VEXAS in association with genetic, histologic, and other clinical findings.
First described in 2020, VEXAS syndrome is an adult-onset multisystem disease that can pose a diagnostic challenge to clinicians, the study’s corresponding author, Edward W. Cowen, MD, MHSc, of the dermatology branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), said in an interview. The disease is caused by pathogenic variants in the UBA1 gene, located on the X chromosome. Affected individuals exhibit a wide range of manifestations, including cytopenia/myelodysplasia, multiorgan systemic inflammation, and cutaneous involvement.
“Patients may present to a variety of disease specialists depending on their symptoms and providers may not immediately consider a genetic etiology in an older individual,” Dr. Cowen said in an interview. “Although skin involvement occurs in more than 80% of patients, it is pleomorphic and may resemble a variety of other conditions such as vasculitis and Sweet syndrome.”
To better understand the cutaneous manifestations of VEXAS syndrome, the researchers evaluated data from 112 patients with VEXAS-defining genetic variants in the UBA1 gene between 2019 and 2023. Of the 112 patients, 73 underwent medical record review only, and 39 were prospectively evaluated at NIH. All but one of the patients were men, 94% were White individuals, and their mean age was 64 years. Skin involvement occurred in 83% of cases and was the most common presenting feature of VEXAS in 61% of cases.
Of the 64 histopathologic reports available from 60 patients, the main skin histopathologic findings were leukocytoclastic vasculitis in 23 patients (36%), neutrophilic dermatosis in 22 patients (34%), and perivascular dermatitis in 19 patients (30%). According to Dr. Cowen, one key histologic finding was a distinct pattern of “histiocytoid” dermal neutrophilic inflammation, which was present in 13 of 15 specimens (86%) that underwent central re-review. “This pattern can occasionally also be seen in patients with Sweet syndrome, unrelated to VEXAS, but was a hallmark feature found in the majority of skin biopsies of patients with VEXAS,” he said.
“Together with another pathologic finding, leukocytoclasia, these features can be useful clues to alert the pathologist to a potential diagnosis of VEXAS. This myeloid predominant pattern of skin inflammation was also most strongly associated with the leucine pathogenic variant of the UBA1 gene.” In contrast, cutaneous vasculitis was most strongly associated with the valine pathogenic variant of UBA1. “This is important because the valine variant has been previously independently linked to decreased survival,” he said.
In findings related to pathogenic genetic variants, the researchers observed that the p.Met41Leu variant was most frequently associated with neutrophilic dermal infiltrates in 14 of 17 patients (82%) with this variant and often resembled histiocytoid Sweet syndrome. In addition, the p.Met41Val variant was associated with vasculitic lesions in 11 of 20 patients (55%) with this variant and with a mixed leukocytic infiltrate in 17 of these 20 patients (85%).
Treatment Outcomes
In the realm of therapies, skin manifestations improved in 67 of 73 patients (92%) treated with oral prednisone, while treatment with the interleukin-1 receptor antagonist anakinra improved cutaneous disease in 9 of the 16 (56%) who received it. However, 12 (75%) of those who received anakinra developed severe injection-site reactions, including ulceration in two patients and abscess formation in one patient.
Dr. Cowen noted that VEXAS is associated with high mortality (22% in this cohort), and a high degree of suspicion is required to diagnose patients with VEXAS before significant end organ damage has occurred. “This diagnosis should be considered in all older male patients who present with neutrophilic dermatosis — particularly histiocytoid Sweet syndrome, vasculitis, or leukocytoclasia without vasculitis. Patients who appear to have isolated skin involvement may have cytopenias and acute phase reactants. Therefore, complete blood count with differential and ESR and CRP should be considered to investigate for macrocytosis, cytopenias, and systemic inflammation.”
He acknowledged certain limitations of the study, including the fact that many patients were first evaluated at the NIH after having disease symptoms for many months or years. “It is possible that patients with VEXAS referred to the NIH, either for genetic testing or in person evaluation, represent a population with more aggressive disease.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the study, emphasized the importance of the UBA1 mutation in the diagnosis of this complex syndrome. “Dermatologists should be aware of VEXAS syndrome as the majority of patients present with skin lesions, which can range from urticarial to Sweet syndrome–like to palpable purpura,” Dr. Ko said.
“Chondritis and periorbital edema, sometimes unilateral, are also associated. Histopathologic clues include a predominantly histiocytoid infiltrate,” she noted. In addition, “the prominent myxoid stroma around blood vessels and adnexal structures as a clue to VEXAS syndrome surprised me; I had not read that before.”
The study was supported by the Intramural Research Program of NIAMS. One of the study authors reported personal fees from Alexion, Novartis, and Sobi outside of the submitted work. No other disclosures were reported. Dr. Ko reported having no disclosures.
A version of this article appeared on Medscape.com .
Additionally, the most common histologic findings include leukocytoclastic vasculitis, neutrophilic dermatosis, and perivascular dermatitis; different variants in the UBA1 gene are associated with specific skin manifestations.
Those are key findings from a cohort study of 112 patients with VEXAS published online in JAMA Dermatology. The study, conducted by researchers at the National Institutes of Health (NIH) and several other institutions, aimed to define the spectrum of cutaneous manifestations in VEXAS in association with genetic, histologic, and other clinical findings.
First described in 2020, VEXAS syndrome is an adult-onset multisystem disease that can pose a diagnostic challenge to clinicians, the study’s corresponding author, Edward W. Cowen, MD, MHSc, of the dermatology branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), said in an interview. The disease is caused by pathogenic variants in the UBA1 gene, located on the X chromosome. Affected individuals exhibit a wide range of manifestations, including cytopenia/myelodysplasia, multiorgan systemic inflammation, and cutaneous involvement.
“Patients may present to a variety of disease specialists depending on their symptoms and providers may not immediately consider a genetic etiology in an older individual,” Dr. Cowen said in an interview. “Although skin involvement occurs in more than 80% of patients, it is pleomorphic and may resemble a variety of other conditions such as vasculitis and Sweet syndrome.”
To better understand the cutaneous manifestations of VEXAS syndrome, the researchers evaluated data from 112 patients with VEXAS-defining genetic variants in the UBA1 gene between 2019 and 2023. Of the 112 patients, 73 underwent medical record review only, and 39 were prospectively evaluated at NIH. All but one of the patients were men, 94% were White individuals, and their mean age was 64 years. Skin involvement occurred in 83% of cases and was the most common presenting feature of VEXAS in 61% of cases.
Of the 64 histopathologic reports available from 60 patients, the main skin histopathologic findings were leukocytoclastic vasculitis in 23 patients (36%), neutrophilic dermatosis in 22 patients (34%), and perivascular dermatitis in 19 patients (30%). According to Dr. Cowen, one key histologic finding was a distinct pattern of “histiocytoid” dermal neutrophilic inflammation, which was present in 13 of 15 specimens (86%) that underwent central re-review. “This pattern can occasionally also be seen in patients with Sweet syndrome, unrelated to VEXAS, but was a hallmark feature found in the majority of skin biopsies of patients with VEXAS,” he said.
“Together with another pathologic finding, leukocytoclasia, these features can be useful clues to alert the pathologist to a potential diagnosis of VEXAS. This myeloid predominant pattern of skin inflammation was also most strongly associated with the leucine pathogenic variant of the UBA1 gene.” In contrast, cutaneous vasculitis was most strongly associated with the valine pathogenic variant of UBA1. “This is important because the valine variant has been previously independently linked to decreased survival,” he said.
In findings related to pathogenic genetic variants, the researchers observed that the p.Met41Leu variant was most frequently associated with neutrophilic dermal infiltrates in 14 of 17 patients (82%) with this variant and often resembled histiocytoid Sweet syndrome. In addition, the p.Met41Val variant was associated with vasculitic lesions in 11 of 20 patients (55%) with this variant and with a mixed leukocytic infiltrate in 17 of these 20 patients (85%).
Treatment Outcomes
In the realm of therapies, skin manifestations improved in 67 of 73 patients (92%) treated with oral prednisone, while treatment with the interleukin-1 receptor antagonist anakinra improved cutaneous disease in 9 of the 16 (56%) who received it. However, 12 (75%) of those who received anakinra developed severe injection-site reactions, including ulceration in two patients and abscess formation in one patient.
Dr. Cowen noted that VEXAS is associated with high mortality (22% in this cohort), and a high degree of suspicion is required to diagnose patients with VEXAS before significant end organ damage has occurred. “This diagnosis should be considered in all older male patients who present with neutrophilic dermatosis — particularly histiocytoid Sweet syndrome, vasculitis, or leukocytoclasia without vasculitis. Patients who appear to have isolated skin involvement may have cytopenias and acute phase reactants. Therefore, complete blood count with differential and ESR and CRP should be considered to investigate for macrocytosis, cytopenias, and systemic inflammation.”
He acknowledged certain limitations of the study, including the fact that many patients were first evaluated at the NIH after having disease symptoms for many months or years. “It is possible that patients with VEXAS referred to the NIH, either for genetic testing or in person evaluation, represent a population with more aggressive disease.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the study, emphasized the importance of the UBA1 mutation in the diagnosis of this complex syndrome. “Dermatologists should be aware of VEXAS syndrome as the majority of patients present with skin lesions, which can range from urticarial to Sweet syndrome–like to palpable purpura,” Dr. Ko said.
“Chondritis and periorbital edema, sometimes unilateral, are also associated. Histopathologic clues include a predominantly histiocytoid infiltrate,” she noted. In addition, “the prominent myxoid stroma around blood vessels and adnexal structures as a clue to VEXAS syndrome surprised me; I had not read that before.”
The study was supported by the Intramural Research Program of NIAMS. One of the study authors reported personal fees from Alexion, Novartis, and Sobi outside of the submitted work. No other disclosures were reported. Dr. Ko reported having no disclosures.
A version of this article appeared on Medscape.com .
Additionally, the most common histologic findings include leukocytoclastic vasculitis, neutrophilic dermatosis, and perivascular dermatitis; different variants in the UBA1 gene are associated with specific skin manifestations.
Those are key findings from a cohort study of 112 patients with VEXAS published online in JAMA Dermatology. The study, conducted by researchers at the National Institutes of Health (NIH) and several other institutions, aimed to define the spectrum of cutaneous manifestations in VEXAS in association with genetic, histologic, and other clinical findings.
First described in 2020, VEXAS syndrome is an adult-onset multisystem disease that can pose a diagnostic challenge to clinicians, the study’s corresponding author, Edward W. Cowen, MD, MHSc, of the dermatology branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), said in an interview. The disease is caused by pathogenic variants in the UBA1 gene, located on the X chromosome. Affected individuals exhibit a wide range of manifestations, including cytopenia/myelodysplasia, multiorgan systemic inflammation, and cutaneous involvement.
“Patients may present to a variety of disease specialists depending on their symptoms and providers may not immediately consider a genetic etiology in an older individual,” Dr. Cowen said in an interview. “Although skin involvement occurs in more than 80% of patients, it is pleomorphic and may resemble a variety of other conditions such as vasculitis and Sweet syndrome.”
To better understand the cutaneous manifestations of VEXAS syndrome, the researchers evaluated data from 112 patients with VEXAS-defining genetic variants in the UBA1 gene between 2019 and 2023. Of the 112 patients, 73 underwent medical record review only, and 39 were prospectively evaluated at NIH. All but one of the patients were men, 94% were White individuals, and their mean age was 64 years. Skin involvement occurred in 83% of cases and was the most common presenting feature of VEXAS in 61% of cases.
Of the 64 histopathologic reports available from 60 patients, the main skin histopathologic findings were leukocytoclastic vasculitis in 23 patients (36%), neutrophilic dermatosis in 22 patients (34%), and perivascular dermatitis in 19 patients (30%). According to Dr. Cowen, one key histologic finding was a distinct pattern of “histiocytoid” dermal neutrophilic inflammation, which was present in 13 of 15 specimens (86%) that underwent central re-review. “This pattern can occasionally also be seen in patients with Sweet syndrome, unrelated to VEXAS, but was a hallmark feature found in the majority of skin biopsies of patients with VEXAS,” he said.
“Together with another pathologic finding, leukocytoclasia, these features can be useful clues to alert the pathologist to a potential diagnosis of VEXAS. This myeloid predominant pattern of skin inflammation was also most strongly associated with the leucine pathogenic variant of the UBA1 gene.” In contrast, cutaneous vasculitis was most strongly associated with the valine pathogenic variant of UBA1. “This is important because the valine variant has been previously independently linked to decreased survival,” he said.
In findings related to pathogenic genetic variants, the researchers observed that the p.Met41Leu variant was most frequently associated with neutrophilic dermal infiltrates in 14 of 17 patients (82%) with this variant and often resembled histiocytoid Sweet syndrome. In addition, the p.Met41Val variant was associated with vasculitic lesions in 11 of 20 patients (55%) with this variant and with a mixed leukocytic infiltrate in 17 of these 20 patients (85%).
Treatment Outcomes
In the realm of therapies, skin manifestations improved in 67 of 73 patients (92%) treated with oral prednisone, while treatment with the interleukin-1 receptor antagonist anakinra improved cutaneous disease in 9 of the 16 (56%) who received it. However, 12 (75%) of those who received anakinra developed severe injection-site reactions, including ulceration in two patients and abscess formation in one patient.
Dr. Cowen noted that VEXAS is associated with high mortality (22% in this cohort), and a high degree of suspicion is required to diagnose patients with VEXAS before significant end organ damage has occurred. “This diagnosis should be considered in all older male patients who present with neutrophilic dermatosis — particularly histiocytoid Sweet syndrome, vasculitis, or leukocytoclasia without vasculitis. Patients who appear to have isolated skin involvement may have cytopenias and acute phase reactants. Therefore, complete blood count with differential and ESR and CRP should be considered to investigate for macrocytosis, cytopenias, and systemic inflammation.”
He acknowledged certain limitations of the study, including the fact that many patients were first evaluated at the NIH after having disease symptoms for many months or years. “It is possible that patients with VEXAS referred to the NIH, either for genetic testing or in person evaluation, represent a population with more aggressive disease.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the study, emphasized the importance of the UBA1 mutation in the diagnosis of this complex syndrome. “Dermatologists should be aware of VEXAS syndrome as the majority of patients present with skin lesions, which can range from urticarial to Sweet syndrome–like to palpable purpura,” Dr. Ko said.
“Chondritis and periorbital edema, sometimes unilateral, are also associated. Histopathologic clues include a predominantly histiocytoid infiltrate,” she noted. In addition, “the prominent myxoid stroma around blood vessels and adnexal structures as a clue to VEXAS syndrome surprised me; I had not read that before.”
The study was supported by the Intramural Research Program of NIAMS. One of the study authors reported personal fees from Alexion, Novartis, and Sobi outside of the submitted work. No other disclosures were reported. Dr. Ko reported having no disclosures.
A version of this article appeared on Medscape.com .
FROM JAMA DERMATOLOGY
Pruritic, violaceous papules in a patient with renal cell carcinoma
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.
Doctors Endorsing Products on X May Not Disclose Company Ties
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
One Patient Changed This Oncologist’s View of Hope. Here’s How.
CHICAGO — Carlos, a 21-year-old, lay in a hospital bed, barely clinging to life. Following a stem cell transplant for leukemia, Carlos had developed a life-threatening case of graft-vs-host disease.
But Carlos’ mother had faith.
“I have hope things will get better,” she said, via interpreter, to Richard Leiter, MD, a palliative care doctor in training at that time.
“I hope they will,” Dr. Leiter told her.
“I should have stopped there,” said Dr. Leiter, recounting an early-career lesson on hope during the ASCO Voices session at the American Society of Clinical Oncology annual meeting. “But in my eagerness to show my attending and myself that I could handle this conversation, I kept going, mistakenly.”
“But none of us think they will,” Dr. Leiter continued.
Carlos’ mother looked Dr. Leiter in the eye. “You want him to die,” she said.
“I knew, even then, that she was right,” recalled Dr. Leiter, now a palliative care physician at Dana-Farber Cancer Institute and Brigham and Women’s Hospital and an assistant professor of medicine at Harvard Medical School, Boston.
Although there was nothing he could do to save Carlos, Dr. Leiter also couldn’t sit with the extreme suffering. “The pain was too great,” Dr. Leiter said. “I needed her to adopt our narrative that we had done everything we could to help him live, and now, we would do everything we could to help his death be a comfortable one.”
But looking back, Dr. Leiter realized, “How could we have asked her to accept what was fundamentally unacceptable, to comprehend the incomprehensible?”
The Importance of Hope
Alan B. Astrow, MD, said during an ASCO symposium on “The Art and Science of Hope.”
“How we think about hope directly influences patient care,” said Dr. Astrow, chief of hematology and medical oncology at NewYork-Presbyterian Brooklyn Methodist Hospital and a professor of clinical medicine at Weill Cornell Medicine in New York City.
Hope, whatever it turns out to be neurobiologically, is “very much a gift” that underlies human existence, he said.
Physicians have the capacity to restore or shatter a patient’s hopes, and those who come to understand the importance of hope will wish to extend the gift to others, Dr. Astrow said.
Asking patients about their hopes is the “golden question,” Steven Z. Pantilat, MD, said at the symposium. “When you think about the future, what do you hope for?”
Often, the answers reveal not only “things beyond a cure that matter tremendously to the patient but things that we can help with,” said Dr. Pantilat, professor and chief of the Division of Palliative Medicine at the University of California San Francisco.
Dr. Pantilat recalled a patient with advanced pancreatic cancer who wished to see her daughter’s wedding in 10 months. He knew that was unlikely, but the discussion led to another solution.
Her daughter moved the wedding to the ICU.
Hope can persist and uplift even in the darkest of times, and “as clinicians, we need to be in the true hope business,” he said.
While some patients may wish for a cure, others may want more time with family or comfort in the face of suffering. People can “hope for all the things that can still be, despite the fact that there’s a lot of things that can’t,” he said.
However, fear that a patient will hope for a cure, and that the difficult discussions to follow might destroy hope or lead to false hope, sometimes means physicians won’t begin the conversation.
“We want to be honest with our patients — compassionate and kind, but honest — when we talk about their hopes,” Dr. Pantilat explained. Sometimes that means he needs to tell patients, “I wish that could happen. I wish I had a treatment that could make your cancer go away, but unfortunately, I don’t. So let’s think about what else we can do to help you.”
Having these difficult discussions matters. The evidence, although limited, indicates that feeling hopeful can improve patients’ well-being and may even boost their cancer outcomes.
One recent study found, for instance, that patients who reported feeling more hopeful also had lower levels of depression and anxiety. Early research also suggests that greater levels of hope may have a hand in reducing inflammation in patients with ovarian cancer and could even improve survival in some patients with advanced cancer.
For Dr. Leiter, while these lessons came early in his career as a palliative care physician, they persist and influence his practice today.
“I know that I could not have prevented Carlos’ death. None of us could have, and none of us could have protected his mother from the unimaginable grief that will stay with her for the rest of her life,” he said. “But I could have made things just a little bit less difficult for her.
“I could have acted as her guide rather than her cross-examiner,” he continued, explaining that he now sees hope as “a generous collaborator” that can coexist with rising creatinine levels, failing livers, and fears about intubation.
“As clinicians, we can always find space to hope with our patients and their families,” he said. “So now, years later when I sit with a terrified and grieving family and they tell me they hope their loved one gets better, I remember Carlos’ mother’s eyes piercing mine ... and I know how to respond: ‘I hope so, too.’ And I do.”
A version of this article appeared on Medscape.com.
CHICAGO — Carlos, a 21-year-old, lay in a hospital bed, barely clinging to life. Following a stem cell transplant for leukemia, Carlos had developed a life-threatening case of graft-vs-host disease.
But Carlos’ mother had faith.
“I have hope things will get better,” she said, via interpreter, to Richard Leiter, MD, a palliative care doctor in training at that time.
“I hope they will,” Dr. Leiter told her.
“I should have stopped there,” said Dr. Leiter, recounting an early-career lesson on hope during the ASCO Voices session at the American Society of Clinical Oncology annual meeting. “But in my eagerness to show my attending and myself that I could handle this conversation, I kept going, mistakenly.”
“But none of us think they will,” Dr. Leiter continued.
Carlos’ mother looked Dr. Leiter in the eye. “You want him to die,” she said.
“I knew, even then, that she was right,” recalled Dr. Leiter, now a palliative care physician at Dana-Farber Cancer Institute and Brigham and Women’s Hospital and an assistant professor of medicine at Harvard Medical School, Boston.
Although there was nothing he could do to save Carlos, Dr. Leiter also couldn’t sit with the extreme suffering. “The pain was too great,” Dr. Leiter said. “I needed her to adopt our narrative that we had done everything we could to help him live, and now, we would do everything we could to help his death be a comfortable one.”
But looking back, Dr. Leiter realized, “How could we have asked her to accept what was fundamentally unacceptable, to comprehend the incomprehensible?”
The Importance of Hope
Alan B. Astrow, MD, said during an ASCO symposium on “The Art and Science of Hope.”
“How we think about hope directly influences patient care,” said Dr. Astrow, chief of hematology and medical oncology at NewYork-Presbyterian Brooklyn Methodist Hospital and a professor of clinical medicine at Weill Cornell Medicine in New York City.
Hope, whatever it turns out to be neurobiologically, is “very much a gift” that underlies human existence, he said.
Physicians have the capacity to restore or shatter a patient’s hopes, and those who come to understand the importance of hope will wish to extend the gift to others, Dr. Astrow said.
Asking patients about their hopes is the “golden question,” Steven Z. Pantilat, MD, said at the symposium. “When you think about the future, what do you hope for?”
Often, the answers reveal not only “things beyond a cure that matter tremendously to the patient but things that we can help with,” said Dr. Pantilat, professor and chief of the Division of Palliative Medicine at the University of California San Francisco.
Dr. Pantilat recalled a patient with advanced pancreatic cancer who wished to see her daughter’s wedding in 10 months. He knew that was unlikely, but the discussion led to another solution.
Her daughter moved the wedding to the ICU.
Hope can persist and uplift even in the darkest of times, and “as clinicians, we need to be in the true hope business,” he said.
While some patients may wish for a cure, others may want more time with family or comfort in the face of suffering. People can “hope for all the things that can still be, despite the fact that there’s a lot of things that can’t,” he said.
However, fear that a patient will hope for a cure, and that the difficult discussions to follow might destroy hope or lead to false hope, sometimes means physicians won’t begin the conversation.
“We want to be honest with our patients — compassionate and kind, but honest — when we talk about their hopes,” Dr. Pantilat explained. Sometimes that means he needs to tell patients, “I wish that could happen. I wish I had a treatment that could make your cancer go away, but unfortunately, I don’t. So let’s think about what else we can do to help you.”
Having these difficult discussions matters. The evidence, although limited, indicates that feeling hopeful can improve patients’ well-being and may even boost their cancer outcomes.
One recent study found, for instance, that patients who reported feeling more hopeful also had lower levels of depression and anxiety. Early research also suggests that greater levels of hope may have a hand in reducing inflammation in patients with ovarian cancer and could even improve survival in some patients with advanced cancer.
For Dr. Leiter, while these lessons came early in his career as a palliative care physician, they persist and influence his practice today.
“I know that I could not have prevented Carlos’ death. None of us could have, and none of us could have protected his mother from the unimaginable grief that will stay with her for the rest of her life,” he said. “But I could have made things just a little bit less difficult for her.
“I could have acted as her guide rather than her cross-examiner,” he continued, explaining that he now sees hope as “a generous collaborator” that can coexist with rising creatinine levels, failing livers, and fears about intubation.
“As clinicians, we can always find space to hope with our patients and their families,” he said. “So now, years later when I sit with a terrified and grieving family and they tell me they hope their loved one gets better, I remember Carlos’ mother’s eyes piercing mine ... and I know how to respond: ‘I hope so, too.’ And I do.”
A version of this article appeared on Medscape.com.
CHICAGO — Carlos, a 21-year-old, lay in a hospital bed, barely clinging to life. Following a stem cell transplant for leukemia, Carlos had developed a life-threatening case of graft-vs-host disease.
But Carlos’ mother had faith.
“I have hope things will get better,” she said, via interpreter, to Richard Leiter, MD, a palliative care doctor in training at that time.
“I hope they will,” Dr. Leiter told her.
“I should have stopped there,” said Dr. Leiter, recounting an early-career lesson on hope during the ASCO Voices session at the American Society of Clinical Oncology annual meeting. “But in my eagerness to show my attending and myself that I could handle this conversation, I kept going, mistakenly.”
“But none of us think they will,” Dr. Leiter continued.
Carlos’ mother looked Dr. Leiter in the eye. “You want him to die,” she said.
“I knew, even then, that she was right,” recalled Dr. Leiter, now a palliative care physician at Dana-Farber Cancer Institute and Brigham and Women’s Hospital and an assistant professor of medicine at Harvard Medical School, Boston.
Although there was nothing he could do to save Carlos, Dr. Leiter also couldn’t sit with the extreme suffering. “The pain was too great,” Dr. Leiter said. “I needed her to adopt our narrative that we had done everything we could to help him live, and now, we would do everything we could to help his death be a comfortable one.”
But looking back, Dr. Leiter realized, “How could we have asked her to accept what was fundamentally unacceptable, to comprehend the incomprehensible?”
The Importance of Hope
Alan B. Astrow, MD, said during an ASCO symposium on “The Art and Science of Hope.”
“How we think about hope directly influences patient care,” said Dr. Astrow, chief of hematology and medical oncology at NewYork-Presbyterian Brooklyn Methodist Hospital and a professor of clinical medicine at Weill Cornell Medicine in New York City.
Hope, whatever it turns out to be neurobiologically, is “very much a gift” that underlies human existence, he said.
Physicians have the capacity to restore or shatter a patient’s hopes, and those who come to understand the importance of hope will wish to extend the gift to others, Dr. Astrow said.
Asking patients about their hopes is the “golden question,” Steven Z. Pantilat, MD, said at the symposium. “When you think about the future, what do you hope for?”
Often, the answers reveal not only “things beyond a cure that matter tremendously to the patient but things that we can help with,” said Dr. Pantilat, professor and chief of the Division of Palliative Medicine at the University of California San Francisco.
Dr. Pantilat recalled a patient with advanced pancreatic cancer who wished to see her daughter’s wedding in 10 months. He knew that was unlikely, but the discussion led to another solution.
Her daughter moved the wedding to the ICU.
Hope can persist and uplift even in the darkest of times, and “as clinicians, we need to be in the true hope business,” he said.
While some patients may wish for a cure, others may want more time with family or comfort in the face of suffering. People can “hope for all the things that can still be, despite the fact that there’s a lot of things that can’t,” he said.
However, fear that a patient will hope for a cure, and that the difficult discussions to follow might destroy hope or lead to false hope, sometimes means physicians won’t begin the conversation.
“We want to be honest with our patients — compassionate and kind, but honest — when we talk about their hopes,” Dr. Pantilat explained. Sometimes that means he needs to tell patients, “I wish that could happen. I wish I had a treatment that could make your cancer go away, but unfortunately, I don’t. So let’s think about what else we can do to help you.”
Having these difficult discussions matters. The evidence, although limited, indicates that feeling hopeful can improve patients’ well-being and may even boost their cancer outcomes.
One recent study found, for instance, that patients who reported feeling more hopeful also had lower levels of depression and anxiety. Early research also suggests that greater levels of hope may have a hand in reducing inflammation in patients with ovarian cancer and could even improve survival in some patients with advanced cancer.
For Dr. Leiter, while these lessons came early in his career as a palliative care physician, they persist and influence his practice today.
“I know that I could not have prevented Carlos’ death. None of us could have, and none of us could have protected his mother from the unimaginable grief that will stay with her for the rest of her life,” he said. “But I could have made things just a little bit less difficult for her.
“I could have acted as her guide rather than her cross-examiner,” he continued, explaining that he now sees hope as “a generous collaborator” that can coexist with rising creatinine levels, failing livers, and fears about intubation.
“As clinicians, we can always find space to hope with our patients and their families,” he said. “So now, years later when I sit with a terrified and grieving family and they tell me they hope their loved one gets better, I remember Carlos’ mother’s eyes piercing mine ... and I know how to respond: ‘I hope so, too.’ And I do.”
A version of this article appeared on Medscape.com.
FROM ASCO 2024