User login
For MD-IQ use only
Prior authorizations interfere with recommended cancer care
Of 178 respondents with a prior authorization (PA) experience, 39 (22%) did not receive the care recommended by their treatment team because of a PA requirement, and 123 (69%) experienced a delay in receiving the recommended care, Fumiko Chino, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues reported.
Reasons for not receiving recommended care included complete denial by the insurance company (26 of 39 patients), and a change in treatment plan because of initial denial (13 of 39 patients). Delays in receiving recommended care were 2 or more weeks for 90 of 123 patients, and 1 month or more in 40 of 123 patients.
Delays in receiving recommended care were associated with increased patient anxiety, a negative perception of the PA process, and patient administrative burden, the investigators noted.
The findings, which capture patient-based perspectives in the ongoing PA debacle, were reported online in JAMA Network Open.
“Prior authorization requires clinicians and patients to navigate a complex approval pathway. Resultant delays and denial can be particularly problematic for patients with cancer, who often need urgent treatment or symptom management,” the investigators explained. “Focusing on patient experiences with PA highlights a missing perspective in policy discussions and suggests another potential factor associated with eroding trust in the health care system.”
To assess the impact of PA, they conducted an anonymous survey using a convenience sample of patients with any cancer-related PA experience from July 1 to Oct. 6, 2022. Mean self-reported PA-related anxiety scores were 74.7 on a scale of 0-100, whereas usual anxiety scores were 37.5.
PA-related anxiety scores were significantly correlated with the length of treatment delay (P = .04), time spent on PA (P < .001), and overall PA experience (P < .001).
“Dealing with PA issues adds an extra layer of stress, which is known to increase anxiety and can worsen treatment-related and disease-related symptoms and adverse effects,” the investigators noted.
PA issues also eroded trust: 89% of respondents trusted their insurance company less, and 83% trusted the health care system less after a PA experience. Patient involvement in the PA process increased the likelihood of such distrust and of having a negative experience.
Of the 178 respondents, most were women (88%), non-Hispanic White individuals (84%), college graduates (84%), and young (18-39 years, 41%; 40-54 years, 33%). Most (67%) had to personally become involved in the PA process by calling their insurance or filing an appeal.
The investigators noted that “efforts to create national health policy solutions that streamline PA and make the process more transparent have been a major lobbying effort of large oncology societies,” and that bipartisan legislation to “establish regulations on the quality and timeliness of PA in the Medicare Advantage population” has stalled.
“In the meantime, the Centers for Medicare & Medicaid Services acted directly by issuing a final rule in April 2023 aimed at improving PA processes within the Medicare Advantage population by 2024,” they wrote, adding that “streamlining the PA process is key to optimizing the quality of care delivered and improving patients’ experience with cancer care.
“Policy interventions will be necessary to reform the PA process, as will advocacy efforts at the patient, clinician, and hospital level,” they concluded.
Chino reported funding through a National Institutes of Health/National Cancer Institute Cancer Center Support Grant.
Of 178 respondents with a prior authorization (PA) experience, 39 (22%) did not receive the care recommended by their treatment team because of a PA requirement, and 123 (69%) experienced a delay in receiving the recommended care, Fumiko Chino, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues reported.
Reasons for not receiving recommended care included complete denial by the insurance company (26 of 39 patients), and a change in treatment plan because of initial denial (13 of 39 patients). Delays in receiving recommended care were 2 or more weeks for 90 of 123 patients, and 1 month or more in 40 of 123 patients.
Delays in receiving recommended care were associated with increased patient anxiety, a negative perception of the PA process, and patient administrative burden, the investigators noted.
The findings, which capture patient-based perspectives in the ongoing PA debacle, were reported online in JAMA Network Open.
“Prior authorization requires clinicians and patients to navigate a complex approval pathway. Resultant delays and denial can be particularly problematic for patients with cancer, who often need urgent treatment or symptom management,” the investigators explained. “Focusing on patient experiences with PA highlights a missing perspective in policy discussions and suggests another potential factor associated with eroding trust in the health care system.”
To assess the impact of PA, they conducted an anonymous survey using a convenience sample of patients with any cancer-related PA experience from July 1 to Oct. 6, 2022. Mean self-reported PA-related anxiety scores were 74.7 on a scale of 0-100, whereas usual anxiety scores were 37.5.
PA-related anxiety scores were significantly correlated with the length of treatment delay (P = .04), time spent on PA (P < .001), and overall PA experience (P < .001).
“Dealing with PA issues adds an extra layer of stress, which is known to increase anxiety and can worsen treatment-related and disease-related symptoms and adverse effects,” the investigators noted.
PA issues also eroded trust: 89% of respondents trusted their insurance company less, and 83% trusted the health care system less after a PA experience. Patient involvement in the PA process increased the likelihood of such distrust and of having a negative experience.
Of the 178 respondents, most were women (88%), non-Hispanic White individuals (84%), college graduates (84%), and young (18-39 years, 41%; 40-54 years, 33%). Most (67%) had to personally become involved in the PA process by calling their insurance or filing an appeal.
The investigators noted that “efforts to create national health policy solutions that streamline PA and make the process more transparent have been a major lobbying effort of large oncology societies,” and that bipartisan legislation to “establish regulations on the quality and timeliness of PA in the Medicare Advantage population” has stalled.
“In the meantime, the Centers for Medicare & Medicaid Services acted directly by issuing a final rule in April 2023 aimed at improving PA processes within the Medicare Advantage population by 2024,” they wrote, adding that “streamlining the PA process is key to optimizing the quality of care delivered and improving patients’ experience with cancer care.
“Policy interventions will be necessary to reform the PA process, as will advocacy efforts at the patient, clinician, and hospital level,” they concluded.
Chino reported funding through a National Institutes of Health/National Cancer Institute Cancer Center Support Grant.
Of 178 respondents with a prior authorization (PA) experience, 39 (22%) did not receive the care recommended by their treatment team because of a PA requirement, and 123 (69%) experienced a delay in receiving the recommended care, Fumiko Chino, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues reported.
Reasons for not receiving recommended care included complete denial by the insurance company (26 of 39 patients), and a change in treatment plan because of initial denial (13 of 39 patients). Delays in receiving recommended care were 2 or more weeks for 90 of 123 patients, and 1 month or more in 40 of 123 patients.
Delays in receiving recommended care were associated with increased patient anxiety, a negative perception of the PA process, and patient administrative burden, the investigators noted.
The findings, which capture patient-based perspectives in the ongoing PA debacle, were reported online in JAMA Network Open.
“Prior authorization requires clinicians and patients to navigate a complex approval pathway. Resultant delays and denial can be particularly problematic for patients with cancer, who often need urgent treatment or symptom management,” the investigators explained. “Focusing on patient experiences with PA highlights a missing perspective in policy discussions and suggests another potential factor associated with eroding trust in the health care system.”
To assess the impact of PA, they conducted an anonymous survey using a convenience sample of patients with any cancer-related PA experience from July 1 to Oct. 6, 2022. Mean self-reported PA-related anxiety scores were 74.7 on a scale of 0-100, whereas usual anxiety scores were 37.5.
PA-related anxiety scores were significantly correlated with the length of treatment delay (P = .04), time spent on PA (P < .001), and overall PA experience (P < .001).
“Dealing with PA issues adds an extra layer of stress, which is known to increase anxiety and can worsen treatment-related and disease-related symptoms and adverse effects,” the investigators noted.
PA issues also eroded trust: 89% of respondents trusted their insurance company less, and 83% trusted the health care system less after a PA experience. Patient involvement in the PA process increased the likelihood of such distrust and of having a negative experience.
Of the 178 respondents, most were women (88%), non-Hispanic White individuals (84%), college graduates (84%), and young (18-39 years, 41%; 40-54 years, 33%). Most (67%) had to personally become involved in the PA process by calling their insurance or filing an appeal.
The investigators noted that “efforts to create national health policy solutions that streamline PA and make the process more transparent have been a major lobbying effort of large oncology societies,” and that bipartisan legislation to “establish regulations on the quality and timeliness of PA in the Medicare Advantage population” has stalled.
“In the meantime, the Centers for Medicare & Medicaid Services acted directly by issuing a final rule in April 2023 aimed at improving PA processes within the Medicare Advantage population by 2024,” they wrote, adding that “streamlining the PA process is key to optimizing the quality of care delivered and improving patients’ experience with cancer care.
“Policy interventions will be necessary to reform the PA process, as will advocacy efforts at the patient, clinician, and hospital level,” they concluded.
Chino reported funding through a National Institutes of Health/National Cancer Institute Cancer Center Support Grant.
FROM JAMA NETWORK OPEN
Studies address primary care oral health screening and prevention for children
Both were published online in JAMA.
In one report, the United States Preventive Services Task Force (USPSTF) concludes that there is not enough evidence to assess harms versus benefits of routine screening or interventions for oral health conditions, including dental caries, in primary care for asymptomatic children and adolescents aged 5-17 years.
The evidence report on administering fluoride supplements, fluoride gels, sealants and varnish finds evidence that they improve outcomes. The report was done to inform the USPSTF for a new recommendation on primary care screening, dental referral, behavioral counseling, and preventive interventions for oral health in children and adolescents aged 5-17.
Primary care physicians’ role
One problem the USPSTF identified in its report was limited evidence on available clinical screening tools or assessments to identify which children have oral health conditions in the primary care setting.
The USPSTF’s team, led by Michael J. Barry, MD, of Harvard Medical School in Boston, calls for more research to fill in the gaps before it can reassess.
Michael S. Reddy, DMD, DMSc, with University of California San Francisco School of Dentistry, Oral Health Affairs, said in an accompanying editorial that the current lack of data should not keep primary care physicians from considering oral health during routine medical exams or keep dentists from finding ways to collaborate with primary care physicians. “Medical primary care must partner with dentistry,” they wrote.
Until there is enough evidence for a USPSTF reevaluation on the topic, primary care clinicians should ask patients about their oral hygiene routines, whether they have any dental symptoms, and when they last saw a dentist, as well as referring to a dentist as necessary, the editorialists wrote.
That works both ways, the editorialists added. “Equally important, oral health professionals are encouraged to collaborate and be a resource for their primary care colleagues. Prevention is one of the best tools clinicians have, and it is promoted by integrated, whole-person health effort, “ wrote Dr. Reddy and colleagues.
When oral health stays separate from medical care, patients are left vulnerable, and referrals between medical and dental offices should be a stronger two-way system, the editorialists said.
“[N]ot every primary care patient has access to a dentist,” they wrote. “Oral health screening and referral by medical primary care clinicians can help ensure that individuals get to the dental chair to receive needed interventions that can benefit both oral and potentially overall health. Likewise, medical challenges and oral mucosal manifestations of chronic health conditions detected at a dental visit should result in medical referral, allowing prompt evaluation and treatment.”
Evidence that gels, varnish, sealants are effective
In a companion paper, done to inform the USPSTF, Roger Chou, MD, with Pacific Northwest Evidence-based Practice Center, Department of Medical Informatics and Clinical Epidemiology at Oregon Health & Science University in Portland, and colleagues found that when administered by a dental professional or in school settings, fluoride supplements, gels and varnish, and resin-based sealants improved health outcomes.
The findings were based on three systematic reviews (20,684 participants) and 19 randomized clinical trials; three nonrandomized trials; and one observational study (total 15,026 participants.)
With fluoride versus placebo or no intervention, researchers found a decrease from baseline in the number of decayed, missing, or filled permanent teeth (DMFT index) or decayed or filled permanent teeth (DFT index). The average difference was −0.73 [95% confidence interval [CI], −1.30 to −0.19]) at 1.5 to 3 years (six trials; n = 1,395).
Fluoride gels were associated with a DMFT- or DFT-prevented fraction of 0.18 (95% CI, 0.09-0.27) at outcomes closest to 3 years (four trials; n = 1,525).
Researchers found an association between fluoride varnish and a DMFT- or DFT-prevented fraction of 0.44 (95% CI, 0.11-0.76) at 1 to 4.5 years (five trials; n = 3,902). The sealants tested were associated with decreased risk of caries in first molars (odds ratio, 0.21 [95% CI, 0.16-0.28]) at 48-54 months (four trials; n = 440).
They noted that the feasibility of administering preventive measures in primary care is unknown; the effectiveness shown here was based on administration in dental and supervised school settings.
Barriers in primary care settings may include lack of training and equipment (particularly for sealants), uncertain reimbursement and lack of acceptance and uptake.
USPSTF working to close evidence gaps
Wanda Nicholson, MD, MPH, Prevention and Community Health, George Washington Milken Institute of Public Health in Washington, wrote in an accompanying editorial that to speed necessary research to facilitate recommendations, “the USPSTF and its stakeholders need a transparent, easily implementable communication tool that will systematically describe the research necessary to be directly responsive to the evidence gaps.”
The editorialists noted that the USPSTF in trying to update recommendations often has few, if any, high-quality additional studies to consider since its previous recommendation.
To address that, meetings were conducted in November of 2022 involving USPSTF members, Agency for Healthcare Research and Quality (AHRQ) staff, and leadership from the Office of Disease Prevention and the National Institutes of Health. Members formed a working group “to develop a standardized template for communicating research gaps” according to a framework developed by the National Academies of Sciences, Engineering, and Medicine.
Dr. Nicholson and colleagues wrote, “classifying evidence gaps and calling for specific research needs is a prudent, collaborative step in addressing missing evidence,” particularly for underserved populations.
The authors and editorialists declared no relevant conflicts of interest.
Both were published online in JAMA.
In one report, the United States Preventive Services Task Force (USPSTF) concludes that there is not enough evidence to assess harms versus benefits of routine screening or interventions for oral health conditions, including dental caries, in primary care for asymptomatic children and adolescents aged 5-17 years.
The evidence report on administering fluoride supplements, fluoride gels, sealants and varnish finds evidence that they improve outcomes. The report was done to inform the USPSTF for a new recommendation on primary care screening, dental referral, behavioral counseling, and preventive interventions for oral health in children and adolescents aged 5-17.
Primary care physicians’ role
One problem the USPSTF identified in its report was limited evidence on available clinical screening tools or assessments to identify which children have oral health conditions in the primary care setting.
The USPSTF’s team, led by Michael J. Barry, MD, of Harvard Medical School in Boston, calls for more research to fill in the gaps before it can reassess.
Michael S. Reddy, DMD, DMSc, with University of California San Francisco School of Dentistry, Oral Health Affairs, said in an accompanying editorial that the current lack of data should not keep primary care physicians from considering oral health during routine medical exams or keep dentists from finding ways to collaborate with primary care physicians. “Medical primary care must partner with dentistry,” they wrote.
Until there is enough evidence for a USPSTF reevaluation on the topic, primary care clinicians should ask patients about their oral hygiene routines, whether they have any dental symptoms, and when they last saw a dentist, as well as referring to a dentist as necessary, the editorialists wrote.
That works both ways, the editorialists added. “Equally important, oral health professionals are encouraged to collaborate and be a resource for their primary care colleagues. Prevention is one of the best tools clinicians have, and it is promoted by integrated, whole-person health effort, “ wrote Dr. Reddy and colleagues.
When oral health stays separate from medical care, patients are left vulnerable, and referrals between medical and dental offices should be a stronger two-way system, the editorialists said.
“[N]ot every primary care patient has access to a dentist,” they wrote. “Oral health screening and referral by medical primary care clinicians can help ensure that individuals get to the dental chair to receive needed interventions that can benefit both oral and potentially overall health. Likewise, medical challenges and oral mucosal manifestations of chronic health conditions detected at a dental visit should result in medical referral, allowing prompt evaluation and treatment.”
Evidence that gels, varnish, sealants are effective
In a companion paper, done to inform the USPSTF, Roger Chou, MD, with Pacific Northwest Evidence-based Practice Center, Department of Medical Informatics and Clinical Epidemiology at Oregon Health & Science University in Portland, and colleagues found that when administered by a dental professional or in school settings, fluoride supplements, gels and varnish, and resin-based sealants improved health outcomes.
The findings were based on three systematic reviews (20,684 participants) and 19 randomized clinical trials; three nonrandomized trials; and one observational study (total 15,026 participants.)
With fluoride versus placebo or no intervention, researchers found a decrease from baseline in the number of decayed, missing, or filled permanent teeth (DMFT index) or decayed or filled permanent teeth (DFT index). The average difference was −0.73 [95% confidence interval [CI], −1.30 to −0.19]) at 1.5 to 3 years (six trials; n = 1,395).
Fluoride gels were associated with a DMFT- or DFT-prevented fraction of 0.18 (95% CI, 0.09-0.27) at outcomes closest to 3 years (four trials; n = 1,525).
Researchers found an association between fluoride varnish and a DMFT- or DFT-prevented fraction of 0.44 (95% CI, 0.11-0.76) at 1 to 4.5 years (five trials; n = 3,902). The sealants tested were associated with decreased risk of caries in first molars (odds ratio, 0.21 [95% CI, 0.16-0.28]) at 48-54 months (four trials; n = 440).
They noted that the feasibility of administering preventive measures in primary care is unknown; the effectiveness shown here was based on administration in dental and supervised school settings.
Barriers in primary care settings may include lack of training and equipment (particularly for sealants), uncertain reimbursement and lack of acceptance and uptake.
USPSTF working to close evidence gaps
Wanda Nicholson, MD, MPH, Prevention and Community Health, George Washington Milken Institute of Public Health in Washington, wrote in an accompanying editorial that to speed necessary research to facilitate recommendations, “the USPSTF and its stakeholders need a transparent, easily implementable communication tool that will systematically describe the research necessary to be directly responsive to the evidence gaps.”
The editorialists noted that the USPSTF in trying to update recommendations often has few, if any, high-quality additional studies to consider since its previous recommendation.
To address that, meetings were conducted in November of 2022 involving USPSTF members, Agency for Healthcare Research and Quality (AHRQ) staff, and leadership from the Office of Disease Prevention and the National Institutes of Health. Members formed a working group “to develop a standardized template for communicating research gaps” according to a framework developed by the National Academies of Sciences, Engineering, and Medicine.
Dr. Nicholson and colleagues wrote, “classifying evidence gaps and calling for specific research needs is a prudent, collaborative step in addressing missing evidence,” particularly for underserved populations.
The authors and editorialists declared no relevant conflicts of interest.
Both were published online in JAMA.
In one report, the United States Preventive Services Task Force (USPSTF) concludes that there is not enough evidence to assess harms versus benefits of routine screening or interventions for oral health conditions, including dental caries, in primary care for asymptomatic children and adolescents aged 5-17 years.
The evidence report on administering fluoride supplements, fluoride gels, sealants and varnish finds evidence that they improve outcomes. The report was done to inform the USPSTF for a new recommendation on primary care screening, dental referral, behavioral counseling, and preventive interventions for oral health in children and adolescents aged 5-17.
Primary care physicians’ role
One problem the USPSTF identified in its report was limited evidence on available clinical screening tools or assessments to identify which children have oral health conditions in the primary care setting.
The USPSTF’s team, led by Michael J. Barry, MD, of Harvard Medical School in Boston, calls for more research to fill in the gaps before it can reassess.
Michael S. Reddy, DMD, DMSc, with University of California San Francisco School of Dentistry, Oral Health Affairs, said in an accompanying editorial that the current lack of data should not keep primary care physicians from considering oral health during routine medical exams or keep dentists from finding ways to collaborate with primary care physicians. “Medical primary care must partner with dentistry,” they wrote.
Until there is enough evidence for a USPSTF reevaluation on the topic, primary care clinicians should ask patients about their oral hygiene routines, whether they have any dental symptoms, and when they last saw a dentist, as well as referring to a dentist as necessary, the editorialists wrote.
That works both ways, the editorialists added. “Equally important, oral health professionals are encouraged to collaborate and be a resource for their primary care colleagues. Prevention is one of the best tools clinicians have, and it is promoted by integrated, whole-person health effort, “ wrote Dr. Reddy and colleagues.
When oral health stays separate from medical care, patients are left vulnerable, and referrals between medical and dental offices should be a stronger two-way system, the editorialists said.
“[N]ot every primary care patient has access to a dentist,” they wrote. “Oral health screening and referral by medical primary care clinicians can help ensure that individuals get to the dental chair to receive needed interventions that can benefit both oral and potentially overall health. Likewise, medical challenges and oral mucosal manifestations of chronic health conditions detected at a dental visit should result in medical referral, allowing prompt evaluation and treatment.”
Evidence that gels, varnish, sealants are effective
In a companion paper, done to inform the USPSTF, Roger Chou, MD, with Pacific Northwest Evidence-based Practice Center, Department of Medical Informatics and Clinical Epidemiology at Oregon Health & Science University in Portland, and colleagues found that when administered by a dental professional or in school settings, fluoride supplements, gels and varnish, and resin-based sealants improved health outcomes.
The findings were based on three systematic reviews (20,684 participants) and 19 randomized clinical trials; three nonrandomized trials; and one observational study (total 15,026 participants.)
With fluoride versus placebo or no intervention, researchers found a decrease from baseline in the number of decayed, missing, or filled permanent teeth (DMFT index) or decayed or filled permanent teeth (DFT index). The average difference was −0.73 [95% confidence interval [CI], −1.30 to −0.19]) at 1.5 to 3 years (six trials; n = 1,395).
Fluoride gels were associated with a DMFT- or DFT-prevented fraction of 0.18 (95% CI, 0.09-0.27) at outcomes closest to 3 years (four trials; n = 1,525).
Researchers found an association between fluoride varnish and a DMFT- or DFT-prevented fraction of 0.44 (95% CI, 0.11-0.76) at 1 to 4.5 years (five trials; n = 3,902). The sealants tested were associated with decreased risk of caries in first molars (odds ratio, 0.21 [95% CI, 0.16-0.28]) at 48-54 months (four trials; n = 440).
They noted that the feasibility of administering preventive measures in primary care is unknown; the effectiveness shown here was based on administration in dental and supervised school settings.
Barriers in primary care settings may include lack of training and equipment (particularly for sealants), uncertain reimbursement and lack of acceptance and uptake.
USPSTF working to close evidence gaps
Wanda Nicholson, MD, MPH, Prevention and Community Health, George Washington Milken Institute of Public Health in Washington, wrote in an accompanying editorial that to speed necessary research to facilitate recommendations, “the USPSTF and its stakeholders need a transparent, easily implementable communication tool that will systematically describe the research necessary to be directly responsive to the evidence gaps.”
The editorialists noted that the USPSTF in trying to update recommendations often has few, if any, high-quality additional studies to consider since its previous recommendation.
To address that, meetings were conducted in November of 2022 involving USPSTF members, Agency for Healthcare Research and Quality (AHRQ) staff, and leadership from the Office of Disease Prevention and the National Institutes of Health. Members formed a working group “to develop a standardized template for communicating research gaps” according to a framework developed by the National Academies of Sciences, Engineering, and Medicine.
Dr. Nicholson and colleagues wrote, “classifying evidence gaps and calling for specific research needs is a prudent, collaborative step in addressing missing evidence,” particularly for underserved populations.
The authors and editorialists declared no relevant conflicts of interest.
FROM JAMA
Veterans Get $6 billion in Hearing Loss Settlement
Hearing loss and tinnitus are the top and third most common service-connected disabilities among veterans. According to a Veterans Benefits Administration report, as of fiscal year 2020, more than 1.3 million veterans were receiving disability compensation for hearing loss and more than 2.3 million for tinnitus. Not surprisingly, the US Department of Veterans Affairs (VA) is the largest employer of audiologists and speech-language pathologists in the US.
On the bright side, military hearing losses are at stable levels—but “it’s not improving,” said US Army Lt Col Michael Murphy, chief of the studies and analysis section and Army audiology liaison at the Defense Health Agency Hearing Center of Excellence (HCE), in an interview for Department of Defense news.
Hearing protection is critical to reduce injury. Exposure to firearms, explosives, and other “continuous hazardous noise” puts service members and US Department of Defense (DoD) civilians at risk of permanent hearing loss, said Theresa Schulz, PhD, chief of the HCE prevention and surveillance section. “Good hearing is a key to mission success.”
Hearing protectors, which Shulz calls “the last line of defense from noise-induced hearing loss,” work best when they fit right: protecting against noise and, when necessary, not muffling voices, alarms, and other important sounds. That is why the DoD has updated its requirements for fit testing. All DoD personnel who are exposed to continuous and intermittent noise ≥ 85 decibels (in an 8-hour average) or impulse noise sound pressure ≥ 140 decibels (for ≥ 1 day per year) must be enrolled in a hearing conservation program. Additional criteria are expected for release by December 2023. According to HCE, each service may have more stringent requirements for hearing protector fit testing that better meets the needs of their hearing conservation program.
The question of proper fit was at the root of a recent lawsuit charging 3M with knowingly selling defective earplugs to the US military. The 3M dual-ended Combat Arms Earplug (CAEv2) was designed to eliminate the need for soldiers to carry 2 different sets of earplugs. Worn one way, it was intended to block sound like traditional earplugs; worn in reverse, it would block only certain types of loud battlefield noise while allowing the wearer to hear softer, closer sounds.
However, no 2 ears are the same—even on the same person. According to the HCE, during hearing protection testing, there is a < 2 mm difference in insertion depth between left and right ears for 85% of subjects. A 2016 whistleblower lawsuit accused 3M of not disclosing that the CAEv2 was too short for proper insertion into users’ ears and that it could loosen imperceptibly and fail to form the protective seal.
In 2018, 3M agreed to pay $9.1 million to the Department of Justice to resolve the allegations without admitting liability. That case led to the largest mass tort multidistrict litigation in US history. Last February, Veterans of Foreign Wars (VFW) filed an amicus curiae brief to the Seventh Circuit Court of Appeals in support of claimants seeking relief from 3M for defective ear protection. Approximately 240,000 veterans filed lawsuits against 3M. In September the parties reached a $6 billion settlement—nearly half of 3M’s worth. According to John Muckelbauer, a veteran and general counsel for the VFW in a military.com opinion piece, the settlement achieves balance: not pushing the already financially strapped 3M into bankruptcy, but sending “a strong signal that the safety of our service members can never be compromised.”
Crucially, Muckelbauer notes, the VA says participating in the lawsuit will not result in the loss of health or disability benefits, nor will it adversely affect disability ratings. VA facilities are also barred from recovering any portion of a plaintiff’s award as part of a medical lien.
3M has not admitted responsibility in this settlement either, frustrating the veteran claimants. An admission of guilt was never on the table, says Ronald Miller, Jr., writing for the Lawsuit Information Center, which posts updates on class action lawsuits. “Admitting responsibility would open the door for everyone to opt out and move forward on that admission… Admitting guilt would also be harmful to 3M’s reputation. They have long vigorously denied responsibility, so the optics would be terrible.”
A new twist cropped up almost immediately when claimants began getting cold calls from scammers impersonating employees of Archer Systems LLC, the company designated to administer the settlement. The scammers attempted to extract sensitive personal information, including Social Security numbers. Judge M. Casey Rodgers alerted the Federal Bureau of Investigation and warned claimants to safeguard their data vigilantly and report any fraudulent attempts.
The settlement money will be paid out from 2023 to 2029, with $1 billion in the form of 3M stock, 3M said in a statement. (In August 2023, upon news of the settlement, the price of 3M shares had risen nearly 5%.) Miller says the whole $6 billion will be distributed using a point system that awards amounts according to disability, with, for instance, tinnitus without contemporaneous corroboration getting the least and moderate or greater hearing loss getting the most. “This settlement is a tremendous outcome for veterans of Iraq and Afghanistan who put their lives on the line for our freedom,” said Duane Sarmiento, VFW national commander in a statement. “For those who came home with hearing damage due to 3M’s faulty earplugs, this is not only compensation, it’s a statement that their sacrifices won’t be ignored.”
Hearing loss and tinnitus are the top and third most common service-connected disabilities among veterans. According to a Veterans Benefits Administration report, as of fiscal year 2020, more than 1.3 million veterans were receiving disability compensation for hearing loss and more than 2.3 million for tinnitus. Not surprisingly, the US Department of Veterans Affairs (VA) is the largest employer of audiologists and speech-language pathologists in the US.
On the bright side, military hearing losses are at stable levels—but “it’s not improving,” said US Army Lt Col Michael Murphy, chief of the studies and analysis section and Army audiology liaison at the Defense Health Agency Hearing Center of Excellence (HCE), in an interview for Department of Defense news.
Hearing protection is critical to reduce injury. Exposure to firearms, explosives, and other “continuous hazardous noise” puts service members and US Department of Defense (DoD) civilians at risk of permanent hearing loss, said Theresa Schulz, PhD, chief of the HCE prevention and surveillance section. “Good hearing is a key to mission success.”
Hearing protectors, which Shulz calls “the last line of defense from noise-induced hearing loss,” work best when they fit right: protecting against noise and, when necessary, not muffling voices, alarms, and other important sounds. That is why the DoD has updated its requirements for fit testing. All DoD personnel who are exposed to continuous and intermittent noise ≥ 85 decibels (in an 8-hour average) or impulse noise sound pressure ≥ 140 decibels (for ≥ 1 day per year) must be enrolled in a hearing conservation program. Additional criteria are expected for release by December 2023. According to HCE, each service may have more stringent requirements for hearing protector fit testing that better meets the needs of their hearing conservation program.
The question of proper fit was at the root of a recent lawsuit charging 3M with knowingly selling defective earplugs to the US military. The 3M dual-ended Combat Arms Earplug (CAEv2) was designed to eliminate the need for soldiers to carry 2 different sets of earplugs. Worn one way, it was intended to block sound like traditional earplugs; worn in reverse, it would block only certain types of loud battlefield noise while allowing the wearer to hear softer, closer sounds.
However, no 2 ears are the same—even on the same person. According to the HCE, during hearing protection testing, there is a < 2 mm difference in insertion depth between left and right ears for 85% of subjects. A 2016 whistleblower lawsuit accused 3M of not disclosing that the CAEv2 was too short for proper insertion into users’ ears and that it could loosen imperceptibly and fail to form the protective seal.
In 2018, 3M agreed to pay $9.1 million to the Department of Justice to resolve the allegations without admitting liability. That case led to the largest mass tort multidistrict litigation in US history. Last February, Veterans of Foreign Wars (VFW) filed an amicus curiae brief to the Seventh Circuit Court of Appeals in support of claimants seeking relief from 3M for defective ear protection. Approximately 240,000 veterans filed lawsuits against 3M. In September the parties reached a $6 billion settlement—nearly half of 3M’s worth. According to John Muckelbauer, a veteran and general counsel for the VFW in a military.com opinion piece, the settlement achieves balance: not pushing the already financially strapped 3M into bankruptcy, but sending “a strong signal that the safety of our service members can never be compromised.”
Crucially, Muckelbauer notes, the VA says participating in the lawsuit will not result in the loss of health or disability benefits, nor will it adversely affect disability ratings. VA facilities are also barred from recovering any portion of a plaintiff’s award as part of a medical lien.
3M has not admitted responsibility in this settlement either, frustrating the veteran claimants. An admission of guilt was never on the table, says Ronald Miller, Jr., writing for the Lawsuit Information Center, which posts updates on class action lawsuits. “Admitting responsibility would open the door for everyone to opt out and move forward on that admission… Admitting guilt would also be harmful to 3M’s reputation. They have long vigorously denied responsibility, so the optics would be terrible.”
A new twist cropped up almost immediately when claimants began getting cold calls from scammers impersonating employees of Archer Systems LLC, the company designated to administer the settlement. The scammers attempted to extract sensitive personal information, including Social Security numbers. Judge M. Casey Rodgers alerted the Federal Bureau of Investigation and warned claimants to safeguard their data vigilantly and report any fraudulent attempts.
The settlement money will be paid out from 2023 to 2029, with $1 billion in the form of 3M stock, 3M said in a statement. (In August 2023, upon news of the settlement, the price of 3M shares had risen nearly 5%.) Miller says the whole $6 billion will be distributed using a point system that awards amounts according to disability, with, for instance, tinnitus without contemporaneous corroboration getting the least and moderate or greater hearing loss getting the most. “This settlement is a tremendous outcome for veterans of Iraq and Afghanistan who put their lives on the line for our freedom,” said Duane Sarmiento, VFW national commander in a statement. “For those who came home with hearing damage due to 3M’s faulty earplugs, this is not only compensation, it’s a statement that their sacrifices won’t be ignored.”
Hearing loss and tinnitus are the top and third most common service-connected disabilities among veterans. According to a Veterans Benefits Administration report, as of fiscal year 2020, more than 1.3 million veterans were receiving disability compensation for hearing loss and more than 2.3 million for tinnitus. Not surprisingly, the US Department of Veterans Affairs (VA) is the largest employer of audiologists and speech-language pathologists in the US.
On the bright side, military hearing losses are at stable levels—but “it’s not improving,” said US Army Lt Col Michael Murphy, chief of the studies and analysis section and Army audiology liaison at the Defense Health Agency Hearing Center of Excellence (HCE), in an interview for Department of Defense news.
Hearing protection is critical to reduce injury. Exposure to firearms, explosives, and other “continuous hazardous noise” puts service members and US Department of Defense (DoD) civilians at risk of permanent hearing loss, said Theresa Schulz, PhD, chief of the HCE prevention and surveillance section. “Good hearing is a key to mission success.”
Hearing protectors, which Shulz calls “the last line of defense from noise-induced hearing loss,” work best when they fit right: protecting against noise and, when necessary, not muffling voices, alarms, and other important sounds. That is why the DoD has updated its requirements for fit testing. All DoD personnel who are exposed to continuous and intermittent noise ≥ 85 decibels (in an 8-hour average) or impulse noise sound pressure ≥ 140 decibels (for ≥ 1 day per year) must be enrolled in a hearing conservation program. Additional criteria are expected for release by December 2023. According to HCE, each service may have more stringent requirements for hearing protector fit testing that better meets the needs of their hearing conservation program.
The question of proper fit was at the root of a recent lawsuit charging 3M with knowingly selling defective earplugs to the US military. The 3M dual-ended Combat Arms Earplug (CAEv2) was designed to eliminate the need for soldiers to carry 2 different sets of earplugs. Worn one way, it was intended to block sound like traditional earplugs; worn in reverse, it would block only certain types of loud battlefield noise while allowing the wearer to hear softer, closer sounds.
However, no 2 ears are the same—even on the same person. According to the HCE, during hearing protection testing, there is a < 2 mm difference in insertion depth between left and right ears for 85% of subjects. A 2016 whistleblower lawsuit accused 3M of not disclosing that the CAEv2 was too short for proper insertion into users’ ears and that it could loosen imperceptibly and fail to form the protective seal.
In 2018, 3M agreed to pay $9.1 million to the Department of Justice to resolve the allegations without admitting liability. That case led to the largest mass tort multidistrict litigation in US history. Last February, Veterans of Foreign Wars (VFW) filed an amicus curiae brief to the Seventh Circuit Court of Appeals in support of claimants seeking relief from 3M for defective ear protection. Approximately 240,000 veterans filed lawsuits against 3M. In September the parties reached a $6 billion settlement—nearly half of 3M’s worth. According to John Muckelbauer, a veteran and general counsel for the VFW in a military.com opinion piece, the settlement achieves balance: not pushing the already financially strapped 3M into bankruptcy, but sending “a strong signal that the safety of our service members can never be compromised.”
Crucially, Muckelbauer notes, the VA says participating in the lawsuit will not result in the loss of health or disability benefits, nor will it adversely affect disability ratings. VA facilities are also barred from recovering any portion of a plaintiff’s award as part of a medical lien.
3M has not admitted responsibility in this settlement either, frustrating the veteran claimants. An admission of guilt was never on the table, says Ronald Miller, Jr., writing for the Lawsuit Information Center, which posts updates on class action lawsuits. “Admitting responsibility would open the door for everyone to opt out and move forward on that admission… Admitting guilt would also be harmful to 3M’s reputation. They have long vigorously denied responsibility, so the optics would be terrible.”
A new twist cropped up almost immediately when claimants began getting cold calls from scammers impersonating employees of Archer Systems LLC, the company designated to administer the settlement. The scammers attempted to extract sensitive personal information, including Social Security numbers. Judge M. Casey Rodgers alerted the Federal Bureau of Investigation and warned claimants to safeguard their data vigilantly and report any fraudulent attempts.
The settlement money will be paid out from 2023 to 2029, with $1 billion in the form of 3M stock, 3M said in a statement. (In August 2023, upon news of the settlement, the price of 3M shares had risen nearly 5%.) Miller says the whole $6 billion will be distributed using a point system that awards amounts according to disability, with, for instance, tinnitus without contemporaneous corroboration getting the least and moderate or greater hearing loss getting the most. “This settlement is a tremendous outcome for veterans of Iraq and Afghanistan who put their lives on the line for our freedom,” said Duane Sarmiento, VFW national commander in a statement. “For those who came home with hearing damage due to 3M’s faulty earplugs, this is not only compensation, it’s a statement that their sacrifices won’t be ignored.”
Body dysmorphic disorder diagnosis guidelines completed in Europe
BERLIN – were outlined in a late-breaker presentation at the annual Congress of the European Academy of Dermatology and Venereology.
The development of guidelines for BDD, a disorder familiar to many clinical dermatologists, is intended as a practical tool, according to Maria-Angeliki Gkini, MD, who has appointments at both Bart’s Health NHS Trust in London and the 401 General Army Hospital in Athens.
“BDD is a relatively common disorder in which the patients are preoccupied with a perceived defect or defects,” Dr. Gkini explained. “This affects them so intensely that it affects their mental health and their quality of life.”
In the DSM-5, published by the American Psychiatric Association, BDD is specifically defined as a preoccupation with “one or more perceived defects or flaws in physical appearance that are not observable or appear slight to others.” But Dr. Gkini said that BDD can also develop as a comorbidity of dermatological disorders that are visible.
These patients are challenging because they are difficult to please, added Dr. Gkini, who said they commonly become involved in doctor shopping, leaving negative reviews on social media for the clinicians they have cycled through. The problem is that the defects they seek to resolve typically stem from distorted perceptions.
BDD is related to obsessive-compulsive disorder by the frequency with which patients pursue repetitive behaviors related to their preoccupation, such as intensive grooming, frequent trips to the mirror, or difficulty in focusing on topics other than their own appearance.
The process to develop the soon-to-be-published guidelines began with a literature search. Of the approximately 3,200 articles identified on BDD, only 10 involved randomized controlled trials. Moreover, even the quality of these trials was considered “low to very low” by the experts who reviewed them, Dr. Gkini said.
One explanation is that psychodermatology has only recently started to attract more research interest, and better studies are now underway, she noted.
However, because of the dearth of high quality evidence now available, the guideline development relied on a Delphi method to reach consensus based on expert opinion in discussion of the available data.
Consensus reached by 17 experts
Specifically, 17 experts, all of whom were members of the European Society for Dermatology and Psychiatry proceeded to systematically address a series of clinical questions and recommendations. Consensus was defined as at least 75% of the participants strongly agreeing or agreeing. Several rounds of discussion were often required.
Among the conclusions, the guidelines support uniform screening for BDD in all patients prior to cosmetic procedures. In identifying depression, anxiety, and distorted perceptions, simple tools, such as the Patient Health Questionnaire might be adequate for an initial evaluation, but Dr. Gkini also recommended routinely inquiring about suicidal ideation, which has been reported in up to 80% of individuals with BDD.
Other instruments for screening that can be considered include DSM-5 criteria for BDD and the Body Dysmorphic Disorder Questionnaire–Dermatology Version, which might be particularly useful and appropriate for dermatologists.
One of the reasons to screen for BDD is that these patients often convince themselves that some specific procedure is needed to resolve the source of their obsession. The goal of screening is to verify that it is the dermatologic concern, not an underlying psychiatric disorder that is driving their search for relief. The risk of dermatologic interventions is not only that expectations are not met, but the patient’s perception of a failed intervention “sometimes makes these worse,” Dr. Gkini explained.
Collaboration with psychiatrists recommended
The guidelines include suggestions for treatment of BDD. Of these, SSRIs are recommended at high relative doses, according to Dr. Gkini. Consistent with the consensus recommendation of collaborating with mental health specialists, she said that the recommendations acknowledge evidence of greater benefits when SSRIs are combined with psychotherapy.
Katharine A. Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, has been conducting BDD research for several years and has written numerous books and articles about this topic, including a review in the journal Focus. She cautioned that, because of a normal concern for appearance, BDD is easily missed by dermatologists.
“For BDD to be diagnosed, the preoccupation with a nonexistent or slight defect in appearance must cause clinically significant distress or impairment in functioning,” she said in an interview. “This is necessary to differentiate BDD from more normal and common appearance concerns that do not qualify for the diagnosis”
She specified that patients should be considered for cognitive-behavioral therapy rather than psychotherapy, a generic term that covers many forms of treatment. She said that most other types of psychotherapy “are probably not effective” for BDD.
Dr. Phillips highly endorsed the development of BDD guidelines for dermatologists because of the frequency with which physicians in this specialty encounter BDD – and believes that more attention to this diagnosis is needed.
“I recommend that dermatologists who have a patient with BDD collaborate with a psychiatrist in delivering care with an SSRI,” she said. “High doses of these medications are often needed to effectively treat BDD.”
Dr. Gkini reported financial relationships with AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO, Novartis, Sanofi, and Regenlab. Dr. Phillips reported no relevant financial relationships.
BERLIN – were outlined in a late-breaker presentation at the annual Congress of the European Academy of Dermatology and Venereology.
The development of guidelines for BDD, a disorder familiar to many clinical dermatologists, is intended as a practical tool, according to Maria-Angeliki Gkini, MD, who has appointments at both Bart’s Health NHS Trust in London and the 401 General Army Hospital in Athens.
“BDD is a relatively common disorder in which the patients are preoccupied with a perceived defect or defects,” Dr. Gkini explained. “This affects them so intensely that it affects their mental health and their quality of life.”
In the DSM-5, published by the American Psychiatric Association, BDD is specifically defined as a preoccupation with “one or more perceived defects or flaws in physical appearance that are not observable or appear slight to others.” But Dr. Gkini said that BDD can also develop as a comorbidity of dermatological disorders that are visible.
These patients are challenging because they are difficult to please, added Dr. Gkini, who said they commonly become involved in doctor shopping, leaving negative reviews on social media for the clinicians they have cycled through. The problem is that the defects they seek to resolve typically stem from distorted perceptions.
BDD is related to obsessive-compulsive disorder by the frequency with which patients pursue repetitive behaviors related to their preoccupation, such as intensive grooming, frequent trips to the mirror, or difficulty in focusing on topics other than their own appearance.
The process to develop the soon-to-be-published guidelines began with a literature search. Of the approximately 3,200 articles identified on BDD, only 10 involved randomized controlled trials. Moreover, even the quality of these trials was considered “low to very low” by the experts who reviewed them, Dr. Gkini said.
One explanation is that psychodermatology has only recently started to attract more research interest, and better studies are now underway, she noted.
However, because of the dearth of high quality evidence now available, the guideline development relied on a Delphi method to reach consensus based on expert opinion in discussion of the available data.
Consensus reached by 17 experts
Specifically, 17 experts, all of whom were members of the European Society for Dermatology and Psychiatry proceeded to systematically address a series of clinical questions and recommendations. Consensus was defined as at least 75% of the participants strongly agreeing or agreeing. Several rounds of discussion were often required.
Among the conclusions, the guidelines support uniform screening for BDD in all patients prior to cosmetic procedures. In identifying depression, anxiety, and distorted perceptions, simple tools, such as the Patient Health Questionnaire might be adequate for an initial evaluation, but Dr. Gkini also recommended routinely inquiring about suicidal ideation, which has been reported in up to 80% of individuals with BDD.
Other instruments for screening that can be considered include DSM-5 criteria for BDD and the Body Dysmorphic Disorder Questionnaire–Dermatology Version, which might be particularly useful and appropriate for dermatologists.
One of the reasons to screen for BDD is that these patients often convince themselves that some specific procedure is needed to resolve the source of their obsession. The goal of screening is to verify that it is the dermatologic concern, not an underlying psychiatric disorder that is driving their search for relief. The risk of dermatologic interventions is not only that expectations are not met, but the patient’s perception of a failed intervention “sometimes makes these worse,” Dr. Gkini explained.
Collaboration with psychiatrists recommended
The guidelines include suggestions for treatment of BDD. Of these, SSRIs are recommended at high relative doses, according to Dr. Gkini. Consistent with the consensus recommendation of collaborating with mental health specialists, she said that the recommendations acknowledge evidence of greater benefits when SSRIs are combined with psychotherapy.
Katharine A. Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, has been conducting BDD research for several years and has written numerous books and articles about this topic, including a review in the journal Focus. She cautioned that, because of a normal concern for appearance, BDD is easily missed by dermatologists.
“For BDD to be diagnosed, the preoccupation with a nonexistent or slight defect in appearance must cause clinically significant distress or impairment in functioning,” she said in an interview. “This is necessary to differentiate BDD from more normal and common appearance concerns that do not qualify for the diagnosis”
She specified that patients should be considered for cognitive-behavioral therapy rather than psychotherapy, a generic term that covers many forms of treatment. She said that most other types of psychotherapy “are probably not effective” for BDD.
Dr. Phillips highly endorsed the development of BDD guidelines for dermatologists because of the frequency with which physicians in this specialty encounter BDD – and believes that more attention to this diagnosis is needed.
“I recommend that dermatologists who have a patient with BDD collaborate with a psychiatrist in delivering care with an SSRI,” she said. “High doses of these medications are often needed to effectively treat BDD.”
Dr. Gkini reported financial relationships with AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO, Novartis, Sanofi, and Regenlab. Dr. Phillips reported no relevant financial relationships.
BERLIN – were outlined in a late-breaker presentation at the annual Congress of the European Academy of Dermatology and Venereology.
The development of guidelines for BDD, a disorder familiar to many clinical dermatologists, is intended as a practical tool, according to Maria-Angeliki Gkini, MD, who has appointments at both Bart’s Health NHS Trust in London and the 401 General Army Hospital in Athens.
“BDD is a relatively common disorder in which the patients are preoccupied with a perceived defect or defects,” Dr. Gkini explained. “This affects them so intensely that it affects their mental health and their quality of life.”
In the DSM-5, published by the American Psychiatric Association, BDD is specifically defined as a preoccupation with “one or more perceived defects or flaws in physical appearance that are not observable or appear slight to others.” But Dr. Gkini said that BDD can also develop as a comorbidity of dermatological disorders that are visible.
These patients are challenging because they are difficult to please, added Dr. Gkini, who said they commonly become involved in doctor shopping, leaving negative reviews on social media for the clinicians they have cycled through. The problem is that the defects they seek to resolve typically stem from distorted perceptions.
BDD is related to obsessive-compulsive disorder by the frequency with which patients pursue repetitive behaviors related to their preoccupation, such as intensive grooming, frequent trips to the mirror, or difficulty in focusing on topics other than their own appearance.
The process to develop the soon-to-be-published guidelines began with a literature search. Of the approximately 3,200 articles identified on BDD, only 10 involved randomized controlled trials. Moreover, even the quality of these trials was considered “low to very low” by the experts who reviewed them, Dr. Gkini said.
One explanation is that psychodermatology has only recently started to attract more research interest, and better studies are now underway, she noted.
However, because of the dearth of high quality evidence now available, the guideline development relied on a Delphi method to reach consensus based on expert opinion in discussion of the available data.
Consensus reached by 17 experts
Specifically, 17 experts, all of whom were members of the European Society for Dermatology and Psychiatry proceeded to systematically address a series of clinical questions and recommendations. Consensus was defined as at least 75% of the participants strongly agreeing or agreeing. Several rounds of discussion were often required.
Among the conclusions, the guidelines support uniform screening for BDD in all patients prior to cosmetic procedures. In identifying depression, anxiety, and distorted perceptions, simple tools, such as the Patient Health Questionnaire might be adequate for an initial evaluation, but Dr. Gkini also recommended routinely inquiring about suicidal ideation, which has been reported in up to 80% of individuals with BDD.
Other instruments for screening that can be considered include DSM-5 criteria for BDD and the Body Dysmorphic Disorder Questionnaire–Dermatology Version, which might be particularly useful and appropriate for dermatologists.
One of the reasons to screen for BDD is that these patients often convince themselves that some specific procedure is needed to resolve the source of their obsession. The goal of screening is to verify that it is the dermatologic concern, not an underlying psychiatric disorder that is driving their search for relief. The risk of dermatologic interventions is not only that expectations are not met, but the patient’s perception of a failed intervention “sometimes makes these worse,” Dr. Gkini explained.
Collaboration with psychiatrists recommended
The guidelines include suggestions for treatment of BDD. Of these, SSRIs are recommended at high relative doses, according to Dr. Gkini. Consistent with the consensus recommendation of collaborating with mental health specialists, she said that the recommendations acknowledge evidence of greater benefits when SSRIs are combined with psychotherapy.
Katharine A. Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, has been conducting BDD research for several years and has written numerous books and articles about this topic, including a review in the journal Focus. She cautioned that, because of a normal concern for appearance, BDD is easily missed by dermatologists.
“For BDD to be diagnosed, the preoccupation with a nonexistent or slight defect in appearance must cause clinically significant distress or impairment in functioning,” she said in an interview. “This is necessary to differentiate BDD from more normal and common appearance concerns that do not qualify for the diagnosis”
She specified that patients should be considered for cognitive-behavioral therapy rather than psychotherapy, a generic term that covers many forms of treatment. She said that most other types of psychotherapy “are probably not effective” for BDD.
Dr. Phillips highly endorsed the development of BDD guidelines for dermatologists because of the frequency with which physicians in this specialty encounter BDD – and believes that more attention to this diagnosis is needed.
“I recommend that dermatologists who have a patient with BDD collaborate with a psychiatrist in delivering care with an SSRI,” she said. “High doses of these medications are often needed to effectively treat BDD.”
Dr. Gkini reported financial relationships with AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO, Novartis, Sanofi, and Regenlab. Dr. Phillips reported no relevant financial relationships.
AT THE EADV CONGRESS
Monitoring Thyrotropin in Veterans With Thyroid Nodules
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
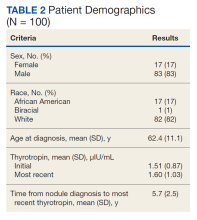
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
Piperacillin-tazobactam poses no renal risk in adults with sepsis
TOPLINE:
METHODOLOGY:
The coadministration of piperacillin-tazobactam and vancomycin may raise the risk for AKI, according to a warning from the Food and Drug Administration.
The ACORN trial included 2,511 adults presenting to emergency department or intensive care unit with suspected infection.
Within 12 hours of presentation, these individuals were prescribed either cefepime (n = 1,214) or piperacillin-tazobactam (n = 1,297).
The primary outcome was the risk for the highest stage of AKI or death within 14 days of randomization.
TAKEAWAY:
The highest stage of AKI or death within 14 days did not differ significantly between the cefepime and piperacillin-tazobactam groups (odds ratio, 0.95; P = .56).
The incidence of major adverse kidney events by day 14 was not significantly different between the two groups (absolute risk difference, 1.4%; 95% confidence interval, −1.0% to 3.8%).
Patients in the cefepime versus piperacillin-tazobactam group had fewer days alive and free of delirium and coma within 14 days (OR, 0.79; 95% CI, 0.65-0.95).
IN PRACTICE:
In an accompanying editorial, Steven Y. C. Tong, department of infectious diseases, University of Melbourne, and colleagues wrote: “Because institutions must make decisions about which antibiotics to position on medical wards for rapid administration in patients meeting sepsis criteria, these data should offer solace that if the choice is made to use piperacillin-tazobactam, there is not an increased risk of AKI.”
SOURCE:
The study was led by Edward T. Qian, MD, of Vanderbilt University Medical Center, Nashville, Tenn. It was published online in JAMA with an accompanying editorial.
LIMITATIONS:
The study was conducted at a single academic center, which may limit the generalizability of findings.
Both patients and clinicians were not blinded to group assignment, which may have influenced clinical assessments like Richmond Agitation-Sedation Scale and CAM-ICU or the frequency of laboratory measurements like creatinine.
DISCLOSURES:
The project was supported by the Vanderbilt Institute for Clinical and Translational Research and several other sources, including grants from the National Center for Advancing Translational Sciences. Some authors declared receiving travel grant, personal fees, honoraria, and unrelated research support from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
The coadministration of piperacillin-tazobactam and vancomycin may raise the risk for AKI, according to a warning from the Food and Drug Administration.
The ACORN trial included 2,511 adults presenting to emergency department or intensive care unit with suspected infection.
Within 12 hours of presentation, these individuals were prescribed either cefepime (n = 1,214) or piperacillin-tazobactam (n = 1,297).
The primary outcome was the risk for the highest stage of AKI or death within 14 days of randomization.
TAKEAWAY:
The highest stage of AKI or death within 14 days did not differ significantly between the cefepime and piperacillin-tazobactam groups (odds ratio, 0.95; P = .56).
The incidence of major adverse kidney events by day 14 was not significantly different between the two groups (absolute risk difference, 1.4%; 95% confidence interval, −1.0% to 3.8%).
Patients in the cefepime versus piperacillin-tazobactam group had fewer days alive and free of delirium and coma within 14 days (OR, 0.79; 95% CI, 0.65-0.95).
IN PRACTICE:
In an accompanying editorial, Steven Y. C. Tong, department of infectious diseases, University of Melbourne, and colleagues wrote: “Because institutions must make decisions about which antibiotics to position on medical wards for rapid administration in patients meeting sepsis criteria, these data should offer solace that if the choice is made to use piperacillin-tazobactam, there is not an increased risk of AKI.”
SOURCE:
The study was led by Edward T. Qian, MD, of Vanderbilt University Medical Center, Nashville, Tenn. It was published online in JAMA with an accompanying editorial.
LIMITATIONS:
The study was conducted at a single academic center, which may limit the generalizability of findings.
Both patients and clinicians were not blinded to group assignment, which may have influenced clinical assessments like Richmond Agitation-Sedation Scale and CAM-ICU or the frequency of laboratory measurements like creatinine.
DISCLOSURES:
The project was supported by the Vanderbilt Institute for Clinical and Translational Research and several other sources, including grants from the National Center for Advancing Translational Sciences. Some authors declared receiving travel grant, personal fees, honoraria, and unrelated research support from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
The coadministration of piperacillin-tazobactam and vancomycin may raise the risk for AKI, according to a warning from the Food and Drug Administration.
The ACORN trial included 2,511 adults presenting to emergency department or intensive care unit with suspected infection.
Within 12 hours of presentation, these individuals were prescribed either cefepime (n = 1,214) or piperacillin-tazobactam (n = 1,297).
The primary outcome was the risk for the highest stage of AKI or death within 14 days of randomization.
TAKEAWAY:
The highest stage of AKI or death within 14 days did not differ significantly between the cefepime and piperacillin-tazobactam groups (odds ratio, 0.95; P = .56).
The incidence of major adverse kidney events by day 14 was not significantly different between the two groups (absolute risk difference, 1.4%; 95% confidence interval, −1.0% to 3.8%).
Patients in the cefepime versus piperacillin-tazobactam group had fewer days alive and free of delirium and coma within 14 days (OR, 0.79; 95% CI, 0.65-0.95).
IN PRACTICE:
In an accompanying editorial, Steven Y. C. Tong, department of infectious diseases, University of Melbourne, and colleagues wrote: “Because institutions must make decisions about which antibiotics to position on medical wards for rapid administration in patients meeting sepsis criteria, these data should offer solace that if the choice is made to use piperacillin-tazobactam, there is not an increased risk of AKI.”
SOURCE:
The study was led by Edward T. Qian, MD, of Vanderbilt University Medical Center, Nashville, Tenn. It was published online in JAMA with an accompanying editorial.
LIMITATIONS:
The study was conducted at a single academic center, which may limit the generalizability of findings.
Both patients and clinicians were not blinded to group assignment, which may have influenced clinical assessments like Richmond Agitation-Sedation Scale and CAM-ICU or the frequency of laboratory measurements like creatinine.
DISCLOSURES:
The project was supported by the Vanderbilt Institute for Clinical and Translational Research and several other sources, including grants from the National Center for Advancing Translational Sciences. Some authors declared receiving travel grant, personal fees, honoraria, and unrelated research support from various sources.
A version of this article appeared on Medscape.com.
One-year anniversary
Dear Friends,
It’s been a year since I have become editor-in-chief of The New Gastroenterologist and I am so grateful for our readers and contributors. Thank you for being a part of the TNG family. , including the increasing armamentarium of inflammatory bowel disease treatments, taking on leadership roles in diversity, equity, and inclusion, and tackling financial planning.
In this issue’s In Focus, Drs. Ariela K. Holmer, Shannon Chang, and Lisa Malter break down the factors that contribute to selecting therapies for moderate to severe IBD, such as disease activity and severity, extraintestinal manifestations, safety, prior anti–tumor necrosis factor exposure, perianal disease, and patient preference.
Drs. Michael G. Rubeiz, Kemmian D. Johnson, and Juan Reyes Genere continue our journey with IBD in the Short Clinical Review section, describing advances in endoscopic therapies in IBD. They review the resection of colitis dysplasia and management of luminal strictures with dilation and stricturotomy.
Early-career faculty are being requested to spearhead diversity, equity, and inclusion (DEI) efforts at their institutions or for their groups. Drs. Cassandra D.L. Fritz and Nicolette Juliana Rodriguez highlight important aspects that should be considered prior to taking on DEI roles.
In the Finance section, Dr. Animesh Jain answers five common questions for young gastroenterologists. He addresses student loans, disability insurance, life insurance, retirement, and buying a first house.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first biologic therapy for IBD, infliximab, was only approved 25 years ago in 1998.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
It’s been a year since I have become editor-in-chief of The New Gastroenterologist and I am so grateful for our readers and contributors. Thank you for being a part of the TNG family. , including the increasing armamentarium of inflammatory bowel disease treatments, taking on leadership roles in diversity, equity, and inclusion, and tackling financial planning.
In this issue’s In Focus, Drs. Ariela K. Holmer, Shannon Chang, and Lisa Malter break down the factors that contribute to selecting therapies for moderate to severe IBD, such as disease activity and severity, extraintestinal manifestations, safety, prior anti–tumor necrosis factor exposure, perianal disease, and patient preference.
Drs. Michael G. Rubeiz, Kemmian D. Johnson, and Juan Reyes Genere continue our journey with IBD in the Short Clinical Review section, describing advances in endoscopic therapies in IBD. They review the resection of colitis dysplasia and management of luminal strictures with dilation and stricturotomy.
Early-career faculty are being requested to spearhead diversity, equity, and inclusion (DEI) efforts at their institutions or for their groups. Drs. Cassandra D.L. Fritz and Nicolette Juliana Rodriguez highlight important aspects that should be considered prior to taking on DEI roles.
In the Finance section, Dr. Animesh Jain answers five common questions for young gastroenterologists. He addresses student loans, disability insurance, life insurance, retirement, and buying a first house.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first biologic therapy for IBD, infliximab, was only approved 25 years ago in 1998.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
It’s been a year since I have become editor-in-chief of The New Gastroenterologist and I am so grateful for our readers and contributors. Thank you for being a part of the TNG family. , including the increasing armamentarium of inflammatory bowel disease treatments, taking on leadership roles in diversity, equity, and inclusion, and tackling financial planning.
In this issue’s In Focus, Drs. Ariela K. Holmer, Shannon Chang, and Lisa Malter break down the factors that contribute to selecting therapies for moderate to severe IBD, such as disease activity and severity, extraintestinal manifestations, safety, prior anti–tumor necrosis factor exposure, perianal disease, and patient preference.
Drs. Michael G. Rubeiz, Kemmian D. Johnson, and Juan Reyes Genere continue our journey with IBD in the Short Clinical Review section, describing advances in endoscopic therapies in IBD. They review the resection of colitis dysplasia and management of luminal strictures with dilation and stricturotomy.
Early-career faculty are being requested to spearhead diversity, equity, and inclusion (DEI) efforts at their institutions or for their groups. Drs. Cassandra D.L. Fritz and Nicolette Juliana Rodriguez highlight important aspects that should be considered prior to taking on DEI roles.
In the Finance section, Dr. Animesh Jain answers five common questions for young gastroenterologists. He addresses student loans, disability insurance, life insurance, retirement, and buying a first house.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first biologic therapy for IBD, infliximab, was only approved 25 years ago in 1998.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
November 2023 - ICYMI
Gastroenterology
July
Newberry C et al. Enhancing Nutrition and Obesity Education in GI Fellowship Through Universal Curriculum Development. Gastroenterology. 2023 Jul;165(1):16-19. doi: 10.1053/j.gastro.2023.04.004. Epub 2023 Apr 13. PMID: 37061170.
Han H et al. Macrophage-derived Osteopontin (SPP1) Protects From Nonalcoholic Steatohepatitis. Gastroenterology. 2023 Jul;165(1):201-17. doi: 10.1053/j.gastro.2023.03.228. Epub 2023 Apr 5. PMID: 37028770.
Deepak P et al. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 Jul;165(1):11-15. doi: 10.1053/j.gastro.2023.05.017. PMID: 37349061.
August
Guo L et al. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology. 2023 Aug;165(2):414-28.e7. doi: 10.1053/j.gastro.2023.04.029. Epub 2023 May 3. PMID: 37146911.
Huang DQ et al. Fibrosis Progression Rate in Biopsy-Proven Nonalcoholic Fatty Liver Disease Among People With Diabetes Versus People Without Diabetes: A Multicenter Study. Gastroenterology. 2023 Aug;165(2):463-72.e5. doi: 10.1053/j.gastro.2023.04.025. Epub 2023 Apr 29. PMID: 37127100.
Teoh AYB et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP With Covered Metallic Stents in Patients With Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology. 2023 Aug;165(2):473-82.e2. doi: 10.1053/j.gastro.2023.04.016. Epub 2023 Apr 28. PMID: 37121331.
September
Mehta RS et al. Association of Proton Pump Inhibitor Use With Incident Dementia and Cognitive Decline in Older Adults: A Prospective Cohort Study. Gastroenterology. 2023 Sep;165(3):564-72.e1. doi: 10.1053/j.gastro.2023.05.052. Epub 2023 Jun 12. PMID: 37315867; PMCID: PMC10527011.
Ballou S et al. Prevalence and Associated Factors of Bloating: Results From the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 Sep;165(3):647-55.e4. doi: 10.1053/j.gastro.2023.05.049. Epub 2023 Jun 13. PMID: 37315866; PMCID: PMC10527500.
CGH
July
Chang JW et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1690-8. doi: 10.1016/j.cgh.2023.03.006. Epub 2023 Mar 16. PMID: 36933603; PMCID: PMC10293042.
Siboni S et al. Improving the Diagnostic Yield of High-Resolution Esophageal Manometry for GERD: The “Straight Leg-Raise” International Study. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1761-70.e1. doi: 10.1016/j.cgh.2022.10.008. Epub 2022 Oct 19. PMID: 36270615.
August
Wechsler EV et al. Up-Front Endoscopy Maximizes Cost-Effectiveness and Cost-Satisfaction in Uninvestigated Dyspepsia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2378-88.e28. doi: 10.1016/j.cgh.2023.01.003. Epub 2023 Jan 13. PMID: 36646234; PMCID: PMC10542651.
Frederiks CN et al. Clinical Relevance of Random Biopsies From the Esophagogastric Junction After Complete Eradication of Barrett’s Esophagus is Low. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2260-9.e9. doi: 10.1016/j.cgh.2022.11.012. Epub 2022 Nov 22. PMID: 36423874.
Rustgi SD et al. Management of Gastric Intestinal Metaplasia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2178-82. doi: 10.1016/j.cgh.2023.03.010. Epub 2023 Apr 19. PMID: 37086748; PMCID: PMC10526696.
September
Baroud S et al. A Protocolized Management of Walled-Off Necrosis (WON) Reduces Time to WON Resolution and Improves Outcomes. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2543-50.e1. doi: 10.1016/j.cgh.2023.04.029. Epub 2023 May 8. PMID: 37164115.
Arnim UV et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2526-33. doi: 10.1016/j.cgh.2022.12.018. Epub 2022 Dec 24. PMID: 36572109.
TIGE
Kaila V et al. Does the Absence of Contrast Passage Into the Duodenum During Intraoperative Cholangiogram Truly Predict Choledocholithiasis? Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.05.002.
O’Keefe SJD et al. Early Enteral Feeding in Severe Acute Pancreatitis: A Randomized Clinical Trial Between Gastric vs Distal Jejunal Feeding. Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.06.002.
Gastro Hep Advances
Mukherjee S et al. Assessing ChatGPT’s ability to reply to queries regarding colon cancer screening based on Multi-Society Guidelines. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.008.
Lopes EW et al. Lochhead P. Improving the Consent Process with an Informed Consent Video Prior to Outpatient Colonoscopy. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.016.
Gastroenterology
July
Newberry C et al. Enhancing Nutrition and Obesity Education in GI Fellowship Through Universal Curriculum Development. Gastroenterology. 2023 Jul;165(1):16-19. doi: 10.1053/j.gastro.2023.04.004. Epub 2023 Apr 13. PMID: 37061170.
Han H et al. Macrophage-derived Osteopontin (SPP1) Protects From Nonalcoholic Steatohepatitis. Gastroenterology. 2023 Jul;165(1):201-17. doi: 10.1053/j.gastro.2023.03.228. Epub 2023 Apr 5. PMID: 37028770.
Deepak P et al. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 Jul;165(1):11-15. doi: 10.1053/j.gastro.2023.05.017. PMID: 37349061.
August
Guo L et al. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology. 2023 Aug;165(2):414-28.e7. doi: 10.1053/j.gastro.2023.04.029. Epub 2023 May 3. PMID: 37146911.
Huang DQ et al. Fibrosis Progression Rate in Biopsy-Proven Nonalcoholic Fatty Liver Disease Among People With Diabetes Versus People Without Diabetes: A Multicenter Study. Gastroenterology. 2023 Aug;165(2):463-72.e5. doi: 10.1053/j.gastro.2023.04.025. Epub 2023 Apr 29. PMID: 37127100.
Teoh AYB et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP With Covered Metallic Stents in Patients With Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology. 2023 Aug;165(2):473-82.e2. doi: 10.1053/j.gastro.2023.04.016. Epub 2023 Apr 28. PMID: 37121331.
September
Mehta RS et al. Association of Proton Pump Inhibitor Use With Incident Dementia and Cognitive Decline in Older Adults: A Prospective Cohort Study. Gastroenterology. 2023 Sep;165(3):564-72.e1. doi: 10.1053/j.gastro.2023.05.052. Epub 2023 Jun 12. PMID: 37315867; PMCID: PMC10527011.
Ballou S et al. Prevalence and Associated Factors of Bloating: Results From the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 Sep;165(3):647-55.e4. doi: 10.1053/j.gastro.2023.05.049. Epub 2023 Jun 13. PMID: 37315866; PMCID: PMC10527500.
CGH
July
Chang JW et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1690-8. doi: 10.1016/j.cgh.2023.03.006. Epub 2023 Mar 16. PMID: 36933603; PMCID: PMC10293042.
Siboni S et al. Improving the Diagnostic Yield of High-Resolution Esophageal Manometry for GERD: The “Straight Leg-Raise” International Study. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1761-70.e1. doi: 10.1016/j.cgh.2022.10.008. Epub 2022 Oct 19. PMID: 36270615.
August
Wechsler EV et al. Up-Front Endoscopy Maximizes Cost-Effectiveness and Cost-Satisfaction in Uninvestigated Dyspepsia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2378-88.e28. doi: 10.1016/j.cgh.2023.01.003. Epub 2023 Jan 13. PMID: 36646234; PMCID: PMC10542651.
Frederiks CN et al. Clinical Relevance of Random Biopsies From the Esophagogastric Junction After Complete Eradication of Barrett’s Esophagus is Low. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2260-9.e9. doi: 10.1016/j.cgh.2022.11.012. Epub 2022 Nov 22. PMID: 36423874.
Rustgi SD et al. Management of Gastric Intestinal Metaplasia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2178-82. doi: 10.1016/j.cgh.2023.03.010. Epub 2023 Apr 19. PMID: 37086748; PMCID: PMC10526696.
September
Baroud S et al. A Protocolized Management of Walled-Off Necrosis (WON) Reduces Time to WON Resolution and Improves Outcomes. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2543-50.e1. doi: 10.1016/j.cgh.2023.04.029. Epub 2023 May 8. PMID: 37164115.
Arnim UV et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2526-33. doi: 10.1016/j.cgh.2022.12.018. Epub 2022 Dec 24. PMID: 36572109.
TIGE
Kaila V et al. Does the Absence of Contrast Passage Into the Duodenum During Intraoperative Cholangiogram Truly Predict Choledocholithiasis? Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.05.002.
O’Keefe SJD et al. Early Enteral Feeding in Severe Acute Pancreatitis: A Randomized Clinical Trial Between Gastric vs Distal Jejunal Feeding. Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.06.002.
Gastro Hep Advances
Mukherjee S et al. Assessing ChatGPT’s ability to reply to queries regarding colon cancer screening based on Multi-Society Guidelines. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.008.
Lopes EW et al. Lochhead P. Improving the Consent Process with an Informed Consent Video Prior to Outpatient Colonoscopy. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.016.
Gastroenterology
July
Newberry C et al. Enhancing Nutrition and Obesity Education in GI Fellowship Through Universal Curriculum Development. Gastroenterology. 2023 Jul;165(1):16-19. doi: 10.1053/j.gastro.2023.04.004. Epub 2023 Apr 13. PMID: 37061170.
Han H et al. Macrophage-derived Osteopontin (SPP1) Protects From Nonalcoholic Steatohepatitis. Gastroenterology. 2023 Jul;165(1):201-17. doi: 10.1053/j.gastro.2023.03.228. Epub 2023 Apr 5. PMID: 37028770.
Deepak P et al. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 Jul;165(1):11-15. doi: 10.1053/j.gastro.2023.05.017. PMID: 37349061.
August
Guo L et al. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology. 2023 Aug;165(2):414-28.e7. doi: 10.1053/j.gastro.2023.04.029. Epub 2023 May 3. PMID: 37146911.
Huang DQ et al. Fibrosis Progression Rate in Biopsy-Proven Nonalcoholic Fatty Liver Disease Among People With Diabetes Versus People Without Diabetes: A Multicenter Study. Gastroenterology. 2023 Aug;165(2):463-72.e5. doi: 10.1053/j.gastro.2023.04.025. Epub 2023 Apr 29. PMID: 37127100.
Teoh AYB et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP With Covered Metallic Stents in Patients With Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology. 2023 Aug;165(2):473-82.e2. doi: 10.1053/j.gastro.2023.04.016. Epub 2023 Apr 28. PMID: 37121331.
September
Mehta RS et al. Association of Proton Pump Inhibitor Use With Incident Dementia and Cognitive Decline in Older Adults: A Prospective Cohort Study. Gastroenterology. 2023 Sep;165(3):564-72.e1. doi: 10.1053/j.gastro.2023.05.052. Epub 2023 Jun 12. PMID: 37315867; PMCID: PMC10527011.
Ballou S et al. Prevalence and Associated Factors of Bloating: Results From the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 Sep;165(3):647-55.e4. doi: 10.1053/j.gastro.2023.05.049. Epub 2023 Jun 13. PMID: 37315866; PMCID: PMC10527500.
CGH
July
Chang JW et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1690-8. doi: 10.1016/j.cgh.2023.03.006. Epub 2023 Mar 16. PMID: 36933603; PMCID: PMC10293042.
Siboni S et al. Improving the Diagnostic Yield of High-Resolution Esophageal Manometry for GERD: The “Straight Leg-Raise” International Study. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1761-70.e1. doi: 10.1016/j.cgh.2022.10.008. Epub 2022 Oct 19. PMID: 36270615.
August
Wechsler EV et al. Up-Front Endoscopy Maximizes Cost-Effectiveness and Cost-Satisfaction in Uninvestigated Dyspepsia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2378-88.e28. doi: 10.1016/j.cgh.2023.01.003. Epub 2023 Jan 13. PMID: 36646234; PMCID: PMC10542651.
Frederiks CN et al. Clinical Relevance of Random Biopsies From the Esophagogastric Junction After Complete Eradication of Barrett’s Esophagus is Low. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2260-9.e9. doi: 10.1016/j.cgh.2022.11.012. Epub 2022 Nov 22. PMID: 36423874.
Rustgi SD et al. Management of Gastric Intestinal Metaplasia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2178-82. doi: 10.1016/j.cgh.2023.03.010. Epub 2023 Apr 19. PMID: 37086748; PMCID: PMC10526696.
September
Baroud S et al. A Protocolized Management of Walled-Off Necrosis (WON) Reduces Time to WON Resolution and Improves Outcomes. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2543-50.e1. doi: 10.1016/j.cgh.2023.04.029. Epub 2023 May 8. PMID: 37164115.
Arnim UV et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2526-33. doi: 10.1016/j.cgh.2022.12.018. Epub 2022 Dec 24. PMID: 36572109.
TIGE
Kaila V et al. Does the Absence of Contrast Passage Into the Duodenum During Intraoperative Cholangiogram Truly Predict Choledocholithiasis? Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.05.002.
O’Keefe SJD et al. Early Enteral Feeding in Severe Acute Pancreatitis: A Randomized Clinical Trial Between Gastric vs Distal Jejunal Feeding. Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.06.002.
Gastro Hep Advances
Mukherjee S et al. Assessing ChatGPT’s ability to reply to queries regarding colon cancer screening based on Multi-Society Guidelines. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.008.
Lopes EW et al. Lochhead P. Improving the Consent Process with an Informed Consent Video Prior to Outpatient Colonoscopy. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.016.
Survey finds oral minoxidil shortage in Washington-area pharmacies
A .
Patients are not finding out until they go to pick up their prescription, which can result in an interruption of treatment – and, potentially a loss of hard-earned hair gain, said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was the lead author of the survey, published online on Oct. 26 as a research letter in the Journal of Drugs in Dermatology.
Going off low-dose oral minoxidil may spark a telogen effluvium event, and that is very disappointing to patients, Dr. Friedman told this news organization.
“There needs to be some system that alerts us,” he said. “Even if it’s a minor shortage, just so we’re aware. We can then prepare patients,” he added, noting that it would be better for someone to be taking a lower-than-normal dose rather than no medication at all while they wait for a refill.
Minoxidil has long been approved in a topical formulation to treat androgenetic alopecia, but a low-dose oral form has gained currency in the wake of findings that it might more effectively treat hair loss, and is without side effects. A New York Times article in August 2022 touting low-dose oral minoxidil as a cheap and effective hair loss drug appeared to ignite interest in this option. In May, 2023, researchers reporting in JAMA Network Open demonstrated a significant uptick in prescriptions for oral minoxidil in the wake of the article’s publication.
Oral minoxidil is approved by the Food and Drug Administration only for hypertension, but dermatologists are prescribing it off-label at a lower dose for hair loss. Dr. Friedman said it’s not clear whether the shortages his team found are national in scope, or whether they are a result of increased demand, or other factors.
After several patients told him they were having trouble filling minoxidil prescriptions, and colleagues said they’d had patients with similar experiences, Dr. Friedman and his colleagues undertook the survey. In the first week of October 2023, they contacted 277 pharmacies by phone in Washington and surrounding Virginia and Maryland counties. The pharmacies were CVS, Giant, Walgreens, and Harris Teeter.
Of the 277 pharmacies they contacted, 40% (111) reported availability of 2.5-mg tablets for a 30-day supply, and just under 30% (82) reported having 10-mg tablets for a 30-day supply.
For treating hair loss, most patients are prescribed 2.5-mg pills, with starting doses ranging from 0.625 mg to 5 mg twice a day, Dr. Friedman said. The 10-mg dose is more frequently prescribed for hypertension.
Only 28% (19 of 67) of the Maryland pharmacies had 30-day supplies of 2.5-mg tablets on hand, and just 22% (15) of the Maryland pharmacies had 30-day supplies of 10-mg tablets. In Northern Virginia, 44% (63 of 143) of the pharmacies had 30-day supplies of the 2.5 mg tablets, as did just 43% (29 of 67) of the Washington pharmacies.
Dr. Friedman said he has started giving patients paper prescriptions they can use to shop around, rather than electronically sending a prescription to a particular pharmacy.
Neither the Food and Drug Administration nor the American Society of Health System Pharmacists lists oral minoxidil as a drug in shortage.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, said the organization received a report from wholesalers in mid-September showing spotty oral minoxidil availability, with the drug on backorder with some manufacturers. ASHP's shortages list is compiled from reports from physicians, manufacturers and wholesalers, he said.
Under what he calls "blue sky conditions," pharmacies using a just-in-time inventory model should be able to fill prescriptions within hours or days, which might explain why some pharmacies in the Washington, DC area survey did not have a 30-day supply on hand, he said. However, Dr. Ganio noted that the causes of drug shortages are complex and multi-factorial. For now, he said there have been no oral minoxidil shortage reports since mid-September.
But Dr. Friedman said some of his patients have waited weeks for a new supply – and that no one is aware of the problem until the last moment.
The lack of alerts or transparency “also erodes the physician-patient relationship because there’s this expectation of the patient that we should have known this,” said Dr. Friedman.
Dr. Friedman reports no relevant financial relationships.
This story was updated on 11/2/2023.
A .
Patients are not finding out until they go to pick up their prescription, which can result in an interruption of treatment – and, potentially a loss of hard-earned hair gain, said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was the lead author of the survey, published online on Oct. 26 as a research letter in the Journal of Drugs in Dermatology.
Going off low-dose oral minoxidil may spark a telogen effluvium event, and that is very disappointing to patients, Dr. Friedman told this news organization.
“There needs to be some system that alerts us,” he said. “Even if it’s a minor shortage, just so we’re aware. We can then prepare patients,” he added, noting that it would be better for someone to be taking a lower-than-normal dose rather than no medication at all while they wait for a refill.
Minoxidil has long been approved in a topical formulation to treat androgenetic alopecia, but a low-dose oral form has gained currency in the wake of findings that it might more effectively treat hair loss, and is without side effects. A New York Times article in August 2022 touting low-dose oral minoxidil as a cheap and effective hair loss drug appeared to ignite interest in this option. In May, 2023, researchers reporting in JAMA Network Open demonstrated a significant uptick in prescriptions for oral minoxidil in the wake of the article’s publication.
Oral minoxidil is approved by the Food and Drug Administration only for hypertension, but dermatologists are prescribing it off-label at a lower dose for hair loss. Dr. Friedman said it’s not clear whether the shortages his team found are national in scope, or whether they are a result of increased demand, or other factors.
After several patients told him they were having trouble filling minoxidil prescriptions, and colleagues said they’d had patients with similar experiences, Dr. Friedman and his colleagues undertook the survey. In the first week of October 2023, they contacted 277 pharmacies by phone in Washington and surrounding Virginia and Maryland counties. The pharmacies were CVS, Giant, Walgreens, and Harris Teeter.
Of the 277 pharmacies they contacted, 40% (111) reported availability of 2.5-mg tablets for a 30-day supply, and just under 30% (82) reported having 10-mg tablets for a 30-day supply.
For treating hair loss, most patients are prescribed 2.5-mg pills, with starting doses ranging from 0.625 mg to 5 mg twice a day, Dr. Friedman said. The 10-mg dose is more frequently prescribed for hypertension.
Only 28% (19 of 67) of the Maryland pharmacies had 30-day supplies of 2.5-mg tablets on hand, and just 22% (15) of the Maryland pharmacies had 30-day supplies of 10-mg tablets. In Northern Virginia, 44% (63 of 143) of the pharmacies had 30-day supplies of the 2.5 mg tablets, as did just 43% (29 of 67) of the Washington pharmacies.
Dr. Friedman said he has started giving patients paper prescriptions they can use to shop around, rather than electronically sending a prescription to a particular pharmacy.
Neither the Food and Drug Administration nor the American Society of Health System Pharmacists lists oral minoxidil as a drug in shortage.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, said the organization received a report from wholesalers in mid-September showing spotty oral minoxidil availability, with the drug on backorder with some manufacturers. ASHP's shortages list is compiled from reports from physicians, manufacturers and wholesalers, he said.
Under what he calls "blue sky conditions," pharmacies using a just-in-time inventory model should be able to fill prescriptions within hours or days, which might explain why some pharmacies in the Washington, DC area survey did not have a 30-day supply on hand, he said. However, Dr. Ganio noted that the causes of drug shortages are complex and multi-factorial. For now, he said there have been no oral minoxidil shortage reports since mid-September.
But Dr. Friedman said some of his patients have waited weeks for a new supply – and that no one is aware of the problem until the last moment.
The lack of alerts or transparency “also erodes the physician-patient relationship because there’s this expectation of the patient that we should have known this,” said Dr. Friedman.
Dr. Friedman reports no relevant financial relationships.
This story was updated on 11/2/2023.
A .
Patients are not finding out until they go to pick up their prescription, which can result in an interruption of treatment – and, potentially a loss of hard-earned hair gain, said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was the lead author of the survey, published online on Oct. 26 as a research letter in the Journal of Drugs in Dermatology.
Going off low-dose oral minoxidil may spark a telogen effluvium event, and that is very disappointing to patients, Dr. Friedman told this news organization.
“There needs to be some system that alerts us,” he said. “Even if it’s a minor shortage, just so we’re aware. We can then prepare patients,” he added, noting that it would be better for someone to be taking a lower-than-normal dose rather than no medication at all while they wait for a refill.
Minoxidil has long been approved in a topical formulation to treat androgenetic alopecia, but a low-dose oral form has gained currency in the wake of findings that it might more effectively treat hair loss, and is without side effects. A New York Times article in August 2022 touting low-dose oral minoxidil as a cheap and effective hair loss drug appeared to ignite interest in this option. In May, 2023, researchers reporting in JAMA Network Open demonstrated a significant uptick in prescriptions for oral minoxidil in the wake of the article’s publication.
Oral minoxidil is approved by the Food and Drug Administration only for hypertension, but dermatologists are prescribing it off-label at a lower dose for hair loss. Dr. Friedman said it’s not clear whether the shortages his team found are national in scope, or whether they are a result of increased demand, or other factors.
After several patients told him they were having trouble filling minoxidil prescriptions, and colleagues said they’d had patients with similar experiences, Dr. Friedman and his colleagues undertook the survey. In the first week of October 2023, they contacted 277 pharmacies by phone in Washington and surrounding Virginia and Maryland counties. The pharmacies were CVS, Giant, Walgreens, and Harris Teeter.
Of the 277 pharmacies they contacted, 40% (111) reported availability of 2.5-mg tablets for a 30-day supply, and just under 30% (82) reported having 10-mg tablets for a 30-day supply.
For treating hair loss, most patients are prescribed 2.5-mg pills, with starting doses ranging from 0.625 mg to 5 mg twice a day, Dr. Friedman said. The 10-mg dose is more frequently prescribed for hypertension.
Only 28% (19 of 67) of the Maryland pharmacies had 30-day supplies of 2.5-mg tablets on hand, and just 22% (15) of the Maryland pharmacies had 30-day supplies of 10-mg tablets. In Northern Virginia, 44% (63 of 143) of the pharmacies had 30-day supplies of the 2.5 mg tablets, as did just 43% (29 of 67) of the Washington pharmacies.
Dr. Friedman said he has started giving patients paper prescriptions they can use to shop around, rather than electronically sending a prescription to a particular pharmacy.
Neither the Food and Drug Administration nor the American Society of Health System Pharmacists lists oral minoxidil as a drug in shortage.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, said the organization received a report from wholesalers in mid-September showing spotty oral minoxidil availability, with the drug on backorder with some manufacturers. ASHP's shortages list is compiled from reports from physicians, manufacturers and wholesalers, he said.
Under what he calls "blue sky conditions," pharmacies using a just-in-time inventory model should be able to fill prescriptions within hours or days, which might explain why some pharmacies in the Washington, DC area survey did not have a 30-day supply on hand, he said. However, Dr. Ganio noted that the causes of drug shortages are complex and multi-factorial. For now, he said there have been no oral minoxidil shortage reports since mid-September.
But Dr. Friedman said some of his patients have waited weeks for a new supply – and that no one is aware of the problem until the last moment.
The lack of alerts or transparency “also erodes the physician-patient relationship because there’s this expectation of the patient that we should have known this,” said Dr. Friedman.
Dr. Friedman reports no relevant financial relationships.
This story was updated on 11/2/2023.
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY