User login
Bariatric surgery may cut cancer in obesity with liver disease
In a large cohort of insured working adults with severe obesity and nonalcoholic fatty liver disease (NAFLD), the rate of incident cancer was lower during a 10-month median follow-up period among those who underwent bariatric surgery. The rate was especially lower with regard to obesity-related cancers. The risk reduction was greater among patients with cirrhosis.
Among almost 100,000 patients with severe obesity (body mass index >40 kg/m2) and NAFLD, those who underwent bariatric surgery had an 18% and 35% lower risk of developing any cancer or obesity-related cancer, respectively.
Bariatric surgery was associated with a significantly lower risk of being diagnosed with colorectal, pancreatic, endometrial, and thyroid cancer, as well as hepatocellular carcinoma and multiple myeloma (all obesity-related cancers). The findings are from an observational study by Vinod K. Rustgi, MD, MBA, and colleagues, which was published online March 17, 2021, in Gastroenterology.
It was not surprising that bariatric surgery is effective in reducing the malignancy rate among patients with cirrhosis, the researchers wrote, because the surgery results in long-term weight loss, resolution of nonalcoholic steatohepatitis (NASH), and regression of fibrosis.
“Cirrhosis can happen from fatty liver disease or NASH,” Dr. Rustgi, a hepatologist at Robert Wood Johnson Medical School, New Brunswick, N.J., explained to this news organization. “It’s becoming the fastest growing indication for liver transplant, but also the reason for increased rates of hepatocellular carcinoma.”
Current treatment for patients with obesity and fatty liver disease begins with lifestyle changes to lose weight, he continued. “As people lose 10% of their weight, they actually start to see regression of fibrosis in the liver that is correlated with [lower rates of] malignancy outcomes and other deleterious outcomes.” But long-lasting weight loss is extremely difficult to achieve.
Future studies “may identify new targets and treatments, such as antidiabetic-, satiety-, or GLP-1-based medications, for chemoprevention in NAFLD/NASH,” the investigators suggested. However, pharmaceutical agents will likely be very expensive when they eventually get marketed, Dr. Rustgi observed.
Although “bariatric surgery is a more aggressive approach than lifestyle modifications, surgery may provide additional benefits, such as improved quality of life and decreased long-term health care costs,” he and his coauthors concluded.
Rising rates of fatty liver disease, obesity
An estimated 30% of the population of the United States has NAFLD, the most common chronic liver disease, the researchers noted in their article. The prevalence of NAFLD increased 2.8-fold in the United States between 2003 and 2011, in parallel with increasing obesity.
NAFLD is more common among male patients with obesity and diabetes and Hispanic patients; “70% of [patients with diabetes] may have fatty liver disease, according to certain surveys,” Dr. Rustgi noted.
Cancer is the second greatest cause of mortality among patients with obesity and NAFLD, he continued, after cardiovascular disease. Cancer mortality is higher than mortality from liver disease.
Obesity-related cancers include adenocarcinoma of the esophagus, cancers of the breast (in postmenopausal women), colon, rectum, endometrium (corpus uterus), gallbladder, gastric cardia, kidney (renal cell), liver, ovary, pancreas, and thyroid, as well as meningioma and multiple myeloma, according to a 2016 report from the International Agency for Research on Cancer working group.
Obesity-related cancer accounted for 40% of all cancer in the United States in 2014 – 55% of cancers in women, and 24% of cancers in men, according to a study published in Morbidity and Mortality Weekly Report in 2017, as previously reported by this news organization.
Several studies, including one presented at Obesity Week in 2019 and later published, have shown that bariatric surgery is linked with a lower risk for cancer in general populations.
One meta-analysis reported that NAFLD is an independent risk factor for cholangiocarcinoma and colorectal, breast, gastric, pancreatic, prostate, and esophageal cancers. In another study, NAFLD was associated with a twofold increased risk for hepatocellular carcinoma and uterine, stomach, pancreatic, and colon cancers, Dr. Rustgi and colleagues noted.
Until now, the impact of bariatric surgery on the risk for cancer among patients with obesity and NAFLD was unknown.
Does bariatric surgery curb cancer risk in liver disease?
The researchers examined insurance claims data from the national MarketScan database from Jan. 1, 2007, to Dec. 31, 2017, for patients aged 18-64 years who had health insurance from 350 employers and 100 insurers. They identified 98,090 patients with severe obesity who were newly diagnosed with NAFLD during 2008-2017.
Roughly a third of the cohort (33,435 patients) underwent bariatric surgery. From 2008 to 2017, laparoscopic sleeve gastrectomies increased from 4% of bariatric procedures to 68% of all surgeries. Laparoscopic adjustable gastric band and laparoscopic Roux-en-Y gastric bypass procedures fell from 35% to less than 1% and from 49% to 28%, respectively.
Patients who underwent bariatric surgery were younger (mean age, 44 vs. 46 years), were more likely to be women (74% vs. 62%), and were less likely to have a history of smoking (6% vs. 10%).
During a mean follow-up of 22 months (and a median follow-up of 10 months), there were 911 incident cases of obesity-related cancers. These included cancer of the colon (116 cases), rectum (15), breast (in postmenopausal women; 131), kidney (120), esophagus (16), gastric cardia (8), gallbladder (4), pancreas (44), ovaries (74), endometrium (135), and thyroid (143), as well as hepatocellular carcinoma (49), multiple myeloma (50), and meningioma (6). There were 1,912 incident cases of other cancers, such as brain and lung cancers and leukemia.
A total of 258 patients who underwent bariatric surgery developed an obesity-related cancer (an incidence of 3.83 per 1,000 person-years), compared with 653 patients who did not have bariatric surgery (an incidence of 5.63 per 1,000 person-years).
The researchers noted that study limitations include the fact that it was restricted to privately insured individuals aged 18-64 years with severe obesity. In addition, “the short median follow-up may underestimate the full effect of bariatric surgery on cancer risk,” they wrote.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large cohort of insured working adults with severe obesity and nonalcoholic fatty liver disease (NAFLD), the rate of incident cancer was lower during a 10-month median follow-up period among those who underwent bariatric surgery. The rate was especially lower with regard to obesity-related cancers. The risk reduction was greater among patients with cirrhosis.
Among almost 100,000 patients with severe obesity (body mass index >40 kg/m2) and NAFLD, those who underwent bariatric surgery had an 18% and 35% lower risk of developing any cancer or obesity-related cancer, respectively.
Bariatric surgery was associated with a significantly lower risk of being diagnosed with colorectal, pancreatic, endometrial, and thyroid cancer, as well as hepatocellular carcinoma and multiple myeloma (all obesity-related cancers). The findings are from an observational study by Vinod K. Rustgi, MD, MBA, and colleagues, which was published online March 17, 2021, in Gastroenterology.
It was not surprising that bariatric surgery is effective in reducing the malignancy rate among patients with cirrhosis, the researchers wrote, because the surgery results in long-term weight loss, resolution of nonalcoholic steatohepatitis (NASH), and regression of fibrosis.
“Cirrhosis can happen from fatty liver disease or NASH,” Dr. Rustgi, a hepatologist at Robert Wood Johnson Medical School, New Brunswick, N.J., explained to this news organization. “It’s becoming the fastest growing indication for liver transplant, but also the reason for increased rates of hepatocellular carcinoma.”
Current treatment for patients with obesity and fatty liver disease begins with lifestyle changes to lose weight, he continued. “As people lose 10% of their weight, they actually start to see regression of fibrosis in the liver that is correlated with [lower rates of] malignancy outcomes and other deleterious outcomes.” But long-lasting weight loss is extremely difficult to achieve.
Future studies “may identify new targets and treatments, such as antidiabetic-, satiety-, or GLP-1-based medications, for chemoprevention in NAFLD/NASH,” the investigators suggested. However, pharmaceutical agents will likely be very expensive when they eventually get marketed, Dr. Rustgi observed.
Although “bariatric surgery is a more aggressive approach than lifestyle modifications, surgery may provide additional benefits, such as improved quality of life and decreased long-term health care costs,” he and his coauthors concluded.
Rising rates of fatty liver disease, obesity
An estimated 30% of the population of the United States has NAFLD, the most common chronic liver disease, the researchers noted in their article. The prevalence of NAFLD increased 2.8-fold in the United States between 2003 and 2011, in parallel with increasing obesity.
NAFLD is more common among male patients with obesity and diabetes and Hispanic patients; “70% of [patients with diabetes] may have fatty liver disease, according to certain surveys,” Dr. Rustgi noted.
Cancer is the second greatest cause of mortality among patients with obesity and NAFLD, he continued, after cardiovascular disease. Cancer mortality is higher than mortality from liver disease.
Obesity-related cancers include adenocarcinoma of the esophagus, cancers of the breast (in postmenopausal women), colon, rectum, endometrium (corpus uterus), gallbladder, gastric cardia, kidney (renal cell), liver, ovary, pancreas, and thyroid, as well as meningioma and multiple myeloma, according to a 2016 report from the International Agency for Research on Cancer working group.
Obesity-related cancer accounted for 40% of all cancer in the United States in 2014 – 55% of cancers in women, and 24% of cancers in men, according to a study published in Morbidity and Mortality Weekly Report in 2017, as previously reported by this news organization.
Several studies, including one presented at Obesity Week in 2019 and later published, have shown that bariatric surgery is linked with a lower risk for cancer in general populations.
One meta-analysis reported that NAFLD is an independent risk factor for cholangiocarcinoma and colorectal, breast, gastric, pancreatic, prostate, and esophageal cancers. In another study, NAFLD was associated with a twofold increased risk for hepatocellular carcinoma and uterine, stomach, pancreatic, and colon cancers, Dr. Rustgi and colleagues noted.
Until now, the impact of bariatric surgery on the risk for cancer among patients with obesity and NAFLD was unknown.
Does bariatric surgery curb cancer risk in liver disease?
The researchers examined insurance claims data from the national MarketScan database from Jan. 1, 2007, to Dec. 31, 2017, for patients aged 18-64 years who had health insurance from 350 employers and 100 insurers. They identified 98,090 patients with severe obesity who were newly diagnosed with NAFLD during 2008-2017.
Roughly a third of the cohort (33,435 patients) underwent bariatric surgery. From 2008 to 2017, laparoscopic sleeve gastrectomies increased from 4% of bariatric procedures to 68% of all surgeries. Laparoscopic adjustable gastric band and laparoscopic Roux-en-Y gastric bypass procedures fell from 35% to less than 1% and from 49% to 28%, respectively.
Patients who underwent bariatric surgery were younger (mean age, 44 vs. 46 years), were more likely to be women (74% vs. 62%), and were less likely to have a history of smoking (6% vs. 10%).
During a mean follow-up of 22 months (and a median follow-up of 10 months), there were 911 incident cases of obesity-related cancers. These included cancer of the colon (116 cases), rectum (15), breast (in postmenopausal women; 131), kidney (120), esophagus (16), gastric cardia (8), gallbladder (4), pancreas (44), ovaries (74), endometrium (135), and thyroid (143), as well as hepatocellular carcinoma (49), multiple myeloma (50), and meningioma (6). There were 1,912 incident cases of other cancers, such as brain and lung cancers and leukemia.
A total of 258 patients who underwent bariatric surgery developed an obesity-related cancer (an incidence of 3.83 per 1,000 person-years), compared with 653 patients who did not have bariatric surgery (an incidence of 5.63 per 1,000 person-years).
The researchers noted that study limitations include the fact that it was restricted to privately insured individuals aged 18-64 years with severe obesity. In addition, “the short median follow-up may underestimate the full effect of bariatric surgery on cancer risk,” they wrote.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large cohort of insured working adults with severe obesity and nonalcoholic fatty liver disease (NAFLD), the rate of incident cancer was lower during a 10-month median follow-up period among those who underwent bariatric surgery. The rate was especially lower with regard to obesity-related cancers. The risk reduction was greater among patients with cirrhosis.
Among almost 100,000 patients with severe obesity (body mass index >40 kg/m2) and NAFLD, those who underwent bariatric surgery had an 18% and 35% lower risk of developing any cancer or obesity-related cancer, respectively.
Bariatric surgery was associated with a significantly lower risk of being diagnosed with colorectal, pancreatic, endometrial, and thyroid cancer, as well as hepatocellular carcinoma and multiple myeloma (all obesity-related cancers). The findings are from an observational study by Vinod K. Rustgi, MD, MBA, and colleagues, which was published online March 17, 2021, in Gastroenterology.
It was not surprising that bariatric surgery is effective in reducing the malignancy rate among patients with cirrhosis, the researchers wrote, because the surgery results in long-term weight loss, resolution of nonalcoholic steatohepatitis (NASH), and regression of fibrosis.
“Cirrhosis can happen from fatty liver disease or NASH,” Dr. Rustgi, a hepatologist at Robert Wood Johnson Medical School, New Brunswick, N.J., explained to this news organization. “It’s becoming the fastest growing indication for liver transplant, but also the reason for increased rates of hepatocellular carcinoma.”
Current treatment for patients with obesity and fatty liver disease begins with lifestyle changes to lose weight, he continued. “As people lose 10% of their weight, they actually start to see regression of fibrosis in the liver that is correlated with [lower rates of] malignancy outcomes and other deleterious outcomes.” But long-lasting weight loss is extremely difficult to achieve.
Future studies “may identify new targets and treatments, such as antidiabetic-, satiety-, or GLP-1-based medications, for chemoprevention in NAFLD/NASH,” the investigators suggested. However, pharmaceutical agents will likely be very expensive when they eventually get marketed, Dr. Rustgi observed.
Although “bariatric surgery is a more aggressive approach than lifestyle modifications, surgery may provide additional benefits, such as improved quality of life and decreased long-term health care costs,” he and his coauthors concluded.
Rising rates of fatty liver disease, obesity
An estimated 30% of the population of the United States has NAFLD, the most common chronic liver disease, the researchers noted in their article. The prevalence of NAFLD increased 2.8-fold in the United States between 2003 and 2011, in parallel with increasing obesity.
NAFLD is more common among male patients with obesity and diabetes and Hispanic patients; “70% of [patients with diabetes] may have fatty liver disease, according to certain surveys,” Dr. Rustgi noted.
Cancer is the second greatest cause of mortality among patients with obesity and NAFLD, he continued, after cardiovascular disease. Cancer mortality is higher than mortality from liver disease.
Obesity-related cancers include adenocarcinoma of the esophagus, cancers of the breast (in postmenopausal women), colon, rectum, endometrium (corpus uterus), gallbladder, gastric cardia, kidney (renal cell), liver, ovary, pancreas, and thyroid, as well as meningioma and multiple myeloma, according to a 2016 report from the International Agency for Research on Cancer working group.
Obesity-related cancer accounted for 40% of all cancer in the United States in 2014 – 55% of cancers in women, and 24% of cancers in men, according to a study published in Morbidity and Mortality Weekly Report in 2017, as previously reported by this news organization.
Several studies, including one presented at Obesity Week in 2019 and later published, have shown that bariatric surgery is linked with a lower risk for cancer in general populations.
One meta-analysis reported that NAFLD is an independent risk factor for cholangiocarcinoma and colorectal, breast, gastric, pancreatic, prostate, and esophageal cancers. In another study, NAFLD was associated with a twofold increased risk for hepatocellular carcinoma and uterine, stomach, pancreatic, and colon cancers, Dr. Rustgi and colleagues noted.
Until now, the impact of bariatric surgery on the risk for cancer among patients with obesity and NAFLD was unknown.
Does bariatric surgery curb cancer risk in liver disease?
The researchers examined insurance claims data from the national MarketScan database from Jan. 1, 2007, to Dec. 31, 2017, for patients aged 18-64 years who had health insurance from 350 employers and 100 insurers. They identified 98,090 patients with severe obesity who were newly diagnosed with NAFLD during 2008-2017.
Roughly a third of the cohort (33,435 patients) underwent bariatric surgery. From 2008 to 2017, laparoscopic sleeve gastrectomies increased from 4% of bariatric procedures to 68% of all surgeries. Laparoscopic adjustable gastric band and laparoscopic Roux-en-Y gastric bypass procedures fell from 35% to less than 1% and from 49% to 28%, respectively.
Patients who underwent bariatric surgery were younger (mean age, 44 vs. 46 years), were more likely to be women (74% vs. 62%), and were less likely to have a history of smoking (6% vs. 10%).
During a mean follow-up of 22 months (and a median follow-up of 10 months), there were 911 incident cases of obesity-related cancers. These included cancer of the colon (116 cases), rectum (15), breast (in postmenopausal women; 131), kidney (120), esophagus (16), gastric cardia (8), gallbladder (4), pancreas (44), ovaries (74), endometrium (135), and thyroid (143), as well as hepatocellular carcinoma (49), multiple myeloma (50), and meningioma (6). There were 1,912 incident cases of other cancers, such as brain and lung cancers and leukemia.
A total of 258 patients who underwent bariatric surgery developed an obesity-related cancer (an incidence of 3.83 per 1,000 person-years), compared with 653 patients who did not have bariatric surgery (an incidence of 5.63 per 1,000 person-years).
The researchers noted that study limitations include the fact that it was restricted to privately insured individuals aged 18-64 years with severe obesity. In addition, “the short median follow-up may underestimate the full effect of bariatric surgery on cancer risk,” they wrote.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Moderate-to-vigorous physical activity is the answer to childhood obesity
There is no question that none of us, not just pediatricians, is doing a very good job of dealing with the obesity problem this nation faces. We can agree that a more active lifestyle that includes spells of vigorous activity is important for weight management. We know that in general overweight people sleep less than do those whose basal metabolic rate is normal. And, of course, we know that a diet high in calorie-dense foods is associated with unhealthy weight gain.
Not surprisingly, overweight individuals are usually struggling with all three of these challenges. They are less active, get too little sleep, and are ingesting a diet that is too calorie dense. In other words, they would benefit from a total lifestyle reboot. But you know as well as I do a change of that magnitude is much easier said than done. Few families can afford nor would they have the appetite for sending their children to a “fat camp” for 6 months with no guarantee of success.
Instead of throwing up our hands in the face of this monumental task or attacking it at close range, maybe we should aim our efforts at the risk associations that will yield the best results for our efforts. A group of researchers at the University of South Australia has just published a study in Pediatrics in which they provide some data that may help us target our interventions with obese and overweight children. The researchers did not investigate diet, but used accelerometers to determine how much time each child spent sleeping and a variety of activity levels. They then determined what effect changes in the child’s allocation of activity had on their adiposity.
The investigators found on a minute-to-minute basis that an increase in a child’s moderate-to-vigorous physical activity (MVPA) was up to six times more effective at influencing adiposity than was a decrease in sedentary time or an increase in sleep duration. For example, 17 minutes of MVPA had the same beneficial effect as 52 minutes more sleep or 56 minutes less sedentary time. Interestingly and somewhat surprisingly, the researchers found that light activity was positively associated with adiposity.
For those of us in primary care, this study from Australia suggests that our time (and the parents’ time) would be best spent figuring out how to include more MVPA in the child’s day and not focus so much on sleep duration and sedentary intervals.
However, before one can make any recommendation one must first have a clear understanding of how the child and his family spend the day. This process can be done in the office by interviewing the family. I have found that this is not as time consuming as one might think and often yields some valuable additional insight into the family’s dynamics. Sending the family home with an hourly log to be filled in or asking them to use a smartphone to record information will also work.
I must admit that at first I found the results of this study ran counter to my intuition. I have always felt that sleep is the linchpin to the solution of a variety of health style related problems. In my construct, more sleep has always been the first and easy answer and decreasing screen time the second. But, it turns out that increasing MVPA may give us the biggest bang for the buck. Which is fine with me.
The problem facing us is how we can be creative in adding that 20 minutes of vigorous activity. In most communities, we have allowed the school system to drop the ball. We can hope that this study will be confirmed or at least widely publicized. It feels like it is time to guarantee that every child gets a robust gym class every school day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
There is no question that none of us, not just pediatricians, is doing a very good job of dealing with the obesity problem this nation faces. We can agree that a more active lifestyle that includes spells of vigorous activity is important for weight management. We know that in general overweight people sleep less than do those whose basal metabolic rate is normal. And, of course, we know that a diet high in calorie-dense foods is associated with unhealthy weight gain.
Not surprisingly, overweight individuals are usually struggling with all three of these challenges. They are less active, get too little sleep, and are ingesting a diet that is too calorie dense. In other words, they would benefit from a total lifestyle reboot. But you know as well as I do a change of that magnitude is much easier said than done. Few families can afford nor would they have the appetite for sending their children to a “fat camp” for 6 months with no guarantee of success.
Instead of throwing up our hands in the face of this monumental task or attacking it at close range, maybe we should aim our efforts at the risk associations that will yield the best results for our efforts. A group of researchers at the University of South Australia has just published a study in Pediatrics in which they provide some data that may help us target our interventions with obese and overweight children. The researchers did not investigate diet, but used accelerometers to determine how much time each child spent sleeping and a variety of activity levels. They then determined what effect changes in the child’s allocation of activity had on their adiposity.
The investigators found on a minute-to-minute basis that an increase in a child’s moderate-to-vigorous physical activity (MVPA) was up to six times more effective at influencing adiposity than was a decrease in sedentary time or an increase in sleep duration. For example, 17 minutes of MVPA had the same beneficial effect as 52 minutes more sleep or 56 minutes less sedentary time. Interestingly and somewhat surprisingly, the researchers found that light activity was positively associated with adiposity.
For those of us in primary care, this study from Australia suggests that our time (and the parents’ time) would be best spent figuring out how to include more MVPA in the child’s day and not focus so much on sleep duration and sedentary intervals.
However, before one can make any recommendation one must first have a clear understanding of how the child and his family spend the day. This process can be done in the office by interviewing the family. I have found that this is not as time consuming as one might think and often yields some valuable additional insight into the family’s dynamics. Sending the family home with an hourly log to be filled in or asking them to use a smartphone to record information will also work.
I must admit that at first I found the results of this study ran counter to my intuition. I have always felt that sleep is the linchpin to the solution of a variety of health style related problems. In my construct, more sleep has always been the first and easy answer and decreasing screen time the second. But, it turns out that increasing MVPA may give us the biggest bang for the buck. Which is fine with me.
The problem facing us is how we can be creative in adding that 20 minutes of vigorous activity. In most communities, we have allowed the school system to drop the ball. We can hope that this study will be confirmed or at least widely publicized. It feels like it is time to guarantee that every child gets a robust gym class every school day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
There is no question that none of us, not just pediatricians, is doing a very good job of dealing with the obesity problem this nation faces. We can agree that a more active lifestyle that includes spells of vigorous activity is important for weight management. We know that in general overweight people sleep less than do those whose basal metabolic rate is normal. And, of course, we know that a diet high in calorie-dense foods is associated with unhealthy weight gain.
Not surprisingly, overweight individuals are usually struggling with all three of these challenges. They are less active, get too little sleep, and are ingesting a diet that is too calorie dense. In other words, they would benefit from a total lifestyle reboot. But you know as well as I do a change of that magnitude is much easier said than done. Few families can afford nor would they have the appetite for sending their children to a “fat camp” for 6 months with no guarantee of success.
Instead of throwing up our hands in the face of this monumental task or attacking it at close range, maybe we should aim our efforts at the risk associations that will yield the best results for our efforts. A group of researchers at the University of South Australia has just published a study in Pediatrics in which they provide some data that may help us target our interventions with obese and overweight children. The researchers did not investigate diet, but used accelerometers to determine how much time each child spent sleeping and a variety of activity levels. They then determined what effect changes in the child’s allocation of activity had on their adiposity.
The investigators found on a minute-to-minute basis that an increase in a child’s moderate-to-vigorous physical activity (MVPA) was up to six times more effective at influencing adiposity than was a decrease in sedentary time or an increase in sleep duration. For example, 17 minutes of MVPA had the same beneficial effect as 52 minutes more sleep or 56 minutes less sedentary time. Interestingly and somewhat surprisingly, the researchers found that light activity was positively associated with adiposity.
For those of us in primary care, this study from Australia suggests that our time (and the parents’ time) would be best spent figuring out how to include more MVPA in the child’s day and not focus so much on sleep duration and sedentary intervals.
However, before one can make any recommendation one must first have a clear understanding of how the child and his family spend the day. This process can be done in the office by interviewing the family. I have found that this is not as time consuming as one might think and often yields some valuable additional insight into the family’s dynamics. Sending the family home with an hourly log to be filled in or asking them to use a smartphone to record information will also work.
I must admit that at first I found the results of this study ran counter to my intuition. I have always felt that sleep is the linchpin to the solution of a variety of health style related problems. In my construct, more sleep has always been the first and easy answer and decreasing screen time the second. But, it turns out that increasing MVPA may give us the biggest bang for the buck. Which is fine with me.
The problem facing us is how we can be creative in adding that 20 minutes of vigorous activity. In most communities, we have allowed the school system to drop the ball. We can hope that this study will be confirmed or at least widely publicized. It feels like it is time to guarantee that every child gets a robust gym class every school day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Integrating primary care into a community mental health center
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
Helping your obese patient achieve a healthier weight
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
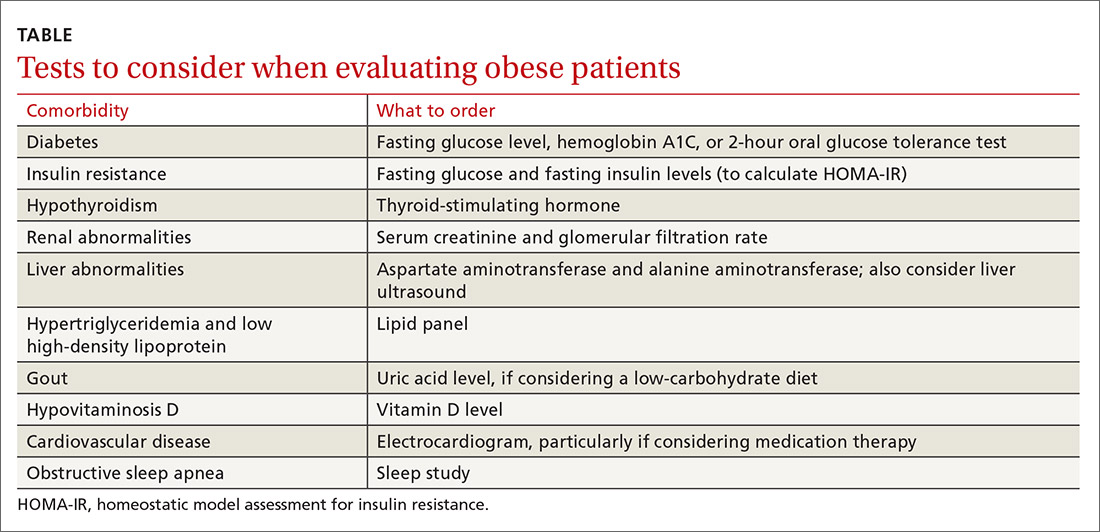
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
PRACTICE RECOMMENDATIONS
› Create an office environment where patients feel comfortable discussing their weight. C
› Screen overweight and obese patients for comorbidities. B
› Focus on nutritional changes more than exercise when working with patients who want to lose weight. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Fit-for-Fertility program boosts births, is cost effective
Incorporation of a nonintensive fitness intervention for women with obesity into a standard fertility treatment program could be cost effective, a new analysis finds.
Financial data for the Canadian Fit-for-Fertility program were presented March 20 at the annual meeting of the Endocrine Society by Matea Belan, PhD, of the division of endocrinology at the University of Sherbrooke (Que.).
Women with obesity and infertility are typically advised to lose 5%-10% of their body weight as first-line fertility treatment, as doing so has been shown to increase rates of ovulation and pregnancy. But most established fertility treatment programs don’t incorporate organized lifestyle modification interventions, Dr. Belan explained during a press briefing.
“Mostly they’re just given general advice, not resources. It’s up to the woman to seek help for lifestyle. Our idea is to give them access to intervention that’s integrated into the setting of a fertility clinic,” she said.
Primary results from the Fit-for-Fertility program, including significant weight loss and a 40% increased live birth rate at 18 months, compared with standard fertility treatment, were presented at ENDO 2019 and reported at the time by this news organization.
In the new analysis, the cost in Canadian dollars per additional newborn achieved with the Fit-for-Fertility program was similar to the willingness-to-pay for in vitro fertilization from a health system perspective.
The final goal, lead investigator Jean-Patrice Baillargeon, MD, said in an interview, “would be to convince stakeholders, and mainly the provincial government, to cover the costs of our lifestyle program. This would not be more costly than funding IVF, but [would provide] more long-term benefits for the whole family and the offspring.”
Chloe A. Zera, MD, said in an interview that she supports the idea in principle, but is concerned that, in the U.S. health care system, women don’t always have access to fertility and obesity treatments to begin with.
“There’s a huge equity issue. People with Medicaid don’t necessarily get coverage for IVF. ... Even many commercially insured people are paying out of pocket, which can be $10,000 to $15,000 for a cycle just for the medications, so the cost to patients on the individual level is huge,” said Dr. Zera, who is associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
She added: “I’m prolifestyle modification. I’m also proequity in health care delivery so I would want to make sure that the way it’s delivered incorporates that as a consideration. ... Is that money better spent on primary prevention of obesity and access to basic services and basic reproductive health care for everybody?”
Primary results: Improvements in overall and spontaneous pregnancy rates
The study included 130 women with infertility and a body mass index of at least 30 kg/m2 (mean, 40), of whom 65 were randomized to the Fit-for-Fitness program and 65 to standard fertility treatment that did not include a lifestyle intervention, although those women could consult professionals on their own. The women in the lifestyle intervention group had to stop medical fertility treatments for the first 6 months but could use them thereafter while the controls continued to use them throughout.
Based on motivational interviewing, the program focused on womens’ individual likes and dislikes, experiences, and perceived capacities, aiming to improve healthful habits gradually and with “low intensity” so as to maintain them in the long run.
The program combined individual sessions with a nutritionist and kinesiologist every 6 weeks and 12 mandatory group sessions. The women were asked to reduce their total caloric intake by about 500 calories/day but weren’t asked to change their diets. They were also advised to increase physical activity by about 150 minutes/week.
“We want to keep it sustainable in time, so they don’t have a relapse when they become pregnant, and to help the newborn and spouse too. It’s about improving and maintaining habits,” Dr. Belan explained during the briefing.
At 6 months, mean weight changes were –3.4% versus –0.89% for the intervention versus control groups (P = .003).
“What is important for women with obesity and infertility is to improve their lifestyle, both physical activity and nutrition, even if the weight loss is minimal,” noted Dr. Baillargeon, professor of medicine, health sciences research and physiology, also at the University of Sherbrooke.
A total of 46 intervention and 52 control patients finished the 18-month study. Pregnancies occurred in 61% of the intervention group versus 39% of the controls, while spontaneous pregnancies – among those not using medical fertility treatments – occurred in 33.3% versus 12.3% (P = .009).
The primary outcome, live births at 18 months, occurred in 51.0% of the intervention group versus 36.8% of controls, which wasn’t a statistically significant difference, but was “highly clinically significant,” Dr. Belan said.
Cost per additional newborn similar to IVF
Costs (in Canadian dollars) considered in the analysis included those related to the management of infertility, obesity, pregnancy, and childbirth. The incremental cost-effectiveness ratios, a standard cost-effectiveness measure, per live birth were $24,393 from a societal perspective, $12,633 for the health system, and $5,980 for the patient.
Because the $12,633 health system cost per additional newborn with the Fit-for-Fertility program is similar to the health system’s willingness-to-pay for IVF of up to $15,000, a lifestyle intervention could be considered cost-efficient compared with the standard of care, Dr. Belan said.
“We think that the Fit-for-Fertility program could be deemed cost effective and could represent an interesting alternative to the usual standard of care for women with obesity seeking fertility treatments,” she commented.
The Canadian Institutes of Health Research is funding a larger randomized, controlled trial of the program at six Canadian centers to validate these results.
Dr. Belan, Dr. Baillargeon, and Dr. Zera reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Incorporation of a nonintensive fitness intervention for women with obesity into a standard fertility treatment program could be cost effective, a new analysis finds.
Financial data for the Canadian Fit-for-Fertility program were presented March 20 at the annual meeting of the Endocrine Society by Matea Belan, PhD, of the division of endocrinology at the University of Sherbrooke (Que.).
Women with obesity and infertility are typically advised to lose 5%-10% of their body weight as first-line fertility treatment, as doing so has been shown to increase rates of ovulation and pregnancy. But most established fertility treatment programs don’t incorporate organized lifestyle modification interventions, Dr. Belan explained during a press briefing.
“Mostly they’re just given general advice, not resources. It’s up to the woman to seek help for lifestyle. Our idea is to give them access to intervention that’s integrated into the setting of a fertility clinic,” she said.
Primary results from the Fit-for-Fertility program, including significant weight loss and a 40% increased live birth rate at 18 months, compared with standard fertility treatment, were presented at ENDO 2019 and reported at the time by this news organization.
In the new analysis, the cost in Canadian dollars per additional newborn achieved with the Fit-for-Fertility program was similar to the willingness-to-pay for in vitro fertilization from a health system perspective.
The final goal, lead investigator Jean-Patrice Baillargeon, MD, said in an interview, “would be to convince stakeholders, and mainly the provincial government, to cover the costs of our lifestyle program. This would not be more costly than funding IVF, but [would provide] more long-term benefits for the whole family and the offspring.”
Chloe A. Zera, MD, said in an interview that she supports the idea in principle, but is concerned that, in the U.S. health care system, women don’t always have access to fertility and obesity treatments to begin with.
“There’s a huge equity issue. People with Medicaid don’t necessarily get coverage for IVF. ... Even many commercially insured people are paying out of pocket, which can be $10,000 to $15,000 for a cycle just for the medications, so the cost to patients on the individual level is huge,” said Dr. Zera, who is associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
She added: “I’m prolifestyle modification. I’m also proequity in health care delivery so I would want to make sure that the way it’s delivered incorporates that as a consideration. ... Is that money better spent on primary prevention of obesity and access to basic services and basic reproductive health care for everybody?”
Primary results: Improvements in overall and spontaneous pregnancy rates
The study included 130 women with infertility and a body mass index of at least 30 kg/m2 (mean, 40), of whom 65 were randomized to the Fit-for-Fitness program and 65 to standard fertility treatment that did not include a lifestyle intervention, although those women could consult professionals on their own. The women in the lifestyle intervention group had to stop medical fertility treatments for the first 6 months but could use them thereafter while the controls continued to use them throughout.
Based on motivational interviewing, the program focused on womens’ individual likes and dislikes, experiences, and perceived capacities, aiming to improve healthful habits gradually and with “low intensity” so as to maintain them in the long run.
The program combined individual sessions with a nutritionist and kinesiologist every 6 weeks and 12 mandatory group sessions. The women were asked to reduce their total caloric intake by about 500 calories/day but weren’t asked to change their diets. They were also advised to increase physical activity by about 150 minutes/week.
“We want to keep it sustainable in time, so they don’t have a relapse when they become pregnant, and to help the newborn and spouse too. It’s about improving and maintaining habits,” Dr. Belan explained during the briefing.
At 6 months, mean weight changes were –3.4% versus –0.89% for the intervention versus control groups (P = .003).
“What is important for women with obesity and infertility is to improve their lifestyle, both physical activity and nutrition, even if the weight loss is minimal,” noted Dr. Baillargeon, professor of medicine, health sciences research and physiology, also at the University of Sherbrooke.
A total of 46 intervention and 52 control patients finished the 18-month study. Pregnancies occurred in 61% of the intervention group versus 39% of the controls, while spontaneous pregnancies – among those not using medical fertility treatments – occurred in 33.3% versus 12.3% (P = .009).
The primary outcome, live births at 18 months, occurred in 51.0% of the intervention group versus 36.8% of controls, which wasn’t a statistically significant difference, but was “highly clinically significant,” Dr. Belan said.
Cost per additional newborn similar to IVF
Costs (in Canadian dollars) considered in the analysis included those related to the management of infertility, obesity, pregnancy, and childbirth. The incremental cost-effectiveness ratios, a standard cost-effectiveness measure, per live birth were $24,393 from a societal perspective, $12,633 for the health system, and $5,980 for the patient.
Because the $12,633 health system cost per additional newborn with the Fit-for-Fertility program is similar to the health system’s willingness-to-pay for IVF of up to $15,000, a lifestyle intervention could be considered cost-efficient compared with the standard of care, Dr. Belan said.
“We think that the Fit-for-Fertility program could be deemed cost effective and could represent an interesting alternative to the usual standard of care for women with obesity seeking fertility treatments,” she commented.
The Canadian Institutes of Health Research is funding a larger randomized, controlled trial of the program at six Canadian centers to validate these results.
Dr. Belan, Dr. Baillargeon, and Dr. Zera reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Incorporation of a nonintensive fitness intervention for women with obesity into a standard fertility treatment program could be cost effective, a new analysis finds.
Financial data for the Canadian Fit-for-Fertility program were presented March 20 at the annual meeting of the Endocrine Society by Matea Belan, PhD, of the division of endocrinology at the University of Sherbrooke (Que.).
Women with obesity and infertility are typically advised to lose 5%-10% of their body weight as first-line fertility treatment, as doing so has been shown to increase rates of ovulation and pregnancy. But most established fertility treatment programs don’t incorporate organized lifestyle modification interventions, Dr. Belan explained during a press briefing.
“Mostly they’re just given general advice, not resources. It’s up to the woman to seek help for lifestyle. Our idea is to give them access to intervention that’s integrated into the setting of a fertility clinic,” she said.
Primary results from the Fit-for-Fertility program, including significant weight loss and a 40% increased live birth rate at 18 months, compared with standard fertility treatment, were presented at ENDO 2019 and reported at the time by this news organization.
In the new analysis, the cost in Canadian dollars per additional newborn achieved with the Fit-for-Fertility program was similar to the willingness-to-pay for in vitro fertilization from a health system perspective.
The final goal, lead investigator Jean-Patrice Baillargeon, MD, said in an interview, “would be to convince stakeholders, and mainly the provincial government, to cover the costs of our lifestyle program. This would not be more costly than funding IVF, but [would provide] more long-term benefits for the whole family and the offspring.”
Chloe A. Zera, MD, said in an interview that she supports the idea in principle, but is concerned that, in the U.S. health care system, women don’t always have access to fertility and obesity treatments to begin with.
“There’s a huge equity issue. People with Medicaid don’t necessarily get coverage for IVF. ... Even many commercially insured people are paying out of pocket, which can be $10,000 to $15,000 for a cycle just for the medications, so the cost to patients on the individual level is huge,” said Dr. Zera, who is associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
She added: “I’m prolifestyle modification. I’m also proequity in health care delivery so I would want to make sure that the way it’s delivered incorporates that as a consideration. ... Is that money better spent on primary prevention of obesity and access to basic services and basic reproductive health care for everybody?”
Primary results: Improvements in overall and spontaneous pregnancy rates
The study included 130 women with infertility and a body mass index of at least 30 kg/m2 (mean, 40), of whom 65 were randomized to the Fit-for-Fitness program and 65 to standard fertility treatment that did not include a lifestyle intervention, although those women could consult professionals on their own. The women in the lifestyle intervention group had to stop medical fertility treatments for the first 6 months but could use them thereafter while the controls continued to use them throughout.
Based on motivational interviewing, the program focused on womens’ individual likes and dislikes, experiences, and perceived capacities, aiming to improve healthful habits gradually and with “low intensity” so as to maintain them in the long run.
The program combined individual sessions with a nutritionist and kinesiologist every 6 weeks and 12 mandatory group sessions. The women were asked to reduce their total caloric intake by about 500 calories/day but weren’t asked to change their diets. They were also advised to increase physical activity by about 150 minutes/week.
“We want to keep it sustainable in time, so they don’t have a relapse when they become pregnant, and to help the newborn and spouse too. It’s about improving and maintaining habits,” Dr. Belan explained during the briefing.
At 6 months, mean weight changes were –3.4% versus –0.89% for the intervention versus control groups (P = .003).
“What is important for women with obesity and infertility is to improve their lifestyle, both physical activity and nutrition, even if the weight loss is minimal,” noted Dr. Baillargeon, professor of medicine, health sciences research and physiology, also at the University of Sherbrooke.
A total of 46 intervention and 52 control patients finished the 18-month study. Pregnancies occurred in 61% of the intervention group versus 39% of the controls, while spontaneous pregnancies – among those not using medical fertility treatments – occurred in 33.3% versus 12.3% (P = .009).
The primary outcome, live births at 18 months, occurred in 51.0% of the intervention group versus 36.8% of controls, which wasn’t a statistically significant difference, but was “highly clinically significant,” Dr. Belan said.
Cost per additional newborn similar to IVF
Costs (in Canadian dollars) considered in the analysis included those related to the management of infertility, obesity, pregnancy, and childbirth. The incremental cost-effectiveness ratios, a standard cost-effectiveness measure, per live birth were $24,393 from a societal perspective, $12,633 for the health system, and $5,980 for the patient.
Because the $12,633 health system cost per additional newborn with the Fit-for-Fertility program is similar to the health system’s willingness-to-pay for IVF of up to $15,000, a lifestyle intervention could be considered cost-efficient compared with the standard of care, Dr. Belan said.
“We think that the Fit-for-Fertility program could be deemed cost effective and could represent an interesting alternative to the usual standard of care for women with obesity seeking fertility treatments,” she commented.
The Canadian Institutes of Health Research is funding a larger randomized, controlled trial of the program at six Canadian centers to validate these results.
Dr. Belan, Dr. Baillargeon, and Dr. Zera reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatric NAFLD almost always stems from excess body weight, not other etiologies
Nonalcoholic fatty liver disease (NAFLD) in children is almost always caused by excess body weight, not other etiologies, based on a retrospective analysis of 900 patients.
Just 2% of children with overweight or obesity and suspected NAFLD had other causes of liver disease, and none tested positive for autoimmune hepatitis (AIH), reported lead author Toshifumi Yodoshi, MD, PhD, of Cincinnati Children’s Hospital Medical Center, and colleagues.
“Currently, recommended testing of patients with suspected NAFLD includes ruling out the following conditions: AIH, Wilson disease, hemochromatosis, alpha-1 antitrypsin [A1AT] deficiency, viral hepatitis, celiac disease, and thyroid dysfunction,” the investigators wrote in Pediatrics.
Yet evidence supporting this particular battery of tests is scant; just one previous pediatric study has estimated the prevalence of other liver diseases among children with suspected NAFLD. The study showed that the second-most common etiology, after NAFLD, was AIH, at a rate of 4%.
But “the generalizability of these findings is uncertain,” noted Dr. Yodoshi and colleagues, as the study was conducted at one tertiary center in the western United States, among a population that was predominantly Hispanic.
This uncertainty spurred the present study, which was conducted at two pediatric centers: Cincinnati Children’s Hospital Medical Center (2009-2017) and Yale New Haven (Conn.) Children’s Hospital (2012-2017).
The final analysis involved 900 patients aged 18 years or younger with suspected NAFLD based on hepatic steatosis detected via imaging and/or elevated serum aminotransferases. Demographically, a slight majority of the patients were boys (63%), and approximately one-quarter (26%) were Hispanic. Median BMI z score was 2.45, with three out of four patients (76%) exhibiting severe obesity. Out of 900 patients, 358 (40%) underwent liver biopsy, among whom 46% had confirmed nonalcoholic steatohepatitis.
All patients underwent testing to exclude the aforementioned conditions using various diagnostics, revealing that just 2% of the population had etiologies other than NAFLD. Specifically, 11 children had thyroid dysfunction (1.2%), 3 had celiac disease (0.4%), 3 had A1AT deficiency (0.4%), 1 had hemophagocytic lymphohistiocytosis, and 1 had Hodgkin’s lymphoma. None of the children had Wilson disease, hepatitis B or C, or AIH.
Dr. Yodoshi and colleagues highlighted the latter finding, noting that 13% of the patients had autoantibodies for AIH, but “none met composite criteria.” This contrasts with the previous study from 2013, which found an AIH rate of 4%.
“Nonetheless,” the investigators went on, “NAFLD remains a diagnosis of exclusion, and key conditions that require specific treatments must be ruled out in the workup of patients with suspected NAFLD. In the future, the cost-effectiveness of this approach will need to be investigated.”
Interpreting the findings, Francis E. Rushton, MD, of Beaufort (S.C.) Memorial Hospital emphasized the implications for preventive and interventional health care.
“This study showing an absence of etiologies other than obesity in overweight children with NAFLD provides further impetus for pediatricians to work on both preventive and treatment regimens for weight issues,” Dr. Rushton said. “Linking community-based initiatives focused on adequate nutritional support with pediatric clinical support services is critical in solving issues related to overweight in children. Tracking BMI over time and developing healthy habit goals for patients are key parts of clinical interventions.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
Nonalcoholic fatty liver disease (NAFLD) in children is almost always caused by excess body weight, not other etiologies, based on a retrospective analysis of 900 patients.
Just 2% of children with overweight or obesity and suspected NAFLD had other causes of liver disease, and none tested positive for autoimmune hepatitis (AIH), reported lead author Toshifumi Yodoshi, MD, PhD, of Cincinnati Children’s Hospital Medical Center, and colleagues.
“Currently, recommended testing of patients with suspected NAFLD includes ruling out the following conditions: AIH, Wilson disease, hemochromatosis, alpha-1 antitrypsin [A1AT] deficiency, viral hepatitis, celiac disease, and thyroid dysfunction,” the investigators wrote in Pediatrics.
Yet evidence supporting this particular battery of tests is scant; just one previous pediatric study has estimated the prevalence of other liver diseases among children with suspected NAFLD. The study showed that the second-most common etiology, after NAFLD, was AIH, at a rate of 4%.
But “the generalizability of these findings is uncertain,” noted Dr. Yodoshi and colleagues, as the study was conducted at one tertiary center in the western United States, among a population that was predominantly Hispanic.
This uncertainty spurred the present study, which was conducted at two pediatric centers: Cincinnati Children’s Hospital Medical Center (2009-2017) and Yale New Haven (Conn.) Children’s Hospital (2012-2017).
The final analysis involved 900 patients aged 18 years or younger with suspected NAFLD based on hepatic steatosis detected via imaging and/or elevated serum aminotransferases. Demographically, a slight majority of the patients were boys (63%), and approximately one-quarter (26%) were Hispanic. Median BMI z score was 2.45, with three out of four patients (76%) exhibiting severe obesity. Out of 900 patients, 358 (40%) underwent liver biopsy, among whom 46% had confirmed nonalcoholic steatohepatitis.
All patients underwent testing to exclude the aforementioned conditions using various diagnostics, revealing that just 2% of the population had etiologies other than NAFLD. Specifically, 11 children had thyroid dysfunction (1.2%), 3 had celiac disease (0.4%), 3 had A1AT deficiency (0.4%), 1 had hemophagocytic lymphohistiocytosis, and 1 had Hodgkin’s lymphoma. None of the children had Wilson disease, hepatitis B or C, or AIH.
Dr. Yodoshi and colleagues highlighted the latter finding, noting that 13% of the patients had autoantibodies for AIH, but “none met composite criteria.” This contrasts with the previous study from 2013, which found an AIH rate of 4%.
“Nonetheless,” the investigators went on, “NAFLD remains a diagnosis of exclusion, and key conditions that require specific treatments must be ruled out in the workup of patients with suspected NAFLD. In the future, the cost-effectiveness of this approach will need to be investigated.”
Interpreting the findings, Francis E. Rushton, MD, of Beaufort (S.C.) Memorial Hospital emphasized the implications for preventive and interventional health care.
“This study showing an absence of etiologies other than obesity in overweight children with NAFLD provides further impetus for pediatricians to work on both preventive and treatment regimens for weight issues,” Dr. Rushton said. “Linking community-based initiatives focused on adequate nutritional support with pediatric clinical support services is critical in solving issues related to overweight in children. Tracking BMI over time and developing healthy habit goals for patients are key parts of clinical interventions.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
Nonalcoholic fatty liver disease (NAFLD) in children is almost always caused by excess body weight, not other etiologies, based on a retrospective analysis of 900 patients.
Just 2% of children with overweight or obesity and suspected NAFLD had other causes of liver disease, and none tested positive for autoimmune hepatitis (AIH), reported lead author Toshifumi Yodoshi, MD, PhD, of Cincinnati Children’s Hospital Medical Center, and colleagues.
“Currently, recommended testing of patients with suspected NAFLD includes ruling out the following conditions: AIH, Wilson disease, hemochromatosis, alpha-1 antitrypsin [A1AT] deficiency, viral hepatitis, celiac disease, and thyroid dysfunction,” the investigators wrote in Pediatrics.
Yet evidence supporting this particular battery of tests is scant; just one previous pediatric study has estimated the prevalence of other liver diseases among children with suspected NAFLD. The study showed that the second-most common etiology, after NAFLD, was AIH, at a rate of 4%.
But “the generalizability of these findings is uncertain,” noted Dr. Yodoshi and colleagues, as the study was conducted at one tertiary center in the western United States, among a population that was predominantly Hispanic.
This uncertainty spurred the present study, which was conducted at two pediatric centers: Cincinnati Children’s Hospital Medical Center (2009-2017) and Yale New Haven (Conn.) Children’s Hospital (2012-2017).
The final analysis involved 900 patients aged 18 years or younger with suspected NAFLD based on hepatic steatosis detected via imaging and/or elevated serum aminotransferases. Demographically, a slight majority of the patients were boys (63%), and approximately one-quarter (26%) were Hispanic. Median BMI z score was 2.45, with three out of four patients (76%) exhibiting severe obesity. Out of 900 patients, 358 (40%) underwent liver biopsy, among whom 46% had confirmed nonalcoholic steatohepatitis.
All patients underwent testing to exclude the aforementioned conditions using various diagnostics, revealing that just 2% of the population had etiologies other than NAFLD. Specifically, 11 children had thyroid dysfunction (1.2%), 3 had celiac disease (0.4%), 3 had A1AT deficiency (0.4%), 1 had hemophagocytic lymphohistiocytosis, and 1 had Hodgkin’s lymphoma. None of the children had Wilson disease, hepatitis B or C, or AIH.
Dr. Yodoshi and colleagues highlighted the latter finding, noting that 13% of the patients had autoantibodies for AIH, but “none met composite criteria.” This contrasts with the previous study from 2013, which found an AIH rate of 4%.
“Nonetheless,” the investigators went on, “NAFLD remains a diagnosis of exclusion, and key conditions that require specific treatments must be ruled out in the workup of patients with suspected NAFLD. In the future, the cost-effectiveness of this approach will need to be investigated.”
Interpreting the findings, Francis E. Rushton, MD, of Beaufort (S.C.) Memorial Hospital emphasized the implications for preventive and interventional health care.
“This study showing an absence of etiologies other than obesity in overweight children with NAFLD provides further impetus for pediatricians to work on both preventive and treatment regimens for weight issues,” Dr. Rushton said. “Linking community-based initiatives focused on adequate nutritional support with pediatric clinical support services is critical in solving issues related to overweight in children. Tracking BMI over time and developing healthy habit goals for patients are key parts of clinical interventions.”
The study was funded by the National Institutes of Health. The investigators reported no conflicts of interest.
FROM PEDIATRICS
Cardiovascular risks elevated in transgender youth
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hyperphagia, anxiety eased with carbetocin in patients with Prader-Willi syndrome
Children and adolescents with Prader-Willi syndrome (PWS) who received three daily, intranasal doses of carbetocin, an investigational, long-acting oxytocin analogue, had significant improvement in hyperphagia and anxiety during 8 weeks on treatment, compared with placebo in a multicenter, phase 3 trial with 119 patients.
The treatment also appeared safe during up to 56 additional weeks on active treatment, with no serious adverse effects nor “unexpected” events, and once completing the study about 95% of enrolled patients opted to remain on active treatment, Cheri L. Deal, MD, PhD, said at the annual meeting of the Endocrine Society.
Based on “the significant results for the placebo-controlled period, as well as for those finishing the 56-week extension, we may well have a new armament for helping these kids and their families deal with the unrelenting hunger of patients with PWS as well as some of the behavioral symptoms,” Dr. Deal, chief of endocrinology and diabetes at the Sainte-Justine Mother-Child University of Montreal Hospital, said in an interview. No treatment currently has labeling for addressing the hyperphagia or anxiety that is characteristic and often problematic for children and adolescents with PWS, an autosomal dominant genetic disease with an incidence of about 1 in 15,000 births and an estimated U.S. prevalence of about 9,000 cases, or about 1 case for every 37,000 people.
‘Gorgeous’ safety
“The results looked pretty positive, and we’re encouraged by what appears to be a good safety profile, so overall I think the PWS community is very excited by the results and is very interested in getting access to this drug,” commented Theresa V. Strong, PhD, director of research programs for the Foundation for Prader-Willi Research in Walnut, Calif., a group not involved with the study. Currently, “we have no effective treatments for these difficult behaviors” of hyperphagia and anxiety. Surveys and studies run by the foundation have documented that hyperphagia and anxiety “were the two most important symptoms that families would like to see treated,” Dr. Strong added in an interview.
PWS “is complex and affects almost every aspect of the lives of affected people and their families. Any treatment that can chip away at some of the problems these patients have can be a huge benefit to the patients and their families,” said Jennifer L. Miller, MD, a professor of pediatric endocrinology at the University of Florida, Gainesville, and a coinvestigator on the study.
But the finding that carbetocin appeared to address, at least in part, this unmet need while compiling a safety record that Dr. Miller called “gorgeous” and “remarkable,” also came with a few limitations.
Fewer patients than planned, and muddled outcomes
The CARE-PWS trial aimed to enroll 175 patients, but fell short once the COVID-19 pandemic hit. Plus the trial had two prespecified primary endpoints – improvements in a measure of hyperphagia, and in a measure of obsessive and compulsive behaviors – specifically in the 40 patients who received the higher of the two dosages studied, 9.6 mg t.i.d. intranasally. Neither endpoint showed significant improvement among the patients on this dosage, compared with the 40 patients who received placebo, although both outcomes trended in the right direction in the actively treated patients.
The study’s positive results came in a secondary treatment group, 39 patients who received 3.2 mg t.i.d., also intranasally. This subgroup had significant benefit, compared with placebo, for reducing hyperphagia symptoms as measured on the Hyperphagia Questionnaire for Clinical Trials (HQ-CT) Total Score. After the first 8 weeks on treatment, patients on the lower carbetocin dosage had an average reduction in their HQ-CT score of greater than 5 points, more than double the reduction seen among control patients who received placebo.
Those on the 3.2-mg t.i.d. dosage also showed significant improvements, compared with placebo, for anxiety, measured by the PWS Anxiety and Distress Questionnaire Total Score, as well as on measures of clinical global impression of severity, and of clinical global impression of change. Like the higher-dosage patients the lower-dosage subgroup did not show a significant difference compared with placebo for the other primary endpoint, change in obsessive and compulsive behaviors as measured by the Children’s Yale-Brown Obsessive-Compulsive Scale Total Score, although also like the higher dosage the effect from the lower dosage trended toward benefit.
A further limitation was that, at the time of her report, presented in abstract OR16-3 at the meeting, Dr. Deal could only present complete 64-week follow-up for 72 patients, although this reassuringly showed that, as time on the 9.6-mg t.i.d. dosage continued beyond 8 weeks, patients gradually improved their HQ-CT response so that by 64 weeks on treatment their hyperphagia score had improved as much as in the patients who received the lower dosage.
In short, documented benefits occurred on the lower dosage, especially for clinically meaningful symptoms like hyperphagia and anxiety, but the study’s overall results were not fully consistent by statistical criteria.
Benefiting an unmet need?
“While it is regrettable that we did not get to 175 patients because of COVID-19, the dataset is significant enough for me to feel that the FDA [Food and Drug Administration] needs to take a very serious look and consider approval,” Dr. Deal said in an interview. “Once safety is assured, which I think it is, I can only hope that regulatory officials understand their unmet needs of this rare disease community and will allow the drug to move to the next stage.”
“This is a very rare disease, and having families participate in trials is really challenging,” especially while the COVID-19 pandemic continues, Dr. Strong said. For the pediatric and adolescent patients targeted in this study “it will take a while for COVID to go away and for families to feel safe again being in a trial, so a real concern is that a need for more clinical trials is not terribly feasible now. Given that the safety profile looked good and one dose seemed to have good efficacy, as long as the long-term data continue to look good we’d love for the FDA to look at the existing data and see whether there is a path forward.”
Dr. Miller highlighted the limitations of what the CARE-PWS findings show.
“Given that it was only an 8-week trial of drug against placebo, and the fact that the primary outcomes weren’t met for the higher dose, my thought is that potentially we need to study more patients for a longer period at the 3.2-mg dose,” she said. She acknowledged that the metric used in the study to assess obsessive and compulsive behaviors is “very difficult” to apply to patients with PWS because of uncertainties in scoring obsessions in patients “who are not very good at telling you what they’re thinking.” Plus, “it’s absolutely not a problem that we did not see an effect on obsession and compulsions if the treatment potentially improves anxiety and hyperphagia, which are very common.” A treatment that reliably reduces these symptoms “would be amazing,” Dr. Miller added.
“PWS is very rare, so it’s very hard to do trials. Maybe the FDA will approve carbetocin because it was safe and gave a signal of efficacy at the lower dose. But my thought is that additional treatment trials are needed with only the lower dose and with longer duration,” she said.
CARE-PWS enrolled patients with nutritional phase 3 PWS who were aged 7-18 years at any of 24 sites in the United States, Canada, or Australia during 2018-2020. They averaged about 12 years of age, and 56% were girls.
The most common adverse effect from carbetocin was flushing, occurring in 14% of those on the lower dose and 21% on the higher dose, but not in any placebo patient. Other adverse effects more common on the lower dose than in the placebo group included headache in 16%, and diarrhea in 9%.
Carbetocin is not only long-lasting in circulation, it also has better affinity for oxytocin receptors than for vasopressin receptors, reducing the potential for causing hyponatremia. The idea to use it in patients with PWS followed prior studies with oxytocin, which had shown dopamine interactions that reduced anxiety and influenced food ingestion behavior. Brain autopsy studies had shown that patients with Prader-Willi syndrome have substantially fewer neurons than usual producing oxytocin. Treatment with intranasal carbetocin had shown efficacy for improving hyperphagia and behavior in a controlled phase 2 study with 37 patients.
Carbetocin is approved for use in reducing excessive bleeding after childbirth, particularly cesarean, in more than 20 countries outside the United States.
CARE-PWS was sponsored by Levo Therapeutics, the company developing carbetocin. Dr. Deal has been an adviser to Levo Therapeutics. Dr. Strong is an employee of the Foundation for Prader-Willi Research, which has received support from Levo Therapeutics as well as from other drug companies, but which receives most of its funding from individuals. Dr. Miller has received research funding from Levo Therapeutics and also from Harmony Biosciences, Rhythm Pharmaceuticals, and Soleno Therapeutics.
Children and adolescents with Prader-Willi syndrome (PWS) who received three daily, intranasal doses of carbetocin, an investigational, long-acting oxytocin analogue, had significant improvement in hyperphagia and anxiety during 8 weeks on treatment, compared with placebo in a multicenter, phase 3 trial with 119 patients.
The treatment also appeared safe during up to 56 additional weeks on active treatment, with no serious adverse effects nor “unexpected” events, and once completing the study about 95% of enrolled patients opted to remain on active treatment, Cheri L. Deal, MD, PhD, said at the annual meeting of the Endocrine Society.
Based on “the significant results for the placebo-controlled period, as well as for those finishing the 56-week extension, we may well have a new armament for helping these kids and their families deal with the unrelenting hunger of patients with PWS as well as some of the behavioral symptoms,” Dr. Deal, chief of endocrinology and diabetes at the Sainte-Justine Mother-Child University of Montreal Hospital, said in an interview. No treatment currently has labeling for addressing the hyperphagia or anxiety that is characteristic and often problematic for children and adolescents with PWS, an autosomal dominant genetic disease with an incidence of about 1 in 15,000 births and an estimated U.S. prevalence of about 9,000 cases, or about 1 case for every 37,000 people.
‘Gorgeous’ safety
“The results looked pretty positive, and we’re encouraged by what appears to be a good safety profile, so overall I think the PWS community is very excited by the results and is very interested in getting access to this drug,” commented Theresa V. Strong, PhD, director of research programs for the Foundation for Prader-Willi Research in Walnut, Calif., a group not involved with the study. Currently, “we have no effective treatments for these difficult behaviors” of hyperphagia and anxiety. Surveys and studies run by the foundation have documented that hyperphagia and anxiety “were the two most important symptoms that families would like to see treated,” Dr. Strong added in an interview.
PWS “is complex and affects almost every aspect of the lives of affected people and their families. Any treatment that can chip away at some of the problems these patients have can be a huge benefit to the patients and their families,” said Jennifer L. Miller, MD, a professor of pediatric endocrinology at the University of Florida, Gainesville, and a coinvestigator on the study.
But the finding that carbetocin appeared to address, at least in part, this unmet need while compiling a safety record that Dr. Miller called “gorgeous” and “remarkable,” also came with a few limitations.
Fewer patients than planned, and muddled outcomes
The CARE-PWS trial aimed to enroll 175 patients, but fell short once the COVID-19 pandemic hit. Plus the trial had two prespecified primary endpoints – improvements in a measure of hyperphagia, and in a measure of obsessive and compulsive behaviors – specifically in the 40 patients who received the higher of the two dosages studied, 9.6 mg t.i.d. intranasally. Neither endpoint showed significant improvement among the patients on this dosage, compared with the 40 patients who received placebo, although both outcomes trended in the right direction in the actively treated patients.
The study’s positive results came in a secondary treatment group, 39 patients who received 3.2 mg t.i.d., also intranasally. This subgroup had significant benefit, compared with placebo, for reducing hyperphagia symptoms as measured on the Hyperphagia Questionnaire for Clinical Trials (HQ-CT) Total Score. After the first 8 weeks on treatment, patients on the lower carbetocin dosage had an average reduction in their HQ-CT score of greater than 5 points, more than double the reduction seen among control patients who received placebo.
Those on the 3.2-mg t.i.d. dosage also showed significant improvements, compared with placebo, for anxiety, measured by the PWS Anxiety and Distress Questionnaire Total Score, as well as on measures of clinical global impression of severity, and of clinical global impression of change. Like the higher-dosage patients the lower-dosage subgroup did not show a significant difference compared with placebo for the other primary endpoint, change in obsessive and compulsive behaviors as measured by the Children’s Yale-Brown Obsessive-Compulsive Scale Total Score, although also like the higher dosage the effect from the lower dosage trended toward benefit.
A further limitation was that, at the time of her report, presented in abstract OR16-3 at the meeting, Dr. Deal could only present complete 64-week follow-up for 72 patients, although this reassuringly showed that, as time on the 9.6-mg t.i.d. dosage continued beyond 8 weeks, patients gradually improved their HQ-CT response so that by 64 weeks on treatment their hyperphagia score had improved as much as in the patients who received the lower dosage.
In short, documented benefits occurred on the lower dosage, especially for clinically meaningful symptoms like hyperphagia and anxiety, but the study’s overall results were not fully consistent by statistical criteria.
Benefiting an unmet need?
“While it is regrettable that we did not get to 175 patients because of COVID-19, the dataset is significant enough for me to feel that the FDA [Food and Drug Administration] needs to take a very serious look and consider approval,” Dr. Deal said in an interview. “Once safety is assured, which I think it is, I can only hope that regulatory officials understand their unmet needs of this rare disease community and will allow the drug to move to the next stage.”
“This is a very rare disease, and having families participate in trials is really challenging,” especially while the COVID-19 pandemic continues, Dr. Strong said. For the pediatric and adolescent patients targeted in this study “it will take a while for COVID to go away and for families to feel safe again being in a trial, so a real concern is that a need for more clinical trials is not terribly feasible now. Given that the safety profile looked good and one dose seemed to have good efficacy, as long as the long-term data continue to look good we’d love for the FDA to look at the existing data and see whether there is a path forward.”
Dr. Miller highlighted the limitations of what the CARE-PWS findings show.
“Given that it was only an 8-week trial of drug against placebo, and the fact that the primary outcomes weren’t met for the higher dose, my thought is that potentially we need to study more patients for a longer period at the 3.2-mg dose,” she said. She acknowledged that the metric used in the study to assess obsessive and compulsive behaviors is “very difficult” to apply to patients with PWS because of uncertainties in scoring obsessions in patients “who are not very good at telling you what they’re thinking.” Plus, “it’s absolutely not a problem that we did not see an effect on obsession and compulsions if the treatment potentially improves anxiety and hyperphagia, which are very common.” A treatment that reliably reduces these symptoms “would be amazing,” Dr. Miller added.
“PWS is very rare, so it’s very hard to do trials. Maybe the FDA will approve carbetocin because it was safe and gave a signal of efficacy at the lower dose. But my thought is that additional treatment trials are needed with only the lower dose and with longer duration,” she said.
CARE-PWS enrolled patients with nutritional phase 3 PWS who were aged 7-18 years at any of 24 sites in the United States, Canada, or Australia during 2018-2020. They averaged about 12 years of age, and 56% were girls.
The most common adverse effect from carbetocin was flushing, occurring in 14% of those on the lower dose and 21% on the higher dose, but not in any placebo patient. Other adverse effects more common on the lower dose than in the placebo group included headache in 16%, and diarrhea in 9%.
Carbetocin is not only long-lasting in circulation, it also has better affinity for oxytocin receptors than for vasopressin receptors, reducing the potential for causing hyponatremia. The idea to use it in patients with PWS followed prior studies with oxytocin, which had shown dopamine interactions that reduced anxiety and influenced food ingestion behavior. Brain autopsy studies had shown that patients with Prader-Willi syndrome have substantially fewer neurons than usual producing oxytocin. Treatment with intranasal carbetocin had shown efficacy for improving hyperphagia and behavior in a controlled phase 2 study with 37 patients.
Carbetocin is approved for use in reducing excessive bleeding after childbirth, particularly cesarean, in more than 20 countries outside the United States.
CARE-PWS was sponsored by Levo Therapeutics, the company developing carbetocin. Dr. Deal has been an adviser to Levo Therapeutics. Dr. Strong is an employee of the Foundation for Prader-Willi Research, which has received support from Levo Therapeutics as well as from other drug companies, but which receives most of its funding from individuals. Dr. Miller has received research funding from Levo Therapeutics and also from Harmony Biosciences, Rhythm Pharmaceuticals, and Soleno Therapeutics.
Children and adolescents with Prader-Willi syndrome (PWS) who received three daily, intranasal doses of carbetocin, an investigational, long-acting oxytocin analogue, had significant improvement in hyperphagia and anxiety during 8 weeks on treatment, compared with placebo in a multicenter, phase 3 trial with 119 patients.
The treatment also appeared safe during up to 56 additional weeks on active treatment, with no serious adverse effects nor “unexpected” events, and once completing the study about 95% of enrolled patients opted to remain on active treatment, Cheri L. Deal, MD, PhD, said at the annual meeting of the Endocrine Society.
Based on “the significant results for the placebo-controlled period, as well as for those finishing the 56-week extension, we may well have a new armament for helping these kids and their families deal with the unrelenting hunger of patients with PWS as well as some of the behavioral symptoms,” Dr. Deal, chief of endocrinology and diabetes at the Sainte-Justine Mother-Child University of Montreal Hospital, said in an interview. No treatment currently has labeling for addressing the hyperphagia or anxiety that is characteristic and often problematic for children and adolescents with PWS, an autosomal dominant genetic disease with an incidence of about 1 in 15,000 births and an estimated U.S. prevalence of about 9,000 cases, or about 1 case for every 37,000 people.
‘Gorgeous’ safety
“The results looked pretty positive, and we’re encouraged by what appears to be a good safety profile, so overall I think the PWS community is very excited by the results and is very interested in getting access to this drug,” commented Theresa V. Strong, PhD, director of research programs for the Foundation for Prader-Willi Research in Walnut, Calif., a group not involved with the study. Currently, “we have no effective treatments for these difficult behaviors” of hyperphagia and anxiety. Surveys and studies run by the foundation have documented that hyperphagia and anxiety “were the two most important symptoms that families would like to see treated,” Dr. Strong added in an interview.
PWS “is complex and affects almost every aspect of the lives of affected people and their families. Any treatment that can chip away at some of the problems these patients have can be a huge benefit to the patients and their families,” said Jennifer L. Miller, MD, a professor of pediatric endocrinology at the University of Florida, Gainesville, and a coinvestigator on the study.
But the finding that carbetocin appeared to address, at least in part, this unmet need while compiling a safety record that Dr. Miller called “gorgeous” and “remarkable,” also came with a few limitations.
Fewer patients than planned, and muddled outcomes
The CARE-PWS trial aimed to enroll 175 patients, but fell short once the COVID-19 pandemic hit. Plus the trial had two prespecified primary endpoints – improvements in a measure of hyperphagia, and in a measure of obsessive and compulsive behaviors – specifically in the 40 patients who received the higher of the two dosages studied, 9.6 mg t.i.d. intranasally. Neither endpoint showed significant improvement among the patients on this dosage, compared with the 40 patients who received placebo, although both outcomes trended in the right direction in the actively treated patients.
The study’s positive results came in a secondary treatment group, 39 patients who received 3.2 mg t.i.d., also intranasally. This subgroup had significant benefit, compared with placebo, for reducing hyperphagia symptoms as measured on the Hyperphagia Questionnaire for Clinical Trials (HQ-CT) Total Score. After the first 8 weeks on treatment, patients on the lower carbetocin dosage had an average reduction in their HQ-CT score of greater than 5 points, more than double the reduction seen among control patients who received placebo.
Those on the 3.2-mg t.i.d. dosage also showed significant improvements, compared with placebo, for anxiety, measured by the PWS Anxiety and Distress Questionnaire Total Score, as well as on measures of clinical global impression of severity, and of clinical global impression of change. Like the higher-dosage patients the lower-dosage subgroup did not show a significant difference compared with placebo for the other primary endpoint, change in obsessive and compulsive behaviors as measured by the Children’s Yale-Brown Obsessive-Compulsive Scale Total Score, although also like the higher dosage the effect from the lower dosage trended toward benefit.
A further limitation was that, at the time of her report, presented in abstract OR16-3 at the meeting, Dr. Deal could only present complete 64-week follow-up for 72 patients, although this reassuringly showed that, as time on the 9.6-mg t.i.d. dosage continued beyond 8 weeks, patients gradually improved their HQ-CT response so that by 64 weeks on treatment their hyperphagia score had improved as much as in the patients who received the lower dosage.
In short, documented benefits occurred on the lower dosage, especially for clinically meaningful symptoms like hyperphagia and anxiety, but the study’s overall results were not fully consistent by statistical criteria.
Benefiting an unmet need?
“While it is regrettable that we did not get to 175 patients because of COVID-19, the dataset is significant enough for me to feel that the FDA [Food and Drug Administration] needs to take a very serious look and consider approval,” Dr. Deal said in an interview. “Once safety is assured, which I think it is, I can only hope that regulatory officials understand their unmet needs of this rare disease community and will allow the drug to move to the next stage.”
“This is a very rare disease, and having families participate in trials is really challenging,” especially while the COVID-19 pandemic continues, Dr. Strong said. For the pediatric and adolescent patients targeted in this study “it will take a while for COVID to go away and for families to feel safe again being in a trial, so a real concern is that a need for more clinical trials is not terribly feasible now. Given that the safety profile looked good and one dose seemed to have good efficacy, as long as the long-term data continue to look good we’d love for the FDA to look at the existing data and see whether there is a path forward.”
Dr. Miller highlighted the limitations of what the CARE-PWS findings show.
“Given that it was only an 8-week trial of drug against placebo, and the fact that the primary outcomes weren’t met for the higher dose, my thought is that potentially we need to study more patients for a longer period at the 3.2-mg dose,” she said. She acknowledged that the metric used in the study to assess obsessive and compulsive behaviors is “very difficult” to apply to patients with PWS because of uncertainties in scoring obsessions in patients “who are not very good at telling you what they’re thinking.” Plus, “it’s absolutely not a problem that we did not see an effect on obsession and compulsions if the treatment potentially improves anxiety and hyperphagia, which are very common.” A treatment that reliably reduces these symptoms “would be amazing,” Dr. Miller added.
“PWS is very rare, so it’s very hard to do trials. Maybe the FDA will approve carbetocin because it was safe and gave a signal of efficacy at the lower dose. But my thought is that additional treatment trials are needed with only the lower dose and with longer duration,” she said.
CARE-PWS enrolled patients with nutritional phase 3 PWS who were aged 7-18 years at any of 24 sites in the United States, Canada, or Australia during 2018-2020. They averaged about 12 years of age, and 56% were girls.
The most common adverse effect from carbetocin was flushing, occurring in 14% of those on the lower dose and 21% on the higher dose, but not in any placebo patient. Other adverse effects more common on the lower dose than in the placebo group included headache in 16%, and diarrhea in 9%.
Carbetocin is not only long-lasting in circulation, it also has better affinity for oxytocin receptors than for vasopressin receptors, reducing the potential for causing hyponatremia. The idea to use it in patients with PWS followed prior studies with oxytocin, which had shown dopamine interactions that reduced anxiety and influenced food ingestion behavior. Brain autopsy studies had shown that patients with Prader-Willi syndrome have substantially fewer neurons than usual producing oxytocin. Treatment with intranasal carbetocin had shown efficacy for improving hyperphagia and behavior in a controlled phase 2 study with 37 patients.
Carbetocin is approved for use in reducing excessive bleeding after childbirth, particularly cesarean, in more than 20 countries outside the United States.
CARE-PWS was sponsored by Levo Therapeutics, the company developing carbetocin. Dr. Deal has been an adviser to Levo Therapeutics. Dr. Strong is an employee of the Foundation for Prader-Willi Research, which has received support from Levo Therapeutics as well as from other drug companies, but which receives most of its funding from individuals. Dr. Miller has received research funding from Levo Therapeutics and also from Harmony Biosciences, Rhythm Pharmaceuticals, and Soleno Therapeutics.
FROM ENDO 2021
Coffee could be the secret weapon against NAFLD
Treatment of obesity through exercise and diet is unquestionably the foundation of care for patients with nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). But drinking at least several cups of coffee a day makes for additional powerful medicine, said Manal F. Abdelmalek, MD, MPH, at the Gastroenterology Updates, IBD, Liver Disease Conference.
“I do recommend at least two to three cups of coffee per day for my patients with NAFLD,” said Dr. Abdelmalek, professor of medicine and a gastroenterologist at Duke University, Durham, N.C.
Her thinking on this recommendation has been influenced by a meta-analysis of 16 studies including more than 3,000 coffee drinkers and 132,000 nonconsumers; the meta-analysis concluded that coffee drinkers were 39% less likely to develop cirrhosis. There was evidence of a dose-response effect: Consumers of two or more cups daily had a 47% reduction in the risk of cirrhosis, compared with the nondrinkers, while more modest consumption was associated with a 34% reduction. Moreover, the investigators found that coffee consumption was also associated with a 27% reduction in the likelihood of developing advanced hepatic fibrosis, compared with that of non–coffee drinkers.
“What’s even more provocative is the evidence that coffee decreases risk of hepatocellular carcinoma,” the gastroenterologist said.
She highlighted a U.K. meta-analysis of 18 cohort studies with 2.27 million participants and 2,905 cases, along with 8 case-control studies featuring a collective 1,825 cases and 4,652 controls. The investigators reported that drinking at least two cups of coffee per day was associated with a 35% reduction in the risk of hepatocellular carcinoma independent of a patient’s stage of liver disease or the presence or absence of high alcohol consumption, smoking, obesity, type 2 diabetes, or hepatitis B or C infection.
“This is very impressive data and certainly not something you should ignore,” according to Dr. Abdelmalek.
There is also “fairly strong” data that coffee reduces the risk of developing type 2 diabetes, she continued. The mechanism of these benefits is unclear.
“It’s not known if it’s caffeine or some other constituent of the bean; a phenol, for example. The story behind tea is not as compelling as for coffee, so it may be something beyond caffeine,” according to Dr. Abdelmalek.
Session moderator Norah A. Terrault, MD, MPH, noted that drinking at least two cups of coffee per day has also been associated with reduced risk of cirrhosis in patients with hepatitis B or hepatitis C infection. So she too is on board the coffee express.
“I’m also a big proponent of recommending coffee. We take so much away from the patients, it’s nice to give them back something, right?” said Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
Diet and exercise
Most of the major gastroenterology professional societies emphasize in their practice guidelines for NAFLD that diet and routine physical activity are mandatory. If sustained, these lifestyle modifications can improve NASH and hepatic fibrosis, as well as reduce the risk of portal hypertension and liver cancer. Dr. Abdelmalek counsels her patients to aim for at least 150 minutes per week of moderate or vigorous aerobic and/or resistance exercise. She doesn’t care about the exercise intensity or type, noting that what she considers to be “a beautifully done intervention trial” in 220 patients over the course of 12 months concluded that both moderate and vigorous exercise achieved a significant reduction in intrahepatic triglyceride content.
“Tailor exercise to what patients can do, what they enjoy, and what they can sustain,” she advised.
She identifies and addresses all modifiable risk factors for NAFLD, including hypertension, diabetes, abdominal obesity, smoking, excessive alcohol intake, obstructive sleep apnea, and an unhealthy diet high in fat, red meat, and fructose.
“The primary message I tell my patients interested in dieting is: I want you to find the right approach for you. There is no right or wrong answer. For some of my patients, it’s intermittent fasting and having their first meal at 2 or 3 o’clock in the afternoon. For others it’s a Weight Watchers approach, or a Mediterranean diet, or it’s high protein. The bottom line of my approach is a gravitation away from excess carbohydrates and fats, and beyond that if I can achieve weight loss through caloric restriction or intermittent fasting, I try to tailor that to my patients’ preferences. I do send them to nutritionists,” the gastroenterologist said.
A 7%-10% weight loss has been shown to result in resolution of NASH in 64%-90% of patients. However, only about 10% of patients who achieve clinically meaningful weight loss short term are able to maintain it at 1 year, so ongoing follow-up is essential.
At present there is no FDA-approved therapy for NAFLD/NASH. Beyond diet and exercise – and coffee – there is the option of antiobesity weight-loss drug therapy, which is about as effective as successful lifestyle modification, and bariatric surgery, which is dramatically effective. French surgeons recently reported in a prospective single-center study of 180 severely obese patients with NASH who underwent bariatric surgery that, at 5 years’ follow-up, 84% of them had resolution of NASH with no worsening of liver fibrosis. Indeed, 63% of patients with mild fibrosis at baseline experienced complete resolution of their fibrosis at follow-up, as did 46% of those with more severe baseline bridging fibrosis.
Dr. Abdelmalek reported having no financial conflicts of interest regarding her presentation.
Treatment of obesity through exercise and diet is unquestionably the foundation of care for patients with nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). But drinking at least several cups of coffee a day makes for additional powerful medicine, said Manal F. Abdelmalek, MD, MPH, at the Gastroenterology Updates, IBD, Liver Disease Conference.
“I do recommend at least two to three cups of coffee per day for my patients with NAFLD,” said Dr. Abdelmalek, professor of medicine and a gastroenterologist at Duke University, Durham, N.C.
Her thinking on this recommendation has been influenced by a meta-analysis of 16 studies including more than 3,000 coffee drinkers and 132,000 nonconsumers; the meta-analysis concluded that coffee drinkers were 39% less likely to develop cirrhosis. There was evidence of a dose-response effect: Consumers of two or more cups daily had a 47% reduction in the risk of cirrhosis, compared with the nondrinkers, while more modest consumption was associated with a 34% reduction. Moreover, the investigators found that coffee consumption was also associated with a 27% reduction in the likelihood of developing advanced hepatic fibrosis, compared with that of non–coffee drinkers.
“What’s even more provocative is the evidence that coffee decreases risk of hepatocellular carcinoma,” the gastroenterologist said.
She highlighted a U.K. meta-analysis of 18 cohort studies with 2.27 million participants and 2,905 cases, along with 8 case-control studies featuring a collective 1,825 cases and 4,652 controls. The investigators reported that drinking at least two cups of coffee per day was associated with a 35% reduction in the risk of hepatocellular carcinoma independent of a patient’s stage of liver disease or the presence or absence of high alcohol consumption, smoking, obesity, type 2 diabetes, or hepatitis B or C infection.
“This is very impressive data and certainly not something you should ignore,” according to Dr. Abdelmalek.
There is also “fairly strong” data that coffee reduces the risk of developing type 2 diabetes, she continued. The mechanism of these benefits is unclear.
“It’s not known if it’s caffeine or some other constituent of the bean; a phenol, for example. The story behind tea is not as compelling as for coffee, so it may be something beyond caffeine,” according to Dr. Abdelmalek.
Session moderator Norah A. Terrault, MD, MPH, noted that drinking at least two cups of coffee per day has also been associated with reduced risk of cirrhosis in patients with hepatitis B or hepatitis C infection. So she too is on board the coffee express.
“I’m also a big proponent of recommending coffee. We take so much away from the patients, it’s nice to give them back something, right?” said Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
Diet and exercise
Most of the major gastroenterology professional societies emphasize in their practice guidelines for NAFLD that diet and routine physical activity are mandatory. If sustained, these lifestyle modifications can improve NASH and hepatic fibrosis, as well as reduce the risk of portal hypertension and liver cancer. Dr. Abdelmalek counsels her patients to aim for at least 150 minutes per week of moderate or vigorous aerobic and/or resistance exercise. She doesn’t care about the exercise intensity or type, noting that what she considers to be “a beautifully done intervention trial” in 220 patients over the course of 12 months concluded that both moderate and vigorous exercise achieved a significant reduction in intrahepatic triglyceride content.
“Tailor exercise to what patients can do, what they enjoy, and what they can sustain,” she advised.
She identifies and addresses all modifiable risk factors for NAFLD, including hypertension, diabetes, abdominal obesity, smoking, excessive alcohol intake, obstructive sleep apnea, and an unhealthy diet high in fat, red meat, and fructose.
“The primary message I tell my patients interested in dieting is: I want you to find the right approach for you. There is no right or wrong answer. For some of my patients, it’s intermittent fasting and having their first meal at 2 or 3 o’clock in the afternoon. For others it’s a Weight Watchers approach, or a Mediterranean diet, or it’s high protein. The bottom line of my approach is a gravitation away from excess carbohydrates and fats, and beyond that if I can achieve weight loss through caloric restriction or intermittent fasting, I try to tailor that to my patients’ preferences. I do send them to nutritionists,” the gastroenterologist said.
A 7%-10% weight loss has been shown to result in resolution of NASH in 64%-90% of patients. However, only about 10% of patients who achieve clinically meaningful weight loss short term are able to maintain it at 1 year, so ongoing follow-up is essential.
At present there is no FDA-approved therapy for NAFLD/NASH. Beyond diet and exercise – and coffee – there is the option of antiobesity weight-loss drug therapy, which is about as effective as successful lifestyle modification, and bariatric surgery, which is dramatically effective. French surgeons recently reported in a prospective single-center study of 180 severely obese patients with NASH who underwent bariatric surgery that, at 5 years’ follow-up, 84% of them had resolution of NASH with no worsening of liver fibrosis. Indeed, 63% of patients with mild fibrosis at baseline experienced complete resolution of their fibrosis at follow-up, as did 46% of those with more severe baseline bridging fibrosis.
Dr. Abdelmalek reported having no financial conflicts of interest regarding her presentation.
Treatment of obesity through exercise and diet is unquestionably the foundation of care for patients with nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). But drinking at least several cups of coffee a day makes for additional powerful medicine, said Manal F. Abdelmalek, MD, MPH, at the Gastroenterology Updates, IBD, Liver Disease Conference.
“I do recommend at least two to three cups of coffee per day for my patients with NAFLD,” said Dr. Abdelmalek, professor of medicine and a gastroenterologist at Duke University, Durham, N.C.
Her thinking on this recommendation has been influenced by a meta-analysis of 16 studies including more than 3,000 coffee drinkers and 132,000 nonconsumers; the meta-analysis concluded that coffee drinkers were 39% less likely to develop cirrhosis. There was evidence of a dose-response effect: Consumers of two or more cups daily had a 47% reduction in the risk of cirrhosis, compared with the nondrinkers, while more modest consumption was associated with a 34% reduction. Moreover, the investigators found that coffee consumption was also associated with a 27% reduction in the likelihood of developing advanced hepatic fibrosis, compared with that of non–coffee drinkers.
“What’s even more provocative is the evidence that coffee decreases risk of hepatocellular carcinoma,” the gastroenterologist said.
She highlighted a U.K. meta-analysis of 18 cohort studies with 2.27 million participants and 2,905 cases, along with 8 case-control studies featuring a collective 1,825 cases and 4,652 controls. The investigators reported that drinking at least two cups of coffee per day was associated with a 35% reduction in the risk of hepatocellular carcinoma independent of a patient’s stage of liver disease or the presence or absence of high alcohol consumption, smoking, obesity, type 2 diabetes, or hepatitis B or C infection.
“This is very impressive data and certainly not something you should ignore,” according to Dr. Abdelmalek.
There is also “fairly strong” data that coffee reduces the risk of developing type 2 diabetes, she continued. The mechanism of these benefits is unclear.
“It’s not known if it’s caffeine or some other constituent of the bean; a phenol, for example. The story behind tea is not as compelling as for coffee, so it may be something beyond caffeine,” according to Dr. Abdelmalek.
Session moderator Norah A. Terrault, MD, MPH, noted that drinking at least two cups of coffee per day has also been associated with reduced risk of cirrhosis in patients with hepatitis B or hepatitis C infection. So she too is on board the coffee express.
“I’m also a big proponent of recommending coffee. We take so much away from the patients, it’s nice to give them back something, right?” said Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
Diet and exercise
Most of the major gastroenterology professional societies emphasize in their practice guidelines for NAFLD that diet and routine physical activity are mandatory. If sustained, these lifestyle modifications can improve NASH and hepatic fibrosis, as well as reduce the risk of portal hypertension and liver cancer. Dr. Abdelmalek counsels her patients to aim for at least 150 minutes per week of moderate or vigorous aerobic and/or resistance exercise. She doesn’t care about the exercise intensity or type, noting that what she considers to be “a beautifully done intervention trial” in 220 patients over the course of 12 months concluded that both moderate and vigorous exercise achieved a significant reduction in intrahepatic triglyceride content.
“Tailor exercise to what patients can do, what they enjoy, and what they can sustain,” she advised.
She identifies and addresses all modifiable risk factors for NAFLD, including hypertension, diabetes, abdominal obesity, smoking, excessive alcohol intake, obstructive sleep apnea, and an unhealthy diet high in fat, red meat, and fructose.
“The primary message I tell my patients interested in dieting is: I want you to find the right approach for you. There is no right or wrong answer. For some of my patients, it’s intermittent fasting and having their first meal at 2 or 3 o’clock in the afternoon. For others it’s a Weight Watchers approach, or a Mediterranean diet, or it’s high protein. The bottom line of my approach is a gravitation away from excess carbohydrates and fats, and beyond that if I can achieve weight loss through caloric restriction or intermittent fasting, I try to tailor that to my patients’ preferences. I do send them to nutritionists,” the gastroenterologist said.
A 7%-10% weight loss has been shown to result in resolution of NASH in 64%-90% of patients. However, only about 10% of patients who achieve clinically meaningful weight loss short term are able to maintain it at 1 year, so ongoing follow-up is essential.
At present there is no FDA-approved therapy for NAFLD/NASH. Beyond diet and exercise – and coffee – there is the option of antiobesity weight-loss drug therapy, which is about as effective as successful lifestyle modification, and bariatric surgery, which is dramatically effective. French surgeons recently reported in a prospective single-center study of 180 severely obese patients with NASH who underwent bariatric surgery that, at 5 years’ follow-up, 84% of them had resolution of NASH with no worsening of liver fibrosis. Indeed, 63% of patients with mild fibrosis at baseline experienced complete resolution of their fibrosis at follow-up, as did 46% of those with more severe baseline bridging fibrosis.
Dr. Abdelmalek reported having no financial conflicts of interest regarding her presentation.
FROM GUILD 2021
‘Striking’ increase in childhood obesity during pandemic
Obesity rates among children jumped substantially in the first months of the COVID-19 pandemic, according to a study published online in Pediatrics. Experts worry the excess weight will be a continuing problem for these children.
“Across the board in the span of a year, there has been a 2% increase in obesity, which is really striking,” lead author Brian P. Jenssen, MD, said in an interview.
The prevalence of obesity in a large pediatric primary care network increased from 13.7% to 15.4%.
Preexisting disparities by race or ethnicity and socioeconomic status worsened, noted Dr. Jenssen, a primary care pediatrician affiliated with Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania, Philadelphia.
Dr. Jenssen and colleagues compared the average obesity rate from June to December 2020 with the rate from June to December 2019 among patients in the CHOP Care Network, which includes 29 urban, suburban, and semirural clinics in the Philadelphia region. In June 2020, the volume of patient visits “returned to near-normal” after a dramatic decline in March 2020, the study authors wrote.
The investigators examined body mass index at all visits for patients aged 2-17 years for whom height and weight were documented. Patients with a BMI at or above the 95th percentile were classified as obese. The analysis included approximately 169,000 visits in 2019 and about 145,000 in 2020.
The average age of the patients was 9.2 years, and 48.9% were girls. In all, 21.4% were non-Hispanic Black, and about 30% were publicly insured.
Increases in obesity rates were more pronounced among patients aged 5-9 years and among patients who were Hispanic/Latino, non-Hispanic Black, publicly insured, or from lower-income neighborhoods.
Whereas the obesity rate increased 1% for patients aged 13-17 years, the rate increased 2.6% for patients aged 5-9 years.
Nearly 25% of Hispanic/Latino or non-Hispanic Black patients seen during the pandemic were obese, compared with 11.3% of non-Hispanic White patients. Before the pandemic, differences by race or ethnicity had been about 10%-11%.
Limiting the analysis to preventive visits did not meaningfully change the results, wrote Dr. Jenssen and colleagues.
“Having any increase in the obesity rates is alarming,” said Sandra Hassink, MD, medical director for the American Academy of Pediatrics’ (AAP’s) Institute for Healthy Childhood Weight. “I think what we’re seeing is what we feared.”
Before the pandemic, children received appropriately portioned breakfasts and lunches at school, but during the pandemic, they had less access to such meals, the academy noted. Disruptions to schooling, easier access to unhealthy snacks, increased screen time, and economic issues such as parents’ job losses were further factors, Hassink said.
Tackling the weight gain
In December 2020, the AAP issued two clinical guidance documents to highlight the importance of addressing obesity during the pandemic. Recommendations included physician counseling of families about maintaining healthy nutrition, minimizing sedentary time, and getting enough sleep and physical activity, as well as the assessment of all patients for onset of obesity and the maintenance of obesity treatment for patients with obesity.
In addition to clinical assessments and guidance, Dr. Jenssen emphasized that a return to routines may be crucial. Prepandemic studies have shown that many children, especially those insured by Medicaid, gain more weight during the summer when they are out of school, he noted. Many of the same factors are present during the pandemic, he said.
“One solution, and probably the most important solution, is getting kids back in school,” Dr. Jenssen said. School disruptions also have affected children’s learning and mental health, but those effects may be harder to quantify than BMI, he said.
Dr. Jenssen suggests that parents do their best to model good routines and habits. For example, they might decide that they and their children will stop drinking soda as a family, or opt for an apple instead of a bag of chips. They can walk around the house or up and down stairs when talking. “Those sorts of little things can make a big difference in the long run,” Dr. Jenssen said.
Clinicians should address obesity in a compassionate and caring way, be aware of community resources to help families adopt healthy lifestyles, and “look for the comorbidities of obesity,” such as type 2 diabetes, liver disease, sleep apnea, knee problems, and hypertension, Dr. Hassink said.
Policies that address other factors, such as the cost of healthy foods and the marketing of unhealthy foods, may also be needed, Dr. Hassink said.
“I’ve always thought of obesity as kind of the canary in the coal mine,” Dr. Hassink said. “It is important to keep our minds on the fact that it is a chronic disease. But it also indicates a lot of things about how we are able to support a healthy population.”
Potato chips, red meat, and sugary drinks
Other researchers have assessed how healthy behaviors tended to take a turn for the worse when routines were disrupted during the pandemic. Steven B. Heymsfield, MD, a professor in the metabolism and body composition laboratory at Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and collaborators documented how diet and activity changed for children during the pandemic.
Dr. Heymsfield worked with researchers in Italy to examine changes in behavior among 41 children and adolescents with obesity in Verona, Italy, during an early lockdown.
As part of a longitudinal observational study, they had baseline data about diet and physical activity from interviews conducted from May to July 2019. They repeated the interviews 3 weeks after a mandatory quarantine.
Intake of potato chips, red meat, and sugary drinks had increased, time spent in sports activities had decreased by more than 2 hours per week, and screen time had increased by more than 4 hours per day, the researchers found. Their study was published in Obesity.
Unpublished follow-up data indicate that “there was further deterioration in the diets and activity patterns” for some but not all of the participants, Dr. Heymsfield said.
He said he was hopeful that children who experienced the onset of obesity during the pandemic may lose weight when routines return to normal, but added that it is unclear whether that will happen.
“My impression from the limited written literature on this question is that for some kids who gain weight during the lockdown or, by analogy, the summer months, the weight doesn’t go back down again. It is not universal, but it is a known phenomenon that it is a bit of a ratchet,” he said. “They just sort of slowly ratchet their weights up, up to adulthood.”
Recognizing weight gain during the pandemic may be an important first step.
“The first thing is not to ignore it,” Dr. Heymsfield said. “Anything that can be done to prevent excess weight gain during childhood – not to promote anorexia or anything like that, but just being careful – is very important, because these behaviors are formed early in life, and they persist.”
CHOP supported the research. Dr. Jenssen and Dr. Hassink have disclosed no relevant financial relationships. Dr. Heymsfield is a medical adviser for Medifast, a weight loss company.
A version of this article first appeared on Medscape.com.
Obesity rates among children jumped substantially in the first months of the COVID-19 pandemic, according to a study published online in Pediatrics. Experts worry the excess weight will be a continuing problem for these children.
“Across the board in the span of a year, there has been a 2% increase in obesity, which is really striking,” lead author Brian P. Jenssen, MD, said in an interview.
The prevalence of obesity in a large pediatric primary care network increased from 13.7% to 15.4%.
Preexisting disparities by race or ethnicity and socioeconomic status worsened, noted Dr. Jenssen, a primary care pediatrician affiliated with Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania, Philadelphia.
Dr. Jenssen and colleagues compared the average obesity rate from June to December 2020 with the rate from June to December 2019 among patients in the CHOP Care Network, which includes 29 urban, suburban, and semirural clinics in the Philadelphia region. In June 2020, the volume of patient visits “returned to near-normal” after a dramatic decline in March 2020, the study authors wrote.
The investigators examined body mass index at all visits for patients aged 2-17 years for whom height and weight were documented. Patients with a BMI at or above the 95th percentile were classified as obese. The analysis included approximately 169,000 visits in 2019 and about 145,000 in 2020.
The average age of the patients was 9.2 years, and 48.9% were girls. In all, 21.4% were non-Hispanic Black, and about 30% were publicly insured.
Increases in obesity rates were more pronounced among patients aged 5-9 years and among patients who were Hispanic/Latino, non-Hispanic Black, publicly insured, or from lower-income neighborhoods.
Whereas the obesity rate increased 1% for patients aged 13-17 years, the rate increased 2.6% for patients aged 5-9 years.
Nearly 25% of Hispanic/Latino or non-Hispanic Black patients seen during the pandemic were obese, compared with 11.3% of non-Hispanic White patients. Before the pandemic, differences by race or ethnicity had been about 10%-11%.
Limiting the analysis to preventive visits did not meaningfully change the results, wrote Dr. Jenssen and colleagues.
“Having any increase in the obesity rates is alarming,” said Sandra Hassink, MD, medical director for the American Academy of Pediatrics’ (AAP’s) Institute for Healthy Childhood Weight. “I think what we’re seeing is what we feared.”
Before the pandemic, children received appropriately portioned breakfasts and lunches at school, but during the pandemic, they had less access to such meals, the academy noted. Disruptions to schooling, easier access to unhealthy snacks, increased screen time, and economic issues such as parents’ job losses were further factors, Hassink said.
Tackling the weight gain
In December 2020, the AAP issued two clinical guidance documents to highlight the importance of addressing obesity during the pandemic. Recommendations included physician counseling of families about maintaining healthy nutrition, minimizing sedentary time, and getting enough sleep and physical activity, as well as the assessment of all patients for onset of obesity and the maintenance of obesity treatment for patients with obesity.
In addition to clinical assessments and guidance, Dr. Jenssen emphasized that a return to routines may be crucial. Prepandemic studies have shown that many children, especially those insured by Medicaid, gain more weight during the summer when they are out of school, he noted. Many of the same factors are present during the pandemic, he said.
“One solution, and probably the most important solution, is getting kids back in school,” Dr. Jenssen said. School disruptions also have affected children’s learning and mental health, but those effects may be harder to quantify than BMI, he said.
Dr. Jenssen suggests that parents do their best to model good routines and habits. For example, they might decide that they and their children will stop drinking soda as a family, or opt for an apple instead of a bag of chips. They can walk around the house or up and down stairs when talking. “Those sorts of little things can make a big difference in the long run,” Dr. Jenssen said.
Clinicians should address obesity in a compassionate and caring way, be aware of community resources to help families adopt healthy lifestyles, and “look for the comorbidities of obesity,” such as type 2 diabetes, liver disease, sleep apnea, knee problems, and hypertension, Dr. Hassink said.
Policies that address other factors, such as the cost of healthy foods and the marketing of unhealthy foods, may also be needed, Dr. Hassink said.
“I’ve always thought of obesity as kind of the canary in the coal mine,” Dr. Hassink said. “It is important to keep our minds on the fact that it is a chronic disease. But it also indicates a lot of things about how we are able to support a healthy population.”
Potato chips, red meat, and sugary drinks
Other researchers have assessed how healthy behaviors tended to take a turn for the worse when routines were disrupted during the pandemic. Steven B. Heymsfield, MD, a professor in the metabolism and body composition laboratory at Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and collaborators documented how diet and activity changed for children during the pandemic.
Dr. Heymsfield worked with researchers in Italy to examine changes in behavior among 41 children and adolescents with obesity in Verona, Italy, during an early lockdown.
As part of a longitudinal observational study, they had baseline data about diet and physical activity from interviews conducted from May to July 2019. They repeated the interviews 3 weeks after a mandatory quarantine.
Intake of potato chips, red meat, and sugary drinks had increased, time spent in sports activities had decreased by more than 2 hours per week, and screen time had increased by more than 4 hours per day, the researchers found. Their study was published in Obesity.
Unpublished follow-up data indicate that “there was further deterioration in the diets and activity patterns” for some but not all of the participants, Dr. Heymsfield said.
He said he was hopeful that children who experienced the onset of obesity during the pandemic may lose weight when routines return to normal, but added that it is unclear whether that will happen.
“My impression from the limited written literature on this question is that for some kids who gain weight during the lockdown or, by analogy, the summer months, the weight doesn’t go back down again. It is not universal, but it is a known phenomenon that it is a bit of a ratchet,” he said. “They just sort of slowly ratchet their weights up, up to adulthood.”
Recognizing weight gain during the pandemic may be an important first step.
“The first thing is not to ignore it,” Dr. Heymsfield said. “Anything that can be done to prevent excess weight gain during childhood – not to promote anorexia or anything like that, but just being careful – is very important, because these behaviors are formed early in life, and they persist.”
CHOP supported the research. Dr. Jenssen and Dr. Hassink have disclosed no relevant financial relationships. Dr. Heymsfield is a medical adviser for Medifast, a weight loss company.
A version of this article first appeared on Medscape.com.
Obesity rates among children jumped substantially in the first months of the COVID-19 pandemic, according to a study published online in Pediatrics. Experts worry the excess weight will be a continuing problem for these children.
“Across the board in the span of a year, there has been a 2% increase in obesity, which is really striking,” lead author Brian P. Jenssen, MD, said in an interview.
The prevalence of obesity in a large pediatric primary care network increased from 13.7% to 15.4%.
Preexisting disparities by race or ethnicity and socioeconomic status worsened, noted Dr. Jenssen, a primary care pediatrician affiliated with Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania, Philadelphia.
Dr. Jenssen and colleagues compared the average obesity rate from June to December 2020 with the rate from June to December 2019 among patients in the CHOP Care Network, which includes 29 urban, suburban, and semirural clinics in the Philadelphia region. In June 2020, the volume of patient visits “returned to near-normal” after a dramatic decline in March 2020, the study authors wrote.
The investigators examined body mass index at all visits for patients aged 2-17 years for whom height and weight were documented. Patients with a BMI at or above the 95th percentile were classified as obese. The analysis included approximately 169,000 visits in 2019 and about 145,000 in 2020.
The average age of the patients was 9.2 years, and 48.9% were girls. In all, 21.4% were non-Hispanic Black, and about 30% were publicly insured.
Increases in obesity rates were more pronounced among patients aged 5-9 years and among patients who were Hispanic/Latino, non-Hispanic Black, publicly insured, or from lower-income neighborhoods.
Whereas the obesity rate increased 1% for patients aged 13-17 years, the rate increased 2.6% for patients aged 5-9 years.
Nearly 25% of Hispanic/Latino or non-Hispanic Black patients seen during the pandemic were obese, compared with 11.3% of non-Hispanic White patients. Before the pandemic, differences by race or ethnicity had been about 10%-11%.
Limiting the analysis to preventive visits did not meaningfully change the results, wrote Dr. Jenssen and colleagues.
“Having any increase in the obesity rates is alarming,” said Sandra Hassink, MD, medical director for the American Academy of Pediatrics’ (AAP’s) Institute for Healthy Childhood Weight. “I think what we’re seeing is what we feared.”
Before the pandemic, children received appropriately portioned breakfasts and lunches at school, but during the pandemic, they had less access to such meals, the academy noted. Disruptions to schooling, easier access to unhealthy snacks, increased screen time, and economic issues such as parents’ job losses were further factors, Hassink said.
Tackling the weight gain
In December 2020, the AAP issued two clinical guidance documents to highlight the importance of addressing obesity during the pandemic. Recommendations included physician counseling of families about maintaining healthy nutrition, minimizing sedentary time, and getting enough sleep and physical activity, as well as the assessment of all patients for onset of obesity and the maintenance of obesity treatment for patients with obesity.
In addition to clinical assessments and guidance, Dr. Jenssen emphasized that a return to routines may be crucial. Prepandemic studies have shown that many children, especially those insured by Medicaid, gain more weight during the summer when they are out of school, he noted. Many of the same factors are present during the pandemic, he said.
“One solution, and probably the most important solution, is getting kids back in school,” Dr. Jenssen said. School disruptions also have affected children’s learning and mental health, but those effects may be harder to quantify than BMI, he said.
Dr. Jenssen suggests that parents do their best to model good routines and habits. For example, they might decide that they and their children will stop drinking soda as a family, or opt for an apple instead of a bag of chips. They can walk around the house or up and down stairs when talking. “Those sorts of little things can make a big difference in the long run,” Dr. Jenssen said.
Clinicians should address obesity in a compassionate and caring way, be aware of community resources to help families adopt healthy lifestyles, and “look for the comorbidities of obesity,” such as type 2 diabetes, liver disease, sleep apnea, knee problems, and hypertension, Dr. Hassink said.
Policies that address other factors, such as the cost of healthy foods and the marketing of unhealthy foods, may also be needed, Dr. Hassink said.
“I’ve always thought of obesity as kind of the canary in the coal mine,” Dr. Hassink said. “It is important to keep our minds on the fact that it is a chronic disease. But it also indicates a lot of things about how we are able to support a healthy population.”
Potato chips, red meat, and sugary drinks
Other researchers have assessed how healthy behaviors tended to take a turn for the worse when routines were disrupted during the pandemic. Steven B. Heymsfield, MD, a professor in the metabolism and body composition laboratory at Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and collaborators documented how diet and activity changed for children during the pandemic.
Dr. Heymsfield worked with researchers in Italy to examine changes in behavior among 41 children and adolescents with obesity in Verona, Italy, during an early lockdown.
As part of a longitudinal observational study, they had baseline data about diet and physical activity from interviews conducted from May to July 2019. They repeated the interviews 3 weeks after a mandatory quarantine.
Intake of potato chips, red meat, and sugary drinks had increased, time spent in sports activities had decreased by more than 2 hours per week, and screen time had increased by more than 4 hours per day, the researchers found. Their study was published in Obesity.
Unpublished follow-up data indicate that “there was further deterioration in the diets and activity patterns” for some but not all of the participants, Dr. Heymsfield said.
He said he was hopeful that children who experienced the onset of obesity during the pandemic may lose weight when routines return to normal, but added that it is unclear whether that will happen.
“My impression from the limited written literature on this question is that for some kids who gain weight during the lockdown or, by analogy, the summer months, the weight doesn’t go back down again. It is not universal, but it is a known phenomenon that it is a bit of a ratchet,” he said. “They just sort of slowly ratchet their weights up, up to adulthood.”
Recognizing weight gain during the pandemic may be an important first step.
“The first thing is not to ignore it,” Dr. Heymsfield said. “Anything that can be done to prevent excess weight gain during childhood – not to promote anorexia or anything like that, but just being careful – is very important, because these behaviors are formed early in life, and they persist.”
CHOP supported the research. Dr. Jenssen and Dr. Hassink have disclosed no relevant financial relationships. Dr. Heymsfield is a medical adviser for Medifast, a weight loss company.
A version of this article first appeared on Medscape.com.