User login
Dolutegravir in pregnant patients with HIV showed more viral suppression at delivery vs. other treatments
“Dolutegravir is increasingly used in pregnancy in the United States,” Kunjal Patel, DSc, one of the investigators, said in an interview. “While its effectiveness and safety in pregnancy have been compared to efavirenz in previous studies, including three randomized trials, efavirenz isn’t really used in the United States and Europe for treatment of HIV; it is mainly used in Africa,” she said. Therefore, it was important to compare dolutegravir use in pregnancy to the other antiretroviral regimens that are listed as being preferred for use in pregnancy in the U.S., including atazanavir/ritonavir, darunavir/ritonavir, and raltegravir, and others often used in the U.S. and Europe, she said.
In the study published in the New England Journal of Medicine, Dr. Patel, of Harvard T.H. Chan School of Public Health, Boston, and colleagues analyzed data from kids enrolled in the Surveillance and Monitoring for ART Toxicities Dynamic (SMARTT) cohort. This group is part of an ongoing research project focused on evaluating ART toxicities during pregnancy in children who were exposed to HIV perinatally but not infected. It included pregnancies from 2007 until January 2020 that involved use of the ARTs listed.
The study population of 1,257 pregnancies with observed birth outcomes included 120 individuals with an initial ART of dolutegravir (DTG), 464 started on atazanavir–ritonavir (ATV/r), 185 on darunavir–ritonavir (DRV/r), 243 on oral rilpivirine (RPV), 86 on raltegravir (RAL), and 159 on elvitegravir–cobicistat (EVG/c). In approximately half of the pregnancies (51%), ART was started before conception, and the initial ART was changed in 27%.
The primary outcomes were viral suppression at delivery, and adverse birth outcomes, including preterm and very preterm birth, low and very low birth weight, and neonatal death within 14 days.
The median age of the patients at conception was 29 years, and 66% were non-Hispanic Black, representative of persons with HIV of childbearing age in the United States, the researchers noted. Overall, 96.7% of the patients who received dolutegravir showed viral suppression at delivery, compared to 90.1% for darunavir–ritonavir, 89.8% for elvitegravir–cobicistat, 89.2% for raltegravir, and 84.0% for atazanavir–ritonavir.
“We expected that dolutegravir to be similar with regards to viral suppression at delivery compared to raltegravir so were surprised that we observed less viral suppression with raltegravir compared to dolutegravir,” Dr. Patel said in an interview. “Our results may be due to the higher pill burden and lower barrier to resistance with RAL compared to dolutegravir, but we did not assess adherence or resistance in our study,” she noted.
Across ART regimens, the observed risks of preterm birth ranged from 13.6% to 17.6%, risks of low birth weight ranged from 11.9% to 16.7%, and risks of being small for gestational age ranged from 9.1% to 12.5%. For the composite of any adverse birth outcome and any severe adverse birth outcome, the observed risks ranged from 22.6% to 27.9% and 0% to 4.2%, respectively.
A total of 20 very preterm births, including 15 infants with very low birth weight, occurred across patients receiving all ART regimens, and no neonatal deaths occurred. The researchers found no apparent patterns of differences in the observed risk of adverse birth outcomes across all groups related to the timing of ART initiation in pregnancy, but the risks were greater among those who began the drugs during pregnancy compared to those who began before conception.
“Our results confirm the recommendation of DTG as “preferred” in U.S. perinatal guidelines, and provide evidence suggesting ATV/r and RAL provides lower HIV viral suppression at delivery compared to DTG, and support DRV/r as a reasonable alternative when DTG use is not feasible,” Dr. Patel said in an interview.
“With regards to next steps, we are interested in comparing the effectiveness and safety of dolutegravir-based regimens that include tenofovir alafenamide (TAF) vs. tenofovir disoproxil fumarate (TDF) in our U.S. setting,” she said.
The study findings were limited by several factors including the lack of data on predictors of preterm birth and low birth weight, such as previous preterm birth and prepregnancy body mass index, the researchers noted.
However, the results indicate that other common ARTs provide less HIV viral suppression at delivery than dolutegravir, with similar adverse birth outcomes; the results also support darunavir–ritonavir as a reasonable alternative when dolutegravir use is not feasible, as it showed the next highest level of viral suppression after dolutegravir, the researchers concluded.
Findings fill a key research gap
The current study is important given the limited data on effectiveness and outcomes in pregnancy with the use of contemporary HIV regimens in the United States, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Pregnancy is still among exclusion criteria for most drug studies,” said Dr. Badell, who was not involved in the current study. “Dolutegravir-based ART is first line in the U.S. today because of its effectiveness, lower side effects, and higher barrier to resistance; therefore understanding the benefits and birth outcomes in pregnancy is critical,” she explained.
Dr. Badell said she was not surprised by the study findings. “However it is very reassuring to see in a large observational study comparing the dolutegravir regimens to other contemporary regimens in pregnancy, such a high level of viral suppression and no increased risk of adverse perinatal outcomes,” she said.
The study findings will impact clinical practice by reaffirming patient counseling regarding the use of dolutegravir in pregnancy, said Dr. Badell. “The use of ART in pregnancy is complex given the number of drug choices, whether the patient was on ART prior to pregnancy or initiated during pregnancy, and the various factors other than ART that affect perinatal outcomes, such as preterm birth and congenital anomalies, she explained.
The finding that the risk of adverse outcomes was higher for those who initiated ART during pregnancy vs. those who were already on ARTs when they became pregnant contradicts some previous research, said Dr. Badell. But this is “reassuring, as we highly recommend ART with viral suppression prior to pregnancy or to start as early as possible in pregnancy.”
Adverse birth outcomes can be affected by many variables such as age, substance abuse, prior adverse birth outcome and other factors, and larger studies that control for these variables will allow better evaluation of the effect of the ART drugs, Dr. Badell added.
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, along with the Office of the Director, National Institutes of Health; National Institute of Dental and Craniofacial Research; National Institute of Allergy and Infectious Diseases; National Institute of Neurological Disorders and Stroke; National Institute on Deafness and Other Communication Disorders; National Institute of Mental Health; National Institute on Drug Abuse; National Cancer Institute; National Institute on Alcohol Abuse and Alcoholism; and National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T.H. Chan School of Public Health and the Tulane University School of Medicine.
The researchers and Dr. Badell had no financial conflicts to disclose.
“Dolutegravir is increasingly used in pregnancy in the United States,” Kunjal Patel, DSc, one of the investigators, said in an interview. “While its effectiveness and safety in pregnancy have been compared to efavirenz in previous studies, including three randomized trials, efavirenz isn’t really used in the United States and Europe for treatment of HIV; it is mainly used in Africa,” she said. Therefore, it was important to compare dolutegravir use in pregnancy to the other antiretroviral regimens that are listed as being preferred for use in pregnancy in the U.S., including atazanavir/ritonavir, darunavir/ritonavir, and raltegravir, and others often used in the U.S. and Europe, she said.
In the study published in the New England Journal of Medicine, Dr. Patel, of Harvard T.H. Chan School of Public Health, Boston, and colleagues analyzed data from kids enrolled in the Surveillance and Monitoring for ART Toxicities Dynamic (SMARTT) cohort. This group is part of an ongoing research project focused on evaluating ART toxicities during pregnancy in children who were exposed to HIV perinatally but not infected. It included pregnancies from 2007 until January 2020 that involved use of the ARTs listed.
The study population of 1,257 pregnancies with observed birth outcomes included 120 individuals with an initial ART of dolutegravir (DTG), 464 started on atazanavir–ritonavir (ATV/r), 185 on darunavir–ritonavir (DRV/r), 243 on oral rilpivirine (RPV), 86 on raltegravir (RAL), and 159 on elvitegravir–cobicistat (EVG/c). In approximately half of the pregnancies (51%), ART was started before conception, and the initial ART was changed in 27%.
The primary outcomes were viral suppression at delivery, and adverse birth outcomes, including preterm and very preterm birth, low and very low birth weight, and neonatal death within 14 days.
The median age of the patients at conception was 29 years, and 66% were non-Hispanic Black, representative of persons with HIV of childbearing age in the United States, the researchers noted. Overall, 96.7% of the patients who received dolutegravir showed viral suppression at delivery, compared to 90.1% for darunavir–ritonavir, 89.8% for elvitegravir–cobicistat, 89.2% for raltegravir, and 84.0% for atazanavir–ritonavir.
“We expected that dolutegravir to be similar with regards to viral suppression at delivery compared to raltegravir so were surprised that we observed less viral suppression with raltegravir compared to dolutegravir,” Dr. Patel said in an interview. “Our results may be due to the higher pill burden and lower barrier to resistance with RAL compared to dolutegravir, but we did not assess adherence or resistance in our study,” she noted.
Across ART regimens, the observed risks of preterm birth ranged from 13.6% to 17.6%, risks of low birth weight ranged from 11.9% to 16.7%, and risks of being small for gestational age ranged from 9.1% to 12.5%. For the composite of any adverse birth outcome and any severe adverse birth outcome, the observed risks ranged from 22.6% to 27.9% and 0% to 4.2%, respectively.
A total of 20 very preterm births, including 15 infants with very low birth weight, occurred across patients receiving all ART regimens, and no neonatal deaths occurred. The researchers found no apparent patterns of differences in the observed risk of adverse birth outcomes across all groups related to the timing of ART initiation in pregnancy, but the risks were greater among those who began the drugs during pregnancy compared to those who began before conception.
“Our results confirm the recommendation of DTG as “preferred” in U.S. perinatal guidelines, and provide evidence suggesting ATV/r and RAL provides lower HIV viral suppression at delivery compared to DTG, and support DRV/r as a reasonable alternative when DTG use is not feasible,” Dr. Patel said in an interview.
“With regards to next steps, we are interested in comparing the effectiveness and safety of dolutegravir-based regimens that include tenofovir alafenamide (TAF) vs. tenofovir disoproxil fumarate (TDF) in our U.S. setting,” she said.
The study findings were limited by several factors including the lack of data on predictors of preterm birth and low birth weight, such as previous preterm birth and prepregnancy body mass index, the researchers noted.
However, the results indicate that other common ARTs provide less HIV viral suppression at delivery than dolutegravir, with similar adverse birth outcomes; the results also support darunavir–ritonavir as a reasonable alternative when dolutegravir use is not feasible, as it showed the next highest level of viral suppression after dolutegravir, the researchers concluded.
Findings fill a key research gap
The current study is important given the limited data on effectiveness and outcomes in pregnancy with the use of contemporary HIV regimens in the United States, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Pregnancy is still among exclusion criteria for most drug studies,” said Dr. Badell, who was not involved in the current study. “Dolutegravir-based ART is first line in the U.S. today because of its effectiveness, lower side effects, and higher barrier to resistance; therefore understanding the benefits and birth outcomes in pregnancy is critical,” she explained.
Dr. Badell said she was not surprised by the study findings. “However it is very reassuring to see in a large observational study comparing the dolutegravir regimens to other contemporary regimens in pregnancy, such a high level of viral suppression and no increased risk of adverse perinatal outcomes,” she said.
The study findings will impact clinical practice by reaffirming patient counseling regarding the use of dolutegravir in pregnancy, said Dr. Badell. “The use of ART in pregnancy is complex given the number of drug choices, whether the patient was on ART prior to pregnancy or initiated during pregnancy, and the various factors other than ART that affect perinatal outcomes, such as preterm birth and congenital anomalies, she explained.
The finding that the risk of adverse outcomes was higher for those who initiated ART during pregnancy vs. those who were already on ARTs when they became pregnant contradicts some previous research, said Dr. Badell. But this is “reassuring, as we highly recommend ART with viral suppression prior to pregnancy or to start as early as possible in pregnancy.”
Adverse birth outcomes can be affected by many variables such as age, substance abuse, prior adverse birth outcome and other factors, and larger studies that control for these variables will allow better evaluation of the effect of the ART drugs, Dr. Badell added.
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, along with the Office of the Director, National Institutes of Health; National Institute of Dental and Craniofacial Research; National Institute of Allergy and Infectious Diseases; National Institute of Neurological Disorders and Stroke; National Institute on Deafness and Other Communication Disorders; National Institute of Mental Health; National Institute on Drug Abuse; National Cancer Institute; National Institute on Alcohol Abuse and Alcoholism; and National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T.H. Chan School of Public Health and the Tulane University School of Medicine.
The researchers and Dr. Badell had no financial conflicts to disclose.
“Dolutegravir is increasingly used in pregnancy in the United States,” Kunjal Patel, DSc, one of the investigators, said in an interview. “While its effectiveness and safety in pregnancy have been compared to efavirenz in previous studies, including three randomized trials, efavirenz isn’t really used in the United States and Europe for treatment of HIV; it is mainly used in Africa,” she said. Therefore, it was important to compare dolutegravir use in pregnancy to the other antiretroviral regimens that are listed as being preferred for use in pregnancy in the U.S., including atazanavir/ritonavir, darunavir/ritonavir, and raltegravir, and others often used in the U.S. and Europe, she said.
In the study published in the New England Journal of Medicine, Dr. Patel, of Harvard T.H. Chan School of Public Health, Boston, and colleagues analyzed data from kids enrolled in the Surveillance and Monitoring for ART Toxicities Dynamic (SMARTT) cohort. This group is part of an ongoing research project focused on evaluating ART toxicities during pregnancy in children who were exposed to HIV perinatally but not infected. It included pregnancies from 2007 until January 2020 that involved use of the ARTs listed.
The study population of 1,257 pregnancies with observed birth outcomes included 120 individuals with an initial ART of dolutegravir (DTG), 464 started on atazanavir–ritonavir (ATV/r), 185 on darunavir–ritonavir (DRV/r), 243 on oral rilpivirine (RPV), 86 on raltegravir (RAL), and 159 on elvitegravir–cobicistat (EVG/c). In approximately half of the pregnancies (51%), ART was started before conception, and the initial ART was changed in 27%.
The primary outcomes were viral suppression at delivery, and adverse birth outcomes, including preterm and very preterm birth, low and very low birth weight, and neonatal death within 14 days.
The median age of the patients at conception was 29 years, and 66% were non-Hispanic Black, representative of persons with HIV of childbearing age in the United States, the researchers noted. Overall, 96.7% of the patients who received dolutegravir showed viral suppression at delivery, compared to 90.1% for darunavir–ritonavir, 89.8% for elvitegravir–cobicistat, 89.2% for raltegravir, and 84.0% for atazanavir–ritonavir.
“We expected that dolutegravir to be similar with regards to viral suppression at delivery compared to raltegravir so were surprised that we observed less viral suppression with raltegravir compared to dolutegravir,” Dr. Patel said in an interview. “Our results may be due to the higher pill burden and lower barrier to resistance with RAL compared to dolutegravir, but we did not assess adherence or resistance in our study,” she noted.
Across ART regimens, the observed risks of preterm birth ranged from 13.6% to 17.6%, risks of low birth weight ranged from 11.9% to 16.7%, and risks of being small for gestational age ranged from 9.1% to 12.5%. For the composite of any adverse birth outcome and any severe adverse birth outcome, the observed risks ranged from 22.6% to 27.9% and 0% to 4.2%, respectively.
A total of 20 very preterm births, including 15 infants with very low birth weight, occurred across patients receiving all ART regimens, and no neonatal deaths occurred. The researchers found no apparent patterns of differences in the observed risk of adverse birth outcomes across all groups related to the timing of ART initiation in pregnancy, but the risks were greater among those who began the drugs during pregnancy compared to those who began before conception.
“Our results confirm the recommendation of DTG as “preferred” in U.S. perinatal guidelines, and provide evidence suggesting ATV/r and RAL provides lower HIV viral suppression at delivery compared to DTG, and support DRV/r as a reasonable alternative when DTG use is not feasible,” Dr. Patel said in an interview.
“With regards to next steps, we are interested in comparing the effectiveness and safety of dolutegravir-based regimens that include tenofovir alafenamide (TAF) vs. tenofovir disoproxil fumarate (TDF) in our U.S. setting,” she said.
The study findings were limited by several factors including the lack of data on predictors of preterm birth and low birth weight, such as previous preterm birth and prepregnancy body mass index, the researchers noted.
However, the results indicate that other common ARTs provide less HIV viral suppression at delivery than dolutegravir, with similar adverse birth outcomes; the results also support darunavir–ritonavir as a reasonable alternative when dolutegravir use is not feasible, as it showed the next highest level of viral suppression after dolutegravir, the researchers concluded.
Findings fill a key research gap
The current study is important given the limited data on effectiveness and outcomes in pregnancy with the use of contemporary HIV regimens in the United States, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Pregnancy is still among exclusion criteria for most drug studies,” said Dr. Badell, who was not involved in the current study. “Dolutegravir-based ART is first line in the U.S. today because of its effectiveness, lower side effects, and higher barrier to resistance; therefore understanding the benefits and birth outcomes in pregnancy is critical,” she explained.
Dr. Badell said she was not surprised by the study findings. “However it is very reassuring to see in a large observational study comparing the dolutegravir regimens to other contemporary regimens in pregnancy, such a high level of viral suppression and no increased risk of adverse perinatal outcomes,” she said.
The study findings will impact clinical practice by reaffirming patient counseling regarding the use of dolutegravir in pregnancy, said Dr. Badell. “The use of ART in pregnancy is complex given the number of drug choices, whether the patient was on ART prior to pregnancy or initiated during pregnancy, and the various factors other than ART that affect perinatal outcomes, such as preterm birth and congenital anomalies, she explained.
The finding that the risk of adverse outcomes was higher for those who initiated ART during pregnancy vs. those who were already on ARTs when they became pregnant contradicts some previous research, said Dr. Badell. But this is “reassuring, as we highly recommend ART with viral suppression prior to pregnancy or to start as early as possible in pregnancy.”
Adverse birth outcomes can be affected by many variables such as age, substance abuse, prior adverse birth outcome and other factors, and larger studies that control for these variables will allow better evaluation of the effect of the ART drugs, Dr. Badell added.
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, along with the Office of the Director, National Institutes of Health; National Institute of Dental and Craniofacial Research; National Institute of Allergy and Infectious Diseases; National Institute of Neurological Disorders and Stroke; National Institute on Deafness and Other Communication Disorders; National Institute of Mental Health; National Institute on Drug Abuse; National Cancer Institute; National Institute on Alcohol Abuse and Alcoholism; and National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T.H. Chan School of Public Health and the Tulane University School of Medicine.
The researchers and Dr. Badell had no financial conflicts to disclose.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Many young kids with COVID may show no symptoms
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
FROM JAMA NETWORK OPEN
Abbott to start making Similac baby formula again
Abbott Nutrition is resuming production of Similac, its leading baby formula, at a Michigan plant that was shut down earlier in 2022 because of contamination concerns.
The company closed the plant in February, which triggered a national shortage of baby formula amid pandemic-related supply chain issues that created a lack of formula ingredients.
“We know that the nationwide infant formula shortage has been difficult for the families we serve, and while restarting Similac production in Michigan is an important milestone, we won’t rest until this product is back on shelves,” Robert Ford, chairman and CEO of Abbott, said in a statement on Aug. 26.
“Making infant formula is a responsibility we take very seriously, and parents can feel confident in the quality and safety of Similac and other Abbott formulas,” he said. “We are committed to re-earning the trust parents and health care providers have placed in us for decades.”
Abbott estimated that it will take about 6 weeks for Similac products to ship to stores. Production has restarted, which will be followed by “enhanced” testing before and after the formula is made.
In February, Abbott voluntarily recalled batches of three formulas after the Food and Drug Administration received consumer complaints about infants becoming sick. Four babies who consumed formulas from the Michigan plant got bacterial infections, and at least two babies died.
The illnesses were linked to Cronobacter sakazakii – bacteria that can lead to life-threatening infections and inflammation of the brain and spine.
After investigations at the plant, Abbott said there is no conclusive evidence to link the formula to the illnesses. No samples of the recalled product tested positive for the bacteria, and in all four cases, unopened containers of formula in the infants’ homes tested negative for the bacteria.
At the same time, FDA officials said in May that the Michigan plant had a leaking roof, water pooling on the floor, and cracks in production equipment that could allow bacteria to grow, according to The New York Times.
Abbott agreed with the federal government to create new safeguards, such as hiring a qualified expert to oversee improvements at the plant and notify the FDA if any issues were identified, the newspaper reported.
On July 1, the company restarted production of EleCare, a specialty formula, and later resumed production of some metabolic formulas. These products will begin to ship in coming weeks, the company said.
Since July, C. sakazakii has been found in a couple of batches of formula.
“In those cases, we found the issue, addressed it and no affected product has been or will be distributed,” Abbott said in the statement. “This confirms our quality systems work.”
In August, Abbott will supply the United States with more than 8 million pounds of infant formula, which is higher than the levels in August 2021, the company said. To ensure that people in the federal Special Supplemental Nutrition Program for Women, Infants and Children have access to formula, the company is extending rebates until the end of October.
“Restarting a large manufacturing facility after a several-month shutdown is a complex process, and it takes time to ensure that equipment, processes and production are functioning smoothly and sustainably,” the company said in the statement. “There have been – and likely will be – stops and starts from time to time. We’ve experienced events like severe weather, we’ve had to make mechanical adjustments, and we’ve had to discard some early production batches that didn’t meet our standards.”
A version of this article first appeared on WebMD.com.
Abbott Nutrition is resuming production of Similac, its leading baby formula, at a Michigan plant that was shut down earlier in 2022 because of contamination concerns.
The company closed the plant in February, which triggered a national shortage of baby formula amid pandemic-related supply chain issues that created a lack of formula ingredients.
“We know that the nationwide infant formula shortage has been difficult for the families we serve, and while restarting Similac production in Michigan is an important milestone, we won’t rest until this product is back on shelves,” Robert Ford, chairman and CEO of Abbott, said in a statement on Aug. 26.
“Making infant formula is a responsibility we take very seriously, and parents can feel confident in the quality and safety of Similac and other Abbott formulas,” he said. “We are committed to re-earning the trust parents and health care providers have placed in us for decades.”
Abbott estimated that it will take about 6 weeks for Similac products to ship to stores. Production has restarted, which will be followed by “enhanced” testing before and after the formula is made.
In February, Abbott voluntarily recalled batches of three formulas after the Food and Drug Administration received consumer complaints about infants becoming sick. Four babies who consumed formulas from the Michigan plant got bacterial infections, and at least two babies died.
The illnesses were linked to Cronobacter sakazakii – bacteria that can lead to life-threatening infections and inflammation of the brain and spine.
After investigations at the plant, Abbott said there is no conclusive evidence to link the formula to the illnesses. No samples of the recalled product tested positive for the bacteria, and in all four cases, unopened containers of formula in the infants’ homes tested negative for the bacteria.
At the same time, FDA officials said in May that the Michigan plant had a leaking roof, water pooling on the floor, and cracks in production equipment that could allow bacteria to grow, according to The New York Times.
Abbott agreed with the federal government to create new safeguards, such as hiring a qualified expert to oversee improvements at the plant and notify the FDA if any issues were identified, the newspaper reported.
On July 1, the company restarted production of EleCare, a specialty formula, and later resumed production of some metabolic formulas. These products will begin to ship in coming weeks, the company said.
Since July, C. sakazakii has been found in a couple of batches of formula.
“In those cases, we found the issue, addressed it and no affected product has been or will be distributed,” Abbott said in the statement. “This confirms our quality systems work.”
In August, Abbott will supply the United States with more than 8 million pounds of infant formula, which is higher than the levels in August 2021, the company said. To ensure that people in the federal Special Supplemental Nutrition Program for Women, Infants and Children have access to formula, the company is extending rebates until the end of October.
“Restarting a large manufacturing facility after a several-month shutdown is a complex process, and it takes time to ensure that equipment, processes and production are functioning smoothly and sustainably,” the company said in the statement. “There have been – and likely will be – stops and starts from time to time. We’ve experienced events like severe weather, we’ve had to make mechanical adjustments, and we’ve had to discard some early production batches that didn’t meet our standards.”
A version of this article first appeared on WebMD.com.
Abbott Nutrition is resuming production of Similac, its leading baby formula, at a Michigan plant that was shut down earlier in 2022 because of contamination concerns.
The company closed the plant in February, which triggered a national shortage of baby formula amid pandemic-related supply chain issues that created a lack of formula ingredients.
“We know that the nationwide infant formula shortage has been difficult for the families we serve, and while restarting Similac production in Michigan is an important milestone, we won’t rest until this product is back on shelves,” Robert Ford, chairman and CEO of Abbott, said in a statement on Aug. 26.
“Making infant formula is a responsibility we take very seriously, and parents can feel confident in the quality and safety of Similac and other Abbott formulas,” he said. “We are committed to re-earning the trust parents and health care providers have placed in us for decades.”
Abbott estimated that it will take about 6 weeks for Similac products to ship to stores. Production has restarted, which will be followed by “enhanced” testing before and after the formula is made.
In February, Abbott voluntarily recalled batches of three formulas after the Food and Drug Administration received consumer complaints about infants becoming sick. Four babies who consumed formulas from the Michigan plant got bacterial infections, and at least two babies died.
The illnesses were linked to Cronobacter sakazakii – bacteria that can lead to life-threatening infections and inflammation of the brain and spine.
After investigations at the plant, Abbott said there is no conclusive evidence to link the formula to the illnesses. No samples of the recalled product tested positive for the bacteria, and in all four cases, unopened containers of formula in the infants’ homes tested negative for the bacteria.
At the same time, FDA officials said in May that the Michigan plant had a leaking roof, water pooling on the floor, and cracks in production equipment that could allow bacteria to grow, according to The New York Times.
Abbott agreed with the federal government to create new safeguards, such as hiring a qualified expert to oversee improvements at the plant and notify the FDA if any issues were identified, the newspaper reported.
On July 1, the company restarted production of EleCare, a specialty formula, and later resumed production of some metabolic formulas. These products will begin to ship in coming weeks, the company said.
Since July, C. sakazakii has been found in a couple of batches of formula.
“In those cases, we found the issue, addressed it and no affected product has been or will be distributed,” Abbott said in the statement. “This confirms our quality systems work.”
In August, Abbott will supply the United States with more than 8 million pounds of infant formula, which is higher than the levels in August 2021, the company said. To ensure that people in the federal Special Supplemental Nutrition Program for Women, Infants and Children have access to formula, the company is extending rebates until the end of October.
“Restarting a large manufacturing facility after a several-month shutdown is a complex process, and it takes time to ensure that equipment, processes and production are functioning smoothly and sustainably,” the company said in the statement. “There have been – and likely will be – stops and starts from time to time. We’ve experienced events like severe weather, we’ve had to make mechanical adjustments, and we’ve had to discard some early production batches that didn’t meet our standards.”
A version of this article first appeared on WebMD.com.
Mother’s fat metabolism in early pregnancy linked to baby’s weight
A baby’s weight and neurodevelopment in the first 2 years of life could be influenced by maternal fat metabolism in the early stages of pregnancy, according to a study.
Patterns of fetal abdominal growth were associated with maternal lipid metabolites that tracked newborn growth, adiposity, and development into childhood and could be identified as early as the 5th month of pregnancy, according to researchers at the University of Oxford, working with colleagues at the University of California.
These fetal growth patterns were also associated with blood flow and nutrient transfer by the placenta, demonstrating a complex interaction between maternal and fetal nutrition early in pregnancy, with implications for postnatal weight and health in later life, they suggested.
Stephen Kennedy, MD, professor of reproductive medicine at the University of Oxford, who co-led the investigation, said it had “provided valuable new insights into the biological origins of childhood obesity, which is one of the most pressing public health issues facing governments around the world.”
International study
The prospective observational study, published in The Lancet Diabetes and Endocrinology, involved 3,598 pregnant women from six countries – Brazil, Kenya, Pakistan, South Africa, Thailand, and the United Kingdom – aged 18 and older and with a BMI of less than 35 kg/m2. The women were monitored using regular fetal ultrasound scans and metabolomic analysis of early pregnancy maternal blood and umbilical cord venous blood at the time of birth.
Their infants, who were singletons, and conceived naturally, were then followed for 2 years to assess their growth and development.
Fetal abdominal circumference growth was found to accelerate or decelerate within “a crucial 20-25 week gestational age window” that followed 4 trajectories of faltering growth, early accelerating growth, late accelerating growth, or median growth. These traits were matched by fetus-placenta blood flow patterns throughout pregnancy and different growth, adiposity, vision, and neurodevelopment outcomes in early childhood, researchers said.
Overall, 709 maternal metabolites had a positive effect for the faltering growth phenotype, and 54 for the early accelerating growth phenotype, whilst 31 had a negative effect for the faltering growth phenotype and 76 for the early accelerating growth phenotype.
The maternal metabolite signatures included 5-hydroxy-eicosatetraenoic acid and 11 phosphatidylcholines linked to oxylipin or saturated fatty acid sidechains. The fungicide, chlorothalonil, was “highly abundant” in the early accelerating growth phenotype group.
‘A unique insight’
Aris Papageorghiou, professor of Fetal Medicine at the University of Oxford, who co-led the research, said: “This study provides evidence of distinct patterns of fetal abdominal growth and placental transfer and how they relate to longer term health. The finding of an association with maternal lipid metabolism early in pregnancy also provides unique insights into how the mother’s health and diet influence her child’s adiposity.”
First author José Villar, MD, professor of perinatal medicine at Oxford, said: “The study complements our previous work that identified fetal head growth trajectories associated with different developmental, behavioral, visual, and growth outcomes at 2 years of age.” Taken together, “the growth of babies’ bodies and brain[s] track separately and early – while still within the womb,” he said.
According to Dr. Kennedy, the latest results “could contribute to earlier identification of infants at risk of obesity” and urged policymakers to “take notice of these findings in their efforts to prevent the oncoming epidemic of obesity, with all its likely adverse social and economic consequences.”
Funding for the study was provided by the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape UK.
A baby’s weight and neurodevelopment in the first 2 years of life could be influenced by maternal fat metabolism in the early stages of pregnancy, according to a study.
Patterns of fetal abdominal growth were associated with maternal lipid metabolites that tracked newborn growth, adiposity, and development into childhood and could be identified as early as the 5th month of pregnancy, according to researchers at the University of Oxford, working with colleagues at the University of California.
These fetal growth patterns were also associated with blood flow and nutrient transfer by the placenta, demonstrating a complex interaction between maternal and fetal nutrition early in pregnancy, with implications for postnatal weight and health in later life, they suggested.
Stephen Kennedy, MD, professor of reproductive medicine at the University of Oxford, who co-led the investigation, said it had “provided valuable new insights into the biological origins of childhood obesity, which is one of the most pressing public health issues facing governments around the world.”
International study
The prospective observational study, published in The Lancet Diabetes and Endocrinology, involved 3,598 pregnant women from six countries – Brazil, Kenya, Pakistan, South Africa, Thailand, and the United Kingdom – aged 18 and older and with a BMI of less than 35 kg/m2. The women were monitored using regular fetal ultrasound scans and metabolomic analysis of early pregnancy maternal blood and umbilical cord venous blood at the time of birth.
Their infants, who were singletons, and conceived naturally, were then followed for 2 years to assess their growth and development.
Fetal abdominal circumference growth was found to accelerate or decelerate within “a crucial 20-25 week gestational age window” that followed 4 trajectories of faltering growth, early accelerating growth, late accelerating growth, or median growth. These traits were matched by fetus-placenta blood flow patterns throughout pregnancy and different growth, adiposity, vision, and neurodevelopment outcomes in early childhood, researchers said.
Overall, 709 maternal metabolites had a positive effect for the faltering growth phenotype, and 54 for the early accelerating growth phenotype, whilst 31 had a negative effect for the faltering growth phenotype and 76 for the early accelerating growth phenotype.
The maternal metabolite signatures included 5-hydroxy-eicosatetraenoic acid and 11 phosphatidylcholines linked to oxylipin or saturated fatty acid sidechains. The fungicide, chlorothalonil, was “highly abundant” in the early accelerating growth phenotype group.
‘A unique insight’
Aris Papageorghiou, professor of Fetal Medicine at the University of Oxford, who co-led the research, said: “This study provides evidence of distinct patterns of fetal abdominal growth and placental transfer and how they relate to longer term health. The finding of an association with maternal lipid metabolism early in pregnancy also provides unique insights into how the mother’s health and diet influence her child’s adiposity.”
First author José Villar, MD, professor of perinatal medicine at Oxford, said: “The study complements our previous work that identified fetal head growth trajectories associated with different developmental, behavioral, visual, and growth outcomes at 2 years of age.” Taken together, “the growth of babies’ bodies and brain[s] track separately and early – while still within the womb,” he said.
According to Dr. Kennedy, the latest results “could contribute to earlier identification of infants at risk of obesity” and urged policymakers to “take notice of these findings in their efforts to prevent the oncoming epidemic of obesity, with all its likely adverse social and economic consequences.”
Funding for the study was provided by the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape UK.
A baby’s weight and neurodevelopment in the first 2 years of life could be influenced by maternal fat metabolism in the early stages of pregnancy, according to a study.
Patterns of fetal abdominal growth were associated with maternal lipid metabolites that tracked newborn growth, adiposity, and development into childhood and could be identified as early as the 5th month of pregnancy, according to researchers at the University of Oxford, working with colleagues at the University of California.
These fetal growth patterns were also associated with blood flow and nutrient transfer by the placenta, demonstrating a complex interaction between maternal and fetal nutrition early in pregnancy, with implications for postnatal weight and health in later life, they suggested.
Stephen Kennedy, MD, professor of reproductive medicine at the University of Oxford, who co-led the investigation, said it had “provided valuable new insights into the biological origins of childhood obesity, which is one of the most pressing public health issues facing governments around the world.”
International study
The prospective observational study, published in The Lancet Diabetes and Endocrinology, involved 3,598 pregnant women from six countries – Brazil, Kenya, Pakistan, South Africa, Thailand, and the United Kingdom – aged 18 and older and with a BMI of less than 35 kg/m2. The women were monitored using regular fetal ultrasound scans and metabolomic analysis of early pregnancy maternal blood and umbilical cord venous blood at the time of birth.
Their infants, who were singletons, and conceived naturally, were then followed for 2 years to assess their growth and development.
Fetal abdominal circumference growth was found to accelerate or decelerate within “a crucial 20-25 week gestational age window” that followed 4 trajectories of faltering growth, early accelerating growth, late accelerating growth, or median growth. These traits were matched by fetus-placenta blood flow patterns throughout pregnancy and different growth, adiposity, vision, and neurodevelopment outcomes in early childhood, researchers said.
Overall, 709 maternal metabolites had a positive effect for the faltering growth phenotype, and 54 for the early accelerating growth phenotype, whilst 31 had a negative effect for the faltering growth phenotype and 76 for the early accelerating growth phenotype.
The maternal metabolite signatures included 5-hydroxy-eicosatetraenoic acid and 11 phosphatidylcholines linked to oxylipin or saturated fatty acid sidechains. The fungicide, chlorothalonil, was “highly abundant” in the early accelerating growth phenotype group.
‘A unique insight’
Aris Papageorghiou, professor of Fetal Medicine at the University of Oxford, who co-led the research, said: “This study provides evidence of distinct patterns of fetal abdominal growth and placental transfer and how they relate to longer term health. The finding of an association with maternal lipid metabolism early in pregnancy also provides unique insights into how the mother’s health and diet influence her child’s adiposity.”
First author José Villar, MD, professor of perinatal medicine at Oxford, said: “The study complements our previous work that identified fetal head growth trajectories associated with different developmental, behavioral, visual, and growth outcomes at 2 years of age.” Taken together, “the growth of babies’ bodies and brain[s] track separately and early – while still within the womb,” he said.
According to Dr. Kennedy, the latest results “could contribute to earlier identification of infants at risk of obesity” and urged policymakers to “take notice of these findings in their efforts to prevent the oncoming epidemic of obesity, with all its likely adverse social and economic consequences.”
Funding for the study was provided by the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape UK.
How much do we really know about gender dysphoria?
At the risk of losing a digit or two I am going to dip my toes into the murky waters of gender-affirming care, sometimes referred to as trans care. Recently, Moira Szilagyi, MD, PhD, president of the American Academy of Pediatrics, released two statements, one in the Aug. 22, 2022, Wall Street Journal, the other summarized in the Aug. 25, 2022, AAP Daily Briefing, in which she attempts to clarify the academy’s position on gender-affirming care. They were well-worded and heroic attempts to clear the air. I fear these explanations will do little to encourage informed and courteous discussions between those entrenched on either side of a disagreement that is unfortunately being played out on media outlets and state legislatures instead of the offices of primary care physicians and specialists where it belongs.
The current mess is an example of what can happen when there is a paucity of reliable data, a superabundance of emotion, and a system that feeds on instant news and sound bites with little understanding of how science should work.
Some of the turmoil is a response to the notion that in certain situations gender dysphoria may be a condition that can be learned or mimicked from exposure to other gender-dysphoric individuals. Two papers anchor either side of the debate. The first paper was published in 2018 by a then–Brown University health expert who hypothesized the existence of a condition which she labeled “rapid-onset gender dysphoria [ROGD]”. One can imagine that “social contagion” might be considered as one of the potential contributors to this hypothesized condition. Unfortunately, the publication of the paper ignited a firestorm of criticism from a segment of the population that advocates for the transgender community, prompting the university and the online publisher to backpedal and reevaluate the quality of the research on which the paper was based.
One of the concerns voiced at the time of publication was that the research could be used to support the transphobic agenda by some state legislatures hoping to ban gender-affirming care. How large a role the paper played in the current spate of legislation in is unclear. I suspect it has been small. But, one can’t deny the potential exists.
Leaping forward to 2022, the second paper was published in the August issue of Pediatrics, in which the authors attempted to test the ROGD hypothesis and question the inference of social contagion.
The investigators found that in 2017 and 2019 the birth ratios of transgender-diverse (TGD) individuals did not favor assigned female-sex-at-birth (AFAB) individuals. They also discovered that in their sample overall there was a decrease in the percentage of adolescents who self-identified as TGD. Not surprisingly, “bullying victimization and suicidality were higher among TGD youth when compared with their cisgender peers.” The authors concluded that their findings were “incongruent with an ROGD hypothesis that posits social contagion” nor should it be used to restrict access to gender-affirming care.
There you have it. Are we any closer to understanding gender dysphoria and its origins? I don’t think so. The media is somewhat less confused. The NBC News online presence headline on Aug. 3, 2022, reads “‘Social contagion’ isn’t causing more youths to be transgender, study finds.”
My sense is that the general population perceives an increase in the prevalence of gender dysphoria. It is very likely that this perception is primarily a reflection of a more compassionate and educated attitude in a significant portion of the population making it less challenging for gender-dysphoric youth to surface. However, it should not surprise us that some parents and observers are concerned that a percentage of this increased prevalence is the result of social contagion. Nor should it surprise us that some advocates for the trans population feel threatened by this hypothesis.
Neither of these studies really answers the question of whether some cases of gender dysphoria are the result of social contagion. Both were small samples using methodology that has been called into question. The bottom line is that we need more studies and must remain open to considering their results. That’s how science should work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
At the risk of losing a digit or two I am going to dip my toes into the murky waters of gender-affirming care, sometimes referred to as trans care. Recently, Moira Szilagyi, MD, PhD, president of the American Academy of Pediatrics, released two statements, one in the Aug. 22, 2022, Wall Street Journal, the other summarized in the Aug. 25, 2022, AAP Daily Briefing, in which she attempts to clarify the academy’s position on gender-affirming care. They were well-worded and heroic attempts to clear the air. I fear these explanations will do little to encourage informed and courteous discussions between those entrenched on either side of a disagreement that is unfortunately being played out on media outlets and state legislatures instead of the offices of primary care physicians and specialists where it belongs.
The current mess is an example of what can happen when there is a paucity of reliable data, a superabundance of emotion, and a system that feeds on instant news and sound bites with little understanding of how science should work.
Some of the turmoil is a response to the notion that in certain situations gender dysphoria may be a condition that can be learned or mimicked from exposure to other gender-dysphoric individuals. Two papers anchor either side of the debate. The first paper was published in 2018 by a then–Brown University health expert who hypothesized the existence of a condition which she labeled “rapid-onset gender dysphoria [ROGD]”. One can imagine that “social contagion” might be considered as one of the potential contributors to this hypothesized condition. Unfortunately, the publication of the paper ignited a firestorm of criticism from a segment of the population that advocates for the transgender community, prompting the university and the online publisher to backpedal and reevaluate the quality of the research on which the paper was based.
One of the concerns voiced at the time of publication was that the research could be used to support the transphobic agenda by some state legislatures hoping to ban gender-affirming care. How large a role the paper played in the current spate of legislation in is unclear. I suspect it has been small. But, one can’t deny the potential exists.
Leaping forward to 2022, the second paper was published in the August issue of Pediatrics, in which the authors attempted to test the ROGD hypothesis and question the inference of social contagion.
The investigators found that in 2017 and 2019 the birth ratios of transgender-diverse (TGD) individuals did not favor assigned female-sex-at-birth (AFAB) individuals. They also discovered that in their sample overall there was a decrease in the percentage of adolescents who self-identified as TGD. Not surprisingly, “bullying victimization and suicidality were higher among TGD youth when compared with their cisgender peers.” The authors concluded that their findings were “incongruent with an ROGD hypothesis that posits social contagion” nor should it be used to restrict access to gender-affirming care.
There you have it. Are we any closer to understanding gender dysphoria and its origins? I don’t think so. The media is somewhat less confused. The NBC News online presence headline on Aug. 3, 2022, reads “‘Social contagion’ isn’t causing more youths to be transgender, study finds.”
My sense is that the general population perceives an increase in the prevalence of gender dysphoria. It is very likely that this perception is primarily a reflection of a more compassionate and educated attitude in a significant portion of the population making it less challenging for gender-dysphoric youth to surface. However, it should not surprise us that some parents and observers are concerned that a percentage of this increased prevalence is the result of social contagion. Nor should it surprise us that some advocates for the trans population feel threatened by this hypothesis.
Neither of these studies really answers the question of whether some cases of gender dysphoria are the result of social contagion. Both were small samples using methodology that has been called into question. The bottom line is that we need more studies and must remain open to considering their results. That’s how science should work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
At the risk of losing a digit or two I am going to dip my toes into the murky waters of gender-affirming care, sometimes referred to as trans care. Recently, Moira Szilagyi, MD, PhD, president of the American Academy of Pediatrics, released two statements, one in the Aug. 22, 2022, Wall Street Journal, the other summarized in the Aug. 25, 2022, AAP Daily Briefing, in which she attempts to clarify the academy’s position on gender-affirming care. They were well-worded and heroic attempts to clear the air. I fear these explanations will do little to encourage informed and courteous discussions between those entrenched on either side of a disagreement that is unfortunately being played out on media outlets and state legislatures instead of the offices of primary care physicians and specialists where it belongs.
The current mess is an example of what can happen when there is a paucity of reliable data, a superabundance of emotion, and a system that feeds on instant news and sound bites with little understanding of how science should work.
Some of the turmoil is a response to the notion that in certain situations gender dysphoria may be a condition that can be learned or mimicked from exposure to other gender-dysphoric individuals. Two papers anchor either side of the debate. The first paper was published in 2018 by a then–Brown University health expert who hypothesized the existence of a condition which she labeled “rapid-onset gender dysphoria [ROGD]”. One can imagine that “social contagion” might be considered as one of the potential contributors to this hypothesized condition. Unfortunately, the publication of the paper ignited a firestorm of criticism from a segment of the population that advocates for the transgender community, prompting the university and the online publisher to backpedal and reevaluate the quality of the research on which the paper was based.
One of the concerns voiced at the time of publication was that the research could be used to support the transphobic agenda by some state legislatures hoping to ban gender-affirming care. How large a role the paper played in the current spate of legislation in is unclear. I suspect it has been small. But, one can’t deny the potential exists.
Leaping forward to 2022, the second paper was published in the August issue of Pediatrics, in which the authors attempted to test the ROGD hypothesis and question the inference of social contagion.
The investigators found that in 2017 and 2019 the birth ratios of transgender-diverse (TGD) individuals did not favor assigned female-sex-at-birth (AFAB) individuals. They also discovered that in their sample overall there was a decrease in the percentage of adolescents who self-identified as TGD. Not surprisingly, “bullying victimization and suicidality were higher among TGD youth when compared with their cisgender peers.” The authors concluded that their findings were “incongruent with an ROGD hypothesis that posits social contagion” nor should it be used to restrict access to gender-affirming care.
There you have it. Are we any closer to understanding gender dysphoria and its origins? I don’t think so. The media is somewhat less confused. The NBC News online presence headline on Aug. 3, 2022, reads “‘Social contagion’ isn’t causing more youths to be transgender, study finds.”
My sense is that the general population perceives an increase in the prevalence of gender dysphoria. It is very likely that this perception is primarily a reflection of a more compassionate and educated attitude in a significant portion of the population making it less challenging for gender-dysphoric youth to surface. However, it should not surprise us that some parents and observers are concerned that a percentage of this increased prevalence is the result of social contagion. Nor should it surprise us that some advocates for the trans population feel threatened by this hypothesis.
Neither of these studies really answers the question of whether some cases of gender dysphoria are the result of social contagion. Both were small samples using methodology that has been called into question. The bottom line is that we need more studies and must remain open to considering their results. That’s how science should work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Children and COVID: New cases increase; hospital admissions could follow
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.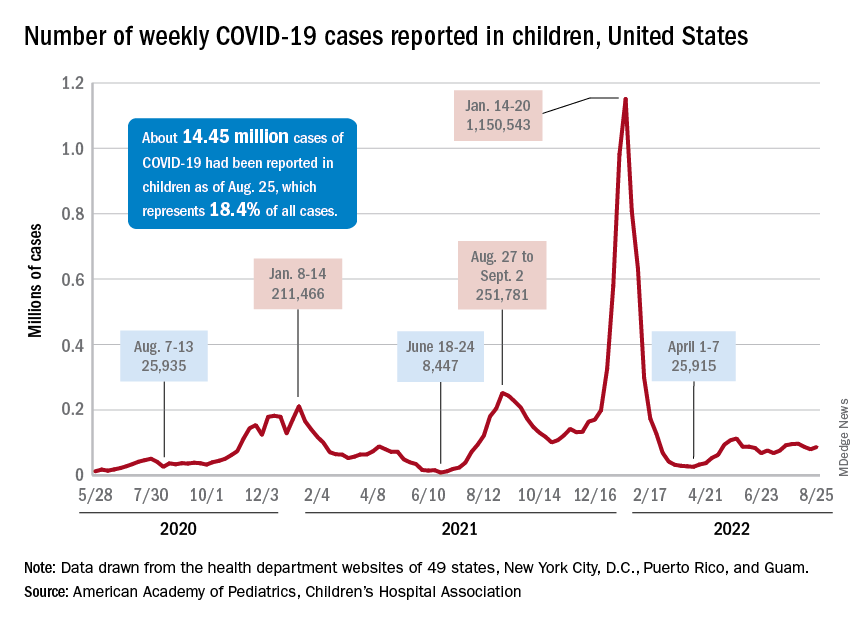
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.
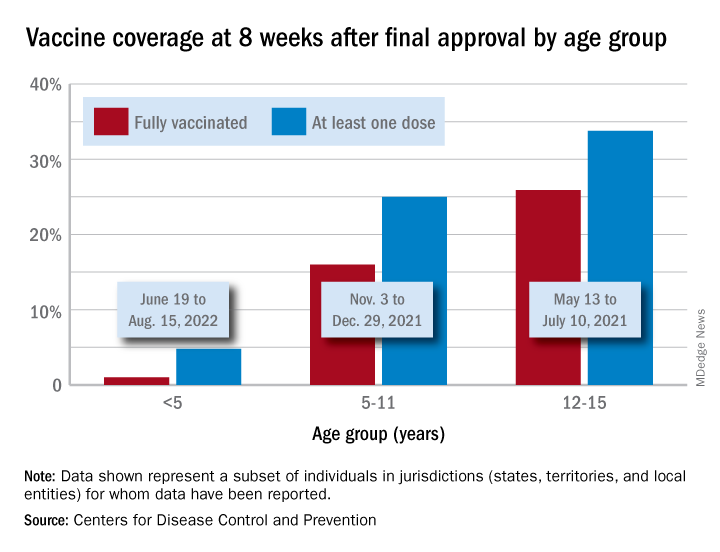
Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
OMERACT continues to set standards on research outcomes, enhancing the patient voice
Clinical research in rheumatology was suffering from an identity crisis of sorts 40 years ago. A lack of consensus across continents resulted in differing views about clinical outcome measures and judgments about treatments.
Patients were not allowed to be the generating source of a clinical outcome, according to Peter Tugwell, MSc, MD. “The only outcomes that were acceptable were clinician assessments, blood tests, and imaging,” said Dr. Tugwell, professor of medicine, epidemiology, and public health at the University of Ottawa (Ont.) and a practicing rheumatologist at Ottawa Hospital.
Clinicians were coming to different conclusions about patient responses to treatment when managing rheumatoid arthritis in clinical practice.
OMERACT sought to address this lack of uniformity. This international group, formed in 1992, leverages stakeholder groups to improve outcome measurement in rheumatology endpoints through a consensus-building, data-driven format.
It was originally known as “Outcome Measures in Rheumatoid Arthritis Clinical Trials,” but its leaders have since broadened its scope to “Outcome Measures in Rheumatology.” Over the years, it has evolved into an international network that assesses measurement across a wide variety of intervention studies. Now 30 years old, the network spans 40 active working groups and has influenced work in patient outcomes across 500 peer-reviewed publications.
The network meets every 2 years to address what is always a challenging agenda, said Dr. Tugwell, one of its founding members and chair. “There’s lots of strong opinions.” Participating in the discussions are individuals from all stages of seniority in rheumatology and clinical epidemiology, patient research partners, industry, approval agencies, and many countries who are committed to the spirit of OMERACT.
“The secret to our success has been getting world leaders to come together and have those discussions, work them through, and identify common ground in such a way that the approval agencies accept these outcome measures in clinical trials,” he added.
“My impression was the founders perceived a problem in the early 1990s and devised a consensus method in an attempt to quantify clinical parameters to define disease activity in rheumatoid arthritis – an important first step to do clinical trials and allow comparisons between them,” said Patricia Woo, CBE, FMedSci, FRCP, emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. At that time, even disease definitions varied between the United States and Europe and other parts of the world, said Dr. Woo, who is not a part of OMERACT. “This was especially true for pediatric rheumatology.”
Fusing the continental divide
OMERACT arose from a need to streamline clinical outcome measures in rheumatology. Research papers during the 1980s demonstrated a lack of coherence in managing patients with rheumatoid arthritis in routine practice. In addition, the measures used to define clinical endpoints in clinical trials operated in silos – they were either too specific to a certain trial, overlapped with other concepts, or didn’t reflect changes in treatment.
Approval agencies in Europe and North America were approving only outcomes measures developed by their respective researchers. This was also true of patients they tested on. “This seemed crazy,” Dr. Tugwell said.
Dr. Tugwell was involved in the Cochrane collaboration, which conducts systematic reviews of best evidence across the world that assesses the magnitude of benefits versus harms.
To achieve this goal, “you need to pull studies from around the world,” he said. Maarten Boers, MD, PhD, a rheumatologist (and later professor of clinical epidemiology at Amsterdam University Medical Center) from the Netherlands, spent a year in Ontario, Canada, to train as a clinical epidemiologist. Together, Dr. Tugwell and Dr. Boers began discussing options to develop more streamlined outcome measures.
They initiated the first OMERACT conference in Maastricht, the Netherlands, in 1992. The Food and Drug Administration and European Medicines Agency participated, along with leaders of outcomes measurement in Europe and in North America.
Discussions centered on methods to develop outcomes in a meaningful fashion. During the first meeting, North American and European approval agencies agreed to accept each other’s studies and endpoints and patient reported outcomes.
Agreement was achieved on a preliminary set of outcome domains and measures that later became known as the WHO-ILAR (World Health Organization–International League of Associations for Rheumatology) core set. The set included seven outcome domains: tender joints, swollen joints, pain, physician global assessment, patient global assessment, physical disability, and acute phase reactants, and one additional outcome domain for studies lasting 1 year or more: radiographs of the joints.
“A proactive program was planned to test not only the validity of these endpoints, but also the methods for their measurement. This was the start of a continuing process,” OMERACT members said in a joint statement for this article. Meetings have since taken place every 2 years.

OMERACT accomplishments
OMERACT now requires buy-in from four continents: Asia, Australia, Europe, and North America.
Its leaders have developed an explicit process for gaining endorsement of core outcome domains and instrument measurement sets. To fully capture the possibilities of “what to measure,” i.e., “measurable aspects of health conditions,” OMERACT has developed a framework of concepts, core areas, and outcome domains. The key concepts are pathophysiology (with a core area termed “manifestations/abnormalities”) and impact (with core areas of “death/lifespan,” and “life impact,” and the optional area of “societal/resource use”). An outcome domain defines an element of a core area to measure the effects of a treatment, such as blood markers, pain intensity, physical function, or emotional well-being.
A core outcome domain set is developed by agreeing to at least one outcome domain within one of the three core areas. Subsequently, a core outcome measurement set is developed by agreeing to at least one applicable measurement instrument for each core outcome domain. This requires documentation of validity, summarized under three metrics: truth, discrimination, and feasibility.
OMERACT’s handbook provides tutelage on establishing and implementing core outcomes, and several workbooks offer guidance on developing core outcome domain sets, selecting instruments for core outcome measurement sets, and OMERACT methodology.
All this work has led to widespread adoption.
Approval agencies have accepted OMERACT’s filter and methods advances, which have been adopted by many research groups in rheumatology and among nonrheumatology research groups. Organizations such as the U.S. National Institutes of Health’s National Institute of Neurological Disorders and Stroke have sought its advice.
Its core outcomes have been adopted and used for approval in the great majority of studies on rheumatoid arthritis, Dr. Tugwell said.
Several BMJ articles underscore the influence and uptake of OMERACT’s core outcome set. One 2017 paper, which analyzed 273 randomized trials of rheumatoid arthritis drug treatments on ClinicalTrials.gov, found that the WHO-ILAR arthritis core outcome set was reported in 81% of the studies. “The adoption of a core outcome set has the potential to increase consistency in outcomes measured across trials and ensure that trials are more likely to measure appropriate outcomes,” the authors concluded.
Since the initial 1992 meeting, OMERACT has broadened its focus from rheumatoid arthritis to 25 other musculoskeletal conditions.
For example, other OMERACT conferences have led to consensus on core sets of measures for osteoarthritis and osteoporosis, psoriasis/psoriatic arthritis, psychosocial measures, and a core set of data for cost-effectiveness evaluations.
‘Speed is a limitation’
OMERACT is a bottom-up volunteer organization. It doesn’t represent any official organization of any clinical society. “We’ve not asked to be adopted by the American College of Rheumatology, EULAR [European Alliance of Associations for Rheumatology], or other international organizations,” Dr. Tugwell said. It offers a chance for patients, users, and doers of research to work together to agree on rigorous criteria accepted by the approval agencies and take the necessary time to work things through.
This is not a fast process, usually taking 4-6 years to initiate and establish an outcome domain set, he emphasized. “It would be beneficial to do it faster if we had the resources to meet every year. The fact is we’re a volunteer organization that meets every 2 years.”
Speed is a limitation, he acknowledged, but it’s an acceptable trade-off for doing things correctly.
The group has faced other challenges during the COVID-19 pandemic, pivoting to a virtual format that had benefits and limitations.
In one respect, moving to a virtual meeting increased uptake in participation and voting, Dr. Tugwell said. Patient participants with severe rheumatoid arthritis no longer faced the challenges of travel. “On the other hand, we didn’t have the same opportunity to achieve common ground virtually,” he said. “Where there are strong disagreements, I’m a great believer that people need to know one another. There needs to be relationship building.”
OMERACT’s emerging leader program has been a cornerstone of its in-person meetings, engaging young rheumatologists to interact with some of the leaders of outcome measurement. The virtual format dampened this process somewhat, eliminating those important “café chats” between the stakeholders.
The hope is to bring people face-to-face once more at the next meeting in May 2023. The agenda will focus on relationship building, identifying controversial areas, and bringing younger people to develop relationships, Dr. Tugwell said. OMERACT will retain a virtual option for the worldwide voting, “which will allow for more buy-in from so many more people,” he added.
A consensus on pain
The onus of developing outcome measures that move with the times is sometimes too great for one group to manage. In 2018, OMERACT became a part of the Red Hat Group (RHG), an organization conceived at the COMET (Core Outcome Measures in Effectiveness Trials) VII meeting in Amsterdam.
RHG aims to improve the choice of outcomes in health research. It includes eight groups: COMET; OMERACT; the Cochrane Skin Core Outcome Set Initiative; Grading of Recommendations, Assessment, Development and Evaluations; Center for Medical Technology Policy; COnsensus-based Standards for the selection of health Measurement Instruments; Clinical Data Interchange Standards Consortium; and Standardized Outcomes in Nephrology.
The collaboration between groups offers a “very interesting interface between consensus building as well as hard evidence,” Dr. Tugwell said. The focus goes beyond rheumatology to other clinical areas of common interest, exploring how one classifies outcome domains in terms of symptoms, life impact, or death.
Pain is an important common denominator that the RHG has evaluated.
“We believe it’s too general. We’re trying to define pain across all Red Hat Groups because it’s clear that the research community has all these different scales for defining pain severity,” Dr. Tugwell said. “We have to find a way to make ruthless decisions and rules for doing it. And of course, it has to be transparent.”
Looking ahead
As part of its ongoing work, OMERACT is evaluating the robustness of instruments that rheumatologists use as outcome measures in clinical trials, which can be a laborious process. The OMERACT Filter 2.0, part of the latest iteration of the handbook, offers strong guidance for researchers but needs a long-term strategy and key methodological support. “To that end, we set up a technical advisory group to help people in the instrument selection work and that remains an ongoing process,” OMERACT leaders said in their joint statement.
OMERACT is looking at opportunities to create benchmark processes for developing core sets outside of rheumatology and a methodology around outcome measures such as contextual factors, composites, and surrogates.
It will also be taking a step back to solicit opinions from the approval agencies represented by the OMERACT membership on the OMERACT handbook.
The goal is to make sure the handbook aligns with everyone else’s approval and labeling requirements.
OMERACT’s patient participants bring important perspectives
OMERACT over the years has sought to become a more patient-centered group. Patients have been involved in OMERACT activities since its sixth meeting, forming an independent, yet integrated, group within the network. They have their own steering committee and produced and helped to update a glossary for OMERACT patients and professionals.
Catherine (McGowan) Hofstetter, who was diagnosed with rheumatoid arthritis 30 years ago, chairs OMERACT’s Patient Research Partners Support Team. In a Q&A, she discussed the importance of patient voices and OMERACT’s plans to further educate and include patients in the dialogue on outcomes.
Question: Have patients always been a part of OMERACT meetings?
Answer: Patients have been involved with OMERACT since 2002. The patient voice adds relevance to all the work that OMERACT does. You can’t begin to talk about outcomes unless there is a patient at the table with lived experience.
Q: Can you cite a few examples of how the patient voice enriches the conversation on outcomes research?
A: Outcomes and priorities that are important to patients are often completely different than those of the clinician. For instance, a work outcome is important to someone who doesn’t have any medical insurance or disability insurance, so that you can ensure that there is food on the table and a roof over your head. Or it may be important to someone because the employment provides medical and disability insurance to provide security for them and their family. These are two different perspectives on work and therefore work priorities and outcomes.
Q: What have been some of the challenges of getting patients to participate?
A: Training patients is one challenge. OMERACT’s work has a very steep learning curve, and while the basics are the same between the groups in terms of looking at what we measure and how we measure it, the nuances of different working groups require a lot of time and energy to be comfortable enough with the work, and then be confident enough to bring your perspective and lived experience to the table. It’s also a very accomplished group, which can be quite intimidating. Self-disclosure is a very personal and intimate undertaking that requires patience, compassion, and respect.
Q: Are there any plans to enhance patient engagement?
A: When we had OMERACT 2020 it was a virtual conference that took place over about 6 months. We had far more patient research partners [PRPs] participate than we have ever had at any OMERACT face-to-face meeting. There is a desire and passion on the part of patients to lend their voices to the work. The working groups meet virtually throughout the year to advance their agendas, and PRPs are a part of each of the working groups.
Hopefully, we can start working toward including more voices at the conferences by enabling a hybrid model. The PRP Support Team will begin engaging patients this fall with education, mentoring, and team-building exercises so by the time we meet in person in May 2023, they will have enough background knowledge and information to give them the confidence that will enhance their experience at the face-to-face meeting.
We also need to ensure that those patients who want to stay engaged can. This means that the education and training should continue long after the face-to-face meeting is over. We need to build capacity in the PRP group and look to succession planning and be a resource to working groups struggling to find PRPs to work with them on a longer-term basis.
Clinical research in rheumatology was suffering from an identity crisis of sorts 40 years ago. A lack of consensus across continents resulted in differing views about clinical outcome measures and judgments about treatments.
Patients were not allowed to be the generating source of a clinical outcome, according to Peter Tugwell, MSc, MD. “The only outcomes that were acceptable were clinician assessments, blood tests, and imaging,” said Dr. Tugwell, professor of medicine, epidemiology, and public health at the University of Ottawa (Ont.) and a practicing rheumatologist at Ottawa Hospital.
Clinicians were coming to different conclusions about patient responses to treatment when managing rheumatoid arthritis in clinical practice.
OMERACT sought to address this lack of uniformity. This international group, formed in 1992, leverages stakeholder groups to improve outcome measurement in rheumatology endpoints through a consensus-building, data-driven format.
It was originally known as “Outcome Measures in Rheumatoid Arthritis Clinical Trials,” but its leaders have since broadened its scope to “Outcome Measures in Rheumatology.” Over the years, it has evolved into an international network that assesses measurement across a wide variety of intervention studies. Now 30 years old, the network spans 40 active working groups and has influenced work in patient outcomes across 500 peer-reviewed publications.
The network meets every 2 years to address what is always a challenging agenda, said Dr. Tugwell, one of its founding members and chair. “There’s lots of strong opinions.” Participating in the discussions are individuals from all stages of seniority in rheumatology and clinical epidemiology, patient research partners, industry, approval agencies, and many countries who are committed to the spirit of OMERACT.
“The secret to our success has been getting world leaders to come together and have those discussions, work them through, and identify common ground in such a way that the approval agencies accept these outcome measures in clinical trials,” he added.
“My impression was the founders perceived a problem in the early 1990s and devised a consensus method in an attempt to quantify clinical parameters to define disease activity in rheumatoid arthritis – an important first step to do clinical trials and allow comparisons between them,” said Patricia Woo, CBE, FMedSci, FRCP, emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. At that time, even disease definitions varied between the United States and Europe and other parts of the world, said Dr. Woo, who is not a part of OMERACT. “This was especially true for pediatric rheumatology.”
Fusing the continental divide
OMERACT arose from a need to streamline clinical outcome measures in rheumatology. Research papers during the 1980s demonstrated a lack of coherence in managing patients with rheumatoid arthritis in routine practice. In addition, the measures used to define clinical endpoints in clinical trials operated in silos – they were either too specific to a certain trial, overlapped with other concepts, or didn’t reflect changes in treatment.
Approval agencies in Europe and North America were approving only outcomes measures developed by their respective researchers. This was also true of patients they tested on. “This seemed crazy,” Dr. Tugwell said.
Dr. Tugwell was involved in the Cochrane collaboration, which conducts systematic reviews of best evidence across the world that assesses the magnitude of benefits versus harms.
To achieve this goal, “you need to pull studies from around the world,” he said. Maarten Boers, MD, PhD, a rheumatologist (and later professor of clinical epidemiology at Amsterdam University Medical Center) from the Netherlands, spent a year in Ontario, Canada, to train as a clinical epidemiologist. Together, Dr. Tugwell and Dr. Boers began discussing options to develop more streamlined outcome measures.
They initiated the first OMERACT conference in Maastricht, the Netherlands, in 1992. The Food and Drug Administration and European Medicines Agency participated, along with leaders of outcomes measurement in Europe and in North America.
Discussions centered on methods to develop outcomes in a meaningful fashion. During the first meeting, North American and European approval agencies agreed to accept each other’s studies and endpoints and patient reported outcomes.
Agreement was achieved on a preliminary set of outcome domains and measures that later became known as the WHO-ILAR (World Health Organization–International League of Associations for Rheumatology) core set. The set included seven outcome domains: tender joints, swollen joints, pain, physician global assessment, patient global assessment, physical disability, and acute phase reactants, and one additional outcome domain for studies lasting 1 year or more: radiographs of the joints.
“A proactive program was planned to test not only the validity of these endpoints, but also the methods for their measurement. This was the start of a continuing process,” OMERACT members said in a joint statement for this article. Meetings have since taken place every 2 years.

OMERACT accomplishments
OMERACT now requires buy-in from four continents: Asia, Australia, Europe, and North America.
Its leaders have developed an explicit process for gaining endorsement of core outcome domains and instrument measurement sets. To fully capture the possibilities of “what to measure,” i.e., “measurable aspects of health conditions,” OMERACT has developed a framework of concepts, core areas, and outcome domains. The key concepts are pathophysiology (with a core area termed “manifestations/abnormalities”) and impact (with core areas of “death/lifespan,” and “life impact,” and the optional area of “societal/resource use”). An outcome domain defines an element of a core area to measure the effects of a treatment, such as blood markers, pain intensity, physical function, or emotional well-being.
A core outcome domain set is developed by agreeing to at least one outcome domain within one of the three core areas. Subsequently, a core outcome measurement set is developed by agreeing to at least one applicable measurement instrument for each core outcome domain. This requires documentation of validity, summarized under three metrics: truth, discrimination, and feasibility.
OMERACT’s handbook provides tutelage on establishing and implementing core outcomes, and several workbooks offer guidance on developing core outcome domain sets, selecting instruments for core outcome measurement sets, and OMERACT methodology.
All this work has led to widespread adoption.
Approval agencies have accepted OMERACT’s filter and methods advances, which have been adopted by many research groups in rheumatology and among nonrheumatology research groups. Organizations such as the U.S. National Institutes of Health’s National Institute of Neurological Disorders and Stroke have sought its advice.
Its core outcomes have been adopted and used for approval in the great majority of studies on rheumatoid arthritis, Dr. Tugwell said.
Several BMJ articles underscore the influence and uptake of OMERACT’s core outcome set. One 2017 paper, which analyzed 273 randomized trials of rheumatoid arthritis drug treatments on ClinicalTrials.gov, found that the WHO-ILAR arthritis core outcome set was reported in 81% of the studies. “The adoption of a core outcome set has the potential to increase consistency in outcomes measured across trials and ensure that trials are more likely to measure appropriate outcomes,” the authors concluded.
Since the initial 1992 meeting, OMERACT has broadened its focus from rheumatoid arthritis to 25 other musculoskeletal conditions.
For example, other OMERACT conferences have led to consensus on core sets of measures for osteoarthritis and osteoporosis, psoriasis/psoriatic arthritis, psychosocial measures, and a core set of data for cost-effectiveness evaluations.
‘Speed is a limitation’
OMERACT is a bottom-up volunteer organization. It doesn’t represent any official organization of any clinical society. “We’ve not asked to be adopted by the American College of Rheumatology, EULAR [European Alliance of Associations for Rheumatology], or other international organizations,” Dr. Tugwell said. It offers a chance for patients, users, and doers of research to work together to agree on rigorous criteria accepted by the approval agencies and take the necessary time to work things through.
This is not a fast process, usually taking 4-6 years to initiate and establish an outcome domain set, he emphasized. “It would be beneficial to do it faster if we had the resources to meet every year. The fact is we’re a volunteer organization that meets every 2 years.”
Speed is a limitation, he acknowledged, but it’s an acceptable trade-off for doing things correctly.
The group has faced other challenges during the COVID-19 pandemic, pivoting to a virtual format that had benefits and limitations.
In one respect, moving to a virtual meeting increased uptake in participation and voting, Dr. Tugwell said. Patient participants with severe rheumatoid arthritis no longer faced the challenges of travel. “On the other hand, we didn’t have the same opportunity to achieve common ground virtually,” he said. “Where there are strong disagreements, I’m a great believer that people need to know one another. There needs to be relationship building.”
OMERACT’s emerging leader program has been a cornerstone of its in-person meetings, engaging young rheumatologists to interact with some of the leaders of outcome measurement. The virtual format dampened this process somewhat, eliminating those important “café chats” between the stakeholders.
The hope is to bring people face-to-face once more at the next meeting in May 2023. The agenda will focus on relationship building, identifying controversial areas, and bringing younger people to develop relationships, Dr. Tugwell said. OMERACT will retain a virtual option for the worldwide voting, “which will allow for more buy-in from so many more people,” he added.
A consensus on pain
The onus of developing outcome measures that move with the times is sometimes too great for one group to manage. In 2018, OMERACT became a part of the Red Hat Group (RHG), an organization conceived at the COMET (Core Outcome Measures in Effectiveness Trials) VII meeting in Amsterdam.
RHG aims to improve the choice of outcomes in health research. It includes eight groups: COMET; OMERACT; the Cochrane Skin Core Outcome Set Initiative; Grading of Recommendations, Assessment, Development and Evaluations; Center for Medical Technology Policy; COnsensus-based Standards for the selection of health Measurement Instruments; Clinical Data Interchange Standards Consortium; and Standardized Outcomes in Nephrology.
The collaboration between groups offers a “very interesting interface between consensus building as well as hard evidence,” Dr. Tugwell said. The focus goes beyond rheumatology to other clinical areas of common interest, exploring how one classifies outcome domains in terms of symptoms, life impact, or death.
Pain is an important common denominator that the RHG has evaluated.
“We believe it’s too general. We’re trying to define pain across all Red Hat Groups because it’s clear that the research community has all these different scales for defining pain severity,” Dr. Tugwell said. “We have to find a way to make ruthless decisions and rules for doing it. And of course, it has to be transparent.”
Looking ahead
As part of its ongoing work, OMERACT is evaluating the robustness of instruments that rheumatologists use as outcome measures in clinical trials, which can be a laborious process. The OMERACT Filter 2.0, part of the latest iteration of the handbook, offers strong guidance for researchers but needs a long-term strategy and key methodological support. “To that end, we set up a technical advisory group to help people in the instrument selection work and that remains an ongoing process,” OMERACT leaders said in their joint statement.
OMERACT is looking at opportunities to create benchmark processes for developing core sets outside of rheumatology and a methodology around outcome measures such as contextual factors, composites, and surrogates.
It will also be taking a step back to solicit opinions from the approval agencies represented by the OMERACT membership on the OMERACT handbook.
The goal is to make sure the handbook aligns with everyone else’s approval and labeling requirements.
OMERACT’s patient participants bring important perspectives
OMERACT over the years has sought to become a more patient-centered group. Patients have been involved in OMERACT activities since its sixth meeting, forming an independent, yet integrated, group within the network. They have their own steering committee and produced and helped to update a glossary for OMERACT patients and professionals.
Catherine (McGowan) Hofstetter, who was diagnosed with rheumatoid arthritis 30 years ago, chairs OMERACT’s Patient Research Partners Support Team. In a Q&A, she discussed the importance of patient voices and OMERACT’s plans to further educate and include patients in the dialogue on outcomes.
Question: Have patients always been a part of OMERACT meetings?
Answer: Patients have been involved with OMERACT since 2002. The patient voice adds relevance to all the work that OMERACT does. You can’t begin to talk about outcomes unless there is a patient at the table with lived experience.
Q: Can you cite a few examples of how the patient voice enriches the conversation on outcomes research?
A: Outcomes and priorities that are important to patients are often completely different than those of the clinician. For instance, a work outcome is important to someone who doesn’t have any medical insurance or disability insurance, so that you can ensure that there is food on the table and a roof over your head. Or it may be important to someone because the employment provides medical and disability insurance to provide security for them and their family. These are two different perspectives on work and therefore work priorities and outcomes.
Q: What have been some of the challenges of getting patients to participate?
A: Training patients is one challenge. OMERACT’s work has a very steep learning curve, and while the basics are the same between the groups in terms of looking at what we measure and how we measure it, the nuances of different working groups require a lot of time and energy to be comfortable enough with the work, and then be confident enough to bring your perspective and lived experience to the table. It’s also a very accomplished group, which can be quite intimidating. Self-disclosure is a very personal and intimate undertaking that requires patience, compassion, and respect.
Q: Are there any plans to enhance patient engagement?
A: When we had OMERACT 2020 it was a virtual conference that took place over about 6 months. We had far more patient research partners [PRPs] participate than we have ever had at any OMERACT face-to-face meeting. There is a desire and passion on the part of patients to lend their voices to the work. The working groups meet virtually throughout the year to advance their agendas, and PRPs are a part of each of the working groups.
Hopefully, we can start working toward including more voices at the conferences by enabling a hybrid model. The PRP Support Team will begin engaging patients this fall with education, mentoring, and team-building exercises so by the time we meet in person in May 2023, they will have enough background knowledge and information to give them the confidence that will enhance their experience at the face-to-face meeting.
We also need to ensure that those patients who want to stay engaged can. This means that the education and training should continue long after the face-to-face meeting is over. We need to build capacity in the PRP group and look to succession planning and be a resource to working groups struggling to find PRPs to work with them on a longer-term basis.
Clinical research in rheumatology was suffering from an identity crisis of sorts 40 years ago. A lack of consensus across continents resulted in differing views about clinical outcome measures and judgments about treatments.
Patients were not allowed to be the generating source of a clinical outcome, according to Peter Tugwell, MSc, MD. “The only outcomes that were acceptable were clinician assessments, blood tests, and imaging,” said Dr. Tugwell, professor of medicine, epidemiology, and public health at the University of Ottawa (Ont.) and a practicing rheumatologist at Ottawa Hospital.
Clinicians were coming to different conclusions about patient responses to treatment when managing rheumatoid arthritis in clinical practice.
OMERACT sought to address this lack of uniformity. This international group, formed in 1992, leverages stakeholder groups to improve outcome measurement in rheumatology endpoints through a consensus-building, data-driven format.
It was originally known as “Outcome Measures in Rheumatoid Arthritis Clinical Trials,” but its leaders have since broadened its scope to “Outcome Measures in Rheumatology.” Over the years, it has evolved into an international network that assesses measurement across a wide variety of intervention studies. Now 30 years old, the network spans 40 active working groups and has influenced work in patient outcomes across 500 peer-reviewed publications.
The network meets every 2 years to address what is always a challenging agenda, said Dr. Tugwell, one of its founding members and chair. “There’s lots of strong opinions.” Participating in the discussions are individuals from all stages of seniority in rheumatology and clinical epidemiology, patient research partners, industry, approval agencies, and many countries who are committed to the spirit of OMERACT.
“The secret to our success has been getting world leaders to come together and have those discussions, work them through, and identify common ground in such a way that the approval agencies accept these outcome measures in clinical trials,” he added.
“My impression was the founders perceived a problem in the early 1990s and devised a consensus method in an attempt to quantify clinical parameters to define disease activity in rheumatoid arthritis – an important first step to do clinical trials and allow comparisons between them,” said Patricia Woo, CBE, FMedSci, FRCP, emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. At that time, even disease definitions varied between the United States and Europe and other parts of the world, said Dr. Woo, who is not a part of OMERACT. “This was especially true for pediatric rheumatology.”
Fusing the continental divide
OMERACT arose from a need to streamline clinical outcome measures in rheumatology. Research papers during the 1980s demonstrated a lack of coherence in managing patients with rheumatoid arthritis in routine practice. In addition, the measures used to define clinical endpoints in clinical trials operated in silos – they were either too specific to a certain trial, overlapped with other concepts, or didn’t reflect changes in treatment.
Approval agencies in Europe and North America were approving only outcomes measures developed by their respective researchers. This was also true of patients they tested on. “This seemed crazy,” Dr. Tugwell said.
Dr. Tugwell was involved in the Cochrane collaboration, which conducts systematic reviews of best evidence across the world that assesses the magnitude of benefits versus harms.
To achieve this goal, “you need to pull studies from around the world,” he said. Maarten Boers, MD, PhD, a rheumatologist (and later professor of clinical epidemiology at Amsterdam University Medical Center) from the Netherlands, spent a year in Ontario, Canada, to train as a clinical epidemiologist. Together, Dr. Tugwell and Dr. Boers began discussing options to develop more streamlined outcome measures.
They initiated the first OMERACT conference in Maastricht, the Netherlands, in 1992. The Food and Drug Administration and European Medicines Agency participated, along with leaders of outcomes measurement in Europe and in North America.
Discussions centered on methods to develop outcomes in a meaningful fashion. During the first meeting, North American and European approval agencies agreed to accept each other’s studies and endpoints and patient reported outcomes.
Agreement was achieved on a preliminary set of outcome domains and measures that later became known as the WHO-ILAR (World Health Organization–International League of Associations for Rheumatology) core set. The set included seven outcome domains: tender joints, swollen joints, pain, physician global assessment, patient global assessment, physical disability, and acute phase reactants, and one additional outcome domain for studies lasting 1 year or more: radiographs of the joints.
“A proactive program was planned to test not only the validity of these endpoints, but also the methods for their measurement. This was the start of a continuing process,” OMERACT members said in a joint statement for this article. Meetings have since taken place every 2 years.

OMERACT accomplishments
OMERACT now requires buy-in from four continents: Asia, Australia, Europe, and North America.
Its leaders have developed an explicit process for gaining endorsement of core outcome domains and instrument measurement sets. To fully capture the possibilities of “what to measure,” i.e., “measurable aspects of health conditions,” OMERACT has developed a framework of concepts, core areas, and outcome domains. The key concepts are pathophysiology (with a core area termed “manifestations/abnormalities”) and impact (with core areas of “death/lifespan,” and “life impact,” and the optional area of “societal/resource use”). An outcome domain defines an element of a core area to measure the effects of a treatment, such as blood markers, pain intensity, physical function, or emotional well-being.
A core outcome domain set is developed by agreeing to at least one outcome domain within one of the three core areas. Subsequently, a core outcome measurement set is developed by agreeing to at least one applicable measurement instrument for each core outcome domain. This requires documentation of validity, summarized under three metrics: truth, discrimination, and feasibility.
OMERACT’s handbook provides tutelage on establishing and implementing core outcomes, and several workbooks offer guidance on developing core outcome domain sets, selecting instruments for core outcome measurement sets, and OMERACT methodology.
All this work has led to widespread adoption.
Approval agencies have accepted OMERACT’s filter and methods advances, which have been adopted by many research groups in rheumatology and among nonrheumatology research groups. Organizations such as the U.S. National Institutes of Health’s National Institute of Neurological Disorders and Stroke have sought its advice.
Its core outcomes have been adopted and used for approval in the great majority of studies on rheumatoid arthritis, Dr. Tugwell said.
Several BMJ articles underscore the influence and uptake of OMERACT’s core outcome set. One 2017 paper, which analyzed 273 randomized trials of rheumatoid arthritis drug treatments on ClinicalTrials.gov, found that the WHO-ILAR arthritis core outcome set was reported in 81% of the studies. “The adoption of a core outcome set has the potential to increase consistency in outcomes measured across trials and ensure that trials are more likely to measure appropriate outcomes,” the authors concluded.
Since the initial 1992 meeting, OMERACT has broadened its focus from rheumatoid arthritis to 25 other musculoskeletal conditions.
For example, other OMERACT conferences have led to consensus on core sets of measures for osteoarthritis and osteoporosis, psoriasis/psoriatic arthritis, psychosocial measures, and a core set of data for cost-effectiveness evaluations.
‘Speed is a limitation’
OMERACT is a bottom-up volunteer organization. It doesn’t represent any official organization of any clinical society. “We’ve not asked to be adopted by the American College of Rheumatology, EULAR [European Alliance of Associations for Rheumatology], or other international organizations,” Dr. Tugwell said. It offers a chance for patients, users, and doers of research to work together to agree on rigorous criteria accepted by the approval agencies and take the necessary time to work things through.
This is not a fast process, usually taking 4-6 years to initiate and establish an outcome domain set, he emphasized. “It would be beneficial to do it faster if we had the resources to meet every year. The fact is we’re a volunteer organization that meets every 2 years.”
Speed is a limitation, he acknowledged, but it’s an acceptable trade-off for doing things correctly.
The group has faced other challenges during the COVID-19 pandemic, pivoting to a virtual format that had benefits and limitations.
In one respect, moving to a virtual meeting increased uptake in participation and voting, Dr. Tugwell said. Patient participants with severe rheumatoid arthritis no longer faced the challenges of travel. “On the other hand, we didn’t have the same opportunity to achieve common ground virtually,” he said. “Where there are strong disagreements, I’m a great believer that people need to know one another. There needs to be relationship building.”
OMERACT’s emerging leader program has been a cornerstone of its in-person meetings, engaging young rheumatologists to interact with some of the leaders of outcome measurement. The virtual format dampened this process somewhat, eliminating those important “café chats” between the stakeholders.
The hope is to bring people face-to-face once more at the next meeting in May 2023. The agenda will focus on relationship building, identifying controversial areas, and bringing younger people to develop relationships, Dr. Tugwell said. OMERACT will retain a virtual option for the worldwide voting, “which will allow for more buy-in from so many more people,” he added.
A consensus on pain
The onus of developing outcome measures that move with the times is sometimes too great for one group to manage. In 2018, OMERACT became a part of the Red Hat Group (RHG), an organization conceived at the COMET (Core Outcome Measures in Effectiveness Trials) VII meeting in Amsterdam.
RHG aims to improve the choice of outcomes in health research. It includes eight groups: COMET; OMERACT; the Cochrane Skin Core Outcome Set Initiative; Grading of Recommendations, Assessment, Development and Evaluations; Center for Medical Technology Policy; COnsensus-based Standards for the selection of health Measurement Instruments; Clinical Data Interchange Standards Consortium; and Standardized Outcomes in Nephrology.
The collaboration between groups offers a “very interesting interface between consensus building as well as hard evidence,” Dr. Tugwell said. The focus goes beyond rheumatology to other clinical areas of common interest, exploring how one classifies outcome domains in terms of symptoms, life impact, or death.
Pain is an important common denominator that the RHG has evaluated.
“We believe it’s too general. We’re trying to define pain across all Red Hat Groups because it’s clear that the research community has all these different scales for defining pain severity,” Dr. Tugwell said. “We have to find a way to make ruthless decisions and rules for doing it. And of course, it has to be transparent.”
Looking ahead
As part of its ongoing work, OMERACT is evaluating the robustness of instruments that rheumatologists use as outcome measures in clinical trials, which can be a laborious process. The OMERACT Filter 2.0, part of the latest iteration of the handbook, offers strong guidance for researchers but needs a long-term strategy and key methodological support. “To that end, we set up a technical advisory group to help people in the instrument selection work and that remains an ongoing process,” OMERACT leaders said in their joint statement.
OMERACT is looking at opportunities to create benchmark processes for developing core sets outside of rheumatology and a methodology around outcome measures such as contextual factors, composites, and surrogates.
It will also be taking a step back to solicit opinions from the approval agencies represented by the OMERACT membership on the OMERACT handbook.
The goal is to make sure the handbook aligns with everyone else’s approval and labeling requirements.
OMERACT’s patient participants bring important perspectives
OMERACT over the years has sought to become a more patient-centered group. Patients have been involved in OMERACT activities since its sixth meeting, forming an independent, yet integrated, group within the network. They have their own steering committee and produced and helped to update a glossary for OMERACT patients and professionals.
Catherine (McGowan) Hofstetter, who was diagnosed with rheumatoid arthritis 30 years ago, chairs OMERACT’s Patient Research Partners Support Team. In a Q&A, she discussed the importance of patient voices and OMERACT’s plans to further educate and include patients in the dialogue on outcomes.
Question: Have patients always been a part of OMERACT meetings?
Answer: Patients have been involved with OMERACT since 2002. The patient voice adds relevance to all the work that OMERACT does. You can’t begin to talk about outcomes unless there is a patient at the table with lived experience.
Q: Can you cite a few examples of how the patient voice enriches the conversation on outcomes research?
A: Outcomes and priorities that are important to patients are often completely different than those of the clinician. For instance, a work outcome is important to someone who doesn’t have any medical insurance or disability insurance, so that you can ensure that there is food on the table and a roof over your head. Or it may be important to someone because the employment provides medical and disability insurance to provide security for them and their family. These are two different perspectives on work and therefore work priorities and outcomes.
Q: What have been some of the challenges of getting patients to participate?
A: Training patients is one challenge. OMERACT’s work has a very steep learning curve, and while the basics are the same between the groups in terms of looking at what we measure and how we measure it, the nuances of different working groups require a lot of time and energy to be comfortable enough with the work, and then be confident enough to bring your perspective and lived experience to the table. It’s also a very accomplished group, which can be quite intimidating. Self-disclosure is a very personal and intimate undertaking that requires patience, compassion, and respect.
Q: Are there any plans to enhance patient engagement?
A: When we had OMERACT 2020 it was a virtual conference that took place over about 6 months. We had far more patient research partners [PRPs] participate than we have ever had at any OMERACT face-to-face meeting. There is a desire and passion on the part of patients to lend their voices to the work. The working groups meet virtually throughout the year to advance their agendas, and PRPs are a part of each of the working groups.
Hopefully, we can start working toward including more voices at the conferences by enabling a hybrid model. The PRP Support Team will begin engaging patients this fall with education, mentoring, and team-building exercises so by the time we meet in person in May 2023, they will have enough background knowledge and information to give them the confidence that will enhance their experience at the face-to-face meeting.
We also need to ensure that those patients who want to stay engaged can. This means that the education and training should continue long after the face-to-face meeting is over. We need to build capacity in the PRP group and look to succession planning and be a resource to working groups struggling to find PRPs to work with them on a longer-term basis.
Chlorophyll water can trigger pseudoporphyria, expert warns
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
AT PDA 2022
VTE risk not elevated in AD patients on JAK inhibitors: Study
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
FROM JAMA DERMATOLOGY
How docs in firearm-friendly states talk gun safety
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.











