User login
In-hospital outcomes are better for vaccinated H1N1 patients
TORONTO – Patients who received an influenza vaccination but still required hospitalization for H1N1 influenza had better outcomes, compared with unvaccinated patients, according to findings from a retrospective study.
In the hospital, vaccinated patients had significantly lower rates of acute kidney injury (6% vs. 35%; P = .038) and were more likely to be satisfactorily managed with noninvasive mechanical ventilation (41% vs. 6%; P = .004).
Dr. Chandak and her colleagues studied 72 cases of seasonal influenza requiring hospitalization from September 2015 to April 2016 at Berkshire Medical Center, a 300-bed teaching hospital in western Massachusetts. Based on rapid polymerase chain reaction testing, 51 of these patients were positive for H1N1, of which 38 had received a seasonal flu vaccine.
H1N1 patients who had received vaccination were significantly older (70.4 years vs. 59.6 years; P = .016) and were more often smokers (76% vs. 38%; P = .017), compared with patients who were unvaccinated.
The finding that the unvaccinated patients were younger and still had poorer outcomes, “emphasizes the need for widespread vaccination,” Dr. Chandak said.
There were several parameters that trended in favor of vaccination, but did not reach statistical significance due to the relatively small sample size, Dr. Chandak said. These included a trend towards more ICU admission in the unvaccinated, compared with vaccinated patients (21% and 12%, respectively; P = .699), a longer ICU stay (1.7 days and 0.2 days; P = .144), more multiorgan dysfunction syndrome (12% and 6%; P = .654), and more acute respiratory distress syndrome (6% and 0%; P = .547). Vasopressors were needed in a similar proportion of patients (12% of both groups).
During the 2009-2010 flu season, H1N1 was the cause of about 61 million cases of influenza in the United States, 274,000 hospitalizations, and 12,470 deaths, Dr. Chandak reported.
Since the 2010-2011 influenza season, the trivalent influenza vaccine has included antigen from the 2009 pandemic H1N1 influenza A virus. This has prevented between 700,000 and 1.5 million cases of H1N1, up to 10,000 hospitalizations, and as many as 500 deaths, according to surveillance data (Emerg Infect Dis. 2013;19[3]:439-48).
The viral subtype made a strong reappearance in the 2015-2016 flu season when it was again the predominant viral subtype of the season, according to the CDC. Most studies have looked at the effectiveness of the vaccine, but have not studied critical care outcomes in vaccinated versus unvaccinated patients, Dr. Chandak noted.
Dr. Chandak reported having no financial disclosures.
Daniel Ouellette, MD, FCCP, comments: “I never take the flu vaccine,” my patient stated, following my suggestion that she be inoculated. “It makes me sick.”
I reflected on the cases of influenza patients that I took care of the previous year in the ICU: the 50-year-old man with no comorbidities who died in respiratory failure; the 32-year-old pregnant woman who survived a 3-month hospitalization during which she was treated with ECMO and suffered irreversible kidney failure. “I take it every year,” I told her.
While the influenza vaccine may not prevent all cases of influenza, those who develop influenza may have an attenuated illness. Data from Chandak and colleagues affirm improved outcomes in patients who receive the vaccine and still develop influenza.
Daniel Ouellette, MD, FCCP, comments: “I never take the flu vaccine,” my patient stated, following my suggestion that she be inoculated. “It makes me sick.”
I reflected on the cases of influenza patients that I took care of the previous year in the ICU: the 50-year-old man with no comorbidities who died in respiratory failure; the 32-year-old pregnant woman who survived a 3-month hospitalization during which she was treated with ECMO and suffered irreversible kidney failure. “I take it every year,” I told her.
While the influenza vaccine may not prevent all cases of influenza, those who develop influenza may have an attenuated illness. Data from Chandak and colleagues affirm improved outcomes in patients who receive the vaccine and still develop influenza.
Daniel Ouellette, MD, FCCP, comments: “I never take the flu vaccine,” my patient stated, following my suggestion that she be inoculated. “It makes me sick.”
I reflected on the cases of influenza patients that I took care of the previous year in the ICU: the 50-year-old man with no comorbidities who died in respiratory failure; the 32-year-old pregnant woman who survived a 3-month hospitalization during which she was treated with ECMO and suffered irreversible kidney failure. “I take it every year,” I told her.
While the influenza vaccine may not prevent all cases of influenza, those who develop influenza may have an attenuated illness. Data from Chandak and colleagues affirm improved outcomes in patients who receive the vaccine and still develop influenza.
TORONTO – Patients who received an influenza vaccination but still required hospitalization for H1N1 influenza had better outcomes, compared with unvaccinated patients, according to findings from a retrospective study.
In the hospital, vaccinated patients had significantly lower rates of acute kidney injury (6% vs. 35%; P = .038) and were more likely to be satisfactorily managed with noninvasive mechanical ventilation (41% vs. 6%; P = .004).
Dr. Chandak and her colleagues studied 72 cases of seasonal influenza requiring hospitalization from September 2015 to April 2016 at Berkshire Medical Center, a 300-bed teaching hospital in western Massachusetts. Based on rapid polymerase chain reaction testing, 51 of these patients were positive for H1N1, of which 38 had received a seasonal flu vaccine.
H1N1 patients who had received vaccination were significantly older (70.4 years vs. 59.6 years; P = .016) and were more often smokers (76% vs. 38%; P = .017), compared with patients who were unvaccinated.
The finding that the unvaccinated patients were younger and still had poorer outcomes, “emphasizes the need for widespread vaccination,” Dr. Chandak said.
There were several parameters that trended in favor of vaccination, but did not reach statistical significance due to the relatively small sample size, Dr. Chandak said. These included a trend towards more ICU admission in the unvaccinated, compared with vaccinated patients (21% and 12%, respectively; P = .699), a longer ICU stay (1.7 days and 0.2 days; P = .144), more multiorgan dysfunction syndrome (12% and 6%; P = .654), and more acute respiratory distress syndrome (6% and 0%; P = .547). Vasopressors were needed in a similar proportion of patients (12% of both groups).
During the 2009-2010 flu season, H1N1 was the cause of about 61 million cases of influenza in the United States, 274,000 hospitalizations, and 12,470 deaths, Dr. Chandak reported.
Since the 2010-2011 influenza season, the trivalent influenza vaccine has included antigen from the 2009 pandemic H1N1 influenza A virus. This has prevented between 700,000 and 1.5 million cases of H1N1, up to 10,000 hospitalizations, and as many as 500 deaths, according to surveillance data (Emerg Infect Dis. 2013;19[3]:439-48).
The viral subtype made a strong reappearance in the 2015-2016 flu season when it was again the predominant viral subtype of the season, according to the CDC. Most studies have looked at the effectiveness of the vaccine, but have not studied critical care outcomes in vaccinated versus unvaccinated patients, Dr. Chandak noted.
Dr. Chandak reported having no financial disclosures.
TORONTO – Patients who received an influenza vaccination but still required hospitalization for H1N1 influenza had better outcomes, compared with unvaccinated patients, according to findings from a retrospective study.
In the hospital, vaccinated patients had significantly lower rates of acute kidney injury (6% vs. 35%; P = .038) and were more likely to be satisfactorily managed with noninvasive mechanical ventilation (41% vs. 6%; P = .004).
Dr. Chandak and her colleagues studied 72 cases of seasonal influenza requiring hospitalization from September 2015 to April 2016 at Berkshire Medical Center, a 300-bed teaching hospital in western Massachusetts. Based on rapid polymerase chain reaction testing, 51 of these patients were positive for H1N1, of which 38 had received a seasonal flu vaccine.
H1N1 patients who had received vaccination were significantly older (70.4 years vs. 59.6 years; P = .016) and were more often smokers (76% vs. 38%; P = .017), compared with patients who were unvaccinated.
The finding that the unvaccinated patients were younger and still had poorer outcomes, “emphasizes the need for widespread vaccination,” Dr. Chandak said.
There were several parameters that trended in favor of vaccination, but did not reach statistical significance due to the relatively small sample size, Dr. Chandak said. These included a trend towards more ICU admission in the unvaccinated, compared with vaccinated patients (21% and 12%, respectively; P = .699), a longer ICU stay (1.7 days and 0.2 days; P = .144), more multiorgan dysfunction syndrome (12% and 6%; P = .654), and more acute respiratory distress syndrome (6% and 0%; P = .547). Vasopressors were needed in a similar proportion of patients (12% of both groups).
During the 2009-2010 flu season, H1N1 was the cause of about 61 million cases of influenza in the United States, 274,000 hospitalizations, and 12,470 deaths, Dr. Chandak reported.
Since the 2010-2011 influenza season, the trivalent influenza vaccine has included antigen from the 2009 pandemic H1N1 influenza A virus. This has prevented between 700,000 and 1.5 million cases of H1N1, up to 10,000 hospitalizations, and as many as 500 deaths, according to surveillance data (Emerg Infect Dis. 2013;19[3]:439-48).
The viral subtype made a strong reappearance in the 2015-2016 flu season when it was again the predominant viral subtype of the season, according to the CDC. Most studies have looked at the effectiveness of the vaccine, but have not studied critical care outcomes in vaccinated versus unvaccinated patients, Dr. Chandak noted.
Dr. Chandak reported having no financial disclosures.
AT CHEST 2017
Key clinical point:
Major finding: Unvaccinated patients had a significantly higher risk of acute kidney injury (35% vs. 6%; P = .038) and were less likely to be managed with noninvasive mechanical ventilation (6% vs. 41%; P = .004).
Data source: Retrospective analysis including 72 reported influenza cases, 51 (71%) testing positive for H1N1.
Disclosures: Dr. Chandak reported having no financial disclosures.
ACIP recommends third MMR dose, if outbreak risk
The Advisory Committee on Immunization Practices voted Oct. 25 to recommend a 3rd dose of measles, mumps, and rubella (MMR) vaccine for individuals at mumps risk from an outbreak.
The recommendation applies to individuals who already have been vaccinated with the usual two doses of MMR “who are identified by public health as at increased risk for mumps because of an outbreak,” according to draft text of the recommendation. This practice would “improve protection against mumps disease and related complications.”
Young adults are at highest risk, she said.
Key evidence supporting the ACIP’s recommendation includes one recent study suggesting a 3rd dose of MMR is effective for mumps outbreak control (N Engl J Med. 2017 Sep 7; doi: 10.1056/NEJMoa1703309).
In that study, Cristina V. Cardemil, MD, of the CDC, and her colleagues looked at college students who received a 3rd MMR dose during an outbreak of at the University of Iowa in Iowa City. Almost a quarter of students (4,783 of 20,496) enrolled in the 2015-2016 academic year received a 3rd dose. Compared with two doses of MMR, students receiving three total doses had a 78% lower risk of mumps at 28 days after vaccination, investigators reported.
“These findings suggest that the campaign to administer a 3rd dose of MMR vaccine improved mumps outbreak control and that waning immunity probably contributed to propagation of the outbreak,” Dr. Cardemil and her colleagues wrote.
The vote in favor of a 3rd dose was unanimous among 15 voting members of ACIP. The committee’s recommendations must be approved by the CDC director before they are considered official recommendations.
The Advisory Committee on Immunization Practices voted Oct. 25 to recommend a 3rd dose of measles, mumps, and rubella (MMR) vaccine for individuals at mumps risk from an outbreak.
The recommendation applies to individuals who already have been vaccinated with the usual two doses of MMR “who are identified by public health as at increased risk for mumps because of an outbreak,” according to draft text of the recommendation. This practice would “improve protection against mumps disease and related complications.”
Young adults are at highest risk, she said.
Key evidence supporting the ACIP’s recommendation includes one recent study suggesting a 3rd dose of MMR is effective for mumps outbreak control (N Engl J Med. 2017 Sep 7; doi: 10.1056/NEJMoa1703309).
In that study, Cristina V. Cardemil, MD, of the CDC, and her colleagues looked at college students who received a 3rd MMR dose during an outbreak of at the University of Iowa in Iowa City. Almost a quarter of students (4,783 of 20,496) enrolled in the 2015-2016 academic year received a 3rd dose. Compared with two doses of MMR, students receiving three total doses had a 78% lower risk of mumps at 28 days after vaccination, investigators reported.
“These findings suggest that the campaign to administer a 3rd dose of MMR vaccine improved mumps outbreak control and that waning immunity probably contributed to propagation of the outbreak,” Dr. Cardemil and her colleagues wrote.
The vote in favor of a 3rd dose was unanimous among 15 voting members of ACIP. The committee’s recommendations must be approved by the CDC director before they are considered official recommendations.
The Advisory Committee on Immunization Practices voted Oct. 25 to recommend a 3rd dose of measles, mumps, and rubella (MMR) vaccine for individuals at mumps risk from an outbreak.
The recommendation applies to individuals who already have been vaccinated with the usual two doses of MMR “who are identified by public health as at increased risk for mumps because of an outbreak,” according to draft text of the recommendation. This practice would “improve protection against mumps disease and related complications.”
Young adults are at highest risk, she said.
Key evidence supporting the ACIP’s recommendation includes one recent study suggesting a 3rd dose of MMR is effective for mumps outbreak control (N Engl J Med. 2017 Sep 7; doi: 10.1056/NEJMoa1703309).
In that study, Cristina V. Cardemil, MD, of the CDC, and her colleagues looked at college students who received a 3rd MMR dose during an outbreak of at the University of Iowa in Iowa City. Almost a quarter of students (4,783 of 20,496) enrolled in the 2015-2016 academic year received a 3rd dose. Compared with two doses of MMR, students receiving three total doses had a 78% lower risk of mumps at 28 days after vaccination, investigators reported.
“These findings suggest that the campaign to administer a 3rd dose of MMR vaccine improved mumps outbreak control and that waning immunity probably contributed to propagation of the outbreak,” Dr. Cardemil and her colleagues wrote.
The vote in favor of a 3rd dose was unanimous among 15 voting members of ACIP. The committee’s recommendations must be approved by the CDC director before they are considered official recommendations.
AT AN ACIP MEETING
Conjugate typhoid vaccine safe and effective in phase 2 trials
A new conjugate typhoid vaccine suitable for administration to infants and young children was efficacious, highly immunogenic, and well tolerated, compared with placebo, in a phase 2 study that tested the vaccine using a human typhoid infection model.
In a study that compared two formulations of typhoid vaccine to a control meningococcal vaccine, the new Vi-conjugate (Vi-TT) vaccine had an efficacy of 54.6% (95% confidence interval, 26.8-71.8) and a 100% seroconversion rate.
The study was not powered for a direct comparison of the efficacy of the Vi-TT with the efficacy of the Vi-polysaccharide (Vi-PS), the other vaccine used in the study. The Vi-PS vaccine had an efficacy of 52.0% (95% CI, 23.2-70.0), and 88.6% of the Vi-PS recipients had seroconversion.
However, “clinical manifestations of typhoid fever seemed less severe among diagnosed participants following Vi-TT vaccination,” Celina Jin, MD, and her colleagues wrote (Lancet. 2017 Sep 28: doi: 10.1016/S0140-6736[17]32149-9). Fever, defined as an oral temperature of 38° C or higher, was seen in 6 of 37 (16%) Vi-TT recipients, 17 of 31 (55%) receiving control, and 11 of 35 (31%) receiving Vi-PS.
Geometric mean titers also were significantly higher in the Vi-TT group than in the Vi-PS group, with an adjusted geometric mean titer of 562.9 EU/mL for Vi-TT and 140.5 EU/mL for Vi-PS (P less than .0001).
The study enrolled 112 healthy adult volunteers who were randomized 1:1:1 to receive Vi-PS, Vi-TT, or control meningococcal vaccine. A total of 103 of the participants eventually received one of the two study vaccines or the control vaccines, and that group was included in the per-protocol analysis.
After vaccination (recipients and investigators were masked as to which formulation participants received), study participants kept an online diary to report any vaccination-related symptoms for 7 days, and also had clinic visits scheduled at days 1, 3, 7, and 10.
Participants received one oral dose of wild-type Salmonella enterica serovar Typhi Quailes strain bacteria about 1 month after vaccination. The dose was 1-5x104 colony forming units, and was administered immediately following a 120-mL oral bolus of sodium bicarbonate (to neutralize stomach acid).
Participants then were seen daily in an outpatient clinic for 2 weeks. At each visit, investigators monitored vital signs, performed a general assessment, and drew blood to assess for typhoid bacteremia. Participants also kept an online diary for 21 days, reporting twice-daily self-measured temperatures as well. No antipyretics were allowed before typhoid diagnosis.
Participants who met the study’s criteria for typhoid diagnosis were treated with a 2-week course of ciprofloxacin or azithromycin; patients who did not become ill were treated 14 days after the oral typhoid challenge. None of the four serious adverse events reported during the study was deemed to be related to vaccination.
That broad definition of typhoid infection was used to determine attack rates for the study’s primary outcome measure. However, Dr. Jin and her colleagues also looked at a less stringent – and perhaps more clinically pertinent – definition of 12 hours of fever of 38° C or higher followed by S. Typhi bacteremia. Using those criteria, the Vi-TT vaccine prevented up to 87% of infections.
Salmonella Typhi is the world’s leading cause of enteric fever, said Dr. Jin, of the Oxford Vaccine Group at the University of Oxford (England). Up to 20.6 million people per year are affected, with children most commonly infected and low-resource populations in Asia and Africa hardest hit.
Both prescription and over-the-counter antibiotics are used worldwide to combat typhoid fever, and S. Typhi strains are becoming increasingly antibiotic resistant in South Asia and Africa, Dr. Jin and her coauthors said.
The typhoid vaccines that are currently licensed are either not suitable for administration to infants and young children, or are insufficiently immunogenic in younger populations.
The typhoid conjugate vaccine used in the study combines the Vi-polysaccharide capsule with a protein carrier, increasing host immunologic response and making the vaccine effective in infancy.
“This human challenge study provides further evidence to support the deployment of Vi-conjugate vaccines as a control measure to reduce the burden of typhoid fever, because those individuals living in endemic regions should not be made to wait another 60 years,” wrote Dr. Jin and her coauthors.
The study was funded by the Bill & Melinda Gates Foundation and the European commission FP7 grant, Advanced Immunization Technologies.
The Oxford Vaccine Group has developed a typhoid challenge model that provides an important bridge in clinical testing and affords the possibility of significant acceleration of the vaccine development process. Despite the controversy human challenge models sometimes engender, previous human typhoid challenge studies contributed to the development of the live attenuated typhoid vaccine Ty21a.
The conjugate vaccine tested by Dr. Jin and her colleagues is a much-needed weapon in the public health armamentarium of typhoid control. Treatment options are limited in regions of South Asia and Africa where endemic typhoid shows increasing antibiotic resistance.
This human challenge study provides the first evidence that the conjugate vaccine reduces the attack rate of typhoid fever, though its use in India has shown it to be safe and immunogenic, even in children as young as 6 months of age.
The stringent definition of typhoid fever attack used in this study may result in a finding of lower efficacy than would be seen in a field trial, and a National Institutes of Health–sponsored study of another conjugate vaccine found efficacy rates of 89% among Vietnamese preschoolers followed for nearly 4 years after vaccination. When the present study’s data were reanalyzed with use of the less stringent case definition of fever followed by typhoid bacteremia, a similar efficacy of 87.1% was seen for the conjugate vaccine. A larger sample size would be needed in a challenge study that included the less stringent definition as a coprimary endpoint, but results might better correlate with real-world field trials.
Phase 3 and 4 trials for the typhoid conjugate vaccine are forthcoming, but final results will not be tallied for many years. The typhoid challenge study reported by Dr. Jin and her colleagues bolsters hopes that the candidate vaccine will help with typhoid control where it’s most needed.
Nicholas A. Feasey, MD , is at the Liverpool (England) School of Tropical Medicine. Myron M. Levine, MD , is at the University of Maryland, Baltimore. Their comments were drawn from an editorial accompanying the study (Lancet. 2017 Sep 28. doi: 10.1016/S0140-6736[17]32407-8 ).
The Oxford Vaccine Group has developed a typhoid challenge model that provides an important bridge in clinical testing and affords the possibility of significant acceleration of the vaccine development process. Despite the controversy human challenge models sometimes engender, previous human typhoid challenge studies contributed to the development of the live attenuated typhoid vaccine Ty21a.
The conjugate vaccine tested by Dr. Jin and her colleagues is a much-needed weapon in the public health armamentarium of typhoid control. Treatment options are limited in regions of South Asia and Africa where endemic typhoid shows increasing antibiotic resistance.
This human challenge study provides the first evidence that the conjugate vaccine reduces the attack rate of typhoid fever, though its use in India has shown it to be safe and immunogenic, even in children as young as 6 months of age.
The stringent definition of typhoid fever attack used in this study may result in a finding of lower efficacy than would be seen in a field trial, and a National Institutes of Health–sponsored study of another conjugate vaccine found efficacy rates of 89% among Vietnamese preschoolers followed for nearly 4 years after vaccination. When the present study’s data were reanalyzed with use of the less stringent case definition of fever followed by typhoid bacteremia, a similar efficacy of 87.1% was seen for the conjugate vaccine. A larger sample size would be needed in a challenge study that included the less stringent definition as a coprimary endpoint, but results might better correlate with real-world field trials.
Phase 3 and 4 trials for the typhoid conjugate vaccine are forthcoming, but final results will not be tallied for many years. The typhoid challenge study reported by Dr. Jin and her colleagues bolsters hopes that the candidate vaccine will help with typhoid control where it’s most needed.
Nicholas A. Feasey, MD , is at the Liverpool (England) School of Tropical Medicine. Myron M. Levine, MD , is at the University of Maryland, Baltimore. Their comments were drawn from an editorial accompanying the study (Lancet. 2017 Sep 28. doi: 10.1016/S0140-6736[17]32407-8 ).
The Oxford Vaccine Group has developed a typhoid challenge model that provides an important bridge in clinical testing and affords the possibility of significant acceleration of the vaccine development process. Despite the controversy human challenge models sometimes engender, previous human typhoid challenge studies contributed to the development of the live attenuated typhoid vaccine Ty21a.
The conjugate vaccine tested by Dr. Jin and her colleagues is a much-needed weapon in the public health armamentarium of typhoid control. Treatment options are limited in regions of South Asia and Africa where endemic typhoid shows increasing antibiotic resistance.
This human challenge study provides the first evidence that the conjugate vaccine reduces the attack rate of typhoid fever, though its use in India has shown it to be safe and immunogenic, even in children as young as 6 months of age.
The stringent definition of typhoid fever attack used in this study may result in a finding of lower efficacy than would be seen in a field trial, and a National Institutes of Health–sponsored study of another conjugate vaccine found efficacy rates of 89% among Vietnamese preschoolers followed for nearly 4 years after vaccination. When the present study’s data were reanalyzed with use of the less stringent case definition of fever followed by typhoid bacteremia, a similar efficacy of 87.1% was seen for the conjugate vaccine. A larger sample size would be needed in a challenge study that included the less stringent definition as a coprimary endpoint, but results might better correlate with real-world field trials.
Phase 3 and 4 trials for the typhoid conjugate vaccine are forthcoming, but final results will not be tallied for many years. The typhoid challenge study reported by Dr. Jin and her colleagues bolsters hopes that the candidate vaccine will help with typhoid control where it’s most needed.
Nicholas A. Feasey, MD , is at the Liverpool (England) School of Tropical Medicine. Myron M. Levine, MD , is at the University of Maryland, Baltimore. Their comments were drawn from an editorial accompanying the study (Lancet. 2017 Sep 28. doi: 10.1016/S0140-6736[17]32407-8 ).
A new conjugate typhoid vaccine suitable for administration to infants and young children was efficacious, highly immunogenic, and well tolerated, compared with placebo, in a phase 2 study that tested the vaccine using a human typhoid infection model.
In a study that compared two formulations of typhoid vaccine to a control meningococcal vaccine, the new Vi-conjugate (Vi-TT) vaccine had an efficacy of 54.6% (95% confidence interval, 26.8-71.8) and a 100% seroconversion rate.
The study was not powered for a direct comparison of the efficacy of the Vi-TT with the efficacy of the Vi-polysaccharide (Vi-PS), the other vaccine used in the study. The Vi-PS vaccine had an efficacy of 52.0% (95% CI, 23.2-70.0), and 88.6% of the Vi-PS recipients had seroconversion.
However, “clinical manifestations of typhoid fever seemed less severe among diagnosed participants following Vi-TT vaccination,” Celina Jin, MD, and her colleagues wrote (Lancet. 2017 Sep 28: doi: 10.1016/S0140-6736[17]32149-9). Fever, defined as an oral temperature of 38° C or higher, was seen in 6 of 37 (16%) Vi-TT recipients, 17 of 31 (55%) receiving control, and 11 of 35 (31%) receiving Vi-PS.
Geometric mean titers also were significantly higher in the Vi-TT group than in the Vi-PS group, with an adjusted geometric mean titer of 562.9 EU/mL for Vi-TT and 140.5 EU/mL for Vi-PS (P less than .0001).
The study enrolled 112 healthy adult volunteers who were randomized 1:1:1 to receive Vi-PS, Vi-TT, or control meningococcal vaccine. A total of 103 of the participants eventually received one of the two study vaccines or the control vaccines, and that group was included in the per-protocol analysis.
After vaccination (recipients and investigators were masked as to which formulation participants received), study participants kept an online diary to report any vaccination-related symptoms for 7 days, and also had clinic visits scheduled at days 1, 3, 7, and 10.
Participants received one oral dose of wild-type Salmonella enterica serovar Typhi Quailes strain bacteria about 1 month after vaccination. The dose was 1-5x104 colony forming units, and was administered immediately following a 120-mL oral bolus of sodium bicarbonate (to neutralize stomach acid).
Participants then were seen daily in an outpatient clinic for 2 weeks. At each visit, investigators monitored vital signs, performed a general assessment, and drew blood to assess for typhoid bacteremia. Participants also kept an online diary for 21 days, reporting twice-daily self-measured temperatures as well. No antipyretics were allowed before typhoid diagnosis.
Participants who met the study’s criteria for typhoid diagnosis were treated with a 2-week course of ciprofloxacin or azithromycin; patients who did not become ill were treated 14 days after the oral typhoid challenge. None of the four serious adverse events reported during the study was deemed to be related to vaccination.
That broad definition of typhoid infection was used to determine attack rates for the study’s primary outcome measure. However, Dr. Jin and her colleagues also looked at a less stringent – and perhaps more clinically pertinent – definition of 12 hours of fever of 38° C or higher followed by S. Typhi bacteremia. Using those criteria, the Vi-TT vaccine prevented up to 87% of infections.
Salmonella Typhi is the world’s leading cause of enteric fever, said Dr. Jin, of the Oxford Vaccine Group at the University of Oxford (England). Up to 20.6 million people per year are affected, with children most commonly infected and low-resource populations in Asia and Africa hardest hit.
Both prescription and over-the-counter antibiotics are used worldwide to combat typhoid fever, and S. Typhi strains are becoming increasingly antibiotic resistant in South Asia and Africa, Dr. Jin and her coauthors said.
The typhoid vaccines that are currently licensed are either not suitable for administration to infants and young children, or are insufficiently immunogenic in younger populations.
The typhoid conjugate vaccine used in the study combines the Vi-polysaccharide capsule with a protein carrier, increasing host immunologic response and making the vaccine effective in infancy.
“This human challenge study provides further evidence to support the deployment of Vi-conjugate vaccines as a control measure to reduce the burden of typhoid fever, because those individuals living in endemic regions should not be made to wait another 60 years,” wrote Dr. Jin and her coauthors.
The study was funded by the Bill & Melinda Gates Foundation and the European commission FP7 grant, Advanced Immunization Technologies.
A new conjugate typhoid vaccine suitable for administration to infants and young children was efficacious, highly immunogenic, and well tolerated, compared with placebo, in a phase 2 study that tested the vaccine using a human typhoid infection model.
In a study that compared two formulations of typhoid vaccine to a control meningococcal vaccine, the new Vi-conjugate (Vi-TT) vaccine had an efficacy of 54.6% (95% confidence interval, 26.8-71.8) and a 100% seroconversion rate.
The study was not powered for a direct comparison of the efficacy of the Vi-TT with the efficacy of the Vi-polysaccharide (Vi-PS), the other vaccine used in the study. The Vi-PS vaccine had an efficacy of 52.0% (95% CI, 23.2-70.0), and 88.6% of the Vi-PS recipients had seroconversion.
However, “clinical manifestations of typhoid fever seemed less severe among diagnosed participants following Vi-TT vaccination,” Celina Jin, MD, and her colleagues wrote (Lancet. 2017 Sep 28: doi: 10.1016/S0140-6736[17]32149-9). Fever, defined as an oral temperature of 38° C or higher, was seen in 6 of 37 (16%) Vi-TT recipients, 17 of 31 (55%) receiving control, and 11 of 35 (31%) receiving Vi-PS.
Geometric mean titers also were significantly higher in the Vi-TT group than in the Vi-PS group, with an adjusted geometric mean titer of 562.9 EU/mL for Vi-TT and 140.5 EU/mL for Vi-PS (P less than .0001).
The study enrolled 112 healthy adult volunteers who were randomized 1:1:1 to receive Vi-PS, Vi-TT, or control meningococcal vaccine. A total of 103 of the participants eventually received one of the two study vaccines or the control vaccines, and that group was included in the per-protocol analysis.
After vaccination (recipients and investigators were masked as to which formulation participants received), study participants kept an online diary to report any vaccination-related symptoms for 7 days, and also had clinic visits scheduled at days 1, 3, 7, and 10.
Participants received one oral dose of wild-type Salmonella enterica serovar Typhi Quailes strain bacteria about 1 month after vaccination. The dose was 1-5x104 colony forming units, and was administered immediately following a 120-mL oral bolus of sodium bicarbonate (to neutralize stomach acid).
Participants then were seen daily in an outpatient clinic for 2 weeks. At each visit, investigators monitored vital signs, performed a general assessment, and drew blood to assess for typhoid bacteremia. Participants also kept an online diary for 21 days, reporting twice-daily self-measured temperatures as well. No antipyretics were allowed before typhoid diagnosis.
Participants who met the study’s criteria for typhoid diagnosis were treated with a 2-week course of ciprofloxacin or azithromycin; patients who did not become ill were treated 14 days after the oral typhoid challenge. None of the four serious adverse events reported during the study was deemed to be related to vaccination.
That broad definition of typhoid infection was used to determine attack rates for the study’s primary outcome measure. However, Dr. Jin and her colleagues also looked at a less stringent – and perhaps more clinically pertinent – definition of 12 hours of fever of 38° C or higher followed by S. Typhi bacteremia. Using those criteria, the Vi-TT vaccine prevented up to 87% of infections.
Salmonella Typhi is the world’s leading cause of enteric fever, said Dr. Jin, of the Oxford Vaccine Group at the University of Oxford (England). Up to 20.6 million people per year are affected, with children most commonly infected and low-resource populations in Asia and Africa hardest hit.
Both prescription and over-the-counter antibiotics are used worldwide to combat typhoid fever, and S. Typhi strains are becoming increasingly antibiotic resistant in South Asia and Africa, Dr. Jin and her coauthors said.
The typhoid vaccines that are currently licensed are either not suitable for administration to infants and young children, or are insufficiently immunogenic in younger populations.
The typhoid conjugate vaccine used in the study combines the Vi-polysaccharide capsule with a protein carrier, increasing host immunologic response and making the vaccine effective in infancy.
“This human challenge study provides further evidence to support the deployment of Vi-conjugate vaccines as a control measure to reduce the burden of typhoid fever, because those individuals living in endemic regions should not be made to wait another 60 years,” wrote Dr. Jin and her coauthors.
The study was funded by the Bill & Melinda Gates Foundation and the European commission FP7 grant, Advanced Immunization Technologies.
FROM THE LANCET
Key clinical point: A conjugate typhoid vaccine significantly reduced typhoid fever rates under a stringent case definition.
Major finding: Efficacy was 54.6% for the Vi-conjugate vaccine, with 100% seroconversion.
Study details: Randomized, controlled phase 2b trial of 112 participants receiving one of two typhoid vaccines, or control meningococcal vaccine.
Disclosures: The study was funded by the Bill & Melinda Gates Foundation and the European Commission FP7 grant, Advanced Immunization Technologies.
In close vote, advisory panel prefers Shingrix over Zostavax
Herpes zoster subunit vaccine (Shingrix) was preferentially recommended over zoster vaccine live (Zostavax) for preventing herpes zoster and related complications Oct. 25 at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Eight committee members voted for the recommendation, and seven voted against it.
In discussions leading up to the vote, some committee members cited potential supply issues, as well as the need for longer-term safety data, among other issues.
“I think it would be nice to see data on a larger population that is not just research-based, especially because we have very little data on ethnic minorities,” said Laura E. Riley, MD, of Harvard Medical School, Boston, who voted against the recommendation.
The vote comes several days after GlaxoSmithKline announced the Food and Drug Administration approval of Shingrix for the prevention of herpes zoster (shingles) in adults aged 50 years or older. In pooled clinical trial results, the vaccine demonstrated greater than 90% efficacy in all age groups, according to a company statement.
Shingrix is a non-live, recombinant subunit vaccine that is given in two doses, intramuscularly. Zostavax, also indicated in individuals aged 50 years or older, is a live attenuated virus vaccine.
In a related decision, ACIP voted 14-1 to recommend Shingrix for prevention of herpes zoster and related complications for immunocompetent adults aged 50 years and older.
They also voted 12-3 to recommend Shingrix to prevent herpes zoster and its complications for immunocompetent adults who previously received Zostavax.
Herpes zoster subunit vaccine (Shingrix) was preferentially recommended over zoster vaccine live (Zostavax) for preventing herpes zoster and related complications Oct. 25 at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Eight committee members voted for the recommendation, and seven voted against it.
In discussions leading up to the vote, some committee members cited potential supply issues, as well as the need for longer-term safety data, among other issues.
“I think it would be nice to see data on a larger population that is not just research-based, especially because we have very little data on ethnic minorities,” said Laura E. Riley, MD, of Harvard Medical School, Boston, who voted against the recommendation.
The vote comes several days after GlaxoSmithKline announced the Food and Drug Administration approval of Shingrix for the prevention of herpes zoster (shingles) in adults aged 50 years or older. In pooled clinical trial results, the vaccine demonstrated greater than 90% efficacy in all age groups, according to a company statement.
Shingrix is a non-live, recombinant subunit vaccine that is given in two doses, intramuscularly. Zostavax, also indicated in individuals aged 50 years or older, is a live attenuated virus vaccine.
In a related decision, ACIP voted 14-1 to recommend Shingrix for prevention of herpes zoster and related complications for immunocompetent adults aged 50 years and older.
They also voted 12-3 to recommend Shingrix to prevent herpes zoster and its complications for immunocompetent adults who previously received Zostavax.
Herpes zoster subunit vaccine (Shingrix) was preferentially recommended over zoster vaccine live (Zostavax) for preventing herpes zoster and related complications Oct. 25 at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Eight committee members voted for the recommendation, and seven voted against it.
In discussions leading up to the vote, some committee members cited potential supply issues, as well as the need for longer-term safety data, among other issues.
“I think it would be nice to see data on a larger population that is not just research-based, especially because we have very little data on ethnic minorities,” said Laura E. Riley, MD, of Harvard Medical School, Boston, who voted against the recommendation.
The vote comes several days after GlaxoSmithKline announced the Food and Drug Administration approval of Shingrix for the prevention of herpes zoster (shingles) in adults aged 50 years or older. In pooled clinical trial results, the vaccine demonstrated greater than 90% efficacy in all age groups, according to a company statement.
Shingrix is a non-live, recombinant subunit vaccine that is given in two doses, intramuscularly. Zostavax, also indicated in individuals aged 50 years or older, is a live attenuated virus vaccine.
In a related decision, ACIP voted 14-1 to recommend Shingrix for prevention of herpes zoster and related complications for immunocompetent adults aged 50 years and older.
They also voted 12-3 to recommend Shingrix to prevent herpes zoster and its complications for immunocompetent adults who previously received Zostavax.
FROM AN ACIP MEETING
Vaccine renaissance
In 1967, pediatric patients were vaccinated routinely against eight diseases with 10 vaccines: smallpox; diphtheria; tetanus and pertussis; polio serotypes 1, 2, and 3; measles; rubella; and mumps. Then in 1989, vaccine discovery took a dramatic upward trend. For the physicians and scientists involved in vaccine discovery, the driving force may have been a passion for scientific discovery and a humanitarian motivation, but what drove this major change in pediatric infectious diseases was economics.
I believe The hiatus of more than 20 years between the introduction of the mumps vaccine in 1967 and that of the Hib vaccine in 1989 in my view was because the economic incentives to develop vaccines were absent. In fact, in the 1970s and early 1980s, vaccine manufacturers were drawing back from making vaccines because they were losing money selling them at a few dollars per dose.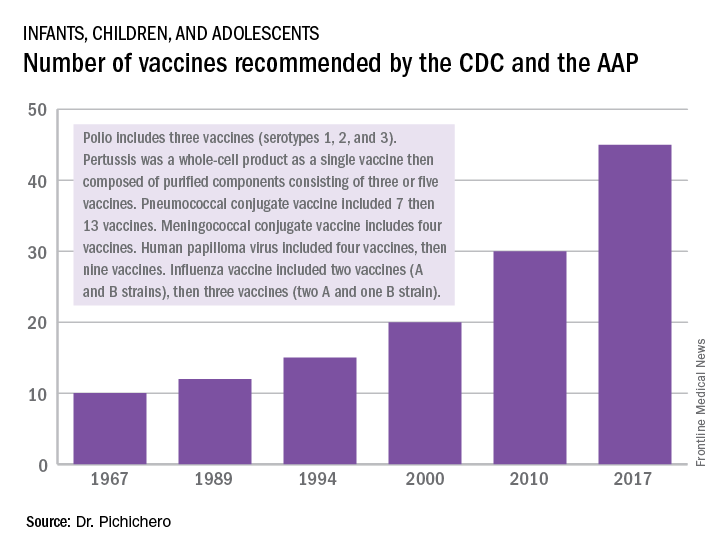
A trailblazing path had been created, and more and more vaccines have been discovered and come to market since then. Combination vaccines and vaccines for adolescents and adults have followed. The biggest blockbuster is Prevnar13 (actually 13 vaccines contained in a single combination), now with annual sales in excess of $7 billion worldwide and growing. Other vaccines with sales of a billion dollars or more are also on the market; anything in excess of $1 billion is considered a blockbuster in the pharmaceutical industry and gets the attention of CEOs (and investors) in a big way.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has received funding awarded to his institution for vaccine research from GlaxoSmithKline, Merck, Pfizer, and Sanofi Pasteur. Email him at pdnews@frontlinemedcom.com.
In 1967, pediatric patients were vaccinated routinely against eight diseases with 10 vaccines: smallpox; diphtheria; tetanus and pertussis; polio serotypes 1, 2, and 3; measles; rubella; and mumps. Then in 1989, vaccine discovery took a dramatic upward trend. For the physicians and scientists involved in vaccine discovery, the driving force may have been a passion for scientific discovery and a humanitarian motivation, but what drove this major change in pediatric infectious diseases was economics.
I believe The hiatus of more than 20 years between the introduction of the mumps vaccine in 1967 and that of the Hib vaccine in 1989 in my view was because the economic incentives to develop vaccines were absent. In fact, in the 1970s and early 1980s, vaccine manufacturers were drawing back from making vaccines because they were losing money selling them at a few dollars per dose.
A trailblazing path had been created, and more and more vaccines have been discovered and come to market since then. Combination vaccines and vaccines for adolescents and adults have followed. The biggest blockbuster is Prevnar13 (actually 13 vaccines contained in a single combination), now with annual sales in excess of $7 billion worldwide and growing. Other vaccines with sales of a billion dollars or more are also on the market; anything in excess of $1 billion is considered a blockbuster in the pharmaceutical industry and gets the attention of CEOs (and investors) in a big way.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has received funding awarded to his institution for vaccine research from GlaxoSmithKline, Merck, Pfizer, and Sanofi Pasteur. Email him at pdnews@frontlinemedcom.com.
In 1967, pediatric patients were vaccinated routinely against eight diseases with 10 vaccines: smallpox; diphtheria; tetanus and pertussis; polio serotypes 1, 2, and 3; measles; rubella; and mumps. Then in 1989, vaccine discovery took a dramatic upward trend. For the physicians and scientists involved in vaccine discovery, the driving force may have been a passion for scientific discovery and a humanitarian motivation, but what drove this major change in pediatric infectious diseases was economics.
I believe The hiatus of more than 20 years between the introduction of the mumps vaccine in 1967 and that of the Hib vaccine in 1989 in my view was because the economic incentives to develop vaccines were absent. In fact, in the 1970s and early 1980s, vaccine manufacturers were drawing back from making vaccines because they were losing money selling them at a few dollars per dose.
A trailblazing path had been created, and more and more vaccines have been discovered and come to market since then. Combination vaccines and vaccines for adolescents and adults have followed. The biggest blockbuster is Prevnar13 (actually 13 vaccines contained in a single combination), now with annual sales in excess of $7 billion worldwide and growing. Other vaccines with sales of a billion dollars or more are also on the market; anything in excess of $1 billion is considered a blockbuster in the pharmaceutical industry and gets the attention of CEOs (and investors) in a big way.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has received funding awarded to his institution for vaccine research from GlaxoSmithKline, Merck, Pfizer, and Sanofi Pasteur. Email him at pdnews@frontlinemedcom.com.
Reminder spurs flu vaccination of chronic disease patients
and because their parents are unaware influenza is risky for their children, reported Gilat Livni, MD, and Alina Wainstein, MD, of Tel Aviv University and their associates.
The investigators surveyed 186 parents of children attending a cardiology institute in Israel regarding flu vaccination. Over a third (37%) of the children had received a flu vaccine during the last flu season. Those who had been vaccinated were significantly more likely to have parents and siblings who were vaccinated (P less than .01).The mean age of the children was 8 years.
The main underlying cardiac diseases in the 186 children were left-to-right shunt defect in 31%, obstructive lesions in 30%, and cyanotic defect in 16%. Other cardiac abnormalities included valvular insufficiency in 11%, cardiomyopathy in 8%, and complete atrioventricular block in 4%.
More than half of parents (59%) reported that a pediatrician had recommended that their child get a flu vaccine; of these parents, 53% complied. Only 13% of parents who did not get such a recommendation had their children vaccinated – a statistically significant difference. Findings were similar regarding recommendations from pediatric cardiologists. “The failure of parents to receive information or advice from a physician regarding vaccination was strongly inversely related to vaccination of the child,” the investigators concluded.
“Our results emphasize the need to raise awareness among physicians and other medical health care personnel dealing with children with heart disease of the importance of properly counseling parents regarding influenza vaccination. Recommending the vaccine should be made part of routine patient visits in fall and winter,” concluded Dr. Livni, Dr. Wainstein, and associates.
The full text is available online (Pediatr Infect Dis J. 2017 Nov;36[11]: e268-71. doi: 10.1097/INF.0000000000001579).
and because their parents are unaware influenza is risky for their children, reported Gilat Livni, MD, and Alina Wainstein, MD, of Tel Aviv University and their associates.
The investigators surveyed 186 parents of children attending a cardiology institute in Israel regarding flu vaccination. Over a third (37%) of the children had received a flu vaccine during the last flu season. Those who had been vaccinated were significantly more likely to have parents and siblings who were vaccinated (P less than .01).The mean age of the children was 8 years.
The main underlying cardiac diseases in the 186 children were left-to-right shunt defect in 31%, obstructive lesions in 30%, and cyanotic defect in 16%. Other cardiac abnormalities included valvular insufficiency in 11%, cardiomyopathy in 8%, and complete atrioventricular block in 4%.
More than half of parents (59%) reported that a pediatrician had recommended that their child get a flu vaccine; of these parents, 53% complied. Only 13% of parents who did not get such a recommendation had their children vaccinated – a statistically significant difference. Findings were similar regarding recommendations from pediatric cardiologists. “The failure of parents to receive information or advice from a physician regarding vaccination was strongly inversely related to vaccination of the child,” the investigators concluded.
“Our results emphasize the need to raise awareness among physicians and other medical health care personnel dealing with children with heart disease of the importance of properly counseling parents regarding influenza vaccination. Recommending the vaccine should be made part of routine patient visits in fall and winter,” concluded Dr. Livni, Dr. Wainstein, and associates.
The full text is available online (Pediatr Infect Dis J. 2017 Nov;36[11]: e268-71. doi: 10.1097/INF.0000000000001579).
and because their parents are unaware influenza is risky for their children, reported Gilat Livni, MD, and Alina Wainstein, MD, of Tel Aviv University and their associates.
The investigators surveyed 186 parents of children attending a cardiology institute in Israel regarding flu vaccination. Over a third (37%) of the children had received a flu vaccine during the last flu season. Those who had been vaccinated were significantly more likely to have parents and siblings who were vaccinated (P less than .01).The mean age of the children was 8 years.
The main underlying cardiac diseases in the 186 children were left-to-right shunt defect in 31%, obstructive lesions in 30%, and cyanotic defect in 16%. Other cardiac abnormalities included valvular insufficiency in 11%, cardiomyopathy in 8%, and complete atrioventricular block in 4%.
More than half of parents (59%) reported that a pediatrician had recommended that their child get a flu vaccine; of these parents, 53% complied. Only 13% of parents who did not get such a recommendation had their children vaccinated – a statistically significant difference. Findings were similar regarding recommendations from pediatric cardiologists. “The failure of parents to receive information or advice from a physician regarding vaccination was strongly inversely related to vaccination of the child,” the investigators concluded.
“Our results emphasize the need to raise awareness among physicians and other medical health care personnel dealing with children with heart disease of the importance of properly counseling parents regarding influenza vaccination. Recommending the vaccine should be made part of routine patient visits in fall and winter,” concluded Dr. Livni, Dr. Wainstein, and associates.
The full text is available online (Pediatr Infect Dis J. 2017 Nov;36[11]: e268-71. doi: 10.1097/INF.0000000000001579).
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Flu study shows overall efficacy of LAIV, but weakness for one strain
Trivalent and quadrivalent inactivated influenza vaccine (IIV) and quadrivalent live attenuated influenza vaccine (LAIV) all gave statistically significant protection against any flu in U.S. children aged 2-17 years in 2015-2016, Katherine A. Poehling, MD, of Wake Forest University, Winston-Salem, N.C., and her associates reported in a study of more than 1,000 children.
“This study also adds to the clinical evidence suggesting that ,” the researchers concluded.
“The 2015-2016 season northern hemisphere trivalent IIV included A/California/7/2009 (H1N1)-like virus, a new A/Switzerland/9715293/2013 (H3N2)-like virus, and a new B/Phuket/3073/2013-like virus (Yamagata lineage),” the investigators noted. “Quadrivalent IIV was similar to trivalent IIV and also included B/Brisbane/60/2008-like virus (Victoria lineage). LAIV was similar to quadrivalent IIV, except that it contained A/Bolivia/559/2013.”
Of the 1,012 children enrolled, 59% were unvaccinated, 10% were given LAIV, 10% received trivalent IIV, 20% were given quadrivalent IIV, and 1% received IIV of “unknown valence.”
Vaccine efficacy against any influenza was 46% for LAIV and 65% for IIV, compared with no vaccination. But only IIV gave “significant protection against influenza A(H1N1)pdm09 strains in the total study population,” Dr. Poehling and her associates said. Vaccine efficacy against influenza A(H1N1)pdm09 strains was 50% for LAIV and 71% for IIV.
Read more in Clinical Infectious Diseases (2017 Oct 4. doi: 10.1093/cid/cix869).
Trivalent and quadrivalent inactivated influenza vaccine (IIV) and quadrivalent live attenuated influenza vaccine (LAIV) all gave statistically significant protection against any flu in U.S. children aged 2-17 years in 2015-2016, Katherine A. Poehling, MD, of Wake Forest University, Winston-Salem, N.C., and her associates reported in a study of more than 1,000 children.
“This study also adds to the clinical evidence suggesting that ,” the researchers concluded.
“The 2015-2016 season northern hemisphere trivalent IIV included A/California/7/2009 (H1N1)-like virus, a new A/Switzerland/9715293/2013 (H3N2)-like virus, and a new B/Phuket/3073/2013-like virus (Yamagata lineage),” the investigators noted. “Quadrivalent IIV was similar to trivalent IIV and also included B/Brisbane/60/2008-like virus (Victoria lineage). LAIV was similar to quadrivalent IIV, except that it contained A/Bolivia/559/2013.”
Of the 1,012 children enrolled, 59% were unvaccinated, 10% were given LAIV, 10% received trivalent IIV, 20% were given quadrivalent IIV, and 1% received IIV of “unknown valence.”
Vaccine efficacy against any influenza was 46% for LAIV and 65% for IIV, compared with no vaccination. But only IIV gave “significant protection against influenza A(H1N1)pdm09 strains in the total study population,” Dr. Poehling and her associates said. Vaccine efficacy against influenza A(H1N1)pdm09 strains was 50% for LAIV and 71% for IIV.
Read more in Clinical Infectious Diseases (2017 Oct 4. doi: 10.1093/cid/cix869).
Trivalent and quadrivalent inactivated influenza vaccine (IIV) and quadrivalent live attenuated influenza vaccine (LAIV) all gave statistically significant protection against any flu in U.S. children aged 2-17 years in 2015-2016, Katherine A. Poehling, MD, of Wake Forest University, Winston-Salem, N.C., and her associates reported in a study of more than 1,000 children.
“This study also adds to the clinical evidence suggesting that ,” the researchers concluded.
“The 2015-2016 season northern hemisphere trivalent IIV included A/California/7/2009 (H1N1)-like virus, a new A/Switzerland/9715293/2013 (H3N2)-like virus, and a new B/Phuket/3073/2013-like virus (Yamagata lineage),” the investigators noted. “Quadrivalent IIV was similar to trivalent IIV and also included B/Brisbane/60/2008-like virus (Victoria lineage). LAIV was similar to quadrivalent IIV, except that it contained A/Bolivia/559/2013.”
Of the 1,012 children enrolled, 59% were unvaccinated, 10% were given LAIV, 10% received trivalent IIV, 20% were given quadrivalent IIV, and 1% received IIV of “unknown valence.”
Vaccine efficacy against any influenza was 46% for LAIV and 65% for IIV, compared with no vaccination. But only IIV gave “significant protection against influenza A(H1N1)pdm09 strains in the total study population,” Dr. Poehling and her associates said. Vaccine efficacy against influenza A(H1N1)pdm09 strains was 50% for LAIV and 71% for IIV.
Read more in Clinical Infectious Diseases (2017 Oct 4. doi: 10.1093/cid/cix869).
FROM CLINICAL INFECTIOUS DISEASES
Vaccination program cut hospital-treated RV gastroenteritis in young children
under 5 years, and it more than adequately pays for itself in secondary health care costs, said Tuija Leino of the National Institute for Health and Welfare, Helsinki, and associates.
Since 2009, all Finnish children younger than 5 years have been offered an RV vaccine.
The investigators conducted a register-based study comparing the RV disease burden before and after introduction of RV vaccination, with the years 1999-2005 as the prevaccine period and the years 2010-2014 as the vaccination period. The study population was all children younger than 5 years living in Finland during the two study periods.
The relative reduction in inpatient RVGE incidence ranged from 86% in the 4-year-old children to 94% in the 0-year-old children, the researchers reported. That amounted to 843 prevented inpatient cases in children under 5 years.
The highest incidence of RVGE, which is rarely treated in hospital outpatients, was 0.4/1,000 person-years in children aged 1 year or younger. The relative reduction in outpatient RVGE incidence was 86% in the 0-year-olds and 100% in the 3- and 4-year-olds. The RV vaccination program prevented only 64 hospital outpatient cases in children younger than 5 years of age in 2014.
RV vaccination also resulted in a reduction of unspecified viral gastroenteritis (UVGE) incidence by 84%. Because the incidence of UVGE in 1-year-olds during the prevaccine era was more than twice the incidence of RVGE, the absolute reduction from 10.7 to 1.7 per 1,000 person-years “reflects a removal of a much larger disease burden than the reduction in the most specific outcome of inpatient RVGE,” the researchers said.
The annually prevented inpatient UVGE cases in children up to 4 years was 1,522, almost twice as many as the prevented inpatient RVGE cases. In the prevaccine era, the UVGE reduction was greatest among 1-year-olds, at 71%. In children younger than 5 years of age, 1,313 UVGE hospital outpatient cases were prevented in 2014.
“Considering secondary health care, the program annually pays for itself almost two times over in Finland,” the investigators concluded.
Read more in Vaccine (2017 Oct 9;35[42]:5611-7).
The researchers had no conflicts of interest.
cnellist@frontlinmedcom.com
under 5 years, and it more than adequately pays for itself in secondary health care costs, said Tuija Leino of the National Institute for Health and Welfare, Helsinki, and associates.
Since 2009, all Finnish children younger than 5 years have been offered an RV vaccine.
The investigators conducted a register-based study comparing the RV disease burden before and after introduction of RV vaccination, with the years 1999-2005 as the prevaccine period and the years 2010-2014 as the vaccination period. The study population was all children younger than 5 years living in Finland during the two study periods.
The relative reduction in inpatient RVGE incidence ranged from 86% in the 4-year-old children to 94% in the 0-year-old children, the researchers reported. That amounted to 843 prevented inpatient cases in children under 5 years.
The highest incidence of RVGE, which is rarely treated in hospital outpatients, was 0.4/1,000 person-years in children aged 1 year or younger. The relative reduction in outpatient RVGE incidence was 86% in the 0-year-olds and 100% in the 3- and 4-year-olds. The RV vaccination program prevented only 64 hospital outpatient cases in children younger than 5 years of age in 2014.
RV vaccination also resulted in a reduction of unspecified viral gastroenteritis (UVGE) incidence by 84%. Because the incidence of UVGE in 1-year-olds during the prevaccine era was more than twice the incidence of RVGE, the absolute reduction from 10.7 to 1.7 per 1,000 person-years “reflects a removal of a much larger disease burden than the reduction in the most specific outcome of inpatient RVGE,” the researchers said.
The annually prevented inpatient UVGE cases in children up to 4 years was 1,522, almost twice as many as the prevented inpatient RVGE cases. In the prevaccine era, the UVGE reduction was greatest among 1-year-olds, at 71%. In children younger than 5 years of age, 1,313 UVGE hospital outpatient cases were prevented in 2014.
“Considering secondary health care, the program annually pays for itself almost two times over in Finland,” the investigators concluded.
Read more in Vaccine (2017 Oct 9;35[42]:5611-7).
The researchers had no conflicts of interest.
cnellist@frontlinmedcom.com
under 5 years, and it more than adequately pays for itself in secondary health care costs, said Tuija Leino of the National Institute for Health and Welfare, Helsinki, and associates.
Since 2009, all Finnish children younger than 5 years have been offered an RV vaccine.
The investigators conducted a register-based study comparing the RV disease burden before and after introduction of RV vaccination, with the years 1999-2005 as the prevaccine period and the years 2010-2014 as the vaccination period. The study population was all children younger than 5 years living in Finland during the two study periods.
The relative reduction in inpatient RVGE incidence ranged from 86% in the 4-year-old children to 94% in the 0-year-old children, the researchers reported. That amounted to 843 prevented inpatient cases in children under 5 years.
The highest incidence of RVGE, which is rarely treated in hospital outpatients, was 0.4/1,000 person-years in children aged 1 year or younger. The relative reduction in outpatient RVGE incidence was 86% in the 0-year-olds and 100% in the 3- and 4-year-olds. The RV vaccination program prevented only 64 hospital outpatient cases in children younger than 5 years of age in 2014.
RV vaccination also resulted in a reduction of unspecified viral gastroenteritis (UVGE) incidence by 84%. Because the incidence of UVGE in 1-year-olds during the prevaccine era was more than twice the incidence of RVGE, the absolute reduction from 10.7 to 1.7 per 1,000 person-years “reflects a removal of a much larger disease burden than the reduction in the most specific outcome of inpatient RVGE,” the researchers said.
The annually prevented inpatient UVGE cases in children up to 4 years was 1,522, almost twice as many as the prevented inpatient RVGE cases. In the prevaccine era, the UVGE reduction was greatest among 1-year-olds, at 71%. In children younger than 5 years of age, 1,313 UVGE hospital outpatient cases were prevented in 2014.
“Considering secondary health care, the program annually pays for itself almost two times over in Finland,” the investigators concluded.
Read more in Vaccine (2017 Oct 9;35[42]:5611-7).
The researchers had no conflicts of interest.
cnellist@frontlinmedcom.com
from vaccine
Maternally derived pneumococcal, meningococcal antibodies may affect vaccine effectiveness
, according to a new study.
That information may be useful in deciding the impact of vaccination programs that use a combination of maternal and infant vaccines and consider schedules with a delayed start, said Merryn Voysey of the University of Oxford (England) and her associates.
In this study, 5,097 children in 16 cohorts from 13 countries had pneumococcal antibody concentrations assessed from blood samples taken before their first dose of vaccine, and 2,925 infants from 5 cohorts in 4 countries had meningococcal antibody concentrations available.
At the time of their first vaccination, the children were ages 5-23 weeks and were from countries in Europe, Africa, Latin America, and South and East Asia. These populations have no routine programs of immunization in pregnancy, the researchers said. So, the maternal antibodies are passively acquired, and the decay rates may differ from those induced by maternal vaccinations.
The seroprevalence of maternal antibodies in infants was 92% for pneumococcal serotype 14 and 80% for serotype 19F; it was 30% for serotype 4 and 34% for serotype 1. Thirteen percent of infants had detectable levels of group C meningococcal antibodies prior to vaccination, and 43% had group A antibodies.
For the pneumococcal antibodies, “there was statistically significant variation in half-life estimates between country cohorts and between serotypes (both P less than .0001),” the researchers said. The half-life estimate was lowest – at 39 days – for serotype 6B, and highest – at 48 days – for serotype 5. The overall estimate across serotypes was 43 days.
“The age of the child was not significantly associated with decay rates (P = .103), confirming the assumption of exponential decay,” they said.
For the meningococcal antibodies, the half-lives were 43 days for group A and 40 days for group C.
“Substantial proportions of infants have antibodies to many vaccine serotypes of pneumococcus at the age when a vaccine program might normally commence,” the investigators noted. “Conversely, antibodies against capsular groups A and C meningococcal polysaccharides were less common, particularly for group C, which was only present in 13% of infants in the four countries contained in this analysis.
“Higher levels of group A meningococcal antibodies than group C have also been seen in unvaccinated adults of childbearing age in the Netherlands, and in mothers in the United Kingdom,” the researchers added. “Passively acquired maternal antibody has been shown to adversely affect the magnitude of the immune response to vaccination with pneumococcal conjugate vaccine, and increase the occurrence of otitis media in infants under 6 months of age.”
The proportion of infants who had maternal antipneumococcal antibodies differed between serotypes, the authors noted. Almost all infants had serotype 14 pneumococcal antibodies, and very high proportions of infants had serotype 19F antibodies.
“We have previously shown that the antibody response to vaccination with pneumococcal conjugate vaccine is adversely affected by the presence of maternal antibody,” the investigators said. “This inhibitory effect is greatest for serotype 14, with children seropositive from maternal antibodies having a response to vaccination that is only three-quarters the magnitude of those with no maternal antibody.”
Read more in Vaccine (2017 Oct 13;35[43]:5850-7).
, according to a new study.
That information may be useful in deciding the impact of vaccination programs that use a combination of maternal and infant vaccines and consider schedules with a delayed start, said Merryn Voysey of the University of Oxford (England) and her associates.
In this study, 5,097 children in 16 cohorts from 13 countries had pneumococcal antibody concentrations assessed from blood samples taken before their first dose of vaccine, and 2,925 infants from 5 cohorts in 4 countries had meningococcal antibody concentrations available.
At the time of their first vaccination, the children were ages 5-23 weeks and were from countries in Europe, Africa, Latin America, and South and East Asia. These populations have no routine programs of immunization in pregnancy, the researchers said. So, the maternal antibodies are passively acquired, and the decay rates may differ from those induced by maternal vaccinations.
The seroprevalence of maternal antibodies in infants was 92% for pneumococcal serotype 14 and 80% for serotype 19F; it was 30% for serotype 4 and 34% for serotype 1. Thirteen percent of infants had detectable levels of group C meningococcal antibodies prior to vaccination, and 43% had group A antibodies.
For the pneumococcal antibodies, “there was statistically significant variation in half-life estimates between country cohorts and between serotypes (both P less than .0001),” the researchers said. The half-life estimate was lowest – at 39 days – for serotype 6B, and highest – at 48 days – for serotype 5. The overall estimate across serotypes was 43 days.
“The age of the child was not significantly associated with decay rates (P = .103), confirming the assumption of exponential decay,” they said.
For the meningococcal antibodies, the half-lives were 43 days for group A and 40 days for group C.
“Substantial proportions of infants have antibodies to many vaccine serotypes of pneumococcus at the age when a vaccine program might normally commence,” the investigators noted. “Conversely, antibodies against capsular groups A and C meningococcal polysaccharides were less common, particularly for group C, which was only present in 13% of infants in the four countries contained in this analysis.
“Higher levels of group A meningococcal antibodies than group C have also been seen in unvaccinated adults of childbearing age in the Netherlands, and in mothers in the United Kingdom,” the researchers added. “Passively acquired maternal antibody has been shown to adversely affect the magnitude of the immune response to vaccination with pneumococcal conjugate vaccine, and increase the occurrence of otitis media in infants under 6 months of age.”
The proportion of infants who had maternal antipneumococcal antibodies differed between serotypes, the authors noted. Almost all infants had serotype 14 pneumococcal antibodies, and very high proportions of infants had serotype 19F antibodies.
“We have previously shown that the antibody response to vaccination with pneumococcal conjugate vaccine is adversely affected by the presence of maternal antibody,” the investigators said. “This inhibitory effect is greatest for serotype 14, with children seropositive from maternal antibodies having a response to vaccination that is only three-quarters the magnitude of those with no maternal antibody.”
Read more in Vaccine (2017 Oct 13;35[43]:5850-7).
, according to a new study.
That information may be useful in deciding the impact of vaccination programs that use a combination of maternal and infant vaccines and consider schedules with a delayed start, said Merryn Voysey of the University of Oxford (England) and her associates.
In this study, 5,097 children in 16 cohorts from 13 countries had pneumococcal antibody concentrations assessed from blood samples taken before their first dose of vaccine, and 2,925 infants from 5 cohorts in 4 countries had meningococcal antibody concentrations available.
At the time of their first vaccination, the children were ages 5-23 weeks and were from countries in Europe, Africa, Latin America, and South and East Asia. These populations have no routine programs of immunization in pregnancy, the researchers said. So, the maternal antibodies are passively acquired, and the decay rates may differ from those induced by maternal vaccinations.
The seroprevalence of maternal antibodies in infants was 92% for pneumococcal serotype 14 and 80% for serotype 19F; it was 30% for serotype 4 and 34% for serotype 1. Thirteen percent of infants had detectable levels of group C meningococcal antibodies prior to vaccination, and 43% had group A antibodies.
For the pneumococcal antibodies, “there was statistically significant variation in half-life estimates between country cohorts and between serotypes (both P less than .0001),” the researchers said. The half-life estimate was lowest – at 39 days – for serotype 6B, and highest – at 48 days – for serotype 5. The overall estimate across serotypes was 43 days.
“The age of the child was not significantly associated with decay rates (P = .103), confirming the assumption of exponential decay,” they said.
For the meningococcal antibodies, the half-lives were 43 days for group A and 40 days for group C.
“Substantial proportions of infants have antibodies to many vaccine serotypes of pneumococcus at the age when a vaccine program might normally commence,” the investigators noted. “Conversely, antibodies against capsular groups A and C meningococcal polysaccharides were less common, particularly for group C, which was only present in 13% of infants in the four countries contained in this analysis.
“Higher levels of group A meningococcal antibodies than group C have also been seen in unvaccinated adults of childbearing age in the Netherlands, and in mothers in the United Kingdom,” the researchers added. “Passively acquired maternal antibody has been shown to adversely affect the magnitude of the immune response to vaccination with pneumococcal conjugate vaccine, and increase the occurrence of otitis media in infants under 6 months of age.”
The proportion of infants who had maternal antipneumococcal antibodies differed between serotypes, the authors noted. Almost all infants had serotype 14 pneumococcal antibodies, and very high proportions of infants had serotype 19F antibodies.
“We have previously shown that the antibody response to vaccination with pneumococcal conjugate vaccine is adversely affected by the presence of maternal antibody,” the investigators said. “This inhibitory effect is greatest for serotype 14, with children seropositive from maternal antibodies having a response to vaccination that is only three-quarters the magnitude of those with no maternal antibody.”
Read more in Vaccine (2017 Oct 13;35[43]:5850-7).
FROM VACCINE
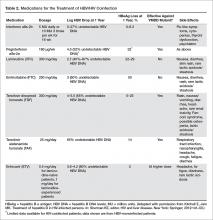
Management of Patients with HIV and Hepatitis B Coinfection
From UT Southwestern Medical Center, Dallas, TX.
Abstract
- Objective: To review the literature on and provide evidence-based recommendations for management of HIV/ hepatitis B (HBV) coinfection.
- Methods: Review of the literature for clinical trials, guidelines, and cohort studies on HIV/HBV disease management.
- Results: HIV patients should be evaluated for viral hepatitis. Those who do not have evidence of immunity should be vaccinated and monitored for response. Those who have HIV/HBV should have additional serologies checked to evaluate for hepatitis B e antigen status and level of viremia. All HIV/HBV coinfected patients should be started on antiretroviral therapy with tenofovir-based regimens. Those with HIV/HBV and cirrhosis should be screened for hepatocellular cancer every 6 months.
- Conclusion: HIV patients should be vaccinated against hepatitis B; those with coinfection should be treated for both viruses. It is important to monitor for treatment response to both HIV and HBV and liver disease complications.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Morbidity and mortality for HIV-infected patients remain high compared to uninfected patients despite effective virologic suppression. Major contributors to illness and death among these patients include cardiovascular disease, non–AIDS-defining malignancies, and chronic liver disease, specifically viral hepatitis [1]. Hepatitis B virus (HBV) infection is one of the main causes of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) globally. Because HIV and HBV can both be acquired through injection drug use and sexual transmission, coinfection occurs frequently. The Joint United Nations Program on HIV/AIDS estimates that 10% of the 33 million HIV-positive patients worldwide have simultaneous chronic HBV infection [2].
HIV/HBV coinfection significantly impacts the natural history, progression, and mortality related to both viruses. HIV infection accelerates HBV-related liver impairment, leading to earlier cirrhosis, end-stage liver disease, and HCC. Conversely, chronic HBV does not have a considerable influence in the progression of HIV; however, antiretroviral treatment (ART) toxicities and/or HBV flares due to immune reconstitution inflammatory syndrome (IRIS) or HBV itself can lead to increased liver-related complications [3,4].
Case Patient 1
Initial Presentation and History
An asymptomatic 38-year-old man diagnosed with HIV infection 1 month ago presents for his initial visit to establish HIV care. The patient is a man who has sex with men (MSM) and is currently sexually active with multiple partners. He reports inconsistent use of condoms. One month ago he underwent routine screening and was found to be HIV-positive. At the time of diagnosis, the patient’s baseline CD4 cell count was 328 cells/µL and his viral load was 182,600 copies/mL. The patient wants to discuss the implications of his new diagnosis of HIV and recommendations for further testing and treatment. He is especially interested in HBV screening, since one of his recent partners was known to be positive. The patient has no relevant past medical history. He does not recall the details of his childhood vaccinations. He denies smoking and injection drug use but reports moderate alcohol consumption.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies show normal complete blood count (CBC) and renal and liver function test results, and a baseline HIV genotype does not show resistance. Hepatitis A total antibody (anti-HAV) testing is positive, while tests for hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), and hepatitis C antibody (anti-HCV) are negative.
• Which screening tests for HBV should be performed in HIV-infected patients?
Routine screening of all HIV-infected patients for hepatitis A virus, HBV, and hepatitis C virus (HCV) is recommended [5]. Routine HBV screening involves obtaining serologies for HBsAg, anti-HBs, and anti-HBc. With these results, patients can be classified into categories of either active infection, immunity, or no evidence of prior exposure (Table 1).
• How effective is HBV vaccination in the HIV population?
Vaccination
Available HBV vaccines in the United States include 2 single-agent vaccines (Recombivax HB [Merck, Whitehouse Station, NJ] and Engerix-B [GlaxoSmithKline, Research Triangle Park, NC]) as well as a combination HAV/HBV vaccine (Twinrix [GlaxoSmithKline]). For adults (age ≥ 20 years) with an immunocompromising condition such as HIV infection, current Centers for Disease Control and Prevention guidelines recommend three 40 µg/mL doses of single-agent vaccine administered at 0, 1, and 6 months (Recombivax HB), or four 40 µg/mL doses of single-agent vaccine (2 doses of 20 µg/mL administered simultaneously) at 0, 1, 2, and 6 months (Engerix-B) [7].
The immunogenicity to HBV vaccination in HIV-positive patients is decreased, reflected by lower antibody titers, waning immunity, and seroconversion rates of 18% to 65% [8–11]. Factors associated with poor response include low CD4 cell counts, detectable HIV RNA, coinfection with HCV, and the general health status of the patient [8]. Ideally, HBV vaccination should occur prior to decline in CD4 cell count below 350 cells/µL. However, guidelines do not recommend deferring vaccination since some patients with advanced HIV disease will seroconvert [6].
Anti-HBs titers should be checked 1 month after completion of the vaccine series to confirm protective antibody titers. For patients with quantitative anti-HBs levels < 10 IU/mL, a second vaccine cycle is recommended. Some specialists may defer revaccination until a sustained increase in CD4 count is achieved on ART.
Two randomized controlled trials have demonstrated that 4 doses of double-dose (40 µg/mL) vaccine generate higher anti-HBs levels than 3 doses of standard-dose (20 µg/mL) vaccine in HIV-infected adults [12,13]. Another study showed that HIV patients with CD4 counts > 350 cells/µL had better responses when immunized with double- dose vaccines on the usual 3-dose series [14]. Currently, the CDC recommends giving a double dose for either a 3-dose schedule or a 4-dose schedule. However, it remains unclear what dosing schedule to use if a patient fails to respond. Likely waiting until the CD4 cell count has increased and HIV viral load is suppressed will be important to seeing a response.
• What approach for HBV prevention should be taken in this patient?
The patient’s serologies confirm no prior exposure to HBV, and he should be offered HBV vaccination. His CD4 cell count is below 350/µL and he has ongoing HIV viremia, which increases his risk for an inadequate response. However, vaccination should not be delayed, particularly given his high risk of sexual transmission. The patient should be counseled regarding all high-risk behaviors. As discussed above, 3 or 4 doses of the higher dose vaccine (40 µg/mL) should be administered depending on what type of recombinant vaccine is available. An anti-HBs level should be checked 1 month after completion. A full repeat vaccine series using the 40 µg/mL dose should be considered for nonresponders who initially received a standard vaccine series. Experts also recommend checking annual anti-HBs levels to monitor for waning immunity, with a booster dose given if the anti-HBs level drops below the protective range.
• What vaccination strategy should be used in patients with isolated positive anti-HBc?
The clinical implications of an isolated positive anti-HBc for vaccination are still uncertain. This serologic pattern may represent a false-positive test, remote HBV infection with loss of anti-HBs, or occult HBV infection with undetectable HBsAg. The latter scenario appears more commonly in HIV-infected patients, particularly with concomitant HCV infection [15].
A recent study suggested that patients with an isolated positive anti-HBc with a negative anamnestic antibody response (anti-HBs titer of < 10 IU/mL 4 weeks after a single 20 µg dose of recombinant HBV vaccine) should be further vaccinated with the double-dose for a 3-dose schedule [6]. Additionally, another study followed HIV/HCV coinfected patients for 9.5 years and found that an isolated positive anti-HBc was not associated with accelerated liver disease progression [16].
Treatment of HIV-1 Infection
Current HIV guidelines recommend initiation of ART for all HIV-infected patients regardless of their CD4 count [17]. ART for a treatment-naïve patient usually consists of 2 nucleotide/nucleoside reverse-transcriptase inhibitors (NRTIs; the “backbone”) combined with a third agent (the “anchor”), which can be a nonnucleoside reverse-transcriptase inhibitor (NNRTI), a protease inhibitor (PI) boosted with a boosting agent, or an integrase strand-transfer inhibitor (INSTI) [18,19].
There are numerous studies indicating that incident HBV risk can be reduced by placing those at risk for HBV acquisition on ART containing a combination of tenofovir disoproxil fumarate (TDF), lamivudine, or emtricitabine [20,21]. Another study in MSM found those on ART with HIV viral load < 400 copies/mL were protected from developing HBV compared to those not on ART [22]. Given this patient’s higher risk for HBV acquisition, placing him on emtricitabine/TDF backbone as part of ART could be protective against incident HBV [23].
Case Patient 2
Initial Presentation and History
A 32-year-old man diagnosed with HIV infection 8 years ago now on ART presents for follow-up. The patient is an MSM with a history of inconsistent condom use. At the time of HIV diagnosis 8 years ago, the patient had a CD4 cell count of 250 cells/µL and an HIV viral load of 648,000 copies/mL. The patient was initiated on lamivudine/zidovudine and lopinavir/ritonavir, and he achieved complete virologic suppression at 20 weeks, with a CD4 cell count of 455 cells/µL at 1 year. The patient has remained on this regimen without major side effects; however, he reports frequent missed doses over the last 2 years due to “pill fatigue.” Previous testing for HBV and HCV at the time of his initial HIV diagnosis was negative, but he failed to complete the HBV vaccine series. He denies alcohol or injection drug use.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies reveal normal electrolytes and renal function, hemoglobin of 11 g/dL, and platelet count of 235,000/µL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are 34 U/L and 44 U/L, respectively, with an INR of 1.1 and albumin level of 3.4 g/dL. CD4 cell count is 320 cells/µL with an HIV viral load of 24,500 copies/mL. Viral hepatitis serologies show anti-HAV positive, anti-HCV negative, HBsAg positive, anti-HBc IgG positive, hepatitis Be antigen (HBeAg) negative, and hepatitis B e antibody (anti-HBe) positive. HBV DNA viral load is 685,000 IU/mL.
• What is the natural history of HIV/HBV coinfection?
Chronic HBV infection affects about 10% of HIV-infected patients globally. Epidemiologic studies indicate that HIV-infected patients have higher rates of reactivation and progression to chronic HBV infection and chronic liver disease than HIV-negative patients [24–26]. Coinfected patients demonstrate higher serum HBV DNA levels, which lead to more rapid progression of hepatic fibrosis and may increase the risk of cirrhosis and HCC [24,27,28]. HIV infection, however, can mediate the necroinflammatory response through blunting of the immune response that drives pathogenesis in HBV infection. Aminotransferase levels may be only slightly elevated or even normal, particularly in the severely immune suppressed. However, elevation in liver enzymes (hepatitis flare) can occur if a patient stops ART, or if HBV resistance develops. Patients being treated for HIV/HBV coinfection should be counseled that stopping HIV treatment puts them at risk of developing a hepatitis flare.
Large cohort studies of HIV/HBV coinfected patients indicate increased risk of liver-related mortality, most pronounced with lower CD4 cell counts [1,28,29]. Introduction of ART appears to increase rather than attenuate this liver-related mortality, possibly by decreasing AIDS-related mortality and allowing more time for liver disease progression. Finally, a recent meta-analysis including 12,382 patients demonstrated a significant effect of HIV/HBV coinfection on all-cause mortality, with a pooled effect estimate of 1.36 [30].
• What diagnostic testing should be done in coinfected patients?
Diagnostic Testing and Evaluation
Persistence of HBsAg for more than 6 months is diagnostic of chronic HBV infection and warrants further serologic evaluation. Patients with chronic HBV infection should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels measured. Elevation of AST and ALT can be seen with untreated HBV. For those on treatment, a hepatitis flare could be caused by abrupt discontinuation of HBV treatment, development of HBV resistance, or superinfection with another viral pathogen.
HBeAg is a marker of active viral replication and is associated with higher levels of HBV DNA and active liver disease. Seroconversion, or loss of HBeAg and development of anti-HBe, heralds a favorable treatment response for those who were initially HBeAg-positive. However, some HBV variants have precore or core promoter mutations that lead to higher levels of HBV DNA in the absence of HBeAg. This highlights the importance of monitoring HBV DNA levels in all patients with chronic HBV infection. Favorable response to therapy in HBeAg-negative patients is marked by normalization of aminotransferases and HBV DNA suppression.Hepatitis D virus (HDV) is a defective virus particle that can only replicate in the presence of HBV. Coinfected patients should be tested for anti-HDV if they are injection drug users or are from a high-prevalence region such as the Mediterranean and Amazon basins. Newly acquired HDV infection should also be considered in the context of hepatitis flares.
Although these routine diagnostic tests are essential for management, studies show low adherence rates to HBV testing guidelines by HIV providers [31,32].
• What is the role of HBV genotype and resistance testing?
HBV can be classified into 10 different genotypes, A through J, based on genomic sequence variations. Each genotype has a distinct ethnic and geographic distribution, with genotypes A and D predominating in North America. HBV genotyping appears to have important prognostic as well as treatment implications [19]. However, data are still preliminary and the guidelines do not recommend routine genotype testing.
Resistance testing in HBV allows for detection of mutations that decrease effectiveness of antivirals. Exposure to lamivudine can lead to mutations in the YMDD region of the HBV DNA polymerase, resulting in drug resistance. Resistance to lamivudine develops at a rate of approximately 25% after 1 year of drug exposure in HIV/HBV coinfection [33]. On the other hand, studies have shown that entecavir is active against HIV and, most importantly, selects for the M184V mutation in HIV. M184V is associated with lamivudine resistance for HIV treatment, thus limiting treatment options [34,35]. After these findings, the FDA advised against monotherapy with entecavir in patients with HIV/HBV coinfection. The case patient was on lamivudine during the time of HBV acquisition, and therefore YMDD mutation must be taken into consideration for therapy purposes. He should be switched to a regimen that suppresses both viruses. The development of drug resistance should be assessed in all patients with persistent or breakthrough HBV viremia on ART, particularly the nucleoside analogues. HIV providers treating HIV/HBV coinfection should regularly monitor both HIV and HBV viral load to assess for therapeutic efficacy.
• What is the role of liver biopsy in this patient?
Liver biopsy should be considered in all coinfected patients as it remains the gold standard for determining the activity and severity of chronic hepatitis B. However, because it carries inherent risks and is not required prior to treatment in all patients, the decision should be individualized. Liver biopsy can be useful to assess baseline liver histology and may be warranted to rule out significant coexisting genetic or metabolic liver disease. Currently, noninvasive methods to assess liver fibrosis either using elastography or various combinations of serum biomarkers are being evaluated [36] and may be considered in lieu of a liver biopsy [37]. One study compared the accuracy of elastography with liver biopsy in HIV/HBV coinfected patients and demonstrated that the former was proficient in discriminating between absence or mild fibrosis and moderate to severe fibrosis [38]. In general, this test has high accuracy in detecting minimal fibrosis from advanced fibrosis or cirrhosis. For the group in the middle, further investigation with additional methods must be considered [37]. Finally, a recent retrospective study involving 70 HIV/HBV coinfected individuals showed fibrosis regression suggesting beneficial effects of long-term ART on liver stiffness, [39] but further studies are needed to confirm these findings.
Case 2 Continued
The patient has now been diagnosed with chronic HBV infection. His diagnostic testing is negative for HBeAg and reveals modest HBV viremia with abnormal aminotransferases less than 2 times the upper limit of normal. Drug resistance testing reveals a mutation in the YMDD region indicative of HBV lamivudine resistance. HIV genotype demonstrates a wild-type virus without resistance. The patient asks what treatment options exist.
• What medications are currently available to treat hepatitis B?
Treatment
All patients with HIV/HBV coinfection should receive treatment to suppress both viruses, and ART needs to include 2 drugs active against HBV, ideally emtricitabine and TDF. This approach prevents drug resistance, slows progression of HBV infection, and reduces the incidence of IRIS [5]. TDF has been associated with decreased renal function and bone mineral density. Recently, tenofovir alafenamide fumarate (TAF) was approved for the treatment of HIV and HBV. A dose of 10 mg daily is given when coadministered with ritonavir, cobicistat, or protease inhibitors, but a dose of 25 mg should be given when administered with NNRTIs or integrase inhibitors. Compared to TDF, TAF shows less accumulation of tenofovir in kidneys and bones and consequently has reduced renal and bone mineral density effects [40]. All patients should be on a TDF- or TAF-based HIV regimen if they have chronic HBV.
Currently, the following antiviral drugs are FDA-approved for the treatment of HBV infection: interferon alfa-2b, pegylated interferon (peginterferon) alfa-2a, lamivudine and emtricitabine, entecavir, adefovir, TDF, TAF, and telbivudine. Telbivudine and adefovir are no longer recommended due to their association with higher incidence of toxicity and higher rates of HBV treatment failure. A summary of available HBV treatment options is outlined in Table 2.
Interferon
Standard interferon alfa-2b blocks HBV replication through interaction with viral proteins and stimulation of host cellular immunity. Peginterferon alfa-2a has proven efficacy in HBV-monoinfected patients, but efficacy data in HIV/HBV coinfected patients is lacking [41,42]. Studies in hepatitis C treatment demonstrate the safety of peginterferon alfa-2a use in HIV-positive patients and indicate that HIV viral suppression occurs with peginterferon without evidence of selection of resistance mutations that affect future ART options [43]. For HIV/HBV coinfected patients not on ART who will receive only therapy for HBV (which is infrequent since all HIV patients should be on ART), pegylated interferon-alfa-2a alone for 48 weeks is the only option that will not cause ART-associated HIV drug resistance [5]. Interferon therapy is contraindicated in patients with decompensated cirrhosis and should be used with caution in patients with active depression, uncontrolled diabetes, and cardiac and pulmonary disease.
Lamivudine and Emtricitabine
Lamivudine is a nucleoside analogue with efficacy against both HIV and HBV. Clinical trials in HIV/HBV-infected patients have shown up to 87% of patients achieve undetectable HBV DNA levels and about 25% achieve HBeAg seroconversion after 1 to 2 years of therapy [44,45]. However, the major issue limiting use of lamivudine is its low genetic barrier to resistance. Mutation of the YMDD motif of the HBV DNA polymerase confers HBV resistance. HIV/HBV coinfected patients develop resistance at rates of up to 94% after 4 years of therapy [33], heralded by rebounds in HBV DNA levels and often hepatitis flares or precipitation of hepatic failure [16]. Because of resistance, lamivudine monotherapy should be avoided; even in patients on ART, abrupt withdrawal of lamivudine or the development of HBV resistance should be closely monitored.
Emtricitabine is another nucleoside analogue with properties and efficacy similar to lamivudine. It is frequently used as a combination pill with TDF (Truvada) in coinfected patients. The same concerns regarding monotherapy and the emergence of resistance that exist for lamivudine apply to emtricitabine.
Tenofovir
TDF, a nucleotide analogue, is one of the preferred first-line agents for HIV treatment and has proven efficacy against both wild-type and lamivudine-resistant HBV. Since it was first used for HIV, TDF has been more extensively studied in coinfected patients compared to most other agents. In a meta-analysis of patients with HIV/HBV coinfection, TDF suppressed HBV viral load to undetectable titers in approximately 90% of patients [46]. Tenofovir is available in 2 preparations: TDF and TAF. TDF has been reported to cause renal impairment as well as bone loss. TAF has shown less renal toxicity and less bone damage [40,47]. In 2016, TAF became available as part as 4 regimens: stand-alone TAF, elvitegravir-cobicistat-emtricitabine-TAF, rilpivirine-emtricitabine-TAF, and TAF-emtricitabine.
TDF and TAF both suppress HIV. Two large randomized trials of HBV monoinfection demonstrate that TAF is noninferior to TDF for the treatment of naïve and treatment-experienced patients [48,49].
Entecavir
Entecavir, a guanosine analogue, is a potent HBV DNA polymerase inhibitor that results in greater virologic suppression compared to lamivudine and retains activity against lamivudine-resistant HBV [50]. Importantly, entecavir shares some cross-resistance with lamivudine, so an entecavir dose of 1 mg daily is recommended in lamivudine-experienced patients compared to 0.5 mg daily in lamivudine-naïve patients. Entecavir requires dose reduction for patients with creatinine clearance less than 50 mL/min, although it is not associated with renal toxicity. A 1-log10 reduction in HIV RNA levels as well as emergence of M184V mutations on entecavir monotherapy has been reported [51,52]. M184V confers HIV resistance to lamivudine and emtricitabine. Therefore, entecavir should not be used as monotherapy in HIV-coinfected patients and/or patients with evidence of lamivudine-resistant HBV.
Combination Therapy
Recent updates in the guidelines recommend that since emtricitabine, lamivudine, TDF, and TAF are active against both viruses, patients with coinfection should start ART with a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual drug combination TDF plus lamivudine [53]. Most experts recommend the use of combination HBV therapy in patients on ART, particularly with lamivudine given the high rates of resistance.
• When should HBV treatment be started in patients with coinfection?
An ART regimen containing TDF (creatinine clearance > 50 mL/min) or TAF (creatinine clearance > 30 mL/min) with lamivudine or emtricitabine should be used in all HIV/HBV patients as soon as the infection is diagnosed. If TDF or TAF cannot be used, the alternate recommended regimen for HBV is entecavir plus a fully suppressive ART. In those with decreased renal function, entecavir should be adjusted to renal function [19].
Although control of viremia is feasible, clearance of infection as marked by loss of HBsAg and development of anti-HBs is unlikely to occur in the majority of patients. Therefore, the goals of treatment focus on prevention of chronic liver disease complications by suppressing viral replication, which can halt disease progression. A suggested algorithm for the management of coinfected patients is provided (Figure).
• What is the duration of therapy for hepatitis B?
Most patients with HIV/HBV coinfection will require lifelong treatment. All patients on HBV therapy as a part of ART should continue HBV therapy, regardless of seroconversion status. Also, patients should be educated and advised against self-discontinuation as it may trigger hepatitis exacerbations and/or hepatic failure.
Case Patient 3
Initial Presentation and History
Two months after starting treatment for HIV and chronic HBV infection, a 46-year-old Hispanic woman presents to clinic with jaundice and right upper quadrant (RUQ) pain. The patient was recently diagnosed with HIV infection and was naïve to treatment with ART. Her CD4 cell count was 50 cells/µL, and her HIV viral load was 743,000 copies/mL, with no baseline mutations on HIV genotype. The patient was also diagnosed with chronic HBV infection with positive HBsAg and HBeAg and negative HBc IgM serologies, as well as an HBV DNA level of 87 million IU/mL. Routine blood work revealed normal renal function and serum transaminases. The decision was made to start the patient on darunavir/ritonavir and TDF/emtricitabine. The patient was also started on sulfamethoxazole/trimethoprim and azithromycin for opportunistic infection prophylaxis.
Physical Examination and Laboratory Testing
Examination is remarkable for mild tenderness in the RUQ and icteric sclera. Laboratory testing demonstrates the following: AST, 1523 U/L; ALT, 795 U/L; albumin, 2.8 mg/dL; and total bilirubin, 3.5 mg/dL. Her CD4 count has increased to 565 cells/µL, and her HIV viral load is 4320 copies/mL. Results of repeat hepatitis serologies are as follows: HBsAg positive, anti-HBc IgM positive, and an HBV DNA level of 4.2 million IU/mL. Testing for hepatitis A, C, and D is negative, and RUQ sonogram reveals no gallstones.
• What monitoring should be done for coinfected patients on HBV therapy?
Monitoring
Providers should routinely monitor patients’ response to HIV/HBV therapy. Initially, all coinfected patients should have liver function tests and HBV DNA levels checked every 12 weeks on therapy. Frequent monitoring allows early detection of HBV drug resistance as well as drug-related hepatotoxicity. In HBeAg-positive coinfected patients who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. In HBeAg-negative patients, only HBV DNA and liver function tests are needed. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy.
Typically, virologic failure results from either the development of drug resistance or abrupt withdrawal of active HBV therapy due to patient nonadherence or changes to the ART regimen. Virologic failure can result in a rise in serum aminotransferases as well as decompensation in patients with significant underlying liver disease. Due to this risk, providers must counsel patients about the importance of adherence to therapy and should continue medications active against HBV when making a change in ART regimens, unless HBV drug resistance dictates a change in HBV therapy.
• What is the likely cause of this patient’s hepatitis “flare”?
Several studies indicate that patients with HIV/HBV coinfection are at increased risk of drug-related hepatotoxicity and grade 4 liver enzyme elevations [54,55].The first 3 months after initiation of ART is a particularly vulnerable time for liver injury. The differential diagnosis for an acute hepatitis “flare” following the initiation of ART is broad and includes the following: development of HBV drug resistance [16]; withdrawal of HBV-active medications due to nonadherence [54]; ART-related hepatotoxicity; superimposed infection with HAV, HCV, or HDV; other opportunistic infections including cytomegalovirus and mycobacterium avium complex; or IRIS, resulting in an exaggerated cytotoxic response by the recovering immune system [56,57]. Complete evaluation is critical to distinguish between the possible causes.
In this case, several clues point toward HBV-related IRIS as the most likely cause for the hepatitis “flare.” A low pretreatment CD4 cell count with a rapid rise after initiation of ART is associated with a higher rate of IRIS [57]. Serologic testing and imaging excluded superinfection with another hepatotropic virus or biliary tract disease. Appropriate declines in HIV viral load and HBV DNA levels imply patient adherence to therapy and argue against the development of HBV drug resistance. Finally, the emergence of anti-HBc IgM positivity signals HBV reactivation, which is commonly seen in patients with HBV-related IRIS [57]. The preferred treatment for HBV-related IRIS involves continuation of therapy, frequently leading to normalization of aminotransferases and subsequent HBeAg seroconversion. Because IRIS usually manifests within the first 6 to 12 weeks after starting ART, liver enzymes should be monitored closely during this period.
• What health maintenance should be done for coinfected patients?
All patients with HIV/HBV coinfection should be monitored for evidence of portal hypertension or cirrhosis and, if these conditions exist, should undergo endoscopic screening for esophageal varices as well as evaluation of ascites and encephalopathy. Patients with HBV are at increased risk for the development of HCC even in the absence of cirrhosis. A recent study showed low rates of HCC screening in HIV/HBV patients by HIV providers [58]. Whether HIV coinfection potentiates HCC risk is uncertain, though coinfected patients present at younger ages and with more symptoms than HIV-negative comparators [59]. Other risk factors for HCC include HCV infection, alcohol abuse, diabetes, obesity, exposure to environmental toxins, and cirrhosis of any etiology (most commonly non-alcoholic fatty liver disease, primary sclerosing cholangitis, primary biliary cirrhosis and hemochromatosis) [60].
The American Association of Liver Diseases (AASLD) guidelines recommend hepatic ultrasound screening every 6 months in all patients with cirrhosis or chronic HBV who are at increased risk (Asian men over the age of 40 years, Asian women over the age of 50 years, African or North American blacks, and patients with family history of HCC) [61]. They should also be referred for an esophagogastroduodenoscopy to evaluate for esophageal varices. In addition, all HIV/HBV coinfected patients with decompensated liver disease should be evaluated for transplantation. HIV infection is not a contraindication for liver transplant with the use of ART. However, since transplantation does not cure HBV infection, post-transplant HBV immune globulin and HBV treatment are required. Contemporary data suggest comparable survival rates after transplant in coinfected patients compared to HBV-monoinfected patients [51].
Summary
Routine screening with HBsAg, anti-HBs, and anti-HBc serologies is recommended for all HIV-positive individuals. Patients without evidence of prior exposure or vaccination and those with isolated anti-HBc should be offered vaccination. HIV-positive adults should receive three or four 40 µg/mL doses of single agent vaccine depending on the recombinant vaccine type available. Anti-HBsAg titers should be checked 1 month after completion of the immunization series. If quantitative anti-HBsAg levels are < 10 IU/mL, patients should receive a second vaccine cycle.
Patients who test positive for HBsAg should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels checked as well. All patients with HIV/HBV coinfection should start treatment as soon as HIV infection is diagnosed. ART needs to include 2 drugs against HBV, and therefore a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual combination of TDF plus lamivudine should be used.
Coinfected patients on treatment should have liver function tests as well as HBV DNA every 12 weeks. In HBeAg-positive coinfected individuals who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy. Those with virologic failure should be tested for HBV resistance thorough HBV genotype. Coinfected patients with cirrhosis should receive ultrasound screening every 6 months for evidence of HCC and esophagogastroduodenoscopy to evaluate for esophageal varices.
1. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–41.
2. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global change. N Engl J Med 2012;366:1749–52.
3. Bellini C, Keise O, Chave JP, et al. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: the Swiss HIV Cohort Study. HIV Med 2009;10:12–8.
4. Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996-2001. AIDS 2003;17:2191–18.
5. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Q1–Q17.
6. Piroth L, Launay O, Michel ML, et al. Vaccination against hepatitis B virus (HBV) in HIV-1-infected patients with isolated anti-HBV core antibody: the ANRS HB EP03 CISOVAC prospective study. J Infect Dis 2016;213:1735–42.
7. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Accessed 22 Dec 2016 at www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
8. Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine 2000;18:1161–5.
9. Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005;41:1045–8.
10. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
11. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
12. Chaiklang K, Wipasa J, Chaiwarith R, et al. Comparison of immunogenicity and safety of four doses and four double doses vs. standard doses of hepatitis B vaccination in HIV-infected adults: a randomized, controlled trial. PLoS One 2013;8:e80409.
13. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
14. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
15. Gandhi RT, Wurcel A, Lee H, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis 2005;191:1435–41.
16. French AL, Hotton A, Young M, et al. Isolated hepatitis B core antibody status is not associated with accelerated liver disease progression in HIV/hepatitis C coinfection. J Acquir Immune Defic Syndr 2016;72:274–80.
17. INSIGHT START Study Group, Lundgren JD, Babiker GA, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
18. Sebaaly JC, Kelley D. Single-tablet regimens for the treatment of HIV-1 infection. Ann Pharmacother 2017;51:332–44.
19. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
20. Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014;28:999–1005.
21. Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on Hepatitis B virus infection. Clin Infect Dis 2013;56:1812–9.
22. Falade-Nwulia O, Seaberg EC, Snider AE, et al. Incident hepatitis B virus infection in HIV-infected and HIV-uninfected men who have sex with men from pre-HAART to HAART periods: a cohort study. Ann Intern Med 2015;163:673–80.
23. Shilaih M, Marzel A, Scherrer AU, et al. Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016;214:599–606.
24. Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999;29:1306–10.
25. Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS 1997;11:597–606.
26. Bodsworth N, Donovan B, Nightingale BN. The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: a
study of 150 homosexual men. J Infect Dis 1989;160:577–82.
27. Di Martino V, Thevenot T, Colin JF, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002;123:1812–22.
28. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360(9349):1921–6.
29. Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005;19:593–601.
30. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009;48:1763–71.
31. Jain MK, Opio CK, Osuagwu CC, et al. Do HIV care providers appropriately manage hepatitis B in coinfected patients treated with antiretroviral therapy? Clin Infect Dis 2007;44:996–1000.
32. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;61:1742–8.
33. Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006;20:863–70.
34. McMahon M, Jilek B, Brennan T, Thio C. The HBV drug entecavir: effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
35. Jain M, Zoellner C. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007; 21:2365–6.
36. Bottero J, Lacombe K, Guechot J, et al. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV coinfected patients. J Hepatol 2009;50:1074–83.
37. Moreno S, Garcia-Samaniego J, Moreno A, et al. Noninvasive diagnosis of liver fibrosis in patients with HIV infection and HCV/HBV co-infection. J Viral Hepat 2009;16:249–58.
38. Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011;18:61–9.
39. Audsley J, Robson C, Aitchison S, et al. Liver fibrosis regression measured by transient elastography in human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfected individuals on long-term HBV-active combination antiretroviral therapy. Open Forum Infect Dis 2016;3:ofw035.
40. Achhra AC, Nugent M, Mocroft A, et al. Chronic kidney disease and antiretroviral therapy in HIV-positive individuals: recent developments. Curr HIV/AIDS Rep 2016;13:149–57.
41. Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–17.
42. Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95.
43. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004;351:438–50.44. Dore GJ, Cooper DA, Barrett C, et al. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis 1999;180:607–13.
45. Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of antihuman immunodeficiency virus regimens. Clin Infect Dis 2001;32:963–9.
46. Price H, Dunn D, PIllary D, et al. Suppresion of HBV by tenofovir in HIV/HBV coinfected patients: a systemic review and meta-analysis. PLoS One 2013;8:e68152.
47. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with eltegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomized, double-blind, phase 3, non-inferiority trial. Lancet 2015;385:2606–15.
48. Chan HLY, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-positive chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:185–95.
49. Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-negative chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:196–206.
50. Chang TT, Lai CL. Hepatitis B virus with primary resistance to adefovir. N Engl J Med 2006;355:322–3.
51. McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir -- effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
52. Jain MK, Zoellner CL. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007;21:2365–6.
53. Nuesch R, Ananworanich J, Srasuebkul P, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS 2008;22:152–4.
54. Gaglio PJ, Sterling R, Daniels E, Tedaldi E. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis 2007;45:618–23.
55. Reisler RB, Han C, Burman WJ, et al. Grade 4 events are as important as AIDS events in the era of HAART. J Acquir Immune Defic Syndr 2003;34:379–86.
56. Drake A, Mijch A, Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis 2004;39:129–32.
57. Jain MK, Parekh NK, Hester J, Lee WM. Aminotransferase elevation in HIV/hepatitis B virus co-infected patients treated with two active hepatitis B virus drugs. AIDS Patient Care STDS 2006;20:817–22.
58. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;611742–8.
59. Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.–Canadian multicenter study. J Hepatol 2007;47:527–37.
60. Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 2011;9:617–23.
61. Bruix J, Sherman M. AASLD Practice Guideline. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2.
From UT Southwestern Medical Center, Dallas, TX.
Abstract
- Objective: To review the literature on and provide evidence-based recommendations for management of HIV/ hepatitis B (HBV) coinfection.
- Methods: Review of the literature for clinical trials, guidelines, and cohort studies on HIV/HBV disease management.
- Results: HIV patients should be evaluated for viral hepatitis. Those who do not have evidence of immunity should be vaccinated and monitored for response. Those who have HIV/HBV should have additional serologies checked to evaluate for hepatitis B e antigen status and level of viremia. All HIV/HBV coinfected patients should be started on antiretroviral therapy with tenofovir-based regimens. Those with HIV/HBV and cirrhosis should be screened for hepatocellular cancer every 6 months.
- Conclusion: HIV patients should be vaccinated against hepatitis B; those with coinfection should be treated for both viruses. It is important to monitor for treatment response to both HIV and HBV and liver disease complications.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Morbidity and mortality for HIV-infected patients remain high compared to uninfected patients despite effective virologic suppression. Major contributors to illness and death among these patients include cardiovascular disease, non–AIDS-defining malignancies, and chronic liver disease, specifically viral hepatitis [1]. Hepatitis B virus (HBV) infection is one of the main causes of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) globally. Because HIV and HBV can both be acquired through injection drug use and sexual transmission, coinfection occurs frequently. The Joint United Nations Program on HIV/AIDS estimates that 10% of the 33 million HIV-positive patients worldwide have simultaneous chronic HBV infection [2].
HIV/HBV coinfection significantly impacts the natural history, progression, and mortality related to both viruses. HIV infection accelerates HBV-related liver impairment, leading to earlier cirrhosis, end-stage liver disease, and HCC. Conversely, chronic HBV does not have a considerable influence in the progression of HIV; however, antiretroviral treatment (ART) toxicities and/or HBV flares due to immune reconstitution inflammatory syndrome (IRIS) or HBV itself can lead to increased liver-related complications [3,4].
Case Patient 1
Initial Presentation and History
An asymptomatic 38-year-old man diagnosed with HIV infection 1 month ago presents for his initial visit to establish HIV care. The patient is a man who has sex with men (MSM) and is currently sexually active with multiple partners. He reports inconsistent use of condoms. One month ago he underwent routine screening and was found to be HIV-positive. At the time of diagnosis, the patient’s baseline CD4 cell count was 328 cells/µL and his viral load was 182,600 copies/mL. The patient wants to discuss the implications of his new diagnosis of HIV and recommendations for further testing and treatment. He is especially interested in HBV screening, since one of his recent partners was known to be positive. The patient has no relevant past medical history. He does not recall the details of his childhood vaccinations. He denies smoking and injection drug use but reports moderate alcohol consumption.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies show normal complete blood count (CBC) and renal and liver function test results, and a baseline HIV genotype does not show resistance. Hepatitis A total antibody (anti-HAV) testing is positive, while tests for hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), and hepatitis C antibody (anti-HCV) are negative.
• Which screening tests for HBV should be performed in HIV-infected patients?
Routine screening of all HIV-infected patients for hepatitis A virus, HBV, and hepatitis C virus (HCV) is recommended [5]. Routine HBV screening involves obtaining serologies for HBsAg, anti-HBs, and anti-HBc. With these results, patients can be classified into categories of either active infection, immunity, or no evidence of prior exposure (Table 1).
• How effective is HBV vaccination in the HIV population?
Vaccination
Available HBV vaccines in the United States include 2 single-agent vaccines (Recombivax HB [Merck, Whitehouse Station, NJ] and Engerix-B [GlaxoSmithKline, Research Triangle Park, NC]) as well as a combination HAV/HBV vaccine (Twinrix [GlaxoSmithKline]). For adults (age ≥ 20 years) with an immunocompromising condition such as HIV infection, current Centers for Disease Control and Prevention guidelines recommend three 40 µg/mL doses of single-agent vaccine administered at 0, 1, and 6 months (Recombivax HB), or four 40 µg/mL doses of single-agent vaccine (2 doses of 20 µg/mL administered simultaneously) at 0, 1, 2, and 6 months (Engerix-B) [7].
The immunogenicity to HBV vaccination in HIV-positive patients is decreased, reflected by lower antibody titers, waning immunity, and seroconversion rates of 18% to 65% [8–11]. Factors associated with poor response include low CD4 cell counts, detectable HIV RNA, coinfection with HCV, and the general health status of the patient [8]. Ideally, HBV vaccination should occur prior to decline in CD4 cell count below 350 cells/µL. However, guidelines do not recommend deferring vaccination since some patients with advanced HIV disease will seroconvert [6].
Anti-HBs titers should be checked 1 month after completion of the vaccine series to confirm protective antibody titers. For patients with quantitative anti-HBs levels < 10 IU/mL, a second vaccine cycle is recommended. Some specialists may defer revaccination until a sustained increase in CD4 count is achieved on ART.
Two randomized controlled trials have demonstrated that 4 doses of double-dose (40 µg/mL) vaccine generate higher anti-HBs levels than 3 doses of standard-dose (20 µg/mL) vaccine in HIV-infected adults [12,13]. Another study showed that HIV patients with CD4 counts > 350 cells/µL had better responses when immunized with double- dose vaccines on the usual 3-dose series [14]. Currently, the CDC recommends giving a double dose for either a 3-dose schedule or a 4-dose schedule. However, it remains unclear what dosing schedule to use if a patient fails to respond. Likely waiting until the CD4 cell count has increased and HIV viral load is suppressed will be important to seeing a response.
• What approach for HBV prevention should be taken in this patient?
The patient’s serologies confirm no prior exposure to HBV, and he should be offered HBV vaccination. His CD4 cell count is below 350/µL and he has ongoing HIV viremia, which increases his risk for an inadequate response. However, vaccination should not be delayed, particularly given his high risk of sexual transmission. The patient should be counseled regarding all high-risk behaviors. As discussed above, 3 or 4 doses of the higher dose vaccine (40 µg/mL) should be administered depending on what type of recombinant vaccine is available. An anti-HBs level should be checked 1 month after completion. A full repeat vaccine series using the 40 µg/mL dose should be considered for nonresponders who initially received a standard vaccine series. Experts also recommend checking annual anti-HBs levels to monitor for waning immunity, with a booster dose given if the anti-HBs level drops below the protective range.
• What vaccination strategy should be used in patients with isolated positive anti-HBc?
The clinical implications of an isolated positive anti-HBc for vaccination are still uncertain. This serologic pattern may represent a false-positive test, remote HBV infection with loss of anti-HBs, or occult HBV infection with undetectable HBsAg. The latter scenario appears more commonly in HIV-infected patients, particularly with concomitant HCV infection [15].
A recent study suggested that patients with an isolated positive anti-HBc with a negative anamnestic antibody response (anti-HBs titer of < 10 IU/mL 4 weeks after a single 20 µg dose of recombinant HBV vaccine) should be further vaccinated with the double-dose for a 3-dose schedule [6]. Additionally, another study followed HIV/HCV coinfected patients for 9.5 years and found that an isolated positive anti-HBc was not associated with accelerated liver disease progression [16].
Treatment of HIV-1 Infection
Current HIV guidelines recommend initiation of ART for all HIV-infected patients regardless of their CD4 count [17]. ART for a treatment-naïve patient usually consists of 2 nucleotide/nucleoside reverse-transcriptase inhibitors (NRTIs; the “backbone”) combined with a third agent (the “anchor”), which can be a nonnucleoside reverse-transcriptase inhibitor (NNRTI), a protease inhibitor (PI) boosted with a boosting agent, or an integrase strand-transfer inhibitor (INSTI) [18,19].
There are numerous studies indicating that incident HBV risk can be reduced by placing those at risk for HBV acquisition on ART containing a combination of tenofovir disoproxil fumarate (TDF), lamivudine, or emtricitabine [20,21]. Another study in MSM found those on ART with HIV viral load < 400 copies/mL were protected from developing HBV compared to those not on ART [22]. Given this patient’s higher risk for HBV acquisition, placing him on emtricitabine/TDF backbone as part of ART could be protective against incident HBV [23].
Case Patient 2
Initial Presentation and History
A 32-year-old man diagnosed with HIV infection 8 years ago now on ART presents for follow-up. The patient is an MSM with a history of inconsistent condom use. At the time of HIV diagnosis 8 years ago, the patient had a CD4 cell count of 250 cells/µL and an HIV viral load of 648,000 copies/mL. The patient was initiated on lamivudine/zidovudine and lopinavir/ritonavir, and he achieved complete virologic suppression at 20 weeks, with a CD4 cell count of 455 cells/µL at 1 year. The patient has remained on this regimen without major side effects; however, he reports frequent missed doses over the last 2 years due to “pill fatigue.” Previous testing for HBV and HCV at the time of his initial HIV diagnosis was negative, but he failed to complete the HBV vaccine series. He denies alcohol or injection drug use.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies reveal normal electrolytes and renal function, hemoglobin of 11 g/dL, and platelet count of 235,000/µL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are 34 U/L and 44 U/L, respectively, with an INR of 1.1 and albumin level of 3.4 g/dL. CD4 cell count is 320 cells/µL with an HIV viral load of 24,500 copies/mL. Viral hepatitis serologies show anti-HAV positive, anti-HCV negative, HBsAg positive, anti-HBc IgG positive, hepatitis Be antigen (HBeAg) negative, and hepatitis B e antibody (anti-HBe) positive. HBV DNA viral load is 685,000 IU/mL.
• What is the natural history of HIV/HBV coinfection?
Chronic HBV infection affects about 10% of HIV-infected patients globally. Epidemiologic studies indicate that HIV-infected patients have higher rates of reactivation and progression to chronic HBV infection and chronic liver disease than HIV-negative patients [24–26]. Coinfected patients demonstrate higher serum HBV DNA levels, which lead to more rapid progression of hepatic fibrosis and may increase the risk of cirrhosis and HCC [24,27,28]. HIV infection, however, can mediate the necroinflammatory response through blunting of the immune response that drives pathogenesis in HBV infection. Aminotransferase levels may be only slightly elevated or even normal, particularly in the severely immune suppressed. However, elevation in liver enzymes (hepatitis flare) can occur if a patient stops ART, or if HBV resistance develops. Patients being treated for HIV/HBV coinfection should be counseled that stopping HIV treatment puts them at risk of developing a hepatitis flare.
Large cohort studies of HIV/HBV coinfected patients indicate increased risk of liver-related mortality, most pronounced with lower CD4 cell counts [1,28,29]. Introduction of ART appears to increase rather than attenuate this liver-related mortality, possibly by decreasing AIDS-related mortality and allowing more time for liver disease progression. Finally, a recent meta-analysis including 12,382 patients demonstrated a significant effect of HIV/HBV coinfection on all-cause mortality, with a pooled effect estimate of 1.36 [30].
• What diagnostic testing should be done in coinfected patients?
Diagnostic Testing and Evaluation
Persistence of HBsAg for more than 6 months is diagnostic of chronic HBV infection and warrants further serologic evaluation. Patients with chronic HBV infection should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels measured. Elevation of AST and ALT can be seen with untreated HBV. For those on treatment, a hepatitis flare could be caused by abrupt discontinuation of HBV treatment, development of HBV resistance, or superinfection with another viral pathogen.
HBeAg is a marker of active viral replication and is associated with higher levels of HBV DNA and active liver disease. Seroconversion, or loss of HBeAg and development of anti-HBe, heralds a favorable treatment response for those who were initially HBeAg-positive. However, some HBV variants have precore or core promoter mutations that lead to higher levels of HBV DNA in the absence of HBeAg. This highlights the importance of monitoring HBV DNA levels in all patients with chronic HBV infection. Favorable response to therapy in HBeAg-negative patients is marked by normalization of aminotransferases and HBV DNA suppression.Hepatitis D virus (HDV) is a defective virus particle that can only replicate in the presence of HBV. Coinfected patients should be tested for anti-HDV if they are injection drug users or are from a high-prevalence region such as the Mediterranean and Amazon basins. Newly acquired HDV infection should also be considered in the context of hepatitis flares.
Although these routine diagnostic tests are essential for management, studies show low adherence rates to HBV testing guidelines by HIV providers [31,32].
• What is the role of HBV genotype and resistance testing?
HBV can be classified into 10 different genotypes, A through J, based on genomic sequence variations. Each genotype has a distinct ethnic and geographic distribution, with genotypes A and D predominating in North America. HBV genotyping appears to have important prognostic as well as treatment implications [19]. However, data are still preliminary and the guidelines do not recommend routine genotype testing.
Resistance testing in HBV allows for detection of mutations that decrease effectiveness of antivirals. Exposure to lamivudine can lead to mutations in the YMDD region of the HBV DNA polymerase, resulting in drug resistance. Resistance to lamivudine develops at a rate of approximately 25% after 1 year of drug exposure in HIV/HBV coinfection [33]. On the other hand, studies have shown that entecavir is active against HIV and, most importantly, selects for the M184V mutation in HIV. M184V is associated with lamivudine resistance for HIV treatment, thus limiting treatment options [34,35]. After these findings, the FDA advised against monotherapy with entecavir in patients with HIV/HBV coinfection. The case patient was on lamivudine during the time of HBV acquisition, and therefore YMDD mutation must be taken into consideration for therapy purposes. He should be switched to a regimen that suppresses both viruses. The development of drug resistance should be assessed in all patients with persistent or breakthrough HBV viremia on ART, particularly the nucleoside analogues. HIV providers treating HIV/HBV coinfection should regularly monitor both HIV and HBV viral load to assess for therapeutic efficacy.
• What is the role of liver biopsy in this patient?
Liver biopsy should be considered in all coinfected patients as it remains the gold standard for determining the activity and severity of chronic hepatitis B. However, because it carries inherent risks and is not required prior to treatment in all patients, the decision should be individualized. Liver biopsy can be useful to assess baseline liver histology and may be warranted to rule out significant coexisting genetic or metabolic liver disease. Currently, noninvasive methods to assess liver fibrosis either using elastography or various combinations of serum biomarkers are being evaluated [36] and may be considered in lieu of a liver biopsy [37]. One study compared the accuracy of elastography with liver biopsy in HIV/HBV coinfected patients and demonstrated that the former was proficient in discriminating between absence or mild fibrosis and moderate to severe fibrosis [38]. In general, this test has high accuracy in detecting minimal fibrosis from advanced fibrosis or cirrhosis. For the group in the middle, further investigation with additional methods must be considered [37]. Finally, a recent retrospective study involving 70 HIV/HBV coinfected individuals showed fibrosis regression suggesting beneficial effects of long-term ART on liver stiffness, [39] but further studies are needed to confirm these findings.
Case 2 Continued
The patient has now been diagnosed with chronic HBV infection. His diagnostic testing is negative for HBeAg and reveals modest HBV viremia with abnormal aminotransferases less than 2 times the upper limit of normal. Drug resistance testing reveals a mutation in the YMDD region indicative of HBV lamivudine resistance. HIV genotype demonstrates a wild-type virus without resistance. The patient asks what treatment options exist.
• What medications are currently available to treat hepatitis B?
Treatment
All patients with HIV/HBV coinfection should receive treatment to suppress both viruses, and ART needs to include 2 drugs active against HBV, ideally emtricitabine and TDF. This approach prevents drug resistance, slows progression of HBV infection, and reduces the incidence of IRIS [5]. TDF has been associated with decreased renal function and bone mineral density. Recently, tenofovir alafenamide fumarate (TAF) was approved for the treatment of HIV and HBV. A dose of 10 mg daily is given when coadministered with ritonavir, cobicistat, or protease inhibitors, but a dose of 25 mg should be given when administered with NNRTIs or integrase inhibitors. Compared to TDF, TAF shows less accumulation of tenofovir in kidneys and bones and consequently has reduced renal and bone mineral density effects [40]. All patients should be on a TDF- or TAF-based HIV regimen if they have chronic HBV.
Currently, the following antiviral drugs are FDA-approved for the treatment of HBV infection: interferon alfa-2b, pegylated interferon (peginterferon) alfa-2a, lamivudine and emtricitabine, entecavir, adefovir, TDF, TAF, and telbivudine. Telbivudine and adefovir are no longer recommended due to their association with higher incidence of toxicity and higher rates of HBV treatment failure. A summary of available HBV treatment options is outlined in Table 2.
Interferon
Standard interferon alfa-2b blocks HBV replication through interaction with viral proteins and stimulation of host cellular immunity. Peginterferon alfa-2a has proven efficacy in HBV-monoinfected patients, but efficacy data in HIV/HBV coinfected patients is lacking [41,42]. Studies in hepatitis C treatment demonstrate the safety of peginterferon alfa-2a use in HIV-positive patients and indicate that HIV viral suppression occurs with peginterferon without evidence of selection of resistance mutations that affect future ART options [43]. For HIV/HBV coinfected patients not on ART who will receive only therapy for HBV (which is infrequent since all HIV patients should be on ART), pegylated interferon-alfa-2a alone for 48 weeks is the only option that will not cause ART-associated HIV drug resistance [5]. Interferon therapy is contraindicated in patients with decompensated cirrhosis and should be used with caution in patients with active depression, uncontrolled diabetes, and cardiac and pulmonary disease.
Lamivudine and Emtricitabine
Lamivudine is a nucleoside analogue with efficacy against both HIV and HBV. Clinical trials in HIV/HBV-infected patients have shown up to 87% of patients achieve undetectable HBV DNA levels and about 25% achieve HBeAg seroconversion after 1 to 2 years of therapy [44,45]. However, the major issue limiting use of lamivudine is its low genetic barrier to resistance. Mutation of the YMDD motif of the HBV DNA polymerase confers HBV resistance. HIV/HBV coinfected patients develop resistance at rates of up to 94% after 4 years of therapy [33], heralded by rebounds in HBV DNA levels and often hepatitis flares or precipitation of hepatic failure [16]. Because of resistance, lamivudine monotherapy should be avoided; even in patients on ART, abrupt withdrawal of lamivudine or the development of HBV resistance should be closely monitored.
Emtricitabine is another nucleoside analogue with properties and efficacy similar to lamivudine. It is frequently used as a combination pill with TDF (Truvada) in coinfected patients. The same concerns regarding monotherapy and the emergence of resistance that exist for lamivudine apply to emtricitabine.
Tenofovir
TDF, a nucleotide analogue, is one of the preferred first-line agents for HIV treatment and has proven efficacy against both wild-type and lamivudine-resistant HBV. Since it was first used for HIV, TDF has been more extensively studied in coinfected patients compared to most other agents. In a meta-analysis of patients with HIV/HBV coinfection, TDF suppressed HBV viral load to undetectable titers in approximately 90% of patients [46]. Tenofovir is available in 2 preparations: TDF and TAF. TDF has been reported to cause renal impairment as well as bone loss. TAF has shown less renal toxicity and less bone damage [40,47]. In 2016, TAF became available as part as 4 regimens: stand-alone TAF, elvitegravir-cobicistat-emtricitabine-TAF, rilpivirine-emtricitabine-TAF, and TAF-emtricitabine.
TDF and TAF both suppress HIV. Two large randomized trials of HBV monoinfection demonstrate that TAF is noninferior to TDF for the treatment of naïve and treatment-experienced patients [48,49].
Entecavir
Entecavir, a guanosine analogue, is a potent HBV DNA polymerase inhibitor that results in greater virologic suppression compared to lamivudine and retains activity against lamivudine-resistant HBV [50]. Importantly, entecavir shares some cross-resistance with lamivudine, so an entecavir dose of 1 mg daily is recommended in lamivudine-experienced patients compared to 0.5 mg daily in lamivudine-naïve patients. Entecavir requires dose reduction for patients with creatinine clearance less than 50 mL/min, although it is not associated with renal toxicity. A 1-log10 reduction in HIV RNA levels as well as emergence of M184V mutations on entecavir monotherapy has been reported [51,52]. M184V confers HIV resistance to lamivudine and emtricitabine. Therefore, entecavir should not be used as monotherapy in HIV-coinfected patients and/or patients with evidence of lamivudine-resistant HBV.
Combination Therapy
Recent updates in the guidelines recommend that since emtricitabine, lamivudine, TDF, and TAF are active against both viruses, patients with coinfection should start ART with a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual drug combination TDF plus lamivudine [53]. Most experts recommend the use of combination HBV therapy in patients on ART, particularly with lamivudine given the high rates of resistance.
• When should HBV treatment be started in patients with coinfection?
An ART regimen containing TDF (creatinine clearance > 50 mL/min) or TAF (creatinine clearance > 30 mL/min) with lamivudine or emtricitabine should be used in all HIV/HBV patients as soon as the infection is diagnosed. If TDF or TAF cannot be used, the alternate recommended regimen for HBV is entecavir plus a fully suppressive ART. In those with decreased renal function, entecavir should be adjusted to renal function [19].
Although control of viremia is feasible, clearance of infection as marked by loss of HBsAg and development of anti-HBs is unlikely to occur in the majority of patients. Therefore, the goals of treatment focus on prevention of chronic liver disease complications by suppressing viral replication, which can halt disease progression. A suggested algorithm for the management of coinfected patients is provided (Figure).
• What is the duration of therapy for hepatitis B?
Most patients with HIV/HBV coinfection will require lifelong treatment. All patients on HBV therapy as a part of ART should continue HBV therapy, regardless of seroconversion status. Also, patients should be educated and advised against self-discontinuation as it may trigger hepatitis exacerbations and/or hepatic failure.
Case Patient 3
Initial Presentation and History
Two months after starting treatment for HIV and chronic HBV infection, a 46-year-old Hispanic woman presents to clinic with jaundice and right upper quadrant (RUQ) pain. The patient was recently diagnosed with HIV infection and was naïve to treatment with ART. Her CD4 cell count was 50 cells/µL, and her HIV viral load was 743,000 copies/mL, with no baseline mutations on HIV genotype. The patient was also diagnosed with chronic HBV infection with positive HBsAg and HBeAg and negative HBc IgM serologies, as well as an HBV DNA level of 87 million IU/mL. Routine blood work revealed normal renal function and serum transaminases. The decision was made to start the patient on darunavir/ritonavir and TDF/emtricitabine. The patient was also started on sulfamethoxazole/trimethoprim and azithromycin for opportunistic infection prophylaxis.
Physical Examination and Laboratory Testing
Examination is remarkable for mild tenderness in the RUQ and icteric sclera. Laboratory testing demonstrates the following: AST, 1523 U/L; ALT, 795 U/L; albumin, 2.8 mg/dL; and total bilirubin, 3.5 mg/dL. Her CD4 count has increased to 565 cells/µL, and her HIV viral load is 4320 copies/mL. Results of repeat hepatitis serologies are as follows: HBsAg positive, anti-HBc IgM positive, and an HBV DNA level of 4.2 million IU/mL. Testing for hepatitis A, C, and D is negative, and RUQ sonogram reveals no gallstones.
• What monitoring should be done for coinfected patients on HBV therapy?
Monitoring
Providers should routinely monitor patients’ response to HIV/HBV therapy. Initially, all coinfected patients should have liver function tests and HBV DNA levels checked every 12 weeks on therapy. Frequent monitoring allows early detection of HBV drug resistance as well as drug-related hepatotoxicity. In HBeAg-positive coinfected patients who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. In HBeAg-negative patients, only HBV DNA and liver function tests are needed. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy.
Typically, virologic failure results from either the development of drug resistance or abrupt withdrawal of active HBV therapy due to patient nonadherence or changes to the ART regimen. Virologic failure can result in a rise in serum aminotransferases as well as decompensation in patients with significant underlying liver disease. Due to this risk, providers must counsel patients about the importance of adherence to therapy and should continue medications active against HBV when making a change in ART regimens, unless HBV drug resistance dictates a change in HBV therapy.
• What is the likely cause of this patient’s hepatitis “flare”?
Several studies indicate that patients with HIV/HBV coinfection are at increased risk of drug-related hepatotoxicity and grade 4 liver enzyme elevations [54,55].The first 3 months after initiation of ART is a particularly vulnerable time for liver injury. The differential diagnosis for an acute hepatitis “flare” following the initiation of ART is broad and includes the following: development of HBV drug resistance [16]; withdrawal of HBV-active medications due to nonadherence [54]; ART-related hepatotoxicity; superimposed infection with HAV, HCV, or HDV; other opportunistic infections including cytomegalovirus and mycobacterium avium complex; or IRIS, resulting in an exaggerated cytotoxic response by the recovering immune system [56,57]. Complete evaluation is critical to distinguish between the possible causes.
In this case, several clues point toward HBV-related IRIS as the most likely cause for the hepatitis “flare.” A low pretreatment CD4 cell count with a rapid rise after initiation of ART is associated with a higher rate of IRIS [57]. Serologic testing and imaging excluded superinfection with another hepatotropic virus or biliary tract disease. Appropriate declines in HIV viral load and HBV DNA levels imply patient adherence to therapy and argue against the development of HBV drug resistance. Finally, the emergence of anti-HBc IgM positivity signals HBV reactivation, which is commonly seen in patients with HBV-related IRIS [57]. The preferred treatment for HBV-related IRIS involves continuation of therapy, frequently leading to normalization of aminotransferases and subsequent HBeAg seroconversion. Because IRIS usually manifests within the first 6 to 12 weeks after starting ART, liver enzymes should be monitored closely during this period.
• What health maintenance should be done for coinfected patients?
All patients with HIV/HBV coinfection should be monitored for evidence of portal hypertension or cirrhosis and, if these conditions exist, should undergo endoscopic screening for esophageal varices as well as evaluation of ascites and encephalopathy. Patients with HBV are at increased risk for the development of HCC even in the absence of cirrhosis. A recent study showed low rates of HCC screening in HIV/HBV patients by HIV providers [58]. Whether HIV coinfection potentiates HCC risk is uncertain, though coinfected patients present at younger ages and with more symptoms than HIV-negative comparators [59]. Other risk factors for HCC include HCV infection, alcohol abuse, diabetes, obesity, exposure to environmental toxins, and cirrhosis of any etiology (most commonly non-alcoholic fatty liver disease, primary sclerosing cholangitis, primary biliary cirrhosis and hemochromatosis) [60].
The American Association of Liver Diseases (AASLD) guidelines recommend hepatic ultrasound screening every 6 months in all patients with cirrhosis or chronic HBV who are at increased risk (Asian men over the age of 40 years, Asian women over the age of 50 years, African or North American blacks, and patients with family history of HCC) [61]. They should also be referred for an esophagogastroduodenoscopy to evaluate for esophageal varices. In addition, all HIV/HBV coinfected patients with decompensated liver disease should be evaluated for transplantation. HIV infection is not a contraindication for liver transplant with the use of ART. However, since transplantation does not cure HBV infection, post-transplant HBV immune globulin and HBV treatment are required. Contemporary data suggest comparable survival rates after transplant in coinfected patients compared to HBV-monoinfected patients [51].
Summary
Routine screening with HBsAg, anti-HBs, and anti-HBc serologies is recommended for all HIV-positive individuals. Patients without evidence of prior exposure or vaccination and those with isolated anti-HBc should be offered vaccination. HIV-positive adults should receive three or four 40 µg/mL doses of single agent vaccine depending on the recombinant vaccine type available. Anti-HBsAg titers should be checked 1 month after completion of the immunization series. If quantitative anti-HBsAg levels are < 10 IU/mL, patients should receive a second vaccine cycle.
Patients who test positive for HBsAg should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels checked as well. All patients with HIV/HBV coinfection should start treatment as soon as HIV infection is diagnosed. ART needs to include 2 drugs against HBV, and therefore a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual combination of TDF plus lamivudine should be used.
Coinfected patients on treatment should have liver function tests as well as HBV DNA every 12 weeks. In HBeAg-positive coinfected individuals who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy. Those with virologic failure should be tested for HBV resistance thorough HBV genotype. Coinfected patients with cirrhosis should receive ultrasound screening every 6 months for evidence of HCC and esophagogastroduodenoscopy to evaluate for esophageal varices.
From UT Southwestern Medical Center, Dallas, TX.
Abstract
- Objective: To review the literature on and provide evidence-based recommendations for management of HIV/ hepatitis B (HBV) coinfection.
- Methods: Review of the literature for clinical trials, guidelines, and cohort studies on HIV/HBV disease management.
- Results: HIV patients should be evaluated for viral hepatitis. Those who do not have evidence of immunity should be vaccinated and monitored for response. Those who have HIV/HBV should have additional serologies checked to evaluate for hepatitis B e antigen status and level of viremia. All HIV/HBV coinfected patients should be started on antiretroviral therapy with tenofovir-based regimens. Those with HIV/HBV and cirrhosis should be screened for hepatocellular cancer every 6 months.
- Conclusion: HIV patients should be vaccinated against hepatitis B; those with coinfection should be treated for both viruses. It is important to monitor for treatment response to both HIV and HBV and liver disease complications.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Morbidity and mortality for HIV-infected patients remain high compared to uninfected patients despite effective virologic suppression. Major contributors to illness and death among these patients include cardiovascular disease, non–AIDS-defining malignancies, and chronic liver disease, specifically viral hepatitis [1]. Hepatitis B virus (HBV) infection is one of the main causes of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) globally. Because HIV and HBV can both be acquired through injection drug use and sexual transmission, coinfection occurs frequently. The Joint United Nations Program on HIV/AIDS estimates that 10% of the 33 million HIV-positive patients worldwide have simultaneous chronic HBV infection [2].
HIV/HBV coinfection significantly impacts the natural history, progression, and mortality related to both viruses. HIV infection accelerates HBV-related liver impairment, leading to earlier cirrhosis, end-stage liver disease, and HCC. Conversely, chronic HBV does not have a considerable influence in the progression of HIV; however, antiretroviral treatment (ART) toxicities and/or HBV flares due to immune reconstitution inflammatory syndrome (IRIS) or HBV itself can lead to increased liver-related complications [3,4].
Case Patient 1
Initial Presentation and History
An asymptomatic 38-year-old man diagnosed with HIV infection 1 month ago presents for his initial visit to establish HIV care. The patient is a man who has sex with men (MSM) and is currently sexually active with multiple partners. He reports inconsistent use of condoms. One month ago he underwent routine screening and was found to be HIV-positive. At the time of diagnosis, the patient’s baseline CD4 cell count was 328 cells/µL and his viral load was 182,600 copies/mL. The patient wants to discuss the implications of his new diagnosis of HIV and recommendations for further testing and treatment. He is especially interested in HBV screening, since one of his recent partners was known to be positive. The patient has no relevant past medical history. He does not recall the details of his childhood vaccinations. He denies smoking and injection drug use but reports moderate alcohol consumption.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies show normal complete blood count (CBC) and renal and liver function test results, and a baseline HIV genotype does not show resistance. Hepatitis A total antibody (anti-HAV) testing is positive, while tests for hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), and hepatitis C antibody (anti-HCV) are negative.
• Which screening tests for HBV should be performed in HIV-infected patients?
Routine screening of all HIV-infected patients for hepatitis A virus, HBV, and hepatitis C virus (HCV) is recommended [5]. Routine HBV screening involves obtaining serologies for HBsAg, anti-HBs, and anti-HBc. With these results, patients can be classified into categories of either active infection, immunity, or no evidence of prior exposure (Table 1).
• How effective is HBV vaccination in the HIV population?
Vaccination
Available HBV vaccines in the United States include 2 single-agent vaccines (Recombivax HB [Merck, Whitehouse Station, NJ] and Engerix-B [GlaxoSmithKline, Research Triangle Park, NC]) as well as a combination HAV/HBV vaccine (Twinrix [GlaxoSmithKline]). For adults (age ≥ 20 years) with an immunocompromising condition such as HIV infection, current Centers for Disease Control and Prevention guidelines recommend three 40 µg/mL doses of single-agent vaccine administered at 0, 1, and 6 months (Recombivax HB), or four 40 µg/mL doses of single-agent vaccine (2 doses of 20 µg/mL administered simultaneously) at 0, 1, 2, and 6 months (Engerix-B) [7].
The immunogenicity to HBV vaccination in HIV-positive patients is decreased, reflected by lower antibody titers, waning immunity, and seroconversion rates of 18% to 65% [8–11]. Factors associated with poor response include low CD4 cell counts, detectable HIV RNA, coinfection with HCV, and the general health status of the patient [8]. Ideally, HBV vaccination should occur prior to decline in CD4 cell count below 350 cells/µL. However, guidelines do not recommend deferring vaccination since some patients with advanced HIV disease will seroconvert [6].
Anti-HBs titers should be checked 1 month after completion of the vaccine series to confirm protective antibody titers. For patients with quantitative anti-HBs levels < 10 IU/mL, a second vaccine cycle is recommended. Some specialists may defer revaccination until a sustained increase in CD4 count is achieved on ART.
Two randomized controlled trials have demonstrated that 4 doses of double-dose (40 µg/mL) vaccine generate higher anti-HBs levels than 3 doses of standard-dose (20 µg/mL) vaccine in HIV-infected adults [12,13]. Another study showed that HIV patients with CD4 counts > 350 cells/µL had better responses when immunized with double- dose vaccines on the usual 3-dose series [14]. Currently, the CDC recommends giving a double dose for either a 3-dose schedule or a 4-dose schedule. However, it remains unclear what dosing schedule to use if a patient fails to respond. Likely waiting until the CD4 cell count has increased and HIV viral load is suppressed will be important to seeing a response.
• What approach for HBV prevention should be taken in this patient?
The patient’s serologies confirm no prior exposure to HBV, and he should be offered HBV vaccination. His CD4 cell count is below 350/µL and he has ongoing HIV viremia, which increases his risk for an inadequate response. However, vaccination should not be delayed, particularly given his high risk of sexual transmission. The patient should be counseled regarding all high-risk behaviors. As discussed above, 3 or 4 doses of the higher dose vaccine (40 µg/mL) should be administered depending on what type of recombinant vaccine is available. An anti-HBs level should be checked 1 month after completion. A full repeat vaccine series using the 40 µg/mL dose should be considered for nonresponders who initially received a standard vaccine series. Experts also recommend checking annual anti-HBs levels to monitor for waning immunity, with a booster dose given if the anti-HBs level drops below the protective range.
• What vaccination strategy should be used in patients with isolated positive anti-HBc?
The clinical implications of an isolated positive anti-HBc for vaccination are still uncertain. This serologic pattern may represent a false-positive test, remote HBV infection with loss of anti-HBs, or occult HBV infection with undetectable HBsAg. The latter scenario appears more commonly in HIV-infected patients, particularly with concomitant HCV infection [15].
A recent study suggested that patients with an isolated positive anti-HBc with a negative anamnestic antibody response (anti-HBs titer of < 10 IU/mL 4 weeks after a single 20 µg dose of recombinant HBV vaccine) should be further vaccinated with the double-dose for a 3-dose schedule [6]. Additionally, another study followed HIV/HCV coinfected patients for 9.5 years and found that an isolated positive anti-HBc was not associated with accelerated liver disease progression [16].
Treatment of HIV-1 Infection
Current HIV guidelines recommend initiation of ART for all HIV-infected patients regardless of their CD4 count [17]. ART for a treatment-naïve patient usually consists of 2 nucleotide/nucleoside reverse-transcriptase inhibitors (NRTIs; the “backbone”) combined with a third agent (the “anchor”), which can be a nonnucleoside reverse-transcriptase inhibitor (NNRTI), a protease inhibitor (PI) boosted with a boosting agent, or an integrase strand-transfer inhibitor (INSTI) [18,19].
There are numerous studies indicating that incident HBV risk can be reduced by placing those at risk for HBV acquisition on ART containing a combination of tenofovir disoproxil fumarate (TDF), lamivudine, or emtricitabine [20,21]. Another study in MSM found those on ART with HIV viral load < 400 copies/mL were protected from developing HBV compared to those not on ART [22]. Given this patient’s higher risk for HBV acquisition, placing him on emtricitabine/TDF backbone as part of ART could be protective against incident HBV [23].
Case Patient 2
Initial Presentation and History
A 32-year-old man diagnosed with HIV infection 8 years ago now on ART presents for follow-up. The patient is an MSM with a history of inconsistent condom use. At the time of HIV diagnosis 8 years ago, the patient had a CD4 cell count of 250 cells/µL and an HIV viral load of 648,000 copies/mL. The patient was initiated on lamivudine/zidovudine and lopinavir/ritonavir, and he achieved complete virologic suppression at 20 weeks, with a CD4 cell count of 455 cells/µL at 1 year. The patient has remained on this regimen without major side effects; however, he reports frequent missed doses over the last 2 years due to “pill fatigue.” Previous testing for HBV and HCV at the time of his initial HIV diagnosis was negative, but he failed to complete the HBV vaccine series. He denies alcohol or injection drug use.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies reveal normal electrolytes and renal function, hemoglobin of 11 g/dL, and platelet count of 235,000/µL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are 34 U/L and 44 U/L, respectively, with an INR of 1.1 and albumin level of 3.4 g/dL. CD4 cell count is 320 cells/µL with an HIV viral load of 24,500 copies/mL. Viral hepatitis serologies show anti-HAV positive, anti-HCV negative, HBsAg positive, anti-HBc IgG positive, hepatitis Be antigen (HBeAg) negative, and hepatitis B e antibody (anti-HBe) positive. HBV DNA viral load is 685,000 IU/mL.
• What is the natural history of HIV/HBV coinfection?
Chronic HBV infection affects about 10% of HIV-infected patients globally. Epidemiologic studies indicate that HIV-infected patients have higher rates of reactivation and progression to chronic HBV infection and chronic liver disease than HIV-negative patients [24–26]. Coinfected patients demonstrate higher serum HBV DNA levels, which lead to more rapid progression of hepatic fibrosis and may increase the risk of cirrhosis and HCC [24,27,28]. HIV infection, however, can mediate the necroinflammatory response through blunting of the immune response that drives pathogenesis in HBV infection. Aminotransferase levels may be only slightly elevated or even normal, particularly in the severely immune suppressed. However, elevation in liver enzymes (hepatitis flare) can occur if a patient stops ART, or if HBV resistance develops. Patients being treated for HIV/HBV coinfection should be counseled that stopping HIV treatment puts them at risk of developing a hepatitis flare.
Large cohort studies of HIV/HBV coinfected patients indicate increased risk of liver-related mortality, most pronounced with lower CD4 cell counts [1,28,29]. Introduction of ART appears to increase rather than attenuate this liver-related mortality, possibly by decreasing AIDS-related mortality and allowing more time for liver disease progression. Finally, a recent meta-analysis including 12,382 patients demonstrated a significant effect of HIV/HBV coinfection on all-cause mortality, with a pooled effect estimate of 1.36 [30].
• What diagnostic testing should be done in coinfected patients?
Diagnostic Testing and Evaluation
Persistence of HBsAg for more than 6 months is diagnostic of chronic HBV infection and warrants further serologic evaluation. Patients with chronic HBV infection should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels measured. Elevation of AST and ALT can be seen with untreated HBV. For those on treatment, a hepatitis flare could be caused by abrupt discontinuation of HBV treatment, development of HBV resistance, or superinfection with another viral pathogen.
HBeAg is a marker of active viral replication and is associated with higher levels of HBV DNA and active liver disease. Seroconversion, or loss of HBeAg and development of anti-HBe, heralds a favorable treatment response for those who were initially HBeAg-positive. However, some HBV variants have precore or core promoter mutations that lead to higher levels of HBV DNA in the absence of HBeAg. This highlights the importance of monitoring HBV DNA levels in all patients with chronic HBV infection. Favorable response to therapy in HBeAg-negative patients is marked by normalization of aminotransferases and HBV DNA suppression.Hepatitis D virus (HDV) is a defective virus particle that can only replicate in the presence of HBV. Coinfected patients should be tested for anti-HDV if they are injection drug users or are from a high-prevalence region such as the Mediterranean and Amazon basins. Newly acquired HDV infection should also be considered in the context of hepatitis flares.
Although these routine diagnostic tests are essential for management, studies show low adherence rates to HBV testing guidelines by HIV providers [31,32].
• What is the role of HBV genotype and resistance testing?
HBV can be classified into 10 different genotypes, A through J, based on genomic sequence variations. Each genotype has a distinct ethnic and geographic distribution, with genotypes A and D predominating in North America. HBV genotyping appears to have important prognostic as well as treatment implications [19]. However, data are still preliminary and the guidelines do not recommend routine genotype testing.
Resistance testing in HBV allows for detection of mutations that decrease effectiveness of antivirals. Exposure to lamivudine can lead to mutations in the YMDD region of the HBV DNA polymerase, resulting in drug resistance. Resistance to lamivudine develops at a rate of approximately 25% after 1 year of drug exposure in HIV/HBV coinfection [33]. On the other hand, studies have shown that entecavir is active against HIV and, most importantly, selects for the M184V mutation in HIV. M184V is associated with lamivudine resistance for HIV treatment, thus limiting treatment options [34,35]. After these findings, the FDA advised against monotherapy with entecavir in patients with HIV/HBV coinfection. The case patient was on lamivudine during the time of HBV acquisition, and therefore YMDD mutation must be taken into consideration for therapy purposes. He should be switched to a regimen that suppresses both viruses. The development of drug resistance should be assessed in all patients with persistent or breakthrough HBV viremia on ART, particularly the nucleoside analogues. HIV providers treating HIV/HBV coinfection should regularly monitor both HIV and HBV viral load to assess for therapeutic efficacy.
• What is the role of liver biopsy in this patient?
Liver biopsy should be considered in all coinfected patients as it remains the gold standard for determining the activity and severity of chronic hepatitis B. However, because it carries inherent risks and is not required prior to treatment in all patients, the decision should be individualized. Liver biopsy can be useful to assess baseline liver histology and may be warranted to rule out significant coexisting genetic or metabolic liver disease. Currently, noninvasive methods to assess liver fibrosis either using elastography or various combinations of serum biomarkers are being evaluated [36] and may be considered in lieu of a liver biopsy [37]. One study compared the accuracy of elastography with liver biopsy in HIV/HBV coinfected patients and demonstrated that the former was proficient in discriminating between absence or mild fibrosis and moderate to severe fibrosis [38]. In general, this test has high accuracy in detecting minimal fibrosis from advanced fibrosis or cirrhosis. For the group in the middle, further investigation with additional methods must be considered [37]. Finally, a recent retrospective study involving 70 HIV/HBV coinfected individuals showed fibrosis regression suggesting beneficial effects of long-term ART on liver stiffness, [39] but further studies are needed to confirm these findings.
Case 2 Continued
The patient has now been diagnosed with chronic HBV infection. His diagnostic testing is negative for HBeAg and reveals modest HBV viremia with abnormal aminotransferases less than 2 times the upper limit of normal. Drug resistance testing reveals a mutation in the YMDD region indicative of HBV lamivudine resistance. HIV genotype demonstrates a wild-type virus without resistance. The patient asks what treatment options exist.
• What medications are currently available to treat hepatitis B?
Treatment
All patients with HIV/HBV coinfection should receive treatment to suppress both viruses, and ART needs to include 2 drugs active against HBV, ideally emtricitabine and TDF. This approach prevents drug resistance, slows progression of HBV infection, and reduces the incidence of IRIS [5]. TDF has been associated with decreased renal function and bone mineral density. Recently, tenofovir alafenamide fumarate (TAF) was approved for the treatment of HIV and HBV. A dose of 10 mg daily is given when coadministered with ritonavir, cobicistat, or protease inhibitors, but a dose of 25 mg should be given when administered with NNRTIs or integrase inhibitors. Compared to TDF, TAF shows less accumulation of tenofovir in kidneys and bones and consequently has reduced renal and bone mineral density effects [40]. All patients should be on a TDF- or TAF-based HIV regimen if they have chronic HBV.
Currently, the following antiviral drugs are FDA-approved for the treatment of HBV infection: interferon alfa-2b, pegylated interferon (peginterferon) alfa-2a, lamivudine and emtricitabine, entecavir, adefovir, TDF, TAF, and telbivudine. Telbivudine and adefovir are no longer recommended due to their association with higher incidence of toxicity and higher rates of HBV treatment failure. A summary of available HBV treatment options is outlined in Table 2.
Interferon
Standard interferon alfa-2b blocks HBV replication through interaction with viral proteins and stimulation of host cellular immunity. Peginterferon alfa-2a has proven efficacy in HBV-monoinfected patients, but efficacy data in HIV/HBV coinfected patients is lacking [41,42]. Studies in hepatitis C treatment demonstrate the safety of peginterferon alfa-2a use in HIV-positive patients and indicate that HIV viral suppression occurs with peginterferon without evidence of selection of resistance mutations that affect future ART options [43]. For HIV/HBV coinfected patients not on ART who will receive only therapy for HBV (which is infrequent since all HIV patients should be on ART), pegylated interferon-alfa-2a alone for 48 weeks is the only option that will not cause ART-associated HIV drug resistance [5]. Interferon therapy is contraindicated in patients with decompensated cirrhosis and should be used with caution in patients with active depression, uncontrolled diabetes, and cardiac and pulmonary disease.
Lamivudine and Emtricitabine
Lamivudine is a nucleoside analogue with efficacy against both HIV and HBV. Clinical trials in HIV/HBV-infected patients have shown up to 87% of patients achieve undetectable HBV DNA levels and about 25% achieve HBeAg seroconversion after 1 to 2 years of therapy [44,45]. However, the major issue limiting use of lamivudine is its low genetic barrier to resistance. Mutation of the YMDD motif of the HBV DNA polymerase confers HBV resistance. HIV/HBV coinfected patients develop resistance at rates of up to 94% after 4 years of therapy [33], heralded by rebounds in HBV DNA levels and often hepatitis flares or precipitation of hepatic failure [16]. Because of resistance, lamivudine monotherapy should be avoided; even in patients on ART, abrupt withdrawal of lamivudine or the development of HBV resistance should be closely monitored.
Emtricitabine is another nucleoside analogue with properties and efficacy similar to lamivudine. It is frequently used as a combination pill with TDF (Truvada) in coinfected patients. The same concerns regarding monotherapy and the emergence of resistance that exist for lamivudine apply to emtricitabine.
Tenofovir
TDF, a nucleotide analogue, is one of the preferred first-line agents for HIV treatment and has proven efficacy against both wild-type and lamivudine-resistant HBV. Since it was first used for HIV, TDF has been more extensively studied in coinfected patients compared to most other agents. In a meta-analysis of patients with HIV/HBV coinfection, TDF suppressed HBV viral load to undetectable titers in approximately 90% of patients [46]. Tenofovir is available in 2 preparations: TDF and TAF. TDF has been reported to cause renal impairment as well as bone loss. TAF has shown less renal toxicity and less bone damage [40,47]. In 2016, TAF became available as part as 4 regimens: stand-alone TAF, elvitegravir-cobicistat-emtricitabine-TAF, rilpivirine-emtricitabine-TAF, and TAF-emtricitabine.
TDF and TAF both suppress HIV. Two large randomized trials of HBV monoinfection demonstrate that TAF is noninferior to TDF for the treatment of naïve and treatment-experienced patients [48,49].
Entecavir
Entecavir, a guanosine analogue, is a potent HBV DNA polymerase inhibitor that results in greater virologic suppression compared to lamivudine and retains activity against lamivudine-resistant HBV [50]. Importantly, entecavir shares some cross-resistance with lamivudine, so an entecavir dose of 1 mg daily is recommended in lamivudine-experienced patients compared to 0.5 mg daily in lamivudine-naïve patients. Entecavir requires dose reduction for patients with creatinine clearance less than 50 mL/min, although it is not associated with renal toxicity. A 1-log10 reduction in HIV RNA levels as well as emergence of M184V mutations on entecavir monotherapy has been reported [51,52]. M184V confers HIV resistance to lamivudine and emtricitabine. Therefore, entecavir should not be used as monotherapy in HIV-coinfected patients and/or patients with evidence of lamivudine-resistant HBV.
Combination Therapy
Recent updates in the guidelines recommend that since emtricitabine, lamivudine, TDF, and TAF are active against both viruses, patients with coinfection should start ART with a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual drug combination TDF plus lamivudine [53]. Most experts recommend the use of combination HBV therapy in patients on ART, particularly with lamivudine given the high rates of resistance.
• When should HBV treatment be started in patients with coinfection?
An ART regimen containing TDF (creatinine clearance > 50 mL/min) or TAF (creatinine clearance > 30 mL/min) with lamivudine or emtricitabine should be used in all HIV/HBV patients as soon as the infection is diagnosed. If TDF or TAF cannot be used, the alternate recommended regimen for HBV is entecavir plus a fully suppressive ART. In those with decreased renal function, entecavir should be adjusted to renal function [19].
Although control of viremia is feasible, clearance of infection as marked by loss of HBsAg and development of anti-HBs is unlikely to occur in the majority of patients. Therefore, the goals of treatment focus on prevention of chronic liver disease complications by suppressing viral replication, which can halt disease progression. A suggested algorithm for the management of coinfected patients is provided (Figure).
• What is the duration of therapy for hepatitis B?
Most patients with HIV/HBV coinfection will require lifelong treatment. All patients on HBV therapy as a part of ART should continue HBV therapy, regardless of seroconversion status. Also, patients should be educated and advised against self-discontinuation as it may trigger hepatitis exacerbations and/or hepatic failure.
Case Patient 3
Initial Presentation and History
Two months after starting treatment for HIV and chronic HBV infection, a 46-year-old Hispanic woman presents to clinic with jaundice and right upper quadrant (RUQ) pain. The patient was recently diagnosed with HIV infection and was naïve to treatment with ART. Her CD4 cell count was 50 cells/µL, and her HIV viral load was 743,000 copies/mL, with no baseline mutations on HIV genotype. The patient was also diagnosed with chronic HBV infection with positive HBsAg and HBeAg and negative HBc IgM serologies, as well as an HBV DNA level of 87 million IU/mL. Routine blood work revealed normal renal function and serum transaminases. The decision was made to start the patient on darunavir/ritonavir and TDF/emtricitabine. The patient was also started on sulfamethoxazole/trimethoprim and azithromycin for opportunistic infection prophylaxis.
Physical Examination and Laboratory Testing
Examination is remarkable for mild tenderness in the RUQ and icteric sclera. Laboratory testing demonstrates the following: AST, 1523 U/L; ALT, 795 U/L; albumin, 2.8 mg/dL; and total bilirubin, 3.5 mg/dL. Her CD4 count has increased to 565 cells/µL, and her HIV viral load is 4320 copies/mL. Results of repeat hepatitis serologies are as follows: HBsAg positive, anti-HBc IgM positive, and an HBV DNA level of 4.2 million IU/mL. Testing for hepatitis A, C, and D is negative, and RUQ sonogram reveals no gallstones.
• What monitoring should be done for coinfected patients on HBV therapy?
Monitoring
Providers should routinely monitor patients’ response to HIV/HBV therapy. Initially, all coinfected patients should have liver function tests and HBV DNA levels checked every 12 weeks on therapy. Frequent monitoring allows early detection of HBV drug resistance as well as drug-related hepatotoxicity. In HBeAg-positive coinfected patients who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. In HBeAg-negative patients, only HBV DNA and liver function tests are needed. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy.
Typically, virologic failure results from either the development of drug resistance or abrupt withdrawal of active HBV therapy due to patient nonadherence or changes to the ART regimen. Virologic failure can result in a rise in serum aminotransferases as well as decompensation in patients with significant underlying liver disease. Due to this risk, providers must counsel patients about the importance of adherence to therapy and should continue medications active against HBV when making a change in ART regimens, unless HBV drug resistance dictates a change in HBV therapy.
• What is the likely cause of this patient’s hepatitis “flare”?
Several studies indicate that patients with HIV/HBV coinfection are at increased risk of drug-related hepatotoxicity and grade 4 liver enzyme elevations [54,55].The first 3 months after initiation of ART is a particularly vulnerable time for liver injury. The differential diagnosis for an acute hepatitis “flare” following the initiation of ART is broad and includes the following: development of HBV drug resistance [16]; withdrawal of HBV-active medications due to nonadherence [54]; ART-related hepatotoxicity; superimposed infection with HAV, HCV, or HDV; other opportunistic infections including cytomegalovirus and mycobacterium avium complex; or IRIS, resulting in an exaggerated cytotoxic response by the recovering immune system [56,57]. Complete evaluation is critical to distinguish between the possible causes.
In this case, several clues point toward HBV-related IRIS as the most likely cause for the hepatitis “flare.” A low pretreatment CD4 cell count with a rapid rise after initiation of ART is associated with a higher rate of IRIS [57]. Serologic testing and imaging excluded superinfection with another hepatotropic virus or biliary tract disease. Appropriate declines in HIV viral load and HBV DNA levels imply patient adherence to therapy and argue against the development of HBV drug resistance. Finally, the emergence of anti-HBc IgM positivity signals HBV reactivation, which is commonly seen in patients with HBV-related IRIS [57]. The preferred treatment for HBV-related IRIS involves continuation of therapy, frequently leading to normalization of aminotransferases and subsequent HBeAg seroconversion. Because IRIS usually manifests within the first 6 to 12 weeks after starting ART, liver enzymes should be monitored closely during this period.
• What health maintenance should be done for coinfected patients?
All patients with HIV/HBV coinfection should be monitored for evidence of portal hypertension or cirrhosis and, if these conditions exist, should undergo endoscopic screening for esophageal varices as well as evaluation of ascites and encephalopathy. Patients with HBV are at increased risk for the development of HCC even in the absence of cirrhosis. A recent study showed low rates of HCC screening in HIV/HBV patients by HIV providers [58]. Whether HIV coinfection potentiates HCC risk is uncertain, though coinfected patients present at younger ages and with more symptoms than HIV-negative comparators [59]. Other risk factors for HCC include HCV infection, alcohol abuse, diabetes, obesity, exposure to environmental toxins, and cirrhosis of any etiology (most commonly non-alcoholic fatty liver disease, primary sclerosing cholangitis, primary biliary cirrhosis and hemochromatosis) [60].
The American Association of Liver Diseases (AASLD) guidelines recommend hepatic ultrasound screening every 6 months in all patients with cirrhosis or chronic HBV who are at increased risk (Asian men over the age of 40 years, Asian women over the age of 50 years, African or North American blacks, and patients with family history of HCC) [61]. They should also be referred for an esophagogastroduodenoscopy to evaluate for esophageal varices. In addition, all HIV/HBV coinfected patients with decompensated liver disease should be evaluated for transplantation. HIV infection is not a contraindication for liver transplant with the use of ART. However, since transplantation does not cure HBV infection, post-transplant HBV immune globulin and HBV treatment are required. Contemporary data suggest comparable survival rates after transplant in coinfected patients compared to HBV-monoinfected patients [51].
Summary
Routine screening with HBsAg, anti-HBs, and anti-HBc serologies is recommended for all HIV-positive individuals. Patients without evidence of prior exposure or vaccination and those with isolated anti-HBc should be offered vaccination. HIV-positive adults should receive three or four 40 µg/mL doses of single agent vaccine depending on the recombinant vaccine type available. Anti-HBsAg titers should be checked 1 month after completion of the immunization series. If quantitative anti-HBsAg levels are < 10 IU/mL, patients should receive a second vaccine cycle.
Patients who test positive for HBsAg should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels checked as well. All patients with HIV/HBV coinfection should start treatment as soon as HIV infection is diagnosed. ART needs to include 2 drugs against HBV, and therefore a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual combination of TDF plus lamivudine should be used.
Coinfected patients on treatment should have liver function tests as well as HBV DNA every 12 weeks. In HBeAg-positive coinfected individuals who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy. Those with virologic failure should be tested for HBV resistance thorough HBV genotype. Coinfected patients with cirrhosis should receive ultrasound screening every 6 months for evidence of HCC and esophagogastroduodenoscopy to evaluate for esophageal varices.
1. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–41.
2. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global change. N Engl J Med 2012;366:1749–52.
3. Bellini C, Keise O, Chave JP, et al. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: the Swiss HIV Cohort Study. HIV Med 2009;10:12–8.
4. Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996-2001. AIDS 2003;17:2191–18.
5. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Q1–Q17.
6. Piroth L, Launay O, Michel ML, et al. Vaccination against hepatitis B virus (HBV) in HIV-1-infected patients with isolated anti-HBV core antibody: the ANRS HB EP03 CISOVAC prospective study. J Infect Dis 2016;213:1735–42.
7. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Accessed 22 Dec 2016 at www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
8. Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine 2000;18:1161–5.
9. Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005;41:1045–8.
10. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
11. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
12. Chaiklang K, Wipasa J, Chaiwarith R, et al. Comparison of immunogenicity and safety of four doses and four double doses vs. standard doses of hepatitis B vaccination in HIV-infected adults: a randomized, controlled trial. PLoS One 2013;8:e80409.
13. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
14. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
15. Gandhi RT, Wurcel A, Lee H, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis 2005;191:1435–41.
16. French AL, Hotton A, Young M, et al. Isolated hepatitis B core antibody status is not associated with accelerated liver disease progression in HIV/hepatitis C coinfection. J Acquir Immune Defic Syndr 2016;72:274–80.
17. INSIGHT START Study Group, Lundgren JD, Babiker GA, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
18. Sebaaly JC, Kelley D. Single-tablet regimens for the treatment of HIV-1 infection. Ann Pharmacother 2017;51:332–44.
19. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
20. Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014;28:999–1005.
21. Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on Hepatitis B virus infection. Clin Infect Dis 2013;56:1812–9.
22. Falade-Nwulia O, Seaberg EC, Snider AE, et al. Incident hepatitis B virus infection in HIV-infected and HIV-uninfected men who have sex with men from pre-HAART to HAART periods: a cohort study. Ann Intern Med 2015;163:673–80.
23. Shilaih M, Marzel A, Scherrer AU, et al. Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016;214:599–606.
24. Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999;29:1306–10.
25. Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS 1997;11:597–606.
26. Bodsworth N, Donovan B, Nightingale BN. The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: a
study of 150 homosexual men. J Infect Dis 1989;160:577–82.
27. Di Martino V, Thevenot T, Colin JF, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002;123:1812–22.
28. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360(9349):1921–6.
29. Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005;19:593–601.
30. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009;48:1763–71.
31. Jain MK, Opio CK, Osuagwu CC, et al. Do HIV care providers appropriately manage hepatitis B in coinfected patients treated with antiretroviral therapy? Clin Infect Dis 2007;44:996–1000.
32. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;61:1742–8.
33. Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006;20:863–70.
34. McMahon M, Jilek B, Brennan T, Thio C. The HBV drug entecavir: effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
35. Jain M, Zoellner C. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007; 21:2365–6.
36. Bottero J, Lacombe K, Guechot J, et al. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV coinfected patients. J Hepatol 2009;50:1074–83.
37. Moreno S, Garcia-Samaniego J, Moreno A, et al. Noninvasive diagnosis of liver fibrosis in patients with HIV infection and HCV/HBV co-infection. J Viral Hepat 2009;16:249–58.
38. Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011;18:61–9.
39. Audsley J, Robson C, Aitchison S, et al. Liver fibrosis regression measured by transient elastography in human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfected individuals on long-term HBV-active combination antiretroviral therapy. Open Forum Infect Dis 2016;3:ofw035.
40. Achhra AC, Nugent M, Mocroft A, et al. Chronic kidney disease and antiretroviral therapy in HIV-positive individuals: recent developments. Curr HIV/AIDS Rep 2016;13:149–57.
41. Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–17.
42. Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95.
43. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004;351:438–50.44. Dore GJ, Cooper DA, Barrett C, et al. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis 1999;180:607–13.
45. Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of antihuman immunodeficiency virus regimens. Clin Infect Dis 2001;32:963–9.
46. Price H, Dunn D, PIllary D, et al. Suppresion of HBV by tenofovir in HIV/HBV coinfected patients: a systemic review and meta-analysis. PLoS One 2013;8:e68152.
47. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with eltegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomized, double-blind, phase 3, non-inferiority trial. Lancet 2015;385:2606–15.
48. Chan HLY, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-positive chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:185–95.
49. Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-negative chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:196–206.
50. Chang TT, Lai CL. Hepatitis B virus with primary resistance to adefovir. N Engl J Med 2006;355:322–3.
51. McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir -- effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
52. Jain MK, Zoellner CL. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007;21:2365–6.
53. Nuesch R, Ananworanich J, Srasuebkul P, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS 2008;22:152–4.
54. Gaglio PJ, Sterling R, Daniels E, Tedaldi E. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis 2007;45:618–23.
55. Reisler RB, Han C, Burman WJ, et al. Grade 4 events are as important as AIDS events in the era of HAART. J Acquir Immune Defic Syndr 2003;34:379–86.
56. Drake A, Mijch A, Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis 2004;39:129–32.
57. Jain MK, Parekh NK, Hester J, Lee WM. Aminotransferase elevation in HIV/hepatitis B virus co-infected patients treated with two active hepatitis B virus drugs. AIDS Patient Care STDS 2006;20:817–22.
58. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;611742–8.
59. Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.–Canadian multicenter study. J Hepatol 2007;47:527–37.
60. Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 2011;9:617–23.
61. Bruix J, Sherman M. AASLD Practice Guideline. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2.
1. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–41.
2. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global change. N Engl J Med 2012;366:1749–52.
3. Bellini C, Keise O, Chave JP, et al. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: the Swiss HIV Cohort Study. HIV Med 2009;10:12–8.
4. Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996-2001. AIDS 2003;17:2191–18.
5. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Q1–Q17.
6. Piroth L, Launay O, Michel ML, et al. Vaccination against hepatitis B virus (HBV) in HIV-1-infected patients with isolated anti-HBV core antibody: the ANRS HB EP03 CISOVAC prospective study. J Infect Dis 2016;213:1735–42.
7. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Accessed 22 Dec 2016 at www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
8. Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine 2000;18:1161–5.
9. Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005;41:1045–8.
10. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
11. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
12. Chaiklang K, Wipasa J, Chaiwarith R, et al. Comparison of immunogenicity and safety of four doses and four double doses vs. standard doses of hepatitis B vaccination in HIV-infected adults: a randomized, controlled trial. PLoS One 2013;8:e80409.
13. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
14. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
15. Gandhi RT, Wurcel A, Lee H, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis 2005;191:1435–41.
16. French AL, Hotton A, Young M, et al. Isolated hepatitis B core antibody status is not associated with accelerated liver disease progression in HIV/hepatitis C coinfection. J Acquir Immune Defic Syndr 2016;72:274–80.
17. INSIGHT START Study Group, Lundgren JD, Babiker GA, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
18. Sebaaly JC, Kelley D. Single-tablet regimens for the treatment of HIV-1 infection. Ann Pharmacother 2017;51:332–44.
19. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
20. Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014;28:999–1005.
21. Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on Hepatitis B virus infection. Clin Infect Dis 2013;56:1812–9.
22. Falade-Nwulia O, Seaberg EC, Snider AE, et al. Incident hepatitis B virus infection in HIV-infected and HIV-uninfected men who have sex with men from pre-HAART to HAART periods: a cohort study. Ann Intern Med 2015;163:673–80.
23. Shilaih M, Marzel A, Scherrer AU, et al. Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016;214:599–606.
24. Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999;29:1306–10.
25. Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS 1997;11:597–606.
26. Bodsworth N, Donovan B, Nightingale BN. The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: a
study of 150 homosexual men. J Infect Dis 1989;160:577–82.
27. Di Martino V, Thevenot T, Colin JF, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002;123:1812–22.
28. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360(9349):1921–6.
29. Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005;19:593–601.
30. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009;48:1763–71.
31. Jain MK, Opio CK, Osuagwu CC, et al. Do HIV care providers appropriately manage hepatitis B in coinfected patients treated with antiretroviral therapy? Clin Infect Dis 2007;44:996–1000.
32. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;61:1742–8.
33. Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006;20:863–70.
34. McMahon M, Jilek B, Brennan T, Thio C. The HBV drug entecavir: effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
35. Jain M, Zoellner C. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007; 21:2365–6.
36. Bottero J, Lacombe K, Guechot J, et al. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV coinfected patients. J Hepatol 2009;50:1074–83.
37. Moreno S, Garcia-Samaniego J, Moreno A, et al. Noninvasive diagnosis of liver fibrosis in patients with HIV infection and HCV/HBV co-infection. J Viral Hepat 2009;16:249–58.
38. Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011;18:61–9.
39. Audsley J, Robson C, Aitchison S, et al. Liver fibrosis regression measured by transient elastography in human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfected individuals on long-term HBV-active combination antiretroviral therapy. Open Forum Infect Dis 2016;3:ofw035.
40. Achhra AC, Nugent M, Mocroft A, et al. Chronic kidney disease and antiretroviral therapy in HIV-positive individuals: recent developments. Curr HIV/AIDS Rep 2016;13:149–57.
41. Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–17.
42. Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95.
43. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004;351:438–50.44. Dore GJ, Cooper DA, Barrett C, et al. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis 1999;180:607–13.
45. Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of antihuman immunodeficiency virus regimens. Clin Infect Dis 2001;32:963–9.
46. Price H, Dunn D, PIllary D, et al. Suppresion of HBV by tenofovir in HIV/HBV coinfected patients: a systemic review and meta-analysis. PLoS One 2013;8:e68152.
47. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with eltegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomized, double-blind, phase 3, non-inferiority trial. Lancet 2015;385:2606–15.
48. Chan HLY, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-positive chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:185–95.
49. Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-negative chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:196–206.
50. Chang TT, Lai CL. Hepatitis B virus with primary resistance to adefovir. N Engl J Med 2006;355:322–3.
51. McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir -- effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
52. Jain MK, Zoellner CL. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007;21:2365–6.
53. Nuesch R, Ananworanich J, Srasuebkul P, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS 2008;22:152–4.
54. Gaglio PJ, Sterling R, Daniels E, Tedaldi E. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis 2007;45:618–23.
55. Reisler RB, Han C, Burman WJ, et al. Grade 4 events are as important as AIDS events in the era of HAART. J Acquir Immune Defic Syndr 2003;34:379–86.
56. Drake A, Mijch A, Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis 2004;39:129–32.
57. Jain MK, Parekh NK, Hester J, Lee WM. Aminotransferase elevation in HIV/hepatitis B virus co-infected patients treated with two active hepatitis B virus drugs. AIDS Patient Care STDS 2006;20:817–22.
58. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;611742–8.
59. Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.–Canadian multicenter study. J Hepatol 2007;47:527–37.
60. Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 2011;9:617–23.
61. Bruix J, Sherman M. AASLD Practice Guideline. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2.