User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Uncombable hair syndrome: One gene, variants responsible for many cases
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Dignity
Queen Elizabeth is everywhere. She was even on the last slide of a presentation on COVID, monkeypox, and influenza vaccines given by our physician in charge of quality. This was odd. The presenter wasn’t English. The Queen had nothing to do with vaccines. Nor apparently would she have said even if she did have an opinion about them. But there we were, an audience of physicians and staff pausing for a moment of remembrance of her.
I’m not a Monarchist – except perhaps for the Kennedys. I grew up in New England. I don’t have an opinion on whether or not the British Crown should endure. But I do marvel at the astounding effect Queen Elizabeth’s passing had on so many around the world. Her personal qualities, particularly her steadiness and humane sympathy, might explain why so many are sad hearing the news. But also I think there was something in her role that we all wished for: Not the owning of palaces and sceptres, but rather, the respect that was given to her.
She was a stateswoman of “unmatched dignity,” the White House wrote. That was true, but it seems being the Queen might have been the last job on earth where such dignity is still possible. Certainly in politics, education, and even health care, there doesn’t seem to be much left lately.
The same day of that presentation I walked into the room of a patient 22 minutes late, she held her arm forth tapping her watch to indicate the time and my tardiness. Unnecessary, if not impertinent. Covering for one of my female physician colleagues, I read an email from a patient which began, “Dear Julie, With all due respect …” Another patient submitted a photo for us to review that was clearly taken from her car while waiting at a stop light. Hardly the consideration a clinical encounter should be given.
Much has been lost for patients. too. There are patients trying to make appointments lately who are told: “There are none. Call back later.” . There is no dignified way to remove exam paper stuck to your backside before introducing yourself to the doctor. Maybe that last slide of Her Majesty was in fact for us to have a moment of silence for what we’ve all lost.
Walter Bagehot (pronounce it “Baj-et” if you tell this story to your Harlan wine friends) was a political writer and editor of The Economist in the 1860s. He famously said that the secret to the English government was having two kinds of institutions, the dignified and the efficient. The efficient, Parliament, was responsible for all the work. The dignified, the Crown, gives significance and holds everyone’s respect. If medicine ever once was both dignified and efficient, we aren’t lately. We push to reduce backlogs, offer same-time virtual care, work to reduce costs. We’ve driven medicine to the efficient and left little of the dignity it seems.
The Queen will be remembered for her lifelong dedication to the laborious service of others. Even though each of us in medicine pledges the same, we also mourn this week the loss of dignity that once came with it.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Queen Elizabeth is everywhere. She was even on the last slide of a presentation on COVID, monkeypox, and influenza vaccines given by our physician in charge of quality. This was odd. The presenter wasn’t English. The Queen had nothing to do with vaccines. Nor apparently would she have said even if she did have an opinion about them. But there we were, an audience of physicians and staff pausing for a moment of remembrance of her.
I’m not a Monarchist – except perhaps for the Kennedys. I grew up in New England. I don’t have an opinion on whether or not the British Crown should endure. But I do marvel at the astounding effect Queen Elizabeth’s passing had on so many around the world. Her personal qualities, particularly her steadiness and humane sympathy, might explain why so many are sad hearing the news. But also I think there was something in her role that we all wished for: Not the owning of palaces and sceptres, but rather, the respect that was given to her.
She was a stateswoman of “unmatched dignity,” the White House wrote. That was true, but it seems being the Queen might have been the last job on earth where such dignity is still possible. Certainly in politics, education, and even health care, there doesn’t seem to be much left lately.
The same day of that presentation I walked into the room of a patient 22 minutes late, she held her arm forth tapping her watch to indicate the time and my tardiness. Unnecessary, if not impertinent. Covering for one of my female physician colleagues, I read an email from a patient which began, “Dear Julie, With all due respect …” Another patient submitted a photo for us to review that was clearly taken from her car while waiting at a stop light. Hardly the consideration a clinical encounter should be given.
Much has been lost for patients. too. There are patients trying to make appointments lately who are told: “There are none. Call back later.” . There is no dignified way to remove exam paper stuck to your backside before introducing yourself to the doctor. Maybe that last slide of Her Majesty was in fact for us to have a moment of silence for what we’ve all lost.
Walter Bagehot (pronounce it “Baj-et” if you tell this story to your Harlan wine friends) was a political writer and editor of The Economist in the 1860s. He famously said that the secret to the English government was having two kinds of institutions, the dignified and the efficient. The efficient, Parliament, was responsible for all the work. The dignified, the Crown, gives significance and holds everyone’s respect. If medicine ever once was both dignified and efficient, we aren’t lately. We push to reduce backlogs, offer same-time virtual care, work to reduce costs. We’ve driven medicine to the efficient and left little of the dignity it seems.
The Queen will be remembered for her lifelong dedication to the laborious service of others. Even though each of us in medicine pledges the same, we also mourn this week the loss of dignity that once came with it.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Queen Elizabeth is everywhere. She was even on the last slide of a presentation on COVID, monkeypox, and influenza vaccines given by our physician in charge of quality. This was odd. The presenter wasn’t English. The Queen had nothing to do with vaccines. Nor apparently would she have said even if she did have an opinion about them. But there we were, an audience of physicians and staff pausing for a moment of remembrance of her.
I’m not a Monarchist – except perhaps for the Kennedys. I grew up in New England. I don’t have an opinion on whether or not the British Crown should endure. But I do marvel at the astounding effect Queen Elizabeth’s passing had on so many around the world. Her personal qualities, particularly her steadiness and humane sympathy, might explain why so many are sad hearing the news. But also I think there was something in her role that we all wished for: Not the owning of palaces and sceptres, but rather, the respect that was given to her.
She was a stateswoman of “unmatched dignity,” the White House wrote. That was true, but it seems being the Queen might have been the last job on earth where such dignity is still possible. Certainly in politics, education, and even health care, there doesn’t seem to be much left lately.
The same day of that presentation I walked into the room of a patient 22 minutes late, she held her arm forth tapping her watch to indicate the time and my tardiness. Unnecessary, if not impertinent. Covering for one of my female physician colleagues, I read an email from a patient which began, “Dear Julie, With all due respect …” Another patient submitted a photo for us to review that was clearly taken from her car while waiting at a stop light. Hardly the consideration a clinical encounter should be given.
Much has been lost for patients. too. There are patients trying to make appointments lately who are told: “There are none. Call back later.” . There is no dignified way to remove exam paper stuck to your backside before introducing yourself to the doctor. Maybe that last slide of Her Majesty was in fact for us to have a moment of silence for what we’ve all lost.
Walter Bagehot (pronounce it “Baj-et” if you tell this story to your Harlan wine friends) was a political writer and editor of The Economist in the 1860s. He famously said that the secret to the English government was having two kinds of institutions, the dignified and the efficient. The efficient, Parliament, was responsible for all the work. The dignified, the Crown, gives significance and holds everyone’s respect. If medicine ever once was both dignified and efficient, we aren’t lately. We push to reduce backlogs, offer same-time virtual care, work to reduce costs. We’ve driven medicine to the efficient and left little of the dignity it seems.
The Queen will be remembered for her lifelong dedication to the laborious service of others. Even though each of us in medicine pledges the same, we also mourn this week the loss of dignity that once came with it.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Tralokinumab earns EU recommendation to expand age range for atopic dermatitis to include adolescents
Tralokinumab has received a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use to extend use to adolescents aged 12 years and older with moderate-to-severe atopic dermatitis (AD) who are candidates for systemic therapy, according to a statement from the manufacturer.
The positive CHMP opinion, issued on Sept. 15, recommends extending the use of tralokinumab (Adtralza), an interleukin-13 antagonist, to adolescents aged 12-17 years in the EU. The positive opinion recommends an initial dose of 600 mg administered subcutaneously followed by 300 mg every other week, the dosing recommended for adults.
In December 2021, tralokinumab was approved for adults with moderate to severe AD in the United States, where it is marketed as Adbry. It is also approved for adults in the EU, Great Britain, Canada, the United Arab Emirates, and Switzerland. It is not currently approved for treatment of adolescents in any country, according to the LEO Pharma statement.
A regulatory filing with the U.S. Food and Drug Administration is in progress, the company said, and an additional study of tralokinumab for individuals aged 12 years and older is underway, according to the manufacturer.
The CHMP opinion was supported by data from a phase 3 study (ECZTRA 6) that assessed safety and efficacy of 150-mg or 300-mg doses of tralokinumab, compared with placebo in adolescents with moderate-to-severe AD, the company statement said. The primary outcomes were an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) and an improvement of at least a 75% on the Eczema Area and Severity Index score (EASI-75). In the study, presented as a poster at a meeting in October 2021, a total of 195 adolescents aged 12-17 with moderate to severe AD who were candidates for systemic therapy were randomly assigned to tralokinumab and 94 to placebo.
At 16 weeks, 21.4% and 17.5% of patients who received 150 mg and 300 mg, respectively, of tralokinumab had IGA scores of 0 or 1, compared with 4.3% of those on placebo (P < .001, P = .002, respectively vs. placebo). In addition, 28.6% and 27.8% of the 150-mg and 300-mg tralokinumab groups, respectively, achieved EASI-75, compared with 6.4% of placebo patients (P < .001, P = .001, respectively, compared with placebo).
Adverse events were similar between the groups, and most were mild or moderate; overall safety profiles were similar to those seen in adult patients.
The European Commission will review the positive opinion and make a final decision.
The research was supported by LEO Pharma.
A version of this article first appeared on Medscape.com.
Tralokinumab has received a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use to extend use to adolescents aged 12 years and older with moderate-to-severe atopic dermatitis (AD) who are candidates for systemic therapy, according to a statement from the manufacturer.
The positive CHMP opinion, issued on Sept. 15, recommends extending the use of tralokinumab (Adtralza), an interleukin-13 antagonist, to adolescents aged 12-17 years in the EU. The positive opinion recommends an initial dose of 600 mg administered subcutaneously followed by 300 mg every other week, the dosing recommended for adults.
In December 2021, tralokinumab was approved for adults with moderate to severe AD in the United States, where it is marketed as Adbry. It is also approved for adults in the EU, Great Britain, Canada, the United Arab Emirates, and Switzerland. It is not currently approved for treatment of adolescents in any country, according to the LEO Pharma statement.
A regulatory filing with the U.S. Food and Drug Administration is in progress, the company said, and an additional study of tralokinumab for individuals aged 12 years and older is underway, according to the manufacturer.
The CHMP opinion was supported by data from a phase 3 study (ECZTRA 6) that assessed safety and efficacy of 150-mg or 300-mg doses of tralokinumab, compared with placebo in adolescents with moderate-to-severe AD, the company statement said. The primary outcomes were an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) and an improvement of at least a 75% on the Eczema Area and Severity Index score (EASI-75). In the study, presented as a poster at a meeting in October 2021, a total of 195 adolescents aged 12-17 with moderate to severe AD who were candidates for systemic therapy were randomly assigned to tralokinumab and 94 to placebo.
At 16 weeks, 21.4% and 17.5% of patients who received 150 mg and 300 mg, respectively, of tralokinumab had IGA scores of 0 or 1, compared with 4.3% of those on placebo (P < .001, P = .002, respectively vs. placebo). In addition, 28.6% and 27.8% of the 150-mg and 300-mg tralokinumab groups, respectively, achieved EASI-75, compared with 6.4% of placebo patients (P < .001, P = .001, respectively, compared with placebo).
Adverse events were similar between the groups, and most were mild or moderate; overall safety profiles were similar to those seen in adult patients.
The European Commission will review the positive opinion and make a final decision.
The research was supported by LEO Pharma.
A version of this article first appeared on Medscape.com.
Tralokinumab has received a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use to extend use to adolescents aged 12 years and older with moderate-to-severe atopic dermatitis (AD) who are candidates for systemic therapy, according to a statement from the manufacturer.
The positive CHMP opinion, issued on Sept. 15, recommends extending the use of tralokinumab (Adtralza), an interleukin-13 antagonist, to adolescents aged 12-17 years in the EU. The positive opinion recommends an initial dose of 600 mg administered subcutaneously followed by 300 mg every other week, the dosing recommended for adults.
In December 2021, tralokinumab was approved for adults with moderate to severe AD in the United States, where it is marketed as Adbry. It is also approved for adults in the EU, Great Britain, Canada, the United Arab Emirates, and Switzerland. It is not currently approved for treatment of adolescents in any country, according to the LEO Pharma statement.
A regulatory filing with the U.S. Food and Drug Administration is in progress, the company said, and an additional study of tralokinumab for individuals aged 12 years and older is underway, according to the manufacturer.
The CHMP opinion was supported by data from a phase 3 study (ECZTRA 6) that assessed safety and efficacy of 150-mg or 300-mg doses of tralokinumab, compared with placebo in adolescents with moderate-to-severe AD, the company statement said. The primary outcomes were an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) and an improvement of at least a 75% on the Eczema Area and Severity Index score (EASI-75). In the study, presented as a poster at a meeting in October 2021, a total of 195 adolescents aged 12-17 with moderate to severe AD who were candidates for systemic therapy were randomly assigned to tralokinumab and 94 to placebo.
At 16 weeks, 21.4% and 17.5% of patients who received 150 mg and 300 mg, respectively, of tralokinumab had IGA scores of 0 or 1, compared with 4.3% of those on placebo (P < .001, P = .002, respectively vs. placebo). In addition, 28.6% and 27.8% of the 150-mg and 300-mg tralokinumab groups, respectively, achieved EASI-75, compared with 6.4% of placebo patients (P < .001, P = .001, respectively, compared with placebo).
Adverse events were similar between the groups, and most were mild or moderate; overall safety profiles were similar to those seen in adult patients.
The European Commission will review the positive opinion and make a final decision.
The research was supported by LEO Pharma.
A version of this article first appeared on Medscape.com.
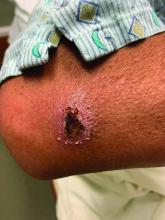
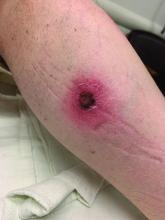
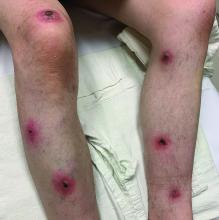
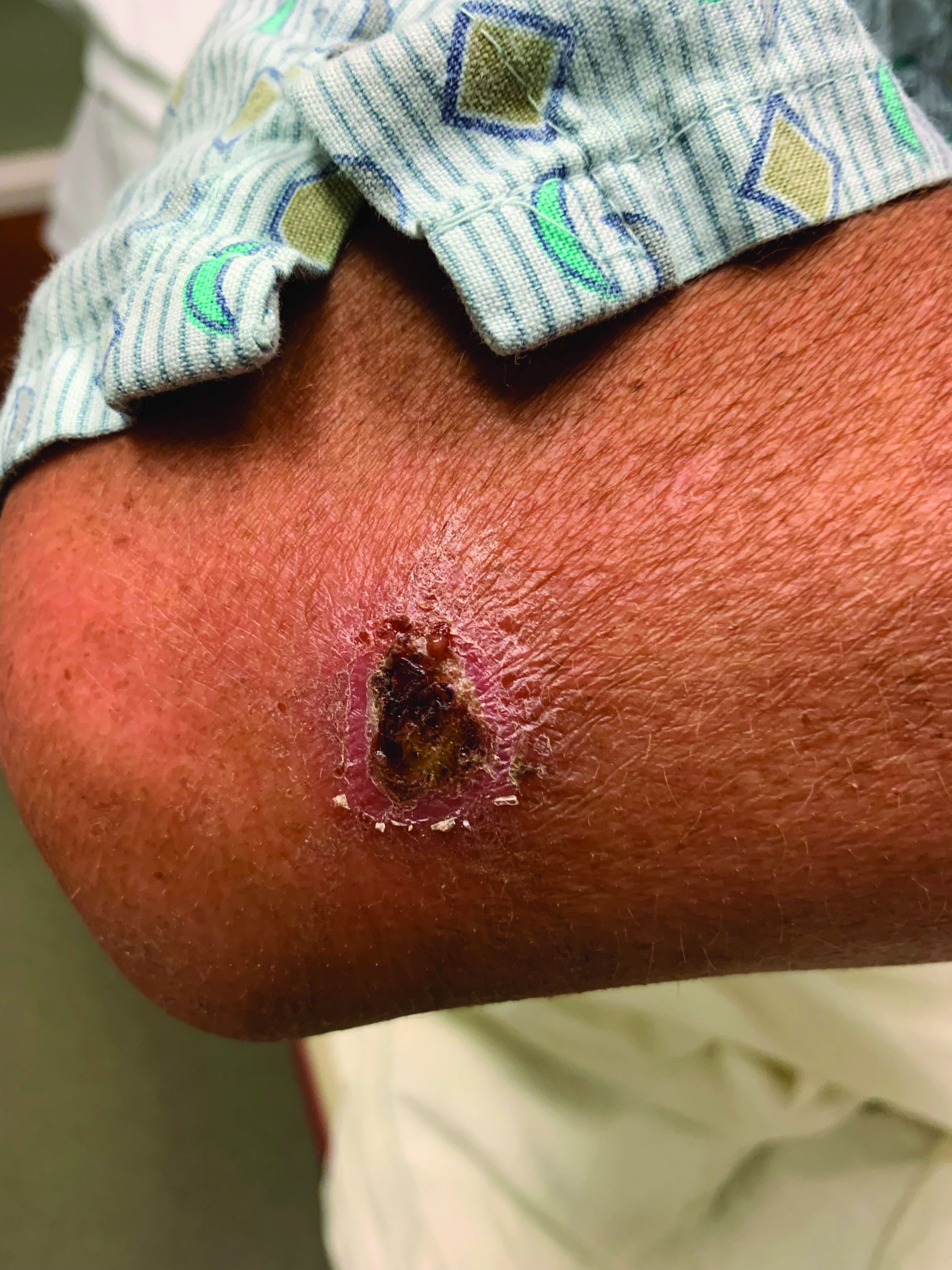
A White male presented with a 1-month history of recurrent, widespread, painful sores
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
House passes prior authorization bill, Senate path unclear
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
Brodalumab suicide risk similar to other biologics, postmarket study finds
.
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
.
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
.
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
Hope shines bright for hidradenitis suppurativa treatments
in separate trials.
Around 40%-50% of patients exhibited a clinical response to these agents at 16 weeks, a leading HS expert reported at the annual congress of the European Academy of Dermatology and Venereology.
Time in the spotlight for HS
Research into HS is “an incredibly active field at this moment,” said Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, and president and chief executive officer of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston.
It’s “been great for advancing our understanding of the biology and the treatments that we will be able to use,” she said.
During the late-breaking sessions at the annual EADV Congress, Dr. Kimball presented data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety and tolerability of the interleukin (IL) 17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS.
“This is only the second phase 3 program we have ever seen in HS and the first one since 2016,” Dr. Kimball said of the trials. It’s also the largest trial program in HS conducted to date, she added, “so it really is a milestone.”
The last big development was when adalimumab, a tumor necrosis factor (TNF) blocker, gained regulatory approval for HS in 2016, observed Neil Patel, PhD, MRCP, who leads the HS service at Imperial College Healthcare NHS Trust in London.
“Adalimumab has been very helpful for many patients, but not all patients respond, and others may respond initially but then the treatment starts to fail after a year or 2,” Dr. Patel said in an interview with this news organization.
“There is definitely a huge need for alternative medication for this condition, which still has a lack of effective treatment options,” added Dr. Patel, who was not involved in either of the studies.
“One major upside for secukinumab is that its safety profile is generally very good and familiarity in the dermatologic community is already well established,” Christopher Sayed, MD, said in a separate interview.
“This will make most providers very comfortable offering it as a potential treatment option sooner rather than later given that its efficacy has now been demonstrated in phase 3 trials,” added Dr. Sayed, associate professor of dermatology at the University of North Carolina at Chapel Hill.
Two identically designed trials
Altogether, SUNSHINE and SUNRISE enrolled just over 1,000 patients at 219 sites in 33 countries. Both trials were identical in their design: A 4-week run-in phase before a randomized, double-blind treatment phase that tested two dosing regimens of secukinumab (300 mg administered subcutaneously) every 2 or 4 weeks vs. placebo for 16 weeks. The trial continued after this time, with patients in the placebo arm re–randomly assigned to treatment with one of the two secukinumab regimens out to a year.
The primary endpoint was the percentage of patients achieving a Hidradenitis Suppurativa Clinical Response (HiSCR) after 16 weeks of treatment, with key secondary endpoints, which were abscess and inflammatory nodule (AN) count, occurrence of flares, and at least a 30% reduction in Patient’s Global Assessment of Skin Pain assessed using a numeric rating scale (NPRS30).
Secukinumab superior to placebo
The HiSCR is defined as at least a 50% decrease in AN count with no increase in the number of abscesses or in the number of draining fistulas relative to baseline. This was achieved by about 42%-45% of patients who received secukinumab every 2 weeks, about 42%-46% of those who received secukinumab every 4 weeks, and about 31%-33% of those on placebo in both studies.
Of note, fewer patients treated with secukinumab (about 15%-20% among those treated every 2 weeks, and about 15% to 23% among those treated every 4 weeks) than those on placebo (27%-29%) experienced flares, defined as at least a 25% increase in AN count and at least a two-point increase relative to baseline values.
Improvement in HS pain can be a difficult parameter to meet, Dr. Kimball noted. “Pain is such an important feature of this disease as it so debilitating for the patients.” More than one-third (almost 36%-39%) of patients given secukinumab vs. just over a quarter (26.9%) given placebo achieved at least a 30% reduction in NPRS30 ratings, she reported. The difference between active and placebo treatment was significant only when secukinumab was given every 2 weeks, however.
“The placebo rates that we see in these studies are exactly parallel to what we saw in other studies, and other disease states when we had a 50% bar of improvement,” Dr. Kimball said when questioned about these results.
“HS is a highly variable disease; it’s maybe not so much the placebo rate or the scoring system used but maybe the 50% bar set for improvement is too low. It’s likely, as data start to mature and a 75% HiSCR can be calculated, that the placebo rates will drop,” she said.
There were no surprises when it came to the safety of secukinumab, being an old player in a new game, she noted. It was “well tolerated” and tolerability was “consistent with the known safety profile,” Dr. Kimball said, “so we expect it to be a new, safe, and effective add to our armamentarium in treating this disease.”
This research involves “basically borrowing drugs from other areas and trying them in HS to see what effect they may have,” Dr. Patel said, noting that drugs such as adalimumab and secukinumab already had a proven track record in other diseases, such as psoriasis. “These early data for secukinumab definitely are very exciting, but we would need to see real-life results” in patients with HS who are not enrolled in trials to see the benefits, he added.
‘Tipping point’ for HS research
“I think we will look back on this meeting and realize that it was an incredibly important tipping point for the treatment of this incredibly debilitating disease,” Dr. Kimball said.
Elsewhere at the meeting, she had presented findings from a phase 2a study that pitted three different kinase inhibitors with different modes of action against each other and compared them with placebo.
The three agents evaluated are an IL-1 receptor–associated kinase 4 inhibitor known as PF-06650833, a tyrosine kinase 2 (TYK2) JAK1 inhibitor brepocitinib, and the TYK2 inhibitor PF-06826647.
“This technique has been used in oncology,” Dr. Kimball said, noting that the ability to test multiple drugs at the same time “means we can really much more efficiently test two different things at the same time, and also put fewer patients at risk for potential problems if drugs don’t work.”
Positive signs for brepocitinib, not the other kinases tested
The results showed that though brepocitinib worked in HS, the other two novel compounds did not appear to have beneficial effects. Just over half (52%) of the 52 patients treated with brepocitinib achieved an HiSCR at 16 weeks, compared with around one-third of those given placebo, PF-06650833, or PF-06826647.
A similar benefit was seen in terms of reduction in flares for brepocitinib but not the other agents, although there was no difference between them all in terms of NPRS30 pain reduction.
“We’ve been able to test three different modalities. This tells us some things about the pathophysiology for HS, which is a very profoundly intensive inflammatory process,” which, Dr. Kimball said, “may require multiple modalities of action to get it under control.” In addition, these “general modalities seem to safe and well tolerated,” she added.
Take-homes for practice and future research
“While it is disappointing that two of the drugs tested did not clearly demonstrate efficacy, it is very possible that these mechanisms of action may be successful targets in the future as new dosing strategies and drugs targeting these pathways are developed,” Dr. Sayed said.
A case in point, he added, was that “adalimumab did not meet treatment endpoints at a dose of 40 mg every other week, but clearly has made a major impact at 40 mg weekly.”
The bottom line is that “both secukinumab and beprocitinib demonstrated efficacy over placebo and are likely to be helpful for a significant number of patients with HS,” Dr. Sayed said. “Hopefully, we’ll see head-to-head trials and more data regarding proportions of patients with deeper responses using criteria such as HiSCR75 and HiSCR90.”
Moreover, “having a larger number of drugs with a range of mechanisms of action is extraordinarily helpful given how difficult the disease can be to manage. We will hopefully continue to see creative approaches and further successes in the current wave of phase 1, 2, and 3 trials that are already underway.”
The SUNSHINE and SUNRISE studies were funded by Novartis Pharma AG, Basel, Switzerland. The phase 2A study Dr. Kimball presented was sponsored by Pfizer.
Dr. Kimball disclosed ties to both Novartis and Pfizer and acts as a consultant and investigator to AbbVie, Bristol-Myers Squibb, Janssen, Eli Lilly, Novartis, and UCB. She is an investigator for Incyte and AnaptysBio; acts as a consultant to Bayer, Boehringer Ingelheim, Ventyz, Moonlake, Lily, Concert, EvoImmune, Sonoma Bio, and Sanofi; receives fellowship funding from Janssen, and serves on the Board of Directors for Almirall.
Dr. Patel had no conflicts of interest to disclose. Dr. Sayed is the director of the HS Foundation, a nonprofit organization, and has acted as an adviser or consultant to, speaker for, and received research funding from multiple drug companies including AbbVie, ChemoCentryx, Incyte, InflaRx, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
in separate trials.
Around 40%-50% of patients exhibited a clinical response to these agents at 16 weeks, a leading HS expert reported at the annual congress of the European Academy of Dermatology and Venereology.
Time in the spotlight for HS
Research into HS is “an incredibly active field at this moment,” said Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, and president and chief executive officer of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston.
It’s “been great for advancing our understanding of the biology and the treatments that we will be able to use,” she said.
During the late-breaking sessions at the annual EADV Congress, Dr. Kimball presented data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety and tolerability of the interleukin (IL) 17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS.
“This is only the second phase 3 program we have ever seen in HS and the first one since 2016,” Dr. Kimball said of the trials. It’s also the largest trial program in HS conducted to date, she added, “so it really is a milestone.”
The last big development was when adalimumab, a tumor necrosis factor (TNF) blocker, gained regulatory approval for HS in 2016, observed Neil Patel, PhD, MRCP, who leads the HS service at Imperial College Healthcare NHS Trust in London.
“Adalimumab has been very helpful for many patients, but not all patients respond, and others may respond initially but then the treatment starts to fail after a year or 2,” Dr. Patel said in an interview with this news organization.
“There is definitely a huge need for alternative medication for this condition, which still has a lack of effective treatment options,” added Dr. Patel, who was not involved in either of the studies.
“One major upside for secukinumab is that its safety profile is generally very good and familiarity in the dermatologic community is already well established,” Christopher Sayed, MD, said in a separate interview.
“This will make most providers very comfortable offering it as a potential treatment option sooner rather than later given that its efficacy has now been demonstrated in phase 3 trials,” added Dr. Sayed, associate professor of dermatology at the University of North Carolina at Chapel Hill.
Two identically designed trials
Altogether, SUNSHINE and SUNRISE enrolled just over 1,000 patients at 219 sites in 33 countries. Both trials were identical in their design: A 4-week run-in phase before a randomized, double-blind treatment phase that tested two dosing regimens of secukinumab (300 mg administered subcutaneously) every 2 or 4 weeks vs. placebo for 16 weeks. The trial continued after this time, with patients in the placebo arm re–randomly assigned to treatment with one of the two secukinumab regimens out to a year.
The primary endpoint was the percentage of patients achieving a Hidradenitis Suppurativa Clinical Response (HiSCR) after 16 weeks of treatment, with key secondary endpoints, which were abscess and inflammatory nodule (AN) count, occurrence of flares, and at least a 30% reduction in Patient’s Global Assessment of Skin Pain assessed using a numeric rating scale (NPRS30).
Secukinumab superior to placebo
The HiSCR is defined as at least a 50% decrease in AN count with no increase in the number of abscesses or in the number of draining fistulas relative to baseline. This was achieved by about 42%-45% of patients who received secukinumab every 2 weeks, about 42%-46% of those who received secukinumab every 4 weeks, and about 31%-33% of those on placebo in both studies.
Of note, fewer patients treated with secukinumab (about 15%-20% among those treated every 2 weeks, and about 15% to 23% among those treated every 4 weeks) than those on placebo (27%-29%) experienced flares, defined as at least a 25% increase in AN count and at least a two-point increase relative to baseline values.
Improvement in HS pain can be a difficult parameter to meet, Dr. Kimball noted. “Pain is such an important feature of this disease as it so debilitating for the patients.” More than one-third (almost 36%-39%) of patients given secukinumab vs. just over a quarter (26.9%) given placebo achieved at least a 30% reduction in NPRS30 ratings, she reported. The difference between active and placebo treatment was significant only when secukinumab was given every 2 weeks, however.
“The placebo rates that we see in these studies are exactly parallel to what we saw in other studies, and other disease states when we had a 50% bar of improvement,” Dr. Kimball said when questioned about these results.
“HS is a highly variable disease; it’s maybe not so much the placebo rate or the scoring system used but maybe the 50% bar set for improvement is too low. It’s likely, as data start to mature and a 75% HiSCR can be calculated, that the placebo rates will drop,” she said.
There were no surprises when it came to the safety of secukinumab, being an old player in a new game, she noted. It was “well tolerated” and tolerability was “consistent with the known safety profile,” Dr. Kimball said, “so we expect it to be a new, safe, and effective add to our armamentarium in treating this disease.”
This research involves “basically borrowing drugs from other areas and trying them in HS to see what effect they may have,” Dr. Patel said, noting that drugs such as adalimumab and secukinumab already had a proven track record in other diseases, such as psoriasis. “These early data for secukinumab definitely are very exciting, but we would need to see real-life results” in patients with HS who are not enrolled in trials to see the benefits, he added.
‘Tipping point’ for HS research
“I think we will look back on this meeting and realize that it was an incredibly important tipping point for the treatment of this incredibly debilitating disease,” Dr. Kimball said.
Elsewhere at the meeting, she had presented findings from a phase 2a study that pitted three different kinase inhibitors with different modes of action against each other and compared them with placebo.
The three agents evaluated are an IL-1 receptor–associated kinase 4 inhibitor known as PF-06650833, a tyrosine kinase 2 (TYK2) JAK1 inhibitor brepocitinib, and the TYK2 inhibitor PF-06826647.
“This technique has been used in oncology,” Dr. Kimball said, noting that the ability to test multiple drugs at the same time “means we can really much more efficiently test two different things at the same time, and also put fewer patients at risk for potential problems if drugs don’t work.”
Positive signs for brepocitinib, not the other kinases tested
The results showed that though brepocitinib worked in HS, the other two novel compounds did not appear to have beneficial effects. Just over half (52%) of the 52 patients treated with brepocitinib achieved an HiSCR at 16 weeks, compared with around one-third of those given placebo, PF-06650833, or PF-06826647.
A similar benefit was seen in terms of reduction in flares for brepocitinib but not the other agents, although there was no difference between them all in terms of NPRS30 pain reduction.
“We’ve been able to test three different modalities. This tells us some things about the pathophysiology for HS, which is a very profoundly intensive inflammatory process,” which, Dr. Kimball said, “may require multiple modalities of action to get it under control.” In addition, these “general modalities seem to safe and well tolerated,” she added.
Take-homes for practice and future research
“While it is disappointing that two of the drugs tested did not clearly demonstrate efficacy, it is very possible that these mechanisms of action may be successful targets in the future as new dosing strategies and drugs targeting these pathways are developed,” Dr. Sayed said.
A case in point, he added, was that “adalimumab did not meet treatment endpoints at a dose of 40 mg every other week, but clearly has made a major impact at 40 mg weekly.”
The bottom line is that “both secukinumab and beprocitinib demonstrated efficacy over placebo and are likely to be helpful for a significant number of patients with HS,” Dr. Sayed said. “Hopefully, we’ll see head-to-head trials and more data regarding proportions of patients with deeper responses using criteria such as HiSCR75 and HiSCR90.”
Moreover, “having a larger number of drugs with a range of mechanisms of action is extraordinarily helpful given how difficult the disease can be to manage. We will hopefully continue to see creative approaches and further successes in the current wave of phase 1, 2, and 3 trials that are already underway.”
The SUNSHINE and SUNRISE studies were funded by Novartis Pharma AG, Basel, Switzerland. The phase 2A study Dr. Kimball presented was sponsored by Pfizer.
Dr. Kimball disclosed ties to both Novartis and Pfizer and acts as a consultant and investigator to AbbVie, Bristol-Myers Squibb, Janssen, Eli Lilly, Novartis, and UCB. She is an investigator for Incyte and AnaptysBio; acts as a consultant to Bayer, Boehringer Ingelheim, Ventyz, Moonlake, Lily, Concert, EvoImmune, Sonoma Bio, and Sanofi; receives fellowship funding from Janssen, and serves on the Board of Directors for Almirall.
Dr. Patel had no conflicts of interest to disclose. Dr. Sayed is the director of the HS Foundation, a nonprofit organization, and has acted as an adviser or consultant to, speaker for, and received research funding from multiple drug companies including AbbVie, ChemoCentryx, Incyte, InflaRx, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
in separate trials.
Around 40%-50% of patients exhibited a clinical response to these agents at 16 weeks, a leading HS expert reported at the annual congress of the European Academy of Dermatology and Venereology.
Time in the spotlight for HS
Research into HS is “an incredibly active field at this moment,” said Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, and president and chief executive officer of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston.
It’s “been great for advancing our understanding of the biology and the treatments that we will be able to use,” she said.
During the late-breaking sessions at the annual EADV Congress, Dr. Kimball presented data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety and tolerability of the interleukin (IL) 17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS.
“This is only the second phase 3 program we have ever seen in HS and the first one since 2016,” Dr. Kimball said of the trials. It’s also the largest trial program in HS conducted to date, she added, “so it really is a milestone.”
The last big development was when adalimumab, a tumor necrosis factor (TNF) blocker, gained regulatory approval for HS in 2016, observed Neil Patel, PhD, MRCP, who leads the HS service at Imperial College Healthcare NHS Trust in London.
“Adalimumab has been very helpful for many patients, but not all patients respond, and others may respond initially but then the treatment starts to fail after a year or 2,” Dr. Patel said in an interview with this news organization.
“There is definitely a huge need for alternative medication for this condition, which still has a lack of effective treatment options,” added Dr. Patel, who was not involved in either of the studies.
“One major upside for secukinumab is that its safety profile is generally very good and familiarity in the dermatologic community is already well established,” Christopher Sayed, MD, said in a separate interview.
“This will make most providers very comfortable offering it as a potential treatment option sooner rather than later given that its efficacy has now been demonstrated in phase 3 trials,” added Dr. Sayed, associate professor of dermatology at the University of North Carolina at Chapel Hill.
Two identically designed trials
Altogether, SUNSHINE and SUNRISE enrolled just over 1,000 patients at 219 sites in 33 countries. Both trials were identical in their design: A 4-week run-in phase before a randomized, double-blind treatment phase that tested two dosing regimens of secukinumab (300 mg administered subcutaneously) every 2 or 4 weeks vs. placebo for 16 weeks. The trial continued after this time, with patients in the placebo arm re–randomly assigned to treatment with one of the two secukinumab regimens out to a year.
The primary endpoint was the percentage of patients achieving a Hidradenitis Suppurativa Clinical Response (HiSCR) after 16 weeks of treatment, with key secondary endpoints, which were abscess and inflammatory nodule (AN) count, occurrence of flares, and at least a 30% reduction in Patient’s Global Assessment of Skin Pain assessed using a numeric rating scale (NPRS30).
Secukinumab superior to placebo
The HiSCR is defined as at least a 50% decrease in AN count with no increase in the number of abscesses or in the number of draining fistulas relative to baseline. This was achieved by about 42%-45% of patients who received secukinumab every 2 weeks, about 42%-46% of those who received secukinumab every 4 weeks, and about 31%-33% of those on placebo in both studies.
Of note, fewer patients treated with secukinumab (about 15%-20% among those treated every 2 weeks, and about 15% to 23% among those treated every 4 weeks) than those on placebo (27%-29%) experienced flares, defined as at least a 25% increase in AN count and at least a two-point increase relative to baseline values.
Improvement in HS pain can be a difficult parameter to meet, Dr. Kimball noted. “Pain is such an important feature of this disease as it so debilitating for the patients.” More than one-third (almost 36%-39%) of patients given secukinumab vs. just over a quarter (26.9%) given placebo achieved at least a 30% reduction in NPRS30 ratings, she reported. The difference between active and placebo treatment was significant only when secukinumab was given every 2 weeks, however.
“The placebo rates that we see in these studies are exactly parallel to what we saw in other studies, and other disease states when we had a 50% bar of improvement,” Dr. Kimball said when questioned about these results.
“HS is a highly variable disease; it’s maybe not so much the placebo rate or the scoring system used but maybe the 50% bar set for improvement is too low. It’s likely, as data start to mature and a 75% HiSCR can be calculated, that the placebo rates will drop,” she said.
There were no surprises when it came to the safety of secukinumab, being an old player in a new game, she noted. It was “well tolerated” and tolerability was “consistent with the known safety profile,” Dr. Kimball said, “so we expect it to be a new, safe, and effective add to our armamentarium in treating this disease.”
This research involves “basically borrowing drugs from other areas and trying them in HS to see what effect they may have,” Dr. Patel said, noting that drugs such as adalimumab and secukinumab already had a proven track record in other diseases, such as psoriasis. “These early data for secukinumab definitely are very exciting, but we would need to see real-life results” in patients with HS who are not enrolled in trials to see the benefits, he added.
‘Tipping point’ for HS research
“I think we will look back on this meeting and realize that it was an incredibly important tipping point for the treatment of this incredibly debilitating disease,” Dr. Kimball said.
Elsewhere at the meeting, she had presented findings from a phase 2a study that pitted three different kinase inhibitors with different modes of action against each other and compared them with placebo.
The three agents evaluated are an IL-1 receptor–associated kinase 4 inhibitor known as PF-06650833, a tyrosine kinase 2 (TYK2) JAK1 inhibitor brepocitinib, and the TYK2 inhibitor PF-06826647.
“This technique has been used in oncology,” Dr. Kimball said, noting that the ability to test multiple drugs at the same time “means we can really much more efficiently test two different things at the same time, and also put fewer patients at risk for potential problems if drugs don’t work.”
Positive signs for brepocitinib, not the other kinases tested
The results showed that though brepocitinib worked in HS, the other two novel compounds did not appear to have beneficial effects. Just over half (52%) of the 52 patients treated with brepocitinib achieved an HiSCR at 16 weeks, compared with around one-third of those given placebo, PF-06650833, or PF-06826647.
A similar benefit was seen in terms of reduction in flares for brepocitinib but not the other agents, although there was no difference between them all in terms of NPRS30 pain reduction.
“We’ve been able to test three different modalities. This tells us some things about the pathophysiology for HS, which is a very profoundly intensive inflammatory process,” which, Dr. Kimball said, “may require multiple modalities of action to get it under control.” In addition, these “general modalities seem to safe and well tolerated,” she added.
Take-homes for practice and future research
“While it is disappointing that two of the drugs tested did not clearly demonstrate efficacy, it is very possible that these mechanisms of action may be successful targets in the future as new dosing strategies and drugs targeting these pathways are developed,” Dr. Sayed said.
A case in point, he added, was that “adalimumab did not meet treatment endpoints at a dose of 40 mg every other week, but clearly has made a major impact at 40 mg weekly.”
The bottom line is that “both secukinumab and beprocitinib demonstrated efficacy over placebo and are likely to be helpful for a significant number of patients with HS,” Dr. Sayed said. “Hopefully, we’ll see head-to-head trials and more data regarding proportions of patients with deeper responses using criteria such as HiSCR75 and HiSCR90.”
Moreover, “having a larger number of drugs with a range of mechanisms of action is extraordinarily helpful given how difficult the disease can be to manage. We will hopefully continue to see creative approaches and further successes in the current wave of phase 1, 2, and 3 trials that are already underway.”
The SUNSHINE and SUNRISE studies were funded by Novartis Pharma AG, Basel, Switzerland. The phase 2A study Dr. Kimball presented was sponsored by Pfizer.
Dr. Kimball disclosed ties to both Novartis and Pfizer and acts as a consultant and investigator to AbbVie, Bristol-Myers Squibb, Janssen, Eli Lilly, Novartis, and UCB. She is an investigator for Incyte and AnaptysBio; acts as a consultant to Bayer, Boehringer Ingelheim, Ventyz, Moonlake, Lily, Concert, EvoImmune, Sonoma Bio, and Sanofi; receives fellowship funding from Janssen, and serves on the Board of Directors for Almirall.
Dr. Patel had no conflicts of interest to disclose. Dr. Sayed is the director of the HS Foundation, a nonprofit organization, and has acted as an adviser or consultant to, speaker for, and received research funding from multiple drug companies including AbbVie, ChemoCentryx, Incyte, InflaRx, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Physicians can’t be bystanders in ‘silent scourge’ of medical bullying
Maya Iyer, MD, MEd, experienced bullying as a faculty member, and she sensed that she wasn’t alone. “The best ideas for research often come from individual experiences, in both personal and the professional academic medicine setting,” she said in an interview.
“And I was correct. I was not the only one who experienced bullying. In fact, the most severe bullying experiences among ... women physician leaders occurred when they were in leadership positions,” said Dr. Iyer, a pediatric emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
She is a coauthor of a study that was published in JAMA Network Open in which investigators surveyed the existence of antibullying policies for faculty at almost 100 U.S. medical schools.
The researchers defined bullying as “a severe form of mistreatment [that] occurs in the medical setting when a power differential allows offenders to consciously target individuals through persistent negative actions to impede the education or career of the target.”
The study included 91 medical schools, of which 4 schools had antibullying policies that included the reporting of procedures. Of the 87 medical schools without antibullying policies, 60 had antiharrassment policies; of those schools, 10 of the schools’ websites cited bullying and antiharassment policies. Five schools required a login to access policies, and one school’s website had a broken webpage link, per the study.
“We need to bring the silent scourge of bullying to the forefront because bullying is causing a brain drain on the medical profession,” said Dr. Iyer. “Bullying has numerous downstream negative effects, including depression, anxiety, burnout stress, decreased patient care satisfaction, increased medical errors, and job attrition.”
She added: “Through bullying, we are losing voices in medicine just at that point in time where we are trying to diversify the workforce to improve representation of all physicians.”
Dr. Iyer’s team sampled the top 25 schools for research and the top 25 schools for primary care. They also took a random sampling from 25 schools for research and a random sampling from top 25 schools for primary care. They assessed antibullying policies, antiharassment policies that mentioned bullying, antiharrassment policies that did not mention bullying, and the absence of policies addressing these issues.
Policy comprehensiveness was another focus for the researchers. They evaluated whether the relevant policies included faculty members and articulated the institution’s commitment to providing a safe and healthy workplace. Other factors included defining bullying and the roles and responsibilities of employees and procedures for reporting bullying.
Physicians can’t be bystanders to bullying
This means transitioning from being a bystander to an upstander.”
She doesn’t let medical schools off the hook, however. Instead, she advocated having institutions “provide safe spaces and opportunities for near-peer mentoring so that targets of bullying can share stories.”
Regarding who is responsible for addressing bullying, Dr. Iyer is emphatic. “I do want to be clear that the onus of disrupting does not fall on the targets. Rather, we need to fix the systems in which such behavior is tolerated.”
Her advice to leaders in academic medicine is to create comprehensive, zero-retaliation bullying policies that include detailed reporting procedures. Dr. Iyer advised leaders to partner with colleagues in human resources, offices of equity, and ombudspersons to develop, implement, and enforce these policies.
The study authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Maya Iyer, MD, MEd, experienced bullying as a faculty member, and she sensed that she wasn’t alone. “The best ideas for research often come from individual experiences, in both personal and the professional academic medicine setting,” she said in an interview.
“And I was correct. I was not the only one who experienced bullying. In fact, the most severe bullying experiences among ... women physician leaders occurred when they were in leadership positions,” said Dr. Iyer, a pediatric emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
She is a coauthor of a study that was published in JAMA Network Open in which investigators surveyed the existence of antibullying policies for faculty at almost 100 U.S. medical schools.
The researchers defined bullying as “a severe form of mistreatment [that] occurs in the medical setting when a power differential allows offenders to consciously target individuals through persistent negative actions to impede the education or career of the target.”
The study included 91 medical schools, of which 4 schools had antibullying policies that included the reporting of procedures. Of the 87 medical schools without antibullying policies, 60 had antiharrassment policies; of those schools, 10 of the schools’ websites cited bullying and antiharassment policies. Five schools required a login to access policies, and one school’s website had a broken webpage link, per the study.
“We need to bring the silent scourge of bullying to the forefront because bullying is causing a brain drain on the medical profession,” said Dr. Iyer. “Bullying has numerous downstream negative effects, including depression, anxiety, burnout stress, decreased patient care satisfaction, increased medical errors, and job attrition.”
She added: “Through bullying, we are losing voices in medicine just at that point in time where we are trying to diversify the workforce to improve representation of all physicians.”
Dr. Iyer’s team sampled the top 25 schools for research and the top 25 schools for primary care. They also took a random sampling from 25 schools for research and a random sampling from top 25 schools for primary care. They assessed antibullying policies, antiharassment policies that mentioned bullying, antiharrassment policies that did not mention bullying, and the absence of policies addressing these issues.
Policy comprehensiveness was another focus for the researchers. They evaluated whether the relevant policies included faculty members and articulated the institution’s commitment to providing a safe and healthy workplace. Other factors included defining bullying and the roles and responsibilities of employees and procedures for reporting bullying.
Physicians can’t be bystanders to bullying
This means transitioning from being a bystander to an upstander.”
She doesn’t let medical schools off the hook, however. Instead, she advocated having institutions “provide safe spaces and opportunities for near-peer mentoring so that targets of bullying can share stories.”
Regarding who is responsible for addressing bullying, Dr. Iyer is emphatic. “I do want to be clear that the onus of disrupting does not fall on the targets. Rather, we need to fix the systems in which such behavior is tolerated.”
Her advice to leaders in academic medicine is to create comprehensive, zero-retaliation bullying policies that include detailed reporting procedures. Dr. Iyer advised leaders to partner with colleagues in human resources, offices of equity, and ombudspersons to develop, implement, and enforce these policies.
The study authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Maya Iyer, MD, MEd, experienced bullying as a faculty member, and she sensed that she wasn’t alone. “The best ideas for research often come from individual experiences, in both personal and the professional academic medicine setting,” she said in an interview.
“And I was correct. I was not the only one who experienced bullying. In fact, the most severe bullying experiences among ... women physician leaders occurred when they were in leadership positions,” said Dr. Iyer, a pediatric emergency medicine physician at Nationwide Children’s Hospital in Columbus, Ohio.
She is a coauthor of a study that was published in JAMA Network Open in which investigators surveyed the existence of antibullying policies for faculty at almost 100 U.S. medical schools.
The researchers defined bullying as “a severe form of mistreatment [that] occurs in the medical setting when a power differential allows offenders to consciously target individuals through persistent negative actions to impede the education or career of the target.”
The study included 91 medical schools, of which 4 schools had antibullying policies that included the reporting of procedures. Of the 87 medical schools without antibullying policies, 60 had antiharrassment policies; of those schools, 10 of the schools’ websites cited bullying and antiharassment policies. Five schools required a login to access policies, and one school’s website had a broken webpage link, per the study.
“We need to bring the silent scourge of bullying to the forefront because bullying is causing a brain drain on the medical profession,” said Dr. Iyer. “Bullying has numerous downstream negative effects, including depression, anxiety, burnout stress, decreased patient care satisfaction, increased medical errors, and job attrition.”
She added: “Through bullying, we are losing voices in medicine just at that point in time where we are trying to diversify the workforce to improve representation of all physicians.”
Dr. Iyer’s team sampled the top 25 schools for research and the top 25 schools for primary care. They also took a random sampling from 25 schools for research and a random sampling from top 25 schools for primary care. They assessed antibullying policies, antiharassment policies that mentioned bullying, antiharrassment policies that did not mention bullying, and the absence of policies addressing these issues.
Policy comprehensiveness was another focus for the researchers. They evaluated whether the relevant policies included faculty members and articulated the institution’s commitment to providing a safe and healthy workplace. Other factors included defining bullying and the roles and responsibilities of employees and procedures for reporting bullying.
Physicians can’t be bystanders to bullying
This means transitioning from being a bystander to an upstander.”
She doesn’t let medical schools off the hook, however. Instead, she advocated having institutions “provide safe spaces and opportunities for near-peer mentoring so that targets of bullying can share stories.”
Regarding who is responsible for addressing bullying, Dr. Iyer is emphatic. “I do want to be clear that the onus of disrupting does not fall on the targets. Rather, we need to fix the systems in which such behavior is tolerated.”
Her advice to leaders in academic medicine is to create comprehensive, zero-retaliation bullying policies that include detailed reporting procedures. Dr. Iyer advised leaders to partner with colleagues in human resources, offices of equity, and ombudspersons to develop, implement, and enforce these policies.
The study authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
EHR: A progress report
I wrote my first column on electronic health records in the mid-1990s. At the time, it seemed like an idea whose time had come. After all, in an era when just about every essential process in medicine had already been computerized, we physicians continued to process clinical data – our key asset – with pen and paper. Most of us were reluctant to make the switch, and for good reason:
Then, the government stepped in. Shortly after his inauguration in 2000, President George W. Bush outlined a plan to ensure that most Americans had electronic health records within 10 years. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” The goal was to eliminate missing charts, duplication of lab testing, ineffective documentation, and inordinate amounts of time spent on paperwork, not to mention illegible handwriting, poor coordination of care between physicians, and many other problems. Studies were quoted, suggesting that EHR shortened inpatient stays, decreased risk of adverse drug interactions, improved the consistency and content of records, and improved continuity of care and follow-up.
The EHR Incentive Program (later renamed the Promoting Interoperability Program) was introduced to encourage physicians and hospitals “to adopt, implement, upgrade, and demonstrate meaningful use of certified electronic health record technology.”
Nearly a quarter-century later, implementation is well behind schedule. According to a 2019 federal study, while nearly all hospitals (96%) have adopted a certified EHR, only 72% of office-based physicians have done so.
There are multiple reasons for this. For one thing, EHR is still by and large slower than pen and paper, because direct data entry is still primarily done by keyboard. Voice recognition, hand-held and wireless devices have been developed, but most work only on specialized tasks. Even the best systems take more clinician time per encounter than the manual processes they replace.
Physicians have been slow to warm to a system that slows them down and forces them to change the way they think and work. In addition, paper systems never crash; the prospect of a server malfunction or Internet failure bringing an entire clinic to a grinding halt is not particularly inviting.
The special needs of dermatology – high patient volumes, multiple diagnoses and prescriptions per patient, the wide variety of procedures we perform, and digital image storage – present further hurdles.
Nevertheless, the march toward electronic record keeping continues, and I continue to receive many questions about choosing a good EHR system. As always, I cannot recommend any specific products since every office has unique needs and requirements.
The key phrase to keep in mind is caveat emptor. Several regulatory bodies exist to test vendor claims and certify system behaviors, but different agencies use different criteria that may or may not be relevant to your requirements. Vaporware is still as common as real software; beware the “feature in the next release” that might never appear, particularly if you need it right now.
Avoid the temptation to buy a flashy new system and then try to adapt it to your office; figure out your needs first, then find a system that meets them.
Unfortunately, there is no easy way around doing the work of comparing one system with another. The most important information a vendor can give you is the names and addresses of two or more offices where you can go watch their system in action. Site visits are time-consuming, but they are only way to pick the best EHR the first time around.
Don’t be the first office using a new system. Let the vendor work out the bugs somewhere else.
Above all, if you have disorganized paper records, don’t count on EHR to automatically solve your problems. Well-designed paper systems usually lend themselves to effective automation, but automating a poorly designed system just increases the chaos. If your paper system is in disarray, solve that problem before considering EHR.
With all of its problems and hurdles, EHRs will inevitably be a part of most of our lives. And for those who take the time to do it right, it will ultimately be an improvement.
Think of information technologies as power tools: They can help you to do things better, but they can also amplify your errors. So choose carefully.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
I wrote my first column on electronic health records in the mid-1990s. At the time, it seemed like an idea whose time had come. After all, in an era when just about every essential process in medicine had already been computerized, we physicians continued to process clinical data – our key asset – with pen and paper. Most of us were reluctant to make the switch, and for good reason:
Then, the government stepped in. Shortly after his inauguration in 2000, President George W. Bush outlined a plan to ensure that most Americans had electronic health records within 10 years. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” The goal was to eliminate missing charts, duplication of lab testing, ineffective documentation, and inordinate amounts of time spent on paperwork, not to mention illegible handwriting, poor coordination of care between physicians, and many other problems. Studies were quoted, suggesting that EHR shortened inpatient stays, decreased risk of adverse drug interactions, improved the consistency and content of records, and improved continuity of care and follow-up.
The EHR Incentive Program (later renamed the Promoting Interoperability Program) was introduced to encourage physicians and hospitals “to adopt, implement, upgrade, and demonstrate meaningful use of certified electronic health record technology.”
Nearly a quarter-century later, implementation is well behind schedule. According to a 2019 federal study, while nearly all hospitals (96%) have adopted a certified EHR, only 72% of office-based physicians have done so.
There are multiple reasons for this. For one thing, EHR is still by and large slower than pen and paper, because direct data entry is still primarily done by keyboard. Voice recognition, hand-held and wireless devices have been developed, but most work only on specialized tasks. Even the best systems take more clinician time per encounter than the manual processes they replace.
Physicians have been slow to warm to a system that slows them down and forces them to change the way they think and work. In addition, paper systems never crash; the prospect of a server malfunction or Internet failure bringing an entire clinic to a grinding halt is not particularly inviting.
The special needs of dermatology – high patient volumes, multiple diagnoses and prescriptions per patient, the wide variety of procedures we perform, and digital image storage – present further hurdles.
Nevertheless, the march toward electronic record keeping continues, and I continue to receive many questions about choosing a good EHR system. As always, I cannot recommend any specific products since every office has unique needs and requirements.
The key phrase to keep in mind is caveat emptor. Several regulatory bodies exist to test vendor claims and certify system behaviors, but different agencies use different criteria that may or may not be relevant to your requirements. Vaporware is still as common as real software; beware the “feature in the next release” that might never appear, particularly if you need it right now.
Avoid the temptation to buy a flashy new system and then try to adapt it to your office; figure out your needs first, then find a system that meets them.
Unfortunately, there is no easy way around doing the work of comparing one system with another. The most important information a vendor can give you is the names and addresses of two or more offices where you can go watch their system in action. Site visits are time-consuming, but they are only way to pick the best EHR the first time around.
Don’t be the first office using a new system. Let the vendor work out the bugs somewhere else.
Above all, if you have disorganized paper records, don’t count on EHR to automatically solve your problems. Well-designed paper systems usually lend themselves to effective automation, but automating a poorly designed system just increases the chaos. If your paper system is in disarray, solve that problem before considering EHR.
With all of its problems and hurdles, EHRs will inevitably be a part of most of our lives. And for those who take the time to do it right, it will ultimately be an improvement.
Think of information technologies as power tools: They can help you to do things better, but they can also amplify your errors. So choose carefully.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
I wrote my first column on electronic health records in the mid-1990s. At the time, it seemed like an idea whose time had come. After all, in an era when just about every essential process in medicine had already been computerized, we physicians continued to process clinical data – our key asset – with pen and paper. Most of us were reluctant to make the switch, and for good reason:
Then, the government stepped in. Shortly after his inauguration in 2000, President George W. Bush outlined a plan to ensure that most Americans had electronic health records within 10 years. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” The goal was to eliminate missing charts, duplication of lab testing, ineffective documentation, and inordinate amounts of time spent on paperwork, not to mention illegible handwriting, poor coordination of care between physicians, and many other problems. Studies were quoted, suggesting that EHR shortened inpatient stays, decreased risk of adverse drug interactions, improved the consistency and content of records, and improved continuity of care and follow-up.
The EHR Incentive Program (later renamed the Promoting Interoperability Program) was introduced to encourage physicians and hospitals “to adopt, implement, upgrade, and demonstrate meaningful use of certified electronic health record technology.”
Nearly a quarter-century later, implementation is well behind schedule. According to a 2019 federal study, while nearly all hospitals (96%) have adopted a certified EHR, only 72% of office-based physicians have done so.
There are multiple reasons for this. For one thing, EHR is still by and large slower than pen and paper, because direct data entry is still primarily done by keyboard. Voice recognition, hand-held and wireless devices have been developed, but most work only on specialized tasks. Even the best systems take more clinician time per encounter than the manual processes they replace.
Physicians have been slow to warm to a system that slows them down and forces them to change the way they think and work. In addition, paper systems never crash; the prospect of a server malfunction or Internet failure bringing an entire clinic to a grinding halt is not particularly inviting.
The special needs of dermatology – high patient volumes, multiple diagnoses and prescriptions per patient, the wide variety of procedures we perform, and digital image storage – present further hurdles.
Nevertheless, the march toward electronic record keeping continues, and I continue to receive many questions about choosing a good EHR system. As always, I cannot recommend any specific products since every office has unique needs and requirements.
The key phrase to keep in mind is caveat emptor. Several regulatory bodies exist to test vendor claims and certify system behaviors, but different agencies use different criteria that may or may not be relevant to your requirements. Vaporware is still as common as real software; beware the “feature in the next release” that might never appear, particularly if you need it right now.
Avoid the temptation to buy a flashy new system and then try to adapt it to your office; figure out your needs first, then find a system that meets them.
Unfortunately, there is no easy way around doing the work of comparing one system with another. The most important information a vendor can give you is the names and addresses of two or more offices where you can go watch their system in action. Site visits are time-consuming, but they are only way to pick the best EHR the first time around.
Don’t be the first office using a new system. Let the vendor work out the bugs somewhere else.
Above all, if you have disorganized paper records, don’t count on EHR to automatically solve your problems. Well-designed paper systems usually lend themselves to effective automation, but automating a poorly designed system just increases the chaos. If your paper system is in disarray, solve that problem before considering EHR.
With all of its problems and hurdles, EHRs will inevitably be a part of most of our lives. And for those who take the time to do it right, it will ultimately be an improvement.
Think of information technologies as power tools: They can help you to do things better, but they can also amplify your errors. So choose carefully.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Quiet quitting: Are physicians dying inside bit by bit? Or setting healthy boundaries?
In the past few months, “quiet quitting” has garnered increasing traction across social media platforms. My morning review of social media revealed thousands of posts ranging from “Why doing less at work could be good for you – and your employer” to “After ‘quiet quitting’ here comes ‘quiet firing.’ ”
But quiet quitting is neither quiet nor quitting.
Quiet quitting is a misnomer. In addition, quiet quitters are firmer with their boundaries, do not take on work above and beyond clearly stated expectations, do not respond after hours, and do not feel like they are “not doing their job” when they are not immediately available.
Individuals who “quiet quit” continue to meet the demands of their job but reject the hustle-culture mentality that you must always be available for more work and, most importantly, that your value as person and self-worth are defined and determined by your work. Quiet quitters believe that it is possible to have good boundaries and yet remain productive, engaged, and active within the workplace.
Earlier this month, NPR’s posted tutorial on how to set better boundaries at work garnered 491,000 views, reflecting employees’ difficulties in communicating their needs, thoughts, and availability to their employers. Quiet quitting refers to not only rejecting the idea of going above and beyond in the workplace but also feeling confident that there will not be negative ramifications for not consistently working beyond the expected requirements.
A focus on balance, life, loves, and family is rarely addressed or emphasized by traditional employers; employees have little skill in addressing boundaries and clarifying their value and availability. For decades, “needing” flexibility of any kind or valuing activities as much as your job were viewed as negative attributes, making those individuals less-desired employees.
Data support the quiet quitting trend. Gallup data reveal that employee engagement has fallen for 2 consecutive years in the U.S. workforce. Across the first quarter of 2022, Generation Z and younger Millennials report the lowest engagement across populations at 31%. More than half of this cohort, 54%, classified as “not engaged” in their workplace.
Why is quiet quitting gaining prominence now? COVID may play a role.
Many suggest that self-evaluation and establishing firmer boundaries is a logical response to emotional sequelae caused by COVID. Quiet quitting appears to have been fueled by the pandemic. Employees were forced into crisis mode by COVID; the lines between work, life, and home evaporated, allowing or forcing workers to evaluate their efficacy and satisfaction. With the structural impact of COVID reducing and a return to more standard work practices, it is expected that the job “rules” once held as truths come under evaluation and scrutiny.
Perhaps COVID has forced, and provided, another opportunity for us to closely examine our routines and habits and take stock of what really matters. Generations expectedly differ in their values and definitions of success. COVID has set prior established rules on fire, by forcing patterns and expectations that were neither expected nor wanted, within the context of a global health crisis. Within this backdrop, should we really believe our worth is determined by our job?
The truth is, we are still grieving what we lost during COVID and we have expectedly not assimilated to “the new normal.” Psychology has long recognized that losing structures and supports, routines and habits, causes symptoms of significant discomfort.
The idea that we would return to prior workplace expectations is naive. The idea we would “return to life as it was” is naive. It seems expected, then, that both employers and employees should evaluate their goals and communicate more openly about how each can be met.
It is incumbent upon the employers to set up clear guidelines regarding expectations, including rewards for performance and expectations for time, both within and outside of the work schedule. Employers must recognize symptoms of detachment in their employees and engage in the process of continuing clarifying roles and expectations while providing necessities for employees to succeed at their highest level. Employees, in turn, must self-examine their goals, communicate their needs, meet their responsibilities fully, and take on the challenge of determining their own definition of balance.
Maybe instead of quiet quitting, we should call it this new movement “self-awareness, growth, and evolution.” Hmmm, there’s an intriguing thought.
Dr. Calvery is professor of pediatrics at the University of Louisville (Ky.) She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In the past few months, “quiet quitting” has garnered increasing traction across social media platforms. My morning review of social media revealed thousands of posts ranging from “Why doing less at work could be good for you – and your employer” to “After ‘quiet quitting’ here comes ‘quiet firing.’ ”
But quiet quitting is neither quiet nor quitting.
Quiet quitting is a misnomer. In addition, quiet quitters are firmer with their boundaries, do not take on work above and beyond clearly stated expectations, do not respond after hours, and do not feel like they are “not doing their job” when they are not immediately available.
Individuals who “quiet quit” continue to meet the demands of their job but reject the hustle-culture mentality that you must always be available for more work and, most importantly, that your value as person and self-worth are defined and determined by your work. Quiet quitters believe that it is possible to have good boundaries and yet remain productive, engaged, and active within the workplace.
Earlier this month, NPR’s posted tutorial on how to set better boundaries at work garnered 491,000 views, reflecting employees’ difficulties in communicating their needs, thoughts, and availability to their employers. Quiet quitting refers to not only rejecting the idea of going above and beyond in the workplace but also feeling confident that there will not be negative ramifications for not consistently working beyond the expected requirements.
A focus on balance, life, loves, and family is rarely addressed or emphasized by traditional employers; employees have little skill in addressing boundaries and clarifying their value and availability. For decades, “needing” flexibility of any kind or valuing activities as much as your job were viewed as negative attributes, making those individuals less-desired employees.
Data support the quiet quitting trend. Gallup data reveal that employee engagement has fallen for 2 consecutive years in the U.S. workforce. Across the first quarter of 2022, Generation Z and younger Millennials report the lowest engagement across populations at 31%. More than half of this cohort, 54%, classified as “not engaged” in their workplace.
Why is quiet quitting gaining prominence now? COVID may play a role.
Many suggest that self-evaluation and establishing firmer boundaries is a logical response to emotional sequelae caused by COVID. Quiet quitting appears to have been fueled by the pandemic. Employees were forced into crisis mode by COVID; the lines between work, life, and home evaporated, allowing or forcing workers to evaluate their efficacy and satisfaction. With the structural impact of COVID reducing and a return to more standard work practices, it is expected that the job “rules” once held as truths come under evaluation and scrutiny.
Perhaps COVID has forced, and provided, another opportunity for us to closely examine our routines and habits and take stock of what really matters. Generations expectedly differ in their values and definitions of success. COVID has set prior established rules on fire, by forcing patterns and expectations that were neither expected nor wanted, within the context of a global health crisis. Within this backdrop, should we really believe our worth is determined by our job?
The truth is, we are still grieving what we lost during COVID and we have expectedly not assimilated to “the new normal.” Psychology has long recognized that losing structures and supports, routines and habits, causes symptoms of significant discomfort.
The idea that we would return to prior workplace expectations is naive. The idea we would “return to life as it was” is naive. It seems expected, then, that both employers and employees should evaluate their goals and communicate more openly about how each can be met.
It is incumbent upon the employers to set up clear guidelines regarding expectations, including rewards for performance and expectations for time, both within and outside of the work schedule. Employers must recognize symptoms of detachment in their employees and engage in the process of continuing clarifying roles and expectations while providing necessities for employees to succeed at their highest level. Employees, in turn, must self-examine their goals, communicate their needs, meet their responsibilities fully, and take on the challenge of determining their own definition of balance.
Maybe instead of quiet quitting, we should call it this new movement “self-awareness, growth, and evolution.” Hmmm, there’s an intriguing thought.
Dr. Calvery is professor of pediatrics at the University of Louisville (Ky.) She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In the past few months, “quiet quitting” has garnered increasing traction across social media platforms. My morning review of social media revealed thousands of posts ranging from “Why doing less at work could be good for you – and your employer” to “After ‘quiet quitting’ here comes ‘quiet firing.’ ”
But quiet quitting is neither quiet nor quitting.
Quiet quitting is a misnomer. In addition, quiet quitters are firmer with their boundaries, do not take on work above and beyond clearly stated expectations, do not respond after hours, and do not feel like they are “not doing their job” when they are not immediately available.
Individuals who “quiet quit” continue to meet the demands of their job but reject the hustle-culture mentality that you must always be available for more work and, most importantly, that your value as person and self-worth are defined and determined by your work. Quiet quitters believe that it is possible to have good boundaries and yet remain productive, engaged, and active within the workplace.
Earlier this month, NPR’s posted tutorial on how to set better boundaries at work garnered 491,000 views, reflecting employees’ difficulties in communicating their needs, thoughts, and availability to their employers. Quiet quitting refers to not only rejecting the idea of going above and beyond in the workplace but also feeling confident that there will not be negative ramifications for not consistently working beyond the expected requirements.
A focus on balance, life, loves, and family is rarely addressed or emphasized by traditional employers; employees have little skill in addressing boundaries and clarifying their value and availability. For decades, “needing” flexibility of any kind or valuing activities as much as your job were viewed as negative attributes, making those individuals less-desired employees.
Data support the quiet quitting trend. Gallup data reveal that employee engagement has fallen for 2 consecutive years in the U.S. workforce. Across the first quarter of 2022, Generation Z and younger Millennials report the lowest engagement across populations at 31%. More than half of this cohort, 54%, classified as “not engaged” in their workplace.
Why is quiet quitting gaining prominence now? COVID may play a role.
Many suggest that self-evaluation and establishing firmer boundaries is a logical response to emotional sequelae caused by COVID. Quiet quitting appears to have been fueled by the pandemic. Employees were forced into crisis mode by COVID; the lines between work, life, and home evaporated, allowing or forcing workers to evaluate their efficacy and satisfaction. With the structural impact of COVID reducing and a return to more standard work practices, it is expected that the job “rules” once held as truths come under evaluation and scrutiny.
Perhaps COVID has forced, and provided, another opportunity for us to closely examine our routines and habits and take stock of what really matters. Generations expectedly differ in their values and definitions of success. COVID has set prior established rules on fire, by forcing patterns and expectations that were neither expected nor wanted, within the context of a global health crisis. Within this backdrop, should we really believe our worth is determined by our job?
The truth is, we are still grieving what we lost during COVID and we have expectedly not assimilated to “the new normal.” Psychology has long recognized that losing structures and supports, routines and habits, causes symptoms of significant discomfort.
The idea that we would return to prior workplace expectations is naive. The idea we would “return to life as it was” is naive. It seems expected, then, that both employers and employees should evaluate their goals and communicate more openly about how each can be met.
It is incumbent upon the employers to set up clear guidelines regarding expectations, including rewards for performance and expectations for time, both within and outside of the work schedule. Employers must recognize symptoms of detachment in their employees and engage in the process of continuing clarifying roles and expectations while providing necessities for employees to succeed at their highest level. Employees, in turn, must self-examine their goals, communicate their needs, meet their responsibilities fully, and take on the challenge of determining their own definition of balance.
Maybe instead of quiet quitting, we should call it this new movement “self-awareness, growth, and evolution.” Hmmm, there’s an intriguing thought.
Dr. Calvery is professor of pediatrics at the University of Louisville (Ky.) She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.