User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
FDA OKs odevixibat for pruritus associated with rare liver disease
The U.S. Food and Drug Administration has approved odevixibat (Bylvay, Albireo Pharma), the first treatment for pruritus associated with all types of progressive familial intrahepatic cholestasis (PFIC).
PFIC is a rare disorder affecting an estimated one to two people per 100,000. The disorder usually appears within the first few months of life and causes progressive, life-threatening liver disease, often leading to cirrhosis and liver failure before age 10.
In PFIC, liver cells are unable to drain bile acids, leading to the buildup of toxic substances in the liver. While the precise cause of severe itching in patients with PFIC is unknown, it may involve increased levels of bile acids in the body and skin.
Odevixibat is a potent, nonsystemic ileal bile acid transport inhibitor that does not need refrigeration and is given as a once-daily capsule or opened and sprinkled onto soft foods, the company said in a news release announcing the approval.
There are at least three types of PFIC; all are inherited genetic conditions caused by gene mutations. Odevixibat is indicated to treat all subtypes.
“Treating children with PFIC can be difficult and frustrating given the current treatment options. Bylvay gives us a nonsurgical option and will change how we treat PFIC,” Richard Thompson, MD, principal investigator for the two trials that led to the approval, said in the news release.
“With this approval, my colleagues and I now have the opportunity to revisit how PFIC patients are being managed, and we are hopeful for better outcomes for these children,” said Dr. Thompson, professor of molecular hepatology at King’s College London.
The approval of odevixibat was supported by data from the PEDFIC 1 and PEDFIC 2 trials.
PEDFIC 1 enrolled 62 children with PFIC and severe itching, with 20 assigned to placebo and 42 to odevixibat, given once daily with a meal in the morning. Odevixibat met both of its primary endpoints, with the drug improving pruritus (P = .004) and reducing serum bile acid responses (P = .003).
In PEDFIC 2, a long-term, open-label extension study, the effects of odevixibat on pruritis and serum bile acids were sustained up to 48 weeks.
Odevixibat was well tolerated in both trials, with the most common treatment-related gastrointestinal adverse events being diarrhea/frequent stools. There were no serious treatment-related adverse events.
Children taking the drug should undergo liver test monitoring periodically during treatment, the FDA said when announcing the approval. Odevixibat may affect absorption of fat-soluble vitamins such as A, D, E, and K. Patients should be monitored for fat-soluble vitamin deficiency while taking the drug.
Full prescribing information is available online.
“Until now, invasive surgery was the only approved treatment option. With the approval of Bylvay, parents may find hope in having a less invasive treatment option available,” Emily Ventura, leader of the PFIC Advocacy and Resource Network and mother to a child with PFIC, said in the news release.
The company said it will launch odevixibat “immediately” to accelerate availability for patients and families affected by PFIC.
Odevixibat is also being studied in other rare pediatric cholestatic liver diseases, including biliary atresia and Alagille syndrome.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved odevixibat (Bylvay, Albireo Pharma), the first treatment for pruritus associated with all types of progressive familial intrahepatic cholestasis (PFIC).
PFIC is a rare disorder affecting an estimated one to two people per 100,000. The disorder usually appears within the first few months of life and causes progressive, life-threatening liver disease, often leading to cirrhosis and liver failure before age 10.
In PFIC, liver cells are unable to drain bile acids, leading to the buildup of toxic substances in the liver. While the precise cause of severe itching in patients with PFIC is unknown, it may involve increased levels of bile acids in the body and skin.
Odevixibat is a potent, nonsystemic ileal bile acid transport inhibitor that does not need refrigeration and is given as a once-daily capsule or opened and sprinkled onto soft foods, the company said in a news release announcing the approval.
There are at least three types of PFIC; all are inherited genetic conditions caused by gene mutations. Odevixibat is indicated to treat all subtypes.
“Treating children with PFIC can be difficult and frustrating given the current treatment options. Bylvay gives us a nonsurgical option and will change how we treat PFIC,” Richard Thompson, MD, principal investigator for the two trials that led to the approval, said in the news release.
“With this approval, my colleagues and I now have the opportunity to revisit how PFIC patients are being managed, and we are hopeful for better outcomes for these children,” said Dr. Thompson, professor of molecular hepatology at King’s College London.
The approval of odevixibat was supported by data from the PEDFIC 1 and PEDFIC 2 trials.
PEDFIC 1 enrolled 62 children with PFIC and severe itching, with 20 assigned to placebo and 42 to odevixibat, given once daily with a meal in the morning. Odevixibat met both of its primary endpoints, with the drug improving pruritus (P = .004) and reducing serum bile acid responses (P = .003).
In PEDFIC 2, a long-term, open-label extension study, the effects of odevixibat on pruritis and serum bile acids were sustained up to 48 weeks.
Odevixibat was well tolerated in both trials, with the most common treatment-related gastrointestinal adverse events being diarrhea/frequent stools. There were no serious treatment-related adverse events.
Children taking the drug should undergo liver test monitoring periodically during treatment, the FDA said when announcing the approval. Odevixibat may affect absorption of fat-soluble vitamins such as A, D, E, and K. Patients should be monitored for fat-soluble vitamin deficiency while taking the drug.
Full prescribing information is available online.
“Until now, invasive surgery was the only approved treatment option. With the approval of Bylvay, parents may find hope in having a less invasive treatment option available,” Emily Ventura, leader of the PFIC Advocacy and Resource Network and mother to a child with PFIC, said in the news release.
The company said it will launch odevixibat “immediately” to accelerate availability for patients and families affected by PFIC.
Odevixibat is also being studied in other rare pediatric cholestatic liver diseases, including biliary atresia and Alagille syndrome.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved odevixibat (Bylvay, Albireo Pharma), the first treatment for pruritus associated with all types of progressive familial intrahepatic cholestasis (PFIC).
PFIC is a rare disorder affecting an estimated one to two people per 100,000. The disorder usually appears within the first few months of life and causes progressive, life-threatening liver disease, often leading to cirrhosis and liver failure before age 10.
In PFIC, liver cells are unable to drain bile acids, leading to the buildup of toxic substances in the liver. While the precise cause of severe itching in patients with PFIC is unknown, it may involve increased levels of bile acids in the body and skin.
Odevixibat is a potent, nonsystemic ileal bile acid transport inhibitor that does not need refrigeration and is given as a once-daily capsule or opened and sprinkled onto soft foods, the company said in a news release announcing the approval.
There are at least three types of PFIC; all are inherited genetic conditions caused by gene mutations. Odevixibat is indicated to treat all subtypes.
“Treating children with PFIC can be difficult and frustrating given the current treatment options. Bylvay gives us a nonsurgical option and will change how we treat PFIC,” Richard Thompson, MD, principal investigator for the two trials that led to the approval, said in the news release.
“With this approval, my colleagues and I now have the opportunity to revisit how PFIC patients are being managed, and we are hopeful for better outcomes for these children,” said Dr. Thompson, professor of molecular hepatology at King’s College London.
The approval of odevixibat was supported by data from the PEDFIC 1 and PEDFIC 2 trials.
PEDFIC 1 enrolled 62 children with PFIC and severe itching, with 20 assigned to placebo and 42 to odevixibat, given once daily with a meal in the morning. Odevixibat met both of its primary endpoints, with the drug improving pruritus (P = .004) and reducing serum bile acid responses (P = .003).
In PEDFIC 2, a long-term, open-label extension study, the effects of odevixibat on pruritis and serum bile acids were sustained up to 48 weeks.
Odevixibat was well tolerated in both trials, with the most common treatment-related gastrointestinal adverse events being diarrhea/frequent stools. There were no serious treatment-related adverse events.
Children taking the drug should undergo liver test monitoring periodically during treatment, the FDA said when announcing the approval. Odevixibat may affect absorption of fat-soluble vitamins such as A, D, E, and K. Patients should be monitored for fat-soluble vitamin deficiency while taking the drug.
Full prescribing information is available online.
“Until now, invasive surgery was the only approved treatment option. With the approval of Bylvay, parents may find hope in having a less invasive treatment option available,” Emily Ventura, leader of the PFIC Advocacy and Resource Network and mother to a child with PFIC, said in the news release.
The company said it will launch odevixibat “immediately” to accelerate availability for patients and families affected by PFIC.
Odevixibat is also being studied in other rare pediatric cholestatic liver diseases, including biliary atresia and Alagille syndrome.
A version of this article first appeared on Medscape.com.
Lucid abductions and Candy Crush addiction
I dream of alien abductions
There he goes! It’s lunchtime and your colleague Tom is going on and on again about that time he was abducted by aliens. It sounds ridiculous, but he does make some convincing arguments. Tom thinks it was real, but could it have all just been in his head?
Lucid dreaming may help explain alleged alien abductions. During a lucid dream, people know that they’re dreaming, and can also have some control over how the dreams play out. During some dream states, a person can feel intense sensations, such as terror and paralysis, so it’s no wonder these dreams feel so real.
In a recent study, scientists encouraged 152 participants who had self-identified as lucid dreamers to dream about aliens. Many (75%) of the participants were able to dream about alien encounters, and 15% “achieved relatively realistic experiences,” the investigators reported.
So cut Tom some slack. He’s not crazy, he might just have lucid dreaming privileges. Tell him he should dream about something more fun, like a vacation in the Bahamas.
Follow your heart: Drink more coffee
It seems like the world is divided into coffee drinkers and non–coffee drinkers. Then there’s decaf and regular drinkers. Whichever camp you fall into, know this: The widespread belief that caffeine consumption has an effect on your heart is all beans.
In what is the largest investigation of its kind, researchers from the University of California, San Francisco, looked into whether drinking caffeinated coffee was linked to a risk for heart arrhythmia. They also researched whether patients with genetic variants that affect their metabolism could change that association. Almost 400,000 people with a mean age of 56 years participated in the study. More than half of the participants were women.
The investigators analyzed the participants’ self-reported coffee consumption using a technique called Mendelian randomization to leverage genetic data with the participants’ relationship with caffeine, making it an even field and not relying on the participant consumption self-reporting for outcomes as in previous studies.
What they found, after the 4-year follow up, was nothing short of myth busting.
“We found no evidence that caffeine consumption leads to a greater risk of arrhythmias,” said senior and corresponding author Gregory Marcus, MD. “Our population-based study provides reassurance that common prohibitions against caffeine to reduce arrhythmia risk are likely unwarranted.”
There was no evidence of a heightened risk of arrhythmias in participants who were genetically predisposed to metabolize caffeine differently from those who were not. And, there was a 3% reduction of arrhythmias in patients who consumed higher amounts of coffee.
We are not lobbying for Big Caffeine, but this study adds to the reported health benefits linked to coffee, which already include reduced risk for cancer, diabetes, and Parkinson’s disease, with an added bonus of anti-inflammatory benefits. So, the next time you’re hesitant to pour that second cup of Joe, just go for it. Your heart can take it.
Bored? Feeling down? Don’t play Candy Crush
Now hang on, aren’t those the perfect times to play video games? If there’s nothing else to do, why not open Candy Crush and mindlessly power through the levels?
Because, according to a study by a group of Canadian researchers, it’s actually the worst thing you can do. Well, maybe not literally, but it’s not helpful. Researchers recruited 60 Candy Crush players who were at various levels in the game. They had the participants play early levels that were far too easy or levels balanced with their gameplay abilities.
Players in the easy-level group got bored and quit far earlier than did those in the advanced-level group. The group playing to their abilities were able to access a “flow” state and focus all their attention on the game. While this is all well and good for their gaming performance, according to the researchers, it confirms the theory that playing to escape boredom or negative emotions is more likely to lead to addiction. As with all addictions, the temporary high can give way to a self-repeating loop, causing patients to ignore real life and deepen depression.
The researchers hope their findings will encourage game developers to “consider implementing responsible video gaming tools directly within their games.” Comedy gold. Perhaps Canadians’ idea of capitalism is a little different from that of those south of the border.
Hiccups and vaccine refusal
Tonight, LOTME News dives into the fetid cesspool that is international politics and comes out with … hiccups?
But first, a word from our sponsor, Fearless Boxing Club of South Etobicoke, Ontario.
Are you looking to flout public health restrictions? Do you want to spend time in an enclosed space with other people who haven’t gotten the COVID-19 vaccine? Do you “feel safer waiting until more research is done on the side effects being discovered right now”? (We are not making this up.)
Then join the Fearless Boxing Club, because we “will not be accepting any vaccinated members.” Our founders, Mohammed Abedeen and Krystal Glazier-Roscoe, are working hard to exclude “those who received the experimental COVID vaccine.” (Still not making it up.)
And now, back to the news.
Brazilian president Jair Bolsonaro was hospitalized recently for a severe case of hiccups that may have been related to a stab wound he received in 2018. [Nope, didn’t make that up, either.]
Mr. Bolsonaro had been hiccuping for 10 days, and was experiencing abdominal pain and difficulty speaking, when he entered the hospital on July 14. Since being stabbed while on the campaign trail, he has undergone several operations, which may have led to the partial intestinal obstruction that caused his latest symptoms.
His medical team advised Mr. Bolsonaro to go on a diet to aid his recovery, but when he was released on July 18 he said, “I hope in 10 days I’ll be eating barbecued ribs.” (Maybe this is all just a lucid dream. Probably shouldn’t have had ribs right before bed.)
I dream of alien abductions
There he goes! It’s lunchtime and your colleague Tom is going on and on again about that time he was abducted by aliens. It sounds ridiculous, but he does make some convincing arguments. Tom thinks it was real, but could it have all just been in his head?
Lucid dreaming may help explain alleged alien abductions. During a lucid dream, people know that they’re dreaming, and can also have some control over how the dreams play out. During some dream states, a person can feel intense sensations, such as terror and paralysis, so it’s no wonder these dreams feel so real.
In a recent study, scientists encouraged 152 participants who had self-identified as lucid dreamers to dream about aliens. Many (75%) of the participants were able to dream about alien encounters, and 15% “achieved relatively realistic experiences,” the investigators reported.
So cut Tom some slack. He’s not crazy, he might just have lucid dreaming privileges. Tell him he should dream about something more fun, like a vacation in the Bahamas.
Follow your heart: Drink more coffee
It seems like the world is divided into coffee drinkers and non–coffee drinkers. Then there’s decaf and regular drinkers. Whichever camp you fall into, know this: The widespread belief that caffeine consumption has an effect on your heart is all beans.
In what is the largest investigation of its kind, researchers from the University of California, San Francisco, looked into whether drinking caffeinated coffee was linked to a risk for heart arrhythmia. They also researched whether patients with genetic variants that affect their metabolism could change that association. Almost 400,000 people with a mean age of 56 years participated in the study. More than half of the participants were women.
The investigators analyzed the participants’ self-reported coffee consumption using a technique called Mendelian randomization to leverage genetic data with the participants’ relationship with caffeine, making it an even field and not relying on the participant consumption self-reporting for outcomes as in previous studies.
What they found, after the 4-year follow up, was nothing short of myth busting.
“We found no evidence that caffeine consumption leads to a greater risk of arrhythmias,” said senior and corresponding author Gregory Marcus, MD. “Our population-based study provides reassurance that common prohibitions against caffeine to reduce arrhythmia risk are likely unwarranted.”
There was no evidence of a heightened risk of arrhythmias in participants who were genetically predisposed to metabolize caffeine differently from those who were not. And, there was a 3% reduction of arrhythmias in patients who consumed higher amounts of coffee.
We are not lobbying for Big Caffeine, but this study adds to the reported health benefits linked to coffee, which already include reduced risk for cancer, diabetes, and Parkinson’s disease, with an added bonus of anti-inflammatory benefits. So, the next time you’re hesitant to pour that second cup of Joe, just go for it. Your heart can take it.
Bored? Feeling down? Don’t play Candy Crush
Now hang on, aren’t those the perfect times to play video games? If there’s nothing else to do, why not open Candy Crush and mindlessly power through the levels?
Because, according to a study by a group of Canadian researchers, it’s actually the worst thing you can do. Well, maybe not literally, but it’s not helpful. Researchers recruited 60 Candy Crush players who were at various levels in the game. They had the participants play early levels that were far too easy or levels balanced with their gameplay abilities.
Players in the easy-level group got bored and quit far earlier than did those in the advanced-level group. The group playing to their abilities were able to access a “flow” state and focus all their attention on the game. While this is all well and good for their gaming performance, according to the researchers, it confirms the theory that playing to escape boredom or negative emotions is more likely to lead to addiction. As with all addictions, the temporary high can give way to a self-repeating loop, causing patients to ignore real life and deepen depression.
The researchers hope their findings will encourage game developers to “consider implementing responsible video gaming tools directly within their games.” Comedy gold. Perhaps Canadians’ idea of capitalism is a little different from that of those south of the border.
Hiccups and vaccine refusal
Tonight, LOTME News dives into the fetid cesspool that is international politics and comes out with … hiccups?
But first, a word from our sponsor, Fearless Boxing Club of South Etobicoke, Ontario.
Are you looking to flout public health restrictions? Do you want to spend time in an enclosed space with other people who haven’t gotten the COVID-19 vaccine? Do you “feel safer waiting until more research is done on the side effects being discovered right now”? (We are not making this up.)
Then join the Fearless Boxing Club, because we “will not be accepting any vaccinated members.” Our founders, Mohammed Abedeen and Krystal Glazier-Roscoe, are working hard to exclude “those who received the experimental COVID vaccine.” (Still not making it up.)
And now, back to the news.
Brazilian president Jair Bolsonaro was hospitalized recently for a severe case of hiccups that may have been related to a stab wound he received in 2018. [Nope, didn’t make that up, either.]
Mr. Bolsonaro had been hiccuping for 10 days, and was experiencing abdominal pain and difficulty speaking, when he entered the hospital on July 14. Since being stabbed while on the campaign trail, he has undergone several operations, which may have led to the partial intestinal obstruction that caused his latest symptoms.
His medical team advised Mr. Bolsonaro to go on a diet to aid his recovery, but when he was released on July 18 he said, “I hope in 10 days I’ll be eating barbecued ribs.” (Maybe this is all just a lucid dream. Probably shouldn’t have had ribs right before bed.)
I dream of alien abductions
There he goes! It’s lunchtime and your colleague Tom is going on and on again about that time he was abducted by aliens. It sounds ridiculous, but he does make some convincing arguments. Tom thinks it was real, but could it have all just been in his head?
Lucid dreaming may help explain alleged alien abductions. During a lucid dream, people know that they’re dreaming, and can also have some control over how the dreams play out. During some dream states, a person can feel intense sensations, such as terror and paralysis, so it’s no wonder these dreams feel so real.
In a recent study, scientists encouraged 152 participants who had self-identified as lucid dreamers to dream about aliens. Many (75%) of the participants were able to dream about alien encounters, and 15% “achieved relatively realistic experiences,” the investigators reported.
So cut Tom some slack. He’s not crazy, he might just have lucid dreaming privileges. Tell him he should dream about something more fun, like a vacation in the Bahamas.
Follow your heart: Drink more coffee
It seems like the world is divided into coffee drinkers and non–coffee drinkers. Then there’s decaf and regular drinkers. Whichever camp you fall into, know this: The widespread belief that caffeine consumption has an effect on your heart is all beans.
In what is the largest investigation of its kind, researchers from the University of California, San Francisco, looked into whether drinking caffeinated coffee was linked to a risk for heart arrhythmia. They also researched whether patients with genetic variants that affect their metabolism could change that association. Almost 400,000 people with a mean age of 56 years participated in the study. More than half of the participants were women.
The investigators analyzed the participants’ self-reported coffee consumption using a technique called Mendelian randomization to leverage genetic data with the participants’ relationship with caffeine, making it an even field and not relying on the participant consumption self-reporting for outcomes as in previous studies.
What they found, after the 4-year follow up, was nothing short of myth busting.
“We found no evidence that caffeine consumption leads to a greater risk of arrhythmias,” said senior and corresponding author Gregory Marcus, MD. “Our population-based study provides reassurance that common prohibitions against caffeine to reduce arrhythmia risk are likely unwarranted.”
There was no evidence of a heightened risk of arrhythmias in participants who were genetically predisposed to metabolize caffeine differently from those who were not. And, there was a 3% reduction of arrhythmias in patients who consumed higher amounts of coffee.
We are not lobbying for Big Caffeine, but this study adds to the reported health benefits linked to coffee, which already include reduced risk for cancer, diabetes, and Parkinson’s disease, with an added bonus of anti-inflammatory benefits. So, the next time you’re hesitant to pour that second cup of Joe, just go for it. Your heart can take it.
Bored? Feeling down? Don’t play Candy Crush
Now hang on, aren’t those the perfect times to play video games? If there’s nothing else to do, why not open Candy Crush and mindlessly power through the levels?
Because, according to a study by a group of Canadian researchers, it’s actually the worst thing you can do. Well, maybe not literally, but it’s not helpful. Researchers recruited 60 Candy Crush players who were at various levels in the game. They had the participants play early levels that were far too easy or levels balanced with their gameplay abilities.
Players in the easy-level group got bored and quit far earlier than did those in the advanced-level group. The group playing to their abilities were able to access a “flow” state and focus all their attention on the game. While this is all well and good for their gaming performance, according to the researchers, it confirms the theory that playing to escape boredom or negative emotions is more likely to lead to addiction. As with all addictions, the temporary high can give way to a self-repeating loop, causing patients to ignore real life and deepen depression.
The researchers hope their findings will encourage game developers to “consider implementing responsible video gaming tools directly within their games.” Comedy gold. Perhaps Canadians’ idea of capitalism is a little different from that of those south of the border.
Hiccups and vaccine refusal
Tonight, LOTME News dives into the fetid cesspool that is international politics and comes out with … hiccups?
But first, a word from our sponsor, Fearless Boxing Club of South Etobicoke, Ontario.
Are you looking to flout public health restrictions? Do you want to spend time in an enclosed space with other people who haven’t gotten the COVID-19 vaccine? Do you “feel safer waiting until more research is done on the side effects being discovered right now”? (We are not making this up.)
Then join the Fearless Boxing Club, because we “will not be accepting any vaccinated members.” Our founders, Mohammed Abedeen and Krystal Glazier-Roscoe, are working hard to exclude “those who received the experimental COVID vaccine.” (Still not making it up.)
And now, back to the news.
Brazilian president Jair Bolsonaro was hospitalized recently for a severe case of hiccups that may have been related to a stab wound he received in 2018. [Nope, didn’t make that up, either.]
Mr. Bolsonaro had been hiccuping for 10 days, and was experiencing abdominal pain and difficulty speaking, when he entered the hospital on July 14. Since being stabbed while on the campaign trail, he has undergone several operations, which may have led to the partial intestinal obstruction that caused his latest symptoms.
His medical team advised Mr. Bolsonaro to go on a diet to aid his recovery, but when he was released on July 18 he said, “I hope in 10 days I’ll be eating barbecued ribs.” (Maybe this is all just a lucid dream. Probably shouldn’t have had ribs right before bed.)
FDA approves intravenous immunoglobulin for dermatomyositis
, according to a statement from manufacturer Octapharma USA.
Dermatomyositis is a rare, idiopathic autoimmune disorder that affects approximately 10 out of every million people in the United States, mainly adults in their late 40s to early 60s, according to the company, but children aged 5-15 years can be affected. The disease is characterized by skin rashes, chronic muscle inflammation, progressive muscle weakness, and risk for mortality that is three times higher than for the general population.
There are no previously approved treatments for dermatomyositis prior to Octagam 10%, which also is indicated for chronic immune thrombocytopenic purpura in adults.
The approval for dermatomyositis was based on the results of a phase 3 randomized, double-blind, placebo-controlled clinical trial (the ProDERM trial) that included 95 adult patients at 36 sites worldwide, with 17 sites in the United States. In the trial, 78.7% of patients with dermatomyositis who were randomized to receive 2 g/kg of Octagam 10% every 4 weeks showed response at 16 weeks, compared with 43.8% of patients who received placebo. Response was based on the 2016 American College of Rheumatology/European Alliance of Associations for Rheumatology myositis response criteria. Placebo patients who switched to intravenous immunoglobulin (IVIG) during a trial extension had response rates at week 40 similar to the original patients at week 16.
“The study gives clinicians much more confidence in the efficacy and safety of intravenous immunoglobulin and provides valuable information about what type of patient is best suited for the treatment,” Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh and a member of the ProDERM study Steering Committee, said in the Octapharma statement.
Safety and tolerability were similar to profiles seen with other IVIG medications, according to the statement. The medication does carry a boxed warning from its chronic ITP approval, cautioning about the potential for thrombosis, renal dysfunction, and acute renal failure.
The most common adverse reactions reported by dermatomyositis patients in the ProDERM trial were headache, fever, nausea, vomiting, increased blood pressure, chills, musculoskeletal pain, increased heart rate, dyspnea, and reactions at the infusion sites.
Read the full prescribing information here.
, according to a statement from manufacturer Octapharma USA.
Dermatomyositis is a rare, idiopathic autoimmune disorder that affects approximately 10 out of every million people in the United States, mainly adults in their late 40s to early 60s, according to the company, but children aged 5-15 years can be affected. The disease is characterized by skin rashes, chronic muscle inflammation, progressive muscle weakness, and risk for mortality that is three times higher than for the general population.
There are no previously approved treatments for dermatomyositis prior to Octagam 10%, which also is indicated for chronic immune thrombocytopenic purpura in adults.
The approval for dermatomyositis was based on the results of a phase 3 randomized, double-blind, placebo-controlled clinical trial (the ProDERM trial) that included 95 adult patients at 36 sites worldwide, with 17 sites in the United States. In the trial, 78.7% of patients with dermatomyositis who were randomized to receive 2 g/kg of Octagam 10% every 4 weeks showed response at 16 weeks, compared with 43.8% of patients who received placebo. Response was based on the 2016 American College of Rheumatology/European Alliance of Associations for Rheumatology myositis response criteria. Placebo patients who switched to intravenous immunoglobulin (IVIG) during a trial extension had response rates at week 40 similar to the original patients at week 16.
“The study gives clinicians much more confidence in the efficacy and safety of intravenous immunoglobulin and provides valuable information about what type of patient is best suited for the treatment,” Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh and a member of the ProDERM study Steering Committee, said in the Octapharma statement.
Safety and tolerability were similar to profiles seen with other IVIG medications, according to the statement. The medication does carry a boxed warning from its chronic ITP approval, cautioning about the potential for thrombosis, renal dysfunction, and acute renal failure.
The most common adverse reactions reported by dermatomyositis patients in the ProDERM trial were headache, fever, nausea, vomiting, increased blood pressure, chills, musculoskeletal pain, increased heart rate, dyspnea, and reactions at the infusion sites.
Read the full prescribing information here.
, according to a statement from manufacturer Octapharma USA.
Dermatomyositis is a rare, idiopathic autoimmune disorder that affects approximately 10 out of every million people in the United States, mainly adults in their late 40s to early 60s, according to the company, but children aged 5-15 years can be affected. The disease is characterized by skin rashes, chronic muscle inflammation, progressive muscle weakness, and risk for mortality that is three times higher than for the general population.
There are no previously approved treatments for dermatomyositis prior to Octagam 10%, which also is indicated for chronic immune thrombocytopenic purpura in adults.
The approval for dermatomyositis was based on the results of a phase 3 randomized, double-blind, placebo-controlled clinical trial (the ProDERM trial) that included 95 adult patients at 36 sites worldwide, with 17 sites in the United States. In the trial, 78.7% of patients with dermatomyositis who were randomized to receive 2 g/kg of Octagam 10% every 4 weeks showed response at 16 weeks, compared with 43.8% of patients who received placebo. Response was based on the 2016 American College of Rheumatology/European Alliance of Associations for Rheumatology myositis response criteria. Placebo patients who switched to intravenous immunoglobulin (IVIG) during a trial extension had response rates at week 40 similar to the original patients at week 16.
“The study gives clinicians much more confidence in the efficacy and safety of intravenous immunoglobulin and provides valuable information about what type of patient is best suited for the treatment,” Rohit Aggarwal, MD, medical director of the Arthritis and Autoimmunity Center at the University of Pittsburgh and a member of the ProDERM study Steering Committee, said in the Octapharma statement.
Safety and tolerability were similar to profiles seen with other IVIG medications, according to the statement. The medication does carry a boxed warning from its chronic ITP approval, cautioning about the potential for thrombosis, renal dysfunction, and acute renal failure.
The most common adverse reactions reported by dermatomyositis patients in the ProDERM trial were headache, fever, nausea, vomiting, increased blood pressure, chills, musculoskeletal pain, increased heart rate, dyspnea, and reactions at the infusion sites.
Read the full prescribing information here.
Recent trend: Melanoma mortality declining rapidly
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.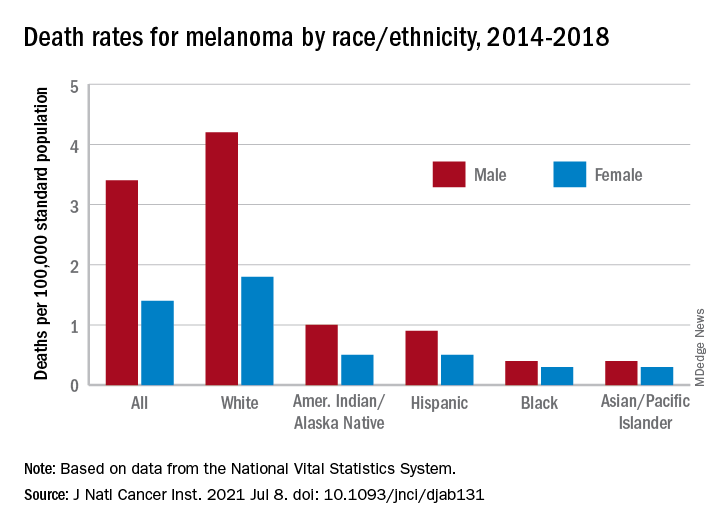
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Artificial intelligence wish list
Dear big-tech AI company,
I do understand, the benefits of artificial intelligence today are already profound and protean. Thanks to AI, I can translate Italian to English in real time in the same voice as an Italian speaker. I can be driven home autonomously by our Tesla. AI helps keep me safe by predicting crimes, on time by predicting traffic, and healthy by designing plant proteins that taste just like beef. I can even use AI to build a sprinkler to keep people off my new lawn.
In medicine, the AI news is so good that a frisson of excitement spreads vertically and horizontally across all health care. AI can detect pulmonary nodules, identify melanomas, develop new drugs – speed vaccine discovery! – and detect malignant cells on a biopsy slide. It can help predict who is going to crash in the ICU and recognize when someone is about to fall out of bed in the surgical unit. Even just this sampling of benefits proves how significant and impactful AI is in improving quality of life for patients and populations.
However, much of what I do every day in medicine cannot be solved with a neat quantitative analysis. The vast majority of my patients do not have a melanoma to be diagnosed or diabetic retinopathy to be scanned. What they want and need is time spent with me, their doctor. Although the schedule says I have 15 minutes (insufficient to begin with), patients are running late and are double booked, and I’ve loads of notes to type, medications to review, and messages to answer. Most days, I have only a fraction of 15 minutes to spend face to face with each patient.
Can AI please help us? How about reviewing the reams of data from my patient’s chart and presenting it to me succinctly? Rather than my tediously clicking through pathology reports, just summarize what skin cancers my patient has had and when. Rather than learning that my patient already failed Protopic a year ago, let me know that before I sign the order and promise: “Now, this ointment will work.” Even better, suggest alternative treatments that I might not be thinking of and which might do just the trick. Oh, and given my EMR has all the data required to determine billing codes, can you just drop that in for me when I’m done? Lastly, if the patient’s insurance is going to reject this claim or that medication, can AI please complete the authorization/paperwork/signed notary document/letter from U.S. senator that will be needed for it to be accepted?
I know this is possible. If we can blast a 70-year-old businessman into space on a private jet, surely you can invent an AI that gives us more time to spend with patients. Proposals postmarked by Dec. 31, 2021, please.
I’m sincerely yours,
Jeff Benabio, MD, MBA
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Dear big-tech AI company,
I do understand, the benefits of artificial intelligence today are already profound and protean. Thanks to AI, I can translate Italian to English in real time in the same voice as an Italian speaker. I can be driven home autonomously by our Tesla. AI helps keep me safe by predicting crimes, on time by predicting traffic, and healthy by designing plant proteins that taste just like beef. I can even use AI to build a sprinkler to keep people off my new lawn.
In medicine, the AI news is so good that a frisson of excitement spreads vertically and horizontally across all health care. AI can detect pulmonary nodules, identify melanomas, develop new drugs – speed vaccine discovery! – and detect malignant cells on a biopsy slide. It can help predict who is going to crash in the ICU and recognize when someone is about to fall out of bed in the surgical unit. Even just this sampling of benefits proves how significant and impactful AI is in improving quality of life for patients and populations.
However, much of what I do every day in medicine cannot be solved with a neat quantitative analysis. The vast majority of my patients do not have a melanoma to be diagnosed or diabetic retinopathy to be scanned. What they want and need is time spent with me, their doctor. Although the schedule says I have 15 minutes (insufficient to begin with), patients are running late and are double booked, and I’ve loads of notes to type, medications to review, and messages to answer. Most days, I have only a fraction of 15 minutes to spend face to face with each patient.
Can AI please help us? How about reviewing the reams of data from my patient’s chart and presenting it to me succinctly? Rather than my tediously clicking through pathology reports, just summarize what skin cancers my patient has had and when. Rather than learning that my patient already failed Protopic a year ago, let me know that before I sign the order and promise: “Now, this ointment will work.” Even better, suggest alternative treatments that I might not be thinking of and which might do just the trick. Oh, and given my EMR has all the data required to determine billing codes, can you just drop that in for me when I’m done? Lastly, if the patient’s insurance is going to reject this claim or that medication, can AI please complete the authorization/paperwork/signed notary document/letter from U.S. senator that will be needed for it to be accepted?
I know this is possible. If we can blast a 70-year-old businessman into space on a private jet, surely you can invent an AI that gives us more time to spend with patients. Proposals postmarked by Dec. 31, 2021, please.
I’m sincerely yours,
Jeff Benabio, MD, MBA
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Dear big-tech AI company,
I do understand, the benefits of artificial intelligence today are already profound and protean. Thanks to AI, I can translate Italian to English in real time in the same voice as an Italian speaker. I can be driven home autonomously by our Tesla. AI helps keep me safe by predicting crimes, on time by predicting traffic, and healthy by designing plant proteins that taste just like beef. I can even use AI to build a sprinkler to keep people off my new lawn.
In medicine, the AI news is so good that a frisson of excitement spreads vertically and horizontally across all health care. AI can detect pulmonary nodules, identify melanomas, develop new drugs – speed vaccine discovery! – and detect malignant cells on a biopsy slide. It can help predict who is going to crash in the ICU and recognize when someone is about to fall out of bed in the surgical unit. Even just this sampling of benefits proves how significant and impactful AI is in improving quality of life for patients and populations.
However, much of what I do every day in medicine cannot be solved with a neat quantitative analysis. The vast majority of my patients do not have a melanoma to be diagnosed or diabetic retinopathy to be scanned. What they want and need is time spent with me, their doctor. Although the schedule says I have 15 minutes (insufficient to begin with), patients are running late and are double booked, and I’ve loads of notes to type, medications to review, and messages to answer. Most days, I have only a fraction of 15 minutes to spend face to face with each patient.
Can AI please help us? How about reviewing the reams of data from my patient’s chart and presenting it to me succinctly? Rather than my tediously clicking through pathology reports, just summarize what skin cancers my patient has had and when. Rather than learning that my patient already failed Protopic a year ago, let me know that before I sign the order and promise: “Now, this ointment will work.” Even better, suggest alternative treatments that I might not be thinking of and which might do just the trick. Oh, and given my EMR has all the data required to determine billing codes, can you just drop that in for me when I’m done? Lastly, if the patient’s insurance is going to reject this claim or that medication, can AI please complete the authorization/paperwork/signed notary document/letter from U.S. senator that will be needed for it to be accepted?
I know this is possible. If we can blast a 70-year-old businessman into space on a private jet, surely you can invent an AI that gives us more time to spend with patients. Proposals postmarked by Dec. 31, 2021, please.
I’m sincerely yours,
Jeff Benabio, MD, MBA
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Synthetic snake venom to the rescue? Potential uses in skin health and rejuvenation
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
The ADA and hearing-impaired patients
A recent claim against a New Jersey physician attracted considerable attention in both the medical and legal communities, not only because it resulted in a substantial jury award, but because that award was not covered by malpractice insurance.
It is a good reminder for the rest of us: This case involved a charge of discrimination against a hearing-impaired patient – which meant the physician not only had to fund his own defense, but was personally responsible for the $400,000 award against him.
The Americans with Disabilities Act (ADA) was designed to protect individuals with various disabilities against discrimination in various public situations – including, specifically, “the professional office of a health care professional.”
When the disability is impaired hearing, the law requires physicians to provide any “auxiliary aids and services” that might be necessary to insure clear communication between doctor and patient. In the vast majority of such situations, a pad and pencil will satisfy that requirement. But occasionally it does not, particularly when complex medical concepts are involved; and in such cases, as the New Jersey trial demonstrated, failure to make the necessary extra effort can be very expensive.
The claim involved a hearing-impaired patient with lupus erythematosus under treatment by a rheumatologist. For almost 2 years the patient’s partner and her daughter provided translation; but that arrangement was inadequate, she testified, because her partner and daughter were unfamiliar with medical terminology and she was “unable to understand and participate in her care,” which left her “unaware of risks and available alternatives.”
She repeatedly requested that the rheumatologist provide an American Sign Language interpreter for her office visits. He refused on grounds that the cost of an interpreter would exceed the payment he would receive for the visits, which made it an “undue financial burden,” and therefore exempt from ADA requirements.
But the undue-burden exemption is not automatic; it must be demonstrated in court. And the jury decided the rheumatologist’s annual income of $425,000 rendered the cost of an interpreter quite affordable.
The lessons are clear: Physicians must take antidiscrimination laws seriously, particularly when uninsurable issues are involved; and we must be constantly aware of the needs of disabled patients, to be sure their care is not substantially different from that of any other patient.
In the case of hearing-impaired or deaf patients, it is important to remember that forms of communication that are quite adequate for most are not appropriate for some. Lip reading, written notes, and the use of family members as interpreters may be perfectly acceptable to one patient and unsuitable for another.
If the patient agrees to written notes and lip reading, as most do, you need to remember to speak slowly, and to write down critical information to avoid any miscommunications. And as always, it is crucial to document all communication, as well as the methods used for that communication – specifically including the fact that the patient agreed to those forms of communication. Documentation, as I’ve often said, is like garlic: There is no such thing as too much of it.
Should a patient not agree that written notes are sufficient, other alternatives can be offered: computer transcription, assistive listening devices, videotext displays (often available in hospitals), and telecommunication devices such as TTY and TDD. But if the patient rejects all of those options and continues to insist on a professional interpreter, the precedent set by the New Jersey case suggests that you need to acquiesce, even if the interpreter’s fee exceeds the visit reimbursement – and the ADA prohibits you from passing your cost along to the patient. But any such cost will be far less than a noninsured judgment against you.
If you must go that route, make sure the interpreter you hire is familiar with medical terminology, and is not acquainted or related to the patient (for HIPAA reasons). Your state may have an online registry of available interpreters, or your hospital may have a sign language interpreter on its staff that they might allow you to “borrow.”
The good news is several states have responded to this issue by introducing legislation that would require health insurance carriers to pay for the cost of interpreters, although none, as of this writing, have yet become law.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
A recent claim against a New Jersey physician attracted considerable attention in both the medical and legal communities, not only because it resulted in a substantial jury award, but because that award was not covered by malpractice insurance.
It is a good reminder for the rest of us: This case involved a charge of discrimination against a hearing-impaired patient – which meant the physician not only had to fund his own defense, but was personally responsible for the $400,000 award against him.
The Americans with Disabilities Act (ADA) was designed to protect individuals with various disabilities against discrimination in various public situations – including, specifically, “the professional office of a health care professional.”
When the disability is impaired hearing, the law requires physicians to provide any “auxiliary aids and services” that might be necessary to insure clear communication between doctor and patient. In the vast majority of such situations, a pad and pencil will satisfy that requirement. But occasionally it does not, particularly when complex medical concepts are involved; and in such cases, as the New Jersey trial demonstrated, failure to make the necessary extra effort can be very expensive.
The claim involved a hearing-impaired patient with lupus erythematosus under treatment by a rheumatologist. For almost 2 years the patient’s partner and her daughter provided translation; but that arrangement was inadequate, she testified, because her partner and daughter were unfamiliar with medical terminology and she was “unable to understand and participate in her care,” which left her “unaware of risks and available alternatives.”
She repeatedly requested that the rheumatologist provide an American Sign Language interpreter for her office visits. He refused on grounds that the cost of an interpreter would exceed the payment he would receive for the visits, which made it an “undue financial burden,” and therefore exempt from ADA requirements.
But the undue-burden exemption is not automatic; it must be demonstrated in court. And the jury decided the rheumatologist’s annual income of $425,000 rendered the cost of an interpreter quite affordable.
The lessons are clear: Physicians must take antidiscrimination laws seriously, particularly when uninsurable issues are involved; and we must be constantly aware of the needs of disabled patients, to be sure their care is not substantially different from that of any other patient.
In the case of hearing-impaired or deaf patients, it is important to remember that forms of communication that are quite adequate for most are not appropriate for some. Lip reading, written notes, and the use of family members as interpreters may be perfectly acceptable to one patient and unsuitable for another.
If the patient agrees to written notes and lip reading, as most do, you need to remember to speak slowly, and to write down critical information to avoid any miscommunications. And as always, it is crucial to document all communication, as well as the methods used for that communication – specifically including the fact that the patient agreed to those forms of communication. Documentation, as I’ve often said, is like garlic: There is no such thing as too much of it.
Should a patient not agree that written notes are sufficient, other alternatives can be offered: computer transcription, assistive listening devices, videotext displays (often available in hospitals), and telecommunication devices such as TTY and TDD. But if the patient rejects all of those options and continues to insist on a professional interpreter, the precedent set by the New Jersey case suggests that you need to acquiesce, even if the interpreter’s fee exceeds the visit reimbursement – and the ADA prohibits you from passing your cost along to the patient. But any such cost will be far less than a noninsured judgment against you.
If you must go that route, make sure the interpreter you hire is familiar with medical terminology, and is not acquainted or related to the patient (for HIPAA reasons). Your state may have an online registry of available interpreters, or your hospital may have a sign language interpreter on its staff that they might allow you to “borrow.”
The good news is several states have responded to this issue by introducing legislation that would require health insurance carriers to pay for the cost of interpreters, although none, as of this writing, have yet become law.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
A recent claim against a New Jersey physician attracted considerable attention in both the medical and legal communities, not only because it resulted in a substantial jury award, but because that award was not covered by malpractice insurance.
It is a good reminder for the rest of us: This case involved a charge of discrimination against a hearing-impaired patient – which meant the physician not only had to fund his own defense, but was personally responsible for the $400,000 award against him.
The Americans with Disabilities Act (ADA) was designed to protect individuals with various disabilities against discrimination in various public situations – including, specifically, “the professional office of a health care professional.”
When the disability is impaired hearing, the law requires physicians to provide any “auxiliary aids and services” that might be necessary to insure clear communication between doctor and patient. In the vast majority of such situations, a pad and pencil will satisfy that requirement. But occasionally it does not, particularly when complex medical concepts are involved; and in such cases, as the New Jersey trial demonstrated, failure to make the necessary extra effort can be very expensive.
The claim involved a hearing-impaired patient with lupus erythematosus under treatment by a rheumatologist. For almost 2 years the patient’s partner and her daughter provided translation; but that arrangement was inadequate, she testified, because her partner and daughter were unfamiliar with medical terminology and she was “unable to understand and participate in her care,” which left her “unaware of risks and available alternatives.”
She repeatedly requested that the rheumatologist provide an American Sign Language interpreter for her office visits. He refused on grounds that the cost of an interpreter would exceed the payment he would receive for the visits, which made it an “undue financial burden,” and therefore exempt from ADA requirements.
But the undue-burden exemption is not automatic; it must be demonstrated in court. And the jury decided the rheumatologist’s annual income of $425,000 rendered the cost of an interpreter quite affordable.
The lessons are clear: Physicians must take antidiscrimination laws seriously, particularly when uninsurable issues are involved; and we must be constantly aware of the needs of disabled patients, to be sure their care is not substantially different from that of any other patient.
In the case of hearing-impaired or deaf patients, it is important to remember that forms of communication that are quite adequate for most are not appropriate for some. Lip reading, written notes, and the use of family members as interpreters may be perfectly acceptable to one patient and unsuitable for another.
If the patient agrees to written notes and lip reading, as most do, you need to remember to speak slowly, and to write down critical information to avoid any miscommunications. And as always, it is crucial to document all communication, as well as the methods used for that communication – specifically including the fact that the patient agreed to those forms of communication. Documentation, as I’ve often said, is like garlic: There is no such thing as too much of it.
Should a patient not agree that written notes are sufficient, other alternatives can be offered: computer transcription, assistive listening devices, videotext displays (often available in hospitals), and telecommunication devices such as TTY and TDD. But if the patient rejects all of those options and continues to insist on a professional interpreter, the precedent set by the New Jersey case suggests that you need to acquiesce, even if the interpreter’s fee exceeds the visit reimbursement – and the ADA prohibits you from passing your cost along to the patient. But any such cost will be far less than a noninsured judgment against you.
If you must go that route, make sure the interpreter you hire is familiar with medical terminology, and is not acquainted or related to the patient (for HIPAA reasons). Your state may have an online registry of available interpreters, or your hospital may have a sign language interpreter on its staff that they might allow you to “borrow.”
The good news is several states have responded to this issue by introducing legislation that would require health insurance carriers to pay for the cost of interpreters, although none, as of this writing, have yet become law.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Syphilis prevalence in MSM 15 times higher than in general population
Worldwide, nearly 8% of men who have sex with men (MSM) may have syphilis, a new systematic review and meta-analysis suggests. This estimate, generated from 275 studies across 77 countries, is 15 times greater than the most recent estimates of syphilis prevalence in men in a general population.
“That disparity is absolutely unacceptable,” Matthew Chico, PhD, associate professor at the London School of Hygiene and Tropical Medicine, and senior author of the review, said in an interview.
Although the World Health Organization (WHO) aims to reduce the global prevalence of syphilis by 90% by 2030, an ambitious goal set in 2016, recent research suggests syphilis numbers are moving in the opposite direction. Cases in the United States rose 74% between 2015 and 2019, and other nations, such as Australia, South Korea, and the United Kingdom, are seeing similar trends.
Syphilis prevalence is generally higher in MSM, largely in subpopulations of men who have multiple sexual partners, Kenneth Mayer, MD, said in an interview. Dr. Mayer is medical research director at the Fenway Institute, Boston, and was not involved with the study.
Health literacy, lack of access to care, and medical mistrust can all be challenges to screening, identifying, and treating the infection in this population.
Reducing syphilis cases will require focusing interventions on higher-risk groups such as MSM, said Dr. Chico; however, there was “a real dearth in knowledge about the most likely prevalence of syphilis among MSM on a global level,” he said.
To help fill in the gaps, Dr. Chico and his research team collected studies that included syphilis prevalence data for MSM published between Jan. 1, 2000, and Feb. 1, 2020. Researchers excluded studies that included only MSM living with HIV, injection drug users, patients who routinely visit sexually transmitted infection (STI) clinics, and people seeking care only for STIs or other genital symptoms, because these studies would have skewed global syphilis prevalence estimates higher.
Their review, published July 8 in The Lancet Global Health, found that the pooled global prevalence of syphilis from 2000-2020 in MSM was 7.5%. It ranged from 1.9% in Australia and New Zealand to 10.6% in Latin America and the Caribbean. In comparison, the WHO estimates that globally, 0.5% of men in a general population have syphilis, a 15-fold difference.
This elevated estimate is not surprising, and the review provides a more international view of syphilis. Earlier attempts to estimate the prevalence of syphilis among MSM were generally conducted in higher-income countries such as the United States, Dr. Mayer said. “It’s important that clinicians recognize that this is a global health issue, so they can do the appropriate screening.”
The review found that regions in which the prevalence of HIV was above 5% had higher rates of syphilis (8.7%) compared to regions in which the prevalence of HIV was below 5% (6.6%). Pooled syphilis prevalence estimates were also higher for lower-middle-income and upper-middle-income countries (8.7% and 8.6%, respectively).
Global syphilis prevalence dipped from 8.9% in studies from 2000 to 2009 to 6.6% in studies from 2010 to 2020. In Europe, Northern America, Latin America, the Caribbean, and Oceania (excluding Australia and New Zealand), syphilis prevalence estimates for 2015-2020 were higher compared with 2010-2014.
The authors acknowledged that there were some limitations to the study, particularly that regions of Eastern and Southeastern Asia contributed more than half (54.5%) of the global data points used in the study and accounted for more than 82% of the study’s participants. This highlights the lack of data from other regions around the world, Dr. Chico said.
Dr. Chico said these findings “serve as a clarion call to action” to focus interventions on groups at higher risk for syphilis, such as MSM, in the effort to drastically reduce syphilis cases around the world. Dr. Mayer agrees. “[Syphilis] is a readily diagnosable and treatable infection,” he said. “It definitely is something that we should be able to get a handle on, but that requires paying attention to the different subgroups who have particularly high rates of the infection.”
A version of this article first appeared on Medscape.com.
Worldwide, nearly 8% of men who have sex with men (MSM) may have syphilis, a new systematic review and meta-analysis suggests. This estimate, generated from 275 studies across 77 countries, is 15 times greater than the most recent estimates of syphilis prevalence in men in a general population.
“That disparity is absolutely unacceptable,” Matthew Chico, PhD, associate professor at the London School of Hygiene and Tropical Medicine, and senior author of the review, said in an interview.
Although the World Health Organization (WHO) aims to reduce the global prevalence of syphilis by 90% by 2030, an ambitious goal set in 2016, recent research suggests syphilis numbers are moving in the opposite direction. Cases in the United States rose 74% between 2015 and 2019, and other nations, such as Australia, South Korea, and the United Kingdom, are seeing similar trends.
Syphilis prevalence is generally higher in MSM, largely in subpopulations of men who have multiple sexual partners, Kenneth Mayer, MD, said in an interview. Dr. Mayer is medical research director at the Fenway Institute, Boston, and was not involved with the study.
Health literacy, lack of access to care, and medical mistrust can all be challenges to screening, identifying, and treating the infection in this population.
Reducing syphilis cases will require focusing interventions on higher-risk groups such as MSM, said Dr. Chico; however, there was “a real dearth in knowledge about the most likely prevalence of syphilis among MSM on a global level,” he said.
To help fill in the gaps, Dr. Chico and his research team collected studies that included syphilis prevalence data for MSM published between Jan. 1, 2000, and Feb. 1, 2020. Researchers excluded studies that included only MSM living with HIV, injection drug users, patients who routinely visit sexually transmitted infection (STI) clinics, and people seeking care only for STIs or other genital symptoms, because these studies would have skewed global syphilis prevalence estimates higher.
Their review, published July 8 in The Lancet Global Health, found that the pooled global prevalence of syphilis from 2000-2020 in MSM was 7.5%. It ranged from 1.9% in Australia and New Zealand to 10.6% in Latin America and the Caribbean. In comparison, the WHO estimates that globally, 0.5% of men in a general population have syphilis, a 15-fold difference.
This elevated estimate is not surprising, and the review provides a more international view of syphilis. Earlier attempts to estimate the prevalence of syphilis among MSM were generally conducted in higher-income countries such as the United States, Dr. Mayer said. “It’s important that clinicians recognize that this is a global health issue, so they can do the appropriate screening.”
The review found that regions in which the prevalence of HIV was above 5% had higher rates of syphilis (8.7%) compared to regions in which the prevalence of HIV was below 5% (6.6%). Pooled syphilis prevalence estimates were also higher for lower-middle-income and upper-middle-income countries (8.7% and 8.6%, respectively).
Global syphilis prevalence dipped from 8.9% in studies from 2000 to 2009 to 6.6% in studies from 2010 to 2020. In Europe, Northern America, Latin America, the Caribbean, and Oceania (excluding Australia and New Zealand), syphilis prevalence estimates for 2015-2020 were higher compared with 2010-2014.
The authors acknowledged that there were some limitations to the study, particularly that regions of Eastern and Southeastern Asia contributed more than half (54.5%) of the global data points used in the study and accounted for more than 82% of the study’s participants. This highlights the lack of data from other regions around the world, Dr. Chico said.
Dr. Chico said these findings “serve as a clarion call to action” to focus interventions on groups at higher risk for syphilis, such as MSM, in the effort to drastically reduce syphilis cases around the world. Dr. Mayer agrees. “[Syphilis] is a readily diagnosable and treatable infection,” he said. “It definitely is something that we should be able to get a handle on, but that requires paying attention to the different subgroups who have particularly high rates of the infection.”
A version of this article first appeared on Medscape.com.
Worldwide, nearly 8% of men who have sex with men (MSM) may have syphilis, a new systematic review and meta-analysis suggests. This estimate, generated from 275 studies across 77 countries, is 15 times greater than the most recent estimates of syphilis prevalence in men in a general population.
“That disparity is absolutely unacceptable,” Matthew Chico, PhD, associate professor at the London School of Hygiene and Tropical Medicine, and senior author of the review, said in an interview.
Although the World Health Organization (WHO) aims to reduce the global prevalence of syphilis by 90% by 2030, an ambitious goal set in 2016, recent research suggests syphilis numbers are moving in the opposite direction. Cases in the United States rose 74% between 2015 and 2019, and other nations, such as Australia, South Korea, and the United Kingdom, are seeing similar trends.
Syphilis prevalence is generally higher in MSM, largely in subpopulations of men who have multiple sexual partners, Kenneth Mayer, MD, said in an interview. Dr. Mayer is medical research director at the Fenway Institute, Boston, and was not involved with the study.
Health literacy, lack of access to care, and medical mistrust can all be challenges to screening, identifying, and treating the infection in this population.
Reducing syphilis cases will require focusing interventions on higher-risk groups such as MSM, said Dr. Chico; however, there was “a real dearth in knowledge about the most likely prevalence of syphilis among MSM on a global level,” he said.
To help fill in the gaps, Dr. Chico and his research team collected studies that included syphilis prevalence data for MSM published between Jan. 1, 2000, and Feb. 1, 2020. Researchers excluded studies that included only MSM living with HIV, injection drug users, patients who routinely visit sexually transmitted infection (STI) clinics, and people seeking care only for STIs or other genital symptoms, because these studies would have skewed global syphilis prevalence estimates higher.
Their review, published July 8 in The Lancet Global Health, found that the pooled global prevalence of syphilis from 2000-2020 in MSM was 7.5%. It ranged from 1.9% in Australia and New Zealand to 10.6% in Latin America and the Caribbean. In comparison, the WHO estimates that globally, 0.5% of men in a general population have syphilis, a 15-fold difference.
This elevated estimate is not surprising, and the review provides a more international view of syphilis. Earlier attempts to estimate the prevalence of syphilis among MSM were generally conducted in higher-income countries such as the United States, Dr. Mayer said. “It’s important that clinicians recognize that this is a global health issue, so they can do the appropriate screening.”
The review found that regions in which the prevalence of HIV was above 5% had higher rates of syphilis (8.7%) compared to regions in which the prevalence of HIV was below 5% (6.6%). Pooled syphilis prevalence estimates were also higher for lower-middle-income and upper-middle-income countries (8.7% and 8.6%, respectively).
Global syphilis prevalence dipped from 8.9% in studies from 2000 to 2009 to 6.6% in studies from 2010 to 2020. In Europe, Northern America, Latin America, the Caribbean, and Oceania (excluding Australia and New Zealand), syphilis prevalence estimates for 2015-2020 were higher compared with 2010-2014.
The authors acknowledged that there were some limitations to the study, particularly that regions of Eastern and Southeastern Asia contributed more than half (54.5%) of the global data points used in the study and accounted for more than 82% of the study’s participants. This highlights the lack of data from other regions around the world, Dr. Chico said.
Dr. Chico said these findings “serve as a clarion call to action” to focus interventions on groups at higher risk for syphilis, such as MSM, in the effort to drastically reduce syphilis cases around the world. Dr. Mayer agrees. “[Syphilis] is a readily diagnosable and treatable infection,” he said. “It definitely is something that we should be able to get a handle on, but that requires paying attention to the different subgroups who have particularly high rates of the infection.”
A version of this article first appeared on Medscape.com.
When patients demand vaccinated health care providers
Should a hospital or medical practice fulfill a patient’s request to be treated or cared for only by vaccinated health care providers?The answer is yes, in a perfect world. Patients should feel assured that their health care providers – clinicians and caregivers – are not exposing them to infectious diseases.But issues are being raised – subquestions that need to be answered to understand the current situation and assist health care employers in their decision-making:
- Must health care employers ensure that their employees are vaccinated?
- Can health care employers require that their employees be vaccinated?
- Do employees have any rights to refuse vaccination or to refuse to supply their employer with their vaccination status?
- Can a health care employer terminate an employee who refuses vaccination?
- Does a patient have a legal right to a vaccinated health care provider?
At present, federal policy says that employers may, but are not required to, insist that employees be vaccinated. The currently prevailing state case law says that hospitals and other employers can require staff to be vaccinated and can terminate employees who refuse vaccination. In June, a Texas court dismissed a case in which 117 employees sued a hospital for requiring that employees be vaccinated. More cases are pending in other states, and there may be differing decisions in other states and on appeal.
State laws enacted years ago also weigh in on employer obligations. In at least one state, Oregon, employers of health care providers may not require vaccination, even though other employers may. Other states have laws about what an employer may or may not require of an employee regarding vaccination, and some have introduced laws which are pending.
So, in most states, health care employers may, not must, require that employees be vaccinated. In most states, hospitals and medical practices may terminate employees who refuse vaccination. However, employers should research the laws of their own states before requiring vaccinations and before terminating employees who are not vaccinated.
The issue of employer mandates is complicated further by the practicality that, in some areas of the country, health care providers are in scarce supply. Employers don’t want to lose the providers they have.
And there are additional questions about how certain federal laws affect the situation. Federal law that may apply includes:
- U.S. Food and Drug Administration regulation on approval of vaccines
- The Americans With Disabilities Act (ADA)
- The Health Insurance Portability and Accountability Act of 1996, which protects sensitive patient health information from being disclosed without the patient’s consent
- Civil rights laws
- Patients’ rights
FDA. Some health care providers who refuse vaccination argue that employers have no legal right to require a vaccine that is not fully approved by the FDA. COVID-19 vaccinations have emergency use authorization – something less than full approval. Courts have not yet ruled on this issue.
ADA. Some attorneys believe that honoring a patient’s request to be attended only by a vaccinated health care provider can implicate the ADA. However, the ADA doesn’t protect healthy individuals who don’t want to be vaccinated. The ADA protects the person who, because of their disability, shouldn’t get the vaccination. If an employer mandates vaccination, the employer must, under the ADA, consider requests for exemptions from disabled individuals. However, even when an employee has a disability that may qualify the employee for an exemption to the vaccination requirement, an employer may argue that giving an exemption would be a direct threat to the safety of others; in that case, the ADA may require that the disabled employee and hospital work something out. A compromise might be that the unvaccinated disabled individual would not provide direct patient care or would wear a mask and maintain physical distance.
HIPAA. Some argue that federal privacy law enters into the discussion, maintaining that health care employers can’t disclose employees’ vaccination status under HIPAA. That is not true. Employers are not “covered entities” under HIPAA. It is health care providers who are precluded under HIPAA from disclosing a patient’s personal information. So, if an employer were to ask an employee’s health care provider about the employee’s vaccination status, the health care provider could disclose that status only if the employee consented to the disclosure. An employer may ask an employee for the employee’s proof of vaccination card. However, employers must not ask for unnecessary details that might reveal disability information protected by the ADA.
Civil rights law. Civil rights laws may protect certain individuals from employment consequences of refusing vaccination. Specifically, individuals with sincerely held religious convictions against vaccinations are protected from retaliation by employers for refusing vaccination, under the Constitutional right of freedom of religion. The individual without sincerely held religious convictions against vaccinations and without a relevant disability doesn’t have legal remedies under civil rights laws.
Civil rights laws may apply if employers don’t apply their vaccination requirements to all employees equally. That is, employers can’t require vaccinations of some employees but not others.
Patients’ rights. Legal protections for patients who want a vaccinated health care provider are nowhere to be seen, at this time. It is unlikely that a single patient will be able to convince a hospital or medical practice to require that its staff be vaccinated. However, if a patient becomes infected with COVID-19 and can prove that the illness is causally related to interacting with an unvaccinated health care worker, the patient may have a case against the employer. The legal theory would be malpractice or negligence under informed consent law: That is, the patient did not consent to be treated by an unvaccinated person.
Employer options
So, what can health care employers do? They have three options:
- Require vaccination of all employees, independent contractors, and other providers who have privileges to see patients. Then, as long as the employer enforces the vaccination mandate, the employer can tell patients that all providers are vaccinated.
- Not require that employees and others with access to patients be vaccinated, and if a patient requests to be seen only by vaccinated providers, provide that patient with a vaccinated provider. It is especially important that health care employers take care with patients who are unvaccinated and who have been advised not to be vaccinated because of a medical condition. Both the patient and the health care employer would be protected best by avoiding having two unvaccinated individuals interact. Masks and physical distancing may decrease the risk.
- Not require that employees be vaccinated and refuse to guarantee that providers are vaccinated. To avoid risk for future lawsuits, employers should inform patients that there is no assurance that providers are vaccinated. That leaves it to each patient to ask individual providers about the provider’s vaccination status. If a patient doesn’t like a provider’s answer, then the patient has the right to leave. It’s not clear that the patient has a legal right to stay and demand a vaccinated provider.
Option three is problematic for a number of reasons. Patients aren’t always in a position to query each provider who enters the room about vaccination status. Patients may be sedated or too ill to exert that effort. And it puts supervisors in the position of having to mediate situations where a patient wants to leave against medical advice but the option of staying may also be dangerous.
Health care employers should discuss the options with their legal counsel before deciding which option to adopt.
A version of this article first appeared on Medscape.com.
Should a hospital or medical practice fulfill a patient’s request to be treated or cared for only by vaccinated health care providers?The answer is yes, in a perfect world. Patients should feel assured that their health care providers – clinicians and caregivers – are not exposing them to infectious diseases.But issues are being raised – subquestions that need to be answered to understand the current situation and assist health care employers in their decision-making:
- Must health care employers ensure that their employees are vaccinated?
- Can health care employers require that their employees be vaccinated?
- Do employees have any rights to refuse vaccination or to refuse to supply their employer with their vaccination status?
- Can a health care employer terminate an employee who refuses vaccination?
- Does a patient have a legal right to a vaccinated health care provider?
At present, federal policy says that employers may, but are not required to, insist that employees be vaccinated. The currently prevailing state case law says that hospitals and other employers can require staff to be vaccinated and can terminate employees who refuse vaccination. In June, a Texas court dismissed a case in which 117 employees sued a hospital for requiring that employees be vaccinated. More cases are pending in other states, and there may be differing decisions in other states and on appeal.
State laws enacted years ago also weigh in on employer obligations. In at least one state, Oregon, employers of health care providers may not require vaccination, even though other employers may. Other states have laws about what an employer may or may not require of an employee regarding vaccination, and some have introduced laws which are pending.
So, in most states, health care employers may, not must, require that employees be vaccinated. In most states, hospitals and medical practices may terminate employees who refuse vaccination. However, employers should research the laws of their own states before requiring vaccinations and before terminating employees who are not vaccinated.
The issue of employer mandates is complicated further by the practicality that, in some areas of the country, health care providers are in scarce supply. Employers don’t want to lose the providers they have.
And there are additional questions about how certain federal laws affect the situation. Federal law that may apply includes:
- U.S. Food and Drug Administration regulation on approval of vaccines
- The Americans With Disabilities Act (ADA)
- The Health Insurance Portability and Accountability Act of 1996, which protects sensitive patient health information from being disclosed without the patient’s consent
- Civil rights laws
- Patients’ rights
FDA. Some health care providers who refuse vaccination argue that employers have no legal right to require a vaccine that is not fully approved by the FDA. COVID-19 vaccinations have emergency use authorization – something less than full approval. Courts have not yet ruled on this issue.
ADA. Some attorneys believe that honoring a patient’s request to be attended only by a vaccinated health care provider can implicate the ADA. However, the ADA doesn’t protect healthy individuals who don’t want to be vaccinated. The ADA protects the person who, because of their disability, shouldn’t get the vaccination. If an employer mandates vaccination, the employer must, under the ADA, consider requests for exemptions from disabled individuals. However, even when an employee has a disability that may qualify the employee for an exemption to the vaccination requirement, an employer may argue that giving an exemption would be a direct threat to the safety of others; in that case, the ADA may require that the disabled employee and hospital work something out. A compromise might be that the unvaccinated disabled individual would not provide direct patient care or would wear a mask and maintain physical distance.
HIPAA. Some argue that federal privacy law enters into the discussion, maintaining that health care employers can’t disclose employees’ vaccination status under HIPAA. That is not true. Employers are not “covered entities” under HIPAA. It is health care providers who are precluded under HIPAA from disclosing a patient’s personal information. So, if an employer were to ask an employee’s health care provider about the employee’s vaccination status, the health care provider could disclose that status only if the employee consented to the disclosure. An employer may ask an employee for the employee’s proof of vaccination card. However, employers must not ask for unnecessary details that might reveal disability information protected by the ADA.
Civil rights law. Civil rights laws may protect certain individuals from employment consequences of refusing vaccination. Specifically, individuals with sincerely held religious convictions against vaccinations are protected from retaliation by employers for refusing vaccination, under the Constitutional right of freedom of religion. The individual without sincerely held religious convictions against vaccinations and without a relevant disability doesn’t have legal remedies under civil rights laws.
Civil rights laws may apply if employers don’t apply their vaccination requirements to all employees equally. That is, employers can’t require vaccinations of some employees but not others.
Patients’ rights. Legal protections for patients who want a vaccinated health care provider are nowhere to be seen, at this time. It is unlikely that a single patient will be able to convince a hospital or medical practice to require that its staff be vaccinated. However, if a patient becomes infected with COVID-19 and can prove that the illness is causally related to interacting with an unvaccinated health care worker, the patient may have a case against the employer. The legal theory would be malpractice or negligence under informed consent law: That is, the patient did not consent to be treated by an unvaccinated person.
Employer options
So, what can health care employers do? They have three options:
- Require vaccination of all employees, independent contractors, and other providers who have privileges to see patients. Then, as long as the employer enforces the vaccination mandate, the employer can tell patients that all providers are vaccinated.
- Not require that employees and others with access to patients be vaccinated, and if a patient requests to be seen only by vaccinated providers, provide that patient with a vaccinated provider. It is especially important that health care employers take care with patients who are unvaccinated and who have been advised not to be vaccinated because of a medical condition. Both the patient and the health care employer would be protected best by avoiding having two unvaccinated individuals interact. Masks and physical distancing may decrease the risk.
- Not require that employees be vaccinated and refuse to guarantee that providers are vaccinated. To avoid risk for future lawsuits, employers should inform patients that there is no assurance that providers are vaccinated. That leaves it to each patient to ask individual providers about the provider’s vaccination status. If a patient doesn’t like a provider’s answer, then the patient has the right to leave. It’s not clear that the patient has a legal right to stay and demand a vaccinated provider.
Option three is problematic for a number of reasons. Patients aren’t always in a position to query each provider who enters the room about vaccination status. Patients may be sedated or too ill to exert that effort. And it puts supervisors in the position of having to mediate situations where a patient wants to leave against medical advice but the option of staying may also be dangerous.
Health care employers should discuss the options with their legal counsel before deciding which option to adopt.
A version of this article first appeared on Medscape.com.
Should a hospital or medical practice fulfill a patient’s request to be treated or cared for only by vaccinated health care providers?The answer is yes, in a perfect world. Patients should feel assured that their health care providers – clinicians and caregivers – are not exposing them to infectious diseases.But issues are being raised – subquestions that need to be answered to understand the current situation and assist health care employers in their decision-making:
- Must health care employers ensure that their employees are vaccinated?
- Can health care employers require that their employees be vaccinated?
- Do employees have any rights to refuse vaccination or to refuse to supply their employer with their vaccination status?
- Can a health care employer terminate an employee who refuses vaccination?
- Does a patient have a legal right to a vaccinated health care provider?
At present, federal policy says that employers may, but are not required to, insist that employees be vaccinated. The currently prevailing state case law says that hospitals and other employers can require staff to be vaccinated and can terminate employees who refuse vaccination. In June, a Texas court dismissed a case in which 117 employees sued a hospital for requiring that employees be vaccinated. More cases are pending in other states, and there may be differing decisions in other states and on appeal.
State laws enacted years ago also weigh in on employer obligations. In at least one state, Oregon, employers of health care providers may not require vaccination, even though other employers may. Other states have laws about what an employer may or may not require of an employee regarding vaccination, and some have introduced laws which are pending.
So, in most states, health care employers may, not must, require that employees be vaccinated. In most states, hospitals and medical practices may terminate employees who refuse vaccination. However, employers should research the laws of their own states before requiring vaccinations and before terminating employees who are not vaccinated.
The issue of employer mandates is complicated further by the practicality that, in some areas of the country, health care providers are in scarce supply. Employers don’t want to lose the providers they have.
And there are additional questions about how certain federal laws affect the situation. Federal law that may apply includes:
- U.S. Food and Drug Administration regulation on approval of vaccines
- The Americans With Disabilities Act (ADA)
- The Health Insurance Portability and Accountability Act of 1996, which protects sensitive patient health information from being disclosed without the patient’s consent
- Civil rights laws
- Patients’ rights
FDA. Some health care providers who refuse vaccination argue that employers have no legal right to require a vaccine that is not fully approved by the FDA. COVID-19 vaccinations have emergency use authorization – something less than full approval. Courts have not yet ruled on this issue.
ADA. Some attorneys believe that honoring a patient’s request to be attended only by a vaccinated health care provider can implicate the ADA. However, the ADA doesn’t protect healthy individuals who don’t want to be vaccinated. The ADA protects the person who, because of their disability, shouldn’t get the vaccination. If an employer mandates vaccination, the employer must, under the ADA, consider requests for exemptions from disabled individuals. However, even when an employee has a disability that may qualify the employee for an exemption to the vaccination requirement, an employer may argue that giving an exemption would be a direct threat to the safety of others; in that case, the ADA may require that the disabled employee and hospital work something out. A compromise might be that the unvaccinated disabled individual would not provide direct patient care or would wear a mask and maintain physical distance.
HIPAA. Some argue that federal privacy law enters into the discussion, maintaining that health care employers can’t disclose employees’ vaccination status under HIPAA. That is not true. Employers are not “covered entities” under HIPAA. It is health care providers who are precluded under HIPAA from disclosing a patient’s personal information. So, if an employer were to ask an employee’s health care provider about the employee’s vaccination status, the health care provider could disclose that status only if the employee consented to the disclosure. An employer may ask an employee for the employee’s proof of vaccination card. However, employers must not ask for unnecessary details that might reveal disability information protected by the ADA.
Civil rights law. Civil rights laws may protect certain individuals from employment consequences of refusing vaccination. Specifically, individuals with sincerely held religious convictions against vaccinations are protected from retaliation by employers for refusing vaccination, under the Constitutional right of freedom of religion. The individual without sincerely held religious convictions against vaccinations and without a relevant disability doesn’t have legal remedies under civil rights laws.
Civil rights laws may apply if employers don’t apply their vaccination requirements to all employees equally. That is, employers can’t require vaccinations of some employees but not others.
Patients’ rights. Legal protections for patients who want a vaccinated health care provider are nowhere to be seen, at this time. It is unlikely that a single patient will be able to convince a hospital or medical practice to require that its staff be vaccinated. However, if a patient becomes infected with COVID-19 and can prove that the illness is causally related to interacting with an unvaccinated health care worker, the patient may have a case against the employer. The legal theory would be malpractice or negligence under informed consent law: That is, the patient did not consent to be treated by an unvaccinated person.
Employer options
So, what can health care employers do? They have three options:
- Require vaccination of all employees, independent contractors, and other providers who have privileges to see patients. Then, as long as the employer enforces the vaccination mandate, the employer can tell patients that all providers are vaccinated.
- Not require that employees and others with access to patients be vaccinated, and if a patient requests to be seen only by vaccinated providers, provide that patient with a vaccinated provider. It is especially important that health care employers take care with patients who are unvaccinated and who have been advised not to be vaccinated because of a medical condition. Both the patient and the health care employer would be protected best by avoiding having two unvaccinated individuals interact. Masks and physical distancing may decrease the risk.
- Not require that employees be vaccinated and refuse to guarantee that providers are vaccinated. To avoid risk for future lawsuits, employers should inform patients that there is no assurance that providers are vaccinated. That leaves it to each patient to ask individual providers about the provider’s vaccination status. If a patient doesn’t like a provider’s answer, then the patient has the right to leave. It’s not clear that the patient has a legal right to stay and demand a vaccinated provider.
Option three is problematic for a number of reasons. Patients aren’t always in a position to query each provider who enters the room about vaccination status. Patients may be sedated or too ill to exert that effort. And it puts supervisors in the position of having to mediate situations where a patient wants to leave against medical advice but the option of staying may also be dangerous.
Health care employers should discuss the options with their legal counsel before deciding which option to adopt.
A version of this article first appeared on Medscape.com.
‘Gold cards’ allow Texas docs to skip prior authorizations
The law was passed in June and will take effect in September. It excuses physicians from having to obtain prior authorization if, during the previous 6 months, 90% of their treatments met medical necessity criteria by the health insurer. Through this law, doctors in the state will spend less time getting approvals for treatments for their patients.
Automatic approval of authorizations for treatments – or what the Texas Medical Association (TMA) calls a “gold card” – “allows patients to get the care they need in a more timely fashion,” says Debra Patt, MD, an Austin, Tex.–based oncologist and former chair of the council on legislation for the TMA.
Eighty-seven percent of Texas physicians reported a “drastic increase over the past five years in the burden of prior authorization on their patients and their practices,” per a 2020 survey by the TMA. Nearly half (48%) of Texas physicians have hired staff whose work focuses on processing requests for prior authorization, according to the survey.
Jack Resneck Jr., MD, a San Francisco–based dermatologist and president-elect of the American Medical Association (AMA), said other states have investigated ways to ease the impact of prior authorizations on physicians, but no other state has passed such a law.
Administrative burdens plague physicians around the country. The Medscape Physician Compensation Report 2021 found that physicians spend on average 15.6 hours per week on paperwork and administrative duties.
Better outcomes, less anxiety for patients
Dr. Patt, who testified in support of the law’s passage in the Texas legislature, says automatic approval of authorizations “is better for patients because it reduces their anxiety about whether they’re able to get the treatments they need now, and they will have better outcomes if they’re able to receive more timely care.”
Recently, a chemotherapy treatment Dr. Patt prescribed for one of her patients was not authorized by an insurer. The result is “a lot of anxiety and potentially health problems” for the patient, said Dr. Patt.
She expects that automatic approval for treatments will be based on prescribing patterns during the preceding 6 months. “It means that when I order a test today, the [health insurer] looks back at my record 6 months previously,” she said. Still, Dr. Patt awaits guidance from the Texas Department of Insurance, which regulates health insurers in the state, regarding the law.
Dr. Resneck said the pharmacy counter is where most patients encounter prior authorization delays. “That’s when the pharmacist looks at them and says, ‘Actually, this isn’t covered by your health insurer’s formulary,’ or it isn’t covered fully on their formulary.”
One of Dr. Resneck’s patients had a life-altering case of eczema that lasted many years. Because of the condition, the patient couldn’t work or maintain meaningful bonds with family members. A biologic treatment transformed his patient’s life. The patient was able to return to work and to re-engage with family, said Dr. Resneck. But a year after his patient started the treatment, the health insurer wouldn’t authorize the treatment because the patient wasn’t experiencing the same symptoms.
The patient didn’t have the same symptoms because the biologic treatment worked, said Dr. Resneck.
Kristine Grow, a spokesperson for America’s Health Insurance Plans, a national association for health insurers, said, “The use of prior authorization is relatively small – typically, less than 15% – and can help ensure safer opioid prescribing, help prevent dangerous drug interactions, and help protect patients from unnecessary exposure to potentially harmful radiation for inappropriate diagnostic imaging. Numerous studies show that Americans frequently receive inappropriate care, and 25% of unnecessary treatments are associated with complications or adverse events.”
Medical management tools, such as prior authorization, are an “an important way” to deliver “safe, high-quality care” to patients, she added.
State and federal efforts to curb prior authorization
In addition to efforts to curb prior authorization in other states, the AMA supports the Improving Seniors’ Timely Access to Care Act (HR 3173). The act includes a provision related to “gold-carding,” said Robert Mills, an AMA spokesperson.
The bill establishes requirements and standards for prior authorization processes related to Medicare Advantage (MA) plans. The requirements and standards for MA plans include the following:
- Establishing an electronic prior authorization program that meets specific standards, such as the ability to provide real-time decisions in response to requests for items and services that are routinely approved.
- Publishing on an annual basis specific prior authorization information, including the percentage of requests approved and the average response time.
- Meeting standards set by the Centers for Medicare & Medicaid Services related to the quality and timeliness of prior authorization determinations.
The act was introduced to the U.S. House of Representatives in May, after which it was referred to two committees for consideration.
A version of this article first appeared on Medscape.com.
The law was passed in June and will take effect in September. It excuses physicians from having to obtain prior authorization if, during the previous 6 months, 90% of their treatments met medical necessity criteria by the health insurer. Through this law, doctors in the state will spend less time getting approvals for treatments for their patients.
Automatic approval of authorizations for treatments – or what the Texas Medical Association (TMA) calls a “gold card” – “allows patients to get the care they need in a more timely fashion,” says Debra Patt, MD, an Austin, Tex.–based oncologist and former chair of the council on legislation for the TMA.
Eighty-seven percent of Texas physicians reported a “drastic increase over the past five years in the burden of prior authorization on their patients and their practices,” per a 2020 survey by the TMA. Nearly half (48%) of Texas physicians have hired staff whose work focuses on processing requests for prior authorization, according to the survey.
Jack Resneck Jr., MD, a San Francisco–based dermatologist and president-elect of the American Medical Association (AMA), said other states have investigated ways to ease the impact of prior authorizations on physicians, but no other state has passed such a law.
Administrative burdens plague physicians around the country. The Medscape Physician Compensation Report 2021 found that physicians spend on average 15.6 hours per week on paperwork and administrative duties.
Better outcomes, less anxiety for patients
Dr. Patt, who testified in support of the law’s passage in the Texas legislature, says automatic approval of authorizations “is better for patients because it reduces their anxiety about whether they’re able to get the treatments they need now, and they will have better outcomes if they’re able to receive more timely care.”
Recently, a chemotherapy treatment Dr. Patt prescribed for one of her patients was not authorized by an insurer. The result is “a lot of anxiety and potentially health problems” for the patient, said Dr. Patt.
She expects that automatic approval for treatments will be based on prescribing patterns during the preceding 6 months. “It means that when I order a test today, the [health insurer] looks back at my record 6 months previously,” she said. Still, Dr. Patt awaits guidance from the Texas Department of Insurance, which regulates health insurers in the state, regarding the law.
Dr. Resneck said the pharmacy counter is where most patients encounter prior authorization delays. “That’s when the pharmacist looks at them and says, ‘Actually, this isn’t covered by your health insurer’s formulary,’ or it isn’t covered fully on their formulary.”
One of Dr. Resneck’s patients had a life-altering case of eczema that lasted many years. Because of the condition, the patient couldn’t work or maintain meaningful bonds with family members. A biologic treatment transformed his patient’s life. The patient was able to return to work and to re-engage with family, said Dr. Resneck. But a year after his patient started the treatment, the health insurer wouldn’t authorize the treatment because the patient wasn’t experiencing the same symptoms.
The patient didn’t have the same symptoms because the biologic treatment worked, said Dr. Resneck.
Kristine Grow, a spokesperson for America’s Health Insurance Plans, a national association for health insurers, said, “The use of prior authorization is relatively small – typically, less than 15% – and can help ensure safer opioid prescribing, help prevent dangerous drug interactions, and help protect patients from unnecessary exposure to potentially harmful radiation for inappropriate diagnostic imaging. Numerous studies show that Americans frequently receive inappropriate care, and 25% of unnecessary treatments are associated with complications or adverse events.”
Medical management tools, such as prior authorization, are an “an important way” to deliver “safe, high-quality care” to patients, she added.
State and federal efforts to curb prior authorization
In addition to efforts to curb prior authorization in other states, the AMA supports the Improving Seniors’ Timely Access to Care Act (HR 3173). The act includes a provision related to “gold-carding,” said Robert Mills, an AMA spokesperson.
The bill establishes requirements and standards for prior authorization processes related to Medicare Advantage (MA) plans. The requirements and standards for MA plans include the following:
- Establishing an electronic prior authorization program that meets specific standards, such as the ability to provide real-time decisions in response to requests for items and services that are routinely approved.
- Publishing on an annual basis specific prior authorization information, including the percentage of requests approved and the average response time.
- Meeting standards set by the Centers for Medicare & Medicaid Services related to the quality and timeliness of prior authorization determinations.
The act was introduced to the U.S. House of Representatives in May, after which it was referred to two committees for consideration.
A version of this article first appeared on Medscape.com.
The law was passed in June and will take effect in September. It excuses physicians from having to obtain prior authorization if, during the previous 6 months, 90% of their treatments met medical necessity criteria by the health insurer. Through this law, doctors in the state will spend less time getting approvals for treatments for their patients.
Automatic approval of authorizations for treatments – or what the Texas Medical Association (TMA) calls a “gold card” – “allows patients to get the care they need in a more timely fashion,” says Debra Patt, MD, an Austin, Tex.–based oncologist and former chair of the council on legislation for the TMA.
Eighty-seven percent of Texas physicians reported a “drastic increase over the past five years in the burden of prior authorization on their patients and their practices,” per a 2020 survey by the TMA. Nearly half (48%) of Texas physicians have hired staff whose work focuses on processing requests for prior authorization, according to the survey.
Jack Resneck Jr., MD, a San Francisco–based dermatologist and president-elect of the American Medical Association (AMA), said other states have investigated ways to ease the impact of prior authorizations on physicians, but no other state has passed such a law.
Administrative burdens plague physicians around the country. The Medscape Physician Compensation Report 2021 found that physicians spend on average 15.6 hours per week on paperwork and administrative duties.
Better outcomes, less anxiety for patients
Dr. Patt, who testified in support of the law’s passage in the Texas legislature, says automatic approval of authorizations “is better for patients because it reduces their anxiety about whether they’re able to get the treatments they need now, and they will have better outcomes if they’re able to receive more timely care.”
Recently, a chemotherapy treatment Dr. Patt prescribed for one of her patients was not authorized by an insurer. The result is “a lot of anxiety and potentially health problems” for the patient, said Dr. Patt.
She expects that automatic approval for treatments will be based on prescribing patterns during the preceding 6 months. “It means that when I order a test today, the [health insurer] looks back at my record 6 months previously,” she said. Still, Dr. Patt awaits guidance from the Texas Department of Insurance, which regulates health insurers in the state, regarding the law.
Dr. Resneck said the pharmacy counter is where most patients encounter prior authorization delays. “That’s when the pharmacist looks at them and says, ‘Actually, this isn’t covered by your health insurer’s formulary,’ or it isn’t covered fully on their formulary.”
One of Dr. Resneck’s patients had a life-altering case of eczema that lasted many years. Because of the condition, the patient couldn’t work or maintain meaningful bonds with family members. A biologic treatment transformed his patient’s life. The patient was able to return to work and to re-engage with family, said Dr. Resneck. But a year after his patient started the treatment, the health insurer wouldn’t authorize the treatment because the patient wasn’t experiencing the same symptoms.
The patient didn’t have the same symptoms because the biologic treatment worked, said Dr. Resneck.
Kristine Grow, a spokesperson for America’s Health Insurance Plans, a national association for health insurers, said, “The use of prior authorization is relatively small – typically, less than 15% – and can help ensure safer opioid prescribing, help prevent dangerous drug interactions, and help protect patients from unnecessary exposure to potentially harmful radiation for inappropriate diagnostic imaging. Numerous studies show that Americans frequently receive inappropriate care, and 25% of unnecessary treatments are associated with complications or adverse events.”
Medical management tools, such as prior authorization, are an “an important way” to deliver “safe, high-quality care” to patients, she added.
State and federal efforts to curb prior authorization
In addition to efforts to curb prior authorization in other states, the AMA supports the Improving Seniors’ Timely Access to Care Act (HR 3173). The act includes a provision related to “gold-carding,” said Robert Mills, an AMA spokesperson.
The bill establishes requirements and standards for prior authorization processes related to Medicare Advantage (MA) plans. The requirements and standards for MA plans include the following:
- Establishing an electronic prior authorization program that meets specific standards, such as the ability to provide real-time decisions in response to requests for items and services that are routinely approved.
- Publishing on an annual basis specific prior authorization information, including the percentage of requests approved and the average response time.
- Meeting standards set by the Centers for Medicare & Medicaid Services related to the quality and timeliness of prior authorization determinations.
The act was introduced to the U.S. House of Representatives in May, after which it was referred to two committees for consideration.
A version of this article first appeared on Medscape.com.










