User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Possible obesity effect detected in cancer death rates

“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.

“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.

“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.
FROM JAMA NETWORK OPEN
Novel hedgehog inhibitor strategies improve BCC outcomes
MD, a Mohs surgeon and chair of the department of dermatology at the Cleveland Clinic.
She and her colleagues have noticed an accelerated and durable response to hedgehog inhibitors after debulking and are studying cell signaling before and after debulking to better understand the issue.
Dr. Vidimos shared a remarkable case to illustrate the point during a clinical pearls talk at the annual meeting of the American College of Mohs Surgery.
An 82-year-old woman presented with a crusted, hemorrhagic, nodular basal cell carcinoma (BCC) that had overgrown over nearly her entire nose and left lower eyelid. A recurrence of a previous BCC, the tumor had been growing for a decade and had invaded her nasal bones but not the periorbital tissue.
An outside surgeon suggested a full rhinectomy and removal of the lower eyelid, but the woman refused.
Dr. Vidimos decided to treat her with vismodegib, but prior to doing so, she debulked the tumor to help with the pain and bleeding. She did not curette the portion of tumor extending through the ala into the nasal vestibule. “I let the vismodegib take care of that,” she said.
After 9 months, the tumor was virtually gone, with no recurrence after 3 years. Surgical debulking prior to hedgehog inhibition “reduces the tumor burden and may increase the efficacy and shorten the course of therapy,” Dr. Vidimos said.
The hedgehog inhibitors vismodegib (Erivedge) and sonidegib Odomzo are both approved for treating locally advanced BCC, with a complete response of 31% of locally advanced disease with vismodegib, according to one report.
But monotherapy is limited by intolerable side effects, most commonly muscle spasms, alopecia, and dysgeusia. To minimize the impact, Dr. Vidimos generally puts patients on treatment with Monday through Friday dosing and gives them the weekends off, a schedule she and her colleagues have reported works as well as daily dosing.
Still, many patients discontinue the drugs because of the side effects. Hedgehog inhibitors are also expensive and responses aren’t always durable. To increase efficacy and shorten the course of therapy, “we need alternative treatment strategies,” Dr. Vidimos said.
Up-front tumor debulking is one such strategy. Altered cell signaling pathways associated with tissue remodeling might improve response, and debulking may reduce the genetic heterogeneity of tumor cells, rendering remaining cells less resistant to hedgehog inhibition, she explained.
“It is exciting to see how tumor debulking may reduce tumor burden and heterogeneity, and thus lead to a durable response in extensive tumors,” said Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who heard the presentation. “More investigation is needed to reproduce these results, but this approach may lead to improved outcomes with targeted therapies,” he said in an interview.
Combination therapy with other agents is another option, and there also seems to be a synergistic effect with radiation, with hedgehog inhibitors increasing cellular response to radiation therapy, Dr. Vidimos said.
Hedgehog inhibitors can also be used to shrink tumors before surgery. One small series found a 27% decrease in the area of the tumor after 3 to 6 months of preoperative vismodegib.
Dr. Vidimos shared another case to illustrate the point.
A 64-year-old woman fainted and presented to the ED with a hemoglobin of 3.2 mg/dL because of chronic blood loss from an ulcerated BCC on her upper back. The lesion measured 25 cm by 9 cm, and was 3.5 cm deep with no bone involvement. The woman was addicted to opioids by the time she presented.
She was started on vismodegib; the ulcer shrunk considerably after 6 months, and the woman underwent a resection. Only one small focus of BCC was found across 78 specimens submitted to Dr. Vidimos for Mohs reading.
Resection was followed by a muscle flap repair and radiation. At 5 and a half years, there is no evidence of disease; the only sign that the lesion had been there was a scar running along the woman’s upper spine.
The approach “was very successful for a very aggressive and worrisome tumor,” Dr. Vidimos said.
Dr. Vidimos did not have any relevant disclosures. Dr. Patel had no relevant disclosures.
MD, a Mohs surgeon and chair of the department of dermatology at the Cleveland Clinic.
She and her colleagues have noticed an accelerated and durable response to hedgehog inhibitors after debulking and are studying cell signaling before and after debulking to better understand the issue.
Dr. Vidimos shared a remarkable case to illustrate the point during a clinical pearls talk at the annual meeting of the American College of Mohs Surgery.
An 82-year-old woman presented with a crusted, hemorrhagic, nodular basal cell carcinoma (BCC) that had overgrown over nearly her entire nose and left lower eyelid. A recurrence of a previous BCC, the tumor had been growing for a decade and had invaded her nasal bones but not the periorbital tissue.
An outside surgeon suggested a full rhinectomy and removal of the lower eyelid, but the woman refused.
Dr. Vidimos decided to treat her with vismodegib, but prior to doing so, she debulked the tumor to help with the pain and bleeding. She did not curette the portion of tumor extending through the ala into the nasal vestibule. “I let the vismodegib take care of that,” she said.
After 9 months, the tumor was virtually gone, with no recurrence after 3 years. Surgical debulking prior to hedgehog inhibition “reduces the tumor burden and may increase the efficacy and shorten the course of therapy,” Dr. Vidimos said.
The hedgehog inhibitors vismodegib (Erivedge) and sonidegib Odomzo are both approved for treating locally advanced BCC, with a complete response of 31% of locally advanced disease with vismodegib, according to one report.
But monotherapy is limited by intolerable side effects, most commonly muscle spasms, alopecia, and dysgeusia. To minimize the impact, Dr. Vidimos generally puts patients on treatment with Monday through Friday dosing and gives them the weekends off, a schedule she and her colleagues have reported works as well as daily dosing.
Still, many patients discontinue the drugs because of the side effects. Hedgehog inhibitors are also expensive and responses aren’t always durable. To increase efficacy and shorten the course of therapy, “we need alternative treatment strategies,” Dr. Vidimos said.
Up-front tumor debulking is one such strategy. Altered cell signaling pathways associated with tissue remodeling might improve response, and debulking may reduce the genetic heterogeneity of tumor cells, rendering remaining cells less resistant to hedgehog inhibition, she explained.
“It is exciting to see how tumor debulking may reduce tumor burden and heterogeneity, and thus lead to a durable response in extensive tumors,” said Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who heard the presentation. “More investigation is needed to reproduce these results, but this approach may lead to improved outcomes with targeted therapies,” he said in an interview.
Combination therapy with other agents is another option, and there also seems to be a synergistic effect with radiation, with hedgehog inhibitors increasing cellular response to radiation therapy, Dr. Vidimos said.
Hedgehog inhibitors can also be used to shrink tumors before surgery. One small series found a 27% decrease in the area of the tumor after 3 to 6 months of preoperative vismodegib.
Dr. Vidimos shared another case to illustrate the point.
A 64-year-old woman fainted and presented to the ED with a hemoglobin of 3.2 mg/dL because of chronic blood loss from an ulcerated BCC on her upper back. The lesion measured 25 cm by 9 cm, and was 3.5 cm deep with no bone involvement. The woman was addicted to opioids by the time she presented.
She was started on vismodegib; the ulcer shrunk considerably after 6 months, and the woman underwent a resection. Only one small focus of BCC was found across 78 specimens submitted to Dr. Vidimos for Mohs reading.
Resection was followed by a muscle flap repair and radiation. At 5 and a half years, there is no evidence of disease; the only sign that the lesion had been there was a scar running along the woman’s upper spine.
The approach “was very successful for a very aggressive and worrisome tumor,” Dr. Vidimos said.
Dr. Vidimos did not have any relevant disclosures. Dr. Patel had no relevant disclosures.
MD, a Mohs surgeon and chair of the department of dermatology at the Cleveland Clinic.
She and her colleagues have noticed an accelerated and durable response to hedgehog inhibitors after debulking and are studying cell signaling before and after debulking to better understand the issue.
Dr. Vidimos shared a remarkable case to illustrate the point during a clinical pearls talk at the annual meeting of the American College of Mohs Surgery.
An 82-year-old woman presented with a crusted, hemorrhagic, nodular basal cell carcinoma (BCC) that had overgrown over nearly her entire nose and left lower eyelid. A recurrence of a previous BCC, the tumor had been growing for a decade and had invaded her nasal bones but not the periorbital tissue.
An outside surgeon suggested a full rhinectomy and removal of the lower eyelid, but the woman refused.
Dr. Vidimos decided to treat her with vismodegib, but prior to doing so, she debulked the tumor to help with the pain and bleeding. She did not curette the portion of tumor extending through the ala into the nasal vestibule. “I let the vismodegib take care of that,” she said.
After 9 months, the tumor was virtually gone, with no recurrence after 3 years. Surgical debulking prior to hedgehog inhibition “reduces the tumor burden and may increase the efficacy and shorten the course of therapy,” Dr. Vidimos said.
The hedgehog inhibitors vismodegib (Erivedge) and sonidegib Odomzo are both approved for treating locally advanced BCC, with a complete response of 31% of locally advanced disease with vismodegib, according to one report.
But monotherapy is limited by intolerable side effects, most commonly muscle spasms, alopecia, and dysgeusia. To minimize the impact, Dr. Vidimos generally puts patients on treatment with Monday through Friday dosing and gives them the weekends off, a schedule she and her colleagues have reported works as well as daily dosing.
Still, many patients discontinue the drugs because of the side effects. Hedgehog inhibitors are also expensive and responses aren’t always durable. To increase efficacy and shorten the course of therapy, “we need alternative treatment strategies,” Dr. Vidimos said.
Up-front tumor debulking is one such strategy. Altered cell signaling pathways associated with tissue remodeling might improve response, and debulking may reduce the genetic heterogeneity of tumor cells, rendering remaining cells less resistant to hedgehog inhibition, she explained.
“It is exciting to see how tumor debulking may reduce tumor burden and heterogeneity, and thus lead to a durable response in extensive tumors,” said Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who heard the presentation. “More investigation is needed to reproduce these results, but this approach may lead to improved outcomes with targeted therapies,” he said in an interview.
Combination therapy with other agents is another option, and there also seems to be a synergistic effect with radiation, with hedgehog inhibitors increasing cellular response to radiation therapy, Dr. Vidimos said.
Hedgehog inhibitors can also be used to shrink tumors before surgery. One small series found a 27% decrease in the area of the tumor after 3 to 6 months of preoperative vismodegib.
Dr. Vidimos shared another case to illustrate the point.
A 64-year-old woman fainted and presented to the ED with a hemoglobin of 3.2 mg/dL because of chronic blood loss from an ulcerated BCC on her upper back. The lesion measured 25 cm by 9 cm, and was 3.5 cm deep with no bone involvement. The woman was addicted to opioids by the time she presented.
She was started on vismodegib; the ulcer shrunk considerably after 6 months, and the woman underwent a resection. Only one small focus of BCC was found across 78 specimens submitted to Dr. Vidimos for Mohs reading.
Resection was followed by a muscle flap repair and radiation. At 5 and a half years, there is no evidence of disease; the only sign that the lesion had been there was a scar running along the woman’s upper spine.
The approach “was very successful for a very aggressive and worrisome tumor,” Dr. Vidimos said.
Dr. Vidimos did not have any relevant disclosures. Dr. Patel had no relevant disclosures.
FROM THE ACMS ANNUAL MEETING
Dr. Topol talks: COVID-19 variants are innocent until proven guilty
Editor in Chief of this news organization Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and professor of molecular medicine, has been closely following COVID-19 data since the pandemic began. He spoke with writer Miriam E. Tucker about the latest on SARS-CoV-2 variants and their impact on vaccine efficacy. The conversation serves as a follow-up to his April 13, 2021, New York Times opinion piece, in which he advised readers that “all variants are innocent until proven guilty.”
You have expressed overall confidence in the efficacy of the vaccines thus far despite the emergence of variants, with some caveats. How do you see the current situation?
The Centers for Disease Control and Prevention has designated five “variants of concern,” but only three of them are real concerns – B.1.1.7, first detected in the United Kingdom; P.1, in Brazil and Japan; and B.1.351, in South Africa. Yet, all three are susceptible to our current vaccines.
The U.K. B.1.1.7 is the worst variant of all because it’s hypertransmissible, so I call it a “superspreader strain.” It also causes more severe illness independent of the spread, so it’s a double whammy. It’s clear that it also causes more deaths. The only arguable point is whether it’s 30% or 50% more deaths, but regardless, it’s more lethal and more transmissible.
The B.1.1.7 is going to be the dominant strain worldwide. It could develop new mutations within it that could come back to haunt us. We must keep watch.
But for now, it’s fully responsive to all the vaccines, which is great because if we didn’t have them, we wouldn’t have gotten through this U.S. pandemic like we have, and neither would Israel and the United Kingdom and other countries that have been able to get out of the crisis. We met the enemy and put it in check.
As for the South Africa variant of concern, B.1.351, we just got some encouraging news showing that it›s very responsive to the Pfizer/BioNTech mRNA vaccine in large numbers of people. The study was conducted in Qatar following that country’s mass immunization campaign in which a total of 385,853 people had received at least one vaccine dose and 265,410 had completed the two doses as of March 31, 2021.
At 2 weeks past the second dose, the vaccine was 75% effective at preventing any documented infection with the B.1.351 variant and 89.5% effective against B.1.1.7. The vaccine’s effectiveness against severe, critical, or fatal COVID-19 was greater than 97.4% for all circulating strains in Qatar, where B.1.1.7 and B.1.351 are most prominent.
We also know that B.1.351 is very responsive to the Johnson & Johnson vaccine and the Novavax [vaccine in development] to a lesser degree. It is the most immune-evading variant we’ve seen thus far, with the highest likelihood of providing some vaccine resistance, yet not enough to interfere with vaccination campaigns. So that’s great news.
The caveats here are that you definitely need two doses of the mRNA vaccines to combat the B.1.351 variant. Also, the AstraZeneca vaccine failed to prevent it in South Africa. However, that study was hard to judge because it was underpowered for number of people with mild infections. So, it didn’t look as if it had any efficacy, but maybe it would if tested in a real trial.
The P.1 (Brazil) variant is the second-highest concern after B.1.1.7 because it’s the only one in the United States that’s still headed up. It seems to be competing a bit with B.1.1.7 here. We know it was associated with the crisis in Brazil, in Chile, and some other South American countries. It has some immune escape, but not as bad as B.1.351. It also appears to have somewhat greater transmissibility but not as much as B.1.1.7.
With P.1, we just don’t know enough yet. It was difficult to assess in Brazil because they were in the midst of a catastrophe – like India is now – and you don’t know how much of it is dragged by the catastrophe vs driving it.
We have to respond to P.1 carefully. There are some good data that it does respond to the Chinese vaccine Sinovac and the AstraZeneca vaccine, and it appears to respond to the others as well, based on serum studies. So it doesn’t look like vaccines will be the worry with this variant. Rather, it could be competing with B.1.1.7 and could lead to breakthrough infections in vaccinated people or reinfections in unvaccinated people who had COVID-19. We need several more weeks to sort it out.
Although the B.1.427 and B.1.429 variants initially seen in California remain on the CDC’s concern list, I’m not worried about them.
You mentioned the current COVID-19 crisis in India, where a new variant has been described as a “double mutant,” but on Twitter you called it a “scariant.” Why?
First of all, the B.1.617 variant isn’t a double mutant. It has 15 mutations. It’s a stupid term, focusing on two mutations which largely have been put aside as to concern. One of them is the L452R, which is the same as one of the California variants, and that hasn’t proved to be particularly serious or concerning. The other is the 484Q, and it’s not clear whether that has any function.
The B.1.617 is not the driver of the catastrophe in India. It may be contributing a small amount, but it has been overhyped as the double mutant that’s causing it all. Adding to that are what I call “scariant” headlines here in the United States when a few cases of that variant have been seen.
I coined the term scariant in early February because it was a pretty clear trend. People don’t know what variants are. They know a little bit about mutations but not variants, and they’re scared. A few variants are concerning, but we keep learning more and more things to decrease the concern. That’s why I wrote the New York Times op-ed, to try to provide some reassurance, since there’s such paranoia.
Do you think booster vaccinations will be necessary? If so, will those be of the original vaccines or new ones that incorporate the variants?
As we go forward, there’s still potential for new variants that we haven’t seen yet that combine the worst of all features – transmissibility and immune evasion – especially since we have a world where COVID-19 is unchecked. So, we’re not out of it yet, but at least for the moment, we have vaccines that are capable of protecting against all variants.
In most people, the immune response against SARS-CoV-2 is very durable and strong and may well last for years. With the most closely related SARS-CoV-1, people still had immune responses up to 18 years later. However, some people will have less robust vaccine responses, including the elderly and the immunocompromised. If they don’t have great responses to the vaccine to start with, over time they’re likely to become more vulnerable, especially if they’re exposed to the variants with some degree of immune evasion.
I think we need to study these individuals post vaccination. A lot of people fit into those categories, including seniors, people being treated for cancer or autoimmune conditions, or post organ transplant. We could set up a prospective study to see whether they develop symptomatic COVID-19 and if so, from what – the original strain, B.1.1.7, or the newer variants.
That’s where I think booster shots may be needed. They may not be necessary across the board, but perhaps just in these special subgroups.
All of the current vaccines can be tweaked to include new variants, but the need for that is uncertain as of now. Moderna is working on a so-called bivalent vaccine that includes the original SARS-CoV-2 strain plus the B.1.351 variant, but it isn’t clear that that’s going to be necessary.
Currently, at least 200 COVID-19 vaccines are in development. There will be vaccines you can inhale, room temperature mRNA vaccines, and potentially even oral vaccines.
In the near future, Novavax is close, and there will likely be a two-dose Johnson & Johnson version that has the same potency as the mRNA vaccines. There are a lot of moving parts here.
There may be a step down in efficacy from mRNA to the others, though, and that shouldn’t be discounted. All of the available vaccines so far protect very well against severe disease and death, but some are less effective against mild to moderate infections, which may then lead to long COVID. We don’t yet know whether those who get mild infection post vaccination can still get long COVID.
What do you think it will take to achieve herd immunity?
I prefer the term “containment.” It’s quantitative. If you get to an infection rate of less than 1 in 100,000 people, as they’ve done in Israel, with 0.8 per 100,000, then you have the virus in check, and there will be very little spread when it’s at that controlled rate, with no outbreaks. The United States is currently at about 15 per 100,000. California is at 4. That still has to get lower.
It will be a challenge to get to President Biden’s goal of having 70% of U.S. adults given at least one dose by July 4. We’re now at about 57%. To get that next 13% of adults is going to take an all-out effort: mobile units, going to homes, making it ultraconvenient, education for people with safety concerns, incentivization, and days off.
We also need to get employers, universities, and health systems to get to the mandatory level. We haven’t done that yet. Some universities have mandated it for students, faculty, and staff. We need it in more health care systems. Right now, we only have a couple. We mandate flu shots, and flu is nothing, compared with COVID-19. And the COVID-19 vaccine is far more efficacious – flu shots are 40% efficacious, while these are 95%. COVID-19 is a tenfold more lethal and serious disease, and much more spreadable.
People are using the lack of full licensure by the Food and Drug Administration – as opposed to emergency use authorization – as an excuse not to get vaccinated. A biologics license application takes time to approve. Meanwhile, we have hundreds of millions of doses that have been well tolerated and incredibly effective.
Another aspect to consider regarding containment is that about 110 million Americans have already had COVID-19, even though only about 30 million cases have been confirmed. Most of these people have immune protection, although it’s not as good as if they have one vaccine dose. But they have enough protection to be part of the story here of the wall against COVID-19 and will help us get through this.
That’s a silver lining of having an unchecked epidemic for the entire year of 2020. The good part is that’s helping to get us to achieve an incredible level of containment when we haven’t even been close. Right now, we’re as good as the country has been in the pandemic, but we still have a long gap to get down to that 1 per 100,000. That’s what we should be working toward, and we can get there.
A version of this article first appeared on Medscape.com.
Editor in Chief of this news organization Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and professor of molecular medicine, has been closely following COVID-19 data since the pandemic began. He spoke with writer Miriam E. Tucker about the latest on SARS-CoV-2 variants and their impact on vaccine efficacy. The conversation serves as a follow-up to his April 13, 2021, New York Times opinion piece, in which he advised readers that “all variants are innocent until proven guilty.”
You have expressed overall confidence in the efficacy of the vaccines thus far despite the emergence of variants, with some caveats. How do you see the current situation?
The Centers for Disease Control and Prevention has designated five “variants of concern,” but only three of them are real concerns – B.1.1.7, first detected in the United Kingdom; P.1, in Brazil and Japan; and B.1.351, in South Africa. Yet, all three are susceptible to our current vaccines.
The U.K. B.1.1.7 is the worst variant of all because it’s hypertransmissible, so I call it a “superspreader strain.” It also causes more severe illness independent of the spread, so it’s a double whammy. It’s clear that it also causes more deaths. The only arguable point is whether it’s 30% or 50% more deaths, but regardless, it’s more lethal and more transmissible.
The B.1.1.7 is going to be the dominant strain worldwide. It could develop new mutations within it that could come back to haunt us. We must keep watch.
But for now, it’s fully responsive to all the vaccines, which is great because if we didn’t have them, we wouldn’t have gotten through this U.S. pandemic like we have, and neither would Israel and the United Kingdom and other countries that have been able to get out of the crisis. We met the enemy and put it in check.
As for the South Africa variant of concern, B.1.351, we just got some encouraging news showing that it›s very responsive to the Pfizer/BioNTech mRNA vaccine in large numbers of people. The study was conducted in Qatar following that country’s mass immunization campaign in which a total of 385,853 people had received at least one vaccine dose and 265,410 had completed the two doses as of March 31, 2021.
At 2 weeks past the second dose, the vaccine was 75% effective at preventing any documented infection with the B.1.351 variant and 89.5% effective against B.1.1.7. The vaccine’s effectiveness against severe, critical, or fatal COVID-19 was greater than 97.4% for all circulating strains in Qatar, where B.1.1.7 and B.1.351 are most prominent.
We also know that B.1.351 is very responsive to the Johnson & Johnson vaccine and the Novavax [vaccine in development] to a lesser degree. It is the most immune-evading variant we’ve seen thus far, with the highest likelihood of providing some vaccine resistance, yet not enough to interfere with vaccination campaigns. So that’s great news.
The caveats here are that you definitely need two doses of the mRNA vaccines to combat the B.1.351 variant. Also, the AstraZeneca vaccine failed to prevent it in South Africa. However, that study was hard to judge because it was underpowered for number of people with mild infections. So, it didn’t look as if it had any efficacy, but maybe it would if tested in a real trial.
The P.1 (Brazil) variant is the second-highest concern after B.1.1.7 because it’s the only one in the United States that’s still headed up. It seems to be competing a bit with B.1.1.7 here. We know it was associated with the crisis in Brazil, in Chile, and some other South American countries. It has some immune escape, but not as bad as B.1.351. It also appears to have somewhat greater transmissibility but not as much as B.1.1.7.
With P.1, we just don’t know enough yet. It was difficult to assess in Brazil because they were in the midst of a catastrophe – like India is now – and you don’t know how much of it is dragged by the catastrophe vs driving it.
We have to respond to P.1 carefully. There are some good data that it does respond to the Chinese vaccine Sinovac and the AstraZeneca vaccine, and it appears to respond to the others as well, based on serum studies. So it doesn’t look like vaccines will be the worry with this variant. Rather, it could be competing with B.1.1.7 and could lead to breakthrough infections in vaccinated people or reinfections in unvaccinated people who had COVID-19. We need several more weeks to sort it out.
Although the B.1.427 and B.1.429 variants initially seen in California remain on the CDC’s concern list, I’m not worried about them.
You mentioned the current COVID-19 crisis in India, where a new variant has been described as a “double mutant,” but on Twitter you called it a “scariant.” Why?
First of all, the B.1.617 variant isn’t a double mutant. It has 15 mutations. It’s a stupid term, focusing on two mutations which largely have been put aside as to concern. One of them is the L452R, which is the same as one of the California variants, and that hasn’t proved to be particularly serious or concerning. The other is the 484Q, and it’s not clear whether that has any function.
The B.1.617 is not the driver of the catastrophe in India. It may be contributing a small amount, but it has been overhyped as the double mutant that’s causing it all. Adding to that are what I call “scariant” headlines here in the United States when a few cases of that variant have been seen.
I coined the term scariant in early February because it was a pretty clear trend. People don’t know what variants are. They know a little bit about mutations but not variants, and they’re scared. A few variants are concerning, but we keep learning more and more things to decrease the concern. That’s why I wrote the New York Times op-ed, to try to provide some reassurance, since there’s such paranoia.
Do you think booster vaccinations will be necessary? If so, will those be of the original vaccines or new ones that incorporate the variants?
As we go forward, there’s still potential for new variants that we haven’t seen yet that combine the worst of all features – transmissibility and immune evasion – especially since we have a world where COVID-19 is unchecked. So, we’re not out of it yet, but at least for the moment, we have vaccines that are capable of protecting against all variants.
In most people, the immune response against SARS-CoV-2 is very durable and strong and may well last for years. With the most closely related SARS-CoV-1, people still had immune responses up to 18 years later. However, some people will have less robust vaccine responses, including the elderly and the immunocompromised. If they don’t have great responses to the vaccine to start with, over time they’re likely to become more vulnerable, especially if they’re exposed to the variants with some degree of immune evasion.
I think we need to study these individuals post vaccination. A lot of people fit into those categories, including seniors, people being treated for cancer or autoimmune conditions, or post organ transplant. We could set up a prospective study to see whether they develop symptomatic COVID-19 and if so, from what – the original strain, B.1.1.7, or the newer variants.
That’s where I think booster shots may be needed. They may not be necessary across the board, but perhaps just in these special subgroups.
All of the current vaccines can be tweaked to include new variants, but the need for that is uncertain as of now. Moderna is working on a so-called bivalent vaccine that includes the original SARS-CoV-2 strain plus the B.1.351 variant, but it isn’t clear that that’s going to be necessary.
Currently, at least 200 COVID-19 vaccines are in development. There will be vaccines you can inhale, room temperature mRNA vaccines, and potentially even oral vaccines.
In the near future, Novavax is close, and there will likely be a two-dose Johnson & Johnson version that has the same potency as the mRNA vaccines. There are a lot of moving parts here.
There may be a step down in efficacy from mRNA to the others, though, and that shouldn’t be discounted. All of the available vaccines so far protect very well against severe disease and death, but some are less effective against mild to moderate infections, which may then lead to long COVID. We don’t yet know whether those who get mild infection post vaccination can still get long COVID.
What do you think it will take to achieve herd immunity?
I prefer the term “containment.” It’s quantitative. If you get to an infection rate of less than 1 in 100,000 people, as they’ve done in Israel, with 0.8 per 100,000, then you have the virus in check, and there will be very little spread when it’s at that controlled rate, with no outbreaks. The United States is currently at about 15 per 100,000. California is at 4. That still has to get lower.
It will be a challenge to get to President Biden’s goal of having 70% of U.S. adults given at least one dose by July 4. We’re now at about 57%. To get that next 13% of adults is going to take an all-out effort: mobile units, going to homes, making it ultraconvenient, education for people with safety concerns, incentivization, and days off.
We also need to get employers, universities, and health systems to get to the mandatory level. We haven’t done that yet. Some universities have mandated it for students, faculty, and staff. We need it in more health care systems. Right now, we only have a couple. We mandate flu shots, and flu is nothing, compared with COVID-19. And the COVID-19 vaccine is far more efficacious – flu shots are 40% efficacious, while these are 95%. COVID-19 is a tenfold more lethal and serious disease, and much more spreadable.
People are using the lack of full licensure by the Food and Drug Administration – as opposed to emergency use authorization – as an excuse not to get vaccinated. A biologics license application takes time to approve. Meanwhile, we have hundreds of millions of doses that have been well tolerated and incredibly effective.
Another aspect to consider regarding containment is that about 110 million Americans have already had COVID-19, even though only about 30 million cases have been confirmed. Most of these people have immune protection, although it’s not as good as if they have one vaccine dose. But they have enough protection to be part of the story here of the wall against COVID-19 and will help us get through this.
That’s a silver lining of having an unchecked epidemic for the entire year of 2020. The good part is that’s helping to get us to achieve an incredible level of containment when we haven’t even been close. Right now, we’re as good as the country has been in the pandemic, but we still have a long gap to get down to that 1 per 100,000. That’s what we should be working toward, and we can get there.
A version of this article first appeared on Medscape.com.
Editor in Chief of this news organization Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and professor of molecular medicine, has been closely following COVID-19 data since the pandemic began. He spoke with writer Miriam E. Tucker about the latest on SARS-CoV-2 variants and their impact on vaccine efficacy. The conversation serves as a follow-up to his April 13, 2021, New York Times opinion piece, in which he advised readers that “all variants are innocent until proven guilty.”
You have expressed overall confidence in the efficacy of the vaccines thus far despite the emergence of variants, with some caveats. How do you see the current situation?
The Centers for Disease Control and Prevention has designated five “variants of concern,” but only three of them are real concerns – B.1.1.7, first detected in the United Kingdom; P.1, in Brazil and Japan; and B.1.351, in South Africa. Yet, all three are susceptible to our current vaccines.
The U.K. B.1.1.7 is the worst variant of all because it’s hypertransmissible, so I call it a “superspreader strain.” It also causes more severe illness independent of the spread, so it’s a double whammy. It’s clear that it also causes more deaths. The only arguable point is whether it’s 30% or 50% more deaths, but regardless, it’s more lethal and more transmissible.
The B.1.1.7 is going to be the dominant strain worldwide. It could develop new mutations within it that could come back to haunt us. We must keep watch.
But for now, it’s fully responsive to all the vaccines, which is great because if we didn’t have them, we wouldn’t have gotten through this U.S. pandemic like we have, and neither would Israel and the United Kingdom and other countries that have been able to get out of the crisis. We met the enemy and put it in check.
As for the South Africa variant of concern, B.1.351, we just got some encouraging news showing that it›s very responsive to the Pfizer/BioNTech mRNA vaccine in large numbers of people. The study was conducted in Qatar following that country’s mass immunization campaign in which a total of 385,853 people had received at least one vaccine dose and 265,410 had completed the two doses as of March 31, 2021.
At 2 weeks past the second dose, the vaccine was 75% effective at preventing any documented infection with the B.1.351 variant and 89.5% effective against B.1.1.7. The vaccine’s effectiveness against severe, critical, or fatal COVID-19 was greater than 97.4% for all circulating strains in Qatar, where B.1.1.7 and B.1.351 are most prominent.
We also know that B.1.351 is very responsive to the Johnson & Johnson vaccine and the Novavax [vaccine in development] to a lesser degree. It is the most immune-evading variant we’ve seen thus far, with the highest likelihood of providing some vaccine resistance, yet not enough to interfere with vaccination campaigns. So that’s great news.
The caveats here are that you definitely need two doses of the mRNA vaccines to combat the B.1.351 variant. Also, the AstraZeneca vaccine failed to prevent it in South Africa. However, that study was hard to judge because it was underpowered for number of people with mild infections. So, it didn’t look as if it had any efficacy, but maybe it would if tested in a real trial.
The P.1 (Brazil) variant is the second-highest concern after B.1.1.7 because it’s the only one in the United States that’s still headed up. It seems to be competing a bit with B.1.1.7 here. We know it was associated with the crisis in Brazil, in Chile, and some other South American countries. It has some immune escape, but not as bad as B.1.351. It also appears to have somewhat greater transmissibility but not as much as B.1.1.7.
With P.1, we just don’t know enough yet. It was difficult to assess in Brazil because they were in the midst of a catastrophe – like India is now – and you don’t know how much of it is dragged by the catastrophe vs driving it.
We have to respond to P.1 carefully. There are some good data that it does respond to the Chinese vaccine Sinovac and the AstraZeneca vaccine, and it appears to respond to the others as well, based on serum studies. So it doesn’t look like vaccines will be the worry with this variant. Rather, it could be competing with B.1.1.7 and could lead to breakthrough infections in vaccinated people or reinfections in unvaccinated people who had COVID-19. We need several more weeks to sort it out.
Although the B.1.427 and B.1.429 variants initially seen in California remain on the CDC’s concern list, I’m not worried about them.
You mentioned the current COVID-19 crisis in India, where a new variant has been described as a “double mutant,” but on Twitter you called it a “scariant.” Why?
First of all, the B.1.617 variant isn’t a double mutant. It has 15 mutations. It’s a stupid term, focusing on two mutations which largely have been put aside as to concern. One of them is the L452R, which is the same as one of the California variants, and that hasn’t proved to be particularly serious or concerning. The other is the 484Q, and it’s not clear whether that has any function.
The B.1.617 is not the driver of the catastrophe in India. It may be contributing a small amount, but it has been overhyped as the double mutant that’s causing it all. Adding to that are what I call “scariant” headlines here in the United States when a few cases of that variant have been seen.
I coined the term scariant in early February because it was a pretty clear trend. People don’t know what variants are. They know a little bit about mutations but not variants, and they’re scared. A few variants are concerning, but we keep learning more and more things to decrease the concern. That’s why I wrote the New York Times op-ed, to try to provide some reassurance, since there’s such paranoia.
Do you think booster vaccinations will be necessary? If so, will those be of the original vaccines or new ones that incorporate the variants?
As we go forward, there’s still potential for new variants that we haven’t seen yet that combine the worst of all features – transmissibility and immune evasion – especially since we have a world where COVID-19 is unchecked. So, we’re not out of it yet, but at least for the moment, we have vaccines that are capable of protecting against all variants.
In most people, the immune response against SARS-CoV-2 is very durable and strong and may well last for years. With the most closely related SARS-CoV-1, people still had immune responses up to 18 years later. However, some people will have less robust vaccine responses, including the elderly and the immunocompromised. If they don’t have great responses to the vaccine to start with, over time they’re likely to become more vulnerable, especially if they’re exposed to the variants with some degree of immune evasion.
I think we need to study these individuals post vaccination. A lot of people fit into those categories, including seniors, people being treated for cancer or autoimmune conditions, or post organ transplant. We could set up a prospective study to see whether they develop symptomatic COVID-19 and if so, from what – the original strain, B.1.1.7, or the newer variants.
That’s where I think booster shots may be needed. They may not be necessary across the board, but perhaps just in these special subgroups.
All of the current vaccines can be tweaked to include new variants, but the need for that is uncertain as of now. Moderna is working on a so-called bivalent vaccine that includes the original SARS-CoV-2 strain plus the B.1.351 variant, but it isn’t clear that that’s going to be necessary.
Currently, at least 200 COVID-19 vaccines are in development. There will be vaccines you can inhale, room temperature mRNA vaccines, and potentially even oral vaccines.
In the near future, Novavax is close, and there will likely be a two-dose Johnson & Johnson version that has the same potency as the mRNA vaccines. There are a lot of moving parts here.
There may be a step down in efficacy from mRNA to the others, though, and that shouldn’t be discounted. All of the available vaccines so far protect very well against severe disease and death, but some are less effective against mild to moderate infections, which may then lead to long COVID. We don’t yet know whether those who get mild infection post vaccination can still get long COVID.
What do you think it will take to achieve herd immunity?
I prefer the term “containment.” It’s quantitative. If you get to an infection rate of less than 1 in 100,000 people, as they’ve done in Israel, with 0.8 per 100,000, then you have the virus in check, and there will be very little spread when it’s at that controlled rate, with no outbreaks. The United States is currently at about 15 per 100,000. California is at 4. That still has to get lower.
It will be a challenge to get to President Biden’s goal of having 70% of U.S. adults given at least one dose by July 4. We’re now at about 57%. To get that next 13% of adults is going to take an all-out effort: mobile units, going to homes, making it ultraconvenient, education for people with safety concerns, incentivization, and days off.
We also need to get employers, universities, and health systems to get to the mandatory level. We haven’t done that yet. Some universities have mandated it for students, faculty, and staff. We need it in more health care systems. Right now, we only have a couple. We mandate flu shots, and flu is nothing, compared with COVID-19. And the COVID-19 vaccine is far more efficacious – flu shots are 40% efficacious, while these are 95%. COVID-19 is a tenfold more lethal and serious disease, and much more spreadable.
People are using the lack of full licensure by the Food and Drug Administration – as opposed to emergency use authorization – as an excuse not to get vaccinated. A biologics license application takes time to approve. Meanwhile, we have hundreds of millions of doses that have been well tolerated and incredibly effective.
Another aspect to consider regarding containment is that about 110 million Americans have already had COVID-19, even though only about 30 million cases have been confirmed. Most of these people have immune protection, although it’s not as good as if they have one vaccine dose. But they have enough protection to be part of the story here of the wall against COVID-19 and will help us get through this.
That’s a silver lining of having an unchecked epidemic for the entire year of 2020. The good part is that’s helping to get us to achieve an incredible level of containment when we haven’t even been close. Right now, we’re as good as the country has been in the pandemic, but we still have a long gap to get down to that 1 per 100,000. That’s what we should be working toward, and we can get there.
A version of this article first appeared on Medscape.com.
Trial: Fecal transplantation safe but ineffective in PsA
The first clinical trial of fecal microbiota transplantation in patients with psoriatic arthritis has found the procedure to be as safe as a sham procedure, but it didn’t show any effectiveness in decreasing PsA symptoms over 6 months, a team of researchers in Denmark reported in Annals of the Rheumatic Diseases (2021 Apr 29. 10.1136/annrheumdis-2020-219511).
Nonetheless, the investigators said the trial indicates fecal microbiota transplantation (FMT) is worthy of further study.
“Overall, we think that the results are very interesting and that the feasibility and safety aspects as well as the clinical results of the trial may encourage more research into the potential of FMT in the treatment of inflammatory arthritis and may help guide the direction of future trials within the field,” lead author Maja S. Kragsnaes, MD, PhD, and principal investigator Torkell Ellingsen, MD, PhD, of Odense (Denmark) University Hospital said together in an interview.
“The most important findings from this trial is that FMT appears to be safe in patients with PsA and that the patients find the treatment acceptable, and it supports future research into the therapeutic potential of FMT in PsA,” they said.
The study evaluated 6-month outcomes of 31 patients randomized to the FMT and sham groups. FMT patients were three times more likely to experience treatment failure – defined by the need for treatment intensification – with failure rates of 60% versus 20% in the sham group.
As a secondary endpoint, the study used 6-month change in the Health Assessment Questionnaire Disability Index (HAQ-DI) and 20% improvement in American College of Rheumatology criteria (ACR20). The sham group demonstrated a greater decrease in HAQ-DI, indicating better physical function (–0.30 vs. –0.07; P = .031). The proportion of ACR20 responders was similar between both groups: 47% for the FMT patients (7 of 15) and 53% for sham (8 of 15).
The study included adults aged 18-75 years with active peripheral disease, defined as three or more swollen joints, who’d been taking at least15 mg methotrexate a week for at least 3 months before enrolling in the study, with a washout period of 12 weeks (26 weeks for those on biologic agents). Four healthy donors provided the stool transplants.
In the study, Dr. Kragsnaes and Dr. Ellingsen acknowledged that FMT has been shown to be safe for Clostridioides difficile infection or inflammatory bowel disease when “thoroughly screened stool” is used. “Hence,” they wrote, “our findings add to the growing body of evidence suggesting a gut-joint axis in the pathogenesis of PsA.”
Factors that may influence the effectiveness of FMT in PsA merit further investigation, Dr. Kragsnaes and Dr. Ellingsen said. “From FMT trials in patients with active ulcerative colitis, higher dose and repeated administration appear to be effective and safe in inducing remission,” they said in their joint statement, pointing to research from China.
“Moreover,” they added, “successes of FMT in inflammatory bowel disease appear to have been driven by ‘superdonors’ characterized by the presence or absence of specific bacteria species.”
They said will continue to investigate the effectiveness of FMT in immune-mediated diseases, including how to characterize superdonors.
“We will conduct new randomized trials using different FMT strategies – by changing the type of administration form, dose, and treatment frequency – to explore whether microbial dysbiosis or specific bacteria are common or decisive mediators of disease activity in inflammatory diseases and whether this proposed relation can be modified without exacerbating the disease,” Dr. Kragsnaes and Dr. Ellingsen said.
Dr. Kragsnaes and Dr. Ellingsen had no relevant financial relationships to disclose.
The first clinical trial of fecal microbiota transplantation in patients with psoriatic arthritis has found the procedure to be as safe as a sham procedure, but it didn’t show any effectiveness in decreasing PsA symptoms over 6 months, a team of researchers in Denmark reported in Annals of the Rheumatic Diseases (2021 Apr 29. 10.1136/annrheumdis-2020-219511).
Nonetheless, the investigators said the trial indicates fecal microbiota transplantation (FMT) is worthy of further study.
“Overall, we think that the results are very interesting and that the feasibility and safety aspects as well as the clinical results of the trial may encourage more research into the potential of FMT in the treatment of inflammatory arthritis and may help guide the direction of future trials within the field,” lead author Maja S. Kragsnaes, MD, PhD, and principal investigator Torkell Ellingsen, MD, PhD, of Odense (Denmark) University Hospital said together in an interview.
“The most important findings from this trial is that FMT appears to be safe in patients with PsA and that the patients find the treatment acceptable, and it supports future research into the therapeutic potential of FMT in PsA,” they said.
The study evaluated 6-month outcomes of 31 patients randomized to the FMT and sham groups. FMT patients were three times more likely to experience treatment failure – defined by the need for treatment intensification – with failure rates of 60% versus 20% in the sham group.
As a secondary endpoint, the study used 6-month change in the Health Assessment Questionnaire Disability Index (HAQ-DI) and 20% improvement in American College of Rheumatology criteria (ACR20). The sham group demonstrated a greater decrease in HAQ-DI, indicating better physical function (–0.30 vs. –0.07; P = .031). The proportion of ACR20 responders was similar between both groups: 47% for the FMT patients (7 of 15) and 53% for sham (8 of 15).
The study included adults aged 18-75 years with active peripheral disease, defined as three or more swollen joints, who’d been taking at least15 mg methotrexate a week for at least 3 months before enrolling in the study, with a washout period of 12 weeks (26 weeks for those on biologic agents). Four healthy donors provided the stool transplants.
In the study, Dr. Kragsnaes and Dr. Ellingsen acknowledged that FMT has been shown to be safe for Clostridioides difficile infection or inflammatory bowel disease when “thoroughly screened stool” is used. “Hence,” they wrote, “our findings add to the growing body of evidence suggesting a gut-joint axis in the pathogenesis of PsA.”
Factors that may influence the effectiveness of FMT in PsA merit further investigation, Dr. Kragsnaes and Dr. Ellingsen said. “From FMT trials in patients with active ulcerative colitis, higher dose and repeated administration appear to be effective and safe in inducing remission,” they said in their joint statement, pointing to research from China.
“Moreover,” they added, “successes of FMT in inflammatory bowel disease appear to have been driven by ‘superdonors’ characterized by the presence or absence of specific bacteria species.”
They said will continue to investigate the effectiveness of FMT in immune-mediated diseases, including how to characterize superdonors.
“We will conduct new randomized trials using different FMT strategies – by changing the type of administration form, dose, and treatment frequency – to explore whether microbial dysbiosis or specific bacteria are common or decisive mediators of disease activity in inflammatory diseases and whether this proposed relation can be modified without exacerbating the disease,” Dr. Kragsnaes and Dr. Ellingsen said.
Dr. Kragsnaes and Dr. Ellingsen had no relevant financial relationships to disclose.
The first clinical trial of fecal microbiota transplantation in patients with psoriatic arthritis has found the procedure to be as safe as a sham procedure, but it didn’t show any effectiveness in decreasing PsA symptoms over 6 months, a team of researchers in Denmark reported in Annals of the Rheumatic Diseases (2021 Apr 29. 10.1136/annrheumdis-2020-219511).
Nonetheless, the investigators said the trial indicates fecal microbiota transplantation (FMT) is worthy of further study.
“Overall, we think that the results are very interesting and that the feasibility and safety aspects as well as the clinical results of the trial may encourage more research into the potential of FMT in the treatment of inflammatory arthritis and may help guide the direction of future trials within the field,” lead author Maja S. Kragsnaes, MD, PhD, and principal investigator Torkell Ellingsen, MD, PhD, of Odense (Denmark) University Hospital said together in an interview.
“The most important findings from this trial is that FMT appears to be safe in patients with PsA and that the patients find the treatment acceptable, and it supports future research into the therapeutic potential of FMT in PsA,” they said.
The study evaluated 6-month outcomes of 31 patients randomized to the FMT and sham groups. FMT patients were three times more likely to experience treatment failure – defined by the need for treatment intensification – with failure rates of 60% versus 20% in the sham group.
As a secondary endpoint, the study used 6-month change in the Health Assessment Questionnaire Disability Index (HAQ-DI) and 20% improvement in American College of Rheumatology criteria (ACR20). The sham group demonstrated a greater decrease in HAQ-DI, indicating better physical function (–0.30 vs. –0.07; P = .031). The proportion of ACR20 responders was similar between both groups: 47% for the FMT patients (7 of 15) and 53% for sham (8 of 15).
The study included adults aged 18-75 years with active peripheral disease, defined as three or more swollen joints, who’d been taking at least15 mg methotrexate a week for at least 3 months before enrolling in the study, with a washout period of 12 weeks (26 weeks for those on biologic agents). Four healthy donors provided the stool transplants.
In the study, Dr. Kragsnaes and Dr. Ellingsen acknowledged that FMT has been shown to be safe for Clostridioides difficile infection or inflammatory bowel disease when “thoroughly screened stool” is used. “Hence,” they wrote, “our findings add to the growing body of evidence suggesting a gut-joint axis in the pathogenesis of PsA.”
Factors that may influence the effectiveness of FMT in PsA merit further investigation, Dr. Kragsnaes and Dr. Ellingsen said. “From FMT trials in patients with active ulcerative colitis, higher dose and repeated administration appear to be effective and safe in inducing remission,” they said in their joint statement, pointing to research from China.
“Moreover,” they added, “successes of FMT in inflammatory bowel disease appear to have been driven by ‘superdonors’ characterized by the presence or absence of specific bacteria species.”
They said will continue to investigate the effectiveness of FMT in immune-mediated diseases, including how to characterize superdonors.
“We will conduct new randomized trials using different FMT strategies – by changing the type of administration form, dose, and treatment frequency – to explore whether microbial dysbiosis or specific bacteria are common or decisive mediators of disease activity in inflammatory diseases and whether this proposed relation can be modified without exacerbating the disease,” Dr. Kragsnaes and Dr. Ellingsen said.
Dr. Kragsnaes and Dr. Ellingsen had no relevant financial relationships to disclose.
FROM ANNALS OF THE RHEUMATIC DISEASES
Genital skin exams in girls: Conduct with care, look for signs of abuse
at the American Academy of Dermatology Virtual Meeting Experience.
“One in four adult women report being childhood victims of sexual abuse, which is just a staggering number. This is an opportunity for us to identify these patients early and give them the terminology to be able to report what is happening to them,” said pediatric dermatologist Kalyani Marathe, MD, MPH, director of the division of dermatology at Cincinnati Children’s Hospital. “We also have the chance to give them a sense of agency over their bodies.”
Dr. Marathe offered the following recommendations when performing a genital skin exam:
- Make sure a “chaperone” is present. “Chaperones are a must when you’re examining children and teens,” she said. “Ask whom they prefer. For prepubertal children, you’re going to usually use the parent who’s there with them. If the parent is their father, they might ask him to step behind the curtain, in which case you can bring over your nurse or medical assistant.” Teens may ask either parent to step out of the room, she said. In that case, a nurse, medical assistant, resident, or trainee can fill in. “If you have male residents or trainees with you and the patient really does not want to be examined by a male, honor their request. Do not force them.”
- Explain why the exam is being performed. Make sure the patient understands why she is being seen, Dr. Marathe advised. For example, say something like “your pediatrician told us that you have an itchy area” or “your mom told us that there’s some loss of color in that area, that you’re having a problem there.” She added that it’s helpful to explain the type of doctor you are, with a comment such as the following: “We’re examining you because we’re doctors who specialize in skin. ... We want to help you feel better and make sure that your skin heals and is healthy.”
- Ask both the child and the parent for permission to perform the exam. While this may seem trivial, “it’s very, very important in setting the right tone for the encounter,” she said. “If the child says yes, we turn to the mom and say: ‘Mom, is it okay for us to do this exam today?’ You can see visible relief on the part of the parent, and as the parent relaxes, the child relaxes. Just saying those few things really makes the encounter so much smoother.” However, “if they say no, you have to honor the response. ... You say: ‘Okay, we’re not going to do the exam today,” and see the patient in a few weeks. If it’s urgent, an exam under anesthesia may be an option, she added.
- Talk to the child about the terms they use for private parts. It can be helpful to ask: “Do you have any terms for your private area?” According to Dr. Marathe, “this is a good chance to educate them on the terms vulva and vagina since they may be using other terminology. Making sure that they have the correct terms will actually help patients identify and report abuse earlier.” Dr. Marathe recalled that a colleague had a patient who’d been calling her private area “pound cake” and had been “reporting to her teacher that someone had been touching her ‘pound cake.’ Her teacher did not know what she meant by that, and this led to a great delay in her childhood abuse being reported.”
- Talk about what will happen during the exam. “I like to show them any instruments that we’re going to be using,” Dr. Marathe said. “If we’re using a flashlight, for example, I like to show them a picture [of a flashlight] or show them that flashlight. If we’re using a camera to do digital photography, show them that. If we’re going to be using a Q-tip or a swab to demonstrate anything or to take a culture, I like to show them that beforehand to make sure that they know what we’re doing.” In regard to photography, “make sure the parent and child know where the photos are going to go, who’s going to see them, what are they going to be used for. If they’re going to be used for educational purposes, make sure they have given explicit permission for that and they know they’ll be deidentified.”
- Make it clear that the exam won’t be painful. It’s important to put both the patient and the parent at ease on this front, Dr. Marathe said. “A lot of parents are concerned that we’re going to do a speculum exam in their prepubertal child. So make sure that it’s clarified ahead of time that we’re not going to be doing a speculum exam.”
Commenting on this topic, Tor Shwayder, MD, a pediatric dermatologist at Henry Ford Health System, Detroit, urged colleagues to take action if they feel suspicious about a possible sign of child abuse, even if they’re far from certain that anything is wrong. “Don’t ignore those feelings in the back of the brain,” he said in an interview.
Most states have child-abuse hotlines for medical professionals, and major hospitals will have child-abuse teams, Dr. Shwayder said. He urged dermatologists to take advantage of these resources when appropriate. “The professionals on the other side of the 800 number or at the hospital will help you. You don’t have to decide immediately whether this is child abuse. You just need to have a suspicion.”
Dr. Marathe and Dr. Shwayder report no disclosures.
at the American Academy of Dermatology Virtual Meeting Experience.
“One in four adult women report being childhood victims of sexual abuse, which is just a staggering number. This is an opportunity for us to identify these patients early and give them the terminology to be able to report what is happening to them,” said pediatric dermatologist Kalyani Marathe, MD, MPH, director of the division of dermatology at Cincinnati Children’s Hospital. “We also have the chance to give them a sense of agency over their bodies.”
Dr. Marathe offered the following recommendations when performing a genital skin exam:
- Make sure a “chaperone” is present. “Chaperones are a must when you’re examining children and teens,” she said. “Ask whom they prefer. For prepubertal children, you’re going to usually use the parent who’s there with them. If the parent is their father, they might ask him to step behind the curtain, in which case you can bring over your nurse or medical assistant.” Teens may ask either parent to step out of the room, she said. In that case, a nurse, medical assistant, resident, or trainee can fill in. “If you have male residents or trainees with you and the patient really does not want to be examined by a male, honor their request. Do not force them.”
- Explain why the exam is being performed. Make sure the patient understands why she is being seen, Dr. Marathe advised. For example, say something like “your pediatrician told us that you have an itchy area” or “your mom told us that there’s some loss of color in that area, that you’re having a problem there.” She added that it’s helpful to explain the type of doctor you are, with a comment such as the following: “We’re examining you because we’re doctors who specialize in skin. ... We want to help you feel better and make sure that your skin heals and is healthy.”
- Ask both the child and the parent for permission to perform the exam. While this may seem trivial, “it’s very, very important in setting the right tone for the encounter,” she said. “If the child says yes, we turn to the mom and say: ‘Mom, is it okay for us to do this exam today?’ You can see visible relief on the part of the parent, and as the parent relaxes, the child relaxes. Just saying those few things really makes the encounter so much smoother.” However, “if they say no, you have to honor the response. ... You say: ‘Okay, we’re not going to do the exam today,” and see the patient in a few weeks. If it’s urgent, an exam under anesthesia may be an option, she added.
- Talk to the child about the terms they use for private parts. It can be helpful to ask: “Do you have any terms for your private area?” According to Dr. Marathe, “this is a good chance to educate them on the terms vulva and vagina since they may be using other terminology. Making sure that they have the correct terms will actually help patients identify and report abuse earlier.” Dr. Marathe recalled that a colleague had a patient who’d been calling her private area “pound cake” and had been “reporting to her teacher that someone had been touching her ‘pound cake.’ Her teacher did not know what she meant by that, and this led to a great delay in her childhood abuse being reported.”
- Talk about what will happen during the exam. “I like to show them any instruments that we’re going to be using,” Dr. Marathe said. “If we’re using a flashlight, for example, I like to show them a picture [of a flashlight] or show them that flashlight. If we’re using a camera to do digital photography, show them that. If we’re going to be using a Q-tip or a swab to demonstrate anything or to take a culture, I like to show them that beforehand to make sure that they know what we’re doing.” In regard to photography, “make sure the parent and child know where the photos are going to go, who’s going to see them, what are they going to be used for. If they’re going to be used for educational purposes, make sure they have given explicit permission for that and they know they’ll be deidentified.”
- Make it clear that the exam won’t be painful. It’s important to put both the patient and the parent at ease on this front, Dr. Marathe said. “A lot of parents are concerned that we’re going to do a speculum exam in their prepubertal child. So make sure that it’s clarified ahead of time that we’re not going to be doing a speculum exam.”
Commenting on this topic, Tor Shwayder, MD, a pediatric dermatologist at Henry Ford Health System, Detroit, urged colleagues to take action if they feel suspicious about a possible sign of child abuse, even if they’re far from certain that anything is wrong. “Don’t ignore those feelings in the back of the brain,” he said in an interview.
Most states have child-abuse hotlines for medical professionals, and major hospitals will have child-abuse teams, Dr. Shwayder said. He urged dermatologists to take advantage of these resources when appropriate. “The professionals on the other side of the 800 number or at the hospital will help you. You don’t have to decide immediately whether this is child abuse. You just need to have a suspicion.”
Dr. Marathe and Dr. Shwayder report no disclosures.
at the American Academy of Dermatology Virtual Meeting Experience.
“One in four adult women report being childhood victims of sexual abuse, which is just a staggering number. This is an opportunity for us to identify these patients early and give them the terminology to be able to report what is happening to them,” said pediatric dermatologist Kalyani Marathe, MD, MPH, director of the division of dermatology at Cincinnati Children’s Hospital. “We also have the chance to give them a sense of agency over their bodies.”
Dr. Marathe offered the following recommendations when performing a genital skin exam:
- Make sure a “chaperone” is present. “Chaperones are a must when you’re examining children and teens,” she said. “Ask whom they prefer. For prepubertal children, you’re going to usually use the parent who’s there with them. If the parent is their father, they might ask him to step behind the curtain, in which case you can bring over your nurse or medical assistant.” Teens may ask either parent to step out of the room, she said. In that case, a nurse, medical assistant, resident, or trainee can fill in. “If you have male residents or trainees with you and the patient really does not want to be examined by a male, honor their request. Do not force them.”
- Explain why the exam is being performed. Make sure the patient understands why she is being seen, Dr. Marathe advised. For example, say something like “your pediatrician told us that you have an itchy area” or “your mom told us that there’s some loss of color in that area, that you’re having a problem there.” She added that it’s helpful to explain the type of doctor you are, with a comment such as the following: “We’re examining you because we’re doctors who specialize in skin. ... We want to help you feel better and make sure that your skin heals and is healthy.”
- Ask both the child and the parent for permission to perform the exam. While this may seem trivial, “it’s very, very important in setting the right tone for the encounter,” she said. “If the child says yes, we turn to the mom and say: ‘Mom, is it okay for us to do this exam today?’ You can see visible relief on the part of the parent, and as the parent relaxes, the child relaxes. Just saying those few things really makes the encounter so much smoother.” However, “if they say no, you have to honor the response. ... You say: ‘Okay, we’re not going to do the exam today,” and see the patient in a few weeks. If it’s urgent, an exam under anesthesia may be an option, she added.
- Talk to the child about the terms they use for private parts. It can be helpful to ask: “Do you have any terms for your private area?” According to Dr. Marathe, “this is a good chance to educate them on the terms vulva and vagina since they may be using other terminology. Making sure that they have the correct terms will actually help patients identify and report abuse earlier.” Dr. Marathe recalled that a colleague had a patient who’d been calling her private area “pound cake” and had been “reporting to her teacher that someone had been touching her ‘pound cake.’ Her teacher did not know what she meant by that, and this led to a great delay in her childhood abuse being reported.”
- Talk about what will happen during the exam. “I like to show them any instruments that we’re going to be using,” Dr. Marathe said. “If we’re using a flashlight, for example, I like to show them a picture [of a flashlight] or show them that flashlight. If we’re using a camera to do digital photography, show them that. If we’re going to be using a Q-tip or a swab to demonstrate anything or to take a culture, I like to show them that beforehand to make sure that they know what we’re doing.” In regard to photography, “make sure the parent and child know where the photos are going to go, who’s going to see them, what are they going to be used for. If they’re going to be used for educational purposes, make sure they have given explicit permission for that and they know they’ll be deidentified.”
- Make it clear that the exam won’t be painful. It’s important to put both the patient and the parent at ease on this front, Dr. Marathe said. “A lot of parents are concerned that we’re going to do a speculum exam in their prepubertal child. So make sure that it’s clarified ahead of time that we’re not going to be doing a speculum exam.”
Commenting on this topic, Tor Shwayder, MD, a pediatric dermatologist at Henry Ford Health System, Detroit, urged colleagues to take action if they feel suspicious about a possible sign of child abuse, even if they’re far from certain that anything is wrong. “Don’t ignore those feelings in the back of the brain,” he said in an interview.
Most states have child-abuse hotlines for medical professionals, and major hospitals will have child-abuse teams, Dr. Shwayder said. He urged dermatologists to take advantage of these resources when appropriate. “The professionals on the other side of the 800 number or at the hospital will help you. You don’t have to decide immediately whether this is child abuse. You just need to have a suspicion.”
Dr. Marathe and Dr. Shwayder report no disclosures.
FROM AAD VMX 2021
AAD unveils new guidelines for actinic keratosis management
. They also conditionally recommend the use of photodynamic therapy (PDT) and diclofenac for the treatment of AK, both individually and as part of combination therapy regimens.
Those are two of 18 recommendations made by 14 members of the multidisciplinary work group that convened to assemble the AAD’s first-ever guidelines on the management of AKs, which were published online April 2 in the Journal of the American Academy of Dermatology. The group, cochaired by Daniel B. Eisen, MD, professor of clinical dermatology at the University of California, Davis, and Todd E. Schlesinger, MD, medical director of the Dermatology and Laser Center of Charleston, S.C., conducted a systematic review to address five clinical questions on the management of AKs in adults. The questions were: What are the efficacy, effectiveness, and adverse effects of surgical and chemical peel treatments for AK; of topically applied agents for AK; of energy devices and other miscellaneous treatments for AK; and of combination therapy for the treatment of AK? And what are the special considerations to be taken into account when treating AK in immunocompromised individuals?
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
“As a participant in the work group, I was impressed by the level of care and detail and the involvement of relevant stakeholders, including a patient advocate, as well as having the draft guidelines go out to the AAD membership at large, and evaluating every comment that came in,” Maryam Asgari, MD, MPH, professor of dermatology at Harvard University, Boston, said in an interview. “The academy sought stakeholder and leadership input in revising and revamping the guidelines. The AAD also made sure the work group had minimal conflicts of interest by requiring that the majority of experts convened did not have relevant financial conflicts of interest. That might not be the case in a publication such as a systematic review, where no threshold for financial conflict of interest for coauthorship is set.”
Of the 18 recommendations the work group made for patients with AKs, only four were ranked as “strong” based on the evidence reviewed, while the rest were ranked as “conditional.”
The strong recommendations include the use of UV protection, field treatment with 5-FU, field treatment with imiquimod, and the use of cryosurgery.
The first four conditional recommendations for patients with AKs include the use of diclofenac, treatment with cryosurgery over CO2 laser ablation, aminolevulinic acid (ALA)–red-light PDT, and 1- to 4-hour 5-ALA incubation time to enhance complete clearance with red-light PDT. The work group also conditionally recommends ALA-daylight PDT as less painful than but equally effective as ALA–red-light PDT.
In the clinical experience of Catherine M. DiGiorgio, MD, who was not involved in the guidelines, daylight PDT with ALA is a viable, cost-effective option. “Patients can come into the office, apply the ALA and then they go outside for 2 hours – not in direct sunlight but in a shady area,” Dr. DiGiorgio, a dermatologist who practices at the Boston Center for Facial Rejuvenation, said in an interview. “That’s a cost-effective treatment for patients who perhaps can’t afford some of the chemotherapy creams. I don’t think we’ve adopted ALA-daylight PDT here in the U.S. very much.”
The work group noted that topical 1% tirbanibulin ointment, a novel microtubule inhibitor, was approved for treatment of AKs on the face and scalp by the Food and Drug Administration after the guidelines had been put together.
Several trials of combination therapy were included in the review of evidence, prompting several recommendations. For example, the work group conditionally recommends combined 5-FU cream and cryosurgery over cryosurgery alone, based on moderate-quality evidence and conditionally recommends combined imiquimod and cryosurgery over cryosurgery alone based on low-quality evidence. In addition, the work group conditionally recommends against the use of 3% diclofenac in addition to cryosurgery, favoring cryosurgery alone based on low-quality evidence, and conditionally recommends against the use of imiquimod typically after ALA–blue-light PDT, based on moderate-quality data.
“The additional treatment with imiquimod was thought to add both expense and burden to the patient, which negates much of the perceived convenience of using PDT as a stand-alone treatment modality and which is not mitigated by the modest increase in lesion reduction,” the authors wrote.
The guidelines emphasize the importance of shared decision-making between patients and clinicians on the choice of therapy, a point that resonates with Dr. DiGiorgio. Success of a treatment can depend on whether a patient is willing to go through with it, she said. “Some patients don’t want to do a therapeutic topical like 5-FU. They prefer to come in and have cryotherapy done. Others prefer to not come in and have the cream at home and treat themselves.”
Assembling the guidelines exposed certain gaps in research, according to the work group. Of the 18 recommendations, seven were based on low-quality evidence, and there were not enough data to make guidelines for the treatment of AKs in immunocompromised individuals.
“I can’t tell you the number of times we in the committee sat back and said, ‘we need to have a randomized trial that looks at this, or compares this to that head on,’” Dr. Asgari said. Such limitations “give researchers direction for where the areas of study need to go to help us answer some of these management conundrums.”
She added that the new guidelines “give clinicians a leg to stand on” when an insurer pushes back on a recommended treatment for AK. “It gives you a way to have dialogue with insurers if you’re prescribing some of these treatments.”
The guidelines authors write that there is “strong theoretic rationale for the treatment of AK to prevent skin cancers” but acknowledge that only a few studies in the review “report the incidence of skin cancer as an outcome measure or have sufficient follow-up to viably measure carcinoma development.” In addition, “more long-term research is needed to validate our current understanding of skin cancer progression from AKs to keratinocyte carcinoma.”
Dr. DiGiorgio thinks about this differently. “I think treatment of AKs does prevent skin cancers,” she said. “We call them precancers as we’re treating our patients because we know a certain percentage of them can develop into skin cancers over time.”
The study was funded by internal funds from the AAD. Dr. Asgari disclosed that she serves as an investigator for Pfizer. Several of the other authors reported having financial disclosures.
Dr. DiGiorgio reported having no financial disclosures.
. They also conditionally recommend the use of photodynamic therapy (PDT) and diclofenac for the treatment of AK, both individually and as part of combination therapy regimens.
Those are two of 18 recommendations made by 14 members of the multidisciplinary work group that convened to assemble the AAD’s first-ever guidelines on the management of AKs, which were published online April 2 in the Journal of the American Academy of Dermatology. The group, cochaired by Daniel B. Eisen, MD, professor of clinical dermatology at the University of California, Davis, and Todd E. Schlesinger, MD, medical director of the Dermatology and Laser Center of Charleston, S.C., conducted a systematic review to address five clinical questions on the management of AKs in adults. The questions were: What are the efficacy, effectiveness, and adverse effects of surgical and chemical peel treatments for AK; of topically applied agents for AK; of energy devices and other miscellaneous treatments for AK; and of combination therapy for the treatment of AK? And what are the special considerations to be taken into account when treating AK in immunocompromised individuals?
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
“As a participant in the work group, I was impressed by the level of care and detail and the involvement of relevant stakeholders, including a patient advocate, as well as having the draft guidelines go out to the AAD membership at large, and evaluating every comment that came in,” Maryam Asgari, MD, MPH, professor of dermatology at Harvard University, Boston, said in an interview. “The academy sought stakeholder and leadership input in revising and revamping the guidelines. The AAD also made sure the work group had minimal conflicts of interest by requiring that the majority of experts convened did not have relevant financial conflicts of interest. That might not be the case in a publication such as a systematic review, where no threshold for financial conflict of interest for coauthorship is set.”
Of the 18 recommendations the work group made for patients with AKs, only four were ranked as “strong” based on the evidence reviewed, while the rest were ranked as “conditional.”
The strong recommendations include the use of UV protection, field treatment with 5-FU, field treatment with imiquimod, and the use of cryosurgery.
The first four conditional recommendations for patients with AKs include the use of diclofenac, treatment with cryosurgery over CO2 laser ablation, aminolevulinic acid (ALA)–red-light PDT, and 1- to 4-hour 5-ALA incubation time to enhance complete clearance with red-light PDT. The work group also conditionally recommends ALA-daylight PDT as less painful than but equally effective as ALA–red-light PDT.
In the clinical experience of Catherine M. DiGiorgio, MD, who was not involved in the guidelines, daylight PDT with ALA is a viable, cost-effective option. “Patients can come into the office, apply the ALA and then they go outside for 2 hours – not in direct sunlight but in a shady area,” Dr. DiGiorgio, a dermatologist who practices at the Boston Center for Facial Rejuvenation, said in an interview. “That’s a cost-effective treatment for patients who perhaps can’t afford some of the chemotherapy creams. I don’t think we’ve adopted ALA-daylight PDT here in the U.S. very much.”
The work group noted that topical 1% tirbanibulin ointment, a novel microtubule inhibitor, was approved for treatment of AKs on the face and scalp by the Food and Drug Administration after the guidelines had been put together.
Several trials of combination therapy were included in the review of evidence, prompting several recommendations. For example, the work group conditionally recommends combined 5-FU cream and cryosurgery over cryosurgery alone, based on moderate-quality evidence and conditionally recommends combined imiquimod and cryosurgery over cryosurgery alone based on low-quality evidence. In addition, the work group conditionally recommends against the use of 3% diclofenac in addition to cryosurgery, favoring cryosurgery alone based on low-quality evidence, and conditionally recommends against the use of imiquimod typically after ALA–blue-light PDT, based on moderate-quality data.
“The additional treatment with imiquimod was thought to add both expense and burden to the patient, which negates much of the perceived convenience of using PDT as a stand-alone treatment modality and which is not mitigated by the modest increase in lesion reduction,” the authors wrote.
The guidelines emphasize the importance of shared decision-making between patients and clinicians on the choice of therapy, a point that resonates with Dr. DiGiorgio. Success of a treatment can depend on whether a patient is willing to go through with it, she said. “Some patients don’t want to do a therapeutic topical like 5-FU. They prefer to come in and have cryotherapy done. Others prefer to not come in and have the cream at home and treat themselves.”
Assembling the guidelines exposed certain gaps in research, according to the work group. Of the 18 recommendations, seven were based on low-quality evidence, and there were not enough data to make guidelines for the treatment of AKs in immunocompromised individuals.
“I can’t tell you the number of times we in the committee sat back and said, ‘we need to have a randomized trial that looks at this, or compares this to that head on,’” Dr. Asgari said. Such limitations “give researchers direction for where the areas of study need to go to help us answer some of these management conundrums.”
She added that the new guidelines “give clinicians a leg to stand on” when an insurer pushes back on a recommended treatment for AK. “It gives you a way to have dialogue with insurers if you’re prescribing some of these treatments.”
The guidelines authors write that there is “strong theoretic rationale for the treatment of AK to prevent skin cancers” but acknowledge that only a few studies in the review “report the incidence of skin cancer as an outcome measure or have sufficient follow-up to viably measure carcinoma development.” In addition, “more long-term research is needed to validate our current understanding of skin cancer progression from AKs to keratinocyte carcinoma.”
Dr. DiGiorgio thinks about this differently. “I think treatment of AKs does prevent skin cancers,” she said. “We call them precancers as we’re treating our patients because we know a certain percentage of them can develop into skin cancers over time.”
The study was funded by internal funds from the AAD. Dr. Asgari disclosed that she serves as an investigator for Pfizer. Several of the other authors reported having financial disclosures.
Dr. DiGiorgio reported having no financial disclosures.
. They also conditionally recommend the use of photodynamic therapy (PDT) and diclofenac for the treatment of AK, both individually and as part of combination therapy regimens.
Those are two of 18 recommendations made by 14 members of the multidisciplinary work group that convened to assemble the AAD’s first-ever guidelines on the management of AKs, which were published online April 2 in the Journal of the American Academy of Dermatology. The group, cochaired by Daniel B. Eisen, MD, professor of clinical dermatology at the University of California, Davis, and Todd E. Schlesinger, MD, medical director of the Dermatology and Laser Center of Charleston, S.C., conducted a systematic review to address five clinical questions on the management of AKs in adults. The questions were: What are the efficacy, effectiveness, and adverse effects of surgical and chemical peel treatments for AK; of topically applied agents for AK; of energy devices and other miscellaneous treatments for AK; and of combination therapy for the treatment of AK? And what are the special considerations to be taken into account when treating AK in immunocompromised individuals?
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
“As a participant in the work group, I was impressed by the level of care and detail and the involvement of relevant stakeholders, including a patient advocate, as well as having the draft guidelines go out to the AAD membership at large, and evaluating every comment that came in,” Maryam Asgari, MD, MPH, professor of dermatology at Harvard University, Boston, said in an interview. “The academy sought stakeholder and leadership input in revising and revamping the guidelines. The AAD also made sure the work group had minimal conflicts of interest by requiring that the majority of experts convened did not have relevant financial conflicts of interest. That might not be the case in a publication such as a systematic review, where no threshold for financial conflict of interest for coauthorship is set.”
Of the 18 recommendations the work group made for patients with AKs, only four were ranked as “strong” based on the evidence reviewed, while the rest were ranked as “conditional.”
The strong recommendations include the use of UV protection, field treatment with 5-FU, field treatment with imiquimod, and the use of cryosurgery.
The first four conditional recommendations for patients with AKs include the use of diclofenac, treatment with cryosurgery over CO2 laser ablation, aminolevulinic acid (ALA)–red-light PDT, and 1- to 4-hour 5-ALA incubation time to enhance complete clearance with red-light PDT. The work group also conditionally recommends ALA-daylight PDT as less painful than but equally effective as ALA–red-light PDT.
In the clinical experience of Catherine M. DiGiorgio, MD, who was not involved in the guidelines, daylight PDT with ALA is a viable, cost-effective option. “Patients can come into the office, apply the ALA and then they go outside for 2 hours – not in direct sunlight but in a shady area,” Dr. DiGiorgio, a dermatologist who practices at the Boston Center for Facial Rejuvenation, said in an interview. “That’s a cost-effective treatment for patients who perhaps can’t afford some of the chemotherapy creams. I don’t think we’ve adopted ALA-daylight PDT here in the U.S. very much.”
The work group noted that topical 1% tirbanibulin ointment, a novel microtubule inhibitor, was approved for treatment of AKs on the face and scalp by the Food and Drug Administration after the guidelines had been put together.
Several trials of combination therapy were included in the review of evidence, prompting several recommendations. For example, the work group conditionally recommends combined 5-FU cream and cryosurgery over cryosurgery alone, based on moderate-quality evidence and conditionally recommends combined imiquimod and cryosurgery over cryosurgery alone based on low-quality evidence. In addition, the work group conditionally recommends against the use of 3% diclofenac in addition to cryosurgery, favoring cryosurgery alone based on low-quality evidence, and conditionally recommends against the use of imiquimod typically after ALA–blue-light PDT, based on moderate-quality data.
“The additional treatment with imiquimod was thought to add both expense and burden to the patient, which negates much of the perceived convenience of using PDT as a stand-alone treatment modality and which is not mitigated by the modest increase in lesion reduction,” the authors wrote.
The guidelines emphasize the importance of shared decision-making between patients and clinicians on the choice of therapy, a point that resonates with Dr. DiGiorgio. Success of a treatment can depend on whether a patient is willing to go through with it, she said. “Some patients don’t want to do a therapeutic topical like 5-FU. They prefer to come in and have cryotherapy done. Others prefer to not come in and have the cream at home and treat themselves.”
Assembling the guidelines exposed certain gaps in research, according to the work group. Of the 18 recommendations, seven were based on low-quality evidence, and there were not enough data to make guidelines for the treatment of AKs in immunocompromised individuals.
“I can’t tell you the number of times we in the committee sat back and said, ‘we need to have a randomized trial that looks at this, or compares this to that head on,’” Dr. Asgari said. Such limitations “give researchers direction for where the areas of study need to go to help us answer some of these management conundrums.”
She added that the new guidelines “give clinicians a leg to stand on” when an insurer pushes back on a recommended treatment for AK. “It gives you a way to have dialogue with insurers if you’re prescribing some of these treatments.”
The guidelines authors write that there is “strong theoretic rationale for the treatment of AK to prevent skin cancers” but acknowledge that only a few studies in the review “report the incidence of skin cancer as an outcome measure or have sufficient follow-up to viably measure carcinoma development.” In addition, “more long-term research is needed to validate our current understanding of skin cancer progression from AKs to keratinocyte carcinoma.”
Dr. DiGiorgio thinks about this differently. “I think treatment of AKs does prevent skin cancers,” she said. “We call them precancers as we’re treating our patients because we know a certain percentage of them can develop into skin cancers over time.”
The study was funded by internal funds from the AAD. Dr. Asgari disclosed that she serves as an investigator for Pfizer. Several of the other authors reported having financial disclosures.
Dr. DiGiorgio reported having no financial disclosures.
FROM JAAD
Pandemic took a cut of cosmetic procedures in 2020
pandemic, according to the American Society of Plastic Surgeons.
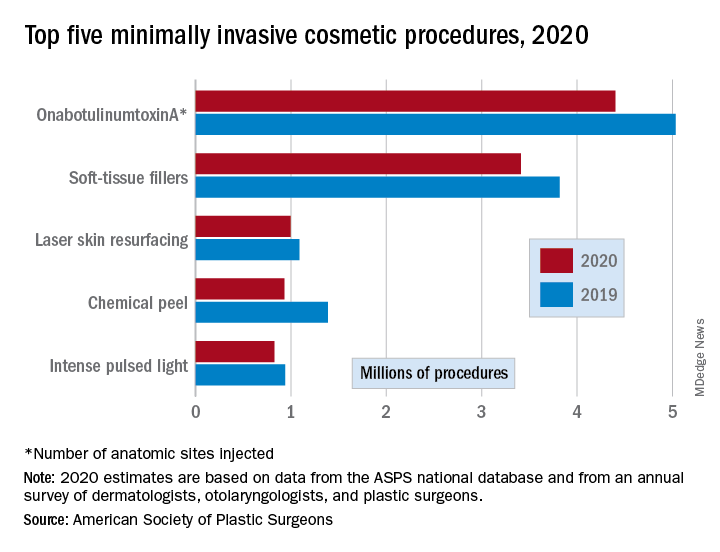
There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
pandemic, according to the American Society of Plastic Surgeons.

There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
pandemic, according to the American Society of Plastic Surgeons.

There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
FDA panel narrowly backs avacopan approval
A panel of federal advisers on May 6 lent support to the ChemoCentryx bid for approval of avacopan for a rare and serious autoimmune condition. But they also flagged concerns about both the evidence supporting claims of a benefit for this experimental drug and its safety.
At a meeting of the Food and Drug Administration’s Arthritis Advisory Committee, panelists voted 10-8 on a question of whether the risk-benefit profile of avacopan is adequate to support approval.
ChemoCentryx is seeking approval of avacopan for antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis in the subtypes of granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
Regardless of their vote on this approval question, the panelists shared an interest in avacopan’s potential to reduce glucocorticoid use among some patients with ANCA-associated vasculitis, also called AAV. Mara L. Becker, MD, MSCE, the chair of the FDA’s panel, was among the panelists who said they reluctantly voted no.
“It pains me because I really want more steroid-sparing” medicines, said Dr. Becker of Duke University, Durham, N.C., who cited a need to gather more data on avacopan.
Margrit Wiesendanger, MD, PhD, of the Icahn School of Medicine at Mount Sinai, New York, who was among the panelists voting yes, spoke of a need for caution if the FDA approves avacopan.
“Judicious use of this new medication will be warranted and perhaps additional guidance could be given to rheumatologists to help them decide for whom this medication is best,” she said.
Panelists had spoken earlier of avacopan as a possible alternative medicine for people with AAV who have conditions that make glucocorticoids riskier for them, such as those who have diabetes.
Close votes on safety profile, efficacy
The panel also voted 10-8 on a question about whether the safety profile of avacopan is adequate to support approval of avacopan for the treatment of adult patients with AAV.
In addition, the panel voted 9-9 on a question about whether efficacy data support approval of avacopan for the treatment of adult patients with AAV.
The FDA considers the recommendations of its advisory panels, but is not bound by them.
The FDA staff clearly expressed the view that ChemoCentryx fell short with the evidence presented for avacopan approval. Shares of San Carlos, Calif.–based ChemoCentryx dropped sharply from a May 3 closing price of $48.82 to a May 4 closing price of $26.63 after the FDA released the staff’s review of avacopan.
In a briefing prepared for the meeting, FDA staff detailed concerns about the evidence ChemoCentryx is using to seek approval. While acknowledging a need for new treatments for AAV as a rare condition, FDA staff honed in on what they described flaws in the testing of this experimental medicine, which is a small-molecule antagonist of the receptor of C5a, an end product of the complement cascade that acts as a potent neutrophil chemoattractant and agonist.
The FDA usually requires two phase 3 studies for approval of a new medicine but will do so with a single trial in cases of exceptional need, the agency staff said. But in these cases, the bar rises for the evidence provided from that single trial.
Difficulties in interpretation of complex study design
In the case of avacopan, though, the data from the key avacopan trial, Study CL010_168, known as ADVOCATE, there were substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.
In the briefing document, the FDA staff noted that it had “communicated many of the concerns” about ChemoCentryx’s research earlier to the company.
“Complexities of the study design, as detailed in the briefing document, raise questions about the interpretability of the data to define a clinically meaningful benefit of avacopan and its role in the management of AAV,” the FDA staff wrote.
“We acknowledge that AAV is a rare and serious disease associated with high morbidity and increased mortality. It is also a disease with high unmet need for new therapies. However, FDA wants to ensure that new products have a defined context of use, i.e., how a product would be used, and a favorable benefit-risk assessment for patients,” the staff added.
In addition, there were differences in the assessments performed by investigators and the adjudication committee, most frequently related to the attribution of persistent vasculitis, the FDA staff noted.
Statistical analyses of the primary endpoint using investigators’ estimates “resulted in more conservative estimates of treatment effect, e.g., statistical significance for superiority would no longer be demonstrated,” the FDA staff noted. “While the prespecified analysis used the Adjudicator assessments, the assessment based on the Investigators, experienced in management of vasculitis, may better reflect real-world use.”
Imbalances in use of glucocorticoids and maintenance therapy
Also among the complications in assessing the ADVOCATE trial data were the glucocorticoids taken by patients in the study, the FDA staff said.
In the avacopan arm of the trial, 86% of patients received non–study-supplied glucocorticoids. In addition, more avacopan‐treated patients experienced adverse events and serious adverse events within the hepatobiliary system leading to discontinuation.
Subgroups given different treatments represented another challenge in interpreting ADVOCATE results for the FDA staff.
At week 26, the proportion of patients in disease remission in the avacopan group (72.3%) was noninferior to the prednisone group (70.1%), the FDA staff said in the briefing document.
But at week 52, a disparity was observed between subgroups that had received rituximab and cyclophosphamide (intravenous and oral) induction treatment. The estimated risk difference for disease remission at week 52 was 15.0% (95% CI, 2.2%-27.7%) in the subgroup receiving induction with rituximab and 3.3% (95% CI, –14.8% to 21.4%) in the cyclophosphamide plus maintenance azathioprine subgroup, the agency’s staff said.
“Based on the data, there is no evidence of clinically meaningful treatment effect in the cyclophosphamide induction subgroup,” the FDA staff wrote. “Further, the treatment comparison in the complementary rituximab induction subgroup may not be considered meaningful because these patients did not receive maintenance therapy, i.e., due to undertreating of patients, the effect observed in the rituximab subgroup may not represent a clinically meaningful treatment effect, compared to standard of care.”
Rachel L. Glaser, MD, clinical team leader in FDA’s division of rheumatology and transplant medicine, reiterated these concerns to the advisory committee at the May 6 meeting.
“Throughout the development program, FDA advised the applicant that a noninferiority comparison would not be sufficient to show that avacopan can replaced glucocorticoids as it would be difficult to establish whether avacopan is effective or whether an effect was due to the rituximab or cyclophosphamide administered to both treatment arms,” she said.
In its briefing for the meeting, ChemoCentryx noted the limits of treatments now available for AAV. It also emphasized the toll of the condition, ranging from skin manifestations to glomerulonephritis to life-threatening pulmonary hemorrhage. If untreated, 80% of patients with GPA or MPA die within 2 years of disease onset, ChemoCentryx said in its briefing materials for the meeting.
The side effects of glucocorticoids were well known to the FDA panelists and the ChemoCentryx presenters. Witnesses at an open public hearing told their own stories of depression, anxiety, and irritability caused by these medicines.
During the ChemoCentryx presentation, a presenter for the company, Peter Merkel, MD, MPH, of the University of Pennsylvania, Philadelphia, said avacopan would provide patients with AAV with an alternative allowing them “to go on a much lower glucocorticoids regimen.”
A similar view was presented in a February 2021 editorial in the New England Journal of Medicine, titled “Avacopan – Time to Replace Glucocorticoids?” Written by Kenneth J. Warrington, MD, of the Mayo Clinic, Rochester, Minn., the opinion article called the ADVOCATE trial “a milestone in the treatment of ANCA-associated vasculitis; complement inhibition with avacopan has glucocorticoid-sparing effects and results in superior disease control.”
Dr. Warrington reported no conflicts in connection with his editorial nor payments from ChemoCentryx. He did report grants from other firms such as Eli Lilly.
Julia Lewis, MD, of Vanderbilt University, Nashville, Tenn., was among the more skeptical members of the FDA panel. She was among the “nays” in all three voting questions put to the panel. Still, she said there were signs of “clinically meaningful benefit” in the data presented, but noted that the nonstudy use of glucocorticoids made it difficult to interpret the ADVOCATE results.
Dr. Lewis noted that the FDA usually requires two studies for a drug approval, particularly with a compound not yet cleared for any use. While ANCA-associated vasculitis is rare, it would be possible to recruit patients for another trial of avacopan, adding to the results reported already for avacopan from ADVOCATE, she said.
“Were there to be another study, this would certainly be a supportive study and maybe qualify as two studies,” she said.
A panel of federal advisers on May 6 lent support to the ChemoCentryx bid for approval of avacopan for a rare and serious autoimmune condition. But they also flagged concerns about both the evidence supporting claims of a benefit for this experimental drug and its safety.
At a meeting of the Food and Drug Administration’s Arthritis Advisory Committee, panelists voted 10-8 on a question of whether the risk-benefit profile of avacopan is adequate to support approval.
ChemoCentryx is seeking approval of avacopan for antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis in the subtypes of granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
Regardless of their vote on this approval question, the panelists shared an interest in avacopan’s potential to reduce glucocorticoid use among some patients with ANCA-associated vasculitis, also called AAV. Mara L. Becker, MD, MSCE, the chair of the FDA’s panel, was among the panelists who said they reluctantly voted no.
“It pains me because I really want more steroid-sparing” medicines, said Dr. Becker of Duke University, Durham, N.C., who cited a need to gather more data on avacopan.
Margrit Wiesendanger, MD, PhD, of the Icahn School of Medicine at Mount Sinai, New York, who was among the panelists voting yes, spoke of a need for caution if the FDA approves avacopan.
“Judicious use of this new medication will be warranted and perhaps additional guidance could be given to rheumatologists to help them decide for whom this medication is best,” she said.
Panelists had spoken earlier of avacopan as a possible alternative medicine for people with AAV who have conditions that make glucocorticoids riskier for them, such as those who have diabetes.
Close votes on safety profile, efficacy
The panel also voted 10-8 on a question about whether the safety profile of avacopan is adequate to support approval of avacopan for the treatment of adult patients with AAV.
In addition, the panel voted 9-9 on a question about whether efficacy data support approval of avacopan for the treatment of adult patients with AAV.
The FDA considers the recommendations of its advisory panels, but is not bound by them.
The FDA staff clearly expressed the view that ChemoCentryx fell short with the evidence presented for avacopan approval. Shares of San Carlos, Calif.–based ChemoCentryx dropped sharply from a May 3 closing price of $48.82 to a May 4 closing price of $26.63 after the FDA released the staff’s review of avacopan.
In a briefing prepared for the meeting, FDA staff detailed concerns about the evidence ChemoCentryx is using to seek approval. While acknowledging a need for new treatments for AAV as a rare condition, FDA staff honed in on what they described flaws in the testing of this experimental medicine, which is a small-molecule antagonist of the receptor of C5a, an end product of the complement cascade that acts as a potent neutrophil chemoattractant and agonist.
The FDA usually requires two phase 3 studies for approval of a new medicine but will do so with a single trial in cases of exceptional need, the agency staff said. But in these cases, the bar rises for the evidence provided from that single trial.
Difficulties in interpretation of complex study design
In the case of avacopan, though, the data from the key avacopan trial, Study CL010_168, known as ADVOCATE, there were substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.
In the briefing document, the FDA staff noted that it had “communicated many of the concerns” about ChemoCentryx’s research earlier to the company.
“Complexities of the study design, as detailed in the briefing document, raise questions about the interpretability of the data to define a clinically meaningful benefit of avacopan and its role in the management of AAV,” the FDA staff wrote.
“We acknowledge that AAV is a rare and serious disease associated with high morbidity and increased mortality. It is also a disease with high unmet need for new therapies. However, FDA wants to ensure that new products have a defined context of use, i.e., how a product would be used, and a favorable benefit-risk assessment for patients,” the staff added.
In addition, there were differences in the assessments performed by investigators and the adjudication committee, most frequently related to the attribution of persistent vasculitis, the FDA staff noted.
Statistical analyses of the primary endpoint using investigators’ estimates “resulted in more conservative estimates of treatment effect, e.g., statistical significance for superiority would no longer be demonstrated,” the FDA staff noted. “While the prespecified analysis used the Adjudicator assessments, the assessment based on the Investigators, experienced in management of vasculitis, may better reflect real-world use.”
Imbalances in use of glucocorticoids and maintenance therapy
Also among the complications in assessing the ADVOCATE trial data were the glucocorticoids taken by patients in the study, the FDA staff said.
In the avacopan arm of the trial, 86% of patients received non–study-supplied glucocorticoids. In addition, more avacopan‐treated patients experienced adverse events and serious adverse events within the hepatobiliary system leading to discontinuation.
Subgroups given different treatments represented another challenge in interpreting ADVOCATE results for the FDA staff.
At week 26, the proportion of patients in disease remission in the avacopan group (72.3%) was noninferior to the prednisone group (70.1%), the FDA staff said in the briefing document.
But at week 52, a disparity was observed between subgroups that had received rituximab and cyclophosphamide (intravenous and oral) induction treatment. The estimated risk difference for disease remission at week 52 was 15.0% (95% CI, 2.2%-27.7%) in the subgroup receiving induction with rituximab and 3.3% (95% CI, –14.8% to 21.4%) in the cyclophosphamide plus maintenance azathioprine subgroup, the agency’s staff said.
“Based on the data, there is no evidence of clinically meaningful treatment effect in the cyclophosphamide induction subgroup,” the FDA staff wrote. “Further, the treatment comparison in the complementary rituximab induction subgroup may not be considered meaningful because these patients did not receive maintenance therapy, i.e., due to undertreating of patients, the effect observed in the rituximab subgroup may not represent a clinically meaningful treatment effect, compared to standard of care.”
Rachel L. Glaser, MD, clinical team leader in FDA’s division of rheumatology and transplant medicine, reiterated these concerns to the advisory committee at the May 6 meeting.
“Throughout the development program, FDA advised the applicant that a noninferiority comparison would not be sufficient to show that avacopan can replaced glucocorticoids as it would be difficult to establish whether avacopan is effective or whether an effect was due to the rituximab or cyclophosphamide administered to both treatment arms,” she said.
In its briefing for the meeting, ChemoCentryx noted the limits of treatments now available for AAV. It also emphasized the toll of the condition, ranging from skin manifestations to glomerulonephritis to life-threatening pulmonary hemorrhage. If untreated, 80% of patients with GPA or MPA die within 2 years of disease onset, ChemoCentryx said in its briefing materials for the meeting.
The side effects of glucocorticoids were well known to the FDA panelists and the ChemoCentryx presenters. Witnesses at an open public hearing told their own stories of depression, anxiety, and irritability caused by these medicines.
During the ChemoCentryx presentation, a presenter for the company, Peter Merkel, MD, MPH, of the University of Pennsylvania, Philadelphia, said avacopan would provide patients with AAV with an alternative allowing them “to go on a much lower glucocorticoids regimen.”
A similar view was presented in a February 2021 editorial in the New England Journal of Medicine, titled “Avacopan – Time to Replace Glucocorticoids?” Written by Kenneth J. Warrington, MD, of the Mayo Clinic, Rochester, Minn., the opinion article called the ADVOCATE trial “a milestone in the treatment of ANCA-associated vasculitis; complement inhibition with avacopan has glucocorticoid-sparing effects and results in superior disease control.”
Dr. Warrington reported no conflicts in connection with his editorial nor payments from ChemoCentryx. He did report grants from other firms such as Eli Lilly.
Julia Lewis, MD, of Vanderbilt University, Nashville, Tenn., was among the more skeptical members of the FDA panel. She was among the “nays” in all three voting questions put to the panel. Still, she said there were signs of “clinically meaningful benefit” in the data presented, but noted that the nonstudy use of glucocorticoids made it difficult to interpret the ADVOCATE results.
Dr. Lewis noted that the FDA usually requires two studies for a drug approval, particularly with a compound not yet cleared for any use. While ANCA-associated vasculitis is rare, it would be possible to recruit patients for another trial of avacopan, adding to the results reported already for avacopan from ADVOCATE, she said.
“Were there to be another study, this would certainly be a supportive study and maybe qualify as two studies,” she said.
A panel of federal advisers on May 6 lent support to the ChemoCentryx bid for approval of avacopan for a rare and serious autoimmune condition. But they also flagged concerns about both the evidence supporting claims of a benefit for this experimental drug and its safety.
At a meeting of the Food and Drug Administration’s Arthritis Advisory Committee, panelists voted 10-8 on a question of whether the risk-benefit profile of avacopan is adequate to support approval.
ChemoCentryx is seeking approval of avacopan for antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis in the subtypes of granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
Regardless of their vote on this approval question, the panelists shared an interest in avacopan’s potential to reduce glucocorticoid use among some patients with ANCA-associated vasculitis, also called AAV. Mara L. Becker, MD, MSCE, the chair of the FDA’s panel, was among the panelists who said they reluctantly voted no.
“It pains me because I really want more steroid-sparing” medicines, said Dr. Becker of Duke University, Durham, N.C., who cited a need to gather more data on avacopan.
Margrit Wiesendanger, MD, PhD, of the Icahn School of Medicine at Mount Sinai, New York, who was among the panelists voting yes, spoke of a need for caution if the FDA approves avacopan.
“Judicious use of this new medication will be warranted and perhaps additional guidance could be given to rheumatologists to help them decide for whom this medication is best,” she said.
Panelists had spoken earlier of avacopan as a possible alternative medicine for people with AAV who have conditions that make glucocorticoids riskier for them, such as those who have diabetes.
Close votes on safety profile, efficacy
The panel also voted 10-8 on a question about whether the safety profile of avacopan is adequate to support approval of avacopan for the treatment of adult patients with AAV.
In addition, the panel voted 9-9 on a question about whether efficacy data support approval of avacopan for the treatment of adult patients with AAV.
The FDA considers the recommendations of its advisory panels, but is not bound by them.
The FDA staff clearly expressed the view that ChemoCentryx fell short with the evidence presented for avacopan approval. Shares of San Carlos, Calif.–based ChemoCentryx dropped sharply from a May 3 closing price of $48.82 to a May 4 closing price of $26.63 after the FDA released the staff’s review of avacopan.
In a briefing prepared for the meeting, FDA staff detailed concerns about the evidence ChemoCentryx is using to seek approval. While acknowledging a need for new treatments for AAV as a rare condition, FDA staff honed in on what they described flaws in the testing of this experimental medicine, which is a small-molecule antagonist of the receptor of C5a, an end product of the complement cascade that acts as a potent neutrophil chemoattractant and agonist.
The FDA usually requires two phase 3 studies for approval of a new medicine but will do so with a single trial in cases of exceptional need, the agency staff said. But in these cases, the bar rises for the evidence provided from that single trial.
Difficulties in interpretation of complex study design
In the case of avacopan, though, the data from the key avacopan trial, Study CL010_168, known as ADVOCATE, there were substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.
In the briefing document, the FDA staff noted that it had “communicated many of the concerns” about ChemoCentryx’s research earlier to the company.
“Complexities of the study design, as detailed in the briefing document, raise questions about the interpretability of the data to define a clinically meaningful benefit of avacopan and its role in the management of AAV,” the FDA staff wrote.
“We acknowledge that AAV is a rare and serious disease associated with high morbidity and increased mortality. It is also a disease with high unmet need for new therapies. However, FDA wants to ensure that new products have a defined context of use, i.e., how a product would be used, and a favorable benefit-risk assessment for patients,” the staff added.
In addition, there were differences in the assessments performed by investigators and the adjudication committee, most frequently related to the attribution of persistent vasculitis, the FDA staff noted.
Statistical analyses of the primary endpoint using investigators’ estimates “resulted in more conservative estimates of treatment effect, e.g., statistical significance for superiority would no longer be demonstrated,” the FDA staff noted. “While the prespecified analysis used the Adjudicator assessments, the assessment based on the Investigators, experienced in management of vasculitis, may better reflect real-world use.”
Imbalances in use of glucocorticoids and maintenance therapy
Also among the complications in assessing the ADVOCATE trial data were the glucocorticoids taken by patients in the study, the FDA staff said.
In the avacopan arm of the trial, 86% of patients received non–study-supplied glucocorticoids. In addition, more avacopan‐treated patients experienced adverse events and serious adverse events within the hepatobiliary system leading to discontinuation.
Subgroups given different treatments represented another challenge in interpreting ADVOCATE results for the FDA staff.
At week 26, the proportion of patients in disease remission in the avacopan group (72.3%) was noninferior to the prednisone group (70.1%), the FDA staff said in the briefing document.
But at week 52, a disparity was observed between subgroups that had received rituximab and cyclophosphamide (intravenous and oral) induction treatment. The estimated risk difference for disease remission at week 52 was 15.0% (95% CI, 2.2%-27.7%) in the subgroup receiving induction with rituximab and 3.3% (95% CI, –14.8% to 21.4%) in the cyclophosphamide plus maintenance azathioprine subgroup, the agency’s staff said.
“Based on the data, there is no evidence of clinically meaningful treatment effect in the cyclophosphamide induction subgroup,” the FDA staff wrote. “Further, the treatment comparison in the complementary rituximab induction subgroup may not be considered meaningful because these patients did not receive maintenance therapy, i.e., due to undertreating of patients, the effect observed in the rituximab subgroup may not represent a clinically meaningful treatment effect, compared to standard of care.”
Rachel L. Glaser, MD, clinical team leader in FDA’s division of rheumatology and transplant medicine, reiterated these concerns to the advisory committee at the May 6 meeting.
“Throughout the development program, FDA advised the applicant that a noninferiority comparison would not be sufficient to show that avacopan can replaced glucocorticoids as it would be difficult to establish whether avacopan is effective or whether an effect was due to the rituximab or cyclophosphamide administered to both treatment arms,” she said.
In its briefing for the meeting, ChemoCentryx noted the limits of treatments now available for AAV. It also emphasized the toll of the condition, ranging from skin manifestations to glomerulonephritis to life-threatening pulmonary hemorrhage. If untreated, 80% of patients with GPA or MPA die within 2 years of disease onset, ChemoCentryx said in its briefing materials for the meeting.
The side effects of glucocorticoids were well known to the FDA panelists and the ChemoCentryx presenters. Witnesses at an open public hearing told their own stories of depression, anxiety, and irritability caused by these medicines.
During the ChemoCentryx presentation, a presenter for the company, Peter Merkel, MD, MPH, of the University of Pennsylvania, Philadelphia, said avacopan would provide patients with AAV with an alternative allowing them “to go on a much lower glucocorticoids regimen.”
A similar view was presented in a February 2021 editorial in the New England Journal of Medicine, titled “Avacopan – Time to Replace Glucocorticoids?” Written by Kenneth J. Warrington, MD, of the Mayo Clinic, Rochester, Minn., the opinion article called the ADVOCATE trial “a milestone in the treatment of ANCA-associated vasculitis; complement inhibition with avacopan has glucocorticoid-sparing effects and results in superior disease control.”
Dr. Warrington reported no conflicts in connection with his editorial nor payments from ChemoCentryx. He did report grants from other firms such as Eli Lilly.
Julia Lewis, MD, of Vanderbilt University, Nashville, Tenn., was among the more skeptical members of the FDA panel. She was among the “nays” in all three voting questions put to the panel. Still, she said there were signs of “clinically meaningful benefit” in the data presented, but noted that the nonstudy use of glucocorticoids made it difficult to interpret the ADVOCATE results.
Dr. Lewis noted that the FDA usually requires two studies for a drug approval, particularly with a compound not yet cleared for any use. While ANCA-associated vasculitis is rare, it would be possible to recruit patients for another trial of avacopan, adding to the results reported already for avacopan from ADVOCATE, she said.
“Were there to be another study, this would certainly be a supportive study and maybe qualify as two studies,” she said.
Atopic dermatitis genes vary with ethnicity
patients, researchers say.
The finding moves researchers another step forward in the effort to figure out which patients are most at risk for the disease and who will respond best to which treatments.
“Because atopic dermatitis is considered a complex trait, we think if there is any method to detect AD gene variations simultaneously, it could be possible to prevent the development of AD and then the atopic march,” said Eung Ho Choi, MD, PhD, a dermatology professor at Yonsei University, Wonju, South Korea.
He presented the finding at the International Society of Atopic Dermatitis (ISAD) 2021 Annual Meeting.
Atopic dermatitis is not caused by a single genetic mutation. But genetic factors play an important role, with about 75% concordance between monozygotic twins versus only 23% for dizygotic twins.
“Genetic biomarkers are needed in predicting the occurrence, severity, and treatment response,” as well as determining the prognosis of atopic dermatitis “and applying it to precision medicine,” Dr. Choi said.
Researchers have identified multiple genetic variations related to atopic dermatitis. One of the most significant genetic contributions found so far is the filaggrin gene variation, which can produce a defective skin barrier, Dr. Choi said. Others are involved in the immune response.
Although variations in the filaggrin gene (FLG ) are the most reliable genetic predictor of atopic dermatitis in Korean patients, they are less common in Korean patients than in Northwestern Europeans, Chinese, and Japanese patients. In Korean patients, the most common reported mutations of this gene are 3321delA and K4022X, Dr. Choi said.
To find out what other gene variants are important in Korean patients with atopic dermatitis, Dr. Choi and his colleagues developed the reverse blot hybridization assay (REBA) to detect skin barrier variations in the FLG, SPINK5 and KLK7 genes, and genes involved in immune response variations, KDR, IL-5RA, IL-9, DEFB1 (Defensin Beta 1), IL-12RB1 (interleukin-12 receptor subunit beta 1), and IL-12RB2.
They compared the prevalence of these variations in 279 Koreans with atopic dermatitis to the prevalence in 224 healthy people without atopic dermatitis and found that the odds ratio for atopic dermatitis increased with the number of these variants: People with three or four variants had a 3.75 times greater risk of AD, and those with 5 or more variants had a 10.3 times greater risk. The number of variants did not correlate to the severity of the disease, however.
The filaggrin variation was present in 13.9% of those with atopic dermatitis. About a quarter (28%) of the patients with AD who had this variation had impetigo, 15% had eczema herpeticum, and 5% had prurigo nodularis. By comparison, 14% of the patients with AD who did not have this variation had impetigo, and 5% had eczema herpeticum, but 19% had prurigo nodularis.
In a separate study, Dr. Choi and his colleagues identified a mutation in IL-17RA, present in 8.1% of 332 patients with AD compared with 3.3% of 245 controls. The patients with IL-17RA mutations all had extrinsic AD.
The variation was associated with longer disease duration, more frequent keratosis pilaris, higher blood eosinophil counts, higher serum total immunoglobulin E (IgE) levels, higher house dust mite allergen-specific IgE levels, and a greater need for systemic treatment than patients without the IL-17RA mutation.
Such findings are important for progress in treating atopic dermatitis because the mechanism differs among patients, said Emma Guttman-Yassky, MD, PhD, director of the Center for Excellence in Eczema and professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“It’s not one size fits all in atopic dermatitis, and we need better biomarkers that will be able to tell us which treatment will work best for each patient,” she said in an interview.
In addition to genetic biomarkers, she and her colleagues are analyzing proteins involved in inflammation. They are using adhesive tape strips to harvest these markers, a less invasive approach than skin biopsies.
A version of this article first appeared on Medscape.com.
patients, researchers say.
The finding moves researchers another step forward in the effort to figure out which patients are most at risk for the disease and who will respond best to which treatments.
“Because atopic dermatitis is considered a complex trait, we think if there is any method to detect AD gene variations simultaneously, it could be possible to prevent the development of AD and then the atopic march,” said Eung Ho Choi, MD, PhD, a dermatology professor at Yonsei University, Wonju, South Korea.
He presented the finding at the International Society of Atopic Dermatitis (ISAD) 2021 Annual Meeting.
Atopic dermatitis is not caused by a single genetic mutation. But genetic factors play an important role, with about 75% concordance between monozygotic twins versus only 23% for dizygotic twins.
“Genetic biomarkers are needed in predicting the occurrence, severity, and treatment response,” as well as determining the prognosis of atopic dermatitis “and applying it to precision medicine,” Dr. Choi said.
Researchers have identified multiple genetic variations related to atopic dermatitis. One of the most significant genetic contributions found so far is the filaggrin gene variation, which can produce a defective skin barrier, Dr. Choi said. Others are involved in the immune response.
Although variations in the filaggrin gene (FLG ) are the most reliable genetic predictor of atopic dermatitis in Korean patients, they are less common in Korean patients than in Northwestern Europeans, Chinese, and Japanese patients. In Korean patients, the most common reported mutations of this gene are 3321delA and K4022X, Dr. Choi said.
To find out what other gene variants are important in Korean patients with atopic dermatitis, Dr. Choi and his colleagues developed the reverse blot hybridization assay (REBA) to detect skin barrier variations in the FLG, SPINK5 and KLK7 genes, and genes involved in immune response variations, KDR, IL-5RA, IL-9, DEFB1 (Defensin Beta 1), IL-12RB1 (interleukin-12 receptor subunit beta 1), and IL-12RB2.
They compared the prevalence of these variations in 279 Koreans with atopic dermatitis to the prevalence in 224 healthy people without atopic dermatitis and found that the odds ratio for atopic dermatitis increased with the number of these variants: People with three or four variants had a 3.75 times greater risk of AD, and those with 5 or more variants had a 10.3 times greater risk. The number of variants did not correlate to the severity of the disease, however.
The filaggrin variation was present in 13.9% of those with atopic dermatitis. About a quarter (28%) of the patients with AD who had this variation had impetigo, 15% had eczema herpeticum, and 5% had prurigo nodularis. By comparison, 14% of the patients with AD who did not have this variation had impetigo, and 5% had eczema herpeticum, but 19% had prurigo nodularis.
In a separate study, Dr. Choi and his colleagues identified a mutation in IL-17RA, present in 8.1% of 332 patients with AD compared with 3.3% of 245 controls. The patients with IL-17RA mutations all had extrinsic AD.
The variation was associated with longer disease duration, more frequent keratosis pilaris, higher blood eosinophil counts, higher serum total immunoglobulin E (IgE) levels, higher house dust mite allergen-specific IgE levels, and a greater need for systemic treatment than patients without the IL-17RA mutation.
Such findings are important for progress in treating atopic dermatitis because the mechanism differs among patients, said Emma Guttman-Yassky, MD, PhD, director of the Center for Excellence in Eczema and professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“It’s not one size fits all in atopic dermatitis, and we need better biomarkers that will be able to tell us which treatment will work best for each patient,” she said in an interview.
In addition to genetic biomarkers, she and her colleagues are analyzing proteins involved in inflammation. They are using adhesive tape strips to harvest these markers, a less invasive approach than skin biopsies.
A version of this article first appeared on Medscape.com.
patients, researchers say.
The finding moves researchers another step forward in the effort to figure out which patients are most at risk for the disease and who will respond best to which treatments.
“Because atopic dermatitis is considered a complex trait, we think if there is any method to detect AD gene variations simultaneously, it could be possible to prevent the development of AD and then the atopic march,” said Eung Ho Choi, MD, PhD, a dermatology professor at Yonsei University, Wonju, South Korea.
He presented the finding at the International Society of Atopic Dermatitis (ISAD) 2021 Annual Meeting.
Atopic dermatitis is not caused by a single genetic mutation. But genetic factors play an important role, with about 75% concordance between monozygotic twins versus only 23% for dizygotic twins.
“Genetic biomarkers are needed in predicting the occurrence, severity, and treatment response,” as well as determining the prognosis of atopic dermatitis “and applying it to precision medicine,” Dr. Choi said.
Researchers have identified multiple genetic variations related to atopic dermatitis. One of the most significant genetic contributions found so far is the filaggrin gene variation, which can produce a defective skin barrier, Dr. Choi said. Others are involved in the immune response.
Although variations in the filaggrin gene (FLG ) are the most reliable genetic predictor of atopic dermatitis in Korean patients, they are less common in Korean patients than in Northwestern Europeans, Chinese, and Japanese patients. In Korean patients, the most common reported mutations of this gene are 3321delA and K4022X, Dr. Choi said.
To find out what other gene variants are important in Korean patients with atopic dermatitis, Dr. Choi and his colleagues developed the reverse blot hybridization assay (REBA) to detect skin barrier variations in the FLG, SPINK5 and KLK7 genes, and genes involved in immune response variations, KDR, IL-5RA, IL-9, DEFB1 (Defensin Beta 1), IL-12RB1 (interleukin-12 receptor subunit beta 1), and IL-12RB2.
They compared the prevalence of these variations in 279 Koreans with atopic dermatitis to the prevalence in 224 healthy people without atopic dermatitis and found that the odds ratio for atopic dermatitis increased with the number of these variants: People with three or four variants had a 3.75 times greater risk of AD, and those with 5 or more variants had a 10.3 times greater risk. The number of variants did not correlate to the severity of the disease, however.
The filaggrin variation was present in 13.9% of those with atopic dermatitis. About a quarter (28%) of the patients with AD who had this variation had impetigo, 15% had eczema herpeticum, and 5% had prurigo nodularis. By comparison, 14% of the patients with AD who did not have this variation had impetigo, and 5% had eczema herpeticum, but 19% had prurigo nodularis.
In a separate study, Dr. Choi and his colleagues identified a mutation in IL-17RA, present in 8.1% of 332 patients with AD compared with 3.3% of 245 controls. The patients with IL-17RA mutations all had extrinsic AD.
The variation was associated with longer disease duration, more frequent keratosis pilaris, higher blood eosinophil counts, higher serum total immunoglobulin E (IgE) levels, higher house dust mite allergen-specific IgE levels, and a greater need for systemic treatment than patients without the IL-17RA mutation.
Such findings are important for progress in treating atopic dermatitis because the mechanism differs among patients, said Emma Guttman-Yassky, MD, PhD, director of the Center for Excellence in Eczema and professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“It’s not one size fits all in atopic dermatitis, and we need better biomarkers that will be able to tell us which treatment will work best for each patient,” she said in an interview.
In addition to genetic biomarkers, she and her colleagues are analyzing proteins involved in inflammation. They are using adhesive tape strips to harvest these markers, a less invasive approach than skin biopsies.
A version of this article first appeared on Medscape.com.
Who can call themselves ‘doctor’? The debate heats up
Who Should Get to Be Called ‘Doctor’? shows. The topic has clearly struck a nerve, since a record number of respondents – over 12,000 – voted in the poll.
Most physicians think it’s appropriate for people with other doctorate degrees such as a PhD or EdD to call themselves ‘doctor,’ although slightly more than half said it depends on the context.
The controversy over who gets to be called a doctor was reignited when a Wall Street Journal opinion piece criticized First Lady Jill Biden, EdD, for wanting to be called “Dr Biden.” The piece also challenged the idea that having a PhD is worth the honorific of ‘doctor.’
Medical ethicist Arthur Caplan, PhD, disagreed with that viewpoint, saying the context matters. For example, he prefers to be called “professor” when he’s introduced to the public rather than “doctor” to avoid any confusion about his professional status.
More than 12,000 clinicians including physicians, medical students, nurses, pharmacists, and other health care professionals responded to the poll. The non-MD clinicians were the most likely to say it was always appropriate to be called “doctor” while physicians were the least likely.
Context matters
Large percentages of clinicians – 54% of doctors, 62% of medical students, and 41% of nurses – said that the context matters for being called “doctor.’’
“I earned my PhD in 1995 and my MD in 2000. I think it is contextual. In a research or University setting, “Dr.” seems appropriate for a PhD. That same person in public should probably not hold themselves out as “Dr.” So, maybe MDs and DOs can choose, while others maintain the title in their specific setting.”
Some readers proposed that people with MDs call themselves physicians rather than doctors. Said one: “Anyone with a terminal doctorate degree has the right to use the word doctor. As a physician when someone asks what I do, I say: ‘I am a physician.’ Problem solved. There can only be one physician but there are many types of doctors.”
Physicians and nurses differed most in their views. Just 24% of physicians said it was always appropriate for people with other doctorate degrees to call themselves doctor whereas about an equal number (22%) thought it was never appropriate.
In contrast, 43% of nurses (including advance practice nurses) said it was always appropriate for people with non-MD doctorates to be called doctor. Only 16% said it’s never appropriate.
This difference may reflect the growing number of nurses with doctorate degrees, either a DNP or PhD, who want to be called doctor in clinical settings.
Age made a difference too. Only 16% of physicians younger than age 45 said it was always appropriate for people with non-MD doctorate degrees to be called doctor, compared with 27% of physicians aged 45 and up.
Medical students (31%) were also more likely than physicians to say it was always appropriate for non-MD doctorates to use the title “doctor” and 64% said it depends on the context. This was noteworthy because twice as many medical students as physicians (16% vs. 8%) said they work in academia, research, or military government settings.
Too many ‘doctors’ confuse the public
Physicians (70%) were also more likely to say it was always or often confusing for the public to hear someone without a medical degree addressed as “doctor.” Only 6% of physicians thought it was never or rarely confusing.
Nurses disagreed. Just 45% said that it was always or often confusing while 16% said it was never or rarely confusing.
Medical students were more aligned with physicians on this issue – 60% said it was always or often confusing to the public and just 10% said it was never or rarely confusing.
One reader commented, “The problem is the confusion the ‘doctor’ title causes for patients, especially in a hospital setting. Is the ‘doctor’ a physician, a pharmacist, a psychologist, a nurse, etc., etc.? We need to think not of our own egos but if and how the confusion about this plethora of titles may be hindering good patient care.”
These concerns are not unfounded. The American Medical Association reported in its Truth in Advertising campaign that “patients mistake physicians with nonphysician providers” based on an online survey of 802 adults in 2018. The participants thought these specialists were MDs: dentists (61%), podiatrists (67%), optometrists (47%), psychologists (43%), doctors of nursing (39%), and chiropractors (27%).
The AMA has advocated that states pass the “Health Care Professional Transparency Act,” which New Jersey has enacted. The law requires all health care professionals dealing with patients to wear a name tag that clearly identifies their licensure. Health care professionals must also display their education, training, and licensure in their office.
A version of this article first appeared on Medscape.com.
Who Should Get to Be Called ‘Doctor’? shows. The topic has clearly struck a nerve, since a record number of respondents – over 12,000 – voted in the poll.
Most physicians think it’s appropriate for people with other doctorate degrees such as a PhD or EdD to call themselves ‘doctor,’ although slightly more than half said it depends on the context.
The controversy over who gets to be called a doctor was reignited when a Wall Street Journal opinion piece criticized First Lady Jill Biden, EdD, for wanting to be called “Dr Biden.” The piece also challenged the idea that having a PhD is worth the honorific of ‘doctor.’
Medical ethicist Arthur Caplan, PhD, disagreed with that viewpoint, saying the context matters. For example, he prefers to be called “professor” when he’s introduced to the public rather than “doctor” to avoid any confusion about his professional status.
More than 12,000 clinicians including physicians, medical students, nurses, pharmacists, and other health care professionals responded to the poll. The non-MD clinicians were the most likely to say it was always appropriate to be called “doctor” while physicians were the least likely.
Context matters
Large percentages of clinicians – 54% of doctors, 62% of medical students, and 41% of nurses – said that the context matters for being called “doctor.’’
“I earned my PhD in 1995 and my MD in 2000. I think it is contextual. In a research or University setting, “Dr.” seems appropriate for a PhD. That same person in public should probably not hold themselves out as “Dr.” So, maybe MDs and DOs can choose, while others maintain the title in their specific setting.”
Some readers proposed that people with MDs call themselves physicians rather than doctors. Said one: “Anyone with a terminal doctorate degree has the right to use the word doctor. As a physician when someone asks what I do, I say: ‘I am a physician.’ Problem solved. There can only be one physician but there are many types of doctors.”
Physicians and nurses differed most in their views. Just 24% of physicians said it was always appropriate for people with other doctorate degrees to call themselves doctor whereas about an equal number (22%) thought it was never appropriate.
In contrast, 43% of nurses (including advance practice nurses) said it was always appropriate for people with non-MD doctorates to be called doctor. Only 16% said it’s never appropriate.
This difference may reflect the growing number of nurses with doctorate degrees, either a DNP or PhD, who want to be called doctor in clinical settings.
Age made a difference too. Only 16% of physicians younger than age 45 said it was always appropriate for people with non-MD doctorate degrees to be called doctor, compared with 27% of physicians aged 45 and up.
Medical students (31%) were also more likely than physicians to say it was always appropriate for non-MD doctorates to use the title “doctor” and 64% said it depends on the context. This was noteworthy because twice as many medical students as physicians (16% vs. 8%) said they work in academia, research, or military government settings.
Too many ‘doctors’ confuse the public
Physicians (70%) were also more likely to say it was always or often confusing for the public to hear someone without a medical degree addressed as “doctor.” Only 6% of physicians thought it was never or rarely confusing.
Nurses disagreed. Just 45% said that it was always or often confusing while 16% said it was never or rarely confusing.
Medical students were more aligned with physicians on this issue – 60% said it was always or often confusing to the public and just 10% said it was never or rarely confusing.
One reader commented, “The problem is the confusion the ‘doctor’ title causes for patients, especially in a hospital setting. Is the ‘doctor’ a physician, a pharmacist, a psychologist, a nurse, etc., etc.? We need to think not of our own egos but if and how the confusion about this plethora of titles may be hindering good patient care.”
These concerns are not unfounded. The American Medical Association reported in its Truth in Advertising campaign that “patients mistake physicians with nonphysician providers” based on an online survey of 802 adults in 2018. The participants thought these specialists were MDs: dentists (61%), podiatrists (67%), optometrists (47%), psychologists (43%), doctors of nursing (39%), and chiropractors (27%).
The AMA has advocated that states pass the “Health Care Professional Transparency Act,” which New Jersey has enacted. The law requires all health care professionals dealing with patients to wear a name tag that clearly identifies their licensure. Health care professionals must also display their education, training, and licensure in their office.
A version of this article first appeared on Medscape.com.
Who Should Get to Be Called ‘Doctor’? shows. The topic has clearly struck a nerve, since a record number of respondents – over 12,000 – voted in the poll.
Most physicians think it’s appropriate for people with other doctorate degrees such as a PhD or EdD to call themselves ‘doctor,’ although slightly more than half said it depends on the context.
The controversy over who gets to be called a doctor was reignited when a Wall Street Journal opinion piece criticized First Lady Jill Biden, EdD, for wanting to be called “Dr Biden.” The piece also challenged the idea that having a PhD is worth the honorific of ‘doctor.’
Medical ethicist Arthur Caplan, PhD, disagreed with that viewpoint, saying the context matters. For example, he prefers to be called “professor” when he’s introduced to the public rather than “doctor” to avoid any confusion about his professional status.
More than 12,000 clinicians including physicians, medical students, nurses, pharmacists, and other health care professionals responded to the poll. The non-MD clinicians were the most likely to say it was always appropriate to be called “doctor” while physicians were the least likely.
Context matters
Large percentages of clinicians – 54% of doctors, 62% of medical students, and 41% of nurses – said that the context matters for being called “doctor.’’
“I earned my PhD in 1995 and my MD in 2000. I think it is contextual. In a research or University setting, “Dr.” seems appropriate for a PhD. That same person in public should probably not hold themselves out as “Dr.” So, maybe MDs and DOs can choose, while others maintain the title in their specific setting.”
Some readers proposed that people with MDs call themselves physicians rather than doctors. Said one: “Anyone with a terminal doctorate degree has the right to use the word doctor. As a physician when someone asks what I do, I say: ‘I am a physician.’ Problem solved. There can only be one physician but there are many types of doctors.”
Physicians and nurses differed most in their views. Just 24% of physicians said it was always appropriate for people with other doctorate degrees to call themselves doctor whereas about an equal number (22%) thought it was never appropriate.
In contrast, 43% of nurses (including advance practice nurses) said it was always appropriate for people with non-MD doctorates to be called doctor. Only 16% said it’s never appropriate.
This difference may reflect the growing number of nurses with doctorate degrees, either a DNP or PhD, who want to be called doctor in clinical settings.
Age made a difference too. Only 16% of physicians younger than age 45 said it was always appropriate for people with non-MD doctorate degrees to be called doctor, compared with 27% of physicians aged 45 and up.
Medical students (31%) were also more likely than physicians to say it was always appropriate for non-MD doctorates to use the title “doctor” and 64% said it depends on the context. This was noteworthy because twice as many medical students as physicians (16% vs. 8%) said they work in academia, research, or military government settings.
Too many ‘doctors’ confuse the public
Physicians (70%) were also more likely to say it was always or often confusing for the public to hear someone without a medical degree addressed as “doctor.” Only 6% of physicians thought it was never or rarely confusing.
Nurses disagreed. Just 45% said that it was always or often confusing while 16% said it was never or rarely confusing.
Medical students were more aligned with physicians on this issue – 60% said it was always or often confusing to the public and just 10% said it was never or rarely confusing.
One reader commented, “The problem is the confusion the ‘doctor’ title causes for patients, especially in a hospital setting. Is the ‘doctor’ a physician, a pharmacist, a psychologist, a nurse, etc., etc.? We need to think not of our own egos but if and how the confusion about this plethora of titles may be hindering good patient care.”
These concerns are not unfounded. The American Medical Association reported in its Truth in Advertising campaign that “patients mistake physicians with nonphysician providers” based on an online survey of 802 adults in 2018. The participants thought these specialists were MDs: dentists (61%), podiatrists (67%), optometrists (47%), psychologists (43%), doctors of nursing (39%), and chiropractors (27%).
The AMA has advocated that states pass the “Health Care Professional Transparency Act,” which New Jersey has enacted. The law requires all health care professionals dealing with patients to wear a name tag that clearly identifies their licensure. Health care professionals must also display their education, training, and licensure in their office.
A version of this article first appeared on Medscape.com.










