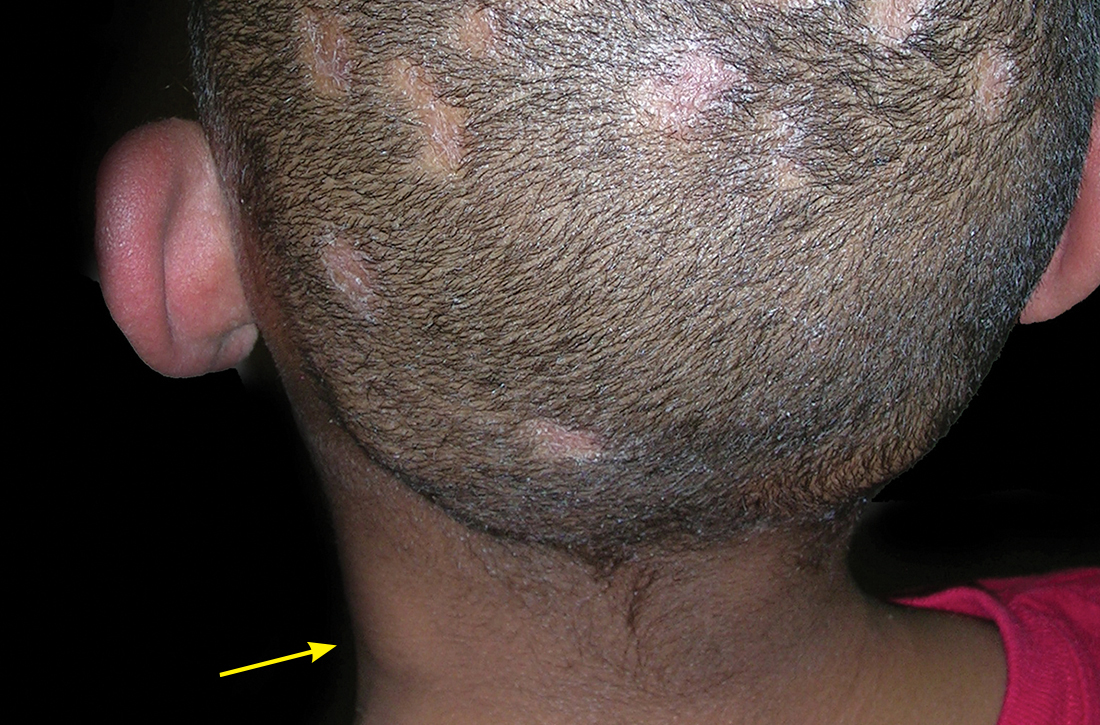
User login
Crohn Disease Medication Overview
Sigmoidoscopy screening cuts CRC mortality, incidence
Although endoscopic screening provides an opportunity for early identification and removal of premalignant polyps, data quantifying the long-term effects of sigmoidoscopy screening are lacking, corresponding author Frederik E. Juul, MD, said in an interview.
“Sigmoidoscopy screening have been shown to reduce colorectal cancer incidence and mortality, but it was unknown how long-lasting the effects were, and whether the effect differed by sex or age,” Dr. Juul said.
“For the first time, we were able to pool data from all four randomized sigmoidoscopy screening trials and include data from recent updates from two of the trials (U.S. and Italy), which means that we were able to answer these questions better than ever before,” he said.
In the pooled analysis, published in Annals of Internal Medicine, researchers from Norway, the United States, Italy, and the United Kingdom reviewed data from four studies with at least 15 years of follow-up. The analysis included 137,493 individuals randomized to at least one sigmoidoscopy screening and 137,459 randomized to usual care.
The primary outcomes were the incidence and mortality of CRC after sigmoidoscopy screening, compared with usual care, in adults with average CRC risk aged 55-64 years. Secondary outcomes included CRC incidence and mortality based on distal versus proximal colon, sex, and older versus younger age group (55-59 years vs. 60-64 years at study enrollment).
After 15 years’ follow-up, the pooled cumulative incidence of CRC was 1.84 cases per 100 persons in the screening group versus 2.35 cases per 100 persons in the usual-care group, representing a 21% reduction in incidence among those who were screened.
The pooled cumulative CRC mortality was 0.51 deaths per 100 persons in the screening group versus 0.65 deaths per 100 persons in the usual-care group, representing a 20% reduction in CRC mortality for those who were screened, the researchers noted. The all-cause mortality was reduced by 2% in the screening group compared with usual care; the pooled cumulative all-cause mortality was 14.3 deaths per 100 persons in the screening group versus 14.6 deaths per 100 persons in the usual-care group.
In terms of secondary outcomes, the significant reductions in CRC incidence and mortality were confined to the distal colon, with no significant differences observed in the proximal colon, the researchers noted. The reasons for this difference are unclear. Previous studies of three of the four trials showed a small reduction in CRC in the proximal colon, but may be related to the longer follow-up in the analysis of four trials.
The incidence of CRC varied by gender, with an incidence reduction of 25% for men versus 16% for women. The reasons for the gender difference are yet to be undetermined, but may include differences in the quality of bowel preparation, the greater technical challenge of screening women, and the higher incidence and proportion of proximal colon cancer versus distal colon cancer in women, the researchers noted.
“The long-term benefit of one single procedure was probably what surprised us the most,” Dr. Juul said in an interview. “Not only were the cumulative incidence and mortality lower in screened individuals 15 years after the procedure, but the yearly incidence was consistently lower in screened individuals compared to usual care, even at the end of the follow-up period.
“Although a previous study in Norway had indicated a sex difference in effect, we were surprised to see this in a pooled analysis across trials in four different countries,” he added.
Data may drive screening guidelines
The main finding of the study is that sigmoidoscopy screening with investigation of the distal colon provides at least 15 years of protection against colorectal cancer; “this may have an impact on how often average-risk individuals needs to be screened,” Dr. Juul said in an interview.
As for additional research, ongoing studies are examining primary colonoscopy screening, including a study recently published in the New England Journal of Medicine, Dr. Juul said.“Our study investigating sigmoidoscopy screening has a longer follow-up and it will be interesting to see if primary colonoscopy screening is equally or more effective as sigmoidoscopy at 15-years follow-up.”
More research is needed on direct comparisons of different colorectal cancer screening methods such as sigmoidoscopy and colonoscopy, said Dr. Juul. In addition, “The optimal surveillance interval in individuals identified at screening to be low- or high-risk of developing colorectal cancer are unknown,” he said.
“Our research group is involved in trials [the EPoS trials] looking into this question, but there are still years until we have the final results,” he added.
The findings were limited by several factors including the variation in methodology among the four trials and the lower number of individuals referred for colonoscopy in the U.K. and Italian trials, lack of analysis of potential confounding variables, and less granular data from the U.K. trial because of privacy regulations, the researchers wrote.
However, the findings were strengthened by the large study population, long-term follow-up, and detailed data, and they indicate a “significant and sustained” effect of screening sigmoidoscopy for the long-term reduction of CRC incidence and mortality, the authors concluded.
Findings can inform shared decision-making
“Colon cancer is the third-leading cause of death in the United States in men and women, and the second-leading cause of cancer deaths if we were to combine both genders,” Noel Deep, MD, said in an interview. “Sigmoidoscopy is more acceptable as a screening tool compared to a colonoscopy because of the lower risk of bowel injury, fewer side effects and less of a bowel prep, and also less need for sedation. This current study confirms prior data, including the 2012 PLCO trial, that it [sigmoidoscopy] reduces the incidence and mortality from colorectal cancer.”
The study findings were not surprising, given the prior knowledge and evidence of the benefits of sigmoidoscopy, Dr. Deep said, who was not involved in the study. However, “the fact that a single sigmoidoscopy led to decreased incidence and decreased mortality at 15 years was surprising to me, as current models suggest increasing incidence of proximal colon adenomas and cancers, which did not seem to be the case in this study.”
The current study can help primary care physicians and advance practice clinicians in patient counseling by supporting sigmoidoscopy as an option for patients who are unwilling to commit to a full colonoscopy, Dr. Deep said. However, “the patients should be advised that abnormal findings on the sigmoidoscopy would necessitate them being referred for a colonoscopy, and also the limitations of a sigmoidoscopy in detecting polyps or cancers in the cecum, ascending colon, transverse colon and descending colon.”
Looking ahead, “I would like to see research into the appropriate age for colorectal cancer screening using sigmoidoscopy and any benefit in offering this option at an earlier age,” Dr. Deep said. He also expressed a wish to know more about the reasons for the decreased benefit of screening sigmoidoscopy in women, and the reasons for the observed difference in all-cause mortality between genders.
“I would also like to see what the results of screening colonoscopies in a general population would reveal, and if it would reveal similar benefits, and also if there would be a gender difference or age-based difference in outcomes,” he said.
The study was supported by the Health Fund of South-East Norway. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
Although endoscopic screening provides an opportunity for early identification and removal of premalignant polyps, data quantifying the long-term effects of sigmoidoscopy screening are lacking, corresponding author Frederik E. Juul, MD, said in an interview.
“Sigmoidoscopy screening have been shown to reduce colorectal cancer incidence and mortality, but it was unknown how long-lasting the effects were, and whether the effect differed by sex or age,” Dr. Juul said.
“For the first time, we were able to pool data from all four randomized sigmoidoscopy screening trials and include data from recent updates from two of the trials (U.S. and Italy), which means that we were able to answer these questions better than ever before,” he said.
In the pooled analysis, published in Annals of Internal Medicine, researchers from Norway, the United States, Italy, and the United Kingdom reviewed data from four studies with at least 15 years of follow-up. The analysis included 137,493 individuals randomized to at least one sigmoidoscopy screening and 137,459 randomized to usual care.
The primary outcomes were the incidence and mortality of CRC after sigmoidoscopy screening, compared with usual care, in adults with average CRC risk aged 55-64 years. Secondary outcomes included CRC incidence and mortality based on distal versus proximal colon, sex, and older versus younger age group (55-59 years vs. 60-64 years at study enrollment).
After 15 years’ follow-up, the pooled cumulative incidence of CRC was 1.84 cases per 100 persons in the screening group versus 2.35 cases per 100 persons in the usual-care group, representing a 21% reduction in incidence among those who were screened.
The pooled cumulative CRC mortality was 0.51 deaths per 100 persons in the screening group versus 0.65 deaths per 100 persons in the usual-care group, representing a 20% reduction in CRC mortality for those who were screened, the researchers noted. The all-cause mortality was reduced by 2% in the screening group compared with usual care; the pooled cumulative all-cause mortality was 14.3 deaths per 100 persons in the screening group versus 14.6 deaths per 100 persons in the usual-care group.
In terms of secondary outcomes, the significant reductions in CRC incidence and mortality were confined to the distal colon, with no significant differences observed in the proximal colon, the researchers noted. The reasons for this difference are unclear. Previous studies of three of the four trials showed a small reduction in CRC in the proximal colon, but may be related to the longer follow-up in the analysis of four trials.
The incidence of CRC varied by gender, with an incidence reduction of 25% for men versus 16% for women. The reasons for the gender difference are yet to be undetermined, but may include differences in the quality of bowel preparation, the greater technical challenge of screening women, and the higher incidence and proportion of proximal colon cancer versus distal colon cancer in women, the researchers noted.
“The long-term benefit of one single procedure was probably what surprised us the most,” Dr. Juul said in an interview. “Not only were the cumulative incidence and mortality lower in screened individuals 15 years after the procedure, but the yearly incidence was consistently lower in screened individuals compared to usual care, even at the end of the follow-up period.
“Although a previous study in Norway had indicated a sex difference in effect, we were surprised to see this in a pooled analysis across trials in four different countries,” he added.
Data may drive screening guidelines
The main finding of the study is that sigmoidoscopy screening with investigation of the distal colon provides at least 15 years of protection against colorectal cancer; “this may have an impact on how often average-risk individuals needs to be screened,” Dr. Juul said in an interview.
As for additional research, ongoing studies are examining primary colonoscopy screening, including a study recently published in the New England Journal of Medicine, Dr. Juul said.“Our study investigating sigmoidoscopy screening has a longer follow-up and it will be interesting to see if primary colonoscopy screening is equally or more effective as sigmoidoscopy at 15-years follow-up.”
More research is needed on direct comparisons of different colorectal cancer screening methods such as sigmoidoscopy and colonoscopy, said Dr. Juul. In addition, “The optimal surveillance interval in individuals identified at screening to be low- or high-risk of developing colorectal cancer are unknown,” he said.
“Our research group is involved in trials [the EPoS trials] looking into this question, but there are still years until we have the final results,” he added.
The findings were limited by several factors including the variation in methodology among the four trials and the lower number of individuals referred for colonoscopy in the U.K. and Italian trials, lack of analysis of potential confounding variables, and less granular data from the U.K. trial because of privacy regulations, the researchers wrote.
However, the findings were strengthened by the large study population, long-term follow-up, and detailed data, and they indicate a “significant and sustained” effect of screening sigmoidoscopy for the long-term reduction of CRC incidence and mortality, the authors concluded.
Findings can inform shared decision-making
“Colon cancer is the third-leading cause of death in the United States in men and women, and the second-leading cause of cancer deaths if we were to combine both genders,” Noel Deep, MD, said in an interview. “Sigmoidoscopy is more acceptable as a screening tool compared to a colonoscopy because of the lower risk of bowel injury, fewer side effects and less of a bowel prep, and also less need for sedation. This current study confirms prior data, including the 2012 PLCO trial, that it [sigmoidoscopy] reduces the incidence and mortality from colorectal cancer.”
The study findings were not surprising, given the prior knowledge and evidence of the benefits of sigmoidoscopy, Dr. Deep said, who was not involved in the study. However, “the fact that a single sigmoidoscopy led to decreased incidence and decreased mortality at 15 years was surprising to me, as current models suggest increasing incidence of proximal colon adenomas and cancers, which did not seem to be the case in this study.”
The current study can help primary care physicians and advance practice clinicians in patient counseling by supporting sigmoidoscopy as an option for patients who are unwilling to commit to a full colonoscopy, Dr. Deep said. However, “the patients should be advised that abnormal findings on the sigmoidoscopy would necessitate them being referred for a colonoscopy, and also the limitations of a sigmoidoscopy in detecting polyps or cancers in the cecum, ascending colon, transverse colon and descending colon.”
Looking ahead, “I would like to see research into the appropriate age for colorectal cancer screening using sigmoidoscopy and any benefit in offering this option at an earlier age,” Dr. Deep said. He also expressed a wish to know more about the reasons for the decreased benefit of screening sigmoidoscopy in women, and the reasons for the observed difference in all-cause mortality between genders.
“I would also like to see what the results of screening colonoscopies in a general population would reveal, and if it would reveal similar benefits, and also if there would be a gender difference or age-based difference in outcomes,” he said.
The study was supported by the Health Fund of South-East Norway. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
Although endoscopic screening provides an opportunity for early identification and removal of premalignant polyps, data quantifying the long-term effects of sigmoidoscopy screening are lacking, corresponding author Frederik E. Juul, MD, said in an interview.
“Sigmoidoscopy screening have been shown to reduce colorectal cancer incidence and mortality, but it was unknown how long-lasting the effects were, and whether the effect differed by sex or age,” Dr. Juul said.
“For the first time, we were able to pool data from all four randomized sigmoidoscopy screening trials and include data from recent updates from two of the trials (U.S. and Italy), which means that we were able to answer these questions better than ever before,” he said.
In the pooled analysis, published in Annals of Internal Medicine, researchers from Norway, the United States, Italy, and the United Kingdom reviewed data from four studies with at least 15 years of follow-up. The analysis included 137,493 individuals randomized to at least one sigmoidoscopy screening and 137,459 randomized to usual care.
The primary outcomes were the incidence and mortality of CRC after sigmoidoscopy screening, compared with usual care, in adults with average CRC risk aged 55-64 years. Secondary outcomes included CRC incidence and mortality based on distal versus proximal colon, sex, and older versus younger age group (55-59 years vs. 60-64 years at study enrollment).
After 15 years’ follow-up, the pooled cumulative incidence of CRC was 1.84 cases per 100 persons in the screening group versus 2.35 cases per 100 persons in the usual-care group, representing a 21% reduction in incidence among those who were screened.
The pooled cumulative CRC mortality was 0.51 deaths per 100 persons in the screening group versus 0.65 deaths per 100 persons in the usual-care group, representing a 20% reduction in CRC mortality for those who were screened, the researchers noted. The all-cause mortality was reduced by 2% in the screening group compared with usual care; the pooled cumulative all-cause mortality was 14.3 deaths per 100 persons in the screening group versus 14.6 deaths per 100 persons in the usual-care group.
In terms of secondary outcomes, the significant reductions in CRC incidence and mortality were confined to the distal colon, with no significant differences observed in the proximal colon, the researchers noted. The reasons for this difference are unclear. Previous studies of three of the four trials showed a small reduction in CRC in the proximal colon, but may be related to the longer follow-up in the analysis of four trials.
The incidence of CRC varied by gender, with an incidence reduction of 25% for men versus 16% for women. The reasons for the gender difference are yet to be undetermined, but may include differences in the quality of bowel preparation, the greater technical challenge of screening women, and the higher incidence and proportion of proximal colon cancer versus distal colon cancer in women, the researchers noted.
“The long-term benefit of one single procedure was probably what surprised us the most,” Dr. Juul said in an interview. “Not only were the cumulative incidence and mortality lower in screened individuals 15 years after the procedure, but the yearly incidence was consistently lower in screened individuals compared to usual care, even at the end of the follow-up period.
“Although a previous study in Norway had indicated a sex difference in effect, we were surprised to see this in a pooled analysis across trials in four different countries,” he added.
Data may drive screening guidelines
The main finding of the study is that sigmoidoscopy screening with investigation of the distal colon provides at least 15 years of protection against colorectal cancer; “this may have an impact on how often average-risk individuals needs to be screened,” Dr. Juul said in an interview.
As for additional research, ongoing studies are examining primary colonoscopy screening, including a study recently published in the New England Journal of Medicine, Dr. Juul said.“Our study investigating sigmoidoscopy screening has a longer follow-up and it will be interesting to see if primary colonoscopy screening is equally or more effective as sigmoidoscopy at 15-years follow-up.”
More research is needed on direct comparisons of different colorectal cancer screening methods such as sigmoidoscopy and colonoscopy, said Dr. Juul. In addition, “The optimal surveillance interval in individuals identified at screening to be low- or high-risk of developing colorectal cancer are unknown,” he said.
“Our research group is involved in trials [the EPoS trials] looking into this question, but there are still years until we have the final results,” he added.
The findings were limited by several factors including the variation in methodology among the four trials and the lower number of individuals referred for colonoscopy in the U.K. and Italian trials, lack of analysis of potential confounding variables, and less granular data from the U.K. trial because of privacy regulations, the researchers wrote.
However, the findings were strengthened by the large study population, long-term follow-up, and detailed data, and they indicate a “significant and sustained” effect of screening sigmoidoscopy for the long-term reduction of CRC incidence and mortality, the authors concluded.
Findings can inform shared decision-making
“Colon cancer is the third-leading cause of death in the United States in men and women, and the second-leading cause of cancer deaths if we were to combine both genders,” Noel Deep, MD, said in an interview. “Sigmoidoscopy is more acceptable as a screening tool compared to a colonoscopy because of the lower risk of bowel injury, fewer side effects and less of a bowel prep, and also less need for sedation. This current study confirms prior data, including the 2012 PLCO trial, that it [sigmoidoscopy] reduces the incidence and mortality from colorectal cancer.”
The study findings were not surprising, given the prior knowledge and evidence of the benefits of sigmoidoscopy, Dr. Deep said, who was not involved in the study. However, “the fact that a single sigmoidoscopy led to decreased incidence and decreased mortality at 15 years was surprising to me, as current models suggest increasing incidence of proximal colon adenomas and cancers, which did not seem to be the case in this study.”
The current study can help primary care physicians and advance practice clinicians in patient counseling by supporting sigmoidoscopy as an option for patients who are unwilling to commit to a full colonoscopy, Dr. Deep said. However, “the patients should be advised that abnormal findings on the sigmoidoscopy would necessitate them being referred for a colonoscopy, and also the limitations of a sigmoidoscopy in detecting polyps or cancers in the cecum, ascending colon, transverse colon and descending colon.”
Looking ahead, “I would like to see research into the appropriate age for colorectal cancer screening using sigmoidoscopy and any benefit in offering this option at an earlier age,” Dr. Deep said. He also expressed a wish to know more about the reasons for the decreased benefit of screening sigmoidoscopy in women, and the reasons for the observed difference in all-cause mortality between genders.
“I would also like to see what the results of screening colonoscopies in a general population would reveal, and if it would reveal similar benefits, and also if there would be a gender difference or age-based difference in outcomes,” he said.
The study was supported by the Health Fund of South-East Norway. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
FROM ANNALS OF INTERNAL MEDICINE
Longer boarding times predict patient processing in ED
on the basis of data from nearly 900 facilities presented at the annual meeting of the American College of Emergency Physicians.
The study was important to conduct at this time because ED boarding is significantly limiting ED physicians to provide optimal care, said Camila Tyminski, MD, of Brown University, Providence, R.I., who presented the findings at the meeting.
“Boarding had steadily been rising prior to the COVID-19 pandemic due to increased ED use. As our data show, boarding had a detrimental impact on ED throughput measures, including increased door to provider time, increased length of stay of the patient discharged from the ED, and increased rate of patients that left before completion of treatment,” she said.
“It was important to understand these trends prior to 2019-2020 because the COVID-19 pandemic and national nursing shortage have drastically worsened boarding. This study provided a framework for future studies on boarding across ED’s nationally since the start of the pandemic,” she added.
“Post-pandemic, we have hit a crisis point,” lead author Anthony Napoli, MD, also of Brown University, said in an interview. “Boarding is largely a hospital capacity problem, but one key fix germane to EM [emergency medicine] is the provider in triage model (PIT). While PIT has been shown to improve efficiency of ED care, a single institution study demonstrated that it was unable to mitigate the effects of boarding. The study of the association of boarding and efficiency of ED operations and intake needed to be shown on a national scale,” he said.
The researchers reviewed cross-sectional ED operational data from the ED Department Benchmarking Alliance (EDBA), a voluntary database that includes self-reports of operational metrics from approximately half of EDs in the United States.
The data set included 892 EDs; freestanding and pediatric EDs, as well as those with missing boarding data, were excluded.
The primary outcome was boarding time, door-to-provider time (D2P), length of stay for discharged patients (LOSD) and the percentage of patients who left the hospital before treatment was complete (LBTC).
In a multivariate analysis, increased boarding time was significantly associated with longer D2P time, LOSD time, and rates of LBTC.
Overall, D2P and LOSD increased by 0.8 minutes and 2.8 minutes, respectively, for each additional 10 minutes of boarding time. LBTC rates increased by 0.1% for each additional 10 minutes of boarding time.
However, boarding did not have a significant impact on operational metrics among hospitals with fewer than 20,000 visits per year.
Although more research is needed, the results indicate that boarding reduces the throughput of nonboarded patients at a ratio of approximately 4:1. The limited impact of ED efficiency measures on operations highlights the need for hospital-based solutions to boarding, Dr. Tyminski concluded.
“Overall, we expected that there would be an association between boarding and reductions in ED intake and operational efficiency,” said Dr. Napoli in an interview. “However, we were surprised the relationship continued to be as strong in a national study of nearly a quarter of all EDs, as it did in our prior local study,” he said. “Every 10 minutes of boarding in an ED is associated with an approximate 0.1% increase in LWBS and a 3-minute increase in LOSD. Extrapolating this association across the country, we predicted that nearly one million patients may have potentially not received ED care due to boarding,” he explained. “Not only does this potentially have a huge impact on hospital finances but also the overall health of our patients,” he added.
The key takeaway from the study is that boarding is a hospital capacity management issue, said Dr. Napoli. Hospital leadership must be directly involved in plans to mitigate or eliminate it to the extent possible; until then, boarding will continue to result in inefficient ED operations, he explained.
“As ED providers, we are limited in what we can do, but one area where we might be able to make the most impact is to optimize the care and throughput of the LOSD patients,” Dr. Tyminski said. More research is needed to see if interventions to reduce boarding correspond with equivalent improvements in emergency department intake and improved ED throughput, she noted.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
on the basis of data from nearly 900 facilities presented at the annual meeting of the American College of Emergency Physicians.
The study was important to conduct at this time because ED boarding is significantly limiting ED physicians to provide optimal care, said Camila Tyminski, MD, of Brown University, Providence, R.I., who presented the findings at the meeting.
“Boarding had steadily been rising prior to the COVID-19 pandemic due to increased ED use. As our data show, boarding had a detrimental impact on ED throughput measures, including increased door to provider time, increased length of stay of the patient discharged from the ED, and increased rate of patients that left before completion of treatment,” she said.
“It was important to understand these trends prior to 2019-2020 because the COVID-19 pandemic and national nursing shortage have drastically worsened boarding. This study provided a framework for future studies on boarding across ED’s nationally since the start of the pandemic,” she added.
“Post-pandemic, we have hit a crisis point,” lead author Anthony Napoli, MD, also of Brown University, said in an interview. “Boarding is largely a hospital capacity problem, but one key fix germane to EM [emergency medicine] is the provider in triage model (PIT). While PIT has been shown to improve efficiency of ED care, a single institution study demonstrated that it was unable to mitigate the effects of boarding. The study of the association of boarding and efficiency of ED operations and intake needed to be shown on a national scale,” he said.
The researchers reviewed cross-sectional ED operational data from the ED Department Benchmarking Alliance (EDBA), a voluntary database that includes self-reports of operational metrics from approximately half of EDs in the United States.
The data set included 892 EDs; freestanding and pediatric EDs, as well as those with missing boarding data, were excluded.
The primary outcome was boarding time, door-to-provider time (D2P), length of stay for discharged patients (LOSD) and the percentage of patients who left the hospital before treatment was complete (LBTC).
In a multivariate analysis, increased boarding time was significantly associated with longer D2P time, LOSD time, and rates of LBTC.
Overall, D2P and LOSD increased by 0.8 minutes and 2.8 minutes, respectively, for each additional 10 minutes of boarding time. LBTC rates increased by 0.1% for each additional 10 minutes of boarding time.
However, boarding did not have a significant impact on operational metrics among hospitals with fewer than 20,000 visits per year.
Although more research is needed, the results indicate that boarding reduces the throughput of nonboarded patients at a ratio of approximately 4:1. The limited impact of ED efficiency measures on operations highlights the need for hospital-based solutions to boarding, Dr. Tyminski concluded.
“Overall, we expected that there would be an association between boarding and reductions in ED intake and operational efficiency,” said Dr. Napoli in an interview. “However, we were surprised the relationship continued to be as strong in a national study of nearly a quarter of all EDs, as it did in our prior local study,” he said. “Every 10 minutes of boarding in an ED is associated with an approximate 0.1% increase in LWBS and a 3-minute increase in LOSD. Extrapolating this association across the country, we predicted that nearly one million patients may have potentially not received ED care due to boarding,” he explained. “Not only does this potentially have a huge impact on hospital finances but also the overall health of our patients,” he added.
The key takeaway from the study is that boarding is a hospital capacity management issue, said Dr. Napoli. Hospital leadership must be directly involved in plans to mitigate or eliminate it to the extent possible; until then, boarding will continue to result in inefficient ED operations, he explained.
“As ED providers, we are limited in what we can do, but one area where we might be able to make the most impact is to optimize the care and throughput of the LOSD patients,” Dr. Tyminski said. More research is needed to see if interventions to reduce boarding correspond with equivalent improvements in emergency department intake and improved ED throughput, she noted.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
on the basis of data from nearly 900 facilities presented at the annual meeting of the American College of Emergency Physicians.
The study was important to conduct at this time because ED boarding is significantly limiting ED physicians to provide optimal care, said Camila Tyminski, MD, of Brown University, Providence, R.I., who presented the findings at the meeting.
“Boarding had steadily been rising prior to the COVID-19 pandemic due to increased ED use. As our data show, boarding had a detrimental impact on ED throughput measures, including increased door to provider time, increased length of stay of the patient discharged from the ED, and increased rate of patients that left before completion of treatment,” she said.
“It was important to understand these trends prior to 2019-2020 because the COVID-19 pandemic and national nursing shortage have drastically worsened boarding. This study provided a framework for future studies on boarding across ED’s nationally since the start of the pandemic,” she added.
“Post-pandemic, we have hit a crisis point,” lead author Anthony Napoli, MD, also of Brown University, said in an interview. “Boarding is largely a hospital capacity problem, but one key fix germane to EM [emergency medicine] is the provider in triage model (PIT). While PIT has been shown to improve efficiency of ED care, a single institution study demonstrated that it was unable to mitigate the effects of boarding. The study of the association of boarding and efficiency of ED operations and intake needed to be shown on a national scale,” he said.
The researchers reviewed cross-sectional ED operational data from the ED Department Benchmarking Alliance (EDBA), a voluntary database that includes self-reports of operational metrics from approximately half of EDs in the United States.
The data set included 892 EDs; freestanding and pediatric EDs, as well as those with missing boarding data, were excluded.
The primary outcome was boarding time, door-to-provider time (D2P), length of stay for discharged patients (LOSD) and the percentage of patients who left the hospital before treatment was complete (LBTC).
In a multivariate analysis, increased boarding time was significantly associated with longer D2P time, LOSD time, and rates of LBTC.
Overall, D2P and LOSD increased by 0.8 minutes and 2.8 minutes, respectively, for each additional 10 minutes of boarding time. LBTC rates increased by 0.1% for each additional 10 minutes of boarding time.
However, boarding did not have a significant impact on operational metrics among hospitals with fewer than 20,000 visits per year.
Although more research is needed, the results indicate that boarding reduces the throughput of nonboarded patients at a ratio of approximately 4:1. The limited impact of ED efficiency measures on operations highlights the need for hospital-based solutions to boarding, Dr. Tyminski concluded.
“Overall, we expected that there would be an association between boarding and reductions in ED intake and operational efficiency,” said Dr. Napoli in an interview. “However, we were surprised the relationship continued to be as strong in a national study of nearly a quarter of all EDs, as it did in our prior local study,” he said. “Every 10 minutes of boarding in an ED is associated with an approximate 0.1% increase in LWBS and a 3-minute increase in LOSD. Extrapolating this association across the country, we predicted that nearly one million patients may have potentially not received ED care due to boarding,” he explained. “Not only does this potentially have a huge impact on hospital finances but also the overall health of our patients,” he added.
The key takeaway from the study is that boarding is a hospital capacity management issue, said Dr. Napoli. Hospital leadership must be directly involved in plans to mitigate or eliminate it to the extent possible; until then, boarding will continue to result in inefficient ED operations, he explained.
“As ED providers, we are limited in what we can do, but one area where we might be able to make the most impact is to optimize the care and throughput of the LOSD patients,” Dr. Tyminski said. More research is needed to see if interventions to reduce boarding correspond with equivalent improvements in emergency department intake and improved ED throughput, she noted.
The study received no outside funding. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
Like texting and driving: The human cost of AI
A recent medical meeting I attended included multiple sessions on the use of artificial intelligence (AI), a mere preview, I suspect, of what is to come for both patients and physicians.
I vow not to be a contrarian, but I have concerns. If we’d known how cell phones would permeate nearly every waking moment of our lives, would we have built in more protections from the onset?
Although anyone can see the enormous potential of AI in medicine, harnessing the wonders of it without guarding against the dangers could be paramount to texting and driving.
A palpable disruption in the common work-a-day human interaction is a given. CEOs who mind the bottom line will seek every opportunity to cut personnel whenever machine learning can deliver. As our dependence on algorithms increases, our need to understand electrocardiogram interpretation and echocardiographic calculations will wane. Subtle case information will go undetected. Nuanced subconscious alerts regarding the patient condition will go unnoticed.
These realities are never reflected in the pronouncements of companies who promote and develop AI.
The 2-minute echo
In September 2020, Carolyn Lam, MBBS, PhD, and James Hare, MBA, founders of the AI tech company US2.AI, told Healthcare Transformers that AI advances in echocardiology will turn “a manual process of 30 minutes, 250 clicks, with up to 21% variability among fully trained sonographers analyzing the same exam, into an AI-automated process taking 2 minutes, 1 click, with 0% variability.”
Let’s contrast this 2-minute human-machine interaction with the standard 20- to 30-minute human-to-human echocardiography procedure.
Take Mrs. Smith, for instance. She is referred for echocardiography for shortness of breath. She’s shown to a room and instructed to lie down on a table, where she undergoes a brief AI-directed acquisition of images and then a cheery dismissal from the imaging lab. Medical corporate chief financial officers will salivate at the efficiency, the decrease in cost for personnel, and the sharp increase in put-through for the echo lab schedule.
But what if Mrs. Smith gets a standard 30-minute sonographer-directed exam and the astute echocardiographer notes a left ventricular ejection fraction of 38%. A conversation with the patient reveals that she lost her son a few weeks ago. Upon completion of the study, the patient stands up and then adds, “I hope I can sleep in my bed tonight.” Thinking there may be more to the patient’s insomnia than grief-driven anxiety, the sonographer asks her to explain. “I had to sleep in a chair last night because I couldn’t breathe,” Mrs. Smith replies.
The sonographer reasons correctly that Mrs. Smith is likely a few weeks past an acute coronary syndrome for which she didn’t seek attention and is now in heart failure. The consulting cardiologist is alerted. Mrs. Smith is worked into the office schedule a week earlier than planned, and a costly in-patient stay for acute heart failure or worse is avoided.
Here’s a true-life example (some details have been changed to protect the patient’s identity): Mr. Rodriquez was referred for echocardiography because of dizziness. The sonographer notes significant mitral regurgitation and a decline in left ventricular ejection fraction from moderately impaired to severely reduced. When the sonographer inquires about a fresh bruise over Mr. Rodriguez’s left eye, he replies that he “must have fallen, but can’t remember.” The sonographer also notes runs of nonsustained ventricular tachycardia on the echo telemetry, and after a phone call from the echo lab to the ordering physician, Mr. Rodriquez is admitted. Instead of chancing a sudden death at home while awaiting follow-up, he undergoes catheterization and gets an implantable cardioverter defibrillator.
These scenarios illustrate that a 2-minute visit for AI-directed acquisition of echocardiogram images will never garner the protections of a conversation with a human. Any attempts at downplaying the importance of these human interactions are misguided.
Sometimes we embrace the latest advances in medicine while failing to tend to the most rudimentary necessities of data analysis and reporting. Catherine M. Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle, is a fan of the basics.
At the recent annual congress of the European Society of Cardiology, she commented on the AI-ENHANCED trial, which used an AI decision support algorithm to identify patients with moderate to severe aortic stenosis, which is associated with poor survival if left untreated. She correctly highlighted that while we are discussing the merits of AI-driven assessment of aortic stenosis, we are doing so in an era when many echo interpreters exclude critical information. The vital findings of aortic valve area, Vmax, and ejection fraction are often nowhere to be seen on reports. We should attend to our basic flaws in interpretation and reporting before we shift our focus to AI.
Flawed algorithms
Incorrect AI algorithms that are broadly adopted could negatively affect the health of millions.
Perhaps the most unsettling claim is made by causaLens: “Causal AI is the only technology that can reason and make choices like humans do,” the website states. A tantalizing tag line that is categorically untrue.
Our mysterious and complex neurophysiological function of reasoning still eludes understanding, but one thing is certain: medical reasoning originates with listening, seeing, and touching.
As AI infiltrates mainstream medicine, opportunities for hearing, observing, and palpating will be greatly reduced.
Folkert Asselbergs from University Medical Center Utrecht, the Netherlands, who has cautioned against overhyping AI, was the discussant for an ESC study on the use of causal AI to improve cardiovascular risk estimation.
He flashed a slide of a 2019 Science article on racial bias in an algorithm that U.S. health care systems use. Remedying that bias “would increase the percentage of Black people receiving additional help from 17.7% to 46.5%,” according to the authors.
Successful integration of AI-driven technology will come only if we build human interaction into every patient encounter.
I hope I don’t live to see the rise of the physician cyborg.
Artificial intelligence could be the greatest boon since the invention of the stethoscope, but it will be our downfall if we stop administering a healthy dose of humanity to every patient encounter.
Melissa Walton-Shirley, MD, is a clinical cardiologist in Nashville, Tenn., who has retired from full-time invasive cardiology. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A recent medical meeting I attended included multiple sessions on the use of artificial intelligence (AI), a mere preview, I suspect, of what is to come for both patients and physicians.
I vow not to be a contrarian, but I have concerns. If we’d known how cell phones would permeate nearly every waking moment of our lives, would we have built in more protections from the onset?
Although anyone can see the enormous potential of AI in medicine, harnessing the wonders of it without guarding against the dangers could be paramount to texting and driving.
A palpable disruption in the common work-a-day human interaction is a given. CEOs who mind the bottom line will seek every opportunity to cut personnel whenever machine learning can deliver. As our dependence on algorithms increases, our need to understand electrocardiogram interpretation and echocardiographic calculations will wane. Subtle case information will go undetected. Nuanced subconscious alerts regarding the patient condition will go unnoticed.
These realities are never reflected in the pronouncements of companies who promote and develop AI.
The 2-minute echo
In September 2020, Carolyn Lam, MBBS, PhD, and James Hare, MBA, founders of the AI tech company US2.AI, told Healthcare Transformers that AI advances in echocardiology will turn “a manual process of 30 minutes, 250 clicks, with up to 21% variability among fully trained sonographers analyzing the same exam, into an AI-automated process taking 2 minutes, 1 click, with 0% variability.”
Let’s contrast this 2-minute human-machine interaction with the standard 20- to 30-minute human-to-human echocardiography procedure.
Take Mrs. Smith, for instance. She is referred for echocardiography for shortness of breath. She’s shown to a room and instructed to lie down on a table, where she undergoes a brief AI-directed acquisition of images and then a cheery dismissal from the imaging lab. Medical corporate chief financial officers will salivate at the efficiency, the decrease in cost for personnel, and the sharp increase in put-through for the echo lab schedule.
But what if Mrs. Smith gets a standard 30-minute sonographer-directed exam and the astute echocardiographer notes a left ventricular ejection fraction of 38%. A conversation with the patient reveals that she lost her son a few weeks ago. Upon completion of the study, the patient stands up and then adds, “I hope I can sleep in my bed tonight.” Thinking there may be more to the patient’s insomnia than grief-driven anxiety, the sonographer asks her to explain. “I had to sleep in a chair last night because I couldn’t breathe,” Mrs. Smith replies.
The sonographer reasons correctly that Mrs. Smith is likely a few weeks past an acute coronary syndrome for which she didn’t seek attention and is now in heart failure. The consulting cardiologist is alerted. Mrs. Smith is worked into the office schedule a week earlier than planned, and a costly in-patient stay for acute heart failure or worse is avoided.
Here’s a true-life example (some details have been changed to protect the patient’s identity): Mr. Rodriquez was referred for echocardiography because of dizziness. The sonographer notes significant mitral regurgitation and a decline in left ventricular ejection fraction from moderately impaired to severely reduced. When the sonographer inquires about a fresh bruise over Mr. Rodriguez’s left eye, he replies that he “must have fallen, but can’t remember.” The sonographer also notes runs of nonsustained ventricular tachycardia on the echo telemetry, and after a phone call from the echo lab to the ordering physician, Mr. Rodriquez is admitted. Instead of chancing a sudden death at home while awaiting follow-up, he undergoes catheterization and gets an implantable cardioverter defibrillator.
These scenarios illustrate that a 2-minute visit for AI-directed acquisition of echocardiogram images will never garner the protections of a conversation with a human. Any attempts at downplaying the importance of these human interactions are misguided.
Sometimes we embrace the latest advances in medicine while failing to tend to the most rudimentary necessities of data analysis and reporting. Catherine M. Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle, is a fan of the basics.
At the recent annual congress of the European Society of Cardiology, she commented on the AI-ENHANCED trial, which used an AI decision support algorithm to identify patients with moderate to severe aortic stenosis, which is associated with poor survival if left untreated. She correctly highlighted that while we are discussing the merits of AI-driven assessment of aortic stenosis, we are doing so in an era when many echo interpreters exclude critical information. The vital findings of aortic valve area, Vmax, and ejection fraction are often nowhere to be seen on reports. We should attend to our basic flaws in interpretation and reporting before we shift our focus to AI.
Flawed algorithms
Incorrect AI algorithms that are broadly adopted could negatively affect the health of millions.
Perhaps the most unsettling claim is made by causaLens: “Causal AI is the only technology that can reason and make choices like humans do,” the website states. A tantalizing tag line that is categorically untrue.
Our mysterious and complex neurophysiological function of reasoning still eludes understanding, but one thing is certain: medical reasoning originates with listening, seeing, and touching.
As AI infiltrates mainstream medicine, opportunities for hearing, observing, and palpating will be greatly reduced.
Folkert Asselbergs from University Medical Center Utrecht, the Netherlands, who has cautioned against overhyping AI, was the discussant for an ESC study on the use of causal AI to improve cardiovascular risk estimation.
He flashed a slide of a 2019 Science article on racial bias in an algorithm that U.S. health care systems use. Remedying that bias “would increase the percentage of Black people receiving additional help from 17.7% to 46.5%,” according to the authors.
Successful integration of AI-driven technology will come only if we build human interaction into every patient encounter.
I hope I don’t live to see the rise of the physician cyborg.
Artificial intelligence could be the greatest boon since the invention of the stethoscope, but it will be our downfall if we stop administering a healthy dose of humanity to every patient encounter.
Melissa Walton-Shirley, MD, is a clinical cardiologist in Nashville, Tenn., who has retired from full-time invasive cardiology. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A recent medical meeting I attended included multiple sessions on the use of artificial intelligence (AI), a mere preview, I suspect, of what is to come for both patients and physicians.
I vow not to be a contrarian, but I have concerns. If we’d known how cell phones would permeate nearly every waking moment of our lives, would we have built in more protections from the onset?
Although anyone can see the enormous potential of AI in medicine, harnessing the wonders of it without guarding against the dangers could be paramount to texting and driving.
A palpable disruption in the common work-a-day human interaction is a given. CEOs who mind the bottom line will seek every opportunity to cut personnel whenever machine learning can deliver. As our dependence on algorithms increases, our need to understand electrocardiogram interpretation and echocardiographic calculations will wane. Subtle case information will go undetected. Nuanced subconscious alerts regarding the patient condition will go unnoticed.
These realities are never reflected in the pronouncements of companies who promote and develop AI.
The 2-minute echo
In September 2020, Carolyn Lam, MBBS, PhD, and James Hare, MBA, founders of the AI tech company US2.AI, told Healthcare Transformers that AI advances in echocardiology will turn “a manual process of 30 minutes, 250 clicks, with up to 21% variability among fully trained sonographers analyzing the same exam, into an AI-automated process taking 2 minutes, 1 click, with 0% variability.”
Let’s contrast this 2-minute human-machine interaction with the standard 20- to 30-minute human-to-human echocardiography procedure.
Take Mrs. Smith, for instance. She is referred for echocardiography for shortness of breath. She’s shown to a room and instructed to lie down on a table, where she undergoes a brief AI-directed acquisition of images and then a cheery dismissal from the imaging lab. Medical corporate chief financial officers will salivate at the efficiency, the decrease in cost for personnel, and the sharp increase in put-through for the echo lab schedule.
But what if Mrs. Smith gets a standard 30-minute sonographer-directed exam and the astute echocardiographer notes a left ventricular ejection fraction of 38%. A conversation with the patient reveals that she lost her son a few weeks ago. Upon completion of the study, the patient stands up and then adds, “I hope I can sleep in my bed tonight.” Thinking there may be more to the patient’s insomnia than grief-driven anxiety, the sonographer asks her to explain. “I had to sleep in a chair last night because I couldn’t breathe,” Mrs. Smith replies.
The sonographer reasons correctly that Mrs. Smith is likely a few weeks past an acute coronary syndrome for which she didn’t seek attention and is now in heart failure. The consulting cardiologist is alerted. Mrs. Smith is worked into the office schedule a week earlier than planned, and a costly in-patient stay for acute heart failure or worse is avoided.
Here’s a true-life example (some details have been changed to protect the patient’s identity): Mr. Rodriquez was referred for echocardiography because of dizziness. The sonographer notes significant mitral regurgitation and a decline in left ventricular ejection fraction from moderately impaired to severely reduced. When the sonographer inquires about a fresh bruise over Mr. Rodriguez’s left eye, he replies that he “must have fallen, but can’t remember.” The sonographer also notes runs of nonsustained ventricular tachycardia on the echo telemetry, and after a phone call from the echo lab to the ordering physician, Mr. Rodriquez is admitted. Instead of chancing a sudden death at home while awaiting follow-up, he undergoes catheterization and gets an implantable cardioverter defibrillator.
These scenarios illustrate that a 2-minute visit for AI-directed acquisition of echocardiogram images will never garner the protections of a conversation with a human. Any attempts at downplaying the importance of these human interactions are misguided.
Sometimes we embrace the latest advances in medicine while failing to tend to the most rudimentary necessities of data analysis and reporting. Catherine M. Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle, is a fan of the basics.
At the recent annual congress of the European Society of Cardiology, she commented on the AI-ENHANCED trial, which used an AI decision support algorithm to identify patients with moderate to severe aortic stenosis, which is associated with poor survival if left untreated. She correctly highlighted that while we are discussing the merits of AI-driven assessment of aortic stenosis, we are doing so in an era when many echo interpreters exclude critical information. The vital findings of aortic valve area, Vmax, and ejection fraction are often nowhere to be seen on reports. We should attend to our basic flaws in interpretation and reporting before we shift our focus to AI.
Flawed algorithms
Incorrect AI algorithms that are broadly adopted could negatively affect the health of millions.
Perhaps the most unsettling claim is made by causaLens: “Causal AI is the only technology that can reason and make choices like humans do,” the website states. A tantalizing tag line that is categorically untrue.
Our mysterious and complex neurophysiological function of reasoning still eludes understanding, but one thing is certain: medical reasoning originates with listening, seeing, and touching.
As AI infiltrates mainstream medicine, opportunities for hearing, observing, and palpating will be greatly reduced.
Folkert Asselbergs from University Medical Center Utrecht, the Netherlands, who has cautioned against overhyping AI, was the discussant for an ESC study on the use of causal AI to improve cardiovascular risk estimation.
He flashed a slide of a 2019 Science article on racial bias in an algorithm that U.S. health care systems use. Remedying that bias “would increase the percentage of Black people receiving additional help from 17.7% to 46.5%,” according to the authors.
Successful integration of AI-driven technology will come only if we build human interaction into every patient encounter.
I hope I don’t live to see the rise of the physician cyborg.
Artificial intelligence could be the greatest boon since the invention of the stethoscope, but it will be our downfall if we stop administering a healthy dose of humanity to every patient encounter.
Melissa Walton-Shirley, MD, is a clinical cardiologist in Nashville, Tenn., who has retired from full-time invasive cardiology. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Dapagliflozin DELIVERs regardless of systolic pressure in HFpEF
Whatever the mechanism of benefit from dapagliflozin (Farxiga) in patients with heart failure (HF) – and potentially also other sodium-glucose cotransporter 2 (SGLT2) inhibitors – its blood pressure lowering effects aren’t likely to contribute much.
Indeed, at least in patients with HF and non-reduced ejection fractions, dapagliflozin has only a modest BP-lowering effect and cuts cardiovascular (CV) risk regardless of baseline pressure or change in systolic BP, suggests a secondary analysis from the large placebo-controlled DELIVER trial.
Systolic BP fell over 1 month by just under 2 mmHg, on average, in trial patients with either mildly reduced or preserved ejection fraction (HFmrEF or HFpEF, respectively) assigned to take dapagliflozin versus placebo.
The effect was achieved without increasing the risk for adverse events from dapagliflozin, even among patients with the lowest baseline systolic pressures. Adverse outcomes overall, however, were more common at the lowest systolic BP level than at higher pressures, researchers reported.
They say the findings should help alleviate long-standing concerns that initiating SGLT2 inhibitors, with their recognized diuretic effects, might present a hazard in patients with HF and low systolic BP.
“It is a consistent theme in heart failure trials that the blood pressure–lowering effect of SGLT2 inhibitors is more modest than it is in non–heart-failure populations,” Senthil Selvaraj, MD, Duke University, Durham, N.C., told this news organization.
Changes to antihypertensive drug therapy throughout the trial, which presumably enhanced BP responses and “might occur more frequently in the placebo group,” Dr. Selvaraj said, “might explain why the blood pressure effect is a little bit more modest in this population.”
Dr. Selvaraj presented the analysis at the Annual Scientific Meeting of the Heart Failure Society of America, held in National Harbor, Md., and is lead author on its same-day publication in JACC: Heart Failure.
The findings “reinforce the clinical benefits of SGLT2 inhibitors in patients with heart failure across the full spectrum of ejection fractions and large range of systolic blood pressures,” said Gregg C. Fonarow, MD, University of California, Los Angeles Medical Center, who was not part of the DELIVER analysis.
The study’s greater adjusted risks for CV and all-cause mortality risks at the lowest baseline systolic pressures “parallels a series of observational analyses from registries, including OPTIMIZE-HF,” Dr. Fonarow observed.
In those prior studies of patients with established HFpEF, “systolic BP less than 120 mmHg or even 130 mmHg was associated with worse outcomes than those with higher systolic BP.”
The current findings, therefore, “highlight how optimal blood pressure targets in patients with established heart failure have not been well established,” Dr. Fonarow said.
The analysis included all 6,263 participants in DELIVER, outpatients or patients hospitalized for worsening HF who were in NYHA class 2-4 with a left ventricular ejection fraction (LVEF) greater than 40%. They averaged 72 in age, and 44% were women. Their mean baseline systolic BP was 128 mmHg.
After 1 month, mean systolic BP had fallen by 1.8 mmHg (P < .001) in patients who had been randomly assigned to dapagliflozin versus placebo. The effect was consistent (interaction P = .16) across all systolic BP categories (less than 120 mmHg, 120-129 mmHg, 130-139 mmHg, and 140 mmHg or higher).
The effect was similarly independent of estimated glomerular filtration rate (eGFR) and LVEF (interaction P = .30 and P = .33, respectively), Dr. Selvaraj reported.
In an analysis adjusted for both baseline and 1-month change in systolic BP, the effect of dapagliflozin on the primary endpoint was “minimally attenuated,” compared with the primary analysis, he said. That suggests the clinical benefits “did not significantly relate to the blood pressure–lowering effect” of the SGLT2 inhibitor.
In that analysis, the hazard ratio for CV death or worsening HF for dapagliflozin versus placebo was 0.85 (95% confidence interval, 0.75-0.96; P = .010). The HR had been 0.82 (95% CI, 0.73-0.92; P < .001) overall in the DELIVER primary analysis.
The current study doesn’t shed further light on the main SGLT2 inhibitor mechanism of clinical benefit in nondiabetics with HF, which remains a mystery.
“There is a diuretic effect, but it’s not incredibly robust,” Dr. Selvaraj observed. It may contribute to the drugs’ benefits, “but it’s definitely more than that – a lot more than that.”
DELIVER was funded by AstraZeneca. Dr. Selvaraj reported no relevant conflicts. Disclosures for the other authors are in the report. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Whatever the mechanism of benefit from dapagliflozin (Farxiga) in patients with heart failure (HF) – and potentially also other sodium-glucose cotransporter 2 (SGLT2) inhibitors – its blood pressure lowering effects aren’t likely to contribute much.
Indeed, at least in patients with HF and non-reduced ejection fractions, dapagliflozin has only a modest BP-lowering effect and cuts cardiovascular (CV) risk regardless of baseline pressure or change in systolic BP, suggests a secondary analysis from the large placebo-controlled DELIVER trial.
Systolic BP fell over 1 month by just under 2 mmHg, on average, in trial patients with either mildly reduced or preserved ejection fraction (HFmrEF or HFpEF, respectively) assigned to take dapagliflozin versus placebo.
The effect was achieved without increasing the risk for adverse events from dapagliflozin, even among patients with the lowest baseline systolic pressures. Adverse outcomes overall, however, were more common at the lowest systolic BP level than at higher pressures, researchers reported.
They say the findings should help alleviate long-standing concerns that initiating SGLT2 inhibitors, with their recognized diuretic effects, might present a hazard in patients with HF and low systolic BP.
“It is a consistent theme in heart failure trials that the blood pressure–lowering effect of SGLT2 inhibitors is more modest than it is in non–heart-failure populations,” Senthil Selvaraj, MD, Duke University, Durham, N.C., told this news organization.
Changes to antihypertensive drug therapy throughout the trial, which presumably enhanced BP responses and “might occur more frequently in the placebo group,” Dr. Selvaraj said, “might explain why the blood pressure effect is a little bit more modest in this population.”
Dr. Selvaraj presented the analysis at the Annual Scientific Meeting of the Heart Failure Society of America, held in National Harbor, Md., and is lead author on its same-day publication in JACC: Heart Failure.
The findings “reinforce the clinical benefits of SGLT2 inhibitors in patients with heart failure across the full spectrum of ejection fractions and large range of systolic blood pressures,” said Gregg C. Fonarow, MD, University of California, Los Angeles Medical Center, who was not part of the DELIVER analysis.
The study’s greater adjusted risks for CV and all-cause mortality risks at the lowest baseline systolic pressures “parallels a series of observational analyses from registries, including OPTIMIZE-HF,” Dr. Fonarow observed.
In those prior studies of patients with established HFpEF, “systolic BP less than 120 mmHg or even 130 mmHg was associated with worse outcomes than those with higher systolic BP.”
The current findings, therefore, “highlight how optimal blood pressure targets in patients with established heart failure have not been well established,” Dr. Fonarow said.
The analysis included all 6,263 participants in DELIVER, outpatients or patients hospitalized for worsening HF who were in NYHA class 2-4 with a left ventricular ejection fraction (LVEF) greater than 40%. They averaged 72 in age, and 44% were women. Their mean baseline systolic BP was 128 mmHg.
After 1 month, mean systolic BP had fallen by 1.8 mmHg (P < .001) in patients who had been randomly assigned to dapagliflozin versus placebo. The effect was consistent (interaction P = .16) across all systolic BP categories (less than 120 mmHg, 120-129 mmHg, 130-139 mmHg, and 140 mmHg or higher).
The effect was similarly independent of estimated glomerular filtration rate (eGFR) and LVEF (interaction P = .30 and P = .33, respectively), Dr. Selvaraj reported.
In an analysis adjusted for both baseline and 1-month change in systolic BP, the effect of dapagliflozin on the primary endpoint was “minimally attenuated,” compared with the primary analysis, he said. That suggests the clinical benefits “did not significantly relate to the blood pressure–lowering effect” of the SGLT2 inhibitor.
In that analysis, the hazard ratio for CV death or worsening HF for dapagliflozin versus placebo was 0.85 (95% confidence interval, 0.75-0.96; P = .010). The HR had been 0.82 (95% CI, 0.73-0.92; P < .001) overall in the DELIVER primary analysis.
The current study doesn’t shed further light on the main SGLT2 inhibitor mechanism of clinical benefit in nondiabetics with HF, which remains a mystery.
“There is a diuretic effect, but it’s not incredibly robust,” Dr. Selvaraj observed. It may contribute to the drugs’ benefits, “but it’s definitely more than that – a lot more than that.”
DELIVER was funded by AstraZeneca. Dr. Selvaraj reported no relevant conflicts. Disclosures for the other authors are in the report. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Whatever the mechanism of benefit from dapagliflozin (Farxiga) in patients with heart failure (HF) – and potentially also other sodium-glucose cotransporter 2 (SGLT2) inhibitors – its blood pressure lowering effects aren’t likely to contribute much.
Indeed, at least in patients with HF and non-reduced ejection fractions, dapagliflozin has only a modest BP-lowering effect and cuts cardiovascular (CV) risk regardless of baseline pressure or change in systolic BP, suggests a secondary analysis from the large placebo-controlled DELIVER trial.
Systolic BP fell over 1 month by just under 2 mmHg, on average, in trial patients with either mildly reduced or preserved ejection fraction (HFmrEF or HFpEF, respectively) assigned to take dapagliflozin versus placebo.
The effect was achieved without increasing the risk for adverse events from dapagliflozin, even among patients with the lowest baseline systolic pressures. Adverse outcomes overall, however, were more common at the lowest systolic BP level than at higher pressures, researchers reported.
They say the findings should help alleviate long-standing concerns that initiating SGLT2 inhibitors, with their recognized diuretic effects, might present a hazard in patients with HF and low systolic BP.
“It is a consistent theme in heart failure trials that the blood pressure–lowering effect of SGLT2 inhibitors is more modest than it is in non–heart-failure populations,” Senthil Selvaraj, MD, Duke University, Durham, N.C., told this news organization.
Changes to antihypertensive drug therapy throughout the trial, which presumably enhanced BP responses and “might occur more frequently in the placebo group,” Dr. Selvaraj said, “might explain why the blood pressure effect is a little bit more modest in this population.”
Dr. Selvaraj presented the analysis at the Annual Scientific Meeting of the Heart Failure Society of America, held in National Harbor, Md., and is lead author on its same-day publication in JACC: Heart Failure.
The findings “reinforce the clinical benefits of SGLT2 inhibitors in patients with heart failure across the full spectrum of ejection fractions and large range of systolic blood pressures,” said Gregg C. Fonarow, MD, University of California, Los Angeles Medical Center, who was not part of the DELIVER analysis.
The study’s greater adjusted risks for CV and all-cause mortality risks at the lowest baseline systolic pressures “parallels a series of observational analyses from registries, including OPTIMIZE-HF,” Dr. Fonarow observed.
In those prior studies of patients with established HFpEF, “systolic BP less than 120 mmHg or even 130 mmHg was associated with worse outcomes than those with higher systolic BP.”
The current findings, therefore, “highlight how optimal blood pressure targets in patients with established heart failure have not been well established,” Dr. Fonarow said.
The analysis included all 6,263 participants in DELIVER, outpatients or patients hospitalized for worsening HF who were in NYHA class 2-4 with a left ventricular ejection fraction (LVEF) greater than 40%. They averaged 72 in age, and 44% were women. Their mean baseline systolic BP was 128 mmHg.
After 1 month, mean systolic BP had fallen by 1.8 mmHg (P < .001) in patients who had been randomly assigned to dapagliflozin versus placebo. The effect was consistent (interaction P = .16) across all systolic BP categories (less than 120 mmHg, 120-129 mmHg, 130-139 mmHg, and 140 mmHg or higher).
The effect was similarly independent of estimated glomerular filtration rate (eGFR) and LVEF (interaction P = .30 and P = .33, respectively), Dr. Selvaraj reported.
In an analysis adjusted for both baseline and 1-month change in systolic BP, the effect of dapagliflozin on the primary endpoint was “minimally attenuated,” compared with the primary analysis, he said. That suggests the clinical benefits “did not significantly relate to the blood pressure–lowering effect” of the SGLT2 inhibitor.
In that analysis, the hazard ratio for CV death or worsening HF for dapagliflozin versus placebo was 0.85 (95% confidence interval, 0.75-0.96; P = .010). The HR had been 0.82 (95% CI, 0.73-0.92; P < .001) overall in the DELIVER primary analysis.
The current study doesn’t shed further light on the main SGLT2 inhibitor mechanism of clinical benefit in nondiabetics with HF, which remains a mystery.
“There is a diuretic effect, but it’s not incredibly robust,” Dr. Selvaraj observed. It may contribute to the drugs’ benefits, “but it’s definitely more than that – a lot more than that.”
DELIVER was funded by AstraZeneca. Dr. Selvaraj reported no relevant conflicts. Disclosures for the other authors are in the report. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Pandemic drove drop in rheumatology payments from industry
Payments to rheumatologists from industry declined early in the COVID-19 pandemic but showed some rebound in 2021, based on information from the Open Payments Database (OPD).
The OPD was established in 2013 to improve transparency in financial relationships between industry and health care professionals in the United States, although many physicians and much of the general public is unaware of the OPD, Anju Murayama of the Medical Governance Research Institute, Tokyo, and colleagues wrote.
The COVID-19 pandemic may have limited rheumatologists’ involvement with industry, but potential changes in financial relationships during the pandemic have not been well studied, they wrote.
In a study published in the Journal of Rheumatology, the researchers reviewed data from 6,047 rheumatologists who received at least one general payment from industry between August 2013 and December 2021. The total value of the payments was $288,326,257.
The data set included all general payments made to the physicians whose primary specialty was categorized as rheumatology in the National Plan and Provider Enumeration System profile. The payment information came from the OPD and included payments between August 2013 and December 2021.
In this analysis, the periods before and after March 2020 were considered as before and after the pandemic, respectively.
At the onset of the pandemic, monthly payments to rheumatologists overall decreased by 65.1%, and the number of rheumatologists who received payments decreased by 39.8%; a decrease occurred across all levels of payment.
“However, the recovery trend in payments during the pandemic was higher among the rheumatologists with lower payments,” the researchers noted.
The most significant decreases across payment types occurred in travel and accommodation, which dropped by 98.2% at the start of the pandemic. Payments for speaking engagements and meals decreased by 72.3% and 72.0%, respectively, at the start of the pandemic; consulting payments decreased by 23.3%.
The number of rheumatologists with payments ranged from 3,547 in 2020 to 4,444 in 2015, and did not change significantly between 2014 and 2019. However, the median total payments increased from $730 in 2014 to $812 in 2019.
Compared with the 2014-2019 period, the number of rheumatologists with payments in 2020-2021 decreased by 21.7% and the payments per rheumatologist decreased by 41.9% (P < .001 for both).
In 2021, general payments to rheumatologists were still below levels from the 2014-2019 period.
The study findings were limited by the exclusion of rheumatologist without payments and the lack of data on confounding factors, the researchers noted. However, the study is the first to show the impact of the COVID-19 pandemic on the financial relationships between U.S. rheumatologists and industry.
“Although there were recovering trends in general payments right after the onset of the COVID-19 pandemic, we observed general payments remaining at low levels between 2020 and 2021,” they noted.
A previous study showed that general payments to rheumatologists between 2013 and 2015 were significantly associated with increased prescription of brand-name rheumatology drugs and health care use. But more long-term studies are needed “to investigate whether this downward trend in general payments [observed in the current study] has contributed to reducing undue influence on rheumatologists’ clinical practice,” the researchers concluded.
The study received no outside funding. One coauthor disclosed personal fees from Medical Network Systems unrelated to the current study. The study authors had no financial conflicts related to the current study, but continue to research financial and nonfinancial conflicts of interest among health care professionals and pharmaceutical companies in Japan and the United States.
Payments to rheumatologists from industry declined early in the COVID-19 pandemic but showed some rebound in 2021, based on information from the Open Payments Database (OPD).
The OPD was established in 2013 to improve transparency in financial relationships between industry and health care professionals in the United States, although many physicians and much of the general public is unaware of the OPD, Anju Murayama of the Medical Governance Research Institute, Tokyo, and colleagues wrote.
The COVID-19 pandemic may have limited rheumatologists’ involvement with industry, but potential changes in financial relationships during the pandemic have not been well studied, they wrote.
In a study published in the Journal of Rheumatology, the researchers reviewed data from 6,047 rheumatologists who received at least one general payment from industry between August 2013 and December 2021. The total value of the payments was $288,326,257.
The data set included all general payments made to the physicians whose primary specialty was categorized as rheumatology in the National Plan and Provider Enumeration System profile. The payment information came from the OPD and included payments between August 2013 and December 2021.
In this analysis, the periods before and after March 2020 were considered as before and after the pandemic, respectively.
At the onset of the pandemic, monthly payments to rheumatologists overall decreased by 65.1%, and the number of rheumatologists who received payments decreased by 39.8%; a decrease occurred across all levels of payment.
“However, the recovery trend in payments during the pandemic was higher among the rheumatologists with lower payments,” the researchers noted.
The most significant decreases across payment types occurred in travel and accommodation, which dropped by 98.2% at the start of the pandemic. Payments for speaking engagements and meals decreased by 72.3% and 72.0%, respectively, at the start of the pandemic; consulting payments decreased by 23.3%.
The number of rheumatologists with payments ranged from 3,547 in 2020 to 4,444 in 2015, and did not change significantly between 2014 and 2019. However, the median total payments increased from $730 in 2014 to $812 in 2019.
Compared with the 2014-2019 period, the number of rheumatologists with payments in 2020-2021 decreased by 21.7% and the payments per rheumatologist decreased by 41.9% (P < .001 for both).
In 2021, general payments to rheumatologists were still below levels from the 2014-2019 period.
The study findings were limited by the exclusion of rheumatologist without payments and the lack of data on confounding factors, the researchers noted. However, the study is the first to show the impact of the COVID-19 pandemic on the financial relationships between U.S. rheumatologists and industry.
“Although there were recovering trends in general payments right after the onset of the COVID-19 pandemic, we observed general payments remaining at low levels between 2020 and 2021,” they noted.
A previous study showed that general payments to rheumatologists between 2013 and 2015 were significantly associated with increased prescription of brand-name rheumatology drugs and health care use. But more long-term studies are needed “to investigate whether this downward trend in general payments [observed in the current study] has contributed to reducing undue influence on rheumatologists’ clinical practice,” the researchers concluded.
The study received no outside funding. One coauthor disclosed personal fees from Medical Network Systems unrelated to the current study. The study authors had no financial conflicts related to the current study, but continue to research financial and nonfinancial conflicts of interest among health care professionals and pharmaceutical companies in Japan and the United States.
Payments to rheumatologists from industry declined early in the COVID-19 pandemic but showed some rebound in 2021, based on information from the Open Payments Database (OPD).
The OPD was established in 2013 to improve transparency in financial relationships between industry and health care professionals in the United States, although many physicians and much of the general public is unaware of the OPD, Anju Murayama of the Medical Governance Research Institute, Tokyo, and colleagues wrote.
The COVID-19 pandemic may have limited rheumatologists’ involvement with industry, but potential changes in financial relationships during the pandemic have not been well studied, they wrote.
In a study published in the Journal of Rheumatology, the researchers reviewed data from 6,047 rheumatologists who received at least one general payment from industry between August 2013 and December 2021. The total value of the payments was $288,326,257.
The data set included all general payments made to the physicians whose primary specialty was categorized as rheumatology in the National Plan and Provider Enumeration System profile. The payment information came from the OPD and included payments between August 2013 and December 2021.
In this analysis, the periods before and after March 2020 were considered as before and after the pandemic, respectively.
At the onset of the pandemic, monthly payments to rheumatologists overall decreased by 65.1%, and the number of rheumatologists who received payments decreased by 39.8%; a decrease occurred across all levels of payment.
“However, the recovery trend in payments during the pandemic was higher among the rheumatologists with lower payments,” the researchers noted.
The most significant decreases across payment types occurred in travel and accommodation, which dropped by 98.2% at the start of the pandemic. Payments for speaking engagements and meals decreased by 72.3% and 72.0%, respectively, at the start of the pandemic; consulting payments decreased by 23.3%.
The number of rheumatologists with payments ranged from 3,547 in 2020 to 4,444 in 2015, and did not change significantly between 2014 and 2019. However, the median total payments increased from $730 in 2014 to $812 in 2019.
Compared with the 2014-2019 period, the number of rheumatologists with payments in 2020-2021 decreased by 21.7% and the payments per rheumatologist decreased by 41.9% (P < .001 for both).
In 2021, general payments to rheumatologists were still below levels from the 2014-2019 period.
The study findings were limited by the exclusion of rheumatologist without payments and the lack of data on confounding factors, the researchers noted. However, the study is the first to show the impact of the COVID-19 pandemic on the financial relationships between U.S. rheumatologists and industry.
“Although there were recovering trends in general payments right after the onset of the COVID-19 pandemic, we observed general payments remaining at low levels between 2020 and 2021,” they noted.
A previous study showed that general payments to rheumatologists between 2013 and 2015 were significantly associated with increased prescription of brand-name rheumatology drugs and health care use. But more long-term studies are needed “to investigate whether this downward trend in general payments [observed in the current study] has contributed to reducing undue influence on rheumatologists’ clinical practice,” the researchers concluded.
The study received no outside funding. One coauthor disclosed personal fees from Medical Network Systems unrelated to the current study. The study authors had no financial conflicts related to the current study, but continue to research financial and nonfinancial conflicts of interest among health care professionals and pharmaceutical companies in Japan and the United States.
FROM THE JOURNAL OF RHEUMATOLOGY
Tinea capitis
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.
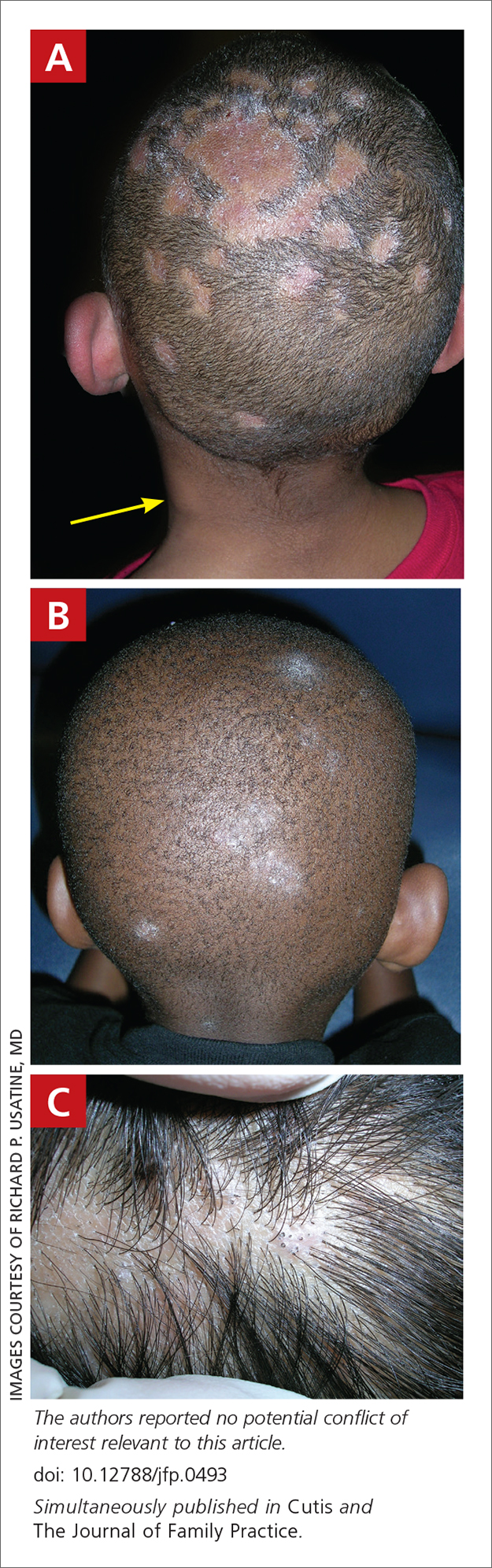
Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
IVIG proves effective for dermatomyositis in phase 3 trial
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Water exchange boosts colonoscopy training experience
A new study finds that colonoscopy trainees had a better experience with and performed better when using water exchange (WE) than when using air insufflation. The new study was published in the Journal of Clinical Gastroenterology.
According to study author Felix W. Leung, MD, from the Veterans Affairs Greater Los Angeles Healthcare System in North Hills, Calif., and the University of California, Los Angeles, WE is less painful than air insufflation and increases cecal intubation rate because it reduces loop formation. He added that it also increases polyp and adenoma detection rates.
Although WE has compared favorably with air insufflation for ADR and pain, there is little evidence regarding how trainees view WE versus air insufflation. Dr. Leung pointed out that the issue could be particularly important among millennial trainees, who may have a different learning style than previous generations. He also noted that previous studies of WE versus air insufflation among trainees measured the perspective of trainers, and did not include the trainees’ opinions of the learning process or trainee outcomes like polyp detection rate.
Seeking to fill this knowledge gap, Dr. Leung conducted a prospective observational study at a Veterans Administration Hospital. Trainees conducted unsedated colonoscopies using WE, as well as WE and air insufflation colonoscopies in alternating order in sedated patients. A total of 83 air insufflation and 119 WE colonoscopies were performed. Trainees rated their experiences on a 1- to 5-point scale, with 1 being “strongly agree” and 5 “strongly disagree” to two statements: “My colonoscopy experience was better than expected” then “I was confident with my technical skills using this method.”
On average, trainees using WE reported a better than expected experience when using WE, compared with air insufflation (2.02 vs. 2.43; P = .0087), but no significant difference in the ensuing confidence in their technical skills (2.76 vs. 2.85; P = .48). There was a longer insertion time for WE (40 minutes vs. 30 minutes; P = .0008). WE was associated with a significantly higher adjusted cecal intubation rate (99% vs. 89%; P = .0031) and a significantly higher polyp detection rate (54% vs. 32%; P = .0447). Overall insertion time was longer with WE than air insufflation (40 minutes vs. 30 minutes; P = .0008), but withdrawal times were similar (22 minutes vs. 20 minutes; P = .3369).
The reduction in pain associated with WE can potentially improve training, in which cases procedures are typically performed on patients under moderate sedation, according to John Allen, MD, who was asked to comment on the study.
He also said that WE can sometimes do a better job than air of opening the lumen. It can help clean the colon surface, and even improve visibility. “Viewing the mucosa under water is like having a lens that helps view the surface and enhance polyp detection,” said Dr. Allen, who is a retired clinical professor of medicine at the University of Michigan, Ann Arbor.
Dr. Allen noted that either air sufflation or WE can be used to overcome the inexperience of the trainee, and that there shouldn’t be much difference between the two methods for sedated colonoscopies. The time of exam is similar, and WE does not require use of carbon dioxide or other gases, which avoids extra costs. “A highly skilled colonoscopist can perform exams using any of the available media. That said, WE is proving to be helpful no matter what your skill level. The only disadvantage I can see is that many trainers do not know how WE works and are unused to this process, although it is easy to learn,” said Dr. Allen.
The study is limited by the fact that it was conducted at a single institution in a nonblinded, nonrandomized population.
Dr. Leung declared there are no conflicts of interest to disclose. Dr. Allen has no relevant financial disclosures.
A new study finds that colonoscopy trainees had a better experience with and performed better when using water exchange (WE) than when using air insufflation. The new study was published in the Journal of Clinical Gastroenterology.
According to study author Felix W. Leung, MD, from the Veterans Affairs Greater Los Angeles Healthcare System in North Hills, Calif., and the University of California, Los Angeles, WE is less painful than air insufflation and increases cecal intubation rate because it reduces loop formation. He added that it also increases polyp and adenoma detection rates.
Although WE has compared favorably with air insufflation for ADR and pain, there is little evidence regarding how trainees view WE versus air insufflation. Dr. Leung pointed out that the issue could be particularly important among millennial trainees, who may have a different learning style than previous generations. He also noted that previous studies of WE versus air insufflation among trainees measured the perspective of trainers, and did not include the trainees’ opinions of the learning process or trainee outcomes like polyp detection rate.
Seeking to fill this knowledge gap, Dr. Leung conducted a prospective observational study at a Veterans Administration Hospital. Trainees conducted unsedated colonoscopies using WE, as well as WE and air insufflation colonoscopies in alternating order in sedated patients. A total of 83 air insufflation and 119 WE colonoscopies were performed. Trainees rated their experiences on a 1- to 5-point scale, with 1 being “strongly agree” and 5 “strongly disagree” to two statements: “My colonoscopy experience was better than expected” then “I was confident with my technical skills using this method.”
On average, trainees using WE reported a better than expected experience when using WE, compared with air insufflation (2.02 vs. 2.43; P = .0087), but no significant difference in the ensuing confidence in their technical skills (2.76 vs. 2.85; P = .48). There was a longer insertion time for WE (40 minutes vs. 30 minutes; P = .0008). WE was associated with a significantly higher adjusted cecal intubation rate (99% vs. 89%; P = .0031) and a significantly higher polyp detection rate (54% vs. 32%; P = .0447). Overall insertion time was longer with WE than air insufflation (40 minutes vs. 30 minutes; P = .0008), but withdrawal times were similar (22 minutes vs. 20 minutes; P = .3369).
The reduction in pain associated with WE can potentially improve training, in which cases procedures are typically performed on patients under moderate sedation, according to John Allen, MD, who was asked to comment on the study.
He also said that WE can sometimes do a better job than air of opening the lumen. It can help clean the colon surface, and even improve visibility. “Viewing the mucosa under water is like having a lens that helps view the surface and enhance polyp detection,” said Dr. Allen, who is a retired clinical professor of medicine at the University of Michigan, Ann Arbor.
Dr. Allen noted that either air sufflation or WE can be used to overcome the inexperience of the trainee, and that there shouldn’t be much difference between the two methods for sedated colonoscopies. The time of exam is similar, and WE does not require use of carbon dioxide or other gases, which avoids extra costs. “A highly skilled colonoscopist can perform exams using any of the available media. That said, WE is proving to be helpful no matter what your skill level. The only disadvantage I can see is that many trainers do not know how WE works and are unused to this process, although it is easy to learn,” said Dr. Allen.
The study is limited by the fact that it was conducted at a single institution in a nonblinded, nonrandomized population.
Dr. Leung declared there are no conflicts of interest to disclose. Dr. Allen has no relevant financial disclosures.
A new study finds that colonoscopy trainees had a better experience with and performed better when using water exchange (WE) than when using air insufflation. The new study was published in the Journal of Clinical Gastroenterology.
According to study author Felix W. Leung, MD, from the Veterans Affairs Greater Los Angeles Healthcare System in North Hills, Calif., and the University of California, Los Angeles, WE is less painful than air insufflation and increases cecal intubation rate because it reduces loop formation. He added that it also increases polyp and adenoma detection rates.
Although WE has compared favorably with air insufflation for ADR and pain, there is little evidence regarding how trainees view WE versus air insufflation. Dr. Leung pointed out that the issue could be particularly important among millennial trainees, who may have a different learning style than previous generations. He also noted that previous studies of WE versus air insufflation among trainees measured the perspective of trainers, and did not include the trainees’ opinions of the learning process or trainee outcomes like polyp detection rate.
Seeking to fill this knowledge gap, Dr. Leung conducted a prospective observational study at a Veterans Administration Hospital. Trainees conducted unsedated colonoscopies using WE, as well as WE and air insufflation colonoscopies in alternating order in sedated patients. A total of 83 air insufflation and 119 WE colonoscopies were performed. Trainees rated their experiences on a 1- to 5-point scale, with 1 being “strongly agree” and 5 “strongly disagree” to two statements: “My colonoscopy experience was better than expected” then “I was confident with my technical skills using this method.”
On average, trainees using WE reported a better than expected experience when using WE, compared with air insufflation (2.02 vs. 2.43; P = .0087), but no significant difference in the ensuing confidence in their technical skills (2.76 vs. 2.85; P = .48). There was a longer insertion time for WE (40 minutes vs. 30 minutes; P = .0008). WE was associated with a significantly higher adjusted cecal intubation rate (99% vs. 89%; P = .0031) and a significantly higher polyp detection rate (54% vs. 32%; P = .0447). Overall insertion time was longer with WE than air insufflation (40 minutes vs. 30 minutes; P = .0008), but withdrawal times were similar (22 minutes vs. 20 minutes; P = .3369).
The reduction in pain associated with WE can potentially improve training, in which cases procedures are typically performed on patients under moderate sedation, according to John Allen, MD, who was asked to comment on the study.
He also said that WE can sometimes do a better job than air of opening the lumen. It can help clean the colon surface, and even improve visibility. “Viewing the mucosa under water is like having a lens that helps view the surface and enhance polyp detection,” said Dr. Allen, who is a retired clinical professor of medicine at the University of Michigan, Ann Arbor.
Dr. Allen noted that either air sufflation or WE can be used to overcome the inexperience of the trainee, and that there shouldn’t be much difference between the two methods for sedated colonoscopies. The time of exam is similar, and WE does not require use of carbon dioxide or other gases, which avoids extra costs. “A highly skilled colonoscopist can perform exams using any of the available media. That said, WE is proving to be helpful no matter what your skill level. The only disadvantage I can see is that many trainers do not know how WE works and are unused to this process, although it is easy to learn,” said Dr. Allen.
The study is limited by the fact that it was conducted at a single institution in a nonblinded, nonrandomized population.
Dr. Leung declared there are no conflicts of interest to disclose. Dr. Allen has no relevant financial disclosures.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY