User login
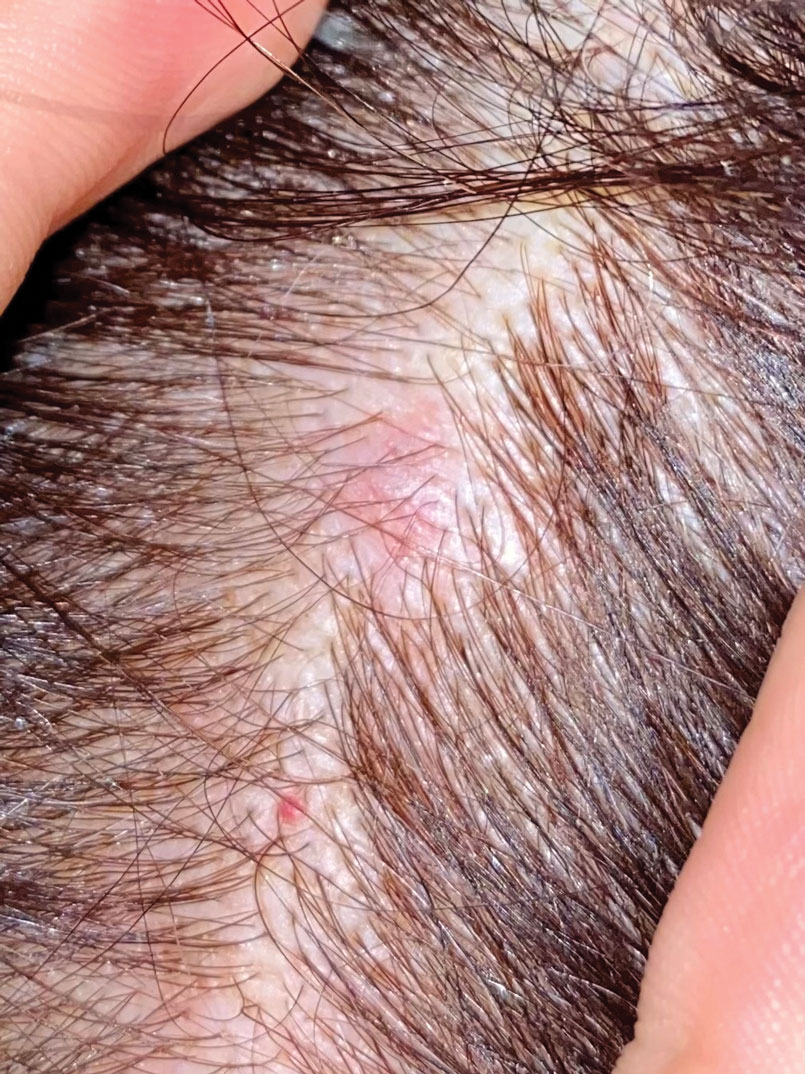
Firm Mobile Nodule on the Scalp
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12
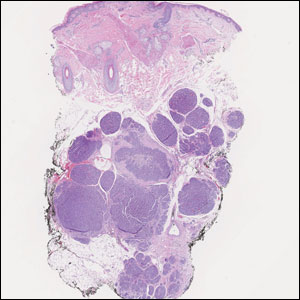
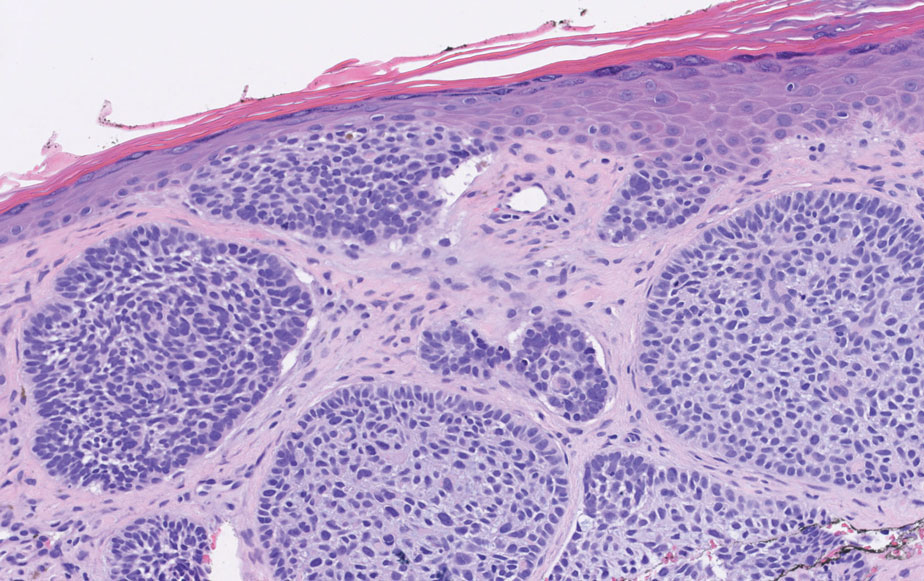
Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.
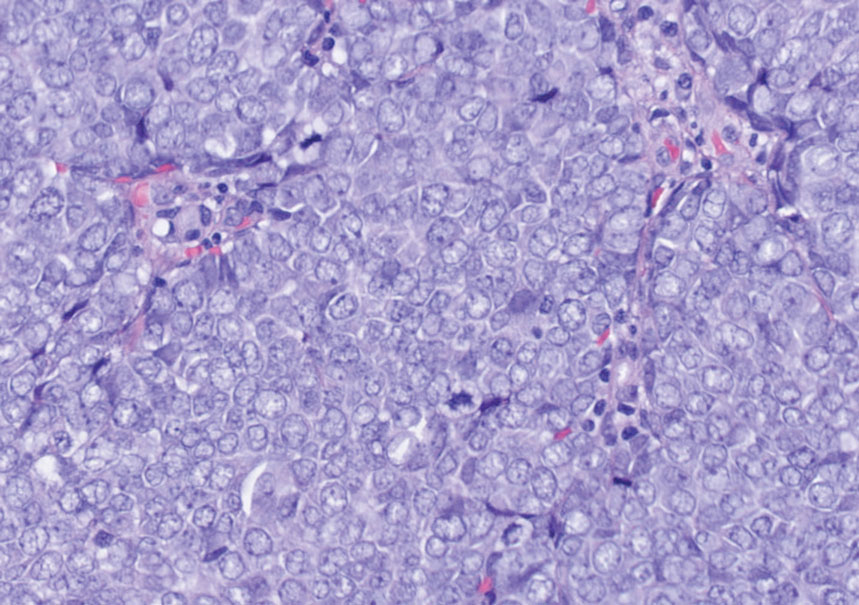
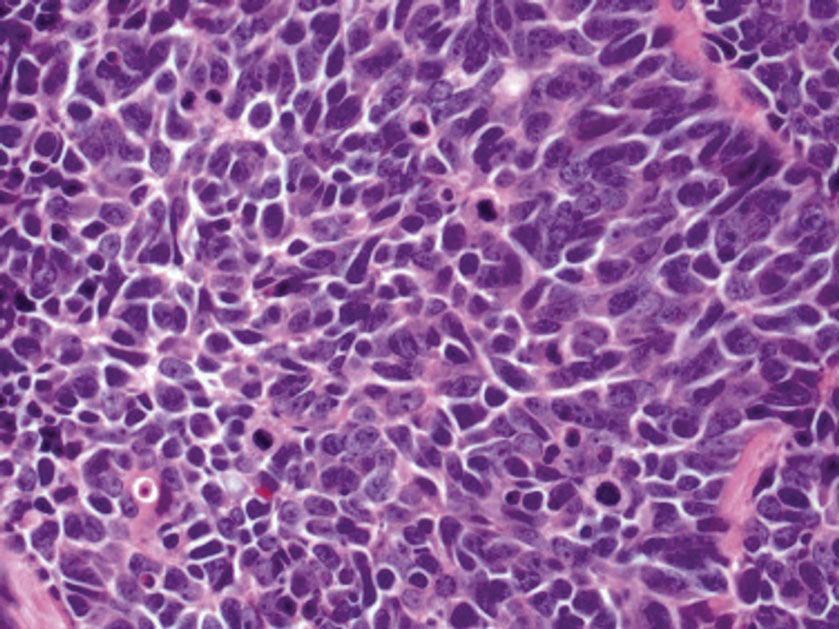
An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14
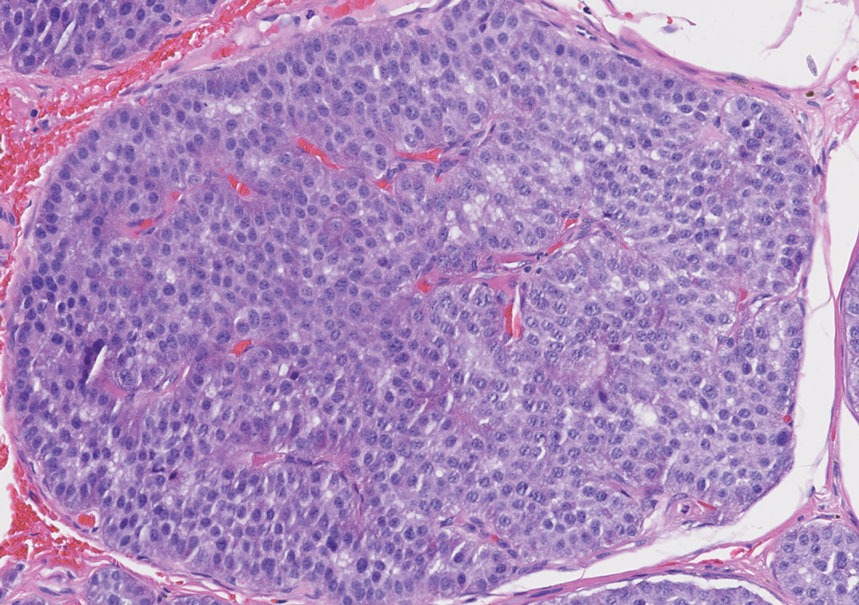
The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19
Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12

Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.

An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14

The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19

Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12

Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.

An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14

The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19

Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
A 47-year-old woman was admitted to the hospital with abdominal pain and flushing. She had a history of a midgut carcinoid that originated in the ileum with metastasis to the colon, liver, and pancreas. Dermatologic examination revealed a firm, nontender, mobile, 7-mm scalp nodule with a pink-purple overlying epidermis. The lesion was associated with a slight decrease in hair density. A 4-mm punch biopsy was performed.

Pumping iron improves longevity in older adults
with the strongest effects observed when the two types of exercise are combined, new research shows.
“The novel finding from our study is that weight lifting is independently associated with lower all-cause and CVD-specific mortality, regardless of aerobic activity,” first author Jessica Gorzelitz, PhD, said in an interview.
“What’s less surprising – but consistent and nonetheless noteworthy – is that weight lifting in combination with aerobic exercise provides the lowest...risk for mortality in older adults,” added Dr. Gorzelitz, an assistant professor of health promotion in the department of health and human physiology at the University of Iowa, Iowa City.
Those who undertook weight lifting and aerobic exercise in combination had around a 40% lower risk of death than those who reported no moderate to vigorous aerobic activity or weight lifting. The findings were recently published online in the British Journal of Sports Medicine.
Physical activity guidelines generally recommend regular moderate to vigorous aerobic physical activity, in addition to at least 2 days per week of muscle-strengthening exercise for all major muscle groups for adults to improve health and boost longevity.
However, few observational studies have examined the association between muscle strengthening and mortality, and even fewer have looked specifically at the benefits of weight lifting, Dr. Gorzelitz said.
Benefit of weight lifting stronger in women than men
To investigate, Dr. Gorzelitz and coauthors evaluated data on participants in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, which, initiated in 1993, and involved adults aged 55-74 at 10 U.S. cancer centers.
Thirteen years into the trial, in 2006, participants completed follow-up questionnaires that included an assessment of weight lifting (not included in a baseline survey).
Among 99,713 participants involved in the current analysis, the mean age at the time of the follow-up questionnaire was 71.3 years. Participants had a mean body mass index of 27.8 kg/m2 and 52.6% were women.
Only about a quarter of adults (23%) reported any weight lifting activity within the previous 12 months, with fewer, at 16%, reporting regular weight lifting of between one and six times per week.
Participants’ physical aerobic activity was also assessed. Physical activity guidelines (2018) recommend at least 150-300 minutes per week of moderate-intensity aerobic physical activity or 75-150 minutes per week of vigorous intensity aerobic activity or an equal combination of the two. Overall, 23.6% of participants reported activity that met the guideline for moderate to vigorous physical activity, and 8% exceeded it.
Over a median follow-up of about 9 years, 28,477 deaths occurred.
Those reporting weight lifting had a 9% lower risk of combined all-cause mortality and CVD mortality, after adjustment for any moderate to vigorous physical activity (each hazard ratio, 0.91).
Adults who met aerobic activity recommendations but did not weight lift had a 32% lower risk of all-cause mortality (HR, 0.68), while those who also reported weight lifting 1-2 times per week in addition to the aerobic activity had as much as a 41% lower risk of death (HR, 0.59), compared with adults reporting no moderate to vigorous aerobic activity or weight lifting.
The benefit of weight lifting in terms of cancer mortality was only observed without adjustment for moderate to vigorous physical activity, and was therefore considered null, which Dr. Gorzelitz said was somewhat surprising. “We will examine this association further because there could still be a signal there,” she said, noting other studies have shown that muscle strengthening activity is associated with lower cancer-specific mortality.
Of note, the benefit of weight lifting appeared stronger in women versus men, Dr. Gorzelitz said.
What are the mechanisms?
Underscoring that the results show only associations and not causation, Dr. Gorzelitz speculated that mechanisms behind a mortality benefit could include known favorable physiological changes of weight lifting.
“If people are weight lifting [to a degree] to reap strength benefits, we generally see improvement in body composition, including reductions in fat and improvements in lean tissue, and we know that those changes are associated with mortality, so it could be that the weight lifting is driving the strength or body composition,” she said.
The full body response involved in weight lifting could also play a key role, she noted.
With weight lifting, “the muscles have to redirect more blood flow, the heart is pumping harder, the lungs breathe more and when the muscles are worked in that fashion, there could be other system-wide adaptations,” she said.
Furthermore, social aspects could play a role, Dr. Gorzelitz observed.
“Unlike muscle strengthening [activities] that can be done in the home setting, weight lifting typically has to be done in recreational facilities or other community centers, and considering that this is an older adult population, that social interaction could be very key for preventing isolation.”
Important limitations include that the study did not determine the nature of the weight lifting, including the duration of the weight lifting sessions or type of weight, which could feasibly range from small hand-held weights to heavier weight lifting.
The study also couldn’t show how long participants had engaged in weight lifting in terms of months or years, hence, the duration needed to see a mortality benefit was not established.
Nevertheless, the study’s finding that the group with the lowest benefits was the one reporting no aerobic or weight lifting exercise underscores the benefits of even small amounts of exercise.
“I think it’s really important to promote the importance of adding muscle strengthening, but also of any physical activity,” Dr. Gorzelitz said. “Start small, but something is better than nothing.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
with the strongest effects observed when the two types of exercise are combined, new research shows.
“The novel finding from our study is that weight lifting is independently associated with lower all-cause and CVD-specific mortality, regardless of aerobic activity,” first author Jessica Gorzelitz, PhD, said in an interview.
“What’s less surprising – but consistent and nonetheless noteworthy – is that weight lifting in combination with aerobic exercise provides the lowest...risk for mortality in older adults,” added Dr. Gorzelitz, an assistant professor of health promotion in the department of health and human physiology at the University of Iowa, Iowa City.
Those who undertook weight lifting and aerobic exercise in combination had around a 40% lower risk of death than those who reported no moderate to vigorous aerobic activity or weight lifting. The findings were recently published online in the British Journal of Sports Medicine.
Physical activity guidelines generally recommend regular moderate to vigorous aerobic physical activity, in addition to at least 2 days per week of muscle-strengthening exercise for all major muscle groups for adults to improve health and boost longevity.
However, few observational studies have examined the association between muscle strengthening and mortality, and even fewer have looked specifically at the benefits of weight lifting, Dr. Gorzelitz said.
Benefit of weight lifting stronger in women than men
To investigate, Dr. Gorzelitz and coauthors evaluated data on participants in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, which, initiated in 1993, and involved adults aged 55-74 at 10 U.S. cancer centers.
Thirteen years into the trial, in 2006, participants completed follow-up questionnaires that included an assessment of weight lifting (not included in a baseline survey).
Among 99,713 participants involved in the current analysis, the mean age at the time of the follow-up questionnaire was 71.3 years. Participants had a mean body mass index of 27.8 kg/m2 and 52.6% were women.
Only about a quarter of adults (23%) reported any weight lifting activity within the previous 12 months, with fewer, at 16%, reporting regular weight lifting of between one and six times per week.
Participants’ physical aerobic activity was also assessed. Physical activity guidelines (2018) recommend at least 150-300 minutes per week of moderate-intensity aerobic physical activity or 75-150 minutes per week of vigorous intensity aerobic activity or an equal combination of the two. Overall, 23.6% of participants reported activity that met the guideline for moderate to vigorous physical activity, and 8% exceeded it.
Over a median follow-up of about 9 years, 28,477 deaths occurred.
Those reporting weight lifting had a 9% lower risk of combined all-cause mortality and CVD mortality, after adjustment for any moderate to vigorous physical activity (each hazard ratio, 0.91).
Adults who met aerobic activity recommendations but did not weight lift had a 32% lower risk of all-cause mortality (HR, 0.68), while those who also reported weight lifting 1-2 times per week in addition to the aerobic activity had as much as a 41% lower risk of death (HR, 0.59), compared with adults reporting no moderate to vigorous aerobic activity or weight lifting.
The benefit of weight lifting in terms of cancer mortality was only observed without adjustment for moderate to vigorous physical activity, and was therefore considered null, which Dr. Gorzelitz said was somewhat surprising. “We will examine this association further because there could still be a signal there,” she said, noting other studies have shown that muscle strengthening activity is associated with lower cancer-specific mortality.
Of note, the benefit of weight lifting appeared stronger in women versus men, Dr. Gorzelitz said.
What are the mechanisms?
Underscoring that the results show only associations and not causation, Dr. Gorzelitz speculated that mechanisms behind a mortality benefit could include known favorable physiological changes of weight lifting.
“If people are weight lifting [to a degree] to reap strength benefits, we generally see improvement in body composition, including reductions in fat and improvements in lean tissue, and we know that those changes are associated with mortality, so it could be that the weight lifting is driving the strength or body composition,” she said.
The full body response involved in weight lifting could also play a key role, she noted.
With weight lifting, “the muscles have to redirect more blood flow, the heart is pumping harder, the lungs breathe more and when the muscles are worked in that fashion, there could be other system-wide adaptations,” she said.
Furthermore, social aspects could play a role, Dr. Gorzelitz observed.
“Unlike muscle strengthening [activities] that can be done in the home setting, weight lifting typically has to be done in recreational facilities or other community centers, and considering that this is an older adult population, that social interaction could be very key for preventing isolation.”
Important limitations include that the study did not determine the nature of the weight lifting, including the duration of the weight lifting sessions or type of weight, which could feasibly range from small hand-held weights to heavier weight lifting.
The study also couldn’t show how long participants had engaged in weight lifting in terms of months or years, hence, the duration needed to see a mortality benefit was not established.
Nevertheless, the study’s finding that the group with the lowest benefits was the one reporting no aerobic or weight lifting exercise underscores the benefits of even small amounts of exercise.
“I think it’s really important to promote the importance of adding muscle strengthening, but also of any physical activity,” Dr. Gorzelitz said. “Start small, but something is better than nothing.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
with the strongest effects observed when the two types of exercise are combined, new research shows.
“The novel finding from our study is that weight lifting is independently associated with lower all-cause and CVD-specific mortality, regardless of aerobic activity,” first author Jessica Gorzelitz, PhD, said in an interview.
“What’s less surprising – but consistent and nonetheless noteworthy – is that weight lifting in combination with aerobic exercise provides the lowest...risk for mortality in older adults,” added Dr. Gorzelitz, an assistant professor of health promotion in the department of health and human physiology at the University of Iowa, Iowa City.
Those who undertook weight lifting and aerobic exercise in combination had around a 40% lower risk of death than those who reported no moderate to vigorous aerobic activity or weight lifting. The findings were recently published online in the British Journal of Sports Medicine.
Physical activity guidelines generally recommend regular moderate to vigorous aerobic physical activity, in addition to at least 2 days per week of muscle-strengthening exercise for all major muscle groups for adults to improve health and boost longevity.
However, few observational studies have examined the association between muscle strengthening and mortality, and even fewer have looked specifically at the benefits of weight lifting, Dr. Gorzelitz said.
Benefit of weight lifting stronger in women than men
To investigate, Dr. Gorzelitz and coauthors evaluated data on participants in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, which, initiated in 1993, and involved adults aged 55-74 at 10 U.S. cancer centers.
Thirteen years into the trial, in 2006, participants completed follow-up questionnaires that included an assessment of weight lifting (not included in a baseline survey).
Among 99,713 participants involved in the current analysis, the mean age at the time of the follow-up questionnaire was 71.3 years. Participants had a mean body mass index of 27.8 kg/m2 and 52.6% were women.
Only about a quarter of adults (23%) reported any weight lifting activity within the previous 12 months, with fewer, at 16%, reporting regular weight lifting of between one and six times per week.
Participants’ physical aerobic activity was also assessed. Physical activity guidelines (2018) recommend at least 150-300 minutes per week of moderate-intensity aerobic physical activity or 75-150 minutes per week of vigorous intensity aerobic activity or an equal combination of the two. Overall, 23.6% of participants reported activity that met the guideline for moderate to vigorous physical activity, and 8% exceeded it.
Over a median follow-up of about 9 years, 28,477 deaths occurred.
Those reporting weight lifting had a 9% lower risk of combined all-cause mortality and CVD mortality, after adjustment for any moderate to vigorous physical activity (each hazard ratio, 0.91).
Adults who met aerobic activity recommendations but did not weight lift had a 32% lower risk of all-cause mortality (HR, 0.68), while those who also reported weight lifting 1-2 times per week in addition to the aerobic activity had as much as a 41% lower risk of death (HR, 0.59), compared with adults reporting no moderate to vigorous aerobic activity or weight lifting.
The benefit of weight lifting in terms of cancer mortality was only observed without adjustment for moderate to vigorous physical activity, and was therefore considered null, which Dr. Gorzelitz said was somewhat surprising. “We will examine this association further because there could still be a signal there,” she said, noting other studies have shown that muscle strengthening activity is associated with lower cancer-specific mortality.
Of note, the benefit of weight lifting appeared stronger in women versus men, Dr. Gorzelitz said.
What are the mechanisms?
Underscoring that the results show only associations and not causation, Dr. Gorzelitz speculated that mechanisms behind a mortality benefit could include known favorable physiological changes of weight lifting.
“If people are weight lifting [to a degree] to reap strength benefits, we generally see improvement in body composition, including reductions in fat and improvements in lean tissue, and we know that those changes are associated with mortality, so it could be that the weight lifting is driving the strength or body composition,” she said.
The full body response involved in weight lifting could also play a key role, she noted.
With weight lifting, “the muscles have to redirect more blood flow, the heart is pumping harder, the lungs breathe more and when the muscles are worked in that fashion, there could be other system-wide adaptations,” she said.
Furthermore, social aspects could play a role, Dr. Gorzelitz observed.
“Unlike muscle strengthening [activities] that can be done in the home setting, weight lifting typically has to be done in recreational facilities or other community centers, and considering that this is an older adult population, that social interaction could be very key for preventing isolation.”
Important limitations include that the study did not determine the nature of the weight lifting, including the duration of the weight lifting sessions or type of weight, which could feasibly range from small hand-held weights to heavier weight lifting.
The study also couldn’t show how long participants had engaged in weight lifting in terms of months or years, hence, the duration needed to see a mortality benefit was not established.
Nevertheless, the study’s finding that the group with the lowest benefits was the one reporting no aerobic or weight lifting exercise underscores the benefits of even small amounts of exercise.
“I think it’s really important to promote the importance of adding muscle strengthening, but also of any physical activity,” Dr. Gorzelitz said. “Start small, but something is better than nothing.”
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF SPORTS MEDICINE
Colonoscopy lowers CRC risk and death, but not by much: NordICC
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM UEG 2022
Gut microbiota disruption a driver of aggression in schizophrenia?
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.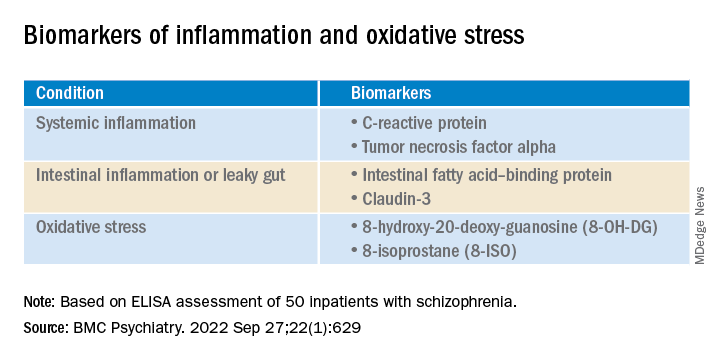
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
FROM BMC PSYCHIATRY
Antioxidant-rich diet may reduce Helicobacter pylori risk
People who eat a balanced diet with sufficient antioxidants from fruits and vegetables may face reduced risks for Heliobacter pylori infections, according to a new report.
In particular, patients with an H. pylori infection were more likely to score lower on the Dietary Antioxidant Index (DAI), which was created to consider a diet’s entire antioxidant profile.
“Available evidence indicates that diet has an important role in developing H. pylori infection. Therefore, protective dietary factors are important from a public health point of view,” Farzad Shidfar, a professor of nutrition at the Iran University of Medical Sciences, Tehran, and member of the university’s colorectal research center, and colleagues write.
“While some nutritional research has widely focused on single nutrients or foods in diet-disease relations, the overall diet could be more informative because humans typically consume a combination of nutrients and foods,” they write. “Dietary indices such as DAI are one of the approaches for this purpose.”
The study was published online in BMC Gastroenterology.
Measuring antioxidant intake
Previous research has indicated an inverse association between the DAI and inflammatory diseases, the study authors write, including gastric cancer, colorectal cancer, nonalcoholic fatty liver disease, and obesity. Studies have also indicated that H. pylori infection is related to deficiencies in vitamins A, C, and E, which have antioxidant properties.
In a case-control study, the research team compared the dietary intake of 148 patients with H. pylori to 302 healthy controls without infection. The patients in the H. pylori–positive group were recruited between June 2021 and November 2021 from the gastroenterology clinic at Rasoul-e-Akram Hospital in Tehran, where they were newly diagnosed with active infection and not yet under treatment.
The researchers calculated the DAI based on dietary intake information from a validated, 168-item food frequency questionnaire used in Iran. The participants were asked about their dietary intake based on the average day, week, month, and year. They also discussed serving sizes of food items, and to increase the accuracy of estimates, interviewers showed household measurements or serving sizes to confirm the measurements with participants.
The average age of the study participants was 39 years, and about 60% were women. Compared with the healthy controls, those with H. pylori were significantly older, had higher body mass index, and smoked more.
Overall, patients with H. pylori had a significantly lower intake of vitamin A, vitamin E, manganese, and selenium. Other differences in dietary intake – for vitamin C and zinc – were not significant.
The average total DAI was significantly higher in the healthy controls, at 7.67, as compared with 3.57 in the patients with H. pylori. The risk for infection decreased as continuous DAI increased.
After adjusting for several variables, the researchers found that participants with less than the median DAI values had an increased risk of developing an H. pylori infection.
“A balanced diet, especially high consumption of fruits and vegetables, might protect people against the consequences of H. pylori infection,” the study authors write. “On the contrary, a diet full of carbohydrates and sweets is related to a higher H. pylori infection prevalence.”
Why a good diet may help combat infection
The findings are consistent with other studies that have noted a higher intake of fruits and vegetables among healthy people compared with those who have H. pylori infections, the study authors write. Animal studies have also indicated that taking vitamins A, C, and E and selenium can lead to a reduction in H. pylori growth.
“Several biologically plausible reasons may explain why dietary antioxidants might be, either directly or indirectly, a protective factor against H. pylori infection,” the researchers write. “It is well-known that antioxidants, with their free radical scavenging activities, can inhibit the growth of H. pylori.”
H. pylori is urease-positive and can synthesize a large amount of urease for ammonia production to neutralize gastric acid, which allows it to colonize in the stomach epithelium, the study authors write. Vitamin C inhibits urease activity and improves the stimulation of granulocytes, macrophages, lymphocytes, and immunoglobulin production. Other nutrients, such as zinc, may inhibit the urease enzyme and prevent H. pylori adhesion to gastric tissues, they write.
“Dietary elements have previously been shown to dramatically alter pathogenic responses to H. pylori infections,” Richard Peek Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
Dr. Peek, who wasn’t involved with this study, and colleagues found that iron deficiency is linked with altered bile metabolism, which can promote H. pylori–induced gastric carcinogenesis.
“The current study is important, as it suggests that shifting to a diet rich in antioxidants may be beneficial in terms of H. pylori infection,” he said.
At the same time, Dr. Peek expressed caution about generalizing the results across populations.
“Most of the persons enrolled in this study were likely infected with H. pylori as children,” he noted. “Therefore, the inverse role of antioxidant-rich diets and H. pylori infection must be interpreted with caution.”
Future studies should confirm the findings in other groups and determine whether antioxidant-rich diets limit the diseases caused by H. pylori infection, Dr. Peek added.
The study was not funded by any research center, and the authors declared no conflicts of interest. Dr. Peek reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
People who eat a balanced diet with sufficient antioxidants from fruits and vegetables may face reduced risks for Heliobacter pylori infections, according to a new report.
In particular, patients with an H. pylori infection were more likely to score lower on the Dietary Antioxidant Index (DAI), which was created to consider a diet’s entire antioxidant profile.
“Available evidence indicates that diet has an important role in developing H. pylori infection. Therefore, protective dietary factors are important from a public health point of view,” Farzad Shidfar, a professor of nutrition at the Iran University of Medical Sciences, Tehran, and member of the university’s colorectal research center, and colleagues write.
“While some nutritional research has widely focused on single nutrients or foods in diet-disease relations, the overall diet could be more informative because humans typically consume a combination of nutrients and foods,” they write. “Dietary indices such as DAI are one of the approaches for this purpose.”
The study was published online in BMC Gastroenterology.
Measuring antioxidant intake
Previous research has indicated an inverse association between the DAI and inflammatory diseases, the study authors write, including gastric cancer, colorectal cancer, nonalcoholic fatty liver disease, and obesity. Studies have also indicated that H. pylori infection is related to deficiencies in vitamins A, C, and E, which have antioxidant properties.
In a case-control study, the research team compared the dietary intake of 148 patients with H. pylori to 302 healthy controls without infection. The patients in the H. pylori–positive group were recruited between June 2021 and November 2021 from the gastroenterology clinic at Rasoul-e-Akram Hospital in Tehran, where they were newly diagnosed with active infection and not yet under treatment.
The researchers calculated the DAI based on dietary intake information from a validated, 168-item food frequency questionnaire used in Iran. The participants were asked about their dietary intake based on the average day, week, month, and year. They also discussed serving sizes of food items, and to increase the accuracy of estimates, interviewers showed household measurements or serving sizes to confirm the measurements with participants.
The average age of the study participants was 39 years, and about 60% were women. Compared with the healthy controls, those with H. pylori were significantly older, had higher body mass index, and smoked more.
Overall, patients with H. pylori had a significantly lower intake of vitamin A, vitamin E, manganese, and selenium. Other differences in dietary intake – for vitamin C and zinc – were not significant.
The average total DAI was significantly higher in the healthy controls, at 7.67, as compared with 3.57 in the patients with H. pylori. The risk for infection decreased as continuous DAI increased.
After adjusting for several variables, the researchers found that participants with less than the median DAI values had an increased risk of developing an H. pylori infection.
“A balanced diet, especially high consumption of fruits and vegetables, might protect people against the consequences of H. pylori infection,” the study authors write. “On the contrary, a diet full of carbohydrates and sweets is related to a higher H. pylori infection prevalence.”
Why a good diet may help combat infection
The findings are consistent with other studies that have noted a higher intake of fruits and vegetables among healthy people compared with those who have H. pylori infections, the study authors write. Animal studies have also indicated that taking vitamins A, C, and E and selenium can lead to a reduction in H. pylori growth.
“Several biologically plausible reasons may explain why dietary antioxidants might be, either directly or indirectly, a protective factor against H. pylori infection,” the researchers write. “It is well-known that antioxidants, with their free radical scavenging activities, can inhibit the growth of H. pylori.”
H. pylori is urease-positive and can synthesize a large amount of urease for ammonia production to neutralize gastric acid, which allows it to colonize in the stomach epithelium, the study authors write. Vitamin C inhibits urease activity and improves the stimulation of granulocytes, macrophages, lymphocytes, and immunoglobulin production. Other nutrients, such as zinc, may inhibit the urease enzyme and prevent H. pylori adhesion to gastric tissues, they write.
“Dietary elements have previously been shown to dramatically alter pathogenic responses to H. pylori infections,” Richard Peek Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
Dr. Peek, who wasn’t involved with this study, and colleagues found that iron deficiency is linked with altered bile metabolism, which can promote H. pylori–induced gastric carcinogenesis.
“The current study is important, as it suggests that shifting to a diet rich in antioxidants may be beneficial in terms of H. pylori infection,” he said.
At the same time, Dr. Peek expressed caution about generalizing the results across populations.
“Most of the persons enrolled in this study were likely infected with H. pylori as children,” he noted. “Therefore, the inverse role of antioxidant-rich diets and H. pylori infection must be interpreted with caution.”
Future studies should confirm the findings in other groups and determine whether antioxidant-rich diets limit the diseases caused by H. pylori infection, Dr. Peek added.
The study was not funded by any research center, and the authors declared no conflicts of interest. Dr. Peek reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
People who eat a balanced diet with sufficient antioxidants from fruits and vegetables may face reduced risks for Heliobacter pylori infections, according to a new report.
In particular, patients with an H. pylori infection were more likely to score lower on the Dietary Antioxidant Index (DAI), which was created to consider a diet’s entire antioxidant profile.
“Available evidence indicates that diet has an important role in developing H. pylori infection. Therefore, protective dietary factors are important from a public health point of view,” Farzad Shidfar, a professor of nutrition at the Iran University of Medical Sciences, Tehran, and member of the university’s colorectal research center, and colleagues write.
“While some nutritional research has widely focused on single nutrients or foods in diet-disease relations, the overall diet could be more informative because humans typically consume a combination of nutrients and foods,” they write. “Dietary indices such as DAI are one of the approaches for this purpose.”
The study was published online in BMC Gastroenterology.
Measuring antioxidant intake
Previous research has indicated an inverse association between the DAI and inflammatory diseases, the study authors write, including gastric cancer, colorectal cancer, nonalcoholic fatty liver disease, and obesity. Studies have also indicated that H. pylori infection is related to deficiencies in vitamins A, C, and E, which have antioxidant properties.
In a case-control study, the research team compared the dietary intake of 148 patients with H. pylori to 302 healthy controls without infection. The patients in the H. pylori–positive group were recruited between June 2021 and November 2021 from the gastroenterology clinic at Rasoul-e-Akram Hospital in Tehran, where they were newly diagnosed with active infection and not yet under treatment.
The researchers calculated the DAI based on dietary intake information from a validated, 168-item food frequency questionnaire used in Iran. The participants were asked about their dietary intake based on the average day, week, month, and year. They also discussed serving sizes of food items, and to increase the accuracy of estimates, interviewers showed household measurements or serving sizes to confirm the measurements with participants.
The average age of the study participants was 39 years, and about 60% were women. Compared with the healthy controls, those with H. pylori were significantly older, had higher body mass index, and smoked more.
Overall, patients with H. pylori had a significantly lower intake of vitamin A, vitamin E, manganese, and selenium. Other differences in dietary intake – for vitamin C and zinc – were not significant.
The average total DAI was significantly higher in the healthy controls, at 7.67, as compared with 3.57 in the patients with H. pylori. The risk for infection decreased as continuous DAI increased.
After adjusting for several variables, the researchers found that participants with less than the median DAI values had an increased risk of developing an H. pylori infection.
“A balanced diet, especially high consumption of fruits and vegetables, might protect people against the consequences of H. pylori infection,” the study authors write. “On the contrary, a diet full of carbohydrates and sweets is related to a higher H. pylori infection prevalence.”
Why a good diet may help combat infection
The findings are consistent with other studies that have noted a higher intake of fruits and vegetables among healthy people compared with those who have H. pylori infections, the study authors write. Animal studies have also indicated that taking vitamins A, C, and E and selenium can lead to a reduction in H. pylori growth.
“Several biologically plausible reasons may explain why dietary antioxidants might be, either directly or indirectly, a protective factor against H. pylori infection,” the researchers write. “It is well-known that antioxidants, with their free radical scavenging activities, can inhibit the growth of H. pylori.”
H. pylori is urease-positive and can synthesize a large amount of urease for ammonia production to neutralize gastric acid, which allows it to colonize in the stomach epithelium, the study authors write. Vitamin C inhibits urease activity and improves the stimulation of granulocytes, macrophages, lymphocytes, and immunoglobulin production. Other nutrients, such as zinc, may inhibit the urease enzyme and prevent H. pylori adhesion to gastric tissues, they write.
“Dietary elements have previously been shown to dramatically alter pathogenic responses to H. pylori infections,” Richard Peek Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
Dr. Peek, who wasn’t involved with this study, and colleagues found that iron deficiency is linked with altered bile metabolism, which can promote H. pylori–induced gastric carcinogenesis.
“The current study is important, as it suggests that shifting to a diet rich in antioxidants may be beneficial in terms of H. pylori infection,” he said.
At the same time, Dr. Peek expressed caution about generalizing the results across populations.
“Most of the persons enrolled in this study were likely infected with H. pylori as children,” he noted. “Therefore, the inverse role of antioxidant-rich diets and H. pylori infection must be interpreted with caution.”
Future studies should confirm the findings in other groups and determine whether antioxidant-rich diets limit the diseases caused by H. pylori infection, Dr. Peek added.
The study was not funded by any research center, and the authors declared no conflicts of interest. Dr. Peek reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM BMC GASTROENTEROLOGY
Ruminations on health care spending
What could you do with $18 billion?
I could pay off my mortgage roughly 60,000 times, or take my wife on a never-ending world cruise so we don’t need a mortgage, or at least hire someone to clean my pool regularly so I don’t have to.
A recent report from the OIG found that, in the last 3 years, the Centers for Medicare & Medicaid Services spent $18 billion on drugs for which there’s no proof of significant clinical benefit.
That’s a lot of money on things that may or may not be placebos, some of which are WAY overdue on Food and Drug Administration–mandated efficacy studies. A few have even been on the market so long that they’ve become equally unproven generics.
Now, if you put this in the big picture, that immense amount of money is still only 2% of their total spending in health care. Hell, probably at least 2% of my personal spending is on pointless things, too. So, realistically, you could say 98% of CMS spending is on worthwhile care, which is as it should be.
But the bottom line is that I’m sure it could be better used in many other programs (refunding it to taxpayers comes out to maybe $55 for each of us, which probably isn’t worth the effort).
As pointed out in the movie “Dave,” shoving that kind of money in even a low-yield savings account would generate at least $180 million in interest each year.
That’s a lot of money, too, that could be used for something. Of course, no one in the government thinks that way. That’s why we all loved the movie.
The problem is that the phrase “no proof of significant clinical benefit” doesn’t mean something doesn’t work. It just means we aren’t sure. Some of those people on one of these drugs may be getting benefit – or not. After all, the placebo effect is remarkably strong. But if they are helping someone, who wants to be the one to tell them “we’re not going to pay for this anymore?”
Another issue is this: Let’s say the drugs only work for 10% of the people who take them ($1.8 billion worth), and for the other 90% it’s iffy ($16.2 billion worth), but the latter want to stay on them anyway, just to be sure. Do we cut them? Or just say that $18 billion is too much money when only 10% are being helped, and cut them all off? I’m sure we could use the money elsewhere (see “Dave” above), so let them find a way to work it out with the manufacturer. The greatest good for the greatest number and all that jazz.
I don’t know, either. Health care dollars are finite, and human suffering is infinite. It’s a balancing act that can’t be won. There are no easy answers.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
What could you do with $18 billion?
I could pay off my mortgage roughly 60,000 times, or take my wife on a never-ending world cruise so we don’t need a mortgage, or at least hire someone to clean my pool regularly so I don’t have to.
A recent report from the OIG found that, in the last 3 years, the Centers for Medicare & Medicaid Services spent $18 billion on drugs for which there’s no proof of significant clinical benefit.
That’s a lot of money on things that may or may not be placebos, some of which are WAY overdue on Food and Drug Administration–mandated efficacy studies. A few have even been on the market so long that they’ve become equally unproven generics.
Now, if you put this in the big picture, that immense amount of money is still only 2% of their total spending in health care. Hell, probably at least 2% of my personal spending is on pointless things, too. So, realistically, you could say 98% of CMS spending is on worthwhile care, which is as it should be.
But the bottom line is that I’m sure it could be better used in many other programs (refunding it to taxpayers comes out to maybe $55 for each of us, which probably isn’t worth the effort).
As pointed out in the movie “Dave,” shoving that kind of money in even a low-yield savings account would generate at least $180 million in interest each year.
That’s a lot of money, too, that could be used for something. Of course, no one in the government thinks that way. That’s why we all loved the movie.
The problem is that the phrase “no proof of significant clinical benefit” doesn’t mean something doesn’t work. It just means we aren’t sure. Some of those people on one of these drugs may be getting benefit – or not. After all, the placebo effect is remarkably strong. But if they are helping someone, who wants to be the one to tell them “we’re not going to pay for this anymore?”
Another issue is this: Let’s say the drugs only work for 10% of the people who take them ($1.8 billion worth), and for the other 90% it’s iffy ($16.2 billion worth), but the latter want to stay on them anyway, just to be sure. Do we cut them? Or just say that $18 billion is too much money when only 10% are being helped, and cut them all off? I’m sure we could use the money elsewhere (see “Dave” above), so let them find a way to work it out with the manufacturer. The greatest good for the greatest number and all that jazz.
I don’t know, either. Health care dollars are finite, and human suffering is infinite. It’s a balancing act that can’t be won. There are no easy answers.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
What could you do with $18 billion?
I could pay off my mortgage roughly 60,000 times, or take my wife on a never-ending world cruise so we don’t need a mortgage, or at least hire someone to clean my pool regularly so I don’t have to.
A recent report from the OIG found that, in the last 3 years, the Centers for Medicare & Medicaid Services spent $18 billion on drugs for which there’s no proof of significant clinical benefit.
That’s a lot of money on things that may or may not be placebos, some of which are WAY overdue on Food and Drug Administration–mandated efficacy studies. A few have even been on the market so long that they’ve become equally unproven generics.
Now, if you put this in the big picture, that immense amount of money is still only 2% of their total spending in health care. Hell, probably at least 2% of my personal spending is on pointless things, too. So, realistically, you could say 98% of CMS spending is on worthwhile care, which is as it should be.
But the bottom line is that I’m sure it could be better used in many other programs (refunding it to taxpayers comes out to maybe $55 for each of us, which probably isn’t worth the effort).
As pointed out in the movie “Dave,” shoving that kind of money in even a low-yield savings account would generate at least $180 million in interest each year.
That’s a lot of money, too, that could be used for something. Of course, no one in the government thinks that way. That’s why we all loved the movie.
The problem is that the phrase “no proof of significant clinical benefit” doesn’t mean something doesn’t work. It just means we aren’t sure. Some of those people on one of these drugs may be getting benefit – or not. After all, the placebo effect is remarkably strong. But if they are helping someone, who wants to be the one to tell them “we’re not going to pay for this anymore?”
Another issue is this: Let’s say the drugs only work for 10% of the people who take them ($1.8 billion worth), and for the other 90% it’s iffy ($16.2 billion worth), but the latter want to stay on them anyway, just to be sure. Do we cut them? Or just say that $18 billion is too much money when only 10% are being helped, and cut them all off? I’m sure we could use the money elsewhere (see “Dave” above), so let them find a way to work it out with the manufacturer. The greatest good for the greatest number and all that jazz.
I don’t know, either. Health care dollars are finite, and human suffering is infinite. It’s a balancing act that can’t be won. There are no easy answers.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
New ICD-10-CM codes a ‘big switch-over’ for neurocognitive disorders
Revised ICD-10-CM codes for neurocognitive disorders are now in effect, the American Psychiatric Association has announced
The coding changes for major and mild neurocognitive disorders represent “the most consequential” coding changes for DSM-5 disorders since the Oct. 1, 2015, changeover from ICD-9-CM to ICD-10-CM,” Michael First, MD, professor of clinical psychiatry at Columbia University, in New York, wrote in a statement published in Psychiatric News.
The updated codes for neurocognitive disorders are “much more specific and indicate all the different types of behavioral problems that could occur with dementia,” First, who served as editor of the DSM-5-TR, added in an interview.
This year, coding changes that affect psychiatry are largely confined to major and mild neurocognitive disorders, but they represent “a big switch-over,” Dr. First said.
What’s new
The first three characters that make up the ICD-10-CM code for major neurocognitive disorder depend on the type of etiologic medical condition and are unchanged:
- F01 for major neurocognitive disorder caused by vascular disease
- F02 for major neurocognitive disorder caused by other medical conditions in which the specific etiologic medical condition is indicated by also listing the ICD-10-CM code for the medical condition
- F03 for major neurocognitive disorder when the medical etiology is unknown
However, DSM-5-TR diagnostic criteria for major neurocognitive disorder include severity specifiers (mild, moderate, severe), but there is no provision for indicating this “clinically important” information in the current ICD-10-CM code for major neurocognitive disorder, Dr. First explained.
The 2022 coding changes for major neurocognitive disorder include the provision of a fourth character code to indicate the severity of the major neurocognitive disorder – “A” indicates mild (difficulties with instrumental activities of daily living, such as housework and managing money); “B,” moderate (difficulties with basic activities of daily living, such as feeding and dressing); and “C,” severe (fully dependent) impairment.
The coding changes for major neurocognitive disorder also now include fifth and sixth characters to indicate the presence of an accompanying behavioral or psychological disturbance, such as agitation, psychotic disturbance, mood symptoms, and anxiety.
The update, which went into effect Oct. 1, also adds to ICD-10-CM two new mental disorder codes, F06.71 and F06.70 for mild neurocognitive disorder caused by a medical condition with or without a behavioral disturbance, respectively.
The coding changes affecting psychiatry are outlined in the APA’s 2022 DSM-5-TR Update: Supplement to the Diagnostic and Statistical Manual of Mental Disorders and DSM-5-TR Neurocognitive Disorders Supplement.
Annual event
Every Oct. 1, ICD-10-CM codes for all of medicine are updated, with new codes being added and others revised or deleted. Only a small fraction of the 68,000 codes is affected. Last year, 159 new codes were added, 25 codes were deleted, and 27 existing codes were revised.
All HIPAA-compliant health care entities are required to use the most up-to-date ICD-10-CM codes.
“I think there’s a grace period where you can still use the old codes, but there will be a point where if you use the old code, it’ll get rejected because it won’t be considered a valid code,” said Dr. First.
A version of this article first appeared on Medscape.com.
Revised ICD-10-CM codes for neurocognitive disorders are now in effect, the American Psychiatric Association has announced
The coding changes for major and mild neurocognitive disorders represent “the most consequential” coding changes for DSM-5 disorders since the Oct. 1, 2015, changeover from ICD-9-CM to ICD-10-CM,” Michael First, MD, professor of clinical psychiatry at Columbia University, in New York, wrote in a statement published in Psychiatric News.
The updated codes for neurocognitive disorders are “much more specific and indicate all the different types of behavioral problems that could occur with dementia,” First, who served as editor of the DSM-5-TR, added in an interview.
This year, coding changes that affect psychiatry are largely confined to major and mild neurocognitive disorders, but they represent “a big switch-over,” Dr. First said.
What’s new
The first three characters that make up the ICD-10-CM code for major neurocognitive disorder depend on the type of etiologic medical condition and are unchanged:
- F01 for major neurocognitive disorder caused by vascular disease
- F02 for major neurocognitive disorder caused by other medical conditions in which the specific etiologic medical condition is indicated by also listing the ICD-10-CM code for the medical condition
- F03 for major neurocognitive disorder when the medical etiology is unknown
However, DSM-5-TR diagnostic criteria for major neurocognitive disorder include severity specifiers (mild, moderate, severe), but there is no provision for indicating this “clinically important” information in the current ICD-10-CM code for major neurocognitive disorder, Dr. First explained.
The 2022 coding changes for major neurocognitive disorder include the provision of a fourth character code to indicate the severity of the major neurocognitive disorder – “A” indicates mild (difficulties with instrumental activities of daily living, such as housework and managing money); “B,” moderate (difficulties with basic activities of daily living, such as feeding and dressing); and “C,” severe (fully dependent) impairment.
The coding changes for major neurocognitive disorder also now include fifth and sixth characters to indicate the presence of an accompanying behavioral or psychological disturbance, such as agitation, psychotic disturbance, mood symptoms, and anxiety.
The update, which went into effect Oct. 1, also adds to ICD-10-CM two new mental disorder codes, F06.71 and F06.70 for mild neurocognitive disorder caused by a medical condition with or without a behavioral disturbance, respectively.
The coding changes affecting psychiatry are outlined in the APA’s 2022 DSM-5-TR Update: Supplement to the Diagnostic and Statistical Manual of Mental Disorders and DSM-5-TR Neurocognitive Disorders Supplement.
Annual event
Every Oct. 1, ICD-10-CM codes for all of medicine are updated, with new codes being added and others revised or deleted. Only a small fraction of the 68,000 codes is affected. Last year, 159 new codes were added, 25 codes were deleted, and 27 existing codes were revised.
All HIPAA-compliant health care entities are required to use the most up-to-date ICD-10-CM codes.
“I think there’s a grace period where you can still use the old codes, but there will be a point where if you use the old code, it’ll get rejected because it won’t be considered a valid code,” said Dr. First.
A version of this article first appeared on Medscape.com.
Revised ICD-10-CM codes for neurocognitive disorders are now in effect, the American Psychiatric Association has announced
The coding changes for major and mild neurocognitive disorders represent “the most consequential” coding changes for DSM-5 disorders since the Oct. 1, 2015, changeover from ICD-9-CM to ICD-10-CM,” Michael First, MD, professor of clinical psychiatry at Columbia University, in New York, wrote in a statement published in Psychiatric News.
The updated codes for neurocognitive disorders are “much more specific and indicate all the different types of behavioral problems that could occur with dementia,” First, who served as editor of the DSM-5-TR, added in an interview.
This year, coding changes that affect psychiatry are largely confined to major and mild neurocognitive disorders, but they represent “a big switch-over,” Dr. First said.
What’s new
The first three characters that make up the ICD-10-CM code for major neurocognitive disorder depend on the type of etiologic medical condition and are unchanged:
- F01 for major neurocognitive disorder caused by vascular disease
- F02 for major neurocognitive disorder caused by other medical conditions in which the specific etiologic medical condition is indicated by also listing the ICD-10-CM code for the medical condition
- F03 for major neurocognitive disorder when the medical etiology is unknown
However, DSM-5-TR diagnostic criteria for major neurocognitive disorder include severity specifiers (mild, moderate, severe), but there is no provision for indicating this “clinically important” information in the current ICD-10-CM code for major neurocognitive disorder, Dr. First explained.
The 2022 coding changes for major neurocognitive disorder include the provision of a fourth character code to indicate the severity of the major neurocognitive disorder – “A” indicates mild (difficulties with instrumental activities of daily living, such as housework and managing money); “B,” moderate (difficulties with basic activities of daily living, such as feeding and dressing); and “C,” severe (fully dependent) impairment.
The coding changes for major neurocognitive disorder also now include fifth and sixth characters to indicate the presence of an accompanying behavioral or psychological disturbance, such as agitation, psychotic disturbance, mood symptoms, and anxiety.
The update, which went into effect Oct. 1, also adds to ICD-10-CM two new mental disorder codes, F06.71 and F06.70 for mild neurocognitive disorder caused by a medical condition with or without a behavioral disturbance, respectively.
The coding changes affecting psychiatry are outlined in the APA’s 2022 DSM-5-TR Update: Supplement to the Diagnostic and Statistical Manual of Mental Disorders and DSM-5-TR Neurocognitive Disorders Supplement.
Annual event
Every Oct. 1, ICD-10-CM codes for all of medicine are updated, with new codes being added and others revised or deleted. Only a small fraction of the 68,000 codes is affected. Last year, 159 new codes were added, 25 codes were deleted, and 27 existing codes were revised.
All HIPAA-compliant health care entities are required to use the most up-to-date ICD-10-CM codes.
“I think there’s a grace period where you can still use the old codes, but there will be a point where if you use the old code, it’ll get rejected because it won’t be considered a valid code,” said Dr. First.
A version of this article first appeared on Medscape.com.
Physicians speak out: Why they love or hate incentive bonuses
Incentive bonuses have long been part and parcel of many physicians’ compensation packages. They allow doctors in some specialties to boost their compensation by tens of thousands of dollars.
Often tied to metrics that doctors must hit,
A recent Medscape poll asked what physicians think about incentive bonuses and whether or not tying metrics to salary is an outdated practice that interferes with the integrity of a physician’s job or contributes to excellence in patient care and increased productivity.
Here is what 406 physicians who answered the poll, which ran from Aug. 17 to Sept. 1, had to say about incentive bonuses:
More than half the physicians polled (58%) received an incentive bonus in 2021. Of those who received a bonus, 44% received up to $25,000. Almost 30% received $25,001-$50,000 in incentive bonus money. Only 14% received more than $100,000.
When we asked physicians which metrics they prefer their bonus to be based on, a large majority (64%) agreed quality of care was most relevant. Other metrics that respondents think appropriate included professionalism (40%), patient outcomes (40%), patient satisfaction (34%), patient volume (26%), market expansion (7%), and other (3%).
The problem with bonuses
Once thought to improve quality and consistency of care, incentive bonuses may be falling out of favor. Developing, administrating, and tracking them may be cumbersome for the institutions that advocate for them. For instance, determining who gave quality care and how to measure that care can be difficult.
What’s more, some top health care employers, Mayo Clinic and Kaiser Permanente, have switched from the incentive bonus model to straight salaries. Data show that the number of tests patients have and the number of treatments they try decreases when doctors receive straight salaries.
In fact, 74% of the polled physicians think that bonuses can result in consequences like unnecessary tests and higher patient costs. Three-fourths of respondents don’t think incentives improve patient care either.
Physicians have long thought incentive bonuses can also have unintended consequences. For example, tying a physician’s monetary reward to metrics such as patient outcomes, like adherence to treatment protocols, may mean that noncompliant patients can jeopardize your metrics and prevent physicians from getting bonuses.
A Merritt Hawkins’ 2019 Review of Physician and Advanced Practitioner Recruiting Incentives found that 56% of bonuses are based in whole or in part on metrics like a patient’s adherence.
Additionally, tying monetary rewards to patient volume encourages some physicians to overbook patients, work more and longer hours, and risk burnout to meet their bonus criteria.
When we asked how hard it was to meet metrics in the Medscape poll, 45% of respondents who receive incentive bonuses said it was somewhat or very difficult. Only 9% consider it very easy. And 71% of physicians say their bonus is at risk because of not meeting their metrics.
Not surprisingly, large pay-for-performance bonuses are only offered to certain specialists and physician specialties in high demand. An orthopedist, for example, can earn up to an average of $126,000 in incentive bonuses, while a pediatrician brings in an average of $28,000, according to the Medscape Physician Compensation Report 2022.
Yet physicians are still torn
Despite these negatives, physicians are split about whether bonuses are good for doctors. The poll shows 51% said no, and 49% said yes. Further, physicians were split 50-50 on whether the bonus makes physicians more productive. Interestingly though, 76% think the bonus compensation method should be phased out in favor of straight salaries.
But many physicians may welcome the “lump sum” nature of receiving large bonuses at certain times of the year to help pay off student loan debt or other expenses, or are just comfortable having a bonus.
Financially speaking
If you have the choice, you may fare better by taking a higher salary and eliminating a bonus. Receiving your pay throughout the year may be preferable to receiving large lump sums only at certain times. Another thing to remember about your incentive bonus is that they are sometimes taxed more heavily based on “supplemental income.” The IRS considers bonuses supplemental to your income, so they may have a higher withholding rate, which can feel penalizing. You may have noticed the extra withholding in your last bonus check.
A version of this article first appeared on Medscape.com.
Incentive bonuses have long been part and parcel of many physicians’ compensation packages. They allow doctors in some specialties to boost their compensation by tens of thousands of dollars.
Often tied to metrics that doctors must hit,
A recent Medscape poll asked what physicians think about incentive bonuses and whether or not tying metrics to salary is an outdated practice that interferes with the integrity of a physician’s job or contributes to excellence in patient care and increased productivity.
Here is what 406 physicians who answered the poll, which ran from Aug. 17 to Sept. 1, had to say about incentive bonuses:
More than half the physicians polled (58%) received an incentive bonus in 2021. Of those who received a bonus, 44% received up to $25,000. Almost 30% received $25,001-$50,000 in incentive bonus money. Only 14% received more than $100,000.
When we asked physicians which metrics they prefer their bonus to be based on, a large majority (64%) agreed quality of care was most relevant. Other metrics that respondents think appropriate included professionalism (40%), patient outcomes (40%), patient satisfaction (34%), patient volume (26%), market expansion (7%), and other (3%).
The problem with bonuses
Once thought to improve quality and consistency of care, incentive bonuses may be falling out of favor. Developing, administrating, and tracking them may be cumbersome for the institutions that advocate for them. For instance, determining who gave quality care and how to measure that care can be difficult.
What’s more, some top health care employers, Mayo Clinic and Kaiser Permanente, have switched from the incentive bonus model to straight salaries. Data show that the number of tests patients have and the number of treatments they try decreases when doctors receive straight salaries.
In fact, 74% of the polled physicians think that bonuses can result in consequences like unnecessary tests and higher patient costs. Three-fourths of respondents don’t think incentives improve patient care either.
Physicians have long thought incentive bonuses can also have unintended consequences. For example, tying a physician’s monetary reward to metrics such as patient outcomes, like adherence to treatment protocols, may mean that noncompliant patients can jeopardize your metrics and prevent physicians from getting bonuses.
A Merritt Hawkins’ 2019 Review of Physician and Advanced Practitioner Recruiting Incentives found that 56% of bonuses are based in whole or in part on metrics like a patient’s adherence.
Additionally, tying monetary rewards to patient volume encourages some physicians to overbook patients, work more and longer hours, and risk burnout to meet their bonus criteria.
When we asked how hard it was to meet metrics in the Medscape poll, 45% of respondents who receive incentive bonuses said it was somewhat or very difficult. Only 9% consider it very easy. And 71% of physicians say their bonus is at risk because of not meeting their metrics.
Not surprisingly, large pay-for-performance bonuses are only offered to certain specialists and physician specialties in high demand. An orthopedist, for example, can earn up to an average of $126,000 in incentive bonuses, while a pediatrician brings in an average of $28,000, according to the Medscape Physician Compensation Report 2022.
Yet physicians are still torn
Despite these negatives, physicians are split about whether bonuses are good for doctors. The poll shows 51% said no, and 49% said yes. Further, physicians were split 50-50 on whether the bonus makes physicians more productive. Interestingly though, 76% think the bonus compensation method should be phased out in favor of straight salaries.
But many physicians may welcome the “lump sum” nature of receiving large bonuses at certain times of the year to help pay off student loan debt or other expenses, or are just comfortable having a bonus.
Financially speaking
If you have the choice, you may fare better by taking a higher salary and eliminating a bonus. Receiving your pay throughout the year may be preferable to receiving large lump sums only at certain times. Another thing to remember about your incentive bonus is that they are sometimes taxed more heavily based on “supplemental income.” The IRS considers bonuses supplemental to your income, so they may have a higher withholding rate, which can feel penalizing. You may have noticed the extra withholding in your last bonus check.
A version of this article first appeared on Medscape.com.
Incentive bonuses have long been part and parcel of many physicians’ compensation packages. They allow doctors in some specialties to boost their compensation by tens of thousands of dollars.
Often tied to metrics that doctors must hit,
A recent Medscape poll asked what physicians think about incentive bonuses and whether or not tying metrics to salary is an outdated practice that interferes with the integrity of a physician’s job or contributes to excellence in patient care and increased productivity.
Here is what 406 physicians who answered the poll, which ran from Aug. 17 to Sept. 1, had to say about incentive bonuses:
More than half the physicians polled (58%) received an incentive bonus in 2021. Of those who received a bonus, 44% received up to $25,000. Almost 30% received $25,001-$50,000 in incentive bonus money. Only 14% received more than $100,000.
When we asked physicians which metrics they prefer their bonus to be based on, a large majority (64%) agreed quality of care was most relevant. Other metrics that respondents think appropriate included professionalism (40%), patient outcomes (40%), patient satisfaction (34%), patient volume (26%), market expansion (7%), and other (3%).
The problem with bonuses
Once thought to improve quality and consistency of care, incentive bonuses may be falling out of favor. Developing, administrating, and tracking them may be cumbersome for the institutions that advocate for them. For instance, determining who gave quality care and how to measure that care can be difficult.
What’s more, some top health care employers, Mayo Clinic and Kaiser Permanente, have switched from the incentive bonus model to straight salaries. Data show that the number of tests patients have and the number of treatments they try decreases when doctors receive straight salaries.
In fact, 74% of the polled physicians think that bonuses can result in consequences like unnecessary tests and higher patient costs. Three-fourths of respondents don’t think incentives improve patient care either.
Physicians have long thought incentive bonuses can also have unintended consequences. For example, tying a physician’s monetary reward to metrics such as patient outcomes, like adherence to treatment protocols, may mean that noncompliant patients can jeopardize your metrics and prevent physicians from getting bonuses.
A Merritt Hawkins’ 2019 Review of Physician and Advanced Practitioner Recruiting Incentives found that 56% of bonuses are based in whole or in part on metrics like a patient’s adherence.
Additionally, tying monetary rewards to patient volume encourages some physicians to overbook patients, work more and longer hours, and risk burnout to meet their bonus criteria.
When we asked how hard it was to meet metrics in the Medscape poll, 45% of respondents who receive incentive bonuses said it was somewhat or very difficult. Only 9% consider it very easy. And 71% of physicians say their bonus is at risk because of not meeting their metrics.
Not surprisingly, large pay-for-performance bonuses are only offered to certain specialists and physician specialties in high demand. An orthopedist, for example, can earn up to an average of $126,000 in incentive bonuses, while a pediatrician brings in an average of $28,000, according to the Medscape Physician Compensation Report 2022.
Yet physicians are still torn
Despite these negatives, physicians are split about whether bonuses are good for doctors. The poll shows 51% said no, and 49% said yes. Further, physicians were split 50-50 on whether the bonus makes physicians more productive. Interestingly though, 76% think the bonus compensation method should be phased out in favor of straight salaries.
But many physicians may welcome the “lump sum” nature of receiving large bonuses at certain times of the year to help pay off student loan debt or other expenses, or are just comfortable having a bonus.
Financially speaking
If you have the choice, you may fare better by taking a higher salary and eliminating a bonus. Receiving your pay throughout the year may be preferable to receiving large lump sums only at certain times. Another thing to remember about your incentive bonus is that they are sometimes taxed more heavily based on “supplemental income.” The IRS considers bonuses supplemental to your income, so they may have a higher withholding rate, which can feel penalizing. You may have noticed the extra withholding in your last bonus check.
A version of this article first appeared on Medscape.com.
COMMENT & CONTROVERSY
Misoprostol: Clinical pharmacology in obstetrics and gynecology
ROBERT L. BARBIERI, MD (JULY 2022)
Outcomes from my practice’s pilot study
In his recent editorial, Dr. Barbieri addressed the important topic of office-based cervical ripening prior to inpatient induction of labor. In order to decrease the length of labor and increase the success of vaginal delivery, the cervical factor is of prime importance. Patients with an unfavorable cervix (Bishop score of ≥6) are more likely to experience longer labor, risk of infection, fetal distress, etc, and may end up with an unwanted cesarean delivery. To prevent the above, numerous approaches (mechanical methods, double-balloon catheter, laminaria, misoprostol among others) have been discussed.
The inclusion criteria for office-based cervical ripening are low-risk patients, singleton pregnancies between 39 and 40 weeks of gestation, and cephalic presentation. The details of inclusion and exclusion criteria have to be determined by each practice individually. Our practice went a step further. We performed a small pilot study to assess the safety and efficacy of office cervical ripening in low-risk primigravid patients with low Bishop scores who were not scheduled for induction in anticipation of labor. Ten primigravid patients with poor Bishop scores (6 or less) were administered 50 µg misoprostol at 39+ weeks of pregnancy in the office setting. Bishop scores were taken twice per week until delivery. In 7 out of 10 patients, the Bishop score became favorable within a week of treatment, and in 3 patients the Bishop score remained the same. Three out of 10 patients experienced self-limited episodes of uterine contractility, and 2 of the patients went into labor within 3 days of using misoprostol. All patients were delivered within 2 weeks of treatment without an induction: 8 delivered vaginally, and 2 by cesarean delivery.2
Cesarean delivery was done for fetal distress (1 case) and prolonged second stage of labor (1 case). All neonates were born in satisfactory condition with Apgar scores between 7 and 10. Our preliminary results demonstrated marked improvement in cervical ripening judged by the Bishop score in 70% of patients.2
A prospective randomized study should be performed with the following agenda:
- Does late pregnancy medical cervical ripening in low-risk patients affect labor course and cesarean delivery rate?
- What is the optimal dose and route of administration of misoprostol?3,4
References
- Barbieri R. Office-based ambulatory cervical ripening prior to in patient induction of labor. OBG Manag. 2021;33:9-13.
- Petrikovsky B. Should cervical ripening become routine in primigravid low risk patients [In press]. Neonat Int Care. 2022:1, 4-6.
- Sharami SH, Milani F, Faraji R. Comparison of 25 µg sublingual and 50 µg intravaginal misoprostol for cervical ripening and labor: a randomized controlled equivalence trial. Arch Med. 2014:10:653-656.
- Barbieri R. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022:34:7, 8-12.
B. Petrikovsky, MD, PhD
New Hyde Park, New York
Dr. Barbieri responds
I appreciate that Dr. Petrikovsky took time from a busy practice to provide our readers with his very innovative idea. I agree with him that a clinical trial is warranted to test the effects of late pregnancy medical cervical ripening in low-risk patients on labor course and birth outcome. Maybe one of our readers will take on the challenge to complete such a trial! ●
Misoprostol: Clinical pharmacology in obstetrics and gynecology
ROBERT L. BARBIERI, MD (JULY 2022)
Outcomes from my practice’s pilot study
In his recent editorial, Dr. Barbieri addressed the important topic of office-based cervical ripening prior to inpatient induction of labor. In order to decrease the length of labor and increase the success of vaginal delivery, the cervical factor is of prime importance. Patients with an unfavorable cervix (Bishop score of ≥6) are more likely to experience longer labor, risk of infection, fetal distress, etc, and may end up with an unwanted cesarean delivery. To prevent the above, numerous approaches (mechanical methods, double-balloon catheter, laminaria, misoprostol among others) have been discussed.
The inclusion criteria for office-based cervical ripening are low-risk patients, singleton pregnancies between 39 and 40 weeks of gestation, and cephalic presentation. The details of inclusion and exclusion criteria have to be determined by each practice individually. Our practice went a step further. We performed a small pilot study to assess the safety and efficacy of office cervical ripening in low-risk primigravid patients with low Bishop scores who were not scheduled for induction in anticipation of labor. Ten primigravid patients with poor Bishop scores (6 or less) were administered 50 µg misoprostol at 39+ weeks of pregnancy in the office setting. Bishop scores were taken twice per week until delivery. In 7 out of 10 patients, the Bishop score became favorable within a week of treatment, and in 3 patients the Bishop score remained the same. Three out of 10 patients experienced self-limited episodes of uterine contractility, and 2 of the patients went into labor within 3 days of using misoprostol. All patients were delivered within 2 weeks of treatment without an induction: 8 delivered vaginally, and 2 by cesarean delivery.2
Cesarean delivery was done for fetal distress (1 case) and prolonged second stage of labor (1 case). All neonates were born in satisfactory condition with Apgar scores between 7 and 10. Our preliminary results demonstrated marked improvement in cervical ripening judged by the Bishop score in 70% of patients.2
A prospective randomized study should be performed with the following agenda:
- Does late pregnancy medical cervical ripening in low-risk patients affect labor course and cesarean delivery rate?
- What is the optimal dose and route of administration of misoprostol?3,4
References
- Barbieri R. Office-based ambulatory cervical ripening prior to in patient induction of labor. OBG Manag. 2021;33:9-13.
- Petrikovsky B. Should cervical ripening become routine in primigravid low risk patients [In press]. Neonat Int Care. 2022:1, 4-6.
- Sharami SH, Milani F, Faraji R. Comparison of 25 µg sublingual and 50 µg intravaginal misoprostol for cervical ripening and labor: a randomized controlled equivalence trial. Arch Med. 2014:10:653-656.
- Barbieri R. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022:34:7, 8-12.
B. Petrikovsky, MD, PhD
New Hyde Park, New York
Dr. Barbieri responds
I appreciate that Dr. Petrikovsky took time from a busy practice to provide our readers with his very innovative idea. I agree with him that a clinical trial is warranted to test the effects of late pregnancy medical cervical ripening in low-risk patients on labor course and birth outcome. Maybe one of our readers will take on the challenge to complete such a trial! ●
Misoprostol: Clinical pharmacology in obstetrics and gynecology
ROBERT L. BARBIERI, MD (JULY 2022)
Outcomes from my practice’s pilot study
In his recent editorial, Dr. Barbieri addressed the important topic of office-based cervical ripening prior to inpatient induction of labor. In order to decrease the length of labor and increase the success of vaginal delivery, the cervical factor is of prime importance. Patients with an unfavorable cervix (Bishop score of ≥6) are more likely to experience longer labor, risk of infection, fetal distress, etc, and may end up with an unwanted cesarean delivery. To prevent the above, numerous approaches (mechanical methods, double-balloon catheter, laminaria, misoprostol among others) have been discussed.
The inclusion criteria for office-based cervical ripening are low-risk patients, singleton pregnancies between 39 and 40 weeks of gestation, and cephalic presentation. The details of inclusion and exclusion criteria have to be determined by each practice individually. Our practice went a step further. We performed a small pilot study to assess the safety and efficacy of office cervical ripening in low-risk primigravid patients with low Bishop scores who were not scheduled for induction in anticipation of labor. Ten primigravid patients with poor Bishop scores (6 or less) were administered 50 µg misoprostol at 39+ weeks of pregnancy in the office setting. Bishop scores were taken twice per week until delivery. In 7 out of 10 patients, the Bishop score became favorable within a week of treatment, and in 3 patients the Bishop score remained the same. Three out of 10 patients experienced self-limited episodes of uterine contractility, and 2 of the patients went into labor within 3 days of using misoprostol. All patients were delivered within 2 weeks of treatment without an induction: 8 delivered vaginally, and 2 by cesarean delivery.2
Cesarean delivery was done for fetal distress (1 case) and prolonged second stage of labor (1 case). All neonates were born in satisfactory condition with Apgar scores between 7 and 10. Our preliminary results demonstrated marked improvement in cervical ripening judged by the Bishop score in 70% of patients.2
A prospective randomized study should be performed with the following agenda:
- Does late pregnancy medical cervical ripening in low-risk patients affect labor course and cesarean delivery rate?
- What is the optimal dose and route of administration of misoprostol?3,4
References
- Barbieri R. Office-based ambulatory cervical ripening prior to in patient induction of labor. OBG Manag. 2021;33:9-13.
- Petrikovsky B. Should cervical ripening become routine in primigravid low risk patients [In press]. Neonat Int Care. 2022:1, 4-6.
- Sharami SH, Milani F, Faraji R. Comparison of 25 µg sublingual and 50 µg intravaginal misoprostol for cervical ripening and labor: a randomized controlled equivalence trial. Arch Med. 2014:10:653-656.
- Barbieri R. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022:34:7, 8-12.
B. Petrikovsky, MD, PhD
New Hyde Park, New York
Dr. Barbieri responds
I appreciate that Dr. Petrikovsky took time from a busy practice to provide our readers with his very innovative idea. I agree with him that a clinical trial is warranted to test the effects of late pregnancy medical cervical ripening in low-risk patients on labor course and birth outcome. Maybe one of our readers will take on the challenge to complete such a trial! ●
Options and outcomes for uterine preservation at the time of prolapse surgery
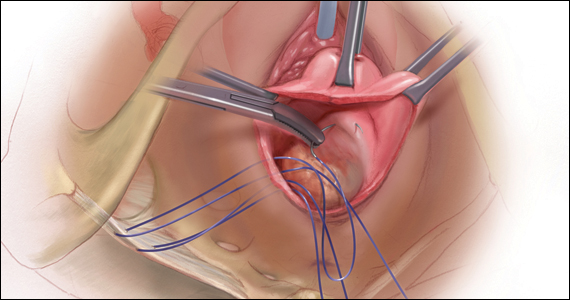
CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7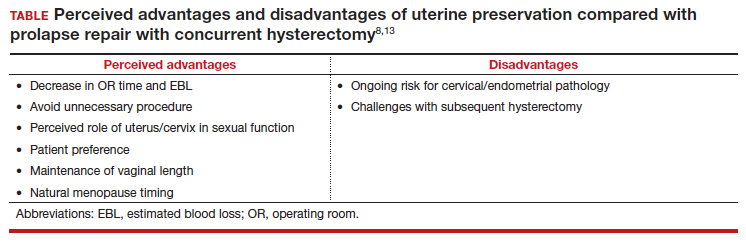
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.

CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●

CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.