User login
How common is IUD perforation, expulsion, and malposition?
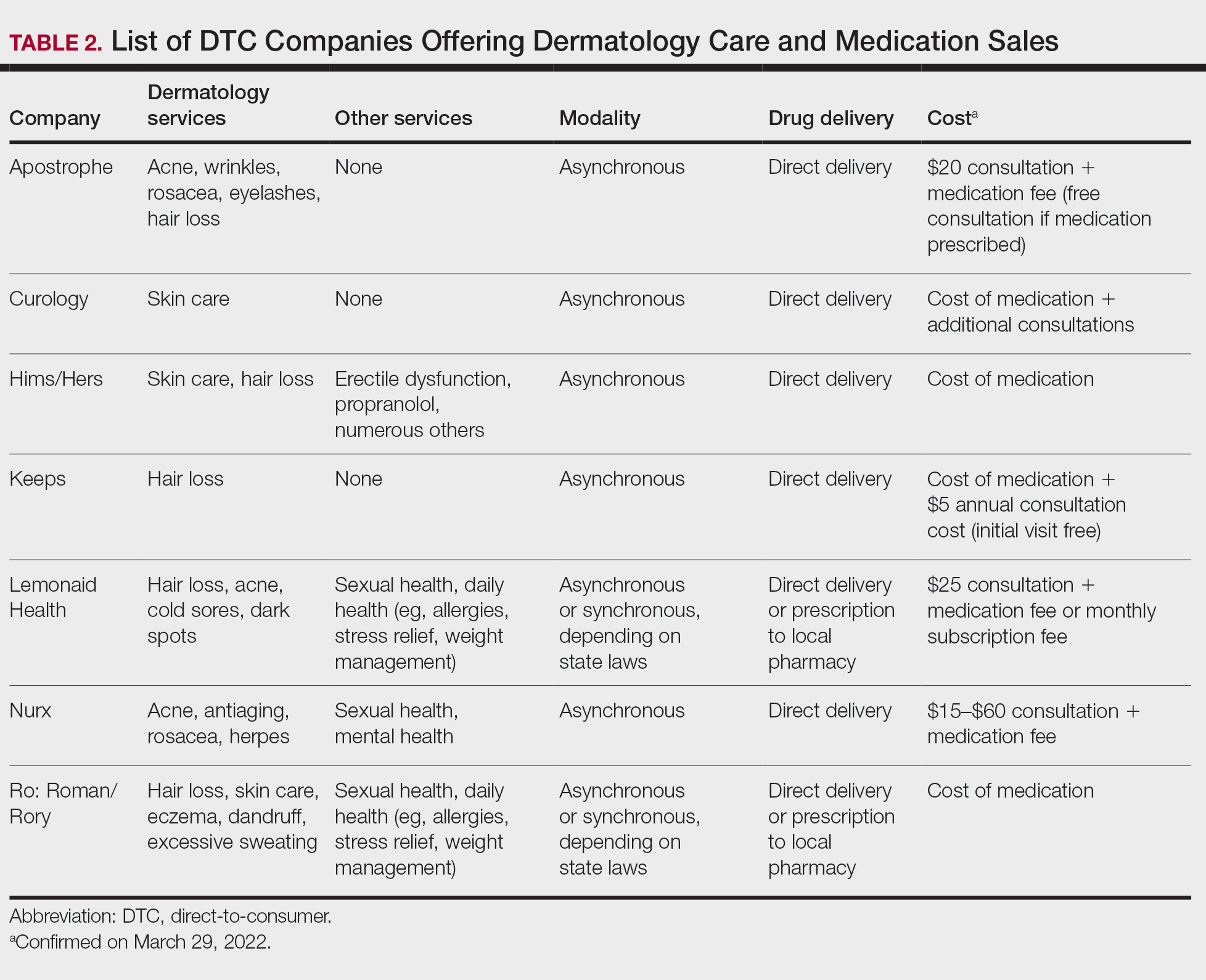
The medicated intrauterine devices (IUDs), including the levonorgestrel-releasing IUD (LNG-IUD) (Mirena, Kyleena, Skyla, and Liletta) and the copper IUD (Cu-IUD; Paragard), are remarkably effective contraceptives. For the 52-mg LNG-IUD (Mirena, Liletta) the pregnancy rate over 6 years of use averaged less than 0.2% per year.1,2 For the Cu-IUD, the pregnancy rate over 10 years of use averaged 0.5% per year for the first 3 years of use and 0.2% per year over the following 7 years of use.3 IUD perforation of the uterus, expulsion, and malposition are recognized complications of IUD use. Our understanding of the prevalence and management of malpositioned IUDs is evolving and the main focus of this editorial.
Complete and partial uterus perforation
A complete uterine perforation occurs when the entire IUD is outside the walls of the uterus. A partial uterine perforation occurs when the IUD is outside the uterine cavity, but a portion of the IUD remains in the myometrium. When uterine perforation is suspected, ultrasound can determine if the IUD is properly sited within the uterus. If ultrasonography does not detect the IUD within the uterus, an x-ray of the pelvis and abdomen should be obtained to determine if the IUD is in the peritoneal cavity. If both an ultrasound and a pelvic-abdominal x-ray do not detect the IUD, the IUD was probably expelled from the patient.
Uterine perforation is uncommon and occurs once in every 500 to 1,000 insertions in non-breastfeeding women.4-8 The most common symptoms reported by patients with a perforated IUD are pain and/or bleeding.8 Investigators in the European Active Surveillance Study on Intrauterine Devices (EURAS) enrolled more than 60,000 patients who had an IUD insertion and followed them for 12 months with more than 39,000 followed for up to 60 months.7,8 The uterine perforation rate per 1,000 IUD insertions in non-breastfeeding women with 60 months of follow-up was 1.6 for the LNG-IUD and 0.8 for the Cu-IUD.8 The rate of uterine perforation was much higher in women who are breastfeeding or recently postpartum. In the EURAS study after 60 months of follow-up, the perforation rate per 1,000 insertions among breastfeeding women was 7.9 for the LNG-IUS and 4.7 for the Cu-IUD.8
Remarkably very few IUD perforations were detected at the time of insertion, including only 2% of the LNG-IUD insertions and 17% of the Cu-IUD insertions.8 Many perforations were not detected until more than 12 months following insertion, including 32% of the LNG-IUD insertions and 22% of the Cu-IUD insertions.8 Obviously, an IUD that has completely perforated the uterus and resides in the peritoneal cavity is not an effective contraceptive. For some patients, the IUD perforation was initially diagnosed after they became pregnant, and imaging studies to locate the IUD and assess the pregnancy were initiated. Complete perforation is usually treated with laparoscopy to remove the IUD and reduce the risk of injury to intra-abdominal organs.
Patients with an IUD partial perforation may present with pelvic pain or abnormal uterine bleeding.9 An ultrasound study to explore the cause of the presenting symptom may detect the partial perforation. It is estimated that approximately 20% of cases of IUD perforation are partial perforation.9 Over time, a partial perforation may progress to a complete perforation. In some cases of partial perforation, the IUD string may still be visible in the cervix, and the IUD may be removed by pulling on the strings.8 Hysteroscopy and/or laparoscopy may be needed to remove a partially perforated IUD. Following a partial or complete IUD perforation, if the patient desires to continue with IUD contraception, it would be wise to insert a new IUD under ultrasound guidance or assess proper placement with a postplacement ultrasound.
Continue to: Expulsion...
Expulsion
IUD expulsion occurs in approximately 3% to 11% of patients.10-13 The age of the patient influences the rate of expulsion. In a study of 2,748 patients with a Cu-IUD, the rate of expulsion by age for patients <20 years, 20–24 years, 25–29 years, 30–34 years, and ≥35 years was 8.2%, 3.2%, 3.0%, 2.3%, and 1.8%, respectively.10 In this study, age did not influence the rate of IUD removal for pelvic pain or abnormal bleeding, which was 4% to 5% across all age groups.10 In a study of 5,403 patients with an IUD, the rate of IUD expulsion by age for patients <20 years, 20–29 years, and 30–45 years was 14.6%, 7.3%, and 7.2%, respectively.12 In this study, the 3-year cumulative rate of expulsion was 10.2%.12 There was no statistically significant difference in the 3-year cumulative rate of expulsion for the 52-mg LNG-IUD (10.1%) and Cu-IUD (10.7%).12
The majority of patients who have an IUD expulsion recognize the event and seek additional contraception care. A few patients first recognize the IUD expulsion when they become pregnant, and imaging studies detect no IUD in the uterus or the peritoneal cavity. In a study of more than 17,000 patients using an LNG-IUD, 108 pregnancies were reported. Seven pregnancies occurred in patients who did not realize their IUD was expelled.14 Patients who have had an IUD expulsion and receive a new IUD are at increased risk for re-expulsion. For these patients, reinsertion of an IUD could be performed under ultrasound guidance to ensure and document optimal initial IUD position within the uterus, or ultrasound can be obtained postinsertion to document appropriate IUD position.
Malposition—prevalence and management
Our understanding of the prevalence and management of a malpositioned IUD is evolving. For the purposes of this discussion a malpositioned IUD is defined as being in the uterus, but not properly positioned within the uterine cavity. Perforation into the peritoneal cavity and complete expulsion of an IUD are considered separate entities. However, a malpositioned IUD within the uterus may eventually perforate the uterus or be expelled from the body. For example, an IUD embedded in the uterine wall may eventually work its way through the wall and become perforated, residing in the peritoneal cavity. An IUD with the stem in the cervix below the internal os may eventually be expelled from the uterus and leave the body through the vagina.
High-quality ultrasonography, including 2-dimensional (2-D) ultrasound with videoclips or 3-dimensional (3-D) ultrasound with coronal views, has greatly advanced our understanding of the prevalence and characteristics of a malpositioned IUD.15-18 Ultrasound features of an IUD correctly placed within the uterus include:
- the IUD is in the uterus
- the shaft is in the midline of the uterine cavity
- the shaft of the IUD is not in the endocervix
- the IUD arms are at a 90-degree angle from the shaft
- the top of the IUD is within 2 cm of the fundus
- the IUD is not rotated outside of the cornual plane, inverted or transverse.
Ultrasound imaging has identified multiple types of malpositioned IUDs, including:
- IUD embedded in the myometrium—a portion of the IUD is embedded in the uterine wall
- low-lying IUD—the IUD is low in the uterine cavity but not in the endocervix
- IUD in the endocervix—the stem is in the endocervical canal
- rotated—the IUD is rotated outside the cornual plane
- malpositioned arms—the arms are not at a 90-degree angle to the stem
- the IUD is inverted, transverse, or laterally displaced.
IUD malposition is highly prevalent and has been identified in 10% to 20% of convenience cohorts in which an ultrasound study was performed.15-18
Benacerraf, Shipp, and Bromley were among the first experts to use ultrasound to detect the high prevalence of malpositioned IUDs among a convenience sample of 167 patients with an IUD undergoing ultrasound for a variety of indications. Using 3-D ultrasound, including reconstructed coronal views, they identified 28 patients (17%) with a malpositioned IUD based on the detection of the IUD “poking into the substance of the uterus or cervix.” Among the patients with a malpositioned IUD, the principal indication for the ultrasound study was pelvic pain (39%) or abnormal uterine bleeding (36%). Among women with a normally sited IUD, pelvic pain (19%) or abnormal uterine bleeding (15%) were less often the principal indication for the ultrasound.15 The malpositioned IUD was removed in 21 of the 28 cases and the symptoms of pelvic pain or abnormal bleeding resolved in 20 of the 21 patients.15
Other investigators have confirmed the observation that IUD malposition is common.16-18 In a retrospective study of 1,748 pelvic ultrasounds performed for any indication where an IUD was present, after excluding 13 patients who were determined to have expelled their IUD (13) and 13 patients with a perforated IUD, 156 patients (8.9%) were diagnosed as having a malpositioned IUD.16 IUD malposition was diagnosed when the IUD was in the uterus but positioned in the lower uterine segment, cervix, rotated or embedded in the uterus. An IUD in the lower uterine segment or cervix was detected in 133 patients, representing 85% of cases. Among these cases, 29 IUDs were also embedded and/or rotated, indicating that some IUDs have multiple causes of the malposition. Twenty-one IUDs were near the fundus but embedded and/or rotated. Controls with a normally-sited IUD were selected for comparison to the case group. Among IUD users, the identification of suspected adenomyosis on the ultrasound was associated with an increased risk of IUD malposition (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08-8.52).16 In this study, removal of a malpositioned LNG-IUD, without initiating a highly reliable contraceptive was associated with an increased risk of pregnancy. It is important to initiate a highly reliable form of contraception if the plan is to remove a malpositioned IUD.16,19
In a study of 1,253 pelvic ultrasounds performed for any indication where an IUD was identified in the uterus, 263 IUDs (19%) were determined to be malpositioned.17 In this study the location of the malpositioned IUDs included17:
- the lower uterine segment not extending into the cervix (38%)
- in the lower uterine segment extending into the cervix (22%)
- in the cervix (26%)
- rotated axis of the IUD (12%)
- other (2%).
Among the 236 malpositioned IUDs, 24% appeared to be embedded in the uterine wall.17 Compared with patients with a normally-sited IUD on ultrasound, patients with a malpositioned IUD more frequently reported vaginal bleeding (30% vs 19%; P<.005) and pelvic pain (43% vs 30%; P<.002), similar to the findings in the Benacerraf et al. study.14
Connolly and Fox18 designed an innovative study to determine the rate of malpositioned IUDs using 2-D ultrasound to ensure proper IUD placement at the time of insertion with a follow-up 3-D ultrasound 8 weeks after insertion to assess IUD position within the uterus. At the 8-week 3-D ultrasound, among 763 women, 16.6% of the IUDs were malpositioned.18 In this study, IUD position was determined to be correct if all the following features were identified:
- the IUD shaft was in the midline of the uterine cavity
- the IUD arms were at 90 degrees from the stem
- the top of the IUD was within 3 to 4 mm of the fundus
- the IUD was not rotated, inverted or transverse.
IUD malpositions were categorized as:
- embedded in the uterine wall
- low in the uterine cavity
- in the endocervical canal
- misaligned
- perforated
- expulsed.
At the 8-week follow-up, 636 patients (83.4%) had an IUD that was correctly positioned.18 In 127 patients (16.6%) IUD malposition was identified, with some patients having more than one type of malposition. The types of malposition identified were:
- embedded in the myometrium (54%)
- misaligned, including rotated, laterally displaced, inverted, transverse or arms not deployed (47%)
- low in the uterine cavity (39%)
- in the endocervical canal (14%)
- perforated (3%)
- expulsion (0%).
Recall that all of these patients had a 2-D ultrasound at the time of insertion that identified the IUD as correctly placed. This suggests that during the 8 weeks following IUD placement there were changes in the location of the IUD or that 2-D ultrasound has lower sensitivity than 3-D ultrasound to detect malposition. Of note, at the 8-week follow-up, bleeding or pain was reported by 36% of the patients with a malpositioned IUD and 20% of patients with a correctly positioned IUD.17 Sixty-seven of the 127 malpositioned IUDs “required” removal, but the precise reasons for the removals were not delineated. The investigators concluded that 3-D ultrasonography is useful for the detection of IUD malposition and could be considered as part of ongoing IUD care, if symptoms of pain or bleeding occur.18
Continue to: IUD malposition following postplacental insertion...
IUD malposition following postplacental insertion
IUD malposition is common in patients who have had a postplacental insertion. Ultrasound imaging plays an important role in detecting IUD expulsion and malposition in these cases. Postplacental IUD insertion is defined as the placement of an IUD within 10 minutes following delivery of the placenta. Postplacental IUD insertion can be performed following a vaginal or cesarean birth and with a Cu-IUD or LNG-IUD. The good news is that postplacental IUD insertion reduces the risk of unplanned pregnancy in the years following birth. However, postplacental IUD insertion is associated with a high rate of IUD malposition.
In a study of 162 patients who had postplacental insertion of a Cu-IUD following a vaginal birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 8%, partial expulsion in 16%, and malposition in 15%.20 The IUD was correctly sited in 56% of patients. Seven patients (4%) had the IUD removed, and 1 patient had a perforated IUD. Among the 25 malpositioned IUDs, 14 were not within 1 cm of the fundus, and 11 were rotated outside of the axis of the cornuas. In this study partial expulsion was defined as an IUD protruding from the external cervical os on physical exam or demonstration of the distal tip of the IUD below the internal os of the cervix on ultrasound. Malposition was defined as an IUD that was >1 cm from the fundus or in an abnormal location or axis, but not partially expelled.
In a study of 69 patients who had postplacental insertion of a Cu-IUD following a cesarean birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 3%, partial expulsion (stem in the cervix below the internal os) in 4% and malposition in 30%.20 The IUD was correctly positioned in 59% of the patients.21 The IUD had been electively removed in 3%. Among the 21 patients with a malpositioned IUD, 10 were rotated within the uterine cavity, 6 were inverted (upside down), 3 were low-lying, and 2 were transverse.21 Given the relatively high rate of IUD malposition following postplacental insertion, it may be useful to perform a pelvic ultrasound at a postpartum visit to assess the location of the IUD, if ultrasonography is available.
Management of the malpositioned IUD
There are no consensus guidelines on how to care for a patient with a malpositioned IUD. Clinicians need to use their best judgment and engage the patient in joint decision making when managing a malpositioned IUD. When an IUD is malpositioned and the patient has bothersome symptoms of pelvic pain or abnormal bleeding that have not responded to standard interventions, consideration may be given to a remove and replace strategy. When the stem of the IUD is below the level of the internal os on ultrasound or visible at the external os on physical examination, consideration should be given to removing and replacing the IUD. However, if the IUD is removed without replacement or the initiation of a highly reliable contraceptive, the risk of unplanned pregnancy is considerable.16,19
IUD totally or partially within the cervix or low-lying. When an IUD is in the cervix, the contraceptive efficacy of the IUD may be diminished, especially with a Cu-IUD.22 In these cases, removing and replacing the IUD is an option. In a survey of 20 expert clinicians, >80% recommended replacing an IUD that was totally or partially in the cervical canal.23 But most of the experts would not replace an IUD that was incidentally noted on ultrasound to be low-lying, being positioned more than 2 cm below the fundus, with no portion of the IUD in the cervical canal. In the same survey, for patients with a low-lying IUD and pelvic pain or bleeding, the majority of experts reported that they would explore other causes of bleeding and pelvic pain not related to the IUD itself and not replace the IUD, but 30% of the experts reported that they would remove and replace the device.23
IUD embedded in the myometrium with pelvic pain. Based on my clinical experience, when a patient has persistent pelvic pain following the insertion of an IUD and the pain does not resolve with standard measures including medication, an ultrasound study is warranted to assess the position of the IUD. If the ultrasound demonstrates that an arm of the IUD is embedded in the myometrium, removal of the IUD may be associated with resolution of the pain. Reinsertion of an IUD under ultrasound guidance may result in a correctly-sited IUD with no recurrence of pelvic pain.
IUD rotated within the uterus with no pain or abnormal bleeding. For an IUD that is near the fundus and rotated on its axis within the uterus, if the patient has no symptoms of pain or abnormal bleeding, my recommendation to the patient would be to leave the device in situ.
Without available guidelines, engage in clinician-patient discussion
It is clear that IUD malposition is common, occurring in 10% to 20% of patients with an IUD. High-quality ultrasound imaging is helpful in detecting IUD malposition, including 2-D ultrasound with videoclips and/or 3-D ultrasound with coronal reconstruction. More data are needed to identify the best options for managing various types of malpositioned IUDs in patients with and without bothersome symptoms such as pain and bleeding. Until consensus guidelines are developed, clinicians need to engage the patient in a discussion of how to best manage the malpositioned IUD. Medicated IUDs and progestin subdermal implants are our two most effective reversible contraceptives. They are among the most important advances in health care over the past half-century. ●
- Mirena FDA approval. , 2022.
- Liletta [package insert]. Allergan USA: Irvine, California; 2019. .
- Paragard [package insert]. CooperSurgical Inc: Trumbull, Connecticut; 2019. .
- Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67:53-56.
- Van Houdenhoven K, van Kaam KJAF, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
- van Grootheest K, Sachs B, Harrison-Woolrych M, et al. Uterine perforation with the levonorgestrel-releasing intrauterine device. Analysis of reports from four national pharmacovigilance centres. Drug Saf. 2011;34:83-88.
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
- Barnett C, Moehner S, Do Minh T, et al. Perforation risk and intra-uterine devices: results of the EURAS-IUD 5-year extension study. Eur J Contracept Reprod Health Care. 2017;22:424-428.
- Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. I. Classification, complications, mechanism, incidence and missing string. Obstet Gynecol Surv. 1981;36:335-353.
- Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Madden T, McNichols, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.
- Keenahan L, Bercaw-Pratt JL, Adeyemi O, et al. Rates of intrauterine device expulsion among adolescents and young women. J Pediatr Adolesc Gynecol. 2021;34:362-365.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
- Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
- Gerkowicz SA, Fiorentino DG, Kovacs AP, et al. Uterine structural abnormality and intrauterine device malposition: analysis of ultrasonographic and demographic variables of 517 patients. Am J Obstet Gynecol. 2019;220:183.e1-e8.
- Connolly CT, Fox NS. Incidence and risk factors for a malpositioned intrauterine device detected on three-dimensional ultrasound within eight weeks of placement. J Ultrasound Med. 2021 ePub Sept 27 2021.
- Golightly E, Gebbie AE. Low-lying or malpositioned intrauterine devices and systems. J Fam Plann Reprod health Care. 2014;40:108-112.
- Gurney EP, Sonalkar S, McAllister A, et al. Six-month expulsion of postplacental copper intrauterine devices placed after vaginal delivery. Am J Obstet Gynecol. 2018;219:183.e1-e9.
- Gurney EP, McAllister A, Lang B, et al. Ultrasound assessment of postplacental copper intrauterine device position 6 months after placement during cesarean delivery. Contraception. 2020;2:100040.
- Anteby E, Revel A, Ben-Chetrit A, et al. Intrauterine device failure: relation to its location with the uterine cavity. Obstet Gynecol. 1993;81:112-114.
- Golightly E, Gebbie AE. Clinicians’ views on low-lying intrauterine devices or systems. J Fam Plann Reprod Health Care. 2014;40:113-116.
The medicated intrauterine devices (IUDs), including the levonorgestrel-releasing IUD (LNG-IUD) (Mirena, Kyleena, Skyla, and Liletta) and the copper IUD (Cu-IUD; Paragard), are remarkably effective contraceptives. For the 52-mg LNG-IUD (Mirena, Liletta) the pregnancy rate over 6 years of use averaged less than 0.2% per year.1,2 For the Cu-IUD, the pregnancy rate over 10 years of use averaged 0.5% per year for the first 3 years of use and 0.2% per year over the following 7 years of use.3 IUD perforation of the uterus, expulsion, and malposition are recognized complications of IUD use. Our understanding of the prevalence and management of malpositioned IUDs is evolving and the main focus of this editorial.
Complete and partial uterus perforation
A complete uterine perforation occurs when the entire IUD is outside the walls of the uterus. A partial uterine perforation occurs when the IUD is outside the uterine cavity, but a portion of the IUD remains in the myometrium. When uterine perforation is suspected, ultrasound can determine if the IUD is properly sited within the uterus. If ultrasonography does not detect the IUD within the uterus, an x-ray of the pelvis and abdomen should be obtained to determine if the IUD is in the peritoneal cavity. If both an ultrasound and a pelvic-abdominal x-ray do not detect the IUD, the IUD was probably expelled from the patient.
Uterine perforation is uncommon and occurs once in every 500 to 1,000 insertions in non-breastfeeding women.4-8 The most common symptoms reported by patients with a perforated IUD are pain and/or bleeding.8 Investigators in the European Active Surveillance Study on Intrauterine Devices (EURAS) enrolled more than 60,000 patients who had an IUD insertion and followed them for 12 months with more than 39,000 followed for up to 60 months.7,8 The uterine perforation rate per 1,000 IUD insertions in non-breastfeeding women with 60 months of follow-up was 1.6 for the LNG-IUD and 0.8 for the Cu-IUD.8 The rate of uterine perforation was much higher in women who are breastfeeding or recently postpartum. In the EURAS study after 60 months of follow-up, the perforation rate per 1,000 insertions among breastfeeding women was 7.9 for the LNG-IUS and 4.7 for the Cu-IUD.8
Remarkably very few IUD perforations were detected at the time of insertion, including only 2% of the LNG-IUD insertions and 17% of the Cu-IUD insertions.8 Many perforations were not detected until more than 12 months following insertion, including 32% of the LNG-IUD insertions and 22% of the Cu-IUD insertions.8 Obviously, an IUD that has completely perforated the uterus and resides in the peritoneal cavity is not an effective contraceptive. For some patients, the IUD perforation was initially diagnosed after they became pregnant, and imaging studies to locate the IUD and assess the pregnancy were initiated. Complete perforation is usually treated with laparoscopy to remove the IUD and reduce the risk of injury to intra-abdominal organs.
Patients with an IUD partial perforation may present with pelvic pain or abnormal uterine bleeding.9 An ultrasound study to explore the cause of the presenting symptom may detect the partial perforation. It is estimated that approximately 20% of cases of IUD perforation are partial perforation.9 Over time, a partial perforation may progress to a complete perforation. In some cases of partial perforation, the IUD string may still be visible in the cervix, and the IUD may be removed by pulling on the strings.8 Hysteroscopy and/or laparoscopy may be needed to remove a partially perforated IUD. Following a partial or complete IUD perforation, if the patient desires to continue with IUD contraception, it would be wise to insert a new IUD under ultrasound guidance or assess proper placement with a postplacement ultrasound.
Continue to: Expulsion...
Expulsion
IUD expulsion occurs in approximately 3% to 11% of patients.10-13 The age of the patient influences the rate of expulsion. In a study of 2,748 patients with a Cu-IUD, the rate of expulsion by age for patients <20 years, 20–24 years, 25–29 years, 30–34 years, and ≥35 years was 8.2%, 3.2%, 3.0%, 2.3%, and 1.8%, respectively.10 In this study, age did not influence the rate of IUD removal for pelvic pain or abnormal bleeding, which was 4% to 5% across all age groups.10 In a study of 5,403 patients with an IUD, the rate of IUD expulsion by age for patients <20 years, 20–29 years, and 30–45 years was 14.6%, 7.3%, and 7.2%, respectively.12 In this study, the 3-year cumulative rate of expulsion was 10.2%.12 There was no statistically significant difference in the 3-year cumulative rate of expulsion for the 52-mg LNG-IUD (10.1%) and Cu-IUD (10.7%).12
The majority of patients who have an IUD expulsion recognize the event and seek additional contraception care. A few patients first recognize the IUD expulsion when they become pregnant, and imaging studies detect no IUD in the uterus or the peritoneal cavity. In a study of more than 17,000 patients using an LNG-IUD, 108 pregnancies were reported. Seven pregnancies occurred in patients who did not realize their IUD was expelled.14 Patients who have had an IUD expulsion and receive a new IUD are at increased risk for re-expulsion. For these patients, reinsertion of an IUD could be performed under ultrasound guidance to ensure and document optimal initial IUD position within the uterus, or ultrasound can be obtained postinsertion to document appropriate IUD position.
Malposition—prevalence and management
Our understanding of the prevalence and management of a malpositioned IUD is evolving. For the purposes of this discussion a malpositioned IUD is defined as being in the uterus, but not properly positioned within the uterine cavity. Perforation into the peritoneal cavity and complete expulsion of an IUD are considered separate entities. However, a malpositioned IUD within the uterus may eventually perforate the uterus or be expelled from the body. For example, an IUD embedded in the uterine wall may eventually work its way through the wall and become perforated, residing in the peritoneal cavity. An IUD with the stem in the cervix below the internal os may eventually be expelled from the uterus and leave the body through the vagina.
High-quality ultrasonography, including 2-dimensional (2-D) ultrasound with videoclips or 3-dimensional (3-D) ultrasound with coronal views, has greatly advanced our understanding of the prevalence and characteristics of a malpositioned IUD.15-18 Ultrasound features of an IUD correctly placed within the uterus include:
- the IUD is in the uterus
- the shaft is in the midline of the uterine cavity
- the shaft of the IUD is not in the endocervix
- the IUD arms are at a 90-degree angle from the shaft
- the top of the IUD is within 2 cm of the fundus
- the IUD is not rotated outside of the cornual plane, inverted or transverse.
Ultrasound imaging has identified multiple types of malpositioned IUDs, including:
- IUD embedded in the myometrium—a portion of the IUD is embedded in the uterine wall
- low-lying IUD—the IUD is low in the uterine cavity but not in the endocervix
- IUD in the endocervix—the stem is in the endocervical canal
- rotated—the IUD is rotated outside the cornual plane
- malpositioned arms—the arms are not at a 90-degree angle to the stem
- the IUD is inverted, transverse, or laterally displaced.
IUD malposition is highly prevalent and has been identified in 10% to 20% of convenience cohorts in which an ultrasound study was performed.15-18
Benacerraf, Shipp, and Bromley were among the first experts to use ultrasound to detect the high prevalence of malpositioned IUDs among a convenience sample of 167 patients with an IUD undergoing ultrasound for a variety of indications. Using 3-D ultrasound, including reconstructed coronal views, they identified 28 patients (17%) with a malpositioned IUD based on the detection of the IUD “poking into the substance of the uterus or cervix.” Among the patients with a malpositioned IUD, the principal indication for the ultrasound study was pelvic pain (39%) or abnormal uterine bleeding (36%). Among women with a normally sited IUD, pelvic pain (19%) or abnormal uterine bleeding (15%) were less often the principal indication for the ultrasound.15 The malpositioned IUD was removed in 21 of the 28 cases and the symptoms of pelvic pain or abnormal bleeding resolved in 20 of the 21 patients.15
Other investigators have confirmed the observation that IUD malposition is common.16-18 In a retrospective study of 1,748 pelvic ultrasounds performed for any indication where an IUD was present, after excluding 13 patients who were determined to have expelled their IUD (13) and 13 patients with a perforated IUD, 156 patients (8.9%) were diagnosed as having a malpositioned IUD.16 IUD malposition was diagnosed when the IUD was in the uterus but positioned in the lower uterine segment, cervix, rotated or embedded in the uterus. An IUD in the lower uterine segment or cervix was detected in 133 patients, representing 85% of cases. Among these cases, 29 IUDs were also embedded and/or rotated, indicating that some IUDs have multiple causes of the malposition. Twenty-one IUDs were near the fundus but embedded and/or rotated. Controls with a normally-sited IUD were selected for comparison to the case group. Among IUD users, the identification of suspected adenomyosis on the ultrasound was associated with an increased risk of IUD malposition (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08-8.52).16 In this study, removal of a malpositioned LNG-IUD, without initiating a highly reliable contraceptive was associated with an increased risk of pregnancy. It is important to initiate a highly reliable form of contraception if the plan is to remove a malpositioned IUD.16,19
In a study of 1,253 pelvic ultrasounds performed for any indication where an IUD was identified in the uterus, 263 IUDs (19%) were determined to be malpositioned.17 In this study the location of the malpositioned IUDs included17:
- the lower uterine segment not extending into the cervix (38%)
- in the lower uterine segment extending into the cervix (22%)
- in the cervix (26%)
- rotated axis of the IUD (12%)
- other (2%).
Among the 236 malpositioned IUDs, 24% appeared to be embedded in the uterine wall.17 Compared with patients with a normally-sited IUD on ultrasound, patients with a malpositioned IUD more frequently reported vaginal bleeding (30% vs 19%; P<.005) and pelvic pain (43% vs 30%; P<.002), similar to the findings in the Benacerraf et al. study.14
Connolly and Fox18 designed an innovative study to determine the rate of malpositioned IUDs using 2-D ultrasound to ensure proper IUD placement at the time of insertion with a follow-up 3-D ultrasound 8 weeks after insertion to assess IUD position within the uterus. At the 8-week 3-D ultrasound, among 763 women, 16.6% of the IUDs were malpositioned.18 In this study, IUD position was determined to be correct if all the following features were identified:
- the IUD shaft was in the midline of the uterine cavity
- the IUD arms were at 90 degrees from the stem
- the top of the IUD was within 3 to 4 mm of the fundus
- the IUD was not rotated, inverted or transverse.
IUD malpositions were categorized as:
- embedded in the uterine wall
- low in the uterine cavity
- in the endocervical canal
- misaligned
- perforated
- expulsed.
At the 8-week follow-up, 636 patients (83.4%) had an IUD that was correctly positioned.18 In 127 patients (16.6%) IUD malposition was identified, with some patients having more than one type of malposition. The types of malposition identified were:
- embedded in the myometrium (54%)
- misaligned, including rotated, laterally displaced, inverted, transverse or arms not deployed (47%)
- low in the uterine cavity (39%)
- in the endocervical canal (14%)
- perforated (3%)
- expulsion (0%).
Recall that all of these patients had a 2-D ultrasound at the time of insertion that identified the IUD as correctly placed. This suggests that during the 8 weeks following IUD placement there were changes in the location of the IUD or that 2-D ultrasound has lower sensitivity than 3-D ultrasound to detect malposition. Of note, at the 8-week follow-up, bleeding or pain was reported by 36% of the patients with a malpositioned IUD and 20% of patients with a correctly positioned IUD.17 Sixty-seven of the 127 malpositioned IUDs “required” removal, but the precise reasons for the removals were not delineated. The investigators concluded that 3-D ultrasonography is useful for the detection of IUD malposition and could be considered as part of ongoing IUD care, if symptoms of pain or bleeding occur.18
Continue to: IUD malposition following postplacental insertion...
IUD malposition following postplacental insertion
IUD malposition is common in patients who have had a postplacental insertion. Ultrasound imaging plays an important role in detecting IUD expulsion and malposition in these cases. Postplacental IUD insertion is defined as the placement of an IUD within 10 minutes following delivery of the placenta. Postplacental IUD insertion can be performed following a vaginal or cesarean birth and with a Cu-IUD or LNG-IUD. The good news is that postplacental IUD insertion reduces the risk of unplanned pregnancy in the years following birth. However, postplacental IUD insertion is associated with a high rate of IUD malposition.
In a study of 162 patients who had postplacental insertion of a Cu-IUD following a vaginal birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 8%, partial expulsion in 16%, and malposition in 15%.20 The IUD was correctly sited in 56% of patients. Seven patients (4%) had the IUD removed, and 1 patient had a perforated IUD. Among the 25 malpositioned IUDs, 14 were not within 1 cm of the fundus, and 11 were rotated outside of the axis of the cornuas. In this study partial expulsion was defined as an IUD protruding from the external cervical os on physical exam or demonstration of the distal tip of the IUD below the internal os of the cervix on ultrasound. Malposition was defined as an IUD that was >1 cm from the fundus or in an abnormal location or axis, but not partially expelled.
In a study of 69 patients who had postplacental insertion of a Cu-IUD following a cesarean birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 3%, partial expulsion (stem in the cervix below the internal os) in 4% and malposition in 30%.20 The IUD was correctly positioned in 59% of the patients.21 The IUD had been electively removed in 3%. Among the 21 patients with a malpositioned IUD, 10 were rotated within the uterine cavity, 6 were inverted (upside down), 3 were low-lying, and 2 were transverse.21 Given the relatively high rate of IUD malposition following postplacental insertion, it may be useful to perform a pelvic ultrasound at a postpartum visit to assess the location of the IUD, if ultrasonography is available.
Management of the malpositioned IUD
There are no consensus guidelines on how to care for a patient with a malpositioned IUD. Clinicians need to use their best judgment and engage the patient in joint decision making when managing a malpositioned IUD. When an IUD is malpositioned and the patient has bothersome symptoms of pelvic pain or abnormal bleeding that have not responded to standard interventions, consideration may be given to a remove and replace strategy. When the stem of the IUD is below the level of the internal os on ultrasound or visible at the external os on physical examination, consideration should be given to removing and replacing the IUD. However, if the IUD is removed without replacement or the initiation of a highly reliable contraceptive, the risk of unplanned pregnancy is considerable.16,19
IUD totally or partially within the cervix or low-lying. When an IUD is in the cervix, the contraceptive efficacy of the IUD may be diminished, especially with a Cu-IUD.22 In these cases, removing and replacing the IUD is an option. In a survey of 20 expert clinicians, >80% recommended replacing an IUD that was totally or partially in the cervical canal.23 But most of the experts would not replace an IUD that was incidentally noted on ultrasound to be low-lying, being positioned more than 2 cm below the fundus, with no portion of the IUD in the cervical canal. In the same survey, for patients with a low-lying IUD and pelvic pain or bleeding, the majority of experts reported that they would explore other causes of bleeding and pelvic pain not related to the IUD itself and not replace the IUD, but 30% of the experts reported that they would remove and replace the device.23
IUD embedded in the myometrium with pelvic pain. Based on my clinical experience, when a patient has persistent pelvic pain following the insertion of an IUD and the pain does not resolve with standard measures including medication, an ultrasound study is warranted to assess the position of the IUD. If the ultrasound demonstrates that an arm of the IUD is embedded in the myometrium, removal of the IUD may be associated with resolution of the pain. Reinsertion of an IUD under ultrasound guidance may result in a correctly-sited IUD with no recurrence of pelvic pain.
IUD rotated within the uterus with no pain or abnormal bleeding. For an IUD that is near the fundus and rotated on its axis within the uterus, if the patient has no symptoms of pain or abnormal bleeding, my recommendation to the patient would be to leave the device in situ.
Without available guidelines, engage in clinician-patient discussion
It is clear that IUD malposition is common, occurring in 10% to 20% of patients with an IUD. High-quality ultrasound imaging is helpful in detecting IUD malposition, including 2-D ultrasound with videoclips and/or 3-D ultrasound with coronal reconstruction. More data are needed to identify the best options for managing various types of malpositioned IUDs in patients with and without bothersome symptoms such as pain and bleeding. Until consensus guidelines are developed, clinicians need to engage the patient in a discussion of how to best manage the malpositioned IUD. Medicated IUDs and progestin subdermal implants are our two most effective reversible contraceptives. They are among the most important advances in health care over the past half-century. ●
The medicated intrauterine devices (IUDs), including the levonorgestrel-releasing IUD (LNG-IUD) (Mirena, Kyleena, Skyla, and Liletta) and the copper IUD (Cu-IUD; Paragard), are remarkably effective contraceptives. For the 52-mg LNG-IUD (Mirena, Liletta) the pregnancy rate over 6 years of use averaged less than 0.2% per year.1,2 For the Cu-IUD, the pregnancy rate over 10 years of use averaged 0.5% per year for the first 3 years of use and 0.2% per year over the following 7 years of use.3 IUD perforation of the uterus, expulsion, and malposition are recognized complications of IUD use. Our understanding of the prevalence and management of malpositioned IUDs is evolving and the main focus of this editorial.
Complete and partial uterus perforation
A complete uterine perforation occurs when the entire IUD is outside the walls of the uterus. A partial uterine perforation occurs when the IUD is outside the uterine cavity, but a portion of the IUD remains in the myometrium. When uterine perforation is suspected, ultrasound can determine if the IUD is properly sited within the uterus. If ultrasonography does not detect the IUD within the uterus, an x-ray of the pelvis and abdomen should be obtained to determine if the IUD is in the peritoneal cavity. If both an ultrasound and a pelvic-abdominal x-ray do not detect the IUD, the IUD was probably expelled from the patient.
Uterine perforation is uncommon and occurs once in every 500 to 1,000 insertions in non-breastfeeding women.4-8 The most common symptoms reported by patients with a perforated IUD are pain and/or bleeding.8 Investigators in the European Active Surveillance Study on Intrauterine Devices (EURAS) enrolled more than 60,000 patients who had an IUD insertion and followed them for 12 months with more than 39,000 followed for up to 60 months.7,8 The uterine perforation rate per 1,000 IUD insertions in non-breastfeeding women with 60 months of follow-up was 1.6 for the LNG-IUD and 0.8 for the Cu-IUD.8 The rate of uterine perforation was much higher in women who are breastfeeding or recently postpartum. In the EURAS study after 60 months of follow-up, the perforation rate per 1,000 insertions among breastfeeding women was 7.9 for the LNG-IUS and 4.7 for the Cu-IUD.8
Remarkably very few IUD perforations were detected at the time of insertion, including only 2% of the LNG-IUD insertions and 17% of the Cu-IUD insertions.8 Many perforations were not detected until more than 12 months following insertion, including 32% of the LNG-IUD insertions and 22% of the Cu-IUD insertions.8 Obviously, an IUD that has completely perforated the uterus and resides in the peritoneal cavity is not an effective contraceptive. For some patients, the IUD perforation was initially diagnosed after they became pregnant, and imaging studies to locate the IUD and assess the pregnancy were initiated. Complete perforation is usually treated with laparoscopy to remove the IUD and reduce the risk of injury to intra-abdominal organs.
Patients with an IUD partial perforation may present with pelvic pain or abnormal uterine bleeding.9 An ultrasound study to explore the cause of the presenting symptom may detect the partial perforation. It is estimated that approximately 20% of cases of IUD perforation are partial perforation.9 Over time, a partial perforation may progress to a complete perforation. In some cases of partial perforation, the IUD string may still be visible in the cervix, and the IUD may be removed by pulling on the strings.8 Hysteroscopy and/or laparoscopy may be needed to remove a partially perforated IUD. Following a partial or complete IUD perforation, if the patient desires to continue with IUD contraception, it would be wise to insert a new IUD under ultrasound guidance or assess proper placement with a postplacement ultrasound.
Continue to: Expulsion...
Expulsion
IUD expulsion occurs in approximately 3% to 11% of patients.10-13 The age of the patient influences the rate of expulsion. In a study of 2,748 patients with a Cu-IUD, the rate of expulsion by age for patients <20 years, 20–24 years, 25–29 years, 30–34 years, and ≥35 years was 8.2%, 3.2%, 3.0%, 2.3%, and 1.8%, respectively.10 In this study, age did not influence the rate of IUD removal for pelvic pain or abnormal bleeding, which was 4% to 5% across all age groups.10 In a study of 5,403 patients with an IUD, the rate of IUD expulsion by age for patients <20 years, 20–29 years, and 30–45 years was 14.6%, 7.3%, and 7.2%, respectively.12 In this study, the 3-year cumulative rate of expulsion was 10.2%.12 There was no statistically significant difference in the 3-year cumulative rate of expulsion for the 52-mg LNG-IUD (10.1%) and Cu-IUD (10.7%).12
The majority of patients who have an IUD expulsion recognize the event and seek additional contraception care. A few patients first recognize the IUD expulsion when they become pregnant, and imaging studies detect no IUD in the uterus or the peritoneal cavity. In a study of more than 17,000 patients using an LNG-IUD, 108 pregnancies were reported. Seven pregnancies occurred in patients who did not realize their IUD was expelled.14 Patients who have had an IUD expulsion and receive a new IUD are at increased risk for re-expulsion. For these patients, reinsertion of an IUD could be performed under ultrasound guidance to ensure and document optimal initial IUD position within the uterus, or ultrasound can be obtained postinsertion to document appropriate IUD position.
Malposition—prevalence and management
Our understanding of the prevalence and management of a malpositioned IUD is evolving. For the purposes of this discussion a malpositioned IUD is defined as being in the uterus, but not properly positioned within the uterine cavity. Perforation into the peritoneal cavity and complete expulsion of an IUD are considered separate entities. However, a malpositioned IUD within the uterus may eventually perforate the uterus or be expelled from the body. For example, an IUD embedded in the uterine wall may eventually work its way through the wall and become perforated, residing in the peritoneal cavity. An IUD with the stem in the cervix below the internal os may eventually be expelled from the uterus and leave the body through the vagina.
High-quality ultrasonography, including 2-dimensional (2-D) ultrasound with videoclips or 3-dimensional (3-D) ultrasound with coronal views, has greatly advanced our understanding of the prevalence and characteristics of a malpositioned IUD.15-18 Ultrasound features of an IUD correctly placed within the uterus include:
- the IUD is in the uterus
- the shaft is in the midline of the uterine cavity
- the shaft of the IUD is not in the endocervix
- the IUD arms are at a 90-degree angle from the shaft
- the top of the IUD is within 2 cm of the fundus
- the IUD is not rotated outside of the cornual plane, inverted or transverse.
Ultrasound imaging has identified multiple types of malpositioned IUDs, including:
- IUD embedded in the myometrium—a portion of the IUD is embedded in the uterine wall
- low-lying IUD—the IUD is low in the uterine cavity but not in the endocervix
- IUD in the endocervix—the stem is in the endocervical canal
- rotated—the IUD is rotated outside the cornual plane
- malpositioned arms—the arms are not at a 90-degree angle to the stem
- the IUD is inverted, transverse, or laterally displaced.
IUD malposition is highly prevalent and has been identified in 10% to 20% of convenience cohorts in which an ultrasound study was performed.15-18
Benacerraf, Shipp, and Bromley were among the first experts to use ultrasound to detect the high prevalence of malpositioned IUDs among a convenience sample of 167 patients with an IUD undergoing ultrasound for a variety of indications. Using 3-D ultrasound, including reconstructed coronal views, they identified 28 patients (17%) with a malpositioned IUD based on the detection of the IUD “poking into the substance of the uterus or cervix.” Among the patients with a malpositioned IUD, the principal indication for the ultrasound study was pelvic pain (39%) or abnormal uterine bleeding (36%). Among women with a normally sited IUD, pelvic pain (19%) or abnormal uterine bleeding (15%) were less often the principal indication for the ultrasound.15 The malpositioned IUD was removed in 21 of the 28 cases and the symptoms of pelvic pain or abnormal bleeding resolved in 20 of the 21 patients.15
Other investigators have confirmed the observation that IUD malposition is common.16-18 In a retrospective study of 1,748 pelvic ultrasounds performed for any indication where an IUD was present, after excluding 13 patients who were determined to have expelled their IUD (13) and 13 patients with a perforated IUD, 156 patients (8.9%) were diagnosed as having a malpositioned IUD.16 IUD malposition was diagnosed when the IUD was in the uterus but positioned in the lower uterine segment, cervix, rotated or embedded in the uterus. An IUD in the lower uterine segment or cervix was detected in 133 patients, representing 85% of cases. Among these cases, 29 IUDs were also embedded and/or rotated, indicating that some IUDs have multiple causes of the malposition. Twenty-one IUDs were near the fundus but embedded and/or rotated. Controls with a normally-sited IUD were selected for comparison to the case group. Among IUD users, the identification of suspected adenomyosis on the ultrasound was associated with an increased risk of IUD malposition (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08-8.52).16 In this study, removal of a malpositioned LNG-IUD, without initiating a highly reliable contraceptive was associated with an increased risk of pregnancy. It is important to initiate a highly reliable form of contraception if the plan is to remove a malpositioned IUD.16,19
In a study of 1,253 pelvic ultrasounds performed for any indication where an IUD was identified in the uterus, 263 IUDs (19%) were determined to be malpositioned.17 In this study the location of the malpositioned IUDs included17:
- the lower uterine segment not extending into the cervix (38%)
- in the lower uterine segment extending into the cervix (22%)
- in the cervix (26%)
- rotated axis of the IUD (12%)
- other (2%).
Among the 236 malpositioned IUDs, 24% appeared to be embedded in the uterine wall.17 Compared with patients with a normally-sited IUD on ultrasound, patients with a malpositioned IUD more frequently reported vaginal bleeding (30% vs 19%; P<.005) and pelvic pain (43% vs 30%; P<.002), similar to the findings in the Benacerraf et al. study.14
Connolly and Fox18 designed an innovative study to determine the rate of malpositioned IUDs using 2-D ultrasound to ensure proper IUD placement at the time of insertion with a follow-up 3-D ultrasound 8 weeks after insertion to assess IUD position within the uterus. At the 8-week 3-D ultrasound, among 763 women, 16.6% of the IUDs were malpositioned.18 In this study, IUD position was determined to be correct if all the following features were identified:
- the IUD shaft was in the midline of the uterine cavity
- the IUD arms were at 90 degrees from the stem
- the top of the IUD was within 3 to 4 mm of the fundus
- the IUD was not rotated, inverted or transverse.
IUD malpositions were categorized as:
- embedded in the uterine wall
- low in the uterine cavity
- in the endocervical canal
- misaligned
- perforated
- expulsed.
At the 8-week follow-up, 636 patients (83.4%) had an IUD that was correctly positioned.18 In 127 patients (16.6%) IUD malposition was identified, with some patients having more than one type of malposition. The types of malposition identified were:
- embedded in the myometrium (54%)
- misaligned, including rotated, laterally displaced, inverted, transverse or arms not deployed (47%)
- low in the uterine cavity (39%)
- in the endocervical canal (14%)
- perforated (3%)
- expulsion (0%).
Recall that all of these patients had a 2-D ultrasound at the time of insertion that identified the IUD as correctly placed. This suggests that during the 8 weeks following IUD placement there were changes in the location of the IUD or that 2-D ultrasound has lower sensitivity than 3-D ultrasound to detect malposition. Of note, at the 8-week follow-up, bleeding or pain was reported by 36% of the patients with a malpositioned IUD and 20% of patients with a correctly positioned IUD.17 Sixty-seven of the 127 malpositioned IUDs “required” removal, but the precise reasons for the removals were not delineated. The investigators concluded that 3-D ultrasonography is useful for the detection of IUD malposition and could be considered as part of ongoing IUD care, if symptoms of pain or bleeding occur.18
Continue to: IUD malposition following postplacental insertion...
IUD malposition following postplacental insertion
IUD malposition is common in patients who have had a postplacental insertion. Ultrasound imaging plays an important role in detecting IUD expulsion and malposition in these cases. Postplacental IUD insertion is defined as the placement of an IUD within 10 minutes following delivery of the placenta. Postplacental IUD insertion can be performed following a vaginal or cesarean birth and with a Cu-IUD or LNG-IUD. The good news is that postplacental IUD insertion reduces the risk of unplanned pregnancy in the years following birth. However, postplacental IUD insertion is associated with a high rate of IUD malposition.
In a study of 162 patients who had postplacental insertion of a Cu-IUD following a vaginal birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 8%, partial expulsion in 16%, and malposition in 15%.20 The IUD was correctly sited in 56% of patients. Seven patients (4%) had the IUD removed, and 1 patient had a perforated IUD. Among the 25 malpositioned IUDs, 14 were not within 1 cm of the fundus, and 11 were rotated outside of the axis of the cornuas. In this study partial expulsion was defined as an IUD protruding from the external cervical os on physical exam or demonstration of the distal tip of the IUD below the internal os of the cervix on ultrasound. Malposition was defined as an IUD that was >1 cm from the fundus or in an abnormal location or axis, but not partially expelled.
In a study of 69 patients who had postplacental insertion of a Cu-IUD following a cesarean birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 3%, partial expulsion (stem in the cervix below the internal os) in 4% and malposition in 30%.20 The IUD was correctly positioned in 59% of the patients.21 The IUD had been electively removed in 3%. Among the 21 patients with a malpositioned IUD, 10 were rotated within the uterine cavity, 6 were inverted (upside down), 3 were low-lying, and 2 were transverse.21 Given the relatively high rate of IUD malposition following postplacental insertion, it may be useful to perform a pelvic ultrasound at a postpartum visit to assess the location of the IUD, if ultrasonography is available.
Management of the malpositioned IUD
There are no consensus guidelines on how to care for a patient with a malpositioned IUD. Clinicians need to use their best judgment and engage the patient in joint decision making when managing a malpositioned IUD. When an IUD is malpositioned and the patient has bothersome symptoms of pelvic pain or abnormal bleeding that have not responded to standard interventions, consideration may be given to a remove and replace strategy. When the stem of the IUD is below the level of the internal os on ultrasound or visible at the external os on physical examination, consideration should be given to removing and replacing the IUD. However, if the IUD is removed without replacement or the initiation of a highly reliable contraceptive, the risk of unplanned pregnancy is considerable.16,19
IUD totally or partially within the cervix or low-lying. When an IUD is in the cervix, the contraceptive efficacy of the IUD may be diminished, especially with a Cu-IUD.22 In these cases, removing and replacing the IUD is an option. In a survey of 20 expert clinicians, >80% recommended replacing an IUD that was totally or partially in the cervical canal.23 But most of the experts would not replace an IUD that was incidentally noted on ultrasound to be low-lying, being positioned more than 2 cm below the fundus, with no portion of the IUD in the cervical canal. In the same survey, for patients with a low-lying IUD and pelvic pain or bleeding, the majority of experts reported that they would explore other causes of bleeding and pelvic pain not related to the IUD itself and not replace the IUD, but 30% of the experts reported that they would remove and replace the device.23
IUD embedded in the myometrium with pelvic pain. Based on my clinical experience, when a patient has persistent pelvic pain following the insertion of an IUD and the pain does not resolve with standard measures including medication, an ultrasound study is warranted to assess the position of the IUD. If the ultrasound demonstrates that an arm of the IUD is embedded in the myometrium, removal of the IUD may be associated with resolution of the pain. Reinsertion of an IUD under ultrasound guidance may result in a correctly-sited IUD with no recurrence of pelvic pain.
IUD rotated within the uterus with no pain or abnormal bleeding. For an IUD that is near the fundus and rotated on its axis within the uterus, if the patient has no symptoms of pain or abnormal bleeding, my recommendation to the patient would be to leave the device in situ.
Without available guidelines, engage in clinician-patient discussion
It is clear that IUD malposition is common, occurring in 10% to 20% of patients with an IUD. High-quality ultrasound imaging is helpful in detecting IUD malposition, including 2-D ultrasound with videoclips and/or 3-D ultrasound with coronal reconstruction. More data are needed to identify the best options for managing various types of malpositioned IUDs in patients with and without bothersome symptoms such as pain and bleeding. Until consensus guidelines are developed, clinicians need to engage the patient in a discussion of how to best manage the malpositioned IUD. Medicated IUDs and progestin subdermal implants are our two most effective reversible contraceptives. They are among the most important advances in health care over the past half-century. ●
- Mirena FDA approval. , 2022.
- Liletta [package insert]. Allergan USA: Irvine, California; 2019. .
- Paragard [package insert]. CooperSurgical Inc: Trumbull, Connecticut; 2019. .
- Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67:53-56.
- Van Houdenhoven K, van Kaam KJAF, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
- van Grootheest K, Sachs B, Harrison-Woolrych M, et al. Uterine perforation with the levonorgestrel-releasing intrauterine device. Analysis of reports from four national pharmacovigilance centres. Drug Saf. 2011;34:83-88.
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
- Barnett C, Moehner S, Do Minh T, et al. Perforation risk and intra-uterine devices: results of the EURAS-IUD 5-year extension study. Eur J Contracept Reprod Health Care. 2017;22:424-428.
- Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. I. Classification, complications, mechanism, incidence and missing string. Obstet Gynecol Surv. 1981;36:335-353.
- Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Madden T, McNichols, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.
- Keenahan L, Bercaw-Pratt JL, Adeyemi O, et al. Rates of intrauterine device expulsion among adolescents and young women. J Pediatr Adolesc Gynecol. 2021;34:362-365.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
- Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
- Gerkowicz SA, Fiorentino DG, Kovacs AP, et al. Uterine structural abnormality and intrauterine device malposition: analysis of ultrasonographic and demographic variables of 517 patients. Am J Obstet Gynecol. 2019;220:183.e1-e8.
- Connolly CT, Fox NS. Incidence and risk factors for a malpositioned intrauterine device detected on three-dimensional ultrasound within eight weeks of placement. J Ultrasound Med. 2021 ePub Sept 27 2021.
- Golightly E, Gebbie AE. Low-lying or malpositioned intrauterine devices and systems. J Fam Plann Reprod health Care. 2014;40:108-112.
- Gurney EP, Sonalkar S, McAllister A, et al. Six-month expulsion of postplacental copper intrauterine devices placed after vaginal delivery. Am J Obstet Gynecol. 2018;219:183.e1-e9.
- Gurney EP, McAllister A, Lang B, et al. Ultrasound assessment of postplacental copper intrauterine device position 6 months after placement during cesarean delivery. Contraception. 2020;2:100040.
- Anteby E, Revel A, Ben-Chetrit A, et al. Intrauterine device failure: relation to its location with the uterine cavity. Obstet Gynecol. 1993;81:112-114.
- Golightly E, Gebbie AE. Clinicians’ views on low-lying intrauterine devices or systems. J Fam Plann Reprod Health Care. 2014;40:113-116.
- Mirena FDA approval. , 2022.
- Liletta [package insert]. Allergan USA: Irvine, California; 2019. .
- Paragard [package insert]. CooperSurgical Inc: Trumbull, Connecticut; 2019. .
- Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67:53-56.
- Van Houdenhoven K, van Kaam KJAF, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
- van Grootheest K, Sachs B, Harrison-Woolrych M, et al. Uterine perforation with the levonorgestrel-releasing intrauterine device. Analysis of reports from four national pharmacovigilance centres. Drug Saf. 2011;34:83-88.
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
- Barnett C, Moehner S, Do Minh T, et al. Perforation risk and intra-uterine devices: results of the EURAS-IUD 5-year extension study. Eur J Contracept Reprod Health Care. 2017;22:424-428.
- Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. I. Classification, complications, mechanism, incidence and missing string. Obstet Gynecol Surv. 1981;36:335-353.
- Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Madden T, McNichols, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.
- Keenahan L, Bercaw-Pratt JL, Adeyemi O, et al. Rates of intrauterine device expulsion among adolescents and young women. J Pediatr Adolesc Gynecol. 2021;34:362-365.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
- Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
- Gerkowicz SA, Fiorentino DG, Kovacs AP, et al. Uterine structural abnormality and intrauterine device malposition: analysis of ultrasonographic and demographic variables of 517 patients. Am J Obstet Gynecol. 2019;220:183.e1-e8.
- Connolly CT, Fox NS. Incidence and risk factors for a malpositioned intrauterine device detected on three-dimensional ultrasound within eight weeks of placement. J Ultrasound Med. 2021 ePub Sept 27 2021.
- Golightly E, Gebbie AE. Low-lying or malpositioned intrauterine devices and systems. J Fam Plann Reprod health Care. 2014;40:108-112.
- Gurney EP, Sonalkar S, McAllister A, et al. Six-month expulsion of postplacental copper intrauterine devices placed after vaginal delivery. Am J Obstet Gynecol. 2018;219:183.e1-e9.
- Gurney EP, McAllister A, Lang B, et al. Ultrasound assessment of postplacental copper intrauterine device position 6 months after placement during cesarean delivery. Contraception. 2020;2:100040.
- Anteby E, Revel A, Ben-Chetrit A, et al. Intrauterine device failure: relation to its location with the uterine cavity. Obstet Gynecol. 1993;81:112-114.
- Golightly E, Gebbie AE. Clinicians’ views on low-lying intrauterine devices or systems. J Fam Plann Reprod Health Care. 2014;40:113-116.
COMMENT & CONTROVERSY
UTIs IN PREGNANCY: MANAGING URETHRITIS, ASYMPTOMATIC BACTERIURIA, CYSTITIS, AND PYELONEPHRITIS
PATRICK DUFF, MD (JANUARY 2022)
Clarification on UTI issues
Regarding the article on urinary tract infections (UTIs) in pregnancy, I have 3 points of clarification. First, in 27 years of practice in which I universally performed screening urine cultures on prenatal patients plus all of those with symptoms, I have seen a total of 2 cultures with Staphylococcus saprophyticus. I see this organism listed in references as a major UTI causative, but is that the case? Second, the clinical case and symptoms discussed are accurate, but costovertebral angle tenderness or fever of 101 °F or higher indicate pyelonephritis and should be treated aggressively. Many of these patients will have nausea and vomiting and will be dehydrated. This decreases urine flow, allowing progressive bacterial growth in renal parenchyma. An initial bolus of intravenous fluids, at least 2 L wide open through a large-bore catheter, rapidly decreases fever, flushes the urinary tract, and improves nausea, headaches, and malaise. Finally, nitrofurantoin is excreted in the urine so rapidly that it does not achieve adequate tissue levels, and it should never be used to treat pyelonephritis or, for that matter, any infection other than uncomplicated cystitis/urethritis.
David Janowitz, MD
Houston, Texas
Dr. Duff responds
I appreciate Dr. Janowitz’s interest and thoughtful comments. The patient presented in the case study has acute cystitis, characterized by a low-grade fever, suprapubic pain, dysuria, frequency, and hesitancy. Patients with pyelonephritis typically have a higher fever and significant costovertebral angle pain and tenderness. I agree completely with Dr. Janowitz’s observations about the seriousness of pyelonephritis in pregnancy. Pyelonephritis is an important cause of preterm labor, bacteremia, and even septic shock. As I point out in the article, women with moderate to severe kidney infections should be hospitalized and treated with intravenous fluids, antipyretics, antiemetics, and intravenous antibiotics. My usual recommendation is ceftriaxone. Intravenous antibiotics should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. Once patients improve, they can be transitioned to oral antibiotics to complete a 10-day course of therapy. Again, I agree with Dr. Janowitz’s statement that nitrofurantoin is not an appropriate drug for treatment of pyelonephritis because it does not reach acceptable concentrations in either the blood or the renal parenchyma. Rather, amoxicillin-clavulanate and trimethoprim-sulfamethoxazole are much better choices for oral therapy. However, once the infection is cleared, nitrofurantoin is an excellent agent for suppression of recurrent infection.
Finally, there is no doubt that the principal pathogens that cause UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, and Proteus species. However, 3 aerobic Gram-positive cocci do, in fact, cause a small percentage of infections: group B streptococci, enterococci, and Staphylococcus saprophyticus. When the latter bacterium is identified as a single organism in high colony count, particularly in a catheterized urine specimen, it should be considered a true pathogen and not simply a contaminant.
CAN WE RETURN TO THE ABCs OF CRAFTING A MEDICAL RECORD NOTE?
ROBERT L. BARBIERI, MD (OCTOBER 2021)
Another suggestion for reducing note bloat in the EMR
Thank you for picking up a topic that is important for all physicians and one that has been annoying me since the introduction of electronic medical records (EMRs). I like the APSO approach, that works well. My idea for reducing “note bloat” is to eliminate all normal and routine findings and to hide them behind a hyperlink or behind a QR code. This would give you a truly short note and, should you need or want more details, you could always scan the QR code for access to the complete (and bloated) note. I would also recommend hiding all details that do not contribute to the immediate pressing issue at hand (for example, routine depression screening) behind a hyperlink or QR code. The same principle should apply to sending faxes to other physicians’ offices. I “love” receiving a chart an inch thick, only to discover that the whole pile of paper could be reduced to a single page of true information. Too few people speak up about this major time and productivity thief. Thank you!
Matthias Muenzer, MD
Rochester, New Hampshire
Dr. Barbieri responds
I thank Dr. Muenzer for his innovative suggestions for improving medical record notes. We spend many hours per week crafting notes in the medical record. Yet, very little attention is given to the development of best practices for improving the value and effectiveness of our notes for our patients and colleagues.
UTIs IN PREGNANCY: MANAGING URETHRITIS, ASYMPTOMATIC BACTERIURIA, CYSTITIS, AND PYELONEPHRITIS
PATRICK DUFF, MD (JANUARY 2022)
Clarification on UTI issues
Regarding the article on urinary tract infections (UTIs) in pregnancy, I have 3 points of clarification. First, in 27 years of practice in which I universally performed screening urine cultures on prenatal patients plus all of those with symptoms, I have seen a total of 2 cultures with Staphylococcus saprophyticus. I see this organism listed in references as a major UTI causative, but is that the case? Second, the clinical case and symptoms discussed are accurate, but costovertebral angle tenderness or fever of 101 °F or higher indicate pyelonephritis and should be treated aggressively. Many of these patients will have nausea and vomiting and will be dehydrated. This decreases urine flow, allowing progressive bacterial growth in renal parenchyma. An initial bolus of intravenous fluids, at least 2 L wide open through a large-bore catheter, rapidly decreases fever, flushes the urinary tract, and improves nausea, headaches, and malaise. Finally, nitrofurantoin is excreted in the urine so rapidly that it does not achieve adequate tissue levels, and it should never be used to treat pyelonephritis or, for that matter, any infection other than uncomplicated cystitis/urethritis.
David Janowitz, MD
Houston, Texas
Dr. Duff responds
I appreciate Dr. Janowitz’s interest and thoughtful comments. The patient presented in the case study has acute cystitis, characterized by a low-grade fever, suprapubic pain, dysuria, frequency, and hesitancy. Patients with pyelonephritis typically have a higher fever and significant costovertebral angle pain and tenderness. I agree completely with Dr. Janowitz’s observations about the seriousness of pyelonephritis in pregnancy. Pyelonephritis is an important cause of preterm labor, bacteremia, and even septic shock. As I point out in the article, women with moderate to severe kidney infections should be hospitalized and treated with intravenous fluids, antipyretics, antiemetics, and intravenous antibiotics. My usual recommendation is ceftriaxone. Intravenous antibiotics should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. Once patients improve, they can be transitioned to oral antibiotics to complete a 10-day course of therapy. Again, I agree with Dr. Janowitz’s statement that nitrofurantoin is not an appropriate drug for treatment of pyelonephritis because it does not reach acceptable concentrations in either the blood or the renal parenchyma. Rather, amoxicillin-clavulanate and trimethoprim-sulfamethoxazole are much better choices for oral therapy. However, once the infection is cleared, nitrofurantoin is an excellent agent for suppression of recurrent infection.
Finally, there is no doubt that the principal pathogens that cause UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, and Proteus species. However, 3 aerobic Gram-positive cocci do, in fact, cause a small percentage of infections: group B streptococci, enterococci, and Staphylococcus saprophyticus. When the latter bacterium is identified as a single organism in high colony count, particularly in a catheterized urine specimen, it should be considered a true pathogen and not simply a contaminant.
CAN WE RETURN TO THE ABCs OF CRAFTING A MEDICAL RECORD NOTE?
ROBERT L. BARBIERI, MD (OCTOBER 2021)
Another suggestion for reducing note bloat in the EMR
Thank you for picking up a topic that is important for all physicians and one that has been annoying me since the introduction of electronic medical records (EMRs). I like the APSO approach, that works well. My idea for reducing “note bloat” is to eliminate all normal and routine findings and to hide them behind a hyperlink or behind a QR code. This would give you a truly short note and, should you need or want more details, you could always scan the QR code for access to the complete (and bloated) note. I would also recommend hiding all details that do not contribute to the immediate pressing issue at hand (for example, routine depression screening) behind a hyperlink or QR code. The same principle should apply to sending faxes to other physicians’ offices. I “love” receiving a chart an inch thick, only to discover that the whole pile of paper could be reduced to a single page of true information. Too few people speak up about this major time and productivity thief. Thank you!
Matthias Muenzer, MD
Rochester, New Hampshire
Dr. Barbieri responds
I thank Dr. Muenzer for his innovative suggestions for improving medical record notes. We spend many hours per week crafting notes in the medical record. Yet, very little attention is given to the development of best practices for improving the value and effectiveness of our notes for our patients and colleagues.
UTIs IN PREGNANCY: MANAGING URETHRITIS, ASYMPTOMATIC BACTERIURIA, CYSTITIS, AND PYELONEPHRITIS
PATRICK DUFF, MD (JANUARY 2022)
Clarification on UTI issues
Regarding the article on urinary tract infections (UTIs) in pregnancy, I have 3 points of clarification. First, in 27 years of practice in which I universally performed screening urine cultures on prenatal patients plus all of those with symptoms, I have seen a total of 2 cultures with Staphylococcus saprophyticus. I see this organism listed in references as a major UTI causative, but is that the case? Second, the clinical case and symptoms discussed are accurate, but costovertebral angle tenderness or fever of 101 °F or higher indicate pyelonephritis and should be treated aggressively. Many of these patients will have nausea and vomiting and will be dehydrated. This decreases urine flow, allowing progressive bacterial growth in renal parenchyma. An initial bolus of intravenous fluids, at least 2 L wide open through a large-bore catheter, rapidly decreases fever, flushes the urinary tract, and improves nausea, headaches, and malaise. Finally, nitrofurantoin is excreted in the urine so rapidly that it does not achieve adequate tissue levels, and it should never be used to treat pyelonephritis or, for that matter, any infection other than uncomplicated cystitis/urethritis.
David Janowitz, MD
Houston, Texas
Dr. Duff responds
I appreciate Dr. Janowitz’s interest and thoughtful comments. The patient presented in the case study has acute cystitis, characterized by a low-grade fever, suprapubic pain, dysuria, frequency, and hesitancy. Patients with pyelonephritis typically have a higher fever and significant costovertebral angle pain and tenderness. I agree completely with Dr. Janowitz’s observations about the seriousness of pyelonephritis in pregnancy. Pyelonephritis is an important cause of preterm labor, bacteremia, and even septic shock. As I point out in the article, women with moderate to severe kidney infections should be hospitalized and treated with intravenous fluids, antipyretics, antiemetics, and intravenous antibiotics. My usual recommendation is ceftriaxone. Intravenous antibiotics should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. Once patients improve, they can be transitioned to oral antibiotics to complete a 10-day course of therapy. Again, I agree with Dr. Janowitz’s statement that nitrofurantoin is not an appropriate drug for treatment of pyelonephritis because it does not reach acceptable concentrations in either the blood or the renal parenchyma. Rather, amoxicillin-clavulanate and trimethoprim-sulfamethoxazole are much better choices for oral therapy. However, once the infection is cleared, nitrofurantoin is an excellent agent for suppression of recurrent infection.
Finally, there is no doubt that the principal pathogens that cause UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, and Proteus species. However, 3 aerobic Gram-positive cocci do, in fact, cause a small percentage of infections: group B streptococci, enterococci, and Staphylococcus saprophyticus. When the latter bacterium is identified as a single organism in high colony count, particularly in a catheterized urine specimen, it should be considered a true pathogen and not simply a contaminant.
CAN WE RETURN TO THE ABCs OF CRAFTING A MEDICAL RECORD NOTE?
ROBERT L. BARBIERI, MD (OCTOBER 2021)
Another suggestion for reducing note bloat in the EMR
Thank you for picking up a topic that is important for all physicians and one that has been annoying me since the introduction of electronic medical records (EMRs). I like the APSO approach, that works well. My idea for reducing “note bloat” is to eliminate all normal and routine findings and to hide them behind a hyperlink or behind a QR code. This would give you a truly short note and, should you need or want more details, you could always scan the QR code for access to the complete (and bloated) note. I would also recommend hiding all details that do not contribute to the immediate pressing issue at hand (for example, routine depression screening) behind a hyperlink or QR code. The same principle should apply to sending faxes to other physicians’ offices. I “love” receiving a chart an inch thick, only to discover that the whole pile of paper could be reduced to a single page of true information. Too few people speak up about this major time and productivity thief. Thank you!
Matthias Muenzer, MD
Rochester, New Hampshire
Dr. Barbieri responds
I thank Dr. Muenzer for his innovative suggestions for improving medical record notes. We spend many hours per week crafting notes in the medical record. Yet, very little attention is given to the development of best practices for improving the value and effectiveness of our notes for our patients and colleagues.
Uterine incision closure: Is it the culprit in the cesarean scar niche and related complications?
While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25
Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.
In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.
Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.

While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25

Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.

In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.

Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●

While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25

Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.

In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.

Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.
About 19% of COVID-19 headaches become chronic
Approximately one in five patients who presented with headache during the acute phase of COVID-19 developed chronic daily headache, according to a study published in Cephalalgia. The greater the headache’s intensity during the acute phase, the greater the likelihood that it would persist.
The research, carried out by members of the Headache Study Group of the Spanish Society of Neurology, evaluated the evolution of headache in more than 900 Spanish patients. Because they found that headache intensity during the acute phase was associated with a more prolonged duration of headache, the team stressed the importance of promptly evaluating patients who have had COVID-19 and who then experience persistent headache.
Long-term evolution unknown
Headache is a common symptom of COVID-19, but its long-term evolution remains unknown. The objective of this study was to evaluate the long-term duration of headache in patients who presented with this symptom during the acute phase of the disease.
Recruitment for this multicenter study took place in March and April 2020. The 905 patients who were enrolled came from six level 3 hospitals in Spain. All completed 9 months of neurologic follow-up.
Their median age was 51 years, 66.5% were women, and more than half (52.7%) had a history of primary headache. About half of the patients required hospitalization (50.5%); the rest were treated as outpatients. The most common headache phenotype was holocranial (67.8%) of severe intensity (50.6%).
Persistent headache common
In the 96.6% cases for which data were available, the median duration of headache was 14 days. The headache persisted at 1 month in 31.1% of patients, at 2 months in 21.5%, at 3 months in 19%, at 6 months in 16.8%, and at 9 months in 16.0%.
“The median duration of COVID-19 headache is around 2 weeks,” David García Azorín, MD, PhD, a member of the Spanish Society of Neurology and one of the coauthors of the study, said in an interview. “However, almost 20% of patients experience it for longer than that. When still present at 2 months, the headache is more likely to follow a chronic daily pattern.” Dr. García Azorín is a neurologist and clinical researcher at the headache unit of the Hospital Clínico Universitario in Valladolid, Spain.
“So, if the headache isn’t letting up, it’s important to make the most of that window of opportunity and provide treatment in that period of 6-12 weeks,” he continued. “To do this, the best option is to carry out preventive treatment so that the patient will have a better chance of recovering.”
Study participants whose headache persisted at 9 months were older and were mostly women. They were less likely to have had pneumonia or to have experienced stabbing pain, photophobia, or phonophobia. They reported that the headache got worse when they engaged in physical activity but less frequently manifested as a throbbing headache.
Secondary tension headaches
On the other hand, Jaime Rodríguez Vico, MD, head of the headache unit at the Jiménez Díaz Foundation Hospital in Madrid, said in an interview that, according to his case studies, the most striking characteristics of post–COVID-19 headaches “in general are secondary, with similarities to tension headaches that patients are able to differentiate from other clinical types of headache. In patients with migraine, very often we see that we’re dealing with a trigger. In other words, more migraines – and more intense ones at that – are brought about.”
He added: “Generally, post–COVID-19 headache usually lasts 1-2 weeks, but we have cases of it lasting several months and even over a year with persistent daily headache. These more persistent cases are probably connected to another type of pathology that makes them more susceptible to becoming chronic, something that occurs in another type of primary headache known as new daily persistent headache.”
Primary headache exacerbation
Dr. García Azorín pointed out that it’s not uncommon that among people who already have primary headache, their condition worsens after they become infected with SARS-CoV-2. However, many people differentiate the headache associated with the infection from their usual headache because after becoming infected, their headache is predominantly frontal, oppressive, and chronic.
“Having a prior history of headache is one of the factors that can increase the likelihood that a headache experienced while suffering from COVID-19 will become chronic,” he noted.
This study also found that, more often than not, patients with persistent headache at 9 months had migraine-like pain.
As for headaches in these patients beyond 9 months, “based on our research, the evolution is quite variable,” said Dr. Rodríguez Vico. “Our unit’s numbers are skewed due to the high number of migraine cases that we follow, and therefore our high volume of migraine patients who’ve gotten worse. The same thing happens with COVID-19 vaccines. Migraine is a polygenic disorder with multiple variants and a pathophysiology that we are just beginning to describe. This is why one patient is completely different from another. It’s a real challenge.”
Infections are a common cause of acute and chronic headache. The persistence of a headache after an infection may be caused by the infection becoming chronic, as happens in some types of chronic meningitis, such as tuberculous meningitis. It may also be caused by the persistence of a certain response and activation of the immune system or to the uncovering or worsening of a primary headache coincident with the infection, added Dr. García Azorín.
“Likewise, there are other people who have a biological predisposition to headache as a multifactorial disorder and polygenic disorder, such that a particular stimulus – from trauma or an infection to alcohol consumption – can cause them to develop a headache very similar to a migraine,” he said.
Providing prognosis and treatment
Certain factors can give an idea of how long the headache might last. The study’s univariate analysis showed that age, female sex, headache intensity, pressure-like quality, the presence of photophobia/phonophobia, and worsening with physical activity were associated with headache of longer duration. But in the multivariate analysis, only headache intensity during the acute phase remained statistically significant (hazard ratio, 0.655; 95% confidence interval, 0.582-0.737; P < .001).
When asked whether they planned to continue the study, Dr. García Azorín commented, “The main questions that have arisen from this study have been, above all: ‘Why does this headache happen?’ and ‘How can it be treated or avoided?’ To answer them, we’re looking into pain: which factors could predispose a person to it and which changes may be associated with its presence.”
In addition, different treatments that may improve patient outcomes are being evaluated, because to date, treatment has been empirical and based on the predominant pain phenotype.
In any case, most doctors currently treat post–COVID-19 headache on the basis of how similar the symptoms are to those of other primary headaches. “Given the impact that headache has on patients’ quality of life, there’s a pressing need for controlled studies on possible treatments and their effectiveness,” noted Patricia Pozo Rosich, MD, PhD, one of the coauthors of the study.
“We at the Spanish Society of Neurology truly believe that if these patients were to have this symptom correctly addressed from the start, they could avoid many of the problems that arise in the situation becoming chronic,” she concluded.
Dr. García Azorín and Dr. Rodríguez Vico disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately one in five patients who presented with headache during the acute phase of COVID-19 developed chronic daily headache, according to a study published in Cephalalgia. The greater the headache’s intensity during the acute phase, the greater the likelihood that it would persist.
The research, carried out by members of the Headache Study Group of the Spanish Society of Neurology, evaluated the evolution of headache in more than 900 Spanish patients. Because they found that headache intensity during the acute phase was associated with a more prolonged duration of headache, the team stressed the importance of promptly evaluating patients who have had COVID-19 and who then experience persistent headache.
Long-term evolution unknown
Headache is a common symptom of COVID-19, but its long-term evolution remains unknown. The objective of this study was to evaluate the long-term duration of headache in patients who presented with this symptom during the acute phase of the disease.
Recruitment for this multicenter study took place in March and April 2020. The 905 patients who were enrolled came from six level 3 hospitals in Spain. All completed 9 months of neurologic follow-up.
Their median age was 51 years, 66.5% were women, and more than half (52.7%) had a history of primary headache. About half of the patients required hospitalization (50.5%); the rest were treated as outpatients. The most common headache phenotype was holocranial (67.8%) of severe intensity (50.6%).
Persistent headache common
In the 96.6% cases for which data were available, the median duration of headache was 14 days. The headache persisted at 1 month in 31.1% of patients, at 2 months in 21.5%, at 3 months in 19%, at 6 months in 16.8%, and at 9 months in 16.0%.
“The median duration of COVID-19 headache is around 2 weeks,” David García Azorín, MD, PhD, a member of the Spanish Society of Neurology and one of the coauthors of the study, said in an interview. “However, almost 20% of patients experience it for longer than that. When still present at 2 months, the headache is more likely to follow a chronic daily pattern.” Dr. García Azorín is a neurologist and clinical researcher at the headache unit of the Hospital Clínico Universitario in Valladolid, Spain.
“So, if the headache isn’t letting up, it’s important to make the most of that window of opportunity and provide treatment in that period of 6-12 weeks,” he continued. “To do this, the best option is to carry out preventive treatment so that the patient will have a better chance of recovering.”
Study participants whose headache persisted at 9 months were older and were mostly women. They were less likely to have had pneumonia or to have experienced stabbing pain, photophobia, or phonophobia. They reported that the headache got worse when they engaged in physical activity but less frequently manifested as a throbbing headache.
Secondary tension headaches
On the other hand, Jaime Rodríguez Vico, MD, head of the headache unit at the Jiménez Díaz Foundation Hospital in Madrid, said in an interview that, according to his case studies, the most striking characteristics of post–COVID-19 headaches “in general are secondary, with similarities to tension headaches that patients are able to differentiate from other clinical types of headache. In patients with migraine, very often we see that we’re dealing with a trigger. In other words, more migraines – and more intense ones at that – are brought about.”
He added: “Generally, post–COVID-19 headache usually lasts 1-2 weeks, but we have cases of it lasting several months and even over a year with persistent daily headache. These more persistent cases are probably connected to another type of pathology that makes them more susceptible to becoming chronic, something that occurs in another type of primary headache known as new daily persistent headache.”
Primary headache exacerbation
Dr. García Azorín pointed out that it’s not uncommon that among people who already have primary headache, their condition worsens after they become infected with SARS-CoV-2. However, many people differentiate the headache associated with the infection from their usual headache because after becoming infected, their headache is predominantly frontal, oppressive, and chronic.
“Having a prior history of headache is one of the factors that can increase the likelihood that a headache experienced while suffering from COVID-19 will become chronic,” he noted.
This study also found that, more often than not, patients with persistent headache at 9 months had migraine-like pain.
As for headaches in these patients beyond 9 months, “based on our research, the evolution is quite variable,” said Dr. Rodríguez Vico. “Our unit’s numbers are skewed due to the high number of migraine cases that we follow, and therefore our high volume of migraine patients who’ve gotten worse. The same thing happens with COVID-19 vaccines. Migraine is a polygenic disorder with multiple variants and a pathophysiology that we are just beginning to describe. This is why one patient is completely different from another. It’s a real challenge.”
Infections are a common cause of acute and chronic headache. The persistence of a headache after an infection may be caused by the infection becoming chronic, as happens in some types of chronic meningitis, such as tuberculous meningitis. It may also be caused by the persistence of a certain response and activation of the immune system or to the uncovering or worsening of a primary headache coincident with the infection, added Dr. García Azorín.
“Likewise, there are other people who have a biological predisposition to headache as a multifactorial disorder and polygenic disorder, such that a particular stimulus – from trauma or an infection to alcohol consumption – can cause them to develop a headache very similar to a migraine,” he said.
Providing prognosis and treatment
Certain factors can give an idea of how long the headache might last. The study’s univariate analysis showed that age, female sex, headache intensity, pressure-like quality, the presence of photophobia/phonophobia, and worsening with physical activity were associated with headache of longer duration. But in the multivariate analysis, only headache intensity during the acute phase remained statistically significant (hazard ratio, 0.655; 95% confidence interval, 0.582-0.737; P < .001).
When asked whether they planned to continue the study, Dr. García Azorín commented, “The main questions that have arisen from this study have been, above all: ‘Why does this headache happen?’ and ‘How can it be treated or avoided?’ To answer them, we’re looking into pain: which factors could predispose a person to it and which changes may be associated with its presence.”
In addition, different treatments that may improve patient outcomes are being evaluated, because to date, treatment has been empirical and based on the predominant pain phenotype.
In any case, most doctors currently treat post–COVID-19 headache on the basis of how similar the symptoms are to those of other primary headaches. “Given the impact that headache has on patients’ quality of life, there’s a pressing need for controlled studies on possible treatments and their effectiveness,” noted Patricia Pozo Rosich, MD, PhD, one of the coauthors of the study.
“We at the Spanish Society of Neurology truly believe that if these patients were to have this symptom correctly addressed from the start, they could avoid many of the problems that arise in the situation becoming chronic,” she concluded.
Dr. García Azorín and Dr. Rodríguez Vico disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately one in five patients who presented with headache during the acute phase of COVID-19 developed chronic daily headache, according to a study published in Cephalalgia. The greater the headache’s intensity during the acute phase, the greater the likelihood that it would persist.
The research, carried out by members of the Headache Study Group of the Spanish Society of Neurology, evaluated the evolution of headache in more than 900 Spanish patients. Because they found that headache intensity during the acute phase was associated with a more prolonged duration of headache, the team stressed the importance of promptly evaluating patients who have had COVID-19 and who then experience persistent headache.
Long-term evolution unknown
Headache is a common symptom of COVID-19, but its long-term evolution remains unknown. The objective of this study was to evaluate the long-term duration of headache in patients who presented with this symptom during the acute phase of the disease.
Recruitment for this multicenter study took place in March and April 2020. The 905 patients who were enrolled came from six level 3 hospitals in Spain. All completed 9 months of neurologic follow-up.
Their median age was 51 years, 66.5% were women, and more than half (52.7%) had a history of primary headache. About half of the patients required hospitalization (50.5%); the rest were treated as outpatients. The most common headache phenotype was holocranial (67.8%) of severe intensity (50.6%).
Persistent headache common
In the 96.6% cases for which data were available, the median duration of headache was 14 days. The headache persisted at 1 month in 31.1% of patients, at 2 months in 21.5%, at 3 months in 19%, at 6 months in 16.8%, and at 9 months in 16.0%.
“The median duration of COVID-19 headache is around 2 weeks,” David García Azorín, MD, PhD, a member of the Spanish Society of Neurology and one of the coauthors of the study, said in an interview. “However, almost 20% of patients experience it for longer than that. When still present at 2 months, the headache is more likely to follow a chronic daily pattern.” Dr. García Azorín is a neurologist and clinical researcher at the headache unit of the Hospital Clínico Universitario in Valladolid, Spain.
“So, if the headache isn’t letting up, it’s important to make the most of that window of opportunity and provide treatment in that period of 6-12 weeks,” he continued. “To do this, the best option is to carry out preventive treatment so that the patient will have a better chance of recovering.”
Study participants whose headache persisted at 9 months were older and were mostly women. They were less likely to have had pneumonia or to have experienced stabbing pain, photophobia, or phonophobia. They reported that the headache got worse when they engaged in physical activity but less frequently manifested as a throbbing headache.
Secondary tension headaches
On the other hand, Jaime Rodríguez Vico, MD, head of the headache unit at the Jiménez Díaz Foundation Hospital in Madrid, said in an interview that, according to his case studies, the most striking characteristics of post–COVID-19 headaches “in general are secondary, with similarities to tension headaches that patients are able to differentiate from other clinical types of headache. In patients with migraine, very often we see that we’re dealing with a trigger. In other words, more migraines – and more intense ones at that – are brought about.”
He added: “Generally, post–COVID-19 headache usually lasts 1-2 weeks, but we have cases of it lasting several months and even over a year with persistent daily headache. These more persistent cases are probably connected to another type of pathology that makes them more susceptible to becoming chronic, something that occurs in another type of primary headache known as new daily persistent headache.”
Primary headache exacerbation
Dr. García Azorín pointed out that it’s not uncommon that among people who already have primary headache, their condition worsens after they become infected with SARS-CoV-2. However, many people differentiate the headache associated with the infection from their usual headache because after becoming infected, their headache is predominantly frontal, oppressive, and chronic.
“Having a prior history of headache is one of the factors that can increase the likelihood that a headache experienced while suffering from COVID-19 will become chronic,” he noted.
This study also found that, more often than not, patients with persistent headache at 9 months had migraine-like pain.
As for headaches in these patients beyond 9 months, “based on our research, the evolution is quite variable,” said Dr. Rodríguez Vico. “Our unit’s numbers are skewed due to the high number of migraine cases that we follow, and therefore our high volume of migraine patients who’ve gotten worse. The same thing happens with COVID-19 vaccines. Migraine is a polygenic disorder with multiple variants and a pathophysiology that we are just beginning to describe. This is why one patient is completely different from another. It’s a real challenge.”
Infections are a common cause of acute and chronic headache. The persistence of a headache after an infection may be caused by the infection becoming chronic, as happens in some types of chronic meningitis, such as tuberculous meningitis. It may also be caused by the persistence of a certain response and activation of the immune system or to the uncovering or worsening of a primary headache coincident with the infection, added Dr. García Azorín.
“Likewise, there are other people who have a biological predisposition to headache as a multifactorial disorder and polygenic disorder, such that a particular stimulus – from trauma or an infection to alcohol consumption – can cause them to develop a headache very similar to a migraine,” he said.
Providing prognosis and treatment
Certain factors can give an idea of how long the headache might last. The study’s univariate analysis showed that age, female sex, headache intensity, pressure-like quality, the presence of photophobia/phonophobia, and worsening with physical activity were associated with headache of longer duration. But in the multivariate analysis, only headache intensity during the acute phase remained statistically significant (hazard ratio, 0.655; 95% confidence interval, 0.582-0.737; P < .001).
When asked whether they planned to continue the study, Dr. García Azorín commented, “The main questions that have arisen from this study have been, above all: ‘Why does this headache happen?’ and ‘How can it be treated or avoided?’ To answer them, we’re looking into pain: which factors could predispose a person to it and which changes may be associated with its presence.”
In addition, different treatments that may improve patient outcomes are being evaluated, because to date, treatment has been empirical and based on the predominant pain phenotype.
In any case, most doctors currently treat post–COVID-19 headache on the basis of how similar the symptoms are to those of other primary headaches. “Given the impact that headache has on patients’ quality of life, there’s a pressing need for controlled studies on possible treatments and their effectiveness,” noted Patricia Pozo Rosich, MD, PhD, one of the coauthors of the study.
“We at the Spanish Society of Neurology truly believe that if these patients were to have this symptom correctly addressed from the start, they could avoid many of the problems that arise in the situation becoming chronic,” she concluded.
Dr. García Azorín and Dr. Rodríguez Vico disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CEPHALALGIA
JIA disease activity, disability linked to social factors
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PEDIATRIC RHEUMATOLOGY
May 2022 - ICYMI
Gastroenterology
February 2022
How to Succeed in Digestive Research
Sonnenberg A, Inadomi JM. Gastroenterology. 2022 Feb;162(2):385-389. doi: 10.1053/j.gastro.2021.12.229.
Incidence and Mortality in Upper Gastrointestinal Cancer After Negative Endoscopy for Gastroesophageal Reflux Disease
Holmberg H et al. Gastroenterology. 2022 Feb;162(2):431-438.e4. doi: 10.1053/j.gastro.2021.10.003.
March 2022
Global Prevalence and Impact of Rumination Syndrome
Josefsson A et al. Gastroenterology. 2022 Mar;162(3):731-742.e9. doi: 10.1053/j.gastro.2021.11.008.
A Clinical Approach to Chronic Diarrhea
Dutra B et al. Gastroenterology. 2022 Mar;162(3):707-709. doi: 10.1053/j.gastro.2021.07.038.
Timeline of Development of Pancreatic Cancer and Implications for Successful Early Detection in High-Risk Individuals
Overbeek KA et al. Gastroenterology. 2022 Mar;162(3):772-785.e4. doi: 10.1053/j.gastro.2021.10.014.
April 2022
Artificial Intelligence and Deep Learning for Upper Gastrointestinal Neoplasia
Sharma P, Hassan C. Gastroenterology. 2022 Apr;162(4):1056-1066. doi: 10.1053/j.gastro.2021.11.040.
Associations of Body Mass Index at Different Ages With Early-Onset Colorectal Cancer
Li H et al. Gastroenterology. 2022 Apr;162(4):1088-1097.e3. doi: 10.1053/j.gastro.2021.12.239.
Inadequate Rectal Pressure and Insufficient Relaxation and Abdominopelvic Coordination in Defecatory Disorders
Deb B et al. Gastroenterology. 2022 Apr;162(4):1111-1122.e2. doi: 10.1053/j.gastro.2021.12.257.
AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review
Targownik LE et al. Gastroenterology. 2022 Apr;162(4):1334-1342. doi: 10.1053/j.gastro.2021.12.247.
Clinical Gastroenterology and Hepatology
February 2022
Restarting Warfarin vs Direct Oral Anticoagulants After Major Gastrointestinal Bleeding and Associated Outcomes in Atrial Fibrillation: A Cohort Study
Tapaskar N et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):381-389.e9. doi: 10.1016/j.cgh.2020.11.029.
Cancer Risk in 47,241 Individuals With Celiac Disease: A Nationwide Cohort Study
Lebwohl B et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):e111-e131. doi: 10.1016/j.cgh.2021.05.034.
Main Duct Thresholds for Malignancy Are Different in Intraductal Papillary Mucinous Neoplasms of the Pancreatic Head and Body-Tail
Crippa S et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):390-399.e7. doi: 10.1016/j.cgh.2020.12.028.
Frequency of Bowel Movements and Risk of Diverticulitis
Jovani M et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):325-333.e5. doi: 10.1016/j.cgh.2021.01.003.
March 2022
AGA Clinical Practice Update on Management of Medically Refractory Gastroparesis: Expert Review
Lacy BE et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):491-500. doi: 10.1016/j.cgh.2021.10.038.
Efficacy and Safety of Tofacitinib in Ulcerative Colitis Based on Prior Tumor Necrosis Factor Inhibitor Failure Status
Sandborn WJ et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):591-601.e8. doi: 10.1016/j.cgh.2021.02.043.
April 2022
What Faculty and Fellows Should Know About Milestones 2.0
Donnangelo JL, Brijen SJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):720-722. doi: 10.1016/j.cgh.2021.12.017.
Patient Experience in the Gastrointestinal Endoscopy Unit
Day LW, Savides TJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):723-726. doi: 10.1016/j.cgh.2021.12.001.
Tailoring Surveillance Colonoscopy in Patients With Advanced Adenomas
Kahi CJ et al. Clin Gastroenterol Hepatol. 2022 Apr;20(4):847-854.e1. doi: 10.1016/j.cgh.2021.03.027.
Techniques and Innovations in Gastrointestinal Endoscopy
Primary CT Angiography Vs Colonoscopy in Acute Lower Gastrointestinal Hemorrhage
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(1):2-9. doi: 10.1016/j.tige.2021.11.004.
Cellular and Molecular Gastroenterology and Hepatology
The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse?
Smet A et al. Cell Mol Gastroenterol Hepatol. 2022;13(3):857-874. doi: 10.1016/j.jcmgh.2021.08.013.
Gastroenterology
February 2022
How to Succeed in Digestive Research
Sonnenberg A, Inadomi JM. Gastroenterology. 2022 Feb;162(2):385-389. doi: 10.1053/j.gastro.2021.12.229.
Incidence and Mortality in Upper Gastrointestinal Cancer After Negative Endoscopy for Gastroesophageal Reflux Disease
Holmberg H et al. Gastroenterology. 2022 Feb;162(2):431-438.e4. doi: 10.1053/j.gastro.2021.10.003.
March 2022
Global Prevalence and Impact of Rumination Syndrome
Josefsson A et al. Gastroenterology. 2022 Mar;162(3):731-742.e9. doi: 10.1053/j.gastro.2021.11.008.
A Clinical Approach to Chronic Diarrhea
Dutra B et al. Gastroenterology. 2022 Mar;162(3):707-709. doi: 10.1053/j.gastro.2021.07.038.
Timeline of Development of Pancreatic Cancer and Implications for Successful Early Detection in High-Risk Individuals
Overbeek KA et al. Gastroenterology. 2022 Mar;162(3):772-785.e4. doi: 10.1053/j.gastro.2021.10.014.
April 2022
Artificial Intelligence and Deep Learning for Upper Gastrointestinal Neoplasia
Sharma P, Hassan C. Gastroenterology. 2022 Apr;162(4):1056-1066. doi: 10.1053/j.gastro.2021.11.040.
Associations of Body Mass Index at Different Ages With Early-Onset Colorectal Cancer
Li H et al. Gastroenterology. 2022 Apr;162(4):1088-1097.e3. doi: 10.1053/j.gastro.2021.12.239.
Inadequate Rectal Pressure and Insufficient Relaxation and Abdominopelvic Coordination in Defecatory Disorders
Deb B et al. Gastroenterology. 2022 Apr;162(4):1111-1122.e2. doi: 10.1053/j.gastro.2021.12.257.
AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review
Targownik LE et al. Gastroenterology. 2022 Apr;162(4):1334-1342. doi: 10.1053/j.gastro.2021.12.247.
Clinical Gastroenterology and Hepatology
February 2022
Restarting Warfarin vs Direct Oral Anticoagulants After Major Gastrointestinal Bleeding and Associated Outcomes in Atrial Fibrillation: A Cohort Study
Tapaskar N et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):381-389.e9. doi: 10.1016/j.cgh.2020.11.029.
Cancer Risk in 47,241 Individuals With Celiac Disease: A Nationwide Cohort Study
Lebwohl B et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):e111-e131. doi: 10.1016/j.cgh.2021.05.034.
Main Duct Thresholds for Malignancy Are Different in Intraductal Papillary Mucinous Neoplasms of the Pancreatic Head and Body-Tail
Crippa S et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):390-399.e7. doi: 10.1016/j.cgh.2020.12.028.
Frequency of Bowel Movements and Risk of Diverticulitis
Jovani M et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):325-333.e5. doi: 10.1016/j.cgh.2021.01.003.
March 2022
AGA Clinical Practice Update on Management of Medically Refractory Gastroparesis: Expert Review
Lacy BE et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):491-500. doi: 10.1016/j.cgh.2021.10.038.
Efficacy and Safety of Tofacitinib in Ulcerative Colitis Based on Prior Tumor Necrosis Factor Inhibitor Failure Status
Sandborn WJ et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):591-601.e8. doi: 10.1016/j.cgh.2021.02.043.
April 2022
What Faculty and Fellows Should Know About Milestones 2.0
Donnangelo JL, Brijen SJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):720-722. doi: 10.1016/j.cgh.2021.12.017.
Patient Experience in the Gastrointestinal Endoscopy Unit
Day LW, Savides TJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):723-726. doi: 10.1016/j.cgh.2021.12.001.
Tailoring Surveillance Colonoscopy in Patients With Advanced Adenomas
Kahi CJ et al. Clin Gastroenterol Hepatol. 2022 Apr;20(4):847-854.e1. doi: 10.1016/j.cgh.2021.03.027.
Techniques and Innovations in Gastrointestinal Endoscopy
Primary CT Angiography Vs Colonoscopy in Acute Lower Gastrointestinal Hemorrhage
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(1):2-9. doi: 10.1016/j.tige.2021.11.004.
Cellular and Molecular Gastroenterology and Hepatology
The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse?
Smet A et al. Cell Mol Gastroenterol Hepatol. 2022;13(3):857-874. doi: 10.1016/j.jcmgh.2021.08.013.
Gastroenterology
February 2022
How to Succeed in Digestive Research
Sonnenberg A, Inadomi JM. Gastroenterology. 2022 Feb;162(2):385-389. doi: 10.1053/j.gastro.2021.12.229.
Incidence and Mortality in Upper Gastrointestinal Cancer After Negative Endoscopy for Gastroesophageal Reflux Disease
Holmberg H et al. Gastroenterology. 2022 Feb;162(2):431-438.e4. doi: 10.1053/j.gastro.2021.10.003.
March 2022
Global Prevalence and Impact of Rumination Syndrome
Josefsson A et al. Gastroenterology. 2022 Mar;162(3):731-742.e9. doi: 10.1053/j.gastro.2021.11.008.
A Clinical Approach to Chronic Diarrhea
Dutra B et al. Gastroenterology. 2022 Mar;162(3):707-709. doi: 10.1053/j.gastro.2021.07.038.
Timeline of Development of Pancreatic Cancer and Implications for Successful Early Detection in High-Risk Individuals
Overbeek KA et al. Gastroenterology. 2022 Mar;162(3):772-785.e4. doi: 10.1053/j.gastro.2021.10.014.
April 2022
Artificial Intelligence and Deep Learning for Upper Gastrointestinal Neoplasia
Sharma P, Hassan C. Gastroenterology. 2022 Apr;162(4):1056-1066. doi: 10.1053/j.gastro.2021.11.040.
Associations of Body Mass Index at Different Ages With Early-Onset Colorectal Cancer
Li H et al. Gastroenterology. 2022 Apr;162(4):1088-1097.e3. doi: 10.1053/j.gastro.2021.12.239.
Inadequate Rectal Pressure and Insufficient Relaxation and Abdominopelvic Coordination in Defecatory Disorders
Deb B et al. Gastroenterology. 2022 Apr;162(4):1111-1122.e2. doi: 10.1053/j.gastro.2021.12.257.
AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review
Targownik LE et al. Gastroenterology. 2022 Apr;162(4):1334-1342. doi: 10.1053/j.gastro.2021.12.247.
Clinical Gastroenterology and Hepatology
February 2022
Restarting Warfarin vs Direct Oral Anticoagulants After Major Gastrointestinal Bleeding and Associated Outcomes in Atrial Fibrillation: A Cohort Study
Tapaskar N et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):381-389.e9. doi: 10.1016/j.cgh.2020.11.029.
Cancer Risk in 47,241 Individuals With Celiac Disease: A Nationwide Cohort Study
Lebwohl B et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):e111-e131. doi: 10.1016/j.cgh.2021.05.034.
Main Duct Thresholds for Malignancy Are Different in Intraductal Papillary Mucinous Neoplasms of the Pancreatic Head and Body-Tail
Crippa S et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):390-399.e7. doi: 10.1016/j.cgh.2020.12.028.
Frequency of Bowel Movements and Risk of Diverticulitis
Jovani M et al. Clin Gastroenterol Hepatol. 2022 Feb;20(2):325-333.e5. doi: 10.1016/j.cgh.2021.01.003.
March 2022
AGA Clinical Practice Update on Management of Medically Refractory Gastroparesis: Expert Review
Lacy BE et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):491-500. doi: 10.1016/j.cgh.2021.10.038.
Efficacy and Safety of Tofacitinib in Ulcerative Colitis Based on Prior Tumor Necrosis Factor Inhibitor Failure Status
Sandborn WJ et al. Clin Gastroenterol Hepatol. 2022 Mar;20(3):591-601.e8. doi: 10.1016/j.cgh.2021.02.043.
April 2022
What Faculty and Fellows Should Know About Milestones 2.0
Donnangelo JL, Brijen SJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):720-722. doi: 10.1016/j.cgh.2021.12.017.
Patient Experience in the Gastrointestinal Endoscopy Unit
Day LW, Savides TJ. Clin Gastroenterol Hepatol. 2022 Apr;20(4):723-726. doi: 10.1016/j.cgh.2021.12.001.
Tailoring Surveillance Colonoscopy in Patients With Advanced Adenomas
Kahi CJ et al. Clin Gastroenterol Hepatol. 2022 Apr;20(4):847-854.e1. doi: 10.1016/j.cgh.2021.03.027.
Techniques and Innovations in Gastrointestinal Endoscopy
Primary CT Angiography Vs Colonoscopy in Acute Lower Gastrointestinal Hemorrhage
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(1):2-9. doi: 10.1016/j.tige.2021.11.004.
Cellular and Molecular Gastroenterology and Hepatology
The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse?
Smet A et al. Cell Mol Gastroenterol Hepatol. 2022;13(3):857-874. doi: 10.1016/j.jcmgh.2021.08.013.
Direct-to-Consumer Teledermatology Growth: A Review and Outlook for the Future
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
Practice Points
- Direct-to-consumer (DTC) teledermatology platforms are for-profit companies that provide telemedicine visits and sell prescription drugs directly to patients.
- Although they are growing in popularity, DTC teledermatology platforms may lead to overdiagnosis, overtreatment, and fragmentation of health care. Knowledge of teledermatology will be vital to counsel patients on the risks and benefits of these platforms.
Live-donor liver transplants for patients with CRC liver mets
These patients usually have a poor prognosis, and for many, palliative chemotherapy is the standard of care.
“For the first time, we have been able to demonstrate [outside of Norway] that liver transplantation for patients with unresectable liver metastases is feasible with good outcomes,” lead author Gonzalo Sapisochin, MD, PhD, an assistant professor of surgery at the University of Toronto, said in an interview.
“Furthermore, this is the first time we are able to prove that living donation may be a good strategy in this setting,” Dr. Sapisochin said of the series of 10 cases that they published in JAMA Surgery.
The series showed “excellent perioperative outcomes for both donors and recipients,” noted the authors of an accompanying commentary. They said the team “should be commended for adding liver-donor live transplantation to the armamentarium of surgical options for patients with CRC liver metastases.”
However, they express concern about the relatively short follow-up of 1.5 years and the “very high” recurrence rate of 30%.
Commenting in an interview, lead editorialist Shimul Shah, MD, an associate professor of surgery and the chief of solid organ transplantation at the University of Cincinnati, said: “I agree that overall survival is an important measure to look at, but it’s hard to look at overall survival with [1.5] years of follow-up.”
Other key areas of concern are the need for more standardized practices and for more data on how liver transplantation compares with patients who just continue to receive chemotherapy.
“I certainly think that there’s a role for liver transplantation in these patients, and I am a big fan of this,” Dr. Shah emphasized, noting that four patients at his own center have recently received liver transplants, including three from deceased donors.
“However, I just think that as a community, we need to be cautious and not get too excited too early,” he said. “We need to keep studying it and take it one step at a time.”
Moving from deceased to living donors
Nearly 70% of patients with CRC develop liver metastases, and when these are unresectable, the prognosis is poor, with 5-year survival rates of less than 10%.
The option of liver transplantation was first reported in 2015 by a group in Norway. Their study included 21 patients with CRC and unresectable liver tumors. They reported a striking improvement in overall survival at 5 years (56% vs. 9% among patients who started first-line chemotherapy).
But with shortages of donor livers, this approach has not caught on. Deceased-donor liver allografts are in short supply in most countries, and recent allocation changes have further shifted available organs away from patients with liver tumors.
An alternative is to use living donors. In a recent study, Dr. Sapisochin and colleagues showed viability and a survival advantage, compared with deceased-donor liver transplantation.
Building on that work, they established treatment protocols at three centers – the University of Rochester (N.Y.) Medical Center, the Cleveland Clinic, , and the University Health Network in Toronto.
Of 91 evaluated patients who were prospectively enrolled with liver-confined, unresectable CRC liver metastases, 10 met all inclusion criteria and received living-donor liver transplants between December 2017 and May 2021. The median age of the patients was 45 years; six were men, and four were women.
These patients all had primary tumors greater than stage T2 (six T3 and four T4b). Lymphovascular invasion was present in two patients, and perineural invasion was present in one patient.
The median time from diagnosis of the liver metastases to liver transplant was 1.7 years (range, 1.1-7.8 years).
At a median follow-up of 1.5 years (range, 0.4-2.9 years), recurrences occurred in three patients, with a rate of recurrence-free survival, using Kaplan-Meier estimates, of 62% and a rate of overall survival of 100%.
Rates of morbidity associated with transplantation were no higher than those observed in established standards for the donors or recipients, the authors noted.
Among transplant recipients, three patients had no Clavien-Dindo complications; three had grade II, and four had grade III complications. Among donors, five had no complications, four had grade I, and one had grade III complications.
All 10 donors were discharged from the hospital 4-7 days after surgery and recovered fully.
All three patients who experienced recurrences were treated with palliative chemotherapy. One died of disease after 3 months of treatment. As of the time of publication of the study, the other two had survived for 2 or more years following their live donor liver transplant.
Patient selection key
The authors are now investigating tumor subtypes, responses in CRC liver metastases, and other factors, with the aim of developing a novel screening method to identify appropriate candidates more quickly.
In the meantime, they emphasized that indicators of disease biology, such as the Oslo Score, the Clinical Risk Score, and sustained clinical response to systemic therapy, “remain the key filters through which to select patients who have sufficient opportunity for long-term cancer control, which is necessary to justify the risk to a living donor.”
Dr. Sapisochin reported receiving grants from Roche and Bayer and personal fees from Integra, Roche, AstraZeneca, and Novartis outside the submitted work. Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
These patients usually have a poor prognosis, and for many, palliative chemotherapy is the standard of care.
“For the first time, we have been able to demonstrate [outside of Norway] that liver transplantation for patients with unresectable liver metastases is feasible with good outcomes,” lead author Gonzalo Sapisochin, MD, PhD, an assistant professor of surgery at the University of Toronto, said in an interview.
“Furthermore, this is the first time we are able to prove that living donation may be a good strategy in this setting,” Dr. Sapisochin said of the series of 10 cases that they published in JAMA Surgery.
The series showed “excellent perioperative outcomes for both donors and recipients,” noted the authors of an accompanying commentary. They said the team “should be commended for adding liver-donor live transplantation to the armamentarium of surgical options for patients with CRC liver metastases.”
However, they express concern about the relatively short follow-up of 1.5 years and the “very high” recurrence rate of 30%.
Commenting in an interview, lead editorialist Shimul Shah, MD, an associate professor of surgery and the chief of solid organ transplantation at the University of Cincinnati, said: “I agree that overall survival is an important measure to look at, but it’s hard to look at overall survival with [1.5] years of follow-up.”
Other key areas of concern are the need for more standardized practices and for more data on how liver transplantation compares with patients who just continue to receive chemotherapy.
“I certainly think that there’s a role for liver transplantation in these patients, and I am a big fan of this,” Dr. Shah emphasized, noting that four patients at his own center have recently received liver transplants, including three from deceased donors.
“However, I just think that as a community, we need to be cautious and not get too excited too early,” he said. “We need to keep studying it and take it one step at a time.”
Moving from deceased to living donors
Nearly 70% of patients with CRC develop liver metastases, and when these are unresectable, the prognosis is poor, with 5-year survival rates of less than 10%.
The option of liver transplantation was first reported in 2015 by a group in Norway. Their study included 21 patients with CRC and unresectable liver tumors. They reported a striking improvement in overall survival at 5 years (56% vs. 9% among patients who started first-line chemotherapy).
But with shortages of donor livers, this approach has not caught on. Deceased-donor liver allografts are in short supply in most countries, and recent allocation changes have further shifted available organs away from patients with liver tumors.
An alternative is to use living donors. In a recent study, Dr. Sapisochin and colleagues showed viability and a survival advantage, compared with deceased-donor liver transplantation.
Building on that work, they established treatment protocols at three centers – the University of Rochester (N.Y.) Medical Center, the Cleveland Clinic, , and the University Health Network in Toronto.
Of 91 evaluated patients who were prospectively enrolled with liver-confined, unresectable CRC liver metastases, 10 met all inclusion criteria and received living-donor liver transplants between December 2017 and May 2021. The median age of the patients was 45 years; six were men, and four were women.
These patients all had primary tumors greater than stage T2 (six T3 and four T4b). Lymphovascular invasion was present in two patients, and perineural invasion was present in one patient.
The median time from diagnosis of the liver metastases to liver transplant was 1.7 years (range, 1.1-7.8 years).
At a median follow-up of 1.5 years (range, 0.4-2.9 years), recurrences occurred in three patients, with a rate of recurrence-free survival, using Kaplan-Meier estimates, of 62% and a rate of overall survival of 100%.
Rates of morbidity associated with transplantation were no higher than those observed in established standards for the donors or recipients, the authors noted.
Among transplant recipients, three patients had no Clavien-Dindo complications; three had grade II, and four had grade III complications. Among donors, five had no complications, four had grade I, and one had grade III complications.
All 10 donors were discharged from the hospital 4-7 days after surgery and recovered fully.
All three patients who experienced recurrences were treated with palliative chemotherapy. One died of disease after 3 months of treatment. As of the time of publication of the study, the other two had survived for 2 or more years following their live donor liver transplant.
Patient selection key
The authors are now investigating tumor subtypes, responses in CRC liver metastases, and other factors, with the aim of developing a novel screening method to identify appropriate candidates more quickly.
In the meantime, they emphasized that indicators of disease biology, such as the Oslo Score, the Clinical Risk Score, and sustained clinical response to systemic therapy, “remain the key filters through which to select patients who have sufficient opportunity for long-term cancer control, which is necessary to justify the risk to a living donor.”
Dr. Sapisochin reported receiving grants from Roche and Bayer and personal fees from Integra, Roche, AstraZeneca, and Novartis outside the submitted work. Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
These patients usually have a poor prognosis, and for many, palliative chemotherapy is the standard of care.
“For the first time, we have been able to demonstrate [outside of Norway] that liver transplantation for patients with unresectable liver metastases is feasible with good outcomes,” lead author Gonzalo Sapisochin, MD, PhD, an assistant professor of surgery at the University of Toronto, said in an interview.
“Furthermore, this is the first time we are able to prove that living donation may be a good strategy in this setting,” Dr. Sapisochin said of the series of 10 cases that they published in JAMA Surgery.
The series showed “excellent perioperative outcomes for both donors and recipients,” noted the authors of an accompanying commentary. They said the team “should be commended for adding liver-donor live transplantation to the armamentarium of surgical options for patients with CRC liver metastases.”
However, they express concern about the relatively short follow-up of 1.5 years and the “very high” recurrence rate of 30%.
Commenting in an interview, lead editorialist Shimul Shah, MD, an associate professor of surgery and the chief of solid organ transplantation at the University of Cincinnati, said: “I agree that overall survival is an important measure to look at, but it’s hard to look at overall survival with [1.5] years of follow-up.”
Other key areas of concern are the need for more standardized practices and for more data on how liver transplantation compares with patients who just continue to receive chemotherapy.
“I certainly think that there’s a role for liver transplantation in these patients, and I am a big fan of this,” Dr. Shah emphasized, noting that four patients at his own center have recently received liver transplants, including three from deceased donors.
“However, I just think that as a community, we need to be cautious and not get too excited too early,” he said. “We need to keep studying it and take it one step at a time.”
Moving from deceased to living donors
Nearly 70% of patients with CRC develop liver metastases, and when these are unresectable, the prognosis is poor, with 5-year survival rates of less than 10%.
The option of liver transplantation was first reported in 2015 by a group in Norway. Their study included 21 patients with CRC and unresectable liver tumors. They reported a striking improvement in overall survival at 5 years (56% vs. 9% among patients who started first-line chemotherapy).
But with shortages of donor livers, this approach has not caught on. Deceased-donor liver allografts are in short supply in most countries, and recent allocation changes have further shifted available organs away from patients with liver tumors.
An alternative is to use living donors. In a recent study, Dr. Sapisochin and colleagues showed viability and a survival advantage, compared with deceased-donor liver transplantation.
Building on that work, they established treatment protocols at three centers – the University of Rochester (N.Y.) Medical Center, the Cleveland Clinic, , and the University Health Network in Toronto.
Of 91 evaluated patients who were prospectively enrolled with liver-confined, unresectable CRC liver metastases, 10 met all inclusion criteria and received living-donor liver transplants between December 2017 and May 2021. The median age of the patients was 45 years; six were men, and four were women.
These patients all had primary tumors greater than stage T2 (six T3 and four T4b). Lymphovascular invasion was present in two patients, and perineural invasion was present in one patient.
The median time from diagnosis of the liver metastases to liver transplant was 1.7 years (range, 1.1-7.8 years).
At a median follow-up of 1.5 years (range, 0.4-2.9 years), recurrences occurred in three patients, with a rate of recurrence-free survival, using Kaplan-Meier estimates, of 62% and a rate of overall survival of 100%.
Rates of morbidity associated with transplantation were no higher than those observed in established standards for the donors or recipients, the authors noted.
Among transplant recipients, three patients had no Clavien-Dindo complications; three had grade II, and four had grade III complications. Among donors, five had no complications, four had grade I, and one had grade III complications.
All 10 donors were discharged from the hospital 4-7 days after surgery and recovered fully.
All three patients who experienced recurrences were treated with palliative chemotherapy. One died of disease after 3 months of treatment. As of the time of publication of the study, the other two had survived for 2 or more years following their live donor liver transplant.
Patient selection key
The authors are now investigating tumor subtypes, responses in CRC liver metastases, and other factors, with the aim of developing a novel screening method to identify appropriate candidates more quickly.
In the meantime, they emphasized that indicators of disease biology, such as the Oslo Score, the Clinical Risk Score, and sustained clinical response to systemic therapy, “remain the key filters through which to select patients who have sufficient opportunity for long-term cancer control, which is necessary to justify the risk to a living donor.”
Dr. Sapisochin reported receiving grants from Roche and Bayer and personal fees from Integra, Roche, AstraZeneca, and Novartis outside the submitted work. Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Acral Papulovesicular Eruption in a Soldier Following Smallpox Vaccination
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
Practice Points
- There are several potential cutaneous adverse reactions associated with smallpox vaccination, ranging from benign self-limited hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
- Acral papulovesicular eruption is a distinct presentation that has been described in the US Military following vaccination with the second-generation live smallpox vaccine (ACAM2000).
Vesicular Eruption Secondary to Bites by Larval Amblyomma americanum
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.
Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.
Comment
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5
Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10
All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.
Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.
Comment
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5
Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10
All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.
Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.
Comment
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5
Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10
All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
Practice Points
- The range of Amblyomma americanum has expanded north in recent years from its core range in the southeastern United States. Warming temperatures also have increased the duration of the ticks’ active season.
- Amblyomma americanum can lay several thousand eggs. A person happening upon a newly hatched nest of larval ticks could sustain a widespread vesicular eruption secondary to tick bites.
- It is important to keep larval tick infestation in the differential when evaluating a patient with a new widespread vesicular eruption to expedite prompt removal of the offending ticks and to begin clinical monitoring.