User login
The central role of informed consent in novel procedures
Mrs. Jones is a 44-year-old woman who has struggled with her weight. She has a body mass index (BMI) of 35 kg/m2 and hypertension requiring daily medication. She has tried various diets over the years and has never been able to exercise consistently. She desperately wants to lose weight to improve her confidence and to avoid developing diabetes and dialysis that her parents required. She has considered weight loss surgery but is afraid after her best friend died following uterine fibroid surgery. She saw a billboard that advertised a new weight loss procedure without surgery. She looked up the procedure, found Dr. Indo on the university medical center’s website, and booked an appointment. Dr. Indo talked about performing an incisionless procedure done with an endoscope through her mouth. It would make her stomach into a tube to reduce the amount of food she could eat as well as prevent some absorption of food in her intestines. When Mrs. Jones asked how many of these the doctor had performed, Dr. Indo remarked she personally had done “several” in the past few years including training. Dr. Indo reassured Mrs. Jones that the procedure has been performed hundreds of times around the country and has been shown to be safe. Dr. Indo also explained that studies were still ongoing, including possibly at the university medical center, but that she had never personally seen any serious complications or death, and only one patient she knew of converted to a traditional bariatric surgery.
Obesity is a large international public health problem, with the World Health Organization estimating that there are 600 million obese adults worldwide.1 Bariatric surgery has been an effective way to improve complications related to obesity and quality of life. Endoscopic approaches to bariatric surgery have appeared since at least the late 1980s and, similarly to their traditional surgical counterparts, work in two main categories: restrictive or malabsorptive.1 Restrictive endoscopic bariatric therapies (EBTs) include intragastric balloons (IGB) that are filled with saline or gas to decrease intragastric luminal size, endoscopic sleeve gastroplasty that makes full-thickness plications of the gastric wall to tubularize the stomach like a sleeve gastrectomy, and AspireAssist where patients use a percutaneous gastrostomy to remove part of an ingested meal.1 Malabsorptive procedures include bypass sleeves that use a stentlike device to bypass absorption of food in the duodenum and proximal jejunum, the incisionless magnetic anastomosis system (IMAS) that creates a gastrojejunal bypass for diverting absorption, and duodenal mucosal resurfacing (DMR) that ablates the duodenal mucosa.1,2
The benefits of EBTs over traditional bariatric surgery are that they have a lower risk profile, there is limited anatomic alternation, and they are potentially reversible.1 Although no formal guidelines exist in the United States for the use of EBTs, the American Society for Gastrointestinal Endoscopy (ASGE) preliminary recommendations describe EBTs as applicable for patients who have failed lifestyle interventions and have BMIs between 30 and 45.1 While some of these techniques were first described in the 1980s, many individual companies and devices still do not have Food and Drug Administration approval and some have even had approval withdrawn. While traditional bariatric surgery may have complication rates up to 17%, EBTs are not without complications.1 Endoscopic barriers can migrate and occlude, cause pancreatitis, cause liver abscesses from biliary occlusion, and more severely cause GI bleeding and perforations.1 Many EBTs are also temporary treatments with IGBs and barrier bypasses placed only for 6-12 months.1 While there have been some studies looking at individual outcomes of the various EBTs, large prospective research trials looking at safety and efficacy, especially when comparing EBT to traditional bariatric surgery or in combination, are lacking.
Continued innovation in medicine and technology is critical to improving patient care. New innovations in medicine have allowed us to treat more disease, save lives, reduce complications, and better care for patients. But what exactly is innovation and when does it become research? The landmark Belmont Report in 1979 distinguishes research from innovative therapy, calling research “an activity designed to test a hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge.”3 Patients in research thus bear the risks while others stand to benefit. The report affirms then that routine medical practice involves interventions designed specifically to benefit the individual patient. The European Association for Endoscopic Surgery defines innovations as any “significant modification of a standard technique, a new application of or new indication for an established technique, or an alternative combination of an established technique with another therapeutic modality.”4 As such, innovations should eventually be formally studied with institutional review board (IRB) approval and protocols to establish safety and efficacy. Another complicating factor is that there is no FDA approval for surgical and procedural techniques as there is for medications and certain devices. Therefore, no robust regulatory mechanisms exist to ensure patient safety and benefit. Further complicating matters is that innovative procedures often start as modifications of techniques and are often done regularly to fit specific situations – for example, an additional stitch in a different location or in a different orientation to what is done in the standard fashion. However, true innovations should be distinguished from these modifications. Perhaps then another way to think about the two is to splinter them into three types of activity: research, routine accepted practice, and innovative medicine.5
Given this potential for blurred lines about novel approaches to medical conditions, how do we communicate this to patients? This is where the role of informed consent becomes essential. Informed consent is key to respecting patients’ autonomy – a central tenet of medical ethics. For patients to make autonomous choices they need basic facts to make informed decisions.6 These facts must be unbiased and free from conflicts, and they must not only be truthful but also be comprehensive and free from omission. It is in this informed consent process that we must explain that a technique or procedure is new, outline the risks and benefits, and share our actual experiences with said procedure especially if it is limited.7 We must also be aware of how certain biases and conflicts can affect our decisions to adapt and recommend innovative therapies. We may have incentives to offer innovative therapies to be on the “cutting edge” and attract patients. We may have explicit financial gain if working directly with device manufacturers or reimbursed by our institutions per procedure. Conflicts of interest are not only financial, but they can also be the prospects of promotion or career advancement.3 Institutions as well are incentivized to advertise the “latest” to bolster their prestige and reputations. Ultimately, we should act to the highest levels of professionalism, and ethics, by ignoring benefit to ourselves as physicians and always focusing on the benefits for our patients.7
What about when patients ask for specific innovative procedures as Mrs. Jones did above? What is our responsibility then? In situations where patients specifically push for a new procedure, it remains our duty to inform patients about the novelty of the procedure and the limited study of its safety and efficacy. When speaking about the “experience” with a novel procedure, it is tempting to speak globally and broadly. For example, Dr. Indo spoke about the procedure being done hundreds of times across the country and being safe in this context. It is our duty to be transparent, disclose our own experiences, and consider our own skills when recommending a novel procedure.7 It should be noted that patients are a vulnerable population and many times at the mercy of our recommendations. We’ve often heard patients say “Whatever you say doc; You’re the doctor;” or “I’ll do what you think is best” when presented with treatment options. This is an incredible amount of power, and we must protect this trust patients place in us by clearly acknowledging the uncertainties of new procedures and placing their benefit over our own potential gain.
Dr. Williams is a general surgery resident at the University of Chicago and a fellow at the MacLean Center for clinical medical ethics. Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, vice chairman for ethics, professional development, and wellness, and chief of endocrine surgery, department of surgery, and the associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago. The authors have no conflicts to disclose.
References
1. Goyal H et al. Ther Adv Gastrointest Endosc. 2021;14. doi: 10.1177/2631774520984627.
2. Machytka E et al. Gastrointestinal Endosc. 2017;86(5):904-12. doi: 10.1016/j.gie.2017.07.009.
3. Eastwood GL. J Gastroenterol Hepatol (Australia). 2015;30(S1):8-11. doi: 10.1111/jgh.12755.
4. Neugebauer EAM et al. Surg Endosc. 2010;24(7):1594-1615. doi: 10.1007/s00464-009-0818-3.
5. Eaton, ML and Kennedy, DL. Innovation in Medical Technology: Ethical Issues and Challenges. Baltimore: Johns Hopkins University Press, 2007.
6. Angelos P. Ann Thorac Surg. 2019;108(6):1611-2. doi: 10.1016/j.athoracsur.2019.08.010.
7. Angelos P. Virtual Mentor. 2011;13(1):6-9. doi: 10.1001/virtualmentor.2011.13.1.ccas1-1101.
Mrs. Jones is a 44-year-old woman who has struggled with her weight. She has a body mass index (BMI) of 35 kg/m2 and hypertension requiring daily medication. She has tried various diets over the years and has never been able to exercise consistently. She desperately wants to lose weight to improve her confidence and to avoid developing diabetes and dialysis that her parents required. She has considered weight loss surgery but is afraid after her best friend died following uterine fibroid surgery. She saw a billboard that advertised a new weight loss procedure without surgery. She looked up the procedure, found Dr. Indo on the university medical center’s website, and booked an appointment. Dr. Indo talked about performing an incisionless procedure done with an endoscope through her mouth. It would make her stomach into a tube to reduce the amount of food she could eat as well as prevent some absorption of food in her intestines. When Mrs. Jones asked how many of these the doctor had performed, Dr. Indo remarked she personally had done “several” in the past few years including training. Dr. Indo reassured Mrs. Jones that the procedure has been performed hundreds of times around the country and has been shown to be safe. Dr. Indo also explained that studies were still ongoing, including possibly at the university medical center, but that she had never personally seen any serious complications or death, and only one patient she knew of converted to a traditional bariatric surgery.
Obesity is a large international public health problem, with the World Health Organization estimating that there are 600 million obese adults worldwide.1 Bariatric surgery has been an effective way to improve complications related to obesity and quality of life. Endoscopic approaches to bariatric surgery have appeared since at least the late 1980s and, similarly to their traditional surgical counterparts, work in two main categories: restrictive or malabsorptive.1 Restrictive endoscopic bariatric therapies (EBTs) include intragastric balloons (IGB) that are filled with saline or gas to decrease intragastric luminal size, endoscopic sleeve gastroplasty that makes full-thickness plications of the gastric wall to tubularize the stomach like a sleeve gastrectomy, and AspireAssist where patients use a percutaneous gastrostomy to remove part of an ingested meal.1 Malabsorptive procedures include bypass sleeves that use a stentlike device to bypass absorption of food in the duodenum and proximal jejunum, the incisionless magnetic anastomosis system (IMAS) that creates a gastrojejunal bypass for diverting absorption, and duodenal mucosal resurfacing (DMR) that ablates the duodenal mucosa.1,2
The benefits of EBTs over traditional bariatric surgery are that they have a lower risk profile, there is limited anatomic alternation, and they are potentially reversible.1 Although no formal guidelines exist in the United States for the use of EBTs, the American Society for Gastrointestinal Endoscopy (ASGE) preliminary recommendations describe EBTs as applicable for patients who have failed lifestyle interventions and have BMIs between 30 and 45.1 While some of these techniques were first described in the 1980s, many individual companies and devices still do not have Food and Drug Administration approval and some have even had approval withdrawn. While traditional bariatric surgery may have complication rates up to 17%, EBTs are not without complications.1 Endoscopic barriers can migrate and occlude, cause pancreatitis, cause liver abscesses from biliary occlusion, and more severely cause GI bleeding and perforations.1 Many EBTs are also temporary treatments with IGBs and barrier bypasses placed only for 6-12 months.1 While there have been some studies looking at individual outcomes of the various EBTs, large prospective research trials looking at safety and efficacy, especially when comparing EBT to traditional bariatric surgery or in combination, are lacking.
Continued innovation in medicine and technology is critical to improving patient care. New innovations in medicine have allowed us to treat more disease, save lives, reduce complications, and better care for patients. But what exactly is innovation and when does it become research? The landmark Belmont Report in 1979 distinguishes research from innovative therapy, calling research “an activity designed to test a hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge.”3 Patients in research thus bear the risks while others stand to benefit. The report affirms then that routine medical practice involves interventions designed specifically to benefit the individual patient. The European Association for Endoscopic Surgery defines innovations as any “significant modification of a standard technique, a new application of or new indication for an established technique, or an alternative combination of an established technique with another therapeutic modality.”4 As such, innovations should eventually be formally studied with institutional review board (IRB) approval and protocols to establish safety and efficacy. Another complicating factor is that there is no FDA approval for surgical and procedural techniques as there is for medications and certain devices. Therefore, no robust regulatory mechanisms exist to ensure patient safety and benefit. Further complicating matters is that innovative procedures often start as modifications of techniques and are often done regularly to fit specific situations – for example, an additional stitch in a different location or in a different orientation to what is done in the standard fashion. However, true innovations should be distinguished from these modifications. Perhaps then another way to think about the two is to splinter them into three types of activity: research, routine accepted practice, and innovative medicine.5
Given this potential for blurred lines about novel approaches to medical conditions, how do we communicate this to patients? This is where the role of informed consent becomes essential. Informed consent is key to respecting patients’ autonomy – a central tenet of medical ethics. For patients to make autonomous choices they need basic facts to make informed decisions.6 These facts must be unbiased and free from conflicts, and they must not only be truthful but also be comprehensive and free from omission. It is in this informed consent process that we must explain that a technique or procedure is new, outline the risks and benefits, and share our actual experiences with said procedure especially if it is limited.7 We must also be aware of how certain biases and conflicts can affect our decisions to adapt and recommend innovative therapies. We may have incentives to offer innovative therapies to be on the “cutting edge” and attract patients. We may have explicit financial gain if working directly with device manufacturers or reimbursed by our institutions per procedure. Conflicts of interest are not only financial, but they can also be the prospects of promotion or career advancement.3 Institutions as well are incentivized to advertise the “latest” to bolster their prestige and reputations. Ultimately, we should act to the highest levels of professionalism, and ethics, by ignoring benefit to ourselves as physicians and always focusing on the benefits for our patients.7
What about when patients ask for specific innovative procedures as Mrs. Jones did above? What is our responsibility then? In situations where patients specifically push for a new procedure, it remains our duty to inform patients about the novelty of the procedure and the limited study of its safety and efficacy. When speaking about the “experience” with a novel procedure, it is tempting to speak globally and broadly. For example, Dr. Indo spoke about the procedure being done hundreds of times across the country and being safe in this context. It is our duty to be transparent, disclose our own experiences, and consider our own skills when recommending a novel procedure.7 It should be noted that patients are a vulnerable population and many times at the mercy of our recommendations. We’ve often heard patients say “Whatever you say doc; You’re the doctor;” or “I’ll do what you think is best” when presented with treatment options. This is an incredible amount of power, and we must protect this trust patients place in us by clearly acknowledging the uncertainties of new procedures and placing their benefit over our own potential gain.
Dr. Williams is a general surgery resident at the University of Chicago and a fellow at the MacLean Center for clinical medical ethics. Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, vice chairman for ethics, professional development, and wellness, and chief of endocrine surgery, department of surgery, and the associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago. The authors have no conflicts to disclose.
References
1. Goyal H et al. Ther Adv Gastrointest Endosc. 2021;14. doi: 10.1177/2631774520984627.
2. Machytka E et al. Gastrointestinal Endosc. 2017;86(5):904-12. doi: 10.1016/j.gie.2017.07.009.
3. Eastwood GL. J Gastroenterol Hepatol (Australia). 2015;30(S1):8-11. doi: 10.1111/jgh.12755.
4. Neugebauer EAM et al. Surg Endosc. 2010;24(7):1594-1615. doi: 10.1007/s00464-009-0818-3.
5. Eaton, ML and Kennedy, DL. Innovation in Medical Technology: Ethical Issues and Challenges. Baltimore: Johns Hopkins University Press, 2007.
6. Angelos P. Ann Thorac Surg. 2019;108(6):1611-2. doi: 10.1016/j.athoracsur.2019.08.010.
7. Angelos P. Virtual Mentor. 2011;13(1):6-9. doi: 10.1001/virtualmentor.2011.13.1.ccas1-1101.
Mrs. Jones is a 44-year-old woman who has struggled with her weight. She has a body mass index (BMI) of 35 kg/m2 and hypertension requiring daily medication. She has tried various diets over the years and has never been able to exercise consistently. She desperately wants to lose weight to improve her confidence and to avoid developing diabetes and dialysis that her parents required. She has considered weight loss surgery but is afraid after her best friend died following uterine fibroid surgery. She saw a billboard that advertised a new weight loss procedure without surgery. She looked up the procedure, found Dr. Indo on the university medical center’s website, and booked an appointment. Dr. Indo talked about performing an incisionless procedure done with an endoscope through her mouth. It would make her stomach into a tube to reduce the amount of food she could eat as well as prevent some absorption of food in her intestines. When Mrs. Jones asked how many of these the doctor had performed, Dr. Indo remarked she personally had done “several” in the past few years including training. Dr. Indo reassured Mrs. Jones that the procedure has been performed hundreds of times around the country and has been shown to be safe. Dr. Indo also explained that studies were still ongoing, including possibly at the university medical center, but that she had never personally seen any serious complications or death, and only one patient she knew of converted to a traditional bariatric surgery.
Obesity is a large international public health problem, with the World Health Organization estimating that there are 600 million obese adults worldwide.1 Bariatric surgery has been an effective way to improve complications related to obesity and quality of life. Endoscopic approaches to bariatric surgery have appeared since at least the late 1980s and, similarly to their traditional surgical counterparts, work in two main categories: restrictive or malabsorptive.1 Restrictive endoscopic bariatric therapies (EBTs) include intragastric balloons (IGB) that are filled with saline or gas to decrease intragastric luminal size, endoscopic sleeve gastroplasty that makes full-thickness plications of the gastric wall to tubularize the stomach like a sleeve gastrectomy, and AspireAssist where patients use a percutaneous gastrostomy to remove part of an ingested meal.1 Malabsorptive procedures include bypass sleeves that use a stentlike device to bypass absorption of food in the duodenum and proximal jejunum, the incisionless magnetic anastomosis system (IMAS) that creates a gastrojejunal bypass for diverting absorption, and duodenal mucosal resurfacing (DMR) that ablates the duodenal mucosa.1,2
The benefits of EBTs over traditional bariatric surgery are that they have a lower risk profile, there is limited anatomic alternation, and they are potentially reversible.1 Although no formal guidelines exist in the United States for the use of EBTs, the American Society for Gastrointestinal Endoscopy (ASGE) preliminary recommendations describe EBTs as applicable for patients who have failed lifestyle interventions and have BMIs between 30 and 45.1 While some of these techniques were first described in the 1980s, many individual companies and devices still do not have Food and Drug Administration approval and some have even had approval withdrawn. While traditional bariatric surgery may have complication rates up to 17%, EBTs are not without complications.1 Endoscopic barriers can migrate and occlude, cause pancreatitis, cause liver abscesses from biliary occlusion, and more severely cause GI bleeding and perforations.1 Many EBTs are also temporary treatments with IGBs and barrier bypasses placed only for 6-12 months.1 While there have been some studies looking at individual outcomes of the various EBTs, large prospective research trials looking at safety and efficacy, especially when comparing EBT to traditional bariatric surgery or in combination, are lacking.
Continued innovation in medicine and technology is critical to improving patient care. New innovations in medicine have allowed us to treat more disease, save lives, reduce complications, and better care for patients. But what exactly is innovation and when does it become research? The landmark Belmont Report in 1979 distinguishes research from innovative therapy, calling research “an activity designed to test a hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge.”3 Patients in research thus bear the risks while others stand to benefit. The report affirms then that routine medical practice involves interventions designed specifically to benefit the individual patient. The European Association for Endoscopic Surgery defines innovations as any “significant modification of a standard technique, a new application of or new indication for an established technique, or an alternative combination of an established technique with another therapeutic modality.”4 As such, innovations should eventually be formally studied with institutional review board (IRB) approval and protocols to establish safety and efficacy. Another complicating factor is that there is no FDA approval for surgical and procedural techniques as there is for medications and certain devices. Therefore, no robust regulatory mechanisms exist to ensure patient safety and benefit. Further complicating matters is that innovative procedures often start as modifications of techniques and are often done regularly to fit specific situations – for example, an additional stitch in a different location or in a different orientation to what is done in the standard fashion. However, true innovations should be distinguished from these modifications. Perhaps then another way to think about the two is to splinter them into three types of activity: research, routine accepted practice, and innovative medicine.5
Given this potential for blurred lines about novel approaches to medical conditions, how do we communicate this to patients? This is where the role of informed consent becomes essential. Informed consent is key to respecting patients’ autonomy – a central tenet of medical ethics. For patients to make autonomous choices they need basic facts to make informed decisions.6 These facts must be unbiased and free from conflicts, and they must not only be truthful but also be comprehensive and free from omission. It is in this informed consent process that we must explain that a technique or procedure is new, outline the risks and benefits, and share our actual experiences with said procedure especially if it is limited.7 We must also be aware of how certain biases and conflicts can affect our decisions to adapt and recommend innovative therapies. We may have incentives to offer innovative therapies to be on the “cutting edge” and attract patients. We may have explicit financial gain if working directly with device manufacturers or reimbursed by our institutions per procedure. Conflicts of interest are not only financial, but they can also be the prospects of promotion or career advancement.3 Institutions as well are incentivized to advertise the “latest” to bolster their prestige and reputations. Ultimately, we should act to the highest levels of professionalism, and ethics, by ignoring benefit to ourselves as physicians and always focusing on the benefits for our patients.7
What about when patients ask for specific innovative procedures as Mrs. Jones did above? What is our responsibility then? In situations where patients specifically push for a new procedure, it remains our duty to inform patients about the novelty of the procedure and the limited study of its safety and efficacy. When speaking about the “experience” with a novel procedure, it is tempting to speak globally and broadly. For example, Dr. Indo spoke about the procedure being done hundreds of times across the country and being safe in this context. It is our duty to be transparent, disclose our own experiences, and consider our own skills when recommending a novel procedure.7 It should be noted that patients are a vulnerable population and many times at the mercy of our recommendations. We’ve often heard patients say “Whatever you say doc; You’re the doctor;” or “I’ll do what you think is best” when presented with treatment options. This is an incredible amount of power, and we must protect this trust patients place in us by clearly acknowledging the uncertainties of new procedures and placing their benefit over our own potential gain.
Dr. Williams is a general surgery resident at the University of Chicago and a fellow at the MacLean Center for clinical medical ethics. Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, vice chairman for ethics, professional development, and wellness, and chief of endocrine surgery, department of surgery, and the associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago. The authors have no conflicts to disclose.
References
1. Goyal H et al. Ther Adv Gastrointest Endosc. 2021;14. doi: 10.1177/2631774520984627.
2. Machytka E et al. Gastrointestinal Endosc. 2017;86(5):904-12. doi: 10.1016/j.gie.2017.07.009.
3. Eastwood GL. J Gastroenterol Hepatol (Australia). 2015;30(S1):8-11. doi: 10.1111/jgh.12755.
4. Neugebauer EAM et al. Surg Endosc. 2010;24(7):1594-1615. doi: 10.1007/s00464-009-0818-3.
5. Eaton, ML and Kennedy, DL. Innovation in Medical Technology: Ethical Issues and Challenges. Baltimore: Johns Hopkins University Press, 2007.
6. Angelos P. Ann Thorac Surg. 2019;108(6):1611-2. doi: 10.1016/j.athoracsur.2019.08.010.
7. Angelos P. Virtual Mentor. 2011;13(1):6-9. doi: 10.1001/virtualmentor.2011.13.1.ccas1-1101.
ACTRIMS 2022: Updates in Multiple Sclerosis Symptom Management
Dr Enrique Alvarez, Associate Professor at the University of Colorado, reviews updates in symptom management that were presented at the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) 2022 meeting.
First, Dr Alvarez highlights two studies of nabiximols — a complex botanical mixture of tetrahydrocannabinol and cannabidiol — in patients with multiple sclerosis (MS). In both the GWSP0604 and SAVANT studies, patients taking nabiximols demonstrated significant spasticity improvement and reductions in spasm frequency.
Next, Dr Alvarez shares study results that compared patient responses to the responses of healthcare practitioners (HCPs) treating these patients for their MS. This analysis, which focused on cases of fatigue, mood, and cognition, found that patients reported significantly higher rates of these symptoms compared with HCP responses.
Another study assessed the importance of shared decision-making between HCPs and patients with MS, drawing from MEDLINE, EMBASE, and CINAHL databases. The researchers identified apparent challenges in patient education and access to information and recommended that shared decision-making be integrated into routine practice.
Dr Alvarez concludes with a review of new resources launched by the National Multiple Sclerosis Society, the goal of which is to inform and empower patients about dietary approaches for self-management and to support clinicians who are facilitating related discussions with their patients.
--
Enrique Alvarez, MD, PhD, Vice Chair of Clinical Research, Associate Professor, Department of Neurology, Division Neuroimmunology, University of Colorado, Rocky Mountain MS Center Anschutz Medical Center, Aurora, Colorado
Enrique Alvarez, MD, PhD, has disclosed the following relevant financial relationships:
Received research grant from: Biogen; Genentech/Roche; Novartis; TG Therapeutics; Patient-Centered Outcomes Research Initiative; National Multiple Sclerosis Society; National Institutes of Health; Rocky Mountain MS Center
Received income in an amount equal to or greater than $250 from: Actelion
Dr Enrique Alvarez, Associate Professor at the University of Colorado, reviews updates in symptom management that were presented at the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) 2022 meeting.
First, Dr Alvarez highlights two studies of nabiximols — a complex botanical mixture of tetrahydrocannabinol and cannabidiol — in patients with multiple sclerosis (MS). In both the GWSP0604 and SAVANT studies, patients taking nabiximols demonstrated significant spasticity improvement and reductions in spasm frequency.
Next, Dr Alvarez shares study results that compared patient responses to the responses of healthcare practitioners (HCPs) treating these patients for their MS. This analysis, which focused on cases of fatigue, mood, and cognition, found that patients reported significantly higher rates of these symptoms compared with HCP responses.
Another study assessed the importance of shared decision-making between HCPs and patients with MS, drawing from MEDLINE, EMBASE, and CINAHL databases. The researchers identified apparent challenges in patient education and access to information and recommended that shared decision-making be integrated into routine practice.
Dr Alvarez concludes with a review of new resources launched by the National Multiple Sclerosis Society, the goal of which is to inform and empower patients about dietary approaches for self-management and to support clinicians who are facilitating related discussions with their patients.
--
Enrique Alvarez, MD, PhD, Vice Chair of Clinical Research, Associate Professor, Department of Neurology, Division Neuroimmunology, University of Colorado, Rocky Mountain MS Center Anschutz Medical Center, Aurora, Colorado
Enrique Alvarez, MD, PhD, has disclosed the following relevant financial relationships:
Received research grant from: Biogen; Genentech/Roche; Novartis; TG Therapeutics; Patient-Centered Outcomes Research Initiative; National Multiple Sclerosis Society; National Institutes of Health; Rocky Mountain MS Center
Received income in an amount equal to or greater than $250 from: Actelion
Dr Enrique Alvarez, Associate Professor at the University of Colorado, reviews updates in symptom management that were presented at the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) 2022 meeting.
First, Dr Alvarez highlights two studies of nabiximols — a complex botanical mixture of tetrahydrocannabinol and cannabidiol — in patients with multiple sclerosis (MS). In both the GWSP0604 and SAVANT studies, patients taking nabiximols demonstrated significant spasticity improvement and reductions in spasm frequency.
Next, Dr Alvarez shares study results that compared patient responses to the responses of healthcare practitioners (HCPs) treating these patients for their MS. This analysis, which focused on cases of fatigue, mood, and cognition, found that patients reported significantly higher rates of these symptoms compared with HCP responses.
Another study assessed the importance of shared decision-making between HCPs and patients with MS, drawing from MEDLINE, EMBASE, and CINAHL databases. The researchers identified apparent challenges in patient education and access to information and recommended that shared decision-making be integrated into routine practice.
Dr Alvarez concludes with a review of new resources launched by the National Multiple Sclerosis Society, the goal of which is to inform and empower patients about dietary approaches for self-management and to support clinicians who are facilitating related discussions with their patients.
--
Enrique Alvarez, MD, PhD, Vice Chair of Clinical Research, Associate Professor, Department of Neurology, Division Neuroimmunology, University of Colorado, Rocky Mountain MS Center Anschutz Medical Center, Aurora, Colorado
Enrique Alvarez, MD, PhD, has disclosed the following relevant financial relationships:
Received research grant from: Biogen; Genentech/Roche; Novartis; TG Therapeutics; Patient-Centered Outcomes Research Initiative; National Multiple Sclerosis Society; National Institutes of Health; Rocky Mountain MS Center
Received income in an amount equal to or greater than $250 from: Actelion

Aerosolized hydrogen peroxide can significantly reduce C. difficile infections
Aerosolized hydrogen peroxide (aHP) can significantly reduce Clostridioides difficile infection (CDI) and is an effective disinfection system, suggests a study published in the American Journal of Infection Control.
C. difficile is the most common cause of health care–associated infection and increasingly occurs outside acute care hospitals. CDI symptoms can range from mild diarrhea to life-threatening colitis and sepsis, sometimes requiring urgent colon removal.
The Centers for Disease Control and Prevention has reported that, in the United States, 223,900 people required hospitalization for CDI and at least 12,800 died in 2017. Because of its large toll, CDI is grouped with antimicrobial-resistant “threat” organisms that often accompany it. People older than age 65 are at particular risk for disease, and at least 20% of patients experience recurrence.
In health care facilities, C. difficile is transmitted by bacterial spores that readily contaminate surfaces in patients’ rooms, from handrails to bedside tables to light switches and knobs. The spores are resistant to disinfectants, and rooms are often cleaned with bleach solutions. But those bleach fumes are irritating and may cause bronchospasm for patients with asthma or chronic obstructive pulmonary disease, and so alternative cleaning agents are needed.
In a retrospective study of an acute-care facility in Philadelphia, researchers compared the incidence of health care–associated CDI (HA-CDI) at the facility before and after adding aHP to other infection control practices. The aHP process produces an aerosolized dry-mist fog that contains a specified percentage of hydrogen peroxide. The fog is used after the room has been physically cleaned, settling on exposed surfaces and killing any remaining C. difficile spores.
The aHP mixture also contains 0.01% ionic silver. The study lead was Christopher L. Truitt, PhD, of Wayland Baptist University. Dr. Truitt told this news organization that hydrogen peroxide affects the endospore layer of the C. difficile organism and allows the “ionic silver to get into the cell and is shown to bind to enzymes and inactivate those inside the cell and actually improve the efficacy.”
Asked whether it’s the silver or the peroxide that disinfects, Dr. Truitt replied: “I can’t answer that. We don’t know if it’s the silver or the hydrogen peroxide. The commercially available chemical that’s used in that machine is a proprietary set-up ... with EPA approval as a sporicidal.”
In the baseline 27-month period, the hospital tallied 120 HA-CDI cases. After aHP was introduced, they counted just 72 cases over 33 months, a 41% decrease in the facility’s HA-CDI rate, from 4.6 per 10,000 patient-days to 2.7 per 10,000 patient-days (P < .001).
There was also a progressive decrease in hospital-onset CDI even after aHP was introduced, from 5.4 per 10,000 patient-days in 2015 to 1.4 per 10,000 patient-days in 2019.
Yoav Golan, MD, of Tufts University, Boston, told this news organization there were two major study limitations. “One is the fact that they did not control for other interventions that may have an effect on C. difficile: antibiotic stewardship and infection control,” he explained. This limitation was noted by the study authors and may explain the continued decline in infections after the introduction of aHP. The other limitation was not using a crossover study design.
“I would argue that they should have provided a little more information about their own practices in their own hospital when it comes to intensification of infection control [and] when it comes to a stewardship and changes that they’ve made to antibiotic usage,” Dr. Golan continued. “The description of changes over time and those practices would have allowed us to better understand the impact of the hydrogen peroxide intervention.”
Despite his criticisms, Dr. Golan concluded: “I think that the study is important. I think their intervention is unique in a way that they’ve been using an aerosolizing system that’s using a relatively high concentration of hydrogen peroxide. I think that there’s enough in this paper to suggest that using such a system may have an impact on the environment, and through that, on dissemination.”
Dr. Truitt added that a next step would be to compare aHP with ultraviolet light, which is commonly used to disinfect hospital rooms.
Dr. Truitt is chief science officer at Infection Controls, dba Germblast, a proprietary service that uses cold-mist hydrogen peroxide and other modalities to disinfect surfaces. Dr. Golan has reported being a consultant for Merck, Seres Therapeutics, Vedanta Biosciences, and Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Aerosolized hydrogen peroxide (aHP) can significantly reduce Clostridioides difficile infection (CDI) and is an effective disinfection system, suggests a study published in the American Journal of Infection Control.
C. difficile is the most common cause of health care–associated infection and increasingly occurs outside acute care hospitals. CDI symptoms can range from mild diarrhea to life-threatening colitis and sepsis, sometimes requiring urgent colon removal.
The Centers for Disease Control and Prevention has reported that, in the United States, 223,900 people required hospitalization for CDI and at least 12,800 died in 2017. Because of its large toll, CDI is grouped with antimicrobial-resistant “threat” organisms that often accompany it. People older than age 65 are at particular risk for disease, and at least 20% of patients experience recurrence.
In health care facilities, C. difficile is transmitted by bacterial spores that readily contaminate surfaces in patients’ rooms, from handrails to bedside tables to light switches and knobs. The spores are resistant to disinfectants, and rooms are often cleaned with bleach solutions. But those bleach fumes are irritating and may cause bronchospasm for patients with asthma or chronic obstructive pulmonary disease, and so alternative cleaning agents are needed.
In a retrospective study of an acute-care facility in Philadelphia, researchers compared the incidence of health care–associated CDI (HA-CDI) at the facility before and after adding aHP to other infection control practices. The aHP process produces an aerosolized dry-mist fog that contains a specified percentage of hydrogen peroxide. The fog is used after the room has been physically cleaned, settling on exposed surfaces and killing any remaining C. difficile spores.
The aHP mixture also contains 0.01% ionic silver. The study lead was Christopher L. Truitt, PhD, of Wayland Baptist University. Dr. Truitt told this news organization that hydrogen peroxide affects the endospore layer of the C. difficile organism and allows the “ionic silver to get into the cell and is shown to bind to enzymes and inactivate those inside the cell and actually improve the efficacy.”
Asked whether it’s the silver or the peroxide that disinfects, Dr. Truitt replied: “I can’t answer that. We don’t know if it’s the silver or the hydrogen peroxide. The commercially available chemical that’s used in that machine is a proprietary set-up ... with EPA approval as a sporicidal.”
In the baseline 27-month period, the hospital tallied 120 HA-CDI cases. After aHP was introduced, they counted just 72 cases over 33 months, a 41% decrease in the facility’s HA-CDI rate, from 4.6 per 10,000 patient-days to 2.7 per 10,000 patient-days (P < .001).
There was also a progressive decrease in hospital-onset CDI even after aHP was introduced, from 5.4 per 10,000 patient-days in 2015 to 1.4 per 10,000 patient-days in 2019.
Yoav Golan, MD, of Tufts University, Boston, told this news organization there were two major study limitations. “One is the fact that they did not control for other interventions that may have an effect on C. difficile: antibiotic stewardship and infection control,” he explained. This limitation was noted by the study authors and may explain the continued decline in infections after the introduction of aHP. The other limitation was not using a crossover study design.
“I would argue that they should have provided a little more information about their own practices in their own hospital when it comes to intensification of infection control [and] when it comes to a stewardship and changes that they’ve made to antibiotic usage,” Dr. Golan continued. “The description of changes over time and those practices would have allowed us to better understand the impact of the hydrogen peroxide intervention.”
Despite his criticisms, Dr. Golan concluded: “I think that the study is important. I think their intervention is unique in a way that they’ve been using an aerosolizing system that’s using a relatively high concentration of hydrogen peroxide. I think that there’s enough in this paper to suggest that using such a system may have an impact on the environment, and through that, on dissemination.”
Dr. Truitt added that a next step would be to compare aHP with ultraviolet light, which is commonly used to disinfect hospital rooms.
Dr. Truitt is chief science officer at Infection Controls, dba Germblast, a proprietary service that uses cold-mist hydrogen peroxide and other modalities to disinfect surfaces. Dr. Golan has reported being a consultant for Merck, Seres Therapeutics, Vedanta Biosciences, and Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Aerosolized hydrogen peroxide (aHP) can significantly reduce Clostridioides difficile infection (CDI) and is an effective disinfection system, suggests a study published in the American Journal of Infection Control.
C. difficile is the most common cause of health care–associated infection and increasingly occurs outside acute care hospitals. CDI symptoms can range from mild diarrhea to life-threatening colitis and sepsis, sometimes requiring urgent colon removal.
The Centers for Disease Control and Prevention has reported that, in the United States, 223,900 people required hospitalization for CDI and at least 12,800 died in 2017. Because of its large toll, CDI is grouped with antimicrobial-resistant “threat” organisms that often accompany it. People older than age 65 are at particular risk for disease, and at least 20% of patients experience recurrence.
In health care facilities, C. difficile is transmitted by bacterial spores that readily contaminate surfaces in patients’ rooms, from handrails to bedside tables to light switches and knobs. The spores are resistant to disinfectants, and rooms are often cleaned with bleach solutions. But those bleach fumes are irritating and may cause bronchospasm for patients with asthma or chronic obstructive pulmonary disease, and so alternative cleaning agents are needed.
In a retrospective study of an acute-care facility in Philadelphia, researchers compared the incidence of health care–associated CDI (HA-CDI) at the facility before and after adding aHP to other infection control practices. The aHP process produces an aerosolized dry-mist fog that contains a specified percentage of hydrogen peroxide. The fog is used after the room has been physically cleaned, settling on exposed surfaces and killing any remaining C. difficile spores.
The aHP mixture also contains 0.01% ionic silver. The study lead was Christopher L. Truitt, PhD, of Wayland Baptist University. Dr. Truitt told this news organization that hydrogen peroxide affects the endospore layer of the C. difficile organism and allows the “ionic silver to get into the cell and is shown to bind to enzymes and inactivate those inside the cell and actually improve the efficacy.”
Asked whether it’s the silver or the peroxide that disinfects, Dr. Truitt replied: “I can’t answer that. We don’t know if it’s the silver or the hydrogen peroxide. The commercially available chemical that’s used in that machine is a proprietary set-up ... with EPA approval as a sporicidal.”
In the baseline 27-month period, the hospital tallied 120 HA-CDI cases. After aHP was introduced, they counted just 72 cases over 33 months, a 41% decrease in the facility’s HA-CDI rate, from 4.6 per 10,000 patient-days to 2.7 per 10,000 patient-days (P < .001).
There was also a progressive decrease in hospital-onset CDI even after aHP was introduced, from 5.4 per 10,000 patient-days in 2015 to 1.4 per 10,000 patient-days in 2019.
Yoav Golan, MD, of Tufts University, Boston, told this news organization there were two major study limitations. “One is the fact that they did not control for other interventions that may have an effect on C. difficile: antibiotic stewardship and infection control,” he explained. This limitation was noted by the study authors and may explain the continued decline in infections after the introduction of aHP. The other limitation was not using a crossover study design.
“I would argue that they should have provided a little more information about their own practices in their own hospital when it comes to intensification of infection control [and] when it comes to a stewardship and changes that they’ve made to antibiotic usage,” Dr. Golan continued. “The description of changes over time and those practices would have allowed us to better understand the impact of the hydrogen peroxide intervention.”
Despite his criticisms, Dr. Golan concluded: “I think that the study is important. I think their intervention is unique in a way that they’ve been using an aerosolizing system that’s using a relatively high concentration of hydrogen peroxide. I think that there’s enough in this paper to suggest that using such a system may have an impact on the environment, and through that, on dissemination.”
Dr. Truitt added that a next step would be to compare aHP with ultraviolet light, which is commonly used to disinfect hospital rooms.
Dr. Truitt is chief science officer at Infection Controls, dba Germblast, a proprietary service that uses cold-mist hydrogen peroxide and other modalities to disinfect surfaces. Dr. Golan has reported being a consultant for Merck, Seres Therapeutics, Vedanta Biosciences, and Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM AMERICAN JOURNAL OF INFECTION CONTROL
‘Vast majority’ of COVID patients wake up after mechanical ventilation
COVID-19 patients who are successfully weaned off a ventilator may take days, or even weeks, to regain consciousness, especially those who experienced episodes of hypoxemia while intubated, a new study shows.
“As we started to see the first patients waking up after successful COVID-19 ICU treatments, we also encountered many patients who remained comatose for days and weeks and then regained consciousness to become fully oriented,” co-senior investigator Nicholas Schiff, MD, with NewYork-Presbyterian/Weill Cornell Medical Center, says in a news release.
The findings have immediate implications regarding life-sustaining therapies for unresponsive COVID-19 patients, the investigators note.
“In critical care medicine, one of our main tasks is to advise families about planning in the event a patient does not regain consciousness,” said co-senior author Jan Claassen, MD, with New York-Presbyterian/Columbia University Irving Medical Center.
“Our findings suggest that for patients with severe COVID, the decision to withdraw life support shouldn’t be based solely on prolonged periods of unconsciousness, as these patients may eventually recover,” Dr. Claassen adds.
The study was published online March 7 in Annals of Neurology.
Slow road back
The researchers examined 795 intubated patients with severe COVID-19 at three medical centers in New York during the first wave of the pandemic (March-July 2020). All patients had impaired consciousness (Glasgow Coma Scale [GCS] motor score less than 6) on day 7 of intubation.
A total of 571 patients (72%) survived and regained consciousness.
The median time to recovery of consciousness was 30 days. One-quarter of the patients recovered consciousness 10 days or longer after they stopped receiving ventilator support and 10% took 23 days or longer to recover.
Time to recovery of consciousness was associated with hypoxemia. The hazard ratio was 0.56 (95% confidence interval, 0.46-0.68) with arterial partial pressure of oxygen (PaO2) less than or equal to 55 mm Hg and 0.88 (95% CI, 0.85-0.91) with a PaO2 less than or equal to 70 mm Hg.
Each additional day of hypoxemia decreased the odds of recovery of consciousness after accounting for confounding factors including sedation.
These findings were confirmed among patients without any imaging evidence of structural brain injury and in a non-overlapping cohort of 427 patients from the second wave of the pandemic (October-April 2021).
“These findings provide us with more accurate information to guide families who are deciding whether to continue life-sustaining therapy in unconscious COVID-19 patients,” co-senior author Brian Edlow, MD, with Massachusetts General Hospital and Harvard Medical School in Boston, says in the news release.
“Encouragingly,” adds Dr. Claassen, “our study shows that the vast majority of unconscious COVID patients recover consciousness, but it is important to consider that we did not look at the quality of recovery. That’s something that should be the focus of long-term follow-up studies.”
The study was supported by the James S. McDonnell Foundation (JSMF). Dr. Schiff, Dr. Claassen, and Dr. Edlow have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 patients who are successfully weaned off a ventilator may take days, or even weeks, to regain consciousness, especially those who experienced episodes of hypoxemia while intubated, a new study shows.
“As we started to see the first patients waking up after successful COVID-19 ICU treatments, we also encountered many patients who remained comatose for days and weeks and then regained consciousness to become fully oriented,” co-senior investigator Nicholas Schiff, MD, with NewYork-Presbyterian/Weill Cornell Medical Center, says in a news release.
The findings have immediate implications regarding life-sustaining therapies for unresponsive COVID-19 patients, the investigators note.
“In critical care medicine, one of our main tasks is to advise families about planning in the event a patient does not regain consciousness,” said co-senior author Jan Claassen, MD, with New York-Presbyterian/Columbia University Irving Medical Center.
“Our findings suggest that for patients with severe COVID, the decision to withdraw life support shouldn’t be based solely on prolonged periods of unconsciousness, as these patients may eventually recover,” Dr. Claassen adds.
The study was published online March 7 in Annals of Neurology.
Slow road back
The researchers examined 795 intubated patients with severe COVID-19 at three medical centers in New York during the first wave of the pandemic (March-July 2020). All patients had impaired consciousness (Glasgow Coma Scale [GCS] motor score less than 6) on day 7 of intubation.
A total of 571 patients (72%) survived and regained consciousness.
The median time to recovery of consciousness was 30 days. One-quarter of the patients recovered consciousness 10 days or longer after they stopped receiving ventilator support and 10% took 23 days or longer to recover.
Time to recovery of consciousness was associated with hypoxemia. The hazard ratio was 0.56 (95% confidence interval, 0.46-0.68) with arterial partial pressure of oxygen (PaO2) less than or equal to 55 mm Hg and 0.88 (95% CI, 0.85-0.91) with a PaO2 less than or equal to 70 mm Hg.
Each additional day of hypoxemia decreased the odds of recovery of consciousness after accounting for confounding factors including sedation.
These findings were confirmed among patients without any imaging evidence of structural brain injury and in a non-overlapping cohort of 427 patients from the second wave of the pandemic (October-April 2021).
“These findings provide us with more accurate information to guide families who are deciding whether to continue life-sustaining therapy in unconscious COVID-19 patients,” co-senior author Brian Edlow, MD, with Massachusetts General Hospital and Harvard Medical School in Boston, says in the news release.
“Encouragingly,” adds Dr. Claassen, “our study shows that the vast majority of unconscious COVID patients recover consciousness, but it is important to consider that we did not look at the quality of recovery. That’s something that should be the focus of long-term follow-up studies.”
The study was supported by the James S. McDonnell Foundation (JSMF). Dr. Schiff, Dr. Claassen, and Dr. Edlow have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 patients who are successfully weaned off a ventilator may take days, or even weeks, to regain consciousness, especially those who experienced episodes of hypoxemia while intubated, a new study shows.
“As we started to see the first patients waking up after successful COVID-19 ICU treatments, we also encountered many patients who remained comatose for days and weeks and then regained consciousness to become fully oriented,” co-senior investigator Nicholas Schiff, MD, with NewYork-Presbyterian/Weill Cornell Medical Center, says in a news release.
The findings have immediate implications regarding life-sustaining therapies for unresponsive COVID-19 patients, the investigators note.
“In critical care medicine, one of our main tasks is to advise families about planning in the event a patient does not regain consciousness,” said co-senior author Jan Claassen, MD, with New York-Presbyterian/Columbia University Irving Medical Center.
“Our findings suggest that for patients with severe COVID, the decision to withdraw life support shouldn’t be based solely on prolonged periods of unconsciousness, as these patients may eventually recover,” Dr. Claassen adds.
The study was published online March 7 in Annals of Neurology.
Slow road back
The researchers examined 795 intubated patients with severe COVID-19 at three medical centers in New York during the first wave of the pandemic (March-July 2020). All patients had impaired consciousness (Glasgow Coma Scale [GCS] motor score less than 6) on day 7 of intubation.
A total of 571 patients (72%) survived and regained consciousness.
The median time to recovery of consciousness was 30 days. One-quarter of the patients recovered consciousness 10 days or longer after they stopped receiving ventilator support and 10% took 23 days or longer to recover.
Time to recovery of consciousness was associated with hypoxemia. The hazard ratio was 0.56 (95% confidence interval, 0.46-0.68) with arterial partial pressure of oxygen (PaO2) less than or equal to 55 mm Hg and 0.88 (95% CI, 0.85-0.91) with a PaO2 less than or equal to 70 mm Hg.
Each additional day of hypoxemia decreased the odds of recovery of consciousness after accounting for confounding factors including sedation.
These findings were confirmed among patients without any imaging evidence of structural brain injury and in a non-overlapping cohort of 427 patients from the second wave of the pandemic (October-April 2021).
“These findings provide us with more accurate information to guide families who are deciding whether to continue life-sustaining therapy in unconscious COVID-19 patients,” co-senior author Brian Edlow, MD, with Massachusetts General Hospital and Harvard Medical School in Boston, says in the news release.
“Encouragingly,” adds Dr. Claassen, “our study shows that the vast majority of unconscious COVID patients recover consciousness, but it is important to consider that we did not look at the quality of recovery. That’s something that should be the focus of long-term follow-up studies.”
The study was supported by the James S. McDonnell Foundation (JSMF). Dr. Schiff, Dr. Claassen, and Dr. Edlow have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF NEUROLOGY
Antiretroviral therapy associated with less risk of preterm birth
Over the past decade, data have suggested that antiretroviral therapy (ART) may be associated with an increased risk for adverse pregnancy outcomes, namely, preterm birth (PTB). But a combination of methodologic challenges, demographic gaps, and spotty clinical data has left the question unresolved, especially for pregnant women with HIV who reside in developed countries.
“Given that a lot of the emerging data has come out of resource-limited settings where patient and clinical characteristics are different from developed world settings like the United States, we felt that this was an important question to address,” Kartik Venkatesh, MD, PhD, a high-risk obstetrician and perinatal epidemiologist at the Ohio State Wexner Medical Center, Columbus, told this news organization.
In a prospective cohort study of U.S. women with or at risk for HIV, Dr. Venkatesh and his colleagues found that ART exposure (including highly active antiretroviral therapy [HAART]) was associated with as much as an 80% decline in the likelihood of PTB (defined as birth less than 34 weeks). The study was published in HIV Medicine.
24 years of data analyzed
Dr. Venkatesh and his team analyzed self-reported birth data of women with singleton live-born pregnancies enrolled in the ongoing, multicenter, prospective observational Women’s Interagency HIV Study (WIHS) from Oct. 1, 1995, to March 31, 2019.
“We first looked at women with HIV versus without HIV, [who were] matched on many clinical and sociodemographic characteristics and at similarly high risk of some of these obstetrical outcomes like PTB,” explained Dr. Venkatesh. “We then looked at the relative impact of antiretroviral therapy amongst women living with HIV compared to no antiretroviral therapy.”
ART regimens were classified as none, monotherapy, dual therapy, or HAART. (HAART was defined as more than three antiretrovirals, including at least one protease inhibitor [PI], nonnucleoside reverse transcriptase inhibitor, integrase inhibitor, or entry inhibitor.) In this cohort, for 63.5% of women receiving ART, therapy was initiated before pregnancy (mean duration of HAART, 6 years), and most were virally suppressed.
Among the 4,944 women assessed in the WIHS trial, 74% (3,646) had HIV. In total, 383 women had 488 singleton deliveries, including 218 women with HIV (272 deliveries) and 165 without HIV (216 deliveries). Sociodemographics in both cohorts were well matched. For most participants, the mean age was 40-41 years at delivery, most were non-Hispanic Black persons, and the mean pregnancy body mass index was greater than or equal to 29 kg/m2. Of the women with HIV, 33% had chronic hypertension; of those without HIV, 42.1% had chronic hypertension; 4.7% and 5.0%, respectively, had pregestational diabetes.
The findings showed that PTB risk less than 34 weeks was similar between women with (10%) and without (8%) HIV (adjusted risk ratio, 1.30; 95% confidence interval, 0.74-2.31). Among deliveries to women with HIV who were receiving ART, PTB risk less than 34 weeks was lower with HAART (7%), compared with not receiving ART (26%) (aRR, 0.19), as well as with monotherapy or dual therapy (3% vs. no ART) (aRR, 0.12). Notably, 67% of deliveries to women receiving HAART included a PI-containing regimen, but these women were not significantly more likely to have a PTB less than 34 weeks, compared with women taking non-PI HAART regimens (aRR, 2.61; 95% CI, 0.65-10.59). Results were similar for secondary outcomes (PTB less than 28 weeks, less than 37 weeks).
Filling in the gaps toward the safest regimen
“This study spans 25 years, so it covers a lot of the history of HIV in pregnancy and is reassuring around using ART in pregnancy,” Shahin Lockman, MD, told this news organization. Dr. Lockman is an associate professor of infectious diseases at Brigham and Women’s Hospital and a co-PI of the Botswana Clinical Trials Unit at the Botswana Harvard AIDS Institute Partnership. She was not involved in the study. “One of the worst things for a mother and for pregnancy outcomes, for the fetus and baby’s health and development, is uncontrolled maternal HIV,’’ she said.
Dr. Lockman also noted potential confounders that drive poor birth outcomes in Southern African women, compared with U.S. women, making comparisons between this and other observational studies difficult. Still, she said that the question is not whether women should be receiving treatment but whether or not there are differences between antiretroviral regimens.
“One of the areas that we did not go deeper into was the subtype of antiretroviral therapy, given the relatively small study numbers [did not] allow us to do a robust analysis,” Dr. Venkatesh said.
Rather, he emphasized that the findings might lend more weight to speculation that immunologic characteristics associated with HIV status and immunotherapy – such as low CD4 cell counts prior to delivery, or duration of HIV infection – may be important drivers of adverse birth outcomes among women with HIV taking ART.
And at least in this cohort, many of these characteristics were similar between the treatment groups.
Both researchers agree that the findings – while reassuring – highlight the importance of collecting robust obstetric and safety data as part of prospective databases of individuals living with HIV, not only in resource-limited settings but also among the domestic U.S. population.
“We’ve learned a lot over the last 10 years,” Dr. Lockman said. “Some regimens (like lopinavir/ritonavir or nevirapine) are associated with significantly worse birth outcomes, whereas efavirenz doesn’t seem to be, or less so, and dolutegravir seems to be associated with even better outcomes. So, I think that where we are moving is to regimens that are the safest.”
Moving forward, Dr. Venkatesh explained, not only should researchers focus on exploring which antiretrovirals are safest in this context but also if the use of preexposure prophylaxis during conception periods affects birth outcomes.
Dr. Venkatesh and Dr. Lockman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over the past decade, data have suggested that antiretroviral therapy (ART) may be associated with an increased risk for adverse pregnancy outcomes, namely, preterm birth (PTB). But a combination of methodologic challenges, demographic gaps, and spotty clinical data has left the question unresolved, especially for pregnant women with HIV who reside in developed countries.
“Given that a lot of the emerging data has come out of resource-limited settings where patient and clinical characteristics are different from developed world settings like the United States, we felt that this was an important question to address,” Kartik Venkatesh, MD, PhD, a high-risk obstetrician and perinatal epidemiologist at the Ohio State Wexner Medical Center, Columbus, told this news organization.
In a prospective cohort study of U.S. women with or at risk for HIV, Dr. Venkatesh and his colleagues found that ART exposure (including highly active antiretroviral therapy [HAART]) was associated with as much as an 80% decline in the likelihood of PTB (defined as birth less than 34 weeks). The study was published in HIV Medicine.
24 years of data analyzed
Dr. Venkatesh and his team analyzed self-reported birth data of women with singleton live-born pregnancies enrolled in the ongoing, multicenter, prospective observational Women’s Interagency HIV Study (WIHS) from Oct. 1, 1995, to March 31, 2019.
“We first looked at women with HIV versus without HIV, [who were] matched on many clinical and sociodemographic characteristics and at similarly high risk of some of these obstetrical outcomes like PTB,” explained Dr. Venkatesh. “We then looked at the relative impact of antiretroviral therapy amongst women living with HIV compared to no antiretroviral therapy.”
ART regimens were classified as none, monotherapy, dual therapy, or HAART. (HAART was defined as more than three antiretrovirals, including at least one protease inhibitor [PI], nonnucleoside reverse transcriptase inhibitor, integrase inhibitor, or entry inhibitor.) In this cohort, for 63.5% of women receiving ART, therapy was initiated before pregnancy (mean duration of HAART, 6 years), and most were virally suppressed.
Among the 4,944 women assessed in the WIHS trial, 74% (3,646) had HIV. In total, 383 women had 488 singleton deliveries, including 218 women with HIV (272 deliveries) and 165 without HIV (216 deliveries). Sociodemographics in both cohorts were well matched. For most participants, the mean age was 40-41 years at delivery, most were non-Hispanic Black persons, and the mean pregnancy body mass index was greater than or equal to 29 kg/m2. Of the women with HIV, 33% had chronic hypertension; of those without HIV, 42.1% had chronic hypertension; 4.7% and 5.0%, respectively, had pregestational diabetes.
The findings showed that PTB risk less than 34 weeks was similar between women with (10%) and without (8%) HIV (adjusted risk ratio, 1.30; 95% confidence interval, 0.74-2.31). Among deliveries to women with HIV who were receiving ART, PTB risk less than 34 weeks was lower with HAART (7%), compared with not receiving ART (26%) (aRR, 0.19), as well as with monotherapy or dual therapy (3% vs. no ART) (aRR, 0.12). Notably, 67% of deliveries to women receiving HAART included a PI-containing regimen, but these women were not significantly more likely to have a PTB less than 34 weeks, compared with women taking non-PI HAART regimens (aRR, 2.61; 95% CI, 0.65-10.59). Results were similar for secondary outcomes (PTB less than 28 weeks, less than 37 weeks).
Filling in the gaps toward the safest regimen
“This study spans 25 years, so it covers a lot of the history of HIV in pregnancy and is reassuring around using ART in pregnancy,” Shahin Lockman, MD, told this news organization. Dr. Lockman is an associate professor of infectious diseases at Brigham and Women’s Hospital and a co-PI of the Botswana Clinical Trials Unit at the Botswana Harvard AIDS Institute Partnership. She was not involved in the study. “One of the worst things for a mother and for pregnancy outcomes, for the fetus and baby’s health and development, is uncontrolled maternal HIV,’’ she said.
Dr. Lockman also noted potential confounders that drive poor birth outcomes in Southern African women, compared with U.S. women, making comparisons between this and other observational studies difficult. Still, she said that the question is not whether women should be receiving treatment but whether or not there are differences between antiretroviral regimens.
“One of the areas that we did not go deeper into was the subtype of antiretroviral therapy, given the relatively small study numbers [did not] allow us to do a robust analysis,” Dr. Venkatesh said.
Rather, he emphasized that the findings might lend more weight to speculation that immunologic characteristics associated with HIV status and immunotherapy – such as low CD4 cell counts prior to delivery, or duration of HIV infection – may be important drivers of adverse birth outcomes among women with HIV taking ART.
And at least in this cohort, many of these characteristics were similar between the treatment groups.
Both researchers agree that the findings – while reassuring – highlight the importance of collecting robust obstetric and safety data as part of prospective databases of individuals living with HIV, not only in resource-limited settings but also among the domestic U.S. population.
“We’ve learned a lot over the last 10 years,” Dr. Lockman said. “Some regimens (like lopinavir/ritonavir or nevirapine) are associated with significantly worse birth outcomes, whereas efavirenz doesn’t seem to be, or less so, and dolutegravir seems to be associated with even better outcomes. So, I think that where we are moving is to regimens that are the safest.”
Moving forward, Dr. Venkatesh explained, not only should researchers focus on exploring which antiretrovirals are safest in this context but also if the use of preexposure prophylaxis during conception periods affects birth outcomes.
Dr. Venkatesh and Dr. Lockman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over the past decade, data have suggested that antiretroviral therapy (ART) may be associated with an increased risk for adverse pregnancy outcomes, namely, preterm birth (PTB). But a combination of methodologic challenges, demographic gaps, and spotty clinical data has left the question unresolved, especially for pregnant women with HIV who reside in developed countries.
“Given that a lot of the emerging data has come out of resource-limited settings where patient and clinical characteristics are different from developed world settings like the United States, we felt that this was an important question to address,” Kartik Venkatesh, MD, PhD, a high-risk obstetrician and perinatal epidemiologist at the Ohio State Wexner Medical Center, Columbus, told this news organization.
In a prospective cohort study of U.S. women with or at risk for HIV, Dr. Venkatesh and his colleagues found that ART exposure (including highly active antiretroviral therapy [HAART]) was associated with as much as an 80% decline in the likelihood of PTB (defined as birth less than 34 weeks). The study was published in HIV Medicine.
24 years of data analyzed
Dr. Venkatesh and his team analyzed self-reported birth data of women with singleton live-born pregnancies enrolled in the ongoing, multicenter, prospective observational Women’s Interagency HIV Study (WIHS) from Oct. 1, 1995, to March 31, 2019.
“We first looked at women with HIV versus without HIV, [who were] matched on many clinical and sociodemographic characteristics and at similarly high risk of some of these obstetrical outcomes like PTB,” explained Dr. Venkatesh. “We then looked at the relative impact of antiretroviral therapy amongst women living with HIV compared to no antiretroviral therapy.”
ART regimens were classified as none, monotherapy, dual therapy, or HAART. (HAART was defined as more than three antiretrovirals, including at least one protease inhibitor [PI], nonnucleoside reverse transcriptase inhibitor, integrase inhibitor, or entry inhibitor.) In this cohort, for 63.5% of women receiving ART, therapy was initiated before pregnancy (mean duration of HAART, 6 years), and most were virally suppressed.
Among the 4,944 women assessed in the WIHS trial, 74% (3,646) had HIV. In total, 383 women had 488 singleton deliveries, including 218 women with HIV (272 deliveries) and 165 without HIV (216 deliveries). Sociodemographics in both cohorts were well matched. For most participants, the mean age was 40-41 years at delivery, most were non-Hispanic Black persons, and the mean pregnancy body mass index was greater than or equal to 29 kg/m2. Of the women with HIV, 33% had chronic hypertension; of those without HIV, 42.1% had chronic hypertension; 4.7% and 5.0%, respectively, had pregestational diabetes.
The findings showed that PTB risk less than 34 weeks was similar between women with (10%) and without (8%) HIV (adjusted risk ratio, 1.30; 95% confidence interval, 0.74-2.31). Among deliveries to women with HIV who were receiving ART, PTB risk less than 34 weeks was lower with HAART (7%), compared with not receiving ART (26%) (aRR, 0.19), as well as with monotherapy or dual therapy (3% vs. no ART) (aRR, 0.12). Notably, 67% of deliveries to women receiving HAART included a PI-containing regimen, but these women were not significantly more likely to have a PTB less than 34 weeks, compared with women taking non-PI HAART regimens (aRR, 2.61; 95% CI, 0.65-10.59). Results were similar for secondary outcomes (PTB less than 28 weeks, less than 37 weeks).
Filling in the gaps toward the safest regimen
“This study spans 25 years, so it covers a lot of the history of HIV in pregnancy and is reassuring around using ART in pregnancy,” Shahin Lockman, MD, told this news organization. Dr. Lockman is an associate professor of infectious diseases at Brigham and Women’s Hospital and a co-PI of the Botswana Clinical Trials Unit at the Botswana Harvard AIDS Institute Partnership. She was not involved in the study. “One of the worst things for a mother and for pregnancy outcomes, for the fetus and baby’s health and development, is uncontrolled maternal HIV,’’ she said.
Dr. Lockman also noted potential confounders that drive poor birth outcomes in Southern African women, compared with U.S. women, making comparisons between this and other observational studies difficult. Still, she said that the question is not whether women should be receiving treatment but whether or not there are differences between antiretroviral regimens.
“One of the areas that we did not go deeper into was the subtype of antiretroviral therapy, given the relatively small study numbers [did not] allow us to do a robust analysis,” Dr. Venkatesh said.
Rather, he emphasized that the findings might lend more weight to speculation that immunologic characteristics associated with HIV status and immunotherapy – such as low CD4 cell counts prior to delivery, or duration of HIV infection – may be important drivers of adverse birth outcomes among women with HIV taking ART.
And at least in this cohort, many of these characteristics were similar between the treatment groups.
Both researchers agree that the findings – while reassuring – highlight the importance of collecting robust obstetric and safety data as part of prospective databases of individuals living with HIV, not only in resource-limited settings but also among the domestic U.S. population.
“We’ve learned a lot over the last 10 years,” Dr. Lockman said. “Some regimens (like lopinavir/ritonavir or nevirapine) are associated with significantly worse birth outcomes, whereas efavirenz doesn’t seem to be, or less so, and dolutegravir seems to be associated with even better outcomes. So, I think that where we are moving is to regimens that are the safest.”
Moving forward, Dr. Venkatesh explained, not only should researchers focus on exploring which antiretrovirals are safest in this context but also if the use of preexposure prophylaxis during conception periods affects birth outcomes.
Dr. Venkatesh and Dr. Lockman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Integrating psychogastroenterology into GI care
Psychogastroenterology, or gastrointestinal psychology, refers to psychosocial research and clinical practice related to GI conditions. This field is situated within a biopsychosocial model of illness and grounded in an understanding of the gut-brain axis. A key feature of GI psychology intervention is behavioral symptom management. Commonly referred to as “brain-gut psychotherapies,” the primary goal of these interventions is to reduce GI symptoms and their impact on those experiencing them. Additionally, GI-focused psychotherapies can help patients with GI disorders cope with their symptoms, diagnosis, or treatment.
GI psychology providers
GI-focused psychotherapies are typically provided by clinical health psychologists (PhDs or PsyDs) with specialized training in GI disorders, although sometimes they are provided by a clinical social worker or advanced-practice nursing provider. Psychologists that identify GI as their primary specialty area often refer to themselves as “GI psychologists.” Psychologists that treat patients with a variety of medical concerns, which may include GI disorders, typically refer to themselves with the broader term, “health psychologists.”
Interventions
A variety of psychological treatments have been applied to GI populations, including cognitive behavioral therapy (CBT), gut-directed hypnotherapy (GDH), psychodynamic interpersonal therapy, relaxation training, and mindfulness-based stress reduction. Psychological therapies have been shown to be useful in a variety of GI disorders, with a number needed to treat of four in IBS.1 Common ingredients of GI-focused psychotherapy interventions include psychoeducation regarding the gut-brain relationship and relaxation strategies to provide in-the-moment tools to deescalate the body’s stress response.
CBT and GDH are the most commonly used interventions across a range of GI conditions, with the bulk of empirical evidence in IBS.2-5 CBT is a theoretical orientation in which thoughts and behaviors are understood to be modifiable factors that impact emotions and physical sensations. When utilized in a GI setting (i.e., GI-CBT), treatment aims to address GI-specific outcomes such as reducing GI symptoms, optimizing health care utilization, and improving quality of life. These interventions target cognitive and behavioral factors common among GI patient populations, such as GI-specific anxiety, symptom hypervigilance, and rigid coping strategies. See Figure 1 for a GI-CBT model.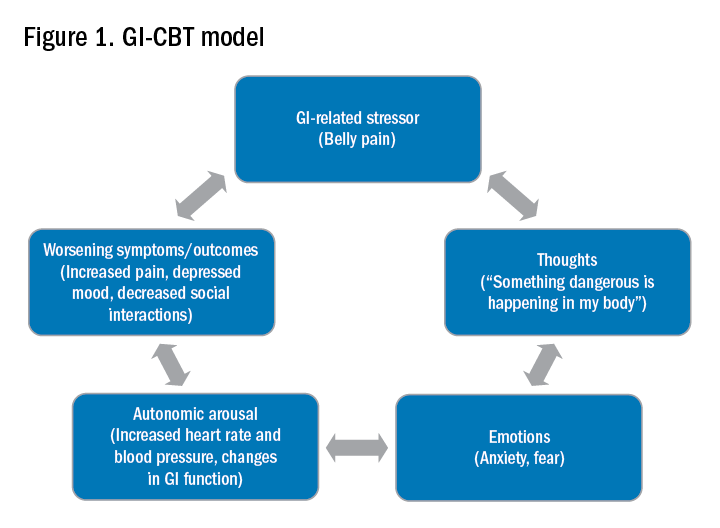
While research studies often implement manualized protocols, in clinical practice many GI psychologists use cognitive-behavioral interventions flexibly to tailor them to each patient’s presentation, while also integrating theory and practice from other types of therapies such as acceptance and commitment therapy (ACT; pronounced as one word). ACT, a “new wave” therapy derived from traditional CBT, emphasizes acceptance of distress (including GI symptoms), with a focus on engaging in values-based activities rather than symptom reduction.
Clinical hypnotherapy is utilized in a variety of medical specialties and has been studied in GI disorders for over 30 years. There are two evidence-based gut-directed hypnotherapy protocols, the Manchester6 and the North Carolina,7 that are widely used by GI psychologists. Though the exact mechanisms of hypnotherapy are unknown, it is thought to improve GI symptoms by modulating autonomic arousal and nerve sensitivity in the GI tract.
Evaluation
GI psychologists typically meet with patients for a 1-hour evaluation to determine appropriateness for psychogastroenterology intervention and develop a treatment plan. If GI-focused psychotherapy is indicated, patients are typically offered a course of treatment ranging from four to eight sessions. Depending on the nature of the patient’s concerns, longer courses of treatment may be offered, such as for with patients with active inflammatory bowel disease undergoing changes in medical treatment.
Appropriateness for psychogastroenterology treatment
Ideal patients are those who are psychologically stable and whose distress is primarily related to GI concerns, as opposed to family, work, or other situational stressors. While these other stressors can certainly impact GI symptoms, general mental health professionals are best suited to assist patients with these concerns. Patients experiencing more severe mental health concerns may be recommended to pursue a different treatment, such as mental health treatment for depression or anxiety or specialized treatments for trauma, eating disorders, or substance use. In both cases, once these general, non-GI, stressors or significant mental health concerns are more optimally managed, patients are likely to benefit from a GI-focused psychological treatment. Note, however, that because a GI psychologist’s particular practice can vary because of interest, experience, and institutional factors, it is best to connect directly with the GI psychologist you work with to clarify the types of referrals they are comfortable seeing and any specific characteristics of their practice.
Best practice recommendations for gastroenterologists
Developing a collaborative relationship with the GI psychologist, as well as any therapists to whom you regularly refer patients, is key to the success of integrated care. When talking to patients about the referral, refer to the GI psychologist as your colleague and a member of the treatment team. Maintain communication with the GI psychologist, and let the patient know that you are doing so.
When referring a patient, do so after you have completed your work-up and have optimized basic medical management for their condition but suspect that psychosocial factors may be negatively impacting their symptoms or ability to cope. Present the referral as an evaluation rather than implying a guarantee of treatment. This is particularly helpful in those cases where the patient is recommended to pursue a different treatment prior to GI-focused psychotherapy. Additionally, avoid telling patients that they are being referred for a specific intervention such as “a referral for CBT” or “a referral for hypnotherapy,” as the GI psychologist will recommend the most appropriate treatment for the patient upon evaluation. See Figure 2 for example scripts to use when referring.
Expect to maintain communication with the GI psychologist after making the referral. GI psychologists typically send the referring provider a written summary following the initial evaluation and conclusion of treatment and, in some cases, provide updates throughout. Be prepared to answer questions or provide input as requested. Not only may the psychologist have questions about the medical diagnosis or treatment, but they may enlist your help for medical expert opinion during treatment to address misinformation, which can often fuel concerns like treatment nonadherence or anxiety.
Identifying a psychogastroenterology provider
In recent years there has been significant growth in the training and hiring of GI psychologists, and it is increasingly common for GI psychologists to be employed at academic medical centers. However, the majority of gastroenterologists do not have access to a fully integrated or co-located GI psychologist. In these cases, gastroenterologists should search for other health psychology options in their area, such as psychologists or clinical social workers with experience with patients with chronic medical conditions and CBT. One positive product of the COVID-19 pandemic is that telemedicine has become increasingly utilized, and in some cases GI psychologists are able to provide virtual therapy to patients across state lines. However, this should be confirmed with the therapy practice as there are numerous factors to consider regarding virtual practice.
Dr. Bedell is assistant professor in the department of psychiatry and behavioral neuroscience at the University of Chicago. She has no conflicts of interest to disclose.
Resources available
To locate a GI psychology provider in your area: Search the Rome Psychogastroenterology directory (https://romegipsych.org/).
To locate general mental health providers: Search the Psychology Today website using the therapist finder function, which allows patients or providers to search by insurance, location, and specialty area (www.psychologytoday.com/us). The patient can also request a list of in-network psychotherapy providers from their insurance company and may find it helpful to cross-check these providers for potential fit by searching them online.
References
1. Ford AC et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: An updated systematic review and meta-analysis. Am J Gastroenterol. 2019 Jan;114(1):21-39. doi: 10.1038/s41395-018-0222-5.
2. Laird KT et al. Short-term and long-term efficacy of psychological therapies for irritable bowel syndrome: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016 Jul;14(7):937-47.e4. doi: 10.1016/j.cgh.2015.11.020.
3. Lackner JM et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. 2018 Jul;155(1):47-57. doi: 10.1053/j.gastro.2018.03.063.
4. Lövdahl J et al. Nurse-administered, gut-directed hypnotherapy in IBS: Efficacy and factors predicting a positive response. Am J Clin Hypn. 2015 Jul;58(1):100-14. doi: 10.1080/00029157.2015.1030492.
5. Smith GD. Effect of nurse-led gut-directed hypnotherapy upon health-related quality of life in patients with irritable bowel syndrome. J Clin Nurs. 2006 Jun;15(6):678-84. doi: 10.1111/j.1365-2702.2006.01356.x.
6. Gonsalkorale WM. Gut-directed hypnotherapy: the Manchester approach for treatment of irritable bowel syndrome. Int J Clin Exp Hypn. 2006 Jan;54(1):27-50. doi: 10.1080/00207140500323030.
7. Palsson OS. Standardized hypnosis treatment for irritable bowel syndrome: The North Carolina protocol. Int J Clin Exp Hypn. 2006 Jan;54(1):51-64. doi: 10.1080/00207140500322933.
Psychogastroenterology, or gastrointestinal psychology, refers to psychosocial research and clinical practice related to GI conditions. This field is situated within a biopsychosocial model of illness and grounded in an understanding of the gut-brain axis. A key feature of GI psychology intervention is behavioral symptom management. Commonly referred to as “brain-gut psychotherapies,” the primary goal of these interventions is to reduce GI symptoms and their impact on those experiencing them. Additionally, GI-focused psychotherapies can help patients with GI disorders cope with their symptoms, diagnosis, or treatment.
GI psychology providers
GI-focused psychotherapies are typically provided by clinical health psychologists (PhDs or PsyDs) with specialized training in GI disorders, although sometimes they are provided by a clinical social worker or advanced-practice nursing provider. Psychologists that identify GI as their primary specialty area often refer to themselves as “GI psychologists.” Psychologists that treat patients with a variety of medical concerns, which may include GI disorders, typically refer to themselves with the broader term, “health psychologists.”
Interventions
A variety of psychological treatments have been applied to GI populations, including cognitive behavioral therapy (CBT), gut-directed hypnotherapy (GDH), psychodynamic interpersonal therapy, relaxation training, and mindfulness-based stress reduction. Psychological therapies have been shown to be useful in a variety of GI disorders, with a number needed to treat of four in IBS.1 Common ingredients of GI-focused psychotherapy interventions include psychoeducation regarding the gut-brain relationship and relaxation strategies to provide in-the-moment tools to deescalate the body’s stress response.
CBT and GDH are the most commonly used interventions across a range of GI conditions, with the bulk of empirical evidence in IBS.2-5 CBT is a theoretical orientation in which thoughts and behaviors are understood to be modifiable factors that impact emotions and physical sensations. When utilized in a GI setting (i.e., GI-CBT), treatment aims to address GI-specific outcomes such as reducing GI symptoms, optimizing health care utilization, and improving quality of life. These interventions target cognitive and behavioral factors common among GI patient populations, such as GI-specific anxiety, symptom hypervigilance, and rigid coping strategies. See Figure 1 for a GI-CBT model.
While research studies often implement manualized protocols, in clinical practice many GI psychologists use cognitive-behavioral interventions flexibly to tailor them to each patient’s presentation, while also integrating theory and practice from other types of therapies such as acceptance and commitment therapy (ACT; pronounced as one word). ACT, a “new wave” therapy derived from traditional CBT, emphasizes acceptance of distress (including GI symptoms), with a focus on engaging in values-based activities rather than symptom reduction.
Clinical hypnotherapy is utilized in a variety of medical specialties and has been studied in GI disorders for over 30 years. There are two evidence-based gut-directed hypnotherapy protocols, the Manchester6 and the North Carolina,7 that are widely used by GI psychologists. Though the exact mechanisms of hypnotherapy are unknown, it is thought to improve GI symptoms by modulating autonomic arousal and nerve sensitivity in the GI tract.
Evaluation
GI psychologists typically meet with patients for a 1-hour evaluation to determine appropriateness for psychogastroenterology intervention and develop a treatment plan. If GI-focused psychotherapy is indicated, patients are typically offered a course of treatment ranging from four to eight sessions. Depending on the nature of the patient’s concerns, longer courses of treatment may be offered, such as for with patients with active inflammatory bowel disease undergoing changes in medical treatment.
Appropriateness for psychogastroenterology treatment
Ideal patients are those who are psychologically stable and whose distress is primarily related to GI concerns, as opposed to family, work, or other situational stressors. While these other stressors can certainly impact GI symptoms, general mental health professionals are best suited to assist patients with these concerns. Patients experiencing more severe mental health concerns may be recommended to pursue a different treatment, such as mental health treatment for depression or anxiety or specialized treatments for trauma, eating disorders, or substance use. In both cases, once these general, non-GI, stressors or significant mental health concerns are more optimally managed, patients are likely to benefit from a GI-focused psychological treatment. Note, however, that because a GI psychologist’s particular practice can vary because of interest, experience, and institutional factors, it is best to connect directly with the GI psychologist you work with to clarify the types of referrals they are comfortable seeing and any specific characteristics of their practice.
Best practice recommendations for gastroenterologists
Developing a collaborative relationship with the GI psychologist, as well as any therapists to whom you regularly refer patients, is key to the success of integrated care. When talking to patients about the referral, refer to the GI psychologist as your colleague and a member of the treatment team. Maintain communication with the GI psychologist, and let the patient know that you are doing so.
When referring a patient, do so after you have completed your work-up and have optimized basic medical management for their condition but suspect that psychosocial factors may be negatively impacting their symptoms or ability to cope. Present the referral as an evaluation rather than implying a guarantee of treatment. This is particularly helpful in those cases where the patient is recommended to pursue a different treatment prior to GI-focused psychotherapy. Additionally, avoid telling patients that they are being referred for a specific intervention such as “a referral for CBT” or “a referral for hypnotherapy,” as the GI psychologist will recommend the most appropriate treatment for the patient upon evaluation. See Figure 2 for example scripts to use when referring.
Expect to maintain communication with the GI psychologist after making the referral. GI psychologists typically send the referring provider a written summary following the initial evaluation and conclusion of treatment and, in some cases, provide updates throughout. Be prepared to answer questions or provide input as requested. Not only may the psychologist have questions about the medical diagnosis or treatment, but they may enlist your help for medical expert opinion during treatment to address misinformation, which can often fuel concerns like treatment nonadherence or anxiety.
Identifying a psychogastroenterology provider
In recent years there has been significant growth in the training and hiring of GI psychologists, and it is increasingly common for GI psychologists to be employed at academic medical centers. However, the majority of gastroenterologists do not have access to a fully integrated or co-located GI psychologist. In these cases, gastroenterologists should search for other health psychology options in their area, such as psychologists or clinical social workers with experience with patients with chronic medical conditions and CBT. One positive product of the COVID-19 pandemic is that telemedicine has become increasingly utilized, and in some cases GI psychologists are able to provide virtual therapy to patients across state lines. However, this should be confirmed with the therapy practice as there are numerous factors to consider regarding virtual practice.
Dr. Bedell is assistant professor in the department of psychiatry and behavioral neuroscience at the University of Chicago. She has no conflicts of interest to disclose.
Resources available
To locate a GI psychology provider in your area: Search the Rome Psychogastroenterology directory (https://romegipsych.org/).
To locate general mental health providers: Search the Psychology Today website using the therapist finder function, which allows patients or providers to search by insurance, location, and specialty area (www.psychologytoday.com/us). The patient can also request a list of in-network psychotherapy providers from their insurance company and may find it helpful to cross-check these providers for potential fit by searching them online.
References
1. Ford AC et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: An updated systematic review and meta-analysis. Am J Gastroenterol. 2019 Jan;114(1):21-39. doi: 10.1038/s41395-018-0222-5.
2. Laird KT et al. Short-term and long-term efficacy of psychological therapies for irritable bowel syndrome: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016 Jul;14(7):937-47.e4. doi: 10.1016/j.cgh.2015.11.020.
3. Lackner JM et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. 2018 Jul;155(1):47-57. doi: 10.1053/j.gastro.2018.03.063.
4. Lövdahl J et al. Nurse-administered, gut-directed hypnotherapy in IBS: Efficacy and factors predicting a positive response. Am J Clin Hypn. 2015 Jul;58(1):100-14. doi: 10.1080/00029157.2015.1030492.
5. Smith GD. Effect of nurse-led gut-directed hypnotherapy upon health-related quality of life in patients with irritable bowel syndrome. J Clin Nurs. 2006 Jun;15(6):678-84. doi: 10.1111/j.1365-2702.2006.01356.x.
6. Gonsalkorale WM. Gut-directed hypnotherapy: the Manchester approach for treatment of irritable bowel syndrome. Int J Clin Exp Hypn. 2006 Jan;54(1):27-50. doi: 10.1080/00207140500323030.
7. Palsson OS. Standardized hypnosis treatment for irritable bowel syndrome: The North Carolina protocol. Int J Clin Exp Hypn. 2006 Jan;54(1):51-64. doi: 10.1080/00207140500322933.
Psychogastroenterology, or gastrointestinal psychology, refers to psychosocial research and clinical practice related to GI conditions. This field is situated within a biopsychosocial model of illness and grounded in an understanding of the gut-brain axis. A key feature of GI psychology intervention is behavioral symptom management. Commonly referred to as “brain-gut psychotherapies,” the primary goal of these interventions is to reduce GI symptoms and their impact on those experiencing them. Additionally, GI-focused psychotherapies can help patients with GI disorders cope with their symptoms, diagnosis, or treatment.
GI psychology providers
GI-focused psychotherapies are typically provided by clinical health psychologists (PhDs or PsyDs) with specialized training in GI disorders, although sometimes they are provided by a clinical social worker or advanced-practice nursing provider. Psychologists that identify GI as their primary specialty area often refer to themselves as “GI psychologists.” Psychologists that treat patients with a variety of medical concerns, which may include GI disorders, typically refer to themselves with the broader term, “health psychologists.”
Interventions
A variety of psychological treatments have been applied to GI populations, including cognitive behavioral therapy (CBT), gut-directed hypnotherapy (GDH), psychodynamic interpersonal therapy, relaxation training, and mindfulness-based stress reduction. Psychological therapies have been shown to be useful in a variety of GI disorders, with a number needed to treat of four in IBS.1 Common ingredients of GI-focused psychotherapy interventions include psychoeducation regarding the gut-brain relationship and relaxation strategies to provide in-the-moment tools to deescalate the body’s stress response.
CBT and GDH are the most commonly used interventions across a range of GI conditions, with the bulk of empirical evidence in IBS.2-5 CBT is a theoretical orientation in which thoughts and behaviors are understood to be modifiable factors that impact emotions and physical sensations. When utilized in a GI setting (i.e., GI-CBT), treatment aims to address GI-specific outcomes such as reducing GI symptoms, optimizing health care utilization, and improving quality of life. These interventions target cognitive and behavioral factors common among GI patient populations, such as GI-specific anxiety, symptom hypervigilance, and rigid coping strategies. See Figure 1 for a GI-CBT model.
While research studies often implement manualized protocols, in clinical practice many GI psychologists use cognitive-behavioral interventions flexibly to tailor them to each patient’s presentation, while also integrating theory and practice from other types of therapies such as acceptance and commitment therapy (ACT; pronounced as one word). ACT, a “new wave” therapy derived from traditional CBT, emphasizes acceptance of distress (including GI symptoms), with a focus on engaging in values-based activities rather than symptom reduction.
Clinical hypnotherapy is utilized in a variety of medical specialties and has been studied in GI disorders for over 30 years. There are two evidence-based gut-directed hypnotherapy protocols, the Manchester6 and the North Carolina,7 that are widely used by GI psychologists. Though the exact mechanisms of hypnotherapy are unknown, it is thought to improve GI symptoms by modulating autonomic arousal and nerve sensitivity in the GI tract.
Evaluation
GI psychologists typically meet with patients for a 1-hour evaluation to determine appropriateness for psychogastroenterology intervention and develop a treatment plan. If GI-focused psychotherapy is indicated, patients are typically offered a course of treatment ranging from four to eight sessions. Depending on the nature of the patient’s concerns, longer courses of treatment may be offered, such as for with patients with active inflammatory bowel disease undergoing changes in medical treatment.
Appropriateness for psychogastroenterology treatment
Ideal patients are those who are psychologically stable and whose distress is primarily related to GI concerns, as opposed to family, work, or other situational stressors. While these other stressors can certainly impact GI symptoms, general mental health professionals are best suited to assist patients with these concerns. Patients experiencing more severe mental health concerns may be recommended to pursue a different treatment, such as mental health treatment for depression or anxiety or specialized treatments for trauma, eating disorders, or substance use. In both cases, once these general, non-GI, stressors or significant mental health concerns are more optimally managed, patients are likely to benefit from a GI-focused psychological treatment. Note, however, that because a GI psychologist’s particular practice can vary because of interest, experience, and institutional factors, it is best to connect directly with the GI psychologist you work with to clarify the types of referrals they are comfortable seeing and any specific characteristics of their practice.
Best practice recommendations for gastroenterologists
Developing a collaborative relationship with the GI psychologist, as well as any therapists to whom you regularly refer patients, is key to the success of integrated care. When talking to patients about the referral, refer to the GI psychologist as your colleague and a member of the treatment team. Maintain communication with the GI psychologist, and let the patient know that you are doing so.
When referring a patient, do so after you have completed your work-up and have optimized basic medical management for their condition but suspect that psychosocial factors may be negatively impacting their symptoms or ability to cope. Present the referral as an evaluation rather than implying a guarantee of treatment. This is particularly helpful in those cases where the patient is recommended to pursue a different treatment prior to GI-focused psychotherapy. Additionally, avoid telling patients that they are being referred for a specific intervention such as “a referral for CBT” or “a referral for hypnotherapy,” as the GI psychologist will recommend the most appropriate treatment for the patient upon evaluation. See Figure 2 for example scripts to use when referring.
Expect to maintain communication with the GI psychologist after making the referral. GI psychologists typically send the referring provider a written summary following the initial evaluation and conclusion of treatment and, in some cases, provide updates throughout. Be prepared to answer questions or provide input as requested. Not only may the psychologist have questions about the medical diagnosis or treatment, but they may enlist your help for medical expert opinion during treatment to address misinformation, which can often fuel concerns like treatment nonadherence or anxiety.
Identifying a psychogastroenterology provider
In recent years there has been significant growth in the training and hiring of GI psychologists, and it is increasingly common for GI psychologists to be employed at academic medical centers. However, the majority of gastroenterologists do not have access to a fully integrated or co-located GI psychologist. In these cases, gastroenterologists should search for other health psychology options in their area, such as psychologists or clinical social workers with experience with patients with chronic medical conditions and CBT. One positive product of the COVID-19 pandemic is that telemedicine has become increasingly utilized, and in some cases GI psychologists are able to provide virtual therapy to patients across state lines. However, this should be confirmed with the therapy practice as there are numerous factors to consider regarding virtual practice.
Dr. Bedell is assistant professor in the department of psychiatry and behavioral neuroscience at the University of Chicago. She has no conflicts of interest to disclose.
Resources available
To locate a GI psychology provider in your area: Search the Rome Psychogastroenterology directory (https://romegipsych.org/).
To locate general mental health providers: Search the Psychology Today website using the therapist finder function, which allows patients or providers to search by insurance, location, and specialty area (www.psychologytoday.com/us). The patient can also request a list of in-network psychotherapy providers from their insurance company and may find it helpful to cross-check these providers for potential fit by searching them online.
References
1. Ford AC et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: An updated systematic review and meta-analysis. Am J Gastroenterol. 2019 Jan;114(1):21-39. doi: 10.1038/s41395-018-0222-5.
2. Laird KT et al. Short-term and long-term efficacy of psychological therapies for irritable bowel syndrome: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016 Jul;14(7):937-47.e4. doi: 10.1016/j.cgh.2015.11.020.
3. Lackner JM et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. 2018 Jul;155(1):47-57. doi: 10.1053/j.gastro.2018.03.063.
4. Lövdahl J et al. Nurse-administered, gut-directed hypnotherapy in IBS: Efficacy and factors predicting a positive response. Am J Clin Hypn. 2015 Jul;58(1):100-14. doi: 10.1080/00029157.2015.1030492.
5. Smith GD. Effect of nurse-led gut-directed hypnotherapy upon health-related quality of life in patients with irritable bowel syndrome. J Clin Nurs. 2006 Jun;15(6):678-84. doi: 10.1111/j.1365-2702.2006.01356.x.
6. Gonsalkorale WM. Gut-directed hypnotherapy: the Manchester approach for treatment of irritable bowel syndrome. Int J Clin Exp Hypn. 2006 Jan;54(1):27-50. doi: 10.1080/00207140500323030.
7. Palsson OS. Standardized hypnosis treatment for irritable bowel syndrome: The North Carolina protocol. Int J Clin Exp Hypn. 2006 Jan;54(1):51-64. doi: 10.1080/00207140500322933.
Adverse skin effects of cancer immunotherapy reviewed
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
HCC risk differs among various liver cirrhosis etiologies
Key clinical point: The risk for hepatocellular carcinoma (HCC) varies with underlying etiologies, with active hepatitis C virus (HCV) cirrhosis posing the highest and alcoholic or nonalcoholic fatty liver disease (NAFLD) cirrhosis posing the lowest risk of developing HCC.
Major finding: Patients with active HCV (3.36%) showed the highest annual HCC incidence rate, followed by those with cured HCV (1.71%), alcoholic liver disease (1.32%), and NAFLD cirrhosis (1.24%). Patients with active HCV vs. NAFLD were at a 2.1-fold higher risk for HCC (adjusted hazard ratio 2.16; 95% CI, 1.16-4.04).
Study details: This multicenter, prospective cohort study analyzed data from two multiethnic cohorts enrolling a total of 2,733 patients with cirrhosis.
Disclosures: The study received financial support from the National Cancer Institute; Cancer Prevention & Research Institute of Texas grant; and Center for Gastrointestinal Development, Infection, and Injury. No conflicts of interest were reported.
Source: Kanwal F et al. Risk factors for hepatocellular cancer in contemporary cohorts of patients with cirrhosis. Hepatology. 2022 (Mar 1). Doi: 10.1002/hep.32434
Key clinical point: The risk for hepatocellular carcinoma (HCC) varies with underlying etiologies, with active hepatitis C virus (HCV) cirrhosis posing the highest and alcoholic or nonalcoholic fatty liver disease (NAFLD) cirrhosis posing the lowest risk of developing HCC.
Major finding: Patients with active HCV (3.36%) showed the highest annual HCC incidence rate, followed by those with cured HCV (1.71%), alcoholic liver disease (1.32%), and NAFLD cirrhosis (1.24%). Patients with active HCV vs. NAFLD were at a 2.1-fold higher risk for HCC (adjusted hazard ratio 2.16; 95% CI, 1.16-4.04).
Study details: This multicenter, prospective cohort study analyzed data from two multiethnic cohorts enrolling a total of 2,733 patients with cirrhosis.
Disclosures: The study received financial support from the National Cancer Institute; Cancer Prevention & Research Institute of Texas grant; and Center for Gastrointestinal Development, Infection, and Injury. No conflicts of interest were reported.
Source: Kanwal F et al. Risk factors for hepatocellular cancer in contemporary cohorts of patients with cirrhosis. Hepatology. 2022 (Mar 1). Doi: 10.1002/hep.32434
Key clinical point: The risk for hepatocellular carcinoma (HCC) varies with underlying etiologies, with active hepatitis C virus (HCV) cirrhosis posing the highest and alcoholic or nonalcoholic fatty liver disease (NAFLD) cirrhosis posing the lowest risk of developing HCC.
Major finding: Patients with active HCV (3.36%) showed the highest annual HCC incidence rate, followed by those with cured HCV (1.71%), alcoholic liver disease (1.32%), and NAFLD cirrhosis (1.24%). Patients with active HCV vs. NAFLD were at a 2.1-fold higher risk for HCC (adjusted hazard ratio 2.16; 95% CI, 1.16-4.04).
Study details: This multicenter, prospective cohort study analyzed data from two multiethnic cohorts enrolling a total of 2,733 patients with cirrhosis.
Disclosures: The study received financial support from the National Cancer Institute; Cancer Prevention & Research Institute of Texas grant; and Center for Gastrointestinal Development, Infection, and Injury. No conflicts of interest were reported.
Source: Kanwal F et al. Risk factors for hepatocellular cancer in contemporary cohorts of patients with cirrhosis. Hepatology. 2022 (Mar 1). Doi: 10.1002/hep.32434
Active HCV infection worsens the prognosis of very early-stage HCC after ablation therapy
Key clinical point: Active hepatitis C virus (HCV) infection negatively affects overall and recurrence-free survival in patients with very early-stage hepatocellular carcinoma (HCC) after curative radiofrequency ablation (RFA).
Major finding: Active HCV infection was a significant risk factor for shorter overall survival (adjusted hazard ratio [aHR] 2.17; P = .003) and early recurrence of HCC (aHR 1.47; P = .022). Patients with vs. without active HCV infection had a shorter median overall (66 months vs. 145 months) and recurrence-free (20 months vs. 31 months) survival (both P < .001).
Study details: Findings are from a single-center retrospective study including 302 patients with very early-stage HCC (Barcelona Clinic Liver Cancer stage 0) who underwent RFA and had follow-up of >6 months, of which 195 had HCV infection, including 132 active infection cases.
Disclosures: M Kurosaki and N Izumi declared funding support from the Japan Agency for Medical Research and Development and Japanese Ministry of Health, Welfare, and Labor, respectively, and along with K Tsuchiya, receiving lecture fees from several sources.
Source: Takaura K et al. The impact of background liver disease on the long-term prognosis of very-early-stage HCC after ablation therapy. PLoS One. 2022;17(2):e0264075 (Feb 23). Doi: 10.1371/journal.pone.0264075
Key clinical point: Active hepatitis C virus (HCV) infection negatively affects overall and recurrence-free survival in patients with very early-stage hepatocellular carcinoma (HCC) after curative radiofrequency ablation (RFA).
Major finding: Active HCV infection was a significant risk factor for shorter overall survival (adjusted hazard ratio [aHR] 2.17; P = .003) and early recurrence of HCC (aHR 1.47; P = .022). Patients with vs. without active HCV infection had a shorter median overall (66 months vs. 145 months) and recurrence-free (20 months vs. 31 months) survival (both P < .001).
Study details: Findings are from a single-center retrospective study including 302 patients with very early-stage HCC (Barcelona Clinic Liver Cancer stage 0) who underwent RFA and had follow-up of >6 months, of which 195 had HCV infection, including 132 active infection cases.
Disclosures: M Kurosaki and N Izumi declared funding support from the Japan Agency for Medical Research and Development and Japanese Ministry of Health, Welfare, and Labor, respectively, and along with K Tsuchiya, receiving lecture fees from several sources.
Source: Takaura K et al. The impact of background liver disease on the long-term prognosis of very-early-stage HCC after ablation therapy. PLoS One. 2022;17(2):e0264075 (Feb 23). Doi: 10.1371/journal.pone.0264075
Key clinical point: Active hepatitis C virus (HCV) infection negatively affects overall and recurrence-free survival in patients with very early-stage hepatocellular carcinoma (HCC) after curative radiofrequency ablation (RFA).
Major finding: Active HCV infection was a significant risk factor for shorter overall survival (adjusted hazard ratio [aHR] 2.17; P = .003) and early recurrence of HCC (aHR 1.47; P = .022). Patients with vs. without active HCV infection had a shorter median overall (66 months vs. 145 months) and recurrence-free (20 months vs. 31 months) survival (both P < .001).
Study details: Findings are from a single-center retrospective study including 302 patients with very early-stage HCC (Barcelona Clinic Liver Cancer stage 0) who underwent RFA and had follow-up of >6 months, of which 195 had HCV infection, including 132 active infection cases.
Disclosures: M Kurosaki and N Izumi declared funding support from the Japan Agency for Medical Research and Development and Japanese Ministry of Health, Welfare, and Labor, respectively, and along with K Tsuchiya, receiving lecture fees from several sources.
Source: Takaura K et al. The impact of background liver disease on the long-term prognosis of very-early-stage HCC after ablation therapy. PLoS One. 2022;17(2):e0264075 (Feb 23). Doi: 10.1371/journal.pone.0264075
Risk factors for recurrence after hepatic resection for early-stage HCC
Key clinical point: Independent risk factors for postoperative recurrence among patients undergoing curative hepatic resection for early-stage hepatocellular carcinoma (HCC) include preoperative alpha-fetoprotein (AFP) level >400 µg/L, tumor size >5 cm, satellite nodules, multiple tumors, and microvascular invasion.
Major finding: Cirrhosis (adjusted hazard ratio [aHR] 1.49; P < .001), preoperative AFP level >400 µg/L (aHR 1.28; P = .004), tumor size >5 cm (aHR 1.74; P < .001), satellite nodules (aHR 1.35; P = .040), multiple tumors (aHR 1.63; P = .015), microvascular invasion (aHR 1.51; P < .001), and intraoperative blood transfusion (aHR 1.50; P = .013) were identified as independent risk factors associated with postoperative recurrence.
Study details: The data come from a large-scale, multicenter retrospective study including 1,424 adult patients who underwent curative hepatic resection for early-stage HCC (Barcelona Clinic Liver Cancer stage 0/A).
Disclosures: The study was supported by the National Natural Science Foundation of China. The authors reported no conflict of interests.
Source: Yao L-Q et al. Clinical features of recurrence after hepatic resection for early-stage hepatocellular carcinoma and long-term survival outcomes of patients with recurrence: A multi-institutional analysis. Ann Surg Oncol. 2022 Feb 22. Doi: 10.1245/s10434-022-11454-y
Key clinical point: Independent risk factors for postoperative recurrence among patients undergoing curative hepatic resection for early-stage hepatocellular carcinoma (HCC) include preoperative alpha-fetoprotein (AFP) level >400 µg/L, tumor size >5 cm, satellite nodules, multiple tumors, and microvascular invasion.
Major finding: Cirrhosis (adjusted hazard ratio [aHR] 1.49; P < .001), preoperative AFP level >400 µg/L (aHR 1.28; P = .004), tumor size >5 cm (aHR 1.74; P < .001), satellite nodules (aHR 1.35; P = .040), multiple tumors (aHR 1.63; P = .015), microvascular invasion (aHR 1.51; P < .001), and intraoperative blood transfusion (aHR 1.50; P = .013) were identified as independent risk factors associated with postoperative recurrence.
Study details: The data come from a large-scale, multicenter retrospective study including 1,424 adult patients who underwent curative hepatic resection for early-stage HCC (Barcelona Clinic Liver Cancer stage 0/A).
Disclosures: The study was supported by the National Natural Science Foundation of China. The authors reported no conflict of interests.
Source: Yao L-Q et al. Clinical features of recurrence after hepatic resection for early-stage hepatocellular carcinoma and long-term survival outcomes of patients with recurrence: A multi-institutional analysis. Ann Surg Oncol. 2022 Feb 22. Doi: 10.1245/s10434-022-11454-y
Key clinical point: Independent risk factors for postoperative recurrence among patients undergoing curative hepatic resection for early-stage hepatocellular carcinoma (HCC) include preoperative alpha-fetoprotein (AFP) level >400 µg/L, tumor size >5 cm, satellite nodules, multiple tumors, and microvascular invasion.
Major finding: Cirrhosis (adjusted hazard ratio [aHR] 1.49; P < .001), preoperative AFP level >400 µg/L (aHR 1.28; P = .004), tumor size >5 cm (aHR 1.74; P < .001), satellite nodules (aHR 1.35; P = .040), multiple tumors (aHR 1.63; P = .015), microvascular invasion (aHR 1.51; P < .001), and intraoperative blood transfusion (aHR 1.50; P = .013) were identified as independent risk factors associated with postoperative recurrence.
Study details: The data come from a large-scale, multicenter retrospective study including 1,424 adult patients who underwent curative hepatic resection for early-stage HCC (Barcelona Clinic Liver Cancer stage 0/A).
Disclosures: The study was supported by the National Natural Science Foundation of China. The authors reported no conflict of interests.
Source: Yao L-Q et al. Clinical features of recurrence after hepatic resection for early-stage hepatocellular carcinoma and long-term survival outcomes of patients with recurrence: A multi-institutional analysis. Ann Surg Oncol. 2022 Feb 22. Doi: 10.1245/s10434-022-11454-y




