User login
Robotic transcranial Doppler improves PFO detection after stroke
in a new study.
Being far easier to perform than regular transcranial Doppler ultrasound, it’s hoped that use of the robotic device will enable many more patients to undergo the more sensitive transcranial screening modality and increase the number of shunts identified.
“I believe robot-assisted transcranial Doppler ultrasound can fill the gap between the gold standard transcranial Doppler and transthoracic echocardiography, which is the current standard of care,” said lead author Mark Rubin, MD.
Dr. Rubin, who is assistant professor of neurology at University of Tennessee Health Science Center, Memphis, presented results of the BUBL study at the International Stroke Conference (ISC) 2022, where they were greeted with applause from the floor.
An improvement in the current standard of care
Dr. Rubin explained that patients with suspected embolic stroke are routinely screened for shunts in the heart, such as patent foramen ovale (PFO), that allow blood to flow from the right chamber to the left chamber and can lead to clots from the venous system, accessing the arterial system, then traveling to the brain and causing a stroke.
The current standard of care in screening for such shunts is the use of transthoracic echocardiography (TTE), a widely available and easy to perform, non-invasive procedure. “But we have known for decades that TTE does not pick up these shunts very well. With a sensitivity of only around 45%, it identifies less than half of the patients affected,” Dr. Rubin noted.
The more sensitive transesophageal echocardiography (TEE) gives much better results, but it is an invasive and unpleasant procedure with the ultrasound probe being passed down the throat, and the patient needing to be sedated, so it’s not appropriate for everyone, he noted.
“Transcranial Doppler ultrasound (TCD) also gives excellent results, with a sensitivity of about 96% for detecting PFO, but this procedure is difficult to perform and requires a great deal of skill in placing the probes in the right position and interpreting the signal,” Dr. Rubin said. “TCD has been around for decades, but it hasn’t caught on, as it is too difficult to do. It takes a lot of time to learn the technique.”
“With the robotic-assisted transcranial Doppler device, we can achieve the sensitivity of TCD without needing expert operators. This should vastly improve accessibility to this technology,” he said. “With such technology we can make significant strides into more accurate diagnoses on the cause of stroke, which should lead to better preventive treatments in those found to have right-to-left shunts.”
Robotic detection of shunts
For the BUBL study, the robotic TCD technique was compared with the standard TTE in 129 patients who had a diagnosis of presumed embolic stroke or transient ischemic attack (TIA), with all patients undergoing both procedures.
The robotic TCD device resembles a giant pair of headphones containing the ultrasound probes, which are attached to a frame. In the study, it was operated by a health care professional without TCD skills. Each ultrasound probe independently scans the temporal area autonomously – with angling and positive pressure against the scalp akin to a sonographer – to find and optimize bilateral middle cerebral artery signals, Dr. Rubin explained.
The primary endpoint was the detection of a right-to-left shunt. This occurred in 82 of the 129 patients (63.6%) with the robotic TCD device but in only 27 patients (20.9%) when TTE was used. This gives an absolute difference of 42.6% (95% confidence interval, 28.6%-56.7%; P < .001), which Dr. Rubin described as “astounding.”
However, he said he was not surprised by these results.
“In my experience with transcranial Doppler, I find shunts in patients every day that have not been seen with transthoracic echo,” he commented.
He noted that a previous meta-analysis has suggested a similar difference between TCD and transthoracic echo, but the current study provides prospectively collected data produced in a clinical trial setting and is therefore more reliable.
“What I hope comes from this is that more patients will be able to undergo transcranial Doppler, which is a far superior screening technique for identifying right-to-left shunts. There is so much evidence to support the use of transcranial Doppler, but with this new artificial-intelligence robotic device, we don’t need an expert to use it,” Dr. Rubin said.
He explained that finding a right-to-left shunt in stroke patients is particularly important, as it can direct treatment strategies to reduce future risk of recurrent strokes.
“If a patient has a large shunt, then they have a high risk of having another stroke, and the PFO should be closed.”
In this study, the robotic-assisted TCD detected three times as many large shunts that were considered “intervenable,” compared with transthoracic echo, identifying these shunts in 35 patients (27%) compared to just 13 (10%) with TTE.
“Of the 35 patients with intervenable shunts detected with robotic transcranial Doppler, TTE was completely negative in 18 of them and only suggested a small shunt in the others. So, the standard of care (TTE) missed half the patients with intervenable PFOs,” Dr. Rubin reported.
Study should ‘dramatically change’ practice
Commenting on the study, Patrick Lyden, MD, professor of physiology and neuroscience and of neurology, University of Southern California, Los Angeles, said: “Most clinicians hesitate to use transcranial Doppler given the need for specialized technical expertise to obtain a reliable result. This study showed that a robotic transcranial Doppler device – which can be applied by any cardiac non-invasive lab technician – provides reliable and rigorous data.”
He added: “This result will dramatically change the typical evaluation of patients with suspected PFO: In place of an invasive transesophageal echo that requires anesthesia and a cardiologist, most patients can have a non-invasive, robotic-guided transcranial Doppler and get the same diagnostic benefit.”
Dr. Lyden also pointed out that the cost of TCD is typically one-tenth that of TEE, although he said the cost of the robotic guided TCD “is not clear.”
A representative of the company that makes the robotic assisted device, NovaSignal, says the cost of the equipment is approximately $250,000, but “understanding the importance of the technology, we work with each hospital to meet their unique needs.”
The company adds that it currently has “over 45 commercial solutions deployed across 25 centers with 3-4 times growth expected year over year.”
The study was supported by NovaSignal, the company which makes the robotic device. Dr. Rubin reports acting as a consultant for the NovaSignal.
A version of this article first appeared on Medscape.com.
in a new study.
Being far easier to perform than regular transcranial Doppler ultrasound, it’s hoped that use of the robotic device will enable many more patients to undergo the more sensitive transcranial screening modality and increase the number of shunts identified.
“I believe robot-assisted transcranial Doppler ultrasound can fill the gap between the gold standard transcranial Doppler and transthoracic echocardiography, which is the current standard of care,” said lead author Mark Rubin, MD.
Dr. Rubin, who is assistant professor of neurology at University of Tennessee Health Science Center, Memphis, presented results of the BUBL study at the International Stroke Conference (ISC) 2022, where they were greeted with applause from the floor.
An improvement in the current standard of care
Dr. Rubin explained that patients with suspected embolic stroke are routinely screened for shunts in the heart, such as patent foramen ovale (PFO), that allow blood to flow from the right chamber to the left chamber and can lead to clots from the venous system, accessing the arterial system, then traveling to the brain and causing a stroke.
The current standard of care in screening for such shunts is the use of transthoracic echocardiography (TTE), a widely available and easy to perform, non-invasive procedure. “But we have known for decades that TTE does not pick up these shunts very well. With a sensitivity of only around 45%, it identifies less than half of the patients affected,” Dr. Rubin noted.
The more sensitive transesophageal echocardiography (TEE) gives much better results, but it is an invasive and unpleasant procedure with the ultrasound probe being passed down the throat, and the patient needing to be sedated, so it’s not appropriate for everyone, he noted.
“Transcranial Doppler ultrasound (TCD) also gives excellent results, with a sensitivity of about 96% for detecting PFO, but this procedure is difficult to perform and requires a great deal of skill in placing the probes in the right position and interpreting the signal,” Dr. Rubin said. “TCD has been around for decades, but it hasn’t caught on, as it is too difficult to do. It takes a lot of time to learn the technique.”
“With the robotic-assisted transcranial Doppler device, we can achieve the sensitivity of TCD without needing expert operators. This should vastly improve accessibility to this technology,” he said. “With such technology we can make significant strides into more accurate diagnoses on the cause of stroke, which should lead to better preventive treatments in those found to have right-to-left shunts.”
Robotic detection of shunts
For the BUBL study, the robotic TCD technique was compared with the standard TTE in 129 patients who had a diagnosis of presumed embolic stroke or transient ischemic attack (TIA), with all patients undergoing both procedures.
The robotic TCD device resembles a giant pair of headphones containing the ultrasound probes, which are attached to a frame. In the study, it was operated by a health care professional without TCD skills. Each ultrasound probe independently scans the temporal area autonomously – with angling and positive pressure against the scalp akin to a sonographer – to find and optimize bilateral middle cerebral artery signals, Dr. Rubin explained.
The primary endpoint was the detection of a right-to-left shunt. This occurred in 82 of the 129 patients (63.6%) with the robotic TCD device but in only 27 patients (20.9%) when TTE was used. This gives an absolute difference of 42.6% (95% confidence interval, 28.6%-56.7%; P < .001), which Dr. Rubin described as “astounding.”
However, he said he was not surprised by these results.
“In my experience with transcranial Doppler, I find shunts in patients every day that have not been seen with transthoracic echo,” he commented.
He noted that a previous meta-analysis has suggested a similar difference between TCD and transthoracic echo, but the current study provides prospectively collected data produced in a clinical trial setting and is therefore more reliable.
“What I hope comes from this is that more patients will be able to undergo transcranial Doppler, which is a far superior screening technique for identifying right-to-left shunts. There is so much evidence to support the use of transcranial Doppler, but with this new artificial-intelligence robotic device, we don’t need an expert to use it,” Dr. Rubin said.
He explained that finding a right-to-left shunt in stroke patients is particularly important, as it can direct treatment strategies to reduce future risk of recurrent strokes.
“If a patient has a large shunt, then they have a high risk of having another stroke, and the PFO should be closed.”
In this study, the robotic-assisted TCD detected three times as many large shunts that were considered “intervenable,” compared with transthoracic echo, identifying these shunts in 35 patients (27%) compared to just 13 (10%) with TTE.
“Of the 35 patients with intervenable shunts detected with robotic transcranial Doppler, TTE was completely negative in 18 of them and only suggested a small shunt in the others. So, the standard of care (TTE) missed half the patients with intervenable PFOs,” Dr. Rubin reported.
Study should ‘dramatically change’ practice
Commenting on the study, Patrick Lyden, MD, professor of physiology and neuroscience and of neurology, University of Southern California, Los Angeles, said: “Most clinicians hesitate to use transcranial Doppler given the need for specialized technical expertise to obtain a reliable result. This study showed that a robotic transcranial Doppler device – which can be applied by any cardiac non-invasive lab technician – provides reliable and rigorous data.”
He added: “This result will dramatically change the typical evaluation of patients with suspected PFO: In place of an invasive transesophageal echo that requires anesthesia and a cardiologist, most patients can have a non-invasive, robotic-guided transcranial Doppler and get the same diagnostic benefit.”
Dr. Lyden also pointed out that the cost of TCD is typically one-tenth that of TEE, although he said the cost of the robotic guided TCD “is not clear.”
A representative of the company that makes the robotic assisted device, NovaSignal, says the cost of the equipment is approximately $250,000, but “understanding the importance of the technology, we work with each hospital to meet their unique needs.”
The company adds that it currently has “over 45 commercial solutions deployed across 25 centers with 3-4 times growth expected year over year.”
The study was supported by NovaSignal, the company which makes the robotic device. Dr. Rubin reports acting as a consultant for the NovaSignal.
A version of this article first appeared on Medscape.com.
in a new study.
Being far easier to perform than regular transcranial Doppler ultrasound, it’s hoped that use of the robotic device will enable many more patients to undergo the more sensitive transcranial screening modality and increase the number of shunts identified.
“I believe robot-assisted transcranial Doppler ultrasound can fill the gap between the gold standard transcranial Doppler and transthoracic echocardiography, which is the current standard of care,” said lead author Mark Rubin, MD.
Dr. Rubin, who is assistant professor of neurology at University of Tennessee Health Science Center, Memphis, presented results of the BUBL study at the International Stroke Conference (ISC) 2022, where they were greeted with applause from the floor.
An improvement in the current standard of care
Dr. Rubin explained that patients with suspected embolic stroke are routinely screened for shunts in the heart, such as patent foramen ovale (PFO), that allow blood to flow from the right chamber to the left chamber and can lead to clots from the venous system, accessing the arterial system, then traveling to the brain and causing a stroke.
The current standard of care in screening for such shunts is the use of transthoracic echocardiography (TTE), a widely available and easy to perform, non-invasive procedure. “But we have known for decades that TTE does not pick up these shunts very well. With a sensitivity of only around 45%, it identifies less than half of the patients affected,” Dr. Rubin noted.
The more sensitive transesophageal echocardiography (TEE) gives much better results, but it is an invasive and unpleasant procedure with the ultrasound probe being passed down the throat, and the patient needing to be sedated, so it’s not appropriate for everyone, he noted.
“Transcranial Doppler ultrasound (TCD) also gives excellent results, with a sensitivity of about 96% for detecting PFO, but this procedure is difficult to perform and requires a great deal of skill in placing the probes in the right position and interpreting the signal,” Dr. Rubin said. “TCD has been around for decades, but it hasn’t caught on, as it is too difficult to do. It takes a lot of time to learn the technique.”
“With the robotic-assisted transcranial Doppler device, we can achieve the sensitivity of TCD without needing expert operators. This should vastly improve accessibility to this technology,” he said. “With such technology we can make significant strides into more accurate diagnoses on the cause of stroke, which should lead to better preventive treatments in those found to have right-to-left shunts.”
Robotic detection of shunts
For the BUBL study, the robotic TCD technique was compared with the standard TTE in 129 patients who had a diagnosis of presumed embolic stroke or transient ischemic attack (TIA), with all patients undergoing both procedures.
The robotic TCD device resembles a giant pair of headphones containing the ultrasound probes, which are attached to a frame. In the study, it was operated by a health care professional without TCD skills. Each ultrasound probe independently scans the temporal area autonomously – with angling and positive pressure against the scalp akin to a sonographer – to find and optimize bilateral middle cerebral artery signals, Dr. Rubin explained.
The primary endpoint was the detection of a right-to-left shunt. This occurred in 82 of the 129 patients (63.6%) with the robotic TCD device but in only 27 patients (20.9%) when TTE was used. This gives an absolute difference of 42.6% (95% confidence interval, 28.6%-56.7%; P < .001), which Dr. Rubin described as “astounding.”
However, he said he was not surprised by these results.
“In my experience with transcranial Doppler, I find shunts in patients every day that have not been seen with transthoracic echo,” he commented.
He noted that a previous meta-analysis has suggested a similar difference between TCD and transthoracic echo, but the current study provides prospectively collected data produced in a clinical trial setting and is therefore more reliable.
“What I hope comes from this is that more patients will be able to undergo transcranial Doppler, which is a far superior screening technique for identifying right-to-left shunts. There is so much evidence to support the use of transcranial Doppler, but with this new artificial-intelligence robotic device, we don’t need an expert to use it,” Dr. Rubin said.
He explained that finding a right-to-left shunt in stroke patients is particularly important, as it can direct treatment strategies to reduce future risk of recurrent strokes.
“If a patient has a large shunt, then they have a high risk of having another stroke, and the PFO should be closed.”
In this study, the robotic-assisted TCD detected three times as many large shunts that were considered “intervenable,” compared with transthoracic echo, identifying these shunts in 35 patients (27%) compared to just 13 (10%) with TTE.
“Of the 35 patients with intervenable shunts detected with robotic transcranial Doppler, TTE was completely negative in 18 of them and only suggested a small shunt in the others. So, the standard of care (TTE) missed half the patients with intervenable PFOs,” Dr. Rubin reported.
Study should ‘dramatically change’ practice
Commenting on the study, Patrick Lyden, MD, professor of physiology and neuroscience and of neurology, University of Southern California, Los Angeles, said: “Most clinicians hesitate to use transcranial Doppler given the need for specialized technical expertise to obtain a reliable result. This study showed that a robotic transcranial Doppler device – which can be applied by any cardiac non-invasive lab technician – provides reliable and rigorous data.”
He added: “This result will dramatically change the typical evaluation of patients with suspected PFO: In place of an invasive transesophageal echo that requires anesthesia and a cardiologist, most patients can have a non-invasive, robotic-guided transcranial Doppler and get the same diagnostic benefit.”
Dr. Lyden also pointed out that the cost of TCD is typically one-tenth that of TEE, although he said the cost of the robotic guided TCD “is not clear.”
A representative of the company that makes the robotic assisted device, NovaSignal, says the cost of the equipment is approximately $250,000, but “understanding the importance of the technology, we work with each hospital to meet their unique needs.”
The company adds that it currently has “over 45 commercial solutions deployed across 25 centers with 3-4 times growth expected year over year.”
The study was supported by NovaSignal, the company which makes the robotic device. Dr. Rubin reports acting as a consultant for the NovaSignal.
A version of this article first appeared on Medscape.com.
FROM ISC 2022
USPSTF tweaks primary prevention statin recommendations in new draft guidance
Given the expansive contemporary role of statins for primary cardiovascular disease (CVD) prevention, the language in the new U.S. Preventive Services Task Force draft guidance on their use in that setting may seem conservative. Even so, the proposed recommendations, open to public comment until March 21, take more recent data into account but don’t substantially vary from the 2016 USPSTF document they are intended to replace.
The task force concluded “with moderate certainty” that a statin prescription will clinically benefit adults aged 40-75 years without CVD but with at least one of several risk factors, such as dyslipidemia or diabetes, who have a 10-year CVD risk of at least 7.5%.
the new report states. That, says an accompanying USPSTF press release, means such people “may benefit from statin use and should decide with their clinician if taking a statin is right for them.”
Also, notes the report, the net benefit of statin therapy is “at least moderate” for individuals with a 10% or greater CVD risk over the next decade who, the press release states, “should take a statin to prevent a first heart attack or stroke.”
The evidence review on which the task force based the guidance, the report says, lacked sufficient basis for determining statin benefit versus risk in adults older than 75 years without a history of CVD. “In the absence of this evidence, clinicians should use their judgment as to whether to offer a statin to a patient in this age group,” according to the press release.
The review focused on 22 clinical trials for data on the statin benefits and saw significantly decreased associated risks for death from any cause, fatal or nonfatal stroke, and fatal or nonfatal myocardial infarction with treatment. The combined trial populations exceeded 85,000 for assessing all-cause mortality and 76,000 for each of the other two endpoints.
To assess any potential statin therapy harms, the evidence review covered 19 clinical trials with a combined enrollment of about 75,000 – two more trials than considered in the 2016 document – plus three observational studies with more than 400,000 participants. Statins were found not to be associated with an increased risk for study withdrawal because of adverse events, nor were there signs of greater risk for myalgia or new-onset diabetes, compared with placebo.
“A majority of the trials reviewed by the USPSTF used moderate-intensity statin therapy,” the report states. “Based on available evidence, use of moderate-intensity statin therapy seems reasonable for the primary prevention of CVD in most persons.”
A version of this article first appeared on Medscape.com.
Given the expansive contemporary role of statins for primary cardiovascular disease (CVD) prevention, the language in the new U.S. Preventive Services Task Force draft guidance on their use in that setting may seem conservative. Even so, the proposed recommendations, open to public comment until March 21, take more recent data into account but don’t substantially vary from the 2016 USPSTF document they are intended to replace.
The task force concluded “with moderate certainty” that a statin prescription will clinically benefit adults aged 40-75 years without CVD but with at least one of several risk factors, such as dyslipidemia or diabetes, who have a 10-year CVD risk of at least 7.5%.
the new report states. That, says an accompanying USPSTF press release, means such people “may benefit from statin use and should decide with their clinician if taking a statin is right for them.”
Also, notes the report, the net benefit of statin therapy is “at least moderate” for individuals with a 10% or greater CVD risk over the next decade who, the press release states, “should take a statin to prevent a first heart attack or stroke.”
The evidence review on which the task force based the guidance, the report says, lacked sufficient basis for determining statin benefit versus risk in adults older than 75 years without a history of CVD. “In the absence of this evidence, clinicians should use their judgment as to whether to offer a statin to a patient in this age group,” according to the press release.
The review focused on 22 clinical trials for data on the statin benefits and saw significantly decreased associated risks for death from any cause, fatal or nonfatal stroke, and fatal or nonfatal myocardial infarction with treatment. The combined trial populations exceeded 85,000 for assessing all-cause mortality and 76,000 for each of the other two endpoints.
To assess any potential statin therapy harms, the evidence review covered 19 clinical trials with a combined enrollment of about 75,000 – two more trials than considered in the 2016 document – plus three observational studies with more than 400,000 participants. Statins were found not to be associated with an increased risk for study withdrawal because of adverse events, nor were there signs of greater risk for myalgia or new-onset diabetes, compared with placebo.
“A majority of the trials reviewed by the USPSTF used moderate-intensity statin therapy,” the report states. “Based on available evidence, use of moderate-intensity statin therapy seems reasonable for the primary prevention of CVD in most persons.”
A version of this article first appeared on Medscape.com.
Given the expansive contemporary role of statins for primary cardiovascular disease (CVD) prevention, the language in the new U.S. Preventive Services Task Force draft guidance on their use in that setting may seem conservative. Even so, the proposed recommendations, open to public comment until March 21, take more recent data into account but don’t substantially vary from the 2016 USPSTF document they are intended to replace.
The task force concluded “with moderate certainty” that a statin prescription will clinically benefit adults aged 40-75 years without CVD but with at least one of several risk factors, such as dyslipidemia or diabetes, who have a 10-year CVD risk of at least 7.5%.
the new report states. That, says an accompanying USPSTF press release, means such people “may benefit from statin use and should decide with their clinician if taking a statin is right for them.”
Also, notes the report, the net benefit of statin therapy is “at least moderate” for individuals with a 10% or greater CVD risk over the next decade who, the press release states, “should take a statin to prevent a first heart attack or stroke.”
The evidence review on which the task force based the guidance, the report says, lacked sufficient basis for determining statin benefit versus risk in adults older than 75 years without a history of CVD. “In the absence of this evidence, clinicians should use their judgment as to whether to offer a statin to a patient in this age group,” according to the press release.
The review focused on 22 clinical trials for data on the statin benefits and saw significantly decreased associated risks for death from any cause, fatal or nonfatal stroke, and fatal or nonfatal myocardial infarction with treatment. The combined trial populations exceeded 85,000 for assessing all-cause mortality and 76,000 for each of the other two endpoints.
To assess any potential statin therapy harms, the evidence review covered 19 clinical trials with a combined enrollment of about 75,000 – two more trials than considered in the 2016 document – plus three observational studies with more than 400,000 participants. Statins were found not to be associated with an increased risk for study withdrawal because of adverse events, nor were there signs of greater risk for myalgia or new-onset diabetes, compared with placebo.
“A majority of the trials reviewed by the USPSTF used moderate-intensity statin therapy,” the report states. “Based on available evidence, use of moderate-intensity statin therapy seems reasonable for the primary prevention of CVD in most persons.”
A version of this article first appeared on Medscape.com.
‘In the presence of kindness’: humanitarian Paul Farmer dies
Renowned infectious disease specialist, humanitarian, and healthcare champion for many of the world’s most vulnerable patient populations, Paul Edward Farmer, MD, died suddenly in his sleep from an acute cardiac event on Feb. 21 in Rwanda, where he had been teaching. He was 62.
Dr. Farmer cofounded the Boston-based global nonprofit Partners In Health and spent decades providing healthcare to impoverished communities worldwide, fighting on the frontline to protect underserved communities against deadly pandemics.
Dr. Farmer was the Kolokotrones University Professor and chair of the department of global health and social medicine in the Blavatnik Institute at Harvard Medical School, Boston. He served as chief of the division of global health equity at Brigham and Women’s Hospital, also in Boston.
“Paul dedicated his life to improving human health and advocating for health equity and social justice on a global scale,” said HMS dean George Q. Daley in a letter to the HMS community. “I am particularly shaken by his passing because he was not only a consummate colleague and a beloved mentor, but a close friend. To me, Paul represented the heart and soul of Harvard Medical School.”
He was also chancellor and cofounder of the University of Global Health Equity in Kigali, Rwanda. Before his death, he spent several weeks teaching at the university.
“Paul Farmer’s loss is devastating, but his vision for the world will live on through Partners In Health,” said Partners In Health CEO Sheila Davis in a statement. “Paul taught all those around him the power of accompaniment, love for one another, and solidarity. Our deepest sympathies are with his family.”
Dr. Farmer was born in North Adams, Mass., and grew up in Florida with his parents and five siblings. He attended Duke University on a Benjamin N. Duke Scholarship and received his medical degree in 1988, followed by his PhD in 1990 from Harvard University.
His humanitarian work began when he was a college student volunteering in Haiti in 1983 working with dispossessed farmers. In 1987, he cofounded Partners In Health with the goal of helping patients in poverty-stricken corners of the world.
Under Dr. Farmer’s leadership, the nonprofit tackled major public health crises: Haiti’s devastating 2010 earthquake, drug-resistant tuberculosis in Peru and other countries, and an Ebola outbreak that tore through West Africa.
Dr. Farmer documented his 2014-2015 experience treating Africa’s Ebola patients in a book called “Fevers, Feuds, and Diamonds: Ebola and the Ravages of History.”
He wrote that by the time he arrived, “western Sierra Leone was ground zero of the epidemic, and Upper West Africa was just about the worst place in the world to be critically ill or injured.”
One of his greatest qualities was his ability to connect with patients – to treat them “not like ones who suffered, but like a pal you’d joke with,” said Pardis Sabeti, MD, PhD, a Harvard University geneticist who also spent time in Africa and famously sequenced samples of the Ebola virus’ genome.
Dr. Sabeti and Dr. Farmer bonded over their love for Sierra Leone, and their grief over losing a close colleague to Ebola, Sheik Humarr Khan, who was one of the area’s leading infectious disease experts.
Dr. Sabeti first met Dr. Farmer years earlier as a first-year Harvard medical student when she enrolled in one of his courses. She said students introduced themselves, one by one, each veering into heartfelt testimonies about what Dr. Farmer’s work had meant to them.
Dr. Farmer and Dr. Sabeti were just texting on Feb. 19, and the two were “goofing around in our usual way, and scheming about how to make the world better, as we always did.”
Dr. Farmer was funny, mischievous, and above all, exactly what you would expect upon meeting him, Dr. Sabeti said.
“It’s cliché, but the energetic kick you get from just being in his presence, it’s almost otherworldly,” she said. “It’s not even otherworldly in the sense of: ‘I just came across – greatness.’ It’s more: ‘I just came across kindness.’ ”
Dr. Farmer’s work has been widely distributed in publications including Bulletin of the World Health Organization, The Lancet, the New England Journal of Medicine, Clinical Infectious Diseases, and Social Science & Medicine.
He was awarded the 2020 Berggruen Prize for Philosophy & Culture, the Margaret Mead Award from the American Anthropological Association, the American Medical Association’s Outstanding International Physician (Nathan Davis) Award, and, with his Partners In Health colleagues, the Hilton Humanitarian Prize.
He is survived by his wife, Didi Bertrand Farmer, and their three children.
A verison of this article first appeared on Medscape.com.
Renowned infectious disease specialist, humanitarian, and healthcare champion for many of the world’s most vulnerable patient populations, Paul Edward Farmer, MD, died suddenly in his sleep from an acute cardiac event on Feb. 21 in Rwanda, where he had been teaching. He was 62.
Dr. Farmer cofounded the Boston-based global nonprofit Partners In Health and spent decades providing healthcare to impoverished communities worldwide, fighting on the frontline to protect underserved communities against deadly pandemics.
Dr. Farmer was the Kolokotrones University Professor and chair of the department of global health and social medicine in the Blavatnik Institute at Harvard Medical School, Boston. He served as chief of the division of global health equity at Brigham and Women’s Hospital, also in Boston.
“Paul dedicated his life to improving human health and advocating for health equity and social justice on a global scale,” said HMS dean George Q. Daley in a letter to the HMS community. “I am particularly shaken by his passing because he was not only a consummate colleague and a beloved mentor, but a close friend. To me, Paul represented the heart and soul of Harvard Medical School.”
He was also chancellor and cofounder of the University of Global Health Equity in Kigali, Rwanda. Before his death, he spent several weeks teaching at the university.
“Paul Farmer’s loss is devastating, but his vision for the world will live on through Partners In Health,” said Partners In Health CEO Sheila Davis in a statement. “Paul taught all those around him the power of accompaniment, love for one another, and solidarity. Our deepest sympathies are with his family.”
Dr. Farmer was born in North Adams, Mass., and grew up in Florida with his parents and five siblings. He attended Duke University on a Benjamin N. Duke Scholarship and received his medical degree in 1988, followed by his PhD in 1990 from Harvard University.
His humanitarian work began when he was a college student volunteering in Haiti in 1983 working with dispossessed farmers. In 1987, he cofounded Partners In Health with the goal of helping patients in poverty-stricken corners of the world.
Under Dr. Farmer’s leadership, the nonprofit tackled major public health crises: Haiti’s devastating 2010 earthquake, drug-resistant tuberculosis in Peru and other countries, and an Ebola outbreak that tore through West Africa.
Dr. Farmer documented his 2014-2015 experience treating Africa’s Ebola patients in a book called “Fevers, Feuds, and Diamonds: Ebola and the Ravages of History.”
He wrote that by the time he arrived, “western Sierra Leone was ground zero of the epidemic, and Upper West Africa was just about the worst place in the world to be critically ill or injured.”
One of his greatest qualities was his ability to connect with patients – to treat them “not like ones who suffered, but like a pal you’d joke with,” said Pardis Sabeti, MD, PhD, a Harvard University geneticist who also spent time in Africa and famously sequenced samples of the Ebola virus’ genome.
Dr. Sabeti and Dr. Farmer bonded over their love for Sierra Leone, and their grief over losing a close colleague to Ebola, Sheik Humarr Khan, who was one of the area’s leading infectious disease experts.
Dr. Sabeti first met Dr. Farmer years earlier as a first-year Harvard medical student when she enrolled in one of his courses. She said students introduced themselves, one by one, each veering into heartfelt testimonies about what Dr. Farmer’s work had meant to them.
Dr. Farmer and Dr. Sabeti were just texting on Feb. 19, and the two were “goofing around in our usual way, and scheming about how to make the world better, as we always did.”
Dr. Farmer was funny, mischievous, and above all, exactly what you would expect upon meeting him, Dr. Sabeti said.
“It’s cliché, but the energetic kick you get from just being in his presence, it’s almost otherworldly,” she said. “It’s not even otherworldly in the sense of: ‘I just came across – greatness.’ It’s more: ‘I just came across kindness.’ ”
Dr. Farmer’s work has been widely distributed in publications including Bulletin of the World Health Organization, The Lancet, the New England Journal of Medicine, Clinical Infectious Diseases, and Social Science & Medicine.
He was awarded the 2020 Berggruen Prize for Philosophy & Culture, the Margaret Mead Award from the American Anthropological Association, the American Medical Association’s Outstanding International Physician (Nathan Davis) Award, and, with his Partners In Health colleagues, the Hilton Humanitarian Prize.
He is survived by his wife, Didi Bertrand Farmer, and their three children.
A verison of this article first appeared on Medscape.com.
Renowned infectious disease specialist, humanitarian, and healthcare champion for many of the world’s most vulnerable patient populations, Paul Edward Farmer, MD, died suddenly in his sleep from an acute cardiac event on Feb. 21 in Rwanda, where he had been teaching. He was 62.
Dr. Farmer cofounded the Boston-based global nonprofit Partners In Health and spent decades providing healthcare to impoverished communities worldwide, fighting on the frontline to protect underserved communities against deadly pandemics.
Dr. Farmer was the Kolokotrones University Professor and chair of the department of global health and social medicine in the Blavatnik Institute at Harvard Medical School, Boston. He served as chief of the division of global health equity at Brigham and Women’s Hospital, also in Boston.
“Paul dedicated his life to improving human health and advocating for health equity and social justice on a global scale,” said HMS dean George Q. Daley in a letter to the HMS community. “I am particularly shaken by his passing because he was not only a consummate colleague and a beloved mentor, but a close friend. To me, Paul represented the heart and soul of Harvard Medical School.”
He was also chancellor and cofounder of the University of Global Health Equity in Kigali, Rwanda. Before his death, he spent several weeks teaching at the university.
“Paul Farmer’s loss is devastating, but his vision for the world will live on through Partners In Health,” said Partners In Health CEO Sheila Davis in a statement. “Paul taught all those around him the power of accompaniment, love for one another, and solidarity. Our deepest sympathies are with his family.”
Dr. Farmer was born in North Adams, Mass., and grew up in Florida with his parents and five siblings. He attended Duke University on a Benjamin N. Duke Scholarship and received his medical degree in 1988, followed by his PhD in 1990 from Harvard University.
His humanitarian work began when he was a college student volunteering in Haiti in 1983 working with dispossessed farmers. In 1987, he cofounded Partners In Health with the goal of helping patients in poverty-stricken corners of the world.
Under Dr. Farmer’s leadership, the nonprofit tackled major public health crises: Haiti’s devastating 2010 earthquake, drug-resistant tuberculosis in Peru and other countries, and an Ebola outbreak that tore through West Africa.
Dr. Farmer documented his 2014-2015 experience treating Africa’s Ebola patients in a book called “Fevers, Feuds, and Diamonds: Ebola and the Ravages of History.”
He wrote that by the time he arrived, “western Sierra Leone was ground zero of the epidemic, and Upper West Africa was just about the worst place in the world to be critically ill or injured.”
One of his greatest qualities was his ability to connect with patients – to treat them “not like ones who suffered, but like a pal you’d joke with,” said Pardis Sabeti, MD, PhD, a Harvard University geneticist who also spent time in Africa and famously sequenced samples of the Ebola virus’ genome.
Dr. Sabeti and Dr. Farmer bonded over their love for Sierra Leone, and their grief over losing a close colleague to Ebola, Sheik Humarr Khan, who was one of the area’s leading infectious disease experts.
Dr. Sabeti first met Dr. Farmer years earlier as a first-year Harvard medical student when she enrolled in one of his courses. She said students introduced themselves, one by one, each veering into heartfelt testimonies about what Dr. Farmer’s work had meant to them.
Dr. Farmer and Dr. Sabeti were just texting on Feb. 19, and the two were “goofing around in our usual way, and scheming about how to make the world better, as we always did.”
Dr. Farmer was funny, mischievous, and above all, exactly what you would expect upon meeting him, Dr. Sabeti said.
“It’s cliché, but the energetic kick you get from just being in his presence, it’s almost otherworldly,” she said. “It’s not even otherworldly in the sense of: ‘I just came across – greatness.’ It’s more: ‘I just came across kindness.’ ”
Dr. Farmer’s work has been widely distributed in publications including Bulletin of the World Health Organization, The Lancet, the New England Journal of Medicine, Clinical Infectious Diseases, and Social Science & Medicine.
He was awarded the 2020 Berggruen Prize for Philosophy & Culture, the Margaret Mead Award from the American Anthropological Association, the American Medical Association’s Outstanding International Physician (Nathan Davis) Award, and, with his Partners In Health colleagues, the Hilton Humanitarian Prize.
He is survived by his wife, Didi Bertrand Farmer, and their three children.
A verison of this article first appeared on Medscape.com.
Pediatrics group stresses benefits of vitamin K shots for infants
After the American Academy of Pediatrics (AAP) began recommending vitamin K shots for newborns in 1961, infant bleeding as a result of vitamin K deficiency plummeted. The life-threatening disorder is so rare that some parents now question the need for injections to safeguard against it.
The situation amounts to “a failure of our success,” Ivan Hand, MD, a coauthor of a new AAP statement on vitamin K, told this news organization. Much like diseases that can be prevented with vaccines, vitamin K deficiency bleeding isn’t top of mind for parents. “It’s not something they’re aware of or afraid of,” he said.
In 2019, however, the AAP listed public education about the importance of the shots in its 10 most important priorities.
The policy update urges clinicians to bone up on the benefits and perceived risks of vitamin K deficiency, which is essential for clotting, and to “strongly advocate” for the shot in discussions with parents who may get competing messages from their social circles, the internet, and other health care professionals.
Dr. Hand, director of neonatology at NYC Health + Hospitals Kings County, Brooklyn, said clinicians walk a line between educating and alienating parents who favor natural birth processes. “We’re hoping that by talking to the families and answering their questions and explaining the risks, parents will accept vitamin K as a necessary treatment for their babies,” he said.
Vitamin K does not easily pass through the placenta and is not plentiful in breast milk, the preferred nutrition source for newborns. It takes months for babies to build their stores through food and gut bacteria.
Infants who do not receive vitamin K at birth are 81 times more likely to develop late-onset vitamin K deficiency bleeding, which occurs a week to 6 months after birth, according to the Centers for Disease Control and Prevention. One in five babies with the disorder dies, and about half have bleeding in the skull that can lead to brain damage.
New dosing for premature infants
The AAP’s new statement, published in the journal Pediatrics, reaffirms the administration of a 1-mg intramuscular dose for infants weighing more than 1,500 grams, or about 3 lb 5 oz, within 6 hours of birth. For premature infants who weigh less, the guidance recommends an intramuscular dose of 0.3 to 0.5 mg/kg.
The group notes that oral preparations of vitamin K have proven less effective because of malabsorption and challenges with adhering to dosing regimens.
The document also warns that breastfed babies can experience vitamin K deficiency bleeding even if they have received the shot, because concentration of vitamin K often wanes before a baby starts eating solid food. The disorder “should be considered when evaluating bleeding in the first 6 months of life, even in infants who received prophylaxis, and especially in exclusively breastfed infants,” it states.
Accounts of parental refusals date back to 2013, when the CDC reported four cases of deficiency bleeding in Tennessee. The infants’ parents said they declined vitamin K because they worried about increased risk of leukemia, thought the injection was unnecessary, or wanted to minimize the baby’s exposure to “toxins.” Leukemia concern stemmed from a 1992 report linking vitamin K to childhood cancer, an association that did not hold up in subsequent studies.
More recent research has documented parental concerns about preservatives and injection pain as well as distrust of medical and public health authorities. Some parents have been accused of neglect for refusing to allow their babies to receive the shots.
Phoebe Danziger, MD, a pediatrician and writer in rural Michigan who has studied parental refusal of standard-of-care interventions, called the document a “welcome update” to the AAP’s last statement on the topic, in 2003. She told this news organization that lower dosing for premature infants may reassure some vitamin K–hesitant parents who worry about one-size-fits-all dosing.
But Dr. Danziger added that “evidence is lacking to support the claim that pediatricians can really move the needle on parental hesitancy and refusal simply through better listening and more persuasive counseling.” She said the AAP should do more to address “the broader social climate of mistrust and misinformation” that fuels refusal.
Dr. Hand and Dr. Danziger have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After the American Academy of Pediatrics (AAP) began recommending vitamin K shots for newborns in 1961, infant bleeding as a result of vitamin K deficiency plummeted. The life-threatening disorder is so rare that some parents now question the need for injections to safeguard against it.
The situation amounts to “a failure of our success,” Ivan Hand, MD, a coauthor of a new AAP statement on vitamin K, told this news organization. Much like diseases that can be prevented with vaccines, vitamin K deficiency bleeding isn’t top of mind for parents. “It’s not something they’re aware of or afraid of,” he said.
In 2019, however, the AAP listed public education about the importance of the shots in its 10 most important priorities.
The policy update urges clinicians to bone up on the benefits and perceived risks of vitamin K deficiency, which is essential for clotting, and to “strongly advocate” for the shot in discussions with parents who may get competing messages from their social circles, the internet, and other health care professionals.
Dr. Hand, director of neonatology at NYC Health + Hospitals Kings County, Brooklyn, said clinicians walk a line between educating and alienating parents who favor natural birth processes. “We’re hoping that by talking to the families and answering their questions and explaining the risks, parents will accept vitamin K as a necessary treatment for their babies,” he said.
Vitamin K does not easily pass through the placenta and is not plentiful in breast milk, the preferred nutrition source for newborns. It takes months for babies to build their stores through food and gut bacteria.
Infants who do not receive vitamin K at birth are 81 times more likely to develop late-onset vitamin K deficiency bleeding, which occurs a week to 6 months after birth, according to the Centers for Disease Control and Prevention. One in five babies with the disorder dies, and about half have bleeding in the skull that can lead to brain damage.
New dosing for premature infants
The AAP’s new statement, published in the journal Pediatrics, reaffirms the administration of a 1-mg intramuscular dose for infants weighing more than 1,500 grams, or about 3 lb 5 oz, within 6 hours of birth. For premature infants who weigh less, the guidance recommends an intramuscular dose of 0.3 to 0.5 mg/kg.
The group notes that oral preparations of vitamin K have proven less effective because of malabsorption and challenges with adhering to dosing regimens.
The document also warns that breastfed babies can experience vitamin K deficiency bleeding even if they have received the shot, because concentration of vitamin K often wanes before a baby starts eating solid food. The disorder “should be considered when evaluating bleeding in the first 6 months of life, even in infants who received prophylaxis, and especially in exclusively breastfed infants,” it states.
Accounts of parental refusals date back to 2013, when the CDC reported four cases of deficiency bleeding in Tennessee. The infants’ parents said they declined vitamin K because they worried about increased risk of leukemia, thought the injection was unnecessary, or wanted to minimize the baby’s exposure to “toxins.” Leukemia concern stemmed from a 1992 report linking vitamin K to childhood cancer, an association that did not hold up in subsequent studies.
More recent research has documented parental concerns about preservatives and injection pain as well as distrust of medical and public health authorities. Some parents have been accused of neglect for refusing to allow their babies to receive the shots.
Phoebe Danziger, MD, a pediatrician and writer in rural Michigan who has studied parental refusal of standard-of-care interventions, called the document a “welcome update” to the AAP’s last statement on the topic, in 2003. She told this news organization that lower dosing for premature infants may reassure some vitamin K–hesitant parents who worry about one-size-fits-all dosing.
But Dr. Danziger added that “evidence is lacking to support the claim that pediatricians can really move the needle on parental hesitancy and refusal simply through better listening and more persuasive counseling.” She said the AAP should do more to address “the broader social climate of mistrust and misinformation” that fuels refusal.
Dr. Hand and Dr. Danziger have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After the American Academy of Pediatrics (AAP) began recommending vitamin K shots for newborns in 1961, infant bleeding as a result of vitamin K deficiency plummeted. The life-threatening disorder is so rare that some parents now question the need for injections to safeguard against it.
The situation amounts to “a failure of our success,” Ivan Hand, MD, a coauthor of a new AAP statement on vitamin K, told this news organization. Much like diseases that can be prevented with vaccines, vitamin K deficiency bleeding isn’t top of mind for parents. “It’s not something they’re aware of or afraid of,” he said.
In 2019, however, the AAP listed public education about the importance of the shots in its 10 most important priorities.
The policy update urges clinicians to bone up on the benefits and perceived risks of vitamin K deficiency, which is essential for clotting, and to “strongly advocate” for the shot in discussions with parents who may get competing messages from their social circles, the internet, and other health care professionals.
Dr. Hand, director of neonatology at NYC Health + Hospitals Kings County, Brooklyn, said clinicians walk a line between educating and alienating parents who favor natural birth processes. “We’re hoping that by talking to the families and answering their questions and explaining the risks, parents will accept vitamin K as a necessary treatment for their babies,” he said.
Vitamin K does not easily pass through the placenta and is not plentiful in breast milk, the preferred nutrition source for newborns. It takes months for babies to build their stores through food and gut bacteria.
Infants who do not receive vitamin K at birth are 81 times more likely to develop late-onset vitamin K deficiency bleeding, which occurs a week to 6 months after birth, according to the Centers for Disease Control and Prevention. One in five babies with the disorder dies, and about half have bleeding in the skull that can lead to brain damage.
New dosing for premature infants
The AAP’s new statement, published in the journal Pediatrics, reaffirms the administration of a 1-mg intramuscular dose for infants weighing more than 1,500 grams, or about 3 lb 5 oz, within 6 hours of birth. For premature infants who weigh less, the guidance recommends an intramuscular dose of 0.3 to 0.5 mg/kg.
The group notes that oral preparations of vitamin K have proven less effective because of malabsorption and challenges with adhering to dosing regimens.
The document also warns that breastfed babies can experience vitamin K deficiency bleeding even if they have received the shot, because concentration of vitamin K often wanes before a baby starts eating solid food. The disorder “should be considered when evaluating bleeding in the first 6 months of life, even in infants who received prophylaxis, and especially in exclusively breastfed infants,” it states.
Accounts of parental refusals date back to 2013, when the CDC reported four cases of deficiency bleeding in Tennessee. The infants’ parents said they declined vitamin K because they worried about increased risk of leukemia, thought the injection was unnecessary, or wanted to minimize the baby’s exposure to “toxins.” Leukemia concern stemmed from a 1992 report linking vitamin K to childhood cancer, an association that did not hold up in subsequent studies.
More recent research has documented parental concerns about preservatives and injection pain as well as distrust of medical and public health authorities. Some parents have been accused of neglect for refusing to allow their babies to receive the shots.
Phoebe Danziger, MD, a pediatrician and writer in rural Michigan who has studied parental refusal of standard-of-care interventions, called the document a “welcome update” to the AAP’s last statement on the topic, in 2003. She told this news organization that lower dosing for premature infants may reassure some vitamin K–hesitant parents who worry about one-size-fits-all dosing.
But Dr. Danziger added that “evidence is lacking to support the claim that pediatricians can really move the needle on parental hesitancy and refusal simply through better listening and more persuasive counseling.” She said the AAP should do more to address “the broader social climate of mistrust and misinformation” that fuels refusal.
Dr. Hand and Dr. Danziger have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers tout new CLL prognostic tool
Researchers report that they’ve confirmed the usefulness of a new tool to help physicians pinpoint prognoses for patients with chronic lymphocytic leukemia (CLL).
“Physicians may use this tool to support decisions regarding supportive care, manage the patient’s and physician’s expectations, and potentially tailor therapy,” study lead author and epidemiologist Emelie Rotbain, MD, PhD, of Rigshospitalet in Copenhagen, said in an interview.
The study appeared Jan. 10 in the journal Blood Advances.
According to Dr. Rotbain,
Researchers developed the questions based on an analysis of categories in the Cumulative Illness Rating Scale that are most linked to event-free survival (EFS) from time of treatment.
The tool looks at three organ systems – vascular, upper GI, and endocrine – and asks about conditions such as diabetes and chronic use of a proton pump inhibitor, study coauthor and hematologist/oncologist Alexey V. Danilov, MD, PhD, codirector of the Toni Stephenson Lymphoma Center at the City of Hope National Medical Center, Duarte, Calif., said in an interview. The tool then generates a score based on the variables.
For the new study, the researchers retrospectively applied the tool to 4,975 patients who appeared in the Danish National CLL Register from 2008 to 2018 (61% male, median age 70.7.). Of those, 1,513 received first-line treatment during follow-up (median = 4.39 years).
At diagnosis, nearly two-thirds (63%) of patients were considered to be low risk. None of these had endocrinological, upper gastrointestinal, or vascular disease. Another 30% were considered to be at intermediate risk. The remaining 7% were at high risk. They had high levels of endocrinological (55.6%), upper gastrointestinal (64.6%), and vascular disease (91.0%).
The high-risk patients had a median survival of 6.0 years. The intermediate-risk patients lived for a median of 8.5 years, while the low-risk patients didn’t reach a median survival level.
Fifty-six percent of high-risk patients were treated within 4 years, compared to 20%-30% of intermediate- and low-risk patients. Median event-free survival from time of treatment was 8.4, 4.4, and 2.2 years for the low-, intermediate-, and high-risk groups, respectively.
The authors cautioned that “differences in survival by type of treatment, particularly in patients treated with targeted therapies who were underrepresented in this study, could influence survival and limit the generalizability of these results.”
They added that “while prognostic factors should remain key for treatment decisions, clinical trial data from pivotal phase 3 trials with novel targeted agents versus chemoimmunotherapy should be reanalyzed with addition of CLL-CI to assess the optimal treatment for patients according to CLL-CI.”
The tool is not yet available online, Dr. Danilov said, “but that is something that we as a group could potentially work on.”
Joanna Rhodes, MD, assistant professor with Northwell Health Cancer Institute/Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y., said in an interview that the tool is easy to use and appropriate to apply at first consultation. It should be used in conjunction with the International Prognostic Index for Chronic Lymphocytic Leukemia (CLL-IPI), she said.
“We often discuss frailty as a factor in types of and timing of treatment for patients with CLL, but often this is not directly measured in clinical practice,” she said. “The CLL-CI is associated with important outcomes, particularly overall survival, which is our most important metric in oncology. Additionally, it provides important information on time to first treatment and overall survival, which are useful when we are counseling patients.”
Like the study authors, Dr. Rhodes cautioned that the CLL-CI has not been validated specifically in patients treated with targeted therapies. “It may not be applicable in this setting, particularly in the front-line setting, as these treatments were underrepresented in this cohort. Further studies in this population are needed to answer this question.”
The study is funded in part by Novo Nordisk Foundation. Several study authors report various disclosures outside the scope of this study. Dr. Rhodes has no disclosures.
Researchers report that they’ve confirmed the usefulness of a new tool to help physicians pinpoint prognoses for patients with chronic lymphocytic leukemia (CLL).
“Physicians may use this tool to support decisions regarding supportive care, manage the patient’s and physician’s expectations, and potentially tailor therapy,” study lead author and epidemiologist Emelie Rotbain, MD, PhD, of Rigshospitalet in Copenhagen, said in an interview.
The study appeared Jan. 10 in the journal Blood Advances.
According to Dr. Rotbain,
Researchers developed the questions based on an analysis of categories in the Cumulative Illness Rating Scale that are most linked to event-free survival (EFS) from time of treatment.
The tool looks at three organ systems – vascular, upper GI, and endocrine – and asks about conditions such as diabetes and chronic use of a proton pump inhibitor, study coauthor and hematologist/oncologist Alexey V. Danilov, MD, PhD, codirector of the Toni Stephenson Lymphoma Center at the City of Hope National Medical Center, Duarte, Calif., said in an interview. The tool then generates a score based on the variables.
For the new study, the researchers retrospectively applied the tool to 4,975 patients who appeared in the Danish National CLL Register from 2008 to 2018 (61% male, median age 70.7.). Of those, 1,513 received first-line treatment during follow-up (median = 4.39 years).
At diagnosis, nearly two-thirds (63%) of patients were considered to be low risk. None of these had endocrinological, upper gastrointestinal, or vascular disease. Another 30% were considered to be at intermediate risk. The remaining 7% were at high risk. They had high levels of endocrinological (55.6%), upper gastrointestinal (64.6%), and vascular disease (91.0%).
The high-risk patients had a median survival of 6.0 years. The intermediate-risk patients lived for a median of 8.5 years, while the low-risk patients didn’t reach a median survival level.
Fifty-six percent of high-risk patients were treated within 4 years, compared to 20%-30% of intermediate- and low-risk patients. Median event-free survival from time of treatment was 8.4, 4.4, and 2.2 years for the low-, intermediate-, and high-risk groups, respectively.
The authors cautioned that “differences in survival by type of treatment, particularly in patients treated with targeted therapies who were underrepresented in this study, could influence survival and limit the generalizability of these results.”
They added that “while prognostic factors should remain key for treatment decisions, clinical trial data from pivotal phase 3 trials with novel targeted agents versus chemoimmunotherapy should be reanalyzed with addition of CLL-CI to assess the optimal treatment for patients according to CLL-CI.”
The tool is not yet available online, Dr. Danilov said, “but that is something that we as a group could potentially work on.”
Joanna Rhodes, MD, assistant professor with Northwell Health Cancer Institute/Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y., said in an interview that the tool is easy to use and appropriate to apply at first consultation. It should be used in conjunction with the International Prognostic Index for Chronic Lymphocytic Leukemia (CLL-IPI), she said.
“We often discuss frailty as a factor in types of and timing of treatment for patients with CLL, but often this is not directly measured in clinical practice,” she said. “The CLL-CI is associated with important outcomes, particularly overall survival, which is our most important metric in oncology. Additionally, it provides important information on time to first treatment and overall survival, which are useful when we are counseling patients.”
Like the study authors, Dr. Rhodes cautioned that the CLL-CI has not been validated specifically in patients treated with targeted therapies. “It may not be applicable in this setting, particularly in the front-line setting, as these treatments were underrepresented in this cohort. Further studies in this population are needed to answer this question.”
The study is funded in part by Novo Nordisk Foundation. Several study authors report various disclosures outside the scope of this study. Dr. Rhodes has no disclosures.
Researchers report that they’ve confirmed the usefulness of a new tool to help physicians pinpoint prognoses for patients with chronic lymphocytic leukemia (CLL).
“Physicians may use this tool to support decisions regarding supportive care, manage the patient’s and physician’s expectations, and potentially tailor therapy,” study lead author and epidemiologist Emelie Rotbain, MD, PhD, of Rigshospitalet in Copenhagen, said in an interview.
The study appeared Jan. 10 in the journal Blood Advances.
According to Dr. Rotbain,
Researchers developed the questions based on an analysis of categories in the Cumulative Illness Rating Scale that are most linked to event-free survival (EFS) from time of treatment.
The tool looks at three organ systems – vascular, upper GI, and endocrine – and asks about conditions such as diabetes and chronic use of a proton pump inhibitor, study coauthor and hematologist/oncologist Alexey V. Danilov, MD, PhD, codirector of the Toni Stephenson Lymphoma Center at the City of Hope National Medical Center, Duarte, Calif., said in an interview. The tool then generates a score based on the variables.
For the new study, the researchers retrospectively applied the tool to 4,975 patients who appeared in the Danish National CLL Register from 2008 to 2018 (61% male, median age 70.7.). Of those, 1,513 received first-line treatment during follow-up (median = 4.39 years).
At diagnosis, nearly two-thirds (63%) of patients were considered to be low risk. None of these had endocrinological, upper gastrointestinal, or vascular disease. Another 30% were considered to be at intermediate risk. The remaining 7% were at high risk. They had high levels of endocrinological (55.6%), upper gastrointestinal (64.6%), and vascular disease (91.0%).
The high-risk patients had a median survival of 6.0 years. The intermediate-risk patients lived for a median of 8.5 years, while the low-risk patients didn’t reach a median survival level.
Fifty-six percent of high-risk patients were treated within 4 years, compared to 20%-30% of intermediate- and low-risk patients. Median event-free survival from time of treatment was 8.4, 4.4, and 2.2 years for the low-, intermediate-, and high-risk groups, respectively.
The authors cautioned that “differences in survival by type of treatment, particularly in patients treated with targeted therapies who were underrepresented in this study, could influence survival and limit the generalizability of these results.”
They added that “while prognostic factors should remain key for treatment decisions, clinical trial data from pivotal phase 3 trials with novel targeted agents versus chemoimmunotherapy should be reanalyzed with addition of CLL-CI to assess the optimal treatment for patients according to CLL-CI.”
The tool is not yet available online, Dr. Danilov said, “but that is something that we as a group could potentially work on.”
Joanna Rhodes, MD, assistant professor with Northwell Health Cancer Institute/Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y., said in an interview that the tool is easy to use and appropriate to apply at first consultation. It should be used in conjunction with the International Prognostic Index for Chronic Lymphocytic Leukemia (CLL-IPI), she said.
“We often discuss frailty as a factor in types of and timing of treatment for patients with CLL, but often this is not directly measured in clinical practice,” she said. “The CLL-CI is associated with important outcomes, particularly overall survival, which is our most important metric in oncology. Additionally, it provides important information on time to first treatment and overall survival, which are useful when we are counseling patients.”
Like the study authors, Dr. Rhodes cautioned that the CLL-CI has not been validated specifically in patients treated with targeted therapies. “It may not be applicable in this setting, particularly in the front-line setting, as these treatments were underrepresented in this cohort. Further studies in this population are needed to answer this question.”
The study is funded in part by Novo Nordisk Foundation. Several study authors report various disclosures outside the scope of this study. Dr. Rhodes has no disclosures.
FROM BLOOD ADVANCES
Children and COVID: The Omicron surge has become a retreat
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 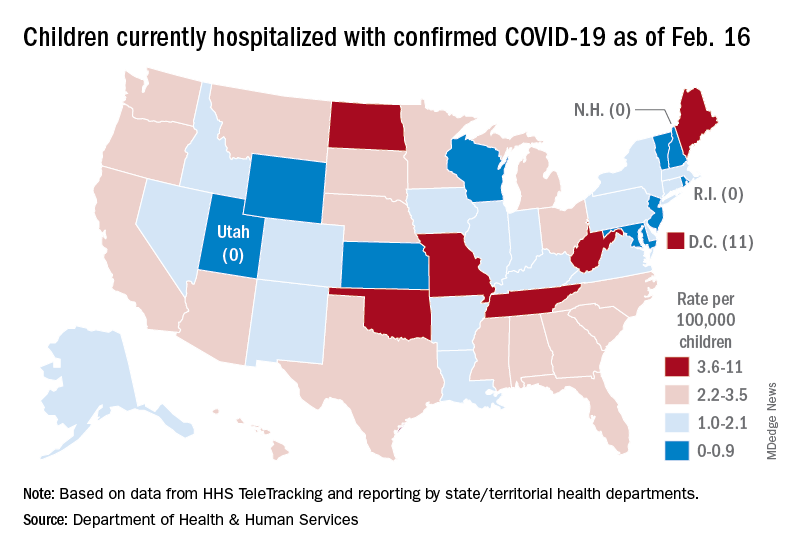
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
Women with von Willebrand disease: Managing menstrual and postpartum bleeding
Women with von Willebrand disease (VWD) experience many obstetric and gynecologic challenges, including higher levels of von Willebrand factor (VWF) in pregnancy, Romina Brignardello-Petersen, PhD, of McMaster University, Hamilton, Ont., and colleagues wrote.
The American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia convened a working group in 2017 to address updated guidelines on VWD with a focus on women, the researchers said.
In an article published in Blood Advances, the researchers described the evidence from three systematic reviews conducted to inform three recommendations for the guidelines: first-line management of heavy menstrual bleeding (HMB), treatment of women requiring or desiring neuraxial analgesia, and management of postpartum hemorrhage. The authors identified studies published through October 2019.
The first systematic review of first-line therapies for HMB included five case series, one retrospective cohort study, and one randomized controlled trial. In the randomized controlled trial of 232 patients, low-certainty evidence suggested less reduction of blood loss with desmopressin, compared with tranexamic acid (TxA), with no significant differences in side effects. Very-low-certainty evidence from an observational study also supported lower effectiveness of desmopressin versus hormonal therapy. Finally, the case series showed very-low-certainty evidence for the comparative effectiveness of hormonal therapy delivered via a levonorgestrel-releasing intrauterine system (LNG-IUS) and other therapies for HMB control.
The second systematic review compared VWF levels in women who received neuraxial anesthesia during labor.
The review included five case series that described outcomes of women with VWF levels greater than 0.50 IU/mL; however, the studies did not describe outcomes according to VWF levels and did not cite the proportion of women with VWF levels greater than 1.50 IU/mL. Consequently, the evidence for the effects of increasing VWF levels was very low certainty, the authors said. In a meta-analysis, the proportion of anesthesia complications in these women was 6% (very low certainty). The complications included hypotension, accidental dural puncture, inadequate analgesia, bloody tap with no further complications, and failed block requiring general anesthesia.
The third systemic review included two retrospective cohort studies on the use of TxA during the postpartum period. In these studies, the authors found very-low-certainty evidence that TxA reduced the risk of severe primary postpartum hemorrhage, primary postpartum hemorrhage, and secondary postpartum hemorrhage (risk ratios, 0.36, 0.25, and 0.42, respectively). The effects of TxA on blood transfusions, vaginal hematoma, blood loss, and thrombotic complications also showed very-low-certainty evidence.
The currently available evidence for treatment options in women with VWD remains very low certainty, the researchers wrote in their discussion. “Because hormonal therapy is effective in controlling HMB (based on data from women without bleeding disorders), we believe the most effective strategy to be hormonal therapy with a LNG-IUS or combined oral contraceptives, followed by TxA and desmopressin.”
The study findings were limited by several factors including scarce evidence, the risk of bias in the observational studies, and lack of comparisons/controls in the case series, the researchers noted. Notable literature gaps included data on outcomes including major bleeding and the need for surgery or additional treatments in the first review; mortality, major bleeding, spinal hematoma, transfusion, and thrombotic events in the second review; and mortality, major bleeding, and the need for other procedures in the third review.
However, the findings were strengthened by the use of broad eligibility criteria to include any studies with potential useful advice, including case series, if these were the only available options. In developing recommendations, “the guideline panel interpreted the evidence adding their experience and knowledge of indirect evidence,” the authors noted.
The current evidence, though mainly very low certainty, “is the best available to inform decisions about management. Clinicians seeking advice on how to manage their patients with VWD should refer to the practice guidelines and assess to what extent they are applicable to their patients,” the researchers concluded.
Meeting the need for evidence-based guidelines
The review is important at this time because current evidence-based guidelines are limited, said coauthor Veronica Flood, MD, a pediatric hematologist at the Medical College of Wisconsin, Milwaukee, and a VWD researcher.
“While we have some guidelines that address von Willebrand disease, these were primarily based on expert opinion and not necessarily based on the best available evidence,” said Dr. Flood.
“Given how many people have von Willebrand disease, it is important that we actually base our recommendations on the data,” she emphasized. The new guidelines also incorporate patient feedback, with the inclusion of multiple panelists who are individuals living with VWD. “The final recommendations looked at not only the evidence, but the cost effectiveness, feasibility, and patient values and preferences,” she added.
“I was surprised we did not have better evidence for some of these common issues for patients with VWD,” said Dr. Flood. “I think that speaks to the need to do more high-quality research in this area.”
From a clinical standpoint, “we now have evidence-based guidelines that support the use of prophylaxis in patients with VWD and significant bleeding, as well as recommendations for surgery and bleeding issues around menstruation,” said Dr. Flood. “I do think it is also important to recognize that many of these are conditional recommendations, meaning there is room for patient preferences in implementation, which is helpful since we know that some people will have different priorities.”
Dr. Flood noted that more research is needed in many aspects of VWD. “We definitely need to better understand best options for surgical treatment, and I consider that a high priority. We are also hoping, along with the National Hemophilia Foundation, to develop some patient decision aids to help with some of these issues.”
Coauthor Nathan T. Connell, MD, an adult hematologist at the Brigham and Women’s Hospital and Harvard Medical School, both in Boston, served as the vice chair for the guideline panel. Dr. Connell agreed with the importance of the reviews and the need for additional research. “I, too, was surprised to see the lack of robust data to answer many of the basic questions about how to manage people living with VWD. Regarding the systematic reviews, I was surprised to see the power of combining the limited data in this way to come up with an evidence base for the panels to review,” he added.
The study was supported by the ASH, ISTH, NHF, and the WFH 2020 Guidelines for Management of VWD. The researchers had no financial conflicts to disclose.
Women with von Willebrand disease (VWD) experience many obstetric and gynecologic challenges, including higher levels of von Willebrand factor (VWF) in pregnancy, Romina Brignardello-Petersen, PhD, of McMaster University, Hamilton, Ont., and colleagues wrote.
The American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia convened a working group in 2017 to address updated guidelines on VWD with a focus on women, the researchers said.
In an article published in Blood Advances, the researchers described the evidence from three systematic reviews conducted to inform three recommendations for the guidelines: first-line management of heavy menstrual bleeding (HMB), treatment of women requiring or desiring neuraxial analgesia, and management of postpartum hemorrhage. The authors identified studies published through October 2019.
The first systematic review of first-line therapies for HMB included five case series, one retrospective cohort study, and one randomized controlled trial. In the randomized controlled trial of 232 patients, low-certainty evidence suggested less reduction of blood loss with desmopressin, compared with tranexamic acid (TxA), with no significant differences in side effects. Very-low-certainty evidence from an observational study also supported lower effectiveness of desmopressin versus hormonal therapy. Finally, the case series showed very-low-certainty evidence for the comparative effectiveness of hormonal therapy delivered via a levonorgestrel-releasing intrauterine system (LNG-IUS) and other therapies for HMB control.
The second systematic review compared VWF levels in women who received neuraxial anesthesia during labor.
The review included five case series that described outcomes of women with VWF levels greater than 0.50 IU/mL; however, the studies did not describe outcomes according to VWF levels and did not cite the proportion of women with VWF levels greater than 1.50 IU/mL. Consequently, the evidence for the effects of increasing VWF levels was very low certainty, the authors said. In a meta-analysis, the proportion of anesthesia complications in these women was 6% (very low certainty). The complications included hypotension, accidental dural puncture, inadequate analgesia, bloody tap with no further complications, and failed block requiring general anesthesia.
The third systemic review included two retrospective cohort studies on the use of TxA during the postpartum period. In these studies, the authors found very-low-certainty evidence that TxA reduced the risk of severe primary postpartum hemorrhage, primary postpartum hemorrhage, and secondary postpartum hemorrhage (risk ratios, 0.36, 0.25, and 0.42, respectively). The effects of TxA on blood transfusions, vaginal hematoma, blood loss, and thrombotic complications also showed very-low-certainty evidence.
The currently available evidence for treatment options in women with VWD remains very low certainty, the researchers wrote in their discussion. “Because hormonal therapy is effective in controlling HMB (based on data from women without bleeding disorders), we believe the most effective strategy to be hormonal therapy with a LNG-IUS or combined oral contraceptives, followed by TxA and desmopressin.”
The study findings were limited by several factors including scarce evidence, the risk of bias in the observational studies, and lack of comparisons/controls in the case series, the researchers noted. Notable literature gaps included data on outcomes including major bleeding and the need for surgery or additional treatments in the first review; mortality, major bleeding, spinal hematoma, transfusion, and thrombotic events in the second review; and mortality, major bleeding, and the need for other procedures in the third review.
However, the findings were strengthened by the use of broad eligibility criteria to include any studies with potential useful advice, including case series, if these were the only available options. In developing recommendations, “the guideline panel interpreted the evidence adding their experience and knowledge of indirect evidence,” the authors noted.
The current evidence, though mainly very low certainty, “is the best available to inform decisions about management. Clinicians seeking advice on how to manage their patients with VWD should refer to the practice guidelines and assess to what extent they are applicable to their patients,” the researchers concluded.
Meeting the need for evidence-based guidelines
The review is important at this time because current evidence-based guidelines are limited, said coauthor Veronica Flood, MD, a pediatric hematologist at the Medical College of Wisconsin, Milwaukee, and a VWD researcher.
“While we have some guidelines that address von Willebrand disease, these were primarily based on expert opinion and not necessarily based on the best available evidence,” said Dr. Flood.
“Given how many people have von Willebrand disease, it is important that we actually base our recommendations on the data,” she emphasized. The new guidelines also incorporate patient feedback, with the inclusion of multiple panelists who are individuals living with VWD. “The final recommendations looked at not only the evidence, but the cost effectiveness, feasibility, and patient values and preferences,” she added.
“I was surprised we did not have better evidence for some of these common issues for patients with VWD,” said Dr. Flood. “I think that speaks to the need to do more high-quality research in this area.”
From a clinical standpoint, “we now have evidence-based guidelines that support the use of prophylaxis in patients with VWD and significant bleeding, as well as recommendations for surgery and bleeding issues around menstruation,” said Dr. Flood. “I do think it is also important to recognize that many of these are conditional recommendations, meaning there is room for patient preferences in implementation, which is helpful since we know that some people will have different priorities.”
Dr. Flood noted that more research is needed in many aspects of VWD. “We definitely need to better understand best options for surgical treatment, and I consider that a high priority. We are also hoping, along with the National Hemophilia Foundation, to develop some patient decision aids to help with some of these issues.”
Coauthor Nathan T. Connell, MD, an adult hematologist at the Brigham and Women’s Hospital and Harvard Medical School, both in Boston, served as the vice chair for the guideline panel. Dr. Connell agreed with the importance of the reviews and the need for additional research. “I, too, was surprised to see the lack of robust data to answer many of the basic questions about how to manage people living with VWD. Regarding the systematic reviews, I was surprised to see the power of combining the limited data in this way to come up with an evidence base for the panels to review,” he added.
The study was supported by the ASH, ISTH, NHF, and the WFH 2020 Guidelines for Management of VWD. The researchers had no financial conflicts to disclose.
Women with von Willebrand disease (VWD) experience many obstetric and gynecologic challenges, including higher levels of von Willebrand factor (VWF) in pregnancy, Romina Brignardello-Petersen, PhD, of McMaster University, Hamilton, Ont., and colleagues wrote.
The American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia convened a working group in 2017 to address updated guidelines on VWD with a focus on women, the researchers said.
In an article published in Blood Advances, the researchers described the evidence from three systematic reviews conducted to inform three recommendations for the guidelines: first-line management of heavy menstrual bleeding (HMB), treatment of women requiring or desiring neuraxial analgesia, and management of postpartum hemorrhage. The authors identified studies published through October 2019.
The first systematic review of first-line therapies for HMB included five case series, one retrospective cohort study, and one randomized controlled trial. In the randomized controlled trial of 232 patients, low-certainty evidence suggested less reduction of blood loss with desmopressin, compared with tranexamic acid (TxA), with no significant differences in side effects. Very-low-certainty evidence from an observational study also supported lower effectiveness of desmopressin versus hormonal therapy. Finally, the case series showed very-low-certainty evidence for the comparative effectiveness of hormonal therapy delivered via a levonorgestrel-releasing intrauterine system (LNG-IUS) and other therapies for HMB control.
The second systematic review compared VWF levels in women who received neuraxial anesthesia during labor.
The review included five case series that described outcomes of women with VWF levels greater than 0.50 IU/mL; however, the studies did not describe outcomes according to VWF levels and did not cite the proportion of women with VWF levels greater than 1.50 IU/mL. Consequently, the evidence for the effects of increasing VWF levels was very low certainty, the authors said. In a meta-analysis, the proportion of anesthesia complications in these women was 6% (very low certainty). The complications included hypotension, accidental dural puncture, inadequate analgesia, bloody tap with no further complications, and failed block requiring general anesthesia.
The third systemic review included two retrospective cohort studies on the use of TxA during the postpartum period. In these studies, the authors found very-low-certainty evidence that TxA reduced the risk of severe primary postpartum hemorrhage, primary postpartum hemorrhage, and secondary postpartum hemorrhage (risk ratios, 0.36, 0.25, and 0.42, respectively). The effects of TxA on blood transfusions, vaginal hematoma, blood loss, and thrombotic complications also showed very-low-certainty evidence.
The currently available evidence for treatment options in women with VWD remains very low certainty, the researchers wrote in their discussion. “Because hormonal therapy is effective in controlling HMB (based on data from women without bleeding disorders), we believe the most effective strategy to be hormonal therapy with a LNG-IUS or combined oral contraceptives, followed by TxA and desmopressin.”
The study findings were limited by several factors including scarce evidence, the risk of bias in the observational studies, and lack of comparisons/controls in the case series, the researchers noted. Notable literature gaps included data on outcomes including major bleeding and the need for surgery or additional treatments in the first review; mortality, major bleeding, spinal hematoma, transfusion, and thrombotic events in the second review; and mortality, major bleeding, and the need for other procedures in the third review.
However, the findings were strengthened by the use of broad eligibility criteria to include any studies with potential useful advice, including case series, if these were the only available options. In developing recommendations, “the guideline panel interpreted the evidence adding their experience and knowledge of indirect evidence,” the authors noted.
The current evidence, though mainly very low certainty, “is the best available to inform decisions about management. Clinicians seeking advice on how to manage their patients with VWD should refer to the practice guidelines and assess to what extent they are applicable to their patients,” the researchers concluded.
Meeting the need for evidence-based guidelines
The review is important at this time because current evidence-based guidelines are limited, said coauthor Veronica Flood, MD, a pediatric hematologist at the Medical College of Wisconsin, Milwaukee, and a VWD researcher.
“While we have some guidelines that address von Willebrand disease, these were primarily based on expert opinion and not necessarily based on the best available evidence,” said Dr. Flood.
“Given how many people have von Willebrand disease, it is important that we actually base our recommendations on the data,” she emphasized. The new guidelines also incorporate patient feedback, with the inclusion of multiple panelists who are individuals living with VWD. “The final recommendations looked at not only the evidence, but the cost effectiveness, feasibility, and patient values and preferences,” she added.
“I was surprised we did not have better evidence for some of these common issues for patients with VWD,” said Dr. Flood. “I think that speaks to the need to do more high-quality research in this area.”
From a clinical standpoint, “we now have evidence-based guidelines that support the use of prophylaxis in patients with VWD and significant bleeding, as well as recommendations for surgery and bleeding issues around menstruation,” said Dr. Flood. “I do think it is also important to recognize that many of these are conditional recommendations, meaning there is room for patient preferences in implementation, which is helpful since we know that some people will have different priorities.”
Dr. Flood noted that more research is needed in many aspects of VWD. “We definitely need to better understand best options for surgical treatment, and I consider that a high priority. We are also hoping, along with the National Hemophilia Foundation, to develop some patient decision aids to help with some of these issues.”
Coauthor Nathan T. Connell, MD, an adult hematologist at the Brigham and Women’s Hospital and Harvard Medical School, both in Boston, served as the vice chair for the guideline panel. Dr. Connell agreed with the importance of the reviews and the need for additional research. “I, too, was surprised to see the lack of robust data to answer many of the basic questions about how to manage people living with VWD. Regarding the systematic reviews, I was surprised to see the power of combining the limited data in this way to come up with an evidence base for the panels to review,” he added.
The study was supported by the ASH, ISTH, NHF, and the WFH 2020 Guidelines for Management of VWD. The researchers had no financial conflicts to disclose.
FROM BLOOD ADVANCES
Mandelic acid
Acids peels are used to elicit a chemical exfoliation of the skin by hydrolyzing amide bonds between keratinocytes, reducing corneocyte adhesion, as well as inducing an inflammatory reaction stimulating tissue remodeling. Release of cytokines such as interleukin (IL)-1 and IL-6 by keratinocytes activates fibroblasts to increase the production of matrix metalloproteinases. These are involved in the production of hyaluronic acid and new collagen formation.
Mandelic acid was derived from bitter almonds (mandel is the German word for almond). It is a white powder originally used as an antibiotic for the treatment of urinary tract infections. Its antibacterial properties make it an excellent product for the topical treatment of acne, as well as for use in topical preparations to treat hyperpigmentation and photoaging. In cosmetic use, mandelic acid is a slow acting chemical peel that can be used in all skin types, including sensitive and rosacea-prone skin, as well as skin of color. Its large molecular size allows for the slow penetration of the acid on the skin and thus it can be carefully titrated.
Studies have shown its efficacy in reducing sebum content, acne, acne scarring, and hyperpigmentation. In clinical practice however, the most effective use of this acid is on sensitive skin. It is a great tool for clinicians to use as an effective exfoliant in less acid tolerant skin types. In commercially available concentrations of 5%-45%, mandelic acid can be used alone or in combination with other beta hydroxy peels, depending on the indication.
Most dermatologists and patients prefer in-office peels that induce noticeable peeling and resurfacing of the skin. Mandelic acid is one of the largest alpha hydroxy acids, a lipophilic acid that penetrates the skin slowly and uniformly, making it an ideal peel in sensitive or aging and thin skin types. Although many mandelic acid peels are available, however, there is a paucity of studies comparing their benefits and efficacies.
Dr. Lily Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
1. Wójcik A et al. Dermatol Alergol. 2013 Jun;30(3):140-5.
2. Soleymani T et al. J Clin Aesthet Dermatol. 2018;11(8):21-8.
Acids peels are used to elicit a chemical exfoliation of the skin by hydrolyzing amide bonds between keratinocytes, reducing corneocyte adhesion, as well as inducing an inflammatory reaction stimulating tissue remodeling. Release of cytokines such as interleukin (IL)-1 and IL-6 by keratinocytes activates fibroblasts to increase the production of matrix metalloproteinases. These are involved in the production of hyaluronic acid and new collagen formation.
Mandelic acid was derived from bitter almonds (mandel is the German word for almond). It is a white powder originally used as an antibiotic for the treatment of urinary tract infections. Its antibacterial properties make it an excellent product for the topical treatment of acne, as well as for use in topical preparations to treat hyperpigmentation and photoaging. In cosmetic use, mandelic acid is a slow acting chemical peel that can be used in all skin types, including sensitive and rosacea-prone skin, as well as skin of color. Its large molecular size allows for the slow penetration of the acid on the skin and thus it can be carefully titrated.
Studies have shown its efficacy in reducing sebum content, acne, acne scarring, and hyperpigmentation. In clinical practice however, the most effective use of this acid is on sensitive skin. It is a great tool for clinicians to use as an effective exfoliant in less acid tolerant skin types. In commercially available concentrations of 5%-45%, mandelic acid can be used alone or in combination with other beta hydroxy peels, depending on the indication.
Most dermatologists and patients prefer in-office peels that induce noticeable peeling and resurfacing of the skin. Mandelic acid is one of the largest alpha hydroxy acids, a lipophilic acid that penetrates the skin slowly and uniformly, making it an ideal peel in sensitive or aging and thin skin types. Although many mandelic acid peels are available, however, there is a paucity of studies comparing their benefits and efficacies.
Dr. Lily Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
1. Wójcik A et al. Dermatol Alergol. 2013 Jun;30(3):140-5.
2. Soleymani T et al. J Clin Aesthet Dermatol. 2018;11(8):21-8.
Acids peels are used to elicit a chemical exfoliation of the skin by hydrolyzing amide bonds between keratinocytes, reducing corneocyte adhesion, as well as inducing an inflammatory reaction stimulating tissue remodeling. Release of cytokines such as interleukin (IL)-1 and IL-6 by keratinocytes activates fibroblasts to increase the production of matrix metalloproteinases. These are involved in the production of hyaluronic acid and new collagen formation.
Mandelic acid was derived from bitter almonds (mandel is the German word for almond). It is a white powder originally used as an antibiotic for the treatment of urinary tract infections. Its antibacterial properties make it an excellent product for the topical treatment of acne, as well as for use in topical preparations to treat hyperpigmentation and photoaging. In cosmetic use, mandelic acid is a slow acting chemical peel that can be used in all skin types, including sensitive and rosacea-prone skin, as well as skin of color. Its large molecular size allows for the slow penetration of the acid on the skin and thus it can be carefully titrated.
Studies have shown its efficacy in reducing sebum content, acne, acne scarring, and hyperpigmentation. In clinical practice however, the most effective use of this acid is on sensitive skin. It is a great tool for clinicians to use as an effective exfoliant in less acid tolerant skin types. In commercially available concentrations of 5%-45%, mandelic acid can be used alone or in combination with other beta hydroxy peels, depending on the indication.
Most dermatologists and patients prefer in-office peels that induce noticeable peeling and resurfacing of the skin. Mandelic acid is one of the largest alpha hydroxy acids, a lipophilic acid that penetrates the skin slowly and uniformly, making it an ideal peel in sensitive or aging and thin skin types. Although many mandelic acid peels are available, however, there is a paucity of studies comparing their benefits and efficacies.
Dr. Lily Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
1. Wójcik A et al. Dermatol Alergol. 2013 Jun;30(3):140-5.
2. Soleymani T et al. J Clin Aesthet Dermatol. 2018;11(8):21-8.
Headache and Covid-19: What clinicians should know
Edoardo Caronna, MD and Patricia Pozo-Rosich, MD, PhD, Neurology Department, Hospital Universitari Vall d’Hebron, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain; and Headache and Neurological Pain Research Group, Vall d’Hebron Research Institute, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain. Dr. Pozo-Rosich also serves on the boards of the International Headache Society and Council of the European Headache Federation and is an editor for various peer-reviewed journals, including Cephalalgia and Headache.
Headache is a symptom of the coronavirus disease 2019 (Covid-19), caused by the novel, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the pandemic began, researchers have tried to describe, understand, and help clinicians manage headache in the setting of Covid-19.
The reason is simple: Headache is common, often debilitating, and difficult to treat.1
Moreover, headache could manifest both in the acute phase of the infection and, once the infection has resolved, in the post-acute phase.1 Therefore, it is critical for clinicians to know more about headache, as headache can be a common reason that patients seek help, both in the specialized and non-specialized medical care setting.
Definitions and manifestations
While the first step in such a communication would be to define headache attributed to Covid-19, no specific definition exists, as this is a new disease. Therefore, headache attributed to Covid-19 should be defined under the diagnostic criteria, as contained in the International Classification of Headache Disorders-3, as headache attributed to a systemic viral infection.2 As this is a secondary headache appearing with an infection, the treating physician needs to rule out possible underlying meningitis and/or encephalitis in the diagnosis. Moreover, other secondary headaches (eg, cerebral venous thrombosis) may appear, so clinicians need to carefully evaluate patients with headache during Covid-19 to detect signs or symptoms that point to other etiologies.
It is also advisable to know the clinical manifestations of headache attributed to Covid-19. Studies published so far have observed two main phenotypes of headache in the acute phase of the infection: one resembles migraine, the other, a tension-type headache.1,3 Although patients with history of migraine who contract Covid-19 report headache that is more similar to primary headache disorder,4 two relevant aspects should be considered. Namely, migraine-like features can be observed in patients without personal migraine history; and Covid-19 patients with such history may perceive that headache they experience in the infection’s acute phase differs from their usual experience, especially regarding increased severity or duration.5,6 Of note, headache can be a prodromal symptom of the SARS-CoV-2 infection.1
Evolution of a headache
Because headache appearing after the acute phase of the infection can persist, often manifesting migraine-like features, it is inordinately helpful for clinicians to know its evolution.1 This persistent headache, sometimes referred to as post-covid headache, is not aptly named because the post-covid headache is not just one type of headache, but instead can manifest as different headache types.
A recently published case series in Headache discussed three Covid patients who all experienced persistent headache during the infection’s post-acute phase.7 These patients experienced a migraine-like phenotype as have others with mild Covid-19, but their personal history of migraine, as well as their experience with Covid-19 related headache, were substantially different. Some patients had personal migraine history while others did not; some patients experienced no headache in the acute phase but did so in the post-acute phase; and the concomitant symptoms of the post-acute phase, such as insomnia, memory loss, dizziness, fatigue, and brain fog, were differentially expressed by patients.7
This case series introduces the concept that patients with no prior history of migraine or any other primary headache disorder can develop a de novo headache because of their SARS-CoV-2 infection. Moreover, it could manifest as a new daily persistent headache. And patients with personal history of migraine may experience sudden chronification in their headache’s characteristics, rather than develop a new type of headache.7
In another study, soon to be published in Cephalalgia, researchers observed that the median duration of headache in the acute phase is 2 weeks. This multicenter Spanish study, in which data on headache duration were available for 874 patients, found that 16% of these particular patients had persistent headache after 9 months. According to this study, headache that does not resolve within the first 3 months is less likely to do so later on.
Treatment
For clinicians, the significance of these findings is straightforward: Patients with headache experienced in the infection’s acute phase that does not seem to resolve post-infection requires continued medical attention. Patients should be monitored, carefully managed, and treated to avoid the onset of a persisting headache. This applies to patients with or without personal migraine history.
But which treatments should be prescribed? As there are no specific therapies for headache attributed to Covid-19, either in the acute or post-acute phase of the infection, clinicians must turn to existing therapies.
As with patients with migraine, patients with persistent headache post-Covid infection need a headache prevention strategy.
The strategy should be based on the following principles:
- treat headache
- treat comorbidities including mood disorders, insomnia, and so on
- avoid complications such as medication overuse, which may be very common in these patients.
Acute medications
Despite the lack of specific literature on this matter, migraine-like phenotypes may respond to triptans and probably, where available, lasmiditan and gepants. These medications probably represent a therapeutic option for Covid patients with headache, but before prescribing them clinicians should carefully evaluate their use.
Before deciding on the prescription, clinicians should consider not only the medications’ most common contraindications, but also those that are related to Covid-19: the phase of the infection (acute/post-acute); the infection’s severity; and the presence of other Covid-related health problems. The concerns over the use of nonsteroidal anti-inflammatory medications (NSAIDs) and corticosteroids, raised when the pandemic first struck, have greatly dissipated.8,9 Some patients with prolonged headache may benefit from a brief cycle of corticosteroids, similar to the treatment given to those patients with status migrainosus. Nerve blocks could also be considered.
Preventive medications
Drugs can be prescribed according to the headache phenotype too, but there are no published studies that specifically evaluate headache prevention treatments in patients with persistent headache post-infection. The case series mentioned earlier in this article recorded that patients whose headaches were treated with amitriptyline and onabotulinumtoxinA had reported variable treatment responses to this regimen, according to the patients’ characteristics.7
However, one important question regarding the safety of Covid patients with migraine – specifically patients on preventive treatments during the infection’s acute phase – has been somewhat resolved.
Medications such as renin-angiotensin system (RAS) blockers, suspected of possible involvement in the SARs-CoV-2 pathogenicity, seem to be safe.8,10 And, in another multicenter Spanish study, researchers found that the use of anti-CGRP monoclonal antibodies did not seem to be associated with worse Covid-19 outcomes despite the possible implication of CGRP in modulating inflammatory responses during a viral infection.11
The study of anti-CGRP monoclonal antibodies could be important in the future for another reason: To see whether these medications could be effective as a preventive treatment in patients with persistent headache after Covid-19, regardless of whether these patients have personal migraine history.
An interesting and important message to close this article. Although headache experienced in the infection’s acute phase could be extremely disabling for patients, the evidence points to the presence of headache as a marker of a better Covid-19 prognosis, in terms of a shorter infection period and a lower risk of mortality among hospitalized patients.1,3,12
This brief communication contains current information to help clinicians treat and inform their patients with Covid-sourced headache. Yet, we must keep in mind that the majority of the data reported here and published in the literature refer to studies conducted during the first wave of the pandemic. The emergence of new SARS-CoV-2 variants and vaccines have enormously changed the disease’s clinical presentation and course, so future studies are warranted to re-assess the validity of these findings under new conditions.
References
1. Caronna E, Ballvé A, Llauradó A, Gallardo VJ, et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020; Nov;40(13):1410-1421.
2. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; Jan;38(1):1-211.
3. Trigo J, García-Azorín D, Planchuelo-Gómez Á, et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: A retrospective cohort study. J Headache Pain. 2020;21(1):94. https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01165-8
4. Porta-Etessam J, Matías-Guiu JA, González-García N, et al. Spectrum of Headaches Associated With SARS-CoV-2 Infection: Study of Healthcare Professionals. Headache. 2020;60(8):1697–1704.
5. Singh J, Ali A. Headache as the Presenting Symptom in 2 Patients With COVID-19 and a History of Migraine: 2 Case Reports. Headache. 2020;60(8):1773–1776.
6. Membrilla JA, de Lorenzo Í, Sastre M, Díaz de Terán J. Headache as a Cardinal Symptom of Coronavirus Disease 2019: A Cross-Sectional Study. Headache. 2020; Nov;60(10):2176-2191.
7. Caronna E, Alpuente A, Torres-Ferrus M, Pozo-Rosich P. Toward a better understanding of persistent headache after mild COVID-19: Three migraine-like yet distinct scenarios. Headache. 2021. https://doi.org/10.1111/head.14197
8. Maassenvandenbrink A, De Vries T, Danser AHJ. Headache medication and the COVID-19 pandemic. J Headache Pain. 2020;21(1). https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01106-5
9. Arca KN, Smith JH, Chiang CC, et al. COVID-19 and Headache Medicine: A Narrative Review of Non-Steroidal Anti-Inflammatory Drug (NSAID) and Corticosteroid Use. Headache. 2020; Sep;60(8): 1558–1568.
10. Hippisley-Cox J, Young D, Coupland C, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart. 2020;Oct;106(19):1503-1511.
11. Caronna E, José Gallardo V, Alpuente A, Torres-Ferrus M, Sánchez-Mateo NM, Viguera-Romero J, et al. Safety of anti-CGRP monoclonal antibodies in patients with migraine during the COVID-19 pandemic: Present and future implications. Neurologia. 2021; Mar 19;36(8):611-617.
12. Gonzalez-Martinez A, Fanjul V, Ramos C, Serrano Ballesteros J, et al. Headache during SARS-CoV-2 infection as an early symptom associated with a more benign course of disease: a case–control study. Eur J Neurol. 2021;28(10):3426–36.
Edoardo Caronna, MD and Patricia Pozo-Rosich, MD, PhD, Neurology Department, Hospital Universitari Vall d’Hebron, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain; and Headache and Neurological Pain Research Group, Vall d’Hebron Research Institute, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain. Dr. Pozo-Rosich also serves on the boards of the International Headache Society and Council of the European Headache Federation and is an editor for various peer-reviewed journals, including Cephalalgia and Headache.
Headache is a symptom of the coronavirus disease 2019 (Covid-19), caused by the novel, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the pandemic began, researchers have tried to describe, understand, and help clinicians manage headache in the setting of Covid-19.
The reason is simple: Headache is common, often debilitating, and difficult to treat.1
Moreover, headache could manifest both in the acute phase of the infection and, once the infection has resolved, in the post-acute phase.1 Therefore, it is critical for clinicians to know more about headache, as headache can be a common reason that patients seek help, both in the specialized and non-specialized medical care setting.
Definitions and manifestations
While the first step in such a communication would be to define headache attributed to Covid-19, no specific definition exists, as this is a new disease. Therefore, headache attributed to Covid-19 should be defined under the diagnostic criteria, as contained in the International Classification of Headache Disorders-3, as headache attributed to a systemic viral infection.2 As this is a secondary headache appearing with an infection, the treating physician needs to rule out possible underlying meningitis and/or encephalitis in the diagnosis. Moreover, other secondary headaches (eg, cerebral venous thrombosis) may appear, so clinicians need to carefully evaluate patients with headache during Covid-19 to detect signs or symptoms that point to other etiologies.
It is also advisable to know the clinical manifestations of headache attributed to Covid-19. Studies published so far have observed two main phenotypes of headache in the acute phase of the infection: one resembles migraine, the other, a tension-type headache.1,3 Although patients with history of migraine who contract Covid-19 report headache that is more similar to primary headache disorder,4 two relevant aspects should be considered. Namely, migraine-like features can be observed in patients without personal migraine history; and Covid-19 patients with such history may perceive that headache they experience in the infection’s acute phase differs from their usual experience, especially regarding increased severity or duration.5,6 Of note, headache can be a prodromal symptom of the SARS-CoV-2 infection.1
Evolution of a headache
Because headache appearing after the acute phase of the infection can persist, often manifesting migraine-like features, it is inordinately helpful for clinicians to know its evolution.1 This persistent headache, sometimes referred to as post-covid headache, is not aptly named because the post-covid headache is not just one type of headache, but instead can manifest as different headache types.
A recently published case series in Headache discussed three Covid patients who all experienced persistent headache during the infection’s post-acute phase.7 These patients experienced a migraine-like phenotype as have others with mild Covid-19, but their personal history of migraine, as well as their experience with Covid-19 related headache, were substantially different. Some patients had personal migraine history while others did not; some patients experienced no headache in the acute phase but did so in the post-acute phase; and the concomitant symptoms of the post-acute phase, such as insomnia, memory loss, dizziness, fatigue, and brain fog, were differentially expressed by patients.7
This case series introduces the concept that patients with no prior history of migraine or any other primary headache disorder can develop a de novo headache because of their SARS-CoV-2 infection. Moreover, it could manifest as a new daily persistent headache. And patients with personal history of migraine may experience sudden chronification in their headache’s characteristics, rather than develop a new type of headache.7
In another study, soon to be published in Cephalalgia, researchers observed that the median duration of headache in the acute phase is 2 weeks. This multicenter Spanish study, in which data on headache duration were available for 874 patients, found that 16% of these particular patients had persistent headache after 9 months. According to this study, headache that does not resolve within the first 3 months is less likely to do so later on.
Treatment
For clinicians, the significance of these findings is straightforward: Patients with headache experienced in the infection’s acute phase that does not seem to resolve post-infection requires continued medical attention. Patients should be monitored, carefully managed, and treated to avoid the onset of a persisting headache. This applies to patients with or without personal migraine history.
But which treatments should be prescribed? As there are no specific therapies for headache attributed to Covid-19, either in the acute or post-acute phase of the infection, clinicians must turn to existing therapies.
As with patients with migraine, patients with persistent headache post-Covid infection need a headache prevention strategy.
The strategy should be based on the following principles:
- treat headache
- treat comorbidities including mood disorders, insomnia, and so on
- avoid complications such as medication overuse, which may be very common in these patients.
Acute medications
Despite the lack of specific literature on this matter, migraine-like phenotypes may respond to triptans and probably, where available, lasmiditan and gepants. These medications probably represent a therapeutic option for Covid patients with headache, but before prescribing them clinicians should carefully evaluate their use.
Before deciding on the prescription, clinicians should consider not only the medications’ most common contraindications, but also those that are related to Covid-19: the phase of the infection (acute/post-acute); the infection’s severity; and the presence of other Covid-related health problems. The concerns over the use of nonsteroidal anti-inflammatory medications (NSAIDs) and corticosteroids, raised when the pandemic first struck, have greatly dissipated.8,9 Some patients with prolonged headache may benefit from a brief cycle of corticosteroids, similar to the treatment given to those patients with status migrainosus. Nerve blocks could also be considered.
Preventive medications
Drugs can be prescribed according to the headache phenotype too, but there are no published studies that specifically evaluate headache prevention treatments in patients with persistent headache post-infection. The case series mentioned earlier in this article recorded that patients whose headaches were treated with amitriptyline and onabotulinumtoxinA had reported variable treatment responses to this regimen, according to the patients’ characteristics.7
However, one important question regarding the safety of Covid patients with migraine – specifically patients on preventive treatments during the infection’s acute phase – has been somewhat resolved.
Medications such as renin-angiotensin system (RAS) blockers, suspected of possible involvement in the SARs-CoV-2 pathogenicity, seem to be safe.8,10 And, in another multicenter Spanish study, researchers found that the use of anti-CGRP monoclonal antibodies did not seem to be associated with worse Covid-19 outcomes despite the possible implication of CGRP in modulating inflammatory responses during a viral infection.11
The study of anti-CGRP monoclonal antibodies could be important in the future for another reason: To see whether these medications could be effective as a preventive treatment in patients with persistent headache after Covid-19, regardless of whether these patients have personal migraine history.
An interesting and important message to close this article. Although headache experienced in the infection’s acute phase could be extremely disabling for patients, the evidence points to the presence of headache as a marker of a better Covid-19 prognosis, in terms of a shorter infection period and a lower risk of mortality among hospitalized patients.1,3,12
This brief communication contains current information to help clinicians treat and inform their patients with Covid-sourced headache. Yet, we must keep in mind that the majority of the data reported here and published in the literature refer to studies conducted during the first wave of the pandemic. The emergence of new SARS-CoV-2 variants and vaccines have enormously changed the disease’s clinical presentation and course, so future studies are warranted to re-assess the validity of these findings under new conditions.
Edoardo Caronna, MD and Patricia Pozo-Rosich, MD, PhD, Neurology Department, Hospital Universitari Vall d’Hebron, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain; and Headache and Neurological Pain Research Group, Vall d’Hebron Research Institute, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain. Dr. Pozo-Rosich also serves on the boards of the International Headache Society and Council of the European Headache Federation and is an editor for various peer-reviewed journals, including Cephalalgia and Headache.
Headache is a symptom of the coronavirus disease 2019 (Covid-19), caused by the novel, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the pandemic began, researchers have tried to describe, understand, and help clinicians manage headache in the setting of Covid-19.
The reason is simple: Headache is common, often debilitating, and difficult to treat.1
Moreover, headache could manifest both in the acute phase of the infection and, once the infection has resolved, in the post-acute phase.1 Therefore, it is critical for clinicians to know more about headache, as headache can be a common reason that patients seek help, both in the specialized and non-specialized medical care setting.
Definitions and manifestations
While the first step in such a communication would be to define headache attributed to Covid-19, no specific definition exists, as this is a new disease. Therefore, headache attributed to Covid-19 should be defined under the diagnostic criteria, as contained in the International Classification of Headache Disorders-3, as headache attributed to a systemic viral infection.2 As this is a secondary headache appearing with an infection, the treating physician needs to rule out possible underlying meningitis and/or encephalitis in the diagnosis. Moreover, other secondary headaches (eg, cerebral venous thrombosis) may appear, so clinicians need to carefully evaluate patients with headache during Covid-19 to detect signs or symptoms that point to other etiologies.
It is also advisable to know the clinical manifestations of headache attributed to Covid-19. Studies published so far have observed two main phenotypes of headache in the acute phase of the infection: one resembles migraine, the other, a tension-type headache.1,3 Although patients with history of migraine who contract Covid-19 report headache that is more similar to primary headache disorder,4 two relevant aspects should be considered. Namely, migraine-like features can be observed in patients without personal migraine history; and Covid-19 patients with such history may perceive that headache they experience in the infection’s acute phase differs from their usual experience, especially regarding increased severity or duration.5,6 Of note, headache can be a prodromal symptom of the SARS-CoV-2 infection.1
Evolution of a headache
Because headache appearing after the acute phase of the infection can persist, often manifesting migraine-like features, it is inordinately helpful for clinicians to know its evolution.1 This persistent headache, sometimes referred to as post-covid headache, is not aptly named because the post-covid headache is not just one type of headache, but instead can manifest as different headache types.
A recently published case series in Headache discussed three Covid patients who all experienced persistent headache during the infection’s post-acute phase.7 These patients experienced a migraine-like phenotype as have others with mild Covid-19, but their personal history of migraine, as well as their experience with Covid-19 related headache, were substantially different. Some patients had personal migraine history while others did not; some patients experienced no headache in the acute phase but did so in the post-acute phase; and the concomitant symptoms of the post-acute phase, such as insomnia, memory loss, dizziness, fatigue, and brain fog, were differentially expressed by patients.7
This case series introduces the concept that patients with no prior history of migraine or any other primary headache disorder can develop a de novo headache because of their SARS-CoV-2 infection. Moreover, it could manifest as a new daily persistent headache. And patients with personal history of migraine may experience sudden chronification in their headache’s characteristics, rather than develop a new type of headache.7
In another study, soon to be published in Cephalalgia, researchers observed that the median duration of headache in the acute phase is 2 weeks. This multicenter Spanish study, in which data on headache duration were available for 874 patients, found that 16% of these particular patients had persistent headache after 9 months. According to this study, headache that does not resolve within the first 3 months is less likely to do so later on.
Treatment
For clinicians, the significance of these findings is straightforward: Patients with headache experienced in the infection’s acute phase that does not seem to resolve post-infection requires continued medical attention. Patients should be monitored, carefully managed, and treated to avoid the onset of a persisting headache. This applies to patients with or without personal migraine history.
But which treatments should be prescribed? As there are no specific therapies for headache attributed to Covid-19, either in the acute or post-acute phase of the infection, clinicians must turn to existing therapies.
As with patients with migraine, patients with persistent headache post-Covid infection need a headache prevention strategy.
The strategy should be based on the following principles:
- treat headache
- treat comorbidities including mood disorders, insomnia, and so on
- avoid complications such as medication overuse, which may be very common in these patients.
Acute medications
Despite the lack of specific literature on this matter, migraine-like phenotypes may respond to triptans and probably, where available, lasmiditan and gepants. These medications probably represent a therapeutic option for Covid patients with headache, but before prescribing them clinicians should carefully evaluate their use.
Before deciding on the prescription, clinicians should consider not only the medications’ most common contraindications, but also those that are related to Covid-19: the phase of the infection (acute/post-acute); the infection’s severity; and the presence of other Covid-related health problems. The concerns over the use of nonsteroidal anti-inflammatory medications (NSAIDs) and corticosteroids, raised when the pandemic first struck, have greatly dissipated.8,9 Some patients with prolonged headache may benefit from a brief cycle of corticosteroids, similar to the treatment given to those patients with status migrainosus. Nerve blocks could also be considered.
Preventive medications
Drugs can be prescribed according to the headache phenotype too, but there are no published studies that specifically evaluate headache prevention treatments in patients with persistent headache post-infection. The case series mentioned earlier in this article recorded that patients whose headaches were treated with amitriptyline and onabotulinumtoxinA had reported variable treatment responses to this regimen, according to the patients’ characteristics.7
However, one important question regarding the safety of Covid patients with migraine – specifically patients on preventive treatments during the infection’s acute phase – has been somewhat resolved.
Medications such as renin-angiotensin system (RAS) blockers, suspected of possible involvement in the SARs-CoV-2 pathogenicity, seem to be safe.8,10 And, in another multicenter Spanish study, researchers found that the use of anti-CGRP monoclonal antibodies did not seem to be associated with worse Covid-19 outcomes despite the possible implication of CGRP in modulating inflammatory responses during a viral infection.11
The study of anti-CGRP monoclonal antibodies could be important in the future for another reason: To see whether these medications could be effective as a preventive treatment in patients with persistent headache after Covid-19, regardless of whether these patients have personal migraine history.
An interesting and important message to close this article. Although headache experienced in the infection’s acute phase could be extremely disabling for patients, the evidence points to the presence of headache as a marker of a better Covid-19 prognosis, in terms of a shorter infection period and a lower risk of mortality among hospitalized patients.1,3,12
This brief communication contains current information to help clinicians treat and inform their patients with Covid-sourced headache. Yet, we must keep in mind that the majority of the data reported here and published in the literature refer to studies conducted during the first wave of the pandemic. The emergence of new SARS-CoV-2 variants and vaccines have enormously changed the disease’s clinical presentation and course, so future studies are warranted to re-assess the validity of these findings under new conditions.
References
1. Caronna E, Ballvé A, Llauradó A, Gallardo VJ, et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020; Nov;40(13):1410-1421.
2. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; Jan;38(1):1-211.
3. Trigo J, García-Azorín D, Planchuelo-Gómez Á, et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: A retrospective cohort study. J Headache Pain. 2020;21(1):94. https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01165-8
4. Porta-Etessam J, Matías-Guiu JA, González-García N, et al. Spectrum of Headaches Associated With SARS-CoV-2 Infection: Study of Healthcare Professionals. Headache. 2020;60(8):1697–1704.
5. Singh J, Ali A. Headache as the Presenting Symptom in 2 Patients With COVID-19 and a History of Migraine: 2 Case Reports. Headache. 2020;60(8):1773–1776.
6. Membrilla JA, de Lorenzo Í, Sastre M, Díaz de Terán J. Headache as a Cardinal Symptom of Coronavirus Disease 2019: A Cross-Sectional Study. Headache. 2020; Nov;60(10):2176-2191.
7. Caronna E, Alpuente A, Torres-Ferrus M, Pozo-Rosich P. Toward a better understanding of persistent headache after mild COVID-19: Three migraine-like yet distinct scenarios. Headache. 2021. https://doi.org/10.1111/head.14197
8. Maassenvandenbrink A, De Vries T, Danser AHJ. Headache medication and the COVID-19 pandemic. J Headache Pain. 2020;21(1). https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01106-5
9. Arca KN, Smith JH, Chiang CC, et al. COVID-19 and Headache Medicine: A Narrative Review of Non-Steroidal Anti-Inflammatory Drug (NSAID) and Corticosteroid Use. Headache. 2020; Sep;60(8): 1558–1568.
10. Hippisley-Cox J, Young D, Coupland C, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart. 2020;Oct;106(19):1503-1511.
11. Caronna E, José Gallardo V, Alpuente A, Torres-Ferrus M, Sánchez-Mateo NM, Viguera-Romero J, et al. Safety of anti-CGRP monoclonal antibodies in patients with migraine during the COVID-19 pandemic: Present and future implications. Neurologia. 2021; Mar 19;36(8):611-617.
12. Gonzalez-Martinez A, Fanjul V, Ramos C, Serrano Ballesteros J, et al. Headache during SARS-CoV-2 infection as an early symptom associated with a more benign course of disease: a case–control study. Eur J Neurol. 2021;28(10):3426–36.
References
1. Caronna E, Ballvé A, Llauradó A, Gallardo VJ, et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020; Nov;40(13):1410-1421.
2. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; Jan;38(1):1-211.
3. Trigo J, García-Azorín D, Planchuelo-Gómez Á, et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: A retrospective cohort study. J Headache Pain. 2020;21(1):94. https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01165-8
4. Porta-Etessam J, Matías-Guiu JA, González-García N, et al. Spectrum of Headaches Associated With SARS-CoV-2 Infection: Study of Healthcare Professionals. Headache. 2020;60(8):1697–1704.
5. Singh J, Ali A. Headache as the Presenting Symptom in 2 Patients With COVID-19 and a History of Migraine: 2 Case Reports. Headache. 2020;60(8):1773–1776.
6. Membrilla JA, de Lorenzo Í, Sastre M, Díaz de Terán J. Headache as a Cardinal Symptom of Coronavirus Disease 2019: A Cross-Sectional Study. Headache. 2020; Nov;60(10):2176-2191.
7. Caronna E, Alpuente A, Torres-Ferrus M, Pozo-Rosich P. Toward a better understanding of persistent headache after mild COVID-19: Three migraine-like yet distinct scenarios. Headache. 2021. https://doi.org/10.1111/head.14197
8. Maassenvandenbrink A, De Vries T, Danser AHJ. Headache medication and the COVID-19 pandemic. J Headache Pain. 2020;21(1). https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01106-5
9. Arca KN, Smith JH, Chiang CC, et al. COVID-19 and Headache Medicine: A Narrative Review of Non-Steroidal Anti-Inflammatory Drug (NSAID) and Corticosteroid Use. Headache. 2020; Sep;60(8): 1558–1568.
10. Hippisley-Cox J, Young D, Coupland C, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart. 2020;Oct;106(19):1503-1511.
11. Caronna E, José Gallardo V, Alpuente A, Torres-Ferrus M, Sánchez-Mateo NM, Viguera-Romero J, et al. Safety of anti-CGRP monoclonal antibodies in patients with migraine during the COVID-19 pandemic: Present and future implications. Neurologia. 2021; Mar 19;36(8):611-617.
12. Gonzalez-Martinez A, Fanjul V, Ramos C, Serrano Ballesteros J, et al. Headache during SARS-CoV-2 infection as an early symptom associated with a more benign course of disease: a case–control study. Eur J Neurol. 2021;28(10):3426–36.
Treat-to-target in RA: Questions remain about adoption, measurement
The roots of treat-to-target (T2T) in rheumatology extend back over 30 years to the development of the 28-joint Disease Activity Score. Although it has been shown to be effective in clinical trials and has been included in guidelines, it has yet to be widely adopted in practice. The question remains: What is the role of T2T in rheumatology?
That’s what Jack Cush, MD, and Martin Bergman, MD, sought to answer in a point-counterpoint session at the 2022 Rheumatology Winter Clinical Symposium.
“I do think that this is a concept we need to keep in mind, and it is a concept whose time is long overdue,” Dr. Bergman said in his presentation arguing in favor of T2T. “As good as you think you are – your ability to see the patient and determine how they’re doing – you’re not.”
But metrics alone are not enough to make clinical decisions, said Dr. Bergman, clinical professor of medicine at Drexel University, Philadelphia, citing a recommendation from 2014 recommendations on T2T published in Annals of the Rheumatic Diseases. “You don’t just follow a number. You have to take into consideration structural changes, functional impairment, comorbidities – and that’s going to adjust how you approach your patient and what you do with them.”
However, implementation of T2T to make changes in clinical practice in RA has been inconsistent. Referencing an abstract from the 2021 American College of Rheumatology annual meeting on 15-year follow-up for changing therapy in RA, Dr. Bergman argued it is not the patient who is unwilling to switch treatments. Between 2006 and 2021, patient unwillingness to change therapies decreased from 64% to 51%.
“What’s driving it mostly here [is] the doctor’s recommendation,” Dr. Bergman said. “And we know this is true because we’ve seen it in other studies.”
Many rheumatologists are “asleep at the wheel” when it comes to administering T2T, he said. “What we need to do is, after we wake up from this nap, we need to get back on the highway and drive to where we should be, which is treat-to-target.”
Dr. Bergman also shared his paradigm for administering T2T, which he noted does not typically take more than a few minutes to administer regardless of the measure chosen. “Pick a measure. I don’t care which measure you take. I personally use two: I like the RAPID3 and the CDAI,” he said. “But then, after you have the measure, evaluate the entire patient. Don’t just look at the number. Look at the patient, what’s going on, solid history, solid physical. And most importantly: Be a doctor, don’t be a computer screen.”
Problems in measurement of RA remission and adoption of T2T
Dr. Cush, who admitted early in his presentation that he is in favor of T2T, delivered his counterpoint somewhat tongue-in-cheek. However, he pointed out that there are several concerns about the goals in measuring outcomes in RA with T2T.
The goal in RA is disease remission, but how you define remission can vary, especially since sometimes there is evidence of synovitis or other disease activity visible through an exam or imaging, said Dr. Cush, a rheumatologist based in Dallas, and executive editor of RheumNow.com. Most of the evidence for T2T is in clinical trials, but adoption is inconsistent in clinical practice, and patients in general appear to be improving without widespread adoption, he argued.
“Are clinical trials the same as clinical practice?” he asked. “I think that this boils down to: Is this a quest for remissions, or the best-you-can-get, low disease activity state? Or is this a quest for physician change, which is actually the path you have to go through to get to remissions?”
“In the end, evidence that the metrics should drive prescribing, especially in private practice is, I think, lacking,” he said.
Roy Fleischmann, MD, agreed with Dr. Cush’s point of how one defines disease remission, as well as Dr. Bergman’s paradigm for T2T. “If you’re a good rheumatologist, you really do examine joints,” but you also “take a look at patient function, patient global [assessment], your global [assessment], joint count,” he said. “You can put a number to it, but you have to take a look at all of that. Really, treat-to-target – it is all of that. It isn’t just looking at a number, it’s looking at everything. And the better that the patient can do, assuming comorbidities and everything else, the better it is.”
“The problem isn’t the patient. The problem isn’t the metric. The problem is the rheumatologist, because the rheumatologist isn’t putting in enough effort in order to reach that goal,” added Dr. Fleischmann, clinical professor of medicine at the University of Texas, Dallas, and codirector of the Metroplex Clinical Research Center, also in in Dallas.
Eric Ruderman, MD, professor of rheumatology at Northwestern University, Chicago, commented that T2T is “great for somebody who’s been doing it for a while, and seeing a lot of patients and has that comfort zone,” but he questioned whether new rheumatologists without a lot of clinical experience could apply the approach. “What information do they use to integrate, and how do they get to that point?” he asked. “I don’t have the answer to that.”
During a rebuttal, Dr. Bergman pushed back on the idea that clinical experience alone was enough. “You need something. You need a benchmark. You need something more than, ‘I say so.’ ”
“The problem is, we still haven’t convinced people to adopt them,” Dr. Bergman said. “And I think it’s failure of training because, in my opinion, I don’t know how you can do a modern current fellowship program and not teach metrics.”
Dr. Cush and Dr. Bergman used the same trials to argue for their side. “I think that us using the same slides, but maybe having different points, speaks to the problem,” Dr. Cush said. “And I choose not to make it the problem of the rheumatologists.”
“You’re in the field of pattern recognition,” Dr. Cush argued. “It’s a visual art. You can have all the numbers you want. You make the most of your decisions based on pattern recognition, which is not rooted in metrics, and that’s why you’re successful at what you do.
“I am a big believer in T2T, but I think you have to measure something, and you have to use it,” Dr. Cush closed. “And the problem is, we can’t be forced into this.”
Dr. Bergman and Dr. Cush reported having financial relationships with numerous pharmaceutical companies.
The roots of treat-to-target (T2T) in rheumatology extend back over 30 years to the development of the 28-joint Disease Activity Score. Although it has been shown to be effective in clinical trials and has been included in guidelines, it has yet to be widely adopted in practice. The question remains: What is the role of T2T in rheumatology?
That’s what Jack Cush, MD, and Martin Bergman, MD, sought to answer in a point-counterpoint session at the 2022 Rheumatology Winter Clinical Symposium.
“I do think that this is a concept we need to keep in mind, and it is a concept whose time is long overdue,” Dr. Bergman said in his presentation arguing in favor of T2T. “As good as you think you are – your ability to see the patient and determine how they’re doing – you’re not.”
But metrics alone are not enough to make clinical decisions, said Dr. Bergman, clinical professor of medicine at Drexel University, Philadelphia, citing a recommendation from 2014 recommendations on T2T published in Annals of the Rheumatic Diseases. “You don’t just follow a number. You have to take into consideration structural changes, functional impairment, comorbidities – and that’s going to adjust how you approach your patient and what you do with them.”
However, implementation of T2T to make changes in clinical practice in RA has been inconsistent. Referencing an abstract from the 2021 American College of Rheumatology annual meeting on 15-year follow-up for changing therapy in RA, Dr. Bergman argued it is not the patient who is unwilling to switch treatments. Between 2006 and 2021, patient unwillingness to change therapies decreased from 64% to 51%.
“What’s driving it mostly here [is] the doctor’s recommendation,” Dr. Bergman said. “And we know this is true because we’ve seen it in other studies.”
Many rheumatologists are “asleep at the wheel” when it comes to administering T2T, he said. “What we need to do is, after we wake up from this nap, we need to get back on the highway and drive to where we should be, which is treat-to-target.”
Dr. Bergman also shared his paradigm for administering T2T, which he noted does not typically take more than a few minutes to administer regardless of the measure chosen. “Pick a measure. I don’t care which measure you take. I personally use two: I like the RAPID3 and the CDAI,” he said. “But then, after you have the measure, evaluate the entire patient. Don’t just look at the number. Look at the patient, what’s going on, solid history, solid physical. And most importantly: Be a doctor, don’t be a computer screen.”
Problems in measurement of RA remission and adoption of T2T
Dr. Cush, who admitted early in his presentation that he is in favor of T2T, delivered his counterpoint somewhat tongue-in-cheek. However, he pointed out that there are several concerns about the goals in measuring outcomes in RA with T2T.
The goal in RA is disease remission, but how you define remission can vary, especially since sometimes there is evidence of synovitis or other disease activity visible through an exam or imaging, said Dr. Cush, a rheumatologist based in Dallas, and executive editor of RheumNow.com. Most of the evidence for T2T is in clinical trials, but adoption is inconsistent in clinical practice, and patients in general appear to be improving without widespread adoption, he argued.
“Are clinical trials the same as clinical practice?” he asked. “I think that this boils down to: Is this a quest for remissions, or the best-you-can-get, low disease activity state? Or is this a quest for physician change, which is actually the path you have to go through to get to remissions?”
“In the end, evidence that the metrics should drive prescribing, especially in private practice is, I think, lacking,” he said.
Roy Fleischmann, MD, agreed with Dr. Cush’s point of how one defines disease remission, as well as Dr. Bergman’s paradigm for T2T. “If you’re a good rheumatologist, you really do examine joints,” but you also “take a look at patient function, patient global [assessment], your global [assessment], joint count,” he said. “You can put a number to it, but you have to take a look at all of that. Really, treat-to-target – it is all of that. It isn’t just looking at a number, it’s looking at everything. And the better that the patient can do, assuming comorbidities and everything else, the better it is.”
“The problem isn’t the patient. The problem isn’t the metric. The problem is the rheumatologist, because the rheumatologist isn’t putting in enough effort in order to reach that goal,” added Dr. Fleischmann, clinical professor of medicine at the University of Texas, Dallas, and codirector of the Metroplex Clinical Research Center, also in in Dallas.
Eric Ruderman, MD, professor of rheumatology at Northwestern University, Chicago, commented that T2T is “great for somebody who’s been doing it for a while, and seeing a lot of patients and has that comfort zone,” but he questioned whether new rheumatologists without a lot of clinical experience could apply the approach. “What information do they use to integrate, and how do they get to that point?” he asked. “I don’t have the answer to that.”
During a rebuttal, Dr. Bergman pushed back on the idea that clinical experience alone was enough. “You need something. You need a benchmark. You need something more than, ‘I say so.’ ”
“The problem is, we still haven’t convinced people to adopt them,” Dr. Bergman said. “And I think it’s failure of training because, in my opinion, I don’t know how you can do a modern current fellowship program and not teach metrics.”
Dr. Cush and Dr. Bergman used the same trials to argue for their side. “I think that us using the same slides, but maybe having different points, speaks to the problem,” Dr. Cush said. “And I choose not to make it the problem of the rheumatologists.”
“You’re in the field of pattern recognition,” Dr. Cush argued. “It’s a visual art. You can have all the numbers you want. You make the most of your decisions based on pattern recognition, which is not rooted in metrics, and that’s why you’re successful at what you do.
“I am a big believer in T2T, but I think you have to measure something, and you have to use it,” Dr. Cush closed. “And the problem is, we can’t be forced into this.”
Dr. Bergman and Dr. Cush reported having financial relationships with numerous pharmaceutical companies.
The roots of treat-to-target (T2T) in rheumatology extend back over 30 years to the development of the 28-joint Disease Activity Score. Although it has been shown to be effective in clinical trials and has been included in guidelines, it has yet to be widely adopted in practice. The question remains: What is the role of T2T in rheumatology?
That’s what Jack Cush, MD, and Martin Bergman, MD, sought to answer in a point-counterpoint session at the 2022 Rheumatology Winter Clinical Symposium.
“I do think that this is a concept we need to keep in mind, and it is a concept whose time is long overdue,” Dr. Bergman said in his presentation arguing in favor of T2T. “As good as you think you are – your ability to see the patient and determine how they’re doing – you’re not.”
But metrics alone are not enough to make clinical decisions, said Dr. Bergman, clinical professor of medicine at Drexel University, Philadelphia, citing a recommendation from 2014 recommendations on T2T published in Annals of the Rheumatic Diseases. “You don’t just follow a number. You have to take into consideration structural changes, functional impairment, comorbidities – and that’s going to adjust how you approach your patient and what you do with them.”
However, implementation of T2T to make changes in clinical practice in RA has been inconsistent. Referencing an abstract from the 2021 American College of Rheumatology annual meeting on 15-year follow-up for changing therapy in RA, Dr. Bergman argued it is not the patient who is unwilling to switch treatments. Between 2006 and 2021, patient unwillingness to change therapies decreased from 64% to 51%.
“What’s driving it mostly here [is] the doctor’s recommendation,” Dr. Bergman said. “And we know this is true because we’ve seen it in other studies.”
Many rheumatologists are “asleep at the wheel” when it comes to administering T2T, he said. “What we need to do is, after we wake up from this nap, we need to get back on the highway and drive to where we should be, which is treat-to-target.”
Dr. Bergman also shared his paradigm for administering T2T, which he noted does not typically take more than a few minutes to administer regardless of the measure chosen. “Pick a measure. I don’t care which measure you take. I personally use two: I like the RAPID3 and the CDAI,” he said. “But then, after you have the measure, evaluate the entire patient. Don’t just look at the number. Look at the patient, what’s going on, solid history, solid physical. And most importantly: Be a doctor, don’t be a computer screen.”
Problems in measurement of RA remission and adoption of T2T
Dr. Cush, who admitted early in his presentation that he is in favor of T2T, delivered his counterpoint somewhat tongue-in-cheek. However, he pointed out that there are several concerns about the goals in measuring outcomes in RA with T2T.
The goal in RA is disease remission, but how you define remission can vary, especially since sometimes there is evidence of synovitis or other disease activity visible through an exam or imaging, said Dr. Cush, a rheumatologist based in Dallas, and executive editor of RheumNow.com. Most of the evidence for T2T is in clinical trials, but adoption is inconsistent in clinical practice, and patients in general appear to be improving without widespread adoption, he argued.
“Are clinical trials the same as clinical practice?” he asked. “I think that this boils down to: Is this a quest for remissions, or the best-you-can-get, low disease activity state? Or is this a quest for physician change, which is actually the path you have to go through to get to remissions?”
“In the end, evidence that the metrics should drive prescribing, especially in private practice is, I think, lacking,” he said.
Roy Fleischmann, MD, agreed with Dr. Cush’s point of how one defines disease remission, as well as Dr. Bergman’s paradigm for T2T. “If you’re a good rheumatologist, you really do examine joints,” but you also “take a look at patient function, patient global [assessment], your global [assessment], joint count,” he said. “You can put a number to it, but you have to take a look at all of that. Really, treat-to-target – it is all of that. It isn’t just looking at a number, it’s looking at everything. And the better that the patient can do, assuming comorbidities and everything else, the better it is.”
“The problem isn’t the patient. The problem isn’t the metric. The problem is the rheumatologist, because the rheumatologist isn’t putting in enough effort in order to reach that goal,” added Dr. Fleischmann, clinical professor of medicine at the University of Texas, Dallas, and codirector of the Metroplex Clinical Research Center, also in in Dallas.
Eric Ruderman, MD, professor of rheumatology at Northwestern University, Chicago, commented that T2T is “great for somebody who’s been doing it for a while, and seeing a lot of patients and has that comfort zone,” but he questioned whether new rheumatologists without a lot of clinical experience could apply the approach. “What information do they use to integrate, and how do they get to that point?” he asked. “I don’t have the answer to that.”
During a rebuttal, Dr. Bergman pushed back on the idea that clinical experience alone was enough. “You need something. You need a benchmark. You need something more than, ‘I say so.’ ”
“The problem is, we still haven’t convinced people to adopt them,” Dr. Bergman said. “And I think it’s failure of training because, in my opinion, I don’t know how you can do a modern current fellowship program and not teach metrics.”
Dr. Cush and Dr. Bergman used the same trials to argue for their side. “I think that us using the same slides, but maybe having different points, speaks to the problem,” Dr. Cush said. “And I choose not to make it the problem of the rheumatologists.”
“You’re in the field of pattern recognition,” Dr. Cush argued. “It’s a visual art. You can have all the numbers you want. You make the most of your decisions based on pattern recognition, which is not rooted in metrics, and that’s why you’re successful at what you do.
“I am a big believer in T2T, but I think you have to measure something, and you have to use it,” Dr. Cush closed. “And the problem is, we can’t be forced into this.”
Dr. Bergman and Dr. Cush reported having financial relationships with numerous pharmaceutical companies.
FROM RWCS 2022