User login
2021 CDC guidelines on sexually transmitted infections
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.
Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1
Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
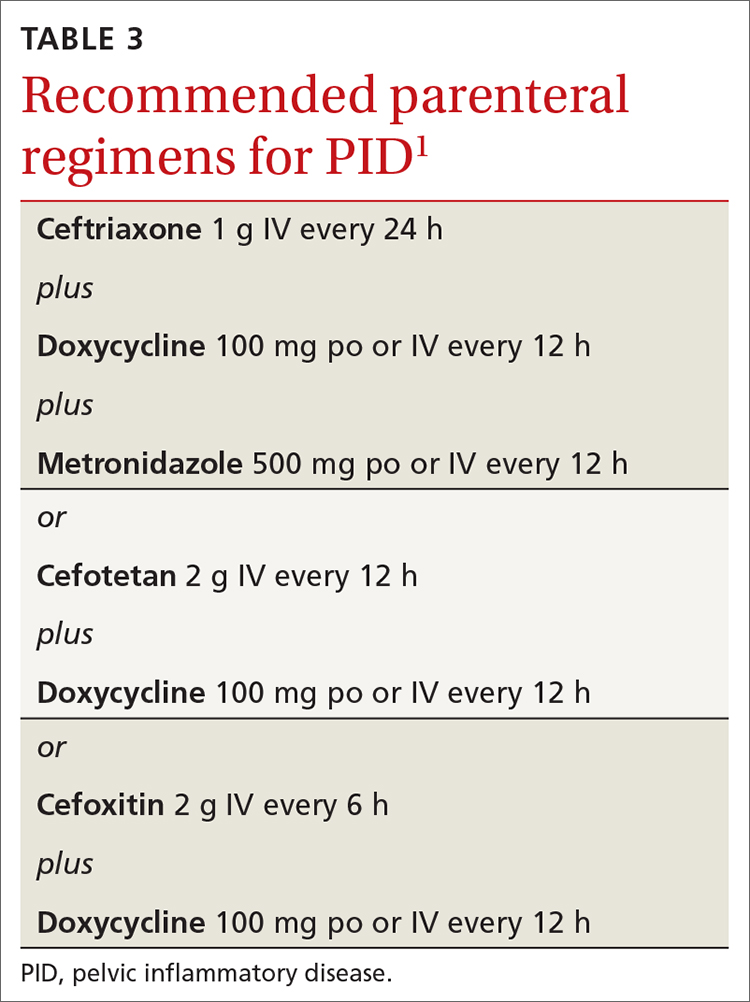
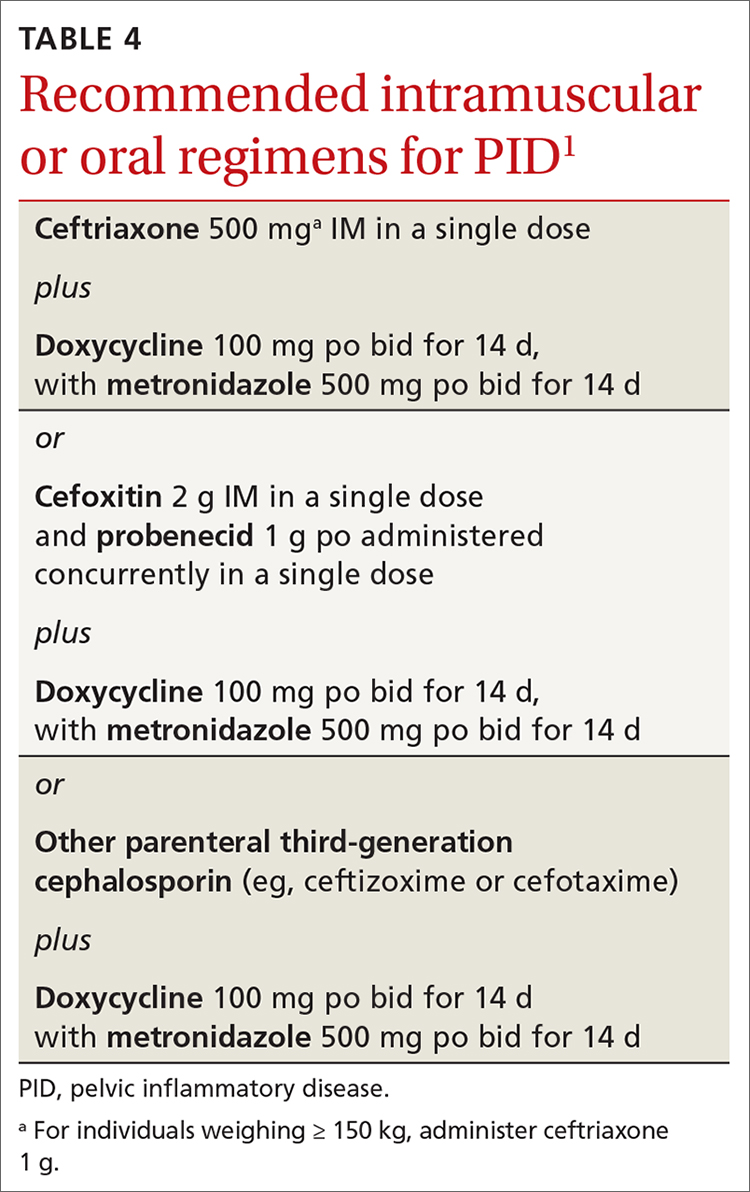
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.
To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.
Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.
Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1
Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.
To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.
Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.
Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1
Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.
To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.
Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
Using biomarkers to quantify problematic alcohol use
CASE A 34-year-old woman presents with fatigue. She appears defensive when asked about her alcohol use. She answers No to all questions on the CAGE (cut down, annoyed, guilty, eye-opener) screening tool, but acknowledges drinking excessively on rare occasions. Her physician has a high suspicion for alcohol use disorder (AUD) and recommends further testing. The patient agrees but denies having used alcohol over the past several days. Which of the following is most likely to help support the suspicion of a heavy drinking pattern?
- Routine lab tests (liver panel and complete blood count).
- Blood or urine alcohol level.
- Phosphatidylethanol (PEth) level in the blood.
- Ethyl glucuronide (EtG) in the urine.
- Carbohydrate-deficient transferrin (CDT) in the blood.
(See "Case answer.").
About 1 in 12 Americans have AUD,1 and 1 in 10 children live in a home with a parent who has a drinking problem.2 While the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) succinctly defines AUD with specific criteria,1 the term generally refers to an inability to control or stop drinking despite adverse social or health consequences. AUD is regarded as > 4 drinks per day for men and > 3 drinks per day for women.3 A “standard drink” would be a 12-oz bottle of beer, a 5-oz glass of wine, or 1.5 oz of distilled spirits. Effects of chronic alcohol use are vast and include malnutrition, alcohol withdrawal syndrome, alcoholic liver disease, pancreatitis/pancreatic cancer, cardiomyopathy, and stroke.4-6 Alcohol use by a pregnant woman can lead to fetal alcohol syndrome in her child.7
AUD may be more prevalent in the wake of COVID-19. Primary care practitioners tend to miss a large fraction of patients with AUD in their practice, especially younger patients and those without somatic comorbidities.8 Systematic screening for AUD can identify many of these people.8 Particularly as the COVID-19 pandemic continues to unfold and increases stress for everyone, risk of worsening drinking increases both in individuals with current AUD and for those in remission.9 Contrary to common belief, patients visiting primary care favor screening for at-risk drinking.10 Thus, awareness of the prevalence of AUD and patient acceptance of screening should encourage wider testing.
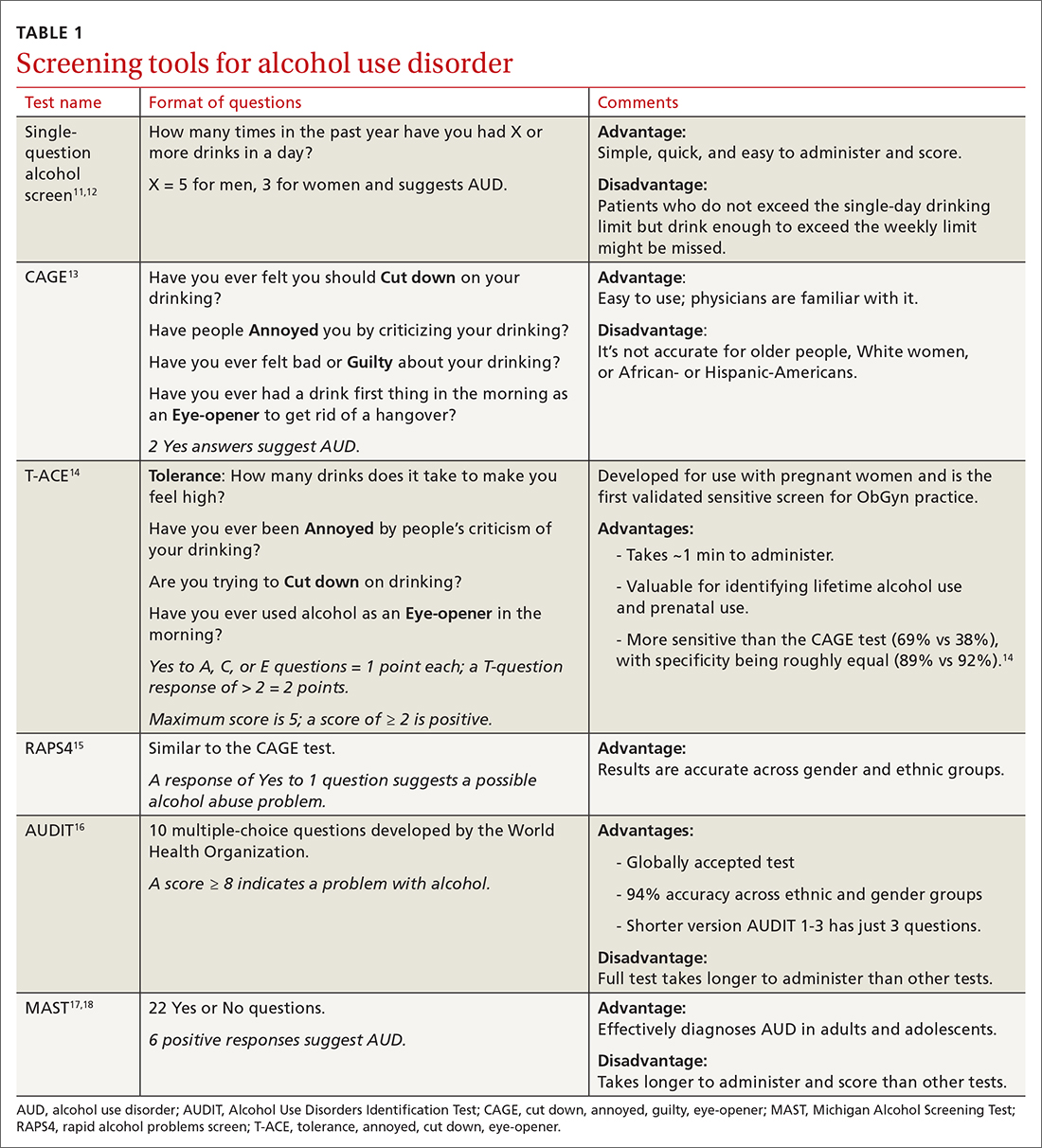
Screening tools. The 2014 guidelines published by the Centers for Disease Control and Prevention recommend using quick screening tools—ie, single question or AUDIT 1-3 (TABLE 111-18)—as an objective means of determining whether patients’ drinking creates a risk for themselves or others.11 Excessive drinking identified using alcohol questionnaires can help reduce medical complications and health care costs.19 The questionnaires we review do not provide a diagnosis but help identify individuals who might benefit from more thorough assessment.20 Following up, as needed, by testing for alcohol biomarkers can provide quantitative insight into problematic alcohol use.2
But before we discuss the utility of biomarkers, it’s important to quickly review how alcohol is eliminated from the body.
Alcohol elimination
The stomach and small intestine are the primary sites for alcohol absorption. Alcohol elimination from the body occurs through 3 pathways. The first involves oxidative metabolism, which eliminates most ethanol (95%) through the actions of alcohol dehydrogenase, cytochrome P4502E1, or catalase. A lesser amount of alcohol (2%-5%) is eliminated, unchanged, via the second pathway, which includes urine, sweat, and breath. Nonoxidative metabolism makes up the third pathway. Nonoxidative metabolism removes a very small amount (0.1%) of alcohol and involves the direct ethanol biomarkers PEth, EtG, ethyl sulfate (EtS), and fatty acid ethyl esters (FAEEs).21 Our emphasis in this article is on assays of direct metabolites of alcohol—particularly PEth.
Continue to: To understand the utility...
To understand the utility of these direct biomarkers, it is helpful to look at the indirect biomarkers first.
Indirect biomarkers have limited sensitivity and specificity
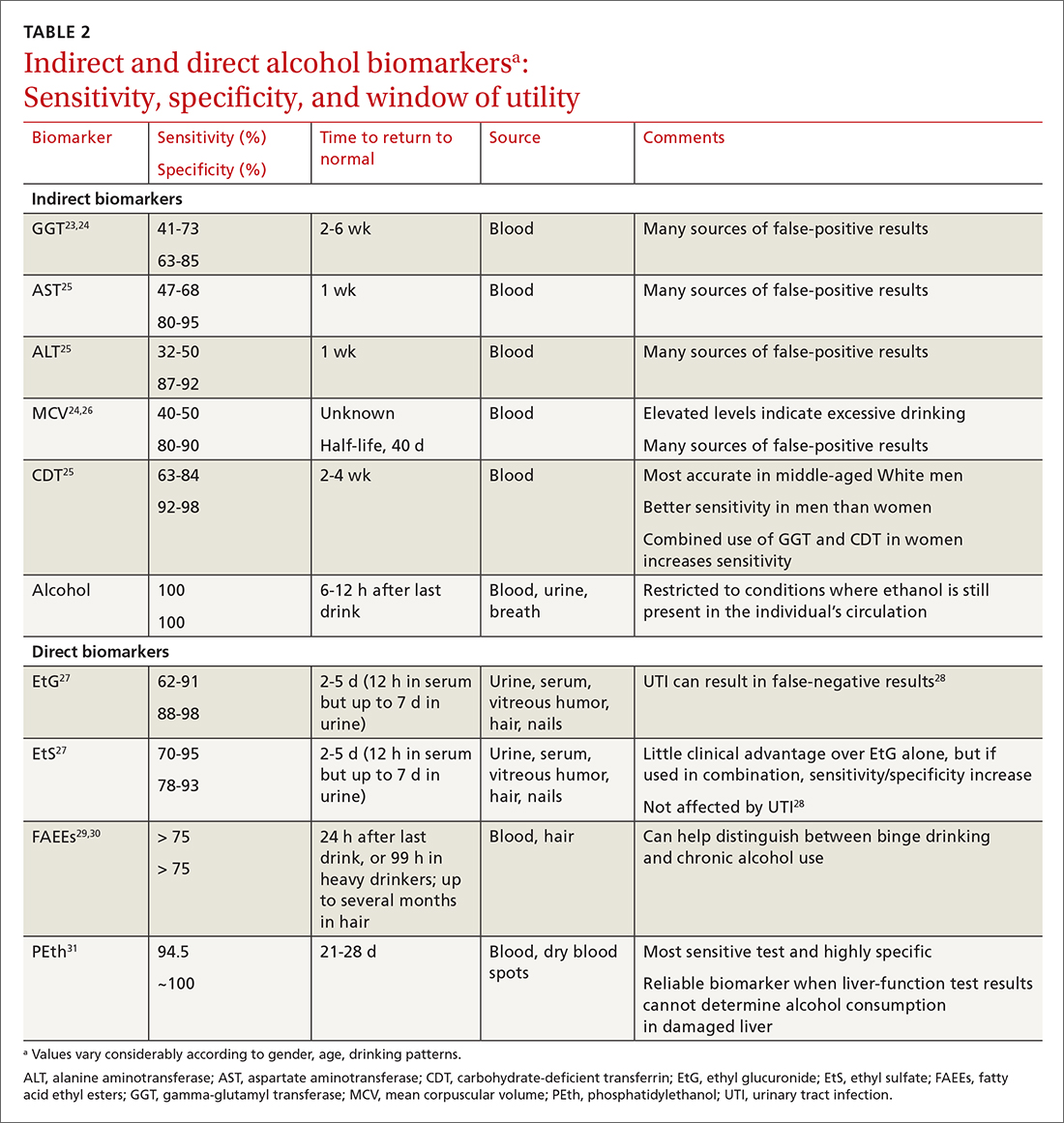
When alcohol is consumed in large enough quantities over time, indirect biomarkers of alcohol can become abnormal.22 The major indirect biomarkers are the liver enzymes aspartate and alanine aminotransferase (AST and ALT), gamma-glutamyl transferase (GGT), mean corpuscular volume (MCV) of red blood cells, and carbohydrate-deficient transferrin (CDT). Indirect biomarkers have limited sensitivity and specificity for AUD. (For specifics on sensitivity and specificity of indirect and direct biomarkers, see TABLE 2.23-31)
Liver enzymes. AST and ALT are also present in the heart, muscle, and kidneys. Elevated levels usually imply injury to hepatocytes, with ALT being more reflective of liver involvement than AST. Both AST and ALT are elevated in other common liver conditions including hepatitis C virus infection and fatty liver disease. In alcoholic liver disease (ALD), AST is elevated more than ALT; an AST-to-ALT ratio > 3 suggests ALD. An elevated GGT often indicates hepatic injury and is used to confirm that elevated alkaline phosphatase is of hepatic origin.3
MCV is the average volume of erythrocytes,33 and an elevated MCV is a potential indicator of excessive alcohol intake. Macrocytosis requires sustained alcohol use, and the test has low sensitivity. Other diseases such as vitamin B12 or folic acid deficiency, hypothyroidism, hematologic diseases (eg, cold agglutinin disease, multiple myeloma, amyloidosis), and certain medications can also increase MCV.34 Moreover, MCV responds slowly to alcohol use, abstinence, and relapse because red cells have a life span of 120 days.35
CDT. Transferrin is a glycoprotein produced in the liver. The level of transferrin with sialic acid chains increases with alcohol consumption as well as in the carbohydrate deficient glycoprotein syndrome, leading to so-called carbohydrate deficient transferrin.36 It is a sensitive marker for detecting alcohol relapse and monitoring sobriety. Moderate-to-heavy alcohol use, averaging ≥ 40 g of alcohol per day for 2 weeks,36 can decrease the amount of carbohydrate attached to transferrin. Two weeks after complete alcohol cessation, CDT levels will return to normal.37
Continue to: CDT is approved...
CDT is approved by the FDA as an assay for alcohol consumption.37 While CDT is felt to be one of the better indirect markers of AUD and can extend the window of detection, there are still issues with its sensitivity and specificity.38 This biomarker can be elevated with other liver diseases and can be affected by the patient’s age, body mass index, gender, and tobacco use.39,40 Testing for CDT has never achieved widespread clinical use and has been largely supplanted by the more accurate PEth test (described in a bit).
Direct biomarkers offer insight into recent alcohol use
Other than ethanol itself, direct biomarkers of alcohol use are minor ethanol metabolites created through biochemical reactions when ethanol is coupled to endogenous compounds. Hence, the presence of these metabolites is usually directly related to ethanol consumption.41 Direct alcohol biomarkers are EtG, EtS, FAEEs, and PEth (TABLE 223-31). They reflect alcohol consumption over a period of several days, making them useful when paired with questionnaire data, especially for identifying young adults who engage in binge drinking.42
Ethanol can be measured in blood, urine, and breath and is detectable a bit longer in urine than in blood. However, alcohol is detectable in the blood only for 6 to 12 hours after drinking. After alcohol consumption, concentrations peak in the blood within 2 hours. The window for detecting ethanol in the blood depends on the amount of alcohol consumed and the elimination rate of alcohol, which is about 12 mg/dL/h (or 0.012%)—approximately the same amount of alcohol contained in a standard drink (14 g).
Checking the blood alcohol level might be helpful in the office if a patient appears intoxicated but denies alcohol use. A blood alcohol level > 300 mg/dL, or > 150 mg/dL without gross evidence of intoxication, or > 100 mg/dL upon routine examination indicates AUD with a high degree of reliability.33,43 But the short half-life of ethanol in blood limits its use as a biomarker,33 and it is not a good indicator of chronic drinking.44
EtG and EtS. Less than 0.1% of ethanol is secreted as the metabolites EtG and EtS, which are generated, respectively, by the enzymes uridine diphosphate glucuronosyltransferase and sulfotransferase.45 They have value in the diagnosis of AUD because of the length of time in which they can be detected. Urinary EtG and EtS have been especially important biomarkers for monitoring relapse in outpatients treated for alcohol-related problems.46 Generally, EtG and EtS can be detected in urine for 13 to 20 hours after a single drink (0.1 g/kg), and for up to 4 to 5 days following ingestion of large amounts of alcohol.47
Continue to: EtG has been detectable...
EtG has been detectable in urine for ≥ 24 hours following only 1 or 2 drinks, and for up to 4 days following heavy consumption.48 Shortly after alcohol intake, even in small amounts, EtG is detectable. Analysis of EtG in urine is helpful in monitoring alcohol consumption during withdrawal treatment, for workplace testing, and to check for abstinence in legal matters. The EtG urine test is useful in detecting alcohol consumption in a person who claims to be abstinent but who drank 2 or 3 days before the evaluation. Although accurate, EtG’s window for detection is narrower than that of the PEth assay.
EtS is a good marker of acute short-term alcohol use, up to 12 hours in the blood (or longer in heavier drinkers) and up to 5 days in urine.49 Its sensitivity is highest in heavy drinkers. Post-sampling formation and degradation of EtS have not been known to occur in urine samples. Testing for this second metabolite of ethanol can slightly improve the sensitivity and specificity of the EtG test. A urine test for EtS has a wider detection window. But it has little practical advantage compared with EtG.50
For better clinical specificity, a combination of both EtG and EtS testing has been recommended. However, the EtS assay is more cumbersome and provides little advantage over EtG. EtG values do not correlate precisely with the amount or frequency of ethanol use, but the magnitude of the EtG finding roughly corresponds to the amount of alcohol recently consumed.
False-positive and false-negative results for EtG and EtS are uncommon in practice. However, false-positive results are possible with the EtG test in certain circumstances: presence of Escherichia coli in the specimen, use of ethanol-based hand sanitizers (> 20 times a day) or mouthwashes, and the consumption of substances like pralines, nonalcoholic beer, pharmaceutical products, and fruit juice. Similarly, false-negative results of EtG can occur from degradation if the samples are contaminated with other bacteria, transported without cooling, or stored improperly.51 In practice, this is uncommon, and the test is believed to be specific with few false-positive results. Commercially available EtG colorimetric test strips permit on-site analysis of urine samples.
FAEEs are a combination of different esters and products of alcohol metabolism through a nonoxidative pathway. They are formed by esterification of endogenous free fatty acids and ethanol in blood and several tissues.29 These are sensitive and specific markers of alcohol ingestion and can differentiate chronic alcohol consumption from binge drinking.29 It is elevated for up to 99 hours in heavy alcohol drinkers.30 It can be detected in hair for a longer period than in blood.52 Detection of FAEEs in meconium can help establish fetal alcohol exposure.53
Continue to: PEth
PEth. Use of the PEth assay has increased in recent years and its accuracy has had a transformative effect on the diagnosis of AUD.54 PEth is a phospholipid found in erythrocyte membranes, formed by an interaction between ethanol and phosphatidylcholine, catalyzed by phospholipase D.55,56 Major advantages of PEth include an unusually long half-life and specificity. Red cells lack enzymes to degrade PEth, therefore PEth accumulates in red cells and has a half-life of 4 to 10 days57,58 allowing for detection of significant ethanol consumption extending back 3 to 4 weeks.59 There is no evidence that PEth is formed in the absence of ethanol, making the test essentially 100% specific, particularly at higher cutoff values of ≥ 150 ng/mL.31,60
PEth levels are not affected by age, gender, or underlying liver or renal disease.61 PEth can differentiate between heavy alcohol use and social drinking and can therefore identify chronic excessive use.62 With chronic excessive alcohol consumption, PEth is detectable in blood up to 28 days after sobriety.63 A correlation exists between PEth concentrations in blood and the amount of consumed ethanol. PEth has increased specificity and sensitivity for the detection of latent ethanol use compared with other direct biomarkers.21 It can identify recent heavy drinking earlier than indirect biomarkers, as it does not rely on hepatic injury.
Using a cutoff level of 20 ng/mL, PEth assays have a sensitivity of 73% for any alcohol use in the past month; at 80 ng/mL, the sensitivity is 91% for > 4 drinks/d.61 PEth is considered semi-quantitative. The World Health Organization defines acceptable social alcohol use at a PEth value < 40 ng/dL for men and < 20 ng/dL for women. Chronic excessive use is defined by a level > 60 ng/dL.55 The cutoff levels tend to be arbitrary and vary with different guidelines.
Although false-positive PEth test results may be possible, most experts believe that dishonesty in self-reporting by test subjects is more likely. That said, the true specificity of PEth remains unknown; a lower value detected should not be regarded as absolute proof of relapse or chronic alcoholism.
Studies have shown a positive correlation between the AUDIT-C score and PEth values combined with self-reported alcohol consumption, indicating that PEth may be a useful marker in difficult-to-assess settings, or in confirming or invalidating self-reported alcohol consumption.61,64,65 The PEth test is now widely available and, in the authors’ experience, usually costs $100 to $200. Analysis typically costs $40 to $100,66 and costs could decrease as the test becomes more widely used. Turnaround time for PEth is 5 to 10 days. It is now the recommended assay by transplant hepatologists for detecting alcohol use.67
Continue to: CASE ANSWER
CASE
CORRESPONDENCE
Frederick Nunes, MD, Pennsylvania Hospital of University of Pennsylvania, 230 West Washington Square, 4th Floor, Philadelphia, PA 19104; frederick.nunes@pennmedicine.upenn.edu
1. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. American Psychiatric Publishing. 2013:490-497.
2. Fleming MF, Smith MJ, Oslakovic E, et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res. 2017;41:857-862.
3. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed November 12, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
4. Herreros-Villanueva M, Hijona E, Bañales JM, et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19:638-647.
5. Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652-14659.
6.
7. Sebastiani G, Borrás-Novell C, Casanova MA, et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. 2018;10:1008.
8. Rehm J, Anderson P, Manthey J, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51:422-427. doi: 10.1093/alcalc/agv127
9. ASAM. Caring for patients during the COVID-19 pandemic. Accessed November 12, 2021. www.asam.org/docs/default-source/covid-19/acute-care_062620.pdf?sfvrsn=e66d54c2_10
10. Miller PM, Thomas SE, Mallin R. Patient attitudes towards self-report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol. 2006;41:306-310. doi: 10.1093/alcalc/agl022
11. Zoorob R, Snell H, Kihlberg C, et al. Screening and brief intervention for risky alcohol use. Curr Probl Pediatr Adolesc Health Care. 2014;44:82-87.
12. Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783-788.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905-1907.
14. Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863-868.
15. Cherpitel CJ. A brief screening instrument for problem drinking in the emergency room: the RAPS4. Rapid Alcohol Problems Screen. J Stud Alcohol. 2000;61:447-449.
16. WHO. AUDIT: The alcohol use identification test. Accessed November 14, 2021. http://apps.who.int/iris/bitstream/handle/10665/67205/WHO_MSD_MSB_01.6a.pdf?sequence=1
17. Westermeyer J, Yargic I, Thuras P. Michigan assessment-screening test for alcohol and drugs (MAST/AD): evaluation in a clinical sample. Am J Addict. 2004;13:151-162.
18. Powers JS, Spickard A. Michigan Alcoholism Screening Test to diagnose early alcoholism in a general practice. South Med J. 1984;77:852-856.
19. NIH. Treatment for alcohol problems: finding and getting help. Accessed November 12, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/treatment-alcohol-problems-finding-and-getting-help
20. Kitchens JM. Does this patient have an alcohol problem? JAMA. 1994;272:1782-1787.
21. Kummer N, Lambert WE, Samyn N, et al. Alternative sampling strategies for the assessment of alcohol intake of living persons. Clin Biochem. 2016;49:1078-1091.
22. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63:1634-1640.
23. Mundle G, Ackermann K, Munkes J, et al. Influence of age, alcohol consumption and abstinence on the sensitivity of carbohydrate‐deficient transferrin, gamma‐glutamyltransferase and mean corpuscular volume. Alcohol Alcohol. 1999;34:760-766.
24. Neumann T, Spies C. Use of biomarkers for alcohol use disorders in clinical practice. Addiction. 2003;98(suppl 2):81-91.
25. Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684-11699.
26. Helander A. Biological markers of alcohol use and abuse in theory and practice. In: Agarwal DP, Seitz HK, eds. Alcohol in Health and Disease. Marcel Dekker. 2001:177-205.
27. Stewart SH, Koch DG, Burgess DM, et al. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcohol Clin Exp Res. 2013;37:150-155.
28. Helander A, Dahl H. Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem. 2005;51:1728-1730.
29. Ghosh S, Jain R, Jhanjee S, et al. Alcohol biomarkers and their relevance in detection of alcohol consumption in clinical settings. Accessed November 12, 2021. https://www.clinmedjournals.org/articles/iasar/international-archives-of-substance-abuse-and-rehabilitation-iasar-1-002.php?jid=iasar
30. Borucki K, Dierkes J, Wartberg J, et al. In heavy drinkers, fatty acid ethyl esters remain elevated for up to 99 hours. Alcohol Clin Exp Res. 2007;31:423-427.
31. Hartmann S, Aradottir S, Graf M, et al. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81-84.
32. Choe YM, Lee BC, Choi IG, et al. Combination of the CAGE and serum gamma-glutamyl transferase: an effective screening tool for alcohol use disorder and alcohol dependence. Neuropsychiatr Dis Treat. 2019 31;15:1507-1515.
33. Niemelä O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39-49.
34. Kauffmann T, Evans DS. Macrocytosis. Accessed November 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK560908/
35. Maenhout TM, De Buyzere ML, Delanghe JR. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chim Acta. 2013;415:322-329.
36. Solomons HD. Carbohydrate deficient transferrin and alcoholism. Germs. 2012;2:75-78.
37. Allen JP, Wurst FM, Thon N, et al. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19:369-376.
38. Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:96-109.
39. Hannuksela ML, Liisanantti MK, Nissinen AE, et al. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953-961.
40. Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001;47:13-27.
41. Cabarcos P, Hassan HM, Tabernero MJ, et al. Analysis of ethyl glucuronide in hair samples by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). J Appl Toxicol. 2013;33:638-643.
42. Piano MR, Mazzuco A, Kang M, et al. Binge drinking episodes in young adults: how should we measure them in a research setting? J Stud Alcohol Drugs. 2017;78:502-511.
43. Adinoff B, Bone GH, Linnoila M. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp. 1988;3:172-196.
44. Cabezas J, Lucey MR, Bataller R. Biomarkers for monitoring alcohol use. Clin Liver Dis (Hoboken). 2016;8:59-63.
45. Wurst FM, Alling C, Aradottir S, et al. Emerging biomarkers: new directions and clinical applications. Alcohol Clin Exp Res. 2005;29:465-473.
46. Helander A, Péter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552-557.
47. Helander A, Böttcher M, Fehr C, et al. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44:55-61.
48. Jatlow P, O’Malley SS. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res. 2010;34:968-975.
49. Jatlow PI, Agro A, Wu R, et al. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin Exp Res. 2014;38:2056-2065.
50. Gonzalo P, Radenne S, Gonzalo S. Biomarkers of chronic alcohol misuse. Curr Biomark Find. 2014;4:9-22.
51. Bornhorst JA, Mbughuni MM. Alcohol biomarkers: clinical issues and analytical methods. In: Critical Issues in Alcohol and Drugs of Abuse Testing. 2nd ed. Academic Press. 2019:25-42.
52. Soderberg BL, Salem RO, Best CA, et al. Fatty acid ethyl esters. Ethanol metabolites that reflect ethanol intake. Am J Clin Pathol. 2003;119(suppl):S94-S99.
53. Cheng CT, Ostrea EM Jr, Alviedo JN, et al. Fatty acid ethyl esters in meconium: a biomarker of fetal alcohol exposure and effect. Exp Biol Med (Maywood). 2021;246:380-386.
54. Andresen-Streichert H, Beres Y, Weinmann W, et al. Improved detection of alcohol consumption using the novel marker phosphatidylethanol in the transplant setting: results of a prospective study. Transpl Int. 2017;30:611-620.
55. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13:14788-14812.
56. Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507-1511.
57. Aradóttir S, Moller K, Alling C. Phosphatidylethanol formation and degradation in human and rat blood. Alcohol Alcohol. 2004;39:8-13.
58. Varga A, Alling C. Formation of phosphatidylethanol in vitro in red blood cells from healthy volunteers and chronic alcoholics. J Lab Clin Med. 2002;140:79-83.
59. Javors MA, Hill-Kapturczak N, Roache JD, et al. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res. 2016;40:1228-1234.
60. Schröck A, Pfäffli M, König S, et al. Application of phosphatidylethanol (PEth) in whole blood in comparison to ethyl glucuronide in hair (hEtG) in driving aptitude assessment (DAA). Int J Legal Med. 2016;130:1527-1533.
61. Stewart SH, Koch DG, Willner IR, et al. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706-1711.
62. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015 29;5:1339-1385.
63. Hill-Kapturczak N, Dougherty DM, Roache JD, et al. Phosphatidylethanol homologs in blood as biomarkers for the time frame and amount of recent alcohol consumption. In: Preedy VR (ed) Neuroscience of Alcohol. Academic Press; 2019:567-576.
64. Jain J, Evans JL, Briceño A, et al. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol Alcohol. 2014;49:520-524.
65. Schröck A, Wurst FM, Thon N, et al. Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test - C (AUDIT-C). Drug Alcohol Depend. 2017;178:80-86.
66. McDonnell MG, Skalisky J, Leickly E, et al. Pilot investigation of a phosphatidylethanol-based contingency management intervention targeting alcohol use. Psychol Addict Behav. 2017;31:608-613.
67. Asrani SK, Trotter J, Lake J, et al. Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis. Liver Transpl. 2020;26:127-140.
68. WHO. International Guide for Monitoring Alcohol Consumption and Harm. 2000. Accessed November 12, 2021. http://apps.who.int/iris/bitstream/handle/10665/66529/WHO_MSD_MSB_00.4.pdf?sequence=1
CASE A 34-year-old woman presents with fatigue. She appears defensive when asked about her alcohol use. She answers No to all questions on the CAGE (cut down, annoyed, guilty, eye-opener) screening tool, but acknowledges drinking excessively on rare occasions. Her physician has a high suspicion for alcohol use disorder (AUD) and recommends further testing. The patient agrees but denies having used alcohol over the past several days. Which of the following is most likely to help support the suspicion of a heavy drinking pattern?
- Routine lab tests (liver panel and complete blood count).
- Blood or urine alcohol level.
- Phosphatidylethanol (PEth) level in the blood.
- Ethyl glucuronide (EtG) in the urine.
- Carbohydrate-deficient transferrin (CDT) in the blood.
(See "Case answer.").
About 1 in 12 Americans have AUD,1 and 1 in 10 children live in a home with a parent who has a drinking problem.2 While the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) succinctly defines AUD with specific criteria,1 the term generally refers to an inability to control or stop drinking despite adverse social or health consequences. AUD is regarded as > 4 drinks per day for men and > 3 drinks per day for women.3 A “standard drink” would be a 12-oz bottle of beer, a 5-oz glass of wine, or 1.5 oz of distilled spirits. Effects of chronic alcohol use are vast and include malnutrition, alcohol withdrawal syndrome, alcoholic liver disease, pancreatitis/pancreatic cancer, cardiomyopathy, and stroke.4-6 Alcohol use by a pregnant woman can lead to fetal alcohol syndrome in her child.7
AUD may be more prevalent in the wake of COVID-19. Primary care practitioners tend to miss a large fraction of patients with AUD in their practice, especially younger patients and those without somatic comorbidities.8 Systematic screening for AUD can identify many of these people.8 Particularly as the COVID-19 pandemic continues to unfold and increases stress for everyone, risk of worsening drinking increases both in individuals with current AUD and for those in remission.9 Contrary to common belief, patients visiting primary care favor screening for at-risk drinking.10 Thus, awareness of the prevalence of AUD and patient acceptance of screening should encourage wider testing.
Screening tools. The 2014 guidelines published by the Centers for Disease Control and Prevention recommend using quick screening tools—ie, single question or AUDIT 1-3 (TABLE 111-18)—as an objective means of determining whether patients’ drinking creates a risk for themselves or others.11 Excessive drinking identified using alcohol questionnaires can help reduce medical complications and health care costs.19 The questionnaires we review do not provide a diagnosis but help identify individuals who might benefit from more thorough assessment.20 Following up, as needed, by testing for alcohol biomarkers can provide quantitative insight into problematic alcohol use.2
But before we discuss the utility of biomarkers, it’s important to quickly review how alcohol is eliminated from the body.
Alcohol elimination
The stomach and small intestine are the primary sites for alcohol absorption. Alcohol elimination from the body occurs through 3 pathways. The first involves oxidative metabolism, which eliminates most ethanol (95%) through the actions of alcohol dehydrogenase, cytochrome P4502E1, or catalase. A lesser amount of alcohol (2%-5%) is eliminated, unchanged, via the second pathway, which includes urine, sweat, and breath. Nonoxidative metabolism makes up the third pathway. Nonoxidative metabolism removes a very small amount (0.1%) of alcohol and involves the direct ethanol biomarkers PEth, EtG, ethyl sulfate (EtS), and fatty acid ethyl esters (FAEEs).21 Our emphasis in this article is on assays of direct metabolites of alcohol—particularly PEth.
Continue to: To understand the utility...
To understand the utility of these direct biomarkers, it is helpful to look at the indirect biomarkers first.
Indirect biomarkers have limited sensitivity and specificity
When alcohol is consumed in large enough quantities over time, indirect biomarkers of alcohol can become abnormal.22 The major indirect biomarkers are the liver enzymes aspartate and alanine aminotransferase (AST and ALT), gamma-glutamyl transferase (GGT), mean corpuscular volume (MCV) of red blood cells, and carbohydrate-deficient transferrin (CDT). Indirect biomarkers have limited sensitivity and specificity for AUD. (For specifics on sensitivity and specificity of indirect and direct biomarkers, see TABLE 2.23-31)
Liver enzymes. AST and ALT are also present in the heart, muscle, and kidneys. Elevated levels usually imply injury to hepatocytes, with ALT being more reflective of liver involvement than AST. Both AST and ALT are elevated in other common liver conditions including hepatitis C virus infection and fatty liver disease. In alcoholic liver disease (ALD), AST is elevated more than ALT; an AST-to-ALT ratio > 3 suggests ALD. An elevated GGT often indicates hepatic injury and is used to confirm that elevated alkaline phosphatase is of hepatic origin.3
MCV is the average volume of erythrocytes,33 and an elevated MCV is a potential indicator of excessive alcohol intake. Macrocytosis requires sustained alcohol use, and the test has low sensitivity. Other diseases such as vitamin B12 or folic acid deficiency, hypothyroidism, hematologic diseases (eg, cold agglutinin disease, multiple myeloma, amyloidosis), and certain medications can also increase MCV.34 Moreover, MCV responds slowly to alcohol use, abstinence, and relapse because red cells have a life span of 120 days.35
CDT. Transferrin is a glycoprotein produced in the liver. The level of transferrin with sialic acid chains increases with alcohol consumption as well as in the carbohydrate deficient glycoprotein syndrome, leading to so-called carbohydrate deficient transferrin.36 It is a sensitive marker for detecting alcohol relapse and monitoring sobriety. Moderate-to-heavy alcohol use, averaging ≥ 40 g of alcohol per day for 2 weeks,36 can decrease the amount of carbohydrate attached to transferrin. Two weeks after complete alcohol cessation, CDT levels will return to normal.37
Continue to: CDT is approved...
CDT is approved by the FDA as an assay for alcohol consumption.37 While CDT is felt to be one of the better indirect markers of AUD and can extend the window of detection, there are still issues with its sensitivity and specificity.38 This biomarker can be elevated with other liver diseases and can be affected by the patient’s age, body mass index, gender, and tobacco use.39,40 Testing for CDT has never achieved widespread clinical use and has been largely supplanted by the more accurate PEth test (described in a bit).
Direct biomarkers offer insight into recent alcohol use
Other than ethanol itself, direct biomarkers of alcohol use are minor ethanol metabolites created through biochemical reactions when ethanol is coupled to endogenous compounds. Hence, the presence of these metabolites is usually directly related to ethanol consumption.41 Direct alcohol biomarkers are EtG, EtS, FAEEs, and PEth (TABLE 223-31). They reflect alcohol consumption over a period of several days, making them useful when paired with questionnaire data, especially for identifying young adults who engage in binge drinking.42
Ethanol can be measured in blood, urine, and breath and is detectable a bit longer in urine than in blood. However, alcohol is detectable in the blood only for 6 to 12 hours after drinking. After alcohol consumption, concentrations peak in the blood within 2 hours. The window for detecting ethanol in the blood depends on the amount of alcohol consumed and the elimination rate of alcohol, which is about 12 mg/dL/h (or 0.012%)—approximately the same amount of alcohol contained in a standard drink (14 g).
Checking the blood alcohol level might be helpful in the office if a patient appears intoxicated but denies alcohol use. A blood alcohol level > 300 mg/dL, or > 150 mg/dL without gross evidence of intoxication, or > 100 mg/dL upon routine examination indicates AUD with a high degree of reliability.33,43 But the short half-life of ethanol in blood limits its use as a biomarker,33 and it is not a good indicator of chronic drinking.44
EtG and EtS. Less than 0.1% of ethanol is secreted as the metabolites EtG and EtS, which are generated, respectively, by the enzymes uridine diphosphate glucuronosyltransferase and sulfotransferase.45 They have value in the diagnosis of AUD because of the length of time in which they can be detected. Urinary EtG and EtS have been especially important biomarkers for monitoring relapse in outpatients treated for alcohol-related problems.46 Generally, EtG and EtS can be detected in urine for 13 to 20 hours after a single drink (0.1 g/kg), and for up to 4 to 5 days following ingestion of large amounts of alcohol.47
Continue to: EtG has been detectable...
EtG has been detectable in urine for ≥ 24 hours following only 1 or 2 drinks, and for up to 4 days following heavy consumption.48 Shortly after alcohol intake, even in small amounts, EtG is detectable. Analysis of EtG in urine is helpful in monitoring alcohol consumption during withdrawal treatment, for workplace testing, and to check for abstinence in legal matters. The EtG urine test is useful in detecting alcohol consumption in a person who claims to be abstinent but who drank 2 or 3 days before the evaluation. Although accurate, EtG’s window for detection is narrower than that of the PEth assay.
EtS is a good marker of acute short-term alcohol use, up to 12 hours in the blood (or longer in heavier drinkers) and up to 5 days in urine.49 Its sensitivity is highest in heavy drinkers. Post-sampling formation and degradation of EtS have not been known to occur in urine samples. Testing for this second metabolite of ethanol can slightly improve the sensitivity and specificity of the EtG test. A urine test for EtS has a wider detection window. But it has little practical advantage compared with EtG.50
For better clinical specificity, a combination of both EtG and EtS testing has been recommended. However, the EtS assay is more cumbersome and provides little advantage over EtG. EtG values do not correlate precisely with the amount or frequency of ethanol use, but the magnitude of the EtG finding roughly corresponds to the amount of alcohol recently consumed.
False-positive and false-negative results for EtG and EtS are uncommon in practice. However, false-positive results are possible with the EtG test in certain circumstances: presence of Escherichia coli in the specimen, use of ethanol-based hand sanitizers (> 20 times a day) or mouthwashes, and the consumption of substances like pralines, nonalcoholic beer, pharmaceutical products, and fruit juice. Similarly, false-negative results of EtG can occur from degradation if the samples are contaminated with other bacteria, transported without cooling, or stored improperly.51 In practice, this is uncommon, and the test is believed to be specific with few false-positive results. Commercially available EtG colorimetric test strips permit on-site analysis of urine samples.
FAEEs are a combination of different esters and products of alcohol metabolism through a nonoxidative pathway. They are formed by esterification of endogenous free fatty acids and ethanol in blood and several tissues.29 These are sensitive and specific markers of alcohol ingestion and can differentiate chronic alcohol consumption from binge drinking.29 It is elevated for up to 99 hours in heavy alcohol drinkers.30 It can be detected in hair for a longer period than in blood.52 Detection of FAEEs in meconium can help establish fetal alcohol exposure.53
Continue to: PEth
PEth. Use of the PEth assay has increased in recent years and its accuracy has had a transformative effect on the diagnosis of AUD.54 PEth is a phospholipid found in erythrocyte membranes, formed by an interaction between ethanol and phosphatidylcholine, catalyzed by phospholipase D.55,56 Major advantages of PEth include an unusually long half-life and specificity. Red cells lack enzymes to degrade PEth, therefore PEth accumulates in red cells and has a half-life of 4 to 10 days57,58 allowing for detection of significant ethanol consumption extending back 3 to 4 weeks.59 There is no evidence that PEth is formed in the absence of ethanol, making the test essentially 100% specific, particularly at higher cutoff values of ≥ 150 ng/mL.31,60
PEth levels are not affected by age, gender, or underlying liver or renal disease.61 PEth can differentiate between heavy alcohol use and social drinking and can therefore identify chronic excessive use.62 With chronic excessive alcohol consumption, PEth is detectable in blood up to 28 days after sobriety.63 A correlation exists between PEth concentrations in blood and the amount of consumed ethanol. PEth has increased specificity and sensitivity for the detection of latent ethanol use compared with other direct biomarkers.21 It can identify recent heavy drinking earlier than indirect biomarkers, as it does not rely on hepatic injury.
Using a cutoff level of 20 ng/mL, PEth assays have a sensitivity of 73% for any alcohol use in the past month; at 80 ng/mL, the sensitivity is 91% for > 4 drinks/d.61 PEth is considered semi-quantitative. The World Health Organization defines acceptable social alcohol use at a PEth value < 40 ng/dL for men and < 20 ng/dL for women. Chronic excessive use is defined by a level > 60 ng/dL.55 The cutoff levels tend to be arbitrary and vary with different guidelines.
Although false-positive PEth test results may be possible, most experts believe that dishonesty in self-reporting by test subjects is more likely. That said, the true specificity of PEth remains unknown; a lower value detected should not be regarded as absolute proof of relapse or chronic alcoholism.
Studies have shown a positive correlation between the AUDIT-C score and PEth values combined with self-reported alcohol consumption, indicating that PEth may be a useful marker in difficult-to-assess settings, or in confirming or invalidating self-reported alcohol consumption.61,64,65 The PEth test is now widely available and, in the authors’ experience, usually costs $100 to $200. Analysis typically costs $40 to $100,66 and costs could decrease as the test becomes more widely used. Turnaround time for PEth is 5 to 10 days. It is now the recommended assay by transplant hepatologists for detecting alcohol use.67
Continue to: CASE ANSWER
CASE
CORRESPONDENCE
Frederick Nunes, MD, Pennsylvania Hospital of University of Pennsylvania, 230 West Washington Square, 4th Floor, Philadelphia, PA 19104; frederick.nunes@pennmedicine.upenn.edu
CASE A 34-year-old woman presents with fatigue. She appears defensive when asked about her alcohol use. She answers No to all questions on the CAGE (cut down, annoyed, guilty, eye-opener) screening tool, but acknowledges drinking excessively on rare occasions. Her physician has a high suspicion for alcohol use disorder (AUD) and recommends further testing. The patient agrees but denies having used alcohol over the past several days. Which of the following is most likely to help support the suspicion of a heavy drinking pattern?
- Routine lab tests (liver panel and complete blood count).
- Blood or urine alcohol level.
- Phosphatidylethanol (PEth) level in the blood.
- Ethyl glucuronide (EtG) in the urine.
- Carbohydrate-deficient transferrin (CDT) in the blood.
(See "Case answer.").
About 1 in 12 Americans have AUD,1 and 1 in 10 children live in a home with a parent who has a drinking problem.2 While the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) succinctly defines AUD with specific criteria,1 the term generally refers to an inability to control or stop drinking despite adverse social or health consequences. AUD is regarded as > 4 drinks per day for men and > 3 drinks per day for women.3 A “standard drink” would be a 12-oz bottle of beer, a 5-oz glass of wine, or 1.5 oz of distilled spirits. Effects of chronic alcohol use are vast and include malnutrition, alcohol withdrawal syndrome, alcoholic liver disease, pancreatitis/pancreatic cancer, cardiomyopathy, and stroke.4-6 Alcohol use by a pregnant woman can lead to fetal alcohol syndrome in her child.7
AUD may be more prevalent in the wake of COVID-19. Primary care practitioners tend to miss a large fraction of patients with AUD in their practice, especially younger patients and those without somatic comorbidities.8 Systematic screening for AUD can identify many of these people.8 Particularly as the COVID-19 pandemic continues to unfold and increases stress for everyone, risk of worsening drinking increases both in individuals with current AUD and for those in remission.9 Contrary to common belief, patients visiting primary care favor screening for at-risk drinking.10 Thus, awareness of the prevalence of AUD and patient acceptance of screening should encourage wider testing.
Screening tools. The 2014 guidelines published by the Centers for Disease Control and Prevention recommend using quick screening tools—ie, single question or AUDIT 1-3 (TABLE 111-18)—as an objective means of determining whether patients’ drinking creates a risk for themselves or others.11 Excessive drinking identified using alcohol questionnaires can help reduce medical complications and health care costs.19 The questionnaires we review do not provide a diagnosis but help identify individuals who might benefit from more thorough assessment.20 Following up, as needed, by testing for alcohol biomarkers can provide quantitative insight into problematic alcohol use.2
But before we discuss the utility of biomarkers, it’s important to quickly review how alcohol is eliminated from the body.
Alcohol elimination
The stomach and small intestine are the primary sites for alcohol absorption. Alcohol elimination from the body occurs through 3 pathways. The first involves oxidative metabolism, which eliminates most ethanol (95%) through the actions of alcohol dehydrogenase, cytochrome P4502E1, or catalase. A lesser amount of alcohol (2%-5%) is eliminated, unchanged, via the second pathway, which includes urine, sweat, and breath. Nonoxidative metabolism makes up the third pathway. Nonoxidative metabolism removes a very small amount (0.1%) of alcohol and involves the direct ethanol biomarkers PEth, EtG, ethyl sulfate (EtS), and fatty acid ethyl esters (FAEEs).21 Our emphasis in this article is on assays of direct metabolites of alcohol—particularly PEth.
Continue to: To understand the utility...
To understand the utility of these direct biomarkers, it is helpful to look at the indirect biomarkers first.
Indirect biomarkers have limited sensitivity and specificity
When alcohol is consumed in large enough quantities over time, indirect biomarkers of alcohol can become abnormal.22 The major indirect biomarkers are the liver enzymes aspartate and alanine aminotransferase (AST and ALT), gamma-glutamyl transferase (GGT), mean corpuscular volume (MCV) of red blood cells, and carbohydrate-deficient transferrin (CDT). Indirect biomarkers have limited sensitivity and specificity for AUD. (For specifics on sensitivity and specificity of indirect and direct biomarkers, see TABLE 2.23-31)
Liver enzymes. AST and ALT are also present in the heart, muscle, and kidneys. Elevated levels usually imply injury to hepatocytes, with ALT being more reflective of liver involvement than AST. Both AST and ALT are elevated in other common liver conditions including hepatitis C virus infection and fatty liver disease. In alcoholic liver disease (ALD), AST is elevated more than ALT; an AST-to-ALT ratio > 3 suggests ALD. An elevated GGT often indicates hepatic injury and is used to confirm that elevated alkaline phosphatase is of hepatic origin.3
MCV is the average volume of erythrocytes,33 and an elevated MCV is a potential indicator of excessive alcohol intake. Macrocytosis requires sustained alcohol use, and the test has low sensitivity. Other diseases such as vitamin B12 or folic acid deficiency, hypothyroidism, hematologic diseases (eg, cold agglutinin disease, multiple myeloma, amyloidosis), and certain medications can also increase MCV.34 Moreover, MCV responds slowly to alcohol use, abstinence, and relapse because red cells have a life span of 120 days.35
CDT. Transferrin is a glycoprotein produced in the liver. The level of transferrin with sialic acid chains increases with alcohol consumption as well as in the carbohydrate deficient glycoprotein syndrome, leading to so-called carbohydrate deficient transferrin.36 It is a sensitive marker for detecting alcohol relapse and monitoring sobriety. Moderate-to-heavy alcohol use, averaging ≥ 40 g of alcohol per day for 2 weeks,36 can decrease the amount of carbohydrate attached to transferrin. Two weeks after complete alcohol cessation, CDT levels will return to normal.37
Continue to: CDT is approved...
CDT is approved by the FDA as an assay for alcohol consumption.37 While CDT is felt to be one of the better indirect markers of AUD and can extend the window of detection, there are still issues with its sensitivity and specificity.38 This biomarker can be elevated with other liver diseases and can be affected by the patient’s age, body mass index, gender, and tobacco use.39,40 Testing for CDT has never achieved widespread clinical use and has been largely supplanted by the more accurate PEth test (described in a bit).
Direct biomarkers offer insight into recent alcohol use
Other than ethanol itself, direct biomarkers of alcohol use are minor ethanol metabolites created through biochemical reactions when ethanol is coupled to endogenous compounds. Hence, the presence of these metabolites is usually directly related to ethanol consumption.41 Direct alcohol biomarkers are EtG, EtS, FAEEs, and PEth (TABLE 223-31). They reflect alcohol consumption over a period of several days, making them useful when paired with questionnaire data, especially for identifying young adults who engage in binge drinking.42
Ethanol can be measured in blood, urine, and breath and is detectable a bit longer in urine than in blood. However, alcohol is detectable in the blood only for 6 to 12 hours after drinking. After alcohol consumption, concentrations peak in the blood within 2 hours. The window for detecting ethanol in the blood depends on the amount of alcohol consumed and the elimination rate of alcohol, which is about 12 mg/dL/h (or 0.012%)—approximately the same amount of alcohol contained in a standard drink (14 g).
Checking the blood alcohol level might be helpful in the office if a patient appears intoxicated but denies alcohol use. A blood alcohol level > 300 mg/dL, or > 150 mg/dL without gross evidence of intoxication, or > 100 mg/dL upon routine examination indicates AUD with a high degree of reliability.33,43 But the short half-life of ethanol in blood limits its use as a biomarker,33 and it is not a good indicator of chronic drinking.44
EtG and EtS. Less than 0.1% of ethanol is secreted as the metabolites EtG and EtS, which are generated, respectively, by the enzymes uridine diphosphate glucuronosyltransferase and sulfotransferase.45 They have value in the diagnosis of AUD because of the length of time in which they can be detected. Urinary EtG and EtS have been especially important biomarkers for monitoring relapse in outpatients treated for alcohol-related problems.46 Generally, EtG and EtS can be detected in urine for 13 to 20 hours after a single drink (0.1 g/kg), and for up to 4 to 5 days following ingestion of large amounts of alcohol.47
Continue to: EtG has been detectable...
EtG has been detectable in urine for ≥ 24 hours following only 1 or 2 drinks, and for up to 4 days following heavy consumption.48 Shortly after alcohol intake, even in small amounts, EtG is detectable. Analysis of EtG in urine is helpful in monitoring alcohol consumption during withdrawal treatment, for workplace testing, and to check for abstinence in legal matters. The EtG urine test is useful in detecting alcohol consumption in a person who claims to be abstinent but who drank 2 or 3 days before the evaluation. Although accurate, EtG’s window for detection is narrower than that of the PEth assay.
EtS is a good marker of acute short-term alcohol use, up to 12 hours in the blood (or longer in heavier drinkers) and up to 5 days in urine.49 Its sensitivity is highest in heavy drinkers. Post-sampling formation and degradation of EtS have not been known to occur in urine samples. Testing for this second metabolite of ethanol can slightly improve the sensitivity and specificity of the EtG test. A urine test for EtS has a wider detection window. But it has little practical advantage compared with EtG.50
For better clinical specificity, a combination of both EtG and EtS testing has been recommended. However, the EtS assay is more cumbersome and provides little advantage over EtG. EtG values do not correlate precisely with the amount or frequency of ethanol use, but the magnitude of the EtG finding roughly corresponds to the amount of alcohol recently consumed.
False-positive and false-negative results for EtG and EtS are uncommon in practice. However, false-positive results are possible with the EtG test in certain circumstances: presence of Escherichia coli in the specimen, use of ethanol-based hand sanitizers (> 20 times a day) or mouthwashes, and the consumption of substances like pralines, nonalcoholic beer, pharmaceutical products, and fruit juice. Similarly, false-negative results of EtG can occur from degradation if the samples are contaminated with other bacteria, transported without cooling, or stored improperly.51 In practice, this is uncommon, and the test is believed to be specific with few false-positive results. Commercially available EtG colorimetric test strips permit on-site analysis of urine samples.
FAEEs are a combination of different esters and products of alcohol metabolism through a nonoxidative pathway. They are formed by esterification of endogenous free fatty acids and ethanol in blood and several tissues.29 These are sensitive and specific markers of alcohol ingestion and can differentiate chronic alcohol consumption from binge drinking.29 It is elevated for up to 99 hours in heavy alcohol drinkers.30 It can be detected in hair for a longer period than in blood.52 Detection of FAEEs in meconium can help establish fetal alcohol exposure.53
Continue to: PEth
PEth. Use of the PEth assay has increased in recent years and its accuracy has had a transformative effect on the diagnosis of AUD.54 PEth is a phospholipid found in erythrocyte membranes, formed by an interaction between ethanol and phosphatidylcholine, catalyzed by phospholipase D.55,56 Major advantages of PEth include an unusually long half-life and specificity. Red cells lack enzymes to degrade PEth, therefore PEth accumulates in red cells and has a half-life of 4 to 10 days57,58 allowing for detection of significant ethanol consumption extending back 3 to 4 weeks.59 There is no evidence that PEth is formed in the absence of ethanol, making the test essentially 100% specific, particularly at higher cutoff values of ≥ 150 ng/mL.31,60
PEth levels are not affected by age, gender, or underlying liver or renal disease.61 PEth can differentiate between heavy alcohol use and social drinking and can therefore identify chronic excessive use.62 With chronic excessive alcohol consumption, PEth is detectable in blood up to 28 days after sobriety.63 A correlation exists between PEth concentrations in blood and the amount of consumed ethanol. PEth has increased specificity and sensitivity for the detection of latent ethanol use compared with other direct biomarkers.21 It can identify recent heavy drinking earlier than indirect biomarkers, as it does not rely on hepatic injury.
Using a cutoff level of 20 ng/mL, PEth assays have a sensitivity of 73% for any alcohol use in the past month; at 80 ng/mL, the sensitivity is 91% for > 4 drinks/d.61 PEth is considered semi-quantitative. The World Health Organization defines acceptable social alcohol use at a PEth value < 40 ng/dL for men and < 20 ng/dL for women. Chronic excessive use is defined by a level > 60 ng/dL.55 The cutoff levels tend to be arbitrary and vary with different guidelines.
Although false-positive PEth test results may be possible, most experts believe that dishonesty in self-reporting by test subjects is more likely. That said, the true specificity of PEth remains unknown; a lower value detected should not be regarded as absolute proof of relapse or chronic alcoholism.
Studies have shown a positive correlation between the AUDIT-C score and PEth values combined with self-reported alcohol consumption, indicating that PEth may be a useful marker in difficult-to-assess settings, or in confirming or invalidating self-reported alcohol consumption.61,64,65 The PEth test is now widely available and, in the authors’ experience, usually costs $100 to $200. Analysis typically costs $40 to $100,66 and costs could decrease as the test becomes more widely used. Turnaround time for PEth is 5 to 10 days. It is now the recommended assay by transplant hepatologists for detecting alcohol use.67
Continue to: CASE ANSWER
CASE
CORRESPONDENCE
Frederick Nunes, MD, Pennsylvania Hospital of University of Pennsylvania, 230 West Washington Square, 4th Floor, Philadelphia, PA 19104; frederick.nunes@pennmedicine.upenn.edu
1. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. American Psychiatric Publishing. 2013:490-497.
2. Fleming MF, Smith MJ, Oslakovic E, et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res. 2017;41:857-862.
3. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed November 12, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
4. Herreros-Villanueva M, Hijona E, Bañales JM, et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19:638-647.
5. Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652-14659.
6.
7. Sebastiani G, Borrás-Novell C, Casanova MA, et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. 2018;10:1008.
8. Rehm J, Anderson P, Manthey J, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51:422-427. doi: 10.1093/alcalc/agv127
9. ASAM. Caring for patients during the COVID-19 pandemic. Accessed November 12, 2021. www.asam.org/docs/default-source/covid-19/acute-care_062620.pdf?sfvrsn=e66d54c2_10
10. Miller PM, Thomas SE, Mallin R. Patient attitudes towards self-report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol. 2006;41:306-310. doi: 10.1093/alcalc/agl022
11. Zoorob R, Snell H, Kihlberg C, et al. Screening and brief intervention for risky alcohol use. Curr Probl Pediatr Adolesc Health Care. 2014;44:82-87.
12. Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783-788.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905-1907.
14. Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863-868.
15. Cherpitel CJ. A brief screening instrument for problem drinking in the emergency room: the RAPS4. Rapid Alcohol Problems Screen. J Stud Alcohol. 2000;61:447-449.
16. WHO. AUDIT: The alcohol use identification test. Accessed November 14, 2021. http://apps.who.int/iris/bitstream/handle/10665/67205/WHO_MSD_MSB_01.6a.pdf?sequence=1
17. Westermeyer J, Yargic I, Thuras P. Michigan assessment-screening test for alcohol and drugs (MAST/AD): evaluation in a clinical sample. Am J Addict. 2004;13:151-162.
18. Powers JS, Spickard A. Michigan Alcoholism Screening Test to diagnose early alcoholism in a general practice. South Med J. 1984;77:852-856.
19. NIH. Treatment for alcohol problems: finding and getting help. Accessed November 12, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/treatment-alcohol-problems-finding-and-getting-help
20. Kitchens JM. Does this patient have an alcohol problem? JAMA. 1994;272:1782-1787.
21. Kummer N, Lambert WE, Samyn N, et al. Alternative sampling strategies for the assessment of alcohol intake of living persons. Clin Biochem. 2016;49:1078-1091.
22. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63:1634-1640.
23. Mundle G, Ackermann K, Munkes J, et al. Influence of age, alcohol consumption and abstinence on the sensitivity of carbohydrate‐deficient transferrin, gamma‐glutamyltransferase and mean corpuscular volume. Alcohol Alcohol. 1999;34:760-766.
24. Neumann T, Spies C. Use of biomarkers for alcohol use disorders in clinical practice. Addiction. 2003;98(suppl 2):81-91.
25. Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684-11699.
26. Helander A. Biological markers of alcohol use and abuse in theory and practice. In: Agarwal DP, Seitz HK, eds. Alcohol in Health and Disease. Marcel Dekker. 2001:177-205.
27. Stewart SH, Koch DG, Burgess DM, et al. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcohol Clin Exp Res. 2013;37:150-155.
28. Helander A, Dahl H. Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem. 2005;51:1728-1730.
29. Ghosh S, Jain R, Jhanjee S, et al. Alcohol biomarkers and their relevance in detection of alcohol consumption in clinical settings. Accessed November 12, 2021. https://www.clinmedjournals.org/articles/iasar/international-archives-of-substance-abuse-and-rehabilitation-iasar-1-002.php?jid=iasar
30. Borucki K, Dierkes J, Wartberg J, et al. In heavy drinkers, fatty acid ethyl esters remain elevated for up to 99 hours. Alcohol Clin Exp Res. 2007;31:423-427.
31. Hartmann S, Aradottir S, Graf M, et al. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81-84.
32. Choe YM, Lee BC, Choi IG, et al. Combination of the CAGE and serum gamma-glutamyl transferase: an effective screening tool for alcohol use disorder and alcohol dependence. Neuropsychiatr Dis Treat. 2019 31;15:1507-1515.
33. Niemelä O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39-49.
34. Kauffmann T, Evans DS. Macrocytosis. Accessed November 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK560908/
35. Maenhout TM, De Buyzere ML, Delanghe JR. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chim Acta. 2013;415:322-329.
36. Solomons HD. Carbohydrate deficient transferrin and alcoholism. Germs. 2012;2:75-78.
37. Allen JP, Wurst FM, Thon N, et al. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19:369-376.
38. Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:96-109.
39. Hannuksela ML, Liisanantti MK, Nissinen AE, et al. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953-961.
40. Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001;47:13-27.
41. Cabarcos P, Hassan HM, Tabernero MJ, et al. Analysis of ethyl glucuronide in hair samples by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). J Appl Toxicol. 2013;33:638-643.
42. Piano MR, Mazzuco A, Kang M, et al. Binge drinking episodes in young adults: how should we measure them in a research setting? J Stud Alcohol Drugs. 2017;78:502-511.
43. Adinoff B, Bone GH, Linnoila M. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp. 1988;3:172-196.
44. Cabezas J, Lucey MR, Bataller R. Biomarkers for monitoring alcohol use. Clin Liver Dis (Hoboken). 2016;8:59-63.
45. Wurst FM, Alling C, Aradottir S, et al. Emerging biomarkers: new directions and clinical applications. Alcohol Clin Exp Res. 2005;29:465-473.
46. Helander A, Péter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552-557.
47. Helander A, Böttcher M, Fehr C, et al. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44:55-61.
48. Jatlow P, O’Malley SS. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res. 2010;34:968-975.
49. Jatlow PI, Agro A, Wu R, et al. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin Exp Res. 2014;38:2056-2065.
50. Gonzalo P, Radenne S, Gonzalo S. Biomarkers of chronic alcohol misuse. Curr Biomark Find. 2014;4:9-22.
51. Bornhorst JA, Mbughuni MM. Alcohol biomarkers: clinical issues and analytical methods. In: Critical Issues in Alcohol and Drugs of Abuse Testing. 2nd ed. Academic Press. 2019:25-42.
52. Soderberg BL, Salem RO, Best CA, et al. Fatty acid ethyl esters. Ethanol metabolites that reflect ethanol intake. Am J Clin Pathol. 2003;119(suppl):S94-S99.
53. Cheng CT, Ostrea EM Jr, Alviedo JN, et al. Fatty acid ethyl esters in meconium: a biomarker of fetal alcohol exposure and effect. Exp Biol Med (Maywood). 2021;246:380-386.
54. Andresen-Streichert H, Beres Y, Weinmann W, et al. Improved detection of alcohol consumption using the novel marker phosphatidylethanol in the transplant setting: results of a prospective study. Transpl Int. 2017;30:611-620.
55. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13:14788-14812.
56. Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507-1511.
57. Aradóttir S, Moller K, Alling C. Phosphatidylethanol formation and degradation in human and rat blood. Alcohol Alcohol. 2004;39:8-13.
58. Varga A, Alling C. Formation of phosphatidylethanol in vitro in red blood cells from healthy volunteers and chronic alcoholics. J Lab Clin Med. 2002;140:79-83.
59. Javors MA, Hill-Kapturczak N, Roache JD, et al. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res. 2016;40:1228-1234.
60. Schröck A, Pfäffli M, König S, et al. Application of phosphatidylethanol (PEth) in whole blood in comparison to ethyl glucuronide in hair (hEtG) in driving aptitude assessment (DAA). Int J Legal Med. 2016;130:1527-1533.
61. Stewart SH, Koch DG, Willner IR, et al. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706-1711.
62. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015 29;5:1339-1385.
63. Hill-Kapturczak N, Dougherty DM, Roache JD, et al. Phosphatidylethanol homologs in blood as biomarkers for the time frame and amount of recent alcohol consumption. In: Preedy VR (ed) Neuroscience of Alcohol. Academic Press; 2019:567-576.
64. Jain J, Evans JL, Briceño A, et al. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol Alcohol. 2014;49:520-524.
65. Schröck A, Wurst FM, Thon N, et al. Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test - C (AUDIT-C). Drug Alcohol Depend. 2017;178:80-86.
66. McDonnell MG, Skalisky J, Leickly E, et al. Pilot investigation of a phosphatidylethanol-based contingency management intervention targeting alcohol use. Psychol Addict Behav. 2017;31:608-613.
67. Asrani SK, Trotter J, Lake J, et al. Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis. Liver Transpl. 2020;26:127-140.
68. WHO. International Guide for Monitoring Alcohol Consumption and Harm. 2000. Accessed November 12, 2021. http://apps.who.int/iris/bitstream/handle/10665/66529/WHO_MSD_MSB_00.4.pdf?sequence=1
1. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. American Psychiatric Publishing. 2013:490-497.
2. Fleming MF, Smith MJ, Oslakovic E, et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res. 2017;41:857-862.
3. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed November 12, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
4. Herreros-Villanueva M, Hijona E, Bañales JM, et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19:638-647.
5. Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652-14659.
6.
7. Sebastiani G, Borrás-Novell C, Casanova MA, et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. 2018;10:1008.
8. Rehm J, Anderson P, Manthey J, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51:422-427. doi: 10.1093/alcalc/agv127
9. ASAM. Caring for patients during the COVID-19 pandemic. Accessed November 12, 2021. www.asam.org/docs/default-source/covid-19/acute-care_062620.pdf?sfvrsn=e66d54c2_10
10. Miller PM, Thomas SE, Mallin R. Patient attitudes towards self-report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol. 2006;41:306-310. doi: 10.1093/alcalc/agl022
11. Zoorob R, Snell H, Kihlberg C, et al. Screening and brief intervention for risky alcohol use. Curr Probl Pediatr Adolesc Health Care. 2014;44:82-87.
12. Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783-788.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905-1907.
14. Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863-868.
15. Cherpitel CJ. A brief screening instrument for problem drinking in the emergency room: the RAPS4. Rapid Alcohol Problems Screen. J Stud Alcohol. 2000;61:447-449.
16. WHO. AUDIT: The alcohol use identification test. Accessed November 14, 2021. http://apps.who.int/iris/bitstream/handle/10665/67205/WHO_MSD_MSB_01.6a.pdf?sequence=1
17. Westermeyer J, Yargic I, Thuras P. Michigan assessment-screening test for alcohol and drugs (MAST/AD): evaluation in a clinical sample. Am J Addict. 2004;13:151-162.
18. Powers JS, Spickard A. Michigan Alcoholism Screening Test to diagnose early alcoholism in a general practice. South Med J. 1984;77:852-856.
19. NIH. Treatment for alcohol problems: finding and getting help. Accessed November 12, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/treatment-alcohol-problems-finding-and-getting-help
20. Kitchens JM. Does this patient have an alcohol problem? JAMA. 1994;272:1782-1787.
21. Kummer N, Lambert WE, Samyn N, et al. Alternative sampling strategies for the assessment of alcohol intake of living persons. Clin Biochem. 2016;49:1078-1091.
22. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63:1634-1640.
23. Mundle G, Ackermann K, Munkes J, et al. Influence of age, alcohol consumption and abstinence on the sensitivity of carbohydrate‐deficient transferrin, gamma‐glutamyltransferase and mean corpuscular volume. Alcohol Alcohol. 1999;34:760-766.
24. Neumann T, Spies C. Use of biomarkers for alcohol use disorders in clinical practice. Addiction. 2003;98(suppl 2):81-91.
25. Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684-11699.
26. Helander A. Biological markers of alcohol use and abuse in theory and practice. In: Agarwal DP, Seitz HK, eds. Alcohol in Health and Disease. Marcel Dekker. 2001:177-205.
27. Stewart SH, Koch DG, Burgess DM, et al. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcohol Clin Exp Res. 2013;37:150-155.
28. Helander A, Dahl H. Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem. 2005;51:1728-1730.
29. Ghosh S, Jain R, Jhanjee S, et al. Alcohol biomarkers and their relevance in detection of alcohol consumption in clinical settings. Accessed November 12, 2021. https://www.clinmedjournals.org/articles/iasar/international-archives-of-substance-abuse-and-rehabilitation-iasar-1-002.php?jid=iasar
30. Borucki K, Dierkes J, Wartberg J, et al. In heavy drinkers, fatty acid ethyl esters remain elevated for up to 99 hours. Alcohol Clin Exp Res. 2007;31:423-427.
31. Hartmann S, Aradottir S, Graf M, et al. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81-84.
32. Choe YM, Lee BC, Choi IG, et al. Combination of the CAGE and serum gamma-glutamyl transferase: an effective screening tool for alcohol use disorder and alcohol dependence. Neuropsychiatr Dis Treat. 2019 31;15:1507-1515.
33. Niemelä O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39-49.
34. Kauffmann T, Evans DS. Macrocytosis. Accessed November 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK560908/
35. Maenhout TM, De Buyzere ML, Delanghe JR. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin Chim Acta. 2013;415:322-329.
36. Solomons HD. Carbohydrate deficient transferrin and alcoholism. Germs. 2012;2:75-78.
37. Allen JP, Wurst FM, Thon N, et al. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19:369-376.
38. Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:96-109.
39. Hannuksela ML, Liisanantti MK, Nissinen AE, et al. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953-961.
40. Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001;47:13-27.
41. Cabarcos P, Hassan HM, Tabernero MJ, et al. Analysis of ethyl glucuronide in hair samples by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). J Appl Toxicol. 2013;33:638-643.
42. Piano MR, Mazzuco A, Kang M, et al. Binge drinking episodes in young adults: how should we measure them in a research setting? J Stud Alcohol Drugs. 2017;78:502-511.
43. Adinoff B, Bone GH, Linnoila M. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp. 1988;3:172-196.
44. Cabezas J, Lucey MR, Bataller R. Biomarkers for monitoring alcohol use. Clin Liver Dis (Hoboken). 2016;8:59-63.
45. Wurst FM, Alling C, Aradottir S, et al. Emerging biomarkers: new directions and clinical applications. Alcohol Clin Exp Res. 2005;29:465-473.
46. Helander A, Péter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552-557.
47. Helander A, Böttcher M, Fehr C, et al. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44:55-61.
48. Jatlow P, O’Malley SS. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res. 2010;34:968-975.
49. Jatlow PI, Agro A, Wu R, et al. Ethyl glucuronide and ethyl sulfate assays in clinical trials, interpretation, and limitations: results of a dose ranging alcohol challenge study and 2 clinical trials. Alcohol Clin Exp Res. 2014;38:2056-2065.
50. Gonzalo P, Radenne S, Gonzalo S. Biomarkers of chronic alcohol misuse. Curr Biomark Find. 2014;4:9-22.
51. Bornhorst JA, Mbughuni MM. Alcohol biomarkers: clinical issues and analytical methods. In: Critical Issues in Alcohol and Drugs of Abuse Testing. 2nd ed. Academic Press. 2019:25-42.
52. Soderberg BL, Salem RO, Best CA, et al. Fatty acid ethyl esters. Ethanol metabolites that reflect ethanol intake. Am J Clin Pathol. 2003;119(suppl):S94-S99.
53. Cheng CT, Ostrea EM Jr, Alviedo JN, et al. Fatty acid ethyl esters in meconium: a biomarker of fetal alcohol exposure and effect. Exp Biol Med (Maywood). 2021;246:380-386.
54. Andresen-Streichert H, Beres Y, Weinmann W, et al. Improved detection of alcohol consumption using the novel marker phosphatidylethanol in the transplant setting: results of a prospective study. Transpl Int. 2017;30:611-620.
55. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13:14788-14812.
56. Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507-1511.
57. Aradóttir S, Moller K, Alling C. Phosphatidylethanol formation and degradation in human and rat blood. Alcohol Alcohol. 2004;39:8-13.
58. Varga A, Alling C. Formation of phosphatidylethanol in vitro in red blood cells from healthy volunteers and chronic alcoholics. J Lab Clin Med. 2002;140:79-83.
59. Javors MA, Hill-Kapturczak N, Roache JD, et al. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res. 2016;40:1228-1234.
60. Schröck A, Pfäffli M, König S, et al. Application of phosphatidylethanol (PEth) in whole blood in comparison to ethyl glucuronide in hair (hEtG) in driving aptitude assessment (DAA). Int J Legal Med. 2016;130:1527-1533.
61. Stewart SH, Koch DG, Willner IR, et al. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706-1711.
62. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015 29;5:1339-1385.
63. Hill-Kapturczak N, Dougherty DM, Roache JD, et al. Phosphatidylethanol homologs in blood as biomarkers for the time frame and amount of recent alcohol consumption. In: Preedy VR (ed) Neuroscience of Alcohol. Academic Press; 2019:567-576.
64. Jain J, Evans JL, Briceño A, et al. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol Alcohol. 2014;49:520-524.
65. Schröck A, Wurst FM, Thon N, et al. Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test - C (AUDIT-C). Drug Alcohol Depend. 2017;178:80-86.
66. McDonnell MG, Skalisky J, Leickly E, et al. Pilot investigation of a phosphatidylethanol-based contingency management intervention targeting alcohol use. Psychol Addict Behav. 2017;31:608-613.
67. Asrani SK, Trotter J, Lake J, et al. Meeting Report: The Dallas Consensus Conference on Liver Transplantation for Alcohol Associated Hepatitis. Liver Transpl. 2020;26:127-140.
68. WHO. International Guide for Monitoring Alcohol Consumption and Harm. 2000. Accessed November 12, 2021. http://apps.who.int/iris/bitstream/handle/10665/66529/WHO_MSD_MSB_00.4.pdf?sequence=1
PRACTICE RECOMMENDATIONS
› Use a quick screening instrument such as the single-question tool or the AUDIT 1-3 to objectively determine whether patients’ drinking is risky for themselves or for others. C
› Suspect alcoholic liver disease if the ratio of aspartate aminotransferase to alanine aminotransferase is > 3. C
› Consider using the PEth assay in high-risk patients to differentiate between heavy alcohol use and social drinking. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Letter counters study that focuses on low-risk home births
A research letter published recently in the American Journal of Obstetrics and Gynecology argues that the methodology of a recent paper on the safety of planned home births presented a biased analysis.
The paper that Amos Grünebaum, MD, and colleagues with the department of obstetrics and gynecology at Lenox Hill Hospital in Hempstead, N.J., referred to is a study in Obstetrics & Gynecology which concluded that planned home births in Washington state are low risk.
In that paper, Elizabeth Nethery, RM, MS, MSM, a midwife and PhD candidate at the University of British Columbia, Vancouver, calculated the outcomes from 2015 to 2020 for “all births attended by members of a statewide midwifery professional association that were within professional association guidelines and met eligibility criteria for planned birth center birth.”
Ms. Nethery’s team concluded: “Rates of adverse outcomes for this cohort in a U.S. state with well-established and integrated community midwifery were low overall. Birth outcomes were similar for births planned at home or at a state-licensed, freestanding birth center.”
This news organization was among the publications that reported the results of that study.
But it’s the exclusion criteria in that study, primarily, that Dr. Grünebaum and colleagues take issue with.
Births excluded from the main analysis of the study by Ms. Nethery and coauthors involved “multifetal pregnancy, prior cesarean delivery, onset of labor at more than 42 0/7 weeks of gestation or preterm (less than 37 weeks), preexisting hypertension or diabetes, known amniotic fluid abnormality, gestational hypertension or preeclampsia, or malpresentation.”
Those are conditions that fall outside guidelines for planned home births. But both Ms. Nethery and Dr. Grünebaum said that sometimes these high-risk conditions are present in home births.
Different conclusion for home birth safety
Dr. Grünebaum and colleagues’ analysis of the risk profiles and outcomes for U.S. planned home births for the years 2016-2020 came to a different conclusion about the safety of home births.
They used a retrospective population-based cohort study that used the Centers for Disease Control and Prevention WONDER natality online database. They included planned home births and compared the outcomes with and without certain risk factors, including some high-risk factors such as twin deliveries, breech births, and previous cesarean.
Dr. Grünebaum’s analysis concluded that “it is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Ms. Nethery said though the high-risk conditions were excluded from their main analysis, they are mentioned in the paper and detailed in the supplement.
She acknowledged in the paper that some midwives practice outside the guidelines and that was the case in 7% of births or for 800 people in the Washington state study. But she told this publication it’s a small number and high-risk births should be handled in a hospital so the team focused its research on low-risk births.
“People plan home births who are outside the guidelines everywhere in the world. There are a lot of reasons why people do it,” she said. Among them are not feeling safe in the hospital, being rejected by an obstetrician for a desired procedure, or, in some cases, because they are misinformed.
She said midwives are sometimes faced with a difficult choice, when a patient wants, for instance, a vaginal birth after cesarean (VBAC), one of the conditions not recommended for home births.
The midwife is left with the choice of saying she will not do a VBAC in the home, or she can explain to the patient why it is not recommended and explain all the reasons it is not recommended, such as an elevated risk of rupture, but honor the patient’s choice.
“Do you tell the person: ‘Sorry, go have the cesarean anyway or do you do your best to support this person?’ Birthing people have the right to autonomy of choice,” Ms. Nethery said.
Dr. Grünebaum and colleagues said: “The recent study by Nethery et al. concluded that planned home births in the state of Washington have good neonatal outcomes by focusing on results of low-risk patients.”
Dr. Grünebaum said in an interview: “It’s like reporting on smoking and lung cancer and saying I’m only going to report on patients who have smoked for less than 5 years. You need to take the whole picture into consideration.”
Ms. Nethery gave this explanation for excluding the high-risk patients: “If you are studying a drug, you exclude people from your study who got the drug even though they had risk factors that were ‘contraindications’ to that drug. Likely there was a reason they got the drug – in consultation with their doctor, the patient and the doctor decided that the potential benefit outweighed the risk – but they are not relevant to understanding how that drug impacts people who were ‘eligible’ for the drug in the first place.”
“That is part of the reason we excluded ‘high-risk’ people from our study,” she said. “The other reason is that that is what is commonly done in most research on this topic – we focus on ‘low-risk’ people who are within standards and eligibility criteria.”
She gave examples such as a 2019 meta-analysis and a 2011 Birthplace in England national prospective cohort study, both of which excluded high-risk home births.
“Third, we wanted to compare apples to apples (for our analysis of home vs. hospital) – and licensed birth centers in Washington state have restrictions based on risk,” Ms. Nethery said.
Dr. Grünebaum said his team supports the right of all women to give birth where they wish. “But you cannot choose unless you are given the right information.”
Dr. Grünebaum also said planned home births in the United States cannot be compared with home births delivered by midwives in other countries. Different from the United States, he said, in countries such as Canada, Germany, and England, midwives are well integrated in the medical system and they are typically affiliated with hospitals and they belong to organizations which support very strong guidelines.
He added that, while Washington state has its own set of guidelines, there are no national guidelines for home births and practice varies greatly by state.
The authors concluded: “It is the professional responsibility of all health care providers, obstetricians, and midwives to present unbiased information. Focusing the reporting of outcomes on low-risk deliveries underreports true adverse outcomes in U.S. home births and provides biased information to patients considering planned home births. It is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Angela Martin, MD, assistant professor of maternal-fetal medicine and medical director of the labor and delivery department at University of Kansas Medical Center, Kansas City, who was not part of either study, said she did not believe it was a problem that Ms. Nethery’s study excluded the high-risk conditions in the main analysis because it was disclosed.
“The authors were clear that they excluded high-risk conditions,” she said. “Therefore, the study should not be extrapolated to women with these conditions.”
“I believe her results do make that case for low-risk women in Washington state,” Dr. Martin said. “Again, it is important that findings are not extrapolated to women outside of those included in the study.”
She said there are several things that make Washington unusual in midwifery care. Consequently, the results should not be seen as representative of the United States.
“It is one of the most integrated states for midwife care in the country,” Dr. Martin said. “Washington has licensure available for midwives, which is not true of all states. It also has a robust state professional association that publishes guidelines for midwives to follow. And midwives in Washington have a wide formulary. For example, they can administer antibiotics, carry and administer hemorrhage medications, they can carry oxygen, and they are allowed to suture.”
Iris Krishna, MD, MPH, director of perinatal quality, Emory Perinatal Center and assistant professor in the division of maternal-fetal medicine at Emory University, Atlanta, said in an interview that the arguments by Ms. Nethery and Dr. Grünebaum illustrate the controversy over home births.
Dr. Krishna, who was not part of either study, said physicians and midwives should counsel patients contemplating a planned community birth that available data is not generalizable to all birth settings or all patients.
“Women should be counseled that delivery in a hospital setting or accredited birth center is safer than home birth,” she said. “Ultimately, each woman has the right to make a medically informed decision about delivery after adequate counseling on the risks and benefits of community birth.”
Dr. Grünebaum and colleagues reported no relevant financial relationships. Ms. Nethery, Dr. Martin, and Dr. Krishna also reported no relevant financial relationships.
A research letter published recently in the American Journal of Obstetrics and Gynecology argues that the methodology of a recent paper on the safety of planned home births presented a biased analysis.
The paper that Amos Grünebaum, MD, and colleagues with the department of obstetrics and gynecology at Lenox Hill Hospital in Hempstead, N.J., referred to is a study in Obstetrics & Gynecology which concluded that planned home births in Washington state are low risk.
In that paper, Elizabeth Nethery, RM, MS, MSM, a midwife and PhD candidate at the University of British Columbia, Vancouver, calculated the outcomes from 2015 to 2020 for “all births attended by members of a statewide midwifery professional association that were within professional association guidelines and met eligibility criteria for planned birth center birth.”
Ms. Nethery’s team concluded: “Rates of adverse outcomes for this cohort in a U.S. state with well-established and integrated community midwifery were low overall. Birth outcomes were similar for births planned at home or at a state-licensed, freestanding birth center.”
This news organization was among the publications that reported the results of that study.
But it’s the exclusion criteria in that study, primarily, that Dr. Grünebaum and colleagues take issue with.
Births excluded from the main analysis of the study by Ms. Nethery and coauthors involved “multifetal pregnancy, prior cesarean delivery, onset of labor at more than 42 0/7 weeks of gestation or preterm (less than 37 weeks), preexisting hypertension or diabetes, known amniotic fluid abnormality, gestational hypertension or preeclampsia, or malpresentation.”
Those are conditions that fall outside guidelines for planned home births. But both Ms. Nethery and Dr. Grünebaum said that sometimes these high-risk conditions are present in home births.
Different conclusion for home birth safety
Dr. Grünebaum and colleagues’ analysis of the risk profiles and outcomes for U.S. planned home births for the years 2016-2020 came to a different conclusion about the safety of home births.
They used a retrospective population-based cohort study that used the Centers for Disease Control and Prevention WONDER natality online database. They included planned home births and compared the outcomes with and without certain risk factors, including some high-risk factors such as twin deliveries, breech births, and previous cesarean.
Dr. Grünebaum’s analysis concluded that “it is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Ms. Nethery said though the high-risk conditions were excluded from their main analysis, they are mentioned in the paper and detailed in the supplement.
She acknowledged in the paper that some midwives practice outside the guidelines and that was the case in 7% of births or for 800 people in the Washington state study. But she told this publication it’s a small number and high-risk births should be handled in a hospital so the team focused its research on low-risk births.
“People plan home births who are outside the guidelines everywhere in the world. There are a lot of reasons why people do it,” she said. Among them are not feeling safe in the hospital, being rejected by an obstetrician for a desired procedure, or, in some cases, because they are misinformed.
She said midwives are sometimes faced with a difficult choice, when a patient wants, for instance, a vaginal birth after cesarean (VBAC), one of the conditions not recommended for home births.
The midwife is left with the choice of saying she will not do a VBAC in the home, or she can explain to the patient why it is not recommended and explain all the reasons it is not recommended, such as an elevated risk of rupture, but honor the patient’s choice.
“Do you tell the person: ‘Sorry, go have the cesarean anyway or do you do your best to support this person?’ Birthing people have the right to autonomy of choice,” Ms. Nethery said.
Dr. Grünebaum and colleagues said: “The recent study by Nethery et al. concluded that planned home births in the state of Washington have good neonatal outcomes by focusing on results of low-risk patients.”
Dr. Grünebaum said in an interview: “It’s like reporting on smoking and lung cancer and saying I’m only going to report on patients who have smoked for less than 5 years. You need to take the whole picture into consideration.”
Ms. Nethery gave this explanation for excluding the high-risk patients: “If you are studying a drug, you exclude people from your study who got the drug even though they had risk factors that were ‘contraindications’ to that drug. Likely there was a reason they got the drug – in consultation with their doctor, the patient and the doctor decided that the potential benefit outweighed the risk – but they are not relevant to understanding how that drug impacts people who were ‘eligible’ for the drug in the first place.”
“That is part of the reason we excluded ‘high-risk’ people from our study,” she said. “The other reason is that that is what is commonly done in most research on this topic – we focus on ‘low-risk’ people who are within standards and eligibility criteria.”
She gave examples such as a 2019 meta-analysis and a 2011 Birthplace in England national prospective cohort study, both of which excluded high-risk home births.
“Third, we wanted to compare apples to apples (for our analysis of home vs. hospital) – and licensed birth centers in Washington state have restrictions based on risk,” Ms. Nethery said.
Dr. Grünebaum said his team supports the right of all women to give birth where they wish. “But you cannot choose unless you are given the right information.”
Dr. Grünebaum also said planned home births in the United States cannot be compared with home births delivered by midwives in other countries. Different from the United States, he said, in countries such as Canada, Germany, and England, midwives are well integrated in the medical system and they are typically affiliated with hospitals and they belong to organizations which support very strong guidelines.
He added that, while Washington state has its own set of guidelines, there are no national guidelines for home births and practice varies greatly by state.
The authors concluded: “It is the professional responsibility of all health care providers, obstetricians, and midwives to present unbiased information. Focusing the reporting of outcomes on low-risk deliveries underreports true adverse outcomes in U.S. home births and provides biased information to patients considering planned home births. It is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Angela Martin, MD, assistant professor of maternal-fetal medicine and medical director of the labor and delivery department at University of Kansas Medical Center, Kansas City, who was not part of either study, said she did not believe it was a problem that Ms. Nethery’s study excluded the high-risk conditions in the main analysis because it was disclosed.
“The authors were clear that they excluded high-risk conditions,” she said. “Therefore, the study should not be extrapolated to women with these conditions.”
“I believe her results do make that case for low-risk women in Washington state,” Dr. Martin said. “Again, it is important that findings are not extrapolated to women outside of those included in the study.”
She said there are several things that make Washington unusual in midwifery care. Consequently, the results should not be seen as representative of the United States.
“It is one of the most integrated states for midwife care in the country,” Dr. Martin said. “Washington has licensure available for midwives, which is not true of all states. It also has a robust state professional association that publishes guidelines for midwives to follow. And midwives in Washington have a wide formulary. For example, they can administer antibiotics, carry and administer hemorrhage medications, they can carry oxygen, and they are allowed to suture.”
Iris Krishna, MD, MPH, director of perinatal quality, Emory Perinatal Center and assistant professor in the division of maternal-fetal medicine at Emory University, Atlanta, said in an interview that the arguments by Ms. Nethery and Dr. Grünebaum illustrate the controversy over home births.
Dr. Krishna, who was not part of either study, said physicians and midwives should counsel patients contemplating a planned community birth that available data is not generalizable to all birth settings or all patients.
“Women should be counseled that delivery in a hospital setting or accredited birth center is safer than home birth,” she said. “Ultimately, each woman has the right to make a medically informed decision about delivery after adequate counseling on the risks and benefits of community birth.”
Dr. Grünebaum and colleagues reported no relevant financial relationships. Ms. Nethery, Dr. Martin, and Dr. Krishna also reported no relevant financial relationships.
A research letter published recently in the American Journal of Obstetrics and Gynecology argues that the methodology of a recent paper on the safety of planned home births presented a biased analysis.
The paper that Amos Grünebaum, MD, and colleagues with the department of obstetrics and gynecology at Lenox Hill Hospital in Hempstead, N.J., referred to is a study in Obstetrics & Gynecology which concluded that planned home births in Washington state are low risk.
In that paper, Elizabeth Nethery, RM, MS, MSM, a midwife and PhD candidate at the University of British Columbia, Vancouver, calculated the outcomes from 2015 to 2020 for “all births attended by members of a statewide midwifery professional association that were within professional association guidelines and met eligibility criteria for planned birth center birth.”
Ms. Nethery’s team concluded: “Rates of adverse outcomes for this cohort in a U.S. state with well-established and integrated community midwifery were low overall. Birth outcomes were similar for births planned at home or at a state-licensed, freestanding birth center.”
This news organization was among the publications that reported the results of that study.
But it’s the exclusion criteria in that study, primarily, that Dr. Grünebaum and colleagues take issue with.
Births excluded from the main analysis of the study by Ms. Nethery and coauthors involved “multifetal pregnancy, prior cesarean delivery, onset of labor at more than 42 0/7 weeks of gestation or preterm (less than 37 weeks), preexisting hypertension or diabetes, known amniotic fluid abnormality, gestational hypertension or preeclampsia, or malpresentation.”
Those are conditions that fall outside guidelines for planned home births. But both Ms. Nethery and Dr. Grünebaum said that sometimes these high-risk conditions are present in home births.
Different conclusion for home birth safety
Dr. Grünebaum and colleagues’ analysis of the risk profiles and outcomes for U.S. planned home births for the years 2016-2020 came to a different conclusion about the safety of home births.
They used a retrospective population-based cohort study that used the Centers for Disease Control and Prevention WONDER natality online database. They included planned home births and compared the outcomes with and without certain risk factors, including some high-risk factors such as twin deliveries, breech births, and previous cesarean.
Dr. Grünebaum’s analysis concluded that “it is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Ms. Nethery said though the high-risk conditions were excluded from their main analysis, they are mentioned in the paper and detailed in the supplement.
She acknowledged in the paper that some midwives practice outside the guidelines and that was the case in 7% of births or for 800 people in the Washington state study. But she told this publication it’s a small number and high-risk births should be handled in a hospital so the team focused its research on low-risk births.
“People plan home births who are outside the guidelines everywhere in the world. There are a lot of reasons why people do it,” she said. Among them are not feeling safe in the hospital, being rejected by an obstetrician for a desired procedure, or, in some cases, because they are misinformed.
She said midwives are sometimes faced with a difficult choice, when a patient wants, for instance, a vaginal birth after cesarean (VBAC), one of the conditions not recommended for home births.
The midwife is left with the choice of saying she will not do a VBAC in the home, or she can explain to the patient why it is not recommended and explain all the reasons it is not recommended, such as an elevated risk of rupture, but honor the patient’s choice.
“Do you tell the person: ‘Sorry, go have the cesarean anyway or do you do your best to support this person?’ Birthing people have the right to autonomy of choice,” Ms. Nethery said.
Dr. Grünebaum and colleagues said: “The recent study by Nethery et al. concluded that planned home births in the state of Washington have good neonatal outcomes by focusing on results of low-risk patients.”
Dr. Grünebaum said in an interview: “It’s like reporting on smoking and lung cancer and saying I’m only going to report on patients who have smoked for less than 5 years. You need to take the whole picture into consideration.”
Ms. Nethery gave this explanation for excluding the high-risk patients: “If you are studying a drug, you exclude people from your study who got the drug even though they had risk factors that were ‘contraindications’ to that drug. Likely there was a reason they got the drug – in consultation with their doctor, the patient and the doctor decided that the potential benefit outweighed the risk – but they are not relevant to understanding how that drug impacts people who were ‘eligible’ for the drug in the first place.”
“That is part of the reason we excluded ‘high-risk’ people from our study,” she said. “The other reason is that that is what is commonly done in most research on this topic – we focus on ‘low-risk’ people who are within standards and eligibility criteria.”
She gave examples such as a 2019 meta-analysis and a 2011 Birthplace in England national prospective cohort study, both of which excluded high-risk home births.
“Third, we wanted to compare apples to apples (for our analysis of home vs. hospital) – and licensed birth centers in Washington state have restrictions based on risk,” Ms. Nethery said.
Dr. Grünebaum said his team supports the right of all women to give birth where they wish. “But you cannot choose unless you are given the right information.”
Dr. Grünebaum also said planned home births in the United States cannot be compared with home births delivered by midwives in other countries. Different from the United States, he said, in countries such as Canada, Germany, and England, midwives are well integrated in the medical system and they are typically affiliated with hospitals and they belong to organizations which support very strong guidelines.
He added that, while Washington state has its own set of guidelines, there are no national guidelines for home births and practice varies greatly by state.
The authors concluded: “It is the professional responsibility of all health care providers, obstetricians, and midwives to present unbiased information. Focusing the reporting of outcomes on low-risk deliveries underreports true adverse outcomes in U.S. home births and provides biased information to patients considering planned home births. It is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Angela Martin, MD, assistant professor of maternal-fetal medicine and medical director of the labor and delivery department at University of Kansas Medical Center, Kansas City, who was not part of either study, said she did not believe it was a problem that Ms. Nethery’s study excluded the high-risk conditions in the main analysis because it was disclosed.
“The authors were clear that they excluded high-risk conditions,” she said. “Therefore, the study should not be extrapolated to women with these conditions.”
“I believe her results do make that case for low-risk women in Washington state,” Dr. Martin said. “Again, it is important that findings are not extrapolated to women outside of those included in the study.”
She said there are several things that make Washington unusual in midwifery care. Consequently, the results should not be seen as representative of the United States.
“It is one of the most integrated states for midwife care in the country,” Dr. Martin said. “Washington has licensure available for midwives, which is not true of all states. It also has a robust state professional association that publishes guidelines for midwives to follow. And midwives in Washington have a wide formulary. For example, they can administer antibiotics, carry and administer hemorrhage medications, they can carry oxygen, and they are allowed to suture.”
Iris Krishna, MD, MPH, director of perinatal quality, Emory Perinatal Center and assistant professor in the division of maternal-fetal medicine at Emory University, Atlanta, said in an interview that the arguments by Ms. Nethery and Dr. Grünebaum illustrate the controversy over home births.
Dr. Krishna, who was not part of either study, said physicians and midwives should counsel patients contemplating a planned community birth that available data is not generalizable to all birth settings or all patients.
“Women should be counseled that delivery in a hospital setting or accredited birth center is safer than home birth,” she said. “Ultimately, each woman has the right to make a medically informed decision about delivery after adequate counseling on the risks and benefits of community birth.”
Dr. Grünebaum and colleagues reported no relevant financial relationships. Ms. Nethery, Dr. Martin, and Dr. Krishna also reported no relevant financial relationships.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Female patients fare worse with male surgeons, study finds
, according to a new analysis of over 1.3 million surgery patients. The study found no difference in adverse outcomes in male patients treated by surgeons of either sex.
While the effect of patient and provider sex discordance (female patient/male physician or male patient/female physician) on care has been explored before, “to the best of our knowledge, this is the first study to assess this in patients undergoing surgery,” Christopher Wallis, MD, PhD, an assistant professor in the division of urology at the University of Toronto, said in an email. The study was published online December 8 in JAMA Surgery.
Past studies in primary care settings have found that sex discordance between a physician and patient can result in “worse rapport, lower certainty of diagnosis, lower likelihood of assessing patient’s conditions as being of high severity, concerns of a hidden agenda, and disagreements regarding advice provided,” the authors write in the paper. Gender discordance has also been shown to negatively affect cancer screening rates and survival after heart attack. Given these past findings, Dr. Wallis and colleagues postulated that gender match between patients and surgeons could affect postoperative outcomes.
To find out, researchers analyzed data from over 1,320,100 patients undergoing one of 21 common elective and emergent surgical procedures in Ontario, Canada, from January 1, 2007, through December 31, 2019. Procedures were performed across the following specialties: cardiothoracic surgery, general surgery, neurosurgery, orthopedic surgery, otolaryngology, plastic surgery, thoracic surgery, urology, and vascular surgery. The investigators compared adverse postoperative outcomes — death, readmission, or complications within 30 days after surgery — in patients of both sexes when treated by male or female surgeons.
The study included 2,937 surgeons, and nearly 46% of patients included in the study were the same sex as their surgeon. Of the remaining 717,548 sex-discordant pairings, 93% were female patients with male surgeons, and 7% were male patients with female surgeons.
Among all patients, 14.9% experienced at least one adverse outcome. The researchers found that sex discordance between patient and surgeon was associated with higher odds of complications (adjusted odds ratio [aOR], 1.09; 95% CI, 1.07 – 1.11) and death (aOR, 1.07; 95% CI, 1.02 – 1.13). There was no statistically significant relationship between sex discordance and readmission in the study.
Using multivariable modeling, the researchers then teased out how patient sex affected this association. They found that female patients treated by male surgeons, compared to those treated by female surgeons, were more likely to have worse outcomes (aOR, 1.15; 95% CI, 1.10 – 1.20); however, there was no difference in outcomes in male patients treated by female surgeons compared with those with male surgeons (aOR, 0.99; 95% CI, 0.95 – 1.03).
While the study did not look at the underlying reasons for this disparity, communication differences between surgeons and patients could be one factor, Dr. Wallis noted. “Prior research has suggested differences in communication style between male and female physicians. Further, there is evidence that female physicians, including surgeons, spend more time with patients,” he wrote in an email. “This, coupled with evidence that female patients may have disparities in the management of their pain, suggest that communication differences may underpin the observed disparity.”
The finding “sounds the alarm for urgent action,” write Andrea Riner, MD, MPH, and Amalia Cochran, MD, both from the department of surgery at the University of Florida College of Medicine in Gainesville, in an accompanying commentary. While recruiting more women into surgical specialties is one way to address this disparity, both Dr. Riner and Dr. Cochran note the importance of identifying unconscious biases in patient care. “Surgeons likely believe they provide the same quality of care to patients irrespective of identity,” they write. “However, these data underscore an underappreciated phenomenon and highlight a measurable repercussion of implicit bias.”
Training programs that work with surgeons to improve communication and care with diverse patients may help counter these biases, they suggest, and incorporating patient identity in surgical outcome metrics could help identify biases. “Female patients with surgical disease should not be disadvantaged because there simply are not enough female surgeons or surgeons who are competent in the care of female patients,” they note. “We owe it to patients to provide them with the best outcomes, regardless of how their identities may align with ours.”
Dr. Riner reports grants from the National Human Genome Research Institute and the National Cancer Institute.Dr. Cochran is a section editor for UpToDate. Dr. Wallis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new analysis of over 1.3 million surgery patients. The study found no difference in adverse outcomes in male patients treated by surgeons of either sex.
While the effect of patient and provider sex discordance (female patient/male physician or male patient/female physician) on care has been explored before, “to the best of our knowledge, this is the first study to assess this in patients undergoing surgery,” Christopher Wallis, MD, PhD, an assistant professor in the division of urology at the University of Toronto, said in an email. The study was published online December 8 in JAMA Surgery.
Past studies in primary care settings have found that sex discordance between a physician and patient can result in “worse rapport, lower certainty of diagnosis, lower likelihood of assessing patient’s conditions as being of high severity, concerns of a hidden agenda, and disagreements regarding advice provided,” the authors write in the paper. Gender discordance has also been shown to negatively affect cancer screening rates and survival after heart attack. Given these past findings, Dr. Wallis and colleagues postulated that gender match between patients and surgeons could affect postoperative outcomes.
To find out, researchers analyzed data from over 1,320,100 patients undergoing one of 21 common elective and emergent surgical procedures in Ontario, Canada, from January 1, 2007, through December 31, 2019. Procedures were performed across the following specialties: cardiothoracic surgery, general surgery, neurosurgery, orthopedic surgery, otolaryngology, plastic surgery, thoracic surgery, urology, and vascular surgery. The investigators compared adverse postoperative outcomes — death, readmission, or complications within 30 days after surgery — in patients of both sexes when treated by male or female surgeons.
The study included 2,937 surgeons, and nearly 46% of patients included in the study were the same sex as their surgeon. Of the remaining 717,548 sex-discordant pairings, 93% were female patients with male surgeons, and 7% were male patients with female surgeons.
Among all patients, 14.9% experienced at least one adverse outcome. The researchers found that sex discordance between patient and surgeon was associated with higher odds of complications (adjusted odds ratio [aOR], 1.09; 95% CI, 1.07 – 1.11) and death (aOR, 1.07; 95% CI, 1.02 – 1.13). There was no statistically significant relationship between sex discordance and readmission in the study.
Using multivariable modeling, the researchers then teased out how patient sex affected this association. They found that female patients treated by male surgeons, compared to those treated by female surgeons, were more likely to have worse outcomes (aOR, 1.15; 95% CI, 1.10 – 1.20); however, there was no difference in outcomes in male patients treated by female surgeons compared with those with male surgeons (aOR, 0.99; 95% CI, 0.95 – 1.03).
While the study did not look at the underlying reasons for this disparity, communication differences between surgeons and patients could be one factor, Dr. Wallis noted. “Prior research has suggested differences in communication style between male and female physicians. Further, there is evidence that female physicians, including surgeons, spend more time with patients,” he wrote in an email. “This, coupled with evidence that female patients may have disparities in the management of their pain, suggest that communication differences may underpin the observed disparity.”
The finding “sounds the alarm for urgent action,” write Andrea Riner, MD, MPH, and Amalia Cochran, MD, both from the department of surgery at the University of Florida College of Medicine in Gainesville, in an accompanying commentary. While recruiting more women into surgical specialties is one way to address this disparity, both Dr. Riner and Dr. Cochran note the importance of identifying unconscious biases in patient care. “Surgeons likely believe they provide the same quality of care to patients irrespective of identity,” they write. “However, these data underscore an underappreciated phenomenon and highlight a measurable repercussion of implicit bias.”
Training programs that work with surgeons to improve communication and care with diverse patients may help counter these biases, they suggest, and incorporating patient identity in surgical outcome metrics could help identify biases. “Female patients with surgical disease should not be disadvantaged because there simply are not enough female surgeons or surgeons who are competent in the care of female patients,” they note. “We owe it to patients to provide them with the best outcomes, regardless of how their identities may align with ours.”
Dr. Riner reports grants from the National Human Genome Research Institute and the National Cancer Institute.Dr. Cochran is a section editor for UpToDate. Dr. Wallis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new analysis of over 1.3 million surgery patients. The study found no difference in adverse outcomes in male patients treated by surgeons of either sex.
While the effect of patient and provider sex discordance (female patient/male physician or male patient/female physician) on care has been explored before, “to the best of our knowledge, this is the first study to assess this in patients undergoing surgery,” Christopher Wallis, MD, PhD, an assistant professor in the division of urology at the University of Toronto, said in an email. The study was published online December 8 in JAMA Surgery.
Past studies in primary care settings have found that sex discordance between a physician and patient can result in “worse rapport, lower certainty of diagnosis, lower likelihood of assessing patient’s conditions as being of high severity, concerns of a hidden agenda, and disagreements regarding advice provided,” the authors write in the paper. Gender discordance has also been shown to negatively affect cancer screening rates and survival after heart attack. Given these past findings, Dr. Wallis and colleagues postulated that gender match between patients and surgeons could affect postoperative outcomes.
To find out, researchers analyzed data from over 1,320,100 patients undergoing one of 21 common elective and emergent surgical procedures in Ontario, Canada, from January 1, 2007, through December 31, 2019. Procedures were performed across the following specialties: cardiothoracic surgery, general surgery, neurosurgery, orthopedic surgery, otolaryngology, plastic surgery, thoracic surgery, urology, and vascular surgery. The investigators compared adverse postoperative outcomes — death, readmission, or complications within 30 days after surgery — in patients of both sexes when treated by male or female surgeons.
The study included 2,937 surgeons, and nearly 46% of patients included in the study were the same sex as their surgeon. Of the remaining 717,548 sex-discordant pairings, 93% were female patients with male surgeons, and 7% were male patients with female surgeons.
Among all patients, 14.9% experienced at least one adverse outcome. The researchers found that sex discordance between patient and surgeon was associated with higher odds of complications (adjusted odds ratio [aOR], 1.09; 95% CI, 1.07 – 1.11) and death (aOR, 1.07; 95% CI, 1.02 – 1.13). There was no statistically significant relationship between sex discordance and readmission in the study.
Using multivariable modeling, the researchers then teased out how patient sex affected this association. They found that female patients treated by male surgeons, compared to those treated by female surgeons, were more likely to have worse outcomes (aOR, 1.15; 95% CI, 1.10 – 1.20); however, there was no difference in outcomes in male patients treated by female surgeons compared with those with male surgeons (aOR, 0.99; 95% CI, 0.95 – 1.03).
While the study did not look at the underlying reasons for this disparity, communication differences between surgeons and patients could be one factor, Dr. Wallis noted. “Prior research has suggested differences in communication style between male and female physicians. Further, there is evidence that female physicians, including surgeons, spend more time with patients,” he wrote in an email. “This, coupled with evidence that female patients may have disparities in the management of their pain, suggest that communication differences may underpin the observed disparity.”
The finding “sounds the alarm for urgent action,” write Andrea Riner, MD, MPH, and Amalia Cochran, MD, both from the department of surgery at the University of Florida College of Medicine in Gainesville, in an accompanying commentary. While recruiting more women into surgical specialties is one way to address this disparity, both Dr. Riner and Dr. Cochran note the importance of identifying unconscious biases in patient care. “Surgeons likely believe they provide the same quality of care to patients irrespective of identity,” they write. “However, these data underscore an underappreciated phenomenon and highlight a measurable repercussion of implicit bias.”
Training programs that work with surgeons to improve communication and care with diverse patients may help counter these biases, they suggest, and incorporating patient identity in surgical outcome metrics could help identify biases. “Female patients with surgical disease should not be disadvantaged because there simply are not enough female surgeons or surgeons who are competent in the care of female patients,” they note. “We owe it to patients to provide them with the best outcomes, regardless of how their identities may align with ours.”
Dr. Riner reports grants from the National Human Genome Research Institute and the National Cancer Institute.Dr. Cochran is a section editor for UpToDate. Dr. Wallis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Bulevirtide shows real-world efficacy versus HDV
A real-world analysis of bulevirtide found a safety and efficacy profile similar to what was seen in earlier clinical trials in the treatment of hepatitis delta virus (HDV) infection.
HDV can only infect patients already carrying hepatitis B virus (HBV), but it causes the most severe form of viral hepatitis as it can progress to cirrhosis within 5 years and to hepatocellular carcinoma within 10 years.
Bulevirtide is a first-in-class medication that mimics the hepatitis B surface antigen, binding to its receptor on hepatocytes and preventing HDV viral particles from binding to it. The drug received conditional marketing approval by the European Medicines Agency in 2020 and has received a breakthrough therapy designation from the U.S. Food and Drug Administration.
The study was presented at the annual meeting of the American Association for the Study of Liver Diseases by Victor De Ledinghen, PhD, who is a professor of hepatology and head of the hepatology and liver transplantation unit at Bordeaux (France) University Hospital.
The early-access program launched after the French National Agency for Medicines and Health Products approved bulevirtide in 2019. It was made available to patients with compensated cirrhosis or severe liver fibrosis (F3) or patients with F2 fibrosis and alanine amino transferase levels more than twice the upper limit of normal for 6 months or more. Patients received bulevirtide alone (n = 77) or in combination with peg-interferon (n = 68), as determined by their physician.
The researchers defined virologic efficacy as HDV RNA levels being undetectable, or decreased by at least 2 log10 from baseline. They defined biochemical efficacy as ALT levels below 40 IU/L.
A per-protocol analysis included all patients in the bulevirtide group, but excluded 12 from the combination group who discontinued peg-interferon (n = 56). Nineteen patients in bulevirtide group had a treatment modification, and seven discontinued treatment. Five in the combination group had a treatment modification, and 14 stopped treatment. At 12 months, there was a greater decline in median log10 IU/mL in the combination group (–5.65 versus –3.64), though the study was not powered to compare the two. At 12 months, the combination group had 93.9% virologic efficacy, compared with 68.3% in the bulevirtide group.
The two groups had similar mean ALT levels at 12 months (48.91 and 48.03 IU/mL, respectively), with more patients in the bulevirtide group having normal ALT levels (<40 IU/L; 48.8% versus 36.4%). At 12 months, 39.0% of the bulevirtide group and 30.3% of the combination group had a combined response, defined as either undetectable HDV RNA or ≥2 log10 from baseline plus normal ALT levels.
Twenty-nine patients in the bulevirtide group had an adverse event, compared with 43 in the combination group. The two groups were similar in the frequency of grade 3-4 adverse events (7 versus 6), discontinuation due to adverse events (2 versus 3), deaths (0 in both), injection-site reactions (2 in both), liver-related adverse events (4 versus 2), and elevated bile acid (76 versus 68).
During the Q&A period following the presentation, Dr. De Ledinghen was asked if he has a preferred regimen for HDV patients. “I think it depends on the tolerance of peg-interferon because of all the side effects with this drug. I think we need to have predictive factors of virological response with or without interferon. At this time, I don’t have a preference, but I think at this time we need to work on predictive factors associated with virologic response,” he said.
The EMA’s conditional bulevirtide approval hinged on results from phase 2 clinical trials, while the phase 3 clinical studies are ongoing. “This was a very unusual step for the EMA to provide what is similar to emergency use approval while the phase 3 clinical trials are still ongoing,” said Anna Lok, MD, who was asked to comment on the study. Dr. Lok is a professor of internal medicine, director of clinical hepatology, and assistant dean for clinical research at the University of Michigan, Ann Arbor.
She noted that the phase 2 studies indicated that the combination with peg-interferon seems to have an additive effect on HDV suppression, while monotherapy with bulevirtide has a greater effect on normalizing ALT levels. The real-world experience confirms these findings.
But the real-world data revealed some concerns. “What really worried me is the large number of patients who required dose modifications or discontinuations, and that seems to be the case in both treatment groups. They didn’t really go into a lot of details [about] why patients needed treatment modifications, but one has to assume that this is due to side effects,” said Dr. Lok.
She also noted that the per-protocol analysis, instead of an intention-to-treat analysis, is a weakness of the study. Additionally, over time, the number of patients analyzed decreased – as many as 40% of patients didn’t have test results at month 12. “It makes you wonder what happened to those patients. Many probably didn’t respond, in which case your overall response rate will be far lower,” said Dr. Lok.
The study was funded by Gilead. Dr. De Ledinghen has financial relationships with Gilead, AbbVie, Echosens, Hologic, Intercept Pharma, Tillotts, Orphalan, Alfasigma, Bristol Myers Squibb, and Siemens Healthineers. Dr. Lok has no relevant financial disclosures.
A real-world analysis of bulevirtide found a safety and efficacy profile similar to what was seen in earlier clinical trials in the treatment of hepatitis delta virus (HDV) infection.
HDV can only infect patients already carrying hepatitis B virus (HBV), but it causes the most severe form of viral hepatitis as it can progress to cirrhosis within 5 years and to hepatocellular carcinoma within 10 years.
Bulevirtide is a first-in-class medication that mimics the hepatitis B surface antigen, binding to its receptor on hepatocytes and preventing HDV viral particles from binding to it. The drug received conditional marketing approval by the European Medicines Agency in 2020 and has received a breakthrough therapy designation from the U.S. Food and Drug Administration.
The study was presented at the annual meeting of the American Association for the Study of Liver Diseases by Victor De Ledinghen, PhD, who is a professor of hepatology and head of the hepatology and liver transplantation unit at Bordeaux (France) University Hospital.
The early-access program launched after the French National Agency for Medicines and Health Products approved bulevirtide in 2019. It was made available to patients with compensated cirrhosis or severe liver fibrosis (F3) or patients with F2 fibrosis and alanine amino transferase levels more than twice the upper limit of normal for 6 months or more. Patients received bulevirtide alone (n = 77) or in combination with peg-interferon (n = 68), as determined by their physician.
The researchers defined virologic efficacy as HDV RNA levels being undetectable, or decreased by at least 2 log10 from baseline. They defined biochemical efficacy as ALT levels below 40 IU/L.
A per-protocol analysis included all patients in the bulevirtide group, but excluded 12 from the combination group who discontinued peg-interferon (n = 56). Nineteen patients in bulevirtide group had a treatment modification, and seven discontinued treatment. Five in the combination group had a treatment modification, and 14 stopped treatment. At 12 months, there was a greater decline in median log10 IU/mL in the combination group (–5.65 versus –3.64), though the study was not powered to compare the two. At 12 months, the combination group had 93.9% virologic efficacy, compared with 68.3% in the bulevirtide group.
The two groups had similar mean ALT levels at 12 months (48.91 and 48.03 IU/mL, respectively), with more patients in the bulevirtide group having normal ALT levels (<40 IU/L; 48.8% versus 36.4%). At 12 months, 39.0% of the bulevirtide group and 30.3% of the combination group had a combined response, defined as either undetectable HDV RNA or ≥2 log10 from baseline plus normal ALT levels.
Twenty-nine patients in the bulevirtide group had an adverse event, compared with 43 in the combination group. The two groups were similar in the frequency of grade 3-4 adverse events (7 versus 6), discontinuation due to adverse events (2 versus 3), deaths (0 in both), injection-site reactions (2 in both), liver-related adverse events (4 versus 2), and elevated bile acid (76 versus 68).
During the Q&A period following the presentation, Dr. De Ledinghen was asked if he has a preferred regimen for HDV patients. “I think it depends on the tolerance of peg-interferon because of all the side effects with this drug. I think we need to have predictive factors of virological response with or without interferon. At this time, I don’t have a preference, but I think at this time we need to work on predictive factors associated with virologic response,” he said.
The EMA’s conditional bulevirtide approval hinged on results from phase 2 clinical trials, while the phase 3 clinical studies are ongoing. “This was a very unusual step for the EMA to provide what is similar to emergency use approval while the phase 3 clinical trials are still ongoing,” said Anna Lok, MD, who was asked to comment on the study. Dr. Lok is a professor of internal medicine, director of clinical hepatology, and assistant dean for clinical research at the University of Michigan, Ann Arbor.
She noted that the phase 2 studies indicated that the combination with peg-interferon seems to have an additive effect on HDV suppression, while monotherapy with bulevirtide has a greater effect on normalizing ALT levels. The real-world experience confirms these findings.
But the real-world data revealed some concerns. “What really worried me is the large number of patients who required dose modifications or discontinuations, and that seems to be the case in both treatment groups. They didn’t really go into a lot of details [about] why patients needed treatment modifications, but one has to assume that this is due to side effects,” said Dr. Lok.
She also noted that the per-protocol analysis, instead of an intention-to-treat analysis, is a weakness of the study. Additionally, over time, the number of patients analyzed decreased – as many as 40% of patients didn’t have test results at month 12. “It makes you wonder what happened to those patients. Many probably didn’t respond, in which case your overall response rate will be far lower,” said Dr. Lok.
The study was funded by Gilead. Dr. De Ledinghen has financial relationships with Gilead, AbbVie, Echosens, Hologic, Intercept Pharma, Tillotts, Orphalan, Alfasigma, Bristol Myers Squibb, and Siemens Healthineers. Dr. Lok has no relevant financial disclosures.
A real-world analysis of bulevirtide found a safety and efficacy profile similar to what was seen in earlier clinical trials in the treatment of hepatitis delta virus (HDV) infection.
HDV can only infect patients already carrying hepatitis B virus (HBV), but it causes the most severe form of viral hepatitis as it can progress to cirrhosis within 5 years and to hepatocellular carcinoma within 10 years.
Bulevirtide is a first-in-class medication that mimics the hepatitis B surface antigen, binding to its receptor on hepatocytes and preventing HDV viral particles from binding to it. The drug received conditional marketing approval by the European Medicines Agency in 2020 and has received a breakthrough therapy designation from the U.S. Food and Drug Administration.
The study was presented at the annual meeting of the American Association for the Study of Liver Diseases by Victor De Ledinghen, PhD, who is a professor of hepatology and head of the hepatology and liver transplantation unit at Bordeaux (France) University Hospital.
The early-access program launched after the French National Agency for Medicines and Health Products approved bulevirtide in 2019. It was made available to patients with compensated cirrhosis or severe liver fibrosis (F3) or patients with F2 fibrosis and alanine amino transferase levels more than twice the upper limit of normal for 6 months or more. Patients received bulevirtide alone (n = 77) or in combination with peg-interferon (n = 68), as determined by their physician.
The researchers defined virologic efficacy as HDV RNA levels being undetectable, or decreased by at least 2 log10 from baseline. They defined biochemical efficacy as ALT levels below 40 IU/L.
A per-protocol analysis included all patients in the bulevirtide group, but excluded 12 from the combination group who discontinued peg-interferon (n = 56). Nineteen patients in bulevirtide group had a treatment modification, and seven discontinued treatment. Five in the combination group had a treatment modification, and 14 stopped treatment. At 12 months, there was a greater decline in median log10 IU/mL in the combination group (–5.65 versus –3.64), though the study was not powered to compare the two. At 12 months, the combination group had 93.9% virologic efficacy, compared with 68.3% in the bulevirtide group.
The two groups had similar mean ALT levels at 12 months (48.91 and 48.03 IU/mL, respectively), with more patients in the bulevirtide group having normal ALT levels (<40 IU/L; 48.8% versus 36.4%). At 12 months, 39.0% of the bulevirtide group and 30.3% of the combination group had a combined response, defined as either undetectable HDV RNA or ≥2 log10 from baseline plus normal ALT levels.
Twenty-nine patients in the bulevirtide group had an adverse event, compared with 43 in the combination group. The two groups were similar in the frequency of grade 3-4 adverse events (7 versus 6), discontinuation due to adverse events (2 versus 3), deaths (0 in both), injection-site reactions (2 in both), liver-related adverse events (4 versus 2), and elevated bile acid (76 versus 68).
During the Q&A period following the presentation, Dr. De Ledinghen was asked if he has a preferred regimen for HDV patients. “I think it depends on the tolerance of peg-interferon because of all the side effects with this drug. I think we need to have predictive factors of virological response with or without interferon. At this time, I don’t have a preference, but I think at this time we need to work on predictive factors associated with virologic response,” he said.
The EMA’s conditional bulevirtide approval hinged on results from phase 2 clinical trials, while the phase 3 clinical studies are ongoing. “This was a very unusual step for the EMA to provide what is similar to emergency use approval while the phase 3 clinical trials are still ongoing,” said Anna Lok, MD, who was asked to comment on the study. Dr. Lok is a professor of internal medicine, director of clinical hepatology, and assistant dean for clinical research at the University of Michigan, Ann Arbor.
She noted that the phase 2 studies indicated that the combination with peg-interferon seems to have an additive effect on HDV suppression, while monotherapy with bulevirtide has a greater effect on normalizing ALT levels. The real-world experience confirms these findings.
But the real-world data revealed some concerns. “What really worried me is the large number of patients who required dose modifications or discontinuations, and that seems to be the case in both treatment groups. They didn’t really go into a lot of details [about] why patients needed treatment modifications, but one has to assume that this is due to side effects,” said Dr. Lok.
She also noted that the per-protocol analysis, instead of an intention-to-treat analysis, is a weakness of the study. Additionally, over time, the number of patients analyzed decreased – as many as 40% of patients didn’t have test results at month 12. “It makes you wonder what happened to those patients. Many probably didn’t respond, in which case your overall response rate will be far lower,” said Dr. Lok.
The study was funded by Gilead. Dr. De Ledinghen has financial relationships with Gilead, AbbVie, Echosens, Hologic, Intercept Pharma, Tillotts, Orphalan, Alfasigma, Bristol Myers Squibb, and Siemens Healthineers. Dr. Lok has no relevant financial disclosures.
FROM THE LIVER MEETING
New data on rare myocarditis after COVID-19 vaccination
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Adolescents and adults younger than age 21 who develop myocarditis after mRNA COVID-19 vaccination frequently have abnormal findings on cardiac MRI (cMRI) but most have a mild clinical course with rapid resolution of symptoms, a new study concludes.
“This study supports what we’ve been seeing. People identified and treated early and appropriately for the rare complication of COVID-19 vaccine-related myocarditis typically experienced only mild cases and short recovery times,” American Heart Association President Donald M. Lloyd-Jones, MD, said in a podcast.
“Overwhelmingly, the data continue to indicate [that] the benefits of COVID-19 vaccine far outweigh any very rare risks of adverse events from the vaccine, including myocarditis,” Dr. Lloyd-Jones added.
The study was published online Dec. 6 in Circulation.
Using data from 26 pediatric medical centers across the United States and Canada, the researchers reviewed the medical records of 139 patients younger than 21 with suspected myocarditis within 1 month of receiving a COVID-19 vaccination.
They made the following key observations:
- Most patients were male (90.6%), White (66.2%) and with a median age of 15.8 years.
- Suspected myocarditis occurred in 136 patients (97.8%) following mRNA vaccine, with 131 (94.2%) following the Pfizer-BioNTech vaccine; 128 cases (91.4%) occurred after the second dose.
- Symptoms started a median of 2 days (range 0 to 22 days) following vaccination administration.
- Chest pain was the most common symptom (99.3%), with fever present in 30.9% of patients and shortness of breath in 27.3%.
- Patients were treated with nonsteroidal anti-inflammatory drugs (81.3%), intravenous immunoglobulin (21.6%), glucocorticoids (21.6%), colchicine (7.9%) or no anti-inflammatory therapies (8.6%).
- Twenty-six patients (18.7%) were admitted to the intensive care unit; 2 received inotropic/vasoactive support; none required extracorporeal membrane oxygenation or died.
- Median time spent in the hospital was 2 days.
- A total of 111 patients had elevated troponin I (8.12 ng/mL) and 28 had elevated troponin T (0.61 ng/mL).
- More than two-thirds (69.8%) had abnormal electrocardiograms and/or arrhythmias (7 with nonsustained ventricular tachycardia).
- Twenty-six patients (18.7%) had left ventricular ejection fraction (LVEF) less than 55% on echocardiogram; LVEF had returned to normal in the 25 who returned for follow-up.
- 75 of 97 patients (77.3%) who underwent cMRI at a median of 5 days from symptom onset had abnormal findings; 74 (76.3%) had late gadolinium enhancement, 54 (55.7%) had myocardial edema, and 49 (50.5%) met Lake Louise criteria for myocarditis.
“These data suggest that most cases of suspected COVID-19 vaccine–related myocarditis in people younger than 21 are mild and resolve quickly,” corresponding author Dongngan Truong, MD, Division of Pediatric Cardiology, University of Utah and Primary Children’s Hospital, Salt Lake City, said in a statement.
“We were very happy to see that type of recovery. However, we are awaiting further studies to better understand the long-term outcomes of patients who have had COVID-19 vaccination-related myocarditis. We also need to study the risk factors and mechanisms for this rare complication,” Dr. Truong added.
Dr. Lloyd-Jones said these findings support the AHA’s position that COVID-19 vaccines are “safe, highly effective, and fundamental to saving lives, protecting our families and communities against COVID-19, and ending the pandemic.”
The study received no funding. Dr. Truong consults for Pfizer on vaccine-associated myocarditis. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
The top pediatric hospital medicine articles of 2020
The year 2020 was unlike any in recent history, particularly for those working in health care. With the onset of the SARs-CoV-2 pandemic, many physicians were met with increasing clinical demands, and hospitalists served an instrumental role in providing medical care as the world faced an unprecedented need for health care resources.
In addition, 2020 was a year in which many of us reflected on inequities both inside and outside of medicine. Many in health care witnessed the disproportionate burden that the SARs-CoV-2 pandemic placed on communities of color and inequities pertaining to vaccine distribution.
In spite of the challenges of 2020, the field of pediatric hospital medicine (PHM) has continued to grow and evolve, with an incredible amount of new literature published in 2020.
In this article, we identify the top 10 articles published in 2020, 5 of which are summarized below. These articles were presented at the Pediatric Update at SHM Converge 2021.
The top 5 articles
Association between parent comfort with English and adverse events among hospitalized children
Khan A et al. JAMA Pediatrics. December 2020.1
Background: Hospitalized children experience similar rates of medical errors compared to adult patients, but higher rates in areas that could cause harm.1 A major contributor to medical errors is communication failure, which language barriers frequently contribute to. Single-center data suggest that pediatric patients of families with limited comfort with English experience increased adverse events,2 but multicenter data are lacking.
Findings: This prospective cohort study observed adverse event rates among 2,148 patients from seven teaching hospitals from December 2014 to January 2017. Survey data revealed 147 of 1,666 (9%) parents of patient families expressed limited comfort in English, and Spanish was the predominant language in this group (71%). There were 217 adverse events reported, 142 (65%) of which were deemed preventable by study personnel. Nearly twice as many children of parents with limited comfort with English experienced an adverse event when compared to their English-speaking counterparts (26 of 147 [17.7%] vs. 146 of 1,519 [9.6%]; adjusted odds ratio, 2.1; 95% confidence interval, 1.2-3.7). Interpreter use was not measured.
Impact to practice: Children of parents with limited comfort with English are nearly twice as likely to experience adverse events when hospitalized. Hospitals should reflect on current practice and make efforts to improve their ability to identify and communicate with this vulnerable cohort.
Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children
Yeung F et al. Hospital Pediatrics. December 2020.3
Background: Peripheral intravenous catheter (PIV) insertion is performed on most hospitalized children. Unfortunately, PIVs frequently fail and need to be replaced. There is a widespread perception that infusing a crystalloid solution at a low rate through a PIV, a strategy known as “to keep vein open” (TKO) prolongs the patency of PIVs, however there is a lack of evidence to support this practice.4Findings: In this prospective, time-allocated study, 172 children were allocated to either a TKO strategy or a saline-lock strategy with a primary outcome of duration of PIV patency.3 Secondary outcomes included PIV–related complication rates and patient and caregiver satisfaction. The mean duration of PIV patency was 41.68 hours in the TKO group and 44.05 hours in the saline-lock group, which did not meet the prespecified definition of a clinically significant difference. There was no significant difference in prevalence of PIV-associated complications and patient satisfaction was similar between the two groups.
Impact to practice: Running fluid “to keep vein open” does not increase the duration of PIV patency compared to intermittent saline locks. Given that a TKO strategy limits a patient’s mobility, this low-value practice can be discontinued without increasing the risk of PIV failure.
Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol
Coon ER et al. Journal of Hospital Medicine. June 2020.5
Background: High Flow Nasal Cannula (HFNC) has been widely adopted for escalation of respiratory support in patients with bronchiolitis; however, its use is dictated by highly variant local protocols.6 Small-scale randomized control trials and systematic reviews show that early HFNC initiation in mild to moderate disease does not change patient outcomes.7Findings: In this retrospective cohort study of ward-based HFNC, the authors used the Pediatric Health Information System database to identify 12 hospitals that had adopted ward-based HFNC protocols. The study used an interrupted time series analysis to compare outcomes for patients ages 3-24 months hospitalized with bronchiolitis (n = 32,809) in the three seasons before and after protocol adoption. Ward-based HFNC adoption paradoxically increased ICU admission (absolute increase 3.1%, 95% confidence interval, 2.8-3.4%) and ICU length of stay (absolute difference 9.1 days/100 patients, 95% CI, 5.1-13.2). Total length of stay and rates of mechanical ventilation were similar between groups.5Impact to practice: Ward-based HFNC protocols are associated with increased ICU utilization. As bronchiolitis is the leading diagnosis in pediatrics, pediatric hospitals can lead ward-based quality efforts to decrease HFNC overutilization focused on decreased initiation or deimplementation.
Lower versus traditional treatment threshold for neonatal hypoglycemia
Van Kempen AAMW et al. New England Journal of Medicine. February 2020.8
Background: Hypoglycemia is the most common metabolic abnormality in newborns, and up to 30% of newborns are routinely monitored for hypoglycemia. There is no consensus regarding the appropriate threshold at which hypoglycemia should be treated in order to prevent neurologic injury. Prior studies of neonatal hypoglycemia have largely been observation and have yielded conflicting results.8Findings: In this multicenter, randomized, noninferiority trial, 689 infants born at 35 weeks gestational age or later with risk factors for hypoglycemia and a measured blood glucose of 36-46 mg/dL were randomized to either a lower glucose treatment threshold (36 mg/dL) or traditional glucose treatment threshold (47 mg/dL). The primary outcome was psychomotor development at 18 months, assessed via the Bayley Scale of Infant and Toddler Development, third edition. There was no significant difference in cognitive or motor scores at 18 months. The lower treatment threshold group had a higher frequency of severe hypoglycemia (< 36 mg/dL) and were more likely to have four or more episodes of hypoglycemia. The traditional treatment threshold group had more supplemental feeding and more IV glucose administration. Length of stay for the mother and baby did not differ between groups.8
Impact to practice: This prospective, randomized study suggests that reducing the treatment threshold for neonatal hypoglycemia did not affect neurodevelopmental at 18 months of age. In contrast, a recent meta-analysis by Shah et al. suggested that neonatal hypoglycemia was not associated with adverse neurodevelopmental outcomes in early childhood; however, differences in rates of neurodevelopmental impairment, low literacy, and low numeracy were detectable by age five.9
Factors associated with family experience in pediatric inpatient care
Feng JY et al. Pediatrics. March 2020 Mar.10
Background: Positive patient experience is associated with better health care outcomes and reduced health care use.11 Consequently, patient experience surveys have played a larger role in public reporting, financial risk sharing arrangements, and pay for performance programs. While adult studies have examined the importance of specific care dimensions for patient experience, data are lacking for inpatient pediatric populations.
Findings: A retrospective study collected Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys from 17,727 patients in 69 hospitals within the United States over a 14-month period.10 Of the 10 care dimensions analyzed, child comfort (aOR 1.50; 95% CI, 1.41-1.60) and nurse-parent communication (aOR 1.50; 95% CI, 1.42-1.58) were most strongly associated with a family’s willingness to recommend a hospital. Additional associated indices included preparing to leave the hospital (aOR 1.34; 95% CI, 1.27-1.41), doctor-parent communication (aOR 1.28; 95% CI, 1.21–1.35), and keeping parents informed (aOR 1.25; 95% CI, 1.18-1.33). Privacy and quietness, which are associated with positive patient experience in adult studies, were not significantly associated with willingness to recommend in this cohort.
Impact to practice: Hospitals seeking to improve patient experience will benefit most by focusing on improving patient comfort and nurse-parent communication. Factors that increase adult patient satisfaction may not be as important to the pediatric population and their families.
The other five articles that comprised the top 10 are listed below:
Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis
Coon ER et al. JAMA Pediatrics. September 2020.12
Clinical prediction rule for distinguishing bacterial from aseptic meningitis
Mintegi S et al. Pediatrics. September 2020.13
The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC Ullman AJ et al. Pediatrics. June 2020.14
A structured neonatal parenting elective: An approach for parenting leave during residency
Cree-Green M et al. Academic Pediatrics. Aug 2020.15
The KidzMed project: Teaching children to swallow tablet medication
Tse Y et al. Archives of Disease in Childhood. November 2020.16
Dr. Steed is an internal medicine and pediatrics hospitalist at Northwestern Memorial Hospital and Ann and Robert H. Lurie’s Children’s Hospital of Chicago. Dr. Fisher is a current fellow in hospice and palliative medicine and a clinical assistant professor at Michigan State University. Dr. Money is an assistant professor of pediatrics at the University of Utah and a fellowship-trained pediatric hospitalist at Utah Valley Hospital and Primary Children’s Hospital.
References
1. Khan A et al. Association between parent comfort with english and adverse events among hospitalized children. JAMA Pediatr. 2020 Dec 1;174(12):e203215. doi: 10.1001/jamapediatrics.2020.3215.
2. Wasserman M et al. Identifying and preventing medical errors in patients with limited English proficiency: Key findings and tools for the field. J Healthc Qual. May-Jun 2014;36(3):5-16. doi: 10.1111/jhq.12065.
3. Yeung F et al. Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children. Hosp Pediatr. 2020 Dec;10(12):1038-43. doi: 10.1542/hpeds.2020-0137.
4. Mok E et al. A randomized controlled trial for maintaining peripheral intravenous lock in children. Int J Nurs Pract. 2007 Feb;13(1):33-45. doi: 10.1111/j.1440-172X.2006.00607.x.
5. Coon ER et al. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020 Jun;15(6):325-30. doi: 10.12788/jhm.3417.
6. Kalburgi S and Halley T. High-flow nasal cannula use outside of the ICU setting. Pediatrics. 2020;146(5):e20194083. doi: 10.1542/peds.2019-4083.
7. Leyenaar JK and Ralston SL. Widespread adoption of low-value therapy: The case of bronchiolitis and high-flow oxygen. Pediatrics. 2020 Nov;146(5):e2020021188. doi: 10.1542/peds.2020-021188.
8. Van Kempen AAMW et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. 2020 Feb 6;382(6):534-44. doi: 10.1056/NEJMoa1905593.
9. Shah R et al. Neonatal glycaemia and neurodevelopmental outcomes: A systematic review and meta-analysis. Neonatology. 2019;115(2):116-26. doi: 10.1159/000492859.
10. Feng JY et al. Factors associated with family experience in pediatric inpatient care. Pediatrics. 2020 Mar;145(3):e20191264. doi: 10.1542/peds.2019-1264.
11. Anhang Price R et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev. 2014 Oct;71(5):522-54. doi: 10.1177/1077558714541480.
12. Coon ER et al. Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis: The Bronchiolitis Follow-up Intervention Trial (BeneFIT) randomized clinical trial. JAMA Pediatr. 2020 Sep 1;174(9):e201937. doi: 10.1001/jamapediatrics.2020.1937.
13. Mintegi S et al. Clinical prediction rule for distinguishing bacterial from aseptic meningitis. Pediatrics. 2020 Sept;146(3): e20201126. doi: 10.1542/peds.2020-1126.
14. Ullman AJ et al. The Michigan Appropriateness Guide for Intravenous Catheters in pediatrics: miniMAGIC. Pediatrics. 2020 Jun;145(Suppl 3):S269-S284. doi: 10.1542/peds.2019-3474I.
15. Cree-Green M et al. A structured neonatal parenting elective: an approach for parenting leave during residency. Acad Pediatr. 2021 Jan-Feb;21(1):16-18. doi: 10.1016/j.acap.2020.02.008.
16. Tse Y et al. The KidzMed project: Teaching children to swallow tablet medication. Arch Dis Child. 2020 Nov;105(11):1105-7. doi: 10.1136/archdischild-2019-317512.
The year 2020 was unlike any in recent history, particularly for those working in health care. With the onset of the SARs-CoV-2 pandemic, many physicians were met with increasing clinical demands, and hospitalists served an instrumental role in providing medical care as the world faced an unprecedented need for health care resources.
In addition, 2020 was a year in which many of us reflected on inequities both inside and outside of medicine. Many in health care witnessed the disproportionate burden that the SARs-CoV-2 pandemic placed on communities of color and inequities pertaining to vaccine distribution.
In spite of the challenges of 2020, the field of pediatric hospital medicine (PHM) has continued to grow and evolve, with an incredible amount of new literature published in 2020.
In this article, we identify the top 10 articles published in 2020, 5 of which are summarized below. These articles were presented at the Pediatric Update at SHM Converge 2021.
The top 5 articles
Association between parent comfort with English and adverse events among hospitalized children
Khan A et al. JAMA Pediatrics. December 2020.1
Background: Hospitalized children experience similar rates of medical errors compared to adult patients, but higher rates in areas that could cause harm.1 A major contributor to medical errors is communication failure, which language barriers frequently contribute to. Single-center data suggest that pediatric patients of families with limited comfort with English experience increased adverse events,2 but multicenter data are lacking.
Findings: This prospective cohort study observed adverse event rates among 2,148 patients from seven teaching hospitals from December 2014 to January 2017. Survey data revealed 147 of 1,666 (9%) parents of patient families expressed limited comfort in English, and Spanish was the predominant language in this group (71%). There were 217 adverse events reported, 142 (65%) of which were deemed preventable by study personnel. Nearly twice as many children of parents with limited comfort with English experienced an adverse event when compared to their English-speaking counterparts (26 of 147 [17.7%] vs. 146 of 1,519 [9.6%]; adjusted odds ratio, 2.1; 95% confidence interval, 1.2-3.7). Interpreter use was not measured.
Impact to practice: Children of parents with limited comfort with English are nearly twice as likely to experience adverse events when hospitalized. Hospitals should reflect on current practice and make efforts to improve their ability to identify and communicate with this vulnerable cohort.
Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children
Yeung F et al. Hospital Pediatrics. December 2020.3
Background: Peripheral intravenous catheter (PIV) insertion is performed on most hospitalized children. Unfortunately, PIVs frequently fail and need to be replaced. There is a widespread perception that infusing a crystalloid solution at a low rate through a PIV, a strategy known as “to keep vein open” (TKO) prolongs the patency of PIVs, however there is a lack of evidence to support this practice.4Findings: In this prospective, time-allocated study, 172 children were allocated to either a TKO strategy or a saline-lock strategy with a primary outcome of duration of PIV patency.3 Secondary outcomes included PIV–related complication rates and patient and caregiver satisfaction. The mean duration of PIV patency was 41.68 hours in the TKO group and 44.05 hours in the saline-lock group, which did not meet the prespecified definition of a clinically significant difference. There was no significant difference in prevalence of PIV-associated complications and patient satisfaction was similar between the two groups.
Impact to practice: Running fluid “to keep vein open” does not increase the duration of PIV patency compared to intermittent saline locks. Given that a TKO strategy limits a patient’s mobility, this low-value practice can be discontinued without increasing the risk of PIV failure.
Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol
Coon ER et al. Journal of Hospital Medicine. June 2020.5
Background: High Flow Nasal Cannula (HFNC) has been widely adopted for escalation of respiratory support in patients with bronchiolitis; however, its use is dictated by highly variant local protocols.6 Small-scale randomized control trials and systematic reviews show that early HFNC initiation in mild to moderate disease does not change patient outcomes.7Findings: In this retrospective cohort study of ward-based HFNC, the authors used the Pediatric Health Information System database to identify 12 hospitals that had adopted ward-based HFNC protocols. The study used an interrupted time series analysis to compare outcomes for patients ages 3-24 months hospitalized with bronchiolitis (n = 32,809) in the three seasons before and after protocol adoption. Ward-based HFNC adoption paradoxically increased ICU admission (absolute increase 3.1%, 95% confidence interval, 2.8-3.4%) and ICU length of stay (absolute difference 9.1 days/100 patients, 95% CI, 5.1-13.2). Total length of stay and rates of mechanical ventilation were similar between groups.5Impact to practice: Ward-based HFNC protocols are associated with increased ICU utilization. As bronchiolitis is the leading diagnosis in pediatrics, pediatric hospitals can lead ward-based quality efforts to decrease HFNC overutilization focused on decreased initiation or deimplementation.
Lower versus traditional treatment threshold for neonatal hypoglycemia
Van Kempen AAMW et al. New England Journal of Medicine. February 2020.8
Background: Hypoglycemia is the most common metabolic abnormality in newborns, and up to 30% of newborns are routinely monitored for hypoglycemia. There is no consensus regarding the appropriate threshold at which hypoglycemia should be treated in order to prevent neurologic injury. Prior studies of neonatal hypoglycemia have largely been observation and have yielded conflicting results.8Findings: In this multicenter, randomized, noninferiority trial, 689 infants born at 35 weeks gestational age or later with risk factors for hypoglycemia and a measured blood glucose of 36-46 mg/dL were randomized to either a lower glucose treatment threshold (36 mg/dL) or traditional glucose treatment threshold (47 mg/dL). The primary outcome was psychomotor development at 18 months, assessed via the Bayley Scale of Infant and Toddler Development, third edition. There was no significant difference in cognitive or motor scores at 18 months. The lower treatment threshold group had a higher frequency of severe hypoglycemia (< 36 mg/dL) and were more likely to have four or more episodes of hypoglycemia. The traditional treatment threshold group had more supplemental feeding and more IV glucose administration. Length of stay for the mother and baby did not differ between groups.8
Impact to practice: This prospective, randomized study suggests that reducing the treatment threshold for neonatal hypoglycemia did not affect neurodevelopmental at 18 months of age. In contrast, a recent meta-analysis by Shah et al. suggested that neonatal hypoglycemia was not associated with adverse neurodevelopmental outcomes in early childhood; however, differences in rates of neurodevelopmental impairment, low literacy, and low numeracy were detectable by age five.9
Factors associated with family experience in pediatric inpatient care
Feng JY et al. Pediatrics. March 2020 Mar.10
Background: Positive patient experience is associated with better health care outcomes and reduced health care use.11 Consequently, patient experience surveys have played a larger role in public reporting, financial risk sharing arrangements, and pay for performance programs. While adult studies have examined the importance of specific care dimensions for patient experience, data are lacking for inpatient pediatric populations.
Findings: A retrospective study collected Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys from 17,727 patients in 69 hospitals within the United States over a 14-month period.10 Of the 10 care dimensions analyzed, child comfort (aOR 1.50; 95% CI, 1.41-1.60) and nurse-parent communication (aOR 1.50; 95% CI, 1.42-1.58) were most strongly associated with a family’s willingness to recommend a hospital. Additional associated indices included preparing to leave the hospital (aOR 1.34; 95% CI, 1.27-1.41), doctor-parent communication (aOR 1.28; 95% CI, 1.21–1.35), and keeping parents informed (aOR 1.25; 95% CI, 1.18-1.33). Privacy and quietness, which are associated with positive patient experience in adult studies, were not significantly associated with willingness to recommend in this cohort.
Impact to practice: Hospitals seeking to improve patient experience will benefit most by focusing on improving patient comfort and nurse-parent communication. Factors that increase adult patient satisfaction may not be as important to the pediatric population and their families.
The other five articles that comprised the top 10 are listed below:
Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis
Coon ER et al. JAMA Pediatrics. September 2020.12
Clinical prediction rule for distinguishing bacterial from aseptic meningitis
Mintegi S et al. Pediatrics. September 2020.13
The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC Ullman AJ et al. Pediatrics. June 2020.14
A structured neonatal parenting elective: An approach for parenting leave during residency
Cree-Green M et al. Academic Pediatrics. Aug 2020.15
The KidzMed project: Teaching children to swallow tablet medication
Tse Y et al. Archives of Disease in Childhood. November 2020.16
Dr. Steed is an internal medicine and pediatrics hospitalist at Northwestern Memorial Hospital and Ann and Robert H. Lurie’s Children’s Hospital of Chicago. Dr. Fisher is a current fellow in hospice and palliative medicine and a clinical assistant professor at Michigan State University. Dr. Money is an assistant professor of pediatrics at the University of Utah and a fellowship-trained pediatric hospitalist at Utah Valley Hospital and Primary Children’s Hospital.
References
1. Khan A et al. Association between parent comfort with english and adverse events among hospitalized children. JAMA Pediatr. 2020 Dec 1;174(12):e203215. doi: 10.1001/jamapediatrics.2020.3215.
2. Wasserman M et al. Identifying and preventing medical errors in patients with limited English proficiency: Key findings and tools for the field. J Healthc Qual. May-Jun 2014;36(3):5-16. doi: 10.1111/jhq.12065.
3. Yeung F et al. Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children. Hosp Pediatr. 2020 Dec;10(12):1038-43. doi: 10.1542/hpeds.2020-0137.
4. Mok E et al. A randomized controlled trial for maintaining peripheral intravenous lock in children. Int J Nurs Pract. 2007 Feb;13(1):33-45. doi: 10.1111/j.1440-172X.2006.00607.x.
5. Coon ER et al. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020 Jun;15(6):325-30. doi: 10.12788/jhm.3417.
6. Kalburgi S and Halley T. High-flow nasal cannula use outside of the ICU setting. Pediatrics. 2020;146(5):e20194083. doi: 10.1542/peds.2019-4083.
7. Leyenaar JK and Ralston SL. Widespread adoption of low-value therapy: The case of bronchiolitis and high-flow oxygen. Pediatrics. 2020 Nov;146(5):e2020021188. doi: 10.1542/peds.2020-021188.
8. Van Kempen AAMW et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. 2020 Feb 6;382(6):534-44. doi: 10.1056/NEJMoa1905593.
9. Shah R et al. Neonatal glycaemia and neurodevelopmental outcomes: A systematic review and meta-analysis. Neonatology. 2019;115(2):116-26. doi: 10.1159/000492859.
10. Feng JY et al. Factors associated with family experience in pediatric inpatient care. Pediatrics. 2020 Mar;145(3):e20191264. doi: 10.1542/peds.2019-1264.
11. Anhang Price R et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev. 2014 Oct;71(5):522-54. doi: 10.1177/1077558714541480.
12. Coon ER et al. Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis: The Bronchiolitis Follow-up Intervention Trial (BeneFIT) randomized clinical trial. JAMA Pediatr. 2020 Sep 1;174(9):e201937. doi: 10.1001/jamapediatrics.2020.1937.
13. Mintegi S et al. Clinical prediction rule for distinguishing bacterial from aseptic meningitis. Pediatrics. 2020 Sept;146(3): e20201126. doi: 10.1542/peds.2020-1126.
14. Ullman AJ et al. The Michigan Appropriateness Guide for Intravenous Catheters in pediatrics: miniMAGIC. Pediatrics. 2020 Jun;145(Suppl 3):S269-S284. doi: 10.1542/peds.2019-3474I.
15. Cree-Green M et al. A structured neonatal parenting elective: an approach for parenting leave during residency. Acad Pediatr. 2021 Jan-Feb;21(1):16-18. doi: 10.1016/j.acap.2020.02.008.
16. Tse Y et al. The KidzMed project: Teaching children to swallow tablet medication. Arch Dis Child. 2020 Nov;105(11):1105-7. doi: 10.1136/archdischild-2019-317512.
The year 2020 was unlike any in recent history, particularly for those working in health care. With the onset of the SARs-CoV-2 pandemic, many physicians were met with increasing clinical demands, and hospitalists served an instrumental role in providing medical care as the world faced an unprecedented need for health care resources.
In addition, 2020 was a year in which many of us reflected on inequities both inside and outside of medicine. Many in health care witnessed the disproportionate burden that the SARs-CoV-2 pandemic placed on communities of color and inequities pertaining to vaccine distribution.
In spite of the challenges of 2020, the field of pediatric hospital medicine (PHM) has continued to grow and evolve, with an incredible amount of new literature published in 2020.
In this article, we identify the top 10 articles published in 2020, 5 of which are summarized below. These articles were presented at the Pediatric Update at SHM Converge 2021.
The top 5 articles
Association between parent comfort with English and adverse events among hospitalized children
Khan A et al. JAMA Pediatrics. December 2020.1
Background: Hospitalized children experience similar rates of medical errors compared to adult patients, but higher rates in areas that could cause harm.1 A major contributor to medical errors is communication failure, which language barriers frequently contribute to. Single-center data suggest that pediatric patients of families with limited comfort with English experience increased adverse events,2 but multicenter data are lacking.
Findings: This prospective cohort study observed adverse event rates among 2,148 patients from seven teaching hospitals from December 2014 to January 2017. Survey data revealed 147 of 1,666 (9%) parents of patient families expressed limited comfort in English, and Spanish was the predominant language in this group (71%). There were 217 adverse events reported, 142 (65%) of which were deemed preventable by study personnel. Nearly twice as many children of parents with limited comfort with English experienced an adverse event when compared to their English-speaking counterparts (26 of 147 [17.7%] vs. 146 of 1,519 [9.6%]; adjusted odds ratio, 2.1; 95% confidence interval, 1.2-3.7). Interpreter use was not measured.
Impact to practice: Children of parents with limited comfort with English are nearly twice as likely to experience adverse events when hospitalized. Hospitals should reflect on current practice and make efforts to improve their ability to identify and communicate with this vulnerable cohort.
Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children
Yeung F et al. Hospital Pediatrics. December 2020.3
Background: Peripheral intravenous catheter (PIV) insertion is performed on most hospitalized children. Unfortunately, PIVs frequently fail and need to be replaced. There is a widespread perception that infusing a crystalloid solution at a low rate through a PIV, a strategy known as “to keep vein open” (TKO) prolongs the patency of PIVs, however there is a lack of evidence to support this practice.4Findings: In this prospective, time-allocated study, 172 children were allocated to either a TKO strategy or a saline-lock strategy with a primary outcome of duration of PIV patency.3 Secondary outcomes included PIV–related complication rates and patient and caregiver satisfaction. The mean duration of PIV patency was 41.68 hours in the TKO group and 44.05 hours in the saline-lock group, which did not meet the prespecified definition of a clinically significant difference. There was no significant difference in prevalence of PIV-associated complications and patient satisfaction was similar between the two groups.
Impact to practice: Running fluid “to keep vein open” does not increase the duration of PIV patency compared to intermittent saline locks. Given that a TKO strategy limits a patient’s mobility, this low-value practice can be discontinued without increasing the risk of PIV failure.
Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol
Coon ER et al. Journal of Hospital Medicine. June 2020.5
Background: High Flow Nasal Cannula (HFNC) has been widely adopted for escalation of respiratory support in patients with bronchiolitis; however, its use is dictated by highly variant local protocols.6 Small-scale randomized control trials and systematic reviews show that early HFNC initiation in mild to moderate disease does not change patient outcomes.7Findings: In this retrospective cohort study of ward-based HFNC, the authors used the Pediatric Health Information System database to identify 12 hospitals that had adopted ward-based HFNC protocols. The study used an interrupted time series analysis to compare outcomes for patients ages 3-24 months hospitalized with bronchiolitis (n = 32,809) in the three seasons before and after protocol adoption. Ward-based HFNC adoption paradoxically increased ICU admission (absolute increase 3.1%, 95% confidence interval, 2.8-3.4%) and ICU length of stay (absolute difference 9.1 days/100 patients, 95% CI, 5.1-13.2). Total length of stay and rates of mechanical ventilation were similar between groups.5Impact to practice: Ward-based HFNC protocols are associated with increased ICU utilization. As bronchiolitis is the leading diagnosis in pediatrics, pediatric hospitals can lead ward-based quality efforts to decrease HFNC overutilization focused on decreased initiation or deimplementation.
Lower versus traditional treatment threshold for neonatal hypoglycemia
Van Kempen AAMW et al. New England Journal of Medicine. February 2020.8
Background: Hypoglycemia is the most common metabolic abnormality in newborns, and up to 30% of newborns are routinely monitored for hypoglycemia. There is no consensus regarding the appropriate threshold at which hypoglycemia should be treated in order to prevent neurologic injury. Prior studies of neonatal hypoglycemia have largely been observation and have yielded conflicting results.8Findings: In this multicenter, randomized, noninferiority trial, 689 infants born at 35 weeks gestational age or later with risk factors for hypoglycemia and a measured blood glucose of 36-46 mg/dL were randomized to either a lower glucose treatment threshold (36 mg/dL) or traditional glucose treatment threshold (47 mg/dL). The primary outcome was psychomotor development at 18 months, assessed via the Bayley Scale of Infant and Toddler Development, third edition. There was no significant difference in cognitive or motor scores at 18 months. The lower treatment threshold group had a higher frequency of severe hypoglycemia (< 36 mg/dL) and were more likely to have four or more episodes of hypoglycemia. The traditional treatment threshold group had more supplemental feeding and more IV glucose administration. Length of stay for the mother and baby did not differ between groups.8
Impact to practice: This prospective, randomized study suggests that reducing the treatment threshold for neonatal hypoglycemia did not affect neurodevelopmental at 18 months of age. In contrast, a recent meta-analysis by Shah et al. suggested that neonatal hypoglycemia was not associated with adverse neurodevelopmental outcomes in early childhood; however, differences in rates of neurodevelopmental impairment, low literacy, and low numeracy were detectable by age five.9
Factors associated with family experience in pediatric inpatient care
Feng JY et al. Pediatrics. March 2020 Mar.10
Background: Positive patient experience is associated with better health care outcomes and reduced health care use.11 Consequently, patient experience surveys have played a larger role in public reporting, financial risk sharing arrangements, and pay for performance programs. While adult studies have examined the importance of specific care dimensions for patient experience, data are lacking for inpatient pediatric populations.
Findings: A retrospective study collected Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys from 17,727 patients in 69 hospitals within the United States over a 14-month period.10 Of the 10 care dimensions analyzed, child comfort (aOR 1.50; 95% CI, 1.41-1.60) and nurse-parent communication (aOR 1.50; 95% CI, 1.42-1.58) were most strongly associated with a family’s willingness to recommend a hospital. Additional associated indices included preparing to leave the hospital (aOR 1.34; 95% CI, 1.27-1.41), doctor-parent communication (aOR 1.28; 95% CI, 1.21–1.35), and keeping parents informed (aOR 1.25; 95% CI, 1.18-1.33). Privacy and quietness, which are associated with positive patient experience in adult studies, were not significantly associated with willingness to recommend in this cohort.
Impact to practice: Hospitals seeking to improve patient experience will benefit most by focusing on improving patient comfort and nurse-parent communication. Factors that increase adult patient satisfaction may not be as important to the pediatric population and their families.
The other five articles that comprised the top 10 are listed below:
Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis
Coon ER et al. JAMA Pediatrics. September 2020.12
Clinical prediction rule for distinguishing bacterial from aseptic meningitis
Mintegi S et al. Pediatrics. September 2020.13
The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics: miniMAGIC Ullman AJ et al. Pediatrics. June 2020.14
A structured neonatal parenting elective: An approach for parenting leave during residency
Cree-Green M et al. Academic Pediatrics. Aug 2020.15
The KidzMed project: Teaching children to swallow tablet medication
Tse Y et al. Archives of Disease in Childhood. November 2020.16
Dr. Steed is an internal medicine and pediatrics hospitalist at Northwestern Memorial Hospital and Ann and Robert H. Lurie’s Children’s Hospital of Chicago. Dr. Fisher is a current fellow in hospice and palliative medicine and a clinical assistant professor at Michigan State University. Dr. Money is an assistant professor of pediatrics at the University of Utah and a fellowship-trained pediatric hospitalist at Utah Valley Hospital and Primary Children’s Hospital.
References
1. Khan A et al. Association between parent comfort with english and adverse events among hospitalized children. JAMA Pediatr. 2020 Dec 1;174(12):e203215. doi: 10.1001/jamapediatrics.2020.3215.
2. Wasserman M et al. Identifying and preventing medical errors in patients with limited English proficiency: Key findings and tools for the field. J Healthc Qual. May-Jun 2014;36(3):5-16. doi: 10.1111/jhq.12065.
3. Yeung F et al. Saline-lock versus continuous infusion: Maintaining peripheral intravenous catheter access in children. Hosp Pediatr. 2020 Dec;10(12):1038-43. doi: 10.1542/hpeds.2020-0137.
4. Mok E et al. A randomized controlled trial for maintaining peripheral intravenous lock in children. Int J Nurs Pract. 2007 Feb;13(1):33-45. doi: 10.1111/j.1440-172X.2006.00607.x.
5. Coon ER et al. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020 Jun;15(6):325-30. doi: 10.12788/jhm.3417.
6. Kalburgi S and Halley T. High-flow nasal cannula use outside of the ICU setting. Pediatrics. 2020;146(5):e20194083. doi: 10.1542/peds.2019-4083.
7. Leyenaar JK and Ralston SL. Widespread adoption of low-value therapy: The case of bronchiolitis and high-flow oxygen. Pediatrics. 2020 Nov;146(5):e2020021188. doi: 10.1542/peds.2020-021188.
8. Van Kempen AAMW et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. 2020 Feb 6;382(6):534-44. doi: 10.1056/NEJMoa1905593.
9. Shah R et al. Neonatal glycaemia and neurodevelopmental outcomes: A systematic review and meta-analysis. Neonatology. 2019;115(2):116-26. doi: 10.1159/000492859.
10. Feng JY et al. Factors associated with family experience in pediatric inpatient care. Pediatrics. 2020 Mar;145(3):e20191264. doi: 10.1542/peds.2019-1264.
11. Anhang Price R et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev. 2014 Oct;71(5):522-54. doi: 10.1177/1077558714541480.
12. Coon ER et al. Comparison of as-needed and scheduled posthospitalization follow-up for children hospitalized for bronchiolitis: The Bronchiolitis Follow-up Intervention Trial (BeneFIT) randomized clinical trial. JAMA Pediatr. 2020 Sep 1;174(9):e201937. doi: 10.1001/jamapediatrics.2020.1937.
13. Mintegi S et al. Clinical prediction rule for distinguishing bacterial from aseptic meningitis. Pediatrics. 2020 Sept;146(3): e20201126. doi: 10.1542/peds.2020-1126.
14. Ullman AJ et al. The Michigan Appropriateness Guide for Intravenous Catheters in pediatrics: miniMAGIC. Pediatrics. 2020 Jun;145(Suppl 3):S269-S284. doi: 10.1542/peds.2019-3474I.
15. Cree-Green M et al. A structured neonatal parenting elective: an approach for parenting leave during residency. Acad Pediatr. 2021 Jan-Feb;21(1):16-18. doi: 10.1016/j.acap.2020.02.008.
16. Tse Y et al. The KidzMed project: Teaching children to swallow tablet medication. Arch Dis Child. 2020 Nov;105(11):1105-7. doi: 10.1136/archdischild-2019-317512.
FDA expands pembrolizumab approval for advanced melanoma
The over age 12 years. The FDA also extended the approval to those with stage III disease.
The FDA approval on Dec. 3 was based on first interim findings from the randomized, placebo-controlled KEYNOTE-716 trial, which evaluated patients with stage IIB and IIC disease.
Since the anti-PD-1 therapy was approved in metastatic melanoma 7 years ago, “we have built on this foundation in melanoma and have expanded the use of KEYTRUDA into earlier stages of this disease,” said Scot Ebbinghaus, MD, vice president, clinical research, Merck Research Laboratories, in a press release. “With today’s approval, we can now offer health care providers and patients 12 years and older the opportunity to help prevent melanoma recurrence with Keytruda across resected stage IIB, stage IIC, and stage III melanoma.”
In KEYNOTE-716, patients with completely resected stage IIB or IIC melanoma were randomly assigned to receive 200 mg of intravenous pembrolizumab, the pediatric dose 2 mg/kg (up to a maximum of 200 mg) every 3 weeks, or placebo for up to 1 year until disease recurrence or unacceptable toxicity.
After a median follow-up of 14.4 months, investigators reported a statistically significant 35% improvement in recurrence-free survival (RFS) in those treated with pembrolizumab, compared with those who received placebo (hazard ratio, 0.65).
The most common adverse reactions reported in patients receiving pembrolizumab in KEYNOTE-716 were fatigue, diarrhea, pruritus, and arthralgia, each occurring in at least 20% of patients.
“Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda,” according to Merck.
A version of this article first appeared on Medscape.com.
The over age 12 years. The FDA also extended the approval to those with stage III disease.
The FDA approval on Dec. 3 was based on first interim findings from the randomized, placebo-controlled KEYNOTE-716 trial, which evaluated patients with stage IIB and IIC disease.
Since the anti-PD-1 therapy was approved in metastatic melanoma 7 years ago, “we have built on this foundation in melanoma and have expanded the use of KEYTRUDA into earlier stages of this disease,” said Scot Ebbinghaus, MD, vice president, clinical research, Merck Research Laboratories, in a press release. “With today’s approval, we can now offer health care providers and patients 12 years and older the opportunity to help prevent melanoma recurrence with Keytruda across resected stage IIB, stage IIC, and stage III melanoma.”
In KEYNOTE-716, patients with completely resected stage IIB or IIC melanoma were randomly assigned to receive 200 mg of intravenous pembrolizumab, the pediatric dose 2 mg/kg (up to a maximum of 200 mg) every 3 weeks, or placebo for up to 1 year until disease recurrence or unacceptable toxicity.
After a median follow-up of 14.4 months, investigators reported a statistically significant 35% improvement in recurrence-free survival (RFS) in those treated with pembrolizumab, compared with those who received placebo (hazard ratio, 0.65).
The most common adverse reactions reported in patients receiving pembrolizumab in KEYNOTE-716 were fatigue, diarrhea, pruritus, and arthralgia, each occurring in at least 20% of patients.
“Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda,” according to Merck.
A version of this article first appeared on Medscape.com.
The over age 12 years. The FDA also extended the approval to those with stage III disease.
The FDA approval on Dec. 3 was based on first interim findings from the randomized, placebo-controlled KEYNOTE-716 trial, which evaluated patients with stage IIB and IIC disease.
Since the anti-PD-1 therapy was approved in metastatic melanoma 7 years ago, “we have built on this foundation in melanoma and have expanded the use of KEYTRUDA into earlier stages of this disease,” said Scot Ebbinghaus, MD, vice president, clinical research, Merck Research Laboratories, in a press release. “With today’s approval, we can now offer health care providers and patients 12 years and older the opportunity to help prevent melanoma recurrence with Keytruda across resected stage IIB, stage IIC, and stage III melanoma.”
In KEYNOTE-716, patients with completely resected stage IIB or IIC melanoma were randomly assigned to receive 200 mg of intravenous pembrolizumab, the pediatric dose 2 mg/kg (up to a maximum of 200 mg) every 3 weeks, or placebo for up to 1 year until disease recurrence or unacceptable toxicity.
After a median follow-up of 14.4 months, investigators reported a statistically significant 35% improvement in recurrence-free survival (RFS) in those treated with pembrolizumab, compared with those who received placebo (hazard ratio, 0.65).
The most common adverse reactions reported in patients receiving pembrolizumab in KEYNOTE-716 were fatigue, diarrhea, pruritus, and arthralgia, each occurring in at least 20% of patients.
“Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda,” according to Merck.
A version of this article first appeared on Medscape.com.
AHA challenges diet doctor’s study alleging COVID vax risks
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
Louisiana to require the COVID-19 vaccine for students
Louisiana Gov. John Bel Edwards says the state government plans to make the COVID-19 vaccine a required immunization for students 16 and older in the state’s public school system.
“I just think it’s really, really important to embrace the science and really it’s also important to not engage in misinformation,” said Gov. Edwards, a Democrat, according to The Advocate. “Absent some compelling reason, which I at present have not seen, I fully expect that we will be adding the vaccine to the schedule.”
Parents could opt out their children from the requirement with a letter from a medical provider or a simple signature in dissent, The Advocate reported. The new rule would go into effect at the start of the 2022 school year and at first would apply to students aged 16 and older.
Republican legislators voiced their opposition to the COVID-19 vaccine requirement at a hearing on Dec. 6, calling it unneeded and an example of governmental overreach.
“I believe the vaccine should be highly recommended but not mandated,” state Rep. Laurie Schlegel said, according to TV station WDSU.
State Sen. Cameron Henry of Metairie said he received “hundreds of emails” from parents asking him to prevent the rule from going into effect, WDSU said.
WDSU said the governor can overrule the committee if it rejects the proposed vaccine rule.
Louisiana State Health Officer Joseph Kanter, MD, testified on Dec. 6 that 18 children had died of COVID-19 in Louisiana and many others had become sick because of it.
“I can’t think of another disease on that childhood schedule that we’ve lost that many kids from. In my mind, it’s very much in the public interest. But it’s the family and the parents’ decision,” Dr. Kanter said.
The addition of the vaccine is being proposed by the Louisiana Department of Health, which has added other vaccines to the required list over the years. In 2015, the legislature added meningitis as a required shot with no controversy, The Advocate said.
A version of this article first appeared on WebMD.com.
Louisiana Gov. John Bel Edwards says the state government plans to make the COVID-19 vaccine a required immunization for students 16 and older in the state’s public school system.
“I just think it’s really, really important to embrace the science and really it’s also important to not engage in misinformation,” said Gov. Edwards, a Democrat, according to The Advocate. “Absent some compelling reason, which I at present have not seen, I fully expect that we will be adding the vaccine to the schedule.”
Parents could opt out their children from the requirement with a letter from a medical provider or a simple signature in dissent, The Advocate reported. The new rule would go into effect at the start of the 2022 school year and at first would apply to students aged 16 and older.
Republican legislators voiced their opposition to the COVID-19 vaccine requirement at a hearing on Dec. 6, calling it unneeded and an example of governmental overreach.
“I believe the vaccine should be highly recommended but not mandated,” state Rep. Laurie Schlegel said, according to TV station WDSU.
State Sen. Cameron Henry of Metairie said he received “hundreds of emails” from parents asking him to prevent the rule from going into effect, WDSU said.
WDSU said the governor can overrule the committee if it rejects the proposed vaccine rule.
Louisiana State Health Officer Joseph Kanter, MD, testified on Dec. 6 that 18 children had died of COVID-19 in Louisiana and many others had become sick because of it.
“I can’t think of another disease on that childhood schedule that we’ve lost that many kids from. In my mind, it’s very much in the public interest. But it’s the family and the parents’ decision,” Dr. Kanter said.
The addition of the vaccine is being proposed by the Louisiana Department of Health, which has added other vaccines to the required list over the years. In 2015, the legislature added meningitis as a required shot with no controversy, The Advocate said.
A version of this article first appeared on WebMD.com.
Louisiana Gov. John Bel Edwards says the state government plans to make the COVID-19 vaccine a required immunization for students 16 and older in the state’s public school system.
“I just think it’s really, really important to embrace the science and really it’s also important to not engage in misinformation,” said Gov. Edwards, a Democrat, according to The Advocate. “Absent some compelling reason, which I at present have not seen, I fully expect that we will be adding the vaccine to the schedule.”
Parents could opt out their children from the requirement with a letter from a medical provider or a simple signature in dissent, The Advocate reported. The new rule would go into effect at the start of the 2022 school year and at first would apply to students aged 16 and older.
Republican legislators voiced their opposition to the COVID-19 vaccine requirement at a hearing on Dec. 6, calling it unneeded and an example of governmental overreach.
“I believe the vaccine should be highly recommended but not mandated,” state Rep. Laurie Schlegel said, according to TV station WDSU.
State Sen. Cameron Henry of Metairie said he received “hundreds of emails” from parents asking him to prevent the rule from going into effect, WDSU said.
WDSU said the governor can overrule the committee if it rejects the proposed vaccine rule.
Louisiana State Health Officer Joseph Kanter, MD, testified on Dec. 6 that 18 children had died of COVID-19 in Louisiana and many others had become sick because of it.
“I can’t think of another disease on that childhood schedule that we’ve lost that many kids from. In my mind, it’s very much in the public interest. But it’s the family and the parents’ decision,” Dr. Kanter said.
The addition of the vaccine is being proposed by the Louisiana Department of Health, which has added other vaccines to the required list over the years. In 2015, the legislature added meningitis as a required shot with no controversy, The Advocate said.
A version of this article first appeared on WebMD.com.