User login
Colorectal cancer screening, 2021: An update
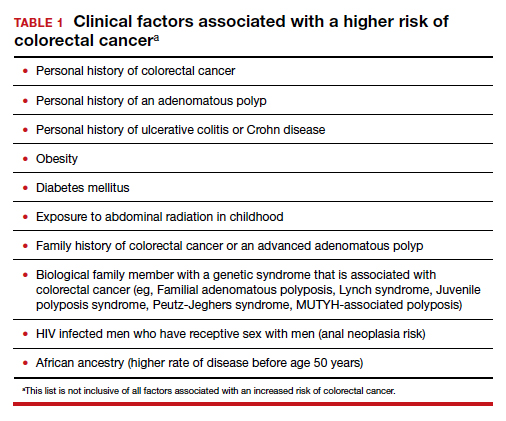
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.
Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
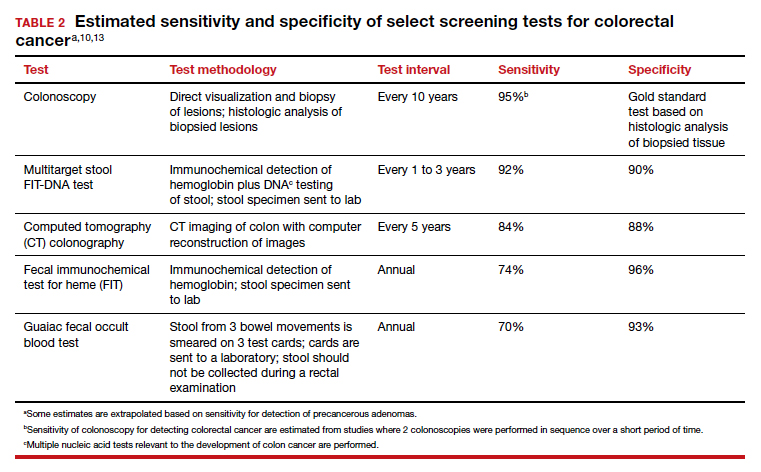
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).
In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.

Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).

In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.

Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).

In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
Endocrinologists’ wealth remains steady, despite pandemic
Despite ongoing pandemic-related economic challenges, endocrinologists report stability in their overall wealth in the past year, with more than a third of the specialists having a net worth between $1 million and $5 million, according to the Medscape Endocrinologist Wealth & Debt Report 2021.
The findings regarding wealth and debt among endocrinologists, along with 28 other specialties, were reported as part of Medscape’s Physician Compensation Report 2021, which included nearly 18,000 physicians.
According to the report, endocrinologists had an upswing in their income, compared with the prior year, with average annual earnings of $245,000 versus $236,000 in 2020. The earnings tie them with infectious disease specialists at fourth from the bottom of the list of specialties.
In the latest report, 38% reported a net worth between $1 million and $5 million, down 1% from 39% in last year’s report.
Nine percent of endocrinologists had a net worth of over $5 million, matching last year’s rate.
That puts endocrinologists and rheumatologists near the middle of specialists earning more than $5 million. Dermatologists rank the highest, with 28% worth over $5 million. Allergy and immunology specialists are at the bottom of the list, with just 2%.
Joel Greenwald, MD, a wealth management advisor to physicians based in St. Louis Park, Minn., said the reasons for the stability in wealth are multifactorial.
“The rise in home prices is certainly a factor,” he said. “Definitely the rise in the stock market played a large role; the S&P 500 finished the year up over 18%.
“I’ve seen clients accumulate cash, which has added to their net worth,” Dr. Greenwald added. “They cut back on spending because they were worried about big declines in income and also because there was simply less to spend money on [during lockdowns].”
The percentage of endocrinologists reporting a net worth below $500,000 decreased from 37% in 2020 to 31% for the current report, placing them fifth from the top of the list of specialists with a net worth below $500,000. Family medicine was at the top of the list, at 40%.
Gender disparities in net worth are striking
The gender disparities in net worth among endocrinologists are substantial. Although only 15% of male endocrinologists have a net worth of less than $500,000, that rate is nearly three times higher – 44% – for female endocrinologists.
Twenty-seven percent of male endocrinologists have a net worth between $1 million and $2 million, compared with just 13% among women. Although 14% of men have a net worth of more than $5 million, only 4% of female endocrinologists fall in that category.
Of note, 61% of those who responded to the poll were men; 36% were women.
Expenses, savings
Only 6% of endocrinologists reported being unable to pay their mortgage as a result of the pandemic; 8% said they were unable to pay other bills because of COVID-19.
The vast majority, however – 91% – said the pandemic did not affect their ability to pay bills or their mortgage. U.S. Census Bureau data from last July show that about a quarter of adults (25.3%) missed a mortgage or rent payment because of challenges related to COVID-19.
Approximately three-quarters of endocrinologists (72%) reported having not made any changes to reduce major expenses in 2020, despite the pandemic. About 25% took significant measures to reduce expenses, including refinancing their house or moving to a different home.
Seventeen percent say they are still paying off their school loans, similar to the rate last year.
The report notes that, according to the Association of American Medical Colleges, the average medical school debt for students who graduated in 2019 was $201,490, compared with an average student loan debt for all graduating students in the same year of $28,950.
Although 65% of endocrinologists said they added the same amount to their 401(k) plan in the past year, 28% put less into their fund, and although 53% put the same amount into their taxable savings account, 23% reported not using the taxable savings accounts at all.
Although earnings were steady in the past year, 12% of endocrinologists report having losses from practice problems, compared with 5% the previous year. COVID-19 was the most common cause. The proportion reporting no financial losses declined to 65%, versus 75% in the last report.
A version of this article first appeared on Medscape.com.
Despite ongoing pandemic-related economic challenges, endocrinologists report stability in their overall wealth in the past year, with more than a third of the specialists having a net worth between $1 million and $5 million, according to the Medscape Endocrinologist Wealth & Debt Report 2021.
The findings regarding wealth and debt among endocrinologists, along with 28 other specialties, were reported as part of Medscape’s Physician Compensation Report 2021, which included nearly 18,000 physicians.
According to the report, endocrinologists had an upswing in their income, compared with the prior year, with average annual earnings of $245,000 versus $236,000 in 2020. The earnings tie them with infectious disease specialists at fourth from the bottom of the list of specialties.
In the latest report, 38% reported a net worth between $1 million and $5 million, down 1% from 39% in last year’s report.
Nine percent of endocrinologists had a net worth of over $5 million, matching last year’s rate.
That puts endocrinologists and rheumatologists near the middle of specialists earning more than $5 million. Dermatologists rank the highest, with 28% worth over $5 million. Allergy and immunology specialists are at the bottom of the list, with just 2%.
Joel Greenwald, MD, a wealth management advisor to physicians based in St. Louis Park, Minn., said the reasons for the stability in wealth are multifactorial.
“The rise in home prices is certainly a factor,” he said. “Definitely the rise in the stock market played a large role; the S&P 500 finished the year up over 18%.
“I’ve seen clients accumulate cash, which has added to their net worth,” Dr. Greenwald added. “They cut back on spending because they were worried about big declines in income and also because there was simply less to spend money on [during lockdowns].”
The percentage of endocrinologists reporting a net worth below $500,000 decreased from 37% in 2020 to 31% for the current report, placing them fifth from the top of the list of specialists with a net worth below $500,000. Family medicine was at the top of the list, at 40%.
Gender disparities in net worth are striking
The gender disparities in net worth among endocrinologists are substantial. Although only 15% of male endocrinologists have a net worth of less than $500,000, that rate is nearly three times higher – 44% – for female endocrinologists.
Twenty-seven percent of male endocrinologists have a net worth between $1 million and $2 million, compared with just 13% among women. Although 14% of men have a net worth of more than $5 million, only 4% of female endocrinologists fall in that category.
Of note, 61% of those who responded to the poll were men; 36% were women.
Expenses, savings
Only 6% of endocrinologists reported being unable to pay their mortgage as a result of the pandemic; 8% said they were unable to pay other bills because of COVID-19.
The vast majority, however – 91% – said the pandemic did not affect their ability to pay bills or their mortgage. U.S. Census Bureau data from last July show that about a quarter of adults (25.3%) missed a mortgage or rent payment because of challenges related to COVID-19.
Approximately three-quarters of endocrinologists (72%) reported having not made any changes to reduce major expenses in 2020, despite the pandemic. About 25% took significant measures to reduce expenses, including refinancing their house or moving to a different home.
Seventeen percent say they are still paying off their school loans, similar to the rate last year.
The report notes that, according to the Association of American Medical Colleges, the average medical school debt for students who graduated in 2019 was $201,490, compared with an average student loan debt for all graduating students in the same year of $28,950.
Although 65% of endocrinologists said they added the same amount to their 401(k) plan in the past year, 28% put less into their fund, and although 53% put the same amount into their taxable savings account, 23% reported not using the taxable savings accounts at all.
Although earnings were steady in the past year, 12% of endocrinologists report having losses from practice problems, compared with 5% the previous year. COVID-19 was the most common cause. The proportion reporting no financial losses declined to 65%, versus 75% in the last report.
A version of this article first appeared on Medscape.com.
Despite ongoing pandemic-related economic challenges, endocrinologists report stability in their overall wealth in the past year, with more than a third of the specialists having a net worth between $1 million and $5 million, according to the Medscape Endocrinologist Wealth & Debt Report 2021.
The findings regarding wealth and debt among endocrinologists, along with 28 other specialties, were reported as part of Medscape’s Physician Compensation Report 2021, which included nearly 18,000 physicians.
According to the report, endocrinologists had an upswing in their income, compared with the prior year, with average annual earnings of $245,000 versus $236,000 in 2020. The earnings tie them with infectious disease specialists at fourth from the bottom of the list of specialties.
In the latest report, 38% reported a net worth between $1 million and $5 million, down 1% from 39% in last year’s report.
Nine percent of endocrinologists had a net worth of over $5 million, matching last year’s rate.
That puts endocrinologists and rheumatologists near the middle of specialists earning more than $5 million. Dermatologists rank the highest, with 28% worth over $5 million. Allergy and immunology specialists are at the bottom of the list, with just 2%.
Joel Greenwald, MD, a wealth management advisor to physicians based in St. Louis Park, Minn., said the reasons for the stability in wealth are multifactorial.
“The rise in home prices is certainly a factor,” he said. “Definitely the rise in the stock market played a large role; the S&P 500 finished the year up over 18%.
“I’ve seen clients accumulate cash, which has added to their net worth,” Dr. Greenwald added. “They cut back on spending because they were worried about big declines in income and also because there was simply less to spend money on [during lockdowns].”
The percentage of endocrinologists reporting a net worth below $500,000 decreased from 37% in 2020 to 31% for the current report, placing them fifth from the top of the list of specialists with a net worth below $500,000. Family medicine was at the top of the list, at 40%.
Gender disparities in net worth are striking
The gender disparities in net worth among endocrinologists are substantial. Although only 15% of male endocrinologists have a net worth of less than $500,000, that rate is nearly three times higher – 44% – for female endocrinologists.
Twenty-seven percent of male endocrinologists have a net worth between $1 million and $2 million, compared with just 13% among women. Although 14% of men have a net worth of more than $5 million, only 4% of female endocrinologists fall in that category.
Of note, 61% of those who responded to the poll were men; 36% were women.
Expenses, savings
Only 6% of endocrinologists reported being unable to pay their mortgage as a result of the pandemic; 8% said they were unable to pay other bills because of COVID-19.
The vast majority, however – 91% – said the pandemic did not affect their ability to pay bills or their mortgage. U.S. Census Bureau data from last July show that about a quarter of adults (25.3%) missed a mortgage or rent payment because of challenges related to COVID-19.
Approximately three-quarters of endocrinologists (72%) reported having not made any changes to reduce major expenses in 2020, despite the pandemic. About 25% took significant measures to reduce expenses, including refinancing their house or moving to a different home.
Seventeen percent say they are still paying off their school loans, similar to the rate last year.
The report notes that, according to the Association of American Medical Colleges, the average medical school debt for students who graduated in 2019 was $201,490, compared with an average student loan debt for all graduating students in the same year of $28,950.
Although 65% of endocrinologists said they added the same amount to their 401(k) plan in the past year, 28% put less into their fund, and although 53% put the same amount into their taxable savings account, 23% reported not using the taxable savings accounts at all.
Although earnings were steady in the past year, 12% of endocrinologists report having losses from practice problems, compared with 5% the previous year. COVID-19 was the most common cause. The proportion reporting no financial losses declined to 65%, versus 75% in the last report.
A version of this article first appeared on Medscape.com.
Brain memory signals appear to regulate metabolism
Rhythmic brain signals that help encode memories also appear to influence blood sugar levels and may regulate the timing of the release of hormones, early, pre-clinical research shows.
“Our study is the first to show how clusters of brain cell firing in the hippocampus may directly regulate metabolism,” senior author György Buzsáki, MD, PhD, professor, department of neuroscience and physiology, NYU Grossman School of Medicine and NYU Langone Health, said in a news release.
“Evidence suggests that the brain evolved, for reasons of efficiency, to use the same signals to achieve two very different functions in terms of memory and hormonal regulation,” added corresponding author David Tingley, PhD, a post-doctoral scholar in Dr. Buzsáki’s lab.
The study was published online August 11 in Nature.
It’s recently been discovered that populations of hippocampal neurons fire within milliseconds of each other in cycles. This firing pattern is called a “sharp wave ripple” for the shape it takes when captured graphically by electroencephalogram.
In their study, Dr. Buzsáki, Dr. Tingley, and colleagues observed that clusters of sharp wave ripples recorded from the hippocampus of rats were “reliably” and rapidly, followed by decreases in blood sugar concentrations in the animals.
“This correlation was not dependent on circadian, ultradian, or meal-triggered fluctuations; it could be mimicked with optogenetically induced ripples in the hippocampus, but not in the parietal cortex, and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum (LS), the major conduit between the hippocampus and hypothalamus,” the researchers report.
These observations suggest that hippocampal sharp wave ripples may regulate the timing of the release of hormones, possibly including insulin, by the pancreas and liver, as well as other hormones by the pituitary gland, the researchers note.
As sharp wave ripples mostly occur during non-rapid eye movement sleep, the impact of sleep disturbance on sharp wave ripples may provide a mechanistic link between poor sleep and high blood sugar levels seen in type 2 diabetes, they suggest.
“There are a couple of experimental studies showing that if you deprive a young healthy person from sleep [for 48 hours], their glucose tolerance resembles” that of a person with diabetes, Dr. Buzsáki noted in an interview.
Moving forward, the researchers will seek to extend their theory that several hormones could be affected by nightly sharp wave ripples.
The research was funded by National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rhythmic brain signals that help encode memories also appear to influence blood sugar levels and may regulate the timing of the release of hormones, early, pre-clinical research shows.
“Our study is the first to show how clusters of brain cell firing in the hippocampus may directly regulate metabolism,” senior author György Buzsáki, MD, PhD, professor, department of neuroscience and physiology, NYU Grossman School of Medicine and NYU Langone Health, said in a news release.
“Evidence suggests that the brain evolved, for reasons of efficiency, to use the same signals to achieve two very different functions in terms of memory and hormonal regulation,” added corresponding author David Tingley, PhD, a post-doctoral scholar in Dr. Buzsáki’s lab.
The study was published online August 11 in Nature.
It’s recently been discovered that populations of hippocampal neurons fire within milliseconds of each other in cycles. This firing pattern is called a “sharp wave ripple” for the shape it takes when captured graphically by electroencephalogram.
In their study, Dr. Buzsáki, Dr. Tingley, and colleagues observed that clusters of sharp wave ripples recorded from the hippocampus of rats were “reliably” and rapidly, followed by decreases in blood sugar concentrations in the animals.
“This correlation was not dependent on circadian, ultradian, or meal-triggered fluctuations; it could be mimicked with optogenetically induced ripples in the hippocampus, but not in the parietal cortex, and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum (LS), the major conduit between the hippocampus and hypothalamus,” the researchers report.
These observations suggest that hippocampal sharp wave ripples may regulate the timing of the release of hormones, possibly including insulin, by the pancreas and liver, as well as other hormones by the pituitary gland, the researchers note.
As sharp wave ripples mostly occur during non-rapid eye movement sleep, the impact of sleep disturbance on sharp wave ripples may provide a mechanistic link between poor sleep and high blood sugar levels seen in type 2 diabetes, they suggest.
“There are a couple of experimental studies showing that if you deprive a young healthy person from sleep [for 48 hours], their glucose tolerance resembles” that of a person with diabetes, Dr. Buzsáki noted in an interview.
Moving forward, the researchers will seek to extend their theory that several hormones could be affected by nightly sharp wave ripples.
The research was funded by National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rhythmic brain signals that help encode memories also appear to influence blood sugar levels and may regulate the timing of the release of hormones, early, pre-clinical research shows.
“Our study is the first to show how clusters of brain cell firing in the hippocampus may directly regulate metabolism,” senior author György Buzsáki, MD, PhD, professor, department of neuroscience and physiology, NYU Grossman School of Medicine and NYU Langone Health, said in a news release.
“Evidence suggests that the brain evolved, for reasons of efficiency, to use the same signals to achieve two very different functions in terms of memory and hormonal regulation,” added corresponding author David Tingley, PhD, a post-doctoral scholar in Dr. Buzsáki’s lab.
The study was published online August 11 in Nature.
It’s recently been discovered that populations of hippocampal neurons fire within milliseconds of each other in cycles. This firing pattern is called a “sharp wave ripple” for the shape it takes when captured graphically by electroencephalogram.
In their study, Dr. Buzsáki, Dr. Tingley, and colleagues observed that clusters of sharp wave ripples recorded from the hippocampus of rats were “reliably” and rapidly, followed by decreases in blood sugar concentrations in the animals.
“This correlation was not dependent on circadian, ultradian, or meal-triggered fluctuations; it could be mimicked with optogenetically induced ripples in the hippocampus, but not in the parietal cortex, and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum (LS), the major conduit between the hippocampus and hypothalamus,” the researchers report.
These observations suggest that hippocampal sharp wave ripples may regulate the timing of the release of hormones, possibly including insulin, by the pancreas and liver, as well as other hormones by the pituitary gland, the researchers note.
As sharp wave ripples mostly occur during non-rapid eye movement sleep, the impact of sleep disturbance on sharp wave ripples may provide a mechanistic link between poor sleep and high blood sugar levels seen in type 2 diabetes, they suggest.
“There are a couple of experimental studies showing that if you deprive a young healthy person from sleep [for 48 hours], their glucose tolerance resembles” that of a person with diabetes, Dr. Buzsáki noted in an interview.
Moving forward, the researchers will seek to extend their theory that several hormones could be affected by nightly sharp wave ripples.
The research was funded by National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves first drug for idiopathic hypersomnia
, the company announced in a news release.
It marks the second approval for Xywav. The FDA approved it last year for the treatment of cataplexy or excessive daytime sleepiness in patients with narcolepsy as young as 7 years of age.
This recent approval is the first for a treatment for idiopathic hypersomnia.
“Idiopathic hypersomnia can have a significant impact on the social, educational, and occupational functioning of people living with the condition,” Diane Powell, board chair and CEO of the Hypersomnia Foundation, noted in the release.
This FDA approval “is a major milestone for the entire idiopathic hypersomnia community as Xywav becomes the first medicine approved to manage this chronic sleep disorder,” said Ms. Powell.
Low sodium oxybate product
Xywav is a novel oxybate product with a unique composition of cations. It contains 92% less sodium than sodium oxybate (Xyrem) at the recommended adult dosage range of 6 to 9 g, the company noted in a news release.
An estimated 37,000 people in the United States have been diagnosed with idiopathic hypersomnia, a neurologic sleep disorder characterized by chronic excessive daytime sleepiness.
Other symptoms of the disorder may include severe sleep inertia or sleep drunkenness (prolonged difficulty waking with frequent re-entries into sleep, confusion, and irritability), as well as prolonged, nonrestorative night-time sleep, cognitive impairment, and long and unrefreshing naps.
The approval was based on findings from a phase 3, double-blind, multicenter, placebo-controlled, randomized withdrawal study.
Results showed “statistically significant and clinically meaningful” differences compared with placebo in change in the primary endpoint of Epworth Sleepiness Scale score (P < .0001) and the secondary endpoints of Patient Global Impression of Change (P < .0001) and the Idiopathic Hypersomnia Severity Scale (P < .0001), the company reported.
The most common adverse reactions were nausea, headache, dizziness, anxiety, insomnia, decreased appetite, hyperhidrosis, vomiting, diarrhea, dry mouth, parasomnia, somnolence, fatigue, and tremor.
The novel agent can be administered once or twice nightly for the treatment of idiopathic hypersomnia in adults.
“To optimize response, a patient’s health care provider may consider prescribing a twice-nightly regimen in equally or unequally divided doses at bedtime and 2.5 to 4 hours later and gradually titrate Xywav so that a patient may receive an individualized dose and regimen based on efficacy and tolerability,” the company said.
Xywav carries a boxed warning because it is a central nervous system depressant and because there is potential for abuse and misuse. The drug is only available through a risk evaluation and mitigation strategy (REMS) program.
The company plans to make Xywav available to patients with idiopathic hypersomnia later this year following implementation of the REMS program.
A version of this article first appeared on Medscape.com.
, the company announced in a news release.
It marks the second approval for Xywav. The FDA approved it last year for the treatment of cataplexy or excessive daytime sleepiness in patients with narcolepsy as young as 7 years of age.
This recent approval is the first for a treatment for idiopathic hypersomnia.
“Idiopathic hypersomnia can have a significant impact on the social, educational, and occupational functioning of people living with the condition,” Diane Powell, board chair and CEO of the Hypersomnia Foundation, noted in the release.
This FDA approval “is a major milestone for the entire idiopathic hypersomnia community as Xywav becomes the first medicine approved to manage this chronic sleep disorder,” said Ms. Powell.
Low sodium oxybate product
Xywav is a novel oxybate product with a unique composition of cations. It contains 92% less sodium than sodium oxybate (Xyrem) at the recommended adult dosage range of 6 to 9 g, the company noted in a news release.
An estimated 37,000 people in the United States have been diagnosed with idiopathic hypersomnia, a neurologic sleep disorder characterized by chronic excessive daytime sleepiness.
Other symptoms of the disorder may include severe sleep inertia or sleep drunkenness (prolonged difficulty waking with frequent re-entries into sleep, confusion, and irritability), as well as prolonged, nonrestorative night-time sleep, cognitive impairment, and long and unrefreshing naps.
The approval was based on findings from a phase 3, double-blind, multicenter, placebo-controlled, randomized withdrawal study.
Results showed “statistically significant and clinically meaningful” differences compared with placebo in change in the primary endpoint of Epworth Sleepiness Scale score (P < .0001) and the secondary endpoints of Patient Global Impression of Change (P < .0001) and the Idiopathic Hypersomnia Severity Scale (P < .0001), the company reported.
The most common adverse reactions were nausea, headache, dizziness, anxiety, insomnia, decreased appetite, hyperhidrosis, vomiting, diarrhea, dry mouth, parasomnia, somnolence, fatigue, and tremor.
The novel agent can be administered once or twice nightly for the treatment of idiopathic hypersomnia in adults.
“To optimize response, a patient’s health care provider may consider prescribing a twice-nightly regimen in equally or unequally divided doses at bedtime and 2.5 to 4 hours later and gradually titrate Xywav so that a patient may receive an individualized dose and regimen based on efficacy and tolerability,” the company said.
Xywav carries a boxed warning because it is a central nervous system depressant and because there is potential for abuse and misuse. The drug is only available through a risk evaluation and mitigation strategy (REMS) program.
The company plans to make Xywav available to patients with idiopathic hypersomnia later this year following implementation of the REMS program.
A version of this article first appeared on Medscape.com.
, the company announced in a news release.
It marks the second approval for Xywav. The FDA approved it last year for the treatment of cataplexy or excessive daytime sleepiness in patients with narcolepsy as young as 7 years of age.
This recent approval is the first for a treatment for idiopathic hypersomnia.
“Idiopathic hypersomnia can have a significant impact on the social, educational, and occupational functioning of people living with the condition,” Diane Powell, board chair and CEO of the Hypersomnia Foundation, noted in the release.
This FDA approval “is a major milestone for the entire idiopathic hypersomnia community as Xywav becomes the first medicine approved to manage this chronic sleep disorder,” said Ms. Powell.
Low sodium oxybate product
Xywav is a novel oxybate product with a unique composition of cations. It contains 92% less sodium than sodium oxybate (Xyrem) at the recommended adult dosage range of 6 to 9 g, the company noted in a news release.
An estimated 37,000 people in the United States have been diagnosed with idiopathic hypersomnia, a neurologic sleep disorder characterized by chronic excessive daytime sleepiness.
Other symptoms of the disorder may include severe sleep inertia or sleep drunkenness (prolonged difficulty waking with frequent re-entries into sleep, confusion, and irritability), as well as prolonged, nonrestorative night-time sleep, cognitive impairment, and long and unrefreshing naps.
The approval was based on findings from a phase 3, double-blind, multicenter, placebo-controlled, randomized withdrawal study.
Results showed “statistically significant and clinically meaningful” differences compared with placebo in change in the primary endpoint of Epworth Sleepiness Scale score (P < .0001) and the secondary endpoints of Patient Global Impression of Change (P < .0001) and the Idiopathic Hypersomnia Severity Scale (P < .0001), the company reported.
The most common adverse reactions were nausea, headache, dizziness, anxiety, insomnia, decreased appetite, hyperhidrosis, vomiting, diarrhea, dry mouth, parasomnia, somnolence, fatigue, and tremor.
The novel agent can be administered once or twice nightly for the treatment of idiopathic hypersomnia in adults.
“To optimize response, a patient’s health care provider may consider prescribing a twice-nightly regimen in equally or unequally divided doses at bedtime and 2.5 to 4 hours later and gradually titrate Xywav so that a patient may receive an individualized dose and regimen based on efficacy and tolerability,” the company said.
Xywav carries a boxed warning because it is a central nervous system depressant and because there is potential for abuse and misuse. The drug is only available through a risk evaluation and mitigation strategy (REMS) program.
The company plans to make Xywav available to patients with idiopathic hypersomnia later this year following implementation of the REMS program.
A version of this article first appeared on Medscape.com.
MR elastography could predict cirrhosis in NAFLD
Liver stiffness measurement with magnetic resonance elastography (MRE) may prove predictive of future cirrhosis risk in patients with nonalcoholic fatty liver disease (NAFLD), according to researchers from the Mayo Clinic in Rochester, Minn.
“These data expand the role of MRE from an accurate diagnostic method to a prognostic noninvasive imaging biomarker that can risk-stratify patients with NAFLD and guide the timing of surveillance and further refine their clinical management,” wrote Tolga Gidener, MD, and colleagues. The study authors added that the research further expands “the role of MRE beyond liver fibrosis estimation by adding a predictive feature to improve individualized disease monitoring and patient counseling.” Their study was published in Clinical Gastroenterology and Hepatology.
Currently, there are no established noninvasive strategies that can effectively identify patients with NAFLD who are at high risk of progression to cirrhosis and liver-related complications. While fibrosis stage on histology may predict liver-associated outcomes in these patients, this approach is invasive, time consuming, and is generally not well tolerated by patients.
Although the technique has been noted for its high success rate and excellent levels of reliability and reproducibility, a possible limitation of MRE is its cost. That said, standalone MRE is reimbursed under Medicare Category I Current Procedural Terminology code 76391 with a cost of $240.02. However, there is also a lack of data on whether baseline liver stiffness measurement by MRE can predict progression of NAFLD to cirrhosis.
To gauge the role of baseline liver stiffness measurement by MRE, Dr. Gidener and colleagues performed a retrospective cohort study that evaluated hard liver–related outcomes in 829 adult patients with NAFLD with or without cirrhosis (median age, 58 years; 54% female) who underwent MRE during 2007-2019.
Patients in the study were followed from the first MRE until death, last clinical encounter, or the end of the study. Clinical outcomes assessed in individual chart review included cirrhosis, hepatic decompensation, and death.
At baseline, the median liver stiffness measurement was 2.8 kPa in 639 patients with NAFLD but without cirrhosis. Over a median 4-year follow-up period, a total of 20 patients developed cirrhosis, with an overall annual incidence rate of 1%.
Baseline liver stiffness measurement by MRE was significantly predictive of subsequent cirrhosis (hazard ratio, 2.93; 95% confidence interval, 1.86-4.62; P < .0001) per 1-kPa difference in liver stiffness measurement at baseline.
According to the researchers, the probability of future cirrhosis development can be ascertained using current liver stiffness measurement. As such, a greater than 1% probability threshold can be reached in 5 years in patients with a measurement of 2 kPa, 3 years in patients with a measurement of 3 kPA, and 1 year in patients with 4-5 kPa. “These time frames inform about estimated time to progression to hard outcomes and provide guidance for subsequent noninvasive monitoring for disease progression,” wrote the researchers.
The baseline liver stiffness measurement by MRE was also significantly predictive of future hepatic decompensation or death (HR, 1.32; 95% CI, 1.13-1.56; P = .0007) per 1-kPa increment in the liver stiffness measurement. Likewise, the 1-year probability of subsequent hepatic decompensation or death in patients with cirrhosis and baseline liver stiffness measurement of 5 kPa versus 8 kPa was 9% versus 20%, respectively. In terms of covariates, age was the only factor that increased the risk of hepatic decompensation or death.
While the current study offers a glimpse into the potential clinical implications of liver stiffness measurement by MRE in NAFLD, the researchers suggest the applicability of the findings are limited by the study’s small sample size, relatively short follow-up duration, and the small number of cirrhosis events.
The researchers received study funding from the National Institute of Diabetes and Digestive and Kidney Diseases, American College of Gastroenterology, National Institutes of Health, and the Department of Defense. The researchers disclosed no other relevant conflicts of interest.
NAFLD is rapidly becoming one of the most common causes of liver disease. While most patients have a benign course, approximately 20% of patients develop nonalcoholic steatohepatitis, the progressive form of the disease. Given the high prevalence (30% of the U.S. population), it is vital to determine which patients are at risk for progression, cirrhosis, and decompensation. Although liver biopsy is the preferred method, this procedure is invasive and carries substantial risks, including severe bleeding. Noninvasive tests that measure liver stiffness have emerged: Examples are controlled elastography (VCTE), such as Fibroscan, and magnetic resonance elastography (MRE). Data support the use of liver stiffness as a surrogate measure of fibrosis; MRE has demonstrated higher fidelity and accuracy, compared with VCTE, while being limited because of cost and availability. However, there is a paucity of data regarding the use of liver stiffness to predict progression to cirrhosis or liver-related events.
This study by Dr. Gidener and colleagues highlights the use of MRE to evaluate liver stiffness measurements as a predictor for cirrhosis and decompensation. Baseline measurements more than 4-5 kPa should alert clinicians regarding increased risk of progression to cirrhosis. Patients with cirrhosis and baseline measurements of 8 kPa or higher have a high risk of decompensation/death, suggesting that they should be followed more closely. Given the burgeoning number of patients with NAFLD and NASH, this study demonstrates the importance of identifying high-risk patients in order to optimize use of resources and improve clinical outcomes.
Yamini Natarajan, MD, is an investigator at the Center for Innovations in Quality, Effectiveness and Safety at the Michael E. DeBakey VA Medical Center, Houston, and an assistant professor, department of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. She has no conflicts.
NAFLD is rapidly becoming one of the most common causes of liver disease. While most patients have a benign course, approximately 20% of patients develop nonalcoholic steatohepatitis, the progressive form of the disease. Given the high prevalence (30% of the U.S. population), it is vital to determine which patients are at risk for progression, cirrhosis, and decompensation. Although liver biopsy is the preferred method, this procedure is invasive and carries substantial risks, including severe bleeding. Noninvasive tests that measure liver stiffness have emerged: Examples are controlled elastography (VCTE), such as Fibroscan, and magnetic resonance elastography (MRE). Data support the use of liver stiffness as a surrogate measure of fibrosis; MRE has demonstrated higher fidelity and accuracy, compared with VCTE, while being limited because of cost and availability. However, there is a paucity of data regarding the use of liver stiffness to predict progression to cirrhosis or liver-related events.
This study by Dr. Gidener and colleagues highlights the use of MRE to evaluate liver stiffness measurements as a predictor for cirrhosis and decompensation. Baseline measurements more than 4-5 kPa should alert clinicians regarding increased risk of progression to cirrhosis. Patients with cirrhosis and baseline measurements of 8 kPa or higher have a high risk of decompensation/death, suggesting that they should be followed more closely. Given the burgeoning number of patients with NAFLD and NASH, this study demonstrates the importance of identifying high-risk patients in order to optimize use of resources and improve clinical outcomes.
Yamini Natarajan, MD, is an investigator at the Center for Innovations in Quality, Effectiveness and Safety at the Michael E. DeBakey VA Medical Center, Houston, and an assistant professor, department of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. She has no conflicts.
NAFLD is rapidly becoming one of the most common causes of liver disease. While most patients have a benign course, approximately 20% of patients develop nonalcoholic steatohepatitis, the progressive form of the disease. Given the high prevalence (30% of the U.S. population), it is vital to determine which patients are at risk for progression, cirrhosis, and decompensation. Although liver biopsy is the preferred method, this procedure is invasive and carries substantial risks, including severe bleeding. Noninvasive tests that measure liver stiffness have emerged: Examples are controlled elastography (VCTE), such as Fibroscan, and magnetic resonance elastography (MRE). Data support the use of liver stiffness as a surrogate measure of fibrosis; MRE has demonstrated higher fidelity and accuracy, compared with VCTE, while being limited because of cost and availability. However, there is a paucity of data regarding the use of liver stiffness to predict progression to cirrhosis or liver-related events.
This study by Dr. Gidener and colleagues highlights the use of MRE to evaluate liver stiffness measurements as a predictor for cirrhosis and decompensation. Baseline measurements more than 4-5 kPa should alert clinicians regarding increased risk of progression to cirrhosis. Patients with cirrhosis and baseline measurements of 8 kPa or higher have a high risk of decompensation/death, suggesting that they should be followed more closely. Given the burgeoning number of patients with NAFLD and NASH, this study demonstrates the importance of identifying high-risk patients in order to optimize use of resources and improve clinical outcomes.
Yamini Natarajan, MD, is an investigator at the Center for Innovations in Quality, Effectiveness and Safety at the Michael E. DeBakey VA Medical Center, Houston, and an assistant professor, department of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. She has no conflicts.
Liver stiffness measurement with magnetic resonance elastography (MRE) may prove predictive of future cirrhosis risk in patients with nonalcoholic fatty liver disease (NAFLD), according to researchers from the Mayo Clinic in Rochester, Minn.
“These data expand the role of MRE from an accurate diagnostic method to a prognostic noninvasive imaging biomarker that can risk-stratify patients with NAFLD and guide the timing of surveillance and further refine their clinical management,” wrote Tolga Gidener, MD, and colleagues. The study authors added that the research further expands “the role of MRE beyond liver fibrosis estimation by adding a predictive feature to improve individualized disease monitoring and patient counseling.” Their study was published in Clinical Gastroenterology and Hepatology.
Currently, there are no established noninvasive strategies that can effectively identify patients with NAFLD who are at high risk of progression to cirrhosis and liver-related complications. While fibrosis stage on histology may predict liver-associated outcomes in these patients, this approach is invasive, time consuming, and is generally not well tolerated by patients.
Although the technique has been noted for its high success rate and excellent levels of reliability and reproducibility, a possible limitation of MRE is its cost. That said, standalone MRE is reimbursed under Medicare Category I Current Procedural Terminology code 76391 with a cost of $240.02. However, there is also a lack of data on whether baseline liver stiffness measurement by MRE can predict progression of NAFLD to cirrhosis.
To gauge the role of baseline liver stiffness measurement by MRE, Dr. Gidener and colleagues performed a retrospective cohort study that evaluated hard liver–related outcomes in 829 adult patients with NAFLD with or without cirrhosis (median age, 58 years; 54% female) who underwent MRE during 2007-2019.
Patients in the study were followed from the first MRE until death, last clinical encounter, or the end of the study. Clinical outcomes assessed in individual chart review included cirrhosis, hepatic decompensation, and death.
At baseline, the median liver stiffness measurement was 2.8 kPa in 639 patients with NAFLD but without cirrhosis. Over a median 4-year follow-up period, a total of 20 patients developed cirrhosis, with an overall annual incidence rate of 1%.
Baseline liver stiffness measurement by MRE was significantly predictive of subsequent cirrhosis (hazard ratio, 2.93; 95% confidence interval, 1.86-4.62; P < .0001) per 1-kPa difference in liver stiffness measurement at baseline.
According to the researchers, the probability of future cirrhosis development can be ascertained using current liver stiffness measurement. As such, a greater than 1% probability threshold can be reached in 5 years in patients with a measurement of 2 kPa, 3 years in patients with a measurement of 3 kPA, and 1 year in patients with 4-5 kPa. “These time frames inform about estimated time to progression to hard outcomes and provide guidance for subsequent noninvasive monitoring for disease progression,” wrote the researchers.
The baseline liver stiffness measurement by MRE was also significantly predictive of future hepatic decompensation or death (HR, 1.32; 95% CI, 1.13-1.56; P = .0007) per 1-kPa increment in the liver stiffness measurement. Likewise, the 1-year probability of subsequent hepatic decompensation or death in patients with cirrhosis and baseline liver stiffness measurement of 5 kPa versus 8 kPa was 9% versus 20%, respectively. In terms of covariates, age was the only factor that increased the risk of hepatic decompensation or death.
While the current study offers a glimpse into the potential clinical implications of liver stiffness measurement by MRE in NAFLD, the researchers suggest the applicability of the findings are limited by the study’s small sample size, relatively short follow-up duration, and the small number of cirrhosis events.
The researchers received study funding from the National Institute of Diabetes and Digestive and Kidney Diseases, American College of Gastroenterology, National Institutes of Health, and the Department of Defense. The researchers disclosed no other relevant conflicts of interest.
Liver stiffness measurement with magnetic resonance elastography (MRE) may prove predictive of future cirrhosis risk in patients with nonalcoholic fatty liver disease (NAFLD), according to researchers from the Mayo Clinic in Rochester, Minn.
“These data expand the role of MRE from an accurate diagnostic method to a prognostic noninvasive imaging biomarker that can risk-stratify patients with NAFLD and guide the timing of surveillance and further refine their clinical management,” wrote Tolga Gidener, MD, and colleagues. The study authors added that the research further expands “the role of MRE beyond liver fibrosis estimation by adding a predictive feature to improve individualized disease monitoring and patient counseling.” Their study was published in Clinical Gastroenterology and Hepatology.
Currently, there are no established noninvasive strategies that can effectively identify patients with NAFLD who are at high risk of progression to cirrhosis and liver-related complications. While fibrosis stage on histology may predict liver-associated outcomes in these patients, this approach is invasive, time consuming, and is generally not well tolerated by patients.
Although the technique has been noted for its high success rate and excellent levels of reliability and reproducibility, a possible limitation of MRE is its cost. That said, standalone MRE is reimbursed under Medicare Category I Current Procedural Terminology code 76391 with a cost of $240.02. However, there is also a lack of data on whether baseline liver stiffness measurement by MRE can predict progression of NAFLD to cirrhosis.
To gauge the role of baseline liver stiffness measurement by MRE, Dr. Gidener and colleagues performed a retrospective cohort study that evaluated hard liver–related outcomes in 829 adult patients with NAFLD with or without cirrhosis (median age, 58 years; 54% female) who underwent MRE during 2007-2019.
Patients in the study were followed from the first MRE until death, last clinical encounter, or the end of the study. Clinical outcomes assessed in individual chart review included cirrhosis, hepatic decompensation, and death.
At baseline, the median liver stiffness measurement was 2.8 kPa in 639 patients with NAFLD but without cirrhosis. Over a median 4-year follow-up period, a total of 20 patients developed cirrhosis, with an overall annual incidence rate of 1%.
Baseline liver stiffness measurement by MRE was significantly predictive of subsequent cirrhosis (hazard ratio, 2.93; 95% confidence interval, 1.86-4.62; P < .0001) per 1-kPa difference in liver stiffness measurement at baseline.
According to the researchers, the probability of future cirrhosis development can be ascertained using current liver stiffness measurement. As such, a greater than 1% probability threshold can be reached in 5 years in patients with a measurement of 2 kPa, 3 years in patients with a measurement of 3 kPA, and 1 year in patients with 4-5 kPa. “These time frames inform about estimated time to progression to hard outcomes and provide guidance for subsequent noninvasive monitoring for disease progression,” wrote the researchers.
The baseline liver stiffness measurement by MRE was also significantly predictive of future hepatic decompensation or death (HR, 1.32; 95% CI, 1.13-1.56; P = .0007) per 1-kPa increment in the liver stiffness measurement. Likewise, the 1-year probability of subsequent hepatic decompensation or death in patients with cirrhosis and baseline liver stiffness measurement of 5 kPa versus 8 kPa was 9% versus 20%, respectively. In terms of covariates, age was the only factor that increased the risk of hepatic decompensation or death.
While the current study offers a glimpse into the potential clinical implications of liver stiffness measurement by MRE in NAFLD, the researchers suggest the applicability of the findings are limited by the study’s small sample size, relatively short follow-up duration, and the small number of cirrhosis events.
The researchers received study funding from the National Institute of Diabetes and Digestive and Kidney Diseases, American College of Gastroenterology, National Institutes of Health, and the Department of Defense. The researchers disclosed no other relevant conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
25% of patients with cancer lack immunity against measles
Before the onslaught of COVID-19, researchers at the Fred Hutchinson Cancer Research Center in Seattle had another infectious disease worry: an “unprecedented” outbreak of measles.
“In 2019, we saw the most measles cases in any year since the 1990s,” said Sara Marquis, MPH, a clinical research coordinator at the center. The worry, she says, was that various oncology treatments, such as bone marrow transplantations and assorted biologics, “may leave cancer patients severely immunosuppressed” and thus vulnerable to infectious diseases.
Measles-related illness is typically not severe but can lead to pneumonia, deafness, and death, even in immunocompetent people, Ms. Marquis added.
So in 2019, a team at Fred Hutchinson initiated a study to get a sense of immunity to measles among patients with cancer.
They now report that of a group of 900-plus patients, 25% lacked protective antibodies for measles. That’s “significantly more” than the general population, in which about 8% of people lack these antibodies, Ms. Marquis said.
The study, published online in JAMA Network Open, also found that 38% lacked protection against the less-worrisome infectious disease of mumps, which is more than the 13% found in the general population.
“The scary thing about measles is that it is one of the most contagious diseases known,” Ms. Marquis told this news organization, adding that it is about twice as contagious as the COVID-19 Delta variant.
And it’s not just in the state of Washington. “We’re seeing it more and more in the community,” as various outbreaks continue to happen, she said.
“Deficits in protective antibodies underscore patients’ increased risk during outbreaks and emphasize the need for community-based efforts to increase herd immunity to protect this population,” the study authors conclude.
In short, administration of the measles-mumps-rubella (MMR) vaccine, introduced in 1963, must continue universally, they said
“We’ve had so many incredible advances in cancer treatment in recent years. … it would be devastating to see something like measles, which is a vaccine-preventable disease, come through and negate those efforts,” said study coauthor Elizabeth Krantz, MS, a biostatistician at Fred Hutchinson.
The health care teams and family caregivers of patients with cancer should also make sure they are vaccinated, said Ms. Marquis. However, some patients may not be able to get a measles booster vaccine because it is a live vaccine or because they cannot generate enough antibodies for it to be protective, she explained.
Three subgroups more likely to have deficits
The new study, which is one of the first to measure measles and mumps seroprevalence among patients with cancer in the modern era of cancer treatment, also identified three subgroups that more commonly had immunity deficits: those aged 30-59 years; those with hematologic malignant neoplasms, and those who had received a hematopoietic cell transplant.
In the study, residual clinical plasma samples were obtained from 959 consecutive patients with cancer at Seattle Cancer Care Alliance and Fred Hutchinson in August 2019. These samples were tested for measles and mumps IgG by using a commercial enzyme-linked immunosorbent assay. In all, 60% of patients had a solid tumor and 40% had a blood cancer.
As noted above, the seroprevalence of measles antibodies was 0.75 and the seroprevalence of mumps antibodies was 0.62.
A study author explained why the study included mumps, a less threatening infection.
“We assessed mumps in this study out of interest to compare response in the MMR vaccine component – particularly as we could assess a potent vaccine (measles) versus one that has a weaker immunologic response (mumps). We remain worried about outbreaks of mumps as MMR vaccination rates drop across the U.S.,” wrote Steven Pergam, MD, MPH, infectious disease specialist at Fred Hutchinson, in an email.
Vaccination vigilance is one of the study’s messages. “We all need to do our part to make sure we are up to date with our vaccinations so we can make sure we protect those who are vulnerable,” said Ms. Krantz.
The study was funded by the National Cancer Institute and Seattle Cancer Care Alliance. Multiple study authors have ties to pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Before the onslaught of COVID-19, researchers at the Fred Hutchinson Cancer Research Center in Seattle had another infectious disease worry: an “unprecedented” outbreak of measles.
“In 2019, we saw the most measles cases in any year since the 1990s,” said Sara Marquis, MPH, a clinical research coordinator at the center. The worry, she says, was that various oncology treatments, such as bone marrow transplantations and assorted biologics, “may leave cancer patients severely immunosuppressed” and thus vulnerable to infectious diseases.
Measles-related illness is typically not severe but can lead to pneumonia, deafness, and death, even in immunocompetent people, Ms. Marquis added.
So in 2019, a team at Fred Hutchinson initiated a study to get a sense of immunity to measles among patients with cancer.
They now report that of a group of 900-plus patients, 25% lacked protective antibodies for measles. That’s “significantly more” than the general population, in which about 8% of people lack these antibodies, Ms. Marquis said.
The study, published online in JAMA Network Open, also found that 38% lacked protection against the less-worrisome infectious disease of mumps, which is more than the 13% found in the general population.
“The scary thing about measles is that it is one of the most contagious diseases known,” Ms. Marquis told this news organization, adding that it is about twice as contagious as the COVID-19 Delta variant.
And it’s not just in the state of Washington. “We’re seeing it more and more in the community,” as various outbreaks continue to happen, she said.
“Deficits in protective antibodies underscore patients’ increased risk during outbreaks and emphasize the need for community-based efforts to increase herd immunity to protect this population,” the study authors conclude.
In short, administration of the measles-mumps-rubella (MMR) vaccine, introduced in 1963, must continue universally, they said
“We’ve had so many incredible advances in cancer treatment in recent years. … it would be devastating to see something like measles, which is a vaccine-preventable disease, come through and negate those efforts,” said study coauthor Elizabeth Krantz, MS, a biostatistician at Fred Hutchinson.
The health care teams and family caregivers of patients with cancer should also make sure they are vaccinated, said Ms. Marquis. However, some patients may not be able to get a measles booster vaccine because it is a live vaccine or because they cannot generate enough antibodies for it to be protective, she explained.
Three subgroups more likely to have deficits
The new study, which is one of the first to measure measles and mumps seroprevalence among patients with cancer in the modern era of cancer treatment, also identified three subgroups that more commonly had immunity deficits: those aged 30-59 years; those with hematologic malignant neoplasms, and those who had received a hematopoietic cell transplant.
In the study, residual clinical plasma samples were obtained from 959 consecutive patients with cancer at Seattle Cancer Care Alliance and Fred Hutchinson in August 2019. These samples were tested for measles and mumps IgG by using a commercial enzyme-linked immunosorbent assay. In all, 60% of patients had a solid tumor and 40% had a blood cancer.
As noted above, the seroprevalence of measles antibodies was 0.75 and the seroprevalence of mumps antibodies was 0.62.
A study author explained why the study included mumps, a less threatening infection.
“We assessed mumps in this study out of interest to compare response in the MMR vaccine component – particularly as we could assess a potent vaccine (measles) versus one that has a weaker immunologic response (mumps). We remain worried about outbreaks of mumps as MMR vaccination rates drop across the U.S.,” wrote Steven Pergam, MD, MPH, infectious disease specialist at Fred Hutchinson, in an email.
Vaccination vigilance is one of the study’s messages. “We all need to do our part to make sure we are up to date with our vaccinations so we can make sure we protect those who are vulnerable,” said Ms. Krantz.
The study was funded by the National Cancer Institute and Seattle Cancer Care Alliance. Multiple study authors have ties to pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Before the onslaught of COVID-19, researchers at the Fred Hutchinson Cancer Research Center in Seattle had another infectious disease worry: an “unprecedented” outbreak of measles.
“In 2019, we saw the most measles cases in any year since the 1990s,” said Sara Marquis, MPH, a clinical research coordinator at the center. The worry, she says, was that various oncology treatments, such as bone marrow transplantations and assorted biologics, “may leave cancer patients severely immunosuppressed” and thus vulnerable to infectious diseases.
Measles-related illness is typically not severe but can lead to pneumonia, deafness, and death, even in immunocompetent people, Ms. Marquis added.
So in 2019, a team at Fred Hutchinson initiated a study to get a sense of immunity to measles among patients with cancer.
They now report that of a group of 900-plus patients, 25% lacked protective antibodies for measles. That’s “significantly more” than the general population, in which about 8% of people lack these antibodies, Ms. Marquis said.
The study, published online in JAMA Network Open, also found that 38% lacked protection against the less-worrisome infectious disease of mumps, which is more than the 13% found in the general population.
“The scary thing about measles is that it is one of the most contagious diseases known,” Ms. Marquis told this news organization, adding that it is about twice as contagious as the COVID-19 Delta variant.
And it’s not just in the state of Washington. “We’re seeing it more and more in the community,” as various outbreaks continue to happen, she said.
“Deficits in protective antibodies underscore patients’ increased risk during outbreaks and emphasize the need for community-based efforts to increase herd immunity to protect this population,” the study authors conclude.
In short, administration of the measles-mumps-rubella (MMR) vaccine, introduced in 1963, must continue universally, they said
“We’ve had so many incredible advances in cancer treatment in recent years. … it would be devastating to see something like measles, which is a vaccine-preventable disease, come through and negate those efforts,” said study coauthor Elizabeth Krantz, MS, a biostatistician at Fred Hutchinson.
The health care teams and family caregivers of patients with cancer should also make sure they are vaccinated, said Ms. Marquis. However, some patients may not be able to get a measles booster vaccine because it is a live vaccine or because they cannot generate enough antibodies for it to be protective, she explained.
Three subgroups more likely to have deficits
The new study, which is one of the first to measure measles and mumps seroprevalence among patients with cancer in the modern era of cancer treatment, also identified three subgroups that more commonly had immunity deficits: those aged 30-59 years; those with hematologic malignant neoplasms, and those who had received a hematopoietic cell transplant.
In the study, residual clinical plasma samples were obtained from 959 consecutive patients with cancer at Seattle Cancer Care Alliance and Fred Hutchinson in August 2019. These samples were tested for measles and mumps IgG by using a commercial enzyme-linked immunosorbent assay. In all, 60% of patients had a solid tumor and 40% had a blood cancer.
As noted above, the seroprevalence of measles antibodies was 0.75 and the seroprevalence of mumps antibodies was 0.62.
A study author explained why the study included mumps, a less threatening infection.
“We assessed mumps in this study out of interest to compare response in the MMR vaccine component – particularly as we could assess a potent vaccine (measles) versus one that has a weaker immunologic response (mumps). We remain worried about outbreaks of mumps as MMR vaccination rates drop across the U.S.,” wrote Steven Pergam, MD, MPH, infectious disease specialist at Fred Hutchinson, in an email.
Vaccination vigilance is one of the study’s messages. “We all need to do our part to make sure we are up to date with our vaccinations so we can make sure we protect those who are vulnerable,” said Ms. Krantz.
The study was funded by the National Cancer Institute and Seattle Cancer Care Alliance. Multiple study authors have ties to pharmaceutical companies.
A version of this article first appeared on Medscape.com.
CDC reports Burkholderia cepacia and B. pseudomallei outbreaks
The Centers for Disease Control and Prevention and Food and Drug Administration have announced an outbreak of at least 15 Burkholderia cepacia infections associated with contaminated ultrasound gel used to guide invasive procedures as well as an unrelated outbreak of Burkholderia pseudomallei that caused two deaths.
The procedures involved in the B. cepacia outbreak included placement of both central and peripheral intravenous catheters and paracentesis (removal of peritoneal fluid from the abdominal cavity). Cases have occurred in several states.
Further testing has shown the presence of Burkholderia stabilis, a member of B. cepacia complex (Bcc), in four lots of unopened bottles of MediChoice M500812 ultrasound gel. Eco-Med Pharmaceuticals of Etobicoke, Ont., the parent manufacturer, has issued a recall of MediChoice M500812 or Eco-Gel 200 with the following lot numbers: B029, B030, B031, B032, B040, B041, B048, B055. A similar outbreak occurred in Canada.
Some of these cases resulted in bloodstream infections. Further details are not yet available. Bcc infections have ranged from asymptomatic to life-threatening pneumonias, particularly in patients with cystic fibrosis. Other risk factors include immunosuppression, mechanical ventilation, and the use of other invasive venous or urinary catheters.
Kiran M. Perkins, MD, MPH, outbreak lead with the CDC’s Prevention Research Branch, said in an interview via email that automated systems such as Vitek might have trouble identifying the organism as “the system may only reveal the microbial species at the genus level but not at the species level, and/or it may have difficulty distinguishing between members of closely related group members.”
In the CDC’s experience, “most facilities do not conduct further species identification.” The agency added that it cannot tell if there has been any increase in cases associated with COVID-19, as they are not notifiable diseases and the “CDC does not systematically collect information on B. cepacia complex infections.”
Rodney Rohde, PhD, professor of clinical laboratory science and chair of the clinical laboratory science program, Texas State University, San Marcos, told this news organization via email that Burkholderia’s “detection in the manufacturing process is difficult, and product recalls are frequent.” He added, “A recent review by the Food and Drug Administration in the U.S. found that almost 40% of contamination reports in both sterile and nonsterile pharmaceutical products were caused by Bcc bacteria.” Another problem is that they often create biofilms, so “they are tenacious environmental colonizers of medical equipment and surfaces in general.”
There have been many other outbreaks as a result to B. cepacia complex. Because it is often in the water supply used in pharmaceutical manufacturing and is resistant to preservatives, the FDA cautions that it poses a risk of contamination in all nonsterile, water-based drug products.
Recalls have included contaminated antiseptics, such as povidone iodine, benzalkonium chloride, and chlorhexidine gluconate. Contamination in manufacturing may not be uniform, and only some samples may be affected. Antiseptic mouthwashes have also been affected. So have nonbacterial soaps and docusate (a stool softener) solutions, and various personal care products, including nasal sprays, lotions, simethicone gas relief drops (Mylicon), and baby wipes.
Although Bcc are considered “objectionable organisms,” there have been no strong or consistent standards for their detection from the U.S. Pharmacopeia, and some manufacturers reportedly underestimate the consequences of contamination. The FDA issued a guidance to manufacturers in 2017 on quality assurance and cleaning procedures. This is particularly important since preservatives are ineffective against Bcc, and sterility has to be insured at each step of production.
Burkholderia isolates are generally resistant to commonly used antibiotics. Treatment might therefore include a combination of two drugs (to try to limit the emergence of more resistance) such as ceftazidime, piperacillin, meropenem with trimethoprim-sulfamethoxazole, or a beta-lactam plus aminoglycoside.
Interestingly, an outbreak of Burkholderia pseudomallei was just reported by the CDC as well. This is a related gram-negative bacillus which is quite uncommon in the United States. It causes melioidosis, usually a tropical infection, which presents with nonspecific symptoms or serious pneumonia, abscesses, or bloodstream infections.
Four cases have been identified this year in Georgia, Kansas, Minnesota, and Texas, two of them fatal. It is usually acquired from soil or water. By genomic analysis, the four cases are felt to be related, but no common source of exposure has been identified. They also appear to be closely related to South Asian strains, although none of the patients had traveled internationally. Prolonged antibiotic therapy with ceftazidime or meropenem, followed by 3-6 months of trimethoprim-sulfamethoxazole, is often required.
In his email, Dr. Rohde stated, “Melioidosis causes cough, chest pain, high fever, headache or unexplained weight loss, but it may take 2-3 weeks for symptoms of melioidosis to appear after a person’s initial exposure to the bacteria. So, one could see how this might be overlooked as COVID per symptoms and per the limitations of laboratory identification.”
It’s essential for clinicians to recognize that automated microbiology identification systems can misidentify B. pseudomallei as B. cepacia and to ask the lab for more specialized molecular diagnostics, particularly when relatively unusual organisms are isolated.
Candice Hoffmann, a public affairs specialist at the CDC, told this news organization that “clinicians should consider melioidosis as a differential diagnosis in both adult and pediatric patients who are suspected to have a bacterial infection (pneumonia, sepsis, meningitis, wound) and are not responding to antibacterial treatment, even if they have not traveled outside of the continental United States.”
Dr. Rohde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and Food and Drug Administration have announced an outbreak of at least 15 Burkholderia cepacia infections associated with contaminated ultrasound gel used to guide invasive procedures as well as an unrelated outbreak of Burkholderia pseudomallei that caused two deaths.
The procedures involved in the B. cepacia outbreak included placement of both central and peripheral intravenous catheters and paracentesis (removal of peritoneal fluid from the abdominal cavity). Cases have occurred in several states.
Further testing has shown the presence of Burkholderia stabilis, a member of B. cepacia complex (Bcc), in four lots of unopened bottles of MediChoice M500812 ultrasound gel. Eco-Med Pharmaceuticals of Etobicoke, Ont., the parent manufacturer, has issued a recall of MediChoice M500812 or Eco-Gel 200 with the following lot numbers: B029, B030, B031, B032, B040, B041, B048, B055. A similar outbreak occurred in Canada.
Some of these cases resulted in bloodstream infections. Further details are not yet available. Bcc infections have ranged from asymptomatic to life-threatening pneumonias, particularly in patients with cystic fibrosis. Other risk factors include immunosuppression, mechanical ventilation, and the use of other invasive venous or urinary catheters.
Kiran M. Perkins, MD, MPH, outbreak lead with the CDC’s Prevention Research Branch, said in an interview via email that automated systems such as Vitek might have trouble identifying the organism as “the system may only reveal the microbial species at the genus level but not at the species level, and/or it may have difficulty distinguishing between members of closely related group members.”
In the CDC’s experience, “most facilities do not conduct further species identification.” The agency added that it cannot tell if there has been any increase in cases associated with COVID-19, as they are not notifiable diseases and the “CDC does not systematically collect information on B. cepacia complex infections.”
Rodney Rohde, PhD, professor of clinical laboratory science and chair of the clinical laboratory science program, Texas State University, San Marcos, told this news organization via email that Burkholderia’s “detection in the manufacturing process is difficult, and product recalls are frequent.” He added, “A recent review by the Food and Drug Administration in the U.S. found that almost 40% of contamination reports in both sterile and nonsterile pharmaceutical products were caused by Bcc bacteria.” Another problem is that they often create biofilms, so “they are tenacious environmental colonizers of medical equipment and surfaces in general.”
There have been many other outbreaks as a result to B. cepacia complex. Because it is often in the water supply used in pharmaceutical manufacturing and is resistant to preservatives, the FDA cautions that it poses a risk of contamination in all nonsterile, water-based drug products.
Recalls have included contaminated antiseptics, such as povidone iodine, benzalkonium chloride, and chlorhexidine gluconate. Contamination in manufacturing may not be uniform, and only some samples may be affected. Antiseptic mouthwashes have also been affected. So have nonbacterial soaps and docusate (a stool softener) solutions, and various personal care products, including nasal sprays, lotions, simethicone gas relief drops (Mylicon), and baby wipes.
Although Bcc are considered “objectionable organisms,” there have been no strong or consistent standards for their detection from the U.S. Pharmacopeia, and some manufacturers reportedly underestimate the consequences of contamination. The FDA issued a guidance to manufacturers in 2017 on quality assurance and cleaning procedures. This is particularly important since preservatives are ineffective against Bcc, and sterility has to be insured at each step of production.
Burkholderia isolates are generally resistant to commonly used antibiotics. Treatment might therefore include a combination of two drugs (to try to limit the emergence of more resistance) such as ceftazidime, piperacillin, meropenem with trimethoprim-sulfamethoxazole, or a beta-lactam plus aminoglycoside.
Interestingly, an outbreak of Burkholderia pseudomallei was just reported by the CDC as well. This is a related gram-negative bacillus which is quite uncommon in the United States. It causes melioidosis, usually a tropical infection, which presents with nonspecific symptoms or serious pneumonia, abscesses, or bloodstream infections.
Four cases have been identified this year in Georgia, Kansas, Minnesota, and Texas, two of them fatal. It is usually acquired from soil or water. By genomic analysis, the four cases are felt to be related, but no common source of exposure has been identified. They also appear to be closely related to South Asian strains, although none of the patients had traveled internationally. Prolonged antibiotic therapy with ceftazidime or meropenem, followed by 3-6 months of trimethoprim-sulfamethoxazole, is often required.
In his email, Dr. Rohde stated, “Melioidosis causes cough, chest pain, high fever, headache or unexplained weight loss, but it may take 2-3 weeks for symptoms of melioidosis to appear after a person’s initial exposure to the bacteria. So, one could see how this might be overlooked as COVID per symptoms and per the limitations of laboratory identification.”
It’s essential for clinicians to recognize that automated microbiology identification systems can misidentify B. pseudomallei as B. cepacia and to ask the lab for more specialized molecular diagnostics, particularly when relatively unusual organisms are isolated.
Candice Hoffmann, a public affairs specialist at the CDC, told this news organization that “clinicians should consider melioidosis as a differential diagnosis in both adult and pediatric patients who are suspected to have a bacterial infection (pneumonia, sepsis, meningitis, wound) and are not responding to antibacterial treatment, even if they have not traveled outside of the continental United States.”
Dr. Rohde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and Food and Drug Administration have announced an outbreak of at least 15 Burkholderia cepacia infections associated with contaminated ultrasound gel used to guide invasive procedures as well as an unrelated outbreak of Burkholderia pseudomallei that caused two deaths.
The procedures involved in the B. cepacia outbreak included placement of both central and peripheral intravenous catheters and paracentesis (removal of peritoneal fluid from the abdominal cavity). Cases have occurred in several states.
Further testing has shown the presence of Burkholderia stabilis, a member of B. cepacia complex (Bcc), in four lots of unopened bottles of MediChoice M500812 ultrasound gel. Eco-Med Pharmaceuticals of Etobicoke, Ont., the parent manufacturer, has issued a recall of MediChoice M500812 or Eco-Gel 200 with the following lot numbers: B029, B030, B031, B032, B040, B041, B048, B055. A similar outbreak occurred in Canada.
Some of these cases resulted in bloodstream infections. Further details are not yet available. Bcc infections have ranged from asymptomatic to life-threatening pneumonias, particularly in patients with cystic fibrosis. Other risk factors include immunosuppression, mechanical ventilation, and the use of other invasive venous or urinary catheters.
Kiran M. Perkins, MD, MPH, outbreak lead with the CDC’s Prevention Research Branch, said in an interview via email that automated systems such as Vitek might have trouble identifying the organism as “the system may only reveal the microbial species at the genus level but not at the species level, and/or it may have difficulty distinguishing between members of closely related group members.”
In the CDC’s experience, “most facilities do not conduct further species identification.” The agency added that it cannot tell if there has been any increase in cases associated with COVID-19, as they are not notifiable diseases and the “CDC does not systematically collect information on B. cepacia complex infections.”
Rodney Rohde, PhD, professor of clinical laboratory science and chair of the clinical laboratory science program, Texas State University, San Marcos, told this news organization via email that Burkholderia’s “detection in the manufacturing process is difficult, and product recalls are frequent.” He added, “A recent review by the Food and Drug Administration in the U.S. found that almost 40% of contamination reports in both sterile and nonsterile pharmaceutical products were caused by Bcc bacteria.” Another problem is that they often create biofilms, so “they are tenacious environmental colonizers of medical equipment and surfaces in general.”
There have been many other outbreaks as a result to B. cepacia complex. Because it is often in the water supply used in pharmaceutical manufacturing and is resistant to preservatives, the FDA cautions that it poses a risk of contamination in all nonsterile, water-based drug products.
Recalls have included contaminated antiseptics, such as povidone iodine, benzalkonium chloride, and chlorhexidine gluconate. Contamination in manufacturing may not be uniform, and only some samples may be affected. Antiseptic mouthwashes have also been affected. So have nonbacterial soaps and docusate (a stool softener) solutions, and various personal care products, including nasal sprays, lotions, simethicone gas relief drops (Mylicon), and baby wipes.
Although Bcc are considered “objectionable organisms,” there have been no strong or consistent standards for their detection from the U.S. Pharmacopeia, and some manufacturers reportedly underestimate the consequences of contamination. The FDA issued a guidance to manufacturers in 2017 on quality assurance and cleaning procedures. This is particularly important since preservatives are ineffective against Bcc, and sterility has to be insured at each step of production.
Burkholderia isolates are generally resistant to commonly used antibiotics. Treatment might therefore include a combination of two drugs (to try to limit the emergence of more resistance) such as ceftazidime, piperacillin, meropenem with trimethoprim-sulfamethoxazole, or a beta-lactam plus aminoglycoside.
Interestingly, an outbreak of Burkholderia pseudomallei was just reported by the CDC as well. This is a related gram-negative bacillus which is quite uncommon in the United States. It causes melioidosis, usually a tropical infection, which presents with nonspecific symptoms or serious pneumonia, abscesses, or bloodstream infections.
Four cases have been identified this year in Georgia, Kansas, Minnesota, and Texas, two of them fatal. It is usually acquired from soil or water. By genomic analysis, the four cases are felt to be related, but no common source of exposure has been identified. They also appear to be closely related to South Asian strains, although none of the patients had traveled internationally. Prolonged antibiotic therapy with ceftazidime or meropenem, followed by 3-6 months of trimethoprim-sulfamethoxazole, is often required.
In his email, Dr. Rohde stated, “Melioidosis causes cough, chest pain, high fever, headache or unexplained weight loss, but it may take 2-3 weeks for symptoms of melioidosis to appear after a person’s initial exposure to the bacteria. So, one could see how this might be overlooked as COVID per symptoms and per the limitations of laboratory identification.”
It’s essential for clinicians to recognize that automated microbiology identification systems can misidentify B. pseudomallei as B. cepacia and to ask the lab for more specialized molecular diagnostics, particularly when relatively unusual organisms are isolated.
Candice Hoffmann, a public affairs specialist at the CDC, told this news organization that “clinicians should consider melioidosis as a differential diagnosis in both adult and pediatric patients who are suspected to have a bacterial infection (pneumonia, sepsis, meningitis, wound) and are not responding to antibacterial treatment, even if they have not traveled outside of the continental United States.”
Dr. Rohde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pathology society first to call for nationwide vaccination mandate
The American Society for Clinical Pathology (ASCP), which represents over 100,000 pathologists and medical laboratory professionals, has called for a nationwide vaccination mandate. It is the first medical specialty society to do so, ASCP chief executive officer Blair Holladay, PhD, said in an interview.
However, the American Lung Association this week said it supports President Biden’s call for businesses to require their employees to be vaccinated. In addition, more than 50 medical societies, including ASCP, recently said they support vaccination mandates for health care workers.
In a position statement released Wednesday, ASCP recommended that every eligible American be vaccinated. “The U.S. Food and Drug Administration is soon expected to fully approve at least one COVID-19 vaccine, and when it does, we urge that vaccination requirements become the norm,” the society said.
Second, ASCP noted that at least 16 states have enacted some form of a ban on COVID-19 vaccine mandates or related requirements. These include blocking employment-based mandates, school vaccination or mask requirements, and vaccine passport requirements.
“These laws prolong the pandemic and threaten the health and safety of every American. They should be repealed or overturned immediately,” the association stated.
Third, ASCP said, it supports the guidance of the Centers for Disease Control and Prevention that masks should be worn indoors in public places in areas of substantial or high COVID-19 transmission.
“Before more people die, our elected leaders need to take serious and aggressive action to ensure that Americans get vaccinated, so we can end the pandemic, end patient and family suffering, end the fatalities, and get back to the lives we had before COVID-19,” the statement concluded.
Laboratories have to focus on COVID again
In his interview, Dr. Holladay noted that the eruption of the Delta variant across the country has again forced laboratories to focus on COVID-19 testing at the expense of necessary tests related to other diseases.
“Because 7 of 10 medical decisions depend on the laboratory, anything that interferes with that interferes with the needs of patient care, including preventive, chronic, and acute care services,” he said.
This is a major reason, he said, for ASCP to support a national vaccination mandate. “People have postponed treatment because of the inability to access medical care [for other conditions],” he noted. The same is true for preventive or diagnostic care such as biopsies for breast cancer and colonoscopies, he added.
“In many parts of the country, the throughput of COVID tests made it difficult for us to focus on tests for other acute conditions. It overwhelmed the laboratory personnel in terms of the number of tests being run.”
Returning to the ‘dark days’
This was a significant issue in the earlier part of the pandemic, Dr. Holladay recalled. The shortage of non-COVID lab capacity eased in the spring and early summer of 2021, when COVID-19 vaccines became widely available.
“But with the Delta variant, we’re going back to those dark days and creating the same bottleneck that we saw in the beginning,” he said.
Although the situation is worse in some states than others, Dr. Holladay added, some of the hardest-hit states like Florida and Texas have very large populations.
“This is not just about doctors, nurses, pathologists, and laboratory personnel being exhausted,” commented Kimberly Sanford, MD, president of ASCP, in a press release. “Laboratory medicine is absolutely necessary for accurate and timely diagnosis of disease, infection control, and effective treatment planning. It is an essential part of the health care system and often overwhelmed by the increasing number of coronavirus tests requiring immediate analysis.
“Such testing takes time and disproportionately consumes scarce equipment and other resources. It means those with cancer and other life-threatening conditions face serious delays in diagnosis and treatment. It delays medical diagnoses, erects barriers to preventative care, and prevents us from focusing on the significant health care needs of the population at large.”
A version of this article first appeared on Medscape.com.
The American Society for Clinical Pathology (ASCP), which represents over 100,000 pathologists and medical laboratory professionals, has called for a nationwide vaccination mandate. It is the first medical specialty society to do so, ASCP chief executive officer Blair Holladay, PhD, said in an interview.
However, the American Lung Association this week said it supports President Biden’s call for businesses to require their employees to be vaccinated. In addition, more than 50 medical societies, including ASCP, recently said they support vaccination mandates for health care workers.
In a position statement released Wednesday, ASCP recommended that every eligible American be vaccinated. “The U.S. Food and Drug Administration is soon expected to fully approve at least one COVID-19 vaccine, and when it does, we urge that vaccination requirements become the norm,” the society said.
Second, ASCP noted that at least 16 states have enacted some form of a ban on COVID-19 vaccine mandates or related requirements. These include blocking employment-based mandates, school vaccination or mask requirements, and vaccine passport requirements.
“These laws prolong the pandemic and threaten the health and safety of every American. They should be repealed or overturned immediately,” the association stated.
Third, ASCP said, it supports the guidance of the Centers for Disease Control and Prevention that masks should be worn indoors in public places in areas of substantial or high COVID-19 transmission.
“Before more people die, our elected leaders need to take serious and aggressive action to ensure that Americans get vaccinated, so we can end the pandemic, end patient and family suffering, end the fatalities, and get back to the lives we had before COVID-19,” the statement concluded.
Laboratories have to focus on COVID again
In his interview, Dr. Holladay noted that the eruption of the Delta variant across the country has again forced laboratories to focus on COVID-19 testing at the expense of necessary tests related to other diseases.
“Because 7 of 10 medical decisions depend on the laboratory, anything that interferes with that interferes with the needs of patient care, including preventive, chronic, and acute care services,” he said.
This is a major reason, he said, for ASCP to support a national vaccination mandate. “People have postponed treatment because of the inability to access medical care [for other conditions],” he noted. The same is true for preventive or diagnostic care such as biopsies for breast cancer and colonoscopies, he added.
“In many parts of the country, the throughput of COVID tests made it difficult for us to focus on tests for other acute conditions. It overwhelmed the laboratory personnel in terms of the number of tests being run.”
Returning to the ‘dark days’
This was a significant issue in the earlier part of the pandemic, Dr. Holladay recalled. The shortage of non-COVID lab capacity eased in the spring and early summer of 2021, when COVID-19 vaccines became widely available.
“But with the Delta variant, we’re going back to those dark days and creating the same bottleneck that we saw in the beginning,” he said.
Although the situation is worse in some states than others, Dr. Holladay added, some of the hardest-hit states like Florida and Texas have very large populations.
“This is not just about doctors, nurses, pathologists, and laboratory personnel being exhausted,” commented Kimberly Sanford, MD, president of ASCP, in a press release. “Laboratory medicine is absolutely necessary for accurate and timely diagnosis of disease, infection control, and effective treatment planning. It is an essential part of the health care system and often overwhelmed by the increasing number of coronavirus tests requiring immediate analysis.
“Such testing takes time and disproportionately consumes scarce equipment and other resources. It means those with cancer and other life-threatening conditions face serious delays in diagnosis and treatment. It delays medical diagnoses, erects barriers to preventative care, and prevents us from focusing on the significant health care needs of the population at large.”
A version of this article first appeared on Medscape.com.
The American Society for Clinical Pathology (ASCP), which represents over 100,000 pathologists and medical laboratory professionals, has called for a nationwide vaccination mandate. It is the first medical specialty society to do so, ASCP chief executive officer Blair Holladay, PhD, said in an interview.
However, the American Lung Association this week said it supports President Biden’s call for businesses to require their employees to be vaccinated. In addition, more than 50 medical societies, including ASCP, recently said they support vaccination mandates for health care workers.
In a position statement released Wednesday, ASCP recommended that every eligible American be vaccinated. “The U.S. Food and Drug Administration is soon expected to fully approve at least one COVID-19 vaccine, and when it does, we urge that vaccination requirements become the norm,” the society said.
Second, ASCP noted that at least 16 states have enacted some form of a ban on COVID-19 vaccine mandates or related requirements. These include blocking employment-based mandates, school vaccination or mask requirements, and vaccine passport requirements.
“These laws prolong the pandemic and threaten the health and safety of every American. They should be repealed or overturned immediately,” the association stated.
Third, ASCP said, it supports the guidance of the Centers for Disease Control and Prevention that masks should be worn indoors in public places in areas of substantial or high COVID-19 transmission.
“Before more people die, our elected leaders need to take serious and aggressive action to ensure that Americans get vaccinated, so we can end the pandemic, end patient and family suffering, end the fatalities, and get back to the lives we had before COVID-19,” the statement concluded.
Laboratories have to focus on COVID again
In his interview, Dr. Holladay noted that the eruption of the Delta variant across the country has again forced laboratories to focus on COVID-19 testing at the expense of necessary tests related to other diseases.
“Because 7 of 10 medical decisions depend on the laboratory, anything that interferes with that interferes with the needs of patient care, including preventive, chronic, and acute care services,” he said.
This is a major reason, he said, for ASCP to support a national vaccination mandate. “People have postponed treatment because of the inability to access medical care [for other conditions],” he noted. The same is true for preventive or diagnostic care such as biopsies for breast cancer and colonoscopies, he added.
“In many parts of the country, the throughput of COVID tests made it difficult for us to focus on tests for other acute conditions. It overwhelmed the laboratory personnel in terms of the number of tests being run.”
Returning to the ‘dark days’
This was a significant issue in the earlier part of the pandemic, Dr. Holladay recalled. The shortage of non-COVID lab capacity eased in the spring and early summer of 2021, when COVID-19 vaccines became widely available.
“But with the Delta variant, we’re going back to those dark days and creating the same bottleneck that we saw in the beginning,” he said.
Although the situation is worse in some states than others, Dr. Holladay added, some of the hardest-hit states like Florida and Texas have very large populations.
“This is not just about doctors, nurses, pathologists, and laboratory personnel being exhausted,” commented Kimberly Sanford, MD, president of ASCP, in a press release. “Laboratory medicine is absolutely necessary for accurate and timely diagnosis of disease, infection control, and effective treatment planning. It is an essential part of the health care system and often overwhelmed by the increasing number of coronavirus tests requiring immediate analysis.
“Such testing takes time and disproportionately consumes scarce equipment and other resources. It means those with cancer and other life-threatening conditions face serious delays in diagnosis and treatment. It delays medical diagnoses, erects barriers to preventative care, and prevents us from focusing on the significant health care needs of the population at large.”
A version of this article first appeared on Medscape.com.
Procedureless intragastric balloon may cut costs as well as weight
Using a procedureless intragastric balloon (PIGB) as a first-line treatment for obesity is cost effective as either a standalone intervention or a bridge to bariatric surgery, according to a new simulation model study published in PLOS One.
PIGB boasts a noninvasive delivery mechanism in the form of a swallowable capsule. Upon reaching the stomach, the capsule is filled with fluid via a catheter. The clinician uses x-ray or fluoroscopy to confirm correct positioning of the balloon. After 4 months, the balloon’s release valve opens to drain the fluid, and the balloon is excreted naturally. If presented with a major complication, clinicians can typically remove PIGB endoscopically. This not only translates into much lower costs than bariatric surgery but also fewer adverse events.
The available evidence surrounding PIGB’s relative efficacy is less clear. Prior studies have shown that PIGB produces an average weight loss of 14.2% after a single, 4-month treatment episode, compared with 32% after bariatric surgery. When compared against other intragastric balloon devices, however, PIGB has been shown to lead to comparable or superior levels of weight loss. There is also limited evidence about PIGB’s long-term efficacy, but some data suggest that weight lost is generally regained after removal of the balloon.
To date, though, there had been no analysis of whether PIBG’s proposed advantages would make it more cost effective when measured against the superior outcomes of commonly performed bariatric surgeries.
Assessing the cost of PIGB
Researchers compared the cost-effectiveness of six regimens: PIGB; standalone gastric bypass or sleeve gastrectomy; PIGB as a bridge to gastric bypass or sleeve gastrectomy; and no treatment. The specific PIGB device the investigators assessed was the Elipse balloon (Allurion Technologies), which is approved in Europe, Asia, and Latin America, and is in the premarket approval process in the United States.
They then applied an individual patient-level Markov microsimulation model to compare these separate regimens in terms of costs and quality-adjusted life years (QALYs). The simulation incorporated data from 10,000 adults aged 18-64 with body mass index (BMI) ≥ 35, of which 44% had a BMI ≥ 40. The model assumed patients initially underwent treatment with PIGB, gastric bypass, or sleeve gastrectomy. Based on the predicted weight loss resulting from that intervention, the model then estimated how PIGB-only, gastric bypass–only, and sleeve gastrectomy–only patients transitioned to a new health state, ranging from no obesity to death. It also incorporated a hybrid strategy in which patients underwent bariatric surgery if their BMI was still ≥ 35. The researchers modeled complications in all groups as chance events, with a probability of occurrence based on BMI state.
The model determined that the most cost-effective approach was using PIGB as a bridge to sleeve gastrectomy, which had an incremental cost-effectiveness ratio (ICER) of $3,781 per QALY. PIGB alone was not cost effective versus bariatric surgery, but it did outperform no treatment (ICER, $21,711 per QALY).
The study investigators noted that there was a counterintuitive aspect to finding that PIGB was most cost effective when used as a bridge to surgery.
“Contrary to expectations that an add-on treatment to already expensive bariatric surgery would further increase health care costs, our results show that using PIGB as an add-on treatment reduces total costs and improves health outcomes, compared with bariatric surgery alone,” they wrote. “Consequently, as decision-makers look for ways to curb rising health care costs, it will be worthwhile to consider incorporating PIGB prior to bariatric surgery within the clinical care pathway.”
They also noted that initial PIGB may help patients achieve a lower BMI following surgery.
An appealing option
“This technique is very appealing to a lot of patients because you don’t need sedation, you can do it fairly quickly, and the risks and complications of endoscopy or surgery aren’t there with the procedureless balloon, at least on implantation,” said Reem Sharaiha, MD, associate professor of medicine and director of Bariatric & Metabolic Endoscopy at Weill Cornell Medicine, when asked to comment on the study’s results. “I believe that you need to offer a lot of options to tackle obesity as an epidemic and to give patients multiple treatment options, because it’s not going to be a one and done. It’s going to be multiple procedures in their lifetime.”
Dr. Sharaiha added that PIGB’s noninvasive qualities may make it a viable option for addressing a notable gap in obesity treatment; only about 2% of individuals who would qualify for surgery actually do so each year.
“A lot of people are reluctant to undergo it because of the fear of complications or the fear of invasiveness. They do not want to be off work for many weeks,” she said. “Many people come to see me and say, ‘I don’t want to tell anyone that I’ve had it done.’ Or, ‘I don’t want any scars.’ So, a lot of these [factors] come into play as well.”
Dr. Sharaiha is a consultant for Boston Scientific and has participated in trials conducted to seek Food and Drug Administration approval for the Elipse device.
Using a procedureless intragastric balloon (PIGB) as a first-line treatment for obesity is cost effective as either a standalone intervention or a bridge to bariatric surgery, according to a new simulation model study published in PLOS One.
PIGB boasts a noninvasive delivery mechanism in the form of a swallowable capsule. Upon reaching the stomach, the capsule is filled with fluid via a catheter. The clinician uses x-ray or fluoroscopy to confirm correct positioning of the balloon. After 4 months, the balloon’s release valve opens to drain the fluid, and the balloon is excreted naturally. If presented with a major complication, clinicians can typically remove PIGB endoscopically. This not only translates into much lower costs than bariatric surgery but also fewer adverse events.
The available evidence surrounding PIGB’s relative efficacy is less clear. Prior studies have shown that PIGB produces an average weight loss of 14.2% after a single, 4-month treatment episode, compared with 32% after bariatric surgery. When compared against other intragastric balloon devices, however, PIGB has been shown to lead to comparable or superior levels of weight loss. There is also limited evidence about PIGB’s long-term efficacy, but some data suggest that weight lost is generally regained after removal of the balloon.
To date, though, there had been no analysis of whether PIBG’s proposed advantages would make it more cost effective when measured against the superior outcomes of commonly performed bariatric surgeries.
Assessing the cost of PIGB
Researchers compared the cost-effectiveness of six regimens: PIGB; standalone gastric bypass or sleeve gastrectomy; PIGB as a bridge to gastric bypass or sleeve gastrectomy; and no treatment. The specific PIGB device the investigators assessed was the Elipse balloon (Allurion Technologies), which is approved in Europe, Asia, and Latin America, and is in the premarket approval process in the United States.
They then applied an individual patient-level Markov microsimulation model to compare these separate regimens in terms of costs and quality-adjusted life years (QALYs). The simulation incorporated data from 10,000 adults aged 18-64 with body mass index (BMI) ≥ 35, of which 44% had a BMI ≥ 40. The model assumed patients initially underwent treatment with PIGB, gastric bypass, or sleeve gastrectomy. Based on the predicted weight loss resulting from that intervention, the model then estimated how PIGB-only, gastric bypass–only, and sleeve gastrectomy–only patients transitioned to a new health state, ranging from no obesity to death. It also incorporated a hybrid strategy in which patients underwent bariatric surgery if their BMI was still ≥ 35. The researchers modeled complications in all groups as chance events, with a probability of occurrence based on BMI state.
The model determined that the most cost-effective approach was using PIGB as a bridge to sleeve gastrectomy, which had an incremental cost-effectiveness ratio (ICER) of $3,781 per QALY. PIGB alone was not cost effective versus bariatric surgery, but it did outperform no treatment (ICER, $21,711 per QALY).
The study investigators noted that there was a counterintuitive aspect to finding that PIGB was most cost effective when used as a bridge to surgery.
“Contrary to expectations that an add-on treatment to already expensive bariatric surgery would further increase health care costs, our results show that using PIGB as an add-on treatment reduces total costs and improves health outcomes, compared with bariatric surgery alone,” they wrote. “Consequently, as decision-makers look for ways to curb rising health care costs, it will be worthwhile to consider incorporating PIGB prior to bariatric surgery within the clinical care pathway.”
They also noted that initial PIGB may help patients achieve a lower BMI following surgery.
An appealing option
“This technique is very appealing to a lot of patients because you don’t need sedation, you can do it fairly quickly, and the risks and complications of endoscopy or surgery aren’t there with the procedureless balloon, at least on implantation,” said Reem Sharaiha, MD, associate professor of medicine and director of Bariatric & Metabolic Endoscopy at Weill Cornell Medicine, when asked to comment on the study’s results. “I believe that you need to offer a lot of options to tackle obesity as an epidemic and to give patients multiple treatment options, because it’s not going to be a one and done. It’s going to be multiple procedures in their lifetime.”
Dr. Sharaiha added that PIGB’s noninvasive qualities may make it a viable option for addressing a notable gap in obesity treatment; only about 2% of individuals who would qualify for surgery actually do so each year.
“A lot of people are reluctant to undergo it because of the fear of complications or the fear of invasiveness. They do not want to be off work for many weeks,” she said. “Many people come to see me and say, ‘I don’t want to tell anyone that I’ve had it done.’ Or, ‘I don’t want any scars.’ So, a lot of these [factors] come into play as well.”
Dr. Sharaiha is a consultant for Boston Scientific and has participated in trials conducted to seek Food and Drug Administration approval for the Elipse device.
Using a procedureless intragastric balloon (PIGB) as a first-line treatment for obesity is cost effective as either a standalone intervention or a bridge to bariatric surgery, according to a new simulation model study published in PLOS One.
PIGB boasts a noninvasive delivery mechanism in the form of a swallowable capsule. Upon reaching the stomach, the capsule is filled with fluid via a catheter. The clinician uses x-ray or fluoroscopy to confirm correct positioning of the balloon. After 4 months, the balloon’s release valve opens to drain the fluid, and the balloon is excreted naturally. If presented with a major complication, clinicians can typically remove PIGB endoscopically. This not only translates into much lower costs than bariatric surgery but also fewer adverse events.
The available evidence surrounding PIGB’s relative efficacy is less clear. Prior studies have shown that PIGB produces an average weight loss of 14.2% after a single, 4-month treatment episode, compared with 32% after bariatric surgery. When compared against other intragastric balloon devices, however, PIGB has been shown to lead to comparable or superior levels of weight loss. There is also limited evidence about PIGB’s long-term efficacy, but some data suggest that weight lost is generally regained after removal of the balloon.
To date, though, there had been no analysis of whether PIBG’s proposed advantages would make it more cost effective when measured against the superior outcomes of commonly performed bariatric surgeries.
Assessing the cost of PIGB
Researchers compared the cost-effectiveness of six regimens: PIGB; standalone gastric bypass or sleeve gastrectomy; PIGB as a bridge to gastric bypass or sleeve gastrectomy; and no treatment. The specific PIGB device the investigators assessed was the Elipse balloon (Allurion Technologies), which is approved in Europe, Asia, and Latin America, and is in the premarket approval process in the United States.
They then applied an individual patient-level Markov microsimulation model to compare these separate regimens in terms of costs and quality-adjusted life years (QALYs). The simulation incorporated data from 10,000 adults aged 18-64 with body mass index (BMI) ≥ 35, of which 44% had a BMI ≥ 40. The model assumed patients initially underwent treatment with PIGB, gastric bypass, or sleeve gastrectomy. Based on the predicted weight loss resulting from that intervention, the model then estimated how PIGB-only, gastric bypass–only, and sleeve gastrectomy–only patients transitioned to a new health state, ranging from no obesity to death. It also incorporated a hybrid strategy in which patients underwent bariatric surgery if their BMI was still ≥ 35. The researchers modeled complications in all groups as chance events, with a probability of occurrence based on BMI state.
The model determined that the most cost-effective approach was using PIGB as a bridge to sleeve gastrectomy, which had an incremental cost-effectiveness ratio (ICER) of $3,781 per QALY. PIGB alone was not cost effective versus bariatric surgery, but it did outperform no treatment (ICER, $21,711 per QALY).
The study investigators noted that there was a counterintuitive aspect to finding that PIGB was most cost effective when used as a bridge to surgery.
“Contrary to expectations that an add-on treatment to already expensive bariatric surgery would further increase health care costs, our results show that using PIGB as an add-on treatment reduces total costs and improves health outcomes, compared with bariatric surgery alone,” they wrote. “Consequently, as decision-makers look for ways to curb rising health care costs, it will be worthwhile to consider incorporating PIGB prior to bariatric surgery within the clinical care pathway.”
They also noted that initial PIGB may help patients achieve a lower BMI following surgery.
An appealing option
“This technique is very appealing to a lot of patients because you don’t need sedation, you can do it fairly quickly, and the risks and complications of endoscopy or surgery aren’t there with the procedureless balloon, at least on implantation,” said Reem Sharaiha, MD, associate professor of medicine and director of Bariatric & Metabolic Endoscopy at Weill Cornell Medicine, when asked to comment on the study’s results. “I believe that you need to offer a lot of options to tackle obesity as an epidemic and to give patients multiple treatment options, because it’s not going to be a one and done. It’s going to be multiple procedures in their lifetime.”
Dr. Sharaiha added that PIGB’s noninvasive qualities may make it a viable option for addressing a notable gap in obesity treatment; only about 2% of individuals who would qualify for surgery actually do so each year.
“A lot of people are reluctant to undergo it because of the fear of complications or the fear of invasiveness. They do not want to be off work for many weeks,” she said. “Many people come to see me and say, ‘I don’t want to tell anyone that I’ve had it done.’ Or, ‘I don’t want any scars.’ So, a lot of these [factors] come into play as well.”
Dr. Sharaiha is a consultant for Boston Scientific and has participated in trials conducted to seek Food and Drug Administration approval for the Elipse device.
FROM PLOS ONE
Heparin’s COVID-19 benefit greatest in moderately ill patients
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Critically ill derive no benefit
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE