User login
Flavonoids dietary ‘powerhouses’ for cognitive decline prevention
, new research shows.
Among the different types of flavonoids, flavones (found in some spices and yellow or orange fruits and vegetables) and anthocyanins (found in blueberries, blackberries, and cherries) seem to have most protective effect, the researchers report.
“There is mounting evidence suggesting flavonoids are powerhouses when it comes to preventing your thinking skills from declining as you get older,” study investigator Walter Willett, MD, DrPH, Harvard University, Boston, said in a statement.
“Our results are exciting because they show that making simple changes to your diet could help prevent cognitive decline,” said Dr. Willett.
The study was published online July 28 in the journal Neurology.
Antioxidant punch
Flavonoids, naturally occurring phytochemicals found in plants, are strong antioxidants. Considering the likely role of oxidative stress in age-related cognitive decline, flavonoids have been proposed as a potentially important preventive.
For the study, Dr. Willett and colleagues prospectively examined associations between long-term dietary flavonoids (flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, polymeric flavonoids, and proanthocyanidins) and subjective cognitive decline in 49,493 women from the Nurses’ Health Study (1984-2006) and 27,842 men from the Health Professionals Follow-up Study (1986-2002).
Those in the highest quintile of flavonoid consumption consumed about 600 mg daily on average while those in the lowest quintile got only about 150 mg daily.
After adjusting for age, total energy intake, major nondietary factors, and specific dietary factors, a higher intake of total flavonoids was associated with lower likelihood of self-reported subjective cognitive decline during follow up.
Individuals in the highest quintile of daily consumption had about a 20% lower risk of subjective cognitive decline relative to peers in the lowest quintile (pooled multivariable-adjusted odds ratio: 0.81; 95% confidence interval, 0.76-0.89).
The strongest protective associations were found for flavones (OR, 0.62; 95% confidence interval, 0.57-0.68), flavanones (OR, 0.64; 95% CI, 0.58-0.68), and anthocyanins (OR, 0.76; 95% CI, 0.72-0.84) (P trend < .0001 for all groups).
“The people in our study who did the best over time ate an average of at least half a serving per day of foods like orange juice, oranges, peppers, celery, grapefruits, grapefruit juice, apples, and pears,” Dr. Willett said.
“While it is possible other phytochemicals are at work here, a colorful diet rich in flavonoids – and specifically flavones and anthocyanins – seems to be a good bet for promoting long-term brain health,” he added.
A limitation of the study is that participants reported on their diets and may not recall perfectly what they ate or how much.
Healthy diet best bet for brain health
Reached for comment, Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association, said this study “adds to our understanding of which elements of a healthy diet may be important in reducing dementia risk; flavonols may be one of those elements.”
“However, at this point, people should not put too much stock in specific nutrients – including subsets of flavonols – for reducing dementia risk until more research is done. Rather, they should focus on eating an overall healthy diet,” he said.
“It would be wonderful if a particular food or supplement could delay or prevent Alzheimer’s disease, but we do not have scientific evidence to support such claims. Randomized controlled clinical trials are necessary to evaluate whether any food or supplement has a scientifically proven beneficial effect,” Dr. Weber added.
For now, the Alzheimer’s Association “encourages everyone to eat a healthy and balanced diet as a way to help reduce the risk of cognitive decline,” Dr. Weber said.
“With more than 6 million Americans living with Alzheimer’s disease and other dementia today, there is a pressing need to test the effectiveness of a healthy lifestyle regimen to reduce risk of cognitive decline in a large and diverse population,” he added.
The Alzheimer’s Association has launched a 2-year clinical trial, called the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), to do just that.
“While we research that definitive lifestyle ‘recipe,’ there are things we can do today that may decrease our risk of cognitive decline as we age. Eating a heart-healthy diet, exercising regularly, and staying cognitively engaged are just a few,” Dr. Weber added.
Also weighing in, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said the study results are not surprising.
“Scientific data on the ability of flavonoids to prevent age-related chronic diseases, including cognitive decline, has accumulated immensely over the last decade. This epidemiological study reinforces findings from smaller shorter-duration clinical trials and mechanistic studies,” said Dr. Wallace, who was not involved in the study.
“Flavonoids show great potential in reducing inflammation and oxidative stress in the body. They are also vasodilators that help improve blood flow, which is important for the cardiovascular and cerebrovascular systems,” he noted.
“Typically, foods rich in flavonoids are also nutrient-dense in vitamins, minerals, and dietary fiber (eg, fruits and vegetables). Anthocyanins in blueberries have long been known to prevent cognitive decline with age,” Dr. Wallace said.
Dr. Wallace was part of a 14-member panel of nutrition scientists who recently reviewed available evidence around fruit and vegetable intake and health.
“Our findings are consistent with this study in regard to cognitive decline and other disease states. Cruciferous vegetables, dark-green leafy vegetables, citrus fruits, and dark-colored berries seem to have superior effects on health promotion and disease prevention in general,” said Dr. Wallace.
This work was supported by grants from the National Institutes of Health. The authors have disclosed no relevant financial relationships. Dr. Weber has no relevant disclosures. Dr. Wallace is principal and chief executive officer of the Think Healthy Group; editor of the Journal of Dietary Supplements; and deputy editor-in-chief of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
, new research shows.
Among the different types of flavonoids, flavones (found in some spices and yellow or orange fruits and vegetables) and anthocyanins (found in blueberries, blackberries, and cherries) seem to have most protective effect, the researchers report.
“There is mounting evidence suggesting flavonoids are powerhouses when it comes to preventing your thinking skills from declining as you get older,” study investigator Walter Willett, MD, DrPH, Harvard University, Boston, said in a statement.
“Our results are exciting because they show that making simple changes to your diet could help prevent cognitive decline,” said Dr. Willett.
The study was published online July 28 in the journal Neurology.
Antioxidant punch
Flavonoids, naturally occurring phytochemicals found in plants, are strong antioxidants. Considering the likely role of oxidative stress in age-related cognitive decline, flavonoids have been proposed as a potentially important preventive.
For the study, Dr. Willett and colleagues prospectively examined associations between long-term dietary flavonoids (flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, polymeric flavonoids, and proanthocyanidins) and subjective cognitive decline in 49,493 women from the Nurses’ Health Study (1984-2006) and 27,842 men from the Health Professionals Follow-up Study (1986-2002).
Those in the highest quintile of flavonoid consumption consumed about 600 mg daily on average while those in the lowest quintile got only about 150 mg daily.
After adjusting for age, total energy intake, major nondietary factors, and specific dietary factors, a higher intake of total flavonoids was associated with lower likelihood of self-reported subjective cognitive decline during follow up.
Individuals in the highest quintile of daily consumption had about a 20% lower risk of subjective cognitive decline relative to peers in the lowest quintile (pooled multivariable-adjusted odds ratio: 0.81; 95% confidence interval, 0.76-0.89).
The strongest protective associations were found for flavones (OR, 0.62; 95% confidence interval, 0.57-0.68), flavanones (OR, 0.64; 95% CI, 0.58-0.68), and anthocyanins (OR, 0.76; 95% CI, 0.72-0.84) (P trend < .0001 for all groups).
“The people in our study who did the best over time ate an average of at least half a serving per day of foods like orange juice, oranges, peppers, celery, grapefruits, grapefruit juice, apples, and pears,” Dr. Willett said.
“While it is possible other phytochemicals are at work here, a colorful diet rich in flavonoids – and specifically flavones and anthocyanins – seems to be a good bet for promoting long-term brain health,” he added.
A limitation of the study is that participants reported on their diets and may not recall perfectly what they ate or how much.
Healthy diet best bet for brain health
Reached for comment, Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association, said this study “adds to our understanding of which elements of a healthy diet may be important in reducing dementia risk; flavonols may be one of those elements.”
“However, at this point, people should not put too much stock in specific nutrients – including subsets of flavonols – for reducing dementia risk until more research is done. Rather, they should focus on eating an overall healthy diet,” he said.
“It would be wonderful if a particular food or supplement could delay or prevent Alzheimer’s disease, but we do not have scientific evidence to support such claims. Randomized controlled clinical trials are necessary to evaluate whether any food or supplement has a scientifically proven beneficial effect,” Dr. Weber added.
For now, the Alzheimer’s Association “encourages everyone to eat a healthy and balanced diet as a way to help reduce the risk of cognitive decline,” Dr. Weber said.
“With more than 6 million Americans living with Alzheimer’s disease and other dementia today, there is a pressing need to test the effectiveness of a healthy lifestyle regimen to reduce risk of cognitive decline in a large and diverse population,” he added.
The Alzheimer’s Association has launched a 2-year clinical trial, called the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), to do just that.
“While we research that definitive lifestyle ‘recipe,’ there are things we can do today that may decrease our risk of cognitive decline as we age. Eating a heart-healthy diet, exercising regularly, and staying cognitively engaged are just a few,” Dr. Weber added.
Also weighing in, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said the study results are not surprising.
“Scientific data on the ability of flavonoids to prevent age-related chronic diseases, including cognitive decline, has accumulated immensely over the last decade. This epidemiological study reinforces findings from smaller shorter-duration clinical trials and mechanistic studies,” said Dr. Wallace, who was not involved in the study.
“Flavonoids show great potential in reducing inflammation and oxidative stress in the body. They are also vasodilators that help improve blood flow, which is important for the cardiovascular and cerebrovascular systems,” he noted.
“Typically, foods rich in flavonoids are also nutrient-dense in vitamins, minerals, and dietary fiber (eg, fruits and vegetables). Anthocyanins in blueberries have long been known to prevent cognitive decline with age,” Dr. Wallace said.
Dr. Wallace was part of a 14-member panel of nutrition scientists who recently reviewed available evidence around fruit and vegetable intake and health.
“Our findings are consistent with this study in regard to cognitive decline and other disease states. Cruciferous vegetables, dark-green leafy vegetables, citrus fruits, and dark-colored berries seem to have superior effects on health promotion and disease prevention in general,” said Dr. Wallace.
This work was supported by grants from the National Institutes of Health. The authors have disclosed no relevant financial relationships. Dr. Weber has no relevant disclosures. Dr. Wallace is principal and chief executive officer of the Think Healthy Group; editor of the Journal of Dietary Supplements; and deputy editor-in-chief of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
, new research shows.
Among the different types of flavonoids, flavones (found in some spices and yellow or orange fruits and vegetables) and anthocyanins (found in blueberries, blackberries, and cherries) seem to have most protective effect, the researchers report.
“There is mounting evidence suggesting flavonoids are powerhouses when it comes to preventing your thinking skills from declining as you get older,” study investigator Walter Willett, MD, DrPH, Harvard University, Boston, said in a statement.
“Our results are exciting because they show that making simple changes to your diet could help prevent cognitive decline,” said Dr. Willett.
The study was published online July 28 in the journal Neurology.
Antioxidant punch
Flavonoids, naturally occurring phytochemicals found in plants, are strong antioxidants. Considering the likely role of oxidative stress in age-related cognitive decline, flavonoids have been proposed as a potentially important preventive.
For the study, Dr. Willett and colleagues prospectively examined associations between long-term dietary flavonoids (flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, polymeric flavonoids, and proanthocyanidins) and subjective cognitive decline in 49,493 women from the Nurses’ Health Study (1984-2006) and 27,842 men from the Health Professionals Follow-up Study (1986-2002).
Those in the highest quintile of flavonoid consumption consumed about 600 mg daily on average while those in the lowest quintile got only about 150 mg daily.
After adjusting for age, total energy intake, major nondietary factors, and specific dietary factors, a higher intake of total flavonoids was associated with lower likelihood of self-reported subjective cognitive decline during follow up.
Individuals in the highest quintile of daily consumption had about a 20% lower risk of subjective cognitive decline relative to peers in the lowest quintile (pooled multivariable-adjusted odds ratio: 0.81; 95% confidence interval, 0.76-0.89).
The strongest protective associations were found for flavones (OR, 0.62; 95% confidence interval, 0.57-0.68), flavanones (OR, 0.64; 95% CI, 0.58-0.68), and anthocyanins (OR, 0.76; 95% CI, 0.72-0.84) (P trend < .0001 for all groups).
“The people in our study who did the best over time ate an average of at least half a serving per day of foods like orange juice, oranges, peppers, celery, grapefruits, grapefruit juice, apples, and pears,” Dr. Willett said.
“While it is possible other phytochemicals are at work here, a colorful diet rich in flavonoids – and specifically flavones and anthocyanins – seems to be a good bet for promoting long-term brain health,” he added.
A limitation of the study is that participants reported on their diets and may not recall perfectly what they ate or how much.
Healthy diet best bet for brain health
Reached for comment, Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association, said this study “adds to our understanding of which elements of a healthy diet may be important in reducing dementia risk; flavonols may be one of those elements.”
“However, at this point, people should not put too much stock in specific nutrients – including subsets of flavonols – for reducing dementia risk until more research is done. Rather, they should focus on eating an overall healthy diet,” he said.
“It would be wonderful if a particular food or supplement could delay or prevent Alzheimer’s disease, but we do not have scientific evidence to support such claims. Randomized controlled clinical trials are necessary to evaluate whether any food or supplement has a scientifically proven beneficial effect,” Dr. Weber added.
For now, the Alzheimer’s Association “encourages everyone to eat a healthy and balanced diet as a way to help reduce the risk of cognitive decline,” Dr. Weber said.
“With more than 6 million Americans living with Alzheimer’s disease and other dementia today, there is a pressing need to test the effectiveness of a healthy lifestyle regimen to reduce risk of cognitive decline in a large and diverse population,” he added.
The Alzheimer’s Association has launched a 2-year clinical trial, called the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), to do just that.
“While we research that definitive lifestyle ‘recipe,’ there are things we can do today that may decrease our risk of cognitive decline as we age. Eating a heart-healthy diet, exercising regularly, and staying cognitively engaged are just a few,” Dr. Weber added.
Also weighing in, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said the study results are not surprising.
“Scientific data on the ability of flavonoids to prevent age-related chronic diseases, including cognitive decline, has accumulated immensely over the last decade. This epidemiological study reinforces findings from smaller shorter-duration clinical trials and mechanistic studies,” said Dr. Wallace, who was not involved in the study.
“Flavonoids show great potential in reducing inflammation and oxidative stress in the body. They are also vasodilators that help improve blood flow, which is important for the cardiovascular and cerebrovascular systems,” he noted.
“Typically, foods rich in flavonoids are also nutrient-dense in vitamins, minerals, and dietary fiber (eg, fruits and vegetables). Anthocyanins in blueberries have long been known to prevent cognitive decline with age,” Dr. Wallace said.
Dr. Wallace was part of a 14-member panel of nutrition scientists who recently reviewed available evidence around fruit and vegetable intake and health.
“Our findings are consistent with this study in regard to cognitive decline and other disease states. Cruciferous vegetables, dark-green leafy vegetables, citrus fruits, and dark-colored berries seem to have superior effects on health promotion and disease prevention in general,” said Dr. Wallace.
This work was supported by grants from the National Institutes of Health. The authors have disclosed no relevant financial relationships. Dr. Weber has no relevant disclosures. Dr. Wallace is principal and chief executive officer of the Think Healthy Group; editor of the Journal of Dietary Supplements; and deputy editor-in-chief of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Exploring the Utility of Artificial Intelligence During COVID-19 in Dermatology Practice
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
Practice Points
- Dermatologists should amass pictures of dermatologic conditions in skin of color to contribute to growing awareness and knowledge of presentation of disease in this population.
- Dermatologists should use artificial intelligence as a tool for delivering more efficient and beneficial patient care.
Mobile App Usage Among Dermatology Residents in America
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
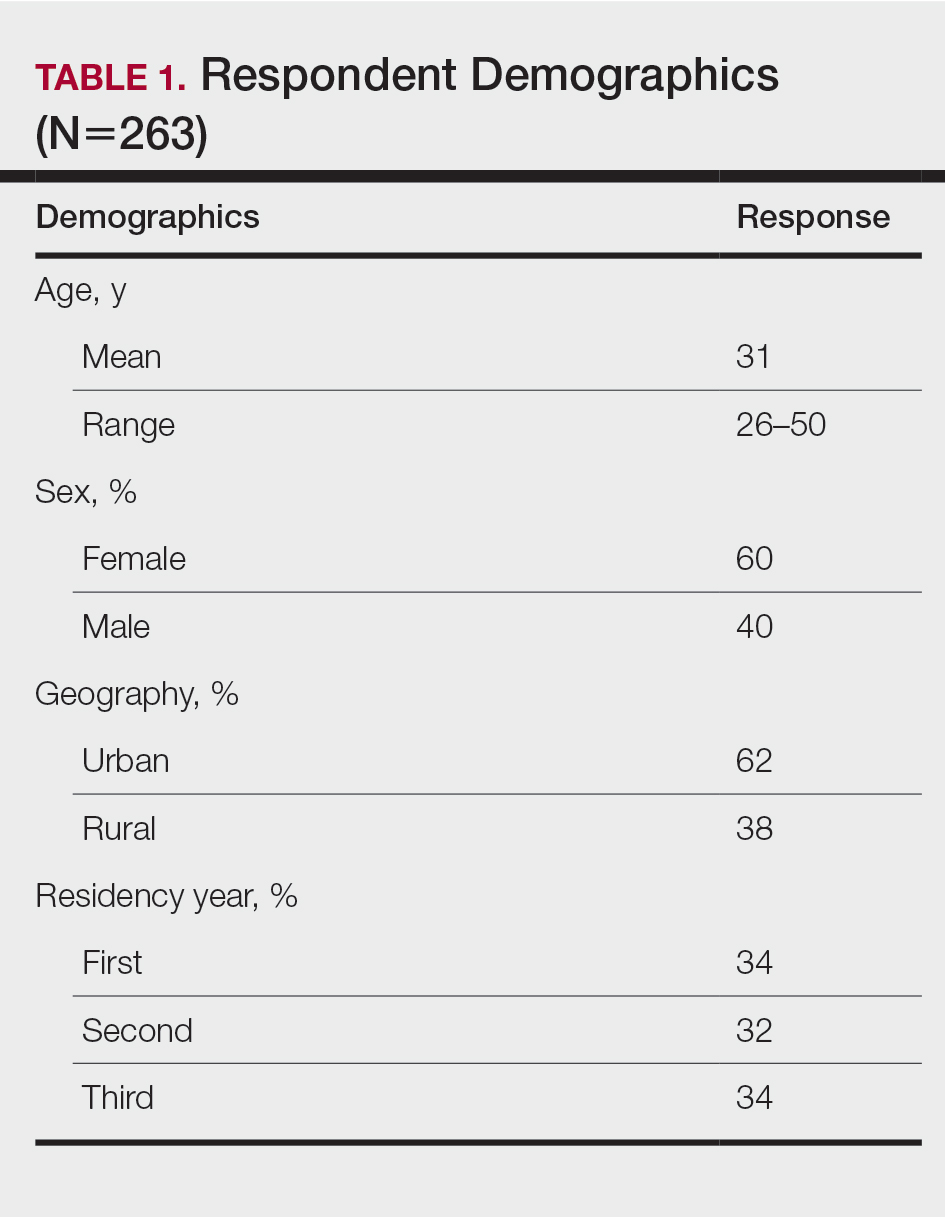
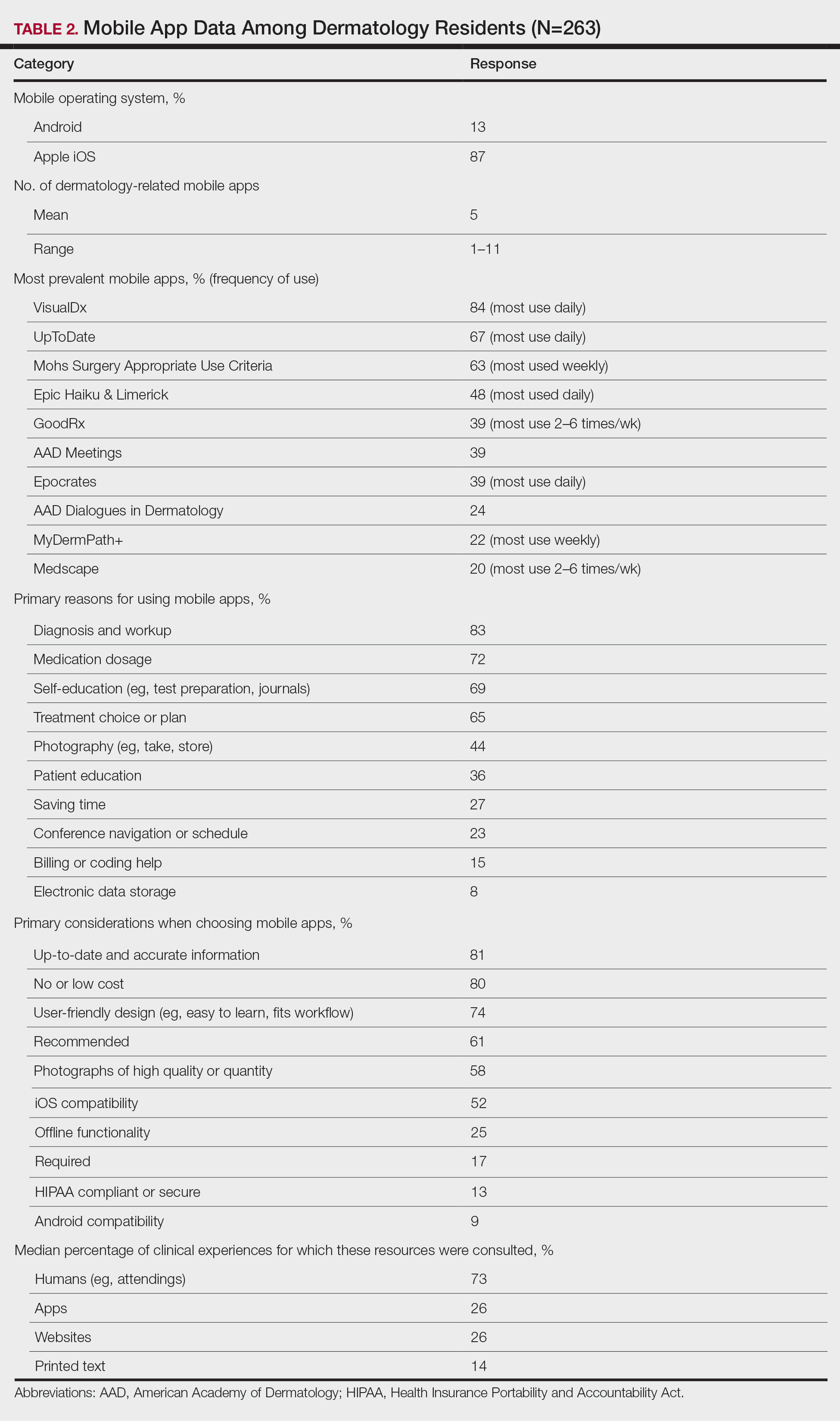
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
Practice Points
- Virtual resources, including mobile apps, have become critical tools for learning and patient care during dermatology resident training for reasons that should be elucidated.
- Dermatology residents of different years and sexes utilize mobile apps in different amounts and for different purposes.
Aquatic Antagonists: Sea Cucumbers (Holothuroidea)
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Practice Points
- Sea cucumbers produce a toxin known as holothurin, which is contained in specialized structures called Cuvierian tubules and secreted onto the outer surface of the body wall. Some species also eject portions of their toxic inner organs through the anus as a defensive mechanism.
- In humans, the holothurin toxins cause an acute irritant dermatitis upon contact with the skin and a painful chemical conjunctivitis upon contact with the eyes.
- In addition to their own toxin, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through their integument, causing additional effects on human skin.
- The dermatologic effects of sea cucumbers can be prevented with the use of gloves and swim masks or goggles.
Tirzepatide questions persist despite serial phase 3 success in type 2 diabetes
The streak of positive phase 3 trial results for the novel “twincretin” tirzepatide when treating patients with type 2 diabetes continued in a report in The Lancet on results from the SURPASS-3 trial, which compared weekly subcutaneous injections of tirzepatide against daily treatment with insulin degludec in patients inadequately controlled on metformin alone or on metformin plus a sodium-glucose cotransporter 2 inhibitor.
Despite positive results in SURPASS-3, as well as in four other pivotal trials that are in the process of releasing full results, the safety and efficacy picture of tirzepatide still includes several as-yet unresolved issues, including the true incidence rate of gastrointestinal adverse effects, the role these effects play in weight loss during tirzepatide treatment, and the drug’s effect on important endpoints beyond weight loss and glycemic control such as cardiovascular outcomes and renal function, said two Australian experts who coauthored a comment on the new SURPASS-3 report.
Tirzepatide is called a “twincretin” because the molecule acts as both a glucagonlike peptide–1 receptor agonist, the drug class that includes semaglutide (Ozempic, Rybelsus, Wegovy) and liraglutide (Saxenda, Victoza), and also as a glucose-dependent insulinotropic polypeptide (GIP). Trial results reported to date suggest that tirzepatide “might be more potent than available GLP-1 receptor agonists,” based on evidence of superior glycemic control it produced relative to semaglutide in results from the SURPASS-2 phase 3 trial reported in August 2021, wrote Christopher K. Rayner, MD, and Michael Horowitz, MD, in their comment.
Uncertainty about gastrointestinal adverse effects
“Limitations of SURPASS-3 include the relatively small number of Asian and Black” patients enrolled, “and an open-label design that carries a risk for bias” when tallying the incidence of gastrointestinal adverse effects, which the trial recorded based on self-reports by enrolled patients.
A better design would use validated questionnaires geared to discerning gastrointestinal symptoms like the ones used in trials involving patients with functional gastrointestinal disorders, wrote Dr. Rayner, a professor of gastroenterology at the University of Adelaide, and Dr. Horowitz, a professor at the same institution and also director of the endocrine and metabolic unit at Royal Adelaide Hospital.
This approach would “allow for more robust evaluation of whether gastrointestinal symptoms are associated with increased weight loss,” they proposed, a possible partial explanation for the weight loss of some patients treated with a GLP-1 receptor agonist.
Additional outstanding questions about tirzepatide include the contribution resulting from the drug’s stimulation of the GIP receptor, as well as the role of GLP-1 receptor stimulation by tirzepatide in slowing gastric emptying. And they also cite the still-unreported effects of tirzepatide on cardiovascular events, fatty liver disease, and kidney function, and its longer-term effects with chronic treatment beyond a year.
All five of the recently completed SURPASS trials ran for 40-52 weeks.
Tirzepatide surpasses insulin degludec’s glycemic control
SURPASS-3 enrolled 1,444 patients with type 2 diabetes at 122 sites in 13 countries during 2019. The study’s primary endpoint was mean change in hemoglobin A1c from baseline after 52 weeks on treatment. The results showed that the A1c reduction with tirzepatide treatment significantly exceeded the drop produced by insulin degludec by 0.59%, 0.86%, and 1.04%, respectively, across the three tirzepatide dosages tested in a dose-response fashion, according to the recent publication.
The most common treatment-emergent adverse effects were gastrointestinal, which decreased with continued treatment, and tirzepatide produced fewer episodes of hypoglycemia, compared with insulin degludec (Tresiba).
In addition to full reports now out from SURPASS-2 and SURPASS-3, researchers also recently published full primary results from SURPASS-1. Results from SURPASS-5 appeared in a poster presented at the American Diabetes Association scientific sessions in June 2021 but have not yet been published in a full report, and the primary results from SURPASS-4are expected in a report during the European Association for the Study of Diabetes in September 2021.
SURPASS-3 and the other trials of tirzepatide were funded by Lilly, the company developing the drug. Dr. Rayner has been an adviser to Allergen and Glyscend, and has received research funding from Sanofi and Novartis. Dr. Horowitz has received symposia fees from Lilly, as well as from AstraZeneca and Boehringer Ingelheim.
The streak of positive phase 3 trial results for the novel “twincretin” tirzepatide when treating patients with type 2 diabetes continued in a report in The Lancet on results from the SURPASS-3 trial, which compared weekly subcutaneous injections of tirzepatide against daily treatment with insulin degludec in patients inadequately controlled on metformin alone or on metformin plus a sodium-glucose cotransporter 2 inhibitor.
Despite positive results in SURPASS-3, as well as in four other pivotal trials that are in the process of releasing full results, the safety and efficacy picture of tirzepatide still includes several as-yet unresolved issues, including the true incidence rate of gastrointestinal adverse effects, the role these effects play in weight loss during tirzepatide treatment, and the drug’s effect on important endpoints beyond weight loss and glycemic control such as cardiovascular outcomes and renal function, said two Australian experts who coauthored a comment on the new SURPASS-3 report.
Tirzepatide is called a “twincretin” because the molecule acts as both a glucagonlike peptide–1 receptor agonist, the drug class that includes semaglutide (Ozempic, Rybelsus, Wegovy) and liraglutide (Saxenda, Victoza), and also as a glucose-dependent insulinotropic polypeptide (GIP). Trial results reported to date suggest that tirzepatide “might be more potent than available GLP-1 receptor agonists,” based on evidence of superior glycemic control it produced relative to semaglutide in results from the SURPASS-2 phase 3 trial reported in August 2021, wrote Christopher K. Rayner, MD, and Michael Horowitz, MD, in their comment.
Uncertainty about gastrointestinal adverse effects
“Limitations of SURPASS-3 include the relatively small number of Asian and Black” patients enrolled, “and an open-label design that carries a risk for bias” when tallying the incidence of gastrointestinal adverse effects, which the trial recorded based on self-reports by enrolled patients.
A better design would use validated questionnaires geared to discerning gastrointestinal symptoms like the ones used in trials involving patients with functional gastrointestinal disorders, wrote Dr. Rayner, a professor of gastroenterology at the University of Adelaide, and Dr. Horowitz, a professor at the same institution and also director of the endocrine and metabolic unit at Royal Adelaide Hospital.
This approach would “allow for more robust evaluation of whether gastrointestinal symptoms are associated with increased weight loss,” they proposed, a possible partial explanation for the weight loss of some patients treated with a GLP-1 receptor agonist.
Additional outstanding questions about tirzepatide include the contribution resulting from the drug’s stimulation of the GIP receptor, as well as the role of GLP-1 receptor stimulation by tirzepatide in slowing gastric emptying. And they also cite the still-unreported effects of tirzepatide on cardiovascular events, fatty liver disease, and kidney function, and its longer-term effects with chronic treatment beyond a year.
All five of the recently completed SURPASS trials ran for 40-52 weeks.
Tirzepatide surpasses insulin degludec’s glycemic control
SURPASS-3 enrolled 1,444 patients with type 2 diabetes at 122 sites in 13 countries during 2019. The study’s primary endpoint was mean change in hemoglobin A1c from baseline after 52 weeks on treatment. The results showed that the A1c reduction with tirzepatide treatment significantly exceeded the drop produced by insulin degludec by 0.59%, 0.86%, and 1.04%, respectively, across the three tirzepatide dosages tested in a dose-response fashion, according to the recent publication.
The most common treatment-emergent adverse effects were gastrointestinal, which decreased with continued treatment, and tirzepatide produced fewer episodes of hypoglycemia, compared with insulin degludec (Tresiba).
In addition to full reports now out from SURPASS-2 and SURPASS-3, researchers also recently published full primary results from SURPASS-1. Results from SURPASS-5 appeared in a poster presented at the American Diabetes Association scientific sessions in June 2021 but have not yet been published in a full report, and the primary results from SURPASS-4are expected in a report during the European Association for the Study of Diabetes in September 2021.
SURPASS-3 and the other trials of tirzepatide were funded by Lilly, the company developing the drug. Dr. Rayner has been an adviser to Allergen and Glyscend, and has received research funding from Sanofi and Novartis. Dr. Horowitz has received symposia fees from Lilly, as well as from AstraZeneca and Boehringer Ingelheim.
The streak of positive phase 3 trial results for the novel “twincretin” tirzepatide when treating patients with type 2 diabetes continued in a report in The Lancet on results from the SURPASS-3 trial, which compared weekly subcutaneous injections of tirzepatide against daily treatment with insulin degludec in patients inadequately controlled on metformin alone or on metformin plus a sodium-glucose cotransporter 2 inhibitor.
Despite positive results in SURPASS-3, as well as in four other pivotal trials that are in the process of releasing full results, the safety and efficacy picture of tirzepatide still includes several as-yet unresolved issues, including the true incidence rate of gastrointestinal adverse effects, the role these effects play in weight loss during tirzepatide treatment, and the drug’s effect on important endpoints beyond weight loss and glycemic control such as cardiovascular outcomes and renal function, said two Australian experts who coauthored a comment on the new SURPASS-3 report.
Tirzepatide is called a “twincretin” because the molecule acts as both a glucagonlike peptide–1 receptor agonist, the drug class that includes semaglutide (Ozempic, Rybelsus, Wegovy) and liraglutide (Saxenda, Victoza), and also as a glucose-dependent insulinotropic polypeptide (GIP). Trial results reported to date suggest that tirzepatide “might be more potent than available GLP-1 receptor agonists,” based on evidence of superior glycemic control it produced relative to semaglutide in results from the SURPASS-2 phase 3 trial reported in August 2021, wrote Christopher K. Rayner, MD, and Michael Horowitz, MD, in their comment.
Uncertainty about gastrointestinal adverse effects
“Limitations of SURPASS-3 include the relatively small number of Asian and Black” patients enrolled, “and an open-label design that carries a risk for bias” when tallying the incidence of gastrointestinal adverse effects, which the trial recorded based on self-reports by enrolled patients.
A better design would use validated questionnaires geared to discerning gastrointestinal symptoms like the ones used in trials involving patients with functional gastrointestinal disorders, wrote Dr. Rayner, a professor of gastroenterology at the University of Adelaide, and Dr. Horowitz, a professor at the same institution and also director of the endocrine and metabolic unit at Royal Adelaide Hospital.
This approach would “allow for more robust evaluation of whether gastrointestinal symptoms are associated with increased weight loss,” they proposed, a possible partial explanation for the weight loss of some patients treated with a GLP-1 receptor agonist.
Additional outstanding questions about tirzepatide include the contribution resulting from the drug’s stimulation of the GIP receptor, as well as the role of GLP-1 receptor stimulation by tirzepatide in slowing gastric emptying. And they also cite the still-unreported effects of tirzepatide on cardiovascular events, fatty liver disease, and kidney function, and its longer-term effects with chronic treatment beyond a year.
All five of the recently completed SURPASS trials ran for 40-52 weeks.
Tirzepatide surpasses insulin degludec’s glycemic control
SURPASS-3 enrolled 1,444 patients with type 2 diabetes at 122 sites in 13 countries during 2019. The study’s primary endpoint was mean change in hemoglobin A1c from baseline after 52 weeks on treatment. The results showed that the A1c reduction with tirzepatide treatment significantly exceeded the drop produced by insulin degludec by 0.59%, 0.86%, and 1.04%, respectively, across the three tirzepatide dosages tested in a dose-response fashion, according to the recent publication.
The most common treatment-emergent adverse effects were gastrointestinal, which decreased with continued treatment, and tirzepatide produced fewer episodes of hypoglycemia, compared with insulin degludec (Tresiba).
In addition to full reports now out from SURPASS-2 and SURPASS-3, researchers also recently published full primary results from SURPASS-1. Results from SURPASS-5 appeared in a poster presented at the American Diabetes Association scientific sessions in June 2021 but have not yet been published in a full report, and the primary results from SURPASS-4are expected in a report during the European Association for the Study of Diabetes in September 2021.
SURPASS-3 and the other trials of tirzepatide were funded by Lilly, the company developing the drug. Dr. Rayner has been an adviser to Allergen and Glyscend, and has received research funding from Sanofi and Novartis. Dr. Horowitz has received symposia fees from Lilly, as well as from AstraZeneca and Boehringer Ingelheim.
FROM THE LANCET
The Top 100 Most-Cited Articles on Nail Psoriasis: A Bibliometric Analysis
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
FDA approves new enzyme replacement therapy for Pompe disease
Pompe disease is a rare genetic disease that occurs in an estimated 1 in 40,000 births. It is caused by a genetic deficiency or dysfunction of the lysosomal enzyme acid alpha-glucosidase (GAA), which leads to a buildup of glycogen in skeletal and cardiac muscle cells, causing muscle weakness and premature death from respiratory failure or heart failure.
Nexviazyme, administered by intravenous infusion every 2 weeks, supplements GAA and helps reduce glycogen accumulation.
The approval of this product “brings patients with Pompe disease another enzyme replacement therapy option for this rare disease,” said Janet Maynard, MD, deputy director, Office of Rare Diseases, Pediatrics, Urologic and Reproductive Medicine, in the FDA’s Center for Drug Evaluation and Research, in a news release.
In 2010, the FDA approved alglucosidase alfa (Lumizyme) for the treatment of late-onset Pompe disease.
“The FDA will continue to work with stakeholders to advance the development of additional new, effective, and safe therapies for rare diseases, including Pompe disease,” said Dr. Maynard.
The approval is based on positive phase 3 data that demonstrated improvements in key disease burden measures, including respiratory function and walking disease, and that established the drug’s safety profile, Genzyme said in a news release.
The most common side effects were headache, fatigue, diarrhea, nausea, joint pain, dizziness, myalgia, pruritus, vomiting, dyspnea, erythema, paresthesia, and urticaria.
Serious reactions included hypersensitivity reactions, such as anaphylaxis, and infusion-associated reactions, including respiratory distress, chills, and pyrexia.
Patients susceptible to fluid volume overload or those with compromised cardiac or respiratory function may be at risk for serious acute cardiorespiratory failure.
The FDA granted Nexviazyme orphan drug designation, priority review, and breakthrough status.
Genzyme expects the new therapy to be available in the United States in the coming weeks and said it will be priced on par with Lumizyme.
A version of this article first appeared on Medscape.com.
Pompe disease is a rare genetic disease that occurs in an estimated 1 in 40,000 births. It is caused by a genetic deficiency or dysfunction of the lysosomal enzyme acid alpha-glucosidase (GAA), which leads to a buildup of glycogen in skeletal and cardiac muscle cells, causing muscle weakness and premature death from respiratory failure or heart failure.
Nexviazyme, administered by intravenous infusion every 2 weeks, supplements GAA and helps reduce glycogen accumulation.
The approval of this product “brings patients with Pompe disease another enzyme replacement therapy option for this rare disease,” said Janet Maynard, MD, deputy director, Office of Rare Diseases, Pediatrics, Urologic and Reproductive Medicine, in the FDA’s Center for Drug Evaluation and Research, in a news release.
In 2010, the FDA approved alglucosidase alfa (Lumizyme) for the treatment of late-onset Pompe disease.
“The FDA will continue to work with stakeholders to advance the development of additional new, effective, and safe therapies for rare diseases, including Pompe disease,” said Dr. Maynard.
The approval is based on positive phase 3 data that demonstrated improvements in key disease burden measures, including respiratory function and walking disease, and that established the drug’s safety profile, Genzyme said in a news release.
The most common side effects were headache, fatigue, diarrhea, nausea, joint pain, dizziness, myalgia, pruritus, vomiting, dyspnea, erythema, paresthesia, and urticaria.
Serious reactions included hypersensitivity reactions, such as anaphylaxis, and infusion-associated reactions, including respiratory distress, chills, and pyrexia.
Patients susceptible to fluid volume overload or those with compromised cardiac or respiratory function may be at risk for serious acute cardiorespiratory failure.
The FDA granted Nexviazyme orphan drug designation, priority review, and breakthrough status.
Genzyme expects the new therapy to be available in the United States in the coming weeks and said it will be priced on par with Lumizyme.
A version of this article first appeared on Medscape.com.
Pompe disease is a rare genetic disease that occurs in an estimated 1 in 40,000 births. It is caused by a genetic deficiency or dysfunction of the lysosomal enzyme acid alpha-glucosidase (GAA), which leads to a buildup of glycogen in skeletal and cardiac muscle cells, causing muscle weakness and premature death from respiratory failure or heart failure.
Nexviazyme, administered by intravenous infusion every 2 weeks, supplements GAA and helps reduce glycogen accumulation.
The approval of this product “brings patients with Pompe disease another enzyme replacement therapy option for this rare disease,” said Janet Maynard, MD, deputy director, Office of Rare Diseases, Pediatrics, Urologic and Reproductive Medicine, in the FDA’s Center for Drug Evaluation and Research, in a news release.
In 2010, the FDA approved alglucosidase alfa (Lumizyme) for the treatment of late-onset Pompe disease.
“The FDA will continue to work with stakeholders to advance the development of additional new, effective, and safe therapies for rare diseases, including Pompe disease,” said Dr. Maynard.
The approval is based on positive phase 3 data that demonstrated improvements in key disease burden measures, including respiratory function and walking disease, and that established the drug’s safety profile, Genzyme said in a news release.
The most common side effects were headache, fatigue, diarrhea, nausea, joint pain, dizziness, myalgia, pruritus, vomiting, dyspnea, erythema, paresthesia, and urticaria.
Serious reactions included hypersensitivity reactions, such as anaphylaxis, and infusion-associated reactions, including respiratory distress, chills, and pyrexia.
Patients susceptible to fluid volume overload or those with compromised cardiac or respiratory function may be at risk for serious acute cardiorespiratory failure.
The FDA granted Nexviazyme orphan drug designation, priority review, and breakthrough status.
Genzyme expects the new therapy to be available in the United States in the coming weeks and said it will be priced on par with Lumizyme.
A version of this article first appeared on Medscape.com.
Internal mammary lymph node radiation safe over the long term
After a median follow-up of 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer related mortality, even before introducing heart-sparing techniques,” say investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp, Belgium.
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery, and decreased doses to non-target tissues,” the team says.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers comment.
The study was published online on July 28 in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweighs the benefits of better disease control, noted Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explains. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White says the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal (IMN) radiation.”
She notes that since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy, but cardiopulmonary complications are still possible even with improved techniques, she writes.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women, versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1% without.
The incidence of any cardiac disease was 11.1% in the radiation arm, versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors note that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and collegues note, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer related mortality with trials that began after 1988.
Still, “it seems logical to take the pre-existing cardiac comorbidity of patients into consideration,” the investigators conclude. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up, they write.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After a median follow-up of 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer related mortality, even before introducing heart-sparing techniques,” say investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp, Belgium.
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery, and decreased doses to non-target tissues,” the team says.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers comment.
The study was published online on July 28 in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweighs the benefits of better disease control, noted Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explains. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White says the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal (IMN) radiation.”
She notes that since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy, but cardiopulmonary complications are still possible even with improved techniques, she writes.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women, versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1% without.
The incidence of any cardiac disease was 11.1% in the radiation arm, versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors note that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and collegues note, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer related mortality with trials that began after 1988.
Still, “it seems logical to take the pre-existing cardiac comorbidity of patients into consideration,” the investigators conclude. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up, they write.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After a median follow-up of 15.7 years among almost 4,000 women, for half of patients who received postoperative internal mammary and medial supraclavicular (IM-MS) lymph node irradiation, the “absolute rates and differences” of heart and lung complications “were very low, with no increased non–breast cancer related mortality, even before introducing heart-sparing techniques,” say investigators.
The findings come from the European Organization for Research and Treatment of Cancer (EORTC) trial. The investigators were led by Philip Poortmans, MD, PhD, a radiation oncologist at the University of Antwerp, Belgium.
The team had previously reported lower breast cancer mortality and breast cancer recurrence rates in the radiation group.
Women in the trial were treated from 1996 to 2004. “We expect that with contemporary volume-based radiation therapy outcomes will be even better, by improved coverage of target volumes, more homogeneous dose delivery, and decreased doses to non-target tissues,” the team says.
In the end, “our findings ... have important – reassuring – consequences for decision-making concerning elective lymph node treatment in breast cancer,” the researchers comment.
The study was published online on July 28 in the Journal of the National Cancer Institute.
Resolving the debate
There’s been debate for decades on whether the long-term risk associated with nodal irradiation, particularly collateral heart and lung damage from internal mammary irradiation, outweighs the benefits of better disease control, noted Julia White, MD, a radiation oncologist at the Ohio State University Breast Center, Columbus, in an accompanying editorial.
Concerns stem originally from trials conducted from the 1950s to the 1970s. In those trials, higher doses of radiation were delivered to the internal mammary node with far less precision than today. Subsequent studies have not laid the worry to rest, and protocols vary across institutions, Dr. White explains. Some treat IM nodes in high-risk patients, but others only treat the axilla and the medial supraclavicular lymph nodes.
Dr. White says the new EORTC trial “moves us one step closer to resolving the debate about the value of internal mammary nodal (IMN) radiation.”
She notes that since 2014, advances in the field have led to an almost 50% reduction in cardiac radiation exposure during breast cancer treatment. Current guidelines recommend that internal mammary nodes “should generally be treated” as part of postmastectomy radiotherapy, but cardiopulmonary complications are still possible even with improved techniques, she writes.
Mostly grade 1 morbidity
Women in the study had stage I-III breast cancer with axillary node involvement and/or medially located primary tumors. The median age at study entry was 54 years. The patients were treated at 46 centers in 13 countries.
The group that received IM-MS irradiation after surgery received 50 Gy in 25 fractions over 5 weeks.
The cumulative 15-year incidence of lung fibrosis was 5.7% among treated women, versus 2.9% among control patients. The incidence of cardiac fibrosis was 1.9% with treatment, versus 1.1% without.
The incidence of any cardiac disease was 11.1% in the radiation arm, versus 9.4% in the control group.
Complications were mostly of grade 1. The only statistically significant difference in rates of events of grade 2 or higher was in the incidence of pulmonary morbidity, which was 0.8% with radiation versus 0.1% without. There were no differences in the incidence of second malignancies, contralateral breast cancer cases, or cardiovascular deaths with IMN irradiation.
The authors note that their results conflict with a 2013 study that found a relative increase in major coronary events of 7.4% per Gy mean heart dose. The women in that trial were treated in Sweden and Denmark between 1958 and 2001.
Dr. Poortmans and collegues note, however, that this 2013 study and others found a proportional and not an absolute increase in risk. With a baseline risk of 10%, for instance, a 7% increase per 1 Gy translates to a total risk of 10.07%.
Also, no increased risk has been reported in more recently published trials, and a meta-analysis found no increase in non–breast cancer related mortality with trials that began after 1988.
Still, “it seems logical to take the pre-existing cardiac comorbidity of patients into consideration,” the investigators conclude. For patients with higher baseline cardiopulmonary risk factors, lower mean heart doses should be used, and such patients should undergo longer-term follow-up, they write.
The study was funded by La Ligue Nationale Contre Le Cancer and the KWF Kanker Bestrijding from the Netherlands. The investigators and Dr. White have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Routine’ use of focal therapy for prostate cancer in next 5 years
They maintain that focal therapy (FT) offers a “middle ground” between two extremes: Treating the whole gland with radical prostatectomy or radiotherapy and not treating immediately via active surveillance or watchful waiting.
Focal therapy typically treats the primary lesion within the prostate, while leaving the rest of the gland intact. Most often performed with cryoablation or high-intensity focused ultrasound (HIFU), it can also be carried out with a variety of technologies, including transurethral ultrasound ablation and focal laser ablation.
The shift to focal therapy will coincide with maturing, long-term data from studies with various technologies, predict the authors, led by Amir Lebastchi, MD, a urologist at the University of Southern California.
“Standard adoption of focal therapy is ultimately dependent on the availability of robust level I evidence, which in turn will drive medical societies and payees,” the authors also write.
But payees are already making changes, even without such data, they add.
For example, in January the American Medical Association announced a new code for high-intensity focal ultrasound (HIFU): This approach now has a Current Procedural Terminology (CPT) code from the U.S. Centers for Medicare & Medicaid Services
This news organization reached out to Matthew Cooperberg, MD, MPH, a urologist at the University of California, San Francisco (UCSF), for comments about the essay’s optimism; he has questioned focal therapy in the past because of a lack of strong supporting evidence.
“While ‘routine’ is a bit of a vague term, now that HIFU has a CPT code, I do expect its use will in fact increase in the next 5 years,” Dr. Cooperberg wrote in an email. “The question is whether its use will increase appropriately.”
The challenge with focal therapy – regardless of energy modality – remains patient selection and accurate ablation zone definition, he added.
Notably, UCSF has launched a new HIFU program – and Dr. Cooperberg has referred selected patients. “I’m both enthusiastic and cautious about the future, and we need to track our outcomes very closely across various practice settings,” he said.
While waiting for CHRONOS, select wisely
The goal of focal therapy is to treat only the area with the most aggressive tumor, known as the index tumor, while leaving the remaining gland and its surrounding structures alone, according to Derek Lomas, MD, PharmD, a urologist at the Mayo Clinic in Rochester, Minn., in an explanatory article. “This approach is widely accepted in other types of cancer. For example, we commonly treat kidney cancers by removing or ablating only the tumor while leaving the rest of the kidney intact.”
However, some focal therapies also include approaches known as hemiablations, in which a full half of the prostate is destroyed, and approaches that leave very little of the gland behind.
Each of the modalities used for focal therapy has “unique indications, risks, and benefits and uses a different energy source for ablation,” Dr. Lebastchi and colleagues write in their essay.
They assert that focal therapy can provide oncological efficacy similar to radical prostatectomy or radiotherapy “while considerably reducing or even eliminating functional morbidities, such as incontinence and erectile dysfunction.”
Overall, they say focal therapy offers an opportunity for improved care because there is “an increasing body of emerging evidence demonstrating a favorable adverse effect profile with oncological control similar to whole-gland treatment options.”
What is that evidence?
In the essay, Dr. Lebastchi and colleagues point to a number of single-arm studies with encouraging efficacy and safety results. They also highlight a phase 3, randomized trial that they were involved in: This compared focal therapy (partial gland ablation with vascular-targeted photodynamic therapy) with active surveillance in early-stage disease and uniformly showed better post-treatment biopsy (disease/no disease) and conversion-to-prostatectomy results with the focal therapy out to 4 years (J Urol. 2018;200:786-793).
However, that study did not have an active treatment comparator. For that gold standard, there is now anticipation for results from the CHRONOS trial in the United Kingdom, especially part A of the trial, which compares radical therapy to focal therapy (HIFU or cryotherapy), with 5-year progression-free survival as the primary outcome. That trial is slated for completion in 2027.
Until then, the lack of prospective randomized clinical trials and long-term follow-up “hinders acceptance [of focal therapy] in the urology community,” the essay authors comment.
Meanwhile, careful patient selection is very important, they say.
The latest relevant guidelines state that appropriate candidates are men with a solitary, well-defined index lesion; patients with bilateral multifocal lesions; or very advanced tumors that are not appropriate for the focal approach.
A multidisciplinary international expert panel recently convened to establish guidance for clinicians offering focal therapies and then published a consensus statement to advise practitioners and researchers.
UCSF’s Dr. Cooperberg sees plenty of room for improvement among focal therapy practitioners and investigators. “From an outcomes standpoint, follow-up protocols and definitions of success remain inconsistent. I believe we’re making progress in all these areas, but we’re not there yet,” he says.
To date, some patients have been managed poorly, Dr. Cooperberg added. “We certainly see many patients who have been inadequately counseled as to HIFU’s advantages and disadvantages, with sometimes disastrous results.”
Some of those unfortunate results may have arisen from the U.S. Food and Drug Administration’s initial approval of HIFU in 2015, which was for use in ablating prostate tissue in general and not cancer specifically. This approval generated confusion, one expert commented at the time: “The FDA doesn’t specify whether it’s for benign or malignant disease; it’s a bit vague, like saying you can drive this car, but we’re not going to tell you how to drive it,” said Manoj Monga, MD, from the Cleveland Clinic.
Dr. Lebastchi has disclosed no relevant financial relationships; co-author Inderbir Gill, MD, is an unpaid consultant for Steba Biotech, and co-author Andre Luis Abreu, MD, is a consultant for Koelis and was a proctor in training for Steba Biotech. Dr. Cooperberg is a consultant for Alessa Therapeutics.
A version of this article first appeared on Medscape.com.
They maintain that focal therapy (FT) offers a “middle ground” between two extremes: Treating the whole gland with radical prostatectomy or radiotherapy and not treating immediately via active surveillance or watchful waiting.
Focal therapy typically treats the primary lesion within the prostate, while leaving the rest of the gland intact. Most often performed with cryoablation or high-intensity focused ultrasound (HIFU), it can also be carried out with a variety of technologies, including transurethral ultrasound ablation and focal laser ablation.
The shift to focal therapy will coincide with maturing, long-term data from studies with various technologies, predict the authors, led by Amir Lebastchi, MD, a urologist at the University of Southern California.
“Standard adoption of focal therapy is ultimately dependent on the availability of robust level I evidence, which in turn will drive medical societies and payees,” the authors also write.
But payees are already making changes, even without such data, they add.
For example, in January the American Medical Association announced a new code for high-intensity focal ultrasound (HIFU): This approach now has a Current Procedural Terminology (CPT) code from the U.S. Centers for Medicare & Medicaid Services
This news organization reached out to Matthew Cooperberg, MD, MPH, a urologist at the University of California, San Francisco (UCSF), for comments about the essay’s optimism; he has questioned focal therapy in the past because of a lack of strong supporting evidence.
“While ‘routine’ is a bit of a vague term, now that HIFU has a CPT code, I do expect its use will in fact increase in the next 5 years,” Dr. Cooperberg wrote in an email. “The question is whether its use will increase appropriately.”
The challenge with focal therapy – regardless of energy modality – remains patient selection and accurate ablation zone definition, he added.
Notably, UCSF has launched a new HIFU program – and Dr. Cooperberg has referred selected patients. “I’m both enthusiastic and cautious about the future, and we need to track our outcomes very closely across various practice settings,” he said.
While waiting for CHRONOS, select wisely
The goal of focal therapy is to treat only the area with the most aggressive tumor, known as the index tumor, while leaving the remaining gland and its surrounding structures alone, according to Derek Lomas, MD, PharmD, a urologist at the Mayo Clinic in Rochester, Minn., in an explanatory article. “This approach is widely accepted in other types of cancer. For example, we commonly treat kidney cancers by removing or ablating only the tumor while leaving the rest of the kidney intact.”
However, some focal therapies also include approaches known as hemiablations, in which a full half of the prostate is destroyed, and approaches that leave very little of the gland behind.
Each of the modalities used for focal therapy has “unique indications, risks, and benefits and uses a different energy source for ablation,” Dr. Lebastchi and colleagues write in their essay.
They assert that focal therapy can provide oncological efficacy similar to radical prostatectomy or radiotherapy “while considerably reducing or even eliminating functional morbidities, such as incontinence and erectile dysfunction.”
Overall, they say focal therapy offers an opportunity for improved care because there is “an increasing body of emerging evidence demonstrating a favorable adverse effect profile with oncological control similar to whole-gland treatment options.”
What is that evidence?
In the essay, Dr. Lebastchi and colleagues point to a number of single-arm studies with encouraging efficacy and safety results. They also highlight a phase 3, randomized trial that they were involved in: This compared focal therapy (partial gland ablation with vascular-targeted photodynamic therapy) with active surveillance in early-stage disease and uniformly showed better post-treatment biopsy (disease/no disease) and conversion-to-prostatectomy results with the focal therapy out to 4 years (J Urol. 2018;200:786-793).
However, that study did not have an active treatment comparator. For that gold standard, there is now anticipation for results from the CHRONOS trial in the United Kingdom, especially part A of the trial, which compares radical therapy to focal therapy (HIFU or cryotherapy), with 5-year progression-free survival as the primary outcome. That trial is slated for completion in 2027.
Until then, the lack of prospective randomized clinical trials and long-term follow-up “hinders acceptance [of focal therapy] in the urology community,” the essay authors comment.
Meanwhile, careful patient selection is very important, they say.
The latest relevant guidelines state that appropriate candidates are men with a solitary, well-defined index lesion; patients with bilateral multifocal lesions; or very advanced tumors that are not appropriate for the focal approach.
A multidisciplinary international expert panel recently convened to establish guidance for clinicians offering focal therapies and then published a consensus statement to advise practitioners and researchers.
UCSF’s Dr. Cooperberg sees plenty of room for improvement among focal therapy practitioners and investigators. “From an outcomes standpoint, follow-up protocols and definitions of success remain inconsistent. I believe we’re making progress in all these areas, but we’re not there yet,” he says.
To date, some patients have been managed poorly, Dr. Cooperberg added. “We certainly see many patients who have been inadequately counseled as to HIFU’s advantages and disadvantages, with sometimes disastrous results.”
Some of those unfortunate results may have arisen from the U.S. Food and Drug Administration’s initial approval of HIFU in 2015, which was for use in ablating prostate tissue in general and not cancer specifically. This approval generated confusion, one expert commented at the time: “The FDA doesn’t specify whether it’s for benign or malignant disease; it’s a bit vague, like saying you can drive this car, but we’re not going to tell you how to drive it,” said Manoj Monga, MD, from the Cleveland Clinic.
Dr. Lebastchi has disclosed no relevant financial relationships; co-author Inderbir Gill, MD, is an unpaid consultant for Steba Biotech, and co-author Andre Luis Abreu, MD, is a consultant for Koelis and was a proctor in training for Steba Biotech. Dr. Cooperberg is a consultant for Alessa Therapeutics.
A version of this article first appeared on Medscape.com.
They maintain that focal therapy (FT) offers a “middle ground” between two extremes: Treating the whole gland with radical prostatectomy or radiotherapy and not treating immediately via active surveillance or watchful waiting.
Focal therapy typically treats the primary lesion within the prostate, while leaving the rest of the gland intact. Most often performed with cryoablation or high-intensity focused ultrasound (HIFU), it can also be carried out with a variety of technologies, including transurethral ultrasound ablation and focal laser ablation.
The shift to focal therapy will coincide with maturing, long-term data from studies with various technologies, predict the authors, led by Amir Lebastchi, MD, a urologist at the University of Southern California.
“Standard adoption of focal therapy is ultimately dependent on the availability of robust level I evidence, which in turn will drive medical societies and payees,” the authors also write.
But payees are already making changes, even without such data, they add.
For example, in January the American Medical Association announced a new code for high-intensity focal ultrasound (HIFU): This approach now has a Current Procedural Terminology (CPT) code from the U.S. Centers for Medicare & Medicaid Services
This news organization reached out to Matthew Cooperberg, MD, MPH, a urologist at the University of California, San Francisco (UCSF), for comments about the essay’s optimism; he has questioned focal therapy in the past because of a lack of strong supporting evidence.
“While ‘routine’ is a bit of a vague term, now that HIFU has a CPT code, I do expect its use will in fact increase in the next 5 years,” Dr. Cooperberg wrote in an email. “The question is whether its use will increase appropriately.”
The challenge with focal therapy – regardless of energy modality – remains patient selection and accurate ablation zone definition, he added.
Notably, UCSF has launched a new HIFU program – and Dr. Cooperberg has referred selected patients. “I’m both enthusiastic and cautious about the future, and we need to track our outcomes very closely across various practice settings,” he said.
While waiting for CHRONOS, select wisely
The goal of focal therapy is to treat only the area with the most aggressive tumor, known as the index tumor, while leaving the remaining gland and its surrounding structures alone, according to Derek Lomas, MD, PharmD, a urologist at the Mayo Clinic in Rochester, Minn., in an explanatory article. “This approach is widely accepted in other types of cancer. For example, we commonly treat kidney cancers by removing or ablating only the tumor while leaving the rest of the kidney intact.”
However, some focal therapies also include approaches known as hemiablations, in which a full half of the prostate is destroyed, and approaches that leave very little of the gland behind.
Each of the modalities used for focal therapy has “unique indications, risks, and benefits and uses a different energy source for ablation,” Dr. Lebastchi and colleagues write in their essay.
They assert that focal therapy can provide oncological efficacy similar to radical prostatectomy or radiotherapy “while considerably reducing or even eliminating functional morbidities, such as incontinence and erectile dysfunction.”
Overall, they say focal therapy offers an opportunity for improved care because there is “an increasing body of emerging evidence demonstrating a favorable adverse effect profile with oncological control similar to whole-gland treatment options.”
What is that evidence?
In the essay, Dr. Lebastchi and colleagues point to a number of single-arm studies with encouraging efficacy and safety results. They also highlight a phase 3, randomized trial that they were involved in: This compared focal therapy (partial gland ablation with vascular-targeted photodynamic therapy) with active surveillance in early-stage disease and uniformly showed better post-treatment biopsy (disease/no disease) and conversion-to-prostatectomy results with the focal therapy out to 4 years (J Urol. 2018;200:786-793).
However, that study did not have an active treatment comparator. For that gold standard, there is now anticipation for results from the CHRONOS trial in the United Kingdom, especially part A of the trial, which compares radical therapy to focal therapy (HIFU or cryotherapy), with 5-year progression-free survival as the primary outcome. That trial is slated for completion in 2027.
Until then, the lack of prospective randomized clinical trials and long-term follow-up “hinders acceptance [of focal therapy] in the urology community,” the essay authors comment.
Meanwhile, careful patient selection is very important, they say.
The latest relevant guidelines state that appropriate candidates are men with a solitary, well-defined index lesion; patients with bilateral multifocal lesions; or very advanced tumors that are not appropriate for the focal approach.
A multidisciplinary international expert panel recently convened to establish guidance for clinicians offering focal therapies and then published a consensus statement to advise practitioners and researchers.
UCSF’s Dr. Cooperberg sees plenty of room for improvement among focal therapy practitioners and investigators. “From an outcomes standpoint, follow-up protocols and definitions of success remain inconsistent. I believe we’re making progress in all these areas, but we’re not there yet,” he says.
To date, some patients have been managed poorly, Dr. Cooperberg added. “We certainly see many patients who have been inadequately counseled as to HIFU’s advantages and disadvantages, with sometimes disastrous results.”
Some of those unfortunate results may have arisen from the U.S. Food and Drug Administration’s initial approval of HIFU in 2015, which was for use in ablating prostate tissue in general and not cancer specifically. This approval generated confusion, one expert commented at the time: “The FDA doesn’t specify whether it’s for benign or malignant disease; it’s a bit vague, like saying you can drive this car, but we’re not going to tell you how to drive it,” said Manoj Monga, MD, from the Cleveland Clinic.
Dr. Lebastchi has disclosed no relevant financial relationships; co-author Inderbir Gill, MD, is an unpaid consultant for Steba Biotech, and co-author Andre Luis Abreu, MD, is a consultant for Koelis and was a proctor in training for Steba Biotech. Dr. Cooperberg is a consultant for Alessa Therapeutics.
A version of this article first appeared on Medscape.com.
AGA Clinical Practice Update: Expert Review on colonoscopy quality improvement
The American Gastroenterological Association recently issued a clinical practice update expert review outlining tenets of high-quality colonoscopy screening and surveillance.
The update includes 15 best practice advice statements aimed at the endoscopist and/or endoscopy unit, reported lead author Rajesh N. Keswani, MD, of Northwestern University, Chicago, and colleagues.
“The efficacy of colonoscopy varies widely among endoscopists, and lower-quality colonoscopies are associated with higher interval CRC [colorectal cancer] incidence and mortality,” the investigators wrote in Gastroenterology.
According to Dr. Keswani and colleagues, quality of colonoscopy screening and surveillance is shaped by three parameters: safety, effectiveness, and value. Some metrics may be best measured at a unit level, they noted, while others are more clinician specific.
“For uncommon outcomes (e.g., adverse events) or metrics that reflect system-based practice (e.g., bowel preparation quality), measurement of aggregate unit-level performance is best,” the investigators wrote. “In contrast, for metrics that primarily reflect colonoscopist skill (e.g., adenoma detection rate), endoscopist-level measurement is preferred to enable individual feedback.”
Endoscopy unit best practice advice
According to the update, endoscopy units should prepare patients for, and monitor, adverse events. Prior to the procedure, patients should be informed about possible adverse events and warning symptoms, and emergency contact information should be recorded. Following the procedure, systematic monitoring of delayed adverse events may be considered, including “postprocedure bleeding, perforation, hospital readmission, 30-day mortality, and/or interval colorectal cancer cases,” with rates reported at the unit level.
Ensuring high-quality bowel preparation is also the responsibility of the endoscopy unit, according to Dr. Keswani and colleagues, and should be measured at least annually. Units should aim for a Boston Bowel Preparation Scale score of at least 6, with each segment scoring at least 2, in at least 90% of colonoscopies. The update provides best practice advice on split-dose bowel prep, with patient instructions written at a sixth-grade level in their native language. If routine quality measurement reveals suboptimal bowel prep quality, instruction revision may be needed, as well as further patient education and support.
During the actual procedure, a high-definition colonoscope should be used, the expert panel wrote. They called for measurement of endoscopist performance via four parameters: cecal intubation rate, which should be at least 90%; mean withdrawal time, which should be at least 6 minutes (aspirational, ≥9 minutes); adenoma detection rate, measured annually or when a given endoscopist has accrued 250 screening colonoscopies; and serrated lesion detection rate.
Endoscopist best practice advice
Both adenoma detection rate and serrated lesion detection rate should also be measured at an endoscopist level, with rates of at least 30% for adenomas and at least 7% for serrated lesions (aspirational, ≥35% and ≥10%, respectively).
“If rates are low, improvement efforts should be oriented toward both colonoscopists and pathologists,” the investigators noted.
A variety of strategies are advised to improve outcomes at the endoscopist level, including a second look at the right colon to detect polyps, either in forward or retroflexed view; use of cold-snare polypectomy for nonpedunculated polyps 3-9 mm in size and avoidance of forceps in polyps greater than 2 mm in size; evaluation by an expert in polypectomy with attempted resection for patients with complex polyps lacking “overt malignant endoscopic features or pathology consistent with invasive adenocarcinoma”; and thorough documentation of all findings.
More broadly, the update advises endoscopists to follow guideline-recommended intervals for screening and surveillance, including repeat colonoscopy in 3 years for all patients with advanced adenomas versus a 10-year interval for patients with normal risk or “only distal hyperplastic polyps.”
Resource-limited institutions and a look ahead
Dr. Keswani and colleagues concluded the clinical practice update with a nod to the challenges of real-world practice, noting that some institutions may not have the resources to comply with all the best practice advice statements.
“If limited resources are available, measurement of cecal intubation rates, bowel preparation quality, and adenoma detection rate should be prioritized,” they wrote.
They also offered a succinct summary of outstanding research needs, saying “we anticipate future work to clarify optimal polyp resection techniques, refine surveillance intervals based on provider skill and patient risk, and highlight the benefits of artificial intelligence in improving colonoscopy quality.”
This clinical practice update was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. Dr. Keswani consults for Boston Scientific. The other authors had no disclosures.
This article was updated Aug. 20, 2021.
The American Gastroenterological Association recently issued a clinical practice update expert review outlining tenets of high-quality colonoscopy screening and surveillance.
The update includes 15 best practice advice statements aimed at the endoscopist and/or endoscopy unit, reported lead author Rajesh N. Keswani, MD, of Northwestern University, Chicago, and colleagues.
“The efficacy of colonoscopy varies widely among endoscopists, and lower-quality colonoscopies are associated with higher interval CRC [colorectal cancer] incidence and mortality,” the investigators wrote in Gastroenterology.
According to Dr. Keswani and colleagues, quality of colonoscopy screening and surveillance is shaped by three parameters: safety, effectiveness, and value. Some metrics may be best measured at a unit level, they noted, while others are more clinician specific.
“For uncommon outcomes (e.g., adverse events) or metrics that reflect system-based practice (e.g., bowel preparation quality), measurement of aggregate unit-level performance is best,” the investigators wrote. “In contrast, for metrics that primarily reflect colonoscopist skill (e.g., adenoma detection rate), endoscopist-level measurement is preferred to enable individual feedback.”
Endoscopy unit best practice advice
According to the update, endoscopy units should prepare patients for, and monitor, adverse events. Prior to the procedure, patients should be informed about possible adverse events and warning symptoms, and emergency contact information should be recorded. Following the procedure, systematic monitoring of delayed adverse events may be considered, including “postprocedure bleeding, perforation, hospital readmission, 30-day mortality, and/or interval colorectal cancer cases,” with rates reported at the unit level.
Ensuring high-quality bowel preparation is also the responsibility of the endoscopy unit, according to Dr. Keswani and colleagues, and should be measured at least annually. Units should aim for a Boston Bowel Preparation Scale score of at least 6, with each segment scoring at least 2, in at least 90% of colonoscopies. The update provides best practice advice on split-dose bowel prep, with patient instructions written at a sixth-grade level in their native language. If routine quality measurement reveals suboptimal bowel prep quality, instruction revision may be needed, as well as further patient education and support.
During the actual procedure, a high-definition colonoscope should be used, the expert panel wrote. They called for measurement of endoscopist performance via four parameters: cecal intubation rate, which should be at least 90%; mean withdrawal time, which should be at least 6 minutes (aspirational, ≥9 minutes); adenoma detection rate, measured annually or when a given endoscopist has accrued 250 screening colonoscopies; and serrated lesion detection rate.
Endoscopist best practice advice
Both adenoma detection rate and serrated lesion detection rate should also be measured at an endoscopist level, with rates of at least 30% for adenomas and at least 7% for serrated lesions (aspirational, ≥35% and ≥10%, respectively).
“If rates are low, improvement efforts should be oriented toward both colonoscopists and pathologists,” the investigators noted.
A variety of strategies are advised to improve outcomes at the endoscopist level, including a second look at the right colon to detect polyps, either in forward or retroflexed view; use of cold-snare polypectomy for nonpedunculated polyps 3-9 mm in size and avoidance of forceps in polyps greater than 2 mm in size; evaluation by an expert in polypectomy with attempted resection for patients with complex polyps lacking “overt malignant endoscopic features or pathology consistent with invasive adenocarcinoma”; and thorough documentation of all findings.
More broadly, the update advises endoscopists to follow guideline-recommended intervals for screening and surveillance, including repeat colonoscopy in 3 years for all patients with advanced adenomas versus a 10-year interval for patients with normal risk or “only distal hyperplastic polyps.”
Resource-limited institutions and a look ahead
Dr. Keswani and colleagues concluded the clinical practice update with a nod to the challenges of real-world practice, noting that some institutions may not have the resources to comply with all the best practice advice statements.
“If limited resources are available, measurement of cecal intubation rates, bowel preparation quality, and adenoma detection rate should be prioritized,” they wrote.
They also offered a succinct summary of outstanding research needs, saying “we anticipate future work to clarify optimal polyp resection techniques, refine surveillance intervals based on provider skill and patient risk, and highlight the benefits of artificial intelligence in improving colonoscopy quality.”
This clinical practice update was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. Dr. Keswani consults for Boston Scientific. The other authors had no disclosures.
This article was updated Aug. 20, 2021.
The American Gastroenterological Association recently issued a clinical practice update expert review outlining tenets of high-quality colonoscopy screening and surveillance.
The update includes 15 best practice advice statements aimed at the endoscopist and/or endoscopy unit, reported lead author Rajesh N. Keswani, MD, of Northwestern University, Chicago, and colleagues.
“The efficacy of colonoscopy varies widely among endoscopists, and lower-quality colonoscopies are associated with higher interval CRC [colorectal cancer] incidence and mortality,” the investigators wrote in Gastroenterology.
According to Dr. Keswani and colleagues, quality of colonoscopy screening and surveillance is shaped by three parameters: safety, effectiveness, and value. Some metrics may be best measured at a unit level, they noted, while others are more clinician specific.
“For uncommon outcomes (e.g., adverse events) or metrics that reflect system-based practice (e.g., bowel preparation quality), measurement of aggregate unit-level performance is best,” the investigators wrote. “In contrast, for metrics that primarily reflect colonoscopist skill (e.g., adenoma detection rate), endoscopist-level measurement is preferred to enable individual feedback.”
Endoscopy unit best practice advice
According to the update, endoscopy units should prepare patients for, and monitor, adverse events. Prior to the procedure, patients should be informed about possible adverse events and warning symptoms, and emergency contact information should be recorded. Following the procedure, systematic monitoring of delayed adverse events may be considered, including “postprocedure bleeding, perforation, hospital readmission, 30-day mortality, and/or interval colorectal cancer cases,” with rates reported at the unit level.
Ensuring high-quality bowel preparation is also the responsibility of the endoscopy unit, according to Dr. Keswani and colleagues, and should be measured at least annually. Units should aim for a Boston Bowel Preparation Scale score of at least 6, with each segment scoring at least 2, in at least 90% of colonoscopies. The update provides best practice advice on split-dose bowel prep, with patient instructions written at a sixth-grade level in their native language. If routine quality measurement reveals suboptimal bowel prep quality, instruction revision may be needed, as well as further patient education and support.
During the actual procedure, a high-definition colonoscope should be used, the expert panel wrote. They called for measurement of endoscopist performance via four parameters: cecal intubation rate, which should be at least 90%; mean withdrawal time, which should be at least 6 minutes (aspirational, ≥9 minutes); adenoma detection rate, measured annually or when a given endoscopist has accrued 250 screening colonoscopies; and serrated lesion detection rate.
Endoscopist best practice advice
Both adenoma detection rate and serrated lesion detection rate should also be measured at an endoscopist level, with rates of at least 30% for adenomas and at least 7% for serrated lesions (aspirational, ≥35% and ≥10%, respectively).
“If rates are low, improvement efforts should be oriented toward both colonoscopists and pathologists,” the investigators noted.
A variety of strategies are advised to improve outcomes at the endoscopist level, including a second look at the right colon to detect polyps, either in forward or retroflexed view; use of cold-snare polypectomy for nonpedunculated polyps 3-9 mm in size and avoidance of forceps in polyps greater than 2 mm in size; evaluation by an expert in polypectomy with attempted resection for patients with complex polyps lacking “overt malignant endoscopic features or pathology consistent with invasive adenocarcinoma”; and thorough documentation of all findings.
More broadly, the update advises endoscopists to follow guideline-recommended intervals for screening and surveillance, including repeat colonoscopy in 3 years for all patients with advanced adenomas versus a 10-year interval for patients with normal risk or “only distal hyperplastic polyps.”
Resource-limited institutions and a look ahead
Dr. Keswani and colleagues concluded the clinical practice update with a nod to the challenges of real-world practice, noting that some institutions may not have the resources to comply with all the best practice advice statements.
“If limited resources are available, measurement of cecal intubation rates, bowel preparation quality, and adenoma detection rate should be prioritized,” they wrote.
They also offered a succinct summary of outstanding research needs, saying “we anticipate future work to clarify optimal polyp resection techniques, refine surveillance intervals based on provider skill and patient risk, and highlight the benefits of artificial intelligence in improving colonoscopy quality.”
This clinical practice update was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. Dr. Keswani consults for Boston Scientific. The other authors had no disclosures.
This article was updated Aug. 20, 2021.
FROM GASTROENTEROLOGY