User login
The first signs of elusive dysautonomia may appear on the skin
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
FROM SPD 2021
Dissolving pacemaker impressive in early research
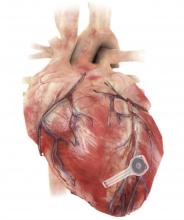
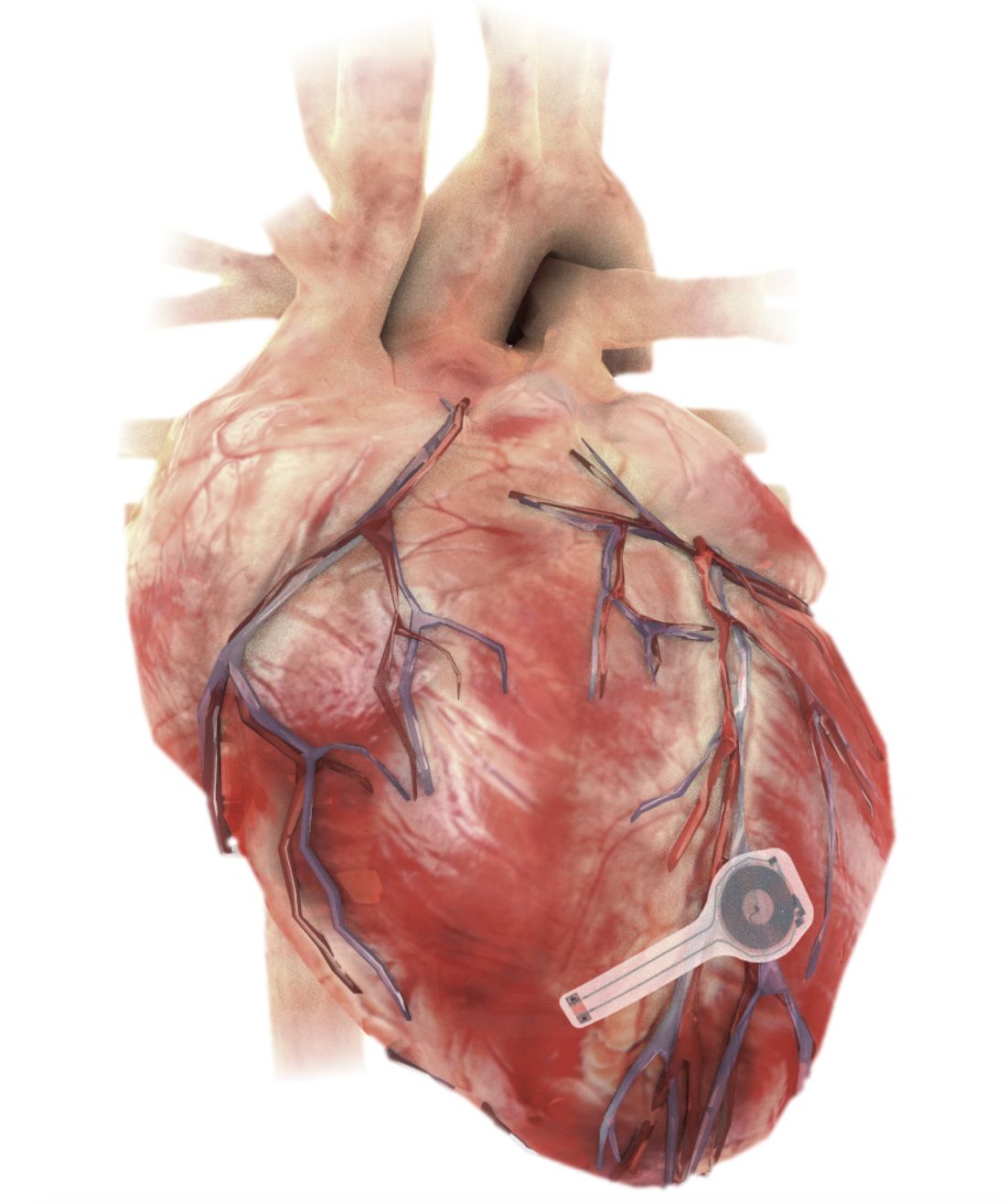
A fully implantable, bioresorbable pacemaker has been developed that’s capable of sustaining heart rhythms in animal and human donor hearts before disappearing over 5-7 weeks.
Temporary pacing devices are frequently used after cardiac surgery but rely on bulky external generators and transcutaneous pacing leads that run the risk of becoming infected or dislodged and can damage the heart when removed if they’re enveloped in fibrotic tissue.
The experimental device is thin, powered without leads or batteries, and made of water-soluble, biocompatible materials, thereby bypassing many of the disadvantages of conventional temporary pacing devices, according to John A. Rogers, PhD, who led the device’s development and directs the Querrey Simpson Institute for Bioelectronics at Northwestern University in Chicago.
“The total material load on the body is very minimal,” he said in an interview. “The amount of silicon and magnesium in a multivitamin tablet is about 3,000 times more than the amount of those materials in our electronics. So you can think of them as a very tiny vitamin pill, in a sense, but configured with electronic functionality.”
Dr. Rogers and his team have a reputation for innovation in bioelectronic medicine, having recently constructed transient wireless devices to accelerate neuroregeneration associated with damaged peripheral nerves, to monitor critically ill neonates, and to detect early signs and symptoms associated with COVID-19.
Shortly after Dr. Rogers joined Northwestern, Rishi Arora, MD, a cardiac electrophysiologist and professor of medicine at Northwestern, reached out to discuss how they could leverage wireless electronics for patients needing temporary pacing.
“It was a natural marriage,” Dr. Arora said in an interview. “Part of the reason to go into the heart was because the cardiology group here at Northwestern, especially on the electrophysiology side, has been very involved in translational research, and John also had a very strong collaboration before he came here with Igor Efimov, [PhD, of George Washington University, Washington], a giant in the field in terms of heart rhythm research.”
Dr. Arora noted that the incidence of temporary pacing after cardiac surgery is at least 10% but can reach 20%. Current devices work well in most patients, but temporary pacing with epicardial wires can cause complications and, typically, work well only for a few days after cardiac surgery. Clinically, though, several patients need postoperative pacing support for 1-2 weeks.
“So if something like this were available where you could tack it onto the surface and forget it for a week or 10 days or 2 weeks, you’d be doing those 20% of patients a huge service,” he said.
Bioresorbable scaffold déjà vu?
The philosophy of “leave nothing behind” is nothing new in cardiology, with bioresorbable vascular scaffolds (BVS) gaining initial support as a potential solution to neoatherosclerosis and late-stent thrombosis in permanent metal stents. Failure to show advantages, and safety concerns such as in-scaffold thrombosis, however, led Abbott to stop global sales of the first approved BVS and Boston Scientific to halt its BVS program in 2017.
The wireless pacemaker, however, is an electrical device, not a mechanical one, observed Dr. Rogers. “The fact that it’s not in the bloodstream greatly lowers risks and, as I mentioned before, everything is super thin, low-mass quantities of materials. So, I guess there’s a relationship there, but it’s different in a couple of very important ways.”
As Dr. Rogers, Dr. Arora, Dr. Efimov, and colleagues recently reported in Nature Biotechnology, the electronic part of the pacemaker contains three layers: A loop antenna with a bilayer tungsten-coated magnesium inductive coil, a radiofrequency PIN diode based on a monocrystalline silicon nanomembrane, and a poly (lactide-co-glycolide) (PLGA) dielectric interlayer.
The electronic components rest between two encapsulation layers of PLGA to isolate the active materials from the surrounding biofluids during implantation, and connect to a pair of flexible extension electrodes that deliver the electrical stimuli to a contact pad sutured onto the heart. The entire system is about 16 mm in width and 15 mm in length, and weighs in at about 0.3 g.
The pacemaker receives power and control commands through a wireless inductive power transfer – the same technology used in implanted medical devices, smartphones, and radio-frequency identification tags – between the receiver coil in the device and a wand-shaped, external transmission coil placed on top of or within a few inches of the heart.
“Right now we’re almost at 15 inches, which I think is a very respectable distance for this particular piece of hardware, and clinically very doable,” observed Dr. Arora.
Competing considerations
Testing thus far shows effective ventricular capture across a range of frequencies in mouse and rabbit hearts and successful pacing and activation of human cardiac tissue.
In vivo tests in dogs also suggest that the system can “achieve the power necessary for operation of bioresorbable pacemakers in adult human patients,” the authors say.
Electrodes placed on the dogs’ legs showed a change in ECG signals from a narrow QRS complex (consistent with a normal rate sinus rhythm of 350-400 bpm) to a widened QRS complex with a shortened R-R interval (consistent with a paced rhythm of 400-450 bpm) – indicating successful ventricular capture.
The device successfully paced the dogs through postoperative day 4 but couldn’t provide enough energy to capture the ventricular myocardium on day 5 and failed to pace the heart on day 6, even when transmitting voltages were increased from 1 Vpp to more than 10 Vpp.
Dr. Rogers pointed out that a transient device of theirs that uses very thin films of silica provides stable intracranial pressure monitoring for traumatic brain injury recovery for 3 weeks before dissolving. The problem with the polymers used as encapsulating layers in the pacemaker is that even if they haven’t completely dissolved, there’s a finite rate of water permeation through the film.
“It turns out that’s what’s become the limiting factor, rather than the chemistry of bioresorption,” he said. “So, what we’re seeing with these devices beginning to degrade electrically in terms of performance around 5-6 days is due to that water permeation.”
Although it is not part of the current study, there’s no reason thin silica layers couldn’t be incorporated into the pacemaker to make it less water permeable, Dr. Rogers said. Still, this will have to be weighed against the competing consideration of stable operating life.
The researchers specifically chose materials that would naturally bioresorb via hydrolysis and metabolic action in the body. PLGA degrades into glycolic and lactic acid, the tungsten-coated magnesium inductive coil into Wox and Mg(OH)2, and the silicon nanomembrane radiofrequency PIN diode into Si(OH)4.
CT imaging in rat models shows the device is enveloped in fibrotic tissue and completely decouples from the heart at 4 weeks, while images of explanted devices suggest the pacemaker largely dissolves within 3 weeks and the remaining residues disappear after 12 weeks.
The researchers have started an investigational device exemption process to allow the device to be used in clinical trials, and they plan to dig deeper into the potential for fragments to form at various stages of resorption, which some imaging suggests may occur.
“Because these devices are made out of pure materials and they’re in a heterogeneous environment, both mechanically and biomechanically, the devices don’t resorb in a perfectly uniform way and, as a result, at the tail end of the process you can end up with small fragments that eventually bioresorb, but before they’re gone, they are potentially mobile within the body cavity,” Dr. Rogers said.
“We feel that because the devices aren’t in the bloodstream, the risk associated with those fragments is probably manageable but at the same time, these are the sorts of details that must be thoroughly addressed before trials in humans,” he said, adding that one solution, if needed, would be to encapsulate the entire device in a thin bioresorbable hydrogel as a containment vehicle.
Dr. Arora said they hope the pacemaker “will make patients’ lives a lot easier in the postoperative setting but, even there, I think one must remember current pacing technology in this setting is actually very good. So there’s a word of caution not to get ahead of ourselves.”
Looking forward, the excitement of this approach is not only in the immediate postop setting but in the transvenous setting, he said. “If we can get to the point where we can actually do this transvenously, that opens up a huge window of opportunity because there we’re talking about post-TAVR [transcatheter aortic valve replacement], post–myocardial infarction, etc.”
Currently, temporary transvenous pacing can be quite unreliable because of a high risk of dislodgement and infection – much higher than for surgical pacing wires, he noted.
“In terms of translatability to larger numbers of patients, the value would be huge. But again, a lot needs to be done before we can get there. But if it can get to that point, then I think you have a real therapy that could potentially be transformative,” Dr. Arora said.
Dr. Rogers reported support from the Leducq Foundation projects RHYTHM and ROI-HL121270. Dr. Arora has disclosed no relevant financial relationships. Coauthor disclosures are listed in the original article.
A version of this article first appeared on Medscape.com.
A fully implantable, bioresorbable pacemaker has been developed that’s capable of sustaining heart rhythms in animal and human donor hearts before disappearing over 5-7 weeks.
Temporary pacing devices are frequently used after cardiac surgery but rely on bulky external generators and transcutaneous pacing leads that run the risk of becoming infected or dislodged and can damage the heart when removed if they’re enveloped in fibrotic tissue.
The experimental device is thin, powered without leads or batteries, and made of water-soluble, biocompatible materials, thereby bypassing many of the disadvantages of conventional temporary pacing devices, according to John A. Rogers, PhD, who led the device’s development and directs the Querrey Simpson Institute for Bioelectronics at Northwestern University in Chicago.
“The total material load on the body is very minimal,” he said in an interview. “The amount of silicon and magnesium in a multivitamin tablet is about 3,000 times more than the amount of those materials in our electronics. So you can think of them as a very tiny vitamin pill, in a sense, but configured with electronic functionality.”
Dr. Rogers and his team have a reputation for innovation in bioelectronic medicine, having recently constructed transient wireless devices to accelerate neuroregeneration associated with damaged peripheral nerves, to monitor critically ill neonates, and to detect early signs and symptoms associated with COVID-19.
Shortly after Dr. Rogers joined Northwestern, Rishi Arora, MD, a cardiac electrophysiologist and professor of medicine at Northwestern, reached out to discuss how they could leverage wireless electronics for patients needing temporary pacing.
“It was a natural marriage,” Dr. Arora said in an interview. “Part of the reason to go into the heart was because the cardiology group here at Northwestern, especially on the electrophysiology side, has been very involved in translational research, and John also had a very strong collaboration before he came here with Igor Efimov, [PhD, of George Washington University, Washington], a giant in the field in terms of heart rhythm research.”
Dr. Arora noted that the incidence of temporary pacing after cardiac surgery is at least 10% but can reach 20%. Current devices work well in most patients, but temporary pacing with epicardial wires can cause complications and, typically, work well only for a few days after cardiac surgery. Clinically, though, several patients need postoperative pacing support for 1-2 weeks.
“So if something like this were available where you could tack it onto the surface and forget it for a week or 10 days or 2 weeks, you’d be doing those 20% of patients a huge service,” he said.
Bioresorbable scaffold déjà vu?
The philosophy of “leave nothing behind” is nothing new in cardiology, with bioresorbable vascular scaffolds (BVS) gaining initial support as a potential solution to neoatherosclerosis and late-stent thrombosis in permanent metal stents. Failure to show advantages, and safety concerns such as in-scaffold thrombosis, however, led Abbott to stop global sales of the first approved BVS and Boston Scientific to halt its BVS program in 2017.
The wireless pacemaker, however, is an electrical device, not a mechanical one, observed Dr. Rogers. “The fact that it’s not in the bloodstream greatly lowers risks and, as I mentioned before, everything is super thin, low-mass quantities of materials. So, I guess there’s a relationship there, but it’s different in a couple of very important ways.”
As Dr. Rogers, Dr. Arora, Dr. Efimov, and colleagues recently reported in Nature Biotechnology, the electronic part of the pacemaker contains three layers: A loop antenna with a bilayer tungsten-coated magnesium inductive coil, a radiofrequency PIN diode based on a monocrystalline silicon nanomembrane, and a poly (lactide-co-glycolide) (PLGA) dielectric interlayer.
The electronic components rest between two encapsulation layers of PLGA to isolate the active materials from the surrounding biofluids during implantation, and connect to a pair of flexible extension electrodes that deliver the electrical stimuli to a contact pad sutured onto the heart. The entire system is about 16 mm in width and 15 mm in length, and weighs in at about 0.3 g.
The pacemaker receives power and control commands through a wireless inductive power transfer – the same technology used in implanted medical devices, smartphones, and radio-frequency identification tags – between the receiver coil in the device and a wand-shaped, external transmission coil placed on top of or within a few inches of the heart.
“Right now we’re almost at 15 inches, which I think is a very respectable distance for this particular piece of hardware, and clinically very doable,” observed Dr. Arora.
Competing considerations
Testing thus far shows effective ventricular capture across a range of frequencies in mouse and rabbit hearts and successful pacing and activation of human cardiac tissue.
In vivo tests in dogs also suggest that the system can “achieve the power necessary for operation of bioresorbable pacemakers in adult human patients,” the authors say.
Electrodes placed on the dogs’ legs showed a change in ECG signals from a narrow QRS complex (consistent with a normal rate sinus rhythm of 350-400 bpm) to a widened QRS complex with a shortened R-R interval (consistent with a paced rhythm of 400-450 bpm) – indicating successful ventricular capture.
The device successfully paced the dogs through postoperative day 4 but couldn’t provide enough energy to capture the ventricular myocardium on day 5 and failed to pace the heart on day 6, even when transmitting voltages were increased from 1 Vpp to more than 10 Vpp.
Dr. Rogers pointed out that a transient device of theirs that uses very thin films of silica provides stable intracranial pressure monitoring for traumatic brain injury recovery for 3 weeks before dissolving. The problem with the polymers used as encapsulating layers in the pacemaker is that even if they haven’t completely dissolved, there’s a finite rate of water permeation through the film.
“It turns out that’s what’s become the limiting factor, rather than the chemistry of bioresorption,” he said. “So, what we’re seeing with these devices beginning to degrade electrically in terms of performance around 5-6 days is due to that water permeation.”
Although it is not part of the current study, there’s no reason thin silica layers couldn’t be incorporated into the pacemaker to make it less water permeable, Dr. Rogers said. Still, this will have to be weighed against the competing consideration of stable operating life.
The researchers specifically chose materials that would naturally bioresorb via hydrolysis and metabolic action in the body. PLGA degrades into glycolic and lactic acid, the tungsten-coated magnesium inductive coil into Wox and Mg(OH)2, and the silicon nanomembrane radiofrequency PIN diode into Si(OH)4.
CT imaging in rat models shows the device is enveloped in fibrotic tissue and completely decouples from the heart at 4 weeks, while images of explanted devices suggest the pacemaker largely dissolves within 3 weeks and the remaining residues disappear after 12 weeks.
The researchers have started an investigational device exemption process to allow the device to be used in clinical trials, and they plan to dig deeper into the potential for fragments to form at various stages of resorption, which some imaging suggests may occur.
“Because these devices are made out of pure materials and they’re in a heterogeneous environment, both mechanically and biomechanically, the devices don’t resorb in a perfectly uniform way and, as a result, at the tail end of the process you can end up with small fragments that eventually bioresorb, but before they’re gone, they are potentially mobile within the body cavity,” Dr. Rogers said.
“We feel that because the devices aren’t in the bloodstream, the risk associated with those fragments is probably manageable but at the same time, these are the sorts of details that must be thoroughly addressed before trials in humans,” he said, adding that one solution, if needed, would be to encapsulate the entire device in a thin bioresorbable hydrogel as a containment vehicle.
Dr. Arora said they hope the pacemaker “will make patients’ lives a lot easier in the postoperative setting but, even there, I think one must remember current pacing technology in this setting is actually very good. So there’s a word of caution not to get ahead of ourselves.”
Looking forward, the excitement of this approach is not only in the immediate postop setting but in the transvenous setting, he said. “If we can get to the point where we can actually do this transvenously, that opens up a huge window of opportunity because there we’re talking about post-TAVR [transcatheter aortic valve replacement], post–myocardial infarction, etc.”
Currently, temporary transvenous pacing can be quite unreliable because of a high risk of dislodgement and infection – much higher than for surgical pacing wires, he noted.
“In terms of translatability to larger numbers of patients, the value would be huge. But again, a lot needs to be done before we can get there. But if it can get to that point, then I think you have a real therapy that could potentially be transformative,” Dr. Arora said.
Dr. Rogers reported support from the Leducq Foundation projects RHYTHM and ROI-HL121270. Dr. Arora has disclosed no relevant financial relationships. Coauthor disclosures are listed in the original article.
A version of this article first appeared on Medscape.com.
A fully implantable, bioresorbable pacemaker has been developed that’s capable of sustaining heart rhythms in animal and human donor hearts before disappearing over 5-7 weeks.
Temporary pacing devices are frequently used after cardiac surgery but rely on bulky external generators and transcutaneous pacing leads that run the risk of becoming infected or dislodged and can damage the heart when removed if they’re enveloped in fibrotic tissue.
The experimental device is thin, powered without leads or batteries, and made of water-soluble, biocompatible materials, thereby bypassing many of the disadvantages of conventional temporary pacing devices, according to John A. Rogers, PhD, who led the device’s development and directs the Querrey Simpson Institute for Bioelectronics at Northwestern University in Chicago.
“The total material load on the body is very minimal,” he said in an interview. “The amount of silicon and magnesium in a multivitamin tablet is about 3,000 times more than the amount of those materials in our electronics. So you can think of them as a very tiny vitamin pill, in a sense, but configured with electronic functionality.”
Dr. Rogers and his team have a reputation for innovation in bioelectronic medicine, having recently constructed transient wireless devices to accelerate neuroregeneration associated with damaged peripheral nerves, to monitor critically ill neonates, and to detect early signs and symptoms associated with COVID-19.
Shortly after Dr. Rogers joined Northwestern, Rishi Arora, MD, a cardiac electrophysiologist and professor of medicine at Northwestern, reached out to discuss how they could leverage wireless electronics for patients needing temporary pacing.
“It was a natural marriage,” Dr. Arora said in an interview. “Part of the reason to go into the heart was because the cardiology group here at Northwestern, especially on the electrophysiology side, has been very involved in translational research, and John also had a very strong collaboration before he came here with Igor Efimov, [PhD, of George Washington University, Washington], a giant in the field in terms of heart rhythm research.”
Dr. Arora noted that the incidence of temporary pacing after cardiac surgery is at least 10% but can reach 20%. Current devices work well in most patients, but temporary pacing with epicardial wires can cause complications and, typically, work well only for a few days after cardiac surgery. Clinically, though, several patients need postoperative pacing support for 1-2 weeks.
“So if something like this were available where you could tack it onto the surface and forget it for a week or 10 days or 2 weeks, you’d be doing those 20% of patients a huge service,” he said.
Bioresorbable scaffold déjà vu?
The philosophy of “leave nothing behind” is nothing new in cardiology, with bioresorbable vascular scaffolds (BVS) gaining initial support as a potential solution to neoatherosclerosis and late-stent thrombosis in permanent metal stents. Failure to show advantages, and safety concerns such as in-scaffold thrombosis, however, led Abbott to stop global sales of the first approved BVS and Boston Scientific to halt its BVS program in 2017.
The wireless pacemaker, however, is an electrical device, not a mechanical one, observed Dr. Rogers. “The fact that it’s not in the bloodstream greatly lowers risks and, as I mentioned before, everything is super thin, low-mass quantities of materials. So, I guess there’s a relationship there, but it’s different in a couple of very important ways.”
As Dr. Rogers, Dr. Arora, Dr. Efimov, and colleagues recently reported in Nature Biotechnology, the electronic part of the pacemaker contains three layers: A loop antenna with a bilayer tungsten-coated magnesium inductive coil, a radiofrequency PIN diode based on a monocrystalline silicon nanomembrane, and a poly (lactide-co-glycolide) (PLGA) dielectric interlayer.
The electronic components rest between two encapsulation layers of PLGA to isolate the active materials from the surrounding biofluids during implantation, and connect to a pair of flexible extension electrodes that deliver the electrical stimuli to a contact pad sutured onto the heart. The entire system is about 16 mm in width and 15 mm in length, and weighs in at about 0.3 g.
The pacemaker receives power and control commands through a wireless inductive power transfer – the same technology used in implanted medical devices, smartphones, and radio-frequency identification tags – between the receiver coil in the device and a wand-shaped, external transmission coil placed on top of or within a few inches of the heart.
“Right now we’re almost at 15 inches, which I think is a very respectable distance for this particular piece of hardware, and clinically very doable,” observed Dr. Arora.
Competing considerations
Testing thus far shows effective ventricular capture across a range of frequencies in mouse and rabbit hearts and successful pacing and activation of human cardiac tissue.
In vivo tests in dogs also suggest that the system can “achieve the power necessary for operation of bioresorbable pacemakers in adult human patients,” the authors say.
Electrodes placed on the dogs’ legs showed a change in ECG signals from a narrow QRS complex (consistent with a normal rate sinus rhythm of 350-400 bpm) to a widened QRS complex with a shortened R-R interval (consistent with a paced rhythm of 400-450 bpm) – indicating successful ventricular capture.
The device successfully paced the dogs through postoperative day 4 but couldn’t provide enough energy to capture the ventricular myocardium on day 5 and failed to pace the heart on day 6, even when transmitting voltages were increased from 1 Vpp to more than 10 Vpp.
Dr. Rogers pointed out that a transient device of theirs that uses very thin films of silica provides stable intracranial pressure monitoring for traumatic brain injury recovery for 3 weeks before dissolving. The problem with the polymers used as encapsulating layers in the pacemaker is that even if they haven’t completely dissolved, there’s a finite rate of water permeation through the film.
“It turns out that’s what’s become the limiting factor, rather than the chemistry of bioresorption,” he said. “So, what we’re seeing with these devices beginning to degrade electrically in terms of performance around 5-6 days is due to that water permeation.”
Although it is not part of the current study, there’s no reason thin silica layers couldn’t be incorporated into the pacemaker to make it less water permeable, Dr. Rogers said. Still, this will have to be weighed against the competing consideration of stable operating life.
The researchers specifically chose materials that would naturally bioresorb via hydrolysis and metabolic action in the body. PLGA degrades into glycolic and lactic acid, the tungsten-coated magnesium inductive coil into Wox and Mg(OH)2, and the silicon nanomembrane radiofrequency PIN diode into Si(OH)4.
CT imaging in rat models shows the device is enveloped in fibrotic tissue and completely decouples from the heart at 4 weeks, while images of explanted devices suggest the pacemaker largely dissolves within 3 weeks and the remaining residues disappear after 12 weeks.
The researchers have started an investigational device exemption process to allow the device to be used in clinical trials, and they plan to dig deeper into the potential for fragments to form at various stages of resorption, which some imaging suggests may occur.
“Because these devices are made out of pure materials and they’re in a heterogeneous environment, both mechanically and biomechanically, the devices don’t resorb in a perfectly uniform way and, as a result, at the tail end of the process you can end up with small fragments that eventually bioresorb, but before they’re gone, they are potentially mobile within the body cavity,” Dr. Rogers said.
“We feel that because the devices aren’t in the bloodstream, the risk associated with those fragments is probably manageable but at the same time, these are the sorts of details that must be thoroughly addressed before trials in humans,” he said, adding that one solution, if needed, would be to encapsulate the entire device in a thin bioresorbable hydrogel as a containment vehicle.
Dr. Arora said they hope the pacemaker “will make patients’ lives a lot easier in the postoperative setting but, even there, I think one must remember current pacing technology in this setting is actually very good. So there’s a word of caution not to get ahead of ourselves.”
Looking forward, the excitement of this approach is not only in the immediate postop setting but in the transvenous setting, he said. “If we can get to the point where we can actually do this transvenously, that opens up a huge window of opportunity because there we’re talking about post-TAVR [transcatheter aortic valve replacement], post–myocardial infarction, etc.”
Currently, temporary transvenous pacing can be quite unreliable because of a high risk of dislodgement and infection – much higher than for surgical pacing wires, he noted.
“In terms of translatability to larger numbers of patients, the value would be huge. But again, a lot needs to be done before we can get there. But if it can get to that point, then I think you have a real therapy that could potentially be transformative,” Dr. Arora said.
Dr. Rogers reported support from the Leducq Foundation projects RHYTHM and ROI-HL121270. Dr. Arora has disclosed no relevant financial relationships. Coauthor disclosures are listed in the original article.
A version of this article first appeared on Medscape.com.
Aspirin efficacious and safe for VTE prophylaxis in total hip and knee replacement
Background: Most patients undergoing total hip replacement (THR) and total knee replacement (TKR) require anticoagulant therapy to reduce venous thromboembolism (VTE) risk. Compared with injectable low-molecular-weight heparin (LMWH), warfarin, and newer oral agents, aspirin is easily administered, inexpensive, and well tolerated and requires no monitoring. There are observational data to support aspirin as VTE prophylaxis after THR and TKR. However, high-quality randomized, clinical trials (RCT) in favor of aspirin have been limited. Recently, a large RCT (n = 3,224) that compared aspirin to rivaroxaban after THR and TKR has been published that supports aspirin use for VTE prophylaxis.
Study design: Systematic review and meta-analysis.
Setting: Seven studies from North America, four from Asia, and two from Europe.
Synopsis: In a meta-analysis comprising 13 RCT including 6,060 participants (2,969 aspirin and 3,091 comparator), there was no statistically significant difference in the risk of venous thromboembolism (including deep-vein thrombosis and pulmonary embolism) when comparing aspirin with other anticoagulants (LMWH, rivaroxaban) in patients undergoing THR and TKR. Also, there were no differences in the risk of adverse events, such as bleeding, wound complications, MI, and death, when aspirin was compared with other anticoagulants.
This systematic review and meta-analysis included trials from around the world, including the most recent and largest in this area. However, because of the heterogeneity and high risk of bias encountered in most RCTs included in this analysis, additional large, well-designed RCTs are needed to validate findings of this review.
Bottom line: Findings of the current meta-analysis support the use of aspirin for VTE prophylaxis after THR and TKR, in line with the 2012 recommendations of the American College of Chest Physicians.
Citation: Matharu GS et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement. JAMA Intern Med. 2020 Feb 3;180(3):376-84.
Dr. Mehta is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Most patients undergoing total hip replacement (THR) and total knee replacement (TKR) require anticoagulant therapy to reduce venous thromboembolism (VTE) risk. Compared with injectable low-molecular-weight heparin (LMWH), warfarin, and newer oral agents, aspirin is easily administered, inexpensive, and well tolerated and requires no monitoring. There are observational data to support aspirin as VTE prophylaxis after THR and TKR. However, high-quality randomized, clinical trials (RCT) in favor of aspirin have been limited. Recently, a large RCT (n = 3,224) that compared aspirin to rivaroxaban after THR and TKR has been published that supports aspirin use for VTE prophylaxis.
Study design: Systematic review and meta-analysis.
Setting: Seven studies from North America, four from Asia, and two from Europe.
Synopsis: In a meta-analysis comprising 13 RCT including 6,060 participants (2,969 aspirin and 3,091 comparator), there was no statistically significant difference in the risk of venous thromboembolism (including deep-vein thrombosis and pulmonary embolism) when comparing aspirin with other anticoagulants (LMWH, rivaroxaban) in patients undergoing THR and TKR. Also, there were no differences in the risk of adverse events, such as bleeding, wound complications, MI, and death, when aspirin was compared with other anticoagulants.
This systematic review and meta-analysis included trials from around the world, including the most recent and largest in this area. However, because of the heterogeneity and high risk of bias encountered in most RCTs included in this analysis, additional large, well-designed RCTs are needed to validate findings of this review.
Bottom line: Findings of the current meta-analysis support the use of aspirin for VTE prophylaxis after THR and TKR, in line with the 2012 recommendations of the American College of Chest Physicians.
Citation: Matharu GS et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement. JAMA Intern Med. 2020 Feb 3;180(3):376-84.
Dr. Mehta is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Most patients undergoing total hip replacement (THR) and total knee replacement (TKR) require anticoagulant therapy to reduce venous thromboembolism (VTE) risk. Compared with injectable low-molecular-weight heparin (LMWH), warfarin, and newer oral agents, aspirin is easily administered, inexpensive, and well tolerated and requires no monitoring. There are observational data to support aspirin as VTE prophylaxis after THR and TKR. However, high-quality randomized, clinical trials (RCT) in favor of aspirin have been limited. Recently, a large RCT (n = 3,224) that compared aspirin to rivaroxaban after THR and TKR has been published that supports aspirin use for VTE prophylaxis.
Study design: Systematic review and meta-analysis.
Setting: Seven studies from North America, four from Asia, and two from Europe.
Synopsis: In a meta-analysis comprising 13 RCT including 6,060 participants (2,969 aspirin and 3,091 comparator), there was no statistically significant difference in the risk of venous thromboembolism (including deep-vein thrombosis and pulmonary embolism) when comparing aspirin with other anticoagulants (LMWH, rivaroxaban) in patients undergoing THR and TKR. Also, there were no differences in the risk of adverse events, such as bleeding, wound complications, MI, and death, when aspirin was compared with other anticoagulants.
This systematic review and meta-analysis included trials from around the world, including the most recent and largest in this area. However, because of the heterogeneity and high risk of bias encountered in most RCTs included in this analysis, additional large, well-designed RCTs are needed to validate findings of this review.
Bottom line: Findings of the current meta-analysis support the use of aspirin for VTE prophylaxis after THR and TKR, in line with the 2012 recommendations of the American College of Chest Physicians.
Citation: Matharu GS et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement. JAMA Intern Med. 2020 Feb 3;180(3):376-84.
Dr. Mehta is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
CDC panel updates info on rare side effect after J&J vaccine
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Exploring your fishpond: Steps toward managing anxiety in the age of COVID
COVID-19’s ever-changing trajectory has led to a notable rise in anxiety-related disorders in the United States. The average share of U.S. adults reporting symptoms of anxiety and or depressive disorder rose from 11% in 2019 to more than 41% in January 2021, according to a report from the Kaiser Family Foundation.
With the arrival of vaccines, Elspeth Cameron Ritchie, MD, MPH, chair of psychiatry at Medstar Washington (D.C.) Hospital Center, has noticed a shift in patients’ fears and concerns. In an interview, she explained how anxiety in patients has evolved along with the pandemic. She also offered strategies for gaining control, engaging with community, and managing anxiety.
Question: When you see patients at this point in the pandemic, what do you ask them?
Answer: I ask them how the pandemic has affected them. Responses have changed over time. In the beginning, I saw a lot of fear, dread of the unknown, a lot of frustration about being in lockdown. As the vaccines have come in and taken hold, there is both a sense of relief, but still a lot of anxiety. Part of that is we’re getting different messages and very much changing messages over time. Then there’s the people who are unvaccinated, and we’re also seeing the Delta variant taking hold in the rest of the world. There’s a lot of anxiety, fear, and some depression, although that’s gotten better with the vaccine.
Q: How do we distinguish between reasonable or rational anxiety and excessive or irrational anxiety?
A: There’s not a bright line between them. What’s rational for one person is not rational for another. What we’ve seen is a spectrum. A rational anxiety is: “I’m not ready to go to a party.” Irrational represents all these crazy theories that are made up, such as putting a microchip into your arm with the vaccine so that the government can track you.
Q: How do you talk to these people thinking irrational thoughts?
A: You must listen to them and not just shut them down. Work with them. Many people with irrational thoughts, or believe in conspiracy theories, may not want to go near a psychiatrist. But there’s also the patients in the psychiatric ward who believe COVID doesn’t exist and there’s government plots. Like any other delusional material, we work with this by talking to these patients and using medication as appropriate.
Q: Do you support prescribing medication for those patients who continue to experience anxiety that is irrational?
A: Patients based in inpatient psychiatry are usually delusional. The medication we usually prescribe for these patients is antipsychotics. If it’s an outpatient who’s anxious about COVID, but has rational anxiety, we usually use antidepressants or antianxiety agents such as Zoloft, Paxil, or Lexapro.
Q: What other strategies can psychiatrists share with patients?
A: What I’ve seen throughout COVID is often an overwhelming sense of dread and inability to control the situation. I tell patients to do things they can control. You can go out and get exercise. Especially during the winter, I recommend that people take a walk and get some sunshine.
It also helps with anxiety to reach out and help someone else. Is there a neighbor you’re concerned about? By and large, this is something many communities have done well. The challenge is we’ve been avoiding each other physically for a long time. So, some of the standard ways of helping each other out, like volunteering at a food bank, have been a little problematic. But there are ways to have minimal people on staff to reduce exposure.
One thing I recommend with any type of anxiety is to learn how to control your breathing. Take breaths through the nose several times a day and teach yourself how to slow down. Another thing that helps many people is contact with animals – especially horses, dogs, and cats. You may not be able to adopt an animal, but you could work at a rescue shelter or other facilities. People can benefit from the nonverbal cues of an animal. A friend of mine got a shelter cat. It sleeps with her and licks her when she feels anxious.
Meditation and yoga are also useful. This is not for everyone, but it’s a way to turn down the level of “buzz” or anxiety. Don’t overdo it on caffeine or other things that increase anxiety. I would stay away from illicit drugs, as they increase anxiety.
Q: What do you say to patients to give them a sense of hope?
A: A lot of people aren’t ready to return to normal; they want to keep the social isolation, the masks, the working from home. We need to show patients what they have control over to minimize their own risk. For example, if they want to wear a mask, then they should wear one. Patients also really like the option of telehealth appointments.
Another way to cope is to identify what’s better about the way things are now and concentrate on those improvements. Here in Maryland, the traffic is so much better in the morning than it once was. There are things I don’t miss, like going to the airport and waiting 5 hours for a flight.
Q: What advice can you give psychiatrists who are experiencing anxiety?
A: We must manage our own anxiety so we can help our patients. Strategies I’ve mentioned are also helpful to psychiatrists or other health care professionals (such as) taking a walk, getting exercise, controlling what you can control. For me, it’s getting dressed, going to work, seeing patients. Having a daily structure, a routine, is important. Many people struggled with this at first. They were working from home and didn’t get much done; they did too much videogaming. It helps to set regular appointments if you’re working from home.
Pre-COVID, many of us got a lot out of our professional meetings. We saw friends there. Now they’re either canceled or we’re doing them virtually, which isn’t the same thing. I think our profession could do a better job of reaching out to each other. We’re used to seeing each other once or twice a year at conventions. I’ve since found it hard to reach out to my colleagues via email. And everyone is tired of Zoom.
If they’re local, ask them to do a safe outdoor activity, a happy hour, a walk. If they’re not, maybe engage with them through a postcard or a phone call.
My colleagues and I go for walks at lunch. There’s a fishpond nearby and we talk to the fish and get a little silly. We sometimes take fish nets with us. People ask what the fish nets are for and we’ll say, “we’re chasing COVID away.”
Dr. Ritchie reported no conflicts of interest.
COVID-19’s ever-changing trajectory has led to a notable rise in anxiety-related disorders in the United States. The average share of U.S. adults reporting symptoms of anxiety and or depressive disorder rose from 11% in 2019 to more than 41% in January 2021, according to a report from the Kaiser Family Foundation.
With the arrival of vaccines, Elspeth Cameron Ritchie, MD, MPH, chair of psychiatry at Medstar Washington (D.C.) Hospital Center, has noticed a shift in patients’ fears and concerns. In an interview, she explained how anxiety in patients has evolved along with the pandemic. She also offered strategies for gaining control, engaging with community, and managing anxiety.
Question: When you see patients at this point in the pandemic, what do you ask them?
Answer: I ask them how the pandemic has affected them. Responses have changed over time. In the beginning, I saw a lot of fear, dread of the unknown, a lot of frustration about being in lockdown. As the vaccines have come in and taken hold, there is both a sense of relief, but still a lot of anxiety. Part of that is we’re getting different messages and very much changing messages over time. Then there’s the people who are unvaccinated, and we’re also seeing the Delta variant taking hold in the rest of the world. There’s a lot of anxiety, fear, and some depression, although that’s gotten better with the vaccine.
Q: How do we distinguish between reasonable or rational anxiety and excessive or irrational anxiety?
A: There’s not a bright line between them. What’s rational for one person is not rational for another. What we’ve seen is a spectrum. A rational anxiety is: “I’m not ready to go to a party.” Irrational represents all these crazy theories that are made up, such as putting a microchip into your arm with the vaccine so that the government can track you.
Q: How do you talk to these people thinking irrational thoughts?
A: You must listen to them and not just shut them down. Work with them. Many people with irrational thoughts, or believe in conspiracy theories, may not want to go near a psychiatrist. But there’s also the patients in the psychiatric ward who believe COVID doesn’t exist and there’s government plots. Like any other delusional material, we work with this by talking to these patients and using medication as appropriate.
Q: Do you support prescribing medication for those patients who continue to experience anxiety that is irrational?
A: Patients based in inpatient psychiatry are usually delusional. The medication we usually prescribe for these patients is antipsychotics. If it’s an outpatient who’s anxious about COVID, but has rational anxiety, we usually use antidepressants or antianxiety agents such as Zoloft, Paxil, or Lexapro.
Q: What other strategies can psychiatrists share with patients?
A: What I’ve seen throughout COVID is often an overwhelming sense of dread and inability to control the situation. I tell patients to do things they can control. You can go out and get exercise. Especially during the winter, I recommend that people take a walk and get some sunshine.
It also helps with anxiety to reach out and help someone else. Is there a neighbor you’re concerned about? By and large, this is something many communities have done well. The challenge is we’ve been avoiding each other physically for a long time. So, some of the standard ways of helping each other out, like volunteering at a food bank, have been a little problematic. But there are ways to have minimal people on staff to reduce exposure.
One thing I recommend with any type of anxiety is to learn how to control your breathing. Take breaths through the nose several times a day and teach yourself how to slow down. Another thing that helps many people is contact with animals – especially horses, dogs, and cats. You may not be able to adopt an animal, but you could work at a rescue shelter or other facilities. People can benefit from the nonverbal cues of an animal. A friend of mine got a shelter cat. It sleeps with her and licks her when she feels anxious.
Meditation and yoga are also useful. This is not for everyone, but it’s a way to turn down the level of “buzz” or anxiety. Don’t overdo it on caffeine or other things that increase anxiety. I would stay away from illicit drugs, as they increase anxiety.
Q: What do you say to patients to give them a sense of hope?
A: A lot of people aren’t ready to return to normal; they want to keep the social isolation, the masks, the working from home. We need to show patients what they have control over to minimize their own risk. For example, if they want to wear a mask, then they should wear one. Patients also really like the option of telehealth appointments.
Another way to cope is to identify what’s better about the way things are now and concentrate on those improvements. Here in Maryland, the traffic is so much better in the morning than it once was. There are things I don’t miss, like going to the airport and waiting 5 hours for a flight.
Q: What advice can you give psychiatrists who are experiencing anxiety?
A: We must manage our own anxiety so we can help our patients. Strategies I’ve mentioned are also helpful to psychiatrists or other health care professionals (such as) taking a walk, getting exercise, controlling what you can control. For me, it’s getting dressed, going to work, seeing patients. Having a daily structure, a routine, is important. Many people struggled with this at first. They were working from home and didn’t get much done; they did too much videogaming. It helps to set regular appointments if you’re working from home.
Pre-COVID, many of us got a lot out of our professional meetings. We saw friends there. Now they’re either canceled or we’re doing them virtually, which isn’t the same thing. I think our profession could do a better job of reaching out to each other. We’re used to seeing each other once or twice a year at conventions. I’ve since found it hard to reach out to my colleagues via email. And everyone is tired of Zoom.
If they’re local, ask them to do a safe outdoor activity, a happy hour, a walk. If they’re not, maybe engage with them through a postcard or a phone call.
My colleagues and I go for walks at lunch. There’s a fishpond nearby and we talk to the fish and get a little silly. We sometimes take fish nets with us. People ask what the fish nets are for and we’ll say, “we’re chasing COVID away.”
Dr. Ritchie reported no conflicts of interest.
COVID-19’s ever-changing trajectory has led to a notable rise in anxiety-related disorders in the United States. The average share of U.S. adults reporting symptoms of anxiety and or depressive disorder rose from 11% in 2019 to more than 41% in January 2021, according to a report from the Kaiser Family Foundation.
With the arrival of vaccines, Elspeth Cameron Ritchie, MD, MPH, chair of psychiatry at Medstar Washington (D.C.) Hospital Center, has noticed a shift in patients’ fears and concerns. In an interview, she explained how anxiety in patients has evolved along with the pandemic. She also offered strategies for gaining control, engaging with community, and managing anxiety.
Question: When you see patients at this point in the pandemic, what do you ask them?
Answer: I ask them how the pandemic has affected them. Responses have changed over time. In the beginning, I saw a lot of fear, dread of the unknown, a lot of frustration about being in lockdown. As the vaccines have come in and taken hold, there is both a sense of relief, but still a lot of anxiety. Part of that is we’re getting different messages and very much changing messages over time. Then there’s the people who are unvaccinated, and we’re also seeing the Delta variant taking hold in the rest of the world. There’s a lot of anxiety, fear, and some depression, although that’s gotten better with the vaccine.
Q: How do we distinguish between reasonable or rational anxiety and excessive or irrational anxiety?
A: There’s not a bright line between them. What’s rational for one person is not rational for another. What we’ve seen is a spectrum. A rational anxiety is: “I’m not ready to go to a party.” Irrational represents all these crazy theories that are made up, such as putting a microchip into your arm with the vaccine so that the government can track you.
Q: How do you talk to these people thinking irrational thoughts?
A: You must listen to them and not just shut them down. Work with them. Many people with irrational thoughts, or believe in conspiracy theories, may not want to go near a psychiatrist. But there’s also the patients in the psychiatric ward who believe COVID doesn’t exist and there’s government plots. Like any other delusional material, we work with this by talking to these patients and using medication as appropriate.
Q: Do you support prescribing medication for those patients who continue to experience anxiety that is irrational?
A: Patients based in inpatient psychiatry are usually delusional. The medication we usually prescribe for these patients is antipsychotics. If it’s an outpatient who’s anxious about COVID, but has rational anxiety, we usually use antidepressants or antianxiety agents such as Zoloft, Paxil, or Lexapro.
Q: What other strategies can psychiatrists share with patients?
A: What I’ve seen throughout COVID is often an overwhelming sense of dread and inability to control the situation. I tell patients to do things they can control. You can go out and get exercise. Especially during the winter, I recommend that people take a walk and get some sunshine.
It also helps with anxiety to reach out and help someone else. Is there a neighbor you’re concerned about? By and large, this is something many communities have done well. The challenge is we’ve been avoiding each other physically for a long time. So, some of the standard ways of helping each other out, like volunteering at a food bank, have been a little problematic. But there are ways to have minimal people on staff to reduce exposure.
One thing I recommend with any type of anxiety is to learn how to control your breathing. Take breaths through the nose several times a day and teach yourself how to slow down. Another thing that helps many people is contact with animals – especially horses, dogs, and cats. You may not be able to adopt an animal, but you could work at a rescue shelter or other facilities. People can benefit from the nonverbal cues of an animal. A friend of mine got a shelter cat. It sleeps with her and licks her when she feels anxious.
Meditation and yoga are also useful. This is not for everyone, but it’s a way to turn down the level of “buzz” or anxiety. Don’t overdo it on caffeine or other things that increase anxiety. I would stay away from illicit drugs, as they increase anxiety.
Q: What do you say to patients to give them a sense of hope?
A: A lot of people aren’t ready to return to normal; they want to keep the social isolation, the masks, the working from home. We need to show patients what they have control over to minimize their own risk. For example, if they want to wear a mask, then they should wear one. Patients also really like the option of telehealth appointments.
Another way to cope is to identify what’s better about the way things are now and concentrate on those improvements. Here in Maryland, the traffic is so much better in the morning than it once was. There are things I don’t miss, like going to the airport and waiting 5 hours for a flight.
Q: What advice can you give psychiatrists who are experiencing anxiety?
A: We must manage our own anxiety so we can help our patients. Strategies I’ve mentioned are also helpful to psychiatrists or other health care professionals (such as) taking a walk, getting exercise, controlling what you can control. For me, it’s getting dressed, going to work, seeing patients. Having a daily structure, a routine, is important. Many people struggled with this at first. They were working from home and didn’t get much done; they did too much videogaming. It helps to set regular appointments if you’re working from home.
Pre-COVID, many of us got a lot out of our professional meetings. We saw friends there. Now they’re either canceled or we’re doing them virtually, which isn’t the same thing. I think our profession could do a better job of reaching out to each other. We’re used to seeing each other once or twice a year at conventions. I’ve since found it hard to reach out to my colleagues via email. And everyone is tired of Zoom.
If they’re local, ask them to do a safe outdoor activity, a happy hour, a walk. If they’re not, maybe engage with them through a postcard or a phone call.
My colleagues and I go for walks at lunch. There’s a fishpond nearby and we talk to the fish and get a little silly. We sometimes take fish nets with us. People ask what the fish nets are for and we’ll say, “we’re chasing COVID away.”
Dr. Ritchie reported no conflicts of interest.
Younger adults with HIV have higher CVD risk but low ASCVD scores
People age 40 and younger living with HIV have a higher risk for heart disease than even their over-40 peers living with HIV – and that risk was 54% higher than the general public.
And this was among people without traditional heart disease risks, such as smoking and obesity.
“What’s surprising is that not only do we see that, yes, they do have increased risk, but this is after controlling for all of that – which means the mechanism underlying this risk,” said Tiffany Gooden, MPH and a PhD candidate at the University of Birmingham, England, who presented the data at the 11th International AIDS Society Conference on HIV Science (IAS 2021).
“If we’re using a non–HIV-validated assessment tool, you should always know that there could be a risk that you are under-recognizing,” she added.
Right now, there’s not a lot to aid clinicians in ferreting out this increased risk. Traditional cardiovascular risk assessment tools, like Framingham risk scores and atherosclerotic cardiovascular disease risk score from the American College of Cardiology, have been found to overlook the real risk of cardiovascular disease in people living with HIV. Plus, most guidelines, including those from the British HIV Medical Association and the American College of Cardiology, primarily focus screening on people 40 or older.
Ms. Gooden’s study drew data from The Health Improvement Network (THIN) database, which combines data from 800 primary care practices in the United Kingdom. Looking at data between January 2000 and January 2020, the investigators compared each person living with HIV with four peers not living with HIV, matched for age, gender, and practice. In total, 9,233 people living with HIV and 35,721 people without HIV were included in the analysis. Median age of participants was 41 years in people living with HIV and 40.4 years in people without HIV. About 35% of participants in both arms were women, and a greater proportion of participants living with HIV were Black, accounting for 22.5% of people living with HIV, versus 3.8% of the general population. Fewer people living with HIV were overweight or obese compared to people without HIV.
Researchers then tracked participants over time to identify the incidence of heart attack, stroke, peripheral vascular disease, and heart failure, as well as common risk factors for heart problems, such as high blood pressure, type 2 diabetes, chronic kidney disease, atrial fibrillation, and use of a lipid-lowering drug such as a statin.
The investigators then sectioned the data on heart disease risk by decade – 2000-2009 and 2010-2019 – in order to separate the potential impact of antiretroviral treatment (ART) drugs, from early combinations that have been associated with cardiovascular disease, to current drugs that are less likely to have that effect.
Overall risk for any kind of cardiovascular disease was 54% higher among people living with HIV of any age, compared to their age- and risk-matched peers. And when they broke the data down by age, they found that people younger than 40 had nearly twice the risk for any heart disease as their HIV-negative peers, which was a numerically higher risk than for people older than 40 – though not significantly so.
People living with HIV also had a 49% increased risk for stroke and a 59% increased risk for ischemic heart disease but no increased risk for peripheral vascular disease, heart failure, or heart attack. But the confidence intervals here were wide, “which may indicate lack of power and therefore not be conclusive,” Ms. Gooden said.
People living with HIV also had a 37% increased risk for hypertension, were 96% more likely to be prescribed lipid-lowering drugs, 2.4-times more likely to have chronic kidney disease, and 2.68-times more likely to experience all-cause mortality. The study couldn’t account for the type of HIV medications people living with the virus used, their viral load, or their CD4 counts – all of which have been found in previous studies to contribute to heart disease in people with HIV.
“That was the biggest limitation of our study,” Ms. Gooden said in an interview. “The fact that the risk of cardiovascular disease remains the same in the [first decade] and the later decade goes to show that even if antiretroviral therapies contributed to that … now or 20 years ago, it’s still not the entire reason for the risk.”
Steven Grinspoon, MD, of Harvard Medical School, Boston, is the lead author on the REPRIEVE trial, now testing statins as a treatment for people like those in this study. He told this news organization that this large analysis had one of the youngest cohorts of people living with HIV he’d seen to explore these issues. Additionally, it backs up what the team recently reported in the Journal of the American Medical Association – that plaque was present in 49% of 755 people living with HIV, despite having risk scores for cardiovascular disease in the normal range. This was as true for people younger than 40 as those older than 40.
For primary care clinicians, the message is that even relatively young people with HIV should be counseled early and often about amending traditional risk factors, while we wait for the results of REPRIEVE to say whether statins improve outcomes for people living with HIV, Dr. Grinspoon said in an interview.
“Sometimes physicians and primary care providers say, ‘Well I’ll focus my hypertension efforts on older people, who are closer to having heart attacks,’” Dr. Grinspoon said. “But this data suggests we should pay attention even in young people … and pay particular attention to women who wouldn’t have traditional risk scores that were very high at all, largely because they are women.”
The study was funded by Merck. Ms. Gooden has disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
People age 40 and younger living with HIV have a higher risk for heart disease than even their over-40 peers living with HIV – and that risk was 54% higher than the general public.
And this was among people without traditional heart disease risks, such as smoking and obesity.
“What’s surprising is that not only do we see that, yes, they do have increased risk, but this is after controlling for all of that – which means the mechanism underlying this risk,” said Tiffany Gooden, MPH and a PhD candidate at the University of Birmingham, England, who presented the data at the 11th International AIDS Society Conference on HIV Science (IAS 2021).
“If we’re using a non–HIV-validated assessment tool, you should always know that there could be a risk that you are under-recognizing,” she added.
Right now, there’s not a lot to aid clinicians in ferreting out this increased risk. Traditional cardiovascular risk assessment tools, like Framingham risk scores and atherosclerotic cardiovascular disease risk score from the American College of Cardiology, have been found to overlook the real risk of cardiovascular disease in people living with HIV. Plus, most guidelines, including those from the British HIV Medical Association and the American College of Cardiology, primarily focus screening on people 40 or older.
Ms. Gooden’s study drew data from The Health Improvement Network (THIN) database, which combines data from 800 primary care practices in the United Kingdom. Looking at data between January 2000 and January 2020, the investigators compared each person living with HIV with four peers not living with HIV, matched for age, gender, and practice. In total, 9,233 people living with HIV and 35,721 people without HIV were included in the analysis. Median age of participants was 41 years in people living with HIV and 40.4 years in people without HIV. About 35% of participants in both arms were women, and a greater proportion of participants living with HIV were Black, accounting for 22.5% of people living with HIV, versus 3.8% of the general population. Fewer people living with HIV were overweight or obese compared to people without HIV.
Researchers then tracked participants over time to identify the incidence of heart attack, stroke, peripheral vascular disease, and heart failure, as well as common risk factors for heart problems, such as high blood pressure, type 2 diabetes, chronic kidney disease, atrial fibrillation, and use of a lipid-lowering drug such as a statin.
The investigators then sectioned the data on heart disease risk by decade – 2000-2009 and 2010-2019 – in order to separate the potential impact of antiretroviral treatment (ART) drugs, from early combinations that have been associated with cardiovascular disease, to current drugs that are less likely to have that effect.
Overall risk for any kind of cardiovascular disease was 54% higher among people living with HIV of any age, compared to their age- and risk-matched peers. And when they broke the data down by age, they found that people younger than 40 had nearly twice the risk for any heart disease as their HIV-negative peers, which was a numerically higher risk than for people older than 40 – though not significantly so.
People living with HIV also had a 49% increased risk for stroke and a 59% increased risk for ischemic heart disease but no increased risk for peripheral vascular disease, heart failure, or heart attack. But the confidence intervals here were wide, “which may indicate lack of power and therefore not be conclusive,” Ms. Gooden said.
People living with HIV also had a 37% increased risk for hypertension, were 96% more likely to be prescribed lipid-lowering drugs, 2.4-times more likely to have chronic kidney disease, and 2.68-times more likely to experience all-cause mortality. The study couldn’t account for the type of HIV medications people living with the virus used, their viral load, or their CD4 counts – all of which have been found in previous studies to contribute to heart disease in people with HIV.
“That was the biggest limitation of our study,” Ms. Gooden said in an interview. “The fact that the risk of cardiovascular disease remains the same in the [first decade] and the later decade goes to show that even if antiretroviral therapies contributed to that … now or 20 years ago, it’s still not the entire reason for the risk.”
Steven Grinspoon, MD, of Harvard Medical School, Boston, is the lead author on the REPRIEVE trial, now testing statins as a treatment for people like those in this study. He told this news organization that this large analysis had one of the youngest cohorts of people living with HIV he’d seen to explore these issues. Additionally, it backs up what the team recently reported in the Journal of the American Medical Association – that plaque was present in 49% of 755 people living with HIV, despite having risk scores for cardiovascular disease in the normal range. This was as true for people younger than 40 as those older than 40.
For primary care clinicians, the message is that even relatively young people with HIV should be counseled early and often about amending traditional risk factors, while we wait for the results of REPRIEVE to say whether statins improve outcomes for people living with HIV, Dr. Grinspoon said in an interview.
“Sometimes physicians and primary care providers say, ‘Well I’ll focus my hypertension efforts on older people, who are closer to having heart attacks,’” Dr. Grinspoon said. “But this data suggests we should pay attention even in young people … and pay particular attention to women who wouldn’t have traditional risk scores that were very high at all, largely because they are women.”
The study was funded by Merck. Ms. Gooden has disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
People age 40 and younger living with HIV have a higher risk for heart disease than even their over-40 peers living with HIV – and that risk was 54% higher than the general public.
And this was among people without traditional heart disease risks, such as smoking and obesity.
“What’s surprising is that not only do we see that, yes, they do have increased risk, but this is after controlling for all of that – which means the mechanism underlying this risk,” said Tiffany Gooden, MPH and a PhD candidate at the University of Birmingham, England, who presented the data at the 11th International AIDS Society Conference on HIV Science (IAS 2021).
“If we’re using a non–HIV-validated assessment tool, you should always know that there could be a risk that you are under-recognizing,” she added.
Right now, there’s not a lot to aid clinicians in ferreting out this increased risk. Traditional cardiovascular risk assessment tools, like Framingham risk scores and atherosclerotic cardiovascular disease risk score from the American College of Cardiology, have been found to overlook the real risk of cardiovascular disease in people living with HIV. Plus, most guidelines, including those from the British HIV Medical Association and the American College of Cardiology, primarily focus screening on people 40 or older.
Ms. Gooden’s study drew data from The Health Improvement Network (THIN) database, which combines data from 800 primary care practices in the United Kingdom. Looking at data between January 2000 and January 2020, the investigators compared each person living with HIV with four peers not living with HIV, matched for age, gender, and practice. In total, 9,233 people living with HIV and 35,721 people without HIV were included in the analysis. Median age of participants was 41 years in people living with HIV and 40.4 years in people without HIV. About 35% of participants in both arms were women, and a greater proportion of participants living with HIV were Black, accounting for 22.5% of people living with HIV, versus 3.8% of the general population. Fewer people living with HIV were overweight or obese compared to people without HIV.
Researchers then tracked participants over time to identify the incidence of heart attack, stroke, peripheral vascular disease, and heart failure, as well as common risk factors for heart problems, such as high blood pressure, type 2 diabetes, chronic kidney disease, atrial fibrillation, and use of a lipid-lowering drug such as a statin.
The investigators then sectioned the data on heart disease risk by decade – 2000-2009 and 2010-2019 – in order to separate the potential impact of antiretroviral treatment (ART) drugs, from early combinations that have been associated with cardiovascular disease, to current drugs that are less likely to have that effect.
Overall risk for any kind of cardiovascular disease was 54% higher among people living with HIV of any age, compared to their age- and risk-matched peers. And when they broke the data down by age, they found that people younger than 40 had nearly twice the risk for any heart disease as their HIV-negative peers, which was a numerically higher risk than for people older than 40 – though not significantly so.
People living with HIV also had a 49% increased risk for stroke and a 59% increased risk for ischemic heart disease but no increased risk for peripheral vascular disease, heart failure, or heart attack. But the confidence intervals here were wide, “which may indicate lack of power and therefore not be conclusive,” Ms. Gooden said.
People living with HIV also had a 37% increased risk for hypertension, were 96% more likely to be prescribed lipid-lowering drugs, 2.4-times more likely to have chronic kidney disease, and 2.68-times more likely to experience all-cause mortality. The study couldn’t account for the type of HIV medications people living with the virus used, their viral load, or their CD4 counts – all of which have been found in previous studies to contribute to heart disease in people with HIV.
“That was the biggest limitation of our study,” Ms. Gooden said in an interview. “The fact that the risk of cardiovascular disease remains the same in the [first decade] and the later decade goes to show that even if antiretroviral therapies contributed to that … now or 20 years ago, it’s still not the entire reason for the risk.”
Steven Grinspoon, MD, of Harvard Medical School, Boston, is the lead author on the REPRIEVE trial, now testing statins as a treatment for people like those in this study. He told this news organization that this large analysis had one of the youngest cohorts of people living with HIV he’d seen to explore these issues. Additionally, it backs up what the team recently reported in the Journal of the American Medical Association – that plaque was present in 49% of 755 people living with HIV, despite having risk scores for cardiovascular disease in the normal range. This was as true for people younger than 40 as those older than 40.
For primary care clinicians, the message is that even relatively young people with HIV should be counseled early and often about amending traditional risk factors, while we wait for the results of REPRIEVE to say whether statins improve outcomes for people living with HIV, Dr. Grinspoon said in an interview.
“Sometimes physicians and primary care providers say, ‘Well I’ll focus my hypertension efforts on older people, who are closer to having heart attacks,’” Dr. Grinspoon said. “But this data suggests we should pay attention even in young people … and pay particular attention to women who wouldn’t have traditional risk scores that were very high at all, largely because they are women.”
The study was funded by Merck. Ms. Gooden has disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Rising meth-related heart failure admissions a ‘crisis,’ costly for society
Rates of heart failure (HF) caused by methamphetamine abuse are climbing quickly in the western United States, at great financial and societal cost, suggests an analysis that documents the trends in California over a recent decade.
In the new study, methamphetamine-associated HF (meth-HF) admissions in the state rose by 585% between 2008 and 2018, and charges related those hospitalizations jumped 840%. Cases of HF unrelated to meth fell by 6% during the same period.
The recent explosion in meth-HF hospitalizations has also been costly for society in general, because most cases are younger adults in their most productive, prime earning years, Susan X. Zhao, MD, Santa Clara Valley Medical Center, San Jose, Calif., said in an interview.
“Over the past 11 years, especially since 2018, it has really started to take off, with a pretty dramatic rise. And it happened without much attention, because when we think about drugs, we think about acute overdose and not so much about the chronic, smoldering, long-term effects,” said Dr. Zhao, who is lead author on the study published July 13, 2021, in Circulation: Cardiovascular Quality and Outcomes.
“It’s really affecting a section of the population that is not supposed to be having heart failure problems. I think it is going to continue for the next decade until we put a stop to the parent problem, which is methamphetamine,” Dr. Zhao said. “We’re at the beginning, even though the rise has been pretty dramatic. The worst is yet to come.”
Under the radar
Methamphetamine-associated HF has been a growing problem for many years but has largely been “flying under the radar” because HF hospitalization data focus on Medicare-age patients, not the overwhelmingly younger meth-HF population, the report notes.
“We have to get this message out. Many of my patients with meth heart failure had no idea this would happen to them. They didn’t know,” Dr. Zhao said. “Once I tell them that this is what methamphetamines will do to you after years and years of use, they say they wish someone had told them.”
Dr. Zhao and colleagues looked at HF admission data collected by California’s Health and Human Services Agency to assess meth-HF trends and disease burden. They identified 1,033,076 HF hospitalizations during the decade, of which 42,565 (4.12%) were for meth-HF.
Patients hospitalized with meth-HF had a mean age of 49.6 years, compared with 72.2 for the other patients admitted with HF (P < .001). Virtually all of the patients hospitalized for meth-HF were younger than 65 years: 94.5%, compared with 30% for the other HF patients (P < .001).
Hospitalized patients with meth-HF were mostly men, their prevalence of 80% contrasting with 52.4% for patients with non–meth-related HF (P < .001).
Rates of hospitalization for meth-HF steadily increased during the study period. The age-adjusted rate of meth-HF hospitalization per 100,000 rose from 4.1 in 2008 to 28.1 in 2018. The rate of hospitalization for HF unrelated to meth actually declined, going from 342.3 in 2008 to 321.6 in 2018.
Charges for hospitalizations related to meth-HF shot up more than eight times, from $41.5 million in 2008 to $390.2 million in 2018. In contrast, charges for other HF hospitalizations rose by only 82%, from $3.5 billion to $6.3 billion.
Multiple layers of prevention
Dr. Zhao proposed ways that clinicians can communicate with their patients who are using or considering to use meth. “There are multiple layers of prevention. For people who are thinking of using meth, they need to get the message that something really bad can happen to them years down the road. They’re not going to die from it overnight, but it will damage the heart slowly,” she said.
The next layer of prevention can potentially help meth users who have not yet developed heart problems, Dr. Zhao said. “This would be the time to say, ‘you’re so lucky, your heart is still good. It’s time to stop because people like you, a few years from now are going to die prematurely from a very horrible, very suffering kind of death’.”
Importantly, in meth users who have already developed HF, even then it may not be too late to reverse the cardiomyopathy and symptoms. For up to a third of people with established meth-HF, “if they stop using meth, if they take good cardiac medications, and if the heart failure is in an early enough course, their heart can entirely revert to normal,” Dr. Zhao said, citing an earlier work from her and her colleagues.
Currently, methamphetamine abuse has taken especially strong root in rural areas in California and the Midwest. But Dr. Zhao predicts it will soon become prevalent throughout the United States.
Spotlight on an ‘epidemic’
The rapid growth of the methamphetamine “epidemic” has been well-documented in the United States and around the world, observed an accompanying editorial from Pavan Reddy, MD, Icahn School of Medicine at Mount Sinai Morningside, New York, and Uri Elkayam, MD, University of Southern California, Los Angeles.
They contend that more attention has been given to opioid overdose deaths; meth abuse does not seem to command the same attention, likely because meth is not as strongly associated with acute overdose.
But meth, wrote Dr. Reddy and Dr. Elkayam, “is a different drug with its own M.O., equally dangerous and costly to society but more insidious in nature, its effects potentially causing decades of mental and physical debilitation before ending in premature death.”
The current study “has turned a spotlight on a public health crisis that has grown unfettered for over 2 decades,” and is a call for the “medical community to recognize and manage cases of meth-HF with a comprehensive approach that addresses both mental and physical illness,” they concluded. “Only then can we hope to properly help these patients and with that, reduce the socioeconomic burden of meth-HF.”
A quietly building crisis
The sharp rise in meth-HF hospitalizations is an expected reflection of the methamphetamine crisis, which has been quietly building over the last few years, addiction psychiatrist Corneliu N. Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview.
“This new version of methamphetamines looks like ice and is more potent and toxic than former versions traditionally made in home-built labs,” he said. Lately the vast majority of methamphetamines in the United States have come from Mexico, are less expensive with higher purity, “and can be manufactured in greater quantities.”
Some patients with opioid use disorder (OUD) also inject methamphetamines, which can make OUD treatment clinics good places to screen for meth abuse and educate about its cardiovascular implications, Dr. Stanciu said.
“Just as addiction treatment centers present an opportunity to implement cardiac screening and referrals,” he said, “cardiology visits and hospitalizations such as those for meth-HF also present a golden opportunity for involvement of substance use disorder interventions and referrals to get patients into treatment and prevent further damage through ongoing use.”
Dr. Zhao, Dr. Reddy, Dr. Eklayam, and Dr. Stanciu report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of heart failure (HF) caused by methamphetamine abuse are climbing quickly in the western United States, at great financial and societal cost, suggests an analysis that documents the trends in California over a recent decade.
In the new study, methamphetamine-associated HF (meth-HF) admissions in the state rose by 585% between 2008 and 2018, and charges related those hospitalizations jumped 840%. Cases of HF unrelated to meth fell by 6% during the same period.
The recent explosion in meth-HF hospitalizations has also been costly for society in general, because most cases are younger adults in their most productive, prime earning years, Susan X. Zhao, MD, Santa Clara Valley Medical Center, San Jose, Calif., said in an interview.
“Over the past 11 years, especially since 2018, it has really started to take off, with a pretty dramatic rise. And it happened without much attention, because when we think about drugs, we think about acute overdose and not so much about the chronic, smoldering, long-term effects,” said Dr. Zhao, who is lead author on the study published July 13, 2021, in Circulation: Cardiovascular Quality and Outcomes.
“It’s really affecting a section of the population that is not supposed to be having heart failure problems. I think it is going to continue for the next decade until we put a stop to the parent problem, which is methamphetamine,” Dr. Zhao said. “We’re at the beginning, even though the rise has been pretty dramatic. The worst is yet to come.”
Under the radar
Methamphetamine-associated HF has been a growing problem for many years but has largely been “flying under the radar” because HF hospitalization data focus on Medicare-age patients, not the overwhelmingly younger meth-HF population, the report notes.
“We have to get this message out. Many of my patients with meth heart failure had no idea this would happen to them. They didn’t know,” Dr. Zhao said. “Once I tell them that this is what methamphetamines will do to you after years and years of use, they say they wish someone had told them.”
Dr. Zhao and colleagues looked at HF admission data collected by California’s Health and Human Services Agency to assess meth-HF trends and disease burden. They identified 1,033,076 HF hospitalizations during the decade, of which 42,565 (4.12%) were for meth-HF.
Patients hospitalized with meth-HF had a mean age of 49.6 years, compared with 72.2 for the other patients admitted with HF (P < .001). Virtually all of the patients hospitalized for meth-HF were younger than 65 years: 94.5%, compared with 30% for the other HF patients (P < .001).
Hospitalized patients with meth-HF were mostly men, their prevalence of 80% contrasting with 52.4% for patients with non–meth-related HF (P < .001).
Rates of hospitalization for meth-HF steadily increased during the study period. The age-adjusted rate of meth-HF hospitalization per 100,000 rose from 4.1 in 2008 to 28.1 in 2018. The rate of hospitalization for HF unrelated to meth actually declined, going from 342.3 in 2008 to 321.6 in 2018.
Charges for hospitalizations related to meth-HF shot up more than eight times, from $41.5 million in 2008 to $390.2 million in 2018. In contrast, charges for other HF hospitalizations rose by only 82%, from $3.5 billion to $6.3 billion.
Multiple layers of prevention
Dr. Zhao proposed ways that clinicians can communicate with their patients who are using or considering to use meth. “There are multiple layers of prevention. For people who are thinking of using meth, they need to get the message that something really bad can happen to them years down the road. They’re not going to die from it overnight, but it will damage the heart slowly,” she said.
The next layer of prevention can potentially help meth users who have not yet developed heart problems, Dr. Zhao said. “This would be the time to say, ‘you’re so lucky, your heart is still good. It’s time to stop because people like you, a few years from now are going to die prematurely from a very horrible, very suffering kind of death’.”
Importantly, in meth users who have already developed HF, even then it may not be too late to reverse the cardiomyopathy and symptoms. For up to a third of people with established meth-HF, “if they stop using meth, if they take good cardiac medications, and if the heart failure is in an early enough course, their heart can entirely revert to normal,” Dr. Zhao said, citing an earlier work from her and her colleagues.
Currently, methamphetamine abuse has taken especially strong root in rural areas in California and the Midwest. But Dr. Zhao predicts it will soon become prevalent throughout the United States.
Spotlight on an ‘epidemic’
The rapid growth of the methamphetamine “epidemic” has been well-documented in the United States and around the world, observed an accompanying editorial from Pavan Reddy, MD, Icahn School of Medicine at Mount Sinai Morningside, New York, and Uri Elkayam, MD, University of Southern California, Los Angeles.
They contend that more attention has been given to opioid overdose deaths; meth abuse does not seem to command the same attention, likely because meth is not as strongly associated with acute overdose.
But meth, wrote Dr. Reddy and Dr. Elkayam, “is a different drug with its own M.O., equally dangerous and costly to society but more insidious in nature, its effects potentially causing decades of mental and physical debilitation before ending in premature death.”
The current study “has turned a spotlight on a public health crisis that has grown unfettered for over 2 decades,” and is a call for the “medical community to recognize and manage cases of meth-HF with a comprehensive approach that addresses both mental and physical illness,” they concluded. “Only then can we hope to properly help these patients and with that, reduce the socioeconomic burden of meth-HF.”
A quietly building crisis
The sharp rise in meth-HF hospitalizations is an expected reflection of the methamphetamine crisis, which has been quietly building over the last few years, addiction psychiatrist Corneliu N. Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview.
“This new version of methamphetamines looks like ice and is more potent and toxic than former versions traditionally made in home-built labs,” he said. Lately the vast majority of methamphetamines in the United States have come from Mexico, are less expensive with higher purity, “and can be manufactured in greater quantities.”
Some patients with opioid use disorder (OUD) also inject methamphetamines, which can make OUD treatment clinics good places to screen for meth abuse and educate about its cardiovascular implications, Dr. Stanciu said.
“Just as addiction treatment centers present an opportunity to implement cardiac screening and referrals,” he said, “cardiology visits and hospitalizations such as those for meth-HF also present a golden opportunity for involvement of substance use disorder interventions and referrals to get patients into treatment and prevent further damage through ongoing use.”
Dr. Zhao, Dr. Reddy, Dr. Eklayam, and Dr. Stanciu report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of heart failure (HF) caused by methamphetamine abuse are climbing quickly in the western United States, at great financial and societal cost, suggests an analysis that documents the trends in California over a recent decade.
In the new study, methamphetamine-associated HF (meth-HF) admissions in the state rose by 585% between 2008 and 2018, and charges related those hospitalizations jumped 840%. Cases of HF unrelated to meth fell by 6% during the same period.
The recent explosion in meth-HF hospitalizations has also been costly for society in general, because most cases are younger adults in their most productive, prime earning years, Susan X. Zhao, MD, Santa Clara Valley Medical Center, San Jose, Calif., said in an interview.
“Over the past 11 years, especially since 2018, it has really started to take off, with a pretty dramatic rise. And it happened without much attention, because when we think about drugs, we think about acute overdose and not so much about the chronic, smoldering, long-term effects,” said Dr. Zhao, who is lead author on the study published July 13, 2021, in Circulation: Cardiovascular Quality and Outcomes.
“It’s really affecting a section of the population that is not supposed to be having heart failure problems. I think it is going to continue for the next decade until we put a stop to the parent problem, which is methamphetamine,” Dr. Zhao said. “We’re at the beginning, even though the rise has been pretty dramatic. The worst is yet to come.”
Under the radar
Methamphetamine-associated HF has been a growing problem for many years but has largely been “flying under the radar” because HF hospitalization data focus on Medicare-age patients, not the overwhelmingly younger meth-HF population, the report notes.
“We have to get this message out. Many of my patients with meth heart failure had no idea this would happen to them. They didn’t know,” Dr. Zhao said. “Once I tell them that this is what methamphetamines will do to you after years and years of use, they say they wish someone had told them.”
Dr. Zhao and colleagues looked at HF admission data collected by California’s Health and Human Services Agency to assess meth-HF trends and disease burden. They identified 1,033,076 HF hospitalizations during the decade, of which 42,565 (4.12%) were for meth-HF.
Patients hospitalized with meth-HF had a mean age of 49.6 years, compared with 72.2 for the other patients admitted with HF (P < .001). Virtually all of the patients hospitalized for meth-HF were younger than 65 years: 94.5%, compared with 30% for the other HF patients (P < .001).
Hospitalized patients with meth-HF were mostly men, their prevalence of 80% contrasting with 52.4% for patients with non–meth-related HF (P < .001).
Rates of hospitalization for meth-HF steadily increased during the study period. The age-adjusted rate of meth-HF hospitalization per 100,000 rose from 4.1 in 2008 to 28.1 in 2018. The rate of hospitalization for HF unrelated to meth actually declined, going from 342.3 in 2008 to 321.6 in 2018.
Charges for hospitalizations related to meth-HF shot up more than eight times, from $41.5 million in 2008 to $390.2 million in 2018. In contrast, charges for other HF hospitalizations rose by only 82%, from $3.5 billion to $6.3 billion.
Multiple layers of prevention
Dr. Zhao proposed ways that clinicians can communicate with their patients who are using or considering to use meth. “There are multiple layers of prevention. For people who are thinking of using meth, they need to get the message that something really bad can happen to them years down the road. They’re not going to die from it overnight, but it will damage the heart slowly,” she said.
The next layer of prevention can potentially help meth users who have not yet developed heart problems, Dr. Zhao said. “This would be the time to say, ‘you’re so lucky, your heart is still good. It’s time to stop because people like you, a few years from now are going to die prematurely from a very horrible, very suffering kind of death’.”
Importantly, in meth users who have already developed HF, even then it may not be too late to reverse the cardiomyopathy and symptoms. For up to a third of people with established meth-HF, “if they stop using meth, if they take good cardiac medications, and if the heart failure is in an early enough course, their heart can entirely revert to normal,” Dr. Zhao said, citing an earlier work from her and her colleagues.
Currently, methamphetamine abuse has taken especially strong root in rural areas in California and the Midwest. But Dr. Zhao predicts it will soon become prevalent throughout the United States.
Spotlight on an ‘epidemic’
The rapid growth of the methamphetamine “epidemic” has been well-documented in the United States and around the world, observed an accompanying editorial from Pavan Reddy, MD, Icahn School of Medicine at Mount Sinai Morningside, New York, and Uri Elkayam, MD, University of Southern California, Los Angeles.
They contend that more attention has been given to opioid overdose deaths; meth abuse does not seem to command the same attention, likely because meth is not as strongly associated with acute overdose.
But meth, wrote Dr. Reddy and Dr. Elkayam, “is a different drug with its own M.O., equally dangerous and costly to society but more insidious in nature, its effects potentially causing decades of mental and physical debilitation before ending in premature death.”
The current study “has turned a spotlight on a public health crisis that has grown unfettered for over 2 decades,” and is a call for the “medical community to recognize and manage cases of meth-HF with a comprehensive approach that addresses both mental and physical illness,” they concluded. “Only then can we hope to properly help these patients and with that, reduce the socioeconomic burden of meth-HF.”
A quietly building crisis
The sharp rise in meth-HF hospitalizations is an expected reflection of the methamphetamine crisis, which has been quietly building over the last few years, addiction psychiatrist Corneliu N. Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview.
“This new version of methamphetamines looks like ice and is more potent and toxic than former versions traditionally made in home-built labs,” he said. Lately the vast majority of methamphetamines in the United States have come from Mexico, are less expensive with higher purity, “and can be manufactured in greater quantities.”
Some patients with opioid use disorder (OUD) also inject methamphetamines, which can make OUD treatment clinics good places to screen for meth abuse and educate about its cardiovascular implications, Dr. Stanciu said.
“Just as addiction treatment centers present an opportunity to implement cardiac screening and referrals,” he said, “cardiology visits and hospitalizations such as those for meth-HF also present a golden opportunity for involvement of substance use disorder interventions and referrals to get patients into treatment and prevent further damage through ongoing use.”
Dr. Zhao, Dr. Reddy, Dr. Eklayam, and Dr. Stanciu report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sharp decrease in opioid access for dying U.S. cancer patients
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.