User login
COVID-19 linked to novel epileptic seizures
, new research shows. In a retrospective study of more than 900 patients admitted to the hospital with COVID-19, those without a known history of epilepsy had three times greater odds of experiencing novel seizures than those with a known history of epilepsy.
In addition, among patients with new-onset seizures, hospital stays were about 15 days longer – and mortality rates were significantly higher.
“We’re finding that there are many neurological consequences that can happen with COVID-19 infections, and it’s important for clinicians to keep that in mind as they monitor people long term,” said study investigator Neeraj Singh, MD, neurologist and epileptologist with Northwell Health System, Great Neck, New York.
Dr. Singh noted that although seizures “might not be the most common thing we see in people with COVID-19, they seem to be new seizures and not just a seizure we knew would happen in someone with epilepsy.”
“So there’s definitely a need now for more prospective research and following people over time to fully understand all the different things that might be newly a problem for them in the long term,” he added.
Dr. Singh and Hardik Bhaskar, an undergraduate student at Hunter College, New York, presented the study findings at the American Academy of Neurology’s 2021 annual meeting.
Largest sample to date
“This study explores the relationship between the incidences of COVID-19 infections and [novel] epileptic seizures in the largest sample to date in a single New York–based hospital system,” the investigators noted. Novel seizures included both new-onset and breakthrough seizures.
Dr. Singh told meeting attendees that the “early epicenter” of the COVID pandemic was in New York and occurred from Feb. 29, 2020 to June 1, 2020. Patients with COVID-19 “had multiple neurological sequelae, including seizures, strokes, and encephalopathy,” he said.
However, the effects of COVID-19 on individuals with epilepsy “remain unclear,” Dr. Singh said.
For their study, the researchers assessed 917 patients in 13 New York City metropolitan hospitals. All participants had received a confirmed positive test result on PCR for COVID and had received an antiepileptic medication upon admission. The patients were admitted between Feb. 14 and June 14, 2020.
For the study, the patients were first divided into two groups: those with a history of epilepsy (n = 451), and those without such a history (n = 466).
The first group was further divided on the basis of those who presented with breakthrough seizures and those who presented without them. The second group was further divided on the basis of those who presented with new-onset seizures and those who presented without them.
Significant adverse outcomes
Results showed that 27% of the patients without a history of epilepsy experienced a novel/new-onset seizure and that 11% of the patients with a history of epilepsy experienced a novel/breakthrough seizure (odds ratio, 3.15; P < .0001).
In addition, participants with new-onset seizures had a longer stay in the hospital (mean, 26.9 days) than the subgroup with a history of epilepsy and no breakthrough seizures (10.9 days) and the subgroup with a history of epilepsy who did experience breakthrough seizures (12.8 days; P < .0001 for both comparisons).
In the group of patients with a history of epilepsy, there were no significant differences in lengths of stay between those with and those without breakthrough seizures (P = .68).
Although mortality rates did not differ significantly between the full group with a history of epilepsy versus the full group without epilepsy (23% vs. 25%; OR, 0.9), the mortality rate was significantly higher among patients who experienced novel seizures than among those who did not experience such seizures (29% vs. 23%; OR, 1.4; P = .045).
Mr. Bhaskar noted that there are “many hypotheses for the mechanism by which COVID-19 might cause seizures.” Those mechanisms include proinflammatory cytokine storms, which may increase the rate of apoptosis, neuronal necrosis, and glutamate concentrations and may disrupt the blood-brain barrier. Another hypothesis is that SARS-CoV-2 infection may lead to hypoxia and abnormal coagulation, resulting in stroke and a subsequent increase in the risk for seizures.
Interestingly, “the presence of antiepileptic medications in patients with epilepsy may confer a protective effect against breakthrough seizures,” Dr. Singh said. “However, some subclinical seizures may be misdiagnosed as encephalopathy when patients present with COVID-19 infections.”
He added that further research is needed into the mechanisms linking these infections and new-onset seizures and to “identify subclinical seizures in encephalopathic patients.”
Asked during the question-and-answer session whether the investigators had assessed differences by demographics, such as age or sex, Dr. Singh said, “We have not subdivided them that way yet,” but he said he would like to do so in the future. He also plans to look further into which specific medications were used by the participants.
The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. In a retrospective study of more than 900 patients admitted to the hospital with COVID-19, those without a known history of epilepsy had three times greater odds of experiencing novel seizures than those with a known history of epilepsy.
In addition, among patients with new-onset seizures, hospital stays were about 15 days longer – and mortality rates were significantly higher.
“We’re finding that there are many neurological consequences that can happen with COVID-19 infections, and it’s important for clinicians to keep that in mind as they monitor people long term,” said study investigator Neeraj Singh, MD, neurologist and epileptologist with Northwell Health System, Great Neck, New York.
Dr. Singh noted that although seizures “might not be the most common thing we see in people with COVID-19, they seem to be new seizures and not just a seizure we knew would happen in someone with epilepsy.”
“So there’s definitely a need now for more prospective research and following people over time to fully understand all the different things that might be newly a problem for them in the long term,” he added.
Dr. Singh and Hardik Bhaskar, an undergraduate student at Hunter College, New York, presented the study findings at the American Academy of Neurology’s 2021 annual meeting.
Largest sample to date
“This study explores the relationship between the incidences of COVID-19 infections and [novel] epileptic seizures in the largest sample to date in a single New York–based hospital system,” the investigators noted. Novel seizures included both new-onset and breakthrough seizures.
Dr. Singh told meeting attendees that the “early epicenter” of the COVID pandemic was in New York and occurred from Feb. 29, 2020 to June 1, 2020. Patients with COVID-19 “had multiple neurological sequelae, including seizures, strokes, and encephalopathy,” he said.
However, the effects of COVID-19 on individuals with epilepsy “remain unclear,” Dr. Singh said.
For their study, the researchers assessed 917 patients in 13 New York City metropolitan hospitals. All participants had received a confirmed positive test result on PCR for COVID and had received an antiepileptic medication upon admission. The patients were admitted between Feb. 14 and June 14, 2020.
For the study, the patients were first divided into two groups: those with a history of epilepsy (n = 451), and those without such a history (n = 466).
The first group was further divided on the basis of those who presented with breakthrough seizures and those who presented without them. The second group was further divided on the basis of those who presented with new-onset seizures and those who presented without them.
Significant adverse outcomes
Results showed that 27% of the patients without a history of epilepsy experienced a novel/new-onset seizure and that 11% of the patients with a history of epilepsy experienced a novel/breakthrough seizure (odds ratio, 3.15; P < .0001).
In addition, participants with new-onset seizures had a longer stay in the hospital (mean, 26.9 days) than the subgroup with a history of epilepsy and no breakthrough seizures (10.9 days) and the subgroup with a history of epilepsy who did experience breakthrough seizures (12.8 days; P < .0001 for both comparisons).
In the group of patients with a history of epilepsy, there were no significant differences in lengths of stay between those with and those without breakthrough seizures (P = .68).
Although mortality rates did not differ significantly between the full group with a history of epilepsy versus the full group without epilepsy (23% vs. 25%; OR, 0.9), the mortality rate was significantly higher among patients who experienced novel seizures than among those who did not experience such seizures (29% vs. 23%; OR, 1.4; P = .045).
Mr. Bhaskar noted that there are “many hypotheses for the mechanism by which COVID-19 might cause seizures.” Those mechanisms include proinflammatory cytokine storms, which may increase the rate of apoptosis, neuronal necrosis, and glutamate concentrations and may disrupt the blood-brain barrier. Another hypothesis is that SARS-CoV-2 infection may lead to hypoxia and abnormal coagulation, resulting in stroke and a subsequent increase in the risk for seizures.
Interestingly, “the presence of antiepileptic medications in patients with epilepsy may confer a protective effect against breakthrough seizures,” Dr. Singh said. “However, some subclinical seizures may be misdiagnosed as encephalopathy when patients present with COVID-19 infections.”
He added that further research is needed into the mechanisms linking these infections and new-onset seizures and to “identify subclinical seizures in encephalopathic patients.”
Asked during the question-and-answer session whether the investigators had assessed differences by demographics, such as age or sex, Dr. Singh said, “We have not subdivided them that way yet,” but he said he would like to do so in the future. He also plans to look further into which specific medications were used by the participants.
The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. In a retrospective study of more than 900 patients admitted to the hospital with COVID-19, those without a known history of epilepsy had three times greater odds of experiencing novel seizures than those with a known history of epilepsy.
In addition, among patients with new-onset seizures, hospital stays were about 15 days longer – and mortality rates were significantly higher.
“We’re finding that there are many neurological consequences that can happen with COVID-19 infections, and it’s important for clinicians to keep that in mind as they monitor people long term,” said study investigator Neeraj Singh, MD, neurologist and epileptologist with Northwell Health System, Great Neck, New York.
Dr. Singh noted that although seizures “might not be the most common thing we see in people with COVID-19, they seem to be new seizures and not just a seizure we knew would happen in someone with epilepsy.”
“So there’s definitely a need now for more prospective research and following people over time to fully understand all the different things that might be newly a problem for them in the long term,” he added.
Dr. Singh and Hardik Bhaskar, an undergraduate student at Hunter College, New York, presented the study findings at the American Academy of Neurology’s 2021 annual meeting.
Largest sample to date
“This study explores the relationship between the incidences of COVID-19 infections and [novel] epileptic seizures in the largest sample to date in a single New York–based hospital system,” the investigators noted. Novel seizures included both new-onset and breakthrough seizures.
Dr. Singh told meeting attendees that the “early epicenter” of the COVID pandemic was in New York and occurred from Feb. 29, 2020 to June 1, 2020. Patients with COVID-19 “had multiple neurological sequelae, including seizures, strokes, and encephalopathy,” he said.
However, the effects of COVID-19 on individuals with epilepsy “remain unclear,” Dr. Singh said.
For their study, the researchers assessed 917 patients in 13 New York City metropolitan hospitals. All participants had received a confirmed positive test result on PCR for COVID and had received an antiepileptic medication upon admission. The patients were admitted between Feb. 14 and June 14, 2020.
For the study, the patients were first divided into two groups: those with a history of epilepsy (n = 451), and those without such a history (n = 466).
The first group was further divided on the basis of those who presented with breakthrough seizures and those who presented without them. The second group was further divided on the basis of those who presented with new-onset seizures and those who presented without them.
Significant adverse outcomes
Results showed that 27% of the patients without a history of epilepsy experienced a novel/new-onset seizure and that 11% of the patients with a history of epilepsy experienced a novel/breakthrough seizure (odds ratio, 3.15; P < .0001).
In addition, participants with new-onset seizures had a longer stay in the hospital (mean, 26.9 days) than the subgroup with a history of epilepsy and no breakthrough seizures (10.9 days) and the subgroup with a history of epilepsy who did experience breakthrough seizures (12.8 days; P < .0001 for both comparisons).
In the group of patients with a history of epilepsy, there were no significant differences in lengths of stay between those with and those without breakthrough seizures (P = .68).
Although mortality rates did not differ significantly between the full group with a history of epilepsy versus the full group without epilepsy (23% vs. 25%; OR, 0.9), the mortality rate was significantly higher among patients who experienced novel seizures than among those who did not experience such seizures (29% vs. 23%; OR, 1.4; P = .045).
Mr. Bhaskar noted that there are “many hypotheses for the mechanism by which COVID-19 might cause seizures.” Those mechanisms include proinflammatory cytokine storms, which may increase the rate of apoptosis, neuronal necrosis, and glutamate concentrations and may disrupt the blood-brain barrier. Another hypothesis is that SARS-CoV-2 infection may lead to hypoxia and abnormal coagulation, resulting in stroke and a subsequent increase in the risk for seizures.
Interestingly, “the presence of antiepileptic medications in patients with epilepsy may confer a protective effect against breakthrough seizures,” Dr. Singh said. “However, some subclinical seizures may be misdiagnosed as encephalopathy when patients present with COVID-19 infections.”
He added that further research is needed into the mechanisms linking these infections and new-onset seizures and to “identify subclinical seizures in encephalopathic patients.”
Asked during the question-and-answer session whether the investigators had assessed differences by demographics, such as age or sex, Dr. Singh said, “We have not subdivided them that way yet,” but he said he would like to do so in the future. He also plans to look further into which specific medications were used by the participants.
The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From AAN 2021
Survival benefit with nivolumab extends to 5 years in NSCLC
Across the two studies – CheckMate 017 and 057 – 854 patients were randomized 1:1 following progression on platinum therapy to either nivolumab at 3 mg/kg once every 2 weeks or docetaxel at 75 mg/m2 once every 3 weeks until further progression or unacceptable toxicity. Previously reported overall survival (OS) outcomes favored nivolumab.
At a minimum follow-up of 5.4 years, 50 nivolumab-treated patients and 9 docetaxel-treated patients were still alive.
The 5-year OS rates were 13.4% in the nivolumab arm and 2.6% in the docetaxel arm. The 5-year progression-free survival (PFS) rates were 8% and 0%, respectively.
There were no new safety signals with nivolumab, and there was no evidence of select late-onset grade 3-4 adverse events.
According to the study authors, this analysis is the longest phase 3 follow-up to date of a PD-1 inhibitor in previously treated, advanced NSCLC, and it suggests that “long-term survival beyond 5 years may ... be possible in NSCLC.”
“The results indicate that some patients with NSCLC can have long-lasting benefit from checkpoint inhibitors. We have seen similar results in terms of long-term OS with pembrolizumab,” said investigator Hossein Borghaei, DO, chief of thoracic medical oncology at the Fox Chase Cancer Center in Philadelphia.
Dr. Borghaei said the question now is “how to identify the population that really benefits from these treatments. We think PD-L1–high [patients] have a better chance, [as do patients with] tumors that have a higher percentage of tumor-infiltrating lymphocytes, but there’s nothing concrete beyond that.”
No baseline clinical or tumor factors emerged to distinguish between long-and short-term survivors, but the 5-year OS rate was 18.3% among nivolumab-treated patients with PD-L1 expression at or above 1% versus 8% among patients with expression below 1%.
The optimal duration of nivolumab treatment beyond 1 year is also uncertain.
The median duration of therapy was 36.9 months in the 5-year survivors treated with nivolumab, and 36% of patients (18/50) were still on nivolumab at the 5-year mark.
The median duration of time off treatment was 41.9 months among patients who discontinued nivolumab. Five patients (10%) were off treatment with no subsequent therapy and had not progressed at 5 years, “suggesting benefit even for patients who stopped nivolumab treatment,” the researchers wrote.
They also found that nivolumab-treated patients who remained alive at 3 years appeared to stabilize and plateau thereafter, with early response suggesting better long-term outcomes. The majority of patients without disease progression at 2, 3, and 4 years, for instance, remained progression free at 5 years. Nearly one-third of patients who achieved an objective response with nivolumab – but none of the patients who responded to docetaxel – had ongoing responses at 5 years.
Similarly, nivolumab-treated patients without disease progression at 2 years and 3 years had an 82% and 93% chance of survival, respectively, and a 59.6% and 78.3% chance of remaining progression free at 5 years.
This research was funded by Bristol-Myers Squibb. Dr. Borghaei and coauthors disclosed numerous ties to the company, including employment.
Across the two studies – CheckMate 017 and 057 – 854 patients were randomized 1:1 following progression on platinum therapy to either nivolumab at 3 mg/kg once every 2 weeks or docetaxel at 75 mg/m2 once every 3 weeks until further progression or unacceptable toxicity. Previously reported overall survival (OS) outcomes favored nivolumab.
At a minimum follow-up of 5.4 years, 50 nivolumab-treated patients and 9 docetaxel-treated patients were still alive.
The 5-year OS rates were 13.4% in the nivolumab arm and 2.6% in the docetaxel arm. The 5-year progression-free survival (PFS) rates were 8% and 0%, respectively.
There were no new safety signals with nivolumab, and there was no evidence of select late-onset grade 3-4 adverse events.
According to the study authors, this analysis is the longest phase 3 follow-up to date of a PD-1 inhibitor in previously treated, advanced NSCLC, and it suggests that “long-term survival beyond 5 years may ... be possible in NSCLC.”
“The results indicate that some patients with NSCLC can have long-lasting benefit from checkpoint inhibitors. We have seen similar results in terms of long-term OS with pembrolizumab,” said investigator Hossein Borghaei, DO, chief of thoracic medical oncology at the Fox Chase Cancer Center in Philadelphia.
Dr. Borghaei said the question now is “how to identify the population that really benefits from these treatments. We think PD-L1–high [patients] have a better chance, [as do patients with] tumors that have a higher percentage of tumor-infiltrating lymphocytes, but there’s nothing concrete beyond that.”
No baseline clinical or tumor factors emerged to distinguish between long-and short-term survivors, but the 5-year OS rate was 18.3% among nivolumab-treated patients with PD-L1 expression at or above 1% versus 8% among patients with expression below 1%.
The optimal duration of nivolumab treatment beyond 1 year is also uncertain.
The median duration of therapy was 36.9 months in the 5-year survivors treated with nivolumab, and 36% of patients (18/50) were still on nivolumab at the 5-year mark.
The median duration of time off treatment was 41.9 months among patients who discontinued nivolumab. Five patients (10%) were off treatment with no subsequent therapy and had not progressed at 5 years, “suggesting benefit even for patients who stopped nivolumab treatment,” the researchers wrote.
They also found that nivolumab-treated patients who remained alive at 3 years appeared to stabilize and plateau thereafter, with early response suggesting better long-term outcomes. The majority of patients without disease progression at 2, 3, and 4 years, for instance, remained progression free at 5 years. Nearly one-third of patients who achieved an objective response with nivolumab – but none of the patients who responded to docetaxel – had ongoing responses at 5 years.
Similarly, nivolumab-treated patients without disease progression at 2 years and 3 years had an 82% and 93% chance of survival, respectively, and a 59.6% and 78.3% chance of remaining progression free at 5 years.
This research was funded by Bristol-Myers Squibb. Dr. Borghaei and coauthors disclosed numerous ties to the company, including employment.
Across the two studies – CheckMate 017 and 057 – 854 patients were randomized 1:1 following progression on platinum therapy to either nivolumab at 3 mg/kg once every 2 weeks or docetaxel at 75 mg/m2 once every 3 weeks until further progression or unacceptable toxicity. Previously reported overall survival (OS) outcomes favored nivolumab.
At a minimum follow-up of 5.4 years, 50 nivolumab-treated patients and 9 docetaxel-treated patients were still alive.
The 5-year OS rates were 13.4% in the nivolumab arm and 2.6% in the docetaxel arm. The 5-year progression-free survival (PFS) rates were 8% and 0%, respectively.
There were no new safety signals with nivolumab, and there was no evidence of select late-onset grade 3-4 adverse events.
According to the study authors, this analysis is the longest phase 3 follow-up to date of a PD-1 inhibitor in previously treated, advanced NSCLC, and it suggests that “long-term survival beyond 5 years may ... be possible in NSCLC.”
“The results indicate that some patients with NSCLC can have long-lasting benefit from checkpoint inhibitors. We have seen similar results in terms of long-term OS with pembrolizumab,” said investigator Hossein Borghaei, DO, chief of thoracic medical oncology at the Fox Chase Cancer Center in Philadelphia.
Dr. Borghaei said the question now is “how to identify the population that really benefits from these treatments. We think PD-L1–high [patients] have a better chance, [as do patients with] tumors that have a higher percentage of tumor-infiltrating lymphocytes, but there’s nothing concrete beyond that.”
No baseline clinical or tumor factors emerged to distinguish between long-and short-term survivors, but the 5-year OS rate was 18.3% among nivolumab-treated patients with PD-L1 expression at or above 1% versus 8% among patients with expression below 1%.
The optimal duration of nivolumab treatment beyond 1 year is also uncertain.
The median duration of therapy was 36.9 months in the 5-year survivors treated with nivolumab, and 36% of patients (18/50) were still on nivolumab at the 5-year mark.
The median duration of time off treatment was 41.9 months among patients who discontinued nivolumab. Five patients (10%) were off treatment with no subsequent therapy and had not progressed at 5 years, “suggesting benefit even for patients who stopped nivolumab treatment,” the researchers wrote.
They also found that nivolumab-treated patients who remained alive at 3 years appeared to stabilize and plateau thereafter, with early response suggesting better long-term outcomes. The majority of patients without disease progression at 2, 3, and 4 years, for instance, remained progression free at 5 years. Nearly one-third of patients who achieved an objective response with nivolumab – but none of the patients who responded to docetaxel – had ongoing responses at 5 years.
Similarly, nivolumab-treated patients without disease progression at 2 years and 3 years had an 82% and 93% chance of survival, respectively, and a 59.6% and 78.3% chance of remaining progression free at 5 years.
This research was funded by Bristol-Myers Squibb. Dr. Borghaei and coauthors disclosed numerous ties to the company, including employment.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Debate: Should biologics be used for milder cases of psoriasis?
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
Tofacitinib: Small study shows big cutaneous sarcoidosis response
Researchers are reporting impressive results in a small, , and all patients improved by an average of 83% via a scoring system.
“Not only did patients get better, but they were in many cases able to come off their baseline immunosuppressive regimen, including prednisone and methotrexate. They’d get off prednisone entirely or, in some cases, decrease it substantially,” study investigator William Damsky, MD, PhD, reported at the American Academy of Dermatology Virtual Meeting Experience.
Sarcoidosis is a common disease that affects an estimated 1 in 25 Black women and is believed to contribute to the deaths of about 4,000 people in the United States each year, noted Dr. Damsky of the department of dermatology, Yale University, New Haven, Conn. One famous patient is comedian Bernie Mac, who died from the condition in 2008.
“Approximately one third of patients have cutaneous involvement,” Dr. Damsky said, and skin may be the only manifestation of the disease. There is no Food and Drug Administration-approved therapy for cutaneous sarcoidosis, he added. Prednisone, the first-line therapy in skin manifestations, is approved only for pulmonary sarcoidosis.
“Oftentimes, there’s an attempt to transition either partially or fully to other therapies, including methotrexate and TNF-alpha blockers. But there’s been mixed success in doing that,” he said. This is not always possible, “so a lot of patients end up on prednisone.”
Earlier, a team at Yale prescribed 5 mg tofacitinib (Xeljanz) for several patients with severe cutaneous sarcoidosis and saw impressive results, Dr. Damsky said, including a patient with pulmonary sarcoidosis that also improved. He noted that there are case reports in the medical literature with similar findings.
Those positive results inspired the new study. Researchers recruited 10 patients with cutaneous sarcoidosis (9 with internal organ involvement) with a Cutaneous Sarcoidosis Activity and Morphology Instrument ( CSAMI ) score of 10 or higher. Nine patients were in their 50s, one was aged 63 years, and five were men. Skin colors of the patients ranged from Fitzpatrick skin types I to VI, and all had been taking at least two medications, typically methotrexate and prednisone.
The patients received 5 mg of tofacitinib twice a day for 6 months. “Everyone got better during the study, and six patients had a complete response, which we defined as a CSAMI score of zero activity,” Dr. Damsky said. “It’s really quite remarkable to see that.” Overall, the patients saw an 83% improvement in CSAMI scores.
In regard to safety, “all patients completed the study,” he said. “Tofacitinib was well tolerated, and there were no serious adverse effects or events.”
Tofacitinib is approved for treating rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis.
A month’s supply of twice-daily 5 mg tofacitinib pills would cost $4,900-$5,100 with free coupons, according to information accessed on April 24, 2021, on GoodRx.com. Generics are not available.
In an interview, Sotonye Imadojemu, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, praised the study, and said “tofacitinib is a reasonable treatment for treatment-refractory or extensive cutaneous sarcoidosis,” although it will be helpful to get results from randomized-controlled trials.
She cautioned that the drug “is a powerful immunosuppressant, so the risk of infection must be discussed with patients before prescribing. Screening for chronic infections such as viral hepatitis, tuberculosis, and HIV should be completed prior to treatment initiation. Blood counts, liver function, and lipid panels should be regularly monitored. The vaccines necessary for those who are immunosuppressed should be administered as able, and age-appropriate cancer screening must be kept up to date.”
The study was funded by Pfizer, the Dermatology Foundation, and the Yale Department of Dermatology. Dr. Damsky disclosed research support (Pfizer), consulting fees (Eli Lilly, Pfizer, TWi Biotechnology), and licensing fees (EMD Millipore/MillporeSigma). Dr. Imadojemu has no disclosures.
This article was updated 5/5/21.
Researchers are reporting impressive results in a small, , and all patients improved by an average of 83% via a scoring system.
“Not only did patients get better, but they were in many cases able to come off their baseline immunosuppressive regimen, including prednisone and methotrexate. They’d get off prednisone entirely or, in some cases, decrease it substantially,” study investigator William Damsky, MD, PhD, reported at the American Academy of Dermatology Virtual Meeting Experience.
Sarcoidosis is a common disease that affects an estimated 1 in 25 Black women and is believed to contribute to the deaths of about 4,000 people in the United States each year, noted Dr. Damsky of the department of dermatology, Yale University, New Haven, Conn. One famous patient is comedian Bernie Mac, who died from the condition in 2008.
“Approximately one third of patients have cutaneous involvement,” Dr. Damsky said, and skin may be the only manifestation of the disease. There is no Food and Drug Administration-approved therapy for cutaneous sarcoidosis, he added. Prednisone, the first-line therapy in skin manifestations, is approved only for pulmonary sarcoidosis.
“Oftentimes, there’s an attempt to transition either partially or fully to other therapies, including methotrexate and TNF-alpha blockers. But there’s been mixed success in doing that,” he said. This is not always possible, “so a lot of patients end up on prednisone.”
Earlier, a team at Yale prescribed 5 mg tofacitinib (Xeljanz) for several patients with severe cutaneous sarcoidosis and saw impressive results, Dr. Damsky said, including a patient with pulmonary sarcoidosis that also improved. He noted that there are case reports in the medical literature with similar findings.
Those positive results inspired the new study. Researchers recruited 10 patients with cutaneous sarcoidosis (9 with internal organ involvement) with a Cutaneous Sarcoidosis Activity and Morphology Instrument ( CSAMI ) score of 10 or higher. Nine patients were in their 50s, one was aged 63 years, and five were men. Skin colors of the patients ranged from Fitzpatrick skin types I to VI, and all had been taking at least two medications, typically methotrexate and prednisone.
The patients received 5 mg of tofacitinib twice a day for 6 months. “Everyone got better during the study, and six patients had a complete response, which we defined as a CSAMI score of zero activity,” Dr. Damsky said. “It’s really quite remarkable to see that.” Overall, the patients saw an 83% improvement in CSAMI scores.
In regard to safety, “all patients completed the study,” he said. “Tofacitinib was well tolerated, and there were no serious adverse effects or events.”
Tofacitinib is approved for treating rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis.
A month’s supply of twice-daily 5 mg tofacitinib pills would cost $4,900-$5,100 with free coupons, according to information accessed on April 24, 2021, on GoodRx.com. Generics are not available.
In an interview, Sotonye Imadojemu, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, praised the study, and said “tofacitinib is a reasonable treatment for treatment-refractory or extensive cutaneous sarcoidosis,” although it will be helpful to get results from randomized-controlled trials.
She cautioned that the drug “is a powerful immunosuppressant, so the risk of infection must be discussed with patients before prescribing. Screening for chronic infections such as viral hepatitis, tuberculosis, and HIV should be completed prior to treatment initiation. Blood counts, liver function, and lipid panels should be regularly monitored. The vaccines necessary for those who are immunosuppressed should be administered as able, and age-appropriate cancer screening must be kept up to date.”
The study was funded by Pfizer, the Dermatology Foundation, and the Yale Department of Dermatology. Dr. Damsky disclosed research support (Pfizer), consulting fees (Eli Lilly, Pfizer, TWi Biotechnology), and licensing fees (EMD Millipore/MillporeSigma). Dr. Imadojemu has no disclosures.
This article was updated 5/5/21.
Researchers are reporting impressive results in a small, , and all patients improved by an average of 83% via a scoring system.
“Not only did patients get better, but they were in many cases able to come off their baseline immunosuppressive regimen, including prednisone and methotrexate. They’d get off prednisone entirely or, in some cases, decrease it substantially,” study investigator William Damsky, MD, PhD, reported at the American Academy of Dermatology Virtual Meeting Experience.
Sarcoidosis is a common disease that affects an estimated 1 in 25 Black women and is believed to contribute to the deaths of about 4,000 people in the United States each year, noted Dr. Damsky of the department of dermatology, Yale University, New Haven, Conn. One famous patient is comedian Bernie Mac, who died from the condition in 2008.
“Approximately one third of patients have cutaneous involvement,” Dr. Damsky said, and skin may be the only manifestation of the disease. There is no Food and Drug Administration-approved therapy for cutaneous sarcoidosis, he added. Prednisone, the first-line therapy in skin manifestations, is approved only for pulmonary sarcoidosis.
“Oftentimes, there’s an attempt to transition either partially or fully to other therapies, including methotrexate and TNF-alpha blockers. But there’s been mixed success in doing that,” he said. This is not always possible, “so a lot of patients end up on prednisone.”
Earlier, a team at Yale prescribed 5 mg tofacitinib (Xeljanz) for several patients with severe cutaneous sarcoidosis and saw impressive results, Dr. Damsky said, including a patient with pulmonary sarcoidosis that also improved. He noted that there are case reports in the medical literature with similar findings.
Those positive results inspired the new study. Researchers recruited 10 patients with cutaneous sarcoidosis (9 with internal organ involvement) with a Cutaneous Sarcoidosis Activity and Morphology Instrument ( CSAMI ) score of 10 or higher. Nine patients were in their 50s, one was aged 63 years, and five were men. Skin colors of the patients ranged from Fitzpatrick skin types I to VI, and all had been taking at least two medications, typically methotrexate and prednisone.
The patients received 5 mg of tofacitinib twice a day for 6 months. “Everyone got better during the study, and six patients had a complete response, which we defined as a CSAMI score of zero activity,” Dr. Damsky said. “It’s really quite remarkable to see that.” Overall, the patients saw an 83% improvement in CSAMI scores.
In regard to safety, “all patients completed the study,” he said. “Tofacitinib was well tolerated, and there were no serious adverse effects or events.”
Tofacitinib is approved for treating rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis.
A month’s supply of twice-daily 5 mg tofacitinib pills would cost $4,900-$5,100 with free coupons, according to information accessed on April 24, 2021, on GoodRx.com. Generics are not available.
In an interview, Sotonye Imadojemu, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, praised the study, and said “tofacitinib is a reasonable treatment for treatment-refractory or extensive cutaneous sarcoidosis,” although it will be helpful to get results from randomized-controlled trials.
She cautioned that the drug “is a powerful immunosuppressant, so the risk of infection must be discussed with patients before prescribing. Screening for chronic infections such as viral hepatitis, tuberculosis, and HIV should be completed prior to treatment initiation. Blood counts, liver function, and lipid panels should be regularly monitored. The vaccines necessary for those who are immunosuppressed should be administered as able, and age-appropriate cancer screening must be kept up to date.”
The study was funded by Pfizer, the Dermatology Foundation, and the Yale Department of Dermatology. Dr. Damsky disclosed research support (Pfizer), consulting fees (Eli Lilly, Pfizer, TWi Biotechnology), and licensing fees (EMD Millipore/MillporeSigma). Dr. Imadojemu has no disclosures.
This article was updated 5/5/21.
REPORTING FROM AAD VMX 2021
Topical anticholinergic for axillary hyperhidrosis shows fewer side effects
according to 48-week safety and outcome data.
A structural analogue of glycopyrrolate working through the same mechanism, sofpironium bromide was developed as a retrometabolic agent. This means it is rapidly transformed into an inactive metabolite after application, reducing risk of systemic effects, study investigator Stacy Smith, MD, explained in the late-breaking research session at the American Academy of Dermatology Virtual Meeting Experience.
The anticholinergic glycopyrrolate, which currently is the most commonly used therapy for hyperhidrosis, is absorbed through the skin and excreted through the urine. The systemic exposure to the active agent after topical application explains the substantial risk of adverse effects, said Dr. Smith, a clinician and researcher affiliated with the California Dermatology and Clinical Research Institute, Encinitas.
In contrast,“sofpironium bromide is the ideal topical medication, because it has strong activity at the application site but then reduced systemic activity due to the retrometabolism,” Dr. Smith said.
The 52-week data from the open-label, phase 3 trial supports the premise. In this study of 299 patients randomized to the 5% (102 patients) or 15% (197 patients) topical sofpironium bromide gel formulations, most anticholinergic adverse events were mild or moderate and transient, with complaints concentrated in the first 3 months of the trial.
“The retrometabolic pathway seems to work,” Dr. Smith said. He acknowledged that the treatment-naive patients who entered the study “had to get used to the drug over time,” but the data “show they did.”
The phase 3 trial of sofpironium bromide, which is already approved to treat axillary hyperhidrosis in Japan, did not have a placebo control. It was focused primarily on safety, but outcomes were assessed with the Hyperhidrosis Disease Severity Measure–Axillary (HDSM-Ax).
At least a 1-point improvement in the 7-point HDSM-Ax scale, which is considered clinically meaningful, was achieved by 86.1% and 85.8% of those treated with the 5% and 15% gels, respectively. A 2-point or greater improvement at the end of the study was observed in 69.4% and 61.9%, respectively.
“The medication works well and there was improved efficacy over time. About two-thirds of the patients had at least a 2-point improvement in the HDSM-Ax score at the end of 48 weeks,” Dr. Smith reported.
While response rates climbed over the course of the study, rates of adverse events fell markedly.
After 2 weeks of treatment, the proportions of patients with a treatment-related adverse event were 6% and just under 15% for the 5% and 15% topical-gel groups, respectively. At each 2-week interval when reassessed, the rates fell. By week 12, the rates were less than 2% and about 4% in the two groups, respectively.
The discontinuation rates overall for anticholinergic side effects were 3% and 8.1% for the lower and higher doses. Blurred vision accounted for the vast majority of these discontinuations in both groups. The other discontinuations, which included those for dry mouth, urinary retention, and mydriasis, occurred in one patient each. Again, discontinuations were most common in the first few months of the study.
For the total study population, mild (10.8% vs. 24%) and moderate (10.8% vs. 20.3%) side effects accounted for almost all side effects with the lower and higher doses of the topical drug. Only one patient in the low-dose group had a severe adverse event. At 6.1%, the proportion of the high-dose group with a severe adverse event was higher, but none of the adverse events were considered serious. All were transient.
These rates of adverse events are lower than those reported historically with effective doses of glycopyrrolate, Dr. Smith said.
The data presented by Dr. Smith are part of a phase 3 pivotal trials program designed to gain FDA approval. Going forward, these trials, which are enrolling patients as young as 9 years old, are expected to focus on clinical development of the 15% gel, he added.
The gel is delivered with a metered-dose pump that has an applicator, according to Brickell Biotech, the company developing the treatment in the United States. The 5% formulation was approved in Japan in September 2020, for the treatment of primary axillary hyperhidrosis.
In an interview, David M. Pariser, MD, professor of dermatology, Eastern Virginia Medical School, Norfolk, said that he believes that this drug has could be helpful if the pivotal studies confirm efficacy with a lower risk of adverse events relative to glycopyrrolate. “If it is true that, in phase 3, placebo-controlled trials, there are fewer systemic anticholinergic effects, then this drug will be very useful,” said Dr. Pariser, cofounder of the International Hyperhidrosis Society and an investigator on a previously published dose-ranging, phase 2 study of sofpironium bromide.
The trial was sponsored by Brickell Biotech, which compensated Dr. Smith and other coauthors for their participation. Dr. Pariser has financial relationships with multiple pharmaceutical companies with dermatologic products, including Brickell Biotech.
This article was updated 4/26/21.
according to 48-week safety and outcome data.
A structural analogue of glycopyrrolate working through the same mechanism, sofpironium bromide was developed as a retrometabolic agent. This means it is rapidly transformed into an inactive metabolite after application, reducing risk of systemic effects, study investigator Stacy Smith, MD, explained in the late-breaking research session at the American Academy of Dermatology Virtual Meeting Experience.
The anticholinergic glycopyrrolate, which currently is the most commonly used therapy for hyperhidrosis, is absorbed through the skin and excreted through the urine. The systemic exposure to the active agent after topical application explains the substantial risk of adverse effects, said Dr. Smith, a clinician and researcher affiliated with the California Dermatology and Clinical Research Institute, Encinitas.
In contrast,“sofpironium bromide is the ideal topical medication, because it has strong activity at the application site but then reduced systemic activity due to the retrometabolism,” Dr. Smith said.
The 52-week data from the open-label, phase 3 trial supports the premise. In this study of 299 patients randomized to the 5% (102 patients) or 15% (197 patients) topical sofpironium bromide gel formulations, most anticholinergic adverse events were mild or moderate and transient, with complaints concentrated in the first 3 months of the trial.
“The retrometabolic pathway seems to work,” Dr. Smith said. He acknowledged that the treatment-naive patients who entered the study “had to get used to the drug over time,” but the data “show they did.”
The phase 3 trial of sofpironium bromide, which is already approved to treat axillary hyperhidrosis in Japan, did not have a placebo control. It was focused primarily on safety, but outcomes were assessed with the Hyperhidrosis Disease Severity Measure–Axillary (HDSM-Ax).
At least a 1-point improvement in the 7-point HDSM-Ax scale, which is considered clinically meaningful, was achieved by 86.1% and 85.8% of those treated with the 5% and 15% gels, respectively. A 2-point or greater improvement at the end of the study was observed in 69.4% and 61.9%, respectively.
“The medication works well and there was improved efficacy over time. About two-thirds of the patients had at least a 2-point improvement in the HDSM-Ax score at the end of 48 weeks,” Dr. Smith reported.
While response rates climbed over the course of the study, rates of adverse events fell markedly.
After 2 weeks of treatment, the proportions of patients with a treatment-related adverse event were 6% and just under 15% for the 5% and 15% topical-gel groups, respectively. At each 2-week interval when reassessed, the rates fell. By week 12, the rates were less than 2% and about 4% in the two groups, respectively.
The discontinuation rates overall for anticholinergic side effects were 3% and 8.1% for the lower and higher doses. Blurred vision accounted for the vast majority of these discontinuations in both groups. The other discontinuations, which included those for dry mouth, urinary retention, and mydriasis, occurred in one patient each. Again, discontinuations were most common in the first few months of the study.
For the total study population, mild (10.8% vs. 24%) and moderate (10.8% vs. 20.3%) side effects accounted for almost all side effects with the lower and higher doses of the topical drug. Only one patient in the low-dose group had a severe adverse event. At 6.1%, the proportion of the high-dose group with a severe adverse event was higher, but none of the adverse events were considered serious. All were transient.
These rates of adverse events are lower than those reported historically with effective doses of glycopyrrolate, Dr. Smith said.
The data presented by Dr. Smith are part of a phase 3 pivotal trials program designed to gain FDA approval. Going forward, these trials, which are enrolling patients as young as 9 years old, are expected to focus on clinical development of the 15% gel, he added.
The gel is delivered with a metered-dose pump that has an applicator, according to Brickell Biotech, the company developing the treatment in the United States. The 5% formulation was approved in Japan in September 2020, for the treatment of primary axillary hyperhidrosis.
In an interview, David M. Pariser, MD, professor of dermatology, Eastern Virginia Medical School, Norfolk, said that he believes that this drug has could be helpful if the pivotal studies confirm efficacy with a lower risk of adverse events relative to glycopyrrolate. “If it is true that, in phase 3, placebo-controlled trials, there are fewer systemic anticholinergic effects, then this drug will be very useful,” said Dr. Pariser, cofounder of the International Hyperhidrosis Society and an investigator on a previously published dose-ranging, phase 2 study of sofpironium bromide.
The trial was sponsored by Brickell Biotech, which compensated Dr. Smith and other coauthors for their participation. Dr. Pariser has financial relationships with multiple pharmaceutical companies with dermatologic products, including Brickell Biotech.
This article was updated 4/26/21.
according to 48-week safety and outcome data.
A structural analogue of glycopyrrolate working through the same mechanism, sofpironium bromide was developed as a retrometabolic agent. This means it is rapidly transformed into an inactive metabolite after application, reducing risk of systemic effects, study investigator Stacy Smith, MD, explained in the late-breaking research session at the American Academy of Dermatology Virtual Meeting Experience.
The anticholinergic glycopyrrolate, which currently is the most commonly used therapy for hyperhidrosis, is absorbed through the skin and excreted through the urine. The systemic exposure to the active agent after topical application explains the substantial risk of adverse effects, said Dr. Smith, a clinician and researcher affiliated with the California Dermatology and Clinical Research Institute, Encinitas.
In contrast,“sofpironium bromide is the ideal topical medication, because it has strong activity at the application site but then reduced systemic activity due to the retrometabolism,” Dr. Smith said.
The 52-week data from the open-label, phase 3 trial supports the premise. In this study of 299 patients randomized to the 5% (102 patients) or 15% (197 patients) topical sofpironium bromide gel formulations, most anticholinergic adverse events were mild or moderate and transient, with complaints concentrated in the first 3 months of the trial.
“The retrometabolic pathway seems to work,” Dr. Smith said. He acknowledged that the treatment-naive patients who entered the study “had to get used to the drug over time,” but the data “show they did.”
The phase 3 trial of sofpironium bromide, which is already approved to treat axillary hyperhidrosis in Japan, did not have a placebo control. It was focused primarily on safety, but outcomes were assessed with the Hyperhidrosis Disease Severity Measure–Axillary (HDSM-Ax).
At least a 1-point improvement in the 7-point HDSM-Ax scale, which is considered clinically meaningful, was achieved by 86.1% and 85.8% of those treated with the 5% and 15% gels, respectively. A 2-point or greater improvement at the end of the study was observed in 69.4% and 61.9%, respectively.
“The medication works well and there was improved efficacy over time. About two-thirds of the patients had at least a 2-point improvement in the HDSM-Ax score at the end of 48 weeks,” Dr. Smith reported.
While response rates climbed over the course of the study, rates of adverse events fell markedly.
After 2 weeks of treatment, the proportions of patients with a treatment-related adverse event were 6% and just under 15% for the 5% and 15% topical-gel groups, respectively. At each 2-week interval when reassessed, the rates fell. By week 12, the rates were less than 2% and about 4% in the two groups, respectively.
The discontinuation rates overall for anticholinergic side effects were 3% and 8.1% for the lower and higher doses. Blurred vision accounted for the vast majority of these discontinuations in both groups. The other discontinuations, which included those for dry mouth, urinary retention, and mydriasis, occurred in one patient each. Again, discontinuations were most common in the first few months of the study.
For the total study population, mild (10.8% vs. 24%) and moderate (10.8% vs. 20.3%) side effects accounted for almost all side effects with the lower and higher doses of the topical drug. Only one patient in the low-dose group had a severe adverse event. At 6.1%, the proportion of the high-dose group with a severe adverse event was higher, but none of the adverse events were considered serious. All were transient.
These rates of adverse events are lower than those reported historically with effective doses of glycopyrrolate, Dr. Smith said.
The data presented by Dr. Smith are part of a phase 3 pivotal trials program designed to gain FDA approval. Going forward, these trials, which are enrolling patients as young as 9 years old, are expected to focus on clinical development of the 15% gel, he added.
The gel is delivered with a metered-dose pump that has an applicator, according to Brickell Biotech, the company developing the treatment in the United States. The 5% formulation was approved in Japan in September 2020, for the treatment of primary axillary hyperhidrosis.
In an interview, David M. Pariser, MD, professor of dermatology, Eastern Virginia Medical School, Norfolk, said that he believes that this drug has could be helpful if the pivotal studies confirm efficacy with a lower risk of adverse events relative to glycopyrrolate. “If it is true that, in phase 3, placebo-controlled trials, there are fewer systemic anticholinergic effects, then this drug will be very useful,” said Dr. Pariser, cofounder of the International Hyperhidrosis Society and an investigator on a previously published dose-ranging, phase 2 study of sofpironium bromide.
The trial was sponsored by Brickell Biotech, which compensated Dr. Smith and other coauthors for their participation. Dr. Pariser has financial relationships with multiple pharmaceutical companies with dermatologic products, including Brickell Biotech.
This article was updated 4/26/21.
FROM AAD VMX 2021
MPL, microaggressions, and more
Dear colleagues,
Welcome to the May edition of The New Gastroenterologist, which is packed with a fantastic line-up of articles! The 1-year mark of the pandemic recently passed, and we now are gearing up for our second virtual Digestive Disease Week (DDW®). While we are all anxious to return to lives that have some semblance of normalcy, we continue to endure the ebbs and flows that characterize life in a pandemic. For over a year now, we spend our days caught in a constant battle between the risk and reward of activities we previously took for granted or considered mundane. Our moods vacillate with the continued rise and fall of COVID-19 cases, but the advent and distribution of vaccines have offered palpable hope for better outcomes.
I’m pleased to introduce this quarter’s content – beginning with our legal section. Dr. John Azizian (UCLA-Olive-View), Dr. James Tabibian (UCLA-Olive-View), Dr. Camellia Dalai (UCLA-Olive-View/University of New Mexico), and Dr. Megan Adams (University of Michigan) contribute a comprehensive piece on medical professional liability (MPL), a topic that is seldom discussed in training but has important implications in clinical practice. This article reviews basic legal concepts, recent trends and details on gastroenterology specific claims, and most importantly, advice on how to mitigate MPL risk as gastroenterologists.
Many trainees and early career gastroenterologists face microaggressions for a variety of different reasons. Dr. Oveia Aktopaire and Dr. Rachel Issaka (University of Washington) present a thought-provoking piece as they delve into structural racism in medicine and how microaggressions are a proxy for bias.
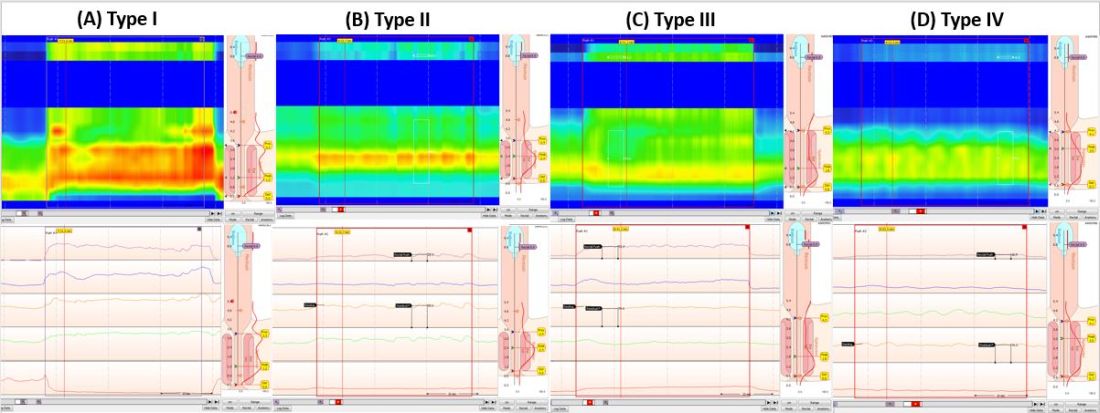
Dyssynergic defecation (DD) affects up to one-half of patients with chronic constipation. The “In Focus” feature for May provides an excellent review of DD written by international expert Dr. Satish Rao and Dr. Asad Jehangir (both, Medical College of Georgia/Augusta University). The article provides guidance on the diagnosis of DD, including high-yield features of physical and digital rectal exams, guidance on interpretation of anorectal manometry testing, and how these can dictate an effective therapeutic plan.
Meaningful mentorship is crucial for young gastroenterologists but at times the nature of the mentor-mentee relationship can be difficult to navigate. Dr. David Fessell and Bridger Rodoni (University of Michigan) explore this dynamic and discuss the notion of mentorship malpractice in a compelling addition to our ethics case series.
Abdominal wall pain is common yet often overlooked diagnosis and a great teaching point for trainees. Dr. Manish Singla (Uniformed Services University/Capital Digestive Care) and Dr. Brian Park (Naval Medical Center) discuss the diagnosis and management and how the early recognition of abdominal wall pain can save both patients and clinicians from a battery of unnecessary diagnostic testing.
The DHPA Private Practice Perspectives article this quarter, written by Dr. Aja McCutchen (Atlanta Gastroenterology Associates), addresses colorectal cancer screening, the disparities that exist, and the important role we have as gastroenterologists in reducing barriers to screening. Lastly, Dr. Bilal Asif (University of Maryland/National Institutes of Health) walks us through a fellow’s perspective on the AGA’s first virtual Advocacy Day – demonstrating that advocacy is still possible even as a trainee and in the setting of a pandemic.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu) or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
Welcome to the May edition of The New Gastroenterologist, which is packed with a fantastic line-up of articles! The 1-year mark of the pandemic recently passed, and we now are gearing up for our second virtual Digestive Disease Week (DDW®). While we are all anxious to return to lives that have some semblance of normalcy, we continue to endure the ebbs and flows that characterize life in a pandemic. For over a year now, we spend our days caught in a constant battle between the risk and reward of activities we previously took for granted or considered mundane. Our moods vacillate with the continued rise and fall of COVID-19 cases, but the advent and distribution of vaccines have offered palpable hope for better outcomes.
I’m pleased to introduce this quarter’s content – beginning with our legal section. Dr. John Azizian (UCLA-Olive-View), Dr. James Tabibian (UCLA-Olive-View), Dr. Camellia Dalai (UCLA-Olive-View/University of New Mexico), and Dr. Megan Adams (University of Michigan) contribute a comprehensive piece on medical professional liability (MPL), a topic that is seldom discussed in training but has important implications in clinical practice. This article reviews basic legal concepts, recent trends and details on gastroenterology specific claims, and most importantly, advice on how to mitigate MPL risk as gastroenterologists.
Many trainees and early career gastroenterologists face microaggressions for a variety of different reasons. Dr. Oveia Aktopaire and Dr. Rachel Issaka (University of Washington) present a thought-provoking piece as they delve into structural racism in medicine and how microaggressions are a proxy for bias.
Dyssynergic defecation (DD) affects up to one-half of patients with chronic constipation. The “In Focus” feature for May provides an excellent review of DD written by international expert Dr. Satish Rao and Dr. Asad Jehangir (both, Medical College of Georgia/Augusta University). The article provides guidance on the diagnosis of DD, including high-yield features of physical and digital rectal exams, guidance on interpretation of anorectal manometry testing, and how these can dictate an effective therapeutic plan.
Meaningful mentorship is crucial for young gastroenterologists but at times the nature of the mentor-mentee relationship can be difficult to navigate. Dr. David Fessell and Bridger Rodoni (University of Michigan) explore this dynamic and discuss the notion of mentorship malpractice in a compelling addition to our ethics case series.
Abdominal wall pain is common yet often overlooked diagnosis and a great teaching point for trainees. Dr. Manish Singla (Uniformed Services University/Capital Digestive Care) and Dr. Brian Park (Naval Medical Center) discuss the diagnosis and management and how the early recognition of abdominal wall pain can save both patients and clinicians from a battery of unnecessary diagnostic testing.
The DHPA Private Practice Perspectives article this quarter, written by Dr. Aja McCutchen (Atlanta Gastroenterology Associates), addresses colorectal cancer screening, the disparities that exist, and the important role we have as gastroenterologists in reducing barriers to screening. Lastly, Dr. Bilal Asif (University of Maryland/National Institutes of Health) walks us through a fellow’s perspective on the AGA’s first virtual Advocacy Day – demonstrating that advocacy is still possible even as a trainee and in the setting of a pandemic.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu) or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
Welcome to the May edition of The New Gastroenterologist, which is packed with a fantastic line-up of articles! The 1-year mark of the pandemic recently passed, and we now are gearing up for our second virtual Digestive Disease Week (DDW®). While we are all anxious to return to lives that have some semblance of normalcy, we continue to endure the ebbs and flows that characterize life in a pandemic. For over a year now, we spend our days caught in a constant battle between the risk and reward of activities we previously took for granted or considered mundane. Our moods vacillate with the continued rise and fall of COVID-19 cases, but the advent and distribution of vaccines have offered palpable hope for better outcomes.
I’m pleased to introduce this quarter’s content – beginning with our legal section. Dr. John Azizian (UCLA-Olive-View), Dr. James Tabibian (UCLA-Olive-View), Dr. Camellia Dalai (UCLA-Olive-View/University of New Mexico), and Dr. Megan Adams (University of Michigan) contribute a comprehensive piece on medical professional liability (MPL), a topic that is seldom discussed in training but has important implications in clinical practice. This article reviews basic legal concepts, recent trends and details on gastroenterology specific claims, and most importantly, advice on how to mitigate MPL risk as gastroenterologists.
Many trainees and early career gastroenterologists face microaggressions for a variety of different reasons. Dr. Oveia Aktopaire and Dr. Rachel Issaka (University of Washington) present a thought-provoking piece as they delve into structural racism in medicine and how microaggressions are a proxy for bias.
Dyssynergic defecation (DD) affects up to one-half of patients with chronic constipation. The “In Focus” feature for May provides an excellent review of DD written by international expert Dr. Satish Rao and Dr. Asad Jehangir (both, Medical College of Georgia/Augusta University). The article provides guidance on the diagnosis of DD, including high-yield features of physical and digital rectal exams, guidance on interpretation of anorectal manometry testing, and how these can dictate an effective therapeutic plan.
Meaningful mentorship is crucial for young gastroenterologists but at times the nature of the mentor-mentee relationship can be difficult to navigate. Dr. David Fessell and Bridger Rodoni (University of Michigan) explore this dynamic and discuss the notion of mentorship malpractice in a compelling addition to our ethics case series.
Abdominal wall pain is common yet often overlooked diagnosis and a great teaching point for trainees. Dr. Manish Singla (Uniformed Services University/Capital Digestive Care) and Dr. Brian Park (Naval Medical Center) discuss the diagnosis and management and how the early recognition of abdominal wall pain can save both patients and clinicians from a battery of unnecessary diagnostic testing.
The DHPA Private Practice Perspectives article this quarter, written by Dr. Aja McCutchen (Atlanta Gastroenterology Associates), addresses colorectal cancer screening, the disparities that exist, and the important role we have as gastroenterologists in reducing barriers to screening. Lastly, Dr. Bilal Asif (University of Maryland/National Institutes of Health) walks us through a fellow’s perspective on the AGA’s first virtual Advocacy Day – demonstrating that advocacy is still possible even as a trainee and in the setting of a pandemic.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu) or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Clearance rates higher with bimekizumab vs. secukinumab in phase 3 psoriasis study
Secukinumab is the latest adult plaque psoriasis treatment to be bested by a newcomer, the interleukin 17A and 17F blocker bimekizumab.
Rates of complete clearance were substantially higher with bimekizumab in a phase 3 trial with 743 patients with moderate-to-severe plaque psoriasis, but oral candidiasis (oral thrush) again emerged as a particular issue with the agent.
Clinical improvements seen with bimekizumab have exceeded those with two standard options for adult plaque psoriasis — the tumor necrosis factor blocker adalimumab and the interleukin (IL) 12/23 inhibitor ustekinumab
— in phase 3 trials from manufacturer UCB Pharma, and it›s under review for the indication by the U.S. Food and Drug Administration and the European Medicines Agency.
The biologic is also being evaluated in phase 3 trials for treating psoriatic arthritis, ankylosing spondylitis, nonradiographic axial spondyloarthritis, and hidradenitis suppurativa.
Results of the trial comparing bimekizumab to secukinumab, dubbed BE RADIANT, were presented at the American Academy of Dermatology Virtual Meeting Experience and published online concurrently April 23 in the New England Journal of Medicine.
The results “suggest that inhibition of both interleukin-17A and interleukin-17F with bimekizumab may provide greater clinical benefit for patients with moderate-to-severe plaque psoriasis than inhibition of interleukin-17A alone,” as with secukinumab, said the investigators, led by Kristian Reich, MD, professor of dermatology at the University Medical Center Hamburg-Eppendorf in Hamburg, Germany.
The trial randomly assigned 373 adults to bimekizumab 320 mg every 4 weeks to week 16, then rerandomized them to maintenance dosing either every 4 weeks or every 8 weeks to week 48; another 370 adults were randomly assigned to secukinumab 300 mg weekly for the first 4 weeks, then every 4 weeks to week 48. Baseline Psoriasis Area and Severity Index (PASI) scores were about 20 points in both treatment groups.
At the 1-month point, 71% in the bimekizumab group, vs 47.3% on secukinumab, had a 75% or greater reduction from their baseline PASI score. At 4 months, 61.7% of those on bimekizumab but 48.9% in the secukinumab group had complete clearance with a PASI score of 100.
At 48 weeks, 67% of those on bimekizumab had a PASI 100 response — which was numerically similar between the two bimekizumab dosing regimens after week 16 — vs 46.2% of the secukinumab group (P for all < .001).
The incidence of serious adverse events was just under 6% in both groups, with adverse events leading to discontinuation in 3.5% of bimekizumab and 2.7% of secukinumab subjects. The rate of serious infections was similar in both groups.
However, as in past trials, oral candidiasis was an issue, occurring in 19.3% of bimekizumab subjects vs 3% on secukinumab. Half of the 72 bimekizumab cases were classified as mild, and all but two of the rest as moderate. Over 40% of affected subjects reported more than one case, but none led to treatment discontinuation.
More than 85% of oral candidiasis cases in the study were treated with antifungal therapy and resolved during the trial. Inflammatory bowel disease is a concern with IL-17 blockade, but this hasn’t emerged as a particular issue with bimekizumab. There was one case each of ulcerative colitis in both the bimekizumab and secukinumab groups, and just one case of ulcerative colitis in three previous phase 3 bimekizumab trials, according to the investigators.
Among the trial limitations: Patients who had been on bimekizumab or secukinumab previously were excluded, as were patients who had no response to an IL-17 biologic or more than one biologic agent of any other class within the previous 12 weeks. The limitations could reduce generalizability, the investigators said.
Patients in the trial were about 45 years old, on average, and about two thirds were men; over 90% were White.
The study was funded by UCB Pharma. The investigators had numerous disclosures, including Reich who reported grants and personal fees from companies including UCB Pharma. The full list of disclosures can be found with the New England Journal of Medicine article.
A version of this article first appeared on Medscape.com .
Secukinumab is the latest adult plaque psoriasis treatment to be bested by a newcomer, the interleukin 17A and 17F blocker bimekizumab.
Rates of complete clearance were substantially higher with bimekizumab in a phase 3 trial with 743 patients with moderate-to-severe plaque psoriasis, but oral candidiasis (oral thrush) again emerged as a particular issue with the agent.
Clinical improvements seen with bimekizumab have exceeded those with two standard options for adult plaque psoriasis — the tumor necrosis factor blocker adalimumab and the interleukin (IL) 12/23 inhibitor ustekinumab
— in phase 3 trials from manufacturer UCB Pharma, and it›s under review for the indication by the U.S. Food and Drug Administration and the European Medicines Agency.
The biologic is also being evaluated in phase 3 trials for treating psoriatic arthritis, ankylosing spondylitis, nonradiographic axial spondyloarthritis, and hidradenitis suppurativa.
Results of the trial comparing bimekizumab to secukinumab, dubbed BE RADIANT, were presented at the American Academy of Dermatology Virtual Meeting Experience and published online concurrently April 23 in the New England Journal of Medicine.
The results “suggest that inhibition of both interleukin-17A and interleukin-17F with bimekizumab may provide greater clinical benefit for patients with moderate-to-severe plaque psoriasis than inhibition of interleukin-17A alone,” as with secukinumab, said the investigators, led by Kristian Reich, MD, professor of dermatology at the University Medical Center Hamburg-Eppendorf in Hamburg, Germany.
The trial randomly assigned 373 adults to bimekizumab 320 mg every 4 weeks to week 16, then rerandomized them to maintenance dosing either every 4 weeks or every 8 weeks to week 48; another 370 adults were randomly assigned to secukinumab 300 mg weekly for the first 4 weeks, then every 4 weeks to week 48. Baseline Psoriasis Area and Severity Index (PASI) scores were about 20 points in both treatment groups.
At the 1-month point, 71% in the bimekizumab group, vs 47.3% on secukinumab, had a 75% or greater reduction from their baseline PASI score. At 4 months, 61.7% of those on bimekizumab but 48.9% in the secukinumab group had complete clearance with a PASI score of 100.
At 48 weeks, 67% of those on bimekizumab had a PASI 100 response — which was numerically similar between the two bimekizumab dosing regimens after week 16 — vs 46.2% of the secukinumab group (P for all < .001).
The incidence of serious adverse events was just under 6% in both groups, with adverse events leading to discontinuation in 3.5% of bimekizumab and 2.7% of secukinumab subjects. The rate of serious infections was similar in both groups.
However, as in past trials, oral candidiasis was an issue, occurring in 19.3% of bimekizumab subjects vs 3% on secukinumab. Half of the 72 bimekizumab cases were classified as mild, and all but two of the rest as moderate. Over 40% of affected subjects reported more than one case, but none led to treatment discontinuation.
More than 85% of oral candidiasis cases in the study were treated with antifungal therapy and resolved during the trial. Inflammatory bowel disease is a concern with IL-17 blockade, but this hasn’t emerged as a particular issue with bimekizumab. There was one case each of ulcerative colitis in both the bimekizumab and secukinumab groups, and just one case of ulcerative colitis in three previous phase 3 bimekizumab trials, according to the investigators.
Among the trial limitations: Patients who had been on bimekizumab or secukinumab previously were excluded, as were patients who had no response to an IL-17 biologic or more than one biologic agent of any other class within the previous 12 weeks. The limitations could reduce generalizability, the investigators said.
Patients in the trial were about 45 years old, on average, and about two thirds were men; over 90% were White.
The study was funded by UCB Pharma. The investigators had numerous disclosures, including Reich who reported grants and personal fees from companies including UCB Pharma. The full list of disclosures can be found with the New England Journal of Medicine article.
A version of this article first appeared on Medscape.com .
Secukinumab is the latest adult plaque psoriasis treatment to be bested by a newcomer, the interleukin 17A and 17F blocker bimekizumab.
Rates of complete clearance were substantially higher with bimekizumab in a phase 3 trial with 743 patients with moderate-to-severe plaque psoriasis, but oral candidiasis (oral thrush) again emerged as a particular issue with the agent.
Clinical improvements seen with bimekizumab have exceeded those with two standard options for adult plaque psoriasis — the tumor necrosis factor blocker adalimumab and the interleukin (IL) 12/23 inhibitor ustekinumab
— in phase 3 trials from manufacturer UCB Pharma, and it›s under review for the indication by the U.S. Food and Drug Administration and the European Medicines Agency.
The biologic is also being evaluated in phase 3 trials for treating psoriatic arthritis, ankylosing spondylitis, nonradiographic axial spondyloarthritis, and hidradenitis suppurativa.
Results of the trial comparing bimekizumab to secukinumab, dubbed BE RADIANT, were presented at the American Academy of Dermatology Virtual Meeting Experience and published online concurrently April 23 in the New England Journal of Medicine.
The results “suggest that inhibition of both interleukin-17A and interleukin-17F with bimekizumab may provide greater clinical benefit for patients with moderate-to-severe plaque psoriasis than inhibition of interleukin-17A alone,” as with secukinumab, said the investigators, led by Kristian Reich, MD, professor of dermatology at the University Medical Center Hamburg-Eppendorf in Hamburg, Germany.
The trial randomly assigned 373 adults to bimekizumab 320 mg every 4 weeks to week 16, then rerandomized them to maintenance dosing either every 4 weeks or every 8 weeks to week 48; another 370 adults were randomly assigned to secukinumab 300 mg weekly for the first 4 weeks, then every 4 weeks to week 48. Baseline Psoriasis Area and Severity Index (PASI) scores were about 20 points in both treatment groups.
At the 1-month point, 71% in the bimekizumab group, vs 47.3% on secukinumab, had a 75% or greater reduction from their baseline PASI score. At 4 months, 61.7% of those on bimekizumab but 48.9% in the secukinumab group had complete clearance with a PASI score of 100.
At 48 weeks, 67% of those on bimekizumab had a PASI 100 response — which was numerically similar between the two bimekizumab dosing regimens after week 16 — vs 46.2% of the secukinumab group (P for all < .001).
The incidence of serious adverse events was just under 6% in both groups, with adverse events leading to discontinuation in 3.5% of bimekizumab and 2.7% of secukinumab subjects. The rate of serious infections was similar in both groups.
However, as in past trials, oral candidiasis was an issue, occurring in 19.3% of bimekizumab subjects vs 3% on secukinumab. Half of the 72 bimekizumab cases were classified as mild, and all but two of the rest as moderate. Over 40% of affected subjects reported more than one case, but none led to treatment discontinuation.
More than 85% of oral candidiasis cases in the study were treated with antifungal therapy and resolved during the trial. Inflammatory bowel disease is a concern with IL-17 blockade, but this hasn’t emerged as a particular issue with bimekizumab. There was one case each of ulcerative colitis in both the bimekizumab and secukinumab groups, and just one case of ulcerative colitis in three previous phase 3 bimekizumab trials, according to the investigators.
Among the trial limitations: Patients who had been on bimekizumab or secukinumab previously were excluded, as were patients who had no response to an IL-17 biologic or more than one biologic agent of any other class within the previous 12 weeks. The limitations could reduce generalizability, the investigators said.
Patients in the trial were about 45 years old, on average, and about two thirds were men; over 90% were White.
The study was funded by UCB Pharma. The investigators had numerous disclosures, including Reich who reported grants and personal fees from companies including UCB Pharma. The full list of disclosures can be found with the New England Journal of Medicine article.
A version of this article first appeared on Medscape.com .
Systematic approach to pain helps avoid opioid issues for dermatologists
, according to an expert who outlined his strategies at the American Academy of Dermatology Virtual Meeting Experience.
The exceptions relate primarily to patients with issues complicating pain control, such as those with psychosocial problems exacerbating the pain response, drug-seeking behavior, or both, according to Robert G. Micheletti, MD, chief of hospital dermatology, University of Pennsylvania, Philadelphia.
To stay out of trouble, Dr. Micheletti advocated a systematic approach to the control of pain that includes documentation, clear expectations, and a sparing use of opioids only at the lowest acceptable dose for periods measured in days.
Using a case of pyoderma gangrenosum to make several points, he recognized that some patients do have a level of pain that warrants a short course of opioids, but this is not his first step. Rather, the initial focus, after administering standard therapies for this disease, is wound care, which often attenuates symptoms. He adds non-pharmacologic treatments, such as ice, heat, and rest when appropriate. The initial pharmacologic approach is alternating doses of an NSAID and acetaminophen.
“If necessary, a short course of opioids is reasonable for patients with acute pain,” he acknowledged. But he wants to avoid providing more opioids than needed to address the initial period of acute pain. In the case of pyoderma gangrenosum, he suggested a typical prescription might be 12 pills of 5 mg oxycodone taken every six hours. A followup appointment within a week provides the opportunity to reassess.
“Set clear expectations,” Dr. Micheletti said. This includes explaining that the goal is manageable pain, not complete pain relief, which is often unobtainable. For painful conditions such as pyoderma gangrenosum, hidradenitis suppurativa, or vasculitis, a short course will generally be sufficient to get past the most significant discomfort.
There are several reasons that Dr. Micheletti encourages dermatologists to take responsibility for pain related to skin diseases. One is the potential for inefficiencies and delays common to referrals, but another is the value of the dermatologist’s expertise in judging pain as a symptom of the disorder. With effective treatment, pain should self-resolve.
“If the patient is not getting better medically, then change therapies,” Dr. Micheletti said. When referred to a non-dermatologist, the pain expert might not recognize what persistent pain is revealing about the underlying condition.
Repeatedly, Dr. Micheletti made the point that dermatologists should manage pain related to skin disorders because of their ability to assess complaints in the context of the disease.
“We are the experts. We should understand when what we are seeing should or should not be painful,” he said. He added that dermatologists are also in the best position to judge “when analgesia is no longer needed.”
With this same logic, dermatologists are in a good position to distinguish nociceptive from neuropathic pain. Some conditions are likely to have both, and this should influence choice of pain relief. Citing a patient with calciphylaxis as an example, Dr. Micheletti suggested that drugs with efficacy against neuropathic pain, such as gabapentin, should be one of the options to consider before moving to opioids. In those with sufficient pain to warrant an opioid, however, Dr. Micheletti would consider tramadol, which acts on both types of pain.
Treating pain is not always straightforward, Dr. Micheletti acknowledged. For example, depression and mood disorders are known to exacerbate pain and are reasonable targets of pain control. The stress related to disruptive psychosocial problems can be another factor in risk of pain.
“Be prepared to acknowledge and address these types of issues,” Dr. Micheletti said. Although these are the types of patients some dermatologists might prefer to refer to a pain specialist, he said that the contribution of factors outside of skin disease should not be allowed to obscure a dermatologic source of pain.
“Just because a patient has psychosocial issues does not mean that there is no pain,” he said.
A systematic approach to the assessment and treatment of pain will help sort out these issues, but Dr. Micheletti also said, “Know your comfort zone.” When patients require opioids, there are several appropriate steps important or mandatory to provide adequate protection for the patient and the physician. In addition to documentation, it is reasonable to verify that the patient is not obtaining opioids from other prescribers, a step that is mandatory in some states.
When opioids are needed, Dr. Micheletti suggested a standard approach that includes short courses without refills. He recommended avoiding long-acting opioids and drugs not commonly used by non-pain specialists, such as codeine, hydrocodone, or fentanyl.
“This is not a prescribe and walk away situation,” he said.
Although the same general approach is employed by Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, he is a little less reluctant to refer patients to pain specialists.
“For complex situations, you need complex solutions. In the case of significant pain and even itch, I will collaborate with the GW Pain Center,” he said. For severe pain, the solutions might include nerve blocks or even intravenous ketamine for in-patients.
He also made the point that dermatologists, even if they are uncomfortable prescribing opioids, “should be equipped to use relevant medications such as topical anesthetics, gabapentinoids, and SSRIs” to control pain related to skin conditions.
Dr. Micheletti reports no relevant conflicts of interest. Dr. Friedman has consulting relationships with several pharmaceutical companies, including Amgen, GlaxoSmithKline, and Valeant.
, according to an expert who outlined his strategies at the American Academy of Dermatology Virtual Meeting Experience.
The exceptions relate primarily to patients with issues complicating pain control, such as those with psychosocial problems exacerbating the pain response, drug-seeking behavior, or both, according to Robert G. Micheletti, MD, chief of hospital dermatology, University of Pennsylvania, Philadelphia.
To stay out of trouble, Dr. Micheletti advocated a systematic approach to the control of pain that includes documentation, clear expectations, and a sparing use of opioids only at the lowest acceptable dose for periods measured in days.
Using a case of pyoderma gangrenosum to make several points, he recognized that some patients do have a level of pain that warrants a short course of opioids, but this is not his first step. Rather, the initial focus, after administering standard therapies for this disease, is wound care, which often attenuates symptoms. He adds non-pharmacologic treatments, such as ice, heat, and rest when appropriate. The initial pharmacologic approach is alternating doses of an NSAID and acetaminophen.
“If necessary, a short course of opioids is reasonable for patients with acute pain,” he acknowledged. But he wants to avoid providing more opioids than needed to address the initial period of acute pain. In the case of pyoderma gangrenosum, he suggested a typical prescription might be 12 pills of 5 mg oxycodone taken every six hours. A followup appointment within a week provides the opportunity to reassess.
“Set clear expectations,” Dr. Micheletti said. This includes explaining that the goal is manageable pain, not complete pain relief, which is often unobtainable. For painful conditions such as pyoderma gangrenosum, hidradenitis suppurativa, or vasculitis, a short course will generally be sufficient to get past the most significant discomfort.
There are several reasons that Dr. Micheletti encourages dermatologists to take responsibility for pain related to skin diseases. One is the potential for inefficiencies and delays common to referrals, but another is the value of the dermatologist’s expertise in judging pain as a symptom of the disorder. With effective treatment, pain should self-resolve.
“If the patient is not getting better medically, then change therapies,” Dr. Micheletti said. When referred to a non-dermatologist, the pain expert might not recognize what persistent pain is revealing about the underlying condition.
Repeatedly, Dr. Micheletti made the point that dermatologists should manage pain related to skin disorders because of their ability to assess complaints in the context of the disease.
“We are the experts. We should understand when what we are seeing should or should not be painful,” he said. He added that dermatologists are also in the best position to judge “when analgesia is no longer needed.”
With this same logic, dermatologists are in a good position to distinguish nociceptive from neuropathic pain. Some conditions are likely to have both, and this should influence choice of pain relief. Citing a patient with calciphylaxis as an example, Dr. Micheletti suggested that drugs with efficacy against neuropathic pain, such as gabapentin, should be one of the options to consider before moving to opioids. In those with sufficient pain to warrant an opioid, however, Dr. Micheletti would consider tramadol, which acts on both types of pain.
Treating pain is not always straightforward, Dr. Micheletti acknowledged. For example, depression and mood disorders are known to exacerbate pain and are reasonable targets of pain control. The stress related to disruptive psychosocial problems can be another factor in risk of pain.
“Be prepared to acknowledge and address these types of issues,” Dr. Micheletti said. Although these are the types of patients some dermatologists might prefer to refer to a pain specialist, he said that the contribution of factors outside of skin disease should not be allowed to obscure a dermatologic source of pain.
“Just because a patient has psychosocial issues does not mean that there is no pain,” he said.
A systematic approach to the assessment and treatment of pain will help sort out these issues, but Dr. Micheletti also said, “Know your comfort zone.” When patients require opioids, there are several appropriate steps important or mandatory to provide adequate protection for the patient and the physician. In addition to documentation, it is reasonable to verify that the patient is not obtaining opioids from other prescribers, a step that is mandatory in some states.
When opioids are needed, Dr. Micheletti suggested a standard approach that includes short courses without refills. He recommended avoiding long-acting opioids and drugs not commonly used by non-pain specialists, such as codeine, hydrocodone, or fentanyl.
“This is not a prescribe and walk away situation,” he said.
Although the same general approach is employed by Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, he is a little less reluctant to refer patients to pain specialists.
“For complex situations, you need complex solutions. In the case of significant pain and even itch, I will collaborate with the GW Pain Center,” he said. For severe pain, the solutions might include nerve blocks or even intravenous ketamine for in-patients.
He also made the point that dermatologists, even if they are uncomfortable prescribing opioids, “should be equipped to use relevant medications such as topical anesthetics, gabapentinoids, and SSRIs” to control pain related to skin conditions.
Dr. Micheletti reports no relevant conflicts of interest. Dr. Friedman has consulting relationships with several pharmaceutical companies, including Amgen, GlaxoSmithKline, and Valeant.
, according to an expert who outlined his strategies at the American Academy of Dermatology Virtual Meeting Experience.
The exceptions relate primarily to patients with issues complicating pain control, such as those with psychosocial problems exacerbating the pain response, drug-seeking behavior, or both, according to Robert G. Micheletti, MD, chief of hospital dermatology, University of Pennsylvania, Philadelphia.
To stay out of trouble, Dr. Micheletti advocated a systematic approach to the control of pain that includes documentation, clear expectations, and a sparing use of opioids only at the lowest acceptable dose for periods measured in days.
Using a case of pyoderma gangrenosum to make several points, he recognized that some patients do have a level of pain that warrants a short course of opioids, but this is not his first step. Rather, the initial focus, after administering standard therapies for this disease, is wound care, which often attenuates symptoms. He adds non-pharmacologic treatments, such as ice, heat, and rest when appropriate. The initial pharmacologic approach is alternating doses of an NSAID and acetaminophen.
“If necessary, a short course of opioids is reasonable for patients with acute pain,” he acknowledged. But he wants to avoid providing more opioids than needed to address the initial period of acute pain. In the case of pyoderma gangrenosum, he suggested a typical prescription might be 12 pills of 5 mg oxycodone taken every six hours. A followup appointment within a week provides the opportunity to reassess.
“Set clear expectations,” Dr. Micheletti said. This includes explaining that the goal is manageable pain, not complete pain relief, which is often unobtainable. For painful conditions such as pyoderma gangrenosum, hidradenitis suppurativa, or vasculitis, a short course will generally be sufficient to get past the most significant discomfort.
There are several reasons that Dr. Micheletti encourages dermatologists to take responsibility for pain related to skin diseases. One is the potential for inefficiencies and delays common to referrals, but another is the value of the dermatologist’s expertise in judging pain as a symptom of the disorder. With effective treatment, pain should self-resolve.
“If the patient is not getting better medically, then change therapies,” Dr. Micheletti said. When referred to a non-dermatologist, the pain expert might not recognize what persistent pain is revealing about the underlying condition.
Repeatedly, Dr. Micheletti made the point that dermatologists should manage pain related to skin disorders because of their ability to assess complaints in the context of the disease.
“We are the experts. We should understand when what we are seeing should or should not be painful,” he said. He added that dermatologists are also in the best position to judge “when analgesia is no longer needed.”
With this same logic, dermatologists are in a good position to distinguish nociceptive from neuropathic pain. Some conditions are likely to have both, and this should influence choice of pain relief. Citing a patient with calciphylaxis as an example, Dr. Micheletti suggested that drugs with efficacy against neuropathic pain, such as gabapentin, should be one of the options to consider before moving to opioids. In those with sufficient pain to warrant an opioid, however, Dr. Micheletti would consider tramadol, which acts on both types of pain.
Treating pain is not always straightforward, Dr. Micheletti acknowledged. For example, depression and mood disorders are known to exacerbate pain and are reasonable targets of pain control. The stress related to disruptive psychosocial problems can be another factor in risk of pain.
“Be prepared to acknowledge and address these types of issues,” Dr. Micheletti said. Although these are the types of patients some dermatologists might prefer to refer to a pain specialist, he said that the contribution of factors outside of skin disease should not be allowed to obscure a dermatologic source of pain.
“Just because a patient has psychosocial issues does not mean that there is no pain,” he said.
A systematic approach to the assessment and treatment of pain will help sort out these issues, but Dr. Micheletti also said, “Know your comfort zone.” When patients require opioids, there are several appropriate steps important or mandatory to provide adequate protection for the patient and the physician. In addition to documentation, it is reasonable to verify that the patient is not obtaining opioids from other prescribers, a step that is mandatory in some states.
When opioids are needed, Dr. Micheletti suggested a standard approach that includes short courses without refills. He recommended avoiding long-acting opioids and drugs not commonly used by non-pain specialists, such as codeine, hydrocodone, or fentanyl.
“This is not a prescribe and walk away situation,” he said.
Although the same general approach is employed by Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, he is a little less reluctant to refer patients to pain specialists.
“For complex situations, you need complex solutions. In the case of significant pain and even itch, I will collaborate with the GW Pain Center,” he said. For severe pain, the solutions might include nerve blocks or even intravenous ketamine for in-patients.
He also made the point that dermatologists, even if they are uncomfortable prescribing opioids, “should be equipped to use relevant medications such as topical anesthetics, gabapentinoids, and SSRIs” to control pain related to skin conditions.
Dr. Micheletti reports no relevant conflicts of interest. Dr. Friedman has consulting relationships with several pharmaceutical companies, including Amgen, GlaxoSmithKline, and Valeant.
FROM AAD VMX 2021
Feds lift pause of J&J COVID vaccine, add new warning
Use of the Johnson & Johnson COVID-19 vaccine should resume in the United States for all adults, the Food and Drug Administration and Centers for Disease Contol and Prevention said April 23, although health care providers should warn patients of the risk of developing the rare and serious blood clots that caused the agencies to pause the vaccine’s distribution earlier this month.
“What we are seeing is the overall rate of events was 1.9 cases per million people. In women 18 to 29 years there was an approximate 7 cases per million. The risk is even lower in women over the age of 50 at .9 cases per million,” CDC Director Rochelle Walensky, MD, said in a news briefing the same day.
In the end, the potential benefits of the vaccine far outweighed its risks.
“In terms of benefits, we found that for every 1 million doses of this vaccine, the J&J vaccine could prevent over 650 hospitalizations and 12 deaths among women ages 18-49,” Dr. Walensky said. The potential benefits to women over 50 were even greater: It could prevent 4,700 hospitalizations and 650 deaths.
“In the end, this vaccine was shown to be safe and effective for the vast majority of people,” Dr. Walensky said.
The recommendation to continue the vaccine’s rollout came barely 2 hours after a CDC Advisory Committee on Immunization Practices voted to recommend the pause be lifted. The vote was 10-4 with one abstention.
The decision also includes instructions for the warning directed at women under 50 who have an increased risk of a rare but serious blood clot disorder called thrombosis with thrombocytopenia syndrome (TTS).
As of April 21, 15 cases of TTS, all in women and 13 of them in women under 50, have been confirmed among 7.98 million doses of the J&J vaccine administered in the United States. Three women have died.
The FDA and CDC recommended the pause on April 13 after reports that 6 women developed a blood clotting disorder 6 to 13 days after they received the J&J vaccine.
William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville, and a non-voting ACIP member, said in an interview the panel made the right recommendation.
He applauded both the decision to restart the vaccine and the updated warning information that “will explain [TTS] more fully to people, particularly women, who are coming to be vaccinated.”
As to women in the risk group needing to have a choice of vaccines, Dr. Schaffner said that will be addressed differently across the country.
“Every provider will not have alternative vaccines in their location so there will be many different ways to do this. You may have to get this information and select which site you’re going to depending on which vaccine is available if this matter is important to you,” he noted.
ACIP made the decision after a 6-hour emergency meeting to hear evidence on the Johnson & Johnson vaccine's protective benefits against COVID-19 vs. risk of TTS.
In the CDC-FDA press briefing, Dr. Walensky pointed out that over the past few days, as regulators have reviewed the rare events, newly identified patients had been treated appropriately, without the use of heparin, which is not advised for treating TTS.
As a result, regulators felt as if their messages had gotten out to doctors who now knew how to take special precautions when treating patients with the disorder.
She said the Johnson & Johnson shot remained an important option because it was convenient to give and easier to store than the other vaccines currently authorized in the United States.
Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the agency had already added information describing the risk of the rare clotting disorder to its fact sheets for patients and doctors.
Janet Woodcock, MD, acting commissioner of the FDA, said vaccination centers could resume giving the “one and done” shots as early as April 24.
This article was updated April 24, 2021, and first appeared on WebMD.com.
Use of the Johnson & Johnson COVID-19 vaccine should resume in the United States for all adults, the Food and Drug Administration and Centers for Disease Contol and Prevention said April 23, although health care providers should warn patients of the risk of developing the rare and serious blood clots that caused the agencies to pause the vaccine’s distribution earlier this month.
“What we are seeing is the overall rate of events was 1.9 cases per million people. In women 18 to 29 years there was an approximate 7 cases per million. The risk is even lower in women over the age of 50 at .9 cases per million,” CDC Director Rochelle Walensky, MD, said in a news briefing the same day.
In the end, the potential benefits of the vaccine far outweighed its risks.
“In terms of benefits, we found that for every 1 million doses of this vaccine, the J&J vaccine could prevent over 650 hospitalizations and 12 deaths among women ages 18-49,” Dr. Walensky said. The potential benefits to women over 50 were even greater: It could prevent 4,700 hospitalizations and 650 deaths.
“In the end, this vaccine was shown to be safe and effective for the vast majority of people,” Dr. Walensky said.
The recommendation to continue the vaccine’s rollout came barely 2 hours after a CDC Advisory Committee on Immunization Practices voted to recommend the pause be lifted. The vote was 10-4 with one abstention.
The decision also includes instructions for the warning directed at women under 50 who have an increased risk of a rare but serious blood clot disorder called thrombosis with thrombocytopenia syndrome (TTS).
As of April 21, 15 cases of TTS, all in women and 13 of them in women under 50, have been confirmed among 7.98 million doses of the J&J vaccine administered in the United States. Three women have died.
The FDA and CDC recommended the pause on April 13 after reports that 6 women developed a blood clotting disorder 6 to 13 days after they received the J&J vaccine.
William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville, and a non-voting ACIP member, said in an interview the panel made the right recommendation.
He applauded both the decision to restart the vaccine and the updated warning information that “will explain [TTS] more fully to people, particularly women, who are coming to be vaccinated.”
As to women in the risk group needing to have a choice of vaccines, Dr. Schaffner said that will be addressed differently across the country.
“Every provider will not have alternative vaccines in their location so there will be many different ways to do this. You may have to get this information and select which site you’re going to depending on which vaccine is available if this matter is important to you,” he noted.
ACIP made the decision after a 6-hour emergency meeting to hear evidence on the Johnson & Johnson vaccine's protective benefits against COVID-19 vs. risk of TTS.
In the CDC-FDA press briefing, Dr. Walensky pointed out that over the past few days, as regulators have reviewed the rare events, newly identified patients had been treated appropriately, without the use of heparin, which is not advised for treating TTS.
As a result, regulators felt as if their messages had gotten out to doctors who now knew how to take special precautions when treating patients with the disorder.
She said the Johnson & Johnson shot remained an important option because it was convenient to give and easier to store than the other vaccines currently authorized in the United States.
Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the agency had already added information describing the risk of the rare clotting disorder to its fact sheets for patients and doctors.
Janet Woodcock, MD, acting commissioner of the FDA, said vaccination centers could resume giving the “one and done” shots as early as April 24.
This article was updated April 24, 2021, and first appeared on WebMD.com.
Use of the Johnson & Johnson COVID-19 vaccine should resume in the United States for all adults, the Food and Drug Administration and Centers for Disease Contol and Prevention said April 23, although health care providers should warn patients of the risk of developing the rare and serious blood clots that caused the agencies to pause the vaccine’s distribution earlier this month.
“What we are seeing is the overall rate of events was 1.9 cases per million people. In women 18 to 29 years there was an approximate 7 cases per million. The risk is even lower in women over the age of 50 at .9 cases per million,” CDC Director Rochelle Walensky, MD, said in a news briefing the same day.
In the end, the potential benefits of the vaccine far outweighed its risks.
“In terms of benefits, we found that for every 1 million doses of this vaccine, the J&J vaccine could prevent over 650 hospitalizations and 12 deaths among women ages 18-49,” Dr. Walensky said. The potential benefits to women over 50 were even greater: It could prevent 4,700 hospitalizations and 650 deaths.
“In the end, this vaccine was shown to be safe and effective for the vast majority of people,” Dr. Walensky said.
The recommendation to continue the vaccine’s rollout came barely 2 hours after a CDC Advisory Committee on Immunization Practices voted to recommend the pause be lifted. The vote was 10-4 with one abstention.
The decision also includes instructions for the warning directed at women under 50 who have an increased risk of a rare but serious blood clot disorder called thrombosis with thrombocytopenia syndrome (TTS).
As of April 21, 15 cases of TTS, all in women and 13 of them in women under 50, have been confirmed among 7.98 million doses of the J&J vaccine administered in the United States. Three women have died.
The FDA and CDC recommended the pause on April 13 after reports that 6 women developed a blood clotting disorder 6 to 13 days after they received the J&J vaccine.
William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville, and a non-voting ACIP member, said in an interview the panel made the right recommendation.
He applauded both the decision to restart the vaccine and the updated warning information that “will explain [TTS] more fully to people, particularly women, who are coming to be vaccinated.”
As to women in the risk group needing to have a choice of vaccines, Dr. Schaffner said that will be addressed differently across the country.
“Every provider will not have alternative vaccines in their location so there will be many different ways to do this. You may have to get this information and select which site you’re going to depending on which vaccine is available if this matter is important to you,” he noted.
ACIP made the decision after a 6-hour emergency meeting to hear evidence on the Johnson & Johnson vaccine's protective benefits against COVID-19 vs. risk of TTS.
In the CDC-FDA press briefing, Dr. Walensky pointed out that over the past few days, as regulators have reviewed the rare events, newly identified patients had been treated appropriately, without the use of heparin, which is not advised for treating TTS.
As a result, regulators felt as if their messages had gotten out to doctors who now knew how to take special precautions when treating patients with the disorder.
She said the Johnson & Johnson shot remained an important option because it was convenient to give and easier to store than the other vaccines currently authorized in the United States.
Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the agency had already added information describing the risk of the rare clotting disorder to its fact sheets for patients and doctors.
Janet Woodcock, MD, acting commissioner of the FDA, said vaccination centers could resume giving the “one and done” shots as early as April 24.
This article was updated April 24, 2021, and first appeared on WebMD.com.
Dyssynergic defecation
Introduction
About 40% of the population experiences lower GI symptoms suggestive of gastrointestinal motility disorders.1,2 The global prevalence of chronic constipation is 18%, and the condition includes multiple overlapping subtypes.3 Evacuation disorders affect over half (59%) of patients and include dyssynergic defecation (DD).4 The inability to coordinate the abdominal, rectal, pelvic floor, and anal/puborectalis muscles to evacuate stools causes DD.5 The etiology of DD remains unclear and is often misdiagnosed. Clinically, the symptoms of DD overlap with other lower GI disorders, often leading to unnecessary and invasive procedures.2 We describe the clinical characteristics, diagnostic tools, treatment options, and evidence-based approach for the management of DD.

Clinical presentation
Over two-thirds of patients with DD acquire this disorder during adulthood, and one-third have symptoms from childhood.6 Though there is not usually an inciting event, 29% of patients report that symptoms began after events such as pregnancy or back injury,6 and opioid users have higher prevalence and severity of DD.7
Over 80% of patients report excessive straining, feelings of incomplete evacuation, and hard stools, and 50% report sensation of anal blockage or use of digital maneuvers.2 Other symptoms include infrequent bowel movements, abdominal pain, anal pain, and stool leakage.2 Evaluation of DD includes obtaining a detailed history utilizing the Bristol Stool Form Scale;8 however, patients’ recall of stool habit is often inaccurate, which results in suboptimal care.9,10 Prospective stool diaries can help to provide more objective assessment of patients’ symptoms, eliminate recall bias, and provide more reliable information. Several useful questionnaires are available for clinical and research purposes to characterize lower-GI symptoms, including the Constipation Scoring System,11 Patient Assessment of Constipation Symptoms (PAC-SYM),12 and Patient Assessment of Constipation Quality of Life (PAC-QOL).2,13 The Constipation Stool digital app enhances accuracy of data capture and offers a reliable and user-friendly method for recording bowel symptoms for patients, clinicians, and clinical investigators.14
Diagnosis
The diagnosis of DD requires careful physical and digital rectal examination together with anorectal manometry and a balloon expulsion test. Defecography and colonic transit studies provide additional assessment.
Physical examination
Abdominal examination should include palpation for stool in the colon and identification of abdominal mass or fecal impaction.2A high-quality digital rectal examination can help to identify patients who could benefit from physiological testing to confirm and treat DD.15 Rectal examination is performed by placing examiner’s lubricated gloved right index finger in a patient’s rectum, with the examiner’s left hand on patient’s abdomen, and asking the patient to push and bear down as if defecating.15 The contraction of the abdominal muscles is felt using the left hand, while the anal sphincter relaxation and degree of perineal descent are felt using the right-hand index finger.15 A diagnosis of dyssynergia is suspected if the digital rectal examination reveals two or more of the following abnormalities: inability to contract abdominal muscles (lack of push effort), inability to relax or paradoxical contraction of the anal sphincter and/or puborectalis, or absence of perineal descent.15 Digital rectal examination has good sensitivity (75%), specificity (87%), and positive predictive value (97%) for DD.16
High resolution anorectal manometry
Anorectal manometry (ARM) is the preferred method for the evaluation of defecatory disorders.17,18 ARM is best performed using the high-resolution anorectal manometry (HRAM) systems19 that consist of a flexible probe – 0.5-cm diameter with multiple circumferential sensors along the anal canal – and another two sensors inside a rectal balloon.18 It provides a topographic and waveform display of manometric pressure data (Figure). The 3D high-definition ARM probe is a rigid 1-cm probe that provides 3D topographic profiles.18 ARM is typically performed in both the left lateral position and in a more physiological seated position.20,21 There is considerable variation amongst different institutions on how to perform HRAM, and a recent International Anorectal Physiology Working Group (IAPWG) has provided consensus recommendations for performing this test.22 The procedure for performing HRAM is reviewed elsewhere, but the key elements are summarized below.18
Push maneuver: On HRAM, after the assessment of resting and squeeze anal sphincter pressures, the patient is asked to push or bear down as if to defecate while lying in left lateral decubitus position. The best of two attempts that closely mimics a normal bearing down maneuver is used for categorizing patient’s defecatory pattern.18 In patients with DD, at least four distinct dyssynergia phenotypes have been recognized (Figure),23 though recent studies suggest eight patterns.24 Defecation index (maximum rectal pressure/minimum residual anal pressure when bearing down) greater than 1.2 is considered normal.18
Simulated defecation on commode: The subject is asked to attempt defecation while seated on a commode with intrarectal balloon filled with 60 cc of air, and both the defecation pattern(s) and defecation index are calculated. A lack of coordinated push effort is highly suggestive of DD.21
Rectoanal Inhibitory Reflex (RAIR): RAIR describes the reflex relaxation of the internal anal sphincter after rectal distension. RAIR is dependent on intact autonomic ganglia and myenteric plexus25and is mediated by the release of nitric oxide and vasoactive intestinal peptide.26 The absence of RAIR suggests Hirschsprung disease.22.27.28
Rectal sensory testing: Intermittent balloon distension of the rectum with incremental volumes of air induces a range of rectal sensations that include first sensation, desire to defecate, urgency to defecate, and maximum tolerable volume. Rectal hyposensitivity is diagnosed when two or more sensory thresholds are higher than those seen in normal subjects29.30 and likely results from disruption of afferent gut-brain pathways, cortical perception/rectal wall dysfunction, or both.29 Rectal hyposensitivity affects 40% of patients with constipation30and is associated with DD but not delayed colonic transit.31 Rectal hyposensitivity may also be seen in patients with diabetes or fecal incontinence.18 About two-thirds of patients with rectal hyposensitivity have rectal hypercompliance, and some have megarectum.32 Some patients with DD have coexisting irritable bowel syndrome (IBS) and may have rectal hypersensitivity.18,33 Rectal compliance is measured alongside rectal sensitivity analysis by plotting a graph between the change in intraballoon volume (mL) and change in intrarectal pressures (mm Hg) during incremental balloon distensions.18.34 Rectal hypercompliance may be seen in megarectum and dyssynergic defecation.34,35 Rectal hypocompliance may be seen in patients with inflammatory bowel disease, postpelvic radiation, chronic ischemia, and advanced age.18
Balloon expulsion test: This test is performed by placing a plastic probe with a balloon in the rectum and filling it with 50 cc of warm water. Patients are given 5 minutes to expel the balloon while sitting on a commode. Balloon expulsion time of more than 1 minute suggests a diagnosis of DD,21 although 2 minutes provides a higher level of agreement with manometric findings.36 Balloon type and body position can influence the results.37 Inability to expel the balloon with normal manometric findings is considered an inconclusive finding per the recent London Classification (i.e., it may be associated with generation of anorectal symptoms, but the clinical relevance of this finding is unclear as it may also be seen in healthy subjects).22
Defecography
Defecography is a dynamic fluoroscopic study performed in the sitting position after injecting 150 mL of barium paste into the patient’s rectum. Defecography provides useful information about structural changes (e.g., rectoceles, enteroceles, rectal prolapse, and intussusception), DD, and descending perineum syndrome.38 Methodological differences, radiation exposure, and poor interobserver agreement have limited its wider use; therefore, anorectal manometry and the balloon expulsion test are recommended for the initial evaluation of DD.39 Magnetic resonance defecography may be more useful.17,38
Colonic transit studies
Colonic transit study can be assessed using radiopaque markers, wireless motility capsule, or scintigraphy. Wireless motility capsule and scintigraphy have the advantage of determining gastric, small bowel, and whole gut transit times as well. About two-thirds of patients with DD have slow transit constipation (STC),6 which improves after treatment of DD.40 Hence, in patients with chronic constipation, evaluation and management of DD is recommended first. If symptoms persist, then consider colonic transit assessment.41 Given the overlapping nature of the conditions, documentation of STC at the outset could facilitate treatment of both.
Diagnostic criteria for DD
Patients should fulfill the following criteria for diagnosis of DD:42,43
- Fulfill symptom(s) diagnostic criteria for functional constipation and/or constipation-predominant IBS.
- Demonstrate dyssynergic pattern (Types I-IV; Figure) during attempted defecation on manometry recordings.
- Meet one or more of the following criteria:
- Inability to expel an artificial stool (50 mL water-filled balloon) within 1 minute.
- Inability to evacuate or retention of 50% or more of barium during defecography. (Some institutions use a prolonged colonic transit time: greater than 5 markers or 20% or higher marker retention on a plain abdominal x-Ray at 120 hours after ingestion of one radio-opaque marker capsule containing 24 radio-opaque markers.)
Treatment of DD
The treatment modalities for DD depend on several factors: patient’s age, comorbidities, underlying pathophysiology, and patient expectations. Treatment options include standard management of constipation, but biofeedback therapy is the mainstay.
Standard management
Medications that cause or worsen constipation should be avoided. The patient should consume adequate fluid and exercise regularly. Patients should receive instructions for timed toilet training (twice daily, 30 minutes after meals). Patients should push at about 50%-70% of their ability for no longer than 5 minutes and avoid postponing defecation or use of digital maneuvers to facilitate defecation.42 The patients should take 25 g of soluble fiber (e.g., psyllium) daily. Of note, the benefits of fiber can take days to weeks44 and may be limited in patients with STC and DD.45 Medications including laxatives and intestinal secretagogues (lubiprostone, linaclotide, plecanatide), and enterokinetic agents (prucalopride) can be used as adjunct therapy for management of DD.42 Their use is titrated during and after biofeedback therapy and may decrease after successful treatment.46
Biofeedback therapy
Biofeedback therapy involves operant conditioning techniques using either a solid state anorectal manometry system, electromyography, simulated balloon, or home biofeedback training devices.42,47 The goals of biofeedback therapy are to correct the abdominal pelvic muscle discoordination during defecation and improve rectal sensation to stool if impaired. Biofeedback therapy involves patient education and active training (typically six sessions, 1-2 weeks apart, with each about 30-60 minutes long), followed by a reinforcement stage (three sessions at 3, 6, and 12 months), though there are variations in training protocols.42
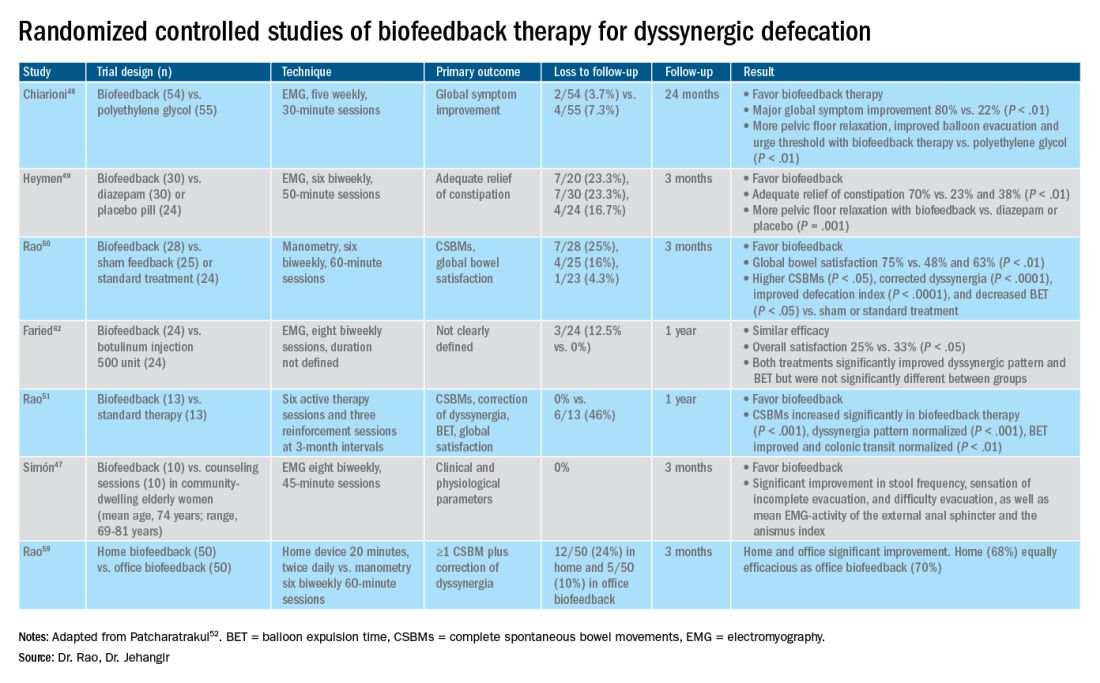
The success of biofeedback therapy depends on the patient’s motivation and the therapist’s skills.42 Compared with standard therapy (diet, exercise, pharmacotherapy), biofeedback therapy provides sustained improvement of bowel symptoms and anorectal function. Up to 70%-80% of DD patients show significant improvement of symptoms in randomized controlled trials (Table).48-52 Biofeedback therapy may also improve dyspeptic symptoms.53 Patients with harder stool consistency, greater willingness to participate, lower baseline bowel satisfaction, lower baseline anal sphincter relaxation, and prolonged balloon expulsion time, as well as patients who used digital maneuvers for defection, more commonly respond to biofeedback therapy.54,55 Longstanding laxative use has been associated with decreased response to biofeedback therapy.56 In patients with rectal hyposensitivity, barostat-assisted sensory training is more effective than a hand-held syringe technique.30 In patients with constipation predominant IBS and rectal hyposensitivity, sensory adaption training is more efficacious and better tolerated than escitalopram.30 Biofeedback therapy was afforded a grade A recommendation for treatment of DD by the American and European Societies of Neurogastroenterology and Motility.57
The access to office-based biofeedback therapy may be limited because of costs and low availability. The time required to attend multiple sessions may be burdensome for some patients, especially if they are taking time off from work. A recent study showed that patients with higher level of education may be less likely to adhere to biofeedback therapy.58 Recently, home biofeedback was shown to be noninferior to office biofeedback and was more cost-effective, which provides an alternative option for treating more patients.59
Endoscopic/surgical options
Other less effective treatment options for DD include botulinum toxin injection and myectomy.60-62 Botulinum toxin injection appears to have mixed effects with less than 50% of patients reporting symptomatic improvement, and it may cause fecal incontinence.60,63
Conclusion
DD is a common yet poorly recognized cause of constipation. Its clinical presentation overlaps with other lower-GI disorders. Its diagnosis requires detailed history, digital rectal examination, prospective stool diaries, anorectal manometry, and balloon expulsion tests. Biofeedback therapy offers excellent and sustained symptomatic improvement; however, access to office-based biofeedback is limited, and there is an urgent need for home-based biofeedback therapy programs.59
Dr. Rao is J. Harold Harrison Distinguished University Chair, professor of medicine, director of neurogastroenterology/motility, and director of digestive health at the Digestive Health Clinical Research Center Augusta (Georgia) University. He is supported by National Institutes of Health grants R01DK121003-02 and U01DK115572. Dr. Jehangir is a gastroenterology and Hepatology Fellow at the Digestive Health Clinical Research Center at Augusta University. They reported having no conflicts of interest.
References
1. Peery AF, et al. Gastroenterology. 2012;143(5):1179-1187.e3 .
2. Curtin B, et al. J Neurogastroenterol Motil. 2020 30;26(4):423-36.
3. Suares NC & Ford AC. Am J Gastroenterol. 2011 Sep;106(9):1582-91.
4. Mertz H, et al. Am J Gastroenterol. 1999;94(3):609-15.
5. Rao SS, et al. Am J Gastroenterol. 1998;93(7):1042-50.
6. Rao SSC, et al. J Clin Gastroenterol. 2004;38(8):680-5.
7. Nojkov B, et al. Am J Gastroenterol. 2019;114(11):1772-7.
8. Heaton KW, et al. Gut. 1992;33(6):818-24.
9. Prichard DO & Bharucha AE. 2018 Oct 15;7:F1000 Faculty Rev-1640.
10. Ashraf W, et al. Am J Gastroenterol. 1996;91(1):26-32.
11. Agachan F, et al.. Dis Colon Rectum. 1996;39(6):681-5.
12. Frank L, et al. Scand J Gastroenterol. 1999;34(9):870-7.
13. Marquis P, et al. Scand J Gastroenterol. 2005;40(5):540-51.
14. Yan Y, et al. Gastroenterology. 2020;158(6):S-400.
15. Rao SSC. Am J Gastroenterol. 2018;113(5):635-8.
16. Tantiphlachiva K, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8(11):955-60.
17. Carrington EV, et al. Nat Rev Gastroenterol Hepatol. 2018;15(5):309-23.
18. Tetangco EP, et al. Performing and analyzing high-resolution anorectal manometry. NeuroGastroLatam Rev. 2018;2:120-32.
19. Lee YY, et al. Curr Gastroenterol Rep. 2013;15(12):360.
20. Sharma M, et al. Neurogastroenterol Motil. 2020;32(10):e13910.
21. Rao SSC, et al.. Am J Gastroenterol. 2006;101(12):2790-6.
22. Carrington EV, et al. Neurogastroenterol Motil. 2020;32(1):e13679.
23. Rao SSC. Gastroenterol Clin North Am. 2008;37(3):569-86, viii.
24. Rao SSC, et al. Gastroenterology. 2016;150(4):S158-9.
25. Guinet A, et al. Int J Colorectal Dis. 2011;26(4):507-13.
26. Rattan S, et al. Gastroenterology. 1992;103(1):43-50.
27. Remes-Troche JM & Rao SSC. 2008;2(3):323-35.
28. Zaafouri H, et al..Int J Surgery. 2015. 2(1):9-17.
29. Remes-Troche JM, et al. Dis Colon Rectum. 2010;53(7):1047-54.
30. Rao SSC, et al. Gastroenterology. 2013;144(5):S-363.
31. Yu T, et al. Medicine (Baltimore). 2016;95(19):e3667.
32. Gladman MA, et al. Neurogastroenterol Motil. 2009;21(5):508-16, e4-5.
33. Lee KJ, et al. Digestion. 2006;73(2-3):133-41 .
34. Rao SSC, et al. Neurogastroenterol Motil. 2002;14(5):553-9.
35. Coss-Adame E, et al.. Clin Gastroenterol Hepatol. 2015;13(6):1143-1150.e1.
36. Chiarioni G, et al. Clin Gastroenterol Hepatol. 2014;12(12):2049-54.
37. Gu G, et al. Gastroenterology. 2018;154(6):S-545–S-546.
38. Savoye-Collet C, et al.. Gastroenterol Clin North Am. 2008;37(3):553-67, viii.
39. Videlock EJ, et al. Neurogastroenterol Motil. 2013;25(6):509-20.
40. Rao SSC, et al. Neurogastroenterol Motil. 2004;16(5):589-96.
41. Wald A, et al. Am J Gastroenterol. 2014;109(8):1141-57 ; (Quiz) 1058.
42. Rao SSC & Patcharatrakul T. J Neurogastroenterol Motil. 2016;22(3):423-35.
43. Rao SS, et al. Functional Anorectal Disorders. Gastroenterology. 2016. S0016-5085(16)00175-X.
44. Bharucha AE, et al.. Gastroenterology. 2013;144(1):218-38.
45. Voderholzer WA, et al. Am J Gastroenterol. 1997;92(1):95-8.
46. Lee HJ, et al. Neurogastroenterol Motil. 2015;27(6):787-95.
47. Simón MA & Bueno AM. J Clin Gastroenterol. 2017;51(10):e90-4.
48. Chiarioni G,et al.. Gastroenterology. 2006;130(3):657-64.
49. Heymen S, et al.. Dis Colon Rectum. 2007;50(4):428-41.
50. Rao SSC, et al. Clin Gastroenterol Hepatol. 2007;5(3):331-8.
51. Rao SSC, et al. Am J Gastroenterol. 2010;105(4):890-6.
52. Patcharatrakul T, et al. Biofeedback therapy. In Clinical and basic neurogastroenterology and motility. India: Stacy Masucci; 2020:517-32.
53. Huaman J-W, et al. Clin Gastroenterol Hepatol. 2020;18(11):2463-2470.e1.
54. Patcharatrakul T, et al. Clin Gastroenterol Hepatol. 2018;16(5):715-21.
55. Chaudhry A, et al. Gastroenterology. 2020;158(6):S-382–S-383.
56. Shim LSE, et al. Aliment Pharmacol Ther. 2011;33(11):1245-51.
57. Rao SSC, et al. Neurogastroenterol Motil. 2015;27(5):594-609.
58. Jangsirikul S, et al. Gastroenterology. 2020;158(6):S-383.
59. Rao SSC, et al. Am J Gastroenterol. 2019;114(6):938-44.
60. Ron Y, et al.. Dis Colon Rectum. 2001;44(12):1821-6.
61. Podzemny V, et al. World J Gastroenterol. 2015;21(4):1053-60.
62. Faried M, et al. J Gastrointest Surg. 2010;14(8):1235-43.
63. Hallan RI, et al. Lancet. 1988;2(8613):714-7.
Introduction
About 40% of the population experiences lower GI symptoms suggestive of gastrointestinal motility disorders.1,2 The global prevalence of chronic constipation is 18%, and the condition includes multiple overlapping subtypes.3 Evacuation disorders affect over half (59%) of patients and include dyssynergic defecation (DD).4 The inability to coordinate the abdominal, rectal, pelvic floor, and anal/puborectalis muscles to evacuate stools causes DD.5 The etiology of DD remains unclear and is often misdiagnosed. Clinically, the symptoms of DD overlap with other lower GI disorders, often leading to unnecessary and invasive procedures.2 We describe the clinical characteristics, diagnostic tools, treatment options, and evidence-based approach for the management of DD.

Clinical presentation
Over two-thirds of patients with DD acquire this disorder during adulthood, and one-third have symptoms from childhood.6 Though there is not usually an inciting event, 29% of patients report that symptoms began after events such as pregnancy or back injury,6 and opioid users have higher prevalence and severity of DD.7
Over 80% of patients report excessive straining, feelings of incomplete evacuation, and hard stools, and 50% report sensation of anal blockage or use of digital maneuvers.2 Other symptoms include infrequent bowel movements, abdominal pain, anal pain, and stool leakage.2 Evaluation of DD includes obtaining a detailed history utilizing the Bristol Stool Form Scale;8 however, patients’ recall of stool habit is often inaccurate, which results in suboptimal care.9,10 Prospective stool diaries can help to provide more objective assessment of patients’ symptoms, eliminate recall bias, and provide more reliable information. Several useful questionnaires are available for clinical and research purposes to characterize lower-GI symptoms, including the Constipation Scoring System,11 Patient Assessment of Constipation Symptoms (PAC-SYM),12 and Patient Assessment of Constipation Quality of Life (PAC-QOL).2,13 The Constipation Stool digital app enhances accuracy of data capture and offers a reliable and user-friendly method for recording bowel symptoms for patients, clinicians, and clinical investigators.14
Diagnosis
The diagnosis of DD requires careful physical and digital rectal examination together with anorectal manometry and a balloon expulsion test. Defecography and colonic transit studies provide additional assessment.
Physical examination
Abdominal examination should include palpation for stool in the colon and identification of abdominal mass or fecal impaction.2A high-quality digital rectal examination can help to identify patients who could benefit from physiological testing to confirm and treat DD.15 Rectal examination is performed by placing examiner’s lubricated gloved right index finger in a patient’s rectum, with the examiner’s left hand on patient’s abdomen, and asking the patient to push and bear down as if defecating.15 The contraction of the abdominal muscles is felt using the left hand, while the anal sphincter relaxation and degree of perineal descent are felt using the right-hand index finger.15 A diagnosis of dyssynergia is suspected if the digital rectal examination reveals two or more of the following abnormalities: inability to contract abdominal muscles (lack of push effort), inability to relax or paradoxical contraction of the anal sphincter and/or puborectalis, or absence of perineal descent.15 Digital rectal examination has good sensitivity (75%), specificity (87%), and positive predictive value (97%) for DD.16
High resolution anorectal manometry
Anorectal manometry (ARM) is the preferred method for the evaluation of defecatory disorders.17,18 ARM is best performed using the high-resolution anorectal manometry (HRAM) systems19 that consist of a flexible probe – 0.5-cm diameter with multiple circumferential sensors along the anal canal – and another two sensors inside a rectal balloon.18 It provides a topographic and waveform display of manometric pressure data (Figure). The 3D high-definition ARM probe is a rigid 1-cm probe that provides 3D topographic profiles.18 ARM is typically performed in both the left lateral position and in a more physiological seated position.20,21 There is considerable variation amongst different institutions on how to perform HRAM, and a recent International Anorectal Physiology Working Group (IAPWG) has provided consensus recommendations for performing this test.22 The procedure for performing HRAM is reviewed elsewhere, but the key elements are summarized below.18
Push maneuver: On HRAM, after the assessment of resting and squeeze anal sphincter pressures, the patient is asked to push or bear down as if to defecate while lying in left lateral decubitus position. The best of two attempts that closely mimics a normal bearing down maneuver is used for categorizing patient’s defecatory pattern.18 In patients with DD, at least four distinct dyssynergia phenotypes have been recognized (Figure),23 though recent studies suggest eight patterns.24 Defecation index (maximum rectal pressure/minimum residual anal pressure when bearing down) greater than 1.2 is considered normal.18
Simulated defecation on commode: The subject is asked to attempt defecation while seated on a commode with intrarectal balloon filled with 60 cc of air, and both the defecation pattern(s) and defecation index are calculated. A lack of coordinated push effort is highly suggestive of DD.21
Rectoanal Inhibitory Reflex (RAIR): RAIR describes the reflex relaxation of the internal anal sphincter after rectal distension. RAIR is dependent on intact autonomic ganglia and myenteric plexus25and is mediated by the release of nitric oxide and vasoactive intestinal peptide.26 The absence of RAIR suggests Hirschsprung disease.22.27.28
Rectal sensory testing: Intermittent balloon distension of the rectum with incremental volumes of air induces a range of rectal sensations that include first sensation, desire to defecate, urgency to defecate, and maximum tolerable volume. Rectal hyposensitivity is diagnosed when two or more sensory thresholds are higher than those seen in normal subjects29.30 and likely results from disruption of afferent gut-brain pathways, cortical perception/rectal wall dysfunction, or both.29 Rectal hyposensitivity affects 40% of patients with constipation30and is associated with DD but not delayed colonic transit.31 Rectal hyposensitivity may also be seen in patients with diabetes or fecal incontinence.18 About two-thirds of patients with rectal hyposensitivity have rectal hypercompliance, and some have megarectum.32 Some patients with DD have coexisting irritable bowel syndrome (IBS) and may have rectal hypersensitivity.18,33 Rectal compliance is measured alongside rectal sensitivity analysis by plotting a graph between the change in intraballoon volume (mL) and change in intrarectal pressures (mm Hg) during incremental balloon distensions.18.34 Rectal hypercompliance may be seen in megarectum and dyssynergic defecation.34,35 Rectal hypocompliance may be seen in patients with inflammatory bowel disease, postpelvic radiation, chronic ischemia, and advanced age.18
Balloon expulsion test: This test is performed by placing a plastic probe with a balloon in the rectum and filling it with 50 cc of warm water. Patients are given 5 minutes to expel the balloon while sitting on a commode. Balloon expulsion time of more than 1 minute suggests a diagnosis of DD,21 although 2 minutes provides a higher level of agreement with manometric findings.36 Balloon type and body position can influence the results.37 Inability to expel the balloon with normal manometric findings is considered an inconclusive finding per the recent London Classification (i.e., it may be associated with generation of anorectal symptoms, but the clinical relevance of this finding is unclear as it may also be seen in healthy subjects).22
Defecography
Defecography is a dynamic fluoroscopic study performed in the sitting position after injecting 150 mL of barium paste into the patient’s rectum. Defecography provides useful information about structural changes (e.g., rectoceles, enteroceles, rectal prolapse, and intussusception), DD, and descending perineum syndrome.38 Methodological differences, radiation exposure, and poor interobserver agreement have limited its wider use; therefore, anorectal manometry and the balloon expulsion test are recommended for the initial evaluation of DD.39 Magnetic resonance defecography may be more useful.17,38
Colonic transit studies
Colonic transit study can be assessed using radiopaque markers, wireless motility capsule, or scintigraphy. Wireless motility capsule and scintigraphy have the advantage of determining gastric, small bowel, and whole gut transit times as well. About two-thirds of patients with DD have slow transit constipation (STC),6 which improves after treatment of DD.40 Hence, in patients with chronic constipation, evaluation and management of DD is recommended first. If symptoms persist, then consider colonic transit assessment.41 Given the overlapping nature of the conditions, documentation of STC at the outset could facilitate treatment of both.
Diagnostic criteria for DD
Patients should fulfill the following criteria for diagnosis of DD:42,43
- Fulfill symptom(s) diagnostic criteria for functional constipation and/or constipation-predominant IBS.
- Demonstrate dyssynergic pattern (Types I-IV; Figure) during attempted defecation on manometry recordings.
- Meet one or more of the following criteria:
- Inability to expel an artificial stool (50 mL water-filled balloon) within 1 minute.
- Inability to evacuate or retention of 50% or more of barium during defecography. (Some institutions use a prolonged colonic transit time: greater than 5 markers or 20% or higher marker retention on a plain abdominal x-Ray at 120 hours after ingestion of one radio-opaque marker capsule containing 24 radio-opaque markers.)
Treatment of DD
The treatment modalities for DD depend on several factors: patient’s age, comorbidities, underlying pathophysiology, and patient expectations. Treatment options include standard management of constipation, but biofeedback therapy is the mainstay.
Standard management
Medications that cause or worsen constipation should be avoided. The patient should consume adequate fluid and exercise regularly. Patients should receive instructions for timed toilet training (twice daily, 30 minutes after meals). Patients should push at about 50%-70% of their ability for no longer than 5 minutes and avoid postponing defecation or use of digital maneuvers to facilitate defecation.42 The patients should take 25 g of soluble fiber (e.g., psyllium) daily. Of note, the benefits of fiber can take days to weeks44 and may be limited in patients with STC and DD.45 Medications including laxatives and intestinal secretagogues (lubiprostone, linaclotide, plecanatide), and enterokinetic agents (prucalopride) can be used as adjunct therapy for management of DD.42 Their use is titrated during and after biofeedback therapy and may decrease after successful treatment.46
Biofeedback therapy
Biofeedback therapy involves operant conditioning techniques using either a solid state anorectal manometry system, electromyography, simulated balloon, or home biofeedback training devices.42,47 The goals of biofeedback therapy are to correct the abdominal pelvic muscle discoordination during defecation and improve rectal sensation to stool if impaired. Biofeedback therapy involves patient education and active training (typically six sessions, 1-2 weeks apart, with each about 30-60 minutes long), followed by a reinforcement stage (three sessions at 3, 6, and 12 months), though there are variations in training protocols.42

The success of biofeedback therapy depends on the patient’s motivation and the therapist’s skills.42 Compared with standard therapy (diet, exercise, pharmacotherapy), biofeedback therapy provides sustained improvement of bowel symptoms and anorectal function. Up to 70%-80% of DD patients show significant improvement of symptoms in randomized controlled trials (Table).48-52 Biofeedback therapy may also improve dyspeptic symptoms.53 Patients with harder stool consistency, greater willingness to participate, lower baseline bowel satisfaction, lower baseline anal sphincter relaxation, and prolonged balloon expulsion time, as well as patients who used digital maneuvers for defection, more commonly respond to biofeedback therapy.54,55 Longstanding laxative use has been associated with decreased response to biofeedback therapy.56 In patients with rectal hyposensitivity, barostat-assisted sensory training is more effective than a hand-held syringe technique.30 In patients with constipation predominant IBS and rectal hyposensitivity, sensory adaption training is more efficacious and better tolerated than escitalopram.30 Biofeedback therapy was afforded a grade A recommendation for treatment of DD by the American and European Societies of Neurogastroenterology and Motility.57
The access to office-based biofeedback therapy may be limited because of costs and low availability. The time required to attend multiple sessions may be burdensome for some patients, especially if they are taking time off from work. A recent study showed that patients with higher level of education may be less likely to adhere to biofeedback therapy.58 Recently, home biofeedback was shown to be noninferior to office biofeedback and was more cost-effective, which provides an alternative option for treating more patients.59
Endoscopic/surgical options
Other less effective treatment options for DD include botulinum toxin injection and myectomy.60-62 Botulinum toxin injection appears to have mixed effects with less than 50% of patients reporting symptomatic improvement, and it may cause fecal incontinence.60,63
Conclusion
DD is a common yet poorly recognized cause of constipation. Its clinical presentation overlaps with other lower-GI disorders. Its diagnosis requires detailed history, digital rectal examination, prospective stool diaries, anorectal manometry, and balloon expulsion tests. Biofeedback therapy offers excellent and sustained symptomatic improvement; however, access to office-based biofeedback is limited, and there is an urgent need for home-based biofeedback therapy programs.59
Dr. Rao is J. Harold Harrison Distinguished University Chair, professor of medicine, director of neurogastroenterology/motility, and director of digestive health at the Digestive Health Clinical Research Center Augusta (Georgia) University. He is supported by National Institutes of Health grants R01DK121003-02 and U01DK115572. Dr. Jehangir is a gastroenterology and Hepatology Fellow at the Digestive Health Clinical Research Center at Augusta University. They reported having no conflicts of interest.
References
1. Peery AF, et al. Gastroenterology. 2012;143(5):1179-1187.e3 .
2. Curtin B, et al. J Neurogastroenterol Motil. 2020 30;26(4):423-36.
3. Suares NC & Ford AC. Am J Gastroenterol. 2011 Sep;106(9):1582-91.
4. Mertz H, et al. Am J Gastroenterol. 1999;94(3):609-15.
5. Rao SS, et al. Am J Gastroenterol. 1998;93(7):1042-50.
6. Rao SSC, et al. J Clin Gastroenterol. 2004;38(8):680-5.
7. Nojkov B, et al. Am J Gastroenterol. 2019;114(11):1772-7.
8. Heaton KW, et al. Gut. 1992;33(6):818-24.
9. Prichard DO & Bharucha AE. 2018 Oct 15;7:F1000 Faculty Rev-1640.
10. Ashraf W, et al. Am J Gastroenterol. 1996;91(1):26-32.
11. Agachan F, et al.. Dis Colon Rectum. 1996;39(6):681-5.
12. Frank L, et al. Scand J Gastroenterol. 1999;34(9):870-7.
13. Marquis P, et al. Scand J Gastroenterol. 2005;40(5):540-51.
14. Yan Y, et al. Gastroenterology. 2020;158(6):S-400.
15. Rao SSC. Am J Gastroenterol. 2018;113(5):635-8.
16. Tantiphlachiva K, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8(11):955-60.
17. Carrington EV, et al. Nat Rev Gastroenterol Hepatol. 2018;15(5):309-23.
18. Tetangco EP, et al. Performing and analyzing high-resolution anorectal manometry. NeuroGastroLatam Rev. 2018;2:120-32.
19. Lee YY, et al. Curr Gastroenterol Rep. 2013;15(12):360.
20. Sharma M, et al. Neurogastroenterol Motil. 2020;32(10):e13910.
21. Rao SSC, et al.. Am J Gastroenterol. 2006;101(12):2790-6.
22. Carrington EV, et al. Neurogastroenterol Motil. 2020;32(1):e13679.
23. Rao SSC. Gastroenterol Clin North Am. 2008;37(3):569-86, viii.
24. Rao SSC, et al. Gastroenterology. 2016;150(4):S158-9.
25. Guinet A, et al. Int J Colorectal Dis. 2011;26(4):507-13.
26. Rattan S, et al. Gastroenterology. 1992;103(1):43-50.
27. Remes-Troche JM & Rao SSC. 2008;2(3):323-35.
28. Zaafouri H, et al..Int J Surgery. 2015. 2(1):9-17.
29. Remes-Troche JM, et al. Dis Colon Rectum. 2010;53(7):1047-54.
30. Rao SSC, et al. Gastroenterology. 2013;144(5):S-363.
31. Yu T, et al. Medicine (Baltimore). 2016;95(19):e3667.
32. Gladman MA, et al. Neurogastroenterol Motil. 2009;21(5):508-16, e4-5.
33. Lee KJ, et al. Digestion. 2006;73(2-3):133-41 .
34. Rao SSC, et al. Neurogastroenterol Motil. 2002;14(5):553-9.
35. Coss-Adame E, et al.. Clin Gastroenterol Hepatol. 2015;13(6):1143-1150.e1.
36. Chiarioni G, et al. Clin Gastroenterol Hepatol. 2014;12(12):2049-54.
37. Gu G, et al. Gastroenterology. 2018;154(6):S-545–S-546.
38. Savoye-Collet C, et al.. Gastroenterol Clin North Am. 2008;37(3):553-67, viii.
39. Videlock EJ, et al. Neurogastroenterol Motil. 2013;25(6):509-20.
40. Rao SSC, et al. Neurogastroenterol Motil. 2004;16(5):589-96.
41. Wald A, et al. Am J Gastroenterol. 2014;109(8):1141-57 ; (Quiz) 1058.
42. Rao SSC & Patcharatrakul T. J Neurogastroenterol Motil. 2016;22(3):423-35.
43. Rao SS, et al. Functional Anorectal Disorders. Gastroenterology. 2016. S0016-5085(16)00175-X.
44. Bharucha AE, et al.. Gastroenterology. 2013;144(1):218-38.
45. Voderholzer WA, et al. Am J Gastroenterol. 1997;92(1):95-8.
46. Lee HJ, et al. Neurogastroenterol Motil. 2015;27(6):787-95.
47. Simón MA & Bueno AM. J Clin Gastroenterol. 2017;51(10):e90-4.
48. Chiarioni G,et al.. Gastroenterology. 2006;130(3):657-64.
49. Heymen S, et al.. Dis Colon Rectum. 2007;50(4):428-41.
50. Rao SSC, et al. Clin Gastroenterol Hepatol. 2007;5(3):331-8.
51. Rao SSC, et al. Am J Gastroenterol. 2010;105(4):890-6.
52. Patcharatrakul T, et al. Biofeedback therapy. In Clinical and basic neurogastroenterology and motility. India: Stacy Masucci; 2020:517-32.
53. Huaman J-W, et al. Clin Gastroenterol Hepatol. 2020;18(11):2463-2470.e1.
54. Patcharatrakul T, et al. Clin Gastroenterol Hepatol. 2018;16(5):715-21.
55. Chaudhry A, et al. Gastroenterology. 2020;158(6):S-382–S-383.
56. Shim LSE, et al. Aliment Pharmacol Ther. 2011;33(11):1245-51.
57. Rao SSC, et al. Neurogastroenterol Motil. 2015;27(5):594-609.
58. Jangsirikul S, et al. Gastroenterology. 2020;158(6):S-383.
59. Rao SSC, et al. Am J Gastroenterol. 2019;114(6):938-44.
60. Ron Y, et al.. Dis Colon Rectum. 2001;44(12):1821-6.
61. Podzemny V, et al. World J Gastroenterol. 2015;21(4):1053-60.
62. Faried M, et al. J Gastrointest Surg. 2010;14(8):1235-43.
63. Hallan RI, et al. Lancet. 1988;2(8613):714-7.
Introduction
About 40% of the population experiences lower GI symptoms suggestive of gastrointestinal motility disorders.1,2 The global prevalence of chronic constipation is 18%, and the condition includes multiple overlapping subtypes.3 Evacuation disorders affect over half (59%) of patients and include dyssynergic defecation (DD).4 The inability to coordinate the abdominal, rectal, pelvic floor, and anal/puborectalis muscles to evacuate stools causes DD.5 The etiology of DD remains unclear and is often misdiagnosed. Clinically, the symptoms of DD overlap with other lower GI disorders, often leading to unnecessary and invasive procedures.2 We describe the clinical characteristics, diagnostic tools, treatment options, and evidence-based approach for the management of DD.

Clinical presentation
Over two-thirds of patients with DD acquire this disorder during adulthood, and one-third have symptoms from childhood.6 Though there is not usually an inciting event, 29% of patients report that symptoms began after events such as pregnancy or back injury,6 and opioid users have higher prevalence and severity of DD.7
Over 80% of patients report excessive straining, feelings of incomplete evacuation, and hard stools, and 50% report sensation of anal blockage or use of digital maneuvers.2 Other symptoms include infrequent bowel movements, abdominal pain, anal pain, and stool leakage.2 Evaluation of DD includes obtaining a detailed history utilizing the Bristol Stool Form Scale;8 however, patients’ recall of stool habit is often inaccurate, which results in suboptimal care.9,10 Prospective stool diaries can help to provide more objective assessment of patients’ symptoms, eliminate recall bias, and provide more reliable information. Several useful questionnaires are available for clinical and research purposes to characterize lower-GI symptoms, including the Constipation Scoring System,11 Patient Assessment of Constipation Symptoms (PAC-SYM),12 and Patient Assessment of Constipation Quality of Life (PAC-QOL).2,13 The Constipation Stool digital app enhances accuracy of data capture and offers a reliable and user-friendly method for recording bowel symptoms for patients, clinicians, and clinical investigators.14
Diagnosis
The diagnosis of DD requires careful physical and digital rectal examination together with anorectal manometry and a balloon expulsion test. Defecography and colonic transit studies provide additional assessment.
Physical examination
Abdominal examination should include palpation for stool in the colon and identification of abdominal mass or fecal impaction.2A high-quality digital rectal examination can help to identify patients who could benefit from physiological testing to confirm and treat DD.15 Rectal examination is performed by placing examiner’s lubricated gloved right index finger in a patient’s rectum, with the examiner’s left hand on patient’s abdomen, and asking the patient to push and bear down as if defecating.15 The contraction of the abdominal muscles is felt using the left hand, while the anal sphincter relaxation and degree of perineal descent are felt using the right-hand index finger.15 A diagnosis of dyssynergia is suspected if the digital rectal examination reveals two or more of the following abnormalities: inability to contract abdominal muscles (lack of push effort), inability to relax or paradoxical contraction of the anal sphincter and/or puborectalis, or absence of perineal descent.15 Digital rectal examination has good sensitivity (75%), specificity (87%), and positive predictive value (97%) for DD.16
High resolution anorectal manometry
Anorectal manometry (ARM) is the preferred method for the evaluation of defecatory disorders.17,18 ARM is best performed using the high-resolution anorectal manometry (HRAM) systems19 that consist of a flexible probe – 0.5-cm diameter with multiple circumferential sensors along the anal canal – and another two sensors inside a rectal balloon.18 It provides a topographic and waveform display of manometric pressure data (Figure). The 3D high-definition ARM probe is a rigid 1-cm probe that provides 3D topographic profiles.18 ARM is typically performed in both the left lateral position and in a more physiological seated position.20,21 There is considerable variation amongst different institutions on how to perform HRAM, and a recent International Anorectal Physiology Working Group (IAPWG) has provided consensus recommendations for performing this test.22 The procedure for performing HRAM is reviewed elsewhere, but the key elements are summarized below.18
Push maneuver: On HRAM, after the assessment of resting and squeeze anal sphincter pressures, the patient is asked to push or bear down as if to defecate while lying in left lateral decubitus position. The best of two attempts that closely mimics a normal bearing down maneuver is used for categorizing patient’s defecatory pattern.18 In patients with DD, at least four distinct dyssynergia phenotypes have been recognized (Figure),23 though recent studies suggest eight patterns.24 Defecation index (maximum rectal pressure/minimum residual anal pressure when bearing down) greater than 1.2 is considered normal.18
Simulated defecation on commode: The subject is asked to attempt defecation while seated on a commode with intrarectal balloon filled with 60 cc of air, and both the defecation pattern(s) and defecation index are calculated. A lack of coordinated push effort is highly suggestive of DD.21
Rectoanal Inhibitory Reflex (RAIR): RAIR describes the reflex relaxation of the internal anal sphincter after rectal distension. RAIR is dependent on intact autonomic ganglia and myenteric plexus25and is mediated by the release of nitric oxide and vasoactive intestinal peptide.26 The absence of RAIR suggests Hirschsprung disease.22.27.28
Rectal sensory testing: Intermittent balloon distension of the rectum with incremental volumes of air induces a range of rectal sensations that include first sensation, desire to defecate, urgency to defecate, and maximum tolerable volume. Rectal hyposensitivity is diagnosed when two or more sensory thresholds are higher than those seen in normal subjects29.30 and likely results from disruption of afferent gut-brain pathways, cortical perception/rectal wall dysfunction, or both.29 Rectal hyposensitivity affects 40% of patients with constipation30and is associated with DD but not delayed colonic transit.31 Rectal hyposensitivity may also be seen in patients with diabetes or fecal incontinence.18 About two-thirds of patients with rectal hyposensitivity have rectal hypercompliance, and some have megarectum.32 Some patients with DD have coexisting irritable bowel syndrome (IBS) and may have rectal hypersensitivity.18,33 Rectal compliance is measured alongside rectal sensitivity analysis by plotting a graph between the change in intraballoon volume (mL) and change in intrarectal pressures (mm Hg) during incremental balloon distensions.18.34 Rectal hypercompliance may be seen in megarectum and dyssynergic defecation.34,35 Rectal hypocompliance may be seen in patients with inflammatory bowel disease, postpelvic radiation, chronic ischemia, and advanced age.18
Balloon expulsion test: This test is performed by placing a plastic probe with a balloon in the rectum and filling it with 50 cc of warm water. Patients are given 5 minutes to expel the balloon while sitting on a commode. Balloon expulsion time of more than 1 minute suggests a diagnosis of DD,21 although 2 minutes provides a higher level of agreement with manometric findings.36 Balloon type and body position can influence the results.37 Inability to expel the balloon with normal manometric findings is considered an inconclusive finding per the recent London Classification (i.e., it may be associated with generation of anorectal symptoms, but the clinical relevance of this finding is unclear as it may also be seen in healthy subjects).22
Defecography
Defecography is a dynamic fluoroscopic study performed in the sitting position after injecting 150 mL of barium paste into the patient’s rectum. Defecography provides useful information about structural changes (e.g., rectoceles, enteroceles, rectal prolapse, and intussusception), DD, and descending perineum syndrome.38 Methodological differences, radiation exposure, and poor interobserver agreement have limited its wider use; therefore, anorectal manometry and the balloon expulsion test are recommended for the initial evaluation of DD.39 Magnetic resonance defecography may be more useful.17,38
Colonic transit studies
Colonic transit study can be assessed using radiopaque markers, wireless motility capsule, or scintigraphy. Wireless motility capsule and scintigraphy have the advantage of determining gastric, small bowel, and whole gut transit times as well. About two-thirds of patients with DD have slow transit constipation (STC),6 which improves after treatment of DD.40 Hence, in patients with chronic constipation, evaluation and management of DD is recommended first. If symptoms persist, then consider colonic transit assessment.41 Given the overlapping nature of the conditions, documentation of STC at the outset could facilitate treatment of both.
Diagnostic criteria for DD
Patients should fulfill the following criteria for diagnosis of DD:42,43
- Fulfill symptom(s) diagnostic criteria for functional constipation and/or constipation-predominant IBS.
- Demonstrate dyssynergic pattern (Types I-IV; Figure) during attempted defecation on manometry recordings.
- Meet one or more of the following criteria:
- Inability to expel an artificial stool (50 mL water-filled balloon) within 1 minute.
- Inability to evacuate or retention of 50% or more of barium during defecography. (Some institutions use a prolonged colonic transit time: greater than 5 markers or 20% or higher marker retention on a plain abdominal x-Ray at 120 hours after ingestion of one radio-opaque marker capsule containing 24 radio-opaque markers.)
Treatment of DD
The treatment modalities for DD depend on several factors: patient’s age, comorbidities, underlying pathophysiology, and patient expectations. Treatment options include standard management of constipation, but biofeedback therapy is the mainstay.
Standard management
Medications that cause or worsen constipation should be avoided. The patient should consume adequate fluid and exercise regularly. Patients should receive instructions for timed toilet training (twice daily, 30 minutes after meals). Patients should push at about 50%-70% of their ability for no longer than 5 minutes and avoid postponing defecation or use of digital maneuvers to facilitate defecation.42 The patients should take 25 g of soluble fiber (e.g., psyllium) daily. Of note, the benefits of fiber can take days to weeks44 and may be limited in patients with STC and DD.45 Medications including laxatives and intestinal secretagogues (lubiprostone, linaclotide, plecanatide), and enterokinetic agents (prucalopride) can be used as adjunct therapy for management of DD.42 Their use is titrated during and after biofeedback therapy and may decrease after successful treatment.46
Biofeedback therapy
Biofeedback therapy involves operant conditioning techniques using either a solid state anorectal manometry system, electromyography, simulated balloon, or home biofeedback training devices.42,47 The goals of biofeedback therapy are to correct the abdominal pelvic muscle discoordination during defecation and improve rectal sensation to stool if impaired. Biofeedback therapy involves patient education and active training (typically six sessions, 1-2 weeks apart, with each about 30-60 minutes long), followed by a reinforcement stage (three sessions at 3, 6, and 12 months), though there are variations in training protocols.42

The success of biofeedback therapy depends on the patient’s motivation and the therapist’s skills.42 Compared with standard therapy (diet, exercise, pharmacotherapy), biofeedback therapy provides sustained improvement of bowel symptoms and anorectal function. Up to 70%-80% of DD patients show significant improvement of symptoms in randomized controlled trials (Table).48-52 Biofeedback therapy may also improve dyspeptic symptoms.53 Patients with harder stool consistency, greater willingness to participate, lower baseline bowel satisfaction, lower baseline anal sphincter relaxation, and prolonged balloon expulsion time, as well as patients who used digital maneuvers for defection, more commonly respond to biofeedback therapy.54,55 Longstanding laxative use has been associated with decreased response to biofeedback therapy.56 In patients with rectal hyposensitivity, barostat-assisted sensory training is more effective than a hand-held syringe technique.30 In patients with constipation predominant IBS and rectal hyposensitivity, sensory adaption training is more efficacious and better tolerated than escitalopram.30 Biofeedback therapy was afforded a grade A recommendation for treatment of DD by the American and European Societies of Neurogastroenterology and Motility.57
The access to office-based biofeedback therapy may be limited because of costs and low availability. The time required to attend multiple sessions may be burdensome for some patients, especially if they are taking time off from work. A recent study showed that patients with higher level of education may be less likely to adhere to biofeedback therapy.58 Recently, home biofeedback was shown to be noninferior to office biofeedback and was more cost-effective, which provides an alternative option for treating more patients.59
Endoscopic/surgical options
Other less effective treatment options for DD include botulinum toxin injection and myectomy.60-62 Botulinum toxin injection appears to have mixed effects with less than 50% of patients reporting symptomatic improvement, and it may cause fecal incontinence.60,63
Conclusion
DD is a common yet poorly recognized cause of constipation. Its clinical presentation overlaps with other lower-GI disorders. Its diagnosis requires detailed history, digital rectal examination, prospective stool diaries, anorectal manometry, and balloon expulsion tests. Biofeedback therapy offers excellent and sustained symptomatic improvement; however, access to office-based biofeedback is limited, and there is an urgent need for home-based biofeedback therapy programs.59
Dr. Rao is J. Harold Harrison Distinguished University Chair, professor of medicine, director of neurogastroenterology/motility, and director of digestive health at the Digestive Health Clinical Research Center Augusta (Georgia) University. He is supported by National Institutes of Health grants R01DK121003-02 and U01DK115572. Dr. Jehangir is a gastroenterology and Hepatology Fellow at the Digestive Health Clinical Research Center at Augusta University. They reported having no conflicts of interest.
References
1. Peery AF, et al. Gastroenterology. 2012;143(5):1179-1187.e3 .
2. Curtin B, et al. J Neurogastroenterol Motil. 2020 30;26(4):423-36.
3. Suares NC & Ford AC. Am J Gastroenterol. 2011 Sep;106(9):1582-91.
4. Mertz H, et al. Am J Gastroenterol. 1999;94(3):609-15.
5. Rao SS, et al. Am J Gastroenterol. 1998;93(7):1042-50.
6. Rao SSC, et al. J Clin Gastroenterol. 2004;38(8):680-5.
7. Nojkov B, et al. Am J Gastroenterol. 2019;114(11):1772-7.
8. Heaton KW, et al. Gut. 1992;33(6):818-24.
9. Prichard DO & Bharucha AE. 2018 Oct 15;7:F1000 Faculty Rev-1640.
10. Ashraf W, et al. Am J Gastroenterol. 1996;91(1):26-32.
11. Agachan F, et al.. Dis Colon Rectum. 1996;39(6):681-5.
12. Frank L, et al. Scand J Gastroenterol. 1999;34(9):870-7.
13. Marquis P, et al. Scand J Gastroenterol. 2005;40(5):540-51.
14. Yan Y, et al. Gastroenterology. 2020;158(6):S-400.
15. Rao SSC. Am J Gastroenterol. 2018;113(5):635-8.
16. Tantiphlachiva K, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8(11):955-60.
17. Carrington EV, et al. Nat Rev Gastroenterol Hepatol. 2018;15(5):309-23.
18. Tetangco EP, et al. Performing and analyzing high-resolution anorectal manometry. NeuroGastroLatam Rev. 2018;2:120-32.
19. Lee YY, et al. Curr Gastroenterol Rep. 2013;15(12):360.
20. Sharma M, et al. Neurogastroenterol Motil. 2020;32(10):e13910.
21. Rao SSC, et al.. Am J Gastroenterol. 2006;101(12):2790-6.
22. Carrington EV, et al. Neurogastroenterol Motil. 2020;32(1):e13679.
23. Rao SSC. Gastroenterol Clin North Am. 2008;37(3):569-86, viii.
24. Rao SSC, et al. Gastroenterology. 2016;150(4):S158-9.
25. Guinet A, et al. Int J Colorectal Dis. 2011;26(4):507-13.
26. Rattan S, et al. Gastroenterology. 1992;103(1):43-50.
27. Remes-Troche JM & Rao SSC. 2008;2(3):323-35.
28. Zaafouri H, et al..Int J Surgery. 2015. 2(1):9-17.
29. Remes-Troche JM, et al. Dis Colon Rectum. 2010;53(7):1047-54.
30. Rao SSC, et al. Gastroenterology. 2013;144(5):S-363.
31. Yu T, et al. Medicine (Baltimore). 2016;95(19):e3667.
32. Gladman MA, et al. Neurogastroenterol Motil. 2009;21(5):508-16, e4-5.
33. Lee KJ, et al. Digestion. 2006;73(2-3):133-41 .
34. Rao SSC, et al. Neurogastroenterol Motil. 2002;14(5):553-9.
35. Coss-Adame E, et al.. Clin Gastroenterol Hepatol. 2015;13(6):1143-1150.e1.
36. Chiarioni G, et al. Clin Gastroenterol Hepatol. 2014;12(12):2049-54.
37. Gu G, et al. Gastroenterology. 2018;154(6):S-545–S-546.
38. Savoye-Collet C, et al.. Gastroenterol Clin North Am. 2008;37(3):553-67, viii.
39. Videlock EJ, et al. Neurogastroenterol Motil. 2013;25(6):509-20.
40. Rao SSC, et al. Neurogastroenterol Motil. 2004;16(5):589-96.
41. Wald A, et al. Am J Gastroenterol. 2014;109(8):1141-57 ; (Quiz) 1058.
42. Rao SSC & Patcharatrakul T. J Neurogastroenterol Motil. 2016;22(3):423-35.
43. Rao SS, et al. Functional Anorectal Disorders. Gastroenterology. 2016. S0016-5085(16)00175-X.
44. Bharucha AE, et al.. Gastroenterology. 2013;144(1):218-38.
45. Voderholzer WA, et al. Am J Gastroenterol. 1997;92(1):95-8.
46. Lee HJ, et al. Neurogastroenterol Motil. 2015;27(6):787-95.
47. Simón MA & Bueno AM. J Clin Gastroenterol. 2017;51(10):e90-4.
48. Chiarioni G,et al.. Gastroenterology. 2006;130(3):657-64.
49. Heymen S, et al.. Dis Colon Rectum. 2007;50(4):428-41.
50. Rao SSC, et al. Clin Gastroenterol Hepatol. 2007;5(3):331-8.
51. Rao SSC, et al. Am J Gastroenterol. 2010;105(4):890-6.
52. Patcharatrakul T, et al. Biofeedback therapy. In Clinical and basic neurogastroenterology and motility. India: Stacy Masucci; 2020:517-32.
53. Huaman J-W, et al. Clin Gastroenterol Hepatol. 2020;18(11):2463-2470.e1.
54. Patcharatrakul T, et al. Clin Gastroenterol Hepatol. 2018;16(5):715-21.
55. Chaudhry A, et al. Gastroenterology. 2020;158(6):S-382–S-383.
56. Shim LSE, et al. Aliment Pharmacol Ther. 2011;33(11):1245-51.
57. Rao SSC, et al. Neurogastroenterol Motil. 2015;27(5):594-609.
58. Jangsirikul S, et al. Gastroenterology. 2020;158(6):S-383.
59. Rao SSC, et al. Am J Gastroenterol. 2019;114(6):938-44.
60. Ron Y, et al.. Dis Colon Rectum. 2001;44(12):1821-6.
61. Podzemny V, et al. World J Gastroenterol. 2015;21(4):1053-60.
62. Faried M, et al. J Gastrointest Surg. 2010;14(8):1235-43.
63. Hallan RI, et al. Lancet. 1988;2(8613):714-7.