User login
Patients dislike prurigo nodularis treatment options, survey finds
.
The eye-opening results of the 406-patient, 12-country European patient survey indicate “high levels of disbelief in currently available treatment options and an overall dissatisfaction with treatment,” Manuel P. Pereira, MD, PhD, said in presenting the findings at the annual congress of the European Academy of Dermatology and Venereology.
Only 5.3% of patients pronounced themselves “very satisfied” with their treatment. Another 28% were “rather satisfied.”
“Remarkably, almost 10% of patients were not being treated for prurigo despite having active disease,” said Dr. Pereira, a dermatologist at the Center for Chronic Pruritus at University Hospital Münster (Germany).
When survey participants were asked to identify their most important unmet treatment needs, 79.5% named improvement of itch, 57.2% sought improvement in skin lesions, and 30.5% wanted better sleep.
The most widely used treatments were emollients, prescribed in 84.5% of patients; topical steroids, in 55.7%; antihistamines, 55.2%; and phototherapy, 42.1%. Far fewer patients were on more potent medications: Cyclosporine, systemic corticosteroids, or other immunosuppressants were prescribed for 21.9% of patients; gabapentin and related compounds in 17%; and topical immunomodulators in 8.6%. Twenty-three percent of patients were on antidepressants.
None of the available treatment options, all of which are off label, received high marks from patients. For example, only 1 in 10 patients on antihistamines during the last 6 months rated the drugs as effective. Topical immunomodulators were deemed effective by 1.1% of patients with active prurigo nodularis; gabapentinoids by 3.1%; phototherapy by 9.9%; and antidepressants were rated as effective for the chronic skin disease by only 2.3% of patients. The top-rated therapies were topical steroids, deemed effective by 12.8% of patients; systemic immunosuppressants, favored by 12.2%; and emollients, deemed effective by 10.5% of patients, even though more than 80% of survey participants were using them.
Dr. Pereira said the survey results highlight a pressing need for guidelines aimed at improving clinical care for patients with chronic prurigo nodularis. The first-ever such guidelines on the diagnosis and management of this debilitating disease, developed by Dr. Pereira and other members of the International Forum for the Study of Itch (IFSI), were recently published in the journal Itch. The new guidelines advocate a multimodal treatment approach incorporating a combination of topical and systemic therapies.
At present, there is no approved treatment for prurigo nodularis. Given the unmet need, however, the pace of research has quickened. Innovative potential treatments in the developmental pipeline include Janus kinase inhibitors, topical phosphodiesterase-4 inhibitors, systemic opioid receptor modulators, and neurokinin-1 receptor antagonists.
The patient survey was funded by the EADV and carried out by the EADV’s Pruritus Task Force as part of the European Prurigo Project. Dr. Pereira reported receiving research funding from the EADV and the German Research Foundation. He is a paid speaker for AbbVie, Galderma, Menlo Therapeutics (now VYNE Therapeutics), Novartis, and Trevi.
.
The eye-opening results of the 406-patient, 12-country European patient survey indicate “high levels of disbelief in currently available treatment options and an overall dissatisfaction with treatment,” Manuel P. Pereira, MD, PhD, said in presenting the findings at the annual congress of the European Academy of Dermatology and Venereology.
Only 5.3% of patients pronounced themselves “very satisfied” with their treatment. Another 28% were “rather satisfied.”
“Remarkably, almost 10% of patients were not being treated for prurigo despite having active disease,” said Dr. Pereira, a dermatologist at the Center for Chronic Pruritus at University Hospital Münster (Germany).
When survey participants were asked to identify their most important unmet treatment needs, 79.5% named improvement of itch, 57.2% sought improvement in skin lesions, and 30.5% wanted better sleep.
The most widely used treatments were emollients, prescribed in 84.5% of patients; topical steroids, in 55.7%; antihistamines, 55.2%; and phototherapy, 42.1%. Far fewer patients were on more potent medications: Cyclosporine, systemic corticosteroids, or other immunosuppressants were prescribed for 21.9% of patients; gabapentin and related compounds in 17%; and topical immunomodulators in 8.6%. Twenty-three percent of patients were on antidepressants.
None of the available treatment options, all of which are off label, received high marks from patients. For example, only 1 in 10 patients on antihistamines during the last 6 months rated the drugs as effective. Topical immunomodulators were deemed effective by 1.1% of patients with active prurigo nodularis; gabapentinoids by 3.1%; phototherapy by 9.9%; and antidepressants were rated as effective for the chronic skin disease by only 2.3% of patients. The top-rated therapies were topical steroids, deemed effective by 12.8% of patients; systemic immunosuppressants, favored by 12.2%; and emollients, deemed effective by 10.5% of patients, even though more than 80% of survey participants were using them.
Dr. Pereira said the survey results highlight a pressing need for guidelines aimed at improving clinical care for patients with chronic prurigo nodularis. The first-ever such guidelines on the diagnosis and management of this debilitating disease, developed by Dr. Pereira and other members of the International Forum for the Study of Itch (IFSI), were recently published in the journal Itch. The new guidelines advocate a multimodal treatment approach incorporating a combination of topical and systemic therapies.
At present, there is no approved treatment for prurigo nodularis. Given the unmet need, however, the pace of research has quickened. Innovative potential treatments in the developmental pipeline include Janus kinase inhibitors, topical phosphodiesterase-4 inhibitors, systemic opioid receptor modulators, and neurokinin-1 receptor antagonists.
The patient survey was funded by the EADV and carried out by the EADV’s Pruritus Task Force as part of the European Prurigo Project. Dr. Pereira reported receiving research funding from the EADV and the German Research Foundation. He is a paid speaker for AbbVie, Galderma, Menlo Therapeutics (now VYNE Therapeutics), Novartis, and Trevi.
.
The eye-opening results of the 406-patient, 12-country European patient survey indicate “high levels of disbelief in currently available treatment options and an overall dissatisfaction with treatment,” Manuel P. Pereira, MD, PhD, said in presenting the findings at the annual congress of the European Academy of Dermatology and Venereology.
Only 5.3% of patients pronounced themselves “very satisfied” with their treatment. Another 28% were “rather satisfied.”
“Remarkably, almost 10% of patients were not being treated for prurigo despite having active disease,” said Dr. Pereira, a dermatologist at the Center for Chronic Pruritus at University Hospital Münster (Germany).
When survey participants were asked to identify their most important unmet treatment needs, 79.5% named improvement of itch, 57.2% sought improvement in skin lesions, and 30.5% wanted better sleep.
The most widely used treatments were emollients, prescribed in 84.5% of patients; topical steroids, in 55.7%; antihistamines, 55.2%; and phototherapy, 42.1%. Far fewer patients were on more potent medications: Cyclosporine, systemic corticosteroids, or other immunosuppressants were prescribed for 21.9% of patients; gabapentin and related compounds in 17%; and topical immunomodulators in 8.6%. Twenty-three percent of patients were on antidepressants.
None of the available treatment options, all of which are off label, received high marks from patients. For example, only 1 in 10 patients on antihistamines during the last 6 months rated the drugs as effective. Topical immunomodulators were deemed effective by 1.1% of patients with active prurigo nodularis; gabapentinoids by 3.1%; phototherapy by 9.9%; and antidepressants were rated as effective for the chronic skin disease by only 2.3% of patients. The top-rated therapies were topical steroids, deemed effective by 12.8% of patients; systemic immunosuppressants, favored by 12.2%; and emollients, deemed effective by 10.5% of patients, even though more than 80% of survey participants were using them.
Dr. Pereira said the survey results highlight a pressing need for guidelines aimed at improving clinical care for patients with chronic prurigo nodularis. The first-ever such guidelines on the diagnosis and management of this debilitating disease, developed by Dr. Pereira and other members of the International Forum for the Study of Itch (IFSI), were recently published in the journal Itch. The new guidelines advocate a multimodal treatment approach incorporating a combination of topical and systemic therapies.
At present, there is no approved treatment for prurigo nodularis. Given the unmet need, however, the pace of research has quickened. Innovative potential treatments in the developmental pipeline include Janus kinase inhibitors, topical phosphodiesterase-4 inhibitors, systemic opioid receptor modulators, and neurokinin-1 receptor antagonists.
The patient survey was funded by the EADV and carried out by the EADV’s Pruritus Task Force as part of the European Prurigo Project. Dr. Pereira reported receiving research funding from the EADV and the German Research Foundation. He is a paid speaker for AbbVie, Galderma, Menlo Therapeutics (now VYNE Therapeutics), Novartis, and Trevi.
FROM THE EADV CONGRESS
Limiting antibiotic therapy after surgical drainage for native joint bacterial arthritis
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Patient Handout: Safe practices during the COVID-19 pandemic
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
The state of inpatient COVID-19 care
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.
At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
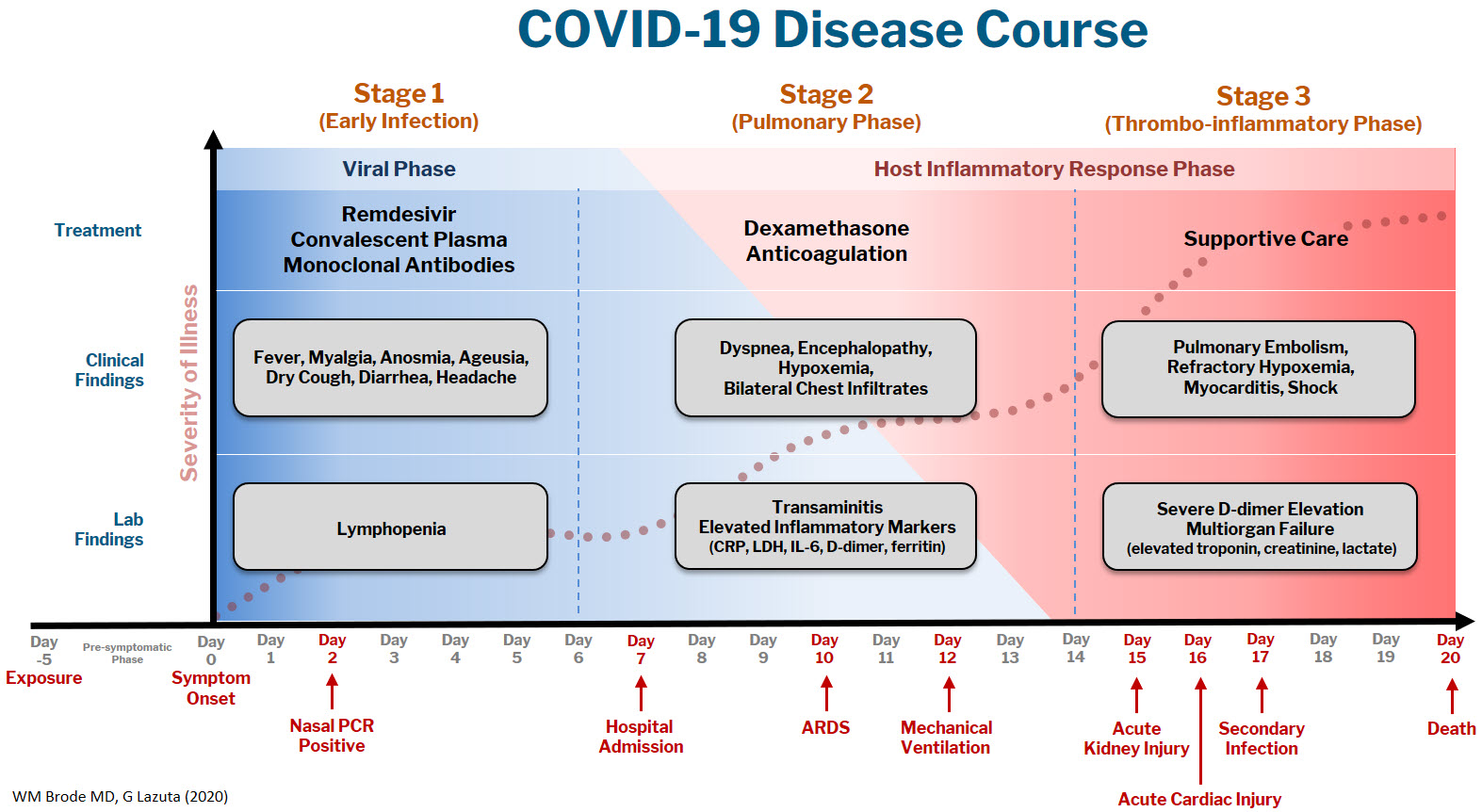
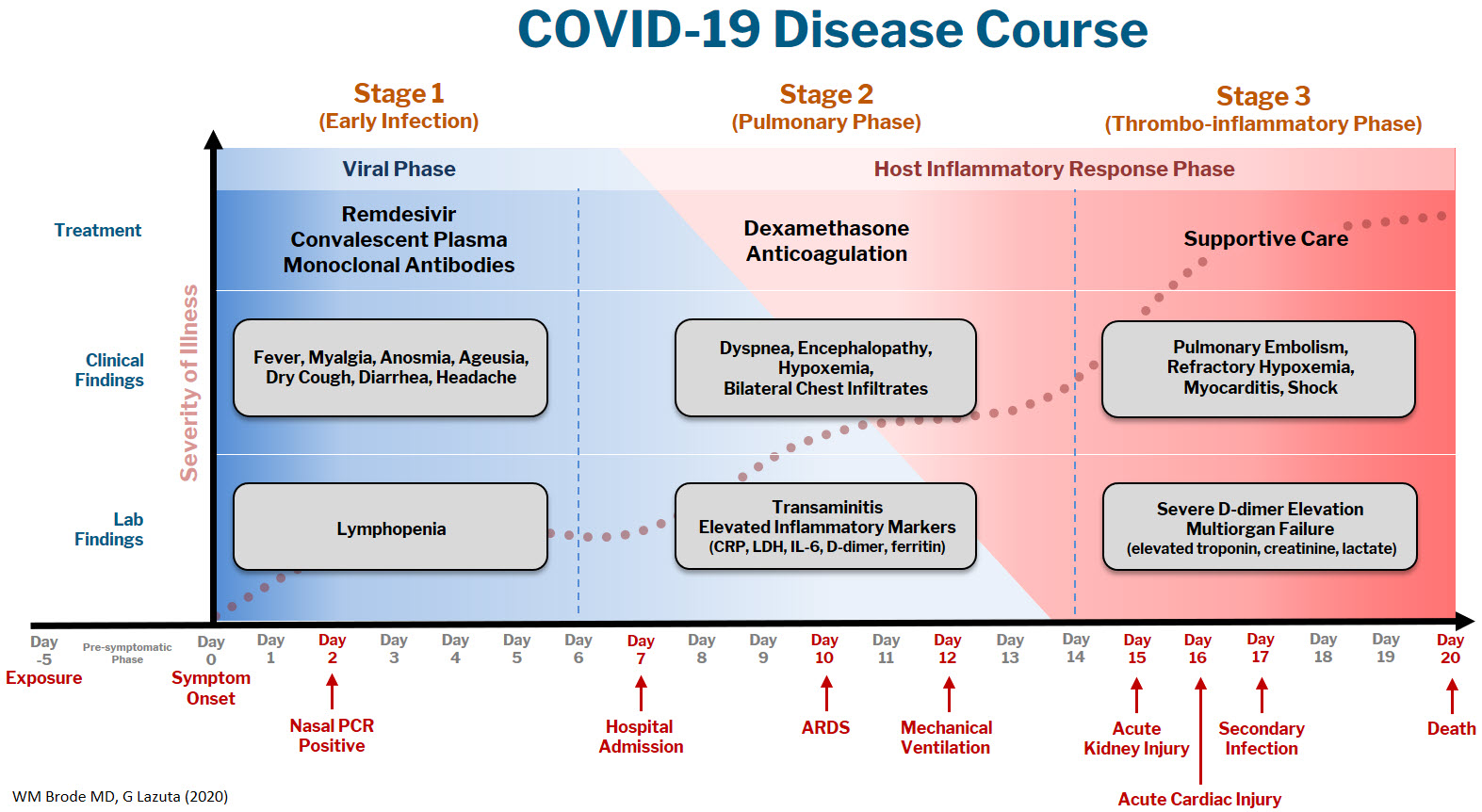
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
A brief evidence-based review of everything we have learned
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
Tool predicts severe toxicity from chemo in older breast cancer patients
They devised and tested the tool, called the Cancer and Aging Research Group–Breast Cancer (CARG-BC) score, in two cohorts of patients aged 65 years or older with stage I-III breast cancer.
The area under the curve for predicting grade 3-5 toxicity was 0.75 in the development cohort and 0.69 in the validation cohort, for a combined AUC of 0.73.
The CARG-BC score outperformed both Karnofsky performance status (AUC, 0.50) and the Cancer and Aging Research Group Chemotherapy Toxicity Tool (AUC, 0.56).
CARG-BC risk groups were also associated with hospitalizations, dose modifications, and early termination of treatment.
Allison Magnuson, DO, of the University of Rochester (N.Y.), and colleagues described these results in the Journal of Clinical Oncology.
About CARG-BC
To calculate a patient’s CARG-BC score, the researchers added up points assigned to eight independent predictors of grade 3-5 chemotherapy toxicity:
- Planned anthracycline use (1 point)
- Stage II or III disease (3 points)
- Planned treatment duration longer than 3 months (4 points)
- Abnormal liver function (3 points)
- Low hemoglobin level (3 points)
- A fall in the previous 6 months (4 points)
- Limited ability to walk more than 1 mile (3 points)
- Lack of social support (3 points)
Patients with scores of 0-5 have a low risk, those with scores of 6-11 have an intermediate risk, and those with scores of 12 or above have a high risk of grade 3-5 toxicity.
Patient characteristics and results
There were 283 patients in the development cohort and 190 in the validation cohort. There were no significant demographic, disease, or treatment differences between the cohorts.
All patients had a mean age of 70.5 years, 36.2% had stage I disease, 42.9% had stage II, and 20.9% had stage III disease. Three-quarters of patients were non-Hispanic White, and 99.4% were women. Roughly a third of patients had received an anthracycline-based regimen.
Overall, about a quarter of patients had an unplanned dose reduction (24%), dose delay (26%), stopped treatment early (24%), or were hospitalized during treatment (23%). All of these occurrences were more likely in intermediate- and high-risk patients versus low-risk patients (P < .001).
In the development cohort, 19% of low-risk patients, 54% of intermediate-risk patients, and 87% of high-risk patients developed grade 3-5 chemotherapy toxicity.
Compared with the 93 patients in the low-risk group, the odds of toxicity was almost 5 times greater for the 159 intermediate-risk subjects, and 28 times greater for the 30 high-risk subjects.
In the validation cohort, grade 3-5 toxicity rates were 27% in the low-risk group, 45% in the intermediate-risk group, and 76% in the high-risk group.
This study had its limitations, including that a majority of subjects (72.2%) had a college education, and the validation cohort was accrued from the same 16 institutions as the development cohort.
“Further validation in a more diverse population should be considered,” the investigators wrote.
A ‘useful’ tool for guiding therapy
The investigators noted that chemotherapy is a complex decision for older adults with stage I-III breast cancer. While it may be indicated, chemotherapy is underused often because of the higher risk of severe toxicity in older people.
“Unfortunately, older adults aged 65 and over, who comprise about half of all breast cancer diagnoses, are significantly less likely to be offered chemotherapy compared to younger patients – sometimes because their doctors fear they won’t be able to tolerate it,” investigator Mina Sedrak, MD, of City of Hope National Medical Center in Duarte, Calif., said in a press release.
The CARG-BC score may be useful to direct therapy in older adults with early-stage breast cancer, the investigators wrote. They noted that intensifying supportive care and developing modified treatment regimens may be appropriate for patients at higher risk for toxicity.
“Although this score should not be used as the only factor in deciding whether to administer and/or alter the dose or schedule of chemotherapy, the CARG-BC score can be used to facilitate this complex decision-making process, along with clinical judgment and patient preferences,” they wrote.
“I think this is a great tool. [It] will be helpful to me to have a more accurate conversation with geriatric patients about the actual risk/benefit ratio for chemotherapy in early breast cancer,” said Amy Tiersten, MD, of Mount Sinai Hospital in New York, when asked for comment.
“If routinely implemented, it may help reduce age bias and also identify older patients who may look well but may be vulnerable and quickly decompensate while undergoing treatment,” said Lidia Schapira, MD, of Stanford (Calif.) University. “Importantly, it can be used to guide conversations about trade-offs and to start a frank conversation about an older patient’s fears and concerns about treatment.”
This research was funded by the National Institute on Aging, the Breast Cancer Research Foundation, and the Center for Cancer and Aging at City of Hope. The investigators, Dr. Schapira, and Dr. Tiersten had no relevant disclosures.
They devised and tested the tool, called the Cancer and Aging Research Group–Breast Cancer (CARG-BC) score, in two cohorts of patients aged 65 years or older with stage I-III breast cancer.
The area under the curve for predicting grade 3-5 toxicity was 0.75 in the development cohort and 0.69 in the validation cohort, for a combined AUC of 0.73.
The CARG-BC score outperformed both Karnofsky performance status (AUC, 0.50) and the Cancer and Aging Research Group Chemotherapy Toxicity Tool (AUC, 0.56).
CARG-BC risk groups were also associated with hospitalizations, dose modifications, and early termination of treatment.
Allison Magnuson, DO, of the University of Rochester (N.Y.), and colleagues described these results in the Journal of Clinical Oncology.
About CARG-BC
To calculate a patient’s CARG-BC score, the researchers added up points assigned to eight independent predictors of grade 3-5 chemotherapy toxicity:
- Planned anthracycline use (1 point)
- Stage II or III disease (3 points)
- Planned treatment duration longer than 3 months (4 points)
- Abnormal liver function (3 points)
- Low hemoglobin level (3 points)
- A fall in the previous 6 months (4 points)
- Limited ability to walk more than 1 mile (3 points)
- Lack of social support (3 points)
Patients with scores of 0-5 have a low risk, those with scores of 6-11 have an intermediate risk, and those with scores of 12 or above have a high risk of grade 3-5 toxicity.
Patient characteristics and results
There were 283 patients in the development cohort and 190 in the validation cohort. There were no significant demographic, disease, or treatment differences between the cohorts.
All patients had a mean age of 70.5 years, 36.2% had stage I disease, 42.9% had stage II, and 20.9% had stage III disease. Three-quarters of patients were non-Hispanic White, and 99.4% were women. Roughly a third of patients had received an anthracycline-based regimen.
Overall, about a quarter of patients had an unplanned dose reduction (24%), dose delay (26%), stopped treatment early (24%), or were hospitalized during treatment (23%). All of these occurrences were more likely in intermediate- and high-risk patients versus low-risk patients (P < .001).
In the development cohort, 19% of low-risk patients, 54% of intermediate-risk patients, and 87% of high-risk patients developed grade 3-5 chemotherapy toxicity.
Compared with the 93 patients in the low-risk group, the odds of toxicity was almost 5 times greater for the 159 intermediate-risk subjects, and 28 times greater for the 30 high-risk subjects.
In the validation cohort, grade 3-5 toxicity rates were 27% in the low-risk group, 45% in the intermediate-risk group, and 76% in the high-risk group.
This study had its limitations, including that a majority of subjects (72.2%) had a college education, and the validation cohort was accrued from the same 16 institutions as the development cohort.
“Further validation in a more diverse population should be considered,” the investigators wrote.
A ‘useful’ tool for guiding therapy
The investigators noted that chemotherapy is a complex decision for older adults with stage I-III breast cancer. While it may be indicated, chemotherapy is underused often because of the higher risk of severe toxicity in older people.
“Unfortunately, older adults aged 65 and over, who comprise about half of all breast cancer diagnoses, are significantly less likely to be offered chemotherapy compared to younger patients – sometimes because their doctors fear they won’t be able to tolerate it,” investigator Mina Sedrak, MD, of City of Hope National Medical Center in Duarte, Calif., said in a press release.
The CARG-BC score may be useful to direct therapy in older adults with early-stage breast cancer, the investigators wrote. They noted that intensifying supportive care and developing modified treatment regimens may be appropriate for patients at higher risk for toxicity.
“Although this score should not be used as the only factor in deciding whether to administer and/or alter the dose or schedule of chemotherapy, the CARG-BC score can be used to facilitate this complex decision-making process, along with clinical judgment and patient preferences,” they wrote.
“I think this is a great tool. [It] will be helpful to me to have a more accurate conversation with geriatric patients about the actual risk/benefit ratio for chemotherapy in early breast cancer,” said Amy Tiersten, MD, of Mount Sinai Hospital in New York, when asked for comment.
“If routinely implemented, it may help reduce age bias and also identify older patients who may look well but may be vulnerable and quickly decompensate while undergoing treatment,” said Lidia Schapira, MD, of Stanford (Calif.) University. “Importantly, it can be used to guide conversations about trade-offs and to start a frank conversation about an older patient’s fears and concerns about treatment.”
This research was funded by the National Institute on Aging, the Breast Cancer Research Foundation, and the Center for Cancer and Aging at City of Hope. The investigators, Dr. Schapira, and Dr. Tiersten had no relevant disclosures.
They devised and tested the tool, called the Cancer and Aging Research Group–Breast Cancer (CARG-BC) score, in two cohorts of patients aged 65 years or older with stage I-III breast cancer.
The area under the curve for predicting grade 3-5 toxicity was 0.75 in the development cohort and 0.69 in the validation cohort, for a combined AUC of 0.73.
The CARG-BC score outperformed both Karnofsky performance status (AUC, 0.50) and the Cancer and Aging Research Group Chemotherapy Toxicity Tool (AUC, 0.56).
CARG-BC risk groups were also associated with hospitalizations, dose modifications, and early termination of treatment.
Allison Magnuson, DO, of the University of Rochester (N.Y.), and colleagues described these results in the Journal of Clinical Oncology.
About CARG-BC
To calculate a patient’s CARG-BC score, the researchers added up points assigned to eight independent predictors of grade 3-5 chemotherapy toxicity:
- Planned anthracycline use (1 point)
- Stage II or III disease (3 points)
- Planned treatment duration longer than 3 months (4 points)
- Abnormal liver function (3 points)
- Low hemoglobin level (3 points)
- A fall in the previous 6 months (4 points)
- Limited ability to walk more than 1 mile (3 points)
- Lack of social support (3 points)
Patients with scores of 0-5 have a low risk, those with scores of 6-11 have an intermediate risk, and those with scores of 12 or above have a high risk of grade 3-5 toxicity.
Patient characteristics and results
There were 283 patients in the development cohort and 190 in the validation cohort. There were no significant demographic, disease, or treatment differences between the cohorts.
All patients had a mean age of 70.5 years, 36.2% had stage I disease, 42.9% had stage II, and 20.9% had stage III disease. Three-quarters of patients were non-Hispanic White, and 99.4% were women. Roughly a third of patients had received an anthracycline-based regimen.
Overall, about a quarter of patients had an unplanned dose reduction (24%), dose delay (26%), stopped treatment early (24%), or were hospitalized during treatment (23%). All of these occurrences were more likely in intermediate- and high-risk patients versus low-risk patients (P < .001).
In the development cohort, 19% of low-risk patients, 54% of intermediate-risk patients, and 87% of high-risk patients developed grade 3-5 chemotherapy toxicity.
Compared with the 93 patients in the low-risk group, the odds of toxicity was almost 5 times greater for the 159 intermediate-risk subjects, and 28 times greater for the 30 high-risk subjects.
In the validation cohort, grade 3-5 toxicity rates were 27% in the low-risk group, 45% in the intermediate-risk group, and 76% in the high-risk group.
This study had its limitations, including that a majority of subjects (72.2%) had a college education, and the validation cohort was accrued from the same 16 institutions as the development cohort.
“Further validation in a more diverse population should be considered,” the investigators wrote.
A ‘useful’ tool for guiding therapy
The investigators noted that chemotherapy is a complex decision for older adults with stage I-III breast cancer. While it may be indicated, chemotherapy is underused often because of the higher risk of severe toxicity in older people.
“Unfortunately, older adults aged 65 and over, who comprise about half of all breast cancer diagnoses, are significantly less likely to be offered chemotherapy compared to younger patients – sometimes because their doctors fear they won’t be able to tolerate it,” investigator Mina Sedrak, MD, of City of Hope National Medical Center in Duarte, Calif., said in a press release.
The CARG-BC score may be useful to direct therapy in older adults with early-stage breast cancer, the investigators wrote. They noted that intensifying supportive care and developing modified treatment regimens may be appropriate for patients at higher risk for toxicity.
“Although this score should not be used as the only factor in deciding whether to administer and/or alter the dose or schedule of chemotherapy, the CARG-BC score can be used to facilitate this complex decision-making process, along with clinical judgment and patient preferences,” they wrote.
“I think this is a great tool. [It] will be helpful to me to have a more accurate conversation with geriatric patients about the actual risk/benefit ratio for chemotherapy in early breast cancer,” said Amy Tiersten, MD, of Mount Sinai Hospital in New York, when asked for comment.
“If routinely implemented, it may help reduce age bias and also identify older patients who may look well but may be vulnerable and quickly decompensate while undergoing treatment,” said Lidia Schapira, MD, of Stanford (Calif.) University. “Importantly, it can be used to guide conversations about trade-offs and to start a frank conversation about an older patient’s fears and concerns about treatment.”
This research was funded by the National Institute on Aging, the Breast Cancer Research Foundation, and the Center for Cancer and Aging at City of Hope. The investigators, Dr. Schapira, and Dr. Tiersten had no relevant disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Low-carb diets boost diabetes remission rates, at least short term
Patients with type 2 diabetes who follow a low-carbohydrate diet (LCD) for at least 6 months appear to have significantly higher remission rates than those following other diets, although the benefits diminish by 12 months, suggests a new analysis of trial data from over 1,300 individuals.
“Based on other evidence, it is likely the degree of weight loss would have been a contributing factor, combined with the lower intake of dietary carbohydrates,” study coauthor Grant D. Brinkworth, PhD, Commonwealth Scientific and Industrial Research Organisation, Sydney, , said in an interview.
He acknowledged, however, that “diets in general can be difficult to sustain over the long term. ... We need to provide patients with easy-to-use support tools and convenient solutions to help them adhere to a low-carb diet long term to gain these greater health improvements.
“In addition, more long-term, well-controlled, randomized trials are needed to determine the effects of low-carb diets on sustained weight loss, diabetes remission, and health outcomes,” Dr. Brinkworth added.
The research was published on Janu. 13 in the BMJ by a consortium of international scientists, led by Joshua Z. Goldenberg, PhD, department of nutrition, Texas A&M University, College Station.
Confusion as to best diet for those with diabetes
Type 2 diabetes is a “significant and worsening” worldwide health problem, wrote Dr. Goldenberg and coauthors, in spite of “many pharmaceutical developments and a global emphasis on glycemic control.”
Although structured diets are “recognized as an essential component of treating diabetes, confusion remains about which diet to choose,” with multiple systemic reviews and meta-analyses of carbohydrate-restricted diets “reporting mixed results,” they noted.
They therefore undertook a systematic review of randomized, controlled trials on the efficacy and safety of LCDs and very-low-carbohydrate diets (VLCDs) using the CENTRAL, Medline, CINAHL, and CAB databases, as well as other literature sources.
Researchers defined LCDs as less than 130 g/day of carbohydrates or less than 26% of calories from carbohydrates as part of a 2,000 kcal/day diet and VLCDs as less than 50 g/day or less than 10% of daily calories. They focused on interventions that lasted at least 12 weeks in adults with type 2 diabetes.
Overall, 23 trials involving 1,357 participants met the inclusion criteria; 52% used VLCDs and the control comparator was a low-fat diet in 78% of the studies. The mean age range of patients was 47-67 years, and treatment duration spanned from 3 months to 2 years.
LCDs were associated with a higher rate of diabetes remission when defined as a hemoglobin A1c level of less than 6.5%, compared with control diets at 6 months, at 57% versus 31% – an increase in remission of 32% associated with LCDs (P < .001 for overall effect).
But when defined more tightly as an A1c level of less than 6.5% in the absence of diabetes medications, remission with LCDs was reduced to a nonsignificant 5% versus control diets at 6 months.
At 12 months, data on remission were sparse, ranging from a small effect to a trivial increased risk of diabetes.
Subgroup analysis demonstrated that patients on an LCD achieved greater weight loss at 6 months than those on a control diet, at a mean reduction of 3.46 kg (approximately 7.6 lb). However, the researchers noted that, at 12 months, any weight-loss benefit was “trivial and nonsignificant.”
A similar pattern was seen for reductions in A1c and fasting glucose levels with LCDs: Notable reductions at 6 months largely disappeared by 12 months.
LCDs were also associated with “greater reductions in diabetes medication and clinically important benefits” in triglycerides and insulin resistance at 6 and 12 months, the team wrote.
VLCDs: Adherence Is key
Finally, the team looked at weight loss achieved with VLCDs.
VLCDs were less effective for weight loss at 6 months than less restrictive LCDs. However, this effect was explained by diet adherence, the researchers noted.
Restricting the analysis to “credible” studies, VLCDs were associated with a larger “clinically important” weight-loss versus control diets when patients were highly adherent to the diet, at a mean reduction of 4.47 kg (9.9 lb) versus a mean increase of 0.55 kg (1.2 lb) among patients who were less adherent.
The team noted that their review has a number of limitations, not least of which is the definition of diabetes remission used, which “is the subject of considerable debate,” as well as the safety concerns raised over LCDs.
Given the latter concerns, “clinicians might consider short-term LCDs for management of type 2 diabetes, while actively monitoring and adjusting diabetes medication as needed,” they concluded.
This study was funded in part by Texas A&M University. One coauthor reported receiving funding from Texas A&M AgriLife Research for a separate research project. Dr. Brinkworth is author of the book “The CSIRO Low Carb Diet,” but does not receive financial royalties or funds either directly or indirectly.
A version of this article first appeared on Medscape.com.
Patients with type 2 diabetes who follow a low-carbohydrate diet (LCD) for at least 6 months appear to have significantly higher remission rates than those following other diets, although the benefits diminish by 12 months, suggests a new analysis of trial data from over 1,300 individuals.
“Based on other evidence, it is likely the degree of weight loss would have been a contributing factor, combined with the lower intake of dietary carbohydrates,” study coauthor Grant D. Brinkworth, PhD, Commonwealth Scientific and Industrial Research Organisation, Sydney, , said in an interview.
He acknowledged, however, that “diets in general can be difficult to sustain over the long term. ... We need to provide patients with easy-to-use support tools and convenient solutions to help them adhere to a low-carb diet long term to gain these greater health improvements.
“In addition, more long-term, well-controlled, randomized trials are needed to determine the effects of low-carb diets on sustained weight loss, diabetes remission, and health outcomes,” Dr. Brinkworth added.
The research was published on Janu. 13 in the BMJ by a consortium of international scientists, led by Joshua Z. Goldenberg, PhD, department of nutrition, Texas A&M University, College Station.
Confusion as to best diet for those with diabetes
Type 2 diabetes is a “significant and worsening” worldwide health problem, wrote Dr. Goldenberg and coauthors, in spite of “many pharmaceutical developments and a global emphasis on glycemic control.”
Although structured diets are “recognized as an essential component of treating diabetes, confusion remains about which diet to choose,” with multiple systemic reviews and meta-analyses of carbohydrate-restricted diets “reporting mixed results,” they noted.
They therefore undertook a systematic review of randomized, controlled trials on the efficacy and safety of LCDs and very-low-carbohydrate diets (VLCDs) using the CENTRAL, Medline, CINAHL, and CAB databases, as well as other literature sources.
Researchers defined LCDs as less than 130 g/day of carbohydrates or less than 26% of calories from carbohydrates as part of a 2,000 kcal/day diet and VLCDs as less than 50 g/day or less than 10% of daily calories. They focused on interventions that lasted at least 12 weeks in adults with type 2 diabetes.
Overall, 23 trials involving 1,357 participants met the inclusion criteria; 52% used VLCDs and the control comparator was a low-fat diet in 78% of the studies. The mean age range of patients was 47-67 years, and treatment duration spanned from 3 months to 2 years.
LCDs were associated with a higher rate of diabetes remission when defined as a hemoglobin A1c level of less than 6.5%, compared with control diets at 6 months, at 57% versus 31% – an increase in remission of 32% associated with LCDs (P < .001 for overall effect).
But when defined more tightly as an A1c level of less than 6.5% in the absence of diabetes medications, remission with LCDs was reduced to a nonsignificant 5% versus control diets at 6 months.
At 12 months, data on remission were sparse, ranging from a small effect to a trivial increased risk of diabetes.
Subgroup analysis demonstrated that patients on an LCD achieved greater weight loss at 6 months than those on a control diet, at a mean reduction of 3.46 kg (approximately 7.6 lb). However, the researchers noted that, at 12 months, any weight-loss benefit was “trivial and nonsignificant.”
A similar pattern was seen for reductions in A1c and fasting glucose levels with LCDs: Notable reductions at 6 months largely disappeared by 12 months.
LCDs were also associated with “greater reductions in diabetes medication and clinically important benefits” in triglycerides and insulin resistance at 6 and 12 months, the team wrote.
VLCDs: Adherence Is key
Finally, the team looked at weight loss achieved with VLCDs.
VLCDs were less effective for weight loss at 6 months than less restrictive LCDs. However, this effect was explained by diet adherence, the researchers noted.
Restricting the analysis to “credible” studies, VLCDs were associated with a larger “clinically important” weight-loss versus control diets when patients were highly adherent to the diet, at a mean reduction of 4.47 kg (9.9 lb) versus a mean increase of 0.55 kg (1.2 lb) among patients who were less adherent.
The team noted that their review has a number of limitations, not least of which is the definition of diabetes remission used, which “is the subject of considerable debate,” as well as the safety concerns raised over LCDs.
Given the latter concerns, “clinicians might consider short-term LCDs for management of type 2 diabetes, while actively monitoring and adjusting diabetes medication as needed,” they concluded.
This study was funded in part by Texas A&M University. One coauthor reported receiving funding from Texas A&M AgriLife Research for a separate research project. Dr. Brinkworth is author of the book “The CSIRO Low Carb Diet,” but does not receive financial royalties or funds either directly or indirectly.
A version of this article first appeared on Medscape.com.
Patients with type 2 diabetes who follow a low-carbohydrate diet (LCD) for at least 6 months appear to have significantly higher remission rates than those following other diets, although the benefits diminish by 12 months, suggests a new analysis of trial data from over 1,300 individuals.
“Based on other evidence, it is likely the degree of weight loss would have been a contributing factor, combined with the lower intake of dietary carbohydrates,” study coauthor Grant D. Brinkworth, PhD, Commonwealth Scientific and Industrial Research Organisation, Sydney, , said in an interview.
He acknowledged, however, that “diets in general can be difficult to sustain over the long term. ... We need to provide patients with easy-to-use support tools and convenient solutions to help them adhere to a low-carb diet long term to gain these greater health improvements.
“In addition, more long-term, well-controlled, randomized trials are needed to determine the effects of low-carb diets on sustained weight loss, diabetes remission, and health outcomes,” Dr. Brinkworth added.
The research was published on Janu. 13 in the BMJ by a consortium of international scientists, led by Joshua Z. Goldenberg, PhD, department of nutrition, Texas A&M University, College Station.
Confusion as to best diet for those with diabetes
Type 2 diabetes is a “significant and worsening” worldwide health problem, wrote Dr. Goldenberg and coauthors, in spite of “many pharmaceutical developments and a global emphasis on glycemic control.”
Although structured diets are “recognized as an essential component of treating diabetes, confusion remains about which diet to choose,” with multiple systemic reviews and meta-analyses of carbohydrate-restricted diets “reporting mixed results,” they noted.
They therefore undertook a systematic review of randomized, controlled trials on the efficacy and safety of LCDs and very-low-carbohydrate diets (VLCDs) using the CENTRAL, Medline, CINAHL, and CAB databases, as well as other literature sources.
Researchers defined LCDs as less than 130 g/day of carbohydrates or less than 26% of calories from carbohydrates as part of a 2,000 kcal/day diet and VLCDs as less than 50 g/day or less than 10% of daily calories. They focused on interventions that lasted at least 12 weeks in adults with type 2 diabetes.
Overall, 23 trials involving 1,357 participants met the inclusion criteria; 52% used VLCDs and the control comparator was a low-fat diet in 78% of the studies. The mean age range of patients was 47-67 years, and treatment duration spanned from 3 months to 2 years.
LCDs were associated with a higher rate of diabetes remission when defined as a hemoglobin A1c level of less than 6.5%, compared with control diets at 6 months, at 57% versus 31% – an increase in remission of 32% associated with LCDs (P < .001 for overall effect).
But when defined more tightly as an A1c level of less than 6.5% in the absence of diabetes medications, remission with LCDs was reduced to a nonsignificant 5% versus control diets at 6 months.
At 12 months, data on remission were sparse, ranging from a small effect to a trivial increased risk of diabetes.
Subgroup analysis demonstrated that patients on an LCD achieved greater weight loss at 6 months than those on a control diet, at a mean reduction of 3.46 kg (approximately 7.6 lb). However, the researchers noted that, at 12 months, any weight-loss benefit was “trivial and nonsignificant.”
A similar pattern was seen for reductions in A1c and fasting glucose levels with LCDs: Notable reductions at 6 months largely disappeared by 12 months.
LCDs were also associated with “greater reductions in diabetes medication and clinically important benefits” in triglycerides and insulin resistance at 6 and 12 months, the team wrote.
VLCDs: Adherence Is key
Finally, the team looked at weight loss achieved with VLCDs.
VLCDs were less effective for weight loss at 6 months than less restrictive LCDs. However, this effect was explained by diet adherence, the researchers noted.
Restricting the analysis to “credible” studies, VLCDs were associated with a larger “clinically important” weight-loss versus control diets when patients were highly adherent to the diet, at a mean reduction of 4.47 kg (9.9 lb) versus a mean increase of 0.55 kg (1.2 lb) among patients who were less adherent.
The team noted that their review has a number of limitations, not least of which is the definition of diabetes remission used, which “is the subject of considerable debate,” as well as the safety concerns raised over LCDs.
Given the latter concerns, “clinicians might consider short-term LCDs for management of type 2 diabetes, while actively monitoring and adjusting diabetes medication as needed,” they concluded.
This study was funded in part by Texas A&M University. One coauthor reported receiving funding from Texas A&M AgriLife Research for a separate research project. Dr. Brinkworth is author of the book “The CSIRO Low Carb Diet,” but does not receive financial royalties or funds either directly or indirectly.
A version of this article first appeared on Medscape.com.
President Biden kicks off health agenda with COVID actions, WHO outreach
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
APA apologizes for past support of racism in psychiatry
The American Psychiatric Association has issued a formal apology for its past support of structural racism in psychiatry.
The apology, issued Jan. 18, coincided with the federal holiday honoring the life and work of civil rights activist Dr. Martin Luther King Jr.
“We apologize for our role in perpetrating structural racism in this country, and we hope to begin to make amends for APA’s and psychiatry’s history of actions, intentional and not, that hurt Black, indigenous, and people of color,” APA President Jeffrey Geller, MD, MPH, said in a statement.
The apology was written and issued by the APA Board of Trustees. It acknowledges practices and events in psychiatry that contributed to racial inequality, and expresses the organization’s commitment to developing antiracist policies that promote equity in mental health for all.
“This apology is one important step we needed to take to move forward to a more equitable future. The board is issuing this document on Martin Luther King Jr. Day, because we hope that it honors his life’s work of reconciliation and equality. We do not take that legacy or his call to action lightly and will continue our important work,” said Dr. Geller.
One involved the Eastern State Hospital in Williamsburg, Va., the nation’s first psychiatric care facility, founded in 1773.
Eastern State, which for a time in the 1800s was called the Eastern Lunatic Asylum, was not segregated when founded. However, 70 years later, when the 13 founders of what is now the APA met to discuss improvements in mental health care delivery, the treatment system they created and the organization they founded aligned with that era’s racist social and political policies. In this system, Black patients received psychiatric care separately from White patients, the APA said.
The APA also acknowledged failing to act in Black Americans’ best interest at critical points in the United States’ sociopolitical evolution throughout the 19th and 20th centuries.
“This inactivity was notably evident while white supremacists lynched Black people during the Reconstruction Era as well as when Jim Crow segregation was in effect, which led to ‘separate but equal’ standards of care starting in 1896,” the APA said.
Later, the APA failed to declare support for Brown v. Board of Education of Topeka in 1954, along with further major civil rights legislation designed to improve social and psychological conditions for Black people, the organization admitted.
Throughout the decades that followed, psychiatric misdiagnosis among Black, indigenous, and people of color populations were also common, the APA acknowledged.
For example, late 20th century psychiatrists commonly attributed their minority patients’ frustrations to schizophrenia, while categorizing similar behaviors as “neuroticism” in White patients.
The APA pointed to one study which found that APA members diagnosed more Black than White patients with schizophrenia, even when both had otherwise identical clinical presentations.
“This reveals the basis for embedded discrimination within psychiatry that has contributed to reduced quality of care” for Black, indigenous, and people of color, and “perpetuation of dangerous stereotypes,” the APA said.
Saul Levin, MD, the APA’s medical director and CEO, said the Board of Trustees has taken “an important step in issuing this apology. The APA administration is committed to working toward inclusion, health equity, and fairness that everyone deserves.”
The APA Board of Trustees began drafting the apology late last year after it concluded that events and persistent inequities in health care and psychiatry had highlighted an organizational need for action.
The APA’s Presidential Task Force on Structural Racism is continuing with efforts to educate and engage members on the issue and implement changes within the organization.
A version of this article first appeared on Medscape.com.
The American Psychiatric Association has issued a formal apology for its past support of structural racism in psychiatry.
The apology, issued Jan. 18, coincided with the federal holiday honoring the life and work of civil rights activist Dr. Martin Luther King Jr.
“We apologize for our role in perpetrating structural racism in this country, and we hope to begin to make amends for APA’s and psychiatry’s history of actions, intentional and not, that hurt Black, indigenous, and people of color,” APA President Jeffrey Geller, MD, MPH, said in a statement.
The apology was written and issued by the APA Board of Trustees. It acknowledges practices and events in psychiatry that contributed to racial inequality, and expresses the organization’s commitment to developing antiracist policies that promote equity in mental health for all.
“This apology is one important step we needed to take to move forward to a more equitable future. The board is issuing this document on Martin Luther King Jr. Day, because we hope that it honors his life’s work of reconciliation and equality. We do not take that legacy or his call to action lightly and will continue our important work,” said Dr. Geller.
One involved the Eastern State Hospital in Williamsburg, Va., the nation’s first psychiatric care facility, founded in 1773.
Eastern State, which for a time in the 1800s was called the Eastern Lunatic Asylum, was not segregated when founded. However, 70 years later, when the 13 founders of what is now the APA met to discuss improvements in mental health care delivery, the treatment system they created and the organization they founded aligned with that era’s racist social and political policies. In this system, Black patients received psychiatric care separately from White patients, the APA said.
The APA also acknowledged failing to act in Black Americans’ best interest at critical points in the United States’ sociopolitical evolution throughout the 19th and 20th centuries.
“This inactivity was notably evident while white supremacists lynched Black people during the Reconstruction Era as well as when Jim Crow segregation was in effect, which led to ‘separate but equal’ standards of care starting in 1896,” the APA said.
Later, the APA failed to declare support for Brown v. Board of Education of Topeka in 1954, along with further major civil rights legislation designed to improve social and psychological conditions for Black people, the organization admitted.
Throughout the decades that followed, psychiatric misdiagnosis among Black, indigenous, and people of color populations were also common, the APA acknowledged.
For example, late 20th century psychiatrists commonly attributed their minority patients’ frustrations to schizophrenia, while categorizing similar behaviors as “neuroticism” in White patients.
The APA pointed to one study which found that APA members diagnosed more Black than White patients with schizophrenia, even when both had otherwise identical clinical presentations.
“This reveals the basis for embedded discrimination within psychiatry that has contributed to reduced quality of care” for Black, indigenous, and people of color, and “perpetuation of dangerous stereotypes,” the APA said.
Saul Levin, MD, the APA’s medical director and CEO, said the Board of Trustees has taken “an important step in issuing this apology. The APA administration is committed to working toward inclusion, health equity, and fairness that everyone deserves.”
The APA Board of Trustees began drafting the apology late last year after it concluded that events and persistent inequities in health care and psychiatry had highlighted an organizational need for action.
The APA’s Presidential Task Force on Structural Racism is continuing with efforts to educate and engage members on the issue and implement changes within the organization.
A version of this article first appeared on Medscape.com.
The American Psychiatric Association has issued a formal apology for its past support of structural racism in psychiatry.
The apology, issued Jan. 18, coincided with the federal holiday honoring the life and work of civil rights activist Dr. Martin Luther King Jr.
“We apologize for our role in perpetrating structural racism in this country, and we hope to begin to make amends for APA’s and psychiatry’s history of actions, intentional and not, that hurt Black, indigenous, and people of color,” APA President Jeffrey Geller, MD, MPH, said in a statement.
The apology was written and issued by the APA Board of Trustees. It acknowledges practices and events in psychiatry that contributed to racial inequality, and expresses the organization’s commitment to developing antiracist policies that promote equity in mental health for all.
“This apology is one important step we needed to take to move forward to a more equitable future. The board is issuing this document on Martin Luther King Jr. Day, because we hope that it honors his life’s work of reconciliation and equality. We do not take that legacy or his call to action lightly and will continue our important work,” said Dr. Geller.
One involved the Eastern State Hospital in Williamsburg, Va., the nation’s first psychiatric care facility, founded in 1773.
Eastern State, which for a time in the 1800s was called the Eastern Lunatic Asylum, was not segregated when founded. However, 70 years later, when the 13 founders of what is now the APA met to discuss improvements in mental health care delivery, the treatment system they created and the organization they founded aligned with that era’s racist social and political policies. In this system, Black patients received psychiatric care separately from White patients, the APA said.
The APA also acknowledged failing to act in Black Americans’ best interest at critical points in the United States’ sociopolitical evolution throughout the 19th and 20th centuries.
“This inactivity was notably evident while white supremacists lynched Black people during the Reconstruction Era as well as when Jim Crow segregation was in effect, which led to ‘separate but equal’ standards of care starting in 1896,” the APA said.
Later, the APA failed to declare support for Brown v. Board of Education of Topeka in 1954, along with further major civil rights legislation designed to improve social and psychological conditions for Black people, the organization admitted.
Throughout the decades that followed, psychiatric misdiagnosis among Black, indigenous, and people of color populations were also common, the APA acknowledged.
For example, late 20th century psychiatrists commonly attributed their minority patients’ frustrations to schizophrenia, while categorizing similar behaviors as “neuroticism” in White patients.
The APA pointed to one study which found that APA members diagnosed more Black than White patients with schizophrenia, even when both had otherwise identical clinical presentations.
“This reveals the basis for embedded discrimination within psychiatry that has contributed to reduced quality of care” for Black, indigenous, and people of color, and “perpetuation of dangerous stereotypes,” the APA said.
Saul Levin, MD, the APA’s medical director and CEO, said the Board of Trustees has taken “an important step in issuing this apology. The APA administration is committed to working toward inclusion, health equity, and fairness that everyone deserves.”
The APA Board of Trustees began drafting the apology late last year after it concluded that events and persistent inequities in health care and psychiatry had highlighted an organizational need for action.
The APA’s Presidential Task Force on Structural Racism is continuing with efforts to educate and engage members on the issue and implement changes within the organization.
A version of this article first appeared on Medscape.com.
Does screening for skin cancer result in melanoma overdiagnosis?
When the COVID-19 pandemic first hit, cancer screening in the United States came to an abrupt halt. That experience, coupled with the financial fallout of the pandemic, has led some doctors to reassess business as usual.
In particular, a trio has taken aim at skin cancer screening – arguing that it should stop – in a ‘sounding board’ commentary published online Jan. 7 in the New England Journal of Medicine.
“The COVID-19 pandemic has functionally stopped skin cancer screening; what is important is not to restart it,” wrote the authors, led by H. Gilbert Welch, MD, MPH, at Brigham and Women’s Hospital, Boston, Massachusetts. Dr. Welch has often raised questions about cancer screening and highlighted the issue of overdiagnosis.
In this latest essay, Dr. Welch teamed up with pathologist Benjamin Mazer, MD, Yale University, New Haven, Conn., who writes commentaries for this news organization, and dermatologist Adewole S. Adamson, MD, University of Texas, Austin, to argue that screening for skin cancer has led to an overdiagnosis of melanoma.
However, two melanoma experts pointed out flaws in some of their arguments, and said the issue is more nuanced than they present.
Arguing that melanoma is overdiagnosed
The incidence of melanoma is six times as high as it was 40 years ago, making it the third most common cancer in the United States, the investigators pointed out. However, while case rates have skyrocketed, death rates from melanoma have remained about the same, which points to overdiagnosis.
They described a cycle of increased diagnostic scrutiny that is driving overdiagnosis of melanoma. This includes heightened awareness (perhaps overly) among patients, widespread skin screenings, lower clinical thresholds for biopsy, and lower thresholds among pathologists for diagnosis of melanoma. Fear of missing cancer, legal concerns, and financial incentives may all contribute.
“We view the rise in the incidence of melanoma as a sentinel event, a warning that an epidemic of inspection, surveillance, and biopsy of pigmented skin lesions is permeating through the general population,” they wrote.
Furthermore, overdiagnosis could contribute to unnecessary intervention.
Between 2004 and 2017, rates of biopsy among fee-for-service Medicare recipients almost doubled (from 5% to 8%), according to coding trends data cited in the article. Overdiagnosis and unnecessary intervention could cause psychological, financial, and physical harm to the patient, and the authors argued for interrupting the cycle.
“The most important step to break the cycle of melanoma overdiagnosis is to stop population-wide screening for skin cancer,” they wrote.
The U.S. Preventive Services Task Force currently states that there is insufficient evidence to weigh the balances versus the harms of skin cancer screening, leaving it open to interpretation.
“[T]he increase in melanoma diagnoses by a factor of 6, with at least an order of magnitude more persons undergoing a biopsy and no apparent effect on mortality, is more than enough to recommend against population-wide screening,” Dr. Welch and colleagues concluded.
But the issue may be more nuanced, argued a melanoma expert.
“Everyone agrees that screening high-risk groups has the greatest chance of reducing cancer mortality. In melanoma, the strongest risk factor is the number of moles and presence of clinically atypical moles,” David Polsky, MD, PhD, commented in an interview. Dr. Polsky is a professor of dermatologic oncology at the Perlmutter Cancer Center at New York University Langone Health.
However, population-based studies have shown that at least half of melanoma patients are not considered high risk based on the appearance of the mole, he explained.
“Studies to identify genetic risk factors for melanoma have not yet progressed to the point where these can be tested in the clinic. We clearly have a knowledge gap that needs to be addressed,” he said.
Moreover, it’s not easy to predict which early melanomas will metastasize, said dermatologist Jennifer Stein, MD, PhD, who specializes in treating patients at high risk for melanoma at NYU Langone.
“This paper suggests that it may not be important to detect and treat melanoma in situ, and that the increase in diagnosis of melanoma in situ has led to more harms than good,” she said. “There is evidence that most melanomas do originate as in situ lesions. Unfortunately, we cannot predict which ones will become more aggressive. For this reason, we treat melanoma in situ.”
Taking issue with some of the arguments
Both Dr. Polsky and Dr. Stein took issue with several of the arguments put forward by Dr. Welch and colleagues.
For instance, Dr. Welch and colleagues cited research suggesting that UV light is a weak risk factor for melanoma, but Dr. Polsky disagreed. “There are many lines of evidence ranging from epidemiological, clinical, and biological studies that prove the causative association between ultraviolet light and melanoma, while acknowledging that other factors, such as genetic predisposition, play an important role,” he said. “Since ultraviolet light in the form of outdoor sunburns or indoor tanning exposure are modifiable risk factors, it is important that we continue with our current public messaging on their causal role in the development of melanoma.”
Furthermore, the 2012 study that the authors cited to support their argument that pathologists today are more likely to diagnose melanoma than in years past is flawed, according to Dr. Stein. The study was very small and included just nine contemporary pathologists. Unlike in real life, pathologists in the study could not diagnose lesions as “atypical,” and may have erred on the side of caution by calling them malignant.
“There were multiple limitations to this study that were acknowledged by its authors, who stated that it was a hypothesis-generating study and may not be generalizable,” Dr. Stein said.
In addition, Dr. Polsky took issue with the suggestion that awareness about melanoma among the general public is overly heightened.
“Reducing melanoma awareness would not be wise,” he said. “Studies have shown that awareness of melanoma is associated with the diagnosis of earlier-stage lesions that can be cured by simple skin surgery, without the need for more costly interventions utilized for more advanced melanomas.”
Dr. Mazer reported receiving travel compensation from Hillcrest Healthcare Systems, and is a commentator for this new organization. Dr. Welch has written three books on the subjects of overdiagnosis and testing for cancer. Dr. Adamson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When the COVID-19 pandemic first hit, cancer screening in the United States came to an abrupt halt. That experience, coupled with the financial fallout of the pandemic, has led some doctors to reassess business as usual.
In particular, a trio has taken aim at skin cancer screening – arguing that it should stop – in a ‘sounding board’ commentary published online Jan. 7 in the New England Journal of Medicine.
“The COVID-19 pandemic has functionally stopped skin cancer screening; what is important is not to restart it,” wrote the authors, led by H. Gilbert Welch, MD, MPH, at Brigham and Women’s Hospital, Boston, Massachusetts. Dr. Welch has often raised questions about cancer screening and highlighted the issue of overdiagnosis.
In this latest essay, Dr. Welch teamed up with pathologist Benjamin Mazer, MD, Yale University, New Haven, Conn., who writes commentaries for this news organization, and dermatologist Adewole S. Adamson, MD, University of Texas, Austin, to argue that screening for skin cancer has led to an overdiagnosis of melanoma.
However, two melanoma experts pointed out flaws in some of their arguments, and said the issue is more nuanced than they present.
Arguing that melanoma is overdiagnosed
The incidence of melanoma is six times as high as it was 40 years ago, making it the third most common cancer in the United States, the investigators pointed out. However, while case rates have skyrocketed, death rates from melanoma have remained about the same, which points to overdiagnosis.
They described a cycle of increased diagnostic scrutiny that is driving overdiagnosis of melanoma. This includes heightened awareness (perhaps overly) among patients, widespread skin screenings, lower clinical thresholds for biopsy, and lower thresholds among pathologists for diagnosis of melanoma. Fear of missing cancer, legal concerns, and financial incentives may all contribute.
“We view the rise in the incidence of melanoma as a sentinel event, a warning that an epidemic of inspection, surveillance, and biopsy of pigmented skin lesions is permeating through the general population,” they wrote.
Furthermore, overdiagnosis could contribute to unnecessary intervention.
Between 2004 and 2017, rates of biopsy among fee-for-service Medicare recipients almost doubled (from 5% to 8%), according to coding trends data cited in the article. Overdiagnosis and unnecessary intervention could cause psychological, financial, and physical harm to the patient, and the authors argued for interrupting the cycle.
“The most important step to break the cycle of melanoma overdiagnosis is to stop population-wide screening for skin cancer,” they wrote.
The U.S. Preventive Services Task Force currently states that there is insufficient evidence to weigh the balances versus the harms of skin cancer screening, leaving it open to interpretation.
“[T]he increase in melanoma diagnoses by a factor of 6, with at least an order of magnitude more persons undergoing a biopsy and no apparent effect on mortality, is more than enough to recommend against population-wide screening,” Dr. Welch and colleagues concluded.
But the issue may be more nuanced, argued a melanoma expert.
“Everyone agrees that screening high-risk groups has the greatest chance of reducing cancer mortality. In melanoma, the strongest risk factor is the number of moles and presence of clinically atypical moles,” David Polsky, MD, PhD, commented in an interview. Dr. Polsky is a professor of dermatologic oncology at the Perlmutter Cancer Center at New York University Langone Health.
However, population-based studies have shown that at least half of melanoma patients are not considered high risk based on the appearance of the mole, he explained.
“Studies to identify genetic risk factors for melanoma have not yet progressed to the point where these can be tested in the clinic. We clearly have a knowledge gap that needs to be addressed,” he said.
Moreover, it’s not easy to predict which early melanomas will metastasize, said dermatologist Jennifer Stein, MD, PhD, who specializes in treating patients at high risk for melanoma at NYU Langone.
“This paper suggests that it may not be important to detect and treat melanoma in situ, and that the increase in diagnosis of melanoma in situ has led to more harms than good,” she said. “There is evidence that most melanomas do originate as in situ lesions. Unfortunately, we cannot predict which ones will become more aggressive. For this reason, we treat melanoma in situ.”
Taking issue with some of the arguments
Both Dr. Polsky and Dr. Stein took issue with several of the arguments put forward by Dr. Welch and colleagues.
For instance, Dr. Welch and colleagues cited research suggesting that UV light is a weak risk factor for melanoma, but Dr. Polsky disagreed. “There are many lines of evidence ranging from epidemiological, clinical, and biological studies that prove the causative association between ultraviolet light and melanoma, while acknowledging that other factors, such as genetic predisposition, play an important role,” he said. “Since ultraviolet light in the form of outdoor sunburns or indoor tanning exposure are modifiable risk factors, it is important that we continue with our current public messaging on their causal role in the development of melanoma.”
Furthermore, the 2012 study that the authors cited to support their argument that pathologists today are more likely to diagnose melanoma than in years past is flawed, according to Dr. Stein. The study was very small and included just nine contemporary pathologists. Unlike in real life, pathologists in the study could not diagnose lesions as “atypical,” and may have erred on the side of caution by calling them malignant.
“There were multiple limitations to this study that were acknowledged by its authors, who stated that it was a hypothesis-generating study and may not be generalizable,” Dr. Stein said.
In addition, Dr. Polsky took issue with the suggestion that awareness about melanoma among the general public is overly heightened.
“Reducing melanoma awareness would not be wise,” he said. “Studies have shown that awareness of melanoma is associated with the diagnosis of earlier-stage lesions that can be cured by simple skin surgery, without the need for more costly interventions utilized for more advanced melanomas.”
Dr. Mazer reported receiving travel compensation from Hillcrest Healthcare Systems, and is a commentator for this new organization. Dr. Welch has written three books on the subjects of overdiagnosis and testing for cancer. Dr. Adamson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When the COVID-19 pandemic first hit, cancer screening in the United States came to an abrupt halt. That experience, coupled with the financial fallout of the pandemic, has led some doctors to reassess business as usual.
In particular, a trio has taken aim at skin cancer screening – arguing that it should stop – in a ‘sounding board’ commentary published online Jan. 7 in the New England Journal of Medicine.
“The COVID-19 pandemic has functionally stopped skin cancer screening; what is important is not to restart it,” wrote the authors, led by H. Gilbert Welch, MD, MPH, at Brigham and Women’s Hospital, Boston, Massachusetts. Dr. Welch has often raised questions about cancer screening and highlighted the issue of overdiagnosis.
In this latest essay, Dr. Welch teamed up with pathologist Benjamin Mazer, MD, Yale University, New Haven, Conn., who writes commentaries for this news organization, and dermatologist Adewole S. Adamson, MD, University of Texas, Austin, to argue that screening for skin cancer has led to an overdiagnosis of melanoma.
However, two melanoma experts pointed out flaws in some of their arguments, and said the issue is more nuanced than they present.
Arguing that melanoma is overdiagnosed
The incidence of melanoma is six times as high as it was 40 years ago, making it the third most common cancer in the United States, the investigators pointed out. However, while case rates have skyrocketed, death rates from melanoma have remained about the same, which points to overdiagnosis.
They described a cycle of increased diagnostic scrutiny that is driving overdiagnosis of melanoma. This includes heightened awareness (perhaps overly) among patients, widespread skin screenings, lower clinical thresholds for biopsy, and lower thresholds among pathologists for diagnosis of melanoma. Fear of missing cancer, legal concerns, and financial incentives may all contribute.
“We view the rise in the incidence of melanoma as a sentinel event, a warning that an epidemic of inspection, surveillance, and biopsy of pigmented skin lesions is permeating through the general population,” they wrote.
Furthermore, overdiagnosis could contribute to unnecessary intervention.
Between 2004 and 2017, rates of biopsy among fee-for-service Medicare recipients almost doubled (from 5% to 8%), according to coding trends data cited in the article. Overdiagnosis and unnecessary intervention could cause psychological, financial, and physical harm to the patient, and the authors argued for interrupting the cycle.
“The most important step to break the cycle of melanoma overdiagnosis is to stop population-wide screening for skin cancer,” they wrote.
The U.S. Preventive Services Task Force currently states that there is insufficient evidence to weigh the balances versus the harms of skin cancer screening, leaving it open to interpretation.
“[T]he increase in melanoma diagnoses by a factor of 6, with at least an order of magnitude more persons undergoing a biopsy and no apparent effect on mortality, is more than enough to recommend against population-wide screening,” Dr. Welch and colleagues concluded.
But the issue may be more nuanced, argued a melanoma expert.
“Everyone agrees that screening high-risk groups has the greatest chance of reducing cancer mortality. In melanoma, the strongest risk factor is the number of moles and presence of clinically atypical moles,” David Polsky, MD, PhD, commented in an interview. Dr. Polsky is a professor of dermatologic oncology at the Perlmutter Cancer Center at New York University Langone Health.
However, population-based studies have shown that at least half of melanoma patients are not considered high risk based on the appearance of the mole, he explained.
“Studies to identify genetic risk factors for melanoma have not yet progressed to the point where these can be tested in the clinic. We clearly have a knowledge gap that needs to be addressed,” he said.
Moreover, it’s not easy to predict which early melanomas will metastasize, said dermatologist Jennifer Stein, MD, PhD, who specializes in treating patients at high risk for melanoma at NYU Langone.
“This paper suggests that it may not be important to detect and treat melanoma in situ, and that the increase in diagnosis of melanoma in situ has led to more harms than good,” she said. “There is evidence that most melanomas do originate as in situ lesions. Unfortunately, we cannot predict which ones will become more aggressive. For this reason, we treat melanoma in situ.”
Taking issue with some of the arguments
Both Dr. Polsky and Dr. Stein took issue with several of the arguments put forward by Dr. Welch and colleagues.
For instance, Dr. Welch and colleagues cited research suggesting that UV light is a weak risk factor for melanoma, but Dr. Polsky disagreed. “There are many lines of evidence ranging from epidemiological, clinical, and biological studies that prove the causative association between ultraviolet light and melanoma, while acknowledging that other factors, such as genetic predisposition, play an important role,” he said. “Since ultraviolet light in the form of outdoor sunburns or indoor tanning exposure are modifiable risk factors, it is important that we continue with our current public messaging on their causal role in the development of melanoma.”
Furthermore, the 2012 study that the authors cited to support their argument that pathologists today are more likely to diagnose melanoma than in years past is flawed, according to Dr. Stein. The study was very small and included just nine contemporary pathologists. Unlike in real life, pathologists in the study could not diagnose lesions as “atypical,” and may have erred on the side of caution by calling them malignant.
“There were multiple limitations to this study that were acknowledged by its authors, who stated that it was a hypothesis-generating study and may not be generalizable,” Dr. Stein said.
In addition, Dr. Polsky took issue with the suggestion that awareness about melanoma among the general public is overly heightened.
“Reducing melanoma awareness would not be wise,” he said. “Studies have shown that awareness of melanoma is associated with the diagnosis of earlier-stage lesions that can be cured by simple skin surgery, without the need for more costly interventions utilized for more advanced melanomas.”
Dr. Mazer reported receiving travel compensation from Hillcrest Healthcare Systems, and is a commentator for this new organization. Dr. Welch has written three books on the subjects of overdiagnosis and testing for cancer. Dr. Adamson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Global Ebola vaccine stockpile established
The International Coordinating Group (ICG) on Vaccine Provision announced the establishment of a global Ebola vaccine stockpile initiative.
The ICG, which was established in 1997, is made up of the World Health Organization, the United Nations Children’s Fund, the International Federation of Red Cross and Red Crescent Societies, and Médecins Sans Frontières.
The stockpile was created in order to make the single-dose Ebola vaccine (rVSV∆G-ZEBOV-GP, live; trade name Everbo) rapidly available at the start of the next Ebola outbreak anywhere in the world. The vaccine was developed and is marketed by Merck Sharp & Dohme, with financial support from the United States.
The stockpile, which is maintained in Switzerland and managed by UNICEF, is designed to be readily deployed to other countries whenever there is an outbreak. The ICG will be the decision-making body for the vaccine’s allocation and release, as is also the case with previously created stockpiles of cholera, meningitis, and yellow fever vaccines.
“The decision to allocate the vaccine will be made within 48 hours of receiving a request from a country; vaccines will be made available together with ultra-cold chain packaging by the manufacturer for shipment to countries within 48 hours of the decision. The targeted overall delivery time from the stockpile to countries is 7 days,” according to the WHO press release.
Currently 6,890 doses are available for outbreak response, with further quantities to be delivered into the stockpile throughout 2021 and beyond. Initial use of the vaccine will be directed to health care and frontline workers. It is expected that it will take 2-3 years to reach the Strategic Advisory Group of Experts on Immunization–recommended level of 500,000 doses for the stockpile of Ebola vaccines.
The International Coordinating Group (ICG) on Vaccine Provision announced the establishment of a global Ebola vaccine stockpile initiative.
The ICG, which was established in 1997, is made up of the World Health Organization, the United Nations Children’s Fund, the International Federation of Red Cross and Red Crescent Societies, and Médecins Sans Frontières.
The stockpile was created in order to make the single-dose Ebola vaccine (rVSV∆G-ZEBOV-GP, live; trade name Everbo) rapidly available at the start of the next Ebola outbreak anywhere in the world. The vaccine was developed and is marketed by Merck Sharp & Dohme, with financial support from the United States.
The stockpile, which is maintained in Switzerland and managed by UNICEF, is designed to be readily deployed to other countries whenever there is an outbreak. The ICG will be the decision-making body for the vaccine’s allocation and release, as is also the case with previously created stockpiles of cholera, meningitis, and yellow fever vaccines.
“The decision to allocate the vaccine will be made within 48 hours of receiving a request from a country; vaccines will be made available together with ultra-cold chain packaging by the manufacturer for shipment to countries within 48 hours of the decision. The targeted overall delivery time from the stockpile to countries is 7 days,” according to the WHO press release.
Currently 6,890 doses are available for outbreak response, with further quantities to be delivered into the stockpile throughout 2021 and beyond. Initial use of the vaccine will be directed to health care and frontline workers. It is expected that it will take 2-3 years to reach the Strategic Advisory Group of Experts on Immunization–recommended level of 500,000 doses for the stockpile of Ebola vaccines.
The International Coordinating Group (ICG) on Vaccine Provision announced the establishment of a global Ebola vaccine stockpile initiative.
The ICG, which was established in 1997, is made up of the World Health Organization, the United Nations Children’s Fund, the International Federation of Red Cross and Red Crescent Societies, and Médecins Sans Frontières.
The stockpile was created in order to make the single-dose Ebola vaccine (rVSV∆G-ZEBOV-GP, live; trade name Everbo) rapidly available at the start of the next Ebola outbreak anywhere in the world. The vaccine was developed and is marketed by Merck Sharp & Dohme, with financial support from the United States.
The stockpile, which is maintained in Switzerland and managed by UNICEF, is designed to be readily deployed to other countries whenever there is an outbreak. The ICG will be the decision-making body for the vaccine’s allocation and release, as is also the case with previously created stockpiles of cholera, meningitis, and yellow fever vaccines.
“The decision to allocate the vaccine will be made within 48 hours of receiving a request from a country; vaccines will be made available together with ultra-cold chain packaging by the manufacturer for shipment to countries within 48 hours of the decision. The targeted overall delivery time from the stockpile to countries is 7 days,” according to the WHO press release.
Currently 6,890 doses are available for outbreak response, with further quantities to be delivered into the stockpile throughout 2021 and beyond. Initial use of the vaccine will be directed to health care and frontline workers. It is expected that it will take 2-3 years to reach the Strategic Advisory Group of Experts on Immunization–recommended level of 500,000 doses for the stockpile of Ebola vaccines.