User login
Combination approach to melasma treatment yields best results
When establishing a treatment plan for patients with melasma, counseling them about realistic expectations is key.
“It’s important that they understand that this is a chronic condition, so it does require long-term maintenance therapy,” Arisa E. Ortiz, MD, said at the virtual annual Masters of Aesthetics Symposium. “We can improve melasma, but it’s difficult to cure melasma.”
While hydroquinone and other bleaching agents are typical treatment mainstays, chemical peels with glycolic acid, trichloroacetic acid, and salicylic acid can benefit some individuals. “For chemical peels, I really like glycolic acid peels because there is no downtime; it peels at the microscopic level,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “This is something they may need to repeat monthly, and having a week of peeling may be difficult to go through every month.”
Other common melasma treatments include lasers, intense pulsed light (IPL), and oral medications. “I personally am not impressed with microdermabrasion for melasma, so I don’t use that very much,” she said. “With laser treatment, you want to make sure you’re using low-energy lasers so that it doesn’t exacerbate or make them relapse or rebound.”
While hydroquinone is a mainstay of therapy, “you can’t use it chronically because of the risk of ochronosis (permanent darkening), so you do need to take drug holidays,” Dr. Ortiz said. “During those drug holidays, you want to make sure patients have a nonhydroquinone bleaching agent so that they don’t flare.” Options include lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, and oral antioxidants.
In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with topical 4% hydroquinone (J Drugs Dermatol 2012 Dec;11[12]:1478-82). They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
In a separate unpublished study of 22 females, investigators assessed the efficacy of the U.SK Advanced Defense Booster, which contains ferulic acid, maslinic acid, peptides, and olive leaf extract. They observed that 98% of patients saw improvement after 28 days of treatment.
When it comes to using lasers for melasma treatment, low-energy devices provide the best outcomes. “I prefer using something like the 1927-nm fractional diode lasers at 3.75% density, really low densities because there’s less risk for rebound,” Dr. Ortiz said. “They also enhance skin permeability for the use of topicals.”
In an observational study of 27 female patients with refractory melasma, Arielle Kauvar, MD, director of New York Laser & Skin Care, combined microdermabrasion with the Q-switched Nd:YAG (Lasers in Surgery and Medicine 2012; 44:117-24). “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” Dr. Ortiz said. Specifically, she used a laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion for 4 weeks. “She got a good improvement using a skin care regimen of sunscreen, hydroquinone, and tretinoin or vitamin C,” she said. “Remission lasted at least 6 months.”
In a study presented at the 2019 annual meeting of the America Society for Laser Medicine and Surgery, Dr. Ortiz and Tanya Greywal, MD, of the University of California, San Diego, used three passes of the 10764-nm Nd:YAG laser to treat 10 subjects with melasma skin types 2-5. The device has a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm3. “There was no downtime with these patients, and they saw a mean improvement of 26%-50% as early as 3 weeks,” she said. “Patients did require multiple treatments to see adequate resolution, but no anesthesia or numbing cream was required. This is a good option for patients who need chronic maintenance treatment.”
Topicals also play a key role following the laser treatment of melasma. Dr. Ortiz characterized clobetasol as “kind of like the magic ointment.” She uses one application immediately post procedure “whenever I’m worried about a patient having postinflammatory hyperpigmentation or if I don’t want melasma patients to rebound. It can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation.”
Researchers have discovered that there is a vascular component to melasma. Paul M. Friedman, MD, of the Dermatology and Laser Surgery Center, Houston, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in 11 patients with melasma (Lasers Surg Med 2017 Jan;49[1]:20-6). They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combined vascular-targeted laser therapy together with fractional low-powered diode laser therapy. “A parallel improvement in telangiectatic erythema suggests a relationship between the underlying vasculature and hyperpigmentation,” said Dr. Ortiz, who was not affiliated with the study. “So, patients who have a vascular component to their melasma actually can get improved efficacy.”
Another strategy for melasma patients involves oral treatment with Polypodium leucotomos extract (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I like to think of it as an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection. It has been shown to significantly reduce the severity of sunburn and decrease the risk of UV radiation–induced skin cancer, as well as prevent skin aging.” The purported mechanism of action includes decreasing UV-mediated oxidative damage to DNA, enhancing the activity of endogenous antioxidant systems, increasing the minimal erythema dose, blocking UV radiation–induced cyclooxygenase-2 expression, reducing UV-induced immune suppression, and promoting p53 suppressor gene expression.
In a pilot placebo-controlled study of melasma patients on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks (J Clin Aesthet Dermatol 2018 Mar;11[3]:14-9). They found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from the first month of treatment, compared with placebo.
Dr. Ortiz next discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and for prevention of hemorrhage in patients with hemophilia undergoing tooth extractions. “It is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule, and it’s a game changer for melasma treatment,” she said. “One of the side effects is that it inhibits melanogenesis and neovascularization. It’s been effective for melasma, but its use is limited by the risk for thromboembolism. It’s a slight increased risk, something patients should be aware of, but not something that should scare us away from prescribing it.”
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects (J Am Acad Dermatol 2016;75:385-92). The most common side effects were abdominal bloating and pain. One patient developed a DVT during treatment, but that person was found to have a protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for melasma has ranged from 500 mg-1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg pill in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of present illness. She does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
She concluded her presentation by noting that she favors a combination approach to treating melasma patients that starts with a broad spectrum sunscreen and PLE. “For bleaching, I like to use 12% hydroquinone with 6% kojic acid in VersaBase,” she said. “Once I get them in better control, then I switch them to 4% hydroquinone for maintenance. I use glycolic peels, low-energy lasers, and tranexamic acid if the melasma is severe, and they have no contraindications. A combination approach really achieves the best results, and counseling is key.”
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of MOA.
When establishing a treatment plan for patients with melasma, counseling them about realistic expectations is key.
“It’s important that they understand that this is a chronic condition, so it does require long-term maintenance therapy,” Arisa E. Ortiz, MD, said at the virtual annual Masters of Aesthetics Symposium. “We can improve melasma, but it’s difficult to cure melasma.”
While hydroquinone and other bleaching agents are typical treatment mainstays, chemical peels with glycolic acid, trichloroacetic acid, and salicylic acid can benefit some individuals. “For chemical peels, I really like glycolic acid peels because there is no downtime; it peels at the microscopic level,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “This is something they may need to repeat monthly, and having a week of peeling may be difficult to go through every month.”
Other common melasma treatments include lasers, intense pulsed light (IPL), and oral medications. “I personally am not impressed with microdermabrasion for melasma, so I don’t use that very much,” she said. “With laser treatment, you want to make sure you’re using low-energy lasers so that it doesn’t exacerbate or make them relapse or rebound.”
While hydroquinone is a mainstay of therapy, “you can’t use it chronically because of the risk of ochronosis (permanent darkening), so you do need to take drug holidays,” Dr. Ortiz said. “During those drug holidays, you want to make sure patients have a nonhydroquinone bleaching agent so that they don’t flare.” Options include lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, and oral antioxidants.
In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with topical 4% hydroquinone (J Drugs Dermatol 2012 Dec;11[12]:1478-82). They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
In a separate unpublished study of 22 females, investigators assessed the efficacy of the U.SK Advanced Defense Booster, which contains ferulic acid, maslinic acid, peptides, and olive leaf extract. They observed that 98% of patients saw improvement after 28 days of treatment.
When it comes to using lasers for melasma treatment, low-energy devices provide the best outcomes. “I prefer using something like the 1927-nm fractional diode lasers at 3.75% density, really low densities because there’s less risk for rebound,” Dr. Ortiz said. “They also enhance skin permeability for the use of topicals.”
In an observational study of 27 female patients with refractory melasma, Arielle Kauvar, MD, director of New York Laser & Skin Care, combined microdermabrasion with the Q-switched Nd:YAG (Lasers in Surgery and Medicine 2012; 44:117-24). “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” Dr. Ortiz said. Specifically, she used a laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion for 4 weeks. “She got a good improvement using a skin care regimen of sunscreen, hydroquinone, and tretinoin or vitamin C,” she said. “Remission lasted at least 6 months.”
In a study presented at the 2019 annual meeting of the America Society for Laser Medicine and Surgery, Dr. Ortiz and Tanya Greywal, MD, of the University of California, San Diego, used three passes of the 10764-nm Nd:YAG laser to treat 10 subjects with melasma skin types 2-5. The device has a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm3. “There was no downtime with these patients, and they saw a mean improvement of 26%-50% as early as 3 weeks,” she said. “Patients did require multiple treatments to see adequate resolution, but no anesthesia or numbing cream was required. This is a good option for patients who need chronic maintenance treatment.”
Topicals also play a key role following the laser treatment of melasma. Dr. Ortiz characterized clobetasol as “kind of like the magic ointment.” She uses one application immediately post procedure “whenever I’m worried about a patient having postinflammatory hyperpigmentation or if I don’t want melasma patients to rebound. It can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation.”
Researchers have discovered that there is a vascular component to melasma. Paul M. Friedman, MD, of the Dermatology and Laser Surgery Center, Houston, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in 11 patients with melasma (Lasers Surg Med 2017 Jan;49[1]:20-6). They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combined vascular-targeted laser therapy together with fractional low-powered diode laser therapy. “A parallel improvement in telangiectatic erythema suggests a relationship between the underlying vasculature and hyperpigmentation,” said Dr. Ortiz, who was not affiliated with the study. “So, patients who have a vascular component to their melasma actually can get improved efficacy.”
Another strategy for melasma patients involves oral treatment with Polypodium leucotomos extract (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I like to think of it as an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection. It has been shown to significantly reduce the severity of sunburn and decrease the risk of UV radiation–induced skin cancer, as well as prevent skin aging.” The purported mechanism of action includes decreasing UV-mediated oxidative damage to DNA, enhancing the activity of endogenous antioxidant systems, increasing the minimal erythema dose, blocking UV radiation–induced cyclooxygenase-2 expression, reducing UV-induced immune suppression, and promoting p53 suppressor gene expression.
In a pilot placebo-controlled study of melasma patients on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks (J Clin Aesthet Dermatol 2018 Mar;11[3]:14-9). They found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from the first month of treatment, compared with placebo.
Dr. Ortiz next discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and for prevention of hemorrhage in patients with hemophilia undergoing tooth extractions. “It is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule, and it’s a game changer for melasma treatment,” she said. “One of the side effects is that it inhibits melanogenesis and neovascularization. It’s been effective for melasma, but its use is limited by the risk for thromboembolism. It’s a slight increased risk, something patients should be aware of, but not something that should scare us away from prescribing it.”
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects (J Am Acad Dermatol 2016;75:385-92). The most common side effects were abdominal bloating and pain. One patient developed a DVT during treatment, but that person was found to have a protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for melasma has ranged from 500 mg-1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg pill in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of present illness. She does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
She concluded her presentation by noting that she favors a combination approach to treating melasma patients that starts with a broad spectrum sunscreen and PLE. “For bleaching, I like to use 12% hydroquinone with 6% kojic acid in VersaBase,” she said. “Once I get them in better control, then I switch them to 4% hydroquinone for maintenance. I use glycolic peels, low-energy lasers, and tranexamic acid if the melasma is severe, and they have no contraindications. A combination approach really achieves the best results, and counseling is key.”
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of MOA.
When establishing a treatment plan for patients with melasma, counseling them about realistic expectations is key.
“It’s important that they understand that this is a chronic condition, so it does require long-term maintenance therapy,” Arisa E. Ortiz, MD, said at the virtual annual Masters of Aesthetics Symposium. “We can improve melasma, but it’s difficult to cure melasma.”
While hydroquinone and other bleaching agents are typical treatment mainstays, chemical peels with glycolic acid, trichloroacetic acid, and salicylic acid can benefit some individuals. “For chemical peels, I really like glycolic acid peels because there is no downtime; it peels at the microscopic level,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “This is something they may need to repeat monthly, and having a week of peeling may be difficult to go through every month.”
Other common melasma treatments include lasers, intense pulsed light (IPL), and oral medications. “I personally am not impressed with microdermabrasion for melasma, so I don’t use that very much,” she said. “With laser treatment, you want to make sure you’re using low-energy lasers so that it doesn’t exacerbate or make them relapse or rebound.”
While hydroquinone is a mainstay of therapy, “you can’t use it chronically because of the risk of ochronosis (permanent darkening), so you do need to take drug holidays,” Dr. Ortiz said. “During those drug holidays, you want to make sure patients have a nonhydroquinone bleaching agent so that they don’t flare.” Options include lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, and oral antioxidants.
In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with topical 4% hydroquinone (J Drugs Dermatol 2012 Dec;11[12]:1478-82). They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
In a separate unpublished study of 22 females, investigators assessed the efficacy of the U.SK Advanced Defense Booster, which contains ferulic acid, maslinic acid, peptides, and olive leaf extract. They observed that 98% of patients saw improvement after 28 days of treatment.
When it comes to using lasers for melasma treatment, low-energy devices provide the best outcomes. “I prefer using something like the 1927-nm fractional diode lasers at 3.75% density, really low densities because there’s less risk for rebound,” Dr. Ortiz said. “They also enhance skin permeability for the use of topicals.”
In an observational study of 27 female patients with refractory melasma, Arielle Kauvar, MD, director of New York Laser & Skin Care, combined microdermabrasion with the Q-switched Nd:YAG (Lasers in Surgery and Medicine 2012; 44:117-24). “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” Dr. Ortiz said. Specifically, she used a laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion for 4 weeks. “She got a good improvement using a skin care regimen of sunscreen, hydroquinone, and tretinoin or vitamin C,” she said. “Remission lasted at least 6 months.”
In a study presented at the 2019 annual meeting of the America Society for Laser Medicine and Surgery, Dr. Ortiz and Tanya Greywal, MD, of the University of California, San Diego, used three passes of the 10764-nm Nd:YAG laser to treat 10 subjects with melasma skin types 2-5. The device has a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm3. “There was no downtime with these patients, and they saw a mean improvement of 26%-50% as early as 3 weeks,” she said. “Patients did require multiple treatments to see adequate resolution, but no anesthesia or numbing cream was required. This is a good option for patients who need chronic maintenance treatment.”
Topicals also play a key role following the laser treatment of melasma. Dr. Ortiz characterized clobetasol as “kind of like the magic ointment.” She uses one application immediately post procedure “whenever I’m worried about a patient having postinflammatory hyperpigmentation or if I don’t want melasma patients to rebound. It can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation.”
Researchers have discovered that there is a vascular component to melasma. Paul M. Friedman, MD, of the Dermatology and Laser Surgery Center, Houston, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in 11 patients with melasma (Lasers Surg Med 2017 Jan;49[1]:20-6). They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combined vascular-targeted laser therapy together with fractional low-powered diode laser therapy. “A parallel improvement in telangiectatic erythema suggests a relationship between the underlying vasculature and hyperpigmentation,” said Dr. Ortiz, who was not affiliated with the study. “So, patients who have a vascular component to their melasma actually can get improved efficacy.”
Another strategy for melasma patients involves oral treatment with Polypodium leucotomos extract (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I like to think of it as an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection. It has been shown to significantly reduce the severity of sunburn and decrease the risk of UV radiation–induced skin cancer, as well as prevent skin aging.” The purported mechanism of action includes decreasing UV-mediated oxidative damage to DNA, enhancing the activity of endogenous antioxidant systems, increasing the minimal erythema dose, blocking UV radiation–induced cyclooxygenase-2 expression, reducing UV-induced immune suppression, and promoting p53 suppressor gene expression.
In a pilot placebo-controlled study of melasma patients on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks (J Clin Aesthet Dermatol 2018 Mar;11[3]:14-9). They found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from the first month of treatment, compared with placebo.
Dr. Ortiz next discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and for prevention of hemorrhage in patients with hemophilia undergoing tooth extractions. “It is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule, and it’s a game changer for melasma treatment,” she said. “One of the side effects is that it inhibits melanogenesis and neovascularization. It’s been effective for melasma, but its use is limited by the risk for thromboembolism. It’s a slight increased risk, something patients should be aware of, but not something that should scare us away from prescribing it.”
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects (J Am Acad Dermatol 2016;75:385-92). The most common side effects were abdominal bloating and pain. One patient developed a DVT during treatment, but that person was found to have a protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for melasma has ranged from 500 mg-1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg pill in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of present illness. She does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
She concluded her presentation by noting that she favors a combination approach to treating melasma patients that starts with a broad spectrum sunscreen and PLE. “For bleaching, I like to use 12% hydroquinone with 6% kojic acid in VersaBase,” she said. “Once I get them in better control, then I switch them to 4% hydroquinone for maintenance. I use glycolic peels, low-energy lasers, and tranexamic acid if the melasma is severe, and they have no contraindications. A combination approach really achieves the best results, and counseling is key.”
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of MOA.
EXPERT ANALYSIS FROM MOA 2020
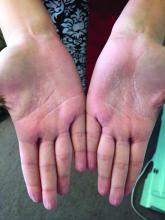
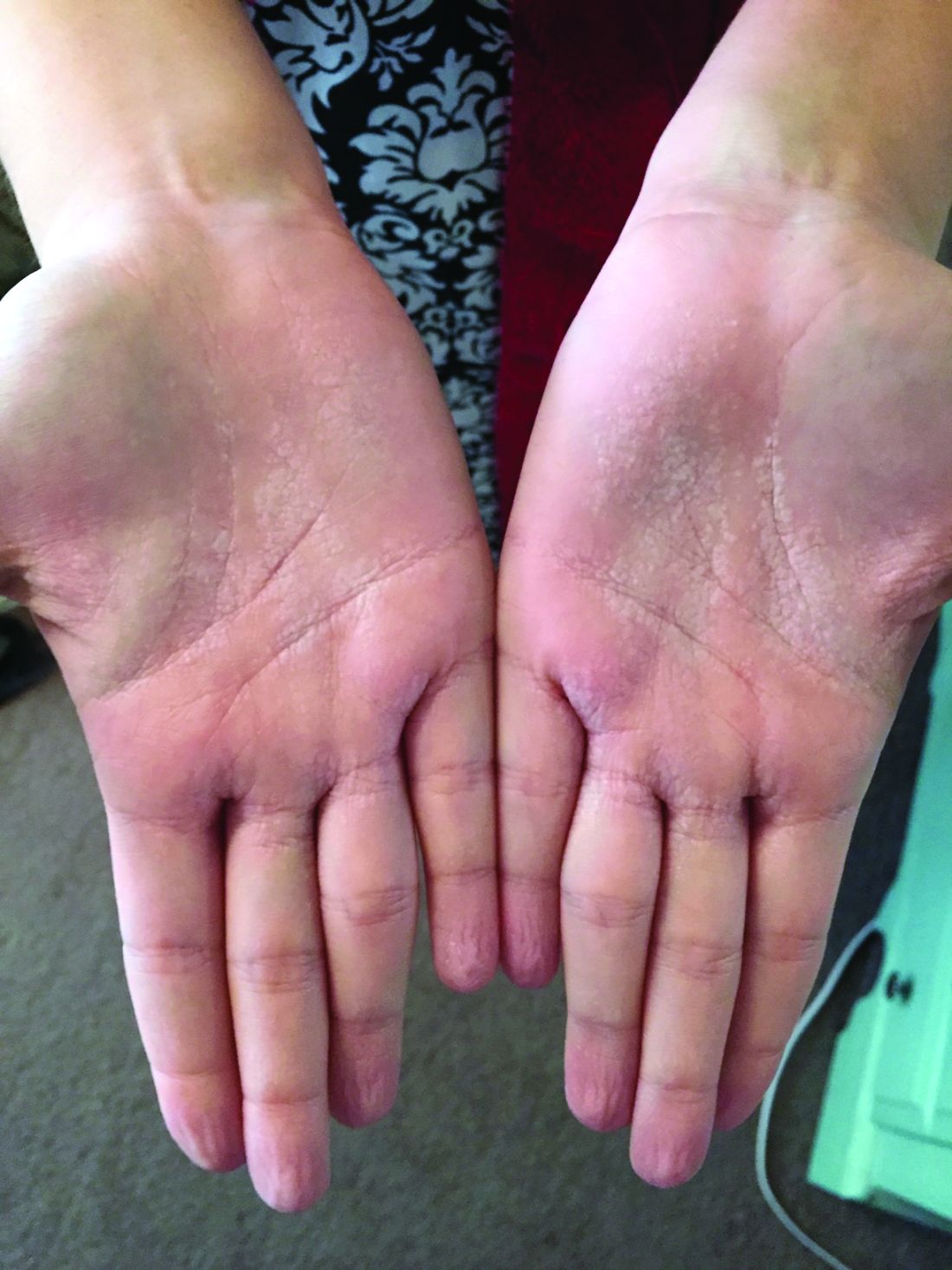
A woman with an asymptomatic eruption on her palms after exposure to water
This eruption can be accompanied by a mild burning or tingling sensation, which will subside with the rest of the symptoms in minutes to hours after drying.1
AWP is most frequently associated with cystic fibrosis (CF).2 It can be observed in up to 80% of CF patients and is considered a clinical sign of the disease. AWP can be present in CF carriers to a lesser extent,2,4 and has also been associated with focal hyperhidrosis, atopic dermatitis, Raynaud phenomenon, and COX-2 inhibitor use.5
While a definitive cause is unknown, it is thought that AWP is caused by dysregulation of sweat glands in the palms through increased expression of aquaporin, a protein crucial in the transport of water between cells.3
AWP is quite rare and benign in nature. However, because of its strong association with CF, genetic screening should be considered in asymptomatic patients. Our patient had been screened in the past and is not a CF carrier. Often, the itching or burning associated with CF is mild and easily controlled. The patient was placed on low dose isotretinoin for treatment of her acne. Interestingly, the patient claimed her eruption no longer appeared after starting isotretinoin therapy. To our knowledge, this is the first reported case of AWP resolving with isotretinoin use.
This case and photo were submitted by Mr. Birk, University of Texas, Austin, Texas; and Dr. Mamelak, Sanova Dermatology, in Austin. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Katz M, Ramot Y. CMAJ. 2015 Dec 8;187(18):E515.
2. Tolland JP et al. Dermatology. 2010;221(4):326-30.
3. Kabashima K et al. J Am Acad Dermatol. 2008 Aug;59(2 Suppl 1):S28-32.
4. Gild R et al. Br J Dermatol. 2010 Nov;163(5):1082-4.
5. Glatz M and Muellegger RR. BMJ Case Rep. 2014. doi: 10.1136/bcr-2014-203929.
This eruption can be accompanied by a mild burning or tingling sensation, which will subside with the rest of the symptoms in minutes to hours after drying.1
AWP is most frequently associated with cystic fibrosis (CF).2 It can be observed in up to 80% of CF patients and is considered a clinical sign of the disease. AWP can be present in CF carriers to a lesser extent,2,4 and has also been associated with focal hyperhidrosis, atopic dermatitis, Raynaud phenomenon, and COX-2 inhibitor use.5
While a definitive cause is unknown, it is thought that AWP is caused by dysregulation of sweat glands in the palms through increased expression of aquaporin, a protein crucial in the transport of water between cells.3
AWP is quite rare and benign in nature. However, because of its strong association with CF, genetic screening should be considered in asymptomatic patients. Our patient had been screened in the past and is not a CF carrier. Often, the itching or burning associated with CF is mild and easily controlled. The patient was placed on low dose isotretinoin for treatment of her acne. Interestingly, the patient claimed her eruption no longer appeared after starting isotretinoin therapy. To our knowledge, this is the first reported case of AWP resolving with isotretinoin use.
This case and photo were submitted by Mr. Birk, University of Texas, Austin, Texas; and Dr. Mamelak, Sanova Dermatology, in Austin. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Katz M, Ramot Y. CMAJ. 2015 Dec 8;187(18):E515.
2. Tolland JP et al. Dermatology. 2010;221(4):326-30.
3. Kabashima K et al. J Am Acad Dermatol. 2008 Aug;59(2 Suppl 1):S28-32.
4. Gild R et al. Br J Dermatol. 2010 Nov;163(5):1082-4.
5. Glatz M and Muellegger RR. BMJ Case Rep. 2014. doi: 10.1136/bcr-2014-203929.
This eruption can be accompanied by a mild burning or tingling sensation, which will subside with the rest of the symptoms in minutes to hours after drying.1
AWP is most frequently associated with cystic fibrosis (CF).2 It can be observed in up to 80% of CF patients and is considered a clinical sign of the disease. AWP can be present in CF carriers to a lesser extent,2,4 and has also been associated with focal hyperhidrosis, atopic dermatitis, Raynaud phenomenon, and COX-2 inhibitor use.5
While a definitive cause is unknown, it is thought that AWP is caused by dysregulation of sweat glands in the palms through increased expression of aquaporin, a protein crucial in the transport of water between cells.3
AWP is quite rare and benign in nature. However, because of its strong association with CF, genetic screening should be considered in asymptomatic patients. Our patient had been screened in the past and is not a CF carrier. Often, the itching or burning associated with CF is mild and easily controlled. The patient was placed on low dose isotretinoin for treatment of her acne. Interestingly, the patient claimed her eruption no longer appeared after starting isotretinoin therapy. To our knowledge, this is the first reported case of AWP resolving with isotretinoin use.
This case and photo were submitted by Mr. Birk, University of Texas, Austin, Texas; and Dr. Mamelak, Sanova Dermatology, in Austin. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Katz M, Ramot Y. CMAJ. 2015 Dec 8;187(18):E515.
2. Tolland JP et al. Dermatology. 2010;221(4):326-30.
3. Kabashima K et al. J Am Acad Dermatol. 2008 Aug;59(2 Suppl 1):S28-32.
4. Gild R et al. Br J Dermatol. 2010 Nov;163(5):1082-4.
5. Glatz M and Muellegger RR. BMJ Case Rep. 2014. doi: 10.1136/bcr-2014-203929.
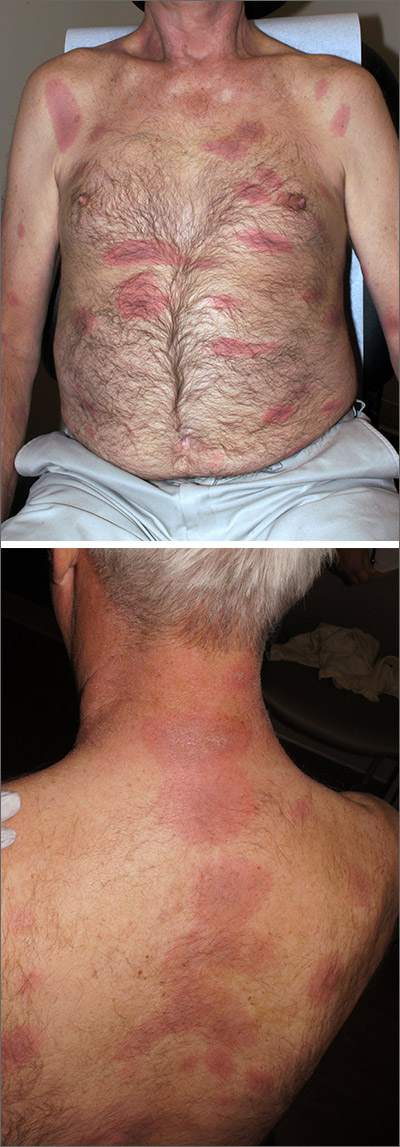
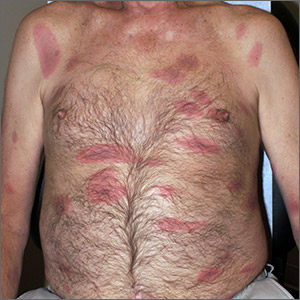
Rash, muscle weakness, and confusion
The constellation of symptoms was suggestive of Lyme disease, although connective tissue disease and syphilis were also considered. Two punch biopsies were performed in the office, and erythrocyte sedimentation rate (ESR), complete blood cell count (CBC), international normalized ratio (INR), comprehensive metabolic panel (CMP), Lyme enzyme-linked immunosorbent assay (ELISA) antibody panel, and rapid plasma reagin (RPR) laboratory tests were ordered.
Immediately available laboratory results included ESR, CBC, INR, and CMP. Findings were notable for elevated INR, as well as elevated alanine aminotransferase and aspartate transaminase. The transaminitis suggested myopathy and was consistent with clinical muscle weakness. RPR testing was negative.
Because of the confusion, severity of muscle weakness, and plausibility of early encephalopathy with Lyme disease, the patient was admitted to the hospital for further work-up. Lumbar puncture was delayed until his INR was reduced, but subsequently was found to be normal. He received intravenous (IV) ceftriaxone (2 g/d) empirically for possible early disseminated disease with neurologic complications. His confusion, muscle weakness, and transaminitis rapidly improved.
His Lyme antibody panel was positive for IgM after his third day of hospitalization. A reflexive confirmatory western blot for IgG was not positive on the initial set of labs but was positive when redrawn 4 weeks after this hospitalization, confirming Lyme disease.
Lyme disease is a vector-borne disease caused by the Borrelia genus of spirochete bacteria, most commonly Borrelia burgdorferi in North America. Transmission occurs through prolonged (typically 36-48 hours) attachment of a blacklegged tick.
The disease can be divided into 3 stages:
- localized (3-30 days): erythema migrans rash and flulike illness
- early disseminated (days to weeks; seen in this patient): multiple erythema migrans rashes, early neuroborreliosis, arthritis, carditis, and rarely hepatitis and uveitis
- late disseminated (months to years): chronic Lyme arthritis, chronic neurological disorders (eg, encephalopathy, radicular pain, and chronic neuropathy).
The initial erythema migrans rash is classically red and targetoid; it expands from the site of attachment. Early disseminated patches tend to be smaller and can occur on any body part. The rash is rarely itchy or painful but may be warm to the touch or sensitive. The rash resolves spontaneously within 3 to 4 weeks of onset.
Treatment of all early and early disseminated Lyme disease typically involves a 14- to 28-day course of doxycycline (100 mg bid for adults, 2.2 mg/kg bid [maximum 100 mg bid] for children). Patients with acute neurologic disease often can be treated with doxycycline, but patients who cannot tolerate doxycycline and those with parenchymal disease such as encephalitis should receive IV therapy with ceftriaxone 2 g/d.
In this case, the patient was discharged home on a 3-week course of doxycycline 100 mg bid and cleared without further symptoms.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Lyme disease. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/lyme/healthcare/index.html. Accessed September 1, 2020.
The constellation of symptoms was suggestive of Lyme disease, although connective tissue disease and syphilis were also considered. Two punch biopsies were performed in the office, and erythrocyte sedimentation rate (ESR), complete blood cell count (CBC), international normalized ratio (INR), comprehensive metabolic panel (CMP), Lyme enzyme-linked immunosorbent assay (ELISA) antibody panel, and rapid plasma reagin (RPR) laboratory tests were ordered.
Immediately available laboratory results included ESR, CBC, INR, and CMP. Findings were notable for elevated INR, as well as elevated alanine aminotransferase and aspartate transaminase. The transaminitis suggested myopathy and was consistent with clinical muscle weakness. RPR testing was negative.
Because of the confusion, severity of muscle weakness, and plausibility of early encephalopathy with Lyme disease, the patient was admitted to the hospital for further work-up. Lumbar puncture was delayed until his INR was reduced, but subsequently was found to be normal. He received intravenous (IV) ceftriaxone (2 g/d) empirically for possible early disseminated disease with neurologic complications. His confusion, muscle weakness, and transaminitis rapidly improved.
His Lyme antibody panel was positive for IgM after his third day of hospitalization. A reflexive confirmatory western blot for IgG was not positive on the initial set of labs but was positive when redrawn 4 weeks after this hospitalization, confirming Lyme disease.
Lyme disease is a vector-borne disease caused by the Borrelia genus of spirochete bacteria, most commonly Borrelia burgdorferi in North America. Transmission occurs through prolonged (typically 36-48 hours) attachment of a blacklegged tick.
The disease can be divided into 3 stages:
- localized (3-30 days): erythema migrans rash and flulike illness
- early disseminated (days to weeks; seen in this patient): multiple erythema migrans rashes, early neuroborreliosis, arthritis, carditis, and rarely hepatitis and uveitis
- late disseminated (months to years): chronic Lyme arthritis, chronic neurological disorders (eg, encephalopathy, radicular pain, and chronic neuropathy).
The initial erythema migrans rash is classically red and targetoid; it expands from the site of attachment. Early disseminated patches tend to be smaller and can occur on any body part. The rash is rarely itchy or painful but may be warm to the touch or sensitive. The rash resolves spontaneously within 3 to 4 weeks of onset.
Treatment of all early and early disseminated Lyme disease typically involves a 14- to 28-day course of doxycycline (100 mg bid for adults, 2.2 mg/kg bid [maximum 100 mg bid] for children). Patients with acute neurologic disease often can be treated with doxycycline, but patients who cannot tolerate doxycycline and those with parenchymal disease such as encephalitis should receive IV therapy with ceftriaxone 2 g/d.
In this case, the patient was discharged home on a 3-week course of doxycycline 100 mg bid and cleared without further symptoms.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
The constellation of symptoms was suggestive of Lyme disease, although connective tissue disease and syphilis were also considered. Two punch biopsies were performed in the office, and erythrocyte sedimentation rate (ESR), complete blood cell count (CBC), international normalized ratio (INR), comprehensive metabolic panel (CMP), Lyme enzyme-linked immunosorbent assay (ELISA) antibody panel, and rapid plasma reagin (RPR) laboratory tests were ordered.
Immediately available laboratory results included ESR, CBC, INR, and CMP. Findings were notable for elevated INR, as well as elevated alanine aminotransferase and aspartate transaminase. The transaminitis suggested myopathy and was consistent with clinical muscle weakness. RPR testing was negative.
Because of the confusion, severity of muscle weakness, and plausibility of early encephalopathy with Lyme disease, the patient was admitted to the hospital for further work-up. Lumbar puncture was delayed until his INR was reduced, but subsequently was found to be normal. He received intravenous (IV) ceftriaxone (2 g/d) empirically for possible early disseminated disease with neurologic complications. His confusion, muscle weakness, and transaminitis rapidly improved.
His Lyme antibody panel was positive for IgM after his third day of hospitalization. A reflexive confirmatory western blot for IgG was not positive on the initial set of labs but was positive when redrawn 4 weeks after this hospitalization, confirming Lyme disease.
Lyme disease is a vector-borne disease caused by the Borrelia genus of spirochete bacteria, most commonly Borrelia burgdorferi in North America. Transmission occurs through prolonged (typically 36-48 hours) attachment of a blacklegged tick.
The disease can be divided into 3 stages:
- localized (3-30 days): erythema migrans rash and flulike illness
- early disseminated (days to weeks; seen in this patient): multiple erythema migrans rashes, early neuroborreliosis, arthritis, carditis, and rarely hepatitis and uveitis
- late disseminated (months to years): chronic Lyme arthritis, chronic neurological disorders (eg, encephalopathy, radicular pain, and chronic neuropathy).
The initial erythema migrans rash is classically red and targetoid; it expands from the site of attachment. Early disseminated patches tend to be smaller and can occur on any body part. The rash is rarely itchy or painful but may be warm to the touch or sensitive. The rash resolves spontaneously within 3 to 4 weeks of onset.
Treatment of all early and early disseminated Lyme disease typically involves a 14- to 28-day course of doxycycline (100 mg bid for adults, 2.2 mg/kg bid [maximum 100 mg bid] for children). Patients with acute neurologic disease often can be treated with doxycycline, but patients who cannot tolerate doxycycline and those with parenchymal disease such as encephalitis should receive IV therapy with ceftriaxone 2 g/d.
In this case, the patient was discharged home on a 3-week course of doxycycline 100 mg bid and cleared without further symptoms.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Lyme disease. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/lyme/healthcare/index.html. Accessed September 1, 2020.
Lyme disease. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/lyme/healthcare/index.html. Accessed September 1, 2020.
Study supports multigene panel testing for all breast cancer patients with second primary cancers
according to a paper published in JCO Precision Oncology.
The authors noted that women with breast cancer have a 4%-16% lifetime risk of a second primary cancer. However, it hasn’t been clear if mutations in genes other than BRCA1/2 are enriched in patients with multiple primary cancers.
“Surprisingly few papers have focused on genetic evaluation of patients with multiple primary cancers,” senior author Katherine L. Nathanson, MD, of the University of Pennsylvania in Philadelphia, said in an interview.
“Ours is one of the first studies to look closely at this issue. We know from clinical experience that these patients are more likely to have more than one genetic mutation,” she added.
For their study, Dr. Nathanson and colleagues identified pathogenic germline mutations in 17 cancer susceptibility genes in patients with BRCA1/2-negative breast cancer in two cohorts.
Cohort 1 consisted of 1,000 high-risk breast cancer patients – 551 with multiple primary cancers and 449 with a single breast cancer.
Cohort 2 included 1,804 familial breast cancer patients – 340 with multiple primaries and 1,464 with a single breast cancer.
The researchers assessed mutations in these cohorts and compared them with mutations in a control data set.
Mutation rates and age
Pathogenic mutation rates were higher in both cohorts in patients with multiple primaries as compared with patients with single primaries.
In cohort 1, the overall panel positive rate was 8.53% in the multiple-primaries group and 4.90% in the single-primary group (P = .024).
In cohort 2, the overall panel positive rate was 7.06% in the multiple-primaries group and 4.23% in the single-primary group (P = .034).
In both cohorts, younger age at first breast cancer was associated with higher mutation rates. However, the age at onset of cancers other than breast cancer was not related to mutation rate.
“Regardless of age, mutations in genes other than BRCA1/2 are found in at least 5% of patients with breast cancer and another primary cancer, with up to 25% in patients with their first breast cancer at age 30 years,” Dr. Nathanson said. “This supports the need for multigene panel testing in all patients with breast cancer and another primary cancer.”
“Once a woman has multiple primaries with breast cancer, it doesn’t matter what her family history is, she is more likely to be at risk,” Dr. Nathanson added.
Genetic susceptibility
The researchers also identified genes associated with multiple primary cancers. TP53 and MSH6 mutations were significantly enriched in patients with multiple primaries but not single primaries. ATM and PALB2 mutations were significantly enriched in both groups when compared with controls.
The researchers noted that high-penetrance cancer genes were responsible for higher mutation rates in the cohort enriched for early-onset breast cancer and non–breast cancer second primaries. Moderate-penetrance cancer genes were responsible for the higher mutation rates in the cohort enriched for familial breast cancer and second breast cancer primaries.
“In multiple primary cancers, we found additional genes with moderate penetrance and some genes with high penetrance associated with TP53 and Lynch syndrome,” Dr. Nathanson said.
Cancer prevention and screening
The results of this study could lead to better implementation of cancer prevention and screening strategies, according to the researchers.
“As we look at guidelines in development and NCCN recommendations, our data suggest that age should not be part of the criteria for genetic testing in patients who have more than one primary cancer. These patients are at high risk and should be recommended for screening,” Dr. Nathanson said.
“If you see a patient with multiple primary cancers, refer for genetic testing. Age does not matter,” she reiterated.
Future research will look at potentially missing mutations.
“With targeted sequencing, structurally rearranged genes might be missed for those at risk. We will try to identify cancer susceptibility genes and define the true risk of penetrance of these genes in the general population,” Dr. Nathanson said.
This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Dr. Nathanson disclosed no conflicts of interest. Other authors disclosed relationships with a range of companies, all listed in the paper.
SOURCE: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
according to a paper published in JCO Precision Oncology.
The authors noted that women with breast cancer have a 4%-16% lifetime risk of a second primary cancer. However, it hasn’t been clear if mutations in genes other than BRCA1/2 are enriched in patients with multiple primary cancers.
“Surprisingly few papers have focused on genetic evaluation of patients with multiple primary cancers,” senior author Katherine L. Nathanson, MD, of the University of Pennsylvania in Philadelphia, said in an interview.
“Ours is one of the first studies to look closely at this issue. We know from clinical experience that these patients are more likely to have more than one genetic mutation,” she added.
For their study, Dr. Nathanson and colleagues identified pathogenic germline mutations in 17 cancer susceptibility genes in patients with BRCA1/2-negative breast cancer in two cohorts.
Cohort 1 consisted of 1,000 high-risk breast cancer patients – 551 with multiple primary cancers and 449 with a single breast cancer.
Cohort 2 included 1,804 familial breast cancer patients – 340 with multiple primaries and 1,464 with a single breast cancer.
The researchers assessed mutations in these cohorts and compared them with mutations in a control data set.
Mutation rates and age
Pathogenic mutation rates were higher in both cohorts in patients with multiple primaries as compared with patients with single primaries.
In cohort 1, the overall panel positive rate was 8.53% in the multiple-primaries group and 4.90% in the single-primary group (P = .024).
In cohort 2, the overall panel positive rate was 7.06% in the multiple-primaries group and 4.23% in the single-primary group (P = .034).
In both cohorts, younger age at first breast cancer was associated with higher mutation rates. However, the age at onset of cancers other than breast cancer was not related to mutation rate.
“Regardless of age, mutations in genes other than BRCA1/2 are found in at least 5% of patients with breast cancer and another primary cancer, with up to 25% in patients with their first breast cancer at age 30 years,” Dr. Nathanson said. “This supports the need for multigene panel testing in all patients with breast cancer and another primary cancer.”
“Once a woman has multiple primaries with breast cancer, it doesn’t matter what her family history is, she is more likely to be at risk,” Dr. Nathanson added.
Genetic susceptibility
The researchers also identified genes associated with multiple primary cancers. TP53 and MSH6 mutations were significantly enriched in patients with multiple primaries but not single primaries. ATM and PALB2 mutations were significantly enriched in both groups when compared with controls.
The researchers noted that high-penetrance cancer genes were responsible for higher mutation rates in the cohort enriched for early-onset breast cancer and non–breast cancer second primaries. Moderate-penetrance cancer genes were responsible for the higher mutation rates in the cohort enriched for familial breast cancer and second breast cancer primaries.
“In multiple primary cancers, we found additional genes with moderate penetrance and some genes with high penetrance associated with TP53 and Lynch syndrome,” Dr. Nathanson said.
Cancer prevention and screening
The results of this study could lead to better implementation of cancer prevention and screening strategies, according to the researchers.
“As we look at guidelines in development and NCCN recommendations, our data suggest that age should not be part of the criteria for genetic testing in patients who have more than one primary cancer. These patients are at high risk and should be recommended for screening,” Dr. Nathanson said.
“If you see a patient with multiple primary cancers, refer for genetic testing. Age does not matter,” she reiterated.
Future research will look at potentially missing mutations.
“With targeted sequencing, structurally rearranged genes might be missed for those at risk. We will try to identify cancer susceptibility genes and define the true risk of penetrance of these genes in the general population,” Dr. Nathanson said.
This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Dr. Nathanson disclosed no conflicts of interest. Other authors disclosed relationships with a range of companies, all listed in the paper.
SOURCE: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
according to a paper published in JCO Precision Oncology.
The authors noted that women with breast cancer have a 4%-16% lifetime risk of a second primary cancer. However, it hasn’t been clear if mutations in genes other than BRCA1/2 are enriched in patients with multiple primary cancers.
“Surprisingly few papers have focused on genetic evaluation of patients with multiple primary cancers,” senior author Katherine L. Nathanson, MD, of the University of Pennsylvania in Philadelphia, said in an interview.
“Ours is one of the first studies to look closely at this issue. We know from clinical experience that these patients are more likely to have more than one genetic mutation,” she added.
For their study, Dr. Nathanson and colleagues identified pathogenic germline mutations in 17 cancer susceptibility genes in patients with BRCA1/2-negative breast cancer in two cohorts.
Cohort 1 consisted of 1,000 high-risk breast cancer patients – 551 with multiple primary cancers and 449 with a single breast cancer.
Cohort 2 included 1,804 familial breast cancer patients – 340 with multiple primaries and 1,464 with a single breast cancer.
The researchers assessed mutations in these cohorts and compared them with mutations in a control data set.
Mutation rates and age
Pathogenic mutation rates were higher in both cohorts in patients with multiple primaries as compared with patients with single primaries.
In cohort 1, the overall panel positive rate was 8.53% in the multiple-primaries group and 4.90% in the single-primary group (P = .024).
In cohort 2, the overall panel positive rate was 7.06% in the multiple-primaries group and 4.23% in the single-primary group (P = .034).
In both cohorts, younger age at first breast cancer was associated with higher mutation rates. However, the age at onset of cancers other than breast cancer was not related to mutation rate.
“Regardless of age, mutations in genes other than BRCA1/2 are found in at least 5% of patients with breast cancer and another primary cancer, with up to 25% in patients with their first breast cancer at age 30 years,” Dr. Nathanson said. “This supports the need for multigene panel testing in all patients with breast cancer and another primary cancer.”
“Once a woman has multiple primaries with breast cancer, it doesn’t matter what her family history is, she is more likely to be at risk,” Dr. Nathanson added.
Genetic susceptibility
The researchers also identified genes associated with multiple primary cancers. TP53 and MSH6 mutations were significantly enriched in patients with multiple primaries but not single primaries. ATM and PALB2 mutations were significantly enriched in both groups when compared with controls.
The researchers noted that high-penetrance cancer genes were responsible for higher mutation rates in the cohort enriched for early-onset breast cancer and non–breast cancer second primaries. Moderate-penetrance cancer genes were responsible for the higher mutation rates in the cohort enriched for familial breast cancer and second breast cancer primaries.
“In multiple primary cancers, we found additional genes with moderate penetrance and some genes with high penetrance associated with TP53 and Lynch syndrome,” Dr. Nathanson said.
Cancer prevention and screening
The results of this study could lead to better implementation of cancer prevention and screening strategies, according to the researchers.
“As we look at guidelines in development and NCCN recommendations, our data suggest that age should not be part of the criteria for genetic testing in patients who have more than one primary cancer. These patients are at high risk and should be recommended for screening,” Dr. Nathanson said.
“If you see a patient with multiple primary cancers, refer for genetic testing. Age does not matter,” she reiterated.
Future research will look at potentially missing mutations.
“With targeted sequencing, structurally rearranged genes might be missed for those at risk. We will try to identify cancer susceptibility genes and define the true risk of penetrance of these genes in the general population,” Dr. Nathanson said.
This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Dr. Nathanson disclosed no conflicts of interest. Other authors disclosed relationships with a range of companies, all listed in the paper.
SOURCE: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
FROM JCO PRECISION ONCOLOGY
U.S. tops 500,000 COVID-19 cases in children
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
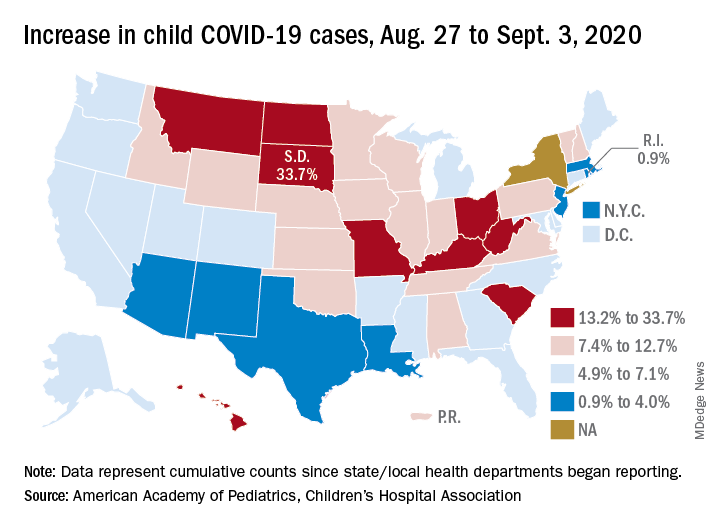
States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
Mounting data support COVID-19 acute pancreatitis
Mounting data support acute pancreatitis as one possible GI manifestation of COVID-19, according to investigators.
While previous case reports suggested that infection with SARS-CoV2 may lead to pancreatitis, this retrospective analysis, which is the largest to date, is the first to offer substantial evidence for this claim, reported lead author Sumant Inamdar, MBBS, of the University of Arkansas, Little Rock, and colleagues.
“It has become increasingly clear that COVID-19 has systemic effects that also includes the gastrointestinal and pancreaticobiliary systems,” the investigators wrote in Gastroenterology. “As islet cells of the pancreas contain ACE2 receptor proteins, SARS-CoV2 can bind to these receptors and cause pancreatic injury.”
For the present analysis, Dr. Inamdar and colleagues reviewed charts from 48,012 patients who were hospitalized in New York between March and June of this year. While pancreatitis is usually diagnosed based on two out of three criteria, disease classification in the study required all three: characteristic upper abdominal pain upon admission, lipase greater than three times the upper limit of normal, and evidence of pancreatitis on cross-sectional imaging.
“[B]y including all three criteria for pancreatitis in our definition, we may be underestimating the rate of pancreatitis,” the investigators wrote. “However, we felt including diagnostic lipase levels and imaging was important for the accuracy of the diagnosis.”
Primary outcomes included mechanical ventilation, length of stay, development of pancreatic necrosis, and mortality. Outcomes were compared between patients with and without COVID-19.
Out of 48,012 hospitalized patients, 11,883 (24.75%) tested positive for SARS-CoV2. Across the entire population, 189 patients had pancreatitis (0.39%), and of these, 32 (17%) also had COVID-19. This translates to a point prevalence for pancreatitis of 0.27% for patients hospitalized with COVID-19.
Among patients with pancreatitis who did not have COVID-19, the most common etiologies for pancreatitis were gallstones (34%) and alcohol (37%), compared with just 16% and 6% of SARS-CoV2-positive cases of pancreatitis, respectively. Idiopathic pancreatitis was significantly more common among patients with COVID-19 than those without (69% vs 21%; P less than .0001).
Black or Hispanic patients with pancreatitis were 4-5 times more likely to have COVID-19 than patients with pancreatitis who were white. Across all races/ethnicities, patients with pancreatitis and COVID-19 more often required mechanical ventilation (odds ratio [OR], 5.65) and longer hospital stays (OR, 3.22), compared with those who had pancreatitis alone. While rates of mortality and pancreatic necrosis showed similar trends, associations with COVID-19 were not statistically significant.
“These findings support the notion that pancreatitis should be included in the list of GI manifestations of COVID-19,” the investigators wrote.
When caring for patients with COVID-19, Dr. Inamdar and colleagues recommended that clinicians pay close attention to any history of abdominal pain, and consider testing serum lipase levels.
“Further large studies are needed to confirm our findings,” they concluded.
Gyanprakash Avinash Ketwaroo, MD, of Baylor College of Medicine in Houston, agreed that more work is needed; in the meantime, he suggested that evidence is now strong enough for clinicians to take notice.
“Overall, this study adds further weight to COVID-19 acute pancreatitis,” he said. “Larger studies, and convincing pathophysiologic data, will be needed to confirm COVID-19 as a cause of acute pancreatitis. However, there appears to be enough circumstantial evidence to consider a COVID-19 diagnosis in patients presenting with acute pancreatitis.”
He noted that the new clinical evidence also stands on a solid theoretical foundation.
“Viruses, especially mumps and measles, have long been known to cause acute pancreatitis,” he said. “Additionally, the ACE2 receptor is present on pancreatic beta-cells and may mediate COVID-19 induced pancreatitis.”
Along with larger observational studies, Dr. Ketwaroo suggested that a number of interventional questions remain unanswered.
“While most acute pancreatitis is treated with supportive care, could proven therapies for COVID-19, such as steroids, also mitigate COVID-19 acute pancreatitis?” he asked. “Is COVID-19 a cofactor for acute pancreatitis caused by alcohol or endoscopic retrograde cholangiopancreatography? We await further information from an active area of research.”
The investigators disclosed relationships with Boston Scientific, Olympus, Fujifilm, and others.
SOURCE: Inamdar S et al. Gastroenterology. 2020 Aug 26. doi: 10.1053/j.gastro.2020.08.044.
Mounting data support acute pancreatitis as one possible GI manifestation of COVID-19, according to investigators.
While previous case reports suggested that infection with SARS-CoV2 may lead to pancreatitis, this retrospective analysis, which is the largest to date, is the first to offer substantial evidence for this claim, reported lead author Sumant Inamdar, MBBS, of the University of Arkansas, Little Rock, and colleagues.
“It has become increasingly clear that COVID-19 has systemic effects that also includes the gastrointestinal and pancreaticobiliary systems,” the investigators wrote in Gastroenterology. “As islet cells of the pancreas contain ACE2 receptor proteins, SARS-CoV2 can bind to these receptors and cause pancreatic injury.”
For the present analysis, Dr. Inamdar and colleagues reviewed charts from 48,012 patients who were hospitalized in New York between March and June of this year. While pancreatitis is usually diagnosed based on two out of three criteria, disease classification in the study required all three: characteristic upper abdominal pain upon admission, lipase greater than three times the upper limit of normal, and evidence of pancreatitis on cross-sectional imaging.
“[B]y including all three criteria for pancreatitis in our definition, we may be underestimating the rate of pancreatitis,” the investigators wrote. “However, we felt including diagnostic lipase levels and imaging was important for the accuracy of the diagnosis.”
Primary outcomes included mechanical ventilation, length of stay, development of pancreatic necrosis, and mortality. Outcomes were compared between patients with and without COVID-19.
Out of 48,012 hospitalized patients, 11,883 (24.75%) tested positive for SARS-CoV2. Across the entire population, 189 patients had pancreatitis (0.39%), and of these, 32 (17%) also had COVID-19. This translates to a point prevalence for pancreatitis of 0.27% for patients hospitalized with COVID-19.
Among patients with pancreatitis who did not have COVID-19, the most common etiologies for pancreatitis were gallstones (34%) and alcohol (37%), compared with just 16% and 6% of SARS-CoV2-positive cases of pancreatitis, respectively. Idiopathic pancreatitis was significantly more common among patients with COVID-19 than those without (69% vs 21%; P less than .0001).
Black or Hispanic patients with pancreatitis were 4-5 times more likely to have COVID-19 than patients with pancreatitis who were white. Across all races/ethnicities, patients with pancreatitis and COVID-19 more often required mechanical ventilation (odds ratio [OR], 5.65) and longer hospital stays (OR, 3.22), compared with those who had pancreatitis alone. While rates of mortality and pancreatic necrosis showed similar trends, associations with COVID-19 were not statistically significant.
“These findings support the notion that pancreatitis should be included in the list of GI manifestations of COVID-19,” the investigators wrote.
When caring for patients with COVID-19, Dr. Inamdar and colleagues recommended that clinicians pay close attention to any history of abdominal pain, and consider testing serum lipase levels.
“Further large studies are needed to confirm our findings,” they concluded.
Gyanprakash Avinash Ketwaroo, MD, of Baylor College of Medicine in Houston, agreed that more work is needed; in the meantime, he suggested that evidence is now strong enough for clinicians to take notice.
“Overall, this study adds further weight to COVID-19 acute pancreatitis,” he said. “Larger studies, and convincing pathophysiologic data, will be needed to confirm COVID-19 as a cause of acute pancreatitis. However, there appears to be enough circumstantial evidence to consider a COVID-19 diagnosis in patients presenting with acute pancreatitis.”
He noted that the new clinical evidence also stands on a solid theoretical foundation.
“Viruses, especially mumps and measles, have long been known to cause acute pancreatitis,” he said. “Additionally, the ACE2 receptor is present on pancreatic beta-cells and may mediate COVID-19 induced pancreatitis.”
Along with larger observational studies, Dr. Ketwaroo suggested that a number of interventional questions remain unanswered.
“While most acute pancreatitis is treated with supportive care, could proven therapies for COVID-19, such as steroids, also mitigate COVID-19 acute pancreatitis?” he asked. “Is COVID-19 a cofactor for acute pancreatitis caused by alcohol or endoscopic retrograde cholangiopancreatography? We await further information from an active area of research.”
The investigators disclosed relationships with Boston Scientific, Olympus, Fujifilm, and others.
SOURCE: Inamdar S et al. Gastroenterology. 2020 Aug 26. doi: 10.1053/j.gastro.2020.08.044.
Mounting data support acute pancreatitis as one possible GI manifestation of COVID-19, according to investigators.
While previous case reports suggested that infection with SARS-CoV2 may lead to pancreatitis, this retrospective analysis, which is the largest to date, is the first to offer substantial evidence for this claim, reported lead author Sumant Inamdar, MBBS, of the University of Arkansas, Little Rock, and colleagues.
“It has become increasingly clear that COVID-19 has systemic effects that also includes the gastrointestinal and pancreaticobiliary systems,” the investigators wrote in Gastroenterology. “As islet cells of the pancreas contain ACE2 receptor proteins, SARS-CoV2 can bind to these receptors and cause pancreatic injury.”
For the present analysis, Dr. Inamdar and colleagues reviewed charts from 48,012 patients who were hospitalized in New York between March and June of this year. While pancreatitis is usually diagnosed based on two out of three criteria, disease classification in the study required all three: characteristic upper abdominal pain upon admission, lipase greater than three times the upper limit of normal, and evidence of pancreatitis on cross-sectional imaging.
“[B]y including all three criteria for pancreatitis in our definition, we may be underestimating the rate of pancreatitis,” the investigators wrote. “However, we felt including diagnostic lipase levels and imaging was important for the accuracy of the diagnosis.”
Primary outcomes included mechanical ventilation, length of stay, development of pancreatic necrosis, and mortality. Outcomes were compared between patients with and without COVID-19.
Out of 48,012 hospitalized patients, 11,883 (24.75%) tested positive for SARS-CoV2. Across the entire population, 189 patients had pancreatitis (0.39%), and of these, 32 (17%) also had COVID-19. This translates to a point prevalence for pancreatitis of 0.27% for patients hospitalized with COVID-19.
Among patients with pancreatitis who did not have COVID-19, the most common etiologies for pancreatitis were gallstones (34%) and alcohol (37%), compared with just 16% and 6% of SARS-CoV2-positive cases of pancreatitis, respectively. Idiopathic pancreatitis was significantly more common among patients with COVID-19 than those without (69% vs 21%; P less than .0001).
Black or Hispanic patients with pancreatitis were 4-5 times more likely to have COVID-19 than patients with pancreatitis who were white. Across all races/ethnicities, patients with pancreatitis and COVID-19 more often required mechanical ventilation (odds ratio [OR], 5.65) and longer hospital stays (OR, 3.22), compared with those who had pancreatitis alone. While rates of mortality and pancreatic necrosis showed similar trends, associations with COVID-19 were not statistically significant.
“These findings support the notion that pancreatitis should be included in the list of GI manifestations of COVID-19,” the investigators wrote.
When caring for patients with COVID-19, Dr. Inamdar and colleagues recommended that clinicians pay close attention to any history of abdominal pain, and consider testing serum lipase levels.
“Further large studies are needed to confirm our findings,” they concluded.
Gyanprakash Avinash Ketwaroo, MD, of Baylor College of Medicine in Houston, agreed that more work is needed; in the meantime, he suggested that evidence is now strong enough for clinicians to take notice.
“Overall, this study adds further weight to COVID-19 acute pancreatitis,” he said. “Larger studies, and convincing pathophysiologic data, will be needed to confirm COVID-19 as a cause of acute pancreatitis. However, there appears to be enough circumstantial evidence to consider a COVID-19 diagnosis in patients presenting with acute pancreatitis.”
He noted that the new clinical evidence also stands on a solid theoretical foundation.
“Viruses, especially mumps and measles, have long been known to cause acute pancreatitis,” he said. “Additionally, the ACE2 receptor is present on pancreatic beta-cells and may mediate COVID-19 induced pancreatitis.”
Along with larger observational studies, Dr. Ketwaroo suggested that a number of interventional questions remain unanswered.
“While most acute pancreatitis is treated with supportive care, could proven therapies for COVID-19, such as steroids, also mitigate COVID-19 acute pancreatitis?” he asked. “Is COVID-19 a cofactor for acute pancreatitis caused by alcohol or endoscopic retrograde cholangiopancreatography? We await further information from an active area of research.”
The investigators disclosed relationships with Boston Scientific, Olympus, Fujifilm, and others.
SOURCE: Inamdar S et al. Gastroenterology. 2020 Aug 26. doi: 10.1053/j.gastro.2020.08.044.
FROM GASTROENTEROLOGY
Five reasons why medical meetings will never be the same
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
Lowering rituximab dose in patients with MS proves safe and effective
“Given its favorable cost-effectiveness profile, [rituximab] remains a valuable treatment option in the current landscape of MS treatments, even at the reduced dose,” wrote Giulio Disanto, MD, PhD, of the Neurocenter of Southern Switzerland in Lugano, and coauthors. The study was published in Multiple Sclerosis Journal.
To determine the clinical and radiologic effectiveness of deescalating rituximab dosage – along with assessing any adverse outcomes – this observational, single-center study examined 59 patients with MS who had been treated with rituximab at 1,000 mg for at least 1 year before the study began. Roughly 63% (n = 37) of the patients had relapsing remitting MS (RRMS), while the rest (n = 22) had secondary progressive disease (SPD). Their median age was 51, and nearly 75% were women.
All patients underwent neurologic examinations at baseline and then every 3 months for 1 year, with new symptoms, infections, or adverse events being assessed via the Expanded Disability Status Scale (EDSS). They also underwent brain and spinal MRI at baseline and at 12 months while blood samples were taken at baseline and then every 3 months for 1 year, with previous data for both collected when available.
Study results
All 59 patients completed 12-month follow-up, and no relapses occurred in the year after lowering rituximab dosage to 500 mg. No significant differences were observed when comparing EDSS scores at the start of the 1,000-mg dose with the start of the 500-mg dose (Wilcoxon P = .131) as well as from the start of the 500-mg dose to the end of follow-up (Wilcoxon P = .284). Analyzing RRMS and SPD patients separately also led to no differences in EDSS scores from the start of the 500-mg dose to the end of follow-up (Wilcoxon P = .531; Wilcoxon P = .408).
During the 1,000-mg treatment period the number of patients who developed at least one new T2 lesion on their brain or spine was 9 and 4, respectively. During the 500-mg period, just one patient developed a new T2 brain lesion and two patients developed new T2 spine lesions. IgG and IgM levels did not change from the start of 500-mg treatment, although total dose of rituximab was inversely associated with IgG concentrations when previous treatment with 1,000 mg was factored in (coefficient, −0.439; P = 0.041).
A total of 33 patients reported at least one adverse event during the 500-mg treatment period, with only three events being classified as serious: one pancreatitis, one coronary stenting, and one neutropenia.
Validating clinical experience
“This randomized trial is an important step,” said Timothy Vollmer, MD, of the Rocky Mountain MS Center in Westminster, Colo., in an interview. “It clearly supports that you can lessen the dose, which will allow us to use this revolutionary drug for a longer period of time in patients.”
Dr. Vollmer noted that, at his center, they have been using 500 mg of rituximab over a 6-month period since 2010 without a formal clinical trial and with no notable difference in adverse outcomes on MRIs or disability scales. “This validates what we’ve been doing, which we appreciate,” he said.
“The next thing you have to do is determine whether you really have to give it every 6 months,” he added, “because the treatment effect in most patients will last, in terms of B-cell depletion, about a year or more. What we should be testing next is giving the 500 mg and waiting until patients begin to recover B cells before we give them the next cycle, to see if that helps decrease the major side effect, which is a drop in IgG levels.”
The authors acknowledged their study’s limitations, including a moderate sample size, a short follow-up period after 500-mg dosage, and an inability to confirm consistency among 1,000-mg dose administration among all patients, which “may well influence efficacy and safety measures.”
The study was supported by the Neurocenter of Southern Switzerland. One author declared numerous potential conflicts of interest, including receiving speaker fees, research fees, and travel support, and serving on advisory boards for various foundations, universities, and pharmaceutical companies.
SOURCE: Disanto G et al. Mult Scler J. 2020 Aug 25. doi: 10.1177/1352458520952036.
“Given its favorable cost-effectiveness profile, [rituximab] remains a valuable treatment option in the current landscape of MS treatments, even at the reduced dose,” wrote Giulio Disanto, MD, PhD, of the Neurocenter of Southern Switzerland in Lugano, and coauthors. The study was published in Multiple Sclerosis Journal.
To determine the clinical and radiologic effectiveness of deescalating rituximab dosage – along with assessing any adverse outcomes – this observational, single-center study examined 59 patients with MS who had been treated with rituximab at 1,000 mg for at least 1 year before the study began. Roughly 63% (n = 37) of the patients had relapsing remitting MS (RRMS), while the rest (n = 22) had secondary progressive disease (SPD). Their median age was 51, and nearly 75% were women.
All patients underwent neurologic examinations at baseline and then every 3 months for 1 year, with new symptoms, infections, or adverse events being assessed via the Expanded Disability Status Scale (EDSS). They also underwent brain and spinal MRI at baseline and at 12 months while blood samples were taken at baseline and then every 3 months for 1 year, with previous data for both collected when available.
Study results
All 59 patients completed 12-month follow-up, and no relapses occurred in the year after lowering rituximab dosage to 500 mg. No significant differences were observed when comparing EDSS scores at the start of the 1,000-mg dose with the start of the 500-mg dose (Wilcoxon P = .131) as well as from the start of the 500-mg dose to the end of follow-up (Wilcoxon P = .284). Analyzing RRMS and SPD patients separately also led to no differences in EDSS scores from the start of the 500-mg dose to the end of follow-up (Wilcoxon P = .531; Wilcoxon P = .408).
During the 1,000-mg treatment period the number of patients who developed at least one new T2 lesion on their brain or spine was 9 and 4, respectively. During the 500-mg period, just one patient developed a new T2 brain lesion and two patients developed new T2 spine lesions. IgG and IgM levels did not change from the start of 500-mg treatment, although total dose of rituximab was inversely associated with IgG concentrations when previous treatment with 1,000 mg was factored in (coefficient, −0.439; P = 0.041).
A total of 33 patients reported at least one adverse event during the 500-mg treatment period, with only three events being classified as serious: one pancreatitis, one coronary stenting, and one neutropenia.
Validating clinical experience
“This randomized trial is an important step,” said Timothy Vollmer, MD, of the Rocky Mountain MS Center in Westminster, Colo., in an interview. “It clearly supports that you can lessen the dose, which will allow us to use this revolutionary drug for a longer period of time in patients.”
Dr. Vollmer noted that, at his center, they have been using 500 mg of rituximab over a 6-month period since 2010 without a formal clinical trial and with no notable difference in adverse outcomes on MRIs or disability scales. “This validates what we’ve been doing, which we appreciate,” he said.
“The next thing you have to do is determine whether you really have to give it every 6 months,” he added, “because the treatment effect in most patients will last, in terms of B-cell depletion, about a year or more. What we should be testing next is giving the 500 mg and waiting until patients begin to recover B cells before we give them the next cycle, to see if that helps decrease the major side effect, which is a drop in IgG levels.”
The authors acknowledged their study’s limitations, including a moderate sample size, a short follow-up period after 500-mg dosage, and an inability to confirm consistency among 1,000-mg dose administration among all patients, which “may well influence efficacy and safety measures.”
The study was supported by the Neurocenter of Southern Switzerland. One author declared numerous potential conflicts of interest, including receiving speaker fees, research fees, and travel support, and serving on advisory boards for various foundations, universities, and pharmaceutical companies.
SOURCE: Disanto G et al. Mult Scler J. 2020 Aug 25. doi: 10.1177/1352458520952036.
“Given its favorable cost-effectiveness profile, [rituximab] remains a valuable treatment option in the current landscape of MS treatments, even at the reduced dose,” wrote Giulio Disanto, MD, PhD, of the Neurocenter of Southern Switzerland in Lugano, and coauthors. The study was published in Multiple Sclerosis Journal.
To determine the clinical and radiologic effectiveness of deescalating rituximab dosage – along with assessing any adverse outcomes – this observational, single-center study examined 59 patients with MS who had been treated with rituximab at 1,000 mg for at least 1 year before the study began. Roughly 63% (n = 37) of the patients had relapsing remitting MS (RRMS), while the rest (n = 22) had secondary progressive disease (SPD). Their median age was 51, and nearly 75% were women.
All patients underwent neurologic examinations at baseline and then every 3 months for 1 year, with new symptoms, infections, or adverse events being assessed via the Expanded Disability Status Scale (EDSS). They also underwent brain and spinal MRI at baseline and at 12 months while blood samples were taken at baseline and then every 3 months for 1 year, with previous data for both collected when available.
Study results
All 59 patients completed 12-month follow-up, and no relapses occurred in the year after lowering rituximab dosage to 500 mg. No significant differences were observed when comparing EDSS scores at the start of the 1,000-mg dose with the start of the 500-mg dose (Wilcoxon P = .131) as well as from the start of the 500-mg dose to the end of follow-up (Wilcoxon P = .284). Analyzing RRMS and SPD patients separately also led to no differences in EDSS scores from the start of the 500-mg dose to the end of follow-up (Wilcoxon P = .531; Wilcoxon P = .408).
During the 1,000-mg treatment period the number of patients who developed at least one new T2 lesion on their brain or spine was 9 and 4, respectively. During the 500-mg period, just one patient developed a new T2 brain lesion and two patients developed new T2 spine lesions. IgG and IgM levels did not change from the start of 500-mg treatment, although total dose of rituximab was inversely associated with IgG concentrations when previous treatment with 1,000 mg was factored in (coefficient, −0.439; P = 0.041).
A total of 33 patients reported at least one adverse event during the 500-mg treatment period, with only three events being classified as serious: one pancreatitis, one coronary stenting, and one neutropenia.
Validating clinical experience
“This randomized trial is an important step,” said Timothy Vollmer, MD, of the Rocky Mountain MS Center in Westminster, Colo., in an interview. “It clearly supports that you can lessen the dose, which will allow us to use this revolutionary drug for a longer period of time in patients.”
Dr. Vollmer noted that, at his center, they have been using 500 mg of rituximab over a 6-month period since 2010 without a formal clinical trial and with no notable difference in adverse outcomes on MRIs or disability scales. “This validates what we’ve been doing, which we appreciate,” he said.
“The next thing you have to do is determine whether you really have to give it every 6 months,” he added, “because the treatment effect in most patients will last, in terms of B-cell depletion, about a year or more. What we should be testing next is giving the 500 mg and waiting until patients begin to recover B cells before we give them the next cycle, to see if that helps decrease the major side effect, which is a drop in IgG levels.”
The authors acknowledged their study’s limitations, including a moderate sample size, a short follow-up period after 500-mg dosage, and an inability to confirm consistency among 1,000-mg dose administration among all patients, which “may well influence efficacy and safety measures.”
The study was supported by the Neurocenter of Southern Switzerland. One author declared numerous potential conflicts of interest, including receiving speaker fees, research fees, and travel support, and serving on advisory boards for various foundations, universities, and pharmaceutical companies.
SOURCE: Disanto G et al. Mult Scler J. 2020 Aug 25. doi: 10.1177/1352458520952036.
FROM MULTIPLE SCLEROSIS JOURNAL
Tools emerging to predict liver failure in cirrhosis
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.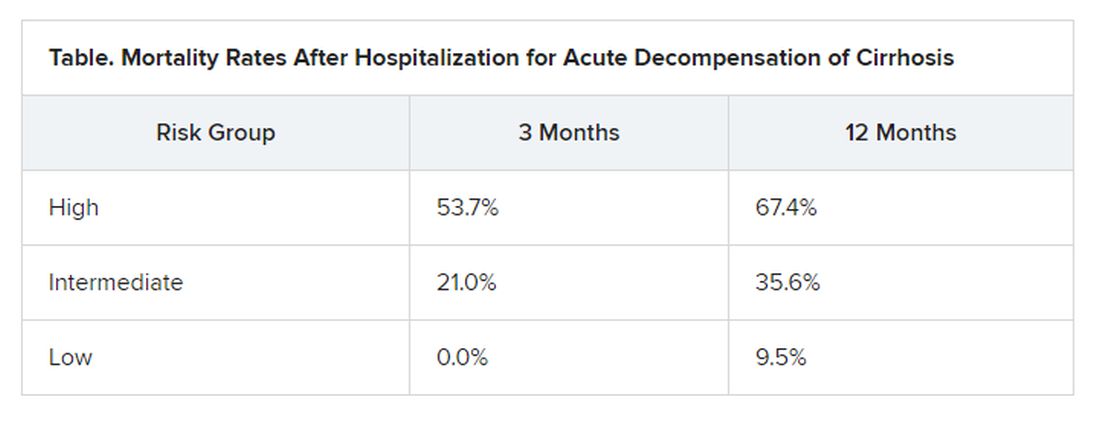
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Vascepa maker loses patent appeal, plans ‘vigorous’ fight
Amarin’s hopes of fending off generic competition for its blockbuster high-strength eicosapentaenoic acid product, icosapent ethyl (Vascepa), have dimmed following a decision by the U.S. Court of Appeals for the Federal Circuit in the company’s ongoing patent litigation.
The court upheld the March ruling by the District Court for the District of Nevada in favor of two generic companies in connection with their abbreviated new drug applications (ANDAs) for the product.
Amarin said it is currently reviewing its legal options and within 30 days expects to file a petition for an en banc review of the current decision by the full panel of 12 active judges at the Court of Appeals for the Federal Circuit.
“We are extremely disappointed with [the] ruling and plan to vigorously pursue available remedies,” John Thero, Amarin president and chief executive officer, said in a statement.
In 2012, Vascepa became the first and only prescription treatment approved by the Food and Drug Administration made up solely of the active ingredient icosapent ethyl, a unique form of eicosapentaenoic acid. It was initially approved for the reduction of very high triglyceride levels (≥500 mg/dL).
In late 2019, the FDA extended the indication to reduce the risk for cardiovascular events in people with elevated triglyceride levels and either established CV disease or diabetes with other CV risk factors.
The extended indication was based on results of the landmark REDUCE-IT trial, which showed a 25% relative risk reduction in major adverse CV events with icosapent ethyl, compared with placebo, in patients with triglyceride levels above 135 mg/dL and who had CV disease (70% of the study population), or who were high-risk primary-prevention patients with diabetes and one additional risk factor (30% of the study population).
According to Amarin, since its launch, Vascepa has been prescribed more than 8 million times.
The company said demand for the product in the United States remains “strong” and indicated that, despite the legal setback, it would continue promotional efforts. The company is also seeking additional regulatory approvals in China, Europe, and additional countries in the Middle East.
“We are particularly excited about the anticipated commercialization opportunities for Vascepa in Europe as we prepare for expected approval and launch in early 2021,” Mr. Thero said.
The company anticipates 10 years of market protection because of regulatory exclusivity in the European Union once approved, and said patent protection could extend into 2039.
Only Vascepa sold in the United States is subject to this litigation and judgment. No generic litigation is pending outside the United States, Amarin said.
A version of this article originally appeared on Medscape.com.
Amarin’s hopes of fending off generic competition for its blockbuster high-strength eicosapentaenoic acid product, icosapent ethyl (Vascepa), have dimmed following a decision by the U.S. Court of Appeals for the Federal Circuit in the company’s ongoing patent litigation.
The court upheld the March ruling by the District Court for the District of Nevada in favor of two generic companies in connection with their abbreviated new drug applications (ANDAs) for the product.
Amarin said it is currently reviewing its legal options and within 30 days expects to file a petition for an en banc review of the current decision by the full panel of 12 active judges at the Court of Appeals for the Federal Circuit.
“We are extremely disappointed with [the] ruling and plan to vigorously pursue available remedies,” John Thero, Amarin president and chief executive officer, said in a statement.
In 2012, Vascepa became the first and only prescription treatment approved by the Food and Drug Administration made up solely of the active ingredient icosapent ethyl, a unique form of eicosapentaenoic acid. It was initially approved for the reduction of very high triglyceride levels (≥500 mg/dL).
In late 2019, the FDA extended the indication to reduce the risk for cardiovascular events in people with elevated triglyceride levels and either established CV disease or diabetes with other CV risk factors.
The extended indication was based on results of the landmark REDUCE-IT trial, which showed a 25% relative risk reduction in major adverse CV events with icosapent ethyl, compared with placebo, in patients with triglyceride levels above 135 mg/dL and who had CV disease (70% of the study population), or who were high-risk primary-prevention patients with diabetes and one additional risk factor (30% of the study population).
According to Amarin, since its launch, Vascepa has been prescribed more than 8 million times.
The company said demand for the product in the United States remains “strong” and indicated that, despite the legal setback, it would continue promotional efforts. The company is also seeking additional regulatory approvals in China, Europe, and additional countries in the Middle East.
“We are particularly excited about the anticipated commercialization opportunities for Vascepa in Europe as we prepare for expected approval and launch in early 2021,” Mr. Thero said.
The company anticipates 10 years of market protection because of regulatory exclusivity in the European Union once approved, and said patent protection could extend into 2039.
Only Vascepa sold in the United States is subject to this litigation and judgment. No generic litigation is pending outside the United States, Amarin said.
A version of this article originally appeared on Medscape.com.
Amarin’s hopes of fending off generic competition for its blockbuster high-strength eicosapentaenoic acid product, icosapent ethyl (Vascepa), have dimmed following a decision by the U.S. Court of Appeals for the Federal Circuit in the company’s ongoing patent litigation.
The court upheld the March ruling by the District Court for the District of Nevada in favor of two generic companies in connection with their abbreviated new drug applications (ANDAs) for the product.
Amarin said it is currently reviewing its legal options and within 30 days expects to file a petition for an en banc review of the current decision by the full panel of 12 active judges at the Court of Appeals for the Federal Circuit.
“We are extremely disappointed with [the] ruling and plan to vigorously pursue available remedies,” John Thero, Amarin president and chief executive officer, said in a statement.
In 2012, Vascepa became the first and only prescription treatment approved by the Food and Drug Administration made up solely of the active ingredient icosapent ethyl, a unique form of eicosapentaenoic acid. It was initially approved for the reduction of very high triglyceride levels (≥500 mg/dL).
In late 2019, the FDA extended the indication to reduce the risk for cardiovascular events in people with elevated triglyceride levels and either established CV disease or diabetes with other CV risk factors.
The extended indication was based on results of the landmark REDUCE-IT trial, which showed a 25% relative risk reduction in major adverse CV events with icosapent ethyl, compared with placebo, in patients with triglyceride levels above 135 mg/dL and who had CV disease (70% of the study population), or who were high-risk primary-prevention patients with diabetes and one additional risk factor (30% of the study population).
According to Amarin, since its launch, Vascepa has been prescribed more than 8 million times.
The company said demand for the product in the United States remains “strong” and indicated that, despite the legal setback, it would continue promotional efforts. The company is also seeking additional regulatory approvals in China, Europe, and additional countries in the Middle East.
“We are particularly excited about the anticipated commercialization opportunities for Vascepa in Europe as we prepare for expected approval and launch in early 2021,” Mr. Thero said.
The company anticipates 10 years of market protection because of regulatory exclusivity in the European Union once approved, and said patent protection could extend into 2039.
Only Vascepa sold in the United States is subject to this litigation and judgment. No generic litigation is pending outside the United States, Amarin said.
A version of this article originally appeared on Medscape.com.