User login
Low VWF levels or blood group O not linked to intracerebral hemorrhage risk
In contrast to findings of previous research, low levels of von Willebrand Factor (VWF) and blood group O were not associated with a first-ever intracerebral hemorrhage (ICH), according to a study published in Thrombosis Research.
The researchers compared 176 cases of ICH with 349 age- and sex-matched controls. The mean patient age was 57 years, and 50% were women. The median time from baseline blood sampling to the first ICH was 5.6 years, according to the study reported by Kristina Johansson of Umeå (Sweden) University and her colleagues.
Complicated picture
The level of VWF differed significantly among blood groups: In individuals with blood group O, the mean VWF level was 1.29 kIU/L; for blood group A, it was 1.52 kIU/L; for blood group AB, 1.59 kIU/L; and in blood group B, 1.76 kIU/L. However, there was no difference in VWF concentration between cases and controls.
The researchers found no association between blood group O and the risk of ICH, a finding previously seen in other studies. They did, however, find that, in the limited number of patients with blood group B there was an association with a lower risk of ICH, compared with blood group A (odds ratio, 0.47; 95% confidence interval, 0.23-0.95).
“To our knowledge this is the largest prospective study investigating the association between VWF, ABO blood group and ICH. We found no association between VWF or blood group O and risk of future ICH,” the researchers concluded.
The study was funded by public institutions in Sweden. The authors declared that they had no conflicts.
SOURCE: Johansson K et al. Thromb Res. 2020 Jul 5;195:77-80.
In contrast to findings of previous research, low levels of von Willebrand Factor (VWF) and blood group O were not associated with a first-ever intracerebral hemorrhage (ICH), according to a study published in Thrombosis Research.
The researchers compared 176 cases of ICH with 349 age- and sex-matched controls. The mean patient age was 57 years, and 50% were women. The median time from baseline blood sampling to the first ICH was 5.6 years, according to the study reported by Kristina Johansson of Umeå (Sweden) University and her colleagues.
Complicated picture
The level of VWF differed significantly among blood groups: In individuals with blood group O, the mean VWF level was 1.29 kIU/L; for blood group A, it was 1.52 kIU/L; for blood group AB, 1.59 kIU/L; and in blood group B, 1.76 kIU/L. However, there was no difference in VWF concentration between cases and controls.
The researchers found no association between blood group O and the risk of ICH, a finding previously seen in other studies. They did, however, find that, in the limited number of patients with blood group B there was an association with a lower risk of ICH, compared with blood group A (odds ratio, 0.47; 95% confidence interval, 0.23-0.95).
“To our knowledge this is the largest prospective study investigating the association between VWF, ABO blood group and ICH. We found no association between VWF or blood group O and risk of future ICH,” the researchers concluded.
The study was funded by public institutions in Sweden. The authors declared that they had no conflicts.
SOURCE: Johansson K et al. Thromb Res. 2020 Jul 5;195:77-80.
In contrast to findings of previous research, low levels of von Willebrand Factor (VWF) and blood group O were not associated with a first-ever intracerebral hemorrhage (ICH), according to a study published in Thrombosis Research.
The researchers compared 176 cases of ICH with 349 age- and sex-matched controls. The mean patient age was 57 years, and 50% were women. The median time from baseline blood sampling to the first ICH was 5.6 years, according to the study reported by Kristina Johansson of Umeå (Sweden) University and her colleagues.
Complicated picture
The level of VWF differed significantly among blood groups: In individuals with blood group O, the mean VWF level was 1.29 kIU/L; for blood group A, it was 1.52 kIU/L; for blood group AB, 1.59 kIU/L; and in blood group B, 1.76 kIU/L. However, there was no difference in VWF concentration between cases and controls.
The researchers found no association between blood group O and the risk of ICH, a finding previously seen in other studies. They did, however, find that, in the limited number of patients with blood group B there was an association with a lower risk of ICH, compared with blood group A (odds ratio, 0.47; 95% confidence interval, 0.23-0.95).
“To our knowledge this is the largest prospective study investigating the association between VWF, ABO blood group and ICH. We found no association between VWF or blood group O and risk of future ICH,” the researchers concluded.
The study was funded by public institutions in Sweden. The authors declared that they had no conflicts.
SOURCE: Johansson K et al. Thromb Res. 2020 Jul 5;195:77-80.
FROM THROMBOSIS RESEARCH
Lifting the restrictions on mifepristone during COVID-19: A step in the right direction
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
4-year-old girl • limited movement & diffuse pain in both arms • pronated hands • Dx?
THE CASE
A 4-year-old girl was triaged to the Pediatric Emergency Department (PED) Fast Track, complaining of pain and limited movement in both arms. For an unknown reason, she had attempted to lift a heavy, 3-person sofa several hours earlier.
Her prior medical history included left nursemaid elbow (NME) at both 15 months and 33 months of age. Neither event had a known mechanism of injury. In both episodes, it was noted in the medical record that the child was not using her arm, “was holding it funny,” and was complaining of pain. Each time, she presented about 24 hours after symptom onset.
During the physical exam in the PED, the patient showed no signs of acute distress. She held both arms close to her body, with a slight flexion at the elbows, and her hands were pronated. She could not pinpoint the location of her discomfort and described diffuse pain in her forearms, elbows, and upper arms. Examination revealed no localized pain or tenderness in her hands, wrists, or clavicles. Radial pulses were easily palpated, and capillary refill was less than 2 seconds. There was no swelling or bruising. The rest of her physical exam was normal.
DIAGNOSIS
The patient was given a diagnosis of self-inflicted bilateral
DISCUSSION
BNME is an uncommon diagnosis; a literature review of reported cases indicates none were self-inflicted.1-4 However, NME is a common injury and is easily reduced. The classic mechanism of injury for NME involves the elbow in extension, while the forearm is pronated, and a sudden brisk axial traction is applied. This combination of motions causes the annular ligament to slip over the head of the radius and become displaced downward into the radiohumeral joint, where it becomes entrapped. In this case, the patient apparently exerted enough longitudinal traction while trying to lift the couch to produce the injury.
NME occurs most commonly in the left arm of girls between the ages of 4 months and 7 years and peaks at around the age of 2 years.5 A 2014 study by Irie et al6 corroborated the findings on left-side predominance and increased incidence with age, noting that frequency of injury peaked at 6 months in those younger than 1 year of age and at 2 years for those 1 year or older. However, the researchers found no significant sex difference.6
NME is radiographically indistinguishable from a healthy elbow.7 To prevent unnecessary expense and radiation exposure in young children, prereduction radiographs should only be used to rule out the possibility of fracture or other injury.7 Krul et al8 recommend restricting x-ray use to cases with an unclear history or those that are due to trauma other than an arm pull.
Continue to: Methods of reduction
Methods of reduction. Once NME is diagnosed, there are 2 methods of reduction: hyper-pronation and supination-flexion. Reduction is best performed with the child sitting in the parent’s lap with the injured arm facing the examiner.
Success rates for both methods of NME reduction are statistically similar; however, first-attempt success rates are significantly higher with the hyper-pronation method than with supination-flexion.9 Furthermore, physicians have deemed the hyper-pronation method significantly easier to perform than supination-flexion.9 A Cochrane review by Krul et al10 concluded that the hyper-pronation method may result in lower failure rates than supination-flexion, but due to limited evidence, the researchers were unable to draw any conclusions on other outcomes, such as pain. Green et al11 noted that hyper-pronation is perceived by parents of children with NME as being less painful. For these reasons, hyper-pronation should be utilized as the first method of reduction, followed by supination-flexion if the former does not work.12
Additional management. In a limited study of 50 children with pulled-elbow injuries, ultrasound revealed that 78% had an intact yet interposed radial annular ligament and 22% had a tear in the radial annular ligament.13 The authors propose that if, after appropriate reduction methods are attempted, no pop is felt, or there is no prompt clinical improvement, and ultrasound is not available to assess the integrity of the annular ligament, the child should be placed in a splint for 7 days
Our patient returned to the PED 3 days later, complaining of pain and an inability to move her left arm after her older sibling pulled her by her outstretched arms. She was once again diagnosed with NME, the injury was reduced, and she was using the arm within minutes. She has not presented to either the PED or the pediatric clinic with a similar complaint since. Discarding outliers, NME recurrence rates fall within a range of 23.7% to 32.9%.14,15
THE TAKEAWAY
Pre-reduction x-rays are not warranted in cases of NME unless there is suspicion for fracture or another injury. The 2 reduction methods, hyper-pronation and supination-flexion, are easily mastered. Any reduction should be quick, easy, and as painless as possible. Hyper-pronation should be utilized first, as this maneuver seems to be the more successful and is perceived by parents as being less painful. However, it is always most helpful to be proficient in both methods. If, after appropriate attempts at reduction, the child has not regained the use of the arm, 7 days of splinting is recommended, along with an orthopedic referral.
CORRESPONDENCE
Robert N. Anderson, DNP, APRN, (F)NP-C, ENP-BC, Vanderbilt Health, 512 Autumn Springs Court, Suite 100 C, Franklin, TN 37067; bob.anderson@vumc.org
1. Quan L, Marcuse EK. The epidemiology and treatment of radial head subluxation. Am J Dis Child. 1985;139:1194-1197.
2. Michaels MG. A case of bilateral nursemaid’s elbow. Pediatr Emerg Care. 1989;5:226-227.
3. Meiner EV, Sama AE, Lee DC, et al. Bilateral nursemaid’s elbow. Am J Emerg Med. 2004;6:502-503.
4. Wang YX, Zhang G, Song B, et al. Radial head subluxation in pediatric clinics and emergency departments in China. Chin J Traum. 2019;22:340-344.
5. Schunk JE. Radial head subluxation: epidemiology and treatment of 87 episodes. Ann Emerg Med. 1990;19:1019-1023.
6. Irie T, Sono T, Hayama Y, et al. Investigation on 2331 cases of pulled elbow over the last 10 years. Pediatr Rep. 2014;6:5090. doi: 10.4081/pr.2014.5090
7. Eismann EA, Cosco ED, Wall EJ. Absence of radiographic abnormalities in nursemaid’s elbows. J Pediatr Orthop. 2014;34:426-431.
8. Krul M, van der Wouden JC, Koes BW, et al. Nursemaid’s Elbow: its diagnostic clues and preferred means of reduction. J Fam Pract. 2010:59:E5-E7.
9. Bek D, Yildiz C, Köse O, et al. Pronation versus supination maneuvers for the reduction of ‘pulled elbow’: a randomized clinical trial. Eur J Emerg Med. 2009;16:135-138.
10. Krul M, van der Wouden JC, Kruithof EJ, et al. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database Syst Rev. 2017.
11. Green DA, Linares MYR, Garcia Peña BM, et al. Randomized comparison of pain perception during radial head subluxation reduction using supination-flexion of forced pronation. Pediatr Emerg Care. 2006;22:235-238.
12. García-Mata S, Hidalgo-Ovejero A. Efficacy of reduction maneuvers for “pulled elbow” in children: a prospective study of 115 cases. J Pediatr Orthop. 2014;34:432-436.
13. Diab HS, Hamed MMS, Allam Y. Obscure pathology of pulled elbow: dynamic high-resolution ultrasound-assisted classification. J Child Orthop. 2010;4:539-543.
14. Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150:164-166.
15. Macias CG, Bothner J, Wiebe R. Comparison of supination/flexion to hyperpronation in the reduction of radial head subluxation. Pediatrics. 1998;102:E10. doi: 10.1542/peds.102.1.e10.
THE CASE
A 4-year-old girl was triaged to the Pediatric Emergency Department (PED) Fast Track, complaining of pain and limited movement in both arms. For an unknown reason, she had attempted to lift a heavy, 3-person sofa several hours earlier.
Her prior medical history included left nursemaid elbow (NME) at both 15 months and 33 months of age. Neither event had a known mechanism of injury. In both episodes, it was noted in the medical record that the child was not using her arm, “was holding it funny,” and was complaining of pain. Each time, she presented about 24 hours after symptom onset.
During the physical exam in the PED, the patient showed no signs of acute distress. She held both arms close to her body, with a slight flexion at the elbows, and her hands were pronated. She could not pinpoint the location of her discomfort and described diffuse pain in her forearms, elbows, and upper arms. Examination revealed no localized pain or tenderness in her hands, wrists, or clavicles. Radial pulses were easily palpated, and capillary refill was less than 2 seconds. There was no swelling or bruising. The rest of her physical exam was normal.
DIAGNOSIS
The patient was given a diagnosis of self-inflicted bilateral
DISCUSSION
BNME is an uncommon diagnosis; a literature review of reported cases indicates none were self-inflicted.1-4 However, NME is a common injury and is easily reduced. The classic mechanism of injury for NME involves the elbow in extension, while the forearm is pronated, and a sudden brisk axial traction is applied. This combination of motions causes the annular ligament to slip over the head of the radius and become displaced downward into the radiohumeral joint, where it becomes entrapped. In this case, the patient apparently exerted enough longitudinal traction while trying to lift the couch to produce the injury.
NME occurs most commonly in the left arm of girls between the ages of 4 months and 7 years and peaks at around the age of 2 years.5 A 2014 study by Irie et al6 corroborated the findings on left-side predominance and increased incidence with age, noting that frequency of injury peaked at 6 months in those younger than 1 year of age and at 2 years for those 1 year or older. However, the researchers found no significant sex difference.6
NME is radiographically indistinguishable from a healthy elbow.7 To prevent unnecessary expense and radiation exposure in young children, prereduction radiographs should only be used to rule out the possibility of fracture or other injury.7 Krul et al8 recommend restricting x-ray use to cases with an unclear history or those that are due to trauma other than an arm pull.
Continue to: Methods of reduction
Methods of reduction. Once NME is diagnosed, there are 2 methods of reduction: hyper-pronation and supination-flexion. Reduction is best performed with the child sitting in the parent’s lap with the injured arm facing the examiner.
Success rates for both methods of NME reduction are statistically similar; however, first-attempt success rates are significantly higher with the hyper-pronation method than with supination-flexion.9 Furthermore, physicians have deemed the hyper-pronation method significantly easier to perform than supination-flexion.9 A Cochrane review by Krul et al10 concluded that the hyper-pronation method may result in lower failure rates than supination-flexion, but due to limited evidence, the researchers were unable to draw any conclusions on other outcomes, such as pain. Green et al11 noted that hyper-pronation is perceived by parents of children with NME as being less painful. For these reasons, hyper-pronation should be utilized as the first method of reduction, followed by supination-flexion if the former does not work.12
Additional management. In a limited study of 50 children with pulled-elbow injuries, ultrasound revealed that 78% had an intact yet interposed radial annular ligament and 22% had a tear in the radial annular ligament.13 The authors propose that if, after appropriate reduction methods are attempted, no pop is felt, or there is no prompt clinical improvement, and ultrasound is not available to assess the integrity of the annular ligament, the child should be placed in a splint for 7 days
Our patient returned to the PED 3 days later, complaining of pain and an inability to move her left arm after her older sibling pulled her by her outstretched arms. She was once again diagnosed with NME, the injury was reduced, and she was using the arm within minutes. She has not presented to either the PED or the pediatric clinic with a similar complaint since. Discarding outliers, NME recurrence rates fall within a range of 23.7% to 32.9%.14,15
THE TAKEAWAY
Pre-reduction x-rays are not warranted in cases of NME unless there is suspicion for fracture or another injury. The 2 reduction methods, hyper-pronation and supination-flexion, are easily mastered. Any reduction should be quick, easy, and as painless as possible. Hyper-pronation should be utilized first, as this maneuver seems to be the more successful and is perceived by parents as being less painful. However, it is always most helpful to be proficient in both methods. If, after appropriate attempts at reduction, the child has not regained the use of the arm, 7 days of splinting is recommended, along with an orthopedic referral.
CORRESPONDENCE
Robert N. Anderson, DNP, APRN, (F)NP-C, ENP-BC, Vanderbilt Health, 512 Autumn Springs Court, Suite 100 C, Franklin, TN 37067; bob.anderson@vumc.org
THE CASE
A 4-year-old girl was triaged to the Pediatric Emergency Department (PED) Fast Track, complaining of pain and limited movement in both arms. For an unknown reason, she had attempted to lift a heavy, 3-person sofa several hours earlier.
Her prior medical history included left nursemaid elbow (NME) at both 15 months and 33 months of age. Neither event had a known mechanism of injury. In both episodes, it was noted in the medical record that the child was not using her arm, “was holding it funny,” and was complaining of pain. Each time, she presented about 24 hours after symptom onset.
During the physical exam in the PED, the patient showed no signs of acute distress. She held both arms close to her body, with a slight flexion at the elbows, and her hands were pronated. She could not pinpoint the location of her discomfort and described diffuse pain in her forearms, elbows, and upper arms. Examination revealed no localized pain or tenderness in her hands, wrists, or clavicles. Radial pulses were easily palpated, and capillary refill was less than 2 seconds. There was no swelling or bruising. The rest of her physical exam was normal.
DIAGNOSIS
The patient was given a diagnosis of self-inflicted bilateral
DISCUSSION
BNME is an uncommon diagnosis; a literature review of reported cases indicates none were self-inflicted.1-4 However, NME is a common injury and is easily reduced. The classic mechanism of injury for NME involves the elbow in extension, while the forearm is pronated, and a sudden brisk axial traction is applied. This combination of motions causes the annular ligament to slip over the head of the radius and become displaced downward into the radiohumeral joint, where it becomes entrapped. In this case, the patient apparently exerted enough longitudinal traction while trying to lift the couch to produce the injury.
NME occurs most commonly in the left arm of girls between the ages of 4 months and 7 years and peaks at around the age of 2 years.5 A 2014 study by Irie et al6 corroborated the findings on left-side predominance and increased incidence with age, noting that frequency of injury peaked at 6 months in those younger than 1 year of age and at 2 years for those 1 year or older. However, the researchers found no significant sex difference.6
NME is radiographically indistinguishable from a healthy elbow.7 To prevent unnecessary expense and radiation exposure in young children, prereduction radiographs should only be used to rule out the possibility of fracture or other injury.7 Krul et al8 recommend restricting x-ray use to cases with an unclear history or those that are due to trauma other than an arm pull.
Continue to: Methods of reduction
Methods of reduction. Once NME is diagnosed, there are 2 methods of reduction: hyper-pronation and supination-flexion. Reduction is best performed with the child sitting in the parent’s lap with the injured arm facing the examiner.
Success rates for both methods of NME reduction are statistically similar; however, first-attempt success rates are significantly higher with the hyper-pronation method than with supination-flexion.9 Furthermore, physicians have deemed the hyper-pronation method significantly easier to perform than supination-flexion.9 A Cochrane review by Krul et al10 concluded that the hyper-pronation method may result in lower failure rates than supination-flexion, but due to limited evidence, the researchers were unable to draw any conclusions on other outcomes, such as pain. Green et al11 noted that hyper-pronation is perceived by parents of children with NME as being less painful. For these reasons, hyper-pronation should be utilized as the first method of reduction, followed by supination-flexion if the former does not work.12
Additional management. In a limited study of 50 children with pulled-elbow injuries, ultrasound revealed that 78% had an intact yet interposed radial annular ligament and 22% had a tear in the radial annular ligament.13 The authors propose that if, after appropriate reduction methods are attempted, no pop is felt, or there is no prompt clinical improvement, and ultrasound is not available to assess the integrity of the annular ligament, the child should be placed in a splint for 7 days
Our patient returned to the PED 3 days later, complaining of pain and an inability to move her left arm after her older sibling pulled her by her outstretched arms. She was once again diagnosed with NME, the injury was reduced, and she was using the arm within minutes. She has not presented to either the PED or the pediatric clinic with a similar complaint since. Discarding outliers, NME recurrence rates fall within a range of 23.7% to 32.9%.14,15
THE TAKEAWAY
Pre-reduction x-rays are not warranted in cases of NME unless there is suspicion for fracture or another injury. The 2 reduction methods, hyper-pronation and supination-flexion, are easily mastered. Any reduction should be quick, easy, and as painless as possible. Hyper-pronation should be utilized first, as this maneuver seems to be the more successful and is perceived by parents as being less painful. However, it is always most helpful to be proficient in both methods. If, after appropriate attempts at reduction, the child has not regained the use of the arm, 7 days of splinting is recommended, along with an orthopedic referral.
CORRESPONDENCE
Robert N. Anderson, DNP, APRN, (F)NP-C, ENP-BC, Vanderbilt Health, 512 Autumn Springs Court, Suite 100 C, Franklin, TN 37067; bob.anderson@vumc.org
1. Quan L, Marcuse EK. The epidemiology and treatment of radial head subluxation. Am J Dis Child. 1985;139:1194-1197.
2. Michaels MG. A case of bilateral nursemaid’s elbow. Pediatr Emerg Care. 1989;5:226-227.
3. Meiner EV, Sama AE, Lee DC, et al. Bilateral nursemaid’s elbow. Am J Emerg Med. 2004;6:502-503.
4. Wang YX, Zhang G, Song B, et al. Radial head subluxation in pediatric clinics and emergency departments in China. Chin J Traum. 2019;22:340-344.
5. Schunk JE. Radial head subluxation: epidemiology and treatment of 87 episodes. Ann Emerg Med. 1990;19:1019-1023.
6. Irie T, Sono T, Hayama Y, et al. Investigation on 2331 cases of pulled elbow over the last 10 years. Pediatr Rep. 2014;6:5090. doi: 10.4081/pr.2014.5090
7. Eismann EA, Cosco ED, Wall EJ. Absence of radiographic abnormalities in nursemaid’s elbows. J Pediatr Orthop. 2014;34:426-431.
8. Krul M, van der Wouden JC, Koes BW, et al. Nursemaid’s Elbow: its diagnostic clues and preferred means of reduction. J Fam Pract. 2010:59:E5-E7.
9. Bek D, Yildiz C, Köse O, et al. Pronation versus supination maneuvers for the reduction of ‘pulled elbow’: a randomized clinical trial. Eur J Emerg Med. 2009;16:135-138.
10. Krul M, van der Wouden JC, Kruithof EJ, et al. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database Syst Rev. 2017.
11. Green DA, Linares MYR, Garcia Peña BM, et al. Randomized comparison of pain perception during radial head subluxation reduction using supination-flexion of forced pronation. Pediatr Emerg Care. 2006;22:235-238.
12. García-Mata S, Hidalgo-Ovejero A. Efficacy of reduction maneuvers for “pulled elbow” in children: a prospective study of 115 cases. J Pediatr Orthop. 2014;34:432-436.
13. Diab HS, Hamed MMS, Allam Y. Obscure pathology of pulled elbow: dynamic high-resolution ultrasound-assisted classification. J Child Orthop. 2010;4:539-543.
14. Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150:164-166.
15. Macias CG, Bothner J, Wiebe R. Comparison of supination/flexion to hyperpronation in the reduction of radial head subluxation. Pediatrics. 1998;102:E10. doi: 10.1542/peds.102.1.e10.
1. Quan L, Marcuse EK. The epidemiology and treatment of radial head subluxation. Am J Dis Child. 1985;139:1194-1197.
2. Michaels MG. A case of bilateral nursemaid’s elbow. Pediatr Emerg Care. 1989;5:226-227.
3. Meiner EV, Sama AE, Lee DC, et al. Bilateral nursemaid’s elbow. Am J Emerg Med. 2004;6:502-503.
4. Wang YX, Zhang G, Song B, et al. Radial head subluxation in pediatric clinics and emergency departments in China. Chin J Traum. 2019;22:340-344.
5. Schunk JE. Radial head subluxation: epidemiology and treatment of 87 episodes. Ann Emerg Med. 1990;19:1019-1023.
6. Irie T, Sono T, Hayama Y, et al. Investigation on 2331 cases of pulled elbow over the last 10 years. Pediatr Rep. 2014;6:5090. doi: 10.4081/pr.2014.5090
7. Eismann EA, Cosco ED, Wall EJ. Absence of radiographic abnormalities in nursemaid’s elbows. J Pediatr Orthop. 2014;34:426-431.
8. Krul M, van der Wouden JC, Koes BW, et al. Nursemaid’s Elbow: its diagnostic clues and preferred means of reduction. J Fam Pract. 2010:59:E5-E7.
9. Bek D, Yildiz C, Köse O, et al. Pronation versus supination maneuvers for the reduction of ‘pulled elbow’: a randomized clinical trial. Eur J Emerg Med. 2009;16:135-138.
10. Krul M, van der Wouden JC, Kruithof EJ, et al. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database Syst Rev. 2017.
11. Green DA, Linares MYR, Garcia Peña BM, et al. Randomized comparison of pain perception during radial head subluxation reduction using supination-flexion of forced pronation. Pediatr Emerg Care. 2006;22:235-238.
12. García-Mata S, Hidalgo-Ovejero A. Efficacy of reduction maneuvers for “pulled elbow” in children: a prospective study of 115 cases. J Pediatr Orthop. 2014;34:432-436.
13. Diab HS, Hamed MMS, Allam Y. Obscure pathology of pulled elbow: dynamic high-resolution ultrasound-assisted classification. J Child Orthop. 2010;4:539-543.
14. Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150:164-166.
15. Macias CG, Bothner J, Wiebe R. Comparison of supination/flexion to hyperpronation in the reduction of radial head subluxation. Pediatrics. 1998;102:E10. doi: 10.1542/peds.102.1.e10.
Your role in early diagnosis & Tx of metastatic bone disease
Since the early 1990s, modern treatments have steadily reduced overall cancer mortality from primary tumors.1 Consequently, more people are at risk of metastatic bone disease, with subsequent pain and pathologic fractures1,2 and death from metastasis.3 Patients who have bone metastases present with a variety of signs and symptoms including pain, fractures, and metabolic derangements. The primary care approach to work-up and diagnosis described in this article enables prompt treatment, either surgical or nonsurgical, to maintain a high quality of life for patients.
Primary tumors determine types of metastases and prognosis
Metastasis, a complex pathologic process in which cancerous cells migrate to distant organs, implant, and grow,3 is a poor prognostic indicator in cancer patients. Bone is the third most common site of metastasis, behind the liver and lungs.4 While the true prevalence of metastatic bone cancer is unknown, studies have estimated it to be > 280,000 cases in the United States.5
Bone metastases interfere with normal bone metabolism and turnover in several different characteristic patterns. These changes—radiographically defined as osteoblastic, osteolytic, or mixed lesions—are determined by the primary tumor type.
- Osteoblastic lesions, comprised of new, disorganized bone formation, often occur secondary to prostate cancer, small cell lung cancer, and carcinoid malignancies, among others.
- Osteolytic lesions, in which bone is destroyed, are more common with breast cancer, renal cell carcinoma, melanoma, and multiple myeloma.
- Mixed lesions, in which areas of bone destruction and growth are simultaneously found, occur with some GI cancers and a few breast cancers.6,7
Most bone metastases result from carcinomas, of which up to 50% eventually spread to bone, although this process can take 10 to 15 years.8,9 The likelihood of bone metastasis depends on the primary tumor and its stage. Breast and prostate cancer account for most skeletal metastases, although these lesions are often asymptomatic.6,9 Other malignancies, such as ovarian and gastrointestinal, metastasize to bone much less frequently.7,10 Virtually any cancer at an advanced stage can spread to bone. These metastases are usually multifocal and incurable, with the patient’s prognosis varying from a few months to years.6,11,12
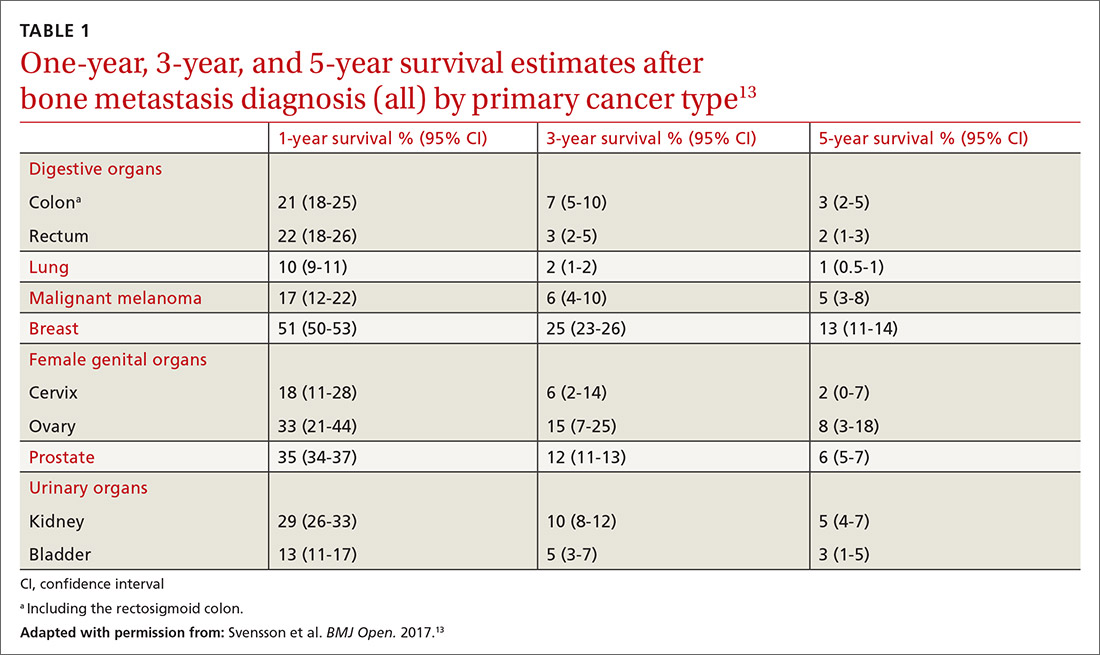
Factors that influence prognosis. Metastatic bone disease arising from melanoma and lung cancers has the shortest life expectancy of roughly 6 months from initial diagnosis; metastasis following prostate, breast, and thyroid cancers has the longest, usually 2 to 4 years.11TABLE 113 shows survival estimates from a large Danish population at various time points following bone metastasis diagnosis for several primary cancer types.
When surgical intervention for bony metastasis is required, prognosis is generally poorer, likely due to more advanced disease. The overall 1-year survival following surgery varies, but several large studies have found a rate of around 40% when considering all primary tumors.14,15 The most common metastases, from breast and prostate cancers, have 1-year survivals of around 50% and 30%, respectively, following surgical intervention.16-18
What you’re likely to see on presentation
Bone metastases are one of the leading causes of morbidity in cancer patients from resultant pain, pathologic fractures, metabolic derangements, and reduced activities of daily living.8,19 The most common cause of cancer pain is bone involvement.6 Patients report pain that is usually worse at night, poorly localized, and not alleviated with rest. They often mistakenly relate the pain to an injury.20 The pathophysiology of bone pain is not completely understood but is likely multifactorial and includes inflammatory and mechanical processes.7,21 Spine involvement can lead to stenosis or nerve root compression, with symptoms dependent on level and severity of nerve or cord compromise.20 Overall, the most common site of bone metastasis is the thoracic spine, followed by the ribs, pelvis, and proximal long bones.20
Continue to: Pathologic fractures
Pathologic fractures occur frequently in cancer patients. Bone destruction leads to a loss of mechanical support which, in turn, causes microfractures and pain. These microfractures can proliferate and coalesce, causing a pathologic fracture, often in weight-bearing bones.6 Breast cancer with lytic lesions is the single leading cause of all pathologic fractures.22 Lung cancer with its short survival time and prostate cancer with blastic lesions are less common causes.23 In the appendicular skeleton, the vast majority of these fractures occur in the femur and humerus.11
Symptomatic metabolic derangements. The most common metabolic disorder is hypercalcemia, found predominantly in patients with hematologic malignancies, squamous cell lung cancer, renal cell cancer, and breast cancer.6,7,12,24 The clinical presentation is nonspecific and can include polyuria, polydipsia, fatigue, constipation, and confusion. The prevalence is estimated to be 13% in breast cancer, 4% in lung cancers, and 1% in prostate cancer, although results in individual studies vary.12 The pathophysiology is multifactorial and often includes osteolytic lesions and an increased circulating level of parathyroid hormone–related peptide, although other mechanisms contribute.25,26 Ultimately, severe hypercalcemia may be fatal secondary to renal failure and cardiac arrhythmias.6,7,12 Paraneoplastic hypercalcemia independently decreases survival; 1 study found the median survival to be 10 to 12 weeks.11
Primary care work-up and diagnosis
When a patient presents with signs and symptoms suggestive of metastatic bone disease, inquire about a history of cancer. Even if such a history is remote, it is important—particularly so if the patient received chemotherapy or radiation, which can lead to secondary cancers such as leukemia or sarcoma.20 If a primary site of malignancy is unknown, pursue a general review of systems. Clues to the primary site of disease could be a history of chest pain, shortness of breath, hemoptysis, heat/cold intolerance, or changes in bowel/bladder habits. Also ask about risk factors such as smoking, chemical exposure, and sun exposure.
Pointers on radiographic imaging. If you suspect a destructive bone lesion, order appropriate radiographic imaging. Arrange for plain radiographs with at least 2 views of the specific area of interest that include the entire bone along with the joints above and below. Importantly, the entire bone must be imaged before any surgical procedure to avoid periprosthetic fractures from undetected bone metastases around hardware.20 Keep in mind that plain films can miss early lesions, and computed tomography (CT) or magnetic resonance imaging (MRI) may be needed if suspicion of a pathologic process is still strong and especially if a primary malignancy is known.27
Working back to a primary diagnosis
If imaging confirms a suspicious lesion and the patient has no known primary tumor, order labs, a CT scan with contrast of the chest, abdomen, and pelvis, and a bone scan, and refer the patient to an oncologist. If the bone lesion is painful, initiate protected weight-bearing and additionally refer the patient to an orthopedic surgeon.
Continue to: Appropriate laboratory evaluation
Appropriate laboratory evaluation entails a complete blood count; metabolic panel that includes serum calcium and phosphorus, vitamin D, alkaline phosphatase, thyroid-stimulating hormone, and parathyroid hormone; and serum protein electrophoresis to rule out multiple myeloma.7,11 Tumor markers are useful to monitor a patient’s response to cancer treatment or to determine recurrence, but they play only a limited role in the initial work-up of an unknown bone tumor.28
Further imaging. A CT scan with intravenous contrast of the chest, abdomen, and pelvis is done to screen for visceral malignancy; however, 15% of bone lesions in patients with an unknown primary lesion never have a source identified.29 Bone scans can be useful in identifying the extent of a single lesion seen on plain films and to assess for additional asymptomatic lesions. Additional imaging—eg, CT or MRI of the lesion, or positron emission tomography (PET)—can be left to the discretion of the oncologist or surgeon.
CT scans have significantly higher sensitivity than radiographs and offer better visualization of bone quality, bone destruction, and soft-tissue extension.30 MRI can be used to assess changes in bone marrow and soft-tissue involvement. PET scans, which detect tumors by quantifying metabolic activity, remain controversial. PET is superior to bone scans in detecting bone metastases from lung and breast cancers, but worse in renal and prostate cancers due to slow growth of metastases.31-33
Caveat.
Treatment options
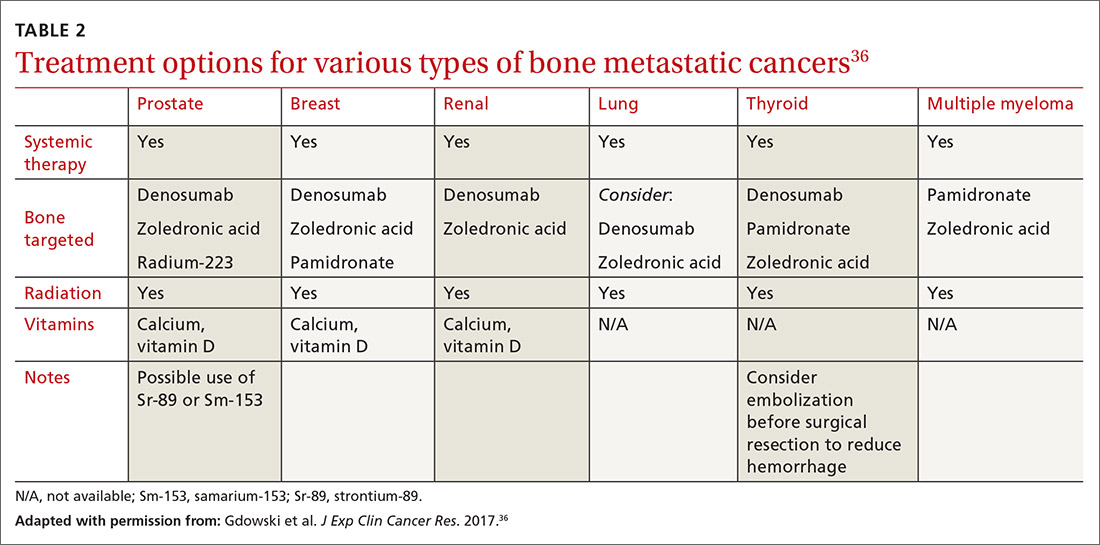
Metastatic bone disease is typically managed nonsurgically with radiation, chemo- or immunotherapies, hormone suppression, bone-modifying agents, or ablation.36 An overview of the cancer treatment guidelines for bone metastasis from the 2017 National Comprehensive Cancer Network is shown in TABLE 2.36
Continue to: Radiotherapy
Radiotherapy can take the form of external-beam or radioisotope radiation. With localized irradiation, most patients who have painful lesions experience at least partial relief, often within a few weeks.12,37 It may be used postoperatively, as well, to decrease the chances of disease progession.20
Systemic therapies include chemo- and hormone therapies. Chemotherapy effectiveness is highly dependent on the primary tumor type. For example, renal cell carcinoma and melanoma are often resistant, while lymphoma and germ-cell tumors may be eliminated and sometimes even cured.7 Hormone therapy can be highly effective in selective cancers, primarily breast and prostate cancers. Immunotherapy options may also be used to specifically target bone metastasis sites.
Bone-modifying agents include bisphosphonates and denosumab (Prolia, Xgeva). These are generally initiated at the discretion of the oncologist, but primary care physicians should be familiar with their use. Bisphosphonates, which includes zoledronic acid, pamidronate, and other agents, are analogues of pyrophosphate that inhibit bone demineralization.38 These agents target bone resorption through incorporation into osteoclasts and have been effective in the treatment of hypercalcemia and bone lesions.6,12,39 Not only do they reduce the incidence of all skeleton-related events, including pathologic fractures and pain, they also appear to have antitumor activity with prolonged survival in certain cancers.7,12
Denosumab, which has a much shorter half-life than bisphosphonates, is a monoclonal antibody that targets the gene RANKL, a key activator of osteoclasts, and thereby prevents the development of osteoclasts and related bone resorption.40
Radiofrequency ablation or cryoablation, using image-guided needle placement, specifically targets individual bone lesions, destroying tumor cells with extreme heat or cold, respectively. This has been shown to reduce pain and opioid consumption.41
Continue to: Managing pain
Managing pain
Pain management can be difficult, especially as patients live longer and undergo additional treatments such as surgery, radiation, and chemotherapy, each with the potential to produce chronic pain.42 A multidisciplinary team with a stepwise and multimodal approach can improve the patient’s function and comfort while decreasing drug adverse effects.43
For mild-to-moderate pain, nonsteroidal anti-inflammatory drugs, acetaminophen, and tramadol may provide effective relief. For more severe pain, narcotics are often required on a fixed-dose schedule along with breakthrough options such as short-acting hydromorphone, oxycodone, or transmucosal fentanyl.42-44 Opioid adverse effects such as constipation and nausea/vomiting must be managed with laxatives and metoclopramide/antidopaminergics, respectively.
Other important non-narcotic therapies are corticosteroids, tricyclic antidepressants, gabapentin, neuroleptics, and nerve blocks.45 Physical therapy and acupuncture may also be useful, depending on the patient’s needs and desires. Despite the wide range of options, most patients continue to have a significant amount of pain that can impact daily activities and even cause them to feel that their quality of life was not an important factor in physician decision making.46
Surgery options
Surgical intervention for metastatic bone disease differs from its use in primary bone tumors in that clinical indications are not clearly defined. In general, surgery for metastatic disease is used in patients who have pathologic fractures, a risk of pathologic fracture, or uncontrolled cancer-induced bone pain. Keep in mind that the overarching goal of surgery is to reduce morbidity, not mortality, although exceptions exist. Metastatic renal cell carcinoma is one such exception: improved survival may be achieved via aggressive surgical resection for solitary or oligometastatic lesions.47
Before deciding on surgery, engage the patient in goals-of-care discussions and take into account factors specific to the individual, as operative complications can be devasting. Risk of postoperative infection is high, given that these patients are often immunocompromised and that irradiated tissue is prone to wound healing issues.8 Complications may require a pause in chemotherapy and a subsequent decrease in life expectancy.
Continue to: Another factor in surgical decision making...
Another factor in surgical decision making is that newer systemic therapies are leading to longer survival for those with various types of metastatic cancer.48 Older methods of fixation designed to last a few years may now fail during the patient’s prolonged lifespan. As novel therapies continue to improve survival and complicate surgical indications, it may be prudent for the surgical management of metastatic bone disease to be handled by fellowship-trained orthopedic oncologists.
Factors that affect timing. Surgical intervention ideally occurs before the development of a pathologic fracture. Outcomes research has shown that intervention before fracture leads to reduced blood loss and length of hospital stay with improved functional recovery and survival.12,49 Despite these improved outcomes, an adequate scoring system to guide surgical intervention has yet to be developed. Mirels’ criteria are cited most often, yet this scoring system fails to account for many important considerations such as primary tumor type, life expectancy, and other factors.50,51
Given the deleterious effects of fractures in cancer patients and the inadequacy of closed reduction and immobilization, surgical intervention is often warranted.52 Surgical technology has continued to progress; however, intramedullary nailing, plating, and endoprostheses are still the most commonly used methods.53
Intramedullary nailing is commonly used in the prophylactic treatment of pathologic lesions and fractures of long bones in patients whose expected survival is as little as 6 to 12 weeks.54 Plate and screw fixation is a viable alternative to intramedullary nailing when tumor resection is desired. Endoprostheses replacement is used when a tumor involves joint surfaces or if biological reconstruction cannot be achieved by nailing or plating.
Explicit communication with patients is critical
Of vital importance is your participation with patients and families in shared decision making throughout the diagnostic and treatment process, ensuring clear communication. Misunderstandings about cancer stages and prognoses are not uncommon and are sometimes due to insufficient explanation.55,56 Additionally, expectations of survival and adverse effects of treatment often differ greatly between physicians and patients, which can lead to patient dissatisfaction.57
Continue to: Finally, the long-term care...
Finally, the long-term care of patients with metastatic cancers necessarily involves multidisciplinary teams, which further complicates communication. To ensure that patients are receiving an appropriate course of treatment, evaluate their health literacy, confirm their understanding of the disease, and acknowledge their desires.
CORRESPONDENCE
Kyle Sweeney, MD, University of Kansas Medical Center, Department of Orthopedic Surgery, 3901 Rainbow Boulevard, MS 3017, Kansas City, KS 66160; ksweeney2@kumc.edu.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289.
3. Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243-255.
4. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165-176.
5. Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87-93.
6. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s.
7. Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
8. Wood TJ, Racano A, Yeung H, et al. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081-4089.
9. Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9(suppl 1):S5.
10. Suva LJ, Washam C, Nicholas RW, et al. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208-218.
11. Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365-378.
12. Shibata H, Kato S, Sekine I, et al. Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open. 2016;1:e000037.
13. Svensson E, Christiansen CF, Ulrichsen SP, et al. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7 e016022.
14. Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132-138.
15. Hansen BH, Keller J, Laitinen M, et al. The Scandinavian Sarcoma Group Skeletal Metastasis Register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11-15.
16. Dürr HR, Müller PE, Lenz T, et al. Surgical treatment of bone metastases in patients with breast cancer. Clin Orthop Relat Res. 2002:191-196.
17. Weiss RJ, Tullberg E, Forsberg JA, et al. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23:286-290.
18. Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83:74-79.
19. Nathan SS, Chan L, Tan WL, et al. The need for a system of prognostication in skeletal metastasis to decide best end-of-life care - a call to arms. Ann Acad Med Singapore. 2010;39:476-481.
20. Weber KL. Evaluation of the adult patient (aged > 40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18:169-179.
21. Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97(3 suppl):866-873.
22. McDuffee LA, Colterjohn N, Singh G. Bone metastasis and pathological fractures. In: Singh G, Rabbani SA, eds. Bone Metastasis. Experimental and Clinical Therapeutics. Totowa, NJ: Humana Press; 2005:229-241.
23. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509-524.
24. Maisano R, Pergolizzi S, Cascinu S. Novel therapeutic approaches to cancer patients with bone metastasis. Crit Rev Oncol Hematol. 2001;40:239-250.
25. Marino MT, Asp AA, Budayer AA, et al. Hypercalcaemia and elevated levels of parathyroid hormone-related protein in cutaneous squamous/basal cell carcinoma. J Intern Med. 1993;233:205-207.
26. Grill V, Ho P, Body JJ, et al. Parathyroid hormone-related protein: elevated levels in both humoral hypercalcemia of malignancy and hypercalcemia complicating metastatic breast cancer. J Clin Endocrinol Metab. 1991;73:1309-1315.
27. Jehn CF, Diel IJ, Overkamp F, et al. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res. 2016;36:2631-2637.
28. Molina R, Bosch X, Auge JM, et al. Utility of serum tumor markers as an aid in the differential diagnosis of patients with clinical suspicion of cancer and in patients with cancer of unknown primary site. Tumour Biol. 2012;33:463-474.
29. Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. a prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75:1276-1281.
30. Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53-64.
31. Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803-809.
32. Yang SN, Liang JA, Lin FJ, et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:325-328.
33. Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623-1629.
34. Adams SC, Potter BK, Mahmood Z, et al. Consequences and prevention of inadvertent internal fixation of primary osseous sarcomas. Clin Orthop Relat Res. 2009;467:519-525.
35. Scolaro JA, Lackman RD. Surgical management of metastatic long bone fractures: principles and techniques. J Am Acad Orthop Surg. 2014;22:90-100.
36. Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J Exp Clin Cancer Res. 2017;36:108.
37. Yoon F, Morton GC. Single fraction radiotherapy versus multiple fraction radiotherapy for bone metastases in prostate cancer patients: comparative effectiveness. Cancer Manag Res. 2014;6:451-457.
38. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336-340.
39. Van Acker HH, Anguille S, Willemen Y, et al. Bisphosphonates for cancer treatment: mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24-40.
40. Castellano D, Sepulveda JM, Garcia-Escobar I, et al. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16:136-145.
41. Guenette JP, Lopez MJ, Kim E, et al. Solitary painful osseous metastases: correlation of imaging features with pain palliation after radiofrequency ablation—a multicenter American College of Radiology imaging network study. Radiology. 2013;268:907-915.
42. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
43. ASATFCPM, ASRAPM. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810-833.
44. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(suppl 4):iv166-iv191.
45. Kvale PA, Simoff M, Prakash UBS, ACCP. Lung cancer. Palliative care. Chest. 2003;123(1 suppl):284S-311S.
46. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420-1433.
47. Kato S, Murakami H, Takeuchi A, et al. Fifteen-year survivor of renal cell carcinoma after metastasectomies for multiple bone metastases. Orthopedics. 2013;36:e1454-e1457.
48. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 suppl):1614-1627.
49. Ristevski B, Jenkinson RJ, Stephen DJG, et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52:302-308.
50. Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. 1989. Clin Orthop Relat Res. 2003(415 suppl):S4-S13.
51. Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;468:2825-2827.
52. Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983:297-302.
53. Bird JE. “Advances in the surgical management of bone tumors.” Curr Oncol Rep. 2014;16:392.
54. Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91:1503-1516.
55. Kim SH, Shin DW, Kim SY, et al. Terminal versus advanced cancer: do the general population and health care professionals share a common language? Cancer Res Treat. 2016;48:759-767.
56. Lee JK, Yun YH, An AR, et al. The understanding of terminal cancer and its relationship with attitudes toward end-of-life care issues. Med Decis Making. 2014;34:720-730.
57. Lux MP, Bayer CM, Loehberg CR, et al. Shared decision-making in metastatic breast cancer: discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat. 2013;139:429-440.
Since the early 1990s, modern treatments have steadily reduced overall cancer mortality from primary tumors.1 Consequently, more people are at risk of metastatic bone disease, with subsequent pain and pathologic fractures1,2 and death from metastasis.3 Patients who have bone metastases present with a variety of signs and symptoms including pain, fractures, and metabolic derangements. The primary care approach to work-up and diagnosis described in this article enables prompt treatment, either surgical or nonsurgical, to maintain a high quality of life for patients.
Primary tumors determine types of metastases and prognosis
Metastasis, a complex pathologic process in which cancerous cells migrate to distant organs, implant, and grow,3 is a poor prognostic indicator in cancer patients. Bone is the third most common site of metastasis, behind the liver and lungs.4 While the true prevalence of metastatic bone cancer is unknown, studies have estimated it to be > 280,000 cases in the United States.5
Bone metastases interfere with normal bone metabolism and turnover in several different characteristic patterns. These changes—radiographically defined as osteoblastic, osteolytic, or mixed lesions—are determined by the primary tumor type.
- Osteoblastic lesions, comprised of new, disorganized bone formation, often occur secondary to prostate cancer, small cell lung cancer, and carcinoid malignancies, among others.
- Osteolytic lesions, in which bone is destroyed, are more common with breast cancer, renal cell carcinoma, melanoma, and multiple myeloma.
- Mixed lesions, in which areas of bone destruction and growth are simultaneously found, occur with some GI cancers and a few breast cancers.6,7
Most bone metastases result from carcinomas, of which up to 50% eventually spread to bone, although this process can take 10 to 15 years.8,9 The likelihood of bone metastasis depends on the primary tumor and its stage. Breast and prostate cancer account for most skeletal metastases, although these lesions are often asymptomatic.6,9 Other malignancies, such as ovarian and gastrointestinal, metastasize to bone much less frequently.7,10 Virtually any cancer at an advanced stage can spread to bone. These metastases are usually multifocal and incurable, with the patient’s prognosis varying from a few months to years.6,11,12
Factors that influence prognosis. Metastatic bone disease arising from melanoma and lung cancers has the shortest life expectancy of roughly 6 months from initial diagnosis; metastasis following prostate, breast, and thyroid cancers has the longest, usually 2 to 4 years.11TABLE 113 shows survival estimates from a large Danish population at various time points following bone metastasis diagnosis for several primary cancer types.
When surgical intervention for bony metastasis is required, prognosis is generally poorer, likely due to more advanced disease. The overall 1-year survival following surgery varies, but several large studies have found a rate of around 40% when considering all primary tumors.14,15 The most common metastases, from breast and prostate cancers, have 1-year survivals of around 50% and 30%, respectively, following surgical intervention.16-18
What you’re likely to see on presentation
Bone metastases are one of the leading causes of morbidity in cancer patients from resultant pain, pathologic fractures, metabolic derangements, and reduced activities of daily living.8,19 The most common cause of cancer pain is bone involvement.6 Patients report pain that is usually worse at night, poorly localized, and not alleviated with rest. They often mistakenly relate the pain to an injury.20 The pathophysiology of bone pain is not completely understood but is likely multifactorial and includes inflammatory and mechanical processes.7,21 Spine involvement can lead to stenosis or nerve root compression, with symptoms dependent on level and severity of nerve or cord compromise.20 Overall, the most common site of bone metastasis is the thoracic spine, followed by the ribs, pelvis, and proximal long bones.20
Continue to: Pathologic fractures
Pathologic fractures occur frequently in cancer patients. Bone destruction leads to a loss of mechanical support which, in turn, causes microfractures and pain. These microfractures can proliferate and coalesce, causing a pathologic fracture, often in weight-bearing bones.6 Breast cancer with lytic lesions is the single leading cause of all pathologic fractures.22 Lung cancer with its short survival time and prostate cancer with blastic lesions are less common causes.23 In the appendicular skeleton, the vast majority of these fractures occur in the femur and humerus.11
Symptomatic metabolic derangements. The most common metabolic disorder is hypercalcemia, found predominantly in patients with hematologic malignancies, squamous cell lung cancer, renal cell cancer, and breast cancer.6,7,12,24 The clinical presentation is nonspecific and can include polyuria, polydipsia, fatigue, constipation, and confusion. The prevalence is estimated to be 13% in breast cancer, 4% in lung cancers, and 1% in prostate cancer, although results in individual studies vary.12 The pathophysiology is multifactorial and often includes osteolytic lesions and an increased circulating level of parathyroid hormone–related peptide, although other mechanisms contribute.25,26 Ultimately, severe hypercalcemia may be fatal secondary to renal failure and cardiac arrhythmias.6,7,12 Paraneoplastic hypercalcemia independently decreases survival; 1 study found the median survival to be 10 to 12 weeks.11
Primary care work-up and diagnosis
When a patient presents with signs and symptoms suggestive of metastatic bone disease, inquire about a history of cancer. Even if such a history is remote, it is important—particularly so if the patient received chemotherapy or radiation, which can lead to secondary cancers such as leukemia or sarcoma.20 If a primary site of malignancy is unknown, pursue a general review of systems. Clues to the primary site of disease could be a history of chest pain, shortness of breath, hemoptysis, heat/cold intolerance, or changes in bowel/bladder habits. Also ask about risk factors such as smoking, chemical exposure, and sun exposure.
Pointers on radiographic imaging. If you suspect a destructive bone lesion, order appropriate radiographic imaging. Arrange for plain radiographs with at least 2 views of the specific area of interest that include the entire bone along with the joints above and below. Importantly, the entire bone must be imaged before any surgical procedure to avoid periprosthetic fractures from undetected bone metastases around hardware.20 Keep in mind that plain films can miss early lesions, and computed tomography (CT) or magnetic resonance imaging (MRI) may be needed if suspicion of a pathologic process is still strong and especially if a primary malignancy is known.27
Working back to a primary diagnosis
If imaging confirms a suspicious lesion and the patient has no known primary tumor, order labs, a CT scan with contrast of the chest, abdomen, and pelvis, and a bone scan, and refer the patient to an oncologist. If the bone lesion is painful, initiate protected weight-bearing and additionally refer the patient to an orthopedic surgeon.
Continue to: Appropriate laboratory evaluation
Appropriate laboratory evaluation entails a complete blood count; metabolic panel that includes serum calcium and phosphorus, vitamin D, alkaline phosphatase, thyroid-stimulating hormone, and parathyroid hormone; and serum protein electrophoresis to rule out multiple myeloma.7,11 Tumor markers are useful to monitor a patient’s response to cancer treatment or to determine recurrence, but they play only a limited role in the initial work-up of an unknown bone tumor.28
Further imaging. A CT scan with intravenous contrast of the chest, abdomen, and pelvis is done to screen for visceral malignancy; however, 15% of bone lesions in patients with an unknown primary lesion never have a source identified.29 Bone scans can be useful in identifying the extent of a single lesion seen on plain films and to assess for additional asymptomatic lesions. Additional imaging—eg, CT or MRI of the lesion, or positron emission tomography (PET)—can be left to the discretion of the oncologist or surgeon.
CT scans have significantly higher sensitivity than radiographs and offer better visualization of bone quality, bone destruction, and soft-tissue extension.30 MRI can be used to assess changes in bone marrow and soft-tissue involvement. PET scans, which detect tumors by quantifying metabolic activity, remain controversial. PET is superior to bone scans in detecting bone metastases from lung and breast cancers, but worse in renal and prostate cancers due to slow growth of metastases.31-33
Caveat.
Treatment options
Metastatic bone disease is typically managed nonsurgically with radiation, chemo- or immunotherapies, hormone suppression, bone-modifying agents, or ablation.36 An overview of the cancer treatment guidelines for bone metastasis from the 2017 National Comprehensive Cancer Network is shown in TABLE 2.36
Continue to: Radiotherapy
Radiotherapy can take the form of external-beam or radioisotope radiation. With localized irradiation, most patients who have painful lesions experience at least partial relief, often within a few weeks.12,37 It may be used postoperatively, as well, to decrease the chances of disease progession.20
Systemic therapies include chemo- and hormone therapies. Chemotherapy effectiveness is highly dependent on the primary tumor type. For example, renal cell carcinoma and melanoma are often resistant, while lymphoma and germ-cell tumors may be eliminated and sometimes even cured.7 Hormone therapy can be highly effective in selective cancers, primarily breast and prostate cancers. Immunotherapy options may also be used to specifically target bone metastasis sites.
Bone-modifying agents include bisphosphonates and denosumab (Prolia, Xgeva). These are generally initiated at the discretion of the oncologist, but primary care physicians should be familiar with their use. Bisphosphonates, which includes zoledronic acid, pamidronate, and other agents, are analogues of pyrophosphate that inhibit bone demineralization.38 These agents target bone resorption through incorporation into osteoclasts and have been effective in the treatment of hypercalcemia and bone lesions.6,12,39 Not only do they reduce the incidence of all skeleton-related events, including pathologic fractures and pain, they also appear to have antitumor activity with prolonged survival in certain cancers.7,12
Denosumab, which has a much shorter half-life than bisphosphonates, is a monoclonal antibody that targets the gene RANKL, a key activator of osteoclasts, and thereby prevents the development of osteoclasts and related bone resorption.40
Radiofrequency ablation or cryoablation, using image-guided needle placement, specifically targets individual bone lesions, destroying tumor cells with extreme heat or cold, respectively. This has been shown to reduce pain and opioid consumption.41
Continue to: Managing pain
Managing pain
Pain management can be difficult, especially as patients live longer and undergo additional treatments such as surgery, radiation, and chemotherapy, each with the potential to produce chronic pain.42 A multidisciplinary team with a stepwise and multimodal approach can improve the patient’s function and comfort while decreasing drug adverse effects.43
For mild-to-moderate pain, nonsteroidal anti-inflammatory drugs, acetaminophen, and tramadol may provide effective relief. For more severe pain, narcotics are often required on a fixed-dose schedule along with breakthrough options such as short-acting hydromorphone, oxycodone, or transmucosal fentanyl.42-44 Opioid adverse effects such as constipation and nausea/vomiting must be managed with laxatives and metoclopramide/antidopaminergics, respectively.
Other important non-narcotic therapies are corticosteroids, tricyclic antidepressants, gabapentin, neuroleptics, and nerve blocks.45 Physical therapy and acupuncture may also be useful, depending on the patient’s needs and desires. Despite the wide range of options, most patients continue to have a significant amount of pain that can impact daily activities and even cause them to feel that their quality of life was not an important factor in physician decision making.46
Surgery options
Surgical intervention for metastatic bone disease differs from its use in primary bone tumors in that clinical indications are not clearly defined. In general, surgery for metastatic disease is used in patients who have pathologic fractures, a risk of pathologic fracture, or uncontrolled cancer-induced bone pain. Keep in mind that the overarching goal of surgery is to reduce morbidity, not mortality, although exceptions exist. Metastatic renal cell carcinoma is one such exception: improved survival may be achieved via aggressive surgical resection for solitary or oligometastatic lesions.47
Before deciding on surgery, engage the patient in goals-of-care discussions and take into account factors specific to the individual, as operative complications can be devasting. Risk of postoperative infection is high, given that these patients are often immunocompromised and that irradiated tissue is prone to wound healing issues.8 Complications may require a pause in chemotherapy and a subsequent decrease in life expectancy.
Continue to: Another factor in surgical decision making...
Another factor in surgical decision making is that newer systemic therapies are leading to longer survival for those with various types of metastatic cancer.48 Older methods of fixation designed to last a few years may now fail during the patient’s prolonged lifespan. As novel therapies continue to improve survival and complicate surgical indications, it may be prudent for the surgical management of metastatic bone disease to be handled by fellowship-trained orthopedic oncologists.
Factors that affect timing. Surgical intervention ideally occurs before the development of a pathologic fracture. Outcomes research has shown that intervention before fracture leads to reduced blood loss and length of hospital stay with improved functional recovery and survival.12,49 Despite these improved outcomes, an adequate scoring system to guide surgical intervention has yet to be developed. Mirels’ criteria are cited most often, yet this scoring system fails to account for many important considerations such as primary tumor type, life expectancy, and other factors.50,51
Given the deleterious effects of fractures in cancer patients and the inadequacy of closed reduction and immobilization, surgical intervention is often warranted.52 Surgical technology has continued to progress; however, intramedullary nailing, plating, and endoprostheses are still the most commonly used methods.53
Intramedullary nailing is commonly used in the prophylactic treatment of pathologic lesions and fractures of long bones in patients whose expected survival is as little as 6 to 12 weeks.54 Plate and screw fixation is a viable alternative to intramedullary nailing when tumor resection is desired. Endoprostheses replacement is used when a tumor involves joint surfaces or if biological reconstruction cannot be achieved by nailing or plating.
Explicit communication with patients is critical
Of vital importance is your participation with patients and families in shared decision making throughout the diagnostic and treatment process, ensuring clear communication. Misunderstandings about cancer stages and prognoses are not uncommon and are sometimes due to insufficient explanation.55,56 Additionally, expectations of survival and adverse effects of treatment often differ greatly between physicians and patients, which can lead to patient dissatisfaction.57
Continue to: Finally, the long-term care...
Finally, the long-term care of patients with metastatic cancers necessarily involves multidisciplinary teams, which further complicates communication. To ensure that patients are receiving an appropriate course of treatment, evaluate their health literacy, confirm their understanding of the disease, and acknowledge their desires.
CORRESPONDENCE
Kyle Sweeney, MD, University of Kansas Medical Center, Department of Orthopedic Surgery, 3901 Rainbow Boulevard, MS 3017, Kansas City, KS 66160; ksweeney2@kumc.edu.
Since the early 1990s, modern treatments have steadily reduced overall cancer mortality from primary tumors.1 Consequently, more people are at risk of metastatic bone disease, with subsequent pain and pathologic fractures1,2 and death from metastasis.3 Patients who have bone metastases present with a variety of signs and symptoms including pain, fractures, and metabolic derangements. The primary care approach to work-up and diagnosis described in this article enables prompt treatment, either surgical or nonsurgical, to maintain a high quality of life for patients.
Primary tumors determine types of metastases and prognosis
Metastasis, a complex pathologic process in which cancerous cells migrate to distant organs, implant, and grow,3 is a poor prognostic indicator in cancer patients. Bone is the third most common site of metastasis, behind the liver and lungs.4 While the true prevalence of metastatic bone cancer is unknown, studies have estimated it to be > 280,000 cases in the United States.5
Bone metastases interfere with normal bone metabolism and turnover in several different characteristic patterns. These changes—radiographically defined as osteoblastic, osteolytic, or mixed lesions—are determined by the primary tumor type.
- Osteoblastic lesions, comprised of new, disorganized bone formation, often occur secondary to prostate cancer, small cell lung cancer, and carcinoid malignancies, among others.
- Osteolytic lesions, in which bone is destroyed, are more common with breast cancer, renal cell carcinoma, melanoma, and multiple myeloma.
- Mixed lesions, in which areas of bone destruction and growth are simultaneously found, occur with some GI cancers and a few breast cancers.6,7
Most bone metastases result from carcinomas, of which up to 50% eventually spread to bone, although this process can take 10 to 15 years.8,9 The likelihood of bone metastasis depends on the primary tumor and its stage. Breast and prostate cancer account for most skeletal metastases, although these lesions are often asymptomatic.6,9 Other malignancies, such as ovarian and gastrointestinal, metastasize to bone much less frequently.7,10 Virtually any cancer at an advanced stage can spread to bone. These metastases are usually multifocal and incurable, with the patient’s prognosis varying from a few months to years.6,11,12
Factors that influence prognosis. Metastatic bone disease arising from melanoma and lung cancers has the shortest life expectancy of roughly 6 months from initial diagnosis; metastasis following prostate, breast, and thyroid cancers has the longest, usually 2 to 4 years.11TABLE 113 shows survival estimates from a large Danish population at various time points following bone metastasis diagnosis for several primary cancer types.
When surgical intervention for bony metastasis is required, prognosis is generally poorer, likely due to more advanced disease. The overall 1-year survival following surgery varies, but several large studies have found a rate of around 40% when considering all primary tumors.14,15 The most common metastases, from breast and prostate cancers, have 1-year survivals of around 50% and 30%, respectively, following surgical intervention.16-18
What you’re likely to see on presentation
Bone metastases are one of the leading causes of morbidity in cancer patients from resultant pain, pathologic fractures, metabolic derangements, and reduced activities of daily living.8,19 The most common cause of cancer pain is bone involvement.6 Patients report pain that is usually worse at night, poorly localized, and not alleviated with rest. They often mistakenly relate the pain to an injury.20 The pathophysiology of bone pain is not completely understood but is likely multifactorial and includes inflammatory and mechanical processes.7,21 Spine involvement can lead to stenosis or nerve root compression, with symptoms dependent on level and severity of nerve or cord compromise.20 Overall, the most common site of bone metastasis is the thoracic spine, followed by the ribs, pelvis, and proximal long bones.20
Continue to: Pathologic fractures
Pathologic fractures occur frequently in cancer patients. Bone destruction leads to a loss of mechanical support which, in turn, causes microfractures and pain. These microfractures can proliferate and coalesce, causing a pathologic fracture, often in weight-bearing bones.6 Breast cancer with lytic lesions is the single leading cause of all pathologic fractures.22 Lung cancer with its short survival time and prostate cancer with blastic lesions are less common causes.23 In the appendicular skeleton, the vast majority of these fractures occur in the femur and humerus.11
Symptomatic metabolic derangements. The most common metabolic disorder is hypercalcemia, found predominantly in patients with hematologic malignancies, squamous cell lung cancer, renal cell cancer, and breast cancer.6,7,12,24 The clinical presentation is nonspecific and can include polyuria, polydipsia, fatigue, constipation, and confusion. The prevalence is estimated to be 13% in breast cancer, 4% in lung cancers, and 1% in prostate cancer, although results in individual studies vary.12 The pathophysiology is multifactorial and often includes osteolytic lesions and an increased circulating level of parathyroid hormone–related peptide, although other mechanisms contribute.25,26 Ultimately, severe hypercalcemia may be fatal secondary to renal failure and cardiac arrhythmias.6,7,12 Paraneoplastic hypercalcemia independently decreases survival; 1 study found the median survival to be 10 to 12 weeks.11
Primary care work-up and diagnosis
When a patient presents with signs and symptoms suggestive of metastatic bone disease, inquire about a history of cancer. Even if such a history is remote, it is important—particularly so if the patient received chemotherapy or radiation, which can lead to secondary cancers such as leukemia or sarcoma.20 If a primary site of malignancy is unknown, pursue a general review of systems. Clues to the primary site of disease could be a history of chest pain, shortness of breath, hemoptysis, heat/cold intolerance, or changes in bowel/bladder habits. Also ask about risk factors such as smoking, chemical exposure, and sun exposure.
Pointers on radiographic imaging. If you suspect a destructive bone lesion, order appropriate radiographic imaging. Arrange for plain radiographs with at least 2 views of the specific area of interest that include the entire bone along with the joints above and below. Importantly, the entire bone must be imaged before any surgical procedure to avoid periprosthetic fractures from undetected bone metastases around hardware.20 Keep in mind that plain films can miss early lesions, and computed tomography (CT) or magnetic resonance imaging (MRI) may be needed if suspicion of a pathologic process is still strong and especially if a primary malignancy is known.27
Working back to a primary diagnosis
If imaging confirms a suspicious lesion and the patient has no known primary tumor, order labs, a CT scan with contrast of the chest, abdomen, and pelvis, and a bone scan, and refer the patient to an oncologist. If the bone lesion is painful, initiate protected weight-bearing and additionally refer the patient to an orthopedic surgeon.
Continue to: Appropriate laboratory evaluation
Appropriate laboratory evaluation entails a complete blood count; metabolic panel that includes serum calcium and phosphorus, vitamin D, alkaline phosphatase, thyroid-stimulating hormone, and parathyroid hormone; and serum protein electrophoresis to rule out multiple myeloma.7,11 Tumor markers are useful to monitor a patient’s response to cancer treatment or to determine recurrence, but they play only a limited role in the initial work-up of an unknown bone tumor.28
Further imaging. A CT scan with intravenous contrast of the chest, abdomen, and pelvis is done to screen for visceral malignancy; however, 15% of bone lesions in patients with an unknown primary lesion never have a source identified.29 Bone scans can be useful in identifying the extent of a single lesion seen on plain films and to assess for additional asymptomatic lesions. Additional imaging—eg, CT or MRI of the lesion, or positron emission tomography (PET)—can be left to the discretion of the oncologist or surgeon.
CT scans have significantly higher sensitivity than radiographs and offer better visualization of bone quality, bone destruction, and soft-tissue extension.30 MRI can be used to assess changes in bone marrow and soft-tissue involvement. PET scans, which detect tumors by quantifying metabolic activity, remain controversial. PET is superior to bone scans in detecting bone metastases from lung and breast cancers, but worse in renal and prostate cancers due to slow growth of metastases.31-33
Caveat.
Treatment options
Metastatic bone disease is typically managed nonsurgically with radiation, chemo- or immunotherapies, hormone suppression, bone-modifying agents, or ablation.36 An overview of the cancer treatment guidelines for bone metastasis from the 2017 National Comprehensive Cancer Network is shown in TABLE 2.36
Continue to: Radiotherapy
Radiotherapy can take the form of external-beam or radioisotope radiation. With localized irradiation, most patients who have painful lesions experience at least partial relief, often within a few weeks.12,37 It may be used postoperatively, as well, to decrease the chances of disease progession.20
Systemic therapies include chemo- and hormone therapies. Chemotherapy effectiveness is highly dependent on the primary tumor type. For example, renal cell carcinoma and melanoma are often resistant, while lymphoma and germ-cell tumors may be eliminated and sometimes even cured.7 Hormone therapy can be highly effective in selective cancers, primarily breast and prostate cancers. Immunotherapy options may also be used to specifically target bone metastasis sites.
Bone-modifying agents include bisphosphonates and denosumab (Prolia, Xgeva). These are generally initiated at the discretion of the oncologist, but primary care physicians should be familiar with their use. Bisphosphonates, which includes zoledronic acid, pamidronate, and other agents, are analogues of pyrophosphate that inhibit bone demineralization.38 These agents target bone resorption through incorporation into osteoclasts and have been effective in the treatment of hypercalcemia and bone lesions.6,12,39 Not only do they reduce the incidence of all skeleton-related events, including pathologic fractures and pain, they also appear to have antitumor activity with prolonged survival in certain cancers.7,12
Denosumab, which has a much shorter half-life than bisphosphonates, is a monoclonal antibody that targets the gene RANKL, a key activator of osteoclasts, and thereby prevents the development of osteoclasts and related bone resorption.40
Radiofrequency ablation or cryoablation, using image-guided needle placement, specifically targets individual bone lesions, destroying tumor cells with extreme heat or cold, respectively. This has been shown to reduce pain and opioid consumption.41
Continue to: Managing pain
Managing pain
Pain management can be difficult, especially as patients live longer and undergo additional treatments such as surgery, radiation, and chemotherapy, each with the potential to produce chronic pain.42 A multidisciplinary team with a stepwise and multimodal approach can improve the patient’s function and comfort while decreasing drug adverse effects.43
For mild-to-moderate pain, nonsteroidal anti-inflammatory drugs, acetaminophen, and tramadol may provide effective relief. For more severe pain, narcotics are often required on a fixed-dose schedule along with breakthrough options such as short-acting hydromorphone, oxycodone, or transmucosal fentanyl.42-44 Opioid adverse effects such as constipation and nausea/vomiting must be managed with laxatives and metoclopramide/antidopaminergics, respectively.
Other important non-narcotic therapies are corticosteroids, tricyclic antidepressants, gabapentin, neuroleptics, and nerve blocks.45 Physical therapy and acupuncture may also be useful, depending on the patient’s needs and desires. Despite the wide range of options, most patients continue to have a significant amount of pain that can impact daily activities and even cause them to feel that their quality of life was not an important factor in physician decision making.46
Surgery options
Surgical intervention for metastatic bone disease differs from its use in primary bone tumors in that clinical indications are not clearly defined. In general, surgery for metastatic disease is used in patients who have pathologic fractures, a risk of pathologic fracture, or uncontrolled cancer-induced bone pain. Keep in mind that the overarching goal of surgery is to reduce morbidity, not mortality, although exceptions exist. Metastatic renal cell carcinoma is one such exception: improved survival may be achieved via aggressive surgical resection for solitary or oligometastatic lesions.47
Before deciding on surgery, engage the patient in goals-of-care discussions and take into account factors specific to the individual, as operative complications can be devasting. Risk of postoperative infection is high, given that these patients are often immunocompromised and that irradiated tissue is prone to wound healing issues.8 Complications may require a pause in chemotherapy and a subsequent decrease in life expectancy.
Continue to: Another factor in surgical decision making...
Another factor in surgical decision making is that newer systemic therapies are leading to longer survival for those with various types of metastatic cancer.48 Older methods of fixation designed to last a few years may now fail during the patient’s prolonged lifespan. As novel therapies continue to improve survival and complicate surgical indications, it may be prudent for the surgical management of metastatic bone disease to be handled by fellowship-trained orthopedic oncologists.
Factors that affect timing. Surgical intervention ideally occurs before the development of a pathologic fracture. Outcomes research has shown that intervention before fracture leads to reduced blood loss and length of hospital stay with improved functional recovery and survival.12,49 Despite these improved outcomes, an adequate scoring system to guide surgical intervention has yet to be developed. Mirels’ criteria are cited most often, yet this scoring system fails to account for many important considerations such as primary tumor type, life expectancy, and other factors.50,51
Given the deleterious effects of fractures in cancer patients and the inadequacy of closed reduction and immobilization, surgical intervention is often warranted.52 Surgical technology has continued to progress; however, intramedullary nailing, plating, and endoprostheses are still the most commonly used methods.53
Intramedullary nailing is commonly used in the prophylactic treatment of pathologic lesions and fractures of long bones in patients whose expected survival is as little as 6 to 12 weeks.54 Plate and screw fixation is a viable alternative to intramedullary nailing when tumor resection is desired. Endoprostheses replacement is used when a tumor involves joint surfaces or if biological reconstruction cannot be achieved by nailing or plating.
Explicit communication with patients is critical
Of vital importance is your participation with patients and families in shared decision making throughout the diagnostic and treatment process, ensuring clear communication. Misunderstandings about cancer stages and prognoses are not uncommon and are sometimes due to insufficient explanation.55,56 Additionally, expectations of survival and adverse effects of treatment often differ greatly between physicians and patients, which can lead to patient dissatisfaction.57
Continue to: Finally, the long-term care...
Finally, the long-term care of patients with metastatic cancers necessarily involves multidisciplinary teams, which further complicates communication. To ensure that patients are receiving an appropriate course of treatment, evaluate their health literacy, confirm their understanding of the disease, and acknowledge their desires.
CORRESPONDENCE
Kyle Sweeney, MD, University of Kansas Medical Center, Department of Orthopedic Surgery, 3901 Rainbow Boulevard, MS 3017, Kansas City, KS 66160; ksweeney2@kumc.edu.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289.
3. Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243-255.
4. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165-176.
5. Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87-93.
6. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s.
7. Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
8. Wood TJ, Racano A, Yeung H, et al. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081-4089.
9. Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9(suppl 1):S5.
10. Suva LJ, Washam C, Nicholas RW, et al. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208-218.
11. Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365-378.
12. Shibata H, Kato S, Sekine I, et al. Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open. 2016;1:e000037.
13. Svensson E, Christiansen CF, Ulrichsen SP, et al. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7 e016022.
14. Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132-138.
15. Hansen BH, Keller J, Laitinen M, et al. The Scandinavian Sarcoma Group Skeletal Metastasis Register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11-15.
16. Dürr HR, Müller PE, Lenz T, et al. Surgical treatment of bone metastases in patients with breast cancer. Clin Orthop Relat Res. 2002:191-196.
17. Weiss RJ, Tullberg E, Forsberg JA, et al. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23:286-290.
18. Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83:74-79.
19. Nathan SS, Chan L, Tan WL, et al. The need for a system of prognostication in skeletal metastasis to decide best end-of-life care - a call to arms. Ann Acad Med Singapore. 2010;39:476-481.
20. Weber KL. Evaluation of the adult patient (aged > 40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18:169-179.
21. Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97(3 suppl):866-873.
22. McDuffee LA, Colterjohn N, Singh G. Bone metastasis and pathological fractures. In: Singh G, Rabbani SA, eds. Bone Metastasis. Experimental and Clinical Therapeutics. Totowa, NJ: Humana Press; 2005:229-241.
23. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509-524.
24. Maisano R, Pergolizzi S, Cascinu S. Novel therapeutic approaches to cancer patients with bone metastasis. Crit Rev Oncol Hematol. 2001;40:239-250.
25. Marino MT, Asp AA, Budayer AA, et al. Hypercalcaemia and elevated levels of parathyroid hormone-related protein in cutaneous squamous/basal cell carcinoma. J Intern Med. 1993;233:205-207.
26. Grill V, Ho P, Body JJ, et al. Parathyroid hormone-related protein: elevated levels in both humoral hypercalcemia of malignancy and hypercalcemia complicating metastatic breast cancer. J Clin Endocrinol Metab. 1991;73:1309-1315.
27. Jehn CF, Diel IJ, Overkamp F, et al. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res. 2016;36:2631-2637.
28. Molina R, Bosch X, Auge JM, et al. Utility of serum tumor markers as an aid in the differential diagnosis of patients with clinical suspicion of cancer and in patients with cancer of unknown primary site. Tumour Biol. 2012;33:463-474.
29. Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. a prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75:1276-1281.
30. Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53-64.
31. Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803-809.
32. Yang SN, Liang JA, Lin FJ, et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:325-328.
33. Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623-1629.
34. Adams SC, Potter BK, Mahmood Z, et al. Consequences and prevention of inadvertent internal fixation of primary osseous sarcomas. Clin Orthop Relat Res. 2009;467:519-525.
35. Scolaro JA, Lackman RD. Surgical management of metastatic long bone fractures: principles and techniques. J Am Acad Orthop Surg. 2014;22:90-100.
36. Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J Exp Clin Cancer Res. 2017;36:108.
37. Yoon F, Morton GC. Single fraction radiotherapy versus multiple fraction radiotherapy for bone metastases in prostate cancer patients: comparative effectiveness. Cancer Manag Res. 2014;6:451-457.
38. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336-340.
39. Van Acker HH, Anguille S, Willemen Y, et al. Bisphosphonates for cancer treatment: mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24-40.
40. Castellano D, Sepulveda JM, Garcia-Escobar I, et al. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16:136-145.
41. Guenette JP, Lopez MJ, Kim E, et al. Solitary painful osseous metastases: correlation of imaging features with pain palliation after radiofrequency ablation—a multicenter American College of Radiology imaging network study. Radiology. 2013;268:907-915.
42. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
43. ASATFCPM, ASRAPM. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810-833.
44. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(suppl 4):iv166-iv191.
45. Kvale PA, Simoff M, Prakash UBS, ACCP. Lung cancer. Palliative care. Chest. 2003;123(1 suppl):284S-311S.
46. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420-1433.
47. Kato S, Murakami H, Takeuchi A, et al. Fifteen-year survivor of renal cell carcinoma after metastasectomies for multiple bone metastases. Orthopedics. 2013;36:e1454-e1457.
48. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 suppl):1614-1627.
49. Ristevski B, Jenkinson RJ, Stephen DJG, et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52:302-308.
50. Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. 1989. Clin Orthop Relat Res. 2003(415 suppl):S4-S13.
51. Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;468:2825-2827.
52. Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983:297-302.
53. Bird JE. “Advances in the surgical management of bone tumors.” Curr Oncol Rep. 2014;16:392.
54. Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91:1503-1516.
55. Kim SH, Shin DW, Kim SY, et al. Terminal versus advanced cancer: do the general population and health care professionals share a common language? Cancer Res Treat. 2016;48:759-767.
56. Lee JK, Yun YH, An AR, et al. The understanding of terminal cancer and its relationship with attitudes toward end-of-life care issues. Med Decis Making. 2014;34:720-730.
57. Lux MP, Bayer CM, Loehberg CR, et al. Shared decision-making in metastatic breast cancer: discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat. 2013;139:429-440.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289.
3. Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243-255.
4. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165-176.
5. Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87-93.
6. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s.
7. Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
8. Wood TJ, Racano A, Yeung H, et al. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081-4089.
9. Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9(suppl 1):S5.
10. Suva LJ, Washam C, Nicholas RW, et al. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208-218.
11. Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365-378.
12. Shibata H, Kato S, Sekine I, et al. Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open. 2016;1:e000037.
13. Svensson E, Christiansen CF, Ulrichsen SP, et al. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7 e016022.
14. Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132-138.
15. Hansen BH, Keller J, Laitinen M, et al. The Scandinavian Sarcoma Group Skeletal Metastasis Register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11-15.
16. Dürr HR, Müller PE, Lenz T, et al. Surgical treatment of bone metastases in patients with breast cancer. Clin Orthop Relat Res. 2002:191-196.
17. Weiss RJ, Tullberg E, Forsberg JA, et al. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23:286-290.
18. Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83:74-79.
19. Nathan SS, Chan L, Tan WL, et al. The need for a system of prognostication in skeletal metastasis to decide best end-of-life care - a call to arms. Ann Acad Med Singapore. 2010;39:476-481.
20. Weber KL. Evaluation of the adult patient (aged > 40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18:169-179.
21. Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97(3 suppl):866-873.
22. McDuffee LA, Colterjohn N, Singh G. Bone metastasis and pathological fractures. In: Singh G, Rabbani SA, eds. Bone Metastasis. Experimental and Clinical Therapeutics. Totowa, NJ: Humana Press; 2005:229-241.
23. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509-524.
24. Maisano R, Pergolizzi S, Cascinu S. Novel therapeutic approaches to cancer patients with bone metastasis. Crit Rev Oncol Hematol. 2001;40:239-250.
25. Marino MT, Asp AA, Budayer AA, et al. Hypercalcaemia and elevated levels of parathyroid hormone-related protein in cutaneous squamous/basal cell carcinoma. J Intern Med. 1993;233:205-207.
26. Grill V, Ho P, Body JJ, et al. Parathyroid hormone-related protein: elevated levels in both humoral hypercalcemia of malignancy and hypercalcemia complicating metastatic breast cancer. J Clin Endocrinol Metab. 1991;73:1309-1315.
27. Jehn CF, Diel IJ, Overkamp F, et al. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res. 2016;36:2631-2637.
28. Molina R, Bosch X, Auge JM, et al. Utility of serum tumor markers as an aid in the differential diagnosis of patients with clinical suspicion of cancer and in patients with cancer of unknown primary site. Tumour Biol. 2012;33:463-474.
29. Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. a prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75:1276-1281.
30. Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53-64.
31. Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803-809.
32. Yang SN, Liang JA, Lin FJ, et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:325-328.
33. Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623-1629.
34. Adams SC, Potter BK, Mahmood Z, et al. Consequences and prevention of inadvertent internal fixation of primary osseous sarcomas. Clin Orthop Relat Res. 2009;467:519-525.
35. Scolaro JA, Lackman RD. Surgical management of metastatic long bone fractures: principles and techniques. J Am Acad Orthop Surg. 2014;22:90-100.
36. Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J Exp Clin Cancer Res. 2017;36:108.
37. Yoon F, Morton GC. Single fraction radiotherapy versus multiple fraction radiotherapy for bone metastases in prostate cancer patients: comparative effectiveness. Cancer Manag Res. 2014;6:451-457.
38. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336-340.
39. Van Acker HH, Anguille S, Willemen Y, et al. Bisphosphonates for cancer treatment: mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24-40.
40. Castellano D, Sepulveda JM, Garcia-Escobar I, et al. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16:136-145.
41. Guenette JP, Lopez MJ, Kim E, et al. Solitary painful osseous metastases: correlation of imaging features with pain palliation after radiofrequency ablation—a multicenter American College of Radiology imaging network study. Radiology. 2013;268:907-915.
42. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
43. ASATFCPM, ASRAPM. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810-833.
44. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(suppl 4):iv166-iv191.
45. Kvale PA, Simoff M, Prakash UBS, ACCP. Lung cancer. Palliative care. Chest. 2003;123(1 suppl):284S-311S.
46. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420-1433.
47. Kato S, Murakami H, Takeuchi A, et al. Fifteen-year survivor of renal cell carcinoma after metastasectomies for multiple bone metastases. Orthopedics. 2013;36:e1454-e1457.
48. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 suppl):1614-1627.
49. Ristevski B, Jenkinson RJ, Stephen DJG, et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52:302-308.
50. Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. 1989. Clin Orthop Relat Res. 2003(415 suppl):S4-S13.
51. Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;468:2825-2827.
52. Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983:297-302.
53. Bird JE. “Advances in the surgical management of bone tumors.” Curr Oncol Rep. 2014;16:392.
54. Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91:1503-1516.
55. Kim SH, Shin DW, Kim SY, et al. Terminal versus advanced cancer: do the general population and health care professionals share a common language? Cancer Res Treat. 2016;48:759-767.
56. Lee JK, Yun YH, An AR, et al. The understanding of terminal cancer and its relationship with attitudes toward end-of-life care issues. Med Decis Making. 2014;34:720-730.
57. Lux MP, Bayer CM, Loehberg CR, et al. Shared decision-making in metastatic breast cancer: discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat. 2013;139:429-440.
PRACTICE RECOMMENDATIONS
› Initiate appropriate lab and imaging work-ups for any patient without known malignancy who has a suspicious bone lesion. C
› Prescribe protected weight-bearing for the patient who has a painful bone lesion, and refer promptly to an orthopedic surgeon to prevent pathologic fracture. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
COVID-19: New guidance to stem mental health crisis in frontline HCPs
A new review offers fresh guidance to help stem the mental health toll of the COVID-19 pandemic on frontline clinicians.
Investigators gathered practice guidelines and resources from a wide range of health care organizations and professional societies to develop a conceptual framework of mental health support for health care professionals (HCPs) caring for COVID-19 patients.
“Support needs to be deployed in multiple dimensions – including individual, organizational, and societal levels – and include training in resilience, stress reduction, emotional awareness, and self-care strategies,” lead author Rachel Schwartz, PhD, health services researcher, Stanford (Calif.) University, said in an interview.
The review was published Aug. 21 in the Annals of Internal Medicine.
An opportune moment
Coauthor Rebecca Margolis, DO, director of well-being in the division of medical education and faculty development, Children’s Hospital of Los Angeles, said that this is “an opportune moment to look at how we treat frontline providers in this country.”
Studies of previous pandemics have shown heightened distress in HCPs, even years after the pandemic, and the unique challenges posed by the COVID-19 pandemic surpass those of previous pandemics, Dr. Margolis said in an interview.
Dr. Schwartz, Dr. Margolis, and coauthors Uma Anand, PhD, LP, and Jina Sinskey, MD, met through the Collaborative for Healing and Renewal in Medicine network, a group of medical educators, leaders in academic medicine, experts in burnout research and interventions, and trainees working together to promote well-being among trainees and practicing physicians.
“We were brought together on a conference call in March, when things were particularly bad in New York, and started looking to see what resources we could get to frontline providers who were suffering. It was great to lean on each other and stand on the shoulders of colleagues in New York, who were the ones we learned from on these calls,” said Dr. Margolis.
The authors recommended addressing clinicians’ basic practical needs, including ensuring essentials like meals and transportation, establishing a “well-being area” within hospitals for staff to rest, and providing well-stocked living quarters so clinicians can safely quarantine from family, as well as personal protective equipment and child care.
Clinicians are often asked to “assume new professional roles to meet evolving needs” during a pandemic, which can increase stress. The authors recommended targeted training, assessment of clinician skills before redeployment to a new clinical role, and clear communication practices around redeployment.
Recognition from hospital and government leaders improves morale and supports clinicians’ ability to continue delivering care. Leadership should “leverage communication strategies to provide clinicians with up-to-date information and reassurance,” they wrote.
‘Uniquely isolated’
Dr. Margolis noted that
“My colleagues feel a sense of moral injury, putting their lives on the line at work, performing the most perilous job, and their kids can’t hang out with other kids, which just puts salt on the wound,” she said.
Additional sources of moral injury are deciding which patients should receive life support in the event of inadequate resources and bearing witness to, or enforcing, policies that lead to patients dying alone.
Leaders should encourage clinicians to “seek informal support from colleagues, managers, or chaplains” and to “provide rapid access to professional help,” the authors noted.
Furthermore, they contended that leaders should “proactively and routinely monitor the psychological well-being of their teams,” since guilt and shame often prevent clinicians from disclosing feelings of moral injury.
“Being provided with routine mental health support should be normalized and it should be part of the job – not only during COVID-19 but in general,” Dr. Schwartz said.
‘Battle buddies’
Dr. Margolis recommended the “battle buddy” model for mutual peer support.
Dr. Anand, a mental health clinician at Mayo Medical School, Rochester, Minn., elaborated.
“We connect residents with each other, and they form pairs to support each other and watch for warning signs such as withdrawal from colleagues, being frequently tearful, not showing up at work or showing up late, missing assignments, making mistakes at work, increased use of alcohol, or verbalizing serious concerns,” Dr. Anand said.
If the buddy shows any of these warning signs, he or she can be directed to appropriate resources to get help.
Since the pandemic has interfered with the ability to connect with colleagues and family members, attention should be paid to addressing the social support needs of clinicians.
Dr. Anand suggested that clinicians maintain contact with counselors, friends, and family, even if they cannot be together in person and must connect “virtually.”
Resilience and strength training are “key” components of reducing clinician distress, but trainings as well as processing groups and support workshops should be offered during protected time, Dr. Margolis advised, since it can be burdensome for clinicians to wake up early or stay late to attend these sessions.
Leaders and administrators should “model self-care and well-being,” she noted. For example, sending emails to clinicians late at night or on weekends creates an expectation of a rapid reply, which leads to additional pressure for the clinician.
“This is of the most powerful unspoken curricula we can develop,” Dr. Margolis emphasized.
Self-care critical
Marcus S. Shaker, MD, MSc, associate professor of pediatrics, medicine, and community and family medicine, Children’s Hospital at Dartmouth-Hitchcock in Lebanon, N.H., and Geisel School of Medicine at Dartmouth, Hanover, N.H., said the study was “a much appreciated, timely reminder of the importance of clinician wellness.”
Moreover, “without self-care, our ability to help our patients withers. This article provides a useful conceptual framework for individuals and organizations to provide the right care at the right time in these unprecedented times,” said Dr. Shaker, who was not involved with the study.
The authors agreed, stating that clinicians “require proactive psychological protection specifically because they are a population known for putting others’ needs before their own.”
They recommended several resources for HCPs, including the Physician Support Line; Headspace, a mindfulness Web-based app for reducing stress and anxiety; the National Suicide Prevention Lifeline; and the Crisis Text Line.
The authors and Dr. Shaker disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new review offers fresh guidance to help stem the mental health toll of the COVID-19 pandemic on frontline clinicians.
Investigators gathered practice guidelines and resources from a wide range of health care organizations and professional societies to develop a conceptual framework of mental health support for health care professionals (HCPs) caring for COVID-19 patients.
“Support needs to be deployed in multiple dimensions – including individual, organizational, and societal levels – and include training in resilience, stress reduction, emotional awareness, and self-care strategies,” lead author Rachel Schwartz, PhD, health services researcher, Stanford (Calif.) University, said in an interview.
The review was published Aug. 21 in the Annals of Internal Medicine.
An opportune moment
Coauthor Rebecca Margolis, DO, director of well-being in the division of medical education and faculty development, Children’s Hospital of Los Angeles, said that this is “an opportune moment to look at how we treat frontline providers in this country.”
Studies of previous pandemics have shown heightened distress in HCPs, even years after the pandemic, and the unique challenges posed by the COVID-19 pandemic surpass those of previous pandemics, Dr. Margolis said in an interview.
Dr. Schwartz, Dr. Margolis, and coauthors Uma Anand, PhD, LP, and Jina Sinskey, MD, met through the Collaborative for Healing and Renewal in Medicine network, a group of medical educators, leaders in academic medicine, experts in burnout research and interventions, and trainees working together to promote well-being among trainees and practicing physicians.
“We were brought together on a conference call in March, when things were particularly bad in New York, and started looking to see what resources we could get to frontline providers who were suffering. It was great to lean on each other and stand on the shoulders of colleagues in New York, who were the ones we learned from on these calls,” said Dr. Margolis.
The authors recommended addressing clinicians’ basic practical needs, including ensuring essentials like meals and transportation, establishing a “well-being area” within hospitals for staff to rest, and providing well-stocked living quarters so clinicians can safely quarantine from family, as well as personal protective equipment and child care.
Clinicians are often asked to “assume new professional roles to meet evolving needs” during a pandemic, which can increase stress. The authors recommended targeted training, assessment of clinician skills before redeployment to a new clinical role, and clear communication practices around redeployment.
Recognition from hospital and government leaders improves morale and supports clinicians’ ability to continue delivering care. Leadership should “leverage communication strategies to provide clinicians with up-to-date information and reassurance,” they wrote.
‘Uniquely isolated’
Dr. Margolis noted that
“My colleagues feel a sense of moral injury, putting their lives on the line at work, performing the most perilous job, and their kids can’t hang out with other kids, which just puts salt on the wound,” she said.
Additional sources of moral injury are deciding which patients should receive life support in the event of inadequate resources and bearing witness to, or enforcing, policies that lead to patients dying alone.
Leaders should encourage clinicians to “seek informal support from colleagues, managers, or chaplains” and to “provide rapid access to professional help,” the authors noted.
Furthermore, they contended that leaders should “proactively and routinely monitor the psychological well-being of their teams,” since guilt and shame often prevent clinicians from disclosing feelings of moral injury.
“Being provided with routine mental health support should be normalized and it should be part of the job – not only during COVID-19 but in general,” Dr. Schwartz said.
‘Battle buddies’
Dr. Margolis recommended the “battle buddy” model for mutual peer support.
Dr. Anand, a mental health clinician at Mayo Medical School, Rochester, Minn., elaborated.
“We connect residents with each other, and they form pairs to support each other and watch for warning signs such as withdrawal from colleagues, being frequently tearful, not showing up at work or showing up late, missing assignments, making mistakes at work, increased use of alcohol, or verbalizing serious concerns,” Dr. Anand said.
If the buddy shows any of these warning signs, he or she can be directed to appropriate resources to get help.
Since the pandemic has interfered with the ability to connect with colleagues and family members, attention should be paid to addressing the social support needs of clinicians.
Dr. Anand suggested that clinicians maintain contact with counselors, friends, and family, even if they cannot be together in person and must connect “virtually.”
Resilience and strength training are “key” components of reducing clinician distress, but trainings as well as processing groups and support workshops should be offered during protected time, Dr. Margolis advised, since it can be burdensome for clinicians to wake up early or stay late to attend these sessions.
Leaders and administrators should “model self-care and well-being,” she noted. For example, sending emails to clinicians late at night or on weekends creates an expectation of a rapid reply, which leads to additional pressure for the clinician.
“This is of the most powerful unspoken curricula we can develop,” Dr. Margolis emphasized.
Self-care critical
Marcus S. Shaker, MD, MSc, associate professor of pediatrics, medicine, and community and family medicine, Children’s Hospital at Dartmouth-Hitchcock in Lebanon, N.H., and Geisel School of Medicine at Dartmouth, Hanover, N.H., said the study was “a much appreciated, timely reminder of the importance of clinician wellness.”
Moreover, “without self-care, our ability to help our patients withers. This article provides a useful conceptual framework for individuals and organizations to provide the right care at the right time in these unprecedented times,” said Dr. Shaker, who was not involved with the study.
The authors agreed, stating that clinicians “require proactive psychological protection specifically because they are a population known for putting others’ needs before their own.”
They recommended several resources for HCPs, including the Physician Support Line; Headspace, a mindfulness Web-based app for reducing stress and anxiety; the National Suicide Prevention Lifeline; and the Crisis Text Line.
The authors and Dr. Shaker disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new review offers fresh guidance to help stem the mental health toll of the COVID-19 pandemic on frontline clinicians.
Investigators gathered practice guidelines and resources from a wide range of health care organizations and professional societies to develop a conceptual framework of mental health support for health care professionals (HCPs) caring for COVID-19 patients.
“Support needs to be deployed in multiple dimensions – including individual, organizational, and societal levels – and include training in resilience, stress reduction, emotional awareness, and self-care strategies,” lead author Rachel Schwartz, PhD, health services researcher, Stanford (Calif.) University, said in an interview.
The review was published Aug. 21 in the Annals of Internal Medicine.
An opportune moment
Coauthor Rebecca Margolis, DO, director of well-being in the division of medical education and faculty development, Children’s Hospital of Los Angeles, said that this is “an opportune moment to look at how we treat frontline providers in this country.”
Studies of previous pandemics have shown heightened distress in HCPs, even years after the pandemic, and the unique challenges posed by the COVID-19 pandemic surpass those of previous pandemics, Dr. Margolis said in an interview.
Dr. Schwartz, Dr. Margolis, and coauthors Uma Anand, PhD, LP, and Jina Sinskey, MD, met through the Collaborative for Healing and Renewal in Medicine network, a group of medical educators, leaders in academic medicine, experts in burnout research and interventions, and trainees working together to promote well-being among trainees and practicing physicians.
“We were brought together on a conference call in March, when things were particularly bad in New York, and started looking to see what resources we could get to frontline providers who were suffering. It was great to lean on each other and stand on the shoulders of colleagues in New York, who were the ones we learned from on these calls,” said Dr. Margolis.
The authors recommended addressing clinicians’ basic practical needs, including ensuring essentials like meals and transportation, establishing a “well-being area” within hospitals for staff to rest, and providing well-stocked living quarters so clinicians can safely quarantine from family, as well as personal protective equipment and child care.
Clinicians are often asked to “assume new professional roles to meet evolving needs” during a pandemic, which can increase stress. The authors recommended targeted training, assessment of clinician skills before redeployment to a new clinical role, and clear communication practices around redeployment.
Recognition from hospital and government leaders improves morale and supports clinicians’ ability to continue delivering care. Leadership should “leverage communication strategies to provide clinicians with up-to-date information and reassurance,” they wrote.
‘Uniquely isolated’
Dr. Margolis noted that
“My colleagues feel a sense of moral injury, putting their lives on the line at work, performing the most perilous job, and their kids can’t hang out with other kids, which just puts salt on the wound,” she said.
Additional sources of moral injury are deciding which patients should receive life support in the event of inadequate resources and bearing witness to, or enforcing, policies that lead to patients dying alone.
Leaders should encourage clinicians to “seek informal support from colleagues, managers, or chaplains” and to “provide rapid access to professional help,” the authors noted.
Furthermore, they contended that leaders should “proactively and routinely monitor the psychological well-being of their teams,” since guilt and shame often prevent clinicians from disclosing feelings of moral injury.
“Being provided with routine mental health support should be normalized and it should be part of the job – not only during COVID-19 but in general,” Dr. Schwartz said.
‘Battle buddies’
Dr. Margolis recommended the “battle buddy” model for mutual peer support.
Dr. Anand, a mental health clinician at Mayo Medical School, Rochester, Minn., elaborated.
“We connect residents with each other, and they form pairs to support each other and watch for warning signs such as withdrawal from colleagues, being frequently tearful, not showing up at work or showing up late, missing assignments, making mistakes at work, increased use of alcohol, or verbalizing serious concerns,” Dr. Anand said.
If the buddy shows any of these warning signs, he or she can be directed to appropriate resources to get help.
Since the pandemic has interfered with the ability to connect with colleagues and family members, attention should be paid to addressing the social support needs of clinicians.
Dr. Anand suggested that clinicians maintain contact with counselors, friends, and family, even if they cannot be together in person and must connect “virtually.”
Resilience and strength training are “key” components of reducing clinician distress, but trainings as well as processing groups and support workshops should be offered during protected time, Dr. Margolis advised, since it can be burdensome for clinicians to wake up early or stay late to attend these sessions.
Leaders and administrators should “model self-care and well-being,” she noted. For example, sending emails to clinicians late at night or on weekends creates an expectation of a rapid reply, which leads to additional pressure for the clinician.
“This is of the most powerful unspoken curricula we can develop,” Dr. Margolis emphasized.
Self-care critical
Marcus S. Shaker, MD, MSc, associate professor of pediatrics, medicine, and community and family medicine, Children’s Hospital at Dartmouth-Hitchcock in Lebanon, N.H., and Geisel School of Medicine at Dartmouth, Hanover, N.H., said the study was “a much appreciated, timely reminder of the importance of clinician wellness.”
Moreover, “without self-care, our ability to help our patients withers. This article provides a useful conceptual framework for individuals and organizations to provide the right care at the right time in these unprecedented times,” said Dr. Shaker, who was not involved with the study.
The authors agreed, stating that clinicians “require proactive psychological protection specifically because they are a population known for putting others’ needs before their own.”
They recommended several resources for HCPs, including the Physician Support Line; Headspace, a mindfulness Web-based app for reducing stress and anxiety; the National Suicide Prevention Lifeline; and the Crisis Text Line.
The authors and Dr. Shaker disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
It's time to change when BP meds are taken
In this issue of JFP, there is an extraordinarily valuable PURL (Priority Updates from the Research Literature) for you. PURLs® are written by academic family physicians who comb through volumes of research to identify and then summarize for JFP important studies we believe should change your practice. After reading a PURL, you may find that you have already implemented this new evidence into your practice. In that case, the PURL confirms that you are doing the right thing.
Here is the good news from this month’s PURL: Having patients take their blood pressure (BP) medication in the evening, rather than in the morning, leads not only to better BP control but also to a reduction in cardiovascular events.1 How large is this effect? This simple intervention nearly cut in half the number of major cardiovascular events—including myocardial infarction, stroke, and congestive heart failure—and the risk for death from a cardiovascular event was reduced 56%. The number needed to treat to prevent 1 major cardiovascular event over the course of 6.3 years was 20. That means this intervention is more powerful than taking a statin!
The investigators, who call this intervention “chronotherapy” since it takes into account the body’s circadian rhythms, have been studying the effect of this simple intervention for many years, and this large randomized trial provides very strong evidence that it’s effective. Despite the excellent trial design and execution, some cardiovascular researchers have questioned the integrity of the trial and believe patients should continue to take their antihypertensives in the morning.2 The main investigator of the study, however, has provided a very strong rebuttal in print.3
I am delighted to see the positive results of this definitive trial of chronotherapy for hypertension. When these investigators published their first randomized trial of chronotherapy in 2010,4 which demonstrated a significant BP reduction with evening dosing of antihypertensives, I began telling all of my patients to take at least 1 of their antihypertensive meds in the evening. Maybe I was jumping the gun at that time, but we should all tell our patients to take their BP medication in the evening from now on. What could be an easier way to reduce cardiovascular morbidity and mortality?
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754
2. Kreutz R, Kjeldsen SE, Burnier M, et al. Blood pressure medication should not be routinely dosed at bedtime. We must disregard the data from the HYGIA project [editorial]. Blood Press. 2020;29:135-136.
3. Crespo JJ, Domínguez-Sardiña M, Otero A, et. al. Bedtime hypertension chronotherapy best reduces cardiovascular disease risk as corroborated by the Hygia Chronotherapy Trial. Rebuttal to European Society of Hypertension officials. Chronobiol Int. 2020;37:771-780.
4. Hermida RC, Ayala DE, Mojón A, Fernández JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
In this issue of JFP, there is an extraordinarily valuable PURL (Priority Updates from the Research Literature) for you. PURLs® are written by academic family physicians who comb through volumes of research to identify and then summarize for JFP important studies we believe should change your practice. After reading a PURL, you may find that you have already implemented this new evidence into your practice. In that case, the PURL confirms that you are doing the right thing.
Here is the good news from this month’s PURL: Having patients take their blood pressure (BP) medication in the evening, rather than in the morning, leads not only to better BP control but also to a reduction in cardiovascular events.1 How large is this effect? This simple intervention nearly cut in half the number of major cardiovascular events—including myocardial infarction, stroke, and congestive heart failure—and the risk for death from a cardiovascular event was reduced 56%. The number needed to treat to prevent 1 major cardiovascular event over the course of 6.3 years was 20. That means this intervention is more powerful than taking a statin!
The investigators, who call this intervention “chronotherapy” since it takes into account the body’s circadian rhythms, have been studying the effect of this simple intervention for many years, and this large randomized trial provides very strong evidence that it’s effective. Despite the excellent trial design and execution, some cardiovascular researchers have questioned the integrity of the trial and believe patients should continue to take their antihypertensives in the morning.2 The main investigator of the study, however, has provided a very strong rebuttal in print.3
I am delighted to see the positive results of this definitive trial of chronotherapy for hypertension. When these investigators published their first randomized trial of chronotherapy in 2010,4 which demonstrated a significant BP reduction with evening dosing of antihypertensives, I began telling all of my patients to take at least 1 of their antihypertensive meds in the evening. Maybe I was jumping the gun at that time, but we should all tell our patients to take their BP medication in the evening from now on. What could be an easier way to reduce cardiovascular morbidity and mortality?
In this issue of JFP, there is an extraordinarily valuable PURL (Priority Updates from the Research Literature) for you. PURLs® are written by academic family physicians who comb through volumes of research to identify and then summarize for JFP important studies we believe should change your practice. After reading a PURL, you may find that you have already implemented this new evidence into your practice. In that case, the PURL confirms that you are doing the right thing.
Here is the good news from this month’s PURL: Having patients take their blood pressure (BP) medication in the evening, rather than in the morning, leads not only to better BP control but also to a reduction in cardiovascular events.1 How large is this effect? This simple intervention nearly cut in half the number of major cardiovascular events—including myocardial infarction, stroke, and congestive heart failure—and the risk for death from a cardiovascular event was reduced 56%. The number needed to treat to prevent 1 major cardiovascular event over the course of 6.3 years was 20. That means this intervention is more powerful than taking a statin!
The investigators, who call this intervention “chronotherapy” since it takes into account the body’s circadian rhythms, have been studying the effect of this simple intervention for many years, and this large randomized trial provides very strong evidence that it’s effective. Despite the excellent trial design and execution, some cardiovascular researchers have questioned the integrity of the trial and believe patients should continue to take their antihypertensives in the morning.2 The main investigator of the study, however, has provided a very strong rebuttal in print.3
I am delighted to see the positive results of this definitive trial of chronotherapy for hypertension. When these investigators published their first randomized trial of chronotherapy in 2010,4 which demonstrated a significant BP reduction with evening dosing of antihypertensives, I began telling all of my patients to take at least 1 of their antihypertensive meds in the evening. Maybe I was jumping the gun at that time, but we should all tell our patients to take their BP medication in the evening from now on. What could be an easier way to reduce cardiovascular morbidity and mortality?
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754
2. Kreutz R, Kjeldsen SE, Burnier M, et al. Blood pressure medication should not be routinely dosed at bedtime. We must disregard the data from the HYGIA project [editorial]. Blood Press. 2020;29:135-136.
3. Crespo JJ, Domínguez-Sardiña M, Otero A, et. al. Bedtime hypertension chronotherapy best reduces cardiovascular disease risk as corroborated by the Hygia Chronotherapy Trial. Rebuttal to European Society of Hypertension officials. Chronobiol Int. 2020;37:771-780.
4. Hermida RC, Ayala DE, Mojón A, Fernández JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754
2. Kreutz R, Kjeldsen SE, Burnier M, et al. Blood pressure medication should not be routinely dosed at bedtime. We must disregard the data from the HYGIA project [editorial]. Blood Press. 2020;29:135-136.
3. Crespo JJ, Domínguez-Sardiña M, Otero A, et. al. Bedtime hypertension chronotherapy best reduces cardiovascular disease risk as corroborated by the Hygia Chronotherapy Trial. Rebuttal to European Society of Hypertension officials. Chronobiol Int. 2020;37:771-780.
4. Hermida RC, Ayala DE, Mojón A, Fernández JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
Is it better to take that antihypertensive at night?
ILLUSTRATIVE CASE
A 54-year-old White woman presents to your office with new-onset hypertension. As you are discussing options for treatment, she mentions she would prefer once-daily dosing to help her remember to take her medication. She also wants to know what the best time of day is to take her medication to reduce her risk of cardiovascular disease (CVD). What do you advise?
The burden of hypertension is significant and growing in the United States. The 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines reported that more than 108 million people were affected in 2015-2016—up from 87 million in 1999-2000.2 Yet control of hypertension is improving among those receiving antihypertension pharmacotherapy. As reported in the ACC/AHA guidelines, data from the 2016 National Health and Nutrition Examination Survey (NHANES) indicate an increase of controlled hypertension among those receiving treatment from 25.6% (1999-2000) to 43.5% (2015-2016).2
Chronotherapy involves the administration of medication in coordination with the body’s circadian rhythms to maximize therapeutic effectiveness and/or minimize adverse effects. It is not a new concept as it applies to hypertension. Circadian rhythm–dependent mechanisms influence the natural rise and fall of blood pressure (BP).1 The renin-angiotensin-aldosterone system, known to be most active at night, is a target mechanism for BP control.1 Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are more effective (alone or in combination with other agents) at reducing BP during sleep and wakefulness when they are taken at night.3,4 Additional prospective clinical trials and systematic reviews have documented improved BP during sleep and on 24-hour ambulatory monitoring when antihypertensives are taken at bedtime.3-5
However, there have been few long-term studies assessing the effects of bedtime administration of antihypertensive medication on CVD risk reduction with patient-oriented outcomes.6,7 Additionally, no studies have evaluated morning vs bedtime administration of antihypertensive medication for CVD risk reduction in a primary care setting. The 2019 ACC/AHA guideline on the primary prevention of CVD offers no recommendation regarding when to take antihypertensive medication.8 Timing of medication administration also is not addressed in the NHANES study of hypertension awareness, treatment, and control in US adults.9
This study sought to determine in a primary care setting whether taking antihypertensives at bedtime, as opposed to upon waking, more effectively reduces CVD risk.
STUDY SUMMARY
PM vs AM antihypertensive dosing reduces CV events
This prospective, randomized, open-label, blinded endpoint trial of antihypertensive medication administration timing was part of a large, multicenter Spanish study investigating ambulatory BP monitoring (ABPM) as a routine diagnostic tool.
Study participants were randomly assigned in a 1:1 ratio to 2 treatment arms; participants either took all of their BP medications in the morning upon waking (n = 9532) or right before bedtime (n = 9552). The study was conducted in a primary care clinical setting. It included adult participants (age ≥ 18 years) with hypertension (defined as having at least 1 of the following benchmarks: awake systolic BP [SBP] mean ≥ 135 mm Hg, awake diastolic BP (DBP) mean ≥ 85 mm Hg, asleep SBP mean ≥ 120 mm Hg, asleep DBP mean ≥ 70 mm Hg as corroborated by 48-hour ABPM) who were taking at least 1 antihypertensive medication.
Continue to: Any antihypertension medication...
Any antihypertension medication included in the Spanish national formulary was allowed (exact agents were not delineated, but the following classes were included: ARB, ACE inhibitor, calcium channel blocker [CCB], beta-blocker, and/or diuretic). All BP medications had to be dosed once daily for inclusion. Exclusion criteria included pregnancy, night or rotating-shift work, alcohol or other substance dependence, acquired immunodeficiency syndrome, preexisting CVD (unstable angina, heart failure, arrhythmia, kidney failure, and retinopathy), inability to tolerate ABPM, and inability to comply with required 1-year follow-up.
Upon enrollment and at every subsequent clinic visit (scheduled at least annually), participants underwent 48-hour ABPM. Those with uncontrolled BP or elevated CVD risk had scheduled follow-up and ABPM more frequently. The primary outcome was a composite of CVD events including new-onset myocardial infarction, coronary revascularization, heart failure, ischemic stroke, hemorrhagic stroke, and CVD death. Secondary endpoints were individually analyzed primary outcomes of CVD events. The typical patient at baseline was 60.5 years of age with a body mass index of 29.7, an almost 9-year duration of hypertension, and a baseline office BP of 149/86 mm Hg. The patient break-out by antihypertensive class (awakening vs bedtime groups) was as follows: ARB (53% vs 53%), ACE inhibitor (25% vs 23%), CCB (33% vs 37%), beta-blocker (22% vs 18%), and diuretic (47% vs 40%).
During the median 6.3-year patient follow-up period, 1752 participants experienced a total of 2454 CVD events. Patients in the bedtime administration group, compared with those in the morning group, showed significantly lower risk for a CVD event (hazard ratio [HR] = 0.55; 95% confidence interval [CI], 0.50-0.61; P < .001). Also, there was a lower risk for individual CVD events in the bedtime administration group: CVD death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63). This difference remained after correction for multiple potential confounders. There were no differences in adverse events, such as sleep-time hypotension, between groups.
WHAT’S NEW
First RCT in primary care to show dosing time change reduces CV risk
This is the first randomized controlled trial (RCT) performed in a primary care setting to compare before-bedtime to upon-waking administration of antihypertensive medications using clinically significant endpoints. The study demonstrates that a simple change in administration time has the potential to significantly improve the lives of our patients by reducing the risk for cardiovascular events and their medication burden.
CAVEATS
Homogenous population and exclusions limit generalizability
Because the study population consisted of white Spanish men and women, the results may not be generalizable beyond that ethnic group. In addition, the study exclusions limit interpretation in night/rotating-shift employees, patients with secondary hypertension, and those with CVD, chronic kidney disease, or severe retinopathy looking to reduce their risk.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Nighttime urination could lead to nonadherence
Taking diuretics at bedtime may result in unwanted nighttime awakenings for visits to the bathroom, which could lead to nonadherence in some patients.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754.
2. Dorans KS, Mills KT, Liu Y, et al. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc. 2018;7:e008888.
3. Hermida RC, Ayala DE, Smolensky MH, et al. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens Res. 2016;39:277-292.
4. Bowles NP, Thosar SS, Herzig MX, et al. Chronotherapy for hypertension. Curr Hypertens Rep. 2018;20:97.
5. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011:CD004184.
6. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145-153.
7. Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073-2082.
8. Arnette DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:e177-e232.
9. Foti K, Wang D, Appel LJ, et al. Hypertension awareness, treatment, and control in US adults: trends in the hypertensive control cascade by population subgroup (National Health and Nutrition Examination Survey, 1999-2016). Am J Epidemiol. 2019;188:2165-2174.
ILLUSTRATIVE CASE
A 54-year-old White woman presents to your office with new-onset hypertension. As you are discussing options for treatment, she mentions she would prefer once-daily dosing to help her remember to take her medication. She also wants to know what the best time of day is to take her medication to reduce her risk of cardiovascular disease (CVD). What do you advise?
The burden of hypertension is significant and growing in the United States. The 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines reported that more than 108 million people were affected in 2015-2016—up from 87 million in 1999-2000.2 Yet control of hypertension is improving among those receiving antihypertension pharmacotherapy. As reported in the ACC/AHA guidelines, data from the 2016 National Health and Nutrition Examination Survey (NHANES) indicate an increase of controlled hypertension among those receiving treatment from 25.6% (1999-2000) to 43.5% (2015-2016).2
Chronotherapy involves the administration of medication in coordination with the body’s circadian rhythms to maximize therapeutic effectiveness and/or minimize adverse effects. It is not a new concept as it applies to hypertension. Circadian rhythm–dependent mechanisms influence the natural rise and fall of blood pressure (BP).1 The renin-angiotensin-aldosterone system, known to be most active at night, is a target mechanism for BP control.1 Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are more effective (alone or in combination with other agents) at reducing BP during sleep and wakefulness when they are taken at night.3,4 Additional prospective clinical trials and systematic reviews have documented improved BP during sleep and on 24-hour ambulatory monitoring when antihypertensives are taken at bedtime.3-5
However, there have been few long-term studies assessing the effects of bedtime administration of antihypertensive medication on CVD risk reduction with patient-oriented outcomes.6,7 Additionally, no studies have evaluated morning vs bedtime administration of antihypertensive medication for CVD risk reduction in a primary care setting. The 2019 ACC/AHA guideline on the primary prevention of CVD offers no recommendation regarding when to take antihypertensive medication.8 Timing of medication administration also is not addressed in the NHANES study of hypertension awareness, treatment, and control in US adults.9
This study sought to determine in a primary care setting whether taking antihypertensives at bedtime, as opposed to upon waking, more effectively reduces CVD risk.
STUDY SUMMARY
PM vs AM antihypertensive dosing reduces CV events
This prospective, randomized, open-label, blinded endpoint trial of antihypertensive medication administration timing was part of a large, multicenter Spanish study investigating ambulatory BP monitoring (ABPM) as a routine diagnostic tool.
Study participants were randomly assigned in a 1:1 ratio to 2 treatment arms; participants either took all of their BP medications in the morning upon waking (n = 9532) or right before bedtime (n = 9552). The study was conducted in a primary care clinical setting. It included adult participants (age ≥ 18 years) with hypertension (defined as having at least 1 of the following benchmarks: awake systolic BP [SBP] mean ≥ 135 mm Hg, awake diastolic BP (DBP) mean ≥ 85 mm Hg, asleep SBP mean ≥ 120 mm Hg, asleep DBP mean ≥ 70 mm Hg as corroborated by 48-hour ABPM) who were taking at least 1 antihypertensive medication.
Continue to: Any antihypertension medication...
Any antihypertension medication included in the Spanish national formulary was allowed (exact agents were not delineated, but the following classes were included: ARB, ACE inhibitor, calcium channel blocker [CCB], beta-blocker, and/or diuretic). All BP medications had to be dosed once daily for inclusion. Exclusion criteria included pregnancy, night or rotating-shift work, alcohol or other substance dependence, acquired immunodeficiency syndrome, preexisting CVD (unstable angina, heart failure, arrhythmia, kidney failure, and retinopathy), inability to tolerate ABPM, and inability to comply with required 1-year follow-up.
Upon enrollment and at every subsequent clinic visit (scheduled at least annually), participants underwent 48-hour ABPM. Those with uncontrolled BP or elevated CVD risk had scheduled follow-up and ABPM more frequently. The primary outcome was a composite of CVD events including new-onset myocardial infarction, coronary revascularization, heart failure, ischemic stroke, hemorrhagic stroke, and CVD death. Secondary endpoints were individually analyzed primary outcomes of CVD events. The typical patient at baseline was 60.5 years of age with a body mass index of 29.7, an almost 9-year duration of hypertension, and a baseline office BP of 149/86 mm Hg. The patient break-out by antihypertensive class (awakening vs bedtime groups) was as follows: ARB (53% vs 53%), ACE inhibitor (25% vs 23%), CCB (33% vs 37%), beta-blocker (22% vs 18%), and diuretic (47% vs 40%).
During the median 6.3-year patient follow-up period, 1752 participants experienced a total of 2454 CVD events. Patients in the bedtime administration group, compared with those in the morning group, showed significantly lower risk for a CVD event (hazard ratio [HR] = 0.55; 95% confidence interval [CI], 0.50-0.61; P < .001). Also, there was a lower risk for individual CVD events in the bedtime administration group: CVD death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63). This difference remained after correction for multiple potential confounders. There were no differences in adverse events, such as sleep-time hypotension, between groups.
WHAT’S NEW
First RCT in primary care to show dosing time change reduces CV risk
This is the first randomized controlled trial (RCT) performed in a primary care setting to compare before-bedtime to upon-waking administration of antihypertensive medications using clinically significant endpoints. The study demonstrates that a simple change in administration time has the potential to significantly improve the lives of our patients by reducing the risk for cardiovascular events and their medication burden.
CAVEATS
Homogenous population and exclusions limit generalizability
Because the study population consisted of white Spanish men and women, the results may not be generalizable beyond that ethnic group. In addition, the study exclusions limit interpretation in night/rotating-shift employees, patients with secondary hypertension, and those with CVD, chronic kidney disease, or severe retinopathy looking to reduce their risk.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Nighttime urination could lead to nonadherence
Taking diuretics at bedtime may result in unwanted nighttime awakenings for visits to the bathroom, which could lead to nonadherence in some patients.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 54-year-old White woman presents to your office with new-onset hypertension. As you are discussing options for treatment, she mentions she would prefer once-daily dosing to help her remember to take her medication. She also wants to know what the best time of day is to take her medication to reduce her risk of cardiovascular disease (CVD). What do you advise?
The burden of hypertension is significant and growing in the United States. The 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines reported that more than 108 million people were affected in 2015-2016—up from 87 million in 1999-2000.2 Yet control of hypertension is improving among those receiving antihypertension pharmacotherapy. As reported in the ACC/AHA guidelines, data from the 2016 National Health and Nutrition Examination Survey (NHANES) indicate an increase of controlled hypertension among those receiving treatment from 25.6% (1999-2000) to 43.5% (2015-2016).2
Chronotherapy involves the administration of medication in coordination with the body’s circadian rhythms to maximize therapeutic effectiveness and/or minimize adverse effects. It is not a new concept as it applies to hypertension. Circadian rhythm–dependent mechanisms influence the natural rise and fall of blood pressure (BP).1 The renin-angiotensin-aldosterone system, known to be most active at night, is a target mechanism for BP control.1 Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are more effective (alone or in combination with other agents) at reducing BP during sleep and wakefulness when they are taken at night.3,4 Additional prospective clinical trials and systematic reviews have documented improved BP during sleep and on 24-hour ambulatory monitoring when antihypertensives are taken at bedtime.3-5
However, there have been few long-term studies assessing the effects of bedtime administration of antihypertensive medication on CVD risk reduction with patient-oriented outcomes.6,7 Additionally, no studies have evaluated morning vs bedtime administration of antihypertensive medication for CVD risk reduction in a primary care setting. The 2019 ACC/AHA guideline on the primary prevention of CVD offers no recommendation regarding when to take antihypertensive medication.8 Timing of medication administration also is not addressed in the NHANES study of hypertension awareness, treatment, and control in US adults.9
This study sought to determine in a primary care setting whether taking antihypertensives at bedtime, as opposed to upon waking, more effectively reduces CVD risk.
STUDY SUMMARY
PM vs AM antihypertensive dosing reduces CV events
This prospective, randomized, open-label, blinded endpoint trial of antihypertensive medication administration timing was part of a large, multicenter Spanish study investigating ambulatory BP monitoring (ABPM) as a routine diagnostic tool.
Study participants were randomly assigned in a 1:1 ratio to 2 treatment arms; participants either took all of their BP medications in the morning upon waking (n = 9532) or right before bedtime (n = 9552). The study was conducted in a primary care clinical setting. It included adult participants (age ≥ 18 years) with hypertension (defined as having at least 1 of the following benchmarks: awake systolic BP [SBP] mean ≥ 135 mm Hg, awake diastolic BP (DBP) mean ≥ 85 mm Hg, asleep SBP mean ≥ 120 mm Hg, asleep DBP mean ≥ 70 mm Hg as corroborated by 48-hour ABPM) who were taking at least 1 antihypertensive medication.
Continue to: Any antihypertension medication...
Any antihypertension medication included in the Spanish national formulary was allowed (exact agents were not delineated, but the following classes were included: ARB, ACE inhibitor, calcium channel blocker [CCB], beta-blocker, and/or diuretic). All BP medications had to be dosed once daily for inclusion. Exclusion criteria included pregnancy, night or rotating-shift work, alcohol or other substance dependence, acquired immunodeficiency syndrome, preexisting CVD (unstable angina, heart failure, arrhythmia, kidney failure, and retinopathy), inability to tolerate ABPM, and inability to comply with required 1-year follow-up.
Upon enrollment and at every subsequent clinic visit (scheduled at least annually), participants underwent 48-hour ABPM. Those with uncontrolled BP or elevated CVD risk had scheduled follow-up and ABPM more frequently. The primary outcome was a composite of CVD events including new-onset myocardial infarction, coronary revascularization, heart failure, ischemic stroke, hemorrhagic stroke, and CVD death. Secondary endpoints were individually analyzed primary outcomes of CVD events. The typical patient at baseline was 60.5 years of age with a body mass index of 29.7, an almost 9-year duration of hypertension, and a baseline office BP of 149/86 mm Hg. The patient break-out by antihypertensive class (awakening vs bedtime groups) was as follows: ARB (53% vs 53%), ACE inhibitor (25% vs 23%), CCB (33% vs 37%), beta-blocker (22% vs 18%), and diuretic (47% vs 40%).
During the median 6.3-year patient follow-up period, 1752 participants experienced a total of 2454 CVD events. Patients in the bedtime administration group, compared with those in the morning group, showed significantly lower risk for a CVD event (hazard ratio [HR] = 0.55; 95% confidence interval [CI], 0.50-0.61; P < .001). Also, there was a lower risk for individual CVD events in the bedtime administration group: CVD death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63). This difference remained after correction for multiple potential confounders. There were no differences in adverse events, such as sleep-time hypotension, between groups.
WHAT’S NEW
First RCT in primary care to show dosing time change reduces CV risk
This is the first randomized controlled trial (RCT) performed in a primary care setting to compare before-bedtime to upon-waking administration of antihypertensive medications using clinically significant endpoints. The study demonstrates that a simple change in administration time has the potential to significantly improve the lives of our patients by reducing the risk for cardiovascular events and their medication burden.
CAVEATS
Homogenous population and exclusions limit generalizability
Because the study population consisted of white Spanish men and women, the results may not be generalizable beyond that ethnic group. In addition, the study exclusions limit interpretation in night/rotating-shift employees, patients with secondary hypertension, and those with CVD, chronic kidney disease, or severe retinopathy looking to reduce their risk.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Nighttime urination could lead to nonadherence
Taking diuretics at bedtime may result in unwanted nighttime awakenings for visits to the bathroom, which could lead to nonadherence in some patients.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754.
2. Dorans KS, Mills KT, Liu Y, et al. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc. 2018;7:e008888.
3. Hermida RC, Ayala DE, Smolensky MH, et al. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens Res. 2016;39:277-292.
4. Bowles NP, Thosar SS, Herzig MX, et al. Chronotherapy for hypertension. Curr Hypertens Rep. 2018;20:97.
5. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011:CD004184.
6. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145-153.
7. Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073-2082.
8. Arnette DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:e177-e232.
9. Foti K, Wang D, Appel LJ, et al. Hypertension awareness, treatment, and control in US adults: trends in the hypertensive control cascade by population subgroup (National Health and Nutrition Examination Survey, 1999-2016). Am J Epidemiol. 2019;188:2165-2174.
1. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754.
2. Dorans KS, Mills KT, Liu Y, et al. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc. 2018;7:e008888.
3. Hermida RC, Ayala DE, Smolensky MH, et al. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens Res. 2016;39:277-292.
4. Bowles NP, Thosar SS, Herzig MX, et al. Chronotherapy for hypertension. Curr Hypertens Rep. 2018;20:97.
5. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011:CD004184.
6. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145-153.
7. Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073-2082.
8. Arnette DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:e177-e232.
9. Foti K, Wang D, Appel LJ, et al. Hypertension awareness, treatment, and control in US adults: trends in the hypertensive control cascade by population subgroup (National Health and Nutrition Examination Survey, 1999-2016). Am J Epidemiol. 2019;188:2165-2174.
PRACTICE CHANGER
Advise patients to take blood pressure (BP) medication at bedtime rather than upon waking because it results in a decrease in major cardiovascular disease events.
STRENGTH OF RECOMMENDATION
B: Based on a single, good-quality, multicenter trial.
Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial [published online ahead of print October 22, 2019]. Eur Heart J. 2019;ehz754. doi:10.1093/eurheartj/ehz754.1
August 2020 Advances in Precision Oncology
Click here to access August 2020 Advances in Precision Oncology
Table of Contents
- Foreword
- Introduction: Precision Oncology Changes the Game for VA Health Care
- VA National Precision Oncology Program
- Prostate Cancer Foundation-Department of Veterans Affairs Partnership: A Model to Advance Treatment and Care of Invasive Cancers
- The Precision Oncology Program for Cancer of the Prostate Network: A VA-Prostate Cancer Foundation Alliance
- Leveraging Veterans Health Administration Clinical and Research Resources to Accelerate Discovery and Testing in Precision Oncology
- Strategic Initiatives for Veterans With Lung Cancer
- Integrating Germline Genetics Into Precision Oncology Practice in the Veterans Health Administration: Challenges and Opportunities

Click here to access August 2020 Advances in Precision Oncology
Table of Contents
- Foreword
- Introduction: Precision Oncology Changes the Game for VA Health Care
- VA National Precision Oncology Program
- Prostate Cancer Foundation-Department of Veterans Affairs Partnership: A Model to Advance Treatment and Care of Invasive Cancers
- The Precision Oncology Program for Cancer of the Prostate Network: A VA-Prostate Cancer Foundation Alliance
- Leveraging Veterans Health Administration Clinical and Research Resources to Accelerate Discovery and Testing in Precision Oncology
- Strategic Initiatives for Veterans With Lung Cancer
- Integrating Germline Genetics Into Precision Oncology Practice in the Veterans Health Administration: Challenges and Opportunities

Click here to access August 2020 Advances in Precision Oncology
Table of Contents
- Foreword
- Introduction: Precision Oncology Changes the Game for VA Health Care
- VA National Precision Oncology Program
- Prostate Cancer Foundation-Department of Veterans Affairs Partnership: A Model to Advance Treatment and Care of Invasive Cancers
- The Precision Oncology Program for Cancer of the Prostate Network: A VA-Prostate Cancer Foundation Alliance
- Leveraging Veterans Health Administration Clinical and Research Resources to Accelerate Discovery and Testing in Precision Oncology
- Strategic Initiatives for Veterans With Lung Cancer
- Integrating Germline Genetics Into Precision Oncology Practice in the Veterans Health Administration: Challenges and Opportunities

One in seven high schoolers is misusing opioids
according to an analysis from the Centers for Disease Control and Prevention.
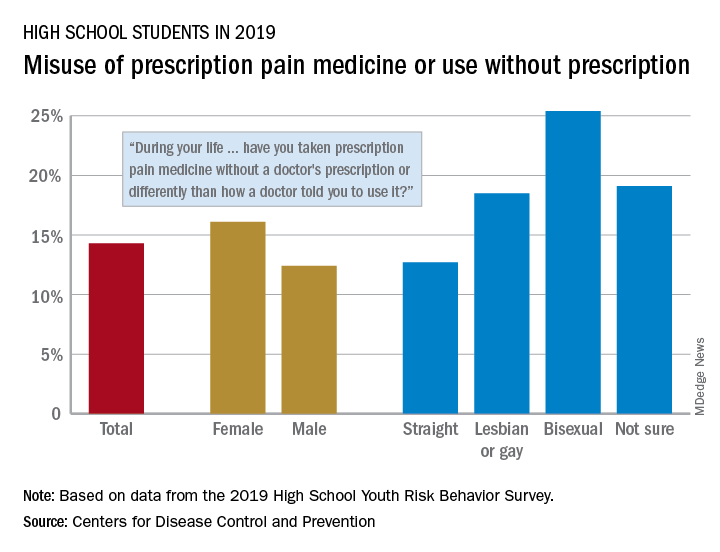
That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
according to an analysis from the Centers for Disease Control and Prevention.

That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
according to an analysis from the Centers for Disease Control and Prevention.

That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
FROM MMWR
Hysteroscopy and COVID-19: Have recommended techniques changed due to the pandemic?
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13
Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.