User login
An Ethical Analysis of Treatment of an Active-Duty Service Member With Limited Follow-up
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
PRACTICE POINTS
- Dermatologic conditions are among the most common concerns reported by active-duty service members.
- The unique considerations of deployments are important for dermatologists to consider in the treatment of skin disease.
Spinal Cord Injury Tied to Greater Risk for Heart Disease
TOPLINE:
Spinal cord injury (SCI) is associated with a significantly greater risk for heart disease than that of the general non-SCI population, especially among those with severe disability, new observational data suggest.
METHODOLOGY:
- Researchers analyzed data from Korea’s National Health Insurance Service on 5083 patients with cervical, thoracic, or lumbar SCI (mean age, 58; 75% men) and 1:3 age- and sex-matched non-SCI controls.
- The study endpoint was new-onset myocardial infarction (MI), heart failure (HF), or atrial fibrillation (AF) during a mean follow-up of 4.3 years.
- Covariates included low income, living in an urban or rural area, alcohol consumption, smoking status, physical activity engagement, body mass index, and blood pressure; comorbidities included hypertension, type 2 diabetes, and dyslipidemia.
TAKEAWAY:
- A total of 169 MI events (7.3 per 1000 person-years), 426 HF events (18.8 per 1000 person-years), and 158 AF events (6.8 per 1000 person-years) occurred among SCI survivors.
- After adjustment, SCI survivors had a higher risk for MI (adjusted hazard ratio [aHR], 2.41), HF (aHR, 2.24), and AF (aHR, 1.84) than that of controls.
- Cervical and lumbar SCI survivors had an increased risk for heart disease compared with controls regardless of disability, and the risk was slightly higher for those with a disability; for cervical SCI survivors with a disability, aHRs for MI, HF, and AF, respectively, were 2.30, 2.05, and 1.73; for lumbar SCI survivors with a disability, aHRs were 2.79, 2.35, and 2.47.
- Thoracic SCI survivors with disability had a higher risk for MI (aHR, 5.62) and HF (aHR, 3.31) than controls.
IN PRACTICE:
“[T]he recognition and treatment of modifiable cardiovascular risk factors must be reinforced in the SCI population, [and] proper rehabilitation and education should be considered to prevent autonomic dysreflexia or orthostatic hypotension,” the authors wrote.
In an accompanying editorial, Christopher R. West, PhD, and Jacquelyn J. Cragg, PhD, both of the University of British Columbia, Vancouver, Canada, noted that clinical guidelines for cardiovascular and cardiometabolic disease after SCI don’t include approaches to help mitigate the risk for cardiac events such as those reported in the study; therefore, they wrote, the findings “should act as ‘call-to-arms’ to researchers and clinicians to shift gears from tradition and begin studying the clinical efficacy of neuraxial therapies that could help restore autonomic balance [in SCI], such as targeted neuromodulation.”
SOURCE:
The study was led by Jung Eun Yoo, MD, PhD of Seoul National University College of Medicine, Seoul, South Korea, and published online on February 12 in the Journal of the American College of Cardiology.
LIMITATIONS:
The database was not designed for the SCI population, so data are incomplete. The incidence of thoracic SCI was particularly low. Because SCI survivors may have impaired perception of chest pain in ischemic heart disease, those with asymptomatic or silent heart disease may not have been captured during follow-up. All study participants were Korean, so the findings may not be generalizable to other ethnicities.
DISCLOSURES:
This research was partially supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, South Korea. The study authors and the editorialists had no relevant relationships to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Spinal cord injury (SCI) is associated with a significantly greater risk for heart disease than that of the general non-SCI population, especially among those with severe disability, new observational data suggest.
METHODOLOGY:
- Researchers analyzed data from Korea’s National Health Insurance Service on 5083 patients with cervical, thoracic, or lumbar SCI (mean age, 58; 75% men) and 1:3 age- and sex-matched non-SCI controls.
- The study endpoint was new-onset myocardial infarction (MI), heart failure (HF), or atrial fibrillation (AF) during a mean follow-up of 4.3 years.
- Covariates included low income, living in an urban or rural area, alcohol consumption, smoking status, physical activity engagement, body mass index, and blood pressure; comorbidities included hypertension, type 2 diabetes, and dyslipidemia.
TAKEAWAY:
- A total of 169 MI events (7.3 per 1000 person-years), 426 HF events (18.8 per 1000 person-years), and 158 AF events (6.8 per 1000 person-years) occurred among SCI survivors.
- After adjustment, SCI survivors had a higher risk for MI (adjusted hazard ratio [aHR], 2.41), HF (aHR, 2.24), and AF (aHR, 1.84) than that of controls.
- Cervical and lumbar SCI survivors had an increased risk for heart disease compared with controls regardless of disability, and the risk was slightly higher for those with a disability; for cervical SCI survivors with a disability, aHRs for MI, HF, and AF, respectively, were 2.30, 2.05, and 1.73; for lumbar SCI survivors with a disability, aHRs were 2.79, 2.35, and 2.47.
- Thoracic SCI survivors with disability had a higher risk for MI (aHR, 5.62) and HF (aHR, 3.31) than controls.
IN PRACTICE:
“[T]he recognition and treatment of modifiable cardiovascular risk factors must be reinforced in the SCI population, [and] proper rehabilitation and education should be considered to prevent autonomic dysreflexia or orthostatic hypotension,” the authors wrote.
In an accompanying editorial, Christopher R. West, PhD, and Jacquelyn J. Cragg, PhD, both of the University of British Columbia, Vancouver, Canada, noted that clinical guidelines for cardiovascular and cardiometabolic disease after SCI don’t include approaches to help mitigate the risk for cardiac events such as those reported in the study; therefore, they wrote, the findings “should act as ‘call-to-arms’ to researchers and clinicians to shift gears from tradition and begin studying the clinical efficacy of neuraxial therapies that could help restore autonomic balance [in SCI], such as targeted neuromodulation.”
SOURCE:
The study was led by Jung Eun Yoo, MD, PhD of Seoul National University College of Medicine, Seoul, South Korea, and published online on February 12 in the Journal of the American College of Cardiology.
LIMITATIONS:
The database was not designed for the SCI population, so data are incomplete. The incidence of thoracic SCI was particularly low. Because SCI survivors may have impaired perception of chest pain in ischemic heart disease, those with asymptomatic or silent heart disease may not have been captured during follow-up. All study participants were Korean, so the findings may not be generalizable to other ethnicities.
DISCLOSURES:
This research was partially supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, South Korea. The study authors and the editorialists had no relevant relationships to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Spinal cord injury (SCI) is associated with a significantly greater risk for heart disease than that of the general non-SCI population, especially among those with severe disability, new observational data suggest.
METHODOLOGY:
- Researchers analyzed data from Korea’s National Health Insurance Service on 5083 patients with cervical, thoracic, or lumbar SCI (mean age, 58; 75% men) and 1:3 age- and sex-matched non-SCI controls.
- The study endpoint was new-onset myocardial infarction (MI), heart failure (HF), or atrial fibrillation (AF) during a mean follow-up of 4.3 years.
- Covariates included low income, living in an urban or rural area, alcohol consumption, smoking status, physical activity engagement, body mass index, and blood pressure; comorbidities included hypertension, type 2 diabetes, and dyslipidemia.
TAKEAWAY:
- A total of 169 MI events (7.3 per 1000 person-years), 426 HF events (18.8 per 1000 person-years), and 158 AF events (6.8 per 1000 person-years) occurred among SCI survivors.
- After adjustment, SCI survivors had a higher risk for MI (adjusted hazard ratio [aHR], 2.41), HF (aHR, 2.24), and AF (aHR, 1.84) than that of controls.
- Cervical and lumbar SCI survivors had an increased risk for heart disease compared with controls regardless of disability, and the risk was slightly higher for those with a disability; for cervical SCI survivors with a disability, aHRs for MI, HF, and AF, respectively, were 2.30, 2.05, and 1.73; for lumbar SCI survivors with a disability, aHRs were 2.79, 2.35, and 2.47.
- Thoracic SCI survivors with disability had a higher risk for MI (aHR, 5.62) and HF (aHR, 3.31) than controls.
IN PRACTICE:
“[T]he recognition and treatment of modifiable cardiovascular risk factors must be reinforced in the SCI population, [and] proper rehabilitation and education should be considered to prevent autonomic dysreflexia or orthostatic hypotension,” the authors wrote.
In an accompanying editorial, Christopher R. West, PhD, and Jacquelyn J. Cragg, PhD, both of the University of British Columbia, Vancouver, Canada, noted that clinical guidelines for cardiovascular and cardiometabolic disease after SCI don’t include approaches to help mitigate the risk for cardiac events such as those reported in the study; therefore, they wrote, the findings “should act as ‘call-to-arms’ to researchers and clinicians to shift gears from tradition and begin studying the clinical efficacy of neuraxial therapies that could help restore autonomic balance [in SCI], such as targeted neuromodulation.”
SOURCE:
The study was led by Jung Eun Yoo, MD, PhD of Seoul National University College of Medicine, Seoul, South Korea, and published online on February 12 in the Journal of the American College of Cardiology.
LIMITATIONS:
The database was not designed for the SCI population, so data are incomplete. The incidence of thoracic SCI was particularly low. Because SCI survivors may have impaired perception of chest pain in ischemic heart disease, those with asymptomatic or silent heart disease may not have been captured during follow-up. All study participants were Korean, so the findings may not be generalizable to other ethnicities.
DISCLOSURES:
This research was partially supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, South Korea. The study authors and the editorialists had no relevant relationships to disclose.
A version of this article appeared on Medscape.com.
FDA Emphasizes Alternative Device Sterilization Strategies
The US Food and Drug Administration has expanded its guidance on medical device sterilization to include vaporized hydrogen peroxide, according to an agency press release issued on January 8.
The update is intended to promote wider use of vaporized hydrogen peroxide (VHP) as a viable alternative to ethylene oxide (EtO). The FDA guidance on sterile devices has been revised to include VHP.
The acceptance of VHP as an Established Category A method of sterilization is another step toward the FDA’s larger goal of reducing EtO, according to the release.
Sterilization is essential for certain medical devices, but the use of EtO, currently the most common method, involves the release of emissions that are potentially harmful to health, and the FDA seeks to identify safe and effective alternatives to reduce risk to the environment and communities where device sterilization occurs. Current Established Category A sterilization methods include moist heat, dry heat, EtO, and radiation.
“Vaporized hydrogen peroxide’s addition as an established sterilization method helps us build a more resilient supply chain for sterilized devices that can help prevent medical device shortages,” Suzanne Schwartz, MD, director of the Office of Strategic Partnerships and Technology Innovation in the FDA’s Center for Devices and Radiological Health, said in the press release. “As innovations in sterilization advance, the FDA will continue to seek additional modalities that deliver safe and effective sterilization methods that best protect public health,” she said.
The FDA has supported the development of EtO alternatives since 2019, and remains committed to reducing EtO emissions and also to avoiding potential device shortages, according to the release.
“Ethylene oxide is highly flammable and carcinogenic and poses exposure-related safety concerns for reprocessing staff, as well as environmental risks,” said Venkataraman R. Muthusamy, MD, AGAF, of the University of California, Los Angeles, in an interview. “These risks have led some states or regions to ban or limit its use, but despite these risks, it is currently the most commonly used sterilization technique for medical devices in the United States,” he said. Therefore, coming up with alternatives has been a high priority for the FDA, he added.
VHP has several advantages over EtO, Dr. Muthusamy said. VHP breaks down safely into water and oxygen, with low residual levels after exposure, and has no known oxidation or discoloration effects. In addition, VHP has a low temperature, and should theoretically be safe to use with endoscopes, although data are lacking, he said.
Dr. Muthusamy said that he was not yet too familiar with VHP as a technique, in part because most accessories in GI are single-use.
Primary issues to expanding the use of vaporized hydrogen peroxide as a sterilizing agent in GI clinical practice include availability and the cost of acquiring the devices needed, Dr. Muthusamy told GI & Hepatology News. “Also, the comparative efficacy of this technique in sterilizing GI endoscopes to ethylene oxide and the impact of VHP on scope durability and performance will need to be assessed, and the impact of VHP on the health and safety of reprocessing staff will need to be assessed and monitored,” he said.
There is an interest in the GI community in “green” endoscopy and reducing waste, Dr. Muthusamy said. If an inexpensive, safe, and cost-effective option for sterilization of other devices beyond endoscopes exists, “perhaps we could reduce our use of some disposables as well,” he said.
Dr. Muthusamy had no financial conflicts to disclose.
The US Food and Drug Administration has expanded its guidance on medical device sterilization to include vaporized hydrogen peroxide, according to an agency press release issued on January 8.
The update is intended to promote wider use of vaporized hydrogen peroxide (VHP) as a viable alternative to ethylene oxide (EtO). The FDA guidance on sterile devices has been revised to include VHP.
The acceptance of VHP as an Established Category A method of sterilization is another step toward the FDA’s larger goal of reducing EtO, according to the release.
Sterilization is essential for certain medical devices, but the use of EtO, currently the most common method, involves the release of emissions that are potentially harmful to health, and the FDA seeks to identify safe and effective alternatives to reduce risk to the environment and communities where device sterilization occurs. Current Established Category A sterilization methods include moist heat, dry heat, EtO, and radiation.
“Vaporized hydrogen peroxide’s addition as an established sterilization method helps us build a more resilient supply chain for sterilized devices that can help prevent medical device shortages,” Suzanne Schwartz, MD, director of the Office of Strategic Partnerships and Technology Innovation in the FDA’s Center for Devices and Radiological Health, said in the press release. “As innovations in sterilization advance, the FDA will continue to seek additional modalities that deliver safe and effective sterilization methods that best protect public health,” she said.
The FDA has supported the development of EtO alternatives since 2019, and remains committed to reducing EtO emissions and also to avoiding potential device shortages, according to the release.
“Ethylene oxide is highly flammable and carcinogenic and poses exposure-related safety concerns for reprocessing staff, as well as environmental risks,” said Venkataraman R. Muthusamy, MD, AGAF, of the University of California, Los Angeles, in an interview. “These risks have led some states or regions to ban or limit its use, but despite these risks, it is currently the most commonly used sterilization technique for medical devices in the United States,” he said. Therefore, coming up with alternatives has been a high priority for the FDA, he added.
VHP has several advantages over EtO, Dr. Muthusamy said. VHP breaks down safely into water and oxygen, with low residual levels after exposure, and has no known oxidation or discoloration effects. In addition, VHP has a low temperature, and should theoretically be safe to use with endoscopes, although data are lacking, he said.
Dr. Muthusamy said that he was not yet too familiar with VHP as a technique, in part because most accessories in GI are single-use.
Primary issues to expanding the use of vaporized hydrogen peroxide as a sterilizing agent in GI clinical practice include availability and the cost of acquiring the devices needed, Dr. Muthusamy told GI & Hepatology News. “Also, the comparative efficacy of this technique in sterilizing GI endoscopes to ethylene oxide and the impact of VHP on scope durability and performance will need to be assessed, and the impact of VHP on the health and safety of reprocessing staff will need to be assessed and monitored,” he said.
There is an interest in the GI community in “green” endoscopy and reducing waste, Dr. Muthusamy said. If an inexpensive, safe, and cost-effective option for sterilization of other devices beyond endoscopes exists, “perhaps we could reduce our use of some disposables as well,” he said.
Dr. Muthusamy had no financial conflicts to disclose.
The US Food and Drug Administration has expanded its guidance on medical device sterilization to include vaporized hydrogen peroxide, according to an agency press release issued on January 8.
The update is intended to promote wider use of vaporized hydrogen peroxide (VHP) as a viable alternative to ethylene oxide (EtO). The FDA guidance on sterile devices has been revised to include VHP.
The acceptance of VHP as an Established Category A method of sterilization is another step toward the FDA’s larger goal of reducing EtO, according to the release.
Sterilization is essential for certain medical devices, but the use of EtO, currently the most common method, involves the release of emissions that are potentially harmful to health, and the FDA seeks to identify safe and effective alternatives to reduce risk to the environment and communities where device sterilization occurs. Current Established Category A sterilization methods include moist heat, dry heat, EtO, and radiation.
“Vaporized hydrogen peroxide’s addition as an established sterilization method helps us build a more resilient supply chain for sterilized devices that can help prevent medical device shortages,” Suzanne Schwartz, MD, director of the Office of Strategic Partnerships and Technology Innovation in the FDA’s Center for Devices and Radiological Health, said in the press release. “As innovations in sterilization advance, the FDA will continue to seek additional modalities that deliver safe and effective sterilization methods that best protect public health,” she said.
The FDA has supported the development of EtO alternatives since 2019, and remains committed to reducing EtO emissions and also to avoiding potential device shortages, according to the release.
“Ethylene oxide is highly flammable and carcinogenic and poses exposure-related safety concerns for reprocessing staff, as well as environmental risks,” said Venkataraman R. Muthusamy, MD, AGAF, of the University of California, Los Angeles, in an interview. “These risks have led some states or regions to ban or limit its use, but despite these risks, it is currently the most commonly used sterilization technique for medical devices in the United States,” he said. Therefore, coming up with alternatives has been a high priority for the FDA, he added.
VHP has several advantages over EtO, Dr. Muthusamy said. VHP breaks down safely into water and oxygen, with low residual levels after exposure, and has no known oxidation or discoloration effects. In addition, VHP has a low temperature, and should theoretically be safe to use with endoscopes, although data are lacking, he said.
Dr. Muthusamy said that he was not yet too familiar with VHP as a technique, in part because most accessories in GI are single-use.
Primary issues to expanding the use of vaporized hydrogen peroxide as a sterilizing agent in GI clinical practice include availability and the cost of acquiring the devices needed, Dr. Muthusamy told GI & Hepatology News. “Also, the comparative efficacy of this technique in sterilizing GI endoscopes to ethylene oxide and the impact of VHP on scope durability and performance will need to be assessed, and the impact of VHP on the health and safety of reprocessing staff will need to be assessed and monitored,” he said.
There is an interest in the GI community in “green” endoscopy and reducing waste, Dr. Muthusamy said. If an inexpensive, safe, and cost-effective option for sterilization of other devices beyond endoscopes exists, “perhaps we could reduce our use of some disposables as well,” he said.
Dr. Muthusamy had no financial conflicts to disclose.
SARS-CoV-2 a Possible Trigger for Achalasia
TOPLINE:
METHODOLOGY:
- The etiology of achalasia is unclear. Studies have suggested an immune reaction to viral infections, including SARS-CoV-2, as a potential cause.
- Researchers studied four adults who developed achalasia within 5 months of SARS-CoV-2 infection (group 1), six with longstanding achalasia predating SARS-CoV-2 infection (group 2), and two with longstanding achalasia with no known SARS-CoV-2 infection (group 3).
- They tested for the presence of SARS-CoV-2 nucleocapsid (N) and spike (S) proteins, as well as inflammatory markers, in esophageal muscle tissue isolated from the participants.
TAKEAWAY:
- Group 1 patients (confirmed or suspected post–COVID-19 achalasia) had the highest levels of the N protein in all four cases and higher levels of the S protein in the two confirmed cases. No N or S protein was detected in group 3.
- The presence of mRNA for SARS-CoV-2 N protein correlated with a significant increase in the inflammatory markers of NOD-like receptor family pyrin domain-containing 3 and tumor necrosis factor. There were no differences in interleukin 18 in groups 1 and 2.
- The S protein was detected in all muscle tissue samples from group 1. It was also detected in some (but not all) samples from group 2 and to a much lesser degree. The presence of S protein was irrespective of the SARS-CoV-2 vaccination status.
IN PRACTICE:
“Our findings not only show the continued presence of SARS-CoV-2 proteins in esophageal muscle tissue isolated from subjects with achalasia post infection, but they further correlate this with the presence of a sustained inflammatory response,” the authors wrote.
SOURCE:
The study, with first author Salih Samo, MD, MS, Division of Gastroenterology, Hepatology, and Motility, University of Kansas School of Medicine, Kansas City, Kansas, was published online on January 24, 2024, in the American Journal of Gastroenterology.
LIMITATIONS:
The sample size was small, and it was not known which SARS-CoV-2 variant each patient had. The study cannot definitively confirm that SARS-CoV-2 is causative for achalasia.
DISCLOSURES:
The study had no specific funding. Samo reported relationships with Castle Biosciences, Sanofi, Evoke, and EndoGastric Solutions.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The etiology of achalasia is unclear. Studies have suggested an immune reaction to viral infections, including SARS-CoV-2, as a potential cause.
- Researchers studied four adults who developed achalasia within 5 months of SARS-CoV-2 infection (group 1), six with longstanding achalasia predating SARS-CoV-2 infection (group 2), and two with longstanding achalasia with no known SARS-CoV-2 infection (group 3).
- They tested for the presence of SARS-CoV-2 nucleocapsid (N) and spike (S) proteins, as well as inflammatory markers, in esophageal muscle tissue isolated from the participants.
TAKEAWAY:
- Group 1 patients (confirmed or suspected post–COVID-19 achalasia) had the highest levels of the N protein in all four cases and higher levels of the S protein in the two confirmed cases. No N or S protein was detected in group 3.
- The presence of mRNA for SARS-CoV-2 N protein correlated with a significant increase in the inflammatory markers of NOD-like receptor family pyrin domain-containing 3 and tumor necrosis factor. There were no differences in interleukin 18 in groups 1 and 2.
- The S protein was detected in all muscle tissue samples from group 1. It was also detected in some (but not all) samples from group 2 and to a much lesser degree. The presence of S protein was irrespective of the SARS-CoV-2 vaccination status.
IN PRACTICE:
“Our findings not only show the continued presence of SARS-CoV-2 proteins in esophageal muscle tissue isolated from subjects with achalasia post infection, but they further correlate this with the presence of a sustained inflammatory response,” the authors wrote.
SOURCE:
The study, with first author Salih Samo, MD, MS, Division of Gastroenterology, Hepatology, and Motility, University of Kansas School of Medicine, Kansas City, Kansas, was published online on January 24, 2024, in the American Journal of Gastroenterology.
LIMITATIONS:
The sample size was small, and it was not known which SARS-CoV-2 variant each patient had. The study cannot definitively confirm that SARS-CoV-2 is causative for achalasia.
DISCLOSURES:
The study had no specific funding. Samo reported relationships with Castle Biosciences, Sanofi, Evoke, and EndoGastric Solutions.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The etiology of achalasia is unclear. Studies have suggested an immune reaction to viral infections, including SARS-CoV-2, as a potential cause.
- Researchers studied four adults who developed achalasia within 5 months of SARS-CoV-2 infection (group 1), six with longstanding achalasia predating SARS-CoV-2 infection (group 2), and two with longstanding achalasia with no known SARS-CoV-2 infection (group 3).
- They tested for the presence of SARS-CoV-2 nucleocapsid (N) and spike (S) proteins, as well as inflammatory markers, in esophageal muscle tissue isolated from the participants.
TAKEAWAY:
- Group 1 patients (confirmed or suspected post–COVID-19 achalasia) had the highest levels of the N protein in all four cases and higher levels of the S protein in the two confirmed cases. No N or S protein was detected in group 3.
- The presence of mRNA for SARS-CoV-2 N protein correlated with a significant increase in the inflammatory markers of NOD-like receptor family pyrin domain-containing 3 and tumor necrosis factor. There were no differences in interleukin 18 in groups 1 and 2.
- The S protein was detected in all muscle tissue samples from group 1. It was also detected in some (but not all) samples from group 2 and to a much lesser degree. The presence of S protein was irrespective of the SARS-CoV-2 vaccination status.
IN PRACTICE:
“Our findings not only show the continued presence of SARS-CoV-2 proteins in esophageal muscle tissue isolated from subjects with achalasia post infection, but they further correlate this with the presence of a sustained inflammatory response,” the authors wrote.
SOURCE:
The study, with first author Salih Samo, MD, MS, Division of Gastroenterology, Hepatology, and Motility, University of Kansas School of Medicine, Kansas City, Kansas, was published online on January 24, 2024, in the American Journal of Gastroenterology.
LIMITATIONS:
The sample size was small, and it was not known which SARS-CoV-2 variant each patient had. The study cannot definitively confirm that SARS-CoV-2 is causative for achalasia.
DISCLOSURES:
The study had no specific funding. Samo reported relationships with Castle Biosciences, Sanofi, Evoke, and EndoGastric Solutions.
A version of this article appeared on Medscape.com.
Can Iron Supplementation Protect Against Celiac Disease?
TOPLINE:
Genetically lower iron levels were associated with an increased risk for celiac disease, pointing to a potential opportunity to prevent the disease, new data suggested.
METHODOLOGY:
- To investigate, researchers conducted a Mendelian randomization study examining the relationship between single nucleotide polymorphisms (SNPs) associated with iron status and the presence of celiac disease.
- SNPs were drawn from a meta-analysis of three genome-wide association studies. Their association with celiac disease was assessed using data from 336,638 White UK Biobank participants, including 1855 with celiac disease.
TAKEAWAY:
- Four SNPs were strongly and independently associated with systemic iron status: rs1800562 and rs1799945 in the hemochromatosis gene, rs855791 in the transmembrane protease serine 6 gene, and rs57659670 predicted to affect the Dual Oxidase 2 gene. None were associated with known celiac disease risk factors.
- Higher iron status was negatively associated with celiac disease risk (odds ratio per 1 SD increase in serum iron: 0.65).
- No single SNP appeared to drive the association in sensitivity analyses.
- By relying on SNPs associated with iron status, and not on iron status itself, this Mendelian randomization analysis suggests a causal effect of iron deficiency on subsequent celiac disease development.
IN PRACTICE:
“These findings suggest that iron supplementation in select individuals may provide a potential protective effect against celiac disease development,” the authors wrote.
SOURCE:
The study, with first author Isabel A. Hujoel, MD, a gastroenterologist with University of Washington, Seattle, was published online on January 4, 2024, in BMJ Open Gastroenterology.
LIMITATIONS:
Researchers used a PheCode to identify celiac disease cases, which could lead to misclassification. Mendelian randomization provides some protection against biases, such as reverse causation, but is not completely invulnerable.
DISCLOSURES:
The study had no specific funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Genetically lower iron levels were associated with an increased risk for celiac disease, pointing to a potential opportunity to prevent the disease, new data suggested.
METHODOLOGY:
- To investigate, researchers conducted a Mendelian randomization study examining the relationship between single nucleotide polymorphisms (SNPs) associated with iron status and the presence of celiac disease.
- SNPs were drawn from a meta-analysis of three genome-wide association studies. Their association with celiac disease was assessed using data from 336,638 White UK Biobank participants, including 1855 with celiac disease.
TAKEAWAY:
- Four SNPs were strongly and independently associated with systemic iron status: rs1800562 and rs1799945 in the hemochromatosis gene, rs855791 in the transmembrane protease serine 6 gene, and rs57659670 predicted to affect the Dual Oxidase 2 gene. None were associated with known celiac disease risk factors.
- Higher iron status was negatively associated with celiac disease risk (odds ratio per 1 SD increase in serum iron: 0.65).
- No single SNP appeared to drive the association in sensitivity analyses.
- By relying on SNPs associated with iron status, and not on iron status itself, this Mendelian randomization analysis suggests a causal effect of iron deficiency on subsequent celiac disease development.
IN PRACTICE:
“These findings suggest that iron supplementation in select individuals may provide a potential protective effect against celiac disease development,” the authors wrote.
SOURCE:
The study, with first author Isabel A. Hujoel, MD, a gastroenterologist with University of Washington, Seattle, was published online on January 4, 2024, in BMJ Open Gastroenterology.
LIMITATIONS:
Researchers used a PheCode to identify celiac disease cases, which could lead to misclassification. Mendelian randomization provides some protection against biases, such as reverse causation, but is not completely invulnerable.
DISCLOSURES:
The study had no specific funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Genetically lower iron levels were associated with an increased risk for celiac disease, pointing to a potential opportunity to prevent the disease, new data suggested.
METHODOLOGY:
- To investigate, researchers conducted a Mendelian randomization study examining the relationship between single nucleotide polymorphisms (SNPs) associated with iron status and the presence of celiac disease.
- SNPs were drawn from a meta-analysis of three genome-wide association studies. Their association with celiac disease was assessed using data from 336,638 White UK Biobank participants, including 1855 with celiac disease.
TAKEAWAY:
- Four SNPs were strongly and independently associated with systemic iron status: rs1800562 and rs1799945 in the hemochromatosis gene, rs855791 in the transmembrane protease serine 6 gene, and rs57659670 predicted to affect the Dual Oxidase 2 gene. None were associated with known celiac disease risk factors.
- Higher iron status was negatively associated with celiac disease risk (odds ratio per 1 SD increase in serum iron: 0.65).
- No single SNP appeared to drive the association in sensitivity analyses.
- By relying on SNPs associated with iron status, and not on iron status itself, this Mendelian randomization analysis suggests a causal effect of iron deficiency on subsequent celiac disease development.
IN PRACTICE:
“These findings suggest that iron supplementation in select individuals may provide a potential protective effect against celiac disease development,” the authors wrote.
SOURCE:
The study, with first author Isabel A. Hujoel, MD, a gastroenterologist with University of Washington, Seattle, was published online on January 4, 2024, in BMJ Open Gastroenterology.
LIMITATIONS:
Researchers used a PheCode to identify celiac disease cases, which could lead to misclassification. Mendelian randomization provides some protection against biases, such as reverse causation, but is not completely invulnerable.
DISCLOSURES:
The study had no specific funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
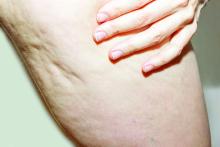
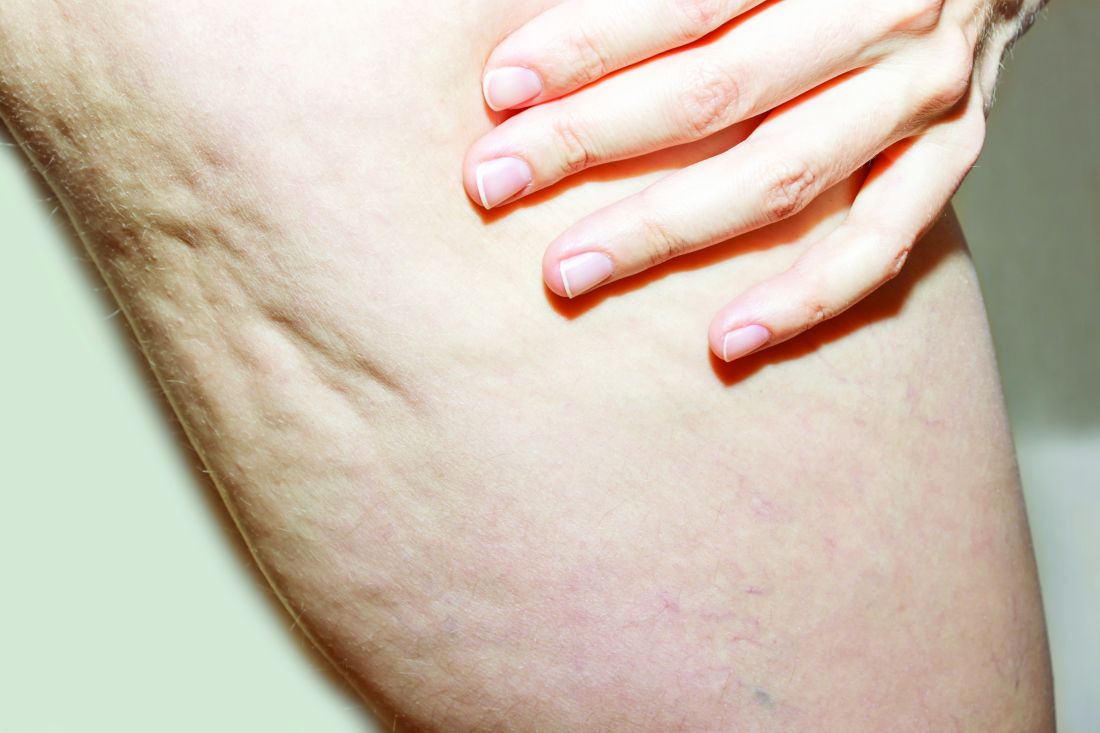
What Do Results from Acoustic Subcision for Cellulite Look Like at One Year?
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
FROM DERMATOLOGIC SURGERY
Migraine Variants
Mood Interventions May Reduce IBD Inflammation
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
, according to a new study.
“IBD is a distressing condition, and current medication that reduces inflammation is expensive and can have side effects,” said Natasha Seaton, first author and a PhD student at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London.
“Our study showed that interventions that treat mental health reduce levels of inflammation in the body,” she said. “This indicates that mood interventions could be a valuable tool in our approach to help those with IBD.”
The study was published online in eBioMedicine.
Analyzing Mood Interventions
Ms. Seaton and colleagues conducted a systematic review and meta-analysis of randomized controlled trials in adults with IBD that measured inflammatory biomarker levels and tested a mood intervention, including those aimed at reducing depression, anxiety, stress, or distress or improving emotional well-being.
Looking at data from 28 randomized controlled trials with 1789 participants, the research team evaluated whether mood interventions affected IBD inflammation, particularly IBD indicators such as C-reactive protein and fecal calprotectin, and other general inflammatory biomarkers.
The researchers found mood interventions significantly reduced levels of inflammatory biomarkers, compared with controls, corresponding to an 18% reduction in inflammatory biomarkers.
Psychological therapies had the best outcomes related to IBD inflammation, compared with antidepressants or exercise. These therapies included cognitive behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction.
Individual analyses of IBD-specific inflammatory markers found small but statistically significant reductions in C-reactive protein and fecal calprotectin after a mood intervention. This could mean mood treatments have positive effects on both inflammation and disease-specific biomarkers, the authors wrote.
In addition, interventions that had a larger positive effect on mood had a greater effect in reducing inflammatory biomarkers. This suggests that a better mood could reduce IBD inflammation, they noted.
“We know stress-related feelings can increase inflammation, and the findings suggest that by improving mood, we can reduce this type of inflammation,” said Valeria Mondelli, MD, PhD, clinical professor of psychoneuroimmunology at King’s IoPPN.
“This adds to the growing body of research demonstrating the role of inflammation in mental health and suggests that interventions working to improve mood could also have direct physical effects on levels of inflammation,” she said. “However, more research is needed to understand exact mechanisms in IBD.”
Cost Benefit
Many IBD interventions and medications can be expensive for patients, have significant negative side effects, and have a lower long-term treatment response, the authors noted. Mood interventions, whether psychological therapy or medication, could potentially reduce costs and improve both mood and inflammation.
Previous studies have indicated that psychosocial factors, as well as mood disorders such as anxiety and depression, affect IBD symptom severity and progression, the authors wrote. However, researchers still need to understand the mechanisms behind this connection, including gut-brain dynamics.
Future research should focus on interventions that have been effective at improving mood in patients with IBD, assess inflammation and disease activity at numerous timepoints, and include potential variables related to illness self-management, the authors wrote.
In addition, implementation of mood interventions for IBD management may require better continuity of care and healthcare integration.
“Integrated mental health support, alongside pharmacological treatments, may offer a more holistic approach to IBD care, potentially leading to reduced disease and healthcare costs,” said Rona Moss-Morris, PhD, senior author and professor of psychology at King’s IoPPN.
Medications taken to reduce inflammation can be costly compared with psychological therapies, she said. “Given this, including psychological interventions, such as cost-effective digital interventions, within IBD management might reduce the need for anti-inflammatory medication, resulting in an overall cost benefit.”
The study was funded by the Medical Research Council (MRC) and National Institute for Health and Care Research Maudsley Biomedical Research Centre, which is hosted by South London and Maudsley NHS Foundation Trust in partnership with King’s College London. Ms. Seaton was funded by an MRC Doctoral Training Partnership. No other interests were declared.
A version of this article appeared on Medscape.com.
Nonepidemic Kaposi Sarcoma: A Case of a Rare Epidemiologic Subtype
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
Practice Points
- Nonepidemic Kaposi sarcoma (KS) is a recently described fifth subtype of the disease that typically occurs in younger men who are HIV-negative without detectable cellular or humoral immune deficiency.
- The cutaneous manifestations of nonepidemic KS are similar to those of classic KS, except that disease extent is limited and the prognosis is favorable in nonepidemic KS.
- Dermatologists should consider KS when a patient presents with clinically representative findings, even in the absence of typical risk factors such as immunosuppression.
FDA OKs First Oral Agent for Eosinophilic Esophagitis
Budesonide oral suspension is a corticosteroid indicated for 12 weeks of treatment of EoE in adults and children as young as 11 years.
It will be available in 2-mg/10-mL single-dose stick packs by the end of February.
“Developed specifically for EoE, Eohilia’s novel formulation of budesonide confers thixotropic properties — flowing more freely when shaken and returning to a more viscous state when swallowed,” the company said in a news release.
“Various formulations of corticosteroids have been used in the past to manage EoE, but in an off-label capacity and using multiple delivery options. With Eohilia, it’s gratifying to now have an FDA-approved treatment specifically formulated for a consistent dose delivery with demonstrated ability to address esophageal inflammation and EoE dysphagia symptoms,” Ikuo Hirano, MD, professor of medicine and director of the Esophageal Center at Northwestern University Feinberg School of Medicine, Chicago, said in the release.
Supporting Data
The FDA approved budesonide oral suspension for EoE based on efficacy and safety data from two multicenter, randomized, double-blind, parallel-group, placebo-controlled 12-week studies.
In study 1, significantly more patients receiving active treatment achieved histologic remission (53.1% vs 1% with placebo). The same was true in study 2, with 38% of patients receiving active treatment achieving histologic remission compared with 2.4% of those receiving placebo.
The absolute change from baseline in the patient-reported Dysphagia Symptom Questionnaire combined score was -10.2 with budesonide vs -6.5 with placebo in Study 1 and -14.5 vs -5.9 in Study 2.
During the last 2 weeks of treatment, more patients receiving budesonide oral suspension experienced no dysphagia or only experienced dysphagia that “got better or cleared up on its own” compared with those receiving placebo, the company said.
The most common adverse reactions seen in the clinical trials of budesonide oral suspension for EoE included respiratory tract infection (13%), gastrointestinal mucosal candidiasis (8%), headache (5%), gastroenteritis (3%), throat irritation (3%), adrenal suppression (2%), and erosive esophagitis (2%).
Complete prescribing information is available on the FDA website.
A version of this article appeared on Medscape.com.
Budesonide oral suspension is a corticosteroid indicated for 12 weeks of treatment of EoE in adults and children as young as 11 years.
It will be available in 2-mg/10-mL single-dose stick packs by the end of February.
“Developed specifically for EoE, Eohilia’s novel formulation of budesonide confers thixotropic properties — flowing more freely when shaken and returning to a more viscous state when swallowed,” the company said in a news release.
“Various formulations of corticosteroids have been used in the past to manage EoE, but in an off-label capacity and using multiple delivery options. With Eohilia, it’s gratifying to now have an FDA-approved treatment specifically formulated for a consistent dose delivery with demonstrated ability to address esophageal inflammation and EoE dysphagia symptoms,” Ikuo Hirano, MD, professor of medicine and director of the Esophageal Center at Northwestern University Feinberg School of Medicine, Chicago, said in the release.
Supporting Data
The FDA approved budesonide oral suspension for EoE based on efficacy and safety data from two multicenter, randomized, double-blind, parallel-group, placebo-controlled 12-week studies.
In study 1, significantly more patients receiving active treatment achieved histologic remission (53.1% vs 1% with placebo). The same was true in study 2, with 38% of patients receiving active treatment achieving histologic remission compared with 2.4% of those receiving placebo.
The absolute change from baseline in the patient-reported Dysphagia Symptom Questionnaire combined score was -10.2 with budesonide vs -6.5 with placebo in Study 1 and -14.5 vs -5.9 in Study 2.
During the last 2 weeks of treatment, more patients receiving budesonide oral suspension experienced no dysphagia or only experienced dysphagia that “got better or cleared up on its own” compared with those receiving placebo, the company said.
The most common adverse reactions seen in the clinical trials of budesonide oral suspension for EoE included respiratory tract infection (13%), gastrointestinal mucosal candidiasis (8%), headache (5%), gastroenteritis (3%), throat irritation (3%), adrenal suppression (2%), and erosive esophagitis (2%).
Complete prescribing information is available on the FDA website.
A version of this article appeared on Medscape.com.
Budesonide oral suspension is a corticosteroid indicated for 12 weeks of treatment of EoE in adults and children as young as 11 years.
It will be available in 2-mg/10-mL single-dose stick packs by the end of February.
“Developed specifically for EoE, Eohilia’s novel formulation of budesonide confers thixotropic properties — flowing more freely when shaken and returning to a more viscous state when swallowed,” the company said in a news release.
“Various formulations of corticosteroids have been used in the past to manage EoE, but in an off-label capacity and using multiple delivery options. With Eohilia, it’s gratifying to now have an FDA-approved treatment specifically formulated for a consistent dose delivery with demonstrated ability to address esophageal inflammation and EoE dysphagia symptoms,” Ikuo Hirano, MD, professor of medicine and director of the Esophageal Center at Northwestern University Feinberg School of Medicine, Chicago, said in the release.
Supporting Data
The FDA approved budesonide oral suspension for EoE based on efficacy and safety data from two multicenter, randomized, double-blind, parallel-group, placebo-controlled 12-week studies.
In study 1, significantly more patients receiving active treatment achieved histologic remission (53.1% vs 1% with placebo). The same was true in study 2, with 38% of patients receiving active treatment achieving histologic remission compared with 2.4% of those receiving placebo.
The absolute change from baseline in the patient-reported Dysphagia Symptom Questionnaire combined score was -10.2 with budesonide vs -6.5 with placebo in Study 1 and -14.5 vs -5.9 in Study 2.
During the last 2 weeks of treatment, more patients receiving budesonide oral suspension experienced no dysphagia or only experienced dysphagia that “got better or cleared up on its own” compared with those receiving placebo, the company said.
The most common adverse reactions seen in the clinical trials of budesonide oral suspension for EoE included respiratory tract infection (13%), gastrointestinal mucosal candidiasis (8%), headache (5%), gastroenteritis (3%), throat irritation (3%), adrenal suppression (2%), and erosive esophagitis (2%).
Complete prescribing information is available on the FDA website.
A version of this article appeared on Medscape.com.