User login
European Commission approves first CAR T-cell therapies
The , two chimeric antigen receptor (CAR) T-cell therapies.
Tisagenlecleucel is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
Axicabtagene ciloleucel is approved for adults with relapsed/refractory DLBCL and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy. The treatment is marketed by Kite, a Gilead company.
The axicabtagene ciloleucel approval is based on results from the single arm, ZUMA-1 trial. During the study of 101 patients who received a single infusion, 72% responded to therapy and 51% achieved a complete response. At 1 year, median overall survival had not been reached.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
Updated results from JULIET were presented at the annual congress of the European Hematology Association in June 2018. The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so because of disease progression or clinical deterioration.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
Updated results from ELIANA were published in New England Journal of Medicine (2018;378:439-48).
The trial included 75 children and young adults with relapsed/refractory ALL. The overall remission rate was 81% (61/75), with 60% of patients (n = 45) achieving a complete remission (CR) and 21% (n = 16) achieving a CR with incomplete hematologic recovery (CRi).
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The , two chimeric antigen receptor (CAR) T-cell therapies.
Tisagenlecleucel is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
Axicabtagene ciloleucel is approved for adults with relapsed/refractory DLBCL and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy. The treatment is marketed by Kite, a Gilead company.
The axicabtagene ciloleucel approval is based on results from the single arm, ZUMA-1 trial. During the study of 101 patients who received a single infusion, 72% responded to therapy and 51% achieved a complete response. At 1 year, median overall survival had not been reached.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
Updated results from JULIET were presented at the annual congress of the European Hematology Association in June 2018. The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so because of disease progression or clinical deterioration.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
Updated results from ELIANA were published in New England Journal of Medicine (2018;378:439-48).
The trial included 75 children and young adults with relapsed/refractory ALL. The overall remission rate was 81% (61/75), with 60% of patients (n = 45) achieving a complete remission (CR) and 21% (n = 16) achieving a CR with incomplete hematologic recovery (CRi).
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The , two chimeric antigen receptor (CAR) T-cell therapies.
Tisagenlecleucel is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
Axicabtagene ciloleucel is approved for adults with relapsed/refractory DLBCL and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy. The treatment is marketed by Kite, a Gilead company.
The axicabtagene ciloleucel approval is based on results from the single arm, ZUMA-1 trial. During the study of 101 patients who received a single infusion, 72% responded to therapy and 51% achieved a complete response. At 1 year, median overall survival had not been reached.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
Updated results from JULIET were presented at the annual congress of the European Hematology Association in June 2018. The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so because of disease progression or clinical deterioration.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
Updated results from ELIANA were published in New England Journal of Medicine (2018;378:439-48).
The trial included 75 children and young adults with relapsed/refractory ALL. The overall remission rate was 81% (61/75), with 60% of patients (n = 45) achieving a complete remission (CR) and 21% (n = 16) achieving a CR with incomplete hematologic recovery (CRi).
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
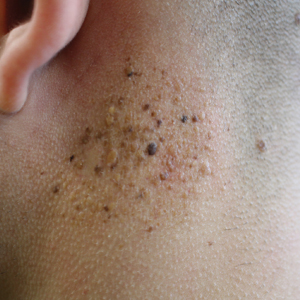
Agminated Heterogeneous Papules on the Neck
The Diagnosis: Eruptive Blue Nevus
All biopsies demonstrated similar histologic features, including an intradermal proliferation of heavily pigmented, spindle-shaped dendritic melanocytes (Figure). The dermal pigment was most pronounced in the grossly darker papules, and there was not a substantial amount of background pigmentation at the stratum basale. Cytologic atypia, foci of necrosis, and mitotic activity were absent from all sections. There was no definitive junctional component identified, no multinucleated giant cells, and there was no overlying epidermal aberration. With some background pigmentation seen histologically, nevus spilus was considered, but because this acute eruption occurred in a young adult without appreciable gross background hyperpigmentation, the clinical context led to a diagnosis of eruptive blue nevus. After communicating the findings to the patient, he declined further treatment.
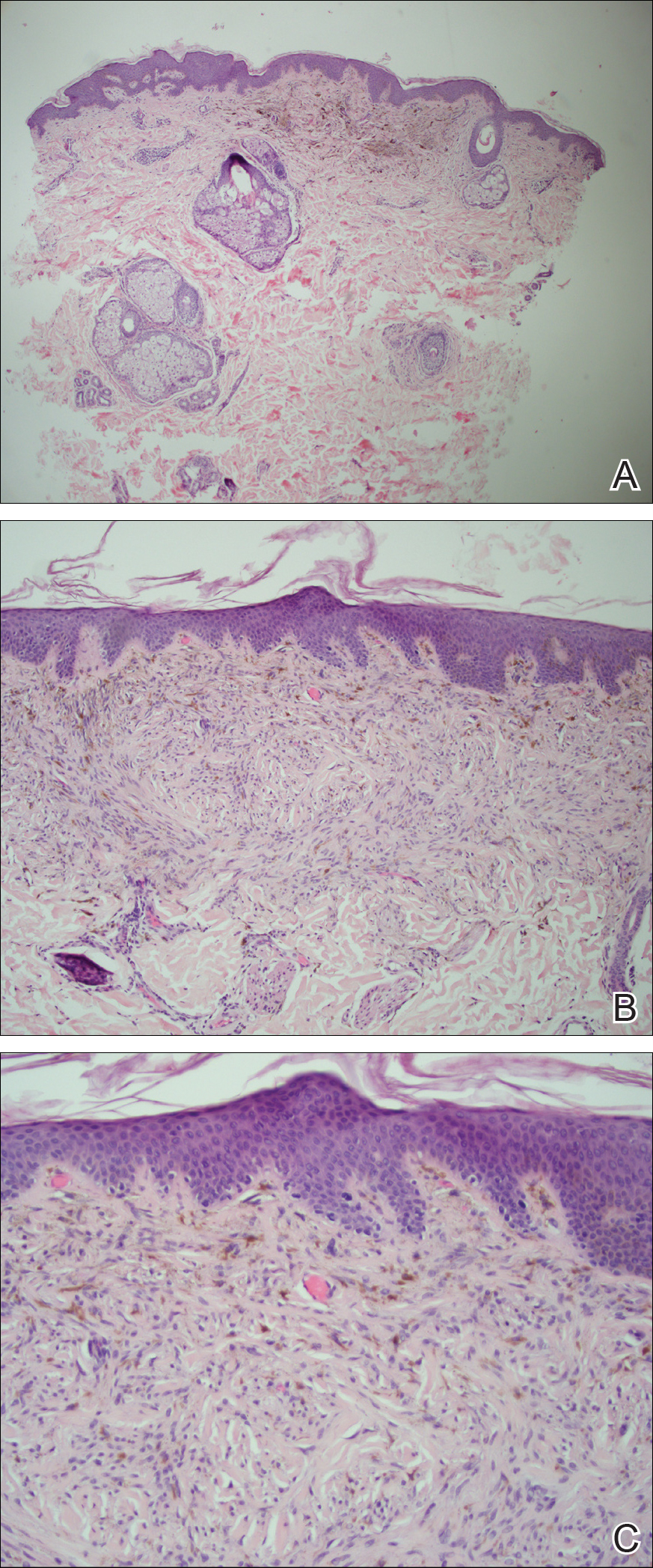
Eruptive blue nevus is an exceptionally rare subtype of blue nevus with few cases reported since the 1940s.1-9 Generally, each case report found a triggering event that could possibly have precipitated the acute proliferation and evolution of nevi. Triggering events can include bullous processes such as erythema multiforme2 and Stevens-Johnson syndrome,3 severe sunburn,4 trauma,5 immunosuppression,6 and a variety of endocrinopathies. No such history could be identified in our patient, except the biopsy.
Common blue nevi are benign, usually congenital, well-circumscribed, solitary, blue-gray macules or papules. Half of blue nevi cases are found on the dorsal aspects of the hands and feet but can present anywhere (eg, face, scalp, wrists, sacrum, buttocks). The blue-gray color appreciated clinically is attributed to the Tyndall effect, which occurs when long-wavelength light--red, orange, and yellow--is absorbed by the melanin deep in the dermis, while short-wavelength visible light--blue, violet, and indigo--is reflected with backscattering. On polarized dermoscopy, a homogeneous blue-gray hue is appreciated, but lighter segments may be present when collagen deposition is robust. Histopathologic findings confirm spindle-shaped dendritic melanocytes in the dermis without epidermal involvement. It generally is accepted that the etiology of these benign nevi is a failed migration of neural crest cells to the epidermis.10,11 Although the common blue nevus may be simple to diagnose, several subtypes have been described in the literature, including combined blue nevus, desmoplastic blue nevus, hypomelanotic/amelanotic blue nevus, and epithelioid blue nevus of Carney complex, and excluding a malignant process is of monumental importance.7,12
Biopsy is recommended for common blue nevi in the evaluation of newly acquired lesions, expansion of previously stable nevi, or for nevi larger than 10 mm in diameter. The nature of eruptive blue nevi warrants a biopsy to exclude melanoma or another malignant process. While the Becker nevus may manifest in adolescent males, it is clinically distinct from an eruptive blue nevus due to the size, relative homogeneity, and presence of hair within the lesion. Cutaneous amyloidosis may appear clinically similar to an eruptive blue nevus, but globular or amorphous material was not present in the papillary dermis of biopsied lesions in our patient. Since there was no cellular atypia or mitotic activity, melanoma and other malignancies were ruled out. Lastly, NAME syndrome by definition must include atrial myxomas, myxoid neurofibromas, and ephelides in addition to the nevi; however, our patient had only nevi and few ephelides. Once the diagnosis is established and benign nature confirmed, treatment is not necessarily required. If the patient elects to remove the lesion for aesthetic reasons, an excision into the subcutaneous fat is required to ensure complete removal of deep dermal melanocytes. Prior excisions of eruptive blue nevi have had no recurrence after more than 10 months.8,9
- Krause M, Bonnekoh B, Weisshaar E, et al. Coincidence of multiple, disseminated, tardive-eruptive blue nevi with cutis marmorata teleangiectatica congenita. Dermatology. 2000;200:134-138.
- Soltani K, Bernstein J, Lorincz A. Eruptive nevocytic nevi after erythema multiforme. J Am Acad Dermatol. 1979;1:503-505.
- Shoji T, Cockerell C, Koff A, et al. Eruptive melanocytic nevi after Stevens-Johnson syndrome. J Am Acad Dermatol. 1997;37:337-339.
- Hendricks W. Eruptive blue nevi. J Am Acad Dermatol. 1981;4:50-53.
- Kesty K, Zargari O. Eruptive blue nevi. Indian J Dermatol Venereol Leprol. 2015;81:198-201.
- Chen T, Kurwa H, Trotter M, et al. Agminated blue nevi in a patient with dermatomyositis. J Am Acad Dermatol. 2013;68:52-53.
- Walsh M. Correspondence: eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;141:581-582.
- Nardini P, De Giorgi V, Massi D, et al. Eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;140:178-180.
- de Giorgi V, Massi D, Brunasso G, et al. Eruptive multiple blue nevi of the penis: a clinical dermoscopic pathologic case study. J Cutan Pathol. 2004;31:185-188.
- Zimmermann AH, Becker SA. Precursors of epidermal melanocytes in the negro fetus. In: Gordon M, ed. Pigment Cell Biology. New York, NY: Academic Press Inc; 1959:159-170.
- Leopold JG, Richards DB. The interrelationship of blue and common naevi. J Pathol Bacteriol. 1968;95:37-46.
- Zembowicz A, Phadke P. Blue nevi and variants: an update. Arch Pathol Lab Med. 2011;135:327-336.
The Diagnosis: Eruptive Blue Nevus
All biopsies demonstrated similar histologic features, including an intradermal proliferation of heavily pigmented, spindle-shaped dendritic melanocytes (Figure). The dermal pigment was most pronounced in the grossly darker papules, and there was not a substantial amount of background pigmentation at the stratum basale. Cytologic atypia, foci of necrosis, and mitotic activity were absent from all sections. There was no definitive junctional component identified, no multinucleated giant cells, and there was no overlying epidermal aberration. With some background pigmentation seen histologically, nevus spilus was considered, but because this acute eruption occurred in a young adult without appreciable gross background hyperpigmentation, the clinical context led to a diagnosis of eruptive blue nevus. After communicating the findings to the patient, he declined further treatment.

Eruptive blue nevus is an exceptionally rare subtype of blue nevus with few cases reported since the 1940s.1-9 Generally, each case report found a triggering event that could possibly have precipitated the acute proliferation and evolution of nevi. Triggering events can include bullous processes such as erythema multiforme2 and Stevens-Johnson syndrome,3 severe sunburn,4 trauma,5 immunosuppression,6 and a variety of endocrinopathies. No such history could be identified in our patient, except the biopsy.
Common blue nevi are benign, usually congenital, well-circumscribed, solitary, blue-gray macules or papules. Half of blue nevi cases are found on the dorsal aspects of the hands and feet but can present anywhere (eg, face, scalp, wrists, sacrum, buttocks). The blue-gray color appreciated clinically is attributed to the Tyndall effect, which occurs when long-wavelength light--red, orange, and yellow--is absorbed by the melanin deep in the dermis, while short-wavelength visible light--blue, violet, and indigo--is reflected with backscattering. On polarized dermoscopy, a homogeneous blue-gray hue is appreciated, but lighter segments may be present when collagen deposition is robust. Histopathologic findings confirm spindle-shaped dendritic melanocytes in the dermis without epidermal involvement. It generally is accepted that the etiology of these benign nevi is a failed migration of neural crest cells to the epidermis.10,11 Although the common blue nevus may be simple to diagnose, several subtypes have been described in the literature, including combined blue nevus, desmoplastic blue nevus, hypomelanotic/amelanotic blue nevus, and epithelioid blue nevus of Carney complex, and excluding a malignant process is of monumental importance.7,12
Biopsy is recommended for common blue nevi in the evaluation of newly acquired lesions, expansion of previously stable nevi, or for nevi larger than 10 mm in diameter. The nature of eruptive blue nevi warrants a biopsy to exclude melanoma or another malignant process. While the Becker nevus may manifest in adolescent males, it is clinically distinct from an eruptive blue nevus due to the size, relative homogeneity, and presence of hair within the lesion. Cutaneous amyloidosis may appear clinically similar to an eruptive blue nevus, but globular or amorphous material was not present in the papillary dermis of biopsied lesions in our patient. Since there was no cellular atypia or mitotic activity, melanoma and other malignancies were ruled out. Lastly, NAME syndrome by definition must include atrial myxomas, myxoid neurofibromas, and ephelides in addition to the nevi; however, our patient had only nevi and few ephelides. Once the diagnosis is established and benign nature confirmed, treatment is not necessarily required. If the patient elects to remove the lesion for aesthetic reasons, an excision into the subcutaneous fat is required to ensure complete removal of deep dermal melanocytes. Prior excisions of eruptive blue nevi have had no recurrence after more than 10 months.8,9
The Diagnosis: Eruptive Blue Nevus
All biopsies demonstrated similar histologic features, including an intradermal proliferation of heavily pigmented, spindle-shaped dendritic melanocytes (Figure). The dermal pigment was most pronounced in the grossly darker papules, and there was not a substantial amount of background pigmentation at the stratum basale. Cytologic atypia, foci of necrosis, and mitotic activity were absent from all sections. There was no definitive junctional component identified, no multinucleated giant cells, and there was no overlying epidermal aberration. With some background pigmentation seen histologically, nevus spilus was considered, but because this acute eruption occurred in a young adult without appreciable gross background hyperpigmentation, the clinical context led to a diagnosis of eruptive blue nevus. After communicating the findings to the patient, he declined further treatment.

Eruptive blue nevus is an exceptionally rare subtype of blue nevus with few cases reported since the 1940s.1-9 Generally, each case report found a triggering event that could possibly have precipitated the acute proliferation and evolution of nevi. Triggering events can include bullous processes such as erythema multiforme2 and Stevens-Johnson syndrome,3 severe sunburn,4 trauma,5 immunosuppression,6 and a variety of endocrinopathies. No such history could be identified in our patient, except the biopsy.
Common blue nevi are benign, usually congenital, well-circumscribed, solitary, blue-gray macules or papules. Half of blue nevi cases are found on the dorsal aspects of the hands and feet but can present anywhere (eg, face, scalp, wrists, sacrum, buttocks). The blue-gray color appreciated clinically is attributed to the Tyndall effect, which occurs when long-wavelength light--red, orange, and yellow--is absorbed by the melanin deep in the dermis, while short-wavelength visible light--blue, violet, and indigo--is reflected with backscattering. On polarized dermoscopy, a homogeneous blue-gray hue is appreciated, but lighter segments may be present when collagen deposition is robust. Histopathologic findings confirm spindle-shaped dendritic melanocytes in the dermis without epidermal involvement. It generally is accepted that the etiology of these benign nevi is a failed migration of neural crest cells to the epidermis.10,11 Although the common blue nevus may be simple to diagnose, several subtypes have been described in the literature, including combined blue nevus, desmoplastic blue nevus, hypomelanotic/amelanotic blue nevus, and epithelioid blue nevus of Carney complex, and excluding a malignant process is of monumental importance.7,12
Biopsy is recommended for common blue nevi in the evaluation of newly acquired lesions, expansion of previously stable nevi, or for nevi larger than 10 mm in diameter. The nature of eruptive blue nevi warrants a biopsy to exclude melanoma or another malignant process. While the Becker nevus may manifest in adolescent males, it is clinically distinct from an eruptive blue nevus due to the size, relative homogeneity, and presence of hair within the lesion. Cutaneous amyloidosis may appear clinically similar to an eruptive blue nevus, but globular or amorphous material was not present in the papillary dermis of biopsied lesions in our patient. Since there was no cellular atypia or mitotic activity, melanoma and other malignancies were ruled out. Lastly, NAME syndrome by definition must include atrial myxomas, myxoid neurofibromas, and ephelides in addition to the nevi; however, our patient had only nevi and few ephelides. Once the diagnosis is established and benign nature confirmed, treatment is not necessarily required. If the patient elects to remove the lesion for aesthetic reasons, an excision into the subcutaneous fat is required to ensure complete removal of deep dermal melanocytes. Prior excisions of eruptive blue nevi have had no recurrence after more than 10 months.8,9
- Krause M, Bonnekoh B, Weisshaar E, et al. Coincidence of multiple, disseminated, tardive-eruptive blue nevi with cutis marmorata teleangiectatica congenita. Dermatology. 2000;200:134-138.
- Soltani K, Bernstein J, Lorincz A. Eruptive nevocytic nevi after erythema multiforme. J Am Acad Dermatol. 1979;1:503-505.
- Shoji T, Cockerell C, Koff A, et al. Eruptive melanocytic nevi after Stevens-Johnson syndrome. J Am Acad Dermatol. 1997;37:337-339.
- Hendricks W. Eruptive blue nevi. J Am Acad Dermatol. 1981;4:50-53.
- Kesty K, Zargari O. Eruptive blue nevi. Indian J Dermatol Venereol Leprol. 2015;81:198-201.
- Chen T, Kurwa H, Trotter M, et al. Agminated blue nevi in a patient with dermatomyositis. J Am Acad Dermatol. 2013;68:52-53.
- Walsh M. Correspondence: eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;141:581-582.
- Nardini P, De Giorgi V, Massi D, et al. Eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;140:178-180.
- de Giorgi V, Massi D, Brunasso G, et al. Eruptive multiple blue nevi of the penis: a clinical dermoscopic pathologic case study. J Cutan Pathol. 2004;31:185-188.
- Zimmermann AH, Becker SA. Precursors of epidermal melanocytes in the negro fetus. In: Gordon M, ed. Pigment Cell Biology. New York, NY: Academic Press Inc; 1959:159-170.
- Leopold JG, Richards DB. The interrelationship of blue and common naevi. J Pathol Bacteriol. 1968;95:37-46.
- Zembowicz A, Phadke P. Blue nevi and variants: an update. Arch Pathol Lab Med. 2011;135:327-336.
- Krause M, Bonnekoh B, Weisshaar E, et al. Coincidence of multiple, disseminated, tardive-eruptive blue nevi with cutis marmorata teleangiectatica congenita. Dermatology. 2000;200:134-138.
- Soltani K, Bernstein J, Lorincz A. Eruptive nevocytic nevi after erythema multiforme. J Am Acad Dermatol. 1979;1:503-505.
- Shoji T, Cockerell C, Koff A, et al. Eruptive melanocytic nevi after Stevens-Johnson syndrome. J Am Acad Dermatol. 1997;37:337-339.
- Hendricks W. Eruptive blue nevi. J Am Acad Dermatol. 1981;4:50-53.
- Kesty K, Zargari O. Eruptive blue nevi. Indian J Dermatol Venereol Leprol. 2015;81:198-201.
- Chen T, Kurwa H, Trotter M, et al. Agminated blue nevi in a patient with dermatomyositis. J Am Acad Dermatol. 2013;68:52-53.
- Walsh M. Correspondence: eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;141:581-582.
- Nardini P, De Giorgi V, Massi D, et al. Eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;140:178-180.
- de Giorgi V, Massi D, Brunasso G, et al. Eruptive multiple blue nevi of the penis: a clinical dermoscopic pathologic case study. J Cutan Pathol. 2004;31:185-188.
- Zimmermann AH, Becker SA. Precursors of epidermal melanocytes in the negro fetus. In: Gordon M, ed. Pigment Cell Biology. New York, NY: Academic Press Inc; 1959:159-170.
- Leopold JG, Richards DB. The interrelationship of blue and common naevi. J Pathol Bacteriol. 1968;95:37-46.
- Zembowicz A, Phadke P. Blue nevi and variants: an update. Arch Pathol Lab Med. 2011;135:327-336.
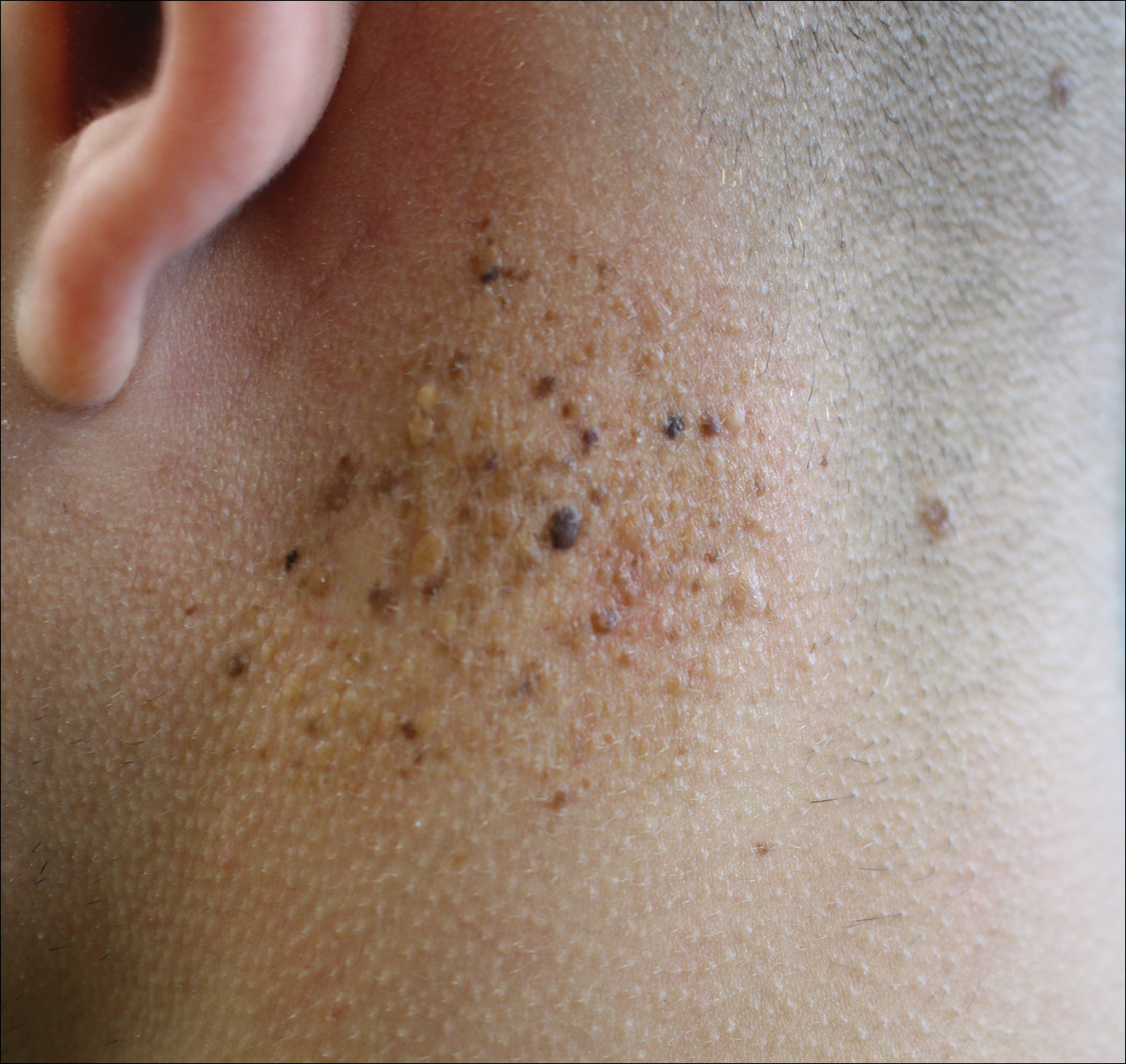
A 19-year-old man presented to the dermatology clinic for evaluation of several new dark papules on the neck of 1 year's duration. He denied any personal or family history of skin cancer, cardiac abnormalities, or endocrine dysfunction. He also denied any recent changes in health or use of medication. A biopsy was performed at the site 2 years prior for a single blue nevus, but the patient denied history of other trauma or cutaneous eruptions localized to the area. Physical examination revealed numerous dark brown, blue, white, and flesh-colored papules and macules agminated into a well-circumscribed plaque on the left posterolateral neck without background hyperpigmentation. The total area of the plaque was roughly 3×4 cm. There was no associated edema or erythema. Cardiac murmur, thyromegaly, exophthalmos, neurologic deficits, regional lymphadenopathy, and similar skin findings on other areas of the body were not appreciated. Three scouting punch biopsies were taken of the various morphologies present.
New MI definition aims to better distinguish infarction from injury
MUNICH – The worldwide cardiology community’s newly revised universal definition of an MI refines the way that cardiologists distinguish between myocardial infarction and myocardial injury, said Joseph S. Alpert, MD, one of the two chairs of the definition-writing panel.
“We had three previous definitions, but there is still a lot of confusion [about distinguishing] between injury and infarction. We definitely hope that this fourth definition will further help people distinguish the two and help people determine whether or not a patient has an MI,” said Dr. Alpert following a session at the annual congress of the European Society of Cardiology that introduced some of the key elements of the new revision.
Days before the ESC congress, a task force formed by the European Society of Cardiology, the American College of Cardiology, the American Heart Association, and the World Heart Federation released the Fourth Universal Definition of Myocardial Infarction (2018) (J Am Coll Cardiol. 2018 Aug 24. doi: 10.1016/j.jacc.2018.08.1038), which follows the series of three prior MI definitions that these groups have issued since the first iteration came out in 2007 (J Am Coll Cardiol. 2007;50[22]:2173-95).
The new revision includes 5 “new concepts,” 14 updated concepts, and 6 new sections since the third universal definition from 2012. The change that topped Dr. Alpert’s list of key messages was the need to determine whether a rise in cardiac troponin, a key biomarker of cardiac damage, resulted from infarction or injury.
These two alternative diagnoses mean “a very different outlook for patients. Treatment is different, and their prognosis is different. It’s important to make the distinction,” said Dr. Alpert, professor of medicine at the University of Arizona in Tucson.
The new changes to making an MI diagnosis will likely help drive a couple of important changes in the way U.S. patients with suspected myocardial injury or infarction get assessed, he said in an interview. The first change will be wide uptake of high sensitivity cardiac troponin (hscTn) assays over the next 5 years or so, as the ability to measure this key diagnostic biomarker progresses from its initial Food and Drug Administration approval for the U.S. market in 2017 to “close to 100% of U.S. hospitals using it,” he predicted. A big issue that is currently slowing even quicker adoption of hscTn is that many hospitals, including the one where Dr. Alpert practices, still have laboratory contracts in place that tether them to older troponin-testing technologies and make it economically unfeasible to change until their contracts expire. The contract in place where Dr. Alpert practices runs out in 2019, and soon after that happens he expects to gain the ability to order a hscTn test.
The new, fourth definition says that hscTn is “recommended for routine clinical use,” but routine U.S. use “won’t be immediate because many hospitals will put in hscTn only when their old contract runs out,” he said.
Another practice-changing impact from the fourth definition may be expanded U.S. availability and use of MR imaging, which the fourth definition identified as the most informative and versatile of the several imaging options used to confirm or rule out an MI.
Cardiac MR “provides both functional and tissue characterization. It’s the technique with the most potential,” able to noninvasively identify “both the nature and extent of myocardial damage,” explained Chiara Bucciarelli-Ducci, MD, a cardiologist and imaging specialist at the Bristol (England) Heart Institute. A single cardiac MR scan “gives many answers,” said Dr. Bucciarelli-Ducci, who also served on the fourth definition task force and spoke at the session about the document’s revised imaging recommendations.
“In the setting of acute MI, cardiac MR can also be used to assess the presence and extent of myocardium at risk (myocardial edema), myocardial salvage, microvascular obstruction, intramyocardial hemorrhage, and infarct size, all markers of myocardial injury that have prognostic value,” according to the fourth definition. “In patients with possible acute MI but unobstructed coronary arteries, cardiac MR can help to diagnose alternative conditions such as myocarditis, Takotsubo syndrome, embolic infarction, or MI with spontaneous recanalization.”
“What’s turning out is that, a large number of patients with chest pain have an infection and not an MI, and cardiac MR can distinguish inflammation and myocarditis from infarction. We’re now doing a lot more MRs,” Dr. Alpert said. Although MR capability is not as widely available today as other imaging methods, like echocardiography and CT, over the next 5 years that will likely change, he said. But Dr. Alpert cautioned that not every patient with a suspected MI needs MR assessment. It’s best focused for selected patients with an uncertain diagnosis based on the core indicators of disease: history, ECG, changes in hscTn levels over time, and a chest x-ray. “MR is for when there are questions,” he said. When patients present with classic MI signs and symptoms the diagnosis can depend just on the basics, perhaps supplemented with a more widely available imaging method like echocardiography to look for wall motion abnormalities, he said. “If echo shows good left ventricular function you probably don’t need MR.” he said.
CT coronary angiography (CTCA) is another useful diagnostic tool, and right now is more widely available than MR. CTCA “may be used to diagnose coronary artery disease in patients with an acute coronary syndrome event in the emergency department or chest pain unit, particularly in low- to intermediate-risk patients with normal hscTn at presentation,” said the fourth definition. But Dr. Alpert cited the radiation dose from CT as a limiting factor. “We have patients who get repeat CT scans, and we know that increases their cancer risk. There is no such thing as a totally safe dose of radiation.” Lack of radiation exposure is another feature that makes MR imaging attractive.
Dr. Alpert had no disclosures. Dr. Bucciarelli-Ducci has had a financial relationship with Circle Cardiovascular Imaging.
MUNICH – The worldwide cardiology community’s newly revised universal definition of an MI refines the way that cardiologists distinguish between myocardial infarction and myocardial injury, said Joseph S. Alpert, MD, one of the two chairs of the definition-writing panel.
“We had three previous definitions, but there is still a lot of confusion [about distinguishing] between injury and infarction. We definitely hope that this fourth definition will further help people distinguish the two and help people determine whether or not a patient has an MI,” said Dr. Alpert following a session at the annual congress of the European Society of Cardiology that introduced some of the key elements of the new revision.
Days before the ESC congress, a task force formed by the European Society of Cardiology, the American College of Cardiology, the American Heart Association, and the World Heart Federation released the Fourth Universal Definition of Myocardial Infarction (2018) (J Am Coll Cardiol. 2018 Aug 24. doi: 10.1016/j.jacc.2018.08.1038), which follows the series of three prior MI definitions that these groups have issued since the first iteration came out in 2007 (J Am Coll Cardiol. 2007;50[22]:2173-95).
The new revision includes 5 “new concepts,” 14 updated concepts, and 6 new sections since the third universal definition from 2012. The change that topped Dr. Alpert’s list of key messages was the need to determine whether a rise in cardiac troponin, a key biomarker of cardiac damage, resulted from infarction or injury.
These two alternative diagnoses mean “a very different outlook for patients. Treatment is different, and their prognosis is different. It’s important to make the distinction,” said Dr. Alpert, professor of medicine at the University of Arizona in Tucson.
The new changes to making an MI diagnosis will likely help drive a couple of important changes in the way U.S. patients with suspected myocardial injury or infarction get assessed, he said in an interview. The first change will be wide uptake of high sensitivity cardiac troponin (hscTn) assays over the next 5 years or so, as the ability to measure this key diagnostic biomarker progresses from its initial Food and Drug Administration approval for the U.S. market in 2017 to “close to 100% of U.S. hospitals using it,” he predicted. A big issue that is currently slowing even quicker adoption of hscTn is that many hospitals, including the one where Dr. Alpert practices, still have laboratory contracts in place that tether them to older troponin-testing technologies and make it economically unfeasible to change until their contracts expire. The contract in place where Dr. Alpert practices runs out in 2019, and soon after that happens he expects to gain the ability to order a hscTn test.
The new, fourth definition says that hscTn is “recommended for routine clinical use,” but routine U.S. use “won’t be immediate because many hospitals will put in hscTn only when their old contract runs out,” he said.
Another practice-changing impact from the fourth definition may be expanded U.S. availability and use of MR imaging, which the fourth definition identified as the most informative and versatile of the several imaging options used to confirm or rule out an MI.
Cardiac MR “provides both functional and tissue characterization. It’s the technique with the most potential,” able to noninvasively identify “both the nature and extent of myocardial damage,” explained Chiara Bucciarelli-Ducci, MD, a cardiologist and imaging specialist at the Bristol (England) Heart Institute. A single cardiac MR scan “gives many answers,” said Dr. Bucciarelli-Ducci, who also served on the fourth definition task force and spoke at the session about the document’s revised imaging recommendations.
“In the setting of acute MI, cardiac MR can also be used to assess the presence and extent of myocardium at risk (myocardial edema), myocardial salvage, microvascular obstruction, intramyocardial hemorrhage, and infarct size, all markers of myocardial injury that have prognostic value,” according to the fourth definition. “In patients with possible acute MI but unobstructed coronary arteries, cardiac MR can help to diagnose alternative conditions such as myocarditis, Takotsubo syndrome, embolic infarction, or MI with spontaneous recanalization.”
“What’s turning out is that, a large number of patients with chest pain have an infection and not an MI, and cardiac MR can distinguish inflammation and myocarditis from infarction. We’re now doing a lot more MRs,” Dr. Alpert said. Although MR capability is not as widely available today as other imaging methods, like echocardiography and CT, over the next 5 years that will likely change, he said. But Dr. Alpert cautioned that not every patient with a suspected MI needs MR assessment. It’s best focused for selected patients with an uncertain diagnosis based on the core indicators of disease: history, ECG, changes in hscTn levels over time, and a chest x-ray. “MR is for when there are questions,” he said. When patients present with classic MI signs and symptoms the diagnosis can depend just on the basics, perhaps supplemented with a more widely available imaging method like echocardiography to look for wall motion abnormalities, he said. “If echo shows good left ventricular function you probably don’t need MR.” he said.
CT coronary angiography (CTCA) is another useful diagnostic tool, and right now is more widely available than MR. CTCA “may be used to diagnose coronary artery disease in patients with an acute coronary syndrome event in the emergency department or chest pain unit, particularly in low- to intermediate-risk patients with normal hscTn at presentation,” said the fourth definition. But Dr. Alpert cited the radiation dose from CT as a limiting factor. “We have patients who get repeat CT scans, and we know that increases their cancer risk. There is no such thing as a totally safe dose of radiation.” Lack of radiation exposure is another feature that makes MR imaging attractive.
Dr. Alpert had no disclosures. Dr. Bucciarelli-Ducci has had a financial relationship with Circle Cardiovascular Imaging.
MUNICH – The worldwide cardiology community’s newly revised universal definition of an MI refines the way that cardiologists distinguish between myocardial infarction and myocardial injury, said Joseph S. Alpert, MD, one of the two chairs of the definition-writing panel.
“We had three previous definitions, but there is still a lot of confusion [about distinguishing] between injury and infarction. We definitely hope that this fourth definition will further help people distinguish the two and help people determine whether or not a patient has an MI,” said Dr. Alpert following a session at the annual congress of the European Society of Cardiology that introduced some of the key elements of the new revision.
Days before the ESC congress, a task force formed by the European Society of Cardiology, the American College of Cardiology, the American Heart Association, and the World Heart Federation released the Fourth Universal Definition of Myocardial Infarction (2018) (J Am Coll Cardiol. 2018 Aug 24. doi: 10.1016/j.jacc.2018.08.1038), which follows the series of three prior MI definitions that these groups have issued since the first iteration came out in 2007 (J Am Coll Cardiol. 2007;50[22]:2173-95).
The new revision includes 5 “new concepts,” 14 updated concepts, and 6 new sections since the third universal definition from 2012. The change that topped Dr. Alpert’s list of key messages was the need to determine whether a rise in cardiac troponin, a key biomarker of cardiac damage, resulted from infarction or injury.
These two alternative diagnoses mean “a very different outlook for patients. Treatment is different, and their prognosis is different. It’s important to make the distinction,” said Dr. Alpert, professor of medicine at the University of Arizona in Tucson.
The new changes to making an MI diagnosis will likely help drive a couple of important changes in the way U.S. patients with suspected myocardial injury or infarction get assessed, he said in an interview. The first change will be wide uptake of high sensitivity cardiac troponin (hscTn) assays over the next 5 years or so, as the ability to measure this key diagnostic biomarker progresses from its initial Food and Drug Administration approval for the U.S. market in 2017 to “close to 100% of U.S. hospitals using it,” he predicted. A big issue that is currently slowing even quicker adoption of hscTn is that many hospitals, including the one where Dr. Alpert practices, still have laboratory contracts in place that tether them to older troponin-testing technologies and make it economically unfeasible to change until their contracts expire. The contract in place where Dr. Alpert practices runs out in 2019, and soon after that happens he expects to gain the ability to order a hscTn test.
The new, fourth definition says that hscTn is “recommended for routine clinical use,” but routine U.S. use “won’t be immediate because many hospitals will put in hscTn only when their old contract runs out,” he said.
Another practice-changing impact from the fourth definition may be expanded U.S. availability and use of MR imaging, which the fourth definition identified as the most informative and versatile of the several imaging options used to confirm or rule out an MI.
Cardiac MR “provides both functional and tissue characterization. It’s the technique with the most potential,” able to noninvasively identify “both the nature and extent of myocardial damage,” explained Chiara Bucciarelli-Ducci, MD, a cardiologist and imaging specialist at the Bristol (England) Heart Institute. A single cardiac MR scan “gives many answers,” said Dr. Bucciarelli-Ducci, who also served on the fourth definition task force and spoke at the session about the document’s revised imaging recommendations.
“In the setting of acute MI, cardiac MR can also be used to assess the presence and extent of myocardium at risk (myocardial edema), myocardial salvage, microvascular obstruction, intramyocardial hemorrhage, and infarct size, all markers of myocardial injury that have prognostic value,” according to the fourth definition. “In patients with possible acute MI but unobstructed coronary arteries, cardiac MR can help to diagnose alternative conditions such as myocarditis, Takotsubo syndrome, embolic infarction, or MI with spontaneous recanalization.”
“What’s turning out is that, a large number of patients with chest pain have an infection and not an MI, and cardiac MR can distinguish inflammation and myocarditis from infarction. We’re now doing a lot more MRs,” Dr. Alpert said. Although MR capability is not as widely available today as other imaging methods, like echocardiography and CT, over the next 5 years that will likely change, he said. But Dr. Alpert cautioned that not every patient with a suspected MI needs MR assessment. It’s best focused for selected patients with an uncertain diagnosis based on the core indicators of disease: history, ECG, changes in hscTn levels over time, and a chest x-ray. “MR is for when there are questions,” he said. When patients present with classic MI signs and symptoms the diagnosis can depend just on the basics, perhaps supplemented with a more widely available imaging method like echocardiography to look for wall motion abnormalities, he said. “If echo shows good left ventricular function you probably don’t need MR.” he said.
CT coronary angiography (CTCA) is another useful diagnostic tool, and right now is more widely available than MR. CTCA “may be used to diagnose coronary artery disease in patients with an acute coronary syndrome event in the emergency department or chest pain unit, particularly in low- to intermediate-risk patients with normal hscTn at presentation,” said the fourth definition. But Dr. Alpert cited the radiation dose from CT as a limiting factor. “We have patients who get repeat CT scans, and we know that increases their cancer risk. There is no such thing as a totally safe dose of radiation.” Lack of radiation exposure is another feature that makes MR imaging attractive.
Dr. Alpert had no disclosures. Dr. Bucciarelli-Ducci has had a financial relationship with Circle Cardiovascular Imaging.
REPORTING FROM THE ESC CONGRESS 2018
Serum Nf-L shows promise as biomarker for BMT response in MS
LOS ANGELES – Serum neurofilament light chain shows promise as a biomarker for disease severity and response to treatment with autologous bone marrow transplant in patients with multiple sclerosis, according to findings from an analysis of paired samples.
Serum neurofilament light chain (Nf-L) levels were found to be significantly elevated in both the serum and cerebrospinal fluid (CSF) of 23 patients with aggressive multiple sclerosis (MS), compared with samples from 5 controls with noninflammatory neurologic conditions such as chronic fatigue syndrome or migraine. The levels in the patients with MS were significantly reduced following autologous bone marrow transplant (BMT), Simon Thebault, MD, reported in a poster at the annual meeting of the American Academy of Neurology.
Nf-L has been shown to be a biomarker for neuronal damage and to have potential for denoting disease severity and treatment response in MS; for this analysis, samples from patients were collected at baseline and annually for 3 years after transplant. Samples from controls were obtained for comparison.
“Our objective, really, was [to determine if we could] detect a treatment response in neurofilament light chain, and secondly, did the degree of neurofilament light chain being high at baseline predict anything about subsequent disease outcome?” Dr. Thebault, a third-year resident at the University of Ottawa, reported in the poster.
Indeed, a treatment response was detected; baseline levels were high, and levels in both serum and CSF were down significantly (P = .05) at both 1 and 3 years following bone marrow transplant, and they stayed down. “In fact, they were so low, they were not significantly different from [the levels] in noninflammatory controls,” he said, noting that serum and CSF NfL levels were highly correlated (r = .833; P less than .001).
Study subjects were patients with aggressive MS, characterized by early disease onset, rapid progression, frequent relapses, and poor treatment responses. Such patients who have received autologous BMT represent an interesting group for examining treatment responses, he said.
At baseline, these patients presumably have a high burden of tissue injury, which would be representative of high levels of Nf-L; good, prospective data show these patients have no evidence of ongoing inflammatory disease following an intensive regimen that includes chemoablation and reinfusion of autologous stem cells, which is representative of a significant reduction of neurofilament levels, he explained.
Importantly, serum and CSF levels were correlated in this study, he said, noting that most prior work has involved CSF, but “the real clinical utility in neurofilament light chain is probably in the serum, because patients don’t like having lumbar punctures too often.”
With respect to the second question pertaining to disease outcomes, the study did show that patients with baseline Nf-L levels above the median for the group had worse T1 lesion volumes at baseline and after transplant (P = .0021 at 36 months), and also had worsening brain atrophy even after transplant (P = .0389 at 60 months).
The curves appeared to be diverging, suggesting that, in the absence of inflammatory disease activity, patients with high Nf-L levels at baseline continue to have ongoing atrophy at a differential rate, compared with those with low baseline levels, Dr. Thebault said.
Other findings included better Expanded Disability Status Scale outcomes after transplant in patients with lower baseline Nf-L, and a trend toward worsening outcomes among those with higher baseline Nf-L levels, although the study may have been underpowered for this. Additionally, lower baseline Nf-L predicted significantly lower T1 and T2 lesion volume, number of gadolinium-enhancing lesions, and a reduction in brain atrophy, whereas higher baseline Nf-L predicted the opposite, he noted, adding that N-acetylaspartate-to-creatinine ratios also tended to vary inversely with Nf-L levels.
The most important findings of the study are “the sustained reduction in Nf-L levels after BMT, and that higher baseline levels predicted worse MRI outcomes,” Dr. Thebault noted. “Together these data add to a growing body of evidence that suggests that serum Nf-L has a role in monitoring treatment responses and even a predictive value in determining disease severity.”
This study was supported by Quanterix, which provided Nf-L assay kits. Dr. Thebault reported having no disclosures.
SOURCE: Thebault S et al. Neurology. 2018 Apr;90(15 Suppl.):P5.036.
LOS ANGELES – Serum neurofilament light chain shows promise as a biomarker for disease severity and response to treatment with autologous bone marrow transplant in patients with multiple sclerosis, according to findings from an analysis of paired samples.
Serum neurofilament light chain (Nf-L) levels were found to be significantly elevated in both the serum and cerebrospinal fluid (CSF) of 23 patients with aggressive multiple sclerosis (MS), compared with samples from 5 controls with noninflammatory neurologic conditions such as chronic fatigue syndrome or migraine. The levels in the patients with MS were significantly reduced following autologous bone marrow transplant (BMT), Simon Thebault, MD, reported in a poster at the annual meeting of the American Academy of Neurology.
Nf-L has been shown to be a biomarker for neuronal damage and to have potential for denoting disease severity and treatment response in MS; for this analysis, samples from patients were collected at baseline and annually for 3 years after transplant. Samples from controls were obtained for comparison.
“Our objective, really, was [to determine if we could] detect a treatment response in neurofilament light chain, and secondly, did the degree of neurofilament light chain being high at baseline predict anything about subsequent disease outcome?” Dr. Thebault, a third-year resident at the University of Ottawa, reported in the poster.
Indeed, a treatment response was detected; baseline levels were high, and levels in both serum and CSF were down significantly (P = .05) at both 1 and 3 years following bone marrow transplant, and they stayed down. “In fact, they were so low, they were not significantly different from [the levels] in noninflammatory controls,” he said, noting that serum and CSF NfL levels were highly correlated (r = .833; P less than .001).
Study subjects were patients with aggressive MS, characterized by early disease onset, rapid progression, frequent relapses, and poor treatment responses. Such patients who have received autologous BMT represent an interesting group for examining treatment responses, he said.
At baseline, these patients presumably have a high burden of tissue injury, which would be representative of high levels of Nf-L; good, prospective data show these patients have no evidence of ongoing inflammatory disease following an intensive regimen that includes chemoablation and reinfusion of autologous stem cells, which is representative of a significant reduction of neurofilament levels, he explained.
Importantly, serum and CSF levels were correlated in this study, he said, noting that most prior work has involved CSF, but “the real clinical utility in neurofilament light chain is probably in the serum, because patients don’t like having lumbar punctures too often.”
With respect to the second question pertaining to disease outcomes, the study did show that patients with baseline Nf-L levels above the median for the group had worse T1 lesion volumes at baseline and after transplant (P = .0021 at 36 months), and also had worsening brain atrophy even after transplant (P = .0389 at 60 months).
The curves appeared to be diverging, suggesting that, in the absence of inflammatory disease activity, patients with high Nf-L levels at baseline continue to have ongoing atrophy at a differential rate, compared with those with low baseline levels, Dr. Thebault said.
Other findings included better Expanded Disability Status Scale outcomes after transplant in patients with lower baseline Nf-L, and a trend toward worsening outcomes among those with higher baseline Nf-L levels, although the study may have been underpowered for this. Additionally, lower baseline Nf-L predicted significantly lower T1 and T2 lesion volume, number of gadolinium-enhancing lesions, and a reduction in brain atrophy, whereas higher baseline Nf-L predicted the opposite, he noted, adding that N-acetylaspartate-to-creatinine ratios also tended to vary inversely with Nf-L levels.
The most important findings of the study are “the sustained reduction in Nf-L levels after BMT, and that higher baseline levels predicted worse MRI outcomes,” Dr. Thebault noted. “Together these data add to a growing body of evidence that suggests that serum Nf-L has a role in monitoring treatment responses and even a predictive value in determining disease severity.”
This study was supported by Quanterix, which provided Nf-L assay kits. Dr. Thebault reported having no disclosures.
SOURCE: Thebault S et al. Neurology. 2018 Apr;90(15 Suppl.):P5.036.
LOS ANGELES – Serum neurofilament light chain shows promise as a biomarker for disease severity and response to treatment with autologous bone marrow transplant in patients with multiple sclerosis, according to findings from an analysis of paired samples.
Serum neurofilament light chain (Nf-L) levels were found to be significantly elevated in both the serum and cerebrospinal fluid (CSF) of 23 patients with aggressive multiple sclerosis (MS), compared with samples from 5 controls with noninflammatory neurologic conditions such as chronic fatigue syndrome or migraine. The levels in the patients with MS were significantly reduced following autologous bone marrow transplant (BMT), Simon Thebault, MD, reported in a poster at the annual meeting of the American Academy of Neurology.
Nf-L has been shown to be a biomarker for neuronal damage and to have potential for denoting disease severity and treatment response in MS; for this analysis, samples from patients were collected at baseline and annually for 3 years after transplant. Samples from controls were obtained for comparison.
“Our objective, really, was [to determine if we could] detect a treatment response in neurofilament light chain, and secondly, did the degree of neurofilament light chain being high at baseline predict anything about subsequent disease outcome?” Dr. Thebault, a third-year resident at the University of Ottawa, reported in the poster.
Indeed, a treatment response was detected; baseline levels were high, and levels in both serum and CSF were down significantly (P = .05) at both 1 and 3 years following bone marrow transplant, and they stayed down. “In fact, they were so low, they were not significantly different from [the levels] in noninflammatory controls,” he said, noting that serum and CSF NfL levels were highly correlated (r = .833; P less than .001).
Study subjects were patients with aggressive MS, characterized by early disease onset, rapid progression, frequent relapses, and poor treatment responses. Such patients who have received autologous BMT represent an interesting group for examining treatment responses, he said.
At baseline, these patients presumably have a high burden of tissue injury, which would be representative of high levels of Nf-L; good, prospective data show these patients have no evidence of ongoing inflammatory disease following an intensive regimen that includes chemoablation and reinfusion of autologous stem cells, which is representative of a significant reduction of neurofilament levels, he explained.
Importantly, serum and CSF levels were correlated in this study, he said, noting that most prior work has involved CSF, but “the real clinical utility in neurofilament light chain is probably in the serum, because patients don’t like having lumbar punctures too often.”
With respect to the second question pertaining to disease outcomes, the study did show that patients with baseline Nf-L levels above the median for the group had worse T1 lesion volumes at baseline and after transplant (P = .0021 at 36 months), and also had worsening brain atrophy even after transplant (P = .0389 at 60 months).
The curves appeared to be diverging, suggesting that, in the absence of inflammatory disease activity, patients with high Nf-L levels at baseline continue to have ongoing atrophy at a differential rate, compared with those with low baseline levels, Dr. Thebault said.
Other findings included better Expanded Disability Status Scale outcomes after transplant in patients with lower baseline Nf-L, and a trend toward worsening outcomes among those with higher baseline Nf-L levels, although the study may have been underpowered for this. Additionally, lower baseline Nf-L predicted significantly lower T1 and T2 lesion volume, number of gadolinium-enhancing lesions, and a reduction in brain atrophy, whereas higher baseline Nf-L predicted the opposite, he noted, adding that N-acetylaspartate-to-creatinine ratios also tended to vary inversely with Nf-L levels.
The most important findings of the study are “the sustained reduction in Nf-L levels after BMT, and that higher baseline levels predicted worse MRI outcomes,” Dr. Thebault noted. “Together these data add to a growing body of evidence that suggests that serum Nf-L has a role in monitoring treatment responses and even a predictive value in determining disease severity.”
This study was supported by Quanterix, which provided Nf-L assay kits. Dr. Thebault reported having no disclosures.
SOURCE: Thebault S et al. Neurology. 2018 Apr;90(15 Suppl.):P5.036.
REPORTING FROM AAN 2018
Key clinical point:
Major finding: Serum and cerebrospinal fluid Nf-L levels declines significantly after bone marrow transplant (P less than .05) and did not differ from the levels in controls.
Study details: An analysis of paired samples from 23 patients with multiple sclerosis and 5 controls.
Disclosures: This study was supported by Quanterix, which provided Nf-L assay kits. Dr. Thebault reported having no disclosures.
Source: Thebault S et al. Neurology. 2018 Apr;90(15 Suppl.):P5.036.
Am I My Brother’s/Sister’s Keeper?
Adherents of the Abrahamic faiths or students of the Bible as literature will easily recall the circumstances of this famous quotation from the Hebrew Scriptures. Cain has killed his brother Abel, earning the disrepute of committing the first murder in biblical history. Scholars of ethics have answered Cain’s sardonic question in the affirmative: avowing that while the individual is primarily responsible for his conduct and its consequences, the community also bears a responsibility.1
Many of you will have seen news clips of yet another Department of Veteran Affairs (VA) scandal: This one involving an impaired pathologist. I have purposely not used the pathologist’s name to emphasize that our shared obligation to protect patients from impaired members of the profession goes far beyond this single outrage. Although we might believe we could never be that individual; none of us are immune from depression, dementia, or physical disabilities that diminish our ability to care for patients. Although it is worth noting that having read or watched everything I could find on the story, the “facts” are few and far between, which I will argue later only underscores the problems with professional accountability especially in a large bureaucratic organization.
This column is not meant to impugn or exculpate anyone but to encourage us to reflect on our ethical awareness and response to hints that there might be an impaired practitioner among us. The Fayetteville VA Medical Center (VAMC) in North Carolina—ground zero for this incident—held several town halls in which anxious and angry veterans demanded an explanation for how an impaired pathologist could have placed in harm’s way nearly 19,000 patients.2 Of this cohort, 5,250 patients have died since 2005. The VA leadership, including the VISN 16 network director and the interim medical director of the Fayetteville VAMC indicated that out of 911 pathology reports that had undergone expert peer review, an incorrect diagnosis was identified in at least 7 with 1 possible associated death.3
The VA officials estimate that the entire review may involve more than 30,000 cases. The allegations are that the pathologist was impaired due to a substance use disorder, in this case alcohol. In interviews, the physician has admitted the alcohol problem and receiving treatment for it but denied he was ever impaired on duty.4 This denial directly contradicts the VA reports that he was intoxicated on duty at least twice.
We do know that the physician in question was enrolled in the Mississippi Physician Health Program (PHP). The Federation of State Physician Health Programs (FSPHP), of which the Mississippi program is a member, provides confidential initial evaluation, ongoing treatment, and monitoring for state licensed health care practitioners with a substance use or other mental health disorder or other condition that could potentially impair their fitness for duty. The PHPs have been career saving for many physicians and other health care professionals. The incentives to return to the practice of medicine are powerful, leading to a higher recovery rate than that of the general population.5
According to FSPHP, “While both PHPs and state licensing boards are engaged in patient safety efforts, PHPs primary focus is on improving the health of the physician, and the licensing board’s primary duty is to protect the public.”6 This potential conflict of interest has been criticized recently.7 News reports suggest that the ambiguity of this relationship between PHP and licensing board may have left a “who’s minding the store” mentality. But here are the facts of this particular case.
In March 2016, an employee reported that the pathologist was intoxicated on duty, and he was immediately removed from service. Of the many elephants in the room, the biggest moral pachyderm is why, given the chronic and progressive nature of substance use disorders did it take 11 years for a formal report leading to action?
Having been in circles of leadership, I know well that often there is much discussed that cannot be disclosed, which frequently contributes to staff demoralization. Dozens of medical center employees over decades must have seen behaviors; some of those employees likely reported their observations. The news reports are silent on this point except for attributing it to “fear of retaliation.” In our current VA climate this is a major intimidator of staff trying to tell the truth and leaders seeking to do what is right. Yet research has identified myriad other motives for not reporting impaired colleagues, including loyalty, collusion, denial, indifference, scarcity of resources, and overwork.8
In October 2017, the pathologist was again found intoxicated on the job, attributing it to a migraine headache. The hospital investigated, but the pathologist may have continued working in some capacity. Finally, after the pathologist was arrested for driving while intoxicated March 1 of this year, the interim VAMC director contacted the Mississippi licensing board, declaring that the pathologist “had failed to meet standards of practice” and indicted that he “constituted an imminent threat to patient welfare.” The Arkansas Medical Foundation then rescinded its support of the pathologist, citing the pathologist’s failure to adhere to monitoring and reporting requirements. The Mississippi program followed suit on June 20, and the medical board rescinded his license.
All employees at whatever level are owed due process and respect for their rights, but Congress recently saw fit to legislate further limits on federal employee protections. Most health care administrators still standing in the chaos of today’s VA would tell you that survival is all about when did you know and what did you do about it? But it is not just administrators, the code of ethics of almost every health care profession includes an obligation to report impaired practitioners. Principle II of the American Medical Association Code of Ethics states, “A physician shall uphold the standards of professionalism, be honest in all professional interactions, and strive to report physicians deficient in character or competence, or engaging in fraud or deception, to appropriate entities.”9
If one is in private practice in a small town, it is easier to pull your friend aside on the golf course and say you seem to be having a problem, counsel your friend to get help, often through a PHP. Then if the gentler approach fails, take the harder action of reporting to the medical board or other authority. To report takes moral courage. It takes seeing that the practitioner is not betraying the “white line” of health care professions but honoring the highest commitment of professionalism to the patient.
The last thing we need in our current suspicious and fearful environment is to turn VAMCs and clinics into a dystopia.Yet reporting in a large organization with rules and red tape can seem terrifying, overwhelming, and confusing. A fair, safe, transparent, clear, and consistent means for staff to discharge their ethical obligations is sorely needed. Or else we will be reading about another tragic scandal and asking each other, “How could this have happened?”
1. Morreim EH. Am I my brother’s warden? Responding to the unethical or incompetent colleague. Hastings Cent Rep. 1993;23(3):19-27
2. Worrell T. Veterans question VA over report of ‘impaired’ pathologist at town hall. http://www.joplinglobe.com/news /local_news/veterans-question-va-over-report-of-impaired-pathologist-at-town/article_9281a9d8-8354-5739-b21e-d38e2f28137c.html. Published July 9, 2018. Accessed July 25, 2018 .
3. Wornell T. Fayetteville VA hospital: ‘impaired’ pathologist misdiagnosed some patients. http://www.joplinglobe.com/news/local_news/fayetteville-va-hospital-impaired-pathologist-misdiagnosed-some-patients/article_519f6adc-2109-5ff1-a9ca-98ba9403d6a7.html. Published June 18, 2018. Accessed July 25, 2018.
4. Merlo LLJ, Teitelbaum SA, Thompson K. Substance use disorders in physicians: assessment and treatment. https://www.uptodate.com/contents/substance-use-disorders-in-physicians-assessment-and-treatment. Last updated July 13, 2017. Accessed July 25, 2018.
5. Grabenstein H. Former VA pathologist denies being impaired on duty. https://www.apnews.com/8ca77da8f7ce40868ffbc091608ee681. Published July 9, 2018. Accessed July 25, 2018.
6. Federation of State Physician Health Programs. Frequently asked questions. https://www.fsphp.org/about/faqs. Accessed July 25, 2018.
7. Anderson P. Physician health programs: more harm than good? https://www.medscape.com/viewarticle/849772. Published August 19, 2015. Accessed July 25, 2018.
8. DesRoches CM, Rao SR, Fromson JA, et al. Physicians’ perceptions, preparedness for reporting, and experiences related to impaired and incompetent colleagues. JAMA. 2010;304(2):187-193.
9. American Medical Association. Code of Medical Ethics 2014-2015. https://commerce.ama-assn.org/store/catalog/productDetail.jsp?product_id=prod2510003&sku_id=sku2510003&navAction=push. Accessed July 25, 2018.
Adherents of the Abrahamic faiths or students of the Bible as literature will easily recall the circumstances of this famous quotation from the Hebrew Scriptures. Cain has killed his brother Abel, earning the disrepute of committing the first murder in biblical history. Scholars of ethics have answered Cain’s sardonic question in the affirmative: avowing that while the individual is primarily responsible for his conduct and its consequences, the community also bears a responsibility.1
Many of you will have seen news clips of yet another Department of Veteran Affairs (VA) scandal: This one involving an impaired pathologist. I have purposely not used the pathologist’s name to emphasize that our shared obligation to protect patients from impaired members of the profession goes far beyond this single outrage. Although we might believe we could never be that individual; none of us are immune from depression, dementia, or physical disabilities that diminish our ability to care for patients. Although it is worth noting that having read or watched everything I could find on the story, the “facts” are few and far between, which I will argue later only underscores the problems with professional accountability especially in a large bureaucratic organization.
This column is not meant to impugn or exculpate anyone but to encourage us to reflect on our ethical awareness and response to hints that there might be an impaired practitioner among us. The Fayetteville VA Medical Center (VAMC) in North Carolina—ground zero for this incident—held several town halls in which anxious and angry veterans demanded an explanation for how an impaired pathologist could have placed in harm’s way nearly 19,000 patients.2 Of this cohort, 5,250 patients have died since 2005. The VA leadership, including the VISN 16 network director and the interim medical director of the Fayetteville VAMC indicated that out of 911 pathology reports that had undergone expert peer review, an incorrect diagnosis was identified in at least 7 with 1 possible associated death.3
The VA officials estimate that the entire review may involve more than 30,000 cases. The allegations are that the pathologist was impaired due to a substance use disorder, in this case alcohol. In interviews, the physician has admitted the alcohol problem and receiving treatment for it but denied he was ever impaired on duty.4 This denial directly contradicts the VA reports that he was intoxicated on duty at least twice.
We do know that the physician in question was enrolled in the Mississippi Physician Health Program (PHP). The Federation of State Physician Health Programs (FSPHP), of which the Mississippi program is a member, provides confidential initial evaluation, ongoing treatment, and monitoring for state licensed health care practitioners with a substance use or other mental health disorder or other condition that could potentially impair their fitness for duty. The PHPs have been career saving for many physicians and other health care professionals. The incentives to return to the practice of medicine are powerful, leading to a higher recovery rate than that of the general population.5
According to FSPHP, “While both PHPs and state licensing boards are engaged in patient safety efforts, PHPs primary focus is on improving the health of the physician, and the licensing board’s primary duty is to protect the public.”6 This potential conflict of interest has been criticized recently.7 News reports suggest that the ambiguity of this relationship between PHP and licensing board may have left a “who’s minding the store” mentality. But here are the facts of this particular case.
In March 2016, an employee reported that the pathologist was intoxicated on duty, and he was immediately removed from service. Of the many elephants in the room, the biggest moral pachyderm is why, given the chronic and progressive nature of substance use disorders did it take 11 years for a formal report leading to action?
Having been in circles of leadership, I know well that often there is much discussed that cannot be disclosed, which frequently contributes to staff demoralization. Dozens of medical center employees over decades must have seen behaviors; some of those employees likely reported their observations. The news reports are silent on this point except for attributing it to “fear of retaliation.” In our current VA climate this is a major intimidator of staff trying to tell the truth and leaders seeking to do what is right. Yet research has identified myriad other motives for not reporting impaired colleagues, including loyalty, collusion, denial, indifference, scarcity of resources, and overwork.8
In October 2017, the pathologist was again found intoxicated on the job, attributing it to a migraine headache. The hospital investigated, but the pathologist may have continued working in some capacity. Finally, after the pathologist was arrested for driving while intoxicated March 1 of this year, the interim VAMC director contacted the Mississippi licensing board, declaring that the pathologist “had failed to meet standards of practice” and indicted that he “constituted an imminent threat to patient welfare.” The Arkansas Medical Foundation then rescinded its support of the pathologist, citing the pathologist’s failure to adhere to monitoring and reporting requirements. The Mississippi program followed suit on June 20, and the medical board rescinded his license.
All employees at whatever level are owed due process and respect for their rights, but Congress recently saw fit to legislate further limits on federal employee protections. Most health care administrators still standing in the chaos of today’s VA would tell you that survival is all about when did you know and what did you do about it? But it is not just administrators, the code of ethics of almost every health care profession includes an obligation to report impaired practitioners. Principle II of the American Medical Association Code of Ethics states, “A physician shall uphold the standards of professionalism, be honest in all professional interactions, and strive to report physicians deficient in character or competence, or engaging in fraud or deception, to appropriate entities.”9
If one is in private practice in a small town, it is easier to pull your friend aside on the golf course and say you seem to be having a problem, counsel your friend to get help, often through a PHP. Then if the gentler approach fails, take the harder action of reporting to the medical board or other authority. To report takes moral courage. It takes seeing that the practitioner is not betraying the “white line” of health care professions but honoring the highest commitment of professionalism to the patient.
The last thing we need in our current suspicious and fearful environment is to turn VAMCs and clinics into a dystopia.Yet reporting in a large organization with rules and red tape can seem terrifying, overwhelming, and confusing. A fair, safe, transparent, clear, and consistent means for staff to discharge their ethical obligations is sorely needed. Or else we will be reading about another tragic scandal and asking each other, “How could this have happened?”
Adherents of the Abrahamic faiths or students of the Bible as literature will easily recall the circumstances of this famous quotation from the Hebrew Scriptures. Cain has killed his brother Abel, earning the disrepute of committing the first murder in biblical history. Scholars of ethics have answered Cain’s sardonic question in the affirmative: avowing that while the individual is primarily responsible for his conduct and its consequences, the community also bears a responsibility.1
Many of you will have seen news clips of yet another Department of Veteran Affairs (VA) scandal: This one involving an impaired pathologist. I have purposely not used the pathologist’s name to emphasize that our shared obligation to protect patients from impaired members of the profession goes far beyond this single outrage. Although we might believe we could never be that individual; none of us are immune from depression, dementia, or physical disabilities that diminish our ability to care for patients. Although it is worth noting that having read or watched everything I could find on the story, the “facts” are few and far between, which I will argue later only underscores the problems with professional accountability especially in a large bureaucratic organization.
This column is not meant to impugn or exculpate anyone but to encourage us to reflect on our ethical awareness and response to hints that there might be an impaired practitioner among us. The Fayetteville VA Medical Center (VAMC) in North Carolina—ground zero for this incident—held several town halls in which anxious and angry veterans demanded an explanation for how an impaired pathologist could have placed in harm’s way nearly 19,000 patients.2 Of this cohort, 5,250 patients have died since 2005. The VA leadership, including the VISN 16 network director and the interim medical director of the Fayetteville VAMC indicated that out of 911 pathology reports that had undergone expert peer review, an incorrect diagnosis was identified in at least 7 with 1 possible associated death.3
The VA officials estimate that the entire review may involve more than 30,000 cases. The allegations are that the pathologist was impaired due to a substance use disorder, in this case alcohol. In interviews, the physician has admitted the alcohol problem and receiving treatment for it but denied he was ever impaired on duty.4 This denial directly contradicts the VA reports that he was intoxicated on duty at least twice.
We do know that the physician in question was enrolled in the Mississippi Physician Health Program (PHP). The Federation of State Physician Health Programs (FSPHP), of which the Mississippi program is a member, provides confidential initial evaluation, ongoing treatment, and monitoring for state licensed health care practitioners with a substance use or other mental health disorder or other condition that could potentially impair their fitness for duty. The PHPs have been career saving for many physicians and other health care professionals. The incentives to return to the practice of medicine are powerful, leading to a higher recovery rate than that of the general population.5
According to FSPHP, “While both PHPs and state licensing boards are engaged in patient safety efforts, PHPs primary focus is on improving the health of the physician, and the licensing board’s primary duty is to protect the public.”6 This potential conflict of interest has been criticized recently.7 News reports suggest that the ambiguity of this relationship between PHP and licensing board may have left a “who’s minding the store” mentality. But here are the facts of this particular case.
In March 2016, an employee reported that the pathologist was intoxicated on duty, and he was immediately removed from service. Of the many elephants in the room, the biggest moral pachyderm is why, given the chronic and progressive nature of substance use disorders did it take 11 years for a formal report leading to action?
Having been in circles of leadership, I know well that often there is much discussed that cannot be disclosed, which frequently contributes to staff demoralization. Dozens of medical center employees over decades must have seen behaviors; some of those employees likely reported their observations. The news reports are silent on this point except for attributing it to “fear of retaliation.” In our current VA climate this is a major intimidator of staff trying to tell the truth and leaders seeking to do what is right. Yet research has identified myriad other motives for not reporting impaired colleagues, including loyalty, collusion, denial, indifference, scarcity of resources, and overwork.8
In October 2017, the pathologist was again found intoxicated on the job, attributing it to a migraine headache. The hospital investigated, but the pathologist may have continued working in some capacity. Finally, after the pathologist was arrested for driving while intoxicated March 1 of this year, the interim VAMC director contacted the Mississippi licensing board, declaring that the pathologist “had failed to meet standards of practice” and indicted that he “constituted an imminent threat to patient welfare.” The Arkansas Medical Foundation then rescinded its support of the pathologist, citing the pathologist’s failure to adhere to monitoring and reporting requirements. The Mississippi program followed suit on June 20, and the medical board rescinded his license.
All employees at whatever level are owed due process and respect for their rights, but Congress recently saw fit to legislate further limits on federal employee protections. Most health care administrators still standing in the chaos of today’s VA would tell you that survival is all about when did you know and what did you do about it? But it is not just administrators, the code of ethics of almost every health care profession includes an obligation to report impaired practitioners. Principle II of the American Medical Association Code of Ethics states, “A physician shall uphold the standards of professionalism, be honest in all professional interactions, and strive to report physicians deficient in character or competence, or engaging in fraud or deception, to appropriate entities.”9
If one is in private practice in a small town, it is easier to pull your friend aside on the golf course and say you seem to be having a problem, counsel your friend to get help, often through a PHP. Then if the gentler approach fails, take the harder action of reporting to the medical board or other authority. To report takes moral courage. It takes seeing that the practitioner is not betraying the “white line” of health care professions but honoring the highest commitment of professionalism to the patient.
The last thing we need in our current suspicious and fearful environment is to turn VAMCs and clinics into a dystopia.Yet reporting in a large organization with rules and red tape can seem terrifying, overwhelming, and confusing. A fair, safe, transparent, clear, and consistent means for staff to discharge their ethical obligations is sorely needed. Or else we will be reading about another tragic scandal and asking each other, “How could this have happened?”
1. Morreim EH. Am I my brother’s warden? Responding to the unethical or incompetent colleague. Hastings Cent Rep. 1993;23(3):19-27
2. Worrell T. Veterans question VA over report of ‘impaired’ pathologist at town hall. http://www.joplinglobe.com/news /local_news/veterans-question-va-over-report-of-impaired-pathologist-at-town/article_9281a9d8-8354-5739-b21e-d38e2f28137c.html. Published July 9, 2018. Accessed July 25, 2018 .
3. Wornell T. Fayetteville VA hospital: ‘impaired’ pathologist misdiagnosed some patients. http://www.joplinglobe.com/news/local_news/fayetteville-va-hospital-impaired-pathologist-misdiagnosed-some-patients/article_519f6adc-2109-5ff1-a9ca-98ba9403d6a7.html. Published June 18, 2018. Accessed July 25, 2018.
4. Merlo LLJ, Teitelbaum SA, Thompson K. Substance use disorders in physicians: assessment and treatment. https://www.uptodate.com/contents/substance-use-disorders-in-physicians-assessment-and-treatment. Last updated July 13, 2017. Accessed July 25, 2018.
5. Grabenstein H. Former VA pathologist denies being impaired on duty. https://www.apnews.com/8ca77da8f7ce40868ffbc091608ee681. Published July 9, 2018. Accessed July 25, 2018.
6. Federation of State Physician Health Programs. Frequently asked questions. https://www.fsphp.org/about/faqs. Accessed July 25, 2018.
7. Anderson P. Physician health programs: more harm than good? https://www.medscape.com/viewarticle/849772. Published August 19, 2015. Accessed July 25, 2018.
8. DesRoches CM, Rao SR, Fromson JA, et al. Physicians’ perceptions, preparedness for reporting, and experiences related to impaired and incompetent colleagues. JAMA. 2010;304(2):187-193.
9. American Medical Association. Code of Medical Ethics 2014-2015. https://commerce.ama-assn.org/store/catalog/productDetail.jsp?product_id=prod2510003&sku_id=sku2510003&navAction=push. Accessed July 25, 2018.
1. Morreim EH. Am I my brother’s warden? Responding to the unethical or incompetent colleague. Hastings Cent Rep. 1993;23(3):19-27
2. Worrell T. Veterans question VA over report of ‘impaired’ pathologist at town hall. http://www.joplinglobe.com/news /local_news/veterans-question-va-over-report-of-impaired-pathologist-at-town/article_9281a9d8-8354-5739-b21e-d38e2f28137c.html. Published July 9, 2018. Accessed July 25, 2018 .
3. Wornell T. Fayetteville VA hospital: ‘impaired’ pathologist misdiagnosed some patients. http://www.joplinglobe.com/news/local_news/fayetteville-va-hospital-impaired-pathologist-misdiagnosed-some-patients/article_519f6adc-2109-5ff1-a9ca-98ba9403d6a7.html. Published June 18, 2018. Accessed July 25, 2018.
4. Merlo LLJ, Teitelbaum SA, Thompson K. Substance use disorders in physicians: assessment and treatment. https://www.uptodate.com/contents/substance-use-disorders-in-physicians-assessment-and-treatment. Last updated July 13, 2017. Accessed July 25, 2018.
5. Grabenstein H. Former VA pathologist denies being impaired on duty. https://www.apnews.com/8ca77da8f7ce40868ffbc091608ee681. Published July 9, 2018. Accessed July 25, 2018.
6. Federation of State Physician Health Programs. Frequently asked questions. https://www.fsphp.org/about/faqs. Accessed July 25, 2018.
7. Anderson P. Physician health programs: more harm than good? https://www.medscape.com/viewarticle/849772. Published August 19, 2015. Accessed July 25, 2018.
8. DesRoches CM, Rao SR, Fromson JA, et al. Physicians’ perceptions, preparedness for reporting, and experiences related to impaired and incompetent colleagues. JAMA. 2010;304(2):187-193.
9. American Medical Association. Code of Medical Ethics 2014-2015. https://commerce.ama-assn.org/store/catalog/productDetail.jsp?product_id=prod2510003&sku_id=sku2510003&navAction=push. Accessed July 25, 2018.
HIV, HBV, and HCV Increase Risks After Joint Replacement
As patients with HIV, hepatitis B (HBV), and hepatitis C (HCV) live longer, more active lives with the help of antiviral treatments, they are now more often having joint replacement surgeries. But HIV, HBV, and HCV can increase the risk of postoperative complications, say researchers from Duke University in North Carolina.
The researchers studied 16,338 patients in 4 cohorts: those with HIV, HBV, HCV, and HIV plus HBV or HCV. They evaluated the patients at 30 days, 90 days, and 2 years after total joint arthroplasty (TJA), comparing their progress with that of a control group.
Patients with HBV and HCV were at risk of both acute and long-term medical and surgical complications. At 90 days and 2 years, the participants had increased risk of pneumonia, sepsis, joint infection, and revision surgery. They also had a greater risk of complications than did patients with HIV, especially for infection, within the first 90 days postsurgery.
Notably, after TJA, patients with HCV had increased risk of acute kidney injury, sepsis, and transfusion at 30 and 90 days. After hip replacement, patients with HBV had a higher risk of acute kidney injury, pneumonia, and transfusion at 30 and 90 days.
Only 364 patients in the study had both HIV and HBV or HBC, but they did have a greater risk of transfusion at 30 and 90 days following both knee and hip surgery.
Following total hip arthroplasty, patients with HIV were more at risk for deep vein thrombosis and transfusion rather than infection. The lower incidence of infection is likely to be related to effective highly active antiretroviral therapy, the researchers say. HIV patients also were more likely to have mechanical complications, such as loosening, periprosthetic fracture, and revision at 90 days, but not at 2 years. Patients with HIV who underwent total knee arthroplasty (TKA) had a higher risk of death at 30 days and medical complications at 90 days. At 2 years, they had a higher risk of periprosthetic joint infection, irrigation and debridement, and revision. The researchers say the higher incidence of mechanical complications can be explained by the younger, more active, and healthy HIV patients in the cohort—the majority were aged younger than 65 years. They also emphasize that the only risk of infection following TJA in their study was 2 years after TKA.
The researchers suggest that their findings could help prompt future guidelines supporting routine screening prior to elective TJA.
Source:
Kildow BJ, Politzer CS, DiLallo M, Bolognesi MP, Seyler TM. J Arthroplasty. 2018;33(suppl 7):S86-S92.
doi: 10.1016/j.arth.2017.10.061.
As patients with HIV, hepatitis B (HBV), and hepatitis C (HCV) live longer, more active lives with the help of antiviral treatments, they are now more often having joint replacement surgeries. But HIV, HBV, and HCV can increase the risk of postoperative complications, say researchers from Duke University in North Carolina.
The researchers studied 16,338 patients in 4 cohorts: those with HIV, HBV, HCV, and HIV plus HBV or HCV. They evaluated the patients at 30 days, 90 days, and 2 years after total joint arthroplasty (TJA), comparing their progress with that of a control group.
Patients with HBV and HCV were at risk of both acute and long-term medical and surgical complications. At 90 days and 2 years, the participants had increased risk of pneumonia, sepsis, joint infection, and revision surgery. They also had a greater risk of complications than did patients with HIV, especially for infection, within the first 90 days postsurgery.
Notably, after TJA, patients with HCV had increased risk of acute kidney injury, sepsis, and transfusion at 30 and 90 days. After hip replacement, patients with HBV had a higher risk of acute kidney injury, pneumonia, and transfusion at 30 and 90 days.
Only 364 patients in the study had both HIV and HBV or HBC, but they did have a greater risk of transfusion at 30 and 90 days following both knee and hip surgery.
Following total hip arthroplasty, patients with HIV were more at risk for deep vein thrombosis and transfusion rather than infection. The lower incidence of infection is likely to be related to effective highly active antiretroviral therapy, the researchers say. HIV patients also were more likely to have mechanical complications, such as loosening, periprosthetic fracture, and revision at 90 days, but not at 2 years. Patients with HIV who underwent total knee arthroplasty (TKA) had a higher risk of death at 30 days and medical complications at 90 days. At 2 years, they had a higher risk of periprosthetic joint infection, irrigation and debridement, and revision. The researchers say the higher incidence of mechanical complications can be explained by the younger, more active, and healthy HIV patients in the cohort—the majority were aged younger than 65 years. They also emphasize that the only risk of infection following TJA in their study was 2 years after TKA.
The researchers suggest that their findings could help prompt future guidelines supporting routine screening prior to elective TJA.
Source:
Kildow BJ, Politzer CS, DiLallo M, Bolognesi MP, Seyler TM. J Arthroplasty. 2018;33(suppl 7):S86-S92.
doi: 10.1016/j.arth.2017.10.061.
As patients with HIV, hepatitis B (HBV), and hepatitis C (HCV) live longer, more active lives with the help of antiviral treatments, they are now more often having joint replacement surgeries. But HIV, HBV, and HCV can increase the risk of postoperative complications, say researchers from Duke University in North Carolina.
The researchers studied 16,338 patients in 4 cohorts: those with HIV, HBV, HCV, and HIV plus HBV or HCV. They evaluated the patients at 30 days, 90 days, and 2 years after total joint arthroplasty (TJA), comparing their progress with that of a control group.
Patients with HBV and HCV were at risk of both acute and long-term medical and surgical complications. At 90 days and 2 years, the participants had increased risk of pneumonia, sepsis, joint infection, and revision surgery. They also had a greater risk of complications than did patients with HIV, especially for infection, within the first 90 days postsurgery.
Notably, after TJA, patients with HCV had increased risk of acute kidney injury, sepsis, and transfusion at 30 and 90 days. After hip replacement, patients with HBV had a higher risk of acute kidney injury, pneumonia, and transfusion at 30 and 90 days.
Only 364 patients in the study had both HIV and HBV or HBC, but they did have a greater risk of transfusion at 30 and 90 days following both knee and hip surgery.
Following total hip arthroplasty, patients with HIV were more at risk for deep vein thrombosis and transfusion rather than infection. The lower incidence of infection is likely to be related to effective highly active antiretroviral therapy, the researchers say. HIV patients also were more likely to have mechanical complications, such as loosening, periprosthetic fracture, and revision at 90 days, but not at 2 years. Patients with HIV who underwent total knee arthroplasty (TKA) had a higher risk of death at 30 days and medical complications at 90 days. At 2 years, they had a higher risk of periprosthetic joint infection, irrigation and debridement, and revision. The researchers say the higher incidence of mechanical complications can be explained by the younger, more active, and healthy HIV patients in the cohort—the majority were aged younger than 65 years. They also emphasize that the only risk of infection following TJA in their study was 2 years after TKA.
The researchers suggest that their findings could help prompt future guidelines supporting routine screening prior to elective TJA.
Source:
Kildow BJ, Politzer CS, DiLallo M, Bolognesi MP, Seyler TM. J Arthroplasty. 2018;33(suppl 7):S86-S92.
doi: 10.1016/j.arth.2017.10.061.
EC approves CAR T-cell therapy for DLBCL, PMBCL
The European Commission (EC) has approved the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®) to treat two types of lymphoma.
Axicabtagene ciloleucel is now approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy.
The approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival rate was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome.
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher cytokine release syndrome occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.
The European Commission (EC) has approved the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®) to treat two types of lymphoma.
Axicabtagene ciloleucel is now approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy.
The approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival rate was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome.
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher cytokine release syndrome occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.
The European Commission (EC) has approved the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®) to treat two types of lymphoma.
Axicabtagene ciloleucel is now approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy.
The approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival rate was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome.
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher cytokine release syndrome occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.
EC approves product for high-risk AML
The European Commission (EC) has approved CPX-351 (Vyxeos), a liposomal formulation that delivers a fixed ratio of daunorubicin and cytarabine (44 mg/100 mg).
CPX-351 is approved to treat adults with newly diagnosed, therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The approval extends to all European Union member states as well as Iceland, Norway, and Liechtenstein.
The EC’s approval is supported by data from 5 studies, including a phase 3 trial.
Data from the phase 3 trial were published in the Journal of Clinical Oncology in July.
The trial enrolled 309 patients, ages 60 to 75, with newly diagnosed, therapy-related AML or AML with myelodysplasia-related changes.
Patients received CPX-351 (n=153) or cytarabine and daunorubicin (7+3; n=156).
The overall remission rate—the rate of complete response (CR) plus CR with incomplete count recovery—was 47.7% in the CPX-351 arm and 33.3% in the 7+3 arm (P=0.016). The CR rate was 37.3% and 25.6%, respectively (P=0.040).
About 30% of patients went on to allogeneic hematopoietic stem cell transplant—34% in the CPX-351 arm and 25% in the 7+3 arm (P=0.098).
The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm (P=0.003). The median event-free survival was 2.53 months and 1.31 months, respectively (P=0.021).
Adverse events (AEs) that led to treatment discontinuation in the CPX-351 arm were cardiac failure (n=1), cardiomyopathy (n=1), and acute renal failure (n=1). AEs that led to treatment discontinuation in the 7+3 arm were decreased ejection fraction in 2 patients.
The most common grade 3 to 5 AEs (in the CPX-351 and 7+3 arms, respectively) were infection-related events (83.7% and 86.1%), febrile neutropenia (68.0% and 70.9%), pneumonia (19.6% and 14.6%), hypoxia (13.1% and 15.2%), and bleeding events (11.8% and 8.6%).
Ultimately, 69.3% of patients in the CPX-351 arm and 84.8% of those in the 7+3 arm died.
Deaths (in the CPX-351 and 7+3 cohorts, respectively) were due to progressive disease (n=65 and 67), AEs (n=15 and 19), cancer-related organ failure in the absence of progressive disease (n=0 and 5), and unknown/other causes (n=26 and 37).
The European Commission (EC) has approved CPX-351 (Vyxeos), a liposomal formulation that delivers a fixed ratio of daunorubicin and cytarabine (44 mg/100 mg).
CPX-351 is approved to treat adults with newly diagnosed, therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The approval extends to all European Union member states as well as Iceland, Norway, and Liechtenstein.
The EC’s approval is supported by data from 5 studies, including a phase 3 trial.
Data from the phase 3 trial were published in the Journal of Clinical Oncology in July.
The trial enrolled 309 patients, ages 60 to 75, with newly diagnosed, therapy-related AML or AML with myelodysplasia-related changes.
Patients received CPX-351 (n=153) or cytarabine and daunorubicin (7+3; n=156).
The overall remission rate—the rate of complete response (CR) plus CR with incomplete count recovery—was 47.7% in the CPX-351 arm and 33.3% in the 7+3 arm (P=0.016). The CR rate was 37.3% and 25.6%, respectively (P=0.040).
About 30% of patients went on to allogeneic hematopoietic stem cell transplant—34% in the CPX-351 arm and 25% in the 7+3 arm (P=0.098).
The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm (P=0.003). The median event-free survival was 2.53 months and 1.31 months, respectively (P=0.021).
Adverse events (AEs) that led to treatment discontinuation in the CPX-351 arm were cardiac failure (n=1), cardiomyopathy (n=1), and acute renal failure (n=1). AEs that led to treatment discontinuation in the 7+3 arm were decreased ejection fraction in 2 patients.
The most common grade 3 to 5 AEs (in the CPX-351 and 7+3 arms, respectively) were infection-related events (83.7% and 86.1%), febrile neutropenia (68.0% and 70.9%), pneumonia (19.6% and 14.6%), hypoxia (13.1% and 15.2%), and bleeding events (11.8% and 8.6%).
Ultimately, 69.3% of patients in the CPX-351 arm and 84.8% of those in the 7+3 arm died.
Deaths (in the CPX-351 and 7+3 cohorts, respectively) were due to progressive disease (n=65 and 67), AEs (n=15 and 19), cancer-related organ failure in the absence of progressive disease (n=0 and 5), and unknown/other causes (n=26 and 37).
The European Commission (EC) has approved CPX-351 (Vyxeos), a liposomal formulation that delivers a fixed ratio of daunorubicin and cytarabine (44 mg/100 mg).
CPX-351 is approved to treat adults with newly diagnosed, therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The approval extends to all European Union member states as well as Iceland, Norway, and Liechtenstein.
The EC’s approval is supported by data from 5 studies, including a phase 3 trial.
Data from the phase 3 trial were published in the Journal of Clinical Oncology in July.
The trial enrolled 309 patients, ages 60 to 75, with newly diagnosed, therapy-related AML or AML with myelodysplasia-related changes.
Patients received CPX-351 (n=153) or cytarabine and daunorubicin (7+3; n=156).
The overall remission rate—the rate of complete response (CR) plus CR with incomplete count recovery—was 47.7% in the CPX-351 arm and 33.3% in the 7+3 arm (P=0.016). The CR rate was 37.3% and 25.6%, respectively (P=0.040).
About 30% of patients went on to allogeneic hematopoietic stem cell transplant—34% in the CPX-351 arm and 25% in the 7+3 arm (P=0.098).
The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm (P=0.003). The median event-free survival was 2.53 months and 1.31 months, respectively (P=0.021).
Adverse events (AEs) that led to treatment discontinuation in the CPX-351 arm were cardiac failure (n=1), cardiomyopathy (n=1), and acute renal failure (n=1). AEs that led to treatment discontinuation in the 7+3 arm were decreased ejection fraction in 2 patients.
The most common grade 3 to 5 AEs (in the CPX-351 and 7+3 arms, respectively) were infection-related events (83.7% and 86.1%), febrile neutropenia (68.0% and 70.9%), pneumonia (19.6% and 14.6%), hypoxia (13.1% and 15.2%), and bleeding events (11.8% and 8.6%).
Ultimately, 69.3% of patients in the CPX-351 arm and 84.8% of those in the 7+3 arm died.
Deaths (in the CPX-351 and 7+3 cohorts, respectively) were due to progressive disease (n=65 and 67), AEs (n=15 and 19), cancer-related organ failure in the absence of progressive disease (n=0 and 5), and unknown/other causes (n=26 and 37).
FDA grants fast track designation to CX-01 for AML
The US Food and Drug Administration (FDA) has granted fast track designation to CX-01 as a treatment for patients older than 60 receiving induction therapy for newly diagnosed acute myeloid leukemia (AML).
CX-01 also has orphan drug designation from the FDA.
CX-01 is a polysaccharide derived from heparin that is thought to enhance chemotherapy by disrupting the adhesion of leukemia cells in the bone marrow.
CX-01 inhibits the activity of HMGB1, disrupts the CXCL12/CXCR4 axis, and neutralizes the activity of platelet factor 4. HMGB1 has been implicated in autophagy, a mechanism by which cells withstand the effects of chemotherapy.
The CXCL12/CXCR4 axis is thought to be involved in protecting leukemia cells from chemotherapy. And platelet factor 4 inhibits bone marrow recovery after chemotherapy.
CX-01 research
Cantex Pharmaceuticals, Inc., is conducting a randomized, phase 2b study to determine whether CX-01 can improve the efficacy of frontline chemotherapy in patients with AML.
This study builds upon results of a pilot study, which were published in Blood Advances in February.
The study enrolled 12 adults with newly diagnosed AML. Patients had good-risk (n=3), intermediate-risk (n=5), and poor-risk (n=4) disease.
They received CX-01 as a 7-day continuous infusion, along with standard induction chemotherapy (cytarabine and idarubicin).
Eleven patients (92%) achieved morphologic complete remission after one cycle of induction. This includes two patients who did not complete induction. All patients received subsequent therapy—consolidation, salvage, or transplant—on- or off-study.
At a median follow-up of 24 months, 8 patients were still alive. Two patients died of transplant-related complications, one died of infectious complications, and one died of cerebral hemorrhage.
The median disease-free survival was 14.8 months, and the median overall survival was not reached.
There were five serious adverse events (AEs) in five patients. Most of these AEs were considered unrelated to CX-01, but a case of grade 4 sepsis was considered possibly related to CX-01.
Transient, asymptomatic, low-grade elevations of liver transaminases observed during induction were considered possibly related to CX-01. There were also transient, asymptomatic, grade 3-4 liver transaminase elevations observed during consolidation that were considered possibly related to CX-01.
The researchers said the most frequent nonserious AEs were hematologic toxicities, infectious complications, and organ toxicity complications resulting from treatment and/or the underlying leukemia.
About fast track, orphan designations
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted fast track designation to CX-01 as a treatment for patients older than 60 receiving induction therapy for newly diagnosed acute myeloid leukemia (AML).
CX-01 also has orphan drug designation from the FDA.
CX-01 is a polysaccharide derived from heparin that is thought to enhance chemotherapy by disrupting the adhesion of leukemia cells in the bone marrow.
CX-01 inhibits the activity of HMGB1, disrupts the CXCL12/CXCR4 axis, and neutralizes the activity of platelet factor 4. HMGB1 has been implicated in autophagy, a mechanism by which cells withstand the effects of chemotherapy.
The CXCL12/CXCR4 axis is thought to be involved in protecting leukemia cells from chemotherapy. And platelet factor 4 inhibits bone marrow recovery after chemotherapy.
CX-01 research
Cantex Pharmaceuticals, Inc., is conducting a randomized, phase 2b study to determine whether CX-01 can improve the efficacy of frontline chemotherapy in patients with AML.
This study builds upon results of a pilot study, which were published in Blood Advances in February.
The study enrolled 12 adults with newly diagnosed AML. Patients had good-risk (n=3), intermediate-risk (n=5), and poor-risk (n=4) disease.
They received CX-01 as a 7-day continuous infusion, along with standard induction chemotherapy (cytarabine and idarubicin).
Eleven patients (92%) achieved morphologic complete remission after one cycle of induction. This includes two patients who did not complete induction. All patients received subsequent therapy—consolidation, salvage, or transplant—on- or off-study.
At a median follow-up of 24 months, 8 patients were still alive. Two patients died of transplant-related complications, one died of infectious complications, and one died of cerebral hemorrhage.
The median disease-free survival was 14.8 months, and the median overall survival was not reached.
There were five serious adverse events (AEs) in five patients. Most of these AEs were considered unrelated to CX-01, but a case of grade 4 sepsis was considered possibly related to CX-01.
Transient, asymptomatic, low-grade elevations of liver transaminases observed during induction were considered possibly related to CX-01. There were also transient, asymptomatic, grade 3-4 liver transaminase elevations observed during consolidation that were considered possibly related to CX-01.
The researchers said the most frequent nonserious AEs were hematologic toxicities, infectious complications, and organ toxicity complications resulting from treatment and/or the underlying leukemia.
About fast track, orphan designations
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted fast track designation to CX-01 as a treatment for patients older than 60 receiving induction therapy for newly diagnosed acute myeloid leukemia (AML).
CX-01 also has orphan drug designation from the FDA.
CX-01 is a polysaccharide derived from heparin that is thought to enhance chemotherapy by disrupting the adhesion of leukemia cells in the bone marrow.
CX-01 inhibits the activity of HMGB1, disrupts the CXCL12/CXCR4 axis, and neutralizes the activity of platelet factor 4. HMGB1 has been implicated in autophagy, a mechanism by which cells withstand the effects of chemotherapy.
The CXCL12/CXCR4 axis is thought to be involved in protecting leukemia cells from chemotherapy. And platelet factor 4 inhibits bone marrow recovery after chemotherapy.
CX-01 research
Cantex Pharmaceuticals, Inc., is conducting a randomized, phase 2b study to determine whether CX-01 can improve the efficacy of frontline chemotherapy in patients with AML.
This study builds upon results of a pilot study, which were published in Blood Advances in February.
The study enrolled 12 adults with newly diagnosed AML. Patients had good-risk (n=3), intermediate-risk (n=5), and poor-risk (n=4) disease.
They received CX-01 as a 7-day continuous infusion, along with standard induction chemotherapy (cytarabine and idarubicin).
Eleven patients (92%) achieved morphologic complete remission after one cycle of induction. This includes two patients who did not complete induction. All patients received subsequent therapy—consolidation, salvage, or transplant—on- or off-study.
At a median follow-up of 24 months, 8 patients were still alive. Two patients died of transplant-related complications, one died of infectious complications, and one died of cerebral hemorrhage.
The median disease-free survival was 14.8 months, and the median overall survival was not reached.
There were five serious adverse events (AEs) in five patients. Most of these AEs were considered unrelated to CX-01, but a case of grade 4 sepsis was considered possibly related to CX-01.
Transient, asymptomatic, low-grade elevations of liver transaminases observed during induction were considered possibly related to CX-01. There were also transient, asymptomatic, grade 3-4 liver transaminase elevations observed during consolidation that were considered possibly related to CX-01.
The researchers said the most frequent nonserious AEs were hematologic toxicities, infectious complications, and organ toxicity complications resulting from treatment and/or the underlying leukemia.
About fast track, orphan designations
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
Negative chest x-ray to rule out pediatric pneumonia
, researchers say.
In a paper published in the September issue of Pediatrics, researchers report the results of a prospective cohort study in 683 children – with a median age of 3.1 years – presenting to emergency departments with suspected pneumonia.
Dr. Susan C. Lipsett, from the division of emergency medicine at Boston Children’s Hospital, and co-authors, wrote that the use of chest radiograph to diagnose pneumonia is thought to have limitations such as its inability to distinguish between bacteria and viral infection, and the possible absence of radiographic presentations early in the disease in patients with dehydration.
In this study, 457 (72.8%) of the children had negative chest radiographs. Of these, 44 were clinically diagnosed with pneumonia, despite the radiograph results, and prescribed antibiotics. These children were more likely to have rales or respiratory distress and less likely to have wheezing compared with the children with negative radiographs who were not initially diagnosed with pneumonia.
Among the remaining 411 children with negative radiographs – who were not prescribed antibiotics – five (1.2%) were subsequently diagnosed with pneumonia within 2 weeks of the radiograph. These five children were all under 3 years of age, but none had been treated with intravenous fluids for dehydration. Only one had radiographic findings of pneumonia on a follow-up visit.
Counting the 44 children diagnosed with pneumonia despite the negative x-ray, chest radiography showed a negative predictive value of 89.2% (95% confidence interval, 85.9%-91.9%). Without those children, the negative predictive value was 98.8% (95% CI, 97%-99.6%).
There were also 113 children (16.5%) with positive chest radiographs, and 72 (10.7%) with equivocal radiographs.
The authors said their results showed that most children with negative chest radiograph would recover fully without needing antibiotics, and argued there was a place for chest radiography in the diagnostic process, to rule out bacterial pneumonia.
“Most clinicians caring for children in the outpatient setting rely on clinical signs and symptoms to determine whether to prescribe an antibiotic for the treatment of pneumonia,” they wrote. “However, given recent literature in which the poor reliability and validity of physical examination findings are cited, reliance on physical examination alone may lead to the overdiagnosis of pneumonia.”
They acknowledged that the lack of a universally accepted gold standard for the diagnosis of pneumonia in children was a significant limitation of the research. In addition, the lack of systematic radiographs meant some children who initially had a negative result and recovered without antibiotics may have shown a positive result on a second scan.
No conflicts of interest were declared.
SOURCE: Lipsett S et al. Pediatrics 2018 142(3):e20180236.
While the results of this study offer reassurance that chest radiograph for suspected pneumonia in children has a high negative predictive value, perhaps a more important question is the accuracy of chest radiography at ruling in bacterial pneumonia – its positive predictive value.
There are reasons to suspect that the positive predictive value of chest radiography may not be as high as the negative predictive value found in this study. This is particularly important given that questions have been raised about the utility of antibiotic therapy in treating Mycoplasma pneumonia infection in children.
Leaving out chest radiography altogether in children with a low clinical suspicion for pneumonia would decreased radi-ation use, cost, and perhaps also unnecessary antibiotic prescriptions.
Matthew D. Garber, MD, is from the department of pediatrics at the University of Florida College of Medicine in Jacksonville and Ricardo A. Quinonez, MD, is from the department of pediatrics at the Baylor College of Medicine and Texas Children’s Hospital. These comments are taken from an accompanying editorial (Pediatrics 2018, 142(3): e20182025. https://doi.org/10.1542/peds.2018-2025). No conflicts of interest were declared.
While the results of this study offer reassurance that chest radiograph for suspected pneumonia in children has a high negative predictive value, perhaps a more important question is the accuracy of chest radiography at ruling in bacterial pneumonia – its positive predictive value.
There are reasons to suspect that the positive predictive value of chest radiography may not be as high as the negative predictive value found in this study. This is particularly important given that questions have been raised about the utility of antibiotic therapy in treating Mycoplasma pneumonia infection in children.
Leaving out chest radiography altogether in children with a low clinical suspicion for pneumonia would decreased radi-ation use, cost, and perhaps also unnecessary antibiotic prescriptions.
Matthew D. Garber, MD, is from the department of pediatrics at the University of Florida College of Medicine in Jacksonville and Ricardo A. Quinonez, MD, is from the department of pediatrics at the Baylor College of Medicine and Texas Children’s Hospital. These comments are taken from an accompanying editorial (Pediatrics 2018, 142(3): e20182025. https://doi.org/10.1542/peds.2018-2025). No conflicts of interest were declared.
While the results of this study offer reassurance that chest radiograph for suspected pneumonia in children has a high negative predictive value, perhaps a more important question is the accuracy of chest radiography at ruling in bacterial pneumonia – its positive predictive value.
There are reasons to suspect that the positive predictive value of chest radiography may not be as high as the negative predictive value found in this study. This is particularly important given that questions have been raised about the utility of antibiotic therapy in treating Mycoplasma pneumonia infection in children.
Leaving out chest radiography altogether in children with a low clinical suspicion for pneumonia would decreased radi-ation use, cost, and perhaps also unnecessary antibiotic prescriptions.
Matthew D. Garber, MD, is from the department of pediatrics at the University of Florida College of Medicine in Jacksonville and Ricardo A. Quinonez, MD, is from the department of pediatrics at the Baylor College of Medicine and Texas Children’s Hospital. These comments are taken from an accompanying editorial (Pediatrics 2018, 142(3): e20182025. https://doi.org/10.1542/peds.2018-2025). No conflicts of interest were declared.
, researchers say.
In a paper published in the September issue of Pediatrics, researchers report the results of a prospective cohort study in 683 children – with a median age of 3.1 years – presenting to emergency departments with suspected pneumonia.
Dr. Susan C. Lipsett, from the division of emergency medicine at Boston Children’s Hospital, and co-authors, wrote that the use of chest radiograph to diagnose pneumonia is thought to have limitations such as its inability to distinguish between bacteria and viral infection, and the possible absence of radiographic presentations early in the disease in patients with dehydration.
In this study, 457 (72.8%) of the children had negative chest radiographs. Of these, 44 were clinically diagnosed with pneumonia, despite the radiograph results, and prescribed antibiotics. These children were more likely to have rales or respiratory distress and less likely to have wheezing compared with the children with negative radiographs who were not initially diagnosed with pneumonia.
Among the remaining 411 children with negative radiographs – who were not prescribed antibiotics – five (1.2%) were subsequently diagnosed with pneumonia within 2 weeks of the radiograph. These five children were all under 3 years of age, but none had been treated with intravenous fluids for dehydration. Only one had radiographic findings of pneumonia on a follow-up visit.
Counting the 44 children diagnosed with pneumonia despite the negative x-ray, chest radiography showed a negative predictive value of 89.2% (95% confidence interval, 85.9%-91.9%). Without those children, the negative predictive value was 98.8% (95% CI, 97%-99.6%).
There were also 113 children (16.5%) with positive chest radiographs, and 72 (10.7%) with equivocal radiographs.
The authors said their results showed that most children with negative chest radiograph would recover fully without needing antibiotics, and argued there was a place for chest radiography in the diagnostic process, to rule out bacterial pneumonia.
“Most clinicians caring for children in the outpatient setting rely on clinical signs and symptoms to determine whether to prescribe an antibiotic for the treatment of pneumonia,” they wrote. “However, given recent literature in which the poor reliability and validity of physical examination findings are cited, reliance on physical examination alone may lead to the overdiagnosis of pneumonia.”
They acknowledged that the lack of a universally accepted gold standard for the diagnosis of pneumonia in children was a significant limitation of the research. In addition, the lack of systematic radiographs meant some children who initially had a negative result and recovered without antibiotics may have shown a positive result on a second scan.
No conflicts of interest were declared.
SOURCE: Lipsett S et al. Pediatrics 2018 142(3):e20180236.
, researchers say.
In a paper published in the September issue of Pediatrics, researchers report the results of a prospective cohort study in 683 children – with a median age of 3.1 years – presenting to emergency departments with suspected pneumonia.
Dr. Susan C. Lipsett, from the division of emergency medicine at Boston Children’s Hospital, and co-authors, wrote that the use of chest radiograph to diagnose pneumonia is thought to have limitations such as its inability to distinguish between bacteria and viral infection, and the possible absence of radiographic presentations early in the disease in patients with dehydration.
In this study, 457 (72.8%) of the children had negative chest radiographs. Of these, 44 were clinically diagnosed with pneumonia, despite the radiograph results, and prescribed antibiotics. These children were more likely to have rales or respiratory distress and less likely to have wheezing compared with the children with negative radiographs who were not initially diagnosed with pneumonia.
Among the remaining 411 children with negative radiographs – who were not prescribed antibiotics – five (1.2%) were subsequently diagnosed with pneumonia within 2 weeks of the radiograph. These five children were all under 3 years of age, but none had been treated with intravenous fluids for dehydration. Only one had radiographic findings of pneumonia on a follow-up visit.
Counting the 44 children diagnosed with pneumonia despite the negative x-ray, chest radiography showed a negative predictive value of 89.2% (95% confidence interval, 85.9%-91.9%). Without those children, the negative predictive value was 98.8% (95% CI, 97%-99.6%).
There were also 113 children (16.5%) with positive chest radiographs, and 72 (10.7%) with equivocal radiographs.
The authors said their results showed that most children with negative chest radiograph would recover fully without needing antibiotics, and argued there was a place for chest radiography in the diagnostic process, to rule out bacterial pneumonia.
“Most clinicians caring for children in the outpatient setting rely on clinical signs and symptoms to determine whether to prescribe an antibiotic for the treatment of pneumonia,” they wrote. “However, given recent literature in which the poor reliability and validity of physical examination findings are cited, reliance on physical examination alone may lead to the overdiagnosis of pneumonia.”
They acknowledged that the lack of a universally accepted gold standard for the diagnosis of pneumonia in children was a significant limitation of the research. In addition, the lack of systematic radiographs meant some children who initially had a negative result and recovered without antibiotics may have shown a positive result on a second scan.
No conflicts of interest were declared.
SOURCE: Lipsett S et al. Pediatrics 2018 142(3):e20180236.
FROM PEDIATRICS
Key clinical point: Negative chest radiograph can rule out pneumonia in children.
Major finding: Chest radiograph has a negative predictive value of 89.2% in children with suspected pneumonia.
Study details: Prospective cohort study in 683 children with suspected pneumonia.
Disclosures: No conflicts of interest were declared.
Source: Lipsett S et al. Pediatrics 2018 142(3):e20180236. https://doi.org/10.1542/peds.2018-0236.