User login
Five-year survival for non-Hodgkin lymphoma tops 71%
The overall 5-year survival rate for non-Hodgkin lymphoma (NHL) is 71.4%, according to the National Cancer Institute.
That number falls neatly into the middle of the range for survival by stage at diagnosis, with stage I (81.8%) and stage II (75.3%) disease on the high side and stage III (69.1%) and stage IV (61.7%) on the low side, the most recent data from the Surveillance, Epidemiology, and End Results (SEER) Program show. Five-year survival for NHL of unknown stage at diagnosis is 76.4%.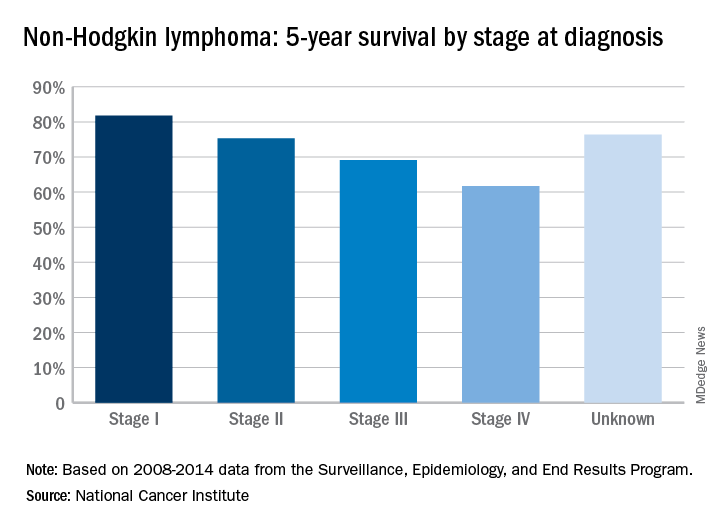
The overall 5-year survival rate for non-Hodgkin lymphoma (NHL) is 71.4%, according to the National Cancer Institute.
That number falls neatly into the middle of the range for survival by stage at diagnosis, with stage I (81.8%) and stage II (75.3%) disease on the high side and stage III (69.1%) and stage IV (61.7%) on the low side, the most recent data from the Surveillance, Epidemiology, and End Results (SEER) Program show. Five-year survival for NHL of unknown stage at diagnosis is 76.4%.
The overall 5-year survival rate for non-Hodgkin lymphoma (NHL) is 71.4%, according to the National Cancer Institute.
That number falls neatly into the middle of the range for survival by stage at diagnosis, with stage I (81.8%) and stage II (75.3%) disease on the high side and stage III (69.1%) and stage IV (61.7%) on the low side, the most recent data from the Surveillance, Epidemiology, and End Results (SEER) Program show. Five-year survival for NHL of unknown stage at diagnosis is 76.4%.
Does warfarin cause acute kidney injury?
SAN DIEGO – Patients with chronic kidney disease (CKD) and those on renin angiotensin system inhibitors and/or diuretics should have their renal function monitored during periods of overanticoagulation, results from a large retrospective study suggest.
“Unfortunately, warfarin-related nephropathy is quite hard to study,” Hugh Traquair, MD, the study’s lead author, said in an interview at the biennial summit of the Thrombosis & Hemostasis Societies of North America. “The best way to establish diagnosis is with a kidney biopsy. No one is very keen to stick a needle into a kidney when someone’s overanticoagulated. It’s been observed previously that acute kidney injury related to over-anticoagulation is more common in people with CKD, but we don’t know more about risk factors.”
The primary outcome was AKI, defined as an acute increase in creatinine of greater than 26.5 micromol/L within 7-14 days of an INR 4 or greater. The secondary outcome was creatinine level within 3 months of the abnormal INR. The researchers excluded patients with AKI due to another cause, and those who lacked a creatinine level at baseline, within 7-14 days of an INR of 4 or greater, and/or at 3 months.
The median age of the 292 patients was 79 years, 55% were male, 30% were taking aspirin, and 77% were taking renin angiotensin inhibitors and/or diuretics. The control group consisted of 93 patients with a 12-month time in therapeutic range of 100%. The median age of controls was 68 years, 67% were male, and 9% had CKD. None of the controls had an AKI, said Dr. Traquair, a second-year internal medicine resident in the department of medicine at McMaster University.
Of the 292 patients with an INR of 4 or greater, 13% had an AKI, and the incidence of AKI was significantly higher in the CKD patients, compared with those who had a normal baseline creatinine level (19% vs. 10%; odds ratio, 2.1; P less than .05).
In a binomial logistic regression model, diuretic use was the only significant predictor of AKI (OR 3.4; P less than .05). The researchers also found that of the 52 patients with an INR of 4 or greater who did not use renin angiotensin system inhibitors and/or diuretics and did not have CKD, only 1 had an AKI (2%).
“We don’t know that all of these episodes of AKI are related to warfarin, but we do see a definite increase of AKI after an episode of overanticoagulation (an INR greater than 4),” Dr. Traquair said. “In patients who are at risk for AKI, monitoring their kidney function after an episode of overanticoagulation is probably warranted.”
Dr. Traquair reported having no financial disclosures.
SOURCE: Traquair H et al. THSNA 2018, Poster 79.
SAN DIEGO – Patients with chronic kidney disease (CKD) and those on renin angiotensin system inhibitors and/or diuretics should have their renal function monitored during periods of overanticoagulation, results from a large retrospective study suggest.
“Unfortunately, warfarin-related nephropathy is quite hard to study,” Hugh Traquair, MD, the study’s lead author, said in an interview at the biennial summit of the Thrombosis & Hemostasis Societies of North America. “The best way to establish diagnosis is with a kidney biopsy. No one is very keen to stick a needle into a kidney when someone’s overanticoagulated. It’s been observed previously that acute kidney injury related to over-anticoagulation is more common in people with CKD, but we don’t know more about risk factors.”
The primary outcome was AKI, defined as an acute increase in creatinine of greater than 26.5 micromol/L within 7-14 days of an INR 4 or greater. The secondary outcome was creatinine level within 3 months of the abnormal INR. The researchers excluded patients with AKI due to another cause, and those who lacked a creatinine level at baseline, within 7-14 days of an INR of 4 or greater, and/or at 3 months.
The median age of the 292 patients was 79 years, 55% were male, 30% were taking aspirin, and 77% were taking renin angiotensin inhibitors and/or diuretics. The control group consisted of 93 patients with a 12-month time in therapeutic range of 100%. The median age of controls was 68 years, 67% were male, and 9% had CKD. None of the controls had an AKI, said Dr. Traquair, a second-year internal medicine resident in the department of medicine at McMaster University.
Of the 292 patients with an INR of 4 or greater, 13% had an AKI, and the incidence of AKI was significantly higher in the CKD patients, compared with those who had a normal baseline creatinine level (19% vs. 10%; odds ratio, 2.1; P less than .05).
In a binomial logistic regression model, diuretic use was the only significant predictor of AKI (OR 3.4; P less than .05). The researchers also found that of the 52 patients with an INR of 4 or greater who did not use renin angiotensin system inhibitors and/or diuretics and did not have CKD, only 1 had an AKI (2%).
“We don’t know that all of these episodes of AKI are related to warfarin, but we do see a definite increase of AKI after an episode of overanticoagulation (an INR greater than 4),” Dr. Traquair said. “In patients who are at risk for AKI, monitoring their kidney function after an episode of overanticoagulation is probably warranted.”
Dr. Traquair reported having no financial disclosures.
SOURCE: Traquair H et al. THSNA 2018, Poster 79.
SAN DIEGO – Patients with chronic kidney disease (CKD) and those on renin angiotensin system inhibitors and/or diuretics should have their renal function monitored during periods of overanticoagulation, results from a large retrospective study suggest.
“Unfortunately, warfarin-related nephropathy is quite hard to study,” Hugh Traquair, MD, the study’s lead author, said in an interview at the biennial summit of the Thrombosis & Hemostasis Societies of North America. “The best way to establish diagnosis is with a kidney biopsy. No one is very keen to stick a needle into a kidney when someone’s overanticoagulated. It’s been observed previously that acute kidney injury related to over-anticoagulation is more common in people with CKD, but we don’t know more about risk factors.”
The primary outcome was AKI, defined as an acute increase in creatinine of greater than 26.5 micromol/L within 7-14 days of an INR 4 or greater. The secondary outcome was creatinine level within 3 months of the abnormal INR. The researchers excluded patients with AKI due to another cause, and those who lacked a creatinine level at baseline, within 7-14 days of an INR of 4 or greater, and/or at 3 months.
The median age of the 292 patients was 79 years, 55% were male, 30% were taking aspirin, and 77% were taking renin angiotensin inhibitors and/or diuretics. The control group consisted of 93 patients with a 12-month time in therapeutic range of 100%. The median age of controls was 68 years, 67% were male, and 9% had CKD. None of the controls had an AKI, said Dr. Traquair, a second-year internal medicine resident in the department of medicine at McMaster University.
Of the 292 patients with an INR of 4 or greater, 13% had an AKI, and the incidence of AKI was significantly higher in the CKD patients, compared with those who had a normal baseline creatinine level (19% vs. 10%; odds ratio, 2.1; P less than .05).
In a binomial logistic regression model, diuretic use was the only significant predictor of AKI (OR 3.4; P less than .05). The researchers also found that of the 52 patients with an INR of 4 or greater who did not use renin angiotensin system inhibitors and/or diuretics and did not have CKD, only 1 had an AKI (2%).
“We don’t know that all of these episodes of AKI are related to warfarin, but we do see a definite increase of AKI after an episode of overanticoagulation (an INR greater than 4),” Dr. Traquair said. “In patients who are at risk for AKI, monitoring their kidney function after an episode of overanticoagulation is probably warranted.”
Dr. Traquair reported having no financial disclosures.
SOURCE: Traquair H et al. THSNA 2018, Poster 79.
REPORTING FROM THSNA 2018
Key clinical point:
Major finding: Among patients with warfarin anticoagulation, 13% had an acute kidney injury.
Study details: A retrospective study of 292 patients with an INR of 4.0 or greater who were treated between 2007 and 2017.
Disclosures: Dr. Traquair reported having no financial disclosures.
Source: Traquair H et al. THSNA 2018, Poster 79.
Association of Dioxin and Dioxin-like Congeners With Hypertension
Persistent organic pollutants (POPs), endocrine-disrupting, lipophilic chemicals that concentrate in adipose tissue, increasingly are being studied for a wide range of health effects.1 Persistent organic pollutants include bisphenol A, phthalates, dioxins, hexachlorobenzene, dichlorodiphenyltrichloroethane (DDT), polybrominated diphenyl ethers, and polychlorinated biphenyls (PCBs). Chlorinated dibenzo-p-dioxins are known as polychlorinated dibenzodioxins (PCDDs), or simply dioxins. Categorization of this group of chemicals is based on the structural chlorinated constituents. Of the 75 congener molecules, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic, and this dioxin, given its more serious health implications, has been studied the most.1,2
Because it was a contaminant in the herbicide Agent Orange, the main defoliant used by the US military in southern Vietnam during the Vietnam War, TCDD is of primary interest. Agent Orange consists of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in equal parts. Like other dioxins, TCDD is lipophilic and retained in adipose tissue.1,3 Contemporaneous sources include occupational and residential exposure from pulp and paper mills, metallurgy, incinerators, industrial waste, fossil fuel combustion, and industrial accidents and poisonings.1-4
Another main class of POPs, polychlorinated benzenes, includes 209 synthetic PCB congener chemicals, a subset of which is referred to as dioxin-like PCBs.1 Organochlorine (OC) pesticides and PCBs were once manufactured as lubricants and coolants for electronics but are now banned; nevertheless, they remain concentrated in fish and mammals and persist in the food chain.3,5,6 These chemicals of interest (COIs) are graded for toxicity based on toxic equivalency factors relative to TCDDs in a 2005 World Health Organization assessment.3
Polychlorinated dibenzofurans (PCDFs), TCDD, PCBs, PCDDs, and other environmental toxins are being studied as possible contributing factors in the development of hypertension. The authors review the results of several recent studies on COI exposure and hypertension.
In 2017, the American College of Cardiology and the American Heart Association lowered the threshold for hypertension to systolic blood pressure (SBP) > 130 mm Hg and diastolic blood pressure (DBP) > 80 mm Hg.7 This new guideline would categorize 46% of the US population as having hypertension, compared with 32% under the former cutoff of 140/90 mm Hg.7 Modifiable factors (eg, diet, body mass index [BMI], smoking, alcohol, physical activity) and nonmodifiable factors (eg, age, family history, sex, race/ethnicity) have a role in the pathophysiology of hypertension. Between 90% and 95% of hypertension is considered primary. Hypertension increases the risk of developing ischemic heart disease, atherosclerosis, aortic dissection, small blood vessel disease, congestive heart failure, and renal failure, and thus results in considerable morbidity and mortality each year.8
Contaminant Exposure and Hypertension Risk
Vietnam-Era Army Chemical Corps
The US Army Chemical Corps (ACC) Vietnam-Era Veterans Health Study (2012-2013) recorded the long-term health burdens imposed by Agent Orange exposure and Vietnam War service.9,10 This cross-sectional study reexamined a subset of 5,609 Vietnam-era ACC veterans for an association of self-reported, physician-diagnosed hypertension (≥ 140/90 mm Hg) and herbicide spraying history confirmed with serum TCDD levels. The 22 Army units that made up the ACC were in charge of spreading Agent Orange and other defoliants on opposition camps between 1965 and 1973. The herbicide was dispersed aerially and on the ground. The ACC was also responsible for dispensing napalm, tear gas, and other chemicals.
A previous phone survey found an association of self-reported hypertension and herbicide spraying in ACC veterans with associated Vietnam service and herbicide spraying history, verified with serum TCDD levels (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.00-1.58).9 Median age of ACC veterans with Vietnam War service at the time of the survey was 53 years.
The 2012-2013 study assessed respondents with a record of their serum TCDD measurements from the time of the survey.10 Most of the respondents were aged in their 60s. The stated purpose of the health study was to examine the association of Vietnam veterans’ occupational herbicide exposure and hypertension risk, rather than isolate a certain responsible chemical, though serum TCDD levels were used to confirm spraying history. After adjustments for age, tobacco use, alcohol use, race, and BMI but not salt intake, family history of hypertension, psychiatric health, or diabetes mellitus (DM) comorbidity, the OR of self-reported, physician-diagnosed hypertension was 1.74 (95% CI, 1.44-2.11) for sprayers and 1.26 (95% CI, 1.05-1.53) for Vietnam veterans.10
Vietnam War Veterans From Korea
Soldiers of the Republic of Korea (ROK) who fought in the Vietnam War also were exposed to Agent Orange and other defoliants and herbicides. In 2013, Yi and colleagues contacted 187,897 ROK Vietnam veterans to analyze their Agent Orange exposure and self-reported diseases decades after the war.11 By mail, the researchers administered a questionnaire of perceived Agent Orange exposure (eg, spraying, handling spray equipment, having contact with COIs). The Korean veterans were classified by military assignment and by their proximity to areas sprayed with Agent Orange, according to the military records of 3 US combat units: Capital Division, 9th Division, and Marine Second Brigade. The ROK veterans in those units presumably would have similar levels of Agent Orange exposure.
The questionnaire response rate was 69%. The 114,562 respondents were divided into groups based on self-perceived exposure (no, low, moderate, high) and qualitative exposure level, derived from service history (battalion/company, division/brigade). After adjusting for BMI, smoking, alcohol use, physical activity, use of nonoccupational herbicides, education, income, and military rank, Yi and colleagues found a statistically significant association of hypertension and self-reported perceived Agent Orange exposure (P < .001) and a statistically significant association of hypertension and exposure in the division/brigade group with the highest exposure level (P < .001).11 The highest ORs were found for high- vs low-exposure and moderate- vs low-exposure subsets in self-reported perceived Agent Orange levels: 1.60 (95% CI, 1.56-1.65) and 1.70 (95% CI, 1.64-1.77), respectively. However, adjusted ORs in proximity-based exposure for all groups were > 1.03.
Inuits in Canada and Greenland
To study total PCBs, non-dioxin-like PCBs, OC pesticides, and their metabolites in plasma, public health researchers Valera and colleagues focused on the Inuit town of Nunavik (in Canada), where there is contamination from foods like fish, a mainstay of the Inuit diet.5 A health survey was sent to 400 households randomly selected from 1,378 households in 14 villages. Data were collected between September and December 1992. In total, 518 people between ages 18 and 74 years agreed to undergo a physical examination, and 492 agreed to have blood drawn. Laboratories measured serum PCB congeners and 13 chlorinated pesticides or their metabolites. Blood pressure (BP) was measured 3 times, and the last 2 measurements averaged. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg.
Of the 518 participants, 315 (134 men, 181 women) had complete BP, serum POP levels, and confounding variables recorded, and these were subsequently analyzed. Mean age was 32.7 years. Polychlorinated biphenyls congeners 105 and 118 were higher in women than in men; no other congeners were quantitatively different. Associations between POP levels and hypertension were analyzed with multiple logistic regression modeling, with adjustments for age, sex, fasting blood glucose, waist circumference, smoking, alcohol use, and physical activity, as well as the common contaminants lead, mercury, and omega-3 polyunsaturated fatty acids (n-3 PUFA).The researchers adjusted for n-3 PUFA because of the posited BP-lowering effects. Inuits consume large amounts of the polyunsaturated fatty acids DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid).5
Using congeners found in at least 70% of the total samples, the study authors found a statistically significant association between certain PCBs, both dioxin-like (DL-PCBs) and non-dioxin-like (NDL-PCBs), and increased risk of hypertension. Congeners 101, 105 (DL-PCB), 138, and 187, as well as p,p'-DDE, were also significantly associated with a higher risk of hypertension. Congener 99 was associated with increased SBP, and congener 118 (DL-PCB) was associated with increased SBP and DBP. Some congeners, such as the OC pesticides, p,p'-DDT, β-hexachlorocyclohexane, and oxychlordane, were inversely associated with hypertension.
In 2012, Valera and colleagues conducted a similar study of Greenland Inuits who also consume marine mammals and fish and present with high POP levels.6 Despite correcting for n-3 PUFA, they found no significant association involving DL-PCBs, NDL-PCBs, or OC pesticides.
Japanese Background Exposures
Nakamoto and colleagues conducted a cross-sectional study of 2,266 Japanese women and men who had been exposed to background (vs occupational or wartime) levels of dioxins, including PCDDs, PCDFs, and DL-PCBs.12 The dioxins likely originated from combustion of chlorinated materials and older manufactured electronics components. The study participants had lived in urban areas or in farming or fishing villages for at least 10 consecutive years and had no concomitant occupational exposure to dioxins. Mean (SD) age was 43.5 (13.6) years for the men and 45.3 (14.0) years for the women. Participants volunteered their disease histories, which included physician-diagnosed hypertension (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg). Logistic regression analyses were adjusted for BMI, sex, age, regional residential area, smoking, alcohol use, and survey year. In fishing regions, PCDDs, PCDFs, and DL-PCBs were significantly higher than in the other regions. Of the 2,266 participants, 647 reported physician-diagnosed hypertension. Dividing the toxic equivalents of serum COI levels into quartiles of concentration, Nakamoto and colleagues found a statistically significant association of hypertension and increased toxic equivalent levels of PCDDs, PCDFs, DL-PCBs, and total dioxins.
Italian Male Steelworkers
In a 2016 retrospective cohort study, Cappelletti and colleagues assessed the health burden of workers at a steel recycling plant in Trento, Italy. The plant, which had been using an electric arc furnace without a coke oven, had been exposing workers to dust containing PCBs, PCDDs, PCDFs, and other metals.13 Each hour, roughly 2 to 5 kg of dust was being released inside the plant (diffuse emissions), and exposure extended to a 2-km radius around the plant. A cohort of 331 plant workers, identified and assessed through company records, had been exposed to diffuse emissions for at least 1 year between 1979 and 2009. This group was compared with a control group of 32 office workers from that company, as identified by company records. The authors found a risk ratio (RR) of 2.23 in cases of noncomplicated hypertension and an RR of 2.01 in cases of complicated hypertension, defined as hypertension with organ damage.
Elderly in Sweden
In a study of 1,016 Swedish men and women who were aged 70 years or older and were living in Uppsala, Sweden, Lind and colleagues calculated average supine BP from 3 sphygmomanometer measurements after 30 minutes of rest.14 The researchers used high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) to measure the serum levels of a set of 23 POPs—16 PCB congeners, 5 OC pesticides, 1 brominated biphenyl ether congener, and octachloro-p-dibenzodioxin—and lipid-normalized the values. They used logistic regression to assess POP levels and prevalent hypertension (BP ≥ 140/90 mm Hg or use of antihypertensives), adjusting for sex, BMI, smoking status, exercise, and education. Among the COIs with the highest circulating lipid-normalized POP levels were PCB congeners 180, 138, and 170 and DDE. There was no clear relationship between toxic equivalents and hypertension; after multivariate adjustments, only DDE showed a statistically significant OR: 1.25 (95% CI, 1.07-1.47).
Organic Pollutants and Hypertension
Using National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2002, Ha and colleagues conducted a cross-sectional study of a 524-adult subset of patients who were exposed to background levels of POPs and had newly diagnosed hypertension (≥ 140/90 mm Hg).15 In the NHANES study, the CDC collected standardized patient history information, physical examination findings, and venous blood sample results. Recorded BP data points were the averages of 3 separate SBP and DBP readings from a sphygmomanometer, as recommended by the AHA. The NHANES study recorded POPs with HRGC/HRMS.
Ha and colleagues selected 12 POPs, and standardized the COI concentrations against lipid concentration. The lipid-standardized POP concentrations used were at a higher level of detection and found in at least 60% of the study patients. The researchers used a logistic regression model to calculate multivariate-adjusted OR separately in men and women, adjusting for race/ethnicity, smoking/alcohol use, physical activity, BMI, cotinine level, and income level. Among the 56 men and 67 women with newly diagnosed prevalent hypertension, PCDD levels in women were positively associated with hypertension but not correlated with higher or lower toxic equivalency factors. Dioxin and NDL-PCBs were positively associated with hypertension in men but negatively in women. Ha and colleagues postulated that this approach of studying a US population subset of patients with background exposure to POPs, instead of groups with high concentrations of exposure (eg, Vietnam War veterans or those exposed occupationally or in industrial accidents), provides an alternative observable effect of long-term, low-dose exposure of a blend of POPs.15
Discussion
In vivo and in vitro studies have found that dioxins induce a subset of 35 genes, including microsomal P450 enzymes, kinases and phosphates, and DNA repair proteins. A microarray profile of cardiovascular murine tissue and cultured vascular smooth muscle cells exposed to TCDD found known dioxin-inducible genes Cyp1b1, a phase 1 drug metabolism enzyme, and Aldh3A1, another drug metabolism gene up-regulated, among lectin-related natural killer cell receptor, insulin-like growth factor binding protein, and cyclin G2.16
Dioxins bind avidly to the aryl hydrocarbon receptor (AhR), a cytosolic transcription factor that also interacts with other xenobiotic compounds with varying affinities. TCDD is one of the most potent ligands for AhR, and other DL compounds have a lower binding affinity. AhR dimerizes in the nucleus with the AhR nuclear translocator and then binds genomic dioxin response elements and induces the expression of cytochrome P450 genes, such as CYP1A1.17
The AhRs are highly expressed in the vascular endothelium.17 Agbor and colleagues found that mice with endothelial AhR knockouts showed decreased baseline SBP and DBP.18 When challenged with angiotensin II, a potent vasoconstrictor, AhR-/- mice failed to show an increase in DBP. AhR-/- exhibited reduced ex vivo aortic contraction in the presence of angiotensin II in aortas with perivascular adipose tissue. Notably, compared with wild-type mice, AhR-/- mice had reduced renin-angiotensinsystem gene expression in the visceral adipose, linking the AhR receptor with the endogenous renin-angiotensin-aldosterone system (RAAS).
Early studies have shown that mice lacking AhR do not demonstrate TCDD toxicity.20 More recently, Kopf and colleagues found that TCDD exposure in mice led to increased BP and cardiac hypertrophy, possibly linked to increased superoxide production in the vasculature.21 When exposed to TCDD, mice showed enhanced CYP1A1 mRNA expression in the left ventricle, kidney, and aorta by day 35 and increased CYP1B1 mRNA expression in the left ventricle after 60 days. Within the first week of TCDD exposure, the mean arterial pressure for the exposure group was statistically significantly increased, showing a trend of peaks and plateaus. Mice exposed to TCDD also showed left ventricular concentric hypertrophy, which is typical of systemic hypertension.8,21 Kerley-Hamilton and colleagues found that AhR ligand activation increased atherosclerosis.22
Most hypertension is idiopathic. Research into the downstream effects of AhR suggests it induces vascular oxidative stress and increases atherosclerosis.22 It is unclear whether this is an initiating or synergistic factor in the development of hypertension. The study results described here indicate that dioxins initiate BP changes through the endothelial AhR receptor, but this mechanism has been proved only in an animal model. Ongoing studies are needed to examine the molecular changes in humans. Clinicians can be advised that dioxin exposure, rather than being an initiating factor, would at most contribute to an accumulating series of assaults, including genetics, lifestyle, and environmental factors, and that these assaults progress to hypertension only after passing a threshold.23 Moreover, many of the studies described here categorized hypertension under the guideline of 140/90 mm Hg. Future studies may use the newer guideline, which will affect their results.
Conclusion
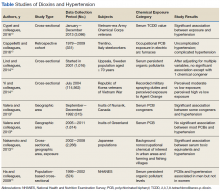
Studies have shown an association between dioxins and endocrine disruption, reproductive and developmental problems, and certain cancers.3,24 The Seveso Women’s Health Study of an industrial accident in Italy linked dioxins to an incidence of DM, obesity, or metabolic syndrome.25 By contrast, evidence of a link between dioxins and hypertension has been limited and inconsistent. Seven of the 8 studies reviewed in this study found moderate evidence of association in patients with at least 1 chemical congener and a certain subset of the study population (Table).
The Vietnam-Era Veterans Health Study found a higher OR of developing hypertension in herbicide sprayers than in its control group. Korean Vietnam War veterans stratified by either self-reported risk or military assignment also had significant associations. For male steelworkers in Italy, occupational exposure had a moderately higher RR in the exposure cohort. In the NHANES study, background levels of POPs were positively associated, but only in men. A nonoccupational study in urban and rural areas of Japan found a significant association between dioxins and hypertension. A nonoccupational study of elderly Swedes found a significant association with only 1 chemical congener. A study of Inuits in Greenland found no significant associations, but a study of Inuits in Canada did yield an association.Recent studies maintain the 2012 veterans update regarding a limited but suggestive association of dioxin and hypertension.4 Despite having high power because of the number of exposed patients, these observational studies can posit only an associative relationship, not a causal one. These studies also are limited by their categorization of dioxin exposure levels—ranging from perceived exposure to proximity and direct serum dioxin measurement. Moreover, chemical levels are measured an inconsistent number of years after exposure, and therefore, as dioxins are primarily metabolized by CYP genes, different metabolic rates could account for different susceptibility to health effects.2
In vivo animal studies could better characterize the effect of time point of exposure and effects on hypertension. Studies could also examine the synergistic effects of dioxins and other toxins, or smoking or alcohol use, on hypertension. New clinical guidelines for hypertension will have an impact on studies. Overall, clinicians who treat patients with known exposure to dioxins can suggest with moderate confidence that it is likely not a primary reason for the development of hypertension. At most, dioxin exposure is a contributing factor in the development of hypertension, with lifestyle, smoking, diet, and genetics playing more compelling roles.
1. Van den Berg M, Birnbaum L, Bosveld AT, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106(12):775-792.
2. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Koplan JP. Toxicological profile for chlorinated dibenzo-p-dioxins. https://www.atsdr.cdc .gov/toxprofiles/tp104.pdf. Published December 1998. Accessed April 3, 2018.
3. Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223-241.
4. Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Ninth Biennial Update); Board of the Health of Select Populations, Institute of Medicine. Veterans and Agent Orange: Update 2012. Washington, DC: National Academies Press; 2014.
5. Valera B, Ayotte P, Poirier P, Dewailly E. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. 2013;59:282-289.
6. Valera B, Jørgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013;122:65-73.
7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;Nov 13:pii:HYP.0000000000000066. [Epub ahead of print.]
8. Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier; 2014.
9. Kang HK, Dalager NA, Needham LL, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006;49(11):875-884.
10. Cypel YS, Kress AM, Eber SM, Schneiderman AI, Davey VJ. Herbicide exposure, Vietnam service, and hypertension risk in Army Chemical Corps veterans. J Occup Environ Med. 2016;58(11):1127-1136.
11. Yi SW, Ohrr H, Hong JS, Yi JJ. Agent Orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J Prev Med Public Health. 2013;46(5):213-225.
12. Nakamoto M, Arisawa K, Uemura H, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. 2013;86(8):849-859.
13. Cappelletti R, Ceppi M, Claudatus J, Gennaro V. Health status of male steel workers at an electric arc furnace (EAF) in Trentino, Italy. J Occup Med Toxicol. 2016;11:7.
14. Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. Circulating levels of p,p’-DDE are related to prevalent hypertension in the elderly. Environ Res. 2014;129:27-31.
15. Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 2009;23(4):274-286.
16. Puga A, Sartor MA, Huang M, et al. Gene expression profiles of mouse aorta and cultured vascular smooth muscle cells differ widely, yet show common responses to dioxin exposure. Cardiovasc Toxicol. 2004;4(4):385-404.
17. Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993;3(5):213-230.
18. Agbor LN, Elased KM, Walker MK. Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol. 2011;82(5):514-523.
19. Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338(1):311-317.
20. Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173-179.
21. Kopf PG, Scott JA, Agbor LN, et al. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117(2):537-546.
22. Kerley-Hamilton JS, Trask HW, Ridley CJ, et al. Inherent and benzo[a]pyrene-induced differential aryl hydrocarbon receptor signaling greatly affects life span, atherosclerosis, cardiac gene expression, and body and heart growth in mice. Toxicol Sci. 2012;126(2):391-404.
23. Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Pressure. 2005;14(2):69-71.
24. Kogevinas M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum Reprod Update. 2001;7(3):331-339.
25. Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso Women’s Health Study. Environ Health Perspect. 2013;121(8):906-911.
Persistent organic pollutants (POPs), endocrine-disrupting, lipophilic chemicals that concentrate in adipose tissue, increasingly are being studied for a wide range of health effects.1 Persistent organic pollutants include bisphenol A, phthalates, dioxins, hexachlorobenzene, dichlorodiphenyltrichloroethane (DDT), polybrominated diphenyl ethers, and polychlorinated biphenyls (PCBs). Chlorinated dibenzo-p-dioxins are known as polychlorinated dibenzodioxins (PCDDs), or simply dioxins. Categorization of this group of chemicals is based on the structural chlorinated constituents. Of the 75 congener molecules, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic, and this dioxin, given its more serious health implications, has been studied the most.1,2
Because it was a contaminant in the herbicide Agent Orange, the main defoliant used by the US military in southern Vietnam during the Vietnam War, TCDD is of primary interest. Agent Orange consists of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in equal parts. Like other dioxins, TCDD is lipophilic and retained in adipose tissue.1,3 Contemporaneous sources include occupational and residential exposure from pulp and paper mills, metallurgy, incinerators, industrial waste, fossil fuel combustion, and industrial accidents and poisonings.1-4
Another main class of POPs, polychlorinated benzenes, includes 209 synthetic PCB congener chemicals, a subset of which is referred to as dioxin-like PCBs.1 Organochlorine (OC) pesticides and PCBs were once manufactured as lubricants and coolants for electronics but are now banned; nevertheless, they remain concentrated in fish and mammals and persist in the food chain.3,5,6 These chemicals of interest (COIs) are graded for toxicity based on toxic equivalency factors relative to TCDDs in a 2005 World Health Organization assessment.3
Polychlorinated dibenzofurans (PCDFs), TCDD, PCBs, PCDDs, and other environmental toxins are being studied as possible contributing factors in the development of hypertension. The authors review the results of several recent studies on COI exposure and hypertension.
In 2017, the American College of Cardiology and the American Heart Association lowered the threshold for hypertension to systolic blood pressure (SBP) > 130 mm Hg and diastolic blood pressure (DBP) > 80 mm Hg.7 This new guideline would categorize 46% of the US population as having hypertension, compared with 32% under the former cutoff of 140/90 mm Hg.7 Modifiable factors (eg, diet, body mass index [BMI], smoking, alcohol, physical activity) and nonmodifiable factors (eg, age, family history, sex, race/ethnicity) have a role in the pathophysiology of hypertension. Between 90% and 95% of hypertension is considered primary. Hypertension increases the risk of developing ischemic heart disease, atherosclerosis, aortic dissection, small blood vessel disease, congestive heart failure, and renal failure, and thus results in considerable morbidity and mortality each year.8
Contaminant Exposure and Hypertension Risk
Vietnam-Era Army Chemical Corps
The US Army Chemical Corps (ACC) Vietnam-Era Veterans Health Study (2012-2013) recorded the long-term health burdens imposed by Agent Orange exposure and Vietnam War service.9,10 This cross-sectional study reexamined a subset of 5,609 Vietnam-era ACC veterans for an association of self-reported, physician-diagnosed hypertension (≥ 140/90 mm Hg) and herbicide spraying history confirmed with serum TCDD levels. The 22 Army units that made up the ACC were in charge of spreading Agent Orange and other defoliants on opposition camps between 1965 and 1973. The herbicide was dispersed aerially and on the ground. The ACC was also responsible for dispensing napalm, tear gas, and other chemicals.
A previous phone survey found an association of self-reported hypertension and herbicide spraying in ACC veterans with associated Vietnam service and herbicide spraying history, verified with serum TCDD levels (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.00-1.58).9 Median age of ACC veterans with Vietnam War service at the time of the survey was 53 years.
The 2012-2013 study assessed respondents with a record of their serum TCDD measurements from the time of the survey.10 Most of the respondents were aged in their 60s. The stated purpose of the health study was to examine the association of Vietnam veterans’ occupational herbicide exposure and hypertension risk, rather than isolate a certain responsible chemical, though serum TCDD levels were used to confirm spraying history. After adjustments for age, tobacco use, alcohol use, race, and BMI but not salt intake, family history of hypertension, psychiatric health, or diabetes mellitus (DM) comorbidity, the OR of self-reported, physician-diagnosed hypertension was 1.74 (95% CI, 1.44-2.11) for sprayers and 1.26 (95% CI, 1.05-1.53) for Vietnam veterans.10
Vietnam War Veterans From Korea
Soldiers of the Republic of Korea (ROK) who fought in the Vietnam War also were exposed to Agent Orange and other defoliants and herbicides. In 2013, Yi and colleagues contacted 187,897 ROK Vietnam veterans to analyze their Agent Orange exposure and self-reported diseases decades after the war.11 By mail, the researchers administered a questionnaire of perceived Agent Orange exposure (eg, spraying, handling spray equipment, having contact with COIs). The Korean veterans were classified by military assignment and by their proximity to areas sprayed with Agent Orange, according to the military records of 3 US combat units: Capital Division, 9th Division, and Marine Second Brigade. The ROK veterans in those units presumably would have similar levels of Agent Orange exposure.
The questionnaire response rate was 69%. The 114,562 respondents were divided into groups based on self-perceived exposure (no, low, moderate, high) and qualitative exposure level, derived from service history (battalion/company, division/brigade). After adjusting for BMI, smoking, alcohol use, physical activity, use of nonoccupational herbicides, education, income, and military rank, Yi and colleagues found a statistically significant association of hypertension and self-reported perceived Agent Orange exposure (P < .001) and a statistically significant association of hypertension and exposure in the division/brigade group with the highest exposure level (P < .001).11 The highest ORs were found for high- vs low-exposure and moderate- vs low-exposure subsets in self-reported perceived Agent Orange levels: 1.60 (95% CI, 1.56-1.65) and 1.70 (95% CI, 1.64-1.77), respectively. However, adjusted ORs in proximity-based exposure for all groups were > 1.03.
Inuits in Canada and Greenland
To study total PCBs, non-dioxin-like PCBs, OC pesticides, and their metabolites in plasma, public health researchers Valera and colleagues focused on the Inuit town of Nunavik (in Canada), where there is contamination from foods like fish, a mainstay of the Inuit diet.5 A health survey was sent to 400 households randomly selected from 1,378 households in 14 villages. Data were collected between September and December 1992. In total, 518 people between ages 18 and 74 years agreed to undergo a physical examination, and 492 agreed to have blood drawn. Laboratories measured serum PCB congeners and 13 chlorinated pesticides or their metabolites. Blood pressure (BP) was measured 3 times, and the last 2 measurements averaged. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg.
Of the 518 participants, 315 (134 men, 181 women) had complete BP, serum POP levels, and confounding variables recorded, and these were subsequently analyzed. Mean age was 32.7 years. Polychlorinated biphenyls congeners 105 and 118 were higher in women than in men; no other congeners were quantitatively different. Associations between POP levels and hypertension were analyzed with multiple logistic regression modeling, with adjustments for age, sex, fasting blood glucose, waist circumference, smoking, alcohol use, and physical activity, as well as the common contaminants lead, mercury, and omega-3 polyunsaturated fatty acids (n-3 PUFA).The researchers adjusted for n-3 PUFA because of the posited BP-lowering effects. Inuits consume large amounts of the polyunsaturated fatty acids DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid).5
Using congeners found in at least 70% of the total samples, the study authors found a statistically significant association between certain PCBs, both dioxin-like (DL-PCBs) and non-dioxin-like (NDL-PCBs), and increased risk of hypertension. Congeners 101, 105 (DL-PCB), 138, and 187, as well as p,p'-DDE, were also significantly associated with a higher risk of hypertension. Congener 99 was associated with increased SBP, and congener 118 (DL-PCB) was associated with increased SBP and DBP. Some congeners, such as the OC pesticides, p,p'-DDT, β-hexachlorocyclohexane, and oxychlordane, were inversely associated with hypertension.
In 2012, Valera and colleagues conducted a similar study of Greenland Inuits who also consume marine mammals and fish and present with high POP levels.6 Despite correcting for n-3 PUFA, they found no significant association involving DL-PCBs, NDL-PCBs, or OC pesticides.
Japanese Background Exposures
Nakamoto and colleagues conducted a cross-sectional study of 2,266 Japanese women and men who had been exposed to background (vs occupational or wartime) levels of dioxins, including PCDDs, PCDFs, and DL-PCBs.12 The dioxins likely originated from combustion of chlorinated materials and older manufactured electronics components. The study participants had lived in urban areas or in farming or fishing villages for at least 10 consecutive years and had no concomitant occupational exposure to dioxins. Mean (SD) age was 43.5 (13.6) years for the men and 45.3 (14.0) years for the women. Participants volunteered their disease histories, which included physician-diagnosed hypertension (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg). Logistic regression analyses were adjusted for BMI, sex, age, regional residential area, smoking, alcohol use, and survey year. In fishing regions, PCDDs, PCDFs, and DL-PCBs were significantly higher than in the other regions. Of the 2,266 participants, 647 reported physician-diagnosed hypertension. Dividing the toxic equivalents of serum COI levels into quartiles of concentration, Nakamoto and colleagues found a statistically significant association of hypertension and increased toxic equivalent levels of PCDDs, PCDFs, DL-PCBs, and total dioxins.
Italian Male Steelworkers
In a 2016 retrospective cohort study, Cappelletti and colleagues assessed the health burden of workers at a steel recycling plant in Trento, Italy. The plant, which had been using an electric arc furnace without a coke oven, had been exposing workers to dust containing PCBs, PCDDs, PCDFs, and other metals.13 Each hour, roughly 2 to 5 kg of dust was being released inside the plant (diffuse emissions), and exposure extended to a 2-km radius around the plant. A cohort of 331 plant workers, identified and assessed through company records, had been exposed to diffuse emissions for at least 1 year between 1979 and 2009. This group was compared with a control group of 32 office workers from that company, as identified by company records. The authors found a risk ratio (RR) of 2.23 in cases of noncomplicated hypertension and an RR of 2.01 in cases of complicated hypertension, defined as hypertension with organ damage.
Elderly in Sweden
In a study of 1,016 Swedish men and women who were aged 70 years or older and were living in Uppsala, Sweden, Lind and colleagues calculated average supine BP from 3 sphygmomanometer measurements after 30 minutes of rest.14 The researchers used high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) to measure the serum levels of a set of 23 POPs—16 PCB congeners, 5 OC pesticides, 1 brominated biphenyl ether congener, and octachloro-p-dibenzodioxin—and lipid-normalized the values. They used logistic regression to assess POP levels and prevalent hypertension (BP ≥ 140/90 mm Hg or use of antihypertensives), adjusting for sex, BMI, smoking status, exercise, and education. Among the COIs with the highest circulating lipid-normalized POP levels were PCB congeners 180, 138, and 170 and DDE. There was no clear relationship between toxic equivalents and hypertension; after multivariate adjustments, only DDE showed a statistically significant OR: 1.25 (95% CI, 1.07-1.47).
Organic Pollutants and Hypertension
Using National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2002, Ha and colleagues conducted a cross-sectional study of a 524-adult subset of patients who were exposed to background levels of POPs and had newly diagnosed hypertension (≥ 140/90 mm Hg).15 In the NHANES study, the CDC collected standardized patient history information, physical examination findings, and venous blood sample results. Recorded BP data points were the averages of 3 separate SBP and DBP readings from a sphygmomanometer, as recommended by the AHA. The NHANES study recorded POPs with HRGC/HRMS.
Ha and colleagues selected 12 POPs, and standardized the COI concentrations against lipid concentration. The lipid-standardized POP concentrations used were at a higher level of detection and found in at least 60% of the study patients. The researchers used a logistic regression model to calculate multivariate-adjusted OR separately in men and women, adjusting for race/ethnicity, smoking/alcohol use, physical activity, BMI, cotinine level, and income level. Among the 56 men and 67 women with newly diagnosed prevalent hypertension, PCDD levels in women were positively associated with hypertension but not correlated with higher or lower toxic equivalency factors. Dioxin and NDL-PCBs were positively associated with hypertension in men but negatively in women. Ha and colleagues postulated that this approach of studying a US population subset of patients with background exposure to POPs, instead of groups with high concentrations of exposure (eg, Vietnam War veterans or those exposed occupationally or in industrial accidents), provides an alternative observable effect of long-term, low-dose exposure of a blend of POPs.15
Discussion
In vivo and in vitro studies have found that dioxins induce a subset of 35 genes, including microsomal P450 enzymes, kinases and phosphates, and DNA repair proteins. A microarray profile of cardiovascular murine tissue and cultured vascular smooth muscle cells exposed to TCDD found known dioxin-inducible genes Cyp1b1, a phase 1 drug metabolism enzyme, and Aldh3A1, another drug metabolism gene up-regulated, among lectin-related natural killer cell receptor, insulin-like growth factor binding protein, and cyclin G2.16
Dioxins bind avidly to the aryl hydrocarbon receptor (AhR), a cytosolic transcription factor that also interacts with other xenobiotic compounds with varying affinities. TCDD is one of the most potent ligands for AhR, and other DL compounds have a lower binding affinity. AhR dimerizes in the nucleus with the AhR nuclear translocator and then binds genomic dioxin response elements and induces the expression of cytochrome P450 genes, such as CYP1A1.17
The AhRs are highly expressed in the vascular endothelium.17 Agbor and colleagues found that mice with endothelial AhR knockouts showed decreased baseline SBP and DBP.18 When challenged with angiotensin II, a potent vasoconstrictor, AhR-/- mice failed to show an increase in DBP. AhR-/- exhibited reduced ex vivo aortic contraction in the presence of angiotensin II in aortas with perivascular adipose tissue. Notably, compared with wild-type mice, AhR-/- mice had reduced renin-angiotensinsystem gene expression in the visceral adipose, linking the AhR receptor with the endogenous renin-angiotensin-aldosterone system (RAAS).
Early studies have shown that mice lacking AhR do not demonstrate TCDD toxicity.20 More recently, Kopf and colleagues found that TCDD exposure in mice led to increased BP and cardiac hypertrophy, possibly linked to increased superoxide production in the vasculature.21 When exposed to TCDD, mice showed enhanced CYP1A1 mRNA expression in the left ventricle, kidney, and aorta by day 35 and increased CYP1B1 mRNA expression in the left ventricle after 60 days. Within the first week of TCDD exposure, the mean arterial pressure for the exposure group was statistically significantly increased, showing a trend of peaks and plateaus. Mice exposed to TCDD also showed left ventricular concentric hypertrophy, which is typical of systemic hypertension.8,21 Kerley-Hamilton and colleagues found that AhR ligand activation increased atherosclerosis.22
Most hypertension is idiopathic. Research into the downstream effects of AhR suggests it induces vascular oxidative stress and increases atherosclerosis.22 It is unclear whether this is an initiating or synergistic factor in the development of hypertension. The study results described here indicate that dioxins initiate BP changes through the endothelial AhR receptor, but this mechanism has been proved only in an animal model. Ongoing studies are needed to examine the molecular changes in humans. Clinicians can be advised that dioxin exposure, rather than being an initiating factor, would at most contribute to an accumulating series of assaults, including genetics, lifestyle, and environmental factors, and that these assaults progress to hypertension only after passing a threshold.23 Moreover, many of the studies described here categorized hypertension under the guideline of 140/90 mm Hg. Future studies may use the newer guideline, which will affect their results.
Conclusion
Studies have shown an association between dioxins and endocrine disruption, reproductive and developmental problems, and certain cancers.3,24 The Seveso Women’s Health Study of an industrial accident in Italy linked dioxins to an incidence of DM, obesity, or metabolic syndrome.25 By contrast, evidence of a link between dioxins and hypertension has been limited and inconsistent. Seven of the 8 studies reviewed in this study found moderate evidence of association in patients with at least 1 chemical congener and a certain subset of the study population (Table).
The Vietnam-Era Veterans Health Study found a higher OR of developing hypertension in herbicide sprayers than in its control group. Korean Vietnam War veterans stratified by either self-reported risk or military assignment also had significant associations. For male steelworkers in Italy, occupational exposure had a moderately higher RR in the exposure cohort. In the NHANES study, background levels of POPs were positively associated, but only in men. A nonoccupational study in urban and rural areas of Japan found a significant association between dioxins and hypertension. A nonoccupational study of elderly Swedes found a significant association with only 1 chemical congener. A study of Inuits in Greenland found no significant associations, but a study of Inuits in Canada did yield an association.Recent studies maintain the 2012 veterans update regarding a limited but suggestive association of dioxin and hypertension.4 Despite having high power because of the number of exposed patients, these observational studies can posit only an associative relationship, not a causal one. These studies also are limited by their categorization of dioxin exposure levels—ranging from perceived exposure to proximity and direct serum dioxin measurement. Moreover, chemical levels are measured an inconsistent number of years after exposure, and therefore, as dioxins are primarily metabolized by CYP genes, different metabolic rates could account for different susceptibility to health effects.2
In vivo animal studies could better characterize the effect of time point of exposure and effects on hypertension. Studies could also examine the synergistic effects of dioxins and other toxins, or smoking or alcohol use, on hypertension. New clinical guidelines for hypertension will have an impact on studies. Overall, clinicians who treat patients with known exposure to dioxins can suggest with moderate confidence that it is likely not a primary reason for the development of hypertension. At most, dioxin exposure is a contributing factor in the development of hypertension, with lifestyle, smoking, diet, and genetics playing more compelling roles.
Persistent organic pollutants (POPs), endocrine-disrupting, lipophilic chemicals that concentrate in adipose tissue, increasingly are being studied for a wide range of health effects.1 Persistent organic pollutants include bisphenol A, phthalates, dioxins, hexachlorobenzene, dichlorodiphenyltrichloroethane (DDT), polybrominated diphenyl ethers, and polychlorinated biphenyls (PCBs). Chlorinated dibenzo-p-dioxins are known as polychlorinated dibenzodioxins (PCDDs), or simply dioxins. Categorization of this group of chemicals is based on the structural chlorinated constituents. Of the 75 congener molecules, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic, and this dioxin, given its more serious health implications, has been studied the most.1,2
Because it was a contaminant in the herbicide Agent Orange, the main defoliant used by the US military in southern Vietnam during the Vietnam War, TCDD is of primary interest. Agent Orange consists of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in equal parts. Like other dioxins, TCDD is lipophilic and retained in adipose tissue.1,3 Contemporaneous sources include occupational and residential exposure from pulp and paper mills, metallurgy, incinerators, industrial waste, fossil fuel combustion, and industrial accidents and poisonings.1-4
Another main class of POPs, polychlorinated benzenes, includes 209 synthetic PCB congener chemicals, a subset of which is referred to as dioxin-like PCBs.1 Organochlorine (OC) pesticides and PCBs were once manufactured as lubricants and coolants for electronics but are now banned; nevertheless, they remain concentrated in fish and mammals and persist in the food chain.3,5,6 These chemicals of interest (COIs) are graded for toxicity based on toxic equivalency factors relative to TCDDs in a 2005 World Health Organization assessment.3
Polychlorinated dibenzofurans (PCDFs), TCDD, PCBs, PCDDs, and other environmental toxins are being studied as possible contributing factors in the development of hypertension. The authors review the results of several recent studies on COI exposure and hypertension.
In 2017, the American College of Cardiology and the American Heart Association lowered the threshold for hypertension to systolic blood pressure (SBP) > 130 mm Hg and diastolic blood pressure (DBP) > 80 mm Hg.7 This new guideline would categorize 46% of the US population as having hypertension, compared with 32% under the former cutoff of 140/90 mm Hg.7 Modifiable factors (eg, diet, body mass index [BMI], smoking, alcohol, physical activity) and nonmodifiable factors (eg, age, family history, sex, race/ethnicity) have a role in the pathophysiology of hypertension. Between 90% and 95% of hypertension is considered primary. Hypertension increases the risk of developing ischemic heart disease, atherosclerosis, aortic dissection, small blood vessel disease, congestive heart failure, and renal failure, and thus results in considerable morbidity and mortality each year.8
Contaminant Exposure and Hypertension Risk
Vietnam-Era Army Chemical Corps
The US Army Chemical Corps (ACC) Vietnam-Era Veterans Health Study (2012-2013) recorded the long-term health burdens imposed by Agent Orange exposure and Vietnam War service.9,10 This cross-sectional study reexamined a subset of 5,609 Vietnam-era ACC veterans for an association of self-reported, physician-diagnosed hypertension (≥ 140/90 mm Hg) and herbicide spraying history confirmed with serum TCDD levels. The 22 Army units that made up the ACC were in charge of spreading Agent Orange and other defoliants on opposition camps between 1965 and 1973. The herbicide was dispersed aerially and on the ground. The ACC was also responsible for dispensing napalm, tear gas, and other chemicals.
A previous phone survey found an association of self-reported hypertension and herbicide spraying in ACC veterans with associated Vietnam service and herbicide spraying history, verified with serum TCDD levels (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.00-1.58).9 Median age of ACC veterans with Vietnam War service at the time of the survey was 53 years.
The 2012-2013 study assessed respondents with a record of their serum TCDD measurements from the time of the survey.10 Most of the respondents were aged in their 60s. The stated purpose of the health study was to examine the association of Vietnam veterans’ occupational herbicide exposure and hypertension risk, rather than isolate a certain responsible chemical, though serum TCDD levels were used to confirm spraying history. After adjustments for age, tobacco use, alcohol use, race, and BMI but not salt intake, family history of hypertension, psychiatric health, or diabetes mellitus (DM) comorbidity, the OR of self-reported, physician-diagnosed hypertension was 1.74 (95% CI, 1.44-2.11) for sprayers and 1.26 (95% CI, 1.05-1.53) for Vietnam veterans.10
Vietnam War Veterans From Korea
Soldiers of the Republic of Korea (ROK) who fought in the Vietnam War also were exposed to Agent Orange and other defoliants and herbicides. In 2013, Yi and colleagues contacted 187,897 ROK Vietnam veterans to analyze their Agent Orange exposure and self-reported diseases decades after the war.11 By mail, the researchers administered a questionnaire of perceived Agent Orange exposure (eg, spraying, handling spray equipment, having contact with COIs). The Korean veterans were classified by military assignment and by their proximity to areas sprayed with Agent Orange, according to the military records of 3 US combat units: Capital Division, 9th Division, and Marine Second Brigade. The ROK veterans in those units presumably would have similar levels of Agent Orange exposure.
The questionnaire response rate was 69%. The 114,562 respondents were divided into groups based on self-perceived exposure (no, low, moderate, high) and qualitative exposure level, derived from service history (battalion/company, division/brigade). After adjusting for BMI, smoking, alcohol use, physical activity, use of nonoccupational herbicides, education, income, and military rank, Yi and colleagues found a statistically significant association of hypertension and self-reported perceived Agent Orange exposure (P < .001) and a statistically significant association of hypertension and exposure in the division/brigade group with the highest exposure level (P < .001).11 The highest ORs were found for high- vs low-exposure and moderate- vs low-exposure subsets in self-reported perceived Agent Orange levels: 1.60 (95% CI, 1.56-1.65) and 1.70 (95% CI, 1.64-1.77), respectively. However, adjusted ORs in proximity-based exposure for all groups were > 1.03.
Inuits in Canada and Greenland
To study total PCBs, non-dioxin-like PCBs, OC pesticides, and their metabolites in plasma, public health researchers Valera and colleagues focused on the Inuit town of Nunavik (in Canada), where there is contamination from foods like fish, a mainstay of the Inuit diet.5 A health survey was sent to 400 households randomly selected from 1,378 households in 14 villages. Data were collected between September and December 1992. In total, 518 people between ages 18 and 74 years agreed to undergo a physical examination, and 492 agreed to have blood drawn. Laboratories measured serum PCB congeners and 13 chlorinated pesticides or their metabolites. Blood pressure (BP) was measured 3 times, and the last 2 measurements averaged. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg.
Of the 518 participants, 315 (134 men, 181 women) had complete BP, serum POP levels, and confounding variables recorded, and these were subsequently analyzed. Mean age was 32.7 years. Polychlorinated biphenyls congeners 105 and 118 were higher in women than in men; no other congeners were quantitatively different. Associations between POP levels and hypertension were analyzed with multiple logistic regression modeling, with adjustments for age, sex, fasting blood glucose, waist circumference, smoking, alcohol use, and physical activity, as well as the common contaminants lead, mercury, and omega-3 polyunsaturated fatty acids (n-3 PUFA).The researchers adjusted for n-3 PUFA because of the posited BP-lowering effects. Inuits consume large amounts of the polyunsaturated fatty acids DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid).5
Using congeners found in at least 70% of the total samples, the study authors found a statistically significant association between certain PCBs, both dioxin-like (DL-PCBs) and non-dioxin-like (NDL-PCBs), and increased risk of hypertension. Congeners 101, 105 (DL-PCB), 138, and 187, as well as p,p'-DDE, were also significantly associated with a higher risk of hypertension. Congener 99 was associated with increased SBP, and congener 118 (DL-PCB) was associated with increased SBP and DBP. Some congeners, such as the OC pesticides, p,p'-DDT, β-hexachlorocyclohexane, and oxychlordane, were inversely associated with hypertension.
In 2012, Valera and colleagues conducted a similar study of Greenland Inuits who also consume marine mammals and fish and present with high POP levels.6 Despite correcting for n-3 PUFA, they found no significant association involving DL-PCBs, NDL-PCBs, or OC pesticides.
Japanese Background Exposures
Nakamoto and colleagues conducted a cross-sectional study of 2,266 Japanese women and men who had been exposed to background (vs occupational or wartime) levels of dioxins, including PCDDs, PCDFs, and DL-PCBs.12 The dioxins likely originated from combustion of chlorinated materials and older manufactured electronics components. The study participants had lived in urban areas or in farming or fishing villages for at least 10 consecutive years and had no concomitant occupational exposure to dioxins. Mean (SD) age was 43.5 (13.6) years for the men and 45.3 (14.0) years for the women. Participants volunteered their disease histories, which included physician-diagnosed hypertension (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg). Logistic regression analyses were adjusted for BMI, sex, age, regional residential area, smoking, alcohol use, and survey year. In fishing regions, PCDDs, PCDFs, and DL-PCBs were significantly higher than in the other regions. Of the 2,266 participants, 647 reported physician-diagnosed hypertension. Dividing the toxic equivalents of serum COI levels into quartiles of concentration, Nakamoto and colleagues found a statistically significant association of hypertension and increased toxic equivalent levels of PCDDs, PCDFs, DL-PCBs, and total dioxins.
Italian Male Steelworkers
In a 2016 retrospective cohort study, Cappelletti and colleagues assessed the health burden of workers at a steel recycling plant in Trento, Italy. The plant, which had been using an electric arc furnace without a coke oven, had been exposing workers to dust containing PCBs, PCDDs, PCDFs, and other metals.13 Each hour, roughly 2 to 5 kg of dust was being released inside the plant (diffuse emissions), and exposure extended to a 2-km radius around the plant. A cohort of 331 plant workers, identified and assessed through company records, had been exposed to diffuse emissions for at least 1 year between 1979 and 2009. This group was compared with a control group of 32 office workers from that company, as identified by company records. The authors found a risk ratio (RR) of 2.23 in cases of noncomplicated hypertension and an RR of 2.01 in cases of complicated hypertension, defined as hypertension with organ damage.
Elderly in Sweden
In a study of 1,016 Swedish men and women who were aged 70 years or older and were living in Uppsala, Sweden, Lind and colleagues calculated average supine BP from 3 sphygmomanometer measurements after 30 minutes of rest.14 The researchers used high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) to measure the serum levels of a set of 23 POPs—16 PCB congeners, 5 OC pesticides, 1 brominated biphenyl ether congener, and octachloro-p-dibenzodioxin—and lipid-normalized the values. They used logistic regression to assess POP levels and prevalent hypertension (BP ≥ 140/90 mm Hg or use of antihypertensives), adjusting for sex, BMI, smoking status, exercise, and education. Among the COIs with the highest circulating lipid-normalized POP levels were PCB congeners 180, 138, and 170 and DDE. There was no clear relationship between toxic equivalents and hypertension; after multivariate adjustments, only DDE showed a statistically significant OR: 1.25 (95% CI, 1.07-1.47).
Organic Pollutants and Hypertension
Using National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2002, Ha and colleagues conducted a cross-sectional study of a 524-adult subset of patients who were exposed to background levels of POPs and had newly diagnosed hypertension (≥ 140/90 mm Hg).15 In the NHANES study, the CDC collected standardized patient history information, physical examination findings, and venous blood sample results. Recorded BP data points were the averages of 3 separate SBP and DBP readings from a sphygmomanometer, as recommended by the AHA. The NHANES study recorded POPs with HRGC/HRMS.
Ha and colleagues selected 12 POPs, and standardized the COI concentrations against lipid concentration. The lipid-standardized POP concentrations used were at a higher level of detection and found in at least 60% of the study patients. The researchers used a logistic regression model to calculate multivariate-adjusted OR separately in men and women, adjusting for race/ethnicity, smoking/alcohol use, physical activity, BMI, cotinine level, and income level. Among the 56 men and 67 women with newly diagnosed prevalent hypertension, PCDD levels in women were positively associated with hypertension but not correlated with higher or lower toxic equivalency factors. Dioxin and NDL-PCBs were positively associated with hypertension in men but negatively in women. Ha and colleagues postulated that this approach of studying a US population subset of patients with background exposure to POPs, instead of groups with high concentrations of exposure (eg, Vietnam War veterans or those exposed occupationally or in industrial accidents), provides an alternative observable effect of long-term, low-dose exposure of a blend of POPs.15
Discussion
In vivo and in vitro studies have found that dioxins induce a subset of 35 genes, including microsomal P450 enzymes, kinases and phosphates, and DNA repair proteins. A microarray profile of cardiovascular murine tissue and cultured vascular smooth muscle cells exposed to TCDD found known dioxin-inducible genes Cyp1b1, a phase 1 drug metabolism enzyme, and Aldh3A1, another drug metabolism gene up-regulated, among lectin-related natural killer cell receptor, insulin-like growth factor binding protein, and cyclin G2.16
Dioxins bind avidly to the aryl hydrocarbon receptor (AhR), a cytosolic transcription factor that also interacts with other xenobiotic compounds with varying affinities. TCDD is one of the most potent ligands for AhR, and other DL compounds have a lower binding affinity. AhR dimerizes in the nucleus with the AhR nuclear translocator and then binds genomic dioxin response elements and induces the expression of cytochrome P450 genes, such as CYP1A1.17
The AhRs are highly expressed in the vascular endothelium.17 Agbor and colleagues found that mice with endothelial AhR knockouts showed decreased baseline SBP and DBP.18 When challenged with angiotensin II, a potent vasoconstrictor, AhR-/- mice failed to show an increase in DBP. AhR-/- exhibited reduced ex vivo aortic contraction in the presence of angiotensin II in aortas with perivascular adipose tissue. Notably, compared with wild-type mice, AhR-/- mice had reduced renin-angiotensinsystem gene expression in the visceral adipose, linking the AhR receptor with the endogenous renin-angiotensin-aldosterone system (RAAS).
Early studies have shown that mice lacking AhR do not demonstrate TCDD toxicity.20 More recently, Kopf and colleagues found that TCDD exposure in mice led to increased BP and cardiac hypertrophy, possibly linked to increased superoxide production in the vasculature.21 When exposed to TCDD, mice showed enhanced CYP1A1 mRNA expression in the left ventricle, kidney, and aorta by day 35 and increased CYP1B1 mRNA expression in the left ventricle after 60 days. Within the first week of TCDD exposure, the mean arterial pressure for the exposure group was statistically significantly increased, showing a trend of peaks and plateaus. Mice exposed to TCDD also showed left ventricular concentric hypertrophy, which is typical of systemic hypertension.8,21 Kerley-Hamilton and colleagues found that AhR ligand activation increased atherosclerosis.22
Most hypertension is idiopathic. Research into the downstream effects of AhR suggests it induces vascular oxidative stress and increases atherosclerosis.22 It is unclear whether this is an initiating or synergistic factor in the development of hypertension. The study results described here indicate that dioxins initiate BP changes through the endothelial AhR receptor, but this mechanism has been proved only in an animal model. Ongoing studies are needed to examine the molecular changes in humans. Clinicians can be advised that dioxin exposure, rather than being an initiating factor, would at most contribute to an accumulating series of assaults, including genetics, lifestyle, and environmental factors, and that these assaults progress to hypertension only after passing a threshold.23 Moreover, many of the studies described here categorized hypertension under the guideline of 140/90 mm Hg. Future studies may use the newer guideline, which will affect their results.
Conclusion
Studies have shown an association between dioxins and endocrine disruption, reproductive and developmental problems, and certain cancers.3,24 The Seveso Women’s Health Study of an industrial accident in Italy linked dioxins to an incidence of DM, obesity, or metabolic syndrome.25 By contrast, evidence of a link between dioxins and hypertension has been limited and inconsistent. Seven of the 8 studies reviewed in this study found moderate evidence of association in patients with at least 1 chemical congener and a certain subset of the study population (Table).
The Vietnam-Era Veterans Health Study found a higher OR of developing hypertension in herbicide sprayers than in its control group. Korean Vietnam War veterans stratified by either self-reported risk or military assignment also had significant associations. For male steelworkers in Italy, occupational exposure had a moderately higher RR in the exposure cohort. In the NHANES study, background levels of POPs were positively associated, but only in men. A nonoccupational study in urban and rural areas of Japan found a significant association between dioxins and hypertension. A nonoccupational study of elderly Swedes found a significant association with only 1 chemical congener. A study of Inuits in Greenland found no significant associations, but a study of Inuits in Canada did yield an association.Recent studies maintain the 2012 veterans update regarding a limited but suggestive association of dioxin and hypertension.4 Despite having high power because of the number of exposed patients, these observational studies can posit only an associative relationship, not a causal one. These studies also are limited by their categorization of dioxin exposure levels—ranging from perceived exposure to proximity and direct serum dioxin measurement. Moreover, chemical levels are measured an inconsistent number of years after exposure, and therefore, as dioxins are primarily metabolized by CYP genes, different metabolic rates could account for different susceptibility to health effects.2
In vivo animal studies could better characterize the effect of time point of exposure and effects on hypertension. Studies could also examine the synergistic effects of dioxins and other toxins, or smoking or alcohol use, on hypertension. New clinical guidelines for hypertension will have an impact on studies. Overall, clinicians who treat patients with known exposure to dioxins can suggest with moderate confidence that it is likely not a primary reason for the development of hypertension. At most, dioxin exposure is a contributing factor in the development of hypertension, with lifestyle, smoking, diet, and genetics playing more compelling roles.
1. Van den Berg M, Birnbaum L, Bosveld AT, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106(12):775-792.
2. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Koplan JP. Toxicological profile for chlorinated dibenzo-p-dioxins. https://www.atsdr.cdc .gov/toxprofiles/tp104.pdf. Published December 1998. Accessed April 3, 2018.
3. Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223-241.
4. Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Ninth Biennial Update); Board of the Health of Select Populations, Institute of Medicine. Veterans and Agent Orange: Update 2012. Washington, DC: National Academies Press; 2014.
5. Valera B, Ayotte P, Poirier P, Dewailly E. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. 2013;59:282-289.
6. Valera B, Jørgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013;122:65-73.
7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;Nov 13:pii:HYP.0000000000000066. [Epub ahead of print.]
8. Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier; 2014.
9. Kang HK, Dalager NA, Needham LL, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006;49(11):875-884.
10. Cypel YS, Kress AM, Eber SM, Schneiderman AI, Davey VJ. Herbicide exposure, Vietnam service, and hypertension risk in Army Chemical Corps veterans. J Occup Environ Med. 2016;58(11):1127-1136.
11. Yi SW, Ohrr H, Hong JS, Yi JJ. Agent Orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J Prev Med Public Health. 2013;46(5):213-225.
12. Nakamoto M, Arisawa K, Uemura H, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. 2013;86(8):849-859.
13. Cappelletti R, Ceppi M, Claudatus J, Gennaro V. Health status of male steel workers at an electric arc furnace (EAF) in Trentino, Italy. J Occup Med Toxicol. 2016;11:7.
14. Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. Circulating levels of p,p’-DDE are related to prevalent hypertension in the elderly. Environ Res. 2014;129:27-31.
15. Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 2009;23(4):274-286.
16. Puga A, Sartor MA, Huang M, et al. Gene expression profiles of mouse aorta and cultured vascular smooth muscle cells differ widely, yet show common responses to dioxin exposure. Cardiovasc Toxicol. 2004;4(4):385-404.
17. Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993;3(5):213-230.
18. Agbor LN, Elased KM, Walker MK. Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol. 2011;82(5):514-523.
19. Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338(1):311-317.
20. Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173-179.
21. Kopf PG, Scott JA, Agbor LN, et al. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117(2):537-546.
22. Kerley-Hamilton JS, Trask HW, Ridley CJ, et al. Inherent and benzo[a]pyrene-induced differential aryl hydrocarbon receptor signaling greatly affects life span, atherosclerosis, cardiac gene expression, and body and heart growth in mice. Toxicol Sci. 2012;126(2):391-404.
23. Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Pressure. 2005;14(2):69-71.
24. Kogevinas M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum Reprod Update. 2001;7(3):331-339.
25. Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso Women’s Health Study. Environ Health Perspect. 2013;121(8):906-911.
1. Van den Berg M, Birnbaum L, Bosveld AT, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106(12):775-792.
2. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Koplan JP. Toxicological profile for chlorinated dibenzo-p-dioxins. https://www.atsdr.cdc .gov/toxprofiles/tp104.pdf. Published December 1998. Accessed April 3, 2018.
3. Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223-241.
4. Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Ninth Biennial Update); Board of the Health of Select Populations, Institute of Medicine. Veterans and Agent Orange: Update 2012. Washington, DC: National Academies Press; 2014.
5. Valera B, Ayotte P, Poirier P, Dewailly E. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. 2013;59:282-289.
6. Valera B, Jørgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013;122:65-73.
7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;Nov 13:pii:HYP.0000000000000066. [Epub ahead of print.]
8. Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier; 2014.
9. Kang HK, Dalager NA, Needham LL, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006;49(11):875-884.
10. Cypel YS, Kress AM, Eber SM, Schneiderman AI, Davey VJ. Herbicide exposure, Vietnam service, and hypertension risk in Army Chemical Corps veterans. J Occup Environ Med. 2016;58(11):1127-1136.
11. Yi SW, Ohrr H, Hong JS, Yi JJ. Agent Orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J Prev Med Public Health. 2013;46(5):213-225.
12. Nakamoto M, Arisawa K, Uemura H, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. 2013;86(8):849-859.
13. Cappelletti R, Ceppi M, Claudatus J, Gennaro V. Health status of male steel workers at an electric arc furnace (EAF) in Trentino, Italy. J Occup Med Toxicol. 2016;11:7.
14. Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. Circulating levels of p,p’-DDE are related to prevalent hypertension in the elderly. Environ Res. 2014;129:27-31.
15. Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 2009;23(4):274-286.
16. Puga A, Sartor MA, Huang M, et al. Gene expression profiles of mouse aorta and cultured vascular smooth muscle cells differ widely, yet show common responses to dioxin exposure. Cardiovasc Toxicol. 2004;4(4):385-404.
17. Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993;3(5):213-230.
18. Agbor LN, Elased KM, Walker MK. Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol. 2011;82(5):514-523.
19. Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338(1):311-317.
20. Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173-179.
21. Kopf PG, Scott JA, Agbor LN, et al. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117(2):537-546.
22. Kerley-Hamilton JS, Trask HW, Ridley CJ, et al. Inherent and benzo[a]pyrene-induced differential aryl hydrocarbon receptor signaling greatly affects life span, atherosclerosis, cardiac gene expression, and body and heart growth in mice. Toxicol Sci. 2012;126(2):391-404.
23. Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Pressure. 2005;14(2):69-71.
24. Kogevinas M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum Reprod Update. 2001;7(3):331-339.
25. Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso Women’s Health Study. Environ Health Perspect. 2013;121(8):906-911.
From the Washington Office: Upcoming Leadership and Advocacy Summit
The seventh annual American College of Surgeons Leadership and Advocacy Summit will be held May 19-22 at the Renaissance Washington, DC Downtown Hotel.
The event will kick off with the Leadership portion on Saturday evening, May 19, with a Welcome Reception open to all registrants and continues with a full-day agenda on Sunday, May 20. The program on Sunday includes sessions addressing important topics such as mentoring for a career in surgical leadership, ethics in surgical leadership, leading in times of crisis, change management, managing complex teams, and more.
1) “Understanding Strategic Advocacy” presented by staff of the Washington office
2) “Regulatory Reform: Past, Present, and Patient-Focused” featuring staff from the Centers for Medicare and Medicaid Services.
3) A historical perspective on health care reform entitled, “Health Care Reform, Then and Now,” presented by long-time Health Affairs columnist, Professor Timothy S. Jost.
4) “The Opioid Epidemic: Long-term Solutions for Sustained Success” featuring staff from the Food and Drug Administration and the Drug Enforcement Administration.
5) A luncheon, sponsored by the ACSPA-SurgeonsPAC, where attendees will hear remarks on the upcoming mid-term elections from the Executive Directors of both the Democratic Congressional Campaign Committee (DCCC) and the National Republican Congressional Committee (NRCC).
The day will also include issue briefings and specific “asks” on topics in preparation for Hill visits. Specifically, attendees will be briefed on the Pandemic and All-Hazards Preparedness Act (PAHPA), the Standardizing Electronic Prior Authorization for Safe Prescribing Act, the Ensuring Access to General Surgery Act, the Removing Barriers to Colorectal Screening Act, the Childhood Cancer STAR Act, and funding for the CDC to conduct research on firearm injury prevention. Following this training, Fellows will be very well prepared to discuss the issues the following day on Capitol Hill.
Pending last minute conflicts, several Members of Congress are also scheduled to address the group, including a member of leadership from the House of Representatives. Monday’s activities will conclude with an evening reception for 2018 SurgeonsPAC members at the historic Willard InterContinental Hotel. On Tuesday, May 22, attendees will then apply the knowledge and skill gained from Monday’s sessions during meetings with their individual Members of Congress and their staff on Capitol Hill.
As I write, nearly three weeks prior to the event, attendance is already projected to be at record levels. We look forward to welcoming all those already registered to DC for this exciting, informative and important event. Though by press time pre-registration will have closed, on-site registration will be available if you would be able to join us.
For questions regarding the Leadership Summit please contact Brian Frankel at bfrankfel@facs.org, or 312-202-5361. For questions regarding the Advocacy Summit please contact Michael Carmody at mcarmody@facs.org, or 202-672-1511.
Until next month ….
The seventh annual American College of Surgeons Leadership and Advocacy Summit will be held May 19-22 at the Renaissance Washington, DC Downtown Hotel.
The event will kick off with the Leadership portion on Saturday evening, May 19, with a Welcome Reception open to all registrants and continues with a full-day agenda on Sunday, May 20. The program on Sunday includes sessions addressing important topics such as mentoring for a career in surgical leadership, ethics in surgical leadership, leading in times of crisis, change management, managing complex teams, and more.
1) “Understanding Strategic Advocacy” presented by staff of the Washington office
2) “Regulatory Reform: Past, Present, and Patient-Focused” featuring staff from the Centers for Medicare and Medicaid Services.
3) A historical perspective on health care reform entitled, “Health Care Reform, Then and Now,” presented by long-time Health Affairs columnist, Professor Timothy S. Jost.
4) “The Opioid Epidemic: Long-term Solutions for Sustained Success” featuring staff from the Food and Drug Administration and the Drug Enforcement Administration.
5) A luncheon, sponsored by the ACSPA-SurgeonsPAC, where attendees will hear remarks on the upcoming mid-term elections from the Executive Directors of both the Democratic Congressional Campaign Committee (DCCC) and the National Republican Congressional Committee (NRCC).
The day will also include issue briefings and specific “asks” on topics in preparation for Hill visits. Specifically, attendees will be briefed on the Pandemic and All-Hazards Preparedness Act (PAHPA), the Standardizing Electronic Prior Authorization for Safe Prescribing Act, the Ensuring Access to General Surgery Act, the Removing Barriers to Colorectal Screening Act, the Childhood Cancer STAR Act, and funding for the CDC to conduct research on firearm injury prevention. Following this training, Fellows will be very well prepared to discuss the issues the following day on Capitol Hill.
Pending last minute conflicts, several Members of Congress are also scheduled to address the group, including a member of leadership from the House of Representatives. Monday’s activities will conclude with an evening reception for 2018 SurgeonsPAC members at the historic Willard InterContinental Hotel. On Tuesday, May 22, attendees will then apply the knowledge and skill gained from Monday’s sessions during meetings with their individual Members of Congress and their staff on Capitol Hill.
As I write, nearly three weeks prior to the event, attendance is already projected to be at record levels. We look forward to welcoming all those already registered to DC for this exciting, informative and important event. Though by press time pre-registration will have closed, on-site registration will be available if you would be able to join us.
For questions regarding the Leadership Summit please contact Brian Frankel at bfrankfel@facs.org, or 312-202-5361. For questions regarding the Advocacy Summit please contact Michael Carmody at mcarmody@facs.org, or 202-672-1511.
Until next month ….
The seventh annual American College of Surgeons Leadership and Advocacy Summit will be held May 19-22 at the Renaissance Washington, DC Downtown Hotel.
The event will kick off with the Leadership portion on Saturday evening, May 19, with a Welcome Reception open to all registrants and continues with a full-day agenda on Sunday, May 20. The program on Sunday includes sessions addressing important topics such as mentoring for a career in surgical leadership, ethics in surgical leadership, leading in times of crisis, change management, managing complex teams, and more.
1) “Understanding Strategic Advocacy” presented by staff of the Washington office
2) “Regulatory Reform: Past, Present, and Patient-Focused” featuring staff from the Centers for Medicare and Medicaid Services.
3) A historical perspective on health care reform entitled, “Health Care Reform, Then and Now,” presented by long-time Health Affairs columnist, Professor Timothy S. Jost.
4) “The Opioid Epidemic: Long-term Solutions for Sustained Success” featuring staff from the Food and Drug Administration and the Drug Enforcement Administration.
5) A luncheon, sponsored by the ACSPA-SurgeonsPAC, where attendees will hear remarks on the upcoming mid-term elections from the Executive Directors of both the Democratic Congressional Campaign Committee (DCCC) and the National Republican Congressional Committee (NRCC).
The day will also include issue briefings and specific “asks” on topics in preparation for Hill visits. Specifically, attendees will be briefed on the Pandemic and All-Hazards Preparedness Act (PAHPA), the Standardizing Electronic Prior Authorization for Safe Prescribing Act, the Ensuring Access to General Surgery Act, the Removing Barriers to Colorectal Screening Act, the Childhood Cancer STAR Act, and funding for the CDC to conduct research on firearm injury prevention. Following this training, Fellows will be very well prepared to discuss the issues the following day on Capitol Hill.
Pending last minute conflicts, several Members of Congress are also scheduled to address the group, including a member of leadership from the House of Representatives. Monday’s activities will conclude with an evening reception for 2018 SurgeonsPAC members at the historic Willard InterContinental Hotel. On Tuesday, May 22, attendees will then apply the knowledge and skill gained from Monday’s sessions during meetings with their individual Members of Congress and their staff on Capitol Hill.
As I write, nearly three weeks prior to the event, attendance is already projected to be at record levels. We look forward to welcoming all those already registered to DC for this exciting, informative and important event. Though by press time pre-registration will have closed, on-site registration will be available if you would be able to join us.
For questions regarding the Leadership Summit please contact Brian Frankel at bfrankfel@facs.org, or 312-202-5361. For questions regarding the Advocacy Summit please contact Michael Carmody at mcarmody@facs.org, or 202-672-1511.
Until next month ….
Glyburide failed to show noninferiority in gestational diabetes
A randomized, multicenter trial failed to find glyburide noninferior to insulin for treatment of gestational diabetes, investigators reported.
The composite rate of macrosomia, neonatal hypoglycemia, and hyperbilirubinemia was 27.6% with oral glyburide and 23.4% with subcutaneous insulin (P = .19) therapy, said Marie-Victoire Sénat, MD, PhD, of Hôpital Bicêtre in Paris, and her associates. The upper limit of the 97.5% confidence interval for the difference between groups was 10.5%, exceeding the prespecified noninferiority margin of 7%. “These findings do not justify the use of glyburide as first-line treatment,” the researchers wrote. The report was published online May 1 in JAMA.
Glyburide is a common add-on therapy for gestational diabetes in the United States but is not used regularly in Europe. The treatments exert similar glycemic control, but meta-analyses and recent studies have linked glyburide to increased rates of neonatal macrosomia and hypoglycemia. However, trials comparing glyburide with insulin focused on maternal glycemic control and thus “were not optimally designed to investigate neonatal complications,” the researchers wrote.
For the study, they randomly assigned 914 women whose gestational diabetes persisted despite dietary intervention to receive either 2.5 mg glyburide once daily or 4 IU to 20 IU insulin one to four times daily. Patients up-titrated treatment as needed based on self-measured blood glucose levels. Glyburide first was increased by 2.5 mg on day 4 and thereafter by 5 mg every 4 days in morning and evening doses to a daily maximum of 20 mg. Prandial insulin was increased by 2 IU every 2 days, while basal or intermediate insulin was dosed at 4 IU to 8 IU at bedtime and increased by 2 IU every 2 days.
The difference in the composite endpoint still exceeded 4% between groups even after the researchers controlled for multiparity and gestational age at treatment. Rates of each individual complication were higher with glyburide than with insulin, although only hypoglycemia reached statistical significance (12.2% for glyburide versus 7.2% for insulin; P = .02).
Maternal hypoglycemia affected 3.8% of the glyburide arm and 1% of the insulin arm (P = .02), and 72% of glyburide patients maintained good fasting glycemic control versus 63% of insulin recipients (P = .003). Also, 58% of glyburide recipients had good postprandial glucose control versus 49% of insulin recipients (P = .051).
Questionnaires indicated that patients were more likely to find glyburide tolerable and to report that they would use it again, if needed, during a future pregnancy (P less than .001 for between-group comparisons). “Although the data do not allow a conclusion that glyburide is not inferior to insulin in the prevention of perinatal complications, the results suggest that the increase in complications may be no more than 10.5% compared with insulin,” the investigators wrote. “This result should be balanced with the ease of use and better satisfaction with glyburide.”
Dr. Sénat reported having no conflicts of interest. One coinvestigator disclosed ties to Ferring Laboratories.
SOURCE: Sénat M-V et al. JAMA. 319(17):1773-80.
The researchers were “reasonable” to conclude that insulin should remain the first-line pharmacotherapy for gestational diabetes, according to Donald R. Coustan, MD, and Linda Barbour, MD, MSPH, whose editorial accompanied the study in JAMA.
“Use of glyburide may be most appropriate when insulin injections are not acceptable or practical,” they wrote. They suggested “frankly” counseling pregnant women about glyburide crossing the placenta and about “unanswered questions regarding long-term effects on offspring.”
Ideally, pregnant women should receive glyburide 1 hour before meals so that its effect peaks 3-4 hours later, according to the experts. But the study authors did not describe treatment timing with respect to meals, did not adjust initial dosing based on fasting or postprandial hyperglycemia, and only increased the dose every 4 days, they noted.
Although insulin was dosed much more flexibly, the glyburide group had better fasting glucose than did controls (72% vs. 63%; P = .003), the editorialists noted. Glyburide is most likely to succeed in younger women without fasting hyperglycemia and whose gestational diabetes begins later in pregnancy. Better dosing and patient selection might make glyburide more effective while also helping prevent maternal hypoglycemia and adverse perinatal outcomes, they contended.
Dr. Coustan is with Brown University, Providence, R.I. Dr. Barbour is with University of Colorado at Denver, Aurora. They reported having no conflicts of interest. These comments paraphrase their editorial ( JAMA. 2018;319[17]:1769-70 ).
The researchers were “reasonable” to conclude that insulin should remain the first-line pharmacotherapy for gestational diabetes, according to Donald R. Coustan, MD, and Linda Barbour, MD, MSPH, whose editorial accompanied the study in JAMA.
“Use of glyburide may be most appropriate when insulin injections are not acceptable or practical,” they wrote. They suggested “frankly” counseling pregnant women about glyburide crossing the placenta and about “unanswered questions regarding long-term effects on offspring.”
Ideally, pregnant women should receive glyburide 1 hour before meals so that its effect peaks 3-4 hours later, according to the experts. But the study authors did not describe treatment timing with respect to meals, did not adjust initial dosing based on fasting or postprandial hyperglycemia, and only increased the dose every 4 days, they noted.
Although insulin was dosed much more flexibly, the glyburide group had better fasting glucose than did controls (72% vs. 63%; P = .003), the editorialists noted. Glyburide is most likely to succeed in younger women without fasting hyperglycemia and whose gestational diabetes begins later in pregnancy. Better dosing and patient selection might make glyburide more effective while also helping prevent maternal hypoglycemia and adverse perinatal outcomes, they contended.
Dr. Coustan is with Brown University, Providence, R.I. Dr. Barbour is with University of Colorado at Denver, Aurora. They reported having no conflicts of interest. These comments paraphrase their editorial ( JAMA. 2018;319[17]:1769-70 ).
The researchers were “reasonable” to conclude that insulin should remain the first-line pharmacotherapy for gestational diabetes, according to Donald R. Coustan, MD, and Linda Barbour, MD, MSPH, whose editorial accompanied the study in JAMA.
“Use of glyburide may be most appropriate when insulin injections are not acceptable or practical,” they wrote. They suggested “frankly” counseling pregnant women about glyburide crossing the placenta and about “unanswered questions regarding long-term effects on offspring.”
Ideally, pregnant women should receive glyburide 1 hour before meals so that its effect peaks 3-4 hours later, according to the experts. But the study authors did not describe treatment timing with respect to meals, did not adjust initial dosing based on fasting or postprandial hyperglycemia, and only increased the dose every 4 days, they noted.
Although insulin was dosed much more flexibly, the glyburide group had better fasting glucose than did controls (72% vs. 63%; P = .003), the editorialists noted. Glyburide is most likely to succeed in younger women without fasting hyperglycemia and whose gestational diabetes begins later in pregnancy. Better dosing and patient selection might make glyburide more effective while also helping prevent maternal hypoglycemia and adverse perinatal outcomes, they contended.
Dr. Coustan is with Brown University, Providence, R.I. Dr. Barbour is with University of Colorado at Denver, Aurora. They reported having no conflicts of interest. These comments paraphrase their editorial ( JAMA. 2018;319[17]:1769-70 ).
A randomized, multicenter trial failed to find glyburide noninferior to insulin for treatment of gestational diabetes, investigators reported.
The composite rate of macrosomia, neonatal hypoglycemia, and hyperbilirubinemia was 27.6% with oral glyburide and 23.4% with subcutaneous insulin (P = .19) therapy, said Marie-Victoire Sénat, MD, PhD, of Hôpital Bicêtre in Paris, and her associates. The upper limit of the 97.5% confidence interval for the difference between groups was 10.5%, exceeding the prespecified noninferiority margin of 7%. “These findings do not justify the use of glyburide as first-line treatment,” the researchers wrote. The report was published online May 1 in JAMA.
Glyburide is a common add-on therapy for gestational diabetes in the United States but is not used regularly in Europe. The treatments exert similar glycemic control, but meta-analyses and recent studies have linked glyburide to increased rates of neonatal macrosomia and hypoglycemia. However, trials comparing glyburide with insulin focused on maternal glycemic control and thus “were not optimally designed to investigate neonatal complications,” the researchers wrote.
For the study, they randomly assigned 914 women whose gestational diabetes persisted despite dietary intervention to receive either 2.5 mg glyburide once daily or 4 IU to 20 IU insulin one to four times daily. Patients up-titrated treatment as needed based on self-measured blood glucose levels. Glyburide first was increased by 2.5 mg on day 4 and thereafter by 5 mg every 4 days in morning and evening doses to a daily maximum of 20 mg. Prandial insulin was increased by 2 IU every 2 days, while basal or intermediate insulin was dosed at 4 IU to 8 IU at bedtime and increased by 2 IU every 2 days.
The difference in the composite endpoint still exceeded 4% between groups even after the researchers controlled for multiparity and gestational age at treatment. Rates of each individual complication were higher with glyburide than with insulin, although only hypoglycemia reached statistical significance (12.2% for glyburide versus 7.2% for insulin; P = .02).
Maternal hypoglycemia affected 3.8% of the glyburide arm and 1% of the insulin arm (P = .02), and 72% of glyburide patients maintained good fasting glycemic control versus 63% of insulin recipients (P = .003). Also, 58% of glyburide recipients had good postprandial glucose control versus 49% of insulin recipients (P = .051).
Questionnaires indicated that patients were more likely to find glyburide tolerable and to report that they would use it again, if needed, during a future pregnancy (P less than .001 for between-group comparisons). “Although the data do not allow a conclusion that glyburide is not inferior to insulin in the prevention of perinatal complications, the results suggest that the increase in complications may be no more than 10.5% compared with insulin,” the investigators wrote. “This result should be balanced with the ease of use and better satisfaction with glyburide.”
Dr. Sénat reported having no conflicts of interest. One coinvestigator disclosed ties to Ferring Laboratories.
SOURCE: Sénat M-V et al. JAMA. 319(17):1773-80.
A randomized, multicenter trial failed to find glyburide noninferior to insulin for treatment of gestational diabetes, investigators reported.
The composite rate of macrosomia, neonatal hypoglycemia, and hyperbilirubinemia was 27.6% with oral glyburide and 23.4% with subcutaneous insulin (P = .19) therapy, said Marie-Victoire Sénat, MD, PhD, of Hôpital Bicêtre in Paris, and her associates. The upper limit of the 97.5% confidence interval for the difference between groups was 10.5%, exceeding the prespecified noninferiority margin of 7%. “These findings do not justify the use of glyburide as first-line treatment,” the researchers wrote. The report was published online May 1 in JAMA.
Glyburide is a common add-on therapy for gestational diabetes in the United States but is not used regularly in Europe. The treatments exert similar glycemic control, but meta-analyses and recent studies have linked glyburide to increased rates of neonatal macrosomia and hypoglycemia. However, trials comparing glyburide with insulin focused on maternal glycemic control and thus “were not optimally designed to investigate neonatal complications,” the researchers wrote.
For the study, they randomly assigned 914 women whose gestational diabetes persisted despite dietary intervention to receive either 2.5 mg glyburide once daily or 4 IU to 20 IU insulin one to four times daily. Patients up-titrated treatment as needed based on self-measured blood glucose levels. Glyburide first was increased by 2.5 mg on day 4 and thereafter by 5 mg every 4 days in morning and evening doses to a daily maximum of 20 mg. Prandial insulin was increased by 2 IU every 2 days, while basal or intermediate insulin was dosed at 4 IU to 8 IU at bedtime and increased by 2 IU every 2 days.
The difference in the composite endpoint still exceeded 4% between groups even after the researchers controlled for multiparity and gestational age at treatment. Rates of each individual complication were higher with glyburide than with insulin, although only hypoglycemia reached statistical significance (12.2% for glyburide versus 7.2% for insulin; P = .02).
Maternal hypoglycemia affected 3.8% of the glyburide arm and 1% of the insulin arm (P = .02), and 72% of glyburide patients maintained good fasting glycemic control versus 63% of insulin recipients (P = .003). Also, 58% of glyburide recipients had good postprandial glucose control versus 49% of insulin recipients (P = .051).
Questionnaires indicated that patients were more likely to find glyburide tolerable and to report that they would use it again, if needed, during a future pregnancy (P less than .001 for between-group comparisons). “Although the data do not allow a conclusion that glyburide is not inferior to insulin in the prevention of perinatal complications, the results suggest that the increase in complications may be no more than 10.5% compared with insulin,” the investigators wrote. “This result should be balanced with the ease of use and better satisfaction with glyburide.”
Dr. Sénat reported having no conflicts of interest. One coinvestigator disclosed ties to Ferring Laboratories.
SOURCE: Sénat M-V et al. JAMA. 319(17):1773-80.
FROM JAMA
Key clinical point: A large trial failed to justify the use of glyburide as first-line therapy for gestational diabetes.
Major finding: Combined rates of macrosomia, neonatal hypoglycemia, and hyperbilirubinemia were 27.6% in the glyburide group and 23.4% in the insulin group (P = .19). The upper limit of the confidence interval for the difference between groups was 10.5%, exceeding the prespecified noninferiority margin of 7%.
Study details: Multicenter randomized trial of 914 women with gestational diabetes.
Disclosures: Dr. Sénat reported having no conflicts of interest. One coinvestigator disclosed ties to Ferring Laboratories.
Source: Sénat M-V et al. JAMA. 319(17):1773-80.
Celiac disease: Can biopsy be avoided?
LAS VEGAS – It may be only a matter of time before the “gold standard” small biopsy is no longer considered mandatory to make a diagnosis of celiac disease in adults, according to Joseph A. Murray, MD, consultant in the division of gastroenterology and hepatology and department of immunology, Mayo Clinic, Rochester, Minn.
“Right now, none of the adult societies support biopsy avoidance, but I predict that it will come to be,” Dr. Murray said at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
In one recently reported study, investigators at Royal Derby Hospital, England, suggested that clinicians could make a reliable diagnosis of celiac disease by looking at serum IgA-tissue transglutaminase antibody levels.
Those investigators retrospectively analyzed an unselected series of 270 adult patients and found that an IgA-tissue transglutaminase antibody cut-off of 45 U/mL, or 8 times the upper limit of normal, had a positive predictive value of 100%.
Biopsy avoidance remains controversial, however. In a published letter to the editor commenting on the Derby study, authors took issue with some of the statistical analysis and remarked that the study included some patients with Marsh 1 histology.
“Studies suggest that the majority of seropositive patients with Marsh 1 histology do not progress to develop villous atrophy while on a gluten-containing diet, raising the question whether all of them are truly celiac,” they wrote.
The first society to endorse skipping the biopsy was the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
In guidelines for the diagnosis of celiac disease, that group said a celiac diagnosis could be made based on symptoms, antibodies, and HLA in children with symptoms suggestive of the disease and high antibody levels (IgA anti-tissue transglutaminase type 2 antibody titers greater than 10 times the upper limit of normal).
“The data [are] now pretty good to support that approach in symptomatic children,” Dr. Murray said. “If we apply these to adult patients, it’s not bad, actually, partly because our biopsies aren’t perfect.”
However, not all adult gastroenterology specialists agreed with the recommendations of the pediatric society. Guidelines from the British Society of Gastroenterology have stated that serology cannot replace biopsy, which “remains essential” for celiac disease diagnosis.
Global Academy and this news organization are owned by the same parent company.
Dr. Murray reported disclosures related to Ardent Mills, DBV Technologies, Evelo, GlaxoSmithKline, Johnson & Johnson, Immunogenix, Innovate, National Center for Complementary and Integrative Health, Takeda, Torax Medical, and UCB.
LAS VEGAS – It may be only a matter of time before the “gold standard” small biopsy is no longer considered mandatory to make a diagnosis of celiac disease in adults, according to Joseph A. Murray, MD, consultant in the division of gastroenterology and hepatology and department of immunology, Mayo Clinic, Rochester, Minn.
“Right now, none of the adult societies support biopsy avoidance, but I predict that it will come to be,” Dr. Murray said at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
In one recently reported study, investigators at Royal Derby Hospital, England, suggested that clinicians could make a reliable diagnosis of celiac disease by looking at serum IgA-tissue transglutaminase antibody levels.
Those investigators retrospectively analyzed an unselected series of 270 adult patients and found that an IgA-tissue transglutaminase antibody cut-off of 45 U/mL, or 8 times the upper limit of normal, had a positive predictive value of 100%.
Biopsy avoidance remains controversial, however. In a published letter to the editor commenting on the Derby study, authors took issue with some of the statistical analysis and remarked that the study included some patients with Marsh 1 histology.
“Studies suggest that the majority of seropositive patients with Marsh 1 histology do not progress to develop villous atrophy while on a gluten-containing diet, raising the question whether all of them are truly celiac,” they wrote.
The first society to endorse skipping the biopsy was the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
In guidelines for the diagnosis of celiac disease, that group said a celiac diagnosis could be made based on symptoms, antibodies, and HLA in children with symptoms suggestive of the disease and high antibody levels (IgA anti-tissue transglutaminase type 2 antibody titers greater than 10 times the upper limit of normal).
“The data [are] now pretty good to support that approach in symptomatic children,” Dr. Murray said. “If we apply these to adult patients, it’s not bad, actually, partly because our biopsies aren’t perfect.”
However, not all adult gastroenterology specialists agreed with the recommendations of the pediatric society. Guidelines from the British Society of Gastroenterology have stated that serology cannot replace biopsy, which “remains essential” for celiac disease diagnosis.
Global Academy and this news organization are owned by the same parent company.
Dr. Murray reported disclosures related to Ardent Mills, DBV Technologies, Evelo, GlaxoSmithKline, Johnson & Johnson, Immunogenix, Innovate, National Center for Complementary and Integrative Health, Takeda, Torax Medical, and UCB.
LAS VEGAS – It may be only a matter of time before the “gold standard” small biopsy is no longer considered mandatory to make a diagnosis of celiac disease in adults, according to Joseph A. Murray, MD, consultant in the division of gastroenterology and hepatology and department of immunology, Mayo Clinic, Rochester, Minn.
“Right now, none of the adult societies support biopsy avoidance, but I predict that it will come to be,” Dr. Murray said at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
In one recently reported study, investigators at Royal Derby Hospital, England, suggested that clinicians could make a reliable diagnosis of celiac disease by looking at serum IgA-tissue transglutaminase antibody levels.
Those investigators retrospectively analyzed an unselected series of 270 adult patients and found that an IgA-tissue transglutaminase antibody cut-off of 45 U/mL, or 8 times the upper limit of normal, had a positive predictive value of 100%.
Biopsy avoidance remains controversial, however. In a published letter to the editor commenting on the Derby study, authors took issue with some of the statistical analysis and remarked that the study included some patients with Marsh 1 histology.
“Studies suggest that the majority of seropositive patients with Marsh 1 histology do not progress to develop villous atrophy while on a gluten-containing diet, raising the question whether all of them are truly celiac,” they wrote.
The first society to endorse skipping the biopsy was the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
In guidelines for the diagnosis of celiac disease, that group said a celiac diagnosis could be made based on symptoms, antibodies, and HLA in children with symptoms suggestive of the disease and high antibody levels (IgA anti-tissue transglutaminase type 2 antibody titers greater than 10 times the upper limit of normal).
“The data [are] now pretty good to support that approach in symptomatic children,” Dr. Murray said. “If we apply these to adult patients, it’s not bad, actually, partly because our biopsies aren’t perfect.”
However, not all adult gastroenterology specialists agreed with the recommendations of the pediatric society. Guidelines from the British Society of Gastroenterology have stated that serology cannot replace biopsy, which “remains essential” for celiac disease diagnosis.
Global Academy and this news organization are owned by the same parent company.
Dr. Murray reported disclosures related to Ardent Mills, DBV Technologies, Evelo, GlaxoSmithKline, Johnson & Johnson, Immunogenix, Innovate, National Center for Complementary and Integrative Health, Takeda, Torax Medical, and UCB.
EXPERT ANALYSIS FROM PERSPECTIVES IN DIGESTIVE DISEASES
Endoscopic therapy for Barrett’s: highly effective, but not perfect
LAS VEGAS – While endoscopic therapy of Barrett’s esophagus is often successful, the risk of recurrence after complete ablation remains considerable, according to Prateek Sharma, MD, of the department of medicine in the division of gastroenterology and hepatology at the University of Kansas, Kansas City.
“This is not perfect therapy,” Dr. Sharma said in a presentation at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
The risk of recurrence for intestinal metaplasia was 7.1% per patient-year, authors of that study found. The risk of dysplastic Barrett’s esophagus was 1.3% per patient-year in the meta-analysis, while the risk of high-grade dysplasia or esophageal adenocarcinoma was 0.8%.
Because of these risks, patients should be followed up regularly with careful examination and biopsies to ensure there is no recurrent intestinal metaplasia, dysplasia, or adenocarcinoma, Dr. Sharma told attendees at the meeting.
“If it comes back, you can still treat it endoscopically,” he continued, “but you have to be aware of the situation, and inform the patient that it can be curative, but at the same time it’s not a 100% success story in all situations.”
When patients do develop early cancers or high-grade lesions, the latest evidence suggests endoscopic therapy is effective and helps avoid esophagectomy in the majority of patients.
“It used to be esophagectomy for all,” Dr. Sharma said. “Now the paradigm has switched, and it is endoscopic therapy for all.”
That paradigm shift is supported in part by a German study showing excellent long-term results in 1,000 consecutive patients receiving endoscopic treatment of mucosal adenocarcinoma of the esophagus.
In that study, nearly all of the patients (963, or 96.3%) had a complete response, with 12 patients undergoing surgery because of failure of endoscopic therapy. Fifteen patients (1.5%) had major complications that were nonetheless managed conservatively, according to investigators. Although new lesions or recurrences were seen in 140 patients (14.5%) over 5 years of follow-up, 115 patients had successful endoscopic retreatment. Based on these data, the investigators calculated a 10-year survival rate of 75%.
On the basis of these findings, investigators said endoscopic therapy should be considered the standard of care for patients with mucosal adenocarcinoma of the esophagus.
“That’s the paradigm shift that I was talking about – now, we are sending less than 4% of our patients for surgery for this condition,” Dr. Sharma said at the meeting.
Global Academy and this news organization are owned by the same parent company.
Dr. Sharma reported disclosures related to Medtronics, National Institutes of Health, and US Endoscopy.
LAS VEGAS – While endoscopic therapy of Barrett’s esophagus is often successful, the risk of recurrence after complete ablation remains considerable, according to Prateek Sharma, MD, of the department of medicine in the division of gastroenterology and hepatology at the University of Kansas, Kansas City.
“This is not perfect therapy,” Dr. Sharma said in a presentation at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
The risk of recurrence for intestinal metaplasia was 7.1% per patient-year, authors of that study found. The risk of dysplastic Barrett’s esophagus was 1.3% per patient-year in the meta-analysis, while the risk of high-grade dysplasia or esophageal adenocarcinoma was 0.8%.
Because of these risks, patients should be followed up regularly with careful examination and biopsies to ensure there is no recurrent intestinal metaplasia, dysplasia, or adenocarcinoma, Dr. Sharma told attendees at the meeting.
“If it comes back, you can still treat it endoscopically,” he continued, “but you have to be aware of the situation, and inform the patient that it can be curative, but at the same time it’s not a 100% success story in all situations.”
When patients do develop early cancers or high-grade lesions, the latest evidence suggests endoscopic therapy is effective and helps avoid esophagectomy in the majority of patients.
“It used to be esophagectomy for all,” Dr. Sharma said. “Now the paradigm has switched, and it is endoscopic therapy for all.”
That paradigm shift is supported in part by a German study showing excellent long-term results in 1,000 consecutive patients receiving endoscopic treatment of mucosal adenocarcinoma of the esophagus.
In that study, nearly all of the patients (963, or 96.3%) had a complete response, with 12 patients undergoing surgery because of failure of endoscopic therapy. Fifteen patients (1.5%) had major complications that were nonetheless managed conservatively, according to investigators. Although new lesions or recurrences were seen in 140 patients (14.5%) over 5 years of follow-up, 115 patients had successful endoscopic retreatment. Based on these data, the investigators calculated a 10-year survival rate of 75%.
On the basis of these findings, investigators said endoscopic therapy should be considered the standard of care for patients with mucosal adenocarcinoma of the esophagus.
“That’s the paradigm shift that I was talking about – now, we are sending less than 4% of our patients for surgery for this condition,” Dr. Sharma said at the meeting.
Global Academy and this news organization are owned by the same parent company.
Dr. Sharma reported disclosures related to Medtronics, National Institutes of Health, and US Endoscopy.
LAS VEGAS – While endoscopic therapy of Barrett’s esophagus is often successful, the risk of recurrence after complete ablation remains considerable, according to Prateek Sharma, MD, of the department of medicine in the division of gastroenterology and hepatology at the University of Kansas, Kansas City.
“This is not perfect therapy,” Dr. Sharma said in a presentation at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
The risk of recurrence for intestinal metaplasia was 7.1% per patient-year, authors of that study found. The risk of dysplastic Barrett’s esophagus was 1.3% per patient-year in the meta-analysis, while the risk of high-grade dysplasia or esophageal adenocarcinoma was 0.8%.
Because of these risks, patients should be followed up regularly with careful examination and biopsies to ensure there is no recurrent intestinal metaplasia, dysplasia, or adenocarcinoma, Dr. Sharma told attendees at the meeting.
“If it comes back, you can still treat it endoscopically,” he continued, “but you have to be aware of the situation, and inform the patient that it can be curative, but at the same time it’s not a 100% success story in all situations.”
When patients do develop early cancers or high-grade lesions, the latest evidence suggests endoscopic therapy is effective and helps avoid esophagectomy in the majority of patients.
“It used to be esophagectomy for all,” Dr. Sharma said. “Now the paradigm has switched, and it is endoscopic therapy for all.”
That paradigm shift is supported in part by a German study showing excellent long-term results in 1,000 consecutive patients receiving endoscopic treatment of mucosal adenocarcinoma of the esophagus.
In that study, nearly all of the patients (963, or 96.3%) had a complete response, with 12 patients undergoing surgery because of failure of endoscopic therapy. Fifteen patients (1.5%) had major complications that were nonetheless managed conservatively, according to investigators. Although new lesions or recurrences were seen in 140 patients (14.5%) over 5 years of follow-up, 115 patients had successful endoscopic retreatment. Based on these data, the investigators calculated a 10-year survival rate of 75%.
On the basis of these findings, investigators said endoscopic therapy should be considered the standard of care for patients with mucosal adenocarcinoma of the esophagus.
“That’s the paradigm shift that I was talking about – now, we are sending less than 4% of our patients for surgery for this condition,” Dr. Sharma said at the meeting.
Global Academy and this news organization are owned by the same parent company.
Dr. Sharma reported disclosures related to Medtronics, National Institutes of Health, and US Endoscopy.
EXPERT ANALYSIS FROM PERSPECTIVES IN DIGESTIVE DISEASES
MDedge Daily News: Is ‘medical aid in dying’ suicide?
PPIs remain suspects in cognitive decline. Which beta-blocker is best for hypertension? And one in five Medicaid kids may have a mental health diagnosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
PPIs remain suspects in cognitive decline. Which beta-blocker is best for hypertension? And one in five Medicaid kids may have a mental health diagnosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
PPIs remain suspects in cognitive decline. Which beta-blocker is best for hypertension? And one in five Medicaid kids may have a mental health diagnosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
FDA approves CAR T-cell therapy for lymphoma
The US Food and Drug Administration (FDA) has approved tisagenlecleucel (Kymriah®) for its second indication.
The chimeric antigen receptor (CAR) T-cell therapy is now approved to treat adults with relapsed or refractory large B-cell lymphoma after 2 or more lines of systemic therapy.
This includes patients with diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
The application for tisagenlecleucel in B-cell lymphoma was granted priority review. The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
Tisagenlecleucel is also FDA-approved to treat patients age 25 and younger who have B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Access to tisagenlecleucel
The prescribing information for tisagenlecleucel includes a boxed warning detailing the risk of cytokine release syndrome (CRS) and neurological toxicities for patients receiving tisagenlecleucel.
Because of these risks, tisagenlecleucel is only available through a Risk Evaluation and Mitigation Strategy (REMS) program. The REMS program serves to inform and educate healthcare professionals about the risks associated with tisagenlecleucel treatment.
Novartis, the company marketing tisagenlecleucel, has established a network of certified treatment centers throughout the US. Staff at these centers are trained on the use of tisagenlecleucel and appropriate patient care.
Tisagenlecleucel is manufactured at a Novartis facility in Morris Plains, New Jersey. In the US, the target turnaround time for manufacturing tisagenlecleucel is 22 days.
Tisagenlecleucel costs $475,000 for a single course of treatment. However, Novartis said it is collaborating with the US Centers for Medicare and Medicaid Services on the creation of an appropriate value-based pricing approach.
The company also has a program called KYMRIAH CARES™, which offers financial assistance to eligible patients to help them gain access to tisagenlecleucel.
Phase 2 trial
The FDA approval of tisagenlecleucel for adults with relapsed/refractory B-cell lymphoma is based on results of the phase 2 JULIET trial.
The prescribing information for tisagenlecleucel includes data on 106 patients treated on this trial.
Only 68 of these patients were evaluable for efficacy. They had a median age of 56 (range, 22 to 74), and 71% were male.
Seventy-eight percent of patients had primary DLBCL not otherwise specified, and 22% had DLBCL following transformation from follicular lymphoma. Seventeen percent had high grade DLBCL.
Fifty-six percent of patients had refractory disease, and 44% had relapsed after their last therapy. The median number of prior therapies was 3 (range, 1 to 6), and 44% of patients had undergone autologous transplant.
Ninety percent of patients received lymphodepleting chemotherapy (66% fludarabine and 24% bendamustine) prior to tisagenlecleucel, and 10% did not. The median dose of tisagenlecleucel was 3.5 × 108 CAR+ T cells (range, 1.0 to 5.2 × 108).
The overall response rate was 50%, with 32% of patients achieving a complete response and 18% achieving a partial response. The median duration of response was not reached with a median follow-up of 9.4 months.
In all 106 patients infused with tisagenlecleucel, the most common grade 3/4 adverse events were infections (25%), CRS (23%), neurologic events (18%), febrile neutropenia (17%), encephalopathy (11%), lymphopenia (94%), neutropenia (81%), leukopenia (77%), anemia (58%), thrombocytopenia (54%), hypophosphatemia (24%), hypokalemia (12%), and hyponatremia (11%).
Three patients died within 30 days of tisagenlecleucel infusion. All of them had CRS and either stable or progressive disease. One of these patients developed bowel necrosis.
One patient died of infection. There were no deaths attributed to neurological events, and no fatal cases of cerebral edema.
The US Food and Drug Administration (FDA) has approved tisagenlecleucel (Kymriah®) for its second indication.
The chimeric antigen receptor (CAR) T-cell therapy is now approved to treat adults with relapsed or refractory large B-cell lymphoma after 2 or more lines of systemic therapy.
This includes patients with diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
The application for tisagenlecleucel in B-cell lymphoma was granted priority review. The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
Tisagenlecleucel is also FDA-approved to treat patients age 25 and younger who have B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Access to tisagenlecleucel
The prescribing information for tisagenlecleucel includes a boxed warning detailing the risk of cytokine release syndrome (CRS) and neurological toxicities for patients receiving tisagenlecleucel.
Because of these risks, tisagenlecleucel is only available through a Risk Evaluation and Mitigation Strategy (REMS) program. The REMS program serves to inform and educate healthcare professionals about the risks associated with tisagenlecleucel treatment.
Novartis, the company marketing tisagenlecleucel, has established a network of certified treatment centers throughout the US. Staff at these centers are trained on the use of tisagenlecleucel and appropriate patient care.
Tisagenlecleucel is manufactured at a Novartis facility in Morris Plains, New Jersey. In the US, the target turnaround time for manufacturing tisagenlecleucel is 22 days.
Tisagenlecleucel costs $475,000 for a single course of treatment. However, Novartis said it is collaborating with the US Centers for Medicare and Medicaid Services on the creation of an appropriate value-based pricing approach.
The company also has a program called KYMRIAH CARES™, which offers financial assistance to eligible patients to help them gain access to tisagenlecleucel.
Phase 2 trial
The FDA approval of tisagenlecleucel for adults with relapsed/refractory B-cell lymphoma is based on results of the phase 2 JULIET trial.
The prescribing information for tisagenlecleucel includes data on 106 patients treated on this trial.
Only 68 of these patients were evaluable for efficacy. They had a median age of 56 (range, 22 to 74), and 71% were male.
Seventy-eight percent of patients had primary DLBCL not otherwise specified, and 22% had DLBCL following transformation from follicular lymphoma. Seventeen percent had high grade DLBCL.
Fifty-six percent of patients had refractory disease, and 44% had relapsed after their last therapy. The median number of prior therapies was 3 (range, 1 to 6), and 44% of patients had undergone autologous transplant.
Ninety percent of patients received lymphodepleting chemotherapy (66% fludarabine and 24% bendamustine) prior to tisagenlecleucel, and 10% did not. The median dose of tisagenlecleucel was 3.5 × 108 CAR+ T cells (range, 1.0 to 5.2 × 108).
The overall response rate was 50%, with 32% of patients achieving a complete response and 18% achieving a partial response. The median duration of response was not reached with a median follow-up of 9.4 months.
In all 106 patients infused with tisagenlecleucel, the most common grade 3/4 adverse events were infections (25%), CRS (23%), neurologic events (18%), febrile neutropenia (17%), encephalopathy (11%), lymphopenia (94%), neutropenia (81%), leukopenia (77%), anemia (58%), thrombocytopenia (54%), hypophosphatemia (24%), hypokalemia (12%), and hyponatremia (11%).
Three patients died within 30 days of tisagenlecleucel infusion. All of them had CRS and either stable or progressive disease. One of these patients developed bowel necrosis.
One patient died of infection. There were no deaths attributed to neurological events, and no fatal cases of cerebral edema.
The US Food and Drug Administration (FDA) has approved tisagenlecleucel (Kymriah®) for its second indication.
The chimeric antigen receptor (CAR) T-cell therapy is now approved to treat adults with relapsed or refractory large B-cell lymphoma after 2 or more lines of systemic therapy.
This includes patients with diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
The application for tisagenlecleucel in B-cell lymphoma was granted priority review. The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
Tisagenlecleucel is also FDA-approved to treat patients age 25 and younger who have B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Access to tisagenlecleucel
The prescribing information for tisagenlecleucel includes a boxed warning detailing the risk of cytokine release syndrome (CRS) and neurological toxicities for patients receiving tisagenlecleucel.
Because of these risks, tisagenlecleucel is only available through a Risk Evaluation and Mitigation Strategy (REMS) program. The REMS program serves to inform and educate healthcare professionals about the risks associated with tisagenlecleucel treatment.
Novartis, the company marketing tisagenlecleucel, has established a network of certified treatment centers throughout the US. Staff at these centers are trained on the use of tisagenlecleucel and appropriate patient care.
Tisagenlecleucel is manufactured at a Novartis facility in Morris Plains, New Jersey. In the US, the target turnaround time for manufacturing tisagenlecleucel is 22 days.
Tisagenlecleucel costs $475,000 for a single course of treatment. However, Novartis said it is collaborating with the US Centers for Medicare and Medicaid Services on the creation of an appropriate value-based pricing approach.
The company also has a program called KYMRIAH CARES™, which offers financial assistance to eligible patients to help them gain access to tisagenlecleucel.
Phase 2 trial
The FDA approval of tisagenlecleucel for adults with relapsed/refractory B-cell lymphoma is based on results of the phase 2 JULIET trial.
The prescribing information for tisagenlecleucel includes data on 106 patients treated on this trial.
Only 68 of these patients were evaluable for efficacy. They had a median age of 56 (range, 22 to 74), and 71% were male.
Seventy-eight percent of patients had primary DLBCL not otherwise specified, and 22% had DLBCL following transformation from follicular lymphoma. Seventeen percent had high grade DLBCL.
Fifty-six percent of patients had refractory disease, and 44% had relapsed after their last therapy. The median number of prior therapies was 3 (range, 1 to 6), and 44% of patients had undergone autologous transplant.
Ninety percent of patients received lymphodepleting chemotherapy (66% fludarabine and 24% bendamustine) prior to tisagenlecleucel, and 10% did not. The median dose of tisagenlecleucel was 3.5 × 108 CAR+ T cells (range, 1.0 to 5.2 × 108).
The overall response rate was 50%, with 32% of patients achieving a complete response and 18% achieving a partial response. The median duration of response was not reached with a median follow-up of 9.4 months.
In all 106 patients infused with tisagenlecleucel, the most common grade 3/4 adverse events were infections (25%), CRS (23%), neurologic events (18%), febrile neutropenia (17%), encephalopathy (11%), lymphopenia (94%), neutropenia (81%), leukopenia (77%), anemia (58%), thrombocytopenia (54%), hypophosphatemia (24%), hypokalemia (12%), and hyponatremia (11%).
Three patients died within 30 days of tisagenlecleucel infusion. All of them had CRS and either stable or progressive disease. One of these patients developed bowel necrosis.
One patient died of infection. There were no deaths attributed to neurological events, and no fatal cases of cerebral edema.
Drug may alleviate CIPN in MM patients
Researchers say they’ve discovered why multiple myeloma (MM) patients may experience chemotherapy-induced peripheral neuropathy (CIPN) when treated with bortezomib.
The group’s study also suggests fingolimod—a drug approved to treat multiple sclerosis—could mitigate CIPN without compromising the efficacy of bortezomib.
Daniela Salvemini, PhD, of the Saint Louis University School of Medicine in St. Louis, Missouri, and her colleagues reported these findings in the Journal of Experimental Medicine.
The researchers said bortezomib causes CIPN in more than 40% of patients, but the reasons for this are unclear.
With their study, Dr Salvemini and her colleagues found that bortezomib accelerates the production of sphingolipids, which have been linked to neuropathic pain.
Rats treated with bortezomib began to accumulate 2 sphingolipid metabolites—sphingosine 1-phosphate and dihydrosphingosine 1-phosphate—in their spinal cords at the time they began to show signs of neuropathic pain.
Blocking the production of these molecules prevented the animals from developing CIPN in response to bortezomib.
Sphingosine 1-phosphate and dihydrosphingosine 1-phosphate can activate a cell surface receptor protein called S1PR1. Dr Salvemini and her colleagues determined that the 2 metabolites cause CIPN by activating S1PR1 on the surface of astrocytes, resulting in neuroinflammation and enhanced release of the excitatory neurotransmitter glutamate.
Drugs that inhibit S1PR1 prevented rats from developing CIPN in response to bortezomib. One such inhibitor was fingolimod, a drug approved by the US Food and Drug Administration (FDA) to treat multiple sclerosis.
In addition to preventing CIPN, fingolimod did not inhibit bortezomib’s ability to kill MM cells. In fact, fingolimod has demonstrated anticancer activity in past studies.
“Because fingolimod shows promising anticancer potential and is already FDA-approved, we think that our findings in rats can be rapidly translated to the clinic to prevent and treat bortezomib-induced neuropathic pain,” Dr Salvemini said.
Researchers say they’ve discovered why multiple myeloma (MM) patients may experience chemotherapy-induced peripheral neuropathy (CIPN) when treated with bortezomib.
The group’s study also suggests fingolimod—a drug approved to treat multiple sclerosis—could mitigate CIPN without compromising the efficacy of bortezomib.
Daniela Salvemini, PhD, of the Saint Louis University School of Medicine in St. Louis, Missouri, and her colleagues reported these findings in the Journal of Experimental Medicine.
The researchers said bortezomib causes CIPN in more than 40% of patients, but the reasons for this are unclear.
With their study, Dr Salvemini and her colleagues found that bortezomib accelerates the production of sphingolipids, which have been linked to neuropathic pain.
Rats treated with bortezomib began to accumulate 2 sphingolipid metabolites—sphingosine 1-phosphate and dihydrosphingosine 1-phosphate—in their spinal cords at the time they began to show signs of neuropathic pain.
Blocking the production of these molecules prevented the animals from developing CIPN in response to bortezomib.
Sphingosine 1-phosphate and dihydrosphingosine 1-phosphate can activate a cell surface receptor protein called S1PR1. Dr Salvemini and her colleagues determined that the 2 metabolites cause CIPN by activating S1PR1 on the surface of astrocytes, resulting in neuroinflammation and enhanced release of the excitatory neurotransmitter glutamate.
Drugs that inhibit S1PR1 prevented rats from developing CIPN in response to bortezomib. One such inhibitor was fingolimod, a drug approved by the US Food and Drug Administration (FDA) to treat multiple sclerosis.
In addition to preventing CIPN, fingolimod did not inhibit bortezomib’s ability to kill MM cells. In fact, fingolimod has demonstrated anticancer activity in past studies.
“Because fingolimod shows promising anticancer potential and is already FDA-approved, we think that our findings in rats can be rapidly translated to the clinic to prevent and treat bortezomib-induced neuropathic pain,” Dr Salvemini said.
Researchers say they’ve discovered why multiple myeloma (MM) patients may experience chemotherapy-induced peripheral neuropathy (CIPN) when treated with bortezomib.
The group’s study also suggests fingolimod—a drug approved to treat multiple sclerosis—could mitigate CIPN without compromising the efficacy of bortezomib.
Daniela Salvemini, PhD, of the Saint Louis University School of Medicine in St. Louis, Missouri, and her colleagues reported these findings in the Journal of Experimental Medicine.
The researchers said bortezomib causes CIPN in more than 40% of patients, but the reasons for this are unclear.
With their study, Dr Salvemini and her colleagues found that bortezomib accelerates the production of sphingolipids, which have been linked to neuropathic pain.
Rats treated with bortezomib began to accumulate 2 sphingolipid metabolites—sphingosine 1-phosphate and dihydrosphingosine 1-phosphate—in their spinal cords at the time they began to show signs of neuropathic pain.
Blocking the production of these molecules prevented the animals from developing CIPN in response to bortezomib.
Sphingosine 1-phosphate and dihydrosphingosine 1-phosphate can activate a cell surface receptor protein called S1PR1. Dr Salvemini and her colleagues determined that the 2 metabolites cause CIPN by activating S1PR1 on the surface of astrocytes, resulting in neuroinflammation and enhanced release of the excitatory neurotransmitter glutamate.
Drugs that inhibit S1PR1 prevented rats from developing CIPN in response to bortezomib. One such inhibitor was fingolimod, a drug approved by the US Food and Drug Administration (FDA) to treat multiple sclerosis.
In addition to preventing CIPN, fingolimod did not inhibit bortezomib’s ability to kill MM cells. In fact, fingolimod has demonstrated anticancer activity in past studies.
“Because fingolimod shows promising anticancer potential and is already FDA-approved, we think that our findings in rats can be rapidly translated to the clinic to prevent and treat bortezomib-induced neuropathic pain,” Dr Salvemini said.