User login
Continued extension of time for thrombolysis in stroke
Background: Current guidelines for ischemic stroke recommend the time to thrombolysis be within 4.5 hours after onset of stroke. Guidelines are based on noncontrasted CT, but CT perfusion and perfusion-diffusion MRI may show salvageable brain tissue beyond the 4.5 hours. Studies have shown better outcomes in patients who were chosen for reperfusion based on tissue viability rather than time from onset of stroke. This has resulted in a disparity between the time windows used for thrombolysis.
Study design: Multicenter, randomized, placebo-controlled trial.
Setting: Hospitalized patients with acute ischemic stroke from 16 centers in Australia, 10 centers in Taiwan, 1 center in New Zealand, and 1 center in Finland.
Synopsis: 225 patients (aged 18 years and older) with acute ischemic stroke with hypoperfused but salvageable areas of brain detected on CT perfusion imaging or perfusion-diffusion MRI were randomly assigned to receive IV alteplase or placebo between 4.5 and 9 hours after onset of stroke or on awakening with stroke. Primary outcome measured on modified Rankin scale was 0 (no neurologic deficit) or 1. Before the trial was fully enrolled, it was terminated because of a loss of equipoise based on positive results from a previous trial. Of the patients enrolled, the primary outcome occurred in 35.4% of the alteplase group and 29.5% in the placebo group (adjusted risk ratio, 1.44). Symptomatic intracerebral hemorrhage was experienced in 6.2% of the patients in the alteplase group and 0.9% of patients in the placebo group (adjusted risk ratio, 7.22).
Not all centers may have access to perfusion imaging, so the study’s findings may not be applicable to multiple sites.
Bottom line: Diffusion-perfusion imaging may be useful in determining salvageable brain tissue in acute ischemic stroke that may benefit from thrombolysis after the standard 4.5-hour window, but further studies need to be conducted before guidelines are changed.
Citation: Ma H et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795-803.
Dr. Rogers is a hospitalist at Ochsner Health System, New Orleans.
Background: Current guidelines for ischemic stroke recommend the time to thrombolysis be within 4.5 hours after onset of stroke. Guidelines are based on noncontrasted CT, but CT perfusion and perfusion-diffusion MRI may show salvageable brain tissue beyond the 4.5 hours. Studies have shown better outcomes in patients who were chosen for reperfusion based on tissue viability rather than time from onset of stroke. This has resulted in a disparity between the time windows used for thrombolysis.
Study design: Multicenter, randomized, placebo-controlled trial.
Setting: Hospitalized patients with acute ischemic stroke from 16 centers in Australia, 10 centers in Taiwan, 1 center in New Zealand, and 1 center in Finland.
Synopsis: 225 patients (aged 18 years and older) with acute ischemic stroke with hypoperfused but salvageable areas of brain detected on CT perfusion imaging or perfusion-diffusion MRI were randomly assigned to receive IV alteplase or placebo between 4.5 and 9 hours after onset of stroke or on awakening with stroke. Primary outcome measured on modified Rankin scale was 0 (no neurologic deficit) or 1. Before the trial was fully enrolled, it was terminated because of a loss of equipoise based on positive results from a previous trial. Of the patients enrolled, the primary outcome occurred in 35.4% of the alteplase group and 29.5% in the placebo group (adjusted risk ratio, 1.44). Symptomatic intracerebral hemorrhage was experienced in 6.2% of the patients in the alteplase group and 0.9% of patients in the placebo group (adjusted risk ratio, 7.22).
Not all centers may have access to perfusion imaging, so the study’s findings may not be applicable to multiple sites.
Bottom line: Diffusion-perfusion imaging may be useful in determining salvageable brain tissue in acute ischemic stroke that may benefit from thrombolysis after the standard 4.5-hour window, but further studies need to be conducted before guidelines are changed.
Citation: Ma H et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795-803.
Dr. Rogers is a hospitalist at Ochsner Health System, New Orleans.
Background: Current guidelines for ischemic stroke recommend the time to thrombolysis be within 4.5 hours after onset of stroke. Guidelines are based on noncontrasted CT, but CT perfusion and perfusion-diffusion MRI may show salvageable brain tissue beyond the 4.5 hours. Studies have shown better outcomes in patients who were chosen for reperfusion based on tissue viability rather than time from onset of stroke. This has resulted in a disparity between the time windows used for thrombolysis.
Study design: Multicenter, randomized, placebo-controlled trial.
Setting: Hospitalized patients with acute ischemic stroke from 16 centers in Australia, 10 centers in Taiwan, 1 center in New Zealand, and 1 center in Finland.
Synopsis: 225 patients (aged 18 years and older) with acute ischemic stroke with hypoperfused but salvageable areas of brain detected on CT perfusion imaging or perfusion-diffusion MRI were randomly assigned to receive IV alteplase or placebo between 4.5 and 9 hours after onset of stroke or on awakening with stroke. Primary outcome measured on modified Rankin scale was 0 (no neurologic deficit) or 1. Before the trial was fully enrolled, it was terminated because of a loss of equipoise based on positive results from a previous trial. Of the patients enrolled, the primary outcome occurred in 35.4% of the alteplase group and 29.5% in the placebo group (adjusted risk ratio, 1.44). Symptomatic intracerebral hemorrhage was experienced in 6.2% of the patients in the alteplase group and 0.9% of patients in the placebo group (adjusted risk ratio, 7.22).
Not all centers may have access to perfusion imaging, so the study’s findings may not be applicable to multiple sites.
Bottom line: Diffusion-perfusion imaging may be useful in determining salvageable brain tissue in acute ischemic stroke that may benefit from thrombolysis after the standard 4.5-hour window, but further studies need to be conducted before guidelines are changed.
Citation: Ma H et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795-803.
Dr. Rogers is a hospitalist at Ochsner Health System, New Orleans.
Consensus document reviews determination of brain death
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
Long-lasting COVID-19 symptoms: Patients want answers
Q&A with Dr. Sachin Gupta
For some patients, a bout of COVID-19 may not be over after hospital discharge, acute symptoms subside, or a couple of tests for SARS-CoV-2 come back negative. Those who have reached these milestones of conquering the disease may find that their recovery journey has only begun. Debilitating symptoms such as fatigue, headache, and dyspnea may linger for weeks or longer. Patients with persistent symptoms, often referred to as “long haulers” in reference to the duration of their recovery, are looking for answers about their condition and when their COVID-19 illness will finally resolve.
Long-haul patients organize
What started as an accumulation of anecdotal evidence in social media, blogs, and the mainstream press about slow recovery and long-lasting symptoms of COVID-19 is now the focus of clinical trials in the population of recovering patients. Projects such as the COVID Symptom Study, initiated by the Massachusetts General Hospital, Boston; the Harvard School of Public Health, Boston; King’s College London; and Stanford (Calif.) University, are collecting data on symptoms from millions of patients and will eventually contribute to a better understanding of prolonged recovery.
Patients looking for answers have created groups on social media such as Facebook to exchange information about their experiences (e.g., Survivor Corps, COVID-19 Support Group, COVID-19 Recovered Survivors). Recovering patients have created patient-led research organizations (Body Politic COVID-19 Support Group) to explore persistent symptoms and begin to create data for research.
Some data on lingering symptoms
A small study of 143 previously hospitalized, recovering patients in Italy found that 87.4% of the cohort had at least one persistent symptom 2 months or longer after initial onset and at more than a month after discharge. In this sample, only 5% had been intubated. (JAMA 2020 Jul 9. doi: 10.1001/jama.2020.12603).
One study found that even patients who have had relatively mild symptoms and were not hospitalized can have persistent symptoms. The Centers for Disease Control and Prevention conducted a survey of adults who tested positive for the positive reverse transcription–polymerase chain reaction test for SARS-CoV-2 and found that, among the 292 respondents, 35% were still feeling the impact of the disease 2-3 weeks after testing. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms. The survey found that delayed recovery was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization (MMWR. 2020 Jul 24. doi: 10.15585/mmwr.mm6930e1).
Sachin Gupta, MD, FCCP, ATSF, a pulmonologist and member of the CHEST Physician editorial advisory board, has treated patients with COVID-19 and shared some of his thoughts on the problem of prolonged symptoms of COVID-19.
Q: Should clinicians expect to see COVID-19 patients who have symptoms persisting weeks after they are diagnosed?
Dr. Gupta: I think clinicians, especially in primary care, are already seeing many patients with lingering symptoms, both respiratory and nonrespiratory related, and debility. A few patients here in the San Francisco Bay Area that I have spoken with 4-6 weeks out from their acute illness have complained of persisting, though improving, fatigue and cough. Early studies are confirming this as a topical issue. There may be other long-lasting sequelae of COVID-19 beyond the common mild lingering symptoms. It will also be important to consider (and get more data on) to what degree asymptomatic patients develop some degree of mild inflammatory and subsequent fibrotic changes in organs like the lungs and heart
Q: How does the recovery phase of COVID-19 compare with recovery from severe influenza or ARDS?
Dr. Gupta: Most prior influenza and acute respiratory distress syndrome (ARDS) studies have provided initial follow-up at 3 months and beyond, so technically speaking, it is a little difficult to compare the symptomatology patterns in the JAMA study of 2 months on follow-up. Nevertheless, the key takeaway is that, even though few patients in the study had ARDS requiring intubation (severe disease), many patients with milder disease had significant lingering symptoms (55% with three or more symptoms) at 2 months.
This fits logically with the premise, which we have some limited data on with ARDS (N Engl J Med. 2003;348:683-93. doi: 10.1056/NEJMoa022450) and severe influenza infection survivors (Nature Sci Rep. 2017;7:17275. doi: 10.1038/s41598-017-17497-6) that varying degrees of the inflammation cascade triggered by certain viruses can lead to changes in important patient-reported outcomes, and objective measures such as pulmonary function over the long term.
Q: What can you do for patients with lingering symptoms of COVID-19 or what can you tell them about their symptoms?
Dr. Gupta: For many patients, there is fear, given the novel nature of the virus/pandemic, that their symptoms may persist long term. Acknowledgment of their symptoms is validating and important for us to recognize as we learn more about the virus. As we are finding, many patients are going online to find answers, after sometimes feeling rushed or dismissed initially in the clinical setting.
In my experience, the bar is fairly high for most patients to reach out to their physicians with complaints of lingering symptoms after acute infection. For the ones who do reach out, they tend to have either a greater constellation of symptoms or higher severity of one or two key symptoms. After assessing and, when appropriate, ruling out secondary infections or newly developed conditions, I shift toward symptom management. I encourage such patients to build up slowly. I suggest they work first on their activities of daily living (bathing, grooming), then their instrumental activities of daily living (cooking, cleaning, checking the mail), and then to engage, based on their tolerance of symptoms, to light purposeful exercise. There are many online resources for at-home exercise activities that I recommend to patients who are more debilitated; some larger centers are beginning to offer some forms of telepulmonary rehab.
Based on what we know about other causes of viral pneumonitis and ARDS, I ask such symptomatic patients to expect a slow, gradual, and in most cases a complete recovery, and depending on the individual case, I recommend pulmonary function tests and imaging that may be helpful to track that progress.
I remind myself, and patients, that our understanding may change as we learn more over time. Checking in at set intervals, even if not in person but through a phone call, can go a long way in a setting where we do not have a specific therapy, other than gradual exercise training, to help these patients recover faster. Reassurance and encouragement are vital for patients who are struggling with the lingering burden of disease and who may find it difficult to return to work or function as usual at home. The final point is to be mindful of our patient’s mental health and, where our reassurance is not enough, to consider appropriate mental health referrals.
Q: Can you handle this kind of problem with telemedicine or which patients with lingering symptoms need to come into the office – or failing that, the ED?
Dr. Gupta: Telemedicine in the outpatient setting provides a helpful tool to assess and manage patients, in my experience, with limited and straightforward complaints. Its scope is limited diagnostically (assessing symptoms and signs) as is its reach (ability to connect with elderly, disabled, or patients without/limited telemedicine access). In many instances, telemedicine limits our ability to connect with patients emotionally and build trust. Many patients who have gone through the acute illness that we see in pulmonary clinic on follow-up are older in age, and for many, video visits are not a practical solution. Telemedicine visits can sometimes present challenges for me as well in terms of thoroughly conveying lifestyle and symptom management strategies. Health literacy is typically easier to gauge and address in person.
For patients with any degree of enduring dyspnea, more so in the acute phase, I recommend home pulse oximetry for monitoring their oxygen saturation if it is financially and technically feasible for them to obtain one. Sending a patient to the ED is an option of last resort, but one that is necessary in some cases. I expect patients with lingering symptoms to tell me that symptoms may be persisting, hopefully gradually improving, and not getting worse. If post–COVID-19 symptoms such as fever, dyspnea, fatigue, or lightheadedness are new or worsening, particularly rapidly, the safest and best option I advise patients is to go to the ED for further assessment and testing. Postviral bacterial pneumonia is something we should consider, and there is some potential for aspergillosis as well.
Q: Do you have any concerns about patients with asthma, chronic obstructive pulmonary disease, or other pulmonary issues having lingering symptoms that may mask exacerbations or may cause exacerbation of their disease?
Dr. Gupta: So far, patients with chronic lung conditions do not appear to have not been disproportionately affected by the pandemic in terms of absolute numbers or percentage wise compared to the general public. I think that sheltering in place has been readily followed by many of these patients, and in addition, I assume better adherence to their maintenance therapies has likely helped. The very few cases of patients with underlying chronic obstructive pulmonary disease and interstitial lung disease that I have seen have fared very poorly when they were diagnosed with COVID-19 in the hospital. There are emerging data about short-term outcomes from severe COVID-19 infection in patients with interstitial lung disease in Europe (medRxiv. 2020 Jul 17. doi: 10.1101/2020.07.15.20152967), and from physicians treating pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (Ann Am Thorac Soc. 2020 Jul 29. doi: 10.1513/AnnalsATS.202005-521OC). But so far, little has been published on the outcomes of mild disease in these patients with chronic lung disease.
Q: It’s still early days to know the significance of lingering symptoms. But at what point do you begin to consider the possibility of some kind of relapse? And what is your next move if the symptoms get worse?
Dr. Gupta: COVID-19 recurrence, whether because of reinfection or relapse, is a potential concern but not one that is very commonly seen so far in my purview. Generally, symptoms of post–COVID-19 infection that are lingering trend toward getting better, even if slowly. If post–COVID-19 infection symptoms are progressing (particularly if rapidly), that would be a strong indication to evaluate that patient in the ED (less likely in clinic), reswab them for SARS-CoV-2, and obtain further testing such as blood work and imaging. A significant challenge from a research perspective will be determining if coinfection with another virus is playing a role as we move closer to the fall season.
Q&A with Dr. Sachin Gupta
Q&A with Dr. Sachin Gupta
For some patients, a bout of COVID-19 may not be over after hospital discharge, acute symptoms subside, or a couple of tests for SARS-CoV-2 come back negative. Those who have reached these milestones of conquering the disease may find that their recovery journey has only begun. Debilitating symptoms such as fatigue, headache, and dyspnea may linger for weeks or longer. Patients with persistent symptoms, often referred to as “long haulers” in reference to the duration of their recovery, are looking for answers about their condition and when their COVID-19 illness will finally resolve.
Long-haul patients organize
What started as an accumulation of anecdotal evidence in social media, blogs, and the mainstream press about slow recovery and long-lasting symptoms of COVID-19 is now the focus of clinical trials in the population of recovering patients. Projects such as the COVID Symptom Study, initiated by the Massachusetts General Hospital, Boston; the Harvard School of Public Health, Boston; King’s College London; and Stanford (Calif.) University, are collecting data on symptoms from millions of patients and will eventually contribute to a better understanding of prolonged recovery.
Patients looking for answers have created groups on social media such as Facebook to exchange information about their experiences (e.g., Survivor Corps, COVID-19 Support Group, COVID-19 Recovered Survivors). Recovering patients have created patient-led research organizations (Body Politic COVID-19 Support Group) to explore persistent symptoms and begin to create data for research.
Some data on lingering symptoms
A small study of 143 previously hospitalized, recovering patients in Italy found that 87.4% of the cohort had at least one persistent symptom 2 months or longer after initial onset and at more than a month after discharge. In this sample, only 5% had been intubated. (JAMA 2020 Jul 9. doi: 10.1001/jama.2020.12603).
One study found that even patients who have had relatively mild symptoms and were not hospitalized can have persistent symptoms. The Centers for Disease Control and Prevention conducted a survey of adults who tested positive for the positive reverse transcription–polymerase chain reaction test for SARS-CoV-2 and found that, among the 292 respondents, 35% were still feeling the impact of the disease 2-3 weeks after testing. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms. The survey found that delayed recovery was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization (MMWR. 2020 Jul 24. doi: 10.15585/mmwr.mm6930e1).
Sachin Gupta, MD, FCCP, ATSF, a pulmonologist and member of the CHEST Physician editorial advisory board, has treated patients with COVID-19 and shared some of his thoughts on the problem of prolonged symptoms of COVID-19.
Q: Should clinicians expect to see COVID-19 patients who have symptoms persisting weeks after they are diagnosed?
Dr. Gupta: I think clinicians, especially in primary care, are already seeing many patients with lingering symptoms, both respiratory and nonrespiratory related, and debility. A few patients here in the San Francisco Bay Area that I have spoken with 4-6 weeks out from their acute illness have complained of persisting, though improving, fatigue and cough. Early studies are confirming this as a topical issue. There may be other long-lasting sequelae of COVID-19 beyond the common mild lingering symptoms. It will also be important to consider (and get more data on) to what degree asymptomatic patients develop some degree of mild inflammatory and subsequent fibrotic changes in organs like the lungs and heart
Q: How does the recovery phase of COVID-19 compare with recovery from severe influenza or ARDS?
Dr. Gupta: Most prior influenza and acute respiratory distress syndrome (ARDS) studies have provided initial follow-up at 3 months and beyond, so technically speaking, it is a little difficult to compare the symptomatology patterns in the JAMA study of 2 months on follow-up. Nevertheless, the key takeaway is that, even though few patients in the study had ARDS requiring intubation (severe disease), many patients with milder disease had significant lingering symptoms (55% with three or more symptoms) at 2 months.
This fits logically with the premise, which we have some limited data on with ARDS (N Engl J Med. 2003;348:683-93. doi: 10.1056/NEJMoa022450) and severe influenza infection survivors (Nature Sci Rep. 2017;7:17275. doi: 10.1038/s41598-017-17497-6) that varying degrees of the inflammation cascade triggered by certain viruses can lead to changes in important patient-reported outcomes, and objective measures such as pulmonary function over the long term.
Q: What can you do for patients with lingering symptoms of COVID-19 or what can you tell them about their symptoms?
Dr. Gupta: For many patients, there is fear, given the novel nature of the virus/pandemic, that their symptoms may persist long term. Acknowledgment of their symptoms is validating and important for us to recognize as we learn more about the virus. As we are finding, many patients are going online to find answers, after sometimes feeling rushed or dismissed initially in the clinical setting.
In my experience, the bar is fairly high for most patients to reach out to their physicians with complaints of lingering symptoms after acute infection. For the ones who do reach out, they tend to have either a greater constellation of symptoms or higher severity of one or two key symptoms. After assessing and, when appropriate, ruling out secondary infections or newly developed conditions, I shift toward symptom management. I encourage such patients to build up slowly. I suggest they work first on their activities of daily living (bathing, grooming), then their instrumental activities of daily living (cooking, cleaning, checking the mail), and then to engage, based on their tolerance of symptoms, to light purposeful exercise. There are many online resources for at-home exercise activities that I recommend to patients who are more debilitated; some larger centers are beginning to offer some forms of telepulmonary rehab.
Based on what we know about other causes of viral pneumonitis and ARDS, I ask such symptomatic patients to expect a slow, gradual, and in most cases a complete recovery, and depending on the individual case, I recommend pulmonary function tests and imaging that may be helpful to track that progress.
I remind myself, and patients, that our understanding may change as we learn more over time. Checking in at set intervals, even if not in person but through a phone call, can go a long way in a setting where we do not have a specific therapy, other than gradual exercise training, to help these patients recover faster. Reassurance and encouragement are vital for patients who are struggling with the lingering burden of disease and who may find it difficult to return to work or function as usual at home. The final point is to be mindful of our patient’s mental health and, where our reassurance is not enough, to consider appropriate mental health referrals.
Q: Can you handle this kind of problem with telemedicine or which patients with lingering symptoms need to come into the office – or failing that, the ED?
Dr. Gupta: Telemedicine in the outpatient setting provides a helpful tool to assess and manage patients, in my experience, with limited and straightforward complaints. Its scope is limited diagnostically (assessing symptoms and signs) as is its reach (ability to connect with elderly, disabled, or patients without/limited telemedicine access). In many instances, telemedicine limits our ability to connect with patients emotionally and build trust. Many patients who have gone through the acute illness that we see in pulmonary clinic on follow-up are older in age, and for many, video visits are not a practical solution. Telemedicine visits can sometimes present challenges for me as well in terms of thoroughly conveying lifestyle and symptom management strategies. Health literacy is typically easier to gauge and address in person.
For patients with any degree of enduring dyspnea, more so in the acute phase, I recommend home pulse oximetry for monitoring their oxygen saturation if it is financially and technically feasible for them to obtain one. Sending a patient to the ED is an option of last resort, but one that is necessary in some cases. I expect patients with lingering symptoms to tell me that symptoms may be persisting, hopefully gradually improving, and not getting worse. If post–COVID-19 symptoms such as fever, dyspnea, fatigue, or lightheadedness are new or worsening, particularly rapidly, the safest and best option I advise patients is to go to the ED for further assessment and testing. Postviral bacterial pneumonia is something we should consider, and there is some potential for aspergillosis as well.
Q: Do you have any concerns about patients with asthma, chronic obstructive pulmonary disease, or other pulmonary issues having lingering symptoms that may mask exacerbations or may cause exacerbation of their disease?
Dr. Gupta: So far, patients with chronic lung conditions do not appear to have not been disproportionately affected by the pandemic in terms of absolute numbers or percentage wise compared to the general public. I think that sheltering in place has been readily followed by many of these patients, and in addition, I assume better adherence to their maintenance therapies has likely helped. The very few cases of patients with underlying chronic obstructive pulmonary disease and interstitial lung disease that I have seen have fared very poorly when they were diagnosed with COVID-19 in the hospital. There are emerging data about short-term outcomes from severe COVID-19 infection in patients with interstitial lung disease in Europe (medRxiv. 2020 Jul 17. doi: 10.1101/2020.07.15.20152967), and from physicians treating pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (Ann Am Thorac Soc. 2020 Jul 29. doi: 10.1513/AnnalsATS.202005-521OC). But so far, little has been published on the outcomes of mild disease in these patients with chronic lung disease.
Q: It’s still early days to know the significance of lingering symptoms. But at what point do you begin to consider the possibility of some kind of relapse? And what is your next move if the symptoms get worse?
Dr. Gupta: COVID-19 recurrence, whether because of reinfection or relapse, is a potential concern but not one that is very commonly seen so far in my purview. Generally, symptoms of post–COVID-19 infection that are lingering trend toward getting better, even if slowly. If post–COVID-19 infection symptoms are progressing (particularly if rapidly), that would be a strong indication to evaluate that patient in the ED (less likely in clinic), reswab them for SARS-CoV-2, and obtain further testing such as blood work and imaging. A significant challenge from a research perspective will be determining if coinfection with another virus is playing a role as we move closer to the fall season.
For some patients, a bout of COVID-19 may not be over after hospital discharge, acute symptoms subside, or a couple of tests for SARS-CoV-2 come back negative. Those who have reached these milestones of conquering the disease may find that their recovery journey has only begun. Debilitating symptoms such as fatigue, headache, and dyspnea may linger for weeks or longer. Patients with persistent symptoms, often referred to as “long haulers” in reference to the duration of their recovery, are looking for answers about their condition and when their COVID-19 illness will finally resolve.
Long-haul patients organize
What started as an accumulation of anecdotal evidence in social media, blogs, and the mainstream press about slow recovery and long-lasting symptoms of COVID-19 is now the focus of clinical trials in the population of recovering patients. Projects such as the COVID Symptom Study, initiated by the Massachusetts General Hospital, Boston; the Harvard School of Public Health, Boston; King’s College London; and Stanford (Calif.) University, are collecting data on symptoms from millions of patients and will eventually contribute to a better understanding of prolonged recovery.
Patients looking for answers have created groups on social media such as Facebook to exchange information about their experiences (e.g., Survivor Corps, COVID-19 Support Group, COVID-19 Recovered Survivors). Recovering patients have created patient-led research organizations (Body Politic COVID-19 Support Group) to explore persistent symptoms and begin to create data for research.
Some data on lingering symptoms
A small study of 143 previously hospitalized, recovering patients in Italy found that 87.4% of the cohort had at least one persistent symptom 2 months or longer after initial onset and at more than a month after discharge. In this sample, only 5% had been intubated. (JAMA 2020 Jul 9. doi: 10.1001/jama.2020.12603).
One study found that even patients who have had relatively mild symptoms and were not hospitalized can have persistent symptoms. The Centers for Disease Control and Prevention conducted a survey of adults who tested positive for the positive reverse transcription–polymerase chain reaction test for SARS-CoV-2 and found that, among the 292 respondents, 35% were still feeling the impact of the disease 2-3 weeks after testing. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms. The survey found that delayed recovery was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization (MMWR. 2020 Jul 24. doi: 10.15585/mmwr.mm6930e1).
Sachin Gupta, MD, FCCP, ATSF, a pulmonologist and member of the CHEST Physician editorial advisory board, has treated patients with COVID-19 and shared some of his thoughts on the problem of prolonged symptoms of COVID-19.
Q: Should clinicians expect to see COVID-19 patients who have symptoms persisting weeks after they are diagnosed?
Dr. Gupta: I think clinicians, especially in primary care, are already seeing many patients with lingering symptoms, both respiratory and nonrespiratory related, and debility. A few patients here in the San Francisco Bay Area that I have spoken with 4-6 weeks out from their acute illness have complained of persisting, though improving, fatigue and cough. Early studies are confirming this as a topical issue. There may be other long-lasting sequelae of COVID-19 beyond the common mild lingering symptoms. It will also be important to consider (and get more data on) to what degree asymptomatic patients develop some degree of mild inflammatory and subsequent fibrotic changes in organs like the lungs and heart
Q: How does the recovery phase of COVID-19 compare with recovery from severe influenza or ARDS?
Dr. Gupta: Most prior influenza and acute respiratory distress syndrome (ARDS) studies have provided initial follow-up at 3 months and beyond, so technically speaking, it is a little difficult to compare the symptomatology patterns in the JAMA study of 2 months on follow-up. Nevertheless, the key takeaway is that, even though few patients in the study had ARDS requiring intubation (severe disease), many patients with milder disease had significant lingering symptoms (55% with three or more symptoms) at 2 months.
This fits logically with the premise, which we have some limited data on with ARDS (N Engl J Med. 2003;348:683-93. doi: 10.1056/NEJMoa022450) and severe influenza infection survivors (Nature Sci Rep. 2017;7:17275. doi: 10.1038/s41598-017-17497-6) that varying degrees of the inflammation cascade triggered by certain viruses can lead to changes in important patient-reported outcomes, and objective measures such as pulmonary function over the long term.
Q: What can you do for patients with lingering symptoms of COVID-19 or what can you tell them about their symptoms?
Dr. Gupta: For many patients, there is fear, given the novel nature of the virus/pandemic, that their symptoms may persist long term. Acknowledgment of their symptoms is validating and important for us to recognize as we learn more about the virus. As we are finding, many patients are going online to find answers, after sometimes feeling rushed or dismissed initially in the clinical setting.
In my experience, the bar is fairly high for most patients to reach out to their physicians with complaints of lingering symptoms after acute infection. For the ones who do reach out, they tend to have either a greater constellation of symptoms or higher severity of one or two key symptoms. After assessing and, when appropriate, ruling out secondary infections or newly developed conditions, I shift toward symptom management. I encourage such patients to build up slowly. I suggest they work first on their activities of daily living (bathing, grooming), then their instrumental activities of daily living (cooking, cleaning, checking the mail), and then to engage, based on their tolerance of symptoms, to light purposeful exercise. There are many online resources for at-home exercise activities that I recommend to patients who are more debilitated; some larger centers are beginning to offer some forms of telepulmonary rehab.
Based on what we know about other causes of viral pneumonitis and ARDS, I ask such symptomatic patients to expect a slow, gradual, and in most cases a complete recovery, and depending on the individual case, I recommend pulmonary function tests and imaging that may be helpful to track that progress.
I remind myself, and patients, that our understanding may change as we learn more over time. Checking in at set intervals, even if not in person but through a phone call, can go a long way in a setting where we do not have a specific therapy, other than gradual exercise training, to help these patients recover faster. Reassurance and encouragement are vital for patients who are struggling with the lingering burden of disease and who may find it difficult to return to work or function as usual at home. The final point is to be mindful of our patient’s mental health and, where our reassurance is not enough, to consider appropriate mental health referrals.
Q: Can you handle this kind of problem with telemedicine or which patients with lingering symptoms need to come into the office – or failing that, the ED?
Dr. Gupta: Telemedicine in the outpatient setting provides a helpful tool to assess and manage patients, in my experience, with limited and straightforward complaints. Its scope is limited diagnostically (assessing symptoms and signs) as is its reach (ability to connect with elderly, disabled, or patients without/limited telemedicine access). In many instances, telemedicine limits our ability to connect with patients emotionally and build trust. Many patients who have gone through the acute illness that we see in pulmonary clinic on follow-up are older in age, and for many, video visits are not a practical solution. Telemedicine visits can sometimes present challenges for me as well in terms of thoroughly conveying lifestyle and symptom management strategies. Health literacy is typically easier to gauge and address in person.
For patients with any degree of enduring dyspnea, more so in the acute phase, I recommend home pulse oximetry for monitoring their oxygen saturation if it is financially and technically feasible for them to obtain one. Sending a patient to the ED is an option of last resort, but one that is necessary in some cases. I expect patients with lingering symptoms to tell me that symptoms may be persisting, hopefully gradually improving, and not getting worse. If post–COVID-19 symptoms such as fever, dyspnea, fatigue, or lightheadedness are new or worsening, particularly rapidly, the safest and best option I advise patients is to go to the ED for further assessment and testing. Postviral bacterial pneumonia is something we should consider, and there is some potential for aspergillosis as well.
Q: Do you have any concerns about patients with asthma, chronic obstructive pulmonary disease, or other pulmonary issues having lingering symptoms that may mask exacerbations or may cause exacerbation of their disease?
Dr. Gupta: So far, patients with chronic lung conditions do not appear to have not been disproportionately affected by the pandemic in terms of absolute numbers or percentage wise compared to the general public. I think that sheltering in place has been readily followed by many of these patients, and in addition, I assume better adherence to their maintenance therapies has likely helped. The very few cases of patients with underlying chronic obstructive pulmonary disease and interstitial lung disease that I have seen have fared very poorly when they were diagnosed with COVID-19 in the hospital. There are emerging data about short-term outcomes from severe COVID-19 infection in patients with interstitial lung disease in Europe (medRxiv. 2020 Jul 17. doi: 10.1101/2020.07.15.20152967), and from physicians treating pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (Ann Am Thorac Soc. 2020 Jul 29. doi: 10.1513/AnnalsATS.202005-521OC). But so far, little has been published on the outcomes of mild disease in these patients with chronic lung disease.
Q: It’s still early days to know the significance of lingering symptoms. But at what point do you begin to consider the possibility of some kind of relapse? And what is your next move if the symptoms get worse?
Dr. Gupta: COVID-19 recurrence, whether because of reinfection or relapse, is a potential concern but not one that is very commonly seen so far in my purview. Generally, symptoms of post–COVID-19 infection that are lingering trend toward getting better, even if slowly. If post–COVID-19 infection symptoms are progressing (particularly if rapidly), that would be a strong indication to evaluate that patient in the ED (less likely in clinic), reswab them for SARS-CoV-2, and obtain further testing such as blood work and imaging. A significant challenge from a research perspective will be determining if coinfection with another virus is playing a role as we move closer to the fall season.
COVID-19 cases in children nearly doubled in just 4 weeks
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.
“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
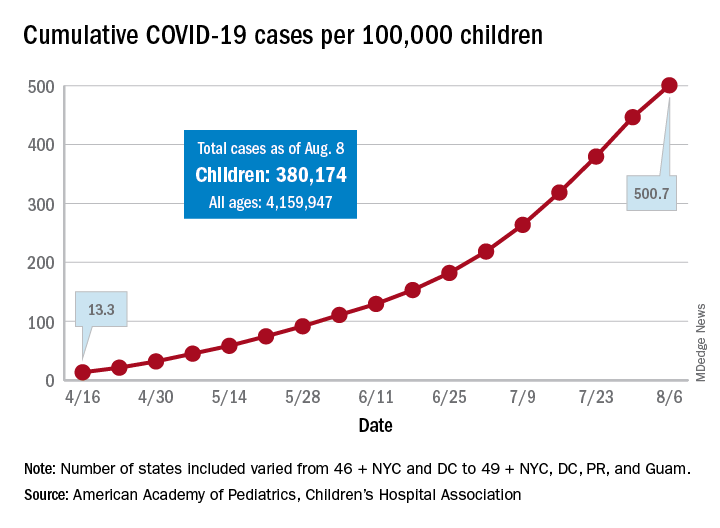
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.

“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.

“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.
Successful bowel preps linked with modifiable risk factors
Background: IBP is very common and associated with increased length of stay and cost of care. Many nonmodifiable risk factors have been identified such as socioeconomic status, male gender, and increased age, but no studies have been done to look at modifiable risk factors such as medication use, timing of colonoscopy, and diet before colonoscopy. Furthermore, no studies have been done to assess the effects of these modifiable factors on IBP.
Study design: Retrospective cohort study using multivariate logistic regression analysis.
Setting: Cleveland Clinic Hospitals in Ohio and Florida.
Synopsis: Records of 8,819 patients (aged greater than 18 years) undergoing colonoscopy at Cleveland Clinic between January 2011 and June 2017 were reviewed. They found that 51% had IBP. Modifiable risk factors, including opiate use within 3 days of colonoscopy, colonoscopy performed before noon, and solid diet the day before colonoscopy, were associated with IBP. After adjustment for these variables, they found the rates of IBP were reduced by 5.6%. They also found that patients who had IBP had increased length of stay by 1 day (6 days vs. 5 days; P less than .001). This translates into 494 unnecessary hospital days or approximately $1 million dollars in unnecessary costs based on the number of patients (almost 9,000).
This study was performed in a single institution so it may be difficult to extrapolate to other institutions. Further studies need to be performed using multicenter institutions to assess accuracy of data.
Bottom line: Liquid diet or nothing by mouth (NPO) 1 day prior to colonoscopy, performing colonoscopy before noon, and avoiding opioids 3 days prior to colonoscopy are modifiable risk factors that may decrease the rate of inadequate bowel preparations in hospitalized patients.
Citation: Garber A et al. Modifiable factors associated with quality of bowel preparation among hospitalized patients undergoing colonoscopy. J Hosp Med. 2019;5:278-83.
Dr. Newsom is a hospitalist at Ochsner Health System, New Orleans.
Background: IBP is very common and associated with increased length of stay and cost of care. Many nonmodifiable risk factors have been identified such as socioeconomic status, male gender, and increased age, but no studies have been done to look at modifiable risk factors such as medication use, timing of colonoscopy, and diet before colonoscopy. Furthermore, no studies have been done to assess the effects of these modifiable factors on IBP.
Study design: Retrospective cohort study using multivariate logistic regression analysis.
Setting: Cleveland Clinic Hospitals in Ohio and Florida.
Synopsis: Records of 8,819 patients (aged greater than 18 years) undergoing colonoscopy at Cleveland Clinic between January 2011 and June 2017 were reviewed. They found that 51% had IBP. Modifiable risk factors, including opiate use within 3 days of colonoscopy, colonoscopy performed before noon, and solid diet the day before colonoscopy, were associated with IBP. After adjustment for these variables, they found the rates of IBP were reduced by 5.6%. They also found that patients who had IBP had increased length of stay by 1 day (6 days vs. 5 days; P less than .001). This translates into 494 unnecessary hospital days or approximately $1 million dollars in unnecessary costs based on the number of patients (almost 9,000).
This study was performed in a single institution so it may be difficult to extrapolate to other institutions. Further studies need to be performed using multicenter institutions to assess accuracy of data.
Bottom line: Liquid diet or nothing by mouth (NPO) 1 day prior to colonoscopy, performing colonoscopy before noon, and avoiding opioids 3 days prior to colonoscopy are modifiable risk factors that may decrease the rate of inadequate bowel preparations in hospitalized patients.
Citation: Garber A et al. Modifiable factors associated with quality of bowel preparation among hospitalized patients undergoing colonoscopy. J Hosp Med. 2019;5:278-83.
Dr. Newsom is a hospitalist at Ochsner Health System, New Orleans.
Background: IBP is very common and associated with increased length of stay and cost of care. Many nonmodifiable risk factors have been identified such as socioeconomic status, male gender, and increased age, but no studies have been done to look at modifiable risk factors such as medication use, timing of colonoscopy, and diet before colonoscopy. Furthermore, no studies have been done to assess the effects of these modifiable factors on IBP.
Study design: Retrospective cohort study using multivariate logistic regression analysis.
Setting: Cleveland Clinic Hospitals in Ohio and Florida.
Synopsis: Records of 8,819 patients (aged greater than 18 years) undergoing colonoscopy at Cleveland Clinic between January 2011 and June 2017 were reviewed. They found that 51% had IBP. Modifiable risk factors, including opiate use within 3 days of colonoscopy, colonoscopy performed before noon, and solid diet the day before colonoscopy, were associated with IBP. After adjustment for these variables, they found the rates of IBP were reduced by 5.6%. They also found that patients who had IBP had increased length of stay by 1 day (6 days vs. 5 days; P less than .001). This translates into 494 unnecessary hospital days or approximately $1 million dollars in unnecessary costs based on the number of patients (almost 9,000).
This study was performed in a single institution so it may be difficult to extrapolate to other institutions. Further studies need to be performed using multicenter institutions to assess accuracy of data.
Bottom line: Liquid diet or nothing by mouth (NPO) 1 day prior to colonoscopy, performing colonoscopy before noon, and avoiding opioids 3 days prior to colonoscopy are modifiable risk factors that may decrease the rate of inadequate bowel preparations in hospitalized patients.
Citation: Garber A et al. Modifiable factors associated with quality of bowel preparation among hospitalized patients undergoing colonoscopy. J Hosp Med. 2019;5:278-83.
Dr. Newsom is a hospitalist at Ochsner Health System, New Orleans.
iResident: Virtual care on hospital medicine teaching services during a pandemic
At the start of each shift on his clinical service with rotating internal medicine residents, Benji Mathews, MD, SFHM, now adds a few components to his usual preparation. First, visiting the Minnesota Department of Health and various organizational websites to review the latest COVID-19 updates and guidelines. Next comes checking to see where he needs to pick up the surgical mask and eye protection that he will need to wear through the day. Last, he evaluates which of his patients are in telemedicine-equipped rooms; this last change has fast become a crucial part of working with his resident learners during a pandemic.
During the COVID-19 pandemic, residents and residency programs find themselves in a unique situation. Balancing the educational needs of a training program with the safety of trainees is a challenging task, specifically when taking care of patients who are COVID-19 positive or patients under investigation (PUI). One increasingly available tool that can help protect trainees while continuing to prioritize patient care and medical education is the use of telemedicine for virtual rounding. For our internal medicine residents through the University of Minnesota Internal Medicine Residency program rotating at Regions Hospital in Saint Paul, Minn., we have used video visits to continue our mandate as both health care and education professionals.
Virtual care decision tree
Virtual care can mitigate exposure risk, minimize use of personal protective equipment (PPE), and improve communications with patients and their families. To guide our teaching teams on the optimal situations for telemedicine, we needed to select those patients who would be most appropriate for a virtual visit.
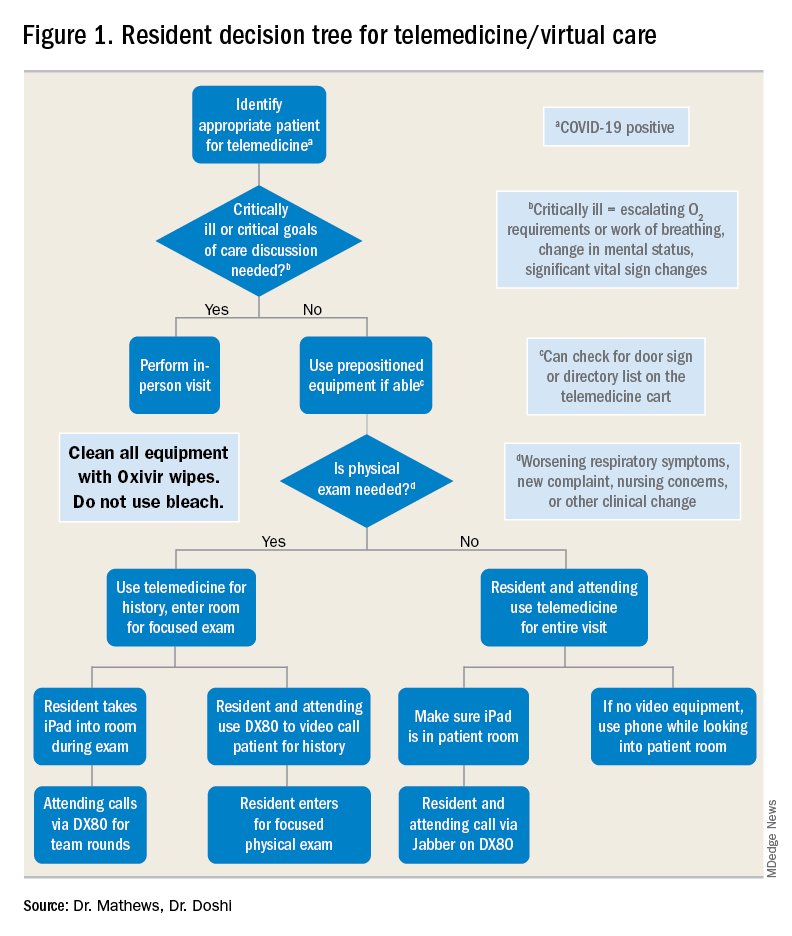
For example, patients with advanced dementia, or intubated in the intensive care unit, would have less utility from a real-time video encounter. Further, we implemented a simple decision tree (Figure 1). First, the team needs to decide whether the patient needs an immediate in-person assessment; for instance, for critically ill patients or those who need end-of-life care discussions, telemedicine would not be an appropriate modality. Next, the decision is made on whether a patient requires an in-person exam at that time. The idea of forgoing the in-person physical exam may run counterintuitive to the core training medical providers undergo, but in certain circumstances telemedicine can still provide the appropriate level of care a patient requires.
Virtual rounding with residents: Pros and cons
Through the course of this pandemic, there have many questions raised regarding how to handle inpatient teaching services: Should resident teams be assigned COVID-19 positives or PUIs? How do you optimize assessing and learning from patients’ conditions that require human touch? Should all members of the teaching team be donning PPE and entering the patient room?
Internal medicine residents in our hospital have been assigned COVID-19 positive and PUI patients. With proper PPE, and donning and doffing practices, residents may continue to learn from this important training opportunity while also optimizing care for patients supplemented by telemedicine. This pandemic has flattened the hierarchy; often residents are teaching their attendings much of the latest literature and best practices around COVID-19. Residents also benefit by joining the organization’s daily virtual interprofessional COVID-19 huddle where they partner with infectious disease, critical care, pharmacy, and other experts to collaborate in the care of these patients.
There have been counterarguments made for residents joining the front lines with COVID-19 patients. Some have conditions that limit them from seeing this subgroup of patients, such as their immune status or other issues. For these residents, we do not assign COVID-19–positive patients. However, they may continue to support in virtually updating COVID-19 patients and their families. A second argument has been the use of PPE. We have implemented telemedicine to limit the total number of exposures and have a protocol for the fewest number of providers possible to see any at-risk or confirmed COVID-19 patient. For example, a resident who sees a COVID-19 patient in person may also be simultaneously virtually supervised by the attending.
Webside manner
The physical exam is only one of several operational considerations when delivering virtual care, whether with a teaching or nonteaching service. One important aspect is the “webside manner” of the provider, the virtual analogue to bedside manner.
Inherent parts of in-person encounters, such as eye contact and allowing for patients to finish their sentences, have added nuances with virtual care. For instance, providers must adjust to looking into the web camera to make eye contact, even though the patient’s face may be on the screen below. Additionally, for patients who are hard of hearing or unfamiliar with video calling, providers must be cognizant of projecting well over an Internet connection and timing responses to avoid overlapping conversation.
Similarly, there are nuances to the virtual physical exam, some specific to care in the COVID-19 era. In our previous virtual care practice, a bedside facilitator assisted in using tools such a digital stethoscope. In contrast, our current practice aims to refine the observational skills of our learners in conjunction with chart review, vital signs, and actively incorporating the patient in the physical exam. This does not mean asking them to auscultate themselves, but is more toward allowing patients to participate in focused evaluations, such as assessing abdominal tenderness or working through range of motion. Remote guidance for virtual exams also extends itself to teaching teams; for example, in our practice, we have been able to conduct bedside ultrasound teaching with in-person team members and a virtual facilitator.
Maskless connections: ‘Face-to-face’ visits with patients
As many hospitalists have witnessed, COVID-19 is so isolating for patients and their families. Patients have limited visitors, and their care team members are aiming to minimize exposures. Those who are entering the rooms wear masks and face shields that limit connecting with patients in a truly “face-to-face” manner. Telemedicine provides a face-to-face encounter that arguably improves upon portions of the traditional in-person encounter during this pandemic, with providers wearing PPE. For medical learners, gaining the interpersonal skills essential for health care professionals has been skewed with pandemic-related limitations; telemedicine can provide a tool to adapt to this unique era and augment this important educational piece.
Limitations, equity, and technological considerations
Realistically, the virtual exam during COVID-19 does have its limitations. An important part of virtual care and teaching services is instilling the appropriate times for use of telemedicine. If a patient has a clinical change (such as increase in FiO2 requirements) or other clinical need, there should be no hesitation for learners to conduct in-person assessments with appropriate PPE.
Nonexam indications are just as important – for example, if a patient requires extensive goals of care counseling, we recommend this not be done virtually. Other indications may vary between organizations; in our practice, we suggest at least one in-person assessment on the initial and discharge hospital days. Regardless of the specific indications, a successful virtual inpatient teaching service must be predicated on outlining the appropriate uses of telemedicine.
In the United States, there are already health care disparities for people of color and non–English speakers. If there is not a careful consideration for these marginalized groups, their health disparities could be further exacerbated – not just around COVID-19, but also for other inpatient conditions where telemedicine is being used. Groups whose equity must be thoughtfully managed include those who do not speak English and those who do not have access to smartphones or the Internet. Our HealthPartners organization has implemented the integration of interpreters for virtual three-way connections with patients and their clinicians to help mitigate this for non–English speakers. Additionally, utilizing easy-to-use tablets and telemedicine-capable carts has helped patients overcome technology barriers.
Last, the members of the teaching team must know the essential technical aspects of the technology they are using. Robust information technology (IT) support is also needed, but no matter how simple the equipment may be, staff and trainees must know how to both operate it and handle basic troubleshooting (such as audio or video disconnections). This also dovetails with the important element of on-boarding other members of the care team. In our practice, nursing staff, chaplains, interpreters, and dietitians also use virtual care as part of their workflow. However, even if it is used only by the teaching team, orienting other care team members will limit technical problems such as equipment being turned off or moved out of position.
Prior to the COVID-19 pandemic, telemedicine adoption was limited because of lack of awareness, barriers in training, understanding, and narrow beliefs regarding the innovation. The COVID-19 pandemic has resulted in a remarkable increase in the provision of telemedicine services in the inpatient hospital medicine services. Importantly, it is, and should be, a developing part of the education and training for health care learners. This pandemic has underscored the need for providing telemedicine services that will likely long outlast this crisis, and to support our health care learners in being effective “iResidents” on our care teams.
Takeaways
- The future of graduate medical education involves virtual care.
The COVID-19 pandemic response has demonstrated that virtual care plays an instrumental part in patient care, and its effects will not dissipate when the pandemic is done. The curriculum for health care trainees should incorporate telemedicine competencies so that they may more effectively leverage this technology for improving care delivery.
- Selection of telemedicine patients must be stratified.
In order to obtain the highest utility for medical learners on telemedicine, there needs to be a clear decision process for which patients can be seen virtually. This involves both clinical criteria, such as avoiding virtual care for end-of-life discussions, and patient criteria, such as those who are hard of hearing.
- Virtual communication requires new communication skills.
Seeing patients via telemedicine mandates a different skill set than in-person communication. Learners must improve their “webside manner” in order to build the patient-provider relationship. Instilling these tools can pay dividends in settings where telemedicine has high yield, such as maskless communication during a pandemic.
- Health disparities could be further exacerbated by telemedicine and should not be overlooked.
Equity in access to health care applies to telemedicine as it does to many other elements. There are multiple groups that can suffer from disparities, such as patients who need interpreters, or those who have lower technological literacy and access to digital devices. Creating awareness of these pitfalls in virtual care can help medical learners recognize and support in creative solutions for these factors.
Dr. Mathews is chief, hospital medicine, at Regions Hospital, HealthPartners, St. Paul, Minn. Dr. Doshi is telemedicine director, hospital medicine, HealthPartners.
At the start of each shift on his clinical service with rotating internal medicine residents, Benji Mathews, MD, SFHM, now adds a few components to his usual preparation. First, visiting the Minnesota Department of Health and various organizational websites to review the latest COVID-19 updates and guidelines. Next comes checking to see where he needs to pick up the surgical mask and eye protection that he will need to wear through the day. Last, he evaluates which of his patients are in telemedicine-equipped rooms; this last change has fast become a crucial part of working with his resident learners during a pandemic.
During the COVID-19 pandemic, residents and residency programs find themselves in a unique situation. Balancing the educational needs of a training program with the safety of trainees is a challenging task, specifically when taking care of patients who are COVID-19 positive or patients under investigation (PUI). One increasingly available tool that can help protect trainees while continuing to prioritize patient care and medical education is the use of telemedicine for virtual rounding. For our internal medicine residents through the University of Minnesota Internal Medicine Residency program rotating at Regions Hospital in Saint Paul, Minn., we have used video visits to continue our mandate as both health care and education professionals.
Virtual care decision tree
Virtual care can mitigate exposure risk, minimize use of personal protective equipment (PPE), and improve communications with patients and their families. To guide our teaching teams on the optimal situations for telemedicine, we needed to select those patients who would be most appropriate for a virtual visit.

For example, patients with advanced dementia, or intubated in the intensive care unit, would have less utility from a real-time video encounter. Further, we implemented a simple decision tree (Figure 1). First, the team needs to decide whether the patient needs an immediate in-person assessment; for instance, for critically ill patients or those who need end-of-life care discussions, telemedicine would not be an appropriate modality. Next, the decision is made on whether a patient requires an in-person exam at that time. The idea of forgoing the in-person physical exam may run counterintuitive to the core training medical providers undergo, but in certain circumstances telemedicine can still provide the appropriate level of care a patient requires.
Virtual rounding with residents: Pros and cons
Through the course of this pandemic, there have many questions raised regarding how to handle inpatient teaching services: Should resident teams be assigned COVID-19 positives or PUIs? How do you optimize assessing and learning from patients’ conditions that require human touch? Should all members of the teaching team be donning PPE and entering the patient room?
Internal medicine residents in our hospital have been assigned COVID-19 positive and PUI patients. With proper PPE, and donning and doffing practices, residents may continue to learn from this important training opportunity while also optimizing care for patients supplemented by telemedicine. This pandemic has flattened the hierarchy; often residents are teaching their attendings much of the latest literature and best practices around COVID-19. Residents also benefit by joining the organization’s daily virtual interprofessional COVID-19 huddle where they partner with infectious disease, critical care, pharmacy, and other experts to collaborate in the care of these patients.
There have been counterarguments made for residents joining the front lines with COVID-19 patients. Some have conditions that limit them from seeing this subgroup of patients, such as their immune status or other issues. For these residents, we do not assign COVID-19–positive patients. However, they may continue to support in virtually updating COVID-19 patients and their families. A second argument has been the use of PPE. We have implemented telemedicine to limit the total number of exposures and have a protocol for the fewest number of providers possible to see any at-risk or confirmed COVID-19 patient. For example, a resident who sees a COVID-19 patient in person may also be simultaneously virtually supervised by the attending.
Webside manner
The physical exam is only one of several operational considerations when delivering virtual care, whether with a teaching or nonteaching service. One important aspect is the “webside manner” of the provider, the virtual analogue to bedside manner.
Inherent parts of in-person encounters, such as eye contact and allowing for patients to finish their sentences, have added nuances with virtual care. For instance, providers must adjust to looking into the web camera to make eye contact, even though the patient’s face may be on the screen below. Additionally, for patients who are hard of hearing or unfamiliar with video calling, providers must be cognizant of projecting well over an Internet connection and timing responses to avoid overlapping conversation.
Similarly, there are nuances to the virtual physical exam, some specific to care in the COVID-19 era. In our previous virtual care practice, a bedside facilitator assisted in using tools such a digital stethoscope. In contrast, our current practice aims to refine the observational skills of our learners in conjunction with chart review, vital signs, and actively incorporating the patient in the physical exam. This does not mean asking them to auscultate themselves, but is more toward allowing patients to participate in focused evaluations, such as assessing abdominal tenderness or working through range of motion. Remote guidance for virtual exams also extends itself to teaching teams; for example, in our practice, we have been able to conduct bedside ultrasound teaching with in-person team members and a virtual facilitator.
Maskless connections: ‘Face-to-face’ visits with patients
As many hospitalists have witnessed, COVID-19 is so isolating for patients and their families. Patients have limited visitors, and their care team members are aiming to minimize exposures. Those who are entering the rooms wear masks and face shields that limit connecting with patients in a truly “face-to-face” manner. Telemedicine provides a face-to-face encounter that arguably improves upon portions of the traditional in-person encounter during this pandemic, with providers wearing PPE. For medical learners, gaining the interpersonal skills essential for health care professionals has been skewed with pandemic-related limitations; telemedicine can provide a tool to adapt to this unique era and augment this important educational piece.
Limitations, equity, and technological considerations
Realistically, the virtual exam during COVID-19 does have its limitations. An important part of virtual care and teaching services is instilling the appropriate times for use of telemedicine. If a patient has a clinical change (such as increase in FiO2 requirements) or other clinical need, there should be no hesitation for learners to conduct in-person assessments with appropriate PPE.
Nonexam indications are just as important – for example, if a patient requires extensive goals of care counseling, we recommend this not be done virtually. Other indications may vary between organizations; in our practice, we suggest at least one in-person assessment on the initial and discharge hospital days. Regardless of the specific indications, a successful virtual inpatient teaching service must be predicated on outlining the appropriate uses of telemedicine.
In the United States, there are already health care disparities for people of color and non–English speakers. If there is not a careful consideration for these marginalized groups, their health disparities could be further exacerbated – not just around COVID-19, but also for other inpatient conditions where telemedicine is being used. Groups whose equity must be thoughtfully managed include those who do not speak English and those who do not have access to smartphones or the Internet. Our HealthPartners organization has implemented the integration of interpreters for virtual three-way connections with patients and their clinicians to help mitigate this for non–English speakers. Additionally, utilizing easy-to-use tablets and telemedicine-capable carts has helped patients overcome technology barriers.
Last, the members of the teaching team must know the essential technical aspects of the technology they are using. Robust information technology (IT) support is also needed, but no matter how simple the equipment may be, staff and trainees must know how to both operate it and handle basic troubleshooting (such as audio or video disconnections). This also dovetails with the important element of on-boarding other members of the care team. In our practice, nursing staff, chaplains, interpreters, and dietitians also use virtual care as part of their workflow. However, even if it is used only by the teaching team, orienting other care team members will limit technical problems such as equipment being turned off or moved out of position.
Prior to the COVID-19 pandemic, telemedicine adoption was limited because of lack of awareness, barriers in training, understanding, and narrow beliefs regarding the innovation. The COVID-19 pandemic has resulted in a remarkable increase in the provision of telemedicine services in the inpatient hospital medicine services. Importantly, it is, and should be, a developing part of the education and training for health care learners. This pandemic has underscored the need for providing telemedicine services that will likely long outlast this crisis, and to support our health care learners in being effective “iResidents” on our care teams.
Takeaways
- The future of graduate medical education involves virtual care.
The COVID-19 pandemic response has demonstrated that virtual care plays an instrumental part in patient care, and its effects will not dissipate when the pandemic is done. The curriculum for health care trainees should incorporate telemedicine competencies so that they may more effectively leverage this technology for improving care delivery.
- Selection of telemedicine patients must be stratified.
In order to obtain the highest utility for medical learners on telemedicine, there needs to be a clear decision process for which patients can be seen virtually. This involves both clinical criteria, such as avoiding virtual care for end-of-life discussions, and patient criteria, such as those who are hard of hearing.
- Virtual communication requires new communication skills.
Seeing patients via telemedicine mandates a different skill set than in-person communication. Learners must improve their “webside manner” in order to build the patient-provider relationship. Instilling these tools can pay dividends in settings where telemedicine has high yield, such as maskless communication during a pandemic.
- Health disparities could be further exacerbated by telemedicine and should not be overlooked.
Equity in access to health care applies to telemedicine as it does to many other elements. There are multiple groups that can suffer from disparities, such as patients who need interpreters, or those who have lower technological literacy and access to digital devices. Creating awareness of these pitfalls in virtual care can help medical learners recognize and support in creative solutions for these factors.
Dr. Mathews is chief, hospital medicine, at Regions Hospital, HealthPartners, St. Paul, Minn. Dr. Doshi is telemedicine director, hospital medicine, HealthPartners.
At the start of each shift on his clinical service with rotating internal medicine residents, Benji Mathews, MD, SFHM, now adds a few components to his usual preparation. First, visiting the Minnesota Department of Health and various organizational websites to review the latest COVID-19 updates and guidelines. Next comes checking to see where he needs to pick up the surgical mask and eye protection that he will need to wear through the day. Last, he evaluates which of his patients are in telemedicine-equipped rooms; this last change has fast become a crucial part of working with his resident learners during a pandemic.
During the COVID-19 pandemic, residents and residency programs find themselves in a unique situation. Balancing the educational needs of a training program with the safety of trainees is a challenging task, specifically when taking care of patients who are COVID-19 positive or patients under investigation (PUI). One increasingly available tool that can help protect trainees while continuing to prioritize patient care and medical education is the use of telemedicine for virtual rounding. For our internal medicine residents through the University of Minnesota Internal Medicine Residency program rotating at Regions Hospital in Saint Paul, Minn., we have used video visits to continue our mandate as both health care and education professionals.
Virtual care decision tree
Virtual care can mitigate exposure risk, minimize use of personal protective equipment (PPE), and improve communications with patients and their families. To guide our teaching teams on the optimal situations for telemedicine, we needed to select those patients who would be most appropriate for a virtual visit.

For example, patients with advanced dementia, or intubated in the intensive care unit, would have less utility from a real-time video encounter. Further, we implemented a simple decision tree (Figure 1). First, the team needs to decide whether the patient needs an immediate in-person assessment; for instance, for critically ill patients or those who need end-of-life care discussions, telemedicine would not be an appropriate modality. Next, the decision is made on whether a patient requires an in-person exam at that time. The idea of forgoing the in-person physical exam may run counterintuitive to the core training medical providers undergo, but in certain circumstances telemedicine can still provide the appropriate level of care a patient requires.
Virtual rounding with residents: Pros and cons
Through the course of this pandemic, there have many questions raised regarding how to handle inpatient teaching services: Should resident teams be assigned COVID-19 positives or PUIs? How do you optimize assessing and learning from patients’ conditions that require human touch? Should all members of the teaching team be donning PPE and entering the patient room?
Internal medicine residents in our hospital have been assigned COVID-19 positive and PUI patients. With proper PPE, and donning and doffing practices, residents may continue to learn from this important training opportunity while also optimizing care for patients supplemented by telemedicine. This pandemic has flattened the hierarchy; often residents are teaching their attendings much of the latest literature and best practices around COVID-19. Residents also benefit by joining the organization’s daily virtual interprofessional COVID-19 huddle where they partner with infectious disease, critical care, pharmacy, and other experts to collaborate in the care of these patients.
There have been counterarguments made for residents joining the front lines with COVID-19 patients. Some have conditions that limit them from seeing this subgroup of patients, such as their immune status or other issues. For these residents, we do not assign COVID-19–positive patients. However, they may continue to support in virtually updating COVID-19 patients and their families. A second argument has been the use of PPE. We have implemented telemedicine to limit the total number of exposures and have a protocol for the fewest number of providers possible to see any at-risk or confirmed COVID-19 patient. For example, a resident who sees a COVID-19 patient in person may also be simultaneously virtually supervised by the attending.
Webside manner
The physical exam is only one of several operational considerations when delivering virtual care, whether with a teaching or nonteaching service. One important aspect is the “webside manner” of the provider, the virtual analogue to bedside manner.
Inherent parts of in-person encounters, such as eye contact and allowing for patients to finish their sentences, have added nuances with virtual care. For instance, providers must adjust to looking into the web camera to make eye contact, even though the patient’s face may be on the screen below. Additionally, for patients who are hard of hearing or unfamiliar with video calling, providers must be cognizant of projecting well over an Internet connection and timing responses to avoid overlapping conversation.
Similarly, there are nuances to the virtual physical exam, some specific to care in the COVID-19 era. In our previous virtual care practice, a bedside facilitator assisted in using tools such a digital stethoscope. In contrast, our current practice aims to refine the observational skills of our learners in conjunction with chart review, vital signs, and actively incorporating the patient in the physical exam. This does not mean asking them to auscultate themselves, but is more toward allowing patients to participate in focused evaluations, such as assessing abdominal tenderness or working through range of motion. Remote guidance for virtual exams also extends itself to teaching teams; for example, in our practice, we have been able to conduct bedside ultrasound teaching with in-person team members and a virtual facilitator.
Maskless connections: ‘Face-to-face’ visits with patients
As many hospitalists have witnessed, COVID-19 is so isolating for patients and their families. Patients have limited visitors, and their care team members are aiming to minimize exposures. Those who are entering the rooms wear masks and face shields that limit connecting with patients in a truly “face-to-face” manner. Telemedicine provides a face-to-face encounter that arguably improves upon portions of the traditional in-person encounter during this pandemic, with providers wearing PPE. For medical learners, gaining the interpersonal skills essential for health care professionals has been skewed with pandemic-related limitations; telemedicine can provide a tool to adapt to this unique era and augment this important educational piece.
Limitations, equity, and technological considerations
Realistically, the virtual exam during COVID-19 does have its limitations. An important part of virtual care and teaching services is instilling the appropriate times for use of telemedicine. If a patient has a clinical change (such as increase in FiO2 requirements) or other clinical need, there should be no hesitation for learners to conduct in-person assessments with appropriate PPE.
Nonexam indications are just as important – for example, if a patient requires extensive goals of care counseling, we recommend this not be done virtually. Other indications may vary between organizations; in our practice, we suggest at least one in-person assessment on the initial and discharge hospital days. Regardless of the specific indications, a successful virtual inpatient teaching service must be predicated on outlining the appropriate uses of telemedicine.
In the United States, there are already health care disparities for people of color and non–English speakers. If there is not a careful consideration for these marginalized groups, their health disparities could be further exacerbated – not just around COVID-19, but also for other inpatient conditions where telemedicine is being used. Groups whose equity must be thoughtfully managed include those who do not speak English and those who do not have access to smartphones or the Internet. Our HealthPartners organization has implemented the integration of interpreters for virtual three-way connections with patients and their clinicians to help mitigate this for non–English speakers. Additionally, utilizing easy-to-use tablets and telemedicine-capable carts has helped patients overcome technology barriers.
Last, the members of the teaching team must know the essential technical aspects of the technology they are using. Robust information technology (IT) support is also needed, but no matter how simple the equipment may be, staff and trainees must know how to both operate it and handle basic troubleshooting (such as audio or video disconnections). This also dovetails with the important element of on-boarding other members of the care team. In our practice, nursing staff, chaplains, interpreters, and dietitians also use virtual care as part of their workflow. However, even if it is used only by the teaching team, orienting other care team members will limit technical problems such as equipment being turned off or moved out of position.
Prior to the COVID-19 pandemic, telemedicine adoption was limited because of lack of awareness, barriers in training, understanding, and narrow beliefs regarding the innovation. The COVID-19 pandemic has resulted in a remarkable increase in the provision of telemedicine services in the inpatient hospital medicine services. Importantly, it is, and should be, a developing part of the education and training for health care learners. This pandemic has underscored the need for providing telemedicine services that will likely long outlast this crisis, and to support our health care learners in being effective “iResidents” on our care teams.
Takeaways
- The future of graduate medical education involves virtual care.
The COVID-19 pandemic response has demonstrated that virtual care plays an instrumental part in patient care, and its effects will not dissipate when the pandemic is done. The curriculum for health care trainees should incorporate telemedicine competencies so that they may more effectively leverage this technology for improving care delivery.
- Selection of telemedicine patients must be stratified.
In order to obtain the highest utility for medical learners on telemedicine, there needs to be a clear decision process for which patients can be seen virtually. This involves both clinical criteria, such as avoiding virtual care for end-of-life discussions, and patient criteria, such as those who are hard of hearing.
- Virtual communication requires new communication skills.
Seeing patients via telemedicine mandates a different skill set than in-person communication. Learners must improve their “webside manner” in order to build the patient-provider relationship. Instilling these tools can pay dividends in settings where telemedicine has high yield, such as maskless communication during a pandemic.
- Health disparities could be further exacerbated by telemedicine and should not be overlooked.
Equity in access to health care applies to telemedicine as it does to many other elements. There are multiple groups that can suffer from disparities, such as patients who need interpreters, or those who have lower technological literacy and access to digital devices. Creating awareness of these pitfalls in virtual care can help medical learners recognize and support in creative solutions for these factors.
Dr. Mathews is chief, hospital medicine, at Regions Hospital, HealthPartners, St. Paul, Minn. Dr. Doshi is telemedicine director, hospital medicine, HealthPartners.
Rapid cycle pediatric simulation exercises promise improved readiness
Focused repetition builds sustained skill
A methodical, constructive, goal-oriented rapid repetition of emergency response simulations has emerged as a dominant strategy for pediatric readiness in the hospital setting, according to a detailed description of one such program at the virtual Pediatric Hospital Medicine.
Rather than a single run-through followed by a lengthy debriefing, which has been a traditional approach, short simulations done rapidly and repeatedly until skills are mastered improve skill development, according to Jeanmarie Schied, MD, of the department of pediatrics, University of Chicago Medicine.
“This method utilizes repetitions to develop muscle memory much like an athlete who ‘practices, practices, practices’ until it becomes second nature,” Dr. Schied explained.
Dr. Schied credited this approach to Elizabeth Hunt, MD, PhD, director of the Johns Hopkins Medicine Simulation Center. The method created by Dr. Hunt is called Rapid Cycle Deliberate Practice (RCDP). At the University of Chicago, where the same principles are being applied, “we have had great success,” Dr. Schied said.
Deficiencies in the traditional approach prompted the change. It has been shown that when experienced residents who have performed multiple simulations are compared to new residents with limited experience or when those certified in Pediatric Advanced Life Support (PAL) are compared to those who are not, they “do not necessarily do better” in the metrics used in simulations to measure competence, according to Dr. Schied.
With the RDCP, learners get multiple chances to master skills.
“Everyone makes mistakes, and letting the participants know this ahead of time puts people at ease,” Dr. Schied said. “People want to know they will have a chance to rewind and do it right.”
In setting up an effective simulation program, the first step is a needs assessment. By first gauging the skill and experience level of those scheduled to participate, Dr. Schied said the program can be tailored to the audience.
The next step is formulating learning objectives. Dr. Schied recommended creating these objectives for the case overall and for each phase of the simulation as it progresses from basic clinical assessments through the specific interventions appropriate for the diagnosis.
Within these objectives there are additional goals. For example, the team should work to administer care within prespecified benchmarks, such as an elapsed time of 60 seconds or less for oxygenation or a time of 180 seconds or less for defibrillation, according to Dr. Schied.
Yet, Dr. Schied suggested that enforcing these goals on initial run-throughs might not be appropriate.
“Let the scenario run longer so you can see the deficits,” Dr. Schied said. If, for example, chest compression is not being done correctly, she recommended interrupting the process to provide immediate and direct feedback. In critiquing the performance, Dr. Schied advised against a critical or punitive tone.
“Inform the learners that they are in a safe environment,” she said. It is essential to identify errors so that they can be corrected on the next run of the practice simulation, but Dr. Schied advised instructors to “be nonjudgmental.” Praise is appropriate when warranted, but she also warned, “don’t sugarcoat” a substandard performance.
During the simulation, team leaders should employ action phrases, meaning that the problem and the action needed are expressed at the same time, according to Dr. Schied. Examples include, “the patient is not breathing, start bagging,” or “there is no pulse, start compression.”
“When the team gets used to these action-linked phrases, studies show that they react in a more timely fashion,” Dr. Schied explained at the event sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
In the study by Dr. Hunt that established the effectiveness of RDCP, 51 pediatric residents who had previously participated in a cardiopulmonary arrest simulation were retested again after being retrained with the RDCP methodology (Resuscitation 2014;85:945-51).
RDCP “was associated with improvement in performance of key measures of quality life support and progressive acquisition of resuscitation skills,” according to Dr. Hunt, who has published frequently on resuscitation training in pediatrics.
Prior to RDCP, traditional methods produced “little improvement” in resuscitation skills when measured over the course of pediatric residency, according to Dr. Hunt. After RDCP, third-year residents were shown to be “significantly more likely than first-years to defibrillate within 2 minutes,” she reported.
However, there are other strategies to improve retention of skills, according to Dr. Schied. For example, it is important to conduct simulations when the staff can focus. Specifically, Dr. Schied recommended conducting simulations immediately after a staff meeting or before a scheduled shift so that clinical responsibilities will not interfere or divert the learner’s attention. She also recommended conducting key simulations quarterly.
“Studies have shown that knowledge deterioration related to resuscitation begins about 4 months after the last simulation,” she said.
In addition to building the skills of individual participants, Dr. Schied emphasized the importance of also developing effective team dynamics and active communication. In the debriefing that should follow every simulation, she recommended encouraging a discussion of strengths and weaknesses of the team response.
Pediatric emergency simulation scenarios are readily available on multiple sites found on the Internet,” Dr. Schied said. She recommended documenting performance so the data are available for subsequent analysis.
Focused repetition builds sustained skill
Focused repetition builds sustained skill
A methodical, constructive, goal-oriented rapid repetition of emergency response simulations has emerged as a dominant strategy for pediatric readiness in the hospital setting, according to a detailed description of one such program at the virtual Pediatric Hospital Medicine.
Rather than a single run-through followed by a lengthy debriefing, which has been a traditional approach, short simulations done rapidly and repeatedly until skills are mastered improve skill development, according to Jeanmarie Schied, MD, of the department of pediatrics, University of Chicago Medicine.
“This method utilizes repetitions to develop muscle memory much like an athlete who ‘practices, practices, practices’ until it becomes second nature,” Dr. Schied explained.
Dr. Schied credited this approach to Elizabeth Hunt, MD, PhD, director of the Johns Hopkins Medicine Simulation Center. The method created by Dr. Hunt is called Rapid Cycle Deliberate Practice (RCDP). At the University of Chicago, where the same principles are being applied, “we have had great success,” Dr. Schied said.
Deficiencies in the traditional approach prompted the change. It has been shown that when experienced residents who have performed multiple simulations are compared to new residents with limited experience or when those certified in Pediatric Advanced Life Support (PAL) are compared to those who are not, they “do not necessarily do better” in the metrics used in simulations to measure competence, according to Dr. Schied.
With the RDCP, learners get multiple chances to master skills.
“Everyone makes mistakes, and letting the participants know this ahead of time puts people at ease,” Dr. Schied said. “People want to know they will have a chance to rewind and do it right.”
In setting up an effective simulation program, the first step is a needs assessment. By first gauging the skill and experience level of those scheduled to participate, Dr. Schied said the program can be tailored to the audience.
The next step is formulating learning objectives. Dr. Schied recommended creating these objectives for the case overall and for each phase of the simulation as it progresses from basic clinical assessments through the specific interventions appropriate for the diagnosis.
Within these objectives there are additional goals. For example, the team should work to administer care within prespecified benchmarks, such as an elapsed time of 60 seconds or less for oxygenation or a time of 180 seconds or less for defibrillation, according to Dr. Schied.
Yet, Dr. Schied suggested that enforcing these goals on initial run-throughs might not be appropriate.
“Let the scenario run longer so you can see the deficits,” Dr. Schied said. If, for example, chest compression is not being done correctly, she recommended interrupting the process to provide immediate and direct feedback. In critiquing the performance, Dr. Schied advised against a critical or punitive tone.
“Inform the learners that they are in a safe environment,” she said. It is essential to identify errors so that they can be corrected on the next run of the practice simulation, but Dr. Schied advised instructors to “be nonjudgmental.” Praise is appropriate when warranted, but she also warned, “don’t sugarcoat” a substandard performance.
During the simulation, team leaders should employ action phrases, meaning that the problem and the action needed are expressed at the same time, according to Dr. Schied. Examples include, “the patient is not breathing, start bagging,” or “there is no pulse, start compression.”
“When the team gets used to these action-linked phrases, studies show that they react in a more timely fashion,” Dr. Schied explained at the event sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
In the study by Dr. Hunt that established the effectiveness of RDCP, 51 pediatric residents who had previously participated in a cardiopulmonary arrest simulation were retested again after being retrained with the RDCP methodology (Resuscitation 2014;85:945-51).
RDCP “was associated with improvement in performance of key measures of quality life support and progressive acquisition of resuscitation skills,” according to Dr. Hunt, who has published frequently on resuscitation training in pediatrics.
Prior to RDCP, traditional methods produced “little improvement” in resuscitation skills when measured over the course of pediatric residency, according to Dr. Hunt. After RDCP, third-year residents were shown to be “significantly more likely than first-years to defibrillate within 2 minutes,” she reported.
However, there are other strategies to improve retention of skills, according to Dr. Schied. For example, it is important to conduct simulations when the staff can focus. Specifically, Dr. Schied recommended conducting simulations immediately after a staff meeting or before a scheduled shift so that clinical responsibilities will not interfere or divert the learner’s attention. She also recommended conducting key simulations quarterly.
“Studies have shown that knowledge deterioration related to resuscitation begins about 4 months after the last simulation,” she said.
In addition to building the skills of individual participants, Dr. Schied emphasized the importance of also developing effective team dynamics and active communication. In the debriefing that should follow every simulation, she recommended encouraging a discussion of strengths and weaknesses of the team response.
Pediatric emergency simulation scenarios are readily available on multiple sites found on the Internet,” Dr. Schied said. She recommended documenting performance so the data are available for subsequent analysis.
A methodical, constructive, goal-oriented rapid repetition of emergency response simulations has emerged as a dominant strategy for pediatric readiness in the hospital setting, according to a detailed description of one such program at the virtual Pediatric Hospital Medicine.
Rather than a single run-through followed by a lengthy debriefing, which has been a traditional approach, short simulations done rapidly and repeatedly until skills are mastered improve skill development, according to Jeanmarie Schied, MD, of the department of pediatrics, University of Chicago Medicine.
“This method utilizes repetitions to develop muscle memory much like an athlete who ‘practices, practices, practices’ until it becomes second nature,” Dr. Schied explained.
Dr. Schied credited this approach to Elizabeth Hunt, MD, PhD, director of the Johns Hopkins Medicine Simulation Center. The method created by Dr. Hunt is called Rapid Cycle Deliberate Practice (RCDP). At the University of Chicago, where the same principles are being applied, “we have had great success,” Dr. Schied said.
Deficiencies in the traditional approach prompted the change. It has been shown that when experienced residents who have performed multiple simulations are compared to new residents with limited experience or when those certified in Pediatric Advanced Life Support (PAL) are compared to those who are not, they “do not necessarily do better” in the metrics used in simulations to measure competence, according to Dr. Schied.
With the RDCP, learners get multiple chances to master skills.
“Everyone makes mistakes, and letting the participants know this ahead of time puts people at ease,” Dr. Schied said. “People want to know they will have a chance to rewind and do it right.”
In setting up an effective simulation program, the first step is a needs assessment. By first gauging the skill and experience level of those scheduled to participate, Dr. Schied said the program can be tailored to the audience.
The next step is formulating learning objectives. Dr. Schied recommended creating these objectives for the case overall and for each phase of the simulation as it progresses from basic clinical assessments through the specific interventions appropriate for the diagnosis.
Within these objectives there are additional goals. For example, the team should work to administer care within prespecified benchmarks, such as an elapsed time of 60 seconds or less for oxygenation or a time of 180 seconds or less for defibrillation, according to Dr. Schied.
Yet, Dr. Schied suggested that enforcing these goals on initial run-throughs might not be appropriate.
“Let the scenario run longer so you can see the deficits,” Dr. Schied said. If, for example, chest compression is not being done correctly, she recommended interrupting the process to provide immediate and direct feedback. In critiquing the performance, Dr. Schied advised against a critical or punitive tone.
“Inform the learners that they are in a safe environment,” she said. It is essential to identify errors so that they can be corrected on the next run of the practice simulation, but Dr. Schied advised instructors to “be nonjudgmental.” Praise is appropriate when warranted, but she also warned, “don’t sugarcoat” a substandard performance.
During the simulation, team leaders should employ action phrases, meaning that the problem and the action needed are expressed at the same time, according to Dr. Schied. Examples include, “the patient is not breathing, start bagging,” or “there is no pulse, start compression.”
“When the team gets used to these action-linked phrases, studies show that they react in a more timely fashion,” Dr. Schied explained at the event sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
In the study by Dr. Hunt that established the effectiveness of RDCP, 51 pediatric residents who had previously participated in a cardiopulmonary arrest simulation were retested again after being retrained with the RDCP methodology (Resuscitation 2014;85:945-51).
RDCP “was associated with improvement in performance of key measures of quality life support and progressive acquisition of resuscitation skills,” according to Dr. Hunt, who has published frequently on resuscitation training in pediatrics.
Prior to RDCP, traditional methods produced “little improvement” in resuscitation skills when measured over the course of pediatric residency, according to Dr. Hunt. After RDCP, third-year residents were shown to be “significantly more likely than first-years to defibrillate within 2 minutes,” she reported.
However, there are other strategies to improve retention of skills, according to Dr. Schied. For example, it is important to conduct simulations when the staff can focus. Specifically, Dr. Schied recommended conducting simulations immediately after a staff meeting or before a scheduled shift so that clinical responsibilities will not interfere or divert the learner’s attention. She also recommended conducting key simulations quarterly.
“Studies have shown that knowledge deterioration related to resuscitation begins about 4 months after the last simulation,” she said.
In addition to building the skills of individual participants, Dr. Schied emphasized the importance of also developing effective team dynamics and active communication. In the debriefing that should follow every simulation, she recommended encouraging a discussion of strengths and weaknesses of the team response.
Pediatric emergency simulation scenarios are readily available on multiple sites found on the Internet,” Dr. Schied said. She recommended documenting performance so the data are available for subsequent analysis.
FROM PHM20 VIRTUAL
Hypertension often goes undertreated in patients with a history of stroke
A new study of hypertension treatment trends found that “To our knowledge, the present study is the first to analyze and report national antihypertensive medication trends exclusively among individuals with a history of stroke in the United States,” wrote Daniel Santos, MD, and Mandip S. Dhamoon, MD, DrPH, of the Icahn School of Medicine at Mount Sinai, New York. Their study was published in JAMA Neurology.
To examine blood pressure control and treatment trends among stroke survivors, the researchers examined more than a decade of data from the National Health and Nutrition Examination Survey (NHANES). The cross-sectional survey is conducted in 2-year cycles; the authors analyzed the results from 2005 to 2016 and uncovered a total of 4,971,136 eligible individuals with a history of both stroke and hypertension.
The mean age of the study population was 67.1 (95% confidence interval, 66.1-68.1), and 2,790,518 (56.1%) were women. Their mean blood pressure was 134/68 mm Hg (95% CI, 133/67–136/69), and the average number of antihypertensive medications they were taking was 1.8 (95% CI, 1.7-1.9). Of the 4,971,136 analyzed individuals, 4,721,409 (95%) were aware of their hypertension diagnosis yet more than 10% of that group had not previously been prescribed an antihypertensive medication.
More than 37% (n = 1,846,470) of the participants had uncontrolled high blood pressure upon examination (95% CI, 33.5%-40.8%), and 15.3% (95% CI, 12.5%-18.0%) were not taking any medication for it at all. The most commonly used antihypertensive medications included ACE inhibitors or angiotensin receptor blockers (59.2%; 95% CI, 54.9%-63.4%), beta-blockers (43.8%; 95% CI, 40.3%-47.3%), diuretics (41.6%; 95% CI, 37.3%-45.9%) and calcium-channel blockers (31.5%; 95% CI, 28.2%-34.8%).* Roughly 57% of the sample was taking more than one antihypertensive medication (95% CI, 52.8%-60.6%) while 28% (95% CI, 24.6%-31.5%) were taking only one.
Continued surveillance is key
“All the studies that have ever been done show that hypertension is inadequately treated,” Louis Caplan, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston, said in an interview. “One of the reasons is that it can be hard to get some of the patients to seek treatment, particularly Black Americans. Also, a lot of the medicines to treat high blood pressure have side effects, so many patients don’t want to take the pills.
“Treating hypertension really requires continued surveillance,” he added. “It’s not one visit where the doctor gives you a pill. It’s taking the pill, following your blood pressure, and seeing if it works. If it doesn’t, then maybe you change the dose, get another pill, and are followed once again. That doesn’t happen as often as it should.”
In regard to next steps, Dr. Caplan urged that hypertension “be evaluated more seriously. Even as home blood pressure kits and monitoring become increasingly available, many doctors are still going by a casual blood pressure test in the office, which doesn’t tell you how serious the problem is. There needs to be more use of technology and more conditioning of patients to monitor their own blood pressure as a guide, and then we go from there.”
The authors acknowledged their study’s limitations, including the NHANES’s reliance on self-reporting a history of stroke and the inability to distinguish between subtypes of stroke. In addition, they noted that many antihypertensive medications have uses beyond treating hypertension, which introduces “another confounding factor to medication trends.”
The authors and Dr. Caplan reported no conflicts of interest.
SOURCE: Santos D et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2499.
Correction, 8/20/20: An earlier version of this article misstated the confidence interval for diuretics.
A new study of hypertension treatment trends found that “To our knowledge, the present study is the first to analyze and report national antihypertensive medication trends exclusively among individuals with a history of stroke in the United States,” wrote Daniel Santos, MD, and Mandip S. Dhamoon, MD, DrPH, of the Icahn School of Medicine at Mount Sinai, New York. Their study was published in JAMA Neurology.
To examine blood pressure control and treatment trends among stroke survivors, the researchers examined more than a decade of data from the National Health and Nutrition Examination Survey (NHANES). The cross-sectional survey is conducted in 2-year cycles; the authors analyzed the results from 2005 to 2016 and uncovered a total of 4,971,136 eligible individuals with a history of both stroke and hypertension.
The mean age of the study population was 67.1 (95% confidence interval, 66.1-68.1), and 2,790,518 (56.1%) were women. Their mean blood pressure was 134/68 mm Hg (95% CI, 133/67–136/69), and the average number of antihypertensive medications they were taking was 1.8 (95% CI, 1.7-1.9). Of the 4,971,136 analyzed individuals, 4,721,409 (95%) were aware of their hypertension diagnosis yet more than 10% of that group had not previously been prescribed an antihypertensive medication.
More than 37% (n = 1,846,470) of the participants had uncontrolled high blood pressure upon examination (95% CI, 33.5%-40.8%), and 15.3% (95% CI, 12.5%-18.0%) were not taking any medication for it at all. The most commonly used antihypertensive medications included ACE inhibitors or angiotensin receptor blockers (59.2%; 95% CI, 54.9%-63.4%), beta-blockers (43.8%; 95% CI, 40.3%-47.3%), diuretics (41.6%; 95% CI, 37.3%-45.9%) and calcium-channel blockers (31.5%; 95% CI, 28.2%-34.8%).* Roughly 57% of the sample was taking more than one antihypertensive medication (95% CI, 52.8%-60.6%) while 28% (95% CI, 24.6%-31.5%) were taking only one.
Continued surveillance is key
“All the studies that have ever been done show that hypertension is inadequately treated,” Louis Caplan, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston, said in an interview. “One of the reasons is that it can be hard to get some of the patients to seek treatment, particularly Black Americans. Also, a lot of the medicines to treat high blood pressure have side effects, so many patients don’t want to take the pills.
“Treating hypertension really requires continued surveillance,” he added. “It’s not one visit where the doctor gives you a pill. It’s taking the pill, following your blood pressure, and seeing if it works. If it doesn’t, then maybe you change the dose, get another pill, and are followed once again. That doesn’t happen as often as it should.”
In regard to next steps, Dr. Caplan urged that hypertension “be evaluated more seriously. Even as home blood pressure kits and monitoring become increasingly available, many doctors are still going by a casual blood pressure test in the office, which doesn’t tell you how serious the problem is. There needs to be more use of technology and more conditioning of patients to monitor their own blood pressure as a guide, and then we go from there.”
The authors acknowledged their study’s limitations, including the NHANES’s reliance on self-reporting a history of stroke and the inability to distinguish between subtypes of stroke. In addition, they noted that many antihypertensive medications have uses beyond treating hypertension, which introduces “another confounding factor to medication trends.”
The authors and Dr. Caplan reported no conflicts of interest.
SOURCE: Santos D et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2499.
Correction, 8/20/20: An earlier version of this article misstated the confidence interval for diuretics.
A new study of hypertension treatment trends found that “To our knowledge, the present study is the first to analyze and report national antihypertensive medication trends exclusively among individuals with a history of stroke in the United States,” wrote Daniel Santos, MD, and Mandip S. Dhamoon, MD, DrPH, of the Icahn School of Medicine at Mount Sinai, New York. Their study was published in JAMA Neurology.
To examine blood pressure control and treatment trends among stroke survivors, the researchers examined more than a decade of data from the National Health and Nutrition Examination Survey (NHANES). The cross-sectional survey is conducted in 2-year cycles; the authors analyzed the results from 2005 to 2016 and uncovered a total of 4,971,136 eligible individuals with a history of both stroke and hypertension.
The mean age of the study population was 67.1 (95% confidence interval, 66.1-68.1), and 2,790,518 (56.1%) were women. Their mean blood pressure was 134/68 mm Hg (95% CI, 133/67–136/69), and the average number of antihypertensive medications they were taking was 1.8 (95% CI, 1.7-1.9). Of the 4,971,136 analyzed individuals, 4,721,409 (95%) were aware of their hypertension diagnosis yet more than 10% of that group had not previously been prescribed an antihypertensive medication.
More than 37% (n = 1,846,470) of the participants had uncontrolled high blood pressure upon examination (95% CI, 33.5%-40.8%), and 15.3% (95% CI, 12.5%-18.0%) were not taking any medication for it at all. The most commonly used antihypertensive medications included ACE inhibitors or angiotensin receptor blockers (59.2%; 95% CI, 54.9%-63.4%), beta-blockers (43.8%; 95% CI, 40.3%-47.3%), diuretics (41.6%; 95% CI, 37.3%-45.9%) and calcium-channel blockers (31.5%; 95% CI, 28.2%-34.8%).* Roughly 57% of the sample was taking more than one antihypertensive medication (95% CI, 52.8%-60.6%) while 28% (95% CI, 24.6%-31.5%) were taking only one.
Continued surveillance is key
“All the studies that have ever been done show that hypertension is inadequately treated,” Louis Caplan, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston, said in an interview. “One of the reasons is that it can be hard to get some of the patients to seek treatment, particularly Black Americans. Also, a lot of the medicines to treat high blood pressure have side effects, so many patients don’t want to take the pills.
“Treating hypertension really requires continued surveillance,” he added. “It’s not one visit where the doctor gives you a pill. It’s taking the pill, following your blood pressure, and seeing if it works. If it doesn’t, then maybe you change the dose, get another pill, and are followed once again. That doesn’t happen as often as it should.”
In regard to next steps, Dr. Caplan urged that hypertension “be evaluated more seriously. Even as home blood pressure kits and monitoring become increasingly available, many doctors are still going by a casual blood pressure test in the office, which doesn’t tell you how serious the problem is. There needs to be more use of technology and more conditioning of patients to monitor their own blood pressure as a guide, and then we go from there.”
The authors acknowledged their study’s limitations, including the NHANES’s reliance on self-reporting a history of stroke and the inability to distinguish between subtypes of stroke. In addition, they noted that many antihypertensive medications have uses beyond treating hypertension, which introduces “another confounding factor to medication trends.”
The authors and Dr. Caplan reported no conflicts of interest.
SOURCE: Santos D et al. JAMA Neurol. 2020 Jul 27. doi: 10.1001/jamaneurol.2020.2499.
Correction, 8/20/20: An earlier version of this article misstated the confidence interval for diuretics.
FROM JAMA NEUROLOGY
Studies gauge role of schools, kids in spread of COVID-19
When officials closed U.S. schools in March to limit the spread of COVID-19, they may have prevented more than 1 million cases over a 26-day period, a new estimate published online July 29 in JAMA suggests.
But school closures also left blind spots in understanding how children and schools affect disease transmission.
“School closures early in pandemic responses thwarted larger-scale investigations of schools as a source of community transmission,” researchers noted in a separate study, published online July 30 in JAMA Pediatrics, that examined levels of viral RNA in children and adults with COVID-19.
“Our analyses suggest children younger than 5 years with mild to moderate COVID-19 have high amounts of SARS-CoV-2 viral RNA in their nasopharynx, compared with older children and adults,” reported Taylor Heald-Sargent, MD, PhD, and colleagues. “Thus, young children can potentially be important drivers of SARS-CoV-2 spread in the general population, as has been demonstrated with respiratory syncytial virus, where children with high viral loads are more likely to transmit.”
Although the study “was not designed to prove that younger children spread COVID-19 as much as adults,” it is a possibility, Dr. Heald-Sargent, a pediatric infectious diseases specialist at Ann and Robert H. Lurie Children’s Hospital and assistant professor of pediatrics at Northwestern University, Chicago, said in a related news release. “We need to take that into account in efforts to reduce transmission as we continue to learn more about this virus.”.
The study included 145 patients with mild or moderate illness who were within 1 week of symptom onset. The researchers used reverse transcriptase–polymerase chain reaction (rt-PCR) on nasopharyngeal swabs collected at inpatient, outpatient, emergency department, or drive-through testing sites to measure SARS-CoV-2 levels. The investigators compared PCR amplification cycle threshold (CT) values for children younger than 5 years (n = 46), children aged 5-17 years (n = 51), and adults aged 18-65 years (n = 48); lower CT values indicate higher amounts of viral nucleic acid.
Median CT values for older children and adults were similar (about 11), whereas the median CT value for young children was significantly lower (6.5). The differences between young children and adults “approximate a 10-fold to 100-fold greater amount of SARS-CoV-2 in the upper respiratory tract of young children,” the researchers wrote.
“Behavioral habits of young children and close quarters in school and day care settings raise concern for SARS-CoV-2 amplification in this population as public health restrictions are eased,” they write.
Modeling the impact of school closures
In the JAMA study, Katherine A. Auger, MD, of Cincinnati Children’s Hospital Medical Center, and colleagues examined at the U.S. population level whether closing schools, as all 50 states did in March, was associated with relative decreases in COVID-19 incidence and mortality.
To isolate the effect of school closures, the researchers used an interrupted time series analysis and included other state-level nonpharmaceutical interventions and variables in their regression models.
“Per week, the incidence was estimated to have been 39% of what it would have been had schools remained open,” Dr. Auger and colleagues wrote. “Extrapolating the absolute differences of 423.9 cases and 12.6 deaths per 100,000 to 322.2 million residents nationally suggests that school closure may have been associated with approximately 1.37 million fewer cases of COVID-19 over a 26-day period and 40,600 fewer deaths over a 16-day period; however, these figures do not account for uncertainty in the model assumptions and the resulting estimates.”
Relative reductions in incidence and mortality were largest in states that closed schools when the incidence of COVID-19 was low, the authors found.
Decisions with high stakes
In an accompanying editorial, Julie M. Donohue, PhD, and Elizabeth Miller, MD, PhD, both affiliated with the University of Pittsburgh, emphasized that the results are estimates. “School closures were enacted in close proximity ... to other physical distancing measures, such as nonessential business closures and stay-at-home orders, making it difficult to disentangle the potential effect of each intervention.”
Although the findings “suggest a role for school closures in virus mitigation, school and health officials must balance this with academic, health, and economic consequences,” Dr. Donohue and Dr. Miller added. “Given the strong connection between education, income, and life expectancy, school closures could have long-term deleterious consequences for child health, likely reaching into adulthood.” Schools provide “meals and nutrition, health care including behavioral health supports, physical activity, social interaction, supports for students with special education needs and disabilities, and other vital resources for healthy development.”
In a viewpoint article also published in JAMA, authors involved in the creation of a National Academies of Sciences, Engineering, and Medicine reported on the reopening of schools recommend that districts “make every effort to prioritize reopening with an emphasis on providing in-person instruction for students in kindergarten through grade 5 as well as those students with special needs who might be best served by in-person instruction.
“To reopen safely, school districts are encouraged to ensure ventilation and air filtration, clean surfaces frequently, provide facilities for regular handwashing, and provide space for physical distancing,” write Kenne A. Dibner, PhD, of the NASEM in Washington, D.C., and coauthors.
Furthermore, districts “need to consider transparent communication of the reality that while measures can be implemented to lower the risk of transmitting COVID-19 when schools reopen, there is no way to eliminate that risk entirely. It is critical to share both the risks and benefits of different scenarios,” they wrote.
The JAMA modeling study received funding from the Agency for Healthcare Research and Quality and the National Institutes of Health. The NASEM report was funded by the Brady Education Foundation and the Spencer Foundation. The authors disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
When officials closed U.S. schools in March to limit the spread of COVID-19, they may have prevented more than 1 million cases over a 26-day period, a new estimate published online July 29 in JAMA suggests.
But school closures also left blind spots in understanding how children and schools affect disease transmission.
“School closures early in pandemic responses thwarted larger-scale investigations of schools as a source of community transmission,” researchers noted in a separate study, published online July 30 in JAMA Pediatrics, that examined levels of viral RNA in children and adults with COVID-19.
“Our analyses suggest children younger than 5 years with mild to moderate COVID-19 have high amounts of SARS-CoV-2 viral RNA in their nasopharynx, compared with older children and adults,” reported Taylor Heald-Sargent, MD, PhD, and colleagues. “Thus, young children can potentially be important drivers of SARS-CoV-2 spread in the general population, as has been demonstrated with respiratory syncytial virus, where children with high viral loads are more likely to transmit.”
Although the study “was not designed to prove that younger children spread COVID-19 as much as adults,” it is a possibility, Dr. Heald-Sargent, a pediatric infectious diseases specialist at Ann and Robert H. Lurie Children’s Hospital and assistant professor of pediatrics at Northwestern University, Chicago, said in a related news release. “We need to take that into account in efforts to reduce transmission as we continue to learn more about this virus.”.
The study included 145 patients with mild or moderate illness who were within 1 week of symptom onset. The researchers used reverse transcriptase–polymerase chain reaction (rt-PCR) on nasopharyngeal swabs collected at inpatient, outpatient, emergency department, or drive-through testing sites to measure SARS-CoV-2 levels. The investigators compared PCR amplification cycle threshold (CT) values for children younger than 5 years (n = 46), children aged 5-17 years (n = 51), and adults aged 18-65 years (n = 48); lower CT values indicate higher amounts of viral nucleic acid.
Median CT values for older children and adults were similar (about 11), whereas the median CT value for young children was significantly lower (6.5). The differences between young children and adults “approximate a 10-fold to 100-fold greater amount of SARS-CoV-2 in the upper respiratory tract of young children,” the researchers wrote.
“Behavioral habits of young children and close quarters in school and day care settings raise concern for SARS-CoV-2 amplification in this population as public health restrictions are eased,” they write.
Modeling the impact of school closures
In the JAMA study, Katherine A. Auger, MD, of Cincinnati Children’s Hospital Medical Center, and colleagues examined at the U.S. population level whether closing schools, as all 50 states did in March, was associated with relative decreases in COVID-19 incidence and mortality.
To isolate the effect of school closures, the researchers used an interrupted time series analysis and included other state-level nonpharmaceutical interventions and variables in their regression models.
“Per week, the incidence was estimated to have been 39% of what it would have been had schools remained open,” Dr. Auger and colleagues wrote. “Extrapolating the absolute differences of 423.9 cases and 12.6 deaths per 100,000 to 322.2 million residents nationally suggests that school closure may have been associated with approximately 1.37 million fewer cases of COVID-19 over a 26-day period and 40,600 fewer deaths over a 16-day period; however, these figures do not account for uncertainty in the model assumptions and the resulting estimates.”
Relative reductions in incidence and mortality were largest in states that closed schools when the incidence of COVID-19 was low, the authors found.
Decisions with high stakes
In an accompanying editorial, Julie M. Donohue, PhD, and Elizabeth Miller, MD, PhD, both affiliated with the University of Pittsburgh, emphasized that the results are estimates. “School closures were enacted in close proximity ... to other physical distancing measures, such as nonessential business closures and stay-at-home orders, making it difficult to disentangle the potential effect of each intervention.”
Although the findings “suggest a role for school closures in virus mitigation, school and health officials must balance this with academic, health, and economic consequences,” Dr. Donohue and Dr. Miller added. “Given the strong connection between education, income, and life expectancy, school closures could have long-term deleterious consequences for child health, likely reaching into adulthood.” Schools provide “meals and nutrition, health care including behavioral health supports, physical activity, social interaction, supports for students with special education needs and disabilities, and other vital resources for healthy development.”
In a viewpoint article also published in JAMA, authors involved in the creation of a National Academies of Sciences, Engineering, and Medicine reported on the reopening of schools recommend that districts “make every effort to prioritize reopening with an emphasis on providing in-person instruction for students in kindergarten through grade 5 as well as those students with special needs who might be best served by in-person instruction.
“To reopen safely, school districts are encouraged to ensure ventilation and air filtration, clean surfaces frequently, provide facilities for regular handwashing, and provide space for physical distancing,” write Kenne A. Dibner, PhD, of the NASEM in Washington, D.C., and coauthors.
Furthermore, districts “need to consider transparent communication of the reality that while measures can be implemented to lower the risk of transmitting COVID-19 when schools reopen, there is no way to eliminate that risk entirely. It is critical to share both the risks and benefits of different scenarios,” they wrote.
The JAMA modeling study received funding from the Agency for Healthcare Research and Quality and the National Institutes of Health. The NASEM report was funded by the Brady Education Foundation and the Spencer Foundation. The authors disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
When officials closed U.S. schools in March to limit the spread of COVID-19, they may have prevented more than 1 million cases over a 26-day period, a new estimate published online July 29 in JAMA suggests.
But school closures also left blind spots in understanding how children and schools affect disease transmission.
“School closures early in pandemic responses thwarted larger-scale investigations of schools as a source of community transmission,” researchers noted in a separate study, published online July 30 in JAMA Pediatrics, that examined levels of viral RNA in children and adults with COVID-19.
“Our analyses suggest children younger than 5 years with mild to moderate COVID-19 have high amounts of SARS-CoV-2 viral RNA in their nasopharynx, compared with older children and adults,” reported Taylor Heald-Sargent, MD, PhD, and colleagues. “Thus, young children can potentially be important drivers of SARS-CoV-2 spread in the general population, as has been demonstrated with respiratory syncytial virus, where children with high viral loads are more likely to transmit.”
Although the study “was not designed to prove that younger children spread COVID-19 as much as adults,” it is a possibility, Dr. Heald-Sargent, a pediatric infectious diseases specialist at Ann and Robert H. Lurie Children’s Hospital and assistant professor of pediatrics at Northwestern University, Chicago, said in a related news release. “We need to take that into account in efforts to reduce transmission as we continue to learn more about this virus.”.
The study included 145 patients with mild or moderate illness who were within 1 week of symptom onset. The researchers used reverse transcriptase–polymerase chain reaction (rt-PCR) on nasopharyngeal swabs collected at inpatient, outpatient, emergency department, or drive-through testing sites to measure SARS-CoV-2 levels. The investigators compared PCR amplification cycle threshold (CT) values for children younger than 5 years (n = 46), children aged 5-17 years (n = 51), and adults aged 18-65 years (n = 48); lower CT values indicate higher amounts of viral nucleic acid.
Median CT values for older children and adults were similar (about 11), whereas the median CT value for young children was significantly lower (6.5). The differences between young children and adults “approximate a 10-fold to 100-fold greater amount of SARS-CoV-2 in the upper respiratory tract of young children,” the researchers wrote.
“Behavioral habits of young children and close quarters in school and day care settings raise concern for SARS-CoV-2 amplification in this population as public health restrictions are eased,” they write.
Modeling the impact of school closures
In the JAMA study, Katherine A. Auger, MD, of Cincinnati Children’s Hospital Medical Center, and colleagues examined at the U.S. population level whether closing schools, as all 50 states did in March, was associated with relative decreases in COVID-19 incidence and mortality.
To isolate the effect of school closures, the researchers used an interrupted time series analysis and included other state-level nonpharmaceutical interventions and variables in their regression models.
“Per week, the incidence was estimated to have been 39% of what it would have been had schools remained open,” Dr. Auger and colleagues wrote. “Extrapolating the absolute differences of 423.9 cases and 12.6 deaths per 100,000 to 322.2 million residents nationally suggests that school closure may have been associated with approximately 1.37 million fewer cases of COVID-19 over a 26-day period and 40,600 fewer deaths over a 16-day period; however, these figures do not account for uncertainty in the model assumptions and the resulting estimates.”
Relative reductions in incidence and mortality were largest in states that closed schools when the incidence of COVID-19 was low, the authors found.
Decisions with high stakes
In an accompanying editorial, Julie M. Donohue, PhD, and Elizabeth Miller, MD, PhD, both affiliated with the University of Pittsburgh, emphasized that the results are estimates. “School closures were enacted in close proximity ... to other physical distancing measures, such as nonessential business closures and stay-at-home orders, making it difficult to disentangle the potential effect of each intervention.”
Although the findings “suggest a role for school closures in virus mitigation, school and health officials must balance this with academic, health, and economic consequences,” Dr. Donohue and Dr. Miller added. “Given the strong connection between education, income, and life expectancy, school closures could have long-term deleterious consequences for child health, likely reaching into adulthood.” Schools provide “meals and nutrition, health care including behavioral health supports, physical activity, social interaction, supports for students with special education needs and disabilities, and other vital resources for healthy development.”
In a viewpoint article also published in JAMA, authors involved in the creation of a National Academies of Sciences, Engineering, and Medicine reported on the reopening of schools recommend that districts “make every effort to prioritize reopening with an emphasis on providing in-person instruction for students in kindergarten through grade 5 as well as those students with special needs who might be best served by in-person instruction.
“To reopen safely, school districts are encouraged to ensure ventilation and air filtration, clean surfaces frequently, provide facilities for regular handwashing, and provide space for physical distancing,” write Kenne A. Dibner, PhD, of the NASEM in Washington, D.C., and coauthors.
Furthermore, districts “need to consider transparent communication of the reality that while measures can be implemented to lower the risk of transmitting COVID-19 when schools reopen, there is no way to eliminate that risk entirely. It is critical to share both the risks and benefits of different scenarios,” they wrote.
The JAMA modeling study received funding from the Agency for Healthcare Research and Quality and the National Institutes of Health. The NASEM report was funded by the Brady Education Foundation and the Spencer Foundation. The authors disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
Guidance covers glycemia in dexamethasone-treated COVID-19 patients
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.