User login
A plane crash interrupts a doctor’s vacation
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Sick call
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com
Keeping up with the evidence (and the residents)
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
New recommendations for hyperglycemia management
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.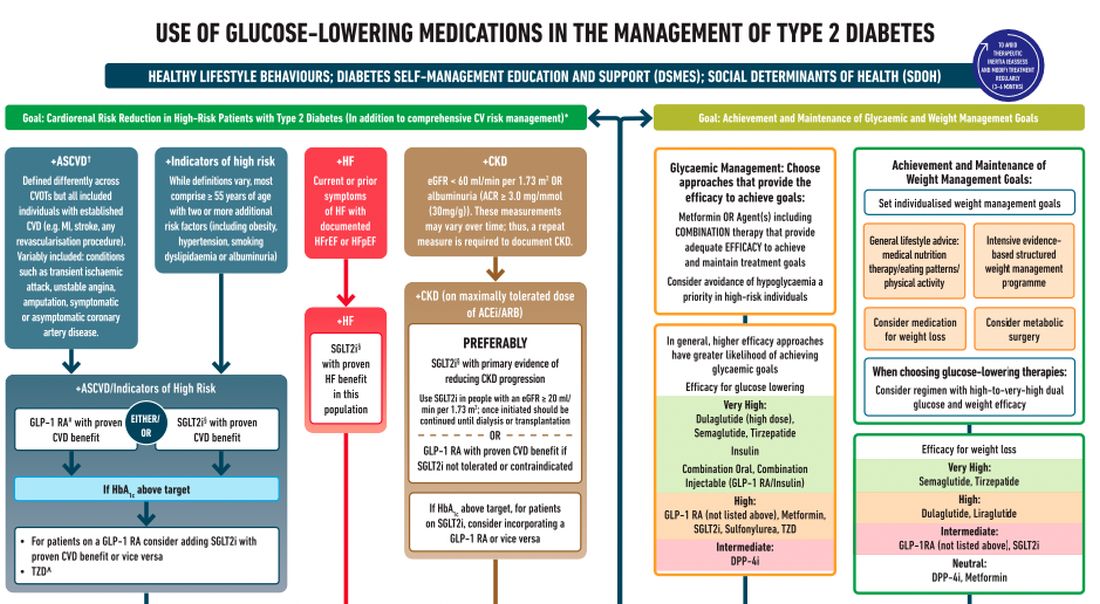
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
Microtox and Mesotox
The terms when they mention one of these terms.
Let’s settle the nomenclature confusion. In this column, I define and outline suggested terminology based on studies and my 15 years of experience using neuromodulators. If any readers or colleagues disagree, please write to me and we can discuss the alternatives in a subsequent article; if you agree, please also write to me so we can collaboratively correct the discrepancies in the literature accordingly.
The term mesotherapy, originating from the Greek “mesos” referring to the early embryonic mesoderm, was identified in the 1950’s by Dr. Michel Pistor, a French physician who administered drugs intradermally. The term was defined as a minimally invasive technique by which drugs or bioactive substances are given in small quantities through dermal micropunctures. Drugs administered intradermally diffuse very slowly and therefore, stay in the tissue longer than those administered intramuscularly.
Thus, Mesotox is defined not by the concentration of the neuromodulator or location, but by the depth of injection in the superficial dermis. It can be delivered through individual injections or through a microneedling pen.
Microtox refers to the dilution of the neuromodulator at concentrations below the proposed dilution guidelines of the manufacturer: Less than 2.5 U per 0.1 mL for onabotulinumtoxinA (OBA), incobotulinumtoxinA (IBA), and prabotulinumtoxinA (PBA); and less than 10 U per 0.1 mL for abobotulinumtoxinA (ABO), This method allows for the injection of superficial cutaneous muscles softening the dynamic rhytids without complete paralysis.
Mesotox is widely used off label for facial lifting, reduction in skin laxity or crepiness, flushing of rosacea, acne, hyperhidrosis of the face, keloids, seborrhea, neck rejuvenation, contouring of the mandibular border, and scalp oiliness. Based on a review of articles using this technique, dilution methods were less than 2.5 U per 1 mL (OBA, IBA) and less than 10 U per 0.1 mL (ABO) depth of injection was the superficial to mid-dermis with injection points 0.5 cm to 1 cm apart.
In a study by Atwa and colleagues, 25 patients with mild facial skin laxity received intradermal Botox-A on one side and saline on the other. This split face study showed a highly significant difference with facial lifting on the treated side. Mesotox injection points vary based on the clinical indication and area being treated.
The treatment of dynamic muscles using standard neuromodulator dosing protocols include the treatment of the glabella, crow’s feet, forehead lines, masseter hypertrophy, bunny lines, gummy smile, perioral lines, mentalis hypertonia, platysmal bands, and marionette lines.
However, hyperdilute neuromodulators or Microtox can effectively be used alone or in combination with standard dosing for the following off-label uses. Used in combination with standard dosing of the forehead lines, I use Microtox in the lateral brow to soften the frontalis muscle without dropping the brow in patients with a low-set brow or lid laxity. I also use it for the jelly roll of the eyes and to open the aperture of the eyes. Along the nose, Microtox can also be used to treat a sagging nasal tip, decrease the width of the ala, and treat overactive facial muscles adjacent to the nose resulting in an overactive nasolabial fold.
Similarly, Microtox can be used to treat lateral smile lines and downward extensions of the crow’s feet. In all of the aforementioned treatment areas, I recommend approximately 0.5-1 U of toxin in each area divided at 1-cm intervals.Mesotox and Microtox are both highly effective strategies to treat the aging face. However, the nomenclature is not interchangeable. I propose that the term Mesotox be used only to articulate or define the superficial injection of a neuromodulator for the improvement of the skin that does not involve the injection into or paralysis of a cutaneous muscle (“tox” being used generically for all neuromodulators). I also propose that the term Microtox should be used to define the dilution of a neuromodulator beyond the manufacturer-recommended dilution protocols – used for the paralysis of a cutaneous muscle. In addition, I recommend that the terms MicroBotox and MesoBotox no longer be used. These procedures all have risks, and adverse events associated with Microtox and Mesotox are similar to those of any neuromodulator injection at FDA-recommended maximum doses, and dilution and storage protocols and proper injection techniques need to be followed. Expertise and training is crucial and treatment by a board-certified dermatologist or plastic surgeon is imperative.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to her at dermnews@mdedge.com. Dr. Talakoub had no relevant disclosures.
References
Awaida CJ et al. Plast Reconstr Surg. 2018 Sep;142(3):640-9.
Calvani F et al. Plast Surg (Oakv). 2019 May;27(2):156-61.
Iranmanesh B et al. J Cosmet Dermatol. 2022 Oct;21(10):4160-70.
Kandhari R et al. J Cutan Aesthet Surg. 2022 Apr-Jun;15(2):101-7.
Lewandowski M et al. Molecules. 2022 May 13;27(10):3143.
Mammucari M et al. Eur Rev Med Pharmacol Sci. 2011 Jun;15(6):682-94.
Park KY et al. Ann Dermatol. 2018 Dec;30(6):688-93.
Pistor M. Chir Dent Fr. 1976;46:59-60.
Rho NK, Gil YC. Toxins (Basel). 2021 Nov 19;13(11):817.
Wu WTL. Plast Reconstr Surg. 2015 Nov;136(5 Suppl):92S-100S.
Zhang H et al. Clin Cosmet Investig Dermatol. 2021 Apr 30;14:407-17.
The terms when they mention one of these terms.
Let’s settle the nomenclature confusion. In this column, I define and outline suggested terminology based on studies and my 15 years of experience using neuromodulators. If any readers or colleagues disagree, please write to me and we can discuss the alternatives in a subsequent article; if you agree, please also write to me so we can collaboratively correct the discrepancies in the literature accordingly.
The term mesotherapy, originating from the Greek “mesos” referring to the early embryonic mesoderm, was identified in the 1950’s by Dr. Michel Pistor, a French physician who administered drugs intradermally. The term was defined as a minimally invasive technique by which drugs or bioactive substances are given in small quantities through dermal micropunctures. Drugs administered intradermally diffuse very slowly and therefore, stay in the tissue longer than those administered intramuscularly.
Thus, Mesotox is defined not by the concentration of the neuromodulator or location, but by the depth of injection in the superficial dermis. It can be delivered through individual injections or through a microneedling pen.
Microtox refers to the dilution of the neuromodulator at concentrations below the proposed dilution guidelines of the manufacturer: Less than 2.5 U per 0.1 mL for onabotulinumtoxinA (OBA), incobotulinumtoxinA (IBA), and prabotulinumtoxinA (PBA); and less than 10 U per 0.1 mL for abobotulinumtoxinA (ABO), This method allows for the injection of superficial cutaneous muscles softening the dynamic rhytids without complete paralysis.
Mesotox is widely used off label for facial lifting, reduction in skin laxity or crepiness, flushing of rosacea, acne, hyperhidrosis of the face, keloids, seborrhea, neck rejuvenation, contouring of the mandibular border, and scalp oiliness. Based on a review of articles using this technique, dilution methods were less than 2.5 U per 1 mL (OBA, IBA) and less than 10 U per 0.1 mL (ABO) depth of injection was the superficial to mid-dermis with injection points 0.5 cm to 1 cm apart.
In a study by Atwa and colleagues, 25 patients with mild facial skin laxity received intradermal Botox-A on one side and saline on the other. This split face study showed a highly significant difference with facial lifting on the treated side. Mesotox injection points vary based on the clinical indication and area being treated.
The treatment of dynamic muscles using standard neuromodulator dosing protocols include the treatment of the glabella, crow’s feet, forehead lines, masseter hypertrophy, bunny lines, gummy smile, perioral lines, mentalis hypertonia, platysmal bands, and marionette lines.
However, hyperdilute neuromodulators or Microtox can effectively be used alone or in combination with standard dosing for the following off-label uses. Used in combination with standard dosing of the forehead lines, I use Microtox in the lateral brow to soften the frontalis muscle without dropping the brow in patients with a low-set brow or lid laxity. I also use it for the jelly roll of the eyes and to open the aperture of the eyes. Along the nose, Microtox can also be used to treat a sagging nasal tip, decrease the width of the ala, and treat overactive facial muscles adjacent to the nose resulting in an overactive nasolabial fold.
Similarly, Microtox can be used to treat lateral smile lines and downward extensions of the crow’s feet. In all of the aforementioned treatment areas, I recommend approximately 0.5-1 U of toxin in each area divided at 1-cm intervals.Mesotox and Microtox are both highly effective strategies to treat the aging face. However, the nomenclature is not interchangeable. I propose that the term Mesotox be used only to articulate or define the superficial injection of a neuromodulator for the improvement of the skin that does not involve the injection into or paralysis of a cutaneous muscle (“tox” being used generically for all neuromodulators). I also propose that the term Microtox should be used to define the dilution of a neuromodulator beyond the manufacturer-recommended dilution protocols – used for the paralysis of a cutaneous muscle. In addition, I recommend that the terms MicroBotox and MesoBotox no longer be used. These procedures all have risks, and adverse events associated with Microtox and Mesotox are similar to those of any neuromodulator injection at FDA-recommended maximum doses, and dilution and storage protocols and proper injection techniques need to be followed. Expertise and training is crucial and treatment by a board-certified dermatologist or plastic surgeon is imperative.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to her at dermnews@mdedge.com. Dr. Talakoub had no relevant disclosures.
References
Awaida CJ et al. Plast Reconstr Surg. 2018 Sep;142(3):640-9.
Calvani F et al. Plast Surg (Oakv). 2019 May;27(2):156-61.
Iranmanesh B et al. J Cosmet Dermatol. 2022 Oct;21(10):4160-70.
Kandhari R et al. J Cutan Aesthet Surg. 2022 Apr-Jun;15(2):101-7.
Lewandowski M et al. Molecules. 2022 May 13;27(10):3143.
Mammucari M et al. Eur Rev Med Pharmacol Sci. 2011 Jun;15(6):682-94.
Park KY et al. Ann Dermatol. 2018 Dec;30(6):688-93.
Pistor M. Chir Dent Fr. 1976;46:59-60.
Rho NK, Gil YC. Toxins (Basel). 2021 Nov 19;13(11):817.
Wu WTL. Plast Reconstr Surg. 2015 Nov;136(5 Suppl):92S-100S.
Zhang H et al. Clin Cosmet Investig Dermatol. 2021 Apr 30;14:407-17.
The terms when they mention one of these terms.
Let’s settle the nomenclature confusion. In this column, I define and outline suggested terminology based on studies and my 15 years of experience using neuromodulators. If any readers or colleagues disagree, please write to me and we can discuss the alternatives in a subsequent article; if you agree, please also write to me so we can collaboratively correct the discrepancies in the literature accordingly.
The term mesotherapy, originating from the Greek “mesos” referring to the early embryonic mesoderm, was identified in the 1950’s by Dr. Michel Pistor, a French physician who administered drugs intradermally. The term was defined as a minimally invasive technique by which drugs or bioactive substances are given in small quantities through dermal micropunctures. Drugs administered intradermally diffuse very slowly and therefore, stay in the tissue longer than those administered intramuscularly.
Thus, Mesotox is defined not by the concentration of the neuromodulator or location, but by the depth of injection in the superficial dermis. It can be delivered through individual injections or through a microneedling pen.
Microtox refers to the dilution of the neuromodulator at concentrations below the proposed dilution guidelines of the manufacturer: Less than 2.5 U per 0.1 mL for onabotulinumtoxinA (OBA), incobotulinumtoxinA (IBA), and prabotulinumtoxinA (PBA); and less than 10 U per 0.1 mL for abobotulinumtoxinA (ABO), This method allows for the injection of superficial cutaneous muscles softening the dynamic rhytids without complete paralysis.
Mesotox is widely used off label for facial lifting, reduction in skin laxity or crepiness, flushing of rosacea, acne, hyperhidrosis of the face, keloids, seborrhea, neck rejuvenation, contouring of the mandibular border, and scalp oiliness. Based on a review of articles using this technique, dilution methods were less than 2.5 U per 1 mL (OBA, IBA) and less than 10 U per 0.1 mL (ABO) depth of injection was the superficial to mid-dermis with injection points 0.5 cm to 1 cm apart.
In a study by Atwa and colleagues, 25 patients with mild facial skin laxity received intradermal Botox-A on one side and saline on the other. This split face study showed a highly significant difference with facial lifting on the treated side. Mesotox injection points vary based on the clinical indication and area being treated.
The treatment of dynamic muscles using standard neuromodulator dosing protocols include the treatment of the glabella, crow’s feet, forehead lines, masseter hypertrophy, bunny lines, gummy smile, perioral lines, mentalis hypertonia, platysmal bands, and marionette lines.
However, hyperdilute neuromodulators or Microtox can effectively be used alone or in combination with standard dosing for the following off-label uses. Used in combination with standard dosing of the forehead lines, I use Microtox in the lateral brow to soften the frontalis muscle without dropping the brow in patients with a low-set brow or lid laxity. I also use it for the jelly roll of the eyes and to open the aperture of the eyes. Along the nose, Microtox can also be used to treat a sagging nasal tip, decrease the width of the ala, and treat overactive facial muscles adjacent to the nose resulting in an overactive nasolabial fold.
Similarly, Microtox can be used to treat lateral smile lines and downward extensions of the crow’s feet. In all of the aforementioned treatment areas, I recommend approximately 0.5-1 U of toxin in each area divided at 1-cm intervals.Mesotox and Microtox are both highly effective strategies to treat the aging face. However, the nomenclature is not interchangeable. I propose that the term Mesotox be used only to articulate or define the superficial injection of a neuromodulator for the improvement of the skin that does not involve the injection into or paralysis of a cutaneous muscle (“tox” being used generically for all neuromodulators). I also propose that the term Microtox should be used to define the dilution of a neuromodulator beyond the manufacturer-recommended dilution protocols – used for the paralysis of a cutaneous muscle. In addition, I recommend that the terms MicroBotox and MesoBotox no longer be used. These procedures all have risks, and adverse events associated with Microtox and Mesotox are similar to those of any neuromodulator injection at FDA-recommended maximum doses, and dilution and storage protocols and proper injection techniques need to be followed. Expertise and training is crucial and treatment by a board-certified dermatologist or plastic surgeon is imperative.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to her at dermnews@mdedge.com. Dr. Talakoub had no relevant disclosures.
References
Awaida CJ et al. Plast Reconstr Surg. 2018 Sep;142(3):640-9.
Calvani F et al. Plast Surg (Oakv). 2019 May;27(2):156-61.
Iranmanesh B et al. J Cosmet Dermatol. 2022 Oct;21(10):4160-70.
Kandhari R et al. J Cutan Aesthet Surg. 2022 Apr-Jun;15(2):101-7.
Lewandowski M et al. Molecules. 2022 May 13;27(10):3143.
Mammucari M et al. Eur Rev Med Pharmacol Sci. 2011 Jun;15(6):682-94.
Park KY et al. Ann Dermatol. 2018 Dec;30(6):688-93.
Pistor M. Chir Dent Fr. 1976;46:59-60.
Rho NK, Gil YC. Toxins (Basel). 2021 Nov 19;13(11):817.
Wu WTL. Plast Reconstr Surg. 2015 Nov;136(5 Suppl):92S-100S.
Zhang H et al. Clin Cosmet Investig Dermatol. 2021 Apr 30;14:407-17.
Ulmus davidiana root extract
Ulmus davidiana, commonly known as yugeunpi, has a long history of use in Korea in treating burns, eczema, frostbite, difficulties in urination, inflammation, and psoriasis,1 and has also been used in China for some of these indications, including skin inflammation.2,3 Currently, there are several areas in which the bioactivity of U. davidiana are under investigation, with numerous potential applications in dermatology. This column focuses briefly on the evidence supporting the traditional uses of the plant and potential new applications.
Anti-inflammatory activity
Eom and colleagues studied the potential of a polysaccharide extract from the root bark of U. davidiana to serve as a suitable cosmetic ingredient for conferring moisturizing, anti-inflammatory, and photoprotective activity. In this 2006 investigation, the composition of the polysaccharide extract was found to be primarily rhamnose, galactose, and glucose. The root extract exhibited a similar humectant moisturizing effect as hyaluronic acid, the researchers reported. The U. davidiana root extract was also found to dose-dependently suppress prostaglandin E2. The inhibition of the release of interleukin-6 and IL-8 was also reported to be significant. The use of the U. davidiana extract also stimulated the recovery of human fibroblasts (two times that of positive control) exposed to UVA irradiation. The researchers suggested that their overall results point to the viability of U. davidiana root extract as a cosmetic agent ingredient to protect skin from UV exposure and the inflammation that follows.2
In 2013, Choi and colleagues found that a methanol extract of the stem and root barks of U. davidiana revealed anti-inflammatory properties, with activity attributed to two trihydroxy acids [then-new trihydroxy fatty acid, 9,12,13-trihydroxyoctadeca-10(Z),15(Z)-dienoic acid, and pinellic acid], both of which blocked prostaglandin D₂ production.4
That same year, Lyu and colleagues studied the antiallergic and anti-inflammatory effects of U. davidiana using a 1-fluoro-2,4-dinitrofluorobenzene (DNFB)–induced contact dermatitis mouse model. They found that treatment at a dose of 10 mg/mL successfully prevented skin lesions caused by consistent DNFB application. Further, the researchers observed that topically applied U. davidiana suppressed spongiosis and reduced total serum immunoglobulin and IgG2a levels. Overall, they concluded that the botanical treatment improved contact dermatitis in mice.1
In 2019, So and colleagues studied the chemical components of U. davidiana root bark (isolating a chromane derivative and 22 known substances) and reported data supporting the traditional use of the root bark for gastroenteric and inflammatory indications.3
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, including U. davidiana, is used for its anti-inflammatory properties in traditional Korean medicine.5 Choi and colleagues determined that bakuchiol exhibited robust anti-inflammatory activity in a study of U. davidiana constituents, at least partially accounting for the anti-inflammatory functions of the plant.5
Antifungal activity
In 2021, Alishir and colleagues conducted a phytochemical analysis of the root bark extract of U. davidiana, resulting in the isolation of 10 substances including the novel coumarin glycoside derivative ulmusakidian. Some of the compounds exhibited antifungal activity against Cryptococcus neoformans, though none demonstrated antifungal activity against Candida albicans.6
Wound dressing
Park and colleagues demonstrated in 2020 that superabsorbing hydrogel wound dressings composed of U. davidiana root bark powders, which exhibit gelling activity, performed effectively in speeding up wound closure and cutaneous regeneration in skin-wound mice models. These dressings also displayed thermal stability and superior mechanical properties to pullulan-only gel films. The researchers concluded that gel films composed of U. davidiana have potential to surpass the effectiveness of current products.7
Anti–hair loss activity
Early in 2022, Kwon and colleagues investigated the anti–hair loss mechanism of U. davidiana and determined that supercritical extraction-residues of U. davidiana significantly hinder the secretion of transforming growth factor–beta but dose dependently salvage insulinlike growth factor 1, and substantially decrease dihydrotestosterone synthesis. They concluded that these U. davidiana supercritical fluid extract residues have the potential to halt the loss of human hair.8
Photoprotective potential
Late in 2020, Her and colleagues reported on their development and analysis of a new distillate derived from a fermented mixture of nine anti-inflammatory herbs including U. davidiana. The investigators assessed the effects of the topically applied distillate on UVB-induced skin damage in Institute of Cancer Research mice, finding significant improvements in the dorsal skin photodamage. Application of the distillate also ameliorated collagen production impairment and diminished proinflammatory cytokine levels of tumor necrosis factor (TNF)–alpha and IL-1B. The researchers concluded that this anti-inflammatory herbal distillate, which includes U. davidiana, displays the potential to serve as a photoprotective agent.9
Antiaging activity
In 2011, Yang and colleagues set out to identify constituent substances of the root bark of U. davidiana that have the capacity to suppress cellular senescence in human fibroblasts and human umbilical vein endothelial cells. They isolated 22 compounds, of which epifriedelanol, ssioriside, and catechin-7-O-beta-D-glucopyranoside impeded adriamycin-induced cellular senescence in human dermal fibroblasts and friedelin, epifriedelanol, and catechin-7-O-beta-apiofuranoside in the umbilical vein endothelial cells. Epifriedelanol was the most potent of the substances, leading the researchers to conclude that this U. davidiana component can diminish cellular senescence in human primary cells and has the potential as an oral and/or topical antiaging agent.10
Also that year, in a study on the protective effects of U. davidiana on UVB-irradiated hairless mice, the authors claimed that an ethanol extract of U. davidiana significantly suppressed wrinkle development in mice chronically exposed to UVB.11 This study showed that U. davidiana extract exerts antioxidant activity as evidenced by a decrease in MMP-1 activity. It also demonstrated antielastase activity. The treated mice showed a decrease in wrinkles as compared with water-treated mice.11 Although this is just one study in mice, it may demonstrate a protective effect on elastic fibers on skin exposed to UVB light.
Late in 2020, Lee and colleagues reported on their study of the possible antiaging effects on the skin of (-)-phenolic compounds isolated from the root bark of U. davidiana. The function of collagenase MMP-1 was found to be inhibited by the isolate (-)-catechin, which also halted collagen degradation caused by TNF-alpha in normal human dermal fibroblasts. Further, the investigators demonstrated that the U. davidiana isolate (-)-catechin reduced the expression of proinflammatory cytokines such as IL-1B and IL-6. They concluded that the U. davidiana isolate exhibits the potential to combat intrinsic as well as extrinsic cutaneous aging.12
These findings are particularly intriguing. There is much overlap between intrinsic and extrinsic aging. If U. davidiana can keep collagen intact and inhibit cellular senescence, it may serve as an early intervention toward slowing or preventing skin aging.
Summary
Of greatest interest now, perhaps, is its potential to impede cellular senescence. Senescent cells release a multitude of inflammatory and other factors that hasten intrinsic aging. Blocking cellular senescence is an important approach to the prevention and treatment of skin aging.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Lyu J et al. J Pharmacopuncture. 2013 Jun;16(2):41-5.
2. Eom SY et al. J Cosmet Sci. 2006 Sep-Oct;57(5):355-67.
3. So HM et al. Bioorg Chem. 2019 Oct;91:103145.
4. Choi HG et al. Phytother Res. 2013 Sep;27(9):1376-80.
5. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
6. Alishir A et al. Bioorg Med Chem Lett. 2021 Mar 15;36:127828.
7. Park TH et al. Saudi Pharm J. 2020 Jul;28(7):791-802.
8. Kwon YE et al. Molecules. 2022 Feb 19;27(4):1419.
9. Her Y et al. Molecules. 2020 Dec 29;26(1):124.
10. Yang HH et al. Planta Med. 2011 Mar;77(5):441-9.
11. Kim YO et al. Korean Journal of Medicinal Crop Science. 2011;19(6):508-13.
12. Lee S et al. Antioxidants (Basel). 2020 Oct 13;9(10):981.
Ulmus davidiana, commonly known as yugeunpi, has a long history of use in Korea in treating burns, eczema, frostbite, difficulties in urination, inflammation, and psoriasis,1 and has also been used in China for some of these indications, including skin inflammation.2,3 Currently, there are several areas in which the bioactivity of U. davidiana are under investigation, with numerous potential applications in dermatology. This column focuses briefly on the evidence supporting the traditional uses of the plant and potential new applications.
Anti-inflammatory activity
Eom and colleagues studied the potential of a polysaccharide extract from the root bark of U. davidiana to serve as a suitable cosmetic ingredient for conferring moisturizing, anti-inflammatory, and photoprotective activity. In this 2006 investigation, the composition of the polysaccharide extract was found to be primarily rhamnose, galactose, and glucose. The root extract exhibited a similar humectant moisturizing effect as hyaluronic acid, the researchers reported. The U. davidiana root extract was also found to dose-dependently suppress prostaglandin E2. The inhibition of the release of interleukin-6 and IL-8 was also reported to be significant. The use of the U. davidiana extract also stimulated the recovery of human fibroblasts (two times that of positive control) exposed to UVA irradiation. The researchers suggested that their overall results point to the viability of U. davidiana root extract as a cosmetic agent ingredient to protect skin from UV exposure and the inflammation that follows.2
In 2013, Choi and colleagues found that a methanol extract of the stem and root barks of U. davidiana revealed anti-inflammatory properties, with activity attributed to two trihydroxy acids [then-new trihydroxy fatty acid, 9,12,13-trihydroxyoctadeca-10(Z),15(Z)-dienoic acid, and pinellic acid], both of which blocked prostaglandin D₂ production.4
That same year, Lyu and colleagues studied the antiallergic and anti-inflammatory effects of U. davidiana using a 1-fluoro-2,4-dinitrofluorobenzene (DNFB)–induced contact dermatitis mouse model. They found that treatment at a dose of 10 mg/mL successfully prevented skin lesions caused by consistent DNFB application. Further, the researchers observed that topically applied U. davidiana suppressed spongiosis and reduced total serum immunoglobulin and IgG2a levels. Overall, they concluded that the botanical treatment improved contact dermatitis in mice.1
In 2019, So and colleagues studied the chemical components of U. davidiana root bark (isolating a chromane derivative and 22 known substances) and reported data supporting the traditional use of the root bark for gastroenteric and inflammatory indications.3
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, including U. davidiana, is used for its anti-inflammatory properties in traditional Korean medicine.5 Choi and colleagues determined that bakuchiol exhibited robust anti-inflammatory activity in a study of U. davidiana constituents, at least partially accounting for the anti-inflammatory functions of the plant.5
Antifungal activity
In 2021, Alishir and colleagues conducted a phytochemical analysis of the root bark extract of U. davidiana, resulting in the isolation of 10 substances including the novel coumarin glycoside derivative ulmusakidian. Some of the compounds exhibited antifungal activity against Cryptococcus neoformans, though none demonstrated antifungal activity against Candida albicans.6
Wound dressing
Park and colleagues demonstrated in 2020 that superabsorbing hydrogel wound dressings composed of U. davidiana root bark powders, which exhibit gelling activity, performed effectively in speeding up wound closure and cutaneous regeneration in skin-wound mice models. These dressings also displayed thermal stability and superior mechanical properties to pullulan-only gel films. The researchers concluded that gel films composed of U. davidiana have potential to surpass the effectiveness of current products.7
Anti–hair loss activity
Early in 2022, Kwon and colleagues investigated the anti–hair loss mechanism of U. davidiana and determined that supercritical extraction-residues of U. davidiana significantly hinder the secretion of transforming growth factor–beta but dose dependently salvage insulinlike growth factor 1, and substantially decrease dihydrotestosterone synthesis. They concluded that these U. davidiana supercritical fluid extract residues have the potential to halt the loss of human hair.8
Photoprotective potential
Late in 2020, Her and colleagues reported on their development and analysis of a new distillate derived from a fermented mixture of nine anti-inflammatory herbs including U. davidiana. The investigators assessed the effects of the topically applied distillate on UVB-induced skin damage in Institute of Cancer Research mice, finding significant improvements in the dorsal skin photodamage. Application of the distillate also ameliorated collagen production impairment and diminished proinflammatory cytokine levels of tumor necrosis factor (TNF)–alpha and IL-1B. The researchers concluded that this anti-inflammatory herbal distillate, which includes U. davidiana, displays the potential to serve as a photoprotective agent.9
Antiaging activity
In 2011, Yang and colleagues set out to identify constituent substances of the root bark of U. davidiana that have the capacity to suppress cellular senescence in human fibroblasts and human umbilical vein endothelial cells. They isolated 22 compounds, of which epifriedelanol, ssioriside, and catechin-7-O-beta-D-glucopyranoside impeded adriamycin-induced cellular senescence in human dermal fibroblasts and friedelin, epifriedelanol, and catechin-7-O-beta-apiofuranoside in the umbilical vein endothelial cells. Epifriedelanol was the most potent of the substances, leading the researchers to conclude that this U. davidiana component can diminish cellular senescence in human primary cells and has the potential as an oral and/or topical antiaging agent.10
Also that year, in a study on the protective effects of U. davidiana on UVB-irradiated hairless mice, the authors claimed that an ethanol extract of U. davidiana significantly suppressed wrinkle development in mice chronically exposed to UVB.11 This study showed that U. davidiana extract exerts antioxidant activity as evidenced by a decrease in MMP-1 activity. It also demonstrated antielastase activity. The treated mice showed a decrease in wrinkles as compared with water-treated mice.11 Although this is just one study in mice, it may demonstrate a protective effect on elastic fibers on skin exposed to UVB light.
Late in 2020, Lee and colleagues reported on their study of the possible antiaging effects on the skin of (-)-phenolic compounds isolated from the root bark of U. davidiana. The function of collagenase MMP-1 was found to be inhibited by the isolate (-)-catechin, which also halted collagen degradation caused by TNF-alpha in normal human dermal fibroblasts. Further, the investigators demonstrated that the U. davidiana isolate (-)-catechin reduced the expression of proinflammatory cytokines such as IL-1B and IL-6. They concluded that the U. davidiana isolate exhibits the potential to combat intrinsic as well as extrinsic cutaneous aging.12
These findings are particularly intriguing. There is much overlap between intrinsic and extrinsic aging. If U. davidiana can keep collagen intact and inhibit cellular senescence, it may serve as an early intervention toward slowing or preventing skin aging.
Summary
Of greatest interest now, perhaps, is its potential to impede cellular senescence. Senescent cells release a multitude of inflammatory and other factors that hasten intrinsic aging. Blocking cellular senescence is an important approach to the prevention and treatment of skin aging.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Lyu J et al. J Pharmacopuncture. 2013 Jun;16(2):41-5.
2. Eom SY et al. J Cosmet Sci. 2006 Sep-Oct;57(5):355-67.
3. So HM et al. Bioorg Chem. 2019 Oct;91:103145.
4. Choi HG et al. Phytother Res. 2013 Sep;27(9):1376-80.
5. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
6. Alishir A et al. Bioorg Med Chem Lett. 2021 Mar 15;36:127828.
7. Park TH et al. Saudi Pharm J. 2020 Jul;28(7):791-802.
8. Kwon YE et al. Molecules. 2022 Feb 19;27(4):1419.
9. Her Y et al. Molecules. 2020 Dec 29;26(1):124.
10. Yang HH et al. Planta Med. 2011 Mar;77(5):441-9.
11. Kim YO et al. Korean Journal of Medicinal Crop Science. 2011;19(6):508-13.
12. Lee S et al. Antioxidants (Basel). 2020 Oct 13;9(10):981.
Ulmus davidiana, commonly known as yugeunpi, has a long history of use in Korea in treating burns, eczema, frostbite, difficulties in urination, inflammation, and psoriasis,1 and has also been used in China for some of these indications, including skin inflammation.2,3 Currently, there are several areas in which the bioactivity of U. davidiana are under investigation, with numerous potential applications in dermatology. This column focuses briefly on the evidence supporting the traditional uses of the plant and potential new applications.
Anti-inflammatory activity
Eom and colleagues studied the potential of a polysaccharide extract from the root bark of U. davidiana to serve as a suitable cosmetic ingredient for conferring moisturizing, anti-inflammatory, and photoprotective activity. In this 2006 investigation, the composition of the polysaccharide extract was found to be primarily rhamnose, galactose, and glucose. The root extract exhibited a similar humectant moisturizing effect as hyaluronic acid, the researchers reported. The U. davidiana root extract was also found to dose-dependently suppress prostaglandin E2. The inhibition of the release of interleukin-6 and IL-8 was also reported to be significant. The use of the U. davidiana extract also stimulated the recovery of human fibroblasts (two times that of positive control) exposed to UVA irradiation. The researchers suggested that their overall results point to the viability of U. davidiana root extract as a cosmetic agent ingredient to protect skin from UV exposure and the inflammation that follows.2
In 2013, Choi and colleagues found that a methanol extract of the stem and root barks of U. davidiana revealed anti-inflammatory properties, with activity attributed to two trihydroxy acids [then-new trihydroxy fatty acid, 9,12,13-trihydroxyoctadeca-10(Z),15(Z)-dienoic acid, and pinellic acid], both of which blocked prostaglandin D₂ production.4
That same year, Lyu and colleagues studied the antiallergic and anti-inflammatory effects of U. davidiana using a 1-fluoro-2,4-dinitrofluorobenzene (DNFB)–induced contact dermatitis mouse model. They found that treatment at a dose of 10 mg/mL successfully prevented skin lesions caused by consistent DNFB application. Further, the researchers observed that topically applied U. davidiana suppressed spongiosis and reduced total serum immunoglobulin and IgG2a levels. Overall, they concluded that the botanical treatment improved contact dermatitis in mice.1
In 2019, So and colleagues studied the chemical components of U. davidiana root bark (isolating a chromane derivative and 22 known substances) and reported data supporting the traditional use of the root bark for gastroenteric and inflammatory indications.3
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, including U. davidiana, is used for its anti-inflammatory properties in traditional Korean medicine.5 Choi and colleagues determined that bakuchiol exhibited robust anti-inflammatory activity in a study of U. davidiana constituents, at least partially accounting for the anti-inflammatory functions of the plant.5
Antifungal activity
In 2021, Alishir and colleagues conducted a phytochemical analysis of the root bark extract of U. davidiana, resulting in the isolation of 10 substances including the novel coumarin glycoside derivative ulmusakidian. Some of the compounds exhibited antifungal activity against Cryptococcus neoformans, though none demonstrated antifungal activity against Candida albicans.6
Wound dressing
Park and colleagues demonstrated in 2020 that superabsorbing hydrogel wound dressings composed of U. davidiana root bark powders, which exhibit gelling activity, performed effectively in speeding up wound closure and cutaneous regeneration in skin-wound mice models. These dressings also displayed thermal stability and superior mechanical properties to pullulan-only gel films. The researchers concluded that gel films composed of U. davidiana have potential to surpass the effectiveness of current products.7
Anti–hair loss activity
Early in 2022, Kwon and colleagues investigated the anti–hair loss mechanism of U. davidiana and determined that supercritical extraction-residues of U. davidiana significantly hinder the secretion of transforming growth factor–beta but dose dependently salvage insulinlike growth factor 1, and substantially decrease dihydrotestosterone synthesis. They concluded that these U. davidiana supercritical fluid extract residues have the potential to halt the loss of human hair.8
Photoprotective potential
Late in 2020, Her and colleagues reported on their development and analysis of a new distillate derived from a fermented mixture of nine anti-inflammatory herbs including U. davidiana. The investigators assessed the effects of the topically applied distillate on UVB-induced skin damage in Institute of Cancer Research mice, finding significant improvements in the dorsal skin photodamage. Application of the distillate also ameliorated collagen production impairment and diminished proinflammatory cytokine levels of tumor necrosis factor (TNF)–alpha and IL-1B. The researchers concluded that this anti-inflammatory herbal distillate, which includes U. davidiana, displays the potential to serve as a photoprotective agent.9
Antiaging activity
In 2011, Yang and colleagues set out to identify constituent substances of the root bark of U. davidiana that have the capacity to suppress cellular senescence in human fibroblasts and human umbilical vein endothelial cells. They isolated 22 compounds, of which epifriedelanol, ssioriside, and catechin-7-O-beta-D-glucopyranoside impeded adriamycin-induced cellular senescence in human dermal fibroblasts and friedelin, epifriedelanol, and catechin-7-O-beta-apiofuranoside in the umbilical vein endothelial cells. Epifriedelanol was the most potent of the substances, leading the researchers to conclude that this U. davidiana component can diminish cellular senescence in human primary cells and has the potential as an oral and/or topical antiaging agent.10
Also that year, in a study on the protective effects of U. davidiana on UVB-irradiated hairless mice, the authors claimed that an ethanol extract of U. davidiana significantly suppressed wrinkle development in mice chronically exposed to UVB.11 This study showed that U. davidiana extract exerts antioxidant activity as evidenced by a decrease in MMP-1 activity. It also demonstrated antielastase activity. The treated mice showed a decrease in wrinkles as compared with water-treated mice.11 Although this is just one study in mice, it may demonstrate a protective effect on elastic fibers on skin exposed to UVB light.
Late in 2020, Lee and colleagues reported on their study of the possible antiaging effects on the skin of (-)-phenolic compounds isolated from the root bark of U. davidiana. The function of collagenase MMP-1 was found to be inhibited by the isolate (-)-catechin, which also halted collagen degradation caused by TNF-alpha in normal human dermal fibroblasts. Further, the investigators demonstrated that the U. davidiana isolate (-)-catechin reduced the expression of proinflammatory cytokines such as IL-1B and IL-6. They concluded that the U. davidiana isolate exhibits the potential to combat intrinsic as well as extrinsic cutaneous aging.12
These findings are particularly intriguing. There is much overlap between intrinsic and extrinsic aging. If U. davidiana can keep collagen intact and inhibit cellular senescence, it may serve as an early intervention toward slowing or preventing skin aging.
Summary
Of greatest interest now, perhaps, is its potential to impede cellular senescence. Senescent cells release a multitude of inflammatory and other factors that hasten intrinsic aging. Blocking cellular senescence is an important approach to the prevention and treatment of skin aging.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Lyu J et al. J Pharmacopuncture. 2013 Jun;16(2):41-5.
2. Eom SY et al. J Cosmet Sci. 2006 Sep-Oct;57(5):355-67.
3. So HM et al. Bioorg Chem. 2019 Oct;91:103145.
4. Choi HG et al. Phytother Res. 2013 Sep;27(9):1376-80.
5. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
6. Alishir A et al. Bioorg Med Chem Lett. 2021 Mar 15;36:127828.
7. Park TH et al. Saudi Pharm J. 2020 Jul;28(7):791-802.
8. Kwon YE et al. Molecules. 2022 Feb 19;27(4):1419.
9. Her Y et al. Molecules. 2020 Dec 29;26(1):124.
10. Yang HH et al. Planta Med. 2011 Mar;77(5):441-9.
11. Kim YO et al. Korean Journal of Medicinal Crop Science. 2011;19(6):508-13.
12. Lee S et al. Antioxidants (Basel). 2020 Oct 13;9(10):981.
IBD and pregnancy: What to tell your patients
While many gastroenterologists may be comfortable with inflammatory bowel disease (IBD), most are not experts in women’s concerns about pregnancy. One study found that, although women with IBD may have concerns about the interplay of their disease and reproductive health, many have not had extensive conversations with their gastroenterologist about it. In fact, that same study found most women expect their gastroenterologist to initiate these conversations.
In this roundtable discussion, Uma Mahadevan, MD, professor of medicine and the director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco; Marla C. Dubinsky, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York; and Sunanda V. Kane, MD, professor of medicine at Mayo Clinic in Rochester, Minn., share how they respond to these questions in their clinical practice.
What should a woman with IBD who is interested in having biological children in the future be thinking about now?
Dr. Mahadevan: Because active disease is associated with lower rates of conception and higher rates of pregnancy loss, women with IBD should first ensure they are in remission. I like to document endoscopic healing with a colonoscopy or sigmoidoscopy, but, if this has been done recently, a fecal calprotectin test can be helpful.
Women with IBD, particularly those with small bowel disease, are at risk for nutritional deficiencies, so prior to conception, I also check vitamin B-12, vitamin D, and iron, and repeat as needed. Zinc and folate can be considered. Those who are underweight should work with a nutritionist to ensure adequate caloric intake.
Dr. Dubinsky: I think it’s also important to stress the importance of taking their IBD medications because they can help patients achieve and maintain disease remission. Uncontrolled inflammation is a key risk factor for spontaneous abortion in the first trimester. Medication we would use in pregnancy is not putting them at risk for spontaneous abortion or congenital anomalies, which is what mothers to be are understandably most concerned about.
I am very honest and transparent with my patients: “About the only thing I need to take care of is you. If you are good, the baby is good.”
Dr. Kane: As Dr. Mahadevan mentioned, women with IBD are at higher risk for vitamin deficiencies so those need to be corrected before conception. If they smoke, they should stop before conceiving.
There is no increased risk of infertility unless there has been a history of abdominal surgery.
Also, if women are not actively planning on getting pregnant, that would be important to share because some gastroenterologists will avoid certain effective medications if pregnancy is a possibility.
If a woman has had surgery for her IBD, could that make it harder for her to get pregnant?
Dr. Kane: Yes, it can because scar tissue may develop within the pelvis. However, if surgery is indicated to manage a patient’s IBD, then talk to the surgeon about ways that they might be able to reduce the risk of scar tissue formation.
Dr. Dubinsky: One thing to note is that almost all the data of infertility risk and scarring are based on open surgical techniques that involve dissection of the rectum. On the other hand, we don’t yet have enough prospective data on the impact of the modern era of laparoscopic surgery to suggest whether it affects fertility. More data is needed because providers may be giving women old information that is no longer relevant in the modern era.
If a woman is experiencing IBD symptoms, should she attempt to conceive?
Dr. Kane: Gastrointestinal symptoms in patients with IBD could be from active disease but also other things, so it’s important to have a thorough check-up to assess if there is active disease or not. Active disease can (but does not always) lead to a more complicated pregnancy, and conception is not recommended while a patient has active IBD.
Dr. Dubinsky: Although some patients feel an urgency to conceive regardless of disease activity, we need to do our due diligence and explain that we need to focus on getting them into the deepest remission possible, including endoscopic findings, biomarkers, and symptoms.
The most important gift you can give your future moms is to optimize the therapy they’re on before they conceive.
Is it important for someone who’s working with a gastroenterologist and an obstetrician to also work with a maternal-fetal medicine (MFM) specialist?
Dr. Kane: Having a diagnosis of IBD makes a woman’s pregnancy “high risk” because just having the diagnosis is associated with a higher risk of prematurity and small for gestational age – but importantly, not birth defects. A woman whose IBD is in remission should still have a discussion with an MFM specialist, just so everyone is on the same page.
Dr. Dubinsky: I refer to care with MFM specialists as “tighter monitoring.” I tell my patients that MFM specialists have managed many complex pregnancies and feel confident around the safety of their medications, understand the impact of when the baby may be exposed to certain medications, and will focus on following them more closely.
What are the risks of IBD medications during pregnancy and while breastfeeding? Should women stop their medications during pregnancy and breastfeeding?
Dr. Dubinsky: Organogenesis occurs in the first 10 weeks, so any medicines that cross the placenta during that time are up for discussion and debate. Methotrexate and the newer small molecules, such as Janus kinase (JAK) inhibitors and S1P receptor modulators, do cross the placenta during the first trimester and need to be discontinued before conception, sometimes as early as 3 months before conception.
However, biologics are very large proteins and do not cross the placenta until closer to week 27. We are not advocating stopping biologics in advance of conception, or during pregnancy, or during breastfeeding. There is more risk to stopping than continuing.
Dr. Mahadevan: Methotrexate should be stopped at least 3 months prior to conception and should not be taken during pregnancy.
There are limited antibiotic safety data in pregnancy for the longer periods of time used in IBD. I generally prefer amoxicillin/clavulanic acid over ciprofloxacin or metronidazole, but short term (less than 2 weeks) use of any of those three are not contraindicated.
Mesalamine agents and thiopurine monotherapy can be continued through pregnancy and breastfeeding.
Biologic agents, such as anti–tumor necrosis factor, anti-interleukin 23, anti-integrin, and biosimilars, can be continued through pregnancy and during breastfeeding. Given limited exposure in the first trimester, there is no evidence of increased risk of birth defects. As Dr. Dubinsky pointed out, there is active transfer, particularly in the third trimester and minimal transfer in breast milk, but this has not been associated with harm.
Lastly, small molecules, such as the JAK inhibitors tofacitinib and upadacitinib, as well as ozanimod, have virtually no human safety data during pregnancy, and animal data show harm. The use of these agents in pregnancy is not recommended.
Dr. Kane: As Dr. Dubinsky stated, most of the medications our patients take are low risk to continue through pregnancy if the patients are in remission. Although a woman “in remission” on steroids is not really in remission and should not get pregnant until she is on something else.
As far as breastfeeding goes, that should be stopped if the patient is on methotrexate, cyclosporine, or certain antibiotics. If she is on more than 20 mg of prednisone this can pass to the infant, and a mother should not breastfeed.
Women should avoid fenugreek as a lactation aid, as that contains a compound that can promote bleeding. Lactation cookies are ok.
Otherwise, there are lots of potential benefits to breastfeeding, and I encourage it.
How is a flare treated if it occurs during pregnancy?
Dr. Dubinsky: A flare during pregnancy is treated the same as a flare outside of pregnancy. We want to use noninvasive ways to confirm it, but I think we don’t need to overly investigate in most of our women. If they’re already on a biologic, you may consider changing.
Some women may need corticosteroids. It’s not our favorite move, but there is an urgency to getting a flare under control during pregnancy because of possible complications.
Dr. Mahadevan: Some of this is contingent on when during pregnancy the flare occurs. A patient who has a flare at 38 weeks’ gestation will likely proceed with delivery and the flare will be dealt with separately. Someone at 8 weeks’ gestation is at high risk for pregnancy loss, so treatment should be quick and effective.
As does Dr. Dubinsky, I do try to avoid steroids if possible. For example, I would rather start an effective biologic right away than drag out steroids to see if they will respond.
Dr. Kane: I would add that, if a mother is losing weight, she might need to be hospitalized for additional nutritional support. If surgery is necessary, we usually try to time it for the second or third trimester.
What needs to be taken into consideration regarding mode of delivery? Also, if a woman has undergone prior surgeries, do they increase the risk of delivery complications?
For ulcerative colitis, mode of delivery is based on obstetric, not gastrointestinal, variables. For Crohn’s disease, if there is evidence of perianal disease, then a cesarean is appropriate.
If there is no history of perianal disease, then delivery is based on obstetric variables.
For a woman who has a J pouch, if possible, the surgeon who created it should be contacted to ask about the technical aspects of the pouch and how it lies in the pelvis.
What’s the risk of a postpartum flare if a woman’s IBD remains in clinical remission during pregnancy?
Dr. Mahadevan: There is no increased risk of postpartum flare if a woman continues her IBD medications after delivery. Many of the reports of flare are from stopping medications (mistakenly often) to breastfeed.
Dr. Kane: As Dr. Mahadevan said, the risk of a flare is usually because a woman stops taking her medications because she thinks that medication will be passed to the infant through breastfeeding, which in most cases is not true.
Otherwise, there is not an increased risk of a flare in a 12-month period. However, it is important to monitor for symptoms after delivery; the risk of a flare is not zero.
What symptoms should women watch out for after delivery that may indicate an uptick in disease activity?
Dr. Kane: The same symptoms as before they were pregnant. Diarrhea, abdominal pain, and rectal bleeding are not normal after delivery and should be considered signs of returning disease.
As a gastroenterologist, is there any additional advice you’d offer about conception, fertility, and pregnancy when treating women with IBD?
Dr. Mahadevan: Women with IBD should, when feasible, have a planned pregnancy when in documented remission and under the care of their gastroenterologists, obstetrician, and an MFM specialist. Life happens, and this is not always possible. That said, a woman with IBD has the same chance of getting pregnant as a woman of the same age without IBD, unless she has active disease or a history of pelvic surgery. Women with IBD in remission will generally have healthy pregnancies if they continue appropriate medications.
Dr. Kane: Agreed. The majority of women with IBD will have normal, healthy pregnancies. It is important for them to not stop their IBD therapy without talking to their gastroenterologist first. Well-intentioned but ignorant obstetricians or midwives may recommend stopping, but then panic when disease flares and the mother’s health is at risk. Active inflammation is the worst enemy to a pregnancy, not active therapy.
Dr. Dubinsky: One additional thing to consider is: How do we help women with IBD who have delivered meet the needs of their family and continue to stay on their meds and be in good inflammatory control?
For example, we can give the biologic in the hospital after they’ve had a cesarean or a vaginal delivery and before they leave. We know that that is safe, giving that to them before they leave the hospital is a huge value added.
Another thing is possibly changing their infusions to home infusions. That would be helpful for the moms as well.
Dr. Mahadevan reports being a consultant for AbbVie, Janssen, Pfizer, Gilead, Bristol-Myers Squibb, Takeda, Protagonist, Prometheus, and Boehringer Ingelheim. Dr. Dubinsky is a consultant for AbbVie, Arena, Bristol-Myers Squibb, Janssen, Eli Lilly, Takeda, and Prometheus BioSciences. She is a shareholder and CEO of a publicly traded company, Trellis Health. Dr. Kane is a consultant for Bristol-Myers Squibb, Boehringer Ingelheim, Gilead, Janssen, Takeda, Seres Therapeutics, TechLab, United Healthcare, Predicta-Med, and InveniAI, and is the editor for the IBD section of UptoDate.
While many gastroenterologists may be comfortable with inflammatory bowel disease (IBD), most are not experts in women’s concerns about pregnancy. One study found that, although women with IBD may have concerns about the interplay of their disease and reproductive health, many have not had extensive conversations with their gastroenterologist about it. In fact, that same study found most women expect their gastroenterologist to initiate these conversations.
In this roundtable discussion, Uma Mahadevan, MD, professor of medicine and the director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco; Marla C. Dubinsky, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York; and Sunanda V. Kane, MD, professor of medicine at Mayo Clinic in Rochester, Minn., share how they respond to these questions in their clinical practice.
What should a woman with IBD who is interested in having biological children in the future be thinking about now?
Dr. Mahadevan: Because active disease is associated with lower rates of conception and higher rates of pregnancy loss, women with IBD should first ensure they are in remission. I like to document endoscopic healing with a colonoscopy or sigmoidoscopy, but, if this has been done recently, a fecal calprotectin test can be helpful.
Women with IBD, particularly those with small bowel disease, are at risk for nutritional deficiencies, so prior to conception, I also check vitamin B-12, vitamin D, and iron, and repeat as needed. Zinc and folate can be considered. Those who are underweight should work with a nutritionist to ensure adequate caloric intake.
Dr. Dubinsky: I think it’s also important to stress the importance of taking their IBD medications because they can help patients achieve and maintain disease remission. Uncontrolled inflammation is a key risk factor for spontaneous abortion in the first trimester. Medication we would use in pregnancy is not putting them at risk for spontaneous abortion or congenital anomalies, which is what mothers to be are understandably most concerned about.
I am very honest and transparent with my patients: “About the only thing I need to take care of is you. If you are good, the baby is good.”
Dr. Kane: As Dr. Mahadevan mentioned, women with IBD are at higher risk for vitamin deficiencies so those need to be corrected before conception. If they smoke, they should stop before conceiving.
There is no increased risk of infertility unless there has been a history of abdominal surgery.
Also, if women are not actively planning on getting pregnant, that would be important to share because some gastroenterologists will avoid certain effective medications if pregnancy is a possibility.
If a woman has had surgery for her IBD, could that make it harder for her to get pregnant?
Dr. Kane: Yes, it can because scar tissue may develop within the pelvis. However, if surgery is indicated to manage a patient’s IBD, then talk to the surgeon about ways that they might be able to reduce the risk of scar tissue formation.
Dr. Dubinsky: One thing to note is that almost all the data of infertility risk and scarring are based on open surgical techniques that involve dissection of the rectum. On the other hand, we don’t yet have enough prospective data on the impact of the modern era of laparoscopic surgery to suggest whether it affects fertility. More data is needed because providers may be giving women old information that is no longer relevant in the modern era.
If a woman is experiencing IBD symptoms, should she attempt to conceive?
Dr. Kane: Gastrointestinal symptoms in patients with IBD could be from active disease but also other things, so it’s important to have a thorough check-up to assess if there is active disease or not. Active disease can (but does not always) lead to a more complicated pregnancy, and conception is not recommended while a patient has active IBD.
Dr. Dubinsky: Although some patients feel an urgency to conceive regardless of disease activity, we need to do our due diligence and explain that we need to focus on getting them into the deepest remission possible, including endoscopic findings, biomarkers, and symptoms.
The most important gift you can give your future moms is to optimize the therapy they’re on before they conceive.
Is it important for someone who’s working with a gastroenterologist and an obstetrician to also work with a maternal-fetal medicine (MFM) specialist?
Dr. Kane: Having a diagnosis of IBD makes a woman’s pregnancy “high risk” because just having the diagnosis is associated with a higher risk of prematurity and small for gestational age – but importantly, not birth defects. A woman whose IBD is in remission should still have a discussion with an MFM specialist, just so everyone is on the same page.
Dr. Dubinsky: I refer to care with MFM specialists as “tighter monitoring.” I tell my patients that MFM specialists have managed many complex pregnancies and feel confident around the safety of their medications, understand the impact of when the baby may be exposed to certain medications, and will focus on following them more closely.
What are the risks of IBD medications during pregnancy and while breastfeeding? Should women stop their medications during pregnancy and breastfeeding?
Dr. Dubinsky: Organogenesis occurs in the first 10 weeks, so any medicines that cross the placenta during that time are up for discussion and debate. Methotrexate and the newer small molecules, such as Janus kinase (JAK) inhibitors and S1P receptor modulators, do cross the placenta during the first trimester and need to be discontinued before conception, sometimes as early as 3 months before conception.
However, biologics are very large proteins and do not cross the placenta until closer to week 27. We are not advocating stopping biologics in advance of conception, or during pregnancy, or during breastfeeding. There is more risk to stopping than continuing.
Dr. Mahadevan: Methotrexate should be stopped at least 3 months prior to conception and should not be taken during pregnancy.
There are limited antibiotic safety data in pregnancy for the longer periods of time used in IBD. I generally prefer amoxicillin/clavulanic acid over ciprofloxacin or metronidazole, but short term (less than 2 weeks) use of any of those three are not contraindicated.
Mesalamine agents and thiopurine monotherapy can be continued through pregnancy and breastfeeding.
Biologic agents, such as anti–tumor necrosis factor, anti-interleukin 23, anti-integrin, and biosimilars, can be continued through pregnancy and during breastfeeding. Given limited exposure in the first trimester, there is no evidence of increased risk of birth defects. As Dr. Dubinsky pointed out, there is active transfer, particularly in the third trimester and minimal transfer in breast milk, but this has not been associated with harm.
Lastly, small molecules, such as the JAK inhibitors tofacitinib and upadacitinib, as well as ozanimod, have virtually no human safety data during pregnancy, and animal data show harm. The use of these agents in pregnancy is not recommended.
Dr. Kane: As Dr. Dubinsky stated, most of the medications our patients take are low risk to continue through pregnancy if the patients are in remission. Although a woman “in remission” on steroids is not really in remission and should not get pregnant until she is on something else.
As far as breastfeeding goes, that should be stopped if the patient is on methotrexate, cyclosporine, or certain antibiotics. If she is on more than 20 mg of prednisone this can pass to the infant, and a mother should not breastfeed.
Women should avoid fenugreek as a lactation aid, as that contains a compound that can promote bleeding. Lactation cookies are ok.
Otherwise, there are lots of potential benefits to breastfeeding, and I encourage it.
How is a flare treated if it occurs during pregnancy?
Dr. Dubinsky: A flare during pregnancy is treated the same as a flare outside of pregnancy. We want to use noninvasive ways to confirm it, but I think we don’t need to overly investigate in most of our women. If they’re already on a biologic, you may consider changing.
Some women may need corticosteroids. It’s not our favorite move, but there is an urgency to getting a flare under control during pregnancy because of possible complications.
Dr. Mahadevan: Some of this is contingent on when during pregnancy the flare occurs. A patient who has a flare at 38 weeks’ gestation will likely proceed with delivery and the flare will be dealt with separately. Someone at 8 weeks’ gestation is at high risk for pregnancy loss, so treatment should be quick and effective.
As does Dr. Dubinsky, I do try to avoid steroids if possible. For example, I would rather start an effective biologic right away than drag out steroids to see if they will respond.
Dr. Kane: I would add that, if a mother is losing weight, she might need to be hospitalized for additional nutritional support. If surgery is necessary, we usually try to time it for the second or third trimester.
What needs to be taken into consideration regarding mode of delivery? Also, if a woman has undergone prior surgeries, do they increase the risk of delivery complications?
For ulcerative colitis, mode of delivery is based on obstetric, not gastrointestinal, variables. For Crohn’s disease, if there is evidence of perianal disease, then a cesarean is appropriate.
If there is no history of perianal disease, then delivery is based on obstetric variables.
For a woman who has a J pouch, if possible, the surgeon who created it should be contacted to ask about the technical aspects of the pouch and how it lies in the pelvis.
What’s the risk of a postpartum flare if a woman’s IBD remains in clinical remission during pregnancy?
Dr. Mahadevan: There is no increased risk of postpartum flare if a woman continues her IBD medications after delivery. Many of the reports of flare are from stopping medications (mistakenly often) to breastfeed.
Dr. Kane: As Dr. Mahadevan said, the risk of a flare is usually because a woman stops taking her medications because she thinks that medication will be passed to the infant through breastfeeding, which in most cases is not true.
Otherwise, there is not an increased risk of a flare in a 12-month period. However, it is important to monitor for symptoms after delivery; the risk of a flare is not zero.
What symptoms should women watch out for after delivery that may indicate an uptick in disease activity?
Dr. Kane: The same symptoms as before they were pregnant. Diarrhea, abdominal pain, and rectal bleeding are not normal after delivery and should be considered signs of returning disease.
As a gastroenterologist, is there any additional advice you’d offer about conception, fertility, and pregnancy when treating women with IBD?
Dr. Mahadevan: Women with IBD should, when feasible, have a planned pregnancy when in documented remission and under the care of their gastroenterologists, obstetrician, and an MFM specialist. Life happens, and this is not always possible. That said, a woman with IBD has the same chance of getting pregnant as a woman of the same age without IBD, unless she has active disease or a history of pelvic surgery. Women with IBD in remission will generally have healthy pregnancies if they continue appropriate medications.
Dr. Kane: Agreed. The majority of women with IBD will have normal, healthy pregnancies. It is important for them to not stop their IBD therapy without talking to their gastroenterologist first. Well-intentioned but ignorant obstetricians or midwives may recommend stopping, but then panic when disease flares and the mother’s health is at risk. Active inflammation is the worst enemy to a pregnancy, not active therapy.
Dr. Dubinsky: One additional thing to consider is: How do we help women with IBD who have delivered meet the needs of their family and continue to stay on their meds and be in good inflammatory control?
For example, we can give the biologic in the hospital after they’ve had a cesarean or a vaginal delivery and before they leave. We know that that is safe, giving that to them before they leave the hospital is a huge value added.
Another thing is possibly changing their infusions to home infusions. That would be helpful for the moms as well.
Dr. Mahadevan reports being a consultant for AbbVie, Janssen, Pfizer, Gilead, Bristol-Myers Squibb, Takeda, Protagonist, Prometheus, and Boehringer Ingelheim. Dr. Dubinsky is a consultant for AbbVie, Arena, Bristol-Myers Squibb, Janssen, Eli Lilly, Takeda, and Prometheus BioSciences. She is a shareholder and CEO of a publicly traded company, Trellis Health. Dr. Kane is a consultant for Bristol-Myers Squibb, Boehringer Ingelheim, Gilead, Janssen, Takeda, Seres Therapeutics, TechLab, United Healthcare, Predicta-Med, and InveniAI, and is the editor for the IBD section of UptoDate.
While many gastroenterologists may be comfortable with inflammatory bowel disease (IBD), most are not experts in women’s concerns about pregnancy. One study found that, although women with IBD may have concerns about the interplay of their disease and reproductive health, many have not had extensive conversations with their gastroenterologist about it. In fact, that same study found most women expect their gastroenterologist to initiate these conversations.
In this roundtable discussion, Uma Mahadevan, MD, professor of medicine and the director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco; Marla C. Dubinsky, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York; and Sunanda V. Kane, MD, professor of medicine at Mayo Clinic in Rochester, Minn., share how they respond to these questions in their clinical practice.
What should a woman with IBD who is interested in having biological children in the future be thinking about now?
Dr. Mahadevan: Because active disease is associated with lower rates of conception and higher rates of pregnancy loss, women with IBD should first ensure they are in remission. I like to document endoscopic healing with a colonoscopy or sigmoidoscopy, but, if this has been done recently, a fecal calprotectin test can be helpful.
Women with IBD, particularly those with small bowel disease, are at risk for nutritional deficiencies, so prior to conception, I also check vitamin B-12, vitamin D, and iron, and repeat as needed. Zinc and folate can be considered. Those who are underweight should work with a nutritionist to ensure adequate caloric intake.
Dr. Dubinsky: I think it’s also important to stress the importance of taking their IBD medications because they can help patients achieve and maintain disease remission. Uncontrolled inflammation is a key risk factor for spontaneous abortion in the first trimester. Medication we would use in pregnancy is not putting them at risk for spontaneous abortion or congenital anomalies, which is what mothers to be are understandably most concerned about.
I am very honest and transparent with my patients: “About the only thing I need to take care of is you. If you are good, the baby is good.”
Dr. Kane: As Dr. Mahadevan mentioned, women with IBD are at higher risk for vitamin deficiencies so those need to be corrected before conception. If they smoke, they should stop before conceiving.
There is no increased risk of infertility unless there has been a history of abdominal surgery.
Also, if women are not actively planning on getting pregnant, that would be important to share because some gastroenterologists will avoid certain effective medications if pregnancy is a possibility.
If a woman has had surgery for her IBD, could that make it harder for her to get pregnant?
Dr. Kane: Yes, it can because scar tissue may develop within the pelvis. However, if surgery is indicated to manage a patient’s IBD, then talk to the surgeon about ways that they might be able to reduce the risk of scar tissue formation.
Dr. Dubinsky: One thing to note is that almost all the data of infertility risk and scarring are based on open surgical techniques that involve dissection of the rectum. On the other hand, we don’t yet have enough prospective data on the impact of the modern era of laparoscopic surgery to suggest whether it affects fertility. More data is needed because providers may be giving women old information that is no longer relevant in the modern era.
If a woman is experiencing IBD symptoms, should she attempt to conceive?
Dr. Kane: Gastrointestinal symptoms in patients with IBD could be from active disease but also other things, so it’s important to have a thorough check-up to assess if there is active disease or not. Active disease can (but does not always) lead to a more complicated pregnancy, and conception is not recommended while a patient has active IBD.
Dr. Dubinsky: Although some patients feel an urgency to conceive regardless of disease activity, we need to do our due diligence and explain that we need to focus on getting them into the deepest remission possible, including endoscopic findings, biomarkers, and symptoms.
The most important gift you can give your future moms is to optimize the therapy they’re on before they conceive.
Is it important for someone who’s working with a gastroenterologist and an obstetrician to also work with a maternal-fetal medicine (MFM) specialist?
Dr. Kane: Having a diagnosis of IBD makes a woman’s pregnancy “high risk” because just having the diagnosis is associated with a higher risk of prematurity and small for gestational age – but importantly, not birth defects. A woman whose IBD is in remission should still have a discussion with an MFM specialist, just so everyone is on the same page.
Dr. Dubinsky: I refer to care with MFM specialists as “tighter monitoring.” I tell my patients that MFM specialists have managed many complex pregnancies and feel confident around the safety of their medications, understand the impact of when the baby may be exposed to certain medications, and will focus on following them more closely.
What are the risks of IBD medications during pregnancy and while breastfeeding? Should women stop their medications during pregnancy and breastfeeding?
Dr. Dubinsky: Organogenesis occurs in the first 10 weeks, so any medicines that cross the placenta during that time are up for discussion and debate. Methotrexate and the newer small molecules, such as Janus kinase (JAK) inhibitors and S1P receptor modulators, do cross the placenta during the first trimester and need to be discontinued before conception, sometimes as early as 3 months before conception.
However, biologics are very large proteins and do not cross the placenta until closer to week 27. We are not advocating stopping biologics in advance of conception, or during pregnancy, or during breastfeeding. There is more risk to stopping than continuing.
Dr. Mahadevan: Methotrexate should be stopped at least 3 months prior to conception and should not be taken during pregnancy.
There are limited antibiotic safety data in pregnancy for the longer periods of time used in IBD. I generally prefer amoxicillin/clavulanic acid over ciprofloxacin or metronidazole, but short term (less than 2 weeks) use of any of those three are not contraindicated.
Mesalamine agents and thiopurine monotherapy can be continued through pregnancy and breastfeeding.
Biologic agents, such as anti–tumor necrosis factor, anti-interleukin 23, anti-integrin, and biosimilars, can be continued through pregnancy and during breastfeeding. Given limited exposure in the first trimester, there is no evidence of increased risk of birth defects. As Dr. Dubinsky pointed out, there is active transfer, particularly in the third trimester and minimal transfer in breast milk, but this has not been associated with harm.
Lastly, small molecules, such as the JAK inhibitors tofacitinib and upadacitinib, as well as ozanimod, have virtually no human safety data during pregnancy, and animal data show harm. The use of these agents in pregnancy is not recommended.
Dr. Kane: As Dr. Dubinsky stated, most of the medications our patients take are low risk to continue through pregnancy if the patients are in remission. Although a woman “in remission” on steroids is not really in remission and should not get pregnant until she is on something else.
As far as breastfeeding goes, that should be stopped if the patient is on methotrexate, cyclosporine, or certain antibiotics. If she is on more than 20 mg of prednisone this can pass to the infant, and a mother should not breastfeed.
Women should avoid fenugreek as a lactation aid, as that contains a compound that can promote bleeding. Lactation cookies are ok.
Otherwise, there are lots of potential benefits to breastfeeding, and I encourage it.
How is a flare treated if it occurs during pregnancy?
Dr. Dubinsky: A flare during pregnancy is treated the same as a flare outside of pregnancy. We want to use noninvasive ways to confirm it, but I think we don’t need to overly investigate in most of our women. If they’re already on a biologic, you may consider changing.
Some women may need corticosteroids. It’s not our favorite move, but there is an urgency to getting a flare under control during pregnancy because of possible complications.
Dr. Mahadevan: Some of this is contingent on when during pregnancy the flare occurs. A patient who has a flare at 38 weeks’ gestation will likely proceed with delivery and the flare will be dealt with separately. Someone at 8 weeks’ gestation is at high risk for pregnancy loss, so treatment should be quick and effective.
As does Dr. Dubinsky, I do try to avoid steroids if possible. For example, I would rather start an effective biologic right away than drag out steroids to see if they will respond.
Dr. Kane: I would add that, if a mother is losing weight, she might need to be hospitalized for additional nutritional support. If surgery is necessary, we usually try to time it for the second or third trimester.
What needs to be taken into consideration regarding mode of delivery? Also, if a woman has undergone prior surgeries, do they increase the risk of delivery complications?
For ulcerative colitis, mode of delivery is based on obstetric, not gastrointestinal, variables. For Crohn’s disease, if there is evidence of perianal disease, then a cesarean is appropriate.
If there is no history of perianal disease, then delivery is based on obstetric variables.
For a woman who has a J pouch, if possible, the surgeon who created it should be contacted to ask about the technical aspects of the pouch and how it lies in the pelvis.
What’s the risk of a postpartum flare if a woman’s IBD remains in clinical remission during pregnancy?
Dr. Mahadevan: There is no increased risk of postpartum flare if a woman continues her IBD medications after delivery. Many of the reports of flare are from stopping medications (mistakenly often) to breastfeed.
Dr. Kane: As Dr. Mahadevan said, the risk of a flare is usually because a woman stops taking her medications because she thinks that medication will be passed to the infant through breastfeeding, which in most cases is not true.
Otherwise, there is not an increased risk of a flare in a 12-month period. However, it is important to monitor for symptoms after delivery; the risk of a flare is not zero.
What symptoms should women watch out for after delivery that may indicate an uptick in disease activity?
Dr. Kane: The same symptoms as before they were pregnant. Diarrhea, abdominal pain, and rectal bleeding are not normal after delivery and should be considered signs of returning disease.
As a gastroenterologist, is there any additional advice you’d offer about conception, fertility, and pregnancy when treating women with IBD?
Dr. Mahadevan: Women with IBD should, when feasible, have a planned pregnancy when in documented remission and under the care of their gastroenterologists, obstetrician, and an MFM specialist. Life happens, and this is not always possible. That said, a woman with IBD has the same chance of getting pregnant as a woman of the same age without IBD, unless she has active disease or a history of pelvic surgery. Women with IBD in remission will generally have healthy pregnancies if they continue appropriate medications.
Dr. Kane: Agreed. The majority of women with IBD will have normal, healthy pregnancies. It is important for them to not stop their IBD therapy without talking to their gastroenterologist first. Well-intentioned but ignorant obstetricians or midwives may recommend stopping, but then panic when disease flares and the mother’s health is at risk. Active inflammation is the worst enemy to a pregnancy, not active therapy.
Dr. Dubinsky: One additional thing to consider is: How do we help women with IBD who have delivered meet the needs of their family and continue to stay on their meds and be in good inflammatory control?
For example, we can give the biologic in the hospital after they’ve had a cesarean or a vaginal delivery and before they leave. We know that that is safe, giving that to them before they leave the hospital is a huge value added.
Another thing is possibly changing their infusions to home infusions. That would be helpful for the moms as well.
Dr. Mahadevan reports being a consultant for AbbVie, Janssen, Pfizer, Gilead, Bristol-Myers Squibb, Takeda, Protagonist, Prometheus, and Boehringer Ingelheim. Dr. Dubinsky is a consultant for AbbVie, Arena, Bristol-Myers Squibb, Janssen, Eli Lilly, Takeda, and Prometheus BioSciences. She is a shareholder and CEO of a publicly traded company, Trellis Health. Dr. Kane is a consultant for Bristol-Myers Squibb, Boehringer Ingelheim, Gilead, Janssen, Takeda, Seres Therapeutics, TechLab, United Healthcare, Predicta-Med, and InveniAI, and is the editor for the IBD section of UptoDate.
Starting a podcast
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Love them or hate them, masks in schools work
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.












