User login
Inclusivity needed in PHM fellowships
A year and a half ago, I found myself seated in a crowded hall at the national Pediatric Hospital Medicine (PHM) conference. Throughout the conference, trainees like me were warmly welcomed into small groups and lunch tables. I tried to keep my cool while PHM “celebrities” chatted with me in the elevator. Most sessions were prepared with plenty of chairs, and those that were not encouraged latecomers to grab a spot on the floor or the back wall – the more the merrier.
The intention of this “advice for applicants” meeting was to inspire and guide our next steps toward fellowship, but a discomforting reality loomed over us. It was the first year graduating pediatricians could not choose PHM board certification via the practice pathway – we needed an invitation in the form of a fellowship match.
The “hidden curriculum” was not subtle: People who scored a seat would keep their options open within the field of PHM, and those who did not had a murkier future. This message stood in stark contrast to the PHM inclusivity I had experienced all conference, and planted seeds of doubt: Was I welcome here? Did I “deserve” a seat?
I found the experience as a PHM fellowship applicant to be uncomfortable, and my all-too familiar friend “imposter syndrome” set up camp in my brain and made herself at home. I had no way of knowing how many programs to apply to, how many to interview at, or the chances of my matching at all. Once on the interview trail, I realized I was not alone in my discomfort – most applicants harbored some trepidation, and no one truly knew how the chips would fall on Match Day.
I am thrilled and relieved to have come out the other end in a great position. The team I work with and learn from is phenomenal. I am grateful that ACGME accreditation ensures structures are in place for fellows to be supported in their academic and educational efforts and have full confidence that the skills I gain in fellowship will help me contribute to progression of the field of PHM and improve my performance as a clinician-educator.
Sadly, each year PHM match day celebrations are dampened by the knowledge that a large portion of our colleagues are being left out in the cold with an “unmatched” notification in their inboxes. Approximately 200 graduating pediatricians become pediatric hospitalists each year,1 but only 68 fellowship positions were available in the United States for matriculation in 2020.2 In 2019, PHM fellowship candidates navigated the 6-month application journey with aspirations to further their training in the profession they love. Of the candidates who submitted a rank list committing to 2 or more years in PHM fellowship, 35% were denied.
Unfortunately, despite expansion of PHM fellowship programs and fifteen seats added from last year, we learned this December that there still are not enough positions to welcome qualified applicants with open arms: Thirty-three percent of candidates ranked PHM programs first in the NRMP but did not match – the highest unmatched percentage out of all pediatric subspecialties.3
The NRMP report shared a glimpse of our colleagues who received interview invitations and submitted a rank list, but this is likely an underestimation of pediatric graduates who wanted to obtain PHM board certification and wound up on a different path. Some residents anticipated the stiff competition and delayed their plans to apply for fellowship, while others matched into another subspecialty that was able to accommodate them. Many pediatric graduates joined the workforce directly as pediatric hospitalists knowing the practice pathway to certification is not available to them. Along with other physicians without board certification in PHM, they shoulder concerns of being withheld from professional advancement opportunities.
For the foreseeable future it is clear that pediatric hospitalists without board certification will be a large part of our community, and are crucial to providing high-quality care to hospitalized children nationally. In 2019, a national survey of pediatric hospital medicine groups revealed that 50% of pediatric hospitalist hires came directly out of residency, and only 8% of hires were fellowship trained.4 The same report revealed that 26% of physicians were board-certified.These percentages are likely to change over the next 5 years as the window of practice pathway certification closes and fellowship programs continue to expand. Only time will tell what the national prevalence of board-certified pediatric hospitalists settles out to be.
Historically, PHM fellowship graduates have assumed roles that include teaching and research responsibilities, and ACGME fellowship requirements have ensured that trainees graduate with skills in medical education and scholarship, and need only 4 weeks of training to be done in a community hospital.5 Pediatric hospitalists who do not pursue board certification are seeing the growing pool of PHM fellowship graduates prepared for positions in academic institutions. It is reasonable that they harbor concerns about being siloed toward primarily community hospital roles, and for community hospitalists to feel that this structure undervalues their role within the field of PHM.
At a time when inclusivity and community in medicine are receiving much-needed recognition, the current fellowship application climate has potential to create division within the PHM community. Newly graduating pediatric residents are among the populations disproportionately affected by the practice pathway cutoff. Like other subspecialties with ever-climbing steps up the “ivory tower” of academia and specialization within medicine, the inherent structure of the training pathway makes navigating it more difficult for pediatricians with professional, geographic, and economic diversity or constraints.
Med-Peds–trained colleagues have the added challenge of finding a fellowship position that is willing and able to support their concurrent internal medicine goals. International medical graduates make up about 20% of graduating residents each year, and just 11% of matched PHM fellows.3,6 Similarly, while DO medical graduates make up 20% of pediatric residents in the United States, only 10% of matched PHM fellows were DOs.3,6 New pediatricians with families or financial insecurity may be unable to invest in an expensive application process, move to a new city, and accept less than half of the average starting salary of a pediatric hospitalist for 2-3 years.7
The prevalence of implicit bias in medicine is well documented, and there is growing evidence that it negatively impacts candidate selection in medical education and contributes to minorities being underrepresented in the physician workforce.8 We must recognize the ways that adding a competitive costly hurdle may risk conflict with our mission to encourage diversity of representation within PHM leadership positions.
We have not yet successfully bridged the gap between qualified PHM fellowship candidates and available fellowship positions. I worry that this gap and the lack of transparency surrounding it is resulting in one portion of new pediatricians being welcomed by the subspecialty, and others feeling unsupported and alienated by the larger PHM community as early career physicians.
Right now, the only solution available is expansion of fellowship programs. We see progress with the new addition of fellowship positions every year, but finding funding for each position is often a lengthy endeavor, and the COVID-19 pandemic has tightened the purse strings of many children’s hospitals. It may be many years before the number of available fellowship positions more closely approximates the 200 pediatricians that become hospitalists each year.
The most equitable solution would be offering other avenues to board certification while this gap is being bridged, either by extending the practice pathway option, or making a third pathway that requires less institutional funding per fellow, but still incentivizes institutional investment in fellowship positions and resources (e.g., a pathway requiring some number of years in practice, plus 1 year in fellowship centered around a nonclinical academic curriculum).
In the absence of the solutions above, we collectively hold the responsibility of maintaining inclusivity and support of our PHM colleagues with and without board certification. One important strategy provided by Dr. Gregory Welsh9 is to incorporate community hospital medicine rotations into residency training. Sharing this side of PHM with residents may help some graduates avoid a training pathway they may not want or need. More importantly, it would raise trainee exposure and interest toward a service that is both expansive – approximately 70% of pediatric hospitalists practice in a community hospital – and crucial to children’s health nationally.
Pediatric hospitalists who are not eligible for board certification are vital and valued members of the PHM community, and as such need to maintain representation within PHM leadership. Professional development opportunities need to remain accessible outside of fellowship. The blossoming of virtual conferences and Zoom meet-ups in the face of the COVID-19 pandemic have shown us that with innovation (and a good Internet connection), networking and mentorship can be accomplished across thousands of miles.
While there’s great diversity within PHM, this subspecialty has a history of attracting pediatricians with some common core qualities: Grit, creativity, and the belief that a strong team is far greater than the sum of its parts. I have confidence that if we approach this PHM transition period with transparency about our goals and challenges, this community can emerge from it strong and united.
Dr. Ezzio is a first-year pediatric hospital medicine fellow at Helen DeVos Children’s Hospital in Grand Rapids, Mich. Her interests include medical education and advocacy. Dr. Ezzio would like to thank Dr. Jeri Kessenich and Dr. Rachel “Danielle” Fisher for their assistance in revising the article. To submit to, or for inquiries about, our PHM Fellows Column, please contact our Pediatrics Editor, Dr. Anika Kumar (KumarA4@ccf.org).
References
1. Leyenaar JK and Fritner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018 Mar;18(2):200-7.
2. National Resident Matching Program. Results and data: Specialties matching service 2020 appointment year. Washington, DC 2020.
3. National Resident Matching Program. Results and data: Specialties matching service 2021 appointment year. Washington, DC 2021.
4. 2020 State of Hospital Medicine report. Society of Hospital Medicine. 2020.
5. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
6. National Resident Matching Program. Results and data: 2020 main residency match. Washington, DC 2020.
7. American Academy of Pediatrics Annual Survey of graduating residents 2003-2020.
8. Quinn Capers IV. How clinicians and educators can mitigate implicit bias in patient care and candidate selection in medical education. American Thoracic Society Scholar. 2020 Jun;1(3):211-17.
9. Welsh G. The importance of community pediatric hospital medicine. The Hospitalist. 2021 Jan;25(1):27.
A year and a half ago, I found myself seated in a crowded hall at the national Pediatric Hospital Medicine (PHM) conference. Throughout the conference, trainees like me were warmly welcomed into small groups and lunch tables. I tried to keep my cool while PHM “celebrities” chatted with me in the elevator. Most sessions were prepared with plenty of chairs, and those that were not encouraged latecomers to grab a spot on the floor or the back wall – the more the merrier.
The intention of this “advice for applicants” meeting was to inspire and guide our next steps toward fellowship, but a discomforting reality loomed over us. It was the first year graduating pediatricians could not choose PHM board certification via the practice pathway – we needed an invitation in the form of a fellowship match.
The “hidden curriculum” was not subtle: People who scored a seat would keep their options open within the field of PHM, and those who did not had a murkier future. This message stood in stark contrast to the PHM inclusivity I had experienced all conference, and planted seeds of doubt: Was I welcome here? Did I “deserve” a seat?
I found the experience as a PHM fellowship applicant to be uncomfortable, and my all-too familiar friend “imposter syndrome” set up camp in my brain and made herself at home. I had no way of knowing how many programs to apply to, how many to interview at, or the chances of my matching at all. Once on the interview trail, I realized I was not alone in my discomfort – most applicants harbored some trepidation, and no one truly knew how the chips would fall on Match Day.
I am thrilled and relieved to have come out the other end in a great position. The team I work with and learn from is phenomenal. I am grateful that ACGME accreditation ensures structures are in place for fellows to be supported in their academic and educational efforts and have full confidence that the skills I gain in fellowship will help me contribute to progression of the field of PHM and improve my performance as a clinician-educator.
Sadly, each year PHM match day celebrations are dampened by the knowledge that a large portion of our colleagues are being left out in the cold with an “unmatched” notification in their inboxes. Approximately 200 graduating pediatricians become pediatric hospitalists each year,1 but only 68 fellowship positions were available in the United States for matriculation in 2020.2 In 2019, PHM fellowship candidates navigated the 6-month application journey with aspirations to further their training in the profession they love. Of the candidates who submitted a rank list committing to 2 or more years in PHM fellowship, 35% were denied.
Unfortunately, despite expansion of PHM fellowship programs and fifteen seats added from last year, we learned this December that there still are not enough positions to welcome qualified applicants with open arms: Thirty-three percent of candidates ranked PHM programs first in the NRMP but did not match – the highest unmatched percentage out of all pediatric subspecialties.3
The NRMP report shared a glimpse of our colleagues who received interview invitations and submitted a rank list, but this is likely an underestimation of pediatric graduates who wanted to obtain PHM board certification and wound up on a different path. Some residents anticipated the stiff competition and delayed their plans to apply for fellowship, while others matched into another subspecialty that was able to accommodate them. Many pediatric graduates joined the workforce directly as pediatric hospitalists knowing the practice pathway to certification is not available to them. Along with other physicians without board certification in PHM, they shoulder concerns of being withheld from professional advancement opportunities.
For the foreseeable future it is clear that pediatric hospitalists without board certification will be a large part of our community, and are crucial to providing high-quality care to hospitalized children nationally. In 2019, a national survey of pediatric hospital medicine groups revealed that 50% of pediatric hospitalist hires came directly out of residency, and only 8% of hires were fellowship trained.4 The same report revealed that 26% of physicians were board-certified.These percentages are likely to change over the next 5 years as the window of practice pathway certification closes and fellowship programs continue to expand. Only time will tell what the national prevalence of board-certified pediatric hospitalists settles out to be.
Historically, PHM fellowship graduates have assumed roles that include teaching and research responsibilities, and ACGME fellowship requirements have ensured that trainees graduate with skills in medical education and scholarship, and need only 4 weeks of training to be done in a community hospital.5 Pediatric hospitalists who do not pursue board certification are seeing the growing pool of PHM fellowship graduates prepared for positions in academic institutions. It is reasonable that they harbor concerns about being siloed toward primarily community hospital roles, and for community hospitalists to feel that this structure undervalues their role within the field of PHM.
At a time when inclusivity and community in medicine are receiving much-needed recognition, the current fellowship application climate has potential to create division within the PHM community. Newly graduating pediatric residents are among the populations disproportionately affected by the practice pathway cutoff. Like other subspecialties with ever-climbing steps up the “ivory tower” of academia and specialization within medicine, the inherent structure of the training pathway makes navigating it more difficult for pediatricians with professional, geographic, and economic diversity or constraints.
Med-Peds–trained colleagues have the added challenge of finding a fellowship position that is willing and able to support their concurrent internal medicine goals. International medical graduates make up about 20% of graduating residents each year, and just 11% of matched PHM fellows.3,6 Similarly, while DO medical graduates make up 20% of pediatric residents in the United States, only 10% of matched PHM fellows were DOs.3,6 New pediatricians with families or financial insecurity may be unable to invest in an expensive application process, move to a new city, and accept less than half of the average starting salary of a pediatric hospitalist for 2-3 years.7
The prevalence of implicit bias in medicine is well documented, and there is growing evidence that it negatively impacts candidate selection in medical education and contributes to minorities being underrepresented in the physician workforce.8 We must recognize the ways that adding a competitive costly hurdle may risk conflict with our mission to encourage diversity of representation within PHM leadership positions.
We have not yet successfully bridged the gap between qualified PHM fellowship candidates and available fellowship positions. I worry that this gap and the lack of transparency surrounding it is resulting in one portion of new pediatricians being welcomed by the subspecialty, and others feeling unsupported and alienated by the larger PHM community as early career physicians.
Right now, the only solution available is expansion of fellowship programs. We see progress with the new addition of fellowship positions every year, but finding funding for each position is often a lengthy endeavor, and the COVID-19 pandemic has tightened the purse strings of many children’s hospitals. It may be many years before the number of available fellowship positions more closely approximates the 200 pediatricians that become hospitalists each year.
The most equitable solution would be offering other avenues to board certification while this gap is being bridged, either by extending the practice pathway option, or making a third pathway that requires less institutional funding per fellow, but still incentivizes institutional investment in fellowship positions and resources (e.g., a pathway requiring some number of years in practice, plus 1 year in fellowship centered around a nonclinical academic curriculum).
In the absence of the solutions above, we collectively hold the responsibility of maintaining inclusivity and support of our PHM colleagues with and without board certification. One important strategy provided by Dr. Gregory Welsh9 is to incorporate community hospital medicine rotations into residency training. Sharing this side of PHM with residents may help some graduates avoid a training pathway they may not want or need. More importantly, it would raise trainee exposure and interest toward a service that is both expansive – approximately 70% of pediatric hospitalists practice in a community hospital – and crucial to children’s health nationally.
Pediatric hospitalists who are not eligible for board certification are vital and valued members of the PHM community, and as such need to maintain representation within PHM leadership. Professional development opportunities need to remain accessible outside of fellowship. The blossoming of virtual conferences and Zoom meet-ups in the face of the COVID-19 pandemic have shown us that with innovation (and a good Internet connection), networking and mentorship can be accomplished across thousands of miles.
While there’s great diversity within PHM, this subspecialty has a history of attracting pediatricians with some common core qualities: Grit, creativity, and the belief that a strong team is far greater than the sum of its parts. I have confidence that if we approach this PHM transition period with transparency about our goals and challenges, this community can emerge from it strong and united.
Dr. Ezzio is a first-year pediatric hospital medicine fellow at Helen DeVos Children’s Hospital in Grand Rapids, Mich. Her interests include medical education and advocacy. Dr. Ezzio would like to thank Dr. Jeri Kessenich and Dr. Rachel “Danielle” Fisher for their assistance in revising the article. To submit to, or for inquiries about, our PHM Fellows Column, please contact our Pediatrics Editor, Dr. Anika Kumar (KumarA4@ccf.org).
References
1. Leyenaar JK and Fritner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018 Mar;18(2):200-7.
2. National Resident Matching Program. Results and data: Specialties matching service 2020 appointment year. Washington, DC 2020.
3. National Resident Matching Program. Results and data: Specialties matching service 2021 appointment year. Washington, DC 2021.
4. 2020 State of Hospital Medicine report. Society of Hospital Medicine. 2020.
5. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
6. National Resident Matching Program. Results and data: 2020 main residency match. Washington, DC 2020.
7. American Academy of Pediatrics Annual Survey of graduating residents 2003-2020.
8. Quinn Capers IV. How clinicians and educators can mitigate implicit bias in patient care and candidate selection in medical education. American Thoracic Society Scholar. 2020 Jun;1(3):211-17.
9. Welsh G. The importance of community pediatric hospital medicine. The Hospitalist. 2021 Jan;25(1):27.
A year and a half ago, I found myself seated in a crowded hall at the national Pediatric Hospital Medicine (PHM) conference. Throughout the conference, trainees like me were warmly welcomed into small groups and lunch tables. I tried to keep my cool while PHM “celebrities” chatted with me in the elevator. Most sessions were prepared with plenty of chairs, and those that were not encouraged latecomers to grab a spot on the floor or the back wall – the more the merrier.
The intention of this “advice for applicants” meeting was to inspire and guide our next steps toward fellowship, but a discomforting reality loomed over us. It was the first year graduating pediatricians could not choose PHM board certification via the practice pathway – we needed an invitation in the form of a fellowship match.
The “hidden curriculum” was not subtle: People who scored a seat would keep their options open within the field of PHM, and those who did not had a murkier future. This message stood in stark contrast to the PHM inclusivity I had experienced all conference, and planted seeds of doubt: Was I welcome here? Did I “deserve” a seat?
I found the experience as a PHM fellowship applicant to be uncomfortable, and my all-too familiar friend “imposter syndrome” set up camp in my brain and made herself at home. I had no way of knowing how many programs to apply to, how many to interview at, or the chances of my matching at all. Once on the interview trail, I realized I was not alone in my discomfort – most applicants harbored some trepidation, and no one truly knew how the chips would fall on Match Day.
I am thrilled and relieved to have come out the other end in a great position. The team I work with and learn from is phenomenal. I am grateful that ACGME accreditation ensures structures are in place for fellows to be supported in their academic and educational efforts and have full confidence that the skills I gain in fellowship will help me contribute to progression of the field of PHM and improve my performance as a clinician-educator.
Sadly, each year PHM match day celebrations are dampened by the knowledge that a large portion of our colleagues are being left out in the cold with an “unmatched” notification in their inboxes. Approximately 200 graduating pediatricians become pediatric hospitalists each year,1 but only 68 fellowship positions were available in the United States for matriculation in 2020.2 In 2019, PHM fellowship candidates navigated the 6-month application journey with aspirations to further their training in the profession they love. Of the candidates who submitted a rank list committing to 2 or more years in PHM fellowship, 35% were denied.
Unfortunately, despite expansion of PHM fellowship programs and fifteen seats added from last year, we learned this December that there still are not enough positions to welcome qualified applicants with open arms: Thirty-three percent of candidates ranked PHM programs first in the NRMP but did not match – the highest unmatched percentage out of all pediatric subspecialties.3
The NRMP report shared a glimpse of our colleagues who received interview invitations and submitted a rank list, but this is likely an underestimation of pediatric graduates who wanted to obtain PHM board certification and wound up on a different path. Some residents anticipated the stiff competition and delayed their plans to apply for fellowship, while others matched into another subspecialty that was able to accommodate them. Many pediatric graduates joined the workforce directly as pediatric hospitalists knowing the practice pathway to certification is not available to them. Along with other physicians without board certification in PHM, they shoulder concerns of being withheld from professional advancement opportunities.
For the foreseeable future it is clear that pediatric hospitalists without board certification will be a large part of our community, and are crucial to providing high-quality care to hospitalized children nationally. In 2019, a national survey of pediatric hospital medicine groups revealed that 50% of pediatric hospitalist hires came directly out of residency, and only 8% of hires were fellowship trained.4 The same report revealed that 26% of physicians were board-certified.These percentages are likely to change over the next 5 years as the window of practice pathway certification closes and fellowship programs continue to expand. Only time will tell what the national prevalence of board-certified pediatric hospitalists settles out to be.
Historically, PHM fellowship graduates have assumed roles that include teaching and research responsibilities, and ACGME fellowship requirements have ensured that trainees graduate with skills in medical education and scholarship, and need only 4 weeks of training to be done in a community hospital.5 Pediatric hospitalists who do not pursue board certification are seeing the growing pool of PHM fellowship graduates prepared for positions in academic institutions. It is reasonable that they harbor concerns about being siloed toward primarily community hospital roles, and for community hospitalists to feel that this structure undervalues their role within the field of PHM.
At a time when inclusivity and community in medicine are receiving much-needed recognition, the current fellowship application climate has potential to create division within the PHM community. Newly graduating pediatric residents are among the populations disproportionately affected by the practice pathway cutoff. Like other subspecialties with ever-climbing steps up the “ivory tower” of academia and specialization within medicine, the inherent structure of the training pathway makes navigating it more difficult for pediatricians with professional, geographic, and economic diversity or constraints.
Med-Peds–trained colleagues have the added challenge of finding a fellowship position that is willing and able to support their concurrent internal medicine goals. International medical graduates make up about 20% of graduating residents each year, and just 11% of matched PHM fellows.3,6 Similarly, while DO medical graduates make up 20% of pediatric residents in the United States, only 10% of matched PHM fellows were DOs.3,6 New pediatricians with families or financial insecurity may be unable to invest in an expensive application process, move to a new city, and accept less than half of the average starting salary of a pediatric hospitalist for 2-3 years.7
The prevalence of implicit bias in medicine is well documented, and there is growing evidence that it negatively impacts candidate selection in medical education and contributes to minorities being underrepresented in the physician workforce.8 We must recognize the ways that adding a competitive costly hurdle may risk conflict with our mission to encourage diversity of representation within PHM leadership positions.
We have not yet successfully bridged the gap between qualified PHM fellowship candidates and available fellowship positions. I worry that this gap and the lack of transparency surrounding it is resulting in one portion of new pediatricians being welcomed by the subspecialty, and others feeling unsupported and alienated by the larger PHM community as early career physicians.
Right now, the only solution available is expansion of fellowship programs. We see progress with the new addition of fellowship positions every year, but finding funding for each position is often a lengthy endeavor, and the COVID-19 pandemic has tightened the purse strings of many children’s hospitals. It may be many years before the number of available fellowship positions more closely approximates the 200 pediatricians that become hospitalists each year.
The most equitable solution would be offering other avenues to board certification while this gap is being bridged, either by extending the practice pathway option, or making a third pathway that requires less institutional funding per fellow, but still incentivizes institutional investment in fellowship positions and resources (e.g., a pathway requiring some number of years in practice, plus 1 year in fellowship centered around a nonclinical academic curriculum).
In the absence of the solutions above, we collectively hold the responsibility of maintaining inclusivity and support of our PHM colleagues with and without board certification. One important strategy provided by Dr. Gregory Welsh9 is to incorporate community hospital medicine rotations into residency training. Sharing this side of PHM with residents may help some graduates avoid a training pathway they may not want or need. More importantly, it would raise trainee exposure and interest toward a service that is both expansive – approximately 70% of pediatric hospitalists practice in a community hospital – and crucial to children’s health nationally.
Pediatric hospitalists who are not eligible for board certification are vital and valued members of the PHM community, and as such need to maintain representation within PHM leadership. Professional development opportunities need to remain accessible outside of fellowship. The blossoming of virtual conferences and Zoom meet-ups in the face of the COVID-19 pandemic have shown us that with innovation (and a good Internet connection), networking and mentorship can be accomplished across thousands of miles.
While there’s great diversity within PHM, this subspecialty has a history of attracting pediatricians with some common core qualities: Grit, creativity, and the belief that a strong team is far greater than the sum of its parts. I have confidence that if we approach this PHM transition period with transparency about our goals and challenges, this community can emerge from it strong and united.
Dr. Ezzio is a first-year pediatric hospital medicine fellow at Helen DeVos Children’s Hospital in Grand Rapids, Mich. Her interests include medical education and advocacy. Dr. Ezzio would like to thank Dr. Jeri Kessenich and Dr. Rachel “Danielle” Fisher for their assistance in revising the article. To submit to, or for inquiries about, our PHM Fellows Column, please contact our Pediatrics Editor, Dr. Anika Kumar (KumarA4@ccf.org).
References
1. Leyenaar JK and Fritner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018 Mar;18(2):200-7.
2. National Resident Matching Program. Results and data: Specialties matching service 2020 appointment year. Washington, DC 2020.
3. National Resident Matching Program. Results and data: Specialties matching service 2021 appointment year. Washington, DC 2021.
4. 2020 State of Hospital Medicine report. Society of Hospital Medicine. 2020.
5. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
6. National Resident Matching Program. Results and data: 2020 main residency match. Washington, DC 2020.
7. American Academy of Pediatrics Annual Survey of graduating residents 2003-2020.
8. Quinn Capers IV. How clinicians and educators can mitigate implicit bias in patient care and candidate selection in medical education. American Thoracic Society Scholar. 2020 Jun;1(3):211-17.
9. Welsh G. The importance of community pediatric hospital medicine. The Hospitalist. 2021 Jan;25(1):27.
Myth busting: SARS-CoV-2 vaccine
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
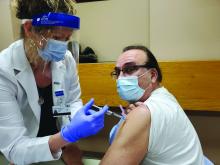
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
Palliative care and hospital medicine partnerships in the pandemic
Patients dying without their loved ones, families forced to remotely decide goals of care without the physical presence or human connection of the care team, overworked staff physically isolated from their critically ill patients, and at-risk community members with uncertain and undocumented goals for care are among the universal challenges confronted by hospitals and hospitalists during this COVID-19 pandemic. Partnerships among hospital medicine (HM) and palliative care (PC) teams at Dell Medical School/Dell Seton Medical Center thrive on mutually shared core values of patient centered care – compassion, empathy, and humanity.
A key PC-HM collaboration was adapting our multidisciplinary huddle to focus on communication effectiveness and efficiency in the medical intensive care unit (MICU). Expanded interprofessional and cross-specialty collaboration promoted streamlined, succinct, and standardized communication with patients’ families while their loved ones were critically ill with COVID-19. The PC team attended daily MICU multidisciplinary huddles, attentive to both the medical and psychosocial updates for each patient. During huddles, residents or HM providers were asked to end their presentation with a clinical status “headline” and solicited feedback from the multidisciplinary team before messaging to the family. The PC team then communicated with families a succinct and cohesive medical update and continuously explored goals of care. This allowed the HM team, often overwhelmed with admissions, co-managing intensive care patients, and facilitating safe discharges, to focus on urgent issues while PC provided continuity and personalized support for patients and families. PC’s ability to synthesize and summarize clinical information from multiple teams and then provide cohesive updates in patient-friendly language modeled important communication skills for learners and simultaneously benefited HM providers.
Our chaplains, too, were central to facilitating timely, proactive conversations and documentation of Medical Power of Attorney (MPOA) for patients with COVID-19 admitted to our hospital. HM prioritized early admission conversations with patients to counsel them on severity of illness, prognosis based on risk factors, to elucidate wishes for intubation or resuscitation, and to capture their desired medical decision maker. HM was notified of all COVID and PUI admissions, allowing us to speak with even critically ill patients in the ER or ICU prior to intubation in order to quickly and accurately capture patients’ wishes for treatment and delegate decision makers. Our chaplains supported and supplemented these efforts by diligently and dutifully soliciting, hearing, and documenting patient MPOA delegates, with over 50% MPOA completion by 24 hours of hospitalization.
Another early PC-HM project, “Meet My Loved One,” was adapted from the University of Alabama at Birmingham Palliative and Comfort Unit. The absence of families visiting the ICU and sharing pictures, stories, anecdotes of our patients left a deeply felt, dehumanizing void in the halls and rooms of our hospital. To fill this space with life and humanity, furloughed medical students on their “transition of care” electives contacted family members of their “continuity” patients focusing primarily on those patients expected to have prolonged ICU or hospital stays and solicited personal, humanizing information about our patients. Questions included: “What is your loved one’s preferred name or nickname?” and “What are three things we should know to take better care of your loved one?” With family permission, we posted this information on the door outside the patient’s room. Nursing staff, in particular, appreciated getting to know their patients more personally and families appreciated the staff’s desire to know their loved one as an individual.
It is also important to acknowledge setbacks. Early efforts to engage technology proved more foe than friend. We continue to struggle with using our iPads for video visits. Most of our families prefer “WhatsApp” for video communication, which is not compatible with operating systems on early versions of the iPad, which were generously and widely donated by local school systems. Desperate to allow families to connect, many providers resorted to using personal devices to facilitate video visits and family meetings. And we discovered that many video visits caused more not less family angst, especially for critically ill patients. Families often required preparation and coaching on what to expect and how to interact with intubated, sedated, proned, and paralyzed loved ones.
Our hospital medicine and palliative care teams have an established strong partnership. The COVID-19 pandemic created novel communication challenges but our shared mission toward patient-centered care allowed us to effectively collaborate to bring the patients goals of care to the forefront aligning patients, families, physicians, nurses, and staff during the COVID-19 surge.
Dr. Johnston is associate professor at Dell Medical School at The University of Texas in Austin. She practices hospital medicine and inpatient palliative care at Dell Seton Medical Center. Dr. Cooremans is a resident physician at Dell Medical School. Dr. Salib is the internal medicine clerkship director and an associate professor at Dell Medical School. Dr. Nieto is an assistant professor and associate chief of the Division of Hospital Medicine at Dell Medical School. Dr. Patel is an assistant professor at Dell Medical School. This article is part of a series written by members of the Division of Hospital Medicine at Dell Medical School, exploring lessons learned from the coronavirus pandemic and outlining an approach for creating COVID-19 Centers of Excellence. The article first appeared in The Hospital Leader, the official blog of SHM.
Patients dying without their loved ones, families forced to remotely decide goals of care without the physical presence or human connection of the care team, overworked staff physically isolated from their critically ill patients, and at-risk community members with uncertain and undocumented goals for care are among the universal challenges confronted by hospitals and hospitalists during this COVID-19 pandemic. Partnerships among hospital medicine (HM) and palliative care (PC) teams at Dell Medical School/Dell Seton Medical Center thrive on mutually shared core values of patient centered care – compassion, empathy, and humanity.
A key PC-HM collaboration was adapting our multidisciplinary huddle to focus on communication effectiveness and efficiency in the medical intensive care unit (MICU). Expanded interprofessional and cross-specialty collaboration promoted streamlined, succinct, and standardized communication with patients’ families while their loved ones were critically ill with COVID-19. The PC team attended daily MICU multidisciplinary huddles, attentive to both the medical and psychosocial updates for each patient. During huddles, residents or HM providers were asked to end their presentation with a clinical status “headline” and solicited feedback from the multidisciplinary team before messaging to the family. The PC team then communicated with families a succinct and cohesive medical update and continuously explored goals of care. This allowed the HM team, often overwhelmed with admissions, co-managing intensive care patients, and facilitating safe discharges, to focus on urgent issues while PC provided continuity and personalized support for patients and families. PC’s ability to synthesize and summarize clinical information from multiple teams and then provide cohesive updates in patient-friendly language modeled important communication skills for learners and simultaneously benefited HM providers.
Our chaplains, too, were central to facilitating timely, proactive conversations and documentation of Medical Power of Attorney (MPOA) for patients with COVID-19 admitted to our hospital. HM prioritized early admission conversations with patients to counsel them on severity of illness, prognosis based on risk factors, to elucidate wishes for intubation or resuscitation, and to capture their desired medical decision maker. HM was notified of all COVID and PUI admissions, allowing us to speak with even critically ill patients in the ER or ICU prior to intubation in order to quickly and accurately capture patients’ wishes for treatment and delegate decision makers. Our chaplains supported and supplemented these efforts by diligently and dutifully soliciting, hearing, and documenting patient MPOA delegates, with over 50% MPOA completion by 24 hours of hospitalization.
Another early PC-HM project, “Meet My Loved One,” was adapted from the University of Alabama at Birmingham Palliative and Comfort Unit. The absence of families visiting the ICU and sharing pictures, stories, anecdotes of our patients left a deeply felt, dehumanizing void in the halls and rooms of our hospital. To fill this space with life and humanity, furloughed medical students on their “transition of care” electives contacted family members of their “continuity” patients focusing primarily on those patients expected to have prolonged ICU or hospital stays and solicited personal, humanizing information about our patients. Questions included: “What is your loved one’s preferred name or nickname?” and “What are three things we should know to take better care of your loved one?” With family permission, we posted this information on the door outside the patient’s room. Nursing staff, in particular, appreciated getting to know their patients more personally and families appreciated the staff’s desire to know their loved one as an individual.
It is also important to acknowledge setbacks. Early efforts to engage technology proved more foe than friend. We continue to struggle with using our iPads for video visits. Most of our families prefer “WhatsApp” for video communication, which is not compatible with operating systems on early versions of the iPad, which were generously and widely donated by local school systems. Desperate to allow families to connect, many providers resorted to using personal devices to facilitate video visits and family meetings. And we discovered that many video visits caused more not less family angst, especially for critically ill patients. Families often required preparation and coaching on what to expect and how to interact with intubated, sedated, proned, and paralyzed loved ones.
Our hospital medicine and palliative care teams have an established strong partnership. The COVID-19 pandemic created novel communication challenges but our shared mission toward patient-centered care allowed us to effectively collaborate to bring the patients goals of care to the forefront aligning patients, families, physicians, nurses, and staff during the COVID-19 surge.
Dr. Johnston is associate professor at Dell Medical School at The University of Texas in Austin. She practices hospital medicine and inpatient palliative care at Dell Seton Medical Center. Dr. Cooremans is a resident physician at Dell Medical School. Dr. Salib is the internal medicine clerkship director and an associate professor at Dell Medical School. Dr. Nieto is an assistant professor and associate chief of the Division of Hospital Medicine at Dell Medical School. Dr. Patel is an assistant professor at Dell Medical School. This article is part of a series written by members of the Division of Hospital Medicine at Dell Medical School, exploring lessons learned from the coronavirus pandemic and outlining an approach for creating COVID-19 Centers of Excellence. The article first appeared in The Hospital Leader, the official blog of SHM.
Patients dying without their loved ones, families forced to remotely decide goals of care without the physical presence or human connection of the care team, overworked staff physically isolated from their critically ill patients, and at-risk community members with uncertain and undocumented goals for care are among the universal challenges confronted by hospitals and hospitalists during this COVID-19 pandemic. Partnerships among hospital medicine (HM) and palliative care (PC) teams at Dell Medical School/Dell Seton Medical Center thrive on mutually shared core values of patient centered care – compassion, empathy, and humanity.
A key PC-HM collaboration was adapting our multidisciplinary huddle to focus on communication effectiveness and efficiency in the medical intensive care unit (MICU). Expanded interprofessional and cross-specialty collaboration promoted streamlined, succinct, and standardized communication with patients’ families while their loved ones were critically ill with COVID-19. The PC team attended daily MICU multidisciplinary huddles, attentive to both the medical and psychosocial updates for each patient. During huddles, residents or HM providers were asked to end their presentation with a clinical status “headline” and solicited feedback from the multidisciplinary team before messaging to the family. The PC team then communicated with families a succinct and cohesive medical update and continuously explored goals of care. This allowed the HM team, often overwhelmed with admissions, co-managing intensive care patients, and facilitating safe discharges, to focus on urgent issues while PC provided continuity and personalized support for patients and families. PC’s ability to synthesize and summarize clinical information from multiple teams and then provide cohesive updates in patient-friendly language modeled important communication skills for learners and simultaneously benefited HM providers.
Our chaplains, too, were central to facilitating timely, proactive conversations and documentation of Medical Power of Attorney (MPOA) for patients with COVID-19 admitted to our hospital. HM prioritized early admission conversations with patients to counsel them on severity of illness, prognosis based on risk factors, to elucidate wishes for intubation or resuscitation, and to capture their desired medical decision maker. HM was notified of all COVID and PUI admissions, allowing us to speak with even critically ill patients in the ER or ICU prior to intubation in order to quickly and accurately capture patients’ wishes for treatment and delegate decision makers. Our chaplains supported and supplemented these efforts by diligently and dutifully soliciting, hearing, and documenting patient MPOA delegates, with over 50% MPOA completion by 24 hours of hospitalization.
Another early PC-HM project, “Meet My Loved One,” was adapted from the University of Alabama at Birmingham Palliative and Comfort Unit. The absence of families visiting the ICU and sharing pictures, stories, anecdotes of our patients left a deeply felt, dehumanizing void in the halls and rooms of our hospital. To fill this space with life and humanity, furloughed medical students on their “transition of care” electives contacted family members of their “continuity” patients focusing primarily on those patients expected to have prolonged ICU or hospital stays and solicited personal, humanizing information about our patients. Questions included: “What is your loved one’s preferred name or nickname?” and “What are three things we should know to take better care of your loved one?” With family permission, we posted this information on the door outside the patient’s room. Nursing staff, in particular, appreciated getting to know their patients more personally and families appreciated the staff’s desire to know their loved one as an individual.
It is also important to acknowledge setbacks. Early efforts to engage technology proved more foe than friend. We continue to struggle with using our iPads for video visits. Most of our families prefer “WhatsApp” for video communication, which is not compatible with operating systems on early versions of the iPad, which were generously and widely donated by local school systems. Desperate to allow families to connect, many providers resorted to using personal devices to facilitate video visits and family meetings. And we discovered that many video visits caused more not less family angst, especially for critically ill patients. Families often required preparation and coaching on what to expect and how to interact with intubated, sedated, proned, and paralyzed loved ones.
Our hospital medicine and palliative care teams have an established strong partnership. The COVID-19 pandemic created novel communication challenges but our shared mission toward patient-centered care allowed us to effectively collaborate to bring the patients goals of care to the forefront aligning patients, families, physicians, nurses, and staff during the COVID-19 surge.
Dr. Johnston is associate professor at Dell Medical School at The University of Texas in Austin. She practices hospital medicine and inpatient palliative care at Dell Seton Medical Center. Dr. Cooremans is a resident physician at Dell Medical School. Dr. Salib is the internal medicine clerkship director and an associate professor at Dell Medical School. Dr. Nieto is an assistant professor and associate chief of the Division of Hospital Medicine at Dell Medical School. Dr. Patel is an assistant professor at Dell Medical School. This article is part of a series written by members of the Division of Hospital Medicine at Dell Medical School, exploring lessons learned from the coronavirus pandemic and outlining an approach for creating COVID-19 Centers of Excellence. The article first appeared in The Hospital Leader, the official blog of SHM.
Who do you call in those late, quiet hours, when all seems lost?
I swear by Apollo Physician and Asclepius and Hygeia and Panacea and all the gods and goddesses, making them my witnesses, that I will fulfill according to my ability and judgment this oath and this covenant.
On my desk sits a bust of Hygeia, a mask from Venice, next to a small sculpture and a figurine of the plague doctor. Nearby, there is a Klimt closeup of Hygeia, a postcard portraying Asclepius, St. Sebastian paintings, and quotes from Maimonides. They whisper secrets and nod to the challenges of the past. These medical specters, ancient voices of the past, keep me grounded. They speak, listen, and elevate me, too. They bring life into my otherwise quiet room.
We all began our careers swearing to Apollo, Asclepius, Hygeia, and Panacea when we recited the Hippocratic Oath. I call upon them, and other gods and totems, and saints and ancient healers, now more than ever. As an atheist, I don’t appeal to them as prayers, but as Hippocrates intended. I look to their supernatural healing powers as a source of strength and as revealers of the natural and observable phenomena.
Apollo was one of the Twelve Olympians, a God of medicine, father of Asclepius. He was a healer, though his arrows also bore the plagues of the Gods.
For centuries, Apollo was found floating above the marble dissection table in the Bologna anatomical theater, guiding students who dove into the secrets of the human body.
Asclepius, son of Apollo, was hailed as a god of medicine. He healed many from plagues at his temples throughout the Ancient Greek and Roman empires. He was mentored in the healing arts by the centaur, Chiron. His many daughters and sons represent various aspects of medicine including cures, healing, recovery, sanitation, and beauty. To Asclepius, temples were places of healing, an ancient ancestor to modern hospitals.
Two of his daughters, Panacea and Hygeia, gave us the healing words of panacea and hygiene. Today, these acts of hygiene, handwashing, mask-wearing, and sanitation are discussed across the world louder than ever. While we’re all wishing for a panacea, we know it will take all the attributes of medicine to get us through this pandemic.
Hospitalists are part of the frontline teams facing this pandemic head-on. Gowning up for MRSA isolation seems quaint nowadays.
My attendings spoke of their fears, up against the unknown while on service in the 1980s, when HIV appeared. 2014 brought the Ebola biocontainment units. Now, this generation works daily against a modern plague, where every day is a risk of exposure. When every patient is in isolation, the garb begins to reflect the PPE that emerged during a 17th-century plague epidemics, the plague doctor outfit.
Godfather II fans recall the famous portrayal of the August 16th festival to San Rocco play out in the streets of New York. For those stricken with COVID-19 and recovered, you emulate San Rocco, in your continued return to service.
The Scuola Grande di San Rocco, in Venice, is the epitome of healing and greatness in one building. Tintoretto, the great Venetian painter, assembled the story of healing through art and portraits of San Rocco. The scuola, a confraternity, was a community of healers, gathered in one place to look after the less fortunate.
Hospitalists march into the hospital risking their lives. We always wear PPE for MRSA, ESBL, or C. diff. And enter reverse isolation rooms wearing N95s for possible TB cases. But those don’t elevate to the volume, to the same fear, as gowning up for COVID-19.
Hospitalists, frontline health care workers, embody the story of San Sebastian, another plague saint who absorbed the arrows, the symbolic plagues, onto his own shoulders so no one else had to bear them. San Sebastian was a Christian persecuted by a Roman emperor once his beliefs were discovered. He is often laden with arrows in spots where buboes would have appeared: the armpits and the groin. His sacrifice for others’ recovery became a symbol of absorbing the plague, the wounds, and the impact of the arrows.
This sacrifice epitomizes the daily work the frontline nurses, ER docs, intensivists, hospitalists, and the entire hospital staff perform daily, bearing the slung arrows of coronavirus.
One of the images I think of frequently during this time lies atop Castel San Angelo in Rome. Built in 161 AD, it has served as a mausoleum, prison, papal residence, and is currently a museum. Atop San’Angelo stands St. Michael, the destroyer of the dragon. He is sheathing his sword in representation of the end of the plague in 590.
The arrows flow, yet the sword will be sheathed. Evil will be halted. The stories of these ancient totems and strength can give us strength as they remind us of the work that was done for centuries: pestilence, famine, war. The great killers never go away completely.
Fast forward to today
These medical specters serve as reminders of what makes the field of medicine so inspiring: the selfless acts, the fortitude of spirit, the healers, the long history, and the shoulders of giants we stand upon. From these stories, we spring the healing waters we bathe in to give us the courage to wake up and care for our patients each day. These specters encourage us to defeat any and all of the scourges that come our way.
I hear and read stories about the frontline heroes, the vaccine makers, the PPE creators, the health care workers, grocery store clerks, and teachers. I’m honored to hear of these stories and your sacrifices. I’m inspired to continue upholding your essence, your fight, and your stories. In keeping with ancient empire metaphors, you are taking the slings of the diseased arrows flying to our brethren as you try to keep yourself and others safe.
The sheathing of this sword will come. These arrows will be silenced. But until then, I lean on these pictures, these stories, and these saints, to give us all the strength to wake up each morning and continue healing.
They serve as reminders of what makes the field of medicine so great: the selfless acts, the fortitude of spirit, the healers, the long history, and the shoulders of giants we stand upon. From these stories spring the healing waters we bathe in to give us the courage to wake up and care for our patients each day and defeat any and all scourges that come our way.
So, who do you call in those late, quiet hours, when all seems lost?
Dr. Messler is the executive director, quality initiatives at Glytec and works as a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. This essay appeared initially on The Hospital Leader, the official blog of SHM.
I swear by Apollo Physician and Asclepius and Hygeia and Panacea and all the gods and goddesses, making them my witnesses, that I will fulfill according to my ability and judgment this oath and this covenant.
On my desk sits a bust of Hygeia, a mask from Venice, next to a small sculpture and a figurine of the plague doctor. Nearby, there is a Klimt closeup of Hygeia, a postcard portraying Asclepius, St. Sebastian paintings, and quotes from Maimonides. They whisper secrets and nod to the challenges of the past. These medical specters, ancient voices of the past, keep me grounded. They speak, listen, and elevate me, too. They bring life into my otherwise quiet room.
We all began our careers swearing to Apollo, Asclepius, Hygeia, and Panacea when we recited the Hippocratic Oath. I call upon them, and other gods and totems, and saints and ancient healers, now more than ever. As an atheist, I don’t appeal to them as prayers, but as Hippocrates intended. I look to their supernatural healing powers as a source of strength and as revealers of the natural and observable phenomena.
Apollo was one of the Twelve Olympians, a God of medicine, father of Asclepius. He was a healer, though his arrows also bore the plagues of the Gods.
For centuries, Apollo was found floating above the marble dissection table in the Bologna anatomical theater, guiding students who dove into the secrets of the human body.
Asclepius, son of Apollo, was hailed as a god of medicine. He healed many from plagues at his temples throughout the Ancient Greek and Roman empires. He was mentored in the healing arts by the centaur, Chiron. His many daughters and sons represent various aspects of medicine including cures, healing, recovery, sanitation, and beauty. To Asclepius, temples were places of healing, an ancient ancestor to modern hospitals.
Two of his daughters, Panacea and Hygeia, gave us the healing words of panacea and hygiene. Today, these acts of hygiene, handwashing, mask-wearing, and sanitation are discussed across the world louder than ever. While we’re all wishing for a panacea, we know it will take all the attributes of medicine to get us through this pandemic.
Hospitalists are part of the frontline teams facing this pandemic head-on. Gowning up for MRSA isolation seems quaint nowadays.
My attendings spoke of their fears, up against the unknown while on service in the 1980s, when HIV appeared. 2014 brought the Ebola biocontainment units. Now, this generation works daily against a modern plague, where every day is a risk of exposure. When every patient is in isolation, the garb begins to reflect the PPE that emerged during a 17th-century plague epidemics, the plague doctor outfit.
Godfather II fans recall the famous portrayal of the August 16th festival to San Rocco play out in the streets of New York. For those stricken with COVID-19 and recovered, you emulate San Rocco, in your continued return to service.
The Scuola Grande di San Rocco, in Venice, is the epitome of healing and greatness in one building. Tintoretto, the great Venetian painter, assembled the story of healing through art and portraits of San Rocco. The scuola, a confraternity, was a community of healers, gathered in one place to look after the less fortunate.
Hospitalists march into the hospital risking their lives. We always wear PPE for MRSA, ESBL, or C. diff. And enter reverse isolation rooms wearing N95s for possible TB cases. But those don’t elevate to the volume, to the same fear, as gowning up for COVID-19.
Hospitalists, frontline health care workers, embody the story of San Sebastian, another plague saint who absorbed the arrows, the symbolic plagues, onto his own shoulders so no one else had to bear them. San Sebastian was a Christian persecuted by a Roman emperor once his beliefs were discovered. He is often laden with arrows in spots where buboes would have appeared: the armpits and the groin. His sacrifice for others’ recovery became a symbol of absorbing the plague, the wounds, and the impact of the arrows.
This sacrifice epitomizes the daily work the frontline nurses, ER docs, intensivists, hospitalists, and the entire hospital staff perform daily, bearing the slung arrows of coronavirus.
One of the images I think of frequently during this time lies atop Castel San Angelo in Rome. Built in 161 AD, it has served as a mausoleum, prison, papal residence, and is currently a museum. Atop San’Angelo stands St. Michael, the destroyer of the dragon. He is sheathing his sword in representation of the end of the plague in 590.
The arrows flow, yet the sword will be sheathed. Evil will be halted. The stories of these ancient totems and strength can give us strength as they remind us of the work that was done for centuries: pestilence, famine, war. The great killers never go away completely.
Fast forward to today
These medical specters serve as reminders of what makes the field of medicine so inspiring: the selfless acts, the fortitude of spirit, the healers, the long history, and the shoulders of giants we stand upon. From these stories, we spring the healing waters we bathe in to give us the courage to wake up and care for our patients each day. These specters encourage us to defeat any and all of the scourges that come our way.
I hear and read stories about the frontline heroes, the vaccine makers, the PPE creators, the health care workers, grocery store clerks, and teachers. I’m honored to hear of these stories and your sacrifices. I’m inspired to continue upholding your essence, your fight, and your stories. In keeping with ancient empire metaphors, you are taking the slings of the diseased arrows flying to our brethren as you try to keep yourself and others safe.
The sheathing of this sword will come. These arrows will be silenced. But until then, I lean on these pictures, these stories, and these saints, to give us all the strength to wake up each morning and continue healing.
They serve as reminders of what makes the field of medicine so great: the selfless acts, the fortitude of spirit, the healers, the long history, and the shoulders of giants we stand upon. From these stories spring the healing waters we bathe in to give us the courage to wake up and care for our patients each day and defeat any and all scourges that come our way.
So, who do you call in those late, quiet hours, when all seems lost?
Dr. Messler is the executive director, quality initiatives at Glytec and works as a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. This essay appeared initially on The Hospital Leader, the official blog of SHM.
I swear by Apollo Physician and Asclepius and Hygeia and Panacea and all the gods and goddesses, making them my witnesses, that I will fulfill according to my ability and judgment this oath and this covenant.
On my desk sits a bust of Hygeia, a mask from Venice, next to a small sculpture and a figurine of the plague doctor. Nearby, there is a Klimt closeup of Hygeia, a postcard portraying Asclepius, St. Sebastian paintings, and quotes from Maimonides. They whisper secrets and nod to the challenges of the past. These medical specters, ancient voices of the past, keep me grounded. They speak, listen, and elevate me, too. They bring life into my otherwise quiet room.
We all began our careers swearing to Apollo, Asclepius, Hygeia, and Panacea when we recited the Hippocratic Oath. I call upon them, and other gods and totems, and saints and ancient healers, now more than ever. As an atheist, I don’t appeal to them as prayers, but as Hippocrates intended. I look to their supernatural healing powers as a source of strength and as revealers of the natural and observable phenomena.
Apollo was one of the Twelve Olympians, a God of medicine, father of Asclepius. He was a healer, though his arrows also bore the plagues of the Gods.
For centuries, Apollo was found floating above the marble dissection table in the Bologna anatomical theater, guiding students who dove into the secrets of the human body.
Asclepius, son of Apollo, was hailed as a god of medicine. He healed many from plagues at his temples throughout the Ancient Greek and Roman empires. He was mentored in the healing arts by the centaur, Chiron. His many daughters and sons represent various aspects of medicine including cures, healing, recovery, sanitation, and beauty. To Asclepius, temples were places of healing, an ancient ancestor to modern hospitals.
Two of his daughters, Panacea and Hygeia, gave us the healing words of panacea and hygiene. Today, these acts of hygiene, handwashing, mask-wearing, and sanitation are discussed across the world louder than ever. While we’re all wishing for a panacea, we know it will take all the attributes of medicine to get us through this pandemic.
Hospitalists are part of the frontline teams facing this pandemic head-on. Gowning up for MRSA isolation seems quaint nowadays.
My attendings spoke of their fears, up against the unknown while on service in the 1980s, when HIV appeared. 2014 brought the Ebola biocontainment units. Now, this generation works daily against a modern plague, where every day is a risk of exposure. When every patient is in isolation, the garb begins to reflect the PPE that emerged during a 17th-century plague epidemics, the plague doctor outfit.
Godfather II fans recall the famous portrayal of the August 16th festival to San Rocco play out in the streets of New York. For those stricken with COVID-19 and recovered, you emulate San Rocco, in your continued return to service.
The Scuola Grande di San Rocco, in Venice, is the epitome of healing and greatness in one building. Tintoretto, the great Venetian painter, assembled the story of healing through art and portraits of San Rocco. The scuola, a confraternity, was a community of healers, gathered in one place to look after the less fortunate.
Hospitalists march into the hospital risking their lives. We always wear PPE for MRSA, ESBL, or C. diff. And enter reverse isolation rooms wearing N95s for possible TB cases. But those don’t elevate to the volume, to the same fear, as gowning up for COVID-19.
Hospitalists, frontline health care workers, embody the story of San Sebastian, another plague saint who absorbed the arrows, the symbolic plagues, onto his own shoulders so no one else had to bear them. San Sebastian was a Christian persecuted by a Roman emperor once his beliefs were discovered. He is often laden with arrows in spots where buboes would have appeared: the armpits and the groin. His sacrifice for others’ recovery became a symbol of absorbing the plague, the wounds, and the impact of the arrows.
This sacrifice epitomizes the daily work the frontline nurses, ER docs, intensivists, hospitalists, and the entire hospital staff perform daily, bearing the slung arrows of coronavirus.
One of the images I think of frequently during this time lies atop Castel San Angelo in Rome. Built in 161 AD, it has served as a mausoleum, prison, papal residence, and is currently a museum. Atop San’Angelo stands St. Michael, the destroyer of the dragon. He is sheathing his sword in representation of the end of the plague in 590.
The arrows flow, yet the sword will be sheathed. Evil will be halted. The stories of these ancient totems and strength can give us strength as they remind us of the work that was done for centuries: pestilence, famine, war. The great killers never go away completely.
Fast forward to today
These medical specters serve as reminders of what makes the field of medicine so inspiring: the selfless acts, the fortitude of spirit, the healers, the long history, and the shoulders of giants we stand upon. From these stories, we spring the healing waters we bathe in to give us the courage to wake up and care for our patients each day. These specters encourage us to defeat any and all of the scourges that come our way.
I hear and read stories about the frontline heroes, the vaccine makers, the PPE creators, the health care workers, grocery store clerks, and teachers. I’m honored to hear of these stories and your sacrifices. I’m inspired to continue upholding your essence, your fight, and your stories. In keeping with ancient empire metaphors, you are taking the slings of the diseased arrows flying to our brethren as you try to keep yourself and others safe.
The sheathing of this sword will come. These arrows will be silenced. But until then, I lean on these pictures, these stories, and these saints, to give us all the strength to wake up each morning and continue healing.
They serve as reminders of what makes the field of medicine so great: the selfless acts, the fortitude of spirit, the healers, the long history, and the shoulders of giants we stand upon. From these stories spring the healing waters we bathe in to give us the courage to wake up and care for our patients each day and defeat any and all scourges that come our way.
So, who do you call in those late, quiet hours, when all seems lost?
Dr. Messler is the executive director, quality initiatives at Glytec and works as a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. This essay appeared initially on The Hospital Leader, the official blog of SHM.
How to make resident mental health care stigma free
Sarah Sofka, MD, FACP, noticed a pattern. As program director for the internal medicine (IM) residency at West Virginia University, Morgantown, she was informed when residents were sent to counseling because they were affected by burnout, depression, or anxiety. When trainees returned from these visits, many told her the same thing: They wished they had sought help sooner.
IM residents and their families had access to free counseling at WVU, but few used the resource, says Dr. Sofka. “So, we thought, let’s just schedule all of our residents for a therapy visit so they can go and see what it’s like,” she said. “This will hopefully decrease the stigma for seeking mental health care. If everybody’s going, it’s not a big deal.”
In July 2015, Dr. Sofka and her colleagues launched a universal well-being assessment program for the IM residents at WVU. The program leaders automatically scheduled first- and second-year residents for a visit to the faculty staff assistance program counselors. The visits were not mandatory, and residents could choose not to go; but if they did go, they received the entire day of their visit off from work.
Five and a half years after launching their program, Dr. Sofka and her colleagues conducted one of the first studies of the efficacy of an opt-out approach for resident mental wellness. They found that , suggesting that residents were seeking help proactively after having to at least consider it.
Opt-out counseling is a recent concept in residency programs – one that’s attracting interest from training programs across the country. Brown University, Providence, R.I.; the University of Colorado at Denver, Aurora; University of Pennsylvania, Philadelphia; and the University of California, San Francisco have at least one residency program that uses the approach.
Lisa Meeks, PhD, an assistant professor of family medicine at Michigan Medicine, in Ann Arbor, and other experts also believe opt-out counseling could decrease stigma and help normalize seeking care for mental health problems in the medical community while lowering the barriers for trainees who need help.
No time, no access, plenty of stigma
Burnout and mental health are known to be major concerns for health care workers, especially trainees. College graduates starting medical education have lower rates of burnout and depression, compared with demographically matched peers; however, once they’ve started training, medical students, residents, and fellows are more likely to be burned out and exhibit symptoms of depression. The ongoing COVID-19 pandemic is further fraying the well-being of overworked and traumatized health care professionals, and experts predict a mental health crisis will follow the viral crisis.
The Accreditation Council for Graduate Medical Education recently mandated that programs offer wellness services to trainees. Yet this doesn’t mean they are always used; well-known barriers stand between residents, medical students, and physicians and their receiving effective mental health treatment.
Two of the most obvious are access and time, given the grueling and often inflexible schedules of most trainees, says Jessica Gold, MD, a psychiatrist at Washington University, St. Louis, who specializes in treating medical professionals. Dr. Gold also points out that, to be done correctly, these programs require institutional support and investment – resources that aren’t always adequate.
“A lack of transparency and clear messaging around what is available, who provides the services, and how to access these services can be a major barrier,” says Erene Stergiopoulos, MD, a second-year psychiatry resident at the University of Toronto. In addition, there can be considerable lag between when a resident realizes they need help and when they manage to find a provider and schedule an appointment, says Dr. Meeks.
Even when these logistical barriers are overcome, trainees and physicians have to contend with the persistent stigma associated with mental health treatment in the culture of medicine, says Dr. Gold. A recent survey by the American College of Emergency Physicians found that 73% of surveyed physicians feel there is stigma in their workplace about seeking mental health treatment. Many state medical licensing boards still require physicians to disclose mental health treatment, which discourages many trainees and providers from seeking proactive care, says Mary Moffit, PhD, associate professor of psychiatry and director of the resident and faculty wellness program at Oregon Health & Science University, Portland.
How the opt-out approach works
“The idea is by making it opt-out, you really normalize it,” says Maneesh Batra, MD, MPH, associate director of the University of Washington, Seattle, Children’s Hospital residency program. Similar approaches have proven effective at shaping human behavior in other health care settings, including boosting testing rates for HIV and increasing immunization rates for childhood vaccines, Dr. Batra says.
In general, opt-out programs acknowledge that people are busy and won’t take that extra step or click that extra button if they don’t have to, says Oana Tomescu, MD, PhD, associate professor of clinical medicine and pediatrics at the University of Pennsylvania, Philadelphia.
In 2018, Dr. Sofka and her colleagues at WVU conducted a survey that showed that a majority of residents thought favorably of their opt-out program and said they would return to counseling for follow-up care. In their most recent study, published in the Journal of Graduate Medical Education in 2021, Dr. Sofka and her colleagues found that residents did just that – only 8 of 239 opted out of universally scheduled visits. Resident-initiated visits increased significantly from zero during the 2014-2015 academic year to 23 in 2018-2019. Between those periods, program-mandated visits decreased significantly from 12 to 3.
The initiative has succeeded in creating a culture of openness and caring at WVU, says 2nd-year internal medicine resident Nistha Modi, MD. “It sets the tone for the program – we talk about mental health openly,” says Dr. Modi.
Crucially, the counselors work out of a different building than the hospital where Dr. Modi and her fellow residents work and use a separate electronic medical record system to protect resident privacy. This is hugely important for medical trainees, note Dr. Tomescu, Dr. Gold, and many other experts. The therapists understand residency and medical education, and there is no limit to the number of visits a resident or fellow can make with the program counselors, says Dr. Modi.
Opt-out programs offer a counterbalance to many negative tendencies in residency, says Dr. Meeks. “We’ve normalized so many things that are not healthy and productive. ... We need to counterbalance that with normalizing help seeking. And it’s really difficult to normalize something that’s not part of a system.”
Costs, concerns, and systematic support
Providing unlimited, free counseling for trainees can be very beneficial, but it requires adequate funding and personnel resources. Offering unlimited access means that an institution has to follow through in making this degree of care available while also ensuring that the system doesn’t get overwhelmed or is unable to accommodate very sick individuals, says Dr. Gold.
Another concern that experts like Dr. Batra, Dr. Moffit, and Dr. Gold share is that residents who go to their scheduled appointments may not completely buy into the experience because it wasn’t their idea in the first place. Participation alone doesn’t necessarily indicate full acceptance. Program personnel don’t intend for these appointments to be thought of as mandatory, yet residents may still experience them that way. Several leading resident well-being programs instead emphasize outreach to trainees, institutional support, and accessible mental health resources that are – and feel – entirely voluntary.
“If I tell someone that they have to do something, it’s very different than if they arrive at that conclusion for themselves,” says Dr. Batra. “That’s how life works.”
When it comes to cost, a recent study published in Academic Medicine provides encouraging data. At the University of Colorado, an opt-out pilot program for IM and pediatrics interns during the 2017-2018 academic year cost just $940 total, equal to $11.75 per intern. As in West Virginia, the program in Colorado covered the cost of the visit, interns were provided a half day off (whether they attended their appointment or not), and the visits and surveys were entirely optional and confidential. During the 1-year pilot program, 29% of 80 interns attended the scheduled appointment, 56% opted out in advance, and 15% didn’t show up. The majority of interns who were surveyed (85%), however, thought the program should continue and that it had a positive effect on their wellness even if they didn’t attend their appointment.
In West Virginia, program costs are higher. The program has $20,000 in annual funding to cover the opt-out program and unlimited counseling visits for residents and fellows. With that funding, Dr. Sofka and her colleagues were also able to expand the program slightly last year to schedule all the critical care faculty for counseling visits. Cost is a barrier to expanding these services to the entire institution, which Dr. Sofka says she hopes to do one day.
Research in this area is still preliminary. The WVU and Colorado studies provide some of the first evidence in support of an opt-out approach. Eventually, it would be beneficial for multicenter studies and longitudinal research to track the effects of such programs over time, say Dr. Sofka and Ajay Major, MD, MBA, one of the study’s coauthors and a hematology/oncology fellow at the University of Chicago.
Whether a program goes with an opt-out approach or not, the systematic supports – protecting resident privacy, providing flexible scheduling, and more – are crucial.
As Dr. Tomescu notes, wellness shouldn’t be just something trainees have to do. “The key with really working on burnout at a huge level is for all programs and schools to recognize that it’s a shared responsibility.”
“I felt very fortunate that I was able to get some help throughout residency,” says Dr. Modi. “About how to be a better daughter. How to be content with things I have in life. How to be happy, and grateful. With the kind of job we have, I think we sometimes forget to be grateful.”
A version of this article first appeared on Medscape.com.
Sarah Sofka, MD, FACP, noticed a pattern. As program director for the internal medicine (IM) residency at West Virginia University, Morgantown, she was informed when residents were sent to counseling because they were affected by burnout, depression, or anxiety. When trainees returned from these visits, many told her the same thing: They wished they had sought help sooner.
IM residents and their families had access to free counseling at WVU, but few used the resource, says Dr. Sofka. “So, we thought, let’s just schedule all of our residents for a therapy visit so they can go and see what it’s like,” she said. “This will hopefully decrease the stigma for seeking mental health care. If everybody’s going, it’s not a big deal.”
In July 2015, Dr. Sofka and her colleagues launched a universal well-being assessment program for the IM residents at WVU. The program leaders automatically scheduled first- and second-year residents for a visit to the faculty staff assistance program counselors. The visits were not mandatory, and residents could choose not to go; but if they did go, they received the entire day of their visit off from work.
Five and a half years after launching their program, Dr. Sofka and her colleagues conducted one of the first studies of the efficacy of an opt-out approach for resident mental wellness. They found that , suggesting that residents were seeking help proactively after having to at least consider it.
Opt-out counseling is a recent concept in residency programs – one that’s attracting interest from training programs across the country. Brown University, Providence, R.I.; the University of Colorado at Denver, Aurora; University of Pennsylvania, Philadelphia; and the University of California, San Francisco have at least one residency program that uses the approach.
Lisa Meeks, PhD, an assistant professor of family medicine at Michigan Medicine, in Ann Arbor, and other experts also believe opt-out counseling could decrease stigma and help normalize seeking care for mental health problems in the medical community while lowering the barriers for trainees who need help.
No time, no access, plenty of stigma
Burnout and mental health are known to be major concerns for health care workers, especially trainees. College graduates starting medical education have lower rates of burnout and depression, compared with demographically matched peers; however, once they’ve started training, medical students, residents, and fellows are more likely to be burned out and exhibit symptoms of depression. The ongoing COVID-19 pandemic is further fraying the well-being of overworked and traumatized health care professionals, and experts predict a mental health crisis will follow the viral crisis.
The Accreditation Council for Graduate Medical Education recently mandated that programs offer wellness services to trainees. Yet this doesn’t mean they are always used; well-known barriers stand between residents, medical students, and physicians and their receiving effective mental health treatment.
Two of the most obvious are access and time, given the grueling and often inflexible schedules of most trainees, says Jessica Gold, MD, a psychiatrist at Washington University, St. Louis, who specializes in treating medical professionals. Dr. Gold also points out that, to be done correctly, these programs require institutional support and investment – resources that aren’t always adequate.
“A lack of transparency and clear messaging around what is available, who provides the services, and how to access these services can be a major barrier,” says Erene Stergiopoulos, MD, a second-year psychiatry resident at the University of Toronto. In addition, there can be considerable lag between when a resident realizes they need help and when they manage to find a provider and schedule an appointment, says Dr. Meeks.
Even when these logistical barriers are overcome, trainees and physicians have to contend with the persistent stigma associated with mental health treatment in the culture of medicine, says Dr. Gold. A recent survey by the American College of Emergency Physicians found that 73% of surveyed physicians feel there is stigma in their workplace about seeking mental health treatment. Many state medical licensing boards still require physicians to disclose mental health treatment, which discourages many trainees and providers from seeking proactive care, says Mary Moffit, PhD, associate professor of psychiatry and director of the resident and faculty wellness program at Oregon Health & Science University, Portland.
How the opt-out approach works
“The idea is by making it opt-out, you really normalize it,” says Maneesh Batra, MD, MPH, associate director of the University of Washington, Seattle, Children’s Hospital residency program. Similar approaches have proven effective at shaping human behavior in other health care settings, including boosting testing rates for HIV and increasing immunization rates for childhood vaccines, Dr. Batra says.
In general, opt-out programs acknowledge that people are busy and won’t take that extra step or click that extra button if they don’t have to, says Oana Tomescu, MD, PhD, associate professor of clinical medicine and pediatrics at the University of Pennsylvania, Philadelphia.
In 2018, Dr. Sofka and her colleagues at WVU conducted a survey that showed that a majority of residents thought favorably of their opt-out program and said they would return to counseling for follow-up care. In their most recent study, published in the Journal of Graduate Medical Education in 2021, Dr. Sofka and her colleagues found that residents did just that – only 8 of 239 opted out of universally scheduled visits. Resident-initiated visits increased significantly from zero during the 2014-2015 academic year to 23 in 2018-2019. Between those periods, program-mandated visits decreased significantly from 12 to 3.
The initiative has succeeded in creating a culture of openness and caring at WVU, says 2nd-year internal medicine resident Nistha Modi, MD. “It sets the tone for the program – we talk about mental health openly,” says Dr. Modi.
Crucially, the counselors work out of a different building than the hospital where Dr. Modi and her fellow residents work and use a separate electronic medical record system to protect resident privacy. This is hugely important for medical trainees, note Dr. Tomescu, Dr. Gold, and many other experts. The therapists understand residency and medical education, and there is no limit to the number of visits a resident or fellow can make with the program counselors, says Dr. Modi.
Opt-out programs offer a counterbalance to many negative tendencies in residency, says Dr. Meeks. “We’ve normalized so many things that are not healthy and productive. ... We need to counterbalance that with normalizing help seeking. And it’s really difficult to normalize something that’s not part of a system.”
Costs, concerns, and systematic support
Providing unlimited, free counseling for trainees can be very beneficial, but it requires adequate funding and personnel resources. Offering unlimited access means that an institution has to follow through in making this degree of care available while also ensuring that the system doesn’t get overwhelmed or is unable to accommodate very sick individuals, says Dr. Gold.
Another concern that experts like Dr. Batra, Dr. Moffit, and Dr. Gold share is that residents who go to their scheduled appointments may not completely buy into the experience because it wasn’t their idea in the first place. Participation alone doesn’t necessarily indicate full acceptance. Program personnel don’t intend for these appointments to be thought of as mandatory, yet residents may still experience them that way. Several leading resident well-being programs instead emphasize outreach to trainees, institutional support, and accessible mental health resources that are – and feel – entirely voluntary.
“If I tell someone that they have to do something, it’s very different than if they arrive at that conclusion for themselves,” says Dr. Batra. “That’s how life works.”
When it comes to cost, a recent study published in Academic Medicine provides encouraging data. At the University of Colorado, an opt-out pilot program for IM and pediatrics interns during the 2017-2018 academic year cost just $940 total, equal to $11.75 per intern. As in West Virginia, the program in Colorado covered the cost of the visit, interns were provided a half day off (whether they attended their appointment or not), and the visits and surveys were entirely optional and confidential. During the 1-year pilot program, 29% of 80 interns attended the scheduled appointment, 56% opted out in advance, and 15% didn’t show up. The majority of interns who were surveyed (85%), however, thought the program should continue and that it had a positive effect on their wellness even if they didn’t attend their appointment.
In West Virginia, program costs are higher. The program has $20,000 in annual funding to cover the opt-out program and unlimited counseling visits for residents and fellows. With that funding, Dr. Sofka and her colleagues were also able to expand the program slightly last year to schedule all the critical care faculty for counseling visits. Cost is a barrier to expanding these services to the entire institution, which Dr. Sofka says she hopes to do one day.
Research in this area is still preliminary. The WVU and Colorado studies provide some of the first evidence in support of an opt-out approach. Eventually, it would be beneficial for multicenter studies and longitudinal research to track the effects of such programs over time, say Dr. Sofka and Ajay Major, MD, MBA, one of the study’s coauthors and a hematology/oncology fellow at the University of Chicago.
Whether a program goes with an opt-out approach or not, the systematic supports – protecting resident privacy, providing flexible scheduling, and more – are crucial.
As Dr. Tomescu notes, wellness shouldn’t be just something trainees have to do. “The key with really working on burnout at a huge level is for all programs and schools to recognize that it’s a shared responsibility.”
“I felt very fortunate that I was able to get some help throughout residency,” says Dr. Modi. “About how to be a better daughter. How to be content with things I have in life. How to be happy, and grateful. With the kind of job we have, I think we sometimes forget to be grateful.”
A version of this article first appeared on Medscape.com.
Sarah Sofka, MD, FACP, noticed a pattern. As program director for the internal medicine (IM) residency at West Virginia University, Morgantown, she was informed when residents were sent to counseling because they were affected by burnout, depression, or anxiety. When trainees returned from these visits, many told her the same thing: They wished they had sought help sooner.
IM residents and their families had access to free counseling at WVU, but few used the resource, says Dr. Sofka. “So, we thought, let’s just schedule all of our residents for a therapy visit so they can go and see what it’s like,” she said. “This will hopefully decrease the stigma for seeking mental health care. If everybody’s going, it’s not a big deal.”
In July 2015, Dr. Sofka and her colleagues launched a universal well-being assessment program for the IM residents at WVU. The program leaders automatically scheduled first- and second-year residents for a visit to the faculty staff assistance program counselors. The visits were not mandatory, and residents could choose not to go; but if they did go, they received the entire day of their visit off from work.
Five and a half years after launching their program, Dr. Sofka and her colleagues conducted one of the first studies of the efficacy of an opt-out approach for resident mental wellness. They found that , suggesting that residents were seeking help proactively after having to at least consider it.
Opt-out counseling is a recent concept in residency programs – one that’s attracting interest from training programs across the country. Brown University, Providence, R.I.; the University of Colorado at Denver, Aurora; University of Pennsylvania, Philadelphia; and the University of California, San Francisco have at least one residency program that uses the approach.
Lisa Meeks, PhD, an assistant professor of family medicine at Michigan Medicine, in Ann Arbor, and other experts also believe opt-out counseling could decrease stigma and help normalize seeking care for mental health problems in the medical community while lowering the barriers for trainees who need help.
No time, no access, plenty of stigma
Burnout and mental health are known to be major concerns for health care workers, especially trainees. College graduates starting medical education have lower rates of burnout and depression, compared with demographically matched peers; however, once they’ve started training, medical students, residents, and fellows are more likely to be burned out and exhibit symptoms of depression. The ongoing COVID-19 pandemic is further fraying the well-being of overworked and traumatized health care professionals, and experts predict a mental health crisis will follow the viral crisis.
The Accreditation Council for Graduate Medical Education recently mandated that programs offer wellness services to trainees. Yet this doesn’t mean they are always used; well-known barriers stand between residents, medical students, and physicians and their receiving effective mental health treatment.
Two of the most obvious are access and time, given the grueling and often inflexible schedules of most trainees, says Jessica Gold, MD, a psychiatrist at Washington University, St. Louis, who specializes in treating medical professionals. Dr. Gold also points out that, to be done correctly, these programs require institutional support and investment – resources that aren’t always adequate.
“A lack of transparency and clear messaging around what is available, who provides the services, and how to access these services can be a major barrier,” says Erene Stergiopoulos, MD, a second-year psychiatry resident at the University of Toronto. In addition, there can be considerable lag between when a resident realizes they need help and when they manage to find a provider and schedule an appointment, says Dr. Meeks.
Even when these logistical barriers are overcome, trainees and physicians have to contend with the persistent stigma associated with mental health treatment in the culture of medicine, says Dr. Gold. A recent survey by the American College of Emergency Physicians found that 73% of surveyed physicians feel there is stigma in their workplace about seeking mental health treatment. Many state medical licensing boards still require physicians to disclose mental health treatment, which discourages many trainees and providers from seeking proactive care, says Mary Moffit, PhD, associate professor of psychiatry and director of the resident and faculty wellness program at Oregon Health & Science University, Portland.
How the opt-out approach works
“The idea is by making it opt-out, you really normalize it,” says Maneesh Batra, MD, MPH, associate director of the University of Washington, Seattle, Children’s Hospital residency program. Similar approaches have proven effective at shaping human behavior in other health care settings, including boosting testing rates for HIV and increasing immunization rates for childhood vaccines, Dr. Batra says.
In general, opt-out programs acknowledge that people are busy and won’t take that extra step or click that extra button if they don’t have to, says Oana Tomescu, MD, PhD, associate professor of clinical medicine and pediatrics at the University of Pennsylvania, Philadelphia.
In 2018, Dr. Sofka and her colleagues at WVU conducted a survey that showed that a majority of residents thought favorably of their opt-out program and said they would return to counseling for follow-up care. In their most recent study, published in the Journal of Graduate Medical Education in 2021, Dr. Sofka and her colleagues found that residents did just that – only 8 of 239 opted out of universally scheduled visits. Resident-initiated visits increased significantly from zero during the 2014-2015 academic year to 23 in 2018-2019. Between those periods, program-mandated visits decreased significantly from 12 to 3.
The initiative has succeeded in creating a culture of openness and caring at WVU, says 2nd-year internal medicine resident Nistha Modi, MD. “It sets the tone for the program – we talk about mental health openly,” says Dr. Modi.
Crucially, the counselors work out of a different building than the hospital where Dr. Modi and her fellow residents work and use a separate electronic medical record system to protect resident privacy. This is hugely important for medical trainees, note Dr. Tomescu, Dr. Gold, and many other experts. The therapists understand residency and medical education, and there is no limit to the number of visits a resident or fellow can make with the program counselors, says Dr. Modi.
Opt-out programs offer a counterbalance to many negative tendencies in residency, says Dr. Meeks. “We’ve normalized so many things that are not healthy and productive. ... We need to counterbalance that with normalizing help seeking. And it’s really difficult to normalize something that’s not part of a system.”
Costs, concerns, and systematic support
Providing unlimited, free counseling for trainees can be very beneficial, but it requires adequate funding and personnel resources. Offering unlimited access means that an institution has to follow through in making this degree of care available while also ensuring that the system doesn’t get overwhelmed or is unable to accommodate very sick individuals, says Dr. Gold.
Another concern that experts like Dr. Batra, Dr. Moffit, and Dr. Gold share is that residents who go to their scheduled appointments may not completely buy into the experience because it wasn’t their idea in the first place. Participation alone doesn’t necessarily indicate full acceptance. Program personnel don’t intend for these appointments to be thought of as mandatory, yet residents may still experience them that way. Several leading resident well-being programs instead emphasize outreach to trainees, institutional support, and accessible mental health resources that are – and feel – entirely voluntary.
“If I tell someone that they have to do something, it’s very different than if they arrive at that conclusion for themselves,” says Dr. Batra. “That’s how life works.”
When it comes to cost, a recent study published in Academic Medicine provides encouraging data. At the University of Colorado, an opt-out pilot program for IM and pediatrics interns during the 2017-2018 academic year cost just $940 total, equal to $11.75 per intern. As in West Virginia, the program in Colorado covered the cost of the visit, interns were provided a half day off (whether they attended their appointment or not), and the visits and surveys were entirely optional and confidential. During the 1-year pilot program, 29% of 80 interns attended the scheduled appointment, 56% opted out in advance, and 15% didn’t show up. The majority of interns who were surveyed (85%), however, thought the program should continue and that it had a positive effect on their wellness even if they didn’t attend their appointment.
In West Virginia, program costs are higher. The program has $20,000 in annual funding to cover the opt-out program and unlimited counseling visits for residents and fellows. With that funding, Dr. Sofka and her colleagues were also able to expand the program slightly last year to schedule all the critical care faculty for counseling visits. Cost is a barrier to expanding these services to the entire institution, which Dr. Sofka says she hopes to do one day.
Research in this area is still preliminary. The WVU and Colorado studies provide some of the first evidence in support of an opt-out approach. Eventually, it would be beneficial for multicenter studies and longitudinal research to track the effects of such programs over time, say Dr. Sofka and Ajay Major, MD, MBA, one of the study’s coauthors and a hematology/oncology fellow at the University of Chicago.
Whether a program goes with an opt-out approach or not, the systematic supports – protecting resident privacy, providing flexible scheduling, and more – are crucial.
As Dr. Tomescu notes, wellness shouldn’t be just something trainees have to do. “The key with really working on burnout at a huge level is for all programs and schools to recognize that it’s a shared responsibility.”
“I felt very fortunate that I was able to get some help throughout residency,” says Dr. Modi. “About how to be a better daughter. How to be content with things I have in life. How to be happy, and grateful. With the kind of job we have, I think we sometimes forget to be grateful.”
A version of this article first appeared on Medscape.com.
Inpatient telemedicine can help address hospitalist pain points
COVID-19 has increased confidence in the technology
Since the advent of COVID-19, health care has seen an unprecedented rise in virtual health. Telemedicine has come to the forefront of our conversations, and there are many speculations around its future state. One such discussion is around the sustainability and expansion of inpatient telemedicine programs post COVID, and if – and how – it is going to be helpful for health care.
Consider the following scenarios:
Scenario 1
A patient presents to an emergency department of a small community hospital. He needs to be seen by a specialist, but (s)he is not available, so patient gets transferred out to the ED of a different hospital several miles away from his hometown.
He is evaluated in the second ED by the specialist, has repeat testing done – some of those tests were already completed at the first hospital. After evaluating him, the specialist recommends that he does not need to be admitted to the hospital and can be safely followed up as an outpatient. The patient does not require any further intervention and is discharged from the ED.
Scenario 2
Dr. N is a hospitalist in a rural hospital that does not have intensivist support at night. She works 7 on/7 off and is on call 24/7 during her “on” week. Dr. N cannot be physically present in the hospital 24/7. She receives messages from the hospital around the clock and feels that this call schedule is no longer sustainable. She doesn’t feel comfortable admitting patients in the ICU who come to the hospital at night without physically seeing them and without ICU backup. Therefore, some of the patients who are sick enough to be admitted in ICU for closer monitoring but can be potentially handled in this rural hospital get transferred out to a different hospital.
Dr. N has been asking the hospital to provide her intensivist back up at night and to give her some flexibility in the call schedule. However, from hospital’s perspective, the volume isn’t high enough to hire a dedicated nocturnist, and because the hospital is in the small rural area, it is having a hard time attracting more intensivists. After multiple conversations between both parties, Dr. N finally resigns.
Scenario 3
Dr. A is a specialist who is on call covering different hospitals and seeing patients in clinic. His call is getting busier. He has received many new consults and also has to follow up on his other patients in hospital who he saw a day prior.
Dr. A started receiving many pages from the hospitals – some of his patients and their families are anxiously waiting on him so that he can let them go home once he sees them, while some are waiting to know what the next steps and plan of action are. He ends up canceling some of his clinic patients who had scheduled an appointment with him 3, 4, or even 5 months ago. It’s already afternoon.
Dr. A now drives to one hospital, sees his new consults, orders tests which may or may not get results the same day, follows up on other patients, reviews their test results, modifies treatment plans for some while clearing other patients for discharge. He then drives to the other hospital and follows the same process. Some of the patients aren’t happy because of the long wait, a few couldn’t arrange for the ride to go home and ended up staying in hospital 1 extra night, while the ER is getting backlogged waiting on discharges.
These scenarios highlight some of the important and prevalent pain points in health care as shown in Figure 1.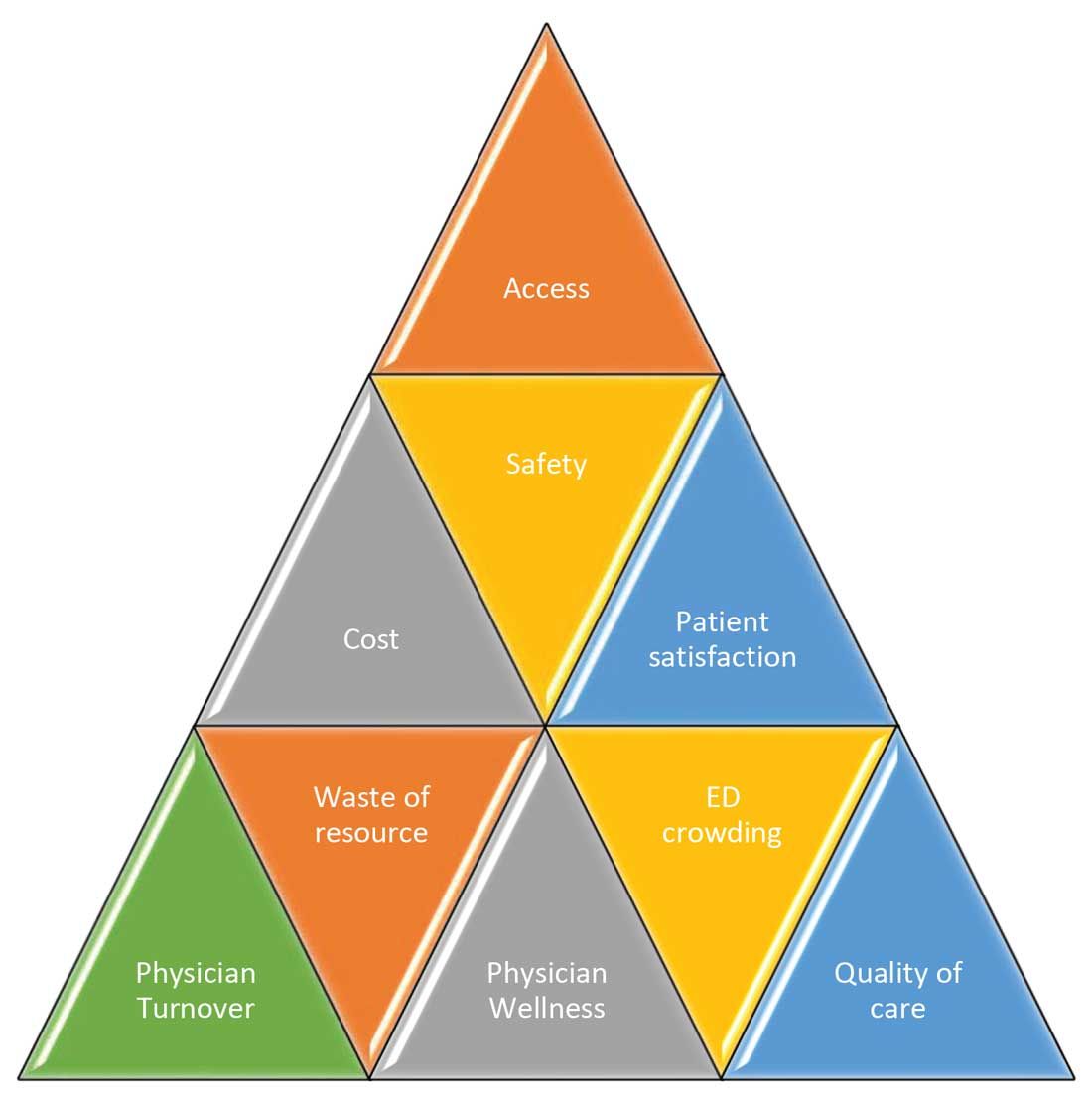
Scenario 1 and part of scenario 2 describe what is called potentially avoidable interfacility transfers. One study showed that around 8% of transferred patients (transferred from one ED to another) were discharged after ED evaluation in the second hospital, meaning they could have been retained locally without necessarily getting transferred if they could have been evaluated by the specialist.1
Transferring a patient from one hospital to another isn’t as simple as picking up a person from point A and dropping him off at point B. Rather it’s a very complicated, high-risk, capital-intensive, and time-consuming process that leads not only to excessive cost involved around transfer but also adds additional stress and burden on the patient and family. In these scenarios, having a specialist available via teleconsult could have eliminated much of this hassle and cost, allowing the patient to stay locally close to family and get access to necessary medical expertise from any part of the country in a timely manner.
Scenario 2 talks about the recruitment and retention challenges in low-volume, low-resourced locations because of call schedule and the lack of specialty support. It is reported in one study that 19% of common hospitalist admissions happen between 7:00 p.m. and 7:00 a.m. Eighty percent of admissions occurred prior to midnight. Nonrural facilities averaged 6.69 hospitalist admissions per night in that study, whereas rural facilities averaged 1.35 admissions.2 It’s like a double-edged sword for such facilities. While having a dedicated nocturnist is not a sustainable model for these hospitals, not having adequate support at night impacts physician wellness, which is already costing hospitals billions of dollars as well as leading to physician turnover: It could cost a hospital somewhere between $500,000 and $1 million to replace just one physician.3 Hence, the potential exists for a telehospitalist program in these settings to address this dilemma.
Scenario 3 sheds light on the operational issues resulting in reduced patient satisfaction and lost revenues, both on the outpatient and inpatient sides by cancellation of office visits and ED backlog. Telemedicine use in these situations can improve the turnaround time of physicians who can see some of those patients while staying at one location as they wait on other patients to show up in the clinic or wait on the operation room crew, or the procedure kit etcetera, hence improving the length of stay, ED throughput, patient satisfaction, and quality of care. This also can improve overall workflow and the wellness of physicians.
One common outcome in all these scenarios is emergency department overcrowding. There have been multiple studies that suggest that ED overcrowding can result in increased costs, lost revenues, and poor clinical outcomes, including delayed administration of antibiotics, delayed administration of analgesics to suffering patients, increased hospital length of stay, and even increased mortality.4-6 A crowded ED limits the ability of an institution to accept referrals and increases medicolegal risks. (See Figure 2.)
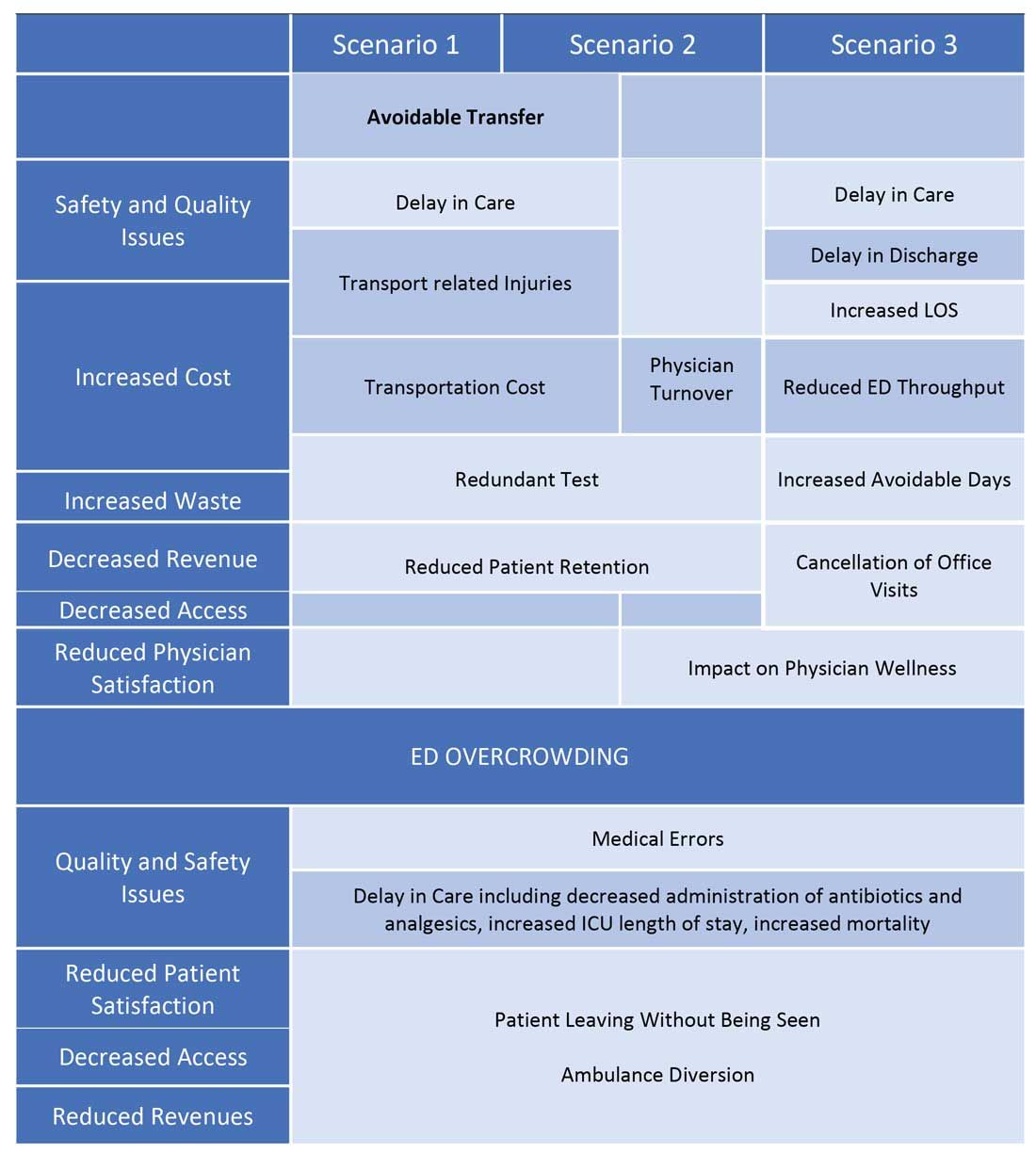
Another study showed that a 1-hour reduction in ED boarding time would result in over $9,000 of additional revenue by reducing ambulance diversion and the number of patients who left without being seen.7 Another found that using tele-emergency services can potentially result in net savings of $3,823 per avoided transfer, while accounting for the costs related to tele-emergency technology, hospital revenues, and patient-associated savings.8
There are other instances where gaps in staffing and cracks in workflow can have a negative impact on hospital operations. For example, the busier hospitals that do have a dedicated nocturnist also struggle with physician retention, since such hospitals have higher volumes and higher cross-coverage needs, and are therefore hard to manage by just one single physician at night. Since these are temporary surges, hiring another full-time nocturnist is not a viable option for the hospitals and is considered an expense in many places.
Similarly, during day shift, if a physician goes on vacation or there are surges in patient volumes, hiring a locum tenens hospitalist can be an expensive option, since the cost also includes travel and lodging. In many instances, hiring locum tenens in a given time frame is also not possible, and it leaves the physicians short staffed, fueling both physicians’ and patients’ dissatisfaction and leading to other operational and safety challenges, which I highlighted above.
Telemedicine services in these situations can provide cross-coverage while nocturnists can focus on admissions and other acute issues. Also, when physicians are on vacation or there is surge capacity (that can be forecast by using various predictive analytics models), hospitals can make plans accordingly and make use of telemedicine services. For example, Providence St. Joseph Health reported improvement in timeliness and efficiency of care after implementation of a telehospitalist program. Their 2-year study at a partner site showed a 59% improvement in patients admitted prior to midnight, about $547,000 improvement in first-day revenue capture, an increase in total revenue days and comparable patient experience scores, and a substantial increase in inpatient census and case mix index.9
Other institutions have successfully implemented some inpatient telemedicine programs – such as telepsych, telestroke, and tele-ICU – and some have also reported positive outcomes in terms of patient satisfaction, improved access, reduced length of stay in the ED, and improved quality metrics. Emory Healthcare in Atlanta reported $4.6 million savings in Medicare costs over a 15-month period from adopting a telemedicine model in the ICU, and a reduction in 60-day readmissions by 2.1%.10 Similarly, another study showed that one large health care center improved its direct contribution margins by 376% (from $7.9 million to $37.7 million) because of increased case volume, shorter lengths of stay, and higher case revenue relative to direct costs. When combined with a logistics center, they reported improved contribution margins by 665% (from $7.9 million to $60.6 million).11
There are barriers to the integration and implementation of inpatient telemedicine, including regulations, reimbursement, physician licensing, adoption of technology, and trust among staff and patients. However, I am cautiously optimistic that increased use of telehealth during the COVID-19 pandemic has allowed patients, physicians, nurses, and health care workers and leaders to gain experience with this technology, which will help them gain confidence and reduce hesitation in adapting to this new digital platform. Ultimately, the extent to which telemedicine is able to positively impact patient care will revolve around overcoming these barriers, likely through an evolution of both the technology itself and the attitudes and regulations surrounding it.
I do not suggest that telemedicine should replace the in-person encounter, but it can be implemented and used successfully in addressing the pain points in U.S. health care. (See Figure 3.)
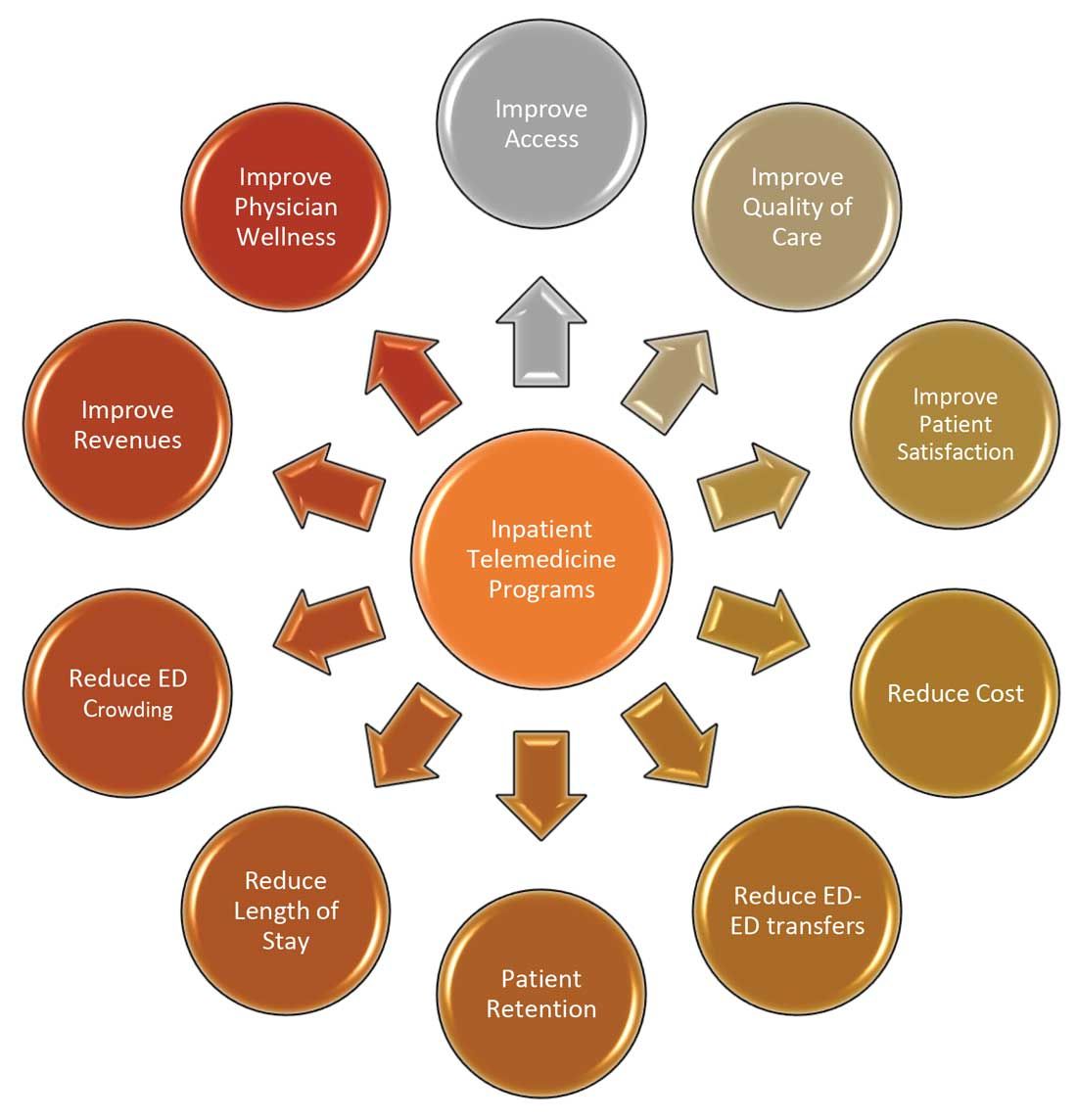
To that end, the purpose of this article is to spark discussion around different ways of implementing telemedicine in inpatient settings to solve many of the challenges that health care faces today.
Dr. Zia is an internal medicine board-certified physician, serving as a hospitalist and physician adviser in a medically underserved area. She has also served as interim medical director of the department of hospital medicine, and medical staff president, at SIH Herrin Hospital, in Herrin, Ill., part of Southern Illinois Healthcare. She has a special interest in improving access to health care in physician shortage areas.
References
1. Kindermann DR et al. Emergency department transfers and transfer relationships in United States hospitals. Acad Emerg Med. 2015 Feb;22(2):157-65.
2. Sanders RB et al. New hospital telemedicine services: Potential market for a nighttime hospitalist service. Telemed J E Health. 2014 Oct 1;20(10):902-8.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Pines JM et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007 Nov;50(5):510-6.
5. Pines JM and Hollander JE. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008 Jan;51(1):1-5.
6. Chalfin DB et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007 Jun;35(6):1477-83.
7. Pines JM et al. The financial consequences of lost demand and reducing boarding in hospital emergency departments. Ann Emerg Med. 2011 Oct;58(4):331-40.
8. Natafgi N et al. Using tele-emergency to avoid patient transfers in rural emergency. J Telemed Telecare. 2018 Apri;24(3):193-201.
9. Providence.org/telehealthhospitalistcasestudy.
10. Woodruff Health Sciences Center. CMS report: eICU program reduced hospital stays, saved millions, eased provider shortage. 2017 Apr 5.
11. Lilly CM et al. ICU telemedicine program financial outcomes. Chest. 2017 Feb;151(2):286-97.
COVID-19 has increased confidence in the technology
COVID-19 has increased confidence in the technology
Since the advent of COVID-19, health care has seen an unprecedented rise in virtual health. Telemedicine has come to the forefront of our conversations, and there are many speculations around its future state. One such discussion is around the sustainability and expansion of inpatient telemedicine programs post COVID, and if – and how – it is going to be helpful for health care.
Consider the following scenarios:
Scenario 1
A patient presents to an emergency department of a small community hospital. He needs to be seen by a specialist, but (s)he is not available, so patient gets transferred out to the ED of a different hospital several miles away from his hometown.
He is evaluated in the second ED by the specialist, has repeat testing done – some of those tests were already completed at the first hospital. After evaluating him, the specialist recommends that he does not need to be admitted to the hospital and can be safely followed up as an outpatient. The patient does not require any further intervention and is discharged from the ED.
Scenario 2
Dr. N is a hospitalist in a rural hospital that does not have intensivist support at night. She works 7 on/7 off and is on call 24/7 during her “on” week. Dr. N cannot be physically present in the hospital 24/7. She receives messages from the hospital around the clock and feels that this call schedule is no longer sustainable. She doesn’t feel comfortable admitting patients in the ICU who come to the hospital at night without physically seeing them and without ICU backup. Therefore, some of the patients who are sick enough to be admitted in ICU for closer monitoring but can be potentially handled in this rural hospital get transferred out to a different hospital.
Dr. N has been asking the hospital to provide her intensivist back up at night and to give her some flexibility in the call schedule. However, from hospital’s perspective, the volume isn’t high enough to hire a dedicated nocturnist, and because the hospital is in the small rural area, it is having a hard time attracting more intensivists. After multiple conversations between both parties, Dr. N finally resigns.
Scenario 3
Dr. A is a specialist who is on call covering different hospitals and seeing patients in clinic. His call is getting busier. He has received many new consults and also has to follow up on his other patients in hospital who he saw a day prior.
Dr. A started receiving many pages from the hospitals – some of his patients and their families are anxiously waiting on him so that he can let them go home once he sees them, while some are waiting to know what the next steps and plan of action are. He ends up canceling some of his clinic patients who had scheduled an appointment with him 3, 4, or even 5 months ago. It’s already afternoon.
Dr. A now drives to one hospital, sees his new consults, orders tests which may or may not get results the same day, follows up on other patients, reviews their test results, modifies treatment plans for some while clearing other patients for discharge. He then drives to the other hospital and follows the same process. Some of the patients aren’t happy because of the long wait, a few couldn’t arrange for the ride to go home and ended up staying in hospital 1 extra night, while the ER is getting backlogged waiting on discharges.
These scenarios highlight some of the important and prevalent pain points in health care as shown in Figure 1.
Scenario 1 and part of scenario 2 describe what is called potentially avoidable interfacility transfers. One study showed that around 8% of transferred patients (transferred from one ED to another) were discharged after ED evaluation in the second hospital, meaning they could have been retained locally without necessarily getting transferred if they could have been evaluated by the specialist.1
Transferring a patient from one hospital to another isn’t as simple as picking up a person from point A and dropping him off at point B. Rather it’s a very complicated, high-risk, capital-intensive, and time-consuming process that leads not only to excessive cost involved around transfer but also adds additional stress and burden on the patient and family. In these scenarios, having a specialist available via teleconsult could have eliminated much of this hassle and cost, allowing the patient to stay locally close to family and get access to necessary medical expertise from any part of the country in a timely manner.
Scenario 2 talks about the recruitment and retention challenges in low-volume, low-resourced locations because of call schedule and the lack of specialty support. It is reported in one study that 19% of common hospitalist admissions happen between 7:00 p.m. and 7:00 a.m. Eighty percent of admissions occurred prior to midnight. Nonrural facilities averaged 6.69 hospitalist admissions per night in that study, whereas rural facilities averaged 1.35 admissions.2 It’s like a double-edged sword for such facilities. While having a dedicated nocturnist is not a sustainable model for these hospitals, not having adequate support at night impacts physician wellness, which is already costing hospitals billions of dollars as well as leading to physician turnover: It could cost a hospital somewhere between $500,000 and $1 million to replace just one physician.3 Hence, the potential exists for a telehospitalist program in these settings to address this dilemma.
Scenario 3 sheds light on the operational issues resulting in reduced patient satisfaction and lost revenues, both on the outpatient and inpatient sides by cancellation of office visits and ED backlog. Telemedicine use in these situations can improve the turnaround time of physicians who can see some of those patients while staying at one location as they wait on other patients to show up in the clinic or wait on the operation room crew, or the procedure kit etcetera, hence improving the length of stay, ED throughput, patient satisfaction, and quality of care. This also can improve overall workflow and the wellness of physicians.
One common outcome in all these scenarios is emergency department overcrowding. There have been multiple studies that suggest that ED overcrowding can result in increased costs, lost revenues, and poor clinical outcomes, including delayed administration of antibiotics, delayed administration of analgesics to suffering patients, increased hospital length of stay, and even increased mortality.4-6 A crowded ED limits the ability of an institution to accept referrals and increases medicolegal risks. (See Figure 2.)

Another study showed that a 1-hour reduction in ED boarding time would result in over $9,000 of additional revenue by reducing ambulance diversion and the number of patients who left without being seen.7 Another found that using tele-emergency services can potentially result in net savings of $3,823 per avoided transfer, while accounting for the costs related to tele-emergency technology, hospital revenues, and patient-associated savings.8
There are other instances where gaps in staffing and cracks in workflow can have a negative impact on hospital operations. For example, the busier hospitals that do have a dedicated nocturnist also struggle with physician retention, since such hospitals have higher volumes and higher cross-coverage needs, and are therefore hard to manage by just one single physician at night. Since these are temporary surges, hiring another full-time nocturnist is not a viable option for the hospitals and is considered an expense in many places.
Similarly, during day shift, if a physician goes on vacation or there are surges in patient volumes, hiring a locum tenens hospitalist can be an expensive option, since the cost also includes travel and lodging. In many instances, hiring locum tenens in a given time frame is also not possible, and it leaves the physicians short staffed, fueling both physicians’ and patients’ dissatisfaction and leading to other operational and safety challenges, which I highlighted above.
Telemedicine services in these situations can provide cross-coverage while nocturnists can focus on admissions and other acute issues. Also, when physicians are on vacation or there is surge capacity (that can be forecast by using various predictive analytics models), hospitals can make plans accordingly and make use of telemedicine services. For example, Providence St. Joseph Health reported improvement in timeliness and efficiency of care after implementation of a telehospitalist program. Their 2-year study at a partner site showed a 59% improvement in patients admitted prior to midnight, about $547,000 improvement in first-day revenue capture, an increase in total revenue days and comparable patient experience scores, and a substantial increase in inpatient census and case mix index.9
Other institutions have successfully implemented some inpatient telemedicine programs – such as telepsych, telestroke, and tele-ICU – and some have also reported positive outcomes in terms of patient satisfaction, improved access, reduced length of stay in the ED, and improved quality metrics. Emory Healthcare in Atlanta reported $4.6 million savings in Medicare costs over a 15-month period from adopting a telemedicine model in the ICU, and a reduction in 60-day readmissions by 2.1%.10 Similarly, another study showed that one large health care center improved its direct contribution margins by 376% (from $7.9 million to $37.7 million) because of increased case volume, shorter lengths of stay, and higher case revenue relative to direct costs. When combined with a logistics center, they reported improved contribution margins by 665% (from $7.9 million to $60.6 million).11
There are barriers to the integration and implementation of inpatient telemedicine, including regulations, reimbursement, physician licensing, adoption of technology, and trust among staff and patients. However, I am cautiously optimistic that increased use of telehealth during the COVID-19 pandemic has allowed patients, physicians, nurses, and health care workers and leaders to gain experience with this technology, which will help them gain confidence and reduce hesitation in adapting to this new digital platform. Ultimately, the extent to which telemedicine is able to positively impact patient care will revolve around overcoming these barriers, likely through an evolution of both the technology itself and the attitudes and regulations surrounding it.
I do not suggest that telemedicine should replace the in-person encounter, but it can be implemented and used successfully in addressing the pain points in U.S. health care. (See Figure 3.)

To that end, the purpose of this article is to spark discussion around different ways of implementing telemedicine in inpatient settings to solve many of the challenges that health care faces today.
Dr. Zia is an internal medicine board-certified physician, serving as a hospitalist and physician adviser in a medically underserved area. She has also served as interim medical director of the department of hospital medicine, and medical staff president, at SIH Herrin Hospital, in Herrin, Ill., part of Southern Illinois Healthcare. She has a special interest in improving access to health care in physician shortage areas.
References
1. Kindermann DR et al. Emergency department transfers and transfer relationships in United States hospitals. Acad Emerg Med. 2015 Feb;22(2):157-65.
2. Sanders RB et al. New hospital telemedicine services: Potential market for a nighttime hospitalist service. Telemed J E Health. 2014 Oct 1;20(10):902-8.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Pines JM et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007 Nov;50(5):510-6.
5. Pines JM and Hollander JE. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008 Jan;51(1):1-5.
6. Chalfin DB et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007 Jun;35(6):1477-83.
7. Pines JM et al. The financial consequences of lost demand and reducing boarding in hospital emergency departments. Ann Emerg Med. 2011 Oct;58(4):331-40.
8. Natafgi N et al. Using tele-emergency to avoid patient transfers in rural emergency. J Telemed Telecare. 2018 Apri;24(3):193-201.
9. Providence.org/telehealthhospitalistcasestudy.
10. Woodruff Health Sciences Center. CMS report: eICU program reduced hospital stays, saved millions, eased provider shortage. 2017 Apr 5.
11. Lilly CM et al. ICU telemedicine program financial outcomes. Chest. 2017 Feb;151(2):286-97.
Since the advent of COVID-19, health care has seen an unprecedented rise in virtual health. Telemedicine has come to the forefront of our conversations, and there are many speculations around its future state. One such discussion is around the sustainability and expansion of inpatient telemedicine programs post COVID, and if – and how – it is going to be helpful for health care.
Consider the following scenarios:
Scenario 1
A patient presents to an emergency department of a small community hospital. He needs to be seen by a specialist, but (s)he is not available, so patient gets transferred out to the ED of a different hospital several miles away from his hometown.
He is evaluated in the second ED by the specialist, has repeat testing done – some of those tests were already completed at the first hospital. After evaluating him, the specialist recommends that he does not need to be admitted to the hospital and can be safely followed up as an outpatient. The patient does not require any further intervention and is discharged from the ED.
Scenario 2
Dr. N is a hospitalist in a rural hospital that does not have intensivist support at night. She works 7 on/7 off and is on call 24/7 during her “on” week. Dr. N cannot be physically present in the hospital 24/7. She receives messages from the hospital around the clock and feels that this call schedule is no longer sustainable. She doesn’t feel comfortable admitting patients in the ICU who come to the hospital at night without physically seeing them and without ICU backup. Therefore, some of the patients who are sick enough to be admitted in ICU for closer monitoring but can be potentially handled in this rural hospital get transferred out to a different hospital.
Dr. N has been asking the hospital to provide her intensivist back up at night and to give her some flexibility in the call schedule. However, from hospital’s perspective, the volume isn’t high enough to hire a dedicated nocturnist, and because the hospital is in the small rural area, it is having a hard time attracting more intensivists. After multiple conversations between both parties, Dr. N finally resigns.
Scenario 3
Dr. A is a specialist who is on call covering different hospitals and seeing patients in clinic. His call is getting busier. He has received many new consults and also has to follow up on his other patients in hospital who he saw a day prior.
Dr. A started receiving many pages from the hospitals – some of his patients and their families are anxiously waiting on him so that he can let them go home once he sees them, while some are waiting to know what the next steps and plan of action are. He ends up canceling some of his clinic patients who had scheduled an appointment with him 3, 4, or even 5 months ago. It’s already afternoon.
Dr. A now drives to one hospital, sees his new consults, orders tests which may or may not get results the same day, follows up on other patients, reviews their test results, modifies treatment plans for some while clearing other patients for discharge. He then drives to the other hospital and follows the same process. Some of the patients aren’t happy because of the long wait, a few couldn’t arrange for the ride to go home and ended up staying in hospital 1 extra night, while the ER is getting backlogged waiting on discharges.
These scenarios highlight some of the important and prevalent pain points in health care as shown in Figure 1.
Scenario 1 and part of scenario 2 describe what is called potentially avoidable interfacility transfers. One study showed that around 8% of transferred patients (transferred from one ED to another) were discharged after ED evaluation in the second hospital, meaning they could have been retained locally without necessarily getting transferred if they could have been evaluated by the specialist.1
Transferring a patient from one hospital to another isn’t as simple as picking up a person from point A and dropping him off at point B. Rather it’s a very complicated, high-risk, capital-intensive, and time-consuming process that leads not only to excessive cost involved around transfer but also adds additional stress and burden on the patient and family. In these scenarios, having a specialist available via teleconsult could have eliminated much of this hassle and cost, allowing the patient to stay locally close to family and get access to necessary medical expertise from any part of the country in a timely manner.
Scenario 2 talks about the recruitment and retention challenges in low-volume, low-resourced locations because of call schedule and the lack of specialty support. It is reported in one study that 19% of common hospitalist admissions happen between 7:00 p.m. and 7:00 a.m. Eighty percent of admissions occurred prior to midnight. Nonrural facilities averaged 6.69 hospitalist admissions per night in that study, whereas rural facilities averaged 1.35 admissions.2 It’s like a double-edged sword for such facilities. While having a dedicated nocturnist is not a sustainable model for these hospitals, not having adequate support at night impacts physician wellness, which is already costing hospitals billions of dollars as well as leading to physician turnover: It could cost a hospital somewhere between $500,000 and $1 million to replace just one physician.3 Hence, the potential exists for a telehospitalist program in these settings to address this dilemma.
Scenario 3 sheds light on the operational issues resulting in reduced patient satisfaction and lost revenues, both on the outpatient and inpatient sides by cancellation of office visits and ED backlog. Telemedicine use in these situations can improve the turnaround time of physicians who can see some of those patients while staying at one location as they wait on other patients to show up in the clinic or wait on the operation room crew, or the procedure kit etcetera, hence improving the length of stay, ED throughput, patient satisfaction, and quality of care. This also can improve overall workflow and the wellness of physicians.
One common outcome in all these scenarios is emergency department overcrowding. There have been multiple studies that suggest that ED overcrowding can result in increased costs, lost revenues, and poor clinical outcomes, including delayed administration of antibiotics, delayed administration of analgesics to suffering patients, increased hospital length of stay, and even increased mortality.4-6 A crowded ED limits the ability of an institution to accept referrals and increases medicolegal risks. (See Figure 2.)

Another study showed that a 1-hour reduction in ED boarding time would result in over $9,000 of additional revenue by reducing ambulance diversion and the number of patients who left without being seen.7 Another found that using tele-emergency services can potentially result in net savings of $3,823 per avoided transfer, while accounting for the costs related to tele-emergency technology, hospital revenues, and patient-associated savings.8
There are other instances where gaps in staffing and cracks in workflow can have a negative impact on hospital operations. For example, the busier hospitals that do have a dedicated nocturnist also struggle with physician retention, since such hospitals have higher volumes and higher cross-coverage needs, and are therefore hard to manage by just one single physician at night. Since these are temporary surges, hiring another full-time nocturnist is not a viable option for the hospitals and is considered an expense in many places.
Similarly, during day shift, if a physician goes on vacation or there are surges in patient volumes, hiring a locum tenens hospitalist can be an expensive option, since the cost also includes travel and lodging. In many instances, hiring locum tenens in a given time frame is also not possible, and it leaves the physicians short staffed, fueling both physicians’ and patients’ dissatisfaction and leading to other operational and safety challenges, which I highlighted above.
Telemedicine services in these situations can provide cross-coverage while nocturnists can focus on admissions and other acute issues. Also, when physicians are on vacation or there is surge capacity (that can be forecast by using various predictive analytics models), hospitals can make plans accordingly and make use of telemedicine services. For example, Providence St. Joseph Health reported improvement in timeliness and efficiency of care after implementation of a telehospitalist program. Their 2-year study at a partner site showed a 59% improvement in patients admitted prior to midnight, about $547,000 improvement in first-day revenue capture, an increase in total revenue days and comparable patient experience scores, and a substantial increase in inpatient census and case mix index.9
Other institutions have successfully implemented some inpatient telemedicine programs – such as telepsych, telestroke, and tele-ICU – and some have also reported positive outcomes in terms of patient satisfaction, improved access, reduced length of stay in the ED, and improved quality metrics. Emory Healthcare in Atlanta reported $4.6 million savings in Medicare costs over a 15-month period from adopting a telemedicine model in the ICU, and a reduction in 60-day readmissions by 2.1%.10 Similarly, another study showed that one large health care center improved its direct contribution margins by 376% (from $7.9 million to $37.7 million) because of increased case volume, shorter lengths of stay, and higher case revenue relative to direct costs. When combined with a logistics center, they reported improved contribution margins by 665% (from $7.9 million to $60.6 million).11
There are barriers to the integration and implementation of inpatient telemedicine, including regulations, reimbursement, physician licensing, adoption of technology, and trust among staff and patients. However, I am cautiously optimistic that increased use of telehealth during the COVID-19 pandemic has allowed patients, physicians, nurses, and health care workers and leaders to gain experience with this technology, which will help them gain confidence and reduce hesitation in adapting to this new digital platform. Ultimately, the extent to which telemedicine is able to positively impact patient care will revolve around overcoming these barriers, likely through an evolution of both the technology itself and the attitudes and regulations surrounding it.
I do not suggest that telemedicine should replace the in-person encounter, but it can be implemented and used successfully in addressing the pain points in U.S. health care. (See Figure 3.)

To that end, the purpose of this article is to spark discussion around different ways of implementing telemedicine in inpatient settings to solve many of the challenges that health care faces today.
Dr. Zia is an internal medicine board-certified physician, serving as a hospitalist and physician adviser in a medically underserved area. She has also served as interim medical director of the department of hospital medicine, and medical staff president, at SIH Herrin Hospital, in Herrin, Ill., part of Southern Illinois Healthcare. She has a special interest in improving access to health care in physician shortage areas.
References
1. Kindermann DR et al. Emergency department transfers and transfer relationships in United States hospitals. Acad Emerg Med. 2015 Feb;22(2):157-65.
2. Sanders RB et al. New hospital telemedicine services: Potential market for a nighttime hospitalist service. Telemed J E Health. 2014 Oct 1;20(10):902-8.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Pines JM et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007 Nov;50(5):510-6.
5. Pines JM and Hollander JE. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008 Jan;51(1):1-5.
6. Chalfin DB et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007 Jun;35(6):1477-83.
7. Pines JM et al. The financial consequences of lost demand and reducing boarding in hospital emergency departments. Ann Emerg Med. 2011 Oct;58(4):331-40.
8. Natafgi N et al. Using tele-emergency to avoid patient transfers in rural emergency. J Telemed Telecare. 2018 Apri;24(3):193-201.
9. Providence.org/telehealthhospitalistcasestudy.
10. Woodruff Health Sciences Center. CMS report: eICU program reduced hospital stays, saved millions, eased provider shortage. 2017 Apr 5.
11. Lilly CM et al. ICU telemedicine program financial outcomes. Chest. 2017 Feb;151(2):286-97.
SHM Converge to be an ‘intellectual feast’
Course director Dr. Daniel Steinberg highlights top content
The weeks leading up to our Annual Conference always trigger certain rituals for me.
Deciding which sessions to attend feels like planning an intellectual feast mixed with an exercise in compromise, as I realize there is just no way to attend every session that I want to. Scheduling all my plans to connect over dinner and drinks with current and former colleagues is a logistical challenge I undertake with anticipation and some stress, especially when I’m the one tasked with making restaurant reservations. Thinking about how to pay for it all means digging out the rules around my CME faculty allowance, after first figuring out if I still even have a CME allowance, of course.
In the years that I am presenting, there are the last-minute emails with my co-presenters to arrange a time to run through our slides together on site. The prospect of seeing cherished colleagues and friends from SHM mixes with the fact that I know I will miss my wife and young son while I am away. Overall though, I am filled with a tremendous sense of excitement, a feeling that I enjoy in a sustained way for weeks before the meeting.
My excitement for SHM Converge is just as strong, but different in some great and important ways. The availability of on-demand content means I won’t have to choose one session over another this year – I can have my cake and eat it, too. Without the need to travel, expenses will be considerably less, and I won’t need to be away from my family.
But what I am most thrilled about when I think about SHM Converge is the content. A year of planning by our outstanding SHM staff, leadership, and Annual Conference Committee has produced a lineup of world-class speakers. Our virtual platform will offer a rich interactive and networking experience. Perennial favorite sessions, such as the Great Debate, Rapid Fire, and Update sessions will provide attendees the chance to update their core clinical knowledge across the breadth of hospital medicine.
Many aspects of health equity will be explored. Over 15 sessions and four special-interest forums covering topics such as racial and gender inequities, implicit bias, vulnerable populations, and ethics will help attendees not only understand the issues but also will show them how they can take action to make a difference.
Clinical and operational aspects of COVID-19 will also be covered at SHM Converge as speakers share the tremendous innovation, triumphs, and challenges that have taken place over the past year. Wellness and resilience are, of course, as relevant as ever, and sessions on balancing parenthood and work, learning from personal failures, and how to handle uncertainty and be resilient are among the topics that will be covered.
The essence of what we will do at SHM Converge in May is captured in our new meeting logo, an animation of nodes connecting with each other through lines that travel short and long, and intersect along the way. It’s a great representation of the togetherness, community, and mutual support that is at the core of who we are as SHM – now, more than ever. Thank you for joining us!
Dr. Steinberg is chief patient safety officer at Mount Sinai Downtown, and associate dean for quality/patient safety in GME, Mount Sinai Health System, New York. He is professor of medicine and medical education at the Icahn School of Medicine at Mount Sinai, and course director of SHM Converge.
Course director Dr. Daniel Steinberg highlights top content
Course director Dr. Daniel Steinberg highlights top content
The weeks leading up to our Annual Conference always trigger certain rituals for me.
Deciding which sessions to attend feels like planning an intellectual feast mixed with an exercise in compromise, as I realize there is just no way to attend every session that I want to. Scheduling all my plans to connect over dinner and drinks with current and former colleagues is a logistical challenge I undertake with anticipation and some stress, especially when I’m the one tasked with making restaurant reservations. Thinking about how to pay for it all means digging out the rules around my CME faculty allowance, after first figuring out if I still even have a CME allowance, of course.
In the years that I am presenting, there are the last-minute emails with my co-presenters to arrange a time to run through our slides together on site. The prospect of seeing cherished colleagues and friends from SHM mixes with the fact that I know I will miss my wife and young son while I am away. Overall though, I am filled with a tremendous sense of excitement, a feeling that I enjoy in a sustained way for weeks before the meeting.
My excitement for SHM Converge is just as strong, but different in some great and important ways. The availability of on-demand content means I won’t have to choose one session over another this year – I can have my cake and eat it, too. Without the need to travel, expenses will be considerably less, and I won’t need to be away from my family.
But what I am most thrilled about when I think about SHM Converge is the content. A year of planning by our outstanding SHM staff, leadership, and Annual Conference Committee has produced a lineup of world-class speakers. Our virtual platform will offer a rich interactive and networking experience. Perennial favorite sessions, such as the Great Debate, Rapid Fire, and Update sessions will provide attendees the chance to update their core clinical knowledge across the breadth of hospital medicine.
Many aspects of health equity will be explored. Over 15 sessions and four special-interest forums covering topics such as racial and gender inequities, implicit bias, vulnerable populations, and ethics will help attendees not only understand the issues but also will show them how they can take action to make a difference.
Clinical and operational aspects of COVID-19 will also be covered at SHM Converge as speakers share the tremendous innovation, triumphs, and challenges that have taken place over the past year. Wellness and resilience are, of course, as relevant as ever, and sessions on balancing parenthood and work, learning from personal failures, and how to handle uncertainty and be resilient are among the topics that will be covered.
The essence of what we will do at SHM Converge in May is captured in our new meeting logo, an animation of nodes connecting with each other through lines that travel short and long, and intersect along the way. It’s a great representation of the togetherness, community, and mutual support that is at the core of who we are as SHM – now, more than ever. Thank you for joining us!
Dr. Steinberg is chief patient safety officer at Mount Sinai Downtown, and associate dean for quality/patient safety in GME, Mount Sinai Health System, New York. He is professor of medicine and medical education at the Icahn School of Medicine at Mount Sinai, and course director of SHM Converge.
The weeks leading up to our Annual Conference always trigger certain rituals for me.
Deciding which sessions to attend feels like planning an intellectual feast mixed with an exercise in compromise, as I realize there is just no way to attend every session that I want to. Scheduling all my plans to connect over dinner and drinks with current and former colleagues is a logistical challenge I undertake with anticipation and some stress, especially when I’m the one tasked with making restaurant reservations. Thinking about how to pay for it all means digging out the rules around my CME faculty allowance, after first figuring out if I still even have a CME allowance, of course.
In the years that I am presenting, there are the last-minute emails with my co-presenters to arrange a time to run through our slides together on site. The prospect of seeing cherished colleagues and friends from SHM mixes with the fact that I know I will miss my wife and young son while I am away. Overall though, I am filled with a tremendous sense of excitement, a feeling that I enjoy in a sustained way for weeks before the meeting.
My excitement for SHM Converge is just as strong, but different in some great and important ways. The availability of on-demand content means I won’t have to choose one session over another this year – I can have my cake and eat it, too. Without the need to travel, expenses will be considerably less, and I won’t need to be away from my family.
But what I am most thrilled about when I think about SHM Converge is the content. A year of planning by our outstanding SHM staff, leadership, and Annual Conference Committee has produced a lineup of world-class speakers. Our virtual platform will offer a rich interactive and networking experience. Perennial favorite sessions, such as the Great Debate, Rapid Fire, and Update sessions will provide attendees the chance to update their core clinical knowledge across the breadth of hospital medicine.
Many aspects of health equity will be explored. Over 15 sessions and four special-interest forums covering topics such as racial and gender inequities, implicit bias, vulnerable populations, and ethics will help attendees not only understand the issues but also will show them how they can take action to make a difference.
Clinical and operational aspects of COVID-19 will also be covered at SHM Converge as speakers share the tremendous innovation, triumphs, and challenges that have taken place over the past year. Wellness and resilience are, of course, as relevant as ever, and sessions on balancing parenthood and work, learning from personal failures, and how to handle uncertainty and be resilient are among the topics that will be covered.
The essence of what we will do at SHM Converge in May is captured in our new meeting logo, an animation of nodes connecting with each other through lines that travel short and long, and intersect along the way. It’s a great representation of the togetherness, community, and mutual support that is at the core of who we are as SHM – now, more than ever. Thank you for joining us!
Dr. Steinberg is chief patient safety officer at Mount Sinai Downtown, and associate dean for quality/patient safety in GME, Mount Sinai Health System, New York. He is professor of medicine and medical education at the Icahn School of Medicine at Mount Sinai, and course director of SHM Converge.
Racial inequity in medical education and psychiatry
The ground trembled, trees shook, and voices echoed throughout the city. I looked around in awe as the dew from my breath settled on the tip of my nose, dampening my face mask. Thousands of people with varying backgrounds, together in recognition that while the arc of the moral universe is long, it cannot bend towards justice without our help. The pain, suffering, and anger of the protestors was palpable, their chants vibrating deep in my chest, all against the backdrop of the historic Los Angeles City Hall, with rows of police officers and National Guard troops on its lawn. The countless recent racially motivated attacks and murders had driven people from all walks of life to protest for an end to systemic racism. I listened to people tell stories and challenge each other to comprehend the depths of the trauma that led us to this moment, and I went home that day curious about the history of racism in medicine.
Medicine’s roots in slavery
The uncomfortable truth is that medicine in America has some of its earliest roots in slavery. In an editorial in the New England Journal of Medicine, Evans et al1 wrote “Slaves provided economic security for physicians and clinical material that permitted the expansion of medical research, improvement of medical care, and enhancement of medical training.”1
In the 1830s, medical schools would publicize abundant access to “black clinical subjects” as a recruitment method. The Savannah Medical Journal, for example, proudly stated that Savannah Medical College had a Black patient census that “provided abundant clinical opportunities for studying disease.”2 The dehumanization of Black people was pervasive, and while racism in medical education today may be less overt because the Black community is no longer sought after as “clinical material,” discrimination continues. Ebede and Papier3 found that patients of color are extremely underrepresented in images used in medical education.
How were trainees learning to recognize clinical findings in dark-skinned patients? Was this ultimately slowing the identification and treatment of diseases in such populations?
Racism in psychiatry
In a 2020 article in Psychiatric News, American Psychiatric Association (APA) president Jeffrey Geller, MD, MPH, provided shocking insight into the history of racism in American psychiatry.4 In 1773, the Public Hospital for Persons of Insane and Disordered Minds in Williamsburg, Virginia, became the first public freestanding psychiatric hospital in British North America.4 The hospital would only accept Black patients if their admission did not interfere with the admission of White patients. Some clinicians also believed that insanity could not occur in Black people due to their “primitive nature.”4 John Galt, physician head of the hospital from 1841 to 1862 and one of the APA’s founding fathers, believed that Black people were “immune” to insanity because they did not experience the “mental excitement” that the free population experienced daily. Further, Benjamin Rush, considered the father of American psychiatry, was adamant that black skin itself was actually a disease, called negritude, and the only treatment involved turning a Black person white.4
The blasphemy is endless. John Calhoun, former vice president of the APA in the 1840s, stated “The African is incapable of self care and sinks into lunacy under the burden of freedom. It is mercy to him to give this guardianship and protection from mental health.”4
How could a population that was owned, sold, beaten, chained, raped, and ultimately dehumanized not develop mental illness? Race was weaponized by the powerful in order to deny the inalienable rights of Black people. Dr. Geller summarized these atrocities perfectly: “…during [the APA’s first 40 years] … Association members did not debate segregation by race. A few members said it shall be so, and the rest were silent—silent for a very long time.”4
While I train as a resident psychiatrist, I am learning the value of cultural sensitivity and the importance of truly understanding the background of all my patients in order to effectively treat mental illness. George Floyd’s murder is the most recent death that has shed light on systemic racism and the challenges that are largely unique to the Black community and their mental health. I recognize that combating disparities in mental health requires an honest and often uncomfortable reckoning with the role that systemic racism has played in creating these health disparities. While the trauma inflicted by centuries of injustice cannot be corrected overnight, it is our responsibility to confront these biases and barriers in medicine on a daily basis as we strive to create a more equitable society.
1. Evans MK, Rosenbaum L, Malina D, et al. Diagnosing and treating systemic racism. N Engl J Med. 2020;353:274-276.
2. Washington HA. Medical apartheid: the dark history of medical experimentation on back Americans from colonial times to the present, 1st ed. Paw Prints; 2010.
3. Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55(4):687-690.
4. Geller J. Structural racism in American psychiatry and APA: part 1. Published June 23, 2020. Accessed January 4, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2020.7a18
The ground trembled, trees shook, and voices echoed throughout the city. I looked around in awe as the dew from my breath settled on the tip of my nose, dampening my face mask. Thousands of people with varying backgrounds, together in recognition that while the arc of the moral universe is long, it cannot bend towards justice without our help. The pain, suffering, and anger of the protestors was palpable, their chants vibrating deep in my chest, all against the backdrop of the historic Los Angeles City Hall, with rows of police officers and National Guard troops on its lawn. The countless recent racially motivated attacks and murders had driven people from all walks of life to protest for an end to systemic racism. I listened to people tell stories and challenge each other to comprehend the depths of the trauma that led us to this moment, and I went home that day curious about the history of racism in medicine.
Medicine’s roots in slavery
The uncomfortable truth is that medicine in America has some of its earliest roots in slavery. In an editorial in the New England Journal of Medicine, Evans et al1 wrote “Slaves provided economic security for physicians and clinical material that permitted the expansion of medical research, improvement of medical care, and enhancement of medical training.”1
In the 1830s, medical schools would publicize abundant access to “black clinical subjects” as a recruitment method. The Savannah Medical Journal, for example, proudly stated that Savannah Medical College had a Black patient census that “provided abundant clinical opportunities for studying disease.”2 The dehumanization of Black people was pervasive, and while racism in medical education today may be less overt because the Black community is no longer sought after as “clinical material,” discrimination continues. Ebede and Papier3 found that patients of color are extremely underrepresented in images used in medical education.
How were trainees learning to recognize clinical findings in dark-skinned patients? Was this ultimately slowing the identification and treatment of diseases in such populations?
Racism in psychiatry
In a 2020 article in Psychiatric News, American Psychiatric Association (APA) president Jeffrey Geller, MD, MPH, provided shocking insight into the history of racism in American psychiatry.4 In 1773, the Public Hospital for Persons of Insane and Disordered Minds in Williamsburg, Virginia, became the first public freestanding psychiatric hospital in British North America.4 The hospital would only accept Black patients if their admission did not interfere with the admission of White patients. Some clinicians also believed that insanity could not occur in Black people due to their “primitive nature.”4 John Galt, physician head of the hospital from 1841 to 1862 and one of the APA’s founding fathers, believed that Black people were “immune” to insanity because they did not experience the “mental excitement” that the free population experienced daily. Further, Benjamin Rush, considered the father of American psychiatry, was adamant that black skin itself was actually a disease, called negritude, and the only treatment involved turning a Black person white.4
The blasphemy is endless. John Calhoun, former vice president of the APA in the 1840s, stated “The African is incapable of self care and sinks into lunacy under the burden of freedom. It is mercy to him to give this guardianship and protection from mental health.”4
How could a population that was owned, sold, beaten, chained, raped, and ultimately dehumanized not develop mental illness? Race was weaponized by the powerful in order to deny the inalienable rights of Black people. Dr. Geller summarized these atrocities perfectly: “…during [the APA’s first 40 years] … Association members did not debate segregation by race. A few members said it shall be so, and the rest were silent—silent for a very long time.”4
While I train as a resident psychiatrist, I am learning the value of cultural sensitivity and the importance of truly understanding the background of all my patients in order to effectively treat mental illness. George Floyd’s murder is the most recent death that has shed light on systemic racism and the challenges that are largely unique to the Black community and their mental health. I recognize that combating disparities in mental health requires an honest and often uncomfortable reckoning with the role that systemic racism has played in creating these health disparities. While the trauma inflicted by centuries of injustice cannot be corrected overnight, it is our responsibility to confront these biases and barriers in medicine on a daily basis as we strive to create a more equitable society.
The ground trembled, trees shook, and voices echoed throughout the city. I looked around in awe as the dew from my breath settled on the tip of my nose, dampening my face mask. Thousands of people with varying backgrounds, together in recognition that while the arc of the moral universe is long, it cannot bend towards justice without our help. The pain, suffering, and anger of the protestors was palpable, their chants vibrating deep in my chest, all against the backdrop of the historic Los Angeles City Hall, with rows of police officers and National Guard troops on its lawn. The countless recent racially motivated attacks and murders had driven people from all walks of life to protest for an end to systemic racism. I listened to people tell stories and challenge each other to comprehend the depths of the trauma that led us to this moment, and I went home that day curious about the history of racism in medicine.
Medicine’s roots in slavery
The uncomfortable truth is that medicine in America has some of its earliest roots in slavery. In an editorial in the New England Journal of Medicine, Evans et al1 wrote “Slaves provided economic security for physicians and clinical material that permitted the expansion of medical research, improvement of medical care, and enhancement of medical training.”1
In the 1830s, medical schools would publicize abundant access to “black clinical subjects” as a recruitment method. The Savannah Medical Journal, for example, proudly stated that Savannah Medical College had a Black patient census that “provided abundant clinical opportunities for studying disease.”2 The dehumanization of Black people was pervasive, and while racism in medical education today may be less overt because the Black community is no longer sought after as “clinical material,” discrimination continues. Ebede and Papier3 found that patients of color are extremely underrepresented in images used in medical education.
How were trainees learning to recognize clinical findings in dark-skinned patients? Was this ultimately slowing the identification and treatment of diseases in such populations?
Racism in psychiatry
In a 2020 article in Psychiatric News, American Psychiatric Association (APA) president Jeffrey Geller, MD, MPH, provided shocking insight into the history of racism in American psychiatry.4 In 1773, the Public Hospital for Persons of Insane and Disordered Minds in Williamsburg, Virginia, became the first public freestanding psychiatric hospital in British North America.4 The hospital would only accept Black patients if their admission did not interfere with the admission of White patients. Some clinicians also believed that insanity could not occur in Black people due to their “primitive nature.”4 John Galt, physician head of the hospital from 1841 to 1862 and one of the APA’s founding fathers, believed that Black people were “immune” to insanity because they did not experience the “mental excitement” that the free population experienced daily. Further, Benjamin Rush, considered the father of American psychiatry, was adamant that black skin itself was actually a disease, called negritude, and the only treatment involved turning a Black person white.4
The blasphemy is endless. John Calhoun, former vice president of the APA in the 1840s, stated “The African is incapable of self care and sinks into lunacy under the burden of freedom. It is mercy to him to give this guardianship and protection from mental health.”4
How could a population that was owned, sold, beaten, chained, raped, and ultimately dehumanized not develop mental illness? Race was weaponized by the powerful in order to deny the inalienable rights of Black people. Dr. Geller summarized these atrocities perfectly: “…during [the APA’s first 40 years] … Association members did not debate segregation by race. A few members said it shall be so, and the rest were silent—silent for a very long time.”4
While I train as a resident psychiatrist, I am learning the value of cultural sensitivity and the importance of truly understanding the background of all my patients in order to effectively treat mental illness. George Floyd’s murder is the most recent death that has shed light on systemic racism and the challenges that are largely unique to the Black community and their mental health. I recognize that combating disparities in mental health requires an honest and often uncomfortable reckoning with the role that systemic racism has played in creating these health disparities. While the trauma inflicted by centuries of injustice cannot be corrected overnight, it is our responsibility to confront these biases and barriers in medicine on a daily basis as we strive to create a more equitable society.
1. Evans MK, Rosenbaum L, Malina D, et al. Diagnosing and treating systemic racism. N Engl J Med. 2020;353:274-276.
2. Washington HA. Medical apartheid: the dark history of medical experimentation on back Americans from colonial times to the present, 1st ed. Paw Prints; 2010.
3. Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55(4):687-690.
4. Geller J. Structural racism in American psychiatry and APA: part 1. Published June 23, 2020. Accessed January 4, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2020.7a18
1. Evans MK, Rosenbaum L, Malina D, et al. Diagnosing and treating systemic racism. N Engl J Med. 2020;353:274-276.
2. Washington HA. Medical apartheid: the dark history of medical experimentation on back Americans from colonial times to the present, 1st ed. Paw Prints; 2010.
3. Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55(4):687-690.
4. Geller J. Structural racism in American psychiatry and APA: part 1. Published June 23, 2020. Accessed January 4, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2020.7a18
Treatment resistance is a myth!
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
Pediatric COVID-19: Data to guide practice
With the daily stream of new information, it is difficult to keep up with data on how the coronavirus epidemic affects children and school attendance, as well as how pediatricians can advise parents. The following is a summary of recently published information about birth and infant outcomes, and symptoms seen in infants and children, along with a review of recent information on transmission in schools.
COVID-19 in newborns
In November 2020, the Centers for Disease Control and Prevention published data from 16 jurisdictions detailing pregnancy and infant outcomes of more than 5,000 women with SARS-CoV-2 infection. The data were collected from March to October 2020. More than 80% of the women found to be positive for SARS-CoV-2 were identified during their third trimester. The surveillance found that 12.9% of infants born to infected mothers were born preterm, compared with an expected rate in the population of approximately 10%, suggesting that third-trimester infection may be associated with an increase in premature birth. Among 610 infants born to infected mothers and tested for SARS-CoV-2 during their nursery stay, 2.6% were positive. The infant positivity rate was as high as 4.3% among infants who were born to women with a documented SARS-CoV-2 infection within 2 weeks of the delivery date. No newborn infections were found among the infants whose mothers’ infection occurred more than 14 days before delivery. Current CDC and American Academy of Pediatrics recommendations are to test infants born to mothers with suspected or confirmed SARS-CoV-2 infection.
Data on clinical characteristics of a series of hospitalized infants in Montreal was published in December 2020. The study identified infants 0-12 months old who were diagnosed or treated at a single Montreal hospital from February until May 2020. In all, 25 (2.0%) of 1,165 infants were confirmed to have SARS-CoV-2, and approximately 8 of those were hospitalized; 85% had gastrointestinal symptoms and 81% had a fever. Upper respiratory tract symptoms were present in 59%, and none of the hospitalized infants required supplemental oxygen. The data overall support the idea that infants are generally only mildly symptomatic when infected, and respiratory symptoms do not appear to be the most prevalent finding.
COVID-19 in children
The lack of prominent respiratory symptoms among children with SARS-CoV-2 infection symptoms was echoed in another study that evaluated more than 2,400 children in Alberta, Canada. Among the 1,987 children who tested positive for SARS-CoV-2, one-third (35.9%) were asymptomatic. Some symptoms were not helpful in differentiating children who tested positive vs. those who tested negative. The frequency of muscle or joint pain, myalgia, malaise, and respiratory symptoms such as nasal congestion, difficulty breathing, and sore throat was indistinguishable between the SARS-CoV-2–infected and –noninfected children. However, anosmia was much more prevalent (7.7%) among those who tested positive for SARS-CoV-2, compared with 1.1% of those who were negative. Headache was present in 15.7% of those who were positive vs. 6.3% of those who were negative. Fever was slightly more prevalent, at 25.5% among the positive patients and 15% of the negative patients.
The authors calculated likelihood ratios for individual symptoms and found that almost all individual symptoms had likelihood ratios of 1:1.8 for testing positive. However, nausea and vomiting had a likelihood ratio of 5.5, and for anosmia it was 7.3. The combination of symptoms of nausea, nausea and vomiting, and headache produced a likelihood ratio of nearly 66. The authors suggest that these data on ambulatory children indicate that, in general, respiratory symptoms are not helpful for distinguishing patients who are likely to be positive, although the symptoms of nausea, headache, and both along with fever can be highly predictive. The authors propose that it may be more helpful for schools to focus on identifying children with combinations of these high-yield symptoms for potential testing and exclusion from school rather than on random or isolated respiratory symptoms.
COVID-19 in schools
Transmission risk in different settings is certainly something parents quiz pediatricians about, so data released in January and February 2021 may help provide some context. A CDC report on the experience of 17 schools in Wisconsin from August to November 2020 is illuminating. In that study, the SARS-CoV-2 case rate in students, school teachers, and staff members was 63% of the rate in the general public at the time, suggesting that the mitigation strategies used by the schools were effective. In addition, among the students who contracted SARS-CoV-2, only 5% of cases were attributable to school exposure. No cases of SARS-CoV-2 among faculty or staff were linked to school exposure.
Indeed, data released on Feb. 2, 2021, demonstrate that younger adults are the largest source of sustaining the epidemic. On the basis of data from August to October 2020, the opening of schools does not appear to be associated with population-level changes in SARS-CoV-2–attributable deaths. For October 2020, the authors estimate that 2.7% of infections were from children 0-9 years old, 7.1% from those ages 10-19 years, but 34% from those 20-34 years old and 38% from those 35-49 years old, by far the largest two groups contributing to spread. It should be noted that ages 20-49 years are the peak working years for adults, but the source of the data did not allow the authors to conclude whether infections were work related or social activity related. Their data do suggest that prioritizing vaccination of younger working-age adults may put more of a dent in the pandemic spread than vaccinating older individuals.
In a similar vein, a systematic review and meta-analysis of recent studies looked at household transmission of SARS-CoV-2 and demonstrated an attack rate within households of 16.6%. Of note, secondary household attack rates were only 0.7% from asymptomatic cases and 18% from symptomatic cases, with spouses and adult household contacts having higher secondary attack rates than children in the household.
COVID-19 in student athletes
A recent MMWR report described a SARS-CoV-2 outbreak associated with a series of wrestling tournaments in Florida, held in December and January 2021. While everyone would like children to be able to participate in sports, such events potentially violate several of the precepts for preventing spread: Avoid close contact and don’t mix contacts from different schools. Moreover, the events occurred during some of the highest incident case rates in the counties where the tournaments took place.
On Dec. 4, 2020, the AAP released updated guidance for athletic activities and recommended cloth face coverings for student athletes during training, in competition, while traveling, and even while waiting on the sidelines and not actively playing. Notable exceptions to the recommendation were competitive cheerleading, gymnastics, wrestling, and water sports, where the risk for entanglement from face coverings was too high or was not practical.
Taken as a whole, the evolving data continue to show that school mitigation practices can be effective in reducing the risk for SARS-CoV-2 infection. In addition, SARS-CoV-2 rates among schoolchildren more closely mirror community rates and are probably more influenced by what happens outside the schools than inside the schools.
A version of this article first appeared on Medscape.com.
With the daily stream of new information, it is difficult to keep up with data on how the coronavirus epidemic affects children and school attendance, as well as how pediatricians can advise parents. The following is a summary of recently published information about birth and infant outcomes, and symptoms seen in infants and children, along with a review of recent information on transmission in schools.
COVID-19 in newborns
In November 2020, the Centers for Disease Control and Prevention published data from 16 jurisdictions detailing pregnancy and infant outcomes of more than 5,000 women with SARS-CoV-2 infection. The data were collected from March to October 2020. More than 80% of the women found to be positive for SARS-CoV-2 were identified during their third trimester. The surveillance found that 12.9% of infants born to infected mothers were born preterm, compared with an expected rate in the population of approximately 10%, suggesting that third-trimester infection may be associated with an increase in premature birth. Among 610 infants born to infected mothers and tested for SARS-CoV-2 during their nursery stay, 2.6% were positive. The infant positivity rate was as high as 4.3% among infants who were born to women with a documented SARS-CoV-2 infection within 2 weeks of the delivery date. No newborn infections were found among the infants whose mothers’ infection occurred more than 14 days before delivery. Current CDC and American Academy of Pediatrics recommendations are to test infants born to mothers with suspected or confirmed SARS-CoV-2 infection.
Data on clinical characteristics of a series of hospitalized infants in Montreal was published in December 2020. The study identified infants 0-12 months old who were diagnosed or treated at a single Montreal hospital from February until May 2020. In all, 25 (2.0%) of 1,165 infants were confirmed to have SARS-CoV-2, and approximately 8 of those were hospitalized; 85% had gastrointestinal symptoms and 81% had a fever. Upper respiratory tract symptoms were present in 59%, and none of the hospitalized infants required supplemental oxygen. The data overall support the idea that infants are generally only mildly symptomatic when infected, and respiratory symptoms do not appear to be the most prevalent finding.
COVID-19 in children
The lack of prominent respiratory symptoms among children with SARS-CoV-2 infection symptoms was echoed in another study that evaluated more than 2,400 children in Alberta, Canada. Among the 1,987 children who tested positive for SARS-CoV-2, one-third (35.9%) were asymptomatic. Some symptoms were not helpful in differentiating children who tested positive vs. those who tested negative. The frequency of muscle or joint pain, myalgia, malaise, and respiratory symptoms such as nasal congestion, difficulty breathing, and sore throat was indistinguishable between the SARS-CoV-2–infected and –noninfected children. However, anosmia was much more prevalent (7.7%) among those who tested positive for SARS-CoV-2, compared with 1.1% of those who were negative. Headache was present in 15.7% of those who were positive vs. 6.3% of those who were negative. Fever was slightly more prevalent, at 25.5% among the positive patients and 15% of the negative patients.
The authors calculated likelihood ratios for individual symptoms and found that almost all individual symptoms had likelihood ratios of 1:1.8 for testing positive. However, nausea and vomiting had a likelihood ratio of 5.5, and for anosmia it was 7.3. The combination of symptoms of nausea, nausea and vomiting, and headache produced a likelihood ratio of nearly 66. The authors suggest that these data on ambulatory children indicate that, in general, respiratory symptoms are not helpful for distinguishing patients who are likely to be positive, although the symptoms of nausea, headache, and both along with fever can be highly predictive. The authors propose that it may be more helpful for schools to focus on identifying children with combinations of these high-yield symptoms for potential testing and exclusion from school rather than on random or isolated respiratory symptoms.
COVID-19 in schools
Transmission risk in different settings is certainly something parents quiz pediatricians about, so data released in January and February 2021 may help provide some context. A CDC report on the experience of 17 schools in Wisconsin from August to November 2020 is illuminating. In that study, the SARS-CoV-2 case rate in students, school teachers, and staff members was 63% of the rate in the general public at the time, suggesting that the mitigation strategies used by the schools were effective. In addition, among the students who contracted SARS-CoV-2, only 5% of cases were attributable to school exposure. No cases of SARS-CoV-2 among faculty or staff were linked to school exposure.
Indeed, data released on Feb. 2, 2021, demonstrate that younger adults are the largest source of sustaining the epidemic. On the basis of data from August to October 2020, the opening of schools does not appear to be associated with population-level changes in SARS-CoV-2–attributable deaths. For October 2020, the authors estimate that 2.7% of infections were from children 0-9 years old, 7.1% from those ages 10-19 years, but 34% from those 20-34 years old and 38% from those 35-49 years old, by far the largest two groups contributing to spread. It should be noted that ages 20-49 years are the peak working years for adults, but the source of the data did not allow the authors to conclude whether infections were work related or social activity related. Their data do suggest that prioritizing vaccination of younger working-age adults may put more of a dent in the pandemic spread than vaccinating older individuals.
In a similar vein, a systematic review and meta-analysis of recent studies looked at household transmission of SARS-CoV-2 and demonstrated an attack rate within households of 16.6%. Of note, secondary household attack rates were only 0.7% from asymptomatic cases and 18% from symptomatic cases, with spouses and adult household contacts having higher secondary attack rates than children in the household.
COVID-19 in student athletes
A recent MMWR report described a SARS-CoV-2 outbreak associated with a series of wrestling tournaments in Florida, held in December and January 2021. While everyone would like children to be able to participate in sports, such events potentially violate several of the precepts for preventing spread: Avoid close contact and don’t mix contacts from different schools. Moreover, the events occurred during some of the highest incident case rates in the counties where the tournaments took place.
On Dec. 4, 2020, the AAP released updated guidance for athletic activities and recommended cloth face coverings for student athletes during training, in competition, while traveling, and even while waiting on the sidelines and not actively playing. Notable exceptions to the recommendation were competitive cheerleading, gymnastics, wrestling, and water sports, where the risk for entanglement from face coverings was too high or was not practical.
Taken as a whole, the evolving data continue to show that school mitigation practices can be effective in reducing the risk for SARS-CoV-2 infection. In addition, SARS-CoV-2 rates among schoolchildren more closely mirror community rates and are probably more influenced by what happens outside the schools than inside the schools.
A version of this article first appeared on Medscape.com.
With the daily stream of new information, it is difficult to keep up with data on how the coronavirus epidemic affects children and school attendance, as well as how pediatricians can advise parents. The following is a summary of recently published information about birth and infant outcomes, and symptoms seen in infants and children, along with a review of recent information on transmission in schools.
COVID-19 in newborns
In November 2020, the Centers for Disease Control and Prevention published data from 16 jurisdictions detailing pregnancy and infant outcomes of more than 5,000 women with SARS-CoV-2 infection. The data were collected from March to October 2020. More than 80% of the women found to be positive for SARS-CoV-2 were identified during their third trimester. The surveillance found that 12.9% of infants born to infected mothers were born preterm, compared with an expected rate in the population of approximately 10%, suggesting that third-trimester infection may be associated with an increase in premature birth. Among 610 infants born to infected mothers and tested for SARS-CoV-2 during their nursery stay, 2.6% were positive. The infant positivity rate was as high as 4.3% among infants who were born to women with a documented SARS-CoV-2 infection within 2 weeks of the delivery date. No newborn infections were found among the infants whose mothers’ infection occurred more than 14 days before delivery. Current CDC and American Academy of Pediatrics recommendations are to test infants born to mothers with suspected or confirmed SARS-CoV-2 infection.
Data on clinical characteristics of a series of hospitalized infants in Montreal was published in December 2020. The study identified infants 0-12 months old who were diagnosed or treated at a single Montreal hospital from February until May 2020. In all, 25 (2.0%) of 1,165 infants were confirmed to have SARS-CoV-2, and approximately 8 of those were hospitalized; 85% had gastrointestinal symptoms and 81% had a fever. Upper respiratory tract symptoms were present in 59%, and none of the hospitalized infants required supplemental oxygen. The data overall support the idea that infants are generally only mildly symptomatic when infected, and respiratory symptoms do not appear to be the most prevalent finding.
COVID-19 in children
The lack of prominent respiratory symptoms among children with SARS-CoV-2 infection symptoms was echoed in another study that evaluated more than 2,400 children in Alberta, Canada. Among the 1,987 children who tested positive for SARS-CoV-2, one-third (35.9%) were asymptomatic. Some symptoms were not helpful in differentiating children who tested positive vs. those who tested negative. The frequency of muscle or joint pain, myalgia, malaise, and respiratory symptoms such as nasal congestion, difficulty breathing, and sore throat was indistinguishable between the SARS-CoV-2–infected and –noninfected children. However, anosmia was much more prevalent (7.7%) among those who tested positive for SARS-CoV-2, compared with 1.1% of those who were negative. Headache was present in 15.7% of those who were positive vs. 6.3% of those who were negative. Fever was slightly more prevalent, at 25.5% among the positive patients and 15% of the negative patients.
The authors calculated likelihood ratios for individual symptoms and found that almost all individual symptoms had likelihood ratios of 1:1.8 for testing positive. However, nausea and vomiting had a likelihood ratio of 5.5, and for anosmia it was 7.3. The combination of symptoms of nausea, nausea and vomiting, and headache produced a likelihood ratio of nearly 66. The authors suggest that these data on ambulatory children indicate that, in general, respiratory symptoms are not helpful for distinguishing patients who are likely to be positive, although the symptoms of nausea, headache, and both along with fever can be highly predictive. The authors propose that it may be more helpful for schools to focus on identifying children with combinations of these high-yield symptoms for potential testing and exclusion from school rather than on random or isolated respiratory symptoms.
COVID-19 in schools
Transmission risk in different settings is certainly something parents quiz pediatricians about, so data released in January and February 2021 may help provide some context. A CDC report on the experience of 17 schools in Wisconsin from August to November 2020 is illuminating. In that study, the SARS-CoV-2 case rate in students, school teachers, and staff members was 63% of the rate in the general public at the time, suggesting that the mitigation strategies used by the schools were effective. In addition, among the students who contracted SARS-CoV-2, only 5% of cases were attributable to school exposure. No cases of SARS-CoV-2 among faculty or staff were linked to school exposure.
Indeed, data released on Feb. 2, 2021, demonstrate that younger adults are the largest source of sustaining the epidemic. On the basis of data from August to October 2020, the opening of schools does not appear to be associated with population-level changes in SARS-CoV-2–attributable deaths. For October 2020, the authors estimate that 2.7% of infections were from children 0-9 years old, 7.1% from those ages 10-19 years, but 34% from those 20-34 years old and 38% from those 35-49 years old, by far the largest two groups contributing to spread. It should be noted that ages 20-49 years are the peak working years for adults, but the source of the data did not allow the authors to conclude whether infections were work related or social activity related. Their data do suggest that prioritizing vaccination of younger working-age adults may put more of a dent in the pandemic spread than vaccinating older individuals.
In a similar vein, a systematic review and meta-analysis of recent studies looked at household transmission of SARS-CoV-2 and demonstrated an attack rate within households of 16.6%. Of note, secondary household attack rates were only 0.7% from asymptomatic cases and 18% from symptomatic cases, with spouses and adult household contacts having higher secondary attack rates than children in the household.
COVID-19 in student athletes
A recent MMWR report described a SARS-CoV-2 outbreak associated with a series of wrestling tournaments in Florida, held in December and January 2021. While everyone would like children to be able to participate in sports, such events potentially violate several of the precepts for preventing spread: Avoid close contact and don’t mix contacts from different schools. Moreover, the events occurred during some of the highest incident case rates in the counties where the tournaments took place.
On Dec. 4, 2020, the AAP released updated guidance for athletic activities and recommended cloth face coverings for student athletes during training, in competition, while traveling, and even while waiting on the sidelines and not actively playing. Notable exceptions to the recommendation were competitive cheerleading, gymnastics, wrestling, and water sports, where the risk for entanglement from face coverings was too high or was not practical.
Taken as a whole, the evolving data continue to show that school mitigation practices can be effective in reducing the risk for SARS-CoV-2 infection. In addition, SARS-CoV-2 rates among schoolchildren more closely mirror community rates and are probably more influenced by what happens outside the schools than inside the schools.
A version of this article first appeared on Medscape.com.