User login
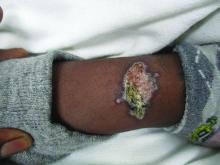
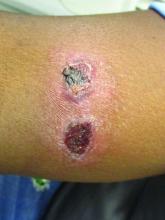
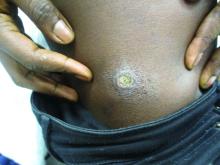
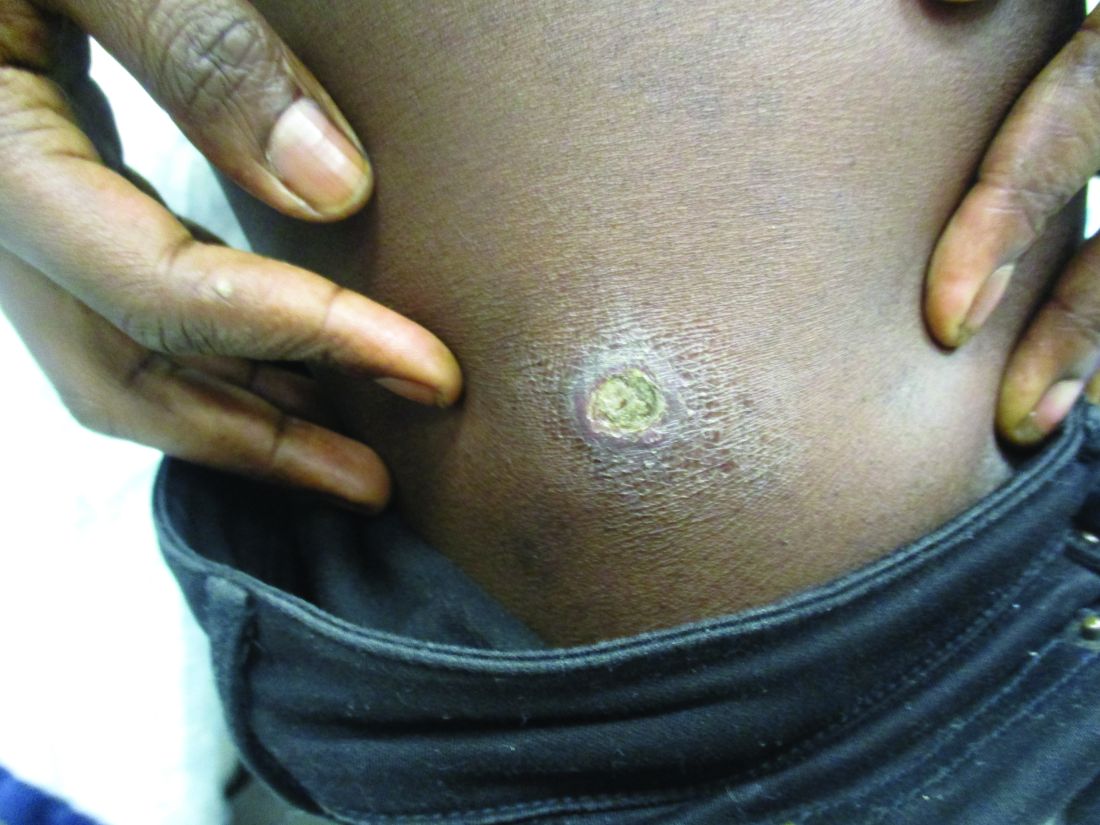
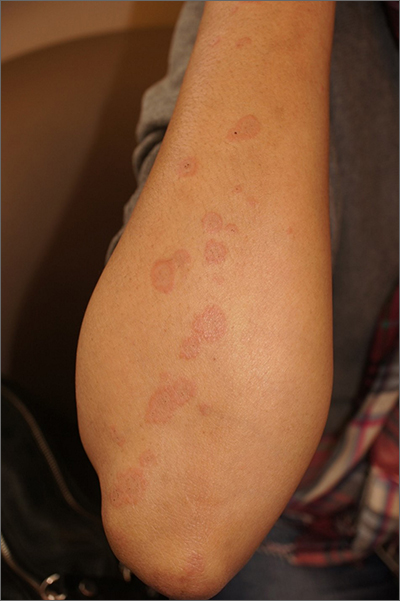
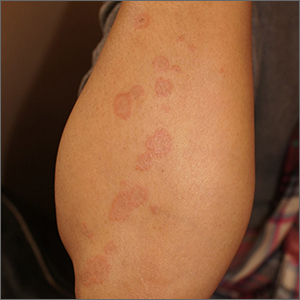
A healthy family who had been living in Brazil presented with crusted plaques on their extremities
Trypanosomatidae
Leishmaniasis is caused by protozoa of the family Trypanosomatidae, called Leishmania. The vector is a sandfly infected with the protozoa.1
The three main forms of leishmaniasis – cutaneous, mucocutaneous, or visceral – varies with the species of organism involved, the geographic distribution, and the immune response of the patient. A majority of the cases seen in the United States are from patients who contracted the disease elsewhere, particularly from Peru and Brazil.2
Lesions can vary from asymptomatic to severe. The initial lesion typically develops within weeks or months, and presents as an erythematous papule that is seen at the bite site.3 The papule evolves into a nodule or plaque that may ulcerate and crust.3 The ulcer can be distinguished by a raised and distinct border. In older stages, atrophic scarring may be seen. In some cases, the lesions may present years after exposure, because of immunosuppression or trauma.
Histology of CL reveals tuberculoid granulomas with parasitized histiocytes present. Amastigotes with distinct nuclei and kinetoplasts characterize Leishmania.2 In addition to histology, the biopsy may be sent for the press-imprint-smear method (PIS). In a study of 75 patients, the PIS method showed a higher sensitivity, as well as being a less costly and more rapid option for diagnosis.5
The treatment depends on the severity of the lesion and the species of the Leishmania genus. Mild lesions may resolve spontaneously. Topical imiquimod, cryotherapy, photodynamic therapy, and heat therapy may aid in the healing process.5 Systemic azole antifungal medications, miltefosine, and amphotericin B, and pentamidine may be used for more persistent lesions. In very severe cases, pentavalent antimonials (sodium stibogluconate, Pentostam) may be administered intravenously, although there is a high occurrence of recorded side effects.2
This case and the photos were submitted by Sabrina Liao, BS, University of California, San Diego; and Brooke Resh Sateesh, MD, San Diego Family Dermatology The case was edited by Donna Bilu Martin, MD.
References
1. Leishmaniasis – Resources for Health Professionals. Centers for Disease Control and Prevention. 2021 Jun 3.
2. Stark CG. Leishmaniasis. Medscape. 2020 Feb 18.
3. Markle WH and Makhoul K. Am Fam Physician. 2004 Mar 15;69(6):1455-604.
4. Ngan V. Leishmaniasis. DermNet NZ. 2017 Jan. 7.
5. Sousa AQ et al. Am J Trop Med Hyg. 2014 Nov;91(5):905-7.
Trypanosomatidae
Leishmaniasis is caused by protozoa of the family Trypanosomatidae, called Leishmania. The vector is a sandfly infected with the protozoa.1
The three main forms of leishmaniasis – cutaneous, mucocutaneous, or visceral – varies with the species of organism involved, the geographic distribution, and the immune response of the patient. A majority of the cases seen in the United States are from patients who contracted the disease elsewhere, particularly from Peru and Brazil.2
Lesions can vary from asymptomatic to severe. The initial lesion typically develops within weeks or months, and presents as an erythematous papule that is seen at the bite site.3 The papule evolves into a nodule or plaque that may ulcerate and crust.3 The ulcer can be distinguished by a raised and distinct border. In older stages, atrophic scarring may be seen. In some cases, the lesions may present years after exposure, because of immunosuppression or trauma.
Histology of CL reveals tuberculoid granulomas with parasitized histiocytes present. Amastigotes with distinct nuclei and kinetoplasts characterize Leishmania.2 In addition to histology, the biopsy may be sent for the press-imprint-smear method (PIS). In a study of 75 patients, the PIS method showed a higher sensitivity, as well as being a less costly and more rapid option for diagnosis.5
The treatment depends on the severity of the lesion and the species of the Leishmania genus. Mild lesions may resolve spontaneously. Topical imiquimod, cryotherapy, photodynamic therapy, and heat therapy may aid in the healing process.5 Systemic azole antifungal medications, miltefosine, and amphotericin B, and pentamidine may be used for more persistent lesions. In very severe cases, pentavalent antimonials (sodium stibogluconate, Pentostam) may be administered intravenously, although there is a high occurrence of recorded side effects.2
This case and the photos were submitted by Sabrina Liao, BS, University of California, San Diego; and Brooke Resh Sateesh, MD, San Diego Family Dermatology The case was edited by Donna Bilu Martin, MD.
References
1. Leishmaniasis – Resources for Health Professionals. Centers for Disease Control and Prevention. 2021 Jun 3.
2. Stark CG. Leishmaniasis. Medscape. 2020 Feb 18.
3. Markle WH and Makhoul K. Am Fam Physician. 2004 Mar 15;69(6):1455-604.
4. Ngan V. Leishmaniasis. DermNet NZ. 2017 Jan. 7.
5. Sousa AQ et al. Am J Trop Med Hyg. 2014 Nov;91(5):905-7.
Trypanosomatidae
Leishmaniasis is caused by protozoa of the family Trypanosomatidae, called Leishmania. The vector is a sandfly infected with the protozoa.1
The three main forms of leishmaniasis – cutaneous, mucocutaneous, or visceral – varies with the species of organism involved, the geographic distribution, and the immune response of the patient. A majority of the cases seen in the United States are from patients who contracted the disease elsewhere, particularly from Peru and Brazil.2
Lesions can vary from asymptomatic to severe. The initial lesion typically develops within weeks or months, and presents as an erythematous papule that is seen at the bite site.3 The papule evolves into a nodule or plaque that may ulcerate and crust.3 The ulcer can be distinguished by a raised and distinct border. In older stages, atrophic scarring may be seen. In some cases, the lesions may present years after exposure, because of immunosuppression or trauma.
Histology of CL reveals tuberculoid granulomas with parasitized histiocytes present. Amastigotes with distinct nuclei and kinetoplasts characterize Leishmania.2 In addition to histology, the biopsy may be sent for the press-imprint-smear method (PIS). In a study of 75 patients, the PIS method showed a higher sensitivity, as well as being a less costly and more rapid option for diagnosis.5
The treatment depends on the severity of the lesion and the species of the Leishmania genus. Mild lesions may resolve spontaneously. Topical imiquimod, cryotherapy, photodynamic therapy, and heat therapy may aid in the healing process.5 Systemic azole antifungal medications, miltefosine, and amphotericin B, and pentamidine may be used for more persistent lesions. In very severe cases, pentavalent antimonials (sodium stibogluconate, Pentostam) may be administered intravenously, although there is a high occurrence of recorded side effects.2
This case and the photos were submitted by Sabrina Liao, BS, University of California, San Diego; and Brooke Resh Sateesh, MD, San Diego Family Dermatology The case was edited by Donna Bilu Martin, MD.
References
1. Leishmaniasis – Resources for Health Professionals. Centers for Disease Control and Prevention. 2021 Jun 3.
2. Stark CG. Leishmaniasis. Medscape. 2020 Feb 18.
3. Markle WH and Makhoul K. Am Fam Physician. 2004 Mar 15;69(6):1455-604.
4. Ngan V. Leishmaniasis. DermNet NZ. 2017 Jan. 7.
5. Sousa AQ et al. Am J Trop Med Hyg. 2014 Nov;91(5):905-7.
Stop using Neutrogena and Aveeno spray sunscreen, J&J warns
Benzene is not an ingredient of sunscreen, and should not be present in these products. The levels detected were low and would not be expected to have an adverse effect on health, but the company says it is recalling the products anyway “out of an abundance of caution.”
The sunscreen products that have been recalled are:
- NEUTROGENA® Beach Defense® aerosol sunscreen.
- NEUTROGENA® Cool Dry Sport aerosol sunscreen.
- NEUTROGENA® Invisible Daily™ defense aerosol sunscreen.
- NEUTROGENA® Ultra Sheer® aerosol sunscreen.
- AVEENO® Protect + Refresh aerosol sunscreen.
These products were distributed nationwide through a variety of retail stores. Consumers should stop using these products and throw them away, the company said.
At the same time, it emphasized the importance of using alternative sunscreen products to protect the skin from excessive sun exposure, which can lead to skin cancer including melanoma.
Johnson & Johnson has launched an investigation into how benzene got into these products.
One of the company’s other spray sunscreen products, Neutrogena Wet Skin, was not included in the recall.
Recently, benzene was found in 78 widely-used sunscreen products in tests conducted by the online pharmacy and laboratory Valisure. Most of the products were aerosol sprays, and the company called on the Food and Drug Administration to recall them all.
That petition suggested that the finding of benzene was the result of contamination somewhere in the manufacturing process.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University. “We don’t want those things to be blurred.”
There is a risk that people take away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview.
He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, he said.
A version of this article first appeared on WebMD.com.
Benzene is not an ingredient of sunscreen, and should not be present in these products. The levels detected were low and would not be expected to have an adverse effect on health, but the company says it is recalling the products anyway “out of an abundance of caution.”
The sunscreen products that have been recalled are:
- NEUTROGENA® Beach Defense® aerosol sunscreen.
- NEUTROGENA® Cool Dry Sport aerosol sunscreen.
- NEUTROGENA® Invisible Daily™ defense aerosol sunscreen.
- NEUTROGENA® Ultra Sheer® aerosol sunscreen.
- AVEENO® Protect + Refresh aerosol sunscreen.
These products were distributed nationwide through a variety of retail stores. Consumers should stop using these products and throw them away, the company said.
At the same time, it emphasized the importance of using alternative sunscreen products to protect the skin from excessive sun exposure, which can lead to skin cancer including melanoma.
Johnson & Johnson has launched an investigation into how benzene got into these products.
One of the company’s other spray sunscreen products, Neutrogena Wet Skin, was not included in the recall.
Recently, benzene was found in 78 widely-used sunscreen products in tests conducted by the online pharmacy and laboratory Valisure. Most of the products were aerosol sprays, and the company called on the Food and Drug Administration to recall them all.
That petition suggested that the finding of benzene was the result of contamination somewhere in the manufacturing process.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University. “We don’t want those things to be blurred.”
There is a risk that people take away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview.
He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, he said.
A version of this article first appeared on WebMD.com.
Benzene is not an ingredient of sunscreen, and should not be present in these products. The levels detected were low and would not be expected to have an adverse effect on health, but the company says it is recalling the products anyway “out of an abundance of caution.”
The sunscreen products that have been recalled are:
- NEUTROGENA® Beach Defense® aerosol sunscreen.
- NEUTROGENA® Cool Dry Sport aerosol sunscreen.
- NEUTROGENA® Invisible Daily™ defense aerosol sunscreen.
- NEUTROGENA® Ultra Sheer® aerosol sunscreen.
- AVEENO® Protect + Refresh aerosol sunscreen.
These products were distributed nationwide through a variety of retail stores. Consumers should stop using these products and throw them away, the company said.
At the same time, it emphasized the importance of using alternative sunscreen products to protect the skin from excessive sun exposure, which can lead to skin cancer including melanoma.
Johnson & Johnson has launched an investigation into how benzene got into these products.
One of the company’s other spray sunscreen products, Neutrogena Wet Skin, was not included in the recall.
Recently, benzene was found in 78 widely-used sunscreen products in tests conducted by the online pharmacy and laboratory Valisure. Most of the products were aerosol sprays, and the company called on the Food and Drug Administration to recall them all.
That petition suggested that the finding of benzene was the result of contamination somewhere in the manufacturing process.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University. “We don’t want those things to be blurred.”
There is a risk that people take away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview.
He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, he said.
A version of this article first appeared on WebMD.com.
Pediatric alopecia areata in the U.S. has increased twofold since 2009, study finds
according to results from the largest study to date on the topic.
“Alopecia areata is a relatively common cause of nonscarring hair loss in children,” Paige McKenzie said during the annual meeting of the Society for Pediatric Dermatology. “The only two epidemiologic studies that have been performed in children have been based on registry or survey data which is inherently at risk for bias,” she added, referring to studies published in 2017 and 2018. “Additionally, epidemiologic descriptions of alopecia areata in adults are limited and overall estimates have varied from 0.2% to 2%. Current understanding is also largely based on population studies in Olmsted County, Minnesota, an area with mostly White racial demographics, so it’s not representative of the U.S. population as a whole.”
To identify the incidence and prevalence of pediatric AA over time, and across age, race/ethnicity, and sex, Ms. McKenzie and colleagues conducted a retrospective cohort study from 2009 to 2020 using PEDSnet, a network of seven U.S. pediatric health institutions with a database of more than 6.5 million children. “PEDSnet is unique because it uses a common data model to standardize EHR data across different health systems and uses SNOMED [Systematized Nomenclature of Medicine]–Clinical Terms to identify specific patient populations,” said Ms. McKenzie, who was a clinical research fellow in the section of dermatology at the Children’s Hospital of Philadelphia during the 2020-2021 academic year.
She and her coauthors limited their analysis to children younger than age 18 who were assigned a SNOMED code for AA during at least one dermatology physician visit or at least two nondermatology physician visits. They also identified an incidence cohort that was a subset of the study cohort who had at least 12 months of follow-up. “To determine the accuracy of AA patient identification, we also reviewed 100 cases at random from one institution with a threshold of greater than 95% accuracy,” said Ms. McKenzie, who is now a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas.
Of 5,409,919 children included in the study, 5,801 had AA, for an overall prevalence of 0.11%. The prevalence doubled from 0.04% in 2009 to 0.08% in 2019. “It fell in 2020, which we believe is a result of the COVID-19 pandemic’s effects on health care utilization,” she said. AA prevalence peaked at 9 years of age and was higher among females, compared with males (0.12% vs. 0.09%, respectively). The prevalence was highest among Hispanic children (0.23%), followed by Asian children (0.17%), Black children (0.12%), and White children (0.08%).
The incidence cohort consisted of 2,896,241 children. Of these, 2,398 had AA between 2009-2020, for an overall incidence of 13.6 cases per 100,000 patient-years. The incidence rate of AA by age was normally distributed and peaked at 6 years of age. Rates were 22.8% higher in female patients than in male patients. In addition, incidence rates were highest among Hispanics (31.5/100,000 person-years), followed by Asians (23.1/100,000 person-years), Blacks (17.0/100,000 person-years), and Whites (8.8/100,000).
Logistic regression analysis showed general agreement with the unadjusted incidence data. Males were less likely to be diagnosed with AA, compared with females (adjusted odds ratio, 0.80; P < .001). Analysis across race/ethnicity revealed significantly increased rates among children from minority backgrounds when compared with white children. Hispanic children had the greatest risk of developing AA (aOR, 3.07), followed by Asian children (aOR, 2.02), and Black children (aOR, 1.73) (P < .001 for all associations). Patients with atopic dermatitis, thyroid disease, psoriasis, vitiligo, and trisomy 21 prior to AA diagnosis all had a significantly higher risk of developing AA, compared with those without those diagnoses.
“This is the largest description of pediatric AA to date,” Ms. McKenzie said. “The prevalence has increased steadily, with a twofold increase over the last 10 years, which mirrors other autoimmune disorders. Children who identify as Hispanic, Asian, and Black have significantly higher incidence rates of alopecia areata compared to those who identify as White.”
Moving forward, she added, “efforts should focus on increasing education and awareness of AA in diverse communities and in community pediatricians so that patients can be diagnosed correctly early on. We can also use this data to ensure that representative populations are included in clinical trials for patients with AA.”
Asked to comment on the results Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said that the study “is a great contribution to our understanding of the epidemiology of pediatric alopecia areata and also highlights how common alopecia areata is in children.” In an interview, she said that it would be interesting to see if this is a worldwide phenomenon or unique to the United States.
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized the work as being “very informative. Looking at a large cohort of pediatric patients with alopecia areata diagnosed by a dermatologist or two or more nondermatologists, the authors found a higher incidence and prevalence in nonwhite children here in the United States. I am worried in fact, the true incidence could be even higher than noted in the searched database because nonwhite children can often come from underserved and undercared for areas.”
The other authors were Christopher B. Forrest, MD, PhD, Mitchell Maltenfort, PhD, and Leslie Castelo-Soccio, MD, PhD, of Children’s Hospital of Philadelphia. Dr. Castelo-Soccio is a consultant for Pfizer; the other authors reported having no financial disclosures. Dr. Hordinsky disclosed receiving grant support for clinical research work on hair diseases from Pfizer, Eli Lilly, Concert Pharmaceuticals, and Target Derm and grant support from the National Alopecia Areata Foundation; and is on an advisory panel for Cassiopea. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
*This story was updated on 7/19/21.
according to results from the largest study to date on the topic.
“Alopecia areata is a relatively common cause of nonscarring hair loss in children,” Paige McKenzie said during the annual meeting of the Society for Pediatric Dermatology. “The only two epidemiologic studies that have been performed in children have been based on registry or survey data which is inherently at risk for bias,” she added, referring to studies published in 2017 and 2018. “Additionally, epidemiologic descriptions of alopecia areata in adults are limited and overall estimates have varied from 0.2% to 2%. Current understanding is also largely based on population studies in Olmsted County, Minnesota, an area with mostly White racial demographics, so it’s not representative of the U.S. population as a whole.”
To identify the incidence and prevalence of pediatric AA over time, and across age, race/ethnicity, and sex, Ms. McKenzie and colleagues conducted a retrospective cohort study from 2009 to 2020 using PEDSnet, a network of seven U.S. pediatric health institutions with a database of more than 6.5 million children. “PEDSnet is unique because it uses a common data model to standardize EHR data across different health systems and uses SNOMED [Systematized Nomenclature of Medicine]–Clinical Terms to identify specific patient populations,” said Ms. McKenzie, who was a clinical research fellow in the section of dermatology at the Children’s Hospital of Philadelphia during the 2020-2021 academic year.
She and her coauthors limited their analysis to children younger than age 18 who were assigned a SNOMED code for AA during at least one dermatology physician visit or at least two nondermatology physician visits. They also identified an incidence cohort that was a subset of the study cohort who had at least 12 months of follow-up. “To determine the accuracy of AA patient identification, we also reviewed 100 cases at random from one institution with a threshold of greater than 95% accuracy,” said Ms. McKenzie, who is now a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas.
Of 5,409,919 children included in the study, 5,801 had AA, for an overall prevalence of 0.11%. The prevalence doubled from 0.04% in 2009 to 0.08% in 2019. “It fell in 2020, which we believe is a result of the COVID-19 pandemic’s effects on health care utilization,” she said. AA prevalence peaked at 9 years of age and was higher among females, compared with males (0.12% vs. 0.09%, respectively). The prevalence was highest among Hispanic children (0.23%), followed by Asian children (0.17%), Black children (0.12%), and White children (0.08%).
The incidence cohort consisted of 2,896,241 children. Of these, 2,398 had AA between 2009-2020, for an overall incidence of 13.6 cases per 100,000 patient-years. The incidence rate of AA by age was normally distributed and peaked at 6 years of age. Rates were 22.8% higher in female patients than in male patients. In addition, incidence rates were highest among Hispanics (31.5/100,000 person-years), followed by Asians (23.1/100,000 person-years), Blacks (17.0/100,000 person-years), and Whites (8.8/100,000).
Logistic regression analysis showed general agreement with the unadjusted incidence data. Males were less likely to be diagnosed with AA, compared with females (adjusted odds ratio, 0.80; P < .001). Analysis across race/ethnicity revealed significantly increased rates among children from minority backgrounds when compared with white children. Hispanic children had the greatest risk of developing AA (aOR, 3.07), followed by Asian children (aOR, 2.02), and Black children (aOR, 1.73) (P < .001 for all associations). Patients with atopic dermatitis, thyroid disease, psoriasis, vitiligo, and trisomy 21 prior to AA diagnosis all had a significantly higher risk of developing AA, compared with those without those diagnoses.
“This is the largest description of pediatric AA to date,” Ms. McKenzie said. “The prevalence has increased steadily, with a twofold increase over the last 10 years, which mirrors other autoimmune disorders. Children who identify as Hispanic, Asian, and Black have significantly higher incidence rates of alopecia areata compared to those who identify as White.”
Moving forward, she added, “efforts should focus on increasing education and awareness of AA in diverse communities and in community pediatricians so that patients can be diagnosed correctly early on. We can also use this data to ensure that representative populations are included in clinical trials for patients with AA.”
Asked to comment on the results Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said that the study “is a great contribution to our understanding of the epidemiology of pediatric alopecia areata and also highlights how common alopecia areata is in children.” In an interview, she said that it would be interesting to see if this is a worldwide phenomenon or unique to the United States.
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized the work as being “very informative. Looking at a large cohort of pediatric patients with alopecia areata diagnosed by a dermatologist or two or more nondermatologists, the authors found a higher incidence and prevalence in nonwhite children here in the United States. I am worried in fact, the true incidence could be even higher than noted in the searched database because nonwhite children can often come from underserved and undercared for areas.”
The other authors were Christopher B. Forrest, MD, PhD, Mitchell Maltenfort, PhD, and Leslie Castelo-Soccio, MD, PhD, of Children’s Hospital of Philadelphia. Dr. Castelo-Soccio is a consultant for Pfizer; the other authors reported having no financial disclosures. Dr. Hordinsky disclosed receiving grant support for clinical research work on hair diseases from Pfizer, Eli Lilly, Concert Pharmaceuticals, and Target Derm and grant support from the National Alopecia Areata Foundation; and is on an advisory panel for Cassiopea. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
*This story was updated on 7/19/21.
according to results from the largest study to date on the topic.
“Alopecia areata is a relatively common cause of nonscarring hair loss in children,” Paige McKenzie said during the annual meeting of the Society for Pediatric Dermatology. “The only two epidemiologic studies that have been performed in children have been based on registry or survey data which is inherently at risk for bias,” she added, referring to studies published in 2017 and 2018. “Additionally, epidemiologic descriptions of alopecia areata in adults are limited and overall estimates have varied from 0.2% to 2%. Current understanding is also largely based on population studies in Olmsted County, Minnesota, an area with mostly White racial demographics, so it’s not representative of the U.S. population as a whole.”
To identify the incidence and prevalence of pediatric AA over time, and across age, race/ethnicity, and sex, Ms. McKenzie and colleagues conducted a retrospective cohort study from 2009 to 2020 using PEDSnet, a network of seven U.S. pediatric health institutions with a database of more than 6.5 million children. “PEDSnet is unique because it uses a common data model to standardize EHR data across different health systems and uses SNOMED [Systematized Nomenclature of Medicine]–Clinical Terms to identify specific patient populations,” said Ms. McKenzie, who was a clinical research fellow in the section of dermatology at the Children’s Hospital of Philadelphia during the 2020-2021 academic year.
She and her coauthors limited their analysis to children younger than age 18 who were assigned a SNOMED code for AA during at least one dermatology physician visit or at least two nondermatology physician visits. They also identified an incidence cohort that was a subset of the study cohort who had at least 12 months of follow-up. “To determine the accuracy of AA patient identification, we also reviewed 100 cases at random from one institution with a threshold of greater than 95% accuracy,” said Ms. McKenzie, who is now a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas.
Of 5,409,919 children included in the study, 5,801 had AA, for an overall prevalence of 0.11%. The prevalence doubled from 0.04% in 2009 to 0.08% in 2019. “It fell in 2020, which we believe is a result of the COVID-19 pandemic’s effects on health care utilization,” she said. AA prevalence peaked at 9 years of age and was higher among females, compared with males (0.12% vs. 0.09%, respectively). The prevalence was highest among Hispanic children (0.23%), followed by Asian children (0.17%), Black children (0.12%), and White children (0.08%).
The incidence cohort consisted of 2,896,241 children. Of these, 2,398 had AA between 2009-2020, for an overall incidence of 13.6 cases per 100,000 patient-years. The incidence rate of AA by age was normally distributed and peaked at 6 years of age. Rates were 22.8% higher in female patients than in male patients. In addition, incidence rates were highest among Hispanics (31.5/100,000 person-years), followed by Asians (23.1/100,000 person-years), Blacks (17.0/100,000 person-years), and Whites (8.8/100,000).
Logistic regression analysis showed general agreement with the unadjusted incidence data. Males were less likely to be diagnosed with AA, compared with females (adjusted odds ratio, 0.80; P < .001). Analysis across race/ethnicity revealed significantly increased rates among children from minority backgrounds when compared with white children. Hispanic children had the greatest risk of developing AA (aOR, 3.07), followed by Asian children (aOR, 2.02), and Black children (aOR, 1.73) (P < .001 for all associations). Patients with atopic dermatitis, thyroid disease, psoriasis, vitiligo, and trisomy 21 prior to AA diagnosis all had a significantly higher risk of developing AA, compared with those without those diagnoses.
“This is the largest description of pediatric AA to date,” Ms. McKenzie said. “The prevalence has increased steadily, with a twofold increase over the last 10 years, which mirrors other autoimmune disorders. Children who identify as Hispanic, Asian, and Black have significantly higher incidence rates of alopecia areata compared to those who identify as White.”
Moving forward, she added, “efforts should focus on increasing education and awareness of AA in diverse communities and in community pediatricians so that patients can be diagnosed correctly early on. We can also use this data to ensure that representative populations are included in clinical trials for patients with AA.”
Asked to comment on the results Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said that the study “is a great contribution to our understanding of the epidemiology of pediatric alopecia areata and also highlights how common alopecia areata is in children.” In an interview, she said that it would be interesting to see if this is a worldwide phenomenon or unique to the United States.
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized the work as being “very informative. Looking at a large cohort of pediatric patients with alopecia areata diagnosed by a dermatologist or two or more nondermatologists, the authors found a higher incidence and prevalence in nonwhite children here in the United States. I am worried in fact, the true incidence could be even higher than noted in the searched database because nonwhite children can often come from underserved and undercared for areas.”
The other authors were Christopher B. Forrest, MD, PhD, Mitchell Maltenfort, PhD, and Leslie Castelo-Soccio, MD, PhD, of Children’s Hospital of Philadelphia. Dr. Castelo-Soccio is a consultant for Pfizer; the other authors reported having no financial disclosures. Dr. Hordinsky disclosed receiving grant support for clinical research work on hair diseases from Pfizer, Eli Lilly, Concert Pharmaceuticals, and Target Derm and grant support from the National Alopecia Areata Foundation; and is on an advisory panel for Cassiopea. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
*This story was updated on 7/19/21.
FROM SPD 2021
Targetoid eruption
The clinical features of targetoid lesions occurring soon after herpes simplex virus (HSV) infection points to a diagnosis of erythema multiforme (EM), which was confirmed by punch biopsy. The differential diagnosis for targetoid small lesions includes granuloma annulare, pityriasis rosea, and linear IgA bullous dermatosis. Larger targetoid lesions would be more concerning for erythema migrans (Lyme disease), tumid lupus, and severe tinea corporis.
Erythema multiforme represents an immune reaction triggered most often by HSV. About 10% of cases are triggered by exposure to various other viruses, drugs, and bacteria—notably, Mycoplasma pneumonia.1 Symptoms vary from mildly uncomfortable crops of annular and targetoid plaques to widespread annular plaques and bullae.
In the past, EM was considered a clinical variant along a continuum with Stevens Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN). Although mucosal involvement may occur with EM, it never progresses to SJS or TEN. The latter 2 diagnoses are associated with significant skin pain, dusky confluent patches, and a positive Nikolsky sign—wherein skin pressure causes superficial separation of the epidermis. Additionally, SJS and TEN tend to involve the trunk, whereas EM typically involves acral surfaces.
EM is self-limited but may recur in patients with additional HSV flares. Patients with frequent recurrences benefit from long-term suppression of HSV with valacyclovir 500 mg bid. Nonsteroidal anti-inflammatory drugs and cool compresses control mild pain. Itching may be relieved with topical, medium-potency steroids or oral antihistamines. Oral ulcers or lesions may be treated with lidocaine oral suspension. Systemic steroids are contraindicated for mild disease, but they have a somewhat controversial role in alleviating severe symptoms.
This patient had mild symptoms and tolerated topical triamcinolone 0.1% cream bid without recurrence at 6 months.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Trayes KP, Love G, Studdiford JS. Erythema multiforme: recognition and management. Am Fam Physician. 2019;100:82-88.
The clinical features of targetoid lesions occurring soon after herpes simplex virus (HSV) infection points to a diagnosis of erythema multiforme (EM), which was confirmed by punch biopsy. The differential diagnosis for targetoid small lesions includes granuloma annulare, pityriasis rosea, and linear IgA bullous dermatosis. Larger targetoid lesions would be more concerning for erythema migrans (Lyme disease), tumid lupus, and severe tinea corporis.
Erythema multiforme represents an immune reaction triggered most often by HSV. About 10% of cases are triggered by exposure to various other viruses, drugs, and bacteria—notably, Mycoplasma pneumonia.1 Symptoms vary from mildly uncomfortable crops of annular and targetoid plaques to widespread annular plaques and bullae.
In the past, EM was considered a clinical variant along a continuum with Stevens Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN). Although mucosal involvement may occur with EM, it never progresses to SJS or TEN. The latter 2 diagnoses are associated with significant skin pain, dusky confluent patches, and a positive Nikolsky sign—wherein skin pressure causes superficial separation of the epidermis. Additionally, SJS and TEN tend to involve the trunk, whereas EM typically involves acral surfaces.
EM is self-limited but may recur in patients with additional HSV flares. Patients with frequent recurrences benefit from long-term suppression of HSV with valacyclovir 500 mg bid. Nonsteroidal anti-inflammatory drugs and cool compresses control mild pain. Itching may be relieved with topical, medium-potency steroids or oral antihistamines. Oral ulcers or lesions may be treated with lidocaine oral suspension. Systemic steroids are contraindicated for mild disease, but they have a somewhat controversial role in alleviating severe symptoms.
This patient had mild symptoms and tolerated topical triamcinolone 0.1% cream bid without recurrence at 6 months.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
The clinical features of targetoid lesions occurring soon after herpes simplex virus (HSV) infection points to a diagnosis of erythema multiforme (EM), which was confirmed by punch biopsy. The differential diagnosis for targetoid small lesions includes granuloma annulare, pityriasis rosea, and linear IgA bullous dermatosis. Larger targetoid lesions would be more concerning for erythema migrans (Lyme disease), tumid lupus, and severe tinea corporis.
Erythema multiforme represents an immune reaction triggered most often by HSV. About 10% of cases are triggered by exposure to various other viruses, drugs, and bacteria—notably, Mycoplasma pneumonia.1 Symptoms vary from mildly uncomfortable crops of annular and targetoid plaques to widespread annular plaques and bullae.
In the past, EM was considered a clinical variant along a continuum with Stevens Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN). Although mucosal involvement may occur with EM, it never progresses to SJS or TEN. The latter 2 diagnoses are associated with significant skin pain, dusky confluent patches, and a positive Nikolsky sign—wherein skin pressure causes superficial separation of the epidermis. Additionally, SJS and TEN tend to involve the trunk, whereas EM typically involves acral surfaces.
EM is self-limited but may recur in patients with additional HSV flares. Patients with frequent recurrences benefit from long-term suppression of HSV with valacyclovir 500 mg bid. Nonsteroidal anti-inflammatory drugs and cool compresses control mild pain. Itching may be relieved with topical, medium-potency steroids or oral antihistamines. Oral ulcers or lesions may be treated with lidocaine oral suspension. Systemic steroids are contraindicated for mild disease, but they have a somewhat controversial role in alleviating severe symptoms.
This patient had mild symptoms and tolerated topical triamcinolone 0.1% cream bid without recurrence at 6 months.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Trayes KP, Love G, Studdiford JS. Erythema multiforme: recognition and management. Am Fam Physician. 2019;100:82-88.
1. Trayes KP, Love G, Studdiford JS. Erythema multiforme: recognition and management. Am Fam Physician. 2019;100:82-88.
UV light linked to prevention of allergic disease in infants
Higher direct ultraviolet light exposure in the first 3 months of life was linked to lower incidence of proinflammatory immune markers and lower incidence of eczema in an early-stage double-blind, randomized controlled trial.
Kristina Rueter, MD, with the University of Western Australia, Perth, who presented her team’s findings on Sunday at the European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress 2021, said their study is the first to demonstrate the association.
“There has been a significant rise in allergic diseases, particularly within the last 20-30 years,” Dr. Rueter noted.
“Changes to the genetic pool take thousands of years to have an impact,” she said, “so the question is why do we have the significant, very recent rise of allergic diseases?”
Suboptimal vitamin D levels during infancy, lifestyle changes, nutritional changes, and living at higher latitudes have emerged as explanations.
In this study, 195 high-risk newborns were randomized to receive oral vitamin D supplements (400 IU/day) or placebo until 6 months of age.
Researchers found that UV light exposure appears more beneficial than vitamin D supplements as an allergy prevention strategy in the critical early years of immune system development.
The researchers used a novel approach of attaching a personal UV dosimeter to the infants’ clothing to measure direct UV light exposure (290-380 nm). Vitamin D levels were measured at 3, 6, 12, and 30 months of age. Immune function was assessed at 6 months of age, and food allergy, eczema, and wheeze were assessed at 6, 12, and 30 months of age.
At 3 (P < .01) and 6 (P = .02) months of age, vitamin D levels were greater in the children who received vitamin D supplements than those who received placebo, but there was no difference in eczema incidence between groups. The finding matched those of previous studies that compared the supplements with placebo, Dr. Rueter said.
However, infants with eczema were found to have had less UV light exposure compared to those without eczema (median interquartile range [IQR], 555 J/m2 vs. 998 J/m2; P = .023).
“We also found an inverse correlation between total UV light exposure and toll-like receptor cytokine production,” Dr. Rueter said.
“The more direct UV light exposure a child got, the less the chance to develop eczema,” she said.
Researchers then extended their analysis to see whether the effect of direct UV light exposure on reduced eczema would be maintained in the first 2.5 years of life, “and we could see again a significant difference, that the children who received higher UV light exposure had less eczema,” Dr. Rueter said.
Barbara Rogala, MD, PhD, professor at the Medical University of Silesia, Katowice, Poland, told this news organization that, just as in studies on vitamin D in adult populations, there must be a balance in infant studies between potential benefit of a therapeutic strategy of vitamin D and sunlight and risk of side effects. (Dr. Rogala was not involved in Dr. Rueter’s study.)
Although vitamin D supplements are a standard part of infant care, exposure to sunlight can come with cancer risk, she noted.
Dr. Rueter agreed caution is necessary.
“You have to follow the cancer guidelines,” she said. “Sunlight may play a role in causing skin cancer, and lots of research needs to be done to find the right balance between what is a good amount which may influence the immune system in a positive way and what, on the other hand, might be too much.”
As for vitamin D supplements, Dr. Rueter said, toxic levels require “extremely high doses,” so with 400 IU/day used in the study, children are likely not being overtreated by combining sunlight and vitamin D supplements.
The study was supported by grants from Telethon–New Children’s Hospital Research Fund, Australia; Asthma Foundation of Western Australia; and the Princess Margaret Hospital Foundation, Australia. Dr. Rueter and Dr. Rogala have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher direct ultraviolet light exposure in the first 3 months of life was linked to lower incidence of proinflammatory immune markers and lower incidence of eczema in an early-stage double-blind, randomized controlled trial.
Kristina Rueter, MD, with the University of Western Australia, Perth, who presented her team’s findings on Sunday at the European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress 2021, said their study is the first to demonstrate the association.
“There has been a significant rise in allergic diseases, particularly within the last 20-30 years,” Dr. Rueter noted.
“Changes to the genetic pool take thousands of years to have an impact,” she said, “so the question is why do we have the significant, very recent rise of allergic diseases?”
Suboptimal vitamin D levels during infancy, lifestyle changes, nutritional changes, and living at higher latitudes have emerged as explanations.
In this study, 195 high-risk newborns were randomized to receive oral vitamin D supplements (400 IU/day) or placebo until 6 months of age.
Researchers found that UV light exposure appears more beneficial than vitamin D supplements as an allergy prevention strategy in the critical early years of immune system development.
The researchers used a novel approach of attaching a personal UV dosimeter to the infants’ clothing to measure direct UV light exposure (290-380 nm). Vitamin D levels were measured at 3, 6, 12, and 30 months of age. Immune function was assessed at 6 months of age, and food allergy, eczema, and wheeze were assessed at 6, 12, and 30 months of age.
At 3 (P < .01) and 6 (P = .02) months of age, vitamin D levels were greater in the children who received vitamin D supplements than those who received placebo, but there was no difference in eczema incidence between groups. The finding matched those of previous studies that compared the supplements with placebo, Dr. Rueter said.
However, infants with eczema were found to have had less UV light exposure compared to those without eczema (median interquartile range [IQR], 555 J/m2 vs. 998 J/m2; P = .023).
“We also found an inverse correlation between total UV light exposure and toll-like receptor cytokine production,” Dr. Rueter said.
“The more direct UV light exposure a child got, the less the chance to develop eczema,” she said.
Researchers then extended their analysis to see whether the effect of direct UV light exposure on reduced eczema would be maintained in the first 2.5 years of life, “and we could see again a significant difference, that the children who received higher UV light exposure had less eczema,” Dr. Rueter said.
Barbara Rogala, MD, PhD, professor at the Medical University of Silesia, Katowice, Poland, told this news organization that, just as in studies on vitamin D in adult populations, there must be a balance in infant studies between potential benefit of a therapeutic strategy of vitamin D and sunlight and risk of side effects. (Dr. Rogala was not involved in Dr. Rueter’s study.)
Although vitamin D supplements are a standard part of infant care, exposure to sunlight can come with cancer risk, she noted.
Dr. Rueter agreed caution is necessary.
“You have to follow the cancer guidelines,” she said. “Sunlight may play a role in causing skin cancer, and lots of research needs to be done to find the right balance between what is a good amount which may influence the immune system in a positive way and what, on the other hand, might be too much.”
As for vitamin D supplements, Dr. Rueter said, toxic levels require “extremely high doses,” so with 400 IU/day used in the study, children are likely not being overtreated by combining sunlight and vitamin D supplements.
The study was supported by grants from Telethon–New Children’s Hospital Research Fund, Australia; Asthma Foundation of Western Australia; and the Princess Margaret Hospital Foundation, Australia. Dr. Rueter and Dr. Rogala have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher direct ultraviolet light exposure in the first 3 months of life was linked to lower incidence of proinflammatory immune markers and lower incidence of eczema in an early-stage double-blind, randomized controlled trial.
Kristina Rueter, MD, with the University of Western Australia, Perth, who presented her team’s findings on Sunday at the European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress 2021, said their study is the first to demonstrate the association.
“There has been a significant rise in allergic diseases, particularly within the last 20-30 years,” Dr. Rueter noted.
“Changes to the genetic pool take thousands of years to have an impact,” she said, “so the question is why do we have the significant, very recent rise of allergic diseases?”
Suboptimal vitamin D levels during infancy, lifestyle changes, nutritional changes, and living at higher latitudes have emerged as explanations.
In this study, 195 high-risk newborns were randomized to receive oral vitamin D supplements (400 IU/day) or placebo until 6 months of age.
Researchers found that UV light exposure appears more beneficial than vitamin D supplements as an allergy prevention strategy in the critical early years of immune system development.
The researchers used a novel approach of attaching a personal UV dosimeter to the infants’ clothing to measure direct UV light exposure (290-380 nm). Vitamin D levels were measured at 3, 6, 12, and 30 months of age. Immune function was assessed at 6 months of age, and food allergy, eczema, and wheeze were assessed at 6, 12, and 30 months of age.
At 3 (P < .01) and 6 (P = .02) months of age, vitamin D levels were greater in the children who received vitamin D supplements than those who received placebo, but there was no difference in eczema incidence between groups. The finding matched those of previous studies that compared the supplements with placebo, Dr. Rueter said.
However, infants with eczema were found to have had less UV light exposure compared to those without eczema (median interquartile range [IQR], 555 J/m2 vs. 998 J/m2; P = .023).
“We also found an inverse correlation between total UV light exposure and toll-like receptor cytokine production,” Dr. Rueter said.
“The more direct UV light exposure a child got, the less the chance to develop eczema,” she said.
Researchers then extended their analysis to see whether the effect of direct UV light exposure on reduced eczema would be maintained in the first 2.5 years of life, “and we could see again a significant difference, that the children who received higher UV light exposure had less eczema,” Dr. Rueter said.
Barbara Rogala, MD, PhD, professor at the Medical University of Silesia, Katowice, Poland, told this news organization that, just as in studies on vitamin D in adult populations, there must be a balance in infant studies between potential benefit of a therapeutic strategy of vitamin D and sunlight and risk of side effects. (Dr. Rogala was not involved in Dr. Rueter’s study.)
Although vitamin D supplements are a standard part of infant care, exposure to sunlight can come with cancer risk, she noted.
Dr. Rueter agreed caution is necessary.
“You have to follow the cancer guidelines,” she said. “Sunlight may play a role in causing skin cancer, and lots of research needs to be done to find the right balance between what is a good amount which may influence the immune system in a positive way and what, on the other hand, might be too much.”
As for vitamin D supplements, Dr. Rueter said, toxic levels require “extremely high doses,” so with 400 IU/day used in the study, children are likely not being overtreated by combining sunlight and vitamin D supplements.
The study was supported by grants from Telethon–New Children’s Hospital Research Fund, Australia; Asthma Foundation of Western Australia; and the Princess Margaret Hospital Foundation, Australia. Dr. Rueter and Dr. Rogala have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Proposed classification framework for atopic dermatitis unveiled
The heterogeneous clinical course of atopic dermatitis (AD) and its differing signs, symptoms, burden, and response to treatment can pose a quandary for physicians.
This is behind facilitate tailoring of therapy to individual patient characteristics, and better identify therapeutically relevant disease subsets.
Dr. Silverberg, director of clinical research in the department of dermatology at George Washington University, Washington, debuted DESCRIBE-AD during the Revolutionizing Atopic Dermatitis symposium. The “D” in the mnemonic stands for dermatitis morphology and phenotype, the “E” for evolution of disease, the “S” for symptom severity, the “C” for comorbid health disorders, the “R” for response to therapy, the “I” for intensity of lesions, the “B” for burden of disease, and the “E” for extent of lesions.
At the meeting, he discussed the concepts behind each letter of the mnemonic.
Dermatitis morphology and phenotype
In the dermatitis morphology and phenotype component of DESCRIBE-AD, “there’s a lot to consider,” he said. “There are chronic signs like lichenification and prurigo nodules, which have treatment ramifications,” such as the length of time patients may need to be treated, and possibly “the use of more potent, targeted options to go after some of these lesions.”
Recent studies suggest that nummular lesions have a different underlying pathogenesis suggesting an overlap between Th2 and Th17 cell–mediated lesions. “How does that impact response to targeted therapies?” he asked. “We have no idea. We need to learn that.” He noted that psoriasiform lesions are not limited to Asian patients, but also appear in elderly patients with AD. “They look different [in elderly patients] and they may respond differently; they have more psoriasiform lesions and it’s not exactly clear why.”
Other morphologic variants of AD to be aware of include follicular eczema, xerosis, and the itch-dominant form, which Dr. Silverberg and colleagues addressed in a recently published study. “There are some patients who have milder-looking lesions, but their itch is just out of control,” he said. “This is a pattern that we need to recognize.”
Evolution of disease, symptom severity
Factors to consider for the evolution of disease component of the proposed classification include age of AD onset or disease recurrence, frequency and duration of flares, disease activity between flares, periods of disease clearance, and the overall disease trajectory. “We do get patients who say that every year their disease seems to get worse over time, for reasons that are not always clear,” Dr. Silverberg said.
Assessment tools he recommends for the symptom severity component of DESCRIBE-AD include the patient-reported global AD severity, numerical rating scale (NRS) worst or average itch in the past 7 days, the Skin Pain NRS, and the Sleep Quality NRS, which each take fewer than 30 seconds to complete. “You can have your nurses do this or can you have the patients fill out the form in the waiting area before they see you,” Dr. Silverberg said.
He also advises asking patients about the number of nights they experience sleep disturbance and if they have difficulty falling asleep or have nighttime awakenings because of their AD. Symptoms of anxiety and depression can be assessed with the Hospital Anxiety and Depression Scale and the Patient-Health Questionnaire–9, which each take 2-3 minutes to complete.
Recommended assessment tools for other symptoms – such as bleeding, oozing, and xerosis – include the Patient-Oriented Eczema Measure, which takes 2-3 minutes to complete, and the Atopic Dermatitis Control Tool or the Recap of Atopic Eczema, which each take 2-3 minutes to complete.
Comorbid health disorders
Comorbid health disorders linked to AD are varied and include atopic comorbidities such as asthma or wheeze, hay fever or oculonasal symptoms, food allergy, recurrent infections such as herpes simplex virus, mental health disorders, alopecia areata, Th1-mediated comorbidities, and adverse events to medication such as venous thromboembolism, hypertension, and impaired renal or liver function. “All of these are important because if the patients have these at baseline, they may not be good candidates for some therapies that cause these types of side effects,” Dr. Silverberg said.
Response to therapy, intensity of lesions
As for response to therapy, clinicians can ask patients, “How do you feel you’re improving?” But it’s also important to assess the signs, symptoms, frequency of flares, and comorbidities as part of that response to therapy, “and of course the adverse events and treatment burden,” he said.
For the intensity of lesions component of DESCRIBE-AD, Dr. Silverberg said that the Investigator’s Global Assessment–AD is an effective tool for clinical use. “You can also use tools like the Eczema Area and Severity Index or the Scoring AD, but recognize these are challenging,” and can be difficult to use if not well trained to use them, he said. “At the very least, do an Investigator’s Global Assessment and do a body surface area measurement.”
In his opinion, four key signs that should be assessed in clinical trials are erythema, edema/papulation, excoriation, and lichenification/prurigo nodules.
Burden of disease
In terms of assessing AD disease burden, guidelines from the American Academy of Dermatology don’t give a specific tool to use, but recommend asking open-ended questions, Dr. Silverberg said. “I would not recommend that, because when you ask an open-ended question, the flood gates open up because most patients are suffering miserably with this disease when it’s uncontrolled.
“That’s why it’s valuable to use structured, validated tools like the Dermatology Life Quality Index and the Patient Reported Outcome Measurement Information System. They don’t take a lot of time to complete, and you can look at the score and determine how burdensome their disease is, even in a busy clinical practice. They’re not going to slow you down; they’re going to speed you up and make you better at your therapeutic decision-making. I can guarantee you that most patients will love you for it. Sometimes patients say to me, ‘you’re the first doctor to ask these questions.’ ”
Extent of disease
Finally, for the extent of disease component of DESCRIBE-AD, he emphasized the importance of doing a full-body exam to appreciate the affected body surface area, flexural versus extensor distribution, and involvement and severity of disease on special sites such as the face, hands, feet, genitals, and scalp.
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
The heterogeneous clinical course of atopic dermatitis (AD) and its differing signs, symptoms, burden, and response to treatment can pose a quandary for physicians.
This is behind facilitate tailoring of therapy to individual patient characteristics, and better identify therapeutically relevant disease subsets.
Dr. Silverberg, director of clinical research in the department of dermatology at George Washington University, Washington, debuted DESCRIBE-AD during the Revolutionizing Atopic Dermatitis symposium. The “D” in the mnemonic stands for dermatitis morphology and phenotype, the “E” for evolution of disease, the “S” for symptom severity, the “C” for comorbid health disorders, the “R” for response to therapy, the “I” for intensity of lesions, the “B” for burden of disease, and the “E” for extent of lesions.
At the meeting, he discussed the concepts behind each letter of the mnemonic.
Dermatitis morphology and phenotype
In the dermatitis morphology and phenotype component of DESCRIBE-AD, “there’s a lot to consider,” he said. “There are chronic signs like lichenification and prurigo nodules, which have treatment ramifications,” such as the length of time patients may need to be treated, and possibly “the use of more potent, targeted options to go after some of these lesions.”
Recent studies suggest that nummular lesions have a different underlying pathogenesis suggesting an overlap between Th2 and Th17 cell–mediated lesions. “How does that impact response to targeted therapies?” he asked. “We have no idea. We need to learn that.” He noted that psoriasiform lesions are not limited to Asian patients, but also appear in elderly patients with AD. “They look different [in elderly patients] and they may respond differently; they have more psoriasiform lesions and it’s not exactly clear why.”
Other morphologic variants of AD to be aware of include follicular eczema, xerosis, and the itch-dominant form, which Dr. Silverberg and colleagues addressed in a recently published study. “There are some patients who have milder-looking lesions, but their itch is just out of control,” he said. “This is a pattern that we need to recognize.”
Evolution of disease, symptom severity
Factors to consider for the evolution of disease component of the proposed classification include age of AD onset or disease recurrence, frequency and duration of flares, disease activity between flares, periods of disease clearance, and the overall disease trajectory. “We do get patients who say that every year their disease seems to get worse over time, for reasons that are not always clear,” Dr. Silverberg said.
Assessment tools he recommends for the symptom severity component of DESCRIBE-AD include the patient-reported global AD severity, numerical rating scale (NRS) worst or average itch in the past 7 days, the Skin Pain NRS, and the Sleep Quality NRS, which each take fewer than 30 seconds to complete. “You can have your nurses do this or can you have the patients fill out the form in the waiting area before they see you,” Dr. Silverberg said.
He also advises asking patients about the number of nights they experience sleep disturbance and if they have difficulty falling asleep or have nighttime awakenings because of their AD. Symptoms of anxiety and depression can be assessed with the Hospital Anxiety and Depression Scale and the Patient-Health Questionnaire–9, which each take 2-3 minutes to complete.
Recommended assessment tools for other symptoms – such as bleeding, oozing, and xerosis – include the Patient-Oriented Eczema Measure, which takes 2-3 minutes to complete, and the Atopic Dermatitis Control Tool or the Recap of Atopic Eczema, which each take 2-3 minutes to complete.
Comorbid health disorders
Comorbid health disorders linked to AD are varied and include atopic comorbidities such as asthma or wheeze, hay fever or oculonasal symptoms, food allergy, recurrent infections such as herpes simplex virus, mental health disorders, alopecia areata, Th1-mediated comorbidities, and adverse events to medication such as venous thromboembolism, hypertension, and impaired renal or liver function. “All of these are important because if the patients have these at baseline, they may not be good candidates for some therapies that cause these types of side effects,” Dr. Silverberg said.
Response to therapy, intensity of lesions
As for response to therapy, clinicians can ask patients, “How do you feel you’re improving?” But it’s also important to assess the signs, symptoms, frequency of flares, and comorbidities as part of that response to therapy, “and of course the adverse events and treatment burden,” he said.
For the intensity of lesions component of DESCRIBE-AD, Dr. Silverberg said that the Investigator’s Global Assessment–AD is an effective tool for clinical use. “You can also use tools like the Eczema Area and Severity Index or the Scoring AD, but recognize these are challenging,” and can be difficult to use if not well trained to use them, he said. “At the very least, do an Investigator’s Global Assessment and do a body surface area measurement.”
In his opinion, four key signs that should be assessed in clinical trials are erythema, edema/papulation, excoriation, and lichenification/prurigo nodules.
Burden of disease
In terms of assessing AD disease burden, guidelines from the American Academy of Dermatology don’t give a specific tool to use, but recommend asking open-ended questions, Dr. Silverberg said. “I would not recommend that, because when you ask an open-ended question, the flood gates open up because most patients are suffering miserably with this disease when it’s uncontrolled.
“That’s why it’s valuable to use structured, validated tools like the Dermatology Life Quality Index and the Patient Reported Outcome Measurement Information System. They don’t take a lot of time to complete, and you can look at the score and determine how burdensome their disease is, even in a busy clinical practice. They’re not going to slow you down; they’re going to speed you up and make you better at your therapeutic decision-making. I can guarantee you that most patients will love you for it. Sometimes patients say to me, ‘you’re the first doctor to ask these questions.’ ”
Extent of disease
Finally, for the extent of disease component of DESCRIBE-AD, he emphasized the importance of doing a full-body exam to appreciate the affected body surface area, flexural versus extensor distribution, and involvement and severity of disease on special sites such as the face, hands, feet, genitals, and scalp.
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
The heterogeneous clinical course of atopic dermatitis (AD) and its differing signs, symptoms, burden, and response to treatment can pose a quandary for physicians.
This is behind facilitate tailoring of therapy to individual patient characteristics, and better identify therapeutically relevant disease subsets.
Dr. Silverberg, director of clinical research in the department of dermatology at George Washington University, Washington, debuted DESCRIBE-AD during the Revolutionizing Atopic Dermatitis symposium. The “D” in the mnemonic stands for dermatitis morphology and phenotype, the “E” for evolution of disease, the “S” for symptom severity, the “C” for comorbid health disorders, the “R” for response to therapy, the “I” for intensity of lesions, the “B” for burden of disease, and the “E” for extent of lesions.
At the meeting, he discussed the concepts behind each letter of the mnemonic.
Dermatitis morphology and phenotype
In the dermatitis morphology and phenotype component of DESCRIBE-AD, “there’s a lot to consider,” he said. “There are chronic signs like lichenification and prurigo nodules, which have treatment ramifications,” such as the length of time patients may need to be treated, and possibly “the use of more potent, targeted options to go after some of these lesions.”
Recent studies suggest that nummular lesions have a different underlying pathogenesis suggesting an overlap between Th2 and Th17 cell–mediated lesions. “How does that impact response to targeted therapies?” he asked. “We have no idea. We need to learn that.” He noted that psoriasiform lesions are not limited to Asian patients, but also appear in elderly patients with AD. “They look different [in elderly patients] and they may respond differently; they have more psoriasiform lesions and it’s not exactly clear why.”
Other morphologic variants of AD to be aware of include follicular eczema, xerosis, and the itch-dominant form, which Dr. Silverberg and colleagues addressed in a recently published study. “There are some patients who have milder-looking lesions, but their itch is just out of control,” he said. “This is a pattern that we need to recognize.”
Evolution of disease, symptom severity
Factors to consider for the evolution of disease component of the proposed classification include age of AD onset or disease recurrence, frequency and duration of flares, disease activity between flares, periods of disease clearance, and the overall disease trajectory. “We do get patients who say that every year their disease seems to get worse over time, for reasons that are not always clear,” Dr. Silverberg said.
Assessment tools he recommends for the symptom severity component of DESCRIBE-AD include the patient-reported global AD severity, numerical rating scale (NRS) worst or average itch in the past 7 days, the Skin Pain NRS, and the Sleep Quality NRS, which each take fewer than 30 seconds to complete. “You can have your nurses do this or can you have the patients fill out the form in the waiting area before they see you,” Dr. Silverberg said.
He also advises asking patients about the number of nights they experience sleep disturbance and if they have difficulty falling asleep or have nighttime awakenings because of their AD. Symptoms of anxiety and depression can be assessed with the Hospital Anxiety and Depression Scale and the Patient-Health Questionnaire–9, which each take 2-3 minutes to complete.
Recommended assessment tools for other symptoms – such as bleeding, oozing, and xerosis – include the Patient-Oriented Eczema Measure, which takes 2-3 minutes to complete, and the Atopic Dermatitis Control Tool or the Recap of Atopic Eczema, which each take 2-3 minutes to complete.
Comorbid health disorders
Comorbid health disorders linked to AD are varied and include atopic comorbidities such as asthma or wheeze, hay fever or oculonasal symptoms, food allergy, recurrent infections such as herpes simplex virus, mental health disorders, alopecia areata, Th1-mediated comorbidities, and adverse events to medication such as venous thromboembolism, hypertension, and impaired renal or liver function. “All of these are important because if the patients have these at baseline, they may not be good candidates for some therapies that cause these types of side effects,” Dr. Silverberg said.
Response to therapy, intensity of lesions
As for response to therapy, clinicians can ask patients, “How do you feel you’re improving?” But it’s also important to assess the signs, symptoms, frequency of flares, and comorbidities as part of that response to therapy, “and of course the adverse events and treatment burden,” he said.
For the intensity of lesions component of DESCRIBE-AD, Dr. Silverberg said that the Investigator’s Global Assessment–AD is an effective tool for clinical use. “You can also use tools like the Eczema Area and Severity Index or the Scoring AD, but recognize these are challenging,” and can be difficult to use if not well trained to use them, he said. “At the very least, do an Investigator’s Global Assessment and do a body surface area measurement.”
In his opinion, four key signs that should be assessed in clinical trials are erythema, edema/papulation, excoriation, and lichenification/prurigo nodules.
Burden of disease
In terms of assessing AD disease burden, guidelines from the American Academy of Dermatology don’t give a specific tool to use, but recommend asking open-ended questions, Dr. Silverberg said. “I would not recommend that, because when you ask an open-ended question, the flood gates open up because most patients are suffering miserably with this disease when it’s uncontrolled.
“That’s why it’s valuable to use structured, validated tools like the Dermatology Life Quality Index and the Patient Reported Outcome Measurement Information System. They don’t take a lot of time to complete, and you can look at the score and determine how burdensome their disease is, even in a busy clinical practice. They’re not going to slow you down; they’re going to speed you up and make you better at your therapeutic decision-making. I can guarantee you that most patients will love you for it. Sometimes patients say to me, ‘you’re the first doctor to ask these questions.’ ”
Extent of disease
Finally, for the extent of disease component of DESCRIBE-AD, he emphasized the importance of doing a full-body exam to appreciate the affected body surface area, flexural versus extensor distribution, and involvement and severity of disease on special sites such as the face, hands, feet, genitals, and scalp.
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2021
Metformin use may curb BCC risk
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Latest FDA pembrolizumab approval expands label to cutaneous SCCs
The
The July 6 approval for the programmed death–1 inhibitor follows a June FDA approval for pembrolizumab monotherapy in patients with recurrent or metastatic cSCC disease not curable by surgery or radiation. Both approvals, pembrolizumab’s first for cSCC, are based on findings from the second interim analysis of the phase 2, multicenter, open-label KEYNOTE-629 trial.
The objective response rate in the cohort of 54 patients with locally advanced disease was 50%, including a complete response rate of 17% and a partial response rate of 33%. Duration of response was 6 months or longer in 81% of the 27 responders, and 12 months or longer in 37% of responders. After a median follow-up of 13.4 months, median duration of response had not yet been reached.
Pembrolizumab has previously received FDA approvals, either as monotherapy or in combination with other agents, for the treatment of numerous cancer types, including certain melanomas, non–small cell lung cancers, head and neck SCCs, classical Hodgkin lymphomas, primary mediastinal large B-cell lymphomas, urothelial carcinomas, microsatellite instability–high or mismatch repair–deficient cancers, and gastric, esophageal, cervical, hepatocellular, Merkel cell, renal cell, tumor mutational burden–high, and triple-negative breast cancers.
Patients in the KEYNOTE-629 trial received pembrolizumab at a dose of 200 mg IV every 3 weeks for 24 months or until documented disease progression or unacceptable toxicity.
Adverse reactions occurring in patients with recurrent or metastatic cSCC or locally advanced cSCC in KEYNOTE-629 were similar to those observed in patients with melanoma or non–small cell lung cancer who were treated with pembrolizumab monotherapy in previous trials.
The checkpoint inhibitor can cause immune-mediated adverse reactions, which may be severe or fatal, according to Merck, the drug’s manufacturer. The reactions can occur in any organ system or tissue and can affect more than one body system simultaneously.
“Immune-mediated adverse reactions can occur at any time during or after treatment with Keytruda, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, dermatologic reactions, solid organ transplant rejection, and complications of allogeneic hematopoietic stem cell transplantation,” Merck explained in a press release, noting that “early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda.”
Depending on the severity of any reaction, treatment should be withheld or permanently discontinued, and corticosteroids administered if appropriate, Merck stated.
The
The July 6 approval for the programmed death–1 inhibitor follows a June FDA approval for pembrolizumab monotherapy in patients with recurrent or metastatic cSCC disease not curable by surgery or radiation. Both approvals, pembrolizumab’s first for cSCC, are based on findings from the second interim analysis of the phase 2, multicenter, open-label KEYNOTE-629 trial.
The objective response rate in the cohort of 54 patients with locally advanced disease was 50%, including a complete response rate of 17% and a partial response rate of 33%. Duration of response was 6 months or longer in 81% of the 27 responders, and 12 months or longer in 37% of responders. After a median follow-up of 13.4 months, median duration of response had not yet been reached.
Pembrolizumab has previously received FDA approvals, either as monotherapy or in combination with other agents, for the treatment of numerous cancer types, including certain melanomas, non–small cell lung cancers, head and neck SCCs, classical Hodgkin lymphomas, primary mediastinal large B-cell lymphomas, urothelial carcinomas, microsatellite instability–high or mismatch repair–deficient cancers, and gastric, esophageal, cervical, hepatocellular, Merkel cell, renal cell, tumor mutational burden–high, and triple-negative breast cancers.
Patients in the KEYNOTE-629 trial received pembrolizumab at a dose of 200 mg IV every 3 weeks for 24 months or until documented disease progression or unacceptable toxicity.
Adverse reactions occurring in patients with recurrent or metastatic cSCC or locally advanced cSCC in KEYNOTE-629 were similar to those observed in patients with melanoma or non–small cell lung cancer who were treated with pembrolizumab monotherapy in previous trials.
The checkpoint inhibitor can cause immune-mediated adverse reactions, which may be severe or fatal, according to Merck, the drug’s manufacturer. The reactions can occur in any organ system or tissue and can affect more than one body system simultaneously.
“Immune-mediated adverse reactions can occur at any time during or after treatment with Keytruda, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, dermatologic reactions, solid organ transplant rejection, and complications of allogeneic hematopoietic stem cell transplantation,” Merck explained in a press release, noting that “early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda.”
Depending on the severity of any reaction, treatment should be withheld or permanently discontinued, and corticosteroids administered if appropriate, Merck stated.
The
The July 6 approval for the programmed death–1 inhibitor follows a June FDA approval for pembrolizumab monotherapy in patients with recurrent or metastatic cSCC disease not curable by surgery or radiation. Both approvals, pembrolizumab’s first for cSCC, are based on findings from the second interim analysis of the phase 2, multicenter, open-label KEYNOTE-629 trial.
The objective response rate in the cohort of 54 patients with locally advanced disease was 50%, including a complete response rate of 17% and a partial response rate of 33%. Duration of response was 6 months or longer in 81% of the 27 responders, and 12 months or longer in 37% of responders. After a median follow-up of 13.4 months, median duration of response had not yet been reached.
Pembrolizumab has previously received FDA approvals, either as monotherapy or in combination with other agents, for the treatment of numerous cancer types, including certain melanomas, non–small cell lung cancers, head and neck SCCs, classical Hodgkin lymphomas, primary mediastinal large B-cell lymphomas, urothelial carcinomas, microsatellite instability–high or mismatch repair–deficient cancers, and gastric, esophageal, cervical, hepatocellular, Merkel cell, renal cell, tumor mutational burden–high, and triple-negative breast cancers.
Patients in the KEYNOTE-629 trial received pembrolizumab at a dose of 200 mg IV every 3 weeks for 24 months or until documented disease progression or unacceptable toxicity.
Adverse reactions occurring in patients with recurrent or metastatic cSCC or locally advanced cSCC in KEYNOTE-629 were similar to those observed in patients with melanoma or non–small cell lung cancer who were treated with pembrolizumab monotherapy in previous trials.
The checkpoint inhibitor can cause immune-mediated adverse reactions, which may be severe or fatal, according to Merck, the drug’s manufacturer. The reactions can occur in any organ system or tissue and can affect more than one body system simultaneously.
“Immune-mediated adverse reactions can occur at any time during or after treatment with Keytruda, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, dermatologic reactions, solid organ transplant rejection, and complications of allogeneic hematopoietic stem cell transplantation,” Merck explained in a press release, noting that “early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda.”
Depending on the severity of any reaction, treatment should be withheld or permanently discontinued, and corticosteroids administered if appropriate, Merck stated.
Patients on methotrexate show T-cell response to Pfizer vaccine
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nadolol bests propranolol for infantile hemangioma treatment out to 52 weeks
of 71 patients showed.
“In clinical practice, we notice that nadolol works very well in terms of controlling the size and the appearance of the hemangioma,” lead study author Elena Pope, MD, MSc, said during the annual meeting of the Society for Pediatric Dermatology. Hence, she and her colleagues were interested in comparing their clinical experience with the standard treatment with propranolol, and designed a prospective, randomized, controlled, double-blinded study, with the aim of proving that “nadolol is noninferior to propranolol, with a margin of noninferiority of 10%.”
Between 2016 and 2020, Dr. Pope and colleagues at two academic Canadian pediatric dermatology centers enrolled 71 infants aged 1-6 months with significant hemangioma that had either the potential for functional impairment or cosmetic deformity, defined as a lesion greater than 1.5 cm on the face or greater than 3 cm on another body part. Treatment consisted of oral propranolol or nadolol in escalating doses up to 2 mg/kg per day. “The blinding portion of the study was for 24 weeks with a follow-up up to 52 weeks,” said Dr. Pope, professor of pediatrics at the University of Toronto and section head of pediatric dermatology at The Hospital for Sick Children, also in Toronto. “After the unblinding at 24 weeks, patients were allowed to switch their intervention if they were not happy with the results.”
Of the 71 patients, 35 received nadolol and 36 received propranolol. The two groups were similar in terms of clinical and demographic characteristics. Their mean age at enrollment was 3.15 months, 80% were female, 61% were White, 20% were Asian, and the rest were from other ethnic backgrounds.
At 24 weeks, the researchers found that the mean size involution was 97.94% in the nadolol group and 89.14% in the propranolol group (P = .005), while the mean color fading on the visual analogue scale (VAS) was 94.47% in the nadolol group and 80.54% in the propranolol group (P < .001). At 52 weeks, the mean size involution was 99.63% in the nadolol group and 93.63% in the propranolol group (P = .001), while the mean VAS color fading was 97.34% in the nadolol group and 87.23% in the propranolol group (P = .001).
According to Dr. Pope, Kaplan-Meir analysis showed that patients in the propranolol group responded slower to treatment (P = .019), while safety data was similar between the two groups. For example, between weeks 25 and 52, 84.2% of patients in the nadolol group experienced an adverse event, compared with 74.2% of patients in the propranolol group (P = .466). The most common respiratory adverse event was upper respiratory tract infection, which affected 87.5% of patients in the nadolol group, compared with 100% of patients in the propranolol group (P = 0.341).
The most common gastrointestinal adverse event was diarrhea, which affected 66.7% of patients in both groups. One patient in the propranolol group was admitted to the hospital with pneumonia and fully recovered. The incident was not suspected to be related to the medication.
“We believe that this data backs up our clinical experience and it may offer an alternative treatment in other centers where patients experience propranolol unresponsiveness, side effects, or intolerance, or where a fast response is needed,” Dr. Pope said. As for the potential cost implications, “nadolol is cheaper than the Hemangiol but comparable with the compounded formulation of propranolol.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics in 2020. Authors from Alberta reported the case of a 10-week-old girl who was started on nadolol for infantile hemangioma, died 7 weeks later, and was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “The infant had no bowel movements for 10 days before her death, which we hypothesize contributed to nadolol toxicity,” the authors wrote.
In a reply to the authors in the same issue of Pediatrics, Dr. Pope, Cathryn Sibbald, MD, and Erin Chung, PhD, pointed out that postmortem redistribution of medications “is complex and measured postmortem cardiac blood concentrations may be significantly higher than the true blood nadolol concentration at the time of death due to significant diffusion from the peripheral tissues.”
They added that the report did not address “other potential errors such as in compounding, dispensing, and administration of the solution,” they wrote, adding: “Finally, we are aware of a Canadian case of death in an infant receiving propranolol, although the cause of death in that case was unable to be determined (ISMP Canada 2016 Safety Bulletin).We agree with the authors that careful consideration of the risks and benefits of beta-blocker therapy should be employed, parents need to be informed when to discontinue therapy and that further research into the pharmacokinetics and pharmacogenetics of beta-blockers are warranted.”
Following publication of the case report in Pediatrics, Dr. Pope said that the only change she made in her practice was to ask families to temporarily discontinue nadolol if their child had constipation for more than 5 days.
The study was supported by a grant from Physician Services, Inc. Dr. Pope reported having no financial disclosures.
of 71 patients showed.
“In clinical practice, we notice that nadolol works very well in terms of controlling the size and the appearance of the hemangioma,” lead study author Elena Pope, MD, MSc, said during the annual meeting of the Society for Pediatric Dermatology. Hence, she and her colleagues were interested in comparing their clinical experience with the standard treatment with propranolol, and designed a prospective, randomized, controlled, double-blinded study, with the aim of proving that “nadolol is noninferior to propranolol, with a margin of noninferiority of 10%.”
Between 2016 and 2020, Dr. Pope and colleagues at two academic Canadian pediatric dermatology centers enrolled 71 infants aged 1-6 months with significant hemangioma that had either the potential for functional impairment or cosmetic deformity, defined as a lesion greater than 1.5 cm on the face or greater than 3 cm on another body part. Treatment consisted of oral propranolol or nadolol in escalating doses up to 2 mg/kg per day. “The blinding portion of the study was for 24 weeks with a follow-up up to 52 weeks,” said Dr. Pope, professor of pediatrics at the University of Toronto and section head of pediatric dermatology at The Hospital for Sick Children, also in Toronto. “After the unblinding at 24 weeks, patients were allowed to switch their intervention if they were not happy with the results.”
Of the 71 patients, 35 received nadolol and 36 received propranolol. The two groups were similar in terms of clinical and demographic characteristics. Their mean age at enrollment was 3.15 months, 80% were female, 61% were White, 20% were Asian, and the rest were from other ethnic backgrounds.
At 24 weeks, the researchers found that the mean size involution was 97.94% in the nadolol group and 89.14% in the propranolol group (P = .005), while the mean color fading on the visual analogue scale (VAS) was 94.47% in the nadolol group and 80.54% in the propranolol group (P < .001). At 52 weeks, the mean size involution was 99.63% in the nadolol group and 93.63% in the propranolol group (P = .001), while the mean VAS color fading was 97.34% in the nadolol group and 87.23% in the propranolol group (P = .001).
According to Dr. Pope, Kaplan-Meir analysis showed that patients in the propranolol group responded slower to treatment (P = .019), while safety data was similar between the two groups. For example, between weeks 25 and 52, 84.2% of patients in the nadolol group experienced an adverse event, compared with 74.2% of patients in the propranolol group (P = .466). The most common respiratory adverse event was upper respiratory tract infection, which affected 87.5% of patients in the nadolol group, compared with 100% of patients in the propranolol group (P = 0.341).
The most common gastrointestinal adverse event was diarrhea, which affected 66.7% of patients in both groups. One patient in the propranolol group was admitted to the hospital with pneumonia and fully recovered. The incident was not suspected to be related to the medication.
“We believe that this data backs up our clinical experience and it may offer an alternative treatment in other centers where patients experience propranolol unresponsiveness, side effects, or intolerance, or where a fast response is needed,” Dr. Pope said. As for the potential cost implications, “nadolol is cheaper than the Hemangiol but comparable with the compounded formulation of propranolol.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics in 2020. Authors from Alberta reported the case of a 10-week-old girl who was started on nadolol for infantile hemangioma, died 7 weeks later, and was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “The infant had no bowel movements for 10 days before her death, which we hypothesize contributed to nadolol toxicity,” the authors wrote.
In a reply to the authors in the same issue of Pediatrics, Dr. Pope, Cathryn Sibbald, MD, and Erin Chung, PhD, pointed out that postmortem redistribution of medications “is complex and measured postmortem cardiac blood concentrations may be significantly higher than the true blood nadolol concentration at the time of death due to significant diffusion from the peripheral tissues.”
They added that the report did not address “other potential errors such as in compounding, dispensing, and administration of the solution,” they wrote, adding: “Finally, we are aware of a Canadian case of death in an infant receiving propranolol, although the cause of death in that case was unable to be determined (ISMP Canada 2016 Safety Bulletin).We agree with the authors that careful consideration of the risks and benefits of beta-blocker therapy should be employed, parents need to be informed when to discontinue therapy and that further research into the pharmacokinetics and pharmacogenetics of beta-blockers are warranted.”
Following publication of the case report in Pediatrics, Dr. Pope said that the only change she made in her practice was to ask families to temporarily discontinue nadolol if their child had constipation for more than 5 days.
The study was supported by a grant from Physician Services, Inc. Dr. Pope reported having no financial disclosures.
of 71 patients showed.
“In clinical practice, we notice that nadolol works very well in terms of controlling the size and the appearance of the hemangioma,” lead study author Elena Pope, MD, MSc, said during the annual meeting of the Society for Pediatric Dermatology. Hence, she and her colleagues were interested in comparing their clinical experience with the standard treatment with propranolol, and designed a prospective, randomized, controlled, double-blinded study, with the aim of proving that “nadolol is noninferior to propranolol, with a margin of noninferiority of 10%.”
Between 2016 and 2020, Dr. Pope and colleagues at two academic Canadian pediatric dermatology centers enrolled 71 infants aged 1-6 months with significant hemangioma that had either the potential for functional impairment or cosmetic deformity, defined as a lesion greater than 1.5 cm on the face or greater than 3 cm on another body part. Treatment consisted of oral propranolol or nadolol in escalating doses up to 2 mg/kg per day. “The blinding portion of the study was for 24 weeks with a follow-up up to 52 weeks,” said Dr. Pope, professor of pediatrics at the University of Toronto and section head of pediatric dermatology at The Hospital for Sick Children, also in Toronto. “After the unblinding at 24 weeks, patients were allowed to switch their intervention if they were not happy with the results.”
Of the 71 patients, 35 received nadolol and 36 received propranolol. The two groups were similar in terms of clinical and demographic characteristics. Their mean age at enrollment was 3.15 months, 80% were female, 61% were White, 20% were Asian, and the rest were from other ethnic backgrounds.
At 24 weeks, the researchers found that the mean size involution was 97.94% in the nadolol group and 89.14% in the propranolol group (P = .005), while the mean color fading on the visual analogue scale (VAS) was 94.47% in the nadolol group and 80.54% in the propranolol group (P < .001). At 52 weeks, the mean size involution was 99.63% in the nadolol group and 93.63% in the propranolol group (P = .001), while the mean VAS color fading was 97.34% in the nadolol group and 87.23% in the propranolol group (P = .001).
According to Dr. Pope, Kaplan-Meir analysis showed that patients in the propranolol group responded slower to treatment (P = .019), while safety data was similar between the two groups. For example, between weeks 25 and 52, 84.2% of patients in the nadolol group experienced an adverse event, compared with 74.2% of patients in the propranolol group (P = .466). The most common respiratory adverse event was upper respiratory tract infection, which affected 87.5% of patients in the nadolol group, compared with 100% of patients in the propranolol group (P = 0.341).
The most common gastrointestinal adverse event was diarrhea, which affected 66.7% of patients in both groups. One patient in the propranolol group was admitted to the hospital with pneumonia and fully recovered. The incident was not suspected to be related to the medication.
“We believe that this data backs up our clinical experience and it may offer an alternative treatment in other centers where patients experience propranolol unresponsiveness, side effects, or intolerance, or where a fast response is needed,” Dr. Pope said. As for the potential cost implications, “nadolol is cheaper than the Hemangiol but comparable with the compounded formulation of propranolol.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics in 2020. Authors from Alberta reported the case of a 10-week-old girl who was started on nadolol for infantile hemangioma, died 7 weeks later, and was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “The infant had no bowel movements for 10 days before her death, which we hypothesize contributed to nadolol toxicity,” the authors wrote.
In a reply to the authors in the same issue of Pediatrics, Dr. Pope, Cathryn Sibbald, MD, and Erin Chung, PhD, pointed out that postmortem redistribution of medications “is complex and measured postmortem cardiac blood concentrations may be significantly higher than the true blood nadolol concentration at the time of death due to significant diffusion from the peripheral tissues.”
They added that the report did not address “other potential errors such as in compounding, dispensing, and administration of the solution,” they wrote, adding: “Finally, we are aware of a Canadian case of death in an infant receiving propranolol, although the cause of death in that case was unable to be determined (ISMP Canada 2016 Safety Bulletin).We agree with the authors that careful consideration of the risks and benefits of beta-blocker therapy should be employed, parents need to be informed when to discontinue therapy and that further research into the pharmacokinetics and pharmacogenetics of beta-blockers are warranted.”
Following publication of the case report in Pediatrics, Dr. Pope said that the only change she made in her practice was to ask families to temporarily discontinue nadolol if their child had constipation for more than 5 days.
The study was supported by a grant from Physician Services, Inc. Dr. Pope reported having no financial disclosures.
FROM SPD 2021