User login
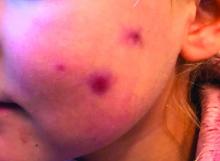
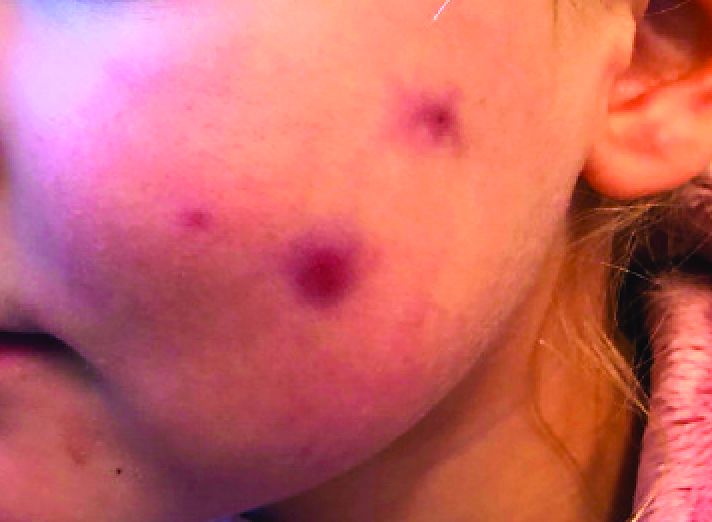
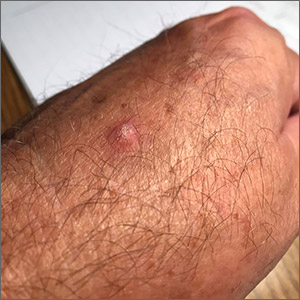
A 4-year-old with a lesion on her cheek, which grew and became firmer over two months
The patient was diagnosed with idiopathic facial aseptic granuloma (IFAG) based on the clinical findings, as well as the associated history of chalazia and erythematous papules seen in childhood rosacea.
She was treated with several months of azithromycin, sulfur wash, and metronidazole cream with improvement of some of the smaller lesions but no change on the larger nodules. Later she was treated with oral and topical ivermectin with no improvement. Some of the nodules slowly resolved except for the larger lesion on the right cheek. She was later treated with a 6-week course of clarithromycin with partial improvement of the nodule. The lesion resolved after 2 months of stopping clarithromycin.
IFAG is a rare condition seen in prepubescent children. The etiology of this condition is not well understood and is thought to be on the spectrum of childhood rosacea.1 From several recent reports, IFAG usually is seen in children with associated conditions including chalazia, conjunctivitis, blepharitis, and telangiectasias, which can be seen in patients with rosacea. These associated findings suggest the possibility of IFAG being a form of granulomatous rosacea in children.
This condition presents in childhood between the ages of 8 months and 13 years. Most of the cases occur in toddlers, and girls appear to be more affected than boys. The lesions appear as pink, rubbery, nontender, nonfluctuant nodules on the cheeks, which can be single or multiple. A large prospective study in 30 children demonstrated that more 70% of the lesions cultured were negative for bacteria. Histologic analysis of some of the lesions showed a chronic dermal lymphohistiocytic granulomatous perifollicular infiltrate with numerous foreign body–type giant cells.2
The differential diagnosis of these lesions should include infectious pyodermas such as mycobacterial infections, cutaneous leishmaniasis, and botryomycosis; deep fungal infections such as sporotrichosis, coccidioidomycosis, and cryptococcosis; childhood nodulocystic acne; pilomatrixoma; epidermoid cyst; vascular tumors or malformations; and leukemia cutis.3
The diagnosis is usually clinical but in atypical cases a skin biopsy with tissue cultures should be performed. The decision to biopsy these lesions will need to be done in a one by one basis, as a biopsy may leave scaring on the area affected.
It has been postulated that a color Doppler ultrasound of the lesion may be a helpful ancillary study. Echographic findings show a well demarcated solid-cystic, hypoechoic dermal lesion, the largest axis of which lies parallel to the skin surface. The lesion lacks calcium deposits. Other findings include increased echogenicity of the underlaying hypodermis. The findings may vary depending on the stage of the lesion.4
The course of the condition may last on average months to years. Some lesions resolve spontaneously and others may respond to courses of oral antibiotics such as clarithromycin, azithromycin, or ivermectin. In our patient, several lesions improved with oral antibiotics, but the larger lesions were more persistent and resolved after a year.
The lesions usually resolve without scarring. In those patients with associated rosacea, maintenance topical treatments may be warranted and also may need follow-up with ophthalmology because they tend to commonly have ocular rosacea as well.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2013 Jan-Feb;30(1):109-11.
2. Br J Dermatol. 2007 Apr;156(4):705-8.
3. Pediatr Dermatol. 2018 Jul;35(4):490-3.
4. Actas Dermosifiliogr. 2019 Oct;110(8):637-41.
The patient was diagnosed with idiopathic facial aseptic granuloma (IFAG) based on the clinical findings, as well as the associated history of chalazia and erythematous papules seen in childhood rosacea.
She was treated with several months of azithromycin, sulfur wash, and metronidazole cream with improvement of some of the smaller lesions but no change on the larger nodules. Later she was treated with oral and topical ivermectin with no improvement. Some of the nodules slowly resolved except for the larger lesion on the right cheek. She was later treated with a 6-week course of clarithromycin with partial improvement of the nodule. The lesion resolved after 2 months of stopping clarithromycin.
IFAG is a rare condition seen in prepubescent children. The etiology of this condition is not well understood and is thought to be on the spectrum of childhood rosacea.1 From several recent reports, IFAG usually is seen in children with associated conditions including chalazia, conjunctivitis, blepharitis, and telangiectasias, which can be seen in patients with rosacea. These associated findings suggest the possibility of IFAG being a form of granulomatous rosacea in children.
This condition presents in childhood between the ages of 8 months and 13 years. Most of the cases occur in toddlers, and girls appear to be more affected than boys. The lesions appear as pink, rubbery, nontender, nonfluctuant nodules on the cheeks, which can be single or multiple. A large prospective study in 30 children demonstrated that more 70% of the lesions cultured were negative for bacteria. Histologic analysis of some of the lesions showed a chronic dermal lymphohistiocytic granulomatous perifollicular infiltrate with numerous foreign body–type giant cells.2
The differential diagnosis of these lesions should include infectious pyodermas such as mycobacterial infections, cutaneous leishmaniasis, and botryomycosis; deep fungal infections such as sporotrichosis, coccidioidomycosis, and cryptococcosis; childhood nodulocystic acne; pilomatrixoma; epidermoid cyst; vascular tumors or malformations; and leukemia cutis.3
The diagnosis is usually clinical but in atypical cases a skin biopsy with tissue cultures should be performed. The decision to biopsy these lesions will need to be done in a one by one basis, as a biopsy may leave scaring on the area affected.
It has been postulated that a color Doppler ultrasound of the lesion may be a helpful ancillary study. Echographic findings show a well demarcated solid-cystic, hypoechoic dermal lesion, the largest axis of which lies parallel to the skin surface. The lesion lacks calcium deposits. Other findings include increased echogenicity of the underlaying hypodermis. The findings may vary depending on the stage of the lesion.4
The course of the condition may last on average months to years. Some lesions resolve spontaneously and others may respond to courses of oral antibiotics such as clarithromycin, azithromycin, or ivermectin. In our patient, several lesions improved with oral antibiotics, but the larger lesions were more persistent and resolved after a year.
The lesions usually resolve without scarring. In those patients with associated rosacea, maintenance topical treatments may be warranted and also may need follow-up with ophthalmology because they tend to commonly have ocular rosacea as well.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2013 Jan-Feb;30(1):109-11.
2. Br J Dermatol. 2007 Apr;156(4):705-8.
3. Pediatr Dermatol. 2018 Jul;35(4):490-3.
4. Actas Dermosifiliogr. 2019 Oct;110(8):637-41.
The patient was diagnosed with idiopathic facial aseptic granuloma (IFAG) based on the clinical findings, as well as the associated history of chalazia and erythematous papules seen in childhood rosacea.
She was treated with several months of azithromycin, sulfur wash, and metronidazole cream with improvement of some of the smaller lesions but no change on the larger nodules. Later she was treated with oral and topical ivermectin with no improvement. Some of the nodules slowly resolved except for the larger lesion on the right cheek. She was later treated with a 6-week course of clarithromycin with partial improvement of the nodule. The lesion resolved after 2 months of stopping clarithromycin.
IFAG is a rare condition seen in prepubescent children. The etiology of this condition is not well understood and is thought to be on the spectrum of childhood rosacea.1 From several recent reports, IFAG usually is seen in children with associated conditions including chalazia, conjunctivitis, blepharitis, and telangiectasias, which can be seen in patients with rosacea. These associated findings suggest the possibility of IFAG being a form of granulomatous rosacea in children.
This condition presents in childhood between the ages of 8 months and 13 years. Most of the cases occur in toddlers, and girls appear to be more affected than boys. The lesions appear as pink, rubbery, nontender, nonfluctuant nodules on the cheeks, which can be single or multiple. A large prospective study in 30 children demonstrated that more 70% of the lesions cultured were negative for bacteria. Histologic analysis of some of the lesions showed a chronic dermal lymphohistiocytic granulomatous perifollicular infiltrate with numerous foreign body–type giant cells.2
The differential diagnosis of these lesions should include infectious pyodermas such as mycobacterial infections, cutaneous leishmaniasis, and botryomycosis; deep fungal infections such as sporotrichosis, coccidioidomycosis, and cryptococcosis; childhood nodulocystic acne; pilomatrixoma; epidermoid cyst; vascular tumors or malformations; and leukemia cutis.3
The diagnosis is usually clinical but in atypical cases a skin biopsy with tissue cultures should be performed. The decision to biopsy these lesions will need to be done in a one by one basis, as a biopsy may leave scaring on the area affected.
It has been postulated that a color Doppler ultrasound of the lesion may be a helpful ancillary study. Echographic findings show a well demarcated solid-cystic, hypoechoic dermal lesion, the largest axis of which lies parallel to the skin surface. The lesion lacks calcium deposits. Other findings include increased echogenicity of the underlaying hypodermis. The findings may vary depending on the stage of the lesion.4
The course of the condition may last on average months to years. Some lesions resolve spontaneously and others may respond to courses of oral antibiotics such as clarithromycin, azithromycin, or ivermectin. In our patient, several lesions improved with oral antibiotics, but the larger lesions were more persistent and resolved after a year.
The lesions usually resolve without scarring. In those patients with associated rosacea, maintenance topical treatments may be warranted and also may need follow-up with ophthalmology because they tend to commonly have ocular rosacea as well.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Pediatr Dermatol. 2013 Jan-Feb;30(1):109-11.
2. Br J Dermatol. 2007 Apr;156(4):705-8.
3. Pediatr Dermatol. 2018 Jul;35(4):490-3.
4. Actas Dermosifiliogr. 2019 Oct;110(8):637-41.
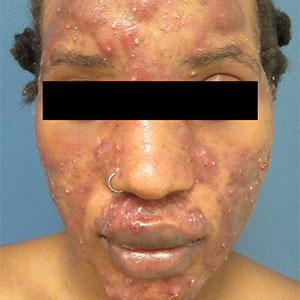
A 4-year-old female is brought to our pediatric dermatology clinic for evaluation of a persistent lesion on the cheek.
The mother of the child reports that the lesion started as a small "bug bite" and then started growing and getting firmer for the past 2 months. The girl has developed other smaller red, pimple-like lesions on the cheeks and one of them is starting to increase in size.
She denies any tenderness on the area or any purulent discharge. She has had no fevers, chills, weight loss, nose bleeds, fatigue, or any other symptoms. The mother has not noted any changes on the child's body odor, any rapid growth, or hair on her axillary or pubic area. She was treated with three different courses of oral antibiotics including cephalexin, trimethoprim/sulfamethoxazole, and clindamycin, as well as topical mupirocin, with no improvement.
Her past medical history is significant for several episodes of eyelid cysts that were treated with warm compresses and topical erythromycin ointment. The family history is significant for the father having severe acne as a teenager. She has two cats, she has not traveled, and she has an older sister who has no lesions.
On physical examination she is a lovely 4-year-old female in no acute distress. Her height is on the 70th percentile and weight on the 40th percentile for her age. Her blood pressure is 95/84 with a heart rate of 96. On skin examination she has several pink macules and papules on her bilateral cheeks. On the left cheek there are two pink nodules: One is 1 cm, and the other is 7 mm. The nodules are not tender. There is no warmth, fluctuance, or discharge from the lesions.
She has no cervical lymphadenopathy. She has no axillary or pubic hair. She is Tanner stage I.
A Roundabout Journey to Diagnosis
ANSWER
The correct answer is all the above (choice “e”).
DISCUSSION
The differential for round or annular, scaly lesions is lengthy. In addition to all 4 conditions listed above, it includes eczema, basal cell carcinoma, and irritant or contact dermatitis.
With this patient’s history, the diagnosis of fungal infection was not unreasonable. However, the total lack of response to antifungal treatment—along with a negative KOH prep—made that diagnosis questionable at best. Then there was the lack of lymphadenopathy, which would almost certainly have been present with such longstanding infection. Given her country of origin, cutaneous New World leishmaniasis (caused by a protozoan delivered to the patient by an insect vector) was a possibility.
For this patient, skin biopsy with a 4-mm punch was the only way to establish the correct diagnosis. The defect from the biopsy was closed with 5-0 nylon sutures to minimize scarring.
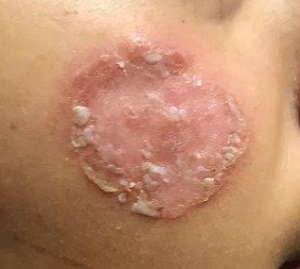
The pathological findings included interface dermatitis, apoptotic keratinocytes, and a brisk periadnexal lymphocytic infiltrate, which—given the morphological and historical context—were entirely consistent with discoid lupus erythematosus (DLE; otherwise known as subacute cutaneous lupus erythematosus). Subsequent bloodwork failed to show any connection with systemic lupus erythematosus (SLE), which is not surprising because only about 18% of DLE cases evolve into SLE.
DLE is more common in women, especially in those with skin of color. The associated lesions are seldom as impressive as this patient’s, manifesting as papulosquamous patches typically found on the ears, neck, face, arms, and other sun-exposed areas. Indeed, it appears that sun exposure is a major trigger for the disease—a clue that can assist with the diagnosis.
TREATMENT
In addition to proscribing excessive sun exposure, providers should encourage the use of sunscreen. DLE is often treated with topical steroids. More advanced cases, such as this patient’s, may require oral hydroxychloroquine (200 mg bid). Though these treatments are effective in most cases, DLE can leave serious scarring and/or discoloration, especially in those with darker skin.
ANSWER
The correct answer is all the above (choice “e”).
DISCUSSION
The differential for round or annular, scaly lesions is lengthy. In addition to all 4 conditions listed above, it includes eczema, basal cell carcinoma, and irritant or contact dermatitis.
With this patient’s history, the diagnosis of fungal infection was not unreasonable. However, the total lack of response to antifungal treatment—along with a negative KOH prep—made that diagnosis questionable at best. Then there was the lack of lymphadenopathy, which would almost certainly have been present with such longstanding infection. Given her country of origin, cutaneous New World leishmaniasis (caused by a protozoan delivered to the patient by an insect vector) was a possibility.
For this patient, skin biopsy with a 4-mm punch was the only way to establish the correct diagnosis. The defect from the biopsy was closed with 5-0 nylon sutures to minimize scarring.
The pathological findings included interface dermatitis, apoptotic keratinocytes, and a brisk periadnexal lymphocytic infiltrate, which—given the morphological and historical context—were entirely consistent with discoid lupus erythematosus (DLE; otherwise known as subacute cutaneous lupus erythematosus). Subsequent bloodwork failed to show any connection with systemic lupus erythematosus (SLE), which is not surprising because only about 18% of DLE cases evolve into SLE.
DLE is more common in women, especially in those with skin of color. The associated lesions are seldom as impressive as this patient’s, manifesting as papulosquamous patches typically found on the ears, neck, face, arms, and other sun-exposed areas. Indeed, it appears that sun exposure is a major trigger for the disease—a clue that can assist with the diagnosis.
TREATMENT
In addition to proscribing excessive sun exposure, providers should encourage the use of sunscreen. DLE is often treated with topical steroids. More advanced cases, such as this patient’s, may require oral hydroxychloroquine (200 mg bid). Though these treatments are effective in most cases, DLE can leave serious scarring and/or discoloration, especially in those with darker skin.
ANSWER
The correct answer is all the above (choice “e”).
DISCUSSION
The differential for round or annular, scaly lesions is lengthy. In addition to all 4 conditions listed above, it includes eczema, basal cell carcinoma, and irritant or contact dermatitis.
With this patient’s history, the diagnosis of fungal infection was not unreasonable. However, the total lack of response to antifungal treatment—along with a negative KOH prep—made that diagnosis questionable at best. Then there was the lack of lymphadenopathy, which would almost certainly have been present with such longstanding infection. Given her country of origin, cutaneous New World leishmaniasis (caused by a protozoan delivered to the patient by an insect vector) was a possibility.
For this patient, skin biopsy with a 4-mm punch was the only way to establish the correct diagnosis. The defect from the biopsy was closed with 5-0 nylon sutures to minimize scarring.
The pathological findings included interface dermatitis, apoptotic keratinocytes, and a brisk periadnexal lymphocytic infiltrate, which—given the morphological and historical context—were entirely consistent with discoid lupus erythematosus (DLE; otherwise known as subacute cutaneous lupus erythematosus). Subsequent bloodwork failed to show any connection with systemic lupus erythematosus (SLE), which is not surprising because only about 18% of DLE cases evolve into SLE.
DLE is more common in women, especially in those with skin of color. The associated lesions are seldom as impressive as this patient’s, manifesting as papulosquamous patches typically found on the ears, neck, face, arms, and other sun-exposed areas. Indeed, it appears that sun exposure is a major trigger for the disease—a clue that can assist with the diagnosis.
TREATMENT
In addition to proscribing excessive sun exposure, providers should encourage the use of sunscreen. DLE is often treated with topical steroids. More advanced cases, such as this patient’s, may require oral hydroxychloroquine (200 mg bid). Though these treatments are effective in most cases, DLE can leave serious scarring and/or discoloration, especially in those with darker skin.
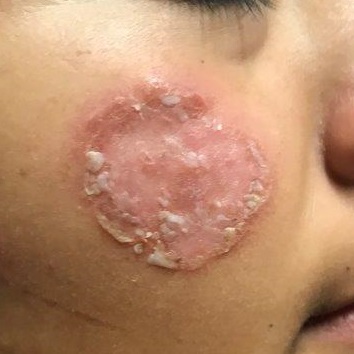
After journeying for several months from Honduras, this 30-year-old woman visits the clinic for evaluation of a lesion that has been growing on her cheek since before she started traveling. She saw several providers—mostly in NGO clinics—along her journey. The diagnosis they gave was consistently “ringworm.” She was offered various topical creams, none of which produced any results.
Though the lesion is not painful, it causes some itching. The patient is much more concerned about its appearance. Through interpreters, she claims to be in otherwise good health. She has no other lesions, joint pain, fever, or malaise. She reports neither her family nor fellow travelers have such lesions.
Examination reveals an impressive 3-cm, round, papulosquamous plaque on the right side of her face (below the malar area). The lesion is neither tender nor notably warm. There are no palpable lymph nodes in the area. The scaling is mostly on the periphery. A KOH prep of the scaling shows no fungal elements.
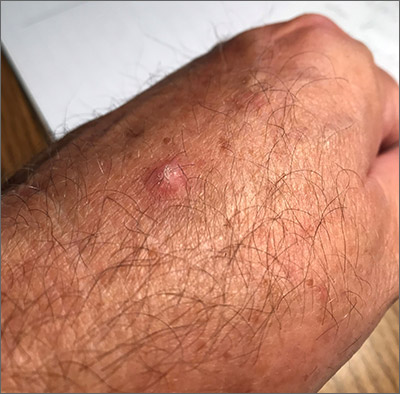
Scaly hand papule
This pink raised nodule underlying a scaly surface was suspicious for squamous cell carcinoma (SCC). Since this was a virtual visit, and the lesion required pathology due to the likelihood of cancer, the patient was brought into the clinic for additional evaluation. A broad-based deep shave biopsy was performed to remove the visible lesion. Pathology showed SCC in situ, with borders uninvolved.
Patients who have had AKs are extremely likely to develop additional AKs. A notable percentage of AKs will, over time, develop into SCC in situ and then invasive SCC if not treated. While cryosurgery of an SK should not result in SCC, it’s most likely that in this case, an AK adjacent to the SK progressed to the SCC in situ.
There are multiple treatments available for SCC in situ. Topical imiquimod has been shown to be somewhat effective in stimulating the immune system, thus leading to resolution of SCC in situ. But there is a significant risk of recurrence. Topical 5-FU can be utilized on a daily or twice daily basis for 2 weeks (or up to several months). The risk of recurrence ranges from 7% to 33%. Electrodesiccation and curettage is often used for SCC in situ, with recurrence rates of 2% to 19%. Cryosurgery for SCC in situ requires an aggressive freeze, with freeze times of up to 30 seconds. Photodynamic therapy also is an option; however, it requires multiple sessions and is more costly than other treatment options.
This patient’s borders were uninvolved on pathology, but it was possible that there was some residual SCC in situ due to the standard “bread loaf slicing” used for routine pathology. To treat possible residual SCC in situ at the wound site and surrounding tissue, the patient was given a prescription for topical 5-FU to apply twice daily for 6 weeks. The patient was instructed to return for follow-up in 6 months, or sooner, if any problems arose.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Shimizu I, Cruz A, Chang KH, et al. Treatment of squamous cell carcinoma in situ: a review. Dermatol Surg. 2011;37:1394-1411.
This pink raised nodule underlying a scaly surface was suspicious for squamous cell carcinoma (SCC). Since this was a virtual visit, and the lesion required pathology due to the likelihood of cancer, the patient was brought into the clinic for additional evaluation. A broad-based deep shave biopsy was performed to remove the visible lesion. Pathology showed SCC in situ, with borders uninvolved.
Patients who have had AKs are extremely likely to develop additional AKs. A notable percentage of AKs will, over time, develop into SCC in situ and then invasive SCC if not treated. While cryosurgery of an SK should not result in SCC, it’s most likely that in this case, an AK adjacent to the SK progressed to the SCC in situ.
There are multiple treatments available for SCC in situ. Topical imiquimod has been shown to be somewhat effective in stimulating the immune system, thus leading to resolution of SCC in situ. But there is a significant risk of recurrence. Topical 5-FU can be utilized on a daily or twice daily basis for 2 weeks (or up to several months). The risk of recurrence ranges from 7% to 33%. Electrodesiccation and curettage is often used for SCC in situ, with recurrence rates of 2% to 19%. Cryosurgery for SCC in situ requires an aggressive freeze, with freeze times of up to 30 seconds. Photodynamic therapy also is an option; however, it requires multiple sessions and is more costly than other treatment options.
This patient’s borders were uninvolved on pathology, but it was possible that there was some residual SCC in situ due to the standard “bread loaf slicing” used for routine pathology. To treat possible residual SCC in situ at the wound site and surrounding tissue, the patient was given a prescription for topical 5-FU to apply twice daily for 6 weeks. The patient was instructed to return for follow-up in 6 months, or sooner, if any problems arose.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
This pink raised nodule underlying a scaly surface was suspicious for squamous cell carcinoma (SCC). Since this was a virtual visit, and the lesion required pathology due to the likelihood of cancer, the patient was brought into the clinic for additional evaluation. A broad-based deep shave biopsy was performed to remove the visible lesion. Pathology showed SCC in situ, with borders uninvolved.
Patients who have had AKs are extremely likely to develop additional AKs. A notable percentage of AKs will, over time, develop into SCC in situ and then invasive SCC if not treated. While cryosurgery of an SK should not result in SCC, it’s most likely that in this case, an AK adjacent to the SK progressed to the SCC in situ.
There are multiple treatments available for SCC in situ. Topical imiquimod has been shown to be somewhat effective in stimulating the immune system, thus leading to resolution of SCC in situ. But there is a significant risk of recurrence. Topical 5-FU can be utilized on a daily or twice daily basis for 2 weeks (or up to several months). The risk of recurrence ranges from 7% to 33%. Electrodesiccation and curettage is often used for SCC in situ, with recurrence rates of 2% to 19%. Cryosurgery for SCC in situ requires an aggressive freeze, with freeze times of up to 30 seconds. Photodynamic therapy also is an option; however, it requires multiple sessions and is more costly than other treatment options.
This patient’s borders were uninvolved on pathology, but it was possible that there was some residual SCC in situ due to the standard “bread loaf slicing” used for routine pathology. To treat possible residual SCC in situ at the wound site and surrounding tissue, the patient was given a prescription for topical 5-FU to apply twice daily for 6 weeks. The patient was instructed to return for follow-up in 6 months, or sooner, if any problems arose.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Shimizu I, Cruz A, Chang KH, et al. Treatment of squamous cell carcinoma in situ: a review. Dermatol Surg. 2011;37:1394-1411.
Shimizu I, Cruz A, Chang KH, et al. Treatment of squamous cell carcinoma in situ: a review. Dermatol Surg. 2011;37:1394-1411.
Most clinicians undertreat childhood lichen sclerosus
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
In the clinical experience of Libby Edwards, MD, the diagnosis of lichen sclerosus in a young girl often triggers worry from patients and parents alike.
“The parents are worried about the ramifications of genital diseases and they’re worried about scarring,” she said during the virtual annual meeting of the Society for Pediatric Dermatology.
Meanwhile, during the initial assessment, physicians tend to think about sexual abuse or sexually transmitted diseases as the primary culprit. “It’s really important that you consider those issues, but they’re not usually what’s going on,” said Dr. Edwards, a dermatologist who practices in Charlotte, N.C. “Also, for some reason we jump to yeast as a cause of diseases in the genital area. If the child is out of diapers and hasn’t reached puberty, it’s almost never yeast. Do a culture. Try and prove yeast. If it doesn’t respond to treatment for yeast, it’s not going to be yeast. Reassure, and don’t forget to reassure.”
. Lichen sclerosus presents classically as white, fragile plaques. “Textbooks say that there is cigarette paper-like crinkling of skin,” Dr. Edwards said. “I think of it being more like cellophane paper. In children, we often see it as smooth, kind of waxy and shiny, compared to adults. Children usually present with pruritus and irritation.”
Lichen sclerosus often starts in the clitoral area and on the perineum, and often with an edematous clitoral hood. “It often eventuates into clitoral phimosis, meaning that there is midline adhesion so that the clitoris is buried,” she said. “In adults, seeing this clitoral phimosis is a reliable sign of a scarring dermatosis – most often lichen sclerosus. But you can’t say that in children, because little girls will often have scarring over the clitoris. It’s just physiologic and means nothing, and it will go away at puberty. Certainly, sometimes this white discoloration can have crinkling. Purpura and tearing are common; if you look at lichen sclerosus histologically it looks like a thin epithelium that’s stretched over gelatin. Any rubbing and scratching can cause bleeding in the skin.”
Clinical appearance of well demarcated white skin with texture change drives the diagnosis. “It can be hard to tell from vitiligo at times, but there always should be texture change – whether it’s crinkling, whether it’s waxy, whether it’s smooth – and it’s symptomatic,” she said.
A biopsy is not usually required. “I think a good picture [of the affected area] or some sort of objective description in the chart is important, because most children do so well that in a few months there’s no sign of it, and the next provider [they see] may not believe that they ever had it,” she said.
The recommended initial treatment for lichen sclerosus in girls is a tiny amount of a superpotent topical corticosteroid ointment such as clobetasol or halobetasol one to two times daily until the skin is clear, which usually takes 2-4 months. “You do not treat these children until they’re comfortable, because that may be a week,” Dr. Edwards said. “You treat these children until the skin looks normal. Then you need to keep treating them, because if you don’t, the skin will relapse, even though they might not have symptoms.”
Following initial treatment, she recommends use of a superpotent corticosteroid once per day three times a week, or a midpotency steroid like triamcinolone ointment 0.1% every day. In her clinical experience, if lesions clear and remain clear with long-term treatment through puberty, the chances are good that they’ll stay clear if the medication is stopped.
“There are no studies on what to do after a patient clears,” said Dr. Edwards, chief of dermatology at Carolinas Medical Center, Charlotte, and adjunct clinical professor of dermatology at the University of North Carolina, Chapel Hill. “We have been informed by trial and error. If a child is totally clear after puberty, I will stop their medication and see them back every 3 months for about a year and a half. If they stay clear after a year and a half, I find that they stay clear. I wonder what happens at menopause. We surely don’t know.”
With consistent topical treatment, many patients will have clearing in one area of affected skin after a month or two, and it will take 3 or 4 months for the remaining area to clear. “I tend to see patients back every 6-8 weeks until they’re clear,” she said. “I do not like the idea of sending people out and saying, ‘use this medication twice a day for a month, then once a day for a month, then three times a week, then as needed.’
For patients concerned about the long-term use of topical steroids, the immunosuppressants tacrolimus and pimecrolimus are options. “They are often irritating on the vulva, but can work better than steroids for extragenital disease,” Dr. Edwards said. “Parents sometimes object to the use of a corticosteroid, but because these produce slower benefit and often burn with application, you can remind the parents that tacrolimus and pimecrolimus are not without side effects and are labeled as being associated with cancer. That often will prompt a parent to be willing to use a topical steroid. You can also point to studies that show the safety of topical steroids.”
Intralesional steroids are useful for thick lesions, but Dr. Edwards said that she has never had to use them in a child with lichen sclerosus. “I have found methotrexate to be useful in some people, but there is not one study on genital lichen sclerosus and methotrexate,” she said. “I find that about one in five patients with recalcitrant vulvar lichen sclerosus has had some benefit from methotrexate,” she added, noting that fractional CO2 laser “is showing promise in these patients.”
Dr. Edwards concluded her remarks by noting that she has never cared for a child with vulvar lichen sclerosus who didn’t respond to topical super potent steroids, “except due to poor compliance.”
She reported having no relevant financial disclosures.
FROM SPD 2020
Beyond PASI 100: striving for molecular clearance
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
FROM AAD 20
Study highlights potential advantages of tape strips over biopsy
for monitoring these and potentially other dermatologic diseases, according to the latest advances with this approach.
“Tape strips are not going to fully replace biopsies, but we think they will have an important role in diagnosing and monitoring response to therapy by avoiding the potential scarring and pain of biopsy,” reported Emma Guttman-Yassky, MD, PhD, professor of dermatology and director of the laboratory inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai Medical Center, New York.
The concept of using adhesive strips to remove surface skin cells for clinical study has been around for more than 20 years, but there has been recent progress. A newly published study, which compared skin from patients with atopic dermatitis (AD) or psoriasis with that of controls, was characterized as “the most comprehensive tape strip molecular profiling in any inflammatory skin disease to date and the first to fully characterize and compare AD to psoriasis,” wrote Dr. Guttman-Yassky, the senior author, and coauthors.
It also appears to be a leap forward. RNA sequencing detected thousands of differentially expressed genes reflecting immune and barrier biomarkers characteristic of the molecular phenotypes of atopic dermatitis and psoriasis. These were not only found to be consistent with biopsy studies but identified additional unique genes and pathways relevant to their pathological signature.
“In the past, the success rate for transcriptome sequencing even for a more limited panel of proteins was approaching 50% when considering both lesional, nonlesional skin, and healthy skin, but we are now approaching 100% for sample recovery and for analysis of RNA and genes,” Dr. Guttman-Yassky said in an interview.
Tissue samples were obtained with tape strips from lesional and nonlesional skin from 20 patients with AD and 20 patients with psoriasis. Compared with 20 tape strips from controls, they were evaluated with RNA sequencing followed by quantitative real-time polymerase chain reaction of immune and barrier biomarkers.
The sample recovery rate was 96% overall and 95% or better regardless of whether the skin was lesional or nonlesional.
With RNA sequencing of more than 20,000 transcripts, including multiple cellular, immune, and barrier biomarkers, an enormous amount of data was generated, but the key finding is that these diseases are readily distinguished with profiling based on tape strips.
Although numerous biomarkers were shared, “tape strips completely discriminate between atopic dermatitis and psoriasis with a degree of reliability that is comparable to skin biopsy,” Dr. Guttman-Yassky said.
One of the biomarkers, expression of nitric oxide synthase 2/inducible nitric oxide synthase, distinguished AD from psoriasis with 100% accuracy. As previously reported in biopsy studies, other biomarkers collectively associated AD with a profile related to a Th2-type inflammatory response and psoriasis with a Th17-type inflammatory response.
Tape strips also confirmed significant pathology in the nonlesional as well as the lesional skin of patients with AD or psoriasis. This included an increase in Th2-type products, such as interleukin-4 and IL-13, in nonlesional skin of atopic dermatitis and Th17-type products, such as IL-17, in nonlesional skin of psoriasis.
Some biomarkers of AD and psoriasis had an even greater differentiation in tape strips than previously reported from biopsy studies, according to Dr. Guttman-Yassky. In this study, tape strips also captured more differentially expressed genes than previously reported with biopsies.
One potential limitation of tape strips is that the RNA isolation process is time consuming, but this might be less of an issue in routine clinical use if there is a more refined number of biomarkers that are targeted or if technological improvements simplify processing, Dr. Guttman-Yassky pointed out.
To develop clinical utility for tape strips beyond AD and psoriasis, more work is needed to standardize the depth of sampling, which is variable with tape strips, she noted. Depth is relevant to the analysis of gene expression and mRNA activity of each dermatologic disease.
“Tape strips remain a research tool for now, but we do think that this technique can be refined and employed for clinical purposes, including diagnosis and monitoring response to treatment,” she said.
Relative to biopsy, the advantages are not difficult to envision. Dr. Guttman-Yassky, who recently published a study of tape strips for evaluating AD in children emphasized that tape strips are generally painless.
“Patients really do not mind tape strips,” she said. Although she believes that tape strips are providing unique insight into the pathology of inflammatory diseases not necessarily available with biopsy, she emphasized the practical value. Not least, “these could really help when the goal is to evaluate response to therapy over time.”
Another investigator who has conducted studies with tape strips, Maja-Lisa Clausen, MD, PhD, also thinks tape strips are likely to become routine clinical tools.
“Once the basis research, validation, and data are out, I think numerous companies will be ready to develop machines for more quick and easy processing, compared to the more labor intensive process that is used today for research,” explained Dr. Clausen, who is in the department of dermatology, Bispebjerb Hospital, University of Copenhagen.
She considers tape strips particularly promising for children, but she thinks the biomarker profiling made possible by these strips might be leading to personalized treatment programs for dermatologic diseases.
“What we need is further validation; which tape to use, how deep, and the importance of storage, which is a big issue in the clinic,” Dr. Clausen said in an interview.
Dr. Guttman-Yassky has financial relationships with multiple pharmaceutical companies, including those with therapies for psoriasis.
SOURCE: Guttman-Yassky E et al. J Allergy Clin Immunol. 2020 Jul 9. doi: 10.1016/j.jaci.2020.05.048.
for monitoring these and potentially other dermatologic diseases, according to the latest advances with this approach.
“Tape strips are not going to fully replace biopsies, but we think they will have an important role in diagnosing and monitoring response to therapy by avoiding the potential scarring and pain of biopsy,” reported Emma Guttman-Yassky, MD, PhD, professor of dermatology and director of the laboratory inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai Medical Center, New York.
The concept of using adhesive strips to remove surface skin cells for clinical study has been around for more than 20 years, but there has been recent progress. A newly published study, which compared skin from patients with atopic dermatitis (AD) or psoriasis with that of controls, was characterized as “the most comprehensive tape strip molecular profiling in any inflammatory skin disease to date and the first to fully characterize and compare AD to psoriasis,” wrote Dr. Guttman-Yassky, the senior author, and coauthors.
It also appears to be a leap forward. RNA sequencing detected thousands of differentially expressed genes reflecting immune and barrier biomarkers characteristic of the molecular phenotypes of atopic dermatitis and psoriasis. These were not only found to be consistent with biopsy studies but identified additional unique genes and pathways relevant to their pathological signature.
“In the past, the success rate for transcriptome sequencing even for a more limited panel of proteins was approaching 50% when considering both lesional, nonlesional skin, and healthy skin, but we are now approaching 100% for sample recovery and for analysis of RNA and genes,” Dr. Guttman-Yassky said in an interview.
Tissue samples were obtained with tape strips from lesional and nonlesional skin from 20 patients with AD and 20 patients with psoriasis. Compared with 20 tape strips from controls, they were evaluated with RNA sequencing followed by quantitative real-time polymerase chain reaction of immune and barrier biomarkers.
The sample recovery rate was 96% overall and 95% or better regardless of whether the skin was lesional or nonlesional.
With RNA sequencing of more than 20,000 transcripts, including multiple cellular, immune, and barrier biomarkers, an enormous amount of data was generated, but the key finding is that these diseases are readily distinguished with profiling based on tape strips.
Although numerous biomarkers were shared, “tape strips completely discriminate between atopic dermatitis and psoriasis with a degree of reliability that is comparable to skin biopsy,” Dr. Guttman-Yassky said.
One of the biomarkers, expression of nitric oxide synthase 2/inducible nitric oxide synthase, distinguished AD from psoriasis with 100% accuracy. As previously reported in biopsy studies, other biomarkers collectively associated AD with a profile related to a Th2-type inflammatory response and psoriasis with a Th17-type inflammatory response.
Tape strips also confirmed significant pathology in the nonlesional as well as the lesional skin of patients with AD or psoriasis. This included an increase in Th2-type products, such as interleukin-4 and IL-13, in nonlesional skin of atopic dermatitis and Th17-type products, such as IL-17, in nonlesional skin of psoriasis.
Some biomarkers of AD and psoriasis had an even greater differentiation in tape strips than previously reported from biopsy studies, according to Dr. Guttman-Yassky. In this study, tape strips also captured more differentially expressed genes than previously reported with biopsies.
One potential limitation of tape strips is that the RNA isolation process is time consuming, but this might be less of an issue in routine clinical use if there is a more refined number of biomarkers that are targeted or if technological improvements simplify processing, Dr. Guttman-Yassky pointed out.
To develop clinical utility for tape strips beyond AD and psoriasis, more work is needed to standardize the depth of sampling, which is variable with tape strips, she noted. Depth is relevant to the analysis of gene expression and mRNA activity of each dermatologic disease.
“Tape strips remain a research tool for now, but we do think that this technique can be refined and employed for clinical purposes, including diagnosis and monitoring response to treatment,” she said.
Relative to biopsy, the advantages are not difficult to envision. Dr. Guttman-Yassky, who recently published a study of tape strips for evaluating AD in children emphasized that tape strips are generally painless.
“Patients really do not mind tape strips,” she said. Although she believes that tape strips are providing unique insight into the pathology of inflammatory diseases not necessarily available with biopsy, she emphasized the practical value. Not least, “these could really help when the goal is to evaluate response to therapy over time.”
Another investigator who has conducted studies with tape strips, Maja-Lisa Clausen, MD, PhD, also thinks tape strips are likely to become routine clinical tools.
“Once the basis research, validation, and data are out, I think numerous companies will be ready to develop machines for more quick and easy processing, compared to the more labor intensive process that is used today for research,” explained Dr. Clausen, who is in the department of dermatology, Bispebjerb Hospital, University of Copenhagen.
She considers tape strips particularly promising for children, but she thinks the biomarker profiling made possible by these strips might be leading to personalized treatment programs for dermatologic diseases.
“What we need is further validation; which tape to use, how deep, and the importance of storage, which is a big issue in the clinic,” Dr. Clausen said in an interview.
Dr. Guttman-Yassky has financial relationships with multiple pharmaceutical companies, including those with therapies for psoriasis.
SOURCE: Guttman-Yassky E et al. J Allergy Clin Immunol. 2020 Jul 9. doi: 10.1016/j.jaci.2020.05.048.
for monitoring these and potentially other dermatologic diseases, according to the latest advances with this approach.
“Tape strips are not going to fully replace biopsies, but we think they will have an important role in diagnosing and monitoring response to therapy by avoiding the potential scarring and pain of biopsy,” reported Emma Guttman-Yassky, MD, PhD, professor of dermatology and director of the laboratory inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai Medical Center, New York.
The concept of using adhesive strips to remove surface skin cells for clinical study has been around for more than 20 years, but there has been recent progress. A newly published study, which compared skin from patients with atopic dermatitis (AD) or psoriasis with that of controls, was characterized as “the most comprehensive tape strip molecular profiling in any inflammatory skin disease to date and the first to fully characterize and compare AD to psoriasis,” wrote Dr. Guttman-Yassky, the senior author, and coauthors.
It also appears to be a leap forward. RNA sequencing detected thousands of differentially expressed genes reflecting immune and barrier biomarkers characteristic of the molecular phenotypes of atopic dermatitis and psoriasis. These were not only found to be consistent with biopsy studies but identified additional unique genes and pathways relevant to their pathological signature.
“In the past, the success rate for transcriptome sequencing even for a more limited panel of proteins was approaching 50% when considering both lesional, nonlesional skin, and healthy skin, but we are now approaching 100% for sample recovery and for analysis of RNA and genes,” Dr. Guttman-Yassky said in an interview.
Tissue samples were obtained with tape strips from lesional and nonlesional skin from 20 patients with AD and 20 patients with psoriasis. Compared with 20 tape strips from controls, they were evaluated with RNA sequencing followed by quantitative real-time polymerase chain reaction of immune and barrier biomarkers.
The sample recovery rate was 96% overall and 95% or better regardless of whether the skin was lesional or nonlesional.
With RNA sequencing of more than 20,000 transcripts, including multiple cellular, immune, and barrier biomarkers, an enormous amount of data was generated, but the key finding is that these diseases are readily distinguished with profiling based on tape strips.
Although numerous biomarkers were shared, “tape strips completely discriminate between atopic dermatitis and psoriasis with a degree of reliability that is comparable to skin biopsy,” Dr. Guttman-Yassky said.
One of the biomarkers, expression of nitric oxide synthase 2/inducible nitric oxide synthase, distinguished AD from psoriasis with 100% accuracy. As previously reported in biopsy studies, other biomarkers collectively associated AD with a profile related to a Th2-type inflammatory response and psoriasis with a Th17-type inflammatory response.
Tape strips also confirmed significant pathology in the nonlesional as well as the lesional skin of patients with AD or psoriasis. This included an increase in Th2-type products, such as interleukin-4 and IL-13, in nonlesional skin of atopic dermatitis and Th17-type products, such as IL-17, in nonlesional skin of psoriasis.
Some biomarkers of AD and psoriasis had an even greater differentiation in tape strips than previously reported from biopsy studies, according to Dr. Guttman-Yassky. In this study, tape strips also captured more differentially expressed genes than previously reported with biopsies.
One potential limitation of tape strips is that the RNA isolation process is time consuming, but this might be less of an issue in routine clinical use if there is a more refined number of biomarkers that are targeted or if technological improvements simplify processing, Dr. Guttman-Yassky pointed out.
To develop clinical utility for tape strips beyond AD and psoriasis, more work is needed to standardize the depth of sampling, which is variable with tape strips, she noted. Depth is relevant to the analysis of gene expression and mRNA activity of each dermatologic disease.
“Tape strips remain a research tool for now, but we do think that this technique can be refined and employed for clinical purposes, including diagnosis and monitoring response to treatment,” she said.
Relative to biopsy, the advantages are not difficult to envision. Dr. Guttman-Yassky, who recently published a study of tape strips for evaluating AD in children emphasized that tape strips are generally painless.
“Patients really do not mind tape strips,” she said. Although she believes that tape strips are providing unique insight into the pathology of inflammatory diseases not necessarily available with biopsy, she emphasized the practical value. Not least, “these could really help when the goal is to evaluate response to therapy over time.”
Another investigator who has conducted studies with tape strips, Maja-Lisa Clausen, MD, PhD, also thinks tape strips are likely to become routine clinical tools.
“Once the basis research, validation, and data are out, I think numerous companies will be ready to develop machines for more quick and easy processing, compared to the more labor intensive process that is used today for research,” explained Dr. Clausen, who is in the department of dermatology, Bispebjerb Hospital, University of Copenhagen.
She considers tape strips particularly promising for children, but she thinks the biomarker profiling made possible by these strips might be leading to personalized treatment programs for dermatologic diseases.
“What we need is further validation; which tape to use, how deep, and the importance of storage, which is a big issue in the clinic,” Dr. Clausen said in an interview.
Dr. Guttman-Yassky has financial relationships with multiple pharmaceutical companies, including those with therapies for psoriasis.
SOURCE: Guttman-Yassky E et al. J Allergy Clin Immunol. 2020 Jul 9. doi: 10.1016/j.jaci.2020.05.048.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Rheumatologist Lindsey Criswell named new NIAMS director
.
Dr. Criswell, vice chancellor of research at the University of California, San Francisco, will replace acting director Robert H. Carter, MD, who has overseen NIAMS since December 2018, following the unexpected death of longtime director Stephen I. Katz, MD, PhD, who had directed the institute since 1995. She will start her new role in early 2021, according to the NIH.
“Dr. Criswell has rich experience as a clinician, researcher, and administrator. Her ability to oversee the research program of one of the country’s top research-intensive medical schools, and her expertise in autoimmune diseases, including rheumatoid arthritis and lupus, make her well-positioned to direct NIAMS,” said NIH director Francis S. Collins, MD, PhD, said in an announcement.
Dr. Criswell, who holds the Kenneth H. Fye, M.D., endowed chair in rheumatology and the Jean S. Engleman Distinguished Professorship in Rheumatology at UCSF, spent most of her career at the university, focusing her research on the genetics and epidemiology of human autoimmune disease, particularly rheumatoid arthritis and systemic lupus erythematosus. Using genome-wide association and other genetic studies, her research team contributed to the identification of more than 30 genes linked to these disorders, according to the NIH.
NIAMS has a budget of nearly $625 million and its extramural research program supports scientific studies and research training and career development throughout the country through grants and contracts to research organizations in fields that include rheumatology, muscle biology, orthopedics, bone and mineral metabolism, and dermatology.
.
Dr. Criswell, vice chancellor of research at the University of California, San Francisco, will replace acting director Robert H. Carter, MD, who has overseen NIAMS since December 2018, following the unexpected death of longtime director Stephen I. Katz, MD, PhD, who had directed the institute since 1995. She will start her new role in early 2021, according to the NIH.
“Dr. Criswell has rich experience as a clinician, researcher, and administrator. Her ability to oversee the research program of one of the country’s top research-intensive medical schools, and her expertise in autoimmune diseases, including rheumatoid arthritis and lupus, make her well-positioned to direct NIAMS,” said NIH director Francis S. Collins, MD, PhD, said in an announcement.
Dr. Criswell, who holds the Kenneth H. Fye, M.D., endowed chair in rheumatology and the Jean S. Engleman Distinguished Professorship in Rheumatology at UCSF, spent most of her career at the university, focusing her research on the genetics and epidemiology of human autoimmune disease, particularly rheumatoid arthritis and systemic lupus erythematosus. Using genome-wide association and other genetic studies, her research team contributed to the identification of more than 30 genes linked to these disorders, according to the NIH.
NIAMS has a budget of nearly $625 million and its extramural research program supports scientific studies and research training and career development throughout the country through grants and contracts to research organizations in fields that include rheumatology, muscle biology, orthopedics, bone and mineral metabolism, and dermatology.
.
Dr. Criswell, vice chancellor of research at the University of California, San Francisco, will replace acting director Robert H. Carter, MD, who has overseen NIAMS since December 2018, following the unexpected death of longtime director Stephen I. Katz, MD, PhD, who had directed the institute since 1995. She will start her new role in early 2021, according to the NIH.
“Dr. Criswell has rich experience as a clinician, researcher, and administrator. Her ability to oversee the research program of one of the country’s top research-intensive medical schools, and her expertise in autoimmune diseases, including rheumatoid arthritis and lupus, make her well-positioned to direct NIAMS,” said NIH director Francis S. Collins, MD, PhD, said in an announcement.
Dr. Criswell, who holds the Kenneth H. Fye, M.D., endowed chair in rheumatology and the Jean S. Engleman Distinguished Professorship in Rheumatology at UCSF, spent most of her career at the university, focusing her research on the genetics and epidemiology of human autoimmune disease, particularly rheumatoid arthritis and systemic lupus erythematosus. Using genome-wide association and other genetic studies, her research team contributed to the identification of more than 30 genes linked to these disorders, according to the NIH.
NIAMS has a budget of nearly $625 million and its extramural research program supports scientific studies and research training and career development throughout the country through grants and contracts to research organizations in fields that include rheumatology, muscle biology, orthopedics, bone and mineral metabolism, and dermatology.
Dermatology atlas will profile disease in all skin types
An atlas that displays the unique nuances between different skin types across the spectrum of dermatologic disease is scheduled for release this coming winter.
Available as an e-book or physical text, Dermatologists need to know what skin diseases look like on all types of skin, said Adam Friedman, MD, who is developing this e-book with Misty Eleryan, MD, MS.
From the SARS-CoV-2 pandemic, multiple nonviral pandemics have rapidly emerged, “most notably the persistent and well-masked racism that maintains disparities in all facets of life, from economics to health care,” Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington, said in an interview.
In dermatology, “clear disparities in workforce representation and trainee/practitioner education have become more apparent than ever before,” he added.
The project is a collaboration of George Washington University and education publishers Sanova Works and Educational Testing & Assessment Systems.
As a person of color who recently completed her residency in dermatology at George Washington University, Dr. Eleryan had noticed a lack of diversity in photos of common dermatoses. This can contribute to misdiagnoses and delays in treatment in patients of color, Dr. Eleryan, who is now a micrographic surgery and dermatologic oncology fellow at the University of California, Los Angeles, said in an interview.
“We recognized the gap, which is the lack of diversity/variation of skin tones in our dermatology textbooks and atlases,” she added.
The project was several years in the making, Dr. Friedman said. To do this right, “you need resources, funding, and a collaborative and galvanized team of experts.” That involved coordinating with several medical publishers and amassing a team of medical photographers and an expert panel that will assist in evaluating and securing difficult-to-access clinical images.
The atlas is one of several initiatives in the dermatology field to address racial disparities in patient care.
Noticing similar information gaps about clinical presentations in darker skin, a medical student in the United Kingdom, Malone Mukwende, created “Mind the Gap,” a handbook that presents side-by-side images of diseases and illnesses in light and dark skin. This project “highlights how far behind we are, that a medical student with minimal dermatology exposure and experience not only recognized the need but was ready to do something about it,” Dr. Friedman noted.
Others in the field have spotlighted disparities in the medical literature. The SARS-CoV-2 pandemic has especially brought this out, Graeme M. Lipper, MD wrote in a recent editorial for this news organization.
He referred to a literature review of 46 articles describing COVID-19–associated skin manifestations, which included mostly (92%) images in patients with skin types I-III (92%) and none in patients with skin types V or VI.
“These investigators have identified a damning lack of images of COVID-19–associated skin manifestations in patients with darker skin,” added Dr. Lipper, an assistant clinical professor at the University of Vermont, Burlington, and a staff physician in the department of dermatology at Danbury (Conn.) Hospital.
For now, Dr. Friedman said that the atlas won’t contain a specific section on COVID-19 skin manifestations, although viral-associated skin reactions like morbilliform eruptions, urticaria, and retiform purpura will be displayed. Overall, the atlas will address 60-70 skin conditions.
Physicians who fail to educate themselves on the variations of skin conditions in all skin types may potentially harm patients of color, Dr. Eleryan said. As Dr. Lipper noted in his editorial, nearly half of all dermatologists feel they haven’t had adequate exposure to diseases in skin of color.
“Our atlas will fill that void and hopefully assist in closing the gap in health disparities among patients of color, who are often misdiagnosed or rendered diagnoses very late in the disease process,” Dr. Eleryan said.
An atlas that displays the unique nuances between different skin types across the spectrum of dermatologic disease is scheduled for release this coming winter.
Available as an e-book or physical text, Dermatologists need to know what skin diseases look like on all types of skin, said Adam Friedman, MD, who is developing this e-book with Misty Eleryan, MD, MS.
From the SARS-CoV-2 pandemic, multiple nonviral pandemics have rapidly emerged, “most notably the persistent and well-masked racism that maintains disparities in all facets of life, from economics to health care,” Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington, said in an interview.
In dermatology, “clear disparities in workforce representation and trainee/practitioner education have become more apparent than ever before,” he added.
The project is a collaboration of George Washington University and education publishers Sanova Works and Educational Testing & Assessment Systems.
As a person of color who recently completed her residency in dermatology at George Washington University, Dr. Eleryan had noticed a lack of diversity in photos of common dermatoses. This can contribute to misdiagnoses and delays in treatment in patients of color, Dr. Eleryan, who is now a micrographic surgery and dermatologic oncology fellow at the University of California, Los Angeles, said in an interview.
“We recognized the gap, which is the lack of diversity/variation of skin tones in our dermatology textbooks and atlases,” she added.
The project was several years in the making, Dr. Friedman said. To do this right, “you need resources, funding, and a collaborative and galvanized team of experts.” That involved coordinating with several medical publishers and amassing a team of medical photographers and an expert panel that will assist in evaluating and securing difficult-to-access clinical images.
The atlas is one of several initiatives in the dermatology field to address racial disparities in patient care.
Noticing similar information gaps about clinical presentations in darker skin, a medical student in the United Kingdom, Malone Mukwende, created “Mind the Gap,” a handbook that presents side-by-side images of diseases and illnesses in light and dark skin. This project “highlights how far behind we are, that a medical student with minimal dermatology exposure and experience not only recognized the need but was ready to do something about it,” Dr. Friedman noted.
Others in the field have spotlighted disparities in the medical literature. The SARS-CoV-2 pandemic has especially brought this out, Graeme M. Lipper, MD wrote in a recent editorial for this news organization.
He referred to a literature review of 46 articles describing COVID-19–associated skin manifestations, which included mostly (92%) images in patients with skin types I-III (92%) and none in patients with skin types V or VI.
“These investigators have identified a damning lack of images of COVID-19–associated skin manifestations in patients with darker skin,” added Dr. Lipper, an assistant clinical professor at the University of Vermont, Burlington, and a staff physician in the department of dermatology at Danbury (Conn.) Hospital.
For now, Dr. Friedman said that the atlas won’t contain a specific section on COVID-19 skin manifestations, although viral-associated skin reactions like morbilliform eruptions, urticaria, and retiform purpura will be displayed. Overall, the atlas will address 60-70 skin conditions.
Physicians who fail to educate themselves on the variations of skin conditions in all skin types may potentially harm patients of color, Dr. Eleryan said. As Dr. Lipper noted in his editorial, nearly half of all dermatologists feel they haven’t had adequate exposure to diseases in skin of color.
“Our atlas will fill that void and hopefully assist in closing the gap in health disparities among patients of color, who are often misdiagnosed or rendered diagnoses very late in the disease process,” Dr. Eleryan said.
An atlas that displays the unique nuances between different skin types across the spectrum of dermatologic disease is scheduled for release this coming winter.
Available as an e-book or physical text, Dermatologists need to know what skin diseases look like on all types of skin, said Adam Friedman, MD, who is developing this e-book with Misty Eleryan, MD, MS.
From the SARS-CoV-2 pandemic, multiple nonviral pandemics have rapidly emerged, “most notably the persistent and well-masked racism that maintains disparities in all facets of life, from economics to health care,” Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington, said in an interview.
In dermatology, “clear disparities in workforce representation and trainee/practitioner education have become more apparent than ever before,” he added.
The project is a collaboration of George Washington University and education publishers Sanova Works and Educational Testing & Assessment Systems.
As a person of color who recently completed her residency in dermatology at George Washington University, Dr. Eleryan had noticed a lack of diversity in photos of common dermatoses. This can contribute to misdiagnoses and delays in treatment in patients of color, Dr. Eleryan, who is now a micrographic surgery and dermatologic oncology fellow at the University of California, Los Angeles, said in an interview.
“We recognized the gap, which is the lack of diversity/variation of skin tones in our dermatology textbooks and atlases,” she added.
The project was several years in the making, Dr. Friedman said. To do this right, “you need resources, funding, and a collaborative and galvanized team of experts.” That involved coordinating with several medical publishers and amassing a team of medical photographers and an expert panel that will assist in evaluating and securing difficult-to-access clinical images.
The atlas is one of several initiatives in the dermatology field to address racial disparities in patient care.
Noticing similar information gaps about clinical presentations in darker skin, a medical student in the United Kingdom, Malone Mukwende, created “Mind the Gap,” a handbook that presents side-by-side images of diseases and illnesses in light and dark skin. This project “highlights how far behind we are, that a medical student with minimal dermatology exposure and experience not only recognized the need but was ready to do something about it,” Dr. Friedman noted.
Others in the field have spotlighted disparities in the medical literature. The SARS-CoV-2 pandemic has especially brought this out, Graeme M. Lipper, MD wrote in a recent editorial for this news organization.
He referred to a literature review of 46 articles describing COVID-19–associated skin manifestations, which included mostly (92%) images in patients with skin types I-III (92%) and none in patients with skin types V or VI.
“These investigators have identified a damning lack of images of COVID-19–associated skin manifestations in patients with darker skin,” added Dr. Lipper, an assistant clinical professor at the University of Vermont, Burlington, and a staff physician in the department of dermatology at Danbury (Conn.) Hospital.
For now, Dr. Friedman said that the atlas won’t contain a specific section on COVID-19 skin manifestations, although viral-associated skin reactions like morbilliform eruptions, urticaria, and retiform purpura will be displayed. Overall, the atlas will address 60-70 skin conditions.
Physicians who fail to educate themselves on the variations of skin conditions in all skin types may potentially harm patients of color, Dr. Eleryan said. As Dr. Lipper noted in his editorial, nearly half of all dermatologists feel they haven’t had adequate exposure to diseases in skin of color.
“Our atlas will fill that void and hopefully assist in closing the gap in health disparities among patients of color, who are often misdiagnosed or rendered diagnoses very late in the disease process,” Dr. Eleryan said.
Cutaneous clues linked to COVID-19 coagulation risk
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Large, painful facial cysts
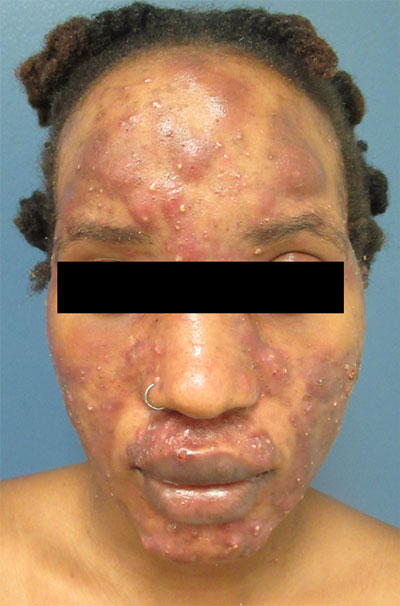
The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.

The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.