User login
FDA Approves Lymphir for R/R Cutaneous T-Cell Lymphoma
The immunotherapy is a reformulation of denileukin diftitox (Ontak), initially approved in 1999 for certain patients with persistent or recurrent cutaneous T-cell lymphoma. In 2014, the original formulation was voluntarily withdrawn from the US market. Citius acquired rights to market a reformulated product outside of Asia in 2021.
This is the first indication for Lymphir, which targets interleukin-2 receptors on malignant T cells.
This approval marks “a significant milestone” for patients with cutaneous T-cell lymphoma, a rare cancer, company CEO Leonard Mazur said in a press release announcing the approval. “The introduction of Lymphir, with its potential to rapidly reduce skin disease and control symptomatic itching without cumulative toxicity, is expected to expand the [cutaneous T-cell lymphoma] treatment landscape and grow the overall market, currently estimated to be $300-$400 million.”
Approval was based on the single-arm, open-label 302 study in 69 patients who had a median of four prior anticancer therapies. Patients received 9 mcg/kg daily from day 1 to day 5 of 21-day cycles until disease progression or unacceptable toxicity.
The objective response rate was 36.2%, including complete responses in 8.7% of patients. Responses lasted 6 months or longer in 52% of patients. Over 80% of subjects had a decrease in skin tumor burden, and almost a third had clinically significant improvements in pruritus.
Adverse events occurring in 20% or more of patients include increased transaminases, decreased albumin, decreased hemoglobin, nausea, edema, fatigue, musculoskeletal pain, rash, chills, constipation, pyrexia, and capillary leak syndrome.
Labeling carries a boxed warning of capillary leak syndrome. Other warnings include visual impairment, infusion reactions, hepatotoxicity, and embryo-fetal toxicity. Citius is under a postmarketing requirement to characterize the risk for visual impairment.
The company expects to launch the agent within 5 months.
A version of this article first appeared on Medscape.com.
The immunotherapy is a reformulation of denileukin diftitox (Ontak), initially approved in 1999 for certain patients with persistent or recurrent cutaneous T-cell lymphoma. In 2014, the original formulation was voluntarily withdrawn from the US market. Citius acquired rights to market a reformulated product outside of Asia in 2021.
This is the first indication for Lymphir, which targets interleukin-2 receptors on malignant T cells.
This approval marks “a significant milestone” for patients with cutaneous T-cell lymphoma, a rare cancer, company CEO Leonard Mazur said in a press release announcing the approval. “The introduction of Lymphir, with its potential to rapidly reduce skin disease and control symptomatic itching without cumulative toxicity, is expected to expand the [cutaneous T-cell lymphoma] treatment landscape and grow the overall market, currently estimated to be $300-$400 million.”
Approval was based on the single-arm, open-label 302 study in 69 patients who had a median of four prior anticancer therapies. Patients received 9 mcg/kg daily from day 1 to day 5 of 21-day cycles until disease progression or unacceptable toxicity.
The objective response rate was 36.2%, including complete responses in 8.7% of patients. Responses lasted 6 months or longer in 52% of patients. Over 80% of subjects had a decrease in skin tumor burden, and almost a third had clinically significant improvements in pruritus.
Adverse events occurring in 20% or more of patients include increased transaminases, decreased albumin, decreased hemoglobin, nausea, edema, fatigue, musculoskeletal pain, rash, chills, constipation, pyrexia, and capillary leak syndrome.
Labeling carries a boxed warning of capillary leak syndrome. Other warnings include visual impairment, infusion reactions, hepatotoxicity, and embryo-fetal toxicity. Citius is under a postmarketing requirement to characterize the risk for visual impairment.
The company expects to launch the agent within 5 months.
A version of this article first appeared on Medscape.com.
The immunotherapy is a reformulation of denileukin diftitox (Ontak), initially approved in 1999 for certain patients with persistent or recurrent cutaneous T-cell lymphoma. In 2014, the original formulation was voluntarily withdrawn from the US market. Citius acquired rights to market a reformulated product outside of Asia in 2021.
This is the first indication for Lymphir, which targets interleukin-2 receptors on malignant T cells.
This approval marks “a significant milestone” for patients with cutaneous T-cell lymphoma, a rare cancer, company CEO Leonard Mazur said in a press release announcing the approval. “The introduction of Lymphir, with its potential to rapidly reduce skin disease and control symptomatic itching without cumulative toxicity, is expected to expand the [cutaneous T-cell lymphoma] treatment landscape and grow the overall market, currently estimated to be $300-$400 million.”
Approval was based on the single-arm, open-label 302 study in 69 patients who had a median of four prior anticancer therapies. Patients received 9 mcg/kg daily from day 1 to day 5 of 21-day cycles until disease progression or unacceptable toxicity.
The objective response rate was 36.2%, including complete responses in 8.7% of patients. Responses lasted 6 months or longer in 52% of patients. Over 80% of subjects had a decrease in skin tumor burden, and almost a third had clinically significant improvements in pruritus.
Adverse events occurring in 20% or more of patients include increased transaminases, decreased albumin, decreased hemoglobin, nausea, edema, fatigue, musculoskeletal pain, rash, chills, constipation, pyrexia, and capillary leak syndrome.
Labeling carries a boxed warning of capillary leak syndrome. Other warnings include visual impairment, infusion reactions, hepatotoxicity, and embryo-fetal toxicity. Citius is under a postmarketing requirement to characterize the risk for visual impairment.
The company expects to launch the agent within 5 months.
A version of this article first appeared on Medscape.com.
Immunotherapy May Be Overused in Dying Patients With Cancer
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Modest Gains Shown in Breast Cancer Immunotherapy Trials
TOPLINE:
particularly among single-center studies which are more likely to go unreported, and many phase 2 studies failing to translate into successful phase 3 trials.
METHODOLOGY:
- Few immunotherapy agents — only pembrolizumab in the United States, as of December 2023, and atezolizumab in Europe — have received approvals for use in patients with breast cancer, indicating low returns on the large number of breast cancer immunotherapy trials launched in the early 2010s.
- In this cross-sectional study, researchers evaluated 331 immunotherapy trials, initiated between January 2004 and April 2023, that enrolled 48,844 patients with breast cancer.
- Of these, 47 were phase 1 trials, 242 were phase 2 trials, and 42 were phase 3 trials.
- A trial was considered reported if the results were posted on ClinicalTrial.gov or reported as an abstract or a manuscript.
- Overall, 120 trials met their completion date up to November 2022; of these, 30 (25%) failed to report outcomes, which included two phase 3 trials.
TAKEAWAY:
- Phase 1 trials had the highest rate of nonreporting (31.8%), followed by phase 2 (23.6%) and phase 3 (22.2%) trials.
- Single-center studies were more likely to be unreported than multicenter studies (35.2% vs 15.0%; P = .02).
- Of 90 reported trials, 47 (52.2%) met their primary endpoints and 43 (47.8%) did not.
- The majority, 17 out of 19 (89.5%), of the reported randomized trials had negative results.
IN PRACTICE:
“The findings of this study suggest that the large number of immunotherapy trials being run have yielded modest clinical impact,” the authors wrote. “More selective initiation of phase 2 trials, grounded in preclinical and biomarker observations and with optimal statistical designs for early efficacy assessment, is needed to increase trial efficiency.”
SOURCE:
The study, led by Marco Mariani, MD, Università Vita-Salute San Raffaele, Milan, Italy, was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ClinicalTrials.gov as the primary source of trial data might have resulted in some trials being overlooked. In addition, manual data extraction could cause inaccuracies and potentially introduced biases in the interpretation of trial results. Primary study completion date cutoff of December 2022 could have excluded significant data from more recent trials.
DISCLOSURES:
This study received support via Susan Komen Leadership Grant and the Fondazione AIRC per la Ricerca sul Cancro. Several authors reported receiving grants and personal fees and having other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly among single-center studies which are more likely to go unreported, and many phase 2 studies failing to translate into successful phase 3 trials.
METHODOLOGY:
- Few immunotherapy agents — only pembrolizumab in the United States, as of December 2023, and atezolizumab in Europe — have received approvals for use in patients with breast cancer, indicating low returns on the large number of breast cancer immunotherapy trials launched in the early 2010s.
- In this cross-sectional study, researchers evaluated 331 immunotherapy trials, initiated between January 2004 and April 2023, that enrolled 48,844 patients with breast cancer.
- Of these, 47 were phase 1 trials, 242 were phase 2 trials, and 42 were phase 3 trials.
- A trial was considered reported if the results were posted on ClinicalTrial.gov or reported as an abstract or a manuscript.
- Overall, 120 trials met their completion date up to November 2022; of these, 30 (25%) failed to report outcomes, which included two phase 3 trials.
TAKEAWAY:
- Phase 1 trials had the highest rate of nonreporting (31.8%), followed by phase 2 (23.6%) and phase 3 (22.2%) trials.
- Single-center studies were more likely to be unreported than multicenter studies (35.2% vs 15.0%; P = .02).
- Of 90 reported trials, 47 (52.2%) met their primary endpoints and 43 (47.8%) did not.
- The majority, 17 out of 19 (89.5%), of the reported randomized trials had negative results.
IN PRACTICE:
“The findings of this study suggest that the large number of immunotherapy trials being run have yielded modest clinical impact,” the authors wrote. “More selective initiation of phase 2 trials, grounded in preclinical and biomarker observations and with optimal statistical designs for early efficacy assessment, is needed to increase trial efficiency.”
SOURCE:
The study, led by Marco Mariani, MD, Università Vita-Salute San Raffaele, Milan, Italy, was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ClinicalTrials.gov as the primary source of trial data might have resulted in some trials being overlooked. In addition, manual data extraction could cause inaccuracies and potentially introduced biases in the interpretation of trial results. Primary study completion date cutoff of December 2022 could have excluded significant data from more recent trials.
DISCLOSURES:
This study received support via Susan Komen Leadership Grant and the Fondazione AIRC per la Ricerca sul Cancro. Several authors reported receiving grants and personal fees and having other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly among single-center studies which are more likely to go unreported, and many phase 2 studies failing to translate into successful phase 3 trials.
METHODOLOGY:
- Few immunotherapy agents — only pembrolizumab in the United States, as of December 2023, and atezolizumab in Europe — have received approvals for use in patients with breast cancer, indicating low returns on the large number of breast cancer immunotherapy trials launched in the early 2010s.
- In this cross-sectional study, researchers evaluated 331 immunotherapy trials, initiated between January 2004 and April 2023, that enrolled 48,844 patients with breast cancer.
- Of these, 47 were phase 1 trials, 242 were phase 2 trials, and 42 were phase 3 trials.
- A trial was considered reported if the results were posted on ClinicalTrial.gov or reported as an abstract or a manuscript.
- Overall, 120 trials met their completion date up to November 2022; of these, 30 (25%) failed to report outcomes, which included two phase 3 trials.
TAKEAWAY:
- Phase 1 trials had the highest rate of nonreporting (31.8%), followed by phase 2 (23.6%) and phase 3 (22.2%) trials.
- Single-center studies were more likely to be unreported than multicenter studies (35.2% vs 15.0%; P = .02).
- Of 90 reported trials, 47 (52.2%) met their primary endpoints and 43 (47.8%) did not.
- The majority, 17 out of 19 (89.5%), of the reported randomized trials had negative results.
IN PRACTICE:
“The findings of this study suggest that the large number of immunotherapy trials being run have yielded modest clinical impact,” the authors wrote. “More selective initiation of phase 2 trials, grounded in preclinical and biomarker observations and with optimal statistical designs for early efficacy assessment, is needed to increase trial efficiency.”
SOURCE:
The study, led by Marco Mariani, MD, Università Vita-Salute San Raffaele, Milan, Italy, was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ClinicalTrials.gov as the primary source of trial data might have resulted in some trials being overlooked. In addition, manual data extraction could cause inaccuracies and potentially introduced biases in the interpretation of trial results. Primary study completion date cutoff of December 2022 could have excluded significant data from more recent trials.
DISCLOSURES:
This study received support via Susan Komen Leadership Grant and the Fondazione AIRC per la Ricerca sul Cancro. Several authors reported receiving grants and personal fees and having other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FDA Approves First Engineered Cell Therapy for a Solid Tumor
Afami-cel — the first engineered cell therapy for a solid tumor — is indicated specifically for adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are positive for several human leukocyte antigens (HLAs), and whose tumors express melanoma-associated antigen A4, as determined by FDA-authorized companion diagnostic devices.
The single-dose treatment targets solid tumors expressing melanoma-associated antigen A4, a protein highly expressed in synovial sarcoma.
Synovial sarcoma is a rare form of cancer, which affects about 1000 people in the US each year. Malignant cells develop and form a tumor in soft tissues, often in the extremities.
“Adults with metastatic synovial sarcoma, a life-threatening form of cancer, often face limited treatment options in addition to the risk of cancer spread or recurrence,” Nicole Verdun, MD, director of the Office of Therapeutic Products in the FDA’s Center for Biologics Evaluation and Research, said in the agency press release announcing the approval. “Today’s approval represents a significant milestone in the development of an innovative, safe and effective therapy for patients with this rare but potentially fatal disease.”
T-cell receptor therapy, like chimeric antigen receptor (CAR) T-cell (CAR-T) therapy, involves altering patient T cells to fight cancer. While CAR-T therapy inserts an artificial receptor to target a specific surface protein on cancer cells, the T-cell receptor therapy modifies existing receptors to recognize an array of antigens on the surface of cancer cells — a promising strategy for targeting solid tumors.
The accelerated approval of afami-cel was based on the phase 2 SPEARHEAD-1 trial in 44 patients with synovial sarcoma who received a single infusion of the therapy. The trial had enrolled 52 patients, but 8 did not receive afami-cel, including 3 who died and 1 who withdrew.
According to the FDA announcement, the overall response rate was 43.2%, with a median time to response of 4.9 weeks. The median duration of response was 6 months (95% CI, 4.6 months to not reached). Among patients who responded, 39% had a duration of response of 12 months or longer.
“These results suggest that a one-time treatment with afami-cel has the potential to extend life while allowing responders to go off chemotherapy,” said lead investigator Sandra D’Angelo, MD, a sarcoma specialist at Memorial Sloan Kettering Cancer Center in New York City, in a company press release.
The prescribing information includes a boxed warning for serious or fatal cytokine release syndrome.
The most common nonlaboratory adverse reactions, occurring in at least 20% of patients, included cytokine release syndrome, nausea, vomiting, fatigue, infections, pyrexia, constipation, dyspnea, tachycardia, hypotension, diarrhea, and edema. The most common grade 3 or 4 laboratory abnormalities, occurring in at least 20% of patients, included decreased lymphocyte count, neutrophil count, white cell blood count, red blood cell, and platelet count.
The recommended dose is between 2.68x109 to 10x109 MAGE-A4 T-cell receptor–positive T-cells. The FDA notice specifies not using a leukodepleting filter or prophylactic systemic corticosteroids.
The list price for the one-time therapy is $727,000, according to Fierce Pharma.
A version of this article first appeared on Medscape.com.
Afami-cel — the first engineered cell therapy for a solid tumor — is indicated specifically for adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are positive for several human leukocyte antigens (HLAs), and whose tumors express melanoma-associated antigen A4, as determined by FDA-authorized companion diagnostic devices.
The single-dose treatment targets solid tumors expressing melanoma-associated antigen A4, a protein highly expressed in synovial sarcoma.
Synovial sarcoma is a rare form of cancer, which affects about 1000 people in the US each year. Malignant cells develop and form a tumor in soft tissues, often in the extremities.
“Adults with metastatic synovial sarcoma, a life-threatening form of cancer, often face limited treatment options in addition to the risk of cancer spread or recurrence,” Nicole Verdun, MD, director of the Office of Therapeutic Products in the FDA’s Center for Biologics Evaluation and Research, said in the agency press release announcing the approval. “Today’s approval represents a significant milestone in the development of an innovative, safe and effective therapy for patients with this rare but potentially fatal disease.”
T-cell receptor therapy, like chimeric antigen receptor (CAR) T-cell (CAR-T) therapy, involves altering patient T cells to fight cancer. While CAR-T therapy inserts an artificial receptor to target a specific surface protein on cancer cells, the T-cell receptor therapy modifies existing receptors to recognize an array of antigens on the surface of cancer cells — a promising strategy for targeting solid tumors.
The accelerated approval of afami-cel was based on the phase 2 SPEARHEAD-1 trial in 44 patients with synovial sarcoma who received a single infusion of the therapy. The trial had enrolled 52 patients, but 8 did not receive afami-cel, including 3 who died and 1 who withdrew.
According to the FDA announcement, the overall response rate was 43.2%, with a median time to response of 4.9 weeks. The median duration of response was 6 months (95% CI, 4.6 months to not reached). Among patients who responded, 39% had a duration of response of 12 months or longer.
“These results suggest that a one-time treatment with afami-cel has the potential to extend life while allowing responders to go off chemotherapy,” said lead investigator Sandra D’Angelo, MD, a sarcoma specialist at Memorial Sloan Kettering Cancer Center in New York City, in a company press release.
The prescribing information includes a boxed warning for serious or fatal cytokine release syndrome.
The most common nonlaboratory adverse reactions, occurring in at least 20% of patients, included cytokine release syndrome, nausea, vomiting, fatigue, infections, pyrexia, constipation, dyspnea, tachycardia, hypotension, diarrhea, and edema. The most common grade 3 or 4 laboratory abnormalities, occurring in at least 20% of patients, included decreased lymphocyte count, neutrophil count, white cell blood count, red blood cell, and platelet count.
The recommended dose is between 2.68x109 to 10x109 MAGE-A4 T-cell receptor–positive T-cells. The FDA notice specifies not using a leukodepleting filter or prophylactic systemic corticosteroids.
The list price for the one-time therapy is $727,000, according to Fierce Pharma.
A version of this article first appeared on Medscape.com.
Afami-cel — the first engineered cell therapy for a solid tumor — is indicated specifically for adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are positive for several human leukocyte antigens (HLAs), and whose tumors express melanoma-associated antigen A4, as determined by FDA-authorized companion diagnostic devices.
The single-dose treatment targets solid tumors expressing melanoma-associated antigen A4, a protein highly expressed in synovial sarcoma.
Synovial sarcoma is a rare form of cancer, which affects about 1000 people in the US each year. Malignant cells develop and form a tumor in soft tissues, often in the extremities.
“Adults with metastatic synovial sarcoma, a life-threatening form of cancer, often face limited treatment options in addition to the risk of cancer spread or recurrence,” Nicole Verdun, MD, director of the Office of Therapeutic Products in the FDA’s Center for Biologics Evaluation and Research, said in the agency press release announcing the approval. “Today’s approval represents a significant milestone in the development of an innovative, safe and effective therapy for patients with this rare but potentially fatal disease.”
T-cell receptor therapy, like chimeric antigen receptor (CAR) T-cell (CAR-T) therapy, involves altering patient T cells to fight cancer. While CAR-T therapy inserts an artificial receptor to target a specific surface protein on cancer cells, the T-cell receptor therapy modifies existing receptors to recognize an array of antigens on the surface of cancer cells — a promising strategy for targeting solid tumors.
The accelerated approval of afami-cel was based on the phase 2 SPEARHEAD-1 trial in 44 patients with synovial sarcoma who received a single infusion of the therapy. The trial had enrolled 52 patients, but 8 did not receive afami-cel, including 3 who died and 1 who withdrew.
According to the FDA announcement, the overall response rate was 43.2%, with a median time to response of 4.9 weeks. The median duration of response was 6 months (95% CI, 4.6 months to not reached). Among patients who responded, 39% had a duration of response of 12 months or longer.
“These results suggest that a one-time treatment with afami-cel has the potential to extend life while allowing responders to go off chemotherapy,” said lead investigator Sandra D’Angelo, MD, a sarcoma specialist at Memorial Sloan Kettering Cancer Center in New York City, in a company press release.
The prescribing information includes a boxed warning for serious or fatal cytokine release syndrome.
The most common nonlaboratory adverse reactions, occurring in at least 20% of patients, included cytokine release syndrome, nausea, vomiting, fatigue, infections, pyrexia, constipation, dyspnea, tachycardia, hypotension, diarrhea, and edema. The most common grade 3 or 4 laboratory abnormalities, occurring in at least 20% of patients, included decreased lymphocyte count, neutrophil count, white cell blood count, red blood cell, and platelet count.
The recommended dose is between 2.68x109 to 10x109 MAGE-A4 T-cell receptor–positive T-cells. The FDA notice specifies not using a leukodepleting filter or prophylactic systemic corticosteroids.
The list price for the one-time therapy is $727,000, according to Fierce Pharma.
A version of this article first appeared on Medscape.com.
Is Immunotherapy Best for Unresectable HCC with Moderate Liver Dysfunction?
In the last 10 years, clinical outcomes have improved for patients with unresectable hepatocellular carcinoma (uHCC). The cancer generally comes with chronic liver inflammation, and liver cirrhosis is present in up to 80% of cases.
Clinical trials that have tested systemic immunotherapies have excluded patients who don’t fall into the Child-Pugh class A criteria (CP-A) for liver disease, which is the least severe of the Child-Pugh classes A-C. Therefore, there has been much debate about whether patients who have more liver disease (moderate liver dysfunction) and fit under CP-B criteria, instead of CP-A, should be treated with immune checkpoint inhibitor (ICI) therapy or best supportive care (BSC).
A new study, led by Claudia Angela Maria Fulgenzi, MD, with the Department of Surgery and Cancer at the Imperial College London, England, published in JAMA Oncology on July 18, uses an alternative way to compare outcomes following two different paths of care for uHCC patients with moderate liver dysfunction.
How was the study done and what did the investigators find?
Researchers performed a retrospective, multicenter, international clinical case series of patients treated in routine practice in tertiary care centers across Europe, the United States, and Asia. They compared data from uHCC patients with CP-B who were receiving first-line ICI-based treatment regimens (n = 187) with a cohort of matched patients with CP-B receiving BSC (n = 156). The first-line immunotherapies were the monotherapy nivolumab or the combination (atezolizumab plus bevacizumab).
Immunotherapy was linked with significantly lower risk of death, compared with best supportive care.
ICI exposure was associated with a reduction of about 50% in the risk of death (hazard ratio, 0.55; 95% CI, 0.35-0.86; P < .001).
Is immunotherapy or best supportive care the superior treatment?
The authors wrote that the results point to “improved survival in association with ICI treatment, compared with BSC in patients with uHCC with CP-B liver dysfunction.”
According to the study’s senior author David Pinato, MD, PhD, “this is the first study to suggest that there might be an advantage [of treatment with immunotherapy] in a proportion of people with Child-Pugh B liver dysfunction and particularly so in those patients with more limited disease and portal vein tumor thrombosis.”
Will the findings of this study make treatment allocation for patients with uHCC and moderate liver dysfunction (CP-B) less controversial?
Because it is a retrospective study, Dr. Pinato said in an interview, that the findings are not definitive, but can be used to inform future randomized controlled trials.
Dr. Pinato, who is also with the Imperial College London, added that the findings may also introduce a new question.
Although the study was not powered to look at survival differences across the two immunotherapy options given to the patients, there did not seem to be a striking difference between using one immunotherapy (nivolumab) or a combination (atezolizumab plus bevacizumab), he said.
“This is quite important because we know that combinations are significantly superior to monotherapy in patients with normal liver function but based on our study we might say that this provides preliminary evidence that [superiority of combination therapy] might not be true if the liver function is worse.”
What do these findings add to the literature about how best to treat patients with uHCC and suboptimal liver function?
Without evidence of efficacy and safety for the group in previous studies, the widespread recommendation for those with moderate dysfunction has been BSC.
These findings “pave the way to select potential patient subgroups in clinical practice,” Dr. Pinato said. It also suggests that the safety level of immunotherapy treatments is acceptable in this patient population, so they are not necessarily disadvantaged compared to patients with more preserved liver function.
“This is the best level of evidence currently available to guide treatment decisions in patients with Child-Pugh B who have been universally excluded by prospective clinical trials and for whom there is no randomized comparison,” Dr. Pinato said.
Dr. Pinato reported personal fees from Roche, AstraZeneca, Eisai, Mina Therapeutics, Starpharma, Lift Biosciences, Boston Scientific, and Avammune, and grants from GSK, MSD, and BMS outside the submitted work. Dr. Fulgenzi has no disclosures. Other authors of the new research have multiple ties with pharmaceutical companies. Complete disclosures are available with the full text of the journal article.
In the last 10 years, clinical outcomes have improved for patients with unresectable hepatocellular carcinoma (uHCC). The cancer generally comes with chronic liver inflammation, and liver cirrhosis is present in up to 80% of cases.
Clinical trials that have tested systemic immunotherapies have excluded patients who don’t fall into the Child-Pugh class A criteria (CP-A) for liver disease, which is the least severe of the Child-Pugh classes A-C. Therefore, there has been much debate about whether patients who have more liver disease (moderate liver dysfunction) and fit under CP-B criteria, instead of CP-A, should be treated with immune checkpoint inhibitor (ICI) therapy or best supportive care (BSC).
A new study, led by Claudia Angela Maria Fulgenzi, MD, with the Department of Surgery and Cancer at the Imperial College London, England, published in JAMA Oncology on July 18, uses an alternative way to compare outcomes following two different paths of care for uHCC patients with moderate liver dysfunction.
How was the study done and what did the investigators find?
Researchers performed a retrospective, multicenter, international clinical case series of patients treated in routine practice in tertiary care centers across Europe, the United States, and Asia. They compared data from uHCC patients with CP-B who were receiving first-line ICI-based treatment regimens (n = 187) with a cohort of matched patients with CP-B receiving BSC (n = 156). The first-line immunotherapies were the monotherapy nivolumab or the combination (atezolizumab plus bevacizumab).
Immunotherapy was linked with significantly lower risk of death, compared with best supportive care.
ICI exposure was associated with a reduction of about 50% in the risk of death (hazard ratio, 0.55; 95% CI, 0.35-0.86; P < .001).
Is immunotherapy or best supportive care the superior treatment?
The authors wrote that the results point to “improved survival in association with ICI treatment, compared with BSC in patients with uHCC with CP-B liver dysfunction.”
According to the study’s senior author David Pinato, MD, PhD, “this is the first study to suggest that there might be an advantage [of treatment with immunotherapy] in a proportion of people with Child-Pugh B liver dysfunction and particularly so in those patients with more limited disease and portal vein tumor thrombosis.”
Will the findings of this study make treatment allocation for patients with uHCC and moderate liver dysfunction (CP-B) less controversial?
Because it is a retrospective study, Dr. Pinato said in an interview, that the findings are not definitive, but can be used to inform future randomized controlled trials.
Dr. Pinato, who is also with the Imperial College London, added that the findings may also introduce a new question.
Although the study was not powered to look at survival differences across the two immunotherapy options given to the patients, there did not seem to be a striking difference between using one immunotherapy (nivolumab) or a combination (atezolizumab plus bevacizumab), he said.
“This is quite important because we know that combinations are significantly superior to monotherapy in patients with normal liver function but based on our study we might say that this provides preliminary evidence that [superiority of combination therapy] might not be true if the liver function is worse.”
What do these findings add to the literature about how best to treat patients with uHCC and suboptimal liver function?
Without evidence of efficacy and safety for the group in previous studies, the widespread recommendation for those with moderate dysfunction has been BSC.
These findings “pave the way to select potential patient subgroups in clinical practice,” Dr. Pinato said. It also suggests that the safety level of immunotherapy treatments is acceptable in this patient population, so they are not necessarily disadvantaged compared to patients with more preserved liver function.
“This is the best level of evidence currently available to guide treatment decisions in patients with Child-Pugh B who have been universally excluded by prospective clinical trials and for whom there is no randomized comparison,” Dr. Pinato said.
Dr. Pinato reported personal fees from Roche, AstraZeneca, Eisai, Mina Therapeutics, Starpharma, Lift Biosciences, Boston Scientific, and Avammune, and grants from GSK, MSD, and BMS outside the submitted work. Dr. Fulgenzi has no disclosures. Other authors of the new research have multiple ties with pharmaceutical companies. Complete disclosures are available with the full text of the journal article.
In the last 10 years, clinical outcomes have improved for patients with unresectable hepatocellular carcinoma (uHCC). The cancer generally comes with chronic liver inflammation, and liver cirrhosis is present in up to 80% of cases.
Clinical trials that have tested systemic immunotherapies have excluded patients who don’t fall into the Child-Pugh class A criteria (CP-A) for liver disease, which is the least severe of the Child-Pugh classes A-C. Therefore, there has been much debate about whether patients who have more liver disease (moderate liver dysfunction) and fit under CP-B criteria, instead of CP-A, should be treated with immune checkpoint inhibitor (ICI) therapy or best supportive care (BSC).
A new study, led by Claudia Angela Maria Fulgenzi, MD, with the Department of Surgery and Cancer at the Imperial College London, England, published in JAMA Oncology on July 18, uses an alternative way to compare outcomes following two different paths of care for uHCC patients with moderate liver dysfunction.
How was the study done and what did the investigators find?
Researchers performed a retrospective, multicenter, international clinical case series of patients treated in routine practice in tertiary care centers across Europe, the United States, and Asia. They compared data from uHCC patients with CP-B who were receiving first-line ICI-based treatment regimens (n = 187) with a cohort of matched patients with CP-B receiving BSC (n = 156). The first-line immunotherapies were the monotherapy nivolumab or the combination (atezolizumab plus bevacizumab).
Immunotherapy was linked with significantly lower risk of death, compared with best supportive care.
ICI exposure was associated with a reduction of about 50% in the risk of death (hazard ratio, 0.55; 95% CI, 0.35-0.86; P < .001).
Is immunotherapy or best supportive care the superior treatment?
The authors wrote that the results point to “improved survival in association with ICI treatment, compared with BSC in patients with uHCC with CP-B liver dysfunction.”
According to the study’s senior author David Pinato, MD, PhD, “this is the first study to suggest that there might be an advantage [of treatment with immunotherapy] in a proportion of people with Child-Pugh B liver dysfunction and particularly so in those patients with more limited disease and portal vein tumor thrombosis.”
Will the findings of this study make treatment allocation for patients with uHCC and moderate liver dysfunction (CP-B) less controversial?
Because it is a retrospective study, Dr. Pinato said in an interview, that the findings are not definitive, but can be used to inform future randomized controlled trials.
Dr. Pinato, who is also with the Imperial College London, added that the findings may also introduce a new question.
Although the study was not powered to look at survival differences across the two immunotherapy options given to the patients, there did not seem to be a striking difference between using one immunotherapy (nivolumab) or a combination (atezolizumab plus bevacizumab), he said.
“This is quite important because we know that combinations are significantly superior to monotherapy in patients with normal liver function but based on our study we might say that this provides preliminary evidence that [superiority of combination therapy] might not be true if the liver function is worse.”
What do these findings add to the literature about how best to treat patients with uHCC and suboptimal liver function?
Without evidence of efficacy and safety for the group in previous studies, the widespread recommendation for those with moderate dysfunction has been BSC.
These findings “pave the way to select potential patient subgroups in clinical practice,” Dr. Pinato said. It also suggests that the safety level of immunotherapy treatments is acceptable in this patient population, so they are not necessarily disadvantaged compared to patients with more preserved liver function.
“This is the best level of evidence currently available to guide treatment decisions in patients with Child-Pugh B who have been universally excluded by prospective clinical trials and for whom there is no randomized comparison,” Dr. Pinato said.
Dr. Pinato reported personal fees from Roche, AstraZeneca, Eisai, Mina Therapeutics, Starpharma, Lift Biosciences, Boston Scientific, and Avammune, and grants from GSK, MSD, and BMS outside the submitted work. Dr. Fulgenzi has no disclosures. Other authors of the new research have multiple ties with pharmaceutical companies. Complete disclosures are available with the full text of the journal article.
FROM JAMA ONCOLOGY
MUC-1 vaccine associated with notable overall survival rates in breast cancer
“This is the first successful study of a breast cancer vaccine to date,” Christian F. Singer, MD, said during an interview. Dr. Singer, the lead author of the new study, presented the results during a poster session at the 2024 annual meeting of the American Society of Clinical Oncology (ASCO).
Previously known as both liposomal BLP25 and Stimuvax, tecemotide is an antigen-specific immunotherapy that targets the cancer therapy–resistant MUC-1 glycoprotein, which is overexpressed in over 90% of breast cancers. Tecemotide also has been shown to moderately improve overall survival rates in non–small cell lung cancer.
“We are not at all surprised by the results of this study in breast cancer,” Gregory T. Wurz, PhD, senior researcher at RCU Labs in Lincoln, California, said in an interview.
Dr. Wurz is coauthor of several studies on peptide vaccines, including a mouse model study of human MUC-1–expressing mammary tumors showing that tecemotide combined with letrozole had additive antitumor activity. Another paper he coauthored showed that ospemifene enhanced the immune response to tecemotide in both tumor-bearing and non–tumor-bearing mice. These findings, combined with other research, led to the creation of a patented method of combining therapies to enhance the efficacy of immunotherapy in the treatment of cancer and infectious diseases. Dr. Wurz was not involved in the new research that Dr. Singer presented at ASCO.
Study Methods and Results
Dr. Singer, head of obstetrics and gynecology at the Medical University of Vienna, Vienna, Austria, and coauthors randomized 400 patients with HER2-negative early breast cancer in a prospective, multicenter, two-arm, phase 2 ABCSG 34 trial to receive preoperative standard of care (SOC) neoadjuvant treatment with or without tecemotide.
Postmenopausal women with luminal A tumors were given 6 months of letrozole as SOC. Postmenopausal patients with triple-negative breast cancer, luminal B tumors, in whom chemotherapy was SOC, as well as all premenopausal study participants, were given four cycles of both epirubicin cyclophosphamide and docetaxel every 3 weeks.
The study’s primary endpoint was the residual cancer burden at the time of surgery.
Long-term outcomes were measured as part of a translational project, while distant relapse-free survival (DRFS) and overall survival (OS) were analyzed with Cox regression models. Long-term outcome data were available for 291 women, of whom 236 had received chemotherapy as SOC.
While tecemotide plus neoadjuvant SOC was not associated with a significant increase in residual cancer burden (RCB) at the time of surgery (36.4% vs 31.5%; P = .42; 40.5% vs 34.8%; P = .37 for the chemotherapy-only cohort), follow-up at 7 years showed 80.8% of patients who had received SOC plus tecemotide were still alive and free from metastasis.
In patients who had received SOC alone, the OS rate at 7 years with no metastasis was 64.7% (hazard ratio [HR] for DRFS, 0.53; 95% CI, 0.34-0.83; P = .005). The OS rate for the study group was 83.0% vs 68.2% in the non-tecemotide cohort (HR for OS, 0.53; 95% CI, 0.33-0.85; P = .008).
The lack of RCB signal at the endpoints, “tells us that pathologic complete response and residual cancer burden simply are not adequate endpoints for cancer vaccination studies and we need to find other predictive/prognostic markers, said Dr. Singer. “We are currently looking into this in exploratory studies.”
The chemotherapy plus tecemotide cohort had a notable outcome with a DRFS of 81.9% vs 65.0% in the SOC group (HR, 0.50; 95% CI, 0.31-0.83; P = .007), and an OS rate of 83.6% vs 67.8% (HR, 0.51; 95% CI, 0.30-0.88; P = .016).
Dr. Singer characterized the HRs as intriguing, saying that they “pave the way for new trials.”
Ideas for Further Study of Tecemotide
“What we would like to see next for tecemotide are clinical studies that explore whether immunomodulatory agents can further enhance the response to tecemotide in lung, breast, and potentially other MUC-1–expressing cancers,” Dr. Wurz said.
Future phase 3 studies of MUC-1 cancer vaccines, possibly those using mRNA technology, are yet to come, according to Dr. Singer. “We also need to find out why the vaccine works sometimes and sometimes not.”
Dr. Singer disclosed financial ties to AstraZeneca/MedImmune, Daiichi Sankyo Europe, Novartis, Gilead Sciences, Sanofi/Aventis, Amgen, Myriad Genetics, and Roche. Dr. Wurz had no disclosures, but his research partner and founder of RCU Labs, Michael De Gregorio, is the sole inventor of the patent referenced in the story. That patent has been assigned to the Regents of the University of California.
“This is the first successful study of a breast cancer vaccine to date,” Christian F. Singer, MD, said during an interview. Dr. Singer, the lead author of the new study, presented the results during a poster session at the 2024 annual meeting of the American Society of Clinical Oncology (ASCO).
Previously known as both liposomal BLP25 and Stimuvax, tecemotide is an antigen-specific immunotherapy that targets the cancer therapy–resistant MUC-1 glycoprotein, which is overexpressed in over 90% of breast cancers. Tecemotide also has been shown to moderately improve overall survival rates in non–small cell lung cancer.
“We are not at all surprised by the results of this study in breast cancer,” Gregory T. Wurz, PhD, senior researcher at RCU Labs in Lincoln, California, said in an interview.
Dr. Wurz is coauthor of several studies on peptide vaccines, including a mouse model study of human MUC-1–expressing mammary tumors showing that tecemotide combined with letrozole had additive antitumor activity. Another paper he coauthored showed that ospemifene enhanced the immune response to tecemotide in both tumor-bearing and non–tumor-bearing mice. These findings, combined with other research, led to the creation of a patented method of combining therapies to enhance the efficacy of immunotherapy in the treatment of cancer and infectious diseases. Dr. Wurz was not involved in the new research that Dr. Singer presented at ASCO.
Study Methods and Results
Dr. Singer, head of obstetrics and gynecology at the Medical University of Vienna, Vienna, Austria, and coauthors randomized 400 patients with HER2-negative early breast cancer in a prospective, multicenter, two-arm, phase 2 ABCSG 34 trial to receive preoperative standard of care (SOC) neoadjuvant treatment with or without tecemotide.
Postmenopausal women with luminal A tumors were given 6 months of letrozole as SOC. Postmenopausal patients with triple-negative breast cancer, luminal B tumors, in whom chemotherapy was SOC, as well as all premenopausal study participants, were given four cycles of both epirubicin cyclophosphamide and docetaxel every 3 weeks.
The study’s primary endpoint was the residual cancer burden at the time of surgery.
Long-term outcomes were measured as part of a translational project, while distant relapse-free survival (DRFS) and overall survival (OS) were analyzed with Cox regression models. Long-term outcome data were available for 291 women, of whom 236 had received chemotherapy as SOC.
While tecemotide plus neoadjuvant SOC was not associated with a significant increase in residual cancer burden (RCB) at the time of surgery (36.4% vs 31.5%; P = .42; 40.5% vs 34.8%; P = .37 for the chemotherapy-only cohort), follow-up at 7 years showed 80.8% of patients who had received SOC plus tecemotide were still alive and free from metastasis.
In patients who had received SOC alone, the OS rate at 7 years with no metastasis was 64.7% (hazard ratio [HR] for DRFS, 0.53; 95% CI, 0.34-0.83; P = .005). The OS rate for the study group was 83.0% vs 68.2% in the non-tecemotide cohort (HR for OS, 0.53; 95% CI, 0.33-0.85; P = .008).
The lack of RCB signal at the endpoints, “tells us that pathologic complete response and residual cancer burden simply are not adequate endpoints for cancer vaccination studies and we need to find other predictive/prognostic markers, said Dr. Singer. “We are currently looking into this in exploratory studies.”
The chemotherapy plus tecemotide cohort had a notable outcome with a DRFS of 81.9% vs 65.0% in the SOC group (HR, 0.50; 95% CI, 0.31-0.83; P = .007), and an OS rate of 83.6% vs 67.8% (HR, 0.51; 95% CI, 0.30-0.88; P = .016).
Dr. Singer characterized the HRs as intriguing, saying that they “pave the way for new trials.”
Ideas for Further Study of Tecemotide
“What we would like to see next for tecemotide are clinical studies that explore whether immunomodulatory agents can further enhance the response to tecemotide in lung, breast, and potentially other MUC-1–expressing cancers,” Dr. Wurz said.
Future phase 3 studies of MUC-1 cancer vaccines, possibly those using mRNA technology, are yet to come, according to Dr. Singer. “We also need to find out why the vaccine works sometimes and sometimes not.”
Dr. Singer disclosed financial ties to AstraZeneca/MedImmune, Daiichi Sankyo Europe, Novartis, Gilead Sciences, Sanofi/Aventis, Amgen, Myriad Genetics, and Roche. Dr. Wurz had no disclosures, but his research partner and founder of RCU Labs, Michael De Gregorio, is the sole inventor of the patent referenced in the story. That patent has been assigned to the Regents of the University of California.
“This is the first successful study of a breast cancer vaccine to date,” Christian F. Singer, MD, said during an interview. Dr. Singer, the lead author of the new study, presented the results during a poster session at the 2024 annual meeting of the American Society of Clinical Oncology (ASCO).
Previously known as both liposomal BLP25 and Stimuvax, tecemotide is an antigen-specific immunotherapy that targets the cancer therapy–resistant MUC-1 glycoprotein, which is overexpressed in over 90% of breast cancers. Tecemotide also has been shown to moderately improve overall survival rates in non–small cell lung cancer.
“We are not at all surprised by the results of this study in breast cancer,” Gregory T. Wurz, PhD, senior researcher at RCU Labs in Lincoln, California, said in an interview.
Dr. Wurz is coauthor of several studies on peptide vaccines, including a mouse model study of human MUC-1–expressing mammary tumors showing that tecemotide combined with letrozole had additive antitumor activity. Another paper he coauthored showed that ospemifene enhanced the immune response to tecemotide in both tumor-bearing and non–tumor-bearing mice. These findings, combined with other research, led to the creation of a patented method of combining therapies to enhance the efficacy of immunotherapy in the treatment of cancer and infectious diseases. Dr. Wurz was not involved in the new research that Dr. Singer presented at ASCO.
Study Methods and Results
Dr. Singer, head of obstetrics and gynecology at the Medical University of Vienna, Vienna, Austria, and coauthors randomized 400 patients with HER2-negative early breast cancer in a prospective, multicenter, two-arm, phase 2 ABCSG 34 trial to receive preoperative standard of care (SOC) neoadjuvant treatment with or without tecemotide.
Postmenopausal women with luminal A tumors were given 6 months of letrozole as SOC. Postmenopausal patients with triple-negative breast cancer, luminal B tumors, in whom chemotherapy was SOC, as well as all premenopausal study participants, were given four cycles of both epirubicin cyclophosphamide and docetaxel every 3 weeks.
The study’s primary endpoint was the residual cancer burden at the time of surgery.
Long-term outcomes were measured as part of a translational project, while distant relapse-free survival (DRFS) and overall survival (OS) were analyzed with Cox regression models. Long-term outcome data were available for 291 women, of whom 236 had received chemotherapy as SOC.
While tecemotide plus neoadjuvant SOC was not associated with a significant increase in residual cancer burden (RCB) at the time of surgery (36.4% vs 31.5%; P = .42; 40.5% vs 34.8%; P = .37 for the chemotherapy-only cohort), follow-up at 7 years showed 80.8% of patients who had received SOC plus tecemotide were still alive and free from metastasis.
In patients who had received SOC alone, the OS rate at 7 years with no metastasis was 64.7% (hazard ratio [HR] for DRFS, 0.53; 95% CI, 0.34-0.83; P = .005). The OS rate for the study group was 83.0% vs 68.2% in the non-tecemotide cohort (HR for OS, 0.53; 95% CI, 0.33-0.85; P = .008).
The lack of RCB signal at the endpoints, “tells us that pathologic complete response and residual cancer burden simply are not adequate endpoints for cancer vaccination studies and we need to find other predictive/prognostic markers, said Dr. Singer. “We are currently looking into this in exploratory studies.”
The chemotherapy plus tecemotide cohort had a notable outcome with a DRFS of 81.9% vs 65.0% in the SOC group (HR, 0.50; 95% CI, 0.31-0.83; P = .007), and an OS rate of 83.6% vs 67.8% (HR, 0.51; 95% CI, 0.30-0.88; P = .016).
Dr. Singer characterized the HRs as intriguing, saying that they “pave the way for new trials.”
Ideas for Further Study of Tecemotide
“What we would like to see next for tecemotide are clinical studies that explore whether immunomodulatory agents can further enhance the response to tecemotide in lung, breast, and potentially other MUC-1–expressing cancers,” Dr. Wurz said.
Future phase 3 studies of MUC-1 cancer vaccines, possibly those using mRNA technology, are yet to come, according to Dr. Singer. “We also need to find out why the vaccine works sometimes and sometimes not.”
Dr. Singer disclosed financial ties to AstraZeneca/MedImmune, Daiichi Sankyo Europe, Novartis, Gilead Sciences, Sanofi/Aventis, Amgen, Myriad Genetics, and Roche. Dr. Wurz had no disclosures, but his research partner and founder of RCU Labs, Michael De Gregorio, is the sole inventor of the patent referenced in the story. That patent has been assigned to the Regents of the University of California.
FROM ASCO 2024
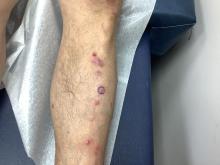
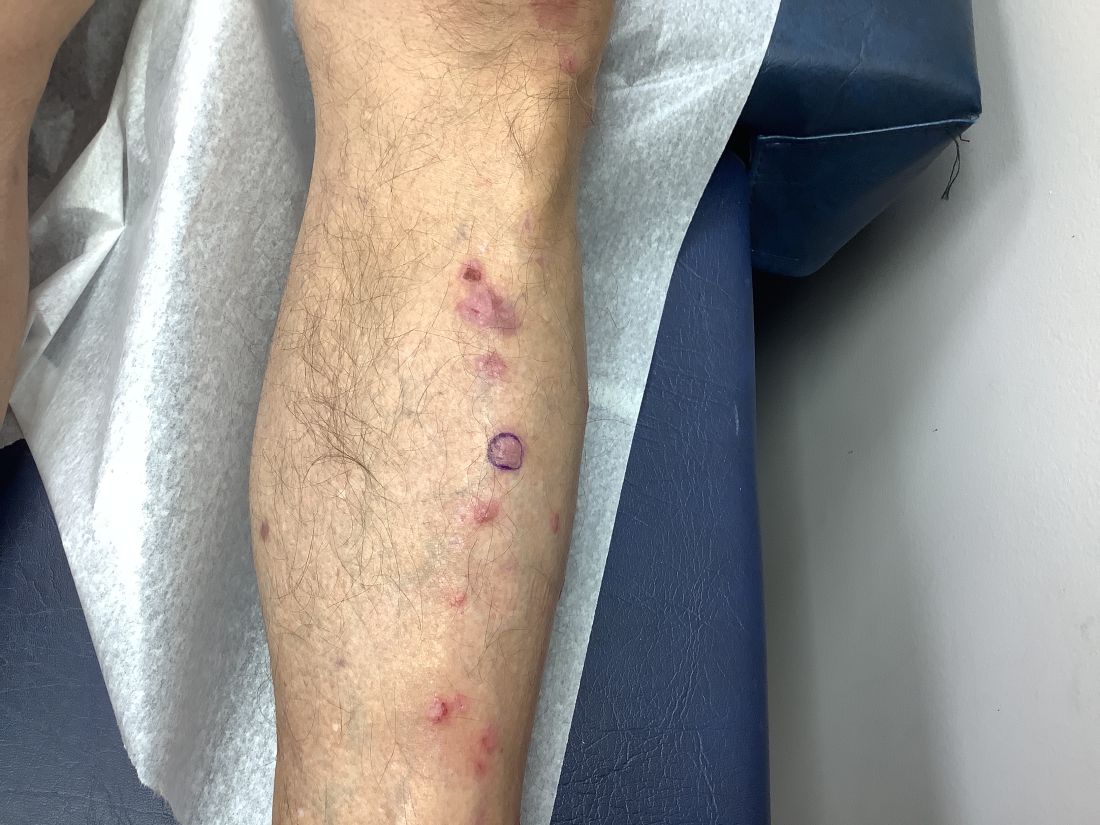
Pruritic, violaceous papules in a patient with renal cell carcinoma
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.
Pembrolizumab (Keytruda) is a programmed cell death protein 1 (PD-1) blocking antibody used to treat different malignancies including melanoma, non–small cell lung cancer, and other advanced solid tumors and hematologic malignancies. and drug rash with eosinophilia and systemic symptoms (DRESS).
Lichen planus-like adverse drug reactions, as seen in this patient, are also referred to as lichenoid drug eruption or drug-induced lichen planus. This cutaneous reaction is one of the more rare side effects of pembrolizumab. It should be noted that in lichenoid reactions, keratinocytes expressing PD-L1 are particularly affected, leading to a dense CD4/CD8 positive lymphocytic infiltration in the basal layer, necrosis of keratinocytes, acanthosis, and hypergranulosis. Subsequently, the cutaneous adverse reaction is a target effect of the PD-1/PD-L1 pathway and not a general hypersensitivity reaction. Clinically, both lichen planus and lichenoid drug eruptions exhibit erythematous papules and plaques. Lichenoid drug eruptions, however, can be scaly, pruritic, and heal with more hyperpigmentation.
A skin biopsy revealed irregular epidermal hyperplasia with jagged rete ridges. Within the dermis, there was a lichenoid inflammatory cell infiltrate obscuring the dermal-epidermal junction. The inflammatory cell infiltrate contained lymphocytes, histiocytes, and eosinophils. A diagnosis of a lichen planus-like adverse drug reaction to pembrolizumab was favored.
If the reaction is mild, topical corticosteroids and oral antihistamines can help with the drug-induced lichen planus. For more severe cases, systemic steroids can be given to help ease the reaction. Physicians should be aware of potential adverse drug effects that can mimic other medical conditions.
The case and photo were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Berke, Three Rivers Dermatology, Coraopolis, Pennsylvania. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Bansal A et al. Indian Dermatol Online J. 2023 Apr 4;14(3):391-4. doi: 10.4103/idoj.idoj_377_22.
Sethi A, Raj M. Cureus. 2021 Mar 8;13(3):e13768. doi: 10.7759/cureus.13768.
Chronotherapy: Why Timing Drugs to Our Body Clocks May Work
Do drugs work better if taken by the clock?
A new analysis published in The Lancet journal’s eClinicalMedicine suggests: Yes, they do — if you consider the patient’s individual body clock. The study is the first to find that timing blood pressure drugs to a person’s personal “chronotype” — that is, whether they are a night owl or an early bird — may reduce the risk for a heart attack.
The findings represent a significant advance in the field of circadian medicine or “chronotherapy” — timing drug administration to circadian rhythms. A growing stack of research suggests this approach could reduce side effects and improve the effectiveness of a wide range of therapies, including vaccines, cancer treatments, and drugs for depression, glaucoma, pain, seizures, and other conditions. Still, despite decades of research, time of day is rarely considered in writing prescriptions.
“We are really just at the beginning of an exciting new way of looking at patient care,” said Kenneth A. Dyar, PhD, whose lab at Helmholtz Zentrum München’s Institute for Diabetes and Cancer focuses on metabolic physiology. Dr. Dyar is co-lead author of the new blood pressure analysis.
“Chronotherapy is a rapidly growing field,” he said, “and I suspect we are soon going to see more and more studies focused on ‘personalized chronotherapy,’ not only in hypertension but also potentially in other clinical areas.”
The ‘Missing Piece’ in Chronotherapy Research
Blood pressure drugs have long been chronotherapy’s battleground. After all, blood pressure follows a circadian rhythm, peaking in the morning and dropping at night.
That healthy overnight dip can disappear in people with diabetes, kidney disease, and obstructive sleep apnea. Some physicians have suggested a bed-time dose to restore that dip. But studies have had mixed results, so “take at bedtime” has become a less common recommendation in recent years.
But the debate continued. After a large 2019 Spanish study found that bedtime doses had benefits so big that the results drew questions, an even larger, 2022 randomized, controlled trial from the University of Dundee in Dundee, Scotland — called the TIME study — aimed to settle the question.
Researchers assigned over 21,000 people to take morning or night hypertension drugs for several years and found no difference in cardiovascular outcomes.
“We did this study thinking nocturnal blood pressure tablets might be better,” said Thomas MacDonald, MD, professor emeritus of clinical pharmacology and pharmacoepidemiology at the University of Dundee and principal investigator for the TIME study and the recent chronotype analysis. “But there was no difference for heart attacks, strokes, or vascular death.”
So, the researchers then looked at participants’ chronotypes, sorting outcomes based on whether the participants were late-to-bed, late-to-rise “night owls” or early-to-bed, early-to-rise “morning larks.”
Their analysis of these 5358 TIME participants found the following results: Risk for hospitalization for a heart attack was at least 34% lower for “owls” who took their drugs at bedtime. By contrast, owls’ heart attack risk was at least 62% higher with morning doses. For “larks,” the opposite was true. Morning doses were associated with an 11% lower heart attack risk and night doses with an 11% higher risk, according to supplemental data.
The personalized approach could explain why some previous chronotherapy studies have failed to show a benefit. Those studies did not individualize drug timing as this one did. But personalization could be key to circadian medicine’s success.
“Our ‘internal personal time’ appears to be an important variable to consider when dosing antihypertensives,” said co-lead author Filippo Pigazzani, MD, PhD, clinical senior lecturer and honorary consultant cardiologist at the University of Dundee School of Medicine. “Chronotherapy research has been going on for decades. We knew there was something important with time of day. But researchers haven’t considered the internal time of individual people. I think that is the missing piece.”
The analysis has several important limitations, the researchers said. A total of 95% of participants were White. And it was an observational study, not a true randomized comparison. “We started it late in the original TIME study,” Dr. MacDonald said. “You could argue we were reporting on those who survived long enough to get into the analysis.” More research is needed, they concluded.
Looking Beyond Blood Pressure
What about the rest of the body? “Almost all the cells of our body contain ‘circadian clocks’ that are synchronized by daily environmental cues, including light-dark, activity-rest, and feeding-fasting cycles,” said Dr. Dyar.
An estimated 50% of prescription drugs hit targets in the body that have circadian patterns. So, experts suspect that syncing a drug with a person’s body clock might increase effectiveness of many drugs.
A handful of US Food and Drug Administration–approved drugs already have time-of-day recommendations on the label for effectiveness or to limit side effects, including bedtime or evening for the insomnia drug Ambien, the HIV antiviral Atripla, and cholesterol-lowering Zocor. Others are intended to be taken with or after your last meal of the day, such as the long-acting insulin Levemir and the cardiovascular drug Xarelto. A morning recommendation comes with the proton pump inhibitor Nexium and the attention-deficit/hyperactivity disorder drug Ritalin.
Interest is expanding. About one third of the papers published about chronotherapy in the past 25 years have come out in the past 5 years. The May 2024 meeting of the Society for Research on Biological Rhythms featured a day-long session aimed at bringing clinicians up to speed. An organization called the International Association of Circadian Health Clinics is trying to bring circadian medicine findings to clinicians and their patients and to support research.
Moreover, while recent research suggests minding the clock could have benefits for a wide range of treatments, ignoring it could cause problems.
In a Massachusetts Institute of Technology study published in April in Science Advances, researchers looked at engineered livers made from human donor cells and found more than 300 genes that operate on a circadian schedule, many with roles in drug metabolism. They also found that circadian patterns affected the toxicity of acetaminophen and atorvastatin. Identifying the time of day to take these drugs could maximize effectiveness and minimize adverse effects, the researchers said.
Timing and the Immune System
Circadian rhythms are also seen in immune processes. In a 2023 study in The Journal of Clinical Investigation of vaccine data from 1.5 million people in Israel, researchers found that children and older adults who got their second dose of the Pfizer mRNA COVID vaccine earlier in the day were about 36% less likely to be hospitalized with SARS-CoV-2 infection than those who got an evening shot.
“The sweet spot in our data was somewhere around late morning to late afternoon,” said lead researcher Jeffrey Haspel, MD, PhD, associate professor of medicine in the division of pulmonary and critical care medicine at Washington University School of Medicine in St. Louis.
In a multicenter, 2024 analysis of 13 studies of immunotherapy for advanced cancers in 1663 people, researchers found treatment earlier in the day was associated with longer survival time and longer survival without cancer progression.
“Patients with selected metastatic cancers seemed to largely benefit from early [time of day] infusions, which is consistent with circadian mechanisms in immune-cell functions and trafficking,” the researchers noted. But “retrospective randomized trials are needed to establish recommendations for optimal circadian timing.”
Other research suggests or is investigating possible chronotherapy benefits for depression, glaucoma, respiratory diseases, stroke treatment, epilepsy, and sedatives used in surgery. So why aren’t healthcare providers adding time of day to more prescriptions? “What’s missing is more reliable data,” Dr. Dyar said.
Should You Use Chronotherapy Now?
Experts emphasize that more research is needed before doctors use chronotherapy and before medical organizations include it in treatment recommendations. But for some patients, circadian dosing may be worth a try:
Night owls whose blood pressure isn’t well controlled. Dr. Dyar and Dr. Pigazzani said night-time blood pressure drugs may be helpful for people with a “late chronotype.” Of course, patients shouldn’t change their medication schedule on their own, they said. And doctors may want to consider other concerns, like more overnight bathroom visits with evening diuretics.
In their study, the researchers determined participants’ chronotype with a few questions from the Munich Chronotype Questionnaire about what time they fell asleep and woke up on workdays and days off and whether they considered themselves “morning types” or “evening types.” (The questions can be found in supplementary data for the study.)
If a physician thinks matching the timing of a dose with chronotype would help, they can consider it, Dr. Pigazzani said. “However, I must add that this was an observational study, so I would advise healthcare practitioners to wait for our data to be confirmed in new RCTs of personalized chronotherapy of hypertension.”
Children and older adults getting vaccines. Timing COVID shots and possibly other vaccines from late morning to mid-afternoon could have a small benefit for individuals and a bigger public-health benefit, Dr. Haspel said. But the most important thing is getting vaccinated. “If you can only get one in the evening, it’s still worthwhile. Timing may add oomph at a public-health level for more vulnerable groups.”
A version of this article appeared on Medscape.com.
Do drugs work better if taken by the clock?
A new analysis published in The Lancet journal’s eClinicalMedicine suggests: Yes, they do — if you consider the patient’s individual body clock. The study is the first to find that timing blood pressure drugs to a person’s personal “chronotype” — that is, whether they are a night owl or an early bird — may reduce the risk for a heart attack.
The findings represent a significant advance in the field of circadian medicine or “chronotherapy” — timing drug administration to circadian rhythms. A growing stack of research suggests this approach could reduce side effects and improve the effectiveness of a wide range of therapies, including vaccines, cancer treatments, and drugs for depression, glaucoma, pain, seizures, and other conditions. Still, despite decades of research, time of day is rarely considered in writing prescriptions.
“We are really just at the beginning of an exciting new way of looking at patient care,” said Kenneth A. Dyar, PhD, whose lab at Helmholtz Zentrum München’s Institute for Diabetes and Cancer focuses on metabolic physiology. Dr. Dyar is co-lead author of the new blood pressure analysis.
“Chronotherapy is a rapidly growing field,” he said, “and I suspect we are soon going to see more and more studies focused on ‘personalized chronotherapy,’ not only in hypertension but also potentially in other clinical areas.”
The ‘Missing Piece’ in Chronotherapy Research
Blood pressure drugs have long been chronotherapy’s battleground. After all, blood pressure follows a circadian rhythm, peaking in the morning and dropping at night.
That healthy overnight dip can disappear in people with diabetes, kidney disease, and obstructive sleep apnea. Some physicians have suggested a bed-time dose to restore that dip. But studies have had mixed results, so “take at bedtime” has become a less common recommendation in recent years.
But the debate continued. After a large 2019 Spanish study found that bedtime doses had benefits so big that the results drew questions, an even larger, 2022 randomized, controlled trial from the University of Dundee in Dundee, Scotland — called the TIME study — aimed to settle the question.
Researchers assigned over 21,000 people to take morning or night hypertension drugs for several years and found no difference in cardiovascular outcomes.
“We did this study thinking nocturnal blood pressure tablets might be better,” said Thomas MacDonald, MD, professor emeritus of clinical pharmacology and pharmacoepidemiology at the University of Dundee and principal investigator for the TIME study and the recent chronotype analysis. “But there was no difference for heart attacks, strokes, or vascular death.”
So, the researchers then looked at participants’ chronotypes, sorting outcomes based on whether the participants were late-to-bed, late-to-rise “night owls” or early-to-bed, early-to-rise “morning larks.”
Their analysis of these 5358 TIME participants found the following results: Risk for hospitalization for a heart attack was at least 34% lower for “owls” who took their drugs at bedtime. By contrast, owls’ heart attack risk was at least 62% higher with morning doses. For “larks,” the opposite was true. Morning doses were associated with an 11% lower heart attack risk and night doses with an 11% higher risk, according to supplemental data.
The personalized approach could explain why some previous chronotherapy studies have failed to show a benefit. Those studies did not individualize drug timing as this one did. But personalization could be key to circadian medicine’s success.
“Our ‘internal personal time’ appears to be an important variable to consider when dosing antihypertensives,” said co-lead author Filippo Pigazzani, MD, PhD, clinical senior lecturer and honorary consultant cardiologist at the University of Dundee School of Medicine. “Chronotherapy research has been going on for decades. We knew there was something important with time of day. But researchers haven’t considered the internal time of individual people. I think that is the missing piece.”
The analysis has several important limitations, the researchers said. A total of 95% of participants were White. And it was an observational study, not a true randomized comparison. “We started it late in the original TIME study,” Dr. MacDonald said. “You could argue we were reporting on those who survived long enough to get into the analysis.” More research is needed, they concluded.
Looking Beyond Blood Pressure
What about the rest of the body? “Almost all the cells of our body contain ‘circadian clocks’ that are synchronized by daily environmental cues, including light-dark, activity-rest, and feeding-fasting cycles,” said Dr. Dyar.
An estimated 50% of prescription drugs hit targets in the body that have circadian patterns. So, experts suspect that syncing a drug with a person’s body clock might increase effectiveness of many drugs.
A handful of US Food and Drug Administration–approved drugs already have time-of-day recommendations on the label for effectiveness or to limit side effects, including bedtime or evening for the insomnia drug Ambien, the HIV antiviral Atripla, and cholesterol-lowering Zocor. Others are intended to be taken with or after your last meal of the day, such as the long-acting insulin Levemir and the cardiovascular drug Xarelto. A morning recommendation comes with the proton pump inhibitor Nexium and the attention-deficit/hyperactivity disorder drug Ritalin.
Interest is expanding. About one third of the papers published about chronotherapy in the past 25 years have come out in the past 5 years. The May 2024 meeting of the Society for Research on Biological Rhythms featured a day-long session aimed at bringing clinicians up to speed. An organization called the International Association of Circadian Health Clinics is trying to bring circadian medicine findings to clinicians and their patients and to support research.
Moreover, while recent research suggests minding the clock could have benefits for a wide range of treatments, ignoring it could cause problems.
In a Massachusetts Institute of Technology study published in April in Science Advances, researchers looked at engineered livers made from human donor cells and found more than 300 genes that operate on a circadian schedule, many with roles in drug metabolism. They also found that circadian patterns affected the toxicity of acetaminophen and atorvastatin. Identifying the time of day to take these drugs could maximize effectiveness and minimize adverse effects, the researchers said.
Timing and the Immune System
Circadian rhythms are also seen in immune processes. In a 2023 study in The Journal of Clinical Investigation of vaccine data from 1.5 million people in Israel, researchers found that children and older adults who got their second dose of the Pfizer mRNA COVID vaccine earlier in the day were about 36% less likely to be hospitalized with SARS-CoV-2 infection than those who got an evening shot.
“The sweet spot in our data was somewhere around late morning to late afternoon,” said lead researcher Jeffrey Haspel, MD, PhD, associate professor of medicine in the division of pulmonary and critical care medicine at Washington University School of Medicine in St. Louis.
In a multicenter, 2024 analysis of 13 studies of immunotherapy for advanced cancers in 1663 people, researchers found treatment earlier in the day was associated with longer survival time and longer survival without cancer progression.
“Patients with selected metastatic cancers seemed to largely benefit from early [time of day] infusions, which is consistent with circadian mechanisms in immune-cell functions and trafficking,” the researchers noted. But “retrospective randomized trials are needed to establish recommendations for optimal circadian timing.”
Other research suggests or is investigating possible chronotherapy benefits for depression, glaucoma, respiratory diseases, stroke treatment, epilepsy, and sedatives used in surgery. So why aren’t healthcare providers adding time of day to more prescriptions? “What’s missing is more reliable data,” Dr. Dyar said.
Should You Use Chronotherapy Now?
Experts emphasize that more research is needed before doctors use chronotherapy and before medical organizations include it in treatment recommendations. But for some patients, circadian dosing may be worth a try:
Night owls whose blood pressure isn’t well controlled. Dr. Dyar and Dr. Pigazzani said night-time blood pressure drugs may be helpful for people with a “late chronotype.” Of course, patients shouldn’t change their medication schedule on their own, they said. And doctors may want to consider other concerns, like more overnight bathroom visits with evening diuretics.
In their study, the researchers determined participants’ chronotype with a few questions from the Munich Chronotype Questionnaire about what time they fell asleep and woke up on workdays and days off and whether they considered themselves “morning types” or “evening types.” (The questions can be found in supplementary data for the study.)
If a physician thinks matching the timing of a dose with chronotype would help, they can consider it, Dr. Pigazzani said. “However, I must add that this was an observational study, so I would advise healthcare practitioners to wait for our data to be confirmed in new RCTs of personalized chronotherapy of hypertension.”
Children and older adults getting vaccines. Timing COVID shots and possibly other vaccines from late morning to mid-afternoon could have a small benefit for individuals and a bigger public-health benefit, Dr. Haspel said. But the most important thing is getting vaccinated. “If you can only get one in the evening, it’s still worthwhile. Timing may add oomph at a public-health level for more vulnerable groups.”
A version of this article appeared on Medscape.com.
Do drugs work better if taken by the clock?
A new analysis published in The Lancet journal’s eClinicalMedicine suggests: Yes, they do — if you consider the patient’s individual body clock. The study is the first to find that timing blood pressure drugs to a person’s personal “chronotype” — that is, whether they are a night owl or an early bird — may reduce the risk for a heart attack.
The findings represent a significant advance in the field of circadian medicine or “chronotherapy” — timing drug administration to circadian rhythms. A growing stack of research suggests this approach could reduce side effects and improve the effectiveness of a wide range of therapies, including vaccines, cancer treatments, and drugs for depression, glaucoma, pain, seizures, and other conditions. Still, despite decades of research, time of day is rarely considered in writing prescriptions.
“We are really just at the beginning of an exciting new way of looking at patient care,” said Kenneth A. Dyar, PhD, whose lab at Helmholtz Zentrum München’s Institute for Diabetes and Cancer focuses on metabolic physiology. Dr. Dyar is co-lead author of the new blood pressure analysis.
“Chronotherapy is a rapidly growing field,” he said, “and I suspect we are soon going to see more and more studies focused on ‘personalized chronotherapy,’ not only in hypertension but also potentially in other clinical areas.”
The ‘Missing Piece’ in Chronotherapy Research
Blood pressure drugs have long been chronotherapy’s battleground. After all, blood pressure follows a circadian rhythm, peaking in the morning and dropping at night.
That healthy overnight dip can disappear in people with diabetes, kidney disease, and obstructive sleep apnea. Some physicians have suggested a bed-time dose to restore that dip. But studies have had mixed results, so “take at bedtime” has become a less common recommendation in recent years.
But the debate continued. After a large 2019 Spanish study found that bedtime doses had benefits so big that the results drew questions, an even larger, 2022 randomized, controlled trial from the University of Dundee in Dundee, Scotland — called the TIME study — aimed to settle the question.
Researchers assigned over 21,000 people to take morning or night hypertension drugs for several years and found no difference in cardiovascular outcomes.
“We did this study thinking nocturnal blood pressure tablets might be better,” said Thomas MacDonald, MD, professor emeritus of clinical pharmacology and pharmacoepidemiology at the University of Dundee and principal investigator for the TIME study and the recent chronotype analysis. “But there was no difference for heart attacks, strokes, or vascular death.”
So, the researchers then looked at participants’ chronotypes, sorting outcomes based on whether the participants were late-to-bed, late-to-rise “night owls” or early-to-bed, early-to-rise “morning larks.”
Their analysis of these 5358 TIME participants found the following results: Risk for hospitalization for a heart attack was at least 34% lower for “owls” who took their drugs at bedtime. By contrast, owls’ heart attack risk was at least 62% higher with morning doses. For “larks,” the opposite was true. Morning doses were associated with an 11% lower heart attack risk and night doses with an 11% higher risk, according to supplemental data.
The personalized approach could explain why some previous chronotherapy studies have failed to show a benefit. Those studies did not individualize drug timing as this one did. But personalization could be key to circadian medicine’s success.
“Our ‘internal personal time’ appears to be an important variable to consider when dosing antihypertensives,” said co-lead author Filippo Pigazzani, MD, PhD, clinical senior lecturer and honorary consultant cardiologist at the University of Dundee School of Medicine. “Chronotherapy research has been going on for decades. We knew there was something important with time of day. But researchers haven’t considered the internal time of individual people. I think that is the missing piece.”
The analysis has several important limitations, the researchers said. A total of 95% of participants were White. And it was an observational study, not a true randomized comparison. “We started it late in the original TIME study,” Dr. MacDonald said. “You could argue we were reporting on those who survived long enough to get into the analysis.” More research is needed, they concluded.
Looking Beyond Blood Pressure
What about the rest of the body? “Almost all the cells of our body contain ‘circadian clocks’ that are synchronized by daily environmental cues, including light-dark, activity-rest, and feeding-fasting cycles,” said Dr. Dyar.
An estimated 50% of prescription drugs hit targets in the body that have circadian patterns. So, experts suspect that syncing a drug with a person’s body clock might increase effectiveness of many drugs.
A handful of US Food and Drug Administration–approved drugs already have time-of-day recommendations on the label for effectiveness or to limit side effects, including bedtime or evening for the insomnia drug Ambien, the HIV antiviral Atripla, and cholesterol-lowering Zocor. Others are intended to be taken with or after your last meal of the day, such as the long-acting insulin Levemir and the cardiovascular drug Xarelto. A morning recommendation comes with the proton pump inhibitor Nexium and the attention-deficit/hyperactivity disorder drug Ritalin.
Interest is expanding. About one third of the papers published about chronotherapy in the past 25 years have come out in the past 5 years. The May 2024 meeting of the Society for Research on Biological Rhythms featured a day-long session aimed at bringing clinicians up to speed. An organization called the International Association of Circadian Health Clinics is trying to bring circadian medicine findings to clinicians and their patients and to support research.
Moreover, while recent research suggests minding the clock could have benefits for a wide range of treatments, ignoring it could cause problems.
In a Massachusetts Institute of Technology study published in April in Science Advances, researchers looked at engineered livers made from human donor cells and found more than 300 genes that operate on a circadian schedule, many with roles in drug metabolism. They also found that circadian patterns affected the toxicity of acetaminophen and atorvastatin. Identifying the time of day to take these drugs could maximize effectiveness and minimize adverse effects, the researchers said.
Timing and the Immune System
Circadian rhythms are also seen in immune processes. In a 2023 study in The Journal of Clinical Investigation of vaccine data from 1.5 million people in Israel, researchers found that children and older adults who got their second dose of the Pfizer mRNA COVID vaccine earlier in the day were about 36% less likely to be hospitalized with SARS-CoV-2 infection than those who got an evening shot.
“The sweet spot in our data was somewhere around late morning to late afternoon,” said lead researcher Jeffrey Haspel, MD, PhD, associate professor of medicine in the division of pulmonary and critical care medicine at Washington University School of Medicine in St. Louis.
In a multicenter, 2024 analysis of 13 studies of immunotherapy for advanced cancers in 1663 people, researchers found treatment earlier in the day was associated with longer survival time and longer survival without cancer progression.
“Patients with selected metastatic cancers seemed to largely benefit from early [time of day] infusions, which is consistent with circadian mechanisms in immune-cell functions and trafficking,” the researchers noted. But “retrospective randomized trials are needed to establish recommendations for optimal circadian timing.”
Other research suggests or is investigating possible chronotherapy benefits for depression, glaucoma, respiratory diseases, stroke treatment, epilepsy, and sedatives used in surgery. So why aren’t healthcare providers adding time of day to more prescriptions? “What’s missing is more reliable data,” Dr. Dyar said.
Should You Use Chronotherapy Now?
Experts emphasize that more research is needed before doctors use chronotherapy and before medical organizations include it in treatment recommendations. But for some patients, circadian dosing may be worth a try:
Night owls whose blood pressure isn’t well controlled. Dr. Dyar and Dr. Pigazzani said night-time blood pressure drugs may be helpful for people with a “late chronotype.” Of course, patients shouldn’t change their medication schedule on their own, they said. And doctors may want to consider other concerns, like more overnight bathroom visits with evening diuretics.
In their study, the researchers determined participants’ chronotype with a few questions from the Munich Chronotype Questionnaire about what time they fell asleep and woke up on workdays and days off and whether they considered themselves “morning types” or “evening types.” (The questions can be found in supplementary data for the study.)
If a physician thinks matching the timing of a dose with chronotype would help, they can consider it, Dr. Pigazzani said. “However, I must add that this was an observational study, so I would advise healthcare practitioners to wait for our data to be confirmed in new RCTs of personalized chronotherapy of hypertension.”
Children and older adults getting vaccines. Timing COVID shots and possibly other vaccines from late morning to mid-afternoon could have a small benefit for individuals and a bigger public-health benefit, Dr. Haspel said. But the most important thing is getting vaccinated. “If you can only get one in the evening, it’s still worthwhile. Timing may add oomph at a public-health level for more vulnerable groups.”
A version of this article appeared on Medscape.com.
Merkel Cell: Immunotherapy Not Used for Many Patients With Metastatic Disease
PHOENIX — Immunotherapy has revolutionized outcomes for patients are better at high-volume centers.
The study has important implications, said study author Shayan Cheraghlou, MD, an incoming fellow in Mohs surgery at New York University, New York City. “We can see that in a real-world setting, these agents have an impact on survival,” he said. “We also found high-volume centers were significantly more likely to use the agents than low-volume centers.” He presented the findings at the annual meeting of the American College of Mohs Surgery.
MCC is a neuroendocrine skin cancer with a high rate of mortality, and even though it remains relatively rare, its incidence has been rising rapidly since the late 1990s and continues to increase. There were no approved treatments available until 2017, when the US Food and Drug Administration (FDA) approved the immunotherapy drug avelumab (Bavencio) to treat advanced MCC. Two years later, pembrolizumab (Keytruda) also received regulatory approval for MCC, and these two agents have revolutionized outcomes.
“In clinical trial settings, these agents led to significant and durable responses, and they are now the recommended treatments in guidelines for metastatic Merkel cell carcinoma,” said Dr. Cheraghlou. “However, we don’t have data as to how they are being used in the real-world setting and if survival outcomes are similar.”
Real World vs Clinical Trials
Real-world outcomes can differ from clinical trial data, and the adoption of novel therapeutics can be gradual. The goal of this study was to see if clinical trial data matched what was being observed in actual clinical use and if the agents were being used uniformly in centers across the United States.
The authors used data from the National Cancer Database that included patients diagnosed with cancer from 2004 to 2019 and identified 1017 adult cases of metastatic MCC. They then looked at the association of a variety of patient characteristics, tumors, and system factors with the likelihood of receiving systemic treatment for their disease.
“Our first finding was maybe the least surprising,” he said. “Patients who received these therapeutic agents had significantly improved survival compared to those who have not.”
Those who received immunotherapy had a 35% decrease in the risk for death per year compared with those who did not. The 1-, 3-, and 5-year survival rates were 47.2%, 21.8%, and 16.5%, respectively, for patients who did not receive immunotherapy compared with 62.7%, 34.4%, and 23.6%, respectively, for those who were treated with these agents.
Dr. Cheraghlou noted that they started to get some “surprising” findings when they looked at utilization data. “While it has been increasing over time, it is not as high as it should be,” he emphasized.
From 2017 to 2019, 54.2% of patients with metastatic MCC received immunotherapy. The data also showed an increase in use from 45.1% in 2017 to 63.0% in 2019. “This is an effective treatment for aggressive malignancy, so we have to ask why more patients aren’t getting them,” said Dr. Cheraghlou.
Their findings did suggest one possible reason, and that was that high-volume centers were significantly more likely to use the agents than low-volume centers. Centers that were in the top percentile for MCC case volume were three times as likely to use immunotherapy for MCC compared with other institutions. “So, if you have metastatic Merkel cell carcinoma and go to a low volume center, you may be less likely to get potential lifesaving treatment,” he noted.
Implications Going Forward
Dr. Cheraghlou concluded his presentation by pointing out that this study has important implications. The data showed that in a real-world setting, these agents have an impact on survival, but all eligible patients do not have access. “In other countries, there are established referral patterns for all patients with aggressive rare malignancies and really all cancers,” he added. “But in the US, cancer care is more decentralized. Studies like this and others show that high-volume centers have much better outcomes for aggressive rare malignancies, and we should be looking at why this is the case and mitigating these disparities and outcomes.”
Commenting on the study results, Jeffrey M. Farma, MD, co-director of the Melanoma and Skin Cancer Program and professor of surgical oncology at Fox Chase Cancer Center, Philadelphia, referred to the two immunotherapies that have been approved for MCC since 2017, which have demonstrated a survival benefit and improved outcomes in patients with metastatic MCC.
“In their study, immunotherapy was associated with improved outcomes,” said Dr. Farma. “This study highlights the continued lag of implementation of guidelines when new therapies are approved, and that for rare cancers like Merkel cell carcinoma, being treated at high-volume centers and the regionalization of care can lead to improved outcomes for patients.”
Dr. Cheraghlou and Dr. Farma had no disclosures.
A version of this article appeared on Medscape.com.
PHOENIX — Immunotherapy has revolutionized outcomes for patients are better at high-volume centers.
The study has important implications, said study author Shayan Cheraghlou, MD, an incoming fellow in Mohs surgery at New York University, New York City. “We can see that in a real-world setting, these agents have an impact on survival,” he said. “We also found high-volume centers were significantly more likely to use the agents than low-volume centers.” He presented the findings at the annual meeting of the American College of Mohs Surgery.
MCC is a neuroendocrine skin cancer with a high rate of mortality, and even though it remains relatively rare, its incidence has been rising rapidly since the late 1990s and continues to increase. There were no approved treatments available until 2017, when the US Food and Drug Administration (FDA) approved the immunotherapy drug avelumab (Bavencio) to treat advanced MCC. Two years later, pembrolizumab (Keytruda) also received regulatory approval for MCC, and these two agents have revolutionized outcomes.
“In clinical trial settings, these agents led to significant and durable responses, and they are now the recommended treatments in guidelines for metastatic Merkel cell carcinoma,” said Dr. Cheraghlou. “However, we don’t have data as to how they are being used in the real-world setting and if survival outcomes are similar.”
Real World vs Clinical Trials
Real-world outcomes can differ from clinical trial data, and the adoption of novel therapeutics can be gradual. The goal of this study was to see if clinical trial data matched what was being observed in actual clinical use and if the agents were being used uniformly in centers across the United States.
The authors used data from the National Cancer Database that included patients diagnosed with cancer from 2004 to 2019 and identified 1017 adult cases of metastatic MCC. They then looked at the association of a variety of patient characteristics, tumors, and system factors with the likelihood of receiving systemic treatment for their disease.
“Our first finding was maybe the least surprising,” he said. “Patients who received these therapeutic agents had significantly improved survival compared to those who have not.”
Those who received immunotherapy had a 35% decrease in the risk for death per year compared with those who did not. The 1-, 3-, and 5-year survival rates were 47.2%, 21.8%, and 16.5%, respectively, for patients who did not receive immunotherapy compared with 62.7%, 34.4%, and 23.6%, respectively, for those who were treated with these agents.
Dr. Cheraghlou noted that they started to get some “surprising” findings when they looked at utilization data. “While it has been increasing over time, it is not as high as it should be,” he emphasized.
From 2017 to 2019, 54.2% of patients with metastatic MCC received immunotherapy. The data also showed an increase in use from 45.1% in 2017 to 63.0% in 2019. “This is an effective treatment for aggressive malignancy, so we have to ask why more patients aren’t getting them,” said Dr. Cheraghlou.
Their findings did suggest one possible reason, and that was that high-volume centers were significantly more likely to use the agents than low-volume centers. Centers that were in the top percentile for MCC case volume were three times as likely to use immunotherapy for MCC compared with other institutions. “So, if you have metastatic Merkel cell carcinoma and go to a low volume center, you may be less likely to get potential lifesaving treatment,” he noted.
Implications Going Forward
Dr. Cheraghlou concluded his presentation by pointing out that this study has important implications. The data showed that in a real-world setting, these agents have an impact on survival, but all eligible patients do not have access. “In other countries, there are established referral patterns for all patients with aggressive rare malignancies and really all cancers,” he added. “But in the US, cancer care is more decentralized. Studies like this and others show that high-volume centers have much better outcomes for aggressive rare malignancies, and we should be looking at why this is the case and mitigating these disparities and outcomes.”
Commenting on the study results, Jeffrey M. Farma, MD, co-director of the Melanoma and Skin Cancer Program and professor of surgical oncology at Fox Chase Cancer Center, Philadelphia, referred to the two immunotherapies that have been approved for MCC since 2017, which have demonstrated a survival benefit and improved outcomes in patients with metastatic MCC.
“In their study, immunotherapy was associated with improved outcomes,” said Dr. Farma. “This study highlights the continued lag of implementation of guidelines when new therapies are approved, and that for rare cancers like Merkel cell carcinoma, being treated at high-volume centers and the regionalization of care can lead to improved outcomes for patients.”
Dr. Cheraghlou and Dr. Farma had no disclosures.
A version of this article appeared on Medscape.com.
PHOENIX — Immunotherapy has revolutionized outcomes for patients are better at high-volume centers.
The study has important implications, said study author Shayan Cheraghlou, MD, an incoming fellow in Mohs surgery at New York University, New York City. “We can see that in a real-world setting, these agents have an impact on survival,” he said. “We also found high-volume centers were significantly more likely to use the agents than low-volume centers.” He presented the findings at the annual meeting of the American College of Mohs Surgery.
MCC is a neuroendocrine skin cancer with a high rate of mortality, and even though it remains relatively rare, its incidence has been rising rapidly since the late 1990s and continues to increase. There were no approved treatments available until 2017, when the US Food and Drug Administration (FDA) approved the immunotherapy drug avelumab (Bavencio) to treat advanced MCC. Two years later, pembrolizumab (Keytruda) also received regulatory approval for MCC, and these two agents have revolutionized outcomes.
“In clinical trial settings, these agents led to significant and durable responses, and they are now the recommended treatments in guidelines for metastatic Merkel cell carcinoma,” said Dr. Cheraghlou. “However, we don’t have data as to how they are being used in the real-world setting and if survival outcomes are similar.”
Real World vs Clinical Trials
Real-world outcomes can differ from clinical trial data, and the adoption of novel therapeutics can be gradual. The goal of this study was to see if clinical trial data matched what was being observed in actual clinical use and if the agents were being used uniformly in centers across the United States.
The authors used data from the National Cancer Database that included patients diagnosed with cancer from 2004 to 2019 and identified 1017 adult cases of metastatic MCC. They then looked at the association of a variety of patient characteristics, tumors, and system factors with the likelihood of receiving systemic treatment for their disease.
“Our first finding was maybe the least surprising,” he said. “Patients who received these therapeutic agents had significantly improved survival compared to those who have not.”
Those who received immunotherapy had a 35% decrease in the risk for death per year compared with those who did not. The 1-, 3-, and 5-year survival rates were 47.2%, 21.8%, and 16.5%, respectively, for patients who did not receive immunotherapy compared with 62.7%, 34.4%, and 23.6%, respectively, for those who were treated with these agents.
Dr. Cheraghlou noted that they started to get some “surprising” findings when they looked at utilization data. “While it has been increasing over time, it is not as high as it should be,” he emphasized.
From 2017 to 2019, 54.2% of patients with metastatic MCC received immunotherapy. The data also showed an increase in use from 45.1% in 2017 to 63.0% in 2019. “This is an effective treatment for aggressive malignancy, so we have to ask why more patients aren’t getting them,” said Dr. Cheraghlou.
Their findings did suggest one possible reason, and that was that high-volume centers were significantly more likely to use the agents than low-volume centers. Centers that were in the top percentile for MCC case volume were three times as likely to use immunotherapy for MCC compared with other institutions. “So, if you have metastatic Merkel cell carcinoma and go to a low volume center, you may be less likely to get potential lifesaving treatment,” he noted.
Implications Going Forward
Dr. Cheraghlou concluded his presentation by pointing out that this study has important implications. The data showed that in a real-world setting, these agents have an impact on survival, but all eligible patients do not have access. “In other countries, there are established referral patterns for all patients with aggressive rare malignancies and really all cancers,” he added. “But in the US, cancer care is more decentralized. Studies like this and others show that high-volume centers have much better outcomes for aggressive rare malignancies, and we should be looking at why this is the case and mitigating these disparities and outcomes.”
Commenting on the study results, Jeffrey M. Farma, MD, co-director of the Melanoma and Skin Cancer Program and professor of surgical oncology at Fox Chase Cancer Center, Philadelphia, referred to the two immunotherapies that have been approved for MCC since 2017, which have demonstrated a survival benefit and improved outcomes in patients with metastatic MCC.
“In their study, immunotherapy was associated with improved outcomes,” said Dr. Farma. “This study highlights the continued lag of implementation of guidelines when new therapies are approved, and that for rare cancers like Merkel cell carcinoma, being treated at high-volume centers and the regionalization of care can lead to improved outcomes for patients.”
Dr. Cheraghlou and Dr. Farma had no disclosures.
A version of this article appeared on Medscape.com.
FROM ACMS 2024
Survey Spotlights Identification of Dermatologic Adverse Events From Cancer Therapies
“New cancer therapies have brought a diversity of treatment-related dermatologic adverse events (dAEs) beyond those experienced with conventional chemotherapy, which has demanded an evolving assessment of toxicities,” researchers led by Nicole R. LeBoeuf, MD, MPH, of the Department of Dermatology at Brigham and Women’s Hospital and the Center for Cutaneous Oncology at the Dana-Farber Brigham Cancer Center, Boston, wrote in a poster presented at the American Academy of Dermatology annual meeting.
The authors noted that “Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5.0)” serves as the current, broadly accepted criteria for classification and grading during routine medical care and clinical trials. But despite extensive utilization of CTCAE, there is little data regarding its application.”
To evaluate how CTCAE is being used in clinical practice, they sent a four-case survey of dAEs to 81 dermatologists and 182 medical oncologists at six US-based academic institutions. For three of the cases, respondents were asked to classify and grade morbilliform, psoriasiform, and papulopustular rashes based on a review of photographs and text descriptions. For the fourth case, respondents were asked to grade a dAE using only a clinic note text description. The researchers used chi-square tests in R software to compare survey responses.
Compared with medical oncologists, dermatologists were significantly more likely to provide correct responses in characterizing morbilliform and psoriasiform eruptions. “As low as 12%” of medical oncologists were correct, and “as low as 87%” of dermatologists were correct (P < .001). Similarly, dermatologists were significantly more likely to grade the psoriasiform, papulopustular, and written cases correctly compared with medical oncologists (P < .001 for all associations).
“These cases demonstrated poor concordance of classification and grading between specialties and across medical oncology,” the authors concluded in their poster, noting that 87% of medical oncologists were interested in additional educational tools on dAEs. “With correct classification as low as 12%, medical oncologists may have more difficulty delivering appropriate, toxicity-specific therapy and may consider banal eruptions dangerous.”
Poor concordance of grading among the two groups of clinicians “raises the question of whether CTCAE v5.0 is an appropriate determinant for patient continuation on therapy or in trials,” they added. “As anticancer therapy becomes more complex — with new toxicities from novel agents and combinations — we must ensure we have a grading system that is valid across investigators and does not harm patients by instituting unnecessary treatment stops.”
Future studies, they said, “can explore what interventions beyond involvement of dermatologists improve classification and grading in practice.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, noted that with the continued expansion and introduction of new targeted and immunotherapies in the oncology space, “you can be sure we will continue to appreciate the importance and value of the field of supportive oncodermatology, as hair, skin, and nails are almost guaranteed collateral damage in this story.
“Ensuring early identification and consistent grading severity is not only important for the plethora of patients who are currently developing the litany of cutaneous adverse events but to evaluate potential mitigation strategies and even push along countermeasures down the FDA approval pathway,” Dr. Friedman said. In this study, the investigators demonstrated that work “is sorely needed, not just in dermatology but even more so for our colleagues across the aisle. A central tenet of supportive oncodermatology must also be education for all stakeholders, and the good news is our oncology partners will welcome it.”
Dr. LeBoeuf disclosed that she is a consultant to and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, Fortress Biotech, and Synox Therapeutics outside the submitted work. No other authors reported having financial disclosures. Dr. Friedman directs the supportive oncodermatology program at GW that received independent funding from La Roche-Posay.
A version of this article first appeared on Medscape.com.
“New cancer therapies have brought a diversity of treatment-related dermatologic adverse events (dAEs) beyond those experienced with conventional chemotherapy, which has demanded an evolving assessment of toxicities,” researchers led by Nicole R. LeBoeuf, MD, MPH, of the Department of Dermatology at Brigham and Women’s Hospital and the Center for Cutaneous Oncology at the Dana-Farber Brigham Cancer Center, Boston, wrote in a poster presented at the American Academy of Dermatology annual meeting.
The authors noted that “Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5.0)” serves as the current, broadly accepted criteria for classification and grading during routine medical care and clinical trials. But despite extensive utilization of CTCAE, there is little data regarding its application.”
To evaluate how CTCAE is being used in clinical practice, they sent a four-case survey of dAEs to 81 dermatologists and 182 medical oncologists at six US-based academic institutions. For three of the cases, respondents were asked to classify and grade morbilliform, psoriasiform, and papulopustular rashes based on a review of photographs and text descriptions. For the fourth case, respondents were asked to grade a dAE using only a clinic note text description. The researchers used chi-square tests in R software to compare survey responses.
Compared with medical oncologists, dermatologists were significantly more likely to provide correct responses in characterizing morbilliform and psoriasiform eruptions. “As low as 12%” of medical oncologists were correct, and “as low as 87%” of dermatologists were correct (P < .001). Similarly, dermatologists were significantly more likely to grade the psoriasiform, papulopustular, and written cases correctly compared with medical oncologists (P < .001 for all associations).
“These cases demonstrated poor concordance of classification and grading between specialties and across medical oncology,” the authors concluded in their poster, noting that 87% of medical oncologists were interested in additional educational tools on dAEs. “With correct classification as low as 12%, medical oncologists may have more difficulty delivering appropriate, toxicity-specific therapy and may consider banal eruptions dangerous.”
Poor concordance of grading among the two groups of clinicians “raises the question of whether CTCAE v5.0 is an appropriate determinant for patient continuation on therapy or in trials,” they added. “As anticancer therapy becomes more complex — with new toxicities from novel agents and combinations — we must ensure we have a grading system that is valid across investigators and does not harm patients by instituting unnecessary treatment stops.”
Future studies, they said, “can explore what interventions beyond involvement of dermatologists improve classification and grading in practice.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, noted that with the continued expansion and introduction of new targeted and immunotherapies in the oncology space, “you can be sure we will continue to appreciate the importance and value of the field of supportive oncodermatology, as hair, skin, and nails are almost guaranteed collateral damage in this story.
“Ensuring early identification and consistent grading severity is not only important for the plethora of patients who are currently developing the litany of cutaneous adverse events but to evaluate potential mitigation strategies and even push along countermeasures down the FDA approval pathway,” Dr. Friedman said. In this study, the investigators demonstrated that work “is sorely needed, not just in dermatology but even more so for our colleagues across the aisle. A central tenet of supportive oncodermatology must also be education for all stakeholders, and the good news is our oncology partners will welcome it.”
Dr. LeBoeuf disclosed that she is a consultant to and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, Fortress Biotech, and Synox Therapeutics outside the submitted work. No other authors reported having financial disclosures. Dr. Friedman directs the supportive oncodermatology program at GW that received independent funding from La Roche-Posay.
A version of this article first appeared on Medscape.com.
“New cancer therapies have brought a diversity of treatment-related dermatologic adverse events (dAEs) beyond those experienced with conventional chemotherapy, which has demanded an evolving assessment of toxicities,” researchers led by Nicole R. LeBoeuf, MD, MPH, of the Department of Dermatology at Brigham and Women’s Hospital and the Center for Cutaneous Oncology at the Dana-Farber Brigham Cancer Center, Boston, wrote in a poster presented at the American Academy of Dermatology annual meeting.
The authors noted that “Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5.0)” serves as the current, broadly accepted criteria for classification and grading during routine medical care and clinical trials. But despite extensive utilization of CTCAE, there is little data regarding its application.”
To evaluate how CTCAE is being used in clinical practice, they sent a four-case survey of dAEs to 81 dermatologists and 182 medical oncologists at six US-based academic institutions. For three of the cases, respondents were asked to classify and grade morbilliform, psoriasiform, and papulopustular rashes based on a review of photographs and text descriptions. For the fourth case, respondents were asked to grade a dAE using only a clinic note text description. The researchers used chi-square tests in R software to compare survey responses.
Compared with medical oncologists, dermatologists were significantly more likely to provide correct responses in characterizing morbilliform and psoriasiform eruptions. “As low as 12%” of medical oncologists were correct, and “as low as 87%” of dermatologists were correct (P < .001). Similarly, dermatologists were significantly more likely to grade the psoriasiform, papulopustular, and written cases correctly compared with medical oncologists (P < .001 for all associations).
“These cases demonstrated poor concordance of classification and grading between specialties and across medical oncology,” the authors concluded in their poster, noting that 87% of medical oncologists were interested in additional educational tools on dAEs. “With correct classification as low as 12%, medical oncologists may have more difficulty delivering appropriate, toxicity-specific therapy and may consider banal eruptions dangerous.”
Poor concordance of grading among the two groups of clinicians “raises the question of whether CTCAE v5.0 is an appropriate determinant for patient continuation on therapy or in trials,” they added. “As anticancer therapy becomes more complex — with new toxicities from novel agents and combinations — we must ensure we have a grading system that is valid across investigators and does not harm patients by instituting unnecessary treatment stops.”
Future studies, they said, “can explore what interventions beyond involvement of dermatologists improve classification and grading in practice.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, noted that with the continued expansion and introduction of new targeted and immunotherapies in the oncology space, “you can be sure we will continue to appreciate the importance and value of the field of supportive oncodermatology, as hair, skin, and nails are almost guaranteed collateral damage in this story.
“Ensuring early identification and consistent grading severity is not only important for the plethora of patients who are currently developing the litany of cutaneous adverse events but to evaluate potential mitigation strategies and even push along countermeasures down the FDA approval pathway,” Dr. Friedman said. In this study, the investigators demonstrated that work “is sorely needed, not just in dermatology but even more so for our colleagues across the aisle. A central tenet of supportive oncodermatology must also be education for all stakeholders, and the good news is our oncology partners will welcome it.”
Dr. LeBoeuf disclosed that she is a consultant to and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, Fortress Biotech, and Synox Therapeutics outside the submitted work. No other authors reported having financial disclosures. Dr. Friedman directs the supportive oncodermatology program at GW that received independent funding from La Roche-Posay.
A version of this article first appeared on Medscape.com.
FROM AAD 2024