User login
For MD-IQ use only
@GiJournal: An online platform to discuss the latest gastroenterology and hepatology publications
The last decade has seen an increased focus on the use of social media for medical education. Twitter, with over 330 million active users, is the most popular social media platform for medical education. We describe here our recent initiative to establish a weekly online gastroenterology-focused journal club on Twitter.
How was the idea conceived?
Sultan Mahmood, MD (@SultanMahmoodMD)
I joined #GITwitter at the end of 2019 and started following some of the leading experts in the field of gastroenterology and hepatology. It was a pleasant surprise to see how easy it was to engage with them and get expert opinions from across the world in real time. #MondayNightIBD, led by Aline Charabaty, MD, had become a phenomenon in the GI community and changed the perception of medical education in the digital world. There were online journal clubs for different medical subspecialties, including #NephroJC, #HOJournalClub, and #DermJC, but none for gastroenterology. Realizing this opportunity, and with guidance from Dr. Charabaty, we started @GiJournal in December of 2019 with weekly discussions.
@GiJournal started off as an informal discussion in which we would post a summary of the article and invite an expert in the field to comment. However, the interest in the journal club quickly took off as we gained more followers and a worldwide audience joined our journal club discussions on a weekly basis. As the COVID-19 pandemic took hold and endoscopy suites around the word closed, interest in online medical education grew. @GIJournal provided a platform for trainees and practicing physicians alike to stay up to date with the latest publications from the comfort of their homes. Needless to say, the journal club has evolved since its inception in that we now work with a team of experts and trainees who run the journal club on a rotating basis.
How does @GiJournal work?
Ijlal Akbar Ali, MD (@IjlalAkbar)
We have a large editorial board with volunteer faculty and trainees, all divided into four special interest groups (general GI/inflammatory bowel disease, interventional endoscopy/bariatric endoscopy, hepatology, and esophageal/motility disorders). Each week, a faculty member and a trainee pick a recently published article from a high-impact GI-focused journal. We also try to invite an expert of international repute (often the authors of the article themselves!) to engage as well. The faculty moderator and invited expert then work with the trainee to plan the session content. We post the topic and article on Monday. At 8 p.m. EST on Wednesday, the trainee posts a series of six to eight tweets summarizing the article. The faculty then asks the invited expert (and audience at large) a series of predetermined questions. Anyone can respond, share their opinion, and direct their own questions toward the moderator and expert who continually check their notifications and respond in real time. This brews into an hour-long discussion which covers not only the methodologic aspects of the article, but clinical practice in general. Discussions often trickle into the next day as people from different time zones participate. Everyone uses #GIJC at the end of their tweets which assists those following the article and facilitates indexing for future review. For those who miss or want to review sessions, we conveniently summarize all articles and corresponding discussions in a monthly publication, @GiJournal Digest, that is posted on Twitter for anyone to download, read and enjoy (Figure 1).
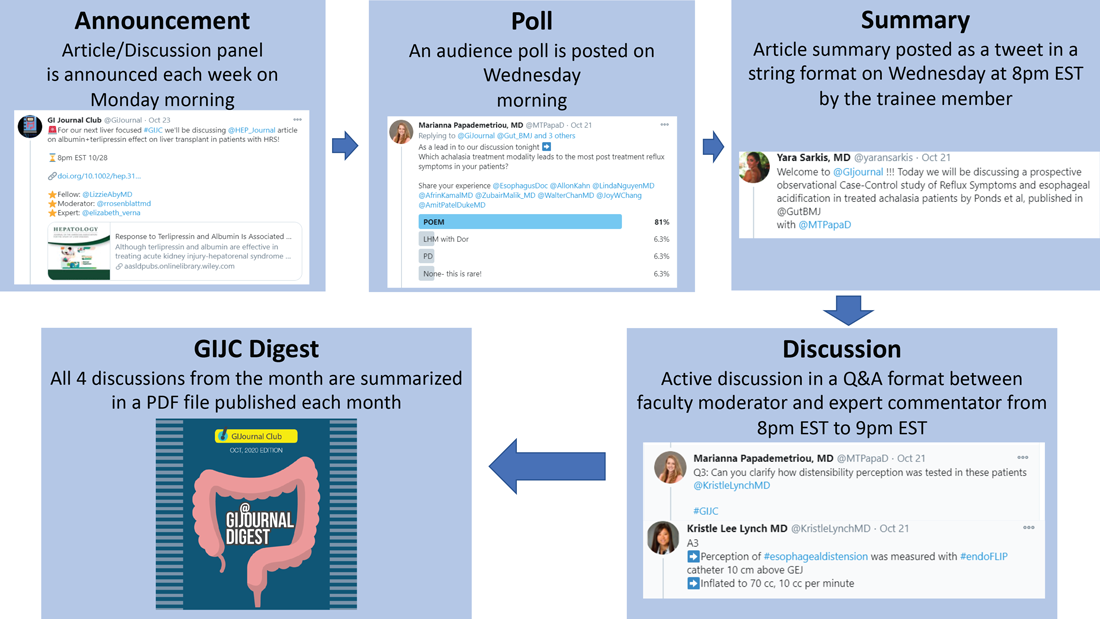
How is this different from any other journal club?
Atoosa Rabiee, MD (@AtoosaRabiee)
@GiJournal is unique in that it provides trainees and practicing gastroenterologists access to interactive discussions with both authors and world-renowned experts in the field. Online journal clubs operate with a flattened hierarchy; as such, they inherently break down access barriers to both the researchers who performed the study and key opinion leaders who commonly participate. There is no boundary as far as institutions or even countries. As a result, our platform has uncovered an unexpected degree of interest in live online discussion, and we have enjoyed collaborating and learning from experts from all over the world. @GiJournal also differs from conventional journal clubs by allowing trainees the opportunity to collaborate and engage with mentors from other institutions. As such, trainees develop relationships with experts in the field outside their home institutions, experts with whom they may not have had contact otherwise.
Although worldwide participation is a key strength of the online @GiJournal platform, it may be challenging for some members to attend the live discussion based on time difference. We account for this in two ways. First, participants are encouraged to continue with comments and questions afterward at their convenience, which allows experts and moderators to continue the conversation, often for several days. Second, to promote inclusivity, we have created a unique, customized publication to summarize and present the key points of conversation for each session. This asynchronous access is a quality not found in more traditional journal club formats. Finally, studies have shown that articles shared on social media tend to have increased citations and higher Altmetric scores.
What are the opportunities for trainees and recent graduates?
Sunil Amin, MD, MPH (@SunilAminMD)
Our surveys have shown that 30%-45% of the @GiJournal discussion participants are trainees. Both gastroenterology fellows and internal medicine residents from around the world are an integral part of each specialty panel for the weekly @GIjournal discussions. Trainees are paired up with a specific faculty mentor and together they choose an article for discussion, create a summary, informal twitter poll, and questions for the discussion. This direct access provides an opportunity for trainees to interact, ask questions, and learn from faculty in an informal atmosphere.
We have heard from multiple trainees who have developed long-term relationships with the experts and faculty mentors they worked with and are now also working on research projects. Additionally, trainees can bring the expertise they have now acquired back to their home institutions to pick articles, add specific teaching points, and enrich their local journal club discussions. Finally, trainees who present on the @GiJournal platform are given unique visibility to the many faculty members and opinion leaders participating in each discussion. This may facilitate future networking opportunities and enhance their CVs for future fellowship or employment applications.
Plans for the future?
Allon Kahn, MD (@AllonKahn)
Despite significant evolution and growth in @GiJournal over the past year, we are still actively working to expand our platform. Modes of online medical education, specifically Twitter-based GI journal club discussions, remain in their infancy. We see this @GiJournal as an opportunity for innovation as we plan for the year ahead. Our top priority for the upcoming year includes obtaining CME approval, which we are currently developing with Integrity CE (an Accreditation Council for Continuing Medical Education–accredited provider of CME for health care professionals). This will give an opportunity for the participants to be awarded CME credit when they participate in our weekly discussions. Other options being explored include starting a podcast and translation of @GiJournal Digest in different languages to reach a wider international audience. Furthermore, with the continued expansion of GI leaders and experts joining and engaging in Twitter, our options for unique and multidisciplinary discussion topics will continue to grow.
How can you join the @GiJournal discussions?
@SultanMahmoodMD
Joining the journal club discussion is easy. Just follow the @GiJournal handle on Twitter and turn on the notifications icon. Although we encourage everyone to “actively” participate in the discussion by asking questions or sharing your personal experience, joining the discussion as an “observer” is also a great way to learn. The discussion starts at 8 p.m. EST every Wednesday. Follow the #GIJC and the @GiJournal handle as questions are posted by the faculty moderator and answered by the experts. Even if you miss the discussion, the @GiJournal Digest is a great way to recap the discussions in an easy-to-read PDF format. The @GiJournal Digest is a monthly publication that archives the four @GiJournal club discussions in the previous month. Follow the link below to access the recent publications: http://ow.ly/uu2550C3RXX
Conclusion
In summary, we believe Twitter-based journal clubs offer an engaging way of virtual learning from the comfort of one’s home and a convenient way to directly interact with the experts. The success of @GiJournal highlights the importance of social media for medical education in the field of gastroenterology and hepatology and we look forward to developing this endeavor further.
Dr. Mahmood is clinical assistant professor of medicine, co–program director of the GI fellowship program, UB division of gastroenterology, hepatology & nutrition, State University of New York at Buffalo; Dr. Rabiee is assistant professor of medicine, director of hepatology, division of gastroenterology and hepatology, Washington DC VA Medical Center, Washington; Dr. Amin is assistant professor of medicine, director of endoscopy, The Lennar Foundation Medical Center, division of digestive health and liver disease, department of medicine, University of Miami; Dr. Kahn is assistant professor of medicine, division of gastroenterology & hepatology, Mayo Clinic, Scottsdale, Ariz.; and Dr. Akbar Ali is a gastroenterology fellow in the division of digestive diseases and nutrition, University of Oklahoma Health Sciences Center, Oklahoma City.
The last decade has seen an increased focus on the use of social media for medical education. Twitter, with over 330 million active users, is the most popular social media platform for medical education. We describe here our recent initiative to establish a weekly online gastroenterology-focused journal club on Twitter.
How was the idea conceived?
Sultan Mahmood, MD (@SultanMahmoodMD)
I joined #GITwitter at the end of 2019 and started following some of the leading experts in the field of gastroenterology and hepatology. It was a pleasant surprise to see how easy it was to engage with them and get expert opinions from across the world in real time. #MondayNightIBD, led by Aline Charabaty, MD, had become a phenomenon in the GI community and changed the perception of medical education in the digital world. There were online journal clubs for different medical subspecialties, including #NephroJC, #HOJournalClub, and #DermJC, but none for gastroenterology. Realizing this opportunity, and with guidance from Dr. Charabaty, we started @GiJournal in December of 2019 with weekly discussions.
@GiJournal started off as an informal discussion in which we would post a summary of the article and invite an expert in the field to comment. However, the interest in the journal club quickly took off as we gained more followers and a worldwide audience joined our journal club discussions on a weekly basis. As the COVID-19 pandemic took hold and endoscopy suites around the word closed, interest in online medical education grew. @GIJournal provided a platform for trainees and practicing physicians alike to stay up to date with the latest publications from the comfort of their homes. Needless to say, the journal club has evolved since its inception in that we now work with a team of experts and trainees who run the journal club on a rotating basis.
How does @GiJournal work?
Ijlal Akbar Ali, MD (@IjlalAkbar)
We have a large editorial board with volunteer faculty and trainees, all divided into four special interest groups (general GI/inflammatory bowel disease, interventional endoscopy/bariatric endoscopy, hepatology, and esophageal/motility disorders). Each week, a faculty member and a trainee pick a recently published article from a high-impact GI-focused journal. We also try to invite an expert of international repute (often the authors of the article themselves!) to engage as well. The faculty moderator and invited expert then work with the trainee to plan the session content. We post the topic and article on Monday. At 8 p.m. EST on Wednesday, the trainee posts a series of six to eight tweets summarizing the article. The faculty then asks the invited expert (and audience at large) a series of predetermined questions. Anyone can respond, share their opinion, and direct their own questions toward the moderator and expert who continually check their notifications and respond in real time. This brews into an hour-long discussion which covers not only the methodologic aspects of the article, but clinical practice in general. Discussions often trickle into the next day as people from different time zones participate. Everyone uses #GIJC at the end of their tweets which assists those following the article and facilitates indexing for future review. For those who miss or want to review sessions, we conveniently summarize all articles and corresponding discussions in a monthly publication, @GiJournal Digest, that is posted on Twitter for anyone to download, read and enjoy (Figure 1).

How is this different from any other journal club?
Atoosa Rabiee, MD (@AtoosaRabiee)
@GiJournal is unique in that it provides trainees and practicing gastroenterologists access to interactive discussions with both authors and world-renowned experts in the field. Online journal clubs operate with a flattened hierarchy; as such, they inherently break down access barriers to both the researchers who performed the study and key opinion leaders who commonly participate. There is no boundary as far as institutions or even countries. As a result, our platform has uncovered an unexpected degree of interest in live online discussion, and we have enjoyed collaborating and learning from experts from all over the world. @GiJournal also differs from conventional journal clubs by allowing trainees the opportunity to collaborate and engage with mentors from other institutions. As such, trainees develop relationships with experts in the field outside their home institutions, experts with whom they may not have had contact otherwise.
Although worldwide participation is a key strength of the online @GiJournal platform, it may be challenging for some members to attend the live discussion based on time difference. We account for this in two ways. First, participants are encouraged to continue with comments and questions afterward at their convenience, which allows experts and moderators to continue the conversation, often for several days. Second, to promote inclusivity, we have created a unique, customized publication to summarize and present the key points of conversation for each session. This asynchronous access is a quality not found in more traditional journal club formats. Finally, studies have shown that articles shared on social media tend to have increased citations and higher Altmetric scores.
What are the opportunities for trainees and recent graduates?
Sunil Amin, MD, MPH (@SunilAminMD)
Our surveys have shown that 30%-45% of the @GiJournal discussion participants are trainees. Both gastroenterology fellows and internal medicine residents from around the world are an integral part of each specialty panel for the weekly @GIjournal discussions. Trainees are paired up with a specific faculty mentor and together they choose an article for discussion, create a summary, informal twitter poll, and questions for the discussion. This direct access provides an opportunity for trainees to interact, ask questions, and learn from faculty in an informal atmosphere.
We have heard from multiple trainees who have developed long-term relationships with the experts and faculty mentors they worked with and are now also working on research projects. Additionally, trainees can bring the expertise they have now acquired back to their home institutions to pick articles, add specific teaching points, and enrich their local journal club discussions. Finally, trainees who present on the @GiJournal platform are given unique visibility to the many faculty members and opinion leaders participating in each discussion. This may facilitate future networking opportunities and enhance their CVs for future fellowship or employment applications.
Plans for the future?
Allon Kahn, MD (@AllonKahn)
Despite significant evolution and growth in @GiJournal over the past year, we are still actively working to expand our platform. Modes of online medical education, specifically Twitter-based GI journal club discussions, remain in their infancy. We see this @GiJournal as an opportunity for innovation as we plan for the year ahead. Our top priority for the upcoming year includes obtaining CME approval, which we are currently developing with Integrity CE (an Accreditation Council for Continuing Medical Education–accredited provider of CME for health care professionals). This will give an opportunity for the participants to be awarded CME credit when they participate in our weekly discussions. Other options being explored include starting a podcast and translation of @GiJournal Digest in different languages to reach a wider international audience. Furthermore, with the continued expansion of GI leaders and experts joining and engaging in Twitter, our options for unique and multidisciplinary discussion topics will continue to grow.
How can you join the @GiJournal discussions?
@SultanMahmoodMD
Joining the journal club discussion is easy. Just follow the @GiJournal handle on Twitter and turn on the notifications icon. Although we encourage everyone to “actively” participate in the discussion by asking questions or sharing your personal experience, joining the discussion as an “observer” is also a great way to learn. The discussion starts at 8 p.m. EST every Wednesday. Follow the #GIJC and the @GiJournal handle as questions are posted by the faculty moderator and answered by the experts. Even if you miss the discussion, the @GiJournal Digest is a great way to recap the discussions in an easy-to-read PDF format. The @GiJournal Digest is a monthly publication that archives the four @GiJournal club discussions in the previous month. Follow the link below to access the recent publications: http://ow.ly/uu2550C3RXX
Conclusion
In summary, we believe Twitter-based journal clubs offer an engaging way of virtual learning from the comfort of one’s home and a convenient way to directly interact with the experts. The success of @GiJournal highlights the importance of social media for medical education in the field of gastroenterology and hepatology and we look forward to developing this endeavor further.
Dr. Mahmood is clinical assistant professor of medicine, co–program director of the GI fellowship program, UB division of gastroenterology, hepatology & nutrition, State University of New York at Buffalo; Dr. Rabiee is assistant professor of medicine, director of hepatology, division of gastroenterology and hepatology, Washington DC VA Medical Center, Washington; Dr. Amin is assistant professor of medicine, director of endoscopy, The Lennar Foundation Medical Center, division of digestive health and liver disease, department of medicine, University of Miami; Dr. Kahn is assistant professor of medicine, division of gastroenterology & hepatology, Mayo Clinic, Scottsdale, Ariz.; and Dr. Akbar Ali is a gastroenterology fellow in the division of digestive diseases and nutrition, University of Oklahoma Health Sciences Center, Oklahoma City.
The last decade has seen an increased focus on the use of social media for medical education. Twitter, with over 330 million active users, is the most popular social media platform for medical education. We describe here our recent initiative to establish a weekly online gastroenterology-focused journal club on Twitter.
How was the idea conceived?
Sultan Mahmood, MD (@SultanMahmoodMD)
I joined #GITwitter at the end of 2019 and started following some of the leading experts in the field of gastroenterology and hepatology. It was a pleasant surprise to see how easy it was to engage with them and get expert opinions from across the world in real time. #MondayNightIBD, led by Aline Charabaty, MD, had become a phenomenon in the GI community and changed the perception of medical education in the digital world. There were online journal clubs for different medical subspecialties, including #NephroJC, #HOJournalClub, and #DermJC, but none for gastroenterology. Realizing this opportunity, and with guidance from Dr. Charabaty, we started @GiJournal in December of 2019 with weekly discussions.
@GiJournal started off as an informal discussion in which we would post a summary of the article and invite an expert in the field to comment. However, the interest in the journal club quickly took off as we gained more followers and a worldwide audience joined our journal club discussions on a weekly basis. As the COVID-19 pandemic took hold and endoscopy suites around the word closed, interest in online medical education grew. @GIJournal provided a platform for trainees and practicing physicians alike to stay up to date with the latest publications from the comfort of their homes. Needless to say, the journal club has evolved since its inception in that we now work with a team of experts and trainees who run the journal club on a rotating basis.
How does @GiJournal work?
Ijlal Akbar Ali, MD (@IjlalAkbar)
We have a large editorial board with volunteer faculty and trainees, all divided into four special interest groups (general GI/inflammatory bowel disease, interventional endoscopy/bariatric endoscopy, hepatology, and esophageal/motility disorders). Each week, a faculty member and a trainee pick a recently published article from a high-impact GI-focused journal. We also try to invite an expert of international repute (often the authors of the article themselves!) to engage as well. The faculty moderator and invited expert then work with the trainee to plan the session content. We post the topic and article on Monday. At 8 p.m. EST on Wednesday, the trainee posts a series of six to eight tweets summarizing the article. The faculty then asks the invited expert (and audience at large) a series of predetermined questions. Anyone can respond, share their opinion, and direct their own questions toward the moderator and expert who continually check their notifications and respond in real time. This brews into an hour-long discussion which covers not only the methodologic aspects of the article, but clinical practice in general. Discussions often trickle into the next day as people from different time zones participate. Everyone uses #GIJC at the end of their tweets which assists those following the article and facilitates indexing for future review. For those who miss or want to review sessions, we conveniently summarize all articles and corresponding discussions in a monthly publication, @GiJournal Digest, that is posted on Twitter for anyone to download, read and enjoy (Figure 1).

How is this different from any other journal club?
Atoosa Rabiee, MD (@AtoosaRabiee)
@GiJournal is unique in that it provides trainees and practicing gastroenterologists access to interactive discussions with both authors and world-renowned experts in the field. Online journal clubs operate with a flattened hierarchy; as such, they inherently break down access barriers to both the researchers who performed the study and key opinion leaders who commonly participate. There is no boundary as far as institutions or even countries. As a result, our platform has uncovered an unexpected degree of interest in live online discussion, and we have enjoyed collaborating and learning from experts from all over the world. @GiJournal also differs from conventional journal clubs by allowing trainees the opportunity to collaborate and engage with mentors from other institutions. As such, trainees develop relationships with experts in the field outside their home institutions, experts with whom they may not have had contact otherwise.
Although worldwide participation is a key strength of the online @GiJournal platform, it may be challenging for some members to attend the live discussion based on time difference. We account for this in two ways. First, participants are encouraged to continue with comments and questions afterward at their convenience, which allows experts and moderators to continue the conversation, often for several days. Second, to promote inclusivity, we have created a unique, customized publication to summarize and present the key points of conversation for each session. This asynchronous access is a quality not found in more traditional journal club formats. Finally, studies have shown that articles shared on social media tend to have increased citations and higher Altmetric scores.
What are the opportunities for trainees and recent graduates?
Sunil Amin, MD, MPH (@SunilAminMD)
Our surveys have shown that 30%-45% of the @GiJournal discussion participants are trainees. Both gastroenterology fellows and internal medicine residents from around the world are an integral part of each specialty panel for the weekly @GIjournal discussions. Trainees are paired up with a specific faculty mentor and together they choose an article for discussion, create a summary, informal twitter poll, and questions for the discussion. This direct access provides an opportunity for trainees to interact, ask questions, and learn from faculty in an informal atmosphere.
We have heard from multiple trainees who have developed long-term relationships with the experts and faculty mentors they worked with and are now also working on research projects. Additionally, trainees can bring the expertise they have now acquired back to their home institutions to pick articles, add specific teaching points, and enrich their local journal club discussions. Finally, trainees who present on the @GiJournal platform are given unique visibility to the many faculty members and opinion leaders participating in each discussion. This may facilitate future networking opportunities and enhance their CVs for future fellowship or employment applications.
Plans for the future?
Allon Kahn, MD (@AllonKahn)
Despite significant evolution and growth in @GiJournal over the past year, we are still actively working to expand our platform. Modes of online medical education, specifically Twitter-based GI journal club discussions, remain in their infancy. We see this @GiJournal as an opportunity for innovation as we plan for the year ahead. Our top priority for the upcoming year includes obtaining CME approval, which we are currently developing with Integrity CE (an Accreditation Council for Continuing Medical Education–accredited provider of CME for health care professionals). This will give an opportunity for the participants to be awarded CME credit when they participate in our weekly discussions. Other options being explored include starting a podcast and translation of @GiJournal Digest in different languages to reach a wider international audience. Furthermore, with the continued expansion of GI leaders and experts joining and engaging in Twitter, our options for unique and multidisciplinary discussion topics will continue to grow.
How can you join the @GiJournal discussions?
@SultanMahmoodMD
Joining the journal club discussion is easy. Just follow the @GiJournal handle on Twitter and turn on the notifications icon. Although we encourage everyone to “actively” participate in the discussion by asking questions or sharing your personal experience, joining the discussion as an “observer” is also a great way to learn. The discussion starts at 8 p.m. EST every Wednesday. Follow the #GIJC and the @GiJournal handle as questions are posted by the faculty moderator and answered by the experts. Even if you miss the discussion, the @GiJournal Digest is a great way to recap the discussions in an easy-to-read PDF format. The @GiJournal Digest is a monthly publication that archives the four @GiJournal club discussions in the previous month. Follow the link below to access the recent publications: http://ow.ly/uu2550C3RXX
Conclusion
In summary, we believe Twitter-based journal clubs offer an engaging way of virtual learning from the comfort of one’s home and a convenient way to directly interact with the experts. The success of @GiJournal highlights the importance of social media for medical education in the field of gastroenterology and hepatology and we look forward to developing this endeavor further.
Dr. Mahmood is clinical assistant professor of medicine, co–program director of the GI fellowship program, UB division of gastroenterology, hepatology & nutrition, State University of New York at Buffalo; Dr. Rabiee is assistant professor of medicine, director of hepatology, division of gastroenterology and hepatology, Washington DC VA Medical Center, Washington; Dr. Amin is assistant professor of medicine, director of endoscopy, The Lennar Foundation Medical Center, division of digestive health and liver disease, department of medicine, University of Miami; Dr. Kahn is assistant professor of medicine, division of gastroenterology & hepatology, Mayo Clinic, Scottsdale, Ariz.; and Dr. Akbar Ali is a gastroenterology fellow in the division of digestive diseases and nutrition, University of Oklahoma Health Sciences Center, Oklahoma City.
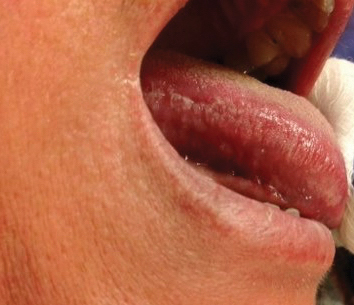
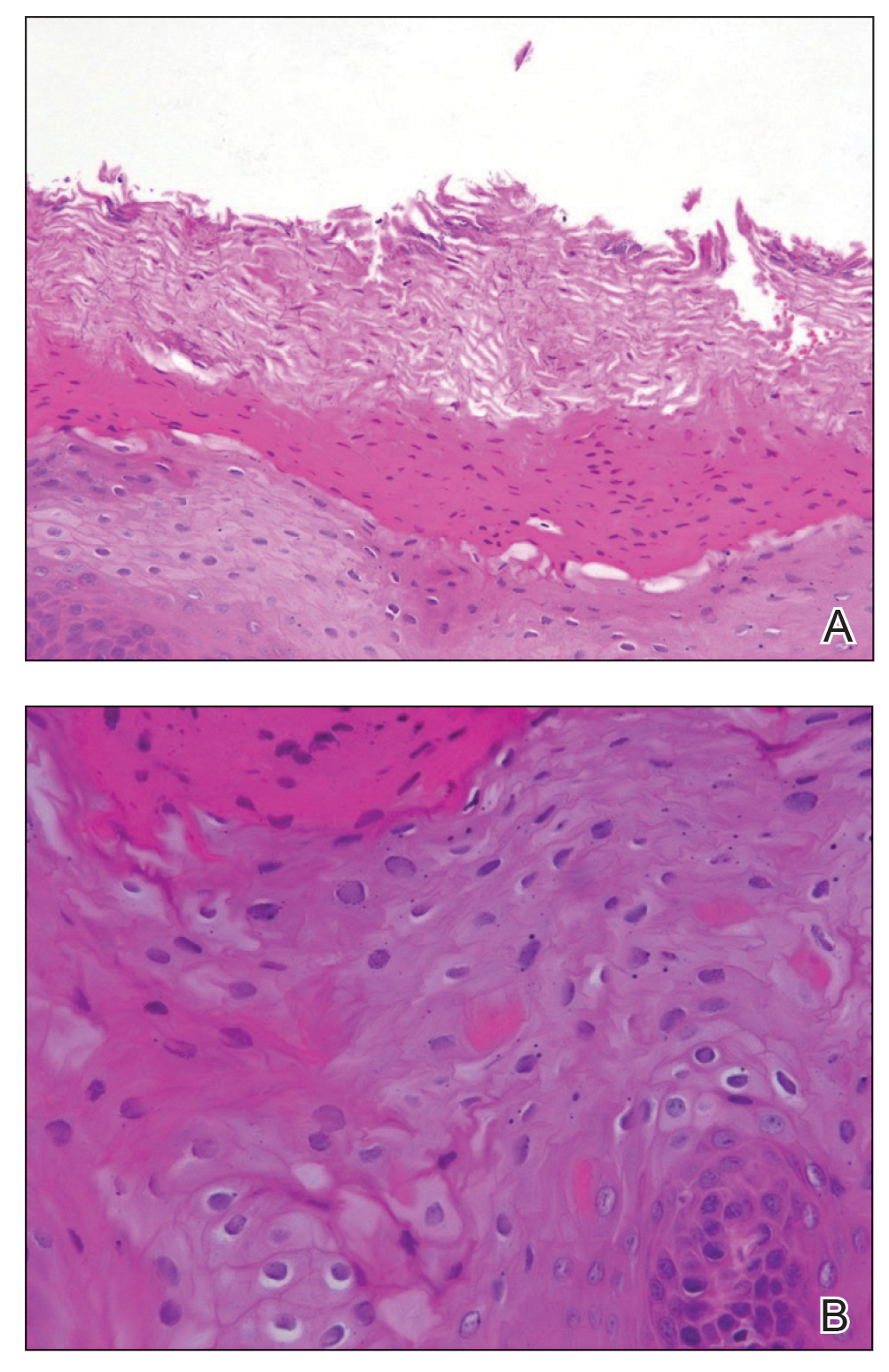
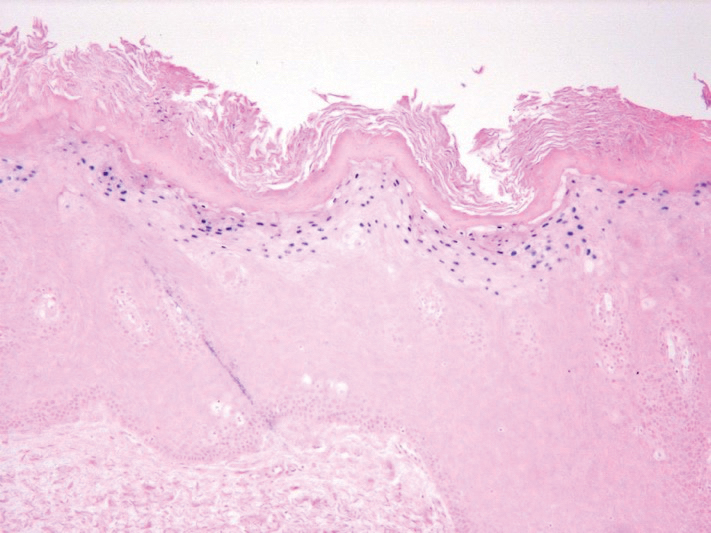
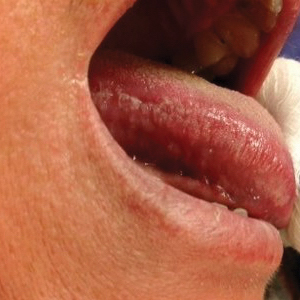
Abnormal anal paps in people with HIV can go more than a year without follow-up
That delay “revealed missed opportunities for a better experience on the patient, clinic, and provider level,” Jessica Wells, PhD, research assistant professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said in an interview. After all, “a lot can happen in that 1 year,” including early development of human papillomavirus (HPV)–associated anal cancer.
Although it’s too soon to say how significant that delay is with respect to the natural history of anal cancer, Dr. Wells said the data are a potential signal of disparities.
“The findings from my study may foreshadow potential disparities if we don’t have the necessary resources in place to promote follow-up care after an abnormal Pap test, similar to the disparities that we see in cervical cancer,” she said during the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
Single-center study
In the United States, people living with HIV are 19 times more likely to develop anal cancer than the general population, according to a 2018 article in the Journal of Clinical Oncology. Another single-center study from Yale University found that, in minority communities, anal cancer rates were 75% higher than in White communities. Anal cancer rates were 72% higher in communities with greater poverty. As a result, many clinics are beginning to administer Pap tests to determine early signs of HPV infection and associated changes.
In Dr. Wells’ study, which was conducted from 2012 to 2015, 150 adults with HIV who were aged 21 and older were recruited from Grady Ponce De Leon Center in Atlanta. According to a 2018 study from that center, a large minority of participants had late-stage HIV and suppressed immune systems.
All participants had been referred for HRA after a recent abnormal anal Pap test. Participants filled out questionnaires on sociodemographics, internalized HIV-related stigma, depression, risk behaviors, social support, and knowledge about HPV and anal cancer.
Participants were disproportionately older (mean age, 50.9 years); cisgender (86.7%), Black (78%); and gay, lesbian, or bisexual (84.3%). Slightly more than 1 in 10 participants (11.3%) were transgender women.
Although for 6% of participants, Pap test results indicated high-grade squamous intraepithelial lesions (HSIL), an additional 8% had atypical Pap findings that couldn’t exclude HSIL – the kinds of results that are one step away from a cancer diagnosis. More than 80% of participants had low-grade or inconclusive results. Nearly half (44%) of participants’ Pap tests revealed low-grade squamous cell intraepithelial cell lesions (LSIL); 42% indicated atypical squamous cells of undetermined significance.
When Dr. Wells looked at how long participants had waited to undergo HRA, she found something that surprised her: although some participants underwent follow-up assessment in 17 days, for many, it took much longer. The longest wait was 2,350 days – more than 6 years.
“There were quite a few patients who had follow-up beyond 1,000-plus days,” Dr. Wells said in an interview. “I didn›t think the delays were that long — at most, I would say that patients will get scheduled and come back within a few weeks or months.”
What’s more, she discovered through the HPV knowledge questionnaire that many participants did not understand why they were having a follow-up appointment. Anecdotally, some confused HPV with HIV.
“There’s education to be done to inform this target population that those living with HIV are more prone or at increased risk of this virus causing cancer later,” she said. “There are a lot of campaigns around women living with HIV, that they need to do cervical cancer screening. I think we need to really expand this campaign to include that HPV can also cause anal cancer.”
Dr. Wells had planned to primarily investigate the impact of psychosocial factors on wait time to follow-up, but none of those factors were associated with longer wait times.
Systems-level factors
That led Ann Gakumo, PhD, chair of nursing at the College of Nursing and Health Sciences of the University of Massachusetts, Boston, to ask what other factors could account for the delay.
There were several, Dr. Wells said. Precarious housing, for example, could have influenced this lag in follow-up. About one in four participants were in transient housing, and one participant reported having been incarcerated. She gathered street addresses and plans to analyze that data to see whether the cases occurred in clusters in specific neighborhoods, as the Yale data indicated.
In addition, the anoscopy clinic was only available to receive patients one day a week and was staffed with only one clinician who was trained to perform HRA. Wait times could stretch for hours. Sometimes, participants had to leave the clinic to attend to other business, and their appointments needed to be rescheduled, Wells said.
In addition to the sometimes poor understanding of the importance of the follow-up test, Dr. Wells said, “we start to see a layering of these barriers. That’s where we start seeing breakdowns. So I’m hoping in a larger study I can address some of these barriers on a multilevel approach.”
This resonated with Dr. Gakumo.
“Oftentimes, we put so much of the responsibility for this on the part of the client and not enough on the part of the provider or on the systems level,” she said.
Guiding guidelines
Guidelines on follow-up for abnormal anal Pap test results are scarce, mostly because, unlike cervical cancer, the natural history of HPV-related anal cancers hasn’t been established. The HIV Medical Association does recommend anal Pap tests, but only in cases in which “access to appropriate referral for follow-up, including high-resolution anoscopy, is available.”
In an interview, Cecile Lahiri, MD, assistant professor of infectious disease at Emory University, said that, at Ponce De Leon Center, they recommend an anal Pap for women with HIV who have a history of cervical dysplasia.
There is a reliable association between high-grade abnormal Pap tests and cervical cancer, although low-grade changes can resolve on their own. In the case of anal cancer, especially in patients with HIV, low-grade cell changes are predictive; moreover, for such patients, anal cancer is more likely to recur and is harder to treat, Dr. Lahiri said.
“The cervical environment and the anal environment are very different,” said Dr. Lahiri, who works at the Grady Ponce De Leon Center but was not involved in Dr. Wells’ study. Dr. Lahiri is also a coinvestigator of the multisite, randomized, controlled Anal Cancer HSIL Outcomes Research (ANCHOR) study, which seeks to establish whether early treatment of high-grade anal Pap changes is better than a watch-and-wait approach.
Dr. Lahiri said that when the results of that trial become available, they are more likely to know how important early anoscopy and treatment are. The findings should inform guidelines and insurance coverage of anal Pap tests and anoscopy.
In the meantime, she said, she suspected that, with the ANCHOR trial in 2015, many sites’ capacity for anoscopy may have increased, and the wait times may have gone down.
“One of the most important pieces of the study is actually the time period in which it was conducted,” said Dr. Lahiri, who in 2015 became the clinic’s second physician trained in anoscopy. Currently, more than 200 people at the Ponce De Leon Center are enrolled in the ANCHOR trial. In addition, the general capacity for performing anoscopies has gone up nationwide as a result of the trial, which required that more providers learn how to properly perform an HRA. Many clinicians are not routinely trained in performing HRA, including gastroenterologists and surgeons, Dr. Lahiri said.
“It would be interesting to look at the differences, with the start of ANCHOR being the time point for before and after,” she said.
This article first appeared on Medscape.com.
That delay “revealed missed opportunities for a better experience on the patient, clinic, and provider level,” Jessica Wells, PhD, research assistant professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said in an interview. After all, “a lot can happen in that 1 year,” including early development of human papillomavirus (HPV)–associated anal cancer.
Although it’s too soon to say how significant that delay is with respect to the natural history of anal cancer, Dr. Wells said the data are a potential signal of disparities.
“The findings from my study may foreshadow potential disparities if we don’t have the necessary resources in place to promote follow-up care after an abnormal Pap test, similar to the disparities that we see in cervical cancer,” she said during the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
Single-center study
In the United States, people living with HIV are 19 times more likely to develop anal cancer than the general population, according to a 2018 article in the Journal of Clinical Oncology. Another single-center study from Yale University found that, in minority communities, anal cancer rates were 75% higher than in White communities. Anal cancer rates were 72% higher in communities with greater poverty. As a result, many clinics are beginning to administer Pap tests to determine early signs of HPV infection and associated changes.
In Dr. Wells’ study, which was conducted from 2012 to 2015, 150 adults with HIV who were aged 21 and older were recruited from Grady Ponce De Leon Center in Atlanta. According to a 2018 study from that center, a large minority of participants had late-stage HIV and suppressed immune systems.
All participants had been referred for HRA after a recent abnormal anal Pap test. Participants filled out questionnaires on sociodemographics, internalized HIV-related stigma, depression, risk behaviors, social support, and knowledge about HPV and anal cancer.
Participants were disproportionately older (mean age, 50.9 years); cisgender (86.7%), Black (78%); and gay, lesbian, or bisexual (84.3%). Slightly more than 1 in 10 participants (11.3%) were transgender women.
Although for 6% of participants, Pap test results indicated high-grade squamous intraepithelial lesions (HSIL), an additional 8% had atypical Pap findings that couldn’t exclude HSIL – the kinds of results that are one step away from a cancer diagnosis. More than 80% of participants had low-grade or inconclusive results. Nearly half (44%) of participants’ Pap tests revealed low-grade squamous cell intraepithelial cell lesions (LSIL); 42% indicated atypical squamous cells of undetermined significance.
When Dr. Wells looked at how long participants had waited to undergo HRA, she found something that surprised her: although some participants underwent follow-up assessment in 17 days, for many, it took much longer. The longest wait was 2,350 days – more than 6 years.
“There were quite a few patients who had follow-up beyond 1,000-plus days,” Dr. Wells said in an interview. “I didn›t think the delays were that long — at most, I would say that patients will get scheduled and come back within a few weeks or months.”
What’s more, she discovered through the HPV knowledge questionnaire that many participants did not understand why they were having a follow-up appointment. Anecdotally, some confused HPV with HIV.
“There’s education to be done to inform this target population that those living with HIV are more prone or at increased risk of this virus causing cancer later,” she said. “There are a lot of campaigns around women living with HIV, that they need to do cervical cancer screening. I think we need to really expand this campaign to include that HPV can also cause anal cancer.”
Dr. Wells had planned to primarily investigate the impact of psychosocial factors on wait time to follow-up, but none of those factors were associated with longer wait times.
Systems-level factors
That led Ann Gakumo, PhD, chair of nursing at the College of Nursing and Health Sciences of the University of Massachusetts, Boston, to ask what other factors could account for the delay.
There were several, Dr. Wells said. Precarious housing, for example, could have influenced this lag in follow-up. About one in four participants were in transient housing, and one participant reported having been incarcerated. She gathered street addresses and plans to analyze that data to see whether the cases occurred in clusters in specific neighborhoods, as the Yale data indicated.
In addition, the anoscopy clinic was only available to receive patients one day a week and was staffed with only one clinician who was trained to perform HRA. Wait times could stretch for hours. Sometimes, participants had to leave the clinic to attend to other business, and their appointments needed to be rescheduled, Wells said.
In addition to the sometimes poor understanding of the importance of the follow-up test, Dr. Wells said, “we start to see a layering of these barriers. That’s where we start seeing breakdowns. So I’m hoping in a larger study I can address some of these barriers on a multilevel approach.”
This resonated with Dr. Gakumo.
“Oftentimes, we put so much of the responsibility for this on the part of the client and not enough on the part of the provider or on the systems level,” she said.
Guiding guidelines
Guidelines on follow-up for abnormal anal Pap test results are scarce, mostly because, unlike cervical cancer, the natural history of HPV-related anal cancers hasn’t been established. The HIV Medical Association does recommend anal Pap tests, but only in cases in which “access to appropriate referral for follow-up, including high-resolution anoscopy, is available.”
In an interview, Cecile Lahiri, MD, assistant professor of infectious disease at Emory University, said that, at Ponce De Leon Center, they recommend an anal Pap for women with HIV who have a history of cervical dysplasia.
There is a reliable association between high-grade abnormal Pap tests and cervical cancer, although low-grade changes can resolve on their own. In the case of anal cancer, especially in patients with HIV, low-grade cell changes are predictive; moreover, for such patients, anal cancer is more likely to recur and is harder to treat, Dr. Lahiri said.
“The cervical environment and the anal environment are very different,” said Dr. Lahiri, who works at the Grady Ponce De Leon Center but was not involved in Dr. Wells’ study. Dr. Lahiri is also a coinvestigator of the multisite, randomized, controlled Anal Cancer HSIL Outcomes Research (ANCHOR) study, which seeks to establish whether early treatment of high-grade anal Pap changes is better than a watch-and-wait approach.
Dr. Lahiri said that when the results of that trial become available, they are more likely to know how important early anoscopy and treatment are. The findings should inform guidelines and insurance coverage of anal Pap tests and anoscopy.
In the meantime, she said, she suspected that, with the ANCHOR trial in 2015, many sites’ capacity for anoscopy may have increased, and the wait times may have gone down.
“One of the most important pieces of the study is actually the time period in which it was conducted,” said Dr. Lahiri, who in 2015 became the clinic’s second physician trained in anoscopy. Currently, more than 200 people at the Ponce De Leon Center are enrolled in the ANCHOR trial. In addition, the general capacity for performing anoscopies has gone up nationwide as a result of the trial, which required that more providers learn how to properly perform an HRA. Many clinicians are not routinely trained in performing HRA, including gastroenterologists and surgeons, Dr. Lahiri said.
“It would be interesting to look at the differences, with the start of ANCHOR being the time point for before and after,” she said.
This article first appeared on Medscape.com.
That delay “revealed missed opportunities for a better experience on the patient, clinic, and provider level,” Jessica Wells, PhD, research assistant professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said in an interview. After all, “a lot can happen in that 1 year,” including early development of human papillomavirus (HPV)–associated anal cancer.
Although it’s too soon to say how significant that delay is with respect to the natural history of anal cancer, Dr. Wells said the data are a potential signal of disparities.
“The findings from my study may foreshadow potential disparities if we don’t have the necessary resources in place to promote follow-up care after an abnormal Pap test, similar to the disparities that we see in cervical cancer,” she said during the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
Single-center study
In the United States, people living with HIV are 19 times more likely to develop anal cancer than the general population, according to a 2018 article in the Journal of Clinical Oncology. Another single-center study from Yale University found that, in minority communities, anal cancer rates were 75% higher than in White communities. Anal cancer rates were 72% higher in communities with greater poverty. As a result, many clinics are beginning to administer Pap tests to determine early signs of HPV infection and associated changes.
In Dr. Wells’ study, which was conducted from 2012 to 2015, 150 adults with HIV who were aged 21 and older were recruited from Grady Ponce De Leon Center in Atlanta. According to a 2018 study from that center, a large minority of participants had late-stage HIV and suppressed immune systems.
All participants had been referred for HRA after a recent abnormal anal Pap test. Participants filled out questionnaires on sociodemographics, internalized HIV-related stigma, depression, risk behaviors, social support, and knowledge about HPV and anal cancer.
Participants were disproportionately older (mean age, 50.9 years); cisgender (86.7%), Black (78%); and gay, lesbian, or bisexual (84.3%). Slightly more than 1 in 10 participants (11.3%) were transgender women.
Although for 6% of participants, Pap test results indicated high-grade squamous intraepithelial lesions (HSIL), an additional 8% had atypical Pap findings that couldn’t exclude HSIL – the kinds of results that are one step away from a cancer diagnosis. More than 80% of participants had low-grade or inconclusive results. Nearly half (44%) of participants’ Pap tests revealed low-grade squamous cell intraepithelial cell lesions (LSIL); 42% indicated atypical squamous cells of undetermined significance.
When Dr. Wells looked at how long participants had waited to undergo HRA, she found something that surprised her: although some participants underwent follow-up assessment in 17 days, for many, it took much longer. The longest wait was 2,350 days – more than 6 years.
“There were quite a few patients who had follow-up beyond 1,000-plus days,” Dr. Wells said in an interview. “I didn›t think the delays were that long — at most, I would say that patients will get scheduled and come back within a few weeks or months.”
What’s more, she discovered through the HPV knowledge questionnaire that many participants did not understand why they were having a follow-up appointment. Anecdotally, some confused HPV with HIV.
“There’s education to be done to inform this target population that those living with HIV are more prone or at increased risk of this virus causing cancer later,” she said. “There are a lot of campaigns around women living with HIV, that they need to do cervical cancer screening. I think we need to really expand this campaign to include that HPV can also cause anal cancer.”
Dr. Wells had planned to primarily investigate the impact of psychosocial factors on wait time to follow-up, but none of those factors were associated with longer wait times.
Systems-level factors
That led Ann Gakumo, PhD, chair of nursing at the College of Nursing and Health Sciences of the University of Massachusetts, Boston, to ask what other factors could account for the delay.
There were several, Dr. Wells said. Precarious housing, for example, could have influenced this lag in follow-up. About one in four participants were in transient housing, and one participant reported having been incarcerated. She gathered street addresses and plans to analyze that data to see whether the cases occurred in clusters in specific neighborhoods, as the Yale data indicated.
In addition, the anoscopy clinic was only available to receive patients one day a week and was staffed with only one clinician who was trained to perform HRA. Wait times could stretch for hours. Sometimes, participants had to leave the clinic to attend to other business, and their appointments needed to be rescheduled, Wells said.
In addition to the sometimes poor understanding of the importance of the follow-up test, Dr. Wells said, “we start to see a layering of these barriers. That’s where we start seeing breakdowns. So I’m hoping in a larger study I can address some of these barriers on a multilevel approach.”
This resonated with Dr. Gakumo.
“Oftentimes, we put so much of the responsibility for this on the part of the client and not enough on the part of the provider or on the systems level,” she said.
Guiding guidelines
Guidelines on follow-up for abnormal anal Pap test results are scarce, mostly because, unlike cervical cancer, the natural history of HPV-related anal cancers hasn’t been established. The HIV Medical Association does recommend anal Pap tests, but only in cases in which “access to appropriate referral for follow-up, including high-resolution anoscopy, is available.”
In an interview, Cecile Lahiri, MD, assistant professor of infectious disease at Emory University, said that, at Ponce De Leon Center, they recommend an anal Pap for women with HIV who have a history of cervical dysplasia.
There is a reliable association between high-grade abnormal Pap tests and cervical cancer, although low-grade changes can resolve on their own. In the case of anal cancer, especially in patients with HIV, low-grade cell changes are predictive; moreover, for such patients, anal cancer is more likely to recur and is harder to treat, Dr. Lahiri said.
“The cervical environment and the anal environment are very different,” said Dr. Lahiri, who works at the Grady Ponce De Leon Center but was not involved in Dr. Wells’ study. Dr. Lahiri is also a coinvestigator of the multisite, randomized, controlled Anal Cancer HSIL Outcomes Research (ANCHOR) study, which seeks to establish whether early treatment of high-grade anal Pap changes is better than a watch-and-wait approach.
Dr. Lahiri said that when the results of that trial become available, they are more likely to know how important early anoscopy and treatment are. The findings should inform guidelines and insurance coverage of anal Pap tests and anoscopy.
In the meantime, she said, she suspected that, with the ANCHOR trial in 2015, many sites’ capacity for anoscopy may have increased, and the wait times may have gone down.
“One of the most important pieces of the study is actually the time period in which it was conducted,” said Dr. Lahiri, who in 2015 became the clinic’s second physician trained in anoscopy. Currently, more than 200 people at the Ponce De Leon Center are enrolled in the ANCHOR trial. In addition, the general capacity for performing anoscopies has gone up nationwide as a result of the trial, which required that more providers learn how to properly perform an HRA. Many clinicians are not routinely trained in performing HRA, including gastroenterologists and surgeons, Dr. Lahiri said.
“It would be interesting to look at the differences, with the start of ANCHOR being the time point for before and after,” she said.
This article first appeared on Medscape.com.
What hospitalists need to know about health care reimbursement and denial prevention
Under a fee-for-service payment model, health care providers get paid by private and public payers for patient services such as physician visits, hospital stays, procedures, and tests. In an ideal world, providers would receive accurate, complete, and timely reimbursements. Unfortunately, the reality is far from ideal, where payment denials and delays are a common occurrence.
According to one study, out of $3 trillion in total claims submitted by health care organizations, an estimated 9% of charges ($262 billion), were initially denied.1 The good news is that 90% of all denials are preventable, and two-thirds of those preventable denials can be successfully appealed.2
Hospitalists are essential in preventing denials for hospital services and should be familiar with the basics of health care reimbursement and common reasons for denials. In this article we will provide an overview of the U.S. health care payment system, revenue cycle management and types of denials, and focus on the role of physician advisors and hospitalists in preventing and combating denials.
Overview of the U.S. health care payment system
In 2018 alone, the U.S. spent $3.6 trillion on health care. Of those dollars, 33% went to payments for hospital care and 20% went to physician and clinical services.3 So where do the nation’s health care dollars come from?
The United States has a complex multiple-payer system that includes private insurance companies and public payers funded by the federal and state governments, such as Medicare and Medicaid. Per the National Association of Insurance Commissioners’ 2018 Market Share Reports, there are 125 private accident and health insurance companies in the U.S., with the top five – UnitedHealth, Kaiser, Anthem, Humana, and CVS – holding a cumulative market share of almost 40%.4
Medicare accounts for 15% of federal budget spending and provides insurance coverage to almost 60 million people who are 65 and older, have end-stage renal disease, or have been approved for Social Security disability insurance benefits.5 Medicare Part A covers hospital, skilled nursing facility, home health, and hospice care. For example, for inpatient stays, Medicare Part A pays hospitals a predetermined rate per discharge according to the Medicare Severity Diagnosis Related Groups (MS-DRGs), which are based on the principal and secondary diagnoses, and performed procedures.6
Medicare Part B covers physician services and outpatient services and supplies, including labs and durable medical equipment, which are paid based on submitted Healthcare Common Procedure Coding System (HCPCS) codes.7 It is important to know that hospital observation stays are considered outpatient services, and are paid by Medicare Part B. Outpatient stays often are reimbursed at a lower rate than inpatient admissions, even in cases with similar utilization of hospital resources.
Medicaid is jointly funded by the states and the federal government and offers insurance coverage to more than 75 million eligible low-income adults, children, pregnant women, elderly adults, and people with disabilities. Over 10 million people are dually eligible for both Medicare and Medicaid.5 Increasingly, government payers, both state and federal, are contracting with private insurance companies to deliver Medicare and Medicaid services, also known as Medicare Advantage and Managed Medicaid Plans.
According to the U.S. Department of Treasury, in the 2019 fiscal year (October 2018 to September 2019), 33% of the nation’s health care dollars came from private insurance, 21% from Medicare, 16% from Medicaid, 15% from other government programs (for example, Veteran Affairs), 10% from out-of-pocket, and 4% from other private sources.5
Understanding revenue cycle management and denials
Providers, such as physicians or hospitals, submit claims to insurance companies that include, among other information, patient demographics and insurance, diagnoses, MS-DRGs and/or HCPCS codes, and charges. Revenue cycle management’s goal is to receive accurate, complete, and timely reimbursement for provided patient services, which is a complex and resource-intensive process.
According to the Healthcare Financial Management Association (HFMA), revenue cycle management includes “all administrative and clinical functions that contribute to the capture, management, and collection of patient service revenue.” These functions could be broken down into four main categories:
- Claims preparation (for example, patient registration, insurance eligibility, benefit verifications, and preauthorization).
- Claims submission (for example, charge capture, medical coding based on medical record documentation and claims transmission).
- Claims management (for example, payment posting, denial management, and patient collections).
- Reporting and analysis.
Claim denial is “the refusal of an insurance company or carrier to honor a request by an individual (or his or her provider) to pay for health care services obtained from a health care professional.”8 Payers can deny an entire claim or provide only a partial payment. Initial denial rate is tracked at the claim level (number of claims denied/number of claims submitted) and at the dollar level (total dollar amount of claims denied/total dollar amount of claims submitted).
Denials are classified as hard versus soft, and clinical versus technical or administrative:
- Hard denials result in lost revenue unless successfully appealed (for example, lack of medical necessity).
- Soft denials do not require appeal and may be paid if a provider corrects the claim or submits additional information (for example, missing or inaccurate patient information, and missing medical records).
- Clinical denials are based on medical necessity, including level of care determination (for example, inpatient versus outpatient) and length of stay. They can be concurrent and retrospective and typically start as soft denials.
- Technical or administrative denials are based on reasons other than clinical (for example, failure to preauthorize care or lack of benefits).
According to the Advisory Board’s 2017 survey of hospitals and health care systems, 50% of initial denials were technical/demographic errors, 20% medical necessity, 16% eligibility, and 14% authorization. Forty seven percent of those denials came from commercial payers, 33% from Medicare/Medicare Advantage, 17% from Medicaid, and 3% from other payers.9
Determination of medical necessity may vary by payer. As an example, let’s look at inpatient admissions. According to the Medicare Two-Midnight Rule, inpatient admission is appropriate “if the admitting practitioner expects the beneficiary to require medically necessary hospital care spanning two or more midnights, and such reasonable expectation is supported by the medical record documentation.”10
Medicare guidelines acknowledge that a physician’s decision to admit a patient is based on complex medical factors including, but not limited to:
- The beneficiary history and comorbidities, and the severity of signs and symptoms (also known as Severity of Illness or SI).
- Current medical needs (also known as Intensity of Service or IS).
- The risk of an adverse event.
Generally, private payers do not follow the Two-Midnight Rule, and instead utilize evidence-based MCG guidelines,11 InterQual® criteria12 or internal criteria to determine if an inpatient admission is “medically necessary.” Hospital utilization review nurses often use MCG and/or InterQual® to aid admission status decisions and may request secondary review by a physician if medical necessity for an inpatient admission is not clear-cut.
The role of physician advisors
Considering the rising financial pressure and growing complexity of private and public payers’ rules and regulations, many hospitals turned to physician advisors to help prevent and reduce denials. Typically, physician advisors perform concurrent secondary reviews to help determine the most appropriate level of care, participate in peer-to-peer discussions with payers, and write formal appeals to overturn clinical denials.
“Physician advisors are generally not in the business of critiquing clinical practice, instead they review whether the chart documentation supports initial and continued hospitalization,” said Charles Locke, MD, senior physician advisor at the Johns Hopkins Hospital and president of the American College of Physician Advisors (ACPA). “However, physician advisors should seek additional information and provide feedback in those cases where the documentation does not support medical necessity for hospitalization.”
Many physician advisors are current or former hospitalists. Chris Shearer, MD, chief medical officer for remote advisory at Sound Physicians Advisory Services, says that “hospitalists are the natural physician advisors as they have a working knowledge of what patients need to be inpatients and which are less sick and likely to be discharged quickly.”
The role of physician advisors extends beyond reviews to include physician engagement and education. Physician advisors are a critical link between physicians, utilization review nurses, case managers, and clinical documentation integrity (CDI) and revenue cycle teams, and are increasingly involved in hospital-wide denial prevention efforts.
Physician advisors are invaluable in identifying and validating root causes for clinical denials and generating potential solutions, as they bring to the table:
- Clinical expertise.
- Understanding of clinical workflows.
- Knowledge of the most current public and private payers’ regulations.
- Insight into hospital-specific clinical documentation opportunities (for example, by diagnosis, procedure, service line, and provider).
- Understanding of payers’ reasons for clinical denials through peer-to-peer discussions
The role of hospitalists in preventing clinical denials
I asked three experienced physician advisors – Dr. Locke, Dr. Shearer, and Deepak Pahuja, MD, chief medical officer at Aerolib Healthcare Solutions – what hospitalists can do to prevent clinical denials. The experts had the following five recommendations:
1. “THINK IN INK.”
The best tool in combating denials is well-documented clinical judgment that outlines:
- WHY the patient requires hospitalization, based on severity of presenting signs and symptoms, comorbidities, and risk of complications.
- WHAT the plan of care is, including diagnostic tests and/or interventions.
- HOW LONG you anticipate the patient will be in the hospital, including potential implications of social determinants (for example homelessness, active drug use) on discharge planning.
2. MASTER THE TWO-MIDNIGHT RULE.
If you expect that a Medicare Part A patient will require two or more midnights in the hospital, document it in the history and physical along with supporting clinical reasoning and sign an inpatient order. If the patient is discharged prior to the second midnight, document the reason in the progress notes and the discharge summary (for example, death, transfer to another hospital, departure against medical advice, faster than expected clinical improvement, or election of hospice in lieu of continued treatment in the hospital). Remember that Medicare Advantage plans may not follow the Two-Midnight rule and instead may use MCG guidelines, InterQual®, or internal criteria.
3. KNOW “SLAM DUNK” MCG CRITERIA FOR TOP DIAGNOSES.
Most large private payers utilize MCG guidelines to determine medical necessity for hospital admissions. Those guidelines are complex and change every year, and it is not required for hospitalists to know them all. However, it might help to remember a few key inpatient admission criteria for the top 5 to 10 diagnoses, such as:
- First episode of heart failure without prior history.
- Upper gastrointestinal bleeding with liver cirrhosis, syncope, or orthostatic hypotension.
- Pneumonia with documented hypoxia, outpatient treatment failure, pneumonia severity index (PSI) class 4 or 5, or CURB-65 score of 3 or greater.
- Cellulitis with outpatient treatment failure or high-risk comorbid conditions (cirrhosis, symptomatic heart failure, immunosuppression, or HbA1c greater than 10%).
4. EACH DAY, DEFEND WHY THE PATIENT NEEDS TO BE IN THE HOSPITAL.
Don’t let your progress notes be swallowed by a “copy-forward” monster and instead provide daily updates, such as:
- Up-to-date clinical status and response to interventions (for example, oxygenation or pain level).
- Updated plan of care: current interventions, additional diagnostic workup, or changes to the intensity of care (for example, increased intravenous pain medication dose or frequency).
- Why the patient cannot be safely discharged to a lower level of care (for example, a skilled nursing facility or home).
5. WORK WITH YOUR UTILIZATION REVIEW NURSES AND PHYSICIAN ADVISORS.
In the end, the two most powerful tools in combating clinical denials for hospital services are good medicine and clear documentation. Armed with an understanding of health care reimbursement and denial prevention, hospitalists can help their hospitals prevent unnecessary clinical denials and receive the reimbursements they deserve.”
Dr. Farah is a hospitalist, physician advisor, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.
References
1. LaPointe J. $262B of Total Hospital Charges in 2016 Initially Claim Denials. RevCycle Intelligence. 2017 June 26.
2. The Advisory Board. An ounce of prevention pays off: 90% of denials are preventable. 2014 Dec 11. [www.advisory.com/research/revenue-cycle-advancement-center/at-the-margins/2014/12/denials-management]
3. Centers for Medicare & Medicaid Services, Office of the Actuary, National Health Statistics Group. The Nation’s Health Dollar: Where It Came From, Where It Went. [www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf]
4. National Association of Insurance Commissioners. 2018 Market Share Reports. [www.naic.org/prod_serv/MSR-HB-19.pdf]
5. Centers for Medicare & Medicaid Services. Transforming the Healthcare System through Competition and Innovation. 2019 Nov. [www.cms.gov/files/document/cms-financial-report-fiscal-year-2019.pdf]
6. Centers for Medicare & Medicaid Services. MS-DRG Classifications and Software. 2020 Oct. [www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software]
7. Centers for Medicare & Medicaid Services. HCPCS Coding Questions. 2020 Feb. [www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/HCPCS_Coding_Questions]
8. Healthinsurance.org. Health insurance and Obamacare terms. [www.healthinsurance.org/glossary/denial-of-claim/]
9. The Advisory Board. Latest Trends in Hospital Revenue Cycle Performance. 2017. [mahamweb.org/images/meeting/112817/maham_2017__latest_trends_in_hospital_rev_cycle_performance_abc.pdf]
10. Centers for Medicare & Medicaid Services. Medicare Program Integrity Manual. Chapter 6: Medicare Contractor Medical Review Guidelines for Specific Services. 2020 July. [www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/pim83c06.pdf]
11. MCG Health. Industry-Leading Evidence-Based Care Guidelines. [www.mcg.com/care-guidelines/care-guidelines/]
12. Change Healthcare. What Is InterQual? [www.changehealthcare.com/solutions/clinical-decision-support/interqual]
Under a fee-for-service payment model, health care providers get paid by private and public payers for patient services such as physician visits, hospital stays, procedures, and tests. In an ideal world, providers would receive accurate, complete, and timely reimbursements. Unfortunately, the reality is far from ideal, where payment denials and delays are a common occurrence.
According to one study, out of $3 trillion in total claims submitted by health care organizations, an estimated 9% of charges ($262 billion), were initially denied.1 The good news is that 90% of all denials are preventable, and two-thirds of those preventable denials can be successfully appealed.2
Hospitalists are essential in preventing denials for hospital services and should be familiar with the basics of health care reimbursement and common reasons for denials. In this article we will provide an overview of the U.S. health care payment system, revenue cycle management and types of denials, and focus on the role of physician advisors and hospitalists in preventing and combating denials.
Overview of the U.S. health care payment system
In 2018 alone, the U.S. spent $3.6 trillion on health care. Of those dollars, 33% went to payments for hospital care and 20% went to physician and clinical services.3 So where do the nation’s health care dollars come from?
The United States has a complex multiple-payer system that includes private insurance companies and public payers funded by the federal and state governments, such as Medicare and Medicaid. Per the National Association of Insurance Commissioners’ 2018 Market Share Reports, there are 125 private accident and health insurance companies in the U.S., with the top five – UnitedHealth, Kaiser, Anthem, Humana, and CVS – holding a cumulative market share of almost 40%.4
Medicare accounts for 15% of federal budget spending and provides insurance coverage to almost 60 million people who are 65 and older, have end-stage renal disease, or have been approved for Social Security disability insurance benefits.5 Medicare Part A covers hospital, skilled nursing facility, home health, and hospice care. For example, for inpatient stays, Medicare Part A pays hospitals a predetermined rate per discharge according to the Medicare Severity Diagnosis Related Groups (MS-DRGs), which are based on the principal and secondary diagnoses, and performed procedures.6
Medicare Part B covers physician services and outpatient services and supplies, including labs and durable medical equipment, which are paid based on submitted Healthcare Common Procedure Coding System (HCPCS) codes.7 It is important to know that hospital observation stays are considered outpatient services, and are paid by Medicare Part B. Outpatient stays often are reimbursed at a lower rate than inpatient admissions, even in cases with similar utilization of hospital resources.
Medicaid is jointly funded by the states and the federal government and offers insurance coverage to more than 75 million eligible low-income adults, children, pregnant women, elderly adults, and people with disabilities. Over 10 million people are dually eligible for both Medicare and Medicaid.5 Increasingly, government payers, both state and federal, are contracting with private insurance companies to deliver Medicare and Medicaid services, also known as Medicare Advantage and Managed Medicaid Plans.
According to the U.S. Department of Treasury, in the 2019 fiscal year (October 2018 to September 2019), 33% of the nation’s health care dollars came from private insurance, 21% from Medicare, 16% from Medicaid, 15% from other government programs (for example, Veteran Affairs), 10% from out-of-pocket, and 4% from other private sources.5
Understanding revenue cycle management and denials
Providers, such as physicians or hospitals, submit claims to insurance companies that include, among other information, patient demographics and insurance, diagnoses, MS-DRGs and/or HCPCS codes, and charges. Revenue cycle management’s goal is to receive accurate, complete, and timely reimbursement for provided patient services, which is a complex and resource-intensive process.
According to the Healthcare Financial Management Association (HFMA), revenue cycle management includes “all administrative and clinical functions that contribute to the capture, management, and collection of patient service revenue.” These functions could be broken down into four main categories:
- Claims preparation (for example, patient registration, insurance eligibility, benefit verifications, and preauthorization).
- Claims submission (for example, charge capture, medical coding based on medical record documentation and claims transmission).
- Claims management (for example, payment posting, denial management, and patient collections).
- Reporting and analysis.
Claim denial is “the refusal of an insurance company or carrier to honor a request by an individual (or his or her provider) to pay for health care services obtained from a health care professional.”8 Payers can deny an entire claim or provide only a partial payment. Initial denial rate is tracked at the claim level (number of claims denied/number of claims submitted) and at the dollar level (total dollar amount of claims denied/total dollar amount of claims submitted).
Denials are classified as hard versus soft, and clinical versus technical or administrative:
- Hard denials result in lost revenue unless successfully appealed (for example, lack of medical necessity).
- Soft denials do not require appeal and may be paid if a provider corrects the claim or submits additional information (for example, missing or inaccurate patient information, and missing medical records).
- Clinical denials are based on medical necessity, including level of care determination (for example, inpatient versus outpatient) and length of stay. They can be concurrent and retrospective and typically start as soft denials.
- Technical or administrative denials are based on reasons other than clinical (for example, failure to preauthorize care or lack of benefits).
According to the Advisory Board’s 2017 survey of hospitals and health care systems, 50% of initial denials were technical/demographic errors, 20% medical necessity, 16% eligibility, and 14% authorization. Forty seven percent of those denials came from commercial payers, 33% from Medicare/Medicare Advantage, 17% from Medicaid, and 3% from other payers.9
Determination of medical necessity may vary by payer. As an example, let’s look at inpatient admissions. According to the Medicare Two-Midnight Rule, inpatient admission is appropriate “if the admitting practitioner expects the beneficiary to require medically necessary hospital care spanning two or more midnights, and such reasonable expectation is supported by the medical record documentation.”10
Medicare guidelines acknowledge that a physician’s decision to admit a patient is based on complex medical factors including, but not limited to:
- The beneficiary history and comorbidities, and the severity of signs and symptoms (also known as Severity of Illness or SI).
- Current medical needs (also known as Intensity of Service or IS).
- The risk of an adverse event.
Generally, private payers do not follow the Two-Midnight Rule, and instead utilize evidence-based MCG guidelines,11 InterQual® criteria12 or internal criteria to determine if an inpatient admission is “medically necessary.” Hospital utilization review nurses often use MCG and/or InterQual® to aid admission status decisions and may request secondary review by a physician if medical necessity for an inpatient admission is not clear-cut.
The role of physician advisors
Considering the rising financial pressure and growing complexity of private and public payers’ rules and regulations, many hospitals turned to physician advisors to help prevent and reduce denials. Typically, physician advisors perform concurrent secondary reviews to help determine the most appropriate level of care, participate in peer-to-peer discussions with payers, and write formal appeals to overturn clinical denials.
“Physician advisors are generally not in the business of critiquing clinical practice, instead they review whether the chart documentation supports initial and continued hospitalization,” said Charles Locke, MD, senior physician advisor at the Johns Hopkins Hospital and president of the American College of Physician Advisors (ACPA). “However, physician advisors should seek additional information and provide feedback in those cases where the documentation does not support medical necessity for hospitalization.”
Many physician advisors are current or former hospitalists. Chris Shearer, MD, chief medical officer for remote advisory at Sound Physicians Advisory Services, says that “hospitalists are the natural physician advisors as they have a working knowledge of what patients need to be inpatients and which are less sick and likely to be discharged quickly.”
The role of physician advisors extends beyond reviews to include physician engagement and education. Physician advisors are a critical link between physicians, utilization review nurses, case managers, and clinical documentation integrity (CDI) and revenue cycle teams, and are increasingly involved in hospital-wide denial prevention efforts.
Physician advisors are invaluable in identifying and validating root causes for clinical denials and generating potential solutions, as they bring to the table:
- Clinical expertise.
- Understanding of clinical workflows.
- Knowledge of the most current public and private payers’ regulations.
- Insight into hospital-specific clinical documentation opportunities (for example, by diagnosis, procedure, service line, and provider).
- Understanding of payers’ reasons for clinical denials through peer-to-peer discussions
The role of hospitalists in preventing clinical denials
I asked three experienced physician advisors – Dr. Locke, Dr. Shearer, and Deepak Pahuja, MD, chief medical officer at Aerolib Healthcare Solutions – what hospitalists can do to prevent clinical denials. The experts had the following five recommendations:
1. “THINK IN INK.”
The best tool in combating denials is well-documented clinical judgment that outlines:
- WHY the patient requires hospitalization, based on severity of presenting signs and symptoms, comorbidities, and risk of complications.
- WHAT the plan of care is, including diagnostic tests and/or interventions.
- HOW LONG you anticipate the patient will be in the hospital, including potential implications of social determinants (for example homelessness, active drug use) on discharge planning.
2. MASTER THE TWO-MIDNIGHT RULE.
If you expect that a Medicare Part A patient will require two or more midnights in the hospital, document it in the history and physical along with supporting clinical reasoning and sign an inpatient order. If the patient is discharged prior to the second midnight, document the reason in the progress notes and the discharge summary (for example, death, transfer to another hospital, departure against medical advice, faster than expected clinical improvement, or election of hospice in lieu of continued treatment in the hospital). Remember that Medicare Advantage plans may not follow the Two-Midnight rule and instead may use MCG guidelines, InterQual®, or internal criteria.
3. KNOW “SLAM DUNK” MCG CRITERIA FOR TOP DIAGNOSES.
Most large private payers utilize MCG guidelines to determine medical necessity for hospital admissions. Those guidelines are complex and change every year, and it is not required for hospitalists to know them all. However, it might help to remember a few key inpatient admission criteria for the top 5 to 10 diagnoses, such as:
- First episode of heart failure without prior history.
- Upper gastrointestinal bleeding with liver cirrhosis, syncope, or orthostatic hypotension.
- Pneumonia with documented hypoxia, outpatient treatment failure, pneumonia severity index (PSI) class 4 or 5, or CURB-65 score of 3 or greater.
- Cellulitis with outpatient treatment failure or high-risk comorbid conditions (cirrhosis, symptomatic heart failure, immunosuppression, or HbA1c greater than 10%).
4. EACH DAY, DEFEND WHY THE PATIENT NEEDS TO BE IN THE HOSPITAL.
Don’t let your progress notes be swallowed by a “copy-forward” monster and instead provide daily updates, such as:
- Up-to-date clinical status and response to interventions (for example, oxygenation or pain level).
- Updated plan of care: current interventions, additional diagnostic workup, or changes to the intensity of care (for example, increased intravenous pain medication dose or frequency).
- Why the patient cannot be safely discharged to a lower level of care (for example, a skilled nursing facility or home).
5. WORK WITH YOUR UTILIZATION REVIEW NURSES AND PHYSICIAN ADVISORS.
In the end, the two most powerful tools in combating clinical denials for hospital services are good medicine and clear documentation. Armed with an understanding of health care reimbursement and denial prevention, hospitalists can help their hospitals prevent unnecessary clinical denials and receive the reimbursements they deserve.”
Dr. Farah is a hospitalist, physician advisor, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.
References
1. LaPointe J. $262B of Total Hospital Charges in 2016 Initially Claim Denials. RevCycle Intelligence. 2017 June 26.
2. The Advisory Board. An ounce of prevention pays off: 90% of denials are preventable. 2014 Dec 11. [www.advisory.com/research/revenue-cycle-advancement-center/at-the-margins/2014/12/denials-management]
3. Centers for Medicare & Medicaid Services, Office of the Actuary, National Health Statistics Group. The Nation’s Health Dollar: Where It Came From, Where It Went. [www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf]
4. National Association of Insurance Commissioners. 2018 Market Share Reports. [www.naic.org/prod_serv/MSR-HB-19.pdf]
5. Centers for Medicare & Medicaid Services. Transforming the Healthcare System through Competition and Innovation. 2019 Nov. [www.cms.gov/files/document/cms-financial-report-fiscal-year-2019.pdf]
6. Centers for Medicare & Medicaid Services. MS-DRG Classifications and Software. 2020 Oct. [www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software]
7. Centers for Medicare & Medicaid Services. HCPCS Coding Questions. 2020 Feb. [www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/HCPCS_Coding_Questions]
8. Healthinsurance.org. Health insurance and Obamacare terms. [www.healthinsurance.org/glossary/denial-of-claim/]
9. The Advisory Board. Latest Trends in Hospital Revenue Cycle Performance. 2017. [mahamweb.org/images/meeting/112817/maham_2017__latest_trends_in_hospital_rev_cycle_performance_abc.pdf]
10. Centers for Medicare & Medicaid Services. Medicare Program Integrity Manual. Chapter 6: Medicare Contractor Medical Review Guidelines for Specific Services. 2020 July. [www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/pim83c06.pdf]
11. MCG Health. Industry-Leading Evidence-Based Care Guidelines. [www.mcg.com/care-guidelines/care-guidelines/]
12. Change Healthcare. What Is InterQual? [www.changehealthcare.com/solutions/clinical-decision-support/interqual]
Under a fee-for-service payment model, health care providers get paid by private and public payers for patient services such as physician visits, hospital stays, procedures, and tests. In an ideal world, providers would receive accurate, complete, and timely reimbursements. Unfortunately, the reality is far from ideal, where payment denials and delays are a common occurrence.
According to one study, out of $3 trillion in total claims submitted by health care organizations, an estimated 9% of charges ($262 billion), were initially denied.1 The good news is that 90% of all denials are preventable, and two-thirds of those preventable denials can be successfully appealed.2
Hospitalists are essential in preventing denials for hospital services and should be familiar with the basics of health care reimbursement and common reasons for denials. In this article we will provide an overview of the U.S. health care payment system, revenue cycle management and types of denials, and focus on the role of physician advisors and hospitalists in preventing and combating denials.
Overview of the U.S. health care payment system
In 2018 alone, the U.S. spent $3.6 trillion on health care. Of those dollars, 33% went to payments for hospital care and 20% went to physician and clinical services.3 So where do the nation’s health care dollars come from?
The United States has a complex multiple-payer system that includes private insurance companies and public payers funded by the federal and state governments, such as Medicare and Medicaid. Per the National Association of Insurance Commissioners’ 2018 Market Share Reports, there are 125 private accident and health insurance companies in the U.S., with the top five – UnitedHealth, Kaiser, Anthem, Humana, and CVS – holding a cumulative market share of almost 40%.4
Medicare accounts for 15% of federal budget spending and provides insurance coverage to almost 60 million people who are 65 and older, have end-stage renal disease, or have been approved for Social Security disability insurance benefits.5 Medicare Part A covers hospital, skilled nursing facility, home health, and hospice care. For example, for inpatient stays, Medicare Part A pays hospitals a predetermined rate per discharge according to the Medicare Severity Diagnosis Related Groups (MS-DRGs), which are based on the principal and secondary diagnoses, and performed procedures.6
Medicare Part B covers physician services and outpatient services and supplies, including labs and durable medical equipment, which are paid based on submitted Healthcare Common Procedure Coding System (HCPCS) codes.7 It is important to know that hospital observation stays are considered outpatient services, and are paid by Medicare Part B. Outpatient stays often are reimbursed at a lower rate than inpatient admissions, even in cases with similar utilization of hospital resources.
Medicaid is jointly funded by the states and the federal government and offers insurance coverage to more than 75 million eligible low-income adults, children, pregnant women, elderly adults, and people with disabilities. Over 10 million people are dually eligible for both Medicare and Medicaid.5 Increasingly, government payers, both state and federal, are contracting with private insurance companies to deliver Medicare and Medicaid services, also known as Medicare Advantage and Managed Medicaid Plans.
According to the U.S. Department of Treasury, in the 2019 fiscal year (October 2018 to September 2019), 33% of the nation’s health care dollars came from private insurance, 21% from Medicare, 16% from Medicaid, 15% from other government programs (for example, Veteran Affairs), 10% from out-of-pocket, and 4% from other private sources.5
Understanding revenue cycle management and denials
Providers, such as physicians or hospitals, submit claims to insurance companies that include, among other information, patient demographics and insurance, diagnoses, MS-DRGs and/or HCPCS codes, and charges. Revenue cycle management’s goal is to receive accurate, complete, and timely reimbursement for provided patient services, which is a complex and resource-intensive process.
According to the Healthcare Financial Management Association (HFMA), revenue cycle management includes “all administrative and clinical functions that contribute to the capture, management, and collection of patient service revenue.” These functions could be broken down into four main categories:
- Claims preparation (for example, patient registration, insurance eligibility, benefit verifications, and preauthorization).
- Claims submission (for example, charge capture, medical coding based on medical record documentation and claims transmission).
- Claims management (for example, payment posting, denial management, and patient collections).
- Reporting and analysis.
Claim denial is “the refusal of an insurance company or carrier to honor a request by an individual (or his or her provider) to pay for health care services obtained from a health care professional.”8 Payers can deny an entire claim or provide only a partial payment. Initial denial rate is tracked at the claim level (number of claims denied/number of claims submitted) and at the dollar level (total dollar amount of claims denied/total dollar amount of claims submitted).
Denials are classified as hard versus soft, and clinical versus technical or administrative:
- Hard denials result in lost revenue unless successfully appealed (for example, lack of medical necessity).
- Soft denials do not require appeal and may be paid if a provider corrects the claim or submits additional information (for example, missing or inaccurate patient information, and missing medical records).
- Clinical denials are based on medical necessity, including level of care determination (for example, inpatient versus outpatient) and length of stay. They can be concurrent and retrospective and typically start as soft denials.
- Technical or administrative denials are based on reasons other than clinical (for example, failure to preauthorize care or lack of benefits).
According to the Advisory Board’s 2017 survey of hospitals and health care systems, 50% of initial denials were technical/demographic errors, 20% medical necessity, 16% eligibility, and 14% authorization. Forty seven percent of those denials came from commercial payers, 33% from Medicare/Medicare Advantage, 17% from Medicaid, and 3% from other payers.9
Determination of medical necessity may vary by payer. As an example, let’s look at inpatient admissions. According to the Medicare Two-Midnight Rule, inpatient admission is appropriate “if the admitting practitioner expects the beneficiary to require medically necessary hospital care spanning two or more midnights, and such reasonable expectation is supported by the medical record documentation.”10
Medicare guidelines acknowledge that a physician’s decision to admit a patient is based on complex medical factors including, but not limited to:
- The beneficiary history and comorbidities, and the severity of signs and symptoms (also known as Severity of Illness or SI).
- Current medical needs (also known as Intensity of Service or IS).
- The risk of an adverse event.
Generally, private payers do not follow the Two-Midnight Rule, and instead utilize evidence-based MCG guidelines,11 InterQual® criteria12 or internal criteria to determine if an inpatient admission is “medically necessary.” Hospital utilization review nurses often use MCG and/or InterQual® to aid admission status decisions and may request secondary review by a physician if medical necessity for an inpatient admission is not clear-cut.
The role of physician advisors
Considering the rising financial pressure and growing complexity of private and public payers’ rules and regulations, many hospitals turned to physician advisors to help prevent and reduce denials. Typically, physician advisors perform concurrent secondary reviews to help determine the most appropriate level of care, participate in peer-to-peer discussions with payers, and write formal appeals to overturn clinical denials.
“Physician advisors are generally not in the business of critiquing clinical practice, instead they review whether the chart documentation supports initial and continued hospitalization,” said Charles Locke, MD, senior physician advisor at the Johns Hopkins Hospital and president of the American College of Physician Advisors (ACPA). “However, physician advisors should seek additional information and provide feedback in those cases where the documentation does not support medical necessity for hospitalization.”
Many physician advisors are current or former hospitalists. Chris Shearer, MD, chief medical officer for remote advisory at Sound Physicians Advisory Services, says that “hospitalists are the natural physician advisors as they have a working knowledge of what patients need to be inpatients and which are less sick and likely to be discharged quickly.”
The role of physician advisors extends beyond reviews to include physician engagement and education. Physician advisors are a critical link between physicians, utilization review nurses, case managers, and clinical documentation integrity (CDI) and revenue cycle teams, and are increasingly involved in hospital-wide denial prevention efforts.
Physician advisors are invaluable in identifying and validating root causes for clinical denials and generating potential solutions, as they bring to the table:
- Clinical expertise.
- Understanding of clinical workflows.
- Knowledge of the most current public and private payers’ regulations.
- Insight into hospital-specific clinical documentation opportunities (for example, by diagnosis, procedure, service line, and provider).
- Understanding of payers’ reasons for clinical denials through peer-to-peer discussions
The role of hospitalists in preventing clinical denials
I asked three experienced physician advisors – Dr. Locke, Dr. Shearer, and Deepak Pahuja, MD, chief medical officer at Aerolib Healthcare Solutions – what hospitalists can do to prevent clinical denials. The experts had the following five recommendations:
1. “THINK IN INK.”
The best tool in combating denials is well-documented clinical judgment that outlines:
- WHY the patient requires hospitalization, based on severity of presenting signs and symptoms, comorbidities, and risk of complications.
- WHAT the plan of care is, including diagnostic tests and/or interventions.
- HOW LONG you anticipate the patient will be in the hospital, including potential implications of social determinants (for example homelessness, active drug use) on discharge planning.
2. MASTER THE TWO-MIDNIGHT RULE.
If you expect that a Medicare Part A patient will require two or more midnights in the hospital, document it in the history and physical along with supporting clinical reasoning and sign an inpatient order. If the patient is discharged prior to the second midnight, document the reason in the progress notes and the discharge summary (for example, death, transfer to another hospital, departure against medical advice, faster than expected clinical improvement, or election of hospice in lieu of continued treatment in the hospital). Remember that Medicare Advantage plans may not follow the Two-Midnight rule and instead may use MCG guidelines, InterQual®, or internal criteria.
3. KNOW “SLAM DUNK” MCG CRITERIA FOR TOP DIAGNOSES.
Most large private payers utilize MCG guidelines to determine medical necessity for hospital admissions. Those guidelines are complex and change every year, and it is not required for hospitalists to know them all. However, it might help to remember a few key inpatient admission criteria for the top 5 to 10 diagnoses, such as:
- First episode of heart failure without prior history.
- Upper gastrointestinal bleeding with liver cirrhosis, syncope, or orthostatic hypotension.
- Pneumonia with documented hypoxia, outpatient treatment failure, pneumonia severity index (PSI) class 4 or 5, or CURB-65 score of 3 or greater.
- Cellulitis with outpatient treatment failure or high-risk comorbid conditions (cirrhosis, symptomatic heart failure, immunosuppression, or HbA1c greater than 10%).
4. EACH DAY, DEFEND WHY THE PATIENT NEEDS TO BE IN THE HOSPITAL.
Don’t let your progress notes be swallowed by a “copy-forward” monster and instead provide daily updates, such as:
- Up-to-date clinical status and response to interventions (for example, oxygenation or pain level).
- Updated plan of care: current interventions, additional diagnostic workup, or changes to the intensity of care (for example, increased intravenous pain medication dose or frequency).
- Why the patient cannot be safely discharged to a lower level of care (for example, a skilled nursing facility or home).
5. WORK WITH YOUR UTILIZATION REVIEW NURSES AND PHYSICIAN ADVISORS.
In the end, the two most powerful tools in combating clinical denials for hospital services are good medicine and clear documentation. Armed with an understanding of health care reimbursement and denial prevention, hospitalists can help their hospitals prevent unnecessary clinical denials and receive the reimbursements they deserve.”
Dr. Farah is a hospitalist, physician advisor, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.
References
1. LaPointe J. $262B of Total Hospital Charges in 2016 Initially Claim Denials. RevCycle Intelligence. 2017 June 26.
2. The Advisory Board. An ounce of prevention pays off: 90% of denials are preventable. 2014 Dec 11. [www.advisory.com/research/revenue-cycle-advancement-center/at-the-margins/2014/12/denials-management]
3. Centers for Medicare & Medicaid Services, Office of the Actuary, National Health Statistics Group. The Nation’s Health Dollar: Where It Came From, Where It Went. [www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf]
4. National Association of Insurance Commissioners. 2018 Market Share Reports. [www.naic.org/prod_serv/MSR-HB-19.pdf]
5. Centers for Medicare & Medicaid Services. Transforming the Healthcare System through Competition and Innovation. 2019 Nov. [www.cms.gov/files/document/cms-financial-report-fiscal-year-2019.pdf]
6. Centers for Medicare & Medicaid Services. MS-DRG Classifications and Software. 2020 Oct. [www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software]
7. Centers for Medicare & Medicaid Services. HCPCS Coding Questions. 2020 Feb. [www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/HCPCS_Coding_Questions]
8. Healthinsurance.org. Health insurance and Obamacare terms. [www.healthinsurance.org/glossary/denial-of-claim/]
9. The Advisory Board. Latest Trends in Hospital Revenue Cycle Performance. 2017. [mahamweb.org/images/meeting/112817/maham_2017__latest_trends_in_hospital_rev_cycle_performance_abc.pdf]
10. Centers for Medicare & Medicaid Services. Medicare Program Integrity Manual. Chapter 6: Medicare Contractor Medical Review Guidelines for Specific Services. 2020 July. [www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/pim83c06.pdf]
11. MCG Health. Industry-Leading Evidence-Based Care Guidelines. [www.mcg.com/care-guidelines/care-guidelines/]
12. Change Healthcare. What Is InterQual? [www.changehealthcare.com/solutions/clinical-decision-support/interqual]
VTEs tied to immune checkpoint inhibitor cancer treatment
Cancer patients who receive an immune checkpoint inhibitor have more than a doubled rate of venous thromboembolism during the subsequent 2 years, compared with their rate during the 2 years before treatment, according to a retrospective analysis of more than 2,800 patients treated at a single U.S. center.
The study focused on cancer patients treated with an immune checkpoint inhibitor (ICI) at Massachusetts General Hospital in Boston. It showed that during the 2 years prior to treatment with any type of ICI, the incidence of venous thromboembolic events (VTE) was 4.85/100 patient-years that then jumped to 11.75/100 patient-years during the 2 years following treatment. This translated into an incidence rate ratio of 2.43 during posttreatment follow-up, compared with pretreatment, Jingyi Gong, MD, said at the virtual American Heart Association scientific sessions.
The increased VTE rate resulted from rises in both the rate of deep vein thrombosis, which had an IRR of 3.23 during the posttreatment period, and for pulmonary embolism, which showed an IRR of 2.24, said Dr. Gong, a physician at Brigham and Women’s Hospital in Boston. She hypothesized that this effect may result from a procoagulant effect of the immune activation and inflammation triggered by ICIs.
Hypothesis-generating results
Cardiologists cautioned that these findings should only be considered hypothesis generating, but raise an important alert for clinicians to have heightened awareness of the potential for VTE following ICI treatment.
“A clear message is to be aware that there is this signal, and be vigilant for patients who might present with VTE following ICI treatment,” commented Richard J. Kovacs, MD, a cardiologist and professor at Indiana University, Indianapolis. The data that Dr. Gong reported are “moderately convincing,” he added in an interview.
“Awareness that patients who receive ICI may be at increased VTE risk is very important,” agreed Umberto Campia, MD, a cardiologist, vascular specialist, and member of the cardio-oncology group at Brigham and Women’s Hospital, who was not involved in the new study.
The potential impact of ICI treatment on VTE risk is slowly emerging, added Dr. Campia. Until recently, the literature primarily was case reports, but recently another retrospective, single-center study came out that reported a 13% incidence of VTE in cancer patients following ICI treatment. On the other hand, a recently published meta-analysis of more than 20,000 patients from 68 ICI studies failed to find a suggestion of increased VTE incidence following ICI interventions.
Attempting to assess the impact of treatment on VTE risk in cancer patients is challenging because cancer itself boosts the risk. Recommendations on the use of VTE prophylaxis in cancer patients most recently came out in 2014 from the American Society of Clinical Oncology, which said that VTE prophylaxis for ambulatory cancer patients “may be considered for highly select high-risk patients.” The impact of cancer therapy on VTE risk and the need for prophylaxis is usually assessed by applying the Khorana score, Dr. Campia said in an interview.
VTE spikes acutely after ICI treatment
Dr. Gong analyzed VTE incidence rates by time during the total 4-year period studied, and found that the rate gradually and steadily rose with time throughout the 2 years preceding treatment, spiked immediately following ICI treatment, and then gradually and steadily fell back to roughly the rate seen just before treatment, reaching that level about a year after treatment. She ran a sensitivity analysis that excluded patients who died during the first year following their ICI treatment, and in this calculation an acute spike in VTE following ICI treatment still occurred but with reduced magnitude.
She also reported the results of several subgroup analyses. The IRRs remained consistent among women and men, among patients who were aged over or under 65 years, and regardless of cancer type or treatment with corticosteroids. But the subgroup analyses identified two parameters that seemed to clearly split VTE rates.
Among patients on treatment with an anticoagulant agent at the time of their ICI treatment, roughly 10% of the patients, the IRR was 0.56, compared with a ratio of 3.86 among the other patients, suggesting possible protection. A second factor that seemed linked with VTE incidence was the number of ICI treatment cycles a patient received. Those who received more than five cycles had a risk ratio of 3.95, while those who received five or fewer cycles had a RR of 1.66.
Her analysis included 2,842 cancer patients who received treatment with an ICI at Massachusetts General Hospital. Patients averaged 64 years of age, slightly more than half were men, and 13% had a prior history of VTE. Patients received an average of 5 ICI treatment cycles, but a quarter of the patients received more than 10 cycles.
During the 2-year follow-up, 244 patients (9%) developed VTE. The patients who developed VTE were significantly younger than those who did not, with an average age of 63 years, compared with 65. And the patients who eventually developed VTE had a significantly higher prevalence of prior VTE at 18%, compared with 12% among the patients who stayed VTE free.
The cancer types patients had were non–small cell lung, 29%; melanoma, 28%; head and neck, 12%; renal genitourinary, 6%; and other, 25%. ICIs have been available for routine U.S. practice since 2011. The class includes agents such as pembrolizumab (Keytruda) and durvalumab (Imfinzi).
Researchers would need to perform a prospective, randomized study to determine whether anticoagulant prophylaxis is clearly beneficial for patients receiving ICI treatment, Dr. Gong said. But both Dr. Kovacs and Dr. Campia said that more data on this topic are first needed.
“We need to confirm that treatment with ICI is associated with VTEs. Retrospective data are not definitive,” said Dr. Campia. “We would need to prospectively assess the impact of ICI,” which will not be easy, as it’s quickly become a cornerstone for treating many cancers. “We need to become more familiar with the adverse effects of these drugs. We are still learning about their toxicities.”
The study had no commercial funding. Dr. Gong, Dr. Kovacs, and Dr. Campia had no disclosures.
Cancer patients who receive an immune checkpoint inhibitor have more than a doubled rate of venous thromboembolism during the subsequent 2 years, compared with their rate during the 2 years before treatment, according to a retrospective analysis of more than 2,800 patients treated at a single U.S. center.
The study focused on cancer patients treated with an immune checkpoint inhibitor (ICI) at Massachusetts General Hospital in Boston. It showed that during the 2 years prior to treatment with any type of ICI, the incidence of venous thromboembolic events (VTE) was 4.85/100 patient-years that then jumped to 11.75/100 patient-years during the 2 years following treatment. This translated into an incidence rate ratio of 2.43 during posttreatment follow-up, compared with pretreatment, Jingyi Gong, MD, said at the virtual American Heart Association scientific sessions.
The increased VTE rate resulted from rises in both the rate of deep vein thrombosis, which had an IRR of 3.23 during the posttreatment period, and for pulmonary embolism, which showed an IRR of 2.24, said Dr. Gong, a physician at Brigham and Women’s Hospital in Boston. She hypothesized that this effect may result from a procoagulant effect of the immune activation and inflammation triggered by ICIs.
Hypothesis-generating results
Cardiologists cautioned that these findings should only be considered hypothesis generating, but raise an important alert for clinicians to have heightened awareness of the potential for VTE following ICI treatment.
“A clear message is to be aware that there is this signal, and be vigilant for patients who might present with VTE following ICI treatment,” commented Richard J. Kovacs, MD, a cardiologist and professor at Indiana University, Indianapolis. The data that Dr. Gong reported are “moderately convincing,” he added in an interview.
“Awareness that patients who receive ICI may be at increased VTE risk is very important,” agreed Umberto Campia, MD, a cardiologist, vascular specialist, and member of the cardio-oncology group at Brigham and Women’s Hospital, who was not involved in the new study.
The potential impact of ICI treatment on VTE risk is slowly emerging, added Dr. Campia. Until recently, the literature primarily was case reports, but recently another retrospective, single-center study came out that reported a 13% incidence of VTE in cancer patients following ICI treatment. On the other hand, a recently published meta-analysis of more than 20,000 patients from 68 ICI studies failed to find a suggestion of increased VTE incidence following ICI interventions.
Attempting to assess the impact of treatment on VTE risk in cancer patients is challenging because cancer itself boosts the risk. Recommendations on the use of VTE prophylaxis in cancer patients most recently came out in 2014 from the American Society of Clinical Oncology, which said that VTE prophylaxis for ambulatory cancer patients “may be considered for highly select high-risk patients.” The impact of cancer therapy on VTE risk and the need for prophylaxis is usually assessed by applying the Khorana score, Dr. Campia said in an interview.
VTE spikes acutely after ICI treatment
Dr. Gong analyzed VTE incidence rates by time during the total 4-year period studied, and found that the rate gradually and steadily rose with time throughout the 2 years preceding treatment, spiked immediately following ICI treatment, and then gradually and steadily fell back to roughly the rate seen just before treatment, reaching that level about a year after treatment. She ran a sensitivity analysis that excluded patients who died during the first year following their ICI treatment, and in this calculation an acute spike in VTE following ICI treatment still occurred but with reduced magnitude.
She also reported the results of several subgroup analyses. The IRRs remained consistent among women and men, among patients who were aged over or under 65 years, and regardless of cancer type or treatment with corticosteroids. But the subgroup analyses identified two parameters that seemed to clearly split VTE rates.
Among patients on treatment with an anticoagulant agent at the time of their ICI treatment, roughly 10% of the patients, the IRR was 0.56, compared with a ratio of 3.86 among the other patients, suggesting possible protection. A second factor that seemed linked with VTE incidence was the number of ICI treatment cycles a patient received. Those who received more than five cycles had a risk ratio of 3.95, while those who received five or fewer cycles had a RR of 1.66.
Her analysis included 2,842 cancer patients who received treatment with an ICI at Massachusetts General Hospital. Patients averaged 64 years of age, slightly more than half were men, and 13% had a prior history of VTE. Patients received an average of 5 ICI treatment cycles, but a quarter of the patients received more than 10 cycles.
During the 2-year follow-up, 244 patients (9%) developed VTE. The patients who developed VTE were significantly younger than those who did not, with an average age of 63 years, compared with 65. And the patients who eventually developed VTE had a significantly higher prevalence of prior VTE at 18%, compared with 12% among the patients who stayed VTE free.
The cancer types patients had were non–small cell lung, 29%; melanoma, 28%; head and neck, 12%; renal genitourinary, 6%; and other, 25%. ICIs have been available for routine U.S. practice since 2011. The class includes agents such as pembrolizumab (Keytruda) and durvalumab (Imfinzi).
Researchers would need to perform a prospective, randomized study to determine whether anticoagulant prophylaxis is clearly beneficial for patients receiving ICI treatment, Dr. Gong said. But both Dr. Kovacs and Dr. Campia said that more data on this topic are first needed.
“We need to confirm that treatment with ICI is associated with VTEs. Retrospective data are not definitive,” said Dr. Campia. “We would need to prospectively assess the impact of ICI,” which will not be easy, as it’s quickly become a cornerstone for treating many cancers. “We need to become more familiar with the adverse effects of these drugs. We are still learning about their toxicities.”
The study had no commercial funding. Dr. Gong, Dr. Kovacs, and Dr. Campia had no disclosures.
Cancer patients who receive an immune checkpoint inhibitor have more than a doubled rate of venous thromboembolism during the subsequent 2 years, compared with their rate during the 2 years before treatment, according to a retrospective analysis of more than 2,800 patients treated at a single U.S. center.
The study focused on cancer patients treated with an immune checkpoint inhibitor (ICI) at Massachusetts General Hospital in Boston. It showed that during the 2 years prior to treatment with any type of ICI, the incidence of venous thromboembolic events (VTE) was 4.85/100 patient-years that then jumped to 11.75/100 patient-years during the 2 years following treatment. This translated into an incidence rate ratio of 2.43 during posttreatment follow-up, compared with pretreatment, Jingyi Gong, MD, said at the virtual American Heart Association scientific sessions.
The increased VTE rate resulted from rises in both the rate of deep vein thrombosis, which had an IRR of 3.23 during the posttreatment period, and for pulmonary embolism, which showed an IRR of 2.24, said Dr. Gong, a physician at Brigham and Women’s Hospital in Boston. She hypothesized that this effect may result from a procoagulant effect of the immune activation and inflammation triggered by ICIs.
Hypothesis-generating results
Cardiologists cautioned that these findings should only be considered hypothesis generating, but raise an important alert for clinicians to have heightened awareness of the potential for VTE following ICI treatment.
“A clear message is to be aware that there is this signal, and be vigilant for patients who might present with VTE following ICI treatment,” commented Richard J. Kovacs, MD, a cardiologist and professor at Indiana University, Indianapolis. The data that Dr. Gong reported are “moderately convincing,” he added in an interview.
“Awareness that patients who receive ICI may be at increased VTE risk is very important,” agreed Umberto Campia, MD, a cardiologist, vascular specialist, and member of the cardio-oncology group at Brigham and Women’s Hospital, who was not involved in the new study.
The potential impact of ICI treatment on VTE risk is slowly emerging, added Dr. Campia. Until recently, the literature primarily was case reports, but recently another retrospective, single-center study came out that reported a 13% incidence of VTE in cancer patients following ICI treatment. On the other hand, a recently published meta-analysis of more than 20,000 patients from 68 ICI studies failed to find a suggestion of increased VTE incidence following ICI interventions.
Attempting to assess the impact of treatment on VTE risk in cancer patients is challenging because cancer itself boosts the risk. Recommendations on the use of VTE prophylaxis in cancer patients most recently came out in 2014 from the American Society of Clinical Oncology, which said that VTE prophylaxis for ambulatory cancer patients “may be considered for highly select high-risk patients.” The impact of cancer therapy on VTE risk and the need for prophylaxis is usually assessed by applying the Khorana score, Dr. Campia said in an interview.
VTE spikes acutely after ICI treatment
Dr. Gong analyzed VTE incidence rates by time during the total 4-year period studied, and found that the rate gradually and steadily rose with time throughout the 2 years preceding treatment, spiked immediately following ICI treatment, and then gradually and steadily fell back to roughly the rate seen just before treatment, reaching that level about a year after treatment. She ran a sensitivity analysis that excluded patients who died during the first year following their ICI treatment, and in this calculation an acute spike in VTE following ICI treatment still occurred but with reduced magnitude.
She also reported the results of several subgroup analyses. The IRRs remained consistent among women and men, among patients who were aged over or under 65 years, and regardless of cancer type or treatment with corticosteroids. But the subgroup analyses identified two parameters that seemed to clearly split VTE rates.
Among patients on treatment with an anticoagulant agent at the time of their ICI treatment, roughly 10% of the patients, the IRR was 0.56, compared with a ratio of 3.86 among the other patients, suggesting possible protection. A second factor that seemed linked with VTE incidence was the number of ICI treatment cycles a patient received. Those who received more than five cycles had a risk ratio of 3.95, while those who received five or fewer cycles had a RR of 1.66.
Her analysis included 2,842 cancer patients who received treatment with an ICI at Massachusetts General Hospital. Patients averaged 64 years of age, slightly more than half were men, and 13% had a prior history of VTE. Patients received an average of 5 ICI treatment cycles, but a quarter of the patients received more than 10 cycles.
During the 2-year follow-up, 244 patients (9%) developed VTE. The patients who developed VTE were significantly younger than those who did not, with an average age of 63 years, compared with 65. And the patients who eventually developed VTE had a significantly higher prevalence of prior VTE at 18%, compared with 12% among the patients who stayed VTE free.
The cancer types patients had were non–small cell lung, 29%; melanoma, 28%; head and neck, 12%; renal genitourinary, 6%; and other, 25%. ICIs have been available for routine U.S. practice since 2011. The class includes agents such as pembrolizumab (Keytruda) and durvalumab (Imfinzi).
Researchers would need to perform a prospective, randomized study to determine whether anticoagulant prophylaxis is clearly beneficial for patients receiving ICI treatment, Dr. Gong said. But both Dr. Kovacs and Dr. Campia said that more data on this topic are first needed.
“We need to confirm that treatment with ICI is associated with VTEs. Retrospective data are not definitive,” said Dr. Campia. “We would need to prospectively assess the impact of ICI,” which will not be easy, as it’s quickly become a cornerstone for treating many cancers. “We need to become more familiar with the adverse effects of these drugs. We are still learning about their toxicities.”
The study had no commercial funding. Dr. Gong, Dr. Kovacs, and Dr. Campia had no disclosures.
FROM AHA 2020
Oral Hairy Leukoplakia Associated With the Use of Adalimumab
To the Editor:
Oral hairy leukoplakia (OHL) is an Epstein-Barr virus (EBV)–mediated mucocutaneous disease that often involves the lingual epithelium. The lateral portions of the tongue are the most commonly affected sites. The lesions often are described as asymptomatic, white, corrugated patches or plaques that are unable to be scraped off.1 Oral hairy leukoplakia was first identified in 1984 and was considered to be associated with AIDS.2 An association between the presence of OHL and the degree of immunosuppression as well as the severity of human immunodeficiency virus (HIV) has been reported.3 Although OHL initially was considered to be pathognomonic for HIV, it has since been described in multiple other immunosuppressive conditions.4 Numerous medical conditions and combinations of immunosuppressive medications have been associated with OHL in patients who were HIV negative.5
Adalimumab is an injectable human IgG1 recombinant antibody to tumor necrosis factor α (TNF-α).6 It currently is approved by the US Food and Drug Administration for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn disease, ulcerative colitis, noninfectious uveitis, hidradenitis suppurativa, and plaque psoriasis.7 We report a case of OHL associated with the use of adalimumab.
A 47-year-old woman initially presented with chronic plaque-type psoriasis. Her medical history was notable for bipolar disorder, migraines, hypertension, and tobacco use. The patient’s psoriasis initially was well controlled on a regimen of topical steroids and methotrexate; however, methotrexate was stopped after 2.5 years due to a mildly elevated alanine aminotransferase level, as well as an abnormal liver biopsy showing mildly active (grade 1 of 3) steatohepatitis with portal chronic inflammation, pericellular fibrosis, and portal and focal periportal fibrosis (stage 1-2 of 4). The patient and her dermatologist were uncomfortable continuing methotrexate with these findings. After baseline screening including a negative purified protein derivative skin test, adalimumab was initiated. A loading dose of 80 mg subcutaneously (SQ) was given, followed by adalimumab 40 mg SQ 1 week later and 40 mg every other week as maintenance.
The patient’s psoriasis was well controlled with adalimumab for 22 months, but she then developed a thin white plaque on the right lateral tongue (Figure 1). An incisional biopsy of the tongue performed by an oral surgeon revealed hyperkeratosis with Candida colonization and viral cytopathic effect (Figure 2). An EBV DNA in situ hybridization stain revealed focal positivity within these cells (Figure 3), leading to a diagnosis of OHL. Laboratory evaluation demonstrated a normal complete blood cell count with differential and liver panel as well as a negative HIV test. The patient otherwise felt well and denied fevers, lymphadenopathy, and weight loss.
We consulted with an infectious disease and immunodeficiency specialist regarding the patient’s case. Before conducting further evaluation beyond HIV screening for immunodeficiency states, adalimumab was discontinued to see if the OHL would spontaneously resolve. Three months after discontinuation of adalimumab, the white plaque on the right lateral tongue was notably improved. The OHL continued to disappear and was completely resolved 1 year after discontinuation of adalimumab. The patient’s psoriasis had subsequently remained well controlled with diet and weight loss, smoking cessation, topical steroids, and apremilast without any recurrence of the OHL.
Oral hairy leukoplakia is associated with upregulated EBV replication and EBV-encoded proteins such as latent membrane protein 1.2 It often presents as white or gray patches on the lateral lingual margins with prominent folds and/or projections, giving a shaggy appearance. Oral hairy leukoplakia often is specific for HIV infection and rarely is associated with other immunodeficiencies.2 Prasad and Bilodeau5 performed a literature review of medical conditions and immunosuppressive medications associated with OHL in patients without HIV. Allogeneic transplant was associated with the highest incidence of OHL in HIV-negative patients (59.2% [45/76]).5 Various combinations of immunosuppressive medications (eg, prednisone, cyclosporine, azathioprine) also may be implicated in cases of HIV-negative patients with OHL. A case of OHL also has been reported with long-standing use of inhaled corticosteroids in an immunocompetent, HIV-negative patient.6 Another case was reported with long-term use of the aromatic antiepileptic lamotrigine, which resolved once stopping the medication.8 Although EBV is an oncovirus and has been associated with lymphoproliferative disorders and nasopharyngeal carcinoma, OHL is not considered to be a premalignant lesion.7 Despite the strong association between OHL and HIV, our patient was HIV negative. The only immunocompromising factor in our patient was the use of adalimumab to treat psoriasis. We did not conduct further testing for immunodeficiency states because the OHL spontaneously resolved when the adalimumab was discontinued.
PubMed and Ovid searches of articles indexed for MEDLINE using the terms adalimumab and oral hairy leukoplakia as well as TNF-alpha inhibitor and oral hairy leukoplakia with humans and English language as limitations revealed that no cases have been reported in the literature demonstrating an association between OHL and adalimumab or any other TNF-α inhibitor. However, Cetkovska et al9 reported a case of EBV hepatitis and subsequently chronic hepatitis as a complication of infliximab used for the treatment of chronic psoriasis. Because TNF-α and IFN-γ play an important role in controlling viral infections, there is an increased risk for reactivating a viral illness when depleting TNF through pharmacologic measures (ie, adalimumab, infliximab).8 Another case of EBV-associated plasmablastic lymphoma was reported after 1 year of adalimumab use in a patient with Crohn disease. The plasmablastic lymphoma resolved after 4 rounds of chemotherapy.10
The only contraindication for the use of adalimumab is a known hypersensitivity to the drug. Relative contraindications for use of adalimumab include active tuberculosis, demyelinating disease, hematologic diseases (ie, thrombocytopenia, pancytopenia), lymphoma, hepatitis C, and hepatitis B.11 The most common adverse effect of adalimumab is an injection-site reaction. Additional reported adverse effects of TNF-α inhibitors as a class are lymphoma, melanoma, nonmelanoma skin cancer, reactivation of latent tuberculosis, congestive heart failure, autoimmunity, and hematologic toxicity.11
This case demonstrates an association between adalimumab and OHL in an HIV-negative patient. Although the mechanism behind OHL and immunosuppression remains to be elucidated, this association is important to keep in mind when using adalimumab or other TNF-α inhibitors for the treatment of psoriasis or other medical conditions.
- Triantos D, Porter SR, Scully C, et al. Oral hairy leukoplakia: clinicopathologic features, pathogenesis, diagnosis, and clinical significance. Clin Infect Dis. 1997;25:1392-1396.
- Greenspan D, Greenspan JS, Conant M, et al. Oral “hairy” leucoplakia in male homosexuals: evidence of association with both papillomavirus and a herpes-group virus. Lancet. 1984;2:831-834.
- Glick M, Muzyka BC, Lurie D, et al. Oral manifestations associated with HIV-related disease as marks for immune suppression and AIDS. Oral Surg Oral Med Oral Pathol. 1994;77:344-349.
- Chambers AE, Conn B, Pemberton M, et al. Twenty-first-century oral hair leukoplakia—a non-HIV-associated entity. Oral Surg Oral Med Oral Patho Oral Radiol. 2015;119:326-332.
- Prasad JL, Bilodeau EA. Oral hairy leukoplakia in patients without HIV: presentation of 2 new cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:E151-E160.
- Moffat M, Jauhar S, Jones ME, et al. Oral hairy leukoplakia in an HIV-negative, immunocompetent patient. Oral Biosci Med. 2005;2:282-284.
- Greenspan JS, Greenspan D. Oral hairy leukoplakia: diagnosis and management. Oral Surg Oral Med Oral Pathol. 1989;67:396-403.
- Gordins P, Sloan P, Spickett GP, et al. Oral hairy leukoplakia in a patient on long-term anticonvulsant treatment with lamotrigine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:E17-E23.
- Cetkovska P, Lomicova I, Mukensnabl P, et al. Anti-tumour necrosis factor treatment of severe psoriasis complicated by Epstein-Barr virus hepatitis and subsequently by chronic hepatitis. Dermatol Ther. 2015;28:369-372.
- Liu L, Charabaty A, Ozdemirli M. EBV-associated plasmablastic lymphoma in a patient with Crohn’s disease after adalimumab treatment. J Crohns Colitis. 2013;7:E118-E119.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2018.
To the Editor:
Oral hairy leukoplakia (OHL) is an Epstein-Barr virus (EBV)–mediated mucocutaneous disease that often involves the lingual epithelium. The lateral portions of the tongue are the most commonly affected sites. The lesions often are described as asymptomatic, white, corrugated patches or plaques that are unable to be scraped off.1 Oral hairy leukoplakia was first identified in 1984 and was considered to be associated with AIDS.2 An association between the presence of OHL and the degree of immunosuppression as well as the severity of human immunodeficiency virus (HIV) has been reported.3 Although OHL initially was considered to be pathognomonic for HIV, it has since been described in multiple other immunosuppressive conditions.4 Numerous medical conditions and combinations of immunosuppressive medications have been associated with OHL in patients who were HIV negative.5
Adalimumab is an injectable human IgG1 recombinant antibody to tumor necrosis factor α (TNF-α).6 It currently is approved by the US Food and Drug Administration for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn disease, ulcerative colitis, noninfectious uveitis, hidradenitis suppurativa, and plaque psoriasis.7 We report a case of OHL associated with the use of adalimumab.
A 47-year-old woman initially presented with chronic plaque-type psoriasis. Her medical history was notable for bipolar disorder, migraines, hypertension, and tobacco use. The patient’s psoriasis initially was well controlled on a regimen of topical steroids and methotrexate; however, methotrexate was stopped after 2.5 years due to a mildly elevated alanine aminotransferase level, as well as an abnormal liver biopsy showing mildly active (grade 1 of 3) steatohepatitis with portal chronic inflammation, pericellular fibrosis, and portal and focal periportal fibrosis (stage 1-2 of 4). The patient and her dermatologist were uncomfortable continuing methotrexate with these findings. After baseline screening including a negative purified protein derivative skin test, adalimumab was initiated. A loading dose of 80 mg subcutaneously (SQ) was given, followed by adalimumab 40 mg SQ 1 week later and 40 mg every other week as maintenance.
The patient’s psoriasis was well controlled with adalimumab for 22 months, but she then developed a thin white plaque on the right lateral tongue (Figure 1). An incisional biopsy of the tongue performed by an oral surgeon revealed hyperkeratosis with Candida colonization and viral cytopathic effect (Figure 2). An EBV DNA in situ hybridization stain revealed focal positivity within these cells (Figure 3), leading to a diagnosis of OHL. Laboratory evaluation demonstrated a normal complete blood cell count with differential and liver panel as well as a negative HIV test. The patient otherwise felt well and denied fevers, lymphadenopathy, and weight loss.
We consulted with an infectious disease and immunodeficiency specialist regarding the patient’s case. Before conducting further evaluation beyond HIV screening for immunodeficiency states, adalimumab was discontinued to see if the OHL would spontaneously resolve. Three months after discontinuation of adalimumab, the white plaque on the right lateral tongue was notably improved. The OHL continued to disappear and was completely resolved 1 year after discontinuation of adalimumab. The patient’s psoriasis had subsequently remained well controlled with diet and weight loss, smoking cessation, topical steroids, and apremilast without any recurrence of the OHL.
Oral hairy leukoplakia is associated with upregulated EBV replication and EBV-encoded proteins such as latent membrane protein 1.2 It often presents as white or gray patches on the lateral lingual margins with prominent folds and/or projections, giving a shaggy appearance. Oral hairy leukoplakia often is specific for HIV infection and rarely is associated with other immunodeficiencies.2 Prasad and Bilodeau5 performed a literature review of medical conditions and immunosuppressive medications associated with OHL in patients without HIV. Allogeneic transplant was associated with the highest incidence of OHL in HIV-negative patients (59.2% [45/76]).5 Various combinations of immunosuppressive medications (eg, prednisone, cyclosporine, azathioprine) also may be implicated in cases of HIV-negative patients with OHL. A case of OHL also has been reported with long-standing use of inhaled corticosteroids in an immunocompetent, HIV-negative patient.6 Another case was reported with long-term use of the aromatic antiepileptic lamotrigine, which resolved once stopping the medication.8 Although EBV is an oncovirus and has been associated with lymphoproliferative disorders and nasopharyngeal carcinoma, OHL is not considered to be a premalignant lesion.7 Despite the strong association between OHL and HIV, our patient was HIV negative. The only immunocompromising factor in our patient was the use of adalimumab to treat psoriasis. We did not conduct further testing for immunodeficiency states because the OHL spontaneously resolved when the adalimumab was discontinued.
PubMed and Ovid searches of articles indexed for MEDLINE using the terms adalimumab and oral hairy leukoplakia as well as TNF-alpha inhibitor and oral hairy leukoplakia with humans and English language as limitations revealed that no cases have been reported in the literature demonstrating an association between OHL and adalimumab or any other TNF-α inhibitor. However, Cetkovska et al9 reported a case of EBV hepatitis and subsequently chronic hepatitis as a complication of infliximab used for the treatment of chronic psoriasis. Because TNF-α and IFN-γ play an important role in controlling viral infections, there is an increased risk for reactivating a viral illness when depleting TNF through pharmacologic measures (ie, adalimumab, infliximab).8 Another case of EBV-associated plasmablastic lymphoma was reported after 1 year of adalimumab use in a patient with Crohn disease. The plasmablastic lymphoma resolved after 4 rounds of chemotherapy.10
The only contraindication for the use of adalimumab is a known hypersensitivity to the drug. Relative contraindications for use of adalimumab include active tuberculosis, demyelinating disease, hematologic diseases (ie, thrombocytopenia, pancytopenia), lymphoma, hepatitis C, and hepatitis B.11 The most common adverse effect of adalimumab is an injection-site reaction. Additional reported adverse effects of TNF-α inhibitors as a class are lymphoma, melanoma, nonmelanoma skin cancer, reactivation of latent tuberculosis, congestive heart failure, autoimmunity, and hematologic toxicity.11
This case demonstrates an association between adalimumab and OHL in an HIV-negative patient. Although the mechanism behind OHL and immunosuppression remains to be elucidated, this association is important to keep in mind when using adalimumab or other TNF-α inhibitors for the treatment of psoriasis or other medical conditions.
To the Editor:
Oral hairy leukoplakia (OHL) is an Epstein-Barr virus (EBV)–mediated mucocutaneous disease that often involves the lingual epithelium. The lateral portions of the tongue are the most commonly affected sites. The lesions often are described as asymptomatic, white, corrugated patches or plaques that are unable to be scraped off.1 Oral hairy leukoplakia was first identified in 1984 and was considered to be associated with AIDS.2 An association between the presence of OHL and the degree of immunosuppression as well as the severity of human immunodeficiency virus (HIV) has been reported.3 Although OHL initially was considered to be pathognomonic for HIV, it has since been described in multiple other immunosuppressive conditions.4 Numerous medical conditions and combinations of immunosuppressive medications have been associated with OHL in patients who were HIV negative.5
Adalimumab is an injectable human IgG1 recombinant antibody to tumor necrosis factor α (TNF-α).6 It currently is approved by the US Food and Drug Administration for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn disease, ulcerative colitis, noninfectious uveitis, hidradenitis suppurativa, and plaque psoriasis.7 We report a case of OHL associated with the use of adalimumab.
A 47-year-old woman initially presented with chronic plaque-type psoriasis. Her medical history was notable for bipolar disorder, migraines, hypertension, and tobacco use. The patient’s psoriasis initially was well controlled on a regimen of topical steroids and methotrexate; however, methotrexate was stopped after 2.5 years due to a mildly elevated alanine aminotransferase level, as well as an abnormal liver biopsy showing mildly active (grade 1 of 3) steatohepatitis with portal chronic inflammation, pericellular fibrosis, and portal and focal periportal fibrosis (stage 1-2 of 4). The patient and her dermatologist were uncomfortable continuing methotrexate with these findings. After baseline screening including a negative purified protein derivative skin test, adalimumab was initiated. A loading dose of 80 mg subcutaneously (SQ) was given, followed by adalimumab 40 mg SQ 1 week later and 40 mg every other week as maintenance.
The patient’s psoriasis was well controlled with adalimumab for 22 months, but she then developed a thin white plaque on the right lateral tongue (Figure 1). An incisional biopsy of the tongue performed by an oral surgeon revealed hyperkeratosis with Candida colonization and viral cytopathic effect (Figure 2). An EBV DNA in situ hybridization stain revealed focal positivity within these cells (Figure 3), leading to a diagnosis of OHL. Laboratory evaluation demonstrated a normal complete blood cell count with differential and liver panel as well as a negative HIV test. The patient otherwise felt well and denied fevers, lymphadenopathy, and weight loss.
We consulted with an infectious disease and immunodeficiency specialist regarding the patient’s case. Before conducting further evaluation beyond HIV screening for immunodeficiency states, adalimumab was discontinued to see if the OHL would spontaneously resolve. Three months after discontinuation of adalimumab, the white plaque on the right lateral tongue was notably improved. The OHL continued to disappear and was completely resolved 1 year after discontinuation of adalimumab. The patient’s psoriasis had subsequently remained well controlled with diet and weight loss, smoking cessation, topical steroids, and apremilast without any recurrence of the OHL.
Oral hairy leukoplakia is associated with upregulated EBV replication and EBV-encoded proteins such as latent membrane protein 1.2 It often presents as white or gray patches on the lateral lingual margins with prominent folds and/or projections, giving a shaggy appearance. Oral hairy leukoplakia often is specific for HIV infection and rarely is associated with other immunodeficiencies.2 Prasad and Bilodeau5 performed a literature review of medical conditions and immunosuppressive medications associated with OHL in patients without HIV. Allogeneic transplant was associated with the highest incidence of OHL in HIV-negative patients (59.2% [45/76]).5 Various combinations of immunosuppressive medications (eg, prednisone, cyclosporine, azathioprine) also may be implicated in cases of HIV-negative patients with OHL. A case of OHL also has been reported with long-standing use of inhaled corticosteroids in an immunocompetent, HIV-negative patient.6 Another case was reported with long-term use of the aromatic antiepileptic lamotrigine, which resolved once stopping the medication.8 Although EBV is an oncovirus and has been associated with lymphoproliferative disorders and nasopharyngeal carcinoma, OHL is not considered to be a premalignant lesion.7 Despite the strong association between OHL and HIV, our patient was HIV negative. The only immunocompromising factor in our patient was the use of adalimumab to treat psoriasis. We did not conduct further testing for immunodeficiency states because the OHL spontaneously resolved when the adalimumab was discontinued.
PubMed and Ovid searches of articles indexed for MEDLINE using the terms adalimumab and oral hairy leukoplakia as well as TNF-alpha inhibitor and oral hairy leukoplakia with humans and English language as limitations revealed that no cases have been reported in the literature demonstrating an association between OHL and adalimumab or any other TNF-α inhibitor. However, Cetkovska et al9 reported a case of EBV hepatitis and subsequently chronic hepatitis as a complication of infliximab used for the treatment of chronic psoriasis. Because TNF-α and IFN-γ play an important role in controlling viral infections, there is an increased risk for reactivating a viral illness when depleting TNF through pharmacologic measures (ie, adalimumab, infliximab).8 Another case of EBV-associated plasmablastic lymphoma was reported after 1 year of adalimumab use in a patient with Crohn disease. The plasmablastic lymphoma resolved after 4 rounds of chemotherapy.10
The only contraindication for the use of adalimumab is a known hypersensitivity to the drug. Relative contraindications for use of adalimumab include active tuberculosis, demyelinating disease, hematologic diseases (ie, thrombocytopenia, pancytopenia), lymphoma, hepatitis C, and hepatitis B.11 The most common adverse effect of adalimumab is an injection-site reaction. Additional reported adverse effects of TNF-α inhibitors as a class are lymphoma, melanoma, nonmelanoma skin cancer, reactivation of latent tuberculosis, congestive heart failure, autoimmunity, and hematologic toxicity.11
This case demonstrates an association between adalimumab and OHL in an HIV-negative patient. Although the mechanism behind OHL and immunosuppression remains to be elucidated, this association is important to keep in mind when using adalimumab or other TNF-α inhibitors for the treatment of psoriasis or other medical conditions.
- Triantos D, Porter SR, Scully C, et al. Oral hairy leukoplakia: clinicopathologic features, pathogenesis, diagnosis, and clinical significance. Clin Infect Dis. 1997;25:1392-1396.
- Greenspan D, Greenspan JS, Conant M, et al. Oral “hairy” leucoplakia in male homosexuals: evidence of association with both papillomavirus and a herpes-group virus. Lancet. 1984;2:831-834.
- Glick M, Muzyka BC, Lurie D, et al. Oral manifestations associated with HIV-related disease as marks for immune suppression and AIDS. Oral Surg Oral Med Oral Pathol. 1994;77:344-349.
- Chambers AE, Conn B, Pemberton M, et al. Twenty-first-century oral hair leukoplakia—a non-HIV-associated entity. Oral Surg Oral Med Oral Patho Oral Radiol. 2015;119:326-332.
- Prasad JL, Bilodeau EA. Oral hairy leukoplakia in patients without HIV: presentation of 2 new cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:E151-E160.
- Moffat M, Jauhar S, Jones ME, et al. Oral hairy leukoplakia in an HIV-negative, immunocompetent patient. Oral Biosci Med. 2005;2:282-284.
- Greenspan JS, Greenspan D. Oral hairy leukoplakia: diagnosis and management. Oral Surg Oral Med Oral Pathol. 1989;67:396-403.
- Gordins P, Sloan P, Spickett GP, et al. Oral hairy leukoplakia in a patient on long-term anticonvulsant treatment with lamotrigine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:E17-E23.
- Cetkovska P, Lomicova I, Mukensnabl P, et al. Anti-tumour necrosis factor treatment of severe psoriasis complicated by Epstein-Barr virus hepatitis and subsequently by chronic hepatitis. Dermatol Ther. 2015;28:369-372.
- Liu L, Charabaty A, Ozdemirli M. EBV-associated plasmablastic lymphoma in a patient with Crohn’s disease after adalimumab treatment. J Crohns Colitis. 2013;7:E118-E119.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Triantos D, Porter SR, Scully C, et al. Oral hairy leukoplakia: clinicopathologic features, pathogenesis, diagnosis, and clinical significance. Clin Infect Dis. 1997;25:1392-1396.
- Greenspan D, Greenspan JS, Conant M, et al. Oral “hairy” leucoplakia in male homosexuals: evidence of association with both papillomavirus and a herpes-group virus. Lancet. 1984;2:831-834.
- Glick M, Muzyka BC, Lurie D, et al. Oral manifestations associated with HIV-related disease as marks for immune suppression and AIDS. Oral Surg Oral Med Oral Pathol. 1994;77:344-349.
- Chambers AE, Conn B, Pemberton M, et al. Twenty-first-century oral hair leukoplakia—a non-HIV-associated entity. Oral Surg Oral Med Oral Patho Oral Radiol. 2015;119:326-332.
- Prasad JL, Bilodeau EA. Oral hairy leukoplakia in patients without HIV: presentation of 2 new cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:E151-E160.
- Moffat M, Jauhar S, Jones ME, et al. Oral hairy leukoplakia in an HIV-negative, immunocompetent patient. Oral Biosci Med. 2005;2:282-284.
- Greenspan JS, Greenspan D. Oral hairy leukoplakia: diagnosis and management. Oral Surg Oral Med Oral Pathol. 1989;67:396-403.
- Gordins P, Sloan P, Spickett GP, et al. Oral hairy leukoplakia in a patient on long-term anticonvulsant treatment with lamotrigine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:E17-E23.
- Cetkovska P, Lomicova I, Mukensnabl P, et al. Anti-tumour necrosis factor treatment of severe psoriasis complicated by Epstein-Barr virus hepatitis and subsequently by chronic hepatitis. Dermatol Ther. 2015;28:369-372.
- Liu L, Charabaty A, Ozdemirli M. EBV-associated plasmablastic lymphoma in a patient with Crohn’s disease after adalimumab treatment. J Crohns Colitis. 2013;7:E118-E119.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2018.
Practice Points
- Workup for new-onset oral hairy leukoplakia should include a comprehensive medication history.
- Oral hairy leukoplakia is an uncommon side effect of adalimumab.
Poor image quality may limit televulvology care
Seeing patients with vulvar problems via telemedicine can lead to efficient and successful care, but there are challenges and limitations with this approach, doctors are finding.
Image quality is one key factor that determines whether a clinician can assess and manage a condition remotely, said Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif. Other issues may be especially relevant to televulvology, including privacy concerns.
“Who is helping with the positioning? Who is the photographer? Is the patient comfortable with having photos taken of this part of their body and submitted, even if they know it is submitted securely? Because they might not be,” Dr. Venkatesan said in a lecture at a virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
When quality photographs from referring providers are available, Dr. Venkatesan has conducted virtual new consultations. “But sometimes I will do a virtual telemedicine visit as the first visit and then figure out, okay, this isn’t really sufficient. I need to see them in person.”
Melissa Mauskar, MD, assistant professor of dermatology and obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas, described a case early on during the COVID-19 pandemic that illustrates a limitation of virtual visits.
A patient sent in a photograph that appeared to show lichen sclerosus. “There looked like some classic lichen sclerosus changes,” Dr. Mauskar said during a discussion at the meeting. “But she was having a lot of pain, and after a week, her pain still was not better.”
Dr. Mauskar brought the patient into the office and ultimately diagnosed a squamous cell carcinoma. “What I thought was a normal erosion was actually an ulcerated plaque,” she said.
Like Dr. Venkatesan, Dr. Mauskar has found that image quality can be uneven. Photographs may be out of focus. Video visits have been a mixed bag. Some are successful. Other times, Dr. Mauskar has to tell the patient she needs to see her in the office.
Certain clinical scenarios require a vaginal exam, Dr. Venkatesan noted. Although some type of assessment may be possible if a patient is with a primary care provider during the telemedicine visit, the examination may not be equivalent. Doctors also should anticipate where a patient might go to have a biopsy if one is necessary.
Another telemedicine caveat pertains to patient counseling. When using store-and-forward telemedicine systems, advising patients in a written report can be challenging. “Is there an easy way ... to counsel patients how to apply their topical medications?” Dr. Venkatesan said.
Excellent care is possible
Vulvology is a small part of Dr. Venkatesan’s general dermatology practice, which has used telemedicine extensively since the pandemic.
In recent years, Dr. Venkatesan’s clinic began encouraging providers in their health system to submit photographs with referrals. “That has really paid off now because we have been able to help provide a lot of excellent quality care for patients without them having to come in,” she said. “We may be able to say: ‘These are excellent photos. We know what this patient has. We can manage it. They don’t need to come see us in person.’ ” That could be the case for certain types of acne, eczema, and psoriasis.
In other cases, they may be able to provide initial advice remotely but still want to see the patient. For a patient with severe acne, “I may be able to tell the referring doctor: ‘Please start the patient on these three medicines. It will take 2 months for those medicines to start working and then we will plan to have an in-person dermatology visit.’ ” In this case, telemedicine essentially replaces one in-person visit.
If photographs are poor, the differential diagnosis is broad, a procedure is required, the doctor needs to touch the lesion, or more involved history taking or counseling are required, the patient may need to go into the office.
Beyond its public health advantages during a pandemic, telemedicine can improve access for patients who live far away, lack transportation, or are unable to take time off from work. It also can decrease patient wait times. “Once we started doing some telemedicine work … we went from having a 5-month wait time for patients to see us in person to a 72-hour wait time for providing some care for patients if they had good photos as part of their referral,” Dr. Venkatesan said.
Telemedicine has been used in inpatient and outpatient dermatology settings. Primary care providers who consult with dermatologists using a store-and-forward telemedicine system may improve their dermatology knowledge and feel more confident in their ability to diagnose and manage dermatologic conditions, research indicates.
In obstetrics and gynecology, telemedicine may play a role in preconception, contraception, and medical abortion care, prenatal visits, well-woman exams, mental health, and pre- and postoperative counseling, a recent review suggests.
Image quality is key
“Quality of the image is so critical for being able to provide good care, especially in such a visual exam field as dermatology,” Dr. Venkatesan said.
To that end, doctors have offered recommendations on how to photograph skin conditions. A guide shared by the mobile telehealth system company ClickMedix suggests focusing on the area of importance, capturing the extent of involvement, and including involved and uninvolved areas.
Good lighting and checking the image resolution can help, Dr. Venkatesan offered. Nevertheless, patients may have difficulty photographing themselves. If a patient is with their primary care doctor, “we are much more likely to be able to get good quality photos,” she said.
Dr. Venkatesan is a paid consultant for DirectDerm, a store-and-forward teledermatology company. Dr. Mauskar had no relevant disclosures.
Seeing patients with vulvar problems via telemedicine can lead to efficient and successful care, but there are challenges and limitations with this approach, doctors are finding.
Image quality is one key factor that determines whether a clinician can assess and manage a condition remotely, said Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif. Other issues may be especially relevant to televulvology, including privacy concerns.
“Who is helping with the positioning? Who is the photographer? Is the patient comfortable with having photos taken of this part of their body and submitted, even if they know it is submitted securely? Because they might not be,” Dr. Venkatesan said in a lecture at a virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
When quality photographs from referring providers are available, Dr. Venkatesan has conducted virtual new consultations. “But sometimes I will do a virtual telemedicine visit as the first visit and then figure out, okay, this isn’t really sufficient. I need to see them in person.”
Melissa Mauskar, MD, assistant professor of dermatology and obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas, described a case early on during the COVID-19 pandemic that illustrates a limitation of virtual visits.
A patient sent in a photograph that appeared to show lichen sclerosus. “There looked like some classic lichen sclerosus changes,” Dr. Mauskar said during a discussion at the meeting. “But she was having a lot of pain, and after a week, her pain still was not better.”
Dr. Mauskar brought the patient into the office and ultimately diagnosed a squamous cell carcinoma. “What I thought was a normal erosion was actually an ulcerated plaque,” she said.
Like Dr. Venkatesan, Dr. Mauskar has found that image quality can be uneven. Photographs may be out of focus. Video visits have been a mixed bag. Some are successful. Other times, Dr. Mauskar has to tell the patient she needs to see her in the office.
Certain clinical scenarios require a vaginal exam, Dr. Venkatesan noted. Although some type of assessment may be possible if a patient is with a primary care provider during the telemedicine visit, the examination may not be equivalent. Doctors also should anticipate where a patient might go to have a biopsy if one is necessary.
Another telemedicine caveat pertains to patient counseling. When using store-and-forward telemedicine systems, advising patients in a written report can be challenging. “Is there an easy way ... to counsel patients how to apply their topical medications?” Dr. Venkatesan said.
Excellent care is possible
Vulvology is a small part of Dr. Venkatesan’s general dermatology practice, which has used telemedicine extensively since the pandemic.
In recent years, Dr. Venkatesan’s clinic began encouraging providers in their health system to submit photographs with referrals. “That has really paid off now because we have been able to help provide a lot of excellent quality care for patients without them having to come in,” she said. “We may be able to say: ‘These are excellent photos. We know what this patient has. We can manage it. They don’t need to come see us in person.’ ” That could be the case for certain types of acne, eczema, and psoriasis.
In other cases, they may be able to provide initial advice remotely but still want to see the patient. For a patient with severe acne, “I may be able to tell the referring doctor: ‘Please start the patient on these three medicines. It will take 2 months for those medicines to start working and then we will plan to have an in-person dermatology visit.’ ” In this case, telemedicine essentially replaces one in-person visit.
If photographs are poor, the differential diagnosis is broad, a procedure is required, the doctor needs to touch the lesion, or more involved history taking or counseling are required, the patient may need to go into the office.
Beyond its public health advantages during a pandemic, telemedicine can improve access for patients who live far away, lack transportation, or are unable to take time off from work. It also can decrease patient wait times. “Once we started doing some telemedicine work … we went from having a 5-month wait time for patients to see us in person to a 72-hour wait time for providing some care for patients if they had good photos as part of their referral,” Dr. Venkatesan said.
Telemedicine has been used in inpatient and outpatient dermatology settings. Primary care providers who consult with dermatologists using a store-and-forward telemedicine system may improve their dermatology knowledge and feel more confident in their ability to diagnose and manage dermatologic conditions, research indicates.
In obstetrics and gynecology, telemedicine may play a role in preconception, contraception, and medical abortion care, prenatal visits, well-woman exams, mental health, and pre- and postoperative counseling, a recent review suggests.
Image quality is key
“Quality of the image is so critical for being able to provide good care, especially in such a visual exam field as dermatology,” Dr. Venkatesan said.
To that end, doctors have offered recommendations on how to photograph skin conditions. A guide shared by the mobile telehealth system company ClickMedix suggests focusing on the area of importance, capturing the extent of involvement, and including involved and uninvolved areas.
Good lighting and checking the image resolution can help, Dr. Venkatesan offered. Nevertheless, patients may have difficulty photographing themselves. If a patient is with their primary care doctor, “we are much more likely to be able to get good quality photos,” she said.
Dr. Venkatesan is a paid consultant for DirectDerm, a store-and-forward teledermatology company. Dr. Mauskar had no relevant disclosures.
Seeing patients with vulvar problems via telemedicine can lead to efficient and successful care, but there are challenges and limitations with this approach, doctors are finding.
Image quality is one key factor that determines whether a clinician can assess and manage a condition remotely, said Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif. Other issues may be especially relevant to televulvology, including privacy concerns.
“Who is helping with the positioning? Who is the photographer? Is the patient comfortable with having photos taken of this part of their body and submitted, even if they know it is submitted securely? Because they might not be,” Dr. Venkatesan said in a lecture at a virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
When quality photographs from referring providers are available, Dr. Venkatesan has conducted virtual new consultations. “But sometimes I will do a virtual telemedicine visit as the first visit and then figure out, okay, this isn’t really sufficient. I need to see them in person.”
Melissa Mauskar, MD, assistant professor of dermatology and obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas, described a case early on during the COVID-19 pandemic that illustrates a limitation of virtual visits.
A patient sent in a photograph that appeared to show lichen sclerosus. “There looked like some classic lichen sclerosus changes,” Dr. Mauskar said during a discussion at the meeting. “But she was having a lot of pain, and after a week, her pain still was not better.”
Dr. Mauskar brought the patient into the office and ultimately diagnosed a squamous cell carcinoma. “What I thought was a normal erosion was actually an ulcerated plaque,” she said.
Like Dr. Venkatesan, Dr. Mauskar has found that image quality can be uneven. Photographs may be out of focus. Video visits have been a mixed bag. Some are successful. Other times, Dr. Mauskar has to tell the patient she needs to see her in the office.
Certain clinical scenarios require a vaginal exam, Dr. Venkatesan noted. Although some type of assessment may be possible if a patient is with a primary care provider during the telemedicine visit, the examination may not be equivalent. Doctors also should anticipate where a patient might go to have a biopsy if one is necessary.
Another telemedicine caveat pertains to patient counseling. When using store-and-forward telemedicine systems, advising patients in a written report can be challenging. “Is there an easy way ... to counsel patients how to apply their topical medications?” Dr. Venkatesan said.
Excellent care is possible
Vulvology is a small part of Dr. Venkatesan’s general dermatology practice, which has used telemedicine extensively since the pandemic.
In recent years, Dr. Venkatesan’s clinic began encouraging providers in their health system to submit photographs with referrals. “That has really paid off now because we have been able to help provide a lot of excellent quality care for patients without them having to come in,” she said. “We may be able to say: ‘These are excellent photos. We know what this patient has. We can manage it. They don’t need to come see us in person.’ ” That could be the case for certain types of acne, eczema, and psoriasis.
In other cases, they may be able to provide initial advice remotely but still want to see the patient. For a patient with severe acne, “I may be able to tell the referring doctor: ‘Please start the patient on these three medicines. It will take 2 months for those medicines to start working and then we will plan to have an in-person dermatology visit.’ ” In this case, telemedicine essentially replaces one in-person visit.
If photographs are poor, the differential diagnosis is broad, a procedure is required, the doctor needs to touch the lesion, or more involved history taking or counseling are required, the patient may need to go into the office.
Beyond its public health advantages during a pandemic, telemedicine can improve access for patients who live far away, lack transportation, or are unable to take time off from work. It also can decrease patient wait times. “Once we started doing some telemedicine work … we went from having a 5-month wait time for patients to see us in person to a 72-hour wait time for providing some care for patients if they had good photos as part of their referral,” Dr. Venkatesan said.
Telemedicine has been used in inpatient and outpatient dermatology settings. Primary care providers who consult with dermatologists using a store-and-forward telemedicine system may improve their dermatology knowledge and feel more confident in their ability to diagnose and manage dermatologic conditions, research indicates.
In obstetrics and gynecology, telemedicine may play a role in preconception, contraception, and medical abortion care, prenatal visits, well-woman exams, mental health, and pre- and postoperative counseling, a recent review suggests.
Image quality is key
“Quality of the image is so critical for being able to provide good care, especially in such a visual exam field as dermatology,” Dr. Venkatesan said.
To that end, doctors have offered recommendations on how to photograph skin conditions. A guide shared by the mobile telehealth system company ClickMedix suggests focusing on the area of importance, capturing the extent of involvement, and including involved and uninvolved areas.
Good lighting and checking the image resolution can help, Dr. Venkatesan offered. Nevertheless, patients may have difficulty photographing themselves. If a patient is with their primary care doctor, “we are much more likely to be able to get good quality photos,” she said.
Dr. Venkatesan is a paid consultant for DirectDerm, a store-and-forward teledermatology company. Dr. Mauskar had no relevant disclosures.
FROM THE ISSVD BIENNIAL CONFERENCE
Mind menders: The future of psychedelic therapy in the United States
After a 50-year hiatus, psychedelic drugs are undergoing a research renaissance. Roland R. Griffiths, PhD, professor in the Departments of Psychiatry and Neuroscience and the Oliver Lee McCabe III, Professor in the Neuropsychopharmacology of Consciousness, and director of the Center for Psychedelic and Consciousness Research at Johns Hopkins University, Baltimore, discusses the status of these drugs in the United States and their potential to treat psychiatric disorders.
Classic psychedelics are compounds that bind to the 5-hydroxytryptamine 2A (5-HT2A) receptor and include the naturally occurring compounds psilocybin, N,N-dimethyltryptamine (DMT, a component of ayahuasca) and mescaline (peyote cactus), as well as the synthesized compound lysergic acid diethylamide (LSD).
Other drugs, such as ketamine, are sometimes referred to as “psychedelics” because they can produce subjective experiences that are similar to those of people who receive classic psychedelics. However, unlike classic psychedelics, the effects of ketamine tend to be short lived. Ketamine also has addictive potential and can be lethal in high doses, which is not the case with psilocybin.
Another compound sometimes referred to as a “psychedelic” is 3,4-methylenedioxymethamphetamine (MDMA), also known as “ecstasy.” The Food and Drug Administration granted breakthrough approval for the study of MDMA for posttraumatic stress disorder (PTSD). FDA-approved registration trials are ongoing. MDMA differs from classic psychedelics in risk profile and pharmacology. In particular, MDMA was widely abused as part of the “rave culture,” while classic psychedelic agents do not lend themselves to that type of misuse.
What is the current legal status of psychedelic agents in the United States? Can clinicians prescribe them, or are they available only in a research setting?
All classic psychedelics are considered to be “Schedule 1” which means they are illegal to possess and use except for research and only if approved by the FDA and under licensure of the Drug Enforcement Administration (DEA), so they are not available for clinical use.
In anticipation of the possibility that phase 3 research may support the efficacy and safety of psilocybin for one or more medical or mental health disorders, our team has reviewed available evidence regarding its abuse liability and concluded that, if psilocybin were approved as medication, it could possibly be included in the Schedule IV category, with additional FDA-mandated risk management provisions. However, this is not yet the case.
Which psychedelic agents are under investigation in the United States, and for which indications?
Psilocybin is under investigation in our center, as well as elsewhere in the United States. We have previously found it to be effective for smoking cessation, and we are conducting another study that is currently recruiting volunteers for this indication. We are also recruiting volunteers for studies on the use of psilocybin for major depression, Alzheimer’s disease, and anorexia nervosa. Further information about our studies can be found on the Web site for our center, the Center for Psychedelic and Consciousness Research.
Two companies – the Usona Institute and COMPASS Pathways – have received FDA Breakthrough Therapy Designation for their programs seeking approval of psilocybin as a treatment major depressive disorder and treatment-resistant depression (TRD), respectively. In addition, an international multicenter study currently underway, which includes US centers in Houston, Baltimore, New York, San Diego, and Atlanta, is investigating psilocybin for TRD.
A number of studies, including one conducted at our center, have investigated psilocybin for depression and anxiety in patients with cancer and found it effective.
Additional research showed that psilocybin alleviated symptoms of cancer-related anxiety and depression, both in the short-term and 5 years later.
LSD has been studied and found promising in the treatment of alcohol use disorder. Additional studies of LSD that are being conducted in Basel Switzerland and at the University of Chicago are examining its impact on mood in healthy volunteers.
Ayahuasca has been studied extensively for depression and anxiety and is also currently under investigation for PTSD. We found that its use in a naturalistic group setting was associated with unintended improvements in depression and anxiety.
Lastly, a lesser-known psychedelic agent is Salvinorin A, which our center has been studying, is the psychoactive constituent of the Salvia divinorum plant. While this is not a “classic” psychedelic compound, it is nevertheless the focus of much scientific interest because its effects are mediated at opioid receptors, rather than 5-HT2A receptors, and may prove to be a novel nonaddictive opioid that may ultimately be a promising treatment for pain and addiction.
What is the typical treatment regimen for psychedelic agents?
It is hard to speak of a “treatment regimen” in agents that are not used in clinical practice. Ongoing clinical trials with psilocybin generally involve one or two 6- to 8-hour sessions involving the oral administration of a moderately high dose under psychologically supported conditions.
Based on the current evidence base, which agents show the most promise?
Psilocybin is currently the most promising classic psychedelic undergoing clinical trials.
Do psychedelics have to be administered in a controlled setting in order to be effective?
Although many people have had meaningful experiences whether inside or outside of a controlled setting, there are serious potential risks associated with use of psilocybin and other classic psychedelics. The safety of psilocybin has been established in clinical studies in which participants have been carefully screened physically and psychologically, are psychologically prepared before their first session, and are psychologically supported during and after sessions. In vulnerable individuals, psilocybin has been associated with enduring psychiatric problems and sometimes persisting visual perceptual conditions. When taken in uncontrolled conditions, classic psychedelics can produce confusion and disorientation resulting in behavior dangerous to the participant and others – including life-threatening risk. Thus, for safety reasons, the optimal environment for using these agents is in a controlled setting.
Do results differ between patients who have used psychedelic agents previously and those who have not?
We have not found any difference between psychedelic-naive volunteers and those who have used psychedelics in the past.
Do you provide patient education prior to treatment initiation?
All of our study participants are thoroughly screened for medical concerns or mental health history such as psychosis, which would preclude their participation. They are educated about the effects of these agents and what they might expect and typically receive several hours of psychological preparation before the first session. They are also provided with psychological support after sessions. Additionally, we spend time developing trust and rapport prior to the first session.
How durable are the effects of psychedelic treatment?
Studies in patients and healthy participants suggest that the positive effects of psilocybin are long lasting, with most individuals reporting positive changes in moods, attitudes, and behavior that they attribute to psilocybin and which endure months or years after the session. The qualities of the acute session experience can vary widely ranging from experiences of transcendence or psychological insight to experiences of intense anxiety or fear.
An enduring shift in worldview and sense of self, as well as psychological insight, may increase psychological flexibility, thereby allowing individuals to subsequently avoid maladaptive patterns of behavior or thought and to make more healthy choices.
Our research has shown that the benefits of these experiences can last as long as 14 months, often longer, and that many participants characterize their psilocybin experience as among the most profound and personally meaningful experience of their lives.
Do participants experience any adverse effects? If so, how are they managed?
Sometimes, despite all the preparation, screening, and support we provide, some participants can have frightening experiences, such as fear and anxiety during the session. When that occurs, it is often shorted lived. The psychological preparation we provide before the session and the psychological support we provide during the session are important for managing such effects.
We provide support and encourage participants to stay with that experience, which may open to experiences of deep meaning or insight. A number of people report that these psychologically challenging states are a valuable part of the overall experience.
We conducted a survey of roughly 2,000 people who took high doses of psilocybin mushrooms and then had a challenging experience. About 10% reported they put themselves or others at risk of physical harm. Of more concern, of those whose experience occurred more than 1 year before, 8% sought treatment for enduring psychological symptoms. These findings underscore potential risks of psilocybin use but do not provide an estimate of the actual incidence of such effects.
Importantly, in our research at Johns Hopkins, we have not observed such effects in over 700 sessions that we have conducted with almost 400 participants, likely because we thoroughly screen and prepare participants and support them after they have completed the study. The potential for serious lasting harm represents a concern and points to the importance of adequate screening and aftercare.
What are the implications for future therapeutics?
We are living in exciting times, in terms of psychedelic research. The potential for a single treatment with a classic psychedelic to produce rapid and sustained therapeutic effects, possibly across a range of psychiatric conditions, is unprecedented in psychiatry. The effect appears to be an “inverse PTSD effect.”
In PTSD, a single exposure to a traumatic event can rewire the nervous system to the point that it produces enduring harm and toxicity. In the case of psychedelics, a single exposure appears to have enduring positive effects in worldview, mood, attitude, behavior, and overall life satisfaction. We can look forward to continued growth and expansion of this research including the refinement of protocols for a variety of therapeutic indications and to the development of a variety of new classic psychedelic compounds.
A version of this article originally appeared on Medscape.com.
After a 50-year hiatus, psychedelic drugs are undergoing a research renaissance. Roland R. Griffiths, PhD, professor in the Departments of Psychiatry and Neuroscience and the Oliver Lee McCabe III, Professor in the Neuropsychopharmacology of Consciousness, and director of the Center for Psychedelic and Consciousness Research at Johns Hopkins University, Baltimore, discusses the status of these drugs in the United States and their potential to treat psychiatric disorders.
Classic psychedelics are compounds that bind to the 5-hydroxytryptamine 2A (5-HT2A) receptor and include the naturally occurring compounds psilocybin, N,N-dimethyltryptamine (DMT, a component of ayahuasca) and mescaline (peyote cactus), as well as the synthesized compound lysergic acid diethylamide (LSD).
Other drugs, such as ketamine, are sometimes referred to as “psychedelics” because they can produce subjective experiences that are similar to those of people who receive classic psychedelics. However, unlike classic psychedelics, the effects of ketamine tend to be short lived. Ketamine also has addictive potential and can be lethal in high doses, which is not the case with psilocybin.
Another compound sometimes referred to as a “psychedelic” is 3,4-methylenedioxymethamphetamine (MDMA), also known as “ecstasy.” The Food and Drug Administration granted breakthrough approval for the study of MDMA for posttraumatic stress disorder (PTSD). FDA-approved registration trials are ongoing. MDMA differs from classic psychedelics in risk profile and pharmacology. In particular, MDMA was widely abused as part of the “rave culture,” while classic psychedelic agents do not lend themselves to that type of misuse.
What is the current legal status of psychedelic agents in the United States? Can clinicians prescribe them, or are they available only in a research setting?
All classic psychedelics are considered to be “Schedule 1” which means they are illegal to possess and use except for research and only if approved by the FDA and under licensure of the Drug Enforcement Administration (DEA), so they are not available for clinical use.
In anticipation of the possibility that phase 3 research may support the efficacy and safety of psilocybin for one or more medical or mental health disorders, our team has reviewed available evidence regarding its abuse liability and concluded that, if psilocybin were approved as medication, it could possibly be included in the Schedule IV category, with additional FDA-mandated risk management provisions. However, this is not yet the case.
Which psychedelic agents are under investigation in the United States, and for which indications?
Psilocybin is under investigation in our center, as well as elsewhere in the United States. We have previously found it to be effective for smoking cessation, and we are conducting another study that is currently recruiting volunteers for this indication. We are also recruiting volunteers for studies on the use of psilocybin for major depression, Alzheimer’s disease, and anorexia nervosa. Further information about our studies can be found on the Web site for our center, the Center for Psychedelic and Consciousness Research.
Two companies – the Usona Institute and COMPASS Pathways – have received FDA Breakthrough Therapy Designation for their programs seeking approval of psilocybin as a treatment major depressive disorder and treatment-resistant depression (TRD), respectively. In addition, an international multicenter study currently underway, which includes US centers in Houston, Baltimore, New York, San Diego, and Atlanta, is investigating psilocybin for TRD.
A number of studies, including one conducted at our center, have investigated psilocybin for depression and anxiety in patients with cancer and found it effective.
Additional research showed that psilocybin alleviated symptoms of cancer-related anxiety and depression, both in the short-term and 5 years later.
LSD has been studied and found promising in the treatment of alcohol use disorder. Additional studies of LSD that are being conducted in Basel Switzerland and at the University of Chicago are examining its impact on mood in healthy volunteers.
Ayahuasca has been studied extensively for depression and anxiety and is also currently under investigation for PTSD. We found that its use in a naturalistic group setting was associated with unintended improvements in depression and anxiety.
Lastly, a lesser-known psychedelic agent is Salvinorin A, which our center has been studying, is the psychoactive constituent of the Salvia divinorum plant. While this is not a “classic” psychedelic compound, it is nevertheless the focus of much scientific interest because its effects are mediated at opioid receptors, rather than 5-HT2A receptors, and may prove to be a novel nonaddictive opioid that may ultimately be a promising treatment for pain and addiction.
What is the typical treatment regimen for psychedelic agents?
It is hard to speak of a “treatment regimen” in agents that are not used in clinical practice. Ongoing clinical trials with psilocybin generally involve one or two 6- to 8-hour sessions involving the oral administration of a moderately high dose under psychologically supported conditions.
Based on the current evidence base, which agents show the most promise?
Psilocybin is currently the most promising classic psychedelic undergoing clinical trials.
Do psychedelics have to be administered in a controlled setting in order to be effective?
Although many people have had meaningful experiences whether inside or outside of a controlled setting, there are serious potential risks associated with use of psilocybin and other classic psychedelics. The safety of psilocybin has been established in clinical studies in which participants have been carefully screened physically and psychologically, are psychologically prepared before their first session, and are psychologically supported during and after sessions. In vulnerable individuals, psilocybin has been associated with enduring psychiatric problems and sometimes persisting visual perceptual conditions. When taken in uncontrolled conditions, classic psychedelics can produce confusion and disorientation resulting in behavior dangerous to the participant and others – including life-threatening risk. Thus, for safety reasons, the optimal environment for using these agents is in a controlled setting.
Do results differ between patients who have used psychedelic agents previously and those who have not?
We have not found any difference between psychedelic-naive volunteers and those who have used psychedelics in the past.
Do you provide patient education prior to treatment initiation?
All of our study participants are thoroughly screened for medical concerns or mental health history such as psychosis, which would preclude their participation. They are educated about the effects of these agents and what they might expect and typically receive several hours of psychological preparation before the first session. They are also provided with psychological support after sessions. Additionally, we spend time developing trust and rapport prior to the first session.
How durable are the effects of psychedelic treatment?
Studies in patients and healthy participants suggest that the positive effects of psilocybin are long lasting, with most individuals reporting positive changes in moods, attitudes, and behavior that they attribute to psilocybin and which endure months or years after the session. The qualities of the acute session experience can vary widely ranging from experiences of transcendence or psychological insight to experiences of intense anxiety or fear.
An enduring shift in worldview and sense of self, as well as psychological insight, may increase psychological flexibility, thereby allowing individuals to subsequently avoid maladaptive patterns of behavior or thought and to make more healthy choices.
Our research has shown that the benefits of these experiences can last as long as 14 months, often longer, and that many participants characterize their psilocybin experience as among the most profound and personally meaningful experience of their lives.
Do participants experience any adverse effects? If so, how are they managed?
Sometimes, despite all the preparation, screening, and support we provide, some participants can have frightening experiences, such as fear and anxiety during the session. When that occurs, it is often shorted lived. The psychological preparation we provide before the session and the psychological support we provide during the session are important for managing such effects.
We provide support and encourage participants to stay with that experience, which may open to experiences of deep meaning or insight. A number of people report that these psychologically challenging states are a valuable part of the overall experience.
We conducted a survey of roughly 2,000 people who took high doses of psilocybin mushrooms and then had a challenging experience. About 10% reported they put themselves or others at risk of physical harm. Of more concern, of those whose experience occurred more than 1 year before, 8% sought treatment for enduring psychological symptoms. These findings underscore potential risks of psilocybin use but do not provide an estimate of the actual incidence of such effects.
Importantly, in our research at Johns Hopkins, we have not observed such effects in over 700 sessions that we have conducted with almost 400 participants, likely because we thoroughly screen and prepare participants and support them after they have completed the study. The potential for serious lasting harm represents a concern and points to the importance of adequate screening and aftercare.
What are the implications for future therapeutics?
We are living in exciting times, in terms of psychedelic research. The potential for a single treatment with a classic psychedelic to produce rapid and sustained therapeutic effects, possibly across a range of psychiatric conditions, is unprecedented in psychiatry. The effect appears to be an “inverse PTSD effect.”
In PTSD, a single exposure to a traumatic event can rewire the nervous system to the point that it produces enduring harm and toxicity. In the case of psychedelics, a single exposure appears to have enduring positive effects in worldview, mood, attitude, behavior, and overall life satisfaction. We can look forward to continued growth and expansion of this research including the refinement of protocols for a variety of therapeutic indications and to the development of a variety of new classic psychedelic compounds.
A version of this article originally appeared on Medscape.com.
After a 50-year hiatus, psychedelic drugs are undergoing a research renaissance. Roland R. Griffiths, PhD, professor in the Departments of Psychiatry and Neuroscience and the Oliver Lee McCabe III, Professor in the Neuropsychopharmacology of Consciousness, and director of the Center for Psychedelic and Consciousness Research at Johns Hopkins University, Baltimore, discusses the status of these drugs in the United States and their potential to treat psychiatric disorders.
Classic psychedelics are compounds that bind to the 5-hydroxytryptamine 2A (5-HT2A) receptor and include the naturally occurring compounds psilocybin, N,N-dimethyltryptamine (DMT, a component of ayahuasca) and mescaline (peyote cactus), as well as the synthesized compound lysergic acid diethylamide (LSD).
Other drugs, such as ketamine, are sometimes referred to as “psychedelics” because they can produce subjective experiences that are similar to those of people who receive classic psychedelics. However, unlike classic psychedelics, the effects of ketamine tend to be short lived. Ketamine also has addictive potential and can be lethal in high doses, which is not the case with psilocybin.
Another compound sometimes referred to as a “psychedelic” is 3,4-methylenedioxymethamphetamine (MDMA), also known as “ecstasy.” The Food and Drug Administration granted breakthrough approval for the study of MDMA for posttraumatic stress disorder (PTSD). FDA-approved registration trials are ongoing. MDMA differs from classic psychedelics in risk profile and pharmacology. In particular, MDMA was widely abused as part of the “rave culture,” while classic psychedelic agents do not lend themselves to that type of misuse.
What is the current legal status of psychedelic agents in the United States? Can clinicians prescribe them, or are they available only in a research setting?
All classic psychedelics are considered to be “Schedule 1” which means they are illegal to possess and use except for research and only if approved by the FDA and under licensure of the Drug Enforcement Administration (DEA), so they are not available for clinical use.
In anticipation of the possibility that phase 3 research may support the efficacy and safety of psilocybin for one or more medical or mental health disorders, our team has reviewed available evidence regarding its abuse liability and concluded that, if psilocybin were approved as medication, it could possibly be included in the Schedule IV category, with additional FDA-mandated risk management provisions. However, this is not yet the case.
Which psychedelic agents are under investigation in the United States, and for which indications?
Psilocybin is under investigation in our center, as well as elsewhere in the United States. We have previously found it to be effective for smoking cessation, and we are conducting another study that is currently recruiting volunteers for this indication. We are also recruiting volunteers for studies on the use of psilocybin for major depression, Alzheimer’s disease, and anorexia nervosa. Further information about our studies can be found on the Web site for our center, the Center for Psychedelic and Consciousness Research.
Two companies – the Usona Institute and COMPASS Pathways – have received FDA Breakthrough Therapy Designation for their programs seeking approval of psilocybin as a treatment major depressive disorder and treatment-resistant depression (TRD), respectively. In addition, an international multicenter study currently underway, which includes US centers in Houston, Baltimore, New York, San Diego, and Atlanta, is investigating psilocybin for TRD.
A number of studies, including one conducted at our center, have investigated psilocybin for depression and anxiety in patients with cancer and found it effective.
Additional research showed that psilocybin alleviated symptoms of cancer-related anxiety and depression, both in the short-term and 5 years later.
LSD has been studied and found promising in the treatment of alcohol use disorder. Additional studies of LSD that are being conducted in Basel Switzerland and at the University of Chicago are examining its impact on mood in healthy volunteers.
Ayahuasca has been studied extensively for depression and anxiety and is also currently under investigation for PTSD. We found that its use in a naturalistic group setting was associated with unintended improvements in depression and anxiety.
Lastly, a lesser-known psychedelic agent is Salvinorin A, which our center has been studying, is the psychoactive constituent of the Salvia divinorum plant. While this is not a “classic” psychedelic compound, it is nevertheless the focus of much scientific interest because its effects are mediated at opioid receptors, rather than 5-HT2A receptors, and may prove to be a novel nonaddictive opioid that may ultimately be a promising treatment for pain and addiction.
What is the typical treatment regimen for psychedelic agents?
It is hard to speak of a “treatment regimen” in agents that are not used in clinical practice. Ongoing clinical trials with psilocybin generally involve one or two 6- to 8-hour sessions involving the oral administration of a moderately high dose under psychologically supported conditions.
Based on the current evidence base, which agents show the most promise?
Psilocybin is currently the most promising classic psychedelic undergoing clinical trials.
Do psychedelics have to be administered in a controlled setting in order to be effective?
Although many people have had meaningful experiences whether inside or outside of a controlled setting, there are serious potential risks associated with use of psilocybin and other classic psychedelics. The safety of psilocybin has been established in clinical studies in which participants have been carefully screened physically and psychologically, are psychologically prepared before their first session, and are psychologically supported during and after sessions. In vulnerable individuals, psilocybin has been associated with enduring psychiatric problems and sometimes persisting visual perceptual conditions. When taken in uncontrolled conditions, classic psychedelics can produce confusion and disorientation resulting in behavior dangerous to the participant and others – including life-threatening risk. Thus, for safety reasons, the optimal environment for using these agents is in a controlled setting.
Do results differ between patients who have used psychedelic agents previously and those who have not?
We have not found any difference between psychedelic-naive volunteers and those who have used psychedelics in the past.
Do you provide patient education prior to treatment initiation?
All of our study participants are thoroughly screened for medical concerns or mental health history such as psychosis, which would preclude their participation. They are educated about the effects of these agents and what they might expect and typically receive several hours of psychological preparation before the first session. They are also provided with psychological support after sessions. Additionally, we spend time developing trust and rapport prior to the first session.
How durable are the effects of psychedelic treatment?
Studies in patients and healthy participants suggest that the positive effects of psilocybin are long lasting, with most individuals reporting positive changes in moods, attitudes, and behavior that they attribute to psilocybin and which endure months or years after the session. The qualities of the acute session experience can vary widely ranging from experiences of transcendence or psychological insight to experiences of intense anxiety or fear.
An enduring shift in worldview and sense of self, as well as psychological insight, may increase psychological flexibility, thereby allowing individuals to subsequently avoid maladaptive patterns of behavior or thought and to make more healthy choices.
Our research has shown that the benefits of these experiences can last as long as 14 months, often longer, and that many participants characterize their psilocybin experience as among the most profound and personally meaningful experience of their lives.
Do participants experience any adverse effects? If so, how are they managed?
Sometimes, despite all the preparation, screening, and support we provide, some participants can have frightening experiences, such as fear and anxiety during the session. When that occurs, it is often shorted lived. The psychological preparation we provide before the session and the psychological support we provide during the session are important for managing such effects.
We provide support and encourage participants to stay with that experience, which may open to experiences of deep meaning or insight. A number of people report that these psychologically challenging states are a valuable part of the overall experience.
We conducted a survey of roughly 2,000 people who took high doses of psilocybin mushrooms and then had a challenging experience. About 10% reported they put themselves or others at risk of physical harm. Of more concern, of those whose experience occurred more than 1 year before, 8% sought treatment for enduring psychological symptoms. These findings underscore potential risks of psilocybin use but do not provide an estimate of the actual incidence of such effects.
Importantly, in our research at Johns Hopkins, we have not observed such effects in over 700 sessions that we have conducted with almost 400 participants, likely because we thoroughly screen and prepare participants and support them after they have completed the study. The potential for serious lasting harm represents a concern and points to the importance of adequate screening and aftercare.
What are the implications for future therapeutics?
We are living in exciting times, in terms of psychedelic research. The potential for a single treatment with a classic psychedelic to produce rapid and sustained therapeutic effects, possibly across a range of psychiatric conditions, is unprecedented in psychiatry. The effect appears to be an “inverse PTSD effect.”
In PTSD, a single exposure to a traumatic event can rewire the nervous system to the point that it produces enduring harm and toxicity. In the case of psychedelics, a single exposure appears to have enduring positive effects in worldview, mood, attitude, behavior, and overall life satisfaction. We can look forward to continued growth and expansion of this research including the refinement of protocols for a variety of therapeutic indications and to the development of a variety of new classic psychedelic compounds.
A version of this article originally appeared on Medscape.com.
Biden plan to lower Medicare eligibility age to 60 faces hostility from hospitals
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Fenway data, the final frontier
Data, as we all know, have taken over the world. ”
Statistical objectivity is in, individuality is out. You may have taught for 30 years and gained a sense for which child has a problem that needs intervention and which one just needs patience and time to develop. You may have managed patients for decades and have a hunch about who needs immediate help and who can be watched. But “senses” and “hunches” can’t be measured and therefore do not exist, or better, don’t count. Numbers count!
Data-obsession reflects what Germans call the Zeitgeist, the spirit of the age. But the Germans will have to come up with a different word for our age, won’t they? Nobody can measure a “spirit.”
Still, you know the spirit’s there, when it knocks you over and stomps on you.
The one sphere of life that has resisted being reduced to numbers is sports. In sports, you don’t need complex analysis to know who’s No. 1 and who’s number everything else. No. 1 crosses the finish line first, wins the most games, knocks out the opponent. The one lying on the mat is No. 2.
Of course, sports always had lots of numbers. Baseball fans have always known about batting averages, runs batted in, earned run averages. But there were always those individual intangibles that goggle the eyes of small boys and keep sportswriters in business: this athlete’s “ferocious drive,” that one’s “will to win,” the way a third “always comes through in the clutch.” Pitchers who couldn’t throw fast anymore were “crafty.” Grizzled, tobacco-chewing scouts could sense which youngster “looked like a ballplayer.”
As if you didn’t already know, you can tell how old I am to talk this way. Bill James and his statistical acolytes put paid to that old kind of thinking a long time ago. Read Moneyball or see the movie. In sports too, it’s now all about the stats.
To generate flagging interest among the young for America’s now-stodgy pastime, Major League Baseball has brought out Statcast 2.0., which adds, according to a recent news story, “Doppler-based tracking of pitch velocity, exit velocity, launch angles, and spin rates, and defensive tracking of players.” Multicamera arrays produce “biomechanical imaging and skeletal models that can help pitchers with delivery issues or batters with swing path quandaries.”
And so we have lots of new data to ponder: exit velocity – how fast a hit ball leaves the bat; launch angle – what angle it leaves at; spin rate – how fast a thrown curveball spins; and defensive tracking – how many feet this shortstop can move left to snag a ground ball, or a right-fielder to catch a fly. And there are new, composite stats, like OPS (on-base plus slugging). I will not try to explain OPS, because it is a mathematical abstraction that I cannot grasp. It signifies a blend of on-base percentage and slugging percentage, which to me is like what you get when you blend a tomato with a broccoli. Or something.
And, stats aside, you do still have to win. Not long ago the Boston Red Sox had a relief pitcher whose spin rate was splendid, but he couldn’t get anybody out.
The real aim of the new broadcast innovations noted above comes at the end of the report:
In an effort to at least reach, if not grow, a younger fan base, MLB from now on will focus on video engagement, gaming, and augmented reality on Snapchat.
You got it: the goal is to reduce baseball to a video game, and its players to gaming characters, perhaps with big contracts and marketing deals. Hey, check out that dude’s OPS!
You can’t measure a Zeitgeist, but you certainly know when it’s sitting on your chest. Your respirations get depressed. Measurably.
Yeah, I sound like every cranky old man in history. But hey – I’m Emeritus! See this column’s title!
In addition, the article has one more detail:
Curiosity about whether a fly ball to deep right field at Fenway Park would be a home run at Yankee Stadium can be satisfied by overlaying the Yankee Stadium footprint on top of Fenway.
Maybe it would satisfy you, buddy, but anything that superimposes Yankee Stadium on top of Fenway Park dissatisfies me by a factor of 6.7!
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available online. Write to him at dermnews@mdedge.com.
Data, as we all know, have taken over the world. ”
Statistical objectivity is in, individuality is out. You may have taught for 30 years and gained a sense for which child has a problem that needs intervention and which one just needs patience and time to develop. You may have managed patients for decades and have a hunch about who needs immediate help and who can be watched. But “senses” and “hunches” can’t be measured and therefore do not exist, or better, don’t count. Numbers count!
Data-obsession reflects what Germans call the Zeitgeist, the spirit of the age. But the Germans will have to come up with a different word for our age, won’t they? Nobody can measure a “spirit.”
Still, you know the spirit’s there, when it knocks you over and stomps on you.
The one sphere of life that has resisted being reduced to numbers is sports. In sports, you don’t need complex analysis to know who’s No. 1 and who’s number everything else. No. 1 crosses the finish line first, wins the most games, knocks out the opponent. The one lying on the mat is No. 2.
Of course, sports always had lots of numbers. Baseball fans have always known about batting averages, runs batted in, earned run averages. But there were always those individual intangibles that goggle the eyes of small boys and keep sportswriters in business: this athlete’s “ferocious drive,” that one’s “will to win,” the way a third “always comes through in the clutch.” Pitchers who couldn’t throw fast anymore were “crafty.” Grizzled, tobacco-chewing scouts could sense which youngster “looked like a ballplayer.”
As if you didn’t already know, you can tell how old I am to talk this way. Bill James and his statistical acolytes put paid to that old kind of thinking a long time ago. Read Moneyball or see the movie. In sports too, it’s now all about the stats.
To generate flagging interest among the young for America’s now-stodgy pastime, Major League Baseball has brought out Statcast 2.0., which adds, according to a recent news story, “Doppler-based tracking of pitch velocity, exit velocity, launch angles, and spin rates, and defensive tracking of players.” Multicamera arrays produce “biomechanical imaging and skeletal models that can help pitchers with delivery issues or batters with swing path quandaries.”
And so we have lots of new data to ponder: exit velocity – how fast a hit ball leaves the bat; launch angle – what angle it leaves at; spin rate – how fast a thrown curveball spins; and defensive tracking – how many feet this shortstop can move left to snag a ground ball, or a right-fielder to catch a fly. And there are new, composite stats, like OPS (on-base plus slugging). I will not try to explain OPS, because it is a mathematical abstraction that I cannot grasp. It signifies a blend of on-base percentage and slugging percentage, which to me is like what you get when you blend a tomato with a broccoli. Or something.
And, stats aside, you do still have to win. Not long ago the Boston Red Sox had a relief pitcher whose spin rate was splendid, but he couldn’t get anybody out.
The real aim of the new broadcast innovations noted above comes at the end of the report:
In an effort to at least reach, if not grow, a younger fan base, MLB from now on will focus on video engagement, gaming, and augmented reality on Snapchat.
You got it: the goal is to reduce baseball to a video game, and its players to gaming characters, perhaps with big contracts and marketing deals. Hey, check out that dude’s OPS!
You can’t measure a Zeitgeist, but you certainly know when it’s sitting on your chest. Your respirations get depressed. Measurably.
Yeah, I sound like every cranky old man in history. But hey – I’m Emeritus! See this column’s title!
In addition, the article has one more detail:
Curiosity about whether a fly ball to deep right field at Fenway Park would be a home run at Yankee Stadium can be satisfied by overlaying the Yankee Stadium footprint on top of Fenway.
Maybe it would satisfy you, buddy, but anything that superimposes Yankee Stadium on top of Fenway Park dissatisfies me by a factor of 6.7!
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available online. Write to him at dermnews@mdedge.com.
Data, as we all know, have taken over the world. ”
Statistical objectivity is in, individuality is out. You may have taught for 30 years and gained a sense for which child has a problem that needs intervention and which one just needs patience and time to develop. You may have managed patients for decades and have a hunch about who needs immediate help and who can be watched. But “senses” and “hunches” can’t be measured and therefore do not exist, or better, don’t count. Numbers count!
Data-obsession reflects what Germans call the Zeitgeist, the spirit of the age. But the Germans will have to come up with a different word for our age, won’t they? Nobody can measure a “spirit.”
Still, you know the spirit’s there, when it knocks you over and stomps on you.
The one sphere of life that has resisted being reduced to numbers is sports. In sports, you don’t need complex analysis to know who’s No. 1 and who’s number everything else. No. 1 crosses the finish line first, wins the most games, knocks out the opponent. The one lying on the mat is No. 2.
Of course, sports always had lots of numbers. Baseball fans have always known about batting averages, runs batted in, earned run averages. But there were always those individual intangibles that goggle the eyes of small boys and keep sportswriters in business: this athlete’s “ferocious drive,” that one’s “will to win,” the way a third “always comes through in the clutch.” Pitchers who couldn’t throw fast anymore were “crafty.” Grizzled, tobacco-chewing scouts could sense which youngster “looked like a ballplayer.”
As if you didn’t already know, you can tell how old I am to talk this way. Bill James and his statistical acolytes put paid to that old kind of thinking a long time ago. Read Moneyball or see the movie. In sports too, it’s now all about the stats.
To generate flagging interest among the young for America’s now-stodgy pastime, Major League Baseball has brought out Statcast 2.0., which adds, according to a recent news story, “Doppler-based tracking of pitch velocity, exit velocity, launch angles, and spin rates, and defensive tracking of players.” Multicamera arrays produce “biomechanical imaging and skeletal models that can help pitchers with delivery issues or batters with swing path quandaries.”
And so we have lots of new data to ponder: exit velocity – how fast a hit ball leaves the bat; launch angle – what angle it leaves at; spin rate – how fast a thrown curveball spins; and defensive tracking – how many feet this shortstop can move left to snag a ground ball, or a right-fielder to catch a fly. And there are new, composite stats, like OPS (on-base plus slugging). I will not try to explain OPS, because it is a mathematical abstraction that I cannot grasp. It signifies a blend of on-base percentage and slugging percentage, which to me is like what you get when you blend a tomato with a broccoli. Or something.
And, stats aside, you do still have to win. Not long ago the Boston Red Sox had a relief pitcher whose spin rate was splendid, but he couldn’t get anybody out.
The real aim of the new broadcast innovations noted above comes at the end of the report:
In an effort to at least reach, if not grow, a younger fan base, MLB from now on will focus on video engagement, gaming, and augmented reality on Snapchat.
You got it: the goal is to reduce baseball to a video game, and its players to gaming characters, perhaps with big contracts and marketing deals. Hey, check out that dude’s OPS!
You can’t measure a Zeitgeist, but you certainly know when it’s sitting on your chest. Your respirations get depressed. Measurably.
Yeah, I sound like every cranky old man in history. But hey – I’m Emeritus! See this column’s title!
In addition, the article has one more detail:
Curiosity about whether a fly ball to deep right field at Fenway Park would be a home run at Yankee Stadium can be satisfied by overlaying the Yankee Stadium footprint on top of Fenway.
Maybe it would satisfy you, buddy, but anything that superimposes Yankee Stadium on top of Fenway Park dissatisfies me by a factor of 6.7!
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available online. Write to him at dermnews@mdedge.com.
Trump could clean house at health agencies
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.