User login
Can a Healthy Gut Microbiome Prevent Kidney Stones?
TOPLINE:
Gut and urinary microbiota alterations appear to play key roles in the development of kidney stones, suggesting that
Kidney stones are commonly formed by calcium oxalate produced by the body, but the findings suggest that in efforts to better understand the formation and prevention of the stones, focus should shift away from the long-held focus on specific gut bacterium, such as Oxalobacter formigenesm,to apparent systems-level microbial imbalances.
METHODOLOGY:
- The authors evaluated the gut, urinary, and oral microbiota of 83 patients having kidney stones surgically removed at St. Joseph’s Hospital in London, Ontario, and compared them to 30 healthy controls recruited between August 2015 and January 2019.
- Analysis using measures including shotgun metagenomic sequencing were used to identify specific gut bacteria and the genetic capabilities of the bacteria, and simpler sequencing was conducted using oral and urinary microbiota samples.
- Patients had no antibiotic exposure within the previous 90 days.
TAKEAWAY:
- Significant differences were observed in disturbances in microbiota in all three microbiomes among those who did and did not form kidney stones.
- Patients who formed kidney stones also showed more antibiotic-resistant genes, suggesting greater exposure to antimicrobials.
- Those who had formed kidney stones had reduced taxonomic and functional diversity compared with healthy controls.
- Core functional bioenergetic pathways had been replaced with virulence-associated gene markers in gut and urinary microbiota in those with kidney stones.
- And community network microbiota had collapsed among those with kidney stones.
- “These multisite microbial community shifts may be the result of deleterious environmental factors including antibiotic exposure,” the authors speculated.
- However, no differences were observed between the cohorts in terms of oxalate metabolism — commonly considered a culprit in kidney stone formation.
IN PRACTICE:
- “We found not only that those who got kidney stones had an unhealthy microbiome, including a gut microbiome that was more likely to excrete toxins to the kidneys, but also that they were antibiotic resistant,” explains senior author Jeremy Burton, PhD, the Lawson Scientist and Research Chair of Human Microbiome and Probiotics at St. Joseph’s Health Care London, in a press statement.
- “We conclude that multisite microbiota alteration is a hallmark of stone formation, and kidney stone disease treatment should consider microbial functional restoration and the avoidance of aberrant modulators such as poor diet and antibiotics, where applicable, to prevent stone recurrence,” the authors further wrote in the study.
- “Based on these findings, we suggest that the historic emphasis put on O formigenes and other direct oxalate-handling taxa should be discontinued in favor of mechanistic study into the apparent systems-level microbial imbalances in kidney stone formers,” they said.
SOURCE:
The study was published in Microbiome.
It was conducted by the first author Kait F. Al, PhD, of the department of microbiology and immunology, The University of Western Ontario, London, and colleagues.
LIMITATIONS:
The sampling techniques used in the study provide relative compositional but not absolute abundance information, and the sequencing methods do not provide taxonomic annotation to the species level in all cases.
“For this reason, as well as the problematic nature of comparison across sequencing methodologies, caution should be taken when comparing these data and taxonomic annotations in future studies,” the authors noted.
Furthermore, as the study was observational, causality of stone disease cannot be established to the differences detected between the microbiota of healthy control individuals and those with kidney stones.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
Gut and urinary microbiota alterations appear to play key roles in the development of kidney stones, suggesting that
Kidney stones are commonly formed by calcium oxalate produced by the body, but the findings suggest that in efforts to better understand the formation and prevention of the stones, focus should shift away from the long-held focus on specific gut bacterium, such as Oxalobacter formigenesm,to apparent systems-level microbial imbalances.
METHODOLOGY:
- The authors evaluated the gut, urinary, and oral microbiota of 83 patients having kidney stones surgically removed at St. Joseph’s Hospital in London, Ontario, and compared them to 30 healthy controls recruited between August 2015 and January 2019.
- Analysis using measures including shotgun metagenomic sequencing were used to identify specific gut bacteria and the genetic capabilities of the bacteria, and simpler sequencing was conducted using oral and urinary microbiota samples.
- Patients had no antibiotic exposure within the previous 90 days.
TAKEAWAY:
- Significant differences were observed in disturbances in microbiota in all three microbiomes among those who did and did not form kidney stones.
- Patients who formed kidney stones also showed more antibiotic-resistant genes, suggesting greater exposure to antimicrobials.
- Those who had formed kidney stones had reduced taxonomic and functional diversity compared with healthy controls.
- Core functional bioenergetic pathways had been replaced with virulence-associated gene markers in gut and urinary microbiota in those with kidney stones.
- And community network microbiota had collapsed among those with kidney stones.
- “These multisite microbial community shifts may be the result of deleterious environmental factors including antibiotic exposure,” the authors speculated.
- However, no differences were observed between the cohorts in terms of oxalate metabolism — commonly considered a culprit in kidney stone formation.
IN PRACTICE:
- “We found not only that those who got kidney stones had an unhealthy microbiome, including a gut microbiome that was more likely to excrete toxins to the kidneys, but also that they were antibiotic resistant,” explains senior author Jeremy Burton, PhD, the Lawson Scientist and Research Chair of Human Microbiome and Probiotics at St. Joseph’s Health Care London, in a press statement.
- “We conclude that multisite microbiota alteration is a hallmark of stone formation, and kidney stone disease treatment should consider microbial functional restoration and the avoidance of aberrant modulators such as poor diet and antibiotics, where applicable, to prevent stone recurrence,” the authors further wrote in the study.
- “Based on these findings, we suggest that the historic emphasis put on O formigenes and other direct oxalate-handling taxa should be discontinued in favor of mechanistic study into the apparent systems-level microbial imbalances in kidney stone formers,” they said.
SOURCE:
The study was published in Microbiome.
It was conducted by the first author Kait F. Al, PhD, of the department of microbiology and immunology, The University of Western Ontario, London, and colleagues.
LIMITATIONS:
The sampling techniques used in the study provide relative compositional but not absolute abundance information, and the sequencing methods do not provide taxonomic annotation to the species level in all cases.
“For this reason, as well as the problematic nature of comparison across sequencing methodologies, caution should be taken when comparing these data and taxonomic annotations in future studies,” the authors noted.
Furthermore, as the study was observational, causality of stone disease cannot be established to the differences detected between the microbiota of healthy control individuals and those with kidney stones.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
Gut and urinary microbiota alterations appear to play key roles in the development of kidney stones, suggesting that
Kidney stones are commonly formed by calcium oxalate produced by the body, but the findings suggest that in efforts to better understand the formation and prevention of the stones, focus should shift away from the long-held focus on specific gut bacterium, such as Oxalobacter formigenesm,to apparent systems-level microbial imbalances.
METHODOLOGY:
- The authors evaluated the gut, urinary, and oral microbiota of 83 patients having kidney stones surgically removed at St. Joseph’s Hospital in London, Ontario, and compared them to 30 healthy controls recruited between August 2015 and January 2019.
- Analysis using measures including shotgun metagenomic sequencing were used to identify specific gut bacteria and the genetic capabilities of the bacteria, and simpler sequencing was conducted using oral and urinary microbiota samples.
- Patients had no antibiotic exposure within the previous 90 days.
TAKEAWAY:
- Significant differences were observed in disturbances in microbiota in all three microbiomes among those who did and did not form kidney stones.
- Patients who formed kidney stones also showed more antibiotic-resistant genes, suggesting greater exposure to antimicrobials.
- Those who had formed kidney stones had reduced taxonomic and functional diversity compared with healthy controls.
- Core functional bioenergetic pathways had been replaced with virulence-associated gene markers in gut and urinary microbiota in those with kidney stones.
- And community network microbiota had collapsed among those with kidney stones.
- “These multisite microbial community shifts may be the result of deleterious environmental factors including antibiotic exposure,” the authors speculated.
- However, no differences were observed between the cohorts in terms of oxalate metabolism — commonly considered a culprit in kidney stone formation.
IN PRACTICE:
- “We found not only that those who got kidney stones had an unhealthy microbiome, including a gut microbiome that was more likely to excrete toxins to the kidneys, but also that they were antibiotic resistant,” explains senior author Jeremy Burton, PhD, the Lawson Scientist and Research Chair of Human Microbiome and Probiotics at St. Joseph’s Health Care London, in a press statement.
- “We conclude that multisite microbiota alteration is a hallmark of stone formation, and kidney stone disease treatment should consider microbial functional restoration and the avoidance of aberrant modulators such as poor diet and antibiotics, where applicable, to prevent stone recurrence,” the authors further wrote in the study.
- “Based on these findings, we suggest that the historic emphasis put on O formigenes and other direct oxalate-handling taxa should be discontinued in favor of mechanistic study into the apparent systems-level microbial imbalances in kidney stone formers,” they said.
SOURCE:
The study was published in Microbiome.
It was conducted by the first author Kait F. Al, PhD, of the department of microbiology and immunology, The University of Western Ontario, London, and colleagues.
LIMITATIONS:
The sampling techniques used in the study provide relative compositional but not absolute abundance information, and the sequencing methods do not provide taxonomic annotation to the species level in all cases.
“For this reason, as well as the problematic nature of comparison across sequencing methodologies, caution should be taken when comparing these data and taxonomic annotations in future studies,” the authors noted.
Furthermore, as the study was observational, causality of stone disease cannot be established to the differences detected between the microbiota of healthy control individuals and those with kidney stones.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
Report: CKD Severity Linked to Thinning of Retina, Choroid Layers
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Children who are overweight at risk for chronic kidney disease
TOPLINE
, with the association, though weaker, still significant among those who do not develop type 2 diabetes or hypertension, in a large cohort study.
METHODOLOGY
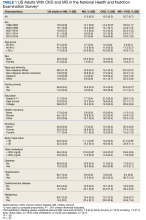
- The study included data on 593,660 adolescents aged 16-20, born after January 1, 1975, who had medical assessments as part of mandatory military service in Israel.
- The mean age at study entry was 17.2 and 54.5% were male.
- Early CKD was defined as stage 1 to 2 CKD with moderately or severely increased albuminuria, with an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher.
- The study excluded those with kidney pathology, albuminuria, hypertension, dysglycemia, or missing blood pressure or BMI data.
- Participants were followed up until early CKD onset, death, the last day insured, or August 23, 2020.
TAKEAWAY
- With a mean follow-up of 13.4 years, 1963 adolescents (0.3%) overall developed early chronic kidney disease. Among males, an increased risk of developing CKD was observed with a high-normal BMI in adolescence (hazard ratio [HR], 1.8); with overweight BMI (HR, 4.0); with mild obesity (HR, 6.7); and severe obesity (HR, 9.4).
- Among females, the increased risk was also observed with high-normal BMI (HR 1.4); overweight (HR, 2.3); mild obesity (HR, 2.7); and severe obesity (HR, 4.3).
- In excluding those who developed diabetes or hypertension, the overall rate of early CKD in the cohort was 0.2%.
- For males without diabetes or hypertension, the adjusted HR for early CKD with high-normal weight was 1.2; for overweight, HR 1.6; for mild obesity, HR 2.2; and for severe obesity, HR 2.7.
- For females without diabetes or hypertension, the corresponding increased risk for early CKD was HR 1.2 for high-normal BMI; HR 1.8 for overweight; 1.5 for mild obesity and 2.3 for severe obesity.
IN PRACTICE
“These findings suggest that adolescent obesity is a major risk factor for early CKD in young adulthood; this underscores the importance of mitigating adolescent obesity rates and managing risk factors for kidney disease in adolescents with high BMI,” the authors report.
“The association was evident even in persons with high-normal BMI in adolescence, was more pronounced in men, and appeared before the age of 30 years,” they say.
“Given the increasing obesity rates among adolescents, our findings are a harbinger of the potentially preventable increasing burden of CKD and subsequent cardiovascular disease.”
SOURCE
The study was conducted by first author Avishai M. Tsur, MD, of the Israel Defense Forces, Medical Corps, Tel Hashomer, Ramat Gan, Israel and Department of Military Medicine, Hebrew University of Jerusalem Faculty of Medicine, Jerusalem, Israel, and colleagues. The study was published online in JAMA Pediatrics.
LIMITATIONS
The study lacked longitudinal data on clinical and lifestyle factors, including stress, diet and physical activity. While adolescents were screened using urine dipstick, a lack of serum creatinine measurements could have missed some adolescents with reduced eGFR at the study entry. The generalizability of the results is limited by the lack of people from West Africa and East Asia in the study population.
DISCLOSURES
Coauthor Josef Coresh, MD, reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE
, with the association, though weaker, still significant among those who do not develop type 2 diabetes or hypertension, in a large cohort study.
METHODOLOGY
- The study included data on 593,660 adolescents aged 16-20, born after January 1, 1975, who had medical assessments as part of mandatory military service in Israel.
- The mean age at study entry was 17.2 and 54.5% were male.
- Early CKD was defined as stage 1 to 2 CKD with moderately or severely increased albuminuria, with an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher.
- The study excluded those with kidney pathology, albuminuria, hypertension, dysglycemia, or missing blood pressure or BMI data.
- Participants were followed up until early CKD onset, death, the last day insured, or August 23, 2020.
TAKEAWAY
- With a mean follow-up of 13.4 years, 1963 adolescents (0.3%) overall developed early chronic kidney disease. Among males, an increased risk of developing CKD was observed with a high-normal BMI in adolescence (hazard ratio [HR], 1.8); with overweight BMI (HR, 4.0); with mild obesity (HR, 6.7); and severe obesity (HR, 9.4).
- Among females, the increased risk was also observed with high-normal BMI (HR 1.4); overweight (HR, 2.3); mild obesity (HR, 2.7); and severe obesity (HR, 4.3).
- In excluding those who developed diabetes or hypertension, the overall rate of early CKD in the cohort was 0.2%.
- For males without diabetes or hypertension, the adjusted HR for early CKD with high-normal weight was 1.2; for overweight, HR 1.6; for mild obesity, HR 2.2; and for severe obesity, HR 2.7.
- For females without diabetes or hypertension, the corresponding increased risk for early CKD was HR 1.2 for high-normal BMI; HR 1.8 for overweight; 1.5 for mild obesity and 2.3 for severe obesity.
IN PRACTICE
“These findings suggest that adolescent obesity is a major risk factor for early CKD in young adulthood; this underscores the importance of mitigating adolescent obesity rates and managing risk factors for kidney disease in adolescents with high BMI,” the authors report.
“The association was evident even in persons with high-normal BMI in adolescence, was more pronounced in men, and appeared before the age of 30 years,” they say.
“Given the increasing obesity rates among adolescents, our findings are a harbinger of the potentially preventable increasing burden of CKD and subsequent cardiovascular disease.”
SOURCE
The study was conducted by first author Avishai M. Tsur, MD, of the Israel Defense Forces, Medical Corps, Tel Hashomer, Ramat Gan, Israel and Department of Military Medicine, Hebrew University of Jerusalem Faculty of Medicine, Jerusalem, Israel, and colleagues. The study was published online in JAMA Pediatrics.
LIMITATIONS
The study lacked longitudinal data on clinical and lifestyle factors, including stress, diet and physical activity. While adolescents were screened using urine dipstick, a lack of serum creatinine measurements could have missed some adolescents with reduced eGFR at the study entry. The generalizability of the results is limited by the lack of people from West Africa and East Asia in the study population.
DISCLOSURES
Coauthor Josef Coresh, MD, reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE
, with the association, though weaker, still significant among those who do not develop type 2 diabetes or hypertension, in a large cohort study.
METHODOLOGY
- The study included data on 593,660 adolescents aged 16-20, born after January 1, 1975, who had medical assessments as part of mandatory military service in Israel.
- The mean age at study entry was 17.2 and 54.5% were male.
- Early CKD was defined as stage 1 to 2 CKD with moderately or severely increased albuminuria, with an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher.
- The study excluded those with kidney pathology, albuminuria, hypertension, dysglycemia, or missing blood pressure or BMI data.
- Participants were followed up until early CKD onset, death, the last day insured, or August 23, 2020.
TAKEAWAY
- With a mean follow-up of 13.4 years, 1963 adolescents (0.3%) overall developed early chronic kidney disease. Among males, an increased risk of developing CKD was observed with a high-normal BMI in adolescence (hazard ratio [HR], 1.8); with overweight BMI (HR, 4.0); with mild obesity (HR, 6.7); and severe obesity (HR, 9.4).
- Among females, the increased risk was also observed with high-normal BMI (HR 1.4); overweight (HR, 2.3); mild obesity (HR, 2.7); and severe obesity (HR, 4.3).
- In excluding those who developed diabetes or hypertension, the overall rate of early CKD in the cohort was 0.2%.
- For males without diabetes or hypertension, the adjusted HR for early CKD with high-normal weight was 1.2; for overweight, HR 1.6; for mild obesity, HR 2.2; and for severe obesity, HR 2.7.
- For females without diabetes or hypertension, the corresponding increased risk for early CKD was HR 1.2 for high-normal BMI; HR 1.8 for overweight; 1.5 for mild obesity and 2.3 for severe obesity.
IN PRACTICE
“These findings suggest that adolescent obesity is a major risk factor for early CKD in young adulthood; this underscores the importance of mitigating adolescent obesity rates and managing risk factors for kidney disease in adolescents with high BMI,” the authors report.
“The association was evident even in persons with high-normal BMI in adolescence, was more pronounced in men, and appeared before the age of 30 years,” they say.
“Given the increasing obesity rates among adolescents, our findings are a harbinger of the potentially preventable increasing burden of CKD and subsequent cardiovascular disease.”
SOURCE
The study was conducted by first author Avishai M. Tsur, MD, of the Israel Defense Forces, Medical Corps, Tel Hashomer, Ramat Gan, Israel and Department of Military Medicine, Hebrew University of Jerusalem Faculty of Medicine, Jerusalem, Israel, and colleagues. The study was published online in JAMA Pediatrics.
LIMITATIONS
The study lacked longitudinal data on clinical and lifestyle factors, including stress, diet and physical activity. While adolescents were screened using urine dipstick, a lack of serum creatinine measurements could have missed some adolescents with reduced eGFR at the study entry. The generalizability of the results is limited by the lack of people from West Africa and East Asia in the study population.
DISCLOSURES
Coauthor Josef Coresh, MD, reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
New KDIGO guideline encourages use of HCV-positive kidneys for HCV-negative recipients
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Meet the newest acronym in primary care: CKM
The advisory, published recently in Circulation introduces the concept of CKM health and reevaluates the relationships between obesity, diabetes, kidney disease, and cardiovascular disease (CVD).
“This approach not only raises awareness, it also empowers PCPs to diagnose and treat these conditions more holistically,” Salim Hayek, MD, associate professor of cardiovascular disease and internal medicine, and medical director of the Frankel Cardiovascular Center Clinics at the University of Michigan in Ann Arbor, said in an interview.
New CKM Staging, Testing, and Care Strategies
The advisory introduces a new scoring system that ranges from stage 0 (patients with no risk factors for CKM) through stage 4 (patients with clinical CVD in CKM syndrome). Each stage requires specific management strategies and may include screening starting at age 30 years for diabetes, hypertension, and heart failure.
“Stage 0 CKM is usually found in young people, and CKM risk factors and scores typically increase as people age,” said Sean M. Drake, MD, a primary care physician at Henry Ford Health in Sterling Heights, Michigan.
Dr. Drake advised PCPs to encourage patients who are at stage 0 to maintain ideal cardiovascular health and to monitor those at risk of progressing through the stages.
While PCPs already perform many of the tests the advisory recommends, the conditions overlap and an abnormality in one system should prompt more testing for other conditions. Additional tests, such as urine albumin-creatinine ratio, and more frequent glomerular filtration rate and lipid profile are advised, according to Dr. Drake.
“There also appears to be a role for additional cardiac testing, including echocardiograms and coronary CT scans, and for liver fibrosis screening,” Dr. Drake said. “Medications such as SGLT2 inhibitors, GLP-1 receptor agonists, and ACE inhibitors, beyond current routine use, are emphasized.”
To better characterize body composition and help diagnose metabolic syndrome, the advisory also recommends measuring waist circumference, which is not routine practice, noted Joshua J. Joseph, MD, MPH, an associate professor of endocrinology, diabetes, and metabolism at The Ohio State University Wexner Medical Center in Columbus, and a co-author of the advisory.
Recognizing the interconnected nature of cardiac, kidney, and metabolic diseases encourages a shift in mindset for clinicians, according to Neha Pagidipati, MD, MPH, a cardiologist at Duke Health in Durham, North Carolina.
“We have often been trained to focus on the specific problem in front of us,” Dr. Pagidipati said. “We need to be hyper-aware that many patients we see are at risk for multiple CKM entities. We need to be proactive about screening for and treating these when appropriate.”
The advisory emphasizes the need for CKM coordinators to support teams of clinicians from primary care, cardiology, endocrinology, nephrology, nursing, and pharmacy, as well as social workers, care navigators, or community health workers, Dr. Joseph said.
“The advisory repositions the PCP at the forefront of CKM care coordination, marking a departure from the traditional model where subspecialists primarily manage complications,” Dr. Hayek added.
Changes to Payment
The new recommendations are consistent with current management guidelines for obesity, hypertriglyceridemia, hypertension, type 2 diabetes, and chronic kidney disease.
“The advisory provides integrated algorithms for cardiovascular prevention and management, with specific therapeutic guidance tied to CKM stages, bringing together the current evidence for best practices from the various guidelines and filling gaps in a unified approach,” Dr. Joseph said.
In addition, the advisory draws attention to the care of younger patients, who may be at increased risk for cardiovascular disease due to lifestyle factors, according to Nishant Shah, MD, assistant professor of medicine at Duke.
“It considers barriers to care that prevent people from optimizing their cardiovascular health,” Dr. Shah said.
Although the advisory does not specify proposed payment changes to support the new care model, the move towards value-based care may require billing practices that accommodate integrated care as well as more frequent and more specialized testing, Dr. Hayek said.
“The advisory is an empowering tool for PCPs, underscoring their critical role in healthcare,” Dr. Hayek said. “It encourages PCPs to advocate for integrated care within their practices and to consider workflow adjustments that enhance the identification and initiation of preventive care for at-risk patients.”
Funding information was not provided.
Dr. Joseph reports no relevant financial involvements; several advisory co-authors report financial involvements with pharmaceutical companies. Dr. Pagidipati reports relevant financial involvement with pharmaceutical companies. Dr. Hayek, Dr. Drake, and Dr. Shah report no relevant financial involvements. Dr. Joseph is an author of the advisory. Dr. Pagidipati, Dr. Hayek, Dr. Drake, and Dr. Shah were not involved in the writing of the advisory.
A version of this article appeared on Medscape.com.
The advisory, published recently in Circulation introduces the concept of CKM health and reevaluates the relationships between obesity, diabetes, kidney disease, and cardiovascular disease (CVD).
“This approach not only raises awareness, it also empowers PCPs to diagnose and treat these conditions more holistically,” Salim Hayek, MD, associate professor of cardiovascular disease and internal medicine, and medical director of the Frankel Cardiovascular Center Clinics at the University of Michigan in Ann Arbor, said in an interview.
New CKM Staging, Testing, and Care Strategies
The advisory introduces a new scoring system that ranges from stage 0 (patients with no risk factors for CKM) through stage 4 (patients with clinical CVD in CKM syndrome). Each stage requires specific management strategies and may include screening starting at age 30 years for diabetes, hypertension, and heart failure.
“Stage 0 CKM is usually found in young people, and CKM risk factors and scores typically increase as people age,” said Sean M. Drake, MD, a primary care physician at Henry Ford Health in Sterling Heights, Michigan.
Dr. Drake advised PCPs to encourage patients who are at stage 0 to maintain ideal cardiovascular health and to monitor those at risk of progressing through the stages.
While PCPs already perform many of the tests the advisory recommends, the conditions overlap and an abnormality in one system should prompt more testing for other conditions. Additional tests, such as urine albumin-creatinine ratio, and more frequent glomerular filtration rate and lipid profile are advised, according to Dr. Drake.
“There also appears to be a role for additional cardiac testing, including echocardiograms and coronary CT scans, and for liver fibrosis screening,” Dr. Drake said. “Medications such as SGLT2 inhibitors, GLP-1 receptor agonists, and ACE inhibitors, beyond current routine use, are emphasized.”
To better characterize body composition and help diagnose metabolic syndrome, the advisory also recommends measuring waist circumference, which is not routine practice, noted Joshua J. Joseph, MD, MPH, an associate professor of endocrinology, diabetes, and metabolism at The Ohio State University Wexner Medical Center in Columbus, and a co-author of the advisory.
Recognizing the interconnected nature of cardiac, kidney, and metabolic diseases encourages a shift in mindset for clinicians, according to Neha Pagidipati, MD, MPH, a cardiologist at Duke Health in Durham, North Carolina.
“We have often been trained to focus on the specific problem in front of us,” Dr. Pagidipati said. “We need to be hyper-aware that many patients we see are at risk for multiple CKM entities. We need to be proactive about screening for and treating these when appropriate.”
The advisory emphasizes the need for CKM coordinators to support teams of clinicians from primary care, cardiology, endocrinology, nephrology, nursing, and pharmacy, as well as social workers, care navigators, or community health workers, Dr. Joseph said.
“The advisory repositions the PCP at the forefront of CKM care coordination, marking a departure from the traditional model where subspecialists primarily manage complications,” Dr. Hayek added.
Changes to Payment
The new recommendations are consistent with current management guidelines for obesity, hypertriglyceridemia, hypertension, type 2 diabetes, and chronic kidney disease.
“The advisory provides integrated algorithms for cardiovascular prevention and management, with specific therapeutic guidance tied to CKM stages, bringing together the current evidence for best practices from the various guidelines and filling gaps in a unified approach,” Dr. Joseph said.
In addition, the advisory draws attention to the care of younger patients, who may be at increased risk for cardiovascular disease due to lifestyle factors, according to Nishant Shah, MD, assistant professor of medicine at Duke.
“It considers barriers to care that prevent people from optimizing their cardiovascular health,” Dr. Shah said.
Although the advisory does not specify proposed payment changes to support the new care model, the move towards value-based care may require billing practices that accommodate integrated care as well as more frequent and more specialized testing, Dr. Hayek said.
“The advisory is an empowering tool for PCPs, underscoring their critical role in healthcare,” Dr. Hayek said. “It encourages PCPs to advocate for integrated care within their practices and to consider workflow adjustments that enhance the identification and initiation of preventive care for at-risk patients.”
Funding information was not provided.
Dr. Joseph reports no relevant financial involvements; several advisory co-authors report financial involvements with pharmaceutical companies. Dr. Pagidipati reports relevant financial involvement with pharmaceutical companies. Dr. Hayek, Dr. Drake, and Dr. Shah report no relevant financial involvements. Dr. Joseph is an author of the advisory. Dr. Pagidipati, Dr. Hayek, Dr. Drake, and Dr. Shah were not involved in the writing of the advisory.
A version of this article appeared on Medscape.com.
The advisory, published recently in Circulation introduces the concept of CKM health and reevaluates the relationships between obesity, diabetes, kidney disease, and cardiovascular disease (CVD).
“This approach not only raises awareness, it also empowers PCPs to diagnose and treat these conditions more holistically,” Salim Hayek, MD, associate professor of cardiovascular disease and internal medicine, and medical director of the Frankel Cardiovascular Center Clinics at the University of Michigan in Ann Arbor, said in an interview.
New CKM Staging, Testing, and Care Strategies
The advisory introduces a new scoring system that ranges from stage 0 (patients with no risk factors for CKM) through stage 4 (patients with clinical CVD in CKM syndrome). Each stage requires specific management strategies and may include screening starting at age 30 years for diabetes, hypertension, and heart failure.
“Stage 0 CKM is usually found in young people, and CKM risk factors and scores typically increase as people age,” said Sean M. Drake, MD, a primary care physician at Henry Ford Health in Sterling Heights, Michigan.
Dr. Drake advised PCPs to encourage patients who are at stage 0 to maintain ideal cardiovascular health and to monitor those at risk of progressing through the stages.
While PCPs already perform many of the tests the advisory recommends, the conditions overlap and an abnormality in one system should prompt more testing for other conditions. Additional tests, such as urine albumin-creatinine ratio, and more frequent glomerular filtration rate and lipid profile are advised, according to Dr. Drake.
“There also appears to be a role for additional cardiac testing, including echocardiograms and coronary CT scans, and for liver fibrosis screening,” Dr. Drake said. “Medications such as SGLT2 inhibitors, GLP-1 receptor agonists, and ACE inhibitors, beyond current routine use, are emphasized.”
To better characterize body composition and help diagnose metabolic syndrome, the advisory also recommends measuring waist circumference, which is not routine practice, noted Joshua J. Joseph, MD, MPH, an associate professor of endocrinology, diabetes, and metabolism at The Ohio State University Wexner Medical Center in Columbus, and a co-author of the advisory.
Recognizing the interconnected nature of cardiac, kidney, and metabolic diseases encourages a shift in mindset for clinicians, according to Neha Pagidipati, MD, MPH, a cardiologist at Duke Health in Durham, North Carolina.
“We have often been trained to focus on the specific problem in front of us,” Dr. Pagidipati said. “We need to be hyper-aware that many patients we see are at risk for multiple CKM entities. We need to be proactive about screening for and treating these when appropriate.”
The advisory emphasizes the need for CKM coordinators to support teams of clinicians from primary care, cardiology, endocrinology, nephrology, nursing, and pharmacy, as well as social workers, care navigators, or community health workers, Dr. Joseph said.
“The advisory repositions the PCP at the forefront of CKM care coordination, marking a departure from the traditional model where subspecialists primarily manage complications,” Dr. Hayek added.
Changes to Payment
The new recommendations are consistent with current management guidelines for obesity, hypertriglyceridemia, hypertension, type 2 diabetes, and chronic kidney disease.
“The advisory provides integrated algorithms for cardiovascular prevention and management, with specific therapeutic guidance tied to CKM stages, bringing together the current evidence for best practices from the various guidelines and filling gaps in a unified approach,” Dr. Joseph said.
In addition, the advisory draws attention to the care of younger patients, who may be at increased risk for cardiovascular disease due to lifestyle factors, according to Nishant Shah, MD, assistant professor of medicine at Duke.
“It considers barriers to care that prevent people from optimizing their cardiovascular health,” Dr. Shah said.
Although the advisory does not specify proposed payment changes to support the new care model, the move towards value-based care may require billing practices that accommodate integrated care as well as more frequent and more specialized testing, Dr. Hayek said.
“The advisory is an empowering tool for PCPs, underscoring their critical role in healthcare,” Dr. Hayek said. “It encourages PCPs to advocate for integrated care within their practices and to consider workflow adjustments that enhance the identification and initiation of preventive care for at-risk patients.”
Funding information was not provided.
Dr. Joseph reports no relevant financial involvements; several advisory co-authors report financial involvements with pharmaceutical companies. Dr. Pagidipati reports relevant financial involvement with pharmaceutical companies. Dr. Hayek, Dr. Drake, and Dr. Shah report no relevant financial involvements. Dr. Joseph is an author of the advisory. Dr. Pagidipati, Dr. Hayek, Dr. Drake, and Dr. Shah were not involved in the writing of the advisory.
A version of this article appeared on Medscape.com.
FROM CIRCULATION
Reducing albumin improves kidney and heart function in people with type 2 diabetes
TOPLINE:
Reducing the urine albumin-to-creatinine ratio (UACR) significantly reduces kidney risk in people with type 2 diabetes, per new research in the Annals of Internal Medicine.
METHODOLOGY:
- Post hoc retrospective analysis of two phase 3 double-blind trials of finerenone in people with type 2 diabetes and chronic kidney disease
- Quantify the long-term health effects of reducing UACR within 4 months of taking finerenone by examining the records of 12,512 participants with an equal chance of receiving finerenone or placebo
- Isolate the impact of UACR reduction on kidney function and cardiovascular function by tracking health indicators related to the kidneys and the heart in participants for up to 4 years
TAKEAWAY:
- Over half of participants who received finerenone had reduced UACR by at least 30% from the baseline of 514 mg/g at the 4-month point after starting treatment, and the median UACR reduction in this group was 33%.
- By 4 months, a little over a quarter of participants who received the placebo had reduced their UACR levels by at least 30%, and the median UACR reduction in this group was 2.6%.
- A UACR reduction of at least 30% reduced kidney risk by 64%, as measured by reductions in kidney failure, sufficient glomerular filtration, and death from kidney disease.
- A UACR reduction of at least 30% reduced cardiovascular risk by 26%, as measured by fewer incidences of cardiovascular death, nonfatal infarction or stroke, and hospitalization for heart failure.
IN PRACTICE:
“Achieving early UACR reduction can lead to tangible benefits for kidney and cardiovascular health,” the authors note.
SOURCE:
The study was published in the Annals of Internal Medicine; the lead author is Rajiv Agarwal, MD, MS.
LIMITATIONS:
The study pertains only to finerenone, so the findings cannot be extrapolated to other drugs with different mechanisms of action.
DISCLOSURES:
Bayer AG Pharmaceuticals, which manufactures finerenone, was the primary funder of the study. The US National Institutes of Health and Veterans Administration also provided funding. Some study authors are full-time employees of Bayer AG. Many authors report consulting relationships with various pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
Reducing the urine albumin-to-creatinine ratio (UACR) significantly reduces kidney risk in people with type 2 diabetes, per new research in the Annals of Internal Medicine.
METHODOLOGY:
- Post hoc retrospective analysis of two phase 3 double-blind trials of finerenone in people with type 2 diabetes and chronic kidney disease
- Quantify the long-term health effects of reducing UACR within 4 months of taking finerenone by examining the records of 12,512 participants with an equal chance of receiving finerenone or placebo
- Isolate the impact of UACR reduction on kidney function and cardiovascular function by tracking health indicators related to the kidneys and the heart in participants for up to 4 years
TAKEAWAY:
- Over half of participants who received finerenone had reduced UACR by at least 30% from the baseline of 514 mg/g at the 4-month point after starting treatment, and the median UACR reduction in this group was 33%.
- By 4 months, a little over a quarter of participants who received the placebo had reduced their UACR levels by at least 30%, and the median UACR reduction in this group was 2.6%.
- A UACR reduction of at least 30% reduced kidney risk by 64%, as measured by reductions in kidney failure, sufficient glomerular filtration, and death from kidney disease.
- A UACR reduction of at least 30% reduced cardiovascular risk by 26%, as measured by fewer incidences of cardiovascular death, nonfatal infarction or stroke, and hospitalization for heart failure.
IN PRACTICE:
“Achieving early UACR reduction can lead to tangible benefits for kidney and cardiovascular health,” the authors note.
SOURCE:
The study was published in the Annals of Internal Medicine; the lead author is Rajiv Agarwal, MD, MS.
LIMITATIONS:
The study pertains only to finerenone, so the findings cannot be extrapolated to other drugs with different mechanisms of action.
DISCLOSURES:
Bayer AG Pharmaceuticals, which manufactures finerenone, was the primary funder of the study. The US National Institutes of Health and Veterans Administration also provided funding. Some study authors are full-time employees of Bayer AG. Many authors report consulting relationships with various pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
Reducing the urine albumin-to-creatinine ratio (UACR) significantly reduces kidney risk in people with type 2 diabetes, per new research in the Annals of Internal Medicine.
METHODOLOGY:
- Post hoc retrospective analysis of two phase 3 double-blind trials of finerenone in people with type 2 diabetes and chronic kidney disease
- Quantify the long-term health effects of reducing UACR within 4 months of taking finerenone by examining the records of 12,512 participants with an equal chance of receiving finerenone or placebo
- Isolate the impact of UACR reduction on kidney function and cardiovascular function by tracking health indicators related to the kidneys and the heart in participants for up to 4 years
TAKEAWAY:
- Over half of participants who received finerenone had reduced UACR by at least 30% from the baseline of 514 mg/g at the 4-month point after starting treatment, and the median UACR reduction in this group was 33%.
- By 4 months, a little over a quarter of participants who received the placebo had reduced their UACR levels by at least 30%, and the median UACR reduction in this group was 2.6%.
- A UACR reduction of at least 30% reduced kidney risk by 64%, as measured by reductions in kidney failure, sufficient glomerular filtration, and death from kidney disease.
- A UACR reduction of at least 30% reduced cardiovascular risk by 26%, as measured by fewer incidences of cardiovascular death, nonfatal infarction or stroke, and hospitalization for heart failure.
IN PRACTICE:
“Achieving early UACR reduction can lead to tangible benefits for kidney and cardiovascular health,” the authors note.
SOURCE:
The study was published in the Annals of Internal Medicine; the lead author is Rajiv Agarwal, MD, MS.
LIMITATIONS:
The study pertains only to finerenone, so the findings cannot be extrapolated to other drugs with different mechanisms of action.
DISCLOSURES:
Bayer AG Pharmaceuticals, which manufactures finerenone, was the primary funder of the study. The US National Institutes of Health and Veterans Administration also provided funding. Some study authors are full-time employees of Bayer AG. Many authors report consulting relationships with various pharmaceutical companies.
A version of this article appeared on Medscape.com.
Chronic Kidney Disease and Military Service in US Adults, 1999-2018
Chronic kidney disease (CKD) affects nearly 37 million people (11%) in the US and is a leading cause of death and morbidity. Due to their older age and higher prevalence of comorbid conditions, the prevalence of CKD among veterans is approximately 34% higher than in the general population and the fourth most common chronic disease diagnosed among US veterans.1,2 US veterans and those with prior military service (MS) may be at a particularly high risk for CKD and associated health care outcomes including increased hospitalization and death. The observed excess burden of CKD is not mirrored in the general population, and it is unclear whether prior MS confers a unique risk profile for CKD.
Current estimates of CKD burden among veterans or those with prior MS are widely variable and have been limited by unique regions, specific exposure profiles, or to single health care systems. As such, there remains a paucity of data examining CKD burden more broadly. We performed a study in the adult population of the US to quantify associations with the extent of CKD, enumerate temporal trends of CKD among those with prior MS, describe risk within subgroups, and compare heterogeneity of risk factors for CKD by MS.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a suite of nationally representative, cross-sectional surveys of the noninstitutionalized US population. It is conducted by the National Center for Health Statistics and uses a stratified, clustered probability design, with surveys carried out without interruption, collated, and made accessible to the public at 2-year intervals.3 The survey consists of a questionnaire, physical examination, and laboratory data.
The inclusion criteria for our study were age ≥ 20 years along with serum creatinine and urinary albumin-creatinine measurements. The following definitions were used for the study:
• CKD: Estimated glomerular filtration rate < 60 mL/min/1.73 m2 calibrated to isotope dilution mass spectrometry (IDMS).
• Traceable: Creatinine-based CKD Epidemiology Collaboration formula or urinary albumin-creatine ratio ≥ 30 mg/g.
• MS: Positive response to the questions “Did you ever serve in the Armed Forces of the United States?” (1999 to 2010) or “Have you ever served on active duty in the US Armed Forces, military Reserves, or National Guard?” (2011 to 2018).
• Diabetes: Self-reported history, medication for diabetes, or glycated hemoglobin ≥ 7%.
• Hypertension: Blood pressure ≥ 140/90 or ≥ 130/40 mm Hg in the presence of diabetes, medication for hypertension, cardiovascular disease, or CKD, myocardial infarction, cardiac failure, or cerebrovascular disease by self-report.2,3
Analysis
Primary sampling unit, stratum, and weight variables were employed throughout to generate parameter estimates that are generalizable to the US population.4,5 The χ2 test and logistic regression, respectively, were employed for comparison of proportions and estimation of odds ratios. R Version 4.1.2 was employed for data analysis.
Results
In the overall sample, the frequencies (95% standard error [SE]) of CKD and prior MS were 15.2% (0.3) and 11.5% (0.3) (Table 1). The proportion (SE) with CKD was significantly higher among those with prior MS vs the overall population: 22.7% (0.7) vs 15.2% (0.3) (P < .001). Significant associations with CKD were observed (P < .05) by age, sex, race and ethnicity, family poverty, school education, health insurance, smoking, body mass index, diabetes, hypertension, cardiovascular disease, and malignancy. Within those reporting prior MS, the proportion (SE) with CKD differed by era: 1999 to 2002, 18.9% (1.1); 2003 to 2006, 24.9% (1.5); 2007 to 2010, 22.3% (1.5); 2011 to 2014, 24.3% (1.7); and 2015 to 2018, 24.0% (1.8) (P = .02) (Figure 1).
Without covariate adjustment, prior MS was significantly associated with an increased risk of CKD (unadjusted odds ratio [OR], 1.78; 95% CI, 1.64-1.93; P < .05) (Table 2). Prior MS was significantly associated with CKD in the following subgroups: 2003 to 2006, 2011 to 2014, 2015 to 2018, age groups of 40 to 64 years and ≥ 65 years, male sex, non-Hispanic White and Hispanic ethnicity, school education of grade 0 to 11, and private or other health insurance. Additional comorbidities strongly associated with CKD included hypertension (OR, 6.37; 95% CI, 5.37-7.55), diabetes (OR, 4.16; 95% CI, 3.45-5.03), and cardiovascular disease (OR, 4.20; 95% CI, 3.57-4.95).
In the population reporting prior MS, the unadjusted OR of CKD vs 1999 to 2002 was greater for all other examined eras; with the greatest likelihood observed for the 2003 to 2006 era. Unadjusted ORs of CKD differed in groups with and without prior MS (P value for interaction < .05) for 2003 to 2006, those aged 40 to 64 years and ≥ 65 years, female sex, non-Hispanic African American and Hispanic race and ethnicity, family poverty, high school education, private health insurance, any smoking history, diabetes, hypertension, and cardiovascular disease (Figure 2A).
Following adjustment for age, sex, and race and ethnicity, MS was associated with a 17% higher likelihood of CKD (adjusted odds ratio [AOR], 1.17; 95% CI, 1.06-1.28; P < .01) (Table 3). Prior MS was significantly associated (P < .05) with CKD in the subgroups: age groups 40 to 64 years and ≥ 65 years, non-Hispanic African American, and body mass index ≥ 30. Among those with prior MS, comorbidities strongly associated with CKD in adjusted models included hypertension (AOR, 3.86; 95% CI, 3.18-4.69), diabetes (AOR, 3.05; 95% CI, 2.44-3.82), and cardiovascular disease (AOR, 2.51; 95% CI, 2.09-3.01). In the population with prior MS, the adjusted likelihood of CKD vs 1999 to 2002 was similar across all eras. Adjusted associations of CKD differed in groups with and without prior MS for age groups 40 to 64 years and ≥ 65 years, female sex, and family poverty (P < .05) (Figure 2B).
Discussion
We observed that prior MS was associated with CKD, all eras were associated with CKD in the subgroup with MS, and risk factors for CKD differed among many subgroups both with and without MS history, a finding that remained present in adjusted models. In addition, the finding of CKD was relatively common among those with prior MS (approximately 15%) and was most strongly associated with increasing age and comorbidities frequently associated with CKD.
Although many studies have demonstrated associations of US veteran status with various comorbidities, including hypertension, obesity, and diabetes, these studies often are limited to those both qualifying and receiving care within the US Department of Veterans Affairs (VA) health care system.6-9 The crude proportion of individuals reporting multiple chronic conditions, which included hypertension, diabetes, and weak or failing kidneys, was 49.7% for US veterans compared with 24.1% for nonveterans.2 Large-scale, nationally representative cohorts for use in this context have been limited by the heterogeneity of definitions of CKD applied with limited timeframes yielding variable estimates.1,10 Moreover, few studies have examined the clinical epidemiology of CKD more broadly in the US among those with prior MS. For example, a PubMed search on March 3, 2022, with the terms “epidemiology”, “military service”, and “chronic kidney disease” produced only 9 citations, one of which examined trends among a non-US cohort and quantifying disease burden another among adolescents.
Whether or not prior MS confers a unique risk profile for CKD is unknown. While our findings of an increased CKD burden among those reporting MS may partially reflect observed increases in baseline comorbidities, the observed excess CKD among those with MS remained across multiple categories even after adjustment for baseline demography. As several studies have demonstrated, enlistment into MS may select for a more diverse population; however those enlisted personnel may be of lower socioeconomic status and possibly at higher risk of CKD.11,12 Our findings of important differences in baseline determinants of health mirror this. The proportion of MS respondents with CKD vs CKD alone reporting a high school education or lower was higher (36.0% vs 21.8%) as well as among those with a history of family poverty (21.1% vs 18.0%).
Limitations
Our study has several limitations, including its cross-sectional study design, a lack of longitudinal data within individuals, and exclusion of institutionalized individuals. Limitations notwithstanding this study has several important aspects. As prior MS is highly variable, we were limited in our inability to stratify by service type or length of service. For example, veteran status is conferred to a “Reservist or member of the National Guard called to federal active duty or disabled from a disease or injury incurred or aggravated in line of duty or while in training status also qualify as a veteran” (13 CFR § 125.11). For the purposes of our study, prior MS would include all active-duty service (veterans) as well as reservists and National Guard members who have not been activated. This may be more representative of the overall effect of MS, as limitation to those receiving care within the VA may select for an older, more multimorbid population of patients, limiting generalizability.
In addition, more detailed information regarding service-related exposures and other service-connected conditions would allow for a more granular risk assessment by service type, era, and military conflict. Our finding of excess CKD burden among those with prior MS compared with the overall population is timely given the recent passage of the Promise to Address Comprehensive Toxics (PACT) Act. Exposure to and injury from Agent Orange—a known service-connected exposure associated with incident hypertension and diabetes—may be a significant contributor to CKD that may have a significant era effect. In addition, water contamination among those stationed in Camp Lejeune in North Carolina has notable genitourinary associations. Finally, burn pit exposures in more recent military conflicts may also have important associations with chronic disease, possibly including CKD. While similar attempts at the creation of large-scale US veteran cohorts have been limited by incomplete capture of creatinine, the large proportion of missing race data, and limited inclusion of additional markers of kidney disease, our use of a well-described, nationally representative survey along with standardized capture of clinical and laboratory elements mitigate the use of various societal or other codified definitions.1
Conclusions
Prior MS is associated with an increased risk of CKD overall and across several important subgroups. This finding was observed in various unadjusted and adjusted models and may constitute a unique risk profile of risk.
1. Ozieh MN, Gebregziabher M, Ward RC, Taber DJ, Egede LE. Creating a 13-year National Longitudinal Cohort of veterans with chronic kidney disease. BMC Nephrol. 2019;20(1):241. doi:10.1186/s12882-019-1430-y
2. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13.
3. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Survey. 2022. Accessed October 31, 2023. www.cdc.gov/nchs/nhanes/index.htm
4. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
5. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918-926. doi:10.1053/j.ajkd.2007.08.020
6. Smoley BA, Smith NL, Runkle GP. Hypertension in a population of active duty service members. J Am Board Fam Med. 2008;21(6):504-511. doi:10.3122/jabfm.2008.06.070182
7. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. doi:10.1056/NEJMoa0808431
8. Smith TJ, Marriott BP, Dotson L, et al. Overweight and obesity in military personnel: sociodemographic predictors. Obesity (Silver Spring). 2012;20(7):1534-1538. doi:10.1038/oby.2012.25
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
11. Wang L, Elder GH, Jr., Spence NJ. Status configurations, military service and higher education. Soc Forces. 2012;91(2):397-422. doi:10.1093/sf/sos174
12. Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72(4):270-279. doi:10.1136/jech-2017-209815
Chronic kidney disease (CKD) affects nearly 37 million people (11%) in the US and is a leading cause of death and morbidity. Due to their older age and higher prevalence of comorbid conditions, the prevalence of CKD among veterans is approximately 34% higher than in the general population and the fourth most common chronic disease diagnosed among US veterans.1,2 US veterans and those with prior military service (MS) may be at a particularly high risk for CKD and associated health care outcomes including increased hospitalization and death. The observed excess burden of CKD is not mirrored in the general population, and it is unclear whether prior MS confers a unique risk profile for CKD.
Current estimates of CKD burden among veterans or those with prior MS are widely variable and have been limited by unique regions, specific exposure profiles, or to single health care systems. As such, there remains a paucity of data examining CKD burden more broadly. We performed a study in the adult population of the US to quantify associations with the extent of CKD, enumerate temporal trends of CKD among those with prior MS, describe risk within subgroups, and compare heterogeneity of risk factors for CKD by MS.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a suite of nationally representative, cross-sectional surveys of the noninstitutionalized US population. It is conducted by the National Center for Health Statistics and uses a stratified, clustered probability design, with surveys carried out without interruption, collated, and made accessible to the public at 2-year intervals.3 The survey consists of a questionnaire, physical examination, and laboratory data.
The inclusion criteria for our study were age ≥ 20 years along with serum creatinine and urinary albumin-creatinine measurements. The following definitions were used for the study:
• CKD: Estimated glomerular filtration rate < 60 mL/min/1.73 m2 calibrated to isotope dilution mass spectrometry (IDMS).
• Traceable: Creatinine-based CKD Epidemiology Collaboration formula or urinary albumin-creatine ratio ≥ 30 mg/g.
• MS: Positive response to the questions “Did you ever serve in the Armed Forces of the United States?” (1999 to 2010) or “Have you ever served on active duty in the US Armed Forces, military Reserves, or National Guard?” (2011 to 2018).
• Diabetes: Self-reported history, medication for diabetes, or glycated hemoglobin ≥ 7%.
• Hypertension: Blood pressure ≥ 140/90 or ≥ 130/40 mm Hg in the presence of diabetes, medication for hypertension, cardiovascular disease, or CKD, myocardial infarction, cardiac failure, or cerebrovascular disease by self-report.2,3
Analysis
Primary sampling unit, stratum, and weight variables were employed throughout to generate parameter estimates that are generalizable to the US population.4,5 The χ2 test and logistic regression, respectively, were employed for comparison of proportions and estimation of odds ratios. R Version 4.1.2 was employed for data analysis.
Results
In the overall sample, the frequencies (95% standard error [SE]) of CKD and prior MS were 15.2% (0.3) and 11.5% (0.3) (Table 1). The proportion (SE) with CKD was significantly higher among those with prior MS vs the overall population: 22.7% (0.7) vs 15.2% (0.3) (P < .001). Significant associations with CKD were observed (P < .05) by age, sex, race and ethnicity, family poverty, school education, health insurance, smoking, body mass index, diabetes, hypertension, cardiovascular disease, and malignancy. Within those reporting prior MS, the proportion (SE) with CKD differed by era: 1999 to 2002, 18.9% (1.1); 2003 to 2006, 24.9% (1.5); 2007 to 2010, 22.3% (1.5); 2011 to 2014, 24.3% (1.7); and 2015 to 2018, 24.0% (1.8) (P = .02) (Figure 1).
Without covariate adjustment, prior MS was significantly associated with an increased risk of CKD (unadjusted odds ratio [OR], 1.78; 95% CI, 1.64-1.93; P < .05) (Table 2). Prior MS was significantly associated with CKD in the following subgroups: 2003 to 2006, 2011 to 2014, 2015 to 2018, age groups of 40 to 64 years and ≥ 65 years, male sex, non-Hispanic White and Hispanic ethnicity, school education of grade 0 to 11, and private or other health insurance. Additional comorbidities strongly associated with CKD included hypertension (OR, 6.37; 95% CI, 5.37-7.55), diabetes (OR, 4.16; 95% CI, 3.45-5.03), and cardiovascular disease (OR, 4.20; 95% CI, 3.57-4.95).
In the population reporting prior MS, the unadjusted OR of CKD vs 1999 to 2002 was greater for all other examined eras; with the greatest likelihood observed for the 2003 to 2006 era. Unadjusted ORs of CKD differed in groups with and without prior MS (P value for interaction < .05) for 2003 to 2006, those aged 40 to 64 years and ≥ 65 years, female sex, non-Hispanic African American and Hispanic race and ethnicity, family poverty, high school education, private health insurance, any smoking history, diabetes, hypertension, and cardiovascular disease (Figure 2A).
Following adjustment for age, sex, and race and ethnicity, MS was associated with a 17% higher likelihood of CKD (adjusted odds ratio [AOR], 1.17; 95% CI, 1.06-1.28; P < .01) (Table 3). Prior MS was significantly associated (P < .05) with CKD in the subgroups: age groups 40 to 64 years and ≥ 65 years, non-Hispanic African American, and body mass index ≥ 30. Among those with prior MS, comorbidities strongly associated with CKD in adjusted models included hypertension (AOR, 3.86; 95% CI, 3.18-4.69), diabetes (AOR, 3.05; 95% CI, 2.44-3.82), and cardiovascular disease (AOR, 2.51; 95% CI, 2.09-3.01). In the population with prior MS, the adjusted likelihood of CKD vs 1999 to 2002 was similar across all eras. Adjusted associations of CKD differed in groups with and without prior MS for age groups 40 to 64 years and ≥ 65 years, female sex, and family poverty (P < .05) (Figure 2B).
Discussion
We observed that prior MS was associated with CKD, all eras were associated with CKD in the subgroup with MS, and risk factors for CKD differed among many subgroups both with and without MS history, a finding that remained present in adjusted models. In addition, the finding of CKD was relatively common among those with prior MS (approximately 15%) and was most strongly associated with increasing age and comorbidities frequently associated with CKD.
Although many studies have demonstrated associations of US veteran status with various comorbidities, including hypertension, obesity, and diabetes, these studies often are limited to those both qualifying and receiving care within the US Department of Veterans Affairs (VA) health care system.6-9 The crude proportion of individuals reporting multiple chronic conditions, which included hypertension, diabetes, and weak or failing kidneys, was 49.7% for US veterans compared with 24.1% for nonveterans.2 Large-scale, nationally representative cohorts for use in this context have been limited by the heterogeneity of definitions of CKD applied with limited timeframes yielding variable estimates.1,10 Moreover, few studies have examined the clinical epidemiology of CKD more broadly in the US among those with prior MS. For example, a PubMed search on March 3, 2022, with the terms “epidemiology”, “military service”, and “chronic kidney disease” produced only 9 citations, one of which examined trends among a non-US cohort and quantifying disease burden another among adolescents.
Whether or not prior MS confers a unique risk profile for CKD is unknown. While our findings of an increased CKD burden among those reporting MS may partially reflect observed increases in baseline comorbidities, the observed excess CKD among those with MS remained across multiple categories even after adjustment for baseline demography. As several studies have demonstrated, enlistment into MS may select for a more diverse population; however those enlisted personnel may be of lower socioeconomic status and possibly at higher risk of CKD.11,12 Our findings of important differences in baseline determinants of health mirror this. The proportion of MS respondents with CKD vs CKD alone reporting a high school education or lower was higher (36.0% vs 21.8%) as well as among those with a history of family poverty (21.1% vs 18.0%).
Limitations
Our study has several limitations, including its cross-sectional study design, a lack of longitudinal data within individuals, and exclusion of institutionalized individuals. Limitations notwithstanding this study has several important aspects. As prior MS is highly variable, we were limited in our inability to stratify by service type or length of service. For example, veteran status is conferred to a “Reservist or member of the National Guard called to federal active duty or disabled from a disease or injury incurred or aggravated in line of duty or while in training status also qualify as a veteran” (13 CFR § 125.11). For the purposes of our study, prior MS would include all active-duty service (veterans) as well as reservists and National Guard members who have not been activated. This may be more representative of the overall effect of MS, as limitation to those receiving care within the VA may select for an older, more multimorbid population of patients, limiting generalizability.
In addition, more detailed information regarding service-related exposures and other service-connected conditions would allow for a more granular risk assessment by service type, era, and military conflict. Our finding of excess CKD burden among those with prior MS compared with the overall population is timely given the recent passage of the Promise to Address Comprehensive Toxics (PACT) Act. Exposure to and injury from Agent Orange—a known service-connected exposure associated with incident hypertension and diabetes—may be a significant contributor to CKD that may have a significant era effect. In addition, water contamination among those stationed in Camp Lejeune in North Carolina has notable genitourinary associations. Finally, burn pit exposures in more recent military conflicts may also have important associations with chronic disease, possibly including CKD. While similar attempts at the creation of large-scale US veteran cohorts have been limited by incomplete capture of creatinine, the large proportion of missing race data, and limited inclusion of additional markers of kidney disease, our use of a well-described, nationally representative survey along with standardized capture of clinical and laboratory elements mitigate the use of various societal or other codified definitions.1
Conclusions
Prior MS is associated with an increased risk of CKD overall and across several important subgroups. This finding was observed in various unadjusted and adjusted models and may constitute a unique risk profile of risk.
Chronic kidney disease (CKD) affects nearly 37 million people (11%) in the US and is a leading cause of death and morbidity. Due to their older age and higher prevalence of comorbid conditions, the prevalence of CKD among veterans is approximately 34% higher than in the general population and the fourth most common chronic disease diagnosed among US veterans.1,2 US veterans and those with prior military service (MS) may be at a particularly high risk for CKD and associated health care outcomes including increased hospitalization and death. The observed excess burden of CKD is not mirrored in the general population, and it is unclear whether prior MS confers a unique risk profile for CKD.
Current estimates of CKD burden among veterans or those with prior MS are widely variable and have been limited by unique regions, specific exposure profiles, or to single health care systems. As such, there remains a paucity of data examining CKD burden more broadly. We performed a study in the adult population of the US to quantify associations with the extent of CKD, enumerate temporal trends of CKD among those with prior MS, describe risk within subgroups, and compare heterogeneity of risk factors for CKD by MS.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a suite of nationally representative, cross-sectional surveys of the noninstitutionalized US population. It is conducted by the National Center for Health Statistics and uses a stratified, clustered probability design, with surveys carried out without interruption, collated, and made accessible to the public at 2-year intervals.3 The survey consists of a questionnaire, physical examination, and laboratory data.
The inclusion criteria for our study were age ≥ 20 years along with serum creatinine and urinary albumin-creatinine measurements. The following definitions were used for the study:
• CKD: Estimated glomerular filtration rate < 60 mL/min/1.73 m2 calibrated to isotope dilution mass spectrometry (IDMS).
• Traceable: Creatinine-based CKD Epidemiology Collaboration formula or urinary albumin-creatine ratio ≥ 30 mg/g.
• MS: Positive response to the questions “Did you ever serve in the Armed Forces of the United States?” (1999 to 2010) or “Have you ever served on active duty in the US Armed Forces, military Reserves, or National Guard?” (2011 to 2018).
• Diabetes: Self-reported history, medication for diabetes, or glycated hemoglobin ≥ 7%.
• Hypertension: Blood pressure ≥ 140/90 or ≥ 130/40 mm Hg in the presence of diabetes, medication for hypertension, cardiovascular disease, or CKD, myocardial infarction, cardiac failure, or cerebrovascular disease by self-report.2,3
Analysis
Primary sampling unit, stratum, and weight variables were employed throughout to generate parameter estimates that are generalizable to the US population.4,5 The χ2 test and logistic regression, respectively, were employed for comparison of proportions and estimation of odds ratios. R Version 4.1.2 was employed for data analysis.
Results
In the overall sample, the frequencies (95% standard error [SE]) of CKD and prior MS were 15.2% (0.3) and 11.5% (0.3) (Table 1). The proportion (SE) with CKD was significantly higher among those with prior MS vs the overall population: 22.7% (0.7) vs 15.2% (0.3) (P < .001). Significant associations with CKD were observed (P < .05) by age, sex, race and ethnicity, family poverty, school education, health insurance, smoking, body mass index, diabetes, hypertension, cardiovascular disease, and malignancy. Within those reporting prior MS, the proportion (SE) with CKD differed by era: 1999 to 2002, 18.9% (1.1); 2003 to 2006, 24.9% (1.5); 2007 to 2010, 22.3% (1.5); 2011 to 2014, 24.3% (1.7); and 2015 to 2018, 24.0% (1.8) (P = .02) (Figure 1).
Without covariate adjustment, prior MS was significantly associated with an increased risk of CKD (unadjusted odds ratio [OR], 1.78; 95% CI, 1.64-1.93; P < .05) (Table 2). Prior MS was significantly associated with CKD in the following subgroups: 2003 to 2006, 2011 to 2014, 2015 to 2018, age groups of 40 to 64 years and ≥ 65 years, male sex, non-Hispanic White and Hispanic ethnicity, school education of grade 0 to 11, and private or other health insurance. Additional comorbidities strongly associated with CKD included hypertension (OR, 6.37; 95% CI, 5.37-7.55), diabetes (OR, 4.16; 95% CI, 3.45-5.03), and cardiovascular disease (OR, 4.20; 95% CI, 3.57-4.95).
In the population reporting prior MS, the unadjusted OR of CKD vs 1999 to 2002 was greater for all other examined eras; with the greatest likelihood observed for the 2003 to 2006 era. Unadjusted ORs of CKD differed in groups with and without prior MS (P value for interaction < .05) for 2003 to 2006, those aged 40 to 64 years and ≥ 65 years, female sex, non-Hispanic African American and Hispanic race and ethnicity, family poverty, high school education, private health insurance, any smoking history, diabetes, hypertension, and cardiovascular disease (Figure 2A).
Following adjustment for age, sex, and race and ethnicity, MS was associated with a 17% higher likelihood of CKD (adjusted odds ratio [AOR], 1.17; 95% CI, 1.06-1.28; P < .01) (Table 3). Prior MS was significantly associated (P < .05) with CKD in the subgroups: age groups 40 to 64 years and ≥ 65 years, non-Hispanic African American, and body mass index ≥ 30. Among those with prior MS, comorbidities strongly associated with CKD in adjusted models included hypertension (AOR, 3.86; 95% CI, 3.18-4.69), diabetes (AOR, 3.05; 95% CI, 2.44-3.82), and cardiovascular disease (AOR, 2.51; 95% CI, 2.09-3.01). In the population with prior MS, the adjusted likelihood of CKD vs 1999 to 2002 was similar across all eras. Adjusted associations of CKD differed in groups with and without prior MS for age groups 40 to 64 years and ≥ 65 years, female sex, and family poverty (P < .05) (Figure 2B).
Discussion
We observed that prior MS was associated with CKD, all eras were associated with CKD in the subgroup with MS, and risk factors for CKD differed among many subgroups both with and without MS history, a finding that remained present in adjusted models. In addition, the finding of CKD was relatively common among those with prior MS (approximately 15%) and was most strongly associated with increasing age and comorbidities frequently associated with CKD.
Although many studies have demonstrated associations of US veteran status with various comorbidities, including hypertension, obesity, and diabetes, these studies often are limited to those both qualifying and receiving care within the US Department of Veterans Affairs (VA) health care system.6-9 The crude proportion of individuals reporting multiple chronic conditions, which included hypertension, diabetes, and weak or failing kidneys, was 49.7% for US veterans compared with 24.1% for nonveterans.2 Large-scale, nationally representative cohorts for use in this context have been limited by the heterogeneity of definitions of CKD applied with limited timeframes yielding variable estimates.1,10 Moreover, few studies have examined the clinical epidemiology of CKD more broadly in the US among those with prior MS. For example, a PubMed search on March 3, 2022, with the terms “epidemiology”, “military service”, and “chronic kidney disease” produced only 9 citations, one of which examined trends among a non-US cohort and quantifying disease burden another among adolescents.
Whether or not prior MS confers a unique risk profile for CKD is unknown. While our findings of an increased CKD burden among those reporting MS may partially reflect observed increases in baseline comorbidities, the observed excess CKD among those with MS remained across multiple categories even after adjustment for baseline demography. As several studies have demonstrated, enlistment into MS may select for a more diverse population; however those enlisted personnel may be of lower socioeconomic status and possibly at higher risk of CKD.11,12 Our findings of important differences in baseline determinants of health mirror this. The proportion of MS respondents with CKD vs CKD alone reporting a high school education or lower was higher (36.0% vs 21.8%) as well as among those with a history of family poverty (21.1% vs 18.0%).
Limitations
Our study has several limitations, including its cross-sectional study design, a lack of longitudinal data within individuals, and exclusion of institutionalized individuals. Limitations notwithstanding this study has several important aspects. As prior MS is highly variable, we were limited in our inability to stratify by service type or length of service. For example, veteran status is conferred to a “Reservist or member of the National Guard called to federal active duty or disabled from a disease or injury incurred or aggravated in line of duty or while in training status also qualify as a veteran” (13 CFR § 125.11). For the purposes of our study, prior MS would include all active-duty service (veterans) as well as reservists and National Guard members who have not been activated. This may be more representative of the overall effect of MS, as limitation to those receiving care within the VA may select for an older, more multimorbid population of patients, limiting generalizability.
In addition, more detailed information regarding service-related exposures and other service-connected conditions would allow for a more granular risk assessment by service type, era, and military conflict. Our finding of excess CKD burden among those with prior MS compared with the overall population is timely given the recent passage of the Promise to Address Comprehensive Toxics (PACT) Act. Exposure to and injury from Agent Orange—a known service-connected exposure associated with incident hypertension and diabetes—may be a significant contributor to CKD that may have a significant era effect. In addition, water contamination among those stationed in Camp Lejeune in North Carolina has notable genitourinary associations. Finally, burn pit exposures in more recent military conflicts may also have important associations with chronic disease, possibly including CKD. While similar attempts at the creation of large-scale US veteran cohorts have been limited by incomplete capture of creatinine, the large proportion of missing race data, and limited inclusion of additional markers of kidney disease, our use of a well-described, nationally representative survey along with standardized capture of clinical and laboratory elements mitigate the use of various societal or other codified definitions.1
Conclusions
Prior MS is associated with an increased risk of CKD overall and across several important subgroups. This finding was observed in various unadjusted and adjusted models and may constitute a unique risk profile of risk.
1. Ozieh MN, Gebregziabher M, Ward RC, Taber DJ, Egede LE. Creating a 13-year National Longitudinal Cohort of veterans with chronic kidney disease. BMC Nephrol. 2019;20(1):241. doi:10.1186/s12882-019-1430-y
2. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13.
3. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Survey. 2022. Accessed October 31, 2023. www.cdc.gov/nchs/nhanes/index.htm
4. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
5. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918-926. doi:10.1053/j.ajkd.2007.08.020
6. Smoley BA, Smith NL, Runkle GP. Hypertension in a population of active duty service members. J Am Board Fam Med. 2008;21(6):504-511. doi:10.3122/jabfm.2008.06.070182
7. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. doi:10.1056/NEJMoa0808431
8. Smith TJ, Marriott BP, Dotson L, et al. Overweight and obesity in military personnel: sociodemographic predictors. Obesity (Silver Spring). 2012;20(7):1534-1538. doi:10.1038/oby.2012.25
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
11. Wang L, Elder GH, Jr., Spence NJ. Status configurations, military service and higher education. Soc Forces. 2012;91(2):397-422. doi:10.1093/sf/sos174
12. Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72(4):270-279. doi:10.1136/jech-2017-209815
1. Ozieh MN, Gebregziabher M, Ward RC, Taber DJ, Egede LE. Creating a 13-year National Longitudinal Cohort of veterans with chronic kidney disease. BMC Nephrol. 2019;20(1):241. doi:10.1186/s12882-019-1430-y
2. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13.
3. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Survey. 2022. Accessed October 31, 2023. www.cdc.gov/nchs/nhanes/index.htm
4. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
5. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918-926. doi:10.1053/j.ajkd.2007.08.020
6. Smoley BA, Smith NL, Runkle GP. Hypertension in a population of active duty service members. J Am Board Fam Med. 2008;21(6):504-511. doi:10.3122/jabfm.2008.06.070182
7. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. doi:10.1056/NEJMoa0808431
8. Smith TJ, Marriott BP, Dotson L, et al. Overweight and obesity in military personnel: sociodemographic predictors. Obesity (Silver Spring). 2012;20(7):1534-1538. doi:10.1038/oby.2012.25
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
11. Wang L, Elder GH, Jr., Spence NJ. Status configurations, military service and higher education. Soc Forces. 2012;91(2):397-422. doi:10.1093/sf/sos174
12. Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72(4):270-279. doi:10.1136/jech-2017-209815
Low-dose methotrexate carries higher risk for older patients with CKD
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Albuminuria reduction fuels finerenone’s kidney benefits
PHILADELPHIA – Reducing albuminuria is a key mediator of the way finerenone (Kerendia, Bayer) reduces adverse renal and cardiovascular events in people with type 2 diabetes and chronic kidney disease (CKD), based on findings from two novel mediation analyses run on data from more than 12,000 people included in the two finerenone pivotal trials.
Results from these analyses showed that that finerenone treatment produced in the FIDELIO-DKD and FIGARO-DKD phase 3 trials. FIDELIO-DKD, which had protection against adverse kidney outcomes as its primary endpoint, supplied the data that led to finerenone’s approval in 2021 by the U.S. Food and Drug Administration for treating people with type 2 diabetes and CKD.
The findings of the mediation analyses underscore the important role that albuminuria plays in the nephropathy and related comorbidities associated with type 2 diabetes and CKD and highlight the importance of ongoing monitoring of albuminuria to guide treatments aimed at minimizing this pathology, said Rajiv Agarwal, MD, who presented a poster on the mediation analyses at Kidney Week 2023, organized by the American Society of Nephrology.
“My hope is that this [report] heightens awareness of UACR” as an important marker of both CKD and of the response by patients with CKD to their treatment, said Dr. Agarwal, a nephrologist and professor at Indiana University in Indianapolis.
“Only about half of people with type 2 diabetes get their UACR measured even though every guideline says measure UACR in people with diabetes. Our findings say that UACR is important not just for CKD diagnosis but also to give feedback” on whether management is working, Dr. Agarwal said in an interview.
Incorporate UACR into clinical decision-making
“My hope is that clinicians will look at UACR as something they should incorporate into clinical decision-making. I measure UACR in my patients [with CKD and type 2 diabetes] at every visit; it’s so inexpensive. Albuminuria is not a good sign. If it’s not reduced in a patient by at least 30% [the recommended minimum reduction by the American Diabetes Association for people who start with a UACR of at least 300 mg/g] clinicians should think of what else they could do to lower albuminuria”: Reduce salt intake, improve blood pressure control, make sure the patient is adherent to treatments, and add additional treatments, Dr. Agarwal advised.
Multiple efforts are now underway or will soon start to boost the rate at which at-risk people get their UACR measured, noted Leslie A. Inker, MD, in a separate talk during Kidney Week. These efforts include the National Kidney Foundation’s CKD Learning Collaborative, which aims to improve clinician awareness of CKD and improve routine testing for CKD. Early results during 2023 from this program in Missouri showed a nearly 8–percentage point increase in the screening rate for UACR levels in at-risk people, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston.
A second advance was introduction in 2018 of the “kidney profile” lab order by the American College of Clinical Pathology that allows clinicians to order as a single test both an estimated glomerular filtration rate (eGFR) and a UACR.
Also, the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance have both taken steps to encourage UACR ordering. The NCQA established a new Healthcare Effectiveness Data and Information Set performance measure for U.S. physicians starting in 2023 that will track measurement of UACR and eGFR in people with diabetes. CMS also has made assessment of kidney health a measure of care quality in programs effective in 2023 and 2024, Dr. Inker noted.
Most subjects had elevated UACRs
The study run by Dr. Agarwal and his associates used data from 12,512 of the more than 13,000 people enrolled in either FIDELITY-DKD or FIGARO-DKD who had UACR measurements recorded at baseline, at 4 months into either study, or both. Their median UACR at the time they began on finerenone or placebo was 514 mg/g, with 67% having a UACR of at least 300 mg/g (macroalbuminuria) and 31% having a UACR of 30-299 mg/g (microalbuminuria). By design, virtually all patients in these two trials were on a renin-angiotensin system inhibitor (either an angiotensin-converting enzyme inhibitor or an angiotensin-receptor blocker), but given the time period when the two trials enrolled participants (during 2015-2018) only 7% of those enrolled were on a sodium-glucose cotransporter 2 inhibitor and only 7% were on a glucagonlike peptide–1 receptor agonist.
Four months after treatment began, 53% of those randomized to finerenone treatment and 27% of those in the placebo arm had their UACR reduced by at least 30% from baseline, the cutpoint chosen by Dr. Agarwal based on the American Diabetes Association guideline.
Kaplan-Meier analyses showed that the incidence of the primary kidney outcome – kidney failure, a sustained ≥ 57% decrease in eGFR from baseline, or kidney death – showed close correlation with at least a 30% reduction in UACR regardless of whether the patients in this subgroup received finerenone or placebo.
A different correlation was found in those with a less than 30% reduction in their UACR from baseline to 4 months, regardless of whether this happened on finerenone or placebo. People in the two finerenone trials who had a lesser reduction from baseline in their UACR also had a significantly higher rate of adverse kidney outcomes whether they received finerenone or placebo.
84% of finerenone’s kidney benefit linked to lowering of UACR
The causal-mediation analysis run by Dr. Agarwal quantified this observation, showing that 84% of finerenone’s effect on the kidney outcome was mediated by the reduction in UACR.
“It seems like the kidney benefit [from finerenone] travels through the level of albuminuria. This has broad implications for treatment of people with type 2 diabetes and CKD,” he said.
The link with reduction in albuminuria was weaker for the primary cardiovascular disease outcome: CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. The strongest effect on this outcome was only seen in Kaplan-Meier analysis in those on finerenone who had at least a 30% reduction in their UACR. Those on placebo and with a similarly robust 4-month reduction in UACR showed a much more modest cardiovascular benefit that resembled those on either finerenone or placebo who had a smaller, less than 30% UACR reduction. The mediation analysis of these data showed that UACR reduction accounted for about 37% of the observed cardiovascular benefit seen during the trials.
“The effect of UACR is much stronger for the kidney outcomes,” summed up Dr. Agarwal. The results suggest that for cardiovascular outcomes finerenone works through factors other than lowering of UACR, but he admitted that no one currently knows what those other factors might be.
Treat aggressively to lower UACR by 30%
“I wouldn’t stop finerenone treatment in people who do not get a 30% reduction in their UACR” because these analyses suggest that a portion of the overall benefits from finerenone occurs via other mechanisms, he said. But in patients whose UACR is not reduced by at least 30% “be more aggressive on other measures to reduce UACR,” he advised.
The mediation analyses he ran are “the first time this has been done in nephrology,” producing a “groundbreaking” analysis and finding, Dr. Agarwal said. He also highlighted that the findings primarily relate to the importance of controlling UACR rather than an endorsement of finerenone as the best way to achieve this.
“All I care about is that people think about UACR as a modifiable risk factor. It doesn’t have to be treated with finerenone. It could be a renin-angiotensin system inhibitor, it could be chlorthalidone [a thiazide diuretic]. It just happened that we had a large dataset of people treated with finerenone or placebo.”
He said that future mediation analyses should look at the link between outcomes and UACR reductions produced by agents from the classes of sodium-glucose cotransporter 2 inhibitors and the glucagonlike peptide–1 receptor agonists.
FIDELIO-DKD and FIGARO-DKD were both sponsored by Bayer, the company that markets finerenone. Dr. Agarwal has received personal fees and nonfinancial support from Bayer. He has also received personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Vifor Pharma, and he is a member of data safety monitoring committees for Chinook and Vertex. Dr. Inker is a consultant to Diamtrix, and her department receives research funding from Chinook, Omeros, Reata, and Tricida.
PHILADELPHIA – Reducing albuminuria is a key mediator of the way finerenone (Kerendia, Bayer) reduces adverse renal and cardiovascular events in people with type 2 diabetes and chronic kidney disease (CKD), based on findings from two novel mediation analyses run on data from more than 12,000 people included in the two finerenone pivotal trials.
Results from these analyses showed that that finerenone treatment produced in the FIDELIO-DKD and FIGARO-DKD phase 3 trials. FIDELIO-DKD, which had protection against adverse kidney outcomes as its primary endpoint, supplied the data that led to finerenone’s approval in 2021 by the U.S. Food and Drug Administration for treating people with type 2 diabetes and CKD.
The findings of the mediation analyses underscore the important role that albuminuria plays in the nephropathy and related comorbidities associated with type 2 diabetes and CKD and highlight the importance of ongoing monitoring of albuminuria to guide treatments aimed at minimizing this pathology, said Rajiv Agarwal, MD, who presented a poster on the mediation analyses at Kidney Week 2023, organized by the American Society of Nephrology.
“My hope is that this [report] heightens awareness of UACR” as an important marker of both CKD and of the response by patients with CKD to their treatment, said Dr. Agarwal, a nephrologist and professor at Indiana University in Indianapolis.
“Only about half of people with type 2 diabetes get their UACR measured even though every guideline says measure UACR in people with diabetes. Our findings say that UACR is important not just for CKD diagnosis but also to give feedback” on whether management is working, Dr. Agarwal said in an interview.
Incorporate UACR into clinical decision-making
“My hope is that clinicians will look at UACR as something they should incorporate into clinical decision-making. I measure UACR in my patients [with CKD and type 2 diabetes] at every visit; it’s so inexpensive. Albuminuria is not a good sign. If it’s not reduced in a patient by at least 30% [the recommended minimum reduction by the American Diabetes Association for people who start with a UACR of at least 300 mg/g] clinicians should think of what else they could do to lower albuminuria”: Reduce salt intake, improve blood pressure control, make sure the patient is adherent to treatments, and add additional treatments, Dr. Agarwal advised.
Multiple efforts are now underway or will soon start to boost the rate at which at-risk people get their UACR measured, noted Leslie A. Inker, MD, in a separate talk during Kidney Week. These efforts include the National Kidney Foundation’s CKD Learning Collaborative, which aims to improve clinician awareness of CKD and improve routine testing for CKD. Early results during 2023 from this program in Missouri showed a nearly 8–percentage point increase in the screening rate for UACR levels in at-risk people, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston.
A second advance was introduction in 2018 of the “kidney profile” lab order by the American College of Clinical Pathology that allows clinicians to order as a single test both an estimated glomerular filtration rate (eGFR) and a UACR.
Also, the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance have both taken steps to encourage UACR ordering. The NCQA established a new Healthcare Effectiveness Data and Information Set performance measure for U.S. physicians starting in 2023 that will track measurement of UACR and eGFR in people with diabetes. CMS also has made assessment of kidney health a measure of care quality in programs effective in 2023 and 2024, Dr. Inker noted.
Most subjects had elevated UACRs
The study run by Dr. Agarwal and his associates used data from 12,512 of the more than 13,000 people enrolled in either FIDELITY-DKD or FIGARO-DKD who had UACR measurements recorded at baseline, at 4 months into either study, or both. Their median UACR at the time they began on finerenone or placebo was 514 mg/g, with 67% having a UACR of at least 300 mg/g (macroalbuminuria) and 31% having a UACR of 30-299 mg/g (microalbuminuria). By design, virtually all patients in these two trials were on a renin-angiotensin system inhibitor (either an angiotensin-converting enzyme inhibitor or an angiotensin-receptor blocker), but given the time period when the two trials enrolled participants (during 2015-2018) only 7% of those enrolled were on a sodium-glucose cotransporter 2 inhibitor and only 7% were on a glucagonlike peptide–1 receptor agonist.
Four months after treatment began, 53% of those randomized to finerenone treatment and 27% of those in the placebo arm had their UACR reduced by at least 30% from baseline, the cutpoint chosen by Dr. Agarwal based on the American Diabetes Association guideline.
Kaplan-Meier analyses showed that the incidence of the primary kidney outcome – kidney failure, a sustained ≥ 57% decrease in eGFR from baseline, or kidney death – showed close correlation with at least a 30% reduction in UACR regardless of whether the patients in this subgroup received finerenone or placebo.
A different correlation was found in those with a less than 30% reduction in their UACR from baseline to 4 months, regardless of whether this happened on finerenone or placebo. People in the two finerenone trials who had a lesser reduction from baseline in their UACR also had a significantly higher rate of adverse kidney outcomes whether they received finerenone or placebo.
84% of finerenone’s kidney benefit linked to lowering of UACR
The causal-mediation analysis run by Dr. Agarwal quantified this observation, showing that 84% of finerenone’s effect on the kidney outcome was mediated by the reduction in UACR.
“It seems like the kidney benefit [from finerenone] travels through the level of albuminuria. This has broad implications for treatment of people with type 2 diabetes and CKD,” he said.
The link with reduction in albuminuria was weaker for the primary cardiovascular disease outcome: CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. The strongest effect on this outcome was only seen in Kaplan-Meier analysis in those on finerenone who had at least a 30% reduction in their UACR. Those on placebo and with a similarly robust 4-month reduction in UACR showed a much more modest cardiovascular benefit that resembled those on either finerenone or placebo who had a smaller, less than 30% UACR reduction. The mediation analysis of these data showed that UACR reduction accounted for about 37% of the observed cardiovascular benefit seen during the trials.
“The effect of UACR is much stronger for the kidney outcomes,” summed up Dr. Agarwal. The results suggest that for cardiovascular outcomes finerenone works through factors other than lowering of UACR, but he admitted that no one currently knows what those other factors might be.
Treat aggressively to lower UACR by 30%
“I wouldn’t stop finerenone treatment in people who do not get a 30% reduction in their UACR” because these analyses suggest that a portion of the overall benefits from finerenone occurs via other mechanisms, he said. But in patients whose UACR is not reduced by at least 30% “be more aggressive on other measures to reduce UACR,” he advised.
The mediation analyses he ran are “the first time this has been done in nephrology,” producing a “groundbreaking” analysis and finding, Dr. Agarwal said. He also highlighted that the findings primarily relate to the importance of controlling UACR rather than an endorsement of finerenone as the best way to achieve this.
“All I care about is that people think about UACR as a modifiable risk factor. It doesn’t have to be treated with finerenone. It could be a renin-angiotensin system inhibitor, it could be chlorthalidone [a thiazide diuretic]. It just happened that we had a large dataset of people treated with finerenone or placebo.”
He said that future mediation analyses should look at the link between outcomes and UACR reductions produced by agents from the classes of sodium-glucose cotransporter 2 inhibitors and the glucagonlike peptide–1 receptor agonists.
FIDELIO-DKD and FIGARO-DKD were both sponsored by Bayer, the company that markets finerenone. Dr. Agarwal has received personal fees and nonfinancial support from Bayer. He has also received personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Vifor Pharma, and he is a member of data safety monitoring committees for Chinook and Vertex. Dr. Inker is a consultant to Diamtrix, and her department receives research funding from Chinook, Omeros, Reata, and Tricida.
PHILADELPHIA – Reducing albuminuria is a key mediator of the way finerenone (Kerendia, Bayer) reduces adverse renal and cardiovascular events in people with type 2 diabetes and chronic kidney disease (CKD), based on findings from two novel mediation analyses run on data from more than 12,000 people included in the two finerenone pivotal trials.
Results from these analyses showed that that finerenone treatment produced in the FIDELIO-DKD and FIGARO-DKD phase 3 trials. FIDELIO-DKD, which had protection against adverse kidney outcomes as its primary endpoint, supplied the data that led to finerenone’s approval in 2021 by the U.S. Food and Drug Administration for treating people with type 2 diabetes and CKD.
The findings of the mediation analyses underscore the important role that albuminuria plays in the nephropathy and related comorbidities associated with type 2 diabetes and CKD and highlight the importance of ongoing monitoring of albuminuria to guide treatments aimed at minimizing this pathology, said Rajiv Agarwal, MD, who presented a poster on the mediation analyses at Kidney Week 2023, organized by the American Society of Nephrology.
“My hope is that this [report] heightens awareness of UACR” as an important marker of both CKD and of the response by patients with CKD to their treatment, said Dr. Agarwal, a nephrologist and professor at Indiana University in Indianapolis.
“Only about half of people with type 2 diabetes get their UACR measured even though every guideline says measure UACR in people with diabetes. Our findings say that UACR is important not just for CKD diagnosis but also to give feedback” on whether management is working, Dr. Agarwal said in an interview.
Incorporate UACR into clinical decision-making
“My hope is that clinicians will look at UACR as something they should incorporate into clinical decision-making. I measure UACR in my patients [with CKD and type 2 diabetes] at every visit; it’s so inexpensive. Albuminuria is not a good sign. If it’s not reduced in a patient by at least 30% [the recommended minimum reduction by the American Diabetes Association for people who start with a UACR of at least 300 mg/g] clinicians should think of what else they could do to lower albuminuria”: Reduce salt intake, improve blood pressure control, make sure the patient is adherent to treatments, and add additional treatments, Dr. Agarwal advised.
Multiple efforts are now underway or will soon start to boost the rate at which at-risk people get their UACR measured, noted Leslie A. Inker, MD, in a separate talk during Kidney Week. These efforts include the National Kidney Foundation’s CKD Learning Collaborative, which aims to improve clinician awareness of CKD and improve routine testing for CKD. Early results during 2023 from this program in Missouri showed a nearly 8–percentage point increase in the screening rate for UACR levels in at-risk people, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston.
A second advance was introduction in 2018 of the “kidney profile” lab order by the American College of Clinical Pathology that allows clinicians to order as a single test both an estimated glomerular filtration rate (eGFR) and a UACR.
Also, the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance have both taken steps to encourage UACR ordering. The NCQA established a new Healthcare Effectiveness Data and Information Set performance measure for U.S. physicians starting in 2023 that will track measurement of UACR and eGFR in people with diabetes. CMS also has made assessment of kidney health a measure of care quality in programs effective in 2023 and 2024, Dr. Inker noted.
Most subjects had elevated UACRs
The study run by Dr. Agarwal and his associates used data from 12,512 of the more than 13,000 people enrolled in either FIDELITY-DKD or FIGARO-DKD who had UACR measurements recorded at baseline, at 4 months into either study, or both. Their median UACR at the time they began on finerenone or placebo was 514 mg/g, with 67% having a UACR of at least 300 mg/g (macroalbuminuria) and 31% having a UACR of 30-299 mg/g (microalbuminuria). By design, virtually all patients in these two trials were on a renin-angiotensin system inhibitor (either an angiotensin-converting enzyme inhibitor or an angiotensin-receptor blocker), but given the time period when the two trials enrolled participants (during 2015-2018) only 7% of those enrolled were on a sodium-glucose cotransporter 2 inhibitor and only 7% were on a glucagonlike peptide–1 receptor agonist.
Four months after treatment began, 53% of those randomized to finerenone treatment and 27% of those in the placebo arm had their UACR reduced by at least 30% from baseline, the cutpoint chosen by Dr. Agarwal based on the American Diabetes Association guideline.
Kaplan-Meier analyses showed that the incidence of the primary kidney outcome – kidney failure, a sustained ≥ 57% decrease in eGFR from baseline, or kidney death – showed close correlation with at least a 30% reduction in UACR regardless of whether the patients in this subgroup received finerenone or placebo.
A different correlation was found in those with a less than 30% reduction in their UACR from baseline to 4 months, regardless of whether this happened on finerenone or placebo. People in the two finerenone trials who had a lesser reduction from baseline in their UACR also had a significantly higher rate of adverse kidney outcomes whether they received finerenone or placebo.
84% of finerenone’s kidney benefit linked to lowering of UACR
The causal-mediation analysis run by Dr. Agarwal quantified this observation, showing that 84% of finerenone’s effect on the kidney outcome was mediated by the reduction in UACR.
“It seems like the kidney benefit [from finerenone] travels through the level of albuminuria. This has broad implications for treatment of people with type 2 diabetes and CKD,” he said.
The link with reduction in albuminuria was weaker for the primary cardiovascular disease outcome: CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. The strongest effect on this outcome was only seen in Kaplan-Meier analysis in those on finerenone who had at least a 30% reduction in their UACR. Those on placebo and with a similarly robust 4-month reduction in UACR showed a much more modest cardiovascular benefit that resembled those on either finerenone or placebo who had a smaller, less than 30% UACR reduction. The mediation analysis of these data showed that UACR reduction accounted for about 37% of the observed cardiovascular benefit seen during the trials.
“The effect of UACR is much stronger for the kidney outcomes,” summed up Dr. Agarwal. The results suggest that for cardiovascular outcomes finerenone works through factors other than lowering of UACR, but he admitted that no one currently knows what those other factors might be.
Treat aggressively to lower UACR by 30%
“I wouldn’t stop finerenone treatment in people who do not get a 30% reduction in their UACR” because these analyses suggest that a portion of the overall benefits from finerenone occurs via other mechanisms, he said. But in patients whose UACR is not reduced by at least 30% “be more aggressive on other measures to reduce UACR,” he advised.
The mediation analyses he ran are “the first time this has been done in nephrology,” producing a “groundbreaking” analysis and finding, Dr. Agarwal said. He also highlighted that the findings primarily relate to the importance of controlling UACR rather than an endorsement of finerenone as the best way to achieve this.
“All I care about is that people think about UACR as a modifiable risk factor. It doesn’t have to be treated with finerenone. It could be a renin-angiotensin system inhibitor, it could be chlorthalidone [a thiazide diuretic]. It just happened that we had a large dataset of people treated with finerenone or placebo.”
He said that future mediation analyses should look at the link between outcomes and UACR reductions produced by agents from the classes of sodium-glucose cotransporter 2 inhibitors and the glucagonlike peptide–1 receptor agonists.
FIDELIO-DKD and FIGARO-DKD were both sponsored by Bayer, the company that markets finerenone. Dr. Agarwal has received personal fees and nonfinancial support from Bayer. He has also received personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Vifor Pharma, and he is a member of data safety monitoring committees for Chinook and Vertex. Dr. Inker is a consultant to Diamtrix, and her department receives research funding from Chinook, Omeros, Reata, and Tricida.
AT KIDNEY WEEK 2023
T2D: Real benefits of new oral antidiabetic drugs
Cardiovascular disease is the most common cause of death in people living with type 2 diabetes (T2D). It is true that patient prognoses have improved with the use of metformin and by addressing cardiovascular risk factors. But the new oral antidiabetic drugs, SGLT2 (sodium glucose cotransporter-2) inhibitors (SGLT2i), and glucagon-like peptide-1 receptor agonists (GLP-1Ra) offer fresh therapeutic approaches.
A cohort of more than 2 million patients with T2D
What about in the real world, far away from the ideal conditions of randomized trials? Could combining SGLT2 inhibitors with GLP-1R agonists be even more effective?
These are the questions answered by a large retrospective cohort study in which 2.2 million patients with T2D receiving insulin were initially enrolled and monitored at 85 specialist centers spread throughout three countries (Denmark, the United Kingdom, and the United States).
Three groups were formed from this cohort according to whether they received monotherapy or combination treatments: SGLT2i (n = 143,600), GLP-1Ra (n = 186,841), and SGLT2i + GLP-1Ra (n = 108,5040). A control group received none of these treatments.
Propensity score matching took into account the following relevant variables: age, sex, ischemic heart disease, hypertension, chronic kidney disease, heart failure, and glycated hemoglobin. The data was analyzed using the Cox’s proportional hazards model, with follow-up at 5 years.
Real-world benefits – Even better when combined
The inter-group comparison suggests that oral antidiabetic agents are effective when taking into account three major events:
All-cause mortality: SGLT2i (hazard ratio, 0.49; confidence interval 95% 0.48-0.50); GLP-1Ra (HR, 0.47; CI 95% 0.46-0.48); SGLT2i + GLP-1Ra (HR, 0.25; CI 95% 0.24-0.26).
Admissions rate: respectively HR: 0.73 (0.72-0.74); 0.69 (0.68-0.69); 0.60 (0.59-0.61).
Myocardial infarction rate: respectively HR: 0.75 (0.72-0.78); 0.70 (0.68-0.73); 0.63 (0.60-0.66).
A complementary sub-analysis also revealed a more significant reduction in all-cause mortality in the event of exposure to the combination of two antidiabetic drugs versus SGLT2i alone (HR, 0.53 [0.50-0.55]) and GLP-1Ra as monotherapy (HR, 0.56 [0.54-0.59]).
This real-world retrospective cohort study involves a large sample size: more than 400,000 patients with T2D treated with new oral antidiabetic drugs and as many control patients. It suggests that SGLT2 inhibitors and GLP-1R agonists have a significant effect on overall mortality, as well as on the risk of myocardial infarction and the admissions rate. Yes, it is retrospective, but its findings are in line with those from the most recent and conclusive randomized trials that suggest a cardio- and nephroprotective effect, at least with regard to SGLT2 inhibitors.
This article was translated from JIM and a version appeared on Medscape.com.
Cardiovascular disease is the most common cause of death in people living with type 2 diabetes (T2D). It is true that patient prognoses have improved with the use of metformin and by addressing cardiovascular risk factors. But the new oral antidiabetic drugs, SGLT2 (sodium glucose cotransporter-2) inhibitors (SGLT2i), and glucagon-like peptide-1 receptor agonists (GLP-1Ra) offer fresh therapeutic approaches.
A cohort of more than 2 million patients with T2D
What about in the real world, far away from the ideal conditions of randomized trials? Could combining SGLT2 inhibitors with GLP-1R agonists be even more effective?
These are the questions answered by a large retrospective cohort study in which 2.2 million patients with T2D receiving insulin were initially enrolled and monitored at 85 specialist centers spread throughout three countries (Denmark, the United Kingdom, and the United States).
Three groups were formed from this cohort according to whether they received monotherapy or combination treatments: SGLT2i (n = 143,600), GLP-1Ra (n = 186,841), and SGLT2i + GLP-1Ra (n = 108,5040). A control group received none of these treatments.
Propensity score matching took into account the following relevant variables: age, sex, ischemic heart disease, hypertension, chronic kidney disease, heart failure, and glycated hemoglobin. The data was analyzed using the Cox’s proportional hazards model, with follow-up at 5 years.
Real-world benefits – Even better when combined
The inter-group comparison suggests that oral antidiabetic agents are effective when taking into account three major events:
All-cause mortality: SGLT2i (hazard ratio, 0.49; confidence interval 95% 0.48-0.50); GLP-1Ra (HR, 0.47; CI 95% 0.46-0.48); SGLT2i + GLP-1Ra (HR, 0.25; CI 95% 0.24-0.26).
Admissions rate: respectively HR: 0.73 (0.72-0.74); 0.69 (0.68-0.69); 0.60 (0.59-0.61).
Myocardial infarction rate: respectively HR: 0.75 (0.72-0.78); 0.70 (0.68-0.73); 0.63 (0.60-0.66).
A complementary sub-analysis also revealed a more significant reduction in all-cause mortality in the event of exposure to the combination of two antidiabetic drugs versus SGLT2i alone (HR, 0.53 [0.50-0.55]) and GLP-1Ra as monotherapy (HR, 0.56 [0.54-0.59]).
This real-world retrospective cohort study involves a large sample size: more than 400,000 patients with T2D treated with new oral antidiabetic drugs and as many control patients. It suggests that SGLT2 inhibitors and GLP-1R agonists have a significant effect on overall mortality, as well as on the risk of myocardial infarction and the admissions rate. Yes, it is retrospective, but its findings are in line with those from the most recent and conclusive randomized trials that suggest a cardio- and nephroprotective effect, at least with regard to SGLT2 inhibitors.
This article was translated from JIM and a version appeared on Medscape.com.
Cardiovascular disease is the most common cause of death in people living with type 2 diabetes (T2D). It is true that patient prognoses have improved with the use of metformin and by addressing cardiovascular risk factors. But the new oral antidiabetic drugs, SGLT2 (sodium glucose cotransporter-2) inhibitors (SGLT2i), and glucagon-like peptide-1 receptor agonists (GLP-1Ra) offer fresh therapeutic approaches.
A cohort of more than 2 million patients with T2D
What about in the real world, far away from the ideal conditions of randomized trials? Could combining SGLT2 inhibitors with GLP-1R agonists be even more effective?
These are the questions answered by a large retrospective cohort study in which 2.2 million patients with T2D receiving insulin were initially enrolled and monitored at 85 specialist centers spread throughout three countries (Denmark, the United Kingdom, and the United States).
Three groups were formed from this cohort according to whether they received monotherapy or combination treatments: SGLT2i (n = 143,600), GLP-1Ra (n = 186,841), and SGLT2i + GLP-1Ra (n = 108,5040). A control group received none of these treatments.
Propensity score matching took into account the following relevant variables: age, sex, ischemic heart disease, hypertension, chronic kidney disease, heart failure, and glycated hemoglobin. The data was analyzed using the Cox’s proportional hazards model, with follow-up at 5 years.
Real-world benefits – Even better when combined
The inter-group comparison suggests that oral antidiabetic agents are effective when taking into account three major events:
All-cause mortality: SGLT2i (hazard ratio, 0.49; confidence interval 95% 0.48-0.50); GLP-1Ra (HR, 0.47; CI 95% 0.46-0.48); SGLT2i + GLP-1Ra (HR, 0.25; CI 95% 0.24-0.26).
Admissions rate: respectively HR: 0.73 (0.72-0.74); 0.69 (0.68-0.69); 0.60 (0.59-0.61).
Myocardial infarction rate: respectively HR: 0.75 (0.72-0.78); 0.70 (0.68-0.73); 0.63 (0.60-0.66).
A complementary sub-analysis also revealed a more significant reduction in all-cause mortality in the event of exposure to the combination of two antidiabetic drugs versus SGLT2i alone (HR, 0.53 [0.50-0.55]) and GLP-1Ra as monotherapy (HR, 0.56 [0.54-0.59]).
This real-world retrospective cohort study involves a large sample size: more than 400,000 patients with T2D treated with new oral antidiabetic drugs and as many control patients. It suggests that SGLT2 inhibitors and GLP-1R agonists have a significant effect on overall mortality, as well as on the risk of myocardial infarction and the admissions rate. Yes, it is retrospective, but its findings are in line with those from the most recent and conclusive randomized trials that suggest a cardio- and nephroprotective effect, at least with regard to SGLT2 inhibitors.
This article was translated from JIM and a version appeared on Medscape.com.