User login
FDA authorizes Pfizer’s COVID-19 vaccine for kids
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
FDA posts new websites on accelerated approvals for cancer drugs
, including a public list detailing cases where accelerated approvals have been rescinded for lack of evidence.
On Oct. 29, the Food and Drug Administration posted new websites detailing the status of oncology medicines given these special clearances:
- Ongoing | Cancer Accelerated Approvals
- Verified Clinical Benefit | Cancer Accelerated Approvals
- Withdrawn | Cancer Accelerated Approvals
The FDA’s cancer center also has created a web page called Project Confirm to provide more information on the way it uses accelerated approvals.
There has been increased concern about medicines cleared by accelerated approvals in recent years, culminating in an uproar over the controversial June approval of aducanumab (Aduhelm) for Alzheimer’s disease. This drew more attention to a debate already underway about how much data supports some of the indications for some cancer drugs.
Federal and state officials and advisers are putting more pressure on pharmaceutical companies to prove that medicines that are put on the market through accelerated approval do deliver meaningful benefits for patients.
In addition, earlier this month two of the top health advisers in Barack Obama’s administration proposed a new model through which Medicare could reduce payments for certain cancer drugs cleared through accelerated approvals – and even cut off reimbursements in cases where companies fail to deliver confirmatory evidence for expected benefits.
This “Pay for Drugs That Work Model” was proposed by Richard Frank, PhD, and Ezekiel Emanuel, MD, PhD, in a recent JAMA article. In their view, the FDA’s accelerated drug approval process allows for too many delays in obtaining answers as to whether medicines cleared this way provide expected benefits.
“The proposed Pay for Drugs That Work model could test a modified approach for incentivizing rapid completion of confirmatory trials to inform clinicians and patients about the true risks and benefits of new drugs and improve the value for money of cancer drugs that receive accelerated approval,” they wrote.
Excel files, regular updates
For the FDA, accelerated approvals require balancing an estimated potential benefit for people facing serious diseases (for example, cancer) against serious risks, including potentially exposing patients to costly, toxic drugs that will later be shown not to work for their conditions.
For many years, there has been significant pressure on the FDA to lean toward speedier approvals, with members of Congress, advocacy groups, and drugmakers advocating for broad use of surrogate data in deciding on clearances. The FDA posts biannual reports on its website that highlight how quickly approvals have been granted. But these biannual reports don’t provide much information on the status of accelerated-approval drugs, other than to say if they have been given full approval or withdrawn.
The newly created websites from the FDA’s oncology division appear to reflect growing public interest in knowing what standards the agency sets for confirmatory trials and what deadlines companies face to deliver evidence of significant benefit for their drugs.
The new sortable websites also include details on trials and have links to Excel files which will help researchers and others seeking to track patterns with accelerated approvals. The FDA said in an interview that it intends to update these sites when there are developments with accelerated approvals for cancer drugs, such as new clearances of this type, conversions to regular approvals, and withdrawn approvals.
Julia Beaver, MD, chief of medical oncology at the FDA’s Oncology Center of Excellence, and acting deputy director of the Office of Oncologic Diseases of the FDA’s Center for Drug Evaluation and Research, described the new websites as part of a “commitment to preserve the integrity” of the accelerated approval program.
“These new web pages will make information on our accelerated approvals more transparent,” Dr. Beaver said in an email to this news organization.
The FDA has been able to speed many medicines to market and clear additional uses for drugs already sold through the program, giving people earlier access in many cases to critical medicines, Dr. Beaver said.
More than 165 oncology indications have received accelerated approval, with almost half converted to regular approval in a median of 3 years. Less than 10% of these indications were withdrawn, Dr. Beaver said.
“Of those accelerated approvals that were converted to regular approval, many demonstrated survival advantages to patients with several types of cancer or provided meaningful therapeutic options where none previously existed,” she said.
However, Dr. Beaver also has made public the FDA’s concerns with what she and Richard Pazdur, MD, director of the Oncology Center of Excellence, have described as “dangling” accelerated approvals.
These are cases where the required trials did not end up confirming benefit for a medicine, yet the manufacturer did not move to withdraw an accelerated approval. The FDA’s cancer center has already announced that it is doing an “industry-wide evaluation of accelerated approvals in oncology in which confirmatory trials did not confirm clinical benefit.”
This stems in part from what can be called the FDA’s “growing pains” in its efforts to manage the rapidly changing landscape for these immunotherapy checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials for an Oncologic Drugs Advisory Committee (ODAC) meeting last April on dangling accelerated approvals.
A newly posted chart on withdrawn oncology accelerated approvals, posted by the FDA’s cancer division, makes it clear that the pace of these rescinded clearances has picked up. The chart lists a total 14 withdrawn indications of oncology accelerated approvals.
Six of these withdrawals happened this year.
There were two withdrawals in 2020, including the December withdrawal of nivolumab, (Opdivo) for a form of metastatic lung cancer.
Then there was a significant gap, with no withdrawals going back to 2013 (when there was one). There were two withdrawals in 2012 and three in 2011.
A version of this article first appeared on Medscape.com.
, including a public list detailing cases where accelerated approvals have been rescinded for lack of evidence.
On Oct. 29, the Food and Drug Administration posted new websites detailing the status of oncology medicines given these special clearances:
- Ongoing | Cancer Accelerated Approvals
- Verified Clinical Benefit | Cancer Accelerated Approvals
- Withdrawn | Cancer Accelerated Approvals
The FDA’s cancer center also has created a web page called Project Confirm to provide more information on the way it uses accelerated approvals.
There has been increased concern about medicines cleared by accelerated approvals in recent years, culminating in an uproar over the controversial June approval of aducanumab (Aduhelm) for Alzheimer’s disease. This drew more attention to a debate already underway about how much data supports some of the indications for some cancer drugs.
Federal and state officials and advisers are putting more pressure on pharmaceutical companies to prove that medicines that are put on the market through accelerated approval do deliver meaningful benefits for patients.
In addition, earlier this month two of the top health advisers in Barack Obama’s administration proposed a new model through which Medicare could reduce payments for certain cancer drugs cleared through accelerated approvals – and even cut off reimbursements in cases where companies fail to deliver confirmatory evidence for expected benefits.
This “Pay for Drugs That Work Model” was proposed by Richard Frank, PhD, and Ezekiel Emanuel, MD, PhD, in a recent JAMA article. In their view, the FDA’s accelerated drug approval process allows for too many delays in obtaining answers as to whether medicines cleared this way provide expected benefits.
“The proposed Pay for Drugs That Work model could test a modified approach for incentivizing rapid completion of confirmatory trials to inform clinicians and patients about the true risks and benefits of new drugs and improve the value for money of cancer drugs that receive accelerated approval,” they wrote.
Excel files, regular updates
For the FDA, accelerated approvals require balancing an estimated potential benefit for people facing serious diseases (for example, cancer) against serious risks, including potentially exposing patients to costly, toxic drugs that will later be shown not to work for their conditions.
For many years, there has been significant pressure on the FDA to lean toward speedier approvals, with members of Congress, advocacy groups, and drugmakers advocating for broad use of surrogate data in deciding on clearances. The FDA posts biannual reports on its website that highlight how quickly approvals have been granted. But these biannual reports don’t provide much information on the status of accelerated-approval drugs, other than to say if they have been given full approval or withdrawn.
The newly created websites from the FDA’s oncology division appear to reflect growing public interest in knowing what standards the agency sets for confirmatory trials and what deadlines companies face to deliver evidence of significant benefit for their drugs.
The new sortable websites also include details on trials and have links to Excel files which will help researchers and others seeking to track patterns with accelerated approvals. The FDA said in an interview that it intends to update these sites when there are developments with accelerated approvals for cancer drugs, such as new clearances of this type, conversions to regular approvals, and withdrawn approvals.
Julia Beaver, MD, chief of medical oncology at the FDA’s Oncology Center of Excellence, and acting deputy director of the Office of Oncologic Diseases of the FDA’s Center for Drug Evaluation and Research, described the new websites as part of a “commitment to preserve the integrity” of the accelerated approval program.
“These new web pages will make information on our accelerated approvals more transparent,” Dr. Beaver said in an email to this news organization.
The FDA has been able to speed many medicines to market and clear additional uses for drugs already sold through the program, giving people earlier access in many cases to critical medicines, Dr. Beaver said.
More than 165 oncology indications have received accelerated approval, with almost half converted to regular approval in a median of 3 years. Less than 10% of these indications were withdrawn, Dr. Beaver said.
“Of those accelerated approvals that were converted to regular approval, many demonstrated survival advantages to patients with several types of cancer or provided meaningful therapeutic options where none previously existed,” she said.
However, Dr. Beaver also has made public the FDA’s concerns with what she and Richard Pazdur, MD, director of the Oncology Center of Excellence, have described as “dangling” accelerated approvals.
These are cases where the required trials did not end up confirming benefit for a medicine, yet the manufacturer did not move to withdraw an accelerated approval. The FDA’s cancer center has already announced that it is doing an “industry-wide evaluation of accelerated approvals in oncology in which confirmatory trials did not confirm clinical benefit.”
This stems in part from what can be called the FDA’s “growing pains” in its efforts to manage the rapidly changing landscape for these immunotherapy checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials for an Oncologic Drugs Advisory Committee (ODAC) meeting last April on dangling accelerated approvals.
A newly posted chart on withdrawn oncology accelerated approvals, posted by the FDA’s cancer division, makes it clear that the pace of these rescinded clearances has picked up. The chart lists a total 14 withdrawn indications of oncology accelerated approvals.
Six of these withdrawals happened this year.
There were two withdrawals in 2020, including the December withdrawal of nivolumab, (Opdivo) for a form of metastatic lung cancer.
Then there was a significant gap, with no withdrawals going back to 2013 (when there was one). There were two withdrawals in 2012 and three in 2011.
A version of this article first appeared on Medscape.com.
, including a public list detailing cases where accelerated approvals have been rescinded for lack of evidence.
On Oct. 29, the Food and Drug Administration posted new websites detailing the status of oncology medicines given these special clearances:
- Ongoing | Cancer Accelerated Approvals
- Verified Clinical Benefit | Cancer Accelerated Approvals
- Withdrawn | Cancer Accelerated Approvals
The FDA’s cancer center also has created a web page called Project Confirm to provide more information on the way it uses accelerated approvals.
There has been increased concern about medicines cleared by accelerated approvals in recent years, culminating in an uproar over the controversial June approval of aducanumab (Aduhelm) for Alzheimer’s disease. This drew more attention to a debate already underway about how much data supports some of the indications for some cancer drugs.
Federal and state officials and advisers are putting more pressure on pharmaceutical companies to prove that medicines that are put on the market through accelerated approval do deliver meaningful benefits for patients.
In addition, earlier this month two of the top health advisers in Barack Obama’s administration proposed a new model through which Medicare could reduce payments for certain cancer drugs cleared through accelerated approvals – and even cut off reimbursements in cases where companies fail to deliver confirmatory evidence for expected benefits.
This “Pay for Drugs That Work Model” was proposed by Richard Frank, PhD, and Ezekiel Emanuel, MD, PhD, in a recent JAMA article. In their view, the FDA’s accelerated drug approval process allows for too many delays in obtaining answers as to whether medicines cleared this way provide expected benefits.
“The proposed Pay for Drugs That Work model could test a modified approach for incentivizing rapid completion of confirmatory trials to inform clinicians and patients about the true risks and benefits of new drugs and improve the value for money of cancer drugs that receive accelerated approval,” they wrote.
Excel files, regular updates
For the FDA, accelerated approvals require balancing an estimated potential benefit for people facing serious diseases (for example, cancer) against serious risks, including potentially exposing patients to costly, toxic drugs that will later be shown not to work for their conditions.
For many years, there has been significant pressure on the FDA to lean toward speedier approvals, with members of Congress, advocacy groups, and drugmakers advocating for broad use of surrogate data in deciding on clearances. The FDA posts biannual reports on its website that highlight how quickly approvals have been granted. But these biannual reports don’t provide much information on the status of accelerated-approval drugs, other than to say if they have been given full approval or withdrawn.
The newly created websites from the FDA’s oncology division appear to reflect growing public interest in knowing what standards the agency sets for confirmatory trials and what deadlines companies face to deliver evidence of significant benefit for their drugs.
The new sortable websites also include details on trials and have links to Excel files which will help researchers and others seeking to track patterns with accelerated approvals. The FDA said in an interview that it intends to update these sites when there are developments with accelerated approvals for cancer drugs, such as new clearances of this type, conversions to regular approvals, and withdrawn approvals.
Julia Beaver, MD, chief of medical oncology at the FDA’s Oncology Center of Excellence, and acting deputy director of the Office of Oncologic Diseases of the FDA’s Center for Drug Evaluation and Research, described the new websites as part of a “commitment to preserve the integrity” of the accelerated approval program.
“These new web pages will make information on our accelerated approvals more transparent,” Dr. Beaver said in an email to this news organization.
The FDA has been able to speed many medicines to market and clear additional uses for drugs already sold through the program, giving people earlier access in many cases to critical medicines, Dr. Beaver said.
More than 165 oncology indications have received accelerated approval, with almost half converted to regular approval in a median of 3 years. Less than 10% of these indications were withdrawn, Dr. Beaver said.
“Of those accelerated approvals that were converted to regular approval, many demonstrated survival advantages to patients with several types of cancer or provided meaningful therapeutic options where none previously existed,” she said.
However, Dr. Beaver also has made public the FDA’s concerns with what she and Richard Pazdur, MD, director of the Oncology Center of Excellence, have described as “dangling” accelerated approvals.
These are cases where the required trials did not end up confirming benefit for a medicine, yet the manufacturer did not move to withdraw an accelerated approval. The FDA’s cancer center has already announced that it is doing an “industry-wide evaluation of accelerated approvals in oncology in which confirmatory trials did not confirm clinical benefit.”
This stems in part from what can be called the FDA’s “growing pains” in its efforts to manage the rapidly changing landscape for these immunotherapy checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials for an Oncologic Drugs Advisory Committee (ODAC) meeting last April on dangling accelerated approvals.
A newly posted chart on withdrawn oncology accelerated approvals, posted by the FDA’s cancer division, makes it clear that the pace of these rescinded clearances has picked up. The chart lists a total 14 withdrawn indications of oncology accelerated approvals.
Six of these withdrawals happened this year.
There were two withdrawals in 2020, including the December withdrawal of nivolumab, (Opdivo) for a form of metastatic lung cancer.
Then there was a significant gap, with no withdrawals going back to 2013 (when there was one). There were two withdrawals in 2012 and three in 2011.
A version of this article first appeared on Medscape.com.
Iatrogenic hyponatremia in a patient with bipolar disorder
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.
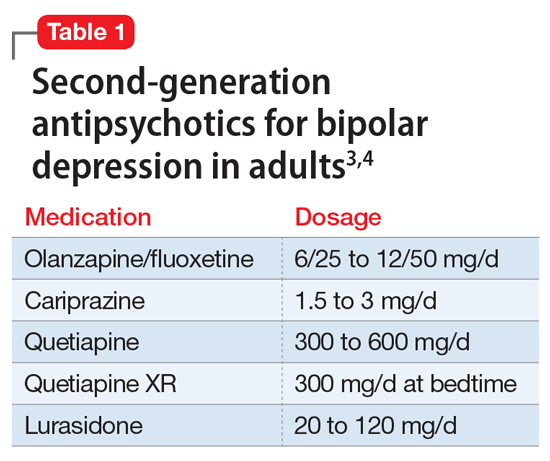
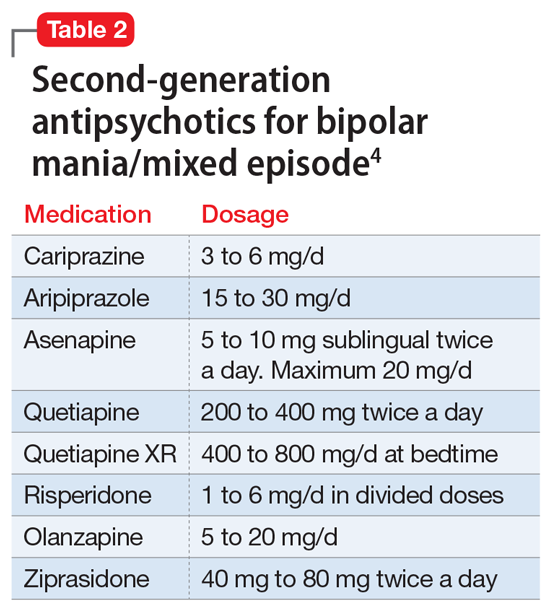
The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.
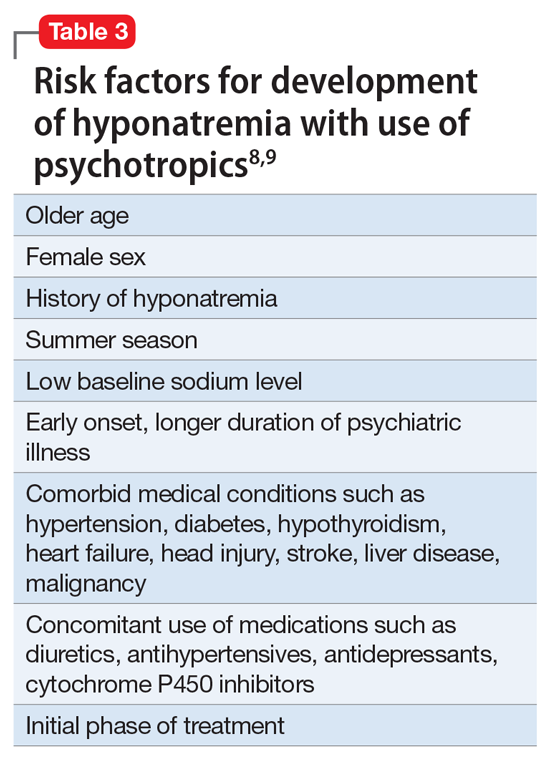
Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.


The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.

Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.


The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.

Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
Antidepressant may cut COVID-19–related hospitalization, mortality: TOGETHER
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Upadacitinib shows potential for ulcerative colitis
LAS VEGAS – An oral Janus kinase 1 inhibitor upadacitinib (Rinvoq, AbbVie) showed high efficacy and good safety as a treatment for ulcerative colitis in a phase 3 trial.
The finding could provide some reassurance after the Food and Drug Administration recently warned of an increased risk of cancer and heart disease associated with medications in the same class as upadacitinib.
“Serious adverse events were numerically lower in patients on upadacitinib, and discontinuations from the study due to adverse events were also lower” than in patients taking a placebo, said Edward Loftus, MD, a gastroenterologist at the Mayo Clinic in Rochester, Minn.
Dr. Loftus presented the findings from the U-ACCOMPLISH study at the annual meeting of the American College of Gastroenterology.
Although other medications are approved for the treatment of ulcerative colitis, including biologics, many patients do not respond. In 2019, tofacitinib (Xeljanz) became the first JAK inhibitor approved for this condition. It works by blocking the JAK1 and JAK3 inflammation pathways, and at high concentrations, it also blocks the tyrosine kinase 2 and JAK2 pathways.
However, adverse events seen in clinical trials of tofacitinib include pneumonia, herpes zoster, anal abscess, and Clostridioides difficile infections. And, as reported by this news organization in September, the FDA required its manufacturer, Pfizer, to add a boxed warning that includes information about the risks of stroke, cancer, blood clots, and death.
Upadacitinib may be more selective and reversible because it preferentially blocks JAK1 or JAK1/3. In August 2019, it received FDA approval at a dose of 15 mg for adult patients with moderately to severely active RA who have had an inadequate response or intolerance to methotrexate.
But the FDA applied the same warnings to upadacitinib – and to a third related drug, baricitinib (Olumiant) – that it required for tofacitinib, even though they are not as well studied.
The FDA also limited approved uses of these three medications to patients who have not responded well to tumor necrosis factor blockers to ensure their benefits outweigh their risks.
A well-tolerated treatment
U-ACCOMPLISH is one of two phase 3 trials induction trials completed on upadacitinib.
Investigators randomized 522 people with moderately to severely active ulcerative colitis, defined as Adapted Mayo Score 5-9 with a centrally read endoscopic score of 2-3. Of those patients, the intent to treat population included 341 in the upadacitinib group (45 mg once daily) and 174 in the placebo group.
The baseline demographics and disease characteristics were similar between groups. More than two-thirds of patients in both groups were White, and more than two-thirds were men. In the upadacitinib group, 50.7% had responded inadequately to biologic treatments, compared with 51.1% in the placebo group.
After 8 weeks, a significantly higher proportion of patients receiving upadacitinib achieved clinical remission as defined by the adapted Mayo Score (stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore of 0, and Mayo endoscopic subscore ≤1).
“In terms of the efficacy, I think it’s very, very promising,” said Derrick Eichele, MD, an assistant professor of gastroenterology-hepatology at the University of Nebraska Medical Center in Omaha, who was not involved in the trial.
The efficacy data were similar to those reported for tofacitinib in clinical trials, he said in an interview. “But I think again, what we’re waiting to see is how is this going to be positioned in relation to tofacitinib in terms of safety profile.”
More patients in the upadacitinib group reported adverse events, including those deemed related to the drug. However, the proportion that were severe, serious, or led to discontinuation was higher in the placebo group. No one in the study died, and no one in the upadacitinib group had an adjudicated major adverse cardiovascular event, tuberculosis, or malignancy.
The most common adverse events were acne, blood creatine phosphokinase elevation, and anemia, which were all more common in the upadacitinib group, and headache and worsening of ulcerative colitis, which were more common in the placebo group.
Among adverse events of special interest, anemia, neutropenia, hepatic disorder, lymphopenia, serious infection, and opportunistic infection were more common in the upadacitinib group than in the placebo group. The four opportunistic infections in the upadacitinib group included two cases of herpes zoster.
In reviewing the poster presented at this meeting, the cases of neutropenia and hepatic disorder in the upadacitinib group stood out for Dr. Eichele. But he said it’s hard to pass judgment based on this amount of data. He is looking forward to a peer-reviewed publication. “I’ll be interested to see what it shows in terms of the details.”
Phase 3 trials of upadacitinib are underway in atopic dermatitis, RA, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, and Takayasu arteritis as well as ulcerative colitis.
In a 52-week maintenance trial, according to a press release, malignancies (excluding nonmelanoma skin cancer) included one event among 148 people taking a 15-mg dose of upadacitinib 15, two events among 154 people taking a 30-mg dose of upadacitinib, and one event among 149 people in the placebo group.
Two cases of pulmonary embolism were reported in the 15-mg group and two cases of deep vein thrombosis were reported in the 30-mg group, compared with one event of ovarian vein thrombosis in the placebo group. One adjudicated major cardiovascular event each were reported in the upadacitinib 30-mg group and the placebo group. No one died.
The study was funded by AbbVie. Dr. Loftus reported that he is a consultant for AbbVie as well as multiple other gastroenterology drug companies. Dr. Eichele disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LAS VEGAS – An oral Janus kinase 1 inhibitor upadacitinib (Rinvoq, AbbVie) showed high efficacy and good safety as a treatment for ulcerative colitis in a phase 3 trial.
The finding could provide some reassurance after the Food and Drug Administration recently warned of an increased risk of cancer and heart disease associated with medications in the same class as upadacitinib.
“Serious adverse events were numerically lower in patients on upadacitinib, and discontinuations from the study due to adverse events were also lower” than in patients taking a placebo, said Edward Loftus, MD, a gastroenterologist at the Mayo Clinic in Rochester, Minn.
Dr. Loftus presented the findings from the U-ACCOMPLISH study at the annual meeting of the American College of Gastroenterology.
Although other medications are approved for the treatment of ulcerative colitis, including biologics, many patients do not respond. In 2019, tofacitinib (Xeljanz) became the first JAK inhibitor approved for this condition. It works by blocking the JAK1 and JAK3 inflammation pathways, and at high concentrations, it also blocks the tyrosine kinase 2 and JAK2 pathways.
However, adverse events seen in clinical trials of tofacitinib include pneumonia, herpes zoster, anal abscess, and Clostridioides difficile infections. And, as reported by this news organization in September, the FDA required its manufacturer, Pfizer, to add a boxed warning that includes information about the risks of stroke, cancer, blood clots, and death.
Upadacitinib may be more selective and reversible because it preferentially blocks JAK1 or JAK1/3. In August 2019, it received FDA approval at a dose of 15 mg for adult patients with moderately to severely active RA who have had an inadequate response or intolerance to methotrexate.
But the FDA applied the same warnings to upadacitinib – and to a third related drug, baricitinib (Olumiant) – that it required for tofacitinib, even though they are not as well studied.
The FDA also limited approved uses of these three medications to patients who have not responded well to tumor necrosis factor blockers to ensure their benefits outweigh their risks.
A well-tolerated treatment
U-ACCOMPLISH is one of two phase 3 trials induction trials completed on upadacitinib.
Investigators randomized 522 people with moderately to severely active ulcerative colitis, defined as Adapted Mayo Score 5-9 with a centrally read endoscopic score of 2-3. Of those patients, the intent to treat population included 341 in the upadacitinib group (45 mg once daily) and 174 in the placebo group.
The baseline demographics and disease characteristics were similar between groups. More than two-thirds of patients in both groups were White, and more than two-thirds were men. In the upadacitinib group, 50.7% had responded inadequately to biologic treatments, compared with 51.1% in the placebo group.
After 8 weeks, a significantly higher proportion of patients receiving upadacitinib achieved clinical remission as defined by the adapted Mayo Score (stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore of 0, and Mayo endoscopic subscore ≤1).
“In terms of the efficacy, I think it’s very, very promising,” said Derrick Eichele, MD, an assistant professor of gastroenterology-hepatology at the University of Nebraska Medical Center in Omaha, who was not involved in the trial.
The efficacy data were similar to those reported for tofacitinib in clinical trials, he said in an interview. “But I think again, what we’re waiting to see is how is this going to be positioned in relation to tofacitinib in terms of safety profile.”
More patients in the upadacitinib group reported adverse events, including those deemed related to the drug. However, the proportion that were severe, serious, or led to discontinuation was higher in the placebo group. No one in the study died, and no one in the upadacitinib group had an adjudicated major adverse cardiovascular event, tuberculosis, or malignancy.
The most common adverse events were acne, blood creatine phosphokinase elevation, and anemia, which were all more common in the upadacitinib group, and headache and worsening of ulcerative colitis, which were more common in the placebo group.
Among adverse events of special interest, anemia, neutropenia, hepatic disorder, lymphopenia, serious infection, and opportunistic infection were more common in the upadacitinib group than in the placebo group. The four opportunistic infections in the upadacitinib group included two cases of herpes zoster.
In reviewing the poster presented at this meeting, the cases of neutropenia and hepatic disorder in the upadacitinib group stood out for Dr. Eichele. But he said it’s hard to pass judgment based on this amount of data. He is looking forward to a peer-reviewed publication. “I’ll be interested to see what it shows in terms of the details.”
Phase 3 trials of upadacitinib are underway in atopic dermatitis, RA, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, and Takayasu arteritis as well as ulcerative colitis.
In a 52-week maintenance trial, according to a press release, malignancies (excluding nonmelanoma skin cancer) included one event among 148 people taking a 15-mg dose of upadacitinib 15, two events among 154 people taking a 30-mg dose of upadacitinib, and one event among 149 people in the placebo group.
Two cases of pulmonary embolism were reported in the 15-mg group and two cases of deep vein thrombosis were reported in the 30-mg group, compared with one event of ovarian vein thrombosis in the placebo group. One adjudicated major cardiovascular event each were reported in the upadacitinib 30-mg group and the placebo group. No one died.
The study was funded by AbbVie. Dr. Loftus reported that he is a consultant for AbbVie as well as multiple other gastroenterology drug companies. Dr. Eichele disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LAS VEGAS – An oral Janus kinase 1 inhibitor upadacitinib (Rinvoq, AbbVie) showed high efficacy and good safety as a treatment for ulcerative colitis in a phase 3 trial.
The finding could provide some reassurance after the Food and Drug Administration recently warned of an increased risk of cancer and heart disease associated with medications in the same class as upadacitinib.
“Serious adverse events were numerically lower in patients on upadacitinib, and discontinuations from the study due to adverse events were also lower” than in patients taking a placebo, said Edward Loftus, MD, a gastroenterologist at the Mayo Clinic in Rochester, Minn.
Dr. Loftus presented the findings from the U-ACCOMPLISH study at the annual meeting of the American College of Gastroenterology.
Although other medications are approved for the treatment of ulcerative colitis, including biologics, many patients do not respond. In 2019, tofacitinib (Xeljanz) became the first JAK inhibitor approved for this condition. It works by blocking the JAK1 and JAK3 inflammation pathways, and at high concentrations, it also blocks the tyrosine kinase 2 and JAK2 pathways.
However, adverse events seen in clinical trials of tofacitinib include pneumonia, herpes zoster, anal abscess, and Clostridioides difficile infections. And, as reported by this news organization in September, the FDA required its manufacturer, Pfizer, to add a boxed warning that includes information about the risks of stroke, cancer, blood clots, and death.
Upadacitinib may be more selective and reversible because it preferentially blocks JAK1 or JAK1/3. In August 2019, it received FDA approval at a dose of 15 mg for adult patients with moderately to severely active RA who have had an inadequate response or intolerance to methotrexate.
But the FDA applied the same warnings to upadacitinib – and to a third related drug, baricitinib (Olumiant) – that it required for tofacitinib, even though they are not as well studied.
The FDA also limited approved uses of these three medications to patients who have not responded well to tumor necrosis factor blockers to ensure their benefits outweigh their risks.
A well-tolerated treatment
U-ACCOMPLISH is one of two phase 3 trials induction trials completed on upadacitinib.
Investigators randomized 522 people with moderately to severely active ulcerative colitis, defined as Adapted Mayo Score 5-9 with a centrally read endoscopic score of 2-3. Of those patients, the intent to treat population included 341 in the upadacitinib group (45 mg once daily) and 174 in the placebo group.
The baseline demographics and disease characteristics were similar between groups. More than two-thirds of patients in both groups were White, and more than two-thirds were men. In the upadacitinib group, 50.7% had responded inadequately to biologic treatments, compared with 51.1% in the placebo group.
After 8 weeks, a significantly higher proportion of patients receiving upadacitinib achieved clinical remission as defined by the adapted Mayo Score (stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore of 0, and Mayo endoscopic subscore ≤1).
“In terms of the efficacy, I think it’s very, very promising,” said Derrick Eichele, MD, an assistant professor of gastroenterology-hepatology at the University of Nebraska Medical Center in Omaha, who was not involved in the trial.
The efficacy data were similar to those reported for tofacitinib in clinical trials, he said in an interview. “But I think again, what we’re waiting to see is how is this going to be positioned in relation to tofacitinib in terms of safety profile.”
More patients in the upadacitinib group reported adverse events, including those deemed related to the drug. However, the proportion that were severe, serious, or led to discontinuation was higher in the placebo group. No one in the study died, and no one in the upadacitinib group had an adjudicated major adverse cardiovascular event, tuberculosis, or malignancy.
The most common adverse events were acne, blood creatine phosphokinase elevation, and anemia, which were all more common in the upadacitinib group, and headache and worsening of ulcerative colitis, which were more common in the placebo group.
Among adverse events of special interest, anemia, neutropenia, hepatic disorder, lymphopenia, serious infection, and opportunistic infection were more common in the upadacitinib group than in the placebo group. The four opportunistic infections in the upadacitinib group included two cases of herpes zoster.
In reviewing the poster presented at this meeting, the cases of neutropenia and hepatic disorder in the upadacitinib group stood out for Dr. Eichele. But he said it’s hard to pass judgment based on this amount of data. He is looking forward to a peer-reviewed publication. “I’ll be interested to see what it shows in terms of the details.”
Phase 3 trials of upadacitinib are underway in atopic dermatitis, RA, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, and Takayasu arteritis as well as ulcerative colitis.
In a 52-week maintenance trial, according to a press release, malignancies (excluding nonmelanoma skin cancer) included one event among 148 people taking a 15-mg dose of upadacitinib 15, two events among 154 people taking a 30-mg dose of upadacitinib, and one event among 149 people in the placebo group.
Two cases of pulmonary embolism were reported in the 15-mg group and two cases of deep vein thrombosis were reported in the 30-mg group, compared with one event of ovarian vein thrombosis in the placebo group. One adjudicated major cardiovascular event each were reported in the upadacitinib 30-mg group and the placebo group. No one died.
The study was funded by AbbVie. Dr. Loftus reported that he is a consultant for AbbVie as well as multiple other gastroenterology drug companies. Dr. Eichele disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACG 2021
Fatal child poisonings linked to common cough and cold meds
A number of fatal child poisonings have been linked to common cough and cold medications, according to a report.
The Pediatric Cough and Cold Safety Surveillance System, which tracks fatal child poisonings, has identified 40 such deaths in recent years and raised particular concern about medications containing diphenhydramine, a common antihistamine that can be sedating.
“There is little evidence that cough and cold medicines make children feel better or reduce their symptoms, but there is evidence they can suffer harm,” says Kevin Osterhoudt, MD, medical director of the Poison Control Center at the Children’s Hospital of Philadelphia.
In recent years, the FDA has advised labeling changes and recommended that cough and cold medications not be given to children younger than 2. Drugmakers also voluntarily relabeled these products to state “do not use in children under 4 years of age.”
Compared to older children or adults, young children have a different physiology when they breathe, so any product containing antihistamines can be a danger to little kids, Dr. Osterhoudt says.
But a recent survey shows about half of American parents gave their child cough and cold medication the last time they were ill, Dr. Osterhoudt says. And the findings suggest that cough and cold medications are in homes where children might find them.
Using the new evidence from the national surveillance system, investigators set up an expert panel to review the results. They found that most of the deaths were in children under the age of 2. The results were reported in the October issue of Pediatrics.
In seven instances, death followed the intentional use of medication to sedate the child, reports lead investigator Laurie Seidel Halmo, MD, from Children’s Hospital Colorado, Aurora.
“It’s not uncommon for parents to use sedatives like diphenhydramine to make their child sleepy for activities like air travel,” Dr. Osterhoudt says.
While antihistamines can be sedating, “an overdose of antihistamines like diphenhydramine can paradoxically become a stimulant,” having the opposite effect, he explains.
Adults and teens who take overdoses will sometimes become delirious, hallucinate, and have a racing heart.
But in young children, “if not careful with your dosing, you could actually give too much and create this stimulant activity,” Dr. Osterhoudt says.
In six other cases, the cough and cold medication was given to murder the child, the investigators reported.
The findings are “concerning,” especially with “more than one-half of nontherapeutic intent cases determined to be malicious in nature,” Michele Burns, MD, from Boston Children’s Hospital, and Madeline Renny, MD, from the Grossman School of Medicine in New York, wrote in a commentary with the report.
This important fatality review shows that despite safety efforts, young children remain at risk for death, they report.
The investigators point out that labeling changes do not seem to have protected vulnerable children, and they recommend that doctors educate parents and caregivers about the risk of cough and cold medications.
Dr. Halmo and her team also recommend that the medical community and child welfare advocates be on the lookout for medication use as a source of child abuse.
At home, preventing accidental ingestion could go along with other practices already ingrained in the minds of many, Dr. Osterhoudt says.
“We know to change the clocks in the spring and fall and make sure your smoke detector and carbon monoxide detector has fresh batteries, but maybe it’s also a good time to look at medicines in the house.”
In other words, after you change the clocks, it’s time to take inventory of medications around the house, and if they’re no longer in use, safely dispose of them.
The American Academy of Pediatrics offers guidelines on the safe home storage of medications to keep them out of reach of children and the use of protective caps on drugs.
A version of this article first appeared on WebMD.com.
A number of fatal child poisonings have been linked to common cough and cold medications, according to a report.
The Pediatric Cough and Cold Safety Surveillance System, which tracks fatal child poisonings, has identified 40 such deaths in recent years and raised particular concern about medications containing diphenhydramine, a common antihistamine that can be sedating.
“There is little evidence that cough and cold medicines make children feel better or reduce their symptoms, but there is evidence they can suffer harm,” says Kevin Osterhoudt, MD, medical director of the Poison Control Center at the Children’s Hospital of Philadelphia.
In recent years, the FDA has advised labeling changes and recommended that cough and cold medications not be given to children younger than 2. Drugmakers also voluntarily relabeled these products to state “do not use in children under 4 years of age.”
Compared to older children or adults, young children have a different physiology when they breathe, so any product containing antihistamines can be a danger to little kids, Dr. Osterhoudt says.
But a recent survey shows about half of American parents gave their child cough and cold medication the last time they were ill, Dr. Osterhoudt says. And the findings suggest that cough and cold medications are in homes where children might find them.
Using the new evidence from the national surveillance system, investigators set up an expert panel to review the results. They found that most of the deaths were in children under the age of 2. The results were reported in the October issue of Pediatrics.
In seven instances, death followed the intentional use of medication to sedate the child, reports lead investigator Laurie Seidel Halmo, MD, from Children’s Hospital Colorado, Aurora.
“It’s not uncommon for parents to use sedatives like diphenhydramine to make their child sleepy for activities like air travel,” Dr. Osterhoudt says.
While antihistamines can be sedating, “an overdose of antihistamines like diphenhydramine can paradoxically become a stimulant,” having the opposite effect, he explains.
Adults and teens who take overdoses will sometimes become delirious, hallucinate, and have a racing heart.
But in young children, “if not careful with your dosing, you could actually give too much and create this stimulant activity,” Dr. Osterhoudt says.
In six other cases, the cough and cold medication was given to murder the child, the investigators reported.
The findings are “concerning,” especially with “more than one-half of nontherapeutic intent cases determined to be malicious in nature,” Michele Burns, MD, from Boston Children’s Hospital, and Madeline Renny, MD, from the Grossman School of Medicine in New York, wrote in a commentary with the report.
This important fatality review shows that despite safety efforts, young children remain at risk for death, they report.
The investigators point out that labeling changes do not seem to have protected vulnerable children, and they recommend that doctors educate parents and caregivers about the risk of cough and cold medications.
Dr. Halmo and her team also recommend that the medical community and child welfare advocates be on the lookout for medication use as a source of child abuse.
At home, preventing accidental ingestion could go along with other practices already ingrained in the minds of many, Dr. Osterhoudt says.
“We know to change the clocks in the spring and fall and make sure your smoke detector and carbon monoxide detector has fresh batteries, but maybe it’s also a good time to look at medicines in the house.”
In other words, after you change the clocks, it’s time to take inventory of medications around the house, and if they’re no longer in use, safely dispose of them.
The American Academy of Pediatrics offers guidelines on the safe home storage of medications to keep them out of reach of children and the use of protective caps on drugs.
A version of this article first appeared on WebMD.com.
A number of fatal child poisonings have been linked to common cough and cold medications, according to a report.
The Pediatric Cough and Cold Safety Surveillance System, which tracks fatal child poisonings, has identified 40 such deaths in recent years and raised particular concern about medications containing diphenhydramine, a common antihistamine that can be sedating.
“There is little evidence that cough and cold medicines make children feel better or reduce their symptoms, but there is evidence they can suffer harm,” says Kevin Osterhoudt, MD, medical director of the Poison Control Center at the Children’s Hospital of Philadelphia.
In recent years, the FDA has advised labeling changes and recommended that cough and cold medications not be given to children younger than 2. Drugmakers also voluntarily relabeled these products to state “do not use in children under 4 years of age.”
Compared to older children or adults, young children have a different physiology when they breathe, so any product containing antihistamines can be a danger to little kids, Dr. Osterhoudt says.
But a recent survey shows about half of American parents gave their child cough and cold medication the last time they were ill, Dr. Osterhoudt says. And the findings suggest that cough and cold medications are in homes where children might find them.
Using the new evidence from the national surveillance system, investigators set up an expert panel to review the results. They found that most of the deaths were in children under the age of 2. The results were reported in the October issue of Pediatrics.
In seven instances, death followed the intentional use of medication to sedate the child, reports lead investigator Laurie Seidel Halmo, MD, from Children’s Hospital Colorado, Aurora.
“It’s not uncommon for parents to use sedatives like diphenhydramine to make their child sleepy for activities like air travel,” Dr. Osterhoudt says.
While antihistamines can be sedating, “an overdose of antihistamines like diphenhydramine can paradoxically become a stimulant,” having the opposite effect, he explains.
Adults and teens who take overdoses will sometimes become delirious, hallucinate, and have a racing heart.
But in young children, “if not careful with your dosing, you could actually give too much and create this stimulant activity,” Dr. Osterhoudt says.
In six other cases, the cough and cold medication was given to murder the child, the investigators reported.
The findings are “concerning,” especially with “more than one-half of nontherapeutic intent cases determined to be malicious in nature,” Michele Burns, MD, from Boston Children’s Hospital, and Madeline Renny, MD, from the Grossman School of Medicine in New York, wrote in a commentary with the report.
This important fatality review shows that despite safety efforts, young children remain at risk for death, they report.
The investigators point out that labeling changes do not seem to have protected vulnerable children, and they recommend that doctors educate parents and caregivers about the risk of cough and cold medications.
Dr. Halmo and her team also recommend that the medical community and child welfare advocates be on the lookout for medication use as a source of child abuse.
At home, preventing accidental ingestion could go along with other practices already ingrained in the minds of many, Dr. Osterhoudt says.
“We know to change the clocks in the spring and fall and make sure your smoke detector and carbon monoxide detector has fresh batteries, but maybe it’s also a good time to look at medicines in the house.”
In other words, after you change the clocks, it’s time to take inventory of medications around the house, and if they’re no longer in use, safely dispose of them.
The American Academy of Pediatrics offers guidelines on the safe home storage of medications to keep them out of reach of children and the use of protective caps on drugs.
A version of this article first appeared on WebMD.com.
In and out surgeries become the norm during pandemic
Urologist Ronney Abaza, MD, a robotic surgery specialist in Dublin, Ohio, and colleagues, reviewed robotic surgeries at their hospital during COVID-19 restrictions on surgery in Ohio between March 17 and June 5, 2020, and compared them with robotic procedures before COVID-19 and after restrictions were lifted. They published their results in Urology.
Since 2016, the hospital has offered the option of same-day discharge (SDD) to all robotic urologic surgery patients, regardless of procedure or patient-specific factors.
Among patients who had surgery during COVID-19 restrictions, 98% (87/89 patients) opted for SDD versus 52% in the group having surgery before the restrictions (P < .00001). After the COVID-19 surgery restrictions were lifted, the higher rate of SDD remained at 98%.
“There were no differences in 30-day complications or readmissions between SDD and overnight patients,” the authors write.
The right patient, the right motivation for successful surgery
Brian Lane, MD, PhD, a urologic oncologist with Spectrum Health in Grand Rapids, Michigan, told this news organization that, for nephrectomies, uptake of same-day discharge will continue to be slow.
“You have to have the right patient, the right patient motivation, and the surgery has to go smoothly,” he said. “If you start sending everyone home the same day, you will certainly see readmissions,” he said.
Dr. Lane is part of the Michigan Urologic Surgery Improvement Collaborative and he said the group recently looked at same-day discharge outcomes after robotic prostatectomies with SDD as compared with 1-2 nights in the hospital.
The work has not yet been published but, “There was a slight signal that there were increased readmissions with same-day discharge vs. 0-1 day,” he said.
A paper on outcomes of same-day discharge in total knee arthroplasty in the Journal of Bone & Joint Surgery found a higher risk of perioperative complications “including component failure, surgical site infection, knee stiffness, and deep vein thrombosis.” Researchers compared outcomes between 4,391 patients who underwent outpatient TKA and 128,951 patients who underwent inpatient TKA.
But for other many surgeries, same-day discharge numbers are increasing without worsening outcomes.
A paper in the Journal of Robotic Surgery found that same-day discharge following robotic-assisted endometrial cancer staging is “safe and feasible.”
Stephen Bradley, MD, MPH, with the Minneapolis Heart Institute in Minneapolis, and colleagues write in the Journal of the American College of Cardiology: Cardiovascular Interventions that they found a large increase in the use of same-day discharge after elective percutaneous coronary intervention (PCI) was not associated with worse 30-day mortality rates or readmission.
In that study, 114,461 patients were discharged the same day they underwent PCI. The proportion of patients who had a same-day discharge increased from 4.5% in 2009 to 28.6% in the fourth quarter of 2017.
Risk-adjusted 30-day mortality did not change in that time, while risk-adjusted rehospitalization decreased over time and more quickly when patients had same-day discharge.
Deepak L. Bhatt, MD, MPH, and Jonathan G. Sung, MBCHB, both of Brigham and Women’s Hospital Heart & Vascular Center, Harvard Medical School, Boston, wrote in an accompanying article that, “Advances in the devices and techniques of PCI have improved the safety and efficacy of the procedure. In selected patients, same-day discharge has become possible, and overnight in-hospital observation can be avoided. By reducing unnecessary hospital stays, both patients and hospitals could benefit.”
Evan Garden, a medical student at Icahn School of Medicine at Mount Sinai in New York, presented findings at the American Urological Association 2021 annual meeting that show patients selected for same-day discharge after partial or radical nephrectomy did not have increased rates of postoperative complications or readmissions in the immediate postoperative period, compared with standard discharge of 1-3 days.
Case studies in nephrectomy
While several case studies have looked at the feasibility and safety of performing partial and radical nephrectomy with same-day discharge in select cases, “this topic has not been addressed on a national level,” Mr. Garden said.
Few patients who have partial or radical nephrectomies have same-day discharges. The researchers found that fewer than 1% of patients who have either procedure in the sample studied were discharged the same day.
Researchers used the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, a nationally representative deidentified database that prospectively tracks patient characteristics and 30-day perioperative outcomes for major inpatient and outpatient surgical procedures at more than 700 hospitals.
They extracted all minimally invasive partial and radical nephrectomies from 2012 to 2019 and refined the cohort to 28,140 patients who were theoretically eligible for same-day discharge: Of those, 237 (0.8%) had SSD, and 27,903 (99.2%) had a standard-length discharge (SLD).
The team found that there were no differences in 30-day complications or readmissions between same-day discharge (Clavien-Dindo [CD] I/II, 4.22%; CD III, 0%; CD IV, 1.27%; readmission, 4.64%); and SLD (CD I/II, 4.11%; CD III, 0.95%; CD IV, 0.79%; readmission, 3.90%; all P > .05).
Controlling for demographic and clinical variables, SDD was not associated with greater risk of 30-day complications or readmissions (CD I/II: odds ratio, 1.08; 95% confidence interval, 0.57-2.048; P = .813; CD IV: OR 1.699; 95% CI, 0.537-5.375; P = .367; readmission: OR, 1.254; 95% CI, 0.681-2.31; P = .467).
Mr. Garden and coauthors report no relevant financial relationships.
Dr. Lane reports no relevant financial relationships.
Urologist Ronney Abaza, MD, a robotic surgery specialist in Dublin, Ohio, and colleagues, reviewed robotic surgeries at their hospital during COVID-19 restrictions on surgery in Ohio between March 17 and June 5, 2020, and compared them with robotic procedures before COVID-19 and after restrictions were lifted. They published their results in Urology.
Since 2016, the hospital has offered the option of same-day discharge (SDD) to all robotic urologic surgery patients, regardless of procedure or patient-specific factors.
Among patients who had surgery during COVID-19 restrictions, 98% (87/89 patients) opted for SDD versus 52% in the group having surgery before the restrictions (P < .00001). After the COVID-19 surgery restrictions were lifted, the higher rate of SDD remained at 98%.
“There were no differences in 30-day complications or readmissions between SDD and overnight patients,” the authors write.
The right patient, the right motivation for successful surgery
Brian Lane, MD, PhD, a urologic oncologist with Spectrum Health in Grand Rapids, Michigan, told this news organization that, for nephrectomies, uptake of same-day discharge will continue to be slow.
“You have to have the right patient, the right patient motivation, and the surgery has to go smoothly,” he said. “If you start sending everyone home the same day, you will certainly see readmissions,” he said.
Dr. Lane is part of the Michigan Urologic Surgery Improvement Collaborative and he said the group recently looked at same-day discharge outcomes after robotic prostatectomies with SDD as compared with 1-2 nights in the hospital.
The work has not yet been published but, “There was a slight signal that there were increased readmissions with same-day discharge vs. 0-1 day,” he said.
A paper on outcomes of same-day discharge in total knee arthroplasty in the Journal of Bone & Joint Surgery found a higher risk of perioperative complications “including component failure, surgical site infection, knee stiffness, and deep vein thrombosis.” Researchers compared outcomes between 4,391 patients who underwent outpatient TKA and 128,951 patients who underwent inpatient TKA.
But for other many surgeries, same-day discharge numbers are increasing without worsening outcomes.
A paper in the Journal of Robotic Surgery found that same-day discharge following robotic-assisted endometrial cancer staging is “safe and feasible.”
Stephen Bradley, MD, MPH, with the Minneapolis Heart Institute in Minneapolis, and colleagues write in the Journal of the American College of Cardiology: Cardiovascular Interventions that they found a large increase in the use of same-day discharge after elective percutaneous coronary intervention (PCI) was not associated with worse 30-day mortality rates or readmission.
In that study, 114,461 patients were discharged the same day they underwent PCI. The proportion of patients who had a same-day discharge increased from 4.5% in 2009 to 28.6% in the fourth quarter of 2017.
Risk-adjusted 30-day mortality did not change in that time, while risk-adjusted rehospitalization decreased over time and more quickly when patients had same-day discharge.
Deepak L. Bhatt, MD, MPH, and Jonathan G. Sung, MBCHB, both of Brigham and Women’s Hospital Heart & Vascular Center, Harvard Medical School, Boston, wrote in an accompanying article that, “Advances in the devices and techniques of PCI have improved the safety and efficacy of the procedure. In selected patients, same-day discharge has become possible, and overnight in-hospital observation can be avoided. By reducing unnecessary hospital stays, both patients and hospitals could benefit.”
Evan Garden, a medical student at Icahn School of Medicine at Mount Sinai in New York, presented findings at the American Urological Association 2021 annual meeting that show patients selected for same-day discharge after partial or radical nephrectomy did not have increased rates of postoperative complications or readmissions in the immediate postoperative period, compared with standard discharge of 1-3 days.
Case studies in nephrectomy
While several case studies have looked at the feasibility and safety of performing partial and radical nephrectomy with same-day discharge in select cases, “this topic has not been addressed on a national level,” Mr. Garden said.
Few patients who have partial or radical nephrectomies have same-day discharges. The researchers found that fewer than 1% of patients who have either procedure in the sample studied were discharged the same day.
Researchers used the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, a nationally representative deidentified database that prospectively tracks patient characteristics and 30-day perioperative outcomes for major inpatient and outpatient surgical procedures at more than 700 hospitals.
They extracted all minimally invasive partial and radical nephrectomies from 2012 to 2019 and refined the cohort to 28,140 patients who were theoretically eligible for same-day discharge: Of those, 237 (0.8%) had SSD, and 27,903 (99.2%) had a standard-length discharge (SLD).
The team found that there were no differences in 30-day complications or readmissions between same-day discharge (Clavien-Dindo [CD] I/II, 4.22%; CD III, 0%; CD IV, 1.27%; readmission, 4.64%); and SLD (CD I/II, 4.11%; CD III, 0.95%; CD IV, 0.79%; readmission, 3.90%; all P > .05).
Controlling for demographic and clinical variables, SDD was not associated with greater risk of 30-day complications or readmissions (CD I/II: odds ratio, 1.08; 95% confidence interval, 0.57-2.048; P = .813; CD IV: OR 1.699; 95% CI, 0.537-5.375; P = .367; readmission: OR, 1.254; 95% CI, 0.681-2.31; P = .467).
Mr. Garden and coauthors report no relevant financial relationships.
Dr. Lane reports no relevant financial relationships.
Urologist Ronney Abaza, MD, a robotic surgery specialist in Dublin, Ohio, and colleagues, reviewed robotic surgeries at their hospital during COVID-19 restrictions on surgery in Ohio between March 17 and June 5, 2020, and compared them with robotic procedures before COVID-19 and after restrictions were lifted. They published their results in Urology.
Since 2016, the hospital has offered the option of same-day discharge (SDD) to all robotic urologic surgery patients, regardless of procedure or patient-specific factors.
Among patients who had surgery during COVID-19 restrictions, 98% (87/89 patients) opted for SDD versus 52% in the group having surgery before the restrictions (P < .00001). After the COVID-19 surgery restrictions were lifted, the higher rate of SDD remained at 98%.
“There were no differences in 30-day complications or readmissions between SDD and overnight patients,” the authors write.
The right patient, the right motivation for successful surgery
Brian Lane, MD, PhD, a urologic oncologist with Spectrum Health in Grand Rapids, Michigan, told this news organization that, for nephrectomies, uptake of same-day discharge will continue to be slow.
“You have to have the right patient, the right patient motivation, and the surgery has to go smoothly,” he said. “If you start sending everyone home the same day, you will certainly see readmissions,” he said.
Dr. Lane is part of the Michigan Urologic Surgery Improvement Collaborative and he said the group recently looked at same-day discharge outcomes after robotic prostatectomies with SDD as compared with 1-2 nights in the hospital.
The work has not yet been published but, “There was a slight signal that there were increased readmissions with same-day discharge vs. 0-1 day,” he said.
A paper on outcomes of same-day discharge in total knee arthroplasty in the Journal of Bone & Joint Surgery found a higher risk of perioperative complications “including component failure, surgical site infection, knee stiffness, and deep vein thrombosis.” Researchers compared outcomes between 4,391 patients who underwent outpatient TKA and 128,951 patients who underwent inpatient TKA.
But for other many surgeries, same-day discharge numbers are increasing without worsening outcomes.
A paper in the Journal of Robotic Surgery found that same-day discharge following robotic-assisted endometrial cancer staging is “safe and feasible.”
Stephen Bradley, MD, MPH, with the Minneapolis Heart Institute in Minneapolis, and colleagues write in the Journal of the American College of Cardiology: Cardiovascular Interventions that they found a large increase in the use of same-day discharge after elective percutaneous coronary intervention (PCI) was not associated with worse 30-day mortality rates or readmission.
In that study, 114,461 patients were discharged the same day they underwent PCI. The proportion of patients who had a same-day discharge increased from 4.5% in 2009 to 28.6% in the fourth quarter of 2017.
Risk-adjusted 30-day mortality did not change in that time, while risk-adjusted rehospitalization decreased over time and more quickly when patients had same-day discharge.
Deepak L. Bhatt, MD, MPH, and Jonathan G. Sung, MBCHB, both of Brigham and Women’s Hospital Heart & Vascular Center, Harvard Medical School, Boston, wrote in an accompanying article that, “Advances in the devices and techniques of PCI have improved the safety and efficacy of the procedure. In selected patients, same-day discharge has become possible, and overnight in-hospital observation can be avoided. By reducing unnecessary hospital stays, both patients and hospitals could benefit.”
Evan Garden, a medical student at Icahn School of Medicine at Mount Sinai in New York, presented findings at the American Urological Association 2021 annual meeting that show patients selected for same-day discharge after partial or radical nephrectomy did not have increased rates of postoperative complications or readmissions in the immediate postoperative period, compared with standard discharge of 1-3 days.
Case studies in nephrectomy
While several case studies have looked at the feasibility and safety of performing partial and radical nephrectomy with same-day discharge in select cases, “this topic has not been addressed on a national level,” Mr. Garden said.
Few patients who have partial or radical nephrectomies have same-day discharges. The researchers found that fewer than 1% of patients who have either procedure in the sample studied were discharged the same day.
Researchers used the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, a nationally representative deidentified database that prospectively tracks patient characteristics and 30-day perioperative outcomes for major inpatient and outpatient surgical procedures at more than 700 hospitals.
They extracted all minimally invasive partial and radical nephrectomies from 2012 to 2019 and refined the cohort to 28,140 patients who were theoretically eligible for same-day discharge: Of those, 237 (0.8%) had SSD, and 27,903 (99.2%) had a standard-length discharge (SLD).
The team found that there were no differences in 30-day complications or readmissions between same-day discharge (Clavien-Dindo [CD] I/II, 4.22%; CD III, 0%; CD IV, 1.27%; readmission, 4.64%); and SLD (CD I/II, 4.11%; CD III, 0.95%; CD IV, 0.79%; readmission, 3.90%; all P > .05).
Controlling for demographic and clinical variables, SDD was not associated with greater risk of 30-day complications or readmissions (CD I/II: odds ratio, 1.08; 95% confidence interval, 0.57-2.048; P = .813; CD IV: OR 1.699; 95% CI, 0.537-5.375; P = .367; readmission: OR, 1.254; 95% CI, 0.681-2.31; P = .467).
Mr. Garden and coauthors report no relevant financial relationships.
Dr. Lane reports no relevant financial relationships.
Which agent is best for neuromyelitis optica?
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).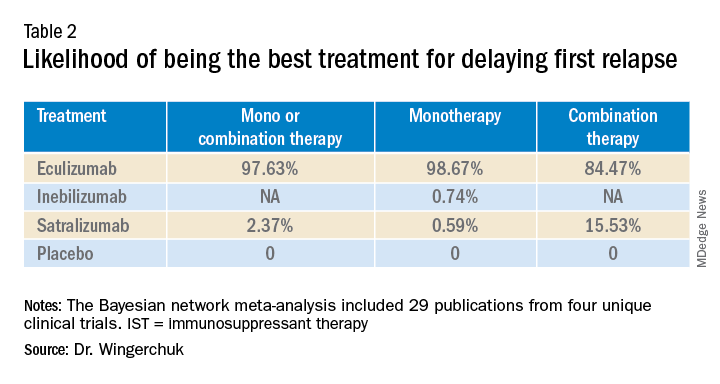
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2021
Clinicians underprescribe behavior therapy for preschool ADHD
The majority of families of preschool children with a diagnosis of attention-deficit/hyperactivity disorder were not offered behavior therapy as a first-line treatment, according to data from nearly 200 children.
The American Academy of Pediatrics’ current clinical practice guidelines recommend parent training in behavior management (PTBM) as a first-line treatment for children aged 4-5 years diagnosed with attention-deficit/hyperactivity disorder (ADHD) or symptoms of ADHD such as hyperactivity or impulsivity, but data on how well primary care providers follow this recommendation in practice are lacking, wrote Yair Bannett, MD, of Stanford (Calif.) University, and colleagues.
To investigate the rates of PTBM recommendations, the researchers reviewed electronic health records for 22,714 children aged 48-71 months who had at least two visits to any 1 of 10 primary care practices in a California pediatric health network between Oct. 1, 2015, and Dec. 31, 2019. Children with an autism diagnosis were excluded; ADHD-related visits were identified via ADHD diagnosis codes or symptom-level diagnosis codes.
In the study, published in JAMA Pediatrics, 192 children (1%) had either an ADHD diagnosis or ADHD symptoms; of these, 21 (11%) received referrals for PTBM during ADHD-related primary care visits. Records showed an additional 55 patients (29%) had a mention of counseling on PTBM by a primary care provider, including handouts.
PCPs prescribed ADHD medications for 32 children; 9 of these had documented PTBM recommendations, and in 4 cases, the PCPs recommended PTBM before prescribing a first medication.
A majority (73%) of the children were male, 64% were privately insured, 56% had subspecialists involved in their care, and 17% were prescribed ADHD medications (88% of which were stimulants).
In a multivariate analysis, children with public insurance were significantly less likely to receive a PTBM recommendation than were those with private insurance (adjusted relative risk 0.87).
The most common recommendation overall was routine/habit modifications (for 79 children), such as reducing sugar or adding supplements to the diet; improving sleep hygiene; and limiting screen time.
The low rates of PTBM among publicly insured patients in particular highlight the need to identify factors behind disparities in recommended treatments, the researchers noted.
The study findings were limited by several factors including the reliance on primary care provider documentation during the study period and the inclusion only of medical record reviews with diagnostic codes for ADHD, the researchers noted. Further studies beyond a single health care system are needed to assess generalizability, they added.
However, the results present an opportunity for primary care providers to improve adherence to clinical practice guidelines and establish behavioral treatment at an early age to mitigate long-term morbidity, they concluded.
Low rates highlight barriers and opportunities
“We were surprised to find very low rates of documented recommendations for behavioral treatment mentioned by PCPs,” Dr. Bannett said in an interview. The researchers were surprised that recommendations for changes in daily routines and habits, such as reduced sugar intake, regular exercise, better sleep, and reduced screen time, were the most common recommendations for families of children presenting with symptoms of ADHD. “Though these are good recommendations that can support the general health of any young child, there is no evidence to support their benefit in alleviating symptoms of ADHD,” he said.
Dr. Bannett acknowledged the challenge for pediatricians to stay current on where and how families can access this type of behavioral treatment, but the evidence supports behavior therapy over medication in preschool children, he said.
“I think that it is important for primary care clinicians to know that there are options for parent training in behavioral management for both privately and publicly insured patients,” said Dr. Bannett. “In California, for example, parent training programs are offered through county mental health services. In some counties, there are other organizations that offer parent training for underserved populations and those with public insurance,” he said.
Dr. Bannett noted that online treatments, including behavioral treatments, may be possible for some families.
He cited Triple P, an evidence-based curriculum for parent training in behavior management, which offers an online course for parents at triplep-parenting.com, and an online parent training course offered through the CHADD website (chadd.org/parent-to-parent/).
Dr. Bannett noted that the researchers are planning a follow-up study to investigate the reasons behind the low referral rates for PTBM. “A known barrier is the limited availability of therapists who can provide this type of therapy,” Dr. Bannett said. “Research is needed on the effectiveness of online versions of parent training, which can overcome some of the access barriers many families experience,” he added.
“Additionally, since behavioral treatment requires a significant effort on the part of the parents and caregivers, who often are not able to complete the therapy, there is a need for research on ways to enhance parent and family engagement and participation in these important evidence-based treatments,” as well as a need to research ways to increase adherence to evidence-based practices, said Dr. Bannett. “We are currently planning intervention studies that will enhance primary care clinicians’ knowledge and clinical practice; for example, decision support tools in the electronic health record, and up-to-date information about available resources and behavioral therapists in their community that they can share with families,” he said.
Barriers make it difficult to adhere to guidelines
The study authors missed a significant element of the AAP guidelines by failing to acknowledge the extensive accompanying section on barriers to adoption, which details why most pediatricians in clinical practice do not prescribe PTBM, Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“Academically, it is a wonderful article,” said Dr. Lessin, who was a member of the authoring committee of the AAP guidelines and a major contributor to the section on barriers. The AAP guidelines recommend PTBM because it is evidence based, but the barrier section is essential to understanding that this evidence-based recommendation is nearly impossible to follow in real-world clinical practice, he emphasized.
The American Academy of Pediatrics’ “Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents,” published in October of 2019 in Pediatrics, included a full subsection on barriers as to why the guidelines might not be followed in many cases in a real-world setting, and the study authors failed to acknowledge this section and its implications, said Dr. Lessin. Notably, the barriers section was originally published in Pediatrics under a Supplemental Data tab that might easily be overlooked by someone reviewing the main practice guideline recommendations, he said.
In most areas of the country, PTBM is simply unavailable, Dr. Lessin said.
There is a dearth of mental health providers in the United States in general, and “a monstrous shortage of mental health practitioners for young children,” he said. Children in underserved areas barely have access to a medical home, let alone mental health subspecialists, he added.
Even in areas where specialized behavior therapy may be available, it can be prohibitively expensive for all but the wealthiest patients, Dr. Lessin noted. Insurance does not cover this type of behavior therapy, and most mental health professionals don’t accept Medicaid, nor commercial insurance, he said.
“I don’t even bother with those referrals, because they are not available,” said Dr. Lessin. The take-home message is that most community-based pediatricians are not following the guidelines because the barriers are so enormous, he said.
The study was supported by a research grant from the Society of Developmental and Behavioral Pediatrics and salary support through the Instructor Support Program at the department of pediatrics, Lucile Packard Children’s Hospital Stanford, to Dr. Bannett. The researchers had no other financial conflicts to disclose. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
The majority of families of preschool children with a diagnosis of attention-deficit/hyperactivity disorder were not offered behavior therapy as a first-line treatment, according to data from nearly 200 children.
The American Academy of Pediatrics’ current clinical practice guidelines recommend parent training in behavior management (PTBM) as a first-line treatment for children aged 4-5 years diagnosed with attention-deficit/hyperactivity disorder (ADHD) or symptoms of ADHD such as hyperactivity or impulsivity, but data on how well primary care providers follow this recommendation in practice are lacking, wrote Yair Bannett, MD, of Stanford (Calif.) University, and colleagues.
To investigate the rates of PTBM recommendations, the researchers reviewed electronic health records for 22,714 children aged 48-71 months who had at least two visits to any 1 of 10 primary care practices in a California pediatric health network between Oct. 1, 2015, and Dec. 31, 2019. Children with an autism diagnosis were excluded; ADHD-related visits were identified via ADHD diagnosis codes or symptom-level diagnosis codes.
In the study, published in JAMA Pediatrics, 192 children (1%) had either an ADHD diagnosis or ADHD symptoms; of these, 21 (11%) received referrals for PTBM during ADHD-related primary care visits. Records showed an additional 55 patients (29%) had a mention of counseling on PTBM by a primary care provider, including handouts.
PCPs prescribed ADHD medications for 32 children; 9 of these had documented PTBM recommendations, and in 4 cases, the PCPs recommended PTBM before prescribing a first medication.
A majority (73%) of the children were male, 64% were privately insured, 56% had subspecialists involved in their care, and 17% were prescribed ADHD medications (88% of which were stimulants).
In a multivariate analysis, children with public insurance were significantly less likely to receive a PTBM recommendation than were those with private insurance (adjusted relative risk 0.87).
The most common recommendation overall was routine/habit modifications (for 79 children), such as reducing sugar or adding supplements to the diet; improving sleep hygiene; and limiting screen time.
The low rates of PTBM among publicly insured patients in particular highlight the need to identify factors behind disparities in recommended treatments, the researchers noted.
The study findings were limited by several factors including the reliance on primary care provider documentation during the study period and the inclusion only of medical record reviews with diagnostic codes for ADHD, the researchers noted. Further studies beyond a single health care system are needed to assess generalizability, they added.
However, the results present an opportunity for primary care providers to improve adherence to clinical practice guidelines and establish behavioral treatment at an early age to mitigate long-term morbidity, they concluded.
Low rates highlight barriers and opportunities
“We were surprised to find very low rates of documented recommendations for behavioral treatment mentioned by PCPs,” Dr. Bannett said in an interview. The researchers were surprised that recommendations for changes in daily routines and habits, such as reduced sugar intake, regular exercise, better sleep, and reduced screen time, were the most common recommendations for families of children presenting with symptoms of ADHD. “Though these are good recommendations that can support the general health of any young child, there is no evidence to support their benefit in alleviating symptoms of ADHD,” he said.
Dr. Bannett acknowledged the challenge for pediatricians to stay current on where and how families can access this type of behavioral treatment, but the evidence supports behavior therapy over medication in preschool children, he said.
“I think that it is important for primary care clinicians to know that there are options for parent training in behavioral management for both privately and publicly insured patients,” said Dr. Bannett. “In California, for example, parent training programs are offered through county mental health services. In some counties, there are other organizations that offer parent training for underserved populations and those with public insurance,” he said.
Dr. Bannett noted that online treatments, including behavioral treatments, may be possible for some families.
He cited Triple P, an evidence-based curriculum for parent training in behavior management, which offers an online course for parents at triplep-parenting.com, and an online parent training course offered through the CHADD website (chadd.org/parent-to-parent/).
Dr. Bannett noted that the researchers are planning a follow-up study to investigate the reasons behind the low referral rates for PTBM. “A known barrier is the limited availability of therapists who can provide this type of therapy,” Dr. Bannett said. “Research is needed on the effectiveness of online versions of parent training, which can overcome some of the access barriers many families experience,” he added.
“Additionally, since behavioral treatment requires a significant effort on the part of the parents and caregivers, who often are not able to complete the therapy, there is a need for research on ways to enhance parent and family engagement and participation in these important evidence-based treatments,” as well as a need to research ways to increase adherence to evidence-based practices, said Dr. Bannett. “We are currently planning intervention studies that will enhance primary care clinicians’ knowledge and clinical practice; for example, decision support tools in the electronic health record, and up-to-date information about available resources and behavioral therapists in their community that they can share with families,” he said.
Barriers make it difficult to adhere to guidelines
The study authors missed a significant element of the AAP guidelines by failing to acknowledge the extensive accompanying section on barriers to adoption, which details why most pediatricians in clinical practice do not prescribe PTBM, Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“Academically, it is a wonderful article,” said Dr. Lessin, who was a member of the authoring committee of the AAP guidelines and a major contributor to the section on barriers. The AAP guidelines recommend PTBM because it is evidence based, but the barrier section is essential to understanding that this evidence-based recommendation is nearly impossible to follow in real-world clinical practice, he emphasized.
The American Academy of Pediatrics’ “Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents,” published in October of 2019 in Pediatrics, included a full subsection on barriers as to why the guidelines might not be followed in many cases in a real-world setting, and the study authors failed to acknowledge this section and its implications, said Dr. Lessin. Notably, the barriers section was originally published in Pediatrics under a Supplemental Data tab that might easily be overlooked by someone reviewing the main practice guideline recommendations, he said.
In most areas of the country, PTBM is simply unavailable, Dr. Lessin said.
There is a dearth of mental health providers in the United States in general, and “a monstrous shortage of mental health practitioners for young children,” he said. Children in underserved areas barely have access to a medical home, let alone mental health subspecialists, he added.
Even in areas where specialized behavior therapy may be available, it can be prohibitively expensive for all but the wealthiest patients, Dr. Lessin noted. Insurance does not cover this type of behavior therapy, and most mental health professionals don’t accept Medicaid, nor commercial insurance, he said.
“I don’t even bother with those referrals, because they are not available,” said Dr. Lessin. The take-home message is that most community-based pediatricians are not following the guidelines because the barriers are so enormous, he said.
The study was supported by a research grant from the Society of Developmental and Behavioral Pediatrics and salary support through the Instructor Support Program at the department of pediatrics, Lucile Packard Children’s Hospital Stanford, to Dr. Bannett. The researchers had no other financial conflicts to disclose. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
The majority of families of preschool children with a diagnosis of attention-deficit/hyperactivity disorder were not offered behavior therapy as a first-line treatment, according to data from nearly 200 children.
The American Academy of Pediatrics’ current clinical practice guidelines recommend parent training in behavior management (PTBM) as a first-line treatment for children aged 4-5 years diagnosed with attention-deficit/hyperactivity disorder (ADHD) or symptoms of ADHD such as hyperactivity or impulsivity, but data on how well primary care providers follow this recommendation in practice are lacking, wrote Yair Bannett, MD, of Stanford (Calif.) University, and colleagues.
To investigate the rates of PTBM recommendations, the researchers reviewed electronic health records for 22,714 children aged 48-71 months who had at least two visits to any 1 of 10 primary care practices in a California pediatric health network between Oct. 1, 2015, and Dec. 31, 2019. Children with an autism diagnosis were excluded; ADHD-related visits were identified via ADHD diagnosis codes or symptom-level diagnosis codes.
In the study, published in JAMA Pediatrics, 192 children (1%) had either an ADHD diagnosis or ADHD symptoms; of these, 21 (11%) received referrals for PTBM during ADHD-related primary care visits. Records showed an additional 55 patients (29%) had a mention of counseling on PTBM by a primary care provider, including handouts.
PCPs prescribed ADHD medications for 32 children; 9 of these had documented PTBM recommendations, and in 4 cases, the PCPs recommended PTBM before prescribing a first medication.
A majority (73%) of the children were male, 64% were privately insured, 56% had subspecialists involved in their care, and 17% were prescribed ADHD medications (88% of which were stimulants).
In a multivariate analysis, children with public insurance were significantly less likely to receive a PTBM recommendation than were those with private insurance (adjusted relative risk 0.87).
The most common recommendation overall was routine/habit modifications (for 79 children), such as reducing sugar or adding supplements to the diet; improving sleep hygiene; and limiting screen time.
The low rates of PTBM among publicly insured patients in particular highlight the need to identify factors behind disparities in recommended treatments, the researchers noted.
The study findings were limited by several factors including the reliance on primary care provider documentation during the study period and the inclusion only of medical record reviews with diagnostic codes for ADHD, the researchers noted. Further studies beyond a single health care system are needed to assess generalizability, they added.
However, the results present an opportunity for primary care providers to improve adherence to clinical practice guidelines and establish behavioral treatment at an early age to mitigate long-term morbidity, they concluded.
Low rates highlight barriers and opportunities
“We were surprised to find very low rates of documented recommendations for behavioral treatment mentioned by PCPs,” Dr. Bannett said in an interview. The researchers were surprised that recommendations for changes in daily routines and habits, such as reduced sugar intake, regular exercise, better sleep, and reduced screen time, were the most common recommendations for families of children presenting with symptoms of ADHD. “Though these are good recommendations that can support the general health of any young child, there is no evidence to support their benefit in alleviating symptoms of ADHD,” he said.
Dr. Bannett acknowledged the challenge for pediatricians to stay current on where and how families can access this type of behavioral treatment, but the evidence supports behavior therapy over medication in preschool children, he said.
“I think that it is important for primary care clinicians to know that there are options for parent training in behavioral management for both privately and publicly insured patients,” said Dr. Bannett. “In California, for example, parent training programs are offered through county mental health services. In some counties, there are other organizations that offer parent training for underserved populations and those with public insurance,” he said.
Dr. Bannett noted that online treatments, including behavioral treatments, may be possible for some families.
He cited Triple P, an evidence-based curriculum for parent training in behavior management, which offers an online course for parents at triplep-parenting.com, and an online parent training course offered through the CHADD website (chadd.org/parent-to-parent/).
Dr. Bannett noted that the researchers are planning a follow-up study to investigate the reasons behind the low referral rates for PTBM. “A known barrier is the limited availability of therapists who can provide this type of therapy,” Dr. Bannett said. “Research is needed on the effectiveness of online versions of parent training, which can overcome some of the access barriers many families experience,” he added.
“Additionally, since behavioral treatment requires a significant effort on the part of the parents and caregivers, who often are not able to complete the therapy, there is a need for research on ways to enhance parent and family engagement and participation in these important evidence-based treatments,” as well as a need to research ways to increase adherence to evidence-based practices, said Dr. Bannett. “We are currently planning intervention studies that will enhance primary care clinicians’ knowledge and clinical practice; for example, decision support tools in the electronic health record, and up-to-date information about available resources and behavioral therapists in their community that they can share with families,” he said.
Barriers make it difficult to adhere to guidelines
The study authors missed a significant element of the AAP guidelines by failing to acknowledge the extensive accompanying section on barriers to adoption, which details why most pediatricians in clinical practice do not prescribe PTBM, Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“Academically, it is a wonderful article,” said Dr. Lessin, who was a member of the authoring committee of the AAP guidelines and a major contributor to the section on barriers. The AAP guidelines recommend PTBM because it is evidence based, but the barrier section is essential to understanding that this evidence-based recommendation is nearly impossible to follow in real-world clinical practice, he emphasized.
The American Academy of Pediatrics’ “Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents,” published in October of 2019 in Pediatrics, included a full subsection on barriers as to why the guidelines might not be followed in many cases in a real-world setting, and the study authors failed to acknowledge this section and its implications, said Dr. Lessin. Notably, the barriers section was originally published in Pediatrics under a Supplemental Data tab that might easily be overlooked by someone reviewing the main practice guideline recommendations, he said.
In most areas of the country, PTBM is simply unavailable, Dr. Lessin said.
There is a dearth of mental health providers in the United States in general, and “a monstrous shortage of mental health practitioners for young children,” he said. Children in underserved areas barely have access to a medical home, let alone mental health subspecialists, he added.
Even in areas where specialized behavior therapy may be available, it can be prohibitively expensive for all but the wealthiest patients, Dr. Lessin noted. Insurance does not cover this type of behavior therapy, and most mental health professionals don’t accept Medicaid, nor commercial insurance, he said.
“I don’t even bother with those referrals, because they are not available,” said Dr. Lessin. The take-home message is that most community-based pediatricians are not following the guidelines because the barriers are so enormous, he said.
The study was supported by a research grant from the Society of Developmental and Behavioral Pediatrics and salary support through the Instructor Support Program at the department of pediatrics, Lucile Packard Children’s Hospital Stanford, to Dr. Bannett. The researchers had no other financial conflicts to disclose. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS
Novel blood-based panel highly effective for early-stage HCC
A blood-based biomarker panel that includes DNA and protein markers featured a 71% sensitivity at 90% specificity for the detection of early-stage hepatocellular carcinoma (HCC) compared with the GALAD (gender, age, a-fetoprotein [AFP], Lens culinaris agglutinin-reactive AFP [AFP-L3], and des-gamma-carboxy-prothrombin [DCP]) score or AFP alone, according to research findings. The panel reportedly performed well in certain subgroups based on sex, presence of cirrhosis, and liver disease etiology.
The study, which included inpatients with HCC and controls without HCC but underlying liver disease, suggests the panel could be utilized in the detection of early stage disease in patients with well-established risk factors for HCC. Ultimately, this may lead to earlier treatment initiation and potentially improved clinical outcomes.
“A blood-based marker panel that detects early-stage HCC with higher sensitivity than current biomarker-based approaches could substantially benefit patients undergoing HCC surveillance,” wrote study authors Naga Chalasani, MD, of Indiana University, Indianapolis, and colleagues. Their report is in Clinical Gastroenterology and Hepatology.
HCC, which accounts for most primary liver cancers, generally occurs in patients with several established risk factors, including alcoholic liver disease or nonalcoholic fatty liver disease as well as chronic hepatitis B virus or hepatitis C virus infection. Current guidelines, such as those from the European Association for the Study of the Liver and those from the American Association for the Study of Liver Diseases, recommend surveillance of at-risk patients every 6 months by ultrasound with or without AFP measurement. When caught early, HCC is typically treatable and is associated with a higher rate of survival compared with late-stage disease. According to Dr. Chalasani and colleagues, however, the effectiveness of current recommended surveillance for very early stage or early stage HCC is poor, characterized by a 45% sensitivity for ultrasound and a 63% sensitivity for ultrasound coupled with AFP measurement.
The investigators of the multicenter, case-control study collected blood specimens from 135 patients with HCC as well as 302 age-matched controls with underlying liver disease but no HCC. Very early or early stage disease was seen in approximately 56.3% of patients with HCC, and intermediate, advanced, or terminal stage disease was seen in 43.7% of patients.
To predict cases of HCC, the researchers used a logistic regression algorithm to analyze 10 methylated DNA markers (MDMs) associated with HCC, methylated B3GALT6 (reference DNA marker), and 3 candidate proteins. Finally, the researchers compared the accuracy of the developed blood-based biomarker panel with other blood-based biomarkers – including the GALAD, AFP, AFP-L3, DCP – for the detection of HCC.
The multitarget HCC panel included 3 MDMs – HOXA1, EMX1, and TSPYL5. In addition, the panel included methylation reference marker B3GALT6 and the protein markers AFP and AFP-L3. The biomarker panel featured a higher sensitivity (71%; 95% confidence interval, 60-81) at 90% specificity for the detection of early stage HCC compared with the GALAD score (41%; 95% CI, 30-53) or AFP ≥ 7.32 ng/mL (45%; 95% CI, 33-57). The area under the curve for the novel HCC panel for the detection of any stage HCC was 0.92 vs. 0.87 for the GALAD and 0.81 for the AFP measurement alone. The researchers found that the performance of the test was similar between men and women in terms of sensitivity (79% and 84%, respectively). Moreover, the panel performed similarly well among subgroups based on presence of cirrhosis and liver disease etiology.
A potential limitation of this study was the inclusion of controls who were largely confirmed HCC negative by ultrasound, a technique that lacks sensitivity for detecting early stage HCC, the researchers noted. Given this limitation, the researchers suggest that some of the control participants may have had underlying HCC that was missed by ultrasound. Furthermore, the findings indicate that the cross-sectional nature of the study may also mean some of the control participants had HCCs that were undetectable at initial screening.
Despite the limitations of the study, the researchers reported that the novel, blood-based marker panel’s sensitivity for detecting early stage HCC likely supports its use “among at-risk patients to enhance HCC surveillance and improve early cancer detection.”
The study was funded by the Exact Sciences Corporation. The researchers reported conflicts of interest with several pharmaceutical companies.
A blood-based biomarker panel that includes DNA and protein markers featured a 71% sensitivity at 90% specificity for the detection of early-stage hepatocellular carcinoma (HCC) compared with the GALAD (gender, age, a-fetoprotein [AFP], Lens culinaris agglutinin-reactive AFP [AFP-L3], and des-gamma-carboxy-prothrombin [DCP]) score or AFP alone, according to research findings. The panel reportedly performed well in certain subgroups based on sex, presence of cirrhosis, and liver disease etiology.
The study, which included inpatients with HCC and controls without HCC but underlying liver disease, suggests the panel could be utilized in the detection of early stage disease in patients with well-established risk factors for HCC. Ultimately, this may lead to earlier treatment initiation and potentially improved clinical outcomes.
“A blood-based marker panel that detects early-stage HCC with higher sensitivity than current biomarker-based approaches could substantially benefit patients undergoing HCC surveillance,” wrote study authors Naga Chalasani, MD, of Indiana University, Indianapolis, and colleagues. Their report is in Clinical Gastroenterology and Hepatology.
HCC, which accounts for most primary liver cancers, generally occurs in patients with several established risk factors, including alcoholic liver disease or nonalcoholic fatty liver disease as well as chronic hepatitis B virus or hepatitis C virus infection. Current guidelines, such as those from the European Association for the Study of the Liver and those from the American Association for the Study of Liver Diseases, recommend surveillance of at-risk patients every 6 months by ultrasound with or without AFP measurement. When caught early, HCC is typically treatable and is associated with a higher rate of survival compared with late-stage disease. According to Dr. Chalasani and colleagues, however, the effectiveness of current recommended surveillance for very early stage or early stage HCC is poor, characterized by a 45% sensitivity for ultrasound and a 63% sensitivity for ultrasound coupled with AFP measurement.
The investigators of the multicenter, case-control study collected blood specimens from 135 patients with HCC as well as 302 age-matched controls with underlying liver disease but no HCC. Very early or early stage disease was seen in approximately 56.3% of patients with HCC, and intermediate, advanced, or terminal stage disease was seen in 43.7% of patients.
To predict cases of HCC, the researchers used a logistic regression algorithm to analyze 10 methylated DNA markers (MDMs) associated with HCC, methylated B3GALT6 (reference DNA marker), and 3 candidate proteins. Finally, the researchers compared the accuracy of the developed blood-based biomarker panel with other blood-based biomarkers – including the GALAD, AFP, AFP-L3, DCP – for the detection of HCC.
The multitarget HCC panel included 3 MDMs – HOXA1, EMX1, and TSPYL5. In addition, the panel included methylation reference marker B3GALT6 and the protein markers AFP and AFP-L3. The biomarker panel featured a higher sensitivity (71%; 95% confidence interval, 60-81) at 90% specificity for the detection of early stage HCC compared with the GALAD score (41%; 95% CI, 30-53) or AFP ≥ 7.32 ng/mL (45%; 95% CI, 33-57). The area under the curve for the novel HCC panel for the detection of any stage HCC was 0.92 vs. 0.87 for the GALAD and 0.81 for the AFP measurement alone. The researchers found that the performance of the test was similar between men and women in terms of sensitivity (79% and 84%, respectively). Moreover, the panel performed similarly well among subgroups based on presence of cirrhosis and liver disease etiology.
A potential limitation of this study was the inclusion of controls who were largely confirmed HCC negative by ultrasound, a technique that lacks sensitivity for detecting early stage HCC, the researchers noted. Given this limitation, the researchers suggest that some of the control participants may have had underlying HCC that was missed by ultrasound. Furthermore, the findings indicate that the cross-sectional nature of the study may also mean some of the control participants had HCCs that were undetectable at initial screening.
Despite the limitations of the study, the researchers reported that the novel, blood-based marker panel’s sensitivity for detecting early stage HCC likely supports its use “among at-risk patients to enhance HCC surveillance and improve early cancer detection.”
The study was funded by the Exact Sciences Corporation. The researchers reported conflicts of interest with several pharmaceutical companies.
A blood-based biomarker panel that includes DNA and protein markers featured a 71% sensitivity at 90% specificity for the detection of early-stage hepatocellular carcinoma (HCC) compared with the GALAD (gender, age, a-fetoprotein [AFP], Lens culinaris agglutinin-reactive AFP [AFP-L3], and des-gamma-carboxy-prothrombin [DCP]) score or AFP alone, according to research findings. The panel reportedly performed well in certain subgroups based on sex, presence of cirrhosis, and liver disease etiology.
The study, which included inpatients with HCC and controls without HCC but underlying liver disease, suggests the panel could be utilized in the detection of early stage disease in patients with well-established risk factors for HCC. Ultimately, this may lead to earlier treatment initiation and potentially improved clinical outcomes.
“A blood-based marker panel that detects early-stage HCC with higher sensitivity than current biomarker-based approaches could substantially benefit patients undergoing HCC surveillance,” wrote study authors Naga Chalasani, MD, of Indiana University, Indianapolis, and colleagues. Their report is in Clinical Gastroenterology and Hepatology.
HCC, which accounts for most primary liver cancers, generally occurs in patients with several established risk factors, including alcoholic liver disease or nonalcoholic fatty liver disease as well as chronic hepatitis B virus or hepatitis C virus infection. Current guidelines, such as those from the European Association for the Study of the Liver and those from the American Association for the Study of Liver Diseases, recommend surveillance of at-risk patients every 6 months by ultrasound with or without AFP measurement. When caught early, HCC is typically treatable and is associated with a higher rate of survival compared with late-stage disease. According to Dr. Chalasani and colleagues, however, the effectiveness of current recommended surveillance for very early stage or early stage HCC is poor, characterized by a 45% sensitivity for ultrasound and a 63% sensitivity for ultrasound coupled with AFP measurement.
The investigators of the multicenter, case-control study collected blood specimens from 135 patients with HCC as well as 302 age-matched controls with underlying liver disease but no HCC. Very early or early stage disease was seen in approximately 56.3% of patients with HCC, and intermediate, advanced, or terminal stage disease was seen in 43.7% of patients.
To predict cases of HCC, the researchers used a logistic regression algorithm to analyze 10 methylated DNA markers (MDMs) associated with HCC, methylated B3GALT6 (reference DNA marker), and 3 candidate proteins. Finally, the researchers compared the accuracy of the developed blood-based biomarker panel with other blood-based biomarkers – including the GALAD, AFP, AFP-L3, DCP – for the detection of HCC.
The multitarget HCC panel included 3 MDMs – HOXA1, EMX1, and TSPYL5. In addition, the panel included methylation reference marker B3GALT6 and the protein markers AFP and AFP-L3. The biomarker panel featured a higher sensitivity (71%; 95% confidence interval, 60-81) at 90% specificity for the detection of early stage HCC compared with the GALAD score (41%; 95% CI, 30-53) or AFP ≥ 7.32 ng/mL (45%; 95% CI, 33-57). The area under the curve for the novel HCC panel for the detection of any stage HCC was 0.92 vs. 0.87 for the GALAD and 0.81 for the AFP measurement alone. The researchers found that the performance of the test was similar between men and women in terms of sensitivity (79% and 84%, respectively). Moreover, the panel performed similarly well among subgroups based on presence of cirrhosis and liver disease etiology.
A potential limitation of this study was the inclusion of controls who were largely confirmed HCC negative by ultrasound, a technique that lacks sensitivity for detecting early stage HCC, the researchers noted. Given this limitation, the researchers suggest that some of the control participants may have had underlying HCC that was missed by ultrasound. Furthermore, the findings indicate that the cross-sectional nature of the study may also mean some of the control participants had HCCs that were undetectable at initial screening.
Despite the limitations of the study, the researchers reported that the novel, blood-based marker panel’s sensitivity for detecting early stage HCC likely supports its use “among at-risk patients to enhance HCC surveillance and improve early cancer detection.”
The study was funded by the Exact Sciences Corporation. The researchers reported conflicts of interest with several pharmaceutical companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY



