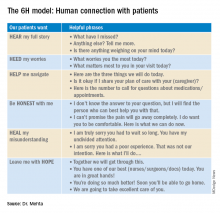
User login
A Roundabout Journey to Diagnosis
ANSWER
The correct answer is all the above (choice “e”).
DISCUSSION
The differential for round or annular, scaly lesions is lengthy. In addition to all 4 conditions listed above, it includes eczema, basal cell carcinoma, and irritant or contact dermatitis.
With this patient’s history, the diagnosis of fungal infection was not unreasonable. However, the total lack of response to antifungal treatment—along with a negative KOH prep—made that diagnosis questionable at best. Then there was the lack of lymphadenopathy, which would almost certainly have been present with such longstanding infection. Given her country of origin, cutaneous New World leishmaniasis (caused by a protozoan delivered to the patient by an insect vector) was a possibility.
For this patient, skin biopsy with a 4-mm punch was the only way to establish the correct diagnosis. The defect from the biopsy was closed with 5-0 nylon sutures to minimize scarring.
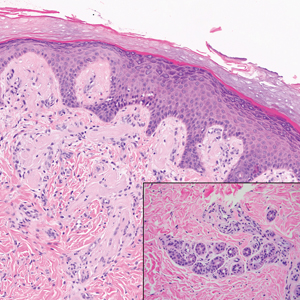
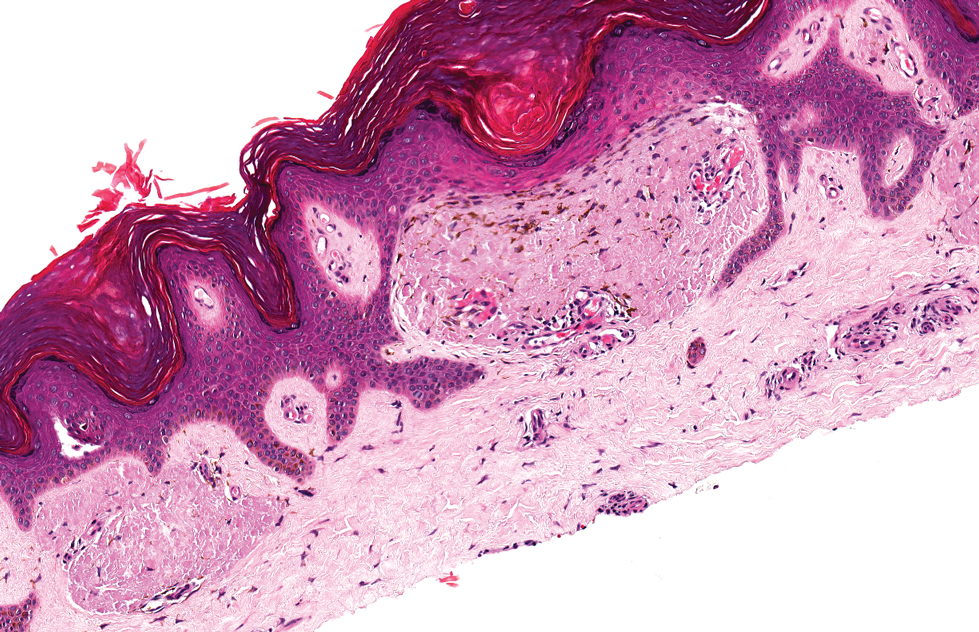
The pathological findings included interface dermatitis, apoptotic keratinocytes, and a brisk periadnexal lymphocytic infiltrate, which—given the morphological and historical context—were entirely consistent with discoid lupus erythematosus (DLE; otherwise known as subacute cutaneous lupus erythematosus). Subsequent bloodwork failed to show any connection with systemic lupus erythematosus (SLE), which is not surprising because only about 18% of DLE cases evolve into SLE.
DLE is more common in women, especially in those with skin of color. The associated lesions are seldom as impressive as this patient’s, manifesting as papulosquamous patches typically found on the ears, neck, face, arms, and other sun-exposed areas. Indeed, it appears that sun exposure is a major trigger for the disease—a clue that can assist with the diagnosis.
TREATMENT
In addition to proscribing excessive sun exposure, providers should encourage the use of sunscreen. DLE is often treated with topical steroids. More advanced cases, such as this patient’s, may require oral hydroxychloroquine (200 mg bid). Though these treatments are effective in most cases, DLE can leave serious scarring and/or discoloration, especially in those with darker skin.
ANSWER
The correct answer is all the above (choice “e”).
DISCUSSION
The differential for round or annular, scaly lesions is lengthy. In addition to all 4 conditions listed above, it includes eczema, basal cell carcinoma, and irritant or contact dermatitis.
With this patient’s history, the diagnosis of fungal infection was not unreasonable. However, the total lack of response to antifungal treatment—along with a negative KOH prep—made that diagnosis questionable at best. Then there was the lack of lymphadenopathy, which would almost certainly have been present with such longstanding infection. Given her country of origin, cutaneous New World leishmaniasis (caused by a protozoan delivered to the patient by an insect vector) was a possibility.
For this patient, skin biopsy with a 4-mm punch was the only way to establish the correct diagnosis. The defect from the biopsy was closed with 5-0 nylon sutures to minimize scarring.
The pathological findings included interface dermatitis, apoptotic keratinocytes, and a brisk periadnexal lymphocytic infiltrate, which—given the morphological and historical context—were entirely consistent with discoid lupus erythematosus (DLE; otherwise known as subacute cutaneous lupus erythematosus). Subsequent bloodwork failed to show any connection with systemic lupus erythematosus (SLE), which is not surprising because only about 18% of DLE cases evolve into SLE.
DLE is more common in women, especially in those with skin of color. The associated lesions are seldom as impressive as this patient’s, manifesting as papulosquamous patches typically found on the ears, neck, face, arms, and other sun-exposed areas. Indeed, it appears that sun exposure is a major trigger for the disease—a clue that can assist with the diagnosis.
TREATMENT
In addition to proscribing excessive sun exposure, providers should encourage the use of sunscreen. DLE is often treated with topical steroids. More advanced cases, such as this patient’s, may require oral hydroxychloroquine (200 mg bid). Though these treatments are effective in most cases, DLE can leave serious scarring and/or discoloration, especially in those with darker skin.
ANSWER
The correct answer is all the above (choice “e”).
DISCUSSION
The differential for round or annular, scaly lesions is lengthy. In addition to all 4 conditions listed above, it includes eczema, basal cell carcinoma, and irritant or contact dermatitis.
With this patient’s history, the diagnosis of fungal infection was not unreasonable. However, the total lack of response to antifungal treatment—along with a negative KOH prep—made that diagnosis questionable at best. Then there was the lack of lymphadenopathy, which would almost certainly have been present with such longstanding infection. Given her country of origin, cutaneous New World leishmaniasis (caused by a protozoan delivered to the patient by an insect vector) was a possibility.
For this patient, skin biopsy with a 4-mm punch was the only way to establish the correct diagnosis. The defect from the biopsy was closed with 5-0 nylon sutures to minimize scarring.
The pathological findings included interface dermatitis, apoptotic keratinocytes, and a brisk periadnexal lymphocytic infiltrate, which—given the morphological and historical context—were entirely consistent with discoid lupus erythematosus (DLE; otherwise known as subacute cutaneous lupus erythematosus). Subsequent bloodwork failed to show any connection with systemic lupus erythematosus (SLE), which is not surprising because only about 18% of DLE cases evolve into SLE.
DLE is more common in women, especially in those with skin of color. The associated lesions are seldom as impressive as this patient’s, manifesting as papulosquamous patches typically found on the ears, neck, face, arms, and other sun-exposed areas. Indeed, it appears that sun exposure is a major trigger for the disease—a clue that can assist with the diagnosis.
TREATMENT
In addition to proscribing excessive sun exposure, providers should encourage the use of sunscreen. DLE is often treated with topical steroids. More advanced cases, such as this patient’s, may require oral hydroxychloroquine (200 mg bid). Though these treatments are effective in most cases, DLE can leave serious scarring and/or discoloration, especially in those with darker skin.
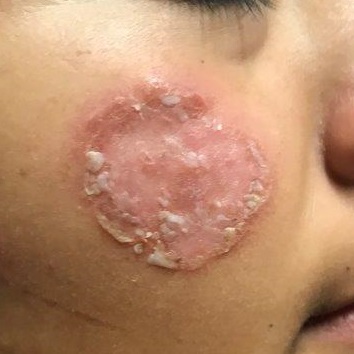
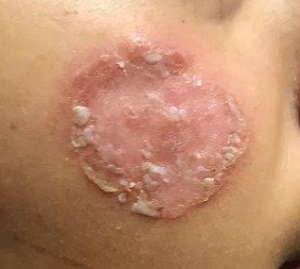
After journeying for several months from Honduras, this 30-year-old woman visits the clinic for evaluation of a lesion that has been growing on her cheek since before she started traveling. She saw several providers—mostly in NGO clinics—along her journey. The diagnosis they gave was consistently “ringworm.” She was offered various topical creams, none of which produced any results.
Though the lesion is not painful, it causes some itching. The patient is much more concerned about its appearance. Through interpreters, she claims to be in otherwise good health. She has no other lesions, joint pain, fever, or malaise. She reports neither her family nor fellow travelers have such lesions.
Examination reveals an impressive 3-cm, round, papulosquamous plaque on the right side of her face (below the malar area). The lesion is neither tender nor notably warm. There are no palpable lymph nodes in the area. The scaling is mostly on the periphery. A KOH prep of the scaling shows no fungal elements.
Mammography starting at 40 cuts risk of breast cancer death
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.
The 2021 Medicare proposed rule: The good, the bad, and the ugly
As most of you know, Medicare publishes its proposed rule, which determines the physician fee schedule, around July 1 each year, accepts comments for 60 days, and then publishes a final rule on or around Nov. 1, which becomes final on Jan. 1 of the following year. The proposed rule is watched closely and has great impact, because not only are Medicare fees based on the rule, but most private insurances are based on Medicare.
This year’s proposed rule, announced in early August, is extraordinary by any past standard. It can be found here.
It cuts the conversion factor (which is what the work, practice expense, and malpractice values are multiplied by to get a payment) by 10.6%, from $36.09 to $32.26. This is necessary to maintain “budget neutrality” since there is a fixed pool of money, and payments for cognitive services are increasing. The overall effect on dermatology is a 2% cut, which is mild, compared with other specialties, such as nurse anesthetists and radiologists, both with an 11% decrease; chiropractors, with a 10% decrease; and interventional radiology, pathology, physical and occupational therapy, and cardiac surgery, all with a 9% decrease. General surgery will see a 7% decrease. Those with major increases are endocrinology, with a 17% increase; rheumatology, with a 16% increase; and hematology/oncology, with a 14% increase.
The overall push by CMS (and the relative value update committee) is to improve the pay for cognitive services, that is evaluation and management (E/M) services. Since dermatology also provides such services, the effect of the proposed rule will vary dramatically depending on your case mix. I must also point out that, since existing overhead is relatively fixed, say at 50%, a 10% decrease in revenue may translate into a 20% loss in physician income.
The good
Simplified coding and billing requirements for E/M visits will go into effect Jan. 1, 2021. For dermatology, any visit where a decision to do a minor procedure or prescribe a medication takes place will become a level 4 visit. Most of the useless documentation requirements and need to examine multiple organ systems will be eliminated. The most common E/M code currently used by dermatologists is a level 3, and this will on average move up to a level 4. Thus, general dermatology will benefit from the new rule. For example, if a dermatologist sees a patient and does a tangential biopsy of the skin, the payment will be $214.52, compared with $178.65 in 2020.
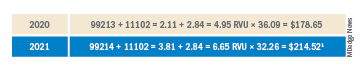
The bad
As mentioned above, the impact will vary by case mix. Those doing a lot of surgery will see a much larger cut. Mohs surgeons, for example will see about a 6.5% decrease.1
Aggravating the cuts to surgery is the fact that, while CMS has bolstered the pay for E/M stand-alone codes, they did not increase the reimbursement level of the built-in follow-up visits inside the 10- and 90-day global periods.

The ugly
Procedure codes with a lot of practice expense built into them, such as Mohs and reconstruction, are not hit as hard by the conversion factor cut because the practice expense is generally spared. There is much less practice expense in a pathology code so dermatopathology faces the most severe cuts. Pathology and other specialties that do not generally bill office/outpatient E/M codes are estimated to see the greatest decrease in payment in 2021.
Code 88305, the most common dermatopathology code, will decrease overall from $71.46 to $66.78 (–6.5%). Digging a little deeper, we find that the technical charge (the payment to process and make the slide) actually increases from $32.12 to $32.26, but the professional component (the interpretation of the slide and report generation) decreases from $39.34 to $34.52 (–12.3%).
I must also point out that this proposed rule allows for nurse practitioners (NPs), clinical nurse specialists (CNSs), physician assistants (PAs), and certified nurse-midwives (CNMs) to supervise the performance of diagnostic tests in addition to physicians. I wonder if we will see an increase in billing of dermatopathology by the untrained.
Adding more confusion – and an additional hit to hospital-based practices – is the federal appeals court decision affirming the ability of the Centers for Medicare & Medicaid Services to mandate site-neutral payments for E/M codes. This means that hospital-affiliated practices, which used to enjoy payment of up to 114% more than offices, will be paid the same as offices. This will save CMS $300 million, but these savings will not be flowing back into the physician fee schedule.
Fixing this will require congressional action since CMS is bound by law to maintain budget neutrality. The specialty societies saw this coming and have already been lobbying furiously to waive budget neutrality requirements, especially in this time of a pandemic that has had an adverse impact on physicians. This is noted in detail on the AADA website, accessible to AAD members.
Since this will take a legislative fix, you should contact your congressional representative or senator and ask them to enact legislation to waive Medicare’s budget neutrality requirements to apply the increased E/M adjustment to all 10- and 90-day global code values. You might also inquire where the $300 million saved by site neutral payment reform will go, and suggest applying it towards restoring the conversion factor to a more normal number.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Reference
1. Calculations and tables courtesy of Brent Moody, M.D., AAD AMA relative value update committee practice expense representative and specialist.
As most of you know, Medicare publishes its proposed rule, which determines the physician fee schedule, around July 1 each year, accepts comments for 60 days, and then publishes a final rule on or around Nov. 1, which becomes final on Jan. 1 of the following year. The proposed rule is watched closely and has great impact, because not only are Medicare fees based on the rule, but most private insurances are based on Medicare.
This year’s proposed rule, announced in early August, is extraordinary by any past standard. It can be found here.
It cuts the conversion factor (which is what the work, practice expense, and malpractice values are multiplied by to get a payment) by 10.6%, from $36.09 to $32.26. This is necessary to maintain “budget neutrality” since there is a fixed pool of money, and payments for cognitive services are increasing. The overall effect on dermatology is a 2% cut, which is mild, compared with other specialties, such as nurse anesthetists and radiologists, both with an 11% decrease; chiropractors, with a 10% decrease; and interventional radiology, pathology, physical and occupational therapy, and cardiac surgery, all with a 9% decrease. General surgery will see a 7% decrease. Those with major increases are endocrinology, with a 17% increase; rheumatology, with a 16% increase; and hematology/oncology, with a 14% increase.
The overall push by CMS (and the relative value update committee) is to improve the pay for cognitive services, that is evaluation and management (E/M) services. Since dermatology also provides such services, the effect of the proposed rule will vary dramatically depending on your case mix. I must also point out that, since existing overhead is relatively fixed, say at 50%, a 10% decrease in revenue may translate into a 20% loss in physician income.
The good
Simplified coding and billing requirements for E/M visits will go into effect Jan. 1, 2021. For dermatology, any visit where a decision to do a minor procedure or prescribe a medication takes place will become a level 4 visit. Most of the useless documentation requirements and need to examine multiple organ systems will be eliminated. The most common E/M code currently used by dermatologists is a level 3, and this will on average move up to a level 4. Thus, general dermatology will benefit from the new rule. For example, if a dermatologist sees a patient and does a tangential biopsy of the skin, the payment will be $214.52, compared with $178.65 in 2020.
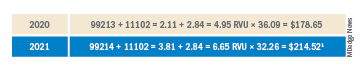
The bad
As mentioned above, the impact will vary by case mix. Those doing a lot of surgery will see a much larger cut. Mohs surgeons, for example will see about a 6.5% decrease.1
Aggravating the cuts to surgery is the fact that, while CMS has bolstered the pay for E/M stand-alone codes, they did not increase the reimbursement level of the built-in follow-up visits inside the 10- and 90-day global periods.

The ugly
Procedure codes with a lot of practice expense built into them, such as Mohs and reconstruction, are not hit as hard by the conversion factor cut because the practice expense is generally spared. There is much less practice expense in a pathology code so dermatopathology faces the most severe cuts. Pathology and other specialties that do not generally bill office/outpatient E/M codes are estimated to see the greatest decrease in payment in 2021.
Code 88305, the most common dermatopathology code, will decrease overall from $71.46 to $66.78 (–6.5%). Digging a little deeper, we find that the technical charge (the payment to process and make the slide) actually increases from $32.12 to $32.26, but the professional component (the interpretation of the slide and report generation) decreases from $39.34 to $34.52 (–12.3%).
I must also point out that this proposed rule allows for nurse practitioners (NPs), clinical nurse specialists (CNSs), physician assistants (PAs), and certified nurse-midwives (CNMs) to supervise the performance of diagnostic tests in addition to physicians. I wonder if we will see an increase in billing of dermatopathology by the untrained.
Adding more confusion – and an additional hit to hospital-based practices – is the federal appeals court decision affirming the ability of the Centers for Medicare & Medicaid Services to mandate site-neutral payments for E/M codes. This means that hospital-affiliated practices, which used to enjoy payment of up to 114% more than offices, will be paid the same as offices. This will save CMS $300 million, but these savings will not be flowing back into the physician fee schedule.
Fixing this will require congressional action since CMS is bound by law to maintain budget neutrality. The specialty societies saw this coming and have already been lobbying furiously to waive budget neutrality requirements, especially in this time of a pandemic that has had an adverse impact on physicians. This is noted in detail on the AADA website, accessible to AAD members.
Since this will take a legislative fix, you should contact your congressional representative or senator and ask them to enact legislation to waive Medicare’s budget neutrality requirements to apply the increased E/M adjustment to all 10- and 90-day global code values. You might also inquire where the $300 million saved by site neutral payment reform will go, and suggest applying it towards restoring the conversion factor to a more normal number.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Reference
1. Calculations and tables courtesy of Brent Moody, M.D., AAD AMA relative value update committee practice expense representative and specialist.
As most of you know, Medicare publishes its proposed rule, which determines the physician fee schedule, around July 1 each year, accepts comments for 60 days, and then publishes a final rule on or around Nov. 1, which becomes final on Jan. 1 of the following year. The proposed rule is watched closely and has great impact, because not only are Medicare fees based on the rule, but most private insurances are based on Medicare.
This year’s proposed rule, announced in early August, is extraordinary by any past standard. It can be found here.
It cuts the conversion factor (which is what the work, practice expense, and malpractice values are multiplied by to get a payment) by 10.6%, from $36.09 to $32.26. This is necessary to maintain “budget neutrality” since there is a fixed pool of money, and payments for cognitive services are increasing. The overall effect on dermatology is a 2% cut, which is mild, compared with other specialties, such as nurse anesthetists and radiologists, both with an 11% decrease; chiropractors, with a 10% decrease; and interventional radiology, pathology, physical and occupational therapy, and cardiac surgery, all with a 9% decrease. General surgery will see a 7% decrease. Those with major increases are endocrinology, with a 17% increase; rheumatology, with a 16% increase; and hematology/oncology, with a 14% increase.
The overall push by CMS (and the relative value update committee) is to improve the pay for cognitive services, that is evaluation and management (E/M) services. Since dermatology also provides such services, the effect of the proposed rule will vary dramatically depending on your case mix. I must also point out that, since existing overhead is relatively fixed, say at 50%, a 10% decrease in revenue may translate into a 20% loss in physician income.
The good
Simplified coding and billing requirements for E/M visits will go into effect Jan. 1, 2021. For dermatology, any visit where a decision to do a minor procedure or prescribe a medication takes place will become a level 4 visit. Most of the useless documentation requirements and need to examine multiple organ systems will be eliminated. The most common E/M code currently used by dermatologists is a level 3, and this will on average move up to a level 4. Thus, general dermatology will benefit from the new rule. For example, if a dermatologist sees a patient and does a tangential biopsy of the skin, the payment will be $214.52, compared with $178.65 in 2020.
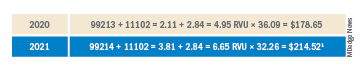
The bad
As mentioned above, the impact will vary by case mix. Those doing a lot of surgery will see a much larger cut. Mohs surgeons, for example will see about a 6.5% decrease.1
Aggravating the cuts to surgery is the fact that, while CMS has bolstered the pay for E/M stand-alone codes, they did not increase the reimbursement level of the built-in follow-up visits inside the 10- and 90-day global periods.

The ugly
Procedure codes with a lot of practice expense built into them, such as Mohs and reconstruction, are not hit as hard by the conversion factor cut because the practice expense is generally spared. There is much less practice expense in a pathology code so dermatopathology faces the most severe cuts. Pathology and other specialties that do not generally bill office/outpatient E/M codes are estimated to see the greatest decrease in payment in 2021.
Code 88305, the most common dermatopathology code, will decrease overall from $71.46 to $66.78 (–6.5%). Digging a little deeper, we find that the technical charge (the payment to process and make the slide) actually increases from $32.12 to $32.26, but the professional component (the interpretation of the slide and report generation) decreases from $39.34 to $34.52 (–12.3%).
I must also point out that this proposed rule allows for nurse practitioners (NPs), clinical nurse specialists (CNSs), physician assistants (PAs), and certified nurse-midwives (CNMs) to supervise the performance of diagnostic tests in addition to physicians. I wonder if we will see an increase in billing of dermatopathology by the untrained.
Adding more confusion – and an additional hit to hospital-based practices – is the federal appeals court decision affirming the ability of the Centers for Medicare & Medicaid Services to mandate site-neutral payments for E/M codes. This means that hospital-affiliated practices, which used to enjoy payment of up to 114% more than offices, will be paid the same as offices. This will save CMS $300 million, but these savings will not be flowing back into the physician fee schedule.
Fixing this will require congressional action since CMS is bound by law to maintain budget neutrality. The specialty societies saw this coming and have already been lobbying furiously to waive budget neutrality requirements, especially in this time of a pandemic that has had an adverse impact on physicians. This is noted in detail on the AADA website, accessible to AAD members.
Since this will take a legislative fix, you should contact your congressional representative or senator and ask them to enact legislation to waive Medicare’s budget neutrality requirements to apply the increased E/M adjustment to all 10- and 90-day global code values. You might also inquire where the $300 million saved by site neutral payment reform will go, and suggest applying it towards restoring the conversion factor to a more normal number.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Reference
1. Calculations and tables courtesy of Brent Moody, M.D., AAD AMA relative value update committee practice expense representative and specialist.
Depressed Shiny Scars and Crusted Erosions
The Diagnosis: Erythropoietic Protoporphyria
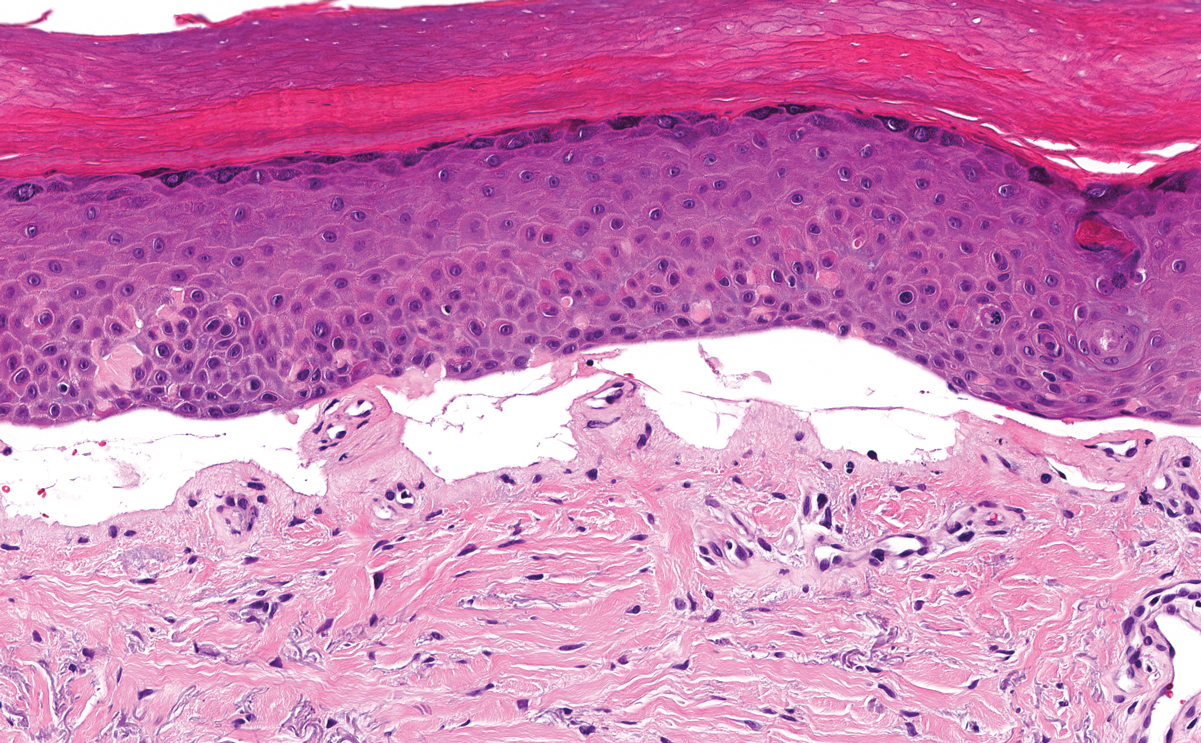
Erythropoietic protoporphyria (EPP) is an autosomal-recessive photodermatosis that results from loss of activity of ferrochelatase, the last enzyme in the heme biosynthetic pathway.1 Erythropoietic protoporphyria normally involves sun-exposed areas of the body. Skin that is exposed to sunlight develops intense burning and stinging pain followed by erythema, edema, crusting, and petechiae that develops into waxy scarring over time. In contrast to other porphyrias, blistering generally is not seen.2 Accurate diagnosis often can be delayed by a decade or more following symptom onset due to the prominence of subjective pain as the presenting sign.
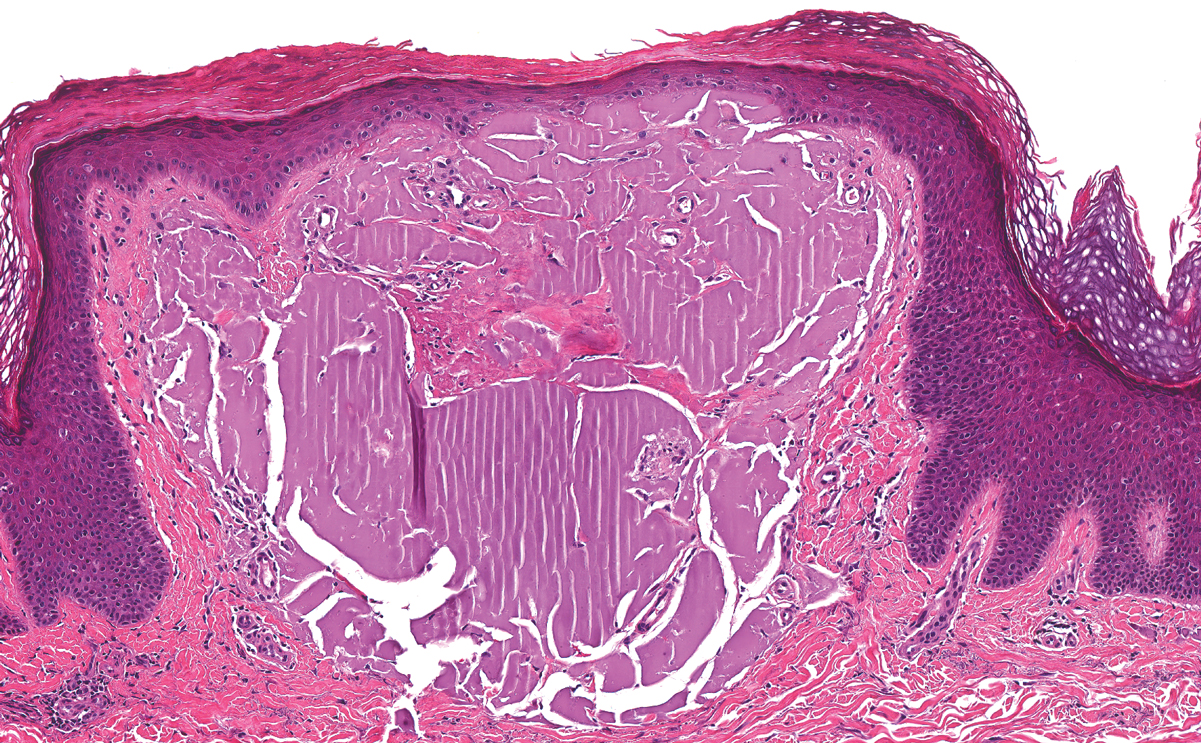
The histologic appearance of EPP differs depending on the chronicity of lesions. Biopsies of acute lesions show vacuolization of epidermal cells with intercellular edema, vacuolization and cytolysis of endothelial cells in superficial blood vessels, and focal red blood cell extravasation.3,4 A largely neutrophilic inflammatory infiltrate can be present.5 Hyaline cuffing develops over time in and around vessels in the papillary and superficial reticular dermis with notable sparing of adnexal structures. The perivascular deposits are strongly periodic acid-Schiff (PAS) positive and diastase resistant (Figure 1). Direct immunofluorescence shows mainly IgG and some IgM, fibrinogen, and C3 outlining characteristic donut-shaped blood vessels in the papillary dermis.6 The prominent thickness of the perivascular hyaline material depositions and the absence of subepidermal blistering can help differentiate EPP from porphyria cutanea tarda (PCT) and pseudoporphyria.6,7 When the deposition is extensive and involves the surrounding dermis, EPP can mimic colloid milium. Additional histologic differential diagnoses of EPP include other dermal depositional diseases such as lipoid proteinosis and amyloidosis.
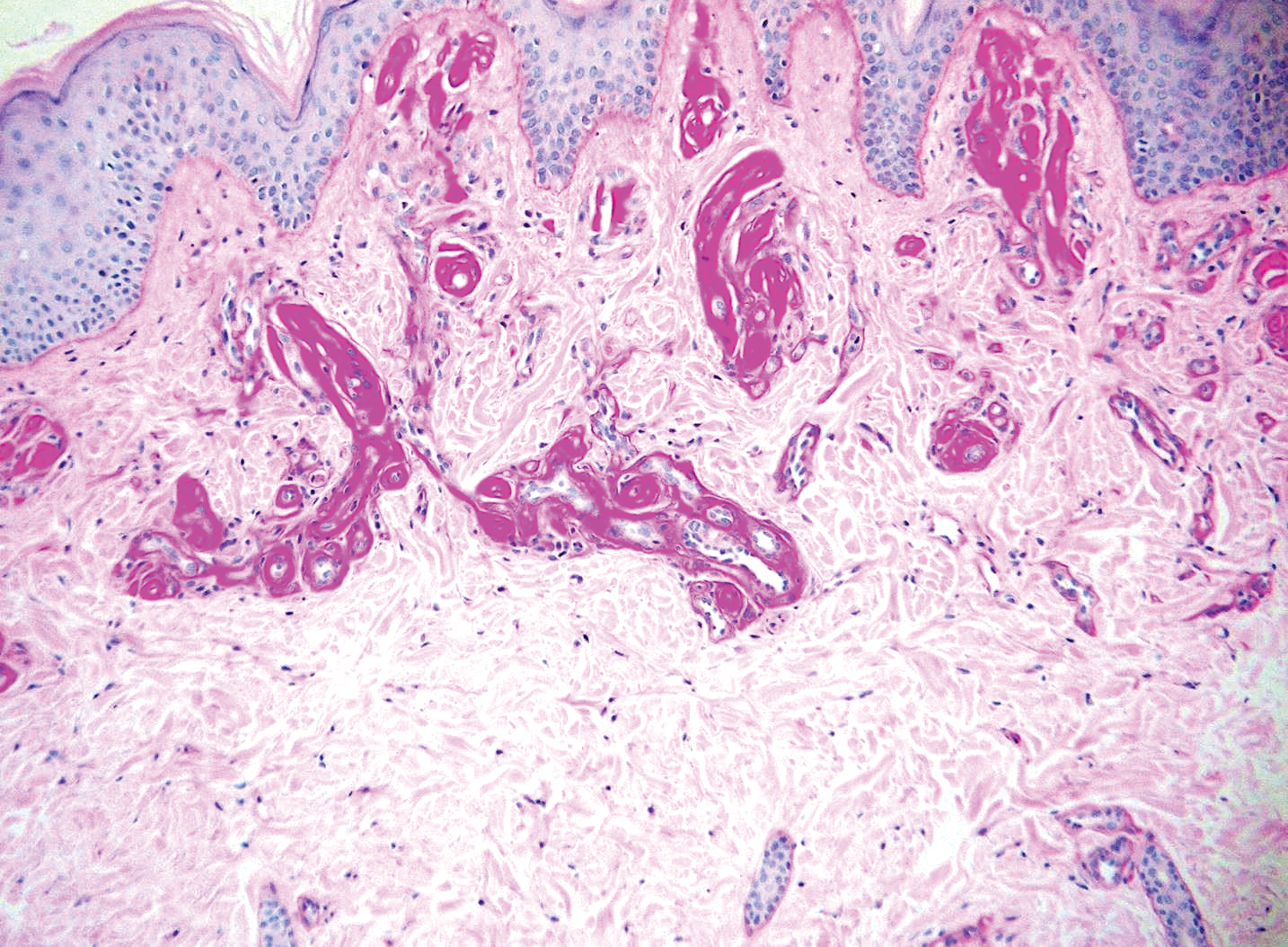
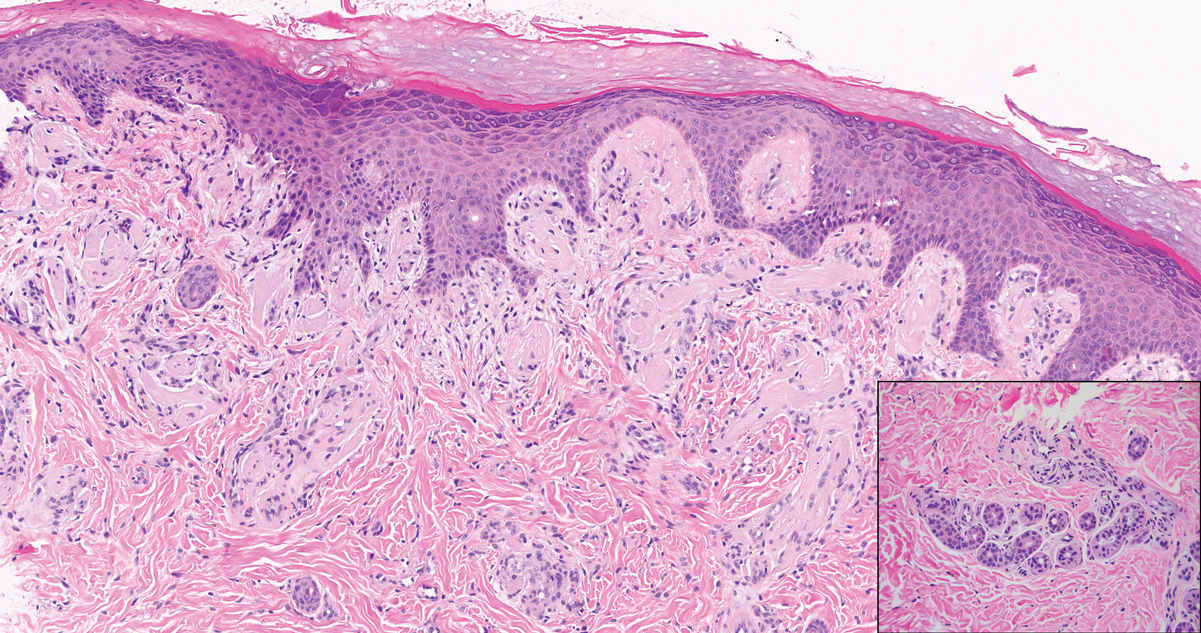
Lipoid proteinosis is an autosomal-recessive multisystem genodermatosis caused by mutations in extracellular matrix gene 1, ECM1. The first clinical sign can be a hoarse cry in infancy due to infiltration of vocal cords.3 Development of papulonodular lesions along the eyelids can result in a string-of-beads appearance called moniliform blepharosis, which is pathognomonic for lipoid proteinosis.6 With chronicity, the involved skin can become yellow, waxy, and thickened, particularly in the flexures or areas of trauma. Histologically, the dermis in lipoid proteinosis becomes diffusely thickened due to deposition of PAS-positive eosinophilic hyaline material that stains weakly with Congo red and thioflavin T.6 Early lesions demonstrate pale pink, hyalinelike thickening of the papillary dermal capillaries. Chronic lesions reveal an acanthotic epidermis, occasional papillomatosis with overlying hyperkeratosis, and a thickened dermis where diffuse thick bundles of pink hyaline deposits are oriented perpendicularly to the dermoepidermal junction.1,6 Lipoid proteinosis can be differentiated from EPP by the involvement of adnexal structures such as hair follicles, sebaceous glands, and arrector pili muscles (Figure 2), as opposed to EPP where adnexal structures are spared.1 Additionally, depositions in lipoid proteinosis are centered around both superficial and deep vessels with an onion skin-like pattern, while EPP involves mainly superficial vessels with more mild and focal hyalinization.
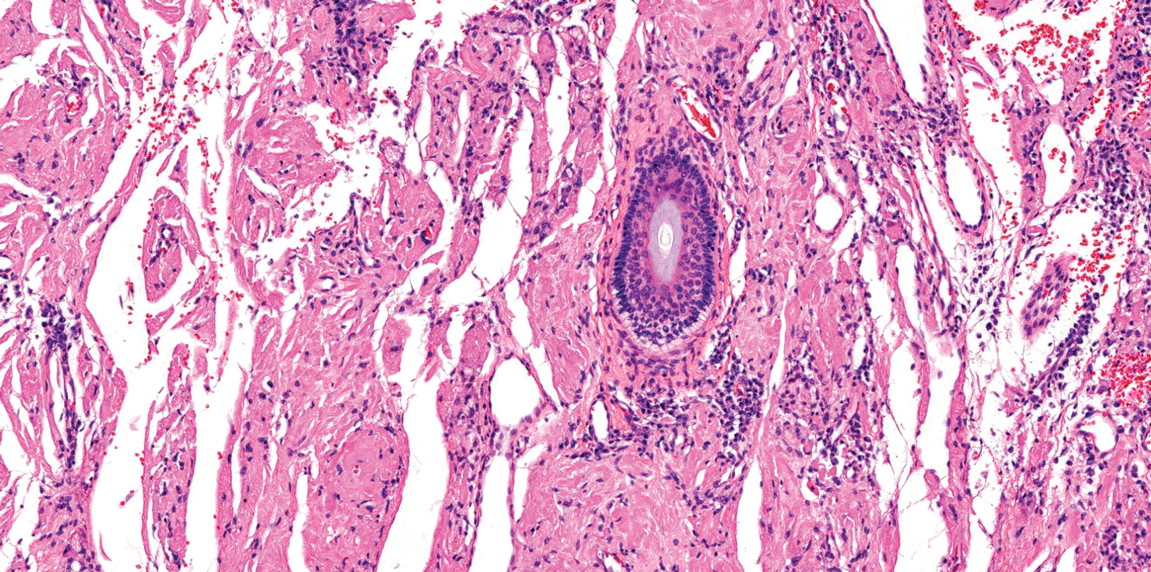
Juvenile colloid milium (JCM) is a rare condition that presents before puberty with discrete, yellow-brown, translucent papules predominantly located on the cheeks and nose and around the mouth. A gelatinous material can be expressed after puncturing a lesion.6 Gingival deposits and ligneous conjunctivitis also can be present. On histopathology, JCM shows degeneration of epidermal keratinocytes that form colloid bodies within the superficial dermis following apoptosis.6 Hematoxylin and eosin staining shows amorphous, fissured, pale pink deposits completely filling and expanding the superficial to mid dermis with clefting and no inflammation (Figure 3). Spindle-shaped fibroblasts may be seen within the lines of colloid fissuring and dispersed throughout the deposits.1 Histologically, JCM can be differentiated from EPP because deposits in EPP are distributed around and within superficial blood vessel walls, causing prominent vascular thickening not seen in JCM.6 The adult variant of colloid milium also can be distinguished from EPP by the presence of solar elastosis, which is absent in EPP due to a history of sun avoidance.3,7
Lichen amyloidosis presents with highly pruritic, red-brown, hyperkeratotic papules that commonly are found on the anterior lower legs and extensor forearms.1 The calves, ankles, dorsal aspects of the feet, thighs, and trunk also may be affected. Excoriations, lichenification, and nodular prurigo-like lesions due to chronic scratching can be present.6 Lichen amyloidosis is characterized by large, pink, amorphous deposits in the papillary dermis with epidermal acanthosis, hypergranulosis, and hyperkeratosis (Figure 4).6 Perivascular deposits are not a feature of primary cutaneous localized amyloid lesions.6 The diagnosis can be confirmed with Congo red staining under polarized light, which classically demonstrates apple green birefringence.1 For cases of amyloid that are not detected by Congo red or are not clear-cut, direct immunofluorescence and immunohistochemistry can be used as adjuncts for diagnosis. Amyloid deposits fluoresce positively for immunoglobulins or complements, particularly IgM and C3,8 and immunohistochemistry confirms the presence of keratin epitopes in deposits.9
Porphyria cutanea tarda can appear histologically similar to EPP. Caterpillar bodies, or linearly arranged eosinophilic PAS-positive globules in the epidermis overlying subepidermal bullae, are a diagnostic histopathologic finding in both PCT and EPP but are seen in less than half of both cases.7,10 Compared to EPP, the perivascular deposits in PCT typically are less pronounced and limited to the vessel wall with smaller hyaline cuffs (Figure 5).7 Additionally, solar elastosis can be seen in PCT lesions but not in EPP, as patients with PCT tend to be older and have increased cumulative sun damage.
- Touart DM, Sau P. Cutaneous deposition diseases. part I. J Am Acad Dermatol. 1998;39(2, pt 1):149-171; quiz 172-144.
- Lim HW. Pathogenesis of photosensitivity in the cutaneous porphyrias. J Invest Dermatol. 2005;124:xvi-xvii.
- In: Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
- Horner ME, Alikhan A, Tintle S, et al. Cutaneous porphyrias part I: epidemiology, pathogenesis, presentation, diagnosis, and histopathology. Int J Dermatol. 2013;52:1464-1480.
- Michaels BD, Del Rosso JQ, Mobini N, et al. Erythropoietic protoporphyria: a case report and literature review. J Clin Aesthet Dermatol. 2010;3:44-48.
- Calonje E, Brenn T, Lazar A, et al, eds. McKee's Pathology of the Skin. 4th ed. China: Elsevier Saunders; 2012.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Elsevier Limited; 2016.
- MacDonald DM, Black MM, Ramnarain N. Immunofluorescence studies in primary localized cutaneous amyloidosis. Br J Dermatol. 1977;96:635-641.
- Ortiz-Romero PL, Ballestin-Carcavilla C, Lopez-Estebaranz JL, et al. Clinicopathologic and immunohistochemical studies on lichen amyloidosis and macular amyloidosis. Arch Dermatol. 1994;130:1559-1560.
- Raso DS, Greene WB, Maize JC, et al. Caterpillar bodies of porphyria cutanea tarda ultrastructurally represent a unique arrangement of colloid and basement membrane bodies. Am J Dermatopathol. 1996;18:24-29.
The Diagnosis: Erythropoietic Protoporphyria
Erythropoietic protoporphyria (EPP) is an autosomal-recessive photodermatosis that results from loss of activity of ferrochelatase, the last enzyme in the heme biosynthetic pathway.1 Erythropoietic protoporphyria normally involves sun-exposed areas of the body. Skin that is exposed to sunlight develops intense burning and stinging pain followed by erythema, edema, crusting, and petechiae that develops into waxy scarring over time. In contrast to other porphyrias, blistering generally is not seen.2 Accurate diagnosis often can be delayed by a decade or more following symptom onset due to the prominence of subjective pain as the presenting sign.
The histologic appearance of EPP differs depending on the chronicity of lesions. Biopsies of acute lesions show vacuolization of epidermal cells with intercellular edema, vacuolization and cytolysis of endothelial cells in superficial blood vessels, and focal red blood cell extravasation.3,4 A largely neutrophilic inflammatory infiltrate can be present.5 Hyaline cuffing develops over time in and around vessels in the papillary and superficial reticular dermis with notable sparing of adnexal structures. The perivascular deposits are strongly periodic acid-Schiff (PAS) positive and diastase resistant (Figure 1). Direct immunofluorescence shows mainly IgG and some IgM, fibrinogen, and C3 outlining characteristic donut-shaped blood vessels in the papillary dermis.6 The prominent thickness of the perivascular hyaline material depositions and the absence of subepidermal blistering can help differentiate EPP from porphyria cutanea tarda (PCT) and pseudoporphyria.6,7 When the deposition is extensive and involves the surrounding dermis, EPP can mimic colloid milium. Additional histologic differential diagnoses of EPP include other dermal depositional diseases such as lipoid proteinosis and amyloidosis.

Lipoid proteinosis is an autosomal-recessive multisystem genodermatosis caused by mutations in extracellular matrix gene 1, ECM1. The first clinical sign can be a hoarse cry in infancy due to infiltration of vocal cords.3 Development of papulonodular lesions along the eyelids can result in a string-of-beads appearance called moniliform blepharosis, which is pathognomonic for lipoid proteinosis.6 With chronicity, the involved skin can become yellow, waxy, and thickened, particularly in the flexures or areas of trauma. Histologically, the dermis in lipoid proteinosis becomes diffusely thickened due to deposition of PAS-positive eosinophilic hyaline material that stains weakly with Congo red and thioflavin T.6 Early lesions demonstrate pale pink, hyalinelike thickening of the papillary dermal capillaries. Chronic lesions reveal an acanthotic epidermis, occasional papillomatosis with overlying hyperkeratosis, and a thickened dermis where diffuse thick bundles of pink hyaline deposits are oriented perpendicularly to the dermoepidermal junction.1,6 Lipoid proteinosis can be differentiated from EPP by the involvement of adnexal structures such as hair follicles, sebaceous glands, and arrector pili muscles (Figure 2), as opposed to EPP where adnexal structures are spared.1 Additionally, depositions in lipoid proteinosis are centered around both superficial and deep vessels with an onion skin-like pattern, while EPP involves mainly superficial vessels with more mild and focal hyalinization.

Juvenile colloid milium (JCM) is a rare condition that presents before puberty with discrete, yellow-brown, translucent papules predominantly located on the cheeks and nose and around the mouth. A gelatinous material can be expressed after puncturing a lesion.6 Gingival deposits and ligneous conjunctivitis also can be present. On histopathology, JCM shows degeneration of epidermal keratinocytes that form colloid bodies within the superficial dermis following apoptosis.6 Hematoxylin and eosin staining shows amorphous, fissured, pale pink deposits completely filling and expanding the superficial to mid dermis with clefting and no inflammation (Figure 3). Spindle-shaped fibroblasts may be seen within the lines of colloid fissuring and dispersed throughout the deposits.1 Histologically, JCM can be differentiated from EPP because deposits in EPP are distributed around and within superficial blood vessel walls, causing prominent vascular thickening not seen in JCM.6 The adult variant of colloid milium also can be distinguished from EPP by the presence of solar elastosis, which is absent in EPP due to a history of sun avoidance.3,7

Lichen amyloidosis presents with highly pruritic, red-brown, hyperkeratotic papules that commonly are found on the anterior lower legs and extensor forearms.1 The calves, ankles, dorsal aspects of the feet, thighs, and trunk also may be affected. Excoriations, lichenification, and nodular prurigo-like lesions due to chronic scratching can be present.6 Lichen amyloidosis is characterized by large, pink, amorphous deposits in the papillary dermis with epidermal acanthosis, hypergranulosis, and hyperkeratosis (Figure 4).6 Perivascular deposits are not a feature of primary cutaneous localized amyloid lesions.6 The diagnosis can be confirmed with Congo red staining under polarized light, which classically demonstrates apple green birefringence.1 For cases of amyloid that are not detected by Congo red or are not clear-cut, direct immunofluorescence and immunohistochemistry can be used as adjuncts for diagnosis. Amyloid deposits fluoresce positively for immunoglobulins or complements, particularly IgM and C3,8 and immunohistochemistry confirms the presence of keratin epitopes in deposits.9

Porphyria cutanea tarda can appear histologically similar to EPP. Caterpillar bodies, or linearly arranged eosinophilic PAS-positive globules in the epidermis overlying subepidermal bullae, are a diagnostic histopathologic finding in both PCT and EPP but are seen in less than half of both cases.7,10 Compared to EPP, the perivascular deposits in PCT typically are less pronounced and limited to the vessel wall with smaller hyaline cuffs (Figure 5).7 Additionally, solar elastosis can be seen in PCT lesions but not in EPP, as patients with PCT tend to be older and have increased cumulative sun damage.

The Diagnosis: Erythropoietic Protoporphyria
Erythropoietic protoporphyria (EPP) is an autosomal-recessive photodermatosis that results from loss of activity of ferrochelatase, the last enzyme in the heme biosynthetic pathway.1 Erythropoietic protoporphyria normally involves sun-exposed areas of the body. Skin that is exposed to sunlight develops intense burning and stinging pain followed by erythema, edema, crusting, and petechiae that develops into waxy scarring over time. In contrast to other porphyrias, blistering generally is not seen.2 Accurate diagnosis often can be delayed by a decade or more following symptom onset due to the prominence of subjective pain as the presenting sign.
The histologic appearance of EPP differs depending on the chronicity of lesions. Biopsies of acute lesions show vacuolization of epidermal cells with intercellular edema, vacuolization and cytolysis of endothelial cells in superficial blood vessels, and focal red blood cell extravasation.3,4 A largely neutrophilic inflammatory infiltrate can be present.5 Hyaline cuffing develops over time in and around vessels in the papillary and superficial reticular dermis with notable sparing of adnexal structures. The perivascular deposits are strongly periodic acid-Schiff (PAS) positive and diastase resistant (Figure 1). Direct immunofluorescence shows mainly IgG and some IgM, fibrinogen, and C3 outlining characteristic donut-shaped blood vessels in the papillary dermis.6 The prominent thickness of the perivascular hyaline material depositions and the absence of subepidermal blistering can help differentiate EPP from porphyria cutanea tarda (PCT) and pseudoporphyria.6,7 When the deposition is extensive and involves the surrounding dermis, EPP can mimic colloid milium. Additional histologic differential diagnoses of EPP include other dermal depositional diseases such as lipoid proteinosis and amyloidosis.

Lipoid proteinosis is an autosomal-recessive multisystem genodermatosis caused by mutations in extracellular matrix gene 1, ECM1. The first clinical sign can be a hoarse cry in infancy due to infiltration of vocal cords.3 Development of papulonodular lesions along the eyelids can result in a string-of-beads appearance called moniliform blepharosis, which is pathognomonic for lipoid proteinosis.6 With chronicity, the involved skin can become yellow, waxy, and thickened, particularly in the flexures or areas of trauma. Histologically, the dermis in lipoid proteinosis becomes diffusely thickened due to deposition of PAS-positive eosinophilic hyaline material that stains weakly with Congo red and thioflavin T.6 Early lesions demonstrate pale pink, hyalinelike thickening of the papillary dermal capillaries. Chronic lesions reveal an acanthotic epidermis, occasional papillomatosis with overlying hyperkeratosis, and a thickened dermis where diffuse thick bundles of pink hyaline deposits are oriented perpendicularly to the dermoepidermal junction.1,6 Lipoid proteinosis can be differentiated from EPP by the involvement of adnexal structures such as hair follicles, sebaceous glands, and arrector pili muscles (Figure 2), as opposed to EPP where adnexal structures are spared.1 Additionally, depositions in lipoid proteinosis are centered around both superficial and deep vessels with an onion skin-like pattern, while EPP involves mainly superficial vessels with more mild and focal hyalinization.

Juvenile colloid milium (JCM) is a rare condition that presents before puberty with discrete, yellow-brown, translucent papules predominantly located on the cheeks and nose and around the mouth. A gelatinous material can be expressed after puncturing a lesion.6 Gingival deposits and ligneous conjunctivitis also can be present. On histopathology, JCM shows degeneration of epidermal keratinocytes that form colloid bodies within the superficial dermis following apoptosis.6 Hematoxylin and eosin staining shows amorphous, fissured, pale pink deposits completely filling and expanding the superficial to mid dermis with clefting and no inflammation (Figure 3). Spindle-shaped fibroblasts may be seen within the lines of colloid fissuring and dispersed throughout the deposits.1 Histologically, JCM can be differentiated from EPP because deposits in EPP are distributed around and within superficial blood vessel walls, causing prominent vascular thickening not seen in JCM.6 The adult variant of colloid milium also can be distinguished from EPP by the presence of solar elastosis, which is absent in EPP due to a history of sun avoidance.3,7

Lichen amyloidosis presents with highly pruritic, red-brown, hyperkeratotic papules that commonly are found on the anterior lower legs and extensor forearms.1 The calves, ankles, dorsal aspects of the feet, thighs, and trunk also may be affected. Excoriations, lichenification, and nodular prurigo-like lesions due to chronic scratching can be present.6 Lichen amyloidosis is characterized by large, pink, amorphous deposits in the papillary dermis with epidermal acanthosis, hypergranulosis, and hyperkeratosis (Figure 4).6 Perivascular deposits are not a feature of primary cutaneous localized amyloid lesions.6 The diagnosis can be confirmed with Congo red staining under polarized light, which classically demonstrates apple green birefringence.1 For cases of amyloid that are not detected by Congo red or are not clear-cut, direct immunofluorescence and immunohistochemistry can be used as adjuncts for diagnosis. Amyloid deposits fluoresce positively for immunoglobulins or complements, particularly IgM and C3,8 and immunohistochemistry confirms the presence of keratin epitopes in deposits.9

Porphyria cutanea tarda can appear histologically similar to EPP. Caterpillar bodies, or linearly arranged eosinophilic PAS-positive globules in the epidermis overlying subepidermal bullae, are a diagnostic histopathologic finding in both PCT and EPP but are seen in less than half of both cases.7,10 Compared to EPP, the perivascular deposits in PCT typically are less pronounced and limited to the vessel wall with smaller hyaline cuffs (Figure 5).7 Additionally, solar elastosis can be seen in PCT lesions but not in EPP, as patients with PCT tend to be older and have increased cumulative sun damage.

- Touart DM, Sau P. Cutaneous deposition diseases. part I. J Am Acad Dermatol. 1998;39(2, pt 1):149-171; quiz 172-144.
- Lim HW. Pathogenesis of photosensitivity in the cutaneous porphyrias. J Invest Dermatol. 2005;124:xvi-xvii.
- In: Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
- Horner ME, Alikhan A, Tintle S, et al. Cutaneous porphyrias part I: epidemiology, pathogenesis, presentation, diagnosis, and histopathology. Int J Dermatol. 2013;52:1464-1480.
- Michaels BD, Del Rosso JQ, Mobini N, et al. Erythropoietic protoporphyria: a case report and literature review. J Clin Aesthet Dermatol. 2010;3:44-48.
- Calonje E, Brenn T, Lazar A, et al, eds. McKee's Pathology of the Skin. 4th ed. China: Elsevier Saunders; 2012.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Elsevier Limited; 2016.
- MacDonald DM, Black MM, Ramnarain N. Immunofluorescence studies in primary localized cutaneous amyloidosis. Br J Dermatol. 1977;96:635-641.
- Ortiz-Romero PL, Ballestin-Carcavilla C, Lopez-Estebaranz JL, et al. Clinicopathologic and immunohistochemical studies on lichen amyloidosis and macular amyloidosis. Arch Dermatol. 1994;130:1559-1560.
- Raso DS, Greene WB, Maize JC, et al. Caterpillar bodies of porphyria cutanea tarda ultrastructurally represent a unique arrangement of colloid and basement membrane bodies. Am J Dermatopathol. 1996;18:24-29.
- Touart DM, Sau P. Cutaneous deposition diseases. part I. J Am Acad Dermatol. 1998;39(2, pt 1):149-171; quiz 172-144.
- Lim HW. Pathogenesis of photosensitivity in the cutaneous porphyrias. J Invest Dermatol. 2005;124:xvi-xvii.
- In: Alikhan A, Hocker TLH, eds. Review of Dermatology. China: Elsevier; 2017.
- Horner ME, Alikhan A, Tintle S, et al. Cutaneous porphyrias part I: epidemiology, pathogenesis, presentation, diagnosis, and histopathology. Int J Dermatol. 2013;52:1464-1480.
- Michaels BD, Del Rosso JQ, Mobini N, et al. Erythropoietic protoporphyria: a case report and literature review. J Clin Aesthet Dermatol. 2010;3:44-48.
- Calonje E, Brenn T, Lazar A, et al, eds. McKee's Pathology of the Skin. 4th ed. China: Elsevier Saunders; 2012.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Elsevier Limited; 2016.
- MacDonald DM, Black MM, Ramnarain N. Immunofluorescence studies in primary localized cutaneous amyloidosis. Br J Dermatol. 1977;96:635-641.
- Ortiz-Romero PL, Ballestin-Carcavilla C, Lopez-Estebaranz JL, et al. Clinicopathologic and immunohistochemical studies on lichen amyloidosis and macular amyloidosis. Arch Dermatol. 1994;130:1559-1560.
- Raso DS, Greene WB, Maize JC, et al. Caterpillar bodies of porphyria cutanea tarda ultrastructurally represent a unique arrangement of colloid and basement membrane bodies. Am J Dermatopathol. 1996;18:24-29.
A 9-year-old girl presented with unexplained burning pain on the face, hands, and feet of 3 years' duration. Physical examination showed depressed shiny scars and crusted erosions on the dorsal aspect of the nose, arms, hands, and fingers. A 3-mm punch biopsy specimen was obtained from the right hand.
Pooled COVID-19 testing feasible, greatly reduces supply use
‘Straightforward, cost effective, and efficient’
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
‘Straightforward, cost effective, and efficient’
‘Straightforward, cost effective, and efficient’
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 and the myth of the super doctor
Let us begin with a thought exercise. Close your eyes and picture the word, “hero.” What comes to mind? A relative, a teacher, a fictional character wielding a hammer or flying gracefully through the air?
Several months ago, our country was introduced to a foe that brought us to our knees. Before that time, the idea of a hero had fluctuated with circumstance and had been guided by aging and maturity; however, since the moment COVID-19 struck, a new image has emerged. Not all heroes wear capes, but some wield stethoscopes.
Over these past months the phrase, “Health Care Heroes” has spread throughout our collective consciousness, highlighted everywhere from talk shows and news media to billboards and journals. Doctors, nurses, and other health care professionals are lauded for their strength, dedication, resilience, and compassion. Citizens line up to clap, honk horns, and shower praise in recognition of those who have risked their health, sacrificed their personal lives, and committed themselves to the greater good. Yet, what does it mean to be a hero, and what is the cost of hero worship?
The focus of medical training has gradually shifted to include the physical as well as mental well-being of future physicians, but the remnants of traditional doctrine linger. Hours of focused training through study and direct clinical interaction reinforce dedication to patient care. Rewards are given for time spent and compassion lent, and research is lauded, but family time is rarely applauded. We are encouraged to do our greatest, work our hardest, be the best, rise and defeat every test. Failure (or the perception thereof) is not an option.
According to Rikinkumar S. Patel, MD, MPH, and associates, physicians have nearly twice the burnout rate of other professionals (Behav Sci. [Basel]. 2018 Nov;8[11]:98). The dedication to our craft propels excellence as well as sacrifice. When COVID-19 entered our lives, many of my colleagues did not hesitate to heed to the call for action. They immersed themselves in the ICU, led triage units, and extended work hours in the service of the sick and dying. Several were years removed from emergency/intensive care, while others were allocated from their chosen residency programs and voluntarily thrust into an environment they had never before traversed.
These individuals are praised as “brave,” “dedicated,” “selfless.” A few even provided insight into their experiences through various publications highlighting their appreciation and gratitude toward such a treacherous, albeit, tremendous experience. Even though their words are an honest perspective of life through one of the worst health care crises in 100 years, in effect, they perpetuate the noble hero; the myth of the super doctor.
In a profession that has borne witness to multiple suicides over the past few months, why do we not encourage open dialogue of our victories as well as our defeats? Our wins as much as our losses? Why does an esteemed veteran physician feel guilt over declining to provide emergency services to patients whom they have long forgotten how to manage? What drives the guilt and the self-doubt? Are we ashamed of what others will think? Is it that the fear of not living up to our cherished medical oath outweighs our own boundaries and acknowledgment of our limitations?
A hero is an entity, a person encompassing a state of being, yet health care professionals are bestowed this title and this burden on a near-daily basis. We are perfectly imperfect. The more in tune we are to vulnerability, the more honest we can become with ourselves and one another.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
Let us begin with a thought exercise. Close your eyes and picture the word, “hero.” What comes to mind? A relative, a teacher, a fictional character wielding a hammer or flying gracefully through the air?
Several months ago, our country was introduced to a foe that brought us to our knees. Before that time, the idea of a hero had fluctuated with circumstance and had been guided by aging and maturity; however, since the moment COVID-19 struck, a new image has emerged. Not all heroes wear capes, but some wield stethoscopes.
Over these past months the phrase, “Health Care Heroes” has spread throughout our collective consciousness, highlighted everywhere from talk shows and news media to billboards and journals. Doctors, nurses, and other health care professionals are lauded for their strength, dedication, resilience, and compassion. Citizens line up to clap, honk horns, and shower praise in recognition of those who have risked their health, sacrificed their personal lives, and committed themselves to the greater good. Yet, what does it mean to be a hero, and what is the cost of hero worship?
The focus of medical training has gradually shifted to include the physical as well as mental well-being of future physicians, but the remnants of traditional doctrine linger. Hours of focused training through study and direct clinical interaction reinforce dedication to patient care. Rewards are given for time spent and compassion lent, and research is lauded, but family time is rarely applauded. We are encouraged to do our greatest, work our hardest, be the best, rise and defeat every test. Failure (or the perception thereof) is not an option.
According to Rikinkumar S. Patel, MD, MPH, and associates, physicians have nearly twice the burnout rate of other professionals (Behav Sci. [Basel]. 2018 Nov;8[11]:98). The dedication to our craft propels excellence as well as sacrifice. When COVID-19 entered our lives, many of my colleagues did not hesitate to heed to the call for action. They immersed themselves in the ICU, led triage units, and extended work hours in the service of the sick and dying. Several were years removed from emergency/intensive care, while others were allocated from their chosen residency programs and voluntarily thrust into an environment they had never before traversed.
These individuals are praised as “brave,” “dedicated,” “selfless.” A few even provided insight into their experiences through various publications highlighting their appreciation and gratitude toward such a treacherous, albeit, tremendous experience. Even though their words are an honest perspective of life through one of the worst health care crises in 100 years, in effect, they perpetuate the noble hero; the myth of the super doctor.
In a profession that has borne witness to multiple suicides over the past few months, why do we not encourage open dialogue of our victories as well as our defeats? Our wins as much as our losses? Why does an esteemed veteran physician feel guilt over declining to provide emergency services to patients whom they have long forgotten how to manage? What drives the guilt and the self-doubt? Are we ashamed of what others will think? Is it that the fear of not living up to our cherished medical oath outweighs our own boundaries and acknowledgment of our limitations?
A hero is an entity, a person encompassing a state of being, yet health care professionals are bestowed this title and this burden on a near-daily basis. We are perfectly imperfect. The more in tune we are to vulnerability, the more honest we can become with ourselves and one another.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
Let us begin with a thought exercise. Close your eyes and picture the word, “hero.” What comes to mind? A relative, a teacher, a fictional character wielding a hammer or flying gracefully through the air?
Several months ago, our country was introduced to a foe that brought us to our knees. Before that time, the idea of a hero had fluctuated with circumstance and had been guided by aging and maturity; however, since the moment COVID-19 struck, a new image has emerged. Not all heroes wear capes, but some wield stethoscopes.
Over these past months the phrase, “Health Care Heroes” has spread throughout our collective consciousness, highlighted everywhere from talk shows and news media to billboards and journals. Doctors, nurses, and other health care professionals are lauded for their strength, dedication, resilience, and compassion. Citizens line up to clap, honk horns, and shower praise in recognition of those who have risked their health, sacrificed their personal lives, and committed themselves to the greater good. Yet, what does it mean to be a hero, and what is the cost of hero worship?
The focus of medical training has gradually shifted to include the physical as well as mental well-being of future physicians, but the remnants of traditional doctrine linger. Hours of focused training through study and direct clinical interaction reinforce dedication to patient care. Rewards are given for time spent and compassion lent, and research is lauded, but family time is rarely applauded. We are encouraged to do our greatest, work our hardest, be the best, rise and defeat every test. Failure (or the perception thereof) is not an option.
According to Rikinkumar S. Patel, MD, MPH, and associates, physicians have nearly twice the burnout rate of other professionals (Behav Sci. [Basel]. 2018 Nov;8[11]:98). The dedication to our craft propels excellence as well as sacrifice. When COVID-19 entered our lives, many of my colleagues did not hesitate to heed to the call for action. They immersed themselves in the ICU, led triage units, and extended work hours in the service of the sick and dying. Several were years removed from emergency/intensive care, while others were allocated from their chosen residency programs and voluntarily thrust into an environment they had never before traversed.
These individuals are praised as “brave,” “dedicated,” “selfless.” A few even provided insight into their experiences through various publications highlighting their appreciation and gratitude toward such a treacherous, albeit, tremendous experience. Even though their words are an honest perspective of life through one of the worst health care crises in 100 years, in effect, they perpetuate the noble hero; the myth of the super doctor.
In a profession that has borne witness to multiple suicides over the past few months, why do we not encourage open dialogue of our victories as well as our defeats? Our wins as much as our losses? Why does an esteemed veteran physician feel guilt over declining to provide emergency services to patients whom they have long forgotten how to manage? What drives the guilt and the self-doubt? Are we ashamed of what others will think? Is it that the fear of not living up to our cherished medical oath outweighs our own boundaries and acknowledgment of our limitations?
A hero is an entity, a person encompassing a state of being, yet health care professionals are bestowed this title and this burden on a near-daily basis. We are perfectly imperfect. The more in tune we are to vulnerability, the more honest we can become with ourselves and one another.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
AGA releases iron-deficiency anemia guideline
The American Gastroenterological Association (AGA) has released a clinical practice guideline for gastrointestinal evaluation of iron-deficiency anemia.
The seven recommendations aim to improve quality of care and reduce practice variability, according to lead author Cynthia W. Ko, MD, of the University of Washington , Seattle, and four copanelists.
First, the panel recommended that iron deficiency be defined by a serum ferritin level less than 45 ng/mL, instead of 15 ng/mL. Data from 55 studies showed that the higher cutoff value had a sensitivity of 85% and a specificity of 92%, compared with respective values of 59% and 99% for the lower threshold.
“Optimizing the threshold ferritin level with high sensitivity will detect the great majority of patients who are truly iron deficient, minimize delays in diagnostic workup, and minimize the number of patients in whom serious underlying etiologies such as gastrointestinal malignancy might be missed,” the panelists wrote. The guideline was published in Gastroenterology.
For asymptomatic postmenopausal women and men with iron-deficiency anemia, the panel recommended bidirectional endoscopy instead of no endoscopy. A similar recommendation was given for premenopausal women, calling for bidirectional endoscopy instead of iron-replacement therapy alone.
Dr. Ko and colleagues noted that these recommendations differ from those issued by the British Society of Gastroenterology (BSG).
For postmenopausal women and men, the BSG suggests that symptoms and local availability of endoscopy should inform diagnostic workup, with either colonoscopy or CT colonography used for assessment. The BSG recommends against bidirectional endoscopy in premenopausal women, unless they are older than 50 years, have a family history of colorectal cancer, or show symptoms of gastrointestinal disease.
“In contrast, the AGA recommends bidirectional endoscopy as the mainstay for gastrointestinal evaluation, particularly in men and in postmenopausal women where no other unequivocal source of iron deficiency has been identified,” the panelists wrote.
When bidirectional endoscopy does not reveal an etiology, the panelists recommended noninvasive testing for Helicobacter pylori.
“An association between H. pylori infection and iron deficiency has been demonstrated in observational studies,” noted Dr. Ko and colleagues.
They also cited three randomized controlled trials in which treating patients with iron-deficiency anemia for H. pylori infection in combination with iron replacement therapy led to a 23.2-ng/mL mean improvement in serum ferritin, compared with patients who received iron-replacement therapy alone.
According to the guideline, if asymptomatic patients with negative bidirectional endoscopy are also negative for H. pylori, then they should receive trial iron supplementation, instead of undergoing video capsule endoscopy.
“[T]he evidence required to evaluate the benefits of video capsule endoscopy in iron-deficiency anemia is not currently available,” the panelists noted.
Dr. Ko and colleagues also recommended against routine gastric biopsies to diagnose autoimmune atrophic gastritis, as earlier diagnosis does not appear to affect outcomes or management of iron-deficiency anemia.
Finally, the panelists recommended that asymptomatic patients with iron-deficiency anemia and suspected celiac disease undergo serologic testing first, with small bowel biopsy only performed if testing is positive.
The only two strong recommendations in the guideline are for the ferritin cutoff value and the use of bidirectional endoscopy in men and postmenopausal women. The other five recommendations are conditional, three of which are based on very low quality evidence. As such, the guideline includes a discussion of research needs.
Dr. Ko and colleagues called for more studies investigating the prevalence of gastrointestinal lesions and risks of bidirectional endoscopy in premenopausal women, role of fecal occult blood testing, timing of serologic testing for H. pylori and celiac disease in relation to endoscopy, patient subgroups, comparative efficacy of small bowel visualization techniques, and other topics.
The investigators disclosed no conflicts of interest. The guideline was funded by the AGA Institute.
SOURCE: Ko CW et al. Gastroenterology 2020 Aug 15. doi: 10.1053/j.gastro.2020.06.046.
The American Gastroenterological Association (AGA) has released a clinical practice guideline for gastrointestinal evaluation of iron-deficiency anemia.
The seven recommendations aim to improve quality of care and reduce practice variability, according to lead author Cynthia W. Ko, MD, of the University of Washington , Seattle, and four copanelists.
First, the panel recommended that iron deficiency be defined by a serum ferritin level less than 45 ng/mL, instead of 15 ng/mL. Data from 55 studies showed that the higher cutoff value had a sensitivity of 85% and a specificity of 92%, compared with respective values of 59% and 99% for the lower threshold.
“Optimizing the threshold ferritin level with high sensitivity will detect the great majority of patients who are truly iron deficient, minimize delays in diagnostic workup, and minimize the number of patients in whom serious underlying etiologies such as gastrointestinal malignancy might be missed,” the panelists wrote. The guideline was published in Gastroenterology.
For asymptomatic postmenopausal women and men with iron-deficiency anemia, the panel recommended bidirectional endoscopy instead of no endoscopy. A similar recommendation was given for premenopausal women, calling for bidirectional endoscopy instead of iron-replacement therapy alone.
Dr. Ko and colleagues noted that these recommendations differ from those issued by the British Society of Gastroenterology (BSG).
For postmenopausal women and men, the BSG suggests that symptoms and local availability of endoscopy should inform diagnostic workup, with either colonoscopy or CT colonography used for assessment. The BSG recommends against bidirectional endoscopy in premenopausal women, unless they are older than 50 years, have a family history of colorectal cancer, or show symptoms of gastrointestinal disease.
“In contrast, the AGA recommends bidirectional endoscopy as the mainstay for gastrointestinal evaluation, particularly in men and in postmenopausal women where no other unequivocal source of iron deficiency has been identified,” the panelists wrote.
When bidirectional endoscopy does not reveal an etiology, the panelists recommended noninvasive testing for Helicobacter pylori.
“An association between H. pylori infection and iron deficiency has been demonstrated in observational studies,” noted Dr. Ko and colleagues.
They also cited three randomized controlled trials in which treating patients with iron-deficiency anemia for H. pylori infection in combination with iron replacement therapy led to a 23.2-ng/mL mean improvement in serum ferritin, compared with patients who received iron-replacement therapy alone.
According to the guideline, if asymptomatic patients with negative bidirectional endoscopy are also negative for H. pylori, then they should receive trial iron supplementation, instead of undergoing video capsule endoscopy.
“[T]he evidence required to evaluate the benefits of video capsule endoscopy in iron-deficiency anemia is not currently available,” the panelists noted.
Dr. Ko and colleagues also recommended against routine gastric biopsies to diagnose autoimmune atrophic gastritis, as earlier diagnosis does not appear to affect outcomes or management of iron-deficiency anemia.
Finally, the panelists recommended that asymptomatic patients with iron-deficiency anemia and suspected celiac disease undergo serologic testing first, with small bowel biopsy only performed if testing is positive.
The only two strong recommendations in the guideline are for the ferritin cutoff value and the use of bidirectional endoscopy in men and postmenopausal women. The other five recommendations are conditional, three of which are based on very low quality evidence. As such, the guideline includes a discussion of research needs.
Dr. Ko and colleagues called for more studies investigating the prevalence of gastrointestinal lesions and risks of bidirectional endoscopy in premenopausal women, role of fecal occult blood testing, timing of serologic testing for H. pylori and celiac disease in relation to endoscopy, patient subgroups, comparative efficacy of small bowel visualization techniques, and other topics.
The investigators disclosed no conflicts of interest. The guideline was funded by the AGA Institute.
SOURCE: Ko CW et al. Gastroenterology 2020 Aug 15. doi: 10.1053/j.gastro.2020.06.046.
The American Gastroenterological Association (AGA) has released a clinical practice guideline for gastrointestinal evaluation of iron-deficiency anemia.
The seven recommendations aim to improve quality of care and reduce practice variability, according to lead author Cynthia W. Ko, MD, of the University of Washington , Seattle, and four copanelists.
First, the panel recommended that iron deficiency be defined by a serum ferritin level less than 45 ng/mL, instead of 15 ng/mL. Data from 55 studies showed that the higher cutoff value had a sensitivity of 85% and a specificity of 92%, compared with respective values of 59% and 99% for the lower threshold.
“Optimizing the threshold ferritin level with high sensitivity will detect the great majority of patients who are truly iron deficient, minimize delays in diagnostic workup, and minimize the number of patients in whom serious underlying etiologies such as gastrointestinal malignancy might be missed,” the panelists wrote. The guideline was published in Gastroenterology.
For asymptomatic postmenopausal women and men with iron-deficiency anemia, the panel recommended bidirectional endoscopy instead of no endoscopy. A similar recommendation was given for premenopausal women, calling for bidirectional endoscopy instead of iron-replacement therapy alone.
Dr. Ko and colleagues noted that these recommendations differ from those issued by the British Society of Gastroenterology (BSG).
For postmenopausal women and men, the BSG suggests that symptoms and local availability of endoscopy should inform diagnostic workup, with either colonoscopy or CT colonography used for assessment. The BSG recommends against bidirectional endoscopy in premenopausal women, unless they are older than 50 years, have a family history of colorectal cancer, or show symptoms of gastrointestinal disease.
“In contrast, the AGA recommends bidirectional endoscopy as the mainstay for gastrointestinal evaluation, particularly in men and in postmenopausal women where no other unequivocal source of iron deficiency has been identified,” the panelists wrote.
When bidirectional endoscopy does not reveal an etiology, the panelists recommended noninvasive testing for Helicobacter pylori.
“An association between H. pylori infection and iron deficiency has been demonstrated in observational studies,” noted Dr. Ko and colleagues.
They also cited three randomized controlled trials in which treating patients with iron-deficiency anemia for H. pylori infection in combination with iron replacement therapy led to a 23.2-ng/mL mean improvement in serum ferritin, compared with patients who received iron-replacement therapy alone.
According to the guideline, if asymptomatic patients with negative bidirectional endoscopy are also negative for H. pylori, then they should receive trial iron supplementation, instead of undergoing video capsule endoscopy.
“[T]he evidence required to evaluate the benefits of video capsule endoscopy in iron-deficiency anemia is not currently available,” the panelists noted.
Dr. Ko and colleagues also recommended against routine gastric biopsies to diagnose autoimmune atrophic gastritis, as earlier diagnosis does not appear to affect outcomes or management of iron-deficiency anemia.
Finally, the panelists recommended that asymptomatic patients with iron-deficiency anemia and suspected celiac disease undergo serologic testing first, with small bowel biopsy only performed if testing is positive.
The only two strong recommendations in the guideline are for the ferritin cutoff value and the use of bidirectional endoscopy in men and postmenopausal women. The other five recommendations are conditional, three of which are based on very low quality evidence. As such, the guideline includes a discussion of research needs.
Dr. Ko and colleagues called for more studies investigating the prevalence of gastrointestinal lesions and risks of bidirectional endoscopy in premenopausal women, role of fecal occult blood testing, timing of serologic testing for H. pylori and celiac disease in relation to endoscopy, patient subgroups, comparative efficacy of small bowel visualization techniques, and other topics.
The investigators disclosed no conflicts of interest. The guideline was funded by the AGA Institute.
SOURCE: Ko CW et al. Gastroenterology 2020 Aug 15. doi: 10.1053/j.gastro.2020.06.046.
FROM GASTROENTEROLOGY
COVID-19 impact: Less chemo, immune checkpoint inhibitors, and steroids
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
FROM JCO GLOBAL ONCOLOGY
Fetal movement education: Time to change the status quo
Every antepartum record, whether it is on paper or EMR, has a space asking whether the patient feels fetal movement at the visit. Every provider inherently knows that fetal movement is important and worth asking about at each visit. Yet the education for patients about fetal movement and when to alert a provider to changes is not currently standardized in the United States. There is no practice bulletin or guideline from the American College of Obstetricians and Gynecologists and, therefore, there is a wide variation in clinical practice. An Australian study found that 97% of women were asked about fetal movement, but only 62% reported formal education regarding fetal movement. More concerning, only 40% were advised to call immediately if concerned about fetal movement change. A quarter were told to call only if baby moved fewer than 10 times in an hour.1
We have a standardized approach to most aspects of prenatal care. We know what to do if the patient has contractions, or protein in their urine, or an increased blood pressure. Our management and education regarding fetal movement must be standardized as well. In this article I will go through the incorrect education that often is given and the data that do not support this. We need a similar care plan or model for fetal movement education in the United States.
Myth one: Kick counts
When education is done, kick counts are far and away what providers and nurses advise in the clinic and hospital triage when women present with complaint of decreased fetal movement. The standard approach to this is advising the patient to perform a kick count several times per day to check in on the baby and call if less than 10 kicks per hour. This is not bad advice as it may help create awareness for the mom about what is “normal” for her baby and may help her to “check in” on the baby when she is occupied at work or with older children. However, advising that a kick count should be done to reassure a patient about a concerning change in fetal movement is not supported in the literature. A meta-analysis in the February 2020 issue of the Green Journal found that advised kick count monitoring did not significantly reduce stillbirth risk.2 Research shows that most moms will get 10 kicks normally within an hour, but there are no data showing what percentage of moms with perceived decreased fetal movement also will get a “passing” result despite their concern. For example, take a patient who normally feels 50 movements in an hour and is not reassured by 10 movements in an hour, but because she is told that 10 movements is okay, she tries not to worry about the concerning change. Many mothers in the stillbirth community report “passing kick counts” in the days leading up to the diagnosis. We need to move away from kick count education to a much simpler plan. We must tell patients if they are worried about a concerning change in fetal movement, they should call their provider.
Myth 2: Fetuses slow down at the end of pregnancy
There is a very common myth that fetuses slow down at the end of pregnancy, especially once labor has started. A study in the Journal of Physiology continuously monitored term fetuses when mom was both awake and asleep. The study also looked at the effect on fetal heart rate and fetal activity based on different maternal positions. The study found the fetuses spent around 90% of the day with active movements and with reactive nonstress tests (NSTs).3 A 2019 study looking at fetal movement at term and preterm in third-trimester patients illustrated that fetal movement does not decrease in frequency or strength at term. It found that only 6% of patients noted decreased strength and 14% decreased frequency of movements at term. Furthermore, 59% reported an increase in strength, and nearly 39% reported an increase in frequency of fetal movements at term.4 We must educate patients that a change in frequency or strength of movements is not normal or expected, and they must call if concerned about a change.
Myth 3: Try juice, ice water, or food before coming in for evaluation
A common set of advice when a patient calls with a complaint of decreased fetal movement is to suggest a meal or something sugary, although there is little or no evidence to support this. A randomized controlled trial found maternal perception of increased fetal movement was similar among the two groups. Giving something sugary at NST also was not shown in this study to improve reactivity.5 Another randomized, double placebo blind study was done to answer the question of whether glucose via IV helped improve fetal movements and decreased the need for admission for induction or further monitoring. In this study, no difference in outcome is found.6
When a patient calls with decreased fetal movement, advice should be to come and be evaluated, not recommendation of measures like ice water, orange juice, or sugary meal because it is not supported by the literature. This incorrect message also may further the false impression that a baby who is not moving is most likely sleeping or is simply in need of sugar, not that the baby may be at risk for impending stillbirth. The Perinatal Society of Australia and New Zealand and Royal College of Obstetricians and Gynecologists have fetal movement protocol that both discourage this advice and encourage immediate evaluation of patients with complaint of concerning fetal movement change.7,8
Myth 4: An increase in fetal movement is not of concern
I used to believe that increased fetal movement is never of concern. However, the STARS study illustrated that a concerning increase in fetal movement often is noted just before the diagnosis of stillbirth. A single episode of excessively vigorous activity which often is described as frantic or crazy is associated with an odds ratio for stillbirth of 4.3. In the study, 30% of cases reported this, compared with 7% of controls.9 In our practice, we manage mothers who call with this concern the same way as a decreased fetal movement complaint, and bring the mother in immediately for evaluation.
Myth 5: Patients all know that a concerning change in fetal movement is a risk factor for stillbirth
Decreased fetal movement has been associated with an increased OR for stillbirth of 4.51.10 However, patients often do not know of this association. A study in the United States of providers and stillbirth families showed fear of anxiety kept providers from talking about stillbirth and that it still happens. Because of this patients were completely surprised by the diagnosis.11 We tell patients that stillbirth still happens because research by Dr Suzanne Pullen found that 77% of families said they never worried their baby could die outside of the first trimester. Our patients have received this information without increased anxiety and are very appreciative and reassured about the education and protocol (based on the U.K. Saving Babies Lives Care Bundle Version 2) that we have implemented in our practice.
Fact: Fetal movement education guidelines exist and are easy to implement
The practice I am a partner at has been using a formalized method for educating patients about fetal movement over the past year. As mentioned earlier the U.K. and Australia have formal fetal movement education and management guidelines.7,8 Both protocols encourage formal education around 20-24 weeks and education for the patient to call immediately with concerns; the patient should be evaluated within 2 hours of the complaint. The formal education we provide is quite simple. The Star Legacy Foundation (United States) and Still Aware (Australia) have created a simple card to educate patients.
These patient-centric materials were devised from the results of the case/control cohort STARS study by Heazell et al. The STARS study demonstrated that patient report of reduced fetal movement in the 2 weeks prior to loss was associated with an OR of 12.9 for stillbirth, that decreased strength of fetal movement was associated with stillbirth OR of 2.83, and that decreased night time activity was strongly associated with impending stillbirth (74% of cases felt their fetuses died at night).12 This card also addresses sleep position data, supported by a 2018 meta-analysis in the journal Sleep Medicine. The study identified an OR for stillbirth of 2.45 for supine sleepers with LGA or average sized babies. Furthermore, if the baby was SGA and the mother slept supine, the OR for stillbirth increased to 15.66.13
Conclusions
When I think about the patients I have cared for who have presented with a stillborn baby, I think often that they usually presented for a complaint other than decreased fetal movement such as labor check or routine prenatal visit. When asked when they last felt fetal movement they will often say days before. This does not need to happen. Protocols in Norway for fetal movement education have shown that patients call sooner with decreased fetal movement when they have received a formal education.14
Not all stillbirth can be prevented but proper education about fetal movement and not perpetuating dangerous myths about fetal movement, may keep presentations like this from happening. I hope we may soon have a formal protocol for fetal movement education, but until then, I hope some will take these educational tips to heart.
Dr. Heather Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, NY. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures.
References
1. Aust N Z J Obstet Gynaecol. 2012 Oct;52(5):445-9.
2. Obstet Gynecol. 2020 Feb;135(2):453-62.
3. J Physiol. 2017 Feb 15;595(4):1213-21.
4. PLOS One. 2019 Jun 12. doi: 10.1371/journal.pone.0217583.
5. J Matern Fetal Neonatal Med. 2013 Jun;26(9):915-9.
6. J Perinatol. 2016 Aug;36(8):598-600.
7. Aust N Z J Obstet Gynaecol. 2018 Aug;58(4):463-8.
8. Reduced fetal movements: Green top #57, Royal College of Obstetricians and Gynaecologists.
9. BMC Pregnancy Childb. 2017. doi: 10.1186/s12884-017-1555-6.
10. BMJ Open. 2018. doi: 10.1136/bmjopen-2017-020031.
11. BMC Pregnancy Childb. 2012. doi: 10.1186/1471-2393-12-137.
12. BMC Pregnancy Childb. 2015. doi: 10.1186/s12884-015-0602-4.
13. EClinicalMedicine. 2019 Apr. doi: 10.1016/j.eclinm.2019.03.014.
14. BMC Pregnancy Childb. 2009. doi: 10.1186/1471-2393-9-32.
Every antepartum record, whether it is on paper or EMR, has a space asking whether the patient feels fetal movement at the visit. Every provider inherently knows that fetal movement is important and worth asking about at each visit. Yet the education for patients about fetal movement and when to alert a provider to changes is not currently standardized in the United States. There is no practice bulletin or guideline from the American College of Obstetricians and Gynecologists and, therefore, there is a wide variation in clinical practice. An Australian study found that 97% of women were asked about fetal movement, but only 62% reported formal education regarding fetal movement. More concerning, only 40% were advised to call immediately if concerned about fetal movement change. A quarter were told to call only if baby moved fewer than 10 times in an hour.1
We have a standardized approach to most aspects of prenatal care. We know what to do if the patient has contractions, or protein in their urine, or an increased blood pressure. Our management and education regarding fetal movement must be standardized as well. In this article I will go through the incorrect education that often is given and the data that do not support this. We need a similar care plan or model for fetal movement education in the United States.
Myth one: Kick counts
When education is done, kick counts are far and away what providers and nurses advise in the clinic and hospital triage when women present with complaint of decreased fetal movement. The standard approach to this is advising the patient to perform a kick count several times per day to check in on the baby and call if less than 10 kicks per hour. This is not bad advice as it may help create awareness for the mom about what is “normal” for her baby and may help her to “check in” on the baby when she is occupied at work or with older children. However, advising that a kick count should be done to reassure a patient about a concerning change in fetal movement is not supported in the literature. A meta-analysis in the February 2020 issue of the Green Journal found that advised kick count monitoring did not significantly reduce stillbirth risk.2 Research shows that most moms will get 10 kicks normally within an hour, but there are no data showing what percentage of moms with perceived decreased fetal movement also will get a “passing” result despite their concern. For example, take a patient who normally feels 50 movements in an hour and is not reassured by 10 movements in an hour, but because she is told that 10 movements is okay, she tries not to worry about the concerning change. Many mothers in the stillbirth community report “passing kick counts” in the days leading up to the diagnosis. We need to move away from kick count education to a much simpler plan. We must tell patients if they are worried about a concerning change in fetal movement, they should call their provider.
Myth 2: Fetuses slow down at the end of pregnancy
There is a very common myth that fetuses slow down at the end of pregnancy, especially once labor has started. A study in the Journal of Physiology continuously monitored term fetuses when mom was both awake and asleep. The study also looked at the effect on fetal heart rate and fetal activity based on different maternal positions. The study found the fetuses spent around 90% of the day with active movements and with reactive nonstress tests (NSTs).3 A 2019 study looking at fetal movement at term and preterm in third-trimester patients illustrated that fetal movement does not decrease in frequency or strength at term. It found that only 6% of patients noted decreased strength and 14% decreased frequency of movements at term. Furthermore, 59% reported an increase in strength, and nearly 39% reported an increase in frequency of fetal movements at term.4 We must educate patients that a change in frequency or strength of movements is not normal or expected, and they must call if concerned about a change.
Myth 3: Try juice, ice water, or food before coming in for evaluation
A common set of advice when a patient calls with a complaint of decreased fetal movement is to suggest a meal or something sugary, although there is little or no evidence to support this. A randomized controlled trial found maternal perception of increased fetal movement was similar among the two groups. Giving something sugary at NST also was not shown in this study to improve reactivity.5 Another randomized, double placebo blind study was done to answer the question of whether glucose via IV helped improve fetal movements and decreased the need for admission for induction or further monitoring. In this study, no difference in outcome is found.6
When a patient calls with decreased fetal movement, advice should be to come and be evaluated, not recommendation of measures like ice water, orange juice, or sugary meal because it is not supported by the literature. This incorrect message also may further the false impression that a baby who is not moving is most likely sleeping or is simply in need of sugar, not that the baby may be at risk for impending stillbirth. The Perinatal Society of Australia and New Zealand and Royal College of Obstetricians and Gynecologists have fetal movement protocol that both discourage this advice and encourage immediate evaluation of patients with complaint of concerning fetal movement change.7,8
Myth 4: An increase in fetal movement is not of concern
I used to believe that increased fetal movement is never of concern. However, the STARS study illustrated that a concerning increase in fetal movement often is noted just before the diagnosis of stillbirth. A single episode of excessively vigorous activity which often is described as frantic or crazy is associated with an odds ratio for stillbirth of 4.3. In the study, 30% of cases reported this, compared with 7% of controls.9 In our practice, we manage mothers who call with this concern the same way as a decreased fetal movement complaint, and bring the mother in immediately for evaluation.
Myth 5: Patients all know that a concerning change in fetal movement is a risk factor for stillbirth
Decreased fetal movement has been associated with an increased OR for stillbirth of 4.51.10 However, patients often do not know of this association. A study in the United States of providers and stillbirth families showed fear of anxiety kept providers from talking about stillbirth and that it still happens. Because of this patients were completely surprised by the diagnosis.11 We tell patients that stillbirth still happens because research by Dr Suzanne Pullen found that 77% of families said they never worried their baby could die outside of the first trimester. Our patients have received this information without increased anxiety and are very appreciative and reassured about the education and protocol (based on the U.K. Saving Babies Lives Care Bundle Version 2) that we have implemented in our practice.
Fact: Fetal movement education guidelines exist and are easy to implement
The practice I am a partner at has been using a formalized method for educating patients about fetal movement over the past year. As mentioned earlier the U.K. and Australia have formal fetal movement education and management guidelines.7,8 Both protocols encourage formal education around 20-24 weeks and education for the patient to call immediately with concerns; the patient should be evaluated within 2 hours of the complaint. The formal education we provide is quite simple. The Star Legacy Foundation (United States) and Still Aware (Australia) have created a simple card to educate patients.
These patient-centric materials were devised from the results of the case/control cohort STARS study by Heazell et al. The STARS study demonstrated that patient report of reduced fetal movement in the 2 weeks prior to loss was associated with an OR of 12.9 for stillbirth, that decreased strength of fetal movement was associated with stillbirth OR of 2.83, and that decreased night time activity was strongly associated with impending stillbirth (74% of cases felt their fetuses died at night).12 This card also addresses sleep position data, supported by a 2018 meta-analysis in the journal Sleep Medicine. The study identified an OR for stillbirth of 2.45 for supine sleepers with LGA or average sized babies. Furthermore, if the baby was SGA and the mother slept supine, the OR for stillbirth increased to 15.66.13
Conclusions
When I think about the patients I have cared for who have presented with a stillborn baby, I think often that they usually presented for a complaint other than decreased fetal movement such as labor check or routine prenatal visit. When asked when they last felt fetal movement they will often say days before. This does not need to happen. Protocols in Norway for fetal movement education have shown that patients call sooner with decreased fetal movement when they have received a formal education.14
Not all stillbirth can be prevented but proper education about fetal movement and not perpetuating dangerous myths about fetal movement, may keep presentations like this from happening. I hope we may soon have a formal protocol for fetal movement education, but until then, I hope some will take these educational tips to heart.
Dr. Heather Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, NY. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures.
References
1. Aust N Z J Obstet Gynaecol. 2012 Oct;52(5):445-9.
2. Obstet Gynecol. 2020 Feb;135(2):453-62.
3. J Physiol. 2017 Feb 15;595(4):1213-21.
4. PLOS One. 2019 Jun 12. doi: 10.1371/journal.pone.0217583.
5. J Matern Fetal Neonatal Med. 2013 Jun;26(9):915-9.
6. J Perinatol. 2016 Aug;36(8):598-600.
7. Aust N Z J Obstet Gynaecol. 2018 Aug;58(4):463-8.
8. Reduced fetal movements: Green top #57, Royal College of Obstetricians and Gynaecologists.
9. BMC Pregnancy Childb. 2017. doi: 10.1186/s12884-017-1555-6.
10. BMJ Open. 2018. doi: 10.1136/bmjopen-2017-020031.
11. BMC Pregnancy Childb. 2012. doi: 10.1186/1471-2393-12-137.
12. BMC Pregnancy Childb. 2015. doi: 10.1186/s12884-015-0602-4.
13. EClinicalMedicine. 2019 Apr. doi: 10.1016/j.eclinm.2019.03.014.
14. BMC Pregnancy Childb. 2009. doi: 10.1186/1471-2393-9-32.
Every antepartum record, whether it is on paper or EMR, has a space asking whether the patient feels fetal movement at the visit. Every provider inherently knows that fetal movement is important and worth asking about at each visit. Yet the education for patients about fetal movement and when to alert a provider to changes is not currently standardized in the United States. There is no practice bulletin or guideline from the American College of Obstetricians and Gynecologists and, therefore, there is a wide variation in clinical practice. An Australian study found that 97% of women were asked about fetal movement, but only 62% reported formal education regarding fetal movement. More concerning, only 40% were advised to call immediately if concerned about fetal movement change. A quarter were told to call only if baby moved fewer than 10 times in an hour.1
We have a standardized approach to most aspects of prenatal care. We know what to do if the patient has contractions, or protein in their urine, or an increased blood pressure. Our management and education regarding fetal movement must be standardized as well. In this article I will go through the incorrect education that often is given and the data that do not support this. We need a similar care plan or model for fetal movement education in the United States.
Myth one: Kick counts
When education is done, kick counts are far and away what providers and nurses advise in the clinic and hospital triage when women present with complaint of decreased fetal movement. The standard approach to this is advising the patient to perform a kick count several times per day to check in on the baby and call if less than 10 kicks per hour. This is not bad advice as it may help create awareness for the mom about what is “normal” for her baby and may help her to “check in” on the baby when she is occupied at work or with older children. However, advising that a kick count should be done to reassure a patient about a concerning change in fetal movement is not supported in the literature. A meta-analysis in the February 2020 issue of the Green Journal found that advised kick count monitoring did not significantly reduce stillbirth risk.2 Research shows that most moms will get 10 kicks normally within an hour, but there are no data showing what percentage of moms with perceived decreased fetal movement also will get a “passing” result despite their concern. For example, take a patient who normally feels 50 movements in an hour and is not reassured by 10 movements in an hour, but because she is told that 10 movements is okay, she tries not to worry about the concerning change. Many mothers in the stillbirth community report “passing kick counts” in the days leading up to the diagnosis. We need to move away from kick count education to a much simpler plan. We must tell patients if they are worried about a concerning change in fetal movement, they should call their provider.
Myth 2: Fetuses slow down at the end of pregnancy
There is a very common myth that fetuses slow down at the end of pregnancy, especially once labor has started. A study in the Journal of Physiology continuously monitored term fetuses when mom was both awake and asleep. The study also looked at the effect on fetal heart rate and fetal activity based on different maternal positions. The study found the fetuses spent around 90% of the day with active movements and with reactive nonstress tests (NSTs).3 A 2019 study looking at fetal movement at term and preterm in third-trimester patients illustrated that fetal movement does not decrease in frequency or strength at term. It found that only 6% of patients noted decreased strength and 14% decreased frequency of movements at term. Furthermore, 59% reported an increase in strength, and nearly 39% reported an increase in frequency of fetal movements at term.4 We must educate patients that a change in frequency or strength of movements is not normal or expected, and they must call if concerned about a change.
Myth 3: Try juice, ice water, or food before coming in for evaluation
A common set of advice when a patient calls with a complaint of decreased fetal movement is to suggest a meal or something sugary, although there is little or no evidence to support this. A randomized controlled trial found maternal perception of increased fetal movement was similar among the two groups. Giving something sugary at NST also was not shown in this study to improve reactivity.5 Another randomized, double placebo blind study was done to answer the question of whether glucose via IV helped improve fetal movements and decreased the need for admission for induction or further monitoring. In this study, no difference in outcome is found.6
When a patient calls with decreased fetal movement, advice should be to come and be evaluated, not recommendation of measures like ice water, orange juice, or sugary meal because it is not supported by the literature. This incorrect message also may further the false impression that a baby who is not moving is most likely sleeping or is simply in need of sugar, not that the baby may be at risk for impending stillbirth. The Perinatal Society of Australia and New Zealand and Royal College of Obstetricians and Gynecologists have fetal movement protocol that both discourage this advice and encourage immediate evaluation of patients with complaint of concerning fetal movement change.7,8
Myth 4: An increase in fetal movement is not of concern
I used to believe that increased fetal movement is never of concern. However, the STARS study illustrated that a concerning increase in fetal movement often is noted just before the diagnosis of stillbirth. A single episode of excessively vigorous activity which often is described as frantic or crazy is associated with an odds ratio for stillbirth of 4.3. In the study, 30% of cases reported this, compared with 7% of controls.9 In our practice, we manage mothers who call with this concern the same way as a decreased fetal movement complaint, and bring the mother in immediately for evaluation.
Myth 5: Patients all know that a concerning change in fetal movement is a risk factor for stillbirth
Decreased fetal movement has been associated with an increased OR for stillbirth of 4.51.10 However, patients often do not know of this association. A study in the United States of providers and stillbirth families showed fear of anxiety kept providers from talking about stillbirth and that it still happens. Because of this patients were completely surprised by the diagnosis.11 We tell patients that stillbirth still happens because research by Dr Suzanne Pullen found that 77% of families said they never worried their baby could die outside of the first trimester. Our patients have received this information without increased anxiety and are very appreciative and reassured about the education and protocol (based on the U.K. Saving Babies Lives Care Bundle Version 2) that we have implemented in our practice.
Fact: Fetal movement education guidelines exist and are easy to implement
The practice I am a partner at has been using a formalized method for educating patients about fetal movement over the past year. As mentioned earlier the U.K. and Australia have formal fetal movement education and management guidelines.7,8 Both protocols encourage formal education around 20-24 weeks and education for the patient to call immediately with concerns; the patient should be evaluated within 2 hours of the complaint. The formal education we provide is quite simple. The Star Legacy Foundation (United States) and Still Aware (Australia) have created a simple card to educate patients.
These patient-centric materials were devised from the results of the case/control cohort STARS study by Heazell et al. The STARS study demonstrated that patient report of reduced fetal movement in the 2 weeks prior to loss was associated with an OR of 12.9 for stillbirth, that decreased strength of fetal movement was associated with stillbirth OR of 2.83, and that decreased night time activity was strongly associated with impending stillbirth (74% of cases felt their fetuses died at night).12 This card also addresses sleep position data, supported by a 2018 meta-analysis in the journal Sleep Medicine. The study identified an OR for stillbirth of 2.45 for supine sleepers with LGA or average sized babies. Furthermore, if the baby was SGA and the mother slept supine, the OR for stillbirth increased to 15.66.13
Conclusions
When I think about the patients I have cared for who have presented with a stillborn baby, I think often that they usually presented for a complaint other than decreased fetal movement such as labor check or routine prenatal visit. When asked when they last felt fetal movement they will often say days before. This does not need to happen. Protocols in Norway for fetal movement education have shown that patients call sooner with decreased fetal movement when they have received a formal education.14
Not all stillbirth can be prevented but proper education about fetal movement and not perpetuating dangerous myths about fetal movement, may keep presentations like this from happening. I hope we may soon have a formal protocol for fetal movement education, but until then, I hope some will take these educational tips to heart.
Dr. Heather Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, NY. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures.
References
1. Aust N Z J Obstet Gynaecol. 2012 Oct;52(5):445-9.
2. Obstet Gynecol. 2020 Feb;135(2):453-62.
3. J Physiol. 2017 Feb 15;595(4):1213-21.
4. PLOS One. 2019 Jun 12. doi: 10.1371/journal.pone.0217583.
5. J Matern Fetal Neonatal Med. 2013 Jun;26(9):915-9.
6. J Perinatol. 2016 Aug;36(8):598-600.
7. Aust N Z J Obstet Gynaecol. 2018 Aug;58(4):463-8.
8. Reduced fetal movements: Green top #57, Royal College of Obstetricians and Gynaecologists.
9. BMC Pregnancy Childb. 2017. doi: 10.1186/s12884-017-1555-6.
10. BMJ Open. 2018. doi: 10.1136/bmjopen-2017-020031.
11. BMC Pregnancy Childb. 2012. doi: 10.1186/1471-2393-12-137.
12. BMC Pregnancy Childb. 2015. doi: 10.1186/s12884-015-0602-4.
13. EClinicalMedicine. 2019 Apr. doi: 10.1016/j.eclinm.2019.03.014.
14. BMC Pregnancy Childb. 2009. doi: 10.1186/1471-2393-9-32.
How to truly connect with your patients
Introducing the ‘6H model’
I vividly remember the conversation that changed the way I practice medicine today.
During my medicine residency rounds, my attending at a Veterans Affairs hospital stated: “Remember Swati, there are three simple steps to gain your patients’ trust. The three questions they have are: No. 1, who are you? No. 2, are you any good? No. 3, do you really care about me?”
The first two questions are easier to address. The third question requires us bare our authentic human self often hiding behind our white coat and medical degree.
Who are you?
- Introduce yourself (everyone is wearing scrubs/white coats – state your full name and title)
- Describe your role in patient’s care plan
- Hand them your card (your name, photo, and a short description of the role of a hospitalist)
Are you any good?
- Briefly address your professional experience
- Explicitly state all the hard work you have done prior to entering the patient’s room (reviewing past medical records, hand off from ED provider or prior hospitalist)
- State your aim to collaborate with all people involved – their primary care provider, nurse, consultant
“Hello Mrs. Jones, my name is Dr. Swati Mehta. I will be your physician today. As a hospitalist, my role is to take care of your medical needs & worries. I will coordinate with your consultants, primary care physician, and other care teams to get you the answers you need. I have been working at XYZ Hospital for 6 years and have over 12 years of experience in medicine taking care of patients. I have reviewed your medical records, blood work, and x-rays before coming in. How are you feeling today? Do you mind if I ask you a few questions?”
Addressing the third question – Do you really care about me? – is the foundation of every human interaction. Answering this question involves addressing our patients’ many fears: Do you care about what I think is going on with my disease? Will you judge me by my socioeconomic status, gender, color of my skin, or addictions? Am I safe to open up and trust you? Are we equal partners in my health care journey? Do you really care?
A successful connection is achieved when we create a space of psychological safety and mutual respect. Once that happens, our patients open up to let us in their world and become more amenable to our opinion and recommendations. That is when true healing begins.
The “6H model” is an aide to form a strong human-centric connection.
The 6H model: Human connection with patients
Looking back at each patient interaction, good or bad, I have had in my almost 2 decades of practicing clinical medicine, the 6H model has brought me closer to my patients. We have formed a bond which has helped them navigate their arduous hospital journey, including medical and financial burdens, social and emotional needs. Utilizing this model, we were fortunate to receive the highest HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) Survey scores for 3 consecutive years while I served as the medical director of a 40-provider hospitalist program in a busy 450-bed hospital in Oregon.
In 2020, we are in the process of embedding the 6H model in several hospitalist programs across California. We are optimistic this intuitive approach will strengthen patient-provider relationships and ultimately improve HCAHPS scores.
To form an authentic connection with our patients doesn’t necessary require a lot of our time. Hardwiring the 6H approach when addressing our patients’ three questions is the key. The answers can change slightly, but the core message remains the same.
While we might not have much influence on all the factors that make or break our patients’ experience, the patient encounter is where we can truly make a difference. Consider using this 6H model in your next clinical shift. Human connection in health care is the need of the hour. Let’s bring “care” back to health care.
Dr. Mehta is director of quality & performance and patient experience at Vituity in Emeryville, Calif., and vice chair of the SHM patient experience committee.
Introducing the ‘6H model’
Introducing the ‘6H model’
I vividly remember the conversation that changed the way I practice medicine today.
During my medicine residency rounds, my attending at a Veterans Affairs hospital stated: “Remember Swati, there are three simple steps to gain your patients’ trust. The three questions they have are: No. 1, who are you? No. 2, are you any good? No. 3, do you really care about me?”
The first two questions are easier to address. The third question requires us bare our authentic human self often hiding behind our white coat and medical degree.
Who are you?
- Introduce yourself (everyone is wearing scrubs/white coats – state your full name and title)
- Describe your role in patient’s care plan
- Hand them your card (your name, photo, and a short description of the role of a hospitalist)
Are you any good?
- Briefly address your professional experience
- Explicitly state all the hard work you have done prior to entering the patient’s room (reviewing past medical records, hand off from ED provider or prior hospitalist)
- State your aim to collaborate with all people involved – their primary care provider, nurse, consultant
“Hello Mrs. Jones, my name is Dr. Swati Mehta. I will be your physician today. As a hospitalist, my role is to take care of your medical needs & worries. I will coordinate with your consultants, primary care physician, and other care teams to get you the answers you need. I have been working at XYZ Hospital for 6 years and have over 12 years of experience in medicine taking care of patients. I have reviewed your medical records, blood work, and x-rays before coming in. How are you feeling today? Do you mind if I ask you a few questions?”
Addressing the third question – Do you really care about me? – is the foundation of every human interaction. Answering this question involves addressing our patients’ many fears: Do you care about what I think is going on with my disease? Will you judge me by my socioeconomic status, gender, color of my skin, or addictions? Am I safe to open up and trust you? Are we equal partners in my health care journey? Do you really care?
A successful connection is achieved when we create a space of psychological safety and mutual respect. Once that happens, our patients open up to let us in their world and become more amenable to our opinion and recommendations. That is when true healing begins.
The “6H model” is an aide to form a strong human-centric connection.
The 6H model: Human connection with patients
Looking back at each patient interaction, good or bad, I have had in my almost 2 decades of practicing clinical medicine, the 6H model has brought me closer to my patients. We have formed a bond which has helped them navigate their arduous hospital journey, including medical and financial burdens, social and emotional needs. Utilizing this model, we were fortunate to receive the highest HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) Survey scores for 3 consecutive years while I served as the medical director of a 40-provider hospitalist program in a busy 450-bed hospital in Oregon.
In 2020, we are in the process of embedding the 6H model in several hospitalist programs across California. We are optimistic this intuitive approach will strengthen patient-provider relationships and ultimately improve HCAHPS scores.
To form an authentic connection with our patients doesn’t necessary require a lot of our time. Hardwiring the 6H approach when addressing our patients’ three questions is the key. The answers can change slightly, but the core message remains the same.
While we might not have much influence on all the factors that make or break our patients’ experience, the patient encounter is where we can truly make a difference. Consider using this 6H model in your next clinical shift. Human connection in health care is the need of the hour. Let’s bring “care” back to health care.
Dr. Mehta is director of quality & performance and patient experience at Vituity in Emeryville, Calif., and vice chair of the SHM patient experience committee.
I vividly remember the conversation that changed the way I practice medicine today.
During my medicine residency rounds, my attending at a Veterans Affairs hospital stated: “Remember Swati, there are three simple steps to gain your patients’ trust. The three questions they have are: No. 1, who are you? No. 2, are you any good? No. 3, do you really care about me?”
The first two questions are easier to address. The third question requires us bare our authentic human self often hiding behind our white coat and medical degree.
Who are you?
- Introduce yourself (everyone is wearing scrubs/white coats – state your full name and title)
- Describe your role in patient’s care plan
- Hand them your card (your name, photo, and a short description of the role of a hospitalist)
Are you any good?
- Briefly address your professional experience
- Explicitly state all the hard work you have done prior to entering the patient’s room (reviewing past medical records, hand off from ED provider or prior hospitalist)
- State your aim to collaborate with all people involved – their primary care provider, nurse, consultant
“Hello Mrs. Jones, my name is Dr. Swati Mehta. I will be your physician today. As a hospitalist, my role is to take care of your medical needs & worries. I will coordinate with your consultants, primary care physician, and other care teams to get you the answers you need. I have been working at XYZ Hospital for 6 years and have over 12 years of experience in medicine taking care of patients. I have reviewed your medical records, blood work, and x-rays before coming in. How are you feeling today? Do you mind if I ask you a few questions?”
Addressing the third question – Do you really care about me? – is the foundation of every human interaction. Answering this question involves addressing our patients’ many fears: Do you care about what I think is going on with my disease? Will you judge me by my socioeconomic status, gender, color of my skin, or addictions? Am I safe to open up and trust you? Are we equal partners in my health care journey? Do you really care?
A successful connection is achieved when we create a space of psychological safety and mutual respect. Once that happens, our patients open up to let us in their world and become more amenable to our opinion and recommendations. That is when true healing begins.
The “6H model” is an aide to form a strong human-centric connection.
The 6H model: Human connection with patients
Looking back at each patient interaction, good or bad, I have had in my almost 2 decades of practicing clinical medicine, the 6H model has brought me closer to my patients. We have formed a bond which has helped them navigate their arduous hospital journey, including medical and financial burdens, social and emotional needs. Utilizing this model, we were fortunate to receive the highest HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) Survey scores for 3 consecutive years while I served as the medical director of a 40-provider hospitalist program in a busy 450-bed hospital in Oregon.
In 2020, we are in the process of embedding the 6H model in several hospitalist programs across California. We are optimistic this intuitive approach will strengthen patient-provider relationships and ultimately improve HCAHPS scores.
To form an authentic connection with our patients doesn’t necessary require a lot of our time. Hardwiring the 6H approach when addressing our patients’ three questions is the key. The answers can change slightly, but the core message remains the same.
While we might not have much influence on all the factors that make or break our patients’ experience, the patient encounter is where we can truly make a difference. Consider using this 6H model in your next clinical shift. Human connection in health care is the need of the hour. Let’s bring “care” back to health care.
Dr. Mehta is director of quality & performance and patient experience at Vituity in Emeryville, Calif., and vice chair of the SHM patient experience committee.